7
Oyster Aquaculture
PRESENT WORLDWIDE STATUS
Based on statistics from the Food and Agriculture Organization (FAO), worldwide oyster production reached a record high of 4.3 million metric tons in 2000; 4 million metric tons (93%) of those landings originated from aquaculture, of which 99.3% consisted of a single species, Crassostrea gigas (FAO, 2001). In contrast, wild harvest fisheries produced 157,409 tons, mainly C. virginica in North America (85%). These statistics indicate a significant shift toward intensive aquaculture practices. Oyster culture has been practiced since ancient times. For instance, the Romans built ponds to stockpile the harvest and collected spat on wooden branches (Clark, 1964; Héral and Deslous-Paoli, 1991). However, the true development of oyster culture in Europe was initiated during the 18th century to sustain the harvest after increased fishing effort depleted the natural beds and fishing regulations failed to halt the precipitous decline in oyster landings. The development of new techniques for using spat collectors to control spat supply was key to the development of aquaculture production during the 19th and 20th centuries. The most recent advance is the introduction of hatchery production in the 1980s (Jones and Jones, 1982; Chew, 1984). Production of oyster seed in hatcheries has allowed greater control of reproductive output and initiated the use of selectively bred oyster strains. Advances in containment system design have facilitated the expansion of aquaculture into offshore waters (Goulletquer and Héral, 1997).
OYSTER CULTURE IN CHESAPEAKE BAY
In Chesapeake Bay, vast stretches of prolific oyster grounds supported a large public fishery until the late 19th century, reducing the interest in developing aquaculture techniques. In 1884, 615,000 tons of oysters were produced in Chesapeake Bay, around 20% of the current worldwide production (Goulletquer et al., 1994a). Maryland and Virginia adopted various approaches and priorities when landings began to decrease. In the 1870s, Virginia oystermen established a system where they harvested seed from public grounds and transferred it to their leases to grow to market size. This may be considered a rudimentary oyster culture system, reliant on natural spatfall originating in public beds but based on private-sector initiative and investment. In Maryland waters only a small portion of the bottom is available for leasing, a total of 11,000 acres in 1892 and 12,000 acres in 1952 (MacKenzie, 1997). From the 1920s to the 1950s, annual harvests from leased grounds were about 100,000 bushels, an insignificant amount when compared to landings from public fisheries (F. Sieling, Maryland Department of Natural Resources, Annapolis, personal communication, 1994). In 1960, the state of Maryland started a repletion program based on shell deployment to maximize recruitment and support the oyster fishery (MacKenzie, 1997). Although this may also be considered a foray into oyster husbandry, it is based on the public fishery rather than the private efforts undertaken in Virginia. Most of the seed supplying both Virginia and Maryland has originated from public beds (e.g., James River). Most leaseholders on Virginia’s Eastern Shore collected their own seed by spreading shell material in parallel rows on intertidal grounds and transplanting the seeded shell on tidal leases (Haven, 1972). Oyster culture in the Chesapeake Bay has been a technologically unsophisticated practice, relying mainly on natural spatfall in public beds and using extensive on-bottom culture. Aquaculture methods have not been employed to any appreciable extent in the Chesapeake Bay, not even methods to maximize spat recruitment using artificial spat collectors.
OYSTER CULTURE WORLDWIDE
Worldwide aquaculture practices for shellfish have been highly variable, depending on a range of internal and external constraints. The wide variety of options for culture practices reflects the physiological flexibility of oysters, such as tolerance of low oxygen conditions, relatively high turbidity, and various salinity and temperature regimes. Oysters filter feed on microscopic algae that are typically abundant in coastal waters. Because oysters do not need additional food to sustain growth, this species is relatively inexpensive and easy to culture. In some productive
coastal areas it is possible to achieve high-density yields characteristic of artificial intensive culture systems. The wide range of techniques employed in oyster aquaculture can be characterized by the source of seed and the grow-out methods or facilities (see Table 7.1). Specific culture practices are highly dependent on internal and external determinants. Key internal determinants include:
-
choice of culture site to maximize yield (survival and growth rates are dependent on water temperature, oxygen, salinity, turbidity, currents [flow], primary productivity [food supply], disease prevalence, and pollutant levels);
-
protection from or removal of predators and fouling organisms;
-
ease and maintenance of equipment used, including suitability for extreme weather conditions, such as ice or severe storms, and amount of labor required for maintenance and harvesting; and
-
targeting production for either the half-shell or shucked market.
External constraints may be factors facilitating or impeding aquaculture; these include a long-term leasing system to guarantee use and investment, acceptance by the community with regard to aesthetics and enforcement, user conflicts with recreational activities, and impediments to navigation. These constraints underlie current regulations, such as no use of the water column in the Chesapeake Bay, which prevents deployment of suspended longlines. It should be noted that in several countries the development of integrated coastal zone management planning has facilitated the use of coastal resources through spatial allocation of re-
TABLE 7.1 Simplified Description of Various Methods for Oyster Culture Production
|
Seed Supply |
Natural Spatfall |
Hatchery Products |
||
|
|
|
Natural strains |
Selected strains |
|
|
Harvest (public beds) |
Spat collectors (oyster shell to plastic PVC tubes and dishes) |
Remote setting |
||
|
|
||||
|
Increasing Technology |
||||||
|
Grow-Out Facilities |
Intertidal |
Subtidal (offshore) |
||||
|
On bottom (directly or in bag |
Off bottom (tables, iron trestles, oyster bags, and racks) |
On bottom |
Tables, fixed rafts (not mobile) |
Floating rafts, suspended bags |
Longlines, cages, racks, lanterns |
|
sources for various uses such as aquaculture and tourism. Zoning helps to prevent conflicts among different user groups. By considering these factors an aquaculture manager can select the optimal site and culture practices and therefore decide what level of investment will be necessary to achieve cost-effective oyster production.
Seed Supply
Traditionally, natural oyster spat has been collected by dredging and by hand picking in tidal and intertidal beds, respectively. Dredges, hand tongs, patent tongs, and rakes are common tools for this purpose. Artificial structures are often used to collect spat to enhance recruitment because they can be deployed at the optimal time and location. Cultch (the substrate for larval settlement) is cleaned to maximize spat survival. Moreover, since larval survival rates are drastically affected by temperature and salinity, environmental monitoring and consideration of interannual variability are used to decide when and where to deploy spat collectors (Lough, 1975; Goulletquer et al., 1994a, b). In most countries that rely on natural spatfall, larval abundance is monitored to determine the appropriate timing (Héral and Deslous-Paoli, 1991). Because oyster larvae only require a clean substrate that is not fouled or covered by silt, many types of materials can be used for spatcollecting operations: oyster shell, limed tiles, tiles, slate, wood, or iron. Plastic polyvinyl chloride (PVC) tubes and dishes with roughened surfaces have recently become favored in several countries because this material is lightweight (reduces field labor), maximizes spat-collecting area (modular deployment), and facilitates the removal of spat using automated equipment (Goulletquer and Héral, 1997).
The development of commercial methods for remote setting of hatchery-produced seed dates to the early 1980s on the U.S. West Coast (Jones and Jones, 1982). Hatchery-produced oyster larvae made oyster farmers independent of natural seed sources and paved the way for the development of broodstock management and genetic improvement programs. Hatchery and nursery facilities on the U.S. West Coast produce cultchless (single) oyster seed as well as the more traditional cultched spat and larvae for remote setting (see Figure 7.1). Brood stock from natural populations is gradually being replaced with selected strains or crossbred varieties, especially polyploid strains. The sterile triploid oysters offer the advantage of year-round harvest because their meats do not become depleted during the annual reproductive cycle (see section on triploidy below). West Coast hatcheries produce 37.5 billion eyed larvae annually, of which 12 billion are triploid (Nell, 2002). The bulk of these 37.5 billion larvae are produced by three large commercial hatcheries.
Oysters raised predominantly for shucked meat production are set onto oyster shells from the processing plants after the shell has been aged for at least a year. The shells are usually washed and placed into plastic mesh bags that are roughly a meter long and 20 to 25 cm in diameter. These bags, referred to as “cultch bags,” are placed in large tanks, usually at or near the farm where the seed will ultimately be planted. The tanks are filled with seawater heated to 22oC and larvae from the hatcheries are added. The larvae complete metamorphosis in the tanks, attaching to the oyster shells in the bags. Generally, farmers aim for about 40 spat per shell, adding enough larvae to the tank to compensate for the roughly 30 to 40% mortality associated with metamorphosis. The cultch bags remain in the tanks for a few days and are then transported to protected nursery locations in adjacent bays. This process is referred to as “remote setting” and has become the norm on the West Coast for shucked meat production.
After several weeks in the summer (or months in the winter) when the young oysters have reached 1 to 2 cm, the shells are removed from the bags and planted on the bottom or longlined intertidally. Longlining is used in
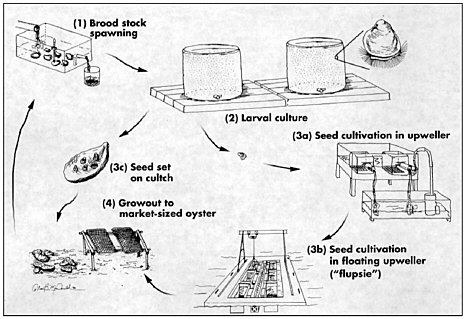
FIGURE 7.1 Diagrammatic of steps in oyster aquaculture for cultched (3c) or cultchless (3a and b) oyster production.
SOURCE: (Elston, 1999); reprinted with permission from the World Aquaculture Society.
areas where the bottom is too soft to support the oysters as they grow. It involves stringing the mother shell (with attached seed) into the braid of a poly rope and supporting it on 19-mm-diameter, 75-cm-long PVC pipe stakes. The stakes are stuck in the mud every half to three-quarter meters.
For cultchless oyster production, farms generally purchase seed from nursery facilities rather than purchasing larvae and setting it themselves. Larvae are set on microscopic shell fragments or induced to metamorphose without shell by the addition of epinephrine. The tiny young oysters require weeks to months in a variety of nursery systems before they can be planted on farms. The nursery systems require high maintenance and are expensive to operate. As a consequence, many farms opt to purchase seed from nursery facilities at sizes ranging from 2 to 20 mm. Growth to harvestable size is accomplished in a variety of systems such as rigid plastic mesh bags attached to ropes anchored on the bottom or secured to intertidal racks or suspended in trays or lantern nets suspended from floating longline or raft structures.
Grow-Out Facilities
Traditionally, oysters have been grown intertidally where they are directly exposed to air during each low tide. Intertidal methods include both bottom (beach) and near-bottom deployments. Oysters are either scattered directly on the bottom or placed in grow-out systems such as stacks, racks, bags, or intertidal longlines. Typically, the oyster grounds are leased, although there are some privately owned tidelands. Grow-out systems may be deployed directly on the bottom and anchored or supported by a variety of systems (iron trestles, posts and cables, and trellis) that suspend the crop a short distance above the bottom. The use of semirigid plastic mesh bags and cages has been highly successful in aquaculture systems around the world. Bags are made of extruded polyethylene material and are available in various forms and mesh sizes to match oyster size (from seed to market size).
In subtidal areas (areas not exposed at low tide), on-bottom culture is common because it requires limited investment for a boat, dredge, and oyster seed. More recently, this technique has been significantly improved by using ground enhancement and management, hydraulic dredges, global positioning systems, and automatic harvesters to improve efficiency. Seed is scattered directly on the bottom, reducing costs for both containment systems and labor. Fixed rafts or tables may be used in areas where tides are small and there are no conflicting uses of the tidewaters. Fast growth rates may be achieved using suspended down lines or trays deployed underneath the fixed structure.
In the Pacific Northwest, on-bottom culture was complicated by two species of indigenous burrowing shrimp that undermine the stability of
oyster bottom culture beds. Since the late 1950s, the shrimp populations in Northwest coastal estuaries have burgeoned, causing severe damage to oyster beds. To maintain oyster beds, the industry had to reduce the impacts of the burrowing shrimp. The only effective treatment to date has been the use of the pesticide carbaryl. Recently, the industry has intensified efforts to find nonchemical alternative controls and is developing an integrated pest management plan for burrowing shrimp to phase out the use of carbaryl.
Floating rafts and surface or subsurface longlines are the most recent deployment methods for culturing oysters. Suspended longlines are flexible enough to handle various grow-out and harvest methods and can be used in high exposure areas. When deployed offshore, oysters are farther away from pollutant sources and increased water flow through the containment system helps maximize growth. Japanese lanterns, trays, and tray stacks can be hung vertically from rafts and longlines. Although market size can be reached rapidly, a large initial investment is required for longlines and specific boats that must be geared with hydraulic arms tailored to handle the lines.
In the Chesapeake Bay there are few intertidal areas, limited to Virginia’s Eastern Shore, and the tidal range is small (1 to 3 feet; see Table 2.1). Most of the available areas for oyster culture are distributed subtidally in both Maryland and Virginia. The subtidal culture practices include on bottom, submerged on-bottom cages, fixed tables and rafts, floating rafts, and longlines. Unless policies are developed to manage concurrent uses of bay waters, spatial conflicts are likely to arise from the use of raft and floating systems that impede navigation and will consequently limit the amount of area available for oyster culture. Container-based oyster culture, whether on bottom or suspended from the sea surface, is more capital intensive and typically limits the farmer to the higher-value half-shell market. Shucked oyster meat prices have been too low generally to make container-based culture economically feasible. On-bottom culture may be the most feasible large-scale method for the Chesapeake Bay because it requires lower infrastructure investment and produces product for the existing shucked market. If C. ariakensis is more susceptible to predation by blue crabs or other predators than C. virginica, direct on-bottom culture without containment may not be practical. On a smaller scale, oyster culture operations can use oyster bags and immersed systems such as a flexible belt, chub ladder, and Taylor float (Luckenbach et al., 1999).
TRIPLOIDY AND REVERSION
As mentioned above, hatchery technology has made it possible to produce nonreproductive triploid oysters. Triploidy is the state in an in-
dividual cell or an organism of having three sets of chromosomes in each cell nucleus, rather than the two sets (diploidy) typically found in most animal cells. Triploidy was first artificially induced in the Eastern oyster, C. virginica, more than 20 years ago by chemical inhibition of meiosis in fertilized eggs (Stanley et al., 1981). Since then triploidy has been induced in several species of oysters, although, to date, it is commercially exploited mostly with the Pacific oyster, C. gigas, (Nell, 2002). More recently, a method has been developed for producing triploids by mating tetraploid and diploid oysters, described in detail below. Currently, hatcheries on the U.S. West Coast produce about 37.5 billion Pacific oyster “eyed larvae” each year, of which about 12 billion are triploid (Nell, 2002). The commercial value of triploid Pacific oysters comes from reduced sexual maturation, which results in retention of better meat quality through the spawning season and superior growth, at least in productive waters (Davis, 1988; Garnier-Gere et al., 2002).
Triploids may also reduce the risk for reproduction and spread of a nonnative species introduced for aquaculture, owing to their nearcomplete sterility (Allen, 1993). For this reason, limited trial introductions of triploid nonnative oysters into the Chesapeake Bay have been carried out since 1997, when Pacific oysters were first introduced by scientists from the Virginia Institute of Marine Sciences (VIMS). After triploid Pacific oysters performed poorly in these early trials, attention shifted to the Suminoe oyster, C. ariakensis, as a potential candidate for introduction. Triploidy had already been chemically induced in the Suminoe oyster for commercial testing on the West Coast (Langdon and Robinson, 1996). To evaluate the growth and survival of C. ariakensis in the Chesapeake Bay, VIMS researchers initially deployed individually certified, chemically induced triploids at six locations (Calvo et al., 2001). In 2001, VIMS researchers, having reared a small number of tetraploid C. ariakensis brood stock, conducted a second trial at 13 sites with 60,000 mated triploids.
Three properties of triploids need to be considered to assess risks associated with an introduction of triploid Suminoe or other nonnative oysters into the Chesapeake Bay: the fidelity with which triploids are produced, the stability of the triploid state, and the sterility of triploid adults. Although there is literature on the fidelity of triploid induction for oysters and other bivalves, there are fewer published data on stability and even fewer on sterility.
Fidelity of triploid production differs between chemical and mated methods of induction. Initially, triploidy was routinely induced in Pacific oysters by inhibiting the formation of the second polar body with cytochalasin B (Allen et al., 1989), so that eggs retained two sets of maternal chromosomes in addition to the one set of paternal chromosomes. Be-
cause eggs develop at slightly different rates, however, chemical inhibition of meiosis is usually not 100% effective. Experienced commercial hatcheries routinely obtain ~80% triploids after chemical inhibition, although percentages as high as 90 to 100% are possible (Downing and Allen, 1987).
In 2002 the Virginia Seafood Council (VSC) proposed introducing a million chemical triploids at 39 locations in the lower Chesapeake Bay, estimating that 99% of the treated seed would be triploid. Such a high percentage of triploids appears to be optimistic based on the reported rates of triploid induction in Pacific oysters by commercial hatcheries (Guo et al., 1996). Even at 99% triploidy, an introduction of this size could potentially introduce 10,000 reproductively competent, nonnative oysters to bay waters, as critics and an ad hoc review panel of the Chesapeake Bay Program argued. However, the issue of the fidelity of chemical triploids has been resolved by the availability of tetraploid C. ariakensis stocks developed by VIMS researchers. Indeed, the 2002 VSC application, which was withdrawn, was subsequently modified for use of mated triploids, resubmitted, and approved in 2003 by the Virginia Marine Resources Commission and the U.S. Army Corps of Engineers.
Because chemical induction of triploidy is much less than 100% and because cytocholasin B is toxic to both humans and oyster larvae, concerted efforts were made in the early 1990s to induce tetraploidy (four chromosome sets) in Pacific oysters (Guo et al., 1994; Guo and Allen, 1994a). In principle, 100% triploidy could be induced simply by fertilizing eggs from normal diploid females, which carry one set of chromosomes (haploid), with diploid sperm from tetraploid males. Guo and Allen (1994a) obtained the first successful induction of viable tetraploid Pacific oysters by inhibiting the first meiotic division of eggs from triploid females. Guo et al. (1996) subsequently demonstrated production of triploid larvae and spat from crosses of tetraploid males with diploid females. Triploids induced in this manner are called mated triploids to distinguish them from chemically induced triploids.
What, then, is the fidelity of mated triploids? Theoretically, 100% of the progeny of a tetraploid by diploid cross (4n×2n) should be triploid. In practice, however, the percentage of triploidy may fall short of this theoretical expectation, as shown by unpublished studies on the Pacific oyster (S. K. Allen, Jr., Virginia Institute of Marine Science, Gloucester Point, personal communication, 2003). Examination of 2- and 4-day-old larvae produced from 178 4n×2n commercial crosses of Pacific oysters in 1999 showed that 93% of the matings were 100% triploid, and of the remaining 7% that were not entirely triploid, 2% had more than 99% triploid larvae, but 5% had less than 95% triploid larvae. The latter were attributed to errors in the commercial spawning trials, perhaps the inadvertent con-
tamination of a 4n×2n cross with sperm from a 2n male. The number of diploid progeny in many spawns (about 1 in 1,000) approaches the limit of detection by flow cytometry, the standard method for distinguishing triploid and diploid cells. Analysis of 2,148 mated Pacific oyster triploid larvae produced in 2000 revealed 2,133 triploids, 13 individuals that appeared to be mosaics of triploid and diploid cells, and only 2 (0.09%) individuals that were diploid. If these results are typical of mated triploids, it can be inferred that a batch of 1 million mated triploids would contain about 900 normal reproductive diploids. This assumes that the relatively larger risk of hatchery error can be managed and reduced through careful certification of hatchery protocols and products. The risk of introducing a small percentage of diploids from true 4n×2n crosses can be managed by considering the density of triploid planting and the distance over which broadcast spawning might be affective (e.g., Levitan et al., 1992).
A second question is whether tissues in triploid individuals remain triploid. Some triploid Pacific and Suminoe oysters have shown signs of reversion to the diploid state. Diploid somatic (i.e., nonreproductive) cells have been found in oysters that were previously confirmed as triploid. Both Pacific and Suminoe oysters have been observed to develop into a mosaic of diploid and triploid cells (S. K. Allen, Jr., Virginia Institute of Marine Science, Gloucester Point, personal communication, 1999, 2000). The percentage of diploid cells is higher for chemically-induced triploids and varies among individuals, as a function of tissue type, and possibly among species. The risk that reversion presents to any proposed introduction of triploid nonnative oysters is that the germinal tissue of triploids may revert to the diploid state, making the oyster reproductively competent. However, the prevalence of mosaics is low in chemically induced C. ariakensis triploids that have been reared in the Chesapeake Bay, averaging 2.5% (range from 1 to 5%) over the first 6 to 7 months and reaching as high as 10% by 18 months. The prevalence of mosaics in mated triploids is reported to be lower and more stable over time, averaging only 0.6% even in post-harvest-size animals (S. K. Allen, Jr., Virginia Institute of Marine Science, Gloucester Point, personal communication, 2003). The percentage of diploid cells in these mosaics is usually less than 10% but can range as high as 25 to 70% in some individuals at 9 months of age. The percentage of diploid cells increases with age, so that an individual with 25 to 30% diploid cells at 9 months of age can have up to 70 to 84% diploid cells by 18 months. Thus, based on these limited preliminary studies, probably much less than 1% of a mated triploid population would be able to produce normal gametes if they remained in the field for more than 3 or 4 years.
There are few data on the ability of mosaics to produce normal hap-
loid gametes, and detection methods can only resolve the status of male oysters. Reversion of reproductive tissue has been observed in a C. gigas male that produced haploid sperm at an age of 4 years (S. K. Allen, Jr., Virginia Institute of Marine Science, Gloucester Point, personal communication, 2003). Another element of the risk of using triploids, then, is the escape of individuals from containment and from harvest, leaving them with sufficient time for reversion of gonadal tissue to the diploid state and recovery of reproductive potential. There are insufficient data to quantify this element of risk at present, but it appears to be quite low and manageable through constraints on methods for containment and harvest of triploids introduced into the Chesapeake Bay.
Finally, one must consider the sterility of triploids even without reversion. The reproductive potential of triploid Pacific oysters has been evaluated in detail (Allen and Downing, 1990; Guo and Allen, 1994b). These authors showed that triploid Pacific oysters are not completely sterile and that progeny, mostly 2n or 3n, are obtained in all laboratory crosses. Fecundity of triploid females was reduced on average to 1.2 million eggs, about 2% of the fecundity of diploid females, but with a considerable range, from 19,000 to 21.5 million eggs per female. The relative fecundity of triploid males could not be ascertained but appeared to be lower than that of females. Fertilization of triploid gametes was only slightly lower than that of diploids, suggesting normal gamete interactions between the eggs produced by triploids and sperm. Survival of progeny from crosses of triploid males with triploid females to the spat stage was less than 1 in 100,000 compared to 1 in 5 survival in diploid crosses. Guo and Allen (1994b) estimate the reproductive potential of triploid relative to diploid Pacific oysters as the product of the relative fecundity and relative survival of progeny, 0.02 × 0.0004 = 0.0008%. However, most progeny of triploid crosses (90%) are themselves triploid; the probability of producing diploid progeny is very low but not zero. The relative reproductive potential of triploid females crossed with diploid males is 0.0045%, which suggests that inadvertent introductions of diploid males with triploid females might raise the reproductive potential of nonnative triploid introductions by an order of magnitude. Similar data on the reproductive potential of triploid Suminoe oysters have not yet been published.
SUMMARY
Triploidy, in which cells have three sets of chromosomes rather than the more typical two sets (diploidy), reduces the risk of reproduction and spread of a nonnative species because they are almost completely sterile. Triploidy induced by chemical means is effective in producing about 80%
triploid larvae in commercial hatcheries; triploidy induced by mating tetraploids with diploid oysters approaches 100% effectiveness, but the small percentage of diploids occurring in mated triploid offspring (0.09%) becomes a significant number in commercial-scale operations involving millions of offspring. Triploids may also revert to the diploid state as they age, but probably less than 1% of a mated triploid cohort might eventually produce normal gametes if they remain in the field more than 3 or 4 years. Triploids are not always completely sterile. Careful examination of C. gigas triploids indicates that the fecundity of triploid females averages 2% of diploids, but survival of progeny of triploid × triploid and triploid × diploid crosses is extremely small.












