3
Seven Criteria for Evaluating the Patent System
PATENTS AND INNOVATION
Ultimately, the test of a patent system is whether it enhances social welfare, not only by encouraging invention and the dissemination of useful technical information but also by providing incentives for investment in the commercialization of new technologies that promote economic growth, create jobs, promote health, and advance other social goals. Assessing the system’s overall economic impact is no simple task, perhaps an impossible one. For one thing, the dual functions of patents are in some degree at odds with each other. The exclusivity that a patent confers is undermined by its publication, which may help others circumvent the patent. Furthermore, patents entail a trade-off between the incentives provided for innovation and the costs resulting from a monopoly that may curtail competition and raise consumer prices or hinder further incentive efforts. Both sides of that ledger are exceedingly complex. Innovation in any technology area may benefit from the incentive created by a patent on a new product or process development, but it may suffer if patents discourage the combining and recombining of inventions that would have been made absent the patent or inhibit follow-on discovery. Competition may suffer when an inventor is granted a temporary monopoly right or a combination of patents is used to bar entry or to maintain a cartel in an industry. On the other hand, competition will benefit if this right facilitates investment by new, innovative firms lacking assets other than intellectual property. Patents can also foster the creation of markets for technology, enabling efficiencies in the research and development (R&D) process and promoting the transfer of discoveries from entities skilled at conducting R&D to firms potentially better suited to commercializing and marketing innovations.
We have previously cited evidence that patents function differently in different industrial sectors. There is also a growing body of research on the relationship between patents and innovation across countries and time. Using mainly 19th-century data, Lerner (2002) and Moser (2003) find that instituting a patent system or strengthening an existing patent system does not produce more domestic innovation although the latter does induce inventors from other countries to patent more in the country making the change. It may also induce foreign multinationals to transfer more technology to affiliates in the country (Branstetter et al., 2003). Sakakibara and Branstetter (2001) studied the effects of a statutory change in Japan allowing multiple claims per patent, as has always been the case in the United States. They found that the effective broadening of patent scope had a very small positive effect on R&D activity by Japanese firms. Lanjouw and Cockburn (2000) found some limited evidence for attributing an increase in Indian research addressing developing country needs to patent reforms of the 1980s, which provided increased protection.1 The effect leveled off, however, in the following decade. Scherer and colleagues (1959) investigated the consequences of Italy’s moving from a no-patent to a patent regime in pharmaceuticals; they did not find a significant effect. Using firm-level survey data for Canada, Baldwin and colleagues (2000) found a much stronger relationship running from innovation to patenting than in the reverse direction. Firms that innovate take out patents, but firms and industries that make more intensive use of patents do not tend to produce more innovation. In the United States manufacturing sector, however, in a model that explicitly controls for mutual causation between patenting and R&D, Arora and colleagues (2002) find evidence that patenting is an important stimulus for R&D.
Other positive results are those of Park and Ginarte (1997) using data across 60 countries for the period 1960-1990. They found that the strength of intellectual property (IP) protection (an index of pharmaceutical coverage, participation in international agreements, lack of compulsory licensing, strength of enforcement, and patent duration) was positively associated with R&D investment in the 30 countries with the highest median incomes. Elsewhere, the relationship was positive but not significant. These results, however, are cross-sectional and fail to account for the reverse causality between conducting R&D and having a robust patent system.
The conclusions from this body of empirical research on the effects of patents are several but mostly tentative (Hall, 2003b). In developed countries, at least in manufacturing, patenting stimulates innovative activity broadly, but the stimulus varies among industries. Introducing or strengthening a patent system, however, unambiguously results in an increase in patenting and may encourage the strategic and tactical use of patents with attendant costs and possibly adverse
impacts on innovation and competition. One may legitimately question whether the impact of patenting on innovation and its consequences for social welfare are, on balance, positive outside of the handful of industries, such as pharmaceuticals, biotechnology, medical devices, and specialty chemicals where the benefits are well established, and possibly to a lesser extent, computers and auto parts.
More subtle effects are suggested by recent economic studies and deserve more attention. Patents may enable the creation and affect the organization of knowledge-based industries by allowing trade in knowledge and facilitating the entry of firms with only intangible assets. As this abbreviated literature review suggests, the empirical economic research on the uses and impacts of patenting is more robust than it was nearly 20 years ago when George Priest (1986) complained about the dearth of useful economic evidence on the impact of intellectual property: “Economists know almost nothing about the effect on social welfare of the patent system or … other intellectual property.” Nevertheless, knowledge is still quite limited and the range of industries examined in any detail is quite narrow.
EVALUATION CRITERIA
In circumstances that at this stage defy a comprehensive evaluation, the committee posits a series of criteria for evaluating the patent system in terms of its impact on innovation rather than addressing its competitive or overall welfare effects. These criteria, although requiring judgments, can in varying degrees be assessed empirically and tracked over time to observe significant changes. In most cases they relate to factors widely thought to be important if not necessary and sufficient conditions for innovation.
First criterion: The patent system should accommodate new technologies. A system granting even temporary monopoly rights to developers of one technology but providing no incentives to developers of other, including substitute, technologies obviously would be hostile to innovation over the long run.
Second criterion: The system should reward only those inventions that meet the statutory tests of novelty and utility, that would not at the time they were made be obvious to people skilled in the respective technologies, and that are adequately disclosed. In the extreme case where an invention is already accessible to the public, or the full scope of what is patented cannot be carried out in practice, there is nothing to be gained and potentially a great deal to be lost by granting a monopoly.
Third criterion: The patent system should serve its second function of disseminating technical information. That means that descriptions of patented inventions should be as complete, clear, and accessible as possible and disclosed in a reasonably timely manner, and there should not be deterrents to consulting the patent or any other technical literature.
Fourth criterion: Administrative and judicial decisions entailed in the patent system should be timely, and the costs associated with them should be reasonable and proportionate. Protracted uncertainty about whether a patent on an application will issue or about whether a patent that is challenged in an infringement dispute will be upheld or found not infringed is not conducive to the investments necessary to innovate. In the same vein, high transaction costs entailed in obtaining or defending a patent are likely to discourage innovation. Such costs tend to escalate the longer the resolution of the issue, whether patentability or infringement, is delayed.
Fifth criterion: In scientific research and in the development of complex or cumulative technologies, where one advance builds upon one or more previous discoveries or inventions and full exploitation of the technology is beyond the capacity of any single entity, reasonably broad access to patented inventions is important. Access depends upon at least three factors: (1) the scope of the patent claims, (2) the availability of licenses on reasonable terms, and (3) the complexity of the patent landscape. Of course, technology must first be created for access to be an issue. Thus, access must be balanced against the incentive to invent and disseminate technology.
Sixth criterion: In an economy where a significant share of its technology-intensive products are bought and sold internationally, the compatibility of national patent systems can be a facilitator of trade and investment and therefore innovation. Indeed, there is an efficiency argument for the integration of the U.S., European, and Japanese patent systems to reduce public and private transaction costs.
Seventh criterion: There should be a level field, with intellectual property rights holders who are similarly situated (e.g., state and private institutions performing research) enjoying the same benefits, while being subject to the same obligations.
Accommodating New Technologies
As the examples of the extensions of patenting in Chapter 2 illustrate, the patent system has proven highly adaptable to changes in technology. This includes not only emergent technologies in advance of or in tandem with their commercial application—for example, biotechnology and nanotechnology—but also technologies that at least in their early stages exhibited rapid progress and substantial commercial success without patents, such as software.
The flexibility of the patent system is a function of at least three features. First, it is a unitary system with few a priori exclusions. Second, the initiative to extend patenting to a new area lies in the first instance with inventors and commercial developers, not with legislators, administrators, or judges. Third, some statutory features of the patent system, as well as administrative and court-
interpreted case law, allow for somewhat specialized treatment in some fields of technology.
The Patent Act of 1952 states that
Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefore, subject to the conditions and requirements of this title.2
The most expansive Supreme Court interpretation of this section was in Diamond v. Chakrabarty,3 the case that held a genetically modified microorganism to be patentable subject matter. In the course of its decision the Court stated that
the Committee Reports accompanying the 1952 Act inform us that Congress intended statutory subject matter to “include anything under the sun that is made by man.”
Sometimes these extensions occur readily. The first patent on a flying machine was issued to Orville and Wilbur Wright within 30 months of the flight at Kitty Hawk, North Carolina. In other cases the federal courts have played a prominent role. Particularly when the emergence of a new domain—for example, genetically modified life forms—is obvious and sensitive, the patent office has been hesitant to move in aggressively, and the courts have been asked to recognize patent eligibility. But even in these cases, the lag, if any, can be quite short. The Supreme Court’s Chakrabarty decision preceded by two years the introduction of the first commercial product, human insulin, made with recombinant DNA techniques.
In other instances the judges have changed their minds over time. With respect to computer software and related inventions, the law changed radically during the latter decades of the 20th century. In the 1970s the Supreme Court held unanimously in Gottschalk v. Benson4 that a computer program was not patentable subject matter. Following two later Supreme Court decisions that suggested a shift in this position,5 the Court of Appeals for the Federal Circuit (“Federal Circuit”) felt comfortable in holding in 1994 that an abstract mathematical algorithm was not patentable, but a computer programmed to run such an algorithm was patentable.6 This may have been a nearly inevitable development, considering that innovations in the design of the software to run a computer and mechanical devices controlled by internal computer chips seem very close to traditional
inventions.7 But the courts have gone even further. The case that has received the most attention is State Street Bank & Trust v. Signature Financial Group,8 which contradicted the prevailing assumption that business methods were not patentable. State Street was followed by AT&T v. Excel Communications, Inc.,9 which, in essence, removed the requirement that software could be patented only as embodied in a computer program and therefore effectively permitted patents on algorithms themselves.10
Thus, the path toward incorporating new technologies in the patent system is not always rapid and seamless. Even less is it free of controversy. The wisdom of permitting the patenting of inventions involving genetic material, computer software, and especially methods of transacting business, where there is long history of innovations without patent protection, is still very much a matter of debate.11
Moreover, the courts have recognized limits to patenting. Historically, patent law has supported the public domain of fundamental scientific research results and other ineligible subject matter not expressed as a product or a method. In its decision in Chakrabarty the Supreme Court qualified its “anything under the sun by the hand of man” dictum as follows:
This is not to suggest that [Section] 101 has no limits or that it embraces every discovery. The laws of nature, physical phenomena, and abstract ideas have been held not patentable. Thus, a new mineral discovered in the earth or a new plant found in the wild is not patentable subject matter. Likewise, Einstein could not patent his celebrated law that E=mc2; nor could Newton have patented the law of gravity. Such discoveries are manifestations of nature, free to all men and reserved exclusively to none.
The recent extension of patenting has led to the granting of quite abstract patents, some of them representing intersections of biotechnology, software, and business methods. Examples include the use of a specific genetic characteristic to infer a specific phenotypic characteristic,12 a technique of statistical analysis on arrays13 and databases,14 and the use of specific protein coordinates in a computer program to search for protein complexes.15 It is of concern to some members of this committee but not clear to a majority that the line between practical invention and pure information is being breached. If it is being crossed in a few cases it is not clear that they represent precedents that the USPTO is continuing to follow, or if the patents were challenged, how the courts would construe these claims or whether the claims so construed would be held valid.16 That there is disagreement should not be surprising given that the line between ideas and inventions is indistinct.
Notwithstanding its unitary character, the U.S. patent system is differentiated in transparent and subtle ways that accommodate differences in technologies or that affect technologies differently. An example of the former is the requirement for patent holders to pay maintenance fees periodically to take advantage of the full statutory patent term. As we discussed in Chapter 2, that means that many patents are allowed to lapse if the cost of keeping them in force exceeds their value. That is much more frequently the case in information technology, where the product cycle is as short as a few months, than in pharmaceuticals, where the returns to patents are concentrated in the last few years of their terms because the early years are consumed with clinical testing and achieving regulatory approval. The patent prosecution process also varies in duration and other characteristics from one major technology class to another (Allison and Lemley, 1998).
Less obvious but important, the patentability rules applied to different technologies show some divergence. According to legal scholars Dan Burk and Mark Lemley (2003a), the ability to calibrate the patent system to industries and technologies derives from a large kit of policy levers available to the USPTO and the courts. These include or could include all of the following rules and patent doctrines—the rule against patenting abstract ideas, the standard of utility, the exception for experimental use, the test for obviousness of the “person having
ordinary skill in the art,” so-called secondary considerations of non-obviousness (for example, commercial success, long-felt need), the written description requirement, the doctrine of equivalents, the principle of pioneering patents, the presumption of validity, patent misuse, and injunctive relief.17 Often their application, not just the technology, is controversial, but they give the patent system a flexibility that would be lacking if it were necessary to amend the patent law every time a new technology presented itself.
Ensuring High-Quality Patents
In 1790 when Congress enacted the first patent statute it stipulated two substantive requirements—novelty and utility—for an invention or discovery to qualify for a patent. From the outset it was recognized that patents ought not to be granted for any trivial advance in an art, that some more substantial improvement should be shown. In 1851 the Supreme Court distinguished the “work of the skillful mechanic,” not justifying protection, from the “work of the inventor”; but for a century, courts struggled without statutory guidance to define an “invention.” Finally, in 1952, Congress adopted an alternative formulation, excluding from patentable subject matter what “would have been obvious at the time the invention was made to a person having ordinary skill in the art.” Thus, the third substantive requirement for patentability became known as the “non-obviousness” standard.18
The importance of these three conditions in the abstract is uncontested. Patents on known or only trivially modified inventions would confer potential market power to restrict access and raise prices and enable the patent holder to use litigation as a competitive weapon without providing incentives for making genuine advances or disclosing such advances to the public. They offer no public benefit in exchange for the benefit given to the patentee. Granting patents for inventions that are not new or useful or that are obvious unjustly rewards the patent holder at the expense of consumer welfare (Levin and Levin, 2003).
A second theoretical argument against poor patents is that because of doubts about their validity they are likely to encourage more infringement and more litigation, raising the transaction costs of the system and discouraging some investment (Merges, 1999; Meurer, 1989). Poor patents may induce investment
in product development that is abandoned later when the patents are invalidated. Hunt (1999) and O’Donoghue and colleagues (1998) conclude from slightly different models of innovation that raising or lowering the standards of patenting could affect the character of R&D. If the standard is high, firms may be more likely to pursue larger innovations.
Over the past decade the quality of issued patents has come under sharp attack.19 The conjecture that patent quality is declining or is simply too low has been characterized in two ways. First, some legal scholars have suggested that the standards of patentability—especially the non-obviousness standard—have been relaxed as a result of court decisions (Barton, 2000; Dreyfuss, 1989; Lunney, 2001). Other observers have suggested that the USPTO too frequently—or more frequently than in the past—issues patents for inventions that do not conform to generally accepted standards for patentability, especially in technology areas that are newly patentable, notably genomics, software, and business methods (Barton, 2000; Hall, 2003b).20 This alleged decline in USPTO performance is variously attributed to the quantity and quality of relevant resources, examiner qualifications, experience and incentives, the time devoted to searching and evaluating each application, and the information available to examiners (for example, access to automated data bases incorporating prior art). Although logically distinct, the notion that standards for patentability are slipping and the notion that USPTO examiners are failing to apply the legal standards appropriately are obviously difficult to distinguish in practice (Cohen et al., 2002).
There is no lack of examples of issued patents that appear dubious on their face. One such list (Hall, 2003b) includes a patent on a computer algorithm for searching a mathematical textbook table to determine the sine or cosine of an angle,21 a patent for cutting or styling hair using scissors or combs in both hands,22 a patent on storing music on a server and letting users access it by clicking on a list of the music available,23 and a patent on initiating forward motion on a child’s swing by pulling on the ropes and swinging sideways (the last subsequently ordered to be re-examined by the director of the USPTO).24 Whether these are products of the office’s interpretation of court decisions or of internally generated guidance given to examiners or of less than thorough examination of applications
or, indeed, whether some of them could withstand challenges in the courts is an open question. Further, whether the examples are aberrant or typical or, for that matter, increasing or declining in frequency is impossible to determine on the basis of a few handpicked examples of apparently bad results. But a nontrivial number of errors in judgment are inevitable in a system whose output by 3,000 individual examiners is 167,000 patents annually.
In the late 1990s the U.S. Department of Commerce inspector general’s (IG) office investigated the growing backlog of applications awaiting decisions before the USPTO Board of Patent Appeals and Interferences (U.S. Department of Commerce, 1998). The IG reported that board personnel attributed declining production to the poor quality of cases being appealed.
Board personnel whom we interviewed stated that cases they receive from the examining corps often contain administrative errors, inadequate support for the examiner’s final rejection, and other unanswered questions or omitted information about the patent’s claim that should have been addressed. As a result, APJs [administrative patent judges] are spending time searching prior art (technical literature including prior-issued patents and foreign patents, related documents, and non-patent literature such as journal articles and abstracts), a task which is normally examiner responsibility. Board workload data supports their assertions. Reversals of examiner decisions and remands for additional examiner review combined for 41 percent of the board’s total disposals in FY 1994, but 54 percent in FY 1997.25 Furthermore, rejections due to examiners having overlooked prior art have averaged 12 percent of the board’s decisions over the same period. In effect, overall production is cut because APJs are spending more time processing appeals in order to make these determinations.
Nevertheless, the claim that quality has deteriorated in a broad and systematic way has not been empirically tested. Three seemingly direct measures of quality are (1) the ratio of invalid to valid patent determinations in infringement lawsuits, (2) the error rate in USPTO quality assurance reviews of allowed patent applications, and (3) the rate of claim cancellation or amendment or outright patent revocation in re-examination proceedings in the USPTO.26 These indicators show mixed results. The rate of invalidity findings in district (trial) court judgments has declined over time. P. J. Federico (1956), using data for 1925-1954, and Gloria Koenig (1980), using data for 1953-1978, found that before 1982 district courts and circuit courts upheld only about one-third of the patents litigated. At the appeals level the rate increased to about 55 percent with the advent of the Federal Circuit (Dunner et al., 1995), as did the validity rates in the district courts as a whole (Lemley, 2002, using data from 1994; and Allison and
|
25 |
The USPTO Annual Report stated the combined reversal/remand rate was slightly less in FY 1997—51 percent. |
|
26 |
See Appendix A for a description of the re-examination procedure. |
Lemley, 1998, using data from 1989 through 1996).27 Although it may seem surprising that the probability that a patent will hold up under challenge is just over 50 percent, it should not be unexpected. Both parties exercise enormous care in deciding whether to run the risk of litigating a patent dispute rather than abandoning or settling it, the much more frequently exercised options. In most cases, not only is the commercial value high but also the validity issues are finely balanced. Consequently, one should be very cautious in interpreting the results of courts’ validity decisions.
The error rate reported in USPTO quality assessment audits has fluctuated between 3.6 and 7 percent since 1980. There was a slight upward trend through the 1990s until 2000, but it has declined in recent years to around 4 percent. Only about 10 percent of patents subject to re-examination in the United States are completely revoked, although nearly two-thirds undergo some adjustment to their claims, often because the patent holders themselves sought re-examination to modify their claims in light of newly discovered prior art.
All three indicators suffer from serious deficiencies, however. In addition to selection effects, the numbers of patents subject to any of these procedures are extremely small. The litigation rate of issued patents is just over 1 percent (Lanjouw and Schankermann, 2003); re-examined patents represent about 0.3 percent of the total (Graham et al., 2003); and about 2 to 3 percent of a year’s patents are reviewed by the USPTO for quality control purposes.
Ostensibly, the USPTO’s audits come closest to producing a measure of quality and therefore deserve closer examination. The patents reviewed are not randomly chosen to assess overall system performance nor is the selection weighted toward technologies in which examination quality may be problematic. Currently, the protocol is designed to take a specific number of applications from each examiner depending upon examiner experience level and certification status.28 Because of the small percentage of allowed applications that are reviewed, the error rates are statistically significant only at the level of the seven technology centers, not the art units.29
In any case, the history of the USPTO’s quality review function does not inspire confidence that its results are meaningful and consistent over time. Created in 1974 in response to earlier criticisms of patent quality, the Office of Patent Quality Review was twice reviewed harshly by the inspector general of the Department of Commerce. In 1990 the IG faulted the USPTO for failing to reduce error rates by using data from the quality review process. Although rates did decline from Fiscal Year (FY) 1991 to FY 1996, the quality review staff was reduced by one-half, as was the sample rate, from nearly 4 percent of patents to just over 2 percent, too low to provide valid results for any of the art units, according to the IG. Meanwhile, the USPTO management proposed to eliminate the quality review auditing of issued patents and “in process” reviewing as well as to substitute a survey of “customer” (that is, patentees’) satisfaction. A second IG report (1997) criticized both the deterioration in the auditing function and the unreliability of the proposed alternative. The USPTO agreed to reestablish a “strong, independent” Office of Patent Quality Review. As a result of the 21st Century Strategic Plan, quality assurance specialists with principal responsibility for the auditing function have been deployed to the technology centers although they report to the Office of Patent Quality Assurance, reporting in turn to the deputy commissioner for patent operations. Previously, the Office of Patent Quality Review was entirely independent of the patent administration. Whether this shift changes auditors’ incentives remains to be seen. It may facilitate communication with examiners and managers.
Another way to test empirically whether there has been a change in patent quality would be to “peer review” a representative sample of patents in different technical areas from different time periods. A group of experts independent of the USPTO could rate the patents on novelty, utility, obviousness, and quality of the description. That has not been done because it is a substantial undertaking but one worth consideration.
What about indirect measures of quality? In research supported by this project Allison and Tiller (2003) examine prior art references in Internet business method patents, one of the categories of patents whose quality is most suspect. They compare the number of references (that is, backward citations) in their sample to those found in a random sample of all other patents. They find that the business method patents contained substantially more total references and patent and nonpatent references than the patents in the general sample. This finding runs counter to the widely held assumption that the USPTO has consistently overlooked nonpatent prior art in the examination of business method applications. Nevertheless, Allison and Tiller’s data cannot answer several intriguing questions. For example, is the body of nonpatented prior art in the area of business methods so large or diverse that examiners are still missing a good share of it? Does the examination process overlook some business methods that are in common use but not documented in written sources?
There are several reasons to suspect that more issued patents are deviating from previous or at least desirable standards of utility, novelty, and especially non-obviousness and that this problem is more pronounced in fast-moving areas of technology newly subject to patenting than in established, less rapidly changing fields.
Workload Pressures on the USPTO
One reason for suspecting that quality has suffered is that even before taking examiner qualifications and experience into account, the number of patent examiners in recent years has not kept pace with the increase in workload represented not only by the enormous growth in the number of applications (doubling in 10 years, between 1991 and 2001) but also by the growing complexity of applications as represented by the growth in the number of claims and prior art citations per application (Allison and Lemley, 2002).30 The number of examiners per 1,000 patent applications is down about 20 percent over the last four or five years (see Figure 3-1) in part because of congressional reluctance to increase personnel. At the same time, the Congress has for several years appropriated a portion of the fees collected by the USPTO to other governmental activities.
It may be that examiner productivity has improved somewhat with access to scientific and technical literature databases capable of automated search for prior art, but a potentially more important source of productivity gains—automated filing and processing of applications—is only now being implemented on a large scale.
The principal result of holding employment growth well below the growth in applications has been longer pendency, rising from an average of 18.3 months in 1990 to 24 months in 2002. The average time an examiner spends on an application has remained largely unchanged,31 meaning that the volume of work may have been accommodated without serious detriment to examination thoroughness, but there has been no apparent adjustment across all technologies to compensate for the greater complexity of the average application.
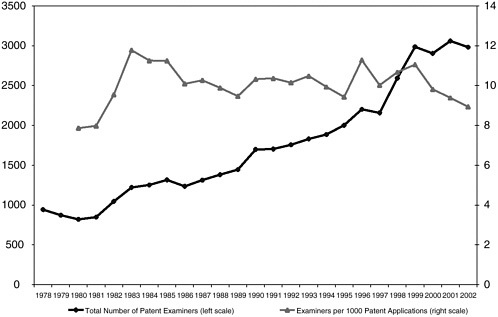
FIGURE 3-1 USPTO examiner workforce. SOURCE: USPTO.
The number and time allocation of examiners says nothing about their training, qualifications, experience, length of tenure, compensation, and performance evaluation criteria. It may well be that thorough examination of these organizational and workforce characteristics—which we were unable to undertake—would reveal other reasons to be concerned about patent quality as well as important ways to improve it.32
Patent Approval Rates
A second reason for concern about changes in quality is that patent approval rates may be significantly higher than officially reported by the USPTO. For a number of years the USPTO has reported that approximately two-thirds of patent applications result in patent grants. In a recent study Quillen and Webster (2001) argued that calculations of allowance rates from USPTO reported numbers of applications filed, abandonments, and total allowances or issued patents have led to a consistent underestimate of actual allowance rates because the calculations did not take into account the effect of U.S. continuation practice. By statute the
United States allows applicants to refile applications to obtain continued examination of the invention claimed in the original application (see Appendix A). Since more than one application claiming a specific invention may be filed before a patent is granted, calculations that do not correct for continuation applications underestimate the allowance rate. Quillen and Webster concluded that once continuation, continuation-in-part, and divisional applications33 are accounted for as renewed attempts to protect the subject matter of their applications, the USPTO eventually issued patents on between 85 percent and 97 percent of applications filed between 1993 and 1998—20 to 30 percent higher than official estimates.
Quillen and Webster noted the possibility that more than one patent could issue from a single disclosure, but because they did not have the data to correct for such occurrences, they based their calculations on the assumption that “parent” patent applications are abandoned when a continuation application is filed. In a follow-up paper Quillen, Webster, and Eichmann (2002) attempted to account for applications that give rise to more than one patent by using a random sample of 1,000 patents developed by John Allison and Mark Lemley to determine the percent of those that were granted on continuations whose parents were also issued as patents. When they incorporated a correction for all continuing applications, including divisionals and continuations-in-part, they calculated an allowance rate of 83 percent.
Last year Robert Clarke (2003) of the USPTO published a review of Quillen and Webster’s original findings along with his own analysis of USPTO allowance rates. Like Quillen and Webster, Clarke subtracted continuation applications from the total applications to derive the number of “original applications,” but he also subtracted from the total pool of patents issued during the relevant time period all patents issued from applications with an ancestor that had also issued into a patent. Clarke’s analysis benefited from additional USPTO data not available to Quillen and Webster. Clarke concluded that the likelihood of a U.S. patent grant from an original application for applications filed during the five-year period from 1994 through 1998 was slightly less than 75 percent. Clarke attempted to validate his calculations by also determining the percentage of applications that go abandoned without being refiled. The percentage of applications that do not issue as patents or give rise to further continuing applications was found to be slightly greater than 25 percent, complementing the percentage of allowed applications determined by counting only those patents that issued from applications that were not continuations of applications that also issued into patents.
The methods of Clarke and those of Quillen and colleagues necessarily rely on certain assumptions, mainly to account for their inability to follow individual
|
33 |
See Appendix A for definitions of these terms. |
applications and application families from original filing to final disposition of all members. For this reason arriving at a consensus on a precise patent approval rate may be elusive. Nevertheless, we can infer from these efforts that the ability to file continuation applications with the USPTO gives applicants a higher probability of obtaining patents on some version of their inventions.
Acceptance rates by themselves ignore how patent claims are modified, nearly always by narrowing their scope, in the course of the examination, surely a key determinant of quality. Moreover, rigor of examination is only one of several factors that may affect allowance rates. The fact is that the examination procedure, allowing an applicant multiple attempts to persuade a critical examiner to approve a patent (see Appendix A), is designed to yield a high “success” rate, at least for persistent applicants.34 The predictability of the standards in a particular technology and the perceived economic value of the patent are some of the factors that affect motivation to pay the costs associated with that iterative process.
The committee believes that high acceptance rates, especially if increasing over time relative to comparable rates in other industrialized countries would be reason to look more closely at examination quality. Under either Quillen and Webster’s or Clarke’s assumptions the USPTO patent approval rate gradually increased in the early 1990s and then declined after 1998 (see Figure 3-2). The European Patent Office (EPO) and Japanese Patent Office (JPO) approval rates peaked at approximately the same time but then declined more rapidly, so that in 2000 the USPTO rate was higher although by a substantial margin only under Quillen and Webster’s assumptions. On the other hand, the Organisation for Economic Co-operation and Development (OECD, 2003) estimates that the USPTO grant rate for U.S. priority applications with at least one subsequent EPO application was consistently higher than the EPO grant rate for U.S. priority applications throughout the 1980s and early 1990s—80 to 90 percent versus 50 to 60 percent.35
These analyses have given the USPTO tools to make more realistic comparisons than the officially reported statistics. These tools should be applied to determine acceptable rates in different technology classes, especially ones newly subject to patenting. If increases in allowance rates are found, other potential causes need to be considered, of course. For example, it is possible that the higher cost of obtaining patents has caused firms to be more rigorous in screening inventions for which they file applications, or that greater predictability in the applications
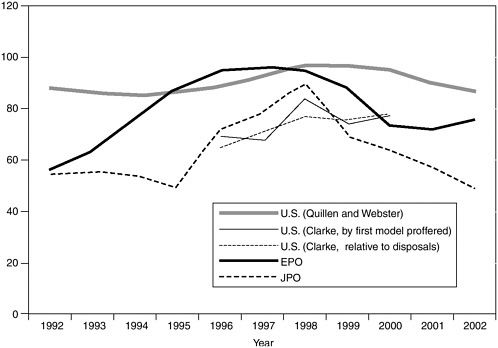
FIGURE 3-2 USPTO, EPO, and JPO patent approval rates. SOURCE: Quillen and Webster (2001) and Clarke (2003).
of patentability criteria by the USPTO means that firms are better at weeding out inventions that will not result in granted patents.36
Changes in Treatment of Genomic and Business Method Inventions
Partially in response to criticisms of the standards being applied to business method and genomic patent applications, the USPTO conducted a broad review of those categories and instituted significant changes in procedures and standards. In March 2000 the Patent Office announced the “Business Methods Patent Initiative” focused on Class 705 (“Data Processing: Financial, business practice, management, or cost/price determination”), encompassing the bulk of the business method applications filed in the wake of the State Street Bank decision and many of the well-known Internet patents including Amazon’s “one-click” shopping
method37 and Open Market’s “on-line shopping cart.”38 The initiative consisted of four steps: (1) improved technical training of Class 705 examiners, (2) revised examination guidelines, (3) mandatory search of specified sources of prior art, and (4) a new “second review” of all allowed applications to ensure compliance with the search guidelines and the appropriateness of allowed claims.
In the following January 2001 the USPTO responded to similar criticisms of the patents being allowed on human genetic sequences by releasing new guidelines clarifying the written description and utility requirements. The guidelines are written to be generic to all technologies, but most affected are claims involving DNA and proteins, and most of the training examples are in biotechnology. The written description guidelines were intended to bring USPTO practice into line with the Federal Circuit’s decision in Regents of the University of California v. Eli Lilly and Co.,39 stating that simply describing a method for isolating a gene or other sequence of DNA is insufficient to show possession, and the complete sequence or other identifying features must be disclosed. The utility guidelines declared that the claimed utility of the invention must be “specific, substantial, and credible” and extend beyond merely describing its biological activity. The guidelines were widely interpreted as raising the bar to patents on genomic inventions.
The new policies reflected a recognition by USPTO management that standards needed to be tightened, at least in two technologies attracting large investments and a great deal of publicity and exhibiting a controversial surge in patenting activity. The question of what practical effect the measures had on examiners’ behavior and USPTO output is difficult to answer. It is complicated by the lag between application filings and patent grants, the downturn in the economy and in technology investments that occurred in 2000, and other nearly simultaneous developments affecting patenting activity in these fields. For example, at the same time that DNA patent applications were accelerating, the international Human Genome Project was rapidly depositing human DNA sequence data in the public domain, where it became prior art. A “working draft” of the genome was published in February 2001.
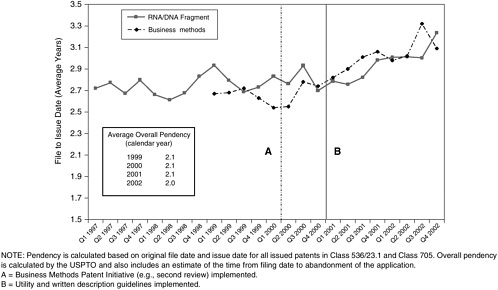
Class 705 patent grants peaked in the last quarter of 1999 and fell sharply in the first quarter of 2000, coincident with the institution of the second review and other measures.40 The decline continued throughout 2000 before leveling off.
Classification 536/23.1 [“DNA or RNA fragments or modified forms thereof (e.g., genes, etc.)”] showed a more modest decline in patent grants beginning in 2001, coincident with the new examination guidelines (see Figure 3-3).
For certain companies known to be patenting large numbers of DNA sequences, however, the decline began at least one year earlier (see Figure 3-4). A full assessment of the effect of the written description and utility guidelines on patents on nucleic acids would require an analysis of the scope of issued claims and the types of nucleic acids claimed (e.g., full-length coding sequences, ESTs, antisense fragments with therapeutic potential) in addition to the numerical
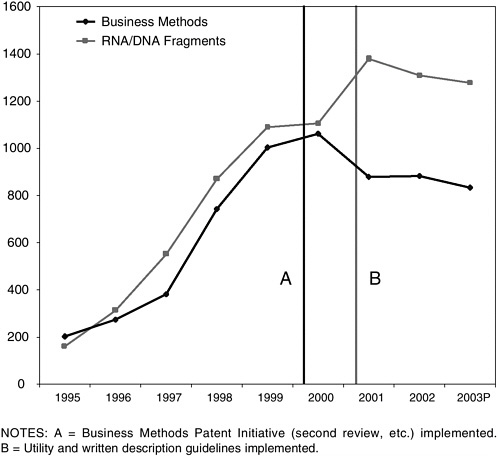
FIGURE 3-3 Business method (USPTO Classification 705) and DNA/RNA fragment (USPTO Classification 536/23.1) patent grants.a SOURCE: USPTO.
aIf data on all patents in the DNA Patent Database, Georgetown University, are used, the same break in the upward trend of patent grants occurs early in 2001. The database is a product of screening several relevant patent classes in USPTO data to yield a set of DNA-related patents.
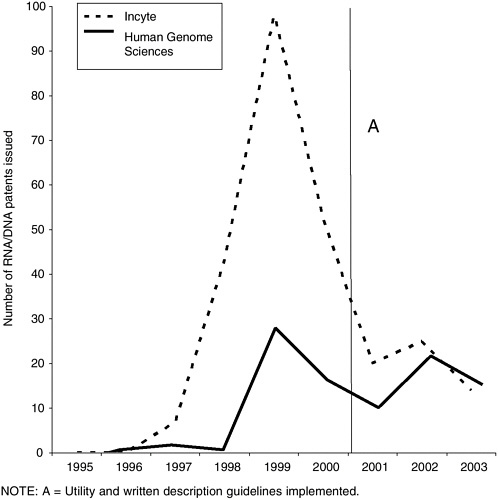
FIGURE 3-4 DNA/RNA fragment patent grants to genomic companies (USPTO Classification 536/23.1). SOURCE: DNA Patent Database, Georgetown University. The database is a product of screening several relevant patent classes in USPTO data to yield a set of DNA-related patents.
analysis shown in Figure 3-3. With respect to DNA patents other factors to be weighed in interpreting patent grants over time is the finite nature of the human genome (an estimated 30,000 genes in all) and the USPTO’s “restriction” practice of forcing patent applicants to separate DNA sequences into different applications. The latter is controversial in the biotechnology industry because it raises the cost of obtaining patents, but by simplifying the task of examiners it is more likely to enhance the quality of the results than to degrade it.
It is clear that in neither case did the high-tech economic collapse play a significant role in the slowdown in patent approvals, at least initially. That is
because the patents in both categories that were issued in 2000 and 2001 derived from applications filed at least two years earlier, at the height of the boom. It is nevertheless conceivable that the principal effect of the new policies in both cases was to make long pendencies even longer. By the end of 2002 applications in both Class 705 and Class 536/23.1 were taking more than three years to yield patents (see Figure 3-5).
Application of the Non-Obviousness Standard
A fourth reason to be concerned about patent quality is that there may have been some dilution of the non-obviousness standard as a result of court decisions and their incorporation in the examination guidance compiled in the USPTO’s Manual of Patent Examining Procedure (MPEP).
Added to the patent statutes in 1952, the standard is stated as follows:41
A patent may not be obtained though the invention is not identically disclosed or described as set forth in section 102 of the title, if the difference between the subject matter sought to be patented and the prior art such that the subject matter as a whole would have been obvious at the time the invention was made to a person having ordinary skill in the art to which the said subject matter pertains.
The enactment of Section 103 was in part a reaction to a line of Supreme Court cases in which patents were held to be invalid because they lacked “invention.” In one case Justice William O. Douglas maintained that for an invention to be patentable it “must reveal the flash of creative genius.”42 Justice Robert H. Jackson in a dissenting opinion complained about this trend in decisions by observing that “the only patent that is valid is one which this Court has not been able to get its hands on.”43
Although it may have been adopted to moderate the antipatent tendency of the Court, Section 103 establishes a level of development beyond not only the documented prior art but also the practice of people of ordinary skill in that art that must be accomplished before a patent can issue. Merges and Duffy (2002) characterize it as the “nontriviality” requirement of the patent law. The Supreme Court did not address the question of how to interpret Section 103 until 14 years after its enactment, when it decided three patent cases frequently referred to as the “Graham trilogy.”44 The Court confirmed the abandonment of the notion of
“invention” as leading to conceptual confusion but said that Section 103 did not and constitutionally could not lower the patentability standard. Indeed, in ruling invalid two of the three patents at issue in the cases, the Court provided the following guidance for evaluating a patent for obviousness:
[T]he scope and content of the prior art are to be determined, differences between the prior art and the claims at issue are to be ascertained; and the level of ordinary skill in the pertinent art resolved. Against this background, the obviousness or non-obviousness of the subject matter is determined. Such secondary considerations as commercial success, long felt but unsolved needs, failures of others, etc. might be utilized to give light to the circumstances surrounding the origin of the subject matter sought to be patented.45
How this blueprint is applied can affect the incentives of both initial and follow-on innovators and the benefits and costs of the patent system. If the required step is too small, the pioneering inventor must share royalties with improvers who might otherwise be excluded. For subsequent inventors the step required affects the choice between seeking ambitious or marginal improvements. Moreover, if the required step is very small, there may result a proliferation of patents that entail costly licensing negotiations and payments and limit firms’ future freedom of action. Patents on trivial inventions may confer or help to sustain significant market power. At the same time, an overly restrictive non-obviousness standard could discourage investment and delay new entrants to a market.
Although not in complete agreement about which aspects of which decisions are responsible, a number of legal scholars view the evolution of the law over the last generation as reducing the size of the step required for patentability under the non-obviousness standard and as allowing the issuance of patents on obvious inventions (Barton, 2003; Desmond, 1993; Kastriner, 1991; Lunney, 2001; Merges, 1999; and Vermont, 2001). Since Graham there have been four cases in which the Supreme Court has considered obviousness decisions by the circuit courts of appeal. In all four cases the Court found obvious patents that the lower court had held valid, although one of the cases was decided on procedural grounds (Barton, 2003).46 The Court, however, has not revisited obviousness for nearly two decades.
A 1995 study of Federal Circuit decisions rendered on cases originating in lower courts shows that the court upheld 86.8 percent of decisions holding valid patents faced with non-obviousness challenges, but upheld only 59.9 percent of
those decisions holding patents invalid on non-obviousness grounds (Dunner et al., 1995).47 Both rates are higher than the appeals court’s overall rate of affirmance in patent cases, which is around 50 percent. With respect to decisions on appeal from the USPTO, the study shows that the Federal Circuit became slightly stricter with respect to non-obviousness and upheld more USPTO rejections during the late 1980s, but then reversed a greater share of USPTO non-obviousness rejections during the early 1990s (Dunner et al., 1995). A more recent study finds a decline in invalidity decisions based on obviousness by the Federal Circuit (Lunney, 2001).48 There is also evidence that the Federal Circuit judges appointed more recently are more likely to uphold a patent against a non-obviousness argument.49 Taking into account trial courts as well as the Federal Circuit, non-obviousness is the leading basis of patent invalidity, providing a basis in 42 percent of invalidity findings, and non-obviousness arguments are accepted 36.3 percent of the time (Allison and Lemley, 1998).
Although the committee considered these analyses, it did not reach a position on their significance with respect to non-obviousness generally. Nevertheless, we are concerned about trends in the application of the obviousness standard to business method and genetic sequence inventions. As the problem in each of these areas is different and the recommended solutions are different, both are addressed in Chapter 4.
Neither USPTO resources in relation to its workload, nor patent approval rates, nor changes in the treatment of genomic and business method inventions and the non-obviousness standard are, separately, conclusive evidence that patent quality is too low or declining. However, together they lead the committee to conclude that there are reasons to be concerned about both the courts’ interpretations of the substantive patent standards, particularly non-obviousness, and the USPTO’s application of the standards in examination. This may be primarily an issue in emerging technologies, where fairly broad patents may be granted early on, and fewer but narrower patents are granted as the field matures, more prior art becomes available, and examiners become more familiar with it. Does this mean that the system automatically adjusts without any need for examiners to be more cautious in issuing patents and the courts more cautious in ruling on validity in a
new technology? That is perhaps the pattern, but the cost of waiting for an evolutionary process to run its course may be too high. As the examples of the Internet technology and biotechnology illustrate, because of the efficiency of U.S. capital markets and the growth of early-stage financing, the stakes become very high very early in the development of new commercial technologies.
Disseminating Technical Information
Disclosure is the quid pro quo for patenting, but patents appear to be a relatively minor means of diffusing technological know-how, possibly less important in the United States than in other countries (Cohen et al., 2000). There are a number of reasons for this, some of them either of little concern or unavoidable. In the United States especially, there is an enormous scientific and technical literature, a tradition of personal communication through technical meetings and conferences, a pattern of interaction between the Academy and industry by means of consultancies, liaison programs, and funding arrangements, and in some geographical regions even a culture that encourages informal exchange of proprietary information between employees of competing firms.50
While alternative means of technological diffusion or, in economists’ terms, channels of spillovers, are exceedingly robust, some features of the legal system make a patent a less than ideal vehicle for communicating technical information in a timely way despite the requirement that it be written to enable a person of ordinary skill in the art to practice the invention. First, a patent is written by an attorney or a patent agent to persuade an examiner to grant and a court to uphold a property right of the desired scope. Beyond the minimum disclosure required by the patent statute, the applicant has no incentive to disclose information that would be useful to a potential competitor. Second, there is a delay of indeterminate length, sometimes quite long, between the characterization of the invention and its disclosure in an issued patent or a published patent application.51 Undoubtedly, however, the ability to file for patent protection permits the early communication of inventions through the other sources noted above long in advance of the corresponding patent’s publication.
There are nevertheless some features peculiar to the U.S. patent system that concern the committee. The first has to do with the publication of patent applications. As part of the international Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement concluded in 1994 the United States acceded to the
general practice elsewhere of publishing patent applications after 18 months.52 This was recognition that large numbers of U.S. applications became public anyway as a result of foreign filing. But along with the 20-year patent term from first application, it also was intended to foil the practice of “submarine” patenting, whereby an applicant could continue prosecuting a patent in secret indefinitely until it was worth having a patent issued to sue an unsuspecting infringer. Publication has the added benefit of making the technical information available earlier, sometimes considerably earlier, than would otherwise be the case. For applications that never result in patents, publication makes available information that might not otherwise be disclosed at all.
Congress, in implementing the agreement, responded to complaints of some independent inventors that early disclosure of their inventions would expose them to predatory behavior by large companies. The legislation left applicants an option to maintain the secrecy of their applications if they declared that they did not intend to seek protection in any country other than the United States. It may be that many of the applications withheld pertain to marginal inventions not seen to be worth patenting abroad, but by sheer numbers of applications, the exclusion is not insignificant. Overall, the withholding rate was just over 11 percent in fiscal year 2002, up slightly from the previous year (see Table 3-1). However, in computer architecture and software, not a patenting domain dominated by small entities, the opt-out rate was 18.2 percent. Biotechnology and chemicals and materials had the lowest, but not negligible, rates of withholding applications from publication. This discrepancy is not surprising. More patent applications are being kept confidential in fields with the shortest product cycles.53
A second unusual feature of the U.S. legal system that may undermine the utility of patents as sources of technical information is the doctrine of willful infringement. Awareness of a patent subjects an accused infringer to the possibility of having to pay triple the amount of damages awarded by a jury finding infringement. Although the committee has no basis for assessing how prevalent the concern is, in the course of our deliberations a number of corporate presenters, particularly in the information technology sector, claimed that this liability is a substantial disincentive to consulting the patent literature.
TABLE 3-1 U.S. Patent Applications Withheld from Publication, FY 2001 and 2002
|
|
Total Perfecteda Applications |
Applications Requesting Nonpublication |
Nonpublication Percentage |
|
FY 2001 |
|||
|
Totals |
145,578 |
14,432 |
9.9 |
|
FY 2002 |
|||
|
Biotechnology and organic chemistry |
28,718 |
1,722 |
6.0 |
|
Chemical and materials engineering |
36,482 |
2,470 |
6.8 |
|
Computer architecture and software |
27,786 |
5,064 |
18.2 |
|
Communications |
35,513 |
4,521 |
12.7 |
|
Semiconductor, electrical, optical systems and components |
61,367 |
5,880 |
9.6 |
|
Transportation, construction, commerce, agriculture |
36,041 |
5,177 |
14.4 |
|
Mechanical engineering, manufacturing, products, and designs |
42,197 |
4,949 |
11.7 |
|
Other |
11 |
0 |
|
|
Totals |
268,115 |
29,783 |
11.1 |
|
aPerfected utility and plant applications filed on November 29, 2000, through September 30, 2002. SOURCE: USPTO. |
|||
Ensuring the Timeliness and Containing the Costs of Decisions
Innovation frequently entails high risk and expense. Patents may help induce the investment by providing the patentee with a means of minimizing one source of risk, free use by others of innovation. But if decisions about whether a patent will be allowed or upheld in a dispute, are long delayed, or if the costs associated with those decisions are very high, that alone may tip the balance against investing in an innovation.
Patent pendency, or the elapsed time between the filing of an application and its abandonment or the issuance of a patent, is often cited as the sole measure of USPTO management efficiency. That is misleading. As described and illustrated by the figure in the accompanying “Patent Primer” (see Appendix A), applicants have substantial although not complete control over how long it takes to process a patent application, and they sometimes endeavor to draw the process out even though, for patent applications filed after 1995, delay reduces the lifetime of the eventual patent. A better measure of USPTO performance is the interval between the filing of an application to the office’s first response, known as the “First Office Action (FOA),” commonly accepting some claims but denying others. Like average pendency (in 2001, 24.7 months), the time to FOA (14.4 months in
2001) has been slowly increasing, as one would expect where a slowly expanding, or in some technologies, shrinking workforce is coping with a mushrooming workload.
As is often the case, however, averages conceal trends of greater concern. The averages are being held down by processing times for patents in relatively mature technologies, while the most rapidly advancing fields, where the current state of the art is likely to be surpassed in a matter of months, are experiencing lengthening pendency. Applications covering DNA and RNA segments were on average taking well over three years to process by the end of 2002, up by more than six months in three years. Average pendency for Internet business method applicants increased more than eight months in a similar period of time (see Figure 3-5). And the pattern is not confined to technologies where the USPTO, under criticism, took announced steps to be more conservative in its screening of applications.
In the committee’s view it takes an inordinately long time to resolve questions of patent validity, whether administratively or in the courts. For patent re-examinations initiated by third-party challengers in the USPTO,54 the median length of time between the date of application and the final outcome is 7.54 years (Graham et al., 2003).55 (See Table 3-2.)
There is a longer time lag for settling patent validity challenges through the courts. For a population of cases litigated between 1989 and 1996, Allison and Lemley (1998) found that the average period between the filing of a patent application and a final ruling on the patent’s validity was 12.26 years; the average time between the issuance of the patent and resolution of its validity was 8.61 years.56 (See Table 3-3.)
In these respects the U.S. patent system performs no worse and in some cases better than its European and Japanese counterparts. Average pendency periods were an astonishing 21.4 months shorter in the United States than in the European Patent Office in 2001 and 3 months shorter than in the Japanese Patent Office. The average times to first office action were 14.4 months in the USPTO, 20.7
|
54 |
The types of re-examinations are described in Chapter 3 and in Appendix A. |
|
55 |
This estimate is for cases involving patents filed before 1991 to minimize the effects of lag truncation. |
|
56 |
This length of time may be influenced by the fact that under 35 U.S.C. 286, damages for patent infringement may be accrued for six years after patent issuance before a suit is filed. Allison and Lemley observe that most patents litigated to judgment involve fairly old technologies on which the patents have existed for some time before they are challenged or enforced. They infer that many firms patent with no immediate intention of enforcing their rights but rather to fence out potential competitors. An alternative explanation is that the rapid developmental pace of some technologies militates against investing the time and resources in lengthy and expensive patent litigation. |
TABLE 3-2 Lags in Years Between Patent Application, Grant, Challenge, and Final Outcomes of USPTO Patent Re-examinations Initiated by Third Parties
TABLE 3-3 Lags in Years Between Patent Application, Grant, and Resolution of Validity Challenges in U.S. Litigation
|
Lag Between Patent Application Filing and Resolution |
|||
|
|
All |
Valid |
Invalid |
|
Mean |
12.26 |
12.14 |
12.36 |
|
Median |
11.3 |
11.05 |
11.5 |
|
Standard Deviation |
5.68 |
5.29 |
6.12 |
|
Lag Between Patent Issuance and Resolution |
|||
|
|
All |
Valid |
Invalid |
|
Mean |
8.61 |
8.69 |
8.49 |
|
Median |
7.8 |
8.0 |
7.5 |
|
Standard Deviation |
5.08 |
5.02 |
5.16 |
|
Pendency of Application (time in prosecution) |
|||
|
|
All |
Valid |
Invalid |
|
Mean |
3.64 |
3.45 |
3.87 |
|
Median |
2.7 |
2.65 |
2.75 |
|
Standard Deviation |
2.98 |
2.56 |
3.39 |
|
SOURCE: Allison and Lemley (1998). |
|||
months in the EPO, and 22 months in the JPO (Japan Patent Office et al., 2001). U.S. patent re-examinations take less time to resolve than do challenges in the European patent opposition procedure, given that the window to request an opposition is open for only nine months after a patent issues while a U.S. re-examination may be requested at any time in the life of the patent (Graham et al., 2003).
Application filing fees and fees to maintain patents in force are also lower in the United States than in Japan. It is the cost of legal counsel that puts transaction costs in the United States far beyond the range of those in other industrial countries, and they are rising at a rate much in excess of inflation. The American Intellectual Property Law Association (AIPLA), from its biannual survey of practitioners, estimates that processing a relatively simple U.S.-origin patent application that progresses through examination without amendment or negotiation costs the applicant at least $7,500 in administrative and legal fees in 2002.57 A complex biotechnology or computer patent subject to multiple amendments could cost tens of thousands of dollars. Albeit with only three data points, 1998, 2000, and 2002,58 the association estimates that the cost is increasing at an annual rate of 6 to 12 percent (AIPLA, 2003). Estimated costs of various steps in prosecution of different types of patents, compared over five years, are shown in Table 3-4.
The costs of patent conflicts, which almost invariably combine issues of infringement and patent validity, have also increased rapidly, especially for complex lawsuits involving very high stakes, according the AIPLA survey (see Table 3-5).
The median costs to each party of proceeding through a patent infringement suit to a trial verdict are at least $500,000 when the stakes are relatively modest. When more than $25 million is at risk in a patent suit, the median litigation costs for the plaintiff and the defendant average $4 million each, and in the highest stakes, patent suit costs can exceed this amount by more than fivefold. Since relatively few infringement disputes reach trial, almost certainly the more significant transaction costs are the time and attention business managers and counsel spend considering raising a patent challenge, evaluating and responding to others’ challenges, devising and carrying out negotiation strategies, and arriving at and implementing settlements.
What is clear is that the burden of costs and uncertainties entailed in challenging and defending patents falls disproportionately on smaller, less experienced
TABLE 3-4 Increase in the Cost of Prosecuting Patent Applications
|
U.S. Utility Patents |
1998 |
2000 |
2002 |
Percent Change, 1998-2002 |
|
Novelty search |
$ 999 |
$1,250 |
$ 1,500 |
50.2 |
|
Original nonprovisional application on invention of minimal complexity |
$4,008 |
$5,002 |
$ 5,504 |
37.3 |
|
Provisional application |
$2,000 |
$2,501 |
$ 2,993 |
49.7 |
|
Original application, relatively complex biotechnology or chemical |
$8,000 |
$9,967 |
$10,001 |
25.0 |
|
Original application, relatively complex electrical or computer |
$7,993 |
$9,970 |
$ 9,995 |
25.0 |
|
Original application, relatively complex mechanical |
$6,007 |
$7,996 |
$ 8,001 |
33.2 |
|
Application amendment or argument of minimal complexity |
$1,000 |
$1,200 |
$ 1,499 |
49.9 |
|
Application amendment or argument, relatively complex biotechnology or chemical |
$1,999 |
$2,499 |
$ 2,806 |
40.4 |
|
Application amendment or argument, relatively complex electrical or computer |
$1,995 |
$2,497 |
$ 2,501 |
25.4 |
|
Application amendment or argument, relatively complex mechanical |
$1,503 |
$1,999 |
$ 2,199 |
46.3 |
|
Issuing an allowed application |
$ 302 |
$ 400 |
$ 499 |
65.2 |
|
SOURCE: AIPLA (2003). |
||||
firms. For example, Lanjouw and Schankerman (2003), in a paper prepared for this project, find large economies of scale in resolving patent disputes. Having a large patent portfolio significantly reduces the probability of filing a suit on any individual patent, conditional upon its observed characteristics. For a small domestic company with a portfolio of 100 patents, the average probability of litigating a given patent is 2 percent. For a larger company with 500 patents, the probability drops to 0.5 percent, a quarter of the rate for smaller firms. The disadvantage borne by individuals and small firms extends to settlement of patent suits out of court. Large firms with substantial portfolios more readily and more quickly settle their infringement disputes. Cohen and colleagues (2000) also find that research and development managers in large firms report patents to be more
TABLE 3-5 Estimated Median Litigation Costs for Each Party of Litigation (thousands of dollars)
|
|
2001 |
2003 |
Percent Change, 2001 to 2003 |
|
Less than $1 million at risk |
|||
|
End of discovery |
$250 |
$290 |
16.0 |
|
Inclusive of discovery, motions, pretrial, trial, post-trial, and appeal |
$499 |
$500 |
0.2 |
|
$1-$25 million at risk |
|||
|
End of discovery |
$797 |
$1,001 |
25.6 |
|
Inclusive of discovery, motions, pretrial, trial, post-trial, and appeal |
$1,499 |
$2,000 |
33.4 |
|
More than $25 million at risk |
|||
|
End of discovery |
$1,508 |
$2,500 |
65.8 |
|
Inclusive of discovery, motions, pretrial, trial, post-trial, and appeal |
$2,992 |
$3,995 |
33.5 |
|
SOURCE: AIPLA (2003). |
|||
effective in protecting the competitive advantage derived from their innovations than do small firms’ respondents; and outside the pharmaceutical industry, small firms disproportionately report that the expected cost of defending patents dissuade them from patenting altogether.
Accessing Technologies for Research and Development
In a variety of contexts the feasibility and terms of access to patented technology, usually by means of licenses, are crucial to further research, technology development, commercialization, and diffusion of new technologies, for example,
-
cross-licenses on the myriad elements in semiconductor devices, without which multi-billion dollar investments in fabrication operations would not occur or could be held hostage;
-
pooled licenses to technologies underlying technical standards permitting interoperability of electronic and communications equipment;
-
licenses to multifunctional research tools that are crucial to progress in biomedical research.
Concerns about access to patented technology, whether from the perspective of innovation or competition, tend to be quite specific to industries and firms. We
would have a better general understanding of how markets for technology arise, how they work, under what circumstances impediments to innovation arise, and how they could be reduced if we had data on patent-related licenses, but so far, disclosure and data collection are very limited.59 Evidence has for the most part been limited to anecdotes, case studies, and occasional court cases.
In all of the panel’s deliberations there was only one area—biotechnology research and development, primarily where applied to human health—where it was repeatedly suggested that there might be a significant problem of access to patented technology. This is obviously a field of great public interest. It is also a priority of the scientific community, medical products industries, and clinicians to sustain the remarkable productivity of biomedical research and to achieve its promise to yield highly beneficial and lucrative therapeutic and diagnostic products. The role of intellectual property in promoting and perhaps in some instances impeding this progress has already been the subject of a National Academies’ public workshop (NRC, 1997) and an aspect of several studies (Institute of Medicine, 2003; Institute of Medicine, forthcoming), and it has received attention from many other organizations (Nuffield Council on Bioethics, 2002; United Kingdom Royal Society, 2003; and Korn and Heinig, 2002).
As we described in Chapter 2, three concerns have been articulated. The first concern, stated in general terms by Merges and Nelson (1990) and Scotchmer (1991) over a decade ago, is that patents on upstream discoveries, if sufficiently broad in scope, can impede follow-on research and development if access to the foundational intellectual property is restricted. The second concern is specific to biotechnology. In a 1998 Science article, attorney Michael Heller and legal scholar Rebecca Eisenberg hypothesized the emergence of what they termed an “anticommons” in biotechnology, which could result if assembling the rights to use the numerous separate patented building blocks necessary to pursue a particular line of research or product development proved to be prohibitively costly and time consuming or simply impossible, causing a promising prospect to be avoided or abandoned. The authors speculated that the diversity of players with different objectives and commercial experience—university administrations, research faculty, biotechnology research firms, large pharmaceutical companies, and government laboratories—increased the likelihood that gridlock would occur. Some might overvalue their upstream research tool inventions from the perspective of downstream product developers faced with the enormous costs of bringing medical products to market. Others might insist on conditions (for example, reach-through rights, downstream royalties) unacceptable to potential licensees. The third concern is specific to university and other nonprofit sector research per
formers. It is that they could be more adversely affected by the potentially high cost of competing in this arena.60
Faced with these conjectures and a few anecdotes, the committee decided to take the unusual step of initiating a modest interview-based survey of firms, intellectual property practitioners, researchers, and government personnel to derive the first empirical data on whether any of these conditions is occurring or emerging. Drawing upon approximately 70 interviews with people in all of these categories, Walsh and colleagues (2003) found that the preconditions for these results appear to exist. More than in the past, therapeutic products tend to be associated with multiple patents; and public research institutions, the locus of many upstream discoveries, are patenting and licensing more aggressively. With important caveats, however, the authors do not find that these developments are yet impeding research and drug development in a significant way. This is in part because the number of patents required for most R&D projects remains manageable and in part because the various players have improvised arrangements or followed norms that mitigate the intellectual property complexities that exist.
What the authors term “working solutions” include, as one would expect, negotiated licenses and royalty payments.61 Patents are also circumvented by inventing around them, using substitute research tools, and locating research activity offshore. Institutional responses include the National Institutes of Health guidelines encouraging research grantees to facilitate access to patented research tools and the steps taken by several research organizations to place results in the public domain, where they become patent-defeating prior art.
According to many university and corporate respondents to the survey, one of the most pervasive working solutions is infringement of patents, especially on tools of precommercial laboratory research, in some cases on the presumption that research is legally shielded from infringement liability by a “research exception,” and in other cases on the assumption that patent holders will not sue over research uses. In particular, there is a widely held belief that private firms will not sue university investigators over patent infringement because there is little to be gained financially and a high risk of adverse publicity.
The first caveat concerns access to patented research tools that are keys to progress in one or more broad therapeutic areas and “rival-in-use,” that is, they are tools that are primarily used to develop innovations that will compete with one another in the marketplace. Holders of intellectual property on nonrival
research tools tend to charge prices that permit broad access, and frequently charge lower prices to university researchers who intend to use the tools for noncommercial purposes. But when tools are rival-in-use, it is in the interest of owners either to exploit the technology themselves or grant exclusive licenses. The concern here is that when such tools are important inputs into the discovery and development of commercial therapies, and there exists uncertainty about the best way to pursue a given application—no less a range of applications—no one firm’s efforts at downstream development are likely to realize the full potential of the tool. This is because no one firm is likely to see or be able to develop all the different ways that the discovery might be exploited.
One example of exclusive access to a foundational discovery that has raised concern—where it has been argued that broad rather than exclusive access to the discovery would better serve society’s interests—is Geron’s exclusive rights to pursue human embryonic stem cell research for three cell types. Other cases of rival-in-use patented technologies that are potentially important inputs into the discovery and development of therapies where exclusive use or licensing has raised similar concerns are described in the sidebar (see Box 3-1). We are not suggesting that these cases represent inappropriate exploitation of the technologies involved. The cost and risk of the technologies’ development would need to be considered. But they do illustrate the kinds of access issues that arise.
Although there may be only a few identified controversial instances where restricted access may potentially impede subsequent discovery and development, the consequences for research and medicine of even a rare such occurrence could be large. On the other hand, neither is it clear that less exclusive, low-cost access would on balance serve society’s interests if such access dampened the incentive to develop the research tools from the outset. At this point we can say that concern about access to them is not misplaced.
A second caveat relates to university researchers’ use in clinical research of diagnostic tests involving patented technologies. Merz, Cho, and their colleagues (2002) have conducted several studies of the impact on clinical laboratories of royalty rates on patented tests, infringement claims, and refusal to license some tests at all. One study found that 25 percent of laboratory physicians reported abandoning a clinical test because of patents. They also reported royalty rates ranging from 9 percent for polymerase chain reaction to 75 percent for the human chorionic gonadotropin patent. A number of laboratories ceased using the genetic test for hemochromatosis once the patent issued and it was exclusively licensed to SmithKline Beecham. Here, too, the issue is not straightforward because clinical laboratories charge patients or their insurers for conducting diagnostic tests, earning revenue that distinguishes the provision of clinical services from noncommercial research. Further, there has been no evidence that patients lacked access to these tests.
The third important caveat is that one of the most prevalent “working solutions”—knowing or unknowing infringement often done or condoned in the belief
|
BOX 3-1 NF-kB (NF-kappa B) Laboratories at Harvard College, the Massachusetts Institute of Technology, and the Whitehead Institute for Biomedical Research discovered a cell-signaling pathway called NF-kB in the 1980s and were awarded a patent (U.S. Patent No. 6,410,516) in 2002 that may cover almost every clinical application of this fundamental signaling pathway. The patent was exclusively licensed to Ariad Pharmaceuticals. Although investigators “at academic and not-for-profit institutions conduct[ing] non-commercial research” may continue working with the technology without a license, according to Ariad, commercial entities must obtain a license. Ariad has sold international nonexclusive sub-licenses to Bristol-Meyers Squibb and DiscoveRx Corporation. In addition to one-time and annual license fees, these licenses also include milestone and royalty payments on products based upon the NF-kB pathway. Furthermore, corporations using products sold by licensed companies may also need to obtain additional licenses from Ariad itself. In 2002 Ariad and the three research institutions sued Eli Lilly, arguing that Lilly’s Evista and Xigris products for osteoporosis and sepsis, respectively, infringe upon their patents since the drugs work via the NF-kB pathway. In support of its lawsuit, Ariad cited several peer-reviewed papers written by Lilly scientists. On May 13, 2003, the U.S. District Court for the District of Massachusetts denied Lilly’s motions to dismiss and for summary judgment. Ariad has approached some 50 other companies for royalty payments on current or future products that function via the NF-kB pathway (Rai and Eisenberg, 2003). COX-2 Enzyme The University of Rochester patented the COX-2 enzyme (U.S. Patent No. 6,048,850), claiming all drugs that inhibit the enzyme and routes for administering such drugs. The university sued Searle/Pharmacia for patent infringement. The U.S. District Court for Western New York dismissed the university’s complaint on the grounds that the discovery in the patent was invalid for lack of “written description” and therefore could not support an infringement claim (University of Rochester v. G.D. Searle & Co., Inc., W.D.N.Y., March 5, 2003). The Court of Appeals for the Federal Circuit affirmed that the patent was invalid (University of Rochester v. G.D. Searle and Co., Inc., 358 F.3d 916, available at 2004 U.S. App. LEXIS 2458, 69 U.S P.Q.2d 1886 (BNA) (Fed. Cir. February 13, 2004)). |
|
CD34 Johns Hopkins University was awarded a patent claiming all antibodies recognizing CD34, an antigen found on stem cells but not on more differentiated cells. The patentee awarded an exclusive license to Baxter. A rival firm, CellPro, combined two discoveries, one a method for using selectively binding antibodies to enrich bone marrow stem cells and the other an antibody that binds to CD34 (although in a different class of antibodies and recognizing a different binding site on CD34) to produce a cell separator instrument for use in cancer therapies. CellPro declined Baxter’s offer of a $750,000/16 percent royalty nonexclusive license, while other firms accepted these licensing terms. CellPro instead chose to sue to invalidate the patent. CellPro ultimately lost the case, was ordered to pay damages (including willfulness damages, because it was found to have lacked a good faith belief the patent was invalid) and legal fees, and went bankrupt (Bar-Shalom and Cook-Deegan, 2002). OncoMouse Harvard University patented a mouse containing a recombinant activated oncogene sequence that permitted it to be employed as a model system for studying cancer and permitting early-stage testing of potential anticancer drugs. The invention was licensed exclusively to DuPont. After years of negotiations, the National Institutes of Health and DuPont signed a memorandum of understanding (MOU) permitting, among other things, relatively unencumbered distribution of the technology among academic institutions, although under specific conditions. Recently DuPont imposed new conditions on academic licensees (for example, barring use of the technology in industry-sponsored research without taking a commercial license) and began asserting its patent against research institutions that have not accepted the new conditions (A. Neighbour. Presentation to the National Cancer Policy Board, Institute of Medicine, April 23, 2002). Embryonic Stem Cells The University of Wisconsin received a broad patent on its embryonic stem cell discovery in 1998. Its affiliate, the Wisconsin Alumni Research Foundation (WARF), licensed the technology exclusively to Geron, Inc., to develop the cells into six tissue types that might be used to treat diseases and gave Geron options to acquire rights to other issue types. When Geron sought to extend its rights to 12 other tissue types, WARF sued the company in order to offer licenses to other firms. Geron and |
|
WARF reached a settlement in January 2002, narrowing Geron’s exclusive rights to three cell types, removing its option to acquire other exclusive rights, and granting rights free of charge to academic and government scientists for noncommercial research (Stolberg, 2001; Pollack, 2002). BRCA1 Myriad patented a test for the gene, BRCA1, linked to breast cancer. It allows licensees to perform the tests provided that no fees are charged and the tests are not used for clinical purposes. It also provides reduced-fee tests ($1,200 versus $2,680) for use in NIH-funded projects. Nevertheless, the firm takes the position that giving test results to patients crosses the line from a research test to a clinical test even if other conditions of the license are observed (Blanton, 2002). |
that the research in question was shielded from liability—appears to have been undercut by a decision of the Federal Circuit Court of Appeals,62 handed down in October 2002 after our survey was completed. Ruling on a claim of a common law research exemption from patent infringement liability, the court in a case brought against Duke University agreed that research “solely for amusement, to satisfy idle curiosity, or for strictly philosophical inquiry” is protected; but it held that the protection does not extend to organized scientific research activity pursued as part of the legitimate business of an institution, whether nonprofit or for-profit. The “business” of a university, according to the opinion, is research, education, and reputation enhancement. A few months later the Supreme Court declined to hear Duke University’s appeal, allowing the decision to stand. The case involved circumstances very different from those arousing concern in the research community. The plaintiff is a former Duke faculty member, the field is laser research, and the patented technology is laboratory equipment. Nevertheless, the holding is in no way confined to those facts.
It is difficult to anticipate the effects of this decision. An informal poll of research institutions reported to a September 30, 2002, meeting organized by the Association of American Universities, American Association of Medical Col
leges, Council on Government Relations, and National Association of State Universities and Land Grant Colleges revealed that a number of institutions were receiving more notification letters with respect to patent infringement in the aftermath of the decision.63 University administrators and legal counsel are uncertain what precautions to take to avoid infringement. An increase in full-fledged litigation against research institutions may be unlikely, but it is clear that investigators and their institutions must now pay closer attention to the intellectual property issues involved in their work, with an attendant increase in its cost.
Reducing Redundancies and Inconsistencies Among National Patent Systems
Although significant international progress has been made on standardizing the length of patent terms, establishing common rules for the publication of patent applications, and reconciling other national differences, important differences in standards and procedures remain among the U.S., European, and Japanese patent systems, ensuring a burdensome redundancy that imposes high costs on users and hampers market integration. With respect to any economically important invention, at least three sets of examiners analyze essentially the same application and search more or less the same prior art.64 This drives up the costs of obtaining and maintaining worldwide patent protection to a level that can be afforded only by the largest multinational corporations. It is estimated to cost as much as $750,000 to $1 million to obtain comprehensive worldwide patent protection for an important invention, and that figure is increasing at a rate of 10 percent per year (Mossinghoff and Kuo, 1998). Equally important, duplication of effort also impacts all three governments, which are coping with the surge in patent applications with at best slowly growing and at worst reduced resources.
Impeding full reciprocity or mutual recognition, let alone uniform enforcement of patent rights, are a host of subtle and overt differences in approach, procedures, and standards, some of them technology-specific, many of them subject to ongoing negotiations in the World Intellectual Property Organization
|
63 |
The organizations have arranged with the American Association for the Advancement of Science to continue to monitor universities’ experience in this regard. |
|
64 |
The Patent Cooperation Treaty (PCT), implemented in 1978 under the World Intellectual Property Organization (see description in Appendix A), has created a division of labor chiefly between the industrial countries and nonindustrial countries with limited or no patent examination capabilities by providing for USPTO or EPO advisory searches and, at an applicant’s option, examinations that are frequently accepted by developing countries. Such searches and examinations are available among the trilateral patent offices but are often repeated or duplicated, since applicants frequently file simultaneously under the PCT and in national offices in major markets. The PCT offers applicants an efficient means of filing applications in multiple countries. |
(WIPO).65 Among the principal substantive differences in patent law are the following:
-
Priority of invention. As between two true inventors—as contrasted with copiers—every nation in the world except the United States provides patents to the inventors who first undertake to use the patent system to disclose their inventions and gain protection.66 This is conventionally known as the first-inventor-to-file system of priority. The United States provides a patent to the first person to “conceive” and/or “reduce the invention to practice” (first-to-invent system). The latter gives rise to a number of priority disputes, known as “interferences,” over the timing and identity of invention that are difficult to adjudicate, whether administratively in the patent office or in the courts. The U.S. system nevertheless has strong adherents among individual inventors and small companies.
-
Best mode requirement. U.S. law requires that a patent application disclose the “best mode” of implementing an invention to prevent the applicant from concealing the invention’s significance by describing a trivial or remotely related application. No other country has such a requirement. The best mode requirement is frequently raised as a defense in patent infringement litigation. In other words, an accused infringer asserts that the patent should be invalidated because of the patent owner’s failure to disclose the best mode. Judicial inquiries into best mode require access to inventor records and testimony that are often inconclusive.
-
Grace period. Under U.S. law inventors can disclose their inventions publicly or commercialize them before filing patent applications as long as the applications are filed within one year. The grace period encourages early disclosure, for example, of research results in scientific publications or conferences, or commercialization of an invention without causing inventors to forfeit their rights to protection. Japan has a more limited grace period in time and scope; Europe provides none.
Maintaining a Level Field Among Rights Holders
Uniform application of the patent law’s rights and obligations was not questioned until the U.S. Supreme Court, in June 1999, struck down a federal law that had denied a state from claiming immunity under the Eleventh Amendment of the Constitution when sued in federal court for patent or other federal intellectual property infringement. In Florida Prepaid Postsecondary Education Expense Board v. College Savings Bank67 the Court said that Congress had not shown
such a pattern of state agency infringement or an absence of state remedies that would have justified a removal of immunity. As a result of the decision a public university could be in the position of asserting its patent rights against an alleged infringer while successfully barring a patent holder from recovering damages for its own past infringement. College Savings Bank does not prevent the patentee from enjoining future use of the patented invention. Still, the partial immunity is not available to a private party, including other universities in the same state. As a result, it could have distorting effects. For example, if investigators in a state institution used a patented research tool one time without license to find a profitable pharmaceutical product, the patentee could sue for an injunction to bar future use of the tool, but it would be pointless. Sovereign immunity prevents the patent holder from suing for past damages, even if they turned out to be substantial.
Like many other issues arising from recent policy changes, it is not clear how serious a problem the disparity represents. It is not enormous. A state could not set up a systematic program of infringement, for example, to produce low-cost prescription drugs for its Medicaid patients. It could be enjoined in federal court and also sued in a state court for an unconstitutional taking of property. Furthermore, if states began to infringe patents systematically, Congress would have the factual predicate the Supreme Court said was necessary to support a waiver of immunity in federal court. Nevertheless, the committee believes the disparity created by the decisions is not negligible. It puts the United States in the position of being out of compliance with the TRIPS agreement, which provides no exceptions for subunits of government. Further, it may over time affect the choices private firms make in supporting research at public or private institutions.
In an analysis for the Senate Judiciary Committee the U.S. General Accounting Office (GAO) reported in 2001 that before the Supreme Court’s decision, state entities were rarely sued in federal court for patent or copyright violations; there had been 58 cases during a 15-year period, less than 0.05 percent of the total number of cases in federal district courts. On the other hand, two-thirds of state universities responded to the GAO that they had received accusations of infringement, usually in the form of cease-and-desist letters, during the same period. Seven of nine institutions responding reported receiving 11-15 complaints, and one institution reported receiving more than 16 complaints. Almost certainly, the number of complaints of university infringement and conceivably the number of lawsuits will increase in the aftermath of the Madey v. Duke ruling that universities in general may not claim a research exemption defense under common law. On the one hand, private university administrations may conclude that they need to make a much more vigorous effort, which could be burdensome for researchers, to guard against infringement suits than do public university administrators. On the other hand, there may develop a perception that private institutions are more reliable partners in collaborative activities with industrial companies.
SUMMARY
The committee concludes that the U.S. patent system, while functioning reasonably well in many respects, most importantly in its rapid accommodation to technological changes and its flexibility in dealing with differences between technologies, is exhibiting a number of characteristics requiring attention and improvement.
-
Although it is not clear that the quality of most patents has declined significantly, there are reasons to be concerned about whether many patents in leading-edge technologies that are drawing substantial investments represent desirable degrees of novelty, utility, and non-obviousness. This appears to be a function both of pressures on the examination process and of interpretations of some patent standards.
-
There are remediable features of the U.S. patent system that undermine its function in disseminating technical information.
-
Delays and costs entailed in resolving questions of patentability, the validity of issued patents, and infringement, although in some respects comparing favorably to those in Europe and Japan, excessively compound the uncertainty surrounding innovation.
-
Difficulties accessing the patented technology necessary to sustain the progress of biomedical research and therapeutic product development have in some cases raised the cost and modified the character of research and in a very few instances have become a serious obstacle. This may become a more significant problem with the greater complexity of research and proliferation of patents on technologies well upstream of commercial products, and in the aftermath of a recent federal appeals court decision denying fundamental research protection from patent infringement liability.
-
Although progress has been made in harmonizing national patent systems, substantial differences in procedures, standards, and substantive law remain and impede achieving reciprocity or mutual recognition of patent search and examination results among the United States, Europe, and Japan.
-
In interpreting the Eleventh Amendment to the Constitution, the United States Supreme Court recently raised a troublesome disparity between state and private institutions with respect to their obligations under federal intellectual property law.