Advances in Materials Research and Development
WILLIAM O.BAKER
The unprecedented progress of science and engineering in the second half of the twentieth century has advanced many worthy goals—beginning with the defense of freedom—and has fostered understanding of the world, its inhabitants, and the cosmos. In this context, materials research and development stands out—both as a twentieth-century phenomenon and as an endeavor that bridges the often-disparate objectives of understanding nature and of ensuring freedom.
This chapter describes, exemplifies, and analyzes various facets of the complex history and development of materials science and engineering both as a field of endeavor and as a national commitment that came into being in the late 1950s through the National Materials Program. Through this program, knowledge of matter has been augmented by new academic structures and by new connections of government and universities with industry and the national economy. A dominant theme throughout the chapter is the need for this nation to learn from and to apply the experiences of the national materials endeavor in addressing major scientific and technological issues in the coming decades.
The inception of the National Materials Program illustrates one of the first, and best, examples of leadership by a chief of state in twentieth-century science and engineering; it was generated by President Eisenhower through the White House Office of Science and Technology and Science Advisory Committee. And, of course, the enterprise has even deeper roots: the great science faculties of Britain and Germany began to see, from classical physics and chemistry, from the optics of Newton, the ionics of Faraday, the radiation of Röntgen, the atom of Thompson, Aston, and Bohr—and through the
statistics and quantum mechanics in large assemblies of atoms and molecules—that solids and even liquids displayed some simplicities.
Still other origins of the materials science and engineering enterprise lay in the ways in which some industrial laboratories and government centers, such as those developing atomic energy, had been able to purify and modify crystals and glasses to achieve new public and commercial capabilities. (These developments range from isotope matrices for nuclear energy to transistors and solar cells, to space vehicles and their reentry nose cones and capsules, to synthetic polymers like polyethylene—which could be adapted to displace metals for sheathing cables—to nylon and its fiber correlates. Other derivatives of advances in materials science and engineering include modern telecommunications and computers.) The circumstances of the 1940s and 1950s also led to a less tangible development—a bold surmise from the White House Science Office, in its earliest days, that some fields in twentieth-century science and technology should promise enough scientific lure and luster to stimulate the keenest minds and to motivate the most creative thinkers; at the same time, knowledge achieved in such fields would be immediately useful for technical, public purposes and commercial needs.
In the late 1950s there was a very wide gulf between academic science and mathematics, as engendered by the great European traditions, and the engineering and technology of an inventive industrial era. This gap narrowed somewhat in a few urgent wartime projects such as the synthetic rubber program, which also supported the emerging proposition about coupling modern science and technology. Materials science and engineering could indeed bring together fundamental “knowledge for its own sake” and the compelling, restless demands of technology and manufacture. For we found with GR-S rubber, in the production of more than 700,000 tons of a new material within two years, that the sometime “odd couple” could be complementary and even reinforcing.
But no one had said yet that it should be possible for an independent, pluralistic government of a free nation to so modify tradition that it could stimulate industrial and academic materials research and development by request of the national leadership. With helpful but modest use of federal funds, it could only be hoped that a new chapter in the creation and use of new knowledge would be written. And yet that is what happened. The 1974 report of the National Academy of Sciences Committee on the Survey of Materials Science and Engineering (COSMAT) showed the profound impact on U.S. and even world resources that the National Materials Program has had.1 Later studies, such as the analysis by Theodore H.Geballe on behalf of the National Science Foundation, and various related estimates of the intellectual appeal of condensed-matter science, have further established the existence of a worldwide conviction that new fundamentals of nature can now be discerned in condensed matter. Likewise we are seeing that the
engineering and manufacture of commercial products as well as the systems for national security and public service are gaining crucially from basic findings about matter and its synthesis. The dreams of President Eisenhower’s time are coming true.
And today, when we have returned to old arguments (for example, whether there should be a single department of science in Washington) and new challenges, when as a debtor nation we must compete vastly better against the products and innovations of a smartening world, it is wise to review our progress in materials science and engineering. We need to assess the national materials endeavor, and to reconsider its origins as a reference for future decisions. For in this and other fields, we should confer on what we ought to take care about as we face new and unexpected conditions on the planet. Accordingly, the experience with the National Materials Program continues to yield insights applicable to decisions about where we could and should go in the coming decades of pluralistic academic, governmental, and industrial science and technology. Noteworthy qualities of this program can be discerned in its beginnings, in its progress, and in the assumptions and premises underlying it.
ORIGINS OF NATIONWIDE SOLID-STATE SCIENCE AND ENGINEERING
The impetus for developing a national program followed by some years the origins of materials research and development that took place in independent laboratories. The impetus for the program was found in a national opportunity for federal agencies concerned with weapons systems, rocket propulsion, nuclear reactors, spaceflight and reentry, as well as materials conservation and supply. Also, there had arisen postwar needs for a common base of research and development that could accommodate the needs of civilian services and industrial activity (through the National Bureau of Standards) and those of education and basic science (through the National Science Foundation).
Conviction of the values of a national program in materials science and engineering had already come from the surging growth in solid-state science and technology, largely in industrial laboratories from which came the transistor, the solar cell, new polymers, high-performance metals and alloys, and the rudimentary composites. This conviction intensified with the stirrings of interest in the far horizons of understanding condensed matter, for example, through the work of Eugene Wigner, Frederick Seitz, and William Conyers Herring in physics, and of C.P.Smyth in chemistry, at Princeton. Usable theory was forthcoming from Seitz’s continued research at Carnegie Mellon and Illinois, from the wartime-stimulated interests and expert pedagogy of J.C.Slater at MIT, and from J.H.Van Vleck at Harvard.
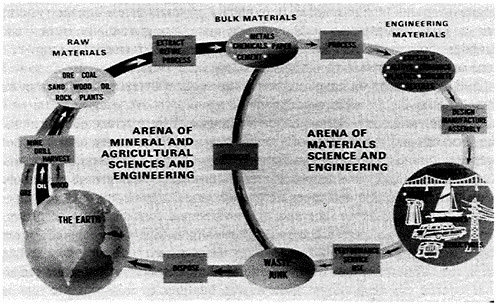
FIGURE 1 Schematic of relations of materials science and engineering to global resources and uses of matter. From the National Academy of Sciences.1
Solid-state work was not devoted to a single objective or goal, although, like the Manhattan Project, GR-S Program, Apollo Program, or National Cancer Plan, it assumed that science and engineering could work together in unprecedented intimacy. But further, science and engineering would support each other, as described in the COSMAT study (Figures 1 and 2).
When the time for federal focus came, the procedures of the National Materials Program (represented especially but not by any means exclusively in the university Materials Research Laboratories [see Schwartz, in this volume]), consisted of institutional unions of unprecedented scope. Yet those program efforts are the basis for the process that we would like to see extended widely in the times ahead. They have united academic and industrial scientists and engineers in joint actions, as foreseen in the Synthetic Rubber Program. They have united a community of users and makers of materials in industrial factories, government contract agencies (especially the Department of Defense), and the whole range of American industry from mining to molding. The program has united teaching and research in extraordinary ways through the interdisciplinary character of the effort. (The uniting of physics, chemistry, mathematics, mechanics, and other engineering fields in novel forms is a task yet uncompleted.) The materials work has united components and materials engineers with systems and electrical and mechanical engineers in unique ways. Here we have only to recall that materials science and engineering, whose early expression in the transistor and the solar cell would soon be succeeded by satellites, new intercontinental cable systems, and
aircraft designs involving novel metals and alloys, now also supports super composites and digital computers and switches. Materials science and engineering provide light guides and composites for prosthetic parts in the human body, and ceramics for gas turbines. Thus, the materials era has dramatically advanced both design and performance engineering in a union not imaginable until only a decade or two ago.
I submit that materials science and engineering are already historic for these new combinations, which is the most important validation of that early surmise of the White House Science Office—that such unions, together with the nature of learning, would bring about an unprecedented speed of conversion of scientific discovery into technologic innovation and commercial and public production. This, too, has happened in electronics, now photonics, in rocketry, and in major refractory structures made of composites. We hope that it will extend into many other competitive and economically decisive domains, including automobiles, buildings, and public facilities.
I believe this strategy is the prescription this country needs for regaining primacy in international trade, in technology, in armaments, in learning, and in quality of living, because it contains sound guidance for the role of science and engineering in those larger and compelling national issues. At this time
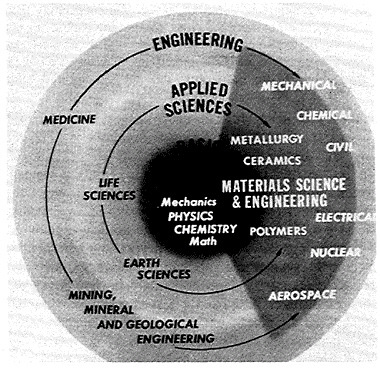
FIGURE 2 Interconnections of physical and life sciences related to engineering, showing the major role of solid matter.
in the advance of science and technology we must find larger values, which involve new ways of thinking and of working and of combining our institutions. That is exactly what we have been fortunate enough, in the era of solid-state science—of materials science and engineering—to do and to assess.
ISSUES EVOLVING FROM ENLARGED MATERIALS TECHNOLOGY
Derivatives of the research and engineering concept embodied in the Interdisciplinary Laboratories (IDL)/Materials Research Laboratories (MRL) program continue to be instructive. Thus, George A.Keyworth II, President Reagan’s Science Advisor and director of the Office of Science and Technology Policy, refers to the Engineering Research Centers organized by the National Science Foundation as “the single most important thing that we’ve done as an Administration in increasing the efficiency and effectiveness of federal R&D dollars.”2 He said the centers address a widely recognized need in various fields of science and technology:
Continued pushing of the frontiers in those fields was constrained by the difficulty of assembling multidisciplinary teams to work on the problems. Our universities are, justifiably and understandably, structured to pursue disciplinary research. On the other hand, we increasingly find ourselves as a nation confronting the solving of problems that have technically based solutions. We need to expose our young people to a problem-solving environment…. These Centers—I’d rather call them Science and Technology Centers—are multidisciplinary mechanisms by which chemists, physicists, neurobiologists, engineers, etc., can get together and solve exciting, intellectually demanding, real-world problems.2
These and other comments about the economic potential of the Engineering Research Centers are almost word-for-word descriptions of the original interdisciplinary laboratories of the National Materials Program. Thus, it is refreshing indeed to find such current agreement on the concept that has involved so significant a portion of our best academic talent.
The national commitment set in motion by President Eisenhower’s call has other facets besides historic industrial and academic gains in materials science and engineering, both within and beyond the federal program. For instance, in the conservation and supplying of strategic materials, we have learned that national security depends heavily on integrated provision of processing and products coming from a host of minerals and related natural sources. We now have synthetic analogs for natural materials ranging from natural rubber to diamonds and platinum. This by no means suggests that we have efficient substitutes for all materials crucial to defense and a viable civilian economy. Rather, we have learned the degrees of alternatives that can be used and how to balance designs, processes, and basic materials
properties with skills whose acquisition alone would justify the modest federal investments in all of our materials research and development.
Indeed, there are many dimensions to such a context. On the one hand, we respect the frustration of Richard A.Reynolds, new director of the Defense Sciences Office of the Defense Advanced Research Projects Agency (DARPA), regarding materials research priorities. Dr. Reynolds notes sparse funding of federal research on “growth of critical electronic materials,” and says, “Furthermore, the United States is losing its international competitive edge with respect to the technology base necessary to support materials self-sufficiency in the manufacture of these man-made strategic materials.”3 In that case, his concerns could have extended beyond semiconductors and integrated-circuit ceramic substrates and the like. He has accurately perceived that the pervasiveness of materials science and engineering in our national and industrial programs does diffuse responsibility, as expected. And in this sense, the new academic centers for study of high-speed integrated circuits, quantum-well semiconductor systems discovered in industrial laboratories, and novel compositions of organic and ionic qualities have shown the need for major new characterization facilities such as synchrotron and neutron radiation sources. Hence others, mostly in industry, must take responsibility for the materials development and leadership that he is properly calling for.
But from another perspective, a recent book, entitled Lost at the Frontier,4 presents one of the most stimulating and critical of many current commentaries about American science and engineering research and development. The senior author is a distinguished materials scientist and engineer, Rustum Roy. The book should remind us that the original precepts of the National Materials Program recognized the need to maintain progress in established fields, such as integrated circuits, ceramics, and other materials identified as priorities by Dr. Reynolds and DARPA. But these original precepts also heeded the larger issues raised in the critique by Shapley and Roy. These authors recognize astutely that the scientific and technical community also has wide responsibility to act with a degree of professionalism and intellectual performance concordant with the high calling from a chief of state, or from a supportive nation.
While messages such as those of Dr. Reynolds need to be considered— and we should note that many of his materials colleagues throughout the Department of Defense are also saying that more ought to be done—the issue is, What more should be done? What more must be done to enable materials science and engineering to fulfill new roles in contributing to the social and economic welfare of the nation? For this is what was intended in the White House initiatives. The National Materials Program was designed for the performance of science and technology in new ways. Industrial ingenuity, time-honored disciplines of academic discovery, the urgent technical requirements for national security, and the overriding need for human talents
would be combined to meet the needs and potentialities of the twentieth century. The drafters of this program for the president in 1958 already knew that such expectations were justified and attainable. The practical origins of the solid-state era had already produced major gains from mutual feedback of technology and science in crystal growth, purification, semiconductor doping and synthesis, and adaptation of polymers as new structural and insulating materials.
For example, the development of high-frequency electronics for radar and microwaves had stimulated extensive and critical use of silicon and germanium point-contact diodes; the work of Scaff and his associates5 and other studies at Purdue University and elsewhere had produced relatively satisfactory materials whose purity and quality could have been further refined by continued detailed pursuit of recognized technology. But the goal of 1015 or fewer foreign atoms per cubic centimeter in a single crystal was so far outside of that conventional pathway that it was spoken of only with a mixture of awe and humor. However, William G.Pfann, then a technologist involved in learning science, sensed that the phase rule worked in all directions. Through his zone-refining method, he achieved both the purity and perfection needed in semiconductor materials, thus opening simultaneously for academic and industrial application an epoch of purity and regularity in matter not two or three times but orders of magnitude greater than had been available. Similarly, investigators at the time saw in the chemical and petrochemical laboratories at Du Pont, Phillips Petroleum, and Union Carbide and in the academic work of Ziegler and co-workers and of Natta and Pasquon,6 A.Morton at MIT, M.Morton at Akron, and others, that when appropriate characterization showed that the qualities required for polymers could be achieved in theory, chemical control could achieve those qualities in practice. For instance, by this pathway polyethylene could replace lead, which is both costly and ecologically sensitive, in the cables supplying electricity and communications around the world.
It should be recalled that these and a few other projects were also subjects of intense technical development in earlier decades. The new industrial tactic, which caused the change, was to combine science and engineering—to promote the interdisciplinary interactions of mechanics and chemistry, of physics and metallurgy. Pfann’s work reduced the characteristic content of dislocations in metallic and semimetallic crystal surfaces from about 3.5 million per square centimeter to near zero. And on the way, the mechanical trauma of even 1,000 dislocations per square centimeter could be tracked. Even with the historic purity of less than 1017 carbon atoms per cubic centimeter of silicon, the solid showed more than five times the stress at yield that a typical “pure” specimen would show.
These and many other signals made clear that mobilization of a new national materials effort would affect vast technical capabilities. But even in
the strength of matter, dominated by dislocation movements, challenge still lies ahead. A current study at the Battelle Memorial Institute finds that the fracture of matter and efforts to contain it now cost the United States no less than $119 billion per year. Even the appropriate basic categorizations of overload, brittle fracture, ductile rupture, fatigue, creep, creep rupture, stress corrosion, threading fatigue, thermal shock, buckling, and delamination require more specific scientific description than has yet been applied. The move toward automated manufacture and robotics processing of materials even accents the ignorance of these factors. As noted below, the appropriate control of dislocations may even provide new networks of conductivity and electronic and photonic responses in suitable crystals.
RECOGNITION OF NEW FRONTIERS
Along with these signals of need, there are signals of knowledge, perhaps as beckoning and as rich with meaning as those once heralding the solid-state and materials endeavors themselves. In polymers, we have long known, and technically and scientifically applied, the close coexistence of ordered and disordered phases. Indeed, a single chain can indulge in both, and many do. Whole classes of important materials, such as the Arnel fiber of Celanese Corporation, came from appropriate adjustment of lateral and longitudinal states of order, in that case, in cellulose triacetate. Annealing and heat treatment affecting such order are crucial factors in the performance of nearly all microcrystalline synthetic fibers, plastics, and films.
However, we have been rightly charmed by the beauty and utility of traditional crystallography. Only recently, computer-assisted study of aluminum alloyed with minor components of manganese, iron, and chromium has shown an icosahedral structure imputing 5-fold symmetry. This, of course, displaces atom groupings from the expected unit-cell behavior. On 29 July 1984, Japanese workers reported in the Physical Review Letters about a nickel-chromium alloy that in electron diffraction by small particles seems to exhibit a 12-fold symmetry. This they interpreted as a dodecagon, which indeed would conform to an intermediate structure between the disorder of glass and the regularity of a crystal. Reexamination of what were termed anomalous diffraction patterns of an aluminum-manganese-silicon alloy from AT&T Bell Laboratories offers a complementary example, where a unit cell would require thousands of atoms, but currently can best be interpreted as icosahedral arrays within such “unit cell.” In India similar icosahedra seem to have been formed in magnesium-zinc-aluminum alloys by rapid cooling. There is also the report of a sheet structure with 10-fold symmetry within the sheet, but a periodic stacking of the sheets themselves.
The point is that conventional structure practices are not really sophisticated enough to deal with the growing diversity of materials science and engi-
neering. Fortunately, this is being heeded by theorists such as P.J.Steinhardt and D.Levine at the University of Pennsylvania (the site of one of the three original Materials Research Laboratories). They have concentrated on the properties of a quasi-periodic translational order, with various degrees of orientational symmetry, leading to a total quasi-crystalline form such as octagonal orientation symmetry, in one of their recent models. At the National Bureau of Standards, Daniel S.Schechtman and his associates have studied an alloy of aluminum and manganese that seems to show some of this structure. Further significant examples of important new directions of study in materials science and engineering, which also reflect the initial concepts of the program, occur where bioscience intersects the study of condensed matter.
The National Materials Program in both its federal and independent forms is probably the only example of a major scientific frontier in which the initiative for study came from technologic and engineering efforts outside of academic centers. Yet the academic centers not only have provided the basic skills and training for the nonacademic work but also continue to organize knowledge and its validation in ways that will prepare for further discovery. The solid-state era in all its semiconducting, magnetic, and superconducting manifestations (the laser, light guides, extraordinary spectroscopy, and photonic circuitry) and polymer plastics, fibers, rubbers, and their growing interplay with the condensed biosystems in living tissue came from practical, usually commercially induced, although sometimes government-stimulated, ventures in their materials aspects. These materials factors are virtually central to the utility of the technical systems in modern life. Indeed, in the case of polymers and their products, they are the system.
The ventures that led to the solid-state era established a new degree of interaction between academic research and learning and certain industrial laboratories seeking commercially to extend human capabilities. This interaction is aided by public policy reflecting a willingness to involve a wide spectrum of independent citizens directly in government. It brings an originality and freshness of organization unknown in bureaucratic rigidities of nondemocratic or more traditional governing mechanisms.
Today, these interactions can go even further than before, as long as we pick the appropriate mutual goals. There are messages in the findings of H.Bock and R.Dammel at the University of Frankfort in work on pyrolysis of triazidosilane to get C6H5NSi (phenylsil isocyanide). J.Michl and G.Gross at Utah confirm and in fact have isolated the linear CNSi group. A familiar but significant footnote is attributed to Michl: “On warming, the product forms an insoluble polymer.” Such comments about organic residues have marked the progress of macromolecular science and biotechnology!
ARRANGEMENTS FOR ONGOING ADVANCES
The special role of the National Materials Program, as represented in the Materials Research Laboratories (MRLs), provides dramatic evidence that there is operational and intellectual unity in materials science and engineering. This appears in the combination of science and engineering, in the combination of research and development, and in the combination of disciplines that are academically and professionally applied. As Schwartz (in this volume) shows, the MRLs themselves have notably enhanced, as well as embodied, this unity.
Beyond this, the national and international significance of advances in materials science and engineering is vast. The present microcosmos of MRLs, with about 400 faculty members remaining from a peak of 600 at the end of the ARPA era in 1971, is an important element in the macrocosmos of modern materials science and engineering. The extraordinary feature is how the purposes, goals, and inspiration of the national program of MRLs have been assimilated by, and reflected in, the large national endeavors.
Indeed, an epochal quality of the total system should be realized. Namely, our planet is mostly silicon, oxygen, and water, although silicates have a marvelous diversity of form. Obviously, materials science and engineering have exploited aspects of this diversity from the Stone Age to the present. But only in this half century have advances at the forefront of physical science and on the frontiers of technology and engineering been combined to bring out the qualities of silicon and its oxides that best serve human wants. These advances are familiar to us through such products as transistors, diodes, solar cells, computers, and all their derivatives. The advances are apparent in important synthetic polymers such as silicones as well as in the mortar and the substance of our buildings, cities, and roads. Moreover, we are seeing how science and engineering can reach far beyond the qualities of basic resources to achieve orders-of-magnitude advances in performance of the stuff of clay and continents.
The purification of silicon by means of Theurer’s chemistry7 and Pfann’s zone refining have resulted in crucial derivatives, such as the production of silicon tetrachloride with less than 10 parts per million (ppm) of trichlorosilane and less than 5 parts per billion (ppb) of iron. Chemical conversion followed by oxidation within modified chemical vapor deposition is now used routinely to produce fibers of silicon containing less than 2 ppb of cobalt ions, less than 20 ppb of iron, less than 30 ppb of copper, and so on. The process is particularly effective also for epitaxial production of silicon films. Thus, the combination of modern chemical analysis, engineering processing, and the physics of characterizing matter has achieved in the oxide of silicon, a cosmic medium of the planet mixed with every other component of the stars, an unsurpassed purity.
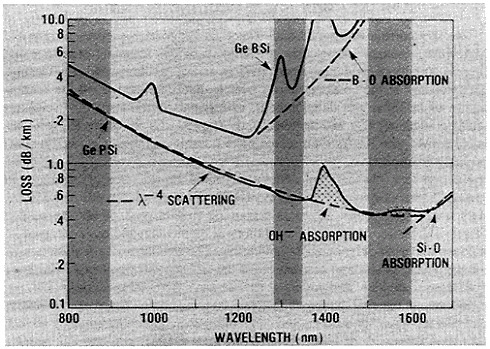
FIGURE 3 Loss of light transmission in decibels per kilometer of various factors in graded-index silica fibers, as a function of wavelength in nanometers. Hyperpure fibers have even half or a quarter of the absorption depicted.
In this pure state, silica transmits 1.2- to 1.6-micron-wavelength photons so well that there is an attenuation of less than 0.15 decibel per kilometer. However, silica-fiber light guides must stay pure, stable, and without significant hydration, since hydroxyl groups degrade their light-carrying ability (Figure 3). Therefore, through still other techniques of materials science and engineering, a composite is formed by deposition of selected and exquisitely controlled polymer films on the silica fiber as it is drawn. The resulting fibers, properly extruded and coated, have strengths far beyond the best fiberglass—a tenacity approaching 0.8 to 1 million pounds per square inch (psi) (Figure 4). Although still short of the 10.5 giganewtons per square meter (1.5 million psi) in a single, idealized silica solid, the tensile strength of modern optical fibers nevertheless approaches the ideal envisioned by Games Slater, the inventor of fiberglass. It is hardly surprising that the skills that make composites of silica with micron dimensions and overlayers of polymers lead one to composites in bulk. These materials are finding new uses in rockets, motor vehicles, boats, and houses.
But then, if our themes hold, the combinations of science and engineering intrinsic to materials programs and cultures should induce still other earth-
matter advances. Carbon is ubiquitous, not just as 1016 atoms per cubic centimeter in hyperpure silicon, but as a chemical chameleon of the earth’s materials. In various forms, carbon provides the major substance of life. In trees and plants its cellulosic form is the basis of tools and buildings, and carbon is the essence of most synthetic polymeric materials. It is the basis for combustible fuels, and, in its elementary form, it is even more spectacular as brightest diamond and darkest graphite.
How have the interaction of materials science and engineering and the intimate connections between research and application led to new carbon materials? The present response is polymer carbon, which can be pyrolyzed
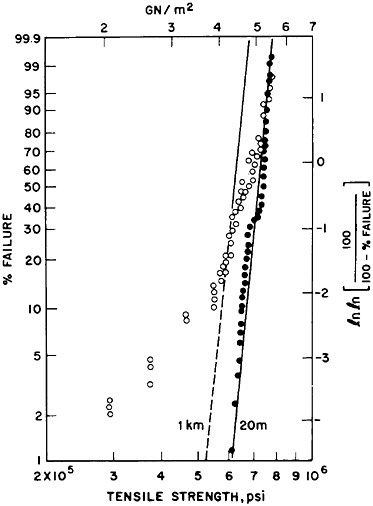
FIGURE 4 Distribution of tensile strengths of pure silica fibers, showing (in solid circles) precise control over 20-meter spans, with somewhat less consistency when characterized over kilometer spans.
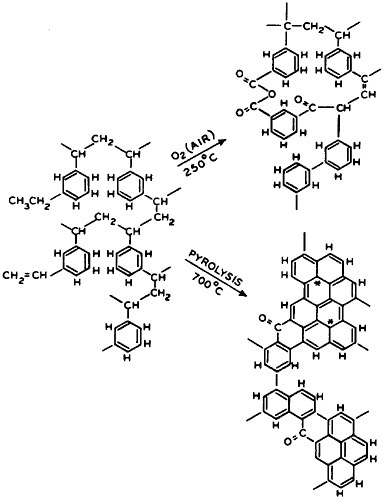
FIGURE 5 Representative effects of controlled oxidation and eventual pyrolysis on cross-linked hydrocarbon polymers, yielding refractory, and also very rigid, forms of polymer carbon. These can be used in composites of high strength and elastic modulus.
to various stages of cross-linked or polymer carbon conversion (Figure 5). The science of polymer carbon formation quickly produced fibers, and also spheres in which it was first studied, with a modulus of rigidity so high that it stirred thoughts of diamond structures. It also stimulated early experiments on how it would behave in place of the classic silicate or fiberglass reinforcement in matrices of casting polymer composites, where it has played a large role in structural uses. This composite evolution was already under way, quite apart from the original polymer carbon research. By 1947 the filament-wound glass composite rocket motor case had been successfully flown, and the associated industrial contractors supported the Navy’s decision
to use fiberglass motor cases for the Polaris missiles. Composites have since served in successive generations of rockets, reducing weight and providing strength and durability (Figure 6). Recently, makers of these composites have found that so-called graphite fiber, actually a polymer-carbon filament composite, outperforms other materials, including fiberglass composites. Using carbon filament instead of traditional metals for a rocket case can increase the range of a ballistic missile by about 600 miles (Figure 7). In the Trident II, for instance, polymer-fiber composites are used in motor parts, motor cases, and indeed throughout the missile system. Fiber strengths in the last few years have doubled and are expected to approach a million pounds per square inch even as the modulus of rigidity remains superlative.
Of course, the promise of future progress exists in systems other than those now known. In systems yielding polymer carbons, copolymers were originally identified with silicon and other elements. These can be converted to novel carbon ceramics, whose properties are beginning to be discussed in various parts of the materials community.
On yet another front, chemists and physicists interested in the process of polymer carbon formation recognized that extensive conjugation of the bonds occurs inside the polymer molecules. Accordingly, a wide span of electrical conductivity was produced. This phenomenon has led to practical applications in lightning arresters, resistor components, and various other devices. In turn, it has also engendered widespread study of other organic conductors such as the charge-transfer agents and their doped derivatives. By 1960, Herbert A.Pohl, then at Princeton University, had dealt extensively with doping of many of these conjugated structures. Thus, stage by stage, this
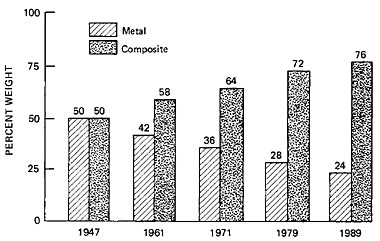
FIGURE 6 Examples of the growth of fibrous composites in rocket cases and motors, since mid century. From Hercules Corporation.
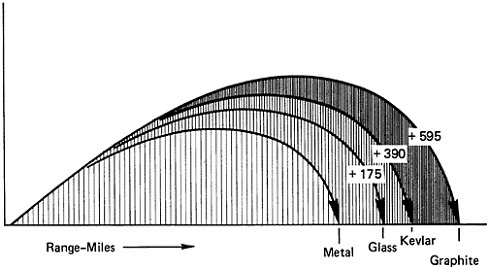
FIGURE 7 Increase in range of typical ballistic missiles through the use of polymer carbon composite materials, with attendant weight saving and efficiency. From Hercules Corporation.
admirable cumulative feature of materials science and engineering has expanded horizons in exceedingly diverse and long-unconnected areas of study, ranging from delicate microcircuitry to massive structures for power and transport.
SCIENTIFIC ADVANCES IN ATOMIC AND MOLECULAR STATE CHANGES
In a different context, other signals are arising. Hans Frauenfelder and E. Shyamsunder at the University of Illinois propose “protein quakes,” in which macromolecular mechanical waves dissipate energy from the fission of myoglobin-iron when photochemically cut from carbon monoxide or oxygen molecules. Their postulates of glass-like relaxation converge with the wide-spectrum relaxation phenomena observed in polymers and identified decades ago with the single molecules themselves in ultrahigh-frequency shear and compressional studies.
Frontiers in materials science and engineering dynamics are steadily being added to the equilibrium and conventional chemical kinetic qualities, already providing productive links between basic science and materials technology. These findings bear heavily, of course, on the basic behavior of phase change, melting, and other processing properties. But again the new dimensions are
striking, and again the interactions of technology, such as the annealing of semiconductor surfaces after ion implantation to restore order, as practiced by Soviet and Italian workers since 1977, have induced more scientific probes of pulsed laser heating. M.Downer, R.Fork, and C.Shank in AT&T’s Holmdel Laboratories have been using 8-femtosecond pulses to generate an electron-hole plasma, which then shifts energy to lattice vibration. This phenomenon, in turn, has recently been studied using Raman scattering of photons. Findings indicated more than simple melting. J.VanVechten at IBM, and later, others, elaborated on the studies as the pulse times decreased to below the picosecond range. By the early 1980s a dozen or more distinguished solid-state and materials centers were pursuing this central question of the mechanism of melting. The femtosecond pulse frequency coupled with reflectivity studies currently demonstrates that a liquid is formed even in the presence of the plasma, and altogether new aspects of liquefaction are appearing.
PATTERNS FOR FURTHER ACTION—EXTENSIONS OF EARLY INITIATIVE
Diverse examples support the conclusion that the 25 years of detailed attention given to materials research and engineering through the National Materials Program has achieved an unprecedented and unsurpassed interaction of science and technology. This interaction appears in research and development in universities and industries and through conscientious government. Such communion has occurred in ways that permit us to build for the future and on a scale that other nations, intensifying their competition in traditional forms, have not yet adopted. Even the large government-industrial organizations supported by the Department of Defense, and to a lesser extent, the Department of Energy and NASA, for materials research and development are small in relation to the total national commitment in materials, as noted in the COSMAT report. Yet the sharing of knowledge from the federal stimulus is large and can be larger still. This is the special message of the National Materials Program.
It may be useful to recall the particular conditions that led to the National Materials Program and what was expected of it. Its current vitality was demonstrated at the 1985 fall meeting of the Materials Research Society (MRS) in Boston, where there were no fewer than 16 symposia, each representing a combination of other scientific society sponsors. The MRS itself played an invaluable professional role by sponsoring sessions on frontiers of materials research and on materials education. Indeed, the growth and quality of the MRS attest to the vigorous response of materials investigators and institutions to the call that went out in 1960 and give cause to believe that
similar calls for scientific and technical achievement today could likewise be expected to succeed.
On the basis of 25 years of experience with the National Materials Program, let us finally look at the pertinent features that propelled it. We should imagine how the next challenges might be phrased if we expect such materials as the high-strength composite structures, unbreakable ceramics, continuous surfaces, and incorruptible metallics to realize their potentials.
The impetus for the National Materials Program was expressed in a short paper of 18 March 1958 from the White House Science Office (see Appendix to this chapter). That paper reflected the pressing interests of those times and also represented a certain coalition that included this author, as member of the President’s Science Advisory Committee (PSAC), and a member of its staff. The paper underscored the need for information centers, which only now are being realized through the new general programs and publications of the MRS, and a few other professional societies, led especially by the American Chemical Society. There was even a paragraph on facilities, including a number of items that then cost no less than $20,000 each! It was said that both those items and buildings had to be supported.
At about the same time, in 1958, PSAC completed a report entitled Strengthening American Science.8 It was not submitted to the President until December 1958, but its invention of the Federal Council for Science and Technology had been in planning during the latter half of that year. The report itself argued for more work on what was still classically known as “metals and materials.” We cast doubt on a very fashionable and popular topic of those times, and one which has arisen repeatedly since. It is the notion of a separate set of institutes for materials science and engineering. Rather, the notion of university participation was forcefully injected into the thinking. Then, as the main outcome of the report, the Federal Council for Science and Technology was implemented on 13 March 1959 by an Executive Order of President Eisenhower. It was activated just two weeks later when James R.Killian, the first chairman of the Federal Council for Science and Technology and the first Special Assistant to the President for those functions, appointed a Coordinating Committee on Materials Research and Development, chaired by John W.Williams, Director of the Division of Research of the Atomic Energy Commission. We pursued the diligent, and by no means silent, doings of that body so that by 11 July 1960 we received an official communique from the Advanced Research Projects Agency of the Department of Defense (which we had designated as the responsible body) saying that “the Department of Defense portion of the Interdisciplinary Laboratory Program has been initiated.”
Much of the operating philosophy, which was then accepted, had been summarized in our letter to Dr. Kelman of 10 June 1960. By July 16 the press had described the designation of the first three universities holding
contracts for interdisciplinary materials research and development. The New York Times account of this action said, “The process by which this decision was reached illustrates the workings of the policy-making machinery built up in the past few years to coordinate the nation’s scientific effort. It also illustrates the time that can elapse between a recognized need and action.” Nevertheless, Cornell University, Northwestern University, and the University of Pennsylvania were moving into action. But files of that time reveal that in his formal notes, President Kennedy had further discussed the onset of the National Materials Program in the fall of 1961, when he appeared in Chapel Hill at the University of North Carolina.
All of these documents emphasize not only the multispecialty, or what we may call polybasic research features, but also a particular ethos of the National Materials Program. Some of this is summarized in a letter I wrote as a member of PSAC to Dr. Kelman on 10 June 1960:
Much of this present federal support outside the National Science Foundation is paid for on the fictional premise that it yields a specific weapons system, irrigation plant, health measure, space vehicle, or the like. This not only deludes the public and the government administrator, but also degrades the university….
A properly coordinated materials program would not require narrow, synthetic, justifications of university study. There would be acceptable probability that the free choice of the investigator would nevertheless advance some phase of needed engineering or procurement. Likewise, there would be important relief of the pressures on individual professors to attach their own studies to the particular project that has the push and the cash at some moment, regardless of its long term scientific values….
It is not yet widely recognized that the materials research and development have a basic generality and thus new knowledge derived from them is almost immediately applicable to an extraordinary range of needs. In this situation, actions of the Federal Council for Science and Technology and specifically those of the Coordinating Committee can be of great value to the national efficiency and economy. Indeed, without such coordinating action it now appears difficult to see how there could be a followup of the original objectives of the Coordinating Committee noted in the minutes of April 8, 1959.9
That program is a major realization of the overall conclusion of the report Strengthening American Science. In this report we said, “The endless frontiers of science, now stretching to the stars, can provide rich opportunities for men to seek a common understanding of the natural forces which all men must obey, and which govern the world in which all men must live together.”8
Advancing materials research and development takes a new but expected shape as we look forward to the next quarter century of materials science and engineering. We and others have recorded in scientific and technical detail the particular vanguard of innovation that we should seek and expect. But we can now add another dimension to the advance.
It is that new links among the traditional divisions of science and engi-
neering can be formed to advance a domain of knowledge and practice, namely, solid-state science and materials technology. The resulting network can mobilize actions in education, industry, and government far beyond the size of the nucleating effort.
In contrast to major programs like the Manhattan Project, the Apollo Program, or the National Cancer Plan, this network does not represent collaboration for a specific goal or mission. Rather it is the generation of a technical capability that applies to nearly every feature of economics and public affairs.
The National Materials Program constitutes a precious American resource of more than a million people devoted to scientific and engineering research and development to advance materials science and technology. It responds to the national need put forward by President Eisenhower in the 1950s. It combines new and once-separate realms of learning and practice and is slowly recasting age-old academic habits. It is intrinsic in industrial and governmental programs to achieve automation of design and process. It is basic to the growth of new technical capabilities such as photonics, bioengineering support and repair of human organs, exploration of space and the oceans, and preservation of the environment. Most of all it is a worthy exercise of the mind.
A national program in materials science and engineering is a new venture in this century of science and technology. Today, almost everything mankind has tried to do with matter through the million years of human evolution can be done not just two or three times better but, through materials science and engineering, a thousand or a thousand thousand times better than we once thought possible.
NOTES
Appendix
COORDINATING MATERIALS RESEARCH IN THE UNITED STATES
Background paper prepared by the staff of The President’s Science Advisory Committee
18 March 1958
Research and development in the field of materials is closely related to the missions and activities of a number of Federal agencies. Unique environmental conditions associated with rocket propulsion, nuclear reactors, space flight, and vehicle re-entry, have established the need for materials which are not currently available. There have been many clever engineering designs which have allowed some progress to be made but these designs have not diminished the urgent need for materials research and development. The use of ablation materials and heat-sinks have allowed progress in high temperature structures. The use of strip-wrap construction for rocket casings and the use of honeycomb structural panels have increased the strength to weight ratio for structures involved in space flight. Yet all of these have meant increases in complexity and cost which would not have been required if new and better materials already existed.
During the last decade significant advances have been made in the sciences of solids. The effect of impurities and surface conditions on physical properties has been partially established. Very pure materials have approached those predicted by calculations of binding energies. Samples of ceramics which are normally brittle have shown ductile properties when prepared under very closely controlled conditions. There is a general feeling, therefore, that such advances in science as these can lead to a technology of materials engineering quite different from the metallurgy, ceramics, and polymer technology of today. But to achieve this result it will be necessary to begin to relate the new fundamental knowledge of matter to the behavior of highly complex materials. This in turn will require scientists and engineers from many different disciplines—organic chemistry, physical chemistry, metallurgy, and solid-state physics—to associate their special knowledge and different points of view.
While problems of high temperature materials, and materials having a high strength to weight ratio, are very urgent matters for Federal agencies, they are of little significance in the civilian economy today. It thus becomes clear that the Federal Government will have to play a leading role in encouraging the research and development which is needed. Most of the agencies represented on the Federal Council have laboratories engaged in materials research and development, and also sponsor such work in universities and industry. A careful coordination of research and applied science programs
among the various agencies can thus increase the effectiveness of the total program. In a situation characterized by a shortage of manpower well-grounded in solid state physics, physical chemistry and crystallography, one naturally turns to the university where funds produce not only the needed research but trained manpower as well. In various universities one finds the faculties engaged in planning interdepartmental efforts to establish a new materials science and engineering. One of their problems is the lack of buildings and equipment. There are many research tools such as electron microscopes, electron diffraction units, high temperature furnaces, and x-ray diffraction units, each of which represents a cost of approximately $20,000. In assisting the universities to do more research and to train more first-rate personnel some way must be found to provide support for interdepartmental and interdisciplinary groups, to provide funds for equipment and buildings, and to assure reasonable continuity of support. In addition, there is a need to define objectives which are suitable for the academic environment. It is extremely important that, whenever possible, engineering departments of universities be supported in the applied science aspects of materials in order that the basic work may have a practical significance as soon as possible.
It is also worthwhile to explore the possibility of an information center which would publish information on the latest research and development accomplishments which are of real significance. Such a center could also serve to coordinate the exchange of samples of known purity and physical properties.
The attention to university programs for research and education is of great importance but is only one step in a program to strengthen our national effort in materials. The many Government laboratories, private research institutes, and industrial laboratories also have significant roles to play. Perhaps the Federal Council can aid in planning a well-coordinated program based on the unique capabilities of these various laboratories and institutions, both public and private.
Materials Research Laboratories: The Early Years
ROBERT L.SPROULL
In examining the origins of the Materials Research Laboratories program in 1960, my purpose is not to evoke nostalgia for that time but to derive lessons for the present and the future by revisiting the program and its antecedents. I shall adopt the point of view that since we are looking back on events of 25 years ago, the proper unit of time is 25 years. Thus, my story starts on the science side, two “time constants” before 1960, in 1910.
THE SCIENTIFIC SETTING
By 1910, chemistry and metallurgy had already hailed many centuries of contributions to the understanding of materials and a transition from art to science that had recently been accelerated by the discovery of x rays. But the contribution from physics had been nearly zero; some descriptive “laws” like Dulong and Petit’s law of specific heats and the Wiedemann-Franz ratio of thermal to electrical conductivity in metals were well known, but they papered over and concealed real understanding. Planck’s introduction of the quantum theory in 1900 and Einstein’s brilliant paper on the photoelectric effect in 1905 were only a gleam in the eye.
By one time constant later, in 1935, the seeds had been sown for a complete revolution in the understanding of matter. The clock really started running only in 1923, and, only four years later, the quantum mechanics developed by the giants Bohr, Schrödinger, Heisenberg, and others was being successfully and widely applied. Heitler and London used wave mechanics to describe the hydrogen molecule in 1927; Pauling extended the theory to molecules generally in the next eight years. Von Neumann introduced mathematical elegance at the same time. Sommerfeld and Bethe’s monumental
Volume 24, Number 2, of the Handbuch der Physik appeared in 1933, full of rich ore that is still being mined. William Hume-Rothery’s The Metallic State came out in 1931, and his seminal The Structure of Metals and Alloys was in manuscript by the end of our first period. A.H.Wilson, R.Peierls, Neville Mott, and many others rapidly advanced the science of the solid state.
I must recount two anecdotes from that period, both with profound implications for the rest of my story. The first concerns Robert Wichert Pohl, the Göttingen giant of experimental solid-state physics. He had borrowed a large diamond from a Berlin bank to measure the Hall effect in photoelectrons. But he, or more likely an assistant, had failed to secure the magnet pole pieces, and when the current was turned on, North and South made instant love at the expense of the brittle diamond. From this experience flowed his concentration on alkali halide crystals.
The second story concerns a very young 1932 graduate of Stanford University, Frederick Seitz. He went to Princeton to do graduate work with E.U.Condon. But Condon, who was then preparing the famous Condon and Shortley Theory of Atomic Spectra, advised him to work with Eugene Wigner instead; Condon remarked, “Solid-state physics is coming, and if you stay with me you’ll just do calculations for my book.”
I need spend little time on the second time constant, since the flowering of understanding and prediction during the period from 1935 to 1960 is well known. Chemistry adopted quantum mechanics with great effectiveness. Metallurgists were beginning to go far beyond their venerable concentration on the austenite-martensite transition. Even geologists were dusting off their hogbacks and cuestas and conducting synthetic mineralogy. Seitz’s The Modern Theory of Solids in 1940 brought understanding to new heights and provided a common language for all workers in materials. William Shockley’s theory of the p-n junction in 1949 and the realization of the junction transistor in 1951 produced immediate visions of a fantastic future for solid-state electronic devices. The complexity of so-called point defects was beginning to be appreciated, and dislocation theory was well advanced. New polymers and new alloys and metals like ductile titanium were being developed.
Thus, by 1960 the stage was set for spectacular advances in materials that would have profound effects on society. Physics was at last bringing something to the party, and metallurgists and chemists needed physicists, if only physicists would rise above their snobbery. Physicists needed chemists and metallurgists, since increasingly sophisticated experiments required detailed knowledge of chemical and physical imperfections and structures. Of even more consequence was the conviction that the design and creation of new materials, such as composites, high-temperature coatings, or catalysts, would require true collaboration among chemists, physicists, and engineers.
THE PROGRAM TAKES SHAPE
I now turn to the second element of the setting for 1960, the currents in and around Washington, and here I shall go back only one time constant, to 1935. The prewar defense establishment had been interested in mechanical properties of solids and in corrosion and coatings, and the Signal Corps contracted for work in vacuum-tube electronics, including work on that enduring mystery, the oxide-coated cathode. But our story really begins with three decisions reached in the months immediately following the war:
-
Science and technology had much to contribute to national defense and prosperity and therefore could appropriately be supported by the federal government;
-
The preparation of a new generation of scientists and engineers and much of federally supported research should be done in the same institutions, the research universities;
-
The federal apparatus and process for this support through contracts (and later, grants) should be quite unlike the prewar “buying brooms” systems and should provide much more scope for the contractor’s imagination and discovery and more harvesting by informal agency-contractor interactions rather than by fulfilling specifications.
The Office of Naval Research (ONR) was the immediate consequence, in late 1945 and early 1946, of these decisions, and it set the pattern for all the later agencies. Two elements of this pattern were especially important: (1) program managers who might be (and occasionally were) principal investigators, and principal investigators who might equally well be (and occasionally were) program managers; and (2) task statements in general terms, with maximum opportunity for creation and discovery. The other defense agencies, the Atomic Energy Commission (AEC), National Science Foundation (NSF), and National Aeronautics and Space Administration (NASA) warmly embraced this tradition.
Another consequence of the war was the Washington realization that the field of materials was far more complex and open-ended than it had appeared in 1935. The AEC had to contend with radiation-produced embrittlement and the Wigner disease (with the Szilard complications). The Department of Defense (DOD) had to contend with nose cones and a fascinating array of materials covered up by the nose cones, as well as the burgeoning opportunities in electronic and optical materials.
There were several Washington rays that converged in 1959–1960, but to begin with they were essentially independent, coming from AEC, ONR, the National Academy of Sciences (NAS), the White House, and DOD. One of the most important was the ray from AEC. The 1945 euphoria concerning
the peaceful uses of atomic energy brought forth the innocent suggestion that electricity produced by fission reactors would soon be so cheap that it would not pay to meter it. (This suggestion, grossly uninformed about materials problems and their implication for Carnot efficiency, was on a par with the insertion by nuclear physicists of an anthracene crystal into one of the early synchrotrons to locate the beam by its fluorescence; it took weeks to clean up the gunk so that a reasonable vacuum could be maintained.) This extreme position was never seriously maintained by AEC, but realization of the compelling influence of materials limitations on nuclear reactor cost, efficiency, and safety was, let us say, slow to develop.
John von Neumann, who brought Hilbert space to bear on quantum mechanics, was especially upset that time and time again what he wanted to do was prevented by an inadequate science of materials. When he asked what limited the growth of that science, he was told, “Lack of people.” And why not produce more capable people? “Lack of university facilities.” He thus began pushing in the AEC General Advisory Committee (GAC) for a substantial program in sponsoring university facilities for materials research and graduate education. By the time he became ill and died in early 1957, Willard Libby had already effectively taken up the torch in the GAC. The AEC Metallurgy and Materials Branch Advisory Panel, of which Seitz was chairman, in its first report in 1956 called for new buildings and research facilities in universities for materials research and education. Although Edward Epremian, who was chief of AEC’s Metallurgy and Materials Branch, recommended to the GAC that the AEC establish “Materials Research Institutes” at universities, the GAC would not commit the funds. After the launch of Sputnik I on 4 October 1957, however, this ray was revitalized by Donald K.Stevens, who succeeded Epremian in December 1957.
Another ray came from ONR with its Solid State Sciences Advisory Panel, a group affectionately known as the Navy’s “chowder and marching society.” Under the leadership of Seitz and Harvey Brooks, it had been studying Navy materials problems and helpfully visiting Navy laboratories since 1950. Its report on opportunities in solid-state science research appeared so soon before Sputnik that its authors were accused (jocularly, I hope) of being privy to Soviet secrets. Julius Harwood and others in ONR urged more materials work in universities.
A third ray was a National Academy of Sciences study sponsored by the Air Force in 1957.1 This study, led by J.Herbert Hollomon, recommended in 1958 and 1959 the creation of a National Materials Laboratory. Opposition was quick and nearly universal, mostly because of the realization that such a move would only reassign people already in the field and would do nothing to enlarge the supply of trained scientists and engineers. It did, however, reinforce the need for action in materials research, and it documented advantages of interdisciplinary approaches.
A fourth ray emanated from the White House. James Killian was appointed Science Advisor to the President on 7 November 1957 and quickly organized the President’s Scientific Advisory Committee (PSAC). Killian and PSAC member William O.Baker identified materials research and training as matters of top priority in the post-Sputnik environment. The Federal Council for Science and Technology, consisting of the heads of all the agencies involved in science and technology, was created as the administrative counterpart to PSAC. Its first and highly effective instrument was the Coordinating Committee on Materials Research and Development, made up of the materials heads in each agency, initially chaired by John H.Williams of AEC and later by Stevens.
A fifth ray started in the Department of Defense, encouraged by the White House interest. Herbert York, director of Defense Research and Engineering, Roy Johnson, director of the Advanced Research Projects Agency (ARPA) until November 1959, and John F.Kincaid of ARPA participated.
The focusing of these rays began in late 1958. A key meeting occurred when Libby, accompanied by Stevens, descended on York, buttressed by Kincaid. Stevens convinced York that his AEC investigations documented a great opportunity for the creation of interdisciplinary materials laboratories in universities and the universities’ need for support for buildings. York asked Kincaid to evaluate Stevens’s findings; Kincaid quickly agreed they were sound.
In the late winter and spring of 1959, Kincaid, Stevens, and others visited a number of research universities at which DOD and AEC work showed promise for development of interdisciplinary laboratories. This team again verified the need for space and modern research equipment and central facilities and the willingness on the part of the universities to take the risks of expansion if appropriate contracts could be worked out.
By early June of 1959, AEC had decided that its one-year contracting authority would not be sufficient for universities to commit space and borrow money to create facilities and to justify expansion by tenure-track professorial appointments. York agreed to DOD’s taking the prime responsibility and assigned it to ARPA on 8 June 1959. DOD had the authority to write five-year contracts; the contracts were for four years, but an additional year was negotiated each year, so there were always at least three years ahead under the contracts. AEC never really left the program, however, and participated positively in the creation of facilities at Berkeley and Urbana and through individual research grants at what became ARPA Interdisciplinary Laboratories (IDLs). NASA joined later.
The selection of the first three laboratories proceeded through the academic year 1959–1960. Assisting Kincaid in ARPA was a group of consultants, including Morris Tanenbaum, G.J.Dienes, M.E.Hebb, J.Herbert Hollomon, and J.P.Howe. Charles Yost in the Air Force Office of Scientific
Research, Harwood, and others from the armed services also participated. All of the recent accomplishments of research and experience in educating Ph.D. students were considered, but the selection was not a prize for past performance; rather it was a judgment of the promise of a university for a significant expansion and a truly joint, cooperative attack on materials research across disciplines.
All of the rays finally came into focus with the first contracts in July 1960. (Coincidentally, 1960 was the year the most monumental real-light focusing of all time occurred in the first demonstration of the laser.) The three universities chosen by ARPA in 1960 were joined in later years by nine more ARPA contracts, three AEC contracts, and two NASA contracts. After a thoroughgoing review in 1971, the program, now called the Materials Research Laboratories program, was transferred to NSF in 1972.
Was this program a success? I believe it was a spectacular success, but I am probably one of the poorest possible evaluators. There is a theorem that says: “All education experiments are successful.” The proof is simple: All education experiments are evaluated by their promoters. So, others must be asked for a dispassionate judgment. I do suggest, however, the way that the question “Success compared to what?” should be answered. The comparison should be with spending the same amounts on materials research by federal agencies through routine individual grants and contracts.
ASSESSING THE EXPERIENCE
Up to this point, I have given a quick look at the early history of the materials laboratories, announced their success, and acknowledged my negative credentials for making that announcement credible. There remains only to give my view as to why the program was successful and to draw any lessons we can from the experience. There is now renewed interest in Washington in “hyphenated” science, as evidenced by a recent report on new interdisciplinary research arrangements by the Government-University-Industry Research Roundtable and the Academy Industry Program of the National Academy of Sciences, National Academy of Engineering, and Institute of Medicine.2 I believe the materials laboratories have much to teach us. I will therefore describe briefly what I believe to be the important features of this program, dividing them into features of the field of materials research, those at the universities, and those in Washington. Of course, the latter two are intimately connected and in many cases (e.g., building support) require looking at the same feature from two perspectives.
The important features of the field of materials research were, first, its immense variety and open-endedness. The preceding description of the situation in 1960 shows how intellectually auspicious it was. The second feature
was the richness of connections among the disciplines, including not just chemistry, physics, and metallurgy, but mathematics, geology, and nearly all branches of engineering; and, of course, the connection throughout between theory and experiment. The third feature was the richness of applications across the vast area from consumer products to national defense. The Ph.D.s educated in the program had a marvelous choice of jobs, as near to or as far from immediately useful products as they chose. Since all product-oriented development is necessarily interdisciplinary, these young people were especially in demand for work, with direct benefits to society. The fourth feature was that most research projects in materials are of human scale, not requiring the huge team efforts associated with particle physics. Of course, cooperation and collaboration were essential. In later work in industry, exstudents might be part of sizable teams (on alloy development, for example), but Ph.D. research projects were naturally of human size, with maximum opportunity for individual initiative, for an individual’s learning just how capable a scientist or engineer he could be.
The important features at each university were, first, that an umbrella contract provided for continuity of support and for the ability to buy large quanta of equipment and facilities. Second, a local director committed a substantial fraction of his career to making the program succeed. He could use the longevity of support to extract concessions from the university and departmental administrations. Third, the contract provided, in most cases, reimbursement over 10 years for the new construction required to do modern experimentation on materials. Fourth, the longevity of the contract induced the university to allocate to the project scarce and prime space in the middle of the campus, thereby establishing the maximum informal connections among disciplines. Fifth, central experimental facilities (such as those for electron microscopy or crystal growth) could have state-of-the-art equipment, even if it was very expensive, and they served as a mixing ground for students and faculty from several disciplines. Sixth, an executive committee composed of people with power and influence in the individual disciplines but oriented toward the success of the program helped the director over the rough spots with department chairmen, people who often were overly protective of their bishoprics and palatinates. Seventh, a contract was not given to an institution unless it had a strong disciplinary base on which to build. Interdisciplinary programs perched on weak disciplines are dangerous; interdisciplinary work already had a bad name on many campuses because of programs alleged to be interdisciplinary but without disciplines (on many campuses home economics was the example cited). Eighth, individual grants and contracts with federal agencies continued; most well-established principal investigators received the majority of their support from some other agency and might enjoy help from the program only in the central facilities or the building space. Thus, when the executive committee and director found that they had to say
“No” to a local high priest, it was not really “No” but only “No with the umbrella contract’s money,” and that made life easier.
This list may be incomplete, but perhaps more importantly it does not quite capture the flavor of the informal interaction among young and old, among electrical engineers and chemists, among administrators and bench scientists that was fostered by the umbrella contracts and was, in my view, at the heart of the success of the program. That ambience would have been different if research institutes had been built. I might illustrate this important point by mentioning Morris Tanenbaum’s history of the development of hard superconductors, described in a report by the National Research Council’s Materials Advisory Board at about the time of the birth of these laboratories.3 Although Tanenbaum explained how the formal interdisciplinary nature of Bell Telephone Laboratories (BTL) “produced” this development, his text permits the conclusion that the most important part of BTL was the lunchroom! The unplanned interactions in various materials facilities in the midst of other campus activities played a key role in the success of the laboratories.
The important features of the program related to Washington were, first, in 1959–1960 and for a few years afterward, Washington was in an expansionist mood; initiatives were welcomed. Second, the flowing together of currents and conviction from NAS, PSAC, the Federal Council for Science and Technology, the Coordinating Committee on Materials Research and Development, and from AEC, DOD, and other agencies gave a solid, joint base to the program. Third, ARPA was a young agency with little doctrine and almost a passion for innovation; it held the profound conviction that the United States should never be only second best in any consequential technology. Fourth, ARPA was willing to write four-year contracts with a four-year renewal each year, thereby getting much more for the taxpayers’ money than if it had insisted on year-by-year contracting. Further, ARPA gave convincing evidence that the program would last at least 10 years, and thereby induced universities to take the risks of borrowing money and building new facilities with a 10-year payback. Although several university presidents were very nervous about placing such confidence in the federal government, in the end all commitments were honored. Fifth, ARPA and other agencies exercised exemplary self-restraint in eschewing micromanagement; they left the allocation of funds to the local management, which resulted in enormous enhancement of efficiency and effectiveness. The work statement was extremely broad, speaking only to “the properties of materials,” “fundamental relationships,” and “theoretical and experimental studies in such fields as metallurgy, ceramic science, solid-state physics, chemistry, solid-state mechanics, surface phenomena, and polymer sciences.” Sixth, the Bureau of the Budget and Capitol Hill were not as concerned about the details of programs as they are now. Seventh, the continuation of individual project
support by the other agencies permitted these agencies to take justifiable pride in their part of the program and to connect it to agency missions.
These features of the Materials Research Laboratories program outlined above helped the program overcome oppositions and concerns, of which there were many. There was, of course, envy on the part of nonparticipating universities; only 3 of the 45 proposals in the first round were funded in 1960. The negative consequences were, of course, mitigated by subsequent rounds of ARPA, AEC, and NASA contracts and by a $6-million ARPA equipment grant program in 1960 and 1961, the funds going to the unsuccessful competitors. In Washington there was nervousness that the whole program was a packaging gimmick, getting wholesale what would not have been possible to get retail. On campuses, there was nervousness that “interdisciplinary” would become a buzzword that would dominate Washington allocation practice. Later, the Mansfield amendment (114 Cong. Rec. 29332 [1968]), which limited indirect costs that could be added to the base cost of a defense research grant on contract, played into the hands of those who equate “relevance” with “immediate applicability.” The intended intimidation of program managers never quite came off, and only the most timid of universities gave credence to those who would have had one believe that the ARPA contracts were helping to napalm the Vietnamese. The program and its leaders were strong enough to shrug off these irritants.
Of course, not all of the features of the MRLs, especially the benign budgetary oversight by Congress and the executive branch, can be re-created for any program proposed today. However, the Materials Research Laboratories tell us that to the extent that these features can be adopted, a new program will be more auspicious.
Since the first Materials Research Laboratories were established, a generation of scientists and engineers has done its work. Some of these individuals have spent nearly their entire professional lives in these laboratories and have led spectacular careers in research and in the guidance of Ph.D. students. I am sure many would give a good deal of the credit to the early support from the ARPA program, to the central facilities, to the fine building, library, and connections with chemistry and metallurgy nourished by the program, and to the colleagues provided in part by the program. Of course, much is also due to their own imagination, energy, physical insight, drive, and generosity of spirit. Thus, although I do not suggest that I am competent to evaluate the materials laboratories, I do claim some part of the success of individuals as a success of the program.
ACKNOWLEDGMENT
I should like to thank D.K.Hess, R.E.Hughes, and D.K.Stevens for help in the preparation of this chapter.
NOTES
Materials Research Laboratories: Reviewing the First Twenty-Five Years
LYLE H.SCHWARTZ
Creativity is a singular effort. It is often said that no original idea has ever come from a committee. And yet, increasingly, group efforts are devoted to the solution of technical problems. Industrial research laboratories first, and then universities, turned to collaborative research teams that cross rigid departmental boundaries and use systems approaches to attack complex problems. The efforts leading to establishment of Materials Research Laboratories have been in the vanguard of this transition.
In 1960 the Advanced Research Projects Agency (ARPA) of the U.S. Department of Defense established the Interdisciplinary Laboratories (IDLs), later known as Materials Research Laboratories (MRLs). Their impact on materials research, on the universities in which they are housed, and on the very manner in which university research is organized has been profound and is still growing. The purpose of this chapter is to survey the brief but significant history of these laboratories, looking back to the time of their origin, tracing their evolution, and then summarizing some of their accomplishments.1
INTERDISCIPLINARY LABORATORIES
In the atmosphere of international competition symptomatic of the cold war and dramatized by the Soviet launching of Sputnik I in October 1957, an interagency Coordinating Committee on Materials Research and Development (CCMRD) was convened in 1958 at the urging of the U.S. Department of Defense (DOD). It was clear then as it is now that the national economy and security would depend increasingly on new technology, which in many cases would require new, more reliable materials. It was not as
TABLE 1 Year of Establishment and Termination of Interdisciplinary Laboratories (IDLs)/Materials Research Laboratories (MRLs)
clear then as it is today that a broad interdisciplinary education was necessary to make progress in materials research, but the farsighted members of the CCMRD did recognize that need. In 1959 they recommended the establishment of interdisciplinary laboratories for materials research to be built on university campuses to carry on research and to train graduate students. Their intention was to foster research in which the pertinent scientific and engineering disciplines would be brought to bear in a collective and cooperative manner on common problem areas in materials science.
The CCMRD recommendation was adopted by the Federal Council for Science and Technology and was assigned to ARPA in June 1959 for execution. During the next three years, 12 IDLs were established, as shown in Table 1. Coincidentally, the Atomic Energy Commission (now the Department of Energy [DOE]) supported analogous laboratories in three universities (University of California, Berkeley; University of Iowa; and University of Illinois), and the National Aeronautics and Space Administration followed
with a smaller program. (Details of the events leading to the establishment of these various laboratories are presented in a 1960 address by William O. Baker.2) This presentation is limited to a discussion of the ARPA IDLs, which were renamed Materials Research Laboratories when the National Science Foundation (NSF) took over the program in 1972.
It is instructive to quote directly from the work statement in ARPA IDL contracts from 1960:
The Contractor shall establish an interdisciplinary materials research program and shall furnish the necessary personnel and facilities for the conduct of research in the science of materials with the objective of furthering the understanding of the factors which influence the properties of materials and the fundamental relationships which exist between composition and structure and the behavior of materials [emphasis added].
In looking back, it should be recalled that in 1960 few academic departments at universities had sufficient breadth of coverage to justify the title “Materials Science,” and none would even have considered the title “Materials Science and Engineering.” Instead, there were many departments in which mining, process metallurgy, physical and mechanical metallurgy, and the physics of metals were the principal, and largely separate, areas of materials research. An occasional individual effort in ceramic engineering could be found, and polymer science, if available at all, was a topical course in advanced chemistry. Yet, 12 years later when the IDLs were transferred to NSF, materials science was a recognized discipline at many major research universities, and the change in emphasis in academia could be clearly demonstrated by the names selected by materials departments (see Table 2). The trend toward more general “materials” departments is continuing, as shown in the table.
TABLE 2 Trends in Titles of Materials Departments at U.S. Universities, 1964–1985
|
|
Number of Departments, by Year |
||
|
Department Title |
1964a |
1970b |
1985b |
|
Minerals and Mining |
9 |
7 |
5 |
|
Metallurgy |
31 |
21 |
17 |
|
Materials |
11 |
29 |
51 |
|
Other |
18 |
21 |
17 |
|
Total |
69 |
78 |
90 |
|
aCompiled from 1964–1970 ASM Metallurgy Materials Education Yearbook, ed., J.P.Nielsen (American Society for Metals, Metals Park, Ohio). bCompiled from 1985 ASM Metallurgy/Materials Education Yearbook, ed., K.Mukherjee (American Society for Metals, Metals Park, Ohio, 1985). |
|||
The establishment of the ARPA IDLs was the first attempt on U.S. university campuses to create a new style of research organization and to accelerate the processes of academic curricular change resulting from federal recognition of a specific national need. It was clear to the organizers of this experiment that this new mode for funding university research would require changes in the way universities do business—in particular, the traditional departmental organization would be threatened.
During the dramatic first years (see Sproull, in this volume), many of the IDL program goals were realized. Buildings were built, paid for by ARPA through a building use fee incorporated into the contract; interdisciplinary research facilities were established in these buildings, with sophisticated equipment operated by trained technicians and available to the entire materials community at the IDL. Graduate students were trained in large numbers and went on to populate the universities and federal and industrial laboratories, which were growing rapidly during this period. Research was carried out in myriad materials fields using federal funds assigned as block grants to the universities and administered locally.
A brief statistical view of the IDLs at their height is informative. In Fiscal Year 1969, for example, the funding for the IDL program reached its maximum—$18.97 million,3 including $1.8 million in building use charges. In that same year a total of nearly 600 faculty members and 2,385 graduate students participated in the program, 360 doctoral degrees were awarded, and more than 2,000 papers by program participants were published. The research efforts of these students and faculty members were grouped into 134 “work units,” or identifiable research project areas, and each participating university engaged in 6 to 20 such units. Significantly missing from the program at that time was the strong interactive team approach, which we now identify as a dominant feature of the Materials Research Laboratories and which would be left to the National Science Foundation to foster.
In the report Materials and Man’s Needs, the National Academy of Sciences Committee on the Survey of Materials Science and Engineering (COSMAT) devoted extensive study to the materials centers (including all ARPA, AEC, and NASA block-funded institutions). The committee’s list of successes for the materials center concept included the following:
-
Drawing “attention to the emergence of coupled materials science and engineering as a new interdisciplinary focus of activity in a way which could not have been achieved otherwise.”
-
Demonstration “that block funding is perfectly feasible on a campus.”
-
Development of “excellent research groupings of faculty members, the building-up of a reputation and attraction for good students, and the training of first-rate materials scientists, physicists, chemists, and other professionals.”
-
Administrative efficiency achieved “through faculty saving their time
-
in writing proposals and seeking support, and the agency officials likewise saving a great deal of administrative time.”
-
“A large number of students were trained in an excellent environment for advanced degrees.”4
Not surprisingly, the COSMAT report found some deficiencies in the program, the most important of which was the limited evidence of interdisciplinary effort as measured principally by the limited number of joint publications. (Of course, the number of joint publications should not be the only criterion in such an evaluation.) One could say of this period that the seeds for interdisciplinary cooperation had been sown but that the young plant needed cultivation. At its best, interdisciplinary activity at the IDLs consisted of the development of a community of scholars brought together by a block grant, which they administered jointly; of research facilities developed by expert disciplinarians but designed and operated in a manner conducive to shared use by students from other disciplines; of additions to faculties in related departments with expertise intended to complement existing strengths throughout the fields of materials research; and of seminar series and internal meetings organized expressly for educating colleagues in other departments. Without these beginnings, the next critical steps toward fully collaborative cross-disciplinarity could not have been achieved in the NSF program.
The COSMAT report was also critical of the effectiveness of the ARPA IDL effort to increase graduate education in materials research more rapidly than in other disciplines. It found that the number of M.S. and Ph.D. degrees granted in the traditional materials departments (Figure 1) grew at 12 to 13 percent per year through the 1960s, the same rate as that for the engineering field as a whole. It seems clear in retrospect that substantial government funding in many forms led to a general expansion of graduate education in all engineering fields. Instead of focusing on any failure of ARPA to do better in some quantitative sense in materials education than was done in other fields by other DOD agencies, DOE, and NASA, one should focus on the quality of the education received by the students. Both graduate and undergraduate students at the institutions where IDLs had been established benefited from the broad interdisciplinary view of materials research that entered the curriculum as faculties grew in size and diversity. By the early 1970s it was no longer uncommon at these schools to find ceramics (and even polymer science) taught as an integral part of the curriculum. Although the IDLs were not alone in this trend, they certainly led the way. Thus, much of the interdisciplinarity sought in the original CCMRD concept was realized through evolutionary changes in the traditional materials departments rather than by dramatic changes in interaction across university departmental lines. This cross-departmental interaction would come only with the group research concept introduced by NSF.
FIGURE 1 Number of degrees awarded by (a) materials-designated departments and (b) engineering departments in all fields (U.S. Engineering Council for Professional Development Schools), 1950–1970. (Percentages indicate average annual rate of growth during the 1960s.)4
TRANSFER OF IDLs TO NSF
The political complexities of the late 1960s led to reevaluation of the role of DOD in sponsoring non-mission-oriented research at universities. In 1970 it was decided that the appropriate agency to which responsibility for this program of research centers should be transferred was the National Science Foundation. However, the concepts of block funding, delegation of authority to local management, and shared experimental facilities differed markedly from those characterizing the traditional single-investigator, discipline-oriented mode of operation at NSF. After an extensive review of the program in 1971, NSF assumed responsibility for the IDLs in 1972, accepting the operational modes built into the program but adding a critical new component. As described in NSF’s program policy statement, the laboratories—now renamed Materials Research Laboratories—would retain locally administered block (or “core”) funding intended to “facilitate research in materials science and engineering which is either difficult or unfeasible to carry out under traditional funding of individual research.” Most importantly, the new component added by NSF was that “scientific excellence is viewed as a necessary, but no longer sufficient, condition to qualify for MRL core support.” In addition, the MRLs would be judged by their ability to foster “coherent,
multidisciplinary and multi-investigator projects in major thrust areas requiring the expertise of two or more materials-related disciplines.” These so-called thrust groups are the heart of the current core funding at MRLs; at their best they have achieved a transformation in the way materials research is done at universities and in the way graduate education proceeds.
The transfer of the MRLs to NSF required an organizational change and led to the establishment of the Materials Research Division, grouping the MRLs with programs that had concentrated on traditional materials departments as well as on areas of physics and chemistry clearly dealing with materials research.
At the time of its move from ARPA to NSF, the program included 600 faculty members at 12 universities. Of these faculty members, some 35 percent were physicists, 25 percent were chemists, 19 percent were metallurgists or members of materials science and engineering departments, 16 percent were from other engineering departments (mainly electrical), and the remaining 5 percent were from other fields. The transition from ARPA to NSF was rather complex, taking place over a two-year period; not all projects (or funds) were finally transferred. The total MRL budget for FY 1974 was $12.1 million, reduced from the $17.2 million for operations of the FY 1971 IDL budget. When the program was examined in 1975 by the MITRE Corporation on contract from the NSF,5 the number of faculty members had been reduced to 532 and the distribution had shifted slightly from physics toward materials and engineering (Table 3). Inflation and limited NSF budgets in subsequent years led to further reduction in effort as measured in constant dollars (Figure 2) and a consequent further reduction in faculty to the 1985 level of 400. Furthermore, additions of new schools to the MRL program and phaseout of others led to the 1985 faculty distribution shown in Table 3. It is significant, and disturbing, that these trends seem counter to the increased emphasis on materials processing and chemistry in materials research. Balance in NSF funding is being achieved by other funding modes.
TABLE 3 Faculty Distribution in IDLs/MRLs, 1970, 1975, and 1985
|
Faculty |
1970a |
1975b |
|
1985c |
|
Materials science and engineering (includes metallurgy, geosciences, etc.) |
19% |
27% |
|
35% |
|
Physicists |
35% |
31% |
35% |
|
|
Chemists |
25% |
19% |
17% |
|
|
Other engineering |
16% |
23% |
|
12% |
|
Other |
5% |
2% |
||
|
Total number |
600 |
532 |
|
400 |
|
aNote 1 of this chapter. bNote 4. cNote 6. |
||||
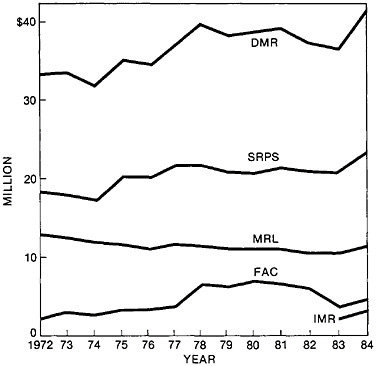
FIGURE 2 NSF-DMR budget, FY 1972-FY 1984, in constant 1982 dollars. DMR, Division of Materials Research; SRPS, single-investigator research project support; MRL, Materials Research Laboratories; FAC, national user facilities; IMR, instrumentation for materials research.
THRUST GROUPS
The formation of the interdisciplinary thrust groups referred to above varies among institutions. It often begins when several faculty members with common interests, challenged by the opportunity to attack complex problems and encouraged by the availability of a funding mode for group effort, come together for brainstorming. This exercise may be stimulated by an MRL director or may result from the efforts and ideas of a single faculty member, but it leads to the definition of a program area in which the university has substantial current or developing capability. As the research program concept is refined, other faculty members with complementary talents may be attracted to the group. In the formative years of the NSF MRL program, most projects proposed by well-conceived thrust groups were accepted, replacing single-investigator activity in the MRL.
By the mid-1970s, this new form of endeavor would compete with existing programs for limited core funding. New thrust groups are commonly given
minimal (seed) funding to encourage continued effort and program refinement before full funding. The decision to fund is made by the MRL director in consultation with his advisory committee (and often with the further advice of an external visiting committee). Until 1985 the start-up of a major new thrust group activity began with a three-year grant from NSF after a site visit and further program evaluation. A recent change in NSF management procedure has opened the way for substantial redistribution of funds among MRLs, allowing for rapid response to new opportunities for research by thrust groups.
As the thrust group’s activity develops, several forms of interaction may be found. For example, graduate students and postdoctoral associates who have the same faculty adviser usually work on common problems, some characterizing the structure of a given material, some studying its properties, others studying problems of theory. Group meetings permit interchange of information about progress as well as plans for the future. Major equipment facilities commonly are used for collaborative projects, and in other instances new, specialized laboratories are designed to support the research needs of the thrust group. At their best, thrust groups create an educational environment that differs radically from that of 25 years ago when each student associated almost exclusively with his adviser and peers. Thrust groups produce materials research unlike that carried out at universities even a decade ago when collaborative efforts by more than two investigators were difficult to stimulate and, once stimulated, difficult to fund.
SUMMARY OF MRL ACCOMPLISHMENTS
The current status of the MRL program may be summarized as follows. The NSF budget for the MRL program in FY 1985 was $27 million. MRL awards range from about $0.75 million to nearly $4 million, with the average award at about $2 million. About 60 percent of the budget is spent on thrust group research, 30 percent on facilities, and 10 percent on seed projects. The average number of faculty members participating in an MRL is about 30, with about 6 postdoctoral scholars, 25 graduate students, and 6 technicians. Since their inception in 1960, the MRLs have produced an estimated 3,000 Ph.D.s in materials research, funded primarily by block grants. Since 1972, when NSF took over the program, five new MRLs have been started and seven have been phased out (see Table 1).
In evaluating the research programs of the MRLs, issues of quality and character must be addressed. In its study of the MRL program, the MITRE Corporation evaluated quality using a complex peer review process. Reviewers compared publications of the faculty and students at the MRLs with those from peer non-MRL universities. Significant achievements identified and submitted to MITRE by MRLs were compared with those submitted
from non-MRL “control” schools. The results of this analysis led the MITRE study group to conclude that the quality of research at the MRLs “is high with a greater number of major achievements at MRL’s than at non-MRL institutions.”5
The character of the research at MRLs is more difficult to describe. In the mid-1970s when data were being gathered for the MITRE study, only one graduate-student cycle (4 years) had passed since the introduction of the thrust group concept, and most thrust group efforts were only at their initiation stage. Now, 10 years later, the accomplishments of those thrust groups are the measure of the MRLs’ character and effectiveness. The following list of research accomplishments of the MRLs was compiled in 1984 by Roman J. Wasilewski, head of NSF’s MRL program for 10 years. His intention was to identify those developments that would be difficult or infeasible to achieve under traditional disciplinary project support.1 The list omits numerous accomplishments of comparable caliber in which project support might just as readily have led to their success.
Organic Metals The research field of organic metals opened as the result of the University of Pennsylvania group’s early findings on tetrathiofulvalene-tetracyanoquinodimethane (TTF-TCNQ), which led to an unprecedented degree of collaboration between organic chemists and physicists. Most of the materials initially investigated were not new. Rather, it was the collaboration between researchers in synthesis chemistry and solid-state physics that led to exciting findings. The development of new, related materials followed quickly at the MRL at Northwestern University and elsewhere.
Ultralow Temperatures Cornell University has pioneered in research in the millidegree range for over a decade. Support for this research had originally been requested from—and was declined by—a traditional disciplinary NSF program. The first disciplinary project support was in fact provided only several years after establishment of the MRL at Cornell—after the facilities and the technique had been developed and the first experimental observations of unexpected phase transitions were observed. Since then most of the low-temperature physics aspects of the program have been project-supported, while the parts of the research primarily concerned with phase transitions, which expanded rapidly to metallurgical transitions, remain core-supported.
Lower-Dimensionality Materials The field of lower-dimensionality materials shows how rapidly progress can be achieved by cross-disciplinary involvement. Although materials like liquid crystals and intercalation compounds had long been known, they were of limited scientific interest for decades, viewed as curiosities with little scientific or technological potential. A collaborative MRL program combining research in synthesis and physics developed early at Harvard University and provided a major stimulus to the
field. Similarly, MRL programs on intercalated compounds at MIT and the University of Pennsylvania and on molecularly stacked organic crystals at Northwestern University developed rapidly.
Surfaces and Interfaces With the current availability of sophisticated equipment and techniques, the area of surfaces and interfaces may be viewed as largely disciplinary and no longer requiring cross-disciplinary collaboration. Nevertheless, many of the original techniques, kinds of instrumentation, and approaches were adopted by groups at several of the MRLs. Areas in which major—and at times definitive—contributions came from the MRL surface science programs include the development of ultraviolet spectroscopy at the University of Pennsylvania and Cornell University (the Wisconsin Synchrotron Radiation Center was used); the application of synchrotron radiation to surface studies at Stanford University, where the MRL provided a significant input at the early stages; and the technique for spectroscopic analysis of adsorbed species at Purdue University. These contributions were primarily due to the close though seldom formalized collaboration between chemists and physicists and—equally significantly—between theorists and experimentalists.
Phase Transitions Although phase-transition phenomena have been of major interest to physicists over the last decade, the extension of the classical approaches to other than model materials was initiated at MRLs. The statistical mechanics approach to structural transitions in polyvinyl fluoride, successfully developed at Case Western Reserve University and already largely experimentally verified, has opened a new way of treating “real”—and quite complex—phase-transformation phenomena in polymeric materials. Theoretical calculations of the highly complex process of solidification in welding are now partly verified by research at Carnegie Mellon University and have similarly provided both a new approach and a potential for better control of this technologically important process. Studies of sol-gel (solution-to-gellation) transition across the transition temperature at Brown University and MIT, as well as the studies of spin glasses at the University of Chicago, all benefited markedly from the participation of cross-disciplinary expertise from the earliest stages of the programs.
New Materials According to the MITRE report, MRLs were unique in academia in 1975 in developing significant new materials, and they continue to dominate this field today. To mention only a few of these materials, nonlinear optical crystals of lithium niobate have been developed at Stanford University, and urea crystals at Cornell. Organic metals have been developed at the University of Pennsylvania and Northwestern University, block copolymers have been synthesized and studied at the University of Massachusetts, and highly reproducible fiber-polymer composites have been developed at Case Western Reserve University. Uniquely characterized transition metal oxides have been developed at Purdue University. All of these new materials
represent sophistication in preparation and characterization of relatively common materials. Modulated materials with layer thicknesses varying from 5 to 50 angstroms are now almost routinely prepared at Northwestern University, Stanford University, and the University of Illinois. Quaternary and semimagnetic semiconductors are prepared at the University of Illinois and Purdue University, respectively. Submicron composite materials prepared at Cornell University for possible solar energy applications are some of the more novel, unorthodox materials originating at the MRLs. In each case of the preparation of a new material, the ultimate success demands a sustained collaboration of individuals with expertise in materials synthesis and characterization techniques at a level of sophistication seldom available in traditional disciplinary research.
To this list of accomplishments compiled by Wasilewski, I wish to add the following MRL programs:
-
Mechanical behavior of metals fracture and high-strain-rate behavior (Brown University); fatigue and high-temperature behavior (Northwestern University and University of Pennsylvania); intergranular fracture (University of Pennsylvania)
-
Fabrication of single-crystal optical fibers (Stanford University)
-
Preparation, characterization, and understanding of amorphous materials (MIT, Harvard University, University of Pennsylvania, Stanford University, and the University of Chicago)
-
Rapid solidification (MIT)
-
Polymer science—dependence of crystallization properties on molecular weight (Northwestern University); high-modulus polyethylene (University of Massachusetts)
-
New techniques for nondestructive testing (Stanford University)
The investment by ARPA and NSF in the IDL/MRL program has been substantial; ARPA contributed $158 million to the IDL program between 1960 and 1972, and NSF contributed $261 million to the MRL program between 1972 and 1985. The continued health of the program, the accomplishments in research and education, and the development of the MRL universities as major national resources have justified the continuation of the program in its evolved form.
To look only at the importance of the MRLs to the fields of materials research, however, is to miss some of their most profound effects. The IDLs were among the first examples of an interdisciplinary research center at their respective institutions. Their success and the perceived value of cross-disciplinary research set an example for other faculties and, perhaps even more importantly, for university administrations. Today’s university register is incomplete without its list of study centers—in areas as diverse as information
and technology, design, urban studies, robotics, environment, teaching, art, and history. There may be no cause and effect operating here, but it is certain that the IDL/MRL concept helped pave the way toward reorganization of the university research environment.
Significant in another area, the success of the MRL concept has encouraged new funding patterns by federal agencies. One can point to the significant research opportunity grants that have been made by the Office of Naval Research and those that have gone to the Centers for Super-Computers, the University/Industry Centers, the new Engineering Research Centers, and the new program for Materials Research Groups (MRGs) at NSF. The Engineering Research Centers share some of the same interdisciplinary goals for attacking complex problems that are the central theme of the MRLs. Of the first six Engineering Research Centers, two deal with materials—the Center for Composites Manufacturing Science and Engineering at the University of Delaware and Rutgers University, and the Center for Robotics Systems in Microelectronics at the University of California, Santa Barbara.
The Materials Research Groups, a new program in the Materials Research Division at NSF, create opportunities for multi-investigator efforts by individual thrust groups. In most cases, the universities awarded an MRG do not have an MRL, although MRGs have been established at Purdue, Carnegie Mellon, Pennsylvania State, and Case Western Reserve universities, where the MRLs have been or are being phased out (Table 1). MRGs should expand the variety of cross-disciplinary collaboration and further stimulate the trend toward interdisciplinary organization of the traditional materials departments. The following list of proposed areas of study in the first five MRGs shows the kinds of exciting new programs made possible by the MRG concept:
-
Rensselaer Polytechnic Institute will investigate the various aspects of glass stability—chemical, mechanical, and microstructural—in order to understand the causes of glass degradation and provide a basis for developing more stable glasses.
-
The Polytechnic Institute of New York will launch a program to gain a better understanding of chemical, physical, and processing effects on the aging of polymer blends, an important emerging class of materials.
-
Pennsylvania State University will focus on the molecular engineering of new, chemically bonded ceramics. The materials will be consolidated without resorting to thermal diffusion, relying instead on chemical reactions at relatively low temperatures to cause the bonding.
-
The University of Texas at Austin will seek answers to questions associated with the synthesis of new materials for photoelectrochemical devices and the underlying mechanisms of photochemical processes at interfaces.
-
The California Institute of Technology will develop a program dealing with the motions of atoms and molecules at interfaces and their relationship to the synthesis and characterization of new materials.
The MRGs should be viewed as a logical intermediate stage between the traditional single-investigator research programs and the MRLs. Taken together, the MRLs and MRGs represent an increasing fraction of the budget of the NSF Materials Research Division and demonstrate a recognition of the trend toward greater cross-departmental interaction in materials research. Furthermore, as the project titles indicate, these Materials Research Groups, along with the new Engineering Research Centers, will bring more chemistry and engineering into the NSF group research program in materials.
The concept of block funding was originally viewed as an experiment. The experiment led to radical measures intended to eliminate barriers to the solution of complex problems in the study of materials. It is fair to conclude, 25 years later, that the experiment was successful and that materials science has fared much better than it might have otherwise.
















































