1
Aniline1
Acute Exposure Guideline Levels
SUMMARY
ANILINE is an aromatic amine used chiefly in the chemical industry in the manufacture of dyes, dye intermediates, rubber accelerators, antioxidants, drugs, photographic chemicals, isocyanates, herbicides, and fungicides. Production of aniline oil in 1993 was approximately 1 billion pounds. The primary effect of an acute exposure to aniline is the oxidation of the hemoglobin in red blood cells (RBCs), resulting in the formation of methemoglobin. The effect may occur following inhalation, ingestion, or dermal absorption. In conjunction with methemoglobinemia, chronic exposures or exposures to high concentrations may produce signs and symptoms of headache, paresthesia, tremor, pain, narcosis/coma, cardiac arrhythmia, and possibly death.
No reliable data on human exposures via the inhalation route were located.
All acute exposure guideline level (AEGL) values are based on a study in which rats were exposed to aniline at concentrations of 0, 10, 30, 50, 100, or 150 parts per million (ppm) for 8 or 12 h (Kim and Carlson 1986). The only reported effect was methemoglobin formation. The relationship between aniline concentration and methemoglobin formation appeared to be linear. Furthermore, at a constant concentration (100 ppm), the formation of methemoglobin between 3 and 8 h was basically linear, reaching an asymptote at 8 h. Based on the linear relationship between aniline concentration and methemoglobin formation and between methemoglobin formation and time at a constant aniline concentration, a linear relationship between concentration and exposure duration (C1×t=k, where C=exposure concentration, t=exposure duration, and k=a constant) was chosen for time-scaling aniline concentrations to the appropriate AEGL exposure durations.
The AEGL-1 was based on an exposure of rats to a concentration of 100 ppm for 8 h, which resulted in elevation of methemoglobin from a control value of 1.1% (range, 0.4% to 2.1%) to 22%. A review of the published data indicates that methemoglobin levels of 15–20% in humans results in clinical cyanosis but no hypoxic symptoms. Although inhalation data for comparison purposes are not available, oral ingestion data suggest that humans may be considerably more sensitive to methemoglobin-forming chemicals than rats. Therefore, a default uncertainty factor of 10-fold was used for interspecies extrapolation (NRC 1993). Several sources also indicate that newborns may be more sensitive to methemoglobin-forming chemicals than adults. Because of the absence of specific quantitative data on sensitive human subpopulations and the fact that there are data suggesting greater susceptibility of infants, a default uncertainty factor of 10-fold was used for intraspecies extrapolation (NRC 1993). It is believed that an intraspecies uncertainty factor of 10 is protective of the general population including susceptible individuals. The default uncertainty factors of 10 for each of the interspecies and intraspecies variabilities are also supported by the small database of information and the lack of reliable human inhalation studies. The data were scaled across time using C1×t=k because of data indicating a linear relationship between concentration and exposure duration as related to methemoglobin formation.
The AEGL-2 was based on the same study with rats in which a concentration of 150 ppm for 8 h resulted in elevation of methemoglobin from a control value of 1.1% to 41%. This level of methemoglobin is associated with fatigue, lethargy, exertional dyspnea, and headache in humans and was considered the threshold for disabling effects. Since the same mode of action applies to AEGL-2 effects, the 150-ppm concentration was divided by a combined uncertainty factor of 100 and scaled across time using the same reasons and relationships as those used for the AEGL-1 above.
Data on concentrations of aniline inducing methemoglobin levels at the threshold for lethality were not available. Based on the fact that the relationship between the concentration of aniline and methemoglobin formation is linear, the dose-response curve from the study on which the AEGL-1 and AEGL-2 values were based was extrapolated to a concentration resulting in >70% formation of methemoglobin, the threshold for lethality. The concentration of 250 ppm for 8 h was chosen as the threshold for lethality, according to Kiese (1974) and Seger (1992). Since the same mode of action applies to AEGL-3 effects, the 250-ppm concentration was divided by a combined uncertainty factor of 100 and scaled across time using the same reasons and relationships as those used for the AEGL-1 above.
Several studies with rats support the AEGL-3 values. A 10-min exposure to aniline at 15,302 ppm resulted in no toxic effects, and a 4-h exposure at 359 ppm resulted in severe toxic effects but no deaths. Dividing these values by a total uncertainty factor of 100 and scaling across time using C1×t=k results in values similar to those derived from the Kim and Carlson (1986) study. Studies with repeated exposures of rats resulted in additional effects on the blood and spleen, but concentrations up to 87 ppm, 6 h/d, 5 d/w for 2 w were not disabling or life-threatening.
The derived AEGLs are listed in Table 1–1. Because aniline is absorbed through the skin in quantities sufficient to induce systemic toxicity, a skin notation was added to the summary table. The reported odor threshold for aniline ranges from 0.012 to 10 ppm. Therefore, the odor of aniline will be noticeable by most individuals at the AEGL-1 concentrations. The odor is somewhat pungent but not necessarily unpleasant.
1. INTRODUCTION
Aniline is an aromatic amine used in the manufacture of dyes, dye intermediates, rubber accelerators, and antioxidants. It has also been used as a solvent, in printing inks, and as an intermediate in the manufacture of pharmaceuticals, photographic developers, plastics, isocyanates, hydroquinones, herbicides, fungicides, and ion-exchange resins. It is produced commercially by catalytic vapor phase hydrogenation of nitrobenzene (Benya and Cornish 1994; HSDB 1996). Production of aniline oil was listed at approximately 1 billion pounds in 1993 (U.S. ITC 1994). Chemical and physical properties are listed in Table 1–2.
Aniline may be absorbed following inhalation, ingestion, and dermal exposures. The inhalation toxicity of aniline was studied in several animal species, but only one study that utilized multiple exposure concentrations for sublethal effects was located. Data from human studies lack specific details or exposures
TABLE 1–1 Summary of AEGL Values for Anilinea
|
Classification |
30 min |
1 h |
4 h |
8 h |
Endpoint (Reference) |
|
AEGL-1b (Nondisabling) |
16 ppm (61 mg/m3) |
8.0 ppm (30 mg/m3) |
2.0 ppm (7.6 mg/m3) |
1.0 ppm (3.8 mg/m3) |
22% Methemoglobin—cyanosis (Kim and Carlson 1986) |
|
AEGL-2 (Disabling) |
24 ppm (91 mg/m3) |
12 ppm (46 mg/m3) |
3.0 ppm (11 mg/m3) |
1.5 ppm (5.7 mg/m3) |
41% Methemoglobin—lethargy (Kim and Carlson 1986) |
|
AEGL-3 (Lethal) |
40 ppm (152 mg/m3) |
20 ppm (76 mg/m3) |
5.0 ppm (19 mg/m3) |
2.5 ppm (9.5 mg/m3) |
>70% Methemoglobin—lethality (extrapolated from data of Kim and Carlson 1986) |
|
aCutaneous absorption of the neat material may occur, adding to the systemic toxicity. bThe aromatic, amine-like odor of aniline will be noticeable by most individuals at these concentrations. Abbreviations: ppm, parts per million; mg/m3, milligrams per cubic meter. |
|||||
TABLE 1-2 Chemical and Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
Benzenamine, aniline oil, phenylamine, aminobenzene, aminophen, arylamine |
Budavari et al. 1996, Benya and Cornish 1994 |
|
Molecular formula |
C6H5NH2 |
Benya and Cornish 1994 |
|
Molecular weight |
93.13 |
Budavari et al. 1996 |
|
CAS Registry No. |
62–53–3 |
HSDB 1996 |
|
Physical description |
Colorless oily liquid (freshly distilled); darkens on exposure to air and light |
Budavari et al. 1996 |
|
Solubility in water |
1 g in 28.6 mL |
Budavari et al. 1996 |
|
Vapor pressure |
15 mm Hg at 77°C 7.6 torr at 20°C 0.67 mm Hg at 25°C |
Benya and Cornish 1994 ACGIH 1991 U.S. EPA 1987 |
|
Vapor density (air=1) |
3.22 |
Benya and Cornish 1994 |
|
Density (water=1) |
1.002 (20/4°C) |
Benya and Cornish 1994 |
|
Melting point |
–6.3°C |
Benya and Cornish 1994 |
|
Boiling point |
184–186°C |
Budavari et al. 1996 |
|
Odor |
aromatic amine-like pungent, oily |
NIOSH 1997 U.S. EPA 1992 |
|
Odor threshold |
0.012 to 10 ppm 0.5 ppm 1.0 ppm |
U.S. EPA 1992 DOT 1985 Billings and Jones 1981 |
|
Conversion factors |
1 ppm=3.8 mg/m3 1 mg/m3=0.26 ppm |
ACGIH 1991 |
were oral or percutaneous to the liquid or an aniline dye. The primary effect of inhalation exposure to aniline vapor is the formation of methemoglobin in the RBCs. Hemolysis of the red cells and effects on the spleen occur following daily repeated or long-term exposures.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No information on acute lethal concentrations for humans by the inhalation route was located. According to Bodansky (1951) and Kiese (1974), methemo-
globin (the primary effect of inhalation exposure) levels above 85% may be lethal if treatment is not initiated. Seger (1992) cites a concentration of >70% as a potentially lethal level. Deaths of adults have occurred from ingestion, and infant deaths have occurred from absorption of aniline from diapers stenciled with ink containing aniline (Gosselin et al. 1984). Incidences of aniline intoxication in infants attributed to aniline dye through dermal exposure are numerous: (Graubarth et al. 1945; Kagan et al. 1949; Etteldorf 1951; Pickup and Eeles 1953; Ramsay and Harvey 1959; Smith 1992). In one study, most of the infants were visibly cyanotic and methemoglobin levels in these infants ranged from 30% to 60% (Etteldorf 1951). Complete recovery followed treatment with methylene blue. In a summary of these and several other reports, an overall infant mortality of 5–10% was reported (Gosselin et al. 1984).
2.2. Nonlethal Toxicity
The reported odor threshold for aniline ranges from 0.012 to 10 ppm (Table 1–2). Although the odor may be somewhat pungent, no adverse effects are predicted to occur at the odor threshold.
With increasing concentrations of aniline, exposure can cause headaches, methemoglobinemia, paresthesias, tremor, pain, narcosis/coma, cardiac arrhythmia, and possibly death (Benya and Cornish 1994). However, according to Bodansky (1951), Kiese (1974), and Seger (1992), the formation of methemoglobin concentrations of <15% are asymptomatic; methemoglobin levels exceeding 15% of the circulating blood pigment result in clinical cyanosis; and hypoxic symptoms including lethargy and semistupor are associated with serum levels of 55–60% or greater. Signs and symptoms associated with methemoglobin formation are summarized in Table 1–3.
In sampling data from 18 workplace sites provided by the Occupational Safety and Health Administration (OSHA 1997), measurable concentrations were present in 3 of 18 samples; these concentrations were 0.070, 0.14, and 0.177 ppm.
2.2.1. Experimental Studies
Two papers cited older data. However, symptoms at specific concentrations were not defined in the summary papers and details of the studies were not available. Flury and Zernik (1931) cited the following human data: a concentration of approximately 130 ppm was tolerated for 1/2 to 1 h without immediate or late sequalae and a concentration of 40–53 ppm was tolerated for 6 h without
TABLE 1–3 Signs and Symptoms Associated with Methemoglobin Concentrations in Humans
|
Methemoglobin Concentration (%) |
Signs and Symptoms |
|
1.1 |
Normal level |
|
1–15 |
None |
|
15–20 |
Clinical cyanosis (chocolate brown blood); no hypoxic symptoms |
|
30 |
Fatigue; recovery without treatment |
|
20–45 |
Anxiety, exertional dyspnea, weakness, fatigue, dizziness, lethargy, headache, syncope, tachycardia |
|
45–55 |
Decreased level of consciousness |
|
55–70, ~60 |
Hypoxic symptoms: semistupor, lethargy, seizures, coma, bradycardia, cardiac arrhythmias |
|
>70 |
Heart failure from hypoxia, High incidence of mortality |
|
>85 |
Lethal |
|
Sources: Kiese 1974; Seger 1992. |
|
distinct symptoms. Henderson and Haggard (1943) cited the following data: a concentration of 5 ppm was considered safe for daily exposure, concentrations of 7 to 53 ppm produced slight symptoms after several hours, and 100 to 160 ppm as the maximum concentration that could be inhaled for 1 h without serious disturbance. The statements by Henderson and Haggard were based on several studies including those of Flury and Zernik (1931).
2.2.2. Epidemiology Studies
No epidemiology studies in which exposure concentrations were measured were identified in the available literature.
2.2.3.
Accidents
No accidental inhalation exposures to aniline in which concentrations were known were identified in the available literature. However, methemoglobin levels were measured after accidental exposures to liquid aniline or aromatic
nitro or amino compounds (Hamblin and Mangelsdorff 1938; Mangelsdorff 1956). In these occupational exposures, methemoglobin levels reached 50–72%; the subjects were cyanotic and complained of headache, dizziness, and weakness. In some cases, oxygen therapy was instituted and intravenous dextrose solutions were administered; in one case, methylene blue was administered intravenously. Regardless of whether or not treatment was given, the half-life of methemoglobin ranged between 3 and 10 h following cessation of exposure, and levels were below 10% in less than 20 h. No deaths occurred.
2.3. Developmental and Reproductive Effects
No developmental and reproductive toxicity data on humans concerning aniline were identified in the available literature.
2.4. Genotoxicity
In an in vitro assay with cultured human fibroblasts, aniline produced only marginal increases in sister chromatid exchanges at the highest dose tested, 10 mM; whereas two metabolites of aniline, 2-aminophenol and N-phenylhydroxylamine, doubled the frequency of sister chromatid exchanges at the highest tested nontoxic concentration, 0.1 mM (Wilmer et al. 1981).
2.5. Carcinogenicity
Historically, bladder tumors have been associated with exposures in the aniline dye industry. However, conclusive evidence for any one particular exposure could not be obtained in these studies since the workers were exposed to many chemicals within the same work area. For example, Case et al. (1954) investigated the incidence of bladder tumors among British workers in the chemical dye industry. In addition to aniline, the workers were exposed to other aromatic amines, including α- and β-naphthylamine, benzidine, and auramine. Although exposures could not be quantified, there was insufficient evidence to suggest that aniline was a cause of bladder cancers. More recent studies indicate that β-naphthylamine, 4-aminodiphenyl, 4-nitrodiphenyl, 4,4'-diaminodiphenyl, or o-toluidine may be involved in increased cancers in the dye industry (Ward et al. 1991; Benya and Cornish 1994).
On the basis of inadequate human data and sufficient animal data, U.S. EPA (1994) in their Integrated Risk Information System (IRIS) classified aniline as
B2, a probable human carcinogen. The International Agency for Research on Cancer has classified the evidence for carcinogenicity of aniline in humans as inadequate and in animals as limited (IARC 1987). Based on a high-dose feeding study with rats (NCI1978), the National Institute for Occupational Safety and Health (NIOSH 1997) considers aniline and its homologues occupational carcinogens; however, OSHA (1995) has not classified aniline as an occupational carcinogen. ACGIH (1999) categorized aniline as A3, a confirmed animal carcinogen with unknown relevance to humans. Animal feeding studies (NCI 1978; CIIT 1982) indicate that aniline may be a very weak carcinogen in male and female rats (i.e., 3,000 and 2,000 ppm dietary threshold in the two studies, respectively) but not in male or female mice. Animal studies are summarized in Section 3.5 and a quantitative cancer risk assessment is performed in Appendix A.
2.6. Summary
Human toxicity data are limited to secondary citations. Because these citations provided no experimental details, they cannot be considered reliable. Deaths have occurred from aniline ingestion and skin absorption, but doses were unknown. Reviews of the older literature indicate that a concentration of 5 ppm was considered safe for daily exposures, concentrations of 7 to 53 ppm produced slight symptoms after several hours, a concentration of 40 to 53 ppm was tolerated for 6 h without distinct symptoms, a concentration of 130 ppm may be tolerated for 0.5 to 1 h without immediate or late sequalae, and 100 to 160 ppm was the maximum concentration that could be inhaled for 1 h without serious disturbance. In studies of accidents with unknown exposure concentrations, methemoglobin levels of up to 72% were measured. Recoveries occurred with a minimum of medical intervention following cessation of exposure.
There is no conclusive evidence from studies of cancers in dye workers that aniline is the causative agent. Two known metabolites of aniline induced sister chromatid exchange in the single study with cultured human fibroblasts. No studies on possible reproductive or developmental effects in humans associated with aniline exposures were located.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
Acute lethality data are summarized in Table 1–4 and discussed below.
TABLE 1–4 Summary of Acute Lethal Inhalation Data in Laboratory Animalsa
|
Species |
Concentration (ppm) |
Exposure Time |
Effect |
Reference |
|
Rat |
839b |
4 h |
LC50 |
E.I.du Pont de Nemours 1982a |
|
Rat |
478c |
4 h |
LC50 |
E.I.du Pont de Nemours 1982a |
|
Rat |
250d |
4 h |
Approximate LC50 |
Carpenter et al. 1949 |
|
Rat |
550 |
8 h |
82% mortality |
Comstock and Oberst 1952, as cited in Oberst et al. 1956 |
|
Mouse |
~175 |
7 h |
LC50 |
von Oettingen et al. 1947 |
|
aLC50 (lethal concentration for 50% of the animals) values were obtained 14 d post-exposure (Carpenter et al. 1949; E.I.du Pont de Nemours 1982a). bHead-only exposure. cWhole-body exposure. dConcentrations not measured. |
||||
3.1.1. Rats
Six Sherman rats (gender not specified) were exposed to graded concentrations of aniline vapor for 4 h and observed for 14 d post-exposure (Carpenter et al. 1949). The concentration that killed approximately half of the rats (exact number not stated) was 250 ppm. Concentrations were based upon empirical calculation and were not measured. An 8-h exposure to 550 ppm killed 82% of an unreported number of rats (Comstock and Oberst 1952, as cited in Oberst et al. 1956). Methemoglobinemia was the only pathologic change cited; no further details were reported.
Groups of 10 8-w-old Crl:CD rats were exposed to various concentrations of aniline vapor/aerosol for 4 h (E.I.du Pont de Nemours 1982a). The atmospheres were generated by passing nitrogen over liquid aniline in a heated flask. The vapor/aerosol was diluted with humidified (45%) and oxygen-enriched (21%) air; the temperature of the exposure chamber was maintained at 27°C. Air samples were analyzed by gas chromatography. Two routes of exposure, head-only, using wire mesh restrainers, and whole-body, were compared to assess the significance of skin absorption and restraint on mortality. LC50 (lethal concentration for 50% of the animals) values for head-only and whole-body exposures were 839 ppm (95% confidence limit (CL), 802–882 ppm) and
TABLE 1–5 Mortality of Rats Exposed to Aniline via Head-Only or Whole-Body Exposures for 4 h
|
Head-Only Exposures |
Whole-Body Exposures |
||
|
Concentration (ppm) |
Mortality |
Concentration |
Mortality |
|
681 |
0/10 |
359 |
0/10 |
|
790 |
2/10 |
400 |
2/10 |
|
834 |
5/10 |
453 |
4/10 |
|
896 |
8/10 |
530 |
7/10 |
|
|
786 |
10/10 |
|
|
Source: E.I.du Pont de Nemours 1982a. |
|||
478 ppm (95% CL, 442–540 ppm), respectively. The lower value for whole-body exposure suggests significant dermal absorption. Mortality at each exposure concentration is listed in Table 1–5.
All deaths occurred by d 4 post-exposure. "Signs observed during exposures by both routes were similar and included cyanosis, prostration, tremors, pallor, clear to reddish-brown eye, mouth, and nasal discharges, corneal clouding, tachypnea, and hair loss" (E.I.du Pont de Nemours 1982a). The severity of the signs was generally dose-related. An initial weight loss at 24–72 h post-exposure was followed by a normal weight gain.
3.1.2. Mice
A 7-h LC50 for the mouse of approximately 175 ppm was reported by von Oettingen et al. (1947). Deaths occurred at all tested concentrations, which ranged from approximately 115 to 390 ppm; however, analytical determinations (both colorimetric and spectrophotometric) of calculated concentrations showed substantial variations, ranging from 49% to 81% of calculated concentrations. The discrepancy between calculated and analyzed values was probably due to condensation of aniline on the sides of the exposure chamber. The authors stated that the actual lethal values probably were within the range of the calculated and analyzed concentrations. In that case, the 7-h LC50 value for the mouse lies within the range of 175 to 288 ppm. Mice exposed to aniline became restless and cyanotic (ears and tails), and their eyes showed signs of irritation. Tremors, followed by convulsions and then depression, preceded death. Histologic examinations revealed hepatic fatty infiltrations.
3.2. Nonlethal Toxicity
3.2.1. Dogs
Except for cyanosis of the mucous membranes, dogs failed to show any signs of methemoglobinemia at methemoglobin concentrations of less than about 60%. At levels of 60–70% the predominant signs were salivation, ataxia and vomiting. Ataxia and vomiting occurred at 71–80% and loss of consciousness occurred at 81–90% (Bodansky 1951).
Oberst et al. (1956) exposed two male beagle dogs to aniline at 5 ppm for 6 h/d, 5 d/w for up to 26 w. Prior to daily exposure, the dogs were exercised on a treadmill for 5 min. Blood and urine analyses were performed and body weights were measured pre-exposure and weekly during exposure. Animals were observed daily for toxic signs. Aside from an increase in free chromogen content of the urine, there were no signs of exposure. Pathologic examinations at sacrifice revealed no adverse effects.
3.2.2. Rats
Kakkar et al. (1992) exposed six male Wistar rats to a single nominal concentration of 15,302 ppm for 10 min; the animals were sacrificed 24 h later. Earlier studies (not presented) had shown this to be the highest concentration tolerated without any mortality or acute toxicity. Biochemical changes in the brains of these rats suggested impairment of antioxidant defenses. No other signs of toxicity were reported. The exposure was performed under static conditions, and the measurement method was not described.
As discussed in Section 3.1.1, groups of 10 8-w-old Crl:CD rats were exposed to aniline vapor/aerosol at concentrations of 359, 400, 453, 530, or 786 ppm for 4 h (E.I.du Pont de Nemours 1982a). No deaths occurred at the lowest concentration. Signs at 359 ppm included cyanosis, tremors, lacrimation, salivation, semi-prostration, an initial body-weight loss followed by normal gain, and a reddish-brown perineal area.
Groups of five adult male Sprague-Dawley rats (200–250 g) were exposed to reagent grade aniline at concentrations of 0, 10, 30, 50, 100, or 150 ppm for 8 h/d for 5 d or 12 h/d for 4 d (Kim and Carlson 1986). Exposure concentrations were achieved by passing air through a bottle containing aniline; concentrations were monitored continuously using a gas chromotograph. Blood samples were taken for methemoglobin measurements prior to and following each daily exposure. The mean pre-exposure (control) methemoglobin level was approximately 1.1% (range of 0.4% to 1.7%). All results were presented
graphically; thus, the levels of methemoglobin following 8 or 12 h of exposure on d 1, presented in Table 1–6, are estimates read from the graphs.
Statistical analyses were not performed on the data; however, it can be seen from the data in Table 1–6 that the methemoglobin levels following exposures to 0, 10, and 30 ppm for 8 h are not different. An additional 4 h of exposure (12 h of exposure) at the higher concentrations resulted in only slightly higher methemoglobin levels.
In rats exposed at concentrations of 30 or 50 ppm for 8 h for up to 5 d, methemoglobin levels returned to control values after overnight recovery (10 ppm was identified by the authors as a no-effect level), whereas in the groups exposed at 150 ppm for 8 h or 50 or 150 ppm for 12 h for a maximum of 4 d, methemoglobin levels increased with increasing days of exposure. Hematocrit levels, measured 1 w after the start of exposure, were reduced at concentrations of ≥30 ppm. Signs of aniline intoxication either did not occur or were not reported.
Kim and Carlson (1986) also studied the formation and disappearance of methemoglobin from rat blood over time. The methemoglobin level in groups of five rats was measured at 3, 6, 8, 10, and 12 h during exposure to 100 ppm (Figure 1–1). Following exposure for 8 or 12 h, there was no difference in the maximum methemoglobin level (i.e., the increase with time begins tapering off between 6 and 8 h and reaches an asymptote at 8 h). In addition, there were no differences in the peak aniline levels in blood and fat or the rate of aniline elimination from fat and blood. The methemoglobin levels read from the original graph are approximately 10.5%, 18%, 22%, 22%, and 23% at the 3-, 6-, 8-, 10-, and 12-h exposure durations, respectively. Following 8 or 12 h of exposure, recovery was rapid as shown in the graph (Figure 1–1).
TABLE 1–6 Methemoglobin Levels in Rats Following 8 or 12 h of Exposure to Aniline
|
Concentration (ppm) |
Methemoglobin % at 8 ha |
Methemoglobin % at 12 ha |
|
0 |
1.1 (0.4–1.7) |
1.1 (0.4–1.7) |
|
10 |
0.4–1.7 |
0.4–1.7 |
|
30 |
1.6 |
3.3 |
|
50 |
4.7 |
6.5 |
|
100 |
22 |
23 |
|
150 |
41 |
46 |
|
aValues shown represent estimates from graphic representations of the data. Source: Kim and Carlson 1986. |
||
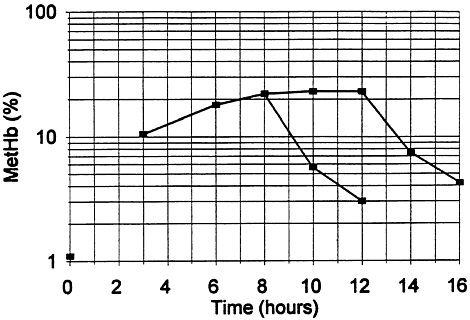
FIGURE 1–1 Formation and disappearance of methemoglobin from blood of rats exposed at 100 ppm for 8 or 12 h. Source: Modified from Kim and Carlson 1986.
In a similar study, groups of 14 male rats were exposed to aniline at concentrations of 0, 10, 30, or 90 ppm for 3, 6, or 12 h daily, 5 d/w for 2 w (Burgess et al. 1984). Methemoglobin levels were measured daily (not reported), and hematology and pathology were evaluated after the tenth exposure and after 14 d of recovery. Ten ppm was a no-effect level for all exposure durations. At 30 and 90 ppm, methemoglobin levels plateaued after four exposures (level not stated), remained at a steady-state level to the tenth day of exposure, and decreased to normal 14 d after exposure. Hemolysis (decreased erythrocyte counts, splenic congestion, and hemosiderin deposition) was seen at 30 and 90 ppm at all exposure durations after the tenth exposure (time of onset could not be determined). Hemolysis was accompanied by compensatory increases in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCHb). After the 14-d recovery period, spleens were nearly normal, but MCV and MCHb remained elevated in the 90-ppm exposure group. The authors noted that effects were predominantly concentration, not time, dependent. Body weights and clinical signs were unaffected. The study was reported in an abstract, and no further details were available.
Groups of 16 male Crl:CD rats were placed in restraints and exposed head-
only to aniline vapor at 17, 45, or 87 ppm for 6 h/d, 5 d/w for 2 w (E.I.du Pont de Nemours 1982b; O’Neal 1982). Concentrations were generated and analyzed as in the E.I.du Pont de Nemours 1982a study. Clinical and histopathologic evaluations were made after 2 w. The 17-ppm concentration for 2 w was considered a minimal effect level—no-observed-adverse-effect level (LOAEL) —on hematologic parameters and histopathology of the spleen. Cyanosis occurred only in the group exposed at 87 ppm for 2 w.
Further details of the above study were discussed in U.S. EPA (1994). After the last exposure, methemoglobin levels were elevated in a dose-dependent manner: 17 ppm, 0% to 2.9% (no different from controls); 45 ppm, 2.2% to 5.4%; and 87 ppm, 4.2% to 23%. The animals exposed at 45 and 87 ppm were anemic with decreases in RBC counts, hemoglobin content, MCHb concentration, and hematocrit, and accompanying increases in erythropoietin foci, reticuloendothelial cell hypertrophy, and hemosiderin deposition in the spleen. The animals in the 87-ppm exposure group were judged cyanotic. In the 17-ppm exposure group, effects were limited to mild splenic congestion.
Oberst et al. (1956) exposed nine male Wistar rats to aniline at 5 ppm for 6 h/d, 5 d/w for up to 26 w. Exposed rats developed a mild hemoglobinemia (0.6%) with some blueness of the skin during w 23 of exposure. Based on the slight increase of methemoglobin content and the absence of spleen toxicity, U.S. EPA (1994) considered this concentration a free-standing no-observed-adverse-effect level (NOAEL).
Acute nonlethal studies using the rat are summarized in Table 1–7.
TABLE 1–7 Summary of Acute Sublethal Inhalation Data in Rats
|
Species |
Concentration (ppm) |
Exposure Time |
Effect |
Reference |
|
Rat |
15,302 |
10 min |
No deaths; oxidative changes in brain |
Kakkar et al. 1992 |
|
Rat |
359 |
4 h |
No deaths; cyanosis, tremors, lacrimation, salivation, semi-prostration, initial weight loss followed by normal gain, reddish-brown perineal area |
E.I.du Pont de Nemours 1982a |
|
Rat |
150 |
8 h |
No deaths; 41% methemoglobin |
Kim and Carlson 1986 |
|
Rat |
150 |
12 h |
No deaths; 46% methemoglobin |
Kim and Carlson 1986 |
3.2.3. Mice
Oberst et al. (1956) exposed 20 female mice to aniline at 5 ppm for 6 h/d, 5 d/w for 20 w. Blood and urine analyses conducted prior to and during exposure and pathologic examinations made at sacrifice revealed no effects.
3.2.4 Guinea pigs
Oberst et al. (1956) exposed 10 guinea pigs to ainline at 5 ppm for 6 h/d, 5 d/w for 20 w. Blood and urine analyses conducted prior to and during exposure and pathologic examinations made at sacrifice revealed no effects.
3.3. Developmental and Reproductive Effects
No studies addressing developmental or reproductive effects following acute inhalation exposure to aniline were located. However, because effects on development and reproduction arise after systemic uptake, oral administration of aniline can be considered for evaluating potential developmental and reproductive toxicity. Aniline (administered as aniline hydrochloride) readily crosses the placental barrier in rodents (Price et al. 1985).
Price et al. (1985) administered aniline hydrochloride by gavage at doses of 10, 30, or 100 milligrams per kilogram per day (mg/kg/d) to timed-pregnant Fischer 344 (F344) rats. Intubation was on gestation d 7 through 20 (group 1) or gestation d 7 through parturition (group 2). Both a reference teratogen (hydroxyurea) and vehicle control group were included in the study protocol. All exposed females survived to scheduled termination, although signs of aniline toxicity—decreased body-weight gain, methemoglobinemia, increased relative spleen weight, decreased erythrocyte count, and hematologic changes indicative of increased hematopoietic activity—were evident in dams exposed at 100 mg/kg/d. Effects on the hematologic profile were not described for the lower doses. In group 1, maternal absolute weight gain was decreased only in the high-dose group, whereas maternal relative spleen weights were increased in all dose groups (in a dose-dependent manner); the other factors were examined only in the 100-mg/kg/d group. On d 20, fetuses from dams in group 1 exposed at 100 mg/kg/d exhibited increased relative liver weights and enhanced hematopoietic activity, but there was no evidence of embryolethality or teratogenicity. Effects in pups observed from post-natal d 0 to 60 (group 2) included transient decreased body weights (dose-related; significant only in the 100-mg/kg/d group), elevated relative liver weights (not dose related), and elevated relative spleen weights (dose-related trend, only on post-natal d 25). A statistically nonsignificant, but exposure-related, increase in the number of exposed
litters with one or more neonatal deaths was observed; the deaths were observed in conjunction with mild but persistent signs of maternal toxicity through postnatal d 30. No evidence of toxicity was observed in pups surviving to post-natal d 60. Price et al. (1985) concluded that doses of aniline that were maternally toxic but nonlethal did not present a selective risk for developmental toxicity to the fetus in the F344 rat.
3.4. Genotoxicity
Aniline and its hydrochloride were tested in standard mutagenicity, cell-transforming, and DNA-damaging tests with mixed results. Results of reverse mutagen assays using Salmonella typhimurium both in the presence and in the absence of an activating system and at doses of up to 2,500 microgram (µg) per plate in studies by McCann et al. (1975), Simon (1979a), and Haworth et al. (1983) were generally negative (U.S. EPA 1994). Positive responses were obtained in the two L5178Y mouse lymphoma cell mutation assays (Amacher et al. 1980; McGregor et al. 1991). Aniline was negative with and without metabolic activation in a mitotic recombination test with Saccharomyces cerevisiae (Simon 1979b).
An increased frequency of sister chromatid exchanges was obtained in vivo in bone-marrow cells of male Swiss mice at intraperitoneal doses of 210 and 420 mg/kg (Parodi et al. 1982, 1983) and in vitro Chinese hamster cells (Abe and Sasaki 1977), although in the latter study, no chromosomal aberrations were observed.
Aniline gave positive responses in the mouse bone-marrow micronucleus assay when administered via ingestion or intraperitoneal injection (Ashby et al. 1991; Westmoreland and Gatehouse 1991). However, the positive responses occurred only at a specific time after administration and at what the authors considered high doses (1,000 mg/kg orally and 300 mg/kg intraperitoneally).
Aniline transformed the Balb/3T3 mouse cell line at doses of 0.8 to 100 µg/mL (without a clear dose-response effect), but not the Syrian hamster embryo cells (Dunkel et al. 1981). Results were negative in DNA damage assays in Escherichia coli (Mamber et al. 1983) and Bacillus subtilis (McCarroll et al. 1981).
3.5. Carcinogenicity
The National Cancer Institute conducted a bioassay of aniline hydrochloride for possible carcinogenicity using F344 rats and B6C3F1 mice (NCI 1978). Aniline hydrochloride was administered in the feed to groups of approximately
50 male and 50 female animals of each species at concentrations of 0.3% (3,000 ppm) and 0.6% (6,000 ppm) of the diet for rats and 0.6% (6,000 ppm) and 1.2% (12,000 ppm) for mice. Groups of 25 or 50 animals were used as concurrent controls. The exposure duration was 103 w; this was followed by a 5-w observation period. Hemangiosarcomas of the spleen and the combined incidence of fibrosarcomas and sarcomas (NOS (not otherwise specified)) of the spleen were each elevated (p<0.05) in male rats. The combined incidence of fibrosarcomas and sarcomas NOS of multiple body organs was also significant in male rats. Incidences in the 0, 3,000- and 6,000-ppm dose groups were 0/24, 1/50, and 7/50, respectively. A compound-related (but statistically nonsignificant) increase in fibrosarcomas or sarcomas NOS of either the spleen alone or multiple organs of the body cavity was observed in female rats. These incidences were statistically significant and associated with increased dietary concentrations of aniline hydrochloride. These was no evidence of compound-related carcinogenicity in mice of either sex. There were no effects on survival for either species.
In the above study, the origin of the tumors was the spleen, a rare site for F344 rats. No bladder tumors were observed. The sequence of pathologic events for this type of tumor is methemoglobinemia, splenic hemosiderosis, splenic fibrosis, splenic sarcoma, and metastatic sarcoma (Goodman et al. 1984). This sequence of events is unlikely to occur until the capacity of the erythrocyte to cope with the insult from continuous high-dose aniline exposure is exceeded (Bus and Popp 1987). Although this sequence of events is unlikely to occur with a single acute exposure, Khan et al. (1997) observed changes in the spleen of rats, including congestion of splenic blood vessels, marked expansion of red pulp, splenic weight change, increased lipid peroxidation, and malondialdehyde-protein adducts 24 h after a single high-dose oral exposure of aniline hydrochloride at 259 mg/kg.
In a second carcinogenicity study, aniline hydrochloride was administered in the diet to CD-F (F344) rats (130/sex/group) at levels of 0, 200, 600, or 2,000 ppm (CIIT 1982). There was an increased incidence of primary splenic sarcomas in male rats in the high-dose group (incidence of 31/90 compared with incidences of 0/64, 0/90, and 1/90 in the 0-, 200-, and 600-ppm groups, respectively). Stromal hyperplasia and fibrosis of the splenic red pulp also occurred in males in the high-dose group and, to a lesser extent, in females in the high-dose group. U.S. EPA (1994) notes that the stromal hyperplasia and fibrosis of the spleen may represent a precursor lesion of sarcoma.
On the basis of induction of tumors of the spleen and the body cavity in two studies with rats, U.S. EPA (1994) in their IRIS document classified aniline as B2, a probable human carcinogen. Evidence is inadequate in humans and
sufficient in animals. Although aniline is a relatively weak carcinogen, a quantitative cancer risk assessment was performed to demonstrate that aniline does not pose a significant cancer risk at the calculated AEGLs (Appendix A).
3.6. Summary
The primary consequence of acute inhalation exposure to aniline is formation of methemoglobin. In rats exposed to aniline, formation of methemoglobin occurred rapidly after exposure, reaching a steady-state in 6 to 8 h. Methemoglobin was removed from the blood with a measurable half-life following termination of exposure. The only reported effect of 8-h exposures of rats at 30, 50, 100, or 150 ppm was the induction of methemoglobin at levels of 1.6%, 4.7%, 22%, and 41%, respectively. The concentration of 30 ppm appears to be a threshold for methemoglobin formation in the rat. No deaths occurred from a 4-h exposure at 359 ppm or a 10-min exposure at 15,302 ppm. Aniline was not a developmental toxicant at doses that were maternally toxic. No information on the reproductive toxicity of aniline was located. Results of genotoxicity tests were mixed or equivocal, most mutagenicity studies being negative. In a 2-y feeding study, daily ingestion of aniline hydrochloride produced increased sarcomas of the spleen in male and female rats but not in male or female mice. The sarcomas were of a rare type and appeared to be related to chronic administration of aniline.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Aniline is lipophilic (pKa of 4.6) and is expected to be rapidly and completely absorbed in the small intestine (Kao et al. 1978). No information on relative bioavailability following inhalation exposure was located, but as indicated by methemoglobin formation during inhalation experiments, systemic absorption by both the inhalation and the percutaneous routes is extensive. Percutaneous absorption of aniline in hairless mice was 4.7% of the nominal applied doses (Susten et al. 1990).
Aromatic amines are initially metabolized by aromatic and N-hydroxylation (oxidation reactions) and N-acetylation. Following aromatic ring hydroxylation, the ring structure may be further conjugated with glucuronic acid or sulfate (Parkinson 1996). N-hydroxylation results in the potential methemoglobin-generating metabolite, phenylhydroxylamine.
Aniline is rapidly and extensively metabolized following oral administration. In the pig and sheep, approximately 30% of a 50-mg/kg dose of 14C-labeled aniline was excreted in the urine, as measured by 14C activity, within 3 h after administration, whereas approximately 50% of the dose was excreted in rats. Within 24 h, more than half the administered dose was excreted by pigs and sheep and 96% of the dose was excreted by rats. Fecal radioactivity was low. N-acetylated metabolites accounted for most of the excretion—N-acetyl-p-aminophenyl glucuronide being the primary metabolite in sheep and pig urine and N-acetyl-p-aminophenyl sulfate being the primary metabolite in the rat (Kao et al. 1978). Biologic monitoring of workers exposed to aniline showed that p-aminophenol constituted 15–55% of the parent compound in the urine; the o- and m-isomers were also formed (Piotrowski 1984).
4.2. Mechanism of Toxicity
Many of the aromatic amines have the ability to convert the ferrous (Fe+2) iron in hemoglobin to the oxidized ferric form (Fe+3), resulting in the formation of methemoglobin. Methemoglobin is unable to transport oxygen, resulting in signs and symptoms of oxygen deficiency. Aniline does not readily oxidize hemoglobin in vitro; it must be metabolized to an active form to induce methemoglobinemia (Smith 1996). Phenylhydroxylamine has been identified as the potential active metabolite, because it produces methemoglobin following administration to dogs (Kiese 1974) and in vitro (Jenkins et al. 1972). Recycling of phenylhydroxylamine may occur: following the reaction of phenylhydroxylamine and hemoglobin to form methemoglobin and nitrosobenzene, nitrosobenzene may be reduced by cell processes to regenerate phenylhydroxylamine. This process would account for the greater potency of phenylhydroxylamine compared with nitrite as a methemoglobin-generating chemical.
In an in vitro study in which phenylhydroxylamine (0.5 milligram per milliliter (mg/mL)) was added to samples of rat and human blood, blood from the human subjects produced less methemoglobin in the human subjects than in the rats (approximately 35% in human blood and 60% in rat blood) (Jenkins et al. 1972). There was no more variation in methemoglobin levels among the cells from different humans than among the cells from different rats.
4.3. Structure-Activity Relationships
As previously noted, many aromatic amines and their metabolites and derivatives are methemoglobin-generating chemicals (Smith 1996).
4.4. Other Relevant Information
In the absence of a known chemical condition such as hemoglobin M, elevated levels of methemoglobin are impossible to maintain without constant infusion of a methemoglobin-inducing chemical. Hemoglobin autoxidation occurs spontaneously in the presence of oxygen and is probably responsible for the low percent (<2%) of methemoglobin normally found in human blood and in blood of most other mammals (Smith 1996). A variety of intraerythrocytic mechanisms reduce methemoglobin to hemoglobin, the most important being methemoglobin reductase, which accounts for 95% of the reducing activity.
4.4.1. Susceptible Subpopulations
Infants are more sensitive to methemoglobin-generating chemicals than adults, as they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase) and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more susceptible to oxidation than adult hemoglobin) (Seger 1992). NADH lacks full activity until infants are 4 mon of age. Human fetal livers are weakly capable of hydroxylating aniline by about 6 w after conception (Pelkonen and Karki 1973). Instances of cyanosis or methemoglobinemia in infants due to percutaneous absorption of aniline dyes from ink were reported in Section 2.1
In rare instances, humans may suffer from hereditary deficiencies of enzymes responsible for reducing methemoglobin. "Rare individuals" with an inherited deficiency of NADH-methemoglobin reductase have 10–50% of their circulating blood pigment in the form of methemoglobin. The effect is primarily cosmetic as these individuals have a compensatory polycythemia, although symptoms may occur during exercise. Other individuals may have a deficiency of erythrocyte NADPH-glucose-6-phosphate dehydrogenase, an enzyme responsible, via the pentose phosphate shunt, for generating an alternate source of energy for the cell; these individuals do not have elevated levels of methemoglobin, as this is a minor methemoglobin-reducing system (Kiese 1974; Smith 1996; Seger 1992). Individuals with hemoglobin M, caused by a substitution of amino acids on the hemoglobin molecule, maintain methemoglobin levels of 25–30% and are clinically cyanotic (Seger 1992).
4.4.2. Species Differences
There are large species differences in the response of hemoglobin to the administration of aniline. Differences appear to be related to the rate of metabo-
lism and formation of specific metabolites as well as to the level of enzymes responsible for reducing methemoglobin (Kiese 1974; Calabrese 1991). Spicer (1950) compared the methemoglobin response in dogs, cats, and rabbits injected intravenously with aniline. Injections of 15 mg/kg of body weight produced an average methemoglobin response of 28.3% in dogs and 56.4% in cats, whereas an injection of 30 mg/kg produced a response of only 3.2% in rabbits. However, on the basis of blood volume, the response in the dog and cat were more similar, 25% and 32%, respectively, following injection of aniline at 1.1 mg/g of hemoglobin. Jenkins et al. (1972) found that an intravenous injection of 20 mg/kg to rats induced a methemoglobin level of 10.9%. For two other methemoglobin-forming chemicals, acetanilide and acetophenetidine, humans were half as susceptible as the cat and one-tenth as susceptible as the rat (Calabrese 1991).
At the low concentration used in the Oberst et al (1956) study with dogs, rats, mice, and guinea pigs (5 ppm for 6 h/d, 5 d/w), no well-defined clinical signs of intoxication occurred in any species; rats showed a slight deviation from pre-exposure methemoglobin levels (maximum, 0.6%), and dogs had an increase of chromogen in their urine, although circulating methemoglobin was not elevated. No clear species differences could be distinguished among these minor effects.
A single oral administration of aniline hydrochloride to male Sprague-Dawley rats at 2 millimole per kilogram (mmole/kg) (259 mg/kg; presumably 186 mg/kg of aniline) resulted in a peak methemoglobin level of 37% at 0.5 h following administration (Khan et al. 1997). A single oral dose of 100 mg of aniline hydrochloride (presumably about 1.0 mg of aniline/kg of body weight) to two human subjects resulted in an increase in methemoglobin content to 11% (Brodie and Axelrod 1948). In another study, oral administration of aniline at 40 mg/kg to two rats produced a mean maximum increase of 16.6% in methemoglobin within 1 to 4 h. Administration of 65 mg (presumably about 0.9 mg/kg) produced a maximum increase of 16.1% in an adult male volunteer (Jenkins et al. 1972). The maximum level in the volunteer was reached 2 h after administration and returned to normal 1 h later. The no-effect dose in 20 male and female volunteers in this study was 15 mg (0.2 mg/kg). The 20 volunteers were given an oral dose mid-morning (10 a.m.) of 5, 15, and 25 mg on successive mornings followed by treatment of some of these volunteers with 35, 45, 55, and 65 mg, whereas the rats, which were fed ad libitum, were administered a single treatment by gastric intubation. It should be noted that an intravenous dose of 40 mg/kg to rats produced a lower increase in methemoglobin (11.7%) than the oral dose (16.6%) and that the increase in methemoglobin formation in rats plateaued between oral doses of 40 and 300 mg/kg. The authors noted that the greater sensitivity may be due to differences in the extent to which aniline
is metabolized or to differences in the activities of enzymes that promote the reduction of methemoglobin.
Mier (1988) reported on the ingestion of aniline by a 4.5-y-old child weighing 16 kg. Ingestion of approximately 1 teaspoon (approximately 0.3125 mg/kg) produced a methemoglobin level of 68% by 6 h after ingestion. At this time, treatment consisted of intravenous methylene blue to which she was poorly responsive followed by blood exchange 13 h after ingestion.
Smith (1996) summarized data on the spontaneous methemoglobin reductase activity of mammalian erythrocytes. Using nitrated RBCs with glucose as a substrate, the data reflect the ratio of the activity of the species to the activity in human RBCs. Activity in rat cells and human cells ranged from 1.3 to 5.0. Activity in cells of the cat and dog was similar to that in human cells, and that of the rabbit was 3.3 to 7.5 times greater. Most studies show that the spontaneous methemoglobin reductase activity of human erythrocytes is within an order of magnitude of that of other mammals (Smith 1996).
Differential spontaneous methemoglobin reductase activity among species is not the sole determining factor for interspecies differences. The inherent sensitivity of the hemoglobin molecule to oxidation; the presence of other reducing agents, such as reduced glutathione, cysteine, and ascorbic acid; and the extent to which the methemoglobin-forming metabolite is formed and its biologic half-life are interacting factors (Calabrese 1991).
4.4.3. Concentration-Exposure Duration Relationship
No single study clearly addressed various exposure durations and concentrations or their relationship. However, the relationship between concentration of aniline and methemoglobin formation at a fixed exposure duration (8 h) is linear (Table 1–6), and although the data are limited, methemoglobin formation increased by less than a factor of 2, i.e., was linear when comparing the 3- and 6-h exposure durations at a constant concentration of 100 ppm before reaching an asymptote at 8 h (Figure 1–1). Based on the linear relationship between concentration and methemoglobin formation in the Kim and Carlson (1986) study, a value of n=1 for scaling across time was selected (C1×t=k) for AEGL development.
As noted, during exposure to a constant concentration, the level of methemoglobin does not approach equilibrium until 6–8 h after initiation of exposure (Figure 1–1). Therefore, methemoglobin levels at the shorter exposure durations are lower than those at 8 h (e.g., the level is 10.5% at 3 h for a constant exposure to 100 ppm), and any effect used as an endpoint at 8 h may not be present at the shorter exposure duration. Because of the 6–8 h lag time before
the methemoglobin plateau (and consequential effect) is reached, the value of n=1 for scaling is considered appropriate, and a more conservative value for scaling to the shorter time periods is unnecessary.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
Henderson and Haggard (1943), in citing older reports, listed 5 ppm as a maximum concentration considered safe for daily exposures but did not give the basis for their statement. No additional human data are available for the derivation of AEGL-1 values for aniline.
5.2. Animal Data Relevant to AEGL-1
Kim and Carlson (1986) exposed animals to several concentrations within the time periods relevant to development of the AEGLs. The authors also followed the increase in methemoglobin (the primary effect of aniline exposure) over time during exposure to a single concentration. Their study determined that a single 8-h exposure to a concentration of 50 ppm was a LOAEL for generation of methemoglobin in the rat (4.7%) but a NOAEL for any clinical effects. Their study also determined that a concentration of 100 ppm for 8 h resulted in a methemoglobin level of 22%. In humans, this level is characterized by clinical cyanosis but no evidence for hypoxia. Furthermore, this level is not reached in rats until completion of a full 8 h of exposure. In the study by Burgess et al. (1984), clinical signs were unaffected by exposure at 90 ppm for up to 12 h daily, 5 d/w for 2 w. Details of the study were not reported. The study by E.I. du Pont de Nemours (1982b) used head-only exposures of rats; whole-body exposures are considered more relevant to AEGL development, inasmuch as head-only exposures do not account for potential percutaneous absorption.
5.3. Derivation of AEGL-1
The concentration of 100 ppm for 8 h in the study by Kim and Carlson (1986) was used as the basis for the AEGL-1. This exposure results in a methemoglobin level of 22% but no hypoxic signs in rats. A review of the literature revealed that methemoglobin levels of 15–20% in humans results in clinical cyanosis, but no sign of clinical hypoxia (Kiese 1974; Seger 1992). Although inhalation data for comparison purposes are not available, oral
ingestion data suggest that humans may be considerably more sensitive to methemoglobin-forming chemicals than rats. Oral administration of aniline at 40 mg/kg to rats produced a maximum increase of 16.6% in methemoglobin, whereas oral administration of aniline at 0.9 mg/kg to a human volunteer produced a maximum increase of 16.1%. A 10-fold uncertainty factor is generally applied when extrapolating from valid results of studies on experimental animals to humans (NRC 1993). Thus, an uncertainty factor of 10 was used for interspecies extrapolation. Differences in sensitivity to aniline among human subpopulations are known to occur, but the extent of the differences in the general population (excluding rare inherited disorders) is unknown. Infants are more sensitive to methemoglobin-generating chemicals than adults as they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase) and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). When quantitative data on a sensitive subpopulation are lacking, a 10-fold uncertainty factor is generally applied to account for the variation in sensitivity in the human population (NRC 1993). Thus, an intraspecies uncertainty factor of 10 was applied to account for the difference in sensitivity between infants and adults. It is believed that an intraspecies uncertainty factor of 10 is protective of infants. The uncertainty factors of 10 for each of the interspecies and intraspecies variabilities are dictated by the small database and the lack of reliable human inhalation studies. The data were scaled across time using C1×t=k and k=480 ppm·min. (The relationship between concentration of aniline and methemoglobin formation at a fixed time (8 h) is linear.) Although an n value of 1 is not the most conservative when scaling to shorter time periods, it is believed that the total uncertainty factor of 100 is protective of human health. The calculated values are listed in Table 1–8; calculations are in Appendix B.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No human data relevant to the calculation of an AEGL-2 were located. Using the descriptions of Kiese (1974) and Seger (1992), concentrations of
TABLE 1–8 AEGL-1 Values for Aniline
|
AEGL Level |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
16 ppm (61 mg/m3) |
8.0 ppm (30 mg/m3) |
2.0 ppm (7.6 mg/m3) |
1.0 ppm (3.8 mg/m3) |
aniline that induce methemoglobinemia levels greater than 30% (fatigue) and less than about 60% of the circulating hemoglobin (lethargy and semistupor) would be applicable to derivation of an AEGL-2.
6.2. Animal Data Relevant to AEGL-2
The study by Kim and Carlson (1986) determined that a concentration of 150 ppm for 8 h resulted in 41% methemoglobinemia. No report of clinical signs was included by these authors. According to Bodansky (1951), dogs failed to show any clinical signs at methemoglobin concentrations of less than about 60%.
6.3. Derivation of AEGL-2
The 8-h exposure at 150 ppm to rats resulted in elevation of methemoglobin to 41% with no reported clinical signs. A review of the literature revealed that methemoglobin levels of 30–45% in humans are associated with fatigue, lethargy, exertional dyspnea, and headache. These signs or symptoms were considered the threshold for disabling effects. The 8-h exposure at 150 ppm was chosen as the basis for the AEGL-2 calculations. The level of methemoglobin attained after 8 h of exposure, 41%, may produce anxiety and signs of fatigue; these signs are below the definition of the AEGL-2. Although inhalation data for comparison purposes are not available, ingestion data suggest that humans may be considerably more sensitive to methemoglobin-forming chemicals than rats. Oral administration of aniline at 40 mg/kg to rats produced a maximum increase of 16.6% in methemoglobin, whereas oral administration of 0.9 mg/kg to a human volunteer produced a maximum increase of 16.1%. A 10-fold uncertainty factor is generally applied when extrapolating from valid results of studies on experimental animals to humans (NRC 1993). Thus, an uncertainty factor of 10 was used for interspecies extrapolation. Differences in sensitivity to aniline among human subpopulations are known to occur, but the extent of the differences in the general population (excluding rare inherited disorders) is unknown. Infants are more sensitive to methemoglobin-generating chemicals than adults, as they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase) and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). When data on a sensitive subpopulation are lacking, a 10-fold uncertainty factor is generally applied to account for the variation in sensitivity among the human population (NRC 1993). Thus, an intraspecies uncertainty factor of 10 was applied to account for
TABLE 1–9 AEGL-2 Values for Aniline
|
AEGL Level |
30 min |
1 h |
4 h |
8 h |
|
AEGL-2 |
24 ppm (91 mg/m3) |
12 ppm (46 mg/m3) |
3.0 ppm (11 mg/m3) |
1.5 ppm (5.7 mg/m3) |
the difference in sensitivity between infants and adults. It is believed that the intraspecies uncertainty factor of 10 is protective of infants. Based on the linear relationship between methemoglobin formation and aniline concentration, the data were scaled to the relevant time periods using the relationship C1×t=k and k=720 ppm·min. Calculations are in Appendix B, and results are listed in Table 1–9.
The 8-h AEGL-2 is 1.5 ppm. Flury and Zernik (1931) cite human data in which a concentration of 40–53 ppm was tolerated for 6 h without distinct symptoms. Although of questionable reliability, their citation indicates that sensitive individuals should be protected during an 8-h exposure to 1.5 ppm.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No human data relevant to the calculation of an AEGL-3 were located. Using the descriptions of Bodansky (1951), Kiese (1974), and Seger (1992), concentrations of about 60% are associated with lethargy and semi-stupor, concentrations of 70% are considered the threshold for lethality, and concentrations exceeding 85% may be lethal if treatment is not initiated. Hamblin and Mangelsdorff (1938) and Mangelsdorff (1956) cite recovery of workers from methemoglobin levels up to 72% with little or no medical treatment.
7.2. Animal Data Relevant to AEGL-3
No studies resulting in a methemoglobin level relevant to the definition of the AEGL-3 were available. The study by E.I.du Pont de Nemours (1982a) with Crl:CD rats did not report methemoglobin levels but did report that no deaths occurred after exposure to a concentration of 359 ppm for 4 h. Kakkar et al. (1992) reported a 10-min no-adverse-effect concentration of 15,302 ppm. The study by Kim and Carlson (1986) with Sprague-Dawley rats did not address methemoglobin levels greater than 41%; those data showed that the methemoglobin level (after 8 h of exposure) varies directly with the concentration of
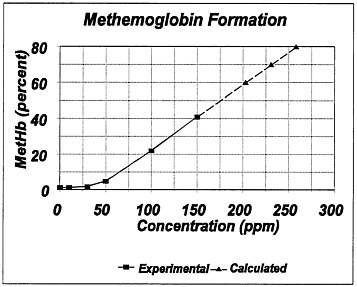
FIGURE 1–2 Measured and projected methemoglobin levels in rats exposed to aniline for 8 h. Source: Data from Kim and Carlson 1986.
aniline. Therefore, the graph of concentration versus methemoglobin level at 8 h can be extrapolated to attain a concentration resulting in a methemoglobin level of 70–80%, the defined threshold for lethality in humans (Figure 1–2).
7.3. Derivation of AEGL-3
An aniline concentration of 250 ppm, which is projected to result in a methemoglobin level between 70% and 80% after an 8-h exposure was identified as the basis for the AEGL-3. The same uncertainty factors and scaling procedure (the value of k in the formula C1×t=k is 1,200 ppm·min) as used for the AEGL-1 were applied to calculations of the AEGL-3. Calculations are in Appendix B, and values appear in Table 1–10.
The 1-h AEGL-3 value is 20 ppm and is considered safe for sensitive individuals when compared with generalizations in older references. Henderson and Haggard (1943) cited human data in which a concentration of 100 to 160 ppm was the maximum concentration that could be inhaled for 1 h without serious disturbance. The American Industrial Hygiene Association (AIHA 1955) stated that 50–100 ppm could probably be tolerated for 60 min. Two additional studies with rats support the AEGL-3 values. The 4-h exposure of rats to 359 ppm resulted in serious signs but no deaths (E.I.du Pont de Nemours 1982a). Using the combined interspecies and intraspecies uncertainty factor of
TABLE 1–10 AEGL-3 Values for Aniline
|
AEGL Level |
30 min |
1 h |
4 h |
8 h |
|
AEGL-3 |
40 ppm (152 mg/m3) |
20 ppm (76 mg/m3) |
5.0 ppm (19 mg/m3) |
2.5 ppm (9.5 mg/m3) |
100 and extrapolating across time, the 1-h AEGL-3 value from this study would be 14 ppm. Using the same combined uncertainty factor and extrapolation across time, the 10-min concentration of 15,302 ppm in the study by Kakkar et al. (1992) results in a 1-h AEGL-3 value of 25.5 ppm. It should also be noted that 5 ppm, 6 h/d, 5 d/w for 20–26 w was a NOAEL for dogs, rats, mice and guinea pigs (Oberst et al. 1956).
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity Endpoints
The AEGL values and toxicity endpoints are summarized in Table 1–11. Because aniline is absorbed through the skin, a skin notation was added to the table of values.
8.2. Comparisons with Other Standards and Guidelines
Standards and guidance levels for workplace and community exposures are listed in Table 1–12. The American Industrial Hygiene Association (AIHA 1955) stated that 50–100 ppm could probably be tolerated for 60 min based on
TABLE 1–11 Summary and Relationship of AEGL Valuesa
TABLE 1–12 Extant Standards and Guidelines for Aniline
|
|
Exposure Duration |
|||
|
Guideline |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
16 ppm |
8 ppm |
2 ppm |
1 ppm |
|
AEGL-2 |
24 ppm |
12 ppm |
3 ppm |
1.5 ppm |
|
AEGL-3 |
40 ppm |
20 ppm |
5 ppm |
2.5 ppm |
|
ERPG-1 |
|
Not derived |
|
|
|
ERPG-2 |
|
Not derived |
|
|
|
ERPG-3 |
|
Not derived |
|
|
|
NIOSH IDLHa |
100 ppm |
|
||
|
NIOSH RELb |
|
—c |
||
|
OSHA PELb |
|
5 ppm |
||
|
ACGIH TLV-TWAd |
|
2 ppme |
||
|
MAK (German)f |
10 ppm |
|
2 ppme |
|
|
MAC (Netherlands)g |
|
1 mg/m3e |
||
|
aNIOSH 1994, 1997. bNIOSH 1997. cPotential occupational carcinogen; occupational exposure should be limited to the lowest feasible concentration. dACGIH 1999. eSkin notation; caution against cutaneous and mucous membrane exposures (aniline and homologues). fGerman Reasearch Association 1999. gMinistry of Social Affairs and Employment 1999. |
||||
a Manufacturing Chemists Association chemical safety data sheet. Because most of these standards are protective of any adverse health effect, they are comparable only to the AEGL-1 levels. The ACGIH time-weighted average (TWA) of 2 ppm is based on the slight increase in methemoglobin in rats exposed at 5 ppm for 6 h/d, 5 d/w for up to 26 w (Oberst et al. 1956) and the fact that skin absorption can contribute to aniline systemic toxicity in humans. The ACGIH Threshold Limit Value (TLV) is intended for repeated daily exposure of the healthy adult worker and is not necessarily comparable to a single 8-h exposure. However, it should be noted that both the 8-h AEGL-1 and AEGL-2 are below the 8-h ACGIH TWA, and the 8-h AEGL-3 is only slightly
above the 8-h ACGIH TWA. The OSHA permissible exposure limit (PEL) is 5 ppm. The German and the Dutch 8-h maximum workplace concentrations are slightly lower than the 8-h AEGL-1. Emergency response planning guidelines (ERPGs) have not been derived.
The NIOSH immediately dangerous to life and health (IDLH) is based on Henderson and Haggard (1943), AIHA (1955), and von Oettingen (1941). Henderson and Haggard, in turn, is based on the animal studies of Flury and Zernik (1931). The statement by Henderson and Haggard that 100 to 160 ppm is the maximum concentration that could be inhaled for 1 h without serious disturbance appears to be the basis for the IDLH of 100 ppm. This 30-min guideline concentration is greater than the 30-min AEGL-3.
The ACGIH TLV-TWA is the time-weighted average concentration for a conventional 8-h workday and a 40-h workweek to which it is believed that nearly all workers may be repeatedly exposed, day after day, without adverse effects.
The NIOSH IDLH is defined by the NIOSH-OSHA Standard Completions Program only for the purpose of respirator selection and represents a maximum concentration from which, in the event of respiratory failure, one could escape within 30 min without experiencing any escape-impairing or irreversible health.
The OSHA PEL is a time-weighted average (8 h/d, 40 h/w).
8.3. Data Adequacy and Research Needs
Recent or definitive inhalation exposure-response data for aniline in humans are lacking. However, accidental human exposures to liquid aniline or aniline-containing dyes confirm that the primary effect is on the blood and consists of the conversion of hemoglobin to methemoglobin. Accidental human exposures also provide qualitative as well as quantitative information on symptoms and effects associated with specific blood methemoglobin concentrations. Recent animal studies which utilized reliable measurement techniques provided good concentration-response data and confirmed the primary toxicologic endpoint of methemoglobin formation. The key study was well designed, conducted and documented.
Data indicate that human infants are more sensitive to methemoglobin-generating chemicals than adults since they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase) and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin). Oral exposures of humans and animals to low doses indicate that humans may be considerably more sensitive to aniline-induced methemoglobin formation than laboratory
rats. The differences in sensitivity between human infants and adults and between humans and laboratory animals are reflected in the uncertainty factor adjustments used in the development of the AEGL values. It is believed that the interspecies and intraspecies default values of 10 each for a total uncertainty factor of 100 will be protective of human health. The margin of safety of the AEGL values derived from acute animal exposures is supported by the gradual uptake/effect in the key study in that maximal methemoglobin formation was reached only after 6–8 h and by the only marginally greater effects in animal studies following repeated exposures at concentrations similar to those of the acute studies.
The available data from oral bioassays with aniline suggest that a tumorigenic response may occur following long-term, repeated high-dose exposures that cause repetitive tissue damage in the spleen as a consequence of physiologic adaptation to the chronic damage to erythrocytes (Bus and Popp 1987). The AEGL values were not based on carcinogenicity in rats, because formation of methemoglobin was a more sensitive endpoint than induction of tumors of the spleen. In addition, the endpoint of carcinogenicity was not used because the route-to-route extrapolation used in the carcinogenicity risk assessment adds additional uncertainty to the calculated values.
9. REFERENCES
Abe, S., and M.Sasaki. 1977. Chromosome aberrations and sister chromatid exchanges in Chinese hamster cells exposed to various chemicals. J. Natl. Cancer Inst. 58:1635–1641.
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices: Aniline. Sixth Ed. Cincinnati, OH: ACGIH.
ACGIH (American Conference of Governmental Industrial Hygienists). 1999. Threshold Limit Values (TLVs) for Chemical and Physical Agents and Biological Exposure Indices (BEIs). Cincinnati, OH: ACGIH.
AIHA (American Industrial Hygiene Association). 1955. Hygienic guide series. Am. Ind. Hyg. Assoc. Q. 16:331–332.
AIHA (American Industrial Hygiene Association). 1999. The AIHA 1999 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guidelines Handbook. Fairfax, VA: AIHA.
Amacher, D.E., S.C.Paillet, G.N.Turner, V.A.Ray, and D.S.Salsburg. 1980. Point mutations at the thymidine kinase locus in L5178Y mouse lymphoma cells. II. Test validation and interpretation. Mutat. Res. 72:447–474.
Ashby, J., D.A.Vlachos, and H.Tinwell. 1991. Activity of aniline in the mouse bone marrow micronucleus assay. Mutat. Res. 263:115–117.
Billings, C.E. and L.C.Jones. 1981. Odor thresholds in air as compared to threshold limit values. Am. Ind. Hyg. Assoc. J. 42:479.
Benya, T.J. and H.H.Cornish. 1994. Aniline. Pp. 982–984 in Patty’s Industrial Hygiene and Toxicology, 4th Ed. New York: John Wiley & Sons.
Bodansky, O. 1951. Methemoglobinemia and methemoglobin producing compounds. Pharmacol. Rev. 3:144–196.
Brodie, B.B. and J.Axelrod. 1948. The fate of acetanilide in man. J. Pharmacol. Exp. Ther. 94:29.
Budavari, S. M.J.O’Neil, A.Smith, P.E.Heckelman, and J.F.Kinneary, eds. 1996. The Merck Index, 12th Ed. Rahway, NJ: Merck & Co., Inc.
Burgess, B.A., T.P.Pastoor, and G.L.Kennedy, Jr. 1984. Aniline induced methemoglobinemia and hemolysis as a function of exposure concentration and duration. Toxicologist 4:64.
Bus, J.S., and J.A.Popp. 1987. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds. Food Chem. Toxicol. 25:619–626.
Calabrese, E.J. 1991. Principles of Animal Extrapolation. New York: John Wiley & Sons. pp. 307–320.
Carpenter, C.P., H.F.Smith, Jr., and U.C.Pozzani. 1949. The assay of acute vapor toxicity, and the grading and interpretation of results on 96 chemical compounds. J. Ind. Hyg. Toxicol. 31:343–346.
Case, R.A.M., M.E.Hosker, D.B.McDonald, and J.T.Pearson. 1954. Tumors of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. Br. J. Ind. Med. 11:75–104.
CIIT (Chemical Industry Institute of Toxicology). 1982. 104-week chronic toxicity study in rats: Aniline hydrochloride. Final report. CIIT, Research Triangle Park, NC.
Comstock, C.C., and F.W.Oberst. 1952. Inhalation toxicity of aniline, furfuryl alcohol and their mixtures in rats and mice. Medical Laboratories Research Report 139, Chemical Corps Medical Laboratories, Army Chemical Center.
Crump, K.S., and R.B.Howe. 1984. The multistage model with a time-dependent dose pattern: Applications to carcinogenic risk assessment. Risk Anal 4:163–176.
DOT (U.S. Department of Transportation), U.S. Coast Guard. 1985. CHRIS—Hazardous Chemical Data, Washington, DC: U.S. Government Printing Office.
Dunkel, V.C., R.J.Pienta, A.Sivak, and K.A.Traul. 1981. Comparative neoplastic transformation responses of Balb/3T3 cells, Syrian hamster embryo cells, and Rauscher murine leukemia virus-infected Fischer 344 rat embryo cells to chemical carcinogens. J. Natl. Cancer Inst. 67:1303–1315.
E.I.du Pont de Nemours. 1982a. Inhalation Median Lethal Concentration (LC50). OTS 84003A, Docket 878220239. E.I.du Pont de Nemours and Co., Inc., Wilmington, DE.
E.I.du Pont de Nemours. 1982b. Subacute Inhalation Toxicity Study of Aniline in Rats. OTS 84003A, Document 878220240. E.I.du Pont de Nemours and Co., Inc., Wilmington, DE.
Etteldorf, J.N. 1951. Methylene blue in the treatment of methemoglobinemia in premature infants caused by marking ink. J. Pediat. 38:24–27.
Flury, F. and F.Zernik. 1931. Aniline. In Noxious Gases—Vapors, Mist, Smoke, and Dust Particles. Berlin: Springer-Verlag.
German Research Association (Deutsche Forschungsgemeinschaft). 1999. List of
MAK and BAT Values, 1999. Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 35. Federal Republic of Germany: Wiley.
Goodman, D.G., J.M.Ward, and W.D.Reichardt. 1984. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4'-sulfonyldianiline, or D & C red no. 9. J. Natl. Cancer Inst. 73:265–273.
Gosselin, R.E., R.P.Smith, and H.C.Hodge. 1984. Aniline. Pp. 31–36, Section 3, in Clinical Toxicology of Commercial Products, 5th Ed. Baltimore, MD: Williams & Wilkins.
Graubarth, J., C.J.Bloom, F.C.Coleman, and H.N.Solomon. 1945. Dye poisoning in the nursery, a review of seventeen cases. J. Am. Med. Assoc. 128:1155–1157.
Hamblin, D.O., and A.F.Mangelsdorff. 1938. Methemoglobinemia and its measurement. J. Ind. Hyg. Toxicol. 20:523–539.
Haworth, S., T.Lawlor, K.Mortelmans, W.Speck, and E.Zeiger. 1983. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. (Suppl.) 1:3–142.
Henderson, Y., and H.W.Haggard. 1943. Noxious Gases, 2nd Ed. New York: Reinhold, p. 228.
HSDB (Hazardous Substances Data Bank). 1996. Aniline. [Online] Available: http://chem.sis.nlm.nih.gov/hsdb/results.html [09/19/96].
IARC (International Agency for Research on Cancer). 1987. IARC monographs on the evaluation of carcinogenic risks to humans: Overall evaluations of carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. IARC Monographs Supplement 7. Lyon, France: International Agency for Research on Cancer.
Jenkins, F.P., J.A.Robinson, J.B.M.Gellatly, and G.W.A.Salmond. 1972. The no-effect dose of aniline in human subjects and a comparison of aniline toxicity in man and the rat. Fd. Cosmet. Toxicol. 10:671–697.
Kagan, B.M., B.Mirman, J.Calvin, and E.Lundeen. 1949. Cyanosis in premature infants due to aniline dye intoxication. J. Pediat. 34:574–578.
Kakkar, P., S.Awasthi, and P.N.Viswanathan. 1992. Oxidative changes in brain of aniline-exposed rats. Arch. Environ. Contam. Toxicol. 23:307–309.
Kao, J., J.Faulkner, and J.W.Bridges. 1978. Metabolism of aniline in rats, pigs, and sheep. Drug Metab. Dispos. 6:549–555.
Khan, M.F., X.Wu, B.S.Kaphalia, P.J.Boor, and G.A.S.Ansari. 1997. Acute hematopoietic toxicity of aniline in rats. Toxicol. Lett. 92:31–37.
Kiese, M. 1974. Methemoglobinemia: A Comprehensive Treatise. Cleveland, OH: CBC Press.
Kim, Y.C., and G.P.Carlson. 1986. The effect of an unusual workshift on chemical toxicity. II. Studies on the exposure of rats to aniline. Fundam. Appl. Toxicol. 7:144–152.
Mamber, S.W., V.Bryson, and S.E.Katz. 1983. The Escherichia coli WP2/WP100 rec assay for detection of potential chemical carcinogens. Mutat. Res. 119:135–144.
Mangelsdorff, A.F. 1956. Treatment of methemoglobinemia. Arch. Ind. Health 14:148–153.
McCann, J., E.Choi, E.Yamasaki, and B.N.Ames. 1975. Detection of carcinogens
as mutagens in the Salmonella/microsome test: Assay of 300 chemicals. Proc. Natl. Acad. Sci. USA 72:5135–5139.
McCarroll, N.E., C.E.Piper, and B.H.Keech. 1981. An E. coli microsuspension assay for the detection of DNA damage induced by direct-acting agents and promutagens. Environ. Mutagen. 3:429–444.
McGregor, D.B., A.G.Brown, S.Howgate, D.McBride, C.Riach, and W.J.Caspary. 1991. Responses of the L5178Y mouse lymphoma cell forward mutation assay. V. 27 coded chemicals. Environ. Mol. Mutagen. 17:196–219.
Mier, R.J. 1988. Treatment of aniline poisoning with exchange transfusion. Clin. Toxicol. 26:357–364.
Ministry of Social Affairs and Employment (SDU Uitgevers). 1999. Nationale MAC (Maximum Allowable Concentration) List, 1999. The Hague, The Netherlands.
NCI (National Cancer Institute). 1978. Bioassay of aniline hydrochloride for possible carcinogenicity. CAS No. 142–04–1. NCI-CG-TR-130, PB 287 539. National Cancer Institute, Bethesda, MD.
NIOSH (National Institute for Occupational Safety and Health). 1997. NIOSH Pocket Guide to Chemical Hazards. NIOSH Publ. No. 94–116, U.S. Department of Health and Human Services. Washington, DC: U.S. Government Printing Office.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHS). National Institute for Occupational Safety and Health, Cincinnati, OH. Available from NTIS, Springfield, VA, as document PB94195047.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). In press. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Airborne Chemicals. Washington, DC: National Academy Press.
Oberst, F.W., E.B.Hackley, and C.C.Comstock. 1956. Chronic toxicity of aniline vapor (5 ppm) by inhalation. AMA Arch. Ind. Health 13:379–384.
O’Neal, F.O. 1982. Subacute inhalation toxicity of aniline: The relationship between methemoglobin formation and toxicity. Toxicologist 2:164.
OSHA (Occupational Safety and Health Administration). 1995. Code of Federal Regulations, CFR 29, Part 1910, Table Z-1.
OSHA (Occupational Safety and Health Administration). 1997. Workplace exposure levels for aniline. Printout received from Paul Tobin, U.S. Environmental Protection Agency. Jan. 13.
Parkinson, A. 1996. Biotransformation of xenobiotics. Chapter 6 in Casarett and Doull’s Toxicology: The Basic Science of Poisons . New York: McGraw-Hill.
Parodi, S., M.Taningher, P.Russo, M.Pala, M.Tamaro, and C.Monti-Bragadin. 1982. DNA damage in liver, kidney, bone marrow and spleen of rats and mice treated with commercial and purified aniline as determined by alkaline elution assay and sister chromatid exchange induction. Cancer Res. 42:2277–2283.
Parodi, S., A.Zunino, L.Ottaggio, M.DeFerrari, and L.Santi. 1983. Lack of correlation between the capability of inducing sister-chromatid exchanges in vivo and
carcinogenic potency for 16 aromatic amines and azo derivatives. Mutat. Res. 108:225–238.
Pelkonen, O., and N.T.Karki. 1973. 3,4-Benzpyrene and aniline are hydroxylated by human fetal liver but not by placenta at 6–7 weeks of fetal age. Biochem. Pharmacol. 22:1538–1540.
Pickup, J.D., and J.Eeles. 1953. Cyanosis in newborn babies caused by aniline-dye poisoning. Lancet 2:118.
Piotrowski, J.K. 1984. Phenol, aniline, and nitrobenzene. Pp. 165–175 in Biological monitoring and surveillance of workers exposed to chemicals, A.Aitio, V. Riihimaki, and H.Vanio, eds. New York: Hemisphere.
Price, C.J., R.W.Tyl, T.A.Marks, L.L.Paschke, T.A.Ledoux, and J.R.Reel. 1985. Teratologic and postnatal evaluation of aniline hydrochloride in the Fischer 344 rat. Toxicol. Appl. Pharmacol. 77:465–478.
Ramsay, D.H.E., and C.C.Harvey. 1959. Marking-ink poisoning: An outbreak of methaemoglobin cyanosis in newborn babies. Lancet 1:910–912.
Seger, D.L. 1992. Methemoglobin-forming chemicals. Pp. 800–806 in Hazardous Materials Toxicology: Clinical Principles of Environmental Health, J.B.Sullivan and G.R.Krieger, eds. Baltimore, MD: Williams & Wilkins.
Simon, V.F. 1979a. In vitro mutagenicity assays of chemical carcinogens and related compounds with Salmonella typhimurium. J. Natl. Cancer Inst. 62:893–899.
Simon, V.F. 1979b. In vitro assays for recombinogenic activity of chemical carcinogens and related compounds with Saccharomyces cerevisiae D3. J. Natl. Cancer Inst. 62:901–909.
Smith, D.S. 1992. Unforgettable patients. J. Pediat. 121:986–988.
Smith, R.P. 1996. Toxic responses of the blood. Pp. 335–354 in Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York: McGraw-Hill.
Spicer, S.S. 1950. Species differences in susceptibility to methemoglobin formation. J. Pharmacol. Exp. Ther. 99:185–194.
Susten, A.S., R.W.Niemier, and S.D.Simon. 1990. In vivo percutaneous absorption studies of volatile organic solvents in hairless mice: II. Toluene, ethylbenzene and aniline. J. Appl. Toxicol. 10:217–226.
U.S. EPA (U.S. Environmental Protection Agency). 1987. Technical Guidance for Hazards Analysis: Emergency Planning for Extremely Hazardous Substances. EPA-OSWER-88–0001, U.S. Environmental Protection Agency, Federal Emergency Management Agency, U.S. Department of Transportation, Washington, DC.
U.S. EPA (U.S. Environmental Protection Agency). 1992. Reference Guide to Odor Thresholds for Hazardous Air Pollutants Listed in the Clean Air Act Amendments of 1990. EPA/600/R-92/014. Air Risk Information Support Center, EPA, Washington, DC.
U.S. EPA (U.S. Environmental Protection Agency). 1994. Aniline. Integrated Risk information System (IRIS). [Online] Available: http://www.epa.gov/ncea/iris.htm. [10/011/96].
U.S. ITC (U.S. International Trade Commission). 1994. Synthetic Organic Chemicals: United States Production and Sales, 1993. U.S. International Trade Commission, Washington, DC.
von Oettingen, W.F. 1941. The aromatic amines and nitro compounds, their toxicity and potential dangers. A review of the literature. U.S. Public Health Bulletin No. 271. Washington, DC: Government Printing Office.
von Oettingen, W.F., P.A.Neal, R.F.Sievers, J.L.Svirbely, A.R.Monaco, B.L. Horecker, H.Yagoda, T.R.Sweeney, D.C.Peterson, W.C.Alford, V.B.Hauff, and H.Gay. 1947. Xylidine (C,C-dimethylaniline): Its toxicity and potential dangers as compared with those of aniline and an appraisal of the potential hazards from its use in blending gasoline. National Institutes of Health Bulletin No. 188. National Institutes of Health, Bethesda, MD.
Ward, E., A.Carpenter, S.Markowitz, D.Roberts and W.Halperin. 1991. Excess number of bladder cancers in workers exposed to ortho-toluidine and aniline. J. Natl. Cancer Inst. 83:501–506.
Westmoreland, C. and D.G.Gatehouse. 1991. Effects of aniline hydrochloride in the mouse bone marrow micronucleus test after oral administration. Carcinogenesis 12:1057–1059.
Wilmer, J.L., A.D.Kligerman, and G.L.Erexson. 1981. Sister chromatid exchange induction and cell cycle inhibition by aniline and its metabolites in human fibroblasts. Environ. Mutagen. 3:627–638.
APPENDIX A
CARCINOGENICITY ASSESSMENT FOR ANILINE
No inhalation slope factor is available for aniline, and the available inhalation studies did not examine the endpoint of carcinogenicity. Based on the chronic oral administration of aniline hydrochloride to CD-F rats (CIIT 1982), U.S. EPA in its Integrated Risk Information Systems (IRIS) has estimated an oral slope factor of 5.7×10P–3/mg/kg/d (U.S. EPA 1994). In that study, spleen tumor incidences in rats administered 0, 200, 600, or 2,000 ppm in the diet were 0/64, 0/90, 1/90, and 31/90, respectively. Aniline also has genotoxic action.
The inhalation slope factor can be estimated by dividing the oral slope factor by 70 kg and multiplying by the inhalation rate of 20 m3/d:
Inhalation slope factor=oral slope factor×1/70 kg×20 m3/d
=5.7×10–3/mg/kg/d×1/70 kg×20 m3/d
=1.6×10–3/mg/m3.
To convert to a dose or concentration of aniline that would cause an excess cancer risk of 10–4 (a virtually safe dose), the risk is divided by the slope factor:
dose=risk/slope
dose=(risk of 1×10–4)/(1.6×10–3 (mg/m3)–1)
=6.3×10–2 mg/m3.
To convert a 70-y exposure to a 24-h exposure, the virtually safe dose is multiplied by the number of days in 70 yr:
24-h exposure=6.3×10–2 mg/m3×25,600 d
=1,613 mg/m3.
To adjust for uncertainties in assessing potential cancer risks for short-term exposures under the multistage model, the 24-h exposure is divided by an adjustment factor of 6 (Crump and Howe 1984).
(1,613 mg/m3)/6=269 mg/m3 (71 ppm).
The 24-h exposure can be converted to the shorter AEGL time points:
24-h exposure=269 mg/m3 (71 ppm)
8-h exposure=806 mg/m3 (212 ppm)
4-h exposure=1,613 mg/m3 (425 ppm)
1-h exposure=6,451 mg/m3 (1698 ppm)
30-min exposure=12,900 mg/m3 (3395 ppm).
For 10–5 and 10–6 risk levels, the 10–4 values are reduced by 10-fold and 100-fold, respectively. Because the cancer risk from a short-term exposure to aniline at the AEGL concentrations is estimated to be well below 1 in 10,000, even for individuals at a sensitive age, the AEGL values are based on the more stringent requirements for methemoglobin formation.
APPENDIX B
DERIVATION OF AEGL VALUES
Derivation of AEGL-1
|
Key study: |
Kim and Carlson (1986) |
|
Toxicity endpoint: |
Methemoglobin level of 22% at exposure of 100 ppm for 8 h |
|
Scaling: |
C1×t=k, based on the linear relationship between concentration of aniline and methemoglobin formation |
|
Uncertainty factors: |
10 for interspecies 10 for intraspecies |
|
Calculations: |
100 ppm/(10×10)=1 ppm C1×t=k 1 ppm×480 min=480 ppm·min |
|
30-min AEGL-1: |
480 ppm·min/30 min=16 ppm |
|
1-h AEGL-1: |
480 ppm·min/60 min=8.0 ppm |
|
4-h AEGL-1: |
480 ppm·min/240 min=2.0 ppm |
|
8-h AEGL-1: |
480 ppm·min/480 min=1.0 ppm |
Derivation of AEGL-2
|
Key study: |
Kim and Carlson (1986) |
|
Toxicity endpoint: |
Methemoglobin level of 41% at exposure of 150 ppm for 8 h |
|
Scaling: |
C1×t=k, based on the linear relationship between concentration of aniline and methemoglobin formation |
|
Uncertainty factors: |
10 for interspecies 10 for intraspecies |
|
Calculations: |
150 ppm/(10×10)=1.5 ppm C1×t=k 1.5 ppm×480 min=720 ppm·min |
|
30-min AEGL-1: |
720 ppm·min/30 min=24 ppm |
|
1-h AEGL-1: |
720 ppm·min/60 min=12 ppm |
|
4-h AEGL-1: |
720 ppm·min/240 min=3.0 ppm |
|
8-h AEGL-1: |
720 ppm·min/480 min=1.5 ppm |
Derivation of AEGL-3
|
Key study: |
Kim and Carlson (1986) |
|
Toxicity endpoint: |
Projected methemoglobin level of 70–80% at exposure of 250 ppm for 8 h |
|
Scaling: |
C1×t=k, based on the linear relationship between concentration of aniline and methemoglobin formation |
|
Uncertainty factors: |
10 for interspecies 10 for intraspecies |
|
Calculations: |
250 ppm/(10×10)=2.5 ppm C1×t=k 2.5 ppm×480 min=1,200 ppm·min |
|
30-min AEGL-1: |
1,200 ppm·min/30 min=40 ppm |
|
1-h AEGL-1: |
1,200 ppm·min/60 min=20 ppm |
|
4-h AEGL-1: |
1,200 ppm·min/240 min=5.0 ppm |
|
8-h AEGL-1: |
1,200 ppm·min/480 min=2.5 ppm |
APPENDIX C
DERIVATION SUMMARY FOR ACUTE EXPOSURE GUIDELINE LEVELS FOR ANILINE (CAS No. 62–53–3)
|
AEGL-1 Values-Aniline |
|||
|
30 min |
1 h |
4 h |
8 h |
|
16 ppm |
8.0 ppm |
2.0 ppm |
1.0 ppm |
|
Key reference: Kim, Y.C., and G.P.Carlson. 1986. The effect of an unusual workshift on chemical toxicity. II. Studies on the exposure of rats to aniline. Fundam. Appl. Toxicol. 7:144–152 |
|||
|
Test Species/Strain/Number: Adult male Sprague-Dawley rats, 5/exposure group |
|||
|
Exposure Route/Concentrations/Durations: Inhalation: 0–150 ppm for 8 h |
|||
|
Effects: |
Concentration (ppm) |
Methemoglobin Formation (%)a |
|
|
|
0 |
1.1 (0.4–1.7) |
|
|
|
10 |
1.1 (0.4–1.7) |
|
|
|
30 |
1.6 |
|
|
|
50 |
4.7 |
|
|
|
100 |
22 |
|
|
|
150 |
41 |
|
|
aValues are estimates from data presented as graphs. |
|||
|
Endpoint/Concentration/Rationale: The only effect of aniline administration was formation of methemoglobin. Administration of 100 ppm for 8 h to rats resulted in elevation of methemoglobin to 22% but no hypoxic signs. A review of the literature revealed that methemoglobin levels of 15–20% in humans result in clinical cyanosis but no hypoxic symptoms. This effect was considered to be mild and reversible and, therefore, within the definition of the AEGL-1. The 8-h exposure to 100 ppm was chosen as the basis for the AEGL-1 calculations. Uncertainty Factors/Rationale: Total uncertainty factor: 100 Interspecies: 10—A review of oral administration studies suggested that humans may be considerably more sensitive to methemoglobin formation than rats. Oral administration of aniline to rats at 40 mg/kg produced a maximum increase of 16.6% in methemoglobin, whereas oral administration of 0.9 mg/kg to a human volunteer produced a maximum increase of 16.1%. |
|
Intraspecies: 10—Infants are more sensitive to methemoglobin-generating chemicals than adults, because they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase), and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). |
|
Modifying Factor: Not applicable |
|
Animal to Human Dosimetric Adjustment: Not applied; insufficient data. |
|
Time Scaling: Cn×t=k, where n=1 and k=480 ppm·min; based on the linear relationship between concentration and methemoglobin formation (Kim and Carlson 1986) |
|
Data Adequacy: The key study was well designed, conducted, and documented. Values were presented graphically. Supporting data were sparse, probably because aniline is not a vapor at room temperature, and poisonings have involved contact with the liquid. Although human data are sparse, it is believed that a total uncertainty factor of 100 is protective of human health. Because aniline is absorbed through the skin, which increases the systemic toxicity, direct skin contact with the liquid would be additive and result in onset of adverse effects at airborne concentrations below the respective AEGL values. Therefore, direct skin contact with the liquid should be avoided. |
|
AEGL-2 Values-Aniline |
|||
|
30 min |
1 h |
4 h |
8 h |
|
24 ppm |
12 ppm |
3.0 ppm |
1.5 ppm |
|
Key reference: Kim, Y.C., and G.P.Carlson. 1986. The effect of an unusual workshift on chemical toxicity. II. Studies on the exposure of rats to aniline. Fundam. Appl. Toxicol. 7:144–152 |
|||
|
Test Species/Strain/Sex/Number: Adult male Sprague-Dawley rats, 5/exposure group |
|||
|
Exposure Route/Concentrations/Durations: Inhalation: 0–150 ppm for 8 h |
|||
|
Effects: |
Concentration (ppm) |
Methemoglobin Formation (%)a |
|
|
|
0 |
1.1 (0.4–1.7) |
|
|
|
10 |
1.1 (0.4–1.7) |
|
|
|
30 |
1.6 |
|
|
|
50 |
4.7 |
|
|
|
100 |
22 |
|
|
|
150 |
41 |
|
|
aValues are estimates from data presented as graphs. |
|||
|
Endpoint/Concentration/Rationale: Administration of 150 ppm for 8 h to rats resulted in elevation of methemoglobin to 41% with no reported toxic signs. A review of the literature revealed that methemoglobin levels of 30–45% in humans are associated with fatigue, lethargy, exertional dyspnea, and headache. These signs and symptoms were considered the threshold for disabling effects. The 8-h exposure to 150 ppm was chosen as the basis for the AEGL-2 calculations. |
|
Uncertainty Factors/Rationale: Total uncertainty factor: 100 Interspecies: 10—A review of oral administration studies suggested that humans may be considerably more sensitive to methemoglobin formation than rats. Oral administration of aniline to rats at 40 mg/kg produced a maximum increase of 16.6% in methemoglobin, whereas oral administration of 0.9 mg/kg to a human volunteer produced a maximum increase of 16.1%. Intraspecies: 10—Infants are more sensitive to methemoglobin-generating chemicals than adults, because they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase), and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). |
|
Modifying Factor: Not applicable |
|
Animal to Human Dosimetric Adjustment: Not applied; insufficient data |
|
Time Scaling: Cn×t=k, where n=1 and k=720 ppm·min; based on the linear relationship between concentration and methemoglobin formation (Kim and Carlson 1986) |
|
Data Adequacy: The key study was well designed, conducted, and documented. Values were presented graphically. Supporting data were sparse, probably because aniline is not a vapor at room temperature, and poisonings have involved contact with the liquid. Although human data are sparse, it is believed that a total uncertainty factor of 100 is protective of human health. Because aniline is absorbed through the skin, which increases the systemic toxicity, direct skin contact with the liquid would be additive and result in onset of adverse effects at airborne concentrations below the respective AEGL values. Therefore, direct skin contact with the liquid should be avoided. |
|
AEGL-3 Values-Aniline |
|||
|
30 min |
1 h |
4 h |
8 h |
|
40 ppm |
20 ppm |
5.0 ppm |
2.5 ppm |
|
Key reference: Kim, Y.C., and G.P.Carlson. 1986. The effect of an unusual workshift on chemical toxicity. II. Studies on the exposure of rats to aniline. Fundam. Appl. Toxicol. 7:144–152 |
|||
|
Test Species/Strain/Sex/Number: Adult male Sprague-Dawley rats, 5/exposure group |
|||
|
Exposure Route/Concentrations/Durations: 0–150 ppm for 8 h |
|||
|
Effects: |
Concentration (ppm) |
Methemoglobin Formation (%)a |
|
|
|
0 |
1.1 (0.4–1.7) |
|
|
|
10 |
1.1 (0.4–1.7) |
|
|
|
30 |
1.6 |
|
|
|
50 |
4.7 |
|
|
|
100 |
22 |
|
|
|
150 |
41 |
|
|
aValues are estimates from data presented as graphs. |
|||
|
Endpoint/Concentration/Rationale: Because the exposures did not result in effects consistent with the definition of an AEGL-3, the concentration vs percent hemoglobin formation data presented by the authors was graphed and projected to a methemoglobin level of 70–80%, which was considered the threshold for lethality in humans. This value was approximately 250 ppm. An 8-h exposure to 250 ppm was chosen as the basis for the AEGL-3 calculations. |
|
Uncertainty Factors/Rationale: Total uncertainty factor: 100 Interspecies: 10—A review of oral administration studies suggested that humans may be considerably more sensitive to methemoglobin formation than rats. Oral administration of aniline to rats at 40 mg/kg produced a maximum increase of 16.6% in methemoglobin, whereas oral administration of 0.9 mg/kg to a human volunteer produced a maximum increase of 16.1%. Intraspecies: 10—Infants are more sensitive to methemoglobin-generating chemicals than adults, because they have reduced levels of nicotine adenine dinucleotide (NADH, the cofactor (electron donor) for methemoglobin reductase), and a high concentration of fetal hemoglobin in their erythrocytes (fetal hemoglobin is more oxidizable than adult hemoglobin) (Seger 1992). |
|
Modifying Factor: Not applicable |
|
Animal to Human Dosimetric Adjustment: Not applied; insufficient data |
|
Time Scaling: Cn×t=k, where n=1 and k=1,200 ppm·min; based on the linear relationship between concentration and methemoglobin formation (Kim and Carlson 1986) |
|
Data Adequacy: The key study was well designed, conducted, and documented. Values were presented graphically. Supporting data were sparse, probably because aniline is not a vapor at room temperature and poisonings have involved contact with the liquid. Although human data are sparse, it is believed that a total uncertainty factor of 100 is protective of human health. Because aniline is absorbed through the skin, which increases the systemic toxicity, direct skin contact with the liquid would be additive and result in onset of adverse effects at airborne concentrations below the respective AEGL values. Therefore, direct skin contact with the liquid should be avoided. |


















































