Page 57
3
The Evaluative Process: Part II. Integration of Toxicity and Exposure Information
The integration step of the evaluative process is conducted in three stages. In the first stage, the evaluators examine the data for relevance to potential human toxicity. Then, if the data are determined to be relevant to human exposures, a quantitative assessment is conducted. Finally, the concluding step of the evaluative process is the integration of toxicity and exposure information to characterize the risk of potential reproductive and developmental toxicity. A narrative summary communicates to Navy environmental health practitioners the scientific judgment on a chemical's risk for reproductive and developmental toxicity.
INTERPRETATION OF TOXICITY DATA
The interpretive section of the evaluative process considers all relevant information in the course of reaching a judgment about whether an exposure has the potential to cause developmental or reproductive toxicity in humans. In most cases, animal data are considered relevant indicators of human risk, unless there is modifying information that suggests otherwise. The most common reason for
Page 58
concluding that no hazard exists for humans is the availability of sufficient experimental data that reveal no adverse effects in animal studies. Some experimental data might demonstrate toxicity of limited relevance to humans because of species differences in metabolism or sensitivity, lack of probable human exposure, or human evidence of no effect. Animal data in which no adverse effects are observed do not always preclude human effects, nor do adverse effects in animals inevitably predict human toxicity.
The evaluative process requires an integrated consideration of a variety of data. Integration involves combining the summary statements formulated during the review of animal and human reproductive and developmental toxicity data and considering them in the context of systemic toxicity parameters and pharmacokinetic data. A weight-of-evidence approach is then used to formulate judgments about the potential for human hazard. In this process, the evaluators develop separate statements to address developmental toxicity, female reproductive toxicity, and male reproductive toxicity. In each case, the basis for the judgment is articulated and makes particular note of such critical factors as replication of effect across species, exposure routes, dose-response parameters, relationship of effective dose to doses that cause other forms of toxicity, and comparative metabolic data.
To achieve a degree of consistency in the interpretation of experimental animal data, this report uses “relevance” terms:
-
Irrelevant means that pharmacokinetic or mechanistic features of the experimental animal model are known in detail and are demonstrably inconsistent with human exposure or response.
-
Relevant identifies a data set in an experimental animal species for which pharmacokinetic and mechanism information is adequate to demonstrate a particular similarity to humans.
-
Assumed relevant indicates there is no modifying supplemental information.
For many agents, there is no detailed understanding of absorption, distribution, biotransformation, or excretion in experimental animals or humans. In these cases, studies of the most sensitive experimental animal species are assumed to be relevant, and would thus drive the judgment of potential risk to humans.
Page 59
Where possible, an evaluation should use pharmacokinetic data, including metabolic and mechanism-of-action information, to determine the relevance of experimental data to humans. Should the available data for a particular species demonstrate a pharmacokinetic pattern similar to that found in humans, the data from that species will be considered relevant. But if, for example, an agent given to an experimental animal requires biotransformation to produce toxicity, and if humans are known to be incapable of that biotransformation pathway, then toxicity data from that experimental animal species would be considered irrelevant to humans.
Toxicity always depends on exposure conditions, such as route of administration, timing and duration of administration, and dose. The conservative default assumption is that, without data to the contrary, treatment of an experimental animal by any route is assumed relevant to human exposure by any route. That default assumption can be dropped when adequate modifying information is available. If, for example, an experimental animal study uses oral dosing, and humans are known not to absorb the agent by the oral route, then the experimental data are irrelevant for human oral exposure (but not necessarily for other routes of human exposure). In another example, if experimental animals are exposed to an agent via the oral route and adverse effects are observed, but exposure to humans is topical and the agent is not absorbed through the skin, then the experimental data are irrelevant to humans.
The use of a template ( Box 3-1) for summarizing the available data is advised as a guide for ensuring consistency in the characterization of reproductive and developmental hazards.
Default Assumptions To Be Considered in Assessing Reproductive and Developmental Toxicity Risk
Certainty of judgment about toxicity is in large part proportional to the quality and amount of chemical-specific data. In many instances, the desired data are not available; in such circumstances, it has been traditional to adopt default assumptions and proceed with the assessment. Default assumptions should incorporate all available information to reduce the level of uncertainty as much as possible. It
Page 60
might be necessary to choose from a range of reasonably plausible default values, such as the volume of inhaled air for the sedentary individual, the worker who performs physically demanding tasks, or the active jogger or marathon runner. In such instances, the common practice is to choose assumptions that would estimate the upper range of exposure in individuals who constitute the exposed population. In cases in which there is little or no information available, the assumption selected might deliberately represent a worst-case value. In every case, default assumptions should be used sparingly and openly, with full disclosure of the degree of certainty. The general default assumptions proposed for use in this evaluative process are summarized below.
Box 3-1 Template for Summarizing Reproductive and Developmental Toxicity Using the Evaluative Process
There is [sufficient, insufficient] evidence in [humans and/or animals] that [chemical/agent] [does or does not] cause [reproductive toxicity in males/females, developmental toxicity] when exposure is [route, dose range, timing, duration]. Relationship to adult toxicity stated. The data are [relevant, assumed relevant, irrelevant] to consideration of human risk. |
Absorption
Rates of absorption and elimination are assumed by default to be comparable among species. If experimental animal absorption has been determined but human absorption is unknown, human absorp-
Page 61
tion is assumed to be the same as that in the species with the highest degree of absorption.
When quantitative absorption data for a route of exposure indicate differences between humans and the relevant test species, the no-observed-adverse-effect level (NOAEL) might need to be adjusted proportionately.
Cross-Species Extrapolation
When assessing manifestations of toxicity, evaluators might base their conclusions about relevance on the mechanism that produces a toxicological effect; however, a basic default assumption is that any manifestation of reproductive or developmental toxicity is relevant to humans unless the mechanism by which it occurs is impossible in humans. For example, if a toxic effect occurs in animals through an inhibition of folic acid synthesis, that effect would not be considered relevant for humans because humans do not synthesize folic acid. It is unusual, however, to have such detailed knowledge about mechanisms of toxicity from experimental animal studies.
It should be noted that the particular effect produced in an experimental animal study does not generally have a bearing on determinations of relevance. If an agent causes tail defects in the offspring of treated mice, for example, this effect is not automatically considered irrelevant to humans simply because humans do not have tails. Instead, the assumption is that the mouse study demonstrated that the chemical substance interfered with vertebral development and therefore has relevance for vertebral or other features of human development.
Where there are experimental data from more than one species, the default assumption is that humans are at least as sensitive as the most sensitive animal species. If the data indicate, however, that some particular species is a more relevant surrogate for humans, either because of physiological similarity at the site of action or because of the pharmacokinetic parameters associated with the substance under review, such information will preempt that general assumption.
In the absence of data, activation and detoxification pathways in
Page 62
animals and humans are assumed by default to be qualitatively and quantitatively similar.
Adjustments of NOAELs from inhalation exposure studies to a human equivalent concentration, based on adjustments for minute volume, respiratory rate, and other factors, are appropriate for reproductive and developmental toxicity (EPA 1994). Toxicity data are scaled directly from experimental animals to humans on the basis of minute volume per kilogram of body weight for inhaled materials and by weight (or volume) of the dose per body weight or surface area for other routes of exposure. The first priority is to use the internal dose at the target site, if available.
Exposure Duration
Evaluators must assume that a single exposure at a critical time in development or in the reproductive cycle might produce an adverse effect; that is, repeated exposure is not necessary for reproductive or developmental toxicity. The fact that toxicity might be cumulative with repeated exposure is another important consideration. In most cases, the data available for reproductive and developmental toxicity risk assessment are from studies that use repeated exposures. The NOAELs and lowest-observed-adverse-effect levels (LOAELs) for reproductive and developmental effects are, however, usually based on a daily dose (e.g., milligrams per kilogram body weight per day (mg/kg/d)). When extrapolating duration data from animals to humans, it is important to consider not only the percentage of the life span during which exposure occurs, but also physiological (including developmental) time of the life span. Rodent development is much more rapid than in humans and maturity at birth differs considerably in rodent species and humans. For example, the duration of exposure in a prenatal developmental toxicity study that extends from gestation day 6 to gestation day 15 in rats might be expressed as equivalent to exposure from approximately 1 week to 8 weeks gestation in humans. Developmental stage also varies among organ systems for experimental animals and humans, both pre- and postnatally; therefore, relative developmental stage must be considered for the organ system(s) of
Page 63
concern. For example, nervous system development at birth in humans is approximately equivalent to that 5 days postnatally in rat pups, while respiratory and cardiovascular function are more similar at birth due to the demands of the ex utero environment.
Windows of Vulnerability
The concept of windows of vulnerability for developmental toxicity is generally known and accepted (Wilson 1973; Moore 1988). For example, thalidomide causes abnormal ear development, autism, duplication of thumbs, and heart and renal defects after exposure on gestation days 20-24 in humans; shortened or missing limbs after exposure on gestation days 24-33; and rectal stenosis and triphalangism of the thumbs on gestation days 35-46 (Schardein 2000; Strömland et al. 1994; Miller and Strömland 1999 ). Screening studies in animal models usually involve treatment throughout organogenesis and, in some cases, throughout fetal and early postnatal life, so that critical periods of vulnerability can not always be discerned. When effects are seen, additional follow-up studies may be conducted to more clearly delineate the time period during development when the effects are produced. For example, exposure of humans to angiotensin converting enzyme (ACE) inhibitors results in oligohydramnios, renal pathology, fetal growth restriction, hypoplastic ossification of the skull, and, possibly, patent ductus arteriosus–unexpected findings based on the standard animal testing studies. When follow-up studies were conducted, it was shown that these effects resulted from exposure during the second and third trimester in humans, equivalent to the late fetal and early neonatal stages in rodents (Barr 1997). Screening studies conducted in animals using repeated exposures throughout organogenesis may also be followed up with more discrete exposures to determine critical windows. For example, the finding that boric acid caused a number of skeletal alterations in studies in rats, mice, and rabbits with exposure throughout organogenesis (Heindel et al. 1994; Price et al. 1996), was followed up with studies by Narotsky et al. (1998) that show the specific times for the induction of skeletal alterations at different sites in the axial skeleton. Information on critical
Page 64
windows of exposure for several organ systems has recently been summarized (Selevan et al. 2000).
Additivity
Exposure by multiple routes is assumed to be additive. The default assumption is that simultaneous exposure to multiple agents having the same site or mode of action results in additive effects. Thus, for example, estimates of the developmental toxicity of chlorinated dibenzodioxins and dibenzofurans should consider the use of toxic equivalency factors (Safe 1993), provided the quantitative value assigned to each congener is relevant to the toxic effect under consideration.
QUANTITATIVE EVALUATION
Once an assessment has determined that the data indicate human risk potential, the next step is to perform a quantitative evaluation. Here, dose-response data from human and animal reproductive and developmental toxicity studies are analyzed to select LOAELs and NOAELs or to calculate a BMD. The assessment should use quantitative human dose-response data if the data span a sufficient range of exposure. Because data on human dose-response relationships are rarely available, the dose-response evaluation is usually based on an assessment of data from tests performed in experimental animals.
Box 3-2 defines terms commonly used in quantitative evaluations.
Identification of the NOAEL and LOAEL
The dose-response evaluation defines the range of doses that produce reproductive and developmental toxicity, the routes of exposure, the timing and duration of exposure, the species specificity of effects, and any pharmacokinetic or other considerations that might influence comparison with human exposure. Much of the focus is on identification of the adverse effect observed at the LOAEL and the NOAEL for the study.
Page 65
Box 3-2. Definitions
No-observed-adverse-effect level (NOAEL). The NOAEL is the highest dose at which there is no biologically significant increase in the frequency of an adverse reproductive or developmental effect when compared with an appropriate control group. Biological significance is based on expert judgment and consideration of statistical analyses. Lowest-observed-adverse-effect level (LOAEL). The LOAEL is the lowest dose at which there is a biologically significant increase in the frequency of adverse developmental or reproductive effects when compared with the appropriate control group. Biological significance is based on expert judgment and consideration of statistical analyses. Uncertainty factors (UF). A UF is a value applied to a NOAEL to account for variability in response across species and among humans. It usually is a factor of 10 for each area of variability (uncertainty), although each factor might be reduced or enlarged according to the quality and amount of data. Additional factors may be applied to account for uncertainty due to missing or inadequate data. A factor of 10 is also commonly applied when the data identify only a LOAEL instead of a NOAEL. Unlikely-effect level (UEL). This is an estimate of the daily exposure of a human population that is assumed to be without appreciable risk of causing reproductive or developmental effects. The duration can vary, depending on the human exposure scenario of interest. Margin of exposure (MOE). The MOE expresses the magnitude of difference between a level of anticipated human exposure and the highest level at which there is no significant increase in the frequency of an adverse effect (NOAEL). The MOE is the ratio of the NOAEL for a specific toxic effect to the estimated human exposure. Benchmark dose (BMD). The BMD is used as an alternative to the NOAEL for reference dose calculations. The dose response is modeled and the lower confidence bound for a dose at a specified response level is calculated. For a further description, see the section on BMD calculation. Human equivalent concentration (HEC). The HEC is used to describe the dose of an agent to which humans are exposed through inhalation. The HEC is the estimated concentration that is equivalent to that used in an experimental animal species. The HEC is estimated using adjustment factors that account for such species-dosimetric differences as ventilatory parameters and lung surface areas, as well as factors related to the gas, aerosol, or particulate nature of the agent. |
Page 66
Determination of the NOAEL is based in part on the statistical evaluation of the data from relevant studies. The statistical procedures used should reflect both the design of the experiment and structure of the data (for example, a blocked experiment in which the observed individuals are fetuses, but exposed individuals are their dams), and the type of data (dichotomous, categorical, or continuous). An overall evaluation of the statistical significance of treatment effect should be carried out first, ideally using a trend test such as Williams's Test (1971, 1972) or the NOSTASOT (no statistical significance of trend) procedure (Tukey et al. 1985), because such tests tend to be more powerful than analysis of variance (ANOVA)-like tests when the alternative to the null hypothesis (of no treatment effect) is a monotonically increasing or decreasing response. In the presence of a significant overall treatment effect, a NOAEL is determined to be the highest dose level at which there are no significantly different responses from the control group. This can be determined using sequential trend tests, sequentially dropping the highest dose in the remaining set of doses until there is no significant trend, as in the NOSTASOT procedure (Tukey et al. 1985; Faustman et al. 1994), or Williams's test (Williams 1971,1972), or by conducting pairwise tests that appropriately control the overall Type I error, such as Dunnett's test for independent, continuous data (Dunnett 1955, 1964). It should be recognized that the power of reproductive and developmental toxicity studies to detect effects with typically 20 animals per dose group varies for endpoints within and between studies. For example, the likelihood of detecting significant changes in continuous endpoints such as fetal weight is much greater than the likelihood of detecting significant changes in malformations (Nelson and Holson 1978). It is beyond the scope of this document to discuss statistical methods for evaluation of data in detail. Study types can be of varied design, thereby influencing statistical approaches for evaluation. Statistical methods for a variety of toxicology study designs are available (Gad 1998).
The NOAEL and LOAEL are constrained by the exposure concentrations used in a given experiment. For example, consider the case in which the administered doses used in an experiment were 1, 10, 100, and 1,000 mg/kg/d and the highest dose at which no increase in adverse effect (the NOAEL) was seen was 10/mg/kg/d. The exposure concentration at which no toxicity would have occurred might have been at any dose between 10 mg/kg/d and just below 100 mg/kg/d,
Page 67
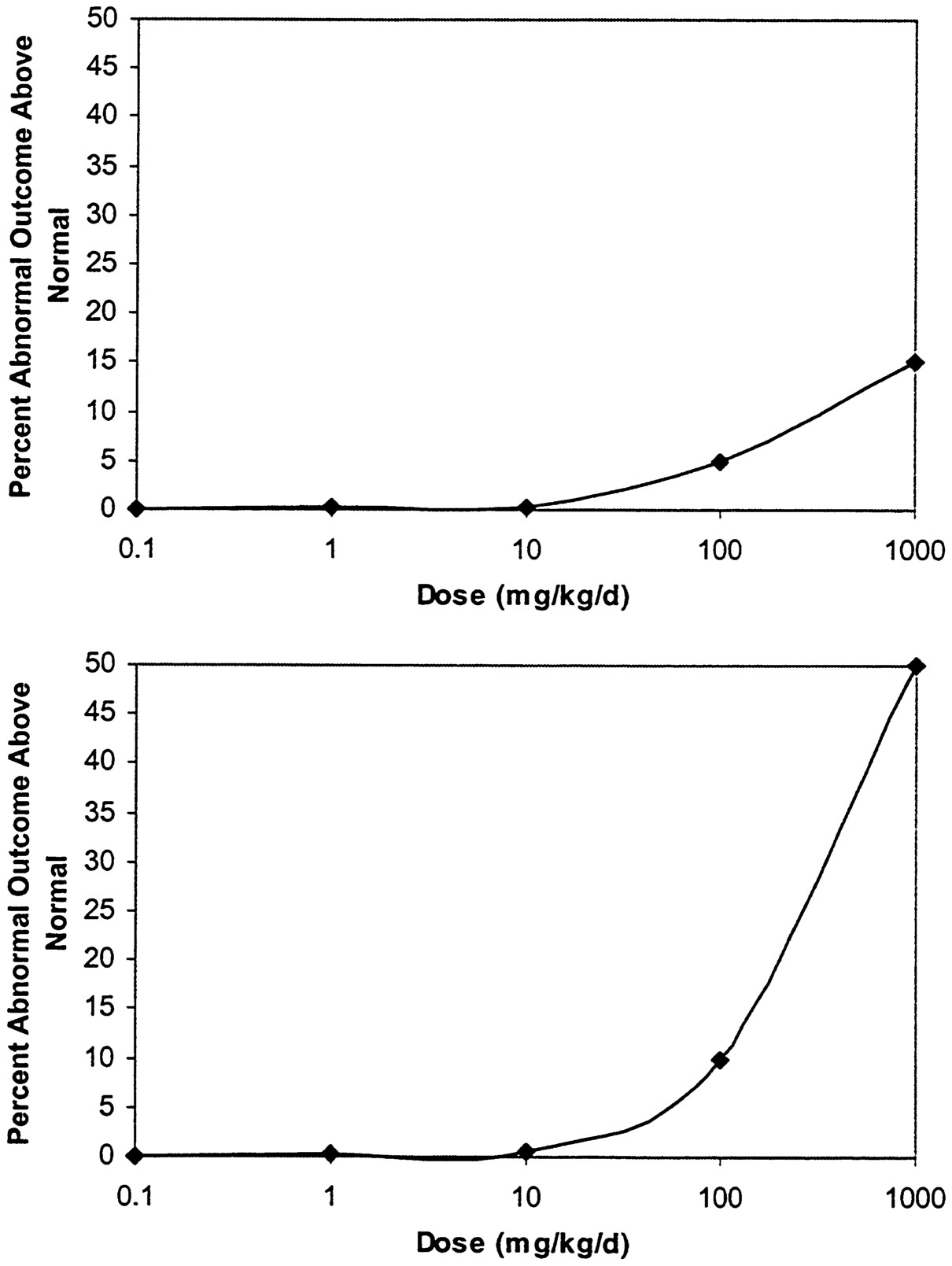
but the NOAEL would still be 10 mg/kg/d, because no other doses between 10 and 100 mg/kg/d were tested. The NOAEL approach also neglects the shape of the dose-response curve and ignores information about responses that were obtained at higher doses ( Figure 3-1), because only the dose at the NOAEL is used. Finally, the NOAEL is dependent on the statistical power of the study, so that using larger numbers of animals might have allowed for an effect to be detected at 10 mg/kg/d, leading to a lower NOAEL.
Calculation of the Benchmark Dose
Because the literature describes several limitations in the use of NOAELs (Gaylor 1983; Crump 1984; Kimmel and Gaylor 1988), the evaluative process considers other methods for expressing quantitative dose-response evaluations. In particular, the BMD approach originally proposed by Crump (1984) is used to model data in the observed range. That approach was recently endorsed for use in quantitative risk assessment for developmental toxicity and other noncancer health effects (Barnes et al. 1995). The BMD can be useful for interpreting dose-response relationships because it accounts for all the data and, unlike the determination of the NOAEL or LOAEL, is not limited to the doses used in the experiment. The BMD approach is especially helpful when a NOAEL is not available because it makes the use of a default uncertainty factor for LOAEL to NOAEL extrapolation unnecessary.
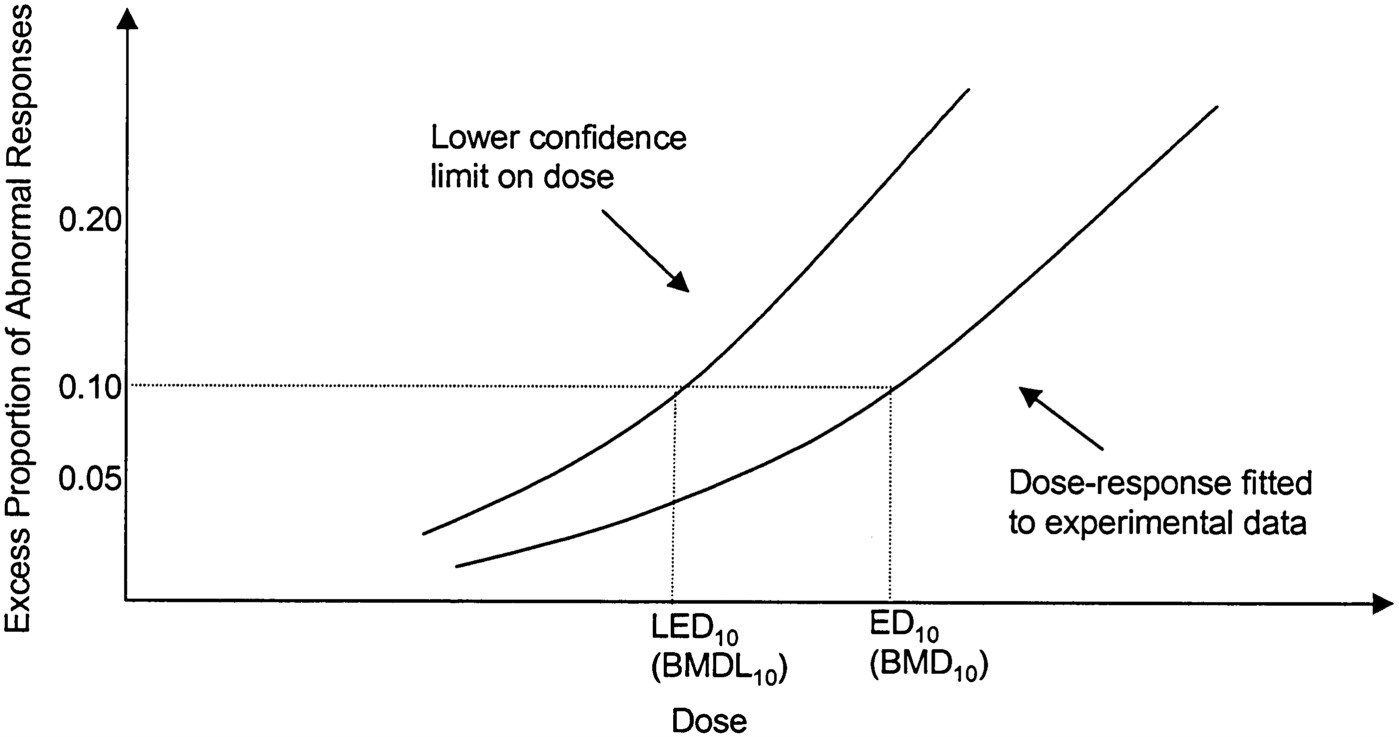
The BMD is a model-derived estimate of a particular level of response above background for dichotomous endpoints that is near the lower limit of the range of experimentally detectable effects, the benchmark response (BMR; e.g., 5% or 10%). To obtain the BMD, one begins by modeling the data in the observed range, resulting in a curve that, for dichotomous data, gives the probability of response for the experimental dose corresponding to the BMR. The BMDL is the lower confidence limit on that dose and is the value used for calculating reference levels.1Figure 3-2 illustrates the relationship between the dose-re
1 The convention of using BMDL as the lower confidence limit follows the terminology proposed in the paper by Crump (1995); this has also been adopted for use in the EPA BMDS software, since it refers explicitly to the lower confidence limit value.
Page 68
~ enlarge ~
FIGURE 3-1 Sample dose-response curves. In each case, the NOAEL is 10 mg/kg/d and the LOAEL is 100 mg/kg/d, assuming that the increase above control at these exposures is significant.
sponse model, the BMD and the BMDL for a BMR of 10% above background for a dichotomous endpoint.
Using the BMD approach, one can calculate a value for each effect of an agent for which sufficient data are available. A level between the
Page 69
~ enlarge ~
FIGURE 3-2 Illustration of the BMD. LED10 = BMDL10, lower confidence limit on the dose resulting in a 10% response; ED10 = BMD10, best estimate of the dose at a 10% level of response derived from the model. Source: Adapted from Moore et al. (1995a).
BMD01 and the BMD10 usually corresponds to the lowest level of observed risk that can be estimated for dichotomous endpoints without extrapolating to lower levels. The articles by Allen et al. (1994a,b) and an EPA background document (1995) provide a broader discussion of these issues. The U.S. Environmental Protection Agency (EPA) has free software for modeling the BMD and BMDL ( http://www.epa.gov/ncea/bmds.htm).
For continuous data, there are several options for deriving the BMR: (1) the degree of change considered adverse for that effect is used as the BMR and the data are modeled as continuous data; (2) if individual data are available and there is an accepted level of change considered adverse, the data can be “dichotomized” (number above or below the cutoff value, perhaps based on some quantile of the distribution), and modeled as for dichotomous data; or (3) in the absence of any knowledge of what to consider adverse, a standard can be applied (e.g., one standard deviation of the control mean), and data handled as in 1 or 2 above for the degree of change. An alternative to modeling continuous data directly is to use the so-called “hybrid” approach, such as that described by Gaylor and Slikker (1990), Kodell et al. (1995),
Page 70
and Crump (1995). This approach fits continuous data using continuous models, then presuming a distribution of the data, a BMD and BMDL can be calculated in terms of the fraction affected, resulting in a probability (risk) of an individual being affected as for dichotomous responses.
Duration Adjustment
Adjustments are often made in the NOAEL or BMD to account for the exposure scenario of concern. In the case of inhalation exposure, for example, if a study involved exposure to 500 parts per million (ppm) for 6 hours per day (h/d), and there are no modifying pharmacokinetic data, the adjusted NOAEL or BMD for a continuous exposure would be calculated by multiplying by 6/24, yielding 125 ppm. Adjustment to account for the duration of exposure has not been applied routinely in assessments of developmental toxicity; such an adjustment is made in the case of assessments for reproductive toxicity. The Subcommittee on Reproductive and Developmental Toxicology recommends that exposure duration should be considered in developmental and reproductive toxicity assessments alike. The reason for this recommendation is that adjusting for duration of exposure is likely to be more conservative with repeated exposures than with single exposures, even for developmental toxicity data (Weller et al. 1999). In the case of occupational exposure during a 6-8 h workday, this adjustment could be unnecessary. However, if pharmacokinetic data indicate accumulation with repeated exposure, an adjustment would be appropriate.
Pharmacokinetic information that relates blood concentration to toxic response is critical in defining such dose-response relationships, but information on peak blood concentrations or blood concentrations over time (area under the curve (AUC)) is seldom available. One agent for which such information has been published is 2-methoxyacetic acid (2-MAA), the active metabolite of 2-methoxyethanol. Terry et al. (1994) showed that peak concentration was related to neural tube defects observed after exposure in mice on gestation day 8, whereas area under the curve was shown to be related to limb defects after exposure to 2-MAA on gestation day 11 (Clarke et al. 1992), suggesting that the time of exposure and pattern of development of the susceptible organ
Page 71
may be as important as the dose metric. Kimmel and Young (1983) showed that a combination of peak exposure and area under the curve for a single dose of salicylic acid was important in defining the dose-response relationship for malformations and other effects. Less has been done to examine the relationship with longer-term, repeated dosing. Pharmacokinetic data may be used to adjust exposure concentrations for such agents. As more pharmacokinetic information becomes available, it is important to minimize the dependence on default assumptions and to encourage the use of pharmacokinetic data to determine the appropriate dosimeters to use in adjusting exposure levels and determining internal dose.
Uncertainty Factors
Factors to account for various uncertainties are applied to the NOAEL, LOAEL, or BMD to derive a UEL. The total size of the uncertainty factor (UF) varies, accounting for assumed or known interspecies differences, variability within humans, quality and quantity of the data, consistency, slope of the dose-response curve, background incidence of the effects, and pharmacokinetic data. The relevance of the species, type of effect, dose, route, timing, and duration of exposure are additional factors that might influence its size. A discussion of UFs is provided in several papers (e.g., Lewis et al. 1990; Renwick 1991,1998; Dourson et al. 1996; Renwick and Lazarus 1998).
UFs for reproductive and developmental toxicity applied to the NOAEL often include 10-fold factors for interspecies and intraspecies variation. Additional factors might be applied to account for other uncertainties or for additional information that might exist in a database. For example, in circumstances in which only a LOAEL is available, it might be necessary to use an additional UF uncertainty factor of up to 10, depending on the sensitivity of the endpoints evaluated, the adequacy of the tested dose, or general confidence in the LOAEL. An additional uncertainty factor of 3-10 has been used by EPA (1996a) to account for database deficiencies, particularly the lack of reproductive and developmental toxicity studies.
The experience gained from assessing the data-rich chemicals lithium and boric acid using the evaluative process described by J.A.
Page 72
Moore et al. (1995b, 1997) showed that the expert review groups did not routinely apply factors of 10 for interspecies and 10 for intraspecies variability to the NOAEL. In each case, interspecies and intraspecies factors were reduced by half a log (J.A. Moore et al. 1995b, 1997); knowledge of pharmacokinetics was useful in reducing uncertainty in the data for predicting human risk.
Calculation of the Unlikely Effect Level
The UEL for reproductive and developmental toxicity is derived by applying uncertainty factors to the NOAEL, LOAEL, or BMDL. To calculate the UEL, the selected UF is divided into the NOAEL, LOAEL, or BMDL for the critical effect in the most appropriate or sensitive mammalian species. This approach is similar to the one used to derive the acute and chronic reference doses (RfD) or Acceptable Daily Intake (ADI) except that it is specific for reproductive and developmental effects and is derived specifically for the exposure duration of concern in the human. The evaluative process uses the UEL both to avoid the connotation that it is the RfD or reference concentration (RfC) value derived by EPA or the ADI derived for food additives by the Food and Drug Administration, both of which consider all types of noncancer toxicity data. Other approaches for more quantitative dose-response evaluations can be used when sufficient data are available. When more extensive data are available (for example, on pharmacokinetics, mechanisms, or biological markers of exposure and effect), one might use more sophisticated quantitative modeling approaches (e.g., a physiologically based pharmacokinetic or pharmacodynamic model) to estimate low levels of risk. Unfortunately, the data sets required for such modeling are rare.
Calculation of the Margin of Exposure(MOE)
The MOE is the ratio of the NOAEL or BMDL to the anticipated human exposure. The higher the ratio, the greater the numerical distance between the human exposure estimate and the dose that is at the lower end of the range of concern from animal studies. The ade-
Page 73
quacy of the MOE should be evaluated on the same basis as for the UEL. For example, if the NOAEL in a rat developmental toxicology study is 10 mg/kg/d and the human exposure anticipated is 0.1 mg/kg/d, the MOE is 100. The adequacy of the MOE should consider interspecies and intraspecies variability and any other uncertainties accounted for in the UFs applied to derive the UEL. If the MOE is less than the total UF applied in calculating the UEL and is judged to be inadequate, then exposure must be reduced either by applying controls or by removing the exposure.
Because human exposure might differ in different settings, there can be different MOEs for different circumstances. For example, the MOE for an occupational setting might differ from the MOE for environmental exposure. An MOE of 100 is equivalent to exposure at the UEL if the UEL is adjusted by 2 orders of magnitude for uncertainty factors that represent interspecies and intraspecies variability. It should be emphasized, though, that the use of those factors is based on judgment rather than on the default assumption that they are appropriate in all instances. In some cases, a small MOE can be considered protective of health. For example, the lithium assessment conducted using a similar evaluative process (J.A. Moore et al. 1995b) resulted in MOEs of 11-108 for exposures other than the therapeutic use of lithium. Because the size of the total UF applied to the NOAEL was 10, these MOEs were judged to be protective.
Some regulatory agencies use the MOE as an action level. Which MOE is selected is a matter of policy rather than science. For example, California's Proposition 65 relies on an MOE of 1,000 to select exposures to a particular agent for regulatory action. The institution of action based on any given MOE does not mean that toxicity will occur at a lower MOE, only that the chosen MOE is believed to be protective. The subcommittee recommends against using a particular value as an action level because it is arbitrary and does not take into account the complexity and uncertainties inherent in such assessment processes or the variability in differing exposure situations.
As in the case for the choice of UFs and modifying factors in the calculation of an UEL, the choice of an MOE for regulatory action can be based on the level of confidence in the underlying data and on judgment about other factors that might influence risk.
Page 74
Assessing a Degree of Concern
EPA (1991, 1996a) and the International Programme on Chemical Safety (IPCS 1995) have proposed approaches for characterizing the database concerning potential reproductive and developmental toxicity and provided a basis for how to assess the degree of concern. In its developmental toxicity (EPA 1991) and reproductive toxicity (EPA 1996a) risk-assessment guidelines, EPA uses a weight-of-evidence approach to determine whether a substance poses a risk to humans based on an overall evaluation of reproductive and developmental toxicity and exposure data.
Some aspects of degree of concern currently can be considered in a quantitative evaluation. For example, EPA considers human and animal data in the process of calculating the RfD, and these data are used as the critical effect when they indicate that developmental effects are the most sensitive endpoints. When a complete database is not available, a database UF is recommended to account for inadequate or missing data. The dose-response nature of the data is considered to an extent in the RfD process, especially when the BMD approach is used to model data and to estimate a low level of response; however, there is no approach for including concerns about the slope of the dose-response curve. Because concerns about the slope of the dose-response curve are related to some extent to human exposure estimates, this issue must be considered in risk characterization. (If the MOE is small and the slope of the dose-response curve is very steep, there could be residual uncertainties that must be dealt with to account for the concern that even a small increase in exposure could result in a marked increase in response.) On the other hand, a very shallow slope could be a concern even with a large MOE, because definition of the “true” biological threshold will be more difficult and an additional factor might be needed to ensure that the RfD is below that threshold.
As an example, consider two compounds that are candidates for use in an occupational setting. Both compounds have an MOE of 100, but one is an alkylating agent with a steep dose-response curve. Although the MOEs are the same in this setting, it is reasonable to select the compound that is not an alkylating agent because it is associated with concern at a higher dose. The NOAEL approach does not allow for considerations of variability in the data, but use of the lower confidence limit on dose in the BMD approach does account for vari-
Page 75
ability in the animal or human data on which it is based, even though it does not account for all intraspecies variability.
Both the interspecies and intraspecies UFs include consideration of potential toxicokinetic and toxicodynamic similarities and differences among species and within humans, and those factors can be adjusted when data are available to account more appropriately for similarities and differences among species and within human subpopulations, including different age groups. For example, if it is assumed that a portion of interspecies and intraspecies variability is the result of differences in kinetics, information about the kinetics of a substance in the experimental animal model and in humans might indicate less uncertainty about the extrapolation of effect levels. When there is less uncertainty, lower UFs can be considered. An example of an evaluation in which lower UFs were used is the evaluation of lithium by Moore et al. (1995b). Lower UFs were used because the risk assessment was performed using achieved serum concentrations, rather than administered doses, obviating the influence of absorption and distribution differences among and within species, and because biotransformation of lithium does not occur. Although various means of accounting for degree of concern are described here, there is no formal process for doing so, and this issue should be further considered for the appropriate calculation of the UEL.
Although it is tempting to use default values for UFs or MOEs in the regulation of human exposures, the importance of professional judgment in evaluating the data set for a given substance makes it advisable to avoid inflexible approaches to regulating exposures. Consideration of the reliability of the reproductive and developmental data set and of non-reproductive toxicity data can be an important part of the evaluative process. The inclusion of all available information in the evaluation and the use of scientific judgment are recommended as most likely to lead to the most informed estimate of the risk of anticipated human exposure.
Application of Reproductive and Developmental Toxicity Data to Various Exposure Scenarios
Although the product of the evaluative process is an effect level that can be manipulated in different ways to estimate the risk posed by
Page 76
human exposures, considerable judgment is necessary in the evaluation of the confidence with which the estimation can be performed. There are instances in which the quality of a data set is sufficient to permit the determination of a NOAEL or BMD, but characteristics of the data set detract from the certainty that the NOAEL or BMD will give rise to an appropriate UEL or MOE. A difference between the dosing schedule and route of exposure used in an experimental study and the anticipated human dose and route of exposure are examples of factors that might undermine confidence in the predictive value of the experimental data. Even when the dose and route of exposure are the same as the anticipated human dose and route of exposure, the exposure pattern might be sufficiently different to decrease confidence in extrapolation from the data.
The data available on reproductive and developmental toxicity usually come from studies using repeated dosing regimens that can be characterized as short-term or subchronic exposures. For example, in the prenatal developmental toxicity study, dosing covers a period of development equivalent to the first and part of the second trimester of human gestation. In the developmental neurotoxicity study, the dosing period is both prenatal and early postnatal to cover most of nervous system development. In the two-generation reproduction study, animals are exposed continuously through both generations. The NOAELs and BMDs for all developmental toxicity studies should be compared with all other toxicity data so that, in the cases where the NOAEL or BMD for developmental toxicity is lower than the NOAEL or BMD for chronic toxicity, they can be used as the basis for the UEL and be protective of children's health.
Although there are no developmental studies in which an acute (single) dosing regimen is used to meet regulatory requirements, a central premise in developmental toxicology is that adverse developmental outcomes can result from a single pre- or postnatal exposure. An experimental animal study that uses once-daily gavage might not produce the same exposure profile as human dietary exposure to an agricultural chemical, for example. Although it has been customary to use effect levels from experimental studies without regard to differences in dosing profiles, supplemental information about the activity of a substance might suggest that dosing profile differences should be considered.
Page 77
If, for example, it is clear that a substance produces toxicity by reaching a peak concentration in the plasma, a single-dose gavage study will be more likely to reach that peak than will administration in the diet. A NOAEL from a single-dose gavage study might be regarded with a different level of confidence under those conditions than would a NOAEL from a dietary study. Data are available to show that most of the types of developmental endpoints from studies used to evaluate pre- and postnatal toxicity (prenatal developmental toxicity, developmental neurotoxicity, two-generation reproduction studies) can result from single exposures. It is recognized that some outcomes might result only from repeated exposure to a given substance and the degree of reversibility of the effect might depend on the duration of exposure. For example, plasma concentrations of agents that induce their own metabolism are lower after a few days of exposure than on the first day of dosing. Thus, a single exposure on a critical day might be of concern. However, some agents might require repeated exposures to reach steady-state plasma concentrations. Therefore, a singleexposure study would underestimate the toxicity of repeated exposures. Determining whether a particular developmental outcome results from a single acute exposure or from repeated exposures requires additional studies that are not often available. Information on toxicokinetics or mechanisms of action might be helpful in interpreting the data but, again, such information is not typically available. As a default, data from all studies that evaluate reproductive and developmental toxicity should be considered in determining UELs for acute, short-term, and longer-term exposure scenarios.
Most developmental toxicity studies (of all types) are conducted using the oral route of exposure. In some cases, dermal exposure is used and, rarely, inhalation exposure. Route-to-route extrapolation is sometimes done to allow consideration of developmental toxicity data. Pharmacokinetic data on different routes of exposure can be extremely useful in the extrapolation of data between routes.
Critical Data Needs
A primary objective of the evaluative process is to use data to formulate and express judgments about reproductive and developmen-
Page 78
tal risk potential for humans. Flawed or nonexistent data compromise the certainty of scientific judgment. Although guidance or regulations promulgated by government agencies serve definite needs, they are somewhat rigid. For the evaluative process that is proposed here, it seems best to determine the adequacy of the database in a case-specific manner.
It is better to ascertain toxicity and estimate dosimetry using a species in which the metabolism pathway for that agent parallels that of humans than it is to try to assess toxicity and dosimetry in two randomly selected species in which metabolism of the substance is either unknown or is dissimilar to that of humans. For example, methanol, which is acutely toxic to humans and nonhuman primates, is metabolized via a folate-dependent pathway. However, rodents use a different folate-dependent pathway, and the rate at which rodents detoxify formate (a metabolite of methanol and the agent that causes toxicity to humans exposed to high doses) is more rapid than that in primates. That increased rate can be attributed to higher levels of hepatic tetrahydrofolate, the enzyme responsible for the oxidation of formate (Tephly and McMartin 1984; Johlin et al. 1987; Medinsky and Dorman 1995). Therefore, rodent species would not be the best to use for extrapolating acute, high-dose methanol toxicity in humans. On the other hand, for human exposures to methanol at concentrations below the threshold for formate accumulation, rodent models can be useful because they provide the advantage of allowing dose-response studies in which the animals do not experience formate build-up (Rogers et al. 1993).
During the review of existing information, evaluators might identify some data as insufficient for judging human risks, either because the data do not exist or because they are compromised in some way for risk assessment. In another chemical evaluation, data might be judged sufficient to determine human risk potential, but in the judgment of the evaluators there might be large degrees of uncertainty because of reliance on default assumptions or because of the inherent uncertainty in some of the data that are central to the evaluation. In each instance, evaluators will cite specific data needs if they determine that the data will materially improve the certainty of an existing judgment about human risk.
Page 79
SUMMARY
The selection of the term “summary,” instead of “risk characterization,” to describe the concluding step in the evaluative process is deliberate. The evaluative process described here focuses on reproductive and developmental effects; it does not account for all the effects that should be included in a risk characterization. Moreover, a lack of detailed exposure information will be common in this type of evaluative process but is necessary for a risk characterization.
In this case, the summary communicates to Navy environmental health practitioners scientific judgment on chemical risk for reproductive and developmental toxicity. The degree of certainty of the judgment must be expressed in terms that are meaningful to those with a general science background in toxicology and risk assessment. The key to achieving that goal is candor in explaining the basis of the judgment, its breadth of support, and, especially, the degree to which the judgment reflects actual information, confident extensions from closely related data, or the invoking of assumptions when no information is available.
The summary is written from statements developed in the integrated evaluation and quantitative assessment steps of the evaluative process. The summary will review the following elements:
-
Background. This section provides a brief, readable review of the general chemical, toxicological, and biological characteristics of the substance.
-
Human exposure. This section gives a clear statement of the conditions of use or ambient concentrations that might produce different doses, routes, or frequencies of human exposure. It describes how different patterns of use produce differences in the magnitude of exposure.
-
Toxicology. Summaries of developmental toxicity, and of male and female reproductive toxicity, appear in this section. The discussion also contains statements about the sufficiency and relevance of the data.
-
Quantitative evaluation. This section lists the quantitative values derived in the evaluative process and states the degree to
Page 80
-
Certainty of judgment and data needs. The use of default assumptions, while often necessary, represents a tangible expression of uncertainty. To clarify that point, this section discusses the magnitude of an assumption's influence on the judgments made in the evaluation. Where the effect is large and the uncertainty great, the evaluators might sometimes defer a judgment. Where a default assumption has a major effect on the evaluative judgment, the evaluative summary clearly defines the kind of data needed to supplant the default and identities that as a critical data need.
Only some aspects of the assessment might involve uncertainty of judgment. For example, although there might be great certainty that the data qualitatively predict human health risk potential, the nature and degree of exposure might be poorly understood. In that case, the evaluative summary will clearly state that there is reasonable certainty of human risk potential and explain why the quantitative uncertainty (missing, inadequate exposure data) leads to the use of a conservative default assumption that is likely to overestimate the degree of exposure and risk.
-
References. In any evaluation of this nature, a bibliography is imperative. All literature reviewed should appear in a reference list. A separate listing of references reviewed but not used in the evaluation also should appear in the document.
which the values are derived from actual data or reflect the use of default assumptions.
























