Page 166
11
Commodity Polymers from Renewable Resources: Polylactic Acid
Patrick R. Gruber
Cargill Dow LLC
Of all of the potential products made from annually renewable resources, polylactic acid (PLA) is currently the most interesting. PLA has the properties and value to create a viable business opportunity that will enable Cargill Dow to create new market opportunities. These new markets value material properties, as well as environmental attributes.
In business, perceptions of commercial opportunity drive investment. In research, I believe the same to be true. Cargill Dow LLC is building a world-scale manufacturing facility for PLA with a capacity of 300 million pounds a year at a cost of several hundred million dollars. This investment was made because (1) the product performance is adequate and (2) the price delivered to the marketplace is reasonable. The fortunate consequence is that because of these two factors, customers are lining up to buy the PLA. 1 We had a very strong opinion that our products must provide value as a material. As a result, we invested tens of millions of dollars and focused our R&D to discover this value. While it is true that our products have interesting environmental benefits, these benefits alone do not drive investment in either research or manufacturing facilities. There is an important principle here. Investment to solve CO2 in the atmosphere ought to focus on products that add value to society—meaning products that work well, are competitively priced, and address the CO2 problem. PLA provides the first real-world example that does all of these things in this new market of renewable resource-based chemical products.
However, many questions are being asked about products made from renewable resources. Can products with the performance of conventional thermoplastics actually be made economically? Do these materials really have the performance required? Are these products really more environmentally friendly and how do you know? What are the issues? This chapter addresses those questions and draws generalizations that can be applied to other chemical products made from renewable resources.
MANUFACTURING OF PLA
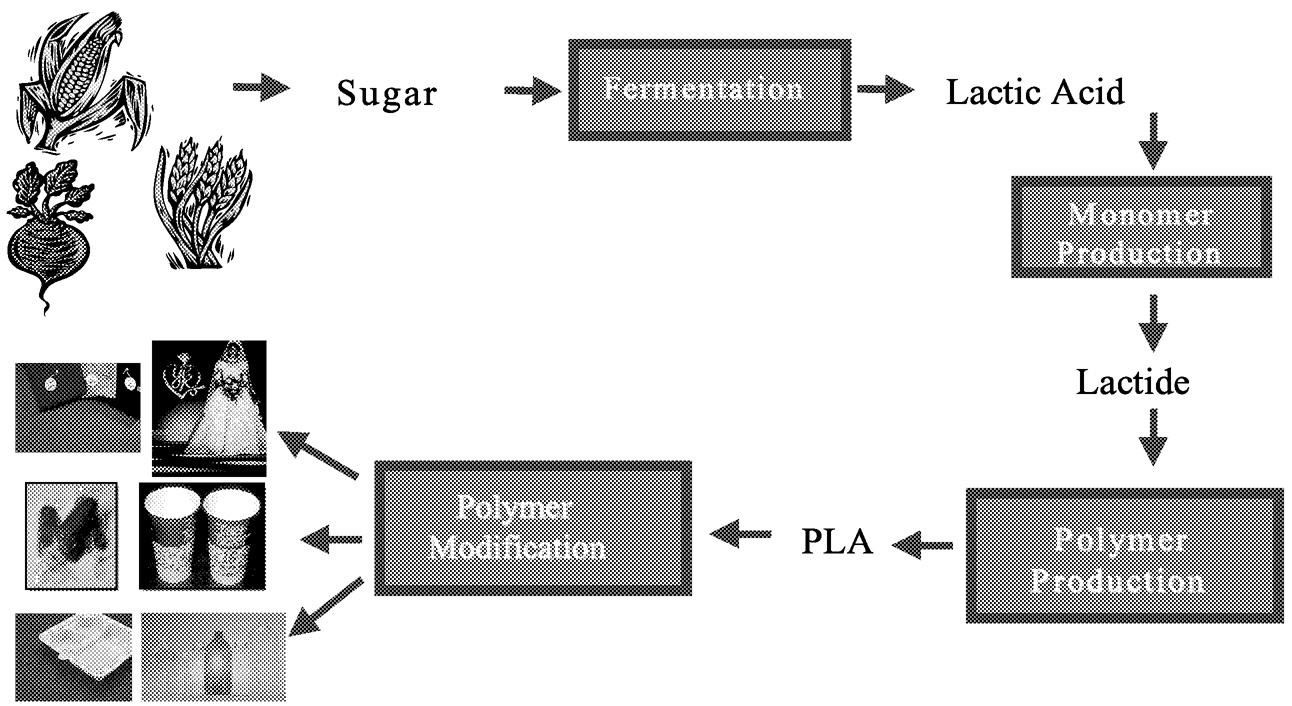
The raw material for PLA manufacturing is any fermentable sugar. The cheapest and most abundant source of sugar in the world is currently corn. Hence, Cargill Dow LLC (CD) is planning to start with
Page 167
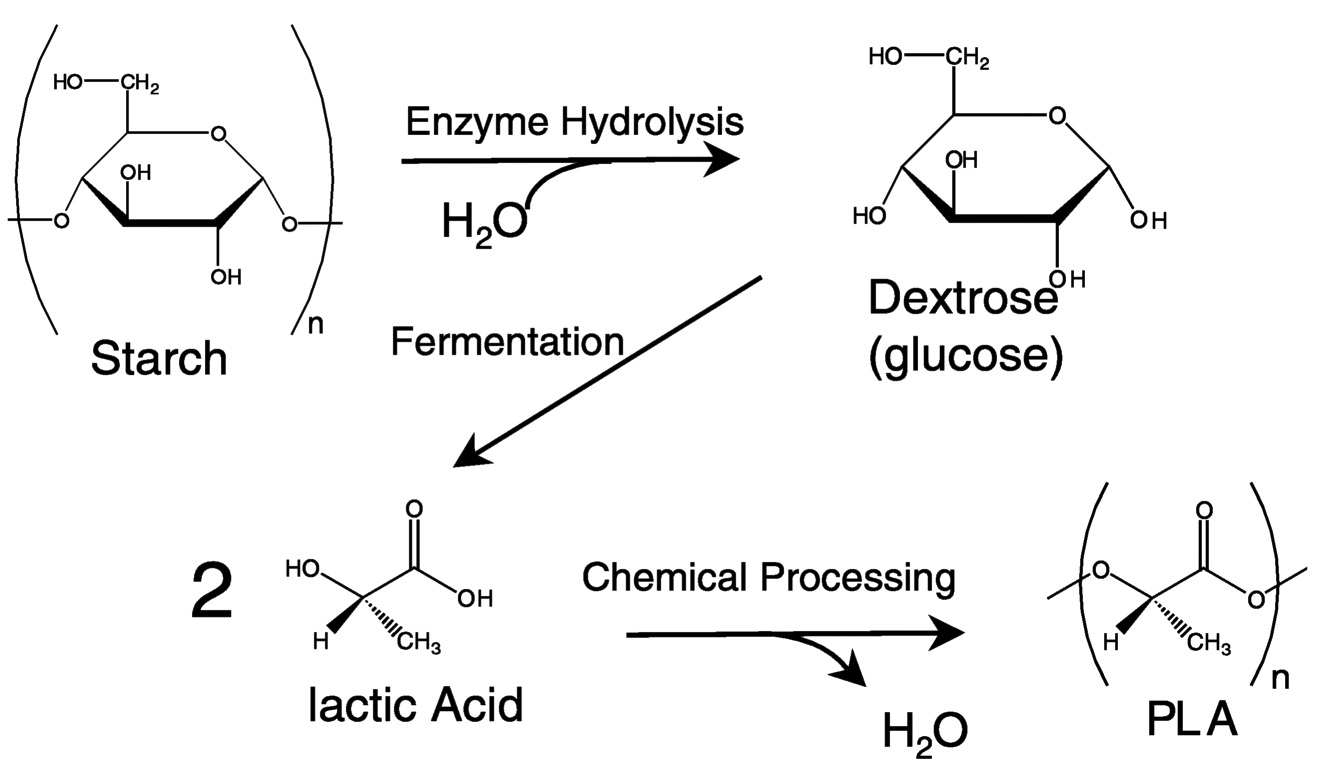
corn. In the long run, I expect that as technologies geared toward biomass-to-sugar conversion mature, CD will use these technologies to generate fermentable feedstocks. Figure 11.1 and Figure 11.2 outline the manufacturing steps of PLA. Carbon dioxide is fixed in crops to make starches. Agriprocessing businesses like corn wet-milling convert the starches to simple sugars. CD buys the sugar and uses it to ferment lactic acid. Using chemical processing techniques, lactic acid is converted efficiently to lactide, a ring-form dimer of lactic acid. Lactides are excellent polymerization starting materials because they are reactive and anhydrous, and they polymerize in the melt. PLA is made through ring-opening melt polymerization. The overall process is sufficiently efficient in terms of yield and energy that the products are economically viable.
Market Opportunities
PLA currently finds demand in three market areas: fibers, packaging, and chemical products. Significant research investment in product development has revealed product attributes that are valuable and the knowledge of how to best to use them in the marketplace. Fibers and packaging provide the strongest examples of how PLA attributes bring value to a market.
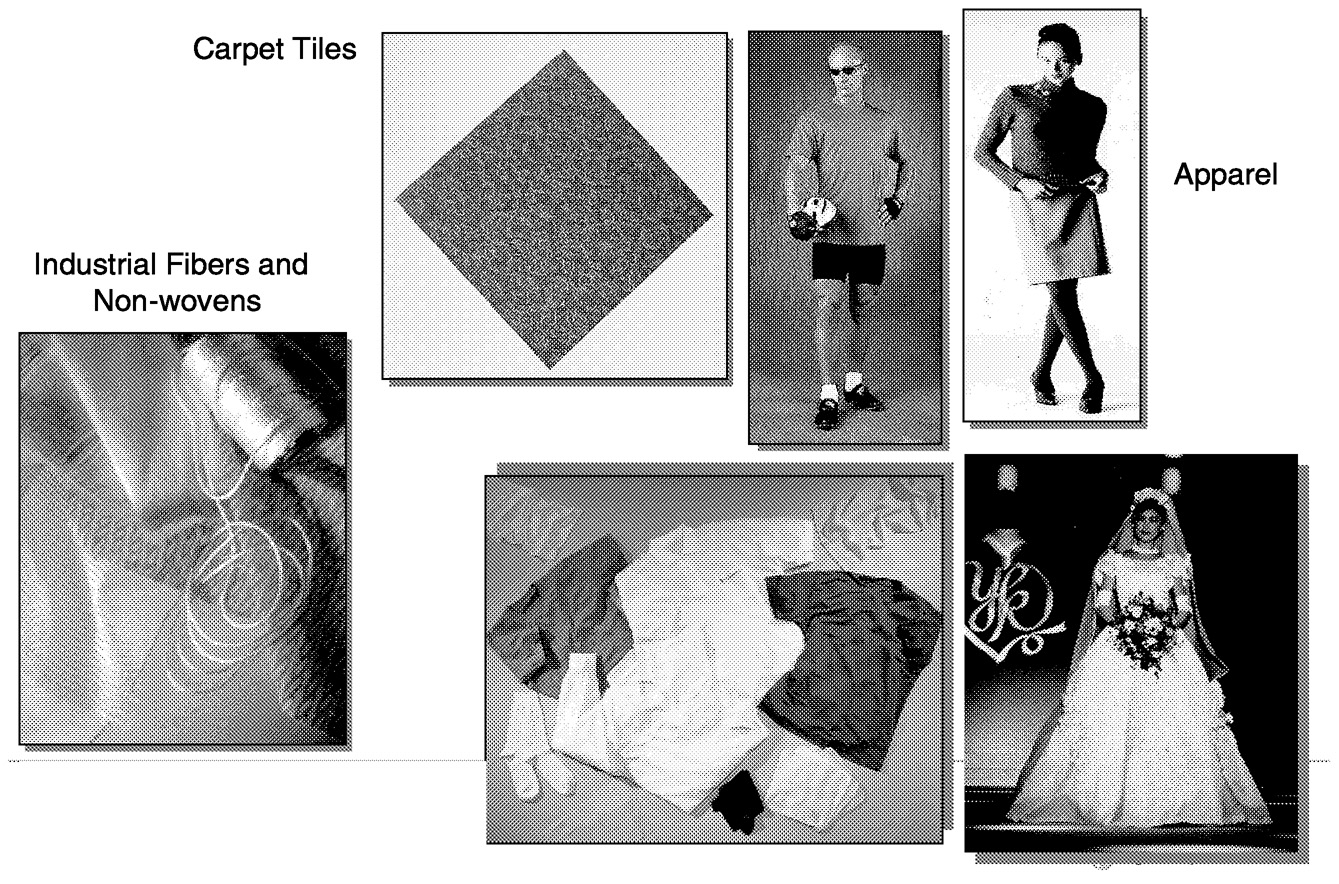
PLA fibers combine the comfort and feel of natural fibers with the performance of synthetics ( Table 11.1). The unique property spectrum of PLA fibers allows the creation of products with superior hand and touch, drape, comfort, moisture management, ultraviolet (UV) resistance, and resilience. Combining these performance features with the features of natural fibers enables PLA to be used in a wide spectrum of products including apparel, carpet, nonwoven fiberfill, and household and industrial markets ( Figure 11.3).
PLA apparel, carpets, and nonwovens are already in test market. Consumers' reports indicate that the products actually work well, and they appreciate the products being made from renewable resources. Of course when consumers indicate that they appreciate a product made from renewable resources, they expect that there should be some measurable advantage regarding the environment compared to traditional petroleum-based products.
PLA Packaging
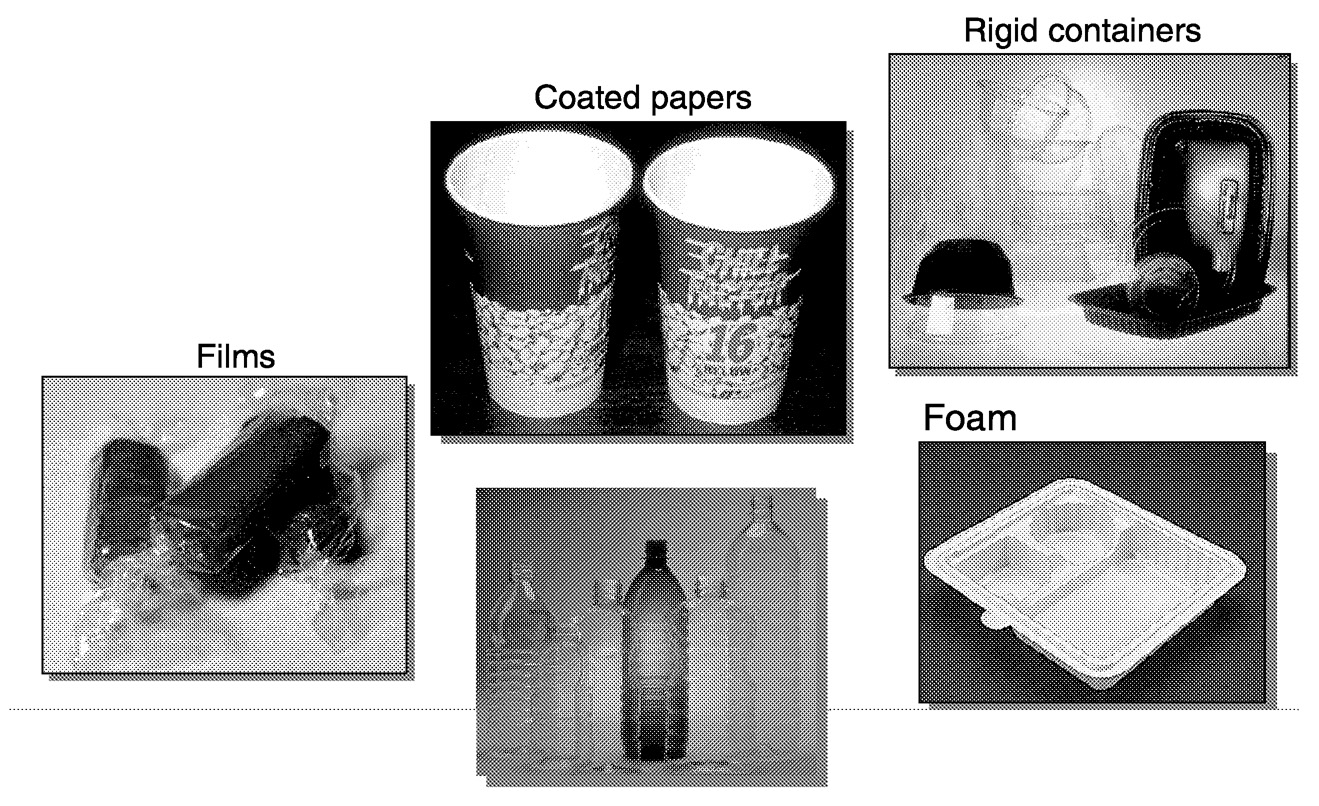
PLA polymers for packaging applications exhibit a balance of performance properties that are comparable and in certain cases superior, to traditional thermoplastics. PLA is useful in coated paper, films, rigid containers, bottles, and a variety of other packaging applications ( Figure 11.4). However, there are two specific packaging areas that have received initial focus—high-value films and rigid thermoformed containers. Functional properties and their benefits are listed in Table 11.2.
The combination of functional properties provides the commercial drive for PLA. A close look at the properties listed in Table 11.2 reveals that the technical attributes primarily benefit manufacturers and converters. The exceptions, renewable resources and compostability, are end-user and consumer-oriented attributes.
The market development for packaging is quite different than for fibers. PLA fibers benefit the consumer directly. For example, not only are the products—a shirt, for example—more comfortable (I can detect it myself as a consumer), they are also made starting from a natural product (a perception). So in fibers, the combination of direct consumer benefit and easily communicated perceptions helps to drive the potential of PLA.
In packaging market segments, consumers' concern for the environment has driven manufacturers to want to adopt new technologies. Led by Europe and Japan where environmental concerns receive a
Page 168
~ enlarge ~
FIGURE 11.1 Overview of manufacturing processes for PLA.
higher priority than in the United States, converters and manufacturers are actively developing packaging products with improved environmental performance.
In contrast to fibers, with packaging market segments consumers will probably not directly detect many of the technical attributes and benefits. Although it is true that PLA can make a better package, consumers don't buy packages. They buy the products in the packages. We find, however, that consumers expect that if technology exists to make a package more “environmentally friendly,” companies should use it—as long as it doesn't increase the price of the product too much. This market insight is critical when investing in technology aimed at environmental attributes. Being environmentally friendly is worthwhile, but only at a certain cost, and it must provide clear benefit.
I believe this insight should be extrapolated to the type of research investment necessary to resolve environmental problems. The answers to the questions, What is the measurable benefit to consumers? and How much should be spent? depend on the benefit provided. Research for CO2 in the atmosphere
~ enlarge ~
FIGURE 11.2 The processing route to PLA combines bioprocessing and chemical processing
Page 169
|
Synthetics |
PLA |
Natural Fiber |
||||||
|
Fibers Property |
Nylon 6 |
PET |
Acrylics |
PLA |
Rayon |
Cotton |
Silk |
Wool |
|
Specific gravity |
1.14 |
1.39 |
1.18 |
1.25 |
1.52 |
1.52 |
1.34 |
1.31 |
|
Tenacity (g/d) |
5.5 |
6.0 |
4.0 |
6.0 |
2.5 |
4.0 |
4.0 |
1.6 |
|
Moisture regain (%) |
4.1 |
0.2-0.4 |
1.0-2.0 |
0.4-0.6 |
11 |
7.5 |
10 |
14-18 |
|
Elastic recovery (5% strain) |
89 |
65 |
50 |
93 |
32 |
52 |
52 |
69 |
|
Flammability |
Medium |
High smoke |
Medium |
Low smoke |
Burns |
Burns |
Burns |
Burns slowly |
|
UV resistance |
Poor |
Fair |
Excellent |
Excellent |
Poor |
Fair-poor |
Fair-poor |
Fair |
|
Wicking (L-W slope; higher slope, more wicking) |
— |
0.7-0.8 (no finish) |
— |
6.3-7.5 (no finish) 19-26 (with finish) |
— |
— |
NOTE: g/d=grams per denier; L-W=Lucas-Washburn Equations; PET=poly(ethyleneterphalate).
should take a product approach. Products must provide value to consumers. The product could be a reforested desert wasteland or it could be PLA—these are tangible to the people who pay—the consumers.
Market Potential
In the long term, PLA can compete successfully in several markets with an annual volume of more than 6.6 billion pounds. With technology improvements in manufacturing and processing, these markets
~ enlarge ~
FIGURE 11.3 Typical PLA fiber applications.
Page 170
~ enlarge ~
FIGURE 11.4 Examples of PLA packaging applications.
could expand to about 10 billion pounds of PLA annually. The market value of annually renewable resource-based thermoplastics from PLA would be at least $6 billion to $10 billion per year.
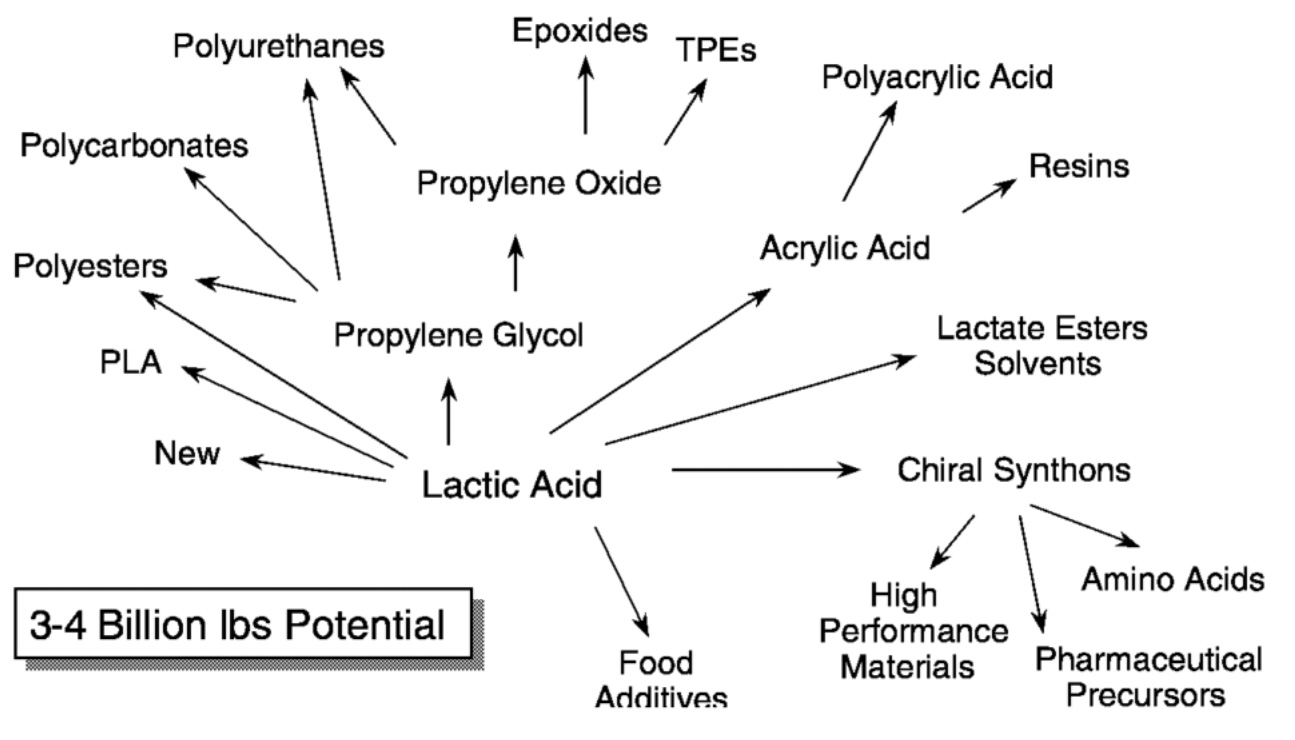
In addition, lactic acid can serve as a chemical intermediate. As our scale increases and the costs of the lactic acid manufacturing process are reduced, we expect that lactic acid will be inexpensive enough to enable several other end markets in the chemical industry. This concept is illustrated in Figure 11.5, which shows the wide variety of chemicals other than PLA that could be made from lactic acid. These chemicals add an additional 3 billion to 4 billion pounds and market value of $1 billion to $4 billion per year to the estimated PLA value.
Why didn't CD initially target traditional chemicals based on renewable resources? The answer is simple: we couldn't convince ourselves that the research investment was justified because the target markets were not big enough. We could not create a hypothetical process that would produce lactic acid cheaply enough to create the profit necessary to justify the research funding. We instead developed a
|
Functional Property |
Packaging Improvement |
|
Deadfold, twist, and crimp |
Improved folding and sealing |
|
High gloss & clarity |
Package aesthetics |
|
Barrier properties |
Grease and oil resistance |
|
Renewable resource |
Made from CO2 |
|
Flavor and aroma |
Reduced taste and odor issues |
|
Low-temperature heat seal |
Stronger seals at lower temperatures |
|
High tensile and modulus |
Wet paper strength, ability to down-gauge |
|
Low COF, polarity |
Printability |
|
GRAS status |
Food contact approved |
|
Compostable |
Compostable, low “green” tax |
NOTE: COF=Coefficient of Friction; GRAS=Generally Recognized As Safe.
Page 171
~ enlarge ~
FIGURE 11.5 Chemicals that could be made from lactic acid.
new product (PLA) in a value-added, large market category that, if it succeeds, allows scale and scope economies. This approach combines more risk, more reward, more complicated market development, and more rapid advancement of our knowledge.
The commodity chemical market dictates that value is created when performance is equal at lower cost. When faced with a project choice where the target molecule is an exact replacement of a commodity, I look for technology that provides at least a 50% cost reduction or the project is not worth pursuing. I've reviewed many proposals where process research work is being justified because it is more environmentally friendly—usually meaning lower energy and/or CO2 emissions—but at a process economic cost close to the incumbent commodity product. This strikes me as underachieving. With modern biotechnology, bioprocessing, and chemical processing technology, more focus should be on creating environmentally friendly processes and 50% cost reductions. The lesson is that just because something is made from renewable resources does not make it better. It must provide value in the marketplace.
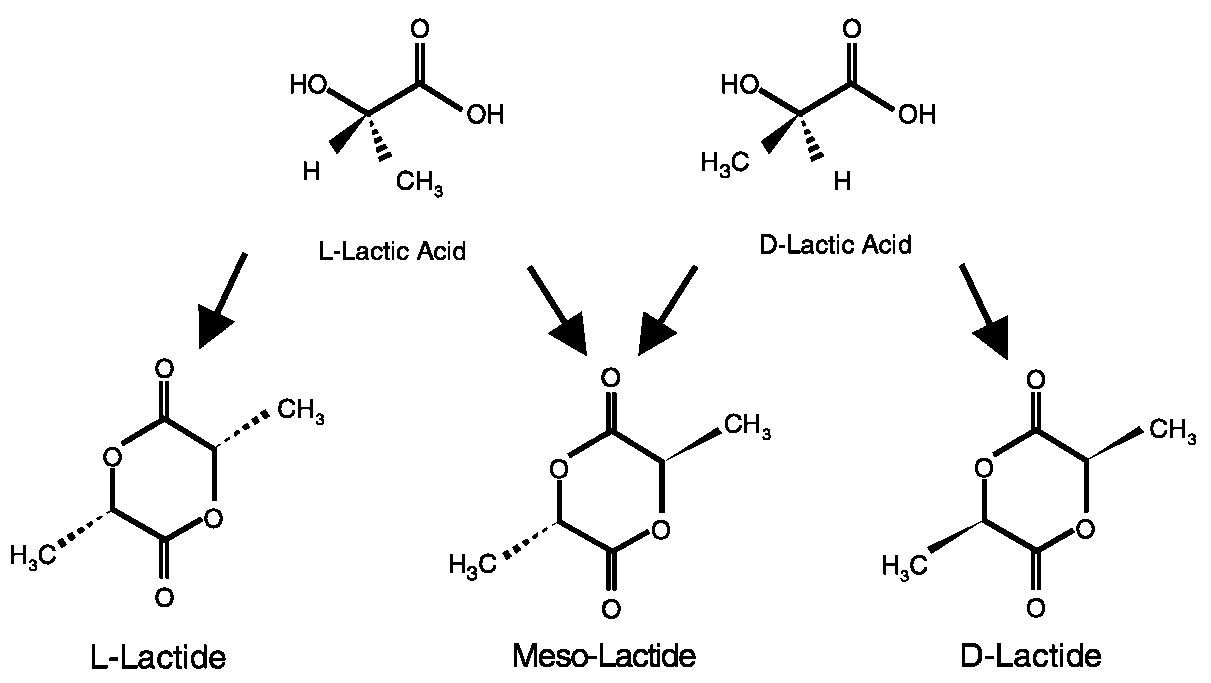
PLA takes advantage of a biological system to do chemistry that traditional chemical techniques can't. Fermentation of sugar to lactic acid does something that we cannot do by chemistry, namely, produce chiral lactic acid in high yield. Figure 11.6 shows the optical isomers of lactic acid.
Not only do bioprocessing and fermentation provide chiral lactic acid, they do so inexpensively. Control of chirality allows us to change the polymer performance by changing the optical activity of the lactic acid units in the polymer backbone. The result is a family of products made from lactic acid, with properties that can reach the wide range of applications previously discussed.
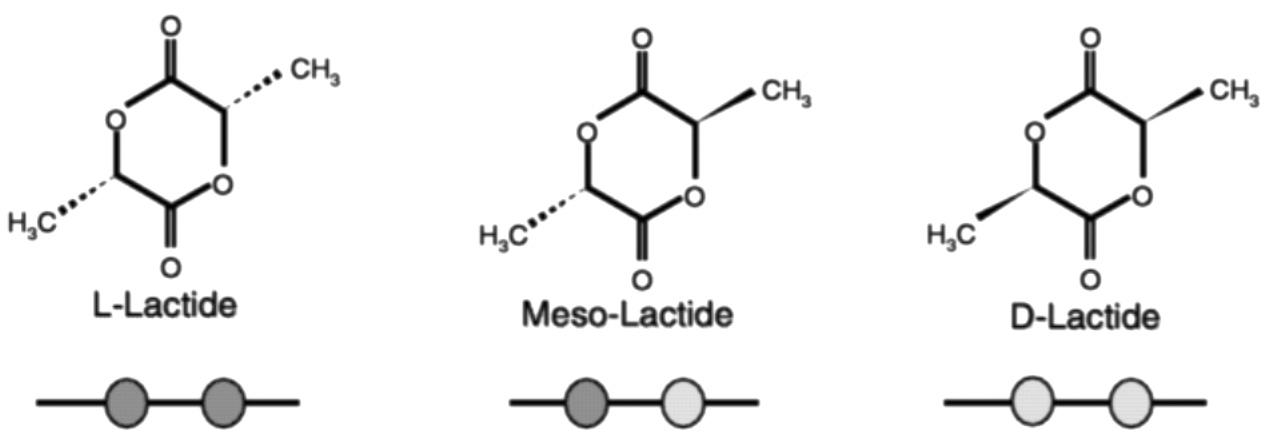
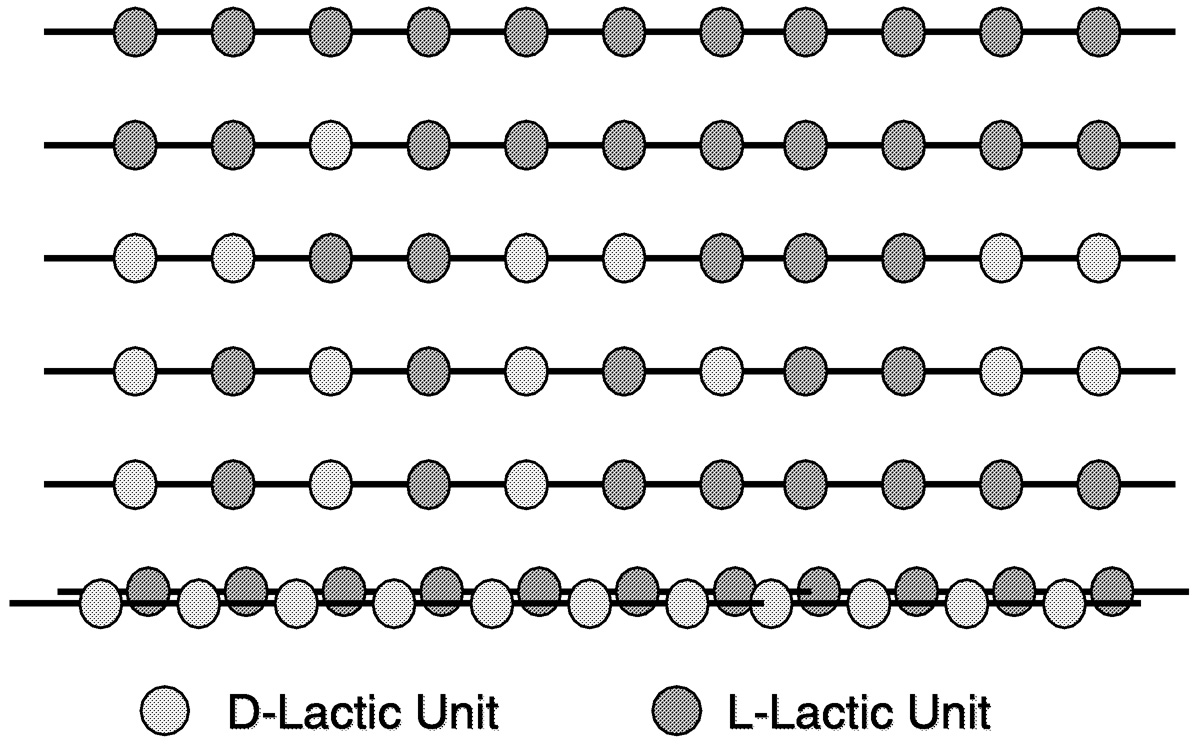
The two isomers of lactic acid can give rise to three lactides: l-, d-, and meso. The fermentation process makes l-lactic acid exclusively. The chemical processing steps allow us to racemize small amounts of l-lactic acid to d-lactic acid. This provides us with three dimers for polymerization. Figure 11.7 and Figure 11.8 are cartoon representations of the polymer structures we can achieve from the three lactides. By varying the amount and sequence of d-lactic units in the polymer backbone, we can change product properties, such as melt behavior, thermal properties, barrier properties, and ductility. 2
Our market opportunity results from the combination of fermentation, bioprocessing, and conventional chemical processing. The optical specificity of the biological system provides value that cannot yet be achieved through chemical processing. The chemical processing steps allow us to specifically combine the chiral lactic acid units to make valuable materials. The combination of fermentation or
Page 172
~ enlarge ~
FIGURE 11.6 The optical isomers of lactic acid.
bioprocessing and chemical processing provides specific value that can't be achieved alone. Using the chirality of the molecules, we are able to create a wider range of performance in a wider range of products. Our market opportunity is therefore bigger. I believe that this insight is general and would extend to other products made from renewable resources.
Renewable Resources, CO2, and PLA
Whenever someone hears that PLA is made from renewable resources, there is an immediate expectation that it must be better for the environment. Two questions soon follow: (1) How does one know it is better? and (2) Better than what? Energy and CO2 are the focus of this discussion. PLA provides an example of what is required to answer these questions successfully.
All of the carbon in PLA comes from carbon dioxide in the atmosphere. How? All of the carbon in lactic acid comes from glucose, which is made by plants via photosynthesis. However, like all manufacturing processes, the production of PLA requires energy. The question is, Does the energy required to drive the processing cause more carbon dioxide emissions than the amount of carbon dioxide fixed in PLA? This question can be addressed using a complete “cradle-to-grave” life-cycle inventory (LCI).
LCIs were designed to provide an accounting of all of the inputs and outputs across the whole business system. LCIs should be used internally by companies to gain insight into where to effectively
~ enlarge ~
FIGURE 11.7 Possible lactide structures. The dark color represents an l-lactic acid residue. The light color represents a d-lactic acid residue.
Page 173
~ enlarge ~
FIGURE 11.8 Examples of PLA structures. The dark color represents an l-lactic acid residue. The light color represents a d-lactic acid residue.
improve their processes by reducing energy and emissions. LCI also causes companies like ours to take a very keen interest in what our suppliers and customers do—they are part of the business system.
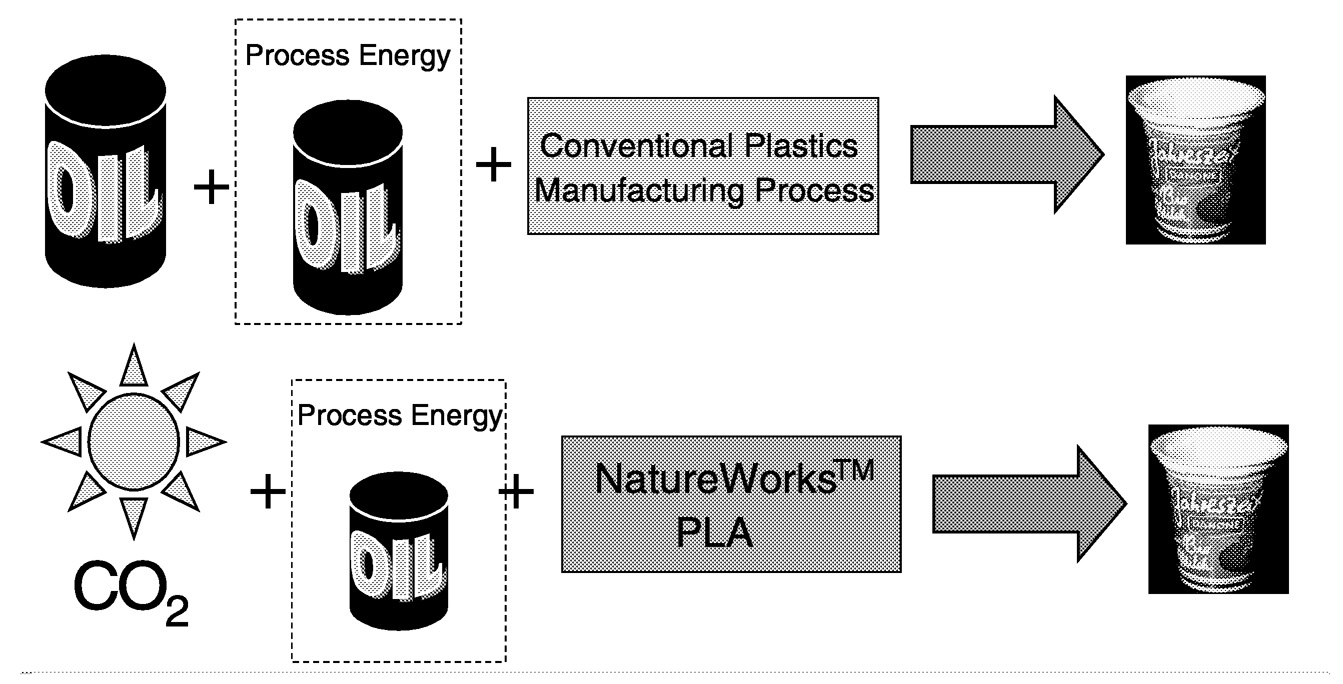
The business system for thermoplastics is illustrated in Figure 11.9, where both a renewable-based system and a petrochemical-based system are shown. The major difference in these two systems is that in the renewable-based system the raw material is corn, while in the petroleum-based system it is petroleum. Both systems use fossil resources for process energy. Both systems make a plastic product that needs to be disposed at the end of its useful life. Another difference between a PLA and a petrochemical-based system is that PLA can be conventionally recycled, re-monomerized back to lactic acid, or composted.
~ enlarge ~
FIGURE 11.9 The business system for thermoplastics. With renewable-resource based products such as PLA all of the mass of the polymer originates from carbon dioxide. Conventional plastic materials use fossil resources. Both petrochemical-based plastics and PLA require process energy. The expectation is that the overall amount of fossil resources is less for PLA compared to petrochemical based products.
Page 174
Each time an LCI is put forth, it needs to be specific for products and end fate to make valid comparisons and interpret the cradle-to-grave energy use and emissions.
LCI techniques are problematic because the data for raw materials are often not specific enough and cradle-to-grave systems are complicated. Raw data for inputs tends to be historical rather than forward looking. Agricultural data are frequently old, and the sample sizes often represent large regions. In the future, as we begin purchasing sugar from Cargill in Blair, Nebraska, we need to understand the agricultural practices used to grow corn. However, agricultural practices are changing rapidly and the data available aren't current or specific enough to represent the agricultural practices around Blair. What does this mean for PLA's LCI? We'll have to collect data and project it.
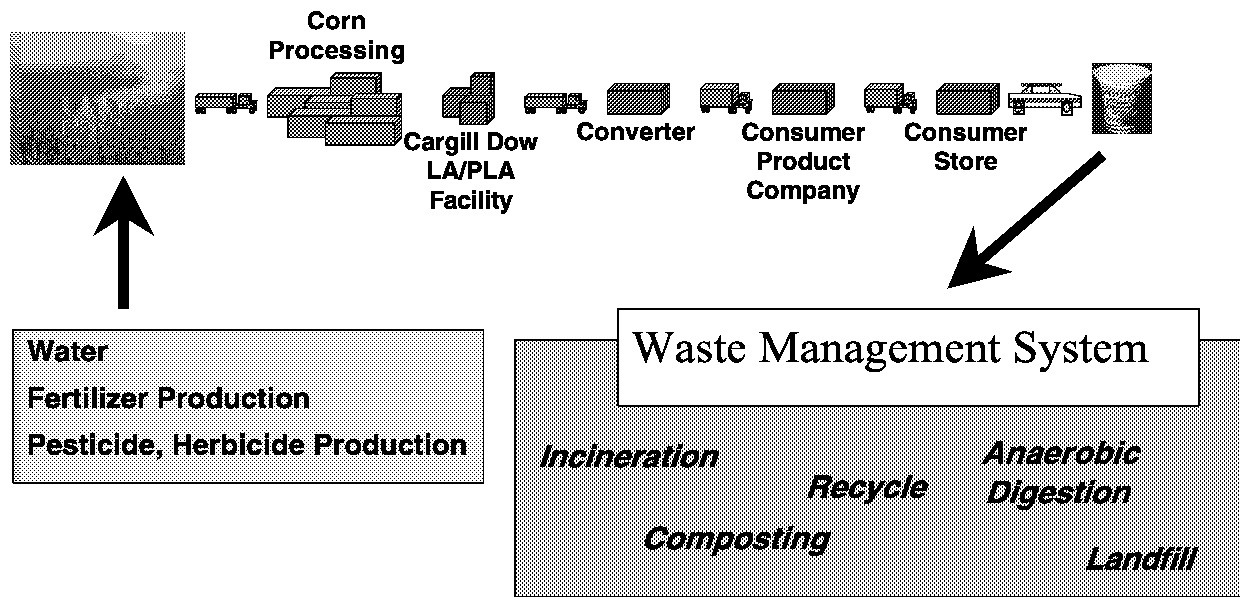
We have analyzed Cargill's wet-milling operation as well as our lactic acid and PLA processes and converted the data for use in our LCI. Our raw data are proprietary because they use real engineering data wherever possible. Figure 11.10 shows all of the inputs and outputs that are considered for an LCI of PLA. We have attributed all of the agriculture inputs to dextrose in our analysis to avoid debate over carbon dioxide capture by crop residues left on the field. We have taken into consideration all of the transportation to ship our products around the world. We have even taken into account the manufacturing of the agricultural machinery (it turns out not to be significant to the analysis).
Another complication in LCI comes from the time horizon considered in the business system. For example, in most developed countries of the world the fate of plastic material is likely to be incineration. Whether the plastic is a “disposable” or a “permanent” product, if it makes it to a waste disposal system, it is most likely incinerated. If the plastic is made from petrochemicals and incinerated, then fossil resources are being converted to carbon dioxide emissions. If the plastic is PLA and it is incinerated, then the carbon is converted back to CO2. If the product does not enter the waste disposal system, then the time horizon becomes important. In the case of permanent products, for a relatively short time frame such as a human lifetime, I suppose the products are permanent. For long time horizons, such as hundreds, thousands, or millions of years, then my assumptions regarding permanent products may change. At these longer time scales, I suspect that even permanent thermoplastics may get converted to carbon dioxide. In our LCI analysis, we have used shorter time frames, on the scale of decades, and made conservative assumptions.
In our LCI, we have tried to be as complete as possible, but all must realize that we are, in fact, using LCI as a tool to guide our developments. Our LCI profile will change depending on what we learn and the resulting new directions we take, and our energy use could even be higher for a period of time.
~ enlarge ~
FIGURE 11.10 Cradle-to-grave understanding requires knowledge across all parts of the business system.
Page 175
However, the general trend over time will be lower energy and lower carbon dioxide emissions. Our long-term goal over the long run is to eliminate petrochemicals from our business systems. We believe we will be rewarded in the marketplace.
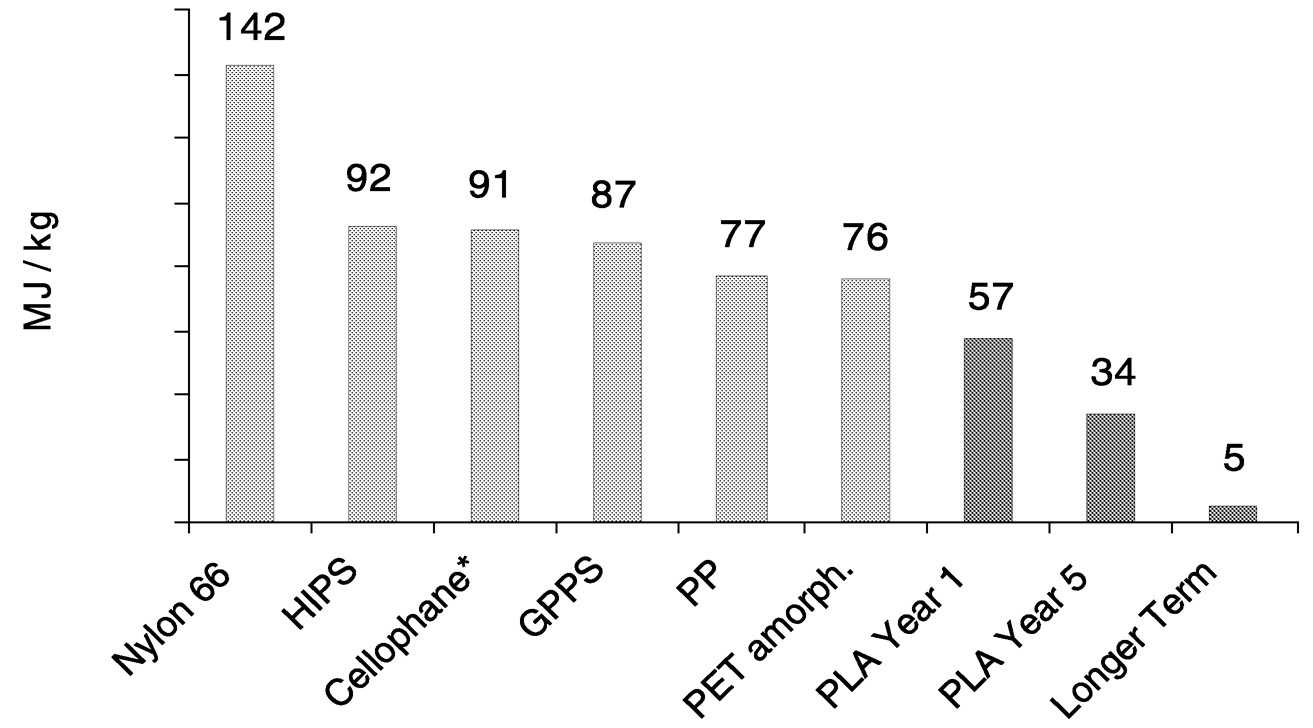
Figure 11.11 compares total petroleum (feedstock + process energy) for production of conventional polymer pellets versus projected total petroleum used for production of PLA polymer pellets. The polymers listed are expected to compete with PLA in the marketplace in some specific applications. “PLA year 1” data were generated using assumptions based on the process technology to be deployed in Cargill Dow's production facility currently under construction. “PLA year 5” and “long-term” data assume improvements in process technology and alternative energy sources that further optimize production efficiencies for PLA. CD is already investing in the technology to reach the “year 5” goals.
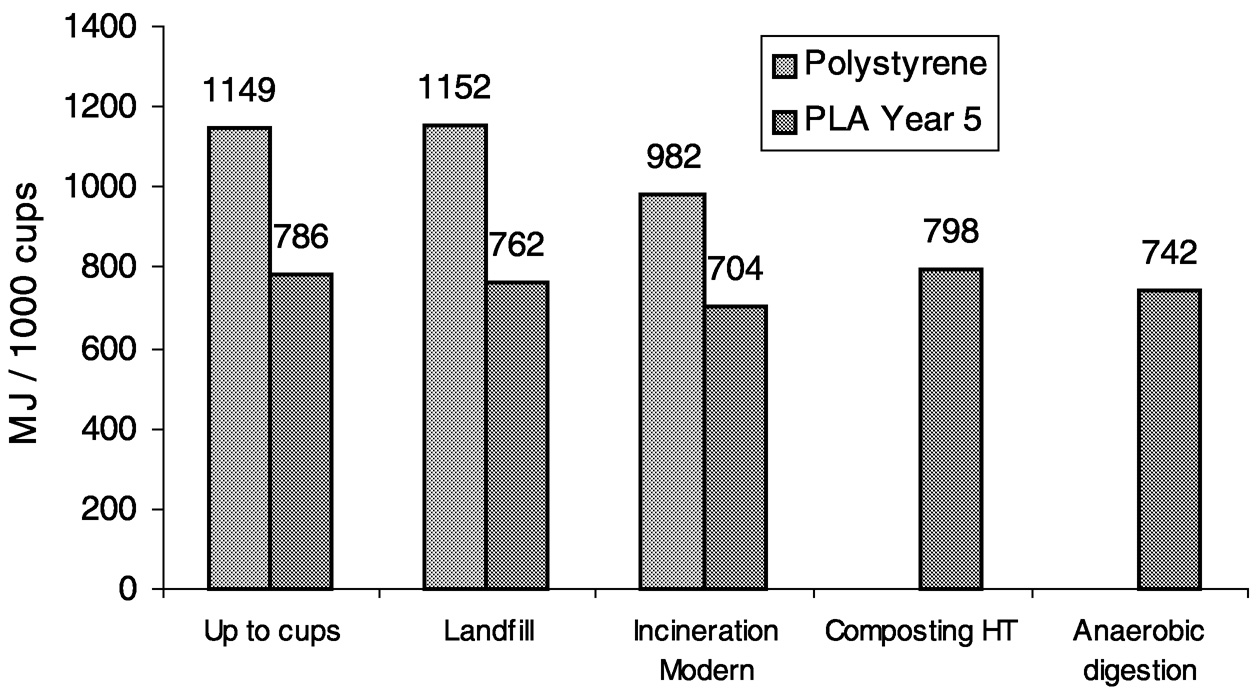
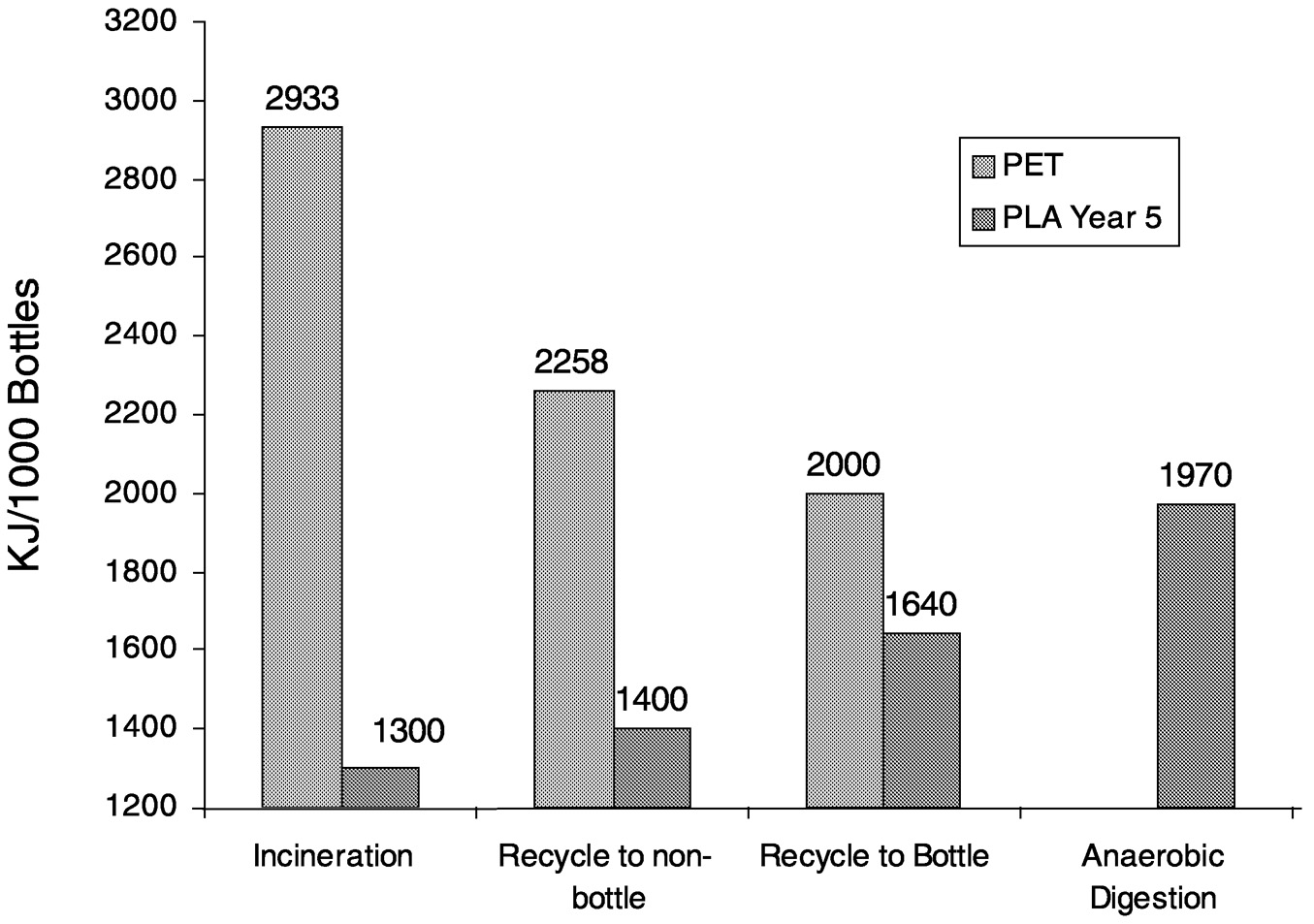
It is clear in Figure 11.11 that PLA has the advantage in total fossil resource use. Note that although cellophane is made from renewable resources, its fossil resource use is higher than many of the other products. However, assessing fossil resource use only up to polymer pellet production is incomplete. For a complete LCI comparison, converted-product and end fate data must be included. The results of fossil resource use in this type of analysis are shown for PLA versus polystyrene cups and PLA versus polyethylene terephthalate (PET) bottles in Figure 11.12 and Figure 11.13, respectively.
PLA is advantaged in each comparison. It is interesting to note that the fossil resource differs with each of the disposal methods. Neither polystyrene nor PET can be composted or degraded in anaerobic digestion.
Fossil resource use drives CO2 emission. The more process energy used, the more carbon dioxide is emitted. If incineration is the end fate, then the carbon of the material itself is emitted as carbon dioxide. If landfill is the end fate, then CO2 emissions on disposal arise primarily from transportation. If composting is the end fate, then most of the carbon is converted to CO2 (we've assumed 95%), and the rest is humic substance.
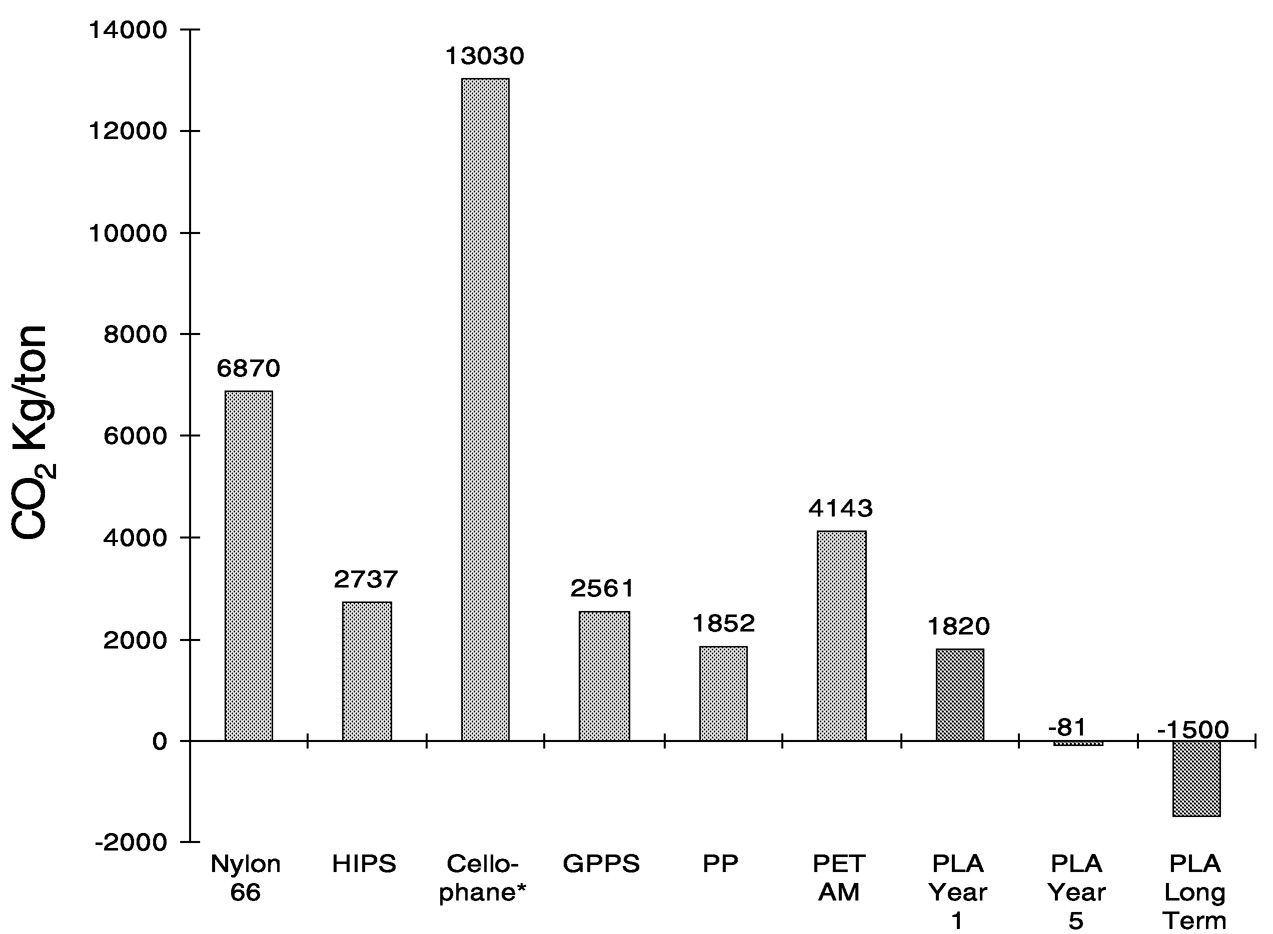
The results of the cradle-to-pellets analysis for CO2 emissions from conventional plastics and PLA are summarized in Figure 11.14. “PLA year 1” data represent the production facility currently under construction by Cargill Dow and projected to be on-line in late 2001. “PLA year 5” and “long-term data”
~ enlarge ~
FIGURE 11.11 Total fossil resource use in common plastics measured in energy. All of the LCI results are based on the Boustad model. 3
Page 176
~ enlarge ~
FIGURE 11.12 Fossil resource use in PLA cups compared to polystyrene cups through various waste disposal systems.
assume improvements in process technology and alternative energy sources to further reduce CO2 contributions by PLA.
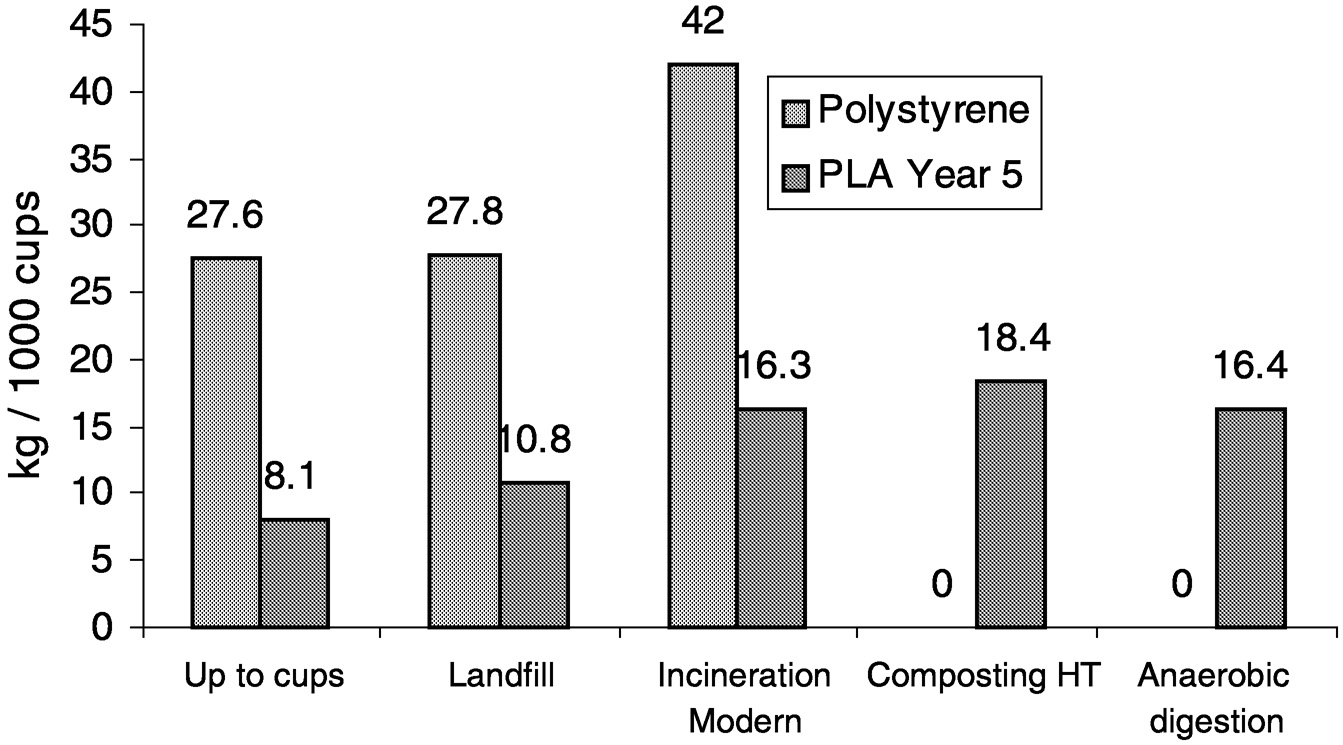
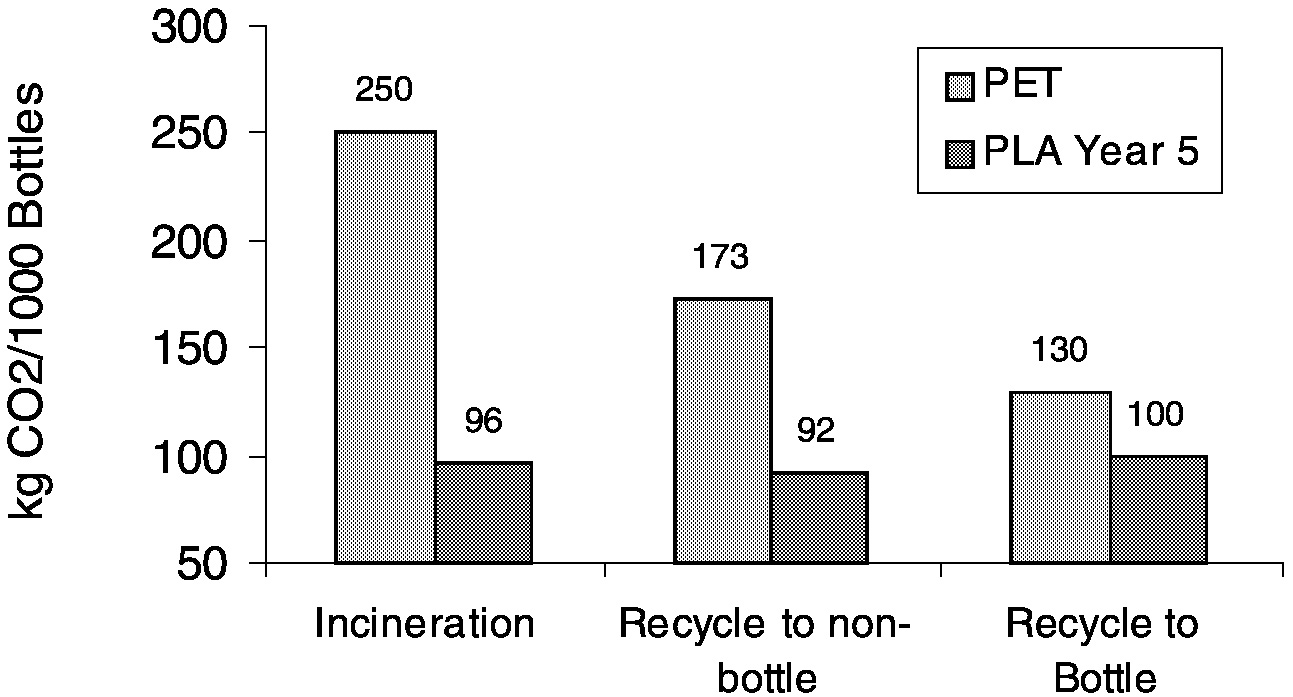
Again, using the analysis only through pellets is incomplete. Figure 11.14 shows that at a pellet level PLA can be a carbon dioxide sink! However, when the complete comparison of PLA to polystyrene cups ( Figure 11.15) and PLA to PET bottles ( Figure 11.16) is done, we find that PLA is still advantaged, with a greater than 50% reduction in CO2 emissions relative to conventional plastics.
The primary reason that PLA has a favorable carbon dioxide profile is that the fermentation and chemical processing steps are extremely efficient. In our analysis, we charged all of the agricultural
~ enlarge ~
FIGURE 11.13 Fossil resource use in PLA bottles compared to polystyrene bottles in various waste disposal systems.
Page 177
~ enlarge ~
FIGURE 11.14 Net CO2 emissions of PLA compared to other polymers: cradle to pellets.
inputs and emissions to dextrose. If our PLA yield was lower, not only would the per-cup process energy inside our fence increase, but all of the upstream inputs would increase due to the additional corn and farming needed. The take-home lesson is that yield is particularly important.
High concentration of product in the fermentation is also important for efficient bioprocessing. In the case of lactic acid, the concentration (titer) is typically greater than 100 g/L. This means that less water has to be removed from the product—hence, less energy input to evaporation and lower carbon dioxide emissions.
To my thinking, a general rule would be that a commercially viable industrial product produced from renewable resources using fermentation and biotechnology must have yields greater than about
~ enlarge ~
FIGURE 11.15 Carbon dioxide emissions of PLA cups compared to polystyrene cups on a cradle-to-grave basis.
Page 178
~ enlarge ~
FIGURE 11.16 Carbon dioxide emissions of PLA bottles compared to PET bottles on a cradle-to-grave basis.
85% and a titer of about 90-100 g/L in order to have a carbon dioxide and fossil resource advantage compared to petrochemical-based products.
Composting and Biodegradation of PLA
PLA is unusual in that it is stable under normal-use conditions but degrades quickly in environments of high temperature, high moisture, and high microbial activity. This feature may allow accelerated development of alternative waste disposal systems, such as composting and anaerobic digestion.
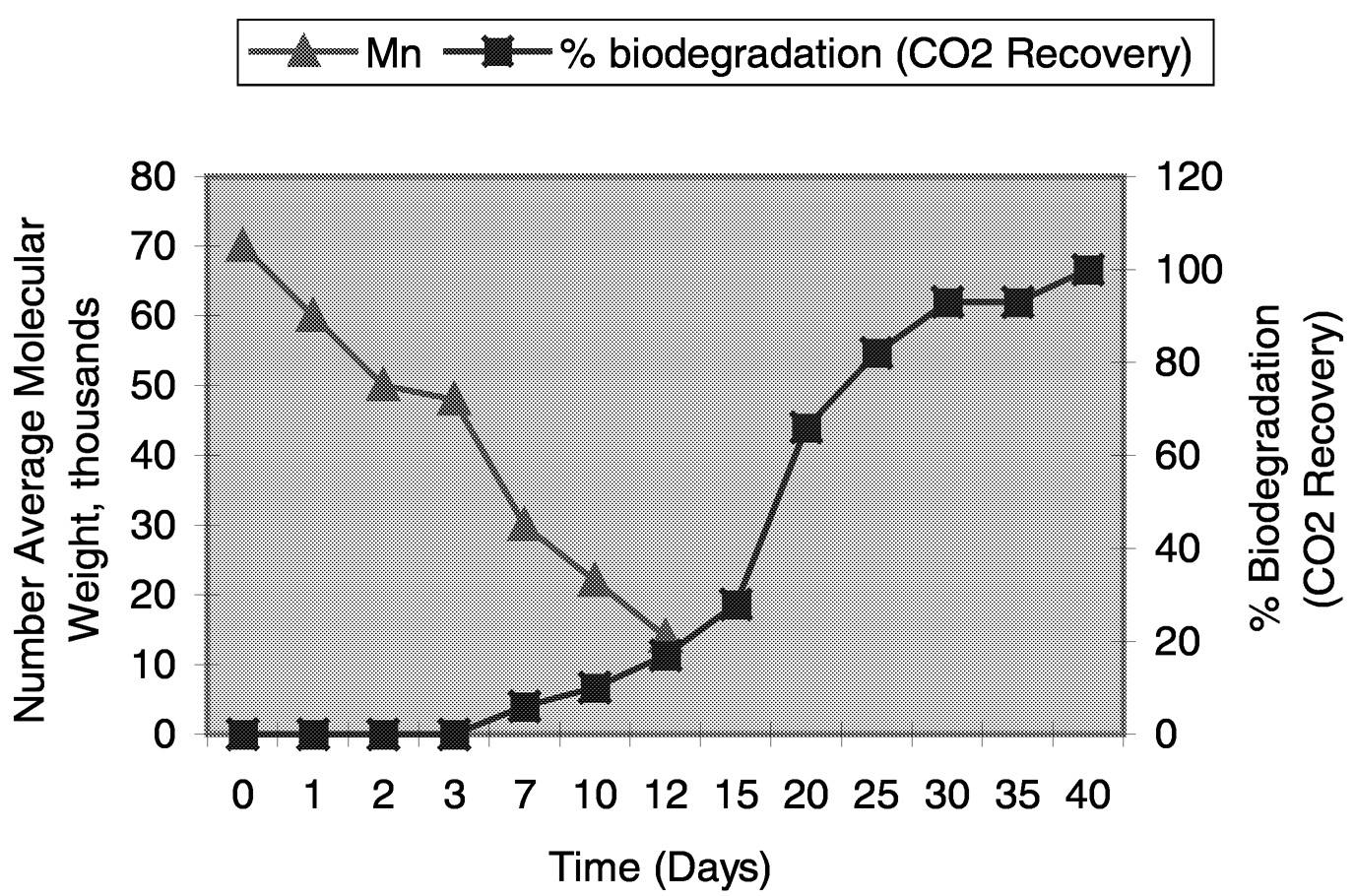
The primary mechanism of degradation is hydrolysis, catalyzed by temperature, followed by bacterial attack on the fragmented residues. The environmental degradation of PLA occurs by a two-step process. During the initial phases of degradation, the high-molecular-weight polyester chains hydrolyze to lower-molecular-weight oligomers. This reaction can be accelerated by acids or bases and is affected by both temperature and moisture levels. Embrittlement of the plastic occurs during this step at a point where the number average molecular weight (Mn) decreases to less than about 40,000. At about this Mn, microorganisms in the environment continue the degradation process by converting these lower molecular-weight components to carbon dioxide, water, and humus. The structural integrity of molded PLA articles decreases as the molecular weight drops, and eventually the article disintegrates. A typical degradation curve of PLA under composting conditions is shown in Figure 11.17.
Under typical use and storage conditions, PLA products are stable. In addition, certain additives can be used to retard hydrolysis. Continuing studies in this area will lead to increased PLA stability for more extreme applications.
Research and Technology
PLA is interesting in that in everything we do to lower the cost of our product, we gain an enhancement in our LCI profile. If we lower our energy use, our LCI profile gets even better. If we use alternative feedstock's (biomass) as a raw material, we eliminate the processing energy and chemical use required in wet milling of corn. Our long-term goal is to eliminate fossil fuel resource use, the source of carbon dioxide emissions.
Page 179
~ enlarge ~
FIGURE 11.17 Biodegradation of PLA in compost at 60° C. NOTE: Mn = number average molecular weight.
On the product side of technology development, we have room to expand our product performance and market potential. The stereoisomers of PLA allow a very wide variety of performance—we have not yet explored this fully. We also will begin to target other molecules made from lactic acid. In our view, lactic acid will become a very inexpensive raw material used in a wide range of products.
We will continue to modify our LCI and use the data to assist our customers to make choices to reduce their environmental impact. We find ourselves forced to develop appropriate agricultural data, because the practices that farmers employ impact our environmental profile.
SUMMARY
PLA uses fewer fossil resources and emits less carbon dioxide in its manufacturing than the petrochemical-based products it replaces. The differences are measurable and significant according to the standard methodology for LCI. PLA fits into any waste management system, including composting or other managed biodegradation systems.
The expectation and perception associated with renewable resources are that the products should be advantaged compared to the petrochemical products they are replacing. Our data suggest that this perception applies—at least for PLA made by our process.
Here are some lessons we have learned with PLA:
1. Research and development of environmentally friendly technology must result in products that have value in the marketplace. The products must perform in the intended application. No one will buy a product that doesn't perform, even if it is environmentally friendly. This ought to be obvious, but it is often overlooked. PLA has an opportunity because it performs well at competitive price. It has a very large market potential that should develop rapidly because it uses fewer fossil resources, emits less carbon dioxide, and is compostable. Furthermore, if we market it properly, PLA will help consumers understand that high-performing products can be made from renewable resources and that they are, in
Page 180
fact, better for the environment. Companies like ours are changing the paradigms and expectations of consumers, while creating more demand for products with improved environmental performance.
2. CO2 is a raw material, not a waste product. PLA uses it successfully as a raw material because the fermentation and chemical processing technologies are very efficient and have high yields. What additional technologies can create other useful products from CO2? Can fermentation technology be developed to fix CO2?
3. Successful renewable resource-based products will have to combine fermentation and biotechnology with chemistry. Combining fermentation and biotechnology with chemistry can create special advantages and new opportunities because nature is very good at making compounds that are difficult to synthesize by traditional chemistry. Chiral lactic acid is a simple example of this dual requirement. Lactic acid has only limited market potential as lactic acid, but through chemistry we can create significant market opportunities that take advantage of the chirality that the natural system provides.
4. We need scientists who can move effectively across disciplines and who know chemistry. Chemists can be extremely effective in a wide variety of technology areas. Chemists are trained in the first principles of reactions and reaction mechanisms. In my experience, they are the ones who understand and are most effective at determining what the critical issues are and what the targets should be. We have been most successful with people who have a depth of chemistry experience and a breadth across related disciplines such as biochemistry, molecular biology, chemical engineering, and business. We need more people who understand the fundamentals of chemistry. I wonder if these basic skills are being lost because of the emphasis on biotechnology.
5. In order for us to reach our long-term environmental goals, we need advancement in biomass processing and alternative energy sources. The economics are easy to see in biomass processing, but the technologies need to be developed. For successful development, a wide variety of companies and institutions will be required to collaborate. Technologies such as wind power have to be fully developed and deployed. We need more renewable energy sources for electricity and steam. We need alternative fuels for transportation. Then we can really reduce carbon dioxide emissions.
6. PLA typifies technology and product development for thermoplastics and other commodity chemicals made from renewable resources. Uncovering performance attributes and value across these business systems takes large investments in R&D. The market developments are long and complicated. The process technology development cuts across many fields and requires an integration of knowledge. Very few companies can undertake and sustain the effort. Development of more renewable resource-based products requires large investments in R&D and in manufacturing facilities. Government funding is critical for identifying issues and developing basic technologies to resolve these issues. In our case, both the Department of Energy (DOE) and the National Institute of Standards and Technology (NIST) funded basic technology that helped us. However, if Cargill, Incorporated, and the Dow Chemical Company had not funded us, I doubt that we would have been able to sustain the effort. Significant government funding will be required if we want to see more products like PLA developed and commercialized.
ACKNOWLEDGMENTS
Mark Hartmann, Nicole Whitemann, Erwin Vink, James Lunt, Kevin McCarthy, Lang Phommahaxay, Ray Drumright, David E. Henton, Patrick Smith, and Jed Randall, Tom Bremel, NIST, and DOE. Nature Works PLA is a registered trademark of Cargill Dow LLC. The graphics included in this chapter are used with the permission of Cargill Dow LLC.
Page 181
REFERENCES
1. Cargill Dow LLC's Web site provides a summary at www.cdpoly.com.
2. There are many references that discuss the material properties of PLA and PLA stereo-polymers. The following list provides good background reading: , , and , 1991 , Polym. Bull 26: 71. and , 1983 , Polymer 24 : 175. and , 1980 , Polymer 21: 607. , and , U.S. Patent 4902515, 1990 . , U.S. Patent 5317064, 1994 . and , 1993 , Macromolecules 26: 6918. , , and , 1987 , Macromolecules 20 : 904. , 1996 , J. Applied Polym. Sci. 62 : 1079. , , , , , , and , 1998 , Macromolecules 31 : 2593. , , and , 1973 , Kolloid-Z.u.Z. Polymere 251: 980. and , 1992 , Clin. Mat. 10 : 21. , , , , and , 1999 , Annu. Tech. Conf. – Soc. Plast. Eng. 57(2) : 2190. , 1996 , J. Macromol. Sci. Pure Appl. Chem. A33(5) : 585. , U.S. Patent 5424346, 1995 . , , and , U.S. Patent 5756651, 1998 . , , and , U.S. Patent 5883199, 1999 . , , , and , 1996 , J.M.S. – Pure Appl. Chem. A33(10) : 1497. , , and , U.S. Patent 5633342, 1997 . , U.S. Patent 5032671, 1991 . and , 1999 , J. of Rheology 43(5): 1141. , , , , and , U.S. Patent 5594095, 1997 . , , , , and , U.S. Patent 5798435, 1998 . , , , , and , U.S. Patent 5998552, 1999 . , , , 1997 , Polymers, Laminations, and Coatings Conf. 139.
3. , “Eco-Profiles of Plastics and Related Intermediates, Methodology,” The Association of Plastics Manufacturers in Europe, April 1999 . Report is available at http://www.apme.org.
4. , , and . 1994 . Wicking of spin finishes and related liquids into continuous filament yarns. Textile Res. J., 64(1) 33-40.
DISCUSSION
Tobin Marks, Northwestern University: When you go from glucose by fermentation to lactic acid, is that a continuous process or a batch process?
Patrick Gruber: We can do it either way. We haven't described the way we will do it to anyone yet. It can be done effectively either way. Parts of the process will probably be done in batches, but some of it depends on how “batch” is defined. Overall, though, it is a continuous process.
The lactic acid must be isolated and purified by removing water and taking out impurities. Then to get to PLA from there involves straight chemistry. As the water is being evaporated from the lactic acid, it starts to do a condensation reaction, because you have both a hydroxyl group and an acid group to give what we call “pre-polymer oligomers.” These start to form an equilibrium mixture with the lactide through a back-biting reaction. In contrast, if we wanted to make conventional polyester, we would heat it and apply a vacuum to condense it. If, at this point, we tried to condense lactic acid directly to make high-molecular-weight material, a dimer, lactide, would be clipped off. We cross over the conditions where the lactide is vaporized—high enough temperatures and low enough pressures. We purify the lactide ring in a distillation column. This allows us to control even the optical isomers in the lactates. We then collect the lactides. Lactide can be thought of as an anhydrous lactic equivalence in a ring form. We simply do a ring opening polymerization to form the polymer. It is quite an efficient process.
Tom Baker, Los Alamos National Laboratory: Can you tell us something about the kind of footprint you would need for plants when PLA cup production becomes very large? In other words, if you are going to make a hundred thousand tons of polymer a year using biocatalysis, what kind of footprint are you going to need?
Page 182
Patrick Gruber: I think making a billion pounds of polymer would require a farm producing corn that is about 10 miles on any side. For us to make 300 million pounds of plastic would require about 400 million pounds of lactic acid. Assuming 120 bushels per acre, this equates to 117,000 acres of corn.
Rosemarie Szostak, Department of the Army: To follow up on that and address the aspect of food security, what percentage of the corn market are you anticipating this to be?
Patrick Gruber: The percentage of corn that we would impact is virtually insignificant. The amount of corn produced in the United States is so massive—7 to 9 billion bushels per year (56 pounds per bushel)—and the amount used for making these polymers is so small that it doesn't even register. A fraction of one corn plant could supply us a billion pounds. It is minuscule in the grand scheme of corn.
Rosemarie Szostak: How do you anticipate this in an international market with regard to agriculturally at-risk areas and deforestation—for example, in developing countries?
Patrick Gruber: Third World countries are trying to figure out what infrastructure they should build. This includes chemical processing systems, bio-processing systems, food processing systems and, of course, waste disposal systems. What can't be done is to take land required for food production, or to further degrade marginal land. This is an issue we need to pay attention to. For example, the amount of material that would feed our PLA plant, would take all the export sugar of Australia. I know they are not a third world country, but that gives you an idea of size. Australia is a good sugar producing country. We would need all their export sugar capacity just to feed our little plant. Our manufacturing plant and raw materials could impact agriculture land and its use. We have to pay attention.
The idea of using energy crops in third world countries as raw materials is hard for me. From an economic point of view it is disadvantaged because you wind up with a low energy concentration in the agricultural product, and therefore, more land. In Third Worlds, you need food production, hence it can only make sense in parts of the world where food is not an issue.
David Keith, Carnegie Mellon University: I want to sound a somewhat cautionary note about the life-cycle analysis. You implied that if you could do a life-cycle analysis and show that some process was producing less carbon, you might get a tax credit for it.
Even with a lot of work, in the absence of bias, it is extraordinarily difficult to be sure that the results of a life-cycle analysis are correct. This is one of the reasons that many folks would argue very strongly that we should not institute a system of tax credits in that way. The accounting challenges are formidable; firms would have an incentive to exaggerate the results, and there is no gold standard to hold them accountable to. I think it makes more sense to tax the relevant environmental insults directly—carbon dioxide emissions or nitrous oxide emissions, or whatever. If you do it that way, you get a very different answer. No longer does a company have to produce some giant statement that the government then has to audit, but the company just has to minimize cost, the way it always did, under appropriate prices.
To give you one example, corn production yields a lot of N2O and methane. So, it is a big net greenhouse gas emitter. Maybe that effect was included, but there are probably seven others that were not.
Patrick Gruber: To clarify: what I said was that if there were a credit available, we would take advantage of it. The market opportunity is created solely because the product works well, customers are
Page 183
willing to buy it, and it is economical. This fundamental principle is too often lost. We are going to take advantage of tax credits or of other markets who are penalized through taxes.
This is the way it actually will unfold. Your point about the life-cycle studies is well taken. It is extremely problematic to sort through these data. You saw me actually cast the argument through this global system of comparing oil, agricultural use, getting it down to a yogurt cup. If we are going to talk about a thousand yogurt cups, this is actually the difference. Now, I am more confident about the data from inside my fence line, but it gets complicated outside the fence line.
Alan M. Wolsky, Argonne National Laboratory: You remarked that the next target would be to make styrene and related compounds from lignin. I am confused about whether or not that input lignin would otherwise be used as fuel. If so, as is the case in most pulp manufacture, is there a benefit to the climate in forgoing the use of lignin as a renewable fuel, and, instead, turning it into benzene, toluene, and xylenes?
Patrick Gruber: That is a good question and ought to be answered, but I don't know the answer to it. My guess is that there will be a set of chemicals that can be made from lignin that have particular value, and these ought to be the ones that get made. The others ought to be burned if the better value is to burn them.
Richard Wool, University of Delaware: Where, along the chain of reactions, are you expending your highest fossil fuel energy, in terms of conversion processes?
Patrick Gruber: In the fermentation process and, more specifically, dealing with the water that is there. That is why I talked about the criteria of what a good fermentation looks like. You can have either too low titer of product or too low yield.
Richard Wool: Is that mostly from the dextrose itself or is that from the starch? Are you talking about starch to lactic acid going through a glucose breakdown process?
Patrick Gruber: The glucose systems are pretty effective. For fermentation products, the same goes for the lactic acid system. It could be improved by moving up the finished product titer. The energy use is determined by the amount of water that has to be evaporated.
Richard Wool: Is that a very tight system or do you think it is open to biotechnological improvements in the future?
Patrick Gruber: It is open to biotechnology improvements, for sure. When I show how we will progress to the future, there is no doubt in my mind that we can impact all critical parameters.
Richard Wool: Is the bottom line, even right now, that you have a considerable CO2 advantage over the fossil fuel?
Patrick Gruber: If we compare them that way, with the caveats we talked about, but following the rules, then yes it does. So I would say that we start off in a good place. We can start to establish this category in the marketplace, and we can learn how to do the LCI or whatever the measurement tech-
Page 184
nique is. There is no doubt that as we apply new biological techniques, catalyst programs, and chemistry, we will make it even better.
Richard Wool: How much fossil fuel energy is expended in breaking the starch down into sugars?
Patrick Gruber: I have the numbers for total percent of the PLA, but I don't remember exactly. It is very small, because it is a highly efficient enzymatic process that takes place under mild conditions.



















