1
Chlorine1
Acute Exposure Guideline Levels
SUMMARY
Chlorine is a greenish-yellow, highly reactive halogen gas that has a pungent, suffocating odor. The vapor is heavier than air and will form a cloud in the vicinity of a spill. Like other halogens, chlorine exists in the diatomic state in nature. Chlorine is extremely reactive and rapidly combines with both inorganic and organic substances. Chlorine is used in the manufacture of a wide variety of chemicals, as a bleaching agent in industry and household products, and as a biocide in water and waste treatment plants.
Chlorine is an irritant to the eyes and respiratory tract; reaction with moist surfaces produces hydrochloric and hypochlorous acids. Its irritant properties have been studied in human volunteers, and its acute inhalation toxicity has been studied in several laboratory animal species. The data from the human and laboratory animal studies were sufficient for developing acute exposure guideline levels (AEGLs) for the five exposure durations (i.e., 10 and 30 minutes [min] and 1, 4, and 8 hours [h]). Regression analysis of human data on nuisance irritation responses (itching or burning of the eyes, nose, or throat) for durations of 30–120 min and during exposures to chlorine at 0–2 parts per million (ppm) determined that the relationship between concentration and time is approximately C2×t=k (where C= concentration, t=time, and k is a constant) (ten Berge and Vis van Heemst 1983).
The AEGL-1 was based on a combination of studies that tested healthy human subjects as well as atopic individuals (Rotman et al. 1983; Shusterman et al. 1998) and asthmatic patients (D’Alessandro et al. 1996). Atopic and asthmatic individuals have been identified as susceptible populations for irritant gases. The highest no-observed-adverse-effect level (NOAEL) for notable irritation and significant changes in pulmonary function parameters was 0.5 ppm in two studies. Eight atopic subjects were exposed for 15 min in one study (Shusterman et al. 1998), and eight healthy exercising individuals and an exercising atopic individual were exposed for two consecutive 4-h periods in the other (Rotman et al. 1983). The subjects in the Shusterman et al. (1998) study experienced nasal congestion, but irritation was described as none to slight. The exercising atopic individual in the Rotman et al. (1983) study experienced nondisabling, transient, asymptomatic changes in pulmonary function parameters. The selection of 0.5 ppm is supported by the lack of symptoms and lack of changes in pulmonary air flow and airway resistance in five asthmatic subjects inhaling 0.4 ppm for 1 h (D’Alessandro et al. 1996).
Because susceptible populations comprising atopic and asthmatic individuals were tested at similar concentrations, with incorporation of exercise into the protocol of one study, an intraspecies uncertainty factor (UF) of 1 was applied. The intraspecies UF of 1 is further supported by the fact that pediatric asthmatic subjects do not appear to be more responsive to irritants than adult asthmatic subjects (Avital et al. 1991). The AEGL-1 value was not time scaled for several reasons. First, the Rotman et al. (1983) study was for 8 h with a single 1-h break. Second, the response to chlorine appears to be concentration-dependent rather than time-dependent, as the pulmonary function parameters of individuals tested in this study, including
those for the atopic individual, did not increase between the 4- and 8-h measurements.
The AEGL-2 values were based on two of the studies used to derive the AEGL-1. Both healthy and susceptible human subjects inhaled chlorine at 1.0 ppm for 1 h (D’Alessandro et al. 1996) or 4 h (Rotman et al. 1983). Both healthy and susceptible subjects experienced some sensory irritation and transient changes in pulmonary function measurements. Greater changes were observed in pulmonary parameters among the susceptible subjects compared with the normal groups. In the latter study (Rotman et al. 1983), an atopic individual experienced no respiratory symptoms other than some sensory irritation during the 4-h exposure, but his airway resistance nearly tripled. He experienced shortness of breath and wheezing during a second 4-h exposure. Five individuals with nonspecific airway hyper-reactivity or asthma also experienced a statistically significant fall in pulmonary air flow and an increase in airway resistance during a 1-h exposure at 1.0 ppm (D’Alessandro et al. (1996). There were no respiratory symptoms during the exposure. The susceptible individual in the Rotman et al. (1983) study remained in the exposure chamber for the full 4 h without respiratory symptoms. Therefore, when considering the definition of the AEGL-2, the first 4 h of exposure was a no-effect level in a susceptible individual. Because the subjects were susceptible individuals, one of the subjects was undergoing light exercise during the exposures (making him more vulnerable to sensory effects), and an exercising susceptible individual exhibited effects that did not impede escape for the 4-h exposure duration (consistent with the definition of the AEGL-2), an intraspecies UF of 1 was applied.
Chlorine is a highly irritating and corrosive gas that reacts directly with the tissues of the respiratory tract with no pharmacokinetic component involved in toxicity; therefore, effects are not expected to vary greatly among other susceptible populations. Time-scaling was considered appropriate for the AEGL-2 because it is defined as the threshold for irreversible effects, which, in the case of irritants, generally involves tissue damage. Although the end point used in this case—a no-effect concentration for wheezing that was accompanied by a significant increase in airways resistance—has a different mechanism of action than that of direct tissue damage, it is assumed that some biomarkers of tissue irritation would be present in the airways and lungs. The 4-h 1-ppm concentration was scaled to the other time periods using the C2×t=k relationship. The scaling factor was based on regression analyses of concentrations and exposure durations that attained nuisance levels of irritation in human subjects (ten Berge and Vis
van Heemst 1983). The 10-min value was set equal to the 30-min value in order to not exceed the highest exposure of 4.0 ppm in controlled human studies.
In the absence of human data, animal lethality data served as the basis for AEGL-3. The mouse was not chosen as an appropriate model for lethality because mice often showed delayed deaths, which several authors attributed to bronchopneumonia. Because the mouse was shown to be more sensitive to chlorine than the dog and rat, and because the mouse does not provide an appropriate basis for quantitatively predicting mortality in humans, a value below those resulting in no deaths in the rat (213 ppm and 322 ppm) and above that resulting in no deaths in the mouse (150 ppm) for a period of 1 h was chosen (MacEwen and Vernot 1972; Zwart and Woutersen 1988). The AEGL-3 values were derived from a 1-h concentration of 200 ppm. That value was calculated applying a total UF of 10–3 to extrapolate from rats to humans (interspecies values for the same end point differed by a factor of approximately 2 within each of several studies), and 3 to account for differences in human sensitivity. The susceptibility of asthmatic subjects relative to healthy subjects when considering lethality is unknown, but the data from two studies with human subjects showed that doubling a no-effect concentration for irritation and bronchial constriction resulted in potentially serious effects in asthmatic subjects but not in normal individuals. Time-scaling was considered appropriate for the AEGL-3, because tissue damage is involved. (Data in animal studies clearly indicate that time scaling is appropriate when lung damage is observed.) The AEGL-3 values for the other exposure times were calculated using the C2 ×t=k relationship, which was derived based on the end point of irritation from a study with humans.
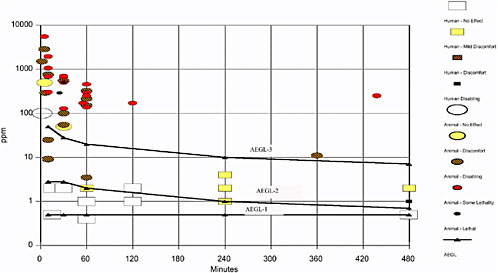
The calculated values are listed in Table 1–1.
1. INTRODUCTION
Chlorine is the most abundant naturally occurring halogen. Halogens do not occur in the elemental state in nature. When formed experimentally, chlorine is a greenish-yellow, diatomic gas (Cl2) with a pungent, suffocating odor. Chlorine is used in the manufacture of a variety of nonagricultural chemicals, such as vinyl chloride and ethylene dichloride; as a bleaching agent in the paper industry (along with chlorine dioxide [ClO2]); as commercial and household bleaching agents (in the form of chlorates [ClO3−] and hypochlorites [OCl−]); and as a biocide in water purification and waste
TABLE 1–1 Summary of AEGLs Values for Chlorine (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1a (Nondisabling) |
0.5 (1.5) |
0.5 (1.5) |
0.5 (1.5) |
0.5 (1.5) |
0.5b (1.5) |
No to slight changes in pulmonary function parameters in humans (Rotman et al. 1983; D’Alessandro et al. 1996; Shusterman et al. 1998) |
|
AEGL-2 (Disabling) |
2.8 (8.1) |
2.8 (8.1) |
2.0 (5.8) |
1.0 (2.9) |
0.7 (2.0) |
1.0 ppm for 4 h was a NOAEL for an asthma-like attack in human subjects; the other values were time-scaled (Rotman et al. 1983; D’Alessandro et al. 1996) |
|
AEGL-3 (Lethal) |
50 (145) |
28 (81) |
20 (58) |
10 (29) |
7.1 (21) |
Threshold for lethality in the rat (MacEwen and Vernot 1972; Zwart and Woutersen 1988) |
|
aThe distinctive, pungent odor of chlorine will be noticeable to most individuals at these concentrations. bBecause effects were not increased following an interrupted 8-h exposure of an atopic individual to 0.5 ppm, the 8-h AEGL-1 was set equal to 0.5 ppm. Abbreviations: mg/m3, milligrams per cubic meter; ppm, parts per million. |
||||||
treatment systems (Perry et al. 1994). Chlorine gas was used as a chemical warfare agent during World War I (Withers and Lees 1987). The vapor is heavier than air and will form a cloud in the vicinity of a spill.
As of January 1999, world annual capacity for chlorine production was estimated at almost 50 million metric tons (CEH 2000). Chlorine is produced at chlor-alkali plants at over 650 sites worldwide, and North America accounts for 32% of capacity (operating rates are greater than 83% of capacity). In the early 1990s, chlorine was produced at 49 facilities, operated by 29 companies, in the United States (Perry et al. 1994). In 1993, U.S.
production was reported at 24 billion pounds (C&EN 1994). The major global market for chlorine is ethylene dichloride production (about 33%) (CEH 2000).
Chlorine is extremely reactive and enters into substitution or addition reactions with both inorganic and organic substances. Moist chlorine unites directly with most elements. Reaction with water produces hydrochloric (HCl) and hypochlorous acid (HClO) (Budavari et al. 1996; Perry et al. 1994). Other relevant chemical and physical properties are listed in Table 1–2. According to Amoore and Hautala (1983), the odor threshold is 0.31 ppm, and a range of 0.2–0.4 ppm was reported in other studies. There is considerable variation in detecting the odor among subjects; for many individuals, the ability to perceive the odor decreases over exposure time (NIOSH 1976).
Chlorine is an eye and respiratory tract irritant and, at high doses, has direct toxic effects on the lungs. It reaches the lungs because it is only moderately soluble in water and it is not totally absorbed in the upper respiratory tract at high concentrations. The acute inhalation toxicity of chlorine has been studied in several laboratory animal species, and its irritant properties have been studied with human volunteers.
2. HUMAN TOXICITY DATA
2.1 Acute Lethality
For humans, a 5-min lethal concentration in 10% of subjects (LC10) of 500 ppm (NTIS 1996) and a possible 30-min lethal exposure of 872 ppm have been reported (Perry et al. 1995). Both of those secondary sources cited data from Prentiss (1937) as well as data from other early sources.
Although accidental releases have resulted in deaths (e.g., Jones et al. 1986), no studies were located in which acute lethal exposure concentrations were measured. Probit analysis of available information on the lethality of chlorine to animals and humans was used by Withers and Lees (1985b) to estimate a concentration lethal to 50% of the population (LC50). Their model incorporates the effects of physical activity, inhalation rate, the effectiveness of medical treatment, and the lethal toxic load function. The estimated 30-min LC50 at a standard level of activity (inhalation rate of 12 liters [L]/min) for the regular, vulnerable, and average (regular plus vulnerable) populations, as described by the authors, were 250,100, and 210 ppm,
TABLE 1–2 Chemical and Physical Properties of Chlorine
|
Parameter |
Value |
Reference |
|
Synonyms |
Bertholite; hypochlorite; hypochlorous acid |
Budavari et al. 1996 |
|
Molecular formula |
Cl2 |
Budavari et al. 1996 |
|
Molecular weight |
70.9 |
Budavari et al. 1996 |
|
CAS registry no. |
7782–50–5 |
Budavari et al. 1996 |
|
Physical state |
Gas |
Budavari et al. 1996 |
|
Color |
Greenish-yellow |
Budavari et al. 1996 |
|
Solubility in water |
0.092 moles/L |
Budavari et al. 1996 |
|
Vapor pressure |
5,025 mm Hg at 20°C |
Matheson Gas Co. 1980 |
|
Vapor density |
1.4085 at 20°C |
AIHA 1988 |
|
Density (water=1) |
1.56 at boiling point |
Perry et al. 1994 |
|
Melting point |
−101°C |
Budavari et al. 1996 |
|
Boiling point |
−34.05°C |
Budavari et al. 1996 |
|
Flammability |
Nonflammable |
Matheson Gas Co. 1980 |
|
Conversion factors in air |
1 ppm=2.9 mg/m3 1 mg/m3=0.34 ppm |
ACGIH 2001 |
respectively. The LC10 for the three populations were 125, 50, and 80 ppm, respectively.
2.2. Nonlethal Toxicity
Exposures at 30 ppm and 40–60 ppm have been reported to cause intense coughing and serious damage, respectively (ILO 1998), but no documentation of those values was given.
2.2.1. Experimental Studies
Five well-conducted and well-documented studies using human volunteers were located. Those studies are summarized in Table 1–3.
TABLE 1–3 Summary of Irritant Effects in Humansa
|
Concentration (ppm) |
Exposure Timeb |
Effect |
References |
|
0.4 |
1 h |
No pulmonary function changes in subjects with airway hyperreactivity/asthma |
D’Alessandro et al. 1996 |
|
0.5 |
15 min |
Change in nasal air resistance in rhinitic subjects (no change in nonrhinitic subjects); no effect on pulmonary peak flow, rhinorrhea, postnasal drip, or headache in either type of subject |
Shusterman et al. 1998 |
|
0.5 |
8 h |
Perception of odor, no discomfort, no effects, no changes in pulmonary function measurements for healthy individuals; some changes for atopic individual |
Anglen 1981; Rotman et al. 1983 |
|
1.0 |
1 h |
Statistically significant but modest changes in FEV1 and Raw for normal and asthmatic subjects |
D’Alessandro et al. 1996 |
|
1.0 |
2 h |
No noticeable effects |
Joosting and Verberk 1974 |
|
1.0 |
4 h |
Irritation for some sensations; no changes in pulmonary function measurements |
Anglen 1981 |
|
1.0 |
4 h |
Transient changes in pulmonary function measurements (airway resistance) |
Rotman et al. 1983 |
|
1.0 |
8 h |
Irritation (itchy eyes, runny nose, mild burning in throat); transient changes in pulmonary function measurements; atopic subject could not complete full 8-h exposure because of wheezing and shortness of breath |
Anglen 1981; Rotman et al. 1983 |
In the first part of a two-part experiment, 31 un-acclimated male and female subjects (age range 20–32 years [y]) were exposed to chlorine at 0.0, 0.5, 1.0, or 2.0 ppm for 4 h or at 0.5 ppm or 1.0 ppm for 8 h (Anglen 1981). Not all subjects were exposed to all concentrations. Exposure days were randomly assigned, and the subjects did not know the test concentration, although the investigator did. In part two, eight nonsmoking males, ages 23–33 y, were exposed to concentrations of chlorine at 0.0, 0.5, or 1.0 ppm for 8 h. The 8-h sessions were broken into two 4-h sessions with a 30- or 60-min lunch break. A 15-min exercise period during each hour of exposure was designed to increase the average heart rate to 100 beats per minute. During the exposures, the subjects filled out a subjective questionnaire concerning 14 sensations ranging from smell to shortness of breath and using a scale of 0 (no sensation) to 5 (unbearable). Eye irritation was documented photographically; other signs of irritation were documented with pre- and post-exposure examinations by a physician. Pulmonary function tests (forced vital capacity [FVC] and forced expiratory volume at 1 second [FEV1) for each subject were measured before, during, and after exposures. The data were analyzed in terms of mean or median response, percent responding to greater than or equal to a set value, and responses to ranges or indices of irritation; the data were analyzed statistically where appropriate (p>0.167, p>0.025). Paired t-tests involving differences in values between pre-exposure and post-exposure times were used to analyze the pulmonary function measurements. Chlorine concentrations in the exposure chamber, measured by a variety of colorimetric and instrumental methods, were consistent.
At the tested concentrations, most of the subjects did not consider the sensations of smell or taste of chlorine unpleasant; therefore, those sensations were not included in the remainder of the analyses. In part one, the greatest number of subjects responded positively to the irritant sensation of itching or burning of the throat (described by the subjects as “feeling as if they had been talking for a long time”). Statistical differences were seen for that sensation at 1 and 2 ppm compared with controls; the level of response was ≥3 (nuisance or greater) for the 2-ppm concentration. A concentration of 0.5 ppm resulted in subjective irritation values between 1 (just perceptible) and 2 (distinctly perceptible) and produced no change in pulmonary functions. In part two, exposure at 1 ppm produced statistically significant changes in pulmonary function and increased subjective irritation at 8 h. No significant differences in pulmonary function measurements were seen at the end of a 4-h exposure at 1 ppm. Some differences in pulmonary function were seen at the end of 4 h at the 2-ppm concentration, but not
at the end of 2 h. Most of the exposed subjects did not report sensations of nausea, headache, dizziness, or drowsiness at any concentration during the exposures. Male subjects were more sensitive to the irritant effects of chlorine than female subjects. The author concluded that exposure at 2 ppm for up to 30 min produced no significant increase in subjective irritation (severity of response not stated) over that seen during control exposures, and 2-h exposures at 2 ppm or 4-h exposures at 1 ppm produced no significant changes in pulmonary function (Anglen 1981).
In a follow-up study, Rotman et al. (1983) reported pulmonary function tests of eight male subjects, ages 19–33 y, exposed at 0.0, 0.5, and 1.0 ppm, like in the Anglen (1981) study. Air samples were collected eight times daily, and analysis for chlorine was accomplished using a modified NIOSH-recommended methyl orange method. The subjects were blind to the exposure concentration, but the study investigators were not. While in the chamber, each subject exercised for 15 min of each hour on an inclined treadmill or a by a simple step test at a rate that produced a heart rate of 100 beats per minute. Subjects exited the exposure chamber after 4 h to undergo the pulmonary function tests and then reentered the chambers. Comparisons of pre- and post-exposure pulmonary functions were made by paired t-tests between the percent change from baseline values obtained at analogous times after a sham exposure. Insignificant differences were observed with the sham versus the 0.5-ppm exposure. Compared with the sham changes from baseline, the changes in the 1.0-ppm exposure group were small, but statistically significant (p<0.05); those changes were in FEV1, peak expiratory flow rate (PEFR), forced expiratory flow rate at 50% and 25% vital capacity (FEF50 and FEF25), total lung capacity (TLC), airway resistance (Raw), and difference in nitrogen concentration between 750 milliliters (mL) and 1,250 mL of exhaled vital capacity (ΔN2). At 8 h, changes were present in FVC, FEV1, forced expired volume in 1 second as %FEV (FEV1%), PEFR, FEF50, FEF25, and Raw. Raw had the greatest response to chlorine exposure with an increase of 31% after 4 h of exposure at 1.0 ppm compared with increases of up to 6% during sham exposures. Most of those parameters had returned to pre-exposure values by the following day. However, a ninth subject whose pre-exposure lung parameters indicated obstructive airway disease (as defined by DuBois et al. [1971]) did not complete the full 8 h of exposure at 1.0 ppm because of shortness of breath and wheezing; his values were not included in the statistical analysis. The atopic individual experienced changes in several pulmonary function parameters after exposure to chlorine at 0.5 ppm. The greatest change for this individual was in Raw, which increased by 40% over the pre-exposure value
after 4 h of exposure at 0.5 ppm and by 33% over the pre-exposure value after 8 h of exposure at 0.5 ppm. Changes in Raw in the healthy subjects were 5% and 15% for the respective time periods. Following the 4-h exposure at 1.0 ppm, Raw increased from a pre-exposure value of 3.3 centimeters (cm) of water per liter per second to 14.4 cm of water per liter per second in the atopic individual. The authors concluded that at the 1-ppm concentration, serious subjective symptoms of irritation were not produced in healthy adults, but transient altered pulmonary function was observed.
The authors discussed the implications of changing baselines and significant changes over time with sham exposures, the latter with respect to diurnal variation in pulmonary function. Baselines differed on different days of the tests (e.g., the pre-exposure baselines for total lung capacities [TLCs] were 7.09 L and 6.55 L on two different days, which is significantly different [p<0.05]; the TLC decreased after the sham exposure, but increased after the 0.5-ppm exposure). However, differences in parameters between baseline and post-exposure for sham exposures were fewer in number and generally smaller in amount than for the 1 ppm treatment. It should be noted that in normal subjects, several pulmonary function tests (FVC, FEV1, and FEF25–75%) may have daily changes of 5% to 13% and week to week changes of 11% to 21% (EPA 1994).
D’Alessandro et al. (1996) exposed 12 male and female volunteers, ages 18–50 y, to chlorine for 1 h. Five of the subjects were without airway hyper-reactivity (defined by baseline methacholine hyper-responsiveness) and seven were diagnosed with airway hyper-reactivity. Five of the seven with airway hyper-reactivity had clinical histories of asthma (one was being treated regularly with corticosteroids). Ten subjects, five normal and five hyper-reactive (three of which had asthma), were exposed to a concentration of chlorine at 1.0 ppm. Five of the subjects with asthma were exposed at 0.4 ppm. They were not blinded to the exposure status. The subjects were exposed to chlorine by mask while in the sitting position; there were no air exposures. Chlorine was measured with a chlorine analyzer. The following pulmonary function parameters were measured or calculated immediately following and 24 h after exposure: FEV1, TLC, carbon monoxide diffusing capacity (Dco), Raw, and FEF25–75%. After asthmatic subjects were exposed at 0.4 ppm, there were no statistically significant changes in any parameters, including FEV1 and Raw, either immediately following or 24 h after exposure. Immediately following the exposure at 1.0 ppm, there were statistically significant changes in FEV1 and Raw for both normal and hyper-reactive subjects compared with baseline values. Hyper-reactive subjects showed a greater relative decrease in FEV1 (16% compared with
4% for normal subjects) and a greater relative increase in Raw (108% compared with 39% for the normal subjects). Although one hyper-responsive subjects’ FEV1 fell by 1,200 mL, and the Raw more than tripled, the mean changes were considered modest by the authors. The hyper-responsive subject with the greatest increase in Raw following exposure at 1.0 ppm showed virtually no change following exposure at 0.4 ppm. Two subjects characterized as hyper-responsive experienced undefined respiratory symptoms following exposure at 1.0 ppm. For all subjects, most values were close to baseline by 24 h post-exposure. The fact that none of the subjects found the odor of chlorine appreciable at either concentration is interesting.
In a single-blind crossover study, Shusterman et al. (1998) measured nasal air resistance via active posterior rhinomanometry in eight subjects with seasonal allergic rhinitis and eight nonrhinitic subjects. Measurements were made before, immediately after, and 15 min after a 15-min exposure to either filtered air or chlorine at 0.5 ppm in filtered air administered through a nasal mask in a climate-controlled chamber. Each subject served as his or her control, and subjects were free of medications for at least 24 h prior to testing. Subjects were between 18 and 40 y of age. The mean percent change in nasal air resistence from baseline to immediately after exposure was +24% in the subjects with allergic rhinitis and +3% in the nonrhinitic group. The mean percent change from baseline to 15 min after exposure was +21% in the subjects with allergic rhinitis and −1% in the nonrhinitic subjects. Differences between groups were significant (p< 0.05) for both post-exposure times. Rhinitic subjects reported greater exposure-related increases in odor intensity, nasal irritation, and nasal congestion than did nonrhinitic subjects, but the relationship between subjective and objective nasal congestion was weak. No significant exposure-related changes were observed for rhinorrhea, postnasal drip, or headache. Pulmonary peak flow was also obtained before and after exposure and none of the subjects exhibited clinically significant changes in peak flow (decreases of ≥10% of baseline), nor did they complain of cough, wheezing, or chest tightness during chlorine exposure days.
Joosting and Verberk (1974) exposed eight subjects, ages 28–52, at 0.5–4 ppm for 2 h. The subjects were all members of a Dutch subcommittee on toxicology. Subjective reactions were noted every 15 min. Subjects exited the chambers every 15 min to perform spirometry tests. Concentrations of chlorine at 0.5 ppm and 1.0 ppm did not produce noticeable effects in 2 h; 2 ppm produced very slight eye, nose, and throat irritation; and 4 ppm resulted in a distinctly perceptible to offensive level of irritation of the nose and throat and desire to cough. The highest score was for irritation of the
throat, for which the average response was nuisance. No sensory irritation scores reached unbearable. No effects on lung function (vital capacity [VC], FEV, and forced inspiratory volume [FIV]) occurred at the lower concentrations; effects on lung function were not reported in the 4-ppm exposure group because only 2–3 subjects completed the exposure.
Older studies were located in the literature; those have been reviewed and critiqued by NIOSH (1976) and OSHA (1989). In several older studies in which measurement techniques and/or ranges of measured values were not given, concentrations as low as 0.027 ppm produced slight sensory effects in humans and concentrations at 0.5–4.0 ppm produced sensory irritation (NIOSH 1976). For example, Rupp and Henschler (1967) reported that human volunteers experienced burning of the eyes after exposure at 0.5 ppm for 15 min. In a separate test, the subjects reported respiratory irritation during exposure at 0.5 ppm and discomfort during exposure at 1 ppm. The study has been criticized for its lack of controls as well as the possible presence of confounding chemicals (OSHA 1989). However, the results of the Rupp and Henschler (1967) study that indicated some irritation at 0.5 ppm and 1 ppm are not all that different from the results of Anglen (1981), Rotman et al. (1983), and D’Alessandro et al. (1996).
2.2.2. Epidemiologic Studies
Few epidemiologic studies document average chlorine exposure concentrations over long periods of time; concentration variations over time; or exposure durations to various concentrations. Interpretation of results is often complicated by unknown previous exposures to chlorine, exposures to other chemicals, and smoking habits. NIOSH (1976) discussed available epidemiological studies conducted prior to 1976. In most of the cited studies, work-room concentrations averaged <1 ppm. The report noted the difficulty in correlating exposures to effects.
Patil et al. (1970) compared the health of 382 workers in 25 chlorine production plants in the United States and Canada with that of unexposed workers in the same plants. All subjects were male, between the ages of 19 and 69. Time-weighted average exposures to chlorine ranged from 0.006 ppm to 1.42 ppm, with a mean of 0.146 ppm; almost all workers were exposed to <1 ppm. The average number of exposure years was 10.9. There were no statistically significant (p<0.05) signs or symptoms on a dose-response relation basis in chest x-rays, electrocardiograms, or pulmonary function tests. Nor were there dose-response relationships with cough,
sputum production, frequency of colds, dyspnea, palpitation, chest pain, fatigue, tremors, gastrointestinal problems, dermatitis, or hematologic parameters. Subjective complaints of tooth decay were dose-related, but that complaint was not borne out by physical examination.
A study of respiratory effects in 52 Italian electrolytic cell workers with an average exposure to chlorine of 0.298±0.181 ppm was undertaken by Capodaglio et al. (1970, as cited in ACGIH 1995, 1996). Of five respiratory function tests (FEV1, VC, DCO, residual volume, and helium concentration gradient in a single breath during washout), only carbon monoxide diffusing capacity showed a slight but significant difference; however, cigarette smoking may have contributed to that observation.
Mortality and morbidity (respiratory symptoms, disease, and functions) of workers exposed to chlorine in the pulp and paper industry over a 10-y period (1963–1973) were similar to those of the general white male population (Ferris et al. 1979). Mean and maximum exposure concentrations in the pulp mill were to trace amounts (<0.0005 ppm). In an earlier study (Ferris et al. 1964), exposures in the pulp mill were more variable (mean, 7.4 ppm; range up to 64 ppm) and slight adverse effects on respiratory ailments were found. Exposures were also to chlorine dioxide.
Between 1984 and 1989, a prospective study was conducted on the effects of chlorine exposure on workers in a chlorine manufacturing plant (Kusch 1994). Chlorine exposures and pulmonary function tests (FVC, FEV1, and FEF25–50%), taken over a period of 5 y were compared with a control group. The average exposure in the control group was 0.058 ppm, and the average exposure in the workers was 0.092 ppm. There were no measurable effects on pulmonary function related to chlorine exposure.
2.2.3. Accidents
An accident at a chemical plant in India resulted in the exposure of 88 workers as well as police and fire-fighting personnel to a measured concentration of 66 ppm for an unspecified amount of time (Shroff et al. 1988). No further details on the exposure concentration or duration were given. The workers, ages 21–60 y, were admitted to the hospital within an hour of exposure with symptoms of dyspnea, coughing, irritation of the throat and eyes, headache, giddiness, chest pain, and abdominal discomfort. Examinations revealed hilar congestion, bronchial vasculature, respiratory incapacitation at the PFT (undefined); bronchoscopy revealed tracheobronchial congestion, chronic bronchitis, scattered hemorrhages, and bronchial ero-
sion. Bronchial smears of 28 patients on day 5 after the accident showed basal-cell and goblet-cell hyperplasia; acute inflammation; and chromatolysis of columnar epithelial cells, multinucleated syncytial respiratory epithelial cells with degenerating cilia, and nonpigmented alveolar macrophages. In some patients these effects progressed to bronchopneumonia, epithelial regeneration, and repair by fibrosis by day 25 post-exposure.
During an industrial accident, a group of workers was presumably exposed to concentrations up to 30 ppm (based on symptoms—actual exposure concentration and duration not known) (Abhyankar et al. 1989). Initial symptoms included watering eyes, sneezing, cough, sputum, retrostenal burning, dyspnea, apprehension, and vomiting. All patients were asymptomatic by 2 weeks (wk) post-exposure; at 6 months (mo) post-exposure, all spirometry tests (FVC, FEV1) were within the normal range. Those patients known to have a pre-existing lung condition did not show any additional evidence of lung damage.
Incidents of acute poisonings at indoor swimming pools have been reported (Decker 1988), but air concentrations during those incidents are unknown. In a study in Spain, the mean air concentration measured during five nonconsecutive days in four enclosed swimming pools was 0.42±0.24 milligrams per cubic meter (mg/m3) (0.14±0.08 ppm) (Drobnic et al. 1996). The samples were taken at <10 cm above the water, the breathing zone of swimmers.
Acute exposure to chlorine/chloramine gas occurs often among the general public through the mixing of domestic home cleaners (Mrvos et al. 1993); swimming pool chlorinator tablets (Wood et al. 1987); and intentional self administration (Rafferty 1980). In the case of home exposures, a review of 216 cases reported to a Regional Poison Information Center showed that symptoms, primarily cough with resulting shortness of breath, resolved within 1 to 6 h without medical intervention. There was no information on exposure concentrations (Mrvos et al. 1993).
2.3. Developmental and Reproductive Effects
No studies on developmental and reproductive effects in humans were located.
2.4. Genotoxicity
No data concerning the genotoxicity of chlorine in humans via inhala-
tion exposures were identified in the available literature. When chlorine (sodium hypochlorite) at concentrations of 20 ppm and above was added to cultures of human lymphocytes, chromosomal aberrations (breaks and rearrangements) and endomitotic figures were observed (Mickey and Holden 1971).
2.5. Carcinogenicity
No increase in neoplasms was reported in an epidemiology study of workers engaged in the production of chlorine (Patil et al. 1970). The range of time-weighted exposures to chlorine was 0.006–1.42 ppm.
2.6. Summary
Anglen (1981) conducted a study on 31 male and female subjects in which slight but statistically significant changes in pulmonary function and subjective irritation resulted from exposure to chlorine at 1 ppm for 8 h (two 4-h sessions). Subjective sensory irritation (itching or burning of the throat) was described as “just perceptible” or “distinctly perceptible.” A 30-min exposure at 2 ppm produced no increase in subjective irritation. An 8-h exposure at 0.5 ppm produced no changes in lung function and no significant sensory irritation. A 4-h exposure at 1 ppm produced no changes in pulmonary function tests, but an 8-h exposure at 1 ppm produced slight declines in some pulmonary function tests. Most of these findings were confirmed in a study by Rotman et al. (1983) in which eight healthy volunteers were exposed at 0.5 ppm or 1.0 ppm for an interrupted 8 h. Transient but statistically significant declines in six of 15 pulmonary function tests were associated with exposure at 1 ppm for 4 or 8 h but not with exposure at 0.5 ppm for 4 or 8 h. These studies reported that there was no effect of chlorine exposure on carbon monoxide diffusing capacity, thus indicating there is no significant pulmonary edema from the exposures. However, an atopic subject in the Rotman et al. study (1983) suffered an asthma-like attack resulting from exposure to chlorine at 1 ppm; that subject withstood exposure, testing, and exercise for the first 4 h, but exited the exposure chamber “before the full 8-h exposure to 1 ppm.” Changes in his pulmonary function measurements were greater than those of the other test subjects. The atopic subject completed the interrupted 8-h exposure at 0.5 ppm.
The Anglen and Rotman studies were supported by two additional studies. In the first study (Angelen 1981), subjects with airway hyper-reactivity, including asthmatic subjects, showed no significant changes in FEV1 or Raw following a 1-h exposure at 0.4 ppm. Exposure of both normal and hyper-reactive subjects at 1.0 ppm significantly decreased FEV1 and significantly increased Raw in both sets of subjects; the hyper-reactive subjects showed a significantly greater response than normal subjects. However, the mean changes were considered modest by the authors. In the second study, subjective sensory irritation of healthy individuals reached “nuisance” level at an exposure concentration of 4 ppm for 2 h (Joosting and Verberk 1974). This study showed that time, as well as concentration, was a factor in subjective response to chlorine inhalation.
No useful exposure data could be derived from epidemiology studies or human exposures to accidental releases of chlorine. No studies were located on developmental and reproductive effects. In an in vitro study, chlorine was genotoxic at 20 ppm.
3. ANIMAL TOXICITY DATA
3.1 Acute Lethality
A summary of the acute lethality data is presented in Table 1–4. Some of those studies were reviewed by Withers and Lees (1985a). According to Withers and Lees (1985a), many of the older studies had deficiencies in gas analysis methods and exposure conditions. More recent studies contradict the results in the older studies (e.g., the 3-h LC50 of 10 ppm in mice reported by Schlagbauer and Henschler [1967, as cited in AIHA 1988] is contradicted by the nonlethal 6-h exposure at 9.3 ppm for 5 d reported in the more recent study by Buckley et al. [1984]). Nevertheless, some of the older studies (Lipton and Rotariu 1941, as cited in Withers and Lees 1985a; Silver et al. 1942, as cited in Withers and Lees 1985a; Schlagbauer and Henschler 1967, as cited in AIHA 1988) are cited in Table 1–4 for comparison purposes. More recent studies are discussed below.
3.1.1. Dogs
In an early study, Underhill (1920) reported on mortality in dogs following 30-min exposures to a range of concentrations (50–2,000 ppm). A total of 112 male and female dogs of several breeds were used. Acute mor-
TABLE 1–4 Summary of Acute Lethal Inhalation Data in Animals
|
Species |
Concentration (ppm) |
Exposure Time |
Effecta |
Reference |
|
Dog |
650 |
30 min |
LC50 |
Underhill 1920; Withers and Lees 1985a |
|
Rat |
5,500 |
5 min |
LC50 |
Zwart and Woutersen 1988 |
|
2,841 |
5 min |
No deaths |
||
|
Rat |
1,946 |
10 min |
LC50 |
Zwart and Woutersen 1988 |
|
Rat |
700 |
30 min |
LC50 |
Zwart and Woutersen 1988 |
|
547 |
30 min |
No deaths |
||
|
Rat |
1,000 |
53 min |
LC50 |
Weedon et al. 1940 |
|
Rat |
455 |
1 h |
LC50 |
Zwart and Woutersen 1988 |
|
Rat |
288b |
1 h |
LC01 |
Zwart and Woutersen 1988 |
|
322 |
1 h |
No deaths |
||
|
Rat |
293c |
1 h |
LC50 |
Back et al. 1972; MacEwen and Vernot 1972; Vernot et al. 1977 |
|
213 |
1 h |
No deaths |
||
|
Rat |
250 |
7.3 h |
LC50 |
Weedon et al. 1940 |
|
Rat |
63 |
>16 h |
LC50 |
Weedon et al. 1940 |
|
Mouse |
290 |
6 min |
No deaths |
Bitron and Aharonson 1978 |
|
Mouse |
1,057 |
10 min |
LC50 |
Zwart and Woutersen 1988 |
|
754 |
10 min |
No deaths |
||
|
Mouse |
676 |
10 min |
LC50 |
Silver et al. 1942d |
|
Mouse |
628 |
10 min |
LC50 |
Lipton and Rotariu 1941d |
|
Mouse |
549 |
10 min |
25–45% mortality |
Silver et al. 1942d |
|
Mouse |
380 |
10 min |
10% mortality |
Silver et al. 1942d |
|
Mouse |
302 |
10 min |
LC50 |
Alarie 1980 |
|
Mouse |
290 |
11 min |
LC50 |
Bitron and Aharonson 1978 |
|
Species |
Concentration (ppm) |
Exposure Time |
Effecta |
Reference |
|
Mouse |
290 |
15 min |
80% mortality |
Bitron and Aharonson 1978 |
|
Mouse |
290 |
25 min |
100% mortality |
Bitron and Aharonson 1978 |
|
Mouse |
1,000 |
28 min |
LC50 |
Weedon et al. 1940 |
|
Mouse |
504 |
30 min |
LC50 |
Zwart and Woutersen 1988 |
|
Mouse |
127 |
30 min |
LC50 |
Schlagbauer and Henschler 1967e |
|
55 |
30 min |
No deaths |
||
|
Mouse |
170 |
55 min |
LC50 |
Bitron and Aharonson 1978 |
|
Mouse |
137c |
1 h |
LC50 |
Back et al. 1972; MacEwen and Vernot 1972; Vernot et al. 1977 |
|
Mouse |
250 |
1 h |
LC80 |
O’Neil 1991 |
|
200 |
1 h |
LC01 |
||
|
150 |
1 h |
No deaths |
||
|
Mouse |
170 |
2 h |
80% mortality |
Bitron and Aharonson 1978 |
|
Mouse |
10 |
3 h |
80% mortalityf |
Schlagbauer and Henschler 1967e |
|
Mouse |
250 |
7.3 h |
LC50 |
Weedon et al. 1940 |
|
Mouse |
63 |
>16 h |
LC50 |
Weedon et al. 1940 |
|
Rabbit |
500 |
30 min |
100% mortality |
Barrow and Smith 1975 |
|
aLC50 and LC100 values were obtained immediately after exposure (Weedon et al. 1940), 3 h post-exposure (Alarie 1980), 10 d post-exposure (Silver et al. 1942), 14 d post-exposure (Back et al. 1972; MacEwen and Vernot 1972; Vernot et al. 1977; Zwart and Woutersen 1988), and 30 d post-exposure (Bitron and Aharonson 1978). bCalculated by Zwart and Woutersen (1988) using probit analysis; note this value is lower than the concentration resulting in no deaths. cAuthors report a 20–30% loss of chlorine in the exposure chambers; the concentrations given are measured concentrations. dAs cited in Withers and Lees 1985a. eAs cited in AIHA 1988. fThese results conflict with results of other studies. |
||||
talities, defined as deaths within 3 d of the exposure, were 0%, 6%, 20%, 43%, 50%, 87%, and 92% at concentration ranges of 50–250 ppm, 400–500 ppm, 500–600 ppm, 600–700 ppm, 700–800 ppm, 800–900 ppm, and 900–2,000 ppm, respectively. However, some delayed deaths, occurring as the result of bronchopneumonia following subsidence of acute pulmonary edema, resulted in all groups; one of nine suffered a delayed death (time not given) at the 50–250 ppm range. Withers and Lees (1985a) analyzed these data using the method of Litchfield and Wilcoxon (1949) and calculated an LC50 of 650 ppm. That value is based on the concentration in ppm at 25°C. Underhill (1920) initially made the conversion from mg/m3 to ppm based on a temperature of 0°C. Dogs exposed to chlorine became excited and displayed signs of respiratory irritation; those signs were followed by labored breathing (Underhill 1920).
3.1.2. Rats
Back et al. (1972), MacEwen and Vernot (1972), and Vernot et al. (1977) reported the same 1-h LC50 of 293 ppm (95% confidence limits, 260–329 ppm) for Sprague-Dawley rats. MacEwen and Vernot (1972) noted a 20–30% loss of chlorine in the exposure chambers, probably due to condensation on the walls. Therefore, the concentrations given are measured concentrations. Rats experienced immediate eye and nose irritation followed by lacrimation, rhinorrhea, and gasping after 1 h of exposure at all tested concentrations (213, 268, 338, and 427 ppm). Rats surviving the 213 ppm and 268-ppm exposures gained less weight than the control group during the 14-d post-exposure period. No deaths occurred in rats exposed at 213 ppm for 1 h. Weedon et al. (1940) exposed groups of eight rats to 63, 240, or 1,000 ppm for 16 h or until death. Times to 50% mortality were >16 h, 7.3 h, and 53 min, respectively.
Zwart and Woutersen (1988) exposed specific pathogen free (SPF) Wistar-derived rats to chlorine at 322–5,793 ppm for exposure durations of 5 min to 1 h to calculate LC50 values. Observations were made over a 14-d period after which the animals were sacrificed and histologic examinations were made; additional groups of rats were exposed and examined 2 d after exposure. Rats exposed at the highest concentrations during the 30- and 60-min exposures showed signs of restlessness, eye and nasal irritation, labored breathing, and reduced respiratory rate. Mortalities occurred during exposure as well as within the first week of the observation period. Increased lung weights were positively correlated with higher concentrations and
longer exposure durations. At the high concentrations, 5,793 ppm for 5 min and 2,248 ppm for 10 min, effects were observed in the nose, larynx, and trachea; at those and the lower concentrations, lung lesions, including focal aggregates of mononuclear inflammatory cells, increased septal cellularity, squamous metaplasia of bronchiolar epithelium, and edema, were observed in one or more animals. Hyperplasia of the larynx and trachea observed at 2 d post-exposure was resolved by 14 d post-exposure in surviving rats. In rats in which minute-volume was measured, death occurred in several animals following a reduction in minute-volume to ≤39% of the pre-exposure level. According to the authors, the 1-h LC01 of 288 ppm (95% confidence interval, 222–345 ppm), estimated by probit analysis, appeared to correspond with the onset of irreversible lung damage. The breathing pattern during the exposures changed from regular inspiration directly followed by a regular expiration to rapid shallow breathing that lasted less than a minute. That was followed by maximal inhalation directly after expiration and a long post-inspiratory pause. No deaths occurred at 2,841 ppm for 5 min, 547 ppm for 30 min, or 322 ppm for 60 min.
In a subchronic study, three of 10 female F-344 rats exposed at 9 ppm for 6 h/d, 5 d/wk died by the 30th exposure (Barrow et al. 1979). In another study, groups of eight 30-wk-old male and female SPF rats were exposed to chlorine at approximately 117 ppm for 3 h/d, 7 d/wk until half of the animals of each gender died (Bell and Elmes 1965). Total exposure time to 50% mortality was 29 h for males and 32 h for females. When the response of conventional rats was compared with that of SPF rats, the response was more severe in the conventional rats, who exhibited proliferation of goblet cells and increased mucus, emphysema, and polymorphonuclear cells in the lungs.
3.1.3. Mice
Back et al. (1972), MacEwen and Vernot (1972), and Vernot et al. (1977) reported a 1-h LC50 of 137 (95% confidence limits, 119–159 ppm). Weedon et al. (1940) exposed groups of four mice to 63,240, or 1,000 ppm for 16 h or until death. Times to 50% mortality were >16 h, 7.3 h, and 28 min, respectively.
Bitron and Aharonson (1978) exposed male albino mice to 170 ppm and 290 ppm for several exposure times and calculated 50% mortality as a function of exposure time (Lt50). Mice were restrained during the exposures. Observations were made over a 30-d period. Lt50 for the 170-ppm
and 290-ppm exposures were 55 min and 11 min, respectively. The results of this work were unusual in that many of the deaths were delayed, occurring during the second week of the observation period, rather than during and immediately following exposure. No mice died within a 30-d observation period following exposure at 290 ppm for 6 min. No deaths occurred in BALB/c mice exposed at 50, 100, or 150 ppm for 1 h (O’Neil 1991). Mice were observed for at least 5 d post-exposure.
Zwart and Woutersen (1988) exposed Swiss mice to chlorine at 579–1,654 ppm for 10 min and 458–645 ppm for 30 min to calculate LC50 values. Mortality observations were made over a 14-d period. Nearly one-third of the mice died during the second week post-exposure, indicating to the authors that the deaths may have been due to secondary infection. Increased lung weights were positively correlated with higher concentrations and longer exposure durations. No deaths occurred at a concentration of chlorine at 754 ppm for 10 min.
Alarie (1980) reported that the 10-min LC50 of male Swiss-Webster mice decreased from 302 ppm in uncannulated mice to 131 ppm when chlorine was delivered directly to the trachea via cannulation.
As part of an experiment on immune response, groups of 10 BALB/c mice were exposed at 50, 100, 150, 200, or 250 ppm for 1 h (O’Neil 1991). Mortality occurred at 200 ppm (two mice, 4–5 d post-exposure) and at 250 ppm (8/10 mice).
3.2. Nonlethal Toxicity
Data on effects following exposures to nonlethal concentrations of chlorine are available for the monkey, rat, mouse, guinea pig, and rabbit. Studies utilizing acute exposure durations are summarized in Table 1–5.
3.2.1. Nonhuman Primates
No studies on single acute exposures were located. Klonne et al. (1987) exposed Rhesus monkeys to chlorine at 0, 0.1, 0.5, or 2.3 ppm for 6 h/d, 5 d/wk for 1 y. In the group exposed at 2.3 ppm, ocular irritation as well as treatment-related histopathologic changes limited to the nasal passages and trachea were observed at 1 y. Those lesions, consisting of focal, epithelial hyperplasia with loss of cilia and decreased numbers of goblet cells, were considered mild in the group exposed at 2.3 ppm and were not present in all
animals. Lesions in the lower exposure groups were minimal. No statistically significant differences were observed between control and exposure groups for pulmonary diffusing capacity of carbon monoxide or distribution of ventilation values (number of breaths to 1% N2).
3.2.2. Rats
Demnati et al. (1995) exposed groups of four male Sprague-Dawley rats (nose-only) to chlorine at 0, 50, 100, 200, 500, or 1,500 ppm for 2–10 min in order to study effects on airway mucosa and lung parenchyma. Histologic examinations were performed at 1, 3, 6, 12, 24, and 72 h after exposure. Exposures to concentrations of ≤500 ppm did not induce significant histologic changes. Lungs from control rats and from rats exposed at 50–100 ppm for 2 min were normal within 72 h; at concentrations of 200 ppm and 500 ppm for 2–5 min, there was only slight perivascular edema in all exposed rats. Exposure at 1,500 ppm for 2 min produced only slight effects, including mild perivascular edema and occasional small clusters of polymorphonuclear leukocytes in the mucosa of large airway. The 10-min exposure at 1,500 ppm caused significant changes that varied with time after exposure—airspace and interstitial edema associated with bronchial epithelial sloughing at 1 h, decreased edema and the appearance of mucosal polymorphonuclear leucocytes at 6–24 h, and epithelial regeneration as evidenced by hyperplasia and goblet cell metaplasia at 72 h. No deaths were reported.
Exposure at 25 ppm lowered the respiratory rate by 50% (RD50) in F-344 rats, presumably during a 10-min test (Barrow and Steinhagen 1982); during exposure for 6 h, the RD50 was 10.9 ppm (Chang and Barrow 1984). Groups of 9–10 male F-344 rats were exposed to a concentration of chlorine at 9.1 ppm (the RD50 of mice) for 1, 3, or 5 d (6 h/d) and examined for respiratory tract pathology (Jiang et al. 1983). Sacrifice took place immediately after exposure. In all animals, lesions were present in the nasal passages with less severe changes in the nasopharynx, larynx, trachea, and lungs. Lesions in the nasal passages involved epithelial degeneration with epithelial cell exfoliation, erosion, and ulceration (respiratory epithelium) and extensive epithelial erosion and ulceration (olfactory epithelium of the dorsal meatus). Electron microscopy examination revealed loss of respiratory and olfactory cilia and cellular exfoliation of the naso- and maxilloturbinates.
TABLE 1–5 Summary of Sublethal Effects in Laboratory Animals
When male and female F-344 rats were exposed to chlorine at 0, 1, 3, or 9 ppm for 6 h/d, 5 d/wk for 6 wk, effects were observed in the upper and lower respiratory tract (Barrow et al. 1979). Lesions in male and female rats exposed at 9 ppm included widespread inflammation throughout the respiratory tract with hyperplasia and hypertrophy of epithelial cells of the respiratory bronchioles, alveolar ducts, and alveoli. Hepatocellular cytoplasmic vacuolation was observed in both genders exposed at 3 ppm or 9 ppm, and renal tubule effects were observed in male rats exposed at 9 ppm. Effects observed in animals exposed at 1 ppm or 3 ppm were much less severe than those observed at 9 ppm. In addition, decreased body weight gains were observed in females at all exposure concentrations and in males at 3 ppm and 9 ppm.
Groups of 14-wk-old male and female SPF rats were exposed to chlorine at approximately 40 ppm for 3 h/d for a total of 42 h (Bell and Elmes 1965). No deaths occurred. Signs and symptoms of exposure included coughing, sneezing, and runny and blood-stained noses after 3 h. Histologic examinations of the lungs revealed recovery by 14 d post-exposure. Exposure of conventional rats for 1 h daily to 14–18 ppm, for a total of 24 exposure hours in 4 wk, also resulted in no mortality (Elmes and Bell 1963). The authors considered the chlorine concentrations overestimates, because the exposed rats huddled together in the exposure cage.
Groups of 70 male and 70 female F-344 rats were exposed to chlorine gas at 0, 0.4, 1.0, or 2.5 ppm for 6 h/d, 5 d/wk (males) or 3 alternate d/wk (females) for 2 y (CIIT 1993; Wolf et al. 1995). Concentration-dependent lesions confined to the nasal passages were observed in all animals. These lesions were most severe in the anterior nasal cavity and included respiratory and olfactory epithelial degeneration, septal fenestration, mucosal inflammation, respiratory epithelial hyperplasia, squamous metaplasia and goblet cell hypertrophy and hyperplasia, and secretory metaplasia of the transitional epithelium of the lateral meatus. Body weights were depressed compared with controls, but no early deaths occurred.
3.2.3. Mice
Groups of four male Swiss-Webster mice were exposed to chlorine concentrations at 0.7–38.4 ppm for 10 min (Barrow et al. 1977). The RD50 was 9.3 ppm. The RD50 of male OF1 mice was calculated at 3.5 ppm by Gagnaire et al. (1994). Although that exposure was for 60 min, the decrease occurred by 10 min. The protocol of ASTM (1991) was followed by Gagnaire et al., but the OF strain is not the strain of mice suggested for use
in measuring sensory irritation. The ASTM (1991) RD50 test calls for male Swiss-Webster mice and a 10-min exposure period.
In a follow-up study to that of Barrow et al. (1977), Buckley et al. (1984) exposed male Swiss-Webster mice to the RD50 (9.3 ppm) for 6 h/d for 5 d. Half of each group was necropsied immediately after the last exposure and the other half was necropsied 72 h post-exposure. No deaths were reported. Lesions in both the anterior respiratory epithelium adjacent to the dorsal meatus and in the respiratory epithelium included exfoliation, inflammation erosion, ulceration, and necrosis. Chlorine reached the lower respiratory tract, as indicated by tracheal lesions and terminal bronchiolitis, with occlusion of the affected bronchioles by serocellular exudate. Recovery was minimal to moderate after 72 h.
Groups of 9–10 male Swiss-Webster mice were exposed at 9.1 ppm, the approximate RD50 for 1, 3, or 5 d (6 h/d) and examined for respiratory tract pathology (Jiang et al. 1983). Sacrifice took place immediately after exposure. Respiratory tract lesions were similar to those of the rat described above (lesions in the nasal passages with less severe changes in the nasopharynx, larynx, trachea, and lungs).
Groups of 70 male and 70 female B6C3F1 mice were exposed to chlorine gas at 0, 0.4,1.0, or 2.5 ppm for 6 h/d, 5 d/wk for 2 y (CIIT 1993; Wolf et al. 1995). Concentration-dependent lesions confined to the nasal passages were observed in all animals. These lesions were most severe in the anterior nasal cavity and included respiratory and olfactory epithelial degeneration, septal fenestration, mucosal inflammation, respiratory epithelial hyperplasia, squamous metaplasia and goblet cell hypertrophy and hyperplasia, and secretory metaplasia of the transitional epithelium of the lateral meatus. Body weights were depressed (males, all exposures; females, 2.5 ppm) compared with controls, but no early deaths occurred.
3.2.4. Guinea pigs
Arlong et al. (1940, as cited in NIOSH 1976) exposed guinea pigs to 1.7 ppm for 5 h daily over 87 d. No deaths were reported during the 300-d observation period. No other details of the study were available.
3.2.5. Rabbits
Groups of two male and two female rabbits were exposed at 0, 50, 100, or 200 ppm for 30 min and tested for lung changes as measured by volume-
pressure relationships and inspiratory-expiratory flow rate at times from 30 min to 60 d post-exposure (Barrow and Smith 1975). Rabbits exposed at 50 ppm showed no changes at any time periods. Recovery of flow rate ratios occurred by 14 d post-exposure in the 100-ppm groups and by 60 d post-exposure in the 200-ppm group. Pulmonary compliance in the 100-ppm and 200-ppm groups did not return to control levels within 60 d. Examinations of the lungs revealed hemorrhages, pneumonitis and anatomic emphysema in the 100-ppm and 200-ppm groups at 3 and 14 d post-exposure; those changes were not present at 60 d post-exposure.
3.3. Developmental and Reproductive Effects
No studies addressing developmental or reproductive effects following inhalation exposure to chlorine were located. However, because effects on development and reproduction would be systemic, due to circulating chlorine, the effects of oral administration of chlorine may have bearing on the chlorine hazard assessment. Those data, reviewed by EPA (1996) and AIHA (1988), demonstrated no or insufficient evidence of reproductive or developmental toxicity.
Groups of 10 male B6C3F1 mice were dosed by oral gavage with 1 mL of test solution containing OCl− or HOCl at 40, 100, or 200 mg/L/d for 5 d (Meier et al. 1985). Animals were sacrificed at 1, 3, or 5 wk after the last treatment, and the caudae epididymides were examined for sperm head abnormalities. No abnormalities were observed at 1 and 5 wk post-treatment in the groups treated with OCl− or at any time in the groups treated with HOCl. A small but statistically significance difference compared with controls was observed in the groups administered OCl− at all dose levels at 3 wk. These results do not clearly indicate an effect on fertility.
Druckrey (1968) administered highly chlorinated drinking water (100 mg/L) to seven consecutive generations of BD II rats. The average daily dose was estimated at 10 mg/kg/d. No treatment-related effects were observed on any generation. Carlton et al. (1986) administered chlorine in deionized water by gavage male and female Long Evans rats at doses of 1.0, 2.0, or 5.0 mg/kg/d to for 66–76 d. Dosing began prior to mating and continued during gestation and lactation. Groups of offspring were necropsied at 21 d after birth or at 40 d of age; the latter group was dosed following weaning. No statistically significant differences were observed between the control and treated rats in litter survival, litter size, or pup weight.
HOCl, formed by bubbling chlorine gas through water, was administered in the drinking water to Sprague-Dawley rats for 2.5 mo, prior to and
throughout gestation (Abdel-Rahman 1982). Concentrations were 0, 1, 10, or 100 mg/L. Rats were sacrificed on day 20 of gestation and fetuses were examined for bone and soft-tissue defects. No increase in resorptions were found in any treatment group. A significant increase in skeletal anomalies at the 100 mg/L concentration (incompletely ossified or missing sternebrae and rudimentary ribs) was interpreted as a nonspecific retardation in growth. The increase in skeletal defects was not significant in the 10 mg/L and 100 mg/L groups compared with the controls. Total defects—skeletal and soft tissue—were increased significantly over the control group in the 100 mg/L group. Maternal toxicity—body weight, food consumption, clinical signs—was not described.
3.4. Genotoxicity
No data on inhalation exposures were located in the available literature. Genotoxicity studies were conducted via oral dosing of groups of 10 male B6C3F1 mice with 1 mL of test solution containing OCl− or HOCl at concentrations of 40, 100, or 200 mg/L for 5 d (Meier et al. 1985). Chlorine was not mutagenic in the bone marrow micronucleus and cytogenetic assays. Sodium hypochlorite produced chromosomal aberrations in several mammalian cell tests (NTP 1992).
3.5. Chronic Toxicity and Carcinogenicity
Groups of male and female F-344 rats were exposed by inhalation to chlorine concentrations at 0, 0.4, 1.0, or 2.5 ppm for 2 y (CIIT 1993; Wolf et al. 1995). Histologic examinations of the nose and major organs revealed no increase in the incidence of neoplasia over that of control groups. F-344 rats and B6C3F1 mice of both genders administered chlorinated or chloraminated drinking water for 2 y showed no increased incidences of neoplasms (NTP 1992).
3.6. Summary
Few animal studies addressed no- or mild-effect levels at exposure times of 10 min to 8 h. No gross or microscopic lung changes occurred in rabbits following a 30-min exposure at 50 ppm (Barrow and Smith 1975). The highest 30-min values resulting in no deaths (LC0) for the rat and rabbit
were 547 ppm (Zwart and Woutersen 1988) and 200 ppm (Barrow and Smith 1975), respectively. The 60-min concentrations resulting in no deaths in the rat and mouse were 322 (Zwart and Woutersen 1988) and 150 ppm (O’Neil 1991), respectively. No deaths, but moderate to severe lesions of the respiratory tract and peribronchiolitis, occurred in rats following a 6-h exposure at 9.1 ppm (Jiang et al. 1983).
Thirty-minute LC50 values ranged from 137 ppm in the mouse (Back et al. 1972) to 700 ppm in the rat (Zwart and Woutersen 1988). The 60-min LC50 and LC01 values for the rat were 455 ppm and 288 ppm (Zwart and Woutersen 1988).
Chlorine administered in the drinking water or by gavage to rats or mice did not cause reproductive or developmental problems (Druckrey 1968; Abdel-Rahman 1982; Meier et al. 1985; Carlton et al. 1986). A 2-y inhalation study with rats showed no evidence of carcinogenicity (CIIT 1993; Wolf et al. 1995). Mutagenicity tests were generally negative (Meier et al. 1985).
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Pharmacokinetic data following acute exposures were not available. Metabolic and kinetic considerations are not relevant regarding the determination of AEGL values because animals die of acute respiratory failure. Chlorine gas reacts at the site of contact, and very little of the chemical is absorbed into the bloodstream (Eaton and Klaassen 1996).
4.2. Mechanism of Toxicity
Although of moderate solubility, chlorine is categorized as a Category I gas because it is so rapidly irreversibly reactive in the surface liquid and tissue of the respiratory tract (EPA 1994). Studies with repeated exposures of laboratory animals indicate that at moderate to high concentrations, chlorine is not effectively scrubbed in the upper respiratory tract and is therefore capable of exerting its effects over the entire respiratory tract (Barrow et al. 1979); however, at low concentrations (≤2.5 ppm for up to 2 y), chlorine is effectively scrubbed in the anterior nasal passages as indicated by the absence of lesions in the lower respiratory tract of rats, mice (Wolf et al. 1995), and monkeys (Klonne et al. 1987).
Chlorine gas combines with tissue water to form hydrochloric and hypochlorous acids (HCl and HClO); the latter spontaneously breaks down into HCl and free O•, which combines with water, releasing oxygen radicals (O−). The oxygen radical produces major tissue damage, which is enhanced by the presence of HCl (Perry et al. 1994; Wolf et al. 1995).
The response to inhalation of chlorine can range from sensory irritation and reflex bronchoconstriction to death, the latter due to pulmonary edema. The sensory irritation response to chlorine is due to stimulation of the trigeminal nerve endings in the respiratory mucosa, which results in a decrease in respiratory rate (Alarie 1981). Reflex bronchoconstriction is a local reaction in which cholinergic-like agents bind to respiratory tract cell surface receptors and trigger an increase in the intracellular concentration of cyclic guanosine monophosphate. That facilitates contraction of the smooth muscles that surround the trachea and bronchi, causing a decrease in airway diameter and a corresponding increase in resistance to airflow that may result in wheezing, coughing, a sensation of chest tightness, and dyspnea (Witschi and Last 1996). Death can occur from lack of oxygen during an asthmatic attack or if chlorine reaches the lungs and causes pulmonary edema; delayed deaths that occur starting 3 d after exposure might be due to bronchial infection (Underhill 1920; Withers and Lees 1985a; Bitron and Aharonson 1978), which may be treatable in humans.
4.3. Structure-Activity Relationships
The combined human and animal data on chlorine are sufficient for derivation of inhalation exposure guidelines and the use of structure-activity comparisons is not necessary. Like hydrogen chloride (HCl) and fluorine (F2), Cl2 is an irritant to the eyes, skin, and respiratory tract. When compared with mortality data for HCl and F2 (Wohlslagel et al. 1976; ATSDR 1993; Perry et al. 1994; NAC 1996), chlorine is more toxic than HCl but slightly less toxic than F2 to laboratory rodents.
4.4. Concentration-Exposure Duration Relationship
When considering low concentrations of chlorine that do not result in tissue damage and the response of atopic and asthmatic individuals, time-scaling might not be relevant. Several studies, such as those of Shusterman et al. (1998) and D’Alessandro et al. (1996), were conducted for short periods of time—15 min and 1 h, respectively—because responses were ex-
pected within those time periods. Furthermore, in the Rotman et al. (1983) study, pulmonary function parameters of the tested individuals, including those for the atopic individual, did not change between the 4- and 8-h measurements, indicating a sustained effect during exposure.
Time-scaling is relevant at the AEGL-2 and AEGL-3 levels where tissue damage is involved. When data are lacking for desired exposure times, scaling across time might be based on the relationship between concentration and exposure duration when a common end point is used. The relationship between concentration and time is described by Cn×t=k, in which the exponent n may be different from 1. ten Berge and Vis van Heemst (1983) analyzed the data of Anglen (1981) on irritation response in humans. Irritation consisted of itching or burning of the eyes, nose, or throat and scores ranged from 0 (no sensation) to 5 (unbearable), with a score of 3 representing a nuisance level or irritation. Regression analysis of the percent of subjects reporting a nuisance irritation response to concentrations at 1 ppm and 2 ppm over exposure durations of 30 min and 120 min resulted in an n value of 1.9.
Several sets of mortality data from animal studies were available for calculating the relationship between concentration and exposure time. Using the probit analysis method often Berge et al. (1986) and/or regression analysis, the data sets and their values for n are Bitron and Aharonson (1978), n=3.5; Zwart and Woutersen (1988), n=1.0; and Weedon et al. (1940), n=1.1. The probit analysis method applied to the data for 11 irritant gases from ten Berge et al. (1986) also results in a range of values for n of 1.0–3.5. In the Bitron and Aharonson (1978) study, the mice were restrained (which usually results in lower lethality values than for unrestrained animals) and deaths were delayed, occurring during the second week of the observation period rather than during and immediately following exposure. Because of the questionable methodology and the delayed deaths (possibly due to bacterial infection) in the Bitron and Aharonson (1978) study, that study will not be considered for calculating a time-scaling relationship for chlorine. Respiratory irritation is an initial step in the progression of irritation that leads to pulmonary edema and death. Based on the evidence for a similar mechanism of action for irritation and death, an n value of 2 will be used for time-scaling for chlorine. An n value of 2 for time-scaling the threshold for lethality for chlorine is supported by data for another halogen. Using the end point of lethality, the concentration-exposure duration relationship for fluorine for several mammalian species was Cn×t=k, where n was approximately slightly less than 2 (NAC 1996). Based on relative toxicity (Section 4.3), the n value for chlorine would be
higher than that of fluorine when extrapolating from longer to shorter exposure durations.
4.5. Other Relevant Information
4.5.1. Susceptible Populations
Chlorine is highly irritating and corrosive to the tissues of the respiratory tract. At low concentrations, the direct action of chlorine on the respiratory tract is not expected to vary greatly among most healthy individuals, including infants, children, and the elderly. For example, at the low concentration of 0.5 ppm, neither healthy nor atopic subjects exhibited clinically significant changes (decreases of ≥10%) in peak air flow (Shusterman et al. 1998). At 0.4 ppm, there were no statistically significant changes in several respiratory parameters in either healthy or asthmatic subjects (D’Alessandro et al. 1996). In the Rotman et al. (1983) study where numerous pulmonary parameters were measured, healthy subjects responded in a similar manner at 1 ppm.
Data from the Rotman et al. (1983) and D’Alessandro et al. (1996) studies on chlorine exposures and individuals with airway hyper-reactivity or asthma indicate that, compared with the general population, the respiratory tracts of those individuals may be very reactive to the presence of chlorine, as reported in Section 2.2.1. Responsiveness to inhaled agents varies among individuals with airway hyper-reactivity and asthma. In children, asthma may be defined as mild, moderate, or severe depending on the response to an inhaled agonist (Larsen 1992). With mild asthma, symptoms are infrequent and brief, and treatment is with inhaled β-agonists as needed. Moderate asthma is defined by symptoms occurring twice weekly, and treatment is with cromolyn sodium or slow-release theophylline. Severe asthmatic patients have daily symptoms, and daily treatment with oral or inhaled steroids is required. One individual in the D’Alessandro et al. (1996) study with airway hyper-reactivity had a clinical history of asthma and “was being treated regularly with inhaled or systemic corticosteroids.”
There is a concern that children with airway hyper-reactivity and asthma may be more sensitive to inhaled irritants and allergens than adult asthmatic subjects. Avital et al. (1991) studied the response to methacholine challenge in 182 asthmatic children (132 males and 50 females) of various ages. All therapy except corticosteroid therapy and slow-release theophylline was discontinued prior to the study. The children were divided
into three age groups—1–6 y, 7–11 y, and 12–17 y—and into three clinical groups according to their minimal therapeutic requirements—mild, moderate, and severe asthma. In older children, responsiveness was measured by the methacholine provocation concentration that produced a 20% fall in FEV1. In younger children, responsiveness was determined by wheezing, persistent cough, or tachypnea following a methacholine challenge. Bronchial reactivity correlated inversely with the severity of bronchial asthma according to minimal drug requirements and was similar over the age range for each severity group. That is, in each severity category (mild, moderate, or severe asthma), the mean concentration of methacholine that evoked the designated response was the same regardless of the age group. Avital et al. (1991) compared their results with the responses of asthmatic adults challenged with histamine from a study by Cockcroft et al. (1977). Mean ages of the adults were between 30 y and 40 y. Although the classes of asthmatic severity in Cockcroft et al. (1977) were only broadly comparable to those in Avital et al. (1991), there was a “striking similarity” in the results. That is, the concentration at which adults in each class of severity responded to histamine was similar to the methacholine concentration at which the children reacted in the respective severity classes.
Adults were tested in the D’Alessandro et al. (1996) study, but the ages of the individuals in the lower range of age (18 y) were close to those of the older children in the Avital et al. (1991) study. The range of provocative concentrations of methacholine in the D’Alessandro et al. (1996) study overlapped the range of the severe asthmatic subjects in the Avital et al. (1991) study, indicating that at least one adult in the former study was as responsive to methacholine as the children with severe asthma. Furthermore, during the exposure to chlorine at 1 ppm, the atopic individual in the Rotman et al. (1983) study, who was not on medication, responded to a greater degree, as measured by a fall in FEV1, than any of the subjects with airway reactivity or asthma in the D’Alessandro et al. (1996) study. Following a 4-h exposure to chlorine at 1 ppm, the FEV1 of the atopic individual was 45% of the pre-exposure value (Rotman et al. 1983), whereas following the 1-h exposure at 1 ppm, the FEV1 of the most sensitive individual with airway hyper-reactivity or asthma was 61% of the pre-exposure value (D’Alessandro et al. 1996). The final FEV1 for both subjects was the same, 1,900 mL.
Another consideration when evaluating the response of individuals with airway hyper-reactivity and asthma to irritants and allergens is the time of response following challenge. Individuals in the D’Alessandro et al. (1996) study were exposed to chlorine for only 1 h; the response of the atopic individual in the Rotman et al. (1983) study may have occurred early, al-
though wheezing did not occur until after 4 h of exposure. According to the literature, asthmatic reactions may be immediate (within minutes of exposure) or delayed (hours after exposure) (Larsen 1992). In the Avital et al. (1991) study, response to provocative concentrations of methacholine occurred within 2 min. In both studies with chlorine (Rotman et al. 1983; D’Alessandro et al. 1996), individuals had responded by the time they were tested for pulmonary function changes (after 1 h and after 4 h of exposure), and there were no reported delayed or greater effects within the 24-h post-exposure period. Although not conclusive, the data appear to indicate that the response to chlorine is concentration-dependent rather than time-dependent. Thus, a 1-h exposure to chlorine is sufficient to elicit a response in susceptible individuals; if a response is not present at 1 h, it is unlikely to occur with continued exposure.
4.5.2. Reactive Airways Dysfunction Syndrome
In humans, the reported long-term effects of accidental exposures at high concentrations of chlorine (evidenced by the presence of a yellow-green cloud) are conflicting, some authors noting residual pulmonary abnormalities (Kowitz et al. 1967; Alberts and do Pico 1996), and others either reporting no significant permanent damage (Weill et al. 1969; Kaufman and Burkons 1971; Jones et al. 1986) or reporting that the presence of permanent damage was questionable (Charan et al. 1985). Several case reports described respiratory hyper-responsiveness following acute exposures to chlorine at high concentrations. This syndrome, called reactive airways disease or reactive airways dysfunction syndrome (RADS), is initiated by one or several exposures to high concentrations of an irritating gas. Case studies were reviewed by Alberts and do Pico (1996) and Lemiere et al. (1996). In several of the studies, a clear interpretation of the results was complicated by the lower values in pulmonary function tests of smokers.
4.5.3. Gender and Species Variability
Several studies indicated gender differences in responses to chlorine exposure, females rats (Barrow et al. 1979; Wolf et al. 1995) and monkeys (Klonne et al. 1987) developing lesions at lower concentrations than males. However, male mice were slightly more sensitive to chlorine than female mice (Wolf et al. 1995).
The data also allow an examination of interspecies differences. In those studies in which investigators tested the lethality of chlorine to two species (rat and mouse), the LC50 values were within a factor of approximately 2 of each other (Weedon et al. 1940; Back et al. 1972; Zwart and Woutersen 1988).
4.5.4. Tolerance to Repeated Exposures
Following repeated exposures, rats developed sensory irritation tolerance to chlorine, as indicated by an increase in the RD50 following pretreatment (Barrow and Steinhagen 1982). The RD50 was 25 ppm in non-pretreated rats, whereas RD50 values increased to 90, 71, and 454 ppm when rats were pretreated at 1, 5, or 10 ppm, respectively, for 6 h/d, 5 d/wk for 2 wk and exposed 16–24 h following the last day of pretreatment.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
NIOSH (1976) discussed available epidemiology studies conducted prior to 1976. In those studies, and in a more recent study (Ferris et al. 1979), work room concentrations averaged <1 ppm, and no effects could be clearly documented. However, those studies generally included a “healthy worker” population.
The studies by Anglen (1981) and Rotman et al. (1983) indicate that there are no significant sensory irritation and no serious pulmonary function changes associated with 4- or 8-h exposures in healthy human subjects of both genders at 0.5 ppm or 1.0 ppm. However, an atopic individual, whose baseline pulmonary parameters were outside of the normal range, as defined by DuBois et al. (1971), experienced changes in several pulmonary function parameters after exposure to chlorine at 0.5 ppm (Rotman et al. 1983). The greatest change for that individual was in Raw, which increased 40% over the pre-exposure value after 4 h of exposure at 0.5 ppm and increased 33% over the pre-exposure value after 8 h of exposure at 0.5 ppm. Changes in Raw in the healthy subjects were 5% and 15% for the respective time periods. Transient changes in pulmonary function (specifically Raw) were seen at 1.0 ppm for 4 h in the Rotman et al. study (1983) and at 2 ppm for 4 h in the Anglen (1981) study; however, sensory irritation reached nuisance
levels at the latter concentration-exposure time combination. In healthy subjects, no lung function measurements were changed at 2.0 ppm for 2 h (Anglen 1981). The FEV1, a particularly reproducible and sensitive measure of obstructive or restrictive flows in the lung (Witschi and Last 1996), changed by less than 10% following exposure at 1 ppm for 4 h. The atopic individual did not tolerate the 1-ppm exposure during a second 4-h exposure period because of serious respiratory effects.
In the study by D’Alessandro et al. (1996), subjects with a clinical history of asthma were tested. In the study by Shusterman et al. (1998), subjects with seasonal allergic rhinitis were tested. A concentration at 0.4 ppm for 1 h elicited no statistically significant response in airflow or resistance in asthmatic subjects (D’Alessandro et al. 1996). In the same study, a concentration at 1.0 ppm for 1 h elicited significant changes in several pulmonary function parameters for both normal subjects and subjects with asthma, but the mean changes were considered modest by the study authors. However, the Raw of one subject with asthma more than tripled during the exposure at 1.0 ppm.
5.2. Summary of Animal Data Relevant to AEGL-1
All short-term animal studies were conducted at concentrations that produced effects greater than those defined by the AEGL-1.
5.3. Derivation of AEGL-1
The studies by Anglen (1981), Rotman et al. (1983), D’Alessandro et al. (1996), and Shusterman et al. (1998) are all relevant to the development of AEGL-1 values. Those studies addressed sensory irritation as well as symptomatic and asymptomatic changes in several pulmonary function parameters. In addition, the studies used a diverse population, including healthy, atopic, and asthmatic subjects. Exercise was incorporated into the protocol of the Rotman et al. (1983) study, simulating conditions of stress. The studies indicate that 0.5 ppm, for 15 min (Shusterman et al. 1998) or for an interrupted 8 h with incorporation of exercise into the protocol (Rotman et al. 1983), is the highest NOAEL consistent with the definition of an AEGL-1 (i.e., a NOAEL for notable discomfort and irritation accompanied by non-disabling, transient, asymptomatic effects). The next highest concentration tested, 1.0 ppm for more than 4 h, resulted in effects, such as shortness of breath and wheezing, greater than those defined by an AEGL-1. The NOAEL of 0.5 ppm was identified from the study of Rotman et al.
subjects in the study of D’Alessandro et al. (1996). That NOAEL supports the choice of 0.5 ppm in the study of Rotman et al. (1983). In fact, the single exercising atopic subject proved to be more sensitive at 0.5 ppm, as indicated by asymptomatic changes in pulmonary function parameters, than were the non-exercising asthmatics exposed at 0.4 ppm.
Because subjects identified as most susceptible to the irritant effects of chlorine were tested (atopic and asthmatic individuals), an intraspecies uncertainty factor (UF) of 1 was applied. The intraspecies UF of 1 is also supported by the fact that bronchoconstriction is induced in pediatric and adult asthmatic subjects at similar levels of challenge (Avital et al. 1991). Therefore, an additional UF to protect pediatric asthmatic subjects is not necessary. Time-scaling was not applied to the AEGL-1 for several reasons. The study conducted by Rotman et al. (1983) actually lasted more than 8 h (two 4-h sessions with a 1-h break between). That reduces the uncertainty usually associated with scaling from shorter to longer time periods. Because effects were not increased following an interrupted 8 h of exposure at 0.5 ppm in the susceptible individual, the 8-h AEGL-1 was also set at 0.5 ppm. The use of the same value across all exposure durations is supported by the fact that the response to the irritant effects of chlorine appears to be concentration-dependent rather than time-dependent. Calculations are presented in Appendix A; results are presented in Table 1–6. Figure 1–1 is a plot of the derived AEGL values and all of the human and animal data on chlorine.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
The studies by Joosting and Verberk (1974), Anglen (1981), Rotman et al. (1983), and D’Alessandro et al. (1996) address sensory irritant effects in humans as well as differences in pulmonary function tests during exposures at 1.0 ppm for up to 8 h and 2.0 ppm and 4.0 ppm for 4 h. As previously noted, Rotman et al. (1983) used an exercising atopic individual, and D’Alessandro et al. (1996) used five subjects with nonspecific airway hyper-reactivity, three of which had clinical histories of asthma. For healthy individuals, the concentrations tested by Rotman et al. (1983) and D’Alessandro et al. (1996) resulted in effects below the definition of the AEGL-2. However, in one atopic subject, the 1-ppm concentration for
TABLE 1–6 AEGL-1 Values for Chlorine (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.5 (1.5) |
0.5 (1.5) |
0.5 (1.5) |
0.5 (1.5) |
0.5 (1.5) |
more than 4 h resulted in shortness of breath and wheezing as well as serious pulmonary function changes (Rotman et al. 1983). In the D’Alessandro et al. (1996) study, there was a significant fall in FEV1 and airway resistance immediately after exposure at 1.0 ppm in both healthy and asthmatic subjects, but the fall among asthmatic subjects was greater. Two of the subjects with airway hyper-reactivity experienced undefined respiratory symptoms following the exposure. Those subjects were not specifically identified as asthmatics. The 2-ppm concentration tested by Anglen (1981) reached nuisance levels by 4 h and was accompanied by transient changes in pulmonary functions. Joosting and Verberk (1974) did not find differences in pulmonary function after exposure at 2 ppm for 2 h; concentrations at 4 ppm for 2 h were irritating, but pulmonary function measurements were not made. Subjective sensory irritation of the throat reached an average level of “nuisance” (distinctly perceptible to offensive) at 4 ppm for 2 h; irritation of the nose and the desire to cough averaged “distinctly perceptible” (range, up to nuisance). No sensory response was reported as unbearable during these exposures. Although airflow resistance, the major pulmonary effect found in the Rotman et al. (1983) study, was not measured by Joosting and Verberk (1974), Anglen (1981) reported that the changes in other pulmonary parameters at 2 ppm for 4 or 8 h were completely reversible by the next day.
6.2. Summary of Animal Data Relevant to AEGL-2
Alarie (1981), the author of the ASTM (1981) RD50 test, considered the RD50 for male Swiss-Webster mice to be intolerable to humans but nonlethal over a period of hours. The RD50 of male Swiss-Webster mice was 9.3 ppm, as reported by Barrow et al. (1977). Lesions were present in the nasal passages, and some changes were present in the lower respiratory tract of rats exposed at 9.1 ppm for 6 h (Jiang et al. 1983). Mice “tolerated” that concentration 6 h/d for 5 d without deaths (Buckley et al. 1984). No gross or microscopic lung changes occurred in rabbits following a 30-min exposure at 50 ppm (Barrow and Smith 1975).
TABLE 1–7 AEGL-2 Values for Chlorine (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.8 (8.1) |
2.8 (8.1) |
2.0 (5.8) |
1.0 (2.9) |
0.71 (2.0) |
6.3. Derivation of AEGL-2
Because human data are available, they should be used to calculate the AEGL-2. In the Rotman et al. (1983) study, the exposure of a susceptible subject to chlorine at 1 ppm for 4 h did not produce respiratory symptoms, but an exposure for more than 4 h resulted in serious asthma-like symptoms and serious pulmonary function changes. The pulmonary function changes were reversible, and the ability to escape was not impaired, but an asthma-like attack that occurred during the longer exposure must be considered a serious health effect. Therefore, the first 4-h exposure, which was without symptoms, can be considered a NOAEL for the symptoms that define AEGL-2. In the D’Alessandro et al. study (1996), two subjects with airway hyper-reactivity and three subjects with asthma also experienced significant changes in several pulmonary function parameters following a 1-h exposure at 1.0 ppm. No respiratory symptoms were experienced during the exposure, although undefined symptoms were experienced later. Because individuals representative of the susceptible population were tested and their reactions met the definition of the AEGL-2, no UF for differences in human sensitivity was applied. Time-scaling was applied to the AEGL-2 values because the 1.0-ppm concentration meets the definition of nuisance irritation, as described by ten Berge and Vis van Heemst (1983), and evacuation or sheltering should take place at the high short-term exposure concentrations that might cause an asthma attack. The 4-h 1-ppm concentration was scaled to the other time periods using the C2×t=k relationship, derived by ten Berge and Vis van Heemst (1983). The 10-min value was set equal to the 30-min value so that the highest exposure of 4.0 ppm in the controlled human studies was not exceeded. Results are presented in Table 1–7 (above), and the calculations are presented in Appendix A.
The 1-y study in which exposure of Rhesus monkeys to a concentration of Cl2 at 2.3 ppm resulted in mild histopathologic changes in the nasal passages and trachea (Klonne et al. 1987) supports the safety of this short-term exposure for humans. Regarding size, anatomy, and tissue distribution, the respiratory tract of the monkey is an appropriate model for humans.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
Although exposure concentrations as high as 30 ppm (actual exposure concentration and duration not known) and 66 ppm for unspecified amounts of time have been reported by Abhyankar et al. (1989) and Shroff et al. (1988), respectively, measurement methods and/or exposure times were not well-documented. No deaths were reported at those exposures. No reliable data on concentrations that caused death in humans were located.
7.2. Summary of Animal Data Relevant to AEGL-3
The most recent available data for relevant exposure times show that 1-h concentrations that resulted in no deaths range from 150 ppm in the mouse (O’Neil 1991) to 322 ppm in the rat (Zwart and Woutersen 1988). On the basis of the rat data, Zwart and Woutersen (1988) calculated an LC01 of 288 ppm. Thirty-minute concentrations that resulted in no deaths were 547 ppm in the rat (Zwart and Woutersen 1988) and 200 ppm in the rabbit (Barrow and Smith 1975). The mouse data of Zwart and Woutersen (1988) were insufficient for calculating an LC01. In addition, lethality measurements in many of the mouse studies were complicated by delayed deaths (Bitron and Aharonson 1978; O’Neil 1991), which were attributed to pneumonia. Mice might not be an appropriate model for extrapolation to humans, as stated by ten Berge et al. (1986) and the NRC (1991) for HCl. The NRC (1991) states that when considering lethal concentrations of respiratory irritants (such as HCl), the mouse “may not be an appropriate model for extrapolation to humans,” because “mice appear to be much more susceptible to the lethal effects of HCl than other rodents or baboons. To some extent, this increased susceptibility may be due to less effective scrubbing of HCl in the upper respiratory tract.”
7.3. Derivation of AEGL-3
Because the experimental data in mice appeared to provide an overly conservative estimate of lethality that was not consistent with the overall preponderance of the data, a value less than the concentration that resulted in no deaths in rats but greater than the value that resulted in no deaths in
mice was chosen as the basis for the AEGL-3 values. The 200-ppm value is below the 1-h highest nonlethal concentrations (213 ppm and 322 ppm) and the LC01 (288 ppm) in two well-conducted studies with rats (MacEwen and Vernot 1972; Zwart and Woutersen 1988) and above the 1-h highest nonlethal concentration in mice, 150 ppm (O’Neil 1991). The 200-ppm concentration is an LC20 for the mouse. A UF of 3 was used to extrapolate from rats to humans (the data show that interspecies differences were within a factor of approximately 2 for lethality). In addition, chlorine is a contact-site, direct-acting toxicant; there is no metabolic or pharmacokinetic component to chlorine-induced effects, and there is likely to be little difference between species in the response of biologic tissues to chlorine exposure. Also, for intraspecies differences, corrosive gases acting at the point of contact would predict low variability in a population; thus a UF of 3 is applied to protect susceptible individuals. The relative sensitivity of asthmatic individuals when considering lethality is unknown, but the data from the Rotman et al. (1983) and D’Alessandro et al. (1996) studies show that doubling a no-effect concentration for irritation and bronchial constriction resulted in potentially serious effects. The data were divided by a combined UF of 10 and were scaled to the 30-min and 4- and 8-h exposure durations using the C2×t=k relationship, which was based on a nuisance level of human irritation (ten Berge and Vis van Heemst 1983). Application of a larger intraspecies UF, such as 10 (for a total of 30), would result in an 8-h AEGL-3 value of 2.3 ppm. It is unlikely that asthmatic subjects would suffer life-threatening symptoms at 2.3 ppm. The values appear in Table 1–8 and the calculations are presented in Appendix A.
It should be noted that the 1 -h AEGL-3 of 20 ppm is below the AEGL-3 derived from the 1-h rat LC01 (the threshold for lethality) of 28.8 ppm (288/10).
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End Points
In summary, the AEGL values for various levels of effects and various exposure periods were derived using the following methods. The AEGL-1 was based on a study with human volunteers, including a susceptible individual, in which a concentration of chlorine at 0.5 ppm for 4 h produced no sensory irritation and resulted in only mild transient effects on pulmonary parameters in the healthy individuals. Pulmonary changes in the susceptible
TABLE 1–8 AEGL-3 Values for Chlorine (ppm [mg/m3])
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
50 (145) |
28 (81) |
20 (58) |
10 (29) |
7.1 (21) |
individual were greater than those in healthy subjects, but did not result in symptoms above the definition of the AEGL-1. Because both genders were tested in one of the studies, a susceptible individual was observed in the other study, and subjects were undergoing light exercise (making them more vulnerable to sensory irritation), no UF to account for differences in human sensitivity was applied. The 0.5-ppm no-effect concentration for susceptible individuals is supported by a 1-h 0.4-ppm no-effect concentration for individuals with airway hyper-reactivity or asthma. The 0.5-ppm exposure was considered a threshold for more severe effects, regardless of exposure duration.
The AEGL-2 values were based on the same studies used to derive the AEGL-1s. In those studies healthy human volunteers experienced transient changes in pulmonary function measurements and a susceptible individual experienced an asthma-like attack (shortness of breath and wheezing) following a more than 4-h exposure to chlorine at 1 ppm. The susceptible individual remained in the exposure chamber for the full 4 h before the symptoms occurred. Because both genders were tested, subjects were undergoing light exercise (making them more vulnerable to sensory irritation), and a susceptible individual was tested, no UF was applied to account for differences in human sensitivity. Similar effects and symptoms in individuals with airway hyper-reactivity or asthma exposed at 1.0 ppm for 1 h supports the application of no intraspecies UF for the 4-h concentration. The 4-h 1-ppm concentration was scaled to the other time periods using the C2 ×t=k relationship.
In the absence of human data, the AEGL-3 values were based on a concentration lower than the highest value that resulted in no deaths for the rat but equal to the LC20 for the mouse. The data resulting from exposure of mice to chlorine were either incomplete or complicated by delayed deaths. The 1-h concentration of 200 ppm was divided by a UF of 3 to extrapolate from animals to humans (interspecies values for the same end point differed by a factor of approximately 2 within each of several studies) and by a UF of 3 to account for differences in human sensitivity (the toxic effect is the result of a chemical reaction with biologic tissue of the respiratory tract, which is unlikely to differ among individuals). The AEGL-3
values for the other exposure times were calculated based on the C2×t= k relationship.
The three AEGL levels for the four exposure times are listed in Table 1–9.
8.2. Extant Standards and Guidelines for Chlorine
Standards and guidance levels for workplace and community exposures are listed in Table 1–10. The 8-h AEGL-1 and the ACGIH TLV-TWA values are the same, and, although not specifically stated, the TLV-TWA is based on the absence of irritation during the 8-h exposure at 0.5 ppm in the Anglen (1981) and Rotman et al. (1983) studies. Those and another, more recent study are also the basis for the AEGL-1 and AEGL-2 values. The AEGL-1 is specifically protective of asthmatic subjects.
The NIOSH IDLH is based on acute inhalation toxicity data in humans, Freitag (1941, as cited in NIOSH 1994a), and several secondary sources, such as ILO (1998). Exposure at 30 ppm is stated to cause intense coughing fits, and exposure at 40–60 ppm for 30–60 min might cause serious damage (ILO 1998). The IDLH is higher than the AEGL-2, but less than the AEGL-3. The AEGL-2 is lower because it is specifically protective of asthmatic patients.
The ERPG-1 is based on an 8-h human exposure at 1 ppm that produced slight transient pulmonary effects in healthy subjects (Anglen 1981; Rotman et al. 1983). The 1-h AEGL-1 is lower than the ERPG-1 because
TABLE 1–9 Summary and Relationship of AEGL Values
|
Classification |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 (Nondisabling) |
0.5 ppm (1.5 mg/m3) |
0.5 ppm (1.5 mg/m3) |
0.5 ppm (1.5 mg/m3) |
0.5 ppm (1.5 mg/m3) |
0.5 ppm (1.5 mg/m3) |
|
AEGL-2 (Disabling) |
2.8 ppm (8.1 mg/m3) |
2.8 ppm (8.1 mg/m3) |
2.0 ppm (5.8 mg/m3) |
1.0 ppm (2.9 mg/m3) |
0.71 ppm (2.0 mg/m3) |
|
AEGL-3 (Lethal) |
50 ppm (145 mg/m3) |
28 ppm (81 mg/m3) |
20 ppm (58 mg/m3) |
10 ppm (29 mg/m3) |
7.1 ppm (21 mg/m3) |
|
Abbreviations: mg/m3, milligrams per cubic meter; ppm, parts per million. |
|||||
TABLE 1–10 Extant Standards and Guidelines for Chlorine (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
AEGL-2 |
2.8 |
2.8 |
2.0 |
1.0 |
0.71 |
|
AEGL-3 |
50 |
28 |
20 |
10 |
7.1 |
|
ERPG-1 (AIHA)a |
|
1 |
|
||
|
ERPG-2 (AIHA) |
3 |
||||
|
ERPG-3 (AIHA) |
20 |
||||
|
EEGL (NRC)b |
|
3 |
|||
|
PEL-Ceiling (OSHA)c |
1 |
||||
|
IDLH (NIOSH)d |
|
10 |
|
||
|
REL-Ceiling (NIOSH)e |
|
0.5 |
|||
|
TLV-TWA (ACGIH)f |
0.5 |
||||
|
TLV-STEL (ACGIH)g |
1.0 |
||||
|
MAK (Germany)h |
0.5 |
||||
|
MAK—Peak Limit (Germany)i |
1.0 |
||||
|
MAC—Ceiling (The Netherlands)j |
1.0 |
||||
|
aERPG (emergency response planning guidelines) of the American Industrial Hygiene Association) (AIHA 2001). ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing symptoms other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for chlorine is based on the transient effects on pulmonary function parameters observed during exposure at 1.0 ppm in the studies of Anglen (1981) and Rotman et al. (1983). The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-2 for chlorine is based on the |
|||||
it is specifically protective of the asthmatic population. The ERPG-2 is based on human data and subchronic and chronic animal data (unreferenced) and is only slightly higher than the 1 -h AEGL-2. The ERPG-3 is based on a preponderance of the animal data (unreferenced) and is the same as the 1-h AEGL-3.
8.3. Data Adequacy and Research Needs
The human database is extensive (Joosting and Verberk 1974; Anglen 1981; Rotman et al. l983; D’Alessandro et al. 1996; Shusterman et al. 1998) and addresses the healthy population as well as asthmatic and atopic individuals. Both genders were tested during exercise in the Anglen (1981) study, and both sensory irritation and pulmonary function parameters were measured. Rotman et al. (1983) measured a range of pulmonary function parameters and included a susceptible individual. D’Alessandro et al. (1996) tested subjects with airway hyper-reactivity or asthma. In addition, in the study by Anglen (1981), exposure concentrations were measured by several different methods, all of which gave similar results. Exposure concentrations were similarly measured in the Rotman et al. (1983) study. Animal studies involved single and multiple species acute tests with multiple dosing regimens and indicated a clear dose-response relationship. Longer-term animal studies that can be used to support the safety of acute exposures were also available. At the higher concentrations, tissue and organ pathology indicated the same toxic mechanism across species.
The mammalian lethality database is extensive and includes four species and exposure times of 5 min to 16 h, as well as chronic studies. However, some of the studies were judged to suffer from methodology and concentration-analysis shortcomings. The mouse appeared to be more sensitive to chlorine than the rat, and in several studies, lethality may have been due to bronchopneumonia, a treatable effect in humans. When developing emergency exposure guidance levels (EEGLs) for hydrogen chloride,
also an irritant chemical, the NRC did not consider the mouse an appropriate model for extrapolations to humans. Therefore, basing the AEGL-3 levels on a concentration higher than the highest nonlethal level in mice but lower than the highest nonlethal concentration in rats is considered appropriate.
9. REFERENCES
Abdel-Rahman, M.S., M.R.Berardi, and R.J.Bull. 1982. Effect of chlorine and monochloramine in drinking water on the developing rat fetus. J. Appl. Toxicol. 2:156–159.
Abhyankar, A., N.Bhambure, N.N.Kamath, S.P.Pajankar, S.T.Nabar, A. Shrenivas, A.C.Shah, and S.N.Deshmukh. 1989. Six month follow-up of fourteen victims with short-term exposure to chlorine gas. J. Soc. Occup. Med. 39:131–132.
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. Documentation of the Threshold Limit Values and Biological Exposure Indices: Chlorine, 6th Ed. Cincinnati, OH: ACGIH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. Threshold Limit Values (TLVs) for Chemical and Physical Agents and Biological Exposure Indices (BEIs). Cincinnati, OH: ACGIH.
AIHA (American Industrial Hygiene Association). 1988. Emergency Response Planning Guidelines, Chlorine. Akron, OH: AIHA.
Alarie, Y. 1980. Toxicological evaluation of airborne chemical irritants and allergens using respiratory reflex reactions. Pp. 207–231 in Proceedings, Symposium on Inhalation Toxicology and Technology. Ann Arbor, MI: Ann Arbor Science.
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health Perspect. 42:9–13.
Alberts, W.M., and G.A.do Pico. 1996. Reactive airways dysfunction syndrome. Chest 109:1618–1626.
Amoore, J.E., and E.Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with Threshold Limit Values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272–290.
Anglen, D.M. 1981. Sensory response of human subjects to chlorine in air. Ph.D. Dissertation, University of Michigan.
Arlong, F., E.Berthet, and J.Viallier. 1940. Action of chronic intoxication by low concentration chlorine fumes on experimental guinea pigs. Presse Med. 48:361.
ASTM (American Society for Testing and Materials). 1991. Standard test method for estimating sensory irritancy of airborne chemicals. In Annual Book of ASTM Standards, Vol. 11.04. Philadelphia, PA: ASTM.
ATSDR (Agency for Toxic Substances and Disease Registry). 1993. Fluorides, Hydrogen Fluoride, and Fluorine (F). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA.
Avital, A., N.Noviski, E.Bar-Yishay, C.Springer, M.Levy, and S.Godfrey. 1991. Nonspecific bronchial reactivity in asthmatic children depends on severity but not on age. Am. Rev. Respir. Dis. 144:36–38.
Back, K.C., A.A.Thomas, and J.D.MacEwen. 1972. Reclassification of Materials Listed as Transportation Health Hazards. Report No. TSA-20–72–3. Aerospace Medical Research Laboratory, Wright-Patterson AFB, Dayton, OH.
Barrow, C.S., and W.H.Steinhagen. 1982. Sensory irritation tolerance development to chlorine in F-344 rats following repeated inhalation. Toxicol. Appl. Pharmacol. 65:383–9.
Barrow, C.S., Y.Alarie, J.C.Warrick, and M.F.Stock. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health 32:68–76.
Barrow, C.S., R.J.Kociba, L.W.Rampy, D.G.Keyes, and R.R.Albee. 1979. An inhalation toxicity study of chlorine in Fischer-344 rats following 30 days of exposure. Toxicol. Appl. Pharmacol. 49:77–88.
Barrow, R.E., and R.G.Smith. 1975. Chlorine induced pulmonary function changes in rabbits. Am. Ind. Hyg. Assoc. J. 36:398–403.
Bell, D.P., and P.C.Elmes. 1965. The effects of chlorine gas on the lungs of rats without spontaneous pulmonary disease. J. Pathol. Bacteriol. 89:307–317.
Bitron, M.D., and E.F.Aharonson. 1978. Delayed mortality of mice following inhalation of acute doses of formaldehyde, sulfur dioxide, chlorine and bromine. Am. Ind. Hyg. Assoc. J. 39:129–138.
Buckley, L.A., X.Z.Jiang, R.A.James, K.T.Morgan, and C.S.Barrow. 1984. Respiratory tract lesions induced by sensory irritants ant the RD50 concentration. Toxicol. Appl. Pharmacol. 74:417–429.
Budavari, S., M.J.O’Neil, A.Smith, P.E.Heckelman, and J.F.Kinneary, eds. 1996. The Merck Index, 12th Ed. Rahway, NJ: Merck & Co., Inc.
C&EN (Chemical & Engineering News). 1994. Chlorine industry running flat out despite persistent health fears. 72:13.
Capodaglio, E., G.Pezzagno, J.C.Bobbio, and F.Cazzoli. 1970. Indagine sulla funzionalita respiratoria di lavoratori addetti a produzioned elettrolitica di chloro e soda [in Italian]. Med. Lav. 60:192–202.
Carlton, B.D., P.Bartlett, A.Basaran, K.Colling, I.Osis, and M.K.Smith. 1986. Reproductive effects of alternative disinfectants. Environ. Health Perspect. 69:237–241.
CEH (Chemical Economics Handbook). 2000. Chlorine/sodium hydroxide [Online]. Available: http://ceh.sric.sri.com/Public/Reports/733.1000/ [October 2001].
Chang, J.C.F., and C.S.Barrow. 1984. Sensory tolerance and cross-tolerance in F-344 rats exposed to chlorine or formaldehyde gas. Toxicol. Appl. Pharmacol. 76:319–327.
Charan, N.B., S.Lakshminarayan, G.C.Myers, and D.D.Smith. 1985. Effects of accidental chlorine inhalation on pulmonary function. West. J. Med. 143:333–336.
CIIT (Chemical Industry Institute of Toxicology). 1993. A Chronic Inhalation Toxicity Study of Chlorine in Female and Male B6C3F1 Mice and Fischer 344 Rats. Research Triangle Park, NC: CIIT.
Cockcroft, D.W., D.N.Killian, J.J.A.Mellon, and F.E.Hargreave. 1977. Bronchial reactivity to inhaled histamine: A method and clinical survey. Clin. Allergy 7:235–243.
D’Alessandro, A., W.Kuschner, H.Wong, H.A.Boushey, and P.D.Blanc. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109:331–337.
Decker, W.J. 1998. Chlorine poisoning at the swimming pool revisited: Anatomy of two minidisasters. Vet. Hum. Toxicol. 30:584–585.
Demnati, R., R.Fraser, G.Plaa, and J.L.Malo. 1995. Histopathological effects of acute exposure to chlorine gas on Sprague-Dawley rat lungs. J. Environ. Pathol. Toxicol. Oncol. 14:15–19.
Drobnic, F., A.Freixa, P.Casan, J.Sanchis, and X.Guardino. 1996. Assessment of chlorine exposure in swimmers during training. Med. Sci. Sports Exerc. 28:271–274.
Druckrey, H. 1968. Chlorinated drinking water toxicity tests involving seven generations of rats. Food Cosmet. Toxicol. 6:147–154.
DuBois, A.B., S.Y.Botelho, and J.H.Comroe. 1971. A new method for measuring airway resistance in man using a body plethysmograph: Values in normal subjects and in patients with respiratory disease. J. Clin. Invest. 35:327–335.
Eaton, D.L., and C.D.Klaassen. 1996. Principles of toxicology. In Casarett and Doul’s Toxicology: The Basic Science of Poisons. New York, NY: McGraw Hill.
Elmes, P.C., and D.P.Bell. 1963. The effects of chlorine gas on the lungs of rats with spontaneous pulmonary disease. J. Pathol. Bacteriol. 86:317–326.
EPA/FEMA/DOT (U.S. Environmental Protection Agency/Federal Emergency Management Association/U.S. Department of Transportation). 1987. Technical Guidance for Hazards Analysis: Emergency Planning for Extremely Hazardous Substances. EPA-OSWER-88–0001. U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8–90/066F. Environmental Criteria and Assessment Office, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1996. Chlorine. Integrated Risk Information System (IRIS) [Online]. Available: http://www.epa.gov/iris/subst/0405.htm [September 2, 1996].
Ferris, B.G., W.A.Burgess, and J.Worchester. 1964. Prevalence of chronic respiratory disease in a pulp mill and a paper mill in the United States. Br. J. Ind. Med. 24:26–37.
Ferris, B.G., S.Puleo, and H.Y.Chen. 1979. Mortality and morbidity in a pulp and a paper mill in the United States: A ten-year follow-up. Br. J. Ind. Med. 36:127–134.
Freitag. 1941. Dangers of chlorine gas [in German]. Z. Gesamte Schiess-Sprengstoffwesen 35:159.
Gagnaire, F., S.Azim, P.Bonnet, G.Hecht, and M.Hery. 1994. Comparison of the sensory irritation response in mice to chlorine and nitrogen trichloride. J. Appl. Toxicol. 14:405–409.
ILO (International Labour Office). 1998. Chlorine and Compounds. In Encyclopaedia of Occupational Health and Safety, 4th Ed., Vol. 1 (A–K). Geneva, Switzerland: ILO.
Jiang, X.Z., L.A.Buckley, and K.T.Morgan. 1983. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol. Appl. Pharmacol. 71:225–236.
Jones, R.N., J.M.Hughes, H.Glindmeyer, and H.Weill. 1986. Lung function after acute chlorine exposure. Am. Rev. Resp. Dis. 134:1190–1195.
Joosting, P., and M.Verberk. 1974. Emergency population exposure: A methodological approach. Recent Adv. Assess. Health Eff. Environ. Pollut. 4:2005–2029.
Kaufman, J., and B.Burkons. 1971. Clinical, roentgenologic, and physiologic effects of acute chlorine exposure. Arch. Environ. Health 23:29–34.
Klonne, D.R., C.E.Ulrich, M.G.Riley, T.E.Hamm Jr., K.T.Morgan, and C.S. Barrow. 1987. One-year inhalation toxicity study of chlorine in rhesus monkeys (Macaca mulatta). Fundam. Appl. Toxicol. 9:557–572.
Kowitz, T.A., R.C.Reba, R.T.Parker, and W.S.Spicer. 1967. Effects of chlorine gas upon respiratory function. Arch. Environ. Health 14:545–558.
Kusch G.D. 1994. Prospective study of the effects of chronic chlorine exposure in manufacturing on respiratory health. In Chlorine Plant Operations Seminar and Workshop Proceedings. Washington, DC: The Chlorine Institute, Inc.
Larsen, G.L. 1992. Asthma in children. N. Engl. J. Med. 326:1540–1545.
Lemiere, C., J.L.Malo, and D.Gautrin. 1996. Nonsensitizing causes of occupational asthma. Obstruct. Lung Dis. 80:749–774.
Lipton, M.A., and G.J.Rotariu. 1941. In Progress report on toxicity of chlorine gas for mice, E.M.K.Gelling and F.C.McLean, eds. Report No. 286. U.S. National Defense Committee, Office of Science Research and Development.
Litchfield, J.T., and F.Wilcoxon. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99.
MacEwen, J.D., and E.H.Vernot. 1972. Toxic Hazards Research Unit Annual Technical Report: 1972. AMRL-TR-72–62. Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, OH; National Technical Information Service, Springfield, VA.
Matheson Gas Co. 1980. Matheson Gas Data Book, 6th Ed. Lyndhurst, NJ: Division Searle Medical Products USA, Inc.
Meier, J.R., R.J.Bull, J.A.Stober, and M.C.Cimino. 1985. Evaluation of chemicals used for drinking water disinfection for production of chromosomal damage and sperm-head abnormalities in mice. Environ. Mut. 7:201–211.
Mickey, G., and H.Holden. 1971. Effects of chlorine on mammalian cells in vitro. EMS Newsletter 4:39–41.
Mrvos, R., B.S.Dean, and E.P.Krenzelok. 1993. Home Exposures to chlorine/chloramine gas: Review of 216 cases. South. Med. J. 86:654–657.
NAC (National Advisory Committee). 1996. Acute Exposure Guideline Levels (AEGLs) for Fluorine. Interim Report.
NIOSH (National Institute for Occupational Safety and Health). 1976. Criteria for a recommended standard: Occupational exposure to chlorine. NIOSH Publication 76–170. U.S. Department of Health and Human Services, Washington, DC.
NIOSH (National Institute for Occupational Safety and Health). 1994a. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). PB94–195047. National Institute for Occupational Safety and Health, Cincinnati, OH; National Technical Information Service, Springfield, VA.
NIOSH (National Institute for Occupational Safety and Health). 1994b. NIOSH Pocket Guide to Chemical Hazards. NIOSH Publication 94–116. U.S. Department of Health and Human Services, U.S. Government Printing Office, Washington, DC.
NRC (National Research Council). 1984. Emergency and Continuous Exposure Limits for Selected Airborne Contaminants, Vol. 2. Washington, DC: National Academy Press.
NRC (National Research Council). 1991. Permissible Exposure Levels and Emergency Exposure Guidance Levels for Selected Airborne Contaminants. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTIS (National Technical Information Service). 1996. Chlorine. Registry of Toxic Effects of Chemical Substances (RTECS) [Online]. Available: Available: http://www.ntis.gov/search/product.asp?ABBR=SUB5363&starDB=GRAHIST [December 1996].
NTP (National Toxicology Program). 1992. Toxicology and Carcinogenesis of Chlorinated Water (CAS Nos. 7782–50–5 and 7681–52–9) and Chloraminated Water (CAS No. 10599–90–3) (Deionized and Charcoal-Filtered) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). NTP TR 392. National Institutes of Health, Bethesda, MD.
O’Neil, C.E. 1991. Immune responsiveness in chlorine exposed rats. PB92–124478. National Institute for Occupational Safety and Health, Cincinnati, OH.
OSHA (Occupational Safety and Health Administration). 1989. 29 CFR Part 1910, Air Contaminants: Final Rule. Fed. Regist. 54(12):2455–2456 (Thursday, January 19, 1989).
Patil, L.R.S., R.G Smith, A.J.Vorwald, and T.F.Mooney. 1970. The health of diaphragm cell workers exposed to chlorine. Am. Ind. Hyg. Assoc. J. 31:678–686.
Perry, W.G., F.A.Smith, and M.B.Kent. 1994. The halogens. Pp. 4482–4505 in Patty’s Industrial Hygiene and Toxicology, Vol. 2, Part F, G.F.Clayton and F.E.Clayton, eds. New York, NY: John Wiley & Sons, Inc.
Prentiss, A.M. 1937. Chemicals in War; a Treatise on Chemical Warfare. New York: McGraw-Hill Book Company.
Rafferty, P. 1980. Voluntary chlorine inhalation: A new form of self-abuse? Br. Med. J. 281:1178–1179.
Rothery, S.P. 1991. Hazards of chlorine to asthmatic patients. Br. J. Gen. Pract. 41:39.
Rotman, H.H., M.J.Fliegelman, T.Moore, R.G.Smith, D.M.Anglen, C.J. Kowalski, and J.G.Weg. 1983. Effects of low concentration of chlorine on pulmonary function in humans. J. Appl. Physiol. 54:1120–1124.
Rupp, H., and D.Henschler. 1967. Effects of low chlorine and bromine concentrations on man. Int. Arch. Gewerbepathol. 23:79–90.
Schlagbauer, M., and D.Henschler. 1967. Toxicity of chlorine and bromine with single and repeated inhalation. Int. Arch. Gewerbepath. Gewerbehyg. 23:91.
Shroff, C.P., M.V.Khade, and M.Srinivasan. 1988. Respiratory cytopathology in chlorine gas toxicity: A study in 28 subjects. Diagn. Cytopathol. 4:28–32.
Shusterman, D.J., M.A.Murphy, and J.R.Balmes. 1998. Subjects with seasonal allergic rhinitis and nonrhinitic subjects react differentially to nasal provocation with chlorine gas. J. Allergy Clin. Immunol. 101:732–740.
Silver, S.D., F.P.McGrath, and R.L.Ferguson. 1942. Chlorine median lethal concentration data for mice. DATR 373. Edgewood Arsenal, MD.
ten Berge, W.F., and M.Vis van Heemst. 1983. Validity and accuracy of a commonly used toxicity-assessment model in risk analysis. IChemE Symposium Series No. 80:I1–I12.
ten Berge, W.F., A.Zwart, and L.M.Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13:301–310.
Underhill, F.P. 1920. The lethal War Gases: Physiology and Experimental Treatment. New Haven, CT: Yale University Press. Pp. 20.
Vernot, E.H., J.D.MacEwen, C.C.Haun, and E.R.Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42:417–423.
Weedon, F.R, A.Hartzell, and C.Setterstrom. 1940. Toxicity of ammonia, chlorine, hydrogen cyanide, hydrogen sulfide and sulfur dioxide gases. V. Animals. Contrib. Boyce Thompson Inst. 11:365–385.
Weill, H., R.George, M.Schwarz, and M.Ziskind. 1969. Late evaluation of pulmonary function after acute exposure to chlorine gas. Amer. Rev. Resp. Dis. 99:374–379.
Withers, R.M.J., and F.P.Lees. 1985a. The assessment of major hazards: The lethal toxicity of chlorine, Part 1: Review of information on toxicity. J. Hazard. Mater. 12:231–282.
Withers, R.M.J., and F.P.Lees. 1985b. The assessment of major hazards: The lethal toxicity of chlorine., Part 2: model of toxicity to man. J. Hazard. Mater. 12:283–302.
Withers, R.M.J., and F.P.Lees. 1987. The assessment of major hazards: The lethal toxicity of chlorine, Part 3: Crosschecks from gas warfare. J. Hazard. Mater. 15:301–342.
Witschi, H.R., and J.A.Last. 1996. Toxic responses of the respiratory system. In Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill.
Wohlslagel, J., L.C.DiPasquale, and E.H.Vernot. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combust. Toxicol. 3:61–69.
Wolf, D.C., K.T.Morgan, E.A.Gross, C.Barrow, O.R.Moss, R.A.James, and J.A. Popp. 1995. Two-year inhalation exposure of female and male B6C3F1 mice and F344 rats to chlorine gas induces lesions confined to the nose. Fundam. Appl. Toxicol. 24:111–131.
Wood, B.R., J.L.Colombo, and B.E.Benson. 1987. Chlorine inhalation toxicity from vapors generated by swimming pool chlorinator tablets. Pediatrics 79:427–430.
Zwart, A., and R.A.Woutersen. 1988. Acute inhalation toxicity of chlorine in rats and mice: Time-concentration-mortality relationships and effects on respiration. J. Hazard. Mater. 19:195–208.
APPENDIX A
Derivation of Chlorine AEGLs
Derivation of AEGL-1
|
Key studies: |
Rotman et al. 1983; D’Alessandro et al. 1996; Shusterman et al. 1998 |
|
Toxicity end point: |
Transient pulmonary function changes in atopic individual exposed at 0.5 ppm for an interrupted 8 h; non-significant changes in pulmonary peak air flow in eight atopic individuals exposed at 0.5 ppm for 15 min; no statistically significant pulmonary parameter changes in asthmatic subjects exposed at 0.4 ppm for 1 h |
|
Time-scaling: |
No time scaling; because there is adaptation to the slight irritation that defines the AEGL-1 end point, the same value (0.5 ppm) was used across all time points |
|
Uncertainty factors: |
1, because susceptible individuals were tested and one of the susceptible individuals was exercising, making him more susceptible to sensory irritation (no-effect level in healthy exercising individuals of both genders) |
|
Calculations: |
Because the 0.5 ppm concentration was indicative of a NOAEL for more serious pulmonary changes, the 0.5 ppm concentration was used for all exposure durations. The susceptible individual underwent an interrupted 8-h exposure at 0.5 ppm without increased symptoms, so that concentration was also used for the 8-h AEGL-1 |
Derivation of AEGL-2
|
Key studies: |
Rotman et al. 1983; D’Alessandro et al. 1996 |
|
Toxicity end point: |
No-effect concentration for serious health effect (asthma-like attack) in a sensitive, exercising individual exposed at 1 ppm for 4 h and in individuals with airway hyper-reactivity (including 3 asthmatic individuals) exposed at 1 ppm for 1 h |
|
Time-scaling: |
C2×t=k (ten Berge and Vis van Heemst 1983) (1 ppm)2×240 min=240 ppm2·min |
|
Uncertainty factors: |
1. The value was based on effects consistent with the AEGL-2 definition in a susceptible, exercising individual and in asthmatics subjects |
|
30-min AEGL-2: |
C2×30 min=240 ppm2·min C=2.8 ppm |
|
1-h AEGL-2: |
C2×60 minutes=240 ppm2·min C=2 ppm |
|
4-h AEGL-2: |
1 ppm for 4 h; basis for derivation of other exposure durations |
|
8-h AEGL-2: |
C2×480 min=240 ppm2·min C=0.71 ppm |
The 10-min AEGL-2 was set equal to the 30-min AEGL-2 so that the highest human test concentration of 4.0 ppm was not exceeded.
Derivation of AEGL-3
|
Key studies: |
Zwart and Woutersen 1988; MacEwen and Vernot 1972 |
|
Toxicity end point: |
1-h lethality value; an end point below the highest concentration resulting in no deaths in the rat and above the highest concentration resulting in no deaths in the mouse was chosen because the mouse was shown to be more sensitive than other mammals to irritant gases, including chlorine, and does not provide an appropriate basis for quantitatively predicting mortality in humans |
|
Time-scaling: |
C2×t=k (ten Berge and Vis van Heemst 1983) (200 ppm/10)2×60 min=24,000 ppm2·min |
|
Uncertainty factors: |
Combined uncertainty factor of 10 |
|
|
3 for interspecies variability (interspecies values for the same end point differed by a factor of approximately 2 in several studies) |
|
|
3 for differences in human sensitivity (the toxic effect is the result of a chemical reaction with biologic tissue of the respiratory tract, which is unlikely to differ among individuals) |
|
10-min AEGL-3: |
C2×10 min=24,000 ppm2·min C=50 ppm |
|
30-min AEGL-3: |
C2×30 min=24,000 ppm2·min C=28.3 ppm |
|
1-h AEGL-3: |
200 ppm/10=20 ppm |
|
4-h AEGL-3: |
C2×240 min=24,000 ppm2·min C=10 ppm |
|
8-h AEGL-3: |
C2×480 min=24,000 ppm2·min C=7.1 ppm |
APPENDIX B ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLORINE (CAS No. 7782–50–5)
DERIVATION SUMMARY
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.5 ppm |
0.5 ppm |
0.5 ppm |
0.50 ppm |
0.50 ppm |
|
Key references: |
(1) Rotman, H.H., M.J.Fliegelman, T.Moore, R.G.Smith, D.M.Anglen, C.J.Kowalski, and J.G.Weg. 1983. Effects of low concentrations of chlorine on pulmonary function in humans. J. Appl. Physiol. 54:1 120–1124. (2) Shusterman, D.J., M.A.Murphy, and J.R.Balmes. 1998. Subjects with seasonal allergic rhinitis and nonrhinitic subjects react differentially to nasal provocation with chlorine gas. J. Allergy Clin. Immunol. 101:732–740. (3) D’Alessandro, A., W.Kuschner, H.Wong, H.A. Boushey, and P.D.Blanc. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109:331–337. |
|||
|
Test species/strain/number: Eight male subjects, one atopic subject (Rotman et al. 1983); eight atopic subjects and eight nonatopic subjects (Shusterman et al. 1998); five asthmatic subjects and five nonasthmatic subjects (D’Alessandro et al. 1996). |
||||
|
Exposure route/concentrations/durations: Inhalation; 0.0, 0.5, 1.0 ppm for 8 h; break at 4 h for an unreported period of time to undergo pulmonary function tests followed by chamber reentry; subjects exercised for 15 min of every hour during exposures; sham exposures were included (Rotman et al. 1983) Inhalation; 0.0 ppm or 0.5 ppm for 15 min (Shusterman et al. 1998) Inhalation; 0.4 ppm or 1.0 ppm for 1 h (D’Alessandro et al. 1996) |
||||
|
Effects: 0.5 ppm for 4 h—no effects in eight of nine subjects; transient changes in pulmonary functions in one of nine subjects. 1.0 ppm for 4 h—some irritation, transient changes in pulmonary functions in nine subjects including an atopic individual; asthma-like episode in one of nine subjects when exposure duration extended to more than 4 h (Rotman et al. 1983). |
||||
|
0.5 ppm for 15 min—nasal congestion; nonsignificant changes in pulmonary peak flow (Shusterman et al. 1998). 0.4 ppm for 1 h—no statistically significant pulmonary function effect in asthmatic individuals (D’Alessandro et al. 1996). |
||||
|
End point/concentration/rationale: 0.5 ppm forr 4 h resulted in no effects in healthy human subjects and transient changes in pulmonary functions for a susceptible individual who had obstructive airways disease prior to the exposure. The 0.5-ppm concentration was chosen as the basis for the AEGL-1 because the next highest concentration produced effects consistent with an AEGL-2 (coughing, wheezing, and a considerable increase in airways resistance) in a susceptible individual. Supported by studies of Shusterman et al. (1998) and D’Alessandro et al. (1996). |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 1 |
||||
|
Interspecies: Not applicable; human subjects tested. |
||||
|
Intraspecies: 1. An atopic individual who had obstructive airways disease prior to the exposure and was considered characteristic of the “susceptible” population was tested. This individual was did not exhibit adverse effects. The choice of an intraspecies uncertainty factor of 1 is supported by another study in which a concentration of 0.4 ppm for 1 h was a no-effect concentration for changes in pulmonary function parameters in individuals with airway hyper-reactivity/asthma and by a study in asthmatic subjects exposed at 0.4 ppm |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable; human data used |
||||
|
Time-scaling: Not applied; because 0.5 ppm appeared to be the threshold for more severe changes in pulmonary parameters in the atopic individual regardless of exposure duration, the 0.5 ppm was used for all AEGL-1 exposure durations. |
||||
|
Data adequacy: The Angelen (1981) study was well conducted and documented and reinforces a study conducted earlier at the same facilities in which 31 male and female subjects were tested for sensory irritation. The Rotman et al. (1983) study went into greater detail than the earlier study, measuring 15 pulmonary function parameters before, during, and after exposures. Subjects were exercising during exposures and this study included a susceptible individual. The choice of intraspecies uncertainty factor was supported by a study of shorter duration with asthmatics. |
||||
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
2.8 ppm |
2.8 ppm |
2.0 ppm |
1.0 ppm |
0.71 ppm |
|
Key references: |
(1) Rotman, H.H., M.J.Fliegelman, T.Moore, R.G. Smith, D.M.Anglen, C.J.Kowalski, and J.G.Weg. 1983. Effects of low concentrations of chlorine on pulmonary function in humans. J. Appl. Physiol. 54:1120–1124. (2) D’Alessandro, A., W.Kuschner, H.Wong, H.A. Boushey, and P.D.Blanc. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109:331–337. |
|||
|
Test species/strain/gender/number: Nine human male subjects, including atopic individual (Rotman et al. 1983); 10 human subjects of which five had airway reactivity/asthma (D’Alessandro et al. 1996) |
||||
|
Exposure route/concentration/duration: Inhalation; 0.0, 0.5, 1.0 ppm for 8 h; break at 4 h for an unreported time period to undergo pulmonary function tests followed by chamber reentry; subjects exercised for 15 min of every hour during exposures; sham exposures were included (Rotman et al. 1983) Inhalation; 0.4 or 1.0 ppm for 1 h (D’Alessandro et al. 1996) |
||||
|
Effects: 0.5 ppm for 4 h—no effects in eight healthy subjects; transient changes in pulmonary functions in one of nine subjects. 1.0 ppm for 4 h—some irritation, transient changes in pulmonary functions nine subjects including an atopic individual; asthma-like episode in one of nine subjects when exposure duration extended beyond 4 h. 1.0 ppm for 1 h—increased airway resistance in asthmatic individuals (D’Alessandro et al. 1996). |
||||
|
End point/concentration/rationale: 1 ppm for 4 h was a no-effect exposure for serious health symptoms in an atopic exercising individual, and 1 ppm for 1 h was a symptomless effect on airway resistance in asthmatic individuals. However, the increase in airways resistance was considered the NOAEL for an AEGL-2 effect. |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 1 |
||||
|
Interspecies: Not applicable; human subjects tested. |
||||
|
Intraspecies: 1. A susceptible exercising individual who had obstructive airways disease prior to the exposure and was considered characteristic of the “susceptible” population was tested. The application of an intraspecies uncertainty factor of 1 is supported by another study in which individuals with airway hyperreactivity/asthma |
||||
|
showed similar pulmonary function changes and some clinical symptoms but no asthma-like attack following exposure at 1.0 ppm for 1 h. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applicable; human data used |
||||
|
Time-scaling: Cn×t=k where n=2. This value describes the concentration-exposure duration relationship for the end point of nuisance irritation (ten Berge and Vis van Heemst 1983, IChemE Symposium Series No. 80:17–21). |
||||
|
Data adequacy: The Angelen (1981) study was well conducted and documented and reinforces a study conducted earlier at the same facilities in which 31 male and female subjects were tested for sensory irritation. The Rotman et al. (1983) study went into greater detail than the earlier study, measuring 15 pulmonary function parameters before, during, and after exposures. Subjects were exercising during exposures, and a susceptible individual was included. The choice of intraspecies uncertainty factor was supported by a study of shorter duration with asthmatic subjects (D’Alessandro et al. 1996). |
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
50 ppm |
28 ppm |
20 ppm |
10 ppm |
7.1 ppm |
|
Key references: |
(1) MacEwen, J.D. and E.H.Vernot. 1972, Toxic Hazards Research Unit Annual Technical Report. 1972. Wright-Patterson Air Force Base, Dayton, OH; (2) Zwart, A. and Woutersen. 1988. Acute inhalation toxicity of chlorine in rats and mice: time-concentration mortality relationships and effects on respiration. J. Hazard. Mater. 19:195–208; (3) O’Neil, C.E. 1991. Immune responsiveness in chlorine exposed mice. PB92-124478, Prepared for NIOSH, Cincinnati, OH. |
|||
|
Test species/strain/gender/number: (1) Sprague-Dawley rats, 10/exposure group; (2) Wistar-derived rats, 10/exposure group; (3) BALB/c mice, 10/exposure group |
||||
|
Exposure route/concentrations/durations: Inhalation; (1) 213–427 ppm for 1 h, (2) 322–595 ppm for 1 h, (3) 50–250 ppm for 1 h Effects: (1) no deaths at 213 ppm for 1 h (Sprague-Dawley rat); (2) no deaths at 322 ppm for 1 h (Wistar-derived rat); (3) no deaths at 150 ppm for 1 h (BALB/c mouse) |
||||
|
End point/concentration/rationale: 200 ppm for 1 h (the estimated mean of highest experimental nonlethal values for the rat and mouse) was chosen as the basis for the 1-h AEGL-3. Mice appeared to be unusually sensitive to chlorine, and in some studies, delayed deaths were attributed to bronchopneumonia rather than direct effects of chlorine. |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 10 |
||||
|
Interspecies: 3. The mouse and rat LC50 values did not differ by more than a factor of 2 to 3, and the mouse was consistently more sensitive. In some mouse studies delayed deaths were attributed to bronchopneumonia rather than direct effects of chlorine exposure. |
||||
|
Intraspecies: 3. Chlorine is a highly reactive, irritating, and corrosive gas whose effect on respiratory tissues is not expected to differ greatly among individuals. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Not applied |
||||
|
Time-scaling: Cn×t=k where n=2. This value describes the concentration-exposure duration relationship for the end point of nuisance irritation (ten Berge and Vis van Heemst 1983, IChemE Symposium Series |
||||
|
No. 80:17–21). The irritation mechanism of action leads to pulmonary edema and potential lethality. An n of 2 is also relevant to animal lethality studies. |
||||
|
Data adequacy: The database for chlorine is extensive with multiple studies of lethality conducted at several exposure durations and involving several species. Studies with multiple dosing regimens showed a clear dose-response relationship. Longer-term studies that support the safety of the values were also available. Tissue and organ pathology indicated that the toxic mechanism was the same across species. |