1
Introduction and Background
Infant formulas are liquids or reconstituted powders fed to infants and young children to serve as substitutes for human milk. Infant formulas have a special role in the diet because they are the only source of nutrients for some infants. In the United States and other industrialized countries, the vast majority of infants receive infant formula at some time during their first year of life (Hediger et al., 2000; Ryan et al., 2002) as the number of infants breastfed after birth rapidly decreases (Figure 1-1). Many infants receive formula in combination with breastfeeding. During these mixed feeding routines there are potential interactions between the components of human milk and those contained in formulas.
Over recent decades ingredients have been added to infant formulas not only to better simulate the composition of human milk, but also to impart health benefits. Examples include fortifying formulas with iron, adding nucleotides, and changing the composition of fat blends. Recently infant formulas containing added sources of arachidonic acid and docosahexenoic acid have been made available in the United States, Europe, and elsewhere. In the United States, new ingredients, such as probiotics and compounds produced by genetic engineering, are currently being considered for addition to formulas.
Infancy is a uniquely vulnerable period of rapid growth and development and as such, feeding changes have the potential to impart benefit or harm in the short term, into early childhood, and even later into adulthood. Thus measurements of safety parameters during infancy need to be equally or even more stringent than at other periods during the life cycle. The introduction of new ingredients to formulas must pose no or minimal risk to infants. In the United States and worldwide there is a paucity of guidelines or recommendations from national and international organizations regarding approaches to assess the safety of ingredients added to infant formulas.
The Committee on the Evaluation of the Addition of Ingredients New to Infant Formula was convened by the Institute of Medicine at the request of the Food and Drug Administration (FDA) and Health Canada to review methods currently used to assess new ingredients to be added to infant formulas, including preclinical and clinical studies and in-market monitoring, and to identify gaps in current safety regulations and guidelines. The committee
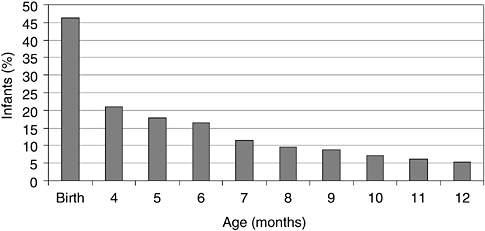
FIGURE 1-1 Percent of infants who were exclusively breastfed from birth to 12 months of age in the United States.
SOURCE: Adapted from Hediger et al. (2000) and Ryan et al. (2002).
met six times over 18 months to fulfill its charge. This report is intended to provide recommendations for regulatory bodies, industry, and basic and clinical investigators involved in determining the safety of new ingredients added to infant formulas.
INFANT FORMULA REGULATIONS AND GUIDELINES
United States
Food products that are designed and marketed for infants are regulated under the Federal Food, Drug and Cosmetic (FD&C) Act of 1938 (21 U.S.C. §301) (Vanderveen, 1991). FDA, an agency in the U.S. Department of Health and Human Services (HHS), regulates infant formulas and evaluates the safety of food and color additives.
Two sections of the FD&C Act, 409 and 412, are the primary laws that relate to infant formulas. Section 409 gives authority to HHS to ensure the safety of new food ingredients (e.g., food additives and Generally Recognized as Safe [GRAS] substances) “under the conditions of its intended use” (e.g., in infant formulas). Manufacturers may propose the addition of new ingredients to infant formulas in the United States by either filing a Food Additive Petition with FDA to request a formal premarket review, or making a GRAS determination. It is important to point out that it is the use of the substance, rather than the substance itself, that is eligible for the GRAS determination (see Chapter 4).
Under Section 412 of the FD&C Act, regulations have been promulgated and ultimately implemented that are intended to ensure proper formulation for infants to thrive. They include:
-
Infant Formula Quality Control Procedures (21 C.F.R. §106),
-
Records and Reports Regulations (21 C.F.R. §106.100),
-
Infant Formula Labeling Requirements (21 C.F.R. §107.10–107.30),
-
Exempt Infant Formulas (21 C.F.R. §107.50),
-
Nutrient Requirements for Infant Formulas (21 C.F.R. §107.100), and
-
Infant Formula Recall Requirements (21 C.F.R. §107.200–107.280).
FDA (1996) has proposed to revise these regulations to establish quality factors, current good manufacturing practices, and revised quality control procedures. Table 1-1 lists some of the U.S., Canadian, and European Union laws and regulations related to adding new ingredients to infant formulas.
Other Countries
Canada
The Canadian Food and Drug Regulations (Canada, 2001) include specific requirements for infant formulas, novel foods,1 and other ingredients. Division 25 of the Regulations provides for the addition to infant formulas of nutritive substances, in addition to specified vitamins and mineral nutrients, found in human milk, provided the nutritive substance is added to the formulas to the level found in human milk (section B.25.056). The Regulations include an inclusive list of those food additives that may be added to infant formulas (section B.25.062). Similar to the process in the United States, a new food additive must undergo premarket approval. The manufacturer must submit a request to the Minister of Health that includes all the details laid out in Division 16 of the Regulations. The request must include “detailed reports of tests made to establish the safety of the food additive under the conditions of use recommended” and “data establishing that the food additive will have the intended physical or other technical effect” (section B.16.002). Health Canada reviews the request and, if accepted, the Minister of Health recommends to the Governor-in-Council that the ingredient be added to the list of food additives in the Food and Drug Regulations. The Food and Drug Regulations also require a premarket notification for novel foods. The manufacturer of a novel food must submit a premarket notification for the food as required under Division 28 of the Regulations. Health Canada issues a written notice to the manufacturer if it is satisfied that information submitted establishes that the novel food is safe for consumption.
European Union
The European Union also has regulations in place for food additives, novel foods, and genetically modified organisms. However the European regulations are not specific for adding new ingredients to infant formulas.
TABLE 1-1 Selected Laws and Regulations Related to New Ingredients Added to Infant Formulas
|
Country or Organization |
Regulations (Reference) |
Identifying Terms |
|
United States |
Federal Food, Drug, and Cosmetic Act (21 U.S.C. §301) |
Food additive, Substances Generally Recognized as Safe (GRAS) |
|
Food Additive (21 C.F.R. §170, 171, and 172) |
Food Additive |
|
|
GRAS (21 C.F.R. §182, 184, and 186) |
GRAS |
|
|
Canada |
Food and Drugs Regulations (Canada, 2001) |
Food additive, novel food |
|
European Union |
European Communities (Infant Formulae and Follow-on Formulae) Regulations, 1998 (FSAI, 1999) |
Established by generally accepted scientific data |
|
Council Directive 89/107/EEC (EEC, 1989) |
Food additive |
|
|
European Parliament and Council Directive 94/34/EC (EEC, 1994) |
Food additive |
|
|
Regulation EC No. 258/97 (EC, 1997) |
Novel foods, novel food ingredients |
|
|
Council Directive 90/220/EEC (EEC, 1990) |
Genetically modified organisms |
In June 2002 representatives of academia, the infant food industry, the European Commission, food regulatory bodies of some European Union member states, and consumer organizations met to discuss opportunities to evaluate the safety of ingredients new to infant formulas (Koletzko et al., 2002). Topics included consumer expectations, preclinical and clinical evaluations, investigations of infant growth and nutrient bioavailability, and in-market surveillance. The participants also discussed a position paper written by members of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition’s Committee on Nutrition (Aggett et al., 2001). The paper provided 13 points for consideration for the evaluation of infant formulas; these points are presented in Box 1-1.
CHARGE TO THE COMMITTEE
FDA and Health Canada asked the committee to review methods currently used to assess new ingredients to be added to infant formulas, including preclinical and clinical studies and in-market monitoring, and to identify gaps in current safety regulations and guidelines. As part of its task, the committee was requested to provide recommendations to strengthen the scientific approaches used in assessing the safety of ingredients added to infant formulas. The committee was asked to focus on:
-
ingredients new to infant formulas that are regulated under the food provisions of the law, not as drugs or therapeutic agents;
-
the health and well-being of healthy term infants (delivered between gestational age of 37 to 42 weeks with birth weights of 2.5 kg or more) from birth to 12 months of age; and
-
the effects that ingredients new to infant formulas could have on metabolism, physiology, neurological function, and normal growth and development, as well as on specific systems, including the immunological, renal, hepatic, hematological, and gastrointestinal systems.
The committee was asked to address the composition of human milk and to evaluate how the presence or absence of a substance in human milk may be factored into the safety
|
BOX 1-1 Key Points of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition’s Committee on Nutrition Commentary on the Nutritional and Safety Assessment of Breast Milk Substitutes and Other Dairy Products for Infants
|
SOURCE: Reprinted, with permission, from Aggett et al. (2001). |
assessment of that substance for use in infant formulas. The committee was also asked to consider how the process of estimating intakes and safety of substances intended for infant formulas has evolved over time and to discuss whether and how this process is changing in light of the current state of clinical science to safeguard the health and well-being of infants enrolled in clinical studies. In addition, the committee was requested to apply its recommended approaches to the specific situation of adding long-chain polyunsaturated fatty acids (LC-PUFAs) to infant formulas for use in term infants. The approaches were to also be applied to other ingredients, if appropriate.
The committee’s primary charge was to provide guidelines for assessing the safety of new ingredients added to infant formulas under U.S. regulations, with possible international applications. Under U.S. regulations, the process that evaluates the safety of ingredients is stated in the FD&C Act, but it does not address issues of efficacy (i.e., health benefits). Although the committee recognizes that efficacy should be considered when assessing new ingredients to be added to infant formulas, efficacy is not a consideration under current or proposed regulations for infant formulas or infant formula ingredients. The committee therefore focused its attention on matters related to safety as delineated in its charge. Similarly, although the committee recognizes that cost can be a factor for expert panels making decisions about the appropriate studies to be used to assess safety, cost issues were not included in the charge and, consequently, were not part of its deliberations.
The committee recognizes that some of its recommendations could not be implemented under the current U.S. laws and may require statutory changes. Even with this limitation, the committee encourages dialogue among members of government agencies, the public, industry, and academia to act on the recommendations set forth by this report in the best interest of our most vulnerable members of society—our infants.
THE COMMITTEE’S APPROACH
Types of Ingredients
The committee focused on new potential ingredients and existing ingredient ratios that could be added to infant formulas to:
-
impart potential health benefits within the first year of life, later in childhood, and perhaps even during adult life; and
-
change color, extend shelf life, or modify marketing or manufacturing processes because they could potentially be associated with harmful short- or long-term effects.
Several compounds have been seriously considered for addition to formulas, but no final decision has been made about their addition for a variety of reasons. For example, cholesterol is present in human milk at levels higher than is present in cow’s milk. A recent article reported that breastfed infants had lower total cholesterol and low-density lipoprotein cholesterol in adulthood and suggested that infant formulas should have added cholesterol to more closely match that of human milk (Owen et al., 2002). On the other hand, animal studies of formulas with added cholesterol found no evidence of any beneficial short- or long-term effects (LSRO, 1998). In addition, lysozyme and lactoferrin are also present in human milk at levels higher than in cow’s milk. These factors may be important in growth or host defense and have been considered for addition to formulas (Lo and Kleinman, 1996). Many substances found in human milk have not been added to infant formulas, perhaps due to the lack of information on their function in human milk or their effect on the infant.
The committee proposes a functional classification of potential target areas of ingredients new to infant formulas, as summarized in Table 1-2, according to potential targeted endpoints. The committee also considered the types of molecules or ingredients that could be considered as new additives, including:
-
new sources of existing ingredients;
-
prebiotics and probiotics;
-
lipids;
-
nitrogen-containing ingredients (e.g., recombinant proteins; single amino acids, such as glutamine, and immunoglobulins; and bioactive peptides/polyamines/proteins, such as lactoferrin, enzymes, and hormones);
-
oligosaccharides;
-
vitamins, minerals, and flavonoids;
-
flavoring and coloring agents; and
-
nonprotein recombinant-derived products.
However the committee did not simply consider molecules or new ingredients as isolated substances, it also considered three important characteristics: (1) the compound itself
TABLE 1-2 Functional Classification of Potential Target Areas of New Ingredients
|
Class |
Examples |
Examples of Possible Adverse Consequences |
|
Anti-allergic |
Prebiotics, probiotics, cytokines |
Gastrointestinal side effects |
|
Anti-infective |
Antiviral/bacterial agents, probiotics, prebiotics, lactoferrin |
Adverse immunological changes, allergic effects |
|
Flavor/color/texture/ stabilizing agents |
Organoleptics, flavors, aeromatics |
Toxicity, carcinogenicity |
|
Immunologic |
Long-chain polyunsaturated fatty acids, enzymes, cytokines, nucleotides |
Immunological, inflammatory effects |
|
Metabolic |
Enzymes, hormones |
Allergic immunological changes, allergic effects |
|
Neurodevelopmental and behavioral |
Long-chain polyunsaturated fatty acids, growth factors, choline, oligosaccharides, precursors of neurotransmitters, neurotransmitters, amino acids |
Decreased growth, decreased cognitive ability |
|
Trophic |
Growth factors, hormones |
Abnormal increase or decrease in growth, endocrine effects |
(the molecule), (2) the matrix in which it is delivered, and (3) its amount and ratio relative to other constituents in the infant formula. The committee also realized that certain potential new additions to infant formulas might be in the form of complex ingredients or biologicals (e.g., probiotics). Some of the recommended chemical, physical, and in vitro characterization steps might not apply to complex ingredients (see Chapter 5).
The committee also reviewed whether ingredients derived from genetically engineered techniques should have the same safety recommendations for use in infant formulas as compared with other classes of new ingredients. Even though compounds derived from genetic engineering techniques would have properties similar to those of natural compounds, they would still have to be tested. Similar to other ingredients, the short- and long-term unintended compositional changes that result directly or indirectly as a result of genetic engineering would need to be considered. In addition, the unintended functional consequences of a successful, specific intentional alteration would need to be considered and monitored over long time periods. Thus the committee recommends that standards for safety should be the same for ingredients derived from genetically engineered techniques as they are for other classes of ingredients. Guidelines should maintain emphasis on a “reasonable certainty of no harm” for all ingredients.
Safety Definitions
The committee reviewed other existing reports and guidelines, including those available from FDA and the Life Sciences Research office (LSRO), and it considered current recommendations relating to safety. While the committee recognized that its recommendations had to be targeted with safety as the ultimate objective, additions of ingredients new to infant formulas need to consider potential efficacy (i.e., health benefits) considerations.
The concept of “safety” refers to a reasonable certainty of no harm per the FD&C Act; to safe and adequate levels of nutrients, including essentiality, stability, history of use, and toxicity per LSRO; or, in some circumstances, a reasonable balance between costs (e.g., risks, harm) and benefits. Safety, therefore, is not an inherent biological property, but rather a point on a continuum that is influenced by intellectual concepts and judgment. Generally, a “hazard” refers to a substance or combination of substances that produce undesired outcomes; in the case of infant formulas, the undesired outcome is health related. “Risk” implies that an adverse event will be expressed under specified conditions, and “harm” refers to the nature of an undesired outcome associated with a hazard. Details about the concept of safety and surrounding issues are provided in Chapter 2.
Use of a Hierarchical Approach to Safety Assessment
The committee recognized that its charge was to provide comprehensive guidelines for evaluating the safety of the addition of ingredients new to infant formulas—not to produce a “how to” document. With this in mind, the committee discussed an initial outline and agreed that for each area it would review the existing systems, identify gaps or limitations of the systems, and make recommendations for revised guidelines. The committee also agreed on the concept of a hierarchical approach to determine levels of safety assessment needed for each ingredient.
To evaluate the safety of the addition of new ingredients to infant formulas, the committee proposes the use of a hierarchical approach, which involves matching the level of safety assessment with the degree of concern about the potential harm. Such an approach needs to
be considered in terms of a new ingredient’s potential: (1) harm (e.g., toxicity), and (2) adverse effects. This hierarchical approach will guide the levels of assessment applied depending on the nature and purpose of the new ingredient.
Considerations about the degree of concern and, therefore, about the level of safety assessment required would include: whether potential harmful effects of a new ingredient would be reversible or irreversible, the severity of the effect, and the consequences. For example, levels of safety assessment might not be as intense for an ingredient with a proven lack of adverse effects (e.g., an ingredient added for formula stability) as compared with a new ingredient added to induce a positive biological effect (e.g., better visual acuity). In its hierarchical approach, the committee includes assessments that consider whether an adverse effect manifests immediately (while the infant is ingesting the formula) or later (even into adulthood and future generations). The committee also recommends assessing the likelihood that a new ingredient could adversely affect a specific system and whether the effect would be common or rare.
To help facilitate the hierarchical approach, the committee recommends the use of algorithms. An algorithm diagrams the process into a step-by-step decision tree. The steps in the algorithm include:
-
recommended observations and assessments,
-
decisions to be considered, and
-
actions to be taken.
Standardized symbols are used in a flowchart to display each step in the algorithm (SMDMC, 1992) (Figure 1-2). Arrows connect the numbered boxes, indicating the order in which the steps should be followed. A letter within a box of an algorithm refers the reader to corresponding text (or sidebar). The sidebar elaborates on the recommendations and statements that are found within each box of the algorithm. Readers should keep in mind that the algorithm depicts the logic of the process, but does not denote a chronology (e.g., a manufacturer may initiate several different studies and procedures at the onset of the process, the results of which are assessed at different steps of the algorithm).
The algorithms presented in Chapters 4 through 7 are designed to encompass a broad spectrum of components for evaluating the safety of new components added to infant formulas. The use of algorithms in applying the hierarchical approach provides several advantages. First, the utilization of algorithms should simplify the process of planning the type and depth of safety assessments for each new ingredient. Second, evidence suggests that an algorithm approach improves data collection, problem solving, and decision making. Third, multiple levels of information are incorporated into a single, unified document. Finally, the algorithmic format allows the regulatory agency and the manufacturer to follow a linear approach to critical information needed at the major decision points.
The algorithms in this report are provided as generic guides and as tools for stepwise approaches to be used in assessing the safety of ingredients new to infant formulas. The committee realizes that it cannot provide specific recommendations for each compound and that some variation in these approaches may be needed for specific ingredients. Thus the committee recommends that the manufacturer and an expert review panel establish the relative importance of potential adverse effects for each specific new ingredient and determine the depth of preclinical and clinical studies and in-market surveillance needed to assess safety.
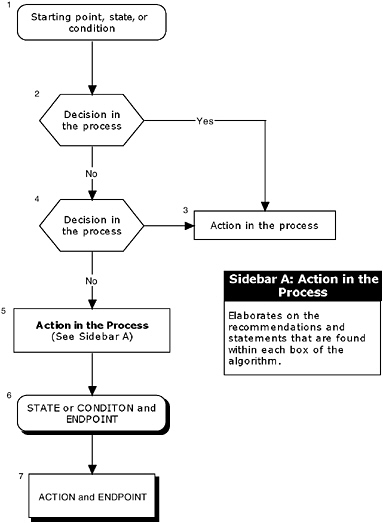
FIGURE 1-2 An example of an algorithm.
Using this flexible, stepwise approach, each potential new ingredient is considered using evidence-based approaches and high-quality scientific data to assess potential adverse effects on:
-
growth and development (including temperament),
-
the immune system (including allergic and infectious risks),
-
metabolism (e.g., acid-base balance, effects related to lipid and carbohydrate processing), and
-
other organ systems (e.g., hematological, gastrointestinal).
For each distinct area of safety assessment (i.e., preclinical, clinical, and in-market monitoring) the committee designed algorithms or stepwise decision trees to be applied to new ingredients for infant formulas. The committee’s approach was based on the uniqueness of the infant population. Therefore each step in the process requires empirical evidence from many disciplines and the application of the highest standards, whether using methods of bioassay, nutritional analysis, or basic chemistry. This approach could be applied to new ingredients to be added to infant formula regardless of the regulatory process used.
ORGANIZATION OF THE REPORT
The committee structured this report so that the first three chapters provide the background information and rationale for the report. Chapter 2 reviews the parameters considered by the committee when defining “safety” and how to approach it from a practical, theoretical, and statistical point of view. Chapter 3 compares the biological and behavioral advantages of human milk with infant formulas and reviews how infant formulas were developed to meet the biological advantages of human milk.
The remainder of the report reviews the current regulatory processes involved in evaluating the safety of ingredients new to infant formulas and provides recommendations for the overall process (Chapter 4), preclinical studies (Chapter 5), clinical studies (Chapter 6), and finally, in-market surveillance (Chapter 7). In Appendix D the committee provides two case studies that have recently utilized the current regulatory process by submitting a GRAS Notification. The addition of LC-PUFAs and probiotics to infant formulas are used as examples of how the recommendations of the committee could be utilized for new ingredients in infant formula. LC-PUFAs may affect diverse functions within the brain and therefore demonstration of safety is critical; probiotics is presented because it is a complex ingredient that could potentially impact diverse organ systems and could potentially raise allergenicity concerns. The purpose of this exercise was not to make conclusions about the completeness of the information or the safety of the new ingredients, but to point out important assessments or steps that could be considered. In doing so, the committee emphasizes the importance of including some steps, such as follow-up surveillance and level 2 assessments, in future assessments of the safety of the addition of new ingredients to infant formulas.
SUMMARY
The Committee on the Evaluation of the Addition of Ingredients New to Infant Formula provides FDA, Health Canada, and other regulatory bodies worldwide with a thorough discussion of the challenging issues surrounding the determination of the safety of new ingredients to be added to infant formulas. The committee encourages dialogue among members of government agencies, the public, industry, and academia to act on the recommendations set forth by this report in the best interest of our most vulnerable members of society—our infants.
REFERENCES
Aggett PJ, Agostini C, Goulet O, Hernell O, Koletzko B, Lafeber HL, Michaelsen KF, Rigo J, Weaver LT. 2001. The nutritional and safety assessment of breast milk substitutes and other dietary products for infants: A commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 32:256–258.
Canada. 2001. Departmental Consolidation of the Food and Drugs Act and the Food and Drug Regulations. Ottawa: Minister of Public Works and Government Services Canada.
EC (European Communities). 1997. Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. Off J Eur Communities L043:1–7.
EEC (European Economic Communities). 1989. Council Directive of 21 December 1988 on the approximation of the laws of the member States concerning food additives authorized for use in food stuffs intended for human consumption (89j/107/EEC). Off J Eur Communities L40:27.
EEC. 1990. Council Directive of 23 April 1990 on the deliberate release into the environment of genetically modified organisms (90/220/EEC). Off J Eur Communities L117:15.
EEC. 1994. European Parliament and Council Directive 94/34/EC of 30 June 1994 amending Directive 89/107/ EEC on the approximation of the laws of the Member States concerning food additives authorized for use in foodstuffs intended for human consumption. Off J Eur Communities L237:1.
FDA (Food and Drug Administration). 1996. Current good manufacturing practice, quality control procedures, quality factors, notification requirements, and records and reports, for the production of infant formula: Proposed rule. Fed Regist 61:36153–36219.
FSAI (Food Safety Authority of Ireland). 1999. Recommendations for a National Infant Feeding Policy. Dublin: FSAI. Pp. 94–121.
Hediger ML, Overpeck MD, Ruan WJ, Troendle JF. 2000. Early infant feeding and growth status of US-born infants and children aged 4–71 mo: Analyses from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 72:159–167.
Koletzko B, Ashwell M, Beck B, Bronner A, Mathioudakis B. 2002. Characterisation of infant food modifications in the European Union. Ann Nutr Metab 46:231–242.
Lo CW, Kleinman RE. 1996. Infant formula, past and future: Opportunities for improvement. Am J Clin Nutr 63:646S–650S.
LSRO (Life Sciences Research Office). 1998. Assessment of Nutrient Requirements for Infant Formulas. Bethesda, MD: LSRO.
Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. 2002. Infant feeding and blood cholesterol: A study in adolescents and a systematic review. Pediatrics 110:597–608.
Ryan AS, Wenjun Z, Acosta A. 2002. Breastfeeding continues to increase into the new millennium. Pediatrics 110:1103–1109.
SMDMC (Society for Medical Decision Making Committee). 1992. Proposal for clinical algorithm standards. Society for Medical Decision Making Committee on Standardization of Clinical Algorithms. Med Decis Making 12:149–154.
Vanderveen JE. 1991. The role of the Food and Drug Administration in regulating food products for children. Ann N Y Acad Sci 623:400–405.












