7
Strategies to Contain the Development and Consequences of Resistance
OVERVIEW
Managing the varied problems associated with antimicrobial resistance will require a coordinated response that includes participation by individuals, organizations, and governments at the local, state, national, and international levels. The focus of this session of the workshop was on examining current management efforts and highlighting additional steps needed to contain the development and consequences of resistance. In light of the increasing magnitude of the problem, participants universally agreed that implementing a comprehensive attack on antimicrobial resistance must proceed without further delay.
The primary blueprint for federal actions in the United States is the Public Health Action Plan to Combat Antimicrobial Resistance, issued in 2001 by a multiagency task force led by the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), and the National Institutes of Health (NIH). The plan has four focus areas: surveillance, prevention and control, research, and product development. Complete implementation of the plan will require adequate funding to support activities across a range of organizations.
Various public agencies and private organizations already are putting parts of the action plan into practice. The FDA is using its regulatory responsibility to ensure that drugs and other chemical agents used in both humans and animals do not pose unacceptable health risks, including risks that may arise as a result of antimicrobial resistance. In addition, the
agency’s Center for Drug Evaluation and Research is exploring ways to enhance available approaches for the development of new antibiotics. Such activities include fostering early communication between the FDA and pharmaceutical companies, using the agency’s product labeling system to help educate physicians and other health care workers about antimicrobial resistance, and exploring methods for using data collected in clinical trials to make reliable inferences about a drug’s potential to trigger antimicrobial resistance.
The CDC is implementing a variety of surveillance efforts and prevention and control activities, and is both undertaking and supporting applied research. In one effort, the agency has initiated the Campaign to Prevent Antimicrobial Resistance in Healthcare Settings, a nationwide program that targets clinicians, patient care partners, health care organizations, purchasers, and patients. The campaign centers around four basic strategies that front-line clinicians can use to prevent antimicrobial resistance. These strategies include preventing infections so as to directly reduce the need for antimicrobial exposure and the emergence and selection of resistant strains; diagnosing and treating infection properly, which will benefit patients and decrease the opportunity for development and selection of resistant microbes; using antimicrobials wisely, since optimal use will ensure proper patient care while avoiding overuse of broad-spectrum antimicrobials and unnecessary treatment; and preventing transmission of resistant organisms from one person to another.
At the international level, the World Health Organization (WHO) in 2001 issued the WHO Global Strategy for Containment of Antimicrobial Resistance. The plan details a comprehensive framework of interventions designed to reduce the disease burden and the spread of infection, improve access to and improve use of appropriate antimicrobial agents, strengthen health systems and their surveillance capabilities, introduce and enforce regulations and legislation, and encourage the development of new drugs and vaccines. In implementing the plan, special priority will be given to educating the distributors, prescribers, and consumers of antimicrobial agents; to infection control measures aimed at preventing the dissemination of resistant strains; to quality assurance programs for antibiotics and other medicines; and to the establishment of functional and sustainable laboratories for antibiotic resistance surveillance.
Much of the responsibility for implementing the WHO plan will fall on individual countries, and some of them—especially in the developing world—will need assistance. Toward this end, the Rational Pharmaceutical Management Plus Program, based at a nongovernmental organization, is helping to develop a systematic approach to designing national-level efforts to contain antimicrobial resistance. This approach will provide a framework by which various stakeholders, working with technical consultants
when necessary, can assess policies, drug use, and levels of resistance in their countries, and then tailor a range of strategies for advocacy, policy development, and systems change. Although this approach is generic, its implementation likely will be country-specific and unfold in distinct ways, according to circumstances in each country.
DEVELOPMENT OF THE PUBLIC HEALTH ACTION PLAN TO COMBAT ANTIMICROBIAL RESISTANCE AND CDC ACTIVITIES RELATED TO ITS IMPLEMENTATION
David M. Bell, M.D.
Office of the Director, National Center for Infectious Diseases Centers for Disease Control and Prevention, Atlanta, GA
The Public Health Action Plan to Combat Antimicrobial Resistance
To provide a blueprint for federal actions to address the emerging threat of antimicrobial resistance (AR), the Public Health Action Plan to Combat Antimicrobial Resistance (Part I Domestic Issues), was developed by a Federal Interagency Task Force on Antimicrobial Resistance and released in January 2001 (http://www.cdc.gov/drugresistance/actionplan/index.htm). The Task Force had been formed in 1999, after hearings held by Senators Frist and Kennedy, in recognition of the fact that addressing the multifaceted problem of antimicrobial resistance (AR) required action by multiple agencies and departments. Co-chaired by CDC, FDA, and NIH, the Task Force also includes the Agency for Healthcare Research and Quality, the Centers for Medicare and Medicaid Services, the Health Resources and Services Administration, the Department of Agriculture, the Department of Defense, the Department of Veterans Affairs, the Environmental Protection Agency, and, since 2001, the U.S. Agency for International Development.
The plan was developed based on input from consultants from state and local health agencies, universities, professional societies, pharmaceutical companies, health care delivery organizations, agricultural producers, consumer groups, and other members of the public. It will be implemented incrementally, in collaboration with these and other partners, as resources become available. The plan has 4 focus areas: surveillance, prevention and control, research, and product development. Of 84 action items, 13 are designated top priority. Seven of the 13 are already underway, and 6 are planned to begin by 2003. Part I of the plan focuses on domestic issues; Part II, under development, will identify federal actions that more specifically address global AR issues in collaboration with the WHO and other part
ners. The Task Force is continuing to meet to monitor implementation of the plan and will release annual progress reports and seek additional input at public meetings.
CDC Activities
CDC activities in 2001–2002 primarily implement action items in the surveillance and prevention and control (which includes prevention research) sections. Of CDC’s $25 million appropriation for AR in fiscal year 2001, about 75 percent was awarded extramurally, primarily to health departments and universities. This appropriation included approximately $12 million in new funds, of which $3.2 million was awarded through a new AR applied research grant program. CDC’s 2002 appropriation for AR, also $25 million, will primarily be used to continue ongoing activities summarized below. More information on these activities is available at www.cdc.gov/drugresistance.
Surveillance
In the United States, disease reporting is mandated by state laws, but most states do not require reporting of drug susceptibility information and the completeness of reporting varies. In collaboration with state health departments and other partners, CDC monitors resistance for several pathogens of public health importance and collects limited data on antimicrobial drug prescribing. For example, resistance in invasive S. pneumoniae infections is monitored on a population basis in 9 states or portions thereof through the Emerging Infections Programs, health care-acquired infections (e.g., S. aureus, enterococci, gram-negative bacteria) in approximately 300 hospitals, and foodborne pathogens such as Salmonella in 27 states in a joint project with the FDA and Department of Agriculture. In this project, resistance in foodborne pathogens and commensal organisms is also monitored in animals (and, beginning in 2001, in retail meat products). CDC-supported projects monitor drug resistance for several other pathogens or infections, for example, community-onset Staphylococcus aureus, tuberculosis, gonorrhea, influenza, Group B streptococci, Neisseria meningitidis, Helicobacter pylori, HIV, and malaria.
These systems need enhancement using updated laboratory and informatics technologies, such as through the National Electronic Disease Surveillance System under development. The major problem, however, is that with the exception of tuberculosis, none provides even close to nationwide coverage. Most communities probably do not need sophisticated systems such as those that provide data to help develop and evaluate national prevention and control strategies. However, awareness of the extent to
which resistance is present locally is helpful in guiding treatment decisions and in generating support for local public health interventions such as appropriate drug use and vaccine campaigns. To implement the action plan, CDC seeks to support coordinated national surveillance of drug resistance and use at two levels. One level would involve surveillance that could be done by most states, communities, and health care systems to meet local needs; the second level would consist of more specialized projects to address specific national needs in more detail and monitor emerging problems. Examples of new projects in 2001 include:
-
Monitoring drug-resistant Staphylococcus aureus in the community in 5 states and a national population sample (the National Health and Nutrition Examination Survey).
-
Increasing surveillance of enteric pathogens (through the National AR Monitoring System: Enteric Bacteria) from 17 to 25 states, characterizing and tracking resistance genes; monitoring drug-resistant bacteria in retail meat, and supporting new joint projects of state public health and veterinary laboratories.
-
Monitoring resistance of influenza virus to newly licensed antiviral drugs.
-
Beginning to develop a coordinated system to monitor antimicrobial drug use, through analyzing existing databases, identifying gaps, and exploring standardization of methods.
-
Improving state, local, and health care system infrastructure to support development of local surveillance and enhance the capacity for electronic reporting.
Reliable surveillance information—as well as patient care and safety— requires that front-line clinical laboratories detect emerging drug resistance accurately. Faced with evidence from surveys that this is frequently not the case (CDC, 2000), CDC is working with partners such as the Association of Public Health Laboratories and the American Society for Microbiology to develop training and proficiency testing programs. For example, in 2001, a new website “MASTER” was introduced that includes discussions of difficult cases in diagnostic microbiology, recommendations and references, and opportunities to question CDC microbiologists (www.phppo.cdc.gov/dls/master/default.asp). In its first year of operation, this site has received approximately 33,000 hits from 20 countries. The need for constant updating of clinical laboratory proficiency offers an opportunity for state public health laboratories to provide important leadership and strengthen their linkages with clinical laboratories. Through the National Laboratory Training Network and other programs, CDC’s goal is to work with partners to
ensure training and proficiency in drug resistance testing and reporting for clinical laboratories in all states and territories.
Prevention and Control
Prevention and control of drug resistance primarily involve promoting appropriate use of antimicrobial drugs1 to extend their useful life and preventing infection transmission (e.g., through appropriate infection control and vaccine use). Prevention and control programs do not obviate the need for a constantly flowing “pipeline” of new drugs, as current drugs will inevitably become less effective with time due to resistance. CDC has been working with a variety of partners to promote appropriate antimicrobial use in the community (outpatient prescribing), in health care settings, and in agriculture (Bell, 2001).
For acute infections in outpatients, a major objective is to reduce antimicrobial drug prescribing for illnesses for which these drugs offer no benefit (e.g., viral respiratory infections). In 1995, CDC launched a National Campaign for Appropriate Antibiotic Use that involves partnerships with state and local health departments, health care delivery organizations, health care purchasers and insurers, professional societies, consumer groups, and others. Often working through state-based coalitions, these partners implement coordinated educational and behavioral interventions directed to patients and clinicians, including public education programs, prescribing principles, clinical training materials, and aids (e.g., “viral prescription pads”) to help clinicians avoid prescribing an antibiotic when not indicated (see Figures 7-1 and 7-2). Data from controlled trials indicate that these interventions can be effective in reducing inappropriate antibiotic prescribing for respiratory infections in the United States, as has been reported in other countries—although resistance rates of respiratory pathogens, having reached a certain level, may not necessarily decline thereafter (Gonzales et al., 1999; Belongia et al., 2001; Finkelstein et al., 2001; Hennessy et al., 2002).
Encouraging data from the National Ambulatory Medical Care Survey indicate that antibiotic prescribing rates for children seen in physician offices declined in the 1990s after having increased in the late 1980s (McCaig et al., 2002) (see Figure 7-3). Initially focused primarily on pediatrics, the
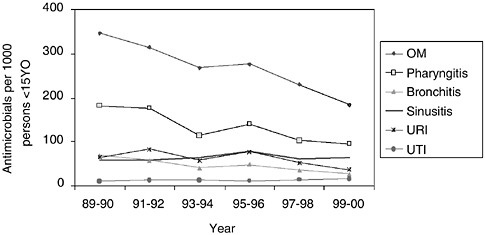
FIGURE 7-3 Trends in annual population-based rates of antimicrobial drug use at physician office visits for children under 15 years of age by selected infectious respiratory diseases and urinary tract infection: United States, 1989–2000. NOTE: Figures are based on 2-year averages. All trends shown are significant (p < 0.001), except sinusitis (p = 0.61) and UTI (p = 0.19). OM denotes otitis media. URI denotes upper respiratory infection. SOURCE: McCaig et al., 2002.
campaign was expanded in 2001 to target prescribing for adults (Gonzales et al., 2001), to increase to 18 the number of state health departments funded to develop coalitions and to develop a national advertising campaign, a model medical curriculum, and HEDIS measures (benchmarks for health plans) for appropriate prescribing. Future goals include expanding the campaign to all states and major health plans and implementing the national advertising campaign.
In health care settings, where infection with multi-drug-resistant organisms is a major patient safety issue, promoting appropriate antimicrobial drug prescribing is complicated by the higher stakes involved in treating sicker patients and the need to develop partnerships with a greater number of medical and surgical specialties involved in their care, as well as with other clinical staff and administrators. Prevent Antimicrobial Resistance, a campaign that emphasizes 12 evidence-based steps for diagnosis of infection, appropriate treatment, appropriate use of antibiotics, and preventing transmission, was launched by the CDC in March 2002, initially focusing on hospital care of adults. Future goals include implementing this campaign in other health care settings, for example, dialysis clinics and long-term care facilities, and for patients in all age groups, and evaluating and facilitating the appropriate use of new informatics technologies, for example, computerized decision support for online prescribing (Evans et al., 1998).
Promoting appropriate antimicrobial drug use in agriculture and veterinary medicine has been complicated by longstanding disagreement between public health and agricultural communities regarding the benefits and risks of these uses, which may have economic implications for major industries. The American Veterinary Medical Association has developed principles for judicious therapeutic use of antimicrobials in veterinary medicine with input from CDC and FDA. CDC has also awarded cooperative agreements to four schools of veterinary medicine to assess the impact of antibiotic use in swine and dairy cattle, develop alternatives to the use of antimicrobials as growth promoters, and evaluate new practices to reduce resistant bacteria in food animals. Finally, CDC strongly supports the FDA’s proposals for a new framework to evaluate antimicrobial drugs used in food animal production and for withdrawal of approval for the use of fluoroquinolones in poultry as important steps toward protecting public health while ensuring the availability of antimicrobials needed for food-producing animals.
Preventing transmission of infections (e.g., through appropriate use of vaccines, infection control in health care, food safety) reduces disease incidence and drug prescribing (Fridkin et al., 1999; Neuzil et al., 2000; Whitney et al., 2001). In 2001 CDC supported projects to evaluate the impact of pneumococcal conjugate vaccine in reducing infections with drug-resistant pneumococci, demonstration programs evaluating comprehensive approaches to infection control in health care settings in Chicago and Pittsburgh, and infection prevention and control research and evaluation programs at seven university-based Centers of Excellence in Healthcare Epidemiology. Regional approaches are important, as illustrated by the early recognition and successful control of the spread of vancomycin-resistant enterococci (VRE) in acute and long-term care facilities in the tri-state area surrounding Sioux City, Iowa. With leadership from the Sioux City health department and support from the Iowa, South Dakota, and Nebraska state health departments and CDC, health care institutions rigorously implemented surveillance and prevention and control guidelines and communi-cated freely. As a result, they were able to eliminate VRE from hospitals and drastically reduce VRE rates in long-term care facilities, an unprecedented success (Ostrowsky et al., 2001). Greater support for comprehensive regional programs such as this is a top priority of the action plan.
Applied Research
In 2001, CDC initiated a new AR applied research grant program. Based on external peer review, four grants totaling $3.2 million were awarded to universities for investigator-initiated research projects addressing: 1) prevention and control of AR in rural settings, and 2) resistance mechanisms and the role of drug use in promoting the spread of resistance
in hospitals and from food animals to humans. Future goals of this research program include addressing additional Action Plan items through peer-reviewed, investigator-initiated proposals, for example, on diagnostic testing, infection control, drug and vaccine use, monitoring spread of resistance genes, impact of drug use in agriculture, clinical and economic outcomes of AR, and novel interventions.
In summary, federal agencies now have a strategy and an action plan to address AR domestically—and a similar plan is being developed to address global resistance. Progress to date is encouraging, indicating that additional investments can be expected to pay dividends in converting AR from an urgent to a routine problem that does not compromise the availability of safe and effective therapy for patients today and in future generations.
ANTIBIOTIC RESISTANCE: ENCOURAGING THE DEVELOPMENT OF NEW THERAPIES, PRESERVING THE USEFULNESS OF CURRENT THERAPIES
Mark J. Goldberger, M.D., M.P.H.
Center for Drug Evaluation and Research U.S. Food and Drug Administration, Rockville, MD
Antibiotic resistance in a broad range of microrganisms has been steadily increasing. This has affected many of the therapies commonly used to treat infections caused by these organisms. This problem is not limited to bacterial infections but also includes fungi, parasites, and viruses. The focus of the following issues will be largely on antibacterial drugs; however, the approaches and issues would also be applicable to therapies for these other organisms.
One obvious part of the solution to this problem is to develop new antimicrobials that are active against resistant organisms. There has already been progress in this area. Two new fluoroquinolone antibiotics, gatifloxacin (Bristol Myers Squibb, 1999) and moxifloxacin (Bayer, 1999), with increased activity against gram-positive bacteria have been recently approved. Members of three new classes of antimicrobials, the oxazolidinones (Pharmacia and Upjohn, 2000), streptogramins (Aventis, 1999), and ketolides (Ketek presented to advisory committee January 2003), have also either recently been approved or are far along in the drug development process. These are all examples of drugs that include some degree of activity against resistant organisms. Nonetheless, despite these advances serious concerns remain about our ability to successfully treat many infections due to either resistant gram-positive or gram-negative organisms. An ongoing focus in the coming months for staff in the Center for Drug Evaluation and
Research (CDER) is to continue to enhance the available approaches to the development of new antibiotics.
The development of new antimicrobials is no easy task, and trying to keep pace with increasing antimicrobial resistance only through the development of new drugs would be a daunting undertaking. It would be highly desirable to link increased development with approaches that preserve the usefulness of these new drugs as well as current antimicrobials. There is an inherent tension in these two approaches that must be reconciled as we move forward. This tension is particularly noteworthy for new antimicrobials that in addition to their activity against resistant organisms also possess broader activity against more susceptible organisms. As part of our efforts at FDA we would certainly also like to encourage the development of drugs whose primary focus would be the treatment of infections due to resistant organisms.
FDA already has a number of regulatory tools to facilitate the development of drugs for serious and life-threatening illness (FDA, 1998). These include our Subpart E and Subpart H regulations and fast track designation. These encourage early communication between FDA and firms, allow us to use data collected earlier in the drug development process as the basis for approval, and permit the use of surrogate markers to form the basis of approval. These approaches have the effect of accelerating drug development by improving the overall quality of the development program, allowing a reduced number of patients in the clinical program, and permitting the use of data collected from an ongoing trial to support approval. They have been used very effectively in the development of new antivirals and drugs for opportunistic infections in patients infected with HIV, as well as for therapies for cancer and other serious illness.
The above methods all have their limitations. Smaller clinical trial programs may leave greater uncertainty as to the efficacy of a product and they unquestionably limit conclusions that can be drawn about safety. Surrogate markers such as CD4 and viral load have been extraordinarily useful in the development of antiretroviral drugs; however, surrogate markers are not always predictive of the ultimate outcome in a clinical trial. In studies of the antiarrythmic drugs, ecanide and flecanide, suppression of ventricular premature depolarizations (the surrogate) was actually associated with a poorer longer-term outcome as measured by mortality (Echt et al., 1991).
Current legislation also provides several options that allow a company developing a product to obtain additional marketing exclusivity beyond that available from an existing patent. The Waxman Hatch Act permits an applicant to recover a portion of patent life that was used during development. This statute also provides for non-patent exclusivity when clinical studies are required for approval. Although not previously eligible, recent changes in legislation have made these benefits applicable to new antibiotic
compounds. Products developed to treat or prevent infection for which the applicable U.S. patient population is less than 200,000 are eligible for Orphan Product exclusivity. This provides seven years of marketing exclusivity for the specific product regardless of whether there is existing patent protection.
Beyond the above it is appropriate to consider how we can improve our ability to make inferences from clinical trials conducted for resistance claims. This would be of particular value for products whose primary emphasis is treatment of resistant infections. In the study of a new agent for treatment of serious gram-positive infections such an approach could include a study of the new agent in smaller numbers of very well characterized patients with a predictable clinical course. Patients with bacterial endocarditis or related infections could be an example of this. Data from such a trial could be supported by a clinical trial in a more conventional situation such as pneumonia to collect additional efficacy and safety data. Collecting pharmacokinetic and pharmacodynamic (PK/PD) data in these settings might also increase our understanding of the product.
As part of these efforts some unresolved scientific and medical issues must also be explored. The definitions and significance of resistance are not always clear. In many circumstances, for instance, penicillins maintain clinically useful activity against penicillin-resistant pneumococci. In other situations pre-existing resistance does not appear to correlate with ultimate outcome.
There are a variety of approaches to studying antimicrobials in both in vitro and in vivo pre-clinical models. Such studies could be extremely useful in defining preliminary activity of a drug as well as providing useful information on dosing regimen. Ideally these approaches would be integrated with PK/PD studies with the overall goal of increasing the efficiency of the development process. There is currently considerable work being done in all these areas. Their ultimate usefulness in facilitating drug development in this area is not yet adequately defined.
It is important to recognize the limits of FDA authority. We cannot, for example, actually develop a drug even though we can facilitate its development by others. There must be some other entity willing and able to do this. Although as previously mentioned existing authority allows us to grant certain types of marketing exclusivity, we have no authority to grant so-called “wildcard exclusivity” that might, for example, apply to another drug of a firm’s choosing in return for development of a drug with more limited economic value. Such authority would require new legislation.
We must recognize that it will not be an easy task to keep up with increasing rates of resistance in many pathogens even if we can improve our approach to the development of new antimicrobials. We must also consider
ways to preserve the usefulness of these new products as well as those already available. There are a variety of approaches that may be of value.
At FDA the product label is the starting point for communicating information to physicians and patients. The information contained within this label is the basis for the promotional material prepared by companies and should also provide a basis for any FDA educational efforts. Our initial efforts with regard to providing additional information in product labeling will include reminders of the value of antimicrobial susceptibility information (when available) in choosing therapy and of the role of antimicrobials in bacterial as opposed to viral infections. These efforts are well under way. It is clear, however, that to effectively communicate information to patients and physicians will require a broad effort from the overall medical community.
Existing regulations provide FDA with the authority to approve drugs with restrictions for safe use (FDA, 1998). This has been done in limited instances, for example, thalidomide. A restricted distribution program can be quite flexible in implementation ranging from statements in labeling, reminding providers who should receive the drug, to programs mandating special testing and limited to certain providers and pharmacies. Such a program could be quite useful particularly for a product that has been approved with limited data on safety and efficacy. Many pharmaceutical firms have limited enthusiasm for such programs. The potential to use such a program may serve as a disincentive to product development. Implementing such a program would also be problematic for a drug that had demonstrated broad antimicrobial activity, including activity against resistant organisms, and for which a more substantial safety database was available.
Antimicrobial therapy is used in a wide variety of clinical situations. In severe infections its value versus no treatment is clearly established. In milder illness the benefits may be more difficult to quantify. In an effort to avoid unnecessary patient exposure to antibiotics and as part of our efforts to rethink our approach to the development of antimicrobial therapy we must, consider with the assistance of our advisory committees and stakeholders, approaches to trial design to deal with this issue.
We also believe that in our approach to product approval and labeling, current issues in medical practice should be considered. As an example, otitis media, an infection of the ear generally seen only in children, is usually caused by one of three bacterial species. It is uncommon however for physicians to routinely do the testing to determine the specific bacterial cause as this involves puncturing the eardrum—a potentially difficult and painful procedure. As a result children are treated empirically. Most drugs approved for this indication are active against the three major pathogens so that this does not pose a problem. However at least one product approved in the past had lower activity against one of the organisms. This problem
was addressed by including such information in product labeling. Given that the physician will not know the actual cause of the infection for a given child it is not clear what he/she could do with such information. Should similar situations occur in the future we will need to reconsider whether an approach using labeling is the most appropriate solution.
There are areas of research that may contribute to better ways to preserve the usefulness of antimicrobial therapy. The availability of rapid diagnostic tests for bacterial and viral infections would allow practitioners to make more informed judgments regarding the need for antimicrobial therapy. Having available additional information on the relationship between dose, duration of therapy, and outcome including development of resistance would also be of value in developing more optimal treatment regimens.
In addition to antibacterial resistance, a worldwide problem, it is worth emphasizing that the broader problem of antimicrobial resistance is also a global one. Drug-resistant malaria and multi-drug-resistant tuberculosis are major health issues throughout much of the world. As the use of antiretroviral therapy increases throughout the world, issues of resistant HIV can be expected to become more widespread.
THE CENTERS FOR DISEASE CONTROL AND PREVENTION’S CAMPAIGN TO PREVENT ANTIMICROBIAL RESISTANCE IN HEALTH CARE SETTINGS
Julie L. Gerberding, M.D., M.P.H.
Centers for Disease Control and Prevention, Atlanta, GA
Each year in the United States, approximately 2 million patients acquire an infection while hospitalized, and about 90,000 of these patients die as a result of their infection (CDC, 1992). Many more people acquire infections in nursing homes and other health care facilities in which vulnerable patients receive care. More than 70 percent of the bacteria that cause hospital-onset infections are resistant to at least one of the drugs most commonly used to treat them (CDC, 1999). Patients infected with antimicrobial-resistant organisms are more likely to have longer hospital stays and require treatment with second- or third-choice drugs that may be less effective, more toxic, and/or more expensive.
The proportion of pathogens that cause hospital-onset infections and are resistant to drugs continues to increase. For example, data collected through the National Nosocomial Infections Surveillance (NNIS) system indicate that more than 50 percent of Staphylococcus aureus isolates causing infections in intensive care units were resistant to methicillin in 2000,
compared to about 30 percent in 1989 (CDC, 2001). (In other hospital units, more than 40 percent of S. aureus isolates were resistant to methicillin in 2000, up from about 20 percent in 1989.) Vancomycin-resistant enterococci, which emerged in the late 1980s, are now endemic in many hospitals, and they accounted for more than 25 percent of enterococcal infections in 2000 (CDC, 2001).
Indeed, the rate of increased prevalence of resistance for some organisms is alarming. Data from NNIS indicate, for example, that fluoroquinolone resistance among Pseudomonas species increased by almost 50 percent between 1994 and 1999. The prevalence of both methicillin-resistant S. aureus and vancomycin-resistant enterococci increased by 40 percent over the same period (CDC, 2001).
Guidelines for preventing antimicrobial-resistant infections in health care settings exist; however, these guidelines often are not read by clinicians and adherence is not optimal. Most data indicate that guidelines alone are not effective in preventing antimicrobial resistance. New approaches are needed to help clinicians who treat patients with infections translate these guidelines into routine practice behaviors that will prevent antimicrobial resistance.
In response to this issue, the CDC has initiated the Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. The campaign is a nationwide effort that targets front-line clinicians, patient care partners, health care organizations, purchasers, and patients. Its general goals include informing clinicians, patients, and other stakeholders about the escalating problem of antimicrobial resistance in health care settings; motivating interest in and acceptance of interventional programs to prevent resistance; and providing clinicians with tools to support needed practice changes. Targeting clinicians at the front end of care is an important step toward preventing the morbidity, mortality, and costs associated with drug resistance. The campaign was developed and is being implemented in collaboration with the CDC Foundation, corporate partners, professional societies, health care organizations, public health agencies, and expert consultants. Additional information about the campaign is available at http://www.cdc.gov/drugresistance/health care.
The campaign centers around four basic strategies that clinicians can use to prevent antimicrobial resistance. These strategies are:
-
Prevent infection. Preventing infections in the first place will directly reduce the need for antimicrobial exposure and the emergence and selection of resistant strains.
-
Diagnose and treat infection effectively. Proper diagnosis and treatment will benefit the patient and decrease the opportunity for development and selection of resistant microbes. This requires rapid diagnosis, identifi
cation of the causative pathogen, and determination of its antimicrobial susceptibility.
-
Use antimicrobials wisely. Optimal use will ensure proper patient care and at the same time avoid overuse of broad-spectrum antimicrobials and unnecessary treatment.
-
Prevent transmission. Preventing transmission of resistant organisms from one person to another is critical to successful prevention efforts.
An integral component of the campaign is the development, dissemination, and implementation of a number of intervention programs for clinicians caring for targeted groups of high-risk patients. These programs, called “12 Steps to Prevent Antimicrobial Resistance,” spell out actions that can be taken immediately to promote patient safety and prevent antimicrobial-resistant infections. The first 12-step program targets hospitalized adults; other programs are being developed for hospitalized children, geriatric patients and nursing home residents, obstetrical patients, critical-care patients, dialysis patients, and surgical patients. The programs are created in close partnership with professional societies and key opinion leaders in the relevant specialties, and all of the action steps are based on scientific evidence and/or published guidelines.
The 12 steps detailed in the programs are framed around the four strategies described above. Each step provides some key facts and background information that serve to buttress the importance of the actions recommended. The steps also include links to other Web-based resources that provide more detailed information.
In the program targeted at hospitalized adults, two steps address the importance of preventing infection. These steps are:
-
Vaccinate. Pre-discharge influenza and pneumococcal vaccination of at-risk hospital patients and influenza vaccination of health care personnel will prevent infections. These infections and their complications are a major cause of hospitalization and exposure to antimicrobials, and they create opportunities for emergence and spread of antimicrobial resistance.
-
Get the catheters out. Catheters and other invasive devices are the leading exogenous cause of hospital-onset infections. Catheters should be used only when essential to patient care, not for convenience or as a “routine” practice. Proper insertion and catheter care may decrease contamination and infection risk, and in some cases antimicrobial-impregnated catheters may be warranted to prevent infections.
Two steps focus on the need to diagnose and treat infection effectively:
-
Target the pathogen. Appropriate antimicrobial therapy saves lives.
When managing patients with known or suspected infection, cultures are almost always indicated. Empiric therapy should be selected to target likely pathogens and be consistent with local antimicrobial susceptibility data. Definitive therapy should target known pathogens once they are identified and their antimicrobial susceptibility test results are known.
-
Access the experts. Infectious diseases expert input improves the outcome of serious infections. Infectious disease specialists are one important resource for providing input, but many other professionals, such as health care epidemiologists, clinical pharmacologists, and surgical infection experts, also can contribute to optimal care for patients with infections. As with all patient safety endeavors, multidisciplinary collaboration is key.
Six steps address the importance of using antimicrobials wisely:
-
Practice antimicrobial control. Programs to improve antimicrobial use are effective. Methods to improve antimicrobial use include passive and interactive prescriber education; use of standardized antimicrobial order forms; formulary restrictions; prior approval to start or switch antimicrobials; multidisciplinary drug utilization evaluation; provider or unit feedback; and computerized decision support (including on-line ordering), which is likely to be the best long-term approach for improving antimicrobial use. Whatever options are available for improving antimicrobial use, the commitment and participation of the prescribing clinician and the institution are essential.
-
Use local data. The prevalence of resistance can vary by time, locale, patient population, hospital unit, and length of stay. Local antimicrobial susceptibility data are the most relevant for predicting the probability of resistance. Thus, clinicians should be fully informed about their local “antibiograms,” which provide a summary picture of common organisms and their susceptibility to many antimicrobial drugs, as well as about the characteristics of their patient populations.
-
Treat infection, not contamination. A major cause of antimicrobial overuse is “treatment” of contaminated cultures. Contamination of blood cultures and other patient specimens is common, often leading to unnecessary antimicrobial use. Proper specimen collection and management is key to preventing contaminated cultures. Clinicians should use proper antisepsis for blood and other cultures; culture the blood, not the skin or catheter hub; and use proper methods to obtain and process all cultures.
-
Treat infection, not colonization. A major cause of antimicrobial overuse is treatment of colonization. Patients often become colonized with new bacterial flora while hospitalized, and when fever or other evidence of infection is mistakenly attributed to these colonizing organisms, unneces
sary broad-spectrum antimicrobial therapy often ensues. Clinical criteria and additional laboratory data can help distinguish infection from colonization. Among specific actions, clinicians should treat pneumonia, not the tracheal aspirate; treat bacteremia, not the catheter tip or hub; and treat urinary tract infection, not the indwelling catheter.
-
Know when to say “no” to vanco. Vancomycin overuse promotes emergence, selection, and spread of resistant pathogens. Emergence of vancomycin resistance among gram-positive organisms is a major threat to patient safety in hospitals. Clinicians should recognize that fever in a patient with an intravenous catheter is not a routine indication for vancomycin. The CDC’s Healthcare Infection Control Practices Advisory Committee has developed a set of guidelines that detail situations in which the use of vancomycin either is acceptable or should be discouraged (CDC, 1995).
-
Stop treatment when infection is cured or unlikely. Failure to stop unnecessary antimicrobial treatment contributes to overuse and resistance. Once antimicrobial therapy is started, it is often difficult to stop, even when there is no indication for ongoing treatment. However, unnecessary treatment adds to treatment costs and may, in some cases, actually harm patients. It is important that treatment be stopped when infection is cured, when cultures are negative and infection is unlikely, and/or when infection is not diagnosed.
The final two steps focus on preventing transmission of resistant organisms:
-
Isolate the pathogen. Patient-to-patient spread of pathogens can be prevented. Adherence to accepted measures to isolate antimicrobial-resistant organisms before they are transferred to other patients or become endemic in a facility is essential. Clinicians and facilities should use standard infection-control procedures and approved techniques for containing infectious body fluids. When in doubt about appropriate isolation procedures, consultation with an infection-control professional is indicated.
-
Break the chain of contagion. Healthcare personnel can spread antimicrobial-resistant pathogens from patient to patient. They also can transmit their own flora and infectious pathogens to patients. Thus, personnel should undertake a number of simple, common-sense measures to prevent the spread of pathogens. These measures include staying home when ill with an infection, covering the mouth when coughing or sneezing, and maintaining appropriate hand hygiene before and after patient contact. Clinicians also should set an example for students, trainees, and colleagues.
This program, and the other 12-step programs when they are ready, will be marketed widely, through Web presentations, “slide sets” detailing
the steps, posters, pocket cards, and other media. CDC also will foster partnerships to implement campaign activities, and will support efforts to evaluate the effectiveness of these programs in preventing antimicrobial resistance in health care settings.
ANTIMICROBIAL RESISTANCE CONTAINMENT STRATEGIES OF THE RATIONAL PHARMACEUTICAL MANAGEMENT PLUS PROGRAM
Terry Green and Anthony Savelli
Rational Pharmaceutical Management Plus (RPM Plus) Program* Management Sciences for Health, Arlington, VA
The use of antimicrobial agents has contributed to the significant decline in infectious diseases over the past half century. However, this achievement in controlling infectious diseases is being undermined by the rapidly growing problem of antimicrobial resistance. Resistance to antimicrobials is increasing, making many infectious diseases more difficult to treat, and raising levels of morbidity, mortality, and health care costs. Major infectious diseases like tuberculosis, gonorrhea, pneumonia, malaria, and dysentery are becoming increasingly difficult and expensive to treat, particularly in developing countries where resources are limited and infection rates are high. With international attention focused on HIV and its treatment, there is much concern about drug resistance and the possibility of future resistance to current antiretrovirals.
Antimicrobial resistance is a natural, progressive reaction that is tied directly to the use and misuse of antimicrobials in humans, animals, and agriculture. As more of the sensitive bacteria are eliminated by antimicrobials, resistant ones will survive and proliferate, transferring their resistance by genes to future generations of bacteria. With time, all antibiotics will encounter resistance development by various bacteria. Depending on the antimicrobial and the bacterium, the degree of resistance can be minimal to almost total.
How Severe Is the AMR Problem?
The increased morbidity, mortality, and treatment costs associated with resistant infections are complicating infectious disease prevention and control efforts worldwide, underscoring the need to preserve the effectiveness of existing antimicrobials. The increased costs for treating resistant infections and new infections that result from the failure of first-line antimicrobials are draining health resources in developing countries. Hospital-acquired infections, which account for thousands of deaths in countries yearly, are frequently caused by antimicrobial-resistant organisms.
A single treatment course for regular tuberculosis (TB) costs about $20, while treatment for multi-drug-resistant TB costs $2000 (WHO, 2000a). Multi-drug-resistant shigella requiring treatment with ciprofloxacin has occurred in 11 countries since 1989. While the cost of ciprofloxacin is high, of more critical importance is the fact that resistance is developing to ciprofloxacin, with no other effective treatment available at this time. Treatment failures due to resistant strains of gonorrhea are a driving force in the HIV epidemic (WHO, 2000b). Persons infected with gonorrhea are more susceptible to HIV infection, and once infected, shed HIV virus at nine times the rate of infected individuals without gonorrhea (WHO, 2000c). Increasing access to antiretroviral therapy for AIDS patients in developing countries should help to reduce opportunistic infections associated with high levels of resistance (i.e., gonorrhea, pneumonia, and TB). However, without proper case management, the benefit of the new drugs may be short-lived. Similar concerns exist for the increased availability of TB and malaria drugs that will occur under the Global Fund to Fight AIDS, Tuberculosis and Malaria. The AMR containment strategies discussed below will increase awareness about this issue and provide opportunities to reduce the risks associated with the Global Fund.
WHO Global Strategy for Containment of AMR
As a major step toward containing AMR, the WHO has recently published a comprehensive set of strategies. WHO recommends a number of interventions, including strategies for use by (1) patients and communities; (2) prescribers and dispensers; (3) hospitals; (4) growers of food-producing animals; (5) national governments and health systems; (6) drug and vaccine developers; (7) pharmaceutical companies and marketers; and (8) international organizations and partnerships concerned with containing AMR. Such a complex, diverse approach is critical in addressing the threats posed by AMR.
RPM Plus AMR Containment Strategies
RPM Plus is a five-year cooperative agreement with USAID to provide technical leadership, assistance, and training on pharmaceutical management in the developing world, and to foster coordination of donor groups to improve health commodity availability and appropriate use. RPM Plus has a portfolio of strategies aimed at managing AMR in various areas of the health care system. These include drug and therapeutics committees (DTC) training, infection-control quality-improvement programs (in the WHO Global Strategies, both are highly recommended for hospitals), national-level implementation strategies, research activities to guide drug use improvement, activities of the International Network for Rational Use of Drugs (INRUD) to provide education for providers/dispensers, and activities to support the national AMR program in Nepal. These strategies are summarized in the discussion below.
Developing and Implementing National AMR Strategies
The WHO global plan is comprehensive and complex, making it difficult to implement in many developing countries. A succinct methodology is needed for implementing the plan at the country level.
To improve the reach of the WHO global initiative, RPM Plus (and our partner the Alliance for Prudent Use of Antibiotics [APUA]), in collaboration with the Academy for Educational Development’s CHANGE project and Boston University’s Applied Research in Child Health (ARCH) project, is supporting the development of a systematic approach to guide the design of national-level efforts to contain AMR. This approach will provide a framework by which stakeholders, working with technical consultants when necessary, can assess policies, drug use, and AMR in their countries, using local data, and can then design strategies for advocacy, policy development, and systems change. For these efforts to succeed, many groups must have a role, including national governments, health care professionals and societies, consumers, non-governmental organizations (NGOs), and the pharmaceutical industry. Although the approach is generic, the implementation process will likely be country-specific and will unfold in distinct ways, according to circumstances in each country. Whatever its specific character, it is anticipated that the systematic approach will result in more active local health organizations, a higher level of awareness about AMR, and stronger policies and programs to monitor and contain the spread of resistance. Figure 7-4 represents the framework for implementing the country strategy for containment of AMR.
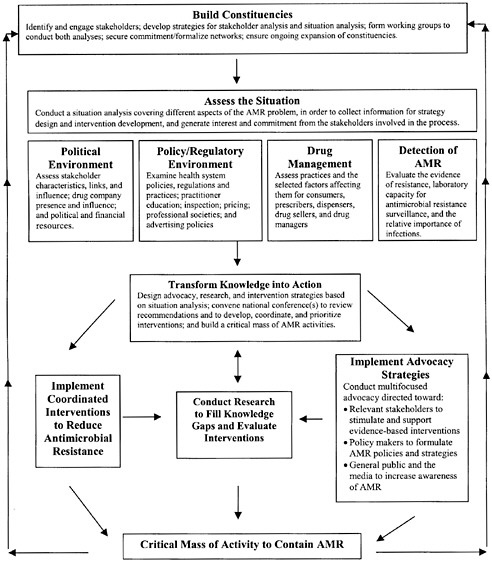
FIGURE 7-4 Outline of a systematic approach for containing antimicrobial resistance.
Establishing and Strengthening Drug and Therapeutics Committees
The DTC is a critical component of a health care organization’s drug selection and use program. This committee evaluates the clinical use of drugs, develops policies for managing drug use and administration, and manages the formulary system.
Many countries spend 30 to 40 percent of their health care budgets on
drugs, and much of that money is wasted on irrational drug use and inefficiencies in procurement and distribution. Other serious drug use problems faced by health care organizations include the overuse of antibiotics, increasing levels of AMR, and increasing rates of adverse drug reaction. DTCs can provide the leadership and structure to help ensure that appropriate drugs are selected for the formulary, rational drug use is promoted, and waste is reduced, thereby reducing costs.
The committee has broad responsibilities in determining what drugs will be available, at what cost, and how they will be used. Some of the important benefits of a functioning DTC are:
-
Selection of effective, safe, high-quality, and cost-effective pharmaceuticals for the formulary
-
Control and management of drug expenditures
-
Identification of drug use problems
-
Rational drug use, including improved antimicrobial utilization
-
Improved drug procurement and inventory management
-
Increased staff- and patient-education levels on matters related to drug use
-
Decreased adverse drug reactions and medication errors.
The functions of a DTC are numerous and will vary with each country and with the size of each health facility. The most important functions are:
-
Advising medical, administrative, and pharmacy departments on drug-related issues
-
Developing drug policies and procedures
-
Evaluating and selecting drugs for the formulary and providing information for its periodic revision
-
Assessing drug use to identify potential problems
-
Promoting and conducting effective interventions to improve drug use (including educational, managerial, and regulatory methods)
-
Managing adverse drug reactions
-
Managing medication errors.
RPM Plus DTC Training Courses
DTCs are weak or non-existent in developing countries and irrational drug use is a common problem. To improve the capacity of committees, RPM Plus (in collaboration with WHO) has developed training materials for a DTC course, which is designed to stimulate the creation of DTCs in hospitals, improve the functioning of existing committees, and train locally based staff to teach the course to others. The training series emphasizes the
technical aspects of a DTC, including selecting drugs for the formulary, managing drug expenditures, identifying drug use problems, and implementing interventions to improve drug use.
An integral part of the DTC training is a field study where participants utilize skills learned in the course and apply them at local hospitals. The field study allows participants to see firsthand how a DTC would work in selecting drugs, identifying drug use problems, and implementing plans to improve drug use.
An important part of the course focuses on developing DTC work plans. The course assists participants in developing comprehensive plans for starting or improving their local DTCs. These plans are monitored by RPM Plus staff members, who also provide technical assistance to help participants complete the plans over the ensuing months.
DTC Website
The DTC Website seeks to encourage former course participants to use what they have learned and to track their progress on work plans. RPM Plus staff monitor the site and provide technical assistance to individuals and teams as necessary. The Website provides course announcements, links to information sources, and access to work plans developed during the courses. The website also provides a discussion board that allows participants and facilitators to keep in contact.
Achievements from DTC Courses
RPM Plus has trained 117 participants from 35 countries in eight training courses since February 2001. DTC course participants have subsequently been active in improving drug management at the local and national level. DTCs have been implemented and are now more effective than previous drug management systems in selecting appropriate drugs for the formulary. Formularies have been evaluated and inappropriate drugs deleted, making procurement activities more efficient and less expensive. Reporting systems for adverse drug reactions (ADRs) have been upgraded and DTCs are reviewing spontaneous reports. Drug use studies and standard treatment guidelines are in use and are guiding strategies to improve drug use. RPM Plus will continue to hold DTC training courses while it monitors the work of past course participants.
Promoting Infection Control Improvement
Hospital-acquired infections are a major cause of preventable morbidity and mortality in developing countries worldwide, putting both patients
and health care workers at risk. These infections also impose a high financial cost by increasing the length of hospitalization and requiring the use of additional, expensive laboratory tests and broad-spectrum antibiotics. They are often caused by AMR organisms that have developed due to poor infection-control and prevention practices and the overuse of antibiotics.
There are many root causes of poor infection control and prevention in hospitals. There is a lack of both an infection-control infrastructure and training in basic infection control, and in many hospitals, basic hand hygiene and sanitation practices are substandard. The use of invasive devices and procedures, such as indwelling lines and catheters and implanted devices, can often be debilitating and even lethal when infection control practices are inadequate.
In addition to the substantial impact on morbidity, mortality, and health care costs, there are other compelling reasons to focus on developing practical strategies for improving infection control and decreasing the emergence of AMR in hospitals:
-
Hospitals are major incubators for resistant bacteria, because antimicrobial agents are commonly prescribed
-
Hospitals can amplify resistance, because resistant bacteria spread quickly among vulnerable patients in facilities that are understaffed, over-crowded, or lack basic infection control practices
-
Patients who acquire resistant infections in hospitals have the potential to disseminate these bacteria in their homes and communities
RPM Plus is developing an infection-control quality improvement program (in collaboration with Harvard University) that will use local assessments, training, and intervention based on assessment results to improve infection control and decrease the rate of nosocomial infections at the hospital level. A distance-learning component will make it possible for many more institutions to take advantage of the program. The program’s objectives are to provide for:
-
Development and use of assessment instruments to identify priority areas for site-specific interventions
-
Training in continuous quality improvement techniques and methodologies
-
Comprehensive training in infection control for teams and other clinical and administrative hospital personnel, available on a multimedia CD-ROM
-
Development of a stand-alone infection control program, using CD-ROM, e-mail, and the Web, to establish or improve infection control
systems by providing assessment instruments, training materials, coaching, and support to local team leaders.
Joint Research Initiative to Improve Use of Medicines in Developing Countries
RPM Plus is a partner with WHO, the Academy for Educational Development (AED), INRUD, and ARCH in the Joint Research Initiative to Improve Use of Medicines. The goal of the initiative is to build a body of evidence on effective drug use interventions in developing countries. Partners provide technical assistance to research groups in applying and testing interventions, as well as in pursuing publication of studies relevant to antimicrobial use.
Current research activities are divided into three categories (see Table 7-1). Phase I studies are nearing completion, and Phase II studies are in the development and funding stage. Phase III studies will begin in late 2002.
Other AMR Containment Support
RPM Plus has developed a manual on hospital antimicrobial indicators, designed to help hospital staff evaluate and improve antimicrobial use in their institutions. The manual is useful in making comparisons of antimicrobial drug use in one hospital over time or between use in two or more hospitals.
The RPM Plus portfolio also includes technical assistance activities for the Nepal national AMR program, which works to enhance AMR sentinel surveillance sites and improve drug management programs in the public and private sectors. Other AMR-related work includes support of INRUD activities including participation in Africa and Asia regional training courses on Promoting Rational Drug Use.
RPM Plus interventions are designed to improve drug selection, appro
TABLE 7-1 Joint Research Initiative to Improve Use of Medicine
|
Phase I |
Phase II |
Phase III |
|
Research focused on improving the prescribing and dispensing behavior of health professionals |
Activities focused on improving the use of antimicrobials in communities and households |
Studies to evaluate the impact of specific national, district, and local policies that affect drug use, including strategies for scaling up |
priate use of drugs, and infection control practices at the local level, and to develop a process to implement these and other WHO global AMR containment strategies at the national level. With implementation of these key drug management programs, AMR containment strategies can be optimized.
ANTIMICROBIAL RESISTANCE AND FUTURE DIRECTIONS
Mary E. Torrence, D.V.M., Ph.D., D.A.C.V.P.M.
USDA, Cooperative State Research Education and Extension Service, Washington, DC
There are several common themes from the last two days. Emerging and re-emerging infectious diseases will continue to occur. These diseases will necessitate the use of current and new antimicrobials for treatment and are an important component of health care. The most important theme is that antimicrobial resistance exists, will continue to exist, and cannot be prevented or stopped. Our goal, therefore, must be to slow down its emergence and continue to develop new antibiotics that will take the place of those drugs that become useless because of resistance.
No one would disagree that the public health action plan is a significant effort among various groups and represents a comprehensive strategy to address antimicrobial resistance (U.S. Government, 2001). However, one may wonder if this document will achieve the goals and action items outlined or whether the plan will remain just a comprehensive paper document. It is important that there is some measure of progress and a means of measuring the outcome or impact of the various actions and strategies. Continual feedback is needed so that revisions can be made and the plan remains responsive to the issues.
In the spirit of the public health action plan, I will make my suggestions within each of the four categories: surveillance, research, prevention and control, and product development.
Surveillance
As we all know, there are multiple, existing surveillance efforts. Some are specifically targeted toward a particular disease or niche, for example, the hospital infectious control program, while others are more general in order to collect data that is representative. The starting question for the design of the best surveillance system has to be, “What is the question that we are trying to answer?” The answer drives the design of the system, the population sampled, and the collection of the data.
The National Antimicrobial Resistance Monitoring System (NARMS) that began in 1996 provides a good framework for antimicrobial resistance surveillance (Tollefson et al., 1998). Would any of the other existing surveillance programs offer additional data? If so, how do we coordinate or integrate the programs or data? In the evaluation of NARMS, limitations must also be considered.
Sampling differs from year to year. For example, isolates from healthy animals are taken from ongoing research projects and from the National Animal Health Monitoring System (NAHMS). The NAHMS studies vary each year as to geographic region represented, the animal species, and the number sampled. Samples taken from slaughter facilities also vary each year. For example, in 1999, campylobacter samples were only taken for 3 months; in 2000, samples were taken the entire year; and for 2001, samples were taken for 10 months (Torrence, 2001). These sampling differences make it difficult to make generalizations about the data and possible trends.
There are currently two laboratories doing testing. Evaluations have been done so that the methods are standardized, and there is validity. If this system expands, standardization of methods is important. Expansion of laboratories may be necessary if this system is to provide quicker feedback of data. Currently results can lag more than a year behind.
Another question is whether there is a role for animal diagnostic laboratories and clinical samples in the surveillance of antimicrobial resistance. Some of the funding available for capacity building for epidemiology and laboratories in public health could be used for animal diagnostic laboratories and epidemiological expertise. This enhancement would be useful for both antimicrobial resistance and for bioterrorism. Funding and human resources are needed for antimicrobial resistance surveillance.
Research
A common response for future directions is the request for more infrastructure, in terms of both expertise and funding. The U.S. Department of Agriculture (USDA) in relation to the CDC and the National Institutes of Health is largely underfunded even though USDA provides the majority of funding for antimicrobial resistance research in agriculture. For example, USDA’s Cooperative State Research Education and Extension Service funded $4 million in 1999 and $4.5 million in 2000. This funding came from food safety programs. The USDA’s Agricultural Research Service continues to budget for research to develop alternatives to antimicrobials, such as competitive exclusion products. The aim of competitive exclusion is to take advantage of the protective effect of normal healthy flora. Competitive exclusion products contain normal healthy microorganisms that compete with and replace pathogenic microorganisms, consequently, lowering the
pathogenic burden. Significant issues of confidentiality of the data and the coordination of research have not been addressed.
Specific Areas of Research
There are basic research projects on the genetics of antimicrobial resistance and whether it occurs through gene transfer (e.g., integrons, plasmids) or through chromosomal mutation. However, it is also important to focus on applied research in an attempt to understand what is occurring in the populations.
It is essential for intervention and mitigation strategies to identify critical control points along the continuum of food production. For example, management changes at the farm level will not prevent the transfer of pathogenic organisms if there is significant contamination at the processing or post-harvest level. Deboning and hide removal are two production steps that have potential for significant bacterial contamination.
The majority of research has been done on important foodborne organisms such as E. coli O157:H7, Salmonella, and Campylobacter. But research is needed to evaluate the role of commensals in the development, persistence, and transference of antimicrobial resistance. Other bacteria that are more difficult to culture, such as anaerobes, must also be studied.
Two primary questions in the study of antimicrobial resistance seem to be two of the simplest; yet, there seems to be no agreement on the answers. First, what is the unit of analysis? Is it the gene, bacterium, host, or population? Second, how do we measure antimicrobial resistance? Is it morbidity, the existence of a gene, or treatment failure? Determining the unit of analysis is also an issue if trying to evaluate drug usage in agriculture. Research is needed to determine which dosage, treatment regimen, or drug might be the most important in the development of resistance. Two recent drug use surveys reported different results because of disagreements in the measurement of drug use (Animal Health Institute, 2000; Lipsitch et al., 2002).
Although economic research has been mentioned, much more needs to be done both in the human and agricultural sectors. For example, what are the costs and the benefits for certain management strategies on the farm?
There has been an increased priority for doing risk assessments in food safety-related areas and in antimicrobial resistance but the methodologies are still primitive. This is particularly true in risk assessments of antimicrobial resistance because data are less available and the unit of measurement not determined.
Finally, as more intervention and mitigation strategies emerge, there needs to be measurement of the outcome or impact of these strategies. This information will help in decision-making about production practices. It is
time to move beyond simple prevalence studies to more applied practical studies.
Prevention and Control
There is still a great need for more rapid diagnostic tests and more rapid, sensitive detection methods. These methods could be used in finished food products and even in fruits and vegetables.
Prudent use needs to be expanded to additional management strategies. There has been a large effort in the development of judicious use guidelines. But evidence of their dissemination and impact is lacking. If professionals have no interest or do not use them, then the existence of guidelines has no impact on antimicrobial resistance.
Within the agricultural and veterinary medical community, the educational efforts need to be more aggressive and creative. For example, there could be information disseminated to pet owners to educate them that viral diseases, such as kennel cough, do not require the use of antibiotics and that less use of antibiotics is better for their pets and themselves.
Educational research methodologies will help with the evaluation of the effect of current and future educational strategies as well as possible management and intervention approaches. There needs to be communication and feedback between education and research so there is continual improvement in both sectors.
Product Development
There are not the same financial incentives for veterinary drugs as human drugs so it is even less likely that new veterinary drugs will be developed or approved. Yet, new drug development for animal drugs may prove useful for human medicine in the common goal of finding new effective drugs. How can incentives be created? Currently most of the thinking is toward alternatives, alternative products and alternative management strategies. A significant effort in agriculture is toward alternative products such as competitive exclusion products, immune modulators, and vaccines. However, it is also important to concentrate on possible alternative management approaches. A more vigorous vaccination program or production design may decrease the amount of bacterial disease and the need for antimicrobials.
Conclusions
Antimicrobial resistance is not easily definable, which makes it more difficult to measure. A unit of analysis is needed. Antimicrobial resistance is
not a product of one source and cannot be isolated in one niche of the world or the environment. It is the result of a confluence of humans, environment, microbes, and animals that are continually interacting with each other. These interactions fluctuate. Therefore, it is more important for researchers, professionals, and policy makers to work together to solve this problem rather than assign blame. Solutions in this area of resistance may come from previous experiences such as insecticide resistance.
There needs to be coordination of the state, local, and federal governments as well as other groups such as industry and professional organizations. How do we better coordinate government, industry, and academia? How do we build trust so we can collaborate? There are many studies in academia that would provide important data and even samples for government initiatives.
In conclusion, I think there are two final questions, “What is our ultimate goal regarding antimicrobial resistance?” Most importantly, “What are we willing to settle for?” I do not think we have answered these questions.
REFERENCES
Animal Health Institute. 2000. Survey indicates most antibiotics used in animals are used for treating and preventing disease. Press Release, February 14, 2000. [Online]. Available: http://www.ahi.org/news%20room/press%20release/2000/feb/antibiotic%20usage%20data.htm.
Aventis. September 21, 1999. Dalfopristin; quinupristin injectable (Synercid). U.S. Patents: no unexpired patents.
Bayer. December 10, 1999. Moxifloxacin tablet (Avelox). U.S. Patents 4,990,517, 5,607,942, 5,849,752.
Bell DM. 2001. Promoting appropriate antimicrobial drug use: perspective from the Centers for Disease Control and Prevention. Clinical Infectious Diseases33 (Suppl 3):S245– S250.
Belongia EA, Sullivan BJ, Chyou PH, Madagame E, Reed KD, Schwartz B. 2001. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics108:575–583.
Bristol Myers Squibb. December 17, 1999. Gatifloxacin injectable (Tequin). U.S. Patents 4,980,470, 5,880,283.
CDC (Centers for Disease Control and Prevention). 1992. Public health focus: surveillance, prevention and control of nosocomial infections. Morbidity and Mortality Weekly Re port41:783–787.
CDC. 1995. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recommendations and Reports44(RR-12):1–13.
CDC. 1999. Antimicrobial resistance: a growing threat to public health. [Online]. Available: http://www.cdc.gov/ncidod/hip/Aresist/am_res.htm.
CDC. 2000. Laboratory capacity to detect antimicrobial resistance, 1998. Morbidity and Mortality Weekly Report48:1167–1171.
CDC. 2001. National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992–June 2001, issued August 2001. American Journal of Infection Control29:404–421.
CDC. 2002. Promoting Appropriate Antibiotic Use in the Community. [Online]. Available: http://www.cdc.gov/drugresistance/community/tools.htm.
Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. 1991. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. New England Journal of Medicine324:781–788.
Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme JF Jr, Lloyd JF, Burke JP. 1998. A computer-assisted management program for antibiotics and other anti-infective agents. New England Journal of Medicine338:232–238.
FDA (Food and Drug Administration). 1998. Guidance for Industry: Fast Track Drug Devel opment Programs—Designation, Development and Application Review. [Online]. Available: http://www.fda.gov/cder/guidance/2112fnl.pdf.
Finkelstein JA, Davis RL, Dowell SF, Metlay JP, Soumerai SB, Rifas-Shiman SL, Higham M, Miller Z, Miroshnik I, Pedan A, Platt R. 2001. Reducing antibiotic use in children: a randomized trial in 12 practices. Pediatrics108:1–7.
Fridkin SK, Edwards JR, Pichette SC, Pryor ER, McGowan JE Jr, Tenover FC, Culver DH, Gaynes RP. 1999. Determinants of vancomycin use in adult intensive care units in 41 United States hospitals. Clinical Infectious Diseases28:1119–1125.
Gonzales R, Bartlett JG, Besser RE, Cooper RJ, Hickner JM, Hoffman JR, Sande MA. 2001. Principles of appropriate antibiotic use for treatment of acute respiratory infections in adults: Background, specific aims, and methods. Annals of Internal Medicine134:479– 486.
Gonzales R, Steiner JF, Lum A, Barrett PH Jr. 1999. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. Journal of the American Medical Association281:1512– 1519.
Hennessy TW, Petersen KM, Bruden D, Parkinson AJ, Hurlburt D, Getty M, Schwartz B, Butler JC. 2002. Changes in antibiotic-prescribing practices and carriage of penicillin-resistant Streptococcus pneumoniae: A controlled intervention trial in rural Alaska. Clinical Infectious Diseases34:1543–1550.
Lipsitch M, Singer RS, Levin BR. 2002. Antibiotics in agriculture: when is it time to close the barn door?Proceedings of the National Academy of Sciences99:5752–5754.
McCaig LF, Besser RE, Hughes JM. 2002. Trends in antimicrobial prescribing rates for children and adolescents. Journal of the American Medical Association287:3096–3102.
Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. 2000. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. New England Journal of Medicine342:225–231.
Ostrowsky BE, Trick WE, Sohn AH, Quirk SB, Holt S, Carson LA, Hill BC, Arduino MJ, Kuehnert MJ, Jarvis WR. 2001. Control of vancomycin-resistant enterococcus in health care facilities in a region. New England Journal of Medicine344:1427–1433.
Pharmacia and Upjohn. April 18, 2000. Linezolid injectable (Zyvox). U.S. Patent: 5,688,792.
Tollefson L, Angulo FJ, Fedorka-Cray PJ. 1998. National surveillance for antibiotic resistance in zoonotic enteric pathogens. Veterinary Clinics of North America: Food Animal Practice14:141–150.
Torrence M. 2001. Activities to address antimicrobial resistance in the United States. Preven tive Veterinary Medicine51:37–49.
U.S. Government. 2001. A Public Health Action Plan to Combat Antimicrobial Resistance. [Online]. Available: http://www.cdc.gov/drugresistance/actionplan.
Whitney CG, Farley MM, Hadler J, et al. 2001. Decline in invasive pneumococcal disease in the U.S. in 2000: an effect of pneumococcal conjugate vaccine?Abstract G-2041 In: Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, December 16–19, 2001.
WHO. 2000a. Antimicrobial resistance: a global threat. Essential Drugs Monitor28&29:1.
WHO. 2000b. Antimicrobial resistance: the facts. Essential Drugs Monitor28&29:7.
WHO. 2000c. World Health Report on Infectious Diseases 2000: Overcoming Antimicrobial Resistance. WHO/CDS/2000.2. Geneva: WHO.





































