Page 10
2
Climate, Atmospheric Chemistry, and Global Air Quality
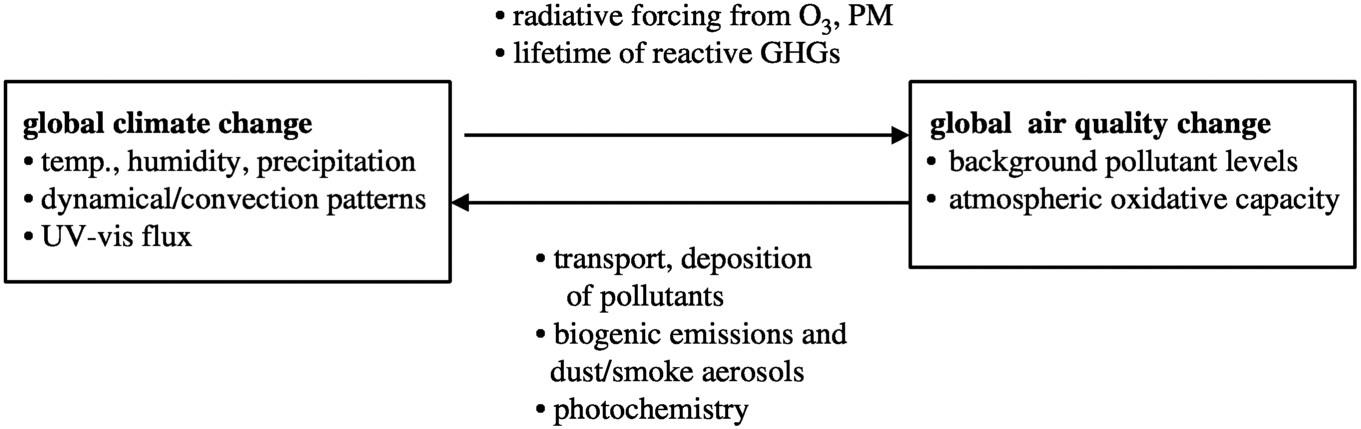
There are numerous linkages among the chemical and physical processes that affect climate change and air quality. Examples of climate/chemistry interactions that must be considered as part of any comprehensive assessment of future atmospheric evolution are discussed in the following list and summarized in Figure 2-1 . Some of these linkages have been the subject of intensive research efforts, while others are still largely unexplored and highly uncertain.
Impacts of Climate Change on Tropospheric / Stratospheric Chemistry
-
Changes in temperature and humidity patterns resulting from global climate change can directly affect the concentrations of many important tropospheric chemical species. For instance, increases in temperature accelerate the rates of reactions involved in the production of tropospheric ozone (e.g., Johnson et al., 1999). At the same time, increases in atmospheric water vapor can affect both the chemical production and the chemical destruction of tropospheric ozone (Stevenson et al., 2000), and thus the net impact of these concurrent changes is difficult to assess.
-
The atmospheric accumulation of greenhouse gases has led to cooling of the stratosphere. This, in turn, may prolong the recovery of the stratospheric ozone layer, since colder winters and more stable polar vortices generally increase polar ozone losses (Shindell et al., 1998). This may also affect stratospheric circulation and alter the lifetime of greenhouse gases such as N2O and CFCs, which are destroyed primarily in the strato-
Page 11
~ enlarge ~
FIGURE 2-1 A schematic summary of the feedbacks between climate change and air quality change.
sphere. Depressed stratospheric ozone levels increase the flux of ultraviolet radiation to the troposphere; under conditions of high ambient NOx levels, this can increase tropospheric OH/HO2 and lead to increased tropospheric ozone production.
Within the troposphere, changes in the vertical temperature structure may affect the evolution and dynamics of the boundary layer and associated mixing processes that influence the dispersion and transport of air pollutants. Changes in the thermal structure of the troposphere and stratosphere may also affect the location of the tropopause and cross-tropopause transport of ozone and its precursors (IPCC, 1996). 1
Impacts of Climate Change on Emissions of Greenhouse Gases and Aerosol/Ozone Precursors
-
Climate can affect the emissions of many biogenic compounds that play an influential role in tropospheric chemistry. For example, changes in temperature, soil moisture, and solar radiation can all lead to changes in the emission of ozone precursors such as isoprene and terpenes (Tingley et al., 1979; Lamb et al., 1985). Changes in temperature could also affect biological activity in the oceans and the resulting emissions of halogenated hydrocarbons such as methyl bromide, and sulfur compounds such as dimethyl sulfide.
1 This reference is to the IPCC's second assessment report. The IPCC's third assessment report is in press and will be available by late 2001. The Summary for Policymakers is currently available online at www.ipcc.ch .
Page 12
Changes in temperature can affect the emission of methane from high-latitude wetlands. As an example, the temporary cooling of the troposphere after the eruption of Mount Pinatubo led to a dramatic decrease in the global methane growth rate, thought to be due in part to a decrease in natural methane emissions (WMO, 1999).
Widespread climatic changes would likely alter the patterns of human activities such as agriculture, biomass burning, and energy consumption (e.g., demand for heating/air conditioning), and thus would affect the emissions of pollutant gases and particles that result from these activities. Likewise, changes in land-use activities and patterns of fires and droughts could influence the level of smoke and mineral dust aerosols in the atmosphere.
Impacts of Atmospheric Chemical Changes on Climate
-
Increases in the atmospheric burden of greenhouse gases (including H2O, O3, CO2, CH4, N2O, and CFCs) can change the earth's radiative balance and the temperature structure of the atmosphere. The potential climatic impacts of greenhouse gas radiative forcing are discussed extensively in international assessment reports such as IPCC (1996) and WMO (1999).
-
Atmospheric aerosols affect climate by scattering and absorbing ultraviolet and visible (UV-Vis) radiation and by altering the formation processes, optical properties, and precipitation efficiency of clouds. Some studies find that pollution aerosols augment cloud albedo and thus exert a cooling influence (Albrecht, 1989; Pincus and Baker, 1994), while other studies find that the dark haze caused by pollution aerosols absorb solar radiation and reduce certain types of cloud coverage (Ackerman et al., 2000). The net impacts of these forcings are highly uncertain because they are dependent upon aerosol composition, and geographical and vertical distribution (IPCC, 1996; NRC, 1996).
-
Large-scale air pollution emissions can significantly affect the concentration of the atmosphere's primary oxidizing agent, the hydroxyl radical (OH). Net changes are difficult to project, however, since increases in NOx tend to elevate OH levels, while increases in CO tend to decrease OH. Large-scale changes in OH, in turn, affect the lifetime and thus the atmospheric abundance of reactive greenhouse gases such as CH4, HFCs, and HCFCs.
The preceding list is not a comprehensive description of all the potentially important linkages between climate and atmospheric chemistry; it is only meant
Page 13
Box 2-1Policy Responses to Global Climate Change
The Kyoto Protocol to the United Nations Framework Convention on Climate Change, adopted in 1997, includes commitments that would limit the emissions of greenhouse gases from industrialized countries to an average of 95% of 1990 levels during the period 2008 through 2012. If the Protocol is ratified by enough countries to enter into force, it would control emissions of carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6). Each Party to the Protocol would have flexibility to select its own mix of control strategies and relative emphasis on each of the controlled greenhouse gases. CO2 is the dominant greenhouse gas emitted by most countries, and thus controlling CO2 emissions is an essential component of any effective strategy for stabilizing radiative forcing. At the same time, non-CO2 GHGs have much higher global warming potentials per unit mass and are also an important target for emission reductions. Tropospheric ozone and PM (in particular, black carbon aerosols) also contribute to global radiative forcing, but control of these species and their precursors is not currently included in the provisions of the Kyoto Protocol. Ozone and PM could be particularly effective targets for emission control efforts, however, as recently underscored by Hansen et al. (2000), since many countries already have domestic regulations that aim to control these species, and since reducing ozone and PM emissions can not only aid in efforts to control global climate, but also can improve local air quality, health, and agricultural productivity. |
to demonstrate the fact that these linkages are complex and involve nonlinear couplings among numerous processes. For example, the atmospheric concentrations and distributions of two important greenhouse gases, O3 and CH4, are affected by anthropogenic and biogenic emissions of NOx, VOCs, and CO, by the tropospheric oxidative capacity (in particular, OH concentrations), and by climatic parameters such as temperature, humidity, and UV flux. Because many of these parameters can change simultaneously, predicting associated atmospheric chemical changes is a complex and scientifically challenging task.
Improving our understanding of the interactions between climate and air quality will depend primarily on developing more sophisticated modeling tools; in particular, it will require the ability to couple local- and regional-scale air quality models (which cover spatial scales of a few hundred meters to hundreds of kilometers) with global-scale climate and chemistry models. In recent years there has been significant progress in developing these types of coupled models. However, one of the primary constraints in developing and testing such models is the lack of comprehensive observational databases, and thus strengthening our long-term observational capabilities emerges as an imperative.




