PANEL MEMBERS
Arlene A.Garrison, University of Tennessee, Chair
James W.Serum, Viaken Systems, Inc., Vice Chair
Thomas M.Baer, Arcturus Engineering, Inc.
Douglas C.Cameron, Cargill, Inc.
Alan Campion, University of Texas at Austin
Robert E.Ellefson, Inficon, Inc.
Walter W.Henslee, The Dow Chemical Company
E.William Kaiser, Ford Motor Company
R.Kenneth Marcus, Clemson University
James D.Olson, The Dow Chemical Company
Athanassios Z.Panagiotopoulos, Princeton University
Frank K.Schweighardt, Air Products and Chemicals, Inc.
Gary S.Selwyn, Los Alamos National Laboratory
Michael L.Shuler, Cornell University
Christine S.Sloane, General Motors Corporation
Anne L.Testoni, KLA-Tencor Corporation
Edward S.Yeung, Iowa State University
Submitted for the panel by its Chair, Arlene A.Garrison, and its Vice Chair, James W.Serum, this assessment of the fiscal year 2001 activities of the Chemical Science and Technology Laboratory is based on site visits by individual panel members, a formal meeting of the panel on February 20–21, 2001, in Gaithersburg, Md., and documents provided by the laboratory.1
LABORATORY-LEVEL REVIEW
Technical Merit
According to laboratory documentation, the mission of the Chemical Science and Technology Laboratory (CSTL) is to provide the chemical measurement infrastructure to enhance U.S. industry’s productivity and competitiveness, assure equity in trade, and improve public health, safety, and environmental quality.
The activities of the CSTL support this mission and are consistent with the NIST mission to strengthen the U.S. economy and improve the quality of life by working with industry to develop and apply technology, measurements, and standards. The panel finds that the diverse array of projects under way in the laboratory successfully impacts a variety of industries. The high-quality technical work done by NIST staff also plays a vital role in the international metrology community. Finally, particularly through its work on health care and environmental standards, the laboratory is improving the lives of the American public.
In all of these areas, the laboratory is careful to concentrate on aspects of industrial, international, or public problems that specifically relate to measurements and standards technologies. With this focus, the laboratory can be assured that it is using the unique expertise and capabilities available at NIST to perform research and provide products that no other organization can. Management is aware of the importance of channeling the laboratory’s scarce resources into areas in which NIST activities can have a unique and optimal impact, and this objective is reflected in strong strategic planning and project selection and evaluation processes. The panel compliments the laboratory on its work in this area. Evidence of good refocusing and reprogramming was observed (e.g., within the Surface Dynamical Processes Group), with a healthy number of programs beginning and ending. This past year, the laboratory identified nine strategic directions to guide the divisions in selecting new program areas and developing new competencies. These areas and competencies include nanotechnology, health-care standards, and data and informatics.
The technical merit of the programs under way in the Chemical Science and Technology Laboratory continues to be excellent. The primary reason for the high caliber of the work at NIST is the impressive collection of staff. These people receive a large number of awards from external organizations as well as from organizations within the federal government; examples include the W.J.Youden Award of the American Statistical Association and the NOAA Environmental Hero Award. They also hold responsible positions in professional societies, on editorial boards, and in standards and trade organizations. In many scientific communities and industries, CSTL and its staff are the primary resource for accurate and useful technical information on relevant measurements and standards. The panel applauds the laboratory for playing a critical role in providing the underpinnings of current industrial practices and for supporting the research that is needed to achieve the technological advances that will allow U.S. companies to continue to participate in and lead the global economy.
The Chemical Science and Technology Laboratory is organized into five divisions: Biotechnology, Process Measurements, Surface and Microanalysis Science, Physical and Chemical Properties, and Analytical Chemistry (see Figure 4.1). Later sections in the chapter detail the panel’s assessment of the goals, technical accomplishments, and impact of the individual divisions. Staff are physically located at a number of sites: about half of the Physical and Chemical Properties Division is in Boulder, the Analytical Chemistry and Biotechnology Divisions is in the new Advanced Chemical Sciences Laboratory in Gaithersburg, a small group from the Analytical Chemistry Division is in the NIST neutron reactor facility, and the remainder of the staff is in the old Chemistry, Physics, and Metrology buildings
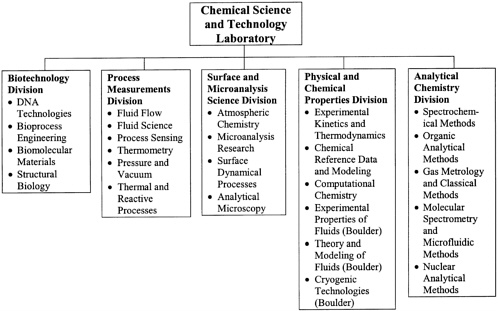
FIGURE 4.1 Organizational structure of the Chemical Science and Technology Laboratory. Listed under each division are the division’s groups.
in the center of the Gaithersburg campus. While all of these placements are a logical consequence of efforts to meet the facilities needs of individual programs, the resulting geographical scattering of personnel is a significant challenge for laboratory and division management. It is important to maintain a sense of unity and cohesion among the laboratory staff, both to keep up morale and to foster the informal relationships that often lead to the most fruitful scientific collaborations. The panel encourages CSTL managers to consider mechanisms to facilitate informal cross-group, cross-division, and cross-building communications and to build awareness across the laboratory of what projects and capabilities exist at NIST and what NIST and laboratory-wide goals are.
One of the important elements of laboratory programs across all of the divisions is an emphasis on international activities. To fulfill the mission of enhancing U.S. industrial competitiveness in light of the globalization of markets and companies, CSTL staff must be aware of and active in measurement and standards discussions throughout the world. The panel applauds the laboratory for its proactive and vigorous efforts in this arena. These efforts ensure that the staff remain knowledgeable about any technical developments related to metrology occurring in the international community and give NIST a chance to demonstrate the technical quality of American standards and measurement methods so that international standards organizations will recognize and include these approaches in any global regulations. The European Union has recently passed directives stating that, in the future, products sold in European markets must be manufactured or tested in ways traceable to “standards of the highest order,” for example, internationally recognized certified reference materials (CRMs). The first products for
which these requirements will be enforced (in 2003) are in vitro diagnostic devices, and CSTL staff are working with the U.S. makers of these devices to put the necessary standards in place. They are also moving proactively to develop CRMs in a number of other areas to support U.S. industry’s effort to freely access global markets. CSTL is also considering an initiative in the area of genetically modified organisms (GMOs); establishing cooperative agreements with relevant U.S. and European organizations and developing SRMs and measurement methods in this field would certainly have global implications. The work on CRMs and the GMOs proposal are just two of the many CSTL efforts in the global standards arena. Others include serving on international standards committees, leading intercomparisons among national measurement institutes, and developing new measurement techniques and methodologies that are adopted by organizations worldwide. These efforts all require a significant financial investment from the laboratory, particularly for staff time and travel expenses. However, the laboratory’s international activities are a vital component of its support of the U.S. economy and cannot be neglected.
Program Relevance and Effectiveness
The panel is pleased by the strong focus on industrial needs and processes in the Chemical Science and Technology Laboratory. The industrial sectors impacted by the laboratory’s work include semiconductors, biotechnology, health care, and chemical processing among others. There are also a number of government agencies that are well served by the activities of the laboratory, including the Environmental Protection Agency (EPA), NIJ, and the Department of Defense (DOD). Specific examples of how laboratory programs affect customers are outlined in the assessments of the individual divisions.
The Chemical Science and Technology Laboratory effectively disseminates information about its results, products, and services to a diverse audience in industry, government, and academia. Some sense of the laboratory’s reach can be gained from the numbers of products sold in the past years: over 18,000 reference materials and over 4300 standard reference databases. Staff produced 604 publications, gave 761 presentations, and filled 504 slots on committees. All of these outputs have been and continue to be important elements of the laboratory’s dissemination strategy, and the panel recognizes the continuing value of staff efforts in these dissemination activities.
In addition to these traditional mechanisms for dissemination, there is also the World Wide Web. The Internet is now the primary means of interaction between the CSTL and its customers from industry and academic institutions. The laboratory’s Web site has been greatly improved since last year’s assessment, but the panel believes that there is room for further improvement, particularly on the divisional Web sites. The panel notes that although the Web certainly can greatly increase the number of people who have access to information about NIST, an effective Web site is not easy to design or maintain. Resources may need to be reallocated to support this effort and to produce a site that meets customer expectations that NIST will provide them with accurate and timely information. One issue is usability for these external groups—that is, the ease with which NIST’s customers can find relevant information. Other issues are internal. Policies and procedures related to Web posting need to be more clearly communicated to the staff. Questions include the following: What are the rules about reviewing information before it is posted? Who is responsible for reviewing posted materials and keeping information and data up to date? How are decisions made about whether fees can or should be charged for access to data? The panel was pleased to hear that the question of charging for data is considered periodically, because the technology for cost recovery for Web-accessible databases is constantly changing, as are CSTL products and the needs of its customers.
A major recent improvement in the Chemical Science and Technology Laboratory’s Web site is that a list of the technical areas in which the laboratory is working is now provided at the top of the main
laboratory Web page (<http://www.cstl.nist.gov/>). These areas are Chemical Characterization of Materials, Process Metrology, Chemical and Biochemical Sensing, Nanotechnology, Healthcare Measurements, Environmental Measurements, Microelectronics, Physical Property Data, Chemical and Biochemical Data, Bio-Molecules and Materials, DNA Technologies, and International Measurement Standards. Clicking on any of these areas leads the user to a pdf document with descriptions of and staff for all laboratory projects relevant to that area. Projects with a variety of applications are listed under multiple areas. This approach is echoed in the annual publication describing the laboratory’s recent achievements.2 The panel believes that organizing the public descriptions of laboratory projects in this manner has several possible benefits. The primary expectation is that the thematic groupings will make it easier for companies and other organizations outside NIST to determine which laboratory programs are relevant to their needs and concerns and to find the right people to approach with questions about current activities or requests for new projects. Another possible advantage is that the cross-divisional listing will help laboratory management and staff recognize areas of potential synergy or opportunities for new collaborative efforts.
Laboratory Resources
Funding sources for the Chemical Science and Technology Laboratory are shown in Table 4.1. As of January 2001, staffing for the Chemical Science and Technology Laboratory included 264 full-time permanent positions, of which 203 were for technical professionals. There were also 106 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
This year, the panel is particularly concerned about a number of issues related to staffing in the Chemical Science and Technology Laboratory. The total number of full-time permanent staff has been decreasing, and the panel observed several areas in which key projects had only a single staff member with the skills and experience to support the work. These cases render the laboratory vulnerable; cross-training of laboratory personnel is necessary to ensure continuity of basic laboratory activities. In other areas, staffing is below critical mass, and progress on important existing programs (such as work in atmospheric chemistry) and new projects (such as the initiative on measurements and standards related to genetically modified organisms) is being impeded. Finally, throughout the laboratory, the number of support staff has declined. The panel notes that the lack of technicians reduces the productivity of professional personnel, who are distracted from their project work by the need to perform routine maintenance on equipment. There are also very few Web programmers, and a great deal of Web-site design and maintenance is done by the technical staff, when they have time. Hiring people with specialized expertise in this area may help address some of the Web-related issues noted above by the panel, such as improving the usability of the sites and the timeliness of updates.
The tight staffing situation is in part a result of relatively flat budgets within the CSTL, but it also reflects a strong economy and a competitive job market, particularly in areas like biotechnology. However, turnover among permanent laboratory staff has been low, and panel members observed high morale in all of the divisions. Therefore, the primary concern of the panel was how CSTL will refresh the staff—that is, attract new young personnel who will support the NIST work on meeting the future measurement needs of industry. National Research Council (NRC) postdoctoral research associates
TABLE 4.1 Sources of Funding for the Chemical Science and Technology Laboratory (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
37.8 |
37.9 |
37.7 |
36.7 |
|
Competence |
2.0 |
2.4 |
2.4 |
2.1 |
|
ATP |
|
|||
|
Measurement Services (SRM production) |
2.3 |
2.4 |
2.2 |
1.7 |
|
OA/NFG/CRADA |
9.6 |
10.9 |
14.2 |
14.3 |
|
Other Reimbursable |
3.0 |
3.4 |
3.4 |
5.2 |
|
Total |
57.7 |
60.0 |
63.2 |
63.0 |
|
Full-time permanent staff (total)a |
280 |
276 |
275 |
264 |
|
NOTE: Funding for the NIST Measurement and Standards Laboratories comes from a variety of sources. The laboratories receive appropriations from Congress, known as Scientific and Technical Research and Services (STRS) funding. Competence funding also comes from NIST’s congressional appropriations but is allocated by the NIST director’s office in multiyear grants for projects that advance NIST’s capabilities in new and emerging areas of measurement science. Advanced Technology Program (ATP) funding reflects support from NIST’s ATP for work done at the NIST laboratories in collaboration with or in support of ATP projects. Funding to support production of Standard Reference Materials (SRMs) is tied to the use of such products and is classified as Measurement Services. NIST laboratories also receive funding through grants or contracts from other government agencies (OA), from nonfederal government (NFG) agencies, and from industry in the form of Cooperative Research and Development Agreements (CRADAs). All other laboratory funding, including that for Calibration Services, is grouped under “Other Reimbursable.” aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
serving at NIST have always been good candidates for potential permanent staff positions, but the panel is concerned that the laboratory might be having trouble recruiting talented members of this group to join the staff. While this is probably a result of tight budgets and more lucrative offers from other institutions, the panel expects laboratory management to track this issue closely.
Another element of laboratory programs that perhaps is being affected by the tight budgets and staffing limitations is the balance between projects with short-term goals and those with longer time scales and more basic research objectives. Both types are necessary to support the current and future measurement and standards needs of NIST’s customers, and the panel is generally pleased with the mix observed in the divisions. However, decisions about relative emphasis should be made strategically, and several cases were observed where pressures on divisional resources were tipping the balance toward shorter-term work in spite of plans for a more even balance. One case is in the Analytical Chemistry Division, where the production and certification of standard reference materials is a major responsibility and that occasionally becomes a significant burden. Another case is in the Biotechnology Division (and elsewhere), where the need to supplement internal funding (STRS monies) with funding from other sources (e.g., other governmental agencies or the NIST ATP) impedes the ability to make long-term hiring or programmatic plans, as external support is often reawarded year to year and can be delayed by processing.
The panel is very pleased to report that CSTL facilities have undergone major improvements. In Boulder, Physical and Chemical Properties Division staff located in rapidly deteriorating Building 3 were moved into much better space in a facility recently vacated by other Department of Commerce personnel. In Gaithersburg, there were major renovations to the work space of some groups in the Surface and Microanalysis Science Division. These two positive steps toward providing laboratory staff with the environments they need to continue to perform measurements and standards activities at worldclass levels build on the occupation of the new Advanced Chemical Sciences Laboratory in 1999 and hopefully will be capped by the completion of the planned Advanced Measurement Laboratory, which is under construction now and due to be finished in 2004. This state-of-the-art building should address the remaining facility issues impeding work in the CSTL.
DIVISIONAL REVIEWS
Biotechnology Division
Technical Merit
According to division documentation, the mission of the Biotechnology Division is to provide the measurement science infrastructure necessary to advance the commercialization of biotechnology by developing the scientific and engineering technical base, reliable measurements, standards, data, and models to enable U.S. industry to quickly and economically produce biochemical products with appropriate quality control.
The Biotechnology Division has four groups: DNA Technologies, Bioprocess Engineering, Biomolecular Materials, and Structural Biology (the last-mentioned forms the NIST segment of the Center for Advanced Research in Biotechnology [CARB], a cooperative venture with the University of Maryland Biotechnology Institute). The division also has a new effort in bioinformatics. The ongoing programs are appropriately aligned with the division mission, and the scientific work carried out in this division is of high quality and comparable to that at research-oriented universities and in leading industrial laboratories. The challenge for NIST will be selecting the projects that are most critical and that will have the greatest impact on this rapidly growing and changing field.
The DNA Technologies Group continues to develop the intellectual and technical base necessary to support and expand NIST’s critical role in supplying DNA-related SRMs that are used for human identity testing and forensics, for DNA diagnostics, and for measuring DNA damage. The efforts in human identification, in collaboration with and supported by NIJ, are clearly world-class. They have yielded SRMs that are widely used, and staff have developed and continue to maintain a popular database on short tandem repeats (STRS).3 Areas in which excellent progress has been made in the past year include the effort to develop higher throughput and more accurate techniques for examining Y chromosome variation; the project on mitochrondrial DNA sequencing, which issued a new SRM in fiscal year 2000; and the development of a heteroplasmic mitochrondrial DNA SRM for detection of heteroplasmy and low-frequency mutation.
NIST has been funded as a biomarker assay validation site for the National Cancer Institute program Early Detection Research Network. Here, the division’s work on chromosome and biomarker validation
|
3 |
The NIST Short Tandem Repeat DNA Internet DataBase is available online at <http://www.cstl.nist.gov/biotech/strbase/>. |
focuses on measurement technologies that use quantitation of chromosomal breakage as a cancer susceptibility test. This work is in the forefront of DNA diagnostics. Similarly, NIST has made significant investments in understanding the effects of oxidative stress on aging DNA, an area in which there are no accurate measurement technologies and standards. Related projects focus on methods to determine the extent and type of DNA damage and on studying mechanisms for DNA repair. To enable accurate measurements, the division is preparing SRM 2396, which consists of 12 stable, isotope-labeled DNA bases; this SRM will allow researchers to use gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry techniques to study damage and repair of oxidatively stressed DNA.
In the area of tissue engineering, the panel commends the Biotechnology Division for correctly recognizing that tissue characterization methods are needed to ensure that tissue-engineered materials are free of mutations and modifications and for focusing on the identification and development of biomarkers, standards, and measurement technologies that will ensure the safety and viability of such materials. This issue is very important to the Food and Drug Administration (FDA) and to industry and is large and complex, but the panel is pleased to note that the Biotechnology Division is carefully targeting its limited resources at specific facets of the problem where its contributions can have the most impact. Given the complexity of the issues, it is extremely important that NIST and FDA efforts be coordinated. The panel was concerned that there did not appear to be any active collaborations with the FDA at this time, but an April meeting between staff from the two institutions may lay the groundwork for a formal agreement that identifies the areas of complementary interest and prevents duplication of effort.
The Bioprocess Engineering Group has had significant successes and is in a transitional period as it refocuses its efforts. Although the work on preparative bioseparations has been productive and staff continue to contribute in the area of DNA separations, the number of personnel on this project is now below the critical level. In biospectroscopy, the panel was impressed by the developmental work on a particle-fluorophore SRM for flow cytometry and genomic microarrays. Current microarray technology is, at best, only semiquantitative, so efforts to increase the rigor and quantitative reliability of microarrays would have a significant and broad impact on research in biotechnology and genomics. In biothermodynamics, the staff have put together a Standard Reference Database (SRD) for thermodynamics of enzyme-catalyzed reactions.4 This database is important for industrial users and has the potential to augment emerging genomic databases with relevant kinetic/thermodynamic information. Some of the users will be biologists with limited math/physics background, and the panel suggests that NIST could make the database more accessible by developing sample problems that demonstrate how the database is used. For example, the development of an updated table such as Table 15 (“Gibbs free energies of formation from the elements for compounds of biological interest”) in the classic microbial energetics article by Thauer et al. would be useful.5 In the biothermodynamics area, the experiences of staff in the Physical and Chemical Properties may be relevant, and if coordination is not already occurring, perhaps more interaction would be useful.
In biocatalytic systems, a recent accomplishment is the synthesis of a highly integrated approach to understanding and characterizing biotransformation in the chorismate pathway. This pathway is of commercial interest, and NIST’s use of a combination of techniques to simultaneously probe the structure and the thermodynamics of the enzymatic transformations is unique. Over the course of this work,
|
4 |
The Thermodynamics of Enzyme-Catalyzed Reactions database (NIST SRD 74) is available online at <http://wwwbmcd.nist.gov:8080/enzyme/enzyme.html>. |
|
5 |
R.K.Thauer, K.Jungermann, and K.Decker, Energy Conservation in Chemotrophic Anaerobic Bacteria, Bacteriology Review 41(1):100–180 (1977). |
expertise from CARB was integrated into this project in support of the goals of the Bioprocess Engineering Group. The panel was impressed by the array of skills developed during this work, and the ability to do an integrated analysis of a metabolic pathway is quite remarkable. The panel believes that this project has been very productive and is nearing completion. The next challenge will be determining how the group can exploit the knowledge and expertise developed in the chorismate pathway work; this will be difficult in part because it is unclear if staff with the relevant computational and nuclear magnetic resonance (NMR) capabilities will continue to be available. In biocatalytic systems, staff also plan to develop an initiative on GMOs. This work would focus on SRM and measurement methods development, and the panel believes that such an effort is timely and completely consistent with the NIST mission. Initial work in this area is progressing well, but staffing levels are below critical mass and are impeding progress.
The work under way in the Biomolecular Materials Group is quite futuristic in nature. It is not as directly connected to customers as work in other groups, and many of the potential applications are years away; however, the panel found the projects scientifically exciting and potentially relevant to a number of NIST programs. For example, the work on nanopores and nanotechnology could contribute to bioMEMS and to techniques for sequencing DNA molecules and could serve as a tool for biosensors. Also, the work on biomimetic membranes is clearly relevant to efforts to understand basic biomolecular assemblies, especially those involving lipid-protein interactions, and it has the potential to serve as a basis for drug screening assays (although this application remains to be validated). Eventually the expertise gained on how to modify and characterize biointerfaces should be useful in the Biotechnology Division’s work on tissue engineering.
The Structural Biology Group forms the NIST component of CARB, a joint undertaking with the University of Maryland Biotechnology Institute. CARB focuses on advanced studies in structural and theoretical molecular biology and seeks to foster the local and national biotechnology industry. Its work is highly complementary to the division’s efforts on the PDB, which are built upon the NIST expertise in structural biology. The panel found that the quality of the science done at CARB is high, as evidenced by several external awards received by staff in recent years. The nature of the work is generally consistent with the NIST mission, although the presence of a university partner changes the environment somewhat. For example, NIST staff at CARB have more responsibility for student training than other NIST personnel and have easier access to funding from the National Science Foundation and the National Institutes of Health (NIH).
The Bioinformatics program is a new effort with several initiatives. The most visible and currently most important is responsibility for NIST’s role in the Research Collaboratory for Structural Bioinformatics, which includes work on software and database support for the Protein Data Bank (PDB).6 The division’s work in this area is a key element of a national resource that will become even more important as the medical, pharmaceutical, and agricultural industries, like academia already, grow increasingly dependent on structural bioinformatics to make the advances and breakthroughs these industries are built on. In its support of the PDB, NIST is well situated to handle the critical issues related to uniformity, data standards, value addition through the compilation of synonyms and citations, and integration of the PDB with data from the international research community. The panel believes that Biotechnology Division staff in this area are discharging their duties with the highest level of scientific rigor. The panel supports the group’s newer initiatives, including the development of specialized databases, such as for HIV proteases, and the development of an application program interface
|
6 |
The Protein Data Bank is available online at <http://www.pdb.org>. |
(API) for NMR/x-ray data. This API will be an important tool for protecting underlying data from the constant format changes associated with the evolution of electronic media.
Program Relevance and Effectiveness
The Biotechnology Division reaches out to its many customers in a variety of ways. Hosting and attending workshops with a targeted list of invitees has proven particularly effective. Staff also interact extensively with individual organizations, including other NIST units, companies, university researchers, and other government agencies. These efforts have been effective, but given the large number (at least several thousand) of biotechnology, biopharmaceutical, and medical device companies, it is unrealistic to expect the staff in this division to be able to make every potentially fruitful connection. The number and variety of potential customers also complicate efforts to disseminate information on NIST results and products, but the division has done what it can in these circumstances, including publishing scientific articles and posting information on the Web. The Protein Data Bank is an obvious example of this last mechanism, but other divisional databases are online, and the DNA Technologies Group has made particularly good use of this approach. The fine record of publication by division staff indicates that NIST is maintaining its commitment to the scientific community, as well as to industry, which is appropriate, given the relevance of basic research to the nurturing of start-up companies in this field.
In general, the panel believes that the Biotechnology Division has done a good job of putting together a set of programs that are directed at meeting current customer needs or laying the groundwork for meeting probable future needs. Current and past projects have impacted a number of industries and research communities. One area in which the effects of NIST work are immediate and obvious is the program with the NIJ on standards and methods for human identification; the division’s results are instantly put to use in the forensics community and can be seen to be having a critical impact on society as a whole. Below the panel discusses other areas in which divisional programs are relevant to and affecting NIST’s customers.
One main field targeted by the Biotechnology Division is genomics and proteomics, which will be a key driver of life-sciences-based technologies over the next several decades. The DNA Technologies Group has done an excellent job of developing programs that deal with important aspects of this field (e.g., human identification, biomarkers and STRs, and mitochrondrial DNA). However, there are many global problems, like technologies for genome-level sequencing, mRNA expression, and proteomics, that remain largely unexplored. When scientists begin to use emerging techniques in these areas, significant issues about data quality and reliability will arise, and tackling these sorts of data-related issues would be consistent with NIST’s mission and suited to the expertise in this division. For example, the Bioprocess Engineering Group has already developed fluorescence standards that can be applied to mRNA arrays. In general, division and laboratory management need to closely monitor developments in the genomics area to allow identification of other opportunities where it is possible for NIST programs to make a difference.
Another NIST program that contributes to general research in genomics is the PDB. This database, which incorporates structures from researchers all over the world, is of ever-increasing importance to the world’s scientific community and is a resource for academic, government, and industry researchers. Staff in this area maintain close ties with the relevant customers through a formal advisory board, an electronic user information line, regular participation in workshops, and involvement in the Research Collaboratory for Structural Bioinformatics. The Bioinformatics Group appears to be very responsive to input from customers and highly committed to providing the best possible services for its user community.
In another high-profile area, scientific results related to GMOs are sought after by industry and
society. The Bioprocess Engineering Group is designing an initiative on the methods and standards necessary to detect the presence of low levels of GMOs in food products, particularly grains. Appropriate groundwork has been laid for customer input and collaborations, as staff have developed cooperative agreements with the Department of Agriculture, four major biotechnology crop companies, and the European Commission Joint Research Centre Institute for Reference Materials and Measurements, the European equivalent of NIST. The panel is enthusiastic about the relevance and potential impact of this program on U.S. industry and the public. Tissue engineering is another area in which there are likely to be significant opportunities for NIST, and CSTL management has identified it as one of nine strategic directions for the laboratory. The DNA Technologies Group has formulated an explicit strategy for developing an SRM for the tissue engineering community, and the Biomolecular Materials Group has also initiated a project in this area.
The Biotechnology Division is also conducting research that is an investment in the future for NIST. For example, scientifically strong work on nanobiotechnology is ongoing in the Biomolecular Materials Group. Investigation of nanopore-based analyte sensors is building NIST expertise in nanobiotechnology while studying a system of potential practical significance for genome-level analyses. The work on biomimetic surfaces should also contribute to this type of technology. These projects are in their early stages and have not yet matured to a point where a strong customer base should be expected.
Defining the customers and the goals for the work at CARB is complex. The University of Maryland and Montgomery County (Maryland) are both partners in and customers for this effort, and Biotechnology Division staff must integrate their work in support of the NIST mission with their responsibilities to support the educational and research goals of the university objectives of the county: to attract industry. Nevertheless, the panel was very impressed by the first-rate work occurring at CARB and the number of collaborations that CARB staff have with other groups in the Biotechnology Division. There are also significant interactions with local companies. In general, though, as might be expected, many of the projects have an academic flavor, and the results are most relevant to an academic audience. In particular, the NIST work on RNA-protein and RNA-ligand complexes could have significant impact in an emerging area where the development and validation of novel and general biophysical measurements will be critical.
The panel has one concern related to the division’s relationships with its customers. The biotechnology sector is evolving rapidly, and intellectual property (IP) is a key component of the valuation of companies. However, as a government-funded research organization, NIST serves the industry as a whole, which means that it disseminates results and technologies as widely as possible. While this is an appropriate overall goal, NIST is responsible for making sure that its results are not just known, but also used. In the biotechnology arena, once information has freely entered the public domain without protection, there is often less incentive to develop these ideas into commercial products that benefit society. This characteristic of the environment in which biotechnology companies currently function should be taken into account when NIST is making decisions about whether to take actions that protect IP (e.g., filing for patents to protect NIST IP or entering into CRADAs to protect companies’ IP). The panel also notes that given the rapidly changing focus of this industry, division staff should receive the legal support needed to execute CRADAs in a timely fashion to ensure that projects and collaborations are not delayed past the potential point of impact.
Division Resources
Funding sources for the Biotechnology Division are shown in Table 4.2. As of January 2001, staffing for the Biotechnology Division included 32 full-time permanent positions, of which 29 were for
TABLE 4.2 Sources of Funding for the Biotechnology Division (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
6.6 |
6.5 |
6.5 |
6.8 |
|
Competence |
0.9 |
0.8 |
0.8 |
0.4 |
|
ATP |
1.9 |
1.7 |
1.9 |
1.9 |
|
Measurement Services (SRM production) |
0.0 |
0.1 |
0.0 |
0.2 |
|
OA/NFG/CRADA |
0.9 |
1.7 |
2.2 |
2.2 |
|
Other Reimbursable |
0.0 |
0.1 |
0.3 |
0.2 |
|
Total |
10.3 |
10.9 |
11.6 |
11.8 |
|
Full-time permanent staff (total)a |
35 |
37 |
35 |
32 |
|
NOTE: Sources of funding are as described in the note accompanying Table 4.1. aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
technical professionals. There were also 32 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
The panel believes that the current resources, particularly human resources, are not adequate to fulfill the mission of the Biotechnology Division. Over the past 2 years, a number of key employees have left NIST, and any organization’s effectiveness would be affected by such departures. From the panel’s perspective, how to attract and retain high-quality staff and how to manage NIST programs in this environment of tight human and fiscal resources are the most critical questions facing the Biotechnology Division. At this time, the panel can point to several areas in which staff departures have left important existing or new projects below critical mass: the tissue engineering work in the DNA Technologies Group and the GMO initiative and the bioseparations project in the Bioprocess Engineering Group. The division has also recently lost three people with expertise in computational chemistry, raising the question of whether NIST can or should maintain a program in this area.
Several things have contributed to the division’s staffing difficulties; all stem from the current funding situation and a hot external job market. Not only do current budget limitations prevent expansion of programs and creation of new positions, but they also impact morale, as the flat funding from Congress over the past 4 years can be interpreted as a lack of appreciation for the quality of NIST’s work and the efforts staff make. Uncertainties about future funding also impact morale, as anxieties about the long-term prospects of their programs may drive staff to look for opportunities elsewhere. The panel was very impressed that, despite these issues, NIST has managed to retain the majority of the key personnel in this division. Since salaries in the private sector are rising (many employees in bioinformatics could easily double their salaries if they left NIST), the people who stay are making financial sacrifices because they believe in the NIST mission and value the supportive environment provided by co-workers and supervisors. It is critical for management to continue to foster an environment in which employee aspirations are supported and accomplishments recognized. Perhaps a system-
atic approach to exit interviews and an analysis of the resulting information could guide the laboratory’s efforts on this problem.
All of the personnel departures will force management to consider the best ways to deploy the available human resources to attain a critical mass in priority areas. The challenge is affected not just by the supply of funds but also by the type of fiscal support currently available in the laboratory. The internal NIST allocation (STRS funds) does not cover the salaries of all of the division staff, which leads to an increasing dependence on support from other agencies and the NIST Advanced Technology Program (although recent changes in rules for ATP spending have decreased the usefulness of these funds). Different programs within the Biotechnology Division appear to have different attitudes to the use of noncore funding and its application to staffing. In some programs, staff aggressively pursue other agency funding in order to hire new personnel and expand into new areas. In others, permanent positions are not created unless they can be supported by NIST core money. The former approach embraces risk and increases the potential impact of the group’s work, while the latter allows program managers to be assured that mission-critical activities are sustained and NIST’s customer base will be served. The panel recognizes that both approaches have value.
The flat internal funds and growing dependence on external support have implications for programmatic planning as well as for staffing levels. To attract funding from other agencies, some projects may be forced to adopt an opportunistic strategy focused on short-term goals. Also, external funds are subject to delays in processing and often must be spent immediately, within the particular fiscal year. These constraints make it difficult to initiate and then sustain long-term programs. As new and important areas emerge within the purview of this division (an example might be nanobiotechnology), a serious and sustained effort will be needed to ensure that NIST work on measurements and standards occurs in a timely fashion and has a critical impact on the growth of a new industry. While the panel believes that the projects in the Biotechnology Division are appropriately directed at serving the needs of NIST’s customers, those customers are so diverse and the relevant technologies are changing so rapidly that it is unrealistic to believe a group of this size can satisfy all of the measurement and standards needs that are or may become apparent. Opportunities in biotechnology are abundant and can emerge quickly, so a strong strategic planning process is crucial to identifying and anticipating future customer needs at the earliest possible moment.
Overall, facilities and equipment do not appear to be limiting the range of projects undertaken by the Biotechnology Division or to be serving as barriers to progress on existing work. The division’s facilities in the new Advanced Chemical Sciences Laboratory are excellent and, in general, equipment is appropriate in quantity and quality for meeting the division’s mission. However, the loss of NMR capabilities on the NIST campus is a potential problem, as a lack of in-house expertise and equipment in this area will hinder research in biocatalysis. A mitigating factor in the panel’s concern is that NMR instrumentation is readily available at CARB, although a larger, faster machine would improve efficiency.
Process Measurements Division
Technical Merit
According to division documentation, the mission of the Process Measurements Division is to pursue research efforts in measurement science as the basis for enhancing measurement standards and services, measurement techniques, recommended practices, sensing technology, instrumentation, and mathematical models required for analysis, control, and optimization of industrial processes. Improvement and dissemination of national measurement standards for temperature, fluid flow, air speed,
pressure and vacuum, humidity, liquid density, and volumetric measurements are core division responsibilities.
The work being done within the division is consistent with this mission. The division is organized into six groups: Fluid Flow; Fluid Science; Process Sensing; Thermometry; Pressure and Vacuum; and Thermal and Reactive Processes. A broad range of research is being carried out in new and traditional fields, including the development of world-class measurement methods, standards, and calibration services and the determination of important physical properties of chemicals of interest to a number of industries. The excellent technical staff are an important resource for the division; their quality is demonstrated by the awards they have received, such as the NASA Lewis Distinguished Publication Award and the NIST Sigma Xi Young Investigator of the Year (2000).
The technical merit of the ongoing projects remains generally very high. The division supports development of state-of-the-art measurement instrumentation and methods, and staff collaborate directly with researchers in industry and academia. In many cases, division personnel are these researchers’ primary source of information on measurement techniques and new technologies relevant to process measurement issues. NIST has assumed a leadership role in many international standards comparisons, and the results of these comparisons demonstrate the quality of NIST data and methods. The division work allows NIST to provide critical standards for pressure, temperature, and flow that are the best in the world. Below the panel briefly describes nine examples of the excellent technical accomplishments of the past year in the Process Measurements Division. These accomplishments are just the highlights and are representative of the balanced mix of world-class measurement technology and industrially relevant research that makes up the division’s portfolio.
-
The Fluid Sciences Group continues to collect data on the thermophysical properties of hazardous gases used in the semiconductor industry, and in 2000 results were published and a database was posted on the Internet.7 These data are needed by industry to model chemical vapor deposition processes and to calibrate mass flow controllers.
-
The measurement phases for four key comparisons of realizations of the International Temperature Scale of 1990 (ITS-90) are finished, and staff in the Thermometry Group completed an exhaustive draft report on the key comparison led by NIST (KC-3, 83.8058 K to 933.473 K).
-
In the Pressure and Vacuum Group, staff are participating in six key comparisons spanning a pressure range from 10–6 Pa to 500 MPa. NIST is the lead on three of these, owing to its recognized position as the world leader in the relevant measurement capabilities.
-
Division staff are leading the new CIPM/CCM (Consultative Committee for Mass and Related Quantities) Working Group for Fluid Flow, which will work with national measurement institutes (NMIs) worldwide. The goal of this working group is to identify areas where key comparisons are needed to resolve measurement-based trade issues and thereby facilitate free trade in the global marketplace.
-
The Thermometry and Fluid Science Groups continue work on a competence project on measuring thermodynamic temperature via acoustic thermometry; lately they have developed the facility and methods needed to extend the range of this technique up to 700 K.
-
In their work on rapid thermal processing for the semiconductor industry, Thermometry Group staff are developing on-wafer thermocouples for the calibration of radiation thermometers. Collabora-
|
7 |
The Database of the Thermophysical Properties of Gases Used in the Semiconductor Industry is available online at <http://properties.nist.gov/fluidsci/semiprop/>. |
-
tions with tool manufacturers and the hosting of a key workshop in 2000 contributed to progress in this area.
-
In the Thermometry Group, the first humidity calibrations were performed using the NIST-developed low frost point generator, which can function at 10 nmol/mol. Now the division is using this instrument to provide a standard for the round-robin tests of commercial low-humidity generators being conducted by members of Semiconductor Equipment and Materials International.
-
In the Process Sensing Group, progress in the work on micro-hotplate arrays for chemical sensing included the demonstration of stable performance of the devices over a 100-hour testing period. The devices can measure 20 to 50 ppm of methanol and ethanol in air while retaining a detection limit as low as 10 ppb for methanol.
-
In the work on standards for Raman spectroscopy, the uranyl glass fluorescence standards for the 514-nm argon wavelength laser have been tested internally. The Thermal and Reactive Processes Group is now organizing a round-robin through the ASTM that will include nine users from industry, instrument manufacturers, and universities. Data from this intercomparison will be used to establish these artifacts as SRMs.
Program Relevance and Effectiveness
An important customer of the Process Measurements Division is the semiconductor industry, but there are many facets to the division’s work and a variety of ways in which its results impact its customers. Staff interact with external organizations through collaborations with industrial and academic researchers and at workshops and conferences, and information about division results and services reaches its audience through publications in refereed journals, technical presentations, citations of NIST work in other literature, and postings on the NIST Web site. The calibrations performed in this division are world-class, cost-effective, and timely; the industrial customer base finds them very valuable and is highly appreciative of the NIST work. These services are a fundamental element of the NIST measurements and standards mission, and the panel notes with approval that calibrations and the international comparison efforts are given a high priority within the division.
One example of an effective interaction with industry can be seen in the work on standards and physical property data for improved mass flow controller performance. In May 2000, NIST held a workshop that brought together the users and manufacturers of mass flow controllers.8 At that workshop, these stakeholders worked with NIST staff to identify what physical property data on hazardous semiconductor gases were needed to support accurate calibration of these instruments and what the relative priorities were for various precise flow measurements of these gases. Many of the requested measurements were completed in 2000, and division staff have published their results in journals, posted them on the NIST Web site, and will report the information in the trade journal Semiconductor International. Work continues on other measurements. NIST results in this area are now leading to the development of meaningful calibration factors for mass flow controllers in which the nitrogen flow calibration achieved at the time of manufacture is related to the actual flow rate of hazardous or toxic gas while the device is in use.
Another program that is producing results of immediate importance to the U.S. semiconductor industry is the work on rapid thermal processing. During the rapid thermal processing of semiconductor wafers,
|
8 |
More information on the NIST Workshop on Mass Flow Control for the Semiconductor Industry, held May 15–16, 2000, and on the work NIST did in response can be found online at <http://www.cstl.nist.gov/div836/workshops/mass_flow.htm>. |
in situ measurements of surface temperatures on the order of 1000 °C must be performed using techniques like optical thermometry. With input they received from industry at the 8th International Conference on Advanced Thermal Processing of Semiconductors (held in September 2000 at NIST) and at other workshops on noncontact thermometry, NIST staff were able to realize a method for in situ calibration of light pipe radiation thermometers that uses wafers instrumented with a combination of thin film and Pt/Pd wire thermocouples of various emissivities. A patent on this approach was issued in March 2001 and licensed to Watlow-Gordon, Inc., for commercial production of these instrumented wafer devices. In preparation for common use of this technology, manufacturers of rapid thermal processing tools are arranging for connections to these thermocouples to be available within the vacuum chambers. Overall, the result of NIST’s work is that the semiconductor manufacturing industry will now have a system to calibrate the optical thermometers used in process measurement to an accuracy of ±2 °C.
There are numerous other areas in which technologies developed in the Process Measurements Division are having an impact on the productivity of U.S. semiconductor-related industries. The low frost-point generator is being used in a new test service that provides direct traceability to a NIST humidity standard. Using secondary standards calibrated with this service, semiconductor gas manufacturers now will be able to more accurately qualify their gas purity. In a collaborative effort with the flow meter manufacturing division of Emerson Electric Co., a prototype ultrasonic gas flow meter was used to develop methods that can recognize different flow classifications and differentiate among various disturbances to the flow. This system will allow companies to more accurately measure average gas flow in geometries where upstream structures alter the flow pattern in the stream at the measurement point.
In plasma processing in semiconductor manufacturing, the division is working with the Electronics and Electrical Engineering Laboratory and the Physics Laboratory on developing measurement and modeling techniques. Current activities include using planar laser-induced fluorescence imaging to investigate species densities in Gaseous Electronics Conference (GEC) Radio-Frequency Reference Cells. The modeling associated with experiments on these chambers is good science, and the panel believes that understanding of the reference system is good enough that staff might move on to modeling chamber designs more closely aligned to current industry needs. NIST has organized a Plasma Processing Common Interest Group to foster interactions with plasma tool manufacturers, and this group met twice last year. Staff are encouraged to build on these relationships in order to get industry input on what experiments with relevance to real-world problems could be done in the GEC chambers.
In addition to supporting semiconductor-related companies, the Process Measurements Division has several projects with the potential to support a broader range of industries. For example, micro-hotplate arrays for chemical sensing and measurement could have a number of industrial applications, such as environmental modeling at hazardous waste sites. NIST work on this “electronic sniffer” approach is built on extensive collaborations with many universities and four commercial manufacturers. Division staff are also working on microfluidic devices, in which a small sample is analyzed via transport through an array of channels and sensors that determine specific characteristics. While other organizations are investigating the use of silicon-based devices, NIST is focusing on methods for forming microchannels in polymer substrates, which would be a more cost-effective platform from which to enable the development of disposable lab-on-a-chip cells. Again, staff are cooperating with relevant players from industry and academia.
The panel commends the division for not assuming that successful impact should automatically result in continuation of a program. NIST work on self-assembled monolayers has been instrumental in the development of techniques that use these materials for chemical and biological sensing. At this point, the biochip industry is self-sustaining and is expected to grow to $1 billion by 2003. Therefore,
NIST has made the decision to terminate research on self-assembled monolayers and redirect the resources to the microfluidics area. The panel applauds this decision.
An area of concern is the underutilization of the division’s reference spray combustion facility. In June 2000, NIST hosted the workshop “Metrology Needs for Multiphase Combustion Data,” at which discussions focused on data needs for multiphase combustion models and industrial metrology needs for development and calibration of instruments and diagnostics. While modelers from academia and industry are using the data produced at NIST, it is not clear that their activities will manage to interest outsiders in using the combustion test facilities. In the meantime, the facility is being used to produce soot under known conditions for an SRM. The panel strongly recommends a concerted push to cultivate industrial use of this unique facility. If outside users cannot be found, it would be practical to redirect the computational fluid dynamic modeling skills devoted to this project to work on a more pressing chemical engineering problem with industrial applications.
Another effort where the panel is concerned that NIST work is not and will not be of value to industry is the effort on modeling and measuring rotating-disk chemical vapor deposition (CVD) processes. The panel continues to question the relevance of this work to mainstream CVD processes. While one commercial CVD unit uses this geometry, the manufacturer is not working with NIST or using the NIST results. The most significant accomplishment of this combined experimental and modeling program is the prediction and demonstration of a stratification layer of SiO2 particles that migrate radially with gas flow above the rotating wafer. The panel cannot encourage further efforts along these lines, as it is unclear what the value of such work would be. In fact, the panel recommends that the considerable modeling skills of the division staff assigned to this project be redirected to more relevant problems, such as studying nanotube growth or gas chemistries for CVD processes. On the experimental side, the techniques being used at NIST are readily available elsewhere. Duplication of external efforts is unnecessary, and in the current budget environment, the division should not allow such activities to drain resources from other projects more relevant to industry or to prevent the start-up of new programs in response to customer needs.
In the areas described in the two preceding paragraphs, the panel has doubts about the quality of the connections to industry and the likelihood of the NIST work successfully impacting industry behavior. In other projects of the Process Measurements Division, there is frequent and direct contact with companies and people familiar with industry needs, but in those two areas, the relationships with external parties are much more general and staff do not appear to have a grasp of how NIST work can be specifically applied to current industrial problems or to increasing fundamental understanding of an area relevant to industry. The panel encourages the division to renew its efforts to establish industrial collaborations in these areas in order to gain input from appropriate industry representatives on which questions the talented NIST researchers might productively address.
The World Wide Web can be an important tool for disseminating results and allowing a wide variety of potential customers to access NIST materials. Yet panel members who used the NIST Web site discovered that it was sometimes difficult to find important data or program information, even when they knew the Web pages existed somewhere on the site. To facilitate use of this dissemination mechanism, the panel suggests that an effort be made to minimize the number of clicks needed to get from the NIST home page to the Web page with a desired piece of information. For example, a page’s keywords for searches should include relevant industries served by the data on the site and should perhaps utilize common industry jargon.
The panel appreciates that it is often difficult to keep on top of all the issues related to building and maintaining an effective Web site. It is important to note that once materials are posted on the Web, they are immediately accessible all over the world. Therefore, to maintain NIST’s reputation for producing
results and data of the highest quality and to ensure that only the information meant to be public becomes available, materials should be reviewed carefully before posting and periodically thereafter.
Division Resources
Funding sources for the Process Measurements Division are shown in Table 4.3. As of January 2001, staffing for the Process Measurements Division included 58 full-time permanent positions, of which 40 were for technical professionals. There were also 18 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
The largest current concern of the panel is staffing. There are some areas within the division where the number of personnel is at or below critical mass, and therefore certain competencies cannot be maintained or expanded to sustain capabilities at the world-class level expected of NIST. The division is losing skilled researchers because of retirements, transfers within NIST, and resignations to take other jobs. The primary goal of management is to bring in young scientists to fill these slots and to provide the new ideas and skills necessary for future growth and impact. However, a number of factors are currently limiting the appeal of NIST as a research organization for young scientists. Normally, candidates for permanent staff positions are drawn from the pool of NRC postdoctoral research associates who serve 2-year terms at NIST. Outstanding associates are recruited during their second year to stay on in a permanent capacity, just as they are usually receiving offers of higher-paying jobs from industry and academia. The decision to remain at NIST, despite other opportunities, is often based on the chance to work on world-class research in the distinctive NIST environment. It is the skilled staff and unique facilities dedicated to developing process and measurement standards and calibration needs that distinguish the Process Measurements Division from industry and other organizations. Division management must therefore maintain the division’s facilities at a high level, creatively provide access to quality
TABLE 4.3 Sources of Funding for the Process Measurements Division (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
8.1 |
7.9 |
8.7 |
8.6 |
|
Competence |
0.6 |
0.8 |
0.9 |
1.2 |
|
ATP |
0.3 |
0.4 |
0.5 |
0.1 |
|
Measurement Services (SRM production) |
0.1 |
0.0 |
0.0 |
0.0 |
|
OA/NFG/CRADA |
0.4 |
0.8 |
1.0 |
1.6 |
|
Other Reimbursable |
1.2 |
1.2 |
1.1 |
0.9 |
|
Total |
10.7 |
11.1 |
12.3 |
12.4 |
|
Full-time permanent staff (total)a |
59 |
59 |
57 |
58 |
|
NOTE: Sources of funding are as described in the note accompanying Table 4.1. aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
capital equipment, and find funding for new and innovative programs to sustain the division’s appeal to talented young scientists.
There is no substitute for permanent staff with a commitment to the NIST mission and expertise in metrology-related issues. However, given the tight budgets for hiring new workers, NIST should consider exploring creative ways to bring in temporary personnel, especially those supported by other organizations. Examples would include apprenticeship programs for people from the armed services or the military academies (like the internships at Los Alamos National Laboratory) and cooperative arrangements with area universities in which academic credit would be given for work done at NIST.
One of the consequences of the staff departures of the past several years has been the turnover in the division management. Within the past 3 years, the division has replaced the division chief and four of six group leaders. (The positions opened up as the result of retirements, reassignments, and one person leaving NIST for a different job.) A new management position, deputy division chief, was also created, meaning that a total of six positions needed to be filled. All of these positions have been filled, and all of the new managers are leaders grown within the organization (three of them had spent a year in the NIST Program Office as a part of their management training). The panel commends the division and laboratory for good succession planning and career development efforts and for the successful execution of a smooth transition, as evidenced by the positive staff morale. The new management team is young, but experienced, and has demonstrated enthusiasm for, knowledge of, and a generally good focus on the division projects and goals. The only potential concern is that the division might become too inwardly focused during program evaluations. The panel notes that the new leadership does seem to be genuinely interested in and receptive to external input on NIST work, as evidenced by their formal approach to developing a strategic plan for the division.
Over the past year, some facilities improvements have been made in division laboratories. For example, the water flow system is being refurbished in the large-bore fluid flow measurement facilities. However, problems still exist. In the pressure calibration laboratory, temperature control is still a problem, and construction of the AML might produce surface vibrations that would affect the accuracy of the calibrations. Staff are considering if conducting the pressure calibration measurements at night would be an adequate work-around, and the panel notes that increased automation of these services would be desirable. The division is currently involved in discussions about developing MEMS fabrication capability at NIST using tools, facilities, and staff that already exist on the Gaithersburg campus. The panel supports the effort to provide such capabilities locally, and notes that the design experience would be useful as NIST prepares to construct a dedicated MEMS fabrication facility that will be located in the new AML.
Surface and Microanalysis Science Division
Technical Merit
According to division documentation, the mission of the Surface and Microanalysis Science Division is to serve as the nation’s reference laboratory for chemical metrology research, standards, and data to characterize the spatial and temporal distribution of chemical species and to improve the accuracy, precision, sensitivity, selectivity and applicability of surface, microanalysis, and advanced isotope measurement techniques.
Since last year’s review, the division has significantly enhanced the content and focus of its mission statement. This statement now clearly and concisely defines the division’s role and responsibilities within the Chemical Science and Technology Laboratory and NIST. A strong mission statement can be
a effective tool to be used both internally to provide focus during strategic planning (as the division is doing) and externally to communicate to customers and collaborators what various parts of NIST are doing and which units’ activities might be most relevant to their needs.
The Surface and Microanalysis Science Division is organized into four technical groups: Atmospheric Chemistry, Microanalysis Research, Surface Dynamical Processes, and Analytical Microscopy. In addition to personnel in these groups, the division staff includes two NIST fellows who pursue very active research programs and provide mentoring and technical guidance to younger staff. The division is fortunate to have its programs supported by these fellows, productive scientists who are internationally recognized as leaders in their fields.
The organization of the Surface and Microanalysis Science Division is sensible and has generally enabled the division to effectively respond to the need for programmatic evolution while maintaining logical groupings of expertise. The boundaries of the groups are transparent in many ways; the panel observed significant collaborations between the groups and with other parts of the laboratory and NIST. Currently, division projects are aligned in support of 7 of the 12 CSTL program directions and play a pivotal role in four: Semiconductor Metrology, Nanotechnology, Chemical Characterization of Materials, and Environmental Measurements.
The technical programs in the Surface and Microanalysis Science Division are world-class. Staff continue to receive significant recognition for their work from within NIST and from the larger scientific community and are much sought after as speakers at major international technical symposia, conferences, and workshops. The panel is impressed with the achievements of the division over the past year and applauds the staff’s efforts to ensure that the high-quality work at NIST is appropriately directed at addressing major customer needs.
The Surface Dynamical Processes Group continues to conduct state-of-the-art research in the molecular spectroscopy of surfaces. The focus of this group has evolved over the past year; several projects were completed and work was initiated in new areas. The emphasis will be on the development and application of chemically sensitive probes of surface structure and dynamics. Staff will look at a variety of techniques with spatial resolutions that range from micron to atomic; examples include vibrationally resonant sum frequency generation, infrared (IR) and Raman near-field scanning optical microscopy (NSOM), spatially resolved dielectric constant measurements, and electron tunneling spectroscopy. These efforts build on past accomplishments and expertise developed within the division. For example, this group pioneered the use of broadband femtosecond infrared lasers as sources for sum frequency generation. The advantage that results from the combination of these sources with multichannel detectors has made this technique sufficiently sensitive that it can be more routinely applied to a wide variety of problems in surface and materials science. The group also has an active research program in vibrational NSOM; key accomplishments of the past year include the development of a constant-height scanning mode that eliminates topographical interferences from the spectra, demonstration of submicron resolution for IR NSOM, and sensitivity improvements of about two orders of magnitude for Raman NSOM. Work in the last area will focus on further increasing the sensitivity by exploiting the surface-enhanced Raman effect.
As part of the programmatic evolution mentioned above, the Surface Dynamical Processes Group’s theoretical activities have been redirected and expanded to include both analytical and computational analysis of electron tunneling phenomena at interfaces. The panel supports this shift in focus and notes that this work will make an important contribution to the Surface and Microanalysis Science Division’s component of the new CSTL competence project on molecular electronics being carried out in collaboration with the Electronics and Electrical Engineering Laboratory. The group is also playing a key role in another new competence project, Polymeric Thin Films: A Test Bed for Combinatorial Methods,
which is a collaboration with the Material Science and Engineering Laboratory. Success in obtaining funding for two competence projects in the highly competitive contest for these awards is clear evidence of the group’s initiative and its ability to form partnerships to imaginatively tackle important interdisciplinary problems. The new proposals probably benefited from the group’s past experience with competence projects; the work on in situ nanoscale probes of catalytic systems was successfully concluded this past year, and work based on this effort will continue as a core program of the group supported by divisional funds.
The Microanalysis Research Group performs research at and beyond the state of the art in techniques for electron and x-ray beam microanalysis. Investigation of transmission electron microscopy (TEM) and analytical electron microscopy systems continues to be a strength of this group. Recent accomplishments include development of a method to allow unbiased calibration of magnification of the images of ultrathin SiO2/Si interfaces that are obtained using cross-sectional TEM. This work on TEM methods is complemented by the study of grazing incidence x-ray photoelectron spectroscopy (GIXPS). While this tool is currently only available at sites with synchrotron radiation sources, GIXPS is worth investigating because it could provide a method for the nondestructive calibration of reference SiO2 films. For other spectroscopy-related research activities, the group finally acquired two state-of-the art Auger electron spectrometers in 2000, and the panel is looking forward to hearing next year about new advances in qualitative and quantitative surface analysis that the division has made using this popular spectroscopy tool.
Recently the Microanalysis Research Group began to reemphasize work on how Monte Carlo methods could be used to model electron microprobe analysis of quantitation on nonideal surfaces. NIST researchers pioneered the use of these methods for analysis of smooth surfaces many years ago, but since few industrially relevant surfaces are smooth, there has been a large gap between the methods and models that work only in ideal cases and what is needed for practical analysis. The panel supports the group’s decision to rededicate resources to this effort and encourages the staff to keep their focus on methods applicable to arbitrary structures.
The Microanalysis Research Group is responsible for the Desktop Spectrum Analyzer, a software product that has been widely used since its release in the early 1990s on a Macintosh platform. Recent efforts in this area have focused on producing three-dimensional “movies” from TEM images, and the panel is impressed by this work because it may provide researchers with a new tool for understanding the structural properties of small particles. The group is also working on novel lattice measurement algorithms for TEM image calibration. While these activities are appropriate, the panel was concerned to learn that the group is developing software based on an uncommon programming system, MacLispix. Macintosh platforms are rare in industrial laboratories, so the ability to run on a standard PC platform is essential for long-term product deployment. Perhaps using a more common language (e.g., Visual Basic, C++, or even FORTRAN) or a standard scientific data analysis platform (e.g., MatLab, MAPLE, Mathematica, or Lab View) would be more practical. Another approach might be that used in the Interactive Standard Test Data Web Site, recently released by the Atmospheric Chemistry Group; this approach uses the World Wide Web to interface with the user and hence is nearly platform-independent.
In the Atmospheric Chemistry Group, staff continue to focus on advanced isotope metrology and chemical measurement processes that are needed to develop and enforce U.S. National Ambient Air Quality Standards (NAAQS) for ozone and particulate matter (PM). The most confounding element of PM is carbonaceous material, which results from particulate emissions and from airborne transformations of emissions from multiple sources. The three key measurement needs in this area are (1) particulate reference standards containing known and quantified mixtures of ambient sources, (2) methods to distinguish the emissions resulting from different types of fossil fuel usage, and (3) methods to
identify natural sources of particulate emissions. For work in these areas, the panel was pleased to learn that NIST had entered into an important interagency agreement with the EPA. This collaboration will focus on the development of a number of reference materials and technologies that are immediately needed by the communities interested in PM standards and measurements. Examples of the expected products include an urban PM-on-filter reference material for carbonaceous species, methods for providing PM calibration materials for organic and elemental carbon, and technology for the large-scale collection of airborne PM less than 2.5 μ in diameter. This collaboration, which involves the EPA, the Atmospheric Chemistry Group of the Surface and Microanalysis Science Division, and the Analytical Chemistry Division, is a fine example of NIST staff working together and reaching out to a key customer for the division’s work on air quality reference materials, and the panel expects that these efforts will produce substantive results in the coming years.
In continuing activities, the group is working on how to extend carbon-dating differentiation of pollution from fossil and natural sources by developing techniques for isotopic profiling of organic and elemental fractions using microanalysis of particles, multi-isotopic speciation via compound-specific isotope ratio mass, and compound-specific infrared/mass spectrometry. The recent purchase of a new thermo-optical PM analyzer should provide staff with an important tool for establishing correspondence between NIST and field-analysis laboratories. Also, the isotopic profiling of organic and elemental fractions, used in conjunction with other tracer analyses, offers the potential to add another analysis dimension to distinguish fossil fuel emission sources, such as the transportation and power generation sectors.
This past year, the Atmospheric Chemistry Group wrapped up work on two significant and successful projects. In the first one, staff established a Web-based tool that calculates carbon-13 and oxygen18 values from measurement data and other information supplied by the user.9 By enabling the standardized processing of measurement data, NIST was able to contribute to a significant improvement in the reproducibility of these measurements, which are made internationally. In the second project, NIST expertise in isotopic analysis was applied to a commercial problem: how to distinguish between the products of two different manufacturing processes when the materials produced were chemically identical. In this work, NIST expertise in isotope analysis was used to develop a measurement method and reference materials based on the levels of carbon-13 and carbon-14 in the final products. While the work was supported by industrial funds from a CRADA and the results allowed the manufacturer to patent the new, more economical, “greener” process, the measurement technique developed at NIST might also prove useful for other sectors of the chemical manufacturing industry that encounter similar barriers to adopting new manufacturing processes. The panel commends the Atmospheric Chemistry Group for not only selecting projects on relevant topics with results that could be immediately and productively applied, but also for completing the work in a timely manner.
The size of the Atmospheric Chemistry Group is cause for significant concern. The June 2000 retirement of the division’s NIST fellow with expertise in this area further reduces the resources devoted to this field. The panel wonders if there might be a way to combine the Chemical Science and Technology Laboratory’s distributed array of atmospheric metrology activities in order to allow a critical mass of personnel working on environmental chemistry measurements to be formed in some part of the laboratory (the Surface and Microanalysis Science Division or elsewhere). An example of the problem can be seen in the area of isotopic profiling; here it is not clear that the current team is large enough to effectively advance the research under way, and the group needs to develop a clear vision of
|
9 |
The tool can be found online at <http://www.acg.nist.gov/outputs/algorithm.html>. |
exactly how this effort might make a significant impact so that future work may be directed toward the most relevant projects. Laboratory and division management are clearly aware of the issue of critical mass, and an effort is being made to define the future direction of atmospheric and environmental metrology work in the laboratory and to move forward on any necessary changes in organization or programmatic focus.
The Analytical Microscopy Group continues to be a premier source of research and applications for ion- and photo-based microscopies. The work on new standard reference materials for dopant profiling is essential for the semiconductor industry, and NIST researchers are viewed as the international leaders in this field. The panel encourages continuation of this work and specifically suggests developing other dopant profile standards of value to industry, such as phosphorus in silicon as well as boron, arsenic, and phosphorus in common metal silicides (e.g., CoSi2, TiSi2). In other work, the group is taking a leading role in the investigations of how to perform very shallow depth profiling using time-of-flight secondaryion mass spectrometry (SIMS) and is developing cluster-ion SIMS techniques that will allow the composition of “soft” surfaces such as biomaterials and polymers to be characterized. These are both important areas of research, and the panel commends the division for its thorough and high-quality work so far.
A collaborative effort to develop Raman spectral intensity calibration standards involves the Analytical Microscopy Group and researchers in the Process Measurements and Analytical Chemistry Divisions. Raman spectroscopy is becoming an increasingly popular tool for materials characterization, but it is impossible to compare results obtained from different spectrometers without intensity standards. NIST staff have a series of luminescent glasses to serve as standards for spectra excited by 488, 514.5, 533, 633, 785 and 1064 nm wavelength radiation. These glasses are stable and not subject to photo-bleaching by the lasers used to excite the Raman spectra. Currently the staff are working through an ASTM committee to evaluate these glasses, and the resulting new standard reference materials are expected to become available in fiscal year 2001. The panel is pleased to see that the group’s efforts in this area are nearing fruition.
Program Relevance and Effectiveness
The Surface and Microanalysis Sciences Division is very visible both nationally and internationally. The staff publish their work extensively in prestigious scientific journals, organize major conferences and present papers at them, and produce and maintain databases on the Web that are used by a wide variety of technical communities. The ongoing division programs are clearly focused on the needs of NIST’s customers in industry and at other government agencies. Staff meet regularly with their colleagues in organizations like the Environmental Protection Agency, the Department of Defense, and the Department of the Treasury to ensure that the division has the input it needs to fulfill the part of its mission directed at supporting national security and the environment.
The Surface and Microanalysis Science Division continues to interact with its customers in a variety of industries, and the panel commends the staff s efforts to seek out industrial partners and their focus on providing critical standards to support U.S. industry. As always, the division utilizes information from contacts with and roadmapping documents of industry groups, such as Vision 2020, the Semiconductor Industry Association (SIA), the National Technology Roadmap for Semiconductors, the International SEMATECH Analytical Laboratory Managers Council, and the Polymer Interphase/Interface Consortium, when determining new areas of focus. Recently, the division increased its efforts to build stronger links with its customer base, and in the past year there have been interactions with a number of
companies, including large corporations such as DuPont Corporation, Dow Chemical Corporation, and General Electric Corporation, as well as smaller, more narrowly focused businesses like instrument manufacturers Photon Imaging, Inc., Millennium Corporation, McCrone Associates, and others. These interactions range from visits of a day or two at NIST or at the company, to NIST hosting of guest workers from industry, to long-term collaborations of NIST staff with industrial scientists that result in coauthored papers. These are all important elements of building a strong relationship with NIST customers, but the panel continues to encourage division management to supplement them with an industrial fellowship program for NIST staff that allows them to spend 3 to 6 months working in an industrial environment. While there are often personal circumstances that make it difficult for staff to relocate for any length of time, the panel notes that NIST hosts a large number of guest researchers from companies and other organizations who have overcome such circumstances and suggests that these researchers may be able to provide practical advice on ways to work around the difficulties associated with temporary displacements. These guest researchers, and indeed all of the people that the division has met through its renewed push for company partners, also should be able to help the division figure out which industrial laboratories would be good hosts and what factors contribute to successful and productive assignments at these laboratories.
The relevance of the Surface and Microanalysis Science Division’s work to customer needs is based on good relationships with those customers, including extensive collaborations, and evidence of past success can be seen in the number of times external groups solicit this division for help with specific problems or with requests for new programs or products from NIST. Overall, there are many examples within the division’s programs of new, ongoing, and recently completed projects that have had impact on a number of technical communities or are directed at their needs. Below, the panel discusses some of these programs.
A good example of how the Surface and Microanalysis Science Division uses input from industry to help determine new programmatic directions in support of emerging customer needs can be found in the work done to prepare for the new competence program on combinatorial methods. First, division staff, in conjunction with the Materials Science and Engineering Laboratory and the Advanced Technology Program at NIST and the Army Research Office in the Department of Defense, organized a workshop at which approximately 140 participants discussed the potential role of combinatorial methods in research, development, and process optimization for a number of fields, such as catalysis and polymers. Second, the division and the Council for Chemical Research worked together to begin a Vision 2020 roadmapping exercise on combinatorial methods; a final report is expected sometime in fiscal year 2001. Now, the division is starting projects relevant to the needs exposed in these meetings; examples include work on polymeric thin films and on high throughput methods for combinatorial chemistry. In this latter area, the Surface Dynamical Processes Group will work with NIST staff from the Materials Science and Engineering Laboratory and the Information Technology Laboratory and will consult with personnel in the NIST Advanced Technology Program for input on industrial issues.
An example of the value of having input from industry during the course of a project can be seen in the Surface Dynamical Processes Group’s work on chemical imaging with NSOM. DuPont was deeply involved in this work, having placed a guest researcher at NIST for the duration of the project. The effort was supported by competence funds and resulted in the development of key new measurement capabilities that will impact traditional fields such as catalysis and particle characterization as well as build expertise that may be applicable to work in new areas such as combinatorial methods and molecular electronics. The technologies are now being transferred to industry with DuPont’s current work on constructing a Raman NSOM facility based on NIST design. The panel members applaud the division’s
work in this area and note that their views are shared by the chief science and technology officer at DuPont Research and Development, who sent a formal letter of appreciation for the opportunities created by the collaborations with NIST.
The division works with industrial consortia as well as with individual companies. For example, International SEMATECH was an active participant in the Microscopy Research Group’s work on TEM techniques relevant to the semiconductor industry. Many industrial laboratories use cross-sectional TEM to measure SiO2 thickness and determine interface quality when studying ultrathin SiO2/Si interfaces, but these methods have not been systematically characterized, and significant variation from laboratory to laboratory can occur. Division staff developed a method to allow unbiased calibration of image magnification, and with this method, 2-nm-thick SiO2 films can nominally be measured to an estimated precision of 0.2 nm. This type of measurement capability is crucial to the development and manufacturing of next-generation complementary metal oxide semiconductor process technologies, and the panel applauds the division for performing this research in cooperation with International SEMATECH to ensure that industrially appropriate materials were used and that information will be disseminated to the scientists who will most benefit from it.
Another Surface and Microanalysis Science Division project with significant impact on the semiconductor industry is the work conducted with the Analytical Chemistry Division on developing techniques that could be used to make a NIST-certified SRM for dopant profiles. Industry has long needed such standards in order to calibrate arsenic ion implantation in silicon, because without standards, measurements can only be used to compare like machines, making it difficult to translate manufacturing processes from the development fabrication stage to the production facility and from one implanter to another. NIST staff used instrumental neutron activation analysis methods to certify the needed SRM, which has an expanded uncertainty of less than 1 percent. This project received the fiscal year 2000 CSTL Technical Achievement Award.
In the Analytical Microscopy Group, individual projects are often applicable to a diverse array of industries. For example, the work on phase mapping of ceramic coatings will help manufacturers of a variety of engine types. The isotope analysis looking at variations in properties due to different feedstock powders and chemical processes demonstrates the division’s ability to respond quickly to an industrial need and develop a tool of general and widespread applicability. The work on new Low-Index Liquid Refractive Index Standard (SRM 1922) is another product with an assortment of customers. This product will meet the standards needs of the different industry sectors that require rapid and simple methods for determining solute concentrations. The uncertainties that will now be obtainable in measuring the temperature dependence of refractive indices are approximately an order of magnitude better than previously achievable on most commercial refractometers. The staff on this project were nominated for the fiscal year 2000 CSTL Technical Achievement Award.
Cooperative efforts and input from external parties are also important when NIST is working with other government agencies. The Atmospheric Chemistry Group is to be congratulated on its progress in this area in the past year. Not only was a formal interagency agreement with EPA signed, but other collaborative work was also initiated. One example is the project on applying carbon-dating techniques to distinguish fossil fuel from biogenic sources of particulate material in California, Houston, and Nashville; this methodology will provide much-needed cross-checks on the inferential methods currently used by EPA. Within the interagency agreement, the group will be working on developing NIST reference materials for particulate matter; these products will be of use to both EPA and industry. Overall, the work of the Surface and Microanalysis Science Division on measurement techniques and calibration standards in atmospheric chemistry has great public value because these products play a key role in enabling meaningful evaluation of compliance with NAAQS.
Another important factor in ensuring that NIST results have impact is mechanisms for effectively disseminating information to interested parties. One approach is posting data on the Web, and very good examples of how this should be done can be seen in the Surface Analysis Data Center collection of three NIST databases and the Interactive Standard Test Data Web site.10 The Data Center provides the large community of academic, government, and industrial scientists who employ x-ray photoelectron and Auger electron spectroscopy with a high-quality source of reference data on line positions, electron elastic-scattering cross sections, and electron inelastic mean free paths. This Web site is extremely popular (it received over 25,000 hits in 2000). The Interactive Standard Test Data site allows users to test their ability to perform appropriate peak fitting procedures on x-ray photoelectron spectroscopy data, and in recognition of outstanding work on the development of this product, two division staff members received a NIST Measurement Services Award in 2000. By making both the Surface Analysis Data Center databases and the Interactive Standard Test Data available at no charge over the Web, NIST is increasing the probability that scientists throughout the United States and the rest of the world will utilize these products and thereby improve the quality of many common surface analysis measurements.
In addition to providing standards and technologies that are utilized in research and manufacturing, Surface and Microanalysis Sciences Division staff also play a significant role in international standards development. For example, a cooperative project between NIST and the International Atomic Energy Agency on isotope reference materials focused on organizing an international comparison in order to improve measurement reproducibility and international consensus on procedures and reference material value assignments. Another activity with international implications is the work on SRM 2806, Particles in Oil. Here the primary customers were the U.S. companies in the National Fluid Power Association, but the goal was to develop a reasonable standard that could be adopted internationally in place of an inappropriate standard that might be used as a barrier to free trade. The NIST SRM was released in 1998 and is now being used by the international hydraulic fluid power and aerospace industries and has been adopted as a requirement by the ISO.
Division Resources
Funding sources for the Surface and Microanalysis Science Division are shown in Table 4.4. As of January 2001, staffing for the Surface and Microanalysis Science Division included 36 full-time permanent positions, of which 32 were for technical professionals. There were also 12 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
The quality of the personnel in the Surface and Microanalysis Science Division is outstanding. The entire staff appear to feel that the work they do is important and interesting and contributes to fulfilling the NIST mission, and morale is high throughout the division. Credit should be given to the strong leadership exhibited by the division chief and group leaders, who provide clear direction, focus, and purpose for the work under way.
The primary issue related to human resources stems from the relatively flat budgets experienced by the division, and NIST as a whole, over the past several years. In this environment, it is difficult to find funds to hire permanent staff. Young scientists, hired now, are needed to lay the foundation that will enable the division to continue to turn out high-quality technical results with impact on industry and the U.S. public. While the panel is particularly impressed by the enthusiasm and qualifications of the
|
10 |
The Surface Analysis Data Center can be accessed online at <http://www.nist.gov/srd/surface.htm>, and the Interactive Standard Test Data Web site is at <http://acg.nist.gov/std/>. |
TABLE 4.4 Sources of Funding for the Surface and Microanalysis Science Division (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
4.5 |
4.6 |
4.7 |
5.1 |
|
Competence |
0.4 |
0.4 |
0.4 |
0.2 |
|
ATP |
0.4 |
0.4 |
0.3 |
0.2 |
|
Measurement Services (SRM production) |
0.0 |
0.1 |
0.1 |
0.0 |
|
OA/NFG/CRADA |
2.1 |
2.9 |
5.7 |
5.5 |
|
Other Reimbursable |
0.3 |
0.3 |
0.3 |
0.2 |
|
Total |
7.7 |
8.7 |
11.5 |
11.1 |
|
Full-time permanent staff (total)a |
37 |
36 |
36 |
36 |
|
NOTE: Sources of funding are as described in the note accompanying Table 4.1. aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
younger people currently on staff, the division must continually freshen its mix of personnel. Turnover is very low, mainly occurring due to retirements, but the limited resources make it a challenge for division management to hire the necessary new people. For example, the division lost several promising postdoctoral researchers at the end of their terms at NIST because funding was not available to support permanent staff positions for these researchers.
Along with making it difficult to take on new young staff, the flat budgets make it hard to maintain or create positions for support staff. The division is currently suffering from a lack of dedicated Web programmers, electronics specialists, machinists, and equipment maintenance specialists. In this situation, there are two possible consequences: (1) important tasks are not being completed or (2) the professional staff is being forced to take time from their primary project-oriented duties. Web programming falls in the first category; the panel notes that there has been little improvement in the division Web site since the last review, although this situation may change with the new push at the laboratory level on improving the overall CSTL Web presence. Caring for laboratory instruments falls in the second category; routine equipment maintenance is being done by technical staff who should be focused on new ways to use the equipment instead of how to keep it going. The panel once again encourages division and laboratory management to consider acquiring dedicated support staff to ensure that key tasks get done and technical staff can maximize their productivity.
In general, the instrumentation resources provided to the Surface and Microanalysis Sciences Division adequately support the technical programs. Capital equipment needs are carefully analyzed, and instruments are acquired in a systematic and timely fashion. As a result, the division has a unique collection of state-of-the-art instrumentation, especially in electron microscopy, secondary-ion mass spectrometry, near-field probes, isotope dilution mass spectrometry, and laser/surface probes. A significant accomplishment of the past year was the acquisition of two state-of-the art Auger electron spectrometers for the Microanalysis Research Group. These pieces have been installed and are undergoing performance testing. One system was transferred from the NIST Physics Laboratory and the other is a
new capital equipment purchase. These instruments are the two most common Auger electron spectroscopy configurations used in surface analysis and together provide an ideal platform on which NIST scientists can perform advanced research into electron and x-ray spectroscopy.
A noticeable gap in equipment available to the division is a focused ion beam (FIB) sample preparation system. In the development and near-production environments of the semiconductor, MEMS, and thin-film-head industries, the FIB technology is increasingly becoming the method of choice for cross-section and thin-section preparation of samples to be used in scanning electron and transmission electron microscopy. However, details of the mechanisms involved in the high-energy (approaching 50 kV) ion sputtering used in FIB and the potentially negative impact of these mechanisms on sample composition and structure are not known. Without research on these issues, there will be little control of the quality of data generated from FIB-prepared samples. This type of work is entirely appropriate for NIST, and the panel encourages the division, in partnership with the Electronics and Electrical Engineering Laboratory and the Office of Microelectronics, to investigate how a FIB system might be acquired by NIST.
While the Surface and Microanalysis Science Division is slated for space in the new Advanced Measurements Laboratory, completion of that facility and the resulting access of the division to high-quality, modern laboratory space are still 4 years away. Therefore, the panel commends the division’s recent effort to improve its current work space by devoting major funds to laboratory renovations that benefited all four of the division’s groups. New space was prepared to accommodate the new Auger electron spectrometers and two electron microprobes, new space was secured and renovated to house the Atmospheric Chemistry Group, and additional modules were added to the Surface Dynamical Processes Group’s work area. In addition to technically enhancing the laboratory environments, the division also emphasized improving safety in the work areas. While these upgrades were made at the expense of other technical activities, the renovations are important investments to ensure the future viability of division programs and the well-being of its staff. The panel applauds the division for performing this work and for its emphasis on safety; these upgrades best serve the division’s long-term goal of making significant technical advancements in relevant fields.
Physical and Chemical Properties Division
Technical Merit
According to division documentation, the mission of the Physical and Chemical Properties Division is to serve as the nation’s reference laboratory for measurements, standards, data, and models in the areas of thermophysics, thermochemistry, and chemical kinetics and to promote U.S. economic growth by providing measurements, standards, data, and models for the thermochemical, thermophysical, and interfacial properties of gases, liquids, and solids, both as pure materials and as mixtures; the rates and mechanisms of chemical reactions in the gas and liquid phases and at surfaces; and the fluid-based physical processes and systems, including separations and low-temperature refrigeration, heat transfer, and flow.
The programs of this division comply with the primary NIST mission to promote U.S. economic growth by working with industry to develop and apply technology, measurements, and standards. Each group in the division has ties to appropriate industries, and the division as a whole maintains a desirable balance between the programs that support shorter-term industrial needs and the research that will allow NIST to fulfill longer-term national science and technology requirements. There is also an excellent balance between experimental and theoretical expertise in the research programs.
The Physical and Chemical Properties Division conducts work of unsurpassed quality in making fundamental measurements of thermophysical and thermochemical properties. A key factor contributing to the value of the division’s work is the outstanding collection of skilled researchers at NIST. The division consists of six groups, three at Gaithersburg (Computational Chemistry, Experimental Kinetics and Thermodynamics, and Chemical Reference Data and Modeling) and three at Boulder (Experimental Properties of Fluids, Theory and Modeling of Fluids, and Cryogenic Technologies). Additionally, three independent projects are also located at Boulder (the Thermodynamics Research Center, Membrane Science and Technology, and Properties for Process Separations). Selected highlights of these programs are presented below.
The Computational Chemistry Group has made remarkable progress since its inception in 1997. Two programs worthy of special note this year are the Computational Chemistry Comparison and Benchmark Database (CCCBDB)11 and the tool for screening potential fire-suppressant compounds. The CCCBDB is a unique resource that provides industrial and academic chemists with the benchmark data they need to evaluate ab initio computational methods in order to make the most effective trade-off between computational cost and desired level of accuracy in a given calculation. The screening tool calculates rates and thermochemical data for critical reactions and is designed to help estimate the reactivity and therefore the potential environmental impact of fire-suppressant compounds. This tool has been completed and is currently being tested against data provided by the Experimental Kinetics and Thermodynamics Group, which has performed measurements on the kinetics of reactions between hydroxyl radicals and halogenated ethers and alkenes. This project is one example of the Computational Chemistry Group’s many productive interactions with other units at NIST Gaithersburg, both within and outside the Physical and Chemical Properties Division. Now that these relationships have been firmly established, the group might wish to explore possible avenues of collaboration with the division groups in Boulder. Another potential area of growth for this group is molecular-based and macroscale simulations; new efforts could be built on the existing expertise of a NIST fellow.
The Experimental Kinetics and Thermodynamics Group provides reliable kinetic and thermodynamic data pertaining to industrial processes, environmental chemistry, energy efficiency, and fire suppression. The group is staffed by a productive set of theorists and experimentalists whose mix of expertise allows them to select the critical experiments that are needed to address important issues in the relevant fields. One example from the past year is the recent project on reactions of the nitrate radical in aqueous solution, which is providing rate constants and solubility data crucial to atmospheric chemistry models. The panel is also pleased with the progress that has been made on the Web-based Chemical Kinetics Database, now in beta testing.12 This important resource will provide industrial scientists with the information they need to design chemical processes more efficiently and will be used by scientists in academia, government, and industry to gain a better understanding of a wide variety of chemical reactions. Future plans on the database call for targeted evaluations of rate constants for chlorination chemistry, to be added next year, and for work to begin on evaluating data related to small hydrocarbon radical chemistry. This database is a high priority for the division, and it is important that NIST provide the funds necessary to complete work on the full public release as quickly as possible and to maintain the database as a national resource. Another funding-related issue is the suspension of research on supercritical water reactors; the panel hopes that this important work can recommence soon.
|
11 |
The latest version of the Computational Chemistry Comparison and Benchmark Database was released in December 2000 and can be found online at <http://srdata.nist.gov/cccbdb/>. |
|
12 |
The beta-level testing version of the Chemical Kinetics Database is available online at <http://kinetics.nist.gov/>. |
The Chemical Reference Data and Modeling Group, which compiles, evaluates, correlates, and disseminates Standard Reference Data, has had another productive year. The sixth edition of the NIST Chemistry WebBook13 is now available and is accessed by between 8000 and 17,000 users each week. The amount of content in this searchable compilation of thermochemical data and spectra also continues to grow; last year the number of chemicals included increased by 13 percent, to over 35,900. New work with academic and industrial partners is focused on ways to provide direct machine access to the data using computer programs that reside on a user’s machine; this approach would support automated chemical reaction design programs. The NIST/EPA/NIH Mass Spectral Library has received a major upgrade to its searching program to incorporate structure similarity searching, and the results from the group’s relatively new experimental program on measuring selected mass spectrometer/mass spectrometer (MS/MS) spectra will be a valuable complement to the existing Mass Spectral database. The group has made substantial progress on these measurements over the past year and is appropriately planning to seek input from the MS/MS community by presenting its results at scientific meetings. A program on how to provide a unique string identifier for any chemical species has begun in collaboration with the International Union of Pure and Applied Chemistry (IUPAC); although this is an old problem, a new and creative solution would be of great value to the international scientific community.
In Boulder, the Experimental Properties of Fluids Group obtains extremely accurate and comprehensive thermophysical and transport property data for pure fluids and mixtures using a unique collection of state-of-the-art laboratory apparatus. The group collaborates closely with the Theory and Modeling of Fluids Group on projects related to alternative refrigerants and natural gas technology. A recent addition to the group’s measurement capabilities is the development and installation of a double-sinker densimeter to measure liquids and dense gases, particularly replacement refrigerants. This unique apparatus provides primary standard density data over a wide range of temperatures and pressures by reference to a silicon specimen whose mass is traceable to the U.S. standard kilogram. Another piece of new equipment that impressed the panel was the heat-of-vaporization/low-vapor-pressure effusion apparatus, now in the commissioning stage. This instrument will provide important regulatory compliance data in the low vapor pressure region over a wide range of temperatures. For example, volatile organic compound requirements in many state and EPA consumer product regulations restrict the use of chemicals that have vapor pressures greater than 0.1 torr (13.3 Pa) at 20 °C. Finally, the panel endorses the exploratory initiative that was taken in an uninvestigated arena: fluid property microanalysis. The goal of this project is to build a “laptop lab” in which microscale analyzers simultaneously measure key properties of very small samples in order to provide the thermophysical property data needed for combinatorial chemistry screening strategies. Recent progress in this area includes the fabrication by micromachining of prototype microscale analyzers.
The Theory and Modeling of Fluids Group continues to make progress in areas related primarily to water and aqueous solutions, electrolyte solutions, and solid-fluid equilibrium. The main techniques are statistical-mechanical and quantum-based models, and the staff play a leading role in setting international standards related to the properties of water. The group cosponsored a workshop with academic and industrial participants in June 2001. The workshop addressed an important problem in the field, namely the lack of appropriate intermolecular potential models for most substances of industrial interest. The panel is concerned that recent shifts in personnel have left the group lacking expertise in simulation approaches using atomistic potentials. Use of these techniques has grown rapidly of late, particularly in academia.
|
13 |
The NIST Chemistry WebBook is available online at <http://webbook.nist.gov/chemistry/>. |
The Cryogenic Technologies Group is well-positioned to continue its recent record of accomplishments. The “pulse tube” refrigerators developed by the group are generating a great deal of external interest owing to their high reliability, which results from the complete absence of moving parts such as pumps and compressors. The interest in this technology is evidenced by the strength of the ongoing collaborations and CRADAs and the number of invited or plenary lectures and short courses being given at conferences by the group’s staff. The panel was also favorably impressed by the work being done on pulse tube neon liquefaction, a project currently being funded by Praxair.
In mid-2000, the Thermodynamics Research Center (TRC) was transferred from Texas A&M University to the NIST Boulder campus. This group adds significant strength to the division as a consequence of its expertise and experience in archiving, organizing, and disseminating thermodynamic property data for fluids. The director of the center at Texas A&M will continue in that role at NIST, and the panel believes that he will bring strong vision and leadership to this undertaking. The major current research project of this group is work on dynamic data compilation methods, which is designed to develop mechanisms for continuous incorporation of new experimental data into databases of chemical properties. This project is of high quality and is supportive of the NIST and division missions. The main challenge for division and center management is ensuring that the transition from Texas to Boulder goes smoothly. Over the next year, the panel expects that efforts will be made to integrate the center’s staff and projects with the other data collection and evaluation activities of the division. For example, there appears to be some overlap in the scope of the Chemistry WebBook and the TRC data collections, so some coordination between these two sources of thermodynamic data might be appropriate. Also, a potential conflict exists between the current NIST approach of disseminating data extensively and freely via the Web and the Center’s tradition of generating income from the sale of its publications.
The Membrane Science and Technology project continues to provide significant resources for industries that seek more efficient chemical and pharmaceutical separations and waste reduction processes through membrane technologies. The group has utilized new laboratory space in Building 24 for work on the application of total reflectance Fourier transform infrared (FTIR) spectroscopy. A recent accomplishment of this project is the measurement of a heretofore undiscovered breakthrough phenomenon by isopropanol through cellulose acetate films. This work is relevant for efforts to measure fundamental diffusion and solubility data and to elucidate transport mechanisms. The new space will also support an important initiative funded by the NIST ATP on the development and demonstration of high-productivity screening of molecular transport through films, membranes, and nanostructures. The panel is pleased to note that the project staff receives feedback from the membrane community by virtue of their advisory roles on other ATP programs, attendance at workshops, involvement with industrial consortia, and participation in the IUPAC committee on membrane characterization.
The Properties for Process Separations project provides critically evaluated data and models for industrial processes such as distillation, adsorption, and supercritical fluid extraction. Work on a novel gas chromatography method for obtaining diffusion data and rate-of-desorption (kinetic) data continues, with the ultimate goal of facilitating predictive models on the transport and partitioning of pollutants in clay. A new initiative is the implementation of in situ FTIR spectroscopy to collect vapor-liquid equilibria data, particularly on mixtures that are reactive, corrosive, or toxic. Initial tests on acetone/ethanol mixtures are in progress, and the project promises to produce important data on industrial systems that could previously not be studied. Now that a full-time NRC postdoctoral research associate is available to work on the testing apparatus, the panel is looking forward to significant progress in this area.
Program Relevance and Effectiveness
The panel continues to applaud the Physical and Chemical Properties Division’s efforts to assure that its programs are relevant to the needs of its customers. The division employs a variety of mechanisms to gather input on current and planned divisional activities and suggestions and requests from external organizations. Examples of events where division personnel interact with people from other institutions include standards committee meetings, technical conferences, roadmapping activities, professional society meetings and committees, and trade organization events. Staff often take a lead role in organizing these gatherings, as in the case of the 14th Symposium on Thermophysical Properties and the 11th Annual Meeting of the North American Membrane Society, and they have informative relationships with a variety of individuals (e.g., guest researchers and collaborators from industry and universities). As discussed in last year’s report, the division has an appropriate process in place for setting priorities, and decisions are made based on external input on needs and potential impact as well as on internal factors, such as the division mission, expertise, and resources. The panel notes that the balance within the current program portfolio between work with short- and long-range goals is a sign that these mechanisms effectively set priorities in project selection and continuation.
The programs of the Physical and Chemical Properties Division have an impact on a wide array of industries and research communities, in part by bridging the gap between research directed at the often short-range goals of industry and the long-range, open-ended inquiries commonly pursued in universities. Just one example of the value of NIST efforts is the receipt by a staff member of the NOAA Environmental Hero Award for “efforts to protect and preserve our nation’s environment.” The primary products of the division are databases that scientists use to develop computational models and analytical techniques for industrial, environmental, and fundamental chemistry applications. The division is to be commended not only for gathering, evaluating, and maintaining the information necessary to produce these databases but also for performing critical experiments and computations in areas such as gas- and liquid-phase kinetics, thermodynamics, and mass spectrometry in order to provide the data and the underlying understanding that allow it to make high-quality information readily available to technical communities.
In last year’s report, the panel expressed a desire to learn if criteria and processes exist for selection, review, and sunsetting of databases. This year, the division explained that such systems are indeed in place; all database activities have 3-year plans and are reviewed annually. One example of phasing out or redirecting is in the Theory and Modeling Fluids Group, where the panel observed a reduction of effort in the study of structured fluids. Another example is the complete restructuring of the Chemical Kinetics Database. When data activities are phased out, the infrastructure and archival nature of the databases are usually maintained.
Another area in which the division’s work has significant impact is international standards activities, such as the efforts on research related to alternative refrigerants. Division staff are active in a number of organizations on this issue, including the International Energy Agency (IEA), IUPAC, and ISO. Also, in addition to data-based products and committee activities, the division provides unique standards and services for fluid flow under cryogenic conditions.
The Physical and Chemical Properties Division makes strong and well-directed efforts to convey NIST results to the relevant scientific and engineering communities. It effectively utilizes basic tools such as publications and presentations; in fiscal year 2000, 166 papers appeared, primarily in peer-review journals, 162 presentations (37 invited) were made at scientific meetings, roughly twice as many as in the previous fiscal year, and staff served on 69 national and international scientific committees. The breadth of the division’s reach into relevant communities can be seen in several other statistics. The
Chemistry WebBook was hit from more than 250,000 unique Internet addresses last year. Roughly 65 percent of all gas chromatography/mass spectrometry instruments manufactured in the United States include the Mass Spectral Database. And when the division organized and hosted the 14th Symposium on Thermophysical Properties, the meeting attracted 500 participants from 40 countries. The impact of all of this work by the division is felt in a variety of ways by different groups, but overall NIST is playing a major role in the cross-fertilization of the field of thermophysical properties of liquids and solids and in integrating the results for the benefit of industrial users throughout the world. As an example of the unique role NIST can play, the division’s activities on production and dissemination of data are increasingly critical, as academic researchers have begun to neglect this field and industrial researchers often will not share their results.
While the above statistics testify to the widespread dissemination occurring in the division, the panel noted in last year’s report that metrics for the quality of the outputs at the project level would also be useful. This year, the division described the various tools used to assess the quality of completed work, including discussions with customers and some formal studies, i.e., economic impact or case studies, and the panel now understands more clearly the entire process for project evaluation.
Division Resources
Funding sources for the Physical and Chemical Properties Division are shown in Table 4.5. As of January 2001, staffing for the Physical and Chemical Properties Division included 56 full-time permanent positions, of which 42 were for technical professionals. There were also 18 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
The congressionally allocated resources (STRS) made available to the Physical and Chemical Properties Division are constant or shrinking. This situation requires division management to obtain additional resources from outside NIST or from other internal sources or to reallocate existing resources so as to maximize the impact of the remaining programs. Either approach has the potential to negatively
TABLE 4.5 Sources of Funding for the Physical and Chemical Properties Division (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
8.7 |
9.1 |
8.7 |
6.9 |
|
Competence |
0.1 |
0.1 |
0.0 |
0.0 |
|
ATP |
0.4 |
0.4 |
0.4 |
0.4 |
|
OA/NFG/CRADA |
4.0 |
3.5 |
2.9 |
2.8 |
|
Other Reimbursable |
0.1 |
0.3 |
0.3 |
2.1 |
|
Total |
13.3 |
13.4 |
12.3 |
12.2 |
|
Full-time permanent staff (total)a |
68 |
65 |
64 |
56 |
|
NOTE: Sources of funding are as described in the note accompanying Table 4.1. aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
affect the quality of the division’s work. However, the panel was encouraged to note that the percentage of the division’s funding received from other government agencies has dropped from 30 percent to 23 percent over the past 4 years. The current level is still high enough to ensure that NIST work is relevant to external parties, but the decrease does allow management to maintain greater control over the division’s complete portfolio of programs. A significant fraction of the current funding arises from sales of the Mass Spectral Database. The panel believes that it is appropriate that, in fiscal year 2000, about 90 percent of the revenue from these sales was returned to the division to support maintaining this important database. Other divisional products are provided to customers free of charge over the Web, and plans call for the new Chemical Kinetics Database to also be disseminated free over the Web. The panel notes that the information in this database will be incredibly useful to many scientists, and NIST might consider imposing a user fee for access, especially since the resources to support the project are already tight. This issue is similar to that of how the division will reconcile the TRC’s approach of selling all of its data products with NIST’s more open dissemination model.
The personnel in Gaithersburg and Boulder are a key asset of the Physical and Chemical Properties Division. The many examples of significant individual accomplishments by staff attest to the high quality of the team of scientists gathered in support of the NIST mission. One member of the staff was elected as a Fellow of the American Physical Society for having developed a world-class research program on the properties of fluids, and another was named as an Honorary Fellow of the International Association for the Properties of Water and Steam for organizing and directing international research efforts into thermophysical properties of water and steam. Division personnel also are lead editors of two major journals (the International Journal of Chemical Kinetics and the International Journal of Thermophysics) and associate editors of 14 other publications. These awards and positions of responsibility demonstrate the regard in which division staff are held by the scientific and industrial communities.
Given the important role high-quality staff play in carrying out the NIST mission, it is necessary to remain alert to the impact of flat budgets on the human resources available to the division. Currently, there is a significant reliance on guest workers and postdoctoral research associates. While these people provide important cross-fertilization with other institutions and industry, the temporary nature of their positions means that they are not adequate substitutes for permanent staff. For example, the potentially important study of kinetics in supercritical water was suspended last year because a temporary staff member departed. The division must be able to select and plan a program portfolio based on a stable core of expertise. For example, the panel believes that work on emerging simulation methods, such as molecular-based and macroscale simulations and approaches using atomistic potentials, would be very valuable for the division, but additional resources would probably be needed to build and sustain a critical and stable mass of researchers in these areas. One reason that the division is not having more difficulties in staffing is that morale is high and there is very low turnover. Recruitment of postdoctoral associates and new staff has been somewhat difficult due to the strong economy, tight job market, and (perhaps) the reduced emphasis on thermophysical property research at the graduate student level. Nonetheless, the division has managed to attract a well-known chemist from industry to assume leadership of the Computational Chemistry Group, and the panel applauds this hiring decision.
Some major improvements have been made to facilities, but serious issues remain. The most significant step forward occurred when staff formerly housed in inadequate facilities of Building 3 in Boulder recently relocated to an area in Building 24 previously occupied by NOAA. This new location is now providing excellent laboratory space for the divisional programs. This move is officially a temporary one, but the panel strongly recommends that it be permanent unless additional space becomes available in Building 2 and the division staff can be consolidated at that location. The facilities of staff
already in Building 2 are adequate, and space is presently under renovation in Building 1 to meet the needs of data entry personnel in the TRC.
In Gaithersburg, staff appear to be generally satisfied with their physical facilities, although the panel continues to be concerned. In some laboratories, air cleanliness, dust control, and air filtration are still insufficient; the quality, capacity, and reliability of the power supply are still problematic; and the exhaust and ventilation systems are still inadequate. While the current quality of the Gaithersburg facilities is generally comparable to that available at research universities, these deficiencies will eventually interfere with the division’s ability to perform the type of high-precision experiments that are needed to supply industrial and academic researchers with the accurate, high-quality data that are the hallmark of this division.
In terms of capital equipment, no funding issues appear to be limiting the initiatives undertaken by division scientists in Gaithersburg or Boulder. In fact, some of the apparatus in the division is not even available in industrial laboratories.
Analytical Chemistry Division
Technical Merit
According to division documentation, the mission of the Analytical Chemistry Division is to serve as the nation’s reference laboratory for chemical measurements and standards to enhance U.S. industry’s productivity and competitiveness, assure equity in trade, and provide quality assurance for chemical measurements used for assessing and improving public health, safety, and the environment.
The panel determined that the activities of the Analytical Chemistry Division are well managed and that the division’s work serves the fundamental role of maintaining U.S. reference standards and standard methodology for analytical chemistry. The ongoing projects fulfill the NIST goal of making a unique contribution commercially and technically, and the panel believes that the division’s programs play a critical role in defining the nation’s measurement infrastructure. For example, last year the division staff certified roughly 18,000 of the more than 34,000 SRMs generated and sold by NIST. These standards touch products in nearly every sector of commerce in the United States, and the measurement infrastructure based on the standards of the Analytical Chemistry Division plays a significant role in supporting the industries that sustain the U.S. economy. Other important elements of the division’s work include helping to ensure regulatory compliance for EPA and the FDA by providing reference materials relevant to health and safety and transferring technical expertise in measurement technologies within and outside the United States. The division uses metrology workshops to train staff of national measurement institutes from countries throughout the Americas, the Middle East, and Africa. Measurement methods and experiences are also disseminated via division staff’s participation on scientific committees; in fiscal year 2000, division personnel filled 121 committee assignments. The international stature of the division was confirmed in a September 2000 report describing the evaluation of the division by an international peer review committee.14 This group, chaired by the president of the CIPM Consultative Committee for the Amount of Substance (CCQM), found that the division conducts “excellent and well-documented work and delivers adequate services to industry and society.”
The primary challenge facing the division in fulfillment of its mission is how to distribute time, effort, and resources among developing new measurement technologies, supplying current SRMs, and assessing the need for maintaining or improving existing SRMs. Work in the first category results in the development of more precise and accurate measurement technologies that allow staff to identify and prepare SRMs previously thought impossible to prepare and to improve the shelf life and accuracy of existing standards. These investigative projects are crucial if the division is to maintain the reputation and quality of the NIST portfolio of standards. Another facet of the division’s activities that competes for staff time and division resources is its growing international responsibilities. Interactions among national measurement institutes and the policies of international standards organizations have become critical components in the global economy, and Analytical Chemistry Division staff have crucial roles to play in leading international intercomparisons and serving on standards committees.
The panel is pleased to note that division management has both a strategic plan to guide decisions about which topics to focus on and a business plan to manage the large number of products (e.g., SRMs) for which the division is responsible. One positive result noted by the panel is the development of a more cohesive strategy for current work and future SRM production in the biomedical area. The new approach takes into account the rapid pace of change in this industry and how the division can make a substantial contribution by defining new certified reference materials, especially for health care and food-related applications. This year the panel also observed improvements in the mission-driven work process used by division staff. There appears to be a greater consideration of what formal elements contribute to the quality process and of how the division might better maintain control of the business metrics that must in part define its work. For example, at the time of last year’s assessment, the division was behind on production of a number of SRMs, but this year the panel was very pleased to note that stocks are up to date. There is room for further progress, which might include a more formal quality process, such as that outlined under ISO/IEC Standard 17025 and recommended by the international peer review committee mentioned above. Elements of such a process could include work practice documentation and records of instrument performance.
The technical merit of the work under way in the Analytical Chemistry Division is very high, even better than the panel has observed in the past, and the panel’s assessment is supported by the many letters the division receives from external customers expressing gratitude and praise for the division’s work. Staff in the Analytical Chemistry Division are organized into five groups: Spectrochemical Methods, Organic Analytical Methods, Gas Metrology and Classical Methods, Molecular Spectrometry and Microfluidic Methods, and Nuclear Analytical Methods. In addition, NIST has a satellite laboratory in Charleston, South Carolina, that focuses on the quality of analytical measurements for contaminants in the marine environment and is managed by the Analytical Chemistry Division. Some of the impressive accomplishments of the past year are highlighted in the individual discussions of each group later in this section.
The division’s programs apply the competencies built up in the groups to provide the measurement standards, accurate and reliable compositional data, and research in new measurement science that are all critical to the overall success of CSTL and NIST. The division focuses on six areas: analytical instrument performance and calibration; chemical characterization of materials; environmental monitoring and technology; forensics, defense, and security applications; health care and clinical chemistry; and nutrition, contamination, and adulteration of foods. All of the groups contribute to multiple program areas, and examples of successful collaborations with both internal and external groups were seen throughout the division. However, the panel encourages division staff to seek out more interactions and partnerships between the various groups in order to gain a broader perspective on current projects. Senior management should investigate ways to encourage and reward such partnerships. One mecha-
nism to support further efforts in this direction could be to upgrade the internal NIST Web site listing staff expertise and laboratory capabilities throughout NIST. Staff should be particularly alert for opportunities in which the unique capabilities available throughout the division, especially in the Nuclear Analytical Methods Group, might complement efforts in other groups.
The Spectrochemical Methods Group conducts research on the development, critical evaluation, and application of methods for the identification and measurement of inorganic chemical species using optical, mass, and x-ray spectrometries. An important element in the success of this group’s programs is its interdisciplinary approach. Major emphasis has been placed on developing methodologies that enhance the traceability of secondary reference materials through both instrumentation and statistical methods. Current technologies of interest include high-performance, inductively coupled plasma optical emission spectroscopy (HP-ICP-OES) and the use of matrix-independent x-ray fluorescence for metals analysis. Both techniques are being evaluated by NIST to determine if practical methodologies can be laid out to enable general applications in the private sector. The goal of the group’s work on statistical methods is to enable more effective and efficient value assignment of SRMs within NIST (and hence expedite the development of new SRMs) and to provide useful tools to enhance traceability between NIST SRM values and reference materials from secondary sources.
The Organic Analytical Methods Group has a long history of productive research on generating new chemistries that can be employed in separation science. In particular, the development of new stationary phases of high selectivity and specificity has been very successful. A very important extension of this research is the effort to develop uniform approaches for characterizing the performance of liquid chromatography columns. One of the major challenges in the field of liquid chromatography is the variability observed between vendors in the performance of what should be equivalent stationary-phase materials. This variability has important consequences in areas such as quality assurance and control in the pharmaceutical sector. The understanding of the fundamental chemistries involved in liquid chromatography separations gained in the division over the last few years has led to the development of a broad set of metrics that can be applied to the evaluation of individual column performance and expected retention behavior. This year’s accomplishments include the completion of two new SRMs that will facilitate column characterization and classification, and the logical extension of this approach to gas chromatography columns is currently under way.
The Gas Metrology and Classical Methods Group conducts research in a variety of areas, including titrimetry, gravimetry, the thermodynamic basis for pH, and wet chemical and electroanalytical methods. This group leads NIST and the global standards-setting community in identifying areas in which SRMs and NIST-Traceable Reference Materials (NTRMs) are needed and develops and distributes the products. Current program areas are directly in line with NIST objectives and are well directed in support of the goals of sponsoring agencies. In the area of pH measurements, the group is very active internationally, participating in a key comparison and directing a pilot study through CCQM. Last year, the group completed an SIM pilot study and played an active role in the revision of an IUPAC document in order to assure continued traceability of pH to sound thermodynamic principles. These activities serve to ensure the quality of measurement methodologies throughout the world. Locally, the group continues to improve its array of SRMs. As they worked on electrolytic conductivity standards, staff realized that new packaging technology could enhance the storage of conductivity solutions. Therefore, during the past year, all conductivity solutions references produced by NIST were packaged in sealed 50-ml ampoules, which solved transpiration problems and allowed NIST to sell SRMs with multiyear shelf lives. The panel commends the group for these efforts, which significantly reduced the SRM reissue rate.
The Gas Metrology and Classical Methods Group houses the NIST-Traceable Reference Material (NTRM) program for gas mixtures. Management of this program, as well as work on regular SRMs for
the specialty gas industry, is a significant burden for the group and the division, and the panel suggests that NIST staff reach out to the specialty gas industry to discuss whether there might be any new approaches by which NIST could meet industrial needs more efficiently while not overwhelming division staff and resources. The NTRM model has proven to be a good approach, and the panel is pleased by laboratory management’s continuing efforts to improve implementation and refine the process. Expansion of the NTRM program to optical filter and metal alloy reference materials is under way in the Analytical Chemistry Division, and perhaps CSTL should consider if the approach would be applicable to work in other divisions.
The Molecular Spectrometry and Microfluidic Methods Group conducts research and develops new technologies for molecular spectrometry standards and applications and for microfluidic devices, methods, and applications. Areas in which standards and traceability are provided include molecular spectrometry for chemical analysis, quantitative forensic analysis, instrument interfacing, and data interchange. A key accomplishment of this group was the development of a new, high-accuracy reference spectrophotometer (HAS-II) for the optical filters program. This unique instrument has many important features, including several automated capabilities, such as automated wavelength selection and light source switching, automated light intensity leveling, and automated environment monitoring. A large number of SRMs were produced and recertified using this spectrophotometer. A significant goal of the group is developing an NTRM program for optical filters, and the panel is pleased that progress in this area is continuing. Three companies have been identified as the initial manufacturers for these NTRM, and they all hope to have products ready for sale some time in 2001.
The group is collaborating with the Process Measurements Division and the NIST Electronics and Electrical Engineering Laboratory on the development of techniques needed to support the development of microanalytical laboratories (labs-on-a-chip based on microfluidic devices). This competence project has allowed the division to branch out into an important new area. A ultraviolet micromachining instrument was installed and numerous collaborations are in place, not only with the other NIST groups but with universities and industrial laboratories as well. Other collaborative work includes a productive relationship with George Washington University in support of the group’s forensic analysis program, which is developing methods for the analysis of gunpowder, gunshot residue, and explosives residue.
The Nuclear Analytical Methods Group focuses on nuclear-based techniques for identification and quantification of chemical species. The measurement capabilities that reside within this group are insensitive to the chemical state of the samples and are nondestructive; these characteristics make the work in this group an excellent complement to the projects under way in the Spectrochemical Methods Group. The Nuclear Analytical Methods Group is located some distance from the rest of the CSTL, but the facilities and expertise available make it a valuable resource for the laboratory. While there are good examples of key collaborations, the panel is concerned that the full potential of this group to support and complement the traditional analytical metrology under way elsewhere in the division has not been realized.
An example of the usefulness of this group’s techniques can be seen in the recent collaboration with the Surface and Microanalysis Science Division. The staff from the Nuclear Analytical Methods Group provided the key expertise in instrumental neutron activation analysis (INAA) that was necessary to produce a new SRM for arsenic levels in silicon that will be of great value to the semiconductor industry. INAA techniques were also used to good effect during the recent international certification of a reference material for chromium in marine sediment. NIST analysis showed that certain procedures used by the Canadian National Research Council were inadequate, allowing the approach to be corrected prior to certification and preventing a potential inaccuracy of over 300 percent. In other areas, the group initiated a pioneering research program on the use of cold neutron beams as analytical probes for both prompt gamma activation analysis and neutron depth profiling. The outstanding technical perfor-
mance of the Nuclear Analytical Methods Group has been recognized by 12 formal letters of acknowledgment; leaders from the electronics industry (e.g., Advanced Micro Devices), the chemical processing sector (e.g., DuPont), interagency customers, and universities all have commended the unique expertise and results that this group provides.
A component of the Analytical Chemistry Division is located in Charleston, South Carolina, as part of the Hollings Marine Laboratory. The mission of this component is to assess and improve the quality of analytical measurements in the marine environment through interlaboratory comparisons and reference material development and to improve the abilities to assess trends in marine environmental quality by expanding the cryogenic banking of environmental samples. In the past year, staff have made significant progress in a number of areas, including coordination of interlaboratory comparison exercises for various environmental monitoring and quality assurance programs in support of marine mammal heath and specimen banking of marine mammal tissues and seabird eggs.
Program Relevance and Effectiveness
The Analytical Chemistry Division produces scientific results and SRMs that are of vital importance to U.S. industry. The measurement methods and reference materials developed in the division enable the manufacture of many products and help companies comply with government regulations. The division is very active internationally in order to ensure that the most advanced and accurate techniques and standards are used throughout the world and that U.S. industry is able to participate in all regions of the global marketplace. While the number of areas in which the division is currently making an impact is quite large, the number of potential topics for programs is gigantic. The panel commends the division for having recently improved its review process for the prioritization and selection of projects. There is now a formal system in place that assesses potential activities based on their relevancy to the NIST mission and commercial and technical impact of potential technological advancements. A strong system of prioritization is very useful for selecting which projects to start and stop in such a way that division activities strengthen the U.S. economy and contribute to public health and welfare in a cost-effective way and that nonproductive efforts are eliminated. The panel notes that in the current environment, where budgets are tight and there is significant emphasis on quantifiable impact, initial project plans could productively be supplemented by both business and technical metrics. The end result of any new reference material or measurement technology development should be described in terms of a quantifiable return to NIST’s customers. Such upfront arguments about future impact can help to support CSTL efforts to explain the importance of current programs to the various organizations that provide NIST’s funding.
The value of the Analytical Chemistry Division’s past work has been quantified in a variety of ways. For example, economic impact studies are done to analyze the effect of a specific NIST product or program on a given industry and to calculate formal rates of return on the NIST investment. This past year, two such studies were completed on division projects. The first looked at the benefits, such as improvements in product quality and production efficiency and reductions in transaction costs, that accrued from the development and distribution of SRMs for sulfur in fossil fuels.15 Industries affected included the fossil fuel extraction and processing industry and the users of the fuels, such as electrical
utilities and the steel industry. The study showed the very positive results of NIST’s work: a significant benefit-cost ratio (113) and a large social rate of return (1056 percent). The panel applauds the technical achievement behind this impact, which is the utilization of a very accurate technique (isotope dilution thermal ionization mass spectrometry) for certification of the SRMs. This approach is unique to NIST and has been at the center of SRM certification activities for over 15 years.
The second economic impact study examined the consequences of the NIST cholesterol standards program.16 Although the analysis was limited to four of the eight SRMs the division has put out in this area and to the first three levels of the supply chain that utilizes these products, the results still indicate a significant social rate of return (154 percent) and benefit-to-cost ratio (4.47). These numbers do not take into account the considerable positive effects associated with reducing the costs of retests and the number of cases in which patients are mistreated as a result of inaccurate measurements of samples. Again, the division’s ability to make an important difference is based on the development and application of specialized methodologies, in this case isotope dilution gas chromatography/mass spectrometry. During the coming year, NIST plans to commission economic impact studies on two more Analytical Chemistry Division programs: optical filters SRMs and NTRMs for gas mixtures. In the latter area, the panel expects the analysis to document significant economic value, as the division’s standards serve a $19 billion specialty and bulk gas production industry, one of the broadest commercial markets in the world.
The division activities that have been or will be formally studied by economists are not the only programs that are impacting industry. As a whole, the division portfolio contains many projects that are closely focused on meeting the metrology needs of U.S. companies. In many cases, a key element of these successful projects is the ability to involve external organizations in the NIST work, as partners, consultants, or just a source of information. For example, in the Spectrochemical Methods Group, the HP-ICP-OES methodology is already routinely used in support of the Spectrometric Solution (3100 Series) SRM program, and the panel believes that the potential impact of this work is significant because about 2000 instruments employing solid-state array detector technology are in use worldwide. The panel applauds the NIST staff’s ability to involve external parties in this project; formal and informal collaborations exist with a number of other U.S. national laboratories, instrument vendors, a commercial producer of certified reference materials, and several national measurement institutes in other countries.
The practical applications of the technical work done in the Organic Analytical Methods Group come in a wide variety of industrial sectors, including the clinical/health, environmental, food/nutrition, and forensic science sectors. The division staff are incredibly active in reaching out to all of these communities to ensure that the relevant external organizations are aware of NIST programs and contribute to and benefit from them. Examples include interagency activities involving NOAA, DOE, EPA, NIJ, and DOD; international comparisons through CCQM, SIM, and the North American Metrology Association (NORAMET); and coordination of quality assurance programs and general outreach activities to facilitate communications with current and potential SRM customers. All of these efforts are excellent examples of how NIST can serve relevant communities by developing new technologies and producing SRMs.
In addition to producing new results, division staff serve industry by continuing to support and upgrade current SRMs and related measurement methods. For example, when the EPA criticized the
fertilizer industry and the NIST-developed SRMs for including excessive amounts of perchlorate, division personnel investigated this charge and demonstrated how the measurement procedures used by the EPA had given incorrect results. This intervention saved the fertilizer industry money and prevented misleading methodologies being used in the future.
Sometimes the true impact of the division’s work cannot begin to be measured in economic terms. The Gas Metrology and Classical Methods Group has worked with the National Institute for Child Health and Human Development to facilitate the safe implementation of inhaled nitric oxide (INO) therapy for newborns. A workshop was organized to test relevant clinical instruments, which measure nitric oxide and nitrogen dioxide, and the results were used by ASTM and industry to draft a standard procedure for the instrument testing that is done before the equipment is put into continued operation in hospitals. The importance and quality of the division’s contribution is evidenced by the fact that the European Union has taken the rare step of adopting this standard procedure, even though it was developed in the United States. The potential impact of this work is huge, as INO therapy could help save the lives of 2000 U.S. newborns annually.
The above paragraphs describe just a few examples of the industrially relevant work ongoing in the Analytical Chemistry Division. This work, which provides tools that impact specific products or sectors, is informed and supported by the diverse activities with international components that are currently under way in the division. These activities give division staff a fuller understanding of the needs of U.S. companies in foreign markets and a greater familiarity with the most advanced technologies, methods, and reference materials in use throughout the world. Demand for reference materials is escalating at a rapid pace worldwide, and the panel notes that the division has made great strides in the past year on its efforts to address the current industrial needs for certified reference materials. Division staff are leading or participating in a number of programs relevant to the chemical measurements needed for international trade. With the organization Cooperation in International Traceability in Analytical Chemistry, NIST is working to establish vertical traceability links between the NMIs and testing labs in every country in the world. The division also has a number of projects under way to put nationally/ internationally recognized certified reference materials into place to enable U.S. manufacturers to comply with European directives. Examples include reference methods and materials for troponin-I, a marker for myocardial infarction; glycated hemoglobin, a marker for diabetes status; homocysteine, a marker for heart attack risk; and prostate specific antigen.
The Analytical Chemistry Division effectively communicates its technical findings to U.S. industry and the network of organizations that support world commerce. A primary element of this dissemination is information about and sales of over 800 reference materials produced or certified within the division. The staff published 140 documents in peer-reviewed journals and presented 171 scientific talks in fiscal year 2000. In addition to the industrial customers that have been discussed in detail, the division also supplies key services to other governmental organizations; for example, last year it provided chemical measurement quality assurance services in support of 25 programs within 11 federal and state agencies on a cost-reimbursable basis.
The panel’s biggest concern about the division’s dissemination efforts is the limited progress that has occurred on utilizing and updating the division’s Web site. The NIST, laboratory, and division Web presence would benefit from a design that reflects an improved strategy for guiding visitors to important information (e.g., via a list of critical links) or helping them find specific data (e.g., via a specialized search engine). A specific focus on meeting the needs of technically directed commercial customers would be appropriate. A useful Web site would provide the division with yet another opportunity to explain and support the value of traceability to NIST, a primary product of the CSTL.
Division Resources
Funding sources for the Analytical Chemistry Division are shown in Table 4.6. As of January 2001, staffing for the Analytical Chemistry Division included 69 full-time permanent positions, of which 56 were for technical professionals. There were also 24 nonpermanent or supplemental personnel, such as postdoctoral research associates and temporary or part-time workers.
The staff of the division form a capable and responsive group of interactive teams with the special measurement expertise needed to fulfill the division’s mission. Members of the technical staff are formally recognized for their achievements by NIST and the Department of Commerce as well as by external organizations. These honors highlight the quality of the division personnel and the importance of the work under way. Examples of recent awards include a 1999 Presidential Early Career Award for Scientists and Engineers for work in collaboration with EPA and the remote sensing community on improving the quantitative database of infrared spectra for use in real-time monitoring of airborne chemical contaminants within and around plant facilities, a 1999 NIST Measurement Services Award for “leadership in the development of measurement methods and standards for the clinical laboratory and nutritional labeling communities,” and the 2000 W.J.Youden Award in Interlaboratory Testing for the work on the micronutrients measurement quality assurance program.
The panel observed a very high level of morale in the Analytical Chemistry Division. A prime source of job satisfaction appears to stem from the international reputation of NIST, which is perceived as a positive reflection of the staff efforts. The phrase “traceable to NIST” carries a great deal of weight in industrial and international standards communities, and the staff takes much pride in contributing to this traceability. The division management (group and project leaders and the division chief) are supportive of staff. However, the panel believed that improvements could be made in one area: more
TABLE 4.6 Sources of Funding for the Analytical Chemistry Division (in millions of dollars), FY 1998 to FY 2001
|
Source of Funding |
Fiscal Year 1998 (actual) |
Fiscal Year 1999 (actual) |
Fiscal Year 2000 (actual) |
Fiscal Year 2001 (estimated) |
|
NIST-STRS, excluding Competence |
8.1 |
8.5 |
8.4 |
8.4 |
|
Competence |
0.0 |
0.3 |
0.3 |
0.3 |
|
ATP |
0.0 |
0.1 |
0.1 |
0.3 |
|
Measurement Services (SRM production) |
2.2 |
2.2 |
2.2 |
1.4 |
|
OA/NFG/CRADA |
2.2 |
2.0 |
2.4 |
2.2 |
|
Other Reimbursable |
1.4 |
1.5 |
1.5 |
1.9 |
|
Total |
13.9 |
14.6 |
14.9 |
14.5 |
|
Full-time permanent staff (total)a |
67 |
66 |
68 |
69 |
|
NOTE: Sources of funding are as described in the note accompanying Table 4.1. aThe number of full-time permanent staff is as of January of that fiscal year. |
||||
timely informal recognition and acknowledgment of individual contributions. Ensuring that the technical staff feel appreciated is a key part of maintaining employees’ enthusiasm for working at NIST. The panel believes that management, particularly senior management at the division and laboratory level, should find informal ways to communicate to the staff that their contributions are valued. Such praise can be a powerful incentive, for example, to encourage the development of intergroup collaborations.
The most important staffing-related issue confronting the Analytical Chemistry Division is the increasing number of responsibilities that need to be met by a constant number of personnel. The demand for new SRMs and advanced measurement technologies continues to increase as the materials, biotechnology, and semiconductor industries grow. In addition to the complex technical projects in support of these new industries, staff also have increased duties related to interfacing with customers and participating in international activities. All of these activities are competing with the basic requirements of established SRM programs in which production and maintenance work cannot be neglected, all of them take human resources away from fundamental research. In addition, the staff is spread so thin that in many programs, only one person has the necessary expertise to address the metrology problems under investigation. While comprehensive cross training is very difficult to arrange in highly specialized technical areas, the panel notes that the unique capabilities the division has developed to service the nation’s measurement and standards needs must be preserved. In addition, a scientist might benefit from having another perspective on his or her project or even just another technical person with whom to consult.
There are many possible responses to the staffing crunch in the Analytical Chemistry Division. A primary tactic is forming partnerships, as the Gas Metrology and Classical Methods Group has done in the NTRM program for gas mixtures. Other options include encouraging long-term guest researchers, hiring selectively to increase the staff complement, and increasing the overall funding of the division. In the last option, one possible mechanism is obtaining money from other government agencies. The panel cautions that NIST’s ability to make independent decisions about implementing projects in support of NIST’s strategic directions must be maintained. However, it would certainly be useful for the division to seek funding in support of its longer-term research.
In the matter of hiring new staff, the NRC postdoctoral research associate program is a good source of temporary workers and of candidates for permanent positions. However, to best attract and retain talented people for this program, the division should be very careful to assure that the associates are exposed to a broad array of opportunities. If students and associates feel they are too much involved in established programs or SRM support, they may become discouraged or the division may miss out on an exciting research opportunity or new, leading-edge metrology development.
As for facilities and equipment, the panel notes that certain very expensive analytical instrumentation is needed by the division in a few areas (e.g., an NMR facility in Gaithersburg and a vibrating cryomill for the new laboratory in South Carolina). These instruments are needed to allow state-of-the-art measurement work to continue and ensure that division staff are able to develop future generations of metrology. Although the acquisition of such capital items impacts the whole division budget, individual groups must document the reasons the items are needed.
MAJOR OBSERVATIONS
The panel presents the following major observations:
-
The technical activities in the Chemical Science and Technology Laboratory continue to be of the highest caliber.
-
The identification of strategic directions will help guide the selection of new programs and allow the laboratory to organize its responses to changing industry needs across its divisions.
-
The panel commends the laboratory on its wide array of important international activities.
-
The World Wide Web now serves as the primary interface between NIST and its diverse array of customers. The laboratory should continue its efforts to enhance the usability of its Web site and should focus on how this tool can best be used to enhance dissemination of NIST results and products.
-
Staffing levels within the laboratory are a concern; the panel observed some projects below critical mass and others with single-point coverage. Tight budgets and a competitive external job market are affecting the laboratory’s ability to hire new staff in key areas.














































