A
Vaccines and Depot Medications for Drug Addiction: Rationale, Mechanisms of Action, and Treatment Implications
Paul R. Pentel
Hennepin County Medical Center and University of Minnesota
OVERVIEW
Immunotherapies and depot medications (dosage forms designed to release a drug gradually over a prolonged period of time) are of particular interest as approaches to treating drug addictions because of their long duration of action. Clinical effects may persist for months, eliminating the need for daily medication and potentially improving patient compliance. At the same time, a long duration of action could help prevent patients from opting out of treatment prematurely and could prolong the duration of any side effects of treatment. These possibilities raise unique questions regarding the therapeutic role for such medications and their ethical implications. The purpose of this appendix is to present the scientific basis for vaccines and depot medications as a background for addressing these unusual and challenging issues.
IMMUNIZATION
The first study of immunotherapy as a treatment for drug dependence was a report in 1974 that a vaccine directed against heroin reduced heroin self-administration in monkeys (Bonese et al., 1974). This new treatment approach was not pursued because of concerns about whether heroin addicts might simply switch to a different opiate. This appendix considers more recent and ongoing efforts directed at cocaine, phencyclidine, nicotine, and methamphetamine dependence. Initial clinical trials have begun on immunotherapies against cocaine and nicotine, but only preliminary
safety data and no efficacy data are available so far. The discussion below is based primarily on data derived from animal studies.
Definitions
There are two general strategies for immunotherapy: active immunization with vaccines and passive immunization with monoclonal antibodies. A vaccine is a chemical that can elicit an immune response consisting of the production of antibodies. Antibodies are large protein molecules that circulate in the blood and that can bind the chemical used in the vaccine. There are other features to an immune response, but they are not important for the treatment of drug addiction and will not be considered here. Vaccination is the process of administering a vaccine repeatedly to elicit an immune response and is sometimes referred to as active immunization. Thus an experimental animal or a person might be vaccinated to elicit antibodies that would potentially be of use as a treatment for drug addiction. It is also possible to vaccinate an experimental animal, remove and purify the antibodies, and administer these to an experimental animal or a person. This is referred to as passive immunization. Antibodies can also be produced in cell cultures rather than whole animals. To accomplish this, a single antibody-producing cell from a mouse is cloned (replicated) in a manner that allows it to grow in a flask and continue to produce antibody. Such antibodies are called monoclonal because they are all identical, in contrast to the antibodies produced by a vaccinated animal, which may have a range of abilities to bind the drug in question. In addition, monoclonal antibodies can be engineered to improve their properties. Because of these potentially advantageous features, monoclonal antibodies are generally considered the most suitable form of antibody for passive immunization.
Vaccination has received the greatest attention as a potential treatment for drug addiction because it requires just a few doses and produces a long-lasting response. Vaccination is easy to perform, relatively inexpensive, is already widely used to prevent infectious diseases, and has an excellent safety record. However, the strength of the immune response varies among individuals and could be inadequate in some. In addition, vaccination requires a series of injections over several weeks to several months before it becomes effective. Passive immunization would likely be more expensive and require more frequent dosing than vaccination but would allow the antibody dose to be controlled and adjusted according to individual needs, and there is no lag time between administration and onset of action. However, clinical experience and safety data with the high antibody doses needed are limited. Both vaccination and passive
immunization may therefore prove to have their own advantages, limitations, and potential uses for the treatment of drug dependence.
For the purposes of this discussion, chemical compounds that produce addiction will be called drugs, and chemical compounds used to treat addiction will be called medications.
Scope of Discussion
The antibodies discussed in this appendix act by binding drug and altering its fate in the body. Immunization can also be used to produce catalytic antibodies, which act by breaking down the drug (Mets et al., 1998; Baird et al., 2000). This appendix considers only binding antibodies because this application is better studied and because the ethical issues raised by catalytic antibodies are analogous.
Rationale
Drugs of abuse produce their addictive effects by acting on specific neural pathways in the brain. Most medications that have been developed or studied as treatments for drug addiction also act in the brain to reduce the effects of addictive drugs or substitute for them in order to reduce withdrawal and cravings (Kreek, LaForge, and Butelman, 2002). While this approach has had substantial successes (nicotine replacement therapy, bupropion, and nortriptyline for tobacco dependence; opiate agonists and antagonists for opiate dependence; naltrexone for alcohol dependence), each of these medications has inherent limitations. The brain pathways targeted by these medications are involved in mediating many normal and essential functions apart from drug addiction, including cognition, emotions, memory, and movement. Medications used to target these pathways can therefore affect these normal functions as well, leading to adverse effects or limits on the usable medication dose.
Immunotherapies represent an attempt to exploit a very different treatment strategy in which the therapeutic target is the drug rather than the brain (Pentel and Keyler, 2004). Vaccines directed against drugs of abuse stimulate the immune system to produce drug-specific antibodies that circulate in the blood and are capable of binding the drug tightly. Antibodies themselves cannot enter the brain because of their large size. Thus any drug bound to antibody also cannot enter the brain. If a sufficient amount of antibody is present when a drug is administered, a substantial fraction of the dose will bind to antibody in the blood so that the fraction of the dose entering the brain is reduced (Figure A-1). Because addictive drugs act in the brain, limiting the amount of drug entering the brain should
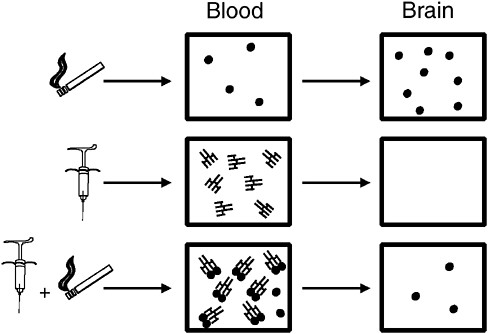
FIGURE A-1 Effects of vaccination on drug distribution to the brain, illustrated for nicotine derived from cigarettes. When nicotine is administered alone (top) it rapidly enters the blood and is delivered to the brain. Vaccination elicits the production of nicotine-specific antibodies in the blood (middle). Because antibodies are large molecules, they are excluded from the brain. If a vaccinated animal is given a dose of nicotine (bottom), a substantial fraction of that dose is bound and sequestered in the blood by the antibody and less nicotine enters brain.
SOURCE: Pentel and Keyler (2004).
also reduce the drug’s effects. The hope in using this strategy is to reduce the rewarding effects of the drug that lead to and sustain addiction. For example, a cocaine addict who is vaccinated and then takes a puff of crack cocaine would feel little effect and therefore be less likely to continue using it.
Attractive Features of Immunotherapy as a Treatment for Drug Dependence
Vaccines or passive immunization have several potential advantages compared to other medications for drug addiction.
Long Duration of Action
Vaccination may elicit therapeutic concentrations (titers) of antibodies in the blood that persists for 3 to 6 months, perhaps longer (Cerny et al., 2002; Kosten et al., 2002a). If needed, satisfactory antibody concentrations could be maintained by periodic booster doses (e.g., every several months). A long duration of action could potentially improve treatment compliance by providing a measure of protection without the need for patients to return frequently to a clinic or remember to take daily medication.
Novel Mechanism of Action
The mechanism of action of vaccines as treatments for drug abuse is unique and distinct from that of currently used medications. Two treatments acting via different mechanisms often have additive effects such that the combination is more effective than either one alone. Vaccines may target different aspects of drug addiction than available medications. For example, nicotine replacement therapy reduces the severity of withdrawal symptoms, while vaccination (based on animal studies) may be more helpful for preventing the rewarding effects of a cigarette that can lead to relapse. Combining medications that have different types of effects may expand the spectrum of therapeutic actions that can be achieved and improve overall results.
Safety
Because the antibodies produced by vaccination do not appreciably enter the brain, vaccination should circumvent the central nervous system side effects that limit the use of other medications (Killian et al., 1978). Because the drug-specific antibodies elicited by vaccination bind to the addictive drug and nothing else, vaccination should also be relatively free of side effects outside of the central nervous system (Owens et al., 1988; Hieda et al., 1997). The generally excellent safety record of vaccines used to prevent infectious diseases supports this notion.
Immunology of Vaccination
Composition of a Vaccine
Small molecules such as drugs of abuse are not by themselves immunogenic and cannot stimulate the immune system to produce antibodies. A commonly used strategy for eliciting antibodies to small molecules such as these is to chemically link the drug to a larger protein molecule
(Figure A-2). The resulting molecule, consisting of drug linked to protein, is called an immunogen because it is now capable of eliciting an immune response. When used as a vaccine, this type of immunogen is referred to as a conjugate vaccine because it represents a small molecule conjugated (linked) to a protein.
Vaccination
Vaccination consists of injecting an immunogen, usually into the muscle of the upper arm, or less commonly administering it as a nasal spray or oral liquid. A single injection of vaccine generally does not stimulate significant immunity, and one or more booster injections at intervals of several weeks to months are needed to achieve a satisfactory response. This response consists of the production of antibody molecules by the cells of the immune system. An immune response may include other components in addition to antibody production, but they do not contribute to the effects of vaccines on drugs of abuse.
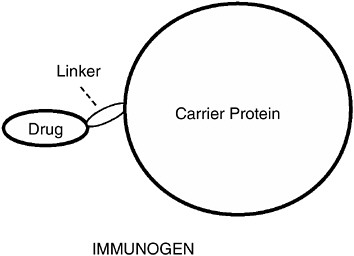
FIGURE A-2 Immunogen structure. Drugs are too small to be recognized by the immune system. To render them immunogenic, drugs must be linked to a large foreign protein. The resulting complex is the complete immunogen. A vaccine consists of the complete immunogen mixed with a chemical adjuvant that enhances the immune response. Because drugs of abuse by themselves are not complete immunogens, they do not elicit antibodies, nor can they boost an immune response in a vaccinated animal or individual. The complete immunogen is needed to boost the immune response.
Antibodies
Antibodies are protein molecules that have the ability to bind tightly to the portions of the immunogen used to stimulate their production. This is achieved through a binding pocket that is complementary in size, shape, and electrical charge to a part of the immunogen, such that the immunogen and antibody fit together in a lock-and-key fashion (Figure A-3). The antibody binding pocket is not large enough to bind the entire immunogen. Rather, immunization produces many antibodies that can bind many different parts of the immunogen. Some of these antibodies may bind the part of the immunogen that has drug linked to it, and these antibodies can also bind drug that is not linked to carrier protein. Thus a portion of the elicited antibodies will be capable of binding the free (unbound) drug.
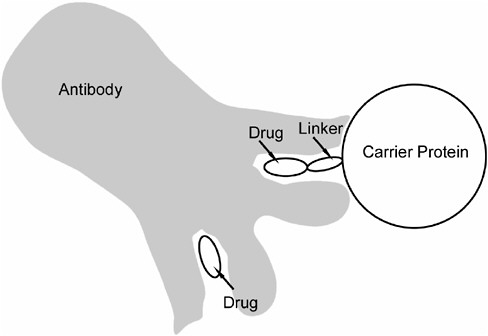
FIGURE A-3 Binding of drug to antibody. The binding site on the antibody consists of a pocket that is complementary to the drug in size, shape, and electrical charge, such that the drug fits into the binding pocket in a lock-and-key fashion. The result is tight (high-affinity) binding that is quite specific for that particular drug. Each antibody molecule has two identical binding sites. The upper site in the figure illustrates antibody binding to the complete immunogen that was used to stimulate antibody production. The lower site illustrates that the drug alone can also bind to this site.
The binding of drug to antibody is typically very tight (high affinity) and very specific. For example, antibodies to nicotine elicited by a vaccine bind only nicotine and do not bind nicotine metabolites (breakdown products), other molecules normally present in the body such as neurotransmitters or hormones, or other drugs or medications (Pentel et al., 2000). This exquisite degree of specificity suggests that the actions of these antibodies should also be quite specific.
Measuring the Response to Vaccination
The three antibody characteristics of greatest interest are the antibody concentration in blood, how tightly the antibodies bind the drug (affinity), and whether the antibodies bind anything other than the drug in question (specificity). All three are readily measured from small blood samples. Antibody concentration is often expressed as a titer, or dilution; higher titers indicate higher antibody concentrations. Measurements are typically obtained from serum or plasma, the liquid portion of blood exclusive of red blood cells.
Initiation of Vaccination
It is likely that a series of two to four injections over 1 to 2 months will be needed for vaccination to produce a satisfactory immune response (Hieda et al., 2000; Byrnes-Blake et al., 2001; Kantak et al., 2001; Kosten et al., 2002a). This 1- to 2-month interval is a potentially important disadvantage since the onset of therapeutic effect would be similarly delayed. However, vaccination can be accomplished even while drug use continues; the presence of drug does not diminish the immune response (Hieda et al., 2000; Byrnes-Blake et al., 2001). Thus individuals could be vaccinated in preparation for stopping drug use by starting 1 to 2 months in advance. When this is not possible, the use of counseling and, when available, other medications with more immediate effects could be used until the vaccine becomes effective.
Duration of Response to Vaccination
Because drugs of abuse by themselves cannot elicit an immune response, drug abusers do not normally have antibodies directed against these drugs. It is only after a series of vaccinations that antibodies are formed. Because the antibody response fades with time, the concentrations of antibodies in serum will fall over a period of months to years. To maintain satisfactory antibody concentrations in serum, it will be necessary to
administer booster doses of vaccine periodically. The frequency of boosting needed is not known, but an interval of approximately every 2 to 6 months is likely (Cerny et al., 2002; Kosten et al., 2002a). It is important to note that vaccines for drug addiction differ in this respect from vaccines for infectious diseases. Infectious microorganisms (bacteria or viruses) are complete immunogens, so exposure to the infectious agent automatically boosts the immune response. In contrast, a drug addict relapsing to drug use would not boost his or her immune response but would require additional vaccination.
Sustained-release vaccines have been studied in animals as a means of reducing the number or frequency of required vaccine injections (Gupta, Singh, and O’Hagan, 1998; Langer, Cleland, and Hanes, 1997). With this technology, one injection might substitute for several monthly injections. This technology has not yet been applied to humans.
The time course of the antibody response to vaccination is critical to determining its duration of action. At some point the concentration of antibody in blood will fall below that needed to have any effect on drug action (Carrera et al., 2000; Kantak et al., 2000; Proksch, Gentry, and Owens, 2000). Thus for practical purposes the effects of vaccination are not permanent. It is difficult at present to estimate the duration of action for any of the vaccines discussed in this chapter. In a Phase I study of a cocaine vaccine, antibody concentrations in serum declined to nearly the prevaccination level by 10 months after the last vaccine dose (Kosten et al., 2002b). It cannot categorically be said, at this point, that these minimal antibody concentrations would have no effect, but the likelihood is very high that this is so. As a result, vaccination can be viewed as a medication with a potentially long duration of action, most likely measured in months, rather than a permanent effect.
While very low levels of antibody persisting months to years after vaccination are unlikely to have any effect on drug use, they may still be detectable. If so, their detection could potentially identify a person as an addict (someone previously treated with vaccination). The length of time that antibodies could be reliably detected at a level sufficient to indicate previous vaccination is unknown.
It is important to note that having a long duration of action, with therapeutic and possible toxic effects that cannot be reversed for periods of weeks to months, is not confined to vaccines, passive immunization, or depot medications for drug addiction. Table A-1 lists several medications in common clinical use that have long durations of action and that have proven to be acceptable and valuable treatments for certain indications.
TABLE A-1 Medications with Long Durations of Action Used to Treat Disorders Other Than Drug Addiction
Fate of Antibody After Vaccination
Antibodies are continually produced and broken down (metabolized and inactivated) in the body. The most common type of antibody (IgG) has a half-life in blood of about 3 weeks (Waldmann and Strober, 1969). That is, about half of the antibody produced on day 1 is eliminated by day 21. Blood levels of antibody after vaccination are maintained because new antibody is continually produced. After passive immunization with monoclonal antibodies, a steady decline in antibody level with a half-life of about 3 weeks is expected, so repeated antibody doses every few months would probably be needed to maintain antibody levels in blood.
Mechanisms of Action
Active immunization (vaccination) and passive immunization for drug addiction act in a similar manner and will be discussed together. Drugs of abuse produce their actions by rapidly entering the brain. When a drug is injected intravenously or smoked, it reaches the brain within 10 to 20 seconds and its rewarding or pleasant effects are equally rapid in onset (Henningfield, Miyasato, and Jasinski, 1985). When an experimental animal is vaccinated, the resulting drug-specific antibodies circulate in the blood and fluid surrounding tissues. When drug is administered, a fraction of that dose binds to the antibody and is prevented from entering the brain (Fox et al., 1996; Valentine and Owens, 1996; Pentel et al., 2000). In this manner the effects of the drug are diminished. If this reduction of effects is sufficiently large, it might lead to a reduction in drug use.
Binding of Drug by Antibody
The brain is protected by the blood-brain barrier, which separates blood in arteries and veins from brain cells. Many small molecules such as drugs of abuse (molecular weights of 200 to 300 Daltons) can readily cross the blood-brain barrier while larger molecules such as antibodies (molecular weights of about 150,000 Daltons) cannot (Bradbury and Lightman, 1990). Thus any drug that is bound to antibody also cannot cross the blood-brain barrier. When a drug is administered to a vaccinated animal, a fraction of the drug becomes bound to antibody while some remains free; the fraction that becomes bound depends on the amount of drug administered and the amount of antibody available to bind it. Only the free (unbound) drug can enter the brain.
Importance of the Amount of Antibody Available
Vaccination is most effective when the available amount of antibody is large compared to the drug dose (Fox et al., 1996; Valentine et al., 1996; Keyler et al., 1999). Surprisingly, vaccination remains effective in reducing drug distribution to the brain even when drug doses exceed the available binding capacity of antibody (Carrera et al., 2001; Tuncok et al., 2001). This is fortunate because the single and daily doses of most drugs of abuse exceed the amount of drug-specific antibody that can be elicited by vaccination. Nevertheless, the amount of antibody elicited by vaccination is very important, and greater amounts confer greater efficacy in altering drug distribution and reducing drug effects. Thus individuals with better responses to a vaccine (higher titers, implying greater total amounts of antibody elicited) would be expected to derive greater benefit from vaccination.
Although the amount of antibody elicited by vaccination has a limit, passive immunization allows the administration of as much antibody as desired. Passive immunization may prove to have advantages in settings where very high antibody doses are needed for clinical efficacy or for individuals who are vaccinated but fail to achieve a satisfactory antibody response.
Effects of Vaccination on Drug Concentrations in Blood and Tissue
When a drug is administered to a vaccinated animal, the drug is bound and retained in the blood by the high concentration of antibody present (Figure A-4). As a result, the total concentration of drug in the blood is higher in a vaccinated animal (Owens and Mayersohn, 1986; Fox et al., 1996). At the same time, the concentration of free (unbound) drug is reduced (Malin et al., 2001). Because only free drug can enter brain, the concentration of drug in the brain is also reduced. The very high total
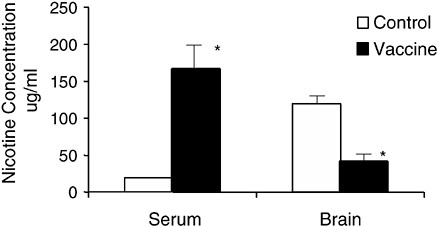
FIGURE A-4 Vaccination effects on nicotine concentration in the blood and brain of rats. Rats were vaccinated over a period of 6 weeks and then given a single dose of nicotine intravenously. Three minutes later the concentrations of nicotine in the blood were substantially higher in the vaccinated rats than in nonvaccinated controls owing to the binding and sequestration of nicotine in the blood. Brain nicotine concentration at the same time was reduced by 65 percent. This very prompt effect is important because the rewarding effects of drugs are also greatest in the first few minutes after a dose. *p <.01.
SOURCE: Adapted from Pentel et al. (2000).
drug concentration in blood is not toxic because the bound drug is unable to interact with tissues.
In animals, immunotherapy reduces drug distribution to the brain within the first few minutes after a single drug dose by up to 80 percent (Fox et al., 1996; Pentel et al., 2000). This is important because the rewarding effects of drugs are also greatest in the first few minutes after a dose. Vaccination is also effective when the drug is administered repeatedly or chronically. In a rat, the ability of vaccination to reduce nicotine distribution to the brain after a single dose equivalent (considering the rat’s size) to two cigarettes is not impaired by the concurrent infusion of nicotine for several weeks at a rate equivalent to smoking three packs of cigarettes daily (Hieda et al., 2000; Cerny et al., 2002). Similarly, a single dose of phencyclidine-specific monoclonal antibody passively administered to rats reduced phencyclidine concentration in the brain despite the continuous infusion of phencyclidine over a period of 4 weeks (Proksch et al., 2000).
Drug-specific antibodies can also slow elimination of a drug from the body because the bound drug is less available for metabolism (conversion to an inactive form) or excretion in urine (Keyler et al., 1999; Proksch et al., 2000). Since bound drug and unbound drug exist in equilibrium, the unbound drug is also eliminated more slowly. The importance of slower drug elimination is not entirely clear. Slowed elimination could lead to drug accumulation and saturation of antibody with drug, rendering it less effective. On the other hand, slowed drug elimination could delay the onset of cravings after a dose by prolonging the drug’s effects, leading to less frequent drug use (Sellers, Kaplan, and Tyndale, 2000).
Table A-2 lists the status of current research on immunotherapies in animals and humans.
Immunization Effects in Animals
Cocaine
Both vaccination and passive immunization have been shown to block or reduce cocaine self-administration in rats (Figure A-5) (Fox et al., 1996; Kantak et al., 2000, 2001). In this model, rats are fitted with a chronic intravenous cannula and can press a level in their cage to receive a dose of cocaine. Rats given access to cocaine in this manner readily learn to self-administer the drug (as well as the other drugs of abuse discussed below), demonstrating its potent reinforcing properties. If rats trained to self-administer cocaine are given single doses of cocaine-specific monoclonal antibody, cocaine self-administration over the next few days can be completely blocked (Fox et al., 1996). That is, lever pressing decreases to the
TABLE A-2 Status of Current Immunotherapy Studies in Animals and Humans
same extent as if it delivered only saline. Vaccination also reduces cocaine self-administration. In this case, vaccination administered during continued daily access to cocaine became maximally effective only after the second booster dose was administered, as would be expected since it takes that long for the maximal antibody concentration in blood to be achieved (Kantak et al., 2001).
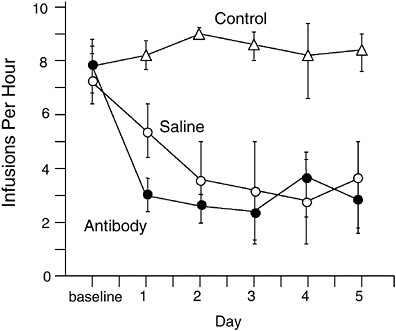
FIGURE A-5 Immunization effects on cocaine self-administration in rats. Rats were trained to self-administer intravenous doses of cocaine by pressing a lever. The top line (control) shows the number of lever presses each day in control rats. One group had saline substituted for cocaine, resulting in decreased lever pressing. A third group continued to have access to cocaine but received an injection of cocaine-specific monoclonal antibodies. Lever pressing in this group decreased to the same extent as the saline substitution group. This experiment illustrates the use of passive immunization. A similar decrease in lever pressing can be obtained with vaccination but requires 6 weeks to become evident because of the gradual rise in antibody levels in vaccinated rats.
SOURCE: Adapted from Fox et al. (1996).
Vaccination has also been shown to be effective in blocking the reacquisition of cocaine self-administration (Carrera et al., 2000; Kantak et al., 2000). Rats were first trained to self-administer cocaine; then their access to cocaine was terminated, and they underwent a 4- to 6-week period of vaccination. When cocaine was again made available, the vaccinated rats showed markedly reduced lever pressing compared to nonvaccinated controls. In a similar protocol, rats were trained to self-administer cocaine, then were vaccinated in the absence of access to cocaine, and then were reexposed to just a single scheduled dose of cocaine. This “reinstatement” procedure is meant to mimic the ability of a single drug exposure to act as
a cue for relapse. In control animals the single dose of cocaine resulted in a burst of lever pressing even though the lever pressing did not result in cocaine infusion. In the vaccinated rats this burst was greatly reduced. Insofar as reacquisition or reinstatement can be considered models of relapse to drug use, these findings suggest that vaccination may be useful in this regard.
The degree to which cocaine self-administration in rats is blocked by immunization depends on the concentration of antibody in the blood (Carrera et al., 2000; Kantak et al., 2000). Efficacy in blocking cocaine self-administration appears to require a certain threshold antibody concentration. Rats with lower antibody concentrations may show either no effect or a paradoxical increase in self-administration, presumably to compensate for the partial blockade of its effects. Whether compensation occurs likely depends on the concentration of antibody in blood and the cocaine dose. Whether compensation occurs may also depend on the immunogen used to elicit antibodies, as it has not been found in all studies (Kantak et al., 2000). These data suggest that the blockade of cocaine effects by vaccination is greatest when antibody concentrations in blood are high and that either loss of efficacy or compensation could occur with lesser antibody concentrations.
A number of other responses to cocaine can be blocked or attenuated in rats. Increases in locomotor activity resulting from very large cocaine doses are reduced by either prior vaccination or passive immunization (Carrera et al., 2001). These data suggest a potential role for passive immunization in the treatment of cocaine overdose, but less expensive therapies are available for this purpose. Vaccination also reduces the preference of rats for cocaine compared to saline, another model of the reinforcing properties of cocaine (Ettinger, Ettinger, and Harless, 1997), and the ability of rats to distinguish cocaine from saline (Johnson and Ettinger, 2000). Only limited studies have been done in other species. Vaccination of monkeys diminished the ability of cocaine to reduce food intake, demonstrating the ability of vaccination to elicit antibodies and affect a cocaine-related behavior in a primate (Koetzner et al., 2001).
Nicotine
Like cocaine, both vaccination and passive immunization have been shown to attenuate a variety of nicotine’s behavioral effects in rats. A number of studies have focused on nicotine withdrawal because the discomfort of withdrawal is one important reason why some smokers who try to quit are unable to do so. If rats are given a continuous infusion of nicotine over a week, they become dependent, as evidenced by developing signs of withdrawal when the nicotine infusion is stopped (Malin, 2001). Rats
passively immunized at the same time they receive the week of nicotine infusion develop less severe withdrawal when the nicotine infusion is stopped (Malin, 2002). When rats develop withdrawal, it can be relieved by administering nicotine, just as smokers who quit and become uncomfortable because of withdrawal can relieve their discomfort by smoking a cigarette. If rats are passively immunized with nicotine-specific antibody given just prior to developing withdrawal, nicotine loses its ability to relieve withdrawal (Malin et al., 2001). Since relief of withdrawal from smoking a cigarette may lead to relapse, blockade of this effect could have a role in relapse prevention (Hughes et al., 1984). Passive immunization also reduces nicotine-induced increases in locomotor activity and the ability to discriminate nicotine from saline in rats (Pentel et al., 2000; Malin et al., 2002; Sanderson et al., 2003).
The effects of immunization on drug self-administration have not been studied as extensively with nicotine as with cocaine. Vaccination reduces the reinstatement of lever pressing in rats after administration of a single low dose of nicotine (Lindblom et al., 2002). A preliminary report suggests that prior vaccination attenuates the acquisition of nicotine self-administration in rats (LeSage et al., 2001). Aside from suggesting that vaccination reduces this important behavioral effect of nicotine, this study introduces the possibility of using vaccination for primary prevention (see below).
Phencyclidine
Studies of immunization against phencyclidine have focused on the use of passive immunization with high-affinity monoclonal antibodies or antibody fragments and on the treatment of phencyclidine toxicity (Valentine, Arnold, and Owens, 1994; Hardin et al., 1998). In contrast to cocaine toxicity, which can be readily managed, the treatment of phencyclidine toxicity is difficult, and better treatment for overdose is needed. Passive immunization of rats with phencyclidine-specific monoclonal antibodies has been shown to markedly reduce the entry of phencyclidine into the brain and to reduce its central nervous system toxicity (Proksch et al., 2000; Hardin et al., 2002). Of particular interest is that a single dose of phencyclidine-specific antibody can reduce phencyclidine toxicity for up to 2 weeks despite repeated phencyclidine challenges at doses that exceed the binding capacity of antibody for the drug (Figure A-6). These data support the feasibility of using passive immunization therapeutically as an alternative or supplement to vaccination. Apart from demonstrating efficacy, they have shown the safety of administering the required high doses of monoclonal antibody. While passive immunization is far more expensive than vaccination, the ability to administer a well-defined anti-
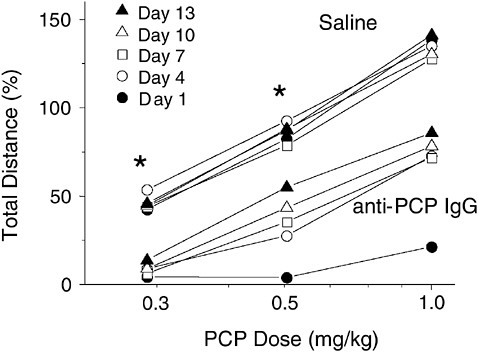
FIGURE A-6 Passive immunization produces long-lasting attenuation of the locomotor activating effects of phencyclidine. Rats received a single intravenous dose of phencyclidine-specific monoclonal IgG (the most common class of antibody) and were challenged with a phencyclidine injection at various times afterward. The top five lines represent control animals, which received saline treatment, showing locomotor activation at all phencyclidine doses. The bottom five lines represent immunized animals that received phencyclidine-specific IgG. Locomotor activity after each phencyclidine challenge dose was attenuated, even 2 weeks after the antibody was administered. *p <.05.
SOURCE: Adapted from Hardin et al. (2002).
body with suitable affinity and specificity and to titrate the antibody dose to produce the desired effect could prove helpful. The immediate onset of effect could also be helpful for facilitating the initiation of treatment for addiction or for the treatment of drug overdose.
Methamphetamine
The initiation of studies on immunization for methamphetamine is more recent, and only limited data are available. A monoclonal antibody directed against methamphetamine has been shown to reduce the ability
of rats or pigeons to discriminate methamphetamine from saline (Byrnes-Blake et al., 2001; McMillan et al., 2002).
Summary of Animal Data
In aggregate the available animal data provide strong proof of principle that both vaccination and passive immunization can block or attenuate a variety of drug effects in animals that are relevant to drug addiction in humans. Because this type of intervention is new and no clinical precedents exist, assessing the clinical potential of immunotherapy for drug abuse will only be possible through clinical trials. Both vaccination and passive immunization appear to be feasible. The success of vaccination will depend in part on whether sufficient blood concentrations of antibody can be elicited. Passive immunization appears to be feasible but is expensive. Both vaccination and passive immunization have advantages and disadvantages in terms of their potential clinical roles and practicality, and both could have a place in therapy.
Clinical Trials of Immunization
Cocaine
A Phase I study of a cocaine vaccine has been reported that demonstrates immunogenicity of the vaccine in humans and a lack of serious adverse effects (Kosten et al., 2002a). Efficacy was not studied in this initial clinical trial. Vaccine was administered at 0, 1, and 2 months intra-muscularly and at three dose levels. Antibody titers in blood were detectable after the second injection, were maximal at 3 months (1 month after the final injection), and had returned to baseline by 1 year. Although antibody titers were not as high as in rats with this vaccine, titers were dose related, so higher vaccine doses or a greater number of injections might result in higher antibody titers. A Phase II clinical trial testing different immunization regimens and efficacy in suppressing cocaine use is under way (Kosten et al., 2002b).
Nicotine
Preliminary data are available from Phase I trials of two distinct nicotine vaccines, both indicating immunogenicity of the vaccine and the absence of serious adverse effects (Lindmayer et al., 2002; St. Clair Roberts et al., 2002). Further clinical trials aimed at establishing suitable vaccination regimens are under way.
Safety: Adverse Effects and Unintended Consequences
Vaccination
Adverse effects Because the drug-specific antibodies elicited by vaccination bind only the drug in question and presumably nothing else in the body, unwanted side effects from the antibodies per se would not be expected. A favorable safety profile is in fact typical of vaccines for infectious diseases. The animal and human data available to date suggest that vaccines against drugs of abuse share this favorable safety profile; no substantial adverse effects have been found other than soreness or irritation at the injection site (Kosten et al., 2002a, 2002b; St. Clair Roberts et al., 2002). However, antibody per se is only one consideration with regard to vaccine safety because vaccines also change the distribution of the abused drug in the body and in some cases its metabolism as well. Vaccines are intended to reduce the amount of drug in the brain, and may reduce the amount of drug in other organs as well, by binding and sequestering it in the blood (Valentine and Owens, 1996). It is conceivable that increased drug in the blood could have adverse effects of its own—for example, by delivering more drug to certain organs. Data presented below argue that such adverse effects are unlikely and have not been observed, but it is important to consider this possibility as novel clinical situations (e.g., pregnancy) are encountered. In addition, a note of caution is appropriate simply because vaccination for drug abuse is a new approach that is without an analogous clinical precedent. Unexpected side effects, such as the binding of antibody to as yet unidentified endogenous compounds or structures, are possible and justify vigilance in looking for such effects in clinical trials.
Preventing or reducing drug effect In the context of this discussion, the ability of vaccination to block or reduce the addictive effects of drugs is the desired therapeutic outcome. However, if a cigarette smoker decided to abandon his or her attempt at cessation and wanted to resume smoking, vaccination could interfere with the ability to do so until antibody concentrations in the blood declined sufficiently. Although this effect would be temporary, patients getting vaccinated would need to be aware of this possibility.
In other circumstances, drugs of abuse may also be used for therapeutic purposes. For example, cocaine is sometimes used for local anesthesia, and nicotine is being studied as a cognitive enhancer in patients with certain cognitive disorders such as Alzheimer’s disease (Lopez-Arrieta, Rodriguez, and Sanz, 2000). If such a therapeutic action were desired, vaccination could potentially block or counteract it. The extent of blocking
effect would depend on the concentration of antibody present in blood and would wane over time but could be present transiently.
Pharmaceutical nicotine is also widely used therapeutically as a treatment for smoking cessation (Fiore, Bailey, and Cohen, 2000). Vaccination could prevent nicotine replacement therapy from being effective, thus eliminating this important therapeutic option. However, rat studies suggest that vaccination and nicotine replacement therapy may in fact be compatible because vaccination has its greatest effect on blocking the early distribution of nicotine to the brain (first few minutes after a puff) but has less effect at later times (Hieda et al., 2000; Tuncok et al., 2001). Thus vaccination may reduce the early rewarding effects of smoking but still allow nicotine administered therapeutically to enter the brain and retain its typical effects of reducing withdrawal and cravings.
Compensation If immunotherapy partially blocks the effect of a drug, it is possible that this blocking effect could be overcome by simply increasing drug intake (the size of each dose or the total daily dose). Some but not all cocaine vaccine studies in rats demonstrate this kind of compensation, primarily in rats with the lowest antibody titers in blood (Carrera et al., 2000; Kantak et al., 2001). Whether compensation takes place in a patient will likely depend on the strength of the antibody response in that individual, the individual’s motivation to remain abstinent, and the use of additional treatment measures, including counseling and other medications. In any event, compensation would remain important only so long as antibody concentrations in the blood remain high and would wane and presumably disappear over time.
One particular concern with compensation, even if transient, is that a drug user might escalate his or her drug use sufficiently to cause an inadvertent overdose. This possibility cannot be discounted and can probably be avoided only with attention to counseling and other adjunctive measures as well as education regarding the dangers of dose escalation in this setting.
Even if drug escalation does not result in overdose, it could increase exposure to other toxins present in the drug formulation. For example, a cigarette smoker could increase his or her rate of smoking to compensate for blockade of nicotine’s effects by vaccination and thereby increase his or her exposure to carbon monoxide as well (Benowitz, Jacob, Kozlowski, and Yu, 1986). Again, this possibility underscores the importance of using vaccination in the context of a comprehensive treatment program with specific education about the risks of compensation.
Pregnancy While potential effects on the fetus are a concern with all new medications, immunotherapy poses specific additional issues. In addition
to the transfer of drug-specific antibodies to the fetus, immunotherapy may alter fetal exposure to the drug that is being targeted. This is particularly important because each of the drugs of abuse considered here have either established or suspected adverse effects on fetal development (Plessinger, 1998; Ernst, 1999; Addis et al., 2001). Women who are immunized in an effort to treat their drug dependence could inadvertently become pregnant. It is therefore important to understand the effects of immunization on fetal risk.
Vaccination could possibly reduce drug distribution to the fetus, just as it reduces drug distribution to the brain and other organs in the mother. On the other hand, maternal antibodies are transferred across the placenta (Simister and Story, 1997) and could act to escort even more drug into the fetus. Only limited data are available addressing this question from one preliminary study of immunization against nicotine (Shoeman, Keyler, and Pentel, 2002). Rats were vaccinated prior to pregnancy, and a single dose of nicotine was administered late in pregnancy. The overall distribution of nicotine to the fetus was not altered. However, nicotine levels in the fetal brain were lower than in controls, which could serve to protect against some of nicotine’s adverse effects on neural development. While these data identify no risk to the fetus from immunization, they are very preliminary and further study is needed in order to assess the safety and acceptability of immunization as a treatment for drug dependence in women with childbearing potential.
Passive Immunization
As with vaccination, the specificity of passive immunization suggests a favorable safety profile. No important adverse effects have been noted to date in animal studies of either polyclonal or monoclonal antibodies to reverse or prevent drug effects. Passive immunization is used for many therapeutic purposes other than drug addiction, but the doses of antibody required are generally lower. Additional safety studies of the higher antibody doses required for treatment of drug addiction or drug overdose are needed. Antibodies considered for clinical use would almost certainly be monoclonal, rather than purified from immunized animals, because antibodies from another species can produce allergic reactions. Monoclonal antibodies can be “humanized” by altering their structure to resemble human antibodies, without compromising their ability to bind drug (Berger, Shankar, and Vafai, 2002). Humanized antibodies used (in smaller doses) for other purposes are generally well tolerated, but allergic reactions, although uncommon, can still occur, and the potential need to administer antibodies repeatedly over long periods of time for the treatment of drug abuse will require specific safety studies.
Each of the considerations mentioned above for vaccine safety apply to passive immunization as well. However, monoclonal antibody concentrations in blood will fall at a predictable rate after the last dose (decreasing by half approximately every 3 weeks), so the duration of action should be quite predictable (Waldmann and Strober, 1969). Moreover, the problem of long-term persistence of very low levels of antibody will not occur with passive immunization. For practical purposes, antibody concentrations in blood should be negligible within about 6 months of the last dose.
Anticipated Clinical Role of Immunization
Expectations
The experience of both health care professionals and the public with vaccines is with those used to prevent infectious diseases. When used for infectious diseases, vaccines often confer complete or nearly complete protection. It is important to realize that vaccination for drug dependence is conceptually different. Rather than supplementing the body’s immune response to an infection, it is simply reducing the access of drug to the brain. Immunization for drug abuse is more likely to reduce than to completely block drug effects and is unlikely confer the complete protection afforded by vaccines for infectious diseases. Immunization is best considered as another medication option for drug dependence, with a range of effects that address some but not all of the features of drug dependence.
Uses
Because immunization for drug abuse has no clinical precedent, anticipating its clinical role is difficult. Immunization’s principal action is to block those drug effects that require the presence of drug in the brain. Thus the pleasure associated with using a drug may be diminished or absent. Immunization would not be expected to directly block withdrawal or craving, since these occur when drug is no longer present. One potential clinical role for immunization is in relapse prevention. In this setting, if a period of abstinence is threatened by a “slip” consisting of just one or a few drug doses, immunization could block or reduce the rewarding effect of those doses and thereby make relapse less likely. Because relapse typically starts with just one or a few drug doses, the ratio of antibody to drug would be high and would maximize the efficacy of immunization. In a comprehensive treatment program, additional measures would be needed to address cravings, withdrawal, and the many psychosocial issues surrounding drug dependence.
Combining
Immunotherapy medications Because immunotherapy acts by a mechanism that is distinct from most other medications for the treatment of drug dependence, immunotherapy should be compatible with concurrent use of these medications. In this case, the combination could have greater efficacy or a broader range of effects. An additional possibility is the combination of vaccination and passive immunization. Vaccination is simpler and less expensive than passive immunization and may require less frequent dosing. However, some patients may not develop an adequate response to vaccination. In this event (which could be determined by a simple blood test to measure antibody concentration in blood), passive immunization with a modest dose of drug-specific monoclonal antibody might be used to supplement vaccination. Passive immunization might also be used to obtain an immediate effect during the 1 to 2 months required to complete vaccination. This strategy should be feasible because passive immunization would not be expected to interfere with the ability of vaccination to stimulate a satisfactory immune response.
Summary of Features of Immunotherapies Requiring Special Consideration
Immunotherapy as a treatment for drug dependence differs from most other medications, even those used for other purposes, because of its unusual mechanism and long duration of action. None of these features are entirely unique to immunization. Nevertheless, their impact in the setting of drug dependence raises issues that may require special consideration. Table A-3 lists some key features of immunotherapies for drug dependence that requires further consideration. Those issues are briefly reviewed below.
Commitment to Therapy
The duration of action of vaccination as a treatment for drug dependence in humans is not known. Animal studies and initial clinical data suggest a duration of at least several months after the last booster dose (Hieda et al., 2000; Kosten et al., 2002a). Antibodies may persist in blood for much longer, but their concentrations would likely be too low to sustain a therapeutic effect. During the several months after vaccination, patients would therefore be committed to this therapy. A similar commitment to therapy occurs with a number of other medications used for other purposes (Table A-1), and is not unique to vaccination. However, drug use (particularly cigarette smoking) is perceived by some as a “choice,”
TABLE A-3 Key Features of Immunotherapy for Drug Dependence That Require Special Consideration
|
Commitment to therapy |
Long duration of action of immunization commits a patient to its effects until antibody levels in the blood decline sufficiently (up to several months for passive immunization, possibly longer for vaccination). |
|
Blockade of therapeutic drug effect |
When a drug of abuse is also used for therapeutic purposes (e.g., nicotine for replacement therapy), immunization may block those actions as well until antibody levels decline. |
|
Compensation |
Attempts to overcome the blockade-of-drug effect from immunization could lead to greater drug use, overdose, or toxicity from adulterants. |
|
Pregnancy |
Immunization could alter the amount or duration of fetal drug exposure. Insufficient data are available to adequately assess risk. |
|
Primary prevention |
The presumed safety and long duration of action of vaccination allow consideration of its use for this purpose. |
|
Privacy |
Detection of antibodies using simple blood tests could identify recipients of vaccination for months or longer after the last booster dose. |
and the decision to resume drug use could be thwarted or made more difficult (requiring higher drug doses) until the effects of vaccination wane. In addition, the duration of persistence of antibody after vaccination is likely to vary among individuals and be difficult to estimate precisely.
The duration of action of passive immunization with monoclonal antibodies is also not known in humans but is likely to be several weeks to several months after the last dose, depending on the dose size (Hardin et al., 2002). Commitment to therapy would be analogous to that following vaccination.
Blockade of Therapeutic Drug Effects
In addition to the use of pharmaceutical nicotine as a treatment to aid smoking cessation, nicotine is being studied as a treatment for dementias and other neurological disorders (Lopez-Arrieta et al., 2000). If it proves to have a therapeutic role, vaccination could temporarily block the ability to gain therapeutic benefit from nicotine. Since the disorders in question
are chronic, their presence would likely be known at the time of vaccination rather than appearing abruptly and requiring immediate treatment.
Compensation
The blockade of drug effect provided by immunization may be incomplete. The concurrent use of counseling and perhaps other medications may be helpful in achieving a therapeutic benefit despite incomplete blockade. However, some patients may try to overcome the partial blockade by using higher or more frequent drug doses. Aside from thwarting the therapeutic intent, increased drug use could be harmful if it is sustained after antibody levels decline. Adverse effects could result if patients do not know how much drug is required to overcome immunization and inadvertently overdose. If a drug is mixed with an adulterant, immunization would reduce the effect of the drug but not the adulterant, and toxicity from the adulterant could result. Targeting immunization to motivated patients who are treated with concurrent counseling would seem the best approach to minimizing such occurrences.
Pregnancy
Vaccines or passive immunization per se are unlikely to harm a fetus, but they could alter the amount of abused drug transferred to a fetus. Limited data suggest, if anything, a protective effect with lesser drug transfer, but these data are very preliminary (Shoeman et al., 2002; Keyler et al., 2003). In addition, antibodies can potentially prolong exposure to a drug because the antibody-bound drug is more slowly eliminated from the body (Keyler et al., 1999; Proksch et al., 2000). Thus a pregnant woman who stops smoking will have unmeasurable nicotine levels in her blood (and presumably in her fetus) within several days, but a woman vaccinated against nicotine who stops smoking could have low levels of nicotine persisting in her blood for weeks. Whether this bound nicotine would be harmful to the fetus is not known. The main point with regard to fetal exposure to a drug is that current data are insufficient to judge whether vaccination or passive immunization will increase, decrease, or have no effect on exposure and harm.
The use of potentially fetotoxic or teratogenic medications during pregnancy is commonly dealt with by recommending that adequate contraceptives be used during the period of exposure. While this strategy could also be applied to immunization for drug dependence, compliance may be lower in drug-dependent women. Thus studying and understanding the potential risks (or benefits) of immunotherapy in women who could become pregnant will be very important.
Primary Prevention
The use of medications for primary prevention (preventing the initial acquisition) of drug dependence has received little consideration because most medications have potential adverse effects, the target population is predominantly young and still undergoing neural development, and the period of risk is protracted, so the duration of treatment and expense would be considerable. In contrast to many other candidate medications, vaccination so far appears free of adverse effects, the resulting antibodies do not enter the brain and therefore should not affect neural development, and the need for only infrequent dosing makes a prolonged period of treatment conceivable. This potential application is of course quite speculative, since efficacy has not yet been demonstrated in humans, and much more toxicity testing would be needed to assure the high level of safety required for administration to teenagers or young adults. However, vaccination could be targeted to high-risk groups—for example, teenagers who already smoke a few cigarettes weekly and who have a very high likelihood of becoming regular smokers over the next 1 to 2 years (Institute of Medicine, 1994). In addition to the issues raised above, this would involve vaccination of minors. While other vaccines are routinely administered to minors, the issue of “choice” discussed above could be raised.
Privacy
Patients who have been vaccinated could potentially be identified as drug abusers by virtue of detection of antibodies from a simple blood test. Because these tests are quite sensitive, antibody from previous vaccination might be detectable long after the therapeutic effect of the vaccination has subsided. This problem is no different from the ability to identify an opiate addict by detecting methadone in urine, or identifying someone as a cardiac patient by detecting the antiarrhythmic agent amiodarone in blood, except that the period during which this may be possible could be considerably longer. Passive immunization would also allow its recipients to be identified by a blood test, but antibody levels would decline in a more predictable manner and probably be undetectable within 6 months.
DEPOT MEDICATIONS
Formulations
Depot medications are formulations of standard medications designed to release a drug slowly and over a long period of time, typically days to weeks. Depot medications can be formulated as a liquid mixture or sus-
pension of small particles that can be injected under the skin (e.g., depot medroxyprogesterone; see Table A-1) (Gupta et al., 1998; Putney and Burke, 1998; Hatefi and Amsden, 2002; Mantripragada, 2002). Slow release of medication can also be achieved by impregnating a device such as a small plastic rod with a drug and placing the device under the skin (e.g., the previously marketed Norplant contraceptive). One important difference between these two technologies is that only the latter is retrievable. An implanted plastic rod can readily be removed to terminate its action, whereas a liquid injected under the skin cannot. In addition, the potential durations of action of these technologies differ. Liquid formulation may release drug for up to several months, while impregnated devices can have durations of years (5 years for Norplant). Thus a wide range of durations is potentially available through the use of depot formulations. Not all medications can be formulated in this manner. Depot formulations are best suited to high-potency medications where the required daily dose is low and only a modest amount of drug needs to be incorporated into the formulation or device. Low-potency medications, requiring higher amounts of drug to be incorporated, may prove too bulky to be practical.
Depot Naltrexone for Opiate or Alcohol Dependence
There are no depot medications currently in clinical use to treat drug dependence. One depot medication being studied for opiate dependence is the opiate antagonist naltrexone. Naltrexone is an effective oral therapy approved by the Food and Drug Administration for opiate dependence that acts by blocking the access of opiates to their brain receptors. It is possible to give a high enough dose of naltrexone orally to block the actions of typical heroin doses, but its duration of action is modest so daily dosing is required (Modesto-Lowe and Van Kirk, 2002). Compliance with naltrexone for the treatment of opiate dependence is lower than with methadone because naltrexone lacks the pleasant receptor-activating effects of methadone. Measures to improve long-term compliance with naltrexone are needed.
Naltrexone has been experimentally formulated as a slow-release suspension of microspheres administered by intramuscular injection that can deliver therapeutic doses over a period of up to 4 weeks after a single injection (Chiang et al., 1985; Comer et al., 2002). Its actions are identical to those of orally administered naltrexone, but daily dosing is not required and substantial blockade of heroin effects is achieved for up to 1 month (Figure A-7). Clinical trials of depot naltrexone for opiate dependence are ongoing (J. Cornish, personal communication, 2003). Once administered, the naltrexone dose cannot be retrieved, so recipients are obligated to its effects for that period of time. The implications of this prolonged effect
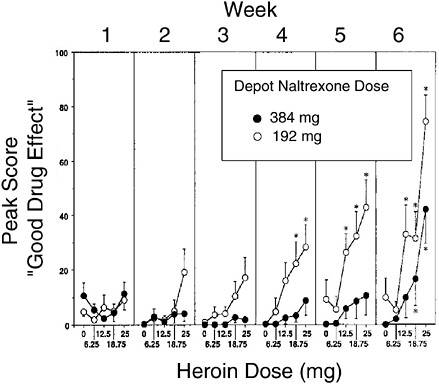
FIGURE A-7 Blockade of heroin effects by depot naltrexone. Subjects were given a single injection of depot naltrexone and were then given increasing doses of heroin at weekly intervals. The “good drug effect” associated with heroin was substantially blocked for a month, more so with the higher naltrexone dose.
SOURCE: Adapted from Comer et al. (2002).
are analogous to those discussed above for immunization, in particular passive immunization, because the dose is controlled and the duration of action is uniform and predictable. The therapeutic effect of naltrexone cannot be readily reversed during the month after dosing (Comer et al., 2002). One difference between depot naltrexone and immunization for other drugs of abuse is that opiates do have an important therapeutic use in the treatment of pain. Naltrexone blocks the pain-relieving ability of all opiates, so the use of this entire class of drugs is difficult after naltrexone is administered. In a hospital setting, higher opiate doses could partially overcome the blockade. In the setting of drug abuse, attempts to overcome the blockade could result in increased drug use, overdose, or toxicity from adulterants.
Naltrexone is also effective in treating alcoholism, and daily doses of oral naltrexone are widely used for this purpose (Streeton and Whelan, 2001). As with its use for opiate dependence, compliance is an issue (Modesto-Lowe and Van Kirk, 2002). Depot naltrexone is therefore being studied for this indication (Alkermes, 2003; Drug Abuse Sciences, 2003).
Many other depot medications are in current clinical use. Depot formulations of several antipsychotic agents are available, with durations of action of several weeks (Adams et al., 2001). Like medications for drug dependence, depot antipsychotic medications are administered to a vulnerable population in order to improve compliance. Thus the clinical and ethical issues presented by depot naltrexone have a precedent in antipsychotic medications. Depot antipsychotic medications have proven to be acceptable to both patients and health care providers when used in select patients (Adams et al., 2001; Walburn et al., 2001).
CONCLUSIONS
The very long duration of action of immunotherapies and depot medications proposed for the treatment of drug dependence makes them attractive as potential treatments for drug dependence. A long duration of action could increase medication compliance and thereby facilitate a comprehensive treatment plan consisting of both medication and counseling. In addition, the unique mechanism of action of immunization may confer both safety and a distinct spectrum of therapeutic effects on this approach. However, a long duration of action raises issues that are not presented by other currently used medications. Patients receiving these long-acting treatments will be obligated to their therapeutic effects for weeks to months, so the decision to undergo treatment may not be readily reversed. Adverse effects could similarly persist for weeks to months. Insofar as some drugs of abuse also have therapeutic uses, these beneficial effects could be blocked during this period as well. The detection of drug-specific antibodies by simple blood tests after immunization, or of treatment medication after use of a depot formulation, could identify patients as drug abusers and compromise their privacy. Immunization may alter drug transfer to the fetus in a woman who subsequently becomes pregnant; present data are insufficient to asses any possible risk. With either immunization or depot antagonist medications, patients could try to overcome the blockade of drug effect by increasing their drug use, leading to overdose or toxicity due to adulterants. The potential use of vaccination for primary prevention of drug dependence is conceivable because of its safety but likely would involve minors. While these issues are new to the field of drug dependence, each has precedents in other areas of pharmacotherapy. The appropriate use of immunization and depot medications
to treat drug dependence will benefit from an understanding of their underlying mechanisms and consideration of approaches adapted for the use of long-acting medications for other purposes.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DA 10714, DA 15668, U19-DA13327, and P50-DA-13333.
REFERENCES
Adams, C.E., Fenton, M.K., Quraishi, S., and David, A.S. (2001). Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. British Journal of Psychiatry, 179, 290-299.
Addis, A., Moretti, M.E., Ahmed, S.F., Einarson, T.R., and Koren, G. (2001). Fetal effects of cocaine: An updated meta-analysis. Reproductive Toxicology, 15(4), 341-369.
Alkermes Inc. (2003). Vivitrex® background kit. Available at http://www.alkermes.com/news/background_kits.asp [August 22, 2003].
Baird, T.J., Deng, S.X., Landry, D.W., Winger, G., and Woods, J.H. (2000). Natural and artificial enzymes against cocaine. I. Monoclonal antibody 15A10 and the reinforcing effects of cocaine in rats. Journal of Pharmacology and Experimental Therapeutics, 295(3), 1127-1134.
Benowitz, N.L., Jacob, P., III, Kozlowski, L.T., and Yu, L. (1986). Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. New England Journal of Medicine, 315(21), 1310-1313.
Berger, M., Shankar, V., and Vafai, A. (2002). Therapeutic applications of monoclonal antibodies. American Journal of the Medical Sciences, 324(1), 14-30.
Bonese, K.F., Wainer, B.H., Fitch, F.W., Rothberg, R.M., and Schuster, C.R. (1974). Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature, 252(5485), 708-710.
Bradbury, M.W., and Lightman, S.L. (1990). The blood-brain interface. Eye, 4(Pt 2), 249-254.
Byrnes-Blake, K.A., Carroll, F.I., Abraham, P., and Owens, S.M. (2001). Generation of anti-(+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. International Immunopharmacology, 1(2), 329-338.
Carrera, M.R., Ashley, J.A., Zhou, B., Wirsching, P., Koob, G.F., and Janda, K.D. (2000). Cocaine vaccines: Antibody protection against relapse in a rat model. Proceedings of the National Academy of Sciences, USA, 97(11), 6202-6206.
Carrera, M.R., Ashley, J.A., Wirsching, P., Koob, G.F., and Janda, K.D. (2001). A second-generation vaccine protects against the psychoactive effects of cocaine. Proceedings of the National Academy of Sciences, USA, 98(4), 1988-1992.
Cerny, E.H., Levy, R., Mauel, J., Mpandi, M., Mutter, M., Henzelin-Nkubana, C., Patiny, L., Tuchscherer, G., and Cerny, T. (2002). Preclinical development of a vaccine against smoking. Onkologie, 25(5), 406-411.
Chiang, C.N., Hollister, L.E., Gillespie, H.K., and Foltz, R.L. (1985). Clinical evaluation of a naltrexone sustained-release preparation. Drug and Alcohol Dependence, 16(1), 1-8.
Comer, S.D., Collins, E.D., Kleber, H.D., Nuwayser, E.S., Kerrigan, J.H., and Fischman, M.W. (2002). Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology, 159(4), 351-360.
Drug Abuse Sciences. (2003). Naltrexone depot for injectable suspension. Available at http:www.drugabusesciences.com/products.asp [August 22, 2003].
Ernst, E. (1999). Second thoughts about safety of St John’s wort [published erratum appears in Lancet, 355(9203), 580]. Lancet, 354(9195), 2014-2016.
Ettinger, R.H., Ettinger, W.F., and Harless, W.E. (1997). Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacology, Biochemistry, and Behavior, 58(1), 215-220.
Fiore, M.C., Bailey, M.C., and Cohen, S.J. (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: Public Health Service, U.S. Department of Health and Human Services.
Fox, B.S., Kantak, K.M., Edwards, M.A., Black, K.M., Bollinger, B.K., Botka, A.J., French, T.L., Thompson, T.L., Schad, V.C., Greenstein, J.L., Gefter, M.L., Exley, M.A., Swain, P.A., and Briner, T.J. (1996). Efficacy of a therapeutic cocaine vaccine in rodent models. Nature Medicine, 2(10), 1129-1132.
Gupta, R.K., Singh, M., and O’Hagan, D.T. (1998). Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Advanced Drug Delivery Reviews, 32(3), 225-246.
Hardin, J.S., Wessinger, W.D., Proksch, J.W., and Owens, S.M. (1998). Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. Journal of Pharmacology and Experimental Therapeutics, 285(3), 1113-1122.
Hardin, J.S., Wessinger, W.D., Wenger, G.R., Proksch, J.W., Laurenzana, E.M., and Owens, S.M. (2002). A single dose of monoclonal anti-phencyclidine IgG offers long-term reductions in phencyclidine behavioral effects in rats. Journal of Pharmacology and Experimental Therapeutics, 302(1), 119-126.
Hatefi, A., and Amsden, B. (2002). Biodegradable injectable in situ forming drug delivery systems. Journal of Controlled Release, 80(1-3), 9-28.
Henningfield, J.E., Miyasato, K., and Jasinski, D.R. (1985). Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics, 234(1), 1-12.
Hieda, Y., Keyler, D.E., Vandevoort, J.T., Kane, J.K., Ross, C.A., Raphael, D.E., Niedbalas, R.S., and Pentel, P.R. (1997). Active immunization alters the plasma nicotine concentration in rats. Journal of Pharmacology and Experimental Therapeutics, 283(3), 1076-1081.
Hieda, Y., Keyler, D.E., Ennifar, S., Fattom, A., and Pentel, P.R. (2000). Vaccination against nicotine during continued nicotine administration in rats: Immunogenicity of the vaccine and effects on nicotine distribution to brain. International Journal of Immunopharmacology, 22(10), 809-819.
Hughes, J.R., Hatsukami, D.K., Pickens, R.W., Krahn, D., Malin, S., and Luknic, A. (1984). Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology, 83(1), 82-87.
Institute of Medicine. (1994). Growing up tobacco free: Preventing nicotine addiction in children and youths. Committee on Preventing Nicotine Addiction in Children and Youths. B.S. Lynch and R.J. Bonnie (Eds.). Washington, DC: National Academy Press.
Johnson, M.W., and Ettinger, R.H. (2000). Active cocaine immunization attenuates the discriminative properties of cocaine. Journal of Experimental and Clinical Psychopharmacology, 8(2), 163-167.
Kantak, K.M., Collins, S.L., Lipman, E.G., Bond, J., Giovanoni, K., and Fox, B.S. (2000). Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology, 148(3), 251-262.
Kantak, K.M., Collins, S.L., Bond, J., and Fox, B.S. (2001). Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology, 153(3), 334-340.
Keyler, D.E., Hieda, Y., St. Peter, J., and Pentel. P.R. (1999). Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine and Tobacco Research, 1(3), 241-249.
Keyler, D.E., Shoeman, D., LeSage, M.G., Calvin, A.D., and Pentel, P.R. (2003). Maternal vaccination against nicotine reduces nicotine distribution to fetal brain in rats. Journal of Experimental and Clinical Psychopharmacology, 305(2), 587-592.
Killian, A., Bonese, K., Rothberg, R.M., Wainer, B.H., and Schuster, C.R. (1978). Effects of passive immunization against morphine on heroin self-administration. Pharmacology, Biochemistry, and Behavior, 9(3), 347-352.
Koetzner, L., Deng, S., Sumpter, T.L., Weisslitz, M., Abner, R.T., Landry, D.W., and Woods, J.H. (2001). Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. Journal of Experimental and Clinical Psychopharmacology, 296(3), 789-796.
Kosten, T.R., Rosen, M., Bond, J., Settles, M., Roberts, J.S., Shields, J., Jack, L., and Fox, B. (2002a). Human therapeutic cocaine vaccine: Safety and immunogenicity. Vaccine, 20(7-8), 1196-1204.
Kosten, T.R., Gonsai, K., St. Clair Roberts, J., Jack, L., Bond, J., Mitchell, E., and Fox, B. (2002b, June). Phase II human study of cocaine vaccine TA-CD. Paper prepared for the College on Problems of Drug Dependence Annual Meeting, June 8-13, Quebec City.
Kreek, M.J., LaForge, K.S., and Butelman, E. (2002). Pharmacotherapy of addictions. Nature Reviews: Drug Discovery, 1(9), 710-726.
Langer, R., Cleland, J.L., and Hanes, J. (1997). New advances in microsphere-based single-dose vaccines. Advanced Drug Delivery Reviews, 28(1), 97-119.
LeSage, M.G., Keyler, D.E., Hieda, Y., Valentine, J., Ross, C., Fattom, A., Ennifar, S., and Pentel, P.R. (2001). Effects of a nicotine conjugate vaccine on the acquisition of nicotine self-administration in rats (abstract). Society for Research on Nicotine and Tobacco Annual Meeting, March, Seattle, WA.
Lindblom, N., De Villiers, S.H., Kalayanov, G., Gordon, S., Johansson, A.M., and Svensson, T.H. (2002). Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration, 69(3), 254-260.
Lindmayer, K., Horwith, G., Fattom, A., Naso, R., Fuller, S.A., Muenz, L., Kennedy, A., and Ennifar, S. (2002). Results of a phase 1, double blinded, controlled safety and immunogenicity trial of NicVAX, a conjugated nicotine vaccine (abstract). Paper presented at the 4th European Conference of the Society for Research on Nicotine and Tobacco, October, 2004, Santander, Spain.
Lopez-Arrieta, J.M., Rodriguez, J.L., and Sanz, F. (2004). Nicotine for Alzheimer’s disease. In The Cochrane Library (Issue 1). Chichester, England: John Wiley & Sons.
Malin, D.H. (2001). Nicotine dependence: Studies with a laboratory model. Pharmacology, Biochemistry, and Behavior, 70(4), 551-559.
Malin, D.H. (2002). Passive immunization against nicotine attenuates dependence as measured by mecamylamine-precipitated withdrawal. Society for Research on Nicotine and Tobacco Proceedings, 8, RP1.
Malin, D.H., Lake, J.R., Lin, A., Saldana, M., Balch, L., Irvin, M.L., Chandrasekara, H., Alvarado, C.L., Hieda, Y., Keyler, D.E., Pentel, P.R., Ennifar, S., Basham, L.E., Naso, R., and Fattom, A. (2001). Passive immunization against nicotine prevents nicotine alleviation of nicotine abstinence syndrome. Pharmacology, Biochemistry, and Behavior, 68(1), 87-92.
Malin, D.H., Alvarado, C.L., Woodhouse, K.S., Karp, H., Urdiales, H., Lay, D., Appleby, P., Moon, W.D., Ennifar, S., Basham, L.E., and Fattom, A. (2002) Passive immunization against nicotine attenuates nicotine discrimination. Life Sciences, 70(23), 2793-2798.
Mantripragada, S. (2002). A lipid based depot (DepoFoam technology) for sustained release drug delivery. Progress in Lipid Research, 41(5), 392-406.
McMillan, D.E., Hardwick, W.C., Li, M., and Owens, S.M. (2002). Pharmacokinetic antagonism of (+)-methamphetamine discrimination by a low-affinity monoclonal anti-methamphetamine antibody. Behavioral Pharmacology, 13(5-6), 465-473.
Mets, B., Winger, G., Cabrera, C., Seo, S., Jamdar, S., Yang, G., Zhao, K., Briscoe, R.J., Almonte, R., Woods, J.H., and Landry, D.W. (1998). A catalytic antibody against cocaine prevents cocaine’s reinforcing and toxic effects in rats. Proceedings of the National Academy of Sciences, USA, 95(17), 10176-10181.
Modesto-Lowe, V., and Van Kirk, J. (2002). Clinical uses of naltrexone: A review of the evidence. Experimental and Clinical Psychopharmacology, 10(3), 213-227.
Owens, S.M., and Mayersohn, M. (1986). Phencyclidine-specific Fab fragments alter phencyclidine disposition in dogs. Drug Metabolism and Disposition, 14(1), 52-58.
Owens, S.M., Zorbas, M., Lattin, D.L., Gunnell, M., and Polk, M. (1988). Antibodies against arylcyclohexylamines and their similarities in binding specificity with the phencyclidine receptor. Journal of Pharmacology and Experimental Therapeutics, 246(2), 472-478.
Pentel, P.R., and Keyler, D.E. (2004). Vaccination as a treatment for drug abuse: Nicotine, cocaine, phencyclidine. In M.M. Levine (Ed.), New generation vaccines. New York: Marcel Dekker.
Pentel, P.R., Malin, D.H., Ennifar, S., Hieda, Y., Keyler, D.E., Lake, J.R., Milstein, J.R., Basham, L.E., Coy, R.T., Moon, J.W., Naso, R., and Fattom, A. (2000). A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacology, Biochemistry, and Behavior, 65(1), 191-198.
Plessinger, M.A. (1998). Prenatal exposure to amphetamines: Risks and adverse outcomes in pregnancy. Obstetrics and Gynecology Clinics of North America, 25(1), 119-138.
Proksch, J.W., Gentry, W.B., and Owens, S.M. (2000). Anti-phencyclidine monoclonal antibodies provide long-term reductions in brain phencyclidine concentrations during chronic phencyclidine administration in rats. Journal of Pharmacology and Experimental Therapeutics, 292(3), 831-837.
Putney, S.D., and Burke, P.A. (1998). Improving protein therapeutics with sustained-release formulations. Nature Biotechnology, 16(2), 153-157.
Sanderson, S.D., Cheruku, S.R., Padmanilayam, M.P., Vennerstrom, J.L., Thiele, G.M., Palmatier, M.I., and Bevins, R.A. (2003). Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. International Immunopharmacology, 3(1), 137-146.
Sellers, E.M., Kaplan, H.L., and Tyndale, R.F. (2000). Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clinical Pharmacology and Therapeutics, 68(1), 35-43.
Shoeman, D., Keyler, D.E., and Pentel, P. (2002). Vaccination of female rats against nicotine reduces nicotine distribution to fetal brain (abstract). Paper presented at the Society for Research on Nicotine and Tobacco Annual Meeting, February, Savannah, GA.
Simister, N.E., and Story, C.M. (1997). Human placental Fc receptors and the transmission of antibodies from mother to fetus. Journal of Reproductive Immunology, 37(1), 1-23.
St. Clair Roberts, J., Dobson, J., Wood, D., and Settles, M. (2002). Safety and immunogenicity of a human nicotine conjugate vaccine (abstract). Paper presented at the College on Problems of Drug Dependence Annual Meeting, June, Quebec City.
Streeton, C., and Whelan, G. (2001). Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: A meta-analysis of randomized controlled trials. Alcohol and Alcoholism, 36(6), 544-552.
Tuncok, Y., Hieda, Y., Keyler, D.E., Brown, S., Ennifar, S., Fattom, A., and Pentel, P.R. (2001). Inhibition of nicotine-induced seizures in rats by combining vaccination against nicotine with chronic nicotine infusion. Experimental and Clinical Psychopharmacology, 9(2), 228-234.
Valentine, J.L., and Owens, S.M. (1996). Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. Journal of Pharmacology and Experimental Therapeutics, 278(2), 717-724.
Valentine, J.L., Arnold, L.W., and Owens, S.M. (1994). Anti-phencyclidine Fab fragments dramatically alter phencyclidine pharmacokinetics in rats. Journal of Pharmacology and Experimental Therapeutics, 269(3), 1079-1085.
Valentine, J.L., Mayersohn, M.M., Wessinger, W.D., Arnold, L.W., and Owens, S.M. (1996). Anti-phencyclidine monoclonal Fab fragments reverse PCP-induced behavioral effects and ataxia in rats. Journal of Pharmacology and Experimental Therapeutics, 278(2), 709-716.
Walburn, J., Gray, R., Gournay, K., Quraishi, S., and David, A.S. (2001). Systematic review of patient and nurse attitudes to depot antipsychotic medication. British Journal of Psychiatry, 179, 300-307.
Waldmann, T.A., and Strober, W. (1969). Metabolism of immunoglobulins. Progress in Allergy, 13, 1-110.





































