4
Oyster Biology
GENERAL BIOLOGY OF OYSTERS
Oysters are members of the family Ostreacea, class Bivalvia, in the phylum Mollusca. Under the current systematic schema, most commercially important species are classified in three major genera: Ostrea, Saccostrea, and Crassostrea and a number of minor genera (Carriker and Gaffney, 1996). Adults are intertidal and subtidal bottom dwellers found worldwide. Most oyster species form the basis of local fisheries or aquaculture operations.
Oysters differ from other bivalves in having a highly irregular shell form. The shape of the shell is typically dictated by environmental constraints, and they are capable of growing over or around adjacent objects, including other oysters. Oysters are plankton feeders; they use their gills to filter microalgae and probably bacteria. During feeding, they relax their single adductor muscle, allowing the two valves of the shell to open slightly. In an action called “pumping,” specialized cilia on the gill draw water into the shell cavity (Newell and Langdon, 1996). Other gill cilia trap particles and funnel them toward the palps—large liplike structures, also covered with cilia that surround the mouth and on which particles are sorted. Some particles, such as microalgae, are sent into the mouth; others, such as sediment, are usually rejected and deposited as “pseudofeces” just outside the shell. Filtration rates are a function of several environmental factors, including temperature, salinity, and suspended particulate concentration. Rates increase with size, although per unit weight, small oysters filter more water than do large individuals
(Shumway, 1996). Powell et al. (1992) reviewed the literature on filtration rates for numerous marine bivalves and found that the relationship between filtration rate and size was similar for all species examined, including several oysters.
Oysters do not regulate their body temperature or the salinity of their body fluids; thus, their metabolic activity is closely tied to the temperature of their surroundings, and the salt content of their blood is the same as that of the ambient water (Shumway, 1996). The ability of oysters to tolerate different environments is species specific. For instance, the European oyster, Ostrea edulis, grows in relatively cool, clear, water of high salinity (Yonge and Thompson, 1976). Crassostrea species, in contrast, are more typically inhabitants of estuaries in which they tolerate wide fluctuations in temperature, salinity, and turbidity.
The oyster’s energetic investment in reproduction is prodigious, with individual females capable of producing many millions of eggs. Oysters typically become reproductively mature as males and may become female in subsequent seasons. Reproductive activity is seasonal and in temperate regions is generally dictated by temperature. Spawning occurs predominantly during the warm season, although other factors, such as phytoplankton blooms, may also play a role. Members of the genus Crassostrea shed their gametes directly into the water where fertilization occurs, and larval life is spent entirely in the water column. In contrast, fertilization and partial larval development in Ostrea take place in the interior of the oyster’s shell. Females release eggs within the shell cavity, and fertilization occurs when sperm shed by nearby male oysters get drawn into the female cavity. The larvae develop partially among the female’s gill filaments, which turn dark and become gritty as the larvae produce shells and become pigmented. The female’s unpleasant appearance and texture at this time are the principal reason that eating Ostrea species is avoided in the summer (months without “R,” or May through August) when reproduction occurs. The larvae of Ostrea species are eventually expelled from the female’s shell cavity and complete their development in the water column. Oysters that are brooders produce smaller numbers of offspring than nonbrooders.
The waterborne larval stage of oysters allows them to disperse from the immediate site of the parental stock, enhances genetic mixing, and allows the colonization of new locations. The larvae are both dispersed and concentrated by water currents and wind. At the end of the larval life, usually 2 to 3 weeks, the oysters “set.” Unlike clams, which can settle into mud and can shift around as adults, oyster larvae cement themselves to a clean, hard substrate and lose their mobility (Yonge and Thompson, 1976). The substrate may be another oyster, a piece of shell, a pebble, a tree root, or any other solid, clean surface. The concentrating effect of wind and
currents, and the fact that larvae prefer to settle where there are other oysters, results in large assemblages on suitable substrates. The mangrove oyster, C. rhizophorae, for instance, congregates on the roots of mangrove trees in shallow water. Species that inhabit deeper water tend to form aggregates known as “reefs” or “beds.” The Eastern oyster, C. virginica, is particularly well known for the large, three-dimensional reefs that it builds as successive generations of oysters settle on each other.
DISEASES OF OYSTERS
Because the terminology is often confused or confusing, this discussion of oyster diseases begins with definitions of key terms. Disease may be caused by an infectious agent or by other factors such as poor diet, exposure to a harmful substance, or a genetic defect. Infection and disease are not synonymous. Infection refers to the establishment of a foreign organism (infectious agent or parasite) in the tissues of another organism, called the host. Disease indicates damage to a body part, organ, or system such that the affected organism no longer functions normally. Infection does not necessarily lead to disease. Many infectious agents cause localized tissue damage but relatively little overall harm to their hosts. Infectious agents capable of causing disease are termed pathogens. Some pathogens are so virulent that they cause disease and mortality in susceptible hosts regardless of the physiological state of the host. Examples include Haplosporidium nelsoni and Perkinsus marinus (the disease agents), which cause MSX and Dermo diseases, respectively, in the Eastern oyster, C. virginica (the host). Other pathogens are described as opportunistic. Host organisms that are otherwise “healthy” can prevent infection by, or control proliferation of, opportunistic pathogens through structural (e.g., shell or epithelial barriers) or biological (physiological activity or the internal defense system) mechanisms. Opportunistic pathogens, however, may proliferate and cause disease if the host is compromised in some manner so that it can no longer effectively defend itself or if the number of opportunistic pathogens in the environment is large enough to overwhelm host defenses. Examples are the various bacterial and fungal species that infect and cause mortalities of cultured molluscan larvae and juveniles (Elston, 1984). Similarly, the herpes viruses associated with mortalities of larval and juvenile stages of a number of molluscan species in commercial hatcheries and nurseries are thought to be promoted by culture conditions, especially high temperature and high density (Farley et al., 1972; LeDeuff et al., 1996; Arzul et al., 2001).
For disease to occur, a potential pathogen must find a susceptible host in a favorable environment. A parasite may infect one species without causing apparent harm but can cause catastrophic disease outbreaks when
it infects another species. The “other species” in such a case can be a resident host infected by an introduced pathogen or an introduced host infected by a resident pathogen. Pathogens may also be present in an environment that inhibits their proliferation. Under these conditions they remain undetectable, either by causing no observable effect (such as death of the host) or because they are too few to be found by standard diagnostic assays. Climate warming, for instance, is hypothesized to have favored outbreaks of Dermo disease from existing undetected foci of P. marinusinfected oysters in the northeastern United States and thus resulted in the apparent range extension of P. marinus (Ford, 1996; Cook et al., 1998).
While all commercial molluscan species examined so far are infected by some parasites, oysters have more reported lethal diseases than any other commercial species (Bower et al., 1994; Ford, 2001; see Table 4.1). As a matter of fact, the molluscan diseases listed as “of concern” by the Office International des Epizooties, an international veterinary body concerned with animal health, are primarily those affecting oysters of various species and are all caused by water-borne protozoan parasites that invade through the gut or external epithelium and proliferate inside the soft tissues, killing the oyster when the parasite burden becomes high. Transmission of some parasites, such as P. marinus and Bonamia ostreae (cause of the disease bonamiasis in Ostrea edulis), is directly from oyster to oyster. The mode of transmission, and indeed the complete life cycle of others, such as H. nelsoni and Marteilia refringens (cause of the disease marteiliosis in O. edulis), remains unknown, although a recent study provides evidence of the involvement of a copepod in the life cycle of M. refringens (Audemard et al., 2002).
Oyster “mass” mortalities have been recorded at least since the early 1900s. Those not attributable to predation, siltation, or freshwater influxes were simply ascribed to unknown causes (Orton, 1924; Roughley, 1926; Sindermann and Rosenfield, 1968), although one such case was later ascribed to a pathogen (Farley et al., 1988). Another early disease outbreak, which killed large numbers of C. virginica in Prince Edward Island, Canada, in 1913 to 1915, has been attributed to an infectious agent (Needler and Logie, 1947). The disease agent is still present but has yet to be identified.
Not until the discovery of P. marinus and H. nelsoni in the late 1940s and late 1950s, respectively, were specific infectious agents clearly identified as the cause of any bivalve mortality. Shortly thereafter, pathogens were associated with catastrophic mortalities of two oyster species (C. angulata and O. edulis) in France. A virus identified in the gills of C. angulata was thought to be the cause of at least some of the mortalities that wiped out commercial production of this species in France in the 1970s (Comps, 1988). The loss of C. angulata prompted the importation of
TABLE 4.1 Important or Common Parasites and Diseases of Oysters
|
Disease/Condition |
Causative agent |
Host(s) |
Region affected |
Comments |
|
Herpes virus disease outbreaks |
Herpes virus |
Numerous bivalve species including oysters |
Worldwide |
Typically found associated with mortalities of larvae and juveniles in commercial culture; has been found in adults and in wild larvae and juveniles, but without observed mortality. |
|
Juvenile Oyster Disease (JOD) |
Probably bacterial |
Crassostrea virginica juveniles grown in culture |
Northeastern United States |
Causative agent unknown, but transmissible; probably has bacterial cause, but may also involve other factors. Caused mortalities from New York to Maine during the 1990s. Problem subsided in most regions in late 1990s. |
|
Summer Mortality |
Vibrio splendidus bacterium (and various other factors) |
C. gigas |
France |
Associated with mortality of juveniles, but adults also suffer. Probably has various causes. |
|
Nocardiosis |
Nodardia crassostreae |
C. gigas |
West Coast of Unitred States |
Associated with summer mortalities. |
|
Maladie du pied |
Ostracoblabe implexa (fungus) |
O. edulis, C. gigas, Saccostrea cucullata |
Europe, Canada, India |
Fungus grows in shells causing “wart-like” protuberances on inner shell. May weaken oyster and diminish marketability. |
|
European oyster haplosporidiosis |
H. armoricanum (protozoan) |
O. edulis and O. angasi |
Northern Europe |
Very low prevalence and no significant impact on population. |
|
Marteiliosis (Aber Disease) |
M. refringens (protozoan) |
O. edulis O. angasi, Tiostrea chilensis (=T. lutaria) |
Western Europe |
In Europe, causes epizootic mortalities in O. edulis; other species have proved susceptible when challenged experimentally, but are not known to be affected in their native ranged. |
|
QX Disease |
M. sydneyi (protozoan) |
Saccostrea glomerata (=S. commercialis) |
Australia |
Causes epizootic mortalities. |
|
Dermo Disease |
P. narinus (protozoan) |
C. virginica, C. gigas, C. ariakensis |
East and Gulf Coast of United States |
Causes epizootic mortalities in C. virginica. C. gigas and C. ariakensis become infected, but do not develop lethal infections. |
|
Bonamiosis |
B. ostrea (protozoan) |
O. edulis (European oyster) and other species Ostrea: O. angasi, O. denselamellosa, O. puelchana, Ostreola conchaphila (= O. lurida), and Tiostrea chilensis (= T. lutaria) |
Western Europe; Maine, United States; Northwestern United States |
In Europe, causes epizootic mortalities in; O. edulis; other species have proved susceptible when challenged experimentally, but are not known to be affected in their native ranges. Not known to caused mortalities in O. edulis in the United States. |
|
|
|
C. ariakensis(?) |
|
|
|
Disease/Condition |
Causative agent |
Host(s) |
Region affected |
Comments |
|
Australian Winter Disease |
Mikrocytos roughleyi (protozoan) |
S. glomerata |
Australia |
Mortalities caused by M. roughleyi apparently first reported in 1926. |
|
Denman Island Disease |
Mikrocytos mackini (protozoan) |
C. gigas |
British Colombia, Canada |
Can be controlled through appropriate aquaculture practices. |
|
MSX Disease |
Haplosporidium nelsoni (protozoan) |
C. virginica, C. gigas |
East Coast of the United States (C. virginica) Pacific Asia and United States (C. gigas) |
Causes epizootic mortalities in C. virginica. C. gigas becomes infected but no mortalities reported. Also found in C. gigas in California. |
|
SSO Disease |
H. costale (protozoan) |
C. virginica |
East Coast of the United States |
Restricted to higher salinity locations compared to H. nelsoni, |
|
Malpeque Disease |
Unknown |
C. virginica |
Atlantic Canada |
First outbreak in 1915-16; oysters in affected areas appear to have developed resistance. |
|
Hemic neoplasia (uncontrolled proliferation of blood cells) |
Etiology unknown, but reported to have genetic or environmental links |
Many species of oysters and other marine bivalves |
Widespread |
Contagious; may be associated with mortality. |
|
Summer mortality |
Various |
C. gigas |
West Coast of United States Japan |
Probably a multifactorial cause. |
|
Infection by Rickettsiales- or Chlamydiales-like organisms |
Intracellular bacteria-like |
All species of oysters examined and most other marine bivalves also |
Global |
Has been associated with mortality of marine bivalves, but not necessarily as the causative agent—more probably opportunist. Found more frequently in dense associations of bivalves (e.g., nurseries or culture parks). |
|
Other parasites |
Trematodespestodes, nematodes, ciliates, and flagellates |
All species of oysters examined and most other marine bivalves also |
Global |
Not deleterious. |
|
SOURCE: Susan Ford, Haskin Shellfish Research Laboratory, Rutger University, Port Norris, New Jersey. |
||||
C. gigas as a replacement species (Grizel and Héral, 1991). The C. angulata mortalities were followed by disease outbreaks in the European oyster, O. edulis, caused by two newly discovered protozoan pathogens, M. refringens and B. ostreae. The resulting mortalities caused a precipitous decline in O. edulis production in France in the 1970s, and accelerated the use of C. gigas, which is not susceptible to the diseases caused by these two pathogens or the viral “gill disease.”
Infections of marine molluscs by other agents, including parasitic worms, protozoans, Rickettsiales- and Chlamydiales-like organisms (RLOs and CLOs), bacteria, and viruses are not uncommon (Bower et al., 1994), and “new” cases continue to be described as more and more host species are grown in culture and examined by an ever-increasing number of scientists. Some are found when mortalities, often of cultured molluscs, are investigated. Culture conditions, in which the molluscs are grown at high density and often using poor animal husbandry practices, favor the proliferation and transmission of opportunistic pathogens, which can then cause or exacerbate disease and mortality in the cultured organisms (Meyers, 1979; Elston, 1984; Bower, 1987; Bricelj et al., 1992; Lacoste et al., 2001). Various bacterial species and the herpes virus are examples of pathogens most commonly associated with disease outbreaks in hatcheries and nurseries (Hine et al., 1998; Renault et al., 2000, 2001; Arzul et al., 2001). Others are encountered during routine surveys or health examinations required for the shipment of molluscs across governmental boundaries. Most occur at low prevalence and intensity and appear to cause no harm to the host. For instance, certain microorganisms, such as the intracellular bacterialike RLOs and CLOs, have been found in all bivalves examined so far, typically without evidence of being harmful. They have often been associated with mortality (Gulka and Chang, 1984; Le Gall et al., 1988; Norton et al., 1993; Villalba et al., 1999; Moore et al., 2000), although it is probable that they are opportunistic pathogens rather than the original cause of death.
Although not exhaustive, Table 4.1 lists the most important or common diseases and parasites reported for oysters, along with the host species and regions where they are found.
CRASSOSTREA VIRGINICA
Life History
C. virginica, the Eastern oyster, inhabits estuarine waters from the Canadian maritime provinces to the Gulf of Mexico, with reports of the species as far south as Brazil and Argentina (Carriker and Gaffney, 1996). Adults are intertidal and subtidal dwellers, typically found in assem-
blages called reefs, bars, or beds that range in size from a few acres to hundreds. The general morphological, physiological, and life history characteristics of oysters described earlier apply equally to C. virginica. This section provides more detailed characteristics of C. virginica.
Reproduction of C. virginica is seasonal and largely influenced by temperature. Gametogenesis begins in the spring and spawning occurs from late May to late September in the mid-Atlantic, with the season contracted or extended to the north and south, respectively (Shumway, 1996; Thompson et al., 1996). C. virginica are either male or female (the reported incidence of hermaphroditism is <0.5%) but may change sex over the winter when they are reproductively inactive. Small oysters (10 to 20 mm) sometimes develop gametes, almost always sperm. Under favorable growth conditions in the mid-Atlantic, this may occur during the late summer after setting, although it is uncertain whether such individuals actually spawn or produce embryos because they do not ripen until after the normal spawning period. In the southeastern United States and the Gulf of Mexico, sexual maturity is typically reached about 3 months after setting, and the prolonged reproductive period in this region increases the probability that these juveniles do participate in the overall reproductive effort of the population.
Males are more sensitive to spawning stimuli, such as temperature and food, than females and tend to spawn first. The presence of sperm in the water stimulates females to release eggs, which are then fertilized externally. Gametes deteriorate within a few hours of spawning and can be rapidly diluted by water currents; thus, the proximity of oysters to one another increases the chances of synchronous spawning and successful fertilization. In the first 24 hours, oyster larvae develop a large ciliated structure, the velum, which acts as both a swimming and food-gathering organ. Initially, the shell is secreted as a single event at about 24 to 48 hours; thereafter, growth occurs through accretion to both thicken and extend the shell. The larval stage lasts for about 2 to 3 weeks, depending on food availability and temperature. Larvae appear to migrate vertically, particularly at later stages, tending to concentrate near the bottom during the outgoing tide and rising in the water column during the incoming tide, thus increasing their chance of being retained in the estuary (Kennedy, 1996; Shumway, 1996). Larval mortality rates are estimated to be close to 99%.
As is the case with all oyster species, C. virginica larvae must eventually find a clean, solid surface on which to cement themselves. Oyster shells meet those criteria if they are not covered with silt or heavily fouled by other epifaunal organisms (although the larvae settle on any type of hard substrate, such as pilings, rocks, and ship bottoms). The suitability of oyster shell for setting, the concentrating mechanisms of wind and
water currents, and the gregarious nature of setting all lead to the formation and persistence of oyster reefs. Without continuous setting and growth of juveniles on the reef, it will usually become covered with silt.
Certain sites within estuaries are known to reliably obtain good “sets” of young oysters. These are locations where clean shell is often spread by management agencies or industry members to “catch” the set, after which it is typically moved to grow-out areas. Despite the knowledge of where the larvae consistently settle, the parental stock for these sets is rarely, if ever, known. The potential obviously exists for oysters to be carried long distances during their larval life, both within and between estuaries; the current state of knowledge is insufficient to predict where larvae originating from oysters in a particular area will be transported or to estimate the likelihood that larvae from one estuary will be carried, along the coast, to another estuary. Oyster larvae are common in summer in water samples collected in East Coast estuaries (Kennedy, 1996); however, investigators sampling nearshore waters off New Jersey for surf clam larvae report seeing only one or two Crassostrea sp.-like larvae during several years of sampling in the 1970s and 1990s (M. Tarnowski, Maryland Department of Natural Resources, Annapolis, personal communication, 2003; J. Grassle, Rutgers University, Port Norris, personal communication, 2003). Further, there is little evidence for a broodstock size/recruitment relationship for oysters, and very large sets can occur even when the stock of oysters is very low, as occurred in Delaware Bay several years after the MSX disease epizootic and heavy oyster drill predation had severely reduced the Chesapeake Bay population (Fegley et al., 1994). For the Gulf of Mexico, Livingston et al. (1999) reported rapid repopulation of Apalachicola Bay after the oyster population was decimated by two hurricanes in 1985. A widespread heavy set occurred in Chesapeake Bay in 1997 even though the oyster population was severely depleted. Unfortunately, most of the oysters suffered disease-caused mortalities before they reached marketable size.
From a few hundred microns in size at the time of setting, C. virginica grow to sizes exceeding 150 mm. They are typically marketed in the United States when they reach about 75 mm (about 3 inches). Growth rates vary with temperature, food, turbidity, and salinity. In the mid-Atlantic, market-sized C. virginica are at least 2 years old but more typically are harvested when 3 to 4 years old. To the south, C. virginica may grow to marketable size in 12 to 15 months. Growth rates of oysters held in floating aquaculture trays are typically much greater than those of oysters on the bottom. The average life span is about 6 to 8 years; the maximum is probably about 25 years.
Oysters provide food for numerous predatory species, including flatworms, crabs, oyster drills, starfish, and certain finfish. Mortality, mostly due to predation, is high on newly set oysters (spat), typically exceeding 40% in the first week (Haskin and Tweed, 1976; Newell et al., 2000) and
sometimes reaching 100% (Roegner and Mann, 1995). Mortality rates diminish as the spat grow but remain high during the first several months after setting. Newell et al. (2000) found that over a period of 14 years less than 0.5% of potential set (measured on clean experimental surfaces during the summer) was found on the bottom by the beginning of winter in a subestuary of central Chesapeake Bay. Higher survival was found in upper Delaware Bay over a 30-year period during which the percentage surviving until winter ranged from <1 to 25 but was mostly <10 (Haskin and Ford, 1986). Predation, especially by oyster drills and crabs, continues on older oysters (MacKenzie, 1970). Ford and Haskin (1982) estimated that predators annually killed 12 to 18% of oysters more than a year old on lower Delaware Bay leased grounds—about half as much as died from other causes, mostly MSX disease.
In addition to providing habitat for numerous other species, a principal ecological function provided by oysters is considered to be their ability to remove, or filter, particles from surrounding water (Newell, 1988). During feeding and respiration, oysters “pump” large quantities of water. A large C. virginica (10 to 12 cm) has been found to transport up to 460 liters per day over its gills (Galtsoff, 1964), although this is probably an extreme value. Powell et al. (1992) reviewed the literature on filtration rates for numerous marine bivalves and found that the relationship between filtration and size was similar for all species. The rates that when used in a numerical growth model most closely fit field observations for C. virginica were about 0.5 to 0.6 liters per hour per gram dry weight, or about 75 liters per day, for a 10-cm oyster. Filtration rates are a function of several environmental factors, including temperature, salinity, and suspended particulate concentration (Shumway, 1996). Rates increase with temperature, decrease with salinity below about 8 ppt, and also decrease with particle load (Powell et al., 1992). The highest rates occur during the summer. In the mid-Atlantic and the northeastern United States, C. virginica are physiologically nearly inactive during the winter; in the Gulf of Mexico they are active much of the time.
Temperature and salinity, and their interaction, are undoubtedly the two most important environmental factors governing survival, growth, and reproduction of C. virginica. Many investigators have attempted to define the temperature and salinity tolerance limits and optimum ranges for C. virginica, with considerable divergence in results (Shumway, 1996). Differences in methodology (laboratory versus field observations), acclimation conditions (Davis, 1958; Davis and Calabrese, 1964), and geographically associated genetic traits (Barber et al., 1991; Dittman et al., 1998) all contribute to observed variations in optimum temperaturesalinity ranges, making it difficult and risky to define limits that apply to all populations. In addition, food and turbidity can confound the interpretation of field observations, especially in the case of salinity, as food availability is often limiting at low-salinity sites.
Despite the variability, it is clear that C. virginica, having evolved in an estuarine environment and having an extremely wide geographic distribution, can tolerate a broad range of both temperatures and salinities (Shumway, 1996). For instance, adults can survive freezing during winters in the Northeast and summer temperatures averaging 36oC in Florida and the Gulf of Mexico, with at least short-term temperatures close to 50oC at intertidal sites. They grow in locations with salinities averaging 5 to 40 ppt, although major oyster aggregations are typically found at 10 to 30 ppt. They can survive for weeks in winter in nearly fresh water. Spat appear to have the same tolerance limits and optimal ranges as adult oysters, but those of larvae are more restricted and, in the case of salinity, depend on the conditions in which their parents developed gametes. In the laboratory, normal larval development has been observed at temperatures of about 15o to 30oC and salinities of 15 to 33 ppt. Over most of their range in the United States, C. virginica spawn when summer temperatures reach between 18o and 25oC; subsequent larval development occurs at somewhat higher temperatures. Larval growth rates increase rapidly with increasing temperature; the fastest rates occur near 30oC.
Although the species has a wide geographic distribution, clear genetic differences have been identified among geographically separated populations of C. virginica, based on both molecular and physiological evidence. C. virginica display geographically and genetically distinct growth, reproduction, and disease susceptibility traits. Enzyme polymorphism (Buroker, 1983) and DNA analyses show a genetic discontinuity between Gulf of Mexico and Atlantic populations (Reeb and Avise, 1990; Cunningham and Collins, 1994). Earlier studies indicated that oysters from higher latitudes spawned at lower temperatures than those from more southern locations (Stauber, 1950; Loosanoff and Nomejko, 1951), a difference later demonstrated to have a genetic basis (Barber et al., 1991). Dittman et al. (1998) subsequently observed that Long Island Sound oysters grew faster than Delaware Bay oysters. Disease resistance also has a regional genetic component. The greater resistance of southern oysters to the pathogen P. marinus was first noted by Andrews and Hewatt (1957) and demonstrated to be heritable by Bushek and Allen (1996). The existence of geographically distinct genetic traits underscores the potential problems of moving individuals, even within the same species, between regions and environments in which they have evolved.
Ecological Value of C. virginica in Chesapeake Bay
Suspension-feeding bivalves are common inhabitants of coastal and estuarine habitats throughout the world. Since these species are capable of capturing large amounts of particulate material from the water column
and transporting it to the benthic system, they are implicated as major biological agents in affecting nutrient cycling and benthic-pelagic coupling in shallow-water marine ecosystems (Wildish and Kristmanson, 1997; Dame, 1996). Suspension feeders also provide other types of ecosystem functions. For instance, their filtering activity may enhance water clarity, increasing light penetration and trapping contaminants entering coastal waters (Cloern, 1982; Newell, 1988). Materials ingested by the bivalves are expelled as feces and pseudofeces, which other groups of organisms living in or on the seafloor may bury or remobilize. Sedimentation of materials by filter feeders can change organic matter decomposition rates and anaerobic or aerobic decomposition processes.
Reef-building bivalve species (e.g., oysters, mussels) also function as “ecosystem engineers” (sensu Jones et al., 1994) through the creation and maintenance of unique habitats that are used by other species as predator refuges and feeding and nesting sites (e.g., Lenihan and Peterson, 1998; Coen and Luckenbach, 2000). As a consequence, the reefs can enhance ecosystem productivity and biodiversity. The structures also stabilize sediments, reduce coastal erosion, and alter the hydrography of shallow-water marsh creeks and embayments (Kirtley and Tanner, 1968; Dame, 1976; Meyer et al., 1997). Some suspension feeding bivalves can attain densities of 4,000 to 10,000 individuals per square meter and, when particularly abundant, have the potential to directly influence resource availability to pelagic species in the ecosystem by causing state changes in abiotic and biotic materials (e.g., Carpenter and Kitchell, 1988; Kimmerer et al., 1994; Dame, 1996).
Over the past 7,000 years the dominant epifaunal suspension-feeding bivalve inhabiting the Chesapeake Bay ecosystem has been the Eastern oyster, C. virginica (Hargis, 1999). During this period, the oysters created large reefs throughout most of the tributary estuaries in the bay with the highest abundances often associated in areas of reduced predation, either in low-salinity (<15 ppt) waters or shallow tidal creeks. But as the C. virginica population declined over 100-fold over the past 150 years, the amount of oyster reef habitat has been reduced by more than 50% (Rothschild et al., 1994). Loss of oysters is generally attributed to overfishing, habitat degradation, disease pressures, and the interaction among these factors, while destruction of reef habitat is the direct result of fishing practices used to harvest the oysters.
Habitat and Resource Provision
Oyster reefs provide essential habitat for the maintenance of oyster populations through provision of substrate for larval settlement, refuge from predators and near-bottom hypoxia, and vertical relief above the
seafloor, which acts to reduce sediment deposition. Reef formation occurs through a chemically mediated cue (Tamburri et al., 1992; Zimmer-Faust and Tamburri, 1994) in which the planktonic oyster larvae gregariously settle on hard substrates. While there is some controversy regarding the source of the settlement cue (i.e., exudates from juvenile and adult oysters: Keck et al., 1971; Veitch and Hidu, 1971) or microbial biofilms (Fitt et al., 1989, 1990; Weiner et al., 1989), the consequence of gregarious larval settlement is that oyster reefs develop as multiple generations of oysters settling one upon another (Coen and Luckenbach, 2000). Reefs can vary in size and shape (hundreds to thousands of square meters) and in subtidal regions can be up to 4 m in height (Ingersoll, 1881; Winslow, 1882; DeAlteris, 1988).
Reefs with higher profiles above the seafloor appear to promote enhanced oyster productivity. Low-profile reefs, frequently a result of harvesting practices (e.g., Marshall, 1954), enhance sediment deposition on the reef surface (DeAlteris, 1988; Seliger and Boggs, 1988). Increased sedimentation reduces the nutritional value of ingested materials, leading to reduced growth and reproduction and increased physiological stress from clogging of the oyster’s filtering mechanism (Mackenzie, 1983). High siltation levels on reefs also impair habitat quantity and quality for settling larvae and attached juveniles (Bahr, 1976). Experimental studies in North Carolina have demonstrated the importance of the interaction of reef morphology (i.e., height above the seafloor) and the abiotic environment (i.e., water flow, dissolved oxygen regime, sedimentation) on oyster productivity (Lenihan and Peterson, 1998). For instance, oyster mortality associated with bottom-water hypoxic/anoxic events was correlated with reef height. In addition to reef morphology, increased amounts of interstitial spaces within artificially constructed reef were found to enhance juvenile oyster survival (Bartol and Mann, 1999), presumably by providing the young life stages with a refuge from predators. While these studies indicate there is a complex interaction between the physical nature of an oyster reef and the physical environment surrounding it, there are few studies examining the direct and indirect impacts of reef destruction over the past century. Experimental studies, similar to those of Lenihan and Peterson (1998) and Coen and Luckenbach (2000), provide a framework for unraveling some of these complexities.
Despite the fact that oyster reefs have been a conspicuous element of the benthic landscape in the Chesapeake Bay for thousands of years, there is surprisingly little information on how reefs provide habitat for other species. The most conspicuous feature is that reefs add habitat complexity relative to the surrounding sediments. Species that enhance structural complexity can be important determinants of population and community dynamics (e.g., coral reefs: Hixon and Beets, 1993; seagrass beds: Heck
and Orth, 1980; kelp forests: Tegner and Dayton, 1987; salt marsh plants: Kneib, 1984). However, it is not well understood whether oyster reefs simply act as physical attractants or actually enhance the overall productivity of species that co-occur on the reefs.
A number of community-based surveys of finfish and macroinverte-brates inhabiting oyster reef habitat have been conducted along the south Atlantic and Gulf coasts. For example, Dame (1979) recorded 37 macrofaunal species on an intertidal oyster reef in South Carolina, and Wells (1961) found a total of 303 species inhabiting several reefs in North Carolina. Others (e.g., Arve, 1960; Bahr and Lanier, 1981) have noted that oyster reefs provide habitat for economically important fish (drums, rock-fish, speckled seatrout) and invertebrates (shrimp, blue crabs). O’Beirn et al. (1999) recorded a wide diversity of species in studies characterizing changes in the composition of benthic plants and animals following the construction of several artificial reefs in lower Chesapeake Bay. Coen et al. (1999) assessed finfish and decapods crustacean species that are found in association with oyster reefs in the bay. Using criteria developed by Breitburg and colleagues (1995, 1999), species were classified into three groups: those that used oyster reefs as their primary habitat (reef residents), those that were generally found on the reefs (facultative residents), and species that were more far-ranging and tended to forage on or near the reefs (transient species). Summarizing data collected in Maryland and Virginia waters, Coen et al. (1999) found five fish species were considered to be reef residents, four were deemed facultative residents, and 48 were considered reef transient species. All of the decapods (three species) associated with reef habitat were considered transient species.
Breitburg and Miller (1999) noted some of the resident reef fish species in the bay (particularly the naked goby, Gobiosoma bosc) were significant zooplankton predators that in turn became prey for larger transient species (e.g., striped bass, Morone saxatilis). High densities of striped bass were regularly found swimming within 1 m of artificial reefs constructed by the authors. Breitburg et al. (1999) also recorded large numbers of larval stages of some resident fish that tended to use the artificial reefs as a hydrodynamic refuge by actively congregating on the down-current side of the structures. Laboratory studies indicate that grass shrimp (Palaemonetes pugio) use oyster reef habitat as a predator refuge (Posey et al., 1999), while other species have been found to temporarily occupy natural and artificial reefs as refuges from hypoxic/anoxic events (Seliger et al., 1985; Breitburg, 1992; Lenihen and Peterson, 1998). Mann and Harding (1998) and Breitburg and Miller (1999) have characterized the trophic links between oyster reefs and selected fish species in the bay. Clearly, additional work is needed to examine the trophic and population
dynamics of reef-dwelling assemblages and the contribution reef habitat plays in supporting the productivity of those assemblages.
Coastal and estuarine ecosystems (e.g., seagrass beds, salt marshes, mangrove forests) are frequently viewed as nursery habitats for a diversity of fish and invertebrate species because of their effects on productivity and biodiversity (Chambers, 1992). While little attention has been paid to the potential value of oyster reefs as nursery habitats (Beck et al., 2001), a number of resident reef fish species have been shown to use specific microhabitats in reefs as nesting sites. Coen and Luckenbach (2000), for example, list 11 fish species common to the bay that potentially could use oyster reefs as sites for reproduction. Of these species, Coen et al. (1999) consider reef habitat essential (i.e., a habitat that is necessary to the fish for breeding, feeding, and/or growth to maturity) for five species in the bay (naked goby, Gobiosoma bosc; striped blenny, Chasmodes bosquianus; feather blenny, Hypsoblennius hentz; skilletfish, Gobiesox strumosus; and oyster toadfish, Opsanus tau).
Pelagic-Benthic Coupling and Nutrient Cycling
Oysters are active suspension feeders, acquiring food by filtering organic materials from the water column and depositing copious quantities of particulate waste materials on the seafloor. Studies in South Carolina and France (Dame et al., 1989; Zurburg et al., 1994a, 1994b) have identified reefs as intense sites of organic matter decomposition and sources of inorganic nutrients. The oysters are functioning as an important feedback loop by catalyzing the flux of particulate nutrients from the water column to sediments through biodeposits of feces and pseudofeces. Deposition fuels remineralization of organic material through complex bacterial bio-geochemical transformations and a reverse flux of organic nutrients from the sediments back into the water column. Added to the positive feedback loop of remineralization of inorganic nutrient pools is the reduced storage of nutrients in phytoplankton biomass as a consequence of bivalve grazing. This process forms another positive feedback that influences inorganic nutrient availability (Prins et al., 1995, 1997) and may also stimulate phytoplankton and macrophyte primary production. Remineralization of the nutrient pools may also influence phytoplankton development as differences in regeneration rates can change nutrient dynamics (Smaal and Prins, 1993). In addition to the positive feedback loops, Newell et al. (2002) demonstrated in laboratory experiments that oyster biodeposits can serve as sites for the removal of nitrate from the ecosystem. Accumulation of phytoplankton-rich oyster biodeposits accumulate on the sea-floor stimulates the conversion of nitrate to nitrogen gas by anaerobic bacterially mediated processes.
By promoting the flux of organic materials from the water column to the sediments, oysters help retain organic matter that may otherwise be lost from the system. Deposited organic matter supplies food to a variety of other species living in and on the reef habitat. In particular, deposit feeders and grazers (i.e., annelid worms, amphipod crustaceans) and microphytobenthos utilize organic matter found in the oyster biodeposits that support a greater diversity and abundance of benthic species than would otherwise occur in the surrounding sedimentary habitat.
Oysters and other suspension feeders can help determine the fate of contaminants through filtration, metabolic modification, and biodeposition. In estuarine waters, trace metals and synthetic organic compounds adhere to particulates and become concentrated by filter feeders such as bivalves. Bivalve tissue often reflects the current (weeks to months) contaminant burden of an ecosystem, whereas sediments reflect the longterm contaminant loading (e.g., Huanxin et al., 2000).
Ecosystem-Level Effects and Trophic Interactions
Studies over the past three decades in a number of estuarine and coastal systems strongly suggest that when benthic suspension feeders are sufficiently abundant, their feeding activities regulate the amount of phytoplankton biomass (see Smaal and Prins, 1993; and Dame, 1996, for reviews). In a theoretical analysis, Officer et al. (1982) concluded that suspension feeder grazing control of phytoplankton is only possible when bivalve biomass is high (~100 g total fresh weight per square meter), water depth is relatively shallow (a few meters), and systemwide bivalve filtration rate is of the same magnitude as the time constant for phytoplankton growth. The strongest evidence for the ability of natural populations of suspension feeders to control phytoplankton populations generally comes from systems in which bivalve biomass has been artificially enhanced or through the introduction and subsequent proliferation of nonnative species. For example, blue mussels and oysters stocked in experimental enclosures or cultured on off-bottom racks or ropes have been shown to reduce chlorophyll levels (e.g., Riemann et al., 1988; Tenore et al., 1982). Also, several species of exotic bivalves introduced into San Francisco Bay and zebra mussels in the Laurentian Great Lakes have been shown to be responsible for controlling phytoplankton stocks (Cloern, 1982, 1996; MacIssac, 1996).
Studies examining the impacts of benthic suspension feeder grazing on phytoplankton are based on in situ experiments and laboratory experiments extrapolated to provide estuarywide estimates of filtering activity. The estimates are used to develop secondary productivity models, which are coupled with primary productivity and hydrodynamic models to de-
velop ecosystem models. The models can be calibrated with field data and used as management tools to assess interactions between environmental parameters and shellfish populations (Bacher et al., 1997a; Dame, 1993; Héral, 1993).
The feeding activities of oysters remove materials in the water column besides phytoplankton that can reduce turbidity and allow more light to reach submerged aquatic plants (e.g., seagrasses, benthic microalgae). Historically, seagrass beds have been an important habitat in the bay, but beds have declined to a fraction of the previous abundances (Orth and Moore, 1983; Brush and Hilgartner, 2000). Seagrass beds are habitats for supporting many species of mobile and sessile benthic species (Orth et al., 1984) and are valuable nursery habitats for economically important shellfish and finfish. Benthic microalgae provide an important food source for benthic herbivorous meiofauna and macrofauna (Miller et al., 1996).
The reduction of oyster populations in the bay over the past 150 years is hypothesized to have removed an important control over phytoplankton blooms and to have led to the damaging consequences of nutrient enrichment and a change in the bay’s trophic structure. Newell (1988) estimated that oyster densities prior to 1870 were abundant enough to filter the entire volume of the bay every 3.3 days. As a consequence of more than a century of oyster harvesting, habitat degradation, and disease, the reduced densities of oysters are now estimated to perform the same task in 325 days (based on Newell’s calculation). Using a carbon flux model, Ulanowicz and Tuttle (1992) expanded on Newell’s work and hypothesized that an increase in oyster abundance in the mesohaline region of the bay should increase benthic diatom production and decrease phytoplankton production and stocks of pelagic microbes, ctenophores, and particulate organic carbon. While the authors cautioned that the magnitude of their predictions were not absolute, they postulated that the combined effects of decreased planktonic primary productivity and increased benthic primary productivity might reduce eutrophication in the bay. Because oysters compete with zooplankton for food, the model also predicted that the numbers of gelatinous predators that feed on zooplankton (e.g., ctenophores, medusa) would dramatically decline with increased oyster abundance.
Most of the information that the bay has changed from a “benthicdominated” to a “pelagic-dominated” system with the reduction in oyster abundance is based on very limited or anecdotal evidence. For example, Baird and Ulanowicz’s (1989) ecosystem model of the mesohaline portion of the bay revealed that zooplankton grazing on summertime primary producers was greatly reduced by the predation activities of gelatinous zooplankton (ctenophores and sea nettles). As a result, some of the
ungrazed phytoplankton fuels water column microbial activity, and the remainder sinks to the bottom and is utilized by a variety of deposit-feeding worms, amphipods, and crabs. The authors conclude their work with the question: “Was this always the case?” While records indicate that benthic filter feeders were far more abundant in the past (e.g., Newell, 1988) and presumably exerted a stronger control on water clarity, Baird and Ulanowicz (1989) note the lack of historical data on phytoplankton, zooplankton, and benthic faunal abundance to confirm whether there were significant changes in phytoplankton or other factors that could have resulted in trophic restructuring in the bay. Coupled with the 100-fold decline in oyster abundance and loss of oyster reef habitat have been dramatic increases in anthropogenic nutrient loading and associated oxygen depletion in the bottom waters during the summer (Seagle et al., 1999). Typically, more eutrophic ecosystems have shorter food chains, few levels of biological organization, lower diversity, and higher phytoplankton productivity. Some reports claim there is a lack of functional redundancy in the bay, and unlike other systems no other suspension-feeding animals have effectively replaced the effects of oyster grazing. There is also anecdotal information that when oyster reefs were abundant along the edges of the deep-water channels of the bay, they may have affected surface water mixing processes by enhancing bed roughness (Newell and Ott, 1999). As a result, less saline and higher oxygenated surface waters may have been mixed downward to deeper regions of the bay and may have helped to ameliorate the effects of hypoxic conditions in the bottom waters.
Gerritsen et al. (1994) developed a model of bivalve suspension feeding by including water mixing parameters, bivalve abundance, and filtering capacities and applied it to the Maryland portion of the Chesapeake Bay. Benthic faunal surveys in the bay (conducted in 1986 and 1987) indicated that despite severely reduced abundances of oysters, 97% of benthic organism biomass consisted of suspension-feeding bivalves (Corbicula fluminea, Macoma spp., Mulinia lateralis, Mya arenaria, Rangia cuneata, Tagelus plebeius). Exceptionally high biomass in the upper shallow reaches and oligohaline regions of the bay were due to the proliferation of nonnative species (Corbicula fluminea and Rangia cuneata). The authors found that most of the primary production appeared to be consumed by the combined actions of the bivalves, zooplankton, and deposit feeders. Phelps (1994) suggested that the major factor influencing reestablishment of submerged aquatic plants in the Potomac River was increased light penetration due to cropping of phytoplankton by filter-feeding bivalves. For the middle bay and in deeper regions, however, Gerritsen et al. (1994) found that less than half of the annual primary production was consumed. The primary factors limiting consumption of primary production by sus-
pension feeders in these portions of the bay were related to low flow rates. They concluded that consumption could be increased by raising suspension feeders in the water column where flow rates are higher.
Diseases
Disease has been among the most important influences on the population dynamics of C. virginica in the United States over the past half century. Two major diseases, MSX and Dermo, both caused by waterborne parasites, have severely reduced the abundance of Eastern oyster populations along the East Coast of the United States (Ford and Tripp, 1996). Although overharvesting had been diminishing the population abundance for decades (Rothschild et al., 1994), diseases have become more widespread and intense during this period (Burreson and Andrews, 1988), and there is no question that disease-caused mortality is largely responsible for the dramatic declines in oyster landings observed since the early 1980s. (Figure 2.1 shows the decline in landings relative to the appearance of the diseases MSX and Dermo).
P. marinus (Dermo) and H. nelsoni (MSX)
The first oyster disease to be recognized in the United States was Dermo disease, caused by P. marinus. Although it was discovered in the late 1940s in the Gulf of Mexico, it had probably been present throughout the southeastern United States and the Gulf of Mexico for many decades at least (Ray, 1996). Between its discovery and 1990, P. marinus was prevalent only in waters south of Delaware Bay. Since then epizootic outbreaks associated with a pronounced winter warming trend have been recorded as far north as Maine (Ford, 1996).
The second disease, MSX, is caused by Haplosporidium nelsoni, a parasite introduced to the East Coast of the United States from Asia, where it infects the Pacific oyster, C. gigas (Burreson et al., 2000). H. nelsoni prevalence in C. gigas is very low and results in no reported mortalities, but it is lethal to C. virginica and began causing epizootic mortalities in Delaware and Chesapeake bays in the late 1950s and early 1960s (Ford and Tripp, 1996). The pathogen is now present along the entire East Coast of the United States, although its major impact has been from Virginia north to Maine. The Long Island Sound oyster industry, which had been spared recurrent disease outbreaks, has recently suffered heavy losses, mostly due to MSX disease (Sunila et al., 1999). The devastating effect of both diseases during the past decade and a half is associated with a period of above-average temperatures and repeated droughts, both of which favor the parasites. Not only is the current depressed level of oyster production
in the Chesapeake Bay strongly linked to recent environmental extremes, but native oyster restoration efforts have been conducted under the same conditions, which so obviously favor the parasites over the oyster throughout most of the bay.
Oysters can become infected by H. nelsoni or P. marinus shortly after they set; however, infection levels in spat are typically very low because the small volume of water pumped by these tiny oysters makes their chances of encountering infective stages extremely small compared to larger oysters. Once infected, however, parasite burdens in spat can become very high. There is no evidence that either parasite can be transmitted directly from parent to offspring via infected gametes or during spawning (Ford et al., 2001). Of the two parasites, H. nelsoni generally infects and kills sooner than does P. marinus. C. virginica can suffer very heavy H. nelsoni-caused mortality during their first year of exposure, whereas P. marinus typically requires 2 or 3 years to attain full epizootic status. Nevertheless, each parasite is capable of killing 90 to 95% of susceptible C. virginica within 2 to 3 years.
The parasites have different environmental limits and different methods of transmission. P. marinus is transmitted directly from oyster to oyster, with most new infections occurring in the late summer when previously infected oysters die and release parasites into the water (Ragone-Calvo et al., 2003a). Transmission of P. marinus is thus dependent on the density of, and proximity to, infected oysters. This parasite develops the heaviest infections and kills most readily at salinities >10 ppt and temperatures >20oC, but it survives at much lower salinities (3 ppt) and temperatures (<5oC) (Chu and La Peyre, 1993; Chu et al., 1993; Ragone-Calvo and Burreson, 1994).
The life cycle and means of transmission of H. nelsoni are unknown. The spore, a life stage common to other members of the same phylogenetic group and one presumed to play a role in transmission, is extremely rare in adult Eastern oysters, although it develops readily in juvenile oysters (Barber et al., 1991; Burreson, 1994). The scarcity of spores suggests that adult Eastern oysters are a dead-end host for H. nelsoni and that there may be another host involved in the life cycle. None has been identified, however, and the possibility of a direct life cycle with juvenile oysters being the source of infective stages cannot be discounted. H. nelsoni is rare in C. virginica living at salinities <10 ppt; in fact, exposure to low salinity can eliminate the parasite from infected individuals (Andrews, 1983; Ford, 1985). This parasite begins proliferating at temperatures of about 10oC and causes most mortalities from early summer into autumn. New infections are acquired during the same period but are not dependent on proximity to infected oysters.
The temperature and salinity constraints on P. marinus and H. nelsoni have largely influenced the historical and present distribution and prevalence of infections in C. virginica. Within Chesapeake Bay, salinity has had the predominant effect. For many years after they were originally discovered in the estuary, both parasites were prevalent mostly in the high-salinity Virginia portion of the bay. Even during the drought of the mid-1960s, only the southern portion of Maryland’s Chesapeake Bay experienced significant mortalities. It was not until the 1980s and the beginning of repeated periods of below-average river flows and elevated salinities (Burreson and Ragone-Calvo, 1996) that both parasites began infecting large numbers of Maryland oysters, causing heavy mortalities and much-reduced harvests. At the same time, the parasites spread upriver in the major tributaries of the Virginia portion of the bay, killing oysters on reefs that had previously been protected from the diseases by low salinity.
A trend toward warmer winters has further favored the parasites (Cook et al., 1998). P. marinus infection intensity in oysters declines over the winter due to the death of the parasites, but some parasites survive and proliferate again as temperatures rise in the spring. When winters are short and mild, more parasites survive and are available to begin proliferating in the spring. Lethal infections are reached earlier in the summer and result in earlier transmission of parasites from dead oysters, leading to another round of infection development, death, and transmission in the same season. Winter temperatures also affect H. nelsoni, although the mechanism is not known because the method of transmission is not known. Cold winters are followed by years of low H. nelsoni infection activity (Ford and Haskin, 1982). Mathematical modeling of H. nelsoni infection cycles predicts that several successive cold winters would substantially diminish infection prevalence and suggests that climate warming, with concomitant relaxation of the cold winter effect, may be responsible for some recent disease outbreaks in the northeastern United States (Hofmann et al., 2001) and perhaps more recently in Nova Scotia, Canada (Office International des Epizooties, 2002).
Few viral or bacterial infections have been reported in adult C. virginica, and no disease outbreaks associated with these organisms have been reported. A disease of suspected bacterial origin, Juvenile Oyster Disease, was a serious problem in C. virginica nurseries in New York and New England for a number of years in the 1990s but has since subsided in most locations. Bacterial and fungal disease outbreaks sometimes develop in hatcheries where larvae are reared at high densities but have not been reported in nature, where larval densities are far less. A lethal viral disease of larvae was detected on the U.S. West Coast in a hatchery rearing the Pacific oyster, C. gigas (Elston and Wilkinson, 1985), and herpes virus has been associated with mortalities in hatcheries and nurseries rearing a
variety of commercial molluscs in numerous places around the world (LeDeuff et al., 1996; Renault et al., 2000, 2001; Arzul et al., 2001). Viruses have not been reported as the cause of problems for C. virginica; however, this may be due more to the difficulty of diagnosing viral infections than to their absence in this species or to a lack of viral involvement in the “unexplained mortalities” that occur from time to time in hatcheries and nurseries rearing C. virginica.
Interaction of Disease and Habitat
The possible influence of oyster habitat on disease is sometimes mentioned in arguments for reef restoration. A paper by Lenihan et al. (2001) is cited to demonstrate that oysters at the base of reefs become less heavily infected with P. marinus than do oysters at the crest of reefs. However, in this study, which took place in a North Carolina river, the oysters were sampled only once, shortly after they had become infected and when infections were still very light. The investigators were unable to continue sampling over time as infections intensified. Another report (Volety et al., 2000) followed oysters at intervals of 2 to 4 weeks over a 2-year period on an artificial reef in a tributary of Chesapeake Bay. P. marinus and H. nelsoni infection levels were determined in oysters near the top of the reef and those near the bottom. When data for all sampling times were pooled, oysters at the bottom of the reef had statistically fewer and lighter infections of both parasites than those near the top, but most differences in P. marinus levels occurred during the first year when infections were relatively few and very light. By the second year, nearly all samples were 100% infected, with the average intensity being moderate to heavy. No H. nelsoni was detected until year two when infection intensities were very low at both locations. Nevertheless, they were clearly fewer and less intense in oysters near the reef crest. The two studies suggest that oysters at the base of reefs may experience heavier infection pressure, perhaps because of a greater concentration of infective stages that may accumulate, along with other suspended particles, at the base of reefs (Lenihan et al., 2001). But the Chesapeake Bay study indicated that the differential in P. marinus infection levels had largely disappeared by the second year of exposure.
In Florida, Quick and Mackin (1971) found that P. marinus prevalence did not change and that intensity actually decreased with increasing depth. The results of the Chesapeake study for H. nelsoni are also at variance with a study in Delaware Bay, which found no difference in the acquisition and intensification of that parasite in oysters suspended in the water column compared to those on the bottom (Ford and Haskin, 1988). Nor do oysters in intertidal locations have fewer infections than subtidal
oysters (Gibbons and Chu, 1989; Littlewood et al., 1992; Burrell et al., 1984). The variable results of these studies, which may be linked to the particular site and methods involved, simply are not consistent enough to support the notion that position in the water would play a significant, long-term role in determining disease levels, especially during the second or third years of growth required to reach market size on reefs in the Chesapeake Bay.
Disease Resistance
Disease Resistance in Wild Oyster Populations
The potential for oysters to develop resistance to a disease was demonstrated in the early 1900s after an outbreak of “Malpeque Bay disease” in eastern Canada (Needler and Logie, 1947). Years after the epizootic, native C. virginica had good survival whereas those transplanted into the area suffered high mortality, indicating that the disease agent, never conclusively identified, was still present and that natural selection had produced a resistant local population. In the 1950s, comparison of South Carolina and Chesapeake Bay oysters exposed to P. marinus showed that the former consistently developed fewer and lighter infections than did the latter, leading to speculation that South Carolina oyster stocks may have experienced greater selective pressure than those from the Chesapeake (Andrews and Hewatt, 1957). More recently, a number of studies have involved Gulf of Mexico oysters, which have been under selective pressure from P. marinus for many decades, if not longer. Gulf stocks consistently show fewer and lighter P. marinus infections than do mid-Atlantic or northeast stocks under the same disease challenge (Bushek and Allen, 1996). Gulf oysters, however, are extremely susceptible to H. nelsoni, which has never been reported in the Gulf of Mexico. The Gulf oysters therefore experience overall higher mortalities than mid-Atlantic/northeast stocks, which have undergone varying selective pressure to both H. nelsoni and P. marinus over the years. In fact, the wild Delaware Bay oyster population appears now to be highly resistant to H. nelsoni. The development of a moderate degree of resistance was documented after the initial epizootic in the late 1950s but did not increase further because most of the surviving oysters were on the upper bay beds where they were protected from lethal infections by low salinity (Haskin and Ford, 1979). Infection prevalences remained high (50 to 80%) even though mortalities were reduced. Since the late 1980s, however, H. nelsoni infection prevalences in native oysters have been very low (<30%) in Delaware Bay, and the parasite has caused essentially no commercially significant oyster mortalities. At the same time, imported non-
Delaware Bay stocks that have not undergone selective mortality still become heavily infected, and molecular detection methods indicate that the parasite is still present and widespread in the bay. Although the putative high resistance of present-day Delaware Bay stocks has not been systematically and rigorously tested, the evidence available suggests that the current level was achieved only after extensive H. nelsoni-caused mortalities occurred on seed beds in the upper bay during two drought years in the mid-1980s. Had the initial epizootic mortalities during the late 1950s been as severe in the upper bay as they were in the mid-1980s, the high degree of resistance apparent now probably would have developed earlier.
A number of papers suggest that some localized oyster stocks in the Chesapeake show selective survival despite disease pressure (Andrews, 1968; Burreson 1991; Ragone-Calvo et al., 2003b), and Farley (1975) reported that some oysters display a histological appearance generally interpreted as evidence of resistance. However, there has been no systematic effort to document resistance in Chesapeake Bay native oysters. The development of resistance through natural selection depends on the degree of selective mortality that a population has experienced, and until recently many Chesapeake Bay populations were protected by low salinity from acquiring and developing lethal infections of either P. marinus or H. nelsoni. As long as unselected oysters survive and reproduce, it is unlikely that the overall level of resistance in the bay will improve measurably. Disease-caused mortalities in the past 2 years have extended into many subestuaries of the upper bay and may increase resistance in the overall population. Whether mortality events will be extensive enough to significantly lessen future epizootics is unknown, as are the time and the number of such widespread mortality episodes that might be needed to establish enough resistance for recovery of the population. Yet another complication is that the repletion program carried out for several decades by Maryland’s Department of Natural Resources has transferred oysters throughout the bay, diluting any acquired genetic resistance in local populations.
Selective Breeding Programs for Disease Resistance
In the early 1960s, after the initial H. nelsoni epizootics, selective breeding programs were begun at Rutgers University and the Virginia Institute of Marine Science (VIMS). Both programs used survivors as initial brood stock, exposed the progeny to natural infections, and then produced another generation from the survivors of that generation. A generation in the Rutgers program was 2.5 years, by which time the oysters were market size. By the fifth generation, on average, about 70% of the oysters were
still living at market size compared to about 7% in unselected controls (see Figure 4.1). Interestingly, the greatest improvement in survival was from the unselected to the first generation; the rate of improvement slowed noticeably after the third generation. The VIMS strains did not achieve as high a level of resistance because selection pressure was not as great at the VIMS exposure site (Haskin and Andrews, 1988). Also, development of resistance may have been less in the VIMS oysters because they were subject to infection by P. marinus. In the late 1980s and early 1990s, some of the Rutgers strains were compared to local stocks at sites from Maryland to Massachusetts. The selected strains survived and grew better than the local stocks when H. nelsoni was prevalent and performed similarly when it was not. Current tests continue to show that the selected stocks outperform unselected and local controls (see Table 4.2).
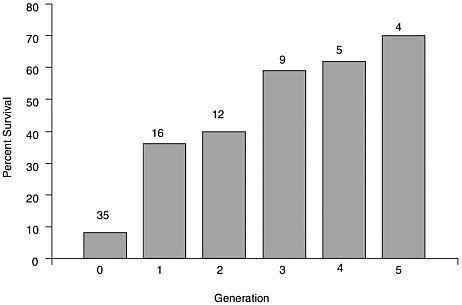
FIGURE 4.1 Percent survival, at age 2.5 years, of C. virginica bred for resistance to MSX disease at the Haskin Shellfish Research Laboratory, Rutgers University over five generations of selection, compared to susceptible controls (Generation 0), 1964 to 1988. Numbers above bars represent the number of different groups in each generation tested. One or more susceptible groups were produced each year, along with the selected groups. The continued poor survival (and high infection levels) of the susceptible oysters verified the sustained presence of the disease agent H. nelsoni.
SOURCE: Unpublished data from S. Ford, Haskin Shellfish Research Laboratory, Rutgers University, Port Norris, NJ.
TABLE 4.2 Cumulative Mortality of Selected virginica Strains and C. ariakensis and Local Control Stocks in Various Tests
|
% Mortality |
Site |
Test Period |
Reference |
|
|
Resistant Strain1 |
Local Control |
|||
|
XB and WHS |
|
|||
|
40-45 |
47 |
Chesapeake Bay, VA |
26 months |
SK Allen, VIMS, per com, 2002 |
|
40-50 |
78 |
Delaware Bay, NJ |
26 months |
SK Allen, VIMS, per com, 2002 |
|
XB and WHS |
|
|||
|
50-70 |
60 |
Chesapeake Bay, VA |
21 months |
SK Allen, VIMS, per com, 2002 |
|
22 |
38 |
Delaware Bay, NJ |
21 months |
SK Allen, VIMS, per com, 2002 |
|
55-58 |
65 |
Delaware Bay, NJ |
36 months |
SK Allen, VIMS, per com, 2002 |
|
NE 1 and NE 2 |
Cape Shore Set |
|
||
|
30-42 |
803 |
Delaware Bay, NJ |
30 months |
4 X Guo and S Ford, per com, 2002 |
|
10-15 |
853 |
Cape May Harbor, NJ |
30 months |
4 X Guo and S Ford, per com, 2002 |
|
DEBY F1 |
Mobjack Bay F |
|
||
|
20 |
82 |
Great Wicomico River, VA |
18 months |
Ragone-Calvo et al., 2003b |
|
52 |
83 |
York River, VA |
18 months |
Ragone-Calvo et al., 2003b |
|
36 |
82 |
Burton Bay, VA |
18 months |
Ragone-Calvo et al., 2003b |
|
C. ariakensis |
Mobjack Bay F |
|
||
|
14 |
80 |
Great Wicomico River, VA |
18 months |
Calvo et al., 2001 |
|
13 |
100 |
York River, VA |
18 months |
Calvo et al., 2001 |
|
16 |
100 |
Burton Bay, VA |
18 months |
Calvo et al., 2001 |
|
1Resistant strain codes: XB = Delaware Bay origin; WHS = Delaware Bay/Long Island Sound hybrid; NE 1 = Long Island Sound Origin, all selected for resistance to MSX and Dermo diseases. NE 2 = hybrid between NE 1 and brood stock from F. M. Flower Company selected for resistance to MSX, Dermo, and juvenile oyster diseases. DEBY = Delaware Bay wild, selected in Chesapeake Bay for resistance to MSX and Dermo diseases 2Infection pressure was much heavier at the Delaware Bay site compared to the Cape May Harbor site. 3Much of this mortality was due to unknown cause(s), not MSX and Dermo diseases. 4 X. Guo, Graduate Institute of Environmental and Occupational Health, Medical College, National Cheng Kung University, personal communication, 2003; S. Ford, Haskin Shellfish Research Laboratory, Rutgers University, 2002. |
||||
The selective breeding programs were crude in comparison with modern plant and animal breeding practices, and inbreeding problems eventually occurred in some lines (Vrijenhoek et al., 1990; Hu et al., 1993), which were later remedied by an improved breeding strategy (S. K. Allen, Virginia Institute of Marine Science, Gloucester Point, personal communication, 2002). Nevertheless, there has been a clear response to selection in the resulting strains, measured as both reduced infection levels and improved survival (Ford and Haskin, 1987; Ragone-Calvo et al., 2003b).
Unfortunately, the strains were not resistant to P. marinus-caused mortality (Burreson, 1991), and mortality was high when this parasite caused an epizootic in Delaware Bay in the early 1990s (S. Ford, Haskin Shellfish Research Laboratory, Rutgers University, 2002). Thus, the selection program evolved to develop Dermo disease resistance in strains already highly resistant to MSX disease. Parallel programs have been conducted at Rutgers and VIMS, and resulting strains have been evaluated at sites from Virginia to Massachusetts.
Oysters “resistant” to MSX and Dermo diseases may still become infected by the causative agents, H. nelsoni and P. marinus, but infections are slower to develop to the stage when they cause disease and mortality. In fact, a negative diagnosis in selected strains may not indicate a lack of infection, but simply infection below the level of detection by standard assays. Over time, with repeated exposures, infections may intensify to a level that is not only detectable but also heavy enough to cause disease and mortality (Ford and Haskin, 1987). Further, the differential between selected and unselected strains may diminish under conditions of very heavy infection pressure (Haskin and Andrews, 1988). Thus, the differential depends on when the comparison is made and how heavy the disease pressure is. Probably the best measure of performance for an oyster stock is survival at market size because it integrates both survival and growth rates to a commercially relevant time. Under infection pressure from both H. nelsoni and P. marinus in Delaware and Chesapeake Bays and coastal embayments of Virginia and New Jersey, the best-performing selected C. virginica strains suffer 25 to 50% the mortalities of local oysters (Table 4.2). In comparison, mortality of C. ariakensis (Chesapeake Bay only) was 15 to 19% that of local stocks. The reduced mortalities were accompanied by lower infection rates of P. marinus and H. nelsoni (Calvo et al., 2001; Ragone-Calvo et al., 2003b; S. K. Allen, Virginia Institute of Marine Science, Gloucester Point, personal communication, 2002; X. Guo, Graduate Institute of Environmental and Occupational Health, Medical College, National Cheng Kung University, Taiwan, personal communication, 2003; S. Ford, Haskin Shellfish Research Laboratory, Rutgers University, Port Norris, New Jersey, personal communication, 2002).
CRASSOSTREA ARIAKENSIS
Life History
C. ariakensis is reported to be found along the entire Chinese coastline (Tschang and Tse-kong, 1956), southern Japan (Rao, 1987), Taiwan, the Philippines, Thailand, Vietnam, and northern Boreno and Malaysia (Zhou and Allen, 2003). There are also reports of C. ariakensis on the northwest coast of India and Pakistan. However, the distributional range outside China and southern Japan has not been genetically confirmed (Allen et al., 2002), and there is considerable taxonomic confusion about the species (Coan et al., 2000; Carriker and Gaffney 1996; Zhou and Allen, 2003). Sometimes C. ariakensis has been identified as Ostrea rivularis, C. rivularis, C. discoidea (e.g., Awati and Rai, 1931; Harry, 1985; Rao, 1987) or C. paulucciae (Carriker and Gaffney, 1996). As a result, biological information on C. ariakensis in its native distribution range is somewhat difficult to unravel because C. ariakensis may have been misidentified.
C. ariakensis was inadvertently introduced to Oregon with shipments of C. gigas and C. sikamea spat from Japan in the 1970s (Breese and Malouf, 1977). Although C. ariakensis seed has been repeatedly outplanted on intertidal mudflats or suspended from floating rafts at several sites from Washington to central California (Breese and Malouf, 1977; Langdon and Robinson, 1996), there are no reports of established wild populations existing on the U.S. West Coast (Coan et al., 2000; J. T. Carlton, Williams College-Mystic Seaport Program, personal communication, 2003; R. Malouf, Oregon State University, personal communication, 2003). Apparently, seawater temperatures are not warm enough for the species to reproduce and maintain self-sustaining populations at these sites.
C. ariakensis has limited aquaculture use in Washington and Oregon because of difficulties in obtaining sufficient quantities of seed for large-scale production (Langdon and Robinson, 1996). Field and laboratory experiments in Virginia (e.g., Calvo et al., 2001) and North Carolina have used the U.S. West Coast strain originally imported from Japan. In addition, scientists at VIMS have imported strains collected from the Yellow River estuary (northern China ariakensis) and from the Guangxi province near Beihai (southern China ariakensis) for use in comparative biological studies (S. K. Allen, Virginia Institute of Marine Science, personal communication, 2003). Laboratory work has also been conducted in France with the strain that was accidentally imported from Japan to the U.S. West Coast. No wild populations currently exist in France.
While C. ariakensis has been extensively cultured throughout southern China and Japan for over 300 years (Cai et al., 1979), there is relatively little information on the ecology or biology of natural populations of the species in its native distributional range. In China the common
name is Jinjiang-muli (meaning “close to river [Jinjiang] oysters [muli]”: Zhou and Allen, 2003). Indeed, Cai et al. (1992) reported that the oyster is cultured in estuarine mid- to shallow subtidal regions of Zhanjiang Bay where water temperatures range from 14 to 31.8oC and salinity varies from 7.5 to 30.2‰. Others report that the species is normally found in areas where the salinity varies between 9 and 30‰ (Cahn, 1950) and that it can tolerate salinities less than 10‰ for short periods of time (Amemiya, 1928). Guo et al. (1999) reported that the species can tolerate a wide salinity range, though settlement is most pronounced in low-salinity estuaries and river beds. Extensive Chinese aquaculture of C. ariakensis is typically found in intertidal and shallow subtidal estuarine habitats, where concrete stakes are transported to lower-salinity waters for spat collection and then moved to more saline areas for growout to marketable sizes (Guo et al., 1999).
Similar to C. virginica, spawning activity in C. ariakensis is seasonal, and the reproductive cycle is influenced by temperature and regional environmental conditions. For China, Cai et al. (1992) reported that the reproductive season was between April and June and that larval settlement was most pronounced on the shady sides of hard surfaces. Moazzam and Rizvi (1983) reported that larval settlement in saltwater creeks in Pakistan was most pronounced from September through October, though recruitment was also observed during July to August. In a saltwater creek near Karachi, Pakistan, Ahmed et al. (1987) reported that larval settlement occurred from April to October, with highest spatfall recorded in April through July. In the Zhujiang River estuary the reproductive season is June to September and spawning is mainly in June to July; a second spawning may occur if environmental conditions are appropriate (Zhou and Allen, 2003). Asif (1979) reported that gonads generally were first present in individuals 2 to 3 months of age (0.4 to 0.6 cm in shell length) and that protandric hermaphrodites were found.
Langdon and Robinson (1996) report that mature oysters collected from Yaquina Bay, Oregon, became available for hatchery spawning in early to mid-May, and female oysters had some percentage of mature eggs until December. From August to November, females were found with more that 50% mature eggs. There was a higher degree of annual variability in the gametogenic cycle of male oysters, though the highest percentages (>50%) of mature individuals were recorded from June to February. For Dabob Bay, Washington, Perdue and Erickson (1984) reported that gonadal proliferation began in late April to early June and the greatest proliferation occurred in mid-June to October. The authors reported that C. ariakensis failed to spawn, however, due to low summer water temperatures. Luckenbach (personal communication, Virginia Institute of Marine Science, Gloucester Point, 2003) has conducted prelimi-
nary laboratory studies which suggest that C. ariakensis is capable of reproducing throughout the same salinity ranges as C. virginica (i.e., 5 TO 35‰). Modeling of larval salinity and temperature tolerances will be needed to predict the dispersal of C. ariakensis both within and beyond the Chesapeake Bay as has been done for other species (Lough, 1975; Goulletquer et al., 1994b).
One concern regarding the potential introduction of C. ariakensis to areas with native populations of C. virginica is that both species may spawn at the same time and place, raising the possibility of interspecific hybridization. Laboratory experiments conducted by Allen et al. (1993) demonstrate that, although fertilization occurs in interspecific crosses, no viable progeny are produced. The larvae do not grow and begin to die a week after fertilization.
Coan et al. (2000) reported that C. ariakensis can reach shell heights up to 20 cm, while Carriker and Gaffney (1996) noted that the oyster can be 20 to 24 cm high. Oysters growth relatively quickly in southern Chinese coastal waters and are typically harvested, when raised in extensive aquaculture conditions, within 2 to 3 years (10- to 15-cm shell length) after larval settlement (Guo et al., 1999). In U.S. waters, growth rates appears to vary with environmental conditions. For example, Langdon and Robinson (1996) reported no differences in the growth rate of C. ariakensis and C. gigas at sites in Puget Sound, Washington, and Coos Bay, Oregon, when planted on intertidal mudflats or when suspended from floating rafts in Yaquina Bay, Oregon. Both species attained shell heights of about 10 cm in roughly 2 years. C. gigas did grow faster than C. ariakensis when placed in mesh bags supported by intertidal trestle in Tomales Bay, California, (central California C. ariakensis grew to only about 6 cm in about 1.5 years, while C. gigas attained an average shell height of 10 cm). Calvo et al. (2001) grew triploid C. ariakensis in mesh bags in floating trays at six sites in the Virginia portion of Chesapeake Bay; two sites were located in low-salinity (<15‰), two in medium-salinity (15-25‰), and two in high-salinity (>25‰) waters. At deployment, oysters averaged 6.4 cm in shell height (2 years old), and growth was followed for about 9 months. It was found that shell growth was significantly slower (2.6 mm/month) in the low-salinity regime when compared to medium- (4.9 mm/month) and high-salinity waters (6.2 mm/month).
A limited number of studies have examined the predators and competitors of C. ariakensis in its natural distribution range. Several species of carnivorous snails and crabs are reported to feed on adult oysters (Zhang and Lou, 1956), while sea urchins and sea stars consume oyster spat (Zhou and Allen, 2003). Barnacles have been noted to compete for food and space with settling oyster larvae (Cai et al., 1992). Calvo et al. (2001) reported that mortalities of oysters suspended in mesh bags above
the bottom at several places in the lower Chesapeake Bay varied from 13 to 16% over a 9-month period, though the agent of mortality was not reported. The oysters were contained in mesh bags placed in suspended trays where benthic predators (e.g., crabs, snails) had reduced access to them. Moreover, the oyster leech (flatworm), Stylochus ellipticus, considered a significant predator on C. virginica, may affect C. ariakensis populations (Daniel et al., 1983; MacKenzie, 1997; Sagasti et al., 2001). Because C. ariakensis is similar in size and shell characteristics, it is likely that the same suite of benthic predators that prey on C. virginica in Chesapeake Bay would also forage on C. araikensis. Luckenbach (personal communication, Virginia Institute of Marine Science, Gloucester Point, 2003) thinks that the shell of C. ariakensis may not as be strong as C. virginica, which may suggest that the effective size refuge from crab predators is larger for C. ariakensis than for Eastern oyster.
Investigations of the larval biology of C. ariakensis are restricted to laboratory studies, most of which were conducted in highly artificial conditions to assess optimal nutritional and environmental conditions for potential aquaculture purposes. Breese and Malouf (1977) reported that maximum larval growth was obtained at 28oC at both 20‰ and 30‰ salinity. The authors found that larval settlement was highest at 28oC and 20‰, with some settlement occurring at 28oC and 15‰ and 26oC and 20‰. Other temperature-salinity combinations yielded poor larval growth and no settlement. No larvae survived at 32oC. Langdon and Robinson (1996) found that larval settlement at salinities of 15 and 20‰ was greater than 25 and 30‰ and that no settlement occurred at 35‰. A similar pattern was found when examining larval growth rate. The authors found that growth of settled oyster spat over a 2-week period (at 20‰ salinity and on a diet of the diatom Chaetoceros calcitrans) was generally fastest at 25o to 30oC, though growth occurred at temperatures ranging from 20o to 35oC. Salinity and temperature conditions were not reported, but Cahn (1950) found that newly settled C. ariakensis could be found as early as June in Japan. Preliminary laboratory studies by Luckenbach (personal communication, Virginia Institute of Marine Science, Gloucester Point, 2003) revealed that swimming larvae of C. ariakensis tended to concentrate nearer the bottoms of culturing tubes while those of C. virginica were more commonly found nearer the surface of the containers. No field observations are available on C. ariakensis larval behavior and mortality rates.
Like other oyster species, larvae of C. ariakensis must settle on solid surfaces. As mentioned earlier, suitable substrates for successful oyster settlement are those not heavily fouled by sediment or organisms (e.g., Osman et al., 1989). While Ahmed et al. (1987) reported that spatfall was primarily in the lower intertidal zone in Pakistan, a much
broader settlement range (low tide to 10-m depth) has been reported in China (Nie, 1991). Larval settlement, 12 to 18 days after spawning (Zhou and Allen, 2003), varies primarily with temperature and other environmental conditions. In southern China, spatfall has been recorded from June to August (Cai and Li, 1990), while in Pakistan larval settlement has been observed from May to December (Moazzam and Rizvi, 1983; Hasan, 1960).
There has been some uncertainty about the reef-building characteristics of C. ariakensis in different areas depending on substrate type. Yao (1988) reported finding an oyster reef primarily composed of fossil shells of the species in the tidal zone of Fujian, China. X. Guo (Graduate Institute of Environmental and Occupational Health, Medical College, National Cheng Kung University, personal communication, 2003) noted, “It is common knowledge among oyster (workers) in China that C. ariakensis is a reef builder. Areas [with] C. ariakensis reefs are north Shandong, Guangzi and Gunangdong [provinces].” There is a brief reference to reef building in the Bohai Sea cited in Wang et al. (1993, referred to by Guo). Lastly, there are some newspaper accounts of using historical reefs found in coastal low-salinity waters of the Bohai Sea in northern China to make concrete and apparently only C. ariakensis is found in this region, according to X. Guo. In Japan, however, the oyster apparently is restricted to lower intertidal muddy bottom habitats (Amemiya, 1928; Hirase, 1930). There are several reports that in India and Pakistan the oyster can be found on both hard substrates and muddy creek bottoms (Mahadevan, 1987; Patel and Jetani, 1991; Ahmed et al., 1987).
Oyster filtration rates are typically related to environmental conditions (e.g., temperature, salinity, hypoxia) as well as the quantity and quality of suspended particulate materials (e.g., Higgins, 1980; Shumway et al., 1985; Riisgard, 1988). Zhang et al. (1959) examined the feeding biology of adult C. ariakensis relative to a number of environmental parameters, and concluded that feeding rate was highest when temperature and salinity were between 10 to 12oC and 15 to 30‰, respectively. While the oyster is known to be able to tolerate reduced salinity conditions, feeding rate was significantly reduced at less than 5‰ salinity. Zhang et al. (1959) also noted that feeding rate was not influenced by high levels of suspended material. In preliminary laboratory experiments conducted by R. Newell (Horn Point Laboratory, University of Maryland Center for Environmental Science, personal communication, 2002), size-specific filtration rates of C. ariakensis appeared similar to those of C. virginica. These results are consistent with Powell et al.’s (1992) conclusion that size-specific filtration rates are quite similar for most marine bivalve species.
Disease and Disease Resistance
Observed Diseases and Parasites in Native Range
There is little information on disease outbreaks in C. ariakensis over its native range. Most C. ariakensis production appears to occur in China, which also encompasses a large portion of this species’ range. A recent publication describes recurrent large-scale mortalities (80 to 90% annually) recorded since 1992 in Guangdong province (Wu and Pan, 2000). The mortalities occur from February to May in 2- to 7-year-old oysters. The authors found heavy concentrations of what they considered to be a Rickettsiales-like organism in moribund oysters collected during one mortality episode. Rickettsiales-like organisms are common and ubiquitous in bivalves worldwide and are generally considered benign. In a few instances they have been associated with mass mortalities of other bivalves (Gulka and Chang, 1984; Gardner et al., 1995; Villalba et al., 1999), although it is not certain in any case whether these organisms are the original cause of the deaths or merely contributed to the deaths of otherwise stressed hosts. Further, the bodies identified by Wu and Pan as rickettsia have morphological and staining characteristics atypical of rickettsia and may have been misidentified. Although Wu and Pan do not mention it, toxic algal blooms are thought by others to cause the occasional large-scale mortalities of C. ariakensis in China (Zhang and Lou, 1956; Zhang et al., 1995). In a survey of molluscan aquaculture in China, Guo et al. (1999) noted reports of mass mortalities in scallops, abalone, and certain clams but none for oysters of any species.
As part of the International Council for the Exploration of the Seas (ICES) protocol being employed in tests of C. ariakensis in Chesapeake Bay, the VIMS has examined tissue sections of 242 individual C. ariakensis from three separate stocks originating from the U.S. West Coast and north and south China. Cells characterized as “unusual” were found in three oysters from the northern China site. One of the oysters contained spores and plasmodia consistent in morphology with members of the Haplosporidia, the phylum containing H. nelsoni; cells in the other two oysters were not identifiable. Neither the haplosporidian infection nor the other cells were abundant in the affected oysters.
VIMS is currently engaged in further testing of C. ariakensis. Samples were obtained from five sites along the Chinese coast in autumn 2002, and another set from the same sites will be collected in spring 2003, with further sampling planned if mortality is observed. Each sample includes 30 small and 30 market-sized cultured oysters and if present, 30 marketsized wild oysters, for a potential total of 900 individuals. The oysters are being examined by tissue slide histopathology for potential pathogens and pathological conditions, and, by polymerase chain reaction (PCR)
using DNA primers specific for Perkinsus spp. and herpes virus. Using PCR, VIMS researchers have detected herpes virus in adult C. ariakensis collected in Japan (Itoki River, Ariaki Bay) but not in two spawns of C. ariakensis made in 2002 at the VIMS hatchery (K. Reece, Virginia Institute of Marine Science, personal communication, 2002). It should be noted that herpes virus is already present in the East Coast oyster population, as reported by Farley et al. (1972) who found it in C. virginica subjected to elevated temperatures in a power plant discharge pipe in Maine.
French researchers at the Institut français de recherche pour l’exploitation de la mer La Tremblade Laboratory examined tissue sections of 15 female C. ariakensis from Hong Kong and found parasites in the egg cells of 7 individuals. The parasite could not be identified with the material available but resembles a Marteilioides-type parasite described as occurring in the egg cells of C. gigas in Japan (Comps et al., 1986).
An “Exotic” Disease
As a possible replacement for C. gigas in France, in the event the species began to have serious mortalities, French scientists imported C. ariakensis (from the Haskin Shellfish Research Laboratory in New Jersey), which they held in a “quarantine” system that treated outgoing, but not incoming, water. A sample was inspected histologically before the importation and found to have no detectable parasites, but after 7 months in the “quarantine” system, the oysters experienced high mortality. Tissues of moribund oysters were examined and found to be heavily parasitized by an organism that resembled, in all morphological and pathological details, B. ostrea, which infects Ostrea edulis, causing the disease “bonamiasis” in the same region (Cochennec et al., 1998). The authors “strongly suspected” that the C. ariakensis became infected through exposure to incoming untreated “waters…endemic for bonamiasis.” They pointed out that the parasite is the first report of a bonamia parasite in a species of Crassostrea; all other findings have been in Ostrea spp.
Resistance to MSX and Dermo Diseases
In several tests conducted since 1998, C. ariakensis has demonstrated rapid growth, high survival, and low infection levels after exposure to H. nelsoni and P. marinus in a number of locations in the Virginia portion of Chesapeake Bay and the coastal bays of Virginia’s Eastern Shore. The oyster has not been tested in Maryland, but the Virginia sites represented a variety of environments that spanned high-, medium-, and low-salinity regimes. The first trial, conducted by VIMS, extended over 18 months in 1998 and 1999. Mortality of C. ariakensis was about 15% compared to 80 to
100% of similar-sized lower Chesapeake Bay wildstock C. virginica (Calvo et al., 2001). Infection prevalence of H. nelsoni was low during the test, with a maximum in C. virginica of only 25%. No H. nelsoni was detected in any C. ariakensis. In contrast, both species developed high prevalences of P. marinus, with peaks of 68 to 84% in C. ariakensis and 100% in C. virginica. Despite the high P. marinus prevalences in C. ariakensis, all infections were categorized as “light” (unlikely to be lethal), whereas a substantial proportion of the infections in C. virginica became advanced and ultimately lethal. Subsequent trials of C. ariakensis, initiated in 2000 and 2001, were conducted by industry and did not include a disease diagnosis component; however, survival and growth of the species were reported to be extremely good. Within a year of the 2000 deployment, the C. ariakensis were of market size with little or no mortality, whereas half of the C. virginica, against which they were compared, were dead (Thompson, 2001).
One concern about the 1998 to 1999 trial (Calvo et al., 2001) was that the C. virginica had been exposed to infection during the year before the trials began, which might have explained their higher infection levels compared to C. ariakensis. Infection prevalence and intensity, especially of P. marinus, typically increase with exposure in C. virginica. Although C. ariakensis did become infected with P. marinus, there was no consistent trend toward increased prevalence or intensity in this species over the 18-month trial, which included two summer infection periods. To investigate the possibility that longer exposure might increase parasite burdens in C. ariakensis, VIMS is now examining residual oysters from the 2000 and 2001 industry trials (E. Burreson, Virginia Institute of Marine Science, personal communication, 2002). Samples collected in June, September, and October 2002, after 2 to 3 years of exposure, showed 20 to 80% prevalence of P. marinus in the C. virginica, depending on test site, but no more than 20% (all very light infections comprising just a few parasites) in the C. ariakensis. No H. nelsoni have been detected in any C. ariakensis examined. A few instances of hemocytic infiltration into the digestive epithelium and lumena have been found, but no recognizable parasites (E. Burreson, Virginia Institute of Marine Science, personal communication, 2002).
All evidence to date, then, indicates that even though C. ariakensis can become infected with P. marinus (and perhaps also with H. nelsoni, although no infections have yet been detected), infections do not develop to the point where they inhibit growth, fattening, and survival. If C. ariakensis actually does become infected with H. nelsoni, infections remain so light and localized that they have so far been undetectable by standard diagnostic methods. This result is similar to that demonstrated for C. gigas in earlier trials (Calvo et al., 1999). One difference, however, is that C.
ariakensis performed well in low salinity, whereas C. gigas grew poorly and had high mortalities. Further, C. ariakensis, under the conditions of the test, did not develop the heavy mud blisters caused by the polychaete Polydora websteri that formed in C. gigas tested in Chesapeake Bay and were also reported in earlier trials in Louisiana (Kavanagh, 1940). More recently however, some 2-year-old C. ariakensis from the industry trials, which had been deployed at a low-salinity site, were observed to have a heavy infestation of Polydora blisters (S. Ford, Haskin Shellfish Research Laboratory, Rutgers University, personal communication, 2002). Infestation by Polydora is probably a site-dependent phenomenon, as the prevalence and intensity of blisters in the 2 to 3-year-old oysters from four sites being monitored by VIMS have not been noted as being heavier than those of C. virginica at the same locations (E. Burreson, Virginia Institute of Marine Science, personal communication, 2002).
SUMMARY
-
Oysters, like other bivalve filter feeders, feed on microalgae and are capable of removing large quantities of particulate matter from the water; they have a larval stage that allows them to be dispersed from the immediate site of the parental stock; and they require a clean, solid substrate on which the larvae can settle and cement themselves.
-
C. virginica spawns during the summer, producing a larval stage that lasts for 2 to 3 weeks, during which time mortality is typically about 99%. Between settlement in mid-summer and winter, mortality of the “spat” due to predators may be nearly as high. Adults can tolerate a wide range of environments: subtidal and intertidal; salinities from <5 to 40 ppt; temperatures from freezing to 36oC, with short-term exposure to 50oC. They display genetically conserved, geographically distinct growth, reproduction, and disease susceptibility traits.
-
Two important ecological functions have been attributed to oysters: habitat and resource provision and filtration of suspended particles from the water. The first function results from habitat created by oyster beds or reefs that provides refuge for the next generations of oysters and structure, refuge, and food resources for other benthic organisms and fish. The second ecological function is the removal of organic material, primarily phytoplankton, from the water and its transformation into inorganic nutrients, thus helping to reduce turbidity. Although these functions are often attributed to oysters, it is unclear how much impact oyster reef habitat and oyster filtering capacity have on the Chesapeake Bay ecosys-
-
tem, especially given other stresses from sedimentation and nutrient overenrichment. Most of the arguments that the loss of oysters from the Chesapeake Bay has changed it from a “benthic”- to a “pelagic”- dominated system are based on very limited or anecdotal evidence.
-
Oysters, like other organisms, become infected by some parasites that cause disease (damage severe enough to cause dysfunction of an organism) and by other parasites that cause little overall harm. The parasites responsible for MSX and Dermo diseases are so virulent that they cause disease in otherwise healthy oysters. Other parasites, including many bacteria and at least one virus (herpes), are “opportunists” that proliferate and cause disease in oysters that are stressed by other factors. Opportunistic parasites are most common in hatcheries and nurseries, where larval and juvenile bivalves are grown at high densities and elevated temperatures.
-
Over the past two decades, above-average temperatures and repeated droughts have favored the parasites that cause MSX and Dermo. Not only have these conditions caused heavy oyster mortalities on existing reefs, they have impeded restoration efforts by causing mortalities on newly created reefs.
-
Resistance to disease has been documented in wild populations of C. virginica after heavy selective mortality. Selective breeding has also produced C. virginica strains that are highly resistant to MSX disease and moderately resistant to Dermo disease; however, when tested under the same conditions, C. ariakensis shows a significantly higher degree of resistance (i.e., lower infection prevalence and intensity). The best C. virginica strains have shown 50 to 75% lower mortality than local (unselected) stocks, whereas C. ariakensis has shown 80 to 85% lower mortality.
-
C. ariakensis is reported from Japan and Korea, south along the coasts of China, Malaysia, the Philippines, and westward to India and Pakistan, although genetic confirmation is lacking for most of this region and the species may have been misidentified in some cases. Relatively little biological and ecological data are available on C. ariakensis in its native range; however, available information indicates that C. ariakensis grows under environmental conditions very similar to those of C. virginica and has the same size-specific filtration rates. It is found intertidally and subtidally on reefs as well as on muddy bottom and is cultured in areas where the temperature ranges from 14o to 32oC and salinities from 7 to 30 ppt. The salinity range under which it reproduces is also similar to that for C. virginica, although it may require higher temperatures to induce spawning. Stocks imported to the West Coast of the United
-
States in the 1970s have not naturalized, presumably because the temperature is too low for reproductive success. C. ariakensis grows faster than C. virginica but has a thinner shell, which may make it more susceptible to predators such as crabs; otherwise, it should be subject to the same predation pressures as C. virginica.
-
Little information is presently available on parasites or diseases of C. ariakensis in its native range. Histological examinations of C. ariakensis at various institutes have found a few protozoan parasites in groups known to cause mortality in oysters, including a haplosporidian (MSX-like) and a Bonamia parasite, which was associated with mortality of C. ariakensis and was probably the same parasite that caused widespread mortalities of the European oyster. Other parasites, including a putative Rickettsiales-like organism and herpes virus, have been found worldwide in numerous marine bivalves.








































