Page 117
Appendix A
Application of the Recommended Evaluative Process to Specific Chemicals
This appendix demonstrates the evaluative process described in Chapter 2 and Chapter 3. To do that, the Subcommittee on Reproductive and Developmental Toxicology evaluated two agents of interest to the Navy, jet fuel JP-8 and 1,1,1,2-tetrafluoroethane (HFC-134a). The Navy is concerned about the health effects, including reproductive and developmental effects, of exposure to these agents. JP-8 was selected because it is a complex mixture and because it illustrates many of the problems that attend characterization of toxic substances: There is a sparse database on the mixture and on many of its individual components, composition varies between lots, and there are few data on human exposure. Because of the wide range of environmental conditions of human exposure (e.g., extreme cold to extreme heat, variable humidity), the actual exposure to aerosolized or vaporized components of the fuel varies with the environmental circumstance. The subcommittee evaluated the toxicity of JP-8 only under standard conditions. A complete assessment by the Navy would require an evaluation of each component under the full range of environmental conditions in which human exposures occur. A number of toxicity studies, including reproductive and developmental toxicity studies, have been conducted on HFC-134a. HFC-134a was selected because more data are available for this compound than for JP8.
Page 118
Additional examples of the application of the evaluative process to specific agents can be found in the literature (Moore et al. 1995b; 1997); An Assessment of Boric Acid and Borax Using the IEHR Evaluative process For Assessing Human Developmental and Reproductive Toxicity of Agents. Reproductive Toxicology, 11(1): 123-160; and An Assessment of Lithium Using the IEHR Evaluative process For Assessing Human Developmental and Reproductive Toxicity of Agents. Reproductive Toxicology, 9(2):175-210.
JP-8 JET FUEL
Jet fuel JP-8 (jet propellant-8) is a kerosene-based distillate selected by the U.S. Air Force to replace JP-4 and other predecessors, which were replaced because JP-8 has a higher flash point, is composed of higher chain hydrocarbons, and does not contain benzene. Profiles for JP-8 list the following classes of compounds exclusive of additives: alkanes (43% by weight); cycloalkanes (11%); alkylbenzenes (12%); naphthalenes (2%); and dicycloparaffins, tetralins, and olefins (% not specified) (USAF 1991). A more detailed list of hydrocarbon components is given in Table A-1.
Another jet fuel, JP-5, is physically and chemically similar to JP-8, and the differences between these fuels are considered minor (ATSDR 1998). Several studies described below were conducted using JP-5.
Exposure Data
Human exposure to JP-8 occurs during refueling and defueling operations and during mechanical activities that deal with storage, transfer, and combustion. Military personnel can be exposed to JP-8 by the inhalation (of aerosolized or vaporized fuel), dermal, and oral routes of exposure.
Occupational standards for JP-8 are primarily based on knowledge about the toxicity of kerosene and naphtha (a petroleum distillate fraction). National Institute for Occupational Safety and Health (NIOSH) guidelines include an 8-hour (hr) time-weighted-average recommended exposure limit (TWA-REL) for naphtha of 400 milligrams per cubic meter (mg/m3) (100 parts per million (ppm)) (NIOSH
Page 119
|
Hydrocarbon Type |
Weight % JP-8a |
|
Isooctane |
3.66 |
|
Methylcyclohexane |
3.51 |
|
m-Xylene |
3.95 |
|
Cyclooctane |
4.54 |
|
Decane |
16.08 |
|
Butylbenzene |
4.72 |
|
1,2,4,5-Tetramethylbenzene |
4.28 |
|
Tetralin |
4.14 |
|
Dodecane |
22.54 |
|
1-Methylnapthalene |
3.49 |
|
Tetradecane |
16.87 |
|
Hexadecane |
12.22 |
a Composition of surrogate JP-8 (USAF 1991).
1999). Naval Occupational Safety and Health recommends a permissible exposure limit (PEL) of 350 mg/m3 and a 15-minute (min) short term exposure limit (STEL) of 1,000 mg/m3 (D.T. Harris et al. 1997). Puhala et al. (1997) reported measurement of jet fuel vapors at three domestic Air Force installations. Breathing-zone samples were collected from workers involved in aircraft maintenance, fuel handling, and flightline positions. Exposures at the base that used only JP-8 are listed in Table A-2. Each exposure fell below the American Conference of Governmental Industrial Hygienists (ACGIH) TWA threshold limit values (TLVs) for the chemicals analyzed. Two recent studies measured exposure of Air Force personnel to jet fuels, including JP-8. Pleil et al. (2000) used newly developed methods to collect exhaled breath from personnel at Air Force bases and then analyzed the samples for certain volatile marker compounds for JP-8 and for aromatic hydrocarbons such as benzene. The study authors found a demonstrable JP-8 exposure for all subjects, ranging from slight elevations to greater than 100-fold when compared with a control cohort. Carlton and Smith (2000) collected breathing zone samples from workers
Page 120
|
Analyte |
Overall Mean |
(n) |
Standard Deviation |
ACGIH TWA-TLV |
|
Naphthas |
0.359 |
(26) |
.556 |
300 |
|
Benzene |
0.003 |
(26) |
.003 |
10b |
|
Heptane |
0.003 |
(26) |
.006 |
400 |
|
m-Xylene |
0.005 |
(26) |
.008 |
100 |
|
o-Xylene |
0.003 |
(26) |
.004 |
100 |
|
p-Xylene |
0.004 |
(26) |
.005 |
100 |
|
Toluene |
0.006 |
(26) |
.012 |
50 |
a From Puhala et al. (1997) for base A where only JP-8 was used.
b The Occupational Safety and Health Administration PEL for benzene is 1 ppm.
during aircraft fuel tank entry and repair at 12 Air Force bases. They report that the highest 8-hr time-weighted average fuel exposure found was 1,304 mg/m3, and the highest 15-min short-term exposure was 10, 295 mg/m3.
General Toxicological and Biological Parameters
Lethality
Several case studies have reported death following accidental ingestion of kerosene by children (reviewed in ATSDR 1998). The primary cause of death is respiratory effects (lipoidal pneumonia). The lowest dose of kerosene associated with death was 1,900 milligrams per kilogram (mg/kg) body weight by a 2-year-old child. Doses ranging from 120 to 870 mg/kg and as high as 1,700 mg/kg did not lead to death in children ranging from 10 months to 5 years old.
No studies have reported death in humans associated with inhalation or dermal exposure to kerosene-based fuels.
The acute oral lethal dose for 50% of the test animals (LD50) of JP-5 in rats (Bogo et al. 1983) and of kerosene in guinea pigs and rabbits (Deichmann et al. 1944) is greater than 10 grams (g) per kg.
Page 121
Acute Studies
Acute exposure to kerosene-based fuels, such as JP-8, has been associated with respiratory, cardiovascular, ocular, neurological, immunological, renal, and dermal effects. Those studies are briefly described below and are summarized in Table A-3.
Human Studies
After inhalation of JP-5 for approximately 1-hr, two individuals experienced mild hypertension, eye irritation, and neurological effects (e.g., coordination and concentration difficulties, fatigue, headache, apparent intoxication, anorexia) and one individual experienced nausea (Porter 1990). All symptoms subsided by 4 days (d) after exposure. The concentration of JP-5 was not known. Six volunteers exposed to kerosene vapor at 140 mg/m3 for 15 min did not experience any respiratory effects (Carpenter et al. 1976).
Ingestion of kerosene by children and adults has been reported to cause pulmonary (e.g., pneumonia, bronchitis) and neurological (e.g., unconsciousness, semiconsciousness, drowsiness, restlessness, irritability) effects, tachycardia, cardiomegaly, vomiting, and increased leukocyte counts (reviewed in ATSDR 1998). Because in many cases ingestion of kerosene is accidental, the concentrations associated with specific effects are not reported. It has been estimated that respiratory distress will result from ingestion of 10-30 milliliters (mL) of kerosene (Zucker etal. 1986). Neurological effects (e.g., convulsions, coma) were observed in 2 of 78 children ingesting approximately 30 mL of kerosene; those effects were not observed in children ingesting from 3 to 20 mL.
There are no studies assessing acute dermal exposure in humans.
Experimental Animal Studies
Respiratory effects, such as bronchoconstriction, were observed in rabbits and guinea pigs exposed by inhalation to kerosene (Casaco et al. 1982; Garcia et al. 1988). The rabbits were exposed to 32,500 mg/m3 for 4-9 min, and the guinea pigs were exposed to 20,400 mg/m3 for 5 min. A study exposing mice to 20 microliters (µL) of kerosene (the only dose tested) by aspiration reported that the animals showed
Page 122
|
Study Type |
Fuel Type |
Species |
Exposure Concentration, Duration, Route |
Observed Effect |
Reference |
|
Acute |
Kerosene |
Rabbit |
32,500 mg/m3, 4-9 min, inhalation (aerosol) |
Reduction in tidal volume and dynamic lung compliance, bronchoconstriction, increase in pulmonary resistance |
Casaco et al. 1982 |
|
Acute |
Kerosene |
Guinea pig |
20,400 mg/m3, 5 min, inhalation (aerosol) |
Bronchoconstriction |
Garcia et al. 1988 |
|
Acute |
Kerosene |
Mouse |
20 µL aspiration |
Pulmonary consolidation and hemorrhage, pneumonitis, decrease in pulmonary clearance of S. aureus, increase in relative lung weight, neurological effects including lack of coordination, drowsiness, behavioral changes |
Nouri et al. 1983 |
|
Acute |
Kerosene |
Guinea pig |
3,200-8,000 mg/kg, gavage |
Mononuclear and polymorphonuclear cell infiltration and unspecified pathological lesion in the lungs |
J. Brown et al. 1974 |
|
Acute |
Kerosene |
Dog |
0.5 mL/kg, aspiration |
Increases in arterial oxygen utilization, intrapulmonary physiological shunt fraction, respiratory rate. Decreases in arterial oxygen tension, heart rate, mean arterial blood pressure |
Goodwin et al. 1988 |
Page 123
|
Acute |
Kerosene |
Rat |
8,000 - 12,000 mg/kg, gavage |
Histopathological changes in kidneys (no change in kidney weight). Neurological effects such as unsteady gait and drowsiness observed at 12,000 mg/m3, but not 8,000 mg/m3. |
Muralidhara et al. 1982 |
|
Acute |
JP-5 |
Rat |
18,912 mg/kg, gavage |
Hematological, hepatic, renal effects |
Parker et al. 1981 |
|
Acute |
JP-5 |
Rat |
24-60 mL/kg, gavage |
Hepatic effects |
Bogo et al. 1983; Mehm and Feser 1984 |
|
Acute |
JP-5 |
Rat |
19,200 mg/kg, gavage |
Renal effects such as hyaline droplets in cytoplasm of epithelial cells in proximal tubules; neurological effects such as reduction in food, water intake; dermal effects such as alopecia, congestion of the subcutis |
Bogo et al.1983 |
|
Acute |
JP-5 or JP-8 |
Rabbit |
0.5 mL, dermal (undiluted fuel) |
No effects observed |
Schultz et al. 1981 |
|
Acute |
JP-8 |
Rabbit |
Dermal, abraded and intact skin |
Slight skin irritation |
Kinkead et al. 1984 |
|
Acute |
JP-5 |
Mouse |
Concentration not reported, dermal |
Dermatitis observed |
NTP/NIH 1986 |
|
Acute |
JP-5 |
Guinea pig |
1% solution, dermal |
Mild dermal sensitization |
Cowan and Jenkins 1981a,b |
Page 124
|
Subchronic |
Kerosene |
Rat and dog |
100 mg/m3, 6 hr/d, 5 d/wk for 13 wk, inhalation |
No respiratory, cardiovascular, gastrointestinal, hematological, musculoskeletal, hepatic, renal, body weight, neurological effects observed |
Carpenter et al. 1976 |
|
Repeated |
Kerosene |
Rat |
1,100 mg/m3, 6 hr/d, 5 d/wk for 30 d, inhalation |
No hepatic effects observed |
Bogo et al. 1983 |
|
Repeated |
JP-8 |
Rat |
497 mg/m3, 1 hr/d for 7 or 28 d, inhalation (nose only) |
Increased alveolar permeability |
USAF 1994; Chen et al. 1992 |
|
Repeated |
JP-8 |
Rat |
500-1,100 mg/m3, 1 hr/d for 7, 28, 56 d, inhalation |
Lung epithelial permeability observed in rats exposed for 56 d |
USAF 1994; Hays et al. 1994 |
|
Repeated |
JP-8 |
Rat |
950 mg/m3, 1 hr/d for 28 d, inhalation |
Disruption of epithelial and endothelial structures, convoluted airways, and alveoli filled with red blood cells and fluid |
USAF 1994; Pfaff et al. 1993 |
|
Repeated |
Kerosene |
Guinea pig |
20,400-34,000 mg/m3, 15 min/d for 21 d, inhalation |
Cardiovascular effects (aortic plaques) observed |
Noa and Illnait 1987a,b |
Page 125
|
Subchronic |
JP-5 |
Rat and Dog |
150-750 mg/m3, continuous exposure for 90 d, inhalation |
Female rats exposed at 150 or 750 mg/m3 and male rats exposed at 750 mg/m3 showed increased creatinine and blood urea nitrogen; dogs exposed at 750 mg/m3 had a slight (statistically significant) decrease in hemoglobin and red blood cell count, significant decreases in serum albumin, sporadic changes in blood urea nitrogen; dogs exposed at 150 or 750 mg/m3, showed hepatic effects such as lesions, mild cloudy swelling of hepatocytes |
USAF 1978b |
|
Repeated |
Kerosene |
Rat |
Average concentrations of 58 mg/m3 and 231 mg/m3, “intermediate duration exposure” inhalation |
At 58 mg/m3, animals had decreased blood glucose; at 231 mg/m3 had increased blood lactate, pyruvate |
Starek and Vojtisek 1986 |
|
Subchronic |
JP-5 |
Mouse |
150 mg/m3, continuous exposure observed in the livers for 90 d, inhalation |
Vacuolization, hepatocellular fatty changes observed in the livers |
Gaworski et al. 1984 |
|
Subchronic |
JP-8 |
Rat and mouse |
500 mg/m3 and 1,000 mg/m3, continuous exposure 90 d, inhalation |
Kidney lesion, α-2-microglobulin protein for droplet nephropathy, observed in male rats. No exposure-related increase in either sex of either species. Condition not considered relevant to humans |
Mattie et al. 1991 |
Page 126
|
Subchronic |
JP-8 |
Rat |
750, 1,500, 3,000 mg/m3/d for 90 d, gavage |
Nephropathy, dose-related decrease in body weight, and dose-related increase in gastritits and perianal dermatitis |
Mattie et al. 1995 |
|
Repeated |
JP-8 |
Mouse |
100, 250, 500, 1,000, 2,500 mg/m3, 1 hr/d for 7 d, inhalation (aerosolized) |
Dose-related decrease in spleen and thymus organ weights and decrease in total viable cells from those organs. At 100 and 250 mg/m3, loss of total cell numbers in the lymph nodes and peripheral blood, but an increase in bone marrow total cell numbers; at 500 and 1,000 mg/m3, increase in total cell numbers in lymph nodes and peripheral blood cell numbers, but a decrease in bone marrow cell numbers. Dose-related decrease in immune function observed in animals exposed to JP-8 and then treated with Concavalin-A |
D.T. Harris et al. 1997 |
|
Repeated |
Kerosene |
Mouse |
0.1 mL/d for 1 wk, dermal |
Dermal (rough skin, edema, inflammation, dermatosis), hematological, immunological (decreases in relative weight of lymph nodes and thymus and decreases in thymus counts, bone marrow nucleated cell counts, thymic cortical lymphocytes, and cellularity of the thymic lobules), and behavioral (tactile stimuli and hyperactivity) effects. |
Upreti et al. 1989 |
Page 127
|
Repeated |
JP-5 |
Mouse |
50% or 100% 3 times/wk for 60 wk, dermal |
Renal lesions |
Easley et al. 1982 |
|
Repeated Subchronic |
JP-5 |
Mouse |
2,500-8,000 mg/kg, for 5 d/wk for 13 or 103 wk, dermal |
Dermal (rough skin, edema, inflammation, dermatosis) and hematological effects. Hepatic effects observed in the 13-wk study. Animals treated at 8,000 mg/kg had slightly decreased body weight. Granulocytic hyperplasia in bone marrow and hyperplasia in lymph nodes. |
NTP/NIH 1986 |
|
Carcinogenicity |
JP-5 |
Rat |
750 mg/m3, continuous exposure for 90 d, inhalation; animals followed for their lifetime |
No renal tumors |
Bruner 1984 |
|
Carcinogenicity |
JP-5 |
Mouse |
22.9 mg and 42.9 mg for 40 wk, dermal |
Skin tumors (type not reported) in animals exposed at 22.9 mg but not at 42.9 mg |
Schultz et al. 1981 |
|
Genotoxicity |
JP-8 |
Salmonella typhimurium |
0.001 µL/plate to 5.0 µL/plate |
Not mutagenic in 5 strains of S. typhimurium with or without metabolic activation |
USAF 1978a |
|
Genotoxicity |
JP-8 |
L5178Y Mouse lymphoma cells |
0.01 µL/mL to 0.16 µL/mL |
Gene mutations not induced in lymphoma cells at thymidine kinase locus |
USAF 1978a |
|
Genotoxicity |
JP-8 |
WI-38 cells |
5.0 µL/mL |
JP-8 induced significant increases in 3H- thymidine incorporation in WI-38 cells |
USAF 1978a |
Page 128
|
Genotoxicity |
JP-8 |
Rat and mouse |
Rat: 0.1 mL/kg, 0.3 mL/kg, 1.0 mL/kg; mouse: 0.13 mL/kg, 0.4 mL/kg, 1.3 mL/kg; in the feed for 5 d |
JP-8 negative in dominant lethal assays |
USAF 1978a |
|
Developmental toxicity |
JP-8 |
Rat |
500, 1,000, 1,500, 2,000 mg/kg/d, given on d 6 to 15 of pregnancy, oral |
Maternal weight gain decreased significantly at 1,000 mg/kg/d. Several deaths among animals exposed to JP-8 were attributed to the presence of JP-8 in the lungs. At 1,500 and 2,000 mg/kg/d, fetal weight was reduced by 12% and 25% and maternal weight gain was reduced by 70% and 85%, respectively. Number and type of fetal malformations and variations observed did not differ significantly between dose groups. Progressive increase in the overall incidence of fetal alterations reported between 500 and 1,500 mg/kg/d, but not 2,000 mg/kg/d. The 2,000 mg/kg/d group had fewer fetuses available for examination than other groups because approximately 1/3 of the dams died. One animal had totally resorbed litter. |
Cooper and Mattie 1996 |
Page 129
respiratory effects (e.g., pulmonary consolidation and hemorrhage, pneumonitis, a decrease in pulmonary clearance of Staphylococcus aureus, an increase in relative lung weight) and neurologic effects (e.g., lack of coordination, drowsiness, behavioral fraction, and respiratory rate and decreases in arterial oxygen tension, heart rate, and mean arterial blood pressure (Goodwin et al. 1988). Rats exposed to kerosene administered by gavage at single doses of up to 12,000 mg/kg were not found to exhibit cardiovascular, gastrointestinal, hematological, hepatic, or endocrine effects (Muralidhara et al. 1982). However, they did exhibit histopathological kidney changes (although no change was observed in kidney weight) and some neurological effects, including unsteady gait and drowsiness. No neurological effects were observed in rats exposed at 8,000 mg/kg. Rats exposed by gavage to a single dose of JP-5 at 18,912 mg/kg did have hematological, hepatic, and renal effects (Parker et al. 1981). Hepatic effects were observed in rats exposed by gavage to single doses of JP-5 at 24-60 mL/kg (Bogo et al. 1983; Mehm and Feser 1984). Renal (e.g., hyaline droplets in the cytoplasm of epithelial cells in the proximal tubules), neurological (e.g., reduction in food and water intake), and dermal (e.g., alopecia and congestion of the subcutis) effects were found in rats exposed to a single dose by gavage at 19,200 mg/kg of JP-5 (Bogo et al. 1983).
Rabbits exposed dermally to undiluted JP-5 or JP-8 at 0.5 mL did not show any signs of dermal effects (Schultz et al. 1981); however, in another study using rabbits dermally exposed to JP-8, slight skin irritation was observed (Kinkead et al. 1984). Also, dermal effects were observed in mice exposed to JP-5 (concentration not reported) (NTP/NIH 1986). Acute dermal exposure of mice to JP-5 at 10,000 mg/kg led to decreased body weight, but exposure at 5,000 mg/kg did not have this effect. Acute dermal exposure of guinea pigs to a 1% solution of JP-5 led to mild dermal sensitization (Cowan and Jenkins 1981a).
Repeated-Dose Studies
Repeated exposure to kerosene-based petroleum distillates, such as JP-8, has been associated with hepatic, renal, cardiovascular, neurological, and pulmonary toxicity in humans and experimental animals. These studies are described briefly and summarized in Table A-3.
Page 130
Human Studies
Struwe et al. (1983) reported that airline industry workers occupationally exposed by inhalation, oral, and dermal routes to jet fuels (type not specified) were examined for neuropsychiatric effects. Thirty employees exposed at an estimated time-weighted average of 250 mg/m3 during work for 4 to 32 years were examined. The study authors concluded that personality changes and emotional dysfunctions are effects of long-term exposures to jet fuels. The usefulness of this study for determining the general toxicity of exposure to JP-8 in the context of Naval operations is unclear for the following reasons: (1) the exposed workers studied were not a random sample but “were selected in collaboration with the management of the factory, the trade unions and the health department of the factory” and the criteria for selection are not reported; (2) the composition of the jet fuel or fuels involved is not reported, and their similarity to JP-8 is uncertain; (3) the average exposure for these workers was estimated to be in the range of the permissible exposure limit, however, 21 of the 30 workers are reported to have had recurrent acute exposures that produced symptoms such as dizziness, headache, nausea, palpitations, and feelings of suffocation; and (4) although the medical history obtained did not indicate that the exposed workers were more likely than controls to have selected confounding factors, possible confounders were not otherwise considered in the data analysis.
In an epidemiological study reported by Knave et al. (1978), factory workers chronically exposed by inhalation, oral, and dermal routes to jet fuel (fuel type not reported) were found to have significant increases in conditions such as fatigue, depression, dizziness, and sleep disturbances. Also, the workers reported a significant increase in “a feeling of heaviness” in their chests. An estimated time-weighted average of 128-423 mg/m3 jet fuel was found in the work area. A limitation of this study is that other exposures were not considered. Factory workers chronically exposed by the dermal route to kerosene for up to 5 hr/d exhibited dermatosis and erythema (Jee et al. 1985). The concentration of kerosene was not reported.
Experimental Animal Studies
Carpenter et al. (1976) exposed rats and dogs to deodorized kero-
Page 131
sene at 100 mg/m3 for 6hr/d, 5d/wk, for 13 wk by inhalation. The animals did not exhibit any respiratory, cardiovascular, gastrointestinal, hematological, musculoskeletal, hepatic, renal, body weight, or neurological effects as a result of exposure. Hepatic effects also were not observed in a study in which rats were exposed by inhalation to 1,100 mg/m3 of JP-5 for 6 hr/d, 5d/wk, for 30 d (Bogo et al. 1983).
However, other studies have reported respiratory, cardiovascular, hematological, hepatic, renal, or body weight effects associated with exposure by inhalation to kerosene-based fuels. Rats exposed nose-only at 497 mg/m3 of JP-8 for 1hr/d for 7d or for 28 d showed increased alveolar epithelial permeability (Chen et al. 1992; USAF 1994). Lung epithelial permeability in rats was affected by exposure by inhalation of JP-8 at 500 and 800 to 1,100 mg/m3 for 56 d, but not for 7 or 28 d (USAF 1994; Hays et al. 1994). Rats exposed to an average concentration of 950 mg/m3 of JP-8 for 28 d exhibited pathological changes, including disruption of epithelial and endothelial structures, convoluted airways, and alveoli filled with red blood cells and fluid (Pfaff et al. 1993; USAF 1994).
Cardiovascular effects (aortic plaques) were observed in guinea pigs exposed by inhalation to kerosene at 20,400-34,000 mg/m3 for 15 min/d for 21 d (Noa and Illnait 1987a,b). Hematological effects were observed in dogs and rats exposed by inhalation to JP-5 (USAF 1978b). The rats were exposed at 150 or 750 mg/m3 (females) and 750 mg/m3 (males) for 90 d. The dogs were exposed at 750 mg/m3 for 90 d. Changes in blood glucose, blood lactate, and pyruvate concentrations were observed in rats exposed by inhalation at an average of 58 mg/m3, 231 mg/m3, and 231 mg/m3, respectively (Starek and Vojtisek 1986). Dogs exposed to JP-5 at 150 or 750 mg/m3 for 90 d showed hepatic effects, including lesions, and mild cloudy swelling of hepatocytes (USAF 1978b). The nature of those lesions was not reported. Hepatic changes also were observed in mice exposed to JP-5 at 150 mg/m3 for 90 d (Gaworski et al. 1984).
In a study by Mattie et al. (1991), Fischer 344 rats and C57B1/6 mice were exposed to vapors of JP-8 at 0, 500, or 1,000 mg/m3 continuously for 90 d and were held for further observation as long as 21 months. The only toxicity observed was a kidney lesion, α-2u-globulin protein droplet nephropathy, specific to male rats. There was no exposure-related increase in the incidence of tumors in either sex of either species. However, it should be noted that a 3-month exposure period is
Page 132
not generally considered adequate for a rigorous evaluation of carcinogenic potential. In a subsequent study, male Sprague-Dawley rats were dosed with JP-8 at 0, 750, 1,500, 3,000 mg/kg/d by gavage for 90 d to further characterize kidney lesion and assess further toxic effects (Mattie et al. 1995). In addition to the α-2u-globulin protein droplet nephropathy observed in male rats, there was a dose-related decrease in body weight, a dose-related increase in gastritis and perianal dermatitis, and an increase in liver enzymes that was not related to the dose of JP-8. Several other studies have reported this type of nephropathy in rats treated via inhalation with jet fuels at 150 or 750 mg/m3 (Cowan and Jenkins 1981a,b; Bruner 1984; Gaworski et al. 1984; USAF 1985). Because the condition is specific to male rats, it is not relevant to humans.
D.T. Harris et al. (1997) exposed C57B1/6 mice to aerosolized JP-8 for 1 hr/d for 7 d at 0, 100, 250, 500, 1,000, 2,500 mg/m3 to determine possible immunotoxicity. Dose-related immunological effects seen at the lowest concentration (100 mg/m3) included a decrease in spleen and thymus organ weight and a decrease in total viable cells recovered from those organs. At low exposure concentrations (100 and 250 mg/m3), there was a loss of total cell numbers in the lymph nodes and peripheral blood; at higher exposure concentrations (500 and 1,000 mg/m3), there was an increase in total cell numbers. Bone marrow analysis showed that exposure to low concentrations resulted in an increase of total cell numbers and that exposure to higher concentrations resulted in a decrease in total cell numbers. To determine whether exposure to JP-8 can cause loss of immune function, splenic immune cells were examined for the ability to undergo functional responses after stimulation by a growth factor and a mitogen. Dose-related decreases in immune function were observed in mice exposed to JP-8 and stimulated with the T-cell mitogen, Concavalin-A. The authors concluded that at concentrations as low as 100 mg/m3, JP-8 can act as an immunosuppressive agent.
No studies have evaluated the toxicity of kerosene-based fuels as a result of multiple oral exposures.
Two studies tested the toxicity in mice exposed by dermal administration of kerosene at 0.1 mL/d for 1 wk (Upreti et al. 1989) and JP-5 at 2,500 to 8,000 mg/kg for 5 d/wk for 13 wk and 103 wk (NTP/NIH 1986). Hematological effects were observed at all concentrations and
Page 133
durations tested, and hepatic effects were observed at all concentrations in the 13-wk study. Dermal effects (rough skin, edema, inflammation, dermatosis) in mice were reported in both studies. No respiratory, cardiovascular, gastrointestinal, musculoskeletal, renal, or endocrine effects were reported in either study. Renal lesions were reported in mice exposed dermally to 100% or 50% JP-5 for 3 times/wk for 60 wk (Easley et al. 1982). Mice treated dermally with JP-5 at 8,000 mg/kg showed small (3-7%) changes in body weight (NTP/NIH 1986). In the study by Upreti et al. (1989), male mice (females were not tested) treated with JP-5 showed decreases in relative lymph node and thymus weight and decreases in thymocyte count, bone marrow nucleated cell count, thymic cortical lymphocytes, and the cellularity of the thymic lobules. The NTP/NIH (1986) study found induced granulocytic hyperplasia in the bone marrow and hyperplasia in the lymph nodes of mice treated with JP-5. Male mice treated with JP-5 dermally to 0.1 mL of kerosene per day for 1 wk had increased response to tactile stimuli and hyperactivity (Upreti et al. 1989).
Cancer
No epidemiology studies have been conducted to determine the carcinogenicity of JP-8 or other kerosene-based fuels.
No oral carcinogenicity studies have been conducted in experimental animals exposed to kerosene, JP-5, or JP-8. No renal tumors were observed in rats after continuous exposure to JP-5 at 750 mg/m3 for 90 d and followed for their lifetime (Bruner 1984). Skin tumors (type not reported) were observed in mice exposed dermally at 22.9 mg of JP-5 for 40 wk; however, tumors were not observed at a dose of 42.4 mg (Schultz et al. 1981).
Genetic Toxicity
The genotoxic potential of JP-8 has been evaluated in a battery of tests (USAF 1978a). JP-8 was not mutagenic in five strains of Salmonella typhimurium with or without metabolic activation. Gene mutations were not induced in L5178Y mouse lymphoma cells at the thymidine
Page 134
kinase locus. In a test for unscheduled DNA synthesis, JP-8 induced significant increases in 3H-thymidine incorporation in WI-38 cells at 5.0 µL/mL. JP-8 was negative in dominant lethal assays in mice and rats. The mice were administered the test compound in the feed for 5 d at concentrations of 0.13 mL/kg, 0.4 mL/kg, and 1.3 mL/kg. The rats were exposed via the same route and duration at concentrations of 0.1 mL/kg, 0.3 mL/kg, and 1.0 mL/kg.
Disposition and Pharmacokinetics
Because JP-8 is a complex mixture of numerous volatile hydrocarbons and other substances, it is difficult to describe the pharmaco-kinetics both of the mixture and of its components as they relate to toxicity. The pharmacokinetics of some JP-8 components are known, but the usefulness of such data is limited because some components of the mixture likely affect the kinetics of uptake, distribution, metabolism, and elimination of others in the mixture. The kinetics of the mixture also would vary by route (e.g., oral versus dermal) and condition of exposure (e.g., aerosol versus vapor).
Riviere et al. (1999) used the isolated perfused porcine skin flap model to study absorption and disposition of JP-8. The percutaneous absorption and cutaneous disposition of topically applied neat Jet-A and JP-8 jet fuels were assessed by monitoring the absorptive flux of the marker components 14C naphthalene and 3H dodecane simultaneously. Absorption of 14C hexadecane was estimated from JP-8. Data were not reported in absolute amounts or concentrations. Instead, the objectives were to determine the relative absorption of the individual marker components from jet fuel, and the effect of a specific jet fuel's composition on the absorption of a specific marker. Having evaluated the absorption of only three of the 228 major nonadditive hydrocarbon constituents of the fuels, the authors stated that this is insufficient information to conduct risk assessments on jet fuels. However, the authors' conclusions are informative. Naphthalene penetrated the skin more rapidly than dodecane or hexadecane, but the latter compounds had a larger fraction of the dose deposited in the skin. There were also differences in naphthalene and dodecane absorption and skin deposition between the fuels. These findings reinforce the difficulty of predicting risk for complex mixtures such as jet fuels.
Page 135
Reproductive and Developmental Toxicity Data
Human Studies
Two studies have been published about possible genotoxicity and male reproductive toxicity in aircraft maintenance personnel exposed to solvents, paints, and fuels (mainly JP-4; Lemasters et al. 1999a,b). A total of 50 men working on aircraft maintenance at an Air Force base were included in the studies. The subjects were divided into subgroups based on work assignment, and therefore related chemical exposure: 6 sheet metal workers, 6 painters, 15 men involved in jet fueling, and 23 flightline workers. Eight unexposed men served as a control group. All measures of chemical exposure were below 6 ppm, well below the Occupational Safety and Health Administration standards for those chemicals. Evaluation of blood lymphocytes for genotoxic changes after 15 and 30 wk exposure as measured by sister chromatid exchanges and micronuclei revealed no significant changes in either parameter among the jet fueling and flight line groups of men (Lemasters et al. 1999a). The reproductive study included measures of sperm production, structure, and function (sperm concentration, sperm motion, viability, morphology, morphometrics, and stability of sperm chromatin) after 15 and 30 wk exposure. There was an increase in sperm concentration in the jet fuel and flightline groups and a decrease in sperm linearity in the jet fuel group, but the authors concluded that exposure to jet fuel did not cause an apparent effect on semen quality for aircraft maintenance personnel (Lemasters et al. 1999b).
No human studies have been conducted to assess female reproductive or developmental toxicity caused by exposure to JP-8 or any other kerosene-based fuel.
Experimental Animal Studies
Developmental Toxicity
Cooper and Mattie (1996) reported the results of a study of the developmental toxicity of JP-8 in Sprague-Dawley rats dosed orally at 0, 500, 1,000, 1,500, 2,000 mg/kg/d on days 6-15 of pregnancy. Dams exposed to doses of 1,000 mg/kg/d or above gained significantly less
Page 136
body weight during pregnancy than did control rats. There were several maternal deaths among exposed animals that were attributed to the presence of JP-8 in the lungs. Fetal body weight at the two highest doses was significantly decreased from control weight, but those doses were associated with even greater reduction of maternal weight gain during pregnancy. Fetal weight was reduced by 12% and 25%, and maternal gestational weight gain was reduced by 70% and 85% at concentrations of 1,500 and 2,000 mg/kg/d, respectively. It is unclear if the fetal weight reduction was causally associated with reduced maternal gestational weight gain.
The number and type of fetal malformations and variations observed did not differ significantly between dose groups. A progressive increase in the overall incidence of fetal alterations (variations and malformations) with increasing dose was reported between the 500 mg/kg/d and 1,500 mg/kg/d dose groups, but not for the 2,000 mg/kg/d dose group. It should be noted that the number of fetuses and litters exposed to the highest dose (2,000 mg/kg/d) and available for examination for abnormal development was much lower than in other dose groups because approximately one-third of the dams died; one animal had a totally resorbed litter. Observed variations included dilated renal pelvis, ureter, and lateral ventricle; unossified sternebra; rudimentary 14th rib; less than four metatarsals; and external and subdural hematomas. Observed malformations included malformed sternum, missing centrum, hydronephrosis, ectopic heart, short tail, no tail, and encephalomyocoele.
No other studies have assessed the developmental toxicity of JP-8 (or other kerosene-based fuels) in experimental animals.
Reproductive Toxicity
No studies of reproductive toxicity of JP-8 (or other kerosene-based fuels) in experimental animals were found. Ancillary data from other toxicity studies do not suggest an adverse reproductive effect (no effect on fertility in dominant lethal studies in mice and rats (USAF 1978a) and no effect on testis weight or histopathology in a 90-d gavage study in rats (Mattie et al. 1995)). An increase in atrophy of seminiferous tubules in testes of male mice exposed to JP-4 by inhalation for 12
Page 137
months was considered by the authors to result from the debilitating effects of chronic skin disease in exposed mice (Bruner et al. 1993).
Integration of Toxicity and Exposure Information
Interpretation of Toxicity Data
Data to assess the potential of JP-8 to adversely affect reproduction and development are sparse. One study (Puhala et al. 1997) reported measurements of human exposures and the values for the components of jet fuels analyzed that were far below the TWA threshold limit values (see Tabel A-2). Data on the absorption of volatile hydrocarbon components of JP-8 suggest that systemic exposure is likely, by any route of exposure. The single published developmental toxicity study (Cooper and Mattie 1996) did not report an adverse effect on embryonic or fetal development in rats with oral treatment at up to 2,000 mg/kg/d on days 6-15 of pregnancy, except for a decrease in body weight of offspring.
No studies of humans or experimental animals have been done to assess reproductive performance after exposure to JP-8. There are human data that demonstrate that exposure to jet fuel (mostly JP-4) at below 6 ppm did not affect semen quality for aircraft maintenance personnel (Lemasters et al. 1999b). Ancillary studies in rats and mice (USAF 1978b; Mattie et al. 1995) did not suggest an adverse effect on reproductive organs or reproductive performance. The testicular atrophy reported in mice exposed to JP-4 (Bruner et al. 1993) might have been secondary to the debilitating effect of chronic skin disease.
Quantitative Evaluation
One study identified a NOAEL (no-observed-adverse-effect level) for a reproductive or developmental endpoint (Cooper and Mattie 1996). Rats were exposed orally at 0, 500, 1,000, 1,500, and 2,000 mg/kg/d on days 6-15 of pregnancy. There was a significant decrease in fetal body weight in rats exposed to JP-8 at high doses (1,500 and 2,000 mg/kg/d). No effect on fetal body weight was observed after
Page 138
exposure at 1,000 mg/kg/day, and the authors identified that dose as the fetal NOAEL.
Maternal toxicity in the form of death and decreased body weight occurred at the same doses at which developmental toxicity (decreased fetal body weight) was observed. No effect on maternal body weight was observed after exposure at 500 mg/kg/d, and that dose was identified as the maternal NOAEL. Whether the developmental toxicity is directly related to the maternal toxicity or is independent of the effects on the mothers is not known.
Data on comparative pharmacokinetics are sparse; there are no data to support a conclusion that adverse reproductive or developmental toxic effects in rats or mice are not predictive of some adverse effect in humans. Thus, it is accepted by default that animal data are relevant to humans.
Estimates of human exposure to JP-8 do not provide documentation of exposures to individual components of JP-8. Studies have not been done to determine which components of JP-8 might account for its toxicity. Thus, the value of calculation of a margin of exposure for JP-8 is questionable because knowledge of the composition of JP-8 might not accurately predict the relative exposure to components of JP-8 at the tissue level.
The subcommittee chose the NOAEL of 1,000 mg/kg/d to calculate an unlikely effect level (UEL) for developmental toxicity. The aggregate uncertainty factor for human sensitivity is 1,000 (10 for inter-individual variation, 10 for extrapolation from rats to humans, 10 for an incomplete data set). The UEL is calculated by dividing the NOAEL by the aggregate uncertainty factor for human sensitivity:

~ enlarge ~
The UEL is only for effects that are observed at birth and only for a short term exposure. No long-term follow-up studies (e.g., on neurotoxicity) have been conducted. UELs for other reproductive endpoints cannot be calculated. A UEL for chronic exposure to JP-8 was not calculated because there are no chronic toxicity studies on JP-8 reported in the literature. Conversion from mg/kg/day by the oral route to the equivalent concentration in inhaled air to achieve the same daily
Page 139
dose determines that the 1 m/kg/d UEL is equivalent to 1.5 ppm for rats (assume 8 hr/d exposure, 100% absorption, 185 g body weight, respiratory minute volume of 0.76 mL/min/g body weight), and 0.8 ppm for humans (assume 8 hr/d exposure, 100% absorption, 69 kg body weight, respiratory minute volume of 0.42 mL/min/kg body weight).
Critical Data Needs
Data on the toxicity and disposition of JP-8 in animals are sparse, and no data are available for humans. No reproductive toxicity studies have been done in experimental animals. One adequate study demonstrated developmental toxicity in rats treated orally at 1,500-2,000 mg/kg/d (Cooper and Mattie 1996). A study in a second species should be supplemented with a multiple-generation reproductive toxicity study in rats or mice, including an evaluation of postnatal endpoints, such as developmental neurotoxicity, immunotoxicity, and hematological, hepatic, and renal effects, that could result from prenatal exposures.
Uncertainty about the toxicity of JP-8 could be reduced as follows:
-
The toxicity of individual components of the fuel should be assessed from the literature to determine whether exposure to any of the components is known to produce reproductive or developmental toxicity in animals or humans.
-
The pharmacokinetics of known components of the fuel should be assessed to better define exposure, placental transfer, bioaccumulation, and other factors relevant to toxicity.
-
Research should determine systemic exposure from the various relevant modes of exposure. Toxicological studies have been done for gavage and inhalation of aerosolized fuel and vapors. Data are not available to determine the comparability of exposure by these routes and modes of exposure. Humans are exposed dermally as well as by inhalation. The contribution to the internal dose from exposure of the skin to JP-8 or its vapors is not known. Measurements of jet-fuel components in blood of exposed workers would permit comparisons to similar data
Page 140
from laboratory animals and would facilitate extrapolation of toxicity data from animals to humans.
Additional data are needed to define exposures of humans and experimental animals better. Because JP-8 is a complex mixture of substances that differ in volatility, solubility, metabolic rates and pathways, and rate and route of elimination from the body, dosimetry of critical components of the mixture at critical sites in the body is crucial. Knowledge of the composition of JP-8 might not be a good surrogate for prediction of risk of some highly toxic minor component of the fuel.
Also, exposure to individual components of JP-8 under desert conditions of high temperature and low humidity would be different from exposures at very low temperatures because of different rates of aerosolization and vaporization. Exposure data should be collected from a variety of environmental conditions.
Summary
Jet propulsion fuel JP-8 is the fuel used by the U.S. Air Force and other services to fuel jets and other military vehicles. JP-8 is a mixture of hundreds of chemicals, mostly alkanes in the C8 to C17 range, and aromatics, including substituted benzenes and naphthalenes. The exact composition of JP-8 varies from batch to batch.
Human Exposure
Human exposure to JP-8 occurs during refueling and defueling operations and mechanical activities that deal with storage, transfer, and combustion. The most likely exposure of military personnel is via inhalation of aerosolized or vaporized fuel; however, topical and oral exposures also are possible. Occupational exposure standards are based on knowledge of the toxicity of components of JP-8. Those few exposure values that are published suggest that human exposures were below ACGIH TWA threshold limit values for those chemicals.
Page 141
Toxicology
Developmental Toxicity
There are no human data on the effects of JP-8 on development. The animal data are sufficient to conclude that prenatal oral exposure at doses of 1,500 mg/kg/d and greater administered on gestation days 6-15 in rats causes developmental toxicity. These toxicity findings in rodents are assumed to be relevant for prediction of risk to humans.
Reproductive Toxicity
There are no human data on the effects of JP-8 on male or female reproduction. There are human data that show that exposure to jet fuel (mostly JP-4) at below 6 ppm did not affect semen quality for aircraft maintenance personnel. Likewise, there are no laboratory animal studies on the effects of JP-8 on male or female reproduction.
Quantitative Evaluation
Developmental Toxicity
There are no human data from which to develop a quantitative evaluation. One laboratory study in rats identified a NOAEL of 1,000 mg/kg for developmental effects. Using an aggregate uncertainty factor of 1,000 (10 for interindividual variation, 10 for extrapolation from rats to humans, 10 for an incomplete data set), the UEL for developmental toxicity for a short term exposure is 1 mg/kg/d.
Reproductive Toxicity
There are no human or animal data from which to develop a quantitative evaluation or calculate UELs for male or female reproductive toxicity endpoints.
Page 142
Certainty of Judgment and Data Needs
Data on the toxicity and disposition of JP-8 in animals are sparse and no data are available for humans. One adequate study demonstrated developmental toxicity in rats. This study has not been replicated and there are no corroborative data from other studies with rats or other species. A multiple-generation reproduction study that examines a variety of postnatal endpoints that result from prenatal exposures, such as developmental neurotoxicity; immunotoxicity; and hematological, hepatic, and renal effects, should be conducted in rats or mice.
Additional data are needed to better define the exposure of humans and, in the context of animal toxicity studies, of laboratory animals. Because JP-8 is a complex mixture of chemicals that differ in volatility, solubility, metabolic rate and pathway, and rate and route of elimination from the body, dosimetry of critical components of the mixture at critical sites in the body is important to enhance the quality of risk assessment. The fact that human exposures can involve liquid fuel, aerosolized fuel, and vapor, by inhalation, dermal, and oral routes of exposure makes it difficult to accurately predict the internal dose of JP-8 and its components.
1,1,1,2-TETRAFLUOROETHANE1
Hydrofluorocarbons (HFCs), including 1,1,1,2-tetrafluoroethane (HFC-134a), have been developed as alternatives to chlorofluorocarbons (CFCs), which are known to contribute to the breakdown of ozone to oxygen in the stratosphere. HFCs do not contribute to the destruction of stratospheric ozone, but some HFCs have global warming potential. They primarily serve as replacements for CFCs in refrig-
1 Subcommittee member Paul Foster was previously employed at a company that conducted reproductive and developmental toxicity studies on HFC-134a. Because Dr. Foster was involved in the review of those studies, he did not participate in the subcommittee's discussions and deliberations on HFC-134a.
Page 143
eration equipment and mobile air conditioning; they also have pharmaceutical applications (e.g., as a propellant for metered dose inhalers used to treat asthma). The physical and chemical properties of HFC-134a are listed in Box A-1.
EPA has developed a chronic reference concentration (RfC) for chronic exposure of 80 mg/m3 (Integrated Risk Information System (IRIS) 1998), based primarily on a 2-year inhalation study in rats (Collins et al. 1995). Briefly, male rats exposed at concentrations of 10,000 ppm and 50,000 ppm had a significant increase in the incidence of Leydig cell hyperplasia compared with controls. The study is described below.
The American Industrial Hygiene Association's (AIHA) Workplace Environmental Exposure Level Committee gave HFC-134a an occupational exposure limit (8-hr time-weighted average) of 4,250 mg/m3 (AIHA 1991, as cited in European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) 1995).
Exposure Data
Human exposure to HFC-134a occurs via inhalation from accidental leaks of air conditioning units and refrigerators, from spills or industrial use, and from use of metered-dose inhalers such as those that deliver medication for the treatment of asthma (Hazardous Substance Data Base (HSDB) 1998; Alexander and Libretto 1995).
The likely maximum exposure from a metered-dose inhaler is 33 ppm hr/m3 lung surface area/d (Alexander et al. 1996).
General Toxicological and Biological Parameters
Acute Studies
No adverse health effects in humans from acute exposure to HFC-134a have been reported.
In experimental animals, HFC-134a has been shown to have low toxicity via inhalation. An approximate lethal concentration in rats ranges from 567,000 to 750,000 ppm after 4-hr and 30-min exposures,
Page 144
Box A-1 Physical and Chemical Properties,HFC-134a
Common name: FC-134a Chemical name: 1,1,1,2-tetrafluoroethane Synonyms: HFC-134a; Norflurane; HFA-134a; 1,2,2,2- tetrafluoroethane; F-134a; R134a; Refrigerant R134a CAS number: 811-97-2 Molecular formula: C2-H2-F4 Description: Colorless gas Molecular weight: 102.03 Boiling point: -26.5 °C at 736 mm Hg Freezing point: -101 °C Density and specific gravity: 1.21 g/mL (liquid under pressure at 25 °C) Vapor pressure: 96 psi at 25 °C Flash point and flammability: Nonflammable Solubility: 0.15% in water; soluble in ether Octanol and water partition coefficient: Pow = 1.06 Conversion factors: 1 mg/L = 238 ppm; 1ppm = 4.2 mg/m3 |
respectively (Rissolo and Zapp 1967; Silber and Kennedy 1979a). Also in rats, a 15-min lethal concentration for 50% of the test animals (LC50) was reported to be 800,000 ppm and a 4-hr LC50 was reported to be 500,000 ppm (Collins 1984). Clinical signs of toxicity included lethargy, labored and rapid respiration, foaming at the nose, tearing, salivation, convulsions, and death. For surviving animals, the effects were reversible. HFC-134a was not lethal to dogs exposed at concentrations of 700,000-800,000 ppm for 3-5 hr (Shulman and Sadove 1967).
Deep narcosis occurred in dogs, cats, and monkeys exposed via inhalation at concentrations of 500,000 ppm within approximately 1 min; the recovery period was approximately 2 min (Shulman and Sadove 1967). In a review of preclinical toxicology studies, Alexander and Libretto (1995) reported no deaths or treatment-related effects on clinical signs, body weight, food and water consumption, or postmortem findings in rats and mice exposed via inhalation at a concentration of 810,000 ppm with oxygen supplementation for 1 hr. Male and
Page 145
female mice exposed to HFC-134a without oxygen supplementation at 150,000 ppm for 1 hr showed respiratory effects, and the female mice were comatose after 15 min exposure (Alexander and Libretto 1995). In that study, there were significant decreases in tidal volume at 74,000 ppm, slight decreases in respiratory rate at 90,500 ppm, and marked reductions in minute volume at 150,000 ppm. In the same study, rats exposed at concentrations of 47,000 ppm had significantly reduced respiratory rates. Dogs exposed via inhalation to HFC-134a at concentrations of 40,000 and 80,000 ppm for 1 hr did not show treatment-related clinical signs. At 160,000 ppm, three of four dogs showed salivation, head shaking, and struggling; at 320,000 ppm the effects were more severe.
HFC-134a was found to be a weak cardiac sensitizer when tested in an epinephrine challenge in dogs (Mullin and Hartgrove 1979). Cardiac arrhythmias were observed at concentrations of 75,000 ppm and above. No effects were observed at 50,000 ppm. In another study, HFC-134a induced cardiac sensitization at concentrations of 80,000 ppm and above, and no effects were observed at 40,000 ppm (Hardy et al. 1991).
Repeated-Dose Studies
No adverse health effects in humans from repeated exposure to HFC-134a have been reported.
Subacute, subchronic, and chronic studies have been conducted in experimental animals to test the toxicity of HFC-134a (Kennedy 1979; Riley et al. 1979; Silber and Kennedy 1979b; Hext and Parr-Dobrzanski 1993; Hext 1989; Alexander and Libretto 1995; Collins et al. 1995). Those studies are summarized in Table A-4.
The NOAEL for subacute exposure in rats ranged from 10,000 to 100,000 ppm (Kennedy 1979; Riley et al. 1979; Silber and Kennedy 1979b). Subchronic exposure using 1 hr/d snout-only exposure for rats and mice resulted in no effects at 50,000 ppm (Alexander and Libretto 1995); whole-body exposure of rats for 6 hr/d to 50,000 ppm also had no effect (Hext 1989; Collins et al. 1995). There were no effects in dogs when 120,000 ppm was administered by face mask for 1 hr/d (Alexander and Libretto 1995). In rats exposed chronically to HFC-134a by
Page 146
|
Study Type |
Species |
Exposure Concentration, Duration, Route |
Observed Effect |
NOAEL (ppm) |
LOAEL (ppm) |
Reference |
|
Subacute |
Rat |
10,000, 50,000, 100,000 ppm; 6 hr/d, 5 d/wk for 14 or 28 d; inhalation |
Pathological changes in lung (focal interstitial pneumonitis) observed in rats exposed at 50,000 and 100,000 ppm; some changes in organ weight observed, but not related to histological changes |
10,000 |
Silber and Kennedy 1979b |
|
|
Subacute |
Rat |
1,000, 10,000, 50,000 ppm; 6 hr/d, 20 times in a 28-d period; inhalation |
Changes in kidney and gonad weight in male rats exposed at 50,000 ppm, changes in liver weight in male rats exposed at 10,000 and 50,000 ppm. No pathological changes found. Reduced organ weights not considered of toxicological significance. |
50,000 |
Riley et al. 1979 |
|
|
Subchronic |
Rat |
2,000, 10,000, 50,000 ppm; 6 hr/d, 5 day/ wk for 13 wk with and without 4-wk recovery period; inhalation (whole body) |
No treatment-related effects |
50,000 |
Hext 1989 |
Page 147
|
Subchronic |
Mouse |
10,000, 25,000, 50,000 ppm; 1 hr/d, 7 d/wk for 13 wk; inhalation (snout-only) |
No clinical signs related to treatment, no effect on body weight gain |
50,000 |
Alexander and Libretto 1995 |
|
|
Subchronic |
Rat |
2,500, 10,000, 50,000 ppm; 1 hr/d, 7 d/ wk for 50 wk; inhalation (snout-only) |
No clinical signs related to treatment, no effect on body weight gain |
50,000 |
Alexander and Libretto 1995 |
|
|
Subchronic |
Dog |
0.225, 0.75, 2.25 g; 3, 10, or 30 metered doses, twice daily for 1 yr (administered by oropharyngeal tube) |
No clinical treatment-related effects |
Alexander and Libretto 1995 |
||
|
Subchronic |
Dog |
120,000 ppm; 1 hr/d for 1 yr; inhalation (face mask) |
No clinical signs, changes in body weight, or changes in postmortem findings related to treatment. Minor effects included trembling, salivation, vomiting, were related to treatment-associated anxiety. |
120,000 |
Alexander and Libretto 1995 |
|
|
Subchronic |
Rat |
2,000, 10,000, 50,000 ppm; 6 hr/d, 5 d/wk for 13 wk; inhalation (whole body) |
No evidence of toxicity or compound-related effects at any exposure |
50,000 |
Collins et al. 1995 |
Page 148
|
Chronic |
Rat |
2,500, 10,000, 50,000 ppm; 6 hr/d, 5 d/wk for 2 yr; inhalation (whole body) |
Survival over 2-yr period not affected by treatment. No changes in clinical condition, clinical chemistry, body weight, food consumption in any exposure group. Histological examination showed statistically significant increase in the incidence of Leydig (interstitial) cell hyperplasia and Leydig cell adenoma in animals exposed to 50,000 ppm |
10,000 |
50,000 |
Collins et al. 1995; Hext and Parr-Dobrzanski 1993 |
|
Chronic |
Rat |
2,500, 10,000, 50,000 ppm; 1 hr/d, 7 d/ wk for treatment, no effect on body weight 108 wk; inhalation (snout only) |
No clinical signs related to treatment, no effect on body weight gain |
50,000 |
Alexander and Libretto 1995 |
NOAEL, no-observed-adverse-effect level; LOAEL, lowest-observed-adverse-effect level; ppm, parts per million.
Page 149
inhalation using snout-only exposure for 1 hr/d, 7d/wk there were no effects at 50,000 ppm (Alexander and Libretto 1995); with whole-body exposure for 6 hr/d, 5 d/wk (Hext and Parr-Dobrzanski 1993; Collins et al. 1995), an increase in Leydig cell hyperplasia and adenomas was seen at 50,000 ppm with a NOAEL of 10,000 ppm.
Genetic Toxicity Studies
There is no evidence to suggest that HFC-134a induces either genetic or chromosomal mutations, and therefore, there is no reason to suspect that HFC-134a exposure would induce heritable effects in humans. HFC-134a is reported to be nonmutagenic when tested in the Ames assay (Litton Bionetics 1976; Callander and Priestly 1990; Collins et al. 1995) or in the microbial mutagenicity assay in Escherichia coli (Alexander and Libretto 1995). It does not alter DNA synthesis in rat hepatocytes (Trueman 1990; Collins et al. 1995), induce chromosomal aberrations in mouse lymphoma L51787 cells in the presence or absence of microsomal-induced liver homogenates (Alexander and Libretto 1995), human lymphocytes or Chinese hamster lung cells (Mackay 1990; Collins et al. 1995), or alter micronucleus formation in the femoral bone marrow of exposed mice (Muller and Hoffmann 1989; Collins et al. 1995). HFC-134a also appears to be nonmutagenic to male mice exposed at 1,000, 10,000, or 50,000 ppm for 6 hr/d for 5 d via inhalation when tested in a dominant lethal assay (Hodge et al. 1979a). The results of a study of chromosomal aberrations in rat bone marrow cells were inconclusive (Anderson and Richardson 1979).
Carcinogenicity Studies
Rats were exposed, whole body, to HFC-134a via inhalation at concentrations of 2,500, 10,000, and 50,000 ppm for 6 hr/d, 5d/wk for up to 104 wk (Hext and Parr-Dobrzanski 1993; Collins et al. 1995). At 50,000 ppm there was an increase in testicular weight, Leydig cell hyperplasia, and Leydig cell tumors. Such tumors are common in rats and are induced by a variety of chemicals. Because HFC-134a does not demonstrate mutagenic activity, the increased incidence of Leydig cell tumors is attributable to a nongenotoxic mechanism. A very low in-
Page 150
cidence of Leydig cell tumors in humans has been reported (Mostofi and Price 1973), and the relevance of extrapolating the findings from rats to humans has been questioned. However, Clegg et al. (1997), in a thorough evaluation of Leydig cell hyperplasia and adenomas, indicated that the incidence in humans is uncertain and that, as a default when the mode of induction is unknown, agents that induced both hyperplasia and adenomas should be considered relevant and of concern for progression to carcinogenesis in humans. Several other HFCs also have been shown to induce Leydig cell hyperplasia and adenomas (summarized in Clegg et al. 1997). When this is the only or the primary effect of an agent and there is no mutagenic activity, the dose-response relationship is assumed to be nonlinear.
In another study, rats were exposed to HFC-134a at 300 mg/kg of body weight by gavage 5 d/wk for 1 year (Longstaff et al. 1984). No carcinogenicity was observed in this investigation. However, only one concentration was used, and it is possible that the route of administration and the dose of the compound used were not capable of detecting carcinogens of low potency. Furthermore, because the onset of Leydig cell adenomas is usually seen in aged animals, chronic studies will be more useful for detecting these effects and predicting their occurrence.
Other Toxicity
HFC-134a was shown to cause slight skin irritation in rabbits, perhaps because of local freezing (Mercier 1989). In that study, 0.5 mL of liquified HFC-134a was applied to scarified and intact skin areas of rabbits and the exposed site was covered for up to 24 hr.
HFC-134a also was shown to produce slight eye irritation in rabbits (Mercier 1990a). The chemical was administered as a gas, sprayed for either 5 or 15 seconds (sec) from a distance of 10 centimeters.
HFC-134a did not produce skin sensitization in one study conducted in guinea pigs (Mercier 1990b). The animals received a single intradermal injection of Freund's complete adjuvant followed by seven consecutive (occlusive) epicutaneous administrations of liquified HFC-134a. The challenge administration was performed after 12 d without treatment by occlusive epicutaneous treatment with liquified HFC-134a.
Page 151
Pharmacokinetics
Two pharmacokinetics studies have been conducted in human volunteers with exposure to HFC-134a. Vinegar et al. (1997) reported on the exposure of two male volunteers to 0.4% (4,000 ppm) HFC-134a via inhalation. The first subject lost consciousness after 4.5 min and exhibited a dramatic increase in blood concentration, which reached 1.29 mg/L by 2.5 min, and decrease to zero in pulse rate and blood pressure. The subject was revived and pulse and blood pressure returned to normal after 1 hr. However, dizziness and balance problems persisted after 6 wk. The second subject showed a rapid rise in blood pressure and pulse after 10.5 min, by which time the blood concentration had reached 0.7 mg/L; he was removed from the exposure and his vital signs returned to normal after 30 sec. The same subject was exposed again after about 1 hr to 0.2% HFC-134a and began having problems after 2.5 min. His blood concentration was 0.16 mg/L at the beginning of exposure and reached 0.38 mg/L by 2.5 min. Most symptoms were gone by the next day, but dizziness and balance problems persisted for 6 wk and he reported persistent ringing in the ears.
The second study (Emmen and Hoogendijk, 1999) involved eight volunteers (four males and four females). This study used whole-body inhalation exposure to CFC-12 or HFC-134a on eight occasions. The exposures to HFC-134a were at 1,000, 2,000, 4,000, or 8,000 ppm for 1 hr. No treatment-related effects of inhalation exposure to HFC-134a were noted on echocardiogram, pulse rate, systolic and diastolic blood pressure, or lung function compared with air control and CFC-12 reference conditions. The maximum concentrations reached 5.95-7.22 µg/mL after exposure to 8000 ppm. The half time (t1/2) for distribution was 8.34-9.44 min, and for elimination it was 38.29-44.45 rain.
Absorption of fluorocarbons and bromofluorocarbons via inhalation in experimental animals is rapid; the maximal blood concentrations of the substances develop within 5 min and equilibrium is achieved within the next 15 rain of exposure (Azar et al. 1973; Trochimowicz et al. 1974; Mullin et al. 1979). Blood concentrations do not increase further with increasing durations of exposure for a given concentration of these substances. In a fertility study (Alexander et al. 1996), blood samples were taken from P (parental generation) male rats after 15 wk exposure, and from P females after 3 wk premating, 3 wk
Page 152
gestation, and 2 wk postpartum exposure. P females from a peri- and postnatal study were sampled after one exposure on gestation day 17, or after repeated exposures in the second week postpartum. The data showed rapid absorption into blood of HFC-134a, increasing concentration with increasing exposure, and rapid excretion with no accumulation on repeated dosing. The mean half-lives ranged from 5.8 to 7 min.
Toxic effects observed in animals following oral and inhalation exposure to HFC-134a indicate it is absorbed by the lungs and gastrointestinal tract (Salmon et al. 1980). Studies conducted in rats exposed to high concentrations of HFC-134a, either orally or via inhalation, indicate it is rapidly excreted, mostly as the unchanged parent compound (Salmon et al. 1980). Analysis of the urine, feces, and expired air of rats exposed to HFC-134a at 10,000 ppm (1.0%) for 1 hr showed that only 0.34%-0.40% was metabolized (Ellis et al. 1993). The study by Salmon et al. (1980) found that some HFC-134a is retained in the liver and that relatively large amounts are retained in the adrenal gland; the study by Ellis et al. (1993) did not report any evidence for specific accumulation in any organ or tissue, including fat.
Studies in rat liver microsomes show that HFC-134a is oxidized by the cytochrome P450 system; this implies that cytochrome P450-containing tissues, such as nasal mucosa, liver, and lungs might convert HFC-134a to trifluoracetic acid, a toxic metabolite (Olson et al. 1990). Another study on the metabolism of HFC-134a in isolated rat hepatocytes found that the chemical undergoes limited metabolism as measured by the release of inorganic fluoride (Reidy et al. 1990). Microsomal metabolism was inhibited by carbon monoxide, was decreased in the presence of low oxygen concentration, and was increased in the presence of hepatic microsomes isolated from Arochlor-treated rats. These results indicate that HFC-134a undergoes a cytochrome P450-catalyzed defluorination reaction.
Reproductive and Developmental Toxicity Data
Human Studies
No data were found on the reproductive and developmental effects of HFC-134a in humans.
Page 153
Experimental Animal Studies
Reproductive Toxicity
Three reproductive toxicity studies testing HFC-134a have been conducted in rats and mice ( Table A-5). Additionally, data from several repeated-dose studies included information on reproductive effects (see Table A-4).
Hodge et al. (1979a) exposed male CD-1 mice (40 per group) in a dominant lethal study design to HFC-134a at 0, 1,000, 10,000 or 50,000 ppm by inhalation for 6 hr/d for 5 consecutive days, then mated them to unexposed females (two females for 4 d/wk) for 8 wk. Fifteen days after the initial date of pairing, the females were killed and their uterine contents examined. Females were not examined for vaginal plugs so there was likely some variability in gestational age when females were killed. The only dose-related effects observed were an increase in early deaths (as a percentage of implants) in the 50,000-ppm group at wk 4 and 8. This could have been because of a low incidence in the controls at these times; the incidence in the HFC-134a-exposed groups was within the control range across all weeks and was unlikely to have been a significant treatment effect.
In a two-generation study by Alexander et al. (1996), 30 male and female rats per group were exposed by snout-only exposure to HFC-134a at 0, 2,500, 10,000 or 50,000 ppm (1 hr/d) for 10 wk (males) or 3 wk (females) before pairing (P generation), the males continued to be exposed for a total of 18 wk, and the females continued through pregnancy and lactation. Estrous cycles were monitored for 14 d before pairing. F1 (first filial) generation fetuses were examined on day 20 of gestation in 14 females per group; the rest were allowed to litter and nurse their young. Twelve F1 males and females per group were selected at weaning and mated at approximately 70 d of age. Survival and development of the F2 (second filial generation) progeny were monitored for 21 d postpartum. One F2 animal of each sex (eight litters per group) was retained to sexual maturity. Physical and reflex development were evaluated in F1 and F2 offspring, as were locomotor coordination, activity, and learning, memory, and reversal. A slight but statistically significant decrease in body weight gain was seen in P males exposed to 10,000 and 50,000 ppm after 2 wk exposure; cumula-
Page 154
|
Study Type |
Species |
Exposure Concentration, Duration, Route |
Observed Effect |
NOAEL (ppm) |
LOAEL (ppm) |
Reference |
|
Dominant lethal study |
Mice (male) |
1,000, 10,000, 50,000 ppm; 6 hr/d for 5 d; inhalation (whole body). After exposure, mated with 2 females each week for 8 wk; females killed 15 d after pairing |
No significant maternal or developmental toxicity |
≥50,000 |
Hodge et al. 1979a |
|
|
Two-generation study (paternal exposure only) |
Rat (male and female) |
2,500, 10,000, 50,000 ppm; 1 hr/d; males treated from 10 wk before mating (period of gametogenesis) through mating (18 wk total exposure), females treated from 3 wk before mating (gametogenesis) through mating, pregnancy, and lactation; inhalation (snout-only). F1 offspring exposed via nursing, not exposed after weaning, F2 offspring not exposed |
No adverse effects on reproductive performance of treated animals. Slight reduction in body weight gain of males exposed at 10,000 and 50,000 ppm; significant reduction of male body weight gain at 50,000 ppm after 5 and 10 wk exposure. No effect on offspring growth offspring showed minor delays survival or body weight. F2 (~1/2 to 1 d) in physical, reflex development; not considered related to exposure |
Adult: 10,000 Offspring: ≥50,000 |
Adult: 50,000 |
Alexander et al. 1996 |
Page 155
|
Testicular endocrine function |
Rat (male) |
10,000, 30,000, 100,000 ppm; 6 hr/d; animals treated through gametogenesis (11 wk) and mating and postmating period (18 weeks total); inhalation, first 9 wk snout-only, final 9 wk whole-body |
Levels of luteinizing hormone measured. No difference between controls and treated groups. Animals exposed at 100,000 ppm showed slight (not statistically significant) increase in testosterone secretion and biosynthesis and a concomitant rise in progesterone secretion when the testis was incubated with human chorionic gonadotrophin, without any qualitative change in androgen biosynthesis |
30,000 |
100,000 |
Barton et al. 1994 |
|
Prenatal developmental toxicity |
Rat |
30,000, 100,000, 300,000 ppm; 6 hr/d from d 5-14 of gestation; inhalation (whole body) |
At 300,000 ppm, significant decrease in fetal weight, significant increase in skeletal variations in fetuses. Maternal toxicity observed at 100,000, 300,000 ppm. No developmental effects in fetuses exposed at 30,000 or 100,000 ppm |
Maternal: 30,000 Fetal: 100,000 |
Maternal: 100,000 Fetal: 300,000 |
Lu and Staples 1981 |
Page 156
|
Prenatal develop mental toxicity |
Rat |
1,000, 10,000, 50,000 ppm; 6 hr/d from days 6-15 of gestation; inhalation (whole body) |
At 50,000 ppm, significantly reduced fetal weight, skeletal ossification. No significant maternal toxicity |
Maternal: 50,000 Fetal: 10,000 |
Fetal: 50,000 |
Hodge et al. 1979b |
|
Prenatal develop mental toxicity |
Rabbit |
Pilot study: 5,000, 20,000, 50,000 ppm (6-9 pregnant animals per group), 6 hr/d days 6-18 gestation; inhalation (whole body) Main study: 2,500, 10,000, 40,000 ppm (18-24 pregnant animals per group, 6 hr/d from days 6-18 gestation; inhalation (whole body) |
Pilot study: Slight decrease in maternal body weight gain at 50,000 ppm. Decreased number of implantations in all groups, significant at 20,000 and 50,000 ppm; decreased number of live fetuses, gravid uterine weight, litter weight, and increased fetal weight at all exposure levels Main study: At 10,000 and 40,000 ppm, maternal body weight and food consumption reduced; effects at 10,000 ppm within historical control range. No treatment-related effects on implants, litter size, fetal weight, or external, visceral, skeletal defects at any exposure level |
Maternal: 10,000 Fetal: ≥40,000 |
Maternal: 40,000 |
Wickramaratne 1989a,b (same as study reported by Collins et al. 1995) |
Page 157
|
Peri- and postnatal |
Rat |
1,800, 9,900, 64,400 ppm; 1 hr/d; days 17-20 pregnancy and 1-21 postpartum; inhalation (snout-only) |
No maternal or developmental effects observed except for a delay in F1 age at pinna detachment, eye opening and startle response at 64,400 ppm (~1/2-d delay). May be related to exposure |
Maternal: 64,400 Fetal: 9,900 |
Fetal: 64,400 |
Alexander et al. 1996 |
NOAEL, no-observed-adverse-effect level; LOAEL, lowest-observed-adverse-effect level; ppm, parts per million.
Page 158
tive body weight gain in males exposed to 50,000 ppm over 5 wk or 10 wk was significantly reduced. There were no effects on body weight or weight gain in P females, or in F1 or F2 offspring. There appeared to be a slight increase in skeletal defects in F1 fetuses, but no significant change was reported, and some might have been cases of decreased ossification (this was unclear from the reported data). In F2 offspring, there was a slight but statistically significant increase in the age at pinna detachment (exposed at 10,000 ppm), startle response (exposed at 10,000 and 50,000 ppm), and air righting (exposed at 2,500 and 10,000 ppm); these changes were not clearly dose related, and on average they represented a ½- to 1-d delay. Because the F2 offspring were never exposed directly or indirectly, there was no change in body weight at birth or weaning, and no changes were seen in the F1 off-spring on these same measures; the changes detected in F2 animals were not considered treatment-related.
As a follow-up to studies showing Leydig cell hyperplasia, Barton et al. (1994) exposed 25 male Sprague-Dawley rats per group to HFC-134a at 0, 10,000, 30,000, or 100,000 ppm for 6 hr/d for a total of 18 wk (11 wk before mating and 7 wk during and after mating). Animals were exposed snout-only for the first 9 wk to reduce the amount of material used; thereafter, whole-body exposure was used. In 10 males per group, luteinizing hormone concentrations were assessed after 16 wk, and again at 17 wk after stimulation with luteinizing hormone releasing hormone (LHRH). In another 10 males per group, at necropsy, the left testis was decapsulated, then incubated with human chorionic gonadotropin to assess androgen release; the right testis was examined histologically. High basal concentrations of luteinizing hormone were seen in all groups including controls, but there was no difference between controls and treated groups in luteinizing hormone levels before or after LHRH stimulation. At 100,000 ppm, there was no statistically significant increase in testosterone secretion and biosynthesis, but an increase in progesterone was observed. The increase in progesterone was consistent with increased Leydig cell function at this exposure level.
Developmental Toxicity Studies
Four developmental toxicity studies testing HFC-134a have been conducted in rats and rabbits ( Table A-5).
Page 159
Lu and Staples (1981) exposed female Sprague-Dawley rats at concentrations of 0, 30,000, 100,000, or 500,000 ppm HFC-134a for 6 hr/d, days 5-14 of gestation (11 pregnant control animals, 6 in each exposed group). All gestational ages are converted to correspond to the day of insemination as gestational day 0. Animals were killed on gestational day 20 and uterine contents were examined. Dams exposed to 100,000 or 300,000 ppm showed a reduced or absent response to sound, respectively, demonstrating the anesthetic action of HFC-134a. Animals exposed to 300,000 ppm consumed significantly less food and gained significantly less weight than did controls. There was a significant decrease in fetal weight at 300,000 ppm, and a significant increase in the incidence of skeletal variations, many of which were related to reduced ossification.
Hodge et al. (1979b) exposed female rats to concentrations of 0, 1,000, 10,000, or 50,000 ppm HFC-134a for 6 hr/d on gestation days 615 and killed them on gestation day 21 for examination of uterine contents (23-29 pregnant animals per group). There were no treatmentrelated effects on maternal animals except for acute pulmonary irritation that increased in severity and incidence with exposure concentration. There was no effect on the number of implants, litter size, or litter weight. At 50,000 ppm, fetal weight was slightly but significantly decreased and there was an increased incidence of skeletal variations, primarily reduced ossification of cervical vertebrae, sternebrae, and digits. An increase in the incidence of abnormal sternebrae also was reported in the 50,000 ppm group, but these effects (bipartite or misaligned sternebrae) are often seen in controls and are likely related to reduced ossification observed in the same groups. All fetal effects reported in this study at the highest exposures can be accounted for by the fact that litter size was greater in this group than in any other, including controls. Reduced fetal weight often is associated with increased litter size, and reduced ossification of skeletal elements often accompanies reduced fetal weight.
Wickramaratne et al. (1989a,b) conducted a prenatal developmental toxicity study in rabbits in two phases. In the pilot study (Wickramaratne et al. 1989a), groups of artificially inseminated rabbits were exposed to 5,000, 20,000 or 50,000 ppm (six to nine pregnant animals per group) for 6 hr/d, on gestation days 6-18, then killed on gestation day 29. Fetuses were examined only for external anomalies and cleft palate. Two animals aborted late in pregnancy, one in the
Page 160
5,000 ppm group and one in the 50,000 ppm group. Maternal body weight loss was observed during the early dosing period in the 50,000 ppm group. The number of implantations was reduced in the 20,000 and 50,000 ppm treated groups compared with controls, with consequent reduction in litter size, gravid uterine weight, and litter weight, but increased fetus weight due to reduced litter size.
In the main study (Wickramaratne et al. 1989b; same study reported in Collins et al. 1995), pregnant rabbits (18-24 per dose group) were exposed 6 hr/d to 2,500, 10,000, and 40,000 ppm gestation days 6-18. Observations were the same as in the pilot study, but fetuses were examined for external, visceral, and skeletal defects, and a single section was made through the head to examine the brain macroscopically. One control animal aborted late in pregnancy. Maternal body weight and food consumption were signficantly reduced at 10,000 and 40,000 ppm, although the effects at 2,500 ppm were within the historical control range. There was no effect on implantation number, litter size, gravid uterine weight, litter weight, or individual fetus weight. The incidence of major and minor defects either did not appear to be dose related or was within historical control ranges. The NOAEL was considered to be 10,000 ppm, based on maternal toxicity, and the NOAEL for developmental toxicity was ≥40,000 ppm.
In a peri- and postnatal study design, Alexander et al. (1996) exposed rats by snout only to HFC-134a at 0, 1,800, 9,900, or 64,400 ppm (1 hr/d). Pregnant females were exposed on gestation day 17-20, then from postnatal day 1-21. F1 pups were weaned at postnatal day 21, and one male and female per litter (20 litters per group) were selected and raised to maturity. F1 animals were mated at approximately 84 d of age, killed on gestation day 20, and the uterine contents were examined. F1 offspring were examined for physical and reflex development as well as locomotor coordination, activity, learning, memory, and reversal. There were no effects on any parameter measured except for a statistically significant delay in the age at pinna detachment, eye opening, and startle response in F1 offspring at 64,400 ppm (approximately half-day delay). Although there was no effect on body weight, these animals were exposed indirectly via the milk during the period when most of these measurements were made. Exposure concentrations and blood concentrations of the dams were somewhat higher in the peri- and postnatal study than in the fertility study reported at the
Page 161
same time. These minor but statistically significant effects could be the result of the exposure to HFC-134a.
Integration of Toxicity and Exposure Information
Interpretation of Toxicity Data
General Toxicity and Pharmacokinetics
Data on the toxicity of HFC-134a indicate that it is nontoxic in most circumstances. Most of the changes reported have been at high concentrations, which also induce narcosis. There is no evidence of genetic toxicity for HFC-134a. The primary effect reported is the induction of Leydig cell hyperplasia and adenomas in male rats exposed at 50,000 ppm for 6 h/d, 5 d/wk over a 2-yr period (Collins et al. 1995; Hext and Parr-Dobrzanski 1993). The NOAEL of 10,000 ppm from these data was the basis of the EPA's RfC.
It should be noted that in a two-generation study (Alexander et al. 1996), the NOAEL for adult toxicity was 10,000 ppm, based on significant changes in body weight in male rats exposed snout-only at a concentration of 50,000 ppm for 1 h/d, 5 d/wk for 18 wk. The difference in results might be attributed to the snout-only exposure protocol, which might have caused stress to the rats and affected their body weight in the Alexander et al. (1996) study.
Data in two human volunteers from one study (Vinegar et al. 1997) indicate severe effects on vital signs after brief exposures at concentrations of 2,000-4,000 ppm HFC-134a by inhalation. However, in a study by Emmen and Hoogendijk (1999), human volunteers exposed whole body at concentrations as high as 8000 ppm for 1 hr did not show any effects of HFC-134a. Absorption of HFC-134a was very rapid and maximal concentrations were achieved within 15-30 min. Repeated exposures in animal studies do not result in accumulation because the half-life is so short. Given the rapid absorption and excretion in rats and humans, the kinetics appear to be similar between the species.
Reproductive and Developmental Toxicity
Interpretation of the experimental animal data described above is
Page 162
complicated by the fact that two exposure regimens were used: 1 hr/d snout-only exposure, to mimic MDI exposures, and 6 hr/d whole-body exposure, to mimic environmental exposures.
Developmental Toxicity. No data were found from studies of developmental toxicity of HFC-134a in humans. The experimental animal data are sufficient to conclude that HFC-134a does not cause prenatal developmental toxicity when pregnant animals were exposed (whole body) for 6 hr/d during major organogenesis to HFC-134a concentrations below those associated with narcosis (<30,000 ppm). The NOAEL for rat maternal toxicity was 30,000 ppm, whereas the NOAEL for rat prenatal toxicity was 50,000 ppm (Hodge et al. 1979a; Lu and Staples 1981). The NOAEL for rabbit maternal toxicity was 10,000 ppm, whereas the NOAEL for rabbit prenatal toxicity was greater than 40,000 ppm (Wickramaratne 1989a,b; Collins et al. 1995). The data are insufficient to determine the extent to which HFC-134a causes postnatal developmental effects, as the only study addressing this issue used 1 hr/d snout-only exposures for 10 wk before mating, throughout gestation to day 20, and from postnatal days 1-21 (fertility study), or from gestation day 17-20 and postnatal days 1-21 (peri- and postnatal study); exposure to pups was not continued beyond postnatal day 21 (Alexander et al. 1996). In these studies, the adult NOAEL was 10,000 ppm (LOAEL, 50,000 ppm) in the fertility study and greater than 64,400 ppm in the peri- and postnatal study. The developmental NOAEL was greater than 50,000 ppm in the fertility study and 9,900 ppm (LOAEL = 64,400 ppm) in the peri- and postnatal study. The effects at 64,400 ppm in the peri- and postnatal study were minimal and might not be related to exposure, so 50,000 ppm is assumed to be the NOAEL for postnatal effects for snout-only exposure for 1 hr/d. The data are insufficient to determine what the extent and types of effects would be with continued exposure to pups after weaning and into the F2 generation. The experimental animal data are assumed relevant to humans.
Reproductive Toxicity. No data were located from studies of the reproductive toxicity of HFC-134a in humans. The animal data were sufficient to show that HFC-134a exposures similar to those metered-dose inhalers did not affect fertility and sexual function. Although there was an effect of exposure to 50,000 ppm on male body weight gain in the fertility study mentioned above (Alexander et al. 1996), fertility and reproductive function were not affected.
Page 163
The data were sufficient to show that whole-body exposure to HFC-134a for 6 hr/d for 2 yr could affect the testis. Although a dominant lethal study showed no effects at concentrations as high as 50,000 ppm (Hodge et al. 1979a), chronic exposure of rats at 50,000 ppm HFC-134a resulted in a statistically significant increase in the incidence of Leydig cell hyperplasia and adenoma (Hext and Parr-Dobrzanski 1993; Collins et al. 1995). The NOAEL was 10,000 ppm for a 6 hr/d exposure. In a follow-up study to Hext and Parr-Dobrzanski (1993), Barton et al. (1994) showed that HFC-134a (6 hr/d, 18 wk) increased the synthesis and release of testosterone and progesterone from testes incubated with human chorionic gonadotropin but did not alter the qualitative aspects of androgen biosynthesis. The changes in testosterone and progesterone secretion were consistent with the increased Leydig cell activity in the chronic study. Given the significant increase in Leydig cell hyperplasia and adenoma in rats, EPA considered these effects adverse and based the RfC for HFC-134a on this effect.
The experimental animal data are assumed relevant to humans, because the data are inadequate to show that the effects are irrelevant.
Default Assumptions
The data on Leydig cell hyperplasia and adenoma are assumed to be relevant to humans. Likewise, the lack of developmental toxicity is also assumed to be relevant to humans. The pharmacokinetics in humans and animals are very similar, as is the lack of toxicity from acute exposures, notwithstanding the data from the two subjects reported by Vinegar et al. (1997). Because of the similarities in human and animal pharmacokinetics, this portion of the interspecies uncertainty factor can be reduced from 10 to 3. The intraspecies uncertainty factor of 10 should be retained because of the unusual findings in the Vinegar et al. (1997) study. A database deficiency factor of 10 should be applied due to the lack of a 6 hr/d exposure in a multigeneration study with exposure continuing throughout the two generations, as well as lack of a developmental neurotoxicity study with a 6 hr/d exposure. It appears that there is little or no effect of HFC-134a in any of the studies reviewed except at doses that induce narcosis, but additional data with longer exposure durations and additional endpoints are needed to confirm this observation.
Page 164
Quantitative Evaluation
In the two-generation study by Alexander et al. (1996), no adverse reproductive or developmental effects were observed in rats exposed at a concentration of 50,000 ppm for 1 hr/d during gametogenesis and mating (males), or gametogenesis, mating, pregnancy, and lactation (females). Since the effects at 64,400 ppm (exposed for 1 hr/d) in the peri- and postnatal study (Alexander et al. 1996) were minimal, and the next lower dose was 9,900 ppm, the subcommittee has identified 50,000 ppm as the NOAEL for reproductive and developmental effects for less than a chronic exposure. The NOAEL of 50,000 ppm for a 1 hr/d exposure converted to a 6 hr/d exposure is 8,300 ppm. The UEL for reproductive and developmental effects is then calculated using the adjusted NOAEL of 8,300 ppm divided by an aggregate uncertainty factor of 300 (3 for interspecies extrapolation, 10 for intraspecies differences, and 10 for deficiencies in the database). Thus, the UEL for reproductive and developmental toxicity is
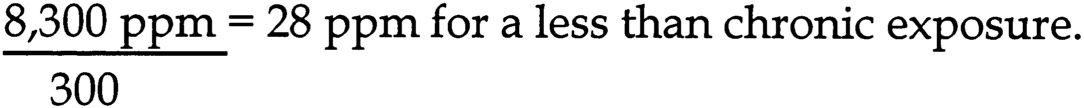
~ enlarge ~
For chronic exposure, the subcommittee chose the NOAEL of 10,000 ppm for a 6-hr/d, 2-yr exposure based on a significant increase in the incidence of Leydig cell hyperplasia in treated rats (Collins et al. 1995; Hext and Parr-Dobrzanski 1993). Applying an aggregate uncertainty factor of 300 (3 for interspecies extrapolation, 10 for intraspecies differences, and 10 for deficiencies in the database), the UEL for reproductive and developmental toxicity is
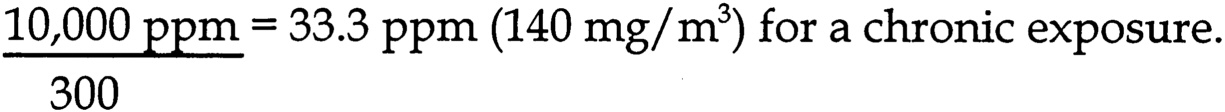
~ enlarge ~
The chronic exposure UEL is higher than the EPA's chronic RfC of 80 mg/m3, which is based on the same study and endpoints (see above). There are several differences in the way the UEL was derived compared with the RfC. To calculate the chronic RfC, EPA derived a BMC10 of 11,030 ppm and adjusted it from a 6-hr/d, 5-d/wk exposure to a continuous exposure by multiplying by 6/24 hr and 5/7 d. A total
Page 165
uncertainty factor of 100 was applied to the BMC10: 3 for interspecies extrapolation, 10 to protect sensitive individuals, and 3 for a database deficiency of a two-generation study. The NOAEL of 10,000 ppm was used as the basis for the chronic UEL and the UEL was not adjusted because it was developed for an occupational exposure scenario. A total uncertainty factor of 300 was applied to the NOAEL: 3 for interspecies extrapolation, 10 to protect sensitive individuals, and 10 for database deficiencies due to the lack of both a two-generation study and a developmental neurotoxicity study. The latter concern was raised by the Alexander et al. (1996) peri- and postnatal study that suggested the possibility of developmental neurotoxicity in offspring of animals exposed for 1 hr/d, 5 d/wk during late gestation and lactation. Had the BMC10 derived by EPA been used in the calculation, the final value would have been slightly higher (i.e., 37 ppm or 155 mg/m3).
Critical Data Needs
A two-generation reproduction study is needed with at least 6 hr/d exposure continuing to pups after weaning and into the F2 generation to determine the effects of long-term exposures. Developmental neurotoxicity endpoints should be included in this study based on the types of effects seen in the peri- and postnatal study with the snout-only 1 hr/d exposure.
Summary
HFCs, including HFC-134a, have been developed as an alternative to CFCs, which are known to contribute to the breakdown of ozone to oxygen in the stratosphere. HFCs do not contribute to the destruction of stratospheric ozone, but some HFCs have global warming potential. They primarily serve as replacements for CFCs in refrigeration equipment and mobile air conditioning; they also have pharmaceutical applications (e.g., as propellants for metered-dose inhalers used to treat asthma).
Page 166
Human Exposure
Human exposure to HFC-134a occurs via inhalation from accidental leaks of air conditioning units and refrigerators, from spills or industrial use, and from use of metered-dose inhalers such as those that deliver medication for the treatment of asthma.
Toxicology
Developmental Toxicity
There are no human data on the effects of HFC-134a on development. The animal data are sufficient to support a conclusion that exposures to HFC-134a does not cause prenatal developmental toxicity when pregnant animals are exposed for 6 hr/d during major organogenesis to concentrations of HFC-134a below those associated with narcosis (less than 30,000 ppm). HFC-134a also did not cause peri- and postnatal effects in rats with snout-only exposure for 1hr/d during gestation days 17-20 and postnatal days 1-21 at 50,000 ppm. However, the data are insufficient to determine the extent to which HFC-134a caused postnatal developmental effects including endpoints of developmental neurotoxicity, as there are no studies with at least a 6 hr/d exposure throughout two generations. These toxicity findings are assumed to be relevant for the prediction of risk to humans.
Reproductive Toxicity
There are no human data on the effects of HFC-134a on male or female reproduction. The animal data are sufficient to conclude that HFC-134a exposures similar to those used in MDIs will not cause affect sexual function and fertility. There was no effect on dominant lethality after treatment of males and mating with unexposed females. However, data are insufficient to determine the effects of whole-body exposure for 6 hr/d, as a multigeneration study with exposure continuing through two generations is not available. Chronic exposure of rats at concentrations as high as 50,000 ppm resulted in a significant in-
Page 167
crease in the incidence of Leydig cell hyperplasia. These toxicity findings are assumed to be relevant for the prediction of human risk.
Quantitative Evaluation
Reproductiveand Developmental Toxicity
There are no human data from which to develop a quantitative evaluation. Laboratory studies in rats identified a NOAEL of 50,000 ppm for reproductive and developmental effects for a less than chronic exposure. The NOAEL of 50,000 ppm was adjusted to convert from a 1hr/d exposure to a 6hr/d exposure; the adjusted NOAEL is 8,300 ppm. Dividing the adjusted NOAEL by an aggregate uncertainty factor of 300 (3 for interspecies extrapolation, 10 for intraindividual differences, and 10 for an incomplete database), the UEL for reproductive and developmental toxicity for less than a chronic exposure is 28 ppm.
A similar UEL is calculated for chronic exposures. Laboratory studies in rats identified a NOAEL of 10,000 ppm based on a significant increase in the incidence of Leydig cell hyperplasia. Applying an aggregate uncertainty factor of 300 (3 for interspecies extrapolation, 10 for intraindividual differences, and 10 for an incomplete database), the UEL for reproductive and developmental toxicity for a chronic exposure is 33.3 ppm.
Certainty of Judgment and Data Needs
Data on the toxicity and disposition of HFC-134a suggest that it is a compound with little or no reproductive or developmental toxicity except at very high exposure concentrations that induce narcosis. However, data are needed from postnatal evaluations and from a multigeneration study with 6 hr/d exposure. The similarities in pharmacokinetics between humans and laboratory animals provide confidence that the data are relevant for predicting human risk.





















































