3
Ecology and Evolution of Waterborne Pathogens and Indicator Organisms
INTRODUCTION
Past efforts to develop and implement indicators of waterborne pathogens have often given little or no consideration to the role of evolution in the ecology and natural history of waterborne pathogens of public health concern. Evolution is a powerful force and can act quickly, even over ecological timeframes, to bring about change in pathogenic and indicator microorganisms. Furthermore, although numerous studies exist on the pathogenicity of various waterborne pathogens few have sought to describe their life history or ecology. The interactions between pathogens and their hosts involve complex and diverse processes at the genetic, biochemical, phenotypic, population, and community levels, while the distribution and abundance of microorganisms in nature and their microbial processes are affected by both biotic and abiotic factors that act at different scales. To develop new and more effective indicators of waterborne pathogens it is important to better understand how both evolution and ecology interact with the genomes and natural history of waterborne pathogens and their indicators, if different from themselves. Failure to consider these effects may result in spurious conclusions that do not truly reflect the abundance and distribution of waterborne pathogens.
Most of the waterborne pathogens discussed in this report (see also Appendix A) are not native to the types of waterbodies addressed herein. Notable exceptions include various species of Vibrio and Legionella bacteria and protozoan parasites such as the free-living amoebae Naegleria and Acanthamoeba. Many microorganisms that are pathogenic to humans and animals enter ambient waters after import from various point and diffuse sources. Upon entry, new selective
|
BOX 3-1
|
forces begin to act on these introduced or exotic microorganisms, whether eukaryotes or prokaryotes.
This chapter describes basic principles of ecology and evolution for waterborne viruses, bacteria, and protozoa (and yeasts and molds to a lesser extent) of public health concern as an aid to better understand how selective forces may alter one’s ability to assess the microbial quality of water. Indeed, indicators of microbial water quality can be the pathogenic organisms themselves, other microorganisms, or other physical or chemical aspects of the aquatic environment (see Chapter 4 for further information), and any biological indicator is subject to evolutionary and ecological changes. The final section is a summary of the chapter and its conclusions and recommendations.
Answers to several sets of related and fundamental questions (summarized in Box 3-1) are imperative to facilitate the understanding of indicators of waterborne pathogens and emerging infectious diseases. These questions include but are not limited to the following:
-
What is the natural distribution and abundance of waterborne pathogens? Are there environmental reservoirs of these microorganisms and, if so, what environmental conditions promote their maintenance or growth? Are these environmental reservoirs biotic or abiotic (i.e., from the living or nonliving)? Can waterborne pathogens colonize and proliferate in sediments or within aquatic systems? The concepts of growth and regrowth are most often applied to water distribution systems and wastewater discharges (and their receiving waters), respectively.
-
Determining whether and how survival and growth occur under natural conditions is important in understanding whether an indicator is indicating “new” contamination. The ecological concept of “source/sink” (Pulliam and Danielson, 1991) needs to be better understood for waterborne pathogens. Are there populations of pathogens or indicator organisms in the environment (sources) that continually feed other habitats where the pathogens or indicators can be found (often at high densities) but cannot grow (sinks)?
-
What is the fate of freshwater pathogens that are transported into brackish or marine habitats and vice versa? The transition from fresh- to saltwater or the reverse is physiologically demanding, and microbial assemblages change both phenotypically and phylogenetically along salinity gradients. Given that freshwater has been imported into U.S. coastal waters for hundreds of years, along with the propensity of microbes to survive in novel environments, some freshwater pathogens might have adapted to increased salinity and some seawater pathogens might have adapted to reduced salinity. If so, flushes of these now “naturally” occurring bacteria may not be indicative of new inputs from either storms or saltwater intrusion but rather indicative of in situ bacterial growth.
-
Is the residence time of waterborne pathogens and indicators within a body of water sufficient for evolutionary mechanisms to alter the genetic composition of the pathogens? If so, could the genetic changes confound the reliability of the indicators or indicator mechanisms? Before selection can alter the genetics of a microorganism, the selective force must be applied for sufficient time and under the right conditions. Imported pathogens or pathogen indicator species gain or lose genetic traits under natural conditions—traits that may be the basis for detecting various indicators (e.g., β-galactosidase activity).
-
What biotic and abiotic factors influence the viability and survivability of waterborne pathogens and their indicators? Are there environmental conditions that promote genetic exchange or the acquisition of genetic elements that confer selective advantage under clinical conditions? For example, the increases in antibiotic and multiple antibiotic resistances may be influenced by physical conditions in the environment. What is the frequency of genetic exchange among native bacteria and introduced or imported bacteria?
-
What are the effects of sampling regime and environmental variation on the efficacy of indicators (see also Chapters 4 and 5)? Population, community, or genetic changes in space or time increase variability. Measures of statistical central tendency (i.e., means, medians, modes) are important in many aspects of science and ecology. However, because exposures at high extremes pose the greatest human health risks—and because of the immense economic component associated with waterborne pathogens and especially outbreaks (see also Chapter 2), including recreational losses and clinical costs—knowledge of simple means, medians, or modes is insufficient for making informed decisions about human health risks. Environmental variability occurs both spatially and temporally, and to understand ecological phenomena such variance must be estimated.
Many human pathogens and candidate indicators of fecal contamination also infect other host animals. Thus, nonhuman hosts may be the natural reservoirs of human pathogens and indicators. These additional ecological niches of pathogens and indicators have major implications for the following:
-
the potential detection, load estimation, and tracking of fecal contamination sources;
-
the ability to distinguish among and track or trace microbes of the same genus and species but from different sources;
-
the ability of pathogens from different sources to cause infection and illness; and
-
the potential for genetic exchange and evolution in microorganisms by coinfection of different strains or genotypes in a host animal or human or in the environment.
Identification of specific sources of pathogens or indicators is impossible unless advanced analytical methods, such as those described in Chapters 4 and 5, are used to genetically or phenotypically characterize the microorganisms. Because the same species of microorganism from different animal hosts or environmental reservoirs can differ greatly in human infectivity and the ability to cause disease, determining risks to human health requires the use of advanced analytical methods that are often well beyond the methods currently used for their detection in environmental waters. Furthermore, the continuous movement of microorganisms through different hosts and abiotic environmental media exerts selective pressures that are opportunities for genetic change leading to the emergence of new strains with different traits and health risks. Current analytical methods used to detect and quantify pathogenic and indicator microbes in water are limited in their ability to distinguish among genetically and phenotypically different organisms and to determine their sources or their human health risks.
Effects of Environmental Change
Environmental change at all scales, from local to global, influences microbial populations and indicator organisms. Large-scale or global changes in weather or climate are predicted to have major effects on waterborne or vectorborne diseases (Patz and Reisen, 2001; Patz et al., 2000). Past and continued alteration of forested areas (e.g., deforestation) and natural waters (e.g., water diversions such as dams and drainages of lakes, river diversions), road construction, commercial and residential development, and other disturbances change the ecological conditions of waterways. These changes often favor introduced over indigenous or “native” organisms at all levels of biological organization and can also result in changes in microbial diversity, the introduction of new or increased levels of pathogens and indicator organisms, and increased opportunities for hu-
|
BOX 3-2 Colwell (1996) described the appearance of a new serogroup of Vibrio cholerae 0139 in 1992 in India. Cholera has had at least seven pandemics since 1817. This disease often disappears for decades and then reemerges with a vengeance. From 1926 to 1960, cholera was expected never to reach pandemic proportions because of the improvement in water supplies worldwide. Yet nature prevails, and in 1961 a new pandemic began and continues to this day. The responsible biotype of V. cholerae was designated El Tor 01. This particular biotype does not cause as severe disease as the classical type. However, in 1992 a new serogroup 0139 emerged in India. Evidence suggests that the new serogroup originates from genetic recombination, horizontal gene transfer, and subsequent acquisition of unique DNA. Furthermore, this new serogroup had completely replaced the V. cholerae 01 in Calcutta by 1993. Various environmental factors have been implicated in the evolution of a new serogroup. The combination of increased inputs of nutrients to eutrophic conditions and association of the organism with shellfish, fish, and zooplankton created environmental reservoirs that could persist for extended periods of time. Thus, reintroduction was not necessary. The association with zooplankton, especially copepods, is central to understanding the dispersal and distribution of cholera. Vibrio cholerae preferentially attach to the chitinous exoskeleton of the copepods and thereby have the potential to be transported with ocean currents. |
man exposure to native pathogens of that environment via water and other routes. Therefore, increases in disease-causing microorganisms would be predicted (see Box 3-2).
For example, certain aquatic ecosystem restoration projects that require construction of wetlands by legislation may affect the growth and distribution of waterborne pathogens. Lake inflows are controlled, in part, by littoral zones or lake margins, and such areas can greatly impact the thermal mediation of small or forested watersheds. Andradottir and Nepf (2000) suggested that littoral wetlands can actually raise the temperature of inflow during the summer and create surface intrusions rather than plunging inflows. In other words, density differences between surface and underlying water would cause warm water to flow above the cooler layers. Consequently, nutrients, contaminants, and pathogens that were previously in the underlying water enter the surface layer, thereby increasing the risk of human exposure in recreational water settings. Furthermore, warmer, nutrient-rich waters may favor growth of pathogens.
Lebaron et al. (1999) have shown that varying nutrient conditions in seawater affect bacterial communities directly and indirectly by stimulating either bacteria or various protozoans that selectively feed on the bacterial assemblage. The stimulation of protozoan fauna may be acute given their interaction with various pathogens (discussed later). In relatively simple mesocosms, bacterial assemblages could be affected by nutrient additions that promote increased growth and productivity. In complex environments, numerous and varied microhabitats (such as organic foams which are described later) exist that may provide the appropriate conditions for changes in microbial assemblages through either direct or indirect selection.
Implications for Indicators
The concept of indicators implies that certain characteristics of an organism (e.g., genes or gene products) are constant under varying environmental conditions. This major assumption is questionable and subject to verification. Although various (primarily bacterial) indicators have been historically effective in detecting and quantifying fecal contamination, they are not always reliable predictors of microbial water quality due largely to our lack of understanding of the basic ecology of waterborne pathogens and indicators. For example, total coliform counts and enterococci have been used as indicators of human fecal contamination for decades (see Chapter 1). However, there are nonhuman and naturally occurring coliforms and enterococci, and their presence confounds the results of the total coliform and enterococci tests.
All coliforms and enterococci do not have the same ecology. If one or more species of coliforms and enterococci had different biotic and abiotic sources and greater or lesser survivability than the indicator species or pathogen of concern, then their presence or absence would not be a reliable indicator of the source or survivability of that pathogen. Similarly, the use of E. coli as an indicator of human fecal contamination in areas where there are high numbers of naturally occurring or introduced E. coli would greatly overestimate a potential microbial contamination problem. Not recognizing alternative sources of indicator organisms could ignore their potential to detect and correctly characterize actual waterborne microbial contamination problems. More specifically, wastewater treatment processes, physical and chemical stressors, and biological antagonists, such as naturally occurring predators, can selectively affect the presence and survival of one “indicator” species, which in turn affects the implied correlation between the indicator and the target pathogen. Furthermore, gene products such as β-galactosidase or β-glucuronidase may not be produced or may be overproduced under various environmental conditions, thereby affecting indicator technologies based on the detection and quantification of these products.
Microbial species can change genetically under natural conditions in ways that can alter their ability to be detected by phenotypic or genotypic methods.
Some of these changes can be profound, with genomes increasing or decreasing in actual DNA content and changing phenotypic properties. Bacteria in aquatic systems have been shown to take up plasmids at fairly high rates. Fry and Day (1990) demonstrated that maximum uptake occurs within 24 hours but that transconjugants could be detected within the first three hours of their experiments. Recently, high mutation rates have been observed in stationary phase E. coli from various natural habitats (Loewe et al., 2003) and stressed aging colonies have also been shown to have increased mutagenesis (Bjedov et al., 2003). Both of these responses could result in increased adaptive responses and emergence of pathogenicity (Loewe et al., 2003). Notably, all of these mechanisms were shown to occur within 24 hours. In natural systems the residence times of introduced bacteria can be much longer than 24 hours, thus providing an opportunity for genetic changes either through acquisition of plasmids or by allowing mutations to take place under the selective pressures of the new habitat.
Various natural history and environmental aspects of pathogens and indicator organisms also contribute to their ability to be detected and monitored. Many of these aspects are discussed below because they directly relate to the ongoing public health challenge of developing and using better indicators for waterborne pathogenic viruses, bacteria, certain parasitic protozoa, and to a lesser extent—yeasts and molds.
VIRUSES
Introduction to Viruses and Their Properties
Virus-host interactions are fundamental to the biology and ecology of viruses because they are obligate intracellular parasites. Viruses are inert outside host cells, despite their persistence in the environment and their ability to infect another host when the opportunity arises. In this section, the ecology and evolution of viruses are considered, particularly for waterborne viruses that are human and animal pathogens or bacterial viruses that are potential indicators of fecal contamination.
Virus Composition, Basic Properties, and Diversity
Viruses are among the smallest and simplest microbes and are obligate intracellular parasites of host cells. They range from about 0.02 to 0.1 μm in size and consist of a nucleic acid surrounded by a protein coat or capsid. The capsid not only is protective but also functions as the structure for host cell attachment leading to infection, because it has specific chemical structures that recognize receptor sites on the host cell. Some viruses, although usually not the ones transmitted by fecally contaminated water, also possess an outermost lipoprotein membrane called the envelope. The envelope is usually a virus-modified host cell membrane
containing virus-specific glycoproteins that is acquired as the virus exits the cell. Some of these glycoproteins in enveloped viruses are the chemical structures for attachment to host cell receptors. Viruses contain relatively small amounts of nucleic acid, usually from a few to several tens of nucleotide kilobases—enough information to encode a few to several tens of proteins. Despite this relative paucity of genetic information, viruses are genetically diverse, sometimes highly genetically variable, and quite capable of adapting to the changing conditions of their host cells and the host environment.
Viral Replication, Virus-Host Interactions, and Viral Evolution
The replication and evolution of viruses and their interactions with their hosts are strongly related to host fitness as both viruses and hosts coevolve. The ability of a virus to infect a particular host cell is primarily a function of the availability of the appropriate chemical structures on the surface of the virus and the host cell that allow for attachment to and penetration of the cell. These receptor-dependent interactions determine the virus host range, tissue tropisms (i.e., ability to infect cells of a particular tissue, such as intestinal, liver, or neurological tissues) for human and animal hosts, and thus the ability to cause certain kinds of infections and diseases. Despite the importance of cell surface receptors in the susceptibility of different cells or tissues to viral infection, the outcomes of viral infection—especially disease—are often mediated by additional events and other molecular interactions during virus replication (Bergelson, 2003; Dimitrov, 2000; Jindrak and Grubhoffer, 1999; McFadden, 1996; Mims et al., 2001; Ohka and Nomoto, 2001; Tyler and Nathanson, 2001).
Several outcomes of viral infection of host cells are possible: (1) virus multiplication leading to many progeny viruses with resulting cell lysis and death; (2) virus multiplication leading to many progeny viruses but cell survival; and (3) development of a stable relationship (at least temporarily) with the host cell with little or no virus multiplication—either as a discrete intracellular genetic element or as an integrated part of the host cell’s genetic material. In the last situation, the virus genetic information is propagated as part of the cell when it divides, and a relationship of co-existence between the cell and the viral genome may form (lysogeny). Under some circumstances, however, the virus genetic material can become capable of initiating replication activities of the viral genome, leading to the production of progeny viruses, lysis, and death of the cell (the lytic cycle). In some cases, the course of the alternative events in viral infection and virus-host interaction, lysogeny (or integration) or the lytic (or cytopathogenic) cycle, are influenced by a number of virus, host, and environmental factors, such as temperature, pH, UV irradiation (sunlight), nutrients, and antagonists (toxicants).
At the human or animal host level, factors influencing the activation of latent viruses to a more active cytopathogenic cycle of events in virus infection and disease can include immune status, hormone levels, chemical (nutritional) cofac-
tors, age, gender, and pregnancy. Therefore, the potential for, or likelihood of, viral infection and the potential outcomes of viral infection are complex and not easily predicted. In fact, some of the most studied viruses (e.g., hepatitis) are still not well understood, making reliable predictions of viral infection and disease outcomes at either the cellular or the population level difficult, if not impossible. Despite the variability and uncertainty of predicting waterborne virus infection and disease outcomes, studies of virus properties, virus-host interactions, virus infection and disease outcomes, and viral ecology and epidemiology have all helped to elucidate the natural history of viruses and virus risks to their hosts.
Virus strains that produce infectious viruses more rapidly and at higher yield are more likely to be successful if fitness is positively correlated with population size of the susceptible host. For many viruses the manifestation of disease in the host is rare, and most infections are unapparent or subclinical. Examples of such viruses are the polioviruses and the rotaviruses. Typically, these viruses infect the youngest members of the population who have previously not been infected. Unfortunately, such infections produce severe disease or death in a small proportion of the humans they infect, and the majority of infections in infants and young children are either subclinical (polioviruses) or mild and self-limiting (rotaviruses). However, the consequences of poliovirus infection are considered sufficiently profound in the small proportion of infected persons who develop paralytic disease or die that vaccination is considered essential and a global eradication for polio is under way by the World Health Organization (Hull and Aylward, 2001). Repeated rotavirus infections are common in infants and young children though most infections are not life-threatening, especially in healthy children in developed countries. However, rotavirus diarrhea does cause severe disease requiring hospitalization in a low proportion of infected infants and children in the United States and other developed countries (<1 percent of rotavirus infections) and there is a very low but non-zero risk of death from rotavirus infections (Parashar et al., 2003).
Hosts that recover from virus infections are immune to future infections, either temporarily or perhaps indefinitely. In the case of rotaviruses, immunity is transient, only partially protective, and even less protective against antigenically different rotaviruses that have considerable antigenic diversity (Jiang et al., 2002). In the case of polioviruses, infection is likely to result in long-lasting immunity that is protective against paralytic disease and mortality, although enteric infections that are subclinical or mild still occur in persons with immunity (Ghendon and Robertson, 1994). If primary (initial) poliovirus infection of a susceptible host does not occur until later in life, as an older child or an adult, the consequences of infection are likely to be severe disease or even death. For polioviruses, infection of infants and children is common in developing countries where poor sanitation and hygiene result in exposure early in life. However, in developed countries with improved hygiene and sanitation, virus exposure often does not occur until later in life so that the likelihood of severe disease and death as a
result of infection is much greater (Evans, 1989; Pallansch and Roos, 2001; White and Fenner, 1994). The above examples serve to highlight the importance of host status and environmental conditions in the ecology and natural history of viruses, and to demonstrate that the “virulence” or pathophysiology of a virus depends on the status of the host and its environment.
Another example of the role of the host and its environment in the outcome of virus infection is hepatitis E virus (HEV). In developing countries, the members of the population at highest risk of severe illness and death are pregnant women. The mortality rate in this group can be as high as 25 percent (Aggarwal and Naik, 1997; Balayan, 1997; Emerson and Purcell, 2003; Hyams, 2002; Krawczynski et al., 2001). Yet, for most of the population in developing countries, HEV infection apparently occurs relatively early in life, with little illness incurred. Children are often asymptomatic and the mortality rate is between 0.1 and 4 percent (Grabow et al., 1994). Seroprevalence of HEV in developing countries ranges from 5 to upwards of 20 percent (Kamel et al., 1995; Mohanavalli, 2003). In developed countries such as the United States, HEV infection is rare and results in very few cases of disease (most traced to probable virus exposures in developing countries); seroprevalence is less than 5 percent (Bernal et al., 1996; Redlinger et al., 1998). Therefore, as with many other viruses, the pathophysiology of HEV varies with the health status of the host and with environmental conditions.
Viral Genetic Variability and Genetic Change
Viruses have evolved a variety of mechanisms that influence their host interactions and their ability to persist over time and in space. Viruses mutate spontaneously and without direct exposure to physical and chemical mutagens during replication in host cells. Mutation rates vary among different virus groups from high rates of 10–3 to 10–4 per incorporated nucleotide in the single-stranded RNA viruses to rates as low as 10–8 to 10–11 per incorporated nucleotide in some of the double-stranded DNA viruses (Domingo et al., 1999).
Genetic changes in viruses that involve relatively minor substitutions, insertions, or deletions of nucleotides as point or frameshift mutations can occur. Such changes are often referred to as genetic drifts, and if they occur in an expressed gene these changes are referred to as antigenic drift. Genetic and antigenic drifts can occur in response to selective pressures from host populations, such as immunity and genetic changes in host cells and whole hosts such as animals and plants. In some cases, genetic drift leads to more benign relationships between viruses and their hosts. At the other extreme, it can result in viruses with properties that have severe consequences, such as the reversion of attenuated poliovirus vaccine strains to a neurovirulence and the ability to cause paralytic disease in human hosts.
Effects of Virus Mutation on Hosts: Poliovirus Virulence, Attenuation, and Reversion to Virulence
Polioviruses are single-stranded RNA viruses belonging to the Picornaviridae family and the Enterovirus genus, and they consist of three genetically distinct types (I, II, and III). These viruses infect the gastrointestinal tract initially and can then spread via the bloodstream and lymphatic system to the central nervous system, thereby causing paralysis in their human hosts. The virus-specific factors responsible for the neurovirulence of polioviruses are still not fully understood at the genetic, protein, or virion (whole virus particle) level. Neurovirulence is mediated by the ability of the virus to successfully infect neurons and cause high levels of virus production and subsequently death of these cells (Ohka and Nomoto, 2001; Pallansch and Roos, 2001; Racaniello, 2001). Paralytic disease depends on the ability of the virus to infect cells of the central nervous system efficiently. The risks of paralytic disease to humans posed by wild-type, neurovirulent polioviruses, led to the selection of avirulent or attenuated polioviruses as vaccine strains in the mid-twentieth century. These live oral poliovirus vaccine strains differ from wild-type viruses because they have several different point mutations that are associated with the ability to infect neural cells. However, despite thorough knowledge of the complete nucleotide sequence of polioviruses for two decades, the cloning and expression of the cell surface receptor of the virus, the development and use of a transgenic (genetically modified) mouse model for neurovirulence, and considerable effort to identify neurovirulence mechanisms in cell culture and animal systems, these mechanisms have not been fully elucidated. However, it is becoming clear that neurovirulence depends on host factors as much as virus-specific factors and that virus-host interactions leading to neurovirulence are probably modulated by the host (Ohka and Nomoto, 2001; Yoneyama et al., 2001).
The attenuated live oral vaccine strains of poliovirus are also subject to back-mutations that cause reversion to wild-type viruses and paralytic poliomyelitis in vaccine recipients. Because virus mutation rates are high, there is rapid reversion of vaccine polioviruses to genotypes with neurovirulent properties among the excreted viruses of vaccine recipients. Serial transmission of vaccine strains of polioviruses among susceptible human hosts results in the accumulation of mutations, which can eventually lead to selection and further serial transmission of neurovirulent vaccine strains. This highly unfortunate outcome occurs when there is inadequate vaccine coverage of susceptible hosts over time, as occurred recently in the Dominican Republic and Haiti on the island of Hispaniola, the Philippines, and several other locations globally (Anonymous, 2002, 2003; Friedrich, 2000; Landaverde et al., 2001). Based on the extent of genetic change (about one to three percent), these viruses had apparently been spreading from person to person over one to two years or more.
Virus Mutation and Evolution by Exchange or Acquisition of Genetic Material
In addition to spontaneous point and frameshift mutations, the genetic composition of viruses can be altered by a number of mechanisms that involve virus-virus-host cell interactions (Domingo et al., 1999; Hendrix et al., 2000; Kaaden et al., 2002). That is, genetic changes in viruses can occur when two or more viruses coinfect host cells and exchange genetic information during replication. The genetic changes can involve major changes or substitutions in whole genes, genomic regions, or genome segments by mechanisms such as recombination and reassortment. Such changes can result in genetic and antigenic shifts that often have profound consequences for the natural history of viruses and their hosts. Examples include (1) the emergence of new strains of pandemic human influenza viruses by the creation of reassortant viruses from avian and human viruses by co-infection of swine, and (2) and the development of new strains of rotaviruses, either in nature or by experimental methods to produce reassortant rotavirus vaccine strains of human and either bovine or monkey origin (Baigent and McCauley, 2003; Bishop, 1996; Jiang et al., 2002; Webby and Webster, 2003).
Although viruses are often viewed as discrete entities that infect and interact with host cells alone, they can engage in genetic exchange and reproduction, directly or indirectly, within their host cells. Through coinfection, there can be evolutionary, cooperative, and competitive interactions among viruses. Intracellular interactions between coinfecting viruses are shown to be important in disease progression (e.g., herpesviruses, HIV; Holmes, 2001; Papathanasopoulos et al., 2003), and entire families of viruses rely on coinfection to complete their life cycle successfully (e.g., geminiviruses that infect plants; Gutierrez, 1999; Hanley-Bowdoin et al., 2000).
Genetic Recombination
In genetic recombination, coinfection with two different viruses results in the formation of new viruses whose genomes contain portions of each infecting virus that were created by the “crossover” event. Genetic recombination of polioviruses with other enteroviruses apparently occurred in the reversion of some vaccine strains to neurovirulence on the island of Hispaniola (Kew et al., 2002). Some of these viruses not only had back-mutations at critical sites associated with neurovirulence, but also had recombinations with other enteroviruses that may have increased their transmission rates in human hosts.
Viral Genetic Change and Evolution by Reassortment
Virus reassortment occurs when two or more viruses with segmented genomes simultaneously coinfect a host cell. The genomic units produced during replication are packaged randomly into virions, resulting in the formation of new
progeny with combinations of genomic segments from each infecting virus. One of the best-studied examples of this phenomenon is the influenza A virus (Hay et al., 2001; Scholtissek, 1995; Webster et al., 1993). Reassortant strains produced when human and avian strains apparently coinfect pigs can have new combinations of surface antigens from each parent virus. These new hybrid viruses periodically emerge as pandemic strains. Similar antigenic shifts created by reassortment also occur in the enteric viruses known as rotaviruses (Bishop, 1996).
Viruses in Human and Animal Wastes and in the Aquatic Environment
Enteric viruses found in human and animal feces, sewage, and fecally contaminated water include not only enteric pathogens but also viruses that infect bacteria residing in the intestinal tracts of humans and other warm-blooded mammals that are called enteric bacteriophages. Some fecally shed viruses are respiratory pathogens that have been swallowed with respiratory exudates, that actually infected the enteric tract, or both. The aquatic environment also contains many other viruses that infect a variety of aquatic and terrestrial life ranging from prokaryotes to protozoans to plants and animals. The viruses shed in feces and present in sewage belong to a diverse range of taxonomic groups that have different genetic, morphological, and functional properties. Of the human enteric viruses, some belong to taxonomic groups containing single-stranded RNA (enteroviruses, caliciviruses, hepatitis A and E viruses, astroviruses, and coronaviruses); double-stranded, segmented RNA (reoviruses and rotaviruses); bisegmented and double-stranded RNA (picobirnaviruses); single-stranded DNA (parvoviruses); or double-stranded DNA (adenoviruses). The bacteriophages found in feces, sewage, and ambient water, while not pathogenic, are genetically and morphologically diverse.
Animal Reservoirs as Sources of Human Enteric Viruses
As noted previously, many human viral pathogens, including some waterborne enteric pathogens, also infect other animals and therefore have animal reservoirs (Enriquez et al., 2001; Weiss, 2003). These animals can potentially be important sources of virus released into aquatic environments leading to human exposure. More often than not, a particular virus infects only one animal species, however, there are some notable exceptions. For example, of the enteric viruses, reovirus type 3 can infect humans as well as a wide range of other mammals, including mice (Cohen et al., 1988). Reovirus 3 is an example of a virus that infects but causes little morbidity or mortality in its human hosts. Other human enteric viruses that infect animals are rotaviruses, hepatitis E virus, and probably caliciviruses (Desselberger et al., 2001; Emerson and Purcell, 2003; Enriquez et al., 2001; Smith et al., 2002). Caliciviruses that infect cattle and swine are geneti-
cally similar to certain subgroups of human caliciviruses. Porcine hepatitis E viruses are very similar to human hepatitis E viruses. Human HEV strains and porcine HEV strains have infected pigs and primates, respectively, in experiments (Clayson et al., 1995; Emerson and Purcell, 2003).
In addition to being reservoirs of human enteric viruses, animals also harbor enteric bacteriophages that are potential indicator viruses of fecal contamination. Somatic and male-specific coliphages (bacteriophages of Eshcerichia coli), Salmonella phages, and Bacteriodes fragilis phages can be found in human and animal feces. The viruses apparently infect the intestinal bacterial flora of a variety of feral, domestic and agricultural animals. The use of bacteriophages as indicators of fecal contamination of water has been considered seriously (see Chapter 4 for further information), and there is evidence that shows their predictive value for enteric viruses and fecal contamination by correlations between presence and levels of enteric viruses and bacteriophages and associations of bacteriophages with increased risks of viral illness (Chung et al., 1998; Havelaar, 1993; Wade et al., 2003).
There also appear to be genetic differences in the host ranges of at least some coliphages, such as the RNA and DNA containing male-specific (F+) coliphages. These differences in host range are dependent in part on host cell factors related to coliphage adsorption to the F pili of the host as well as other host-related factors during later events in virus replication (Miranda et al., 1997; Schuppli et al., 2000; Tomoeda et al., 1972). Of the four major subgroups of the F+ RNA coliphages, two of them (Groups II and III) are found primarily in human feces and sewage, one (Group IV) is found primarily in animal feces, and the last (Group I) is found in both human and animal feces and sewage (Hsu et al., 1995). Therefore, the ecology or natural history of at least some enteric bacteriophages appears to be related to the animal host of their host bacteria. The apparent animal-host specificity of these bacteriophages may be related to the bacterial host ranges of the phages themselves or to the animal host ranges of their bacteria, although the ecological aspects of these relationships have not been adequately studied. Regardless of the mechanisms, the diversity of enteric bacteriophages and their bacteria, and their occurrence in human and animal hosts, pose challenges to the development and application of bacteriophages as indicators of enteric viruses and fecal contamination. This is because the extent to which coliphages are able to reliably and quantitatively indicate the amount of fecal or sewage contamination in water depends on the concentrations and types of coliphages in different sources of fecal contamination or sewage, the absolute and relative stability, persistence, and resistance of the coliphages to water treatment processes, and the extent to which their properties can change depending upon the strain of host bacterium and its human or animal host. These factors influencing coliphage occurrence and properties are still being elucidated.
Stability, Survival, Effects of Physical and Chemical Agents, and Transport of Viruses
Some of the important properties of enteric viruses and bacteriophages that influence their environmental behavior and natural history include their small size, stability over a wide temperature and pH range, resistance to various chemical agents such as oxidants and proteolytic enzymes, and propensity to aggregate and adsorb to particles and surfaces. These properties allow some enteric viruses in feces and sewage to survive conventional sewage treatment processes and persist in environmental waters and their associated sediments.
Conventional sewage treatment systems employing primary and secondary treatment reduce enteric viruses by about 90 to 99 percent in the treated effluent (Leong, 1983). Many of the viruses removed from the effluent remain infectious in the resulting sludge or biosolids, which must be treated further to reduce the viruses and other pathogens (see also NRC, 2002). Chemical and physical disinfection processes vary greatly in their ability to inactivate enteric viruses. Appreciable virus reduction in sewage is achieved only when well-treated effluent is disinfected with free chlorine, ozone, chlorine, or high doses of UV radiation and/ or when viruses are physically removed or inactivated by certain advanced wastewater treatment processes, such as membrane filtration or chemical coagulation. Because municipal sewage is often disinfected only by combined chlorine (a relatively weak oxidant), discharged sewage effluents often still contain relatively high concentrations of viruses (Griffin et al., 2003). Furthermore, sewage treatment plants often must bypass untreated sewage during wet weather by design, and many urban sewage systems still discharge such combined sewer overflows directly to receiving waters.
Because on-site wastewater treatment systems, typically septic tanks and subsurface drainfields, often inadequately reduce viruses and the wastes of feral, domestic, and agricultural animals are either untreated or inadequately treated, they can deliver substantial numbers of enteric viruses and other pathogens to ground- or surface waters (Borchardt et al., 2003; Powell et al., 2003; Scandura and Sobsey, 1996). Enteric viruses have been found on occasion in both surface and groundwaters used as drinking water sources and for primary contact recreation (Bellar et al., 1997; Donaldson et al., 2002; Hot et al., 2003; Jiang et al., 2001; Lipp et al., 2002; van Heerden et al., 2003).
Summary
Despite their relatively small size, limited genetic information, and relatively simple composition and structure, viruses are biologically complex, diverse, and highly adaptable to different environments and hosts. As obligate intracellular parasites, viruses multiply only in specific hosts. However, their host ranges can be either limited or broad and can change over time and space. Some human
viruses also have animal reservoirs, and therefore animals can be sources of human viruses. Viruses evolve over time and do this by coevolving with their hosts. Virus-host interactions are complex and diverse, and they can have different outcomes ranging from virus proliferation with the death of the host to integration of the viral genome into the host cell without virus proliferation beyond cell division. Different viruses have different rates of mutation, but all viruses display genetic variability over time. Mutations can be minor (e.g., point mutation) and lead to genetic drift, or they can be major (gene substitutions or replacements) and lead to a genetic shift. Gene substitutions or replacements can occur by genetic recombination or reassortment when two or more viruses infect the same host cell. Furthermore, animal viruses and human viruses can coinfect cells to create new viruses (by recombination or reassortment) that are infectious to humans and have some properties from each original virus. Both minor genetic changes causing genetic drift and major genetic changes causing genetic shift can have profound effects on the relationships of viruses to their hosts. Such mutations can alter their virulence, either causing virulent viruses to become nonvirulent or the reverse.
Many human viruses can infect the enteric or respiratory tract, or both, and are a concern from exposures to contaminated water and other environmental media. Although they are inert in the environment, viruses can be stable, persist for long periods of time in environmental media, and be resistant to various physical and chemical agents, including disinfectants. In addition, viruses are so small that they are readily transported in water and wastes and can migrate through soils and other porous media. The persistence and transport of human enteric viruses in water and other environmental media constitute a public health concern because the viruses can retain their infectivity and cause human infection if humans ingest or otherwise come in contact with them in environmental media.
BACTERIA
Introduction to Bacteria and Their Properties
Bacterial waterborne pathogens and indicators vary in size from 0.2-2 μm and fall into at least two major groupings: (1) native opportunistic pathogens such as Aeromonas spp. and Mycobacterium spp. and (2) introduced pathogenic bacteria that are not “normally” found in a particular water system (e.g., Shigella) or other bacteria often found only at relatively low concentrations in natural waters and other environmental media (e.g., Legionella, Clostridium). It is important to note that waterborne bacterial pathogens and indicator organisms are only one small component of any aquatic microbial community which may also include heterotrophs, autotrophs, chemotrophs, and saprophytes. Furthermore, certain Gram-positive waterborne bacteria under certain environmental conditions can form endospores. With no metabolic activity, these specialized cells are able to
survive extended periods of time in the environment compared to vegetative bacterial cells.
However, some “introduced” waterborne pathogenic bacteria can often be isolated from nearly pristine systems, thereby suggesting some “natural” low density (Fliermans et al., 1981; Hazen and Fliermans, 1979). Natural densities of pathogens are difficult to ascertain since most systems receive imports of bacteria through surface runoff from precipitation events, atmospheric dryfall, vertebrate and arthropod transport, and human activities. In highly disturbed systems, such as agriculture or water treatment discharges, imports of pathogenic bacteria would be expected to be much higher. For example, Lalitha and Gopakumar (2000) in a study of freshwater and brackish sediments, shellfish, and native fish in India found that 21 percent of all sediment samples contained Clostridium botulinum, 22 percent of the shellfish harbored C. botulinum, and between 2 and 8 percent of indigenous fish had C. botulinum on their surfaces.
Although some pathogenic bacteria exclusively inhabit humans, most also have environmental biotic reservoirs (are zoonotic), and these reservoirs can be important in the transmission of pathogens to other hosts. For example, a bacterial genus that has a substantial biotic habitat is Campylobacter. Both C. jejuni and C. coli are human gastrointestinal pathogens that are the major cause of bacterial diarrheal illness in many developed countries, and such outbreaks can be waterborne or foodborne (Rheinheimer, 1992). Waterborne outbreaks have been associated with community water supplies or untreated spring water, in which Campylobacter cells are viable for months. Outbreaks have also resulted from foods such as raw milk and poultry, although improper food handling is thought to account for the majority of endemic Campylobacter disease in the United States. Campylobacter is carried in a wide range of mammalian hosts, such as rabbits, cows, sheep, pigs, and chickens, as well as wild birds such as crows, gulls, pigeons, and migratory waterfowl. Campylobacter can be transmitted from aquatic sources to animals by direct contact or via carriage by birds or flies, and then spread between animals.
Aerobic Gram-negative bacteria, frequently found in water sources, are a common cause of hospital infection, particularly in intensive care units. Multidrug-resistant Pseudomonas, Enterobacter, Acinetobacter, Klebsiella, and Stenotrophomonas are particularly problematic (Denton and Kerr, 1998; Hanberger et al., 1999). These microorganisms are widespread in aquatic environments and may be introduced into hospitals by patients, staff, or visitors and become established in microenvironments such as sinks, showers, and ice machines. Apart from sporadic infections and outbreaks occurring in recreational or hospital settings, the ultimate sources of these microorganisms are not well known. Antibiotic use for growth promotion in animal agriculture and for treating infections in humans and agricultural animals accounts for the greatest amount of commercial antibiotic production in the United States (Levy, 1997, 1998). Antibiotics and other pharmaceutically active compounds have been found in
ground- and surface waters, especially near human and agricultural animal waste sources, leading to further concerns about the selection of antibiotic-resistant bacteria in the aquatic environment, which is discussed in the next section.
Similarly, potentially pathogenic bacteria such as Aeromonas, Escherichia coli, or Salmonella all have substantial environmental reservoirs. Aeromonas species are frequently found in aquatic environments, and certain pathogenic strains (possessing specific virulence properties) cause human disease. However, the distinctions between nonpathogenic environmental strains of Aeromonas found in water and the pathogenic clinical strains of Aeromonas isolated from humans have not been established adequately, although some pathogenic strains have been isolated from water (Haburchak, 1996; Hazen and Fliermans, 1979). The spread of E. coli and Salmonella among human populations is mediated via foods contaminated by animal products. Notably, a dramatic increase in multidrug-resistant Salmonella typhimurium (phage type DT104) has been observed in the United States and the United Kingdom (CDC, 1997). Like Campylobacter, S. typhimurium is a ubiquitous zoonotic bacterium in nature and is found in wild birds, rodents, foxes, badgers, poultry, cattle, pigs, and sheep.
Bacteria have at least three novel evolutionary mechanisms that can facilitate their rapid response to many environmental changes through alteration of their genetic composition: (1) conjugation, (2) transduction, and (3) transformation (see Box 3-3). Of these, plasmid-mediated conjugation is the most common, though several bacterial genera, including Campylobacter, are naturally competent for DNA uptake through transformation (Wommack and Colwell, 2000). One example of the entry of foreign DNA into Campylobacter is a gene encoding for resistance to the antibiotic kanamycin, which was first identified in an E. coli strain also resistant to the antibiotics ampicillin, tetracycline, chloramphenicol, streptomycin, and erythromycin. The DNA sequence is identical to that from Enterococcus faecalis, and indicated the transfer of this resistance determinant from Gram-positive enterococcal or streptococcal bacteria to the Gram-negative C. coli. The gene was also found in C. jejuni, indicating the subsequent dissemination of kanamycin resistance among Campylobacter species. In some cases, plasmids conferring kanamycin resistance also provided resistance to tetracycline and chloramphenicol. The issue of antibiotic resistance in bacteria is discussed in the following section.
Antibiotic Resistance
Our understanding of the mechanisms that promote the selection and transmission of bacterial genes under various environmental conditions is critical to addressing long-term public health problems. For instance, exposure to heavy metals at concentrations above background may influence the frequency, abundance, and types of antibiotic resistance genes available in the environment, and
these genes could subsequently be transmitted to waterborne pathogens of public health importance (McArthur and Tuckfield, 2000).
Esiobu et al. (2002) have shown that Pseudomonas, Enterococcus-like bacteria, and Enterobacter and Burkholderia species are the dominant reservoirs of certain antibiotic resistance genes in soil and water environments. Patterns of resistance were correlated with the abundance and types of bacterial species found in the various habitats. Movement of genes between and within these taxa has been demonstrated (Davison, 1999). Similarly, movement of resistance genes has been demonstrated between various “native” taxa and introduced bacteria (e.g., opportunistic and frank pathogens such as Aeromonas and Campylobacter, respectively).
While selection for tolerance or resistance to antibiotics from exposure to antibiotics is considerable in clinical and animal agricultural environments, there is increasing evidence that resistant phenotypes are being selected for in natural environments (Seveno et al., 2002). Contributing to the evolution of such resistance are transposons, which allow the movement of genes within cellular genomes and onto plasmids and bacteriophages where they can be more easily spread to neighboring cells (Liebert et al., 1999). Thus, the overall problem of antibiotic resistance and its impact on waterborne pathogens and indicators is one of genetic ecology (Mazel and Davies, 1999). An understanding of genetic ecology would require studies on the transfer of various genes under natural conditions as well as under stressed or disturbed conditions.
Kadavy et al. (2000) found high levels of antibiotic resistance in obligate commensal bacteria associated with flies living in the asphalt seeps of the Le Brea tar pits in California. They suggested that exposure to elevated levels of naturally occurring solvents may have resulted in the indirect selection of antibiotic resistance and that these bacteria are an environmental reservoir of antibiotic resistance genes. Selection acting on one set of genes (e.g., metal tolerance) may indirectly increase levels of other unrelated but linked genes. Such linked genes would then be available for transfer to other bacteria including waterborne pathogens.
Biological Interactions
Environmental Reservoirs
Critical to understanding the ecology of waterborne pathogens and indicators organisms is knowledge of various niches and habitats that promote or safeguard these microorganisms while they reside in a waterbody. Recent studies have shown unique biological interactions between certain prokaryotic and eukaryotic pathogens and other proto- and metazoans (Barker et al., 1999; Steinert et al., 1998). Winiecka-Krusnell and Linder (1999) have shown that free-living amoebae—which are well adapted to harsh or changeable environments such as desic-
|
BOX 3-3 At least three different mechanisms have been observed for the spread of genetic material among environmental and clinically relevant bacteria: conjugation, transformation, and transduction. 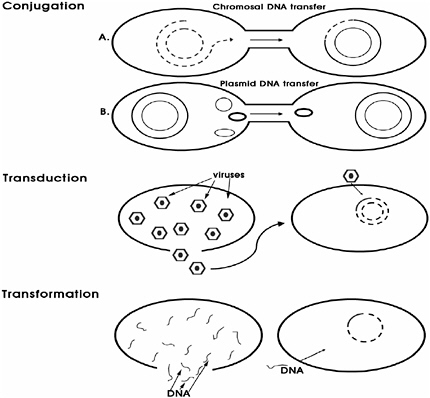 |
cation, elevated temperatures, and disinfectants—harbor bacteria intracellularly. Some bacteria can thus prevent intracellular destruction and can grow and survive within protozoa, finding both protection from adverse environmental conditions and protected modes of transportation. This interaction may also enhance their infectivity in mammals (Harb et al., 2000). For example, endosymbiotic or parasitic relationships between Legionella bacteria and their free-living algal and protozoan hosts allow not only for bacterial proliferation but also for protection from disinfection, thereby increasing their survival and ability to reach human hosts through drinking, recreational, and cooling tower waters. Therefore, proto-
|
zoa play a role in the transition of bacteria from the environment to mammals including humans. In this regard, protozoa may be viewed as “biological gyms” where bacterial pathogens train for encounters with more evolved mammalian cells (Harb et al., 2000).
Bacterial “Trojan horses” thus become a mechanism for immediate survival and long-term reserve. Indeed, some anaerobic bacteria can survive and replicate under aerobic conditions in amoebae. In Tomov’s study (Tomov et al., 1999), free-living Mobiluncus curtisii did not replicate and died in four to seven days whereas those grown with amoebae increased up to 1 × 106 colony forming units
(CFU) per mL over the same duration. If any single amoeba becomes infected with more than one strain or species of bacterium, the probability of gene exchange between bacteria increases considerably because of the increased probability of contact. Furthermore, this unique niche provides a mechanism for bacterial pathogen replication under normally adverse or inhibitory conditions.
Protozoa are not the only biological environmental reservoir for pathogenic organisms. Freshwater and marine mussels have been shown to harbor various bacterial pathogens. Vibrio cholerae non-O1, Salmonella typhi, Escherichia coli, and Vibrio harvey showed differential retention within a marine mussel under different environmental and culturing conditions (Marino et al., 1999). Such associations provide respite from selection imposed on free-living bacteria and increase the probability of gene exchange between strains, species or other taxa. Biological indicators of these pathogens that fail to identify these environmental reservoirs will be of little efficacy in tracking potential impacts or outbreaks.
Ecology of Plasmids
Bacteria in nature can and do acquire and lose genetic material through a variety of novel evolutionary mechanisms. Pathogenic bacteria introduced into aquatic systems could in theory, and do in practice, alter their genetic composition using these same mechanisms (see Box 3-3).
Although numerous papers and reviews have described the roles and exchange of plasmids, little attention has been given to their ecology (Sobecky, 1999). In fact, certain plasmids can be culled after environmental change wherein the benefit provided is no longer selectively advantageous. Plasmids confer varying levels of plasticity on cells and on entire microbial communities (Sobecky et al., 1997, 1998). Given the (re)emergence of new and old pathogens and related diseases (see Appendix A), it seems imperative to understand the acquisition and dissemination of numerous and diverse “natural” plasmids. Do bacteria “sample” available plasmids as an effective “hedge-bet” against future environmental change? What is the evolutionary cost for pathogens that take up environmentally derived plasmids? These and other questions have to be addressed so as to better monitor pathogens and bacterial indicators in the environment and enhance our ability to detect important strains or closely related, but nevertheless significantly different, bacteria.
Effect of Biodiversity on Pathogenic Microorganisms
Numerous studies have been undertaken to determine the effect of biodiversity on emergent properties of various systems. Biodiversity and evenness of bacterial species together may be an indicator of the overall condition of a particular system. For example, certain waterborne pathogens can be expected to be found directly below wastewater outfalls or feedlots. Outfall microbial
biodiversity may be significantly reduced and the evenness may be skewed by pathogens contributed by the discharge. However, “indicator” microorganisms have been found to grow in uncontaminated systems under appropriate conditions (Gauthier and Archibald, 2001), and caution must be used in interpreting results for such indicators. Furthermore, as discussed previously, the biodiversity of higher organisms, especially protozoa, may facilitate the growth of pathogens. Most bacteria in transport in lotic aquatic ecosystems (e.g., stream riffles) are not active (Edwards et al., 1990) because the doubling times of the bacteria are slower than the flow rate of the water and selection would be incapable of causing changes in transported bacteria. Thus, transported bacteria may not be in a given location long enough for selection to act, unless the waterbody is static (i.e., lentic) or the introduced microorganisms are deposited in sediments. Additionally, attached bacteria and endosymbiotic bacteria would be subject to selection for extended time periods.
It is not always clear how long attached bacteria remain. Do strains or species that colonize persist or are they replaced by another species in the same way that terrestrial plant species replace one another in secondary succession? Wise et al. (1997) demonstrated that a particular strain of Burkholderia cepacia was maintained for at least 16 days in the biofilm of a blackwater, organically stained stream, but it is not known for how long that particular strain was present prior to sampling. However, between days 16 and 32 the dominant strain of B. cepacia was replaced by a genetically different strain. Although some evidence shows that biofilm development and maintenance follows a repeatable and predictable pattern, with certain groups of bacteria appearing and supplanting or replacing others, the details have not been adequately elucidated in ecological and evolutionary terms. Waterborne pathogens and indicators can integrate into biofilms under some conditions, and such incorporation could lead to protection, proliferation, and opportunities for gene exchange among different biofilm microbes. Furthermore, if pathogens can become integrated into biofilms and retained for sufficient time, they would be subject to selection.
Bacterial Persistence in the Environment and Detection by Culture Methods
The extent to which pathogenic and bacterial indicator organisms persist outside a vertebrate host is highly variable and depends on the type of pathogen and the environmental conditions (Mitchell, 1972). Evidence shows that there are numerous reservoirs in which these organisms can persist and even increase in number (see discussion above). The problem of new or reemerging diseases is due, in part, to evolution and selection of pathogens, which in turn is caused by changes in water quality. These changes include phenomena such as inputs of novel organic substances, disruption of natural linkages, removal of riparian zones, channelization, and removal of instream habitats (e.g., debris dams)—all of which combine to affect the biotic and abiotic interactions that have evolved
for millennia. Bacteria and eukaryotes alike are then subject to new “harsh” environments. Interactions among species that normally do not occur have resulted in the panmixis of various genes and gene combinations (i.e., integrons and other transposable elements).
Other factors that must be considered regarding bacterial persistence in the environment, especially for bacteria from human or animal reservoirs, is the extent to which they are subjected to environmental stresses (such as extreme temperatures and pH levels, exposure to UV radiation in sunlight and toxic chemicals) that cause physiological stress and damage that is generally termed “injury.” Injury can range in severity and the effects of such injury influence bacterial detection by culture and other methods, as well as bacterial infectivity for human or animal hosts.
Kurath and Morita (1983) called cells that could grow on media viable, but they recognized that most of the bacteria in their samples (>10 times the number of CFUs) had metabolic activity but did not grow on the culture plates. Bacteria that become injured by losing the ability to multiply (form colonies or grow in liquid media), but remain otherwise completely functional as individuals and metabolically active, have been termed “viable but non-culturable” (VBNC; Oliver, 1993). This condition may be due to nutrient deprivation or to the effects of a variety of environmental stresses (Roszak and Colwell, 1987). Many types of bacteria that are injured to varying extents and may be VBNC can be identified from samples using biochemical, immunological, and nucleic acid molecular techniques. Important unresolved questions about VBNC bacteria are what ecological role they play and whether or not they are infectious for human or animal hosts. In this regard, the mere presence of a bacterium, especially when detected by non-culture methods, does not necessarily imply ecological importance (Morita, 1997) or human health risk.
Several species of bacteria including frank human pathogens such as Vibrio spp., Escherichia coli, Campylobacter spp., Salmonella spp., Micrococcus, and Pseudomonas have been found to be VBNC under a variety of conditions. A general concern is that many other waterborne bacterial pathogen and indicator species will be found that express this trait of non-culturablity and that this condition may confound the reliability of various microbial indicators that are based on culture techniques. However, as VBNC cells are metabolically active, indicators that measure some correlate or product of metabolism might be developed that are capable of monitoring these targets even when these cells cannot be cultured. Therefore, detection of bacteria by non-culture methods is both possible and a potentially useful measure of the presence and concentrations of these types of bacteria (see Chapter 5).
It is important to note that the environmental and public health significance of injured bacteria, especially those that are VBNC, remains controversial and uncertain (Bogosian and Bourneuf, 2001). As indicated in Chapter 5, there is considerable evidence that VBNC bacteria are not infectious for human or ani-
mals as well as some evidence that they are. Because of such conflicting evidence and the uncertainties of their public health significance, VBNC bacteria are not addressed or discussed in detail in this report. However, there are good reasons to address the relationships between injured bacteria and their detection by various biochemical, immunological, and nucleic acid methods, and these are covered in Chapter 5 and Appendix C.
Dispersal
Although bacteria and other microbes are widely dispersed in nature, not all bacteria are found everywhere. Whether transported and imported bacteria are capable of survival under new or novel environmental conditions is not known. In freshwater lotic ecosystems, many bacteria in transport are allochthonous, having originated from neighboring terrestrial systems and washed into the aquatic system. Many of these bacteria are not actively growing and presumably contribute little to any ecosystem process (Edwards et al., 1990). Because of the possibility of waterborne pathogens surviving and replicating in various environmental reservoirs however, an understanding of mechanisms of dispersal is important.
Bacteria and other microbes that successfully replicate within a system can take advantage of dispersal mechanisms to both move longitudinally within a waterbody and escape a waterbody. Bacteria can also use dispersal vectors such as formation of aerosols, invection, organic foams, arthropods, and vertebrates either actively or passively.
Abiotic Mechanisms of Dispersal
Long-distance dispersal of waterborne pathogens and bacterial indicators is dependent on the movement of bacteria within waterways and whether they can exit and survive outside the waterbody. Regarding the latter, bacteria can effectively escape the aquatic environment in several ways.
Aerosol Formation The formation of aerosols is a function of the geology of a watercourse. Any turbulence caused by rocks, boulders, and woody structures that make water splash or cause wave action results in the formation of aerosols. Depending on the size of the droplets, the aerosols are transported to varying degrees into the atmosphere. The types of bacterial species found in aerosols should be proportional to those normally found and those transported in the water. Thus, aerosol formation below a sewage treatment plant outfall would be expected to have higher proportions of enteric bacteria than aerosols created either upstream or far downstream of an outfall. Very little research has been conducted in the last two decades on aerosol formation and bacterial transport resulting from sewage treatment practices (e.g., EPA, 1980). However, Rosas et al. (1993) sampled the air over sewage treatment plants and at various distances from the
plants in Mexico City and reported that the highest numbers of pathogenic microorganisms were closest to the plant. Furthermore, Rosas et al. (1997) isolated E. coli from settled dust and air samples in several indoor and outdoor residential environments in Mexico City. Notably, the heterogeneity of E. coli was represented by 89 serotypes, most isolated from settled-dust indoor samples, and 21 percent of these demonstrated antibiotic multiresistance.
Organic Foams Organic foams, foams formed from turbulence or wave action, can be found in both pristine and contaminated streams and beaches. These foams can contain up to three orders of magnitude higher concentrations of bacteria than the underlying water (Hamilton and Lenton, 1998). Bacteria aid in the formation of these foams, and selection may have favored this process as an aid in their dispersal. Air sampled immediately over naturally occurring foams had much higher densities of bacteria than air sampled over open water in two streams in South Carolina (J.V. McArthur, unpublished data), and the proportion of antibiotic-resistant bacteria was much higher in the foam than in the water. Since the numbers of bacteria were 1,000 times higher in the foam, many antibiotic-resistant bacteria were being released into the air as these foams broke apart.
Arthropods and Vertebrates
Both arthropods and vertebrates can assist in the transport and dispersal of bacteria in aquatic systems. The movement of juvenile or adult aquatic insects exiting the water through hatching may be one mechanism of moving waterborne bacteria out of the water and into the air. Insect activity may also increase the release of bacteria from biofilms (Leff et al., 1994), while fish have been shown to have many opportunistic pathogens associated with their surfaces (Pettibone et al., 1996; Son et al., 1997). For example, fish that feed in or disturb sediments have higher proportions of antibiotic-resistant Aeromonas bacteria on their surfaces than fish that feed primarily in the water column (J.V. McArthur, unpublished data).
Summary
Clearly, improved understanding of the ecology of waterborne bacterial pathogens is needed before more effective means of detecting them directly or through the use of indicator organisms can be implemented. Knowledge of environmental reservoirs, movement and dispersal, movement and uptake of various genes, species interactions, and other factors discovered through carefully planned investigations is needed before new and more effective indicators can be developed and implemented. Failure to consider the evolutionary ecology of these organisms may result in the development of methods that are effective under only a few environmental conditions or not at all. Selection has enabled bacteria to adapt
to innumerable habitats and niches and it continues to modify bacterial genomes and genes, thus making the detection and identification of waterborne bacterial pathogens and indicators a moving target.
PROTOZOAN PARASITES
Ecology and Evolution of Parasites
To understand the requirements of indicators or indicator systems for waterborne pathogens, especially parasitic protozoa, it is important to first describe the ecology and evolutionary behavior of parasites.
Parasites and Population Ecology
Parasites, both protozoa and helminths (worms and flukes), have a complex population biology reflecting their diverse species and strains, their hosts, and the environment in which the parasites and the hosts reside. Parasite population ecology is described based on a nested hierarchy that identifies infrapopulations (all of the parasites of a single species in one host); suprapopulations (all of the parasites of a given species, in all stages of development, within all hosts of an ecosystem); and component populations (all of the infrapopulations of a species of parasite within all hosts of a given species in an ecosystem). The complexity of these associations is further complicated by the genetic diversity of the parasites, because many so-called “species” have genetic and phenotypic differences that are not reflected in the current taxonomy of a single genus and species.
Parasite populations are influenced by both density-dependent (i.e., regulated by the survival and reproduction of members of a population, including the immune response of the host and host mortality) and density-independent factors (regulated by external factors such as temperature, climate, and behavior). These density-independent factors are further complicated by both short- and long-term environmental changes that influence the presence and densities of the organisms over time and place.
Suprapopulation dynamics are influenced by both the density and the diversity of reservoir hosts. The impact of parasites on human hosts in a given geographical area will depend on the types and numbers of nonhuman hosts of the same parasite, such as feral, domestic, and agricultural animals. Host-parasite dynamics and host densities of nonhuman reservoir populations of the parasite influence the exposure risks and the flows of parasites through human populations. Parasites have a density-dependent impact on host populations and population dynamics by influencing per capita survival, reproduction, and fitness. The regulation of host populations by parasites has been described in quantitative terms using statistical models for the distributions of the parasites in their host populations and in the environment. A key consideration in these quantitative
relationships is how and to what extent parasite distributions are aggregated (i.e., their nonregular and nonrandom distributions) in the host and in the environment.
Human populations and public health can be strongly influenced by parasite population dynamics, such as the presence and proliferation of schistosomes (flukes causing “swimmers itch”) in surface waters used by humans for aquaculture, agriculture, recreation, and other purposes. There may be epizootic cycles of the parasite in other reservoir hosts that also influence human exposure risks for infection and illness. For example, the prevalence and aggregation of Giardia lamblia and Cryptosporidium parvum are probably influenced by the reproductive cycles of their host cattle, whereby calving season results in high infection rates and increased loads of cysts and oocysts into the environment. In some ecosystems and geographic locations, the extent of risks of human exposure to a parasite may depend on the population dynamics of the definitive host for the parasite. For example, in some locations a major risk for human exposure to Toxoplasma gondii may result from ingestion of the oocysts in the feces of felines, such as domestic and feral cats, as the definitive host or reservoir (see more below).
Non-density-dependent factors also are important in influencing parasite infection, pathogenicity, and virulence. These include age, immunity, nutritional status, sanitation, and behavior (such as eating habits and sexual activities). Age influences susceptibility to infection and severity of illness. Newborn animals are especially susceptible to infections with enteric protozoa such as G. lamblia and C. parvum. They become ill and often shed high concentrations of the parasites in feces. Immunity is important in protecting against parasite infection, at least temporarily. Human volunteer studies on C. parvum infectivity show that previously infected persons have a higher 50 percent infectious dose (shifted dose-response relationship), are protected from infection at lower exposure doses, and shed fewer oocysts when infected (Dann et al., 2000; Okhuysen et al., 1999, 2002; Teunis et al., 2002).
Geography influences host-parasite interactions at all scales ranging from global to very local. At the global scale, land mass fragmentation and movement and bodies of water can divide and separate parasites and their hosts. Such separations or barriers contribute to opportunities for changes in distribution and dispersal patterns and divergences in evolution. As scales decrease however, site-specific factors increasingly influence parasite distribution and host-parasite relationships. These include temperature, precipitation, soil type, vegetation, water quality, seasonal cycles, and availability of intermediate or alternative (nonhuman) hosts. Anthropogenic activities also can influence local loads of parasites. For example, as discussed elsewhere in this report, animal manures and sewage wastes can greatly influence local loads and concentrations of enteric parasites in water.
Evolutionary Aspects of Host-Parasite Relationships
Parasites and their hosts co-evolve under selective pressures that differ from those acting on free-living organisms. These pressures have shaped the ecology of parasites and their hosts over evolutionary time. A variety of interactions influence parasite pathogen effects on a host as well as host effects on the genetic and phenotypic properties of a parasite. The two organisms, parasite and host, live together—often one inside the other, sometimes cell inside cell, or even genome inside genome—and the duration of interactions can be prolonged. According to Combes (2001), two aspects of these interactions have played a major role in evolution. First, genetic information from the parasite can be expressed in the host phenotype, and vice versa. Because of the fundamental unity of the genetic code and the resemblance between signaling molecules in widely divergent organisms, a parasite can manipulate the physiology and behavior of its host to favor its own transmission or survival. Second, DNA can be exchanged between host and parasite, and such exchanges sometimes have occurred on a large scale, for example between eukaryotic cells and bacterial mitochondria. The invasion of genomes by transposable elements is a special case of gene exchange having important consequences for the variability of the host genome.
Phenotypic manipulation and exchange of genetic information can move host-parasite systems toward either symbiosis or greater adverse effects of the parasite on its host (Ewald, 1996). Furthermore, host-parasite associations can involve more than two partners. One host or its genome can use a second to manipulate the phenotype of a third genome. Every host-parasite system exerts pressure on its biotic environment, and thereby, parasites participate in the ecology and evolution of the biosphere.
Bush et al. (2001) state that “the essence of parasitism rests with the nature of host-parasite relationships.” Parasitism is an ecological concept that requires consideration of the parasite, the host, and the environment. Fundamental to parasitism is host resistance and immune response. Complex interactions take place between the host and its many different cells, including those of the immune system. Host recognition of the invading parasite triggers a range of immune responses that influence infectivity and disease outcomes. Furthermore, these immune responses to parasite infection are influenced by the host’s environment and other host-related factors, including genetics, age, gender, diet, physical environment, and behaviors. The host-parasite interaction can have outcomes ranging from successful elimination of the parasite with no adverse effects on the host to continued infection and invasion leading to immune responses that contribute to disease and death. Summarized below are a number of different host-parasite interactions and associations that highlight the importance of the host in the nature and outcomes of host-parasite relationships:
-
Evasion of host responses by mimicry and masking. Parasites have evolved a number of mechanisms to overcome or counteract host immune responses and sustain infectivity. One of the most fundamental mechanisms by which parasites avoid host immune detection and responses is by mimicking key host cell macromolecules or by masking their foreign antigens with a coating of host macromolecules.
-
Intracellular localization. A number of parasites enter host cells as a feature of their pathology and a mechanism of virulence. Examples of protozoan parasites that intracellularly invade the cells of their hosts are the amoebae Entamoeba histolytica and Toxoplasma gondii.
-
Transformation of surface antigens. A number of parasites undergo antigenic variation as a mechanism of their pathology and as a virulence factor. Antigenic variation or change produces immunologically novel parasite strains or variants that enable the parasite to avoid or evade the host’s immune response and also increases the abundance of parasites within an infected individual, thereby enhancing infectivity. Such antigenic variation affects the dynamics of parasite populations at both the between-host and the within-host levels. Thus, antigenic variation has a protective effect on the parasite at individual host, population, and community levels.
-
Direct suppression of host immune responses. Many parasites cause direct suppression of the host immune system. The intimate relationship between parasite and host in immune suppression phenomena is indicated by the dominant role of host cytokines (low molecular weight proteins that function as mediators in immune systems), either through their physiopathological effects on the host or through a direct effect on the parasite.
-
Effects on apoptosis. Programmed cell death (apoptosis) is a recently recognized mechanism of pathology and virulence dependent on host-parasite interactions by intracellular parasites. Apoptosis is an important regulator of the host’s response during infection by a variety of intracellular protozoan parasites, and this phenomenon has recently been reviewed (Luder et al., 2001). Parasitic pathogens have evolved diverse strategies to induce or inhibit host cell apoptosis, thereby modulating the host’s immune response, aiding dissemination within the host, or facilitating intracellular survival. The molecular and cell biological mechanisms of the pathogen-induced modulation of host-cell apoptosis and its effects on the parasite-host interaction and the pathogenesis of parasitic diseases are complex and only now being elucidated (e.g., for Cryptosporidium parvum and Toxoplasma gondii; Luder et al., 2001).
As described above, parasite-host interactions are manifest as both pathological effects and regulatory interactions involving host responses, including immune and other physiological responses, as well as genetic and adaptive responses. Such interactions must be considered not only at the level of individual human or other hosts, but also at the population and community levels and in the
context of the environment and the biosphere. That is, host-parasite interactions must be considered in an ecological context and on an evolutionary basis for both the parasite and the host. Hosts and their parasites interact in ways that can be symbiotic and mutually beneficial at one extreme or deleterious and lethal at the other. In medical and public health parasitology, most of the attention is understandably focused on parasite-host interactions that are debilitating or lethal to the host and on understanding the molecular, biochemical, physiological, and immunological aspects of host-parasite relationships with the goal of designing and implementing prevention and control measures.
Mechanisms of Parasite Pathogenicity
The mechanisms by which parasites cause infection and disease are diverse and complex. The diversity and complexity of the ecology of human parasites and their ability to cause infection and disease constitute a sufficiently substantive area of science for entire books to have been devoted to the subject (e.g., Bogitsh and Cheng, 1998; Bush et al., 2001; Gilles, 2000; Scott and Smith, 1994). As summarized by those authorities, some of the main factors responsible for the pathogenicity and virulence of parasites are (1) direct mechanical effects, (2) biochemical effects, (3) and immunological effects. Not only are the known mechanisms of parasite virulence and pathogenicity diverse and poorly understood, but new mechanisms and factors continue to be discovered or become more fully recognized for their importance. Because of the importance of molecular, biochemical, and immunological factors, genomics and proteomics (the study of all proteins produced by an organism) are contributing greatly to elucidation of the mechanisms of pathogenesis, virulence, and host susceptibility of waterborne and other pathogens. However, the biochemical mechanisms and genetic basis of pathology and virulence are far from being known for the vast majority of parasites and are unlikely to be fully elucidated and quantified for many of them for quite some time.
Introduction to Protozoa and Their Properties
The protozoa are an ancient group of unicellular organisms (single-celled eukaryotes sized 3-30 μm) probably derived from unicellular algae, but most have subsequently lost their photosynthetic capabilities. Movement is accomplished through one of three modes: flagellae, ameboid locomotion, or cilia (Allen, 1987; Stossel, 1994). Although there are numerous free-living protozoa, some can be obligate parasites of humans as well as animals, are zoonotic (spread from animals to humans), and often spread through the fecal-to-oral route. As such, these are important organisms from a public health perspective and are associated with waterborne disease worldwide, including the United States (see also Chapters 1 and 2).
Parasitic protozoa have both a trophozoite (ameboid) and a sporozoite stage within the host (Anderson, 1988). Sporozoites, which are the only stage that can survive outside a host, are called either cysts or oocysts depending on the taxonomic level. Cysts are the sporozoa of parasitic protozoa that reproduce by simple, asexual cell division, whereas oocysts are sporozoa that have both sexual and reproductive stages (Fenchel, 1987). For enteric protozoa, cysts or oocysts are the only stages that can survive outside a host and are excreted in the feces of infected individuals. Water can be contaminated by these supplies of fecally-laden (oo)cysts. Another important parasitic group is the Microsporidia. The Microsporidia are obligate spore-forming parasites in which the only environmentally stable form is the spore (Roberts and Janovy, 1995).
Although several species of waterborne protozoa are of public health concern, this section focuses on the ecology, evolution, and basic biology of the following groups and genera: the free-living amoebae Naegleria and Acanthamoeba; the enteric protozoa Giardia, Cryptosporidium, and Toxoplasma; and a relatively newly recognized group in human infections, the Microsporidia. Entamoeba histolytica and related species are amoeboid enteric protozoans that remain a risk worldwide, but waterborne disease transmission of this parasite appears to be quite rare in the United States during the last 20 years and is not discussed extensively.
Life Cycles, Taxonomy, and Health Effects
While the protozoa (especially those discussed below) may differ in their specific life cycles, they all have in common the production of (oo)cysts or spores, which are the resistant stages found in the environment. The pathway forward to a new host depends on the movement of (oo)cysts and spores through the environment, with water playing a significant role. This movement is accomplished via excretion of large numbers of (oo)cysts and spores over extended periods of time, in some cases chronic infections, survival of resistant forms in the environment, resistance to water treatment, various biotic effects, and a low infectious dose (i.e., few organisms are necessary to initiate a new infection).
The free-living amoebae produce cysts (dormant forms that are characterized by environmentally resistant external coverings) that are the resting stage of these protozoa and are abundant in the environment. As such, amoebae are the main predators of bacteria in soil and in fresh- and marine water sediments (Rodriguezzaragoza, 1994). The reproductive trophozoite stage is released from the cyst (excystation), and the protozoa reproduce by simple, binary cell division. The free-living amoebae, which can be parasitic, include Naegleria and Acanthamoeba. In this case, humans are accidental hosts, via exposure to cysts through the eyes or nose. Free-living amoebae are also capable of harboring other pathogens (e.g., the bacteria Legionella and Vibrio; Harf, 1994). For example, Thom et al. (1992) demonstrated that various strains of V. cholerae survived
better when Naegleria and Acanthamoeba were also present. Furthermore, strains of V. cholerae were isolated from cysts of Naegleria.
Naegleria fowleri is virtually ubiquitous in the aquatic environment and is considered an opportunistic pathogen of great public health concern. However, it is not a pathogen of the fecal-oral route, and the gastrointestinal system is not the primary target; rather, the most serious infections involve the central nervous system. There are two morphological forms of Naegleria, and successful diagnosis and appropriate therapy depend on precise laboratory identification and differentiation of pathogenic from nonpathogenic forms (Szenasi, 1998). Acanthamoeba are also normally found in soil or water and occur worldwide. They are not parasitic per se but can be pathogenic to humans. The most common mode of infection from Naegleria and Acanthamoeba for healthy individuals is swimming or diving into water inhabited by these amoebae and their accidental introduction into the nasal passages.
Naegleria spp. and Acanthamoeba spp. have similar life cycles, and both go through a trophozoite or vegetative stage where the amoebae proliferate asexually by fission. It is at this stage that they are pathogenic. If environmental conditions become adverse, both types of amoebae will encase themselves in a thick-walled cyst containing several ostioles or pores. The cysts are able to withstand desiccation and a host of other environmental stresses. When conditions are again favorable, excystation takes place with the new trophozoite emerging through an ostiole or pore (Martinez, 1985; Schuster, 2002).
There are several manifestations of infection from these free-living amoebae including rare but usually fatal cases of primary amoebic meningoencephalitis (PAM) caused by the genus Naegleria and most commonly the species N. fowleri, and granulomatous amebic encephalitis (GAE) most often caused by Acanthamoeba spp. In PAM, Naegleria trophozoites travel along the olfactory nerves and gain direct access to the central nervous system (CNS) where they quickly multiply and cause extensive damage by way of hemorrhagic necrosis, eventually destroying the olfactory bulb and the cerebral cortex. The victim generally dies within 3 to 10 days after the onset of symptoms and there is no effective treatment (Martinez, 1985; Schuster, 2002; Wiersma, 2002). In contrast to PAM, cases and deaths from GAE usually occur in immunocompromised individuals after weeks or months of CNS symptoms although both diseases are usually confirmed only after autopsy. In addition, the entry of Acanthamoeba can take place outside the nasal passages, and they can enter the bloodstream through a break in the skin or through the lungs before reaching the CNS. In addition to GAE, Acanthamoeba can also cause amoebic keratitis, a condition first noted in individuals with corneal trauma (Ma et al., 1981). It is more commonly associated with contact lens wearers who do not properly disinfect their lenses, thus allowing amoebae to proliferate on the lens. However, amoebic keratitis has not been shown to lead to CNS infection (Schuster, 2002).
Giardia are among the most primitive eukaryotic organisms in existence, being bilaterally symmetrical flagellated amoebae; a characteristic that is unique at their evolutionary level. They are obligate enteric parasites that undergo reproduction only after ingestion of cysts from fecally contaminated water, food, or hands. This parasite was first described by Antony von Leeuwenhoek from his own feces in 1681. Giardia have a broad range of environmental hosts including dogs, cats, sheep, and beavers. Between 1984 and 1990 there were approximately 25 outbreaks of this parasite that infected nearly 3,500 people (Marshall et al., 1997; Steiner et al., 1997). It is estimated that worldwide, 2.8 million people per year in both developed and undeveloped countries are infected with Giardia (Ali and Hill, 2003). Excystation occurs and trophozoites are released in the small intestine, where they attach to the microvillae and begin asexual reproduction. This is followed by the production of both immature sporozoites and more trophozoites in the large intestine, and finally maturation of immature sporozoites to cysts as they travel down the large intestine and exit into the environment in the feces. Giardia lamblia causes diarrhea and abdominal pain in infected persons around the world, in both industrialized and developing countries, and it is an important cause of morbidity in children and adults. However, the basic ecology of this parasite is not well understood.
Microsporidia are eukaryotic spore-forming obligate protozoan parasites that infect all animal groups (especially arthropods), including humans (Weiss, 2001). Microsporidia are usually transmitted by direct human-to-human contact (Mota et al., 2000); however, spores from Microsporidia are common in the environment including surface and drinking waters. These organisms have the smallest known eukaryotic genome and appear to reproduce both sexually and asexually; the latter by a budding process being most common results in a variety of types of spores. Although humans are not their natural hosts, Microsporidia are considered obligate parasites that require a host, and the most common infections in humans are found in the immunocompromised. There are more than 1,000 species of Microsporidia, of which 13 are presently known to infect humans (e.g., Encephalitozoon cuniculi, E. intestinalis, E. hellem, Enterocytozoon bieneusi). Two species of Microsporidia are associated with gastrointestinal disease in humans: Enterocytozoon bieneusi and E. intestinalis (Dowd et al., 1998a).
Cryptosporidium and Toxoplasma are obligate parasites and require a host to reproduce—the former as an intestinal parasite and the latter as a tissue parasite. Cryptosporidium completes its cycle in a single host in the intestinal tract (O’Donoghue, 1995). Oocysts are ingested and the parasite undergoes asexual and sexual reproduction in the intestinal tract. Oocysts are excreted in the feces as a result of reproduction and are extremely hardy. The organism has great genetic recombination abilities through these reproduction strategies and has co-evolved with its various mammalian hosts. Recent analyses of Cryptosporidium show significant host adaptation and the ability of genotypes to expand their host range (Xiao et al., 2002).
Cryptosporidiosis infections are ubiquitous. In industrialized and developing countries, 2.2 percent compared to 6.1 percent of hospital patients admitted for diarrhea pass oocysts, respectively (Guerrant, 1997). However, up to 30 and 50 percent of the U.S. population has antibodies to C. parvum (Frost et al., 2002; Isaac-Renton et al., 1999) compared to upwards of 90 percent of people in impoverished regions of developing countries (Guerrant, 1997; Zu et al., 1994).
Toxoplasma gondii may be one of the most common parasitic infections of man and other warm-blooded animals (Hill and Dubey, 2002). It has a worldwide distribution and can be found from Alaska to Australia. Hill and Dubey (2002) estimated that one-third of humanity has been exposed to this parasite. This parasite completes its entire life cycle in two hosts. Cats (feral and domestic) are the definitive host in they alone produce the oocysts. The parasite completes its life cycle by reproduction in the intestine of cats, and cysts are excreted in their feces. The parasite travels to the next host as an oocyst excreted in feces; however the oocyst (unlike Cryptosporidium) is not infectious upon excretion and requires a maturation phase in the environment that is on the order of days. Other mammals ingest the oocysts and become infected (Kitamoto and Tanabe, 1987). In rodents, the parasite undergoes the reproductive stages in muscle tissue, and upon ingestion by a cat the parasite is able to complete its life cycle. Humans exhibit a “dead-end” infection associated with partial life stages that results in fever. In humans, the primary health concern is for the fetus since the organism can cross the placental barrier in pregnant women and result in severe birth defects such as retardation (Lopez and Wilson, 2003).
Sources, Stability, and Survival
The environmental route of transmission for many protozoan parasites has made it necessary to develop new methods for their early and repeated detection (Smith, 1998). These parasites pose new and emerging threats because of their ability to survive in a variety of moist habitats including surface waters and because protozoan parasites were among the most frequently identified etiologic agents in waterborne disease outbreaks (see Figure 1-1; Marshall et al., 1997).
The parasitic protozoa are of particular interest in this chapter because they may have significant environmental reservoirs that harbor the (oo)cysts, including the free-living forms, in fresh- and marine waters. Furthermore, since ingestion is required for most diseases to develop, knowledge of potential vectors or sources is critical. All of the protozoa discussed in this chapter, except for Naegleria and Acanthamoeba, are zoonotic in nature; thus, a wide range of animals may serve as sources for the parasites.
Species of the genus Naegleria are found in stagnant bodies of freshwater such as lakes, slow-moving rivers, ditches, and non- or poorly chlorinated swimming pools. Naegleria are known to be thermophilic, and the incidence of infection follows a seasonal pattern occurring mostly in the summer months when
water temperatures rise (Martinez, 1985). They have been isolated from environments with temperatures between 26.5 and 28°C and become more virulent between 30 and 37°C (John, 1993). Acanthamoeba spp. are ubiquitous and hardier than Naegleria and have been isolated from many different environments including ocean sediment and even dust. However, their growth is more prolific at lower temperatures (25 to 35°C) than Naegleria (Martinez, 1985).
Research on Naegleria gruberi, which differentiates from the amoebae to the flagellate form in less than 90 minutes, suggests that there are at least two “holdpoints” where the cells hold for up to four hours awaiting additional stimulus (Fulton and Lai, 1998). Changes in temperature greater than 10°C will cause complete differentiation whereas smaller temperature changes result in the intermediate forms. If these hold-points are found for other related species such as N. fowleri, large-scale climate and weather change may affect the forms present in the environment and the potential infection rates.
The ecology of Toxoplasma gondii oocysts is diverse and varied. While the cat is the primary source for Toxoplasma, oocysts from terrestrial land animals (e.g., feral cats) have found their way into surface waters, resulting in major documented outbreaks in humans such as in the Greater Victoria area of British Columbia, Canada (Bowie et al., 1997; Isaac-Renton et al., 1998). Coastal freshwater runoff has been implicated in the infection of southern sea otters (Miller et al., 2002). These researchers found that sea otters sampled near areas of maximal freshwater runoff were three times more likely to be seropositive for T. gondii than otters sampled at low-flow areas. Serological evidence of T. gondii infection of deer mice living in the riparian zone of various watersheds suggests that the oocysts are being shed near the waters’ edge (Aramini et al., 1999).
Besides sea otters, numerous other marine mammals have been infected with T. gondii. Although it is not known how all of these mammals become infected, there is evidence that oocysts can remain viable and infective after removal by eastern oysters (Lindsay et al., 2001). Invertebrates and vertebrates (including humans) may be infected by the handling and eating of oysters. The ability of Toxoplasma to survive under extremely broad environmental conditions has prompted research into the ranges of tolerance of different variables including temperature. The infectivity of T. gondii oocysts showed no loss at 10, 15, 20, and 25°C for 200 days (Dubey, 1998), and oocysts remained infective up to 54 months at 4°C and for 106 days at –5 and –10°C. Some oocysts remained infective at temperatures approaching 55°C. With tolerance ranges that broad, it is easy to see why these protozoa have a global distribution.
Very little is known about the sources of Microsporidia. In France during the summer of 1995, a waterborne outbreak of Microsporidia occurred with approximately 200 cases of disease (Sparfel et al., 1997). The causative species identified was Enterocytozoon bieneusi. Although fecal contamination of the drinking water was never detected, contamination from a nearby lake was suspected. In the United States there are minimal data on the occurrence of human strains of
Microsporidia in surface waters. Dowd et al. (1998b, 1999) described a polymerase chain reaction (PCR) method for detection and identification of Microsporidia (amplifying the small subunit ribosomal DNA of Microsporidia). They found isolates in sewage, surface waters, and groundwaters. The strain that was most often detected was Enterocytozoon bieneusi, which is a cause of diarrhea excreted from infected individuals into wastewater. Microsporidia spores have been shown to be stable in the environment and remain infective for days to weeks outside their hosts (Shadduck, 1989; Shadduck and Polley, 1978; Waller, 1979). Because of their small size (1 to 5 μm), they may be difficult to remove using conventional water filtration techniques, and there is a concern that these microorganisms may have an increased resistance to chlorine disinfection similar to Cryptosporidium. Initial studies using cell culture suggest that the spores may be more susceptible to disinfection (Wolk et al., 2000).
In contrast to Microsporidia, much is known about sources and survivability of Cryptosporidium and Giardia in the environment, especially the aquatic environment (see more below), where their (oo)cysts can survive for weeks or months (Robertson et al., 1992; Rose and Slifko, 1999). Both Cryptosporidium and Giardia are well adapted to environmental extremes, being able to survive temperatures ranging from 4 to 37°C and environments ranging from homeothermic animal bodies to thermally and chemically variable freshwater (Rose and Slifko, 1999). Species of Giardia exhibit a high degree of host specificity and as such have been named based on their normal host (e.g., G. lamblia inhabits humans, G. muris inhabits rodents, and G. ardeae inhabits birds). Recent phylogenetic analysis has shown that G. lamblia infects humans, and many other mammals can potentially become infected with the human type (Marshall et al., 1997). Thus, there is good evidence that G. lamblia has environmental reservoirs such as beavers and possibly muskrats.
Cryptosporidium parvum, also a zoonotic species, can infect an unusually wide range of mammals, including humans (O’Donoghue, 1995). The phylogenic relationships between the whole range of species and genotypes have shed light on the zoonotic transmission potential of Cryptosporidium (Egyed et al., 2003). Typing of isolates from various geographic regions and host origins has relied on direct DNA sequencing or selected genetic loci analysis using PCR. These studies have found mixtures of genotypes and species in feces of infected animals or humans (Morgan et al., 1999).
Two genotypes of C. parvum are of special interest: Genotype 1 only infects humans, whereas Genotype 2 occurs in a wide range of animals, including humans although it is not known if it results in different pathology (Peng et al., 1997; Rose et al., 2002). Indeed, the ability of C. parvum Type 2 to infect not only man, but domesticated animals such as cows, goats, sheep, pigs, horses, dogs, and cats and even wild animals such as mule deer may provide a tremendous evolutionary advantage. DNA-DNA hybridizations reveal little or no mixing between genotypes, suggesting cryptic species. A cryptic species shows little,
if any, phenotypic divergence but differs genetically and as seen in this example shows no recombination occurring between the two genotypes.
The ability of Cryptosporidium to survive outside the host suggests that evolution favors strains that can generate and input many oocysts into the environment without seriously harming the host. Oocysts were found in three species of rodents in Poland (Bajer et al., 2002). Interestingly, there were significant differences between the Cryptosporidium species identified in rodents over time, though fewer older animals carried infection and there were marked seasonal differences. Rickard et al. (1999) tracked both Cryptosporidium and Giardia species in populations of white-tailed deer in the southern United States. These researchers found higher infection rates associated with Cryptosporidium than Giardia. As for rodents, the probability of protozoan infection decreased with increasing age of the deer.
A few studies have tried to determine primary sources of Cryptosporidium and Giardia cysts and oocycts in watersheds. Studies comparing agricultural and wildlife sources have shown that the lowest prevalence of Giardia and Cryptosporidium was found in wildlife (Heitman et al., 2002), while the highest concentrations were found in cattle feces. Given the potential runoff from agricultural sources into watersheds and waterways, this observation is significant. In a 17-month survey (Bodley-Tickell et al., 2002) of Cryptosporidium oocysts in surface waters draining a livestock operation, the parasites were found to be present year-round, with maximum concentrations and highest frequency of occurrence during autumn and winter. Cryptosporidium was also found in an isolated pond (no livestock), indicating that wild animals alone could transport or import oocysts to surface waters. Waterfowl can also disseminate infectious C. parvum and Giardia in the environment (Graczyk et al., 1998). Although this finding is not surprising, the widespread levels of contamination may be, since seven out of nine sites showed occurrence of C. parvum and all nine sites were positive for Giardia in the feces of migrating Canada geese. Given the size of the migrating waterfowl populations in the United States, this observation is of concern.
Cryptosporidium oocysts transported from river waters into estuarine and marine systems have been shown to be taken up and sequestered by filter-feeding invertebrates. Lowery et al. (2001) showed that the marine filter-feeding mussel (Mytilus edulis) collected from the shores of Belfast Lough in Northern Ireland had Genotype 1 oocysts of C. parvum. Thus, these filter-feeding invertebrates serve as environmental reservoirs of this protozoan. While the natural rate of release from these Cryptosporidium reservoirs is not known (because Mytilus edulis is consumed by humans), this aspect of the ecology of C. parvum may have direct public health ramifications.
Terrestrial insects have been implicated as both control agents and vectors of dissemination of Cryptosporidium oocysts (Dumoulin et al., 2000; Follet-Dumoulin et al., 2001; Mathison and Ditrich, 1999). Mathison and Ditrich (1999) demonstrated that many oocysts can pass safely through the mouth parts and
gastrointestinal tracts of dung beetles; however, the majority are destroyed. Predation by coprophagous (dung eating) insects can be important in both reducing oocysts and spreading the oocysts that are not destroyed throughout the environment. In contrast, free-living ciliated protozoa, such as Paramecium caudatum, are capable of consuming up to 170 oocysts per hour (Stott et al., 2001). Thus, predation, under certain conditions, may affect the density of oocysts.
Although Cryptosporidium infects mammals, it is unable to infect other vertebrate groups such as fish, amphibians, and reptiles (Graczyk et al., 1996). This inability greatly limits the spread and dissemination of oocysts in surface waters. However, depending on the characteristics of the watershed, runoff can carry oocysts shed by infected mammals in major pulses. Once in the waterway, oocysts can be maintained until consumed either by humans or by other mammals.
Cryptosporidium and Giardia in Water
Most studies of waterborne protozoa of public health concern have focused on the occurrence of the enteric protozoa Cryptosporidium and Giardia. Although these two enteric protozoan parasites are not related taxonomically, they are closely related from an epidemiological, regulatory, and public health point of view. In addition, methods for their simultaneous detection in water have led to much information on their occurrence, more so than for any of the other protozoan parasites. In surveys from the early 1990s, it was reported that for 66 surface water treatment plants in 14 states and 1 Canadian province, 81 percent of the raw water samples had Giardia cysts and 87 percent tested positive for Cryptosporidium (LeChevallier et al., 1991a,b). Further reviews of the literature have found that most waters contain some level of cysts and oocysts ranging from a high in treated wastewaters (104 per 100 liters) to a low in pristine waters (0.1 per 100 liters) (Rose, 1997; Rose et al., 2001a; Slifko et al., 2000). Similar levels have been found throughout the United States and in Europe (Ong et al., 1996; Smith and Rose, 1998). As noted previously, domestic animals, cattle in particular, and sewage discharges have been identified as some of the primary sources of Cryptosporidium and Giardia in water.
The cysts and oocysts of Giardia and Cryptosporidium may also be transported from irrigation waters to row crops, some of which may be eaten raw. Thurston-Enriquez et al. (2002), using both molecular and immunofluorescent techniques, were able to detect oocysts of Cryptosporidium in 36 percent and cysts of Giardia in 60 percent of all irrigation water samples. They also found that 28 percent of samples had Microsporidia. Average concentrations for Giardia and Cryptosporidium varied from 559 cysts and 229 oocysts per 100 liters in samples from Central America to 25 cysts and <19 oocysts per 100 liters in the United States, respectively. These researchers demonstrated that agricultural irrigation waters may be a significant vector for the transmission of waterborne protozoa and corresponding diseases.
Weather can influence both the transport and the dissemination of these protozoa (Fayer, 2000; Fayer et al., 2000; Rose et al., 2001b). A study conducted in the United States demonstrated similar results with both wildlife and dairy farms contributing to Cryptosporidium oocysts in the watershed and implicated cold seasons as high-risk periods for oocyst contamination of surface waters (Jellison et al., 2002). High concentrations of oocysts during winter and autumn may be indicative of reduced predation. Alternatively, cold temperatures may actually preserve oocysts and thus provide sources during warmer weather. Skerrett and Holland (2000), in a temporal survey at five sites near Dublin, Ireland, showed that the maximum number of oocysts was found after a period of heavy rainfall associated with increased runoff. Knowledge of how changes in rainfall, snow-melt, and runoff affect the transport of protozoan parasites is critical.
Another important aspect of Cryptosporidium and Giardia (oo)cyst ecology that may affect their distribution and survival is their sedimentation velocity. These velocities are much too low to cause significant sedimentation in surface waters or reservoirs (Medema et al., 1998). However, both cysts and oocysts attach readily to organic particles, which greatly increase their sedimentation velocities. Attachment to particles will affect not only the deposition onto sediments but also the potential for consumption. Depending on the size of the particles, ciliated protozoa may not be able to graze the (oo)cysts. Although infection of fish, amphibians, and reptiles has not been shown, bottom-feeding fish may transport the (oo)cysts to new locations.
Summary
Parasite interactions with their hosts and the environment are diverse, complex, and continually evolving. These interactions involve a considerable amount of genetic exchange and selection, as well as host-dependent phenotypic expression. For many parasites, the host immune response is a major factor influencing pathogen-host interaction and its effects on the health of the host. In addition, parasites and their hosts coevolve and show a variety of population-based ecological interactions influencing population density, geographic location, and other factors related to the parasite and host environment.
Factors such as temperature, source of shedding (i.e., from hosts), rainfall, and predation can affect the survivability of these parasites in the aquatic environment. However, other aspects of the ecology of cysts and oocysts are not well understood. For example, why do low temperatures seem to promote survival, and why are the highest concentrations often found in autumn and winter months? Is there more than a proximate answer to this question? Does this observation confer any selective advantage on the parasites and increase their chance of infecting new hosts? In other words, are certain genetic variants better able to adapt to and survive the changing environment than others? If so, these strains would be expected to increase over time. Are there density-independent factors that
affect the survival and chance of future infection? Factors such as genetic background, metabolic activity, or (oo)cyst development are possible examples. Alternatively, are density dependent factors such as predation more important?
While this discussion has focused on the environmental forms (i.e., cysts and [oo]cysts) of protozoans with little mention of the infectious forms, an improved understanding of the ecology of the infectious forms may be crucial in understanding the distribution and dissemination of the environmental forms. For example, Thompson and Lymbery (1996) discussed the genetic variability in parasites in the context of ecological interactions with the host. More information is needed on parasite variability and species recognition as related to parasite or host occurrence. There is a need for accurate parasite characterization to determine fundamental questions about zoonotic relationships and parasite transmissions. Ecological interactions between inter- and intraspecific levels within the host and the impact of these interactions on the evolutionary and clinical outcomes of parasitic infections also have to be studied.
YEASTS AND MOLDS
Introduction and Background
Yeasts and molds are collectively called fungi as a group and possess defined nuclear membranes that contain the chromosomes of the cells. There are more than 100,000 species of known fungi, although only a few are known to be human pathogens. Fungi are more than 10 to 100 times larger than bacteria. Molds are multicellular, complex organisms that produce sexual and asexual spores. They appear as cottony and fuzzy growth in food and other materials due to the growth of hyphae and mycelium, and many are spoilage organisms. Some molds are beneficial to humans because of their production of important antibiotics and fermentation of foods, while others may cause a variety of human diseases ranging from “athlete’s foot” to aspergillosis.
Yeasts are single-celled fungi that are usually oval in shape and divide asexually by budding or sexually through production of spores. They are important in food fermentation, food spoilage, and several human diseases—especially Candida albicans, the common cause of various “yeast infections.” Yeasts and molds are ubiquitous in the environment, including air, soil, food, and water.
Ecology and Evolution of Fungi and Their Role as Human Pathogens
Humans are continuously exposed to fungi from various environmental sources and often are colonized with fungi. On rare occasions, some fungi cause human infection and illness and most of these illnesses occur in immunocompromised hosts. Fungal infections or mycoses are classified according to the degree of tissue involvement and mode of entry into the host. These categories are (1)
superficial (local in skin, hair, and nails); (2) subcutaneous (infection of the dermis, subcutaneous tissue, or adjacent structures); and (3) systemic (deep infections of internal organs). For a fungus to cause serious disease it usually has to actively invade tissues, especially deeper tissues, and become disseminated throughout the body (systemic).
Fungal infections also can be categorized as frank (can infect healthy, immunocompetent hosts) or opportunistic (can infect only the immunocompromised) pathogens. Frank fungal infections in the United States are uncommon, being confined to conditions such as candidiasis (thrush) and dermatophyte skin infections such as athlete’s foot. However, in immunocompromised hosts, a variety of normally mild or nonpathogenic fungi can cause systemic and potentially fatal infections. Thus, the immune state of the host plays an important role in the infectivity and health effects of fungi.
The pathogenic mechanisms of fungi tend to be highly complex. This is because they arise in large part from adaptations of preexisting characteristics of the organisms’ nonparasitic life-styles (van Burik and Magee, 2001). Most of the human pathogenic fungi are dimorphic (i.e., able to reversibly transition between yeast and hyphal forms) (Gow et al., 2002; Rooney and Klein, 2002). This dimorphism is an important attribute of fungi because the morphogenic change from one form to the other is often associated with host invasion and disease. The nature of the association between morphogenic transition, pathogenicity, and virulence is a subject of considerable interest and debate among experts, and efforts are being made to understand both the fungal and the host factors that influence host-fungi interactions and outcomes. Currently, the evidence suggests that the significance of morphological changes by fungi varies considerably in different fungal diseases.
For many fungi, other factors besides morphological changes play an important role in pathogenicity, as do the host’s immune competence, immune response, and other physiological and constitutive states. As noted above, it is well established that the nature and extent of impairment of host defenses influences the pathology, severity, and outcomes of fungal infections, such that the clinical manifestations of disease are contingent upon the hosts’ immune system and other host characteristics. Unlike bacteria, where virulence genes are often organized together into definable “pathogenicity islands” that are co-regulated, most fungal toxins are spread across the genome, unlinked, and independently regulated. The interaction of the fungus with its host and the properties of the host are also critical factors in the expression of virulence and the production of disease. Some protein components of fungi and human cells are functionally interchangeable (Brown, 2001). For example, human proteins with fundamental roles in the cell cycle, stress responses, gene regulation, protein localization, metabolism, and energy generation can functionally replace the corresponding proteins in the budding yeast Saccharomyces cerevisiae.
Fungi and Waterborne Pathogens
Although the potential role of yeasts and fungi as waterborne pathogens has not been systematically assessed, they are regularly isolated from water samples. Of the fungal colonies isolated on agar plates from water, typically about half are yeast and the other half mold colonies. Molds from the families Oomycetes and Chytridiomycetes can virtually always be found in fresh and saline water in the environment. While these molds are not generally pathogenic to humans, they can infect animals and cause disease (Alexopoulos et al., 1996).
Other potential waterborne yeast and mold pathogens include Candida albicans, Geotrichum candidum, and Aspergillus fumigatus. A severe disease called “swamp cancer” can occur when farmers are exposed to Pythium insidiosum in swampy environments (Deneke and Rogers, 1996). Although yeasts and molds are not considered a major source of human waterborne pathogens at the present time, their ubiquitous presence in water should not be ignored.
Summary
Fungi, yeasts, and molds are widely distributed in the environment and most are not human pathogens. For the relatively few that are known to be pathogenic to humans, the interactions between the pathogenic fungi and their hosts are intimate and complex and depend greatly on fungal and host properties as well as environmental conditions. Most experts agree that the properties of the fungus and the host seem to contribute about equally to the outcomes of exposure, infection, and disease and that there are multiple factors at work on the part of both participants (fungus and host). The majority of fungi are opportunistic pathogens, which serves to emphasize the fundamentally important properties of the host in fungal pathogenesis and virulence. A comprehensive analysis that considers the multiple properties of the fungus and the host and the complex manner in which they interact in various environmental settings is necessary in order to understand the host-parasite interaction and gain insights into the factors responsible for exposure, infection, disease, and the resulting health effects in humans.
SUMMARY: CONCLUSIONS AND RECOMMENDATIONS
The ecology and evolution of waterborne pathogens have important implications for the emergence and reemergence of those pathogens of public health concern. The concept of using indicators for waterborne pathogens implies that certain characteristics of microorganisms such as genes and gene products remain constant under varying environmental conditions. As discussed in this chapter however, this assumption cannot always be relied on.
The effectiveness of indicator technologies that are based on the detection of some aspect of the biology or chemistry of a living organism (whether a pathogen
or an indicator microorganism) may decrease over time because of evolutionary changes in the target organism. Natural and artificial selection may alter the structure, function, and production of biological molecules or cause other changes in the organism that affect the ability of the indicator to detect it. Therefore, it is important to understand the effects of the environment on these targets and organisms. In other words, will an indicator be effective in a wide range of geographic locations, habitats, and under different environmental conditions? How rapidly do waterborne infectious microorganisms change in the environment, and are such changes in indicator organisms reflective of changes in the infectious organism (i.e., do they have parallel evolutionary trajectories)?
Existing and candidate indicator organisms should have ecologies and responses to environmental variations similar to those of the pathogenic organisms that they are supposed to be indicating. Furthermore, environmental changes may lead to changes in selective pressures resulting in new strains of pathogens with different traits. These reservoirs can be important in their transmission to other hosts. Genetic materials can be gained or lost during evolution. This gain or loss affects not only the effectiveness of a particular indicator but also one’s ability to detect pathogens directly. The presence and interaction of biotic and abiotic reservoirs that offer environmental refuge may affect the survivability and pathogenicity of the pathogen. Understanding the ecology and evolution of pathogens will provide insights into their pathways of transmission, modes of distribution, potential to reemerge in the future, or emergence in other environments.
The committee makes the following recommendations to improve the understanding of the ecology and evolution of waterborne pathogens and the development of new and effective indicators of microbial contamination:
-
Natural background density of waterborne pathogens should be established to differentiate between native opportunistic pathogens and introduced pathogens.
-
Efforts should be made to differentiate between indicators and pathogens that are native to the environment and those that are introduced from external sources, such as human and animal wastes.
-
Because some waterborne pathogens or indicator organisms may survive and replicate in various environmental reservoirs independently of each other, an improved understanding of the ecology and natural history of microbial indicators and pathogens and the mechanisms of their persistence, proliferation, and dispersal should be sought.
-
Advanced analytical methods should be used to help distinguish between introduced pathogenic and naturally occurring nonpathogenic strains of waterborne microorganisms and to characterize the emergence of new strains of pathogens as a result of genetic change.
-
Bacteria, viruses, and protozoa have evolved mechanisms that facilitate their rapid response to environmental changes. These mechanisms may influence
-
the infectivity and pathogenicity of the organism. Therefore, additional research is needed on microbial evolutionary ecology to address long-term public health issues.
-
The ecology of waterborne pathogens should be assessed in relation to modern agricultural practices and other anthropogenic activities, such as urbanization. Animal wastes from agriculture and urban sewage, runoff, and stormwater are major contributors to both human pathogenic and nonpathogenic strains of microbes, and the wide use of antibiotics in animal agriculture and in human and veterinary therapy leads to selection for antibiotic-resistant phenotypes.
-
Research in genetic ecology is needed to address issues of bacterial resistance to antibiotics, disinfectants, and other chemicals (such as heavy metals) and the regulation and transferability of these resistance traits either independently or together as sets of multiple resistance genes. The factors that select for increased resistance to these agents in natural populations of bacteria need to be elucidated as do the factors influencing the natural transfer of these resistance traits to waterborne pathogens, indicators, and other aquatic microorganisms.
-
Research is needed to develop a better understanding of the ecology and natural history of both the environmental and infectious stages of pathogens and the parallel stages of indicator organisms to grasp how the organisms are distributed in nature; how they persist and accumulate in water, other environmental media, and in animal reservoirs; and how dissemination of the environmental form occurs, especially human exposures.
-
Genetic and phenotypic characterization of pathogenic viral, bacterial, and protozoan parasites is needed to elucidate zoonotic relationships with their hosts and factors influencing waterborne transmission to humans.
-
Given the ubiquity of yeasts and molds in water samples, research should be conducted to clarify their role in the transmission of waterborne diseases.
REFERENCES
Aggarwal, R., and S.R. Naik. 1997. Epidemiology of hepatitis E: Past, present and future. Tropical Gastroenterology 18: 49-56.
Alexopoulos, C.J., C.W. Mims, and M. Blackwell. 1996. Introductory Mycology. New York: John Wiley and Sons.
Ali, S.A., and D.R. Hill. 2003. Giardia intestinalis. Current Opinion in Infectious Diseases 16(5): 453-460.
Allen, R.D. 1987. The microtubulues as an intracellular engine. Scientific American 256: 42-49.
Anderson, O.R. 1988. Comparative Protozoology: Ecology, Physiology, Life History. New York: Springer-Verlag.
Andradottir, H.O., and H.M. Nepf. 2000. Thermal mediation by littoral wetlands and impact on lake intrusion depth. Water Resources Research 36: 725-735.
Anonymous. 2002. From the Centers for Disease Control and Prevention. Acute flaccid paralysis associated with circulating vaccine-derived poliovirus - Philippines, 2001. Journal of the American Medical Association 287(3): 311.
Anonymous. 2003. Laboratory surveillance for wild and vaccine-derived polioviruses, January 2002-June 2003. MMWR 52(38): 913-916.
Aramini, J.J., C. Stephen, J.P. Dubey, C. Engelstofdt, H. Schwantje, and C.S. Ribble. 1999. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiology and Infection 122: 305-315.
Baigent, S.J., and J.W. McCauley. 2003. Influenza type A in humans, mammals and birds: Determinants of virus virulence, host-range and interspecies transmission. Bioessays. 25(7): 657-671.
Bajer, A., M. Bednarska, A. Pawelczyk, J.M. Behnke, F.S. Gilbert, and E. Sinski. 2002. Prevalence and abundance of Cryptosporidium parvum and Giardia spp. in wild rural rodents from the Mazury Lake District region of Poland. Parasitology 125: 21-34.
Balayan, M.S. 1997. Epidemiology of hepatitis E virus infection. Journal of Viral Hepatitis 4: 155-165.
Barker, J., T.J. Humphrey, and M.W.R. Brown. 1999. Survival of Escherichia coli O157 in a soil protozoan: Implications for disease. FEMS Microbiology Letters 173: 291-295.
Bellar, M., A. Ellis, S.H. Lee, M.A. Drebot, S.A. Jenkerson, E. Funk, M.D. Sobsey, O.D. Simmons III, S.S. Monroe, T. Ando, J. Noel, M. Petric, J. Hockin, J.P. Middaugh, and J.S. Spika. 1997. Outbreak of viral gastroenteritis due to a contaminated well: International consequences. Journal of the American Medical Association 278(7): 563-568.
Bergelson, J.M. 2003. Virus interactions with mucosal surfaces: Alternative receptors, alternative pathways. Current Opinion in Microbiology 2003 6(4): 386-391.
Bernal, W., H.M. Smith, and R. Williams. 1996. A community prevalence study of antibodies to hepatitis A and E in inner-city London. Journal of Medical Virology 49(3): 230-234.
Bishop, R.F. 1996. Natural history of human rotavirus infection. Archives of Virology supplementum 12: 119-128.
Bjedov, I., O. Tenaillon, B. Gérard, V. Souza, E. Denamur, M. Radman, F. Taddei, and I. Matic. 2003. Stress-induced mutagenesis in bacteria. Science 300: 1404-1409.
Bodley-Tickell, A.T., S.E. Kitchen, and A.P. Sturdee. 2002. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Research 36: 1880-1886.
Bogitsh, B.J., and T.C. Cheng. 1998. Human Parasitology. New York: Academic Press. Bogosian, G., and E.V. Bourneuf. 2001. A matter of bacteria life and death. EMBO Reports 2(9): 770-774.
Borchardt, M.A., P.D. Bertz, S.K. Spencer, and D.A. Battigelli. 2003. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Applied and Environmental Microbiology 69(2): 1172-1180.
Bowie, W.R., A.S. King, D.H. Werker, J.L. Isaac-Renton, A. Bell, S.B. Eng, and S.A. Marion. 1997. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 350: 173-177.
Brown, A.J.P. 2001. Fungal pathogens—the devil is in the detail. Microbiology Today 29: 120-122.
Bush, A.O., J.C. Fernández, G.W. Esch, and J.R. Seed. 2001. Parasitism: The Diversity and Ecology of Animal Parasites. Cambridge: Cambridge University Press.
CDC (Centers for Disease Control and Prevention). 1997. Multidrug-resistant Salmonella serotype typhimurium—United States, 1996. MMWR 46(14): 308-310.
Chung, H., L.-A. Jaykus, G. Lovelace, and M.D. Sobsey. 1998. Bacteriophages and bacteria as indicators of enteric viruses in oysters and their harvest waters. Water Science and Technology 38(12): 37-44.
Clayson, E.T., B.L. Innis, K.S. Myint, S. Narupiti, D.W. Vaughn, S. Giri, P. Ranabhat, and M.P. Shrestha. 1995. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. American Journal of Tropical Medicine and Hygiene 53(3): 228-232.
Cohen, J.A., W.V. Williams, and M.I. Greene. 1988. Molecular aspects of reovirus-host cell interaction. Microbiological Science 5(9): 265-270.
Colwell, R.R. 1996. Global climate change and infectious disease: The cholera paradigm. Science 274: 2025-2031.
Combes, C. 2001. Parasitism. The Ecology and Evolution of Intimate Interactions. Chicago, Illinois: University of Chicago Press.
Dann, S.M., P.C. Okhuysen, H.L. DuPont, and C.L. Chappell. 2000. Fecal IgA response to reinfection with Cryptosporidium parvum in healthy volunteers. American Journal of Tropical Medicine and Hygiene 62(3): 670.
Davison, J. 1999. Genetic exchange between bacteria in the environment. Plasmid 42(2): 73-91.
Deneke, E.S., and A.L. Rogers. 1996. Medical Mycology and Human Mycoses. Delmonte, California: Star Publication Company.
Denton, M., and K.G. Kerr. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clinical Microbiology Reviews 11: 57-80.
Desselberger, U., M. Iturriza-Gomara, and J.J. Gray. 2001. Rotavirus epidemiology and surveillance. Novartis Foundation Symposium 238: 125-152.
Dimitrov, D.S. 2000. Cell biology of virus entry. Cell 101: 697-702.
Domingo, E., R.G. Webster, and J.J. Holland. 1999. Origin and Evolution of Viruses. New York: Academic Press.
Donaldson, K.A., D.W. Griffin, and J.H. Paul. 2002. Detection, quantitation and identification of enteroviruses from surface waters and sponge tissue from the Florida Keys using real-time RT-PCR. Water Research 36(10): 2505-2514.
Dowd, S., C. Gerba, F. Enriquez, and I. Pepper. 1998a. PCR amplification and species determination of Microsporidia in formalin-fixed feces after immunomagnetic separation. Applied and Environmental Microbiology 64: 333-336.
Dowd, S., C. Gerba, and I. Pepper. 1998b. Confirmation of the human-pathogenic Microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Applied and Environmental Microbiology 64: 3332-3335.
Dowd, S.E., C.P. Gerba, and I. Pepper. 1999. Evaluation of methodologies including immunofluorescent assay (IFA) and the polymerase chain reaction (PCR) for detection of human pathogenic Microsporidia in water. Journal of Microbiological Methods 35(1): 43-52.
Dubey, D.P. 1998. Toxoplasma gondii oocyst survival under defined temperatures. Journal of Parasitology 84: 862-865.
Dumoulin, A., K. Guyot, E. Lelievre, E. Dei-Cas, and J.C. Cailliez. 2000. Cryptosporidium and wildlife: a risk for humans? Parasite-Journal de la Societe Francaise de Parasitologie 7: 167-172.
Edwards, R.T., J.L. Meyer, and S.E.G. Findlay. 1990. The relative contribution of benthic and suspended bacteria to system biomass, production and metabolism in a low-gradient blackwater river. Journal of the North American Benthological Society 9: 216-228.
Egyed, Z., T. Sreter, Z. Szell, and I. Varga. 2003. Characterization of Cryptosporidium spp.—recent developments and future needs. Veterinary Parasitology 111(2): 103-114.
Emerson, S.U., and R.H. Purcell. 2003. Hepatitis E virus. Reviews in Medical Virology 13(3): 145-154.
Enriquez, C., N. Nwachuku, and C.P. Gerba. 2001. Direct exposure to animal enteric pathogens. Reviews in Environmental Health 16(2): 117-131.
EPA (U.S. Environmental Protection Agency). 1980. Wastewater Aerosols and Disease: Proceedings of a Symposium, H. Pahren and W. Jakubowski, eds. Cincinnati, Ohio: Health Effects Research Laboratory. EPA-600-9-80-028.
Esiobu, N., L. Armenta, and J. Ike. 2002. Antibiotic resistance in soil and water environments. International Journal of Environmental Health Research 12: 133-144.
Evans, A.S. 1989. Viral Infection of Humans: Epidemiology and Control, 3rd Edition. New York: Plenum Medical Book Company.
Ewald, P.W. 1996. Evolution of Infectious Disease. Oxford, U.K.: Oxford University Press.
Fayer, R. 2000. Global change and emerging infectious diseases. Journal of Parasitology 86: 1174-1181.
Fayer, R., U. Morgan, and S.J. Upton. 2000. Epidemiology of Cryptosporidium: Transmission, detection and identification. International Journal of Parasitology 30(12-13): 1305-1322.
Fenchel, T. 1987. Ecology of Protozoa: The Biology of Free-Living Phagotrophic Protists. Madison, Wisconsin: Science Tech Publishers.
Fliermans, C.B., W.B. Cherry, L.H. Orrison, S.J. Smith, D.L. Tison, and D.H. Pope. 1981. Ecological distribution of Legionella pneumophila. Applied and Environmental Microbiology 41: 9-16.
Follet-Dumoulin, A., K. Guyot, S. Duchatelle, B. Bourel, F. Guilberrt, E. Dei-Cas, D. Gosset, and J.C. Cailiez. 2001. Involvement of insects in the dissemination of Cryptosporidium in the environment. Journal of Eukaryotic Microbiology 48(Suppl. 1): 36S.
Friedrich, F. 2000. Molecular evolution of oral poliovirus vaccine strains during multiplication in humans and possible implications for global eradication of poliovirus. Acta Virology 44(2): 109-117.
Frost, F.J., T. Muller, G.F. Craun, W.B. Lockwood, and R.L. Calderon. 2002. Serological evidence of endemic waterborne cryptosporidium infections. Annals of Epidemiology 12(4): 222-227.
Fry, J.C., and M.J. Day. 1990. Plasmid transfer in the epilithon. Pp. 55-80 in Bacterial Genetics in Natural Environments, J.C. Fry and M.J. Day, eds. London: Chapman and Hall.
Fulton, C., and E.Y. Lai. 1998. Stable intermediates and holdpoints in the rapid differentiation of Naegleria. Experimental Cell Research 242: 429-438.
Gauthier, F., and F. Archibald. 2001. The ecology of “fecal indicator” bacteria commonly found in pulp and paper mill water systems. Water Research 35: 2207-2218.
Ghendon, Y., and S.E. Robertson. 1994. Interrupting the transmission of wild polioviruses with vaccines: Immunological considerations. Bulletin of the World Health Organization 72(6):973-983.
Gilles, H.M. 2000. Protozoal Diseases. London: Edward Arnold (Hodder Arnold).
Gow, N.A.R., A.J.P. Brown, and F.C. Odds. 2002. Fungal morphogenesis and host invasion. Current Opinion in Microbiology 5(4): 366-371.
Grabow, W.O.K., M.O. Favorov, N.S. Khudyakova, M.B. Taylor, and H.A. Fields. 1994. Hepatitis E seroprevalence in selected individuals in South Africa. Journal of Medical Virology 44:384-388.
Graczyk, T.K., R. Fayer, and M.R. Cranfield. 1996. Cryptosporidium parvum is not transmissible to fish, amphibians, or reptiles. Journal of Parasitology 82(5): 748-751.
Graczyk, T.K., R. Fayer, J.M. Trout, E.J. Lewis, C.A. Farley, I. Sulaiman, and A.A. Lal. 1998. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta candensis). Applied and Environmental Microbiology 64: 2736-2738.
Griffin, D.W., K.A. Donaldson, J.H. Paul, and J.B. Rose. 2003. Pathogenic human viruses in coastal waters. Clinical Microbiology Reviews 16(1): 129-143.
Guerrant, R.L. 1997. Cryptosporidiosis: An emerging, highly infectious threat. Emerging Infectious Diseases 3(1): 51-57.
Gutierrez, C. 1999. Geminivirus DNA replication. Cell and Molecular Life Sciences 56(3-4): 313-329.
Haburchak, D.R. 1996. Aeromonas hydrophilia: An underappreciated danger to fishermen. Infections in Medicine 13: 893-896.
Hamilton, W.D., and T.M. Lenton. 1998. Spora and gaia: How microbes fly with their clouds. Ethology, Ecology, and Evolution 10: 1-16.
Hanberger, H., J. Garcia-Rodriguez, M. Gobernado, H. Goossens, L.E. Nilsson, L. Struelens, and the French and Portuguese ICU Study Groups. 1999. Antibiotic susceptibility among aerobic Gram-negative bacilli in intensive care units in 5 European countries. Journal of the American Medical Association 281: 67-71.
Hanley-Bowdoin, L., S.B. Settlage, B.M. Orozco, S. Nagar, and D. Robertson. 2000. Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. Critical Reviews in Biochemistry and Molecular Biology 35(2): 105-140.
Harb, O.S., L.Y. Gao, and Y. Abu Kwaik. 2000. From protozoa to mammalian cells: A new paradigm in the life cycle of intracellular bacterial pathogens. Environmental Microbiology 2: 251-265.
Harf, C. 1994. Free-living ameba: interactions with environmental pathogenic bacteria. Endocytobiosis and Cell Research 10: 167-183.
Havelaar, A.H. 1993. Bacteriophages as models of human enteric viruses in the environment. American Society for Microbiology News 59: 614-619.
Hay, A.J., V. Gregory, A.R. Douglas, and Y.P. Lin. 2001. The evolution of human influenza viruses. Philosophical Transactions - Royal Society of London Series B Biological Sciences 356(1416): 1861-1870.
Hazen, T.C., and C.B. Fliermans. 1979. Distribution of Aeromonas hydrophila in natural and man-made thermal effluents. Applied and Environmental Microbiology 38: 166-168.
Heitman, T.L., L.M. Frederick, J.R. Viste, N.J. Guselle, U.M. Morgan, R.C.A. Thompson, and M.E. Olson. 2002. Prevalence of Giardia and Cryptosporidium and characterization of Cryptosporidium spp. isolated from wildlife, human, and agricultural sources in the North Saskatchewan River Basin in Alberta, Canada. Canadian Journal of Microbiology 48: 530-541.
Hendrix, R.W., J.G. Lawrence, G.F. Hatfull, and S. Casjens. 2000. The origins and ongoing evolution of viruses. Trends in Microbiology 8(11): 504-508.
Hill, D., and J.P. Dubey. 2002. Toxoplasma gondii: Transmission, diagnosis and prevention. Clinical Microbiology and Infection 8: 634-640.
Holmes, E.C. 2001. On the origin and evolution of the human immunodeficiency virus (HIV). Biological Reviews of the Cambridge Philosophy Society 76(2): 239-254.
Hot, D., O. Legeay, J. Jacques, C. Gantzer, Y. Caudrelier, K. Guyard, M. Lange, and L. Andreoletti. 2003. Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Research 37(19): 4703-4710.
Hsu, F.C., Y.S. Shieh, J. van Duin, M.J. Beekwilder, and M.D. Sobsey. 1995. Genotyping male-specific RNA coliphages by hybridization with oligonucleotide probes. Applied and Environmental Microbiology 61(11): 3960-3966.
Hull, H.F., and R.B. Aylward. 2001. Progress towards global polio eradication. Vaccine 19(31): 4378-4384.
Hyams, K.C. 2002. New perspectives on hepatitis E. Current Gastroenterology Reports 4(4): 302-307.
Hyder, S.L., and M.M. Streitfeld. 1978. Transfer of erythromycin resistance from clinically isolated lysogenic strains of Streptococcus pyogenes via their endogenous phage. Journal of Infectious Diseases 138(3): 281-286.
Issac-Renton, J., W.R. Bowie, A. King, G.S. Irwin, C.S. Ong, C.P. Fung, M.O. Shokeir, and J.P. Dubey. 1998. Detection of Toxoplasma gondii oocysts in drinking water. Applied and Environmental Microbiology 64: 2278-2280.
Isaac-Renton, J., J. Blatherwick, W.R. Bowie, M. Fyfe, M. Khan, A. Li, A. King, M. McLean, L. Medd, W. Moorehead, C.S. Ong, and W. Robertson. 1999. Epidemic and endemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. American Journal of Tropical Medicine and Hygiene 60(4): 578-583.
Jellison, K.L., H.F. Hemond, and D.B. Schauer. 2002. Sources and species of Crypotsporidium oocysts in the Wachusett Reservoir watershed. Applied and Environmental Microbiology 68: 569-575.
Jiang, B., J.R. Gentsch, and R.I. Glass. 2002. The role of serum antibodies in the protection against rotavirus disease: An overview. Clinical Infectious Diseases 34: 1351-1361.
Jiang, S., R. Noble, and W. Chu. 2001. Human adenoviruses and coliphages in urban runoff-impacted coastal waters of Southern California. Applied and Environmental Microbiology 67(1): 179-184.
Jindrak, L., and L. Grubhoffer. 1999. Animal virus receptors. Folia Microbiology (Praha) 44(5): 467-486.
John, D.T. 1993. Opportunistically pathogenic free-living ameba. Pp. 143-246 in Parasitic Protozoa, Volume 3, 2nd Edition, J.P. Kreier and J.R. Baker, eds. New York: Academic Press.
Kaaden, O.R., W. Eichhorn, and S. Essbauer. 2002. Recent developments in the epidemiology of virus diseases. Journal of Veterinary Medicine, Series B. 49(1): 3-6.
Kadavy, D.R., J.M. Hornby, T. Haverkost, and K.W. Nickerson. 2000. Natural antibiotic resistance of bacteria isolated from larvae of the oil fly, Helaeomyia petrolei. Applied and Environmental Microbiology 66: 4615-4619.
Kamel, M.A., H. Troonen, H.P. Kapprell, A. el-Ayady, and F.D. Miller. 1995. Seroepidemiology of hepatitis E virus in the Egyptian Nile Delta. Journal of Medical Virology 47(4): 399-403.
Kew, O., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C.J. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. van Der Avoort, M.S. Oberste, D. Kilpatrick, S. Cochi, M. Pallansch, and C. de Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296(5566): 356-359.
Kitamoto, I., and K. Tanabe. 1987. Secretion by Toxoplasma gondii of an antigen that appears to become associated with the parasitophorous vacuole membrane upon invasion of the host cell. Journal of Cell Science 88: 231-239.
Krawczynski, K., S. Kamili, and R. Aggarwal. 2001. Global epidemiology and medical aspects of hepatitis E. Forum (Genova) 11(2): 166-179.
Kurath, G., and R.Y. Morita. 1983. Starvation-survival physiological studies of a marine Pseudomonas sp. Applied and Environmental Microbiology 45:1206-1211.
Lalitha, K.V., and K. Gopakumar. 2000. Distribution and ecology of Clostridium botulinum in fish and aquatic environments of a tropical region. Food Microbiology 17(5): 535-541.
Landaverde, M., L. Venczel, and C.A. de Quadros. 2001. Poliomyelitis outbreak caused by vaccine-derived virus in Haiti and the Dominican Republic. Pan American Journal of Public Health 9(4): 272-274.
Lebaron, P., P. Servais, M. Troussellier, C. Courties, J Vives-Rego, G. Muyzer, L. Bernard, T. Guindulain, H. Schafer, and E. Stackebrandt. 1999. Changes in bacterial community structure in seawater mesocosms differing in their nutrient status. Aquatic Microbial Ecology 19: 255-267.
LeChevallier, M.W., W.D. Norton, and R.G. Lee. 1991a. Giardia and Cryptosporidium spp. in filtered drinking water supplies. Applied and Environmental Microbiology 57(9):2617-2621.
LeChevallier, M.W., W.D. Norton, and R.G. Lee. 1991b. Occurrence of Giardia and Cryptosporidium spp. in surface-water supplies. Applied and Environmental Microbiology 57(9): 2610-2616.
Leff, L.G., J.V. McArthur, J.L. Meyer, and L.J. Shimkets. 1994. Effect of macroinvertebrates on detachment of bacteria from biofilms in stream microcosms. Journal of the North American Benthological Society 13: 74-79.
Leong, L.Y.C. 1983. Removal and inactivation of viruses by treatment processes for potable water and wastewater—a review. Water Science and Technology 15:91-114.
Levy, S.B. 1997. Antibiotic resistance: An ecological imbalance. Pp. 1-14 in Antibiotic Resistance: Origins, Evolution, Selection and Spread. Ciba Foundation Symposium 207. Chichester, U.K.: Wiley and Sons.
Levy, S.B. 1998. Multidrug resistance—a sign of the times. New England Journal of Medicine 338: 1376-1378.
Liebert, C.A., R.M. Hall, and A.O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiology and Molecular Biology Reviews 63(3): 507-522.
Lindsay, D.S., K.K. Phelps, S.A. Smith, G. Flick, S.S. Sumner, and J.P. Dubey. 2001. Removal of Toxoplasma gondii oocysts from sea water by eastern oysters (Crassostrea virginica). Journal of Eukaryotic Microbiology 48 (Supp. 1): 197S-198S.
Lipp, E.K., J.L. Jarrell, D.W. Griffin, J. Lukasik, J. Jacukiewicz, and J.B. Rose. 2002. Preliminary evidence for human fecal contamination in corals of the Florida Keys, USA. Marine Pollution Bulletin 44(7): 666-670.
Loewe, L., V. Textor, and S. Scherer. 2003. High deleterious genomic mutation rate in stationary phase of Escherichia coli. Science 302: 1558-1559.
Lopez, J.J., and M. Wilson. 2003. Congenital toxoplasmosis. American Family Physician 67(10): 2131-2138.
Lowery, C.J., P. Nugent, J.E. Moore, B.C. Milliar, X. Xiru, and J.S.G. Dooley. 2001. PCR-IMS detection and molecular typing of Cryptosporidium parvum recovered from a recreational river source and an associated mussel (Mytilus edulis) bed in Northern Ireland. Epidemiology and Infection 127: 545-553.
Luder, C.G.K., U. Gross, and M.F. Lopes. 2001. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends in Parasitology 17(10): 480-486.
Ma, P., E. Willart, K.B. Juechter, and A.R. Stevens. 1981. A case of keratitis due to Acanthameba in New York, New York and features of 10 cases. Journal of Infectious Diseases 143: 662-667.
Marino, A., G. Crisafi, T.L. Maugeri, A. Nostro, and V. Alonzo. 1999. Uptake and retention of Vibrio harvey by mussels in seawater. Microbiologica 22: 129-138.
Marshall, M.M., D. Naumovitz, Y. Ortega, and C.R. Sterling. 1997. Waterborne protozoan pathogens. Clinical Microbiology Reviews 10: 67-85.
Martinez, A.J. 1985. Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology and Treatment of the Disease. Boca Raton, Florida: CRC Press.
Mathison, B.A., and O. Ditrich. 1999. The fate of Cryptosporidium parvum oocysts ingested by dung beetles and their possible role in the dissemination of cryptosporidiosis. Journal of Parasitology 85: 678-681.
Mazel, D., and J. Davies. 1999. Antibiotic resistance in microbes. Cellular and Molecular Life Sciences 56: 742-754.
McArthur, J.V., and R.C. Tuckfield. 2000. Spatial patterns in antibiotic resistance among stream bacteria: Effects of industrial pollution. Applied Environmental Microbiology 66: 3722-3726.
McFadden, G. 1996. Viroceptors, Virokines and Related Immune Modulators Encoded by DNA Viruses. New York: Springer-Verlag.
Medema, G.J., F.M. Schets, P.F.M. Teunis, and A.H. Havelaar. 1998. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Applied and Environmental Microbiology 64: 4460-4466.
Miao, E.A., and S.I. Miller. 1999. Bacteriophages in the evolution of pathogen-host interactions. Proceedings of the National Academy of Sciences 96: 9452-9454.
Miller, M.A., I.A. Gardner, C. Kreuder, D.M. Paradies, K.R. Worcester, D.A. Jessup, E. Dodd, M.D. Harris, J.A. Ames, A.E. Packham, and P.A. Conrad. 2002. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). International Journal for Parasitology 32: 997-1006.
Mims, C.A., A. Nash, and J. Stephen. 2001. Mims’ Pathogenesis of Infectious Disease, 5th Edition. New York: Academic Press.
Miranda, G., D. Schuppli, I. Barrera, C. Hausherr, J.M. Sogo, and H. Weber. 1997. Recognition of bacteriophage Qbeta plus strand RNA as a template by Qbeta replicase: Role of RNA interactions mediated by ribosomal proteins S1 and host factor. Journal of Molecular Biology 267(5): 1089-1103.
Mitchell, R. 1972. Ecological control of microbial imbalances. Pp. 273-288 in Water Pollution Microbiology, R. Mitchell, ed. New York: Wiley Interscience.
Mohanavalli, B., E. Dhevahi, T. Menon, S. Malathi, and S.P. Thyagarajan. 2003. Prevalence of antibodies to hepatitis A and hepatitis E virus in urban school children in Chennai. Indian Pediatrics 40(4): 328-331.
Morgan, U.M., L. Xiao, R. Fayer, A.A. Lal, and R.C. Thompson. 1999. Variation in Cryptosporidium: Towards a taxonomic revision of the genus. International Journal of Parasitology 29(11): 1733-1751.
Morita, R.Y. 1997. Bacteria in Oligotrophic Environments. New York: Chapman Hall.
Mota, P., C. Rauch, and S.C. Edberg. 2000. Microsporidia and Cyclospora: Epidemiology and assessment of risk from the environment. Critical Reviews in Microbiology 26: 69-90.
Nielsen, K.M., M.D. van Weerelt, T.N. Berg, A.M. Bones, A.N. Hagler, and J.D. van Elsas. 1997. Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Applied Environmental Microbiology 63(5): 1945-1952.
NRC (National Research Council). 2002. Biosolids Applied to Land: Advancing Standards and Practices. Washington, D.C.: National Academy Press.
O’Donoghue, P.J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. International Journal for Parasitology 25: 139-195.
Ohka, S., and A. Nomoto. 2001. The molecular basis of poliovirus neurovirulence. Developments in Biological Standardization (Basel) 105: 51-58.
Okhuysen, P.C., C.L. Chappell, and H.L. DuPont. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. Journal of Infectious Diseases 180(4): 1275.
Okhuysen, P.C., and C.L. Chappell. 2002. Cryptosporidium virulence determinants—are we there yet? International Journal for Parasitology 32(5): 517-525.
Oliver, J.D. 1993. Formation of viable but nonculturable cells. Pp. 239-276 in Starvation in Bacteria, S. Kjelleberg, ed. New York: Plenum Press.
Ong, C., W. Moorehead, A. Ross, and J. Isaac-Renton. 1996. Studies of Giardia spp. and Cryptosporidium spp. in two adjacent watersheds. Applied and Environmental Microbiology 62(8): 2798-2805.
Pallansch, M.A., and R.P. Roos. 2001. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. Pp. 723-775 in Fields Virology, B.N. Fields, P.M. Howley, D.E. Griffin, R.A. Lamb, M.A. Martin, B. Roizman, S.E. Straus, and D.M. Knipe, eds. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins Publishers.
Papathanasopoulos, M.A., G.M. Hunt, and C.T. Tiemessen. 2003. Evolution and diversity of HIV-1 in Africa—a review. Virus Genes 26(2): 151-163.
Parashar, U.D., E.G. Hummelman, J.S. Bresee, M.A. Miller, and R.I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases 9(5): 565-571.
Patz, J.A., D. Engelberg, and J. Last. 2000. The effects of changing weather on public health. Annual Reviews of Public Health 21: 271-307.
Patz, J.A., and W.K. Reisen. 2001. Immunology, climate change and vector-borne diseases. Trends in Immunology 22: 171-172.
Peng, M.M., L. Xiao, A.R. Freeman, M.J. Arrowood, A.A. Escalante, A.C. Weltman, C.S. Ong, W.R. Mac Kenzie, A.A. Lal, and C.B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: Evidence of two distinct human transmission cycles. Emerging Infectious Disease 3(4): 567-573.
Pettibone, G.W., J.P. Mear, and B.M. Campbell. 1996. Incidence of antibiotic and metal resistance and plasmid carriage in Aeromonas isolated from brown bullhead (Ictalurus nebulosus). Letters in Applied Microbiology 23(4): 234.
Powell, K.L., R.G. Taylor, A.A. Cronin, M.H. Barrett, S. Pedley, J. Sellwood, S.A. Trowsdale, and D.N. Lerner. 2003. Microbial contamination of two urban sandstone aquifers in the UK. Water Research 37(2): 339-352.
Pulliam, H.R., and B.J. Danielson. 1991. Sources, sinks, and habitat selection: A landscape perspective on population-dynamics. American Naturalist 137(Supplement S): S50-S66.
Racaniello, V. 2001. Picornaviridae: The viruses and their replication. Pp. 685-722 in Fields Virology, B.N. Fields, P.M. Howley, D.E. Griffin, R.A. Lamb, M.A. Martin, B. Roizman, S.E. Straus, and D.M. Knipe, eds. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins Publishers.
Redlinger, T., K. O’Rourke, L. Nickey, and G. Martinez. 1998. Elevated hepatitis A and E seroprevalence rates in a Texas/Mexico border community. Texas Medicine 94(5): 68-71.
Rheinheimer, G. 1992. Aquatic Microbiology. 4th Edition. West Sussex, U.K.: John Wiley and Sons, Ltd.
Rickard, L.G., C. Siefker, C.R. Boyle, and E.J. Gentz. 1999. The prevalence of Cryptosporidium and Giardia spp. in fecal samples from free-ranging white-tailed deer (Odocoileus virginianus) in southeastern United States. Journal of Veterinary Diagnostic Investigation 11: 65-72.
Roberts, L.S., and J.J. Janovy, Jr. 1995. Foundations of Parasitology, 5th Edition. Dubuque, Iowa: William C. Brown Publishers.
Robertson, L.J., A.T. Campbell, and H.V. Smith. 1992. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Applied and Environmental Microbiology 58(11): 3494-3500.
Rodriguezzaragoza, S. 1994. Ecology of free-living amebas. Critical Reviews in Microbiology 20: 225-241.
Rooney, P.J., and B.S. Klein. 2002. Linking fungal morphogenesis with virulence. Cellular Microbiology 4(3): 127-138.
Rosas, I., C. Calderon, M. Ulloa, and J. Lacey. 1993. Abundance of airborne Penicillium CFU in relation to urbanization in Mexico City. Applied and Environmental Microbiology 59: 2648-2652.
Rosas, I., E. Salinas, A. Yela, E. Calva, C. Eslava, and A. Cravioto. 1997. Escherichia coli in settled-dust and air samples collected in residential environments in Mexico City. Applied and Environmental Microbiology 63: 4093-4095.
Rose, J.B. 1997. Environmental ecology of Cryptosporidium and public health implications. Annual Review of Public Health 18: 135-161.
Rose, J.B., and T.R. Slifko. 1999. Giardia, Cryptosoridium, and Cyclospora and their impact on foods: A review. Journal of Food Protection 62(9): 1059-1070.
Rose, J.B., D.E. Huffman, K. Riley, S.R. Farrah, J.O. Lukasik, and C.L. Harman. 2001a. Reduction of enteric microorganisms at the Upper Occoquan Sewage Authority Water Reclamation Plant. Water Environmental Resources 73(6): 711-720.
Rose, J.B., P.R. Epstein, E.K. Lipp, B.H. Sherman, S.M. Bernard, and J.A. Patz. 2001b. Climate variability and change in the United States: Potential impacts on water- and foodborne diseases caused by microbiologic agents. Environmental Health Perspectives 109(Supp. 2): 211-221.
Rose, J.B., D.E. Huffman, and A. Gennaccaro. 2002. Risk and control of waterborne cryptosporidiosis. FEMS Microbiology Reviews 26: 113-123.
Roszak, D.B., and R.R. Colwell. 1987. Metabolic activity of bacterial cells enumerated by direct viable count. Applied and Environmental Microbiology 53: 2889-2983.
Scandura, J.E., and M.D. Sobsey. 1996. Viral and bacterial contamination of groundwater from on-site sewage treatment systems. Water Science and Technology 35(11-12): 141-146.
Scholtissek, C. 1995. Molecular evolution of influenza viruses. Virus Genes 11(2-3): 209-215.
Schuppli, D., J. Georgijevic, and H. Weber. 2000. Synergism of mutations in bacteriophage Qbeta RNA affecting host factor dependence of Qbeta replicase. Journal of Molecular Biology 295(2): 149-154.
Schuster, F.L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clinical Microbiology Reviews 13(3): 342-354.
Scott, M.E., and G. Smith. 1994. Parasitic and Infectious Diseases: Epidemiology and Ecology. New York: Academic Press.
Seveno, N.A., D. Kallifidas, K. Smalla, J.D. van Elsas, J.M. Collard, A.D. Karagouni, and E.M.H. Wellington. 2002. Occurrence and reservoirs of antibiotic resistance genes in the environment. Reviews in Medical Microbiology 13: 15-27.
Shadduck, J.A., and M.B. Polley. 1978. Some factors influencing the in vitro infectivity and replication of Encephalitozoon cuniculi. Journal of Protozoology 25: 491-496.
Shadduck, J.A. 1989. Human microsporidiosis and AIDS. Reviews in Infectious Diseases 11: 203-207.
Skerrett, H.E., and C.V. Holland. 2000. The occurrence of Cryptosporidium in environmental waters in the greater Dublin area. Water Research 34: 3755-3760.
Slifko, T.A., H.V. Smith, and J.B. Rose. 2000. Emerging parasite zoonoses associated with water and food. International Journal of Parasitology 30: 1379-1393.
Smith, A.W., D.O. Matson, D.A. Stein, D.E. Skilling, A.D. Kroeker, T. Berke, and P.L. Iversen. 2002. Antisense treatment of Caliciviridae: An emerging disease agent of animals and humans. Current Opinions in Molecular Therapy 4(2): 177-184.
Smith, H.V., and J.B. Rose. 1998. Waterborne cryptosporidiosis current status. Parasitology Today 14(1): 14-22.
Sobecky, P.A., T.J. Mincer, M.C. Chang, and D.R. Helinski. 1997. Plasmids isolated from marine sediment microbial communities contain replicon and incompatibility regions unrelated to those of known plasmid groups. Applied and Environmental Microbiology 63: 888-895.
Sobecky, P.A., T.J. Mincer, M.C. Chang, A. Toukdarian, and D.R. Helinski. 1998. Isolation of broad-host-range replicons from marine sediment bacteria. Applied and Environmental Microbiology 64: 2822-2830.
Sobecky, P.A. 1999. Plasmid ecology of marine sediment microbial communities. Hydrobiologia 401: 9-18.
Son, R., G. Rusul, A.M. Sahilah, A. Zainuri, A.R. Raha, and I. Salmah. 1997. Antibiotic resistance and plasmid profile of Aeromonas hydrophila isolates from cultured fish, telapia (Telapia mossambica). Letters in Applied Microbiology 24(6): 479-482.
Sparfel, J.M., C. Sarafati, O. Ligoury, B. Caroff, N. Dumoutier, B. Gueglio, E. Billaud, F. Raffi, J.M. Molin, M. Miegeville, and F. Derouin. 1997. Detection of Microsporidia and identification of Enterocytozoon bieneusi in surface water by filtration followed by specific PCR. Journal of Eukaryotic Microbiology 44: 78.
Steiner, T.S., N.M. Theilman, and R.L. Guerrant. 1997. Protozoal agents: What are the dangers for the public water supply? Annual Review of Medicine 48: 329-340.
Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in co-culture with Acanthamaoeba polyphaga and survive within cyst walls. Applied and Environmental Microbiology 64: 2256-2261.
Stewart, G.J., and C.D. Sinigalliano. 1991. Exchange of chromosomal markers by natural transformation between the soil isolate, Pseudomonas stutzeri JM300, and the marine isolate, Pseudomonas stutzeri strain ZoBell. Antonie Van Leeuwenhoek 59(1): 19-25.
Stossel, T.F. 1994. The machinery of cell crawling. Scientific American 271: 54-63.
Stott, R., E. May, E. Matsushita, and A. Warren. 2001. Protozoan predation as a mechanism for the removal of Cryptosporidium oocysts from wastewaters in constructed wetlands. Water Science and Technology 44: 191-198.
Szenasi, Z., T. Endo, K. Yagita, and B. Nagy. 1998. Isolation, identification and increasing importance of “free-living” amoebae causing human disease. Journal of Medical Microbiology 47: 5-16.
Teunis, P.F.M., C.L. Chappell, and P.C. Okhuysen. 2002. Cryptosporidium dose-response studies: Variation between hosts. Risk Analysis 22(3): 475-486.
Thom, S., D. Warhurst, and B.S. Drasar. 1992. Association of Vibrio cholerae with freshwater amebas. Journal of Medical Microbiology 36: 303-306.
Thompson, R.C.A., and A.J. Lymbery. 1996. Genetic variability in parasites and host-parasite interactions. Parasitology 112(Supp. S): S7-S22.
Thurston-Enriquez, J.A., P. Watt, S.E. Dowd, J. Enriquez, I.L Pepper, and C.P. Gerba. 2002. Detection of protozoa parasites and Microsporidia in irrigation waters used for crop production. Journal of Food Protection 65: 378-382.
Tomoeda, M., A. Shuta, and M. Inuzuka. 1972. Studies on sex pili: Mutants of the sex factor F in Escherichia coli defective in bacteriophage-adsorbing function of F pili. Journal of Bacteriology 112(3): 1358-1363.
Tomov, A.T., E.D. Tsvetkova, I.A. Tomova, L.I. Michailova, and V.K. Kassovski. 1999. Persistence and multiplication of obligate anaerobe bacteria in amebae under aerobic conditions. Anaerobe 5: 19-23.
Tyler, K.L., and N. Nathanson. 2001. Pathogenesis of viral infections. Pp. 199-243 in Fields Virology, B.N. Fields, P.M. Howley, D.E. Griffin, R.A. Lamb, M.A. Martin, B. Roizman, S.E. Straus, and D.M. Knipe, eds. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins Publishers.
van Burik, J.A.H., and P.T. Magee. 2001. Aspects of fungal pathogenesis in humans. Annual Review of Microbiology 55: 743-772.
Van Heerden, J., M.M. Ehlers, W.B. Van Zyl, and W.O. Grabow. 2003. Incidence of adenoviruses in raw and treated water. Water Research 37(15): 3704-3708.
Wade, T.J., N. Pai, J.N. Eisenberg, J.M. Colford, Jr. 2003. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environmental Health Perspectives 111(8): 1102-1109.
Waller, T. 1979. Sensitivity of Encephalitozoon cuniculi to various temperatures, disinfectants and drugs. Lab Animals 13: 227-230.
Webby, R.J., and R.G. Webster. 2003. Are we ready for pandemic influenza? Science 302(5650): 1519-1522.
Webster, R.G., S.M. Wright, M.R. Castrucci, W.J. Bean, and Y. Kawaoka. 1993. Influenza - a model of an emerging virus disease. Intervirology 35(1-4): 16-25.
Weiss, L.M. 2001. Microsporidia: Emerging pathogenic protists. Acta Tropica 78: 89-102.
Weiss, R.A. 2003. Cross-species infections. Current Topics in Microbiology and Immunology 278: 47-71.
White, D.O., and F.J. Fenner. 1994. Medical Virology, 4th Edition. New York: Academic Press.
Wiersma, S. 2002. Primary Amebic Meningoencephalitis. Tallahassee, Florida: Florida Department of Health.
Williams, H.G., M.J. Day, J.C. Fry, and G.J. Stewart. 1996. Natural transformation in river epilithon. Applied Environmental Microbiology 62: 2994-2998.
Winiecka-Krusnell, J., and E. Linder. 1999. Free-living amoebae protecting Legionella in water: The tip of an iceberg? Scandinavian Journal of Infectious Diseases 31: 383-385.
Wise, M.G., J V. McArthur, C. Wheat, and L.J. Shimkets. 1997. Temporal variation in genetic diversity and structure of a lotic population of Burkholderia (Pseudomonas) cepacia. Applied and Environmental Microbiology 63: 1505-1514.
Wolk, D.M., C.H. Johnson, E.W. Rice, M.M. Marshall, K.F. Grahn, C.B. Plummer, and C.R. Sterling. 2000. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis. Applied and Environmental Microbiology 66: 1266-1273.
Wommack, K.E., and R.R. Colwell. 2000. Virioplankton: Viruses in aquatic ecosystems. Microbiology and Molecular Biology Review 64: 69-114.
Xiao, L., I.M. Sulaiman, U.M. Ryan, L. Zhou, E.R. Atwill, M.L. Tischler, X. Zhang, R. Fayer, and A.A. Lal. 2002. Host adaptation and host-parasite co-evolution in Cryptosporidium: Implications for taxonomy and public health. International Journal of Parasitology 32: 1773-1785.
Yoneyama, T., H. Yoshida, H. Shimizu, K. Yoshii, N. Nagata, O. Kew, and T. Miyamura. 2001. Neurovirulence of Sabin 1-derived poliovirus isolated from an immunodeficient patient with prolonged viral excretion. Developments in Biological Standardization (Basel) 105: 93-98.
Zu, S.X., J.F. Li, L.J. Barrett, R. Fayer, S.Y. Shu, J.F. McAuliffe, J.K. Roche, and R.L. Guerrant. 1994. Seroepidemiologic study of Cryptosporidium infection in children from rural communities of Anhui, China and Fortaleza, Brazil. American Journal of Tropical Medicine and Hygiene 51(1): 1-10.























































