CHAPTER TWO
GENERAL CONSIDERATIONS
ATMOSPHERIC SOURCES, INTERACTIONS AND SINKS
An understanding of the sources, atmospheric reactions, and eventual sinks of sulfur oxides and particles would be of enormous value in studying the health effects of these pollutants and devising appropriate control strategies. Despite a substantial effort, which has been reviewed by a number of authors (Charlson 1974, Goldstein and Nelson 1974, Whitby 1974, Urone and Schroeder, 1969), our knowledge of these processes is far from complete.
Sulfur exists in the atmosphere generally in three forms: hydrogen sulfide, sulfur dioxide, and particulate sulfate. Hydrogen sulfide is almost completely a product of natural sources, such as anaerobic bacteria (Grey and Jensen 1972), but is also to a small extent derived from industrial activity. This gas is relatively rapidly oxidized to sulfur dioxide and, on a global basis, may be the major source of atmospheric sulfur dioxide. However, man-made sources of sulfur dioxide, particularly fossil-fuel combustion, predominate in industrialized areas and their surroundings. Sulfate in the atmosphere mainly results from the oxidation of sulfur dioxide or is derived from sea spray in the form of sea-salt particles. The latter are believed to be
somewhat larger than the oxidation products of sulfur dioxide (Kellogg et al. 1972). If so, sea-salt sulfate particles should be less important toxicologically, because they will tend to settle out of the atmosphere more quickly and will not penetrate as deeply into the respiratory tract. Almost all the sulfur oxide emitted from the stationary combustion of fossil fuels is in the form of the dioxide, but about 2–5 percent is directly released as sulfuric acid.
Fossil fuels used in automobiles generally contain relatively small amounts of sulfur and are thus insignificant as sulfur dioxide sources. However, the advent of catalytic converters will result in the oxidation of gasoline to sulfuric acid which will be released in the exhaust. This could conceivably lead to a considerable ground level of sulfuric acid, particularly in heavily traveled city streets which are often poorly ventilated. This potential problem requires careful consideration before mandating the use of catalytic converters (USEPA 1975).
A number of authors have assessed the total atmospheric sulfur balance, including sources, atmospheric residence time, and eventual sinks. At present, these are estimates, inasmuch as important pertinent information is lacking (Hill 1973). Of particular relevance to the problem of sulfur oxide control is the extent to which natural and man-made sources contribute to airborne sulfur dioxide and sulfate concentrations in the United States. It appears that the bulk of airborne sulfur dioxide and sulfate in industrialized and heavily populated areas, such as the northeastern United States, is derived from fossil-fuel combustion (Altshuller 1973). However, further refinement of the data is necessary.
The atmospheric residence times of the various sulfur oxides are not precisely known. Estimates range from hours to weeks for sulfur dioxide and longer for fine particulate sulfates. Residence time depends on such factors as rainfall, windspeed, and temperature. It should be noted that climatologic conditions affect the deposition of gaseous sulfur dioxide
and of particulate sulfates differently. Furthermore, particle size and density, which also depend in part on weather, will affect the rate at which particles settle out of the atmosphere. Presumably, the height at which pollutants are emitted into the atmosphere will also modify sulfur oxide residence times. Although the exact residence times have not been established, there is ample evidence from European and American studies to indicate that sulfur dioxide and its oxidation products may be distributed up to hundreds of miles downwind from emission sources. With the possible exception of unusual atmospheric conditions in highly polluted areas, the average residence time of sulfuric acid is presumably very short.
The eventual sinks for sulfur oxides are not fully evaluated. There is some evidence that the oceans are major endpoints for the increased global output of sulfur compounds resulting from modern industrialization (Spedding 1972). Rain plays an important role in removing atmospheric sulfur oxides, but the resulting increase in raindrop acidity is believed to have affected vegetation in the United States and northern Europe adversely. Sulfur oxides returned to the soil may be reduced to hydrogen sulfide by anaerobic bacteria and released again into the atmosphere (Abeles and Craker 1971). It is therefore inappropriate to ascribe to natural sources all the sulfur dioxide produced by atmospheric oxidation of hydrogen sulfide until further information is available concerning the extent to which fossil-fuel sulfur oxide deposited in the soil is recycled. This would most likely be inconsequential on a global basis, but it might have some impact in regional air quality considerations.
Sulfur dioxide in the atmosphere is subject to a complex series of chemical reactions. For the purpose of discussion of potential health effects, a simplified reaction scheme can be written:
(1a)
(1b)
(2)
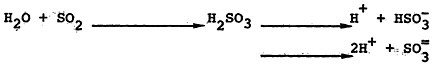
(3)
In reaction 1a, sulfur dioxide, SO2 is oxidized to sulfur trioxide, SO3 the anhydrous form of sulfuric acid, H2SO4. Inasmuch as gaseous sulfur trioxide reacts almost instantaneously with water vapor to form sulfuric acid aerosol (reaction 1b), reactions 1a and 1b can be considered to be a single reaction in which sulfur dioxide is oxidized to sulfuric acid. This process, occurs naturally in clean air and sunlight at the low rate of about 0.1 percent/hr. However, the rate is greatly increased by the presence of other air contaminants through two general mechanisms: catalysis by-trace air ions, particularly metals; and a photochemical oxidation process initiated by light in which hydrocarbons, nitrogen oxides, and activated forms of oxygen play major roles. These two processes will be discussed in more detail below. Once formed, sulfuric acid may react further to form particulate sulfates, as indicated in reaction 2, in which X represents metals or ammonium. This simplified reaction scheme indicates the three forms of sulfur oxides that are usually considered to have potential health effects: sulfur dioxide, sulfuric acid, and particulate sulfates. However, it does not indicate apparent differences in health consequences between various particles, and it ignores other potential sulfur oxide pathogens, such as organic sulfates and particulate sulfites, and possible synergistic effects between sulfur oxides and other pollutants. Furthermore, X-ray photoelectron spectroscopy has reportedly identified seven separate chemical species of sulfur in pollutant aerosols in California.
Sulfur dioxide is highly soluble in water. In the absence of processes leading to its oxidation, sulfur dioxide forms the weakly acidic sulfurous acid, H2SO3, which dissociates to bisulfite ion, ![]() , or sulfite ion,
, or sulfite ion, ![]() ,
,
depending on the pH of the aqueous solution (reaction 3). Two major types of analytic procedures have been used to measure sulfur dioxide in conjunction with the epidemiologic studies to be described below. Earlier studies usually measured the sulfation rate of a lead peroxide candle over relatively long periods. This procedure is somewhat at the mercy of meteorologic conditions and may to some extent include other sulfur oxides. More recent studies have used volumetric measurements of sulfur dioxide that are generally accurate and reliable.
The catalytic oxidation of sulfur dioxide occurs in water droplets or on suspended solid particles that contain absorbed sulfur dioxide and cations capable of accelerating the oxidation of sulfur dioxide to sulfuric acid (Chun and Quon 1973, Cheng et al. 1971, Foster 1969, Gartrell et al. 1963, Johnstone and Moll 1960, Johnstone and Coughanowr 1958, Junge and Ryan 1958). The metallic cations implicated in this process, include vanadium, iron, manganese, copper, lead, and aluminum; many of these are present in effluents with sulfur dioxide. Among the numerous variables that affect this complex reaction are the atomospheric concentrations of sulfur dioxide and the participating cations, the efficiency of the catalyst, the adsorption rate of sulfur dioxide and its diffusion within the aerosol, and the size and pH of the aerosol. These are in turn affected by weather factors, including relative humidity (which has a major influence on droplet size) and temperature (which controls the solubility of sulfur dioxide). Under uniform conditions, the reaction tends to turn itself off, in that it is inhibited by the lower pH resulting from the formation of sulfuric acid, which decreases the solubility of sulfur dioxide. However, at lower pH, water vapor is more likely to add on to the aerosol, thereby decreasing the hydrogen ion concentration (increasing the pH) and allowing the reaction to proceed. In this respect, the presence of alkaline compounds, such as metal oxides and ammonia, is also important, in that they will increase the reaction rate by buffering droplet acidity. Elucidation of the
catalytic oxidation of sulfur dioxide may be important to an understanding of the potentially toxic reactions that occur within the respiratory tract, where the relative humidity is high.
The photochemical oxidation of sulfur dioxide is also a highly complex process that is incompletely understood (James et al. 1973, Atkins et al. 1972). The requirement for sunlight limits this reaction to daylight hours, which is not the case for the catalytic oxidation of sulfur dioxide. Information concerning atmospheric photochemical reactions has been derived mainly from studies of the formation of ozone from oxygen. Superficially, the oxidation of sulfur dioxide to sulfur trioxide appears similar, in that it is predominantly a gas-phase reaction in which free-radical intermediates and excited states of oxygen are central. These reactive species are produced mainly by the action of light on nitrogen oxides in the presence of hydrocarbons. Resulting hydrocarbon radicals, singlet oxygen, and oxygen atoms have all been implicated in sulfur dioxide oxidation. One possible complication is that the formation of sulfuric acid aerosol conceivably decreases sunlight and thereby lessens the rate at which further sulfuric acid is formed. Ozone itself can also oxidize sulfur dioxide in the presence of water droplets, but this reaction occurs very slowly in the gas phase (Cox and Penkett 1972). It has recently been observed that photochemical reactions occur in a power-plant plume, resulting in the production of both ozone and sulfuric acid (Davis et al. 1974). A synergistic effect of ozone and sulfur dioxide on human pulmonary function has also been observed.
The relative contributions of the catalytic and chemical processes to sulfuric acid formation in polluted air are unknown. The concentration and chemical composition of the nonsulfur components of the pollutant mixture seem important. A theoretical approach to this problem has been presented by Judeikis and Siegel (1973).
Much of the available information on rate constants of the individual reactions was obtained from air-chamber studies, and some caution is necessary in extrapolating the results to ambient conditions. The few studies performed in ambient air have led to the estimate of well over 2 percent/hr for the rate of oxidation of sulfur dioxide in polluted air. A number of chamber and ambient studies are tabulated in more detail in the Rall report (1974).
These considerations illustrate the enormous complexity of atmospheric sulfur oxide interactions and highlight the potential importance to air pollution control of ascertaining the extent to which the various contaminants participate in producing adverse health effects or forming the offending agents. Two types of information are obviously necessary: the relative toxicities of the various airborne sulfur oxides, and the sources and atmospheric processes that result in their formation. These subjects are under study. However, available evidence indicates that further elucidation of these processes will not result in the identification of a single sulfur oxide derivative that is both solely responsible for health effects and uniquely controllable. Therefore, although further study is important, the most likely outcome pertinent to air pollution control will probably be the confirmation of the need to decrease atmospheric emission of the precursor compound, sulfur dioxide.
The natural background concentrations of sulfur oxides are difficult to ascertain, because of the widespread dissemination of these compounds from fossil-fuel combustion. For sulfur dioxide, the natural background concentration appears to be less than 0.005 ppm. In comparison, short-term concentrations well over 1 ppm have been reported during severe air pollution episodes. The U.S. standard of 0.03 ppm (annual arithmetic mean) has recently been achieved in most urban areas with the use of low-sulfur fuels. The maximal allowable 24-hr concentration is 0.14 ppm, not to be exceeded more than once a year.
Very little information is available on ambient sulfuric acid concentration, because there has been no adequate analytic method. On the basis of indirect methods, sulfuric acid has been reported to account for 12–60 percent of total atmospheric sulfate. Promising new techniques have recently been developed (Charlson et al. 1974, West et al. 1974). Presumably, the natural background concentration is negligible.
Particulate sulfate concentrations have been measured in a number of localities for more than a decade, so more information is available. But there is some question concerning the validity of the monitoring technique. One problem is the possibility that sulfates detected on the filter paper of a high-volume sampler have been formed in situ by catalytic oxidation of sulfur dioxide drawn through the apparatus (Forrest and Newman 1973). Although this has undoubtedly been a source of error (which it is hoped will be avoided by newer sampling techniques), evaluation of the data suggests that the estimation of the sulfate burden in various communities has been reasonably accurate qualitatively, and perhaps quantitatively. In particular, that possible source of error would not account for the consistently observed higher ratio of sulfates to sulfur dioxide in rural air. The rather significant rural sulfate burden, despite minimal sulfur dioxide content, has been especially evident in the Northeast and has led the Environmental Protection Agency to suggest that long-range transport of sulfur oxides produced by the relatively prevalent fossil-fuel combustion in this area has produced a regional decrease in air quality.
Representative data from the study of Altshuller (1973) indicate that the annual average sulfate concentration in eastern urban areas is about 13.5 ug/m3, compared with 6.4 ug/m3 in western urban areas. Nonurban sulfate concentration in the East averaged 8.1 ug/m3, and in the West, 2.6 ug/m3. Owing to the generally higher sulfur dioxide concentration in eastern urban areas, the ratio of sulfur dioxide to particulate sulfate was higher in eastern than in western cities. Altshuller found that particulate
sulfate and sulfur dioxide concentrations are linearly related only at lower sulfur dioxide concentrations. At higher sulfur dioxide concentrations, sulfate concentrations appear to flatten out. If validated with improved analytic techniques, this observation might indicate a limitation in the oxidation of sulfur dioxide within urban boundaries.
It must be emphasized that particulate sulfates constitute a group of compounds and that there is evidence of a difference in inherent toxicity between individual components of the group. Accordingly, in addition to measuring total sulfates, it is necessary to determine accurately the composition of this particulate fraction. The pH of particulate sulfates also appears to be important, in that acid sulfates have been found to have greater pulmonary effects in guinea pigs than neutral sulfates. The role of the weakly basic ammonia in forming acid sulfates has been evaluated in a few controlled chamber studies; atmospheric ammonia in the parts-per-billion range may have a significant impact on the formation of acid sulfates (McKay 1971, Van Den Heuvel and Mason 1963).
Of utmost importance to sulfur oxide toxicity is the observation that the overwhelming majority of sulfate compounds derived from fossil fuels are in the submicrometer range and can therefore penetrate deeply into the respiratory tract. It is generally accepted that only these smaller particles are responsible for the health effects associated with suspended particles. Although sulfates make up only 10–25 percent of total suspended particles in many areas, they undoutedly form a larger fraction of the small particles responsible for health effects.
Particles are derived from many sources, both natural and man-made. Stationary combustion of fossil fuel is a major source of particles in urban air, both because of its sulfur content and because of the presence of trace elements. However, the exact sources and inventory of particles in polluted air are not known and presumably depend heavily on local factors.
Most of the studies of adverse health effects discussed below have used one of two methods to determine particles. The preferred method in the United States has been the use of a high-volume sampler to draw a standard amount of air through filter paper; the trapped material is weighed and reported as total suspended particles. Europeans have generally used a procedure differing in a number of respects, including the final analysis, which depends on the darkness of the material on the filter paper, referred to as smoke. Both are reported in micrograms per cubic meter (ug/m3) of air. Concentrations of total suspended particles tend to be about 1.5–2.5 times higher than concentrations of smoke shade when the same air is tested, although the difference is smaller at high concentrations (Commins and Waller 1967).
The total-suspended-particles method appears to be preferable, because it does not depend on the color of the particles, but both procedures have serious limitations from the point of view of determining health effects. It is clear that only particles smaller than about 2–3 um in diameter can penetrate deeply into the lung. Furthermore, the exact composition of the particles may be very important, with respect to production of respiratory tract damage. The available data indicate that, for a given concentration of total suspended particles, there may be a wide range in the fraction that is respirable or that is of a specific form, such as sulfates or nitrates. Accordingly, the total suspended particulate or smoke shade procedures result in only an indirect measurement of those particles in polluted air which are injurious. Furthermore, the cation content of respirable particulates has generally not been measured, nor have the potential health effects of such cations been explored in detail.
METHODS OF STUDY OF SULFUR OXIDE HEALTH EFFECTS
In Vitro Studies
Test-tube studies in which cells or cellular constituents are exposed to pollutants have as
their major goal the delineation of the biochemical mechanism of pollution action. These studies are also valuable in ascertaining characteristics that may be useful in further studies of animals or humans exposed to pollutants. It must be emphasized that dose-response data obtained from such studies cannot be readily generalized to humans breathing polluted air. Therefore, although this approach is a necessary part of evaluating pollutant effects, it does not directly provide information concerning acceptable concentrations for population exposure. Whether in vitro studies indicate that low or high doses of a pollutant produce an effect may be meaningless, unless the actual dose delivered in vivo is known. There are a number of mechanisms by which pollutants can be prevented from reaching a given target site or alternatively concentrated to high levels in that area. However, in vitro studies can indicate which of many potential toxic effects might predominate in a given tissue.
Animal Experiments
The exposure of animals to defined concentrations of pollutants is an important and necessary toxicologic approach. Compared with experimental human exposure, animal studies have the significant advantage of allowing the investigator to obtain samples and use concentrations that would otherwise be precluded by ethical considerations. They are generally simpler and much less expensive. In addition, animal studies provide, the only reliable method of ascertaining the long-term effects of controlled pollutant exposures. In the case of sulfur oxide toxicity, important information has been obtained from animal studies that has had pertinent input into the design and understanding of both human-exposure and epidemiologic approaches. The difficulties in extrapolating animal data to man are derived to a large extent from species differences. This is particularly true for pulmonary effects, in view of the relatively marked difference in respiratory tract anatomy and physiology between
man and most laboratory animals. A further difficulty is the inability of the laboratory animal to communicate such subtle effects as discomfort and irritation. It should be noted that the usual conservative toxicologic approach is to assume that any effect observed in animals at a given dose can also be observed in man. In addition, in the extrapolation of standards from animal studies to man, it has generally been considered prudent to use a substantial safety margin below the minimal effective dose in animals. However, a smaller safety factor is required in situations where adequate human dose-response data are available.
Controlled Human-Exposure Experiments
Controlled human-exposure studies in which volunteers inhale defined concentrations of air pollutants, either singly or in mixtures, have provided significant information concerning the effects of sulfur oxides. This approach allows determination of a doseresponse curve and in some cases the establishment of the minimal effective dose.
However, controlled human-exposure experiments have some limitations. As pointed out above, this approach is often cumbersome and costly and is restricted in the measurable response characteristics. The pollutant dose is delivered in an artificial manner, in that the pollutant mixture usually present in ambient air is not duplicated. Accordingly, unless the investigator is specifically aware of possible synergistic pollutant interaction, controlled human exposure to an individual pollutant may provide misleading results. In the case of sulfur dioxide, the failure of usual ambient concentrations to produce effects in normal man has led to the erroneous interpretation that this compound is without harm at these concentrations.
Another limitation of human-exposure experiments is that generally healthy people are studied, rather than sensitive population groups, because the latter are often too sick to be subjected to these studies. A no-effect
exposure of healthy volunteers to pollutants may not be pertinent to susceptible subjects. Furthermore, human-exposure studies usually measure only acute responses to pollutants and have rarely provided any insight into the possible effects of chronic exposure. Inasmuch as pollutant concentrations that produce chronic disease after many decades of exposure do not necessarily result in a measurable acute response, one cannot with complete safety use no-effect concentrations in studies of human acute responses as the sole basis for air quality standards.
Epidemiologic Studies
In comparison with other fields of scientific endeavor, the epidemiologic approach used for the study of air pollutant effects is relatively crude and expensive. However, when properly performed, it has provided very useful information. Furthermore, epidemiologic studies are absolutely necessary to ascertain the minimal concentrations of air pollutants that produce deleterious health effects in human populations.
The ideal information to be extracted from epidemiologic studied would be an accurate dose-response curve for health effects. A major problem pertinent to investigation of sulfur oxides is the great difficulty involved in quantitating dose. The bulk of available epidemiologic studies evaluating sulfur oxides are seriously limited by the lack of accurate dose information for sulfuric acid and respirable particulate sulfates. In most earlier studies, measurements of only sulfur dioxide and total suspended particles are available. However, the evidence indicates that neither of these measurements directly reflects potential health effects, in that oxidation products of sulfur dioxide are probably more responsible for health effects than sulfur dioxide alone and because, of the total suspended particles, only the smaller ones can penetrate into the respiratory tract.
Another frequent difficulty with dosage data in epidemiologic studies is that the number of measuring stations is too small to identify pollutant concentrations over a large area. The reports of Corn and I.Goldstein (Goldstein 1974, Corn 1970) indicate the problems associated with such an approach. However, even if sufficient monitoring stations are used in an area to assess ambient concentrations accurately, the problem is compounded by microenvironmental variations. Exposure of an individual varies greatly, depending on his exact location and activity. Workers might spend a large portion of the day in completely different pollution environments than those who stay at home. There is also a marked difference between indoor sulfur dioxide and outdoor sulfur dioxide, and this is modified further by the exact location (Benson et al. 1972, Wilson 1968, Biersteker et al. 1965). Indoor sulfur dioxide concentrations depend on building material, wall and floor coverings, and the nature of heating and cooling systems. Outdoors, it has been shown that greenery is able to remove substantial amounts of sulfur dioxide (Martin and Barber 1970); thus, there would presumably be a difference in individual exposure, depending on whether a shrub were upwind or downwind from a pollution source. These considerations point out the potential usefulness of developing a personal monitor for epidemiologic studies.
Recent epidemiologic studies of acute health effects have begun to measure concentrations of suspended sulfates and to quantitate more accurately the size distribution and chemical characteristics of particles, so more accurate dose data should be forthcoming. However, the relative lack of such information in the past hampers determination of the role of sulfuric acid and particulate sulfates in causing or aggravating chronic respiratory disease, which presumably reflects the cumulative effects of inhalation of pollutants over a period of decades. Long-term sulfate monitoring data are available for various sites, and reasonably valid assumptions can be made by extrapolating present monitoring and emission information to
the past, so that epidemiologic studies of the prevalence of chronic disease in relation to individual sulfur oxides are not totally precluded.
In terms of the response side of the dose-response curve for sulfur oxides, a relatively large mass of information is available on both acute and chronic effects. Numerous studies have evaluated the effects of short-term exposures on acute responses, such as mortality, and on various indicators of respiratory morbidity. For such studies to provide valid information, other factors must also be evaluated. Unfortunately, variables like temperature and influenza epidemics have often not been taken into account. Furthermore, different analytic and statistical approaches have been used to evaluate the available data, which makes it difficult to compare studies performed by different investigators. The most thorough studies will, of course, develop a highly complex set of data, which will require great sophistication to handle and analyze. In such situations, interpretation of the applicability of the results can be difficult. For instance, the finding that one particular response is statistically related to a particular dose of a pollutant at the 5 percent level of confidence (p=0.05)—i.e., there is a 1-in-20 possibility that the result is due solely to chance—would have questionable value in a study in which 20 sets of response-dose combinations were evaluated.
Another point should be noted on the subject of the relevance of epidemiologic studies of acute nonlethal responses in setting air quality standards: The question of what constitutes a disease effect and what can be considered a normal nonharmful response. As an extreme, it could be argued that there is no need to protect an entire population against a pollutant concentration that causes a few susceptible people to cough once a day. To the extent that measured acute responses can be considered completely reversible, their importance is open to question. However, although a cough itself may be merely a transient symptom, it may also indicate an acute irreversible effect on the
respiratory tract. It is also conceivable that repeated reversible adaptive changes over a period of many decades may lead inexorably to a chronic disease process.
The resolution of this question must ultimately come from epidemiologic studies of the chronic effects of exposure to pollutants. Such studeis have often taken the approach of determining the prevalence of respiratory disease in communities with different degrees of pollution. The prevalence of respiratory disease has usually been measured by administration of a questionnaire, sometimes in conjunction with pulmonary-function tests. Major requirements for the successful completion of such studies include the use of a valid questionnaire, the determination of pulmonary function with equipment that gives reproducible results, and, most important, the standardization and minimization of observer variation. Unfortunately, such requirements have not been fully met in all studies. There is also the problem of using communities that differ only in their pollution. Occupational exposures, socioeconomic and ethnic differences, and time of residence in the area may blur the findings. Cigarette-smoking, the most important factor in respiratory disease, must be carefully evaluated. Recent studies have even suggested that cigarette-smoking by parents may increase the frequency of respiratory disease in their children (Colley 1974, Nelson et al. 1974). Subtle differences in life style may be important and may account for the observed higher prevalence of respiratory disease in urban than in rural areas. To the extent that exercise improves cardiopulmonary function, the rural resident may have an advantage over the sedentary urban resident. In addition, it is possible that some people with chronic respiratory disease move into the city in search of better medical care or out of the city to escape pollution.
Another approach to the determination of the effect of air pollution on the prevalence of respiratory disease is to study communities repetitively as their degree of pollution changes. This has the distinct advantage of
allowing individual communities to serve as their own controls. In retrospect, it is unfortunate that intensive community studies were not started in the middle of the last decade and continued through succeeding years in cities that experienced a marked decline in sulfur dioxide concentration owing to pollution control measures. Recent fluctuations in the availability of lowsulfur fuel may provide an opportunity to perform such studies.
This discussion has focused generally on the difficulties in performing and interpreting epidemiologic studies related to the effects of sulfur oxides. It must be emphasized that careful epidemiologic studies can yield excellent information concerning the health effects of air pollutants. Such studies have clearly indicated a causal role of atmospheric sulfur oxides in human disease. At the present time, the critical considerations discussed above are significant mainly in determining which sulfur oxide contaminant at what concentration produces how much disease.
NORMAL LUNG FUNCTION IN RELATION TO SULFUR OXIDE HEALTH EFFECTS
Respiration is a complex, finely tuned process that results in the delivery of oxygen to the bloodstream and the removal of carbon dioxide. Air is brought into the respiratory system through either of two external passageways, the nose and the mouth. These join at the back of the throat and lead through the larynx into the trachea. In the upper part of the chest, the trachea subdivides into right and left mainstem bronchi, which lead to the lungs. The bronchi further subdivide about 26 times, becoming progressively smaller in diameter until reaching the alveoli. The alveoli are sac-like bags surrounded by blood-containing capillaries in which gases are exchanged between blood and the air brought down the bronchial tree.
Optimal gas exchange, the major function of the lungs, requires an appropriate balance between blood flow and the volume of air reaching the alveoli. Both are influenced by a host of
neuronal, physical, and humoral factors. Sulfur dioxide seems to affect the delivery of gases to and from the alveoli, rather than blood flow itself or the intra-alveolar gas-exchange process. However, there is some evidence that inhalation of aerosols of sulfuric acid, and perhaps particulate sulfates, which are small enough to penetrate far into the lung, may alter alveolar gas exchange. In addition, the susceptibility of patients with cardiac disease to air pollution episodes and the apparent reflex connections between the heart and lungs make it at least possible that sulfur oxide inhalation secondarily affects cardiopulmonary hemodynamics.
The respiratory consequences of the inhalation of sulfur oxides and particles depend greatly on which external orifices are in use. The convoluted and moist nasal passageways are extremely efficient filters for highly soluble gases, such as sulfur dioxide, and for particles (Vaughan et al. 1969, Frank et al. 1967, Frank and Speizer 1965, Dalhamn and Strandberg 1963). A number of studies have indicated that quiet breathing through the nose results in almost total clearance of sulfur dioxide and of larger particles. However, it must be emphasized that there is evidence suggesting a nasal-bronchial reflex whereby stimulation of the nasal mucosa results in constriction of the bronchi. It has been hypothesized that, the nasal mucosal concentration of sulfur dioxide required to produce bronchoconstriction may be lower than the bronchial concentration required (Andersen et al. 1974). Although there is some evidence to support the latter hypothesis, bronchoconstriction in response to nasal sulfur dioxide has been observed only at relatively high pollutant concentrations. It is therefore doubtful whether inhalation of gaseous sulfur dioxide solely through the nose at ordinary ambient concentrations has in itself any direct untoward effect in man. Whether the discharge of nasal receptors at concentrations below the threshold to produce bronchoconstriction has an additive effect with other pollutant and nonpollutant bronchoconstrictor factors is unknown.
The mouth is a far less efficient filter than the nose and permits a much higher concentration of inhaled gaseous or particulate agents to reach the upper airways. A propensity to breathe through the mouth would therefore be expected to lead to an increased rish of pollutant effect in the respiratory tract. Among the causes of increased mouth breathing are exercise, emotional stress, and blockage of the nasal passageways, the latter usually occurring as a consequence of viral upper respiratory infection. The relative susceptibility to air pollutants of children, who frequently mouth breath, and perhaps of chronically ill individuals with upperrespiratory infections may be explainable on this basis.
Although less efficient than the nose, the mouth, pharynx, and larynx would still be the site of deposition of an appreciable amount of inhaled sulfur dioxide and aerosols. The efficiency of removal would be expected to depend to some extent on the rate of air flow into the lung. During exercise, pulmonary ventilation can increase by about a factor of 10; and at high inspiratory flow rates, more pollutants would be expected not only to be brought into the upper airways, but also to penetrate more deeply into the lung before eventual deposition. This may be another reason for the apparent sensitivity to pollution of children, who generally exercise far more often than adults.
An important aspect of pulmonary function is the defense against foreign material inhaled into the trachea and more distal portions of the lungs. The mucoid secretion of cells lining the trachea and larger parts of the bronchi plays a major role in protecting lung parenchyma from exogenous agents. Under normal conditions, respiratory tract mucus can be considered to be a renewable disposable lining that acts to mask underlying structural elements. Fine hair-like structures called cilia, which line the airways, impart the major propulsive force to move mucus up toward the throat, where it is swallowed. Unfortunately, relatively little is known about the basic biologic mechanisms responsible for normal mucociliary transport. Of particular
pertinence would be additional information on ability of mucus to buffer inhaled acid aerosols (Dalhamn 1956) and on the reactions of multiple pollutants—e.g., sulfur dioxide and ozone—within this layer.
As with many other defense mechanisms, exaggeration of the normal response is associated with disease processes. Thus, increased mucus production is a hallmark of chronic bronchitis. Difficulty in the clearance of mucus from airways, resulting in interference with air flow, is a complicating process in a number of respiratory disorders, including chronic obstructive pulmonary disease. This is a problem particularly during expiration when the bronchi are normally relatively constricted.
Very high concentrations of sulfur dioxide have been shown in short-term animal studies to interfere with mucociliary transport (Ferin and Leach 1973, Spiegelman et al. 1968, Dalhamn and Strandberg 1963). Chronic exposure to exceedingly high concentrations has been reported to produce an increase in the number of mucus-secreting cells in the tracheobronchial tree similar to that observed in human chronic bronchitis (Lamb and Reid 1968). However, there is no direct evidence of an effect of sulfur oxides, in concentrations approaching ambient conditions, on mucociliary function in the lower respiratory tract.
Alveolar macrophages are also important in pulmonary defense. These phagocytic mononuclear cells play a major role in destroying inhaled pathogenic microorganisms, and they are also able to phagocytize inhaled particles. Although products of stationary fossil-fuel combustion have been epidemiologically associated with an increased incidence of respiratory infection, very little is known about the effect or realistic concentrations of sulfur oxides or particles on alveolar macrophage function in vivo. The possibility that ingested particles interfere with the bactericidal effects of alveolar macrophages appears worthy of study.
INDIVIDUAL VARIATIONS IN RESPONSE
The air pollution literature contains a refrain citing the enormous individual variability in response to air pollutants. This has been observed not only in epidemiologic studies and controlled human-exposure experiments, but also in investigations involving laboratory animals with similar genetic and environmental backgrounds. Variability in individual response has obvious implications for air pollution control strategy. If the goal, as implied in the Clean Air Act, is to protect every free-living individual in our society, the obviously air quality standards based solely on the response of the average person will not protect hypersensitive groups. The following discussion considers some susceptible groups and the possible underlying basis for their sensitivity.
Groups that have been suggested as more sensitive to the effects of air pollution include young children (possibly because of their higher inspiratory flow rates, more mouth-breathing, and frequent respiratory tract infections), the aged (perhaps because of decreased cardiopulmonary adaptive capacity), people with preexisting chronic bronchitis and emphysema (at least partly because of impaired respiratory tract defense mechanisms), and asthmatics (who tend to hyperrespond to inhaled irritants). Obviously, a substantial portion of the entire population fits into these categories.
There is excellent evidence that ethnic groups are inherently different in their response to respiratory irritants. Such differences might explain some of the wide variation in international chronic bronchitis prevalence rates, although cigarette-smoking, environmental factors, and patterns of medical diagnosis undobtedly play a large role (Holland et al. 1965, Mork 1964, Olsen and Gilson 1960). In Great Britain, there is some suggestion that the Welsh may be inherently more susceptible to environmental respiratory stressors.
Differences between the white and nonwhite populations in the United States have been
observed. Groups with pulmonary-function values lower than usual include blacks, Asians, and Puerto Ricans (Stebbings 1973, Oscherwitz et al. 1972, Stebbings 1972, Densen et al. 1967). There is also fairly good evidence that blacks are less responsive than whites to inhaled toxic agents. Densen et al. evaluated more than 12,000 male New York City postal and transit workers and found that, although the prevalence of chronic bronchitis in nonsmokers was slightly greater in the nonwhite population, the prevalence among smokers was much greater in the nonwhite group (Densen et al. 1967). For any given degree of cigarette consumption, nonwhites were less symptomatic than whites. Similar findings have been observed in other studies (Finklea et al. 1974, Massaro et al. 1965). Review of the U.S. chronic respiratory disease mortality data also tends to support a greater susceptibility to pulmonary irritants among the white population. Although the male death rates for bronchitis and emphysema were similar in whites and nonwhites 25 years ago, the recent steep increase in deaths from these disorders has been much greater in whites, so there is now a substantial difference between the two (HEW 1974).
Differences between the sexes have also been observed, with women appearing to be far less responsive to cigarette-smoking or air pollution. Although a few questionnaire studies have shown equal or greater prevalence rates of respiratory symptoms in women, this is probably because (as pointed out by Ferris) women have filled out the questionnaires for entire families and are more cognizant of their own symptoms (Ferris 1969).
Determination of ethnic differnces may be very important in the interpretation of the many excellent and comprehensive Japanese epidemiologic air pollution studies. In general, these studies have tended to yield evidence of an effect of much lower concentrations of pollutants than have American or European studies. This has been true for photochemical oxidants and nitrogen dioxide, as well as for sulfur dioxide and particles. In addition to possible ethnic variations in
response, this apparent susceptibility might result from some difference in methodologic approach or from synergistic effects due to the presence of significant amounts of both photochemical and stationary-source pollutants in the same areas. Accordingly, it is believed that it would be inappropriate to generalize Japanese dose-response data to the American population until proper comparative studies are performed. These are urgently needed.
The respiratory tract has a complex series of defense mechanisms that allow it to rid itself of inhaled toxic agents. Therefore, it can be predicted that any disease process that interferes with this self-cleansing will sensitize the lungs to the effects of air pollutants. This has been amply demonstrated in a number of population studies in which acute morbidity in response to daily air pollution variations was most readily observed in people who had preexisting lung disease. Similarly, an acute respiratory tract infection might render a person transiently susceptible to pollution (Creasia et al. 1973). Chronic cigarette-smoking also tends to result in a sensitivity to air pollution, and the effects appear to be independent and roughly additive (Finklea et al. 1974, Lambert and Reid 1970).
All those factors, however, do not account completely for the large variability in response to air pollutants among healthy people. Undoubtedly, genetic factors are important. Evidence on this point includes the study of Cammer et al., in which the rate of tracheobronchial clearance of an aerosol was found to differ markedly among normal people, but to be highly similar in monozygotic (identical) twins and somewhat less similar in dizygotic (fraternal) twins (Camner 1972). A familial tendency toward chronic bronchitis has also been suggested, and inherited factors might be responsible for differences in the metabolism of pollutants and in the bronchoconstrictive response to inhaled irritants.
In addition to disease states and smoking, other, more subtle environmental factors might play a role in conditioning the response to sulfur oxides. Cohen et al. (1973) have
reported that the administration of tungsten to rats on a low-molybdenum diet produced a loss in sulfite oxidase activity and an increase in sensitivity to the systemic effects of high sulfur dioxide concentrations. It is unlikely that such gross alterations will occur in normal humans, but the possibility that nutritional or pharmaceutical agents can modify the response to air pollutants cannot be overlooked.
Another mechanism by which responses to air pollutants may be altered is adaption. There is excellent evidence from controlled human-exposure studies and the occupational hygiene literature that the acute response to sulfur dioxide decrease in many if not all people during continued or repetitive exposure. A number of epidemiologic studies have also indirectly suggested that adaption to sulfur oxides and particles may occur during the course of a winter exposure period. Although adaption to ambient concentrations of sulfur oxides and particles is still open to question, two points about this process are worthy of mention:
First, adaption to the acute effects of a toxic agent does not necessarily imply protection against chronic effects; in fact, over a long period, the adaptive response may itself be harmful. Second, if adaption does exist, it is conceivable that a lessening in the response of individuals to the pollutant will be observed during a period of gradually decreasing atmospheric concentration; however, once a new concentration has become stable and is maintained long enough for the adaptive response to wear off, variations around the lower concentration might again produce acute responses. The implications of the latter supposition for the recent period of decreasing air pollution are obvious. Accordingly, more information on the extent and duration of human adaption to sulfur oxides would be of great value.
LITERATURE CITED
Abeles, F.F., and L.E.Craker (1971) Fate of air pollutants: Removal of ethylene, sulfur dioxide, and nitrogen dioxide by soil. Science 173:914–916.
Altshuller, A.P (1973) Atmospheric sulfur dioxide and sulfate. Distribution of concentration at urban and nonurban sites in United States. Environ. Sci. Technol. 7:709–712.
Andersen, Ib., G.R.Lundqvist, P.L.Jensen, and D.F.Proctor (1974) Human response to controlled levels of sulfur dioxide. Arch. Environ. Health 28:31–39.
Atkins, H.F., R.A.Cox, and A.E.J.Eggleton (1972) Photochemical ozone and sulphuric acid aerosol formation in the atmosphere over Southern England. Nature 235:372–376.
Calvert, J.G., D.H.Slater, and J.W.Gall (1971) The methyl radical-sulfur dioxide reaction, pp. 133–158. In C.S.Tuesday, Ed. Chemical Reactions in Urban Atmospheres. Proceedings of the Symposium held at General Motors Research Laboratories, Warren Michigan, 1969. New York: American Elsevier Publishing Company, Inc.
Camner, P., K.Philipson, and L.Friberg (1972) Tracheobronchial clearance in twins. Arch. Environ. Health 24:82–87.
Charlson, R.J. (1974). Personal communication.
Charlson, R.J., A.H.Vanderpol, D.S.Covert, A.P.Waggoner, and N.C.Ahlquist (1974) Sulfuric acid-ammonium sulfate aerosol: Optical detection in the St. Louis region. Science 184:156–158.
Cheng, R.T., M.Corn, and J.O.Frohliger (1971) Contribution to the reaction kinetics of water soluble aerosols and Sulfur dioxide in air at ppm concentrations. Atmos. Environ. 5:987–1008.
Chun, K.C., and J.E.Quon (1973) Capacity of ferric oxide particles to oxidize sulfur dioxide in air. Environ. Sci. Technol. 7:532–538.
Cohen, H.J., R.T.Drew, J.L.Johnson, and K.V.Rajagopalan (1973) Molecular basis of the
biological function of molybdenum. The relationship between sulfite oxidase and the acute toxicity of bisulfite and sulfur dioxide (rat/tungsten/molybedenum deficiency/systemic and respiratory toxicity/enzyme induction). Proc. Nat. Acad. Sci. 70:3655–3659.
Colley, J.R.T. (1974) Respiratory symptoms in children and parental smoking and phlegm production. Brit. Med. J. 2:201–204.
Commins, B.T., and R.E.Waller (1967) Observations from a ten-year study of pollution at a site in the City of London. Atmos. Environ. 1:49–68.
Corn, M. (1970) Measurement of air pollution dosage to human receptors in the community. Environ. Res. 3:218–233.
Cox, R.A., and S.A.Penkett (1970). The photo-oxidation of sulphur dioxide in sunlight. Atmos. Environ. 4:425–433.
Cox, R.A., and S.A.Penkett (1971) Oxidation of atmospheric Sulfur dioxide by products of the ozone-olefin reaction. Nature 230:321–322.
Cox, R.A., and S.A.Penkett (1971) Photooxidation of atmospheric sulfur dioxide. Nature 229:486–488.
Cox, R.A., and S.A.Penkett (1972) Aerosol formation from sulphur dioxide in the presence of ozone and olefinic hydrocarbons. J. Chem. Soc. 68:1735–1753.
Creasia, D.A., P.Nettesheim, and A.S. Hammons. (1973) Impairment of deep lung clearance by influenza virus infection. Arch. Environ. Health 26:197–201.
Dalhamn, T. (1956). Mucous flow and ciliary-activity in the trachea of healthy rats and rats exposed to respiratory irritant gases (Sulfur dioxide, H3N, HCHO). A functional and morphologic (light microscopic and; electron microscopic) study, with special reference to technique. Acta Physiolog. Scand. 36 (Suppl. 123):1–161.
Dalhamn, T., and L.Strandberg (1963) Synergism between sulphur dioxide and carbon particles. Studies on adsorption and on ciliary movements in the rabbit trachea in vivo. Int. J. Air Wat. Pollut. 7:517–529.
Davis, D.D., G.Smith, and G.Klauber (1974). Trace gas analysis of power plant plumes via aircraft measurement: O3 NOx, and SO2 chemistry. Science 186:733–736.
Densen, P.M., E.W.Jones, H.E.Bass, and J.Breuer (1967) A survey of respiratory disease among New York City postal and transit workers. 1. Prevalence of symptoms. Environ. Res. 1:262–286.
Ferin, J., and L.J.Leach (1973) The effect of SO2 on lung clearance of TiO2 particles in rats. Amer. Ind. Hyg. Assoc. J. 34:260–263.
Ferris, B.G., Jr (1969) Chronic low-level air pollution use of general mortality, and chronic disease morbidity and mortality to estimate effects. Environ. Res. 2:79–87.
Finklea, J.F., F.Goldberg, V.Hasselblad, C.M.Shy, and C.G.Hayes. (1974) Prevalence of chronic respiratory disease symptoms in military recruits: Chicago induction center, 1969–1970, pp. 4–23 to 4–36. In U.S. Environmental Protection Agency, Office of Research and Development. Health Consequences of Sulfur Oxides: A Report from CHESS, 1970–1971. EPA-650/1–74–004. Washington, D.C.: U.S. Government Printing Office.
Finklea, J.F., C.M.Shy, G.J.Love, C.G.Hayes, W.C.Nelson, R.S.Chapman, and D.E.House (1974) Health consequences of sulfur oxides; Summary and conclusions based upon CHESS studies of 1970–1971, pp. 7–3 to 7–24 (see p. 7–6). In U.S. Environmental Protection Agency, Office of Research and Development. Health Consequences of Sulfur Oxides: A Report from CHESS, 1970–1971. EPA-650/1–74–004. Washington, D.C.: U.S. Government Printing Office.
Forrest, J., and L.Newman (1973) Ambient air monitoring for sulfur compounds. A critical review. J. Air Pollut. Control Assoc. 23:761–768.
Foster, P.M. (1969) The oxidation of sulphur dioxide in power plant plumes. Atmos. Environ. 3:157.
Frank, N.R., and F.E.Speizer (1965) SO2 effects on the respiratory system in dogs.
Changes in mechanical behavior at different levels of the respiratory system during acute exposure to the gas. Arch. Environ. Health 11:624–634.
Frank, N.R., R.E.Yoder, E.Yokoyama, and F.E.Speizer. (1967) The diffusion of 35SO2 from tissue fluids into the lungs following exposure of dogs to 35SO2. Healthy Phys. 13:31–38.
Gartrell, F.E., F.W.Thomas and S.B.Carpenter (1963) Atmospheric oxidation of Sulfur dioxide in coal-burning power plant plumes. Amer. Ind. Hyg. Assoc. J. 24:113–120.
Goldstein, I.F., F.Landovitz, and G.Block (1974) Air pollution patterns in New York City. J. Air Pollut. Control Assoc. 24:148–152.
Golstein, B., and N.Nelson (1974) Background review: Atmospheric chemistry, pp. 105–108. In Rall, D.P. Review of the health effects of sulfur oxides. Environ. Health Perspect. 8:97–121.
Grey, D.C., and M.L.Jensen (1972) Bacteriogenic sulfur in air pollution. Science 177:1099–1100.
Groblicki, P.J., and G.J.Nevel (1971) The photochemical formation of aerosols in urban atmospheres, pp. 241–267. In C.S.Tuesday, ED. Chemical Reactions in Urban Atmospheres. Proceedings of the Symposium held at General Motors Research Laboratories, Warren, Michigan, 1969. New York: American Elsevier Publishing Company, Inc.
Hill, F.B. (1973) Atmospheric sulfur and its links to the biota, pp. 159–181. In G.M.Woodwell, and E.V.Pecan, Eds. Carbon and the Biosphere. Proceedings of the 24th Brookhaven Symposium in Biology, Upton, New York May 16–18, 1972. CONF-720510. Washington, D.C.: United States Atomic Energy Commission, Technical Information Center, Office of Information Services.
Holland, W.W., D.D.Reid, R.Seltser, and R.W.Stone (1965) Respiratory disease in England and the United States. Studies of comparative prevalence. Arch. Environ. Health 10:338–343.
James, F.C., J.A.Kerr, and J.P.Simons (1973) Direct measurement of the rate of reaction of the methyl radical with sulphur dioxide. J. Chem. Soc. 69:2124–2129.
Johnstone, H.F., and D.R.Coughanowr (1958) Absorption of sulfur dioxide from air: Oxidation in drops containing dissolved catalysts. Ind. Eng. Chem. 50:1169–1172.
Johnstone, H.F., and A.J.Moll (1960) Formation of sulfuric acid in fogs. Ind. Eng. Chem. 52:861–863.
Judeikis, H.S., and S.Siegel (1973) Particle-catalyzed oxidation of atmospheric pollutants. Atmos. Environ. 7:619–631.
Junge, C.E., and T.G.Ryan (1958) Study of the SO2 oxidation in solution and its role in atmospheric chemistry. Quart. J. Roy. Meteor. Soc. 84:46–56.
Kellogg, W.W., R.D.Cadle, E.R.Allen, A.L.Lazrus, and E.A.Martell (1972) The sulfur cycle. Man’s contributions are compared to natural sources of sulfur compounds in the atmosphere and oceans. Science 175:587–596.
Lamb, D., and L.Reid (1968) Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J. Path. Bacteriol. 96:97–111.
Lambert, P.M. and D.C.Reid (1970) Smoking, air pollution, and bronchitis in Britain. Lancet 1:853:857.
Massaro, D., A.Cusick, and S.Katz (1965) Racial differences in incidence of chronic bronchitis. Preliminary report. Amer. Rev. Resp. Dis. 92:94–101.
McKay, H.A.C. (1971) The atmospheric oxidation of sulphur dioxide in water droplets in presence of ammonia. Atmos. Environ. 5:7–14.
Mork, T. (1964) International comparisons of the prevalence of chronic bronchitis. Proc. Roy. Soc. Med. 57:975–978.
Nelson, W.C., J.F.Finklea, D.E.House, D.C.Calafiore, M.Hertz, and D.H.Swanson (1974) Frequency of acute lower respiratory disease in children: Retrospective survey of Salt Lake Basin communities, 1967–1970, pp. 2–55 to 2–73. In U.S. Environmental
Protection Agency, Office of Research and Development. Health Consequences of Sulfur Oxides: A Report from CHESS, 1970–1971. EPA-6501–74–004. Washington, D.C.: U.S. Government Printing Office.
Olsen, H.C., and J.C.Gilson (1960) Respiratory symptoms, bronchitis, and ventilatory capacity in men. An Anglo-Danish comparison, with special reference to differences in smoking habits. Brit. Med. J. 1:450–456.
Oscherwitz, M., S.A.Edlavitch, T.R.Baker, and T.Jarboe (1972) Differences in pulmonary functions in various racial groups. Amer. J. Epidemiol. 96:319–327.
Sidebottom, H.W., C.C.Badcock, G.E.Jackson, J.G.Calvert, G.W.Reinhardt, and E.K.Damon (1972) Photooxidation of sulfur dioxide. Environ. Sci. Technol. 6:72–79.
Spedding, D.J. (1972) Sulphur dioxide absorption by sea water. Atmos. Environ. 6:583–586.
Spiegelman, J.R., G.D.Hanson, A.Lazarus, R.J.Bennett, M.Lippmann, and R.E.Albert. (1968) Effect of acute sulfur dioxide exposure on bronchial clearance in the donkey. Arch. Environ. Health 17:321–326.
Stebbings, J.H., Jr. (1972) A survey of respiratory disease among New York City postal and transit workers. III. Anthropometric, smoking, occupational, and ethnic variables affecting the FEV1, among white males. Environ. Res. 5:451–466.
Stebbings, J.H., Jr. (1973) A survey of respiratory disease among New York City postal and transit workers. IV. Racial differences in the FEV1; Environ. Res. 6:147–158.
Timmons, R.B., H.F.LeFevre, and G.A.Hollinden (1971) Reactions of sulfur dioxide of possible atmospheric significance, pp. 159–190. In C.S.Tuesday, Ed. Chemical Reactions in Urban Atmospheres. Proceedings of the Symposium held at General Motors Research Laboratories, Warren, Michigan, (1969). New York: American Elsevier Publishing Company, Inc., (See p. 185).
Urone, P., and W.H.Schroeder (1969) SO2 in the atmosphere: A wealth of monitoring data, but few reaction rate studies. Study of the reactions of SO2—a vital clue to the chemistry of polluted atmospheres—is beset with difficulties. Environ. Sci. Technol. 3:436–445.
U.S. Department of Health, Education, and Welfare (1974) Mortality Trends for Leading Causes of Death, United States. 1950–1969. (p. 39). Vital and Health Statistics, Series 20, #16 (HRA) 74–1853. Washington, D.C.: U.S. Government Printing Office.
U.S. Environmental Protection Agency (1975) Estimated public health impact as a result of equipping light-duty motor vehicles with oxidation catalysts. Office of Research and Development, Office of Air and Waste Management, January 30.
Van Den Heuvel, A.P., and B.J.Mason (1963) The formation of ammonium sulphate in water droplets exposed to gaseous sulphur dioxide and ammonia. Quart. J. Roy Meteor. Soc. 89:271–275.
Vaughan, T.R., Jr., L.F.Jennelle, and T.R.Lewis (1969) Long-term exposure to low levels of air pollutants. Effects on pulmonary function in the beagle. Arch. Environ. Health 19:45–50.
West, P.W., A.D.Shendrikar, and N.Herrara (1974) The determination of sulfuric acid aerosols. Analyt. Chim. Acta 69:111–116.
Whitby, K.T. (1974) Personal communication.































