5
Testing Ingredients with Preclinical Studies
ABSTRACT
Preclinical studies are a vital first step to assess the safety and quality of ingredients new to infant formulas. They must be performed before an ingredient can be considered for clinical studies in humans in order to determine the potential toxicity of the ingredient, its metabolites, and its matrix. Guidelines for these studies to assess the safety of infant formulas must be based on considerations of the diversity of potential new ingredients and the ingredients’ source and matrix. In the United States, the Food and Drug Administration’s (FDA) Redbook provides comprehensive guidelines for conducting preclinical studies to test the safety of food and color additives, but it often does not take the many special needs and vulnerabilities of infants into consideration. In Canada, there are no comprehensive guidelines for conducting preclinical studies, and decisions are made on a case-by-case basis using internationally accepted guidelines.
The committee recommends implementing a flexible, two-level assessment approach to help guide the expert panel’s decisions on the appropriate preclinical studies (including preanimal tests; absorption, distribution, metabolism, and excretion studies; toxicity studies; and neurological studies) to assess the safety of ingredients new to infant formulas. Level 1 assessments include standard measures for each organ system required for all new ingredients (e.g., commonly used screening tests of cell and organ composition and function). Level 2 assessments include in-depth measures of organ systems that would be used to explicate equivocal level 1 findings or specific theoretical concerns not typically addressed by level 1 tests.
In addition to following established guidelines, a distinct set of procedures using appropriate cellular and animal models at relevant developmental stages should be included in studies to assess safety. The most commonly used animal models for general toxicological studies are the rat and mouse, but they are of limited use for developmental studies involving ingredients new to infant formulas because of the
difficulty of feeding formulas to a preweanling rodent. The nonhuman primate and the piglet are more amenable for these types of studies.
INTRODUCTION
This chapter describes the importance and the unique aspects of conducting preclinical studies to assess the safety of infant formulas. Current regulatory guidelines for preclinical studies are described, and a two-level assessment process is proposed. The committee’s recommended two-level process is a flexible approach that can accommodate a variety of potential ingredients. For example, safety assessment might not be as intense for an ingredient that is not absorbed systemically. The manufacturer, in consultation with an expert panel, determines the types of tests conducted. The extent of this investigation will be determined on the basis of previous experience with the ingredient in other populations and on theoretical concerns based on the putative biological effects of the ingredient, its metabolite, and its matrix. Finally, the committee recommends the following preclinical tests described in detail below:
-
preanimal tests (including structure, stability, and solubility tests of the ingredients; genetic tests; and cellular studies)
-
absorption, distribution, metabolism, and excretion studies
-
toxicity studies (including acute, subchronic, chronic, developmental, and organ studies)
-
neurological studies
Neurological studies are described in a section separate from other toxicity studies because the scope of work defined for this project placed special emphasis on the potential effect of ingredients on the rapidly developing infant brain.
THE IMPORTANCE OF APPROPRIATE PRECLINICAL STUDIES
Preclinical studies must be performed before an ingredient can be considered for clinical studies in humans in order to determine the potential toxicity of the ingredient and its metabolites and their effects in the matrix. Preclinical studies allow researchers to expose cell cultures and experimental animals to doses of ingredients not normally encountered in human consumption. Results of such assessments are used to determine the threshold of toxicity for the given ingredient (i.e., the margin of safety).
Guidelines for preclinical studies to assess the safety of infant formulas must be based on considerations of:
-
The diversity of the potential new ingredients. The new ingredients will possess different chemical characteristics, nutritional contributions, pharmacological activities, and physiological activities. The ingredients may be a conventional synthetic or an extracted single component, a plant extract, or a complex mixture. The ingredients may also be derived from novel sources or processes (e.g., products of fermentation or biotechnology). Such diversity requires preclinical guidelines that are clear but not overly prescriptive because of the disparity in the issues that each class of ingredient may represent. Nevertheless, given the vulnerability of the population to receive the ingredient (infants), it is incumbent upon the manufacturer, in consultation with the expert panel, to be overly conscientious in considering potential safety issues.
-
The ingredient’s source and matrix. The approach to evaluate ingredients new to infant formulas should be driven by the class of the functional substances and by the full characterization of the ingredient in the matrix of the infant formulas. In other words, the general approach is to deal with the targeted ingredient, as well as the nontargeted compounds, such as metabolites of the targeted ingredient, the vehicle required for delivery of the targeted ingredients, and impurities introduced in the manufacturing process.
CURRENT REGULATORY GUIDELINES FOR PRECLINICAL STUDIES
In considering how to construct guidelines for preclinical studies of ingredients new to infant formulas, the committee drew on existing guidelines for the testing of food ingredients and infant formulas. In the United States these are included in Sections 409 and 412 of the Federal Food Drug and Cosmetic (FD&C) Act (21 U.S.C. §301) and in the FDA Redbook (OFAS, 2001, 2003). The committee suggests that the risks of adding ingredients to sole nutrient sources, such as infant formulas, are not adequately addressed; however some aspects of the more stringent guidelines applied to new ingredients as articulated in the Redbook are appropriate for some, if not all, ingredients new to infant formulas.
Sections 409 and 412 of the Federal Food, Drug and Cosmetic Act
There are no explicit requirements for preclinical testing of infant formulas specified under Section 409 of the FD&C Act. The section stipulates that a petition to establish safety of a food additive shall contain “all relevant data bearing on the physical or other technical effect such additive is intended to produce…” (21 U.S.C. §301), but it does not dictate a specific type of preclinical study.
Under Section 412, which applies to infant formulas, a formula shall be deemed to be adulterated if it does not meet the quality factor requirements prescribed by the Secretary of Health under Subsection (b)(1). Subsection (b)(1) then states, “The secretary shall by regulation establish requirements for quality factors for infant formulas to the extent possible consistent with current scientific knowledge, including quality factor requirements for the nutrients required by subsection (i).” Currently, only protein quality is named as a quality factor in FDA regulations but there are no specific requirements to be met regarding quality factors. Subsection (i) specifies levels of certain nutrients (e.g., protein, fat, and specific vitamins) that are required to be met. There is no other requirement for any specific preclinical studies.
FDA Redbook
The FDA Redbook II and Redbook 2000 (OFAS, 2001, 2003) provide comprehensive guidelines for conducting preclinical studies to test the safety of food and color additives. Chapters IV and V in the Redbook II and 2000 describe:
-
general guidelines for designing, conducting, and reporting results of toxicity studies,
-
special considerations in toxicity studies (e.g., pathology, statistics), and
-
guidelines for specific toxicity studies (e.g., genetic, acute, subchronic, chronic, immunotoxicity, and neurotoxicity).
These guidelines, however, do not consider the unique characteristics of having infants as consumers.
Canada’s Food and Drug Regulations
There are no explicit requirements for preclinical testing of infant formulas specified under Canada’s Food and Drug Regulations in Division 16 (Food Additives), Division 25 (Infant Formulas), or Division 28 (Novel Foods) (Canada, 2001). Divisions 16 and 28 require that a notification be submitted to Health Canada that includes information used to establish the safety of a food additive or a novel food. Health Canada refers manufacturers to internationally accepted guidelines for preclinical testing or asks to be consulted because decisions are made on a case-by-case basis.
OVERVIEW OF RECOMMENDED LEVELS OF ASSESSMENT
RECOMMENDATION: A hierarchy of two levels of preclinical assessment, using techniques from cellular-molecular studies through whole-animal studies, should be implemented to assess the safety of ingredients new to infant formulas on developing organ systems:
-
Level 1 assessments. These are suggested standard measures for each organ system (e.g., gastrointestinal, blood, kidney, immune, endocrine, and brain) and are required for any new ingredient.
-
Level 2 assessments. In-depth measures of organ systems or functions that would be performed to explain abnormalities found in level 1 assessments and specific theoretical concerns not typically addressed by level 1 tests. These are suggested measures to assess any new ingredient that primarily interacts with an organ system, has a metabolite that interacts with an organ system, or stimulates or changes the synthesis of factors (e.g., hormones, cytokines, immunoglobulins, endotoxin) that interact with an organ system.
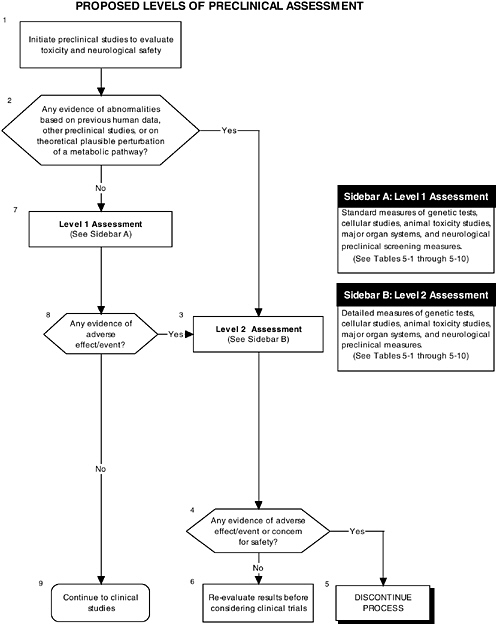
Figure 5-1 describes the overall flow of proposed preclinical studies, from preanimal tests (Boxes 8 and 4) to toxicity tests (Box 4) to neurological tests (Box 7). Figure 5-2 describes the two assessment levels that should be considered when conducting preclinical studies, and it refers to tables in the text for specific measurements that could be conducted.
CONDUCTING PREANIMAL TESTS
Structure, Stability, and Solubility
Chemical and physical characterization is warranted if the targeted and nontargeted ingredients to be added to infant formulas are not well known and if there is no adequate literature about them to determine their safety. Thus the complete chemical structure and functional groups and the purity and stability of the intended and nontargeted ingredients present in the matrix must be determined using well-established physical methods (see Figure 5-1, Box 8). These methods include high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and thin layer chromatography (TLC). LC-MS is a particularly versatile technique. It can be used to assess the structure, molecular mass, and purity of most classes of compounds because derivatization is not necessary. The stability to temperature and ultraviolet light and the solubility properties of the ingredients should be established by standard methodology. The percentage of the unidentifiable materials should be stated in the notification or petition to the regulatory agency. The kind of
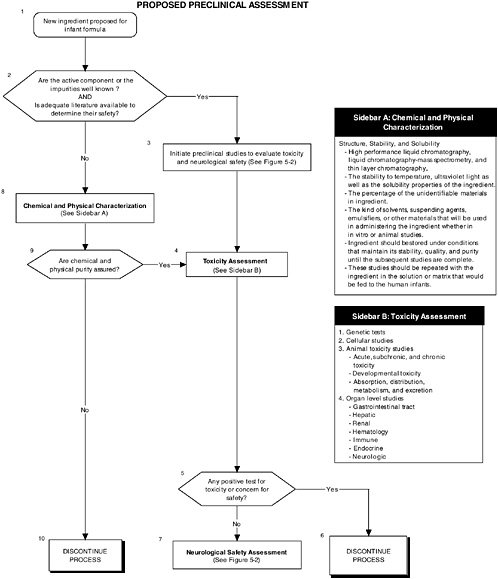
FIGURE 5-1 Proposed preclinical assessment algorithm. ![]() = a state or condition,
= a state or condition, ![]() = a decision point,
= a decision point, ![]() = an action, sidebar = an elaboration of recommendation or statement.
= an action, sidebar = an elaboration of recommendation or statement.
solvents, suspending agents, emulsifiers, or other material that will be used in administering the new ingredients during testing, whether using in vitro or animal studies, should be disclosed. The targeted ingredient should then be stored under conditions that maintain its stability, quality, and purity. The stability and solubility studies should be performed with the ingredient both in the solution and in the matrix that would be fed to human infants.
Genetic Tests
Numerous genetic studies, such as those provided in the Redbook, are available, and the committee recommends evaluating all ingredients, their metabolites, or their secondary effectors for their ability, if any, to cause molecular changes in the deoxyribonucleic acid (DNA) or to cause structural changes in the chromosomes of cells (see Figure 5-1, Box 4). These changes may include forward and reverse mutations, point mutations, deletion mutations, chromosomal aberrations, micronuclei deletions, polymorphisms, DNA strand breaks, or unscheduled DNA synthesis. In vitro assessments use microorganisms and cells from multicellular animals; examples are listed in Table 5-1.
Cellular Studies
It is often most efficient to perform in vitro studies of metabolism before whole-animal (oral dosing) studies to provide information about future in vivo studies and estimate dosages to be used in preclinical animal studies. In vitro work and pharmacokinetic modeling can be used to predict the potential toxicity and in vivo kinetics of the ingredients and the matrix. The in vitro studies can also provide initial evidence of the level of clinical safety or risk.
Because in vitro studies are generally less complex than whole-animal studies, elucidation of an ingredient’s metabolic pathway and toxicity characteristics may be facilitated. Different doses of the solubilized ingredient can be incubated with either confluent or preconfluent cells cultured in 10-cm dishes or 96-wells plates to establish uptake and toxicity levels of both the ingredient and its metabolic byproducts. Time-course and dose-response experiments should be conducted to check the growth characteristics of the cells and the toxicity of the ingredient. The production of metabolites can also be followed upon adding
TABLE 5-1 Examples of Potential in vitro Tests to Assess Genetic Toxicity
|
Test Name |
Function of Test |
Reference |
|
Ames test |
Microsome reverse mutation |
Aeschbacher et al., 1983 |
|
Mouse lymphoma thymidine kinase gene mutation assay |
Genetic forward mutations |
McGregor et al., 1987, 1988a, 1988b; Myhr and Caspary, 1988; Myhr et al., 1990 |
|
Mammalian erythrocyte micronucleus test |
Micronuclei deletions, chromosomal aberrations |
Schlegel and MacGregor, 1982; Schlegel et al., 1986 |
|
Polymerase chain reaction |
Changes in gene expression and deoxyribonucleic (DNA) sequence, polymorphisms, point mutations |
Innis, 1990 |
|
DNA microarraya |
Identifies genes that are up or down regulated |
Cohen et al., 2002; Daniel, 2002; DeRisi and Iyer, 1999; Guengerich, 2001; Lee et al., 2000; Moreno-Aliaga et al., 2001; Perou et al., 1999; Wang, 2000; Williams, 1999 |
|
Proteonomicsa |
Identifies proteins that are altered after exposure to the ingredient |
Anderson and Anderson, 1998; Bogyo and Hurley, 2003; Govorun and Archakov, 2002; Hochstrasser, 1998; Jungblut et al., 1999; MacBeath, 2002 |
|
Bioinformaticsa |
? |
Vihinen, 2001 |
|
aThese techniques are relatively new and are not standardized for clinical use. |
||
the ingredient directly to the cellular extracts. In this case, different concentrations of the solubilized material are incubated with the cellular extract, and the production of metabolites as a function time is determined by analytical methods (e.g., HPLC, LC-MS, TLC). Radioactive and stable isotope studies are useful in tracing nutrient uptake by cells and intracellular metabolic pathways.
The in vitro systems can also be used to measure binding adduct and conjugate formation, transport across membranes, enzyme activity and concentration, enzyme substrate specificity, and other specific objectives. In addition to providing information on the effects of the ingredient and its metabolites on target receptors, cell-culture studies can also indicate subsequent downstream effects. In the future, DNA microarray technologies may be complemented by the capabilities of proteomics and metabolomics to assess the expression of expected and unexpected proteins and metabolites after ingredient exposure to the cells. However, at this point, these techniques are relatively new and are not standardized for clinical use. The principal problems that these advances confront lie in assessing the functional significance of the results of the analyses. Numerous algorithms in dealing with such analyses are available (Lander, 1999; Lee et al., 2000; Soukas et al., 2000) and should be consulted.
CONDUCTING ANIMAL TOXICITY STUDIES
Several toxicity studies must be performed in animals to ensure the safety of ingredients new to infant formulas (see Figure 5-1, Box 4). This section reviews the following issues:
-
considerations when choosing animal models,
-
general considerations when conducting animal studies, and
-
considerations when conducting the following specific animal toxicity studies:
-
-
acute, subchronic, and chronic toxicity studies,
-
developmental toxicity studies,
-
absorption, distribution, metabolism, and excretion studies, and
-
organ-level studies.
-
RECOMMENDATION: In addition to established guidelines and regulations (e.g., the Redbook and regulations under Sections 409 and 412 of the FD&C Act), a distinct set of procedures using appropriate animal models at relevant developmental stages should be included in studies to assess safety.
Choosing Animal Models
Animal models are used as a tool in initial toxicology studies before human clinical trials are conducted. The end points of animal studies include:
-
repeat dose toxicity (target organ evaluation),
-
carcinogenicity (mutagenicity screen),
-
developmental toxicity,
-
reproductive toxicity, and
-
neurotoxicity.
Animal models should be chosen appropriately, and it is especially important to look at the comparative development between the animal model and humans and to choose the
model that can be developmentally extrapolated to humans. For example, the animal model chosen must have digestive and metabolic similarities to the human infant and must be at the same point in development as the human infant who consumes formulas. Investigators should also ensure that the animal model and the reason for the studies have been justified such that there is an understanding of what can and cannot be extrapolated and how the preclinical data generated will be used to interpret the safety profile. All of the following questions must be answered before selecting animal models and moving ahead with a submission to the regulatory agency:
-
What are the appropriate species?
-
What are their ages?
-
Should juvenile animals be assessed, or is age irrelevant for the animal models being considered?
-
What safety factors are needed with these models, and what are going to be the relevant scientific disciplines?
At least two animal models should be selected, keeping in mind that the more animal models used, the better (OFAS, 2001, 2003), because converging lines of biological proof of cause and effect can be established. There are several additional factors to consider when conducting animal studies:
-
The intended ingredient should be added to the animal’s diet during the time in development that corresponds to when the average human infant will consume it.
-
The bioavailability of the ingredient in the human infant must be known.
-
The study must be designed to prevent differences in pharmacokinetics, handling of the ingredient, and dietary imbalance from competing ingredients.
The most commonly used animal models for general toxicological studies are the rat and mouse. However the rat and the mouse are of limited use for developmental studies involving ingredients new to infant formulas because of the difficulty of feeding formulas to a preweanling rodent. The nonhuman primate and the piglet are more amenable for these types of studies because they readily accept infant formulas as a nutrient source. The advantages and disadvantages of using each of these animal models is discussed below and summarized in Table 5-12.
Nonhuman Primate
Nonhuman primates are the closest analog to humans. Their diet is similar to humans and they can be fed infant formulas and followed developmentally. Humans and nonhuman primates are also comparable at a neural systems level. The animals display a wide array of sophisticated cognitive and motor behaviors (for review, see Bachevalier, 2001; Golub and Gershwin, 1984; Overman and Bachevalier, 2001). The nonhuman primate’s neurodevelopmental trajectory is well described, with about a 4:1 ratio in relative time of development (e.g., 4 months of human brain development is equal to 1 month of development in the nonhuman primate).
Nonhuman primates can be more thoroughly assessed than humans through catheters in the blood stream, direct cerebrospinal fluid sampling, deep implanted electrodes, and repeated neuroimaging. These approaches allow a greater correlation of form and function changes.
Nonhuman primates have made substantial contributions to human nutrition studies, including assessment of the neurobiological effects of long-chain polyunsaturated fatty-acid (LC-PUFA) deficiency (Neuringer et al., 1986; Reisbick et al., 1994), iron deficiency (Kriete et al., 1995), and zinc deficiency (Golub et al., 1985). This model could be used to study changes in general behavior and speed of neural processing in response to the addition of ingredients with neurological effects, such as LC-PUFAs. Furthermore, neuroimaging with magnetic resonance imaging (MRI) may reveal differences in myelination or regional brain volumes.
The main disadvantages of using nonhuman primates are that they are very expensive to maintain, and most researchers are reluctant to sacrifice the animals to obtain brain tissue for a single analysis.
Piglet
Piglets are comparable in size to neonatal humans and metabolize fatty acids like humans do. Piglets can be raised on a relatively high-fat infant formulas and are an excellent choice for studies of hypoxia, endocrinology, and lipid metabolism. Piglets can be sacrificed and, thus, tissue can be obtained for analysis.
Pig models have been used extensively to study the role of added LC-PUFAs on the developing brain (Arbuckle et al., 1994; Craig-Schmidt et al., 1996; Hrboticky et al., 1990). These studies generally show that infant formulas enriched with LC-PUFAs and fed to neonatal pigs result in increased incorporation of these fatty acids into neuronal membranes and myelin. However piglets are difficult to work with in behavioral/learning paradigms, and neurodevelopmental form-function relationships are difficult to assess. Thus the inability to test these animals functionally to determine if the fatty acid incorporation results in beneficial or toxic effects is a significant drawback.
Rat
The rat is the most versatile model for toxicological testing for the following reasons:
-
There is a wealth of information available on the rat model, including the nutrient effects on biochemistry, cell biology, neurophysiology, anatomy, and behavior.
-
The developmental trajectory of the rat brain has been extensively compared with the human brain. For example, a postnatal day-7 rat has the structural maturity of a 34-week premature human, a postnatal day-12 animal is similar to a term human (Rice and Barone, 2000), and a postnatal day-28 rat approximates a human toddler.
-
The rat’s short reproductive cycle (21 days gestation) allows for rapid cycle studies and assessment of generational effects.
-
Rats are excellent learners on basic cognitive (medial temporal lobe) tasks. Learning paradigms in rats have been correlated with human behavioral tests, allowing extrapolation of behavioral data between species (for review, see Overman and Bachevalier, 2001).
However the rat also has several limitations. Rats normally consume low-fat diets (approximately 5 percent fat), while human infants consume 45 to 55 percent of their calories as fat. Therefore the rat is a poor model for assessing the effects of altering or supplementing fats (including LC-PUFAs). Studies of LC-PUFA supplementation in the developing rat pup involved supplementing the mother and thus enriching the composition of rat milk (Haubner et
al., 2002; Pasquier et al., 2001). The matrix and source of these LC-PUFAs delivered to the rat pup were therefore different than the matrix and source of LC-PUFAs added to formulas and fed directly to the pup. Although a feeding system for artificial rat-milk substitute (formula) has been devised, it is invasive (i.e., it involves gastrostomy placement), it is difficult to maintain long term, and it is likely nonphysiological in nature.
Rats have been used extensively in brain efficacy and toxicity studies involving LC-PUFAs (Delion et al., 1994; Vazquez et al., 1994; Wainwright et al., 1997). For example, the GRAS Notification by Martek for the addition of LC-PUFAs to infant formulas lists multiple toxicity protocols involving rats (Hahn, 2000). Since it is difficult to feed infant formulas to a preweanling rat, the developmental tenets of timing, dose, and duration cannot be addressed. The literature cited in Martek’s notification provides no mention of neurotoxicological effects in either the developing or the mature rat.
An appropriate approach to study the impact of LC-PUFAs on development would be to assess the animals for evidence of adequate synaptogenesis, myelin biochemistry, and myelin quantity. More specific probes for the effects of these ingredients on regulation of myelination (e.g., microtubule associated protein-2 or synaptophysin messenger ribonucleic acid) would represent targeted approaches to potential physiological processes likely to be affected by LC-PUFAs.
In some instances, such as when rats may possess different tissue metabolism and cortical function compared with humans, the rat model may be inappropriately extrapolated to humans. For example, the newborn human has relatively more cortical activity than the rat, so nutrient effects on cortical structures may be underestimated in the rat model. In contrast, the rat has a large and metabolically active hippocampus at birth relative to the human. Thus nutrient perturbations, such as iron deficiency, protein-energy malnutrition, or hypoglycemia, which profoundly affect the rat’s hippocampus, may overestimate the effect in humans.
Mouse
The mouse is an excellent model because of the ability to manipulate its genetics and to link alterations in genotype to metabolic phenotypes. The mouse genome sequence is almost complete, so it provides a major resource to link gene expression to disease. For example, the ability to manipulate the genome to alter the uptake and processing of nutrients provides valuable information on mechanistic insight. A particularly powerful emerging technology is the conditional knock-out or knock-in model where nutrient dependent genes can be altered in specific regions of the brain at specific times of development (Lee et al., 1999).
Mice have been widely used in LC-PUFA research with respect to regulation of genes of lipid metabolism. In these studies it has been demonstrated that LC-PUFAs are potent inhibitors of lipogenic gene expression (Clarke, 2001; Ntambi, 1999; Shimomura et al., 1998).
The main disadvantages of the mouse model are:
-
The mouse, like the rat, consumes a low-fat diet and cannot be fed infant formulas, making developmental effects difficult to assess.
-
The mouse has different tissue metabolism compared with humans, particularly with respect to adipose tissue and liver lipid metabolism.
-
The mouse has a limited neurobehavioral repertoire compared with the rat, making it more difficult to detect subtle neurocognitive effects after nutrient manipulations.
General Considerations When Conducting Animal Studies
Animal studies should include consideration of bioavailability, nutritional requirements and limitations, metabolic parameters, and developmental stage. They must be conducted in accordance with recommendations for the care and use of laboratory animals. The source and strain of the animals should be justified and stated in the submission. Investigators should choose:
-
healthy animals,
-
animals that have never been exposed to any experimental procedure,
-
male and female animals, and
-
animals that are capable of completing the entire study.
Testing should be performed on suckling animals and should continue after weaning and acclimation. Depending on the test and objective, investigators should choose several animals per sex for each experimental and control group. The animals should be assigned to the experimental and control groups in a randomized manner to minimize bias.
The diets to be used in the study should also be justified and stated in the submission. If the ingredient is to be fed, it must meet the nutritional requirements of the species. The diet fed to the control groups should be equivalent in nutritional value to the diets of the dosed groups. Because the amount of food consumed by each animal in the study cannot be determined when more than one animal is housed in each cage, it is recommended that the test animals be single-caged. Cross-fostering should be considered if a significant effect on feeding or nursing behavior is a potential effect of the added ingredient. For example, all studies of ingredients with potential neurological effects (e.g., LC-PUFAs, choline, oligosaccharides) should be evaluated in this manner. The bedding materials in the cages, ambient temperature, humidity, and lighting conditions also should be stated.
Acute, Subchronic, and Chronic Toxicity Studies
The FDA Redbook (OFAS, 2001, 2003) provides criteria for toxicity assessment of direct food additives and color additives used in food. These criteria are very stringent and it is debatable whether this degree of stringency is necessary for each ingredient new to infant formulas. Nevertheless the Redbook provides a scientifically conservative approach for the riskiest of new ingredients.
For all levels of toxicity studies, the route of administration of the supplement should approximate that of normal human exposure as closely as possible (e.g., through the diet in the case of infant formulas). The diets to be used in toxicological studies have to be properly selected. Natural-ingredient diets, as opposed to purified diets, are preferred because they do not usually affect nutrient balances. However each diet may have advantages and disadvantages that need to be considered.
If there is no information that can be used to determine the appropriate dose levels for short-term or subchronic toxicity levels, toxicity studies should begin with tests of acute toxicity, followed by subchronic, and finally chronic assessments. Table 5-2 provides guidelines for conducting these tests. The committee proposes the addition of developmental toxicity studies to the assessment of ingredients new to infant formulas in addition to the recommended guidance in the Redbook.
TABLE 5-2 Examples of Acute, Subchronic, Chronic, and Developmental Toxicity Studies
|
Study |
Example |
|
|
Acute toxicity |
Single dose known to be toxic to the species, followed by observation of the animals for at least 2 weeks and establishment of the lethal dose for 50% of the animals (LD50) for the ingredient, known bioactive metabolites, and biomass (source) |
|
|
Subchronic toxicity |
Generally conducted for 90 days in rats using doses established with the acute toxicity studies |
|
|
Chronic toxicity |
Can follow the subchronic and are usually carried out beyond the 90-day period and perhaps to adulthood |
|
|
Developmental toxicity |
Multigenerational study endpoints: generation toxicity F0 (parental generation) and F1 (second generation) Reproductive toxicity study endpoints: fertility, live born, weaning, viability indices, and male reproductive indices (e.g., testicular spermatid numbers) |
|
|
SOURCE: OFAS (2001, 2003). |
||
Developmental Toxicity Studies
The purpose of developmental toxicity studies is to evaluate the effects of the ingredient on developing fetuses that result from exposure of either parent prior to conception or to mothers during gestation. In the evaluation of ingredients new to infant formulas, the latter consideration is less relevant since pregnant mothers do not consume these ingredients. The main manifestations of an effect on the developing organism are death, structural abnormality, altered or retarded growth, and functional deficiency. Table 5-2 provides preclinical endpoints for developmental toxicity studies.
Absorption, Distribution, Metabolism, and Excretion Studies
The purpose of conducting absorption, distribution, metabolism, and excretion studies is to address the biological activity or availability of the ingredient when given to the infant. The toxicokinetics of the ingredient should be studied via the following:
-
Absorption studies to assess the possible points of entry into the body (e.g., gastrointestinal tract, nose, mouth, and lung). Many, but not all (e.g., probiotics), new ingredients will be absorbed from the intestinal tract and have the potential to have important positive or negative biological effects. (Even probiotics can stimulate the synthesis of compounds that have important positive or negative biological effects.) It is incumbent on the manufacturer to assay for these effects, identify them, and assess their potential risk to the developing animal and human.
-
Distribution studies to assess the subsequent transport and deposition throughout the body. Some tissues, such as bone, adipose, brain, kidney, liver, and hemotopeotic, may act as reservoirs for the new ingredient.
-
Metabolism studies to assess the organ and cellular response to the presence of the ingredient. A relatively inert ingredient can be metabolized to a biologically potent compound that has extreme toxicity.
-
Excretion studies to assess the removal of the ingredient from both systemic and nonsystemic stores. Organs of excretion include the liver, kidneys, gastrointestinal tract, lungs, and skin.
TABLE 5-3 Examples of Tests for Absorption, Distribution, Metabolism, and Excretion Assessment
|
Function |
Assessmenta |
|
Absorption |
Everted gut sacs and isolated intestinal loops, fecal material analysis, analysis of material in large intestine, radiolabel |
|
Distribution |
Whole body and organ autoradiography |
|
Metabolism |
Radioactive and stable isotopes, LC-MS, HPLC, TLC, DNA microarray,b metabolomics,b proteomics,b bioinformaticsb |
|
Excretion |
Urine and other body fluid chemical analysis, LC-MS, HPLC, scintillation counting if ingredient is radioactive |
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, determines which tests are required based on a thorough analysis of the potential effects of the new ingredient. aLC-MS = liquid chromatography-mass spectrometry, HPLC = high performance liquid chromatography, TLC = thin layer chromatography, DNA = deoxyribonucleic acid. bThese techniques are relatively new and are not standardized for clinical use. |
|
Unlike the organ system analyses described below, absorption, distribution, metabolism, and excretion studies are relatively uniform for any new ingredient and should follow the basic guidelines of the Redbook (OFAS, 2001, 2003). Table 5-3 provides examples of tests that could be conducted.
Organ-Level Studies
Examination of organs from the selected animal models can reveal important information concerning the effects of ingredients at the organ level. Different compounds will have different effects on the different organs and, as discussed earlier in this chapter, the general approach to evaluate the effect of new compounds added to infant formulas, as well as other material, should therefore be driven by the class of the functional substance and by the full characterization of the ingredient. The toxicity studies could be organ driven. All organs should be screened with their appropriate level 1 assessments, while more specific concerns with respect to a particular ingredient can be investigated through level 2 assessments (see Figure 5-2, Box 3).
All test animals should be subjected to complete gross necropsy, including examination of external surfaces, orifices, cranial cavity, carcass, and all organs, in the presence of a qualified pathologist. The ratio of organ weight to body weight should be documented. For a complete list of organs that should be examined and weighed, the reader should consult the Redbook (Chapter IV.B.1.) (OFAS, 2001). This list is extensive, but it is important because long-term adverse effects of the ingredient could later be found to be associated with organs that were not conspicuous or were ignored, and therefore unchecked at the time of the evaluation.
All organs should undergo a general screen or assessment, and then specific screens or assessments should be conducted based on expected biological effects of the new ingredient to be added to infant formulas. The evaluations of some of these key organs systems are detailed in the subsequent sections. Certain organs (e.g., liver, kidney, immune system, bone marrow, and brain) probably deserve greater scrutiny than others because of their functional or metabolic significance. For example, certain histological abnormalities, such as scattered focal mononuclear cell infiltrates in nonlymphoid tissues (e.g., liver and kidney), may indicate autoimmune disease. Also, if the ingredient is shown to either stimulate cell prolifera-
tion or to cause atrophy and cell depletion in any lymphoid organ, the effect is likely to be viewed as potentially immunotoxic and requires more definitive testing.
Gastrointestinal Tract Function
As Chapter 6 describes in detail, the development of the infant’s gastrointestinal tract is essentially complete at birth and, therefore, assessments of its proper development will involve ensuring that its functions (e.g., digestion, absorption, secretion) have not been impaired by the addition of an ingredient new to infant formulas. Using a two-level approach for assessment, level 1 tests will include weighing the organ, performing histology examinations of the organ tissues, and other screening tests. Level 2 tests should be used to explicate equivocal level 1 findings or specific theoretical concerns not typically addressed by level 1 tests. Table 5-4 provides several examples of the types of tests that could be used in level 1 and level 2 assessments of the gastrointestinal tract.
Hepatic Function
The liver is involved in synthesis, metabolism, and excretion. Therefore, along with the above mentioned histology evaluation, tests that account for each of these functions must be performed as part of the level 1 assessment of liver health. Level 2 tests should be used to explicate equivocal level 1 findings or specific theoretical concerns not typically addressed by level 1 tests. Table 5-5 provides several examples of the types of tests that could be used in level 1 and level 2 assessments of liver health.
Renal Function
The kidney is the major excretory organ and also serves a role in blood pressure homeostasis. Ingredients new to infant formulas or their metabolites may be excreted by the kidney and may potentially damage the glomerular or reno-vascular function of the organ. Glomerular health is assessed by serum and urine chemical profiles in addition to the above mentioned histology. Abnormalities in level 1 assessments would lead to level 2 assessments, which should be tailored to the issues raised in level 1. Table 5-6 provides several examples of the types of tests that could be used in level 1 and level 2 assessments of kidney health.
TABLE 5-4 Gastrointestinal Assessment: Examples of Tests in Level 1 and Level 2
TABLE 5-5 Hepatic Assessment: Examples of Tests in Level 1 and Level 2
|
Level |
Assessmenta |
|
Level 1 |
Liver weight/histology, cell culture/mutagenicity Assessment of synthetic function: serum ALAT, ASAT, ornithine carbamyl transferase, albumin:globulin, coagulation profile, prothrombin time, partial thromboplastin time, radioactive amino acids, electrophoresis techniques for serum proteins Assessment of excretion function: gamma-glutyml transferase, LDH, bilirubin, alkaline phosphatase Assessment of metabolic function: total protein, albumin, fasting glucose, urea nitrogen, triglycerides (LDL, VLDL), cholesterol (HDL and LDL) |
|
Level 2 |
Metabolism assessments Microarray/proteonomics Protein electrophoresis Special clotting factor levels Special imaging studies Special stains on histology |
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, determines which tests are required based on a thorough analysis of the potential effects of the new ingredient. aALAT = alanine amino transferase, ASAT = aspartate amino transferase, LDH = lactate dehydrogenase, LDL = low-density lipoprotein, VLDL = very low-density lipoprotein, HDL = high-density lipoprotein. |
|
Hematological Function
Ingredients new to infant formulas or their metabolites may have profound effects on the bone marrow. Numerous tests are available for level 1 assessments in addition to bone marrow histology. Abnormalities in level 1 assessments should lead to level 2 assessments of the relevant system that was perturbed by the addition of the new ingredient. Table 5-7 provides several examples of the types of tests that could be used in level 1 and level 2 assessments of hematological function.
Immunological Function
The immunological system is highly complex and has been shown to be sensitive to nutritional manipulation (Miles and Calder, 1998). The various effects of nutrients in the
TABLE 5-6 Renal Assessment: Examples of Tests in Level 1 and Level 2
TABLE 5-7 Hematology Assessment: Examples of Tests in Level 1 and Level 2
immunological system can be divided into those mediated by antigen-specific immunoglobulin (Ig) E (allergic reactions), other antibodies, T-cells, cytokines, and chemokines, and those mediated by nonimmunological mechanisms. The effects of nutrients, such as LC-PUFAs, on immune response have been extensively examined in laboratory animals and humans (for review, see Miles and Calder, 1998). One could argue that the value of immune system assessments for such nonproteinaceous food ingredients may be questionable since they are unlikely to affect the system or to provoke an allergic reaction. However any residue present in the matrix of an ingredient may be of protein nature and may cause an allergic reaction. Therefore even when assessing nonproteinaceous material, an assessment for allergenicity may be needed depending on the presence and source of impurities.
In addition to histopathological evaluation of the lymphoid tissues, level 1 assessments should include in vivo and in vitro tests with animal models that examine unusual incidences of maternal infections in order to evaluate potential developmental indicators of immunological toxicity (see Table 5-8).
As part of the assessment of the effect of a new ingredient on the immune system, the possibility of a new ingredient being an allergen (i.e., able to provoke an allergic reaction, mostly through IgE mediation) for an infant should also be evaluated. In the case of proteinaceous material with unknown allergenic properties, level 1 and level 2 assessments should be performed to assess their potential for allergenicity (see Table 5-8). A decision tree developed by Metcalfe and colleagues (1996), and later modified by a Food and Agriculture Organization of the United Nations/World Health Organization Expert Consultation (FAO/ WHO, 2001), should serve as the basis for the level 1 assessment. In addition, the committee believes that further recommendations for in vitro evaluation of allergenicity by the Codex Ad Hoc Intergovernmental Task Force on Safety Assessment of Genetically Modified Foods (Codex Alimentarius Commission, 2003) should be followed.
It should be pointed out that development of allergic or immunologically mediated hypersensitivity relative to ingested foods represents a complex spectrum of symptomatology, which is also influenced by a large number of environmental and cultural factors (Sampson, 2002). Because the immune system of the infant is essentially complete, animal models that predict adult allergic responses can also serve as models for infants. Although some animal models, such as the brown Norway rat model, may provide a valuable tool in the future (Knippels and Penninks, 2003), appropriately validated animal models for assess-
TABLE 5-8 Immunology Assessment: Examples of Tests in Level 1 and Level 2
|
Level |
Assessmenta |
|
Level 1 |
T-/B-cell quantitation and function (immunological analysis of B- and T-lymphocytes and T-lymphocytes subsets [Th+Ts or CD4 and CD8]) Thymus, spleen, bone marrow, lymph nodes, tissue histology Electrophoresis (e.g., for changes in levels of γ-globulin fractions [IgG, IgM, IgA, IgE]) Total serum complement and components of complement (e.g., C3 from CH50 determinations) Levels of prostoglandin E2, balance of LTB4 and LTB5 Immunochemical assays of γ-interferons and serum autoantibodies (antinuclear, antimitochondrial, antiparietal antibodies of B-lymphocytes) In vitro assays of activity of natural killer cells Mitogenic stimulation assay of B- and T-lymphocytes Macrophage activity assays Stem cell assays In vitro assays to assess allergenicity (source of the protein, amino acid sequence homology analysis, physicochemical properties) |
|
Level 2 |
Microarray/proteonomics Special histology stains Stimulation tests of immune function using the T-dependent or independent antigens or human vaccines Cell-mediated immune reactivity and host-resistance assays |
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, determines which tests are required based on a thorough analysis of the potential effects of the new ingredient. aTh = T helper cells, Ts = T suppressor cells, CD4 = cell differentiation antigen 4, CD8 = cell differentiation antigen 8, IgG = immunoglobulin G, IgM = immunoglobulin M, IgA = immunoglobulin A, IgE = immunoglobulin E, LTB4 = leukotriene B4, LTB5 = leukotriene B5. |
|
ment of human allergic disease are not currently available. The committee believes that it is of critical importance that animal models for allergenicity assessment in humans be developed. Until such models are validated, the assessment of allergic potential should be approached in vitro on a molecular level (e.g., utilizing sequence homologies, comparative digestive stabilities, functional antigenic determinants, and B- and/or T-cell epitope characteristics), and the nature of the demonstrated immune response to the known allergic products should be determined.
Endocrine Function
Growth abnormalities of the test animal is an important early indication of a possible effect of a new ingredient on the endocrine system. Because endocrine effects may not be immediately apparent in growth changes, nor in other metabolic functions, some screening tests are indicated. Table 5-9 provides several examples of the types of tests that could be used in level 1 and level 2 assessments of endocrine function.
CONDUCTING NEUROLOGICAL TESTS
Background
As explained in detail in Chapter 6, there are important reasons to include neurological tests in safety assessments of new ingredients for infant formulas, including the sensitivity of
TABLE 5-9 Endocrine Assessment: Examples of Tests in Level 1 and Level 2
|
Level |
Assessmenta |
|
Level 1 |
Hormone levels (e.g., T4, TSH, LH, FSH, GH, CCK, NPY, cortisol, leptin), blood sugar, insulin Organ weight/histology (e.g., adrenals, ovaries, testes, pancreas, thyroid) |
|
Level 2 |
Microarray/proteonomics Provocative endocrine tests (e.g., cosyntropin stimulation) Special histology stains |
|
NOTE: The petitioner (or manufacturer), in consultation with the expert panel, determines which tests are required based on a thorough analysis of the potential effects of the new ingredient. aT4 = thyroxine, TSH = thyrotropin, LH = luteinizing hormone, FSH = follicle-stimulating hormone, GH = growth hormone, CCK = cholecystokinin, NPY=neuropeptide Y. |
|
growth and development to toxic substances and the long-term predictive value of behavioral measures. Therefore the scope of work defined for this project placed special emphasis on the potential effects of ingredients to be added to infant formulas on the rapidly developing infant brain. This section discusses the preclinical assessment tools and models that can be utilized to assess ingredients with either positive or negative neurological effects. The approach is decidedly developmental because the effects of added ingredients (or any environmental stressor) in the developing animal are highly dependent on the timing, dose, and duration of ingredient exposure (Kretchmer et al., 1996).
Traditionally, two metrics—composition and performance—are applied as infant formula manufacturers continue to refine their products to match the gold standard of breastfeeding (Benson and Masor, 1994; MacLean and Benson, 1989). With respect to the latter, the impact of infant formulas on neurodevelopment has come to the forefront. It is widely accepted that breastfed infants demonstrate more advanced neurodevelopment (Lucas et al., 1998; Morrow-Tlucak et al., 1988; Mortensen et al., 2002; Wang and Wu, 1996). One potential explanation is that ingredients found in human milk, but not in infant formulas (e.g., LC-PUFAs, nucleotides, growth factors, and oligosaccharides), may be responsible (DeLucchi et al., 1987; Innis, 1992; MacLean and Benson, 1989). As these compounds are identified and added to infant formulas, valid and stringent assessments of their impacts on the developing brain should be undertaken.
Nutrients and growth factors regulate brain development during prenatal and postnatal life. Late fetal and early neonatal life is a period of rapid brain growth and development in most mammals, including humans (Rice and Barone, 2000; for review, see Dobbing, 1990). There is a wealth of information on the critical events that take place in the brain’s development in the early neonatal period. At this stage of development, cell migration is complete, but myelination, synaptogenesis, dendritic arborization and pruning, and apoptosis are highly active. The rapidly growing brain is more vulnerable to restriction or loss of nutrients when compared with the more mature, less actively growing brain in later childhood and adulthood. Arguably, the rapidly developing brain is also more amenable to repair following nutritional perturbations and may be more highly influenced by nutrient supplements (Kretchmer et al., 1996). Researchers have argued that the persistence of neurological abnormalities after repletion of a nutrient deficiency acquired early in life provides evidence that early neurological vulnerability outweighs potential central nervous system (CNS) plasticity in the developing human (Lozoff et al., 2000; Rao, 2000).
Nutrients may have variable effects on the developing brain. Nutrient deficiencies may produce negative effects or may have no effect at all depending on the stage of brain
development (for review, see Georgieff and Rao, 2001). Similarly, nutrient overabundance or supplementation may produce positive effects, negative effects, or no effects, and the magnitude of the effects can be regionally different. For example, iron deficiency in the young rat brain can cause severe energy and structural deficits in the hippocampus (de Ungria et al., 2000; Rao et al., 1999) and can cause abnormalities in dopamine metabolism without energy deficits in the striatum (Erikson et al., 2000). At an older age, the same degree of iron deficiency causes no structural deficits. Thus any nutrient’s effect on the developing brain will be based on the timing, the dose, and the duration of the exposure.
All nutrients are important for neuronal cell growth and development, but manipulation of some nutrients appears to cause more effects than others. Protein, energy, iron, zinc, selenium, iodine, folate, vitamin A, choline, and LC-PUFAs are nutritional components that influence early brain development with measurable clinical effects in humans and animal models (Georgieff and Rao, 2001). A nutrient that promotes normal brain development at one time and concentration may be toxic at another time or concentration. Many nutrients, such as iron, are regulated within a very narrow range because deficiency and toxicity have profound effects in brain development.
The potential effects of nutrients on brain development will determine the types of preclinical testing that are needed. Effects can be neuroanatomical, that is, affecting neuronal cell division and cell growth (e.g., size, complexity, synaptogenesis, dendritic arborization). Furthermore, nutrients can affect developing non-neuronal cells, such as oligodendrocytes, astrocytes, and microglia, in turn influencing myelination, other nutrient delivery, and cell trafficking. Nutrients can affect neurochemistry through alterations of neurotransmitter and receptor expression. They can affect neurophysiology and neurometabolism with subsequent alterations of signal propagation.
The fundamental question that must be answered in the assessment of nutrient effects on neurodevelopment is whether nutritionally induced alterations in brain development result in changes in brain function, loosely defined as behavior. Specific considerations include:
-
Is this effect transient (e.g., only during the time when the nutrient is supplemented or deficient) or are there long-term gains or losses beyond the time of deficiency?
-
How close is the linkage for each nutrient and each time period of development?
-
What accounts for the individual variability in behavioral outcome of nutritional-based alterations in brain development?
-
Does a lack of behavioral effect in spite of a measurable biochemical effect imply plasticity or alternate circuit development?
-
Are there genetic polymorphisms from a change in nutrient status that confer greater or lesser risk or benefit to the host?
Many of these questions can be answered experimentally before a nutrient is added to formulas, but a multilevel, integrated approach is required because of the limitations of human brain analysis (Nelson et al., 2002). The multiple levels of approach are shown in Table 5-10. The integrated approach (see Table 5-11) chosen should be complementary and obey the laws of timing, dose, and duration (Kretchmer et al., 1996). The animal models must be developmentally appropriate with respect to timing of brain events and must coincide with the likely time of nutrient supplementation or deficiency in the human. In other words, for brain-behavior associations to be relevant, the time at which a given nutrient alters brain developmental processes in the animal model should coincide with the time that the nutrient deficit or supplement occurs in the infant population.
TABLE 5-10 Neurological Assessment: Examples of Tests in Level 1 and Level 2
The nonhuman primate, pig, rat, and mouse are the traditional models for neurological research. Table 5-12 and the information provided earlier in this chapter describe the advantages and disadvantages to using each model for neurological research.
Preclinical studies should assess the neurological safety of the nutrients by measuring the physiological and pharmacological levels of the nutrients. The goal is to match structural and biochemical alterations to changes in function (e.g., behavior). It must be recognized, however, that there can be a dissociation between biochemical and cellular changes in the brain and neurodevelopment (defined as behavior).
Environmental effects on the developing brain have been historically divided into effects on the motor and nonmotor systems. The motor system is far better understood than the nonmotor system because it is more discretely localized in the CNS and has a longer history of investigation. Principles learned from the motor system serve as a model for the current approaches to experimentation in the nonmotor system. The nonmotor system includes cognitive and noncognitive behaviors. Within cognition, a number of behaviors can be assessed, including memory and emotion. Memory is further divided into declarative or recognition memory and implicit or procedural memory (Squire, 1992). The neural circuits that subserve these memory systems are quite distinct and are differentially vulnerable to nutrient insults. The difference in ontogeny of the memory systems has been extensively reviewed by Nelson (1995).
It should be noted that many of the neural systems have mixed components. For example, there are connections between structures underlying recognition memory (e.g., hippocampus) and emotion (e.g., amygdala). Thus, for example, it may be difficult to know from behavioral testing alone whether the enhanced ability to perform a recognition memory task following a nutritional supplement is due to direct effects on the hippocampus or through attentional and motivational effects mediated by the amygdala. Certain structures, such as the cerebellum and basal ganglia, are involved in both motor and cognitive events.
TABLE 5-11 Integrated Approach to the Assessment of Neurodevelopment
|
Neurological Function |
System of Assessment |
|
Animal behavior (fit to human) |
Nonhuman primate, rat |
|
Biochemistry (neurochemistry) |
Human, animal model |
|
Brain structure |
Human, animal model |
|
Human behavior |
Human |
|
Physiology, cellular/molecular |
Animal model, cell culture |
TABLE 5-12 Summary of Animal Models Used in Preclinical Studies
|
Animal |
Advantages |
Disadvantages |
|
Chicken |
Immunology |
No relevance to human nutrition, genetics |
|
Dog |
Metabolism, immunology, organs |
Behavior, genetics |
|
Hamster |
Lipid metabolism |
Immunology, genetics |
|
Mouse |
Genetics, molecular analysis, mechanisms, organs |
Learning paradigms, developmental |
|
Nonhuman primate |
Functional/behavioral relationship to humans, similar diet as humans, immunological studies, dermatological studies, renal function, kidney biopsy, invasive assessment |
Basic biochemistry, histopathology, genetics; expense and ethical concerns for neural tests |
|
Pig |
Comparable size to neonatal humans, lipid biochemistry, organs |
Learning paradigms, poor model for genetics |
|
Rabbit |
Biochemistry, immunology |
No relevance to human metabolism |
|
Rat |
Cellular, molecular analysis, behavior, organs, physiological studies; brain development is similar to human infant |
Higher-level learning, genetics, developmental |
The optimal use of an integrated methods approach should allow assessment of nutrient effects on behavior, neuroanatomy, neurochemistry, and form-function relationships (e.g., time-locked combinations of methods, such as functional MRI). It is important to have assessments that tap similar brain areas across species (from human to rodent), allowing extrapolation of CNS effects at the tissue level (rodents) to species where tissue is unavailable (humans, nonhuman primates). Table 5-13 provides examples of the cross-referencing of behavioral tasks across three species.
Neurological Assessment Techniques
Overall there must be a systematic approach to this important aspect of safety effects of ingredients added to infant formulas. Preclinical assessment must include whole-animal work with specific attention to regional brain effects. Here, neuroimaging through histochemistry at autopsy and through MRI in the living animal provides information. Neurochemistry experiments should target plausible biological mechanisms, particularly as to how nutrients might affect neurotransmission. Electrophysiology, using either deep-implanted electrodes or scalp-surface electrodes, provides information about neuronal population functions (Bachevalier, 2001; Fuster and Alexander, 1971) and can be cross-referenced to the assessment of humans conducted by evoked or event-related potentials (see Chapter 6).
The following sections describe various techniques for using preclinical studies to assess the effect of ingredients new to infant formulas on the developing brain. As with the non-
TABLE 5-13 Examples of Cross-Species Developmental-Neural Assessment Tools
|
Measure |
Infant |
Monkey |
Rat |
|
A-not-B |
X |
X |
|
|
Black-white discrimination |
X |
X |
X |
|
Delayed nonmatch to sample test |
X |
X |
X |
|
Maze |
|
X |
X |
|
Paired comparison |
X |
X |
X |
|
Object discrimination |
X |
X |
X |
neurological organs, the committee proposes a two-level hierarchy with respect to application of these techniques in the preclinical studies. Level 1 assessments are required of all proposed ingredients and are designed to survey the neurological system of the developing brain. Level 2 assessments are performed if the ingredient to be added could theoretically affect brain development through a biologically plausible mechanism or if level 1 assessments have previously demonstrated a safety concern for the brain (Table 5-10).
Neuronal Genetics/Mutagenicity
In the future, evaluations should include neuronal and glial-cell culture work to assess the effect of the added ingredient on genomics, metabolomics, and proteomics with the purpose of evaluating mutagenicity and further functional consequences. Genomic techniques (e.g., DNA microarray analysis) are new and promising techniques that, once refined, will likely have great utility in assessing whether there are inductions of desirable or undesirable genes after exposure to an ingredient. These methodologies are still limited because of difficulties in interpreting the functionality of observed changes in gene expression. The parallel fields of proteomics and metabolomics complement the genomics methods by assessing the production of expected and unexpected proteins after nutrient exposures. Furthermore, cell culture work can inform about nutrient effects on target receptors (for the nutrient or its metabolite) (Alcantara et al., 1994) and the subsequent downstream effect on signaling cascades in the neurons (Gietzen and Magrum, 2001; Magrum et al., 1999). An excellent overview of this type of approach can be found in the work of Gietzen (2000) on imbalanced amino acid diet effects on feeding behavior as mediated through the neurons of the anterior piriform cortex. Gietzen’s work serves as a model system for assessing nutrient effects on signaling cascades in the relevant neuronal systems. As noted earlier in this chapter, virtually every new ingredient has the potential to advertently or inadvertently alter basic cellular processes. Therefore neuronal-cell culture techniques should be considered level 1 assessments.
Neuronal- and glial-cell culture techniques were not reported in the GRAS Notification for LC-PUFAs. These techniques may have identified unwanted effects of these ingredients on gene expression through microarray screening analysis. The effect of these added ingredients on the signaling cascade involved in long-term potentiation could have been assessed in hippocampal CA1 cell recordings.
Neuroanatomy
There are a number of techniques that assess neuroanatomy, ranging from direct histological assessment by light, confocal, and electron microscopy to neuroimaging (e.g., MRI). Histological assessment allows a direct view of the brain and should be used as a level 1 tool to assess cytological effects of all proposed ingredients. The approach is used in the pig and rodent models and can visualize general neuronal structure (e.g., myelin, synapses, dendritic arborization, neuronal number), effector proteins (e.g., transporters, receptors), apoptosis/ cell death, and regulatory elements (e.g., iron regulatory proteins). The limitation of the approach is that it only shows structure, and alterations of function may not always follow structural changes.
MRI can be used in the developing human and all typical animal models to assess nutrient effects on total brain volume, regional volumes, myelination, and the visualization of some nutrients (for review, see Casey et al., 2001). In spite of great technical advances, this method is generally insensitive and assesses only relatively large differences in protein-
energy or fatty acid status. Therefore it has poor predictive value for future subtle disability of neurological development in humans. At this time, neuroimaging should be categorized as a level 2 assessment.
Neurochemistry
Neurochemistry can be assessed either directly in the tissue once it is harvested, in the tissue of the living brain through microdialysis catheters (Chen et al., 1995), or in vivo through MRI spectroscopy (Tkáč et al., 2003). The goal is to assess the potential adverse effect of ingredients on neurotransmitters and intermediate metabolism. The advent of MRI spectroscopy allows for the in vivo, real time, repeatable assessment of the chemistry of the developing brain. The technique uses proton, phosphorous, or 13-carbon tracer nuclear magnetic resonance, and it can be used in humans and in animals as small as mice. Its main limitation is the inability to target compounds prospectively; the compounds that can be assessed are dependent on the major peaks that are visualized on the spectra. If the nutritional effect is on compounds that are in a concentration that is too low to result in a major peak, the technique is not useful. This technique is innovative and as yet not standardized. Therefore it should be considered a level 2 assessment in the evaluation of ingredients likely to affect neurochemistry.
The measurement of nutrient effects on neurotransmitter systems is an important part of nutritional research on brain development. The response of neurotransmitters to changes in nutrient availability can be directly measured in the rodent (Chen et al., 1995) and in neuronal cell culture. It is important with the glutamine system that cell cultures contain both neurons and glia since there is an important shuttle of glutamate and glutamine that takes place. It is also important to assess compensatory feedback mechanisms when transmitter concentrations are affected by nutrients. In response to increased transmitter release, there can be alterations in receptor number and affinity and in presynaptic reuptake mechanisms. The effect of iron deficiency on dopamine provides an excellent example (Chen et al., 1995). The assessment of neurotransmitter systems will be a level 2 assessment for most ingredients. However many compounds being considered for addition to infant formulas (e.g., choline) are likely to have a significant impact on these systems and will require this type of assessment.
Neurophysiology
Nutrients have effects on the electrophysiology of neuronal populations. A fundamental question that must be addressed in any nutrient-additive experiment is the plausible biological mechanism by which neuronal output is changed, which could result in a behavioral change. Examples include changes in calcium gating through alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid and N-methyl-D-aspartate receptors in brain areas involved in learning, which could alter long-term potentiation and learning behavior (Magrum et al., 1999). If nutrient supplements have a putative biological effect on myelination, there should be demonstrable effects on the speed of processing of myelinated circuits (Birch et al., 1992; Uauy-Dagach and Mena, 1995). Indirect effects must be considered as well. For example, steroids have significant effects on hippocampal anatomy, physiology, and gene expression (for review, see Sapolsky, 1994). Therefore if a nutrient supplement presents a stress to the developing organism with increased output of corticosteroids or estrogens, secondary effects of these steroids on the hippocampus must be considered. Electrophysiological testing will typically be warranted as a level 2 assessment when there is a reasonable concern that a new ingredient will affect neuronal function.
Behavior
Ultimately the preclinical assessment must shed light on any positive or negative effects on behavior since it is the summation of the efferent expression of brain activity. An assessment of general behavior of the animal model should be performed with any added ingredient. This is part of standard animal care during experiments. Particular attention should be paid to changes in typical behaviors, such as activity level, feeding, and comfort seeking. In the case of suspected specific regional or neurotransmitter effects, a more comprehensive second level evaluation targeting the potential neuropathology is required. Examples include specific motor, cognitive, and behavioral paradigms that map on the brain systems at risk. With any behavioral assessment, cross-referenced behavioral assessments in the motor and cognitive domain are important during the period of nutrient delivery to assess acute effects. Just as importantly, down-stream, long-term effects must also be evaluated to assess whether the added nutrient has permanent or transient effects and whether there are any “sleeper” effects.
SUMMARY
Preclinical studies are a vital first step to assess the safety and quality of ingredients new to infant formulas. Regulatory guidelines for preclinical studies must be based on considerations of the diversity of the potential new ingredients and the ingredients’ source and matrix. In the United States, the FDA Redbook provides comprehensive guidelines for conducting preclinical studies to test the safety of food and color additives, but it often does not take the many special needs and vulnerabilities of infants into consideration.
The committee recommends a two-level preclinical approach with respect to assessing the safety of ingredients new to infant formulas. The approach should take into consideration model systems that are appropriate for the developing infant. All proposed new ingredients require assessments using level 1 techniques (Tables 5-1 through 5-10) at the discretion of the expert panel. Any proposed new ingredient with expected effects on structure or function, either on a theoretical basis or as a result of level 1 tests, will require appropriately targeted level 2 evaluations (Tables 5-1 through 5-10).
REFERENCES
Aeschbacher HU, Finot PA, Wolleb U. 1983. Interactions of histidine-containing test substances and extraction methods with Ames mutagenicity test. Mutat Res 113:103–116.
Alcantara O, Obeid L, Hannun Y, Ponka P, Boldt DH. 1994. Regulation of protein kinase C (PKC) expression by iron: Effect of different iron compounds on PKC-beta and PKC-alpha gene expression and role of the 5'-flanking region of the PKC-beta gene in the response to ferric transferrin. Blood 84:3510–3517.
Anderson NL, Anderson NG. 1998. Proteome and proteomics: New technologies, new concepts and new words. Electrophoresis 19:1853–1861.
Arbuckle LD, MacKinnon MJ, Innis SM. 1994. Formula 18:2(n-6) and 18:3(n-3) content and ratio influence long-chain polyunsaturated fatty acids in the developing piglet liver and central nervous system. J Nutr 124:289–298.
Bachevalier J. 2001. Neural bases of memory development: Insights from neuropsychological studies in nonhuman primates. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press. Pp. 365–379.
Benson JD, Masor ML. 1994. Infant formula development: Past, present and future. Endocr Regul 28:9–16.
Birch DG, Birch EE, Hoffman DR, Uauy RD. 1992. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Invest Ophthalmol Vis Sci 33:2365–2376.
Bogyo M, Hurley JH. 2003. Proteomics and genomics. Curr Opin Chem Biol 7:2–4.
Canada. 2001. Departmental Consolidation of the Food and Drugs Act and the Food and Drug Regulations. (Food and Drugs Act). Ottawa: Minister of Public Works and Government Services Canada.
Casey BJ, Thomas KM, McCandliss B. 2001. Applications of magnetic resonance imaging to the study of development. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press. Pp. 137–147.
Chen Q, Beard JL, Jones BC. 1995. Abnormal rat brain monoamine metabolism in iron deficiency anemia. J Nutr Biochem 6:486–493.
Clarke SD. 2001. Polyunsaturated fatty acid regulation of gene transcription: A molecular mechanism to improve the metabolic syndrome. J Nutr 131:1129–1132.
Codex Alimentarius Commission. 2003. Codex Principles and Guidelines on Foods Derived from Biotechnology. Prepublication. Online. Food and Agriculture Organization, World Health Organization. Available at ftp://ftp.fao.org/codex/2003Prepublicationtexts/Codex_Texts_on_Biotechnology(E).pdf. Accessed January 12, 2004.
Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. 2002. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297:240–243.
Craig-Schmidt MC, Stieh KE, Lien EL. 1996. Retinal fatty acids of piglets fed docosahexaenoic and arachidonic acids from microbial sources. Lipids 31:53–59.
Daniel H. 2002. Genomics and proteomics: Importance for the future of nutrition research. Br J Nutr 87:S305– S311.
de Ungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. 2000. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 48:169–176.
Delion S, Chalon S, Herault J, Guilloteau D, Besnard JC, Durand G. 1994. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr 124:2466–2476.
DeLucchi C, Pita ML, Faus MJ, Molina JA, Uauy R, Gil A. 1987. Effects of dietary nucleotides on the fatty acid composition of erythrocyte membrane lipids in term infants. J Pediatr Gastroenterol Nutr 6:568–574.
DeRisi JL, Iyer VR. 1999. Genomics and array technology. Curr Opin Oncol 11:76–79.
Dobbing J. 1990. Vulnerable periods in developing brain. In: Dobbing J, ed. Brain, Behaviour, and Iron in the Infant Diet. New York: Springer-Verlag. Pp. 1–25.
Erikson KM, Jones BC, Beard JL. 2000. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr 130:2831–2837.
FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). 2001. Evaluation of Allergenicity of Genetically Modified Foods. Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology. Rome: FAO.
Fuster JM, Alexander GE. 1971. Neuron activity related to short-term memory. Science 173:652–654.
Georgieff MK, Rao R. 2001. The role of nutrition in cognitive development. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press. Pp. 491–504.
Gietzen DW. 2000. Amino acid recognition in the central nervous system. In: Berthoud HR, Seeley RJ, eds. Neural and Metabolic Control of Macronutrient Intake. New York: CRC Press. Pp. 339–357.
Gietzen DW, Magrum LJ. 2001. Molecular mechanisms in the brain involved in the anorexia of branched-chain amino acid deficiency. J Nutr 131:851S–855S.
Golub MS, Gershwin ME. 1984. Standardized neonatal assessment in the rhesus monkey. In: Nathanielsz PW, Parer JT, eds. Research in Perinatal Medicine (I). Ithaca, NY: Perinatology Press. Pp. 55–86.
Golub MS, Gershwin ME, Hurley LS, Hendrickx AG, Saito WY. 1985. Studies of marginal zinc deprivation in rhesus monkeys: Infant behavior. Am J Clin Nutr 42:1229–1239.
Govorun VM, Archakov AI. 2002. Proteomic technologies in modern biomedical science. Biochemistry 67:1109–1123.
Guengerich FP. 2001. Functional genomics and proteomics applied to the study of nutritional metabolism. Nut Rev 59:259–263.
Hahn MJ. 2000. Letter to Linda Kahl on February 29, 2000. GRAS notification on behalf of Martek Biosciences Corporation.
Haubner LY, Stockard JE, Saste MD, Benford VJ, Phelps CP, Chen LT, Barness L, Wiener D, Carver JD. 2002. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain Res Bull 58:1–5.
Hochstrasser DF. 1998. Proteome in perspective. Clin Chem Lab Med 36:825–836.
Hrboticky N, MacKinnon MJ, Innis SM. 1990. Effect of a vegetable oil formula rich in linoleic acid on tissue fatty acid accretion in the brain, liver, plasma, and erythrocytes of infant piglets. Am J Clin Nutr 51:173–182.
Innis MA. 1990. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press.
Innis SM. 1992. Human milk and formula fatty acids. J Pediatr 120:S56–S61.
Jungblut PR, Zimny-Arndt U, Zeindl-Eberhart E, Stulik J, Koupilova K, Pleissner KP, Otto A, Muller EC, Sokolowska-Kohler W, Grabher G, Stoffler G. 1999. Proteomics in human disease: Cancer, heart and infectious diseases. Electrophoresis 20:2100–2110.
Knippels LMJ, Penninks AH. 2003. Assessment of the allergic potential of food protein extracts and proteins on oral application using the brown Norway rat model. Environ Health Perspect 111:233–238.
Kretchmer N, Beard JL, Carlson S. 1996. The role of nutrition in the development of normal cognition. Am J Clin Nutr 63:997S–1001S.
Kriete MF, Champoux M, Suomi SJ. 1995. Development of iron deficiency anemia in infant rhesus macaques. Lab Anim Sci 45:15–21.
Lander ES. 1999. Array of hope. Nat Genet 21:3–4.
Lee CK, Weindruch R, Prolla TA. 2000. Gene-expression profile of the ageing brain in mice. Nat Genet 25:294–297.
Lee KH, Calikoglu AS, Ye P, D’Ercole AJ. 1999. Insulin-like growth factor-I (IGF-I) ameliorates and IGF binding protein-1 (IGFBP-1) exacerbates the effects of undernutrition on brain growth during early postnatal life: Studies in IGF-I and IGFBP-1 transgenic mice. Pediatr Res 45:331–336.
Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. 2000. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 105:E51.
Lucas A, Morley R, Cole TJ. 1998. Randomised trial of early diet in preterm babies and later intelligence quotient. Br Med J 317:1481–1487.
MacBeath G. 2002. Protein microarrays and proteomics. Nat Genet 32:526–532.
MacLean WC Jr, Benson JD. 1989. Theory into practice: The incorporation of new knowledge into infant formula. Semin Perinatol 13:104–111.
Magrum LJ, Hickman MA, Gietzen DW. 1999. Increased intracellular calcium in rat anterior piriform cortex in response to threonine after threonine deprivation. J Neurophysiol 81:1147–1149.
McGregor DB, Martin R, Cattanach P, Edwards I, McBride D, Caspary WJ. 1987. Responses of the L5178Y tk+/ tk– mouse lymphoma cell forward mutation assay to coded chemicals. I: Results for nine compounds. Environ Mutagen 9:143–160.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Caspary WJ. 1988a. Responses of the L5178Y tk+/ tk− mouse lymphoma cell forward mutation assay to coded chemicals. II: 18 coded chemicals. Environ Mol Mutagen 11:91–118.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, Caspary WJ. 1988b. Responses of the L5178Y tk+/tk− mouse lymphoma cell forward mutation assay to coded chemicals. III: 72 coded chemicals. Environ Mol Mutagen 12:85–154.
Metcalfe DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL. 1996. Assessment of the allergenic potential of foods derived from genetically engineered crop plants. Crit Rev Food Sci Nutr 36:S165–S186.
Miles EA, Calder PC. 1998. Modulation of immune function by dietary fatty acids. Proc Nutr Soc 5:277–292.
Moreno-Aliaga MJ, Marti A, Garcia-Foncillas J, Alfredo Martinez J. 2001. DNA hybridization arrays: A powerful technology for nutritional and obesity research. Br J Nutr 86:119–122.
Morrow-Tlucak M, Haude RH, Ernhart CB. 1988. Breastfeeding and cognitive development in the first 2 years of life. Soc Sci Med 26:635–639.
Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. 2002. The association between duration of breastfeeding and adult intelligence. J Am Med Assoc 287:2365–2371.
Myhr BC, Caspary WJ. 1988. Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: Intralaboratory results for sixty-three coded chemicals tested at Litton Bionetics, Inc. Environ Mol Mutagen 12:103–194.
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D, Martin R, Caspary WJ. 1990. L5178Y mouse lymphoma cell mutation assay results with 41 compounds. Environ Mol Mutagen 16:138–167.
Nelson CA. 1995. The ontogeny of human memory: A cognitive neuroscience perspective. Devel Psychol 31:723–738.
Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. 2002. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol 14:499–520.
Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. 1986. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA 83:4021–4025.
Ntambi JM. 1999. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40:1549–1558.
OFAS (Office of Food Additive Safety). 2001. Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food. Redbook II-Draft. Washington, DC: OFAS, Center for Food Safety and Applied Nutrition, Food and Drug Administration.
OFAS (Office of Food Additive Safety). 2003. Redbook 2000. Toxicological Principles for the Safety of Food Ingredients. Online. Center for Food Safety and Applied Nutrition, Food and Drug Administration. Available at http://www.cfsan.fda.gov/~redbook/red-toca.html. Accessed November 19, 2003.
Overman WH, Bachevalier J. 2001. Inferences about the functional development of neural systems in children via the application of animal tests of cognition. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press. Pp. 109–124.
Pasquier E, Ratnayake WM, Wolff RL. 2001. Effects of delta5 polyunsaturated fatty acids of maritime pine (Pinus pinaster) seed oil on the fatty acid profile of the developing brain of rats. Lipids 36:567–574.
Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JCF, Lashkari D, Shalon D, Brown PO, Botstein D. 1999. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 96:9212–9217.
Reisbick S, Neuringer M, Hasnain R, Connor WE. 1994. Home cage behavior of rhesus monkeys with long-term deficiency of omega-3 fatty acids. Physiol Behav 55:231–239.
Rao R GM. 2000. Early nutrition and brain development. In: Nelson C, ed. The Minnesota Symposia on Child Psychology. The Effects of Adversity on Neurobehavioral Development. Vol. 31. Mahway, NJ: Lawrence Erlbaum Associates. Pp. 1–30.
Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. 1999. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr 129:199–206.
Rice D, Barone S. 2000. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect 108:511–533.
Sampson HA. 2002. Peanut allergy. New Engl J Med 346:1294–1299.
Sapolsky RM. 1994. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann N Y Acad Sci 746:294–304.
Schlegel R, MacGregor JT. 1982. The persistence of micronuclei in peripheral blood erythrocytes: Detection of chronic chromosome breakage in mice. Mutat Res 104:367–369.
Schlegel R, MacGregor JT, Everson RB. 1986. Assessment of cytogenetic damage by quantitation of micronuclei in human peripheral blood erythrocytes. Cancer Res 46:3717–3721.
Shimomura I, Shimano H, Korn BS, Bashmakov Y, Horton JD. 1998. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem 273:35299–35306.
Soukas A, Cohen P, Socci ND, Friedman JM. 2000. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev 14:963–980.
Squire LR. 1992. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. J Cogn Neurosci 4:232–243.
Tkáč I, Rao R, Georgieff MK, Gruetter R. 2003. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med 50:24–32.
Uauy-Dagach R, Mena P. 1995. Nutritional role of omega-3 fatty acids during the perinatal period. Clin Perinatol 22:157–175.
Vazquez E, Herrero I, Miras-Portugal MT, Sanchez-Prieto J. 1994. Role of arachidonic acid in the facilitation of glutamate release from rat cerebrocortical synaptosomes independent of metabotropic glutamate receptor responses. Neurosci Lett 174:9–13.
Vihinen M. 2001. Bioinformatics in proteomics. Biomol Eng 18:241–248.
Wainwright PE, Xing HC, Mutsaers L, McCutcheon D, Kyle D. 1997. Arachidonic acid offsets the effects on mouse brain and behavior of a diet with a low (n-6):(n-3) ratio and very high levels of docosahexaenoic acid. J Nutr 127:184–193.
Wang J. 2000. From DNA biosensors to gene chips. Nucleic Acids Res 28:3011–3016.
Wang YS, Wu SY. 1996. The effect of exclusive breastfeeding on development and incidence of infection in infants. J Hum Lact 12:27–30.
Williams KL. 1999. Genomes and proteomes: Towards a multidimensional view of biology. Electrophoresis 20:678–688.