7
Framework, Findings, and Recommendations
BACKGROUND
Genetic engineering is one of many genetic modification techniques that can be used to generate foods of altered composition, including novel components. The application of recombinant DNA technology allows a unique opportunity to introduce new genes into plants and animals used for food. However, the products of this technology are not always distinguishable from other methods of genetic modification. Moreover, application of any technique to produce altered levels of or novel food components can result in unintended compositional changes that may in turn result in an adverse health effect.
The safety assessment methods recommended in this chapter for genetically modified (GM) foods—including those that are genetically engineered (GE)—are intended to identify products with a greater likelihood for the potential to introduce unintended adverse health effects. Additional findings and recommendations specifically related to animal genetic manipulation and cloning are contained in the committee’s subreport.
The following framework forms the basis of the committee’s recommendations presented later in this chapter, as well as those presented in the subreport.
FRAMEWORK FOR ASSESSING POTENTIAL UNINTENDED EFFECTS
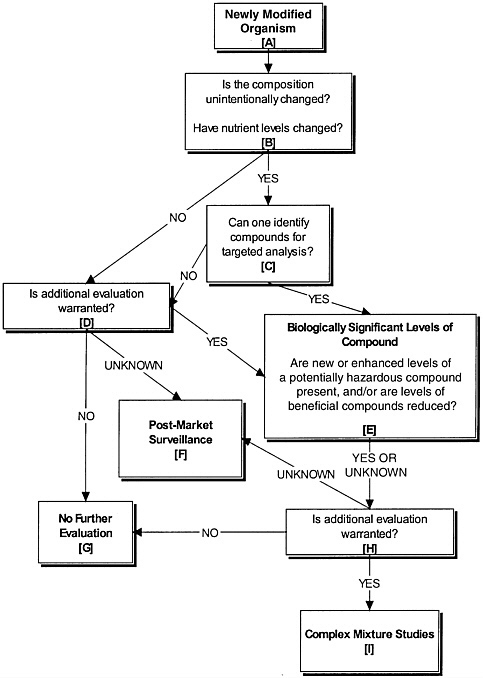
Any GM food has the potential for producing levels of primary and secondary metabolites that differ from its parental counterparts. As part of its task, the committee developed a framework, illustrated by the flow chart in Figure 7-1, as a guide for considering appropriate questions and methods to determine potential
unintended changes in the levels of endogenous nutrients, toxicants, allergens, or other compounds in all types of GM food—including GE food—that may lead to an unintended adverse health effect. It is important to note that this framework does not treat genetic engineering as a technology that is completely separate from other genetic modification techniques; the flow chart can usefully be applied to the full range of genetic modification technologies.
However, there are limitations to the application of this framework—or any other—because technological advances in analytical chemistry have exceeded our ability to interpret the consequences to human health of changes in food composition. Although compositional changes can be detected readily in food, and the power of profiling techniques is rapidly increasing our ability to identify compositional differences between GE food products and their conventional counterparts, methods for determining the biological relevance of these changes and predicting unintended adverse health effects are understudied. As discussed in this report, further advances in analytical technologies and their interpretation are needed to address these limitations.
Nevertheless, the committee believes that useful assessments currently can be made using this framework, giving consideration to questions such those listed below.
1. What differences exist from the progenitor line?
This question should address the known nutrients, toxicants, and antinutritional factors in order to identify and quantify changes introduced to food, whether intentional or unintentional. Two related questions exist:
-
What is the relevant progenitor to use as a comparator?
-
Should all detected differences trigger a requirement for further analytical work?
The selection of the relevant progenitor line is not a trivial issue. Because it is known that the average composition of food crops has changed over time as a result of breeding and changes in agricultural practices, selection of a historical progenitor is not appropriate. The immediate isogenic progenitor line is an appropriate comparator; however, the role of environmental factors on composition must be considered. Similarly, the progenitor’s role in the total diet of target populations must be considered. It is the genome that enables environmental responses, and environmental variables often have been shown to have large effects on composition. The interaction of genotypic and environmental variables must be considered in evaluating compositional effects of genetic modification. Comparisons of the new line with the progenitor, when both are grown under a single set of environmental conditions, would be informative, but not conclusive.
It is proposed that compositional differences attributable to genetic changes be evaluated on a case-by-case basis. In particular, the importance of differences
should be taken into consideration relative to the importance of the particular food as a source of a particular component. For example, the detection of a lower vitamin C content of a GE line of radishes should not be a cause for concern because radishes are not a significant source of vitamin C in human diets. In contrast, lower vitamin C in oranges or derived products, which are an important source of vitamin C in the U.S. diet, should trigger further consideration in that country.
2. What is the biological relevance of an identified compositional change from the perspective of human nutrition and health?
If differences between the progenitor and the new line are detected with respect to nutritionally or toxicologically significant constituents, these differences should be evaluated further to determine the importance of the differences relative to the compositional range and variability of the compound in major commercial varieties. A difference in composition from the progenitor that did not exceed the variability of major commercial varieties should not be considered a cause for concern.
3. What is the biological relevance of a compositional difference with respect to subgroups of the population who have either greater exposure or greater potential susceptibility to an unintended effect?
This question, in essence, constitutes a sensitivity analysis. The issues to be considered include:
-
Is the modified food a large component of average dietary intake?
-
Is the modified food a small component of average dietary intake?
-
What is the anticipated effect on upper-range (niche) consumers?
These questions on the interpretation of analytical data and related issues are discussed more fully in Chapters 5 and 6.
The following examples illustrate how newly modified organisms would proceed through the process presented in the flow chart, using hypothetical scenarios to illustrate the application of various approaches.
In the routine breeding process of crossing—for example, when one wheat variety is crossed with another to transfer a disease resistance gene—the resulting variety is genetically modified, but the desired trait that is obtained is not anticipated to be new to the species or to the food supply. This product would be considered in light of the questions posed in Boxes A through C. If the response to these questions is “no,” and if no other novel substances of concern are present, the products flow from Box D to G; such a variety need not trigger additional concerns.
As an alternate example, a cross of the potatoes Solanum tuberosum and S. brevidans resulting in the production of demissidine—a novel toxic substance in
the tubers—would warrant further scrutiny because the new potato variety is expressing a toxic substance that is new to both the species and the food supply. The identification of demissidine would require further testing (Boxes D and E), such as toxicological or complex mixture studies (Boxes H and I).
In another example, GE rice that expresses a soybean gene but carries no substance new to the species or to the food supply would proceed from Box A through Box C, as in the wheat example above. However, since soybeans are a common allergen, there would be need for additional scrutiny to determine the potential for allergenic response to the product. Once the potential for transfer of a soybean allergen into the rice is determined and, if appropriate, action is taken, the evaluation process would not go any further. Alternatively, a GE soybean that expresses a daffodil gene to enhance production of beta-carotene does warrant additional evaluation because the expression of beta-carotene, a precursor to an essential micronutrient, is substantially enhanced (Box E), even though it is not a novel substance in the food supply. In this case, the answer to the question posed in Box C could be either “yes” or “no,” and if “no,” the process would proceed to Box D. If the level of beta-carotene expressed by the soybean is at a level known to have biological significance, the evaluation process would proceed from Box D to Box E, then through Boxes H and I. If the biological significance is “unknown,” the evaluation process would proceed from Box E to Box H to Box F (postmarket surveillance). Similarly, if unintended compositional changes are accompanied in the introduction of this novel gene to the soybean, the nature of the change could be evaluated within the suggested framework.
FINDINGS AND RECOMMENDATIONS
Overall Findings and Recommendation
Findings
All new crop varieties, animal breeds (see cloning subreport), and microbial strains carry modified deoxyribonucleic acid (DNA) that differs from parental strains. Methods to genetically modify plants, animals, and microbes are mechanistically diverse and include both natural and human-mediated activities. Health outcomes could be associated with the presence or absence of specific substances added or deleted using genetic modification techniques, including genetic engineering, and with unintended compositional changes.
The likelihood that an unintended compositional change will occur can be placed on a continuum that is based on the method of genetic modification used (see Figure 3-1). The genetic modification method used, however, should not be the sole criterion for suspecting and subsequently evaluating possible health effects associated with unintended compositional changes.
All evidence evaluated to date indicates that unexpected and unintended com-
positional changes arise with all forms of genetic modification, including genetic engineering. Whether such compositional changes result in unintended health effects is dependent upon the nature of the substances altered and the biological consequences of the compounds. To date, no adverse health effects attributed to genetic engineering have been documented in the human population.
Recommendation 1
The committee recommends that compositional changes that result from all genetic modification in food, including genetic engineering, undergo an appropriate safety assessment. The extent of an appropriate safety assessment should be determined prior to commercialization. It should be based on the presence of novel compounds or substantial changes in the levels of naturally occurring substances, such as nutrients that are above or below the normal range for that species (see Chapter 3), taking into account the organism modified and the nature of the introduced trait.
Safety Assessment Tools for Assessing Unintended Effects Prior to Commercialization
Findings
Current voluntary and mandated safety assessment approaches focus primarily on intended and predictable effects of novel components of GE foods. Introduction of novel components into food through genetic engineering can pose unique problems in the selection of suitable comparators for the analytical procedures that are crucial to the identification of unintended compositional changes. Other jurisdictions, particularly the European Union, evaluate all GE food products prior to commercialization, but exempt from similar evaluation all other GM foods. As previously discussed in Chapter 3, the policy to assess products based exclusively on their method of breeding is scientifically unjustified.
The most appropriate time for safety assessment of all new food is in the premarket period prior to commercialization, although verification of safety assessments may continue in the postmarket period, generally in cases when a potential problem has been identified or if there is elevated cause for concern. Examples of specific premarket assessments of newly introduced compositional changes to selected GE food are:
-
protein, fat, carbohydrate, fiber, ash, and water in a proximate analysis;
-
essential macro- and micronutrients in a nutritional analysis;
-
known endogenous toxicants and antinutrients in specific species;
-
endogenous allergens;
-
other naturally occurring, species-specific constituents of potential inter-
-
est, such as isoflavones and phytoestrogens in soybean or alkaloids in tomato or potato;
-
gross agronomic characteristics;
-
data derived from domestic animal feeding trials to assess the nutritional quality of new crops; and
-
data derived from toxicological studies in animals.
Recommendation 2
The committee recommends that the appropriate federal agencies determine if evaluation of new GM foods for potential adverse health effects from both intended and unintended compositional changes is warranted by elevated concern, such as identification of a novel substance or levels of a naturally occurring substance that exceeds the range of recommended or tolerable intake.
Recommendation 3
For those foods warranting further evaluation, the committee recommends that a safety assessment be conducted prior to commercialization and continued evaluation postmarket where safety concerns are present. Specifically, the committee recommends the following safety assessment actions.
-
Develop a paradigm for identifying appropriate comparators for GE food.
-
Collect and make publicly available key compositional information on essential nutrients, known toxicants, antinutrients, and allergens of commonly consumed varieties of food (see the Research Needs section, later in this chapter). These should include mean values and ranges that typically occur as a function of genetic makeup, differences in physiological state, and environmental variables.
-
Remove compositional information on GE foods from proprietary do-mains to improve public accessibility.
-
Continue appropriate safety assessments after commercialization to verify premarket evaluations, particularly if the novelty of the introduced substance or the level of a naturally occurring substance leads to increased safety concerns.
Analytical Methodologies
Findings
During the past decade, analytical methodologies for separating and quantifying messenger ribonucleic acids (mRNA), proteins, and metabolites have improved markedly. Applying these methodologies to the targeted analysis of known nutrients and toxicants will improve the knowledge base for these food constituents. The broad application of targeted methods and continuing development of
profiling methods will provide extensive information about food composition and further improve the knowledge base of defined chemical food constituents. The knowledge and understanding needed to relate such compositional information to potential unintended health effects is far from complete, however. Furthermore, currently available bioinformatics and predictive tools are inadequate for correlating compositional analyses with biological effects.
Analytical profiling techniques are appropriate for establishing compositional differences among genotypes, but they must also take into account modification of the profile obtained due to genotype-by-environmental interactions (the influence of the environment on expression of a particular genotype). The knowledge base required to interpret results of profiling methods, however, is insufficiently developed to predict or directly assess potential health effects associated with unintended compositional changes of GM food, as is the necessary associative information (e.g., proteomics, metabolomics, and signaling networks). Additionally, predictive tools to identify the expected behavior of complex and compound structures are limited and require a priori knowledge of their chemical structure, their biological relevance, and their potential interactive targets.
Recommendation 4
The committee recommends the development and employment of standardized sampling methodologies, validation procedures, and performance-based techniques for targeted analyses and profiling of GM food performed in the manner outlined in the flow chart shown in Figure 7-1. Sampling methodology should include suitable comparisons to the near isogenic parental variety of a species, grown under a variety of environmental conditions, as well as ongoing assessment of commonly consumed commercial varieties of food. These include:
-
Reevaluation of current methodologies used to detect and assess the biological consequences of unintended changes in GM food, including better tools for toxicity assessment and a more robust knowledge base for determining which novel or increased naturally occurring components of food have a health impact.
-
Use of data collection programs, such as the Continuing Survey of Food Intakes by Individuals and the National Health and Nutrition Examination Survey (NHANES), to collect information, prior to commercial release of a new GM food, on current food and nutrient intakes and exposure to known toxins or toxicants through food consumption. The information collected should be used to identify food consumption patterns in the general population and susceptible population subgroups that indicate a potential for adverse reactions to novel substances or increased levels of naturally occurring compounds in GM food.
Additional Tools for Postcommercialization: Identification and Assessment of Unintended Effects
Findings
Postcommercialization or postmarket evaluation tools for verifying and validating premarket assessments of novel substances in food or detectable changes in diet composition, including tracking and epidemiological studies, are important components of the overall assessment of food safety. These tools provide a way to check the efficacy of premarket compositional and safety evaluations through a feedback process. In addition, information databases that result from postmarket studies can be valuable assets in the development of future premarket safety assessment tools.
Postmarket surveillance is a commonly accepted procedure, for example, with new pharmaceuticals and has been beneficial in the identification of harmful and unexpected side effects. As a result, pharmacologists accept postmarket surveillance as a part of the process to identify unexpected adverse outcomes from their products. This example is especially pertinent to GE foods because of the unique ability of this process to introduce gene sequences to generate novel products into organisms intended for use as food and especially in situations where the novel products are introduced at levels that have the potential to alter dietary intake patterns (e.g., elevated levels of key nutrients).
Given the possibility that food with unintended changes may enter the marketplace despite premarket safety mechanisms, postmarket surveillance of exposures and effects is needed to validate premarket evaluations. On the other hand, there are many instances in which postmarket surveillance may not be warranted. For example, when compositional comparisons of a new GM crop or food (e.g., Roundup Ready soybeans) with its conventional counterpart indicate they are compositionally very similar, exposure to novel components remains very low. Thus the process of identifying unintended compositional changes in food is best served by combining premarket testing with postmarket surveillance, when compositional changes indicate that it is warranted, in a feedback loop that follows a new GM food or food product long-term, from development through utilization (see Figure 7-1).
Recommendation 5
When warranted by changes such as altered levels of naturally occurring components above those found in the product’s unmodified counterpart, population-specific vulnerabilities, or unexplained clusters of adverse health effects, the committee recommends improving the tracking of potential health consequences from commercially available foods that are genetically modified, including those that are genetically engineered, by actions such as the following:
-
Improve the ability to identify populations that are susceptible to food allergens and develop databases relevant to tracking the prevalence of food allergies and intolerances in the general population, and in susceptible population subgroups.
-
Improve and include other postmarket resources for identifying and tracking unpredicted and unintended health effects from GM foods:
-
Improve the sensitivity of surveys and other analytical methodologies currently used to detect consumer trends in the purchase and use of GM foods after release into the marketplace,
-
Standardize methods for monitoring reports of allergenicity to new foods introduced into the marketplace and apply them to new GM foods,
-
Assure that current food labeling includes relevant nutritional attributes so that consumers can receive more complete information about the nutritional components in GM foods introduced into the marketplace, and
-
Improve utilization of potential traceability technology, such as bar coding of animal carcasses and other relevant foods.
-
-
Develop a database of unique genetic sequences (DNA, polymerase chain reaction sequences) from GE foods entering the marketplace to enable their identification in postmarket surveillance activities.
-
Utilize existing nationwide food intake and health assessment surveys, including NHANES, to:
-
Collect comparative information on diet and consumption patterns of the general population and ethnic subgroups in order to account for anthropological differences among population groups and geographic areas where GM foods may be consumed in skewed quantities, recognizing that this will be possible only under selected circumstances where intakes are not evenly distributed across population subgroups of interest and the relevant outcome data are available, and
-
Provide better representation of the long-term nutritional and other health status information on a full range of children and ethnic groups whose intakes may differ significantly from those of the general population to determine whether changes in health status have occurred as a consequence of consuming novel substances or increased levels of naturally occurring compounds in GM foods released into the marketplace, recognizing again that this will be possible only under selected circumstances that allow one to assess associations between skewed eating patterns and specified health outcomes. Such associations would have to be followed up by other more controlled assessments.
-
Research Needs
Findings
There is a need, in the committee’s judgment, for a broad research and technology development agenda to improve methods for predicting, identifying, and assessing unintended health effects from the genetic modification of food. An additional benefit is that the tools and techniques developed can also be applied to safety assessment and monitoring of foods produced by all methods of genetic modification.
The tools and techniques already developed can be applied to the safety assessment and monitoring of foods produced by all methods of genetic modification. However, although current analytical methods can provide a detailed assessment of food composition, limitations exist in identifying specific differences in composition and interpreting their biological significance.
Recommendation 6
A significant research effort should be made to support analytical methods technology, bioinformatics, and epidemiology and dietary survey tools to detect health changes in the population that could result from genetic modification and, specifically, genetic engineering of food. Specific recommendations to achieve this goal include:
-
Focusing research efforts on improving analytical methodology in the study of food composition to improve nutrient content databases and increase understanding of the relationships among chemical components in foods and their relevance to the safety of the food.
-
Conducting research to provide new information on chemical identification and metabolic profiles of new GM foods and proteomic profiles on individual compounds and complex mixtures in major food crops and use that information to develop and maintain publicly accessible databases.
-
Developing or expanding profiling databases for plants, animals, and microorganisms that are organized by genotype, maturity, growth history, and other relevant environmental variables to improve identification and enhance traceability of GMOs.
-
Developing improved bioinformatics tools to aid in the interpretation of food composition data derived from targeting and profiling methods.
Recommendation 7
Research also is needed to determine the relevance to human health of dietary constituents that arise from or are altered by genetic modification. This effort should include:
-
Focusing research efforts on developing new tools that can be used to assess the potential unintended adverse health effects that result from genetic modification of foods. Such tools should include profiling techniques that relate metabolic components in food with altered gene expression in relevant animal models to specific adverse outcomes identified in GM animal models (animals genetically modified by contemporary biotechnology methods that are proposed to enter the food system).
-
Developing improved DNA-based immunological and biochemical tags for selected GM foods entering the marketplace that could be used as surrogate markers to rapidly identify the presence and relative level of specific foods for postmarket surveillance activities.
-
Developing improved techniques that enable toxicological evaluations of whole foods and complex mixtures, including:
-
microarray analysis,
-
proteomics, and
-
metabolomics.
-
CONCLUDING REMARKS
The committee was charged with the task of identifying appropriate scientific questions and examining methods for determining unintended changes in the levels of nutrients, toxicants, allergens, or other compounds in food from genetically engineered organisms compared with those from other genetic modification processes and outlining methods to assess the potential short- and long-term human health consequences of such changes. To address its charge, the committee took into account the current state of the science for available analytical techniques. These techniques have improved in recent decades, as has knowledge and understanding of food safety. Nevertheless, substantial gaps remain, including our ability to:
-
identify compositional changes in food and other complex mixtures,
-
determine the precise chemical structure of more than a small number of compounds in a tissue,
-
determine the structure-function relationships between compounds in food and their relevance to human health, and
-
predict and assess the potential outcome of unintended changes in food on human health.
In consideration of the advances and limitations to available analytical techniques, the committee developed an appropriate paradigm for:
-
identifying appropriate comparators,
-
increasing understanding of the determinants of compositional variability,
-
increasing understanding of the bioactivity in humans, if any, of secondary metabolites in commonly consumed foods,
-
developing more sensitive tools for assessing potential unintended health effects (e.g., in whole food), and
-
improving methods for tracking and tracing exposure in GE food.
The recommendations presented in this chapter reflect the committee’s application of the framework it has developed to questions of identification and assessment of unintended adverse health effects from foods produced by all forms of genetic modification, including genetic engineering and they can serve as a guide for evaluation of future technologies.