5
Cesium-137 and Strontium-90 Capsules
In the early 1970s operators at the Hanford, Washington, site removed a large fraction of the Cs-137 and Sr-90 from the site’s high-level tank waste in order to reduce the requirements for cooling the tanks. The cesium and strontium were concentrated and sealed in stainless steel (SS) capsules (see Figure 5.1) for potential uses, for example, thermoelectric generators and sterilizers. The expected applications for the Hanford capsules did not materialize and ceased entirely in 1988 after one capsule being used in the commercial sector was found to be leaking (USNRC, 1989). The almost 2,000 capsules contain a total of 67 million curies of radioactivity, which amount to about 37 percent of the total radioactivity at the Hanford site (ROO, 2002). They have been described as the nation’s most lethal single source of radiation other than inside an operating reactor (Long, 2002). According to a recent letter of intent among the state of Washington Department of Ecology, the U.S. Environmental Protection Agency, and the Department of Energy (DOE), dispositioning the capsules is among eight specifically identified priorities for accelerating Hanford Site cleanup (Tri Party, 2002). Currently the capsules are stored at the Waste Encapsulation and Storage Facility (WESF) under 13 feet of water to cool the capsules and provide radiation shielding. DOE plans to move the capsules to passive air storage; however, their final disposition has not been determined (DOE, 2002g).
In the capsules, cesium is in the form of cesium chloride (CsCl) and strontium as strontium fluoride (SrF2). The chemical composition has been described as being relatively uniform (NRC, 1997b). Each cesium capsule contains on average approximately 35,000 Ci of Cs-137 plus an unspecified amount of Cs-135 estimated to be 0.7 Ci and produces approximately 190 W of heat. Each strontium capsule contains approximately 33,000 Ci of Sr-90 and produces approximately 260 W of heat (see Table 5.1).
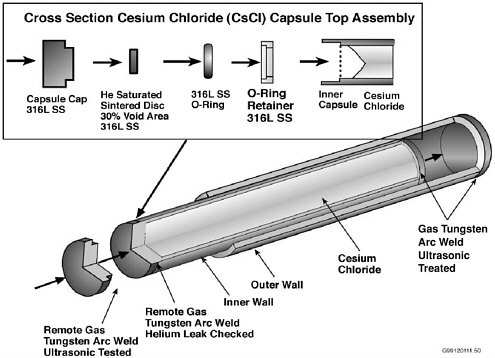
Figure 5.1A typical Cs-137 capsule stored at Hanford may contain up to 40,000 curies of radioactivity. The capsule is made of 316L stainless steel with outside dimensions 20.8 inches long and 2.6 inches in diameter.
Source:DOE Richland.
Table 5.1 Summary of 1995 Cesium and Strontium Capsule Inventory at the Hanford WESF Facility
|
Current inventorya |
1,312 Cesium capsules 23 Cesium capsules in overpacks 601 Strontium capsules |
|
Capsule dimensionsa |
Diameter (outside): 2.6 inches Length (outside): 20.8 inches |
|
Capsule materials of constructionb |
Cesium inner and outer capsule: 316L SS Strontium inner capsule: Hastelloy C-276 Strontium outer capsule: 316L SS |
|
Total volumec |
Cesium capsules, 3.5 m3 Strontium capsules, 1.5 m3 |
|
Total massd |
15 Metric tons of capsules and contents |
|
Radioactivity (as of 8/1/01)a |
Cesium, 47 MCi Strontium, 20 MCi Total including daughter isotopes, 131 Mci |
|
Heat generation (as of 8/1/01)a |
355 kilowatts (1.2 million BTU/hour) |
|
Sources: aROO, 2002; bDNFSB, 1996; cNRC, 1997b; dDOE, 1996b. |
|
Disposition Options and Challenges
The main challenges for managing and dispositioning the capsules are due to the intense radiation and the relatively large amount of heat that they produce. Dose rates range from 8,600 to 18,000 rems/hour for the Cs-137 capsules and from 20 to 420 rems/hour for the Sr-90 capsules.1 Compared to other nuclear materials in DOE’s inventory, Cs-137 and Sr-90 have relatively short half-lives, 30 years and 29 years, respectively. However, the present radioactivity is so great that it will take over 800 years for the Sr-90 to decay to the level currently allowed for disposal as low-level waste, 0.04 Ci/m3. Cs-135, which is present along with the Cs-137, has a half-life of about 2 million years, and it will become the dominant source of radioactivity in the cesium capsules in about 600 years. Cesium and strontium have limited mobility in the environment due to adsorption on clays and other aluminosilicates. However, unlike most other elements, cesium can biomagnify (concentrate) as it moves to higher trophic levels (Whicker and Shultz, 1982).
Capsule integrity is essential for interim storage. Nevertheless, there are gaps in the technical basis for predicting incipient failures and understanding failure mechanisms. Most capsules are considered in good condition, but 23 have had to be overpacked, i.e., sealed in a larger stainless steel container (ROO, 2002). The need for overpacking is typically determined by simply shaking the capsule and listening for the inner capsule to “clunk.” If the inner capsule moves freely, the capsule is deemed to be in good condition. However, if the inner capsule is swollen, it will not move freely and will be a candidate for overpacking. Various mechanisms for capsule failure have been proposed including poor welds and phase changes in the material as a function of temperature (DNFSB, 1996). The report states that the capsules “may have experienced chloride-induced stress corrosion cracking near the outer capsule welds due to lack of water chemistry requirements and control.”
DOE’s current plans (DOE, 2002g) include continuing to provide safe and compliant storage as well as surveillance and maintenance
|
1 |
These are calculated dose rates at 3 feet from a bare capsule based on the contents of individual capsules (Source: Sen Moy, DOE Richland). The radiation is sufficiently intense that the capsules are self-protecting against theft, although the storage facility could be a target for the purpose of scattering radioactive material. The capsules themselves would likely meet the NAS “spent fuel standard” for resisting diversion (see Chapter 3 and NAS, 2000). It is recognized that Cs-137 and Sr-90 from smaller medical or industrial sources could be stolen for the purpose of dispersing radioactive contamination (IAEA, 2003). |
activities for encapsulated cesium and strontium material, and continuing to maintain the WESF and structures associated with capsule storage. Several options are under consideration for disposition of the capsules. These options include
-
continued storage in the pools at the WESF facility,
-
passive storage in air at a new facility,
-
overpacking and disposal of the capsules at a geologic repository, and
-
vitrification into a glass or calcination into an oxide followed by disposal at a geologic repository.
Storage
Intermediate or long-term storage on site has the advantages of allowing monitoring and surveillance, providing physical protection, saving the material as a potential resource, and maintaining the material in disposal-ready condition while avoiding interstate transportation issues. Given their approximately 30-year half-lives, the isotopes could decay significantly during storage thus reducing their hazard and difficulty of eventual disposal. Issues for long-term storage include the commitment to maintain the storage facility and the capsule failure risk due to a lack of understanding of the processes occurring in the capsules.
For dry storage, the capsules would be moved using robotics and stored in air in a special facility designed to convectively exhaust the heat generated. In plans to accelerate the Hanford Site cleanup, the move from wet to passive dry storage is presented as a near-term goal (DOE, 2002g). However, the Tri-City Herald reported that it is unclear the state will agree to the dry storage concept (Herald, 2002). In any event, the dry-storage concept must be demonstrated viable and safe by addressing the aforementioned storage issues.
Disposal
Challenges for permanently disposing of the cesium and strontium involve meeting waste acceptance criteria at an appropriate facility. Under current regulations these materials cannot be disposed in the Waste Isolation Pilot Plant because they are not transuranic isotopes. They exceed U.S. Nuclear Regulatory Commission limits for shallow land disposal, which leaves disposal into the planned geological repository at Yucca Mountain as the only foreseeable option. At a vitrification workshop convened by the National Academy of Sciences in 1997, vitrification was reported to be “likely if overpack is unacceptable” for geological disposal (NRC, 1997b). Conversion to ceramics also appears
attractive for stabilizing these chemically pure materials in a form that would resist dispersion.
Research Needs and Opportunities
The Environmental Management Science Program should support research that will help ensure continued safe storage and potential use or eventual disposal of the Hanford Cs-137 and Sr-90 capsules. Research should lead to understanding potential failure mechanisms of the pre sent capsules, ways to convert the isotopes to stable glass or ceramic forms, and understanding long-term hazards of disposition options.
Stability of the Encapsulated Isotopes
There are research opportunities to understand the chemical and physical behavior of CsCl and SrF2 under intense radiation and localized heating. There is little information for understanding the alterations that accompany the change of valence states during radioactive decay. Cs-137 (monovalent) decays into Ba-137 (divalent), and Sr-90 (divalent) decays into Zr-90 (normally tetravalent) via a short-lived Y-90 intermediate (NRC, 1996). Each of the decay products has very different physical and chemical properties (e.g., melting and phase transition points, ionic radius). Additionally, ionization due to the intense radiation fields is likely to induce other changes.
Topics for research include:
-
the physical and chemical changes that will occur in the CsCl and SrF2 in a confined environment (e.g., in sealed capsules) and intense radiation field,
-
the physical and chemical changes caused by short-lived isotopes decaying to other elements with different valencies,
-
the moisture and other impurity concentration thresholds to keep gas generation to acceptable levels, and
-
the phase changes for these materials as a function of the impurity levels typical in these capsules.
Capsule Integrity
The DOE Office of Science and Technology’s Multiyear Program Plan for FY02-05 (DOE, 2001b) includes two broad research categories for the capsules: (1) capsule integrity assessment methods for WESF at Hanford, and (2) a Cs/Sr capsule leak detection system for WESF.
Beyond these empirical tests there are opportunities for more fundamental research to help assure capsule integrity.
Factors that can affect capsule integrity include defects caused by intense radiation and heat-induced stresses, volume changes due to structural changes as the radioisotopes transmute, volume changes due to phase changes caused by chemical composition and/or temperature changes, and corrosion. Thermal effects will probably be more significant for dry storage since heat convection will be different than in the current water storage. This could induce repeated freeze-thaw cycling in the capsules if the temperatures were near a phase-change temperature. CsCl undergoes a phase change at 469 °C and melts at 645 °C (DNFSB, 1996). At first glance, the phase-change temperatures may seem to be acceptably high, but the presence of only 3 percent FeCl3 causes CsCl to melt at 270 °C. Also, chloride and fluoride attack of the capsule material increases at higher temperatures.
Opportunities for research on capsule integrity include:
-
developing more quantitative capsule surveillance methods than the “clunk” test to determine capsule material and weld integrity or bulging;
-
understanding the fundamental reason for the capsule failures to date, specifically, the mechanical and metallurgical aspects, and applying the knowledge gained to assess the remaining capsules;
-
understanding capsule content phase changes and/or chemical reactions to enable predictions about their behavior if the capsules are moved from storage in pools to passive air storage;
-
understanding the gas-generation potential of the capsule contents over time, especially as the elemental composition changes dramatically in a matter of decades; and
-
determining whether the capsule contents have a unique enough radioisotope signature to be able to identify them in the event of theft.
Matrices for Immobilizing the Isotopes
Unless disposing the capsules in simple overpacks can meet waste acceptance criteria, (e.g., at Yucca Mountain if licensed and constructed), it seems likely that immobilizing these isotopes in a durable matrix will be necessary—either for reuse or disposal. Options include vitrification in glass or incorporation into a crystalline ceramic. Because the cesium and strontium are essentially pure there is opportunity to stabilize these elements in tailored ceramics that are extremely durable. It also seems advantageous to maintain these isotopes in relatively small volumes rather than, for example, to attempt to blend them into
the million-gallon volumes of high-level waste (HLW) in the Hanford tanks for vitrification. However, a long history of basic and applied research supports the vitrification option (Clark et al., 1986; DOE, 1994b). At this point it appears that there is not yet sufficient information to determine whether specialized waste forms or dilution into high-level waste glass is the better disposition path, and there is a clear need for research to help determine the better method.
Although the Hanford capsules appear to be theft resistant, immobilization research would also be applicable for stabilizing cesium and strontium from unused industrial and medical sources worldwide (IAEA, 2003). The chemical and physical form of the radioactive material would have a large effect on its dispersibility. If the cesium were in the form of an appropriate ceramic or other material that was insoluble and that broke up under high strain rates into nonrespirable large particulates (break along grain boundary with grain sizes above the respirable size), its potential for use by terrorists would be greatly reduced.
Research questions for immobilizing the isotopes in either glass or crystalline ceramics include the following:
-
Does the cesium or strontium mix uniformly into the matrix or is there a tendency to segregate?
-
What are the relevant phase transitions, especially with respect to volatilization at the elevated temperatures required to make either glass or ceramics? For example, impurities appear to reduce the phase-transition temperatures of CsCl.
-
Can these salts be vitrified starting with the solid rather than from an aqueous solution?
Hanford’s current plans are to vitrify its high-level tank waste. If the contents of the capsules are uniformly blended with the tank waste, the heat from radioactivity in the vitrified waste product will be increased about 50 percent. (As noted previously the radioactivity of the capsules is about 37 percent of the total radioactivity at the site.) If the blending is not uniform, then some glass with even higher thermal loads will result. High temperatures are detrimental to stability of glass (NRC, 1997b, 2001a), so additional research is needed to ensure long-term stability, especially since the purpose of the glass for immobilizing tank waste is to help sequester long-lived actinides for thousands of years.
Two principal issues for accommodation of cations in crystalline phases are transmutation and radiation damage. With respect to the first, the coordination of oxygen around cations, which stabilizes a given oxide crystal structure, depends on both ion charge and ion size, both of which can change upon transmutation. Monovalent Cs-137 transmutes to divalent Ba-137m and then to Ba-137, accompanied by a
20 percent decrease in ion radius, while divalent Sr-90 transmutes first to trivalent Y-90 and then to tetravalent Zr-90, with a 29 percent overall decrease in final ion radius. There has been little research on predicting the potential destabilizing effects of such cation valency and size changes in insulating crystals, particularly at high isotope loading.
Research is needed to develop novel ways to assess the structural stability of a given crystal type and ways to immobilize cesium and strontium. For example, in the structural tunnels or cages of crystalline zeolitic aluminosilicates or silicotitanates, where changes in ion charge and size may have less structural influence, the Si/Al or Si/Ti ratios could be adjusted to anticipate charge alterations over time to maintain charge neutrality.
The second issue is radiation damage. Radiation fields for both isotopes are extraordinarily intense. It is well known that the CsCl and SrF2 solids currently in storage are acutely susceptible to radiolytic atom displacement (radiolysis). These halides undergo partial radiolytic decomposition to colloidal metal particles and evolvable halogen gas in the temperature range 100-200 °C after accumulated ionization doses in the dose region 108-1010 Gy. Therefore, research opportunities exist to study the stability of the waste type chosen given these large radiation fields.
Opportunities include research to:
-
assess whether or not the current capsule form is viable for either long-term storage or direct disposal in a geologic repository,
-
assess the merits of ceramic versus vitrified waste forms, including processes to produce them,
-
develop methods for uniformly dispersing radionuclides in glass or ceramic and methods for verification,
-
understand how to ensure glass or ceramic stability for time periods of hundreds of years under the intense radiation fields without corroding or leaching, and
-
understand the physical and chemical changes that will occur for these very rapidly decaying compounds in a confined environment (sealed capsules, vitreous or crystalline matrices) under intense radiation fields and with substantial transmutations.








