Page 115
3
Characteristics and Management of Major Symptoms
The signs and symptoms of multiple sclerosis (MS) are generally related to the most heavily myelinated parts of the central nervous system (CNS), but they are notoriously variable. Some symptoms such as dizziness, tingling sensations on the skin, or visual tracking disturbances are easily forgotten and are often hard for patients to describe. The majority of fleeting cerebral abnormalities seen on magnetic resonance imaging (MRI) cannot be correlated with any symptoms; even chronically demyelinated areas of the optic nerve and spinal cord can be symptom free.107
In general, MS patients report mental health as more important than physical impairment and bodily pain in determining their quality of life. This is different from neurologists' beliefs about the most important determinants of health-related quality of life for patients with MS or the beliefs of members of the general public about their own quality of life.146 Nine of the most prominent symptoms are described in this chapter. They are presented roughly in order of the importance that MS patients assign to them as determinants of overall quality of life, although it should be noted that this ranking is based on a small survey and that individual variability is a prominent feature of all aspects of MS.146
COGNITIVE IMPAIRMENT
Fear of mental change is one of the greatest concerns of MS patients when they learn they have the disease. Cognitive dysfunction is one of the most disabling features of MS and, even when subtle, can begin to limit a person's ability
Page 116
to cope, to stay employed, and to carry out family responsibilities and enjoy life.140
Early writers on MS often commented on cognitive and emotional changes, but in the mid-twentieth century, a pattern of denial of these features developed in the medical literature.143 Just as clinicians passed off the frequent symptoms of pain and fatigue as features of the disease, they also ignored the often seen emotional and cognitive changes (Jock Murray, personal communication). Donald Paty (personal communication) noted that there was a negative reaction to his suggestions in the 1970s that cognitive dysfunction should be a focus of study by the National MS Society. At the time, it was estimated that only 5 percent of MS patients might incur cognitive change, and it was argued that cognitive change was relatively unimportant in MS.143 By the 1990s, those views had changed, and in 1992, the MS Society and the International Federation of Multiple Sclerosis Societies jointly held a symposium on “Neurobehavioral Disorders in MS: Diagnosis, Underlying Pathology, Natural History and Therapeutics.” Cognitive changes are now estimated to occur in about 43 percent of MS cases.54
The conviction that cognitive changes must be selectively analyzed and distinguished from other phenomena such as depression and fatigue has emerged only in the last few decades. Standard psychological tests, however, are not very effective in identifying the type of changes that occur in MS. Rao, LaRocca, Fischer, Peyser, and many others have recently made considerable progress in adapting tests that can detect the specific changes seen in this disease,54,57,130 yet much more remains to be done. Paradoxically, as we are learning to separate and more effectively measure the cognitive changes and the affective changes, this separation has made it possible to learn how they are so often linked (Jock Murray, personal communication).
Cognitive changes in MS generally are not global, but are most often circumscribed to specific processes. Learning, recall of new information, and speed of information processing are affected most often; deficits in visuospatial abilities and executive functions such as reasoning, problem solving, and planning are also common.54 Performance accuracy is less affected, but it appears affected if timed tests are used.41 Once cognitive impairment is present, it does not often remit (reviewed in 1999 by Fischer54).
Poor memory is a common complaint among MS patients.3 Depending on sample selection methods and criteria used to define impairment, approximately 20 to 42 percent of MS patients have some deficit in their free recall of recently learned verbal and visual material (reviewed in 1994 by Fischer et al.56). Although memory deficits are common, certain processes remain intact. For example, the rate of learning, the likelihood of remembering a specific item based on when it was presented, and the ability to detect semantic characteristics of the material to be learned are preserved in all but the most impaired MS patients. Implicit memory, or the ability to learn new information or skills without explicitly attending to it, is also preserved. Recognition of recently learned information
Page 117
is generally impaired to a much lesser extent than free recall. Impairment of verbal fluency (the ability to rapidly generate words meeting specific phonological or semantic criteria) and, to a lesser extent, confrontation naming (the ability to retrieve the names of objects) are often associated with memory impairment. Decreases in word fluency are common, whereas decreases in verbal comprehension are less common.4
A study of 44 MS patients found that on tests of cognitive performance designed to measure planning skills tests the MS group performed on average significantly worse than controls.8 However, this was due largely to deficits among chronic progressive, as opposed to relapsing-remitting patients. Another caveat is that this was a timed test, so that in addition to planning skills, information processing speed would have influenced performance, which would likely bias the results since this is often affected in MS patients.
Time Course
The time course of cognitive changes in MS is highly variable, although they appear to occur very early in the disease, often before the onset of other symptoms. Different types of cognitive change can appear in different sequences in different patients, and few studies have documented changes over time in individual patients. In one study, 50 patients were tested early in the disease (on average, 19 months after clinical onset) and again 4.5 years later.4 Initial tests revealed statistically significant deficits in verbal memory and abstract reasoning relative to controls, with similar results in the follow-up tests. The difference in average scores between patients and controls was about 10 percent. However, the difference in variability was much more striking. The variability in scores for the MS group was consistently greater than for controls, and in 7 out of 15 cases the variance of the MS group was more than twice that of the controls. This suggests that the cognitive performance of many of the MS patients was not measurably affected, whereas others were substantially affected. A simple analysis of group differences is not sufficient to answer this question. This study also illustrates the value of using individual patients as their own controls.
Association with Other Symptoms
Cognitive and neurological deficits do not appear to develop in parallel, at least not in patients whose disease is still in its early phase.4 Disease duration is not a good predictor of cognitive function in MS, but disease course influences the likelihood of cognitive impairment. Chronic progressive patients tend to do more poorly on neuropsychological tests than relapsing-remitting patients (reviewed in 2001 by Fischer55). Expanded Disability Status Scale (EDSS) scores and specific neurological symptoms are not correlated with cognitive deficits.8,53,134 The EDSS is shown in Appendix D. Despite this, clinicians consis-
Page 118
tently overestimate the correlation between physical disablity and cognitive impairment in MS patients.56
While some studies report that cognitive function is independent of fatigue,63,180 others raise the possibility that they might be caused by a disruption of the same neural circuits (see Fischer 2000 for discussion). Depression is generally not strongly related to overall cognitive function. One study of 20 MS patients found that cognitive deficits (attention, visuomotor search, and verbal fluency) were independent of depressive symptoms.96 Of these, only frontal function impairment was correlated with depression. However, only 4 percent had significant depression scores, and all of these had secondary progressive MS.53 A cross-sectional study of 24 patients found significant association of cognitive impairment (using tests of abstract verbal and nonverbal memory) with depression, but not with the degree of neurological impairment, specific neurological symptoms, disability, or handicap.68
Neuropathological Correlates
The traditional view that MS is characterized by discrete lesions does not explain the memory and cognitive changes, which would require a more widespread, bilateral change, especially since the complaints often arise early. Recent evidence from the studies of Ian McDonald in Great Britain and Bruce Trapp in the United States indicates that the effects of demyelination and the destruction of axons occur very early in MS and are widespread. Moreover, the process undoubtedly has been going on for a long time before a person experiences the first symptom.
MRI has recently allowed speculation of localization of specific mental changes.139 Although MRI studies of MS patients have reported correlations between cognitive impairment and total lesion burden (the percentage of the brain that shows lesions on MRI scans), neuroimaging techniques have not reached the point where neuropathological changes can be linked to specific aspects of cognitive impairment (reviewed in 2000 by Rovaris and Filippi147). Research on cognitive changes in MS is still in its early stages, and most studies have been relatively small and have not followed changes in individuals. In addition, the application of techniques that allow detection of more specific neuropathological changes, particularly axonal pathology, might provide more useful insights into the causes of cognitive impairment.
Pathological Laughing and Crying
Pathological laughing and crying is a distinctive type of cognitive change that occurs in a variety of neurological disorders including stroke, amyotropic lateral sclerosis (ALS), Alzheimer's disease, cerebral tumors, and MS. The syndrome is defined as a sudden loss of emotional control—for example, laughing, crying, or both in response to nonspecific, often inconsequential stimuli for no
Page 119
apparent reason. The etiology of pathological laughing and crying is unclear. The cortex and, possibly, prefrontal cortex are thought to be involved. In a study of 152 patients with long-standing disease and significant physical disability (unable to walk without assistance), pathological laughing and crying as distinct from emotional lability affected 10 percent of the patients.49 (Emotional lability refers to abrupt changes in mood.) A preliminary study indicates that such patients had relatively greater difficulty with speed of information processing than their MS control subjects without pathological laughing and crying.51
There have been a number of reviews of euphoria, all suggesting that euphoria is a reflection of organic change. Rabins used pre-MRI studies to show that euphoria was associated with greater brain involvement with MS, particularly in the periventricular areas, but occurred in less than 10 percent of patients.136 Recent MRI studies indicate that the cognitive and emotional changes are likely to have specific neuroanatomical correlates.
Management
Initial studies have shown some limited gains by methods of cognitive rehabilitation, and more needs to be known about what approaches would be helpful. Memory failures of MS patients sometimes resemble those found in people with histories of closed-head injury. A study of teaching memory strategies to people with MS found that MS subjects were able to learn the strategies quickly and did not appear to require the lengthy training needed by persons with head injury.3 Two studies of the effects of amantadine, a medication often prescribed for fatigue, showed either no effect on cognitive function63 or a modest benefit on processing speed, and that effect was limited to patients who had MS for 7 years or longer.150 It was recently reported that after a 2-year course of interferon-beta (IFN-β-1a) relapsing MS patients had significantly better cognitive functions than placebo-treated controls.58 Although assessment of cognitive changes in MS clinical trials is challenging, further tests will be important to clarify this effect.
Until ways of stopping or reducing cognitive change are developed, patients could benefit by any methods that at least help them and provide them with techniques to alleviate the problems. For example, people with memory deficits can use portable tape recorders, daily planners, and computer memory aids to keep track of schedules and review discussions during physician visits or education sessions.
Research Needs
The revelation of the prevalence of cognitive changes together with the advent of MRI has stimulated a surge of research over the last decade, which has in turn clarified specific needs for further research.
-
Research on the underlying pathophysiological changes leading to cognitive and pathological emotional change is needed, because the relation
Page 120
-
Further research on more representative populations of MS patients is necessary to reveal the extent and degree of involvement, and long-term studies are needed on this group (community-based versus clinic populations). Previous studies are on groups that may not be representative.
-
We need to know more about the impact of early cognitive changes on the quality of life.140
-
Further research is needed into the MRI, functional MRI, and PET (positron emission tomography) scan correlates of the cognitive change. We need to understand more about why some patients develop severe cognitive change, others mild or moderate change, and others with long-standing disease have no measurable change.
-
Further research is needed into disease-specific neuropsychological tests to better identify early changes in MS, and this should be translated into a standard battery that could be used for the clinic and bedside and as a part of all clinical therapy trials.
-
More data are needed to define the temporal course of cognitive changes and would be invaluable in assessing the impact of new agents used in treating MS.
-
Better measures of the cognitive changes in MS should be developed, although the need for specific neuropsychological tests for the specific changes noted in MS has been recognized, as developed by Stephen Rao.139
ship between pathological changes observed in the disease and the observation of cognitive change is speculative at the present time. Understanding the underlying mechanisms may explain why there isn't a close correlation of cognitive change with disability, disease course, or disease duration.
DEPRESSION
Prevalence and Diagnosis
Depression is the most common mood disorder in MS. Alterations in mood and affective state have long been recognized in MS,35 although estimates of their prevalence vary widely. Estimates of the prevalence of major depression among MS clinic patients at any one time range from about 15 to 30 percent and from 40 to 60 percent for lifetime prevalence (reviewed in 1995 by Nyenhuis et al.,126 and in 1997 by Aikens et al.,1,56), which is three times that found in the general population. Depression is more prevalent in MS than other neurological disorders, such as Parkinson's disease, in which it is one-half to one-third less prevalent than in MS.141 However, these estimates might be deceptive because they are typically based on patients attending MS clinics. For example, the estimated prevalence of major depression among stroke patients depends on setting, in-
Page 121
creasingly linearly from community samples (2 to 4 percent) to primary care settings (5 to 10 percent) to inpatient medical settings (6 to 14 percent).25 The prevalence of depression among the MS population at large has not been well studied and is probably lower than that among patients attending specialized clinics.
Many studies of depression among MS patients have been plagued by methodological difficulties.141 Varying diagnostic criteria have been used, including unstandardized tests. Most importantly, factors that affect mood or its assessment are frequently not taken into account. For example, mood can be affected by exacerbations, psychoactive prescription drugs (for example, corticosteroids), and fatigue.
Research on depression in MS is complicated by the fact that validated depression rating scales rely, in part, on evaluations of fatigue and other bodily symptoms that are common in MS, and can occur independently of depression.126 The Beck Depression Inventory (BDI), which is one of the most widely used depression scales, evaluates depression based on responses to 21 questions, including many that ask about symptoms that overlap with those of MS. For example, subjects are asked whether they get tired more easily than they used to or whether they worry a lot about health problems. Positive answers to such questions from people with MS might have little to do with depressed mood and might simply reflect a realistic appraisal of their condition. Thus, it seems likely that scales such as the BDI would tend to overestimate depression in people with medical conditions that produce certain symptoms. One study that used different scales to measure depression among MS patients found that the apparent prevalence of depression among MS patients varied, depending on the scale used. When only the mood scale was used for the Multiple Depression Inventory (MDI), a self-report depression scale, 18 percent of patients were rated as depressed, in contrast to 31 percent rated with the BDI and 27 percent when the total MDI scale was used.126 Another study found little difference between the scores of MS patients and healthy controls, except for questions about sexual disinterest for which MS patients exceeded healthy controls.2 This potential tendency for spurious increase in depression has been noted for other medical conditions and should be carefully evaluated.23,26
Although much of the research on depression and MS has focused on the existence of major depressive syndromes, many MS patients suffer from mood alterations that are consistent with depression but do not qualify as major depressive disorder.76,126
Association with Other Symptoms
Depression is not clearly related to the severity or type of disability, type of MS, or duration of symptoms.52,134 Measures of depression are not related to EDSS values. However, depressive symptoms interfere with daily functioning in
Page 122
medically well individuals as well as in those with chronic disease. Depressed patients tend to function worse in their work, physical, and social roles compared to patients with a variety of medical conditions, including advanced coronary artery disease, arthritis, diabetes, and lung problems.189 Depression also appears to increase the burden of disability. Smith and Young162 found that MS patients who met criteria for depression on either the Hospital Anxiety and Depression Scale (HADS) or the Beck Depression Inventory were three times more likely than nondepressed patients to perceive their disability as being greater than their physician did.
Depression is often associated with other neurological symptoms of MS, particularly cognitive impairment, fatigue, and pain, although, as discussed earlier in the section on cognitive impairment, the data on the link between depression and cognitive impairment are inconsistent. These other symptoms can be worsened by depression or can themselves increase depression. For example, depressed MS patients have reduced working memory capacity (reading span),7 and it is important to establish which causes which or if both are independently caused by the same factors. Both mental fatigue and total fatigue are correlated with depression.61,93 Depression and disability are significant predictors of fatigue,93 although as noted above, fatigue is used as an indicator of depression and thus the correlation might be overestimated. Depression has been linked to cognitive impairment in numerous studies, but these have generally been cross-sectional studies in which comparisons are made between different groups of patients tested at a single point in time. Interestingly, the only study that compared depression and cognitive function in individual patients who were tested at times when they were not depressed and during bouts of major depression found no significant correlation between depression and cognitive performance.154
While the causes of depression in MS are likely multifactorial, several pathophysiological correlates have been reported. Depression is far less common in patients with lesions that are restricted largely to the spinal cord as opposed to the brain.136 Measures of brain atrophy, such as enlargement of subarachnoid spaces (sulci, fissures, cisterns) and enlargement of ventricles, are associated with depression in MS patients (reviewed in 2000 by Bakshi et al.).10 Recent MRI studies have reported that white matter lesions in the frontal and parietal areas of the brain are correlated with depression, suggesting that those lesions might lead to depression by disconnecting the cortical areas in the brain that regulate mood.10
Suicide
As with depression, suicide rates in MS patients are high. The rate of suicide attempts among a group of MS patients who used hospital services in Nova Scotia was three times that of the general population.59 The suicide rates among Danish women and men with MS are, respectively, 50 and 70 percent greater than those of the general Danish population.169 It is sobering to note that about one in
Page 123
five patients who ended their lives with Dr. Kevorkian had MS. (He is the assistedsuicide advocate who presided over 47 deaths in the United States from 1990 to 1997.)43 Risk factors of suicide for men with MS include mental disorders (which includes depression), recent exacerbations, and moderate disability; risk factors for women with MS are not distinct.168 That study did not include social factors in the analysis of risk factors, but another study reported that people with MS who experience physical decline but have supportive relationships are less likely to commit suicide than those without such relationships.102
Depression is generally associated with an increased risk of suicide; about 15 percent of all people with major depression commit suicide.141 Recognition and treatment of depression thus is an important tool in suicide prevention.168
Treatment
Depression and anxiety among MS patients are often unrecognized and untreated.50 Although there is a general consensus that depression in MS can often be effectively treated (treatments are listed in Table 3.1), there are few controlled clinical trials of antidepressant treatment in MS. A small double-blind study indicated that desipramine was effective in the treatment of depressive symptoms, although anticholinergic side effects limited the dose that could be given.155 However, the study did not examine the effect of antidepressant treatment on the functional abilities or perception of disability in these patients. Another study reported that response to pharmacological treatment for depressive symptoms among MS patients was “extremely high,” as was the relapse rate after discontinuation of the medication.156 In that study, conducted in 1996, 51 out of 228 patients (22 percent) received pharmacological treatment for depression. In addition, treatment of depression improves adherence to beta-interferon (IFN-β) therapy (reviewed in 1999 by Walther and Holfeld185). Even an eight-week treatment of cognitive behavior therapy administered by telephone has been reported to improve adherence.117
Studies conducted in the 1980s reported that as many as 40 percent of MS patients with depression did not receive appropriate treatment (reviewed in 1994 by Fischer et al.56). Paradoxically, a survey of MS practitioners suggested that they tend to overestimate the prevalence of major depression in MS.56 The median estimate made by MS practitioners was that 30 percent of MS patients are depressed at any one time, which is higher than estimates of most studies based on validated depression rating scales.126
Depression as a Side Effect of Interferon Therapy
Based on early clinical trial results, depression is listed as a possible side effect of beta-interferon therapy in MS. However, the data are contradictory (Table 3.2).185 Patients in the first large, controlled North American clinical trial
Page 124
|
TABLE 3.1 Medications Used to Treat Depression |
||
|
Trade Name |
Generic Name |
Mechanism |
|
Elavil153 |
Amitriptyline |
A tricyclic antidepressant. Amitriptyline is metabolized to nortriptyline, which is an active metabolite. Has significant anticholinergic and sedative effects, with moderate orthostatic hypotension. Has very high ability to block serotonin uptake and moderate activity with respect to norepinephrine uptake. |
|
Pamelor69 |
Nortriptyline |
A tricyclic antidepressant. Studies suggest that nortriptyline interferes with the transport, release, and storage of catecholamines. Operant conditioning techniques in rats and pigeons suggest that nortriptyline has a combination of stimulant and depressant properties. (SSRI), meaning that it blocks serotonin from |
|
Paxil123,153 |
Paroxetine |
Paxil is a selective serotonin reuptake inhibitor being reabsorbed into the sender nerve cell. This process increases the amount of serotonin available to be absorbed by the next cell and may help message transmission. |
|
Prozac115,123,153 |
Fluoxetine hydrochloride |
An SSRI that increases serotonin levels in the midbrain. |
|
Tofranil153 |
Imipramine |
A tricyclic antidepressant. In MS it is used to treat bladder symptoms, including urinary frequency and incontinence, and also for the management of neurologic pain. |
|
Wellbutrin153 |
Bupropion hydrochloride |
Mechanism of the antidepressant effect of bupropion is not known. It is a weak blocker of the neuronal uptake of serotonin and norepinephrine; it also inhibits the neuronal reuptake of dopamine to some extent. |
|
Zoloft115,123,153,157 |
Sertraline |
Zoloft is an SSRI that blocks serotonin from being reabsorbed into the sender nerve cell. This process increases the amount of serotonin available to be absorbed by the next cell and may help transmission of nerve cells. |
Page 125
|
Potential Side Effects |
|
Dryness of mouth, constipation, increased appetite and weight gain, dizziness, drowsiness, decreased sexual ability, headache, nausea, unusual tiredness or weakness, unpleasant taste, diarrhea, heartburn, increased sweating, vomiting |
|
Dizziness, drowsiness, headache, decreased sexual ability, increased appetite, nausea, unusual tiredness or weakness, unpleasant taste, diarrhea, heartburn, increased sweating, vomiting |
|
Decrease in sexual drive or ability, headache, nausea, problems urinating, decreased or increased appetite, unusual tiredness or weakness, tremor, trouble sleeping, anxiety, agitation, nervousness or restlessness, changes in vision including blurred vision, fast or irregular heartbeat, tingling, burning, or prickly sensations, vomiting |
|
Anxiety, nervousness, insomnia, fatigue, tremor, sweating, gastrointestinal distress, anorexia, diarrhea, dizziness, decreased libido |
|
Dizziness, drowsiness, headache, decreased sexual ability, increased appetite, nausea, unusual tiredness or weakness, unpleasant taste, diarrhea, heartburn, increased sweating, vomiting |
|
Restlessness, agitation, anxiety, insomnia, delusions, hallucinations, psychotic episodes, confusion, paranoia, weight loss |
|
Nausea, diarrhea or loose stools, tremor, trouble sleeping, drowsiness, dry mouth, decreased appetite, weight loss, sweating, anxiety, or decreased sexual drive |
Page 126
|
TABLE 3.2 Depression and Beta-Interferon |
|||
|
Observation |
Methods Used to Assess Depression |
Study Size a |
Study Group |
|
Four patients on IFN-β1b attempted and one committed suicide. No patients in the placebo group attempted suicide. |
Patient reports |
372 |
North American IFN-β clinical trial174 |
|
Depression rates in different dosage groups of betainterferon-1a were 24% on 44 μg 21% on 22 μg 3. 28% on placebo |
Three different rating scales:
1. Beck's Hopelessness Scale
2. Centre for Epidemiologic Studies' Depression Scale
3. General Health Questionnaire
|
276 |
European IFN-β clinical trial48 |
|
Depression was neither caused nor exacerbated by IFN-β1b. |
Three different rating scales:
1. Hamilton Depression Rating Scale
2. Beck Depression Inventory
3. State-Trait Anxiety Inventory
Patients were interviewed in person at MS clinic by a neuropsychologist |
90 |
Borras and coworkers19 |
|
Patients who were depressed before onset of treatment with IFN-β1a became less depressed at initiation of treatment, but returned to pre-treatment levels within 2 months. |
Depression-Dejection scale of the Profile of Mood States Patients were interviewed by telephone |
56 |
Mohr and coworkers118 |
of beta-interferon therapy reported increased symptoms of depression.174 Four out of 247 patients in the two treatment groups attempted and one committed suicide, but although alarming, this was not statistically significant. In contrast, interferon-treated patients in the comparable European trial showed lower levels of depression than placebo-treated patients, although all were higher than the general population.133 In contrast to the North American trial which relied only on patients' own assessment of depression, depression among patients in the European trial was measured using three different scales.
Treatment with alpha-interferon is also linked to depression (reviewed in 2000 by Menkes and MacDonald114 and Trask178), although there are conflicting reports.121 Alpha-interferon does not cross the blood-brain barrier and the mechanisms by which it induces depression is unknown, although it has been proposed
Page 127
that interferon causes decreased serum tryptophan, a serotonin precursor.114 Decreased serotonin levels are related to the onset of depressed mood.
Other studies have reported varying mood responses to beta-interferon therapy, but the most thorough study indicates that it neither causes nor exacerbates depression in MS patients.19 At the same time, given the variety of reports of depression and the prevalence of depression among MS patients, potential changes in patients taking beta-interferon therapy should be monitored and treated.
Conclusions
Despite the consensus that depression is a prevalent and troubling concern among MS patients, much remains unknown about the interaction of affective and neurological symptoms. In 1990, Minden and Schiffer recommended: “More research, using advanced imaging techniques and standardized psychiatric interviews and diagnostic criteria, is needed to clarify connections between depressive symptoms and the neurological disease. Systematic, controlled and appropriate blinded studies that use reliable and valid instruments to detect changes in mood states to assess the efficacy of psychotherapy and pharmacotherapy among depressed MS patients are also important.”116 This recommendation still rings true today. Trask goes so far as to state that “it is almost certain that individuals treated with IFN will experience fatigue and possibly psychiatric side effects such as depression and anxiety.”178
Although depression is prevalent among MS patients, it is notable that most adapt successfully to the disease. A study that used a variety of standardized scales and interviewing procedures analyzed the psychosocial well-being of 94 people who had lived with MS for more than 10 years on average.44 The majority, approximately two-thirds, had achieved positive psychosocial adjustment to MS. Another study based on a mailed survey to 125 members of a regional chapter of the MS Society indicated similar results.47 Based on their responses to a broad spectrum of measures such as family and social relationships, coping strategies, self-esteem, and emotional functioning, most respondents had adapted to MS successfully. At the same time, about 20 percent reported that they felt a need for professional help in coping with depression, compared to more than twice as many (53 percent) who wished for help identifying ways to adapt to their lives with MS. For this sample, help in coping with depression was seen as an important, but not the most important, need.
Depression is one of the more pervasive complications of MS, yet it is frequently unrecognized. Although its cause is unknown, the availability of effective treatment suggests that the more immediate research priority should be aimed at improving patient and physician education. Insofar as understanding the etiology of depression in MS patients can increase the number of people who are effectively treated, this is also a research priority, but the most far-reaching
Page 128
impact will likely come from simply applying available treatments to more people with MS. Because of their frequent contact with people with MS, local MS Society chapters could plan and coordinate services aimed at recognizing and treating depression. Finally, the role of the family should be considered.
SPASTICITY AND WEAKNESS
Impairments of muscle function are a central feature of MS. They can be manifest in stiffness or involuntary muscle actions as well as in weakness, which limits a muscle's functional capacity. This section focuses on the problems of spasticity, spasms, and weakness. The next major section addresses ataxia and problems of coordination of movement.
Weakness of the limbs is a constant feature of advanced MS and is present in approximately 80 percent of all people with MS (reviewed in Matthews, 1998107). Both lower limbs, usually asymmetrically, are most often affected, followed in frequency by weakness in only one lower limb and then weakness in one lower and one upper limb, usually on the same side. Weakness of one arm without leg weakness is uncommon. Motor disability in the limbs is seldom due to weakness alone. Cerebellar ataxia and tremor, particularly in the arms, and loss of postural sense also contribute to weakness. The initial complaint is often of weakness only after exertion, but then it increases gradually until it is a constant presence.
Spasticity is generally defined as a state of increased muscular tone in which abnormal stretch reflexes intensify muscle resistance to passive movements. In clinical practice, the concept of spasticity extends beyond the resistance to passive movement to include a complex disorder of voluntary movement.107 It is a common symptom of MS. In one survey, 70 percent of 168 MS patients registered with the Northern California Chapter of the MS Society reported that they experienced mild to severe spasticity.72
Many MS patients also experience muscle weakness along with spasticity, but it is possible to have spasticity without weakness or to have weakness without spasticity. Spasticity in MS usually affects the legs more than the arms, and it can even offset muscle weakness and aid in standing, walking, and transferring.86 Spastic paresis, slight or incomplete paralysis due to spasticity, has been cited as the major cause of the loss of ability to work among persons with MS.60
The increase in resting muscle tone that characterizes spasticity is also associated with muscle spasms. In the progressive stages of MS, exaggeration of extensor tone can result in extensor spasms in which there is forceful activation of leg muscles inducing plantar flexion of the ankle, together with hip and knee joint extension. These spasms are most likely to occur while a patient is lying in bed at night or is awakening in the morning. Spasms can be severe enough to eject a seated patient from a wheelchair. Flexor tone becomes more prominent at later stages of MS and initially causes falling without warning. In flexor spasms
Page 129
there is a generalized flexion of muscles at the ankle, knee, and hip, giving rise to limb withdrawal or retraction. Spasms may promote progressive muscle contraction, joint deformities, and ultimately skin damage and breakdown. Both types of spasms may be quite painful, disrupting sleep and adversely affecting activities of daily living.
Origins of Spasticity and Weakness in Voluntary Movement
Muscle groups normally work together: when one is flexed, its opposing muscle is relaxed. This complementary muscle action depends on the transmission of signals along pathways connecting the brain, motor neurons in the spinal cord, and muscles ( Figure 3.1). The CNS damage caused by MS disrupts this
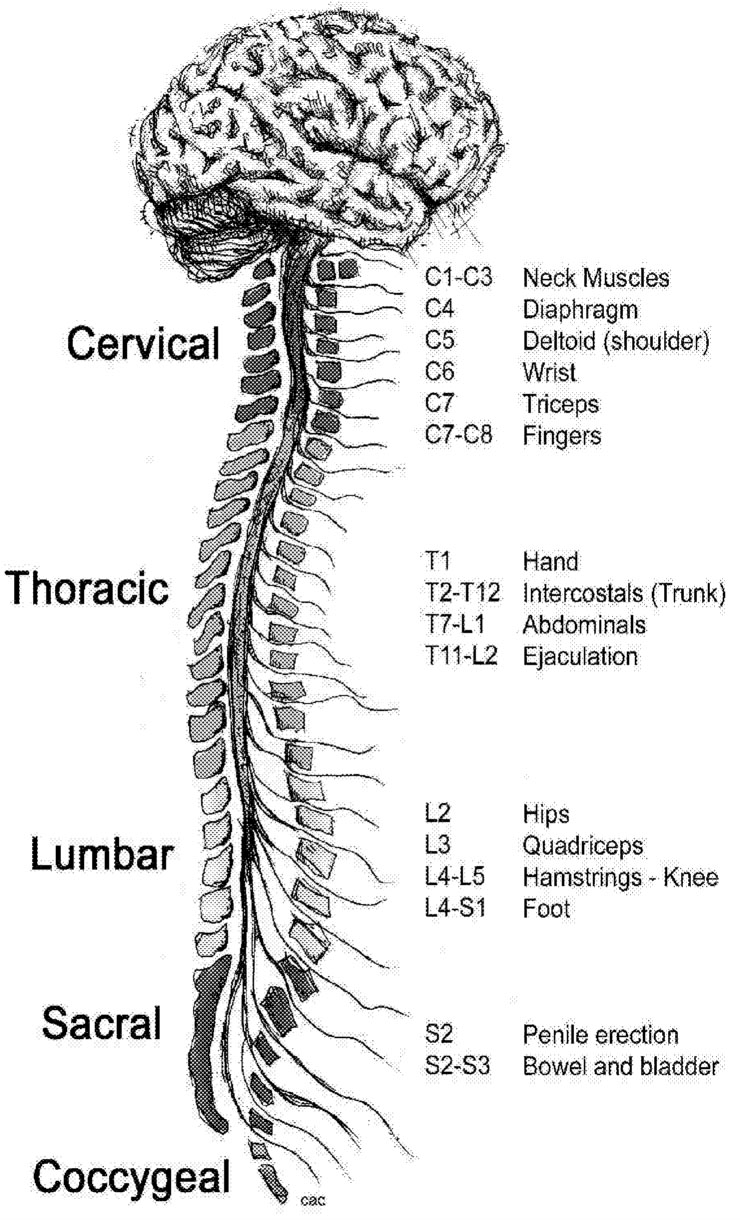
~ enlarge ~
Page 130
communication, allowing the persistent contraction of the muscle fibers that produces spasticity.
Spasticity is associated with sprouting of descending motor pathways to form new synaptic connections with spinal neurons and with hypersensitivity caused by denervation.107 Damage to descending motor pathways also causes slowness of movement and weakness. The spinal plaques occurring in many types of spinal MS are associated with a progressive loss of axons and a reduction in diameter or a loss of myelination of residual axons passing through or close to the plaque.103 There can also be alterations in the numbers and types of excitable sodium membrane channels exposed by the membrane demyelination, further altering the conduction capacity of the damaged axons.
Although the mechanisms leading to spasticity and muscle weakness in MS are not fully understood, knowledge about the mechanisms operating in other conditions may be helpful. For example, the mechanisms related to the spinal cord pathology known to occur in MS may be similar to those in incomplete spinal cord injury. The symptoms of spasticity and weakness in MS can also result from plaques in the supraspinal brain and brainstem, which would suggest a comparison with the results of stroke or intracapsular hemorrhage. However, considerably more is known about the effects of incomplete spinal cord injury. Thus, it is more likely to provide instructive parallels for understanding the pathology and pathophysiology of MS.
Mechanisms of Spasticity and Spasms
The general features of spasticity in MS appear to resemble those observed in incomplete spinal cord injury, with the added complexities of concurrent hemispheric, visual, and cerebellar lesions. It appears that the effects of the distributed axonal loss and demyelination of the spinal cord are mediated primarily by spinal interneuronal systems that are normally held under tight descending control. These interneuronal controls are mediated by reticulospinal actions, including those provided by monoaminergic fibers, whose actions appear to differ on different classes of neurons. Key features of disruptions of these interneuronal systems are briefly reviewed.
Increased Stretch Reflex Responsiveness
The group II excitatory interneurons might have special importance in mediating spastic hypertonia, with exaggerated stretch reflex responses in spinal cord injury or MS. These group II interneurons receive strong inhibitory effects from descending monoaminergic pathways, especially α2 norepinephrine (NE) systems.20,82,124,131 Interruption of these descending pathways releases this interneuron from inhibitory control, providing a strong stretch-evoked excitation. This synapse may be a potential site of action for tizanidine (Zanaflex), an α2 NE agonist used to control spasticity.
Page 131
There is also recent evidence for disynaptic excitation of motoneurons from group Ia afferents released from both extensor and flexor muscles during locomotion.6,40 This system is undoubtedly under tight descending control, possibly indirectly, and can greatly amplify the stretch reflexes in both extensor and flexor muscles.
Conversely, group Ia inhibitory interneurons may show reductions in excitability after spinal injury. This change potentially promotes the emergence of unhelpful muscle contraction by limiting the strength of mutual inhibition between agonist-antagonist muscles.28
Special Roles for Free Nerve Ending Mechanoreceptors
Group III (small myelinated) and group IV (small unmyelinated) fibers are known to exert potent reflex actions in spinal cord injury and are likely to be important in MS-induced cord damage as well. Animal models of incomplete spinal cord injury have shown that stretching the extensor muscles to the point where free nerve ending mechanoreceptors are activated produces a sharp inhibition of the same extensors, accompanied by excitation of flexors.149 This sharp “clasp-knife” inhibition is manifest as a sudden drop in active force and is not causally dependent on the presence of a flexion withdrawal response. The interneurons receiving free nerve ending input are hyperexcitable when spinal cord pathways are interrupted, giving rise to severe extensor inhibition and to concurrent excitation of flexors.
Exaggerated Flexion Reflexes
Loss of monoaminergic fibers in MS potentially releases flexion reflexes from descending inhibitory controls, giving rise to flexor spasms. Under normal conditions, the interneurons mediating the so-called flexor reflex system are closely regulated by monoaminergic pathways, acting in all probability on 5-HT1b/d (serotonin) receptors, or on α2-adrenergic receptors to depress the excitability of several interneuron pathways. Interruption of this monoaminergic innervation as a result of spinal cord damage (as in MS) releases the capacity of these interneurons to generate “plateau potentials.” The plateau potentials in these interneurons may in turn be responsible for the increased responsiveness and the prolonged reflex afterdischarge seen in some flexion responses, especially after long-term spinal cord injury.
Extensor Spasms
The origins of extensor spasms are presently unclear, and there are no published studies examining their origins in human spinal cord injury or MS. One possible explanation for their occurrence is that the extensor spasm is a fragment
Page 132
of the normal locomotion or standing program in the spinal cord, in which the leg extensors are switched on forcefully to provide weight support during the stance phase of locomotion. Indeed, in intensive study in the cat, Pearson and colleagues demonstrated that the stance phase of fictive locomotion is associated with a switch from force-mediated (Ib) inhibition of extensor muscles to a force-mediated excitation.112 This switch presumably happens because Ib inhibitory interneurons are gated out during stance, in favor of a parallel group I excitatory pathway. As with the other interneuronal systems discussed above, this excitatory Ib pathway may be under monoaminergic control, which is disrupted in spinal cord injury or MS.
Treatment of Spasticity
Although little can be done to counter the muscle weakness that occurs in MS, several forms of treatment can be applied to help limit the adverse effects of spasticity. However, treatment plans must be designed to meet individual patient needs and must take into consideration both the benefits and the risks of specific interventions. For some ambulatory patients, for example, treatment of spasticity is less desirable if increased stiffness of the legs facilitates walking by offsetting muscle weakness. Management of spasticity often involves a combination of therapeutic approaches, including control of secondary factors that stimulate spasticity, proper positioning, physiotherapy, and medications. Surgical procedures, such as tendon release, are sometimes used when other interventions are not effective.
Spasticity can be triggered or worsened by a variety of painful or unpleasant stimuli, such as urinary tract infections, fecal impaction, contractures, tight clothing, or ill-fitting footwear. A treatment plan should ensure that these sources of secondary stimulation are eliminated or controlled. Careful attention to balanced and symmetrical positioning when patients are standing, sitting, or lying down is important for preventing fixation in distorted postures induced by spasticity. Proper positioning can help stimulate muscles that are antagonists to those subject to spasticity. Physical therapy helps in maintaining balanced muscle tone, in preventing or treating contractures, and in training muscles in coordinated movement.
Baclofen and tizanidine are the two medications used most often to treat spasticity, with cloazepam and gabapentin usually reserved as secondary medications (Table 3.3). Baclofen, an agonist of γ-aminobutyric acid-B (GABA B) receptors, reduces presynaptic release of excitatory neurotransmitters and, at higher concentrations, acts postsynaptically to antagonize their actions. Generally given orally, baclofen can also be administered intrathecally through subcutaneous pump to treat more severe spasticity in long-standing MS.127 Tizanidine stimulates a2-andrenergic receptors in the spinal cord, which inhibits presynaptic
Page 133
release of excitatory amino acids. Tizanidine reduces flexor reflexes and spasms and relieves pain. It appears to reduce muscle strength less than baclofen but may not result in measurably better function (see Kita and Goodkin92).
Dantrolene acts within the muscles themselves to produce inhibition of the excitation-contraction coupling process, which leads to reduced muscle strength. Because of its action, it may be better suited for treatment of nonambulatory patients for whom added weakness is less likely to impair mobility. Patients receiving dantrolene should be monitored for liver function because of its potential hepatotoxicity. Benzodiazapines, such as diazepam and clonazepam, act to reduce muscle tone through three mechanisms: suppression of sensory impulses from muscles and skin receptors, postsynaptic potentiation of GABA, and inhibition of excitatory descending pathways. They are generally used in combination with baclofen or other medications.
Intramuscular injections of botulinum A toxin can be helpful, especially for patients with severe localized spasms. The chemical denervation produced by botulinum A toxin can last for up to three months. A study of nine patients with long-standing MS showed reduced spasticity and improved ease of nursing care with no adverse effects.92,163
Recommendations for Further Study of Spasticity in MS
Further research is needed to clarify the mechanisms of spasticity and spasms in MS and to identify opportunities for therapeutic intervention.
-
Determine the spectrum of nerve fiber damage in spinal MS and whether this damage involves monoaminergic systems preferentially.
-
Determine whether the extensor spasm is a product of the locomotion pattern generator and whether this interneuronal circuitry is under descending monoaminergic control.
-
Determine the specific monoamine receptors that influence the stance phase of locomotion and, potentially, the extensor spasms of MS and spinal cord injury (see, for example, Kim et al.89).
-
Test the possibility that restoration of 5-HT1b/d or α2 NE action in the spinal cord can lead to restoration of flexion reflex interneuronal excitability and a reduction of flexion spasms.
-
Determine whether flexor spasms can be reduced by reducing the strength of group III and IV afferent input from muscle to spinal interneurons. For example, group III and IV receptors carry substance P and other peptides and make synapses in lamina I of the cord and in the intermediate nucleus as well. It follows that compounds related perhaps to capsaicin may discharge these transmitters from their afferent terminals and reduce the central actions of these afferents.
Page 134
|
TABLE 3.3 Medications Used to Treat Spasticity |
||
|
Trade Name |
Generic Name |
Mechanism |
|
Lioresal64,73,92 |
Baclofen |
Baclofen is a muscle relaxant and antispasmodic that works by inhibiting the nervous system. Its precise mechanism of action is unknown, although it is thought to inhibit the transmission of impulses between nerve cells. It is administered orally in pill form or via an intrathecal delivery system (a surgically implanted pump). |
|
Zanaflex64,73,92 |
Tizanidine |
Tizanidine is an α2-adrenergic agonist that reduces spasticity reducing the release of excitatory neurotransmitters and Substance P in the spinal cord. Its greatest effects are on polysynaptic pathways. |
|
Klonopin64 |
Clonazepam |
Clonazepam is a benzodiazepine and is often prescribed as an anticonvulsant. Benzodiazepines belong to a group of medicines that slow down the central nervous system. |
|
Neurontin37,79 |
Gabapentin |
Gabapentin is structurally related to the neurotransmitter GABA but it does not interact with GABA receptors. The mechanism by which it exerts its anticonvulsant action is unknown. |
|
Botox64,92 |
Botulinum A Toxin |
Intramuscular injection typically results in a dosedependent reduction of hyperactive muscle contraction that lasts for an average of 12.5 weeks and is ultimately reversible. Botox is believed to produce its therapeutic effect by acting selectively on peripheral cholinergic nerve endings to inhibit the release of the neurotransmitter acetylcholine at the neuromuscular junction. |
|
Dantrium64,92 |
Dantrolene |
Acts directly on skeletal muscle, probably by dissociating the excitation-contraction coupling mechanism as a result of interference with the release of calcium from the sarcoplasmic reticulum. This results in a decreased force of reflex muscle contraction. |
Page 135
|
Potential Side Effects |
|
Drowsiness or unusual tiredness, increased weakness, dizziness or lightheadedness, confusion, unusual constipation, new or unusual bladder symptoms, trouble sleeping, unusual unsteadiness or clumsiness, fainting, hallucinations, severe mood changes, skin rash or itching |
|
Sedation, dry mouth, dizziness, hypotension, vasodilation, constipation, diarrhea, flatulence, nervousness, anxiety, paraesthesia, tremor, seizures, vertigo, agitation, euphoria, stupor, urinary frequency and urgency, back pain, sinusitis, flu-like syndrome |
|
Confusion, depression, double vision or abnormal eye movements, hallucinations, lightheadedness or fainting spells, mood changes, excitability, movement difficulty, muscle cramps, tremors, weakness or tiredness, constipation or diarrhea, difficulty sleeping, headache, nausea, vomiting |
|
Somnolence, ataxia, dizziness, fatigue, nausea, vomiting |
|
Transient weakness in injected or neighboring muscles, skin rash, and flu-like syndrome |
|
Diarrhea, dizziness, drowsiness, weakness, nausea, unusual tiredness, abdominal cramps, blurred or double vision, chills and fever, constipation, frequent urination, headache, loss of appetite, speech difficulties, sleep difficulties, nervousness |
Page 136
Mechanisms of Muscular Weakness for Voluntary Movement
As with spasticity, the muscle weakness seen in chronic spinal MS probably results from changes in motoneurons and interneuron electrophysiology that are comparable to those observed in incomplete spinal cord injury. These changes result in disruptions of interneuron and motoneuron pathways and in monoaminergic input to spinal motoneurons. Other peripheral changes that may contribute to muscle weakness include muscle wasting, fiber loss, muscle contracture, and joint contractures.
Loss of Descending Input to Spinal Premotor Interneurons
Communication pathways between the interneurons of the spinal cord and the axons of sensory and motor neurons, which project outside the spinal cord, are essential for generating coordinated muscle commands. Disruption of the axons from corticospinal, rubrospinal, vestibulospinal, and reticulospinal pathways may render these key interneurons less excitable and hence less able to relay motor commands. Loss of descending pathway excitation of key excitatory interneurons can also limit the capacity for other descending or segmental spinal pathways to activate motoneurons and, consequently, to limit voluntary force generation. In addition, some reticulospinal systems control segmental pathways through inhibitory input, and since segmental excitatory and inhibitory systems work in concert to produce normal motor control, disruption of inhibitory input might also contribute to impaired motor function.
Loss of Monosynaptic Corticospinal and Rubrospinal Projections to Motoneurons
Loss of direct or monosynaptic projections to spinal motoneurons, especially those innervating distal limb musculature, contributes to weakness by limiting a patient's capacity to activate the motoneurons. With fewer axons projecting to the motor neurons, residual descending pathways may have greater difficulty reaching the signal threshold of larger motor neurons, thereby limiting the range of voluntary force that a patient can achieve. In experimental work with monkeys, selective loss of these systems can have a greater effect on fine motor control of digits than on the equally voluntary control of more proximal muscle groups.98 With the loss of innervation, muscles are also more likely to fatigue because of the associated increase in the sense of effort, both of which are reported frequently by MS patients.
Loss of Norepinephrine (NE) and Serotonin(5-HT) Input to Spinal Motoneurons.
Damage to spinal pathways projecting from the locus coeruleus (a source of NE projections) and from the raphe nuclei (a source of 5-HT) significantly re-
Page 137
duces measurable 5-HT and NE in the spinal cord and alters the response patterns of many spinal interneurons and motoneurons.* Although not documented in humans, animal models of MS, such as experimental autoimmune encephalomyelitis (EAE, see Chapter 2) have shown loss of these monoaminergic fibers.
For motoneurons, loss of monoaminergic input may reduce their excitability by altering their capacity either to develop plateau potentials or to modulate the characteristics of their current-frequency (I-F) relation. (Plateau potentials are sustained depolarizations of neurons that greatly outlast the duration of the initial depolarizing stimulus. The I-F curve is the relation between injected or synaptic current and the resultant firing of the motoneuron.)
Since these monoaminergic effects are not necessarily uniformly distributed throughout a given motoneuron pool, loss of such innervation could differentially affect the recruitment behavior of motoneurons by compressing the range of synaptic current over which motoneurons are recruited and even reversing the recruitment order in some situations (for example, see Powers and Rymer132). There are also reductions in motoneuron firing rate, both initially at recruitment and over the full span of rate modulation. These rate reductions (which may result from alterations in the locus and slope of the I-F relation) mean that motoneuron firing rates are no longer well matched to the mechanical twitch properties of the active muscle fibers, rendering the overall muscle contraction less “efficient” (also Gemperline et al.65). For a given force level, the higher the level of motoneuronal discharge in the aggregate, the lower the “efficiency” ratio.
Changes in Muscle Tissue
Observations in patients with spinal cord injuries suggest that patients with severe, long-standing MS may also develop weakness through overt changes in muscle tissue (although, note Tilbery et al.177). These changes consist of muscle wasting, shifts in the metabolic properties of muscle fibers (toward glycolytic systems), and a reduction in fatigue resistance. In addition, there may be muscle and joint contractures that are associated with proliferation of connective tissues, shortening of muscle length, loss of muscle fibers, and sometimes joint deformities.
Recommendations for Further Study of Voluntary Muscle Weakness in MS
Research is needed to clarify the mechanisms producing muscle weakness in MS and to test potential therapies to counter weakness.
*The locus coeruleus and raphe nuclei are different points of the motor control relay “stations” in the brain. Neurons projecting from the locus coeruleus transmit their signals largely through norepinephrine; those from the raphe nuclei predominantly use serotonin.
Page 138
-
What is the state of monoaminergic fiber innervation in the spinal cord of chronic MS patients? Is there fiber loss, and if so, is it diffuse or more localized? Is there a disproportionate effect of MS on these nerve fiber types?
-
As a screening tool, what happens to force-EMG (electromyocardiogram) relations in paretic muscles of MS patients? Do they shift in such a way as to indicate a reduction in efficiency of muscle activation?
-
Determine the recruitment and firing rate properties of motoneurons in patients with muscle weakness associated with MS. Do they suggest that there is a loss of monoaminergic innervation?
-
Test whether administration of a 5-HT2 (or α2 NE) agonist improves the performance of motoneurons by increasing their firing rate and normalizing their recruitment patterns. Is this effect associated with recovery of force-EMG relations and a measurable improvement in voluntary force generation?
ATAXIA AND TREMOR
Ataxia refers to a lack of or reduction in coordination and is invariably associated with tremor, which occurs as an involuntary, rhythmic, oscillatory movement of a body part. These symptoms occur in 75 percent of patients with multiple sclerosis and are most frequently manifested as upper limb intention tremor. They are severely disabling and embarrassing, affecting upper limb function, gait, and in severe cases, balance in standing and sitting. The tremor of multiple sclerosis is frequently just one component of a complex movement disorder that includes dysmetria and other ataxic features, and the underlying mechanisms are poorly understood. Although inflammatory demyelination in different parts of the cerebellum and related areas may produce a distinct tremor, it is nonetheless extremely difficult to classify individual tremors in patients. Tremor remains one of the most difficult symptoms to manage and is associated with a poor outcome in rehabilitation.
Management of Ataxia
As with spasticity, there are practical components to the management of ataxia, which must be considered prior to other interventions. These include patient education, improving posture and proximal stability during activities, and provision of equipment. The use of weights to dampen tremor have not proved to be very successful, although they may be slightly better if a computer damping device is incorporated. A small exploratory study of therapy input suggested modest benefit.84 Other treatments involve either drug therapy, which is limited
Page 139
and often not well tolerated, or invasive surgical intervention, which includes thalamotomy and thalamic stimulation.
Drug Therapy
Few drugs have been evaluated and none adequately. Isoniazid (with pyridoxine) has been shown to be of limited benefit in a number of small studies. It showed some effect in 10 of 13 patients, although this did not translate into improved function, while 4 out of 6 patients showed sufficient benefit that they wished to continue the drug. It is thought to be more useful in postural tremor with an intention component rather than pure intention tremor. Up to 1,200 mg a day in divided doses has been used, increasing gradually from 200 mg twice a day. This drug, which was the first to undergo a randomized controlled trial for the treatment of multiple sclerosis, is not well tolerated and causes gastrointestinal disturbance. There has been even less evaluation of other drugs, including carbamazepine, clonazepam and buspirone. Ethyl alcohol and propranolol have not been found to be useful. A single-blind, cross-sectional study evaluated the role of carbamazepine in cerebellar tremor in 10 patients (7 with MS) and suggested some benefit. More recently, the 5-HT3 receptor antagonist, ondansetron, has been evaluated, given by both intravenous and oral routes. Although the I.V. studies looked promising, the more recent placebo-controlled, double-blind parallel group study was negative. Fifty-two patients, the majority of whom had MS, were randomized and the treatment arm received 8 mg per day for one week. Although some benefit in the nine-hole peg test was seen in the treated arm, there was no difference between the groups on the global ataxia rating scale.
Surgical Intervention
Although thalamotomy of the ventral intermediate nucleus (VIN) has been shown to reduce tremor in Parkinson's disease patients, there has been limited evaluation of its role in tremor relating to MS. In general, it is not as effective in this condition. In selected patients with MS, thalamotomy has been reported to alleviate contralateral limb tremor, initially in about 65 to 96 percent of cases, although in about 20 percent, tremor returns within 12 months. Functional improvement is estimated to occur in only 25 to 75 percent of patients. However, these results are not based on controlled studies, and no prospective study has evaluated the influence of this procedure on overall disability, handicap, and quality of life; nor have side effects been quantified, although they may occur in up to 45 percent of cases. Serious side effects, which include hemiparesis, dysphasia, and dysphagia, occur in up to 10 percent of patients. Experience suggests that optimum results are obtained in patients with relatively stable disease, good mobility, and minimal overall disability status—an extremely small group. Three recent papers have suggested that thalamic stimulation can also alleviate tremor
Page 140
in up to 69 percent of patients in studies involving 13, 5, and 15 patients, respectively.66,119,191 These were carefully selected patients; for example, the 5 patients reported by Whittle et al. were from an initial group of 17 patients, and no control study has as yet been carried out.191 Serious side effects were seen in 2 of 15 patients in the study by Montgomery et al.119 No trial has compared thalamic stimulation versus lesioning, although it is suggested that stimulation is associated with fewer side effects. Other approaches, including extracranial application of brief AC (alternating current) pulsed electromagnetic fields, dynamic systems with multidegree of freedom orthoses, and robotic arms based on virtual reality have not been adequately evaluated.
Conclusion
Ataxia and its associated tremor remain among the most resistant and disabling symptoms to manage. Current strategies are not evidence based and are of limited benefit.
BLADDER AND BOWEL DYSFUNCTION
Bladder dysfunction affects up to 90 percent of people with MS at some time during their illness. Bladder dysfunction is the presenting feature in approximately 5 percent of patients, but the incidence increases as the disease progresses. The level of dysfunction relates to the stage of disease, and more than half of patients who cannot walk have bladder complaints (Robert Hamill, personal communication). The symptoms of bladder dysfunction are disruptive to social, vocational, and sexual activities, but they are generally treatable and can be successfully managed (see Table 3.4). Bowel dysfunction is less prevalent than bladder dysfunction in MS, affecting up to 68 percent of patients,29 but it might be related to bladder dysfunction since people with bladder problems are sometimes reluctant to consume enough fluids, which leads to constipation. Chia et al.29 found that 52 percent of patients with urinary symptoms also had some degree of bowel dysfunction. The neurological basis for bowel dysfunction in MS is not as clearly defined as it is for the bladder, and treatment options are limited.
Normal Bladder Control Mechanisms
Neural bladder function control occurs primarily in the sacral spinal cord, as well as the pons, diencephalon, and cerebral cortex in the brain. Activation of the spinal reflex pathway promotes urine retention. Pontine structures ensure that bladder functions are fully integrated. A normal micturition reflex depends on the integrity of the pons and the afferent and efferent pathways active in bladder
Page 141
|
TABLE 3.4 Indications of Bladder Dysfunction |
|||
|
Type |
Definition |
Urodynamic Pattern of Dysfunction |
Prevalence in Patients with MS and Bladder Dysfunction (%) a |
|
Urgency |
Urgent need to urinate; causes the bladder to empty at small volumes |
Detrusor hyperreflexia, excitability, and spasticity |
85 |
|
Frequency |
Frequent need to urinate |
Detrusor hyperreflexia |
83 |
|
Urge Incontinence |
Inability to control the bladder, leading to involuntary urination |
Detrusor hyperreflexia with or without outlet obstruction, and impaired sphincter contractility |
63 |
|
Hesitancy |
Difficulty initiating micturition |
Detrusor sphincter dyssynergia, and detrusor hyperreflexia with impaired contractility |
49 |
|
Interrupted Stream |
Difficulty completing micturition |
Detrusor sphincter dyssynergia |
43 |
|
Sensation of Retention |
Inability to completely empty the bladder |
Detrusor areflexia |
34 |
|
Nocturnal Enuresis |
Incontinence during sleep |
Detrusor hyperreflexia with impaired sphincter contractility |
14 |
function. Activation of the medial aspect of the pons results in detrusor, or bladder muscle, contraction, while activation of the lateral aspect results in urethral sphincter contraction. The ascending afferent pathways to the pontine centers and the descending efferent pathways travel in the anterolateral and lateral columns where demyelinating lesions create dysfunction. Cortical influences permit control of micturition ensuring personal and socially acceptable voiding.
The integrity of central control mechanisms permits bladder filling and maintenance of the filled bladder with little awareness or voluntary participation. Upon reaching normal capacity (350-500 ml), inhibitory pathways provide for suppression of the micturition reflex, activation of sympathetic outflow, and voluntary contraction of the external sphincter. Voiding requires the coordination of a number of control systems such that detrusor muscle activation and relaxation of sphincter mechanisms results in bladder emptying with only a few milliliters of residual urine.
Page 142
Patterns of Bladder Dysfunction
Bladder dysfunction in MS results from interruption of the neural control systems, most often in the sacral nerves.16 As a result, the loss of bladder control is likely to coincide with lower limb spasticity and the diminished ability to respond to urinary urgency by moving to a toilet.
Detrusor Hyperreflexia
The detrusor is the smooth muscle of the bladder that works in concert with internal and external bladder sphincters to allow normal urination. Micturition occurs when the detrusor muscle contracts while the sphincters relax, expelling urine from the bladder into the urethra.
A spastic or hyperreflexic detrusor muscle is usually secondary to lesions in the spinal cord, leading to increased contractility and decreased capacity of the bladder. Urinary urgency, the most common bladder symptom in MS, is caused primarily by detrusor hyperreflexia. Increased detrusor contractility causes the urge to void to be sensed before the bladder has reached normal capacity, resulting in frequent and urgent voiding of small amounts of urine. When these urges can no longer be controlled, urge incontinence results in the forceful expulsion of urine.
Detrusor Sphincter Dyssynergia
Detrusor sphincter dyssynergia occurs when detrusor muscle hyperreflexia coincides with outlet obstruction due to contraction of the sphincters or impaired sphincter contractility. It can also coincide with impaired or ineffective detrusor contractility. Detrusor hyperreflexia with outlet obstruction or impaired detrusor contractility often result in hesitancy, retention, or overflow incontinence. Detrusor hyperreflexia with impaired sphincter contractility may result in overflow incontinence or nocturnal enuresis.
Detrusor Areflexia
Impaired detrusor contractility is another cause of hesitation and retention, which can lead to bladder infections, the reflux of urine from the bladder back into a ureter, and bladder stone formation. Hydronephrosis, the accumulation of urine in the kidney, and renal failure can also occur but are rare in MS.
Therapy for Bladder Dysfunction
The main goals of treatment are to reduce the frequency of voiding, improve emptying, and decrease incontinence. Detrusor hyperreflexia is most frequently
Page 143
treated through the use of anticholinergic medications, of which several are available (Table 3.5). Although some strategies initially work well for some patients, as the disease progresses, treatment is increasingly less effective. Catheterization is sometimes necessary for patients suffering from retention.
Future Research in Bladder Dysfunction
Studies of the central and peripheral neurobiological control of micturition will improve our understanding of the roles of the numerous transmitter systems and permit new ideas of drug therapy. Studies of neuroplasticity should be performed to determine the effect of increased lesion load on micturition. Myelin repair and nerve regeneration studies including stem cell biology and the role of neurotrophins in glial and neuronal function and survival might permit repair and restoration of function. Many of these studies will relate directly to spinal cord injury, and joint funding options may be available.
Clinical research into bladder infections and keeping the urine sterile might be surprisingly fruitful, since infections restrict volume and compromise bladder function and pharmacological treatment. Intrathecal therapy to improve bladder function should be further explored. As mentioned above, motor spasticity is also linked with bladder dysfunction, and therapies developed for motor spasticity may improve bladder function. Clinical pathological spinal cord studies in humans are few and would expand our understanding of the relationship of pathology and pathophysiology in MS.
New and emerging technologies could have an impact on bladder dysfunction. Neural stimulators may be helpful in allowing patients to manually stimulate the nerves involved in bladder or sphincter control. The bladder circuit is ideal for developing computer models. Eventually a “bladder-brain” should be able to be developed to be placed in a pouch under the skin like a cardiac pacemaker and provide an integrated computer circuit (servoloop system) for filling and emptying the bladder without any CNS input.
Normal Bowel Control Mechanisms
Gastrointestinal function, including motility, is controlled primarily by the enteric ganglia, which ensure synchronous contraction of the bowel so that food materials move along the digestive tract. The CNS contributes to bowel function via parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) pathways. The PNS influence occurs via the vagus nerve and the sacral outflow. Vagal input is concentrated in the esophagus, stomach, and proximal small bowel and wanes distally. The SNS influence is via the greater, lesser, and least splanchnic nerves. They are derived from the thoracolumbar outflow and synapse in prevertebral ganglia (coeliac, superior, and inferior mesenteric) before postganglionic sympathetic fibers reach the bowel vasculature and enteric gan-
Page 144
|
TABLE 3.5 Medications Used to Treat Bladder and Bowel Dysfunction |
||
|
Trade Name |
Generic Name |
Indication |
|
Bentyl106 |
Dicyclomine hydrochloride |
Urinary incontinence, gastrointestinal spasms |
|
Cytospaz106 |
Hyoscyamine |
Bladder spasms |
|
DDAVP90,179 |
Desmopressin |
Frequent urination |
|
Ditropan XL106 |
Oxybutynin chloride; atropine |
Overactive bladder, involuntary bladder contractions |
|
Hiprex; Mandelamine87,151 |
Methenamine infection |
Urinary tract |
|
Pro-Banthine106 |
Propantheline bromide |
Bladder spasms, cramps |
|
Detrol24 |
Tolterodine tartrate |
Urinary frequency, urgency, urge incontinence |
|
Tofranil106 |
Imipramine |
Urinary frequency, fecal incontinence |
|
Zostrix38,39 |
Capsaicin |
Hyperreflexia |
Page 145
|
Mechanism |
Potential Side Effects |
|
Cholinergic blocking agent. |
Brief euphoria, slight dizziness, feeling of abdominal distention |
|
Inhibits the actions of specific neurotransmitters on bladder smooth muscle. |
Dry mouth, blurred vision, increased heart rate, dry eyes, headache, nervousness, urinary retention or hesitation, weakness |
|
Hormone that works on the kidneys to reduce urine formation. |
Runny or stuffy nose, abdominal or stomach cramps, flushing of the skin, headache, nausea, pain in the vulva |
|
Relaxes the bladder smooth muscle; increases bladder capacity and delays the initial desire to void. |
Dryness, drowsiness, blurred vision, dizziness, abdominal pain, back pain, flu syndrome, hypertension, palpitation, vasodilatation, constipation, diarrhea, flatulence, gastroesophageal reflux, arthritis, insomnia, nervousness, confusion, impaired urination |
|
An anti-infective, usually prescribed on a long-term basis for repeated or chronic urinary tract infections. |
Nausea, vomiting, skin rash |
|
An antispasmodic-anticholinergic medication; it interferes with contractions and spasms. |
Constipation, decreased sweating, dryness of mouth, nose, and throat, bloated feeling, blurred vision, difficulty swallowing |
|
Acts as a competitive muscarinic receptor antagonist in the bladder to cause increased bladder control. |
Dry mouth, headache, dizziness, somnolence, fatigue |
|
Has moderate anticholinergic and sedative effects and high orthostatic hypotensive effects; imipramine is biotransformed into its active metabolite, desmethylimipramine (desipramine). |
Dizziness, drowsiness, headache, decreased sexual ability, increased appetite, nausea, unusual tiredness or weakness, unpleasant taste, diarrhea, heartburn, increased sweating, vomiting |
|
A neurotoxin that, when instilled within the bladder lumen, injures C fibers, producing partial deafferentiation of the bladder and permitting retention of urine. |
Transient burning following application, stinging, erythema, cough, respiratory irritation |
Page 146
glia. Different sympathetic transmitter systems establish “synaptic input” to the vasculature, submucosal plexus, and myenteric plexus. In turn, efferents from the enteric ganglia project to the prevertebral sympathetic ganglia, providing nicotinic input.
Bowel Dysfunction
Constipation and fecal incontinence are the most common symptoms of bowel dysfunction, with constipation affecting up to 50 percent of people with MS and fecal incontinence affecting approximately 30 percent. Extreme constipation leading to megacolon is a relatively uncommon symptom.
Constipation
The causes of constipation in MS are not clear. Although symptoms tend to increase in later stages, many people experience constipation throughout the course of the disease. One hypothesis is that constipation in MS is caused by decreased parasympathetic input, although this is considered unlikely since bilateral, strategically placed lesions would have to occur. Another hypothesis is that there is little relationship between constipation in MS and the neurological profile. As mentioned above, the treatment of bladder dysfunction can also contribute to constipation. People with bladder dysfunction are less likely to keep adequately hydrated, and the anticholinergic drugs often used to treat detrusor hyperreflexia can cause constipation since sphincter mechanisms are primarily cholinergic.
Fecal Incontinence
Fecal incontinence is usually associated with constipation and occurs due to rectal overloading and overflow or when sphincter control and coordination are diminished due to sphincter muscle weakness. Involuntary sphincter relaxation may occur when the rectum is full due to the rectoanal inhibitory reflex, especially in a chronically distended rectum.
Therapy for Bowel Dysfunction
The main goals of treatment are to enhance gastrointestinal transit and reduce constipation and to prevent or reduce fecal incontinence. Timed evacuation can help with both constipation and fecal incontinence by taking advantage of the gastrocolic reflex, timing bowel movements after food consumption, exercise, or when it is known that a toilet is nearby. Dietary intake can significantly affect constipation. Adequate hydration and dietary fiber promote softer stools. High-fiber foods such as fruits, vegetables, and grains, and supplements such as psyl-
Page 147
lium and Metamucil increase fecal bulk and decrease gastrointestinal transit time. Fruit juices such as apple and prune juice and dietary supplements such as lactulose stimulate the bowel. Finally, foods such as rice, cheese, and excess protein decrease gastrointestinal transit time and should be avoided.
Medicinal treatments for bowel dysfunction in MS are limited. For constipation, stool softeners and laxatives, as well as smooth muscle stimulants, will decrease transit time. Cisapride, a serotonin receptor agonist, partially restores gastrointestinal motility but may cause cardiac complications secondary to its effects on the potassium channel.† Suppositories and enemas can be used to evacuate the rectum to reduce the risk of incontinence or induce defecation.
Devices used to treat fecal incontinence include the rectal bag and the artificial sphincter. After a surgical procedure called a colostomy, a rectal bag is used to catch feces as it is evacuated through an opening in the abdominal wall. An artificial sphincter can be used to control incontinence, as a last resort. To use the sphincter, the person will compress a pump to divert fluid from an anal cuff to a holding balloon, allowing the sphincter to relax and defecation to occur.
Research Areas in Bowel Dysfunction
Studies of fundamental neuroscience should parallel those outlined above for the bladder, with the goals of learning more about central control of bowel function, elucidating the local pharmacology of gastrointestinal transit, and identifying the response and plasticity of the central and peripheral neuronal systems to supraspinal central injury. More specifically, examination of the central control of the enteroenteric reflexes and the sacral outflow to the bowel would directly relate to the clinical problems experienced by MS patients. Determining the role of “channels” (for example, potassium and sodium) in neurotransmission and GI transit and developing drugs with 5-HT4 properties, D2 characteristics, or novel agents that target the bowel would be helpful.
Advances are needed in the understanding of human GI motility. These studies should draw on techniques used by gastroenterologists, such as the use of enteric capsules, intraluminal manometers, microminiature strain gauges, digital logging for prolonged recordings, imaging techniques of GI motility, and sphincter electrophysiology to explore the pharmacology of GI function and control. Also, studies of pelvic floor musculature in spinal MS might reveal new data on how to improve the mechanical aspects of sphincter function. As stated above, studies of spinal cord injury would complement studies on patients with spinal MS.
†Channels are the pores in cell walls that are opened and closed to permit the passage of specific ions, a key process in all aspects of physiological regulation.
Page 148
VISUAL DISTURBANCES
Visual disturbances resulting from demyelination or inflammation in the optic nerve occur in up to 85 percent of people with MS.5 These visual disturbances take a variety of forms, including optic neuritis, abnormal eye movements, blurred or double vision, and color distortion. The optic nerve is involved in the first manifestation of disease in about 35 percent of people with MS.192
Optic Neuritis
Optic neuritis (inflammation of the optic nerve) is the most common visual disturbance in MS. In 17 percent of people with MS, optic neuritis was the first evidence of disease, but it can also occur subsequent to other symptoms.192 Symptoms associated with optic neuritis include rapid vision loss, pain associated with eye movement, dimmed vision, abnormal color vision, altered depth perception, and Uhthoff's phenomenon in which visual loss is associated with increased body temperature.135
Optic neuritis usually worsens for three to seven days before gradually improving over the following weeks and months.192 The prognosis for ultimate recovery of vision following optic neuritis is good, although conduction abnormalities seen by visual evoked potential often persist after recovery.190 Evidence suggests that recovery from optic neuritis might involve cerebral adaptation to persistently abnormal visual input.
Episodes of optic neuritis are frequently but not always followed by a diagnosis of MS. Estimates of the risk for developing MS within 15 years of an acute bout of optic neuritis range from 45 percent to 80 percent.135 MRI is the most powerful predictor of whether MS will develop following optic neuritis, as evidenced by the presence and number of lesions in the brain.135 The Optic Neuritis Treatment Trial is the most extensive study of optic neuritis. This study of 457 subjects with acute optic neuritis found that patients with no initial brain lesions had a 16 percent chance of developing clinically definite MS within the 5-year study period, whereas those with three or more lesions had a 51 percent chance; clinically definite MS developed in 27 percent of all subjects, and probable MS developed in 9 percent.175
The Optic Neuritis Study Group found no benefit of oral or intravenous steroid treatment over nontreatment in ultimate visual outcome or in the development of MS. Within five years of their first bout of optic neuritis, 94 percent of all patients had visual acuity of 20/40 or better, although 56 percent reported that their vision had worsened.175 Patients treated with intravenous methylprednisolone recovered vision more quickly and were half as likely to develop a second event within 2 years, compared to controls and patients treated with oral prednisone.5,14 However, after two years the protective effect wore off. Further, compared to controls and the I.V. steroid group, the oral steroid group was more
Page 149
likely to have recurrences of optic neuritis within two years.5 Steroid treatment for optic neuritis did not influence the development of MS. Within five years of treatment there was no significant difference between the number of steroidtreated patients and nontreated patients who developed MS.175 A small proportion of patients develop progressive visual loss, usually later in the course of the disease. These patients, together with those who fail to recover from an acute episode, might benefit from visual aids.
Abnormal Eye Movements
Abnormal eye movements result from demyelination of afferent nerve pathways to the eye muscles and occur frequently in MS patients. The type of abnormality depends on the lesion site. The most common visual disturbances are nystagmus, saccadic intrusion, and diplopia.5,30
Nystagmus is a back-and-forth eye movement that occurs in 28.5 to 63 percent of people with MS.5 Nystagmus often results from lesions in the brainstem or cerebellum and can take many forms. It can be jerky or pendular, vertical, horizontal, or circular. Patients with nystagmus sometimes experience dizziness and difficulty reading.33
Saccadic intrusion is difficulty in shifting the focus of vision and results in oscillation of the eyes. Smooth pursuit is lost, replaced by a series of saccades. Saccadic intrusion is found in many individuals that do not have MS and is not recommended for routine diagnosis.113 Other saccadic abnormalities can also occur in MS, including delay in the initiation of saccade, decreased velocity, and oscillation of the eyes following saccade and prior to fixation on the target.5
Diplopia, or double vision, is a transient symptom, and estimates of its incidence range from 8 to 22 percent at disease onset and from 29 to 39 percent throughout the course of MS.33
Various drugs are used to treat these conditions with moderate results, including baclofen, clonazepam, memantine, scopolamine, gabapentin, and isoniazid for nystagmus, and clonazepam and propranolol for saccadic oscillations (Table 3.6).30,167 Botulinum toxin injection into extraocular muscles has been shown to decrease the amplitude of nystagmus and improve visual function for about eight weeks in MS patients but is not always well tolerated.142 Finally, prisms have been shown to stabilize images on the retina and may help MS patients with nystagmus or diplopia.
FATIGUE
Fatigue, considered the most common symptom in MS, is experienced by 76 to 92 percent of people with the disease.94,122 Among MS patients, 66 percent report experiencing fatigue on a daily basis,61 and 33 percent report that fatigue is
Page 150
|
TABLE 3.6 Medications Used to Treat Optic Neuritis and Abnormal Eye Movementsa |
|||
|
Trade Name |
Generic Name |
Mechanism |
Potential Side Effects |
|
Solu-Medrol14,85,125 |
Methylprednisolone sodium succinate |
Anti-inflammatory corticosteroid often taken intravenously. |
Fluid retention, weakness, GI upset, increased intracranial pressure, vertigo, headache, ulcers, hypertension |
|
Deltasone14,135 |
Prednisone |
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogues are primarily used for their potent anti-inflammatory effects in disorders of many organ systems. Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli. |
Fluid retention, muscle weakness, steroid myopathy, loss of muscle mass, osteoporosis, aseptic necrosis of femoral and humeral heads, peptic ulcer, pancreatitis, abdominal distention, impaired wound healing, thin fragile skin, petechiae and ecchymoses, facial erythema, increased sweating, convulsions, vertigo, headache, menstrual irregularities, suppression of growth in children |
|
Decadron |
Dexamethasone |
Decadron is a potent anti-inflammatory synthetic adrenocortical steroid used to reduce inflammation that occurs in MS exacerbations. |
Depression, euphoria, hypertension, nausea, anorexia, decreased wound healing, acne, muscle wasting, bone pain, and increased susceptibility to infection |
Page 151
their most troubling symptom.94 Fatigue affects MS patients of all types and all levels of disability and is not related to patient age, level of neurological impairment, or the EDSS.94 Assessment of fatigue in MS is complicated by the many potential contributing factors.176 Antidepressants, beta-blockers, and medications used for spasticity can worsen fatigue, and other problems of MS such as pain, depression, poor sleep, psychological stress, and deconditioning can contribute, as well. For some, fatigue is overwhelming. For others, it is transient, induced by physical activity. It is a particularly pervasive symptom, creating problems for employment, fulfilling family and social roles, getting exercise, and mood—the basic ingredients of life satisfaction.31
Definition and Types of Fatigue
Fatigue can be defined as an overwhelming sense of tiredness, lack of energy, or feeling of exhaustion that is in excess of what might normally be expected.94 Fatigue can occur as a result of a task or in anticipation of a task. It can be influenced by physical features of the task (such as the length of a run) or by psychological aspects of the task (such as how rewarding it will be) or both. Fatigue can also be interpreted as a sense of effort needed to perform a task, and both physical and mental tasks may result in fatigue. In addition to the sensation or perception of fatigue experienced only by the individual, fatigue may also produce observable changes in behavior, especially as a decrement in performance (for example, a decrease in the ability to process information). Acute fatigue typically resolves after completion of the task. Fatigue that is more chronic and pervasive is frequently associated with illness, stress, and sleep disturbances. Fatigue as a distinct clinical symptom in MS must, however, be distinguished from true muscle weakness, sleepiness or drowsiness, and depression. These conditions can coexist with fatigue but are associated with a different array of conditions that may be amenable to separate treatment.
Attempts to establish a clinical definition of fatigue are complicated by patients' varied uses of the term. Sometimes fatigue is a synonym for other complaints, while at other times the term is applied to a collection of symptoms that occur together. Patients might use the term fatigue to refer to weakness, dizziness, lack of coordination or stamina, feeling “spacey”, having poor concentration or cognitive abilities, having “rubber legs”, boredom, lack of motivation, malaise, or feeling blue or depressed.
Causes of Fatigue
The nature of fatigue in MS has yet to be fully characterized, but it is interrelated with physical activity and social engagement.61,176,182 Plausible mechanisms related to impaired motor function include slowing or alteration of neurotransmitter release and increased nerve conduction times. Measurements of
Page 152
physical fatigue are higher in MS patients with pyramidal tract signs and during relapses.46 New onset of fatigue may be a sign of impending relapse.176 Other MS symptoms resulting from impaired motor function, such as spasticity, weakness, and ataxia, may contribute to fatigue by increasing the physical, and psychological, effort required for mobility.86 Symptoms such as pain and spasticity that interfere with sleep may also be a factor in fatigue. Fatigue is worsened by heat, making physical activity particularly difficult.
One finding across a variety of physical disorders has been that the severity of fatigue rarely correlates well with measures of disease activity (for example, in rheumatoid arthritis or hepatitis C).71,75 Functioning is often more closely tied, even in physical conditions, to psychological factors and stressors. Although one study concluded that MS-related fatigue is not associated with depression,95 another suggested that mental fatigue in MS correlates with anxiety and depression but physical fatigue does not.61,173
Although one study suggests that MS fatigue might be associated with frontal cortex and basal ganglia dysfunction resulting from demyelination of frontal white matter,144 other neuroimaging studies have failed to find a significant correlation between fatigue and neuropathology in MS patients.11,13 Neuroimaging of chronic fatigue syndrome, which has been much more widely studied in this regard than MS, has likewise yielded conflicting results.99,105 At this stage, reports of fatigue-associated neuropathology in MS should be interpreted cautiously.
Measurement of Fatigue
Because fatigue is multidimensional, with distinct physical and mental elements, measurement tools must be carefully constructed and carefully used to produce valid, reliable, and meaningful distinctions among the separate elements and differing levels of fatigue. Measurement strategies for fatigue in MS should be closely linked to one or more of the four components of fatigue: behavior, sensation or perception of fatigue, mechanisms of fatigue, and context.
Behavior refers to the physical manifestations of fatigue or a decline in performance, such as making more errors or inability to complete a task. The sensation or perception of fatigue can occur in the absence of any actual physical or mental effort, and it might or might not be in proportion to a particular task. A sense of fatigue can coexist with psychological symptoms (even in the absence of a psychiatric disorder) and with beliefs that result in behaviors—for example, the belief that exertion is harmful and the consequent avoidance of exercise.
The general literature on measurement of mechanisms of fatigue has tended to focus on single causes (for example, infections or psychiatric disorders), a perspective that is difficult to apply to cases where the causes are unknown and possibly many. Physiological mechanisms of fatigue are thought to reflect either peripheral processes operating in the muscles or nerves or central processes oper-
Page 153
ating in the brain. Proposed psychological mechanisms have included beliefs, perceptions, expectations, and symptom amplification. The contextual component of the measurement of fatigue includes an appraisal of the personal, social, occupational, cultural, and physical environment in which the symptom occurs. This component captures the influence of temperature, noise, the family, and stressors on the experience of fatigue.
In studies of MS, various self-report instruments have been used to measure fatigue (summarized in 1998 by Ford and colleagues61). These include the Fatigue Severity Scale and the Fatigue Assessment Instrument. Both scales measure the effect of fatigue on functioning. The Fatigue Assessment Instrument measures fatigue severity, whether fatigue is situation specific, the consequences of fatigue, and the response of fatigue symptoms to rest or sleep. Neither scale identifies distinct mental and physical dimensions of fatigue.
In general, the currently available measurement tools, even the most basic ones, require further evaluation. In particular, they must be tested and validated specifically for use by populations with fatigue. Moreover, the “gold standards” that do exist are often poor. Nonetheless, if carefully selected, some of the currently available measurement tools are adequate for clinical trials. These are primarily self-report instruments, such as the SF-36. For most medical conditions, a suitable biological measure or marker of fatigue has not yet been identified.
Disease-specific instruments may offer the advantage of greater sensitivity in detecting change since they incorporate measurements of phenomena more likely to be experienced by persons with a particular condition (for example, swollen joints in rheumatoid arthritis). With these instruments, however, comparability and generalizability are sacrificed.
Management of Fatigue
Fatigue has a broad impact on functioning for persons with MS. Chapter 4 addresses broader aspects of the management of fatigue in the context of overall strategies for adapting to MS. Noted here are some specific interventions, including medications, that are used in treating fatigue. Exercise programs may help maintain conditioning and improve self-esteem.129,176 One study suggests that aerobic exercise may be beneficial.128 Behavior modification therapy may be helpful for identifying strategies to avoid situations that worsen fatigue.
Pharmacological treatment of fatigue has also been tested (see Table 3.7). Amantadine, an antiviral agent, has been shown to improve fatigue compared to a placebo.95 Pemoline, a CNS stimulant, may also be beneficial, but clinical trials have shown more limited effects than for amantadine.86,95 Fluoxetine hydrochloride, an antidepressant, may help reduce the components of fatigue attributable to depression.
Page 154
|
TABLE 3.7 Medications Used to Treat Fatigue |
|||
|
Trade Name |
Generic Name |
Mechanism |
Potential Side Effects |
|
Symmetrel70,95,115 |
Amantadine |
Mechanism unknown. An antiviral medication used to prevent or treat certain influenza infections, it is also given as an adjunct for the treatment of Parkinson's disease. |
Difficulty concentrating, dizziness, headache, irritability, loss of appetite, nausea, nervousness, purplish red, net-like, blotchy spots on skin, trouble sleeping or nightmares, constipation, dry mouth, vomiting |
|
Provigil104 |
Modafinil |
Precise mechanism unknown. Has wakepromoting effects similar to amphetamine and methylphenidate. Produces psychoactive and euphoric effects; alterations in mood, perception, and thinking; and feelings typical of other CNS stimulants. |
Headache, nervousness, dizziness, depression, anxiety, insomnia, paraesthesia, hypertonia, confusion, emotional lability, ataxia, tremor, diarrhea, dry mouth, anorexia, mouth ulcer, vasodilation, arrhythmia, syncope, urinary retention, abnormal ejaculation |
|
Cylert95,115,171,188 |
Pemoline |
A CNS stimulant used to treat attention deficit disorder. It is used in MS to relieve certain types of fatigue. Krupp et al.95 found that treatment with pemoline was no more beneficial than placebo in patients with MS. |
Insomnia, elevated heart rate, nervousness, agitation, loss of appetite and weight loss, GI upset, including constipation and diarrhea, hallucinations |
|
Prozac115 |
Fluoxetine hydrochloride |
A selective serotonin reuptake inhibitor, it increases serotonin levels in the midbrain. |
Anxiety, nervousness, insomnia, fatigue, tremor, sweating, GI distress, anorexia, diarrhea, dizziness, decreased libido |
Page 155
Recommendations for Further Research
-
Studies are needed to gain a better understanding of the phenomenon of fatigue.
-
A careful review should be made of the possible tools available to assess each domain of fatigue and to assess the relevance of these tools for MS.
-
All studies evaluating physical or mental performance (including trials for treatments such as exercise or cognitive behavioral therapy) should include measures of both perception of effort and actual performance.
-
Attempts to elucidate biological markers of fatigue should focus on specific components (for example, perception, mechanism) of fatigue. Possible markers should make clinical and biological sense.
-
When objective markers for fatigue are sought, care must be taken to determine that observed alterations are due to fatigue, rather than another confounding process (for example, controlling for affective disorders when performing SPECT [simple photon emission computed tomography] scans of the brain).
-
Investigation of biological mechanisms for the perception of fatigue is critical. Given the lack of correlation between perceived fatigue and disease severity in well-recognized diseases, it is crucial to gain a better understanding of perceptual processes.
-
Appropriate challenge paradigms should be developed to examine fatigue in MS and other disorders. Asking subjects to perform tasks under more stressful conditions may help identify biological markers of fatigue that are not normally observed under basal conditions.
SEXUAL DYSFUNCTION
The estimated incidence of sexual dysfunction in MS varies, depending on what source is reviewed. This is not surprising considering the variable nature of the neurologic disorder. Up to 91 percent of men with MS and 72 percent of women have adverse effects on their sexual function, and up to 80 percent of men with MS report erectile dysfunction.17,67,100,181
Desire and Satisfaction
Sexuality has both psychological and physiological components, and both aspects contribute to the level of a person's sexual functioning. However, the incidence of physiological versus psychological sexual dysfunction in men and
Page 156
women with MS is not known. Research addressing this question would be valuable for people living with MS, their partners, and health care providers and also for determining appropriate treatment protocols.
The possibility that the psychological aspects of MS would have a negative impact on sexuality is obvious given the uncertainty of the disorder. Decreased libido has been documented in 22 percent of men with MS12 in addition to 28 to 60 percent80 of women; moreover, sexual dysfunction is associated with depression.109 It has not been determined whether this decrease in libido is related to severity or duration of the disease or is a side effect of medications used. Nor has it been determined whether treatment of depression will improve sexual function. Sixty-four percent of men and 39 percent of women report decreased sexual satisfaction and/or absent sexual activity. Finally, associated marital problems have been noted in 71 percent of MS patients with sexual dysfunction. The relationships of these issues to premorbid psychosexual function are not clearly understood. Furthermore, the impact of MS on performance of sexual activities should be considered. The availability and impact of sexual education and counseling for patients and their partners should also be studied. It is also important to recognize that issues associated with sexuality are different for gay and lesbian populations.81
Sexual Response
One report noted that 58 percent of women and 63 percent of men with MS had problems with anorgasmia.109 The same study noted that 80 percent of men had erectile dysfunction and 40 percent of women had problems with lubrication, but the study did not provide specific information on the subjects' neurological status.
The arousal and orgasm phases of sexual response are also affected by physiological and psychological issues. Lesions affecting the neurological pathways that control sexual response would affect physiological aspects of sexual response. However, these pathways are not yet well understood (Table 3.8). The intracranial control of sexual arousal is not well defined.15,148,160 Better documentation of the normal neurologic pathways that control sexual response will be necessary before the physiologic effects of specific neurological lesions on sexual function can be identified. (See Figure 3.1 for location of spinal nerves.)
Cervical cord plaques are thought to affect some 80 percent of patients, intracranial plaques 60 to 90 percent, and sacral cord (lower back) and conus medullaris (lowermost portion of the spinal cord) lesions are thought to occur in up to 63 percent of patients.101 Thus, some patients will have neurologic lesions affecting the pathways for sexual function whereas others will not. To determine whether there is a physiological reason for altered sexual function, studies that assess the impact of MS on sexual function should provide detailed information regarding the subject's neurological condition. Unfortunately, this is rarely the
Page 157
|
TABLE 3.8 Neurological Pathways That Control Sexual Response in Men and Women |
|
|
Sexual Function |
Probable Neural Pathway |
|
Psychogenic erectile function and lubrication |
The spinal pathways are thought to travel in the lateral white columns that synapse on the autonomic motor cells in the lateral gray columns of the lower thoracic and upper lumbar cord.15,158,160 |
|
Reflex erectile function and lubrication |
Thought to include sensory transmission via the pudendal nerve to stimulate the sacral parasympathetic nerves. |
|
Ejaculation |
Both sacral parasympathetic and thoracolumbar sympathetic contributions, in addition to a sacral somatic component. A spinal cord center has been proposed at the twelfth thoracic and first lumbar nerves.148 |
|
Orgasm |
Postulated to occur as a reflex response of the autonomic nervous system.158 |
case. Rather, the information provided is generally limited to the Expanded Disability Status Scale (see Chapter 4 for discussion of the EDSS).97 Studies published thus far have not found a correlation between level of disability and sexual dysfunction or satisfaction with sexuality.110
Some researchers have attempted to link neurological testing with the status of sexual functioning. In men and women with MS, anorgasmia has been correlated with brainstem and pyramidal abnormalities in addition to the total area of plaques on the brain observed via MRI. Betts and colleagues17 performed posterior tibial and pudendal cortical evoked potentials along with cystometric tests in men with erectile failure and MS. They concluded, as did another group,91 that the neural lesions associated with impotence are generally above the sacral cord. In addition, men with abnormal pudendal or tibial evoked responses had difficulties with ejaculation and orgasmic dysfunction. One report noted that in women with advanced multiple sclerosis, weakness of the pelvic floor and bladder and bowel dysfunction are correlated with changes in lubrication and orgasmic capacity.80 However, that report relied on the EDSS and did not provide detailed neurological information regarding women's sexual arousal and the impact of MS.
Another difficulty in the study of sexual function stems from the inaccuracies associated with self-report data.159 Most importantly, the issues noted in the previous paragraphs should be addressed through the performance of controlled, laboratory-based research to systematically document the physiologic effects of specific neurological dysfunctions associated with MS on sexual function. This should also provide information regarding the incidence of psychological sexual dysfunction affecting persons with MS.
Page 158
Treatment of Sexual Dysfunction
Great strides have been made in the treatment of erectile dysfunction, including the availability of vacuum pumps, injection erections, and oral medications (Table 3.9). Most research has evaluated the effect of therapeutic agents on neurogenic erectile dysfunction in general77,78 or specifically on sexual dysfunction due to spinal cord injury.42 Only minimal research, however, has concentrated on the amelioration of sexual dysfunction specifically associated with MS. While these results are likely relevant to men with MS, the complex interplay between psychological and physiological issues and sexuality must be considered. As a result, new therapeutic interventions for the remediation of erectile dysfunction should be tested to verify their efficacy in men with MS. Patterns of neurological dysfunction with detailed information about psychosexual functioning should be documented. Furthermore, techniques to treat other sexual dysfunctions in men with MS, including orgasmic dysfunction, should be developed and tested.
Female sexual dysfunction has generally received less attention than male sexual dysfunction, particularly erectile dysfunction. However, the development of oral medications to remediate erection dysfunction has led to a heightened awareness of women's sexuality and associated issues. Sildenafil, a drug previously shown to be safe and effective in the treatment of erectile dysfunction, was recently tested in a small study of 19 women with spinal cord injuries.161 The results showed that sildenafil can significantly increase the level of subjective sexual arousal in women with neurogenic sexual dysfunction. Further research is needed with a goal of improving the sexual responsiveness and satisfaction of women with MS. Large-scale studies of the use of sildenafil and other new therapeutics to improve female sexual responsiveness should be conducted.
PAIN
Although multiple sclerosis is not commonly thought of as a painful disease, when MS patients are asked, a surprisingly high proportion report significant pain problems.32,108,137,170 At the time of their initial presentation, pain is a significant complaint in about one in five patients.170 Nearly one-half of individuals with MS experience clinically significant pain at some time during the course of the disease. Significantly more women than men report pain.120 The longer the duration of the disease and the older the patient, the more likely it is that pain will be reported.32,120 The disconnect between the incidence and severity of the pain and the realization by physicians of its importance to the patient is a major problem for the management of MS.
The reasons for this disconnect are unclear. Physicians and patients tend to focus on weakness, poor coordination, visual impairment, and fatigue, and there
Page 159
|
TABLE 3.9 Medications Used to Treat Erectile Dysfunction |
|||
|
Trade Name |
Generic Name |
Mechanism |
Potential Side Effects |
|
Viagra83 |
Sildenafil citrate |
Causes release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation, which produces smooth muscle relaxation in the corpus cavernosum and allows inflow of blood. |
Headache, facial flushing, upset stomach |
|
Muse; Prostin VR78,166 |
Alprostadil |
A vasodilator that causes blood vessels to expand, increasing blood flow. It is a semisolid suppository pellet that is inserted into the urethra, producing an erection by increasing blood flow. |
Burning or aching during erection, erection continuing for more than 4 hours |
|
Pavabid17,184 |
Papaverine |
A vasodilator that is injected into the penis, producing an erection by increasing blood flow. |
Bruising at the injection site, mild burning along the penis, difficulty ejaculating, swelling at the injection site |
Page 160
is a tendency to underestimate the disability that pain can produce. Another problem is the tremendous variety of pain complaints. Different painful manifestations of MS are seen in different individuals and likely have different underlying mechanisms. Little is known about the mechanism for any of the pain complaints in MS. Some of the pain complaints are transient and accompany the acute attack, whereas others appear relatively late in the course of the disease and are unremitting. There is also an understandable bias toward the belief that if the underlying disease process can be stopped or slowed, the symptom of pain will resolve. Unfortunately, current treatments for MS are of limited impact and the accumulated burden of irreversible damage places the patient at risk for persistent pain.
Among the complaints that often accompany MS are headache and back pain, which are so common in the general population that it would be difficult to establish that MS is the cause (see for example, Rae-Grant et al.137). In contrast, painful tonic spasms and tic doloreux in young people are so rarely seen, except in patients with MS, that they probably result directly from the demyelinating process. Optic neuritis is often painful during the acute attack; presumably this is a direct result of the inflammatory process and activation of primary afferent nociceptors.* Sensory changes, such as persistent numbness or tingling of a body part, although not necessarily painful, are experienced by about 80 percent of people with MS.107
The sheer variety of pain complaints, their appearance in subsets of MS patients and the fact that the disease is uncommon have been major impediments to clinical therapeutic trials. There are simply too few eligible patients to conduct clinical trials that would have sufficient statistical power to detect a clear therapeutic effect. There have been no controlled clinical trials specifically for pain treatment in multiple sclerosis. This problem and the lack of a good animal model for pain in MS also make it difficult to investigate the pathophysiology of pain in this disease.
Pain complaints in multiple sclerosis can be divided into four broad categories:
1. Neuropathic pain. This is chronic and/or recurring pains resulting from the destructive process (i.e., due to abnormal function of damaged neuronal or glial elements). This type of pain is either paroxysmal or static depending on the syndrome. The sensory qualities of these painful conditions clearly identify them as neuropathic.
2. Acute pain due to the active inflammatory process. The sensory characteristics of this type of pain are nonspecific, although the pain should temporally track the exacerbations of the underlying demyelinating process.
*Nociceptors are neurons that are preferentially sensitive to noxious stimuli or to stimuli that would become noxious if prolonged.
Page 161
3. Pain secondary to increased muscle tone. This includes cramps and spasticity-related muscle pain.
4. Chronic or recurrent pain of a nonspecific nature such as neck or back pain, possibly due to increased tone or weakness of muscle. Such syndromes are extremely common in normal individuals, so it is usually difficult to be sure that they are caused by MS.
Neuropathic Pain Syndromes
Trigeminal Neuralgia
The occurrence of trigeminal neuralgia in MS patients is well known.27,120 Trigeminal neuralgia is a unique and easily recognized symptom complex that usually affects the elderly. It consists of brief shock-like (tic-like, or lancinating) pains that often have an electrical quality. The key features are very brief duration of individual shocks (a second or less), pain-free intervals, location in the second and third division of the trigeminal sensory distribution, and the presence of trigger points on the face or in the mouth where light touch will trigger an attack. Although trigeminal neuralgia occurs in less than 3 percent of MS patients, it is much more common and tends to occur at a somewhat younger age in MS patients than in the general population.27,120 Not surprisingly, there are often associated sensory changes in MS patients with trigeminal neuralgia. Persistent facial pain may occur in MS patients and can have a lancinating component. Persistent (tonic) pain is not seen in idiopathic trigeminal neuralgia.
The same treatment approaches (anticonvulsants and neurolytic lesions) appear to be effective for trigeminal neuralgia whether in the setting of MS or not.22,88,120 This suggests that MS is a risk factor for trigeminal neuralgia but is insufficient, by itself, to cause the syndrome. One popular theory of idiopathic trigeminal neuralgia is that it represents vascular compression of the central root of the trigeminal ganglion, but this does not explain why trigeminal neuralgia should be overrepresented in MS. It may be that pre-existing demyelination is another risk factor.
In summary, trigeminal neuralgia is an uncommon but severely painful syndrome that occurs significantly more often in MS patients than in the general population. It responds to the usual trigeminal neuralgia therapeutic interventions and appears to share an underlying pathophysiology.
Painful Tonic Spasms
In contrast to trigeminal neuralgia, painful tonic spasms are rarely observed except in MS.108 In MS they seem to be relatively common, occurring in between 10 and 20 percent of patients.183 Their clinical presentation has unique and highly
Page 162
characteristic features. Painful tonic spasms usually occur in the setting of rapidonset but tonic muscle contractions. Individual spasms are localized to a body part or region. They are brief, rarely lasting more than two minutes, but the pain is usually severe. The painful tonic spasms are often triggered by movement or sensory stimulation, rarely by well-localized and reproducible stimulation of the skin, as in trigeminal neuralgia. Sometimes the pain is accompanied by loss of motor function (i.e., weakness or dysarthria). The location can be anywhere on the body. Fortunately, the syndrome tends to be transient, with a duration of two weeks to two months. Electroencephalogram recordings during painful tonic spasms are normal, suggesting a subcortical origin for the underlying neural discharge.108 In a recent report with a literature review and five original cases, Spissu and colleagues165 point out that most patients with painful tonic spasm have at least mild involvement of the pyramidal tract on the side of the pain. In their cases, fMRI studies revealed an acute lesion in the posterior limb of the internal capsule or the cerebral peduncle corresponding somatopically to the affected region.
Although not yet studied in a controlled manner, anticonvulsants such as carbamazepine and gabapentin are reported to provide dramatic relief for many patients with painful tonic spasm in MS.164
Continuous Dysesthetic Pain
This is the most common neuropathic pain in MS.32,120 It is most often located in the lower extremity and is commonly bilateral. In some cases the trunk and/or upper extremity may be involved. This pain is usually described as burning and is typically most pronounced distally. On exam, patients with this complaint have sensory deficits that overlap the painful regions. All of these patients have a deficit in dorsal column sensory function, and about half have a deficit of pain and/or temperature sensation, presumably due to damage to the spinothalamic tract.120,183 The association of spinothalamic tract-type deficits and continuous dysesthetic pain is characteristic of central pain syndromes such as poststroke and spinal cord injury pain. These syndromes are notoriously resistant to analgesic treatments.
There have not been any controlled clinical trials of treatments that specifically address this type of pain. There are anecdotal reports that in some patients it responds to tricyclic antidepressants32,108,120 and anticonvulsants such as gabapentin79,152 and lamotrigine.111
Neuropathic pain can be caused (after nerve injury, for example) by the generation of inappropriate (in some cases, spontaneous) high-frequency bursts of impulses by neurons within the pain pathway. The generation of nerve impulses depends on the activity of specialized proteins called sodium channels, and it is now known that nearly a dozen genes encode distinct sodium channels with different properties, many of which are expressed in sensory neurons.45 It is
Page 163
well established that following injury to their axons, neurons turn off sodium channel genes that are normally active and turn on others that are normally silent.45,186 This produces an abnormal ensemble of sodium channels whose electrical activity can cause less activity.36 Evidence is beginning to emerge suggesting that similar changes in sodium channel expression can occur in neurons whose axons are subjected to inflammation172 or demyelination.18 The identification and characterization of the abnormal ensemble of sodium channel(s) is a promising area for research, that may provide a basis for new pharmacological treatments.
Acute Radicular Pain
Acute radicular pain has been reported as a presenting symptom in MS.138 In several patients there was a report of trauma preceding the onset of the pain. However, in most, there was no obvious trauma. In 2 of 11 patients, there was an MRI-demonstrated plaque at the appropriate site. The quality of the pain, its duration, or its response to treatment was not reported.
Muscle Spasms and Cramps
This problem is mentioned in most large series of pain complaints in MS. This type of pain can be seen in people without any underlying neurological condition but is often present in association with upper motor neuron damage and accompanies spasticity. There are numerous treatment strategies for spasticity, none totally satisfactory. A recently published small controlled study of 20 patients demonstrated a significant benefit of gabapentin for symptoms of spasticity, including painful spasms.37
This type of pain may also be responsive to cannabinoids. In a retrospective, uncontrolled study of 112 MS patients in the United States and the United Kingdom, Consroe and colleagues34 reported that smoking cannabis relieved a variety of pain complaints. More than 90 percent of patients reported improvement in muscle pain, leg pain, and spasticity. A similar percentage reported improvement in their tremor. This report takes on added significance in view of studies in a mouse model of MS (chronic relapsing EAE), which demonstrated significant improvement in tremor and spasticity with a synthetic CB1 receptor agonist.* This evidence warrants controlled clinical trials.
*The CB1 receptor is the primary cannabinoid receptor in the brain and can be stimulated by a variety of agonists, including THC.
Page 164
Acute Pain Due to the Inflammatory Process
Optic Neuritis
This type of pain is most clear-cut when MS is heralded by optic neuritis. In such cases, pain often accompanies the attack. The pain is aching and exacerbated by eye movements. The reported response to glucocorticoids supports the general feeling that this type of pain represents activation of dural nociceptors by the inflammatory process.32 In other situations such as headache and back pain, the association of pain with an acute inflammatory attack is less clear.
Headache
Headaches are such a common occurrence in the general population that it is difficult to prove that MS can cause them. Nonetheless, the weight of evidence suggests that it can. In a prospective study, Rolak and Brown145 found an overall incidence of headache of 52 percent, which is much higher than in the general population. Both tension-type and migraine headaches were common and there was no characteristic “MS headache.” Furthermore, they were not able to establish a higher incidence of headache in correlation with clinical exacerbations based on other symptomatology. In contrast, in a retrospective study of 1,113 MS patients, Freedman and Gray reported 44 patients in whom an attack or exacerbation were heralded by a migraine attack.62 Although their definition of migraine was somewhat loose, 27 of these patients had no prior history of migraine. Headache accompanying attacks seems to be more common in children (Stephen Hauser, personal communication). It is of interest in this regard that Haas et al.74 reported a 16-year-old girl who presented with a severe headache and internuclear ophthalmoplegia but little else neurologically. On imaging, her sole abnormality was in the region of the midbrain periaqueductal gray, an area implicated in migraine pathogenesis.187 Subsequent clinical course and the presence of oligoclonal bands in her cerebrospinal fluid established the diagnosis of MS in this case.
In summary, there seems to be some evidence that headache is a common complaint in MS and may be a direct consequence of disease activity.
Pain Due to Increased Muscle Tone
In any neurological condition characterized by spasticity (i.e., upper motor neuron disease), patients are at risk for muscle pain due to spasm. Spasticity is common in MS, especially over time as the lesion burden increases. Spasticity is most likely to involve the legs. MS patients frequently report leg spasms and cramps.32,120 This type of pain improves if spasticity can be controlled with
Page 165
standard medications such as baclofen, dantrolene, benzodiazepines, and most recently gabapentin.37
Back Pain
Back pain is common in MS.32,120 It is described as aching, usually in the lower back, and is exacerbated by prolonged sitting or standing and the abnormal gait. The most common cause appears to be musculoskeletal. This type of pain, as in patients who are neurologically intact, has two common musculoskeletal causes: degenerative disease around the disks, foramina, and facet joints, and myofascial pain due to disuse and spasticity. This type of pain responds to physical therapy, trigger point injections with stretching, anti-inflammatory agents, and analgesics. In addition, especially in patients treated with glucocorticoids, there is the possibility of vertebral compression fractures secondary to osteoporosis. Urinary tract infection in patients with bladder dysfunction is also a consideration in some patients with subacute back pain.
Summary
Pain is a frequent accompaniment of MS, and for a small group of MS patients, pain is the worst aspect of the disease.137 Despite treatment, many MS patients have ongoing pain. Pain may be due to inflammatory disease activity, in which case it is usually transient and may respond to glucocorticoids. In other cases, the accumulating burden of CNS damage may lead to irreversible lesions that cause neuropathic pain. The neuropathic pain syndromes are of two types: paroxysmal and constant. Paroxysmal phenomena (trigeminal neuralgia and painful tonic spasms) are recurrent and appear to respond well to anticonvulsant medications. Constant dysesthetic pains vary in intensity and appear to be resistant to currently available treatments. However, the committee did not find any satisfying data on this point. Another set of pain types are of musculoskeletal origin: back pain, probably related to weakness, disuse, and immobilization, and limb pains that are apparently due to increased muscle tone and take the form of spasms and cramps. The former seem to respond to aggressive physical therapy, whereas spasm-related pains improve with the treatment of spasticity. Finally, there is the controversial issue of headache, which seems to have an increased incidence in MS patients but no characteristic pattern and no established relationship to disease activity.
Research Recommendations
Attention should be paid to evaluating pain in future clinical trials. Pain relates to both pathophysiology and quality of life, and should be considered in future clinical trials.
Page 166
Multicenter clinical trials are essential to evaluate currently available treatments, especially for the constant dysesthetic pain that is currently the most refractory of the multiple pain syndromes accompanying MS. Cannabinoids might be particularly promising for patients with spasticity but should also be evaluated for other nonparoxysmal pain complaints.
To establish whether acute disease activity is associated with pain, an effort should be made to correlate new-onset clinical signs, MRI with gadolinium, and pain location, quality, and time course. Response to anti-inflammatory agents could be studied as well.
Continued efforts should be made to understand the pathophysiology of a neuropathic pain, including the continuous dysesthetic pain that occurs in patients with demyelinating diseases such as MS. Efforts to understand abnormal expression of ion channels in demyelinated neurons appear promising. The extensive literature on spinal cord injury and pain offers a number of models that could be extended to this type of study so that it can address demyelinating lesions (for example, Brewer and Yezierski, 199821). In addition, although it is difficult to assess continuous pain in animals, the mouse models for inflammatory demyelination offer an area where parallel human and animal work could progress.
Lesion Location
A specific attempt should be made to identify patients with new-onset continuous dysesthetic pain for functional imaging in a concerted effort to locate the relevant pathology. In these patients, quantitative sensory testing should be carried out, especially to assess spinothalamic tract function (heat, heat pain, cold, cold pain). In addition, examination should look for signs of a hyperpathic state (summation, sensory radiation, pain with light mechanical stimulation [allodynia]). With these data in hand, it might be possible to move closer to determining whether the animal model mirrors the human pain problem.
Studies on Nervous System Inflammation and Pain
It appears likely that inflammation per se can lead to abnormal hyperexcitability of pain-signaling neurons. By understanding the underlying molecular mechanisms, it may become possible to devise better interventions.
REFERENCES
1. , , , . 1997. A replicated prospective investigation of life stress, coping, and depressive symptoms in multiple sclerosis. J Behav Med.; 20: 433-45.
Page 167
2. , , , . 1999. Assessing depressive symptoms in multiple sclerosis: is it necessary to omit items from the original Beck Depression Inventory? J Behav Med.; 22: 127-42.
3. , , , . 1998. Teaching memory strategies to persons with multiple sclerosis. J Rehabil Res Dev.; 35: 405-410.
4. , , , , , . 1995. Cognitive impairment in early-onset multiple sclerosis. Pattern, predictors, and impact on everyday life in a 4-year follow-up. Arch Neurol.; 52: 168-72.
5. , . Visual Signs and Symptons. Paty DW, Ebers GC, eds. 1998. Multiple Sclerosis .em>. Philadelphia, PA. F.A. Davis Company. 229-256.
6. , , , . 1996. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol (Lond).; 494: 851-61.
7. , , , . 1999. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology.; 13: 434-46.
8. , , , . 1997. Executive functions in multiple sclerosis: an analysis of temporal ordering, semantic encoding, and planning abilities. Neuropsychology.; 11: 535-44.
9. , , , . 2000. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature.; 404: 84-7.
10. , , , . 2000. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport.; 11: 1153-8.
11. , , , . 1999. Fatigue in multiple sclerosis: cross-sectional correlation with brain MRI findings in 71 patients. Neurology.; 53: 1151-3.
12. , , , , , . 1996. Sexual dysfunction in relapsing-remitting multiple sclerosis: magnetic resonance imaging, clinical, and psychological correlates. J Psychiatry Neurosci.; 21: 255-8.
13. , , , , . 2000. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci.; 15: 314-29.
14. , , , . 1992. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med.; 326: 581-8.
15. , , , . 1988. Sexual dysfunction and electroejaculation in men with spinal cord injury: review. J Urol.; 139: 453-7.
16. , , . 1993. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry.; 56: 245-50.
17. , , , . 1994. Erectile dysfunction in multiple sclerosis. Associated neurological and neurophysiological deficits, and treatment of the condition. Brain.; 117: 1303-10.
18. , , , . 1999. Abnormal expression of SNS/PN3 sodium channel in cerebellar Purkinje cells following loss of myelin in the taiep rat. Neuroreport.; 10: 913-8.
19. , , , , , . 1999. Emotional state of patients with relapsing-remitting MS treated with interferon beta-1b. Neurology.; 52: 1636-9.
20. , , , . 1989. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res.; 76: 27-37.
21. , . 1998. Effects of adrenal medullary transplants on pain-related behaviors following excitotoxic spinal cord inujury. Brain Research.; 798: 83-92.
Page 168
22. . 1987. Trigeminal neuralgia and multiple sclerosis. Archives of Neurology.; 44: 379-81.
23. , , . 1998. Somatic complaints disproportionately contribute to Beck Depression Inventory estimates of depression severity in individuals withm ultiple chemical sensitivity. J Occup Environ Med.; 40: 862-9.
24. , , , . 1997. Pharmacokinetics and pharmacodynamics of tolterodine in man: a new drug for the treatment of urinary bladder overactivity. Int J Clin Pharmacol Ther.; 35: 287-95.
25. , , , , , . 1995. Prevalence of depression after stroke: The Perth Community Stroke Study. Br J Psychiatry.; 166: 320-7.
26. . 1994. Depression in medically ill: Critical issues in diagnostic assessment. Psychosomatics.; 36: 48-49.
27. . 1966. Association of trigeminal neuralgia with multiple sclerosis. Arch Neurol.; 14: 95-9.
28. , , , , , . 2001. Spinal interneurons that receive input from muscle afferents are differentially modulated by dorsolateral descending systems. J Neurophys.; 85: 1005-8.
29. , , , , , . 1995. Prevalence of bowel dysfunction in patients with multiple sclerosis and bladder dysfunction. J Neurol.; 242: 105-8.
30. , . 1997. What is new in the symptomatic management of multiple sclerosis? Thompson AJ, Polman C, Hohlfeld R, eds. Multiple Sclerosis: Clinical Challenges and Controversies.
31. , , . 1999. Answers to unasked questions: writing in the margins. Res Nurs Health.; 22: 512-22.
32. , . 1984. Pain in multiple sclerosis. Arch Neurol.; 41: 1270-2.
33. Compston A, Ebers GC, Lassman H, McDonald WI, Matthews B, Wekerle H, eds. 1998. McAlpine's Multiple Sclerosis. Third Edition ed. New York. Churchill Livingstone.
34. , , , , . 1997. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol.; 38: 44-8.
35. , . 1926. The affective symptomatology of disseminated sclerosis. J. Neurol. Psychopathol.; 7: 1-30.
36. , . 1997. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci.; 17: 3503-14.
37. , , , . 2000. Gabapentin effect on spasticity in multiple sclerosis: a placebo-controlled, randomized trial. Arch Phys Med Rehabil.; 81: 164-9.
38. , , , , , . 1997. Intravesical capsaicin as a treatment for refractory detrusor hyperreflexia: a dual center study with long-term followup. J Urol.; 158: 2087-92.
39. , , , , , . 1998. Capsaicin and neurogenic detrusor hyperreflexia: a double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol Urodyn.; 17: 513-23.
40. , , , . 1998. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol.; 79: 447-63.
41. , , , . 1999. Speed of information processing as a key deficit in multiple sclerosis: implications for rehabilitation. J Neurol Neurosurg Psychiatry.; 67: 661-3.
Page 169
42. , , , . 1998. Efficacy and safety of oral sildenafil (Viagra) in men with erectile dysfunction caused by spinal cord injury. Neurology.; 51: 1629-33.
43. . March 3, 1997. 47 People, 1 Wish: The Individuals Who Chose the Same Final Act. Detroit Free Press.: (NWS);8A.
44. , , , , . 1993. Stability and determinants of psychosocial well-being in multiple sclerosis. Rehabilitation Psychology.; 38.
45. , , , . 1996. Down-regulation of transcripts for Na channel alpha-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci U S A.; 93: 14950-4.
46. , , , . 1996. Fatigue in multiple sclerosis compared with chronic fatigue syndrome: A quantitative assessment. Neurology.; 46: 632-5.
47. , . 1991. Description of persons with multiple sclerosis, with an emphasis on what is needed from psychologists. Professional Psychology: Research and Practice.; 22: 277-284.
48. . 1998. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet.; 352: 1491-7.
49. . 1997. Multiple sclerosis, depression, and suicide. BMJ.; 315: 691-2.
50. , , , . 1999. The effects of anxiety on psychiatric morbidity in patients with multiple sclerosis. Multiple Sclerosis.; 5: 323-326.
51. , , , . 1999. Pathological laughing and crying in multiple sclerosis: A preliminary report suggesting a role for the prefrontal cortex. Multiple Sclerosis.; 5: 69-73.
52. , . 1998. A longitudinal study of psychosis due to a general medical (neurological) condition: establishing predictive and construct validity. J Neuropsychiatry Clin Neurosci.; 10: 448-52.
53. , , , . 1994. Influence of clinical variables on neuropsychological performance in multiple sclerosis. Eur Neurol.; 34: 324-8.
54. . 1999. Assessment of neuropsychological function. Rudick RA, Goodkin DE, Editors. Multiple Sclerosis Therapeutics .em>. London, UK. Martin Dunitz Ltd.. 31-47.
55. . Cognitive impairment in multiple sclerosis. Cook SD. Handbook of Multiple Sclerosis .em>. 3rd ed. New York. Marcel Dekker, Inc.. In Press; Vol. 53: 233-252.
56. , , , , , . 1994. What do we really know about cognitive dysfunction, affective disorders, and stress in multiple sclerosis? A practitioner's guide. J Neuro Rehab.; 8: 141-164.
57. , , , , , . 1999. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis.; 5: 251-9.
58. , , , . 2000. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol.; 48: 885-92.
59. , , , , . 1998. Hospital-based psychiatric service utilization and morbidity in multiple sclerosis. Can J Neurol Sci.; 25: 230-5.
60. , . 1995. Telling your patient he/she has multiple sclerosis. Postgrad Med J.; 71: 449-52.
61. , , . 1998. The nature of fatigue in multiple sclerosis. J Psychosom Res.; 45: 33-8.
62. , . 1989. Vascular headache: a presenting symptom of multiple sclerosis. Can J Neurol Sci Feb.; 16: 63-6.
Page 170
63. , , , , , . 1996. The effects of amantadine and pemoline on cognitive functioning in multiple sclerosis. Arch Neurol.; 53: 185-8.
64. , . 1999. Therapeutics in the Management of Spasticity. Neurorehabilitation and Neural Repair.; 13: 5-14.
65. , , , . 1995. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve.; 18: 1101-14.
66. , , , . 1996. Improvement of severe postural cerebellar tremor in multiple sclerosis by chronic thalamic stimulation. Mov Disord.; 11: 489-94.
67. , , , , . 1995. Erectile impotence in multiple sclerosis: a neurophysiological study. J Neurol.; 242: 123-6.
68. , . 1994. Depression, cognitive impairment and social stress in multiple sclerosis. J Psychosom Res.; 38: 193-201.
69. , . 2000. Antidepressants for depression in people with physical illness (Cochrane Review). Cochrane Database Syst Rev.; 4.
70. , . 1999. Multiple sclerosis and its treatment. J R Coll Physicians Lond.; 33: 315-22.
71. , , , , . 1999. Fatigue does not correlate with the degree of hepatitis or the presence of autoimmune disorders in chronic hepatitis C infection. Eur J Gastroenterol Hepatol.; 11: 833-8.
72. . 1999. Survey of multiple sclerosis in northern California. Northern California MS Study Group. Mult Scler.; 5: 78-88.
73. , , . 1998. Tizanidine treatment of spasticity: a meta-analysis of controlled, double-blind, comparative studies with baclofen and diazepam. Adv Ther.; 15: 241-51.
74. , , . 1993. Headache caused by a single lesion of multiple sclerosis in the periaqueductal gray area. Headache Sep.; 33: 452-5.
75. , , , . 1999. The responsiveness of health status measures in patients with rheumatoid arthritis: comparison of disease-specific and generic instruments. J Rheumatol.; 26: 1474-80.
76. . 1994. Living with multiple sclerosis: the experience of chronic sorrow. J Neurosci Nurs.; 26: 237-40.
77. , , , . 1992. An open trial of vacuum penile tumescence: constriction therapy for neurological impotence. Paraplegia.; 30: 550-3.
78. , , , , , . 1994. Use of intracavernous injection of prostaglandin E1 for neuropathic erectile dysfunction. Paraplegia.; 32: 661-4.
79. , , , . 1997. Open label gabapentin treatment for pain in multiple sclerosis. Mult Scler.; 3: 250-3.
80. , . 1995. Sexual function in women with advanced multiple sclerosis. J Neurol Neurosurg Psychiatry.; 59: 83-6.
81. Institute of Medicine. Solarz AL, ed.. Lesbian Health: Current Assessment and Directions for the Future . Washington, D.C.. National Academy Press. 1999.
82. , , , . 2000. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci.; 12: 701-14.
83. , , . 1999. Clinical efficacy of sildenafil citrate based on etiology and response to prior treatment. J Urol.; 162: 722-5.
84. , , , . 1996. The effectiveness of occupational therapy and physiotherapy in multiple sclerosis patients with ataxia of upper limb and trunk. Clin Rehab.; 10: 277-82.
Page 171
85. , , , . 1998. Effects of intravenous methylprednisolone on outcome in MRI-based prognostic subgroups in acute optic neuritis. Neurology.; 50: 230-7.
86. , . 1997. Spasticity, ataxia and fatigue in multiple sclerosis. Baillieres Clin Neurol.; 6: 429-45.
87. , , . 1984. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc.; 59: 523-9.
88. . 1998. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology.; 51: 611-4.
89. , , , . 1999. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J Neurosci.; 19: 6213-24.
90. , . 1990 . Desmopressin: a new principle for symptomatic treatment of urgency and incontinence in patients with multiple sclerosis. Scand J Urol Nephrol.; 24: 109-12.
91. , , , . 1988. Erectile dysfunction in multiple sclerosis. Neurology.; 38: 1366-71.
92. , . 2000. Drugs used to treat spasticity. Drugs.; 59: 487-95.
93. , , . 2000. Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler.; 6: 131-6.
94. , , , . 1988. Fatigue in multiple sclerosis. Arch Neurol.; 45: 435-7.
95. , , , . 1995. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology .; 45: 1956-61.
96. , , , , . 1994. Cognitive functioning and depression in patients with chronic fatigue syndrome and multiple sclerosis. Arch Neurol.; 51: 705-10.
97. . 1983. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology.; 33: 1444-52.
98. . 1973. Desmedt J, ed. New Developments in Electromyography and Clinical Neurophysiology . Basel. Karger. 36-68.
99. , , , . 1998. Neuroimaging in chronic fatigue syndrome. Am J Med.; 105: 50S-53S.
100. , , . 1976. Sexual problems in patients suffering from multiple sclerosis. J Chronic Dis.; 29: 643-7.
101. , , . 1999. Multiple sclerosis and the urologist. J Urol.; 161: 743-57.
102. , . 1991. Suicidal tendency and multiple sclerosis. Health Soc Work.; 16: 104-9.
103. , , , , . 2000. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain.; 123: 308-17.
104. , . 1991. Modafinil: the unique properties of a new stimulant. Aviat Space Environ Med.; 62: 432-5.
105. , , , . 1999. Fatigue and magnetic resonance imaging activity in multiple sclerosis. J Neurol.; 246: 454-8.
106. , . 1999. Treatment of urge incontinence in Veterans Affairs medical centers. Clin Ther.; 21: 867-77.
107. . 1998. Symptoms and signs of multiple sclerosis. Compston A, Ebers GC, Lassman H, McDonald WI, Matthews B, Wekerle H. McAlpine's Multiple Sclerosis .em>. 3 ed. London. Churchill Livingstone.: 145-190.
108. . 1975. Paroxysmal symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry.; 38: 619-23.
Page 172
109. , , , . 1995. Multiple sclerosis. Sexual dysfunction and its response to medications. Arch Neurol.; 52: 862-8.
110. , , , , . 1996. The Impact of multiple sclerosis on sexuality and relationships. Journal of Sex Research.; 33: 241-248.
111. . 1998. Lamotrigine can reduce neurogenic pain associated with multiple sclerosis. Clin J Pain.; 14: 269-70.
112. , , , . 1995. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol (Lond).; 487: 527-39.
113. , , . 1986. Clinical and oculographic examinations of saccadic eye movements in the diagnosis of multiple sclerosis. Arch Neurol.; 43: 438-43.
114. , . 2000. Interferons, serotonin and neurotoxicity. Psychol Med.; 30: 259-68.
115. , , . 1999. Symptomatic therapies of multiple sclerosis. Biomed Pharmacother.; 53: 371-9.
116. , . 1990. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol.; 47: 98-104.
117. , , , . 2000. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol.; 68: 356-61.
118. , , , , , . 1999. Course of depression during the initiation of interferon beta-1a treatment for multiple sclerosis. Arch Neurol.; 56: 1263-5.
119. , , , . 1999. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology.; 53: 625-8.
120. , , . 1988. Pain syndromes in multiple sclerosis. Neurology.; 38: 1830-4.
121. , , , , , . 2000. Interferon treatment is not associated with a worsening of psychiatric symptoms in patients with hepatitis C. J Gastroenterol Hepatol.; 15: 300-3.
122. . 1998. Fatigue and Multiple Sclerosis: Evidence-based management for fatigue in multiple sclerosis. Paralyzed Veterans of America.
123. , , , , . 1998. Rapid response of emotional incontinence to selective serotonin reuptake inhibitors. J Neuropsychiatry Clin Neurosci.; 10: 453-5.
124. , , . 1992. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kolliker-Fuse and raphe nuclei in the cat. Exp Brain Res.; 88: 502-16.
125. . 1993. Clinical trials in multiple sclerosis. Curr Opin Neurol Neurosurg.; 6: 209-15.
126. , , , , , . 1995. Mood disturbance versus other symptoms of depression in multiple sclerosis. J Int Neuropsychol Soc.; 1: 291-6.
127. , , , . 1996. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg.; 85: 452-7.
128. , , , , , . 1996. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol.; 39: 432-41.
129. , . 1999. Recommendations for physical activity in patients with multiple sclerosis. Sports Med.; 27: 179-91.
130. , , , . 1990. Guidelines for neuropsychological research in multiple sclerosis. Arch Neurol.; 47: 94-7.
Page 173
131. , , , , , . 1998. Frequency and clinical determinants of poststroke depression. Stroke.; 29: 2311-7.
132. , . 1988. Effects of acute dorsal spinal hemisection on motoneuron discharge in the medial gastrocnemius of the decerebrate cat. J Neurophysiol.; 59: 1540-56.
133. . 1998. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet.; 352: 1498-504.
134. , , , , . 1999. A multidimensional assessment of multiple sclerosis: relationships between disability domains. Acta Neurol Scand.; 100: 156-62.
135. . 1998. Optic neuritis. Curr Opin Ophthalmol.; 9: 3-9.
136. , , , . 1986. Structural brain correlates of emotional disorder in multiple sclerosis. Brain.; 109 : 585-97.
137. , , , . 1999. Sensory symptoms of multiple sclerosis: a hidden reservoir of morbidity. Mult Scler.; 5: 179-83.
138. , , , . 1992. Acute radicular pain as a presenting symptom in multiple sclerosis. Arch Neurol.; 49: 255-8.
139. , , , . 1991. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology.; 41: 685-91.
140. , , , , , . 1991. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology.; 41: 692-6.
141. , . Depression and suicide in multiple sclerosis. Thompson AJ, Polman C, Hohlfeld R, eds. 1997. Multiple Sclerosis: Clinical Challenges and Controversies .em>. London. Martin Dunitz Ltd.: 243-251.
142. , , . 1994. Treatment of acquired nystagmus with botulinum neurotoxin A. Arch Ophthalmol.; 112: 1320-4.
143. , , . 1997. Cognition and multiple sclerosis: a historical analysis of medical perceptions. J Hist Neurosci.; 6: 302-319.
144. , , , . 1997. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology.; 48: 1566-71.
145. , . 1990. Headaches and multiple sclerosis: a clinical study and review of the literature. J Neurol.; 237: 300-2.
146. , , , . 1997. Doctors and patients don't agree: cross sectional study of patients' and doctors' perceptions and assessments of disability in multiple sclerosis. BMJ.; 314: 1580-3.
147. , . 2000. MRI correlates of cognitive dysfunction in multiple sclerosis patients. J Neurovirol.; Suppl 2: S172-5.
148. . Sexual and sphincter dysfunction. Bradley WG, Daroff RB, Fenichel GM, Marsden CD, eds. 1991. Neurology in Clinical Practice .em>. Boston. Butterworth-Heinemann. 381-91.
149. , , . 1979. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res.; 37: 93-113.
150. , , , , . 2000. Amantadine influences cognitive processing in patients with multiple sclerosis. Pharmacopsychiatry.; 33: 28-37.
151. , . 1999. Preventing catheter-related bacteriuria: should we? Can we? How? Arch Intern Med.; 159: 800-8.
Page 174
152. , , , . 1997. Amelioration of refractory dysesthetic limb pain in multiple sclerosis by gabapentin. Neurology.; 49: 304-5.
153. . 1999. Medications used in the treatment of multiple sclerosis. Phys Med Rehabil Clin N Am.; 10: 437-46, ix.
154. , . 1991. The interaction between depressive affective disorder and neuropsychological test performance in multiple sclerosis patients. J Neuropsychiatry Clin Neurosci.; 3: 28-32.
155. , . 1990. Antidepressant pharmacotherapy of depression associated with multiple sclerosis. Am J Psychiatry.; 147: 1493-7.
156. , , , , . 1996. Characterization of major depression symptoms in multiple sclerosis patients. J Neuropsychiatry Clin Neurosci.; 8: 318-23.
157. , , , . 1995. Measurement of treatment response to sertraline in depressed multiple sclerosis patients using the Carroll scale. Neurol Res.; 17: 421-2.
158. . 2000. The neurologic basis for sexual arousal and orgasm: effects of spinal cord injury. Ann Neurol.
159. , . 1993. Sexual activities, response and satisfaction in women pre- and post-spinal cord injury. Arch Phys Med Rehabil.; 74: 1025-9.
160. , , . 1999. Sexual response in women with spinal cord injuries: implications for our understanding of the able bodied. J Sex Marital Ther.; 25: 11-22.
161. , , , . 2000. Sildenafil effects on sexual and cardiovascular responses in women with spinal cord injury. Urology.; 55: 812-5.
162. , . 2000. The role of affect on the perception of disability in multiple sclerosis. Clin Rehabil.; 14: 50-4.
163. , , , , , . 1990. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol.; 28: 512-5.
164. , , , . 1998. An open-label trial of gabapentin treatment of paroxysmal symptoms in multiple sclerosis patients. Neurology.; 51: 609-11.
165. , , , , . 1999. Anatomic correlates of painful tonic spasms in multiple sclerosis. Movement Disord.; 14: 331-5.
166. , , , , , . 1996. Value of nocturnal penile tumescence and rigidity (NPTR) recording in impotent patients with multiple sclerosis. Int J Impot Res.; 8: 241-5.
167. , , , , . 1997. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol.; 244: 9-16.
168. , , . 1996. Risk factors for suicide in multiple sclerosis. Psychother Psychosom.; 65: 86-90.
169. , . 1992. Suicide and patients with neurologic diseases. Methodologic problems. Arch Neurol.; 49: 1296-303.
170. , , . 1991. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand.; 84: 197-200.
171. , , , . 1997. Management of impairment, disability, and handicap due to multiple sclerosis. Mayo Clin Proc.; 72: 1184-96.
172. , , , , , . 1998. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport.; 9: 967-72.
173. , . 1998. Neuropsychologic aspects of multiple sclerosis. Phys Med Rehabil Clin N Am.; 9: 643-57, vii-viii.
174. . 1995. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology.; 45: 1277-85.
Page 175
175. . 1997. Visual function 5 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol.; 115: 1545-52.
176. Thompson AJ, Polman C, Hohlfeld R, eds. 1997. Multiple Sclerosis: Clinical Challenges and Controversies. London. Martin Dunitz.
177. , , , , . 1989. [Histochemical study of the skeletal muscle in multiple sclerosis]. Arq Neuropsiquiatr.; 47: 337-45.
178. , , , . 2000. Psychiatric side effects of interferon therapy: Prevalence, proposed mechanisms, and future directions. J. Clin Oncology.; 18: 2316-2326.
179. , , . 1996. Desmopressin in the management of nocturia in patients with multiple sclerosis. A double-blind, crossover trial. Arch Neurol.; 53: 1270-5.
180. , , , , . 1987. Cognitive impairment in patients with multiple sclerosis and mild physical disability. Arch Neurol.; 44: 494-501.
181. . 1969. Sexual impotence and some autonomic disturbances in men with multiple sclerosis. Acta Neurol Scand.; 45: 166-82.
182. , , , . 1996. The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Arch Neurol.; 53(7): 642-649.
183. , , . 1986. Pain in multiple sclerosis patients. A prospective study using the Mc Gill Pain Questionnaire. Clin Neurol Neurosurg.; 88: 87-93.
184. , , , . 1995. Intracavernous pharmacotherapy for management of erectile dysfunction in multiple sclerosis patients. Rev Neurol.; 23: 269-71.
185. , . 1999. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology.; 53: 1622-7.
186. , , . 1994. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol.; 72: 466-70.
187. , , , . 1995. Brain stem activation in spontaneous human migraine attacks. Nat Med.; 1: 658-60.
188. , , , , . 1992. A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology.; 42: 1468-71.
189. , , , . 1989. The functioning and well-being of depressed patients. Results from the medical outcomes study. JAMA.; 262: 914-919.
190. , , , . 2000. Recovery from optic neuritis is associated with a change in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry.; 68: 441-9.
191. , , . 1998. Thalamic deep-brain stimulation for movement disorders due to multiple sclerosis. Lancet.; 351: 109-10.
192. , , . 1980. Optic neuritis as an initial symptom in multiple sclerosis. Acta Neurol Scand.; 61: 178-85.






























































