4
Chemical and Physical Transformations of Matter
|
Some Challenges for Chemists and Chemical Engineers
|
GOALS
A principal goal of the chemical sciences is to understand and manipulate chemical and physical transformations of matter. We are using the term transfor-
mation very generally here to mean a change in composition, state, or organization of matter, or movement or rearrangement of material by flow, heat or diffusion, which describes the very essence of chemical science and technology. We need to understand the mechanisms of chemical reactions that we invent, as well as the transformations that occur in living systems and in the environment. Such an understanding will aid in the design of new transformations.
In this domain of transformations of matter, the full scope of the chemical sciences—from basic to applied to technological innovation—is easily seen. For example, it is a fundamental challenge to understand how efficient catalysts, such as nature’s enzymes, are able to perform transformations with very high speeds and selectivities. Such insight would be useful in applied chemistry to design catalysts or conditions for the transformations. Catalysis has been key to a large number of new chemical products and processes in the last half century. Catalytic chemical reactors are the central elements in chemical process systems in which thermodynamic phase behavior, flow, heat transfer, and diffusion all play key roles in performance. Many processes arising in chemical science and technology involve physical transformations in addition to, or even without, chemical reactions, such as phase change, polymer and particulate processing flows, or diffusion in liquids, solids, and membranes. This chapter provides an overview of chemical and physical transformations of matter that are the heart of the chemical science enterprise.
PROGRESS TO DATE
A chemical transformation can occur when molecules collide with sufficient energy. Typically, reaction happens when the molecules are heated or irradiated to provide the energy necessary to overcome the activation barrier that separates reactants from products. A catalyst reduces the magnitude of the activation barrier by changing the pathway of the reaction. As described in Chapters 3, 7, and 10, catalysis is fundamental to biology, to synthesis, to manufacturing, and to energy saving and generation.
Many intermediates along the path of a reaction cannot be directly observed with current instrumental technology, making this objective one of the long-standing goals of the chemical sciences. In 1999, Ahmed Zewail received the most recent of the several Nobel Prizes recognizing developments of methods to follow fast reactions. He was able to witness the bond-breaking and bond-making process on the time scale of 10−15 seconds, which some say is the limiting time scale for chemical reactions. In 1994, George A Olah received a Nobel prize for work establishing the properties of reactive carbon cations, which are intermediates in many important transformations.
Whereas the detailed molecular structures involved throughout the entire procession from starting materials through the transition states to products in a chemical reaction are so far not directly observable, there are certain classical ways in
which reaction mechanisms are effectively deduced. One route is through the use of kinetics, the observation of how the rate of a reaction depends on concentrations of its various components, and on temperature and sometimes pressure. If the rate doubles when either of two reactants is doubled in concentration, we know that both are together in the transition state, while if the reaction speeds up as the pressure is increased we know that the system has a decreased volume at the transition state. Chemical kinetics studies can also facilitate the design of chemical reactors and processes.
A related technique for revealing mechanisms measures the rates of related reactions, using reacting molecules that differ in a small way from each other. A classical approach involves measurement of isotope effects. For example, when a hydrogen atom in a reactant is replaced by a deuterium atom, the isotope with twice the mass, the rate of the reaction will be affected depending on what that hydrogen atom is doing at the transition state. This technique has contributed to our knowledge of mechanisms for a large number of reactions and has been particularly useful in exploring the mechanisms of enzyme reactions. Enzymes will often have such strict geometric requirements that significant changes in the size of the substrate can change the rate for geometric reasons. However, the change from hydrogen to deuterium does not involve such a geometric problem.
One interesting feature of the hydrogen versus deuterium rate effect is that it can be used to detect a very special phenomenon—quantum mechanical tunneling by the hydrogen. In this process, the hydrogen essentially disappears from one position and then reappears a short distance away without traversing the intermediate space. This is possible because all atoms have the character of both a particle and a wave, and waves can diminish in one spot and build up in another without necessarily rolling across the intervening region. Such tunneling has been demonstrated in some enzymatic reactions and invoked to help explain their very high rates. More detailed understanding of quantum mechanical phenomena is essential to a clearer picture of chemical reaction mechanisms and rates.
Evidence on the mechanism of reactions can also come from examining the exact molecular geometry of the overall process, its stereochemistry. For example, if a reactant with defined handedness (chirality) is converted to a product that is an equal mixture of the left- and right-handed forms, the loss of handedness indicates that a particular geometry must have been involved along the path. If a right-handed molecule is converted only to a left-handed product, we say that an inversion of configuration has occurred, and this also tells us a lot about how the reaction occurred.
Chemists are also interested in developing reactions that produce new asymmetric centers within molecules. There is significant interest in the development of new catalysts that produce centers of asymmetry, or handedness, within molecules that have no preferred handedness to start. Simple chiral molecules generated by these asymmetric catalysts are important building blocks for new medicines and research tools.
|
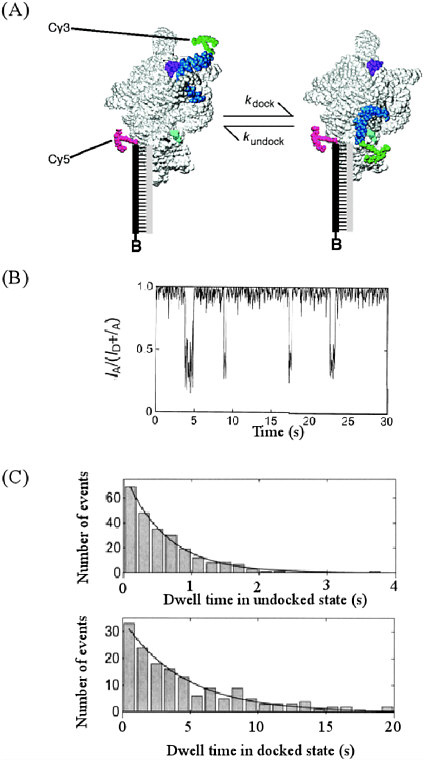
Seeing Chemistry in Motion Chemical processes happen over a range of time scales that challenges our comprehension. Some chemical reactions, such as the change of diamond to graphite under normal conditions of temperature and pressure or the spontaneous conversion of left-handed to right-handed amino acids, are so slow that their rate of change is measured in millions of human lifetimes or longer. Contrast these processes with the direct breaking of a chemical bond when a molecule absorbs light. Such a photodissociation process is so fast that it typically takes 10 to 100 femtoseconds (i.e., between 10 x 10−15 and 100 x 10−15 seconds); this is the time required for light to travel about 3 to 30 microns). As the 20th century came to a close, chemical scientists were delighted that they possessed the tools to make kinetic measurements over the full range of reaction rates. Different changes take place on widely different time scales; consequently, the complex nature of chemical transformations often can be separated into a set of discrete steps. Each step that takes place on a different time scale requires the use of different experimental tools to study it. For example, consider the conformational change and folding of RNA, a key molecule transferring genetic instructions from DNA to build proteins, and a molecule that itself carries out biological transformations. For these molecules conformational changes occur on the time scale of a few seconds. This information has been readily obtained by studying fluorescence resonance energy transfer (FRET) of a single RNA molecule tagged with fluorescent dyes. The energy transfer is a sensitive function of distance between energy donor and acceptor in the molecule. Therefore, by labeling two sites of the RNA by donor and acceptor molecules, it is possible to follow the RNA conformation as a function of time. (A) Conformational change of RNA illustrating motion of the P1 duplex in the Tetrahymena Group I ribozyme. Cy3 dye attached onto P1, and Cy5 attached on the other end of RNA acts as donor and acceptor, respectively. FRET between Cy3 and Cy5 signals conformational change occurring in RNA molecule. (B) FRET time traces from single ribozyme molecules showing P1 docking and undocking. FRET is defined as IA/(ID + IA), where IA and ID are the fluorescence signals from acceptor and donor, respectively. (C) Histograms of the dwell times in the undocked (top) and docked states (bottom) obtained from the FRET time trajectories. The solid lines are single exponential fits of the data giving rate constants for docking (kdock = 1.62 ± 0.08 s-1 ) and undocking (kundock = 0.224 ± 0.015 s-1), respectively. Reprinted with permission from X. Zhuang, L. E. Bartley, H. P. Babcock, R. Russell, T. Ha, D. Herschlag, S. Chu, Science, 288, 2048 (2000). Copyright 2000, American Association for the Advancement of Science. |
|
The binding of calcium ion to calmodulin, a major biochemical regulator of ion pumps and receptors, occurs on a time scale about a thousand times shorter than that observed for RNA conformational change. This Ca2+-calmodulin binding, which can be followed successfully by nuclear magnetic resonance (NMR), occurs in about ten milliseconds. 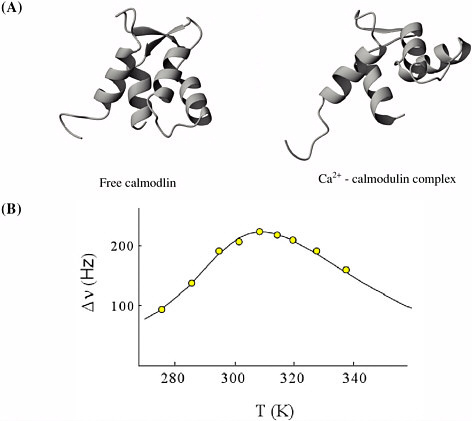 (A) Structures of calmodulin with and without Ca2+ ion binding. Courtesy of Mikael Akke, Lund University, Sweden. (B) Linewidths of 43Ca-NMR spectrum of Ca2+/ calmodulin mixture as a function of temperature (circles), together with best fit to the kinetics (solid line). By analyzing the temperature dependence, the activation barrier is determined. Adapted from T. Drakenberg, S. Forsén, H. Lilja, J. Mag. Res. 53, 412 (1983). Reprinted from Journal of Magnetic Resonance, 53, T. Drakenberg et al., 43Ca NMR Studies of Calcium Binding to Proteins: Interpretation of Experimental Data by Bandshape Analysis, 412-422, (1983), with permission from Elsevier Science. Other chemical changes can be much faster than the RNA conformational changes illustrated here. Photodissociation of sodium iodide (NaI) in the gas phase occurs on the time scale of a few picoseconds (10−12 seconds). To measure this phenomenon, NaI molecules are irradiated by a sub-picosecond ultraviolet pulse of radiation, and the subsequent events are clocked by another short light pulse that detects the newborn |
|
sodium atoms. The illustrations show the time evolution of the Na-atom wave function. 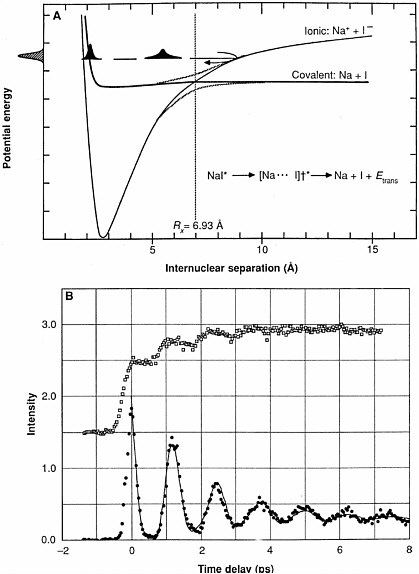 (A) Potential energy curves for the excited states of sodium iodide showing the crossing of the ground state with the excited state (B) Time evolution of free (empty squares), and bound (filled circles) Na fragments observed by laser-induced fluorescence. Motion of the NaI wave packet as a fraction of the molecules cross from the ionic ground state to the covalent repulsive excited state causes oscillations in the observed signals. Reprinted with permission from A. H. Zewail, Science, 242, 1645 (1988). Copyright 1988 American Association for the Advancement of Science. These examples illustrate only a small part of the range of time scales that chemists and chemical engineers examine in their studies of chemical phenomena. By visualizing chemical transformations, chemical scientists obtain a deeper fundamental understanding of how chemical reactions occur and even develop the ability to create favorable conditions to control some of these reactions. |
Usually we talk about reactions in solution, but recently techniques have been developed to follow reactions that occur in a vacuum when a stream of reactant A and a stream of reactant B cross each other in a defined direction, as with molecular beams. From the direction in which the products are ejected and their energies, much fundamental information can be deduced about the details of the molecular processes. Lasers, which emit light-energy in a highly focused beam, are sometimes used to put energy into one of the reactants in a defined way. Such a technique reveals less about the nature of the transition state than about what is called the dynamics of the process—how molecules collide so as to react, and how the products carry away the energy of the overall reaction. The development and application of such techniques were recognized by a Nobel Prize in 1986 to Dudley Herschbach, Yuan Lee, and John Polanyi.
The interaction between experiment and theory is very important in the field of chemical transformations. In 1981 Kenichi Fukui and Roald Hoffmann received a Nobel Prize for their theoretical work on the electronic basis of reaction mechanisms for a number of important reaction types. Theory has also been influential in guiding experimental work toward demonstrating the mechanisms of one of the simplest classes of reactions, electron transfer (movement of an electron from one place to another). Henry Taube received a Nobel prize in 1983 for his studies of electron transfer in inorganic chemistry, and Rudolf Marcus received a Nobel Prize in 1992 for his theoretical work in this area. The state of development of chemical reaction theory is now sufficiently advanced that it can begin to guide the invention of new transformations by synthetic chemists.
Particular interest in recent times has centered on trying to understand the chemical mechanisms by which various biological processes occur. Such complex events as the cleavage of RNA by the enzyme ribonuclease, the multi-step synthesis of ATP in vivo (Paul Boyer and John E. Walker received Nobel prizes in 1997 for working this out), and the activity of molecular motors that power bacterial flagellae are now understood in molecular detail. George Wald received a Nobel Prize in 1967 for discovering the chemical mechanism in the eye by which light is transformed into a signal to the brain that produces vision. The recent sequencing of the human genome has provided a molecular foundation from which other complex biological processes might be tackled at a molecular level.
The basic science of chemical reactions has been put to good use for more than half a century in the fuels, plastics, environmental, and biotechnology fields. One story illustrates well the societal benefit of learning to guide the pathways of chemical reactions, in this case via catalysis and process design. The 1997 Draper Prize of the National Academy of Engineering, the engineering profession’s highest honor, went to Vladimir Haensel for his 1947 invention of platinum-catalyzed reforming of petroleum. The prize citation explains how, while working for Universal Oil Products Co. (now called UOP), Haensel sought to improve the process by which gasoline was produced from crude oil; thermal cracking of petro-
leum over a clay catalyst yielded only modest amounts of very low-octane gasoline. Haensel proposed using platinum as a catalyst for the refining process, despite the challenges posed by the expense and availability of platinum. He developed a simple method that was efficient in producing more gasoline from the same amount of petroleum and that also produced a remarkably higher-grade fuel. Platinum reforming produces vast amounts of aromatic hydrocarbons, the raw materials used in the manufacturing of plastics and synthetic fibers such as polyester. Platinum-catalyzed reforming has reduced U.S. reliance on foreign oil, broadened the world’s long-term energy outlook, helped reduce pollution due to combustion, and saved billions of dollars in transportation costs. These are revolutionary effects on energy, transportation, materials production, and our environment.
For examples such as platinum reforming, designing the reactors is as essential for technological success as is detailing the chemistry. The chemical knowledge and new catalysis are embedded in the process, but other factors are necessary as well. These include transporting materials through the process equipment, mass transfer between the fluid and catalyst, controlling pressure and its effects on physical properties and phase behavior, and mixing and separation processes. Advances in these physical aspects of transformation processes have been essential to modern chemical manufacturing. Effective technological implementation requires integrated knowledge and use of chemical and physical transformation fundamentals.
Important physical transformations between states of matter, not necessarily involving chemical reaction, can occur on changes in temperature or pressure or application of external forces or fields. Such phase transitions have been central to quantitative research in chemical sciences, at least since the time of Robert Boyle in the mid-17th century and, in a more modern context, Sadi Carnot, R.J. Clausius, and J. Willard Gibbs in the 19th century. Suitable choices or changes in temperature, pressure, and other controllable properties can produce abrupt changes (first-order transitions) in the state of a substance—for example, boiling or freezing, or formation of systems having two phases coexisting with distinct phase boundaries between them.
Equations of state relate the phase properties to one another and are an essential part of the full, quantitative description of phase transition phenomena. They are expressions that find their ultimate justification in experimental validation rather than in mathematical rigor. Multiparameter equations of state continue to be developed with parameters tuned for particular applications. This type of applied research has been essential to effective design of many reaction and separation processes.
The last several decades have seen the growing scientific importance of phenomena near critical points, those conditions of system properties where two coexisting phases, such as liquid and vapor, become identical. This is exemplified by the 1982 Nobel Prize awarded to Kenneth Wilson for his theoretical studies of
critical phenomena and continuous or second-order phase transitions. Supercritical fluid processing is exploiting knowledge about this domain of phase behavior for new applications in separations, chemical reactions, and materials production. Supercritical carbon dioxide is now used as a solvent in improved, environmentally benign methods for decaffeination of coffee and for dry cleaning (Chapter 9).
Phase behavior in complex fluids such as polymer blends and block copolymers has been a rich area of the chemical sciences. Near-critical and other transitional phenomena are frequently prominent. Since molecular movement in viscous systems such as these is comparatively slow, phase transitions can be studied more easily in time, and manipulated by quenching and other external influences. Processes for controlled growth of ordered materials are often readily influenced by diffusion, a variety of external fields, and the influence of interacting boundaries, or flow.
Flow processes in materials that contain structural elements, such as polymers and suspensions, can influence important physical and chemical transformations. Thinning or thickening of fluids under shear flow, structural rearrangements that affect physical properties, and flow of granular media are just a few examples where flow produces major changes in a chemical product, often permanent and sometimes helpful. Alignment of polymer molecules in fibers and films is essential to produce high strength. For example, ultra-high molecular weight polyethylene fibers, which appear gel-like when produced, are used in bulletproof vests. Arranging the fibers to cross at 0 degree and 90 degree angles causes the material to have excellent protective properties. Paints, coatings, inks, toners, and adhesives often contain particulate additives precisely for the purpose of producing interactions that respond to the imposition of flow. The science of these interactions is advancing rapidly, through theory and experiment. Extending molecular theories to the length scales of particles has produced detailed descriptions of the behavior of hard spheres, polymer-coated particles, and associating colloids. Experimental insight has come from rheology (the study of the changing form and flow of matter), light scattering, and microscopy, often using national facilities for synchrotron x-rays and neutrons. Frequently systems can be observed under flow to illuminate the “transition states” of these physical transformations.
The importance of physical transformations in making chemical products implies a vast opportunity for understanding structures of many kinds of important chemical products at higher levels and longer length-scales than those of individual molecules. Structured chemical products will be a rapidly growing area, particularly with the investments of financial and intellectual resources that are currently being made in nanoscale science and engineering. In the past 15 years we have seen an accelerating explosion in the ability to formulate, characterize, and predict phase behavior and the resulting transport processes for colloids, surfactant solutions, and block copolymers in particular. Control over syn-
thesis and interparticle forces has allowed selective addition of nonadsorbing, associative, and diblock copolymers, and external electric and magnetic fields have been used to control equilibrium phase behavior and rheology.
Chemists have synthesized a spectacular array of submicron- and nano-particles with well-defined size and atomic structure and very special properties. Examples include CdSe quantum dots and novel spheres and rods. Transport enters the picture via fundamental studies of the physical processes that affect the synthesis, which must be understood for even modest scale-up from the milligram level. Likewise, processes for assembling fascinating face-centered-cubic crystals or ordered multilayers must concentrate on organizing the particles via flow, diffusion, or action of external fields. Near-perfection is possible but requires careful understanding and control of the forces and the rates.
Block copolymers consist of two or more sections of polymer, of which each section is made of a single type of monomer. Block copolymers are representative of a class of structured chemical products where physical chemistry and synthetic chemistry must go hand in hand to create the desired product. The vast effort invested in copolymer microphase separation has recently spawned a number of novel efforts. Studies of the relationship between molecular architecture, ordered-phase morphology, and mechanical properties have shown that multi-block copolymers offer special advantages in product properties that result from the molecular connectivity over the multiple domains that they create. Equally important to mechanical properties are the new transport properties and membrane separation capabilities that structured, composite chemical products can deliver. Another example is the development of block copolymer nanolithography. Ordered monolayers with spherical or cylindrical morphologies are cast, and the minority phase is then removed to permit etching or deposition through the gaps in the remaining continuous phase. The results are holes or posts with lattice spacings on the order of 10 nm over remarkably large areas. In both these examples, transport processes limit the rate of annealing due to thermal motion, but when driven by flow or external fields, may conspire to provide even longer-range order. Application of external fields in physical transformations produces effects analogous to those of catalysis in chemical reactions—enhancing the rates and selecting the products. Transport phenomena play crucial roles in both the creation and performance of structured chemical products. This idea has little parallel in mechanistic studies of reactions to synthesize simpler molecular chemical products.
Transport phenomena present an important challenge in the progression toward smaller length-scales of microscale, nanoscale, and molecular scale. Chemical processing will need to exploit small-scale reactors in new applications. Understanding chemical and physical transformations at these length-scales will be necessary for advances in microfluidics, for progress in developing new chemical and biological sensor applications, and for realizing the promise of self-assembly as a chemical processing method. Molecular models of structured fluids (poly-
mers, suspensions, composites), validated in part by single-molecule mechanical measurements, will be crucial to the growth in this area.
CHALLENGES AND OPPORTUNITIES FOR THE FUTURE
How can we obtain direct information on the molecular details of a reaction path and harness this for the design of new processes? While femtosecond spectroscopy has made it possible to observe reactions on a short time scale, a major challenge is to devise ultrafast techniques, such as superfast electron diffraction, that will permit observation of the actual molecular structure of a transition state, not just its rate of passage. As a general goal, we want to be able to make moving pictures of the reactions themselves, observing all the intermediate states and the rates at which they interconvert. Such moving pictures can be generated even now, by computer simulation of the reaction; the problem is to determine whether those pictures are correct. Thus a second challenge is to interface with theoretical chemistry in getting the best possible calculations, and then devise experimental tests of the major theoretical predictions to see whether experiments confirm the correctness of the calculations.
Most mechanistic work has focused on chemical reactions in solution or extremely simple processes in the gas phase. There is increasing interest in reactions in solids or on solid surfaces, such as the surfaces of solid catalysts in contact with reacting gases. Some such catalysts act inside pores of defined size, such as those in zeolites. In these cases only certain molecules can penetrate the pores to get to the reactive surface, and they are held in defined positions when they react. In fact, the Mobil process for converting methanol to gasoline depends on zeolite-catalyzed reactions.
There is also increasing interest in reactions involving organometallic compounds, at the interface between organic and inorganic chemistry. Many such reactions are useful in synthesis, where the organometallic reagents can have important properties as catalysts. Many details of reaction mechanisms in organometallic chemistry are yet unclear; understanding these mechanisms will allow the development of improved catalysts.
Much work has been done to help understand how metal ions react or catalyze reactions in solution. Many enzymes also use bound metal ions to catalyze their reactions, and there is still need to understand how they work. When we do understand them in detail, we should be able to produce biomimetic catalysts for useful processes in manufacturing.
When molecules react thermally, at room temperature or on heating, they are in their lowest electronic states. However, when reactions occur on irradiation of the molecules with visible or ultraviolet light, the processes involve species in electronic excited states. Some of the details of such processes are known, but there is still much to do. Since photoexcitation is important in many areas— photosynthesis, photography, electronic displays, solar cells, cancer-causing ul-
traviolet interactions with the skin, identification of interstellar molecules, degradation of materials and dyes by sunlight—it is important to investigate these processes. As part of understanding the chemical universe with all the substances and transformations that are possible, or at least learning their major features, basic chemical science needs to understand transformations of excited states, not just of ground states.
The design of multicomponent processes remains an important challenge. Conventional process development has focused on single reactions and their optimization. New processes that involve reaction cascades, where the product of one reaction feeds the next, will permit more efficient production of industrial or biomedical products. A detailed understanding of biological metabolic networks will facilitate the design of similarly complex artificial systems. Computational analysis of such interconnected reaction networks will be important for reaction modeling and design.
It is a continuing challenge to understand the properties of enzymes. They are usually very effective catalysts, able to speed up reactions of many biological substances in both the forward and reverse directions. Also, their activities can be modified by inhibitors that bind to them, and many medicines such as cholesterol-reducing drugs function as enzyme inhibitors. To some extent the details of the catalytic mechanisms of enzymes have been elucidated, or at least guessed at. However, there is not yet a real understanding of the factors that contribute to enzymes’ high effectiveness, and we do not yet have good examples of synthetic analogs of enzymes that catalyze the same reactions with the same or better speeds and selectivities. A full understanding of enzymes will enable the introduction of synthetic catalysts, which will be of great value in manufacturing. The introduction of synthetic catalysts may also contribute to the development of a new class of medicines. Medicines today operate mainly by binding to various biological molecules, but they don’t carry out catalyzed reactions in the body. If we fully understand enzymes well enough to be able to make new catalysts of similar effectiveness, such artificial enzymes could be an exciting class of molecular machines and medicines. They could destroy undesirable species or toxins, or promote the formation of materials that are present in insufficient quantities due to some diseases. We are also learning how to recreate the process of evolution through natural selection in a test tube.1 New enzymes can be evolved by applying selective pressure for improved properties or even for totally different functions.
By manipulating genes, chemists have learned to create new enzymes with new functions. To do this rationally and predictably, it is critical to understand the structures and mechanisms of the genes that are being modified. Enzymes and other biological components often function in clusters, in multicomponent sys-
tems. Thus even when we understand fully the chemical properties of a pure enzyme, a challenge remains—to understand the properties of interacting molecules, including enzymes, assembled into well-defined structures.
WHY ALL THIS IS IMPORTANT
Any list of applications such as the examples presented in this chapter is always too limited. It is more realistic to say that there are still big gaps in our understanding of the molecular details of chemical and biochemical reactions. The chemical universe is filled with transformations, both natural and invented. We apply them in manufacturing, we admire them in biological chemistry, but we do not yet know enough about their details. With understanding, when we have it, will come greatly improved methods for synthesis and manufacturing. Increased understanding of the details and special character of transformations will also lead to applications not yet even thought of, as has happened with every big advance in scientific understanding.