2
Spectrum of Microbial Threats
Microbial threats to health are microbes1 that lead to disease in humans. The challenges posed by microbial threats to health are daunting. Most developing nations have not shared fully in the public health and technological advances that have aided in the fight against infectious disease in the United States—a fight that some had hoped would come close to eliminating these threats in this country (see Box 2-1). In developing countries, clean water is scarce; sewage systems are overwhelmed or nonexistent; the urban metropolis is growing exponentially as the global market economy expands and rural agricultural workers migrate to cities; and economic need, political conflict, and wars are displacing millions of people and creating growing refugee populations (see Chapter 3). Thus, infectious diseases affect poorer nations in the developing world disproportionately, and from thence become a global burden. Infectious diseases are responsible for one in every two deaths in developing countries—and are the leading cause of death for children and young adults (WHO, 1999a, 2000a). It has been estimated that every hour, 1,500 people die from an infectious disease—over half of them are children under 5 years of age. Ninety percent of deaths from infectious diseases worldwide are due to respiratory infec-
|
BOX 2-1 The End of Infectious Diseases in the United States? At the start of the twentieth century, tuberculosis, pneumonia, and diarrheal diseases caused 30 percent of all deaths in the United States. Infectious diseases resulted in such high mortality among infants and children that the average life expectancy at birth in the United States was only 47 years in 1900 (NCHS, 2001). During the first few decades of the 1900s, improved hygiene and sanitation, cleaner water and safer food, improved housing and nutrition, and advanced vector control led to a significant decline in infectious disease mortality in the United States. With the introduction of antibiotics in the middle of the century, the downward trend accelerated even further. Deaths from infectious disease, particularly tuberculosis and pneumonia, declined more than 8 percent each year from 1938 to 1952 (Armstrong et al., 1999). By the mid-1960s, effective pertussis, polio, smallpox, tetanus, and diphtheria vaccines had become widely available. As a result, deaths from numerous infectious diseases were prevented. For example, the number of cases of paralytic poliomyelitis in the United States dropped from more than 57,000 in 1952 to only 72 in 1965 (CDC, 2002a) and the last case of smallpox was documented in the United States in 1949 (CDC, 2002b; IOM, 1999a). These exhilarating accomplishments of the mid–twentieth century were fueled by an unprecedented outlay of government resources, a vast network of field epidemiologists and research scientists, and extensive cooperation from private industry (Garrett, 1995). The eradication of smallpox in 1980 was a testament to the success of comprehensive vaccine coverage, and indeed has been praised by many as the greatest achievement in public health history. From 1900 to 1980, annual deaths from infectious disease had dropped from 797 to 36 per 100,000 persons (Armstrong et al., 1999). By the turn of the 21st century, the average life expectancy in the United States had increased to over 76 years. As a result of this apparent reprieve from infectious diseases, the United States government moved research funding away from infectious diseases toward the “new dimensions” of public health—noncommunicable disorders such as heart disease and lung cancer. The government closed “virtually every tropical and infectious disease outpost run by the U.S. military and Public Health Service” (Garrett, 1989, p. 1). Infectious disease surveillance and control activities were deemphasized. Research, development, and production of new antibiotics and vaccines declined. The potentially devastating impact of infectious diseases was either relegated to the memory of previous generations or left to the imagination of science fiction enthusiasts. Americans could all |
tions, acquired immunodeficiency syndrome (AIDS), diarrheal diseases, tuberculosis (TB), malaria, and measles (see Table 2-1).
This chapter begins by reviewing the global burden imposed by three of today’s most devastating infectious diseases: AIDS, TB, and malaria. Other emerging infectious diseases and antimicrobial-resistant infections are discussed in the subsequent two sections. Chronic diseases with infectious etiology are then reviewed. The chapter ends with a brief discussion of microbes potentially used for intentional harm.
|
look forward to long, healthy lives, free from infectious disease … or could they? The figure below suggests quite otherwise. 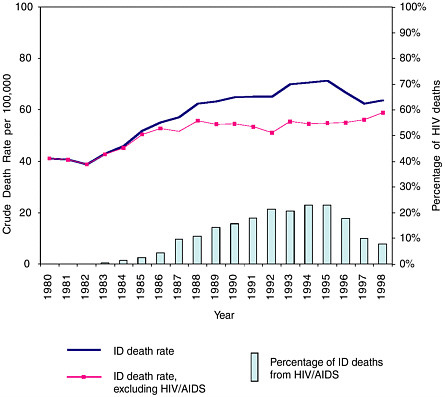 Trends in infectious disease and HIV deaths in the United States: 1980–1998. Reprinted with permission from Pinner RW, Roy K, Shoemake H. Mortality from Infectious Diseases in United States, 1993–1998. Unpublished Manuscript, 2002. SOURCE: NCHS underlying cause of death data. |
THE GLOBAL BURDEN OF AIDS, TUBERCULOSIS, AND MALARIA
Efforts to reduce the global burden of infectious diseases must concentrate on AIDS, TB, and malaria. Combined, these three diseases could account for 500 million or more illnesses a year and at least 6 million deaths (WHO, 2002a).
TABLE 2-1 Leading Infectious Causes of Death Worldwide, 2001
|
Cause |
Rank |
Estimated Number of Deaths |
|
Respiratory infections |
1 |
3,871,000 |
|
HIV/AIDS |
2 |
2,866,000 |
|
Diarrheal diseases |
3 |
2,001,000 |
|
Tuberculosis |
4 |
1,644,000 |
|
Malaria |
5 |
1,124,000 |
|
Measles |
6 |
745,000 |
|
Pertussis |
7 |
285,000 |
|
Tetanus |
8 |
282,000 |
|
Meningitis |
9 |
173,000 |
|
Syphilis |
10 |
167,000 |
|
SOURCE: WHO, 2002b. |
||
Acquired Immunodeficiency Syndrome
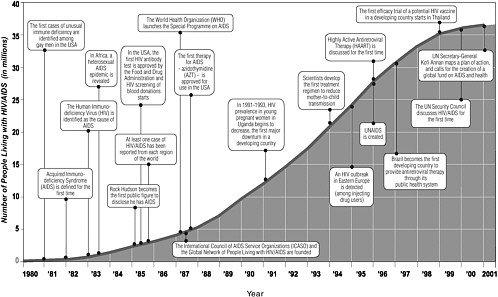
In less than 20 years, AIDS has become a pandemic requiring an unprecedented global response (see Figure 2-1) (UNAIDS and WHO, 2001). More than 60 million people have been infected with the human immunodeficiency virus (HIV) worldwide, and 20 million have died from AIDS, leaving an estimated 40 million adults and children living with HIV. Roughly 14 million children are living bereft of one or both parents who died from the disease. In 2001 alone, it is estimated that 5 million people became HIV-positive worldwide, 800,000 of them children (UNAIDS, 2002). Nearly one-third of those living with HIV/AIDS—11.8 million—are between 15 and 24 years of age (UNAIDS, 2002). Specific projections of the number of anticipated HIV/AIDS cases are difficult because the incidence of HIV infection is declining in some populations and increasing in others, HIV-testing continues to be voluntary, and reporting may be incomplete. Generally, the number of cases is expected to rise in areas where poverty, poor health systems, poor access to health care services, and gender inequality are prevalent; where resources for health care and prevention are limited; and where a high degree of stigma and denial is associated with HIV infection (Monitoring the AIDS Pandemic Network, 2000).
In 2001, the highest incidence of HIV/AIDS worldwide was in Africa, where an estimated 3.5 million adults and children were newly infected with HIV in that year alone (UNAIDS, 2002). Of the 40 million people living with HIV/AIDS worldwide at the end of 2001, 28.5 million were in sub-Saharan Africa (UNAIDS, 2002). The majority of adults living with HIV/AIDS in Africa are women under 25 years of age, whose infection rates are astonishingly high. At the end of 2001, mean HIV prevalence rates for
females aged 15 to 24 in sub-Saharan Africa ranged from 6.4 to 11.4 percent, as compared with 1.0 to 1.8 percent for women of similar ages around the world (UNAIDS, 2002). Ignorance of AIDS is widespread among young people, who are at the greatest risk. Half of all teenage girls in sub-Saharan Africa do not know that a healthy-looking person can be living with HIV/AIDS. A study in Mozambique found that 74 percent of girls aged 15–19 were unaware of any means to protect themselves from infection (WHO, 2002a).
An estimated 1.9 million people were living with HIV in Latin America and the Caribbean in 2002 (UNAIDS, 2002), making it the second most affected region of the world. Recent reports suggest that India, China, Russia, Ethiopia, and Nigeria each are on the cusp of an exploding epidemic. Together, these countries will account for 50 million to 75 million cases of HIV infection by 2010 (National Intelligence Council, 2002). In northeast India, the widespread use of illicit injection drugs accounts for most HIV transmission; in other parts of the country, most cases of infection appear to have been acquired through contact with infected sex workers. By the mid-1990s, approximately one-fourth of the prostitutes in cities such as New Delhi, Hyderabad, Madurai, Pune, Tirupati, and Vellore tested positive for HIV (UNAIDS, 2000). China has recognized these same risky behaviors—intravenous drug use and prostitution—as the primary modes of HIV transmission in that country (Monitoring the AIDS Pandemic Network, 2000). The number of HIV-infected persons in Russia has increased progressively, virtually doubling every year from 1993 to 1998, mainly as a result of an increase in intravenous drug use (Netesov and Conrad, 2001). Furthermore, it is estimated that the official statistics on HIV in Russia may reflect only 10 to 20 percent of the actual number of carriers, since many patients treated in private clinics are not officially reported.
Most infectious diseases have severe consequences at the two ends of the spectrum of life: infants/children and the elderly. In contrast, HIV is spread predominantly among young adults, who represent the most economically active segment of the population (United Nations Economic Commission for Africa, 2000); thus HIV infection dramatically changes a country’s demographics. AIDS is erasing decades of progress made in extending life expectancy; average life expectancy in sub-Saharan Africa is now 47 years, whereas it would have been 62 years without AIDS (UNAIDS, 2002). Of the 14 million orphans resulting from the AIDS pandemic, 80 percent reside in sub-Saharan Africa (UNAIDS, 2002). By 2010, nearly 42 million children in 27 countries are expected to have lost one or both parents to AIDS (National Intelligence Council, 2000). Many countries in South and Southeast Asia are expected to undergo similar demographic changes as HIV/AIDS and associated diseases (e.g., tuberculosis) reduce
human life expectancy (National Intelligence Council, 2000). The vast majority of HIV-infected people are unaware they are infected (WHO, 2002a).
The estimated number of deaths from AIDS in the United States decreased from more than 45,000 in 1993 to less than 17,000 in 1999, largely as the result of access to improved therapies for treating opportunistic infections, the development of antiretrovirals, and decreased perinatal transmission through the diagnosis and treatment of HIV-positive pregnant women (CDC, 2000a). The extent of the decrease varied among different demographic risk groups and was most dramatic from 1993 to 1997; since 1997, however, the annual count of deaths from AIDS in the United States has stabilized or increased slightly. The AIDS epidemic in the United States is not disappearing by any means; rather, it is becoming concentrated in populations who lack easy access to prevention programs and health care services (diagnosis and treatment), including racial and ethnic minorities, women, and the poor (Karon et al., 2001). In addition, increases in unprotected anal sex and in sexually transmitted diseases (STDs) in major U.S. cities among men who have sex with men indicate the potential for the resurgence of HIV infections in this population (Wolitski et al., 2001). Over half a million persons were living with HIV/AIDS in the United States as of December 2001, including the 43,000 new cases that were reported in 2001 (CDC, 2001a).
Tuberculosis
Whereas AIDS emerged only within the latter half of the twentieth century, infection with Mycobacterium tuberculosis has a millennia-long history as a human disease. Spinal column fragments of Egyptian mummies have provided evidence that tuberculosis has been killing humans for at least 3,000 years (Morse et al., 1964; Nerlich et al., 1997). In the early 1600s, the disease grew to epidemic proportions in Europe as cities expanded and human population densities increased. Improved socioeconomic conditions, improved public health services, and the effectiveness of the antituberculosis therapies developed in the mid-twentieth century maintained a steady decline in TB through the early 1980s.
The decreased public health focus on TB in the United States that occurred toward the end of the twentieth century, combined with increasing rates of homelessness and drug abuse, the growing HIV/AIDS epidemic, and increasing immigration rates from countries with high TB prevalence, led to a resurgence of the disease in the United States that peaked in 1992. Transmission of pulmonary TB frequently occurred within institutions such as hospitals, correctional facilities, residential care facilities, and homeless shelters. With the reinstatement of federal funding in 1992, improved casefinding, and the implementation of directly observed therapy (DOT),
the prevalence of TB in the United States decreased 39 percent from 1992 to 2000 (Bloom, 2002). Today, the majority of TB cases in the United States are among foreign-born persons.
Roughly 2 million people die each year from TB worldwide (WHO, 2002c), with the vast majority of these deaths (98 percent) occurring in developing countries (Mukadi et al., 2001). In 2000, approximately 8.7 million new TB cases were reported, of which an estimated 3 to 4 percent were multidrug-resistant (Jaramillo, 2002). In most countries, the average incidence of TB has recently been increasing approximately 3 percent per year; however, the increase is much higher in Eastern Europe (8 percent per year) and those African countries most affected by HIV (10 percent per year). Twenty-three countries account for 80 percent of all new TB cases. In 2000, over half of these cases were concentrated in five countries: India, China, Indonesia, Nigeria, and Bangladesh. Although Zimbabwe and Cambodia report fewer total cases, they possess the highest global rates per 100,000 population (562 and 560, respectively) (WHO, 2001a). If present trends continue, more than 10 million new cases of TB are expected to occur in 2005, mainly in Africa and Southeast Asia; by 2020, nearly 1 billion people will be newly infected, 200 million will develop the disease, and 35 million of them will die (WHO, 2002a).
The global resurgence of TB is not confined to developing countries. From 1990 to 1995, TB rates in Russia increased by 70 percent, with more than 25,000 persons dying from the disease each year (Netesov and Conrad, 2001). The increased incidence is compounded by the spread of multiple drug-resistant TB (MDR-TB), especially in prisons, where patients typically self-administer treatment. Because most prison clinics experience massive shortages of drugs, most patients are unable to complete their full course of treatment, thus fostering the emergence of MDR-TB. Indeed, the rate of MDR-TB among TB isolates in Russian prisons is an astonishing 40 percent, compared with 6 percent in the general population. The overall rate of TB per capita in prison populations (i.e., including both MDR-TB and other forms of the disease) is nearly 100 times higher than in the Russian population at large.
TB is the leading cause of morbidity and mortality among HIV-infected people worldwide (Mukadi et al., 2001), who are at greater risk of developing the disease (Wood et al., 2000). In 1995, approximately one-third of HIV-infected people worldwide were also coinfected with M. tuberculosis; the vast majority of these cases were in sub-Saharan Africa (Harries and Maher, 1996). The incidence and case-fatality rate (i.e., the proportion of patients who die among those diagnosed) for TB in sub-Saharan Africa has increased dramatically since the HIV epidemic first began (Mukadi et al., 2001). In some sub-Saharan countries, the case-fatality rate for HIV-posi-
tive pulmonary TB patients can exceed 50 percent (Dye et al., 1999; Mukadi et al., 2001).
Malaria
Malaria, caused by plasmodia parasites, is responsible for 300–500 million clinical cases and 1.5–2 million deaths each year (Bloland, 2001). Malaria is the most prevalent vector-borne disease and is endemic in 92 countries (Martens and Hall, 2000). It disproportionately affects rural populations living in housing without screens and doors, children under 5 years of age, and pregnant women. Africa accounted for nearly 90 percent of new cases reported worldwide in 1998; of these, 40 percent occurred in children under 5 years of age (WHO, 1999b).
Nearly all people who live in endemic areas are repeatedly exposed to mosquitoes that carry the infective agent, and those who survive malaria develop partial immunity. Endemic areas are subject to irregular rapid increases in incidence as the warm seasons arrive, rainfall and humidity increase, and populations migrate (IOM, 1991; WHO, 1999b). In areas where the infection rate is low and people are rarely exposed to the disease, however, the population is generally much more susceptible to the devastation of epidemic malaria—and the number of malaria epidemics is growing worldwide. Between 1994 and 1996, malaria epidemics in 14 countries of sub-Saharan Africa caused a high number of deaths, many in areas previously free of the disease (Nchinda, 1998). Drug resistance has been implicated as a contributing factor in the spread of malaria to new areas and the reemergence of the disease in areas where it had previously been eliminated, leading to increased morbidity and mortality (Bloland, 2001).
In 1999, the Centers for Disease Control and Prevention (CDC) received 1,227 reports of malaria cases with onset of symptoms in 1998 among persons in the United States and its territories; 98 percent of these cases were classified as imported, primarily from Africa (60 percent), Asia (20 percent), and the Americas (19.1 percent) (Holtz et al., 2001). Western European countries are reporting similar statistics for imported malaria (Fayer, 2000). Between 1990 and 1996, malaria increased as much as 100-fold in certain southern regions of the former Soviet Union; more recently, it has begun to emerge even as far north as Moscow (Fayer, 2000). Only a few isolated cases or small outbreaks have occurred in the United States, in areas where individuals with imported disease have provided a reservoir of infection for local-vector mosquitoes that have subsequently transmitted the infection to persons from that locality (Olliaro et al., 1996). However, increasing global travel, immigration, and the presence of competent anopheline vectors throughout the continental United States all contribute to the growing threat of malaria transmission even in nontropical North
America, as well as other temperate regions of the world. Indeed, two cases of locally acquired malaria were recently discovered in Loudon County, Virginia, 30 miles from Washington, D.C. (CDC, 2002c)
EMERGING INFECTIOUS DISEASES
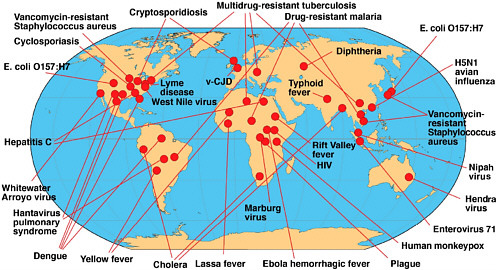
The emergence of a microbial threat is a phenomenon in which something has changed—either our perception of a microbial threat, our recognition of a threat, or the true biological expansion of a microbe. An emerging infectious disease is either a newly recognized, clinically distinct infectious disease, or a known infectious disease whose reported incidence is increasing in a given place or among a specific population. As illustrated in the previous section, HIV, TB, and malaria are certainly emerging infections, even though the latter two diseases have been around for centuries. Figure 2-2 and Table 2-2 provide examples of several emerging infectious diseases identified by scientists in the final decades of the twentieth century. These examples include some diseases that have been known for decades, but have reemerged in new geographic locations and/or in newer, more deadly, drug-resistant forms. These and other examples of emerging infectious diseases, including STDs, nosocomial infections, and vector-borne and zoonotic diseases, are discussed in Chapter 3, along with the major factors in their emergence.
We will inevitably see more emerging infections in the future as the factors that lead to emergence become more prevalent and converge with increased frequency. We can only guess at how many more of the microbes in the environment will eventually be found as human pathogens. Even small, isolated events cannot be readily dismissed because of their potential to expand with time. After all, when the initial handful of cases of what would later be termed AIDS first appeared, few could foresee that their affliction would soon become a global catastrophe, threatening the security of entire nations.
ANTIMICROBIAL-RESISTANT INFECTIONS
Antimicrobial resistance is a paramount microbial threat of the twenty-first century. With the presence of antimicrobial resistance may come a corresponding increase in mortality and morbidity from untreatable disease, an increased risk of the global spread of drug-resistant pathogens, a rise in the health care costs associated with the need for multidrug therapy and longer and more frequent hospital stays, and the costs of research and development of alternative drugs. For example, efforts to control each of the three major global infectious diseases discussed earlier—AIDS, TB, and malaria—are seriously thwarted by the rise of antimicrobial resistance.
TABLE 2-2 Examples of Recent Emerging and Re-Emerging Infectious Diseases
|
Disease/Agent |
Mode of Transmission |
Comment |
|
Bacteria |
||
|
Anthrax (Bacillus anthracis) |
Inhalation of spores; via skin contact with contaminated tissues or materials; ingestion of contaminated food. |
Primarily an infection of animals; long-term persistence in contaminated soil or environment; agent of bioterrorism. |
|
Cholera (Vibrio cholerae O139) |
Ingestion of bacteria in contaminated food or water. |
Emerged in Asia in 1992–1993 in areas with poor sanitation; has caused large outbreaks in India and Bangladesh; prior infection with V. cholerae O1 does not protect against O139; ongoing genetic reassortment in O139. |
|
Diphtheria (Corynebacterium diphtheriae) |
Close contact with person who has diphtheria or who carries Corynebacterium diphtheriae. |
Massive outbreak (>150,000 cases) in the former Soviet Union countries and Mongolia in 1990s at time of decreased vaccination, declining socioeconomic conditions, and breakdown in public health infrastructure; sporadic outbreaks elsewhere in world. |
|
Escherichia coli O157:H7 hemorrhagic colitis (severe bloody diarrhea and kidney failure) |
Ingestion of contaminated food or water; can be spread from person to person via fecal-oral route. |
Healthy cattle are primary reservoir (carried in feces); bacteria survive in acidic environment; small inoculum of bacteria can cause infection; sporadic cases and large outbreaks in U.S. and other countries; vehicles of transmission have included meat, milk, fresh produce, cider, contaminated water ingested during swimming, other; mass processing and wide distribution of contaminated foods has led to widely dispersed outbreaks. |
|
Lyme disease (Borrelia burgdorferi) |
Bite of infective tick. |
Zoonosis; rodents and deer maintain transmission cycle; common in parts of North America, Europe; also found in Asia, Australia; increase in human cases attributed to reforestation and expansion of deer populations; increased human-tick contact. |
|
Plague (Yersinia pestis) |
Bite of infective flea; inhalation of airborne bacilli; close contact with infected animal or tissues. |
Primarily a zoonosis; rodents are reservoir host; most sporadic cases and outbreaks occur in Africa but infections also occur in the Americas (including the U.S.), Asia, and Europe; outbreak in India in 1994; possible agent of bioterrorism. |
|
Staphylococcus aureus, vancomycin-resistant |
Contact with infected person. |
First emerged in U.S. in 2002; consequence of intensive use of antimicrobials; can be spread from patient to patient in health care settings; risk of spread into the community. |
|
Protozoa |
||
|
Cryptosporidiosis (Cryptosporidium parvum) |
Fecal–oral spread; may be food or water borne. |
Massive outbreak in Milwaukee in 1993 (estimated >400,000 cases) linked to contamination of municipal water supply; oocysts are resistant to chlorine and other chemicals used to purify drinking water; infection may be severe in persons with AIDS or otherwise immunocompromised because of drugs or disease. |
|
Cyclospora (Cyclospora cayetanensis) |
Ingestion of contaminated food or water. |
Multiple outbreaks in North America in 1990s linked to imported raspberries from Guatemala; endemic in many countries. |
|
Malaria |
Bite of infective mosquito. |
Increasing morbidity and mortality in many areas, especially in Africa; increase linked to poor vector control and rising resistance to inexpensive antimalarial drugs, lack of resources for other drugs and other control measures. |
|
Viruses |
||
|
Dengue fever (and dengue hemorrhagic fever and shock syndrome); dengue viruses, serotypes 1,2,3,4 |
Bite of infective mosquito, usually Aedes aegypti. |
Found in most tropical and subtropical areas worldwide, including urban areas; outbreak in Hawaii in 2001–2002; epidemics are increasing in size and severity, especially in Asia and Latin America; factors in worsening situation include poor mosquito control, abundant mosquito breeding sites in growing tropical cities, travel of humans who carry the virus, and wide circulation of more than one serotype of virus. |
|
Disease/Agent |
Mode of Transmission |
Comment |
|
Ebola hemorrhagic fever |
Spread from person with acute infection by contact with blood, secretions, or other material. |
Repeated outbreaks with high mortality in sub-Saharan Africa; secondary spread of infection has occurred in health care settings and in households in Africa; reservoir for the virus not yet identified. |
|
Enterovirus 71 |
Direct contact with material from infected persons (nose/ throat discharge or droplets, feces). |
Multiple outbreaks documented since 1974; major epidemic in Taiwan, 1998, with highest incidence in children <1 year, and again in 2000; virus highly mutable. |
|
Hantavirus pulmonary syndrome (Sin Nombre and multiple other hantaviruses) |
Presumed aerosol transmission of excreta from infected rodents. |
Zoonosis; rodent reservoir host; sporadic cases and outbreaks especially in North and South America; rise in reported human cases linked to factors that lead to expansion of rodent population (e.g., rainfall, weather conditions), increased human–rodent contact (changes in land use), and increased recognition. |
|
Hendra virus (related to Nipah virus) |
Humans infected by direct contact with infected horses. |
Zoonosis; 3 human cases in Australia in 1994–1995; fruit bats may be reservoir host. |
|
HIV |
Person to person via blood and body fluids. |
Continued spread and rising rates of infection in some areas; emergence of resistant strains related to antiviral therapy; resistant strains can be transmitted; infected persons can be infected with second strain; HIV-associated immunosuppression contributes to increase in multiple other infections, including TB. |
|
Influenza, avian H5N1 |
Presumed direct spread from chickens or other birds to humans. |
Major epizootics of influenza H5N1 in avian species in Hong Kong in 1997 and spread of avian virus to humans; millions of chickens killed to halt spread of infection to humans; virus infected multiple avian species; no or limited spread of H5N1 from human to human. |
|
Monkeypox (human) Orthopoxvirus related to smallpox virus |
Close contact with infected animal or person. |
Zoonosis; several generations of person-to-person spread documented; outbreaks in central and western Africa; vaccination with vaccinia virus, which is protective, may have limited spread in the past; possible confusion with smallpox. |
|
Nipah virus |
Human cases resulted from close contact with infected, sick pigs. |
Zoonosis; fruit bats are probable reservoir host; outbreaks in Malaysia started in 1996, in areas with intensive pig farming and movement of pigs; large-scale culling of pigs was used to halt outbreaks. |
|
Noroviruses (formerly Norwalk-like viruses, caliciviruses) |
Person to person; food or waterborne; airborne droplets |
Multiple large outbreaks, especially in institutions and shared environments, including nursing homes, schools, cruise ships; multiple modes of transmission, stability of virus in environment and low infectious dose favor transmission; recently emerged strain may be more transmissible. |
|
vCJD (new variant Creutzfeldt-Jakob) |
Presumed via consumption of flesh from cattle with bovine spongiform encephalopathy (BSE). |
A transmissible spongiform encephalopathy; human cases first appeared in UK in 1996 following an epizootic of BSE in cattle in the UK (by 1997, >170,000 head of cattle had been diagnosed with BSE); feeding ruminant animal proteins to animals was thought to have key role; more than 100 human cases of vCJD have been diagnosed, the majority in the UK. |
|
West Nile virus |
Bite of infective mosquito. |
First outbreak in U.S. in 1999 with epicenter in New York. Has subsequently spread through most of the U.S. and into Canada and Mexico; virus infects many species of birds and other animals; migratory birds have facilitated spread of virus. |
|
Whitewater Arroyo virus (an arenavirus) |
Inhalation of virus in aerosolized rat urine |
Presumed zoonosis with rodent reservoir host; caused human deaths in California in 2000. |
|
Yellow fever |
Bite of infective mosquito. |
Recent spread into some urban areas of Africa and South America; poor vector control; increase in urban tropical areas infested with mosquitoes competent to transmit virus increases risk of introduction by infected traveler; many at-risk populations not vaccinated with highly effective vaccine. |
Despite the steadily increasing availability of new drugs against HIV, the management of drug-resistant HIV poses a serious worldwide challenge. Drugs that mitigate opportunistic infections have also encountered an increase in resistance, with a profound effect on the remaining life expectancy of HIV-infected individuals, as well as their quality of life.
Antimicrobial resistance may represent a more profound hindrance to TB prevention and control efforts than is the case with HIV, in that antituberculin drugs can cure the infected individual and also prevent subsequent infection of others. In 1997 more than 50 million cases of TB worldwide were resistant to one or more drugs (WHO, 1998a). Developing countries in which TB is rampant often have limited laboratory resources to test for drug resistance. Individuals infected with drug-resistant strains, therefore, are often treated inappropriately, thus compounding the spread of MDR-TB.
As with the thwarting of efforts to control TB, drug-resistant malaria continues to expand and impair control efforts. Multiple drug-resistant strains of Plasmodium falciparum, the malaria with the highest fatality rate, are common in many parts of the world. Resistance of P. vivax to certain antimalarials has also been described. The spread of resistance results from numerous factors, including incomplete courses of therapy, changes in vector and parasite biology, pharmacokinetics, and economics (Bloland, 2001).
The health challenges created by antimicrobial resistance extend far beyond the management of these three major killers. For example, Staphylococcus aureus was the most common cause of nosocomial infections in the 1990s (Rubin et al., 1999). S. aureus, often referred to simply as “staph,” is normally found on the skin of healthy individuals, but occasionally enters the body to cause infections ranging from minor problems, such as pimples and boils, to life-threatening septicemia or pneumonia. Methicillin or related drugs (i.e., oxacillin, nafcillin) have become the antibiotics of choice for treating serious staph infections, since more than 95 percent of staph-infected patients worldwide fail to respond to first-line antibiotics such as penicillin and ampicillin (Neu, 1992). In 1990, however, 15 percent of all S. aureus isolates were reported to be resistant to methicillin; in critical care units, 22 percent of all nosocomial S. aureus isolates were methicillin-resistant (Wenzel et al., 1991), and this figure had jumped to over 55 percent by 2000 (National Nosocomial Infections Surveillance, 2001). CDC estimates that as many as 80,000 hospital patients are infected with methicillin-resistant S. aureus (MRSA) each year in the United States. Thus MRSA, which first emerged as a nosocomial infection in the early 1960s (shortly after methicillin was introduced to the market), has been increasing in incidence worldwide ever since (O’Brien et al., 1999; Simor et al., 2001; van Belkum and Verbrugh, 2001).
As of this writing (2003), vancomycin remains the mainstay for treatment against staph infection. Yet extensive transfer of antimicrobial resistance can occur among MRSA pathogenic bacteria and normal flora residing in the human colon (Shoemaker et al., 2001). Since 1989 vancomycin resistance has been increasing substantially in enterococci (Enterococcus faecium) isolated from hospitalized patients, and vancomycin resistance is threatening to become a problem in S. aureus as resistant genes can be transferred to S. aureus through horizontal spread. In 1996, the first case of S. aureus infection with intermediate resistance to vancomycin was reported in Japan (CDC, 1997a); the following year, the United States reported two additional cases (CDC, 1997b). In 2002, the first case of vancomycin-resistant S. aureus was isolated from a 40-year-old Michigan resident with diabetes. This newly identified strain underscores the urgent need to prevent the spread of antimicrobial resistance through the appropriate and controlled use of antimicrobials, as well as the need to develop new classes of antibiotics (CDC, 2002d).
Although MRSA infections are much more likely to develop in hospitals and long-term care facilities than in the general healthy population, several recent deaths due to MRSA infection in previously healthy children show that the condition is now circulating outside of hospitals (Groom et al., 2001). Moreover, the incidence of MRSA in prison populations is unexpectedly high for non–health care settings (CDC, 2001b). Public health officials recently reported a foodborne outbreak of community-acquired MRSA infection, which is believed to have been caused by a food handler who had recently been exposed to MRSA while visiting an elderly relative in a nursing home (Jones et al., 2002).
A dramatic increase in resistance among community-acquired bacteria (i.e., pathogens that normally circulate in the general community and outside of hospital settings), including streptococci, pneumococci, and gonococci, has occurred. For example, evidence indicates a very clear relationship between erythromycin use and resistance among Group A streptococci; when erythromycin use is controlled, the prevalence of erythromycin-resistant isolates declines dramatically (Seppala et al., 1997). Resistance to penicillin among isolates of Streptococcus pneumoniae increased from 5 percent in 1991 (Spika et al., 1991) to 24 percent in 1998 (Whitney et al., 2000). Many penicillin-resistant pneumococci are now resistant to multiple other drugs as well; for example, fluoroquinolone-resistant pneumococci emerged as recently as the late 1990s (Chen et al., 1999) (see Figure 2-3). Over the past decade, several countries—including the United States (Kilmarx et al., 1998), Denmark (Su and Lind, 2001), and Greece (Mavroidi et al., 2000)— have reported fluoroquinolone-resistant gonococci as well. The regional surveillance program in the Western Pacific WHO Region documented increases in the proportion of quinolone-resistant gonococci in Hong Kong
(from 3.3 percent in 1994 to 49 percent in 1998), in Singapore (from 0.3 percent in 1993 to 7 percent in 1998), and in Australia (from less than 0.1 percent in 1993 to 5.6 percent in 1997) (WHO, 2001b). Quinolone-resistant gonococci are now highly common (greater than 80 percent) in most large cities in the Peoples Republic of China (Su, 2002). More than 20 percent of gonococci in Hawaii are quinolone-resistant, and similar resistant organisms are being reported in California (see Figure 2-4) (CDC, 2002e). The emergence of fluoroquinolone-resistant gonococci in the Pacific rim, Hawaii, and California is a particularly bad sign because of the historical trend for resistant gonococci to move from those areas across the United States.

FIGURE 2-3 Fluoroquinolone prescriptions per capita (curve) and frequency of pneumococci with reduced susceptibility to fluoroquinolones in Canada according to the patient’s age (bars). No isolates with reduced susceptibility were identified for persons who were younger than 15 years. Solid bars indicate an age of 15 to 64 years, and open bars an age of 65 years or older. Data on per capita fluoroquinolone prescriptions were obtained from 1988 through 1997, and data on the frequency of pneumococci with decreased susceptibility to fluoroquinolones in each age group were obtained in 1988 and in 1993 through 1998. No isolates with reduced susceptibility were identified in 1988 or 1993. Reprinted with permission, from Chen et al., 1999. Copyright 1999 Massachusetts Medical Society.
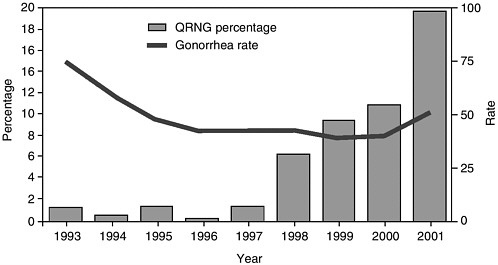
FIGURE 2-4 Percentage of fluoroquinolone-resistant Neisseria gonorrhoeae (QRNG)* among tested gonococcal isolates and gonorrhea rate,† by year§—Hawaii, 1993–2001.
*Defined as N. gonorrhoeae resistant to ciprofloxacin (minimal inhibitory concentration [MIC] ≥ 1.0 μg/mL or disk diffusion zone size ≤ 27 mm) or ofloxacin (MIC ≥ μg/mL or disk diffusion zone size ≤24 mm) by the Committee on Clinical Laboratory Standards.
†Per 100,000 population.
§Data for 1993–2001 include Gonococcal Isolate Surveillance Project (GISP) and non-GISP isolates.
SOURCE: CDC (2002e).
CHRONIC DISEASES WITH INFECTIOUS ETIOLOGY
A growing body of evidence supports the hypothesis that infectious agents cause or contribute to many chronic diseases and cancers previously thought to be caused by genetic, environmental, or lifestyle factors (see Table 2-3) (Cassell, 1998; Pisani et al., 1997). Specific microbes claimed to be associated with chronic conditions may be cofactors with other microbes or other etiologic factors in the disease, sometimes being necessary but perhaps not sufficient elements in the causation pathway. The era of molecular biology and intensive research efforts in the field of AIDS have led to powerful advances in technology for the sensitive detection of infectious agents. These diagnostic tools, plus the realization that organisms of otherwise unimpressive virulence can produce slowly progressive chronic disease with a wide spectrum of clinical manifestations and disease outcomes, have resulted in the discovery of new infectious agents and new concepts in the understanding of infectious diseases.
TABLE 2-3 Infections Associated with Chronic Conditions
|
Infectious Agent |
Chronic Condition |
|
Bacterial |
|
|
Campylobacter jejuni |
Guillain-Barré syndrome |
|
Borrelia burgdorferi |
Lyme arthritis |
|
Helicobacter pylori |
Peptic ulcer disease Gastric adenocarcinoma Gastric lymphoma Immunoproliferative small intestinal disease Mucosa-associated lymphoid tissue lymphoma |
|
Chlamydia trachomatis |
Infertility |
|
Chlamydia pneumoniae |
Atherosclerosis |
|
Enteric bacteria (Shigella, Salmonella, Yersinia, Campylobacter) |
Arthritis Reiter’s syndrome |
|
E. coli O157:H7 |
Hemolytic uremic syndrome |
|
Viral |
|
|
Varicella-zoster |
Post-herpetic neuralgia Congenital mental retardation |
|
Cytomegalovirus |
Congenital mental retardation |
|
Human herpesvirus-8 (HHV-8)/Kaposi’s sarcoma-associated herpesvirus (KSHV) |
Kaposi’s sarcoma Primary effusion lymphoma Castleman’s disease |
|
Hepatitis B |
Hepatocellular carcinoma |
|
Hepatitis C |
Hepatocellular carcinoma |
|
Human papilloma virus (HPV-16, 18, 31, 45) |
Genital carcinomas (cervical, penile, vulvar, anal) |
|
Epstein-Barr virus (EBV) |
Burkitt’s lymphoma Nasopharyngeal carcinoma Hodgkin’s lymphoma |
|
Human T-cell lymphotropic viruses (HTLV-1 and -2) |
Adult T-cell leukemia T-cell lymphoma |
|
Human immunodeficiency viruses (HIV-1, HIV-2) |
Lymphoma Kaposi sarcoma Genital carcinomas related to HPV |
|
Simian virus (SV-40) |
Mesothelioma Ependymomia |
|
Coxsackievirus |
Myocarditis |
|
Infectious Agent |
Chronic Condition |
|
Helminth |
|
|
Schistosoma haematobium |
Urinary bladder carcinoma |
|
Schistosoma japonicum |
Colonic carcinomas |
|
Schistosoma mansoni |
Colonic carcinomas |
|
Opisthorchis viverrini |
Cholangiocarcinoma |
|
SOURCES: Campbell et al., 1998; Danesh et al., 1997; de The, 1995; Epstein et al., 1999; Humphrey et al., 1998; Klein et al., 2002; Persing and Prendergast, 1999; Roivainen et al., 2000. |
|
Cases in point are the proof that many stomach ulcers are due to the bacterium Helicobacter pylori (Parsonnet, 1998; Marshall, 1989; Moller et al., 1995), and the demonstration that at least one form of chronic arthritis and neurologic disorders can be caused by Borrelia burgdorferi, most likely through induction of autoimmunity (Danesh et al., 1997). Recent data obtained in humans and animal models also suggest that mycoplasmas may cause some cases of chronic lung disease in newborns (Cassell et al., 1994; Dyke et al., 1993; Valencia et al., 1993) and chronic asthma in adults (Sabato et al., 1984; Seggev et al., 1986; Yano et al., 1994; Henderson et al., 1979). It has been estimated that more than 15 percent of cancers— including more than 50 percent of stomach and cervical cancers and 80 percent of liver cancers—could be avoided by preventing the associated infectious diseases (WHO, 1996).
Findings such as these raise the possibility that other chronic conditions may also have infectious etiologies. Many of these culprit microbial agents are potentially treatable with existing antibiotics (Cassell, 1998), and they may even be vaccine preventable. For example, the realization that H. pylori causes ulcers revolutionized ulcer treatment. In addition to its causal role in the development of peptic ulcers and gastritis, H. pylori appears to play a role as well in the development of gastric maltomas (i.e., low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue); eradication of the underlying H. pylori infection has been shown to result in tumor regression (Wotherspoon et al., 1993).
The basic biology of those organisms implicated in chronic diseases and cancer is relatively obscure. With rare exceptions, the means by which pathogens suppress, subvert, or evade host defenses and establish chronic and/or latent infection have received little attention. Given that many of these diseases are among the most common in the world, a substantial
impact on improving health and reducing health care costs could be achieved even if only some cases were proven to be of infectious origin, and effective therapies and/or vaccines could be developed. Major advances could be made through the application of functional genomics and integrative biological technologies.
Cardiovascular Disease
Whereas a number of infectious agents—including herpes simplex virus (HSV) and cytomegalovirus (CMV)—have been implicated as causal agents of cardiovascular disease, Chlamydiae pneumoniae has been identified most frequently as a causal infectious agent (Saikku et al., 1988; Campbell et al., 1998). C. pneumoniae is better known for its causal role in community-acquired pneumonia; an estimated 26 percent of all community-acquired pneumonia cases in patients over 65 years of age are due to chlamydial infection (Gant and Parton, 2000). C. pneumoniae infections are usually mild or asymptomatic, but they can be severe, especially in the elderly (Peeling and Brunham, 1996). Prevalence rates of C. pneumoniae antibodies increase from about 50 percent in young adults to 75 percent in the elderly, suggesting that most individuals are infected and reinfected with the bacterium throughout their lives (Kenny and Kuo, 2000).
Compelling evidence of a link between C. pneumoniae and heart disease has been accumulating from a variety of sources, including polymerase chain reaction (PCR), immunocytochemical (ICC) staining, and electron microscopy studies (Campbell et al., 1998). Seroepidemiologic studies have revealed consistent associations between C. pneumoniae antibodies and both coronary heart and cerebrovascular disease, independent of other artherosclerosis risk factors (i.e., hypercholsterolemia, cigarette smoking, hypertension, diabetes, and family history) (Saikku et al., 1992). Insofar as infection does predispose to the development of atherosclerosis, the risk of coronary artery disease is related to the aggregate number of potentially atherogenic pathogens to which an individual has been exposed (Epstein et al., 1999). In addition, infectious agents lead to an increase in other factors, such as C-reactive protein, that may play a causal role in atherosclerosis.
Human Papillomaviruses and Cervical Cancer
Cervical cancer is one of the most common malignant diseases of women. In the United States each year there are approximately 12,800 new cases of invasive cervical cancer and 4,600 deaths due to the disease (CDC, 2001c). Worldwide, an estimated 190,000 deaths occur from cervical cancer, over three-fourths of these in developing countries (Pisani et al., 1999). Fewer than 50 percent of women affected by cervical cancer in developing
countries survive longer than 5 years, whereas the 5-year survival rate in developed countries is about 66 percent (Pisani et al., 1999). Cervical cancer generally affects multiparous women in the early postmenopausal years, although prominent risk factors include the number of sexual partners and age at first intercourse (Brinton, 1992; Herrero, 1996; Schiffman and Brinton, 1995), as well as the sexual behavior of the woman’s male partners (Brinton et al., 1989a).
Human papillomavirus (HPV) is one of the most common causes of STD in the world. Health experts estimate that there are more cases of genital HPV infection than of any other STD in the United States (NIH, 2001). An estimated 5.5 million new cases of sexually transmitted HPV infection occur every year, and at least 20 million Americans are already infected (American Social Health Association, 2001). Scientists have identified more than 100 types of HPV, most of which are not known to cause harm. About 30 types are spread through sexual contact. Some types of HPV that cause genital infections can also cause cervical cancer and other genital cancers. Genital warts (condylomata acuminata, or venereal warts) are the most easily recognized sign of genital HPV infection. Many people, however, have a genital HPV infection without genital warts.
Today, it is well established that infection with certain HPV types is the central causal factor in cervical cancer (Franco et al., 2001; Holly, 1996; International Agency for Research on Cancer Working Group, 1995; Koutsky et al., 1992; Nobbenhuis et al., 1999; Shah, 1997). Relative risks for the association between HPV and cervical cancer are in the 20–70 times range, which is among the strongest statistical relations ever identified in cancer epidemiology. Both retrospective (Munoz et al., 1992; Ylitalo et al., 2000; Zielinski et al., 2001) and prospective (Liaw et al., 1999; Moscicki et al., 2001) epidemiologic studies have demonstrated the unequivocally strong association between infection with the virus and the risk of malignancy, as both cervical intraepithelial neoplasia (CIN) and invasive disease. However, not all infections with high-risk HPVs persist or progress to cervical cancer, thus suggesting that, albeit necessary, HPV infection is not always sufficient to induce cancer; other factors, environmental or host-related, are also involved. Among these cofactors are smoking (Ho et al., 1998); high parity (Brinton et al., 1989b); use of oral contraceptives (Pater et al., 1994); a diet deficient in vitamins A and C (Potischman and Brinton, 1996); and genetic susceptibility traits, such as specific human leukocyte antigen (HLA) alleles and haplotypes (Maciag et al., 2000) and polymorphisms in the p53 gene (Klug et al., 2001). Understanding the role of these cofactors is the objective of much ongoing research on the natural history of HPV infection and cervical cancer.
Scientists are doing research on two types of HPV vaccines. One type would be used to prevent infection or disease (warts or precancerous tissue
changes); the other would be used to treat cervical cancers. Both types of vaccines are currently undergoing clinical trials. Vaccine-induced protection against cervical HPV 16 infection appears to prevent early precancerous changes (Koutsky et al., 2002).
MICROBES INTENTIONALLY USED FOR HARM
Since the terrorist events of September 11, 2001, and the subsequent anthrax attacks through the U.S. mail system, the threat of terrorism has been a prominent subject in the national news. Bioterrorist attacks could occur again at any time, under many circumstances, and at a magnitude far greater than has already been witnessed (IOM, 2002a) (see later discussion in Chapter 3). The knowledge needed for developing biological weapons is accessible to individuals through the open literature and the Internet; the technology is readily available and affordable; and, perhaps most alarming, as the field of molecular genetics advances, an increased capability exists to bioengineer vaccine- or antimicrobial-resistant strains of biological agents. Currently, many terrorist-sponsoring nations or states are suspected of having active bioweapons programs in place.
The United States has been rather complacent about the threat of bioterrorism until recently. A number of factors could account for this complacency, including insufficient intelligence concerning which nations and states have weaponized infectious agents, a naivete regarding the ease with which biological agents can be obtained and weaponized using today’s molecular and biological technology, a general lack of familiarity and skepticism with regard to the dangers of biological weapons, the unpredictable nature of a bioterrorist attack, and little supporting evidence that biological weapons have been widely used thus far. Recent reports have reviewed the threat of bioterrorism (e.g., National Commission on Terrorism, 2000), and in November 2001, the Institute of Medicine (IOM) convened a workshop on Biological Threats and Terrorism: Assessing the Science and Response Capabilities (IOM, 2002a). Experts agree that the United States is vulnerable to bioterrorist attacks and that the likelihood of such an event is increasing. Several agents have been identified and categorized as to their threat potential (see Box 2-2).
The anthrax attacks of 2001 increased awareness that the threat of bioterrorism is real and capable of producing widespread disruption, damage, disease and death. Anthrax is a proven risk and of immediate concern. Smallpox is an equally urgent concern because of its capability for person-to-person transmission and the large numbers of completely susceptible individuals in the United States and worldwide. Three other high-priority potential bioterrorist agents are plague, tularemia, and botulinum toxin. However, these are not the only credible bioterrorist agents. For example,
|
BOX 2-2 Diseases/Agents of Biological Warfare Category A Diseases/Agents The U.S. public health system and primary health care providers must be prepared to address various biological agents, including pathogens that are rarely seen in the United States. High-priority agents include the following organisms that pose a risk to national security because they can easily be disseminated or transmitted from person to person, result in high mortality rates, could cause public panic and social disruption, and require special action for public health preparedness:
Category B Diseases/Agents Agents with the second-highest priority include those that are moderately easy to disseminate, result in moderate morbidity rates and low mortality rates, and require specific enhancements of CDC’s diagnostic capacity and enhanced disease surveillance:
Category C Diseases/Agents Agents with the third-highest priority include emerging pathogens that could be engineered for mass dissemination in the future because of availability, ease of production and dissemination, and potential for high morbidity and mortality rates and major health impacts:
SOURCE: CDC, 2003a. |
the former Soviet Union is known to have weaponized at least 30 biological agents, including several vaccine- or drug-resistant strains.
Many bioterrorist scenarios are possible; two examples are aerosol and foodborne attacks. Aerosols exhibit wide-area coverage, and their small particle size allows them to deposit very deeply in the lung tissue, which is where many agents, including anthrax, induce maximal damage (see Chapter 3). A large amount of agent disseminated under ideal meteorological conditions over a city of substantial size could have considerable downwind reach, resulting in large numbers of casualties. Foodborne bioterrorism, which could encompass a variety of agents, must also be considered a likely threat. Agents that cause foodborne illness are easy to obtain from the environment and often have very low-dose requirements. Foodborne pathogens may in fact be the easiest bioterrorism agent to disseminate.
Consideration of the various bioterrorism agents and some of their properties is the first step in prioritizing defenses against them. Each has unique properties as we see them today, and thus each presents a distinct threat with different opportunities for control.
Anthrax
Bacillus anthracis is a highly stable organism because of its ability to sporulate. Most naturally occurring anthrax cases are cutaneous and are transmitted from agricultural or other occupational exposure. Under natural circumstances, humans become infected through contact with infected animals or contaminated animal products. The incidence of infection is unknown; most cases occur in developing countries.
Several characteristics of B. anthracis make it a potentially very lethal bioweapon (Inglesby et al., 2002). Most important are its stability and infectivity as an aerosol and its large footprint after aerosol release. Anthrax is also widely distributed in nature and thus readily available to terrorists in virulent form. The spores are extraordinarily stable, making them relatively easy to store or transport as an aerosol. An aerosol release of anthrax could potentially affect millions of individuals.
Currently, three types of preventative or therapeutic countermeasures exist against anthrax: vaccination, antibiotics, and various adjunctive antitoxin treatments. Since the late 1930s, attenuated strains of B. anthracis have been used throughout the world as live veterinary vaccines and have proven to be highly effective in controlling disease in domesticated animals. Since the 1950s, one of these strains has been used as a live attenuated vaccine in humans in countries of the former Soviet Union. An inactivated cell-free product is currently used in the United States to vaccinate military personnel and laboratory workers. The molecular pathogenesis of anthrax, including the exact target of its lethal factor, is largely unknown. However,
enough is known that we can begin to predict where second-generation vaccines and various antitoxin modalities might work.
Smallpox
Unlike anthrax, smallpox is a contagious disease with fairly high rates of human-to-human transmission. Consequently, smallpox is considered to pose an even greater threat as an agent of biological terrorism than anthrax (Henderson et al., 1999). Smallpox represents a threat whose consequences are potentially catastrophic; hence it requires careful attention, regardless of the probability of its use. Several features make it an attractive bioterrorist agent: it is moderately stable; it is infectious by droplets and aerosol; it is moderately contagious; and, because vaccination against smallpox ceased after eradication in 1980, most of the world’s population is highly susceptible to infection.
While a smallpox vaccine exists, several bioterrorism-related issues regarding prophylactic smallpox vaccination are unresolved (IOM, 2003). Therefore, we must develop clinician awareness, diagnostic systems, and stockpiles of existing vaccine that will give us a validated countermeasure to deploy in case of the intentional use of this biological agent.
Plague
Yersinia pestis poses a risk to national security because this pathogen can be disseminated by aerosol and/or transmitted from person-to-person, could cause high mortality, and requires special action for public health preparedness (CDC, 2000b). Plague was weaponized in the former Soviet Union for aerosol delivery, and engineered for antimicrobial resistance and possibly enhanced virulence (Inglesby et al., 2000). Plague cultivation in virulent form and its dissemination in stable aerosols, however, are more difficult than is the case for anthrax. Plague generates special concern because of its potential to cause panic, its contagiousness in the pulmonary form, its fulminating clinical course and high fatality, and the possibility that it could be engineered for plasmid-mediated resistance to multiple antimicrobial agents (Galimand et al., 1997). In the WHO modeling scenario that was developed in 1970, a 50 kg release over a city of 5 million would cause about 150,000 cases and 36,000 deaths in the first wave (WHO, 1970). A secondary spread would cause a further 500,000 cases and 100,000 deaths. Plague requires intensive medical and nursing support and isolation for at least the first 48 hours of antibiotic treatment, followed by 2 to 3 weeks of slow convalescence. The hospitalization and isolation that would be required for this number of people in a single city is nearly unimaginable. Pneumonic plague’s contagiousness would require isolation
and possible quarantine, which would complicate medical and public health management.
Currently in the United States, there is no available plague vaccine. The live vaccines that are sometimes used in other countries have unacceptable adverse effects. However, a number of laboratories are attempting to develop a new-generation vaccine, as well as new delivery methods. Several different types of antibiotics that can be used to treat plague are included in the national pharmaceutical stockpile. Antibiotic treatment must be instituted early in the course of infection; otherwise death occurs in 3 to 6 days.
Tularemia
Francisella tularensis was weaponized as an aerosol in both the United States and the former Soviet Union, where it was also engineered for vaccine resistance (Dennis et al., 2001). In the WHO modeling scenario of 1970, a 50 kg release over a city of 5 million would incapacitate 250,000 people and cause 19,000 deaths (WHO, 1970). Tularemia is highly infectious but not contagious. Treatment is similar to that for plague but more extensive, as is the post-prophylaxis to prevent relapse. The tularemia vaccine is a live attenuated vaccine that was previously available as an investigational drug through the U.S. Department of Defense and is now being investigated by the Joint Vaccine Acquisition Program. However, it does not offer full protection against inhalational transmission, and about 14 days is required for protection to develop. The vaccine has been recommended for use in people who work routinely with the organism in the laboratory, but how useful it would be among first responders at high risk for exposure is unknown.
Botulinum Toxin
Botulinum toxin has several features that make it an attractive bioweapon, including its extreme potency and lethality; the ease of its production, transport, and misuse; and its profound impact on its victims as well as the health care infrastructure. Botulinum toxin is readily available as a bioweapon because of the relative ease with which its source, C. botulinum, can be isolated from nature or otherwise obtained. A minimal amount of laboratory equipment and microbiological expertise is needed to cultivate C. botulinum and concentrate its toxin to weaponized material for oral intake. Like tularemia, botulinum toxin can be transmitted through diverse modes: it can spread through foods or beverages or as an aerosol. The toxin, of which there are seven serotypes, kills by paralyzing its victims’ ability to carry out normal respiratory function and is the most poisonous substance known. One gram, evenly aerosolized and inhaled, could gener-
ate more than 1 million lethal doses; 100 grams, evenly distributed in a food or beverage and ingested, could kill a million victims (Arnon et al., 2001).
An investigational vaccine exists, but immunization is really not a viable option for bioweapon defense: the vaccine is still only investigational after 10 years; its components are aging and losing potency; it protects only against serotypes A, B, C, D, and E, and not F and G; it is very painful to receive; it requires a booster at 1 year; and its use deprives the recipient of access to medicinal botulinum toxin for life.
The army has developed an equine antitoxin that provides coverage against all seven serotypes, but the supply is limited, and the drug carries the risk of serious allergic reaction. However, equine antitoxin is inexpensive to produce and could be made in large quantities if a specialized facility were available. A human-derived botulinum antitoxin has been developed as an orphan drug, but is difficult to produce in large quantities and is of limited use because it protects against only five serotypes.