2
Scientific Assumptions and Premises Underpinning the Regulation and Oversight of Environmental Risks of Transgenic Plants
This chapter concentrates on the theoretical and empirical underpinnings of the regulation of environmental risks of transgenic plants and the use of risk analysis to evaluate and manage those risks. The first section summarizes the committee’s approach to risk analysis. Two important roles are identified that risk analysis of transgenic crops must fulfill; it must support the decision-making process of regulatory agencies, and it must legitimize the regulatory process, creating public confidence that human well-being and the environment are protected from unacceptable risks.
The second section examines in detail the scientific and logical bases for regulation. In developing an approach that can be applied to both transgenic and conventional crops, the committee endorses the findings of three previous National Research Council (NRC) reports. Transgenic crops do not pose unique categories or kinds of environmental hazards. The entire set of existing transgenic crops is not so different in kind that they pose environmental hazards unlike those caused by other human activities, including conventional crops and other agricultural activities. The committee finds, however, that specific types of transgenic and conventional crops can pose unique environmental hazards. Also, the committee finds that there are good arguments for regulating all transgenic crops. To be effective, such a regulatory system must have an efficient and accurate method for rapidly evaluating all transgenic plants to separate those that require additional regulatory oversight from those that do not.
The third section examines several technical issues related to the underlying risk assessment models. The concentration is on these because
they provide a basis for risk assessment of transgenic crops and will frame discussion of the case studies in subsequent chapters. Evaluation of the risks of transgenic crops requires specifying a social and environmental context for the assessment. Depending on the choice of context, different risk comparisons may become relevant. Finally, several formalizations are suggested that could help clarify this dependence on context and enable a regulatory agency to develop formal procedures to learn from its experience to improve the regulatory system.
RISK
Risk is both an intuitively easy and a technically difficult concept to understand. On the one hand, people take risks all the time in daily life, and whether explicitly or not, people are constantly balancing these perceived risks against their needs and desires. Everyone knows it is risky to drive a car, have radon leak into the basement, ice skate, play blackjack, or swim in a lake. But the personal and idiosyncratic ways people have of dealing with risk in their own lives have only tenuous connections to how society as a whole should deal with risk. Personal perceptions of risk may not reflect reality. One person might not care about the risks of eating a fish bone, but another may care so much that she will not eat any fish. Because each of us has our own way of dealing with risk, how do we agree as a society?
Volumes have been written on the technical aspects of risk. In the catalog of the National Academy Press alone, there are 184 titles related to risk. Two that are particularly relevant to this report are a 1983 publication, Risk Assessment in the Federal Government: Managing the Process, and a 1996 report, Understanding Risk: Informing Decisions in a Democratic Society. The 1983 report outlines a general approach to characterizing hazards, modeling exposure pathways, and quantifying the probability of injury. The 1996 approach argues that any attempt to assess risk involves a series of interpretive judgments and framing assumptions and suggests that democracy is best served when those affected by regulatory decision making can be as fully involved in making those judgments and assumptions as is practicably possible.
This chapter addresses risk from both perspectives. The 1983 report’s approach is followed in using science to illuminate technical understanding of the environmental risks of transgenic crops. By following this approach, however, the committee has made several implicit interpretive judgments and framing assumptions, which the 1996 report suggests. Several of these are acknowledged as implicit judgments and assumptions, and by doing so, an alternative perspective can be developed for evaluating risk analysis of transgenic crops. Before developing these par-
ticular ideas further, some of the broader assumptions made here to understand risk should be mentioned.
Risk is interpreted primarily as a combination of the probability of occurrence of some hazard and the harm corresponding to that hazard. This is both a highly technical and somewhat vague interpretation, involving the related ideas of hazard, occurrence, and some combining process. A hazard has the potential to produce harm, injury, or some other undesirable consequence. In saying a slippery road is hazardous, it is not meant that any harm or a car accident has occurred. It simply means that the road conditions could potentially cause an accident. In this case the hazard is a car accident, which may or may not happen. Likewise, if a transgenic crop has a hazard, it does not mean that any harm has or will occur. Hazard identification is one of the most subjective and potentially contentious elements of risk analysis. While this report is limited to a consideration of environmental risks, there is some ambiguity in deciding what is and is not an environmental hazard. Does this include or exclude the potential for adverse impacts on human health that are mediated by the environment (not directly by food consumption)? Does it include or exclude the potential for adverse impacts on farming practices and profitability? Is a nonspecific effect on habitats or ecosystems an identifiable hazard? Is an effect on an ecological process an environmental hazard? The characterization of hazards in this chapter reflects an answer to each of these questions.
The occurrence of a hazard is a probability that the hazard would occur. This typically depends on many factors. The probability that an accident will occur on a slippery road will depend on how many cars travel the road, how fast they are going, how much they accelerate and decelerate, the skill of the drivers, and other factors. Clearly, these probabilities will be highly conditional on the environment and other details about the situation, and therefore they will be variable both spatially and temporally. Likewise, the probability that any hazard associated with a transgenic crop would occur is likely to vary spatially and temporally. In much of the literature on risk, the probability of occurrence is called an exposure probability, referring to the probability that people are exposed to the hazard. In this report the term exposure is frequently used as a shorthand notation for the probability that a hazard would occur.
The combining of hazard and occurrence probabilities to characterize risk can be contentious, ranging from simple mathematical formulations to complex deliberative processes involving many people. The committee leaves this process deliberately unspecified, so that the range of formulations can be used as needed in the report. In its simplest form, risk can be understood as a weighted probability in which the probability of occur-
rence is weighted by the magnitude of the hazard. If you are a driver on the slippery road, your risk is a combination of the probability that you will have an accident and the severity of the accident. Presumably (hopefully) the probability of an accident rapidly decreases with the severity of the potential accident, and your risk, which combines across all possible accidents, will be small. If you were a road engineer, you would also average over all combinations of drivers to estimate the total risk for society on that stretch of road. The process of combining hazard and occurrence can become more complicated if some drivers are considered more important than others, so that the risks themselves are weighted by some social criterion reflecting this importance. This can become even more complicated if the weightings are determined by some social deliberative process. Risk characterization is another one of the most subjective and potentially contentious elements of risk analysis.
Risks can be reduced by people’s actions, and risk management is an important concept used in this report. By managing risks, people can reduce them to such an extent that they are considered insignificant. For example, one can reduce his or her risk of an accident on a slippery road by slowing down or simply by staying home during inclement weather. Similarly, planting and harvesting transgenic crops in certain ways can reduce the risks associated with them. These methods are discussed in more detail later in this report.
There are a number of aspects of risk that are not explicitly addressed in this report. The committee does not discuss methods for valuation of hazardous events in either economic or other terms. Consequently the committee does not provide a basis for comparative rankings of risks nor a basis for comparing expectations of cost or loss with expectations of benefit associated with commercialization of transgenic crops. Moreover, by emphasizing the possibility and probability of unwanted outcomes, the approach taken in this chapter excludes an interpretation of risk that lays stress on the novelty or unfamiliarity of actions taken (such as fear of the unknown) except insofar as a novelty or unfamiliarity complicates the estimation of risk. The committee’s concept of risk also does not imply that some hazards are ones for which agents could be held accountable, while others would be considered works of nature. Finally, the distribution of risks among different groups of people is not emphasized. While we are all concerned with the distributional aspects of risks and benefits, and they appear to influence the debates about the utility of transgenic crops, the focus in this report is on issues where this concern has less influence. Specifically, the committee’s focus here is on the roles that risk analysis is expected to fulfill in the discussion of transgenic crops and some of the implications thereof.
Roles of Risk Analysis
Risk analysis has come to play at least two somewhat distinct roles in public discourse. It is often conducted by using scientific information to support decision making in a particular case (referred to as “decision support”). This decision support role presumes that the decision maker (an individual, a group, or an organization) is well defined and has the legitimate and uncontested authority to make a decision. Within this class of risk problems, there are circumstances where decision making consists of selecting from among several well-defined options, such as whether to allow the commercialization of a particular transgenic crop. In these situations, risk analysis often consists of anticipating unwanted outcomes associated with each option and measuring their probability should that option be taken. Much of the present regulatory structure for transgenic organisms in the United States takes this approach to risk analysis.
In circumstances where the decision options are not clearly defined, the role of risk analysis is considerably more ambiguous because its role in helping to inform a decision is not clearly defined. Under such conditions, risk analysis could be used to help inform a decision maker of the general scope of a risk situation and outline potential opportunities for decision making. Risk analysis could be conducted to provide a general survey of hazards and the types of risk management decisions that might confront a decision maker. For example, the World Health Organization (WHO) recently conducted a scientific consultation to determine if indirect human health risks were mediated through the environment from the use of transgenic organisms. This consultation focused on hazard identification because if such hazards could be established, the WHO could use its authority on human health concerns (granted by the United Nations) to justify additional risk analysis.
In addition to its role in decision support, risk analysis can serve to reinforce and legitimize the authority of a particular decision maker to exercise control over a given situation (referred to as “creating legitimacy”). There are a variety of circumstances where the predictive power and presumed value neutrality of scientific risk analysis are implicitly assumed to provide the “best” basis for decision making. A decision maker’s ability to produce scientific risk analysis in such circumstances can play an important role in creating legitimacy to set policy or determine a course of events. For example, member-states of the World Trade Organization (WTO) can exercise their legal authority to restrict trade in a good only when they can produce a scientific risk analysis documenting a need to restrict importation of that good. A case where the risk analysis was insufficient in restricting importation was observed with Australia being forced by the WTO to accept frozen Canadian salmon (or pay a
steep fine) in spite of serious risk of transmission of fish disease, because Australia was unable to produce a sufficiently quantitative risk assessment (Victor 2000).
Risk analysis can create legitimacy in many extralegal contexts as well. Highly contentious, politically charged issues can be decided on the basis of raw power, whether through political power or public influence, with little regard to the facts. Scientific risk analysis, with its philosophical commitment to value neutrality, can be used to legitimize a decision-making process. In some cases, people may agree to support the outcome of such a risk analysis-based decision process even if it contradicts their own desired outcome (NRC 1996). Thus, risk analysis can be a powerful force to garner legitimacy in a decision process from diverse public interests.
There are many points of tension between the use of risk analysis to create legitimacy and its use as a decision support tool. A risk analysis that is expected to help create legitimacy for decision making must meet different burdens of proof than a risk analysis conducted as a decision support tool. When used in decision support, the authoritative agent is already determined and therefore is free to accept, reject, or modify any of the assumptions or parameters that may have been established in developing any component of the analysis. For example, a decision maker could explore the consequences of weighting the effects of a pesticide on farmers and farm workers less than (greater than) the effects on the general public. It is, in part, the flexibility to adjust assumptions and parameters that makes risk analysis particularly useful in a decision support role. This flexibility allows the decision maker to explore the full range of possibilities before coming to a decision. In contrast, when risk analysis is used to create legitimacy, the potential decision-making agent may find that the choice of assumptions and parameters has a profound effect on the willingness of affected parties to recognize the agent’s authority to make a final decision. When it is crucial to gain the acceptance of interested and affected parties, the flexibility to adjust assumptions and parameters may be dramatically reduced or sacrificed altogether. Affected parties know that adjustment of such parameters can influence results in ways that favor one interest over another and may insist on fixing assumptions and parameters in a manner that substantially limits the usefulness of risk analysis techniques. Indeed, the role of risk analysis to create legitimacy becomes particularly perilous when the decision options under consideration are not well defined.
The role of risk analysis in governmental regulatory agencies has become increasingly ambiguous. The legal decision-making authority of such agencies is generally established by specific legislation or administrative findings. Traditionally, officials in these agencies have viewed risk
analyses in the role of decision support for the exercise of this legislatively mandated authority. However, their freedom to adjust assumptions and analytical parameters in risk analyses may be constrained by the language of the authorizing legislation or by successful court challenges from affected parties. This suggests that to some degree risk analysis has already become a component in establishing a government agency’s legal authority to make determinations. A more serious source of ambiguity flows from the extralegal burdens that regulatory decisions are increasingly expected to meet. The regulatory process is often described as the basis for public confidence in the safety, reliability, and fairness of the regulation of transgenic organisms (Carr and Levidow 2000, Ervin et al. 2000). This confidence may be vested in the integrity and judgment of regulatory officials themselves, but many commentators have expressed the view that it is or should be based on the risk analyses alone. This way of describing public confidence implies that risk analyses must be capable of being seen to embody principles of objectivity, fairness, and rigor by the general public. This is a burden of proof that goes considerably beyond the ability to withstand a legal challenge, and it takes risk analysis well beyond its traditional role in decision support (Funtowicz and Ravetz 1990, Von Winterfeldt 1992).
If risk analysis is expected to play any significant role in establishing the public’s confidence in genetically modified crops, it becomes crucial to examine the procedures and methods for conducting a risk analysis with two ends in mind.
Finding 2.1: Risk analysis of transgenic crops must fulfill two distinct roles: (1) technical support for regulatory decision making and (2) establishment and maintenance of the legitimacy of regulatory oversight.
Terminology of Risk Analysis
Although there have been many attempts to standardize the terminology for describing the stages of risk analysis, these attempts seldom gain widespread acceptance. Such stages are only a heuristic device intended to facilitate a collaborative approach to problem solving with respect to risk. Thus, there is no one best set of terminology, and this report shifts among the various sets as the problem merits or demands. However, it is impossible to communicate the committee’s ideas clearly without a discussion of the various approaches to describing the stages of risk analysis.
One of the most influential early studies on risk analysis uses the terminology of risk determination, risk evaluation, and risk acceptance to char-
acterize the stages of risk analysis (Rowe 1977). The general idea is that one must have an initial identification of the problem that the risk analysis is expected to address (i.e., risk determination). With a well-defined problem, techniques derived from the natural sciences and engineering are deployed in a process of risk evaluation (measuring and combining the magnitude of the hazard and the likelihood of its occurrence). Finally a decision maker makes a decision (i.e., risk acceptance).
When risk analysis is used to support policy or contentious decisions, the simple three-stage characterization of risk analysis itself becomes controversial. Some have preferred to call the first stage hazard identification, implying that a hazard does not become a risk until the probability that it might occur has been measured. Some object to the normative connotations of the word evaluation and substitute the term risk measurement or risk assessment for the middle stage. Many have noted that risks may not be simply “accepted,” so the third stage is then relabeled as “risk management.” Because risk analysis of transgenic organisms is itself contentious, the committee chose to adopt the terminology hazard identification, risk assessment, and risk management when discussing risk in its decision support role.
Broadly conceived, the techniques of risk assessment are of five general kinds:
-
Epidemiological analysis. Events of interest are observed, and the statistical relations of these events in the sampled populations are analyzed. This epidemiological approach has been very effective in identifying disease risks among populations such as smokers, industrial workers exposed to certain substances, and persons with a specific genotype. The scientific rationale for this method is that empirical correlations provide a basis for predicting effects and may indicate cause. This method could be used to associate risks with particular transgenic plants that are intensively planted in large or specific areas.
-
Theoretical models. A theoretical model that mimics or simulates the causal interaction of elements in a complex system is used to identify likely sources of system failure. This approach is widely used to study the risk of failure in engineering contexts such as instrumentation and control design. It has also been applied in biology to develop strategies for ecosystem management. The scientific rationale for this method is that it provides the logical consequences of a set of scientific assumptions about risks. It has played a critical role in analysis of the risk of evolution of resistance to transgenic insecticidal plants (Alstad and Andow 1995, Roush 1997, Gould 1998), and could play a role in evaluating community-level non-target effects of transgenic plants (Andow 1994).
-
Experimental studies. Controlled experiments are conducted to iden-
-
tify cause-and-effect relationships. Variations on this approach (often combined with statistical analysis) are used in product testing, including clinical trials for drugs and therapeutic devices. The scientific rationale for this method is that it establishes the cause or causes of a risk. Laboratory experiments can be used to establish a potential hazard (Hilbeck et al. 2000), and field experiments can be used to evaluate potential risks (e.g., Orr and Landis 1997). With field experiments, however, it is necessary to guard against false negatives (type II statistical errors), which would lead to concluding erroneously that there is no risk when, in fact, the experimental design is incapable of detecting any risk except a very large one (Marvier 2001).
-
Expert judgments. A group of experts use personal knowledge of a given system to estimate likely system performance under untested conditions. This is a widely used approach in risk analysis and has become the basis for risk analysis for some invasive species in the United States (Orr et al. 1993). The group is chosen to represent a range in necessary expertise with due consideration to potential conflicts of interest. The scientific rationale for this method is that the consensus of the group of experts represents a synthesis of the best-available scientific knowledge on the risk (Jasonoff 1986). The Environmental Protection Agency (EPA) uses this method frequently to aid in risk assessment of transgenic plants in its SAP (Scientific Advisory Panel) process, and the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service Biotechnology, Biologics, and Environmental Protection unit (USDA-APHIS-BBEP) has used it to evaluate the initial notification system for transgenic plants (USDA-APHIS 1993).
-
Expert regulatory judgment. Regulatory personnel use personal knowledge of a given system to estimate likely system performance under untested conditions. This is the most widely used approach in risk analysis. The scientific rationale for this method is that regulatory personnel have ready access to confidential business information and understand both current scientific knowledge and the process of risk analysis, so their judgments can be rendered with minimal delays and sufficient scientific accuracy. This is the method used by USDA-APHIS-BBEP to evaluate most of the complex, difficult-to-measure potential risks associated with transgenic plants.
The first three of these approaches are generally accepted as scientifically rigorous methods of analysis. Expert judgment, whether by external experts or by regulatory judgments are less rigorous, but often acceptable. A consensus of multiple external experts is likely to be more rigorous than the expert regulatory judgments because disagreements among external experts are likely to lead to more robust risk assessments (Jasanoff 1986).
Finding 2.2: At least five standards of evidence can be used in a risk assessment for decision support. The scientifically rigorous methods include epidemiological, theoretical modeling, and experimental methods. A consensus of multiple external experts is likely to be more rigorous than the expert regulatory judgments.
Finding 2.3: More rigorous methods used in decision support are likely to help risk analysis fulfill its other social role of establishing and maintaining regulatory legitimacy.
Chapter 5 evaluates circumstances in which these various methods should be used to supplement or displace expert regulatory judgment. In addition, for some questions the committee suggests specific types of data that should be collected to support epidemiological analysis, the simulation models that should be developed, the experimental studies that need to be conducted, and when expert panels should be convened. Implementation of any of these approaches (alone or in combination) requires a process to clarify the role that each general technique will play in measuring risk.
Risks may spark public outrage, and a fourth stage of risk communication has been suggested. But in the decision support role of risk analysis, communicating with the public may actually just be a tool of risk management, so the committee treats risk communication as a subset of risk management when discussing risk analysis in its decision support role. However, in its role of creating legitimacy, the greatest source of disagreement over risk often concerns the early stages of conceptualizing and identifying risk. Because some models of risk communication can become a way to exclude affected parties from this crucial part of the process, the 1996 NRC report considers risk communication integral to all stages of risk analysis. While inclusion in all stages blurs the category of risk communication, it must be integrated with all stages of risk analysis, and affected parties must be brought into early discussions.
When this is done, the three-staged framework of risk analysis also becomes less useful. For example, hazard identification is no longer an exercise in listing potential hazards but becomes a deliberative process from which potential hazards are characterized. Moreover, even when science and engineering methods are used to measure the probability that these potential hazards will materialize into bona fide risks, the risk problem is substantially sharpened and redefined by deliberative processes, so it seems appropriate to include this part of risk analysis under the heading of risk characterization as well. Clearly, the stages of risk analysis do not, in fact, represent temporally, analytically, or even conceptually
distinct and discrete elements. These considerations led the 1996 NRC report to describe risk characterization as:
the outcome of an analytic-deliberative process. Its success depends critically on systematic analysis that is appropriate to the problem, responds to the needs of the interested and affected parties, and treats uncertainties of importance of the decision problem in a comprehensible way. Success also depends on deliberations that formulate the decision problem, guide analysis to improve the decision participants’ understanding, seek the meaning of analytic findings and uncertainties, and improve the ability of interested and affected parties to participate effectively in the risk decision process. (158)
In the role of creating legitimacy, deliberation is central in risk characterization. It is important at every step in the process of making risk decisions, and must be incorporated into each stage of risk analysis. Therefore, when discussing risk in its role of creating legitimacy, this committee chooses to adopt the terminology risk characterization, which seamlessly integrates processes involved in hazard identification, risk assessment, and risk management. Hence all these processes are better thought of as integrating subcomponents of risk characterization rather than steps in a risk analysis process.
Risk Analysis as Decision Support in the Regulation of Transgenic Plants
Since the use of transgenic crop plants was first discussed, there has been confusion over the basis for distinguishing between the potential environmental risks associated with these plants and the risks associated with conventionally produced plants. As the power of conventional plant breeding has increased over the past 50 years, there has been a corresponding increase in the kinds and number of traits that can be bred into a commercial variety. As reviewed in Chapter 1, these changes and concomitant changes in agricultural production systems have created opportunities for novel interactions between agriculture and the surrounding organisms, habitats, and ecosystems. Transgenic crops are the latest development in this trend. Consequently, it is important for regulatory agencies to pay heed to the possibility that transgenic crops will be involved in novel ecological interactions resulting in novel environmental risks.
A series of scientific studies and reports have consistently found that the risks associated with a crop variety are independent of the means by which the crop was created. A 1987 NRC report concluded that the environmental risks associated with transgenic organisms are “the same in kind” as those of the unmodified organisms or organisms modified by other means. A 1989 NRC report clarified this statement to argue that this
meant any hazard that could be identified was similar enough to other known environmental hazards that an existing risk assessment methodology could be found to assess the risk. In other words, a new kind of risk would require a new risk assessment methodology. Below the committee reviews the kinds of hazards that can be associated with transgenic crop plants. None of these kinds of hazards is unique to transgenic plants, and hence no new kinds of hazards are identified. However, with the long-term trend toward increased capacity to introduce complex novel traits into plants, the associated potential hazards and risks, while not different in kind, may nonetheless be novel. For example, widespread planting of Bt corn generated concerns over a new potential non-target hazard—that toxic Bt corn pollen could reduce monarch butterfly populations.
The 1989 NRC study also found that transgenic plants should pose risks no different from those modified by classical genetic methods for similar traits. Traits that are unfamiliar (i.e., traits for which there has been little or no prior experience) in a specific plant will require careful evaluation in small-scale field tests. A 2000 NRC (2000c) report amplifies these points. It specifically notes that the magnitude of risk varies on a product-by-product basis and does not depend on the genetic modification process. However, the NRC (1989) report points out that information about the process used to produce a genetically modified organism is important in understanding the characteristics of the end product (NRC 1989).
Finding 2.4: For purposes of decision support, risks must be assessed according to the organism, trait, and environment.
Finding 2.5: For purposes of decision support, the process of production should not enter into the risk assessment.
Finding 2.6: The transgenic process presents no new categories of risk compared to conventional methods of crop improvement, but specific traits introduced by either of the approaches can pose unique risks.
Risk Analysis for Creating Legitimacy
The way Americans think about the relationship between agriculture and environment has evolved over time. For example, there was a time when a farmer’s decision to shift from pasture to crop production would have been thought to be a purely personal economic decision, raising no public concern over environmental issues. Now people recognize that pastures provide habitat for native flora and fauna and, when properly
managed, support more complex agricultural ecosystems than do many modern monocultures (see Chapter 1). As such, a crop genetically modified for a trait such as drought tolerance, which might encourage producers to convert pasture to crops in dryland regions, might today be thought to have environmental implications, whereas in the past it would not have been perceived as a problem. There has been a shift in perspective and values that has led many Americans to take an interest in ecological impacts. This shift is not based only on science (see Chapter 1), yet it clearly affects the expectations Americans have for their regulatory agencies.
As such, it will be important for regulatory agencies to maintain some degree of sensitivity to shifts in cultural values and implicit understandings that frame the public’s expectations. The novelty of recombinant DNA techniques in the public mind, not to mention the degree of discussion and debate that transgenic crops have received in the mass media, means that it may be important for regulatory agencies to be especially sensitive to transgenic crops and their possible impact for the foreseeable future. Maintaining public confidence in the regulatory system almost certainly requires a focus on transgenic crops in addition to any need based solely on scientific grounds.
Finding 2.7: Risk analysis of transgenic crops has played and is likely to continue to play an important role in maintaining the legitimacy of regulatory decision making concerning environmental and food safety in the United States.
The Precautionary Principle
The Precautionary Principle has been used in multilateral, international agreements to help legitimize a regulatory process based on scientific risk analysis. At its core the precautionary principle is based on the well-accepted folk wisdom that haste makes waste and one should look before one leaps. This folk wisdom, however, allows each individual to decide what is haste and how long and carefully to look. Analogous to the problem of risk, much of the controversy in applying the precautionary principle to societal issues relates to setting socially agreed upon standards for sufficient precaution. In one widely used form, the precautionary principle states that the absence of scientific knowledge about a risk should not impede actions to reduce that risk to society. In other words, even in the absence of clear scientific evidence that a risk is likely to occur, actions should be taken to reduce that potential risk. It is because we do this all the time in our daily lives that the precautionary principle makes such intuitive sense. If I think it might rain but do not know for sure, I
often will bring an umbrella. But for the same reasons that risk itself is complicated, and our intuitive understanding of risk has only tenuous connections to scientific risk analysis, the precautionary principle is complicated and our intuitive understanding of it incomplete.
As discussed more thoroughly in Chapter 7, there is no one precautionary principle, and the precautionary principles range from minor procedural changes in risk analysis to major shifts in the burden of proof. The principle is featured prominently in two international agreements about the risks of transgenic organisms—the Biosafety Protocol of the Convention on Biodiversity (CBD 2000) and Directive 90/220 of the European Union (EU), modified in March 2001 (European Commission 2001). In both cases the precautionary principle is specified ambiguously, so its meaning will evolve and become defined by its use. A close reading of the U.S. coordinated framework for regulating transgenic organisms shows that the framework neither excludes the use of a precautionary principle nor endorses one. Thus, the fate of the precautionary principle will be determined in future applications and negotiations among interested parties. As clearly stated in the biosafety protocol and the modified Directive 90/220, science will continue to be the basis of risk assessment.
SCIENTIFIC ASSUMPTIONS UNDERPINNING REGULATION OF TRANSGENIC CROPS
The Categories of Hazards
The initial step in risk analysis and one of the most critical for framing the entire analysis is hazard identification. A hazard is a potential adverse effect (potential harm) from a proposed activity (e.g., release of a transgenic crop plant). Risk is the combination of a hazard and the likelihood that the hazard occurs. This implies that many hazards are not risks, and the existence of a hazard does not imply significant danger from the activity because the hazard might not happen. The set or scope of identified hazards determines the technical capacity and level of deliberation needed to conduct the analysis and therefore is one of the most critical steps.
Four categories of hazards from the release of transgenic crop plants can be identified: (1) hazards associated with the movement of the transgene itself with subsequent expression in a different organism or species, (2) hazards associated directly or indirectly with the transgenic plant as a whole, (3) non-target hazards associated with the transgene product outside the plant, and (4) resistance evolution in the targeted pest populations. A fifth category of hazard, discussed in the other chapters, is that of indirect effects on human health that are mediated by the environment.
Most previous reports have not chosen to classify this as an environmental hazard.
In addition to these categories, the EU recognizes effects on genetic diversity as a separate category of environmental hazard in its modified directive 90/220 (European Commission 2001). The committee has not recognized this category as an environmental hazard because it is the effects of altered genetic diversity, such as increased extinction rate, a compromised genetic resource, inbreeding depression, or increased vulnerability to environmental stresses, that are the actual environmental hazards. These effects are addressed under the effects of movement of transgenes. The EU recognizes this category of potential hazard as a precautionary measure, because the effects of movement of the transgenes are uncertain and are presently incompletely characterized. By recognizing the more easily measured, intermediate effects on genetic diversity as a potential hazard, the EU risk analysis will address and manage all of the effects the committee lists under movement of transgenes without having to assess them specifically.
Hazards Associated with Movement of the Genes
The movement of transgenes does not, in itself, constitute a hazard but can serve as an opportunity for unintentional spread of transgenes in the environment (Nickson 2001). If a specific hazard is associated with that spread, the movement of transgenes constitutes the “exposure” component of a risk. One of three mechanisms—seed dispersal, horizontal transfer, or pollen dispersal—may move transgenes beyond the point of intentional release into environments and organisms other than those intended.
Seed dispersal can be accomplished by (1) unintentional seed spillage during processes that either bring the seed to the field to be planted or involve taking harvested seed from a transgenic crop from the field to market (e.g., in the United Kingdom, some roadside feral oilseed rape populations [Brassica napus] appear to be constantly replenished by seed spilling from vehicles on their way to a major oilseed crushing plant; Crawley and Brown 1995) or (2) dispersal of seeds directly from the transgenic crops into the surrounding environment. The hazard usually associated with seed dispersal is the evolution of increased weediness of the transgenic crop itself. (See “Hazards Associated with the Whole Plant” below.)
Horizontal transfer is the nonsexual transfer of genetic material from one organism into the genome of another. For example, it is becoming clear that plants appear to occasionally acquire genes from other kingdoms of organisms, such as fungi (e.g., Adams et al. 1998). The specific
mechanisms for such transfer are poorly understood. The rate of such acquisition is extremely low relative to within-species gene transfer but is surprisingly high over evolutionary timescales. For example, flowering plants appear to have acquired a certain mitochondrial gene by hundreds of independent horizontal transfer events over the past 10 million years (Palmer et al. 2000). In essence, horizontal transfer is natural genetic engineering. Specific hazards associated with horizontal transfer are rarely posed, and its significance is largely discussed as a source of unanticipated effects. Presently there are no data to suggest that the extremely low rate of natural horizontal transfer should change for transgenic organisms. As Rissler and Mellon (1996) state, “Although currently no evidence exists that genes move horizontally from plants to any other organism, the phenomenon is so recently described that it is too early to conclude that transfers from plants never occur…New discoveries in this area could change the assessment and significance of risk.”
Pollen dispersal provides an opportunity for the sexual transfer of transgenes to relatives of the crop, including other varieties of that crop, related crops, and wild relatives. The specific vectors for pollen dispersal vary with the crop. Wind and insects are the most frequent agents that carry pollen from plant to plant. Almost all crops are expected to disperse some pollen. Although many crops are self-fertilizing (Frankel and Galun 1977), even crops with very high selfing rates are capable of mating with plants many meters away (e.g., Wagner and Allard 1991). Furthermore, although certain crops are typically harvested before flowering (e.g., sugar beets), occasional plants flower prematurely (e.g., “bolters”; Longden 1993) or are missed by harvesting equipment. Very few crops are apparently 100% male sterile (e.g., certain potato clones, certain ornamental varieties).
One hazard associated with pollen dispersal that has received considerable attention is that of sexual transfer of crop alleles to wild relatives, resulting in the evolution of increased weediness (e.g., Goodman and Newell 1985, Ellstrand 1988, Snow and Moran-Palma 1997). When wild relatives are nearby, natural hybridization with them is apparently a common feature of most cultivated plants (Ellstrand et al. 1999). The precedent has been set with traditional crops. Spontaneous hybridization between crops and their wild relatives has already led to the evolution of difficult weeds, such as weed beets in Europe (Boudry et al. 1993, Mücher et al. 2000) and weed rye in California (Suneson et al. 1969, Sun and Corke 1992). One could imagine that certain crop transgenes might also contribute to the evolution of increased weediness.
Another hazard associated with pollen dispersal is that locally common species can overwhelm those that are locally rare with their pollen, increasing the risk of extinction by hybridization (see Chapter 1). Extinc-
tion may occur in one of two not necessarily exclusive ways (Ellstrand and Elam 1993). The rare population may produce so many hybrids that it becomes genetically absorbed into the common species (genetic assimilation). Additionally, hybrids may have reduced fitness (outbreeding depression), and therefore the rarer of the species may be unable to maintain itself. The problem of extinction by hybridization has long been recognized as a conservation problem for animals (e.g., Rhymer and Simberloff 1996) but in plants has only recently received attention (e.g., Levin et al. 1996, Huxel 1999, Wolf et al. 2001). Nonetheless, theoretical models have demonstrated that extinction by hybridization can proceed rapidly, resulting in local extinction of a population in as few as a handful of generations (which, for some plants, could be less than a decade). For example, spontaneous hybridization between crops and their wild relatives has been implicated in increased extinction risk to wild species, ranging from the disappearance of wild coconuts (Harries 1995) to the contamination of California’s wild walnut populations with genes from the cultivated species (Skinner and Pavlik 1994). There is no reason to imagine that this hazard should be any different for transgenic crops.
Hazards associated with the movement of transgenes within a crop, from one variety to another, has rarely been discussed. However, crop-to-crop hybridization may lead to the unanticipated natural “stacking” of transgenes, as in the case of the evolution of triple herbicide resistance in oilseed rape in Canada (Hall et al. 2000). Likewise, it is possible that crops transformed to produce pharmaceutical or other industrial compounds might mate with plantations grown for human consumption, with the unanticipated result of novel chemicals in the human food supply (Ellstrand 2001). While experience with traditional crops appears to offer no precedents for the latter hazards, it seems that if hybridization occurs so readily between crops and their wild relatives, it should occur even more easily between adjacent crops of the same species.
Hazards Associated with the Whole Plant
The transgenic plant itself may become an environmental hazard because the traits it receives may improve its fitness and ecological performance. Many crop plants may pose little hazard, insofar as they are unable to survive without human assistance. Frequently, traits that make them useful to humans also reduce their ability to establish feral populations in either agroecosystems or nonagricultural habitats. For example, lack of seed shattering and seed dormancy greatly reduces the ability of an annual crop to persist without human intervention. Without major changes in its phenotype, corn is unlikely to survive for multiple genera-
tions outside agricultural fields no matter what transgene is added to it (see discussion of epistatic effect in Chapter 1).
However, it is generally the case that most crops have weedy and/or wild populations in close association with cultivated forms in some part of their global distribution (De Wet and Harlan 1975). For example, sugar beets establish wild populations in the United Kingdom (Longden 1993). Depending on the location, certain crops (e.g., tomatoes) evolve to a wild-type phenotype very quickly and could become viable wild populations by the F2 generation. The existence of these populations demonstrates that transgenes that confer adaptation to significant limiting factors can create significant whole-plant hazards, particularly if the ecological effects of transgenic crops are evaluated on global basis. The frequency of feral crop populations also reveals the difficulty of distinguishing gene flow and whole-plant hazards. Gene flow between feral crop populations and transgenic crops may create weeds that bear adaptations derived from the feral plants—such as seed dormancy—that suffice to produce new invasive-plant hazards in an agroecosystem or beyond.
From a U.S. perspective, whole-plant hazards are limited by the fact that many crops are highly unlikely to establish self-reproducing populations in the United States. Potatoes can establish wild populations in South America, but they are not known to establish wild populations in this country. Wheat is established in wild populations in the Middle East but not here. Many tropical plants cannot overwinter in most of the United States, and certain polyploid crops cannot produce viable seed. The factors that limit establishment of many crop species can be subtle and are not well understood.
For example, an annual crop could produce large quantities of viable seed with good seed dormancy characteristics. If, in addition, seedlings establish at high rates and produce viable F1 and F2 populations but germinate at the wrong time relative to weed control measures or herbivory, no viable population may be established. Thus, the mere presence of a transgene should not be taken as prima facie evidence that the weediness of a crop has been altered. Many crops are unlikely to be made more weedy by the addition of a single trait (Keeler 1989).
Some crops are capable of establishing wild populations in the United States. These particularly include crops that are only slightly modified from wild progenitors and that are adapted to U.S. conditions. This class includes many forage grasses, poplars, alfalfa, sunflowers, wild rice, and many horticultural species. Some domesticated species are important weeds of natural plant communities, including birds foot trefoil (Lotus corniculatis) and Bermuda grass (Cynodon dactylon). In these crop species the addition of a single transgene that improves some ecological charac-
teristic could increase the weediness or invasiveness of the species, and these risks merit evaluation.
Non-target Hazards
Non-target organisms are any species that is not the direct target of the transgenic crop. To date, the vast majority of the published studies that examine non-target hazards have focused on Bt crops. For example, Bt corn is presently targeted to control the key pests, European corn borer (Ostrinia nubilalis) and southwestern corn borer (Diatraea grandiosella) (Ostlie et al. 1997) and Bt rice is targeted against striped stem borer (Chilo suppressalis) and yellow stem borer (Scirpophaga incertulas) (Cohen et al. 1996). Any other species affected by Bt corn or Bt rice is a non-target species; consequently, the list of potential non-target species is very long. These organisms can be grouped conveniently into five categories that are not mutually exclusive: (1) beneficial species, including natural enemies of pests (lacewings, ladybird beetles, parasitic wasps, and microbial parasites) and pollinators (bees, flies, beetles, butterflies and moths, birds and bats); (2) non-target pests; (3) soil organisms, which usually are difficult to study and identify to species; (4) species of conservation concern, including endangered species and popular, charismatic species (monarch butterfly); and (5) biodiversity, which may include the species richness in an area.
Transgenic pesticidal crops present a challenge because in some respects they are similar to chemical pesticides that are routinely regulated and in other respects they are similar to conventional breeding and other agricultural technologies that are not regulated at all. Scientific progress has been an awkward accommodation between these perspectives that has yet to reach scientific consensus. This issue is examined in detail later in this chapter.
Hazards can be difficult to demonstrate scientifically because it can be tricky to reproduce experimentally the route by which organisms are exposed. The present status of knowledge indicates the following possible hazards:
Beneficial Species. In well-controlled laboratory studies, Bt toxin similar to that in Bt corn increased mortality in green lacewing larvae (a predator of insect pests; Hilbeck et al. 1998a, 1998b). Both direct consumption of the Bt toxin and indirect consumption by eating caterpillars that had themselves consumed Bt toxin resulted in higher lacewing mortality. Field evaluations of this effect have been inconclusive (e.g., Orr and Landis 1997), although none of the published studies have documented an effect. Herbicide-tolerant crops might cause indirect reductions on beneficial
species that rely on food resources associated with the weeds killed by the herbicides.
Non-target Pests. Most transgenic crops have effects on non-target pests. Although these effects are expected to sometimes be positive and sometimes negative, studies documenting only reductions in non-target pest populations have been published. For example, with Bt rice, it will be very important to confirm that it does not increase attack by rice planthoppers, the most important insect pests of rice (K. Sogawa, personal communication, Chinese National Rice Research Institute, 2000).
Soil Organisms. No effects on soil organisms have been documented in either the laboratory or the field. Surprisingly, however, Bt toxin leaks out of corn roots to detectable levels in the soil and may persist adsorbed to soil particles longer than nine months (Saxena and Stotzky 2000). The consequences of this unusual exposure route are not fully understood, although a number of Bt toxins have been found to be toxic to bacteria-eating soil nematodes (see BOX 4.2). Because there are so many species of soil organisms and their ecology is poorly understood, it is difficult to conduct comprehensive tests of many soil organisms. A different approach to non-target effects that might be considered would be to evaluate the effects of transgenic plants on targeted soil function, such as soil respiration, organic decomposition rates, and emissions of greenhouse and ozone-depleting gases. This has been done using nonisogenic comparisons (Donagan et al. 1995, 1996), but such evaluations probably would require the use of isogenic controls to the transgenic crops so that the effect of the transgene could be clearly evaluated (see discussion below on the need for isogenic varieties). Changes in these soil functions would indicate that some non-target species were being affected in ways that could have significant effects on the environment. Research is needed to evaluate the utility of such an approach.
Species of Conservation Concern. The effect of Bt corn on monarch butterflies (Losey et al. 1999, Jesse and Obrycki 2000) has captured widespread attention in part because it is a species dear to many people. Such charismatic non-target species gain great importance because of their symbolic significance to humans. The scientific data on this issue are still emerging (see BOX 2.1). Because some Bt corn events have higher toxin concentrations in pollen than others, the detrimental effect of some types of Bt pollen on monarchs is less likely for other types of Bt corn. In the upper Midwest, herbicide-tolerant soybeans might cause indirect reductions of monarch populations because their milkweed host plants are killed by the herbicides.
|
BOX 2.1 Effect of Bt Corn on Monarch Butterflies Several types of transgenic Bt corn that are toxic to Lepidoptera have been commercialized in the United States. All have been deregulated by APHIS, but none have received permanent registration from EPA at the time of this writing.
APHIS regulatory authority for evaluating risks to monarch butterflies and other nontarget insects is indirect (see Chapter 3). These non-target species may feed on agricultural weeds, so reducing their populations could indirectly harm crop plants, causing an “indirect plant pest” risk. This differs substantially from EPA regulatory authority, which is directly aimed at protecting non-target species from the effects of a pesticide. In other words, it is possible for APHIS to find that monarch populations are harmed but that there is no increased plant pest risk and therefore no need to manage the risk. This could occur if milkweed abundance were not regulated by monarch density and, even if it were, milkweed might not be competitive enough against other plants to alter plant community composition. Hence, even if monarchs were to disappear, there might be no change in |
|
plant pest risk. This argument, however, has not been used by APHIS in its risk assessments of Bt crops. APHIS evaluations of non-target effects on Bt corn have concentrated on the potential effects on beneficial organisms, such as insect natural enemies and pollinators (honeybees) and threatened or endangered species (see Chapter 4). APHIS did not request information on broader non-target effects, but most petitioners submitted data on potential effects on earthworms, other moths and butterflies, Daphnia, and other species. APHIS concluded that Bt corn could detrimentally affect only a specific group of Lepidoptera, but such effects would cause no additional plant pest risk. Threatened and endangered species were considered not at risk because none of these species feed on corn plants. But the potential toxic effects of Bt corn pollen were not assessed, and effects on monarchs were not considered explicitly in any of the APHIS risk assessments for deregulation up through 1998. EPA granted a conditional registration to all of these Bt crops some time after the APHIS deregulation decision. The main reason for the conditional registration rather than a permanent registration was to enable the registrants to develop and implement scientifically based resistance management strategies prior to permanent registration. The effect of the conditional registration, however, was to maintain all of the Bt crops under active regulatory oversight, so that if any unexpected potential risks were identified, EPA could act to manage those risks. This has given EPA both the authority and the practical ability to make additional risk assessments of Bt corn long after APHIS had decided that it presented no plant pest risk. At the time of its conditional registration of Event 176 (EPA 1995), EPA noted that corn pollen containing Cry1Ab toxin can drift out of cornfields, but the agency considered the amount of such pollen that would drift onto food plants of susceptible endangered species to be very small. Therefore, EPA did not expect that any endangered or threatened species would be adversely affected by Bt pollen-containing Cry1Ab toxin. Significantly, both the EPA and the APHIS evaluations concentrated on only endangered and threatened species. Monarch butterflies are neither endangered nor threatened, so the risk to this species was not considered in risk analyses conducted by either EPA or APHIS. By the end of 1998 APHIS had deregulated five transformation events of Bt corn, finding that none of them created additional plant pest risk, and EPA had granted four of them conditional registration. In May 1999, Nature published a brief communication by Losey et al (1999) that showed that Bt corn pollen was a potential hazard to monarch butterflies. The authors applied large amounts of Bt pollen from Bt corn to milkweed leaves and showed that larval mortality was higher, development was slower, and size was smaller in monarch larvae fed the leaves with Bt pollen compared to leaves with non-Bt pollen or no pollen. The authors suggested that Bt pollen could dust the leaves of milkweed and monarchs could eat it and suffer reduced fitness. The fact that the Bt toxins were harmful to monarchs was not the significant element of the paper. This effect could be anticipated because the toxins were known to be harmful to a specific group of Lepidoptera, of which monarchs were likely to be a member. The significance of the paper was to suggest that the movement of pollen onto other plants could create hazards for the insects feeding on those plants. Previous risk assessments had not given this exposure pathway thorough consideration. This paper stimulated a number of responses during 1999. EPA initiated an internal evaluation of its Bt corn risk assessments to determine what, if anything, it should do in |
|
response to the information. USDA directed the corn insects research group in Ames, Iowa, to conduct research to estimate the risks to monarchs, increasing the laboratory’s budget. At this time, several agricultural biotechnology industries were cooperating informally in a Bt working group. This group initiated a series of studies by selecting and funding several public sector scientists to conduct studies to estimate the risks to monarchs. All of this research was planned and implemented rapidly during the early part of the growing season. During the fall of 1999, EPA issued a data call-in for evaluating the risks of Bt corn to monarchs. This is an infrequently used formal mechanism to require sufficient data from registrants and other interested parties so that EPA can make a science-based risk assessment. A major assumption of the 1999 research was that monarchs do not occur in sufficient numbers in cornfields to merit investigation. The 1999 assumption meant that the researchers focused on estimating the risk that monarchs are killed near Bt corn. These unpublished research results from 1999 were used by the Agricultural Biotechnology Stewardship Technology Committee (ABSTC) to construct an initial evaluation of the risks of Bt corn to monarchs. The essence of the argument was that so few monarch caterpillars encounter Bt pollen near cornfields that monarch populations are not at risk (EPA 2000b). Careful observations at a cornfield in Iowa, coupled with results from two other fields, suggested that Bt corn pollen did not move in quantities sufficient to affect monarch larvae much more than a few meters from a cornfield. Spatial and temporal variations in pollen movement and retention on milkweed leaves were not characterized. The industry group reorganized formally into the ABSTC in January 2000 to provide a mechanism for cooperation among companies to address common interests related to risks of agricultural biotechnology without risking lawsuits on collusion or other monopoly practices (members are Monsanto, Syngenta, Aventis, Dow, and DuPont). Prior to the 2000 field season, the ABSTC and the USDA organized to fund jointly research for the 2000 field season. After considerable negotiation, USDA agreed to match the money contributed by ABSTC (about $200,000) under the condition that a multi-stakeholder advisory panel established by ABSTC would advise the USDA on how the money should be allocated. This advisory panel was comprised of Eric Sachs (Monsanto), Margaret Mellon (Union of Concerned Scientists), J. Mark Scriber (Michigan State University), Adrianna Hewings (USDA), Eldon Ortman (Purdue University), and Richard Hellmich (USDA Agricultural Research Service). One of the major features of the proposed research for 2000 was to challenge the assumption that monarchs did not occur in significant numbers in cornfields. The research also focused on obtaining more accurate toxicity data for the various Bt corn events under both laboratory and field conditions, and further estimation of pollen deposition rates in the field. Early in the summer of 2000, Jesse and Obrycki (2000) published their paper on Bt corn and monarchs in Oecologia. This paper confirmed the finding of Losey et al. (1999)— pollen from Bt corn was toxic to monarchs. Jesse and Obrycki (2000) collected milkweed leaves in and around cornfields and tested the toxicity of these leaves on monarchs in laboratory trials. The new finding was that the level of natural deposition of Bt pollen on milkweed leaves in cornfields was sufficient to kill monarch larvae. The authors also reported that pollen deposition was too low to kill monarchs at distances greater than 5m from a field edge. In addition, preshadowing results to come from the ABSTC/USDA cooperative research, the authors reported variable results from Mon 810 and Bt-11 Bt corn varieties. Also during early summer 2000, Wraight et al. (2000) published a study showing that Bt corn pollen from Mon 810 had little effect on Papilio polyxenes, black swallowtail butterfly caterpillars. In laboratory bioassays, they found that Event 176 pollen, but not |
|
Mon 810 pollen caused significant caterpillar mortality. These results indicate that the different events have different pollen toxicity to swallowtail caterpillars. The results of the ABSTC/USDA funded research was published in September of 2001 in the Proceedings of the National Academy of Sciences (Hellmich et al. 2001, Oberhauser et al. 2001, Pleasants et al. 2001, Sears et al. 2001, Stanley-Horn et al. 2001). A number of significant results were found. Oberhauser et al. (2001) found that monarchs do use cornfields as oviposition sites. In fact, throughout the northern corn belt, more monarchs emerge from cornfields than any other habitat. Cornfields are not the most productive habitat per area, but because they occupy such an extensive area in the monarch’s breeding range, they produce more monarchs. Moreover, monarchs use these cornfields when corn is shedding pollen in the northern part of their breeding range. Thus monarchs are more likely to be at risk inside Bt corn than near it. Hellmich et al. (2001) showed that Event 176 pollen is indeed quite toxic to monarch larvae, but Mon 810 pollen and Bt-11 pollen were not acutely toxic at average pollen densities that were observed in the field (Pleasants et al. 2001). Finally, Stanley-Horn et al. (2001) found that in the field, monarchs protected from natural enemies and exposed to pollen naturally deposited from Event 176 Bt corn had reduced survival and slower growth than monarchs exposed to non-Bt corn pollen, while pollen from Bt-11 and Mon 810 had no detectable effects. Thus, the researchers concluded (Sears et al. 2001) that the risk to the monarch is negligible for the two types of Bt pollen that comprised over 90% of the Bt corn area in the United States in 2001 (Sears et al. 2001). In an independently developed study comparing field survival of monarchs and black swallowtails exposed to naturally deposited pollen, Zangerl et al. (2001) found that Event 176 pollen significantly reduced growth of black swallowtail, but had no detectable effect on monarchs. Mon 810 pollen had no significant effect on either species in the field. This lack of a significant effect of Event 176 pollen on monarchs contrasts with the significant effect observed by Stanley-Horn et al. (2001). Zangerl et al. (2001) did not protect monarch or swallowtail larvae from natural enemies, while Stanley-Horn et al. (2001) did. This raises the speculative possibility that in monarchs, in contrast to black swallowtails, density-dependent mortality from natural enemies compensates for any effects caused by Event 176 pollen. Although the recent articles certainly establish a lack of significant risk of acute toxicity to monarchs, there are a number of questions that should be addressed in follow-up research. While the recent work was able to test for large sublethal effects in early larval development, it would be useful to determine if there are any sublethal effects of Bt corn pollen on monarch caterpillars that result in reduced female fecundity, reduced probability of overwintering survival, or reduced male mating ability. It would also be useful to determine if plant parts other than pollen land on milkweed leaves in cornfields and are eaten by monarch larvae (see Hellmich et al. 2001) because other floral parts have a high concentration of Cry1Ab toxin even in Mon810 and Bt-11 varieties. Event 176 had a sizable share of the Bt corn market when it was first introduced. By the 2001 field season, Ciba Seeds (Novartis) did not even make an effort to sell it. The outcome for monarchs would have been substantially different had Event 176 become the dominant transgenic Bt event in corn given that pollen from Event 176 is over an order of magnitude more toxic to monarchs than that of Mon 810 or Bt-11. There is no reason to expect that a Bt corn using the 35S promoter must necessarily produce a better corn variety than Event 176, which uses other promoters. Indeed, had the toxin content of grain been a significant market force during 1996–1999 and had Event 176 been used in some of the best corn germplasm in the corn belt, it might have become the most popular variety of Bt corn grown because unlike Mon 810 and Bt-11, event 176 does not produce the Bt protein in it kernels. |
Biodiversity is elusive because it embraces many dimensions, including the number of species, their relative abundances, the way they interact, and whether they are indigenous or exotic. From a conservation perspective, preservation of native species is a priority. Relatively little is known about the potential effects of transgenic crops on biodiversity from this perspective. However, herbicide-tolerant crops might reduce biodiversity by eliminating weeds that harbor many species. Such a reduction in biodiversity might then reduce the population densities of birds that rely on this biodiversity for food. Although this topic has been discussed specifically for genetically engineered herbicide-tolerant crops, it is, in fact, a potential impact of all herbicide-tolerant crops, regardless of their origins.
The state of knowledge about non-target effects of transgenic plants is improving slowly, but controversy surrounds each published study. The biggest gap in the research is in establishing scientifically rigorous assessment protocols that take into account the unusual exposure routes of transgene products. Consequently, considerable scientific work remains to be done before evaluation of non-target effects is standardized.
Hazard of Resistance Evolution
Resistance evolution can occur in pests that are targeted for control by or associated with a transgenic crop. This is a potential environmental hazard because if the pest becomes resistant to control, alternative, more environmentally damaging controls may be used. In addition, new control tactics may be rushed into use before their environmental risks are completely assessed. Insects, weeds, and microbial pathogens all have the potential to overcome most control tactics used against them (Barrett 1983, Georghiou 1986, Georghiou and Lagunes 1988, Green et al. 1990, NRC 2000c). Insect resistance to Bt crops is considered inevitable, and efforts are being made by the EPA to manage resistance evolution to these transgenic crops. Virus-resistant transgenic crops have not been used extensively, but many viruses have evolved resistance to conventional virusresistant crops (Fraser 1990). Fungal and bacterial resistance is not yet commercially available in transgenic crops, but both groups of organisms have evolved resistance to conventional crop resistance, sometimes within five years (Delp 1988). Evolution of herbicide-tolerant weeds is an indirect environmental risk. Herbicide-tolerant transgenic crops are designed such that specific herbicides can be used to control weeds, usually after the crop has emerged. These postemergence weed controls might allow herbicides to be used only as needed, reducing herbicide applications to crops. In some crops these postemergence herbicides might replace ones that are more damaging to the environment. As weeds evolve resistance, though, these potential environmental benefits could be lost.
Finding 2.8: There are potential direct environmental hazards associated with the environmental release of transgenic crops. The kinds of direct hazards (or categories of hazards) are those associated with the movement of the transgenes, escape of the whole plant, nontarget effects, and resistance evolution.
Indirect Hazards
Transgenic crops can have indirect environmental hazards, especially when scaled up for commercial production. Although analysis of these indirect hazards is complex, they may in some cases be as important or more important than direct hazards. Some of these indirect hazards are discussed in other chapters.
Environmental Risks of Transgenic Crops and Conventionally Bred Crops
Despite nearly 20 years of scientific discussion, scientific debate continues about whether the risks associated with transgenic crops are similar to or different from those associated with conventional crops. The source of scientific disagreement is that in some ways transgenic crops are just like conventionally bred crops, but in other ways they are quite unlike conventionally bred crops. Clarifying the ways that they are similar and dissimilar can sharpen the scientific dimensions of this issue. This issue is of more than academic interest. Its clarification can provide a scientific justification for the present U.S. regulatory system.
Conventional crop breeding can be broadly understood to consist of two relatively distinct processes. The first is a set of activities to create genetic novelty in the plant population. In the vast majority of cases this is done by crossing several elite varieties together to create a mixed population (see Chapter 1). The second set consists of activities that select out of that genetic novelty genotypes that are useful new crop varieties. These methods include a number of techniques, including recurrent selection, mass selection, marker-assisted selection, and “selfing selection.” Crop breeding must be understood as the integration of these two sets of processes.
Transgenic methodology is a new process for introducing genetic novelty into a crop plant. It is not a process of selection. Transgenic methods as they are applied to crop variety production are only a part of the crop-breeding process. Indeed, it is because transgenic methods provide new ways of introducing genetic novelty into a crop that they are not similar to conventional plant breeding. As pointed out in Chapter 1, new techniques such as wide hybridization, embryo rescue, and functional
genomics that are categorized under the umbrella of conventional breeding are also novel approaches for introducing new qualities into crops.
Transgenic crops bear many similarities to conventionally bred crop varieties because they are both selected by the same conventional methods; crop varieties therefore bear some similarities. It is this confounding of parts of the breeding process with the whole breeding process that has created some of the scientific confusion.
At a deeper level, however, the general comparison of environmental risk of transgenic and conventional crop varieties probably cannot be resolved scientifically at the present. The unresolved scientific challenge is to determine if the range of genetically engineered crop varieties has a similar degree of environmental risk as the range of conventionally produced crop varieties. Theoretically, the most quantitatively robust estimate of environmental risk for transgenic crops would compare the probability of environmental damage associated with transgenic crops to the probability of environmental damage for all crops. This would allow the risk from commercialization of transgenic crops to be expressed as a conditional probability given the presence of transgenes. Such comparison is impossible. There has never been a systematic attempt to measure the probability that a randomly selected agricultural crop will cause environmental damage; hence, the baseline data that would be needed to derive a conditional probability estimate simply do not exist. In addition, because the range of possibilities attainable by both conventional and transgenic methods is expanding rapidly, any scientific determination would be provisional and rapidly outdated. As argued in Chapter 1, while our long experience with some conventional crop breeding techniques indicates that for the most part it has been safe for humans and the environment, there are cases where it has led to environmental harm.
Most inquiries into this issue have focused on one of two narrow questions. First, does a commercial transgenic crop variety with a particular trait have similar ecological risk as an isogenic commercial variety with the same functional trait produced by a conventional method? APHIS expands this argument to assert that the risk of the transgene is not greater than the risk of a comparable conventional variety that is neither isogenic nor possessing the same trait (see Chapter 4). There are no published scientific studies that demonstrate the similarity of risk at either of these levels of comparison (see BOX 1.2).
Second, does a commercial transgenic crop variety with a particular trait have similar ecological risk as an isogenic conventional commercial variety without the trait? Crawley et al. (2001) provide some evidence to this point, although it is not clear if the comparisons were isogenic ones. For the several crop-trait-environment combinations that were tested, no difference in weediness was found within the pairs. Crawley et al. (2001)
warn not to generalize from these cases because in none of the cases would the investigators have hypothesized that the added trait could have increased weediness of the crops tested. Moreover, they evaluated only one hazard within the large class of potential hazards associated with transgenic crops. Similarly, claims that the lack of effects from the tens of millions of hectares of transgenic crops that have been planted in the United States during the past three years are nonscientific. There has been no environmental monitoring of these transgenic crops, so any effects that might have occurred could not have been detected. The absence of evidence of an effect is not evidence of absence of an effect.
The Trigger for Risk Analysis
Although it is generally agreed that the risks associated with any transgenic organisms should be evaluated on the basis of the trait, organism, and environment without reference to the process of transformation, there is considerable disagreement about the scientific basis for triggering regulation of transgenic crops. At one extreme, some might argue that the regulatory trigger should also be based on some ex ante risk evaluation based on the trait, organism, and environment, but at present there is no scientific basis on which to build such an ex ante system. In the absence of such a system, the committee argues below that transformation is both a useful and logically justifiable regulatory trigger. Transgenic organisms have potential environmental risks, but the committee expects that most of them will not produce significant actual environmental risks. Consequently, the committee also suggests that for environmental risk regulatory oversight should be designed to winnow the potentially riskier transgenic crops from the less risky ones before a substantial regulatory burden is imposed on the less risky ones.
This report makes a number of comments about the properties of transgenic and conventionally bred crops that seem, at least superficially, to be in conflict. One of the committee’s key findings (2.6) is that “the transgenic process presents no new categories of risk compared to conventional methods of crop improvement, but specific traits introduced by either of the approaches can pose unique risks.” Chapter 1 points out that any addition of genetic novelty can have ecological impacts and that there is no predictive relationship between the number of new genes and the degree of impact. It also finds that there is no relationship between the taxonomic distance between the donor and recipient of a gene and the environmental risk. In addition, another committee finding (2.4) is that regulation should be based on the trait, organism, and environment. Thus, a transgenic stress-tolerant trait in canola and a conventionally produced allelic trait in canola should receive equal regulatory scrutiny. However,
APHIS would not examine the conventionally bred, stress-tolerant canola because of the process used to breed it. Therefore, it might appear that the APHIS trigger is not justifiable.
Before discussing the scientific details of the issue related to transgenic crops, the committee addresses two related issues. First the Office of Science and Technology Policy (OSTP) scope statement (1992) concerning the regulatory trigger is reviewed. The guidance is found to be ambiguous. Second, another regulatory trigger used by APHIS under the same statutory authority that it regulates transgenic crop plants is reviewed. The committee finds that APHIS has adopted a different process-based trigger to meet these other regulatory responsibilities.
OSTP Scope
The entire coordinated framework (OSTP 1986) is premised on regulating transgenic organisms (i.e., using the process of transformation to trigger the entire regulatory apparatus it describes). Hence, from its very inception, use of a process-based trigger to regulate transgenic organisms has existed. The coordinated framework did not fully address how oversight should be exercised by federal agencies under the various statutes, so in 1992 OSTP issued a statement on the scope of oversight so that common principles would govern decisions on how to regulate transgenic organisms.
The Final Statement of Scope (Section III) states that:
federal agencies shall exercise oversight of planned introductions of biotechnology products into the environment only upon evidence that the risk posed by the introduction is unreasonable. A risk is unreasonable [when] the full value of the reduction in risk obtained by oversight exceeds the full cost of the oversight measure. (6,756)
Any regulatory trigger that adheres to this principle is consistent with the OSTP scope statement. This statement is silent on the use of the transformation process as a trigger for regulatory oversight. While the final statement supersedes all previous discussion on scope, it is nevertheless instructive to examine some of the principles in the 1992 document that the final statement supercedes.
In Section II.A (6,756), three fundamental scope principles are articulated:
-
A determination to exercise oversight within the scope of discretion afforded by statute should not turn on the fact that an organism has been modified by a particular process or technique, because such fact is not alone a sufficient indication of risk.
-
A determination to exercise oversight in the scope of discretion afforded by statute should be based on evidence that the risk presented by
-
introduction of an organism in a particular environment used for a particular type of application is unreasonable.
-
Organisms with new phenotypic trait(s) conferring no greater risk to the target environment than the parental organisms should be subject to a level of oversight no greater than that associated with the unmodified organism.
The first principle is the only one that directly relates to the issue of using a process-based trigger, and it clearly proscribes the use of one if it turns on the fact that an organism is transgenic. The principle does allow the fact that an organism is transgenic to be a part of the basis of a trigger; it simply proscribes its use as the primary basis for a regulatory trigger. More significantly, principle 2 is adopted as the core of the relevant part of the Final Statement on Scope, and principle 3 is mentioned prominently in the implementation of the document, as discussed below. Only principle 1 fails to appear in any form in either the final statement or the implementation. Consequently, principle 1 is fully superseded by the final statement, and a fully process-based trigger is consistent with the 1992 OSTP scope document.
The scope document provides several paragraphs of guidance on implementation (Section IV) of the Final Statement on Scope. Section IV.A (Exercising Discretion Within the Scope of Statutory Authority) clarifies that the final statement governs all federal oversight of transgenic organisms. This section is otherwise silent on the issue of using a process-based trigger. Section IV.B (Evaluating Risks) clearly embraces principle 3 (above) as a core principle (paragraph 4). Section IV.D (Use of Categories of Exclusion/Inclusion) is the only section to mention process-based triggers explicitly. The document notes that:
several commenters suggested that the [previously proposed categories of] exclusions were inconsistent with a risk-based approach because they were “process-based.” … Because this Final Statement is to be a guidance document to the agencies, it is not meant to provide the risk rationales for these examples [the exclusions]. Any agency that wishes to use any of these categories in the context of a specific statute would provide a rationale based on risk. (6,758)
Thus, it is clear that the Final Statement on Scope allows an agency to use a trigger that is process-based as long as it provides a rationale based on risk.
Federal Plant Pest Act and APHIS Regulation
Invasiveness, which is the alteration of community or ecosystem structure or function, is the main environmental risk of nonindigenous species. Invasiveness is a function of both the organism phenotype and
the environment. As discussed in Chapter 1, only a small minority of nonindigenous species is invasive (see Figure 2.1), but at present we are unable to predict ex ante which species will be invasive and which will not. Consequently, it is impossible to construct a scientifically justified regulatory trigger based on the actual risk of invasiveness.
APHIS asserts its regulatory authority over the importation of nonindigenous species under the Federal Plant Pest Act (FPPA). The vast majority of nonindigenous organisms introduced into the United States arrive inadvertently in commercial shipping or deliberately through the trade of living organisms (aquarium fish, pets, horticultural plants). There is also a small but significant possibility that nonindigenous species are brought into the country by people arriving in airplanes and ships. For these passengers, APHIS uses two triggers for additional oversight. If a passenger is carrying animal or plant products, including fruits, meats, and living plants and animals, APHIS inspectors may confiscate or quarantine such products. If a passenger has visited a farm, APHIS inspectors may confiscate or quarantine clothing. While these triggers have a risk-related basis, they are also process-based triggers. Merely visiting a farm does not imply that a passenger is carrying an invasive nonindigenous species. However, as long as there is information indicating that, on average, passengers that have visited farms have a higher probability of carrying such a species, the process of visiting a farm is a useful risk-based trigger. These processes can be logical, operational ways to identify a subset of potentially risk-inducing activities, that will trigger oversight at U.S. borders.
Finding 2.9: A logical scientific argument can be made without reference to conventional crops that all transgenic crops should enter into regulatory oversight.
FIGURE 2.1 Venn diagram illustrating that invasive species are a small subset of all possible nonindigenous species.
Justification: Chapter 1 argues that, although most introduced genes will have no significant adverse environmental effects, any change in genetic composition can cause ecological impacts. (This is especially true for traits that are specifically introduced to change the ecological performance of a plant.) Because we cannot judge, ex ante, which genetic changes will have detrimental effects, it is important to take a case-by-case approach to the review of potential impacts. Most genetic changes are expected to have low risk, so they should be reviewed quickly and determined to have low risk. A smaller fraction will need closer scrutiny and can be judged to have potential for undesirable impacts. Therefore, there is a scientific basis to examining all genetically engineered crops, at least briefly, and then to examine some more carefully when concerns are raised during the quick examination.
Finding 2.10: Even if the risks of all conventionally bred crops are considered to be “acceptable,” there is still a logical scientific justification for genetically engineered crops to enter into regulatory oversight.
Justification: In the 1980s when the United States began to develop its system for regulatory oversight of genetically engineered crops, an assumption was made that, even though conventionally bred crops were not considered to be completely risk free, the risks associated with the entire class of crops could be considered “acceptable” to society (see Figure 2.2). This assumption that any trait produced in a crop by conventional means was acceptable gave a clear baseline for judging traits added by other means. Anytime it could be shown that a trait added to a crop by genetic engineering was substantially equivalent to a trait added by conventional breeding, the genetically engineered trait could be judged to have acceptable risk. Because some transgenic traits that have and could in the future be added to crops that do not have conventional equivalents, some will need to be assessed. It is not possible for a regulatory agency to judge ex ante whether a transgenic crop is similar to a conventional crop variety and therefore has acceptable risks, so some regulatory evaluation must be done. Moreover, because this assessment should be done on a case-by-case basis, all transgenic crops should be reviewed through regulatory oversight. Again, most genetic changes would be expected to have low environmental risk, so all transgenic crops should be reviewed quickly to identify the smaller fraction that will need closer scrutiny. Unlike the risk assessment called for in the argument accompanying Finding 2.9, the initial determination called for here would be an assessment of whether there is a conventional analog to the transgenic plant. Therefore, there is a logical basis to examining all genetically engineered crops
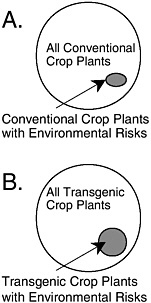
FIGURE 2.2 A. Set of all conventional crop plants and those with unacceptable environmental risk (assumed to be none). B. Similar diagram for transgenic crop plants. Arrow points to the subset of plants with risks.
to determine if they are similar to some conventionally produced variety. If they are dissimilar, a more detailed risk assessment would be called for.
The assumption that all conventional crops have acceptable risk, however, leads to a logical contradiction. If a conventional method, such as mutational breeding, had been used to develop a sulfonyl urea-resistant canola cultivar, then as long as the cultivar produced by genetic engineering could be shown to be similar to the conventional cultivar, the risks of the engineered cultivar would be considered acceptable. If, by chance, no sulfonyl urea-resistant cultivar had been produced by mutational breeding or any other conventional method, the engineered cultivar would not be considered, a priori, to have an acceptable risk but would need greater oversight. In other words, the level of oversight does not depend on the characteristics of the transgenic plant—namely, the trait, plant, and environment—but on the existence of another conventional plant with similar characteristics. This logic also holds if the conventional herbicide resistance was to an environmentally damaging compound, such as atrazine. This could lead to the ludicrous conclusion that a transgenic atrazine-resistant plant would be considered safe for the environment while a transgenic sulfonyl urea-resistant plant would need to undergo considerable regulatory scrutiny.
Finding 2.11: The risks associated with crop cultivars that have been or could be developed through conventional breeding should not be assumed to be acceptable.
Justification: Chapter 1 provides examples of conventionally bred cultivars that have had negative environmental impacts that would have been considered serious enough to question the deregulation of a genetically engineered crop or the introduction of an exotic species. There are also examples of traits such as tolerance to a particular herbicide and drought tolerance in conventional crops that would have been carefully assessed for environmental impacts had they been introduced by genetic engineering. The committee’s finding in Chapter 1 that specific traits developed by both conventional and transgenic methods could have unique risks underscores the fact that the assumption that all conventionally bred crops always have “acceptable risks” is not scientifically justified (see Figure 2.3).
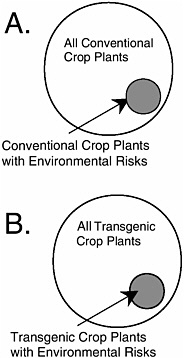
FIGURE 2.3 A. Set of all conventional crop plants with the small subset that have environmental risks, which is the actual situation. B. Similar diagram for all transgenic crop plants. Arrow points to plants with risks.
Finding 2.12: If we reject the assumption that all risks associated with conventionally bred crops are acceptable risks, there is still scientific justification for regulatory oversight of genetically engineered crops. However, substantial equivalence to conventionally bred crops cannot always be used as a reference baseline.
Justification: If we accept the fact that some conventionally bred crops can have significant risks, we cannot automatically conclude that a transgenic crop with a similar trait needs no regulation. For example, just because a sulfonyl urea-tolerant conventionally bred canola exists does not mean that the potential environmental effects of engineered canola with this trait should not be carefully reviewed.
Finding 2.13: The exclusion of all conventionally bred crops from regulatory oversight cannot be justified on a scientific basis. While it would be imprudent to immediately capture all conventionally bred crops under regulatory oversight, there is a need to reexamine the potential environmental impacts of products from traditional and emerging crop improvement technologies that fall under the general category of conventional breeding.
Justification: Because at least some crop varieties produced by conventional breeding can have substantial negative environmental impacts, it is impossible to come to a scientific conclusion that no conventionally bred crops should enter into regulatory oversight. It is nevertheless important to reexamine the safety of conventionally bred cultivars, especially because a number of novel plant-breeding techniques that fall under the broad category of conventional breeding have recently expanded our ability to transfer and accumulate new genetic traits into crop cultivars. The considerable experience and evidence associated with most conventionally produced crop varieties will probably be useful in developing a sound scientific approach to such oversight. Specifically, new conventional varieties of many of the dominant crops in the United States are quite unlikely to cause detrimental environmental effects, so the proportion of risky conventional varieties may be small (see Figure 2.3). As discussed in Chapter 1, there has been an evolution in our concepts of agriculture and the environment over the past 40 years, from a situation where the environmental effects of agriculture were largely ignored to the present recognition that what happens on the farm can affect the health of other ecological systems. In a world with this changed perspective, it is reasonable to reexamine the conventional techniques developed in previous eras. However, a change in regulations that resulted in the environmental assessment of a substantial fraction of conventionally bred crops could have negative effects on conventional plant breeding, so such oversight would need to be developed carefully.
Because transgenic crops are new genotypes introduced into the environment, they fall under the legal and regulatory authority of the Federal Plant Quarantine Act (FPQA) and the FPPA. Clearly, this authority could be extended to regulate any aspect of conventional crop variety production. Because there are no general scientific principles for identifying safe transgenic plants and there is a scientific expectation that most transgenic plants will have little environmental effect, the entire category of transgenic crops has been brought in for regulatory review under the FPQA and FPPA. As outlined above, when this is done, there should be a rapid initial evaluation to separate transgenic crops needing additional regulatory review from those that do not. At first, APHIS had no rapid sieve to separate transgenic crops. Consistent with the scientific and logical rationale discussed above, the agency constructed an early rapid sieve empirically, based on the results of its actual risk assessments. Empirical construction avoids the difficulties and pitfalls of ex ante prediction inherent in many of the scientific arguments about the safety of transgenic crops. However, if it is constructed on an overly broad generalization, the sieve can be faulty. The APHIS notification system is the early rapid sieve. While the committee agrees that the general scientific and logical principles underlying this approach are sound, Chapter 5 evaluates the implementation of these principles.
REFERENCE SCENARIOS— THE COMPARATIVE RISK APPROACH
Reference scenarios or reference values are those situations to which the focal risk is compared to contextualize understanding of the risk situation. This will depend on the risk characterization process. For example, if the context is understanding whether the transgenic crop is likely to increase or decrease risks in the area of introduction, the risks of transgenic crops could be compared to the dominant agricultural practice in the area of adoption or to the best agricultural alternative in the area. Thus, Bt cotton is compared typically to conventional cotton grown with high rates of insecticide application, while Bt corn is typically compared to conventional corn without any insecticides applied. In a broader context, transgenic crops could be compared to alternative technologies or technological systems for reaching similar production and environmental goals. For example, the risk characterization might evaluate the policy issue of which technologies or technological systems have lower environmental risks and should be encouraged for future development. Under this context, transgenic crops could be compared with sustainable agriculture and organic production methods.
The context could be considered even more widely in a developing country where a transgenic crop might replace local varieties and cause
concentration in the seed distribution system so that relatively few people control it. In some of these countries, one instructive reference scenario might be a different agricultural input that changed from being created on farms with relatively little external supplementation to being purchased from a limited number of suppliers. In some parts of the world, soil fertility had been created on farms as animal manure but is now purchased as chemical fertilizers. The kinds of hazards associated with concentration of the seed industry might be paralleled by the hazards that occurred during concentration of the fertilizer industry. A second instructive reference scenario might be a strengthened on-farm seed production system. This would address the policy concern of which systems should be supported for future development. Clearly the reference scenario determines the context for comparison. Thus, there is no one “best” reference scenario because the risk characterizations may need to meet various contexts.
Several key implications of this perspective about reference scenarios for the characterization of risks associated with transgenic crop plants are discussed in the following sections. While some of these implications are relevant to conventionally produced crop varieties, the following discussion concentrates on their application to transgenic crop varieties. The first is that for the evaluation of risks of transgenic crops several reference scenarios can be found, which imply that risk comparisons to conventional breeding are sometime irrelevant, as illustrated by some of the examples described above. Thus, general statements about the relative risk of particular transgenic crops to their conventional counterparts will not be the only basis for risk characterization. Indeed, it is the specifics of the trait, plant, and environment that have the greatest utility in risk characterization, not the specific comparison to conventional crops. The second issue relates to how to characterize the transgenic organism in a way that it can be compared to appropriate reference scenarios. No one method is used worldwide, and two different conceptual approaches are compared here. A third issue concerns a technical component of risk analysis. By introducing some of these more technical methodologies, new questions and issues can be addressed. Specifically, a fault-tree analysis could be helpful for developing a regulatory system that self-corrects itself by learning from its past activities. Finally, the need for closer, more scientifically rigorous experimentation is discussed.
Appropriate Reference Scenarios
Depending on the potential exposure pathway and the potential hazard, various reference scenarios could be chosen. For example, the potential risks from activated Cry toxin in the soil can be evaluated by compari-
sons to the effects of other synthetic pesticides in the soil, of formulated Bt insecticides, or by experimentation using traditional soil toxicology methods. Non-target risks from activated Cry toxin might be evaluated using standard species and methods for non-target testing for insecticides, but these methods may use inappropriate test species and inappropriate exposure methodologies. Typical high-dose toxicology testing is designed to make qualitative assessments of whether a compound has toxicity to an organism, and to measure small quantitative effects. As discussed more fully in Chapter 3, short-term laboratory toxicology testing may be useful in assessing acute effects of a toxin, but when a toxin has more than one mode of action, chronic effects on non-target species fitness may be missed. Furthermore, pesticide toxicology testing was developed for chemicals that had broad effects on the nervous systems of invertebrates and vertebrates. The model organism approach may have been more relevant for these chemicals than for proteinaceous toxins with specific physiological effects on a narrower range of species that are common in transgenic plants.
The potential hazard that antibiotic resistance genes could be transferred from transgenic crops (such as Bt corn with the nptII gene) to soil bacteria is unique to transgenic crops. However, even this unique hazard can be evaluated using appropriate reference models for comparison. A laboratory model optimized so that the bacteria are readily transformed by naked DNA in the culture environment provides a worst-case scenario (Nielsen et al. 2000). The rate of occurrence in the natural environment is assumed to be less than that in the permissive experimental environment. The experimental transformation rate was low, which suggests that gene transfer via transformation in natural environments is correspondingly rare. Another informative comparison is the number of nptII genes in the transgenic crop plant compared to the number in the soil. In several agricultural soils, the nptII gene is already present at 105/g soil (Smalla et al. 1993), a very high rate of occurrence. This high rate of occurrence may be characteristic of only this antibiotic resistance gene, but this comparison suggests that a transgenic crop may not be a significant source for this resistance gene in nature.
It should be clear from these examples that appropriate reference comparisons depend on the nature of the risk. Conventional plant breeding is not always an appropriate reference comparison and for the cases presented here, it was not used as an appropriate reference. Second, there are unique risks associated with certain transgenic crops. The categories that these risks correspond to, however, have been previously identified, and many methods exist to evaluate them. In this sense, transgenic crops present no new types of risks, although specific risks can be unique to specific transgenic crops.
Finding 2.14: There are typically multiple reference scenarios that are appropriate in assessing the risks of transgenic crops.
Characterizing the Transgenic Organism
There are several approaches to characterizing the transgenic organism for comparing risks. In the United States, APHIS divides the transgenic organism into two parts—the unmodified organism and the transgene and its products. The hazards associated with these parts are identified, and the likelihood that the hazard will occur is assessed using a simplified fault-tree analysis (Lewis 1980, Haimes 1998). One alternative to this two-part model is a whole-organism analysis. And in addition to the fault-tree approach, an event-tree analysis also is considered. These appear at first to be very subtle distinctions, but they lead to a different structure in the risk analysis.
Two-Part Model
In the two-part model the transgenic plant is conceptually divided into (1) the unmodified crop and (2) the transgene and its product. The risk assessment then evaluates the risk of the unmodified crop separate from the risks associated with the transgene and its products. For example, a herbicide-tolerant soybean would be evaluated as an untransformed soybean plant for which no environmental risks have been found and as the appropriate herbicide resistance gene and its transcribed product or products (see other examples in Chapter 4). The theoretical rationale for this approach is that the transgene is a small genetic change that is likely to have only a small phenotypic effect. Therefore, the untransformed soybean is unlikely to be changed very much and provides a good baseline comparison for understanding the risk of a transgenic plant. The transgene itself is considered an incremental change in the soybean, and its risks are assumed to be able to be assessed independent of the soybean.
After these assessments are completed, the whole transgenic organism is assessed to determine if there are any additional hazards that would require assessment. If so, appropriate reference comparisons are developed. Conceptually, this reductionist methodology attempts to identify and assess most of the risks in the initial step. The final step is to pick up any residual effects stemming from any interactions between the trait and the untransformed organism that require additional assessment.
The two-part model makes a procedural commitment to making the untransformed crop plant the central reference scenario for contextualizing the risks of a transgenic crop. As discussed below, to evaluate the effect of the transgene, the most scientifically revealing comparison is the
transgenic variety and its near-isogenic non-transformed parent. However, near-isogenic lines are not always available to allow the best scientific comparisons to be made. In addition, the two-part model has not used some alternative reference scenarios, such as a range of cropping systems or alternative approaches to accomplishing similar production or environmental goals as the transgenic crop. Finally, the two-part model assumes that single gene changes have small ecological effects. As discussed in Chapter 1, this is not always the case.
Whole-Organism Model
In a whole-organism model, the reference comparisons to the whole transgenic organism are developed at the beginning of the risk analysis. This opens up the scope of reference comparisons to include some that might not be considered under the first model. This model does not require that all risks are assessed using a whole organism; this is a conceptual model that provides an approach for identifying appropriate hazards and reference scenarios.
For example, under the two-part model, Bt corn was assessed by considering first the unmodified corn plant and the Cry toxin. Because corn was considered to pose insignificant environmental risks, it has been dismissed routinely as a risk. Cry toxins were evaluated for their insecticidal properties during the registration of B. thuringiensis as an insecticide, and these evaluations were used to assess the risks of the toxin. The appropriateness of this comparison can and has been challenged, in that where APHIS found no hazards in its initial assessments (see Chapter 4), EPA identified the evolution of resistance in the target pests to the toxin as a hazard. In posing the question of whether there are any additional hazards arising from the interaction of the transgene and the plant, relatively few hazards have been identified, perhaps in part because the methodology has not been firmly established. In general, as shown in Chapter 4, APHIS relies on an analysis that is based on comparing the transgenic crop to a conventional crop with a similar phenotype. If a similar phenotype can be identified, APHIS contends that because the conventional crop phenotype has a record of safe use, the transgenic crop plant is expected to be safe too. In the case of Bt corn, APHIS compared the transgenic crop to other insect-resistant corn varieties because no conventional Bt variety exists. While the validity of such comparisons can be challenged (see Chapter 5), the method is consistent with the two-part model. A whole-organism model would initially focus on identifying the potential hazards associated with the transgenic crop variety. The appropriate reference scenario for hazard identification may include the untransformed crop and conventionally produced crops with comparable
phenotypes, which are used in the two-part model, but risk comparisons are not restricted to these two scenarios. For example, the transgenic crop will be expected to replace other agricultural production systems and possibly some nonagricultural native or natural habitat, and it may affect neighboring agricultural and nonagricultural habitats. Hence, the risks associated with a Bt corn might be compared to corn sprayed with insecticides in the irrigated regions of northeast Nebraska because it might be expected to replace this production system at that locality. In Minnesota, Iowa, Illinois, and the rest of Nebraska, Bt corn might be logically compared to an unsprayed conventional crop. In a similar way, Bt poplar might be compared to conventional poplar plantations sprayed or not sprayed with insecticide, but because it might lead to an increase in area planted to plantation poplar, it could also be compared to the risks associated with second-growth forest, which it might replace. In addition, because Bt corn might affect neighboring organic farmers through contamination via cross-pollination, and Bt poplar might affect the ecological functioning of neighboring forest and savanna ecosystems, these ecosystems might also be appropriate reference scenarios. The theoretical rationale supporting the whole-organism model is that the risks and hazards associated with a transgenic crop plant occur as a result of the trait in the crop plant in a particular environment. The risks and hazards do not occur from the trait separated from the crop plant separated from the environment.
Comparison of the Two-Part and Whole-Organism Models
In principle, the two approaches should end up with the same characterizations of risk, but in practice they probably will not. The two-part model addresses whole-plant concerns in its second step and should therefore capture all hazards and risks at this stage. The methodology used in the second step of the two-part model, however, must be different from the whole-organism model because if it were the same, the first step would not be necessary. It is in this difference that the models probably diverge. There are potential biases in the two-part model, which may underestimate or overestimate risk. The initial step of dividing the organism and evaluating the risk of the parts may lead to an underestimation of risk when the parts are found to have no significant risk and, vice versa, may lead to an overestimation of risk when the parts are considered risky. This is because these initial findings are likely to influence subsequent investigations used in the second step. If no significant hazards or risks are characterized in the initial step, it may be difficult operationally to justify and sustain a lengthy second step risk analysis. Conversely, an initial finding of risk may unleash a cascade of additional investigations, perhaps leading to an overestimation of risk.
Risk Assessment Models
Risks can be assessed in numerous ways, with various assumptions and biases. Transgenic crops have been assessed in the United States by APHIS utilizing a rigid risk analysis framework that lacks a formal approach for detecting potential for mistakes. Certainly the system is designed to limit mistakes, but mistakes do occur, and their significance is unknown. Two different analytical frameworks are discussed below that could be used to supplement present risk assessment practices—a fault-tree analysis and an event-tree analysis (Lewis 1980, Haimes 1998). Fault-tree analysis can be used to investigate potential risk. For example, one can hypothesize that Bt corn adversely affects some non-target organism. A fault tree could lead the analyst to investigate thoroughly all potential causal pathways.
Fault-Tree Analysis
Fault-tree analysis logically evaluates risk by tracing backwards in time or backwards through a suspected causal chain the many different ways that a particular risk could happen (Lewis 1980, Haimes 1998). The analysis is conditioned on a given hazard, that is, the analyst must have a particular hazard in mind before the analysis can be conducted. This is both its strength and its weakness. It is a strength because the analysis focuses on the ways that risks occur and does not waste time evaluating the ways that risks do not occur. By concentrating on the known hazards, the analysis provides a comprehensive and efficient methodology for assessing risk. It is a weakness because unknown or unanticipated hazards cannot be evaluated simply because they have not been identified. Because the hazards associated with complex systems cannot all be unambiguously identified ex ante, this is its most serious weakness.
Figure 2.4 is a simplified fictitious example of a fault-tree analysis of the risk of Bt corn to a non-target insect species. The total risk is a weighted “or” gate of the risks in cornfields, near cornfields, and far from cornfields, where the weight is the relative proportion of the insect population in each habitat area (in the example in Figure 2.4 these weights are equal). In an “or” gate the risk would occur if any one of the inputs occurs, and in an “and” gate the risk would occur only if all of the inputs occur. This risk calculation is detailed for risks to the species inside cornfields, which is an “or” gate of the risks associated with each of the three most common transformation events in Bt corn. This risk, in turn, is an “and” gate of the probability that monarchs are in Bt corn of the specific event and the probability that they will be killed by pollen from the event. This box is further detailed for Event 176, in which mortality is related via an “and”
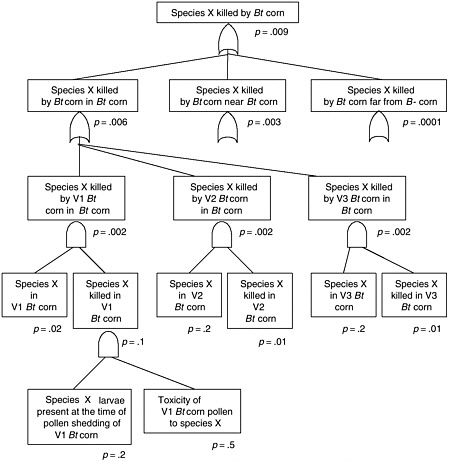
FIGURE 2.4 Simplified fictitious fault-tree analysis of the risk that individuals of species X are killed by the sum of three varieties of Bt corn (V1,V2,V3). This fault-tree traces backwards through the causal chain. In this example the logical structure of the model is Boolean algebra, which consists of “gates” that indicate how the faults lower in the tree should be combined to estimate the probability of occurrence of the higher levels in the tree. The upper levels of the tree consist of “or” gates (the pointed arrowheads). An “or” gate signifies that if any of the input statements are true, the output statement is true and therefore the inverse of the product of the inverse of the input probabilities can be used to compute the output probability, assuming the input statements are independent. The lowest level consists of “and” gates (the half-rounded symbols). An “and” gate signifies that all input statements must be true before the output statement is true, and therefore the product of the input probabilities can be used to compute the output probability. p values in the figure are entirely fictitious and are provided for illustrative purposes.
gate to the probability that the species is exposed to the Bt pollen and the toxicity of Event 176 pollen.
Fault-tree analysis is often used to assess the risk involved in potential failures of the safety system itself. This tradition is well developed in the aeronautics industry, where airplane safety and safety systems are subjected to rigorous fault-tree analyses (Lloyd and Tye 1982). A simplified fault-tree model for the safety system associated with APHIS regulation of transgenic crop plants is illustrated in Figure 2.5. This emphasizes one potential adverse effect that could occur through failure in the present oversight system. Although conducting such an analysis would require too much effort for this committee to complete, the elements of such an analysis are discussed in Chapter 5. Clearly, conducting such an analysis
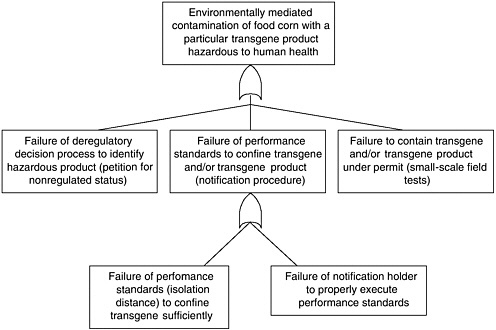
FIGURE 2.5 Simplified fault-tree model of the risk that a commercialized hazardous transgene product enters the human food chain via the environment. The logic is Boolean logic as in Figure 2.4, except that in this tree all the gates are “or” gates. This fault tree focuses on the possibility that the risk occurs because of failures in the safety network. Most industries with safety systems to manage risks analyze the potential failure of the safety system itself. This kind of analysis has led to the development and implementation of “fail-safe” systems, such as in the aeronautics industry.
would provide a regulatory agency with considerable information to improve its regulatory procedures and could contribute to a more formal methodology for a learning system in an agency. The cost of such an analysis is unknown.
Event-Tree Analysis
In contrast to fault-tree analysis, event-tree analysis logically works forward in time or forward through a causal chain to model risk (Lewis 1980, Haimes 1998). Event-tree analysis is not conditioned on the existence of a known hazard. Starting with an initiating event, the next steps in a logical chain of events can be assessed until the risk probability associated with a hazard can be calculated from the probabilities associated with the chain of events. Interestingly, event-tree analysis does not have to be aimed at estimating the risk associated with a particular hazard. Event-tree analysis can be initiated to investigate an entire category of hazards, during which the risk associated with particular hazards is assessed. If the processes in a complex system are understood well enough, event-tree analysis can identify hazards by following the complex sequence of events to their logical conclusions.
For example, an event chain analysis of the potential non-target risks associated with a transgenic crop producing a toxin could begin quite generally, because we know the toxin that would cause any of the nontarget risks that could occur (see Figure 2.6). Consequently, an event-tree analysis of non-target effects can be initiated through a toxin fate and transport analysis, tracking where and when a toxin goes until it is degraded into nontoxic forms. If there is enough information, such a fate and transport analysis could reveal when apparently independent pathways converge to allow toxin to concentrate in some part of the ecosystem.
For Bt corn, the first step in the event-tree analysis requires understanding what toxin is expressed in the plant, where and when it is expressed, and how that toxin might reach the environment. All Bt corn varieties produced a truncated Cry toxin, which is significantly smaller than the protoxin produced by the bacterium. It is hypothesized that truncation may alter the host specificity spectrum, creating novel nontarget risks dissimilar from Bt insecticides (Jepson et al. 1994, Hilbeck 2001, but see MacIntosh et al. 1990). The toxin is expressed in all of the plant tissues when the plant is actively growing, with variation among the transformation events. The toxin moves in the pollen and remains in the dead plant tissue after senescence. In Bt corn, truncated Cry toxin can enter the soil via root exudates (Saxena et al. 1999), exposing soil organisms. The activated toxin readily binds to montmorillic clay particles and
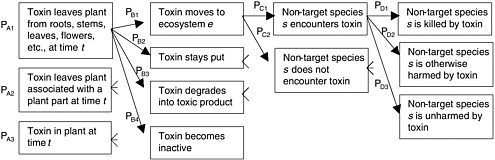
FIGURE 2.6 Simplified event-tree analysis of the non-target risk of a toxin produced in a transgenic plant. Event-tree logic is based on a chain of mutually exclusive events. An event tree begins with an initiating event and follows the consequences of the event through a chain of subsequent events, assigning to each branch a probability of occurrence. The end result is a long list of the possible consequences of the initiating event in which each consequence has associated a certain probability of occurrence. The sum of the probabilities at a level of the tree, SiPWi = 1 (here W = A, B, C, or D), that is, PA1 + PA2 + PA3 = 1. The branches for many of the lower boxes are incomplete. Non-target species s could be affected through multiple causal chains, so the overall effect of the toxin on species s may be a complicated aggregation of these effects. This event-tree analysis also is initiated by a general event that would be common to many non-target hazards. The specific hazards to particular species are evaluated later in the analysis.
can retain insecticidal activity for more than nine months (Saxena et al. 2000). The untruncated protoxin in Bt insecticides does not readily bind to clay and is degraded rapidly in soil (Venkateswerlu and Stotzky 1992). Consequently, Cry toxin from Bt corn has several unique exposure pathways not characteristic of either corn or the toxin from bacteria. Each of the potential pathways can then be assessed to determine if there is a potential hazard associated with them; if so, the risk can be characterized by choosing an appropriate reference comparison for that exposure pathway and potential hazard. Event-tree analysis may become particularly useful when risks associated with transgenic crops with many traits are characterized.
Finding 2.15: The two-part model may bias a risk assessment either toward a finding of no significant risk or a finding of significant risk. As presently implemented, the former bias may predominate.
Finding 2.16. Additional research is needed to evaluate the utility of the whole-organism model and a more formal use of fault-tree and event-tree analysis.
Experimental Comparisons
Plant breeders and geneticists make a variety of comparisons using appropriate statistical designs and analyses. New varieties of crops are not generally released for large-scale use until a variety review committee reviews the data. In the case of transgenics a single gene is introduced into cells growing in tissue culture, and the regenerated plant displaying the trait is backcrossed several generations to an elite line. A reasonable genetic comparison for risk assessment is the untransformed elite line. For example, six backcrosses should produce progeny that are genetically over 99% identical to the elite line used as the recurrent parent. This value of 99%, however, is an average or expectation and is not always reached.
The ideal comparison of a transgenic following backcrossing is to its near-isogenic line derived by self-pollination of plants heterozygous (hemizygous) for the transgene in the most advanced backcross generation. This will give plants homozygous for the transgene as well as plants without the transgene; all unlinked regions of the genome will segregate independently of the transgene locus and should not influence comparison of the two homozygous lines derived from the homozygous plants. This comparison is not absolutely perfect because the transgene chromosome likely will carry a genomic region on each side of the transgene from the transgenic parent. However, this comparison is theoretically the best available.
If near isogenic lines are not available the only possible comparisons are among unrelated plant lines. In such comparisons it will always be difficult or impossible to determine if an observed difference between the plant lines was caused by the insertion of the transgene or was simply due to another genetic difference between the two plant lines. Conversely, if no difference is observed, it will always be difficult, or impossible to determine if the transgene had no effect or if other genetic differences masked the effect of the transgene.
The committee recommends that near-isogenic lines with and without the transgene be developed and evaluated as part of the process of risk analysis. In addition, a way should be found to make such near-isogenic lines available for study by public scientists. The availability of such genetic materials would substantially strengthen comparisons, save time and money, and provide much more legitimate comparisons. The potential to mislead the public—positively or negatively—is too great unless the best-available genetic materials are used. Clonally propagated crops can be readily compared to transgenic versions. Self-pollinated crops raise most of the same concerns expressed above for cases where backcrossing is involved. If backcrossing is not the breeding scheme, the process of advancing the materials must be considered to determine the most appropriate comparisons.
Finding 2.17: For certain questions, the availability of near-isogenic lines can allow scientifically legitimate comparisons and facilitate the assessment of risk.
CONCLUSION
This chapter has reviewed general approaches and roles of risk analysis, the scientific, social, and logical bases for regulation of transgenic plants, and some technical issues related to the risk assessment process itself. It explains that risk analysis typically must fill two roles: providing technical information to decision makers, and creating confidence among stakeholders in the risk analysis process. The specific importance of the second role in the risk analysis of transgenic plants is discussed. The use of the most rigorous scientific approaches in conducting risk analyses is critical for both roles of risk analysis.
Because there is no way to evaluate the risks of transgenic plants without empirical examination, there is a scientific reason to examine the characteristics of transgenic plants on a case-by-case basis. Although there has been a general assumption that the products of conventional plant breeding are safe, while products of transgenic processes could pose envi-
ronmental risks, this chapter and others in the report find no logical or scientific support for this assumption.
Environmental assessment of transgenic crops relies on risks associated with the transgenic variety being compared with multiple appropriate reference scenarios (ways to accomplish the same goal). For example, the risks associated with a transgenic herbicide-tolerant variety could be compared with risks of chemical weed management used on nontransgenic varieties, cultivation practices, crop rotations, and other approaches for weed management. This chapter ends by pointing out that there are a number of general approaches for formally examining a risk analysis to determine where errors could occur. These formal examinations could be helpful in assuring the rigor of the current system used for transgenic plants.

















































