1
Defining the Relationship: An Examination of Infectious Agents Associated with Chronic Diseases
OVERVIEW
Chronic diseases cause 70 percent of all deaths in the United States. Yet the factors that cause many of these conditions have been poorly understood until recently. Advances in numerous detection and diagnostic techniques have revealed that several chronic illnesses result from infectious agents. For example, the human papillomavirus causes more than 90 percent of cervical cancers. The hepatitis B virus accounts for more than 60 percent of liver cancer. The Epstein-Barr virus produces in people simultaneously infected with malaria a cancer known as Burkitt’s lymphoma, a leading cause of childhood cancer deaths globally. The bacterium Helicobacter pylori has been linked to a number of disorders, including duodenal ulcers, gastric cancer, and certain types of lymphomas.
Other connections between infections and chronic diseases are suspected, but not proven. Epstein-Barr virus, for example, has been found in patients with Hodgkin’s disease and with aggressive breast cancers. Multiple sclerosis acts suspiciously like an infection, with patients experiencing high antibody levels as well as exacerbations and remissions. Juvenile-onset diabetes may arise when a Coxsackie B enterovirus elicits an immune response that damages the pancreas.
Identifying and confirming an infectious cause of a chronic disease is complicated by several factors:
-
in some cases, microorganisms may act in a hit-and-run fashion, being undetectable by the time the disease process becomes apparent (e.g., Reiter’s syndrome, Guillian-Barré syndrome, rheumatic heart disease);
-
infection may be in a persistent state at the time of diagnosis;
-
acute, chronic, or recurrent infections may be involved in pathogenesis;
-
detection and culture of microbes in a variety of tissues may be difficult;
-
a number of factors, including environmental and genetic (host and microbe) factors, may be involved in the disease etiology; and
-
adequate methods may be lacking to identify novel or rare microorganisms.
The case studies presented in this chapter were chosen to provide insight into the range of research under way in the field. The chronic diseases covered represent the full spectrum of those that have been linked in some degree, from “clearly proven” to “suspected,” with infectious agents; they are caused by a variety of microorganisms; and their association with disease is supported variously by laboratory and epidemiological studies. Although other diseases and studies might have been included, some limits were imposed by time constraints and the availability of speakers.
Eduardo Franco reviewed the evidence that human papillomavirus (HPV) infection is a cause of cervical cancer. HPV infection precedes lesion development and appears to be necessary for cervical cancer to occur. This is one of the first examples in which an infectious agent has been identified to be necessary for cancer development. This causal relationship was revealed through the use of improved diagnostic tools that enabled more accurate identification of HPV. As the role of infection by certain types of HPV is better elucidated as the cause of cervical cancer, HPV testing in cervical cancer screening programs becomes an important part of a primary prevention strategy. Another component of this strategy may be increased use of a recently developed vaccine. Clinical studies indicate that the new HPV 16 VLP vaccine was 100 percent effective in preventing acquisition of persistent infection with HPV 16, and was 90 percent effective in preventing any incident HPV 16 infection, transient or persistent. Immunization against HPV may have greatest value in developing countries, where 80 percent of the global burden of cervical cancer occurs each year.
William Mason presented the association between hepatitis B virus infection and liver disease. Infection with the virus remains a worldwide problem, with more than 350 million people chronically infected. Although a vaccine has been available for the past 20 years, its high cost prevents universal vaccination. Current research, therefore, has focused on the development of effective therapies to cure those individuals chronically infected with the virus. Mason described the research presently being conducted in a number of animal model systems, including the woodchuck. Along with clinical studies, these models have been able to characterize infections and evaluate therapies, as well as better elucidate the difficulties of treating chronic infections with nucleoside analogs.
Michael Dunne described the relationship between infection and cardiovascular disease. There is a tight association between hypercholesterolemia and atherosclerosis; recent research has examined how inflammation within the plaque
accumulated on arterial walls might drive atherosclerosis. Several pathophysiologic hypotheses have been formulated:
-
Local infection might lead through a variety of pathways to arterial wall atherogenic effects.
-
Local infection might produce a systemic inflammatory mediator that travels to the atherosclerotic plaque and produces expression of adhesion molecules along the endothelium, foam cell formation, and other proinflammatory reactions.
-
Local infection might produce bacteremia or viremia from a variety of pathogens that infect the arterial wall and induce those same inflammatory changes.
There is a long list of potential causes: Chlamydia pneumoniae, cytomegalovirus, various dental disease organisms, H. pylori, and herpes simplex virus. Anything leading to increased foam cell function in the plaque is a potential culprit. This is an example where many different etiologic causes or multiple causes might be involved in the same chronic condition either individually, synergistically, or multifactorially.
Richard Johnson reviewed the various ways that viral infections are associated with demyelinating diseases in animals and humans, including such direct routes as oligodendrocytes or Schwann cells causing demyelination through cell lysis or alteration of cell metabolism; virus-induced immune-mediated reactions, such as incorporation of myelin antigens into the virus envelope or modification of antigenicity of myelin membranes; and viral disruption of regulatory mechanisms of the immune system. Human demyelinating diseases with known viral etiology include postinfectious encephalomyelitis, acute disseminated encephalomyelitis, and progressive multifocal leucoencephalopathy. A viral cause for multiple sclerosis has been postulated for more than 100 years, and epidemiologic studies support this supposition and clearly show an environmental factor. In addition, several studies show multiple sclerosis patients to have elevated levels of various antiviral antibodies compared to controls.
Mark Pallansch discussed some of the difficulties in addressing the association of chronic diseases with infectious diseases, using diabetes and enteroviruses as examples. Type 1 diabetes is clearly a multifactorial disease: there is both a clear genetic predisposition and an autoimmune component. The major manifestation is the loss of beta cells in the pancreas and the associated loss of capacity to produce insulin. There are more than 65 different enteroviruses, which include the most common human viral infections. All individuals may have multiple infections every year with at least one of these viruses. Because the standard enterovirus diagnostics are extremely labor-intensive, efforts are being made to develop diagnostic tools based on reverse transcriptase-polymerase chain reaction (RT-PCR). A semi-nested PCR method is available to determine presence or ab-
sence of enteroviruses, but ways of identifying specific enteroviruses remain to be developed using this technology.
Robert Yolken and Fuller Torrey examined associations between infectious agents and schizophrenia. Epidemiologic studies indicate that environmental events during fetal development and early infancy may contribute to the risk of schizophrenia in some individuals. Yolken and Torrey hypothesized that most cases of schizophrenia are caused by infections and other environmental events occurring in genetically susceptible individuals. The activation of endogenous retroviruses within the central nervous system may possibly be one of several mechanisms by which infections can lead to the disease. If this is the case, then medications controlling these infections could play a major role in treating schizophrenia.
Hung Fan examined evidence from an animal model supporting the possibility that an infectious agent may be involved in human lung adenocarcinoma. Ovine pulmonary adenocarcinoma (OPA) is a contagious lung cancer of sheep. Tumor samples from animals with OPA consistently contain exogenous jaagsiekte sheep retrovirus (JSRV), which has an envelope gene with oncogenic potential. JSRV-induced OPA is histologically very similar to human adenocarcinoma. The lack of association of this cancer with tobacco smoking, together with the disease’s increasing incidence, suggests the possibility of viral involvement.
David Persing discussed the pathogenesis of acne, a dermatologic inflammatory disease unique to humans and the most common dermatological complaint of adolescents and young adults. In addition to the role played by the bacteria Propionibacterium acnes in the development of the inflammatory acne lesion, Persing explained how P. acnes has been implicated as a source of heart valve infections, postoperative implant infections, and prostheses failure. Recently P. acnes has been implicated as a possible cause of chronic inflammation in sciatica. Persing described his approaches to developing a vaccine for acne that could also benefit other P. acnes-related chronic diseases.
Studies in each of these areas are advancing our understanding of the role that infections play in chronic diseases. But the path from suspecting a microorganism to proving its association with a specific disease can be long. The discovery that H. pylori can cause duodenal ulcer disease is often cited as case in point of both the hurdles and the rewards. The medical establishment in the United States and worldwide remained skeptical of this link for years. Finally, the evidence became overwhelming, and the discovery is credited with galvanizing research for the entire field of infection and chronic disease. Medical treatment also has evolved accordingly, with therapies shifting from surgery to blocking hyperacidity and, ultimately, to the use of antibiotics directed against H. pylori.
THE ROLE OF VIRUSES IN ONCOGENESIS: HUMAN PAPILLOMAVIRUSES AND CERVICAL CANCER AS A PARADIGM
Eduardo L. Franco, M.P.H., Dr.P.H.*
Departments of Oncology and Epidemiology,
McGill University, Montreal, Canada
Like other malignant neoplasms of humans, cervical cancer is a disease with multifactorial causes and long latency. Unlike most other cancers, however, in which multiple environmental, biologic, and lifestyle determinants contribute independently or jointly to carcinogenesis, cervical cancer has been shown to have a central causal agent, human papillomavirus (HPV) infection, whose contribution to the risk of the disease is much greater than that of any other recognized determinant (IARC, 1995). Recently, there has been much attention to the fact that it is virtually impossible to find cervical carcinoma specimens devoid of traces of HPV DNA, which strongly suggests that HPV infection could be a necessary cause for this malignancy (Franco et al., 1999a; Walboomers et al., 1999). If this is really the case, then it would be a first in cancer research; no human cancer has yet been shown to have a necessary cause, so clearly identified. Some of the well-studied models in cancer causation, such as tobacco smoking in lung cancer and chronic hepatitis B in liver carcinoma, are among the strongest epidemiologic associations that one can find, but they do not represent causal relations that are necessary. Lung cancers may occur in people who never smoked and had only minimal exposure to environmental tobacco smoke, frequently as a result of exposure to occupation-related carcinogens, and liver cancer may occur in individuals who never had hepatitis B, e.g., via aflatoxin exposure or hepatitis C.
The implications of this finding are substantial and have spawned new approaches to preventing cervical cancer on two fronts: (i) via screening for HPV infection as the biological surrogate that reveals asymptomatic cervical cancer precursor lesions and (ii) via primary immunization against HPV infection to prevent the onset of such precursor lesions. While there is now intense research in these two fronts the debate still continues concerning issues related to the etiologic mechanism whereby HPV infection initiates cervical carcinogenesis. This brief overview addresses the epidemiologic characteristics of HPV infection and cervical cancer and the recent progress using new approaches to preventing cervical cancer.
Global Importance of Cervical Cancer
Cervical cancer is one of the most common malignant diseases of women. In the US each year there are approximately 12,800 new cases of invasive cervical cancer with 4,600 deaths due to this disease (Ries et al., 2000). On average during the last decade, an estimated 371,000 new cases of invasive cervical carcinoma were diagnosed annually worldwide, representing nearly 10 percent of all female cancers. Its incidence is the third among women, after breast and colorectal cancer (Parkin et al., 1999). The highest risk areas are in Central and South America, Southern and Eastern Africa, and the Caribbean, with average incidence rates around 40 per 100,000 women per year. While risk in western Europe and North America is considered relatively low at less than 10 new cases annually per 100,000 women, rates are 10 times higher in some parts of Northeastern Brazil, where the cumulative lifetime risk can approach 10 percent (Muir et al., 1987).
Every year, an estimated 190,000 deaths from cervical cancer occur worldwide, with over three-fourths of them in developing countries, where mortality from this disease is the highest among deaths caused by neoplasms (Pisani et al., 1999). Less than 50 percent of women affected by cervical cancer in developing countries survive longer than five years whereas the 5-year survival rate in developed countries is about 66 percent (Pisani et al., 1999). Moreover, cervical cancer generally affects multiparous women in the early post-menopausal years. In high-fertility developing countries these women are the primary source of moral values and education for their children. The premature loss of these mothers has important social consequences for the community.
Emergence of HPV Infection as the Main Etiologic Factor in Cervical Cancer
Prominent among the risk factors for cervical cancer is the role of two measures of sexual activity, namely number of sexual partners and age at first intercourse (Herrero, 1996), and also the sexual behavior of the woman’s male partners (Brinton et al., 1989a). The consistency of the sexually-transmitted disease model for cervical neoplasia led much of the laboratory and epidemiologic research in attempting to identify the putative microbial agent or agents acting as etiologic factor. Research conducted during the late 1960s and 1970s attempted to unveil an etiologic role for the Herpes simplex viruses (HSV). Although HSV was proven to be carcinogenic, in vitro and in vivo clinical studies eventually demonstrated that only a fraction of cervical carcinomas contained traces (viral DNA) of HSV infection and epidemiologic studies failed to demonstrate that the association between HSV and cervical cancer was the primary causal element (Franco, 1991).
In the 1980s, a solid research base emerged implicating HPV infection as the sexually-transmitted cause of cervical cancer and its precursors. In 1995, the In-
ternational Agency for Research on Cancer at the World Health Organization (WHO), in its monograph series of carcinogenicity evaluation classified HPV types 16 and 18 as carcinogenic to humans, HPV types 31 and 33 as probably carcinogenic, and other HPV types (except 6 and 11) as possibly carcinogenic (IARC, 1995). This classification was conservatively made on the basis of the available published evidence until 1994. Subsequent research has permitted a more inclusive grouping of genital HPV types on the basis of the presumed oncogenic potential. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 are considered to be of high oncogenic risk because of their frequent association with cervical cancer and cervical intraepithelial neoplasia (CIN), the precursor, pre-invasive lesion stage. The remaining genital types, e.g., HPV types 6, 11, 42–44, and some rarer types are considered of low or no oncogenic risk (Bosch et al., 1995). The latter types may cause subclinical and clinically visible benign lesions known as flat and acuminate condylomata, respectively.
Today, it is well established that infection with high oncogenic risk HPV types is the central causal factor in cervical cancer (IARC, 1995; Koutsky et al., 1992; Nobbenhuis et al., 1999). Relative risks for the association between HPV and cervical cancer are in the 20–70 range, which is among the strongest statistical relations ever identified in cancer epidemiology. Both retrospective and prospective epidemiologic studies have demonstrated the unequivocally strong association between viral infection and risk of malignancy, both as CIN or invasive disease (Bosch et al., 2002). Table 1-1 shows that HPV infection satisfies nearly all of standard causal criteria in chronic disease epidemiology. However, not all infections with high risk HPVs persist or progress to cervical cancer, thus suggesting that, albeit necessary, HPV infection is not sufficient to induce this disease; other factors, environmental or host-related, are also involved. Among these co-factors are: smoking (Ho et al., 1998a), high parity (Brinton et al., 1989b), use of oral contraceptives (Moreno et al., 2002), diets deficient in vitamins A and C (Potischman and Brinton, 1996), and genetic susceptibility traits, such as specific HLA alleles and haplotypes (Maciag et al., 2000) and polymorphisms in the p53 gene (Makni et al., 2000). Understanding the role of these cofactors is the subject of much ongoing research on the natural history of HPV infection and cervical cancer (see Figure 1-1).
Human Papillomaviruses
HPVs are small, double-stranded DNA viruses of approximately 55 nanometers (nm) with an icosahedral protein capsid containing 72 capsomers. The genome is circular and contains 7500–8000 base pairs (bp). HPVs have the following characteristics:
-
~8 kilobase (kb) DNA virus from Papillomaviridae family
-
Species- and tissue-specific
TABLE 1-1 Causality Criteria in HPV and Cervical Cancer
|
Causal Criterion |
Degree of Evidence |
Findings |
|
Strength of the association |
++ |
Relative risks among the highest in cancer epidemiology |
|
Consistency |
++ |
Association confirmed in multiple epidemiologic studies |
|
Temporality |
+ |
Infection precedes lesion development |
|
Biological gradient |
+ |
Viral persistence and viral load affect disease risk in dose-dependent manner |
|
Coherence |
++ |
Epidemiology does not conflict with molecular pathogenesis data |
|
Biological plausibility |
++ |
Overwhelming body of evidence from laboratory studies |
|
Experimental evidence |
+ |
HPV vaccination reduces short-term risk of cervical cancer precursor lesions |
|
Necessary factor? |
+ |
HPV DNA found in virtually all cervical cancers |
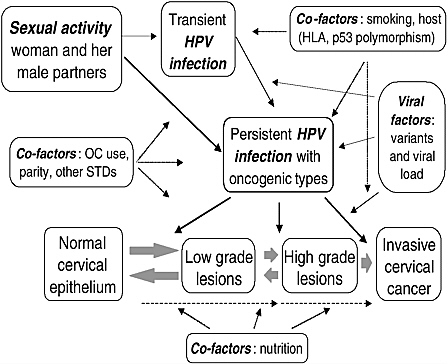
FIGURE 1-1 Etiologic model in cervical carcinogenesis showing the primary role of HPV infection, its relation with sexual activity, and the putative role of cofactors.
-
Cannot be cultivated
-
Over 150 genotypes identified, of which more than 40 infect the anogenital tract
-
High risk (oncogenic) types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68
-
Induces both benign (caused by low risk types) and malignant (caused by high risk types) diseases
-
Two major viral oncogenes: E6 (binds to p53) and E7 (binds to retinoblastoma [Rb] protein)
Taxonomically, papillomaviruses used to be a subfamily in the Papovaviridae family but are now grouped independently as a family, the Papillomaviridae. As infectious agents, they are highly specific to their respective hosts. Different HPVs are classified as types on the basis of DNA sequence homology in the E6, E7, and L1 genes. More than 150 different HPV types have been catalogued so far (zur Hausen, 2000).
The epithelial lining of the anogenital tract is the target for infection by over 40 different mucosotropic HPV types. Clinical, subclinical, and latent HPV infections are the most common sexually-transmitted viral diseases today (Cox, 1995). Latent genital HPV infection can be detected in 5 to 40 percent of sexually active women of reproductive age (IARC, 1995). In most cases, genital HPV infection is transient or intermittent (Hildesheim et al., 1994; Ho et al., 1998b; Moscicki et al., 1998; Franco et al., 1999b; Liaw et al., 2001); the prevalence is highest among young women soon after the onset of sexual activity and falls gradually with age, possibly as a reflection of accrued immunity and decrease in sexual activity (meaning a decrease in number of sexual partners).
The carcinogenic mechanism following HPV infection involves the expression of two major viral oncogenes, E6 and E7, which produce proteins that interfere with tumor suppressor genes controlling the cell cycle. Once viral DNA becomes integrated into the host’s genome, E6 and E7 become upregulated. While E7 complexes with the cell growth regulator Rb protein, causing an uncontrolled cell proliferation (Chellappan et al., 1992), the binding of E6 to p53 protein promotes the degradation of the latter, thus exempting the deregulated cell to undergo p53-mediated control (Thomas et al., 1996). The degradation of p53 by E6 leads to loss of DNA repair function and prevents the cell from undergoing apoptosis. The infected cell can no longer stop further HPV-related damages and becomes susceptible to additional mutations and genomic instability. Interestingly, the effect of the E6 and E7 proteins on p53 and Rb has been shown to occur only with high-risk HPVs but not with low-risk HPVs (Dyson et al., 1989).
Persistent HPV Infection as the Precursor Event in Cervical Carcinogenesis
Most women who engage in sexual activity will probably acquire HPV infection over a lifetime. As mentioned above, the vast majority of these infections will be transient with only a small proportion becoming persistent. We have found in our ongoing cohort study of Brazilian women that only 35 percent of the subjects who were infected at enrollment retain their infections after 12 months, with the mean duration being affected by the viral oncogenic potential (see Figure 1-2). Infections with oncogenic HPVs tend to last longer on average (13.5 months) than those with non-oncogenic types (8.2 months) (Franco et al., 1999b). A substantial increase in risk of CIN (see Figure 1-3) and cancer exists for women who develop persistent, long-term infections with oncogenic HPV types (Koutsky et al., 1992; Ho et al., 1998b; Nobbenhuis et al., 1999; Ylitalo et al., 2000; Moscicki et al., 2001; Schlecht et al., 2001).
There is currently great interest in defining persistent infection and in obtaining additional markers of pathogenesis for predictive purposes. Studies of viral load and intratypic variation of HPVs indicate that persistent infections tend to
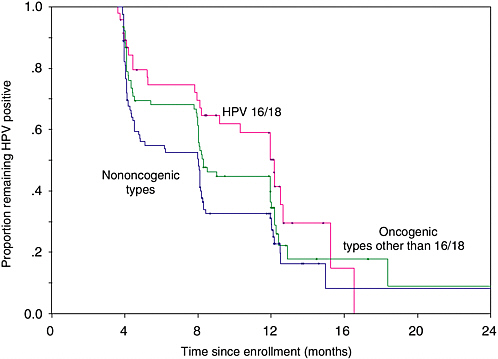
FIGURE 1-2 Actuarial curves showing clearance of prevalent HPV infection according to type present at enrollment in a cohort study of asymptomatic women presenting for cervical cancer screening.
SOURCE: Adapted from Franco et al. (1999b).

FIGURE 1-3 Actuarial curves showing the cumulative incidence of cervical squamous intraepithelial lesions (SIL) according to HPV infection in the first two visits in a cohort study of asymptomatic women presenting for cervical cancer screening.
SOURCE: Adapted from Schlecht et al. (2001).
yield higher viral loads than transient ones (Caballero et al., 1999) and those with non-European variants of HPVs 16 and 18 tend to be associated with higher risk of CIN as compared with those caused by European variants (Villa et al., 2000).
Defining viral persistence is critical because trials of HPV vaccine efficacy rely on the reduction of the risk of persistent infection as one of the primary outcomes. Similarly, concerning screening of cervical cancer by HPV testing, a main drawback is the low positive predictive value of a single test because of the relatively high prevalence of latent HPV infections in the population, particularly among young women. The predictive value would increase substantially if testing were to rely on repeated samplings, about 6 months apart, because of the aforementioned high prognostic value of persistent positivity. However, population screening cannot rely on repeated testing to be cost-effective and realistic as a public health measure. It would be highly desirable if one could, with a single HPV test, collect enough ancillary information on the virus and on the host that would allow determining whether or not a single instance of HPV positivity is likely to represent a persistent infection.
HPV Testing in Cervical Cancer Screening
Detection of HPV DNA in cervical specimens using a commercially available assay has been shown to have greater sensitivity but somewhat lower specificity to detect CIN and cervical cancer as compared with the conventional Pap cytology (Cuzick et al., 2000). This makes HPV testing a suitable alternative to the latter in screening programs in middle- and high-income countries where centralized laboratory resources are available. The costs associated with an increased number of women to be referred for colposcopy (because of the HPV test’s higher false positive rate as compared to cytology) will likely be offset by the increased screening interval that could later be recommended if HPV testing is eventually used to replace cytology screening. The Pap test’s low sensitivity forces screening programs to recommend repeat tests frequently to ensure that lesions will not be missed. In the US, fear of malpractice litigation has led to a conservative recommendation of annual Pap smears by many professional groups. Combination testing of Pap cytology and HPV testing has the potential to allow extending screening intervals (for women who are negative in both tests) to as long as 5 years, although this is yet to be proven a safe alternative in long-term follow-up studies.
Primary Prevention by HPV Vaccination
Two main types of HPV vaccines are currently being developed: prophylactic vaccines to prevent HPV infection and associated diseases, and therapeutic vaccines to induce regression of precancerous lesions or remission of advanced cervical cancer. DNA-free virus-like particles (VLP) synthesized by self-assembly of fusion proteins of the major capsid antigen L1 induce a strong humoral response with neutralizing antibodies. VLPs are thus the best candidate immunogen for HPV vaccine trials. Protection seems to be type-specific so that production of VLPs for a variety of high oncogenic risk types will be required. Such vaccines are already under evaluation in safety and efficacy trials in different populations and are sponsored by pharmaceutical companies and by the National Institutes of Health (Schiller, 1999). The preliminary results of one such a trial were extremely promising (Koutsky et al., 2002). It indicated that an HPV 16 VLP vaccine was 100 percent effective in preventing acquisition of persistent infection with HPV 16 and 90 percent effective in preventing any incident HPV 16 infection, transient or persistent. As a noteworthy secondary finding was the fact that all HPV 16-associated CIN cases occurred in the non-vaccinated group. Immunization against HPV may have greatest value in developing countries, where 80 percent of the global burden of cervical cancer occurs each year and where Pap screening programs have been largely ineffective.
Conclusions
During the last 20 years, the concerted effort among virologists, epidemiologists, and clinical researchers has helped to elucidate the role of infection by certain types of HPV as the necessary cause of cervical cancer. This has opened new frontiers for preventing a disease that is responsible for substantial morbidity and mortality, particularly among women living in resource-poor countries. Research on two prevention fronts has already begun in several populations in the form of preliminary trials assessing the efficacy of HPV vaccines and of studies of the value of HPV testing in cervical cancer screening (see Figure 1-4). Progress on both counts is very promising. While the benefits of vaccination against HPV infection as a cervical cancer prevention tool are at least a decade into the future, the potential benefits of HPV testing in screening for this disease can be realized now in most populations.
Primary prevention of cervical cancer can also be achieved through prevention and control of genital HPV infection. Health promotion strategies geared at a change in sexual behavior targeting all sexually-transmitted infections of public health significance can be effective in preventing genital HPV infection (Franco et al., 2001). Although there is consensus that symptomatic HPV infection (genital warts) should be managed via treatment, counseling, and partner notification, active case-finding of asymptomatic HPV infection is currently not recommended
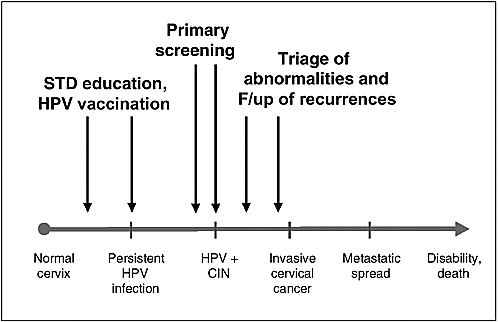
FIGURE 1-4 Opportunities for primary and secondary preventive approaches in the natural history of cervical cancer.
as a control measure. Further research is needed to determine the effectiveness of such a strategy and the significance of such infections concerning a woman’s subsequent cancer risk.
Research on HPVs has progressed at a fast pace and has reached a volume of nearly 1,000 annual publications in Medline. The HPV-cervical cancer model has become a paradigm of progress in cancer research and among neoplastic diseases with infectious roots. After 20 years, we have reached the point where preventing cervical cancer via vaccination against HPV infection is in the foreseeable future. It would be disastrous, however, if countries relaxed their cervical cancer screening programs in anticipation of a successful HPV vaccine. Existing cytologybased screening programs that seem to work need to be constantly assessed for quality and coverage. Ongoing research on the efficacy and cost-effectiveness of HPV testing as a mass screening tool will help countries decide on the best approach for secondary prevention of cervical cancer and will probably lead to reduced morbidity and mortality from this disease.
REFERENCES
Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. Journal of the National Cancer Institute 87:796–802.
Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. 2002. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology 55:244–265.
Brinton LA, Reeves WC, Brenes MM, et al. 1989a. The male factor in the etiology of cervical cancer among sexually monogamous women. International Journal of Cancer 44:199–203.
Brinton LA, Reeves WC, Brenes MM, Herrero R, de Britton RC, Gaitan E, Tenorio F, Garcia M, Rawls WE. 1989b. Parity as a risk factor for cervical cancer. American Journal of Epidemiology 130:486–496.
Caballero OL, Trevisan A, Villa LL, Ferenczy A, Franco EL. 1999. High viral load is associated with persistent HPV infection and risk of cervical dysplasia. 17th International Papillomavirus Conference, Charleston, SC.
Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human Papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proceedings of the National Academy of Sciences 89:4549–4553.
Cox JT. 1995. Epidemiology of cervical intraepithelial neoplasia: the role of human papillomavirus. Baillière’s clinical obstetrics and gynaecology 9:1–37.
Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, van Ballegooijen M, van den Akkervan Marle E. 2000. A systematic review of the role of human papilloma virus (HPV) testing within a cervical screening programme: summary and conclusions. British Journal of Cancer 83:561–565.
Dyson N, Howley PM, Munger K, Harlow ED. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934–937.
Franco EL. 1991. Viral etiology of cervical cancer: a critique of the evidence. Reviews of Infectious Diseases 13:1195–1206.
Franco EL, Rohan TE, Villa LL. 1999a. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. Journal of the National Cancer Institute 91:506–511.
Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, Rohan TE. 1999b. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. The Journal of Infectious Diseases 180:1415–1423.
Franco EL, Duarte-Franco E, Ferenczy A. 2001. Cervical cancer: epidemiology, prevention, and role of human papillomavirus infection. Canadian Medical Association Journal 164:1017–1025.
Herrero R. 1996. Epidemiology of cervical cancer. Journal of the National Cancer Institute Monographs 21:1–6.
Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, Scott DR, Rush BB, Lawler P, Sherman ME, et al. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. The Journal of Infectious Diseases 169:235–240.
Ho GY, Kadish AS, Burk RD, Basu J, Palan PR, Mikhail M, Romney SL. 1998a. HPV 16 and cigarette smoking as risk factors for high-grade cervical intra-epithelial neoplasia. International Journal of Cancer 78:281–285.
Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. 1998b. Natural history of cervicovaginal papillomavirus infection in young women. New England Journal of Medicine 338:423–428.
IARC Working Group. 1995. Human papillomaviruses. IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 64. Lyon: International Agency for Research on Cancer.
Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, DeRouen TA, Galloway DA, Vernon D, Kiviat NB. 1992. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. New England Journal of Medicine 327:1272–1278.
Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. 2002. A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 347:1645–1651.
Liaw KL, Hildesheim A, Burk RD, Gravitt P, Wacholder S, Manos MM, Scott DR, Sherman ME, Kurman RJ, Glass AG, Anderson SM, Schiffman M. 2001. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. The Journal of Infectious Diseases 183:8–15.
Maciag PC, Schlecht NF, Souza PS, Franco EL, Villa LL, Petzl-Erler ML. 2000. Major histocompatibility complex class II polymorphisms and risk of cervical cancer and human papillomavirus infection in Brazilian women. Cancer Epidemiology, Biomarkers and Prevention 9:1183–1191.
Makni H, Franco EL, Kaiano J, Villa LL, Labrecque S, Dudley R, Storey A, Matlashewski G. 2000. p53 polymorphism in codon 72 and risk of human papillomavirus-induced cervical cancer: effect of inter-laboratory variation. International Journal of Cancer 87:528–533.
Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, Herrero R, Franceschi S. 2002. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet 359:1085–1092.
Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, Palefsky J. 1998. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. The Journal of Pediatrics 132:277–284.
Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, Darragh T, Palefsky J. 2001. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. Journal of the American Medical Association 285:2995–3002.
Muir C, Waterhouse J, Mack T, et al. 1987. Cancer incidence in five continents, Vol. V. IARC Scientific Publications No. 88. Lyon: International Agency for Research on Cancer.
Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, Rozendaal L, Remmink AJ, Risse EK, van der Linden HC, Voorhorst FJ, Kenemans P, Meijer CJ. 1999. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet 354:20–25.
Parkin DM, Pisani P, Ferlay J. 1999. Estimates of the worldwide incidence of 25 major cancers in 1990. International Journal of Cancer 80:827–841.
Pisani P, Parkin DM, Bray F, Ferlay J. 1999. Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer 83:18–29.
Potischman N and Brinton LA. 1996. Nutrition and cervical neoplasia. Cancer Causes and Control 7:113–126.
Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Edwards BK, eds. 2000. SEER Cancer Statistics Review: 1973-1997. Bethesda, MD: National Cancer Institute.
Schiller JT. 1999. Papillomavirus-like particle vaccines for cervical cancer. Molecular Medicine Today 5:209–215.
Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. 2001. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. Journal of the American Medical Association 286:3106–3114.
Thomas M, Matlashewski G, Pim D, Banks L. 1996. Induction of apoptosis by p53 is independent of its oligomeric state and can be abolished by HPV-18 E6 through ubiquitin mediated degradation. Oncogene 13:265–273.
Villa LL, Sichero L, Rahal P, Caballero O, Ferenczy A, Rohan T, Franco EL. 2000. Molecular variants of human papillomavirus types 16 and 18 preferentially associated with cervical neoplasia. The Journal of General Virology 81:2959–2968.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology 189:12-9.
Ylitalo N, Josefsson A, Melbye M, Sorensen P, Frisch M, Andersen PK, Sparen P, Gustafsson M, Magnusson P, Ponten J, Gyllensten U, Adami HO. 2000. A prospective study showing long-term infection with human papillomavirus 16 before the development of cervical carcinoma in situ. Cancer Research 60:6027–6032.
zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. 2000. Journal of the National Cancer Institute 92:690–698.
CHRONIC HEPATITIS B VIRUS INFECTIONS
William Mason, Ph.D.
Fox Chase Cancer Center, Philadelphia, PA
Human hepatitis B virus (HBV) is a small, enveloped virus, with a partially double-stranded, relaxed circular DNA genome of 3.3 kilobase pairs. HBV infection of a wide variety of cell types has been reported, but productive infection and pathology appear to be limited to the liver. Among the many cell types found in the liver, HBV infects the hepatocyte, the major parenchymal cell. Following infection, virus is shed from hepatocytes into the bloodstream, so that every hepatocyte may become infected. During the peak of an infection, titers of virus in the blood may reach 1010 per cubic centimeter. Infection of hepatocytes is not typically cytopathic, and the liver pathology results from the immune response to the infected cells. Depending on the strength of the immune response, infections may be either transient or chronic. Transient infections generally resolve in fewer than 6 months, while chronic infections may be lifelong.
Hepatitis B Virus Replication
HBV replication in the liver occurs by reverse transcription (Seeger and Mason, 2000; Summers and Mason, 1982). When a hepatocyte is infected, the viral DNA genome is transported to the nucleus, where it is converted from a relaxed circular DNA to a covalently closed circular form (cccDNA), which serves as the template for viral mRNA synthesis. Though the coding capacity of HBV is limited, it is still capable of encoding three envelope proteins, a nucleocapsid protein, a transcriptional transactivator, and a reverse transcriptase (RT). Encoding of the reverse transcriptase, the largest HBV protein, requires almost the entire viral genome. (To facilitate this, the reverse transcriptase is encoded in different translational reading frames than the other viral gene products, so that overlapping reading frames can be utilized.) mRNA for the RT is, in fact, slightly greater than genome length, with a terminal redundancy of 220 base pairs (bp). When this mRNA is translated, the RT binds near to the 5′ end of its own message. This RNA/RT complex is then packaged into viral nucleocapsids, where the RT transcribes the RNA into DNA, using one of its own tyrosine resides to prime DNA synthesis (Weber et al., 1994; Zoulim and Seeger, 1994a). Following completion of reverse transcription, the RT then synthesizes most, but not all of the second DNA strand, to recreate the partially double stranded virion DNA. Prior to completion of the second strand, nucleocapsids are packaged into viral envelopes by budding into the endoplasmic reticulum, and virions are exported from the cell. Since cccDNA lacks a replication origin, new cccDNA must be created through the reverse transcription pathway (Tuttleman et al., 1986). Early after infection, and probably after division of an infected hepatocyte, extra cccDNA is synthesized, maintaining the copy number at 5 to 50 per cell. cccDNA appears to be stable in non-dividing hepatocytes (Moraleda et al., 1997), but it is unclear how efficiently cccDNA survives through mitosis.
Transmission
Transmission is parenteral, requiring exposure to the blood or blood-contaminated materials of infected individuals. The most common mode of exposure leading to chronic infection occurs at birth when the mother is chronically infected, or during the first year of life. During this period, the risk of an infection becoming chronic is at least 90 percent. In contrast, the risk of chronic infection in adults is greater than 10 percent. According to the CDC, the most common exposure risks in adults in the United States are sexual activity (50 percent of cases) and intravenous drug abuse (15 percent of cases).
Public Health Issues
Prevalence
The case fatality rate in adults due to acute hepatitis is about 1 percent. Individuals with chronic infection, typically acquired in childhood, have a ~25 percent risk of premature death due to either liver cancer or cirrhosis, both resulting from the persistent liver damage associated with infection. According to WHO, there are now 350 million chronically infected individuals worldwide. Of these, 60 million are expected to die prematurely of liver cancer or cirrhosis, at a rate of approximately 1 million per year (5,000 per year in the United States). This does not account for new cases, which will continue to accumulate in the coming decades.
Vaccines
A vaccine comprised of the viral envelope proteins has been available for over 20 years. Due in part to high cost, universal vaccination was not initially feasible in many parts of the world, but lower cost vaccines have subsequently come into use. Universal vaccination of school children is now in effect in the United States. In some parts of the world, especially in Africa and regions of Asia, chronic infection rates exceed 5–10 percent of the population, but vaccination has not yet been economically feasible in all of these areas, even with low-cost vaccines. Although attempts are under way to address this problem (Kane, 2003), for various reasons of cost and delivery, HBV is likely to remain a major public health problem. On top of this problem there is evidence for vaccine escape mutants (He et al., 2001; Torresi et al., 2002; Wilson et al., 2000). Though these do not yet seem to be a major public health problem, they remain a concern even for the large pool of individuals that have already received the current vaccine. In addition, about 5 percent of vaccinated individuals fail to produce a measurable antibody response, suggesting that they also remain at risk for HBV infection.
Current Research
A major goal of current research has thus been the development of therapies to cure chronically infected individuals. A problem in achieving this is that hepatocytes comprise a self-renewing population with a low turnover rate, and this population often appears to be 100 percent infected. This same barrier is confronted and overcome during immune clearance of transient infections, though it remains controversial how the virus is actually destroyed (Guidotti et al. 1999; Guo et al., 2000; Jilbert et al., 1992; Kajino et al., 1994; Thimme et al., 2003). However, in chronic carriers, the immune system is usually unable to mount such a response, especially in those infected as children. Some hope for better immuno-
therapies has however been sustained by the fact that interferon alpha administration induces virus loss in about 20–30 percent of carriers (Hoofnagle and Lau, 1997), typically those with adult-acquired infections. In addition, some carriers experience spontaneous loss of the virus in association with a flare of liver disease. In both instances, clearance is probably due to activation of the same set of immune responses that are active in clearance of transient infections. Key issues now are how this clearance is carried out, whether it requires destruction of all of the infected hepatocytes, if the immune system has the capacity to cure an infected hepatocyte, and if it can be induced in carriers that have failed to respond to interferon therapy with virus clearance.
Treatment
Another approach to treatment of chronic infections is administration of nucleoside analog inhibitors of the HBV reverse transcriptase. Lamivudine was approved by the U.S. Food and Drug Administration (FDA) in 1998 and has been shown in clinical trials to have a treatment success rate similar to interferon alpha (Perrillo, 2002). A significant problem with lamivudine is the emergence of drug-resistant variants of HBV as therapy continues past a year. Another nucleoside, adefovir dipivoxil, recently received FDA approval and to date drug-resistant variants have not been reported. Moreover, this drug retains activity against lamivudine-resistant HBV (Delaney et al., 2001). However, at doses higher than used for HBV carriers, nephrotoxicity has been observed (Tanji et al., 2001). It may be that nephrotoxicity will become a problem in HBV therapy due to a cumulative effect if carriers require treatment indefinitely. A number of other nucleoside analogs are now in Phase II trials. If these compounds are not toxic during long-term administration, and if viral multi-drug resistance does not develop, it should be possible to eliminate over time the viral cccDNA that maintains a cellular infection by a combination of dilution and hepatocyte death. Achieving this would also allow a critical test of the hypothesis that curing a chronic infection would significantly reduce the risk of death due to cirrhosis, which seems likely, and due to liver cancer, which is difficult to predict, because liver cancer may occur in a liver that appears relatively healthy histologically.
Research Models
HBV research generally reflects public health concerns. How can chronic infections be cured? Will eliminating the virus reduce the risk of liver cancer and premature death from liver disease? What is the mechanism of carcinogenesis? (It is speculated that immune-mediated chronic injury, insertional mutagenesis, and viral proteins all may play a role.) These questions have been investigated using clinical samples and a number of model systems.
Woodchucks are naturally infected with woodchuck hepatitis virus (WHV)
(Summers et al., 1978), which is closely related to HBV and, like HBV, induces liver cancer (but not cirrhosis) during a chronic infection (Popper et al., 1987). Similarly, domestic ducks are infected with duck hepatitis B virus (DHBV), a more distant relative of HBV (Mason et al., 1980; Zhou, 1980). Unlike HBV and WHV, chronic DHBV infection has not been associated with either cirrhosis or liver cancer, possibly because of a lower antiviral immune response in carriers. HBV transgenic mice have been powerful tools for studying certain aspects of the antiviral immune response (Guidotti and Chisari, 2001), even though these mice do not support a complete HBV infection cycle (Tang and McLachlan, 2002). On occasion, chimpanzees, which are susceptible to HBV, have been used to address research issues (Guidotti et al., 1999; Thimme et al., 2003).
Among the model systems, the duck has been heavily used to understand the virus life cycle at the molecular level, to study the biology of infection, and to characterize antiviral therapies, primarily with nucleoside analogs. The woodchuck model has been less used to study molecular biology issues, but has been employed extensively in the development of antiviral therapies and in characterization of the link between chronic infection and liver cancer. An unresolved issue arose in the latter studies. It was found that liver cancer in woodchucks is almost always associated with transcriptional activation of N-myc2 expression in the liver by insertion of viral enhancer sequences (Fourel et al., 1994; Wei et al., 1992). Contrary to expectation, insertional activation of N-myc2 does not appear to be a correlate of liver cancer in HBV carriers. Indeed, with a few rare exceptions, it remains unclear if the frequent sporadic integration of viral DNA that characterizes an infection has a role in most liver cancers that occur in individuals chronically infected with HBV (Dejean et al., 1986; Gozuacik et al., 2001).
The HBV transgenic mouse, in contrast to the natural infection models, has been most heavily used to demonstrate the effects of immune cytokines, such as interferons alpha and gamma, on viral replication intermediates. It was found that cytokines can induce the rapid clearance of viral proteins, RNAs, and DNAs from mouse hepatocytes (Guidotti and Chisari, 2001). These observations seem likely to provide part of the explanation for how virus replication is shut down during the clearance of transient HBV infections.
Though the relationship to natural infections is still unclear, a number of studies have shown that mice carrying the HBV transcriptional activator, X, as a transgene, are at increased risk of developing liver cancer (Kim et al., 1991; Madden et al., 2001; Terradillos et al., 1997). These data suggest that X is in fact a viral oncogene, but clinical evidence to support this conclusion is still lacking, and it is difficult to address this issue in the woodchuck model, because X is needed to establish a productive infection (Chen et al., 1993; Zoulim and Seeger, 1994b).
In addition to characterizing infections and therapies, the animal models have also provided, along with clinical studies, a better understanding of the difficulties of treating chronic infections with nucleoside analogs. From such studies, it
has been determined that cccDNA can persist in the liver for months, and probably years, even when virus DNA synthesis is effectively inhibited (Colonno et al., 2001; Foster et al., 2003; Luscombe et al., 1996; Mason et al., 1994; Zhu et al., 2001). Persistence of cccDNA may be attributable to two factors: 1) an inherent stability within non-dividing hepatocytes, and 2) the relatively low turnover (perhaps a few percent per day) of hepatocytes in most carriers. Studies with animal models have also established that the mutation rate of the viruses is quite high, with a single-base mutation prevalence of about 10-4 (Pult et al., 2001). Thus, drug-resistant variants, especially those requiring only one or two base changes, are likely to be present at the start of therapy. The primary factors needed for subsequent emergence of drug-resistant variants are the time required for the hepatocyte population to become susceptible to spread of virus (e.g., for loss of super-infection resistance), the prevalence of a drug-resistant virus at the start of therapy, and its growth rate (Zhang and Summers, 2000). In practice, emergence of mutants can take from months to several years, the variation probably reflecting additional factors, including the effect of nucleoside therapy on the antiviral immune response of the host (Boni et al., 2001).
Outlook
Discovery of an effective HBV vaccine in the 1960s (Blumberg, 1977) led to the hope that HBV would be eliminated, or at least substantially reduced in the human population within the then foreseeable future. This still remains mostly a hope. Two objectives still need to be fulfilled, universal vaccination (Kane, 2003), and development of an effective therapy for chronic infection. Even though not everyone will be protected using the current vaccine, most would be, and the carrier incidence should decline substantially, first among the young. The goal of complete elimination seems unlikely without major advances in the treatment and elimination of chronic infections, particularly treatments that are rapid acting and cost-effective.
REFERENCES
Blumberg BS. 1977. Australia antigen and the biology of hepatitis B. Science 197:17–25.
Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, Cavalli A, Urbani S, Boehme R, Panebianco R, Fiaccadori F, Ferrari C. 2001. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 33:963–971.
Chen HS, Kaneko S, Girones R, Anderson RW, Hornbuckle WE, Tennant BC, Cote PJ, Gerin JL, Purcell RH, Miller RH. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. Journal of Virology 67:1218–1226.
Colonno RJ, Genovesi EV, Medina I, Lamb L, Durham SK, Huang ML, Corey L, Littlejohn M, Locarnini S, Tennant BC, Rose B, Clark JM. 2001. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. The Journal of Infectious Diseases 184:1236–1245.
Dejean A, Bougueleret L, Grzeschik KH, Tiollais P. 1986. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature 322:70–72.
Delaney WE, Edwards R, Colledge D, Shaw T, Torresi J, Miller TG, Isom HC, Bock CT, Manns MP, Trautwein C, Locarnini S. 2001. Cross-resistance testing of antihepadnaviral compounds using novel recombinant baculoviruses which encode drug-resistant strains of hepatitis B virus. Antimicrobial Agents and Chemotherapy 45:1705–1713.
Foster WK, Miller DS, Marion PL, Colonno RJ, Kotlarski I, Jilbert AR. 2003. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrobial Agents and Chemotherapy 47:2624–2635.
Fourel G, Couturier J, Wei Y, Apiou F, Tiollais P, Buendia MA. 1994. Evidence for long-range oncogene activation by hepadnavirus insertion. The European Molecular Biology Organization Journal 13:2526–2534.
Gozuacik D, Murakami Y, Saigo K, Chami M, Mugnier C, Lagorce D, Okanoue T, Urashima T, Brechot C, Paterlini-Brechot P. 2001. Identification of human cancer-related genes by naturally occurring Hepatitis B Virus DNA tagging. Oncogene 20:6233–6240.
Guidotti LG and Chisari FV. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annual Review of Immunology 19:65–91.
Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825–829.
Guo JT, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, Barrasa MI, Mason WS, Seeger C. 2000. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. Journal of Virology 74:1495–1505.
He C, Nomura F, Itoga S, Isobe K, Nakai T. 2001. Prevalence of vaccine-induced escape mutants of hepatitis B virus in the adult population in China: a prospective study in 176 restaurant employees. Journal of Gastroenterology and Hepatology 16:1373–1377.
Hoofnagle JH and Lau D. 1997. New therapies for chronic hepatitis B. Journal of Viral Hepatitis 4:41–50.
Jilbert AR, Wu TT, England JM, Hall PM, Carp NZ, O’Connell AP, Mason WS. 1992. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. Journal of Virology 66:1377–1388.
Kajino, K., A. R. Jilbert, J. Saputelli, C. E. Aldrich, J. Cullen, and W. S. Mason. 1994. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. Journal of Virology 68:5792–5803.
Kane MA. 2003. Global control of primary hepatocellular carcinoma with hepatitis B vaccine: The contributions of research in Taiwan. Cancer Epidemiology, Biomarkers & Prevention 12:2–3.
Kim CM, Koike K, Saito I, Miyamura T, Jay G. 1991. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 351:317–320.
Luscombe C, Pedersen J, Uren E, Locarnini S. 1996. Long-term ganciclovir chemotherapy for congenital duck hepatitis B virus infection in vivo: Effect on intrahepatic-viral DNA, RNA, and protein expression. Hepatology 24:766–773.
Madden CR, Finegold MJ, Slagle BL. 2001. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. Journal of Virology 75:3851–3858.
Mason WS, Seal G, Summers J. 1980. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. Journal of Virology 36:829–836.
Mason WS, Cullen J, Saputelli J, Wu TT, Liu C, London WT, E Lustbader, Schaffer P, O’Connell AP, Fourel I, Aldrich CE, Jilbert AR. 1994. Characterization of the antiviral effects of 2′ carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology 19:398–411.
Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. 1997. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. Journal of Virology 71:9392–9399.
Perrillo RP. 2002. How will we use the new antiviral agents for hepatitis B? Current Gastroenterology Reports 4:63–71.
Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. 1987. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proceedings of the National Academy of Sciences 84:866–870.
Pult I, Abbott N, Zhang YY, Summers JW. 2001. Frequency of spontaneous mutations in an avian hepadnavirus infection. Journal of Virology 75:9623–9632.
Seeger C and Mason WS. 2000. Hepatitis B virus biology. Microbiology and Molecular Biology Reviews 54:51–68.
Summers J and Mason WS. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403–415.
Summers J, Smolec JM, Snyder R. 1978. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proceedings of the National Academy of Sciences 75:4533–4537.
Tang H and McLachlan A. 2002. Avian and mammalian hepadnaviruses have distinct transcription factor requirements for viral replication. Journal of Virology 76:7468–7472.
Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D’Agati VD. 2001. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Human Pathology 32:734–740.
Terradillos O, Billet O, Renard CA, Levy R, Molina T, Briand P, Buendia MA. 1997. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene 14:395–404.
Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. 2003. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. Journal of Virology 77:68–76.
Torresi J, Earnest-Silveira L, Civitico G, Walters TE, Lewin SR, Fyfe J, Locarnini SA, Manns M, Trautwein C, Bock TC. 2002. Restoration of replication phenotype of lamivudine-resistant hepatitis B virus mutants by compensatory changes in the “fingers” subdomain of the viral polymerase selected as a consequence of mutations in the overlapping S gene. Virology 299:88–99.
Tuttleman JS, Pourcel C, Summers J. 1986. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 47:451–460.
Weber M, Bronsema V, Bartos H, Bosserhoff A, Bartenschlager R, Schaller H. 1994. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. Journal of Virology 68:2994–2999.
Wei Y, Fourel G, Ponzetto A, Silvestro M, Tiollais P, Buendia MA. 1992. Hepadnavirus integration: mechanisms of activation of the N-myc2 retrotransposon in woodchuck liver tumors. Journal of Virology 66:5265–5276.
Wilson JN, Nokes DJ, Carman WF. 2000. Predictions of the emergence of vaccine-resistant hepatitis B in The Gambia using a mathematical model. Epidemiology and Infection 124:295–307.
Zhang YY and Summers J. 2000. Low dynamic state of a viral competition in a chronic avian hepadnavirus infection. Journal of Virology 74:5257–5265.
Zhou YZ. 1980. A virus possibly associated with hepatitis and hepatoma in ducks. Shanghai Medical Journal 3:641–644.
Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. 2001. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. Journal of Virology 75:311–322.
Zoulim F and Seeger C. 1994a. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. Journal of Virology 68:6–13.
Zoulim F and Seeger C. 1994b. Woodchuck hepatitis virus X protein is required for viral infection in vivo. Journal of Virology 68:2026–2030.
INFECTIOUS AGENTS AND CARDIOVASCULAR DISEASE
Michael Dunne, M.D.
Pfizer Global Research and Development, New London, CT
Atherosclerosis remains the most significant threat to the health of individuals living in the United States and Europe. Myocardial infarctions, strokes, peripheral vascular disease and premature deaths constitute an enormous burden on the healthcare systems of these regions every year. Risk factors for atherosclerosis have been identified and interventions targeting these risks have helped mitigate its impact. The clinical sequelae of atherosclerosis remain significant, however, justifying continued research efforts to enhance the value of available interventions as well as identify presently unappreciated risk factors.
Examination of an atherosclerotic plaque reveals pools of cholesterol under a fibrous cap and the infiltration of monocytes and T cells at its margins. This concentration of white blood cells within the plaque is consistent with an ongoing inflammatory process, influenced by factors not yet fully understood. One such influence may be infection.
That infection may play a role in atherosclerosis was first suggested over one hundred years ago with the finding that acute infection with Bacillus typhosus resulted in fatty sclerotic changes in the arterial wall (Gilbert and Lion, 1889; Nieto, 1998). Interest in the role of infection in atherosclerosis was renewed with the observation that patients with coronary artery disease were more likely than matched controls to have an elevated antibody titer to Chlamydia pneumoniae (Saikku et al., 1988). Since that observation, a number of additional associations have been identified. The chain of events linking infections to the development of atherosclerosis is outlined in Figure 1-5. A local infection may lead to an arterial response through two different routes. First, local infection may trigger the systemic release of various proinflammatory mediators, including cytokines, bacterial lipopolysaccharide, heat shock proteins, immune complexes and, possibly, activated, but uninfected, mononuclear cells. These mediators move through the systemic circulation and incite an immune response in the arterial wall. This response may include the upregulation of receptors on the endothelial cell surface, enhancement of transendothelial migration of inflammatory cells, or activation of white blood cells already existing within the plaque. These activated WBCs may oxidize LDL cholesterol or release proteinases, which then act to destabilize the overlying fibrous cap of the atheroma.
The second route by which infection may result in progression or initiation of an atherosclerotic lesion involves the dissemination of organisms from local sites of infection directly to the arterial wall itself. The organisms may traffic to the site within an infected monocyte, attach and then diapedese through the endothelial cell layer, taking advantage of secondary host defense mechanisms to
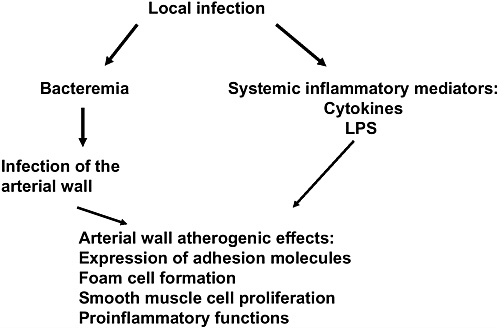
FIGURE 1-5 Pathways through which local infection can lead to atherogenesis.
infect distal tissue. Once at the site, the organisms could drive a local inflammatory process or, in addition, infect other cells within the arterial wall.
A number of potential pathogens have been associated with atherosclerosis (Danesh, 1999). The strength of the association varies with the organism but is based on seroepidemiologic studies, histopathologic evidence of disease, animal model data and various pathophysiologic associations. Among possible viral pathogens are cytomegalovirus and herpes simplex (Nieto, 1999; Dunne, 2000). Among bacterial pathogens are various dental organisms, Helicobacter pylori, and Mycoplasma pneumoniae. The most significant amount of preclinical and clinical investigation, however, has focused on C. pneumoniae; as an example of the types of evidence that can implicate a potential infectious pathogen driving some component of the atherosclerotic process, these data will be reviewed in more detail.
C. Pneumoniae and Atherosclerosis Seroepidemiologic Studies
Since the initial study that identified an association between elevated C. pneumoniae antibody titers and the prevalence of coronary artery disease, over thirty additional studies have been performed and multiple review articles published. These studies used different antibody detection assays with different titer
cutoffs, different case definitions of coronary artery disease, and were performed in different geographic regions. Overall, it appears that elevated antibody titers to C. pneumoniae are associated with a three-fold increase in the likelihood of having coronary artery disease. The association identified in seroepidemiologic studies using titers to predict the incidence, distinct from the prevalence, of heart disease, however, only variably detect an association and, when positive, only in the range of a 20–40 percent increased risk (Dunne, 2000). While the implications of these different findings are being evaluated, the main value of these seroepidemiologic studies may be the attention they have brought to the potential for any association at all.
Histopathology
The next series of studies involve histopathologic examinations of the atheromatous plaque. In the first 15 studies reported in the literature which were conducted in the United States and Europe, approximately 45 percent of the total of 574 samples examined were found to contain evidence of C. pneumoniae by either immunohistochemistry, electron microscopy, in situ polymerase chain reaction (PCR) or, rarely, culture. The primary criticism of these studies has focused on the lack of standardization of the assay techniques but, given the bulk of the observations from these and subsequent studies, it seems likely that this pathogen can be found in the plaque.
Because antibody titers merely suggest historical exposure to the pathogen, there has been recent interest in the use of PCR to identify individuals that may have an active infection with C. pneumoniae. PCR has been used to assess both histopathologic specimens and circulating white blood cells. In four published papers, patients with a history of coronary artery disease were more likely than controls to have C. pneumoniae identified in circulating monocytes by PCR (Dunne, 2000). In a fifth paper, the incidence was not significantly different but the C. pneumoniae rRNA copy number was higher in patients with heart disease (Berger et al., 2000). Of interest, the proportion of individuals with PCR positive cells in these studies ranged from 9 to 60 percent in the patients with heart disease and 2 to 46 percent in the controls. While this range of exposure may be explained by epidemiologic influences, technical concerns about assay methodologies remain and efforts at standardization have been initiated (Dowell et al., 2001). When the technical concerns have been addressed, it will also be important to understand why otherwise normal individuals have evidence of this pathogen circulating in what should be a sterile space.
Animal Models
In addition to serologic and histologic evidence associating C. pneumoniae and atherosclerosis, a number of animal models have been established. Evidence
that C. pneumoniae can either initiate or accelerate the atherosclerotic lesion has come from work with both mice (NIH/s, ApoE-deficient, and LDL-receptor knock-out strains) and New Zealand White rabbits. These animals generally need to consume a high cholesterol diet in order to develop observable changes, though it is possible, in one of the rabbit models, to observe effects without an atherogenic diet (Fong et al., 1999). In the LDL receptor knockout mouse, intranasal inoculation with the C. pneumoniae AR39 strain twice monthly for six months was performed prior to sacrifice of the animals. Uninfected mice fed a high cholesterol diet had a lesion area index (defined as the size of a digitized image of the lesion divided by the aorta luminal surface and multiplied by one hundred) of 18, while infected animals given a high cholesterol diet had an index of 42. This 130 percent increase in lesion size suggests that infection with chlamydia can accelerate the growth of an atherosclerotic plaque (Hu et al., 1999).
There are limitations to the interpretation of animal models of atherosclerosis. In some of these models the atherosclerotic lesions observed are consistent with a very early pathologic process that does not mirror the lesions responsible for causing human disease. The atherosclerotic lesions in these models generally do not rupture or lead to clinical disease in the animal. While these data do support the potential for a contribution of chlamydia to lipid accumulation at the site, they do not provide conclusive evidence that infection will lead to plaque rupture.
Chlamydia Pathogenesis and Atherogenesis
A fourth line of persuasive evidence comes from similarities in the pathophysiology of C. pneumoniae infection and atherogenesis. The generation of an atherosclerotic plaque is generally felt to be a chronic process. To the extent that a chlamydia infection, in addition to any acute effects, has a chronic component to its pathophysiology, an association with atherosclerosis can be more easily defended. The demonstration that chlamydia may exist in a persistent state may serve to explain the latent nature of a chlamydia infection.
Chlamydia exists as elementary bodies in the environment. Upon entry into a host cell the elementary body undergoes a series of transformations that allow it ultimately to replicate. At this stage it is referred to as a reticulate body. After cell division, it again reverts to an elementary body and is released from the host cell. If, however, host cell conditions are not favorable, chlamydia will not progress through cell division and instead moves into what has been referred to as a persistent state, appearing morphologically as a large, aberrant form (Beatty et al., 1994). The organism has been found to persist in cell culture in this state for prolonged periods of time and, in vitro, to be relatively refractory to antibiotic therapy.
While evidence for a persistent state has not been established in clinical specimens, it remains possible that chlamydia could contribute to a chronic condition by remaining relatively dormant, while still influencing the condition of the host
cell. A series of experiments (Zhong et al., 1999; 2000), has offered some insights as to why a chronically infected host cell is not destroyed by the immune system. It appears that chlamydia can selectively inhibit IFN-gamma-inducible MHC class I and II expression and thereby evade antigen presentation on the cell surface. Inhibition of this process by bacterial protein synthesis inhibitors such as chloramphenicol suggests that it is dependent on chlamydial protein synthesis.
Clinically latent infections have been demonstrated with a number of chlamydia species. The blinding eye disease trachoma has occurred decades after exposure to either C. trachomatis or C. pneumoniae. Infertility can result from chronic infection of the upper genital tract with C. trachomatis, a process that can take place over years. C. pneumoniae has also been isolated from the respiratory tract long after resolution of an acute infection.
Atherosclerosis is now considered to be an inflammatory disease (Ross, 1999). The association of C. pneumoniae with atherogenesis is supported by the possibility that C. pneumoniae contributes to this inflammation. Based on data from animal models, and supported by the PCR examinations of circulating white blood cells and histologic examinations of atherosclerotic tissue, a respiratory tract infection could lead to dissemination of C. pneumoniae in monocytes. These monocytes release factors that enhance the likelihood of endothelial infection with chlamydia (Lin et al., 2000). Once infected, the endothelial cells could affect the local arterial environment in three ways. Transendothelial migration of the monocytes is enhanced (Molestina et al., 1999). The infected endothelial cells release tissue factor and platelet aggregation inhibitor, which leads to enhanced coagulability at the site. And thirdly, mitogenic factors are released through an NF-Kβ related mechanism, leading to smooth muscle cell proliferation (Miller et al., 2000). This triad, subendothelial monocyte accumulation, hypercoagulability at the site of the atheroma and smooth muscle cell proliferation, is the hallmark of an atherosclerotic plaque and, as such, provides further support for a contribution of local C. pneumoniae infection to this inflammatory state.
Clinical Trials with Antibiotics
Even with continued gaps in our understanding of the association between infection and atherosclerosis, the significance of coronary artery disease as an unmet medical need has driven interest in conducting antibiotic intervention studies. Based on the various supportive data discussed thus far, a number of clinical trials designed to investigate the role of antibiotic intervention in reducing the incidence of atherosclerotic disease have been initiated. There is certainly more work that needs to be done preclinically, including additional studies outlining the role of C. pneumoniae in atherogenesis, improving the capabilities around diagnostic testing, understanding the influence of antibiotics, alone or in combination, on chlamydia replication, further exploring animal models of in vivo
pathogenesis, and better defining the lifecycle of chlamydia, and specifically the persistent state.
There are a number of challenges to studying the use of antibiotics in clinical coronary artery disease. While several risk factors for coronary artery disease are already well established, the relationship between these risk factors and C. pneumoniae infection has not been fully examined. As such associations become better known, the use of these risk factors as selection criteria may become useful. Clinical studies will need to address this problem of multiple competing risks even while the appropriateness of controlling for these factors in any statistical analyses, or selecting the target group of patients to treat, remains open to debate.
Many questions remain regarding antimicrobial activity within the plaque. While there is clinical evidence that patients with either genitourinary tract or respiratory tract infections due to chlamydia can have the clinical course of their disease positively impacted by antibiotic intervention, it remains unknown whether antibiotic treatment will affect either the replication or pathogenicity of chlamydia infections in the atherosclerotic plaque. It may not be possible to either document infection at the arterial site or substantiate a positive microbiologic outcome. There remain concerns that to the extent that cells contain chlamydia in the persistent state, it may not be possible to fully eradicate the organism. Standard in vitro testing may be inadequate to fully address this issue, given that the contribution of the immune system to clearance of infected cells is not measured.
Specific concerns about the design of clinical trials also exist. The appropriate patient population to treat is not clear. If C. pneumoniae is the target organism, patient selection criteria specific to the organism could be useful. Antibody titers are a crude estimate of previous exposure but may not be adequate to select those patients actively infected. As identification of infection within the atheroma is not presently possible, surrogates of active infection are needed. Perhaps, in the future, there will be a role for the measurement of C. pneumoniae DNA in circulating white blood cells. As is typical with cardiovascular studies of coronary artery disease, the event rates are typically low. Selection of patients likely to have a primary event is critical to ensuring that any treatment effect can be observed. Setting the sample size is made difficult by not having any estimate of the potential treatment effects; in order to avoid missing a potential effect, efficacy rates may need to be assumed to be low. These two issues require that definitive studies be large in order to have sufficient statistical power to determine treatment effects. Interpretation of the results from smaller studies is consequently more problematic.
The results of ongoing clinical trials will be best able to answer questions that are focused on the merits of the antibiotic intervention in the specific population of patients enrolled, and focused on the prespecified endpoints. The results will be compelling to the extent that the studies are adequately powered and the chosen endpoints are clinically relevant. The ongoing trials are less likely to be able to define the mechanism of action underlying any observed treatment effect.
Pre- and post-treatment measurement of such inflammatory indices as C-reactive protein, cytokines, fibrinogen and tissue factor could be performed, but determining whether any changes are a direct result of immune specific activity or an indirect result of reducing the burden of organisms may be problematic.
A number of antibiotic intervention studies have been initiated. The antibiotics used have been either macrolides, doxycycline or a fluoroquinolone, given the in vitro activity of classes of drugs against C. pneumoniae. Of the completed studies, the two earliest reported promising results, both with short-term therapies, but the small sample size of these trials precludes any definitive conclusions (see Table 1-2). In general, the trial design has varied such that no two are the same. They have focused on primary or secondary prevention, different antibiotic
TABLE 1-2 Clinical Trials with Antibiotics for Primary and Secondary Prevention of Atherosclerosis Diseases
|
Study |
Population |
N |
Antibiotic |
Endpoint |
Outcome |
|
St. George’s (Gupta et al., 1997) |
Post-MI |
80 |
Azithromycin |
Heart Diseasea |
Event rate: 8% vs. 28% |
|
ROXIS (Gurfinkel et al., 1997) |
CAD |
200 |
Roxithromycin |
Heart Diseasea |
Event rate: 1% vs. 10% |
|
CROAATS (Reiner, 2002) |
Post-MI |
234 |
Azithromycin |
Heart Diseasea |
Event rate: 7.0% vs. 5.2% |
|
ACADEMIC (Anderson et al., 1999) |
CAD |
300 |
Azithromycin |
Heart Diseasea |
Hazard ratio: 0.89 (0.51, 1.61) |
|
ANTIBIO (Zahn et al., 2003) |
Acute MI |
892 |
Roxithromycin |
Heart Diseasea |
Event rate: 25% vs. 21% |
|
ISAR-3 (Neumann et al., 2001) |
Angioplasty |
1010 |
Roxithromycin |
Restenosis |
Hazard ratio: 1.08 (0.92, 1.26) |
|
Macrolide for PAD (Wiesli et al., 2002) |
Peripheral artery disease |
40 |
Roxithromycin |
Interventions for claudication |
Event rate: 20% vs. 45% |
|
aSome combination of either recurrent MI, hospitalization for angina, coronary artery intervention, stroke, or death. |
|||||
interventions, different durations of therapy, different cardiovascular endpoints and different manifestations of atherosclerosis at baseline. This variability in study design is a consequence of many of the issues noted above and is to be expected at this early stage of the development of a potential new intervention strategy. Results of future study designs that incorporate data-driven refinements in patient selection, duration of dosing and choice of antibiotic will be required before a complete assessment of the value of antibiotic intervention can be made.
Conclusion
Atherosclerosis is an inflammatory disease. That infection may serve as a root cause of this inflammation is supported by a number of different lines of evidence. At present, the most compelling data support the role of C. pneumoniae through pathogens, such as cytomegalovirus and dental organisms, should not be discounted. The macrophage is a critical component in the pathway to atherosclerotic inflammation. To the extent that an infectious process activates a macrophage, either in the local arterial milieu or at a distant site, there is the potential for that macrophage to stimulate both local lipid accumulation and the instability that presages plaque rupture. Given the burden that coronary artery disease imparts on the healthcare system and on society in general, efforts to both understand the role of infection in atherogenesis, and to develop targeted intervention strategies, should continue apace.
REFERENCES
Anderson JL, Muhlestein JB, Carlquist J, Allen A, Trehan S, Nielson C, Hall S, Brady J, Egger M, Horne B, Lim T. 1999. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection: the azithromycin in coronary artery disease: elimination of myocardial infection with chlamydia (ACADEMIC) study. Circulation 99:1540–1547.
Beatty WL, Morrison RP, Byrne GI. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiological Reviews 58:686–699.
Berger M, Schroder B, Daeschlin G, Schneider W, Busjahn A, Buchalow I, Luft FC, Haller H. 2000. Chlamydia pneumoniae DNA in non-coronary atherosclerotic plaques and circulating leokocytes. Journal of Laboratory and Clinical Medicine 136:194–200.
Danesh J. 1999. Coronary heart disease, Helicobacter pylori, dental disease, Chlamydia pneumoniae, and cytomegalovirus: meta-analyses of prospective studies. American Heart Journal 138:S434–437.
Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J, Hammerschlag MR, Jackson LA, Kuo CC, Maass M, Messmer TO, Talkington DF, Tondella ML, Zaki SR. 2001. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clinical Infectious Diseases 33:492–503.
Dunne M. 2000. The evolving relationship between Chlamydia pneumoniae and atherosclerosis. Current Opinion in Infectious Diseases 13(6):583–591.
Fong IG, Chiu B, Viira E, Jang D, Fong MW, Peeling R, Mahony JA. 1999. Can an Antibiotic (macrolide) prevent Chlamydia pneumoniae-induced atherosclerosis in a rabbit model? Clinical and Diagnostic Laboratory Immunology 6:891–894.
Gilbert A and Lion G. 1889. Artérites infectieuses expérimentales. Comptes Rendus Hebdomadaires des Séances et Mémoires de la Société de Biologie. 41:583–584.
Gupta S, Leatham EW, Carrington D, Mendall MA, Kaski JC, Camm AJ. 1997. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 96:404–407.
Gurfinkel EG, Bozovich G, Daroca A, Beck E, Mautner B. 1997. Randomised trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS Pilot Study. ROXIS Study Group. [comment]. Lancet 350:404–407.
Hu H, Pierce GN, Zhong G. 1999. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. Journal of Clinical Investigation 103:747–753.
Lin TM, Campbell LA, Rosenfeld ME, Kuo CC. 2000. Monocyte-endothelial cell coculture enhances infection of endothelial cells with Chlamydia pneumoniae. Journal of Infectious Diseases 181:1096–1100.
Miller SA, Selzman CH, Shames BD, Barton HA, Johnson SM, Harken AH. 2000. Chlamydia pneumoniae activates nuclear factor kappaB and activator protein 1 in human vascular smooth muscle and induces cellular proliferation. Journal of Surgical Research 90:76–81.
Molestina RE, Miller RD, Ramirez, JA Summersgill JT. 1999. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infection & Immunity 67:1323–1330.
Neumann FA, Kastrati A, Miethke T, Pogatsa-Murray G, Mehilli J, Valina C, Jogethaei N, da Costa CP, Wagner H. Schomig A. 2001. Treatment of Chlamydia pneumoniae infection with roxithromycin and effect on neointima proliferation after coronary stent placement (ISAR-3): a randomised, double-blind, placebo-controlled trial. Lancet 357:2085–2089.
Nieto FJ. 1998. Infections and atherosclerosis: new clues from and old hypothesis? American Journal of Epidemiology 48:937–948.
Nieto FJ. 1999. Viruses and atherosclerosis: a critical review of the epidemiologic evidence. American Heart Journal 138:S453–460.
Reiner Z. 2002. Azithromycin in the secondary prevention of adverse cardiovascular events in C. pneumoniae-positive post myocardial infarction patients (CROAATS). Paper presented at the Sixth International Conference on the Macrolides, Azalides, Streptogramins, Ketolides, and Oxazolidinones, Bologna, Italy, January 23-25, 2002.
Ross R. 1999. Atherosclerosis—an inflammatory disease. [comment]. New England Journal of Medicine 340:115–126.
Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, Huttunen JK, Valtonen V. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 2:983–986.
Wiesli P, Czerwenka W, Meniconi A, Maly F, Hoffman U, Vetter W, Schulthess G. 2002. Roxithromycin treatment prevents progression of peripheral arterial occlusive disease in Chlamydia pneumoniae seropositive men: a randomized, double-blind, placebo-controlled trial. Circulation 105:2646–2652.
Zahn R, Schneider S, Frilling B, Seidl K, Tebbe U, Weber M, Gottwik M, Altmann E, Seidel F, Rox J, Hoffler U, Neuhaus KL, Senges J. Working Group of Leading Hospital Cardiologists. 2003. Antibiotic therapy after acute myocardial infarction: a prospective randomized study. Circulation 107:1253–1259.
Zhong G, Fan T, Lui L. 1999. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. Journal of Experimental Medicine 189:1931–1938.
Zhong G, Liu L, Fan T, Fan P, Ji H. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in chlamydia-infected cells. Journal of Experimental Medicine 191:1525–1534.
DEMYELINATING DISEASES
Richard T. Johnson, M.D.
Johns Hopkins University, Baltimore, MD
Viral infections cause a variety of demyelinating diseases in animals and humans. Demyelinating diseases are defined as disorders of the central or peripheral nervous system with destruction of myelin and relative preservation of axons. Other histopathological features do not alter the definition; oligodendrocytes or Schwann cells may or may not be affected, astrocytosis and gliosis may or may not be prominent, and inflammation may or may not be present. All of these features have been described in virus-induced demyelinating disorders. The pathogenesis of the demyelination is different with different infections; these mechanisms range from direct infection and lysis of oligodendrocytes to immune destruction of myelin or supporting cells by cell-mediated immune responses, antibody, or cytokines (see Box 1-1). Many studies of virus-induced demyelinat-
|
Box 1-1
SOURCE: Johnson (1998). |
ing diseases have been pursued in hopes of discovering a role of viruses in multiple sclerosis, but this goal remains elusive.
Animal Models
Animal viruses can produce acute, chronic, and relapsing/remitting demyelinating central nervous system diseases in their natural or experimental hosts (see Table 1-3). The best model for human postinfectious encephalomyelitis (acute disseminated encephalomyelitis), however, is not a viral infection but experimental autoimmune encephalomyelitis (EAE) induced by injection of myelin proteins with Freund’s adjuvant. The latency, clinical disease, pathology and immunological features of these two diseases are similar.
Progressive multifocal leucoencephalopathy (PML) in macaque monkeys
TABLE 1-3 Animal Models of Demyelinating Diseases
|
Virus Family |
Virus |
Host Animal |
Proposed Mechanism |
|
Papoavirus |
SV40 |
Monkeys |
Opportunistic infection of oligodendrocytes in immunodeficient animals |
|
Coronavirus |
Mouse Hepatitis Virus |
Mice |
Persistent oligodendrocyte infection and probable humoral immune responses |
|
|
|
Rats |
|
|
Picornaviruses |
Theiler’s virus |
Mice |
Persistent infection of oligodendrocytes and macrophages and immune responses |
|
|
Encephalomyocarditis |
Mice |
? |
|
Rhabdovirus |
Chandipura |
Mice |
Cell-mediated immune responses |
|
|
Vesicular stomatitis virus |
Mice |
|
|
Togavirus |
Semiliki Forest virus |
Mice |
Neuronal infection and immune |
|
|
Venezuelan equine encephalitis virus |
Mice |
mediated demyelination |
|
|
Ross River virus |
Mice |
Direct lysis of oligodendrocytes |
|
Paramyxovirus |
Canine distemper virus |
Dogs |
Predominantly astrocytic infection with probable indirect demyelination |
|
Lentivirus |
Visna virus |
Sheep |
Macrophage and monocyte infection with cytokine-mediated demyelination |
|
|
Caprine arthritis-encephalitis virus |
Goats |
|
|
SOURCE: Johnson (1998). |
|||
caused by SV40 virus is the animal equivalent of PML in man caused by the related JC virus. As in the human disease, the disease evolves in latently infected animals when other infections or illnesses cause immunodeficiencies. Four naturally occurring infections in their native hosts have been the most widely studied models of virus-induced demyelination. Theiler’s virus, a picornavirus, and JHM virus, a murine coronavirus, were both originally recovered from paralyzed mice; canine distemper, a morbillivirus closely related to measles virus, has long been recognized to cause demyelination in a subacute encephalitis called “old dog disease”; and visna virus, a natural retrovirus infection of sheep, causes relapsing and remitting disease with multifocal demyelinating lesions after a long incubation period. Visna and a related caprine lentivirus (caprine arthritis-ecephalitis virus) best simulate multiple sclerosis, but they have not been widely exploited because of the need to use sheep or goats as the experiment animals. In these lentivirus diseases, infection is limited to macrophages and microglia, and demyelination is thought to result from cytokines released by infected cells.
Human Demyelinating Diseases of Known Viral Etiology
Postinfectious Encephalomyelitis or Acute Disseminated Encephalomyelitis (ADEM)
This is an acute perivenular demyelinating disease of the brain and spinal cord that usually follows viral infections, but on occasions follows some bacterial infections and vaccines, particularly those containing nervous system tissues. Historically, the disorder was also known as post-exanthematous encepahlomyelitis, since it was most frequent after viral diseases characterized by rashes. In the 1950s, ADEM constituted one-third of all cases of encephalitis (see Table 1-4). With the discontinuation of vaccinia virus immunization against smallpox and introduction of vaccines to prevent measles, mumps, rubella, and chickenpox,
TABLE 1-4 Postinfectious Encephalomyelitis Associated with Exanthematous Viral Infections
|
Disease |
Case rate |
Fatality rate |
Sequelae rate |
|
Vaccinia |
1:63 to 1:250,000 |
10% |
Rare |
|
Measles |
1:1,000 |
25% |
Frequent |
|
Varicellaa |
1:10,000 |
5% |
10% |
|
Rubellaa |
<1:20,000 |
20% |
Very rare |
|
aEstimates are difficult to determine because of the frequency of toxic encephalopathy or Reyes syndrome (different pathology) and acute cerebellar ataxia (unknown pathology) and the rare documentation of perivenular demyelinating disease. |
|||
ADEM constitutes less than one-tenth of the cases of acute encephalitis and now is most common after nonspecific upper respiratory infections.
The hazard of ADEM after inoculation of vaccinia virus to protect against smallpox has returned as an issue, since resumption of vaccination is being considered to counter the possible use of smallpox as a terrorist weapon. A very high rate of complications in one Dutch vaccination program was presumably due to use of a more encephalitogenic strain; the low rates during the mass vaccination in New York in 1947 probably reflects poor surveillance. In Great Britain, during the more recent outbreak of smallpox in 1962, a rate of postvaccinal encephalomyelitis of 1 per 20,000 was estimated, and CDC retrospective surveys estimated 1 per 200,000 in the United States prior to the discontinuation of vaccination. However, the risk of ADEM when starting vaccination after a hiatus of 30 years is uncertain, since neurologic complications are more frequent with primary vaccination and higher in persons over the age of 20 years.
The clinical presentation of ADEM usually follows the antecedent exanthem or respiratory or gastrointestinal symptoms by 5 to 21 days. Typically postmeasles encephalomyelitis occurs 5 to 7 days after the rash when the child is returning to normal activity. There is the abrupt recurrence of fever, depression of consciousness, and appearance of multifocal neurological findings. The spinal fluid usually contains myelin basic protein, often shows increased pressure and a mild pleocytosis (but in about one-third of cases, no increased cellularity is found). The MRI may show very dramatic changes with multiple enhancing lesions in the white matter.
The histopathology of fatal cases shows perivenular inflammation and demyelination throughout the brain and spinal cord. In most instances, virus is not found within the nervous system. For example, in measles, virus is seldom recoverable after the rash which corresponds with the humoral immune response. In measles, deaths occurring at or before the time of rash, measles virus has been found in cerebrovascular endothelial cells by in situ PCR; but no virus antigen or nucleic acid has been found in cells of the CNS in patients dying of encephalomyelitis.
The pathogenesis of ADEM is related to infection of immunocompetent cells and the alteration of immune responses. In both postmeasles and postvaricella disease activated peripheral blood lymphocytes responsive to myelin basic protein have been demonstrated. The autoimmune response against CNS myelin appears to occur without the prerequisite of infection of CNS cells. ADEM appears to be an autoimmune disease very similar to experimental autoimmune encephalomyelitis.
Progressive Multifocal Leucoencephalopathy (PML)
PML is a subacute demyelinating disease originally described as a rare complication of leukemia and Hodgkin’s disease. Prior to 1982, PML was an extraor-
dinarily rare disease. With the emergence of AIDS over the past two decades, PML has become a common opportunistic infection causing death in 3–5 percent of AIDS patients.
The clinical presentation is on a background of severe immunosuppression. Multifocal neurological symptoms and signs develop insidiously and usually follow an ingravescent course to death. With introduction of HAART therapy and recovery of T4 counts, stabilization and even improvement has been reported. There is no fever, no nuchal rigidity, and usually no pleocytosis. A very characteristic MRI pattern is seen, however, with nonenhancing multifocal lesions in the subcortical white matter.
The neuropathological changes are unique. Plaques of demyelination are seen preferentially in the grey–white junction. Histologically inflammation is slight or absent. In areas of demyelination, axons are relatively spared and oligodendrocytes are lost. Surrounding these foci, oligodendrocytes are enlarged and contain intranuclear inclusions. Astocytosis is intense, and many astrocytes contain bizarre mitotic figures and multiple nuclei resembling malignant cells.
Electron microscopic examination of the oligodendrocyte inclusions reveal profuse pseudocrystalline arrays of papovaviruses. Only occasional viral particles are seen in astrocytes but they express papovavirus T antigen. JC virus, an ubiquitous human papovavirus, has been associated with almost all cases.
The pathogenesis of demyelination in PML is the opposite of that in ADEM. JC virus causes an asymptomatic persistent infection in most persons. With intense immunosuppression the virus in some patients is transported to brain, probably in B cells. With massive replication in oligodendrocytes these cells are destroyed with secondary loss of myelin. There is no evidence of infection of neurons. Semipermissive infection of astrocytes leads to limited virus production but many astrocytic changes and proliferation resemble transformation. The tat protein of HIV may transactivate JC virus accounting for the unique frequency of PML in HIV-infected patients.
Multiple Sclerosis
A viral cause for multiple sclerosis has been postulated for over 100 years. Over the past half century this speculation has been highlighted by 3 types of studies. First, epidemiological evidence implicates childhood exposure factors (possibly viral infections) in the genesis of multiple sclerosis, and natural history studies have related “virus-like illnesses” to exacerbations of the disease. Second, studies of human and animal viral infections have documented that these infections can have incubation periods of years, cause remitting and relapsing disease and can cause myelin destruction mediated by a variety of mechanisms. Third, laboratory studies of patients with multiple sclerosis consistently show that such patients have greater antibody responses to a variety of viruses than controls and this includes intrathecal antibody synthesis. This is not to deny the clear-cut ge-
netic susceptibility factor (a concordance of over 30 percent in monozygotic twins) or the immunologic abnormalities (which may be caused by infection or be the cause of the unusual viral immune responses in patients).
The unique geographical distribution in temperate zones may in part be explained by Nordic susceptibility genes, but because many immigration studies show that migrants after about age 13 take their risk of early homeland with them and very young migrants acquire the risk of their new land, these findings suggest a childhood exposure. Apparent outbreaks are recorded such as the increase in incidence of multiple sclerosis in the Faroe Islands following the British occupation in World War II. Little evidence is present in these studies to implicate a specific agent; but there are examples of viruses that show different ages of acquisition. For example, varicella occurs at earlier ages in temperate climates and Epstein-Barr virus infections at later ages; in addition the severity or presentation of infection may be age dependent. Early childhood Epstein-Barr virus infection is asymptomatic whereas young adult infection gives rise to infectious mononucleosis.
Specific viral infections have been suggested by serological and virus isolation studies. Over 30 studies have documented the higher levels of antibody to measles in serum and spinal fluid in multiple sclerosis patients than in controls. Although the most striking, measles is not alone as antibodies to many viruses have been found higher in multiple sclerosis patients (see Table 1-5).
Recovery of viruses from tissues or spinal fluid of patients has been repeatedly reported (see Table 1-6), but not with the consistency of serological tests.
TABLE 1-5 Higher Anti-Viral Antibodies in Multiple Sclerosis Than in Controls
|
Serum |
CSF |
|
Measles |
Measles |
|
Parainfluenza 3 |
Parainfluenza 1, 2, 3 |
|
Influenza C |
Influenza A, B |
|
Varicella |
Varicella |
|
Herpes simplex |
Herpes simplex |
|
Human herpes virus – 6 |
Human herpes virus – 6 |
|
Rubella |
Rubella |
|
Epstein-Barr |
Epstein-Barr |
|
|
Mumps |
|
|
Respiratory syncytial |
|
|
Coronaviruses |
|
|
Adenoviruses |
|
HTLV-I (gag) |
HTLV-I (gag) |
|
HTLV-II |
Simian virus-5 |
|
SOURCE: Adapted from Johnson (1998). |
|
TABLE 1-6 Viruses Recovered from Patients with Multiple Sclerosis
|
Virus |
Year |
|
Rabies virus |
1946 |
|
|
1964 |
|
Herpes simplex virus, type 2 |
1964 |
|
Scrapie agent |
1965 |
|
MS-associated agent |
1972 |
|
Parainfluenza virus 1 |
1972 |
|
Measles virus |
1978 |
|
Simian virus 5 |
1979 |
|
Chimpanzee cytomegalovirus |
1980 |
|
Coronavirus |
1982 |
|
SMON-like virus (subacute myelo-optico-neuropathy) |
1982 |
|
Tick-borne encephalitis flavivirus |
1986 |
|
HTLV-1 (human T-cell lymphotrophic virus) |
1989 |
|
LM7 (retrovirus) |
1989 |
|
Herpes simplex virus, type 1 |
1989 |
|
Human herpesvirus 6 |
1994 |
|
Endogenous retroviruses |
1998 |
Indeed most have proved to be contaminants picked up from cell cultures or laboratory animals.
Recent interest has focused on Chlamydia pneumoniae, herpesvirus 6, Epstein-Barr virus, and endogenous retroviruses as latent or persistent agents implicated in multiple sclerosis. While they are all normal flora of the human body, they seem to change in quantity or topography in multiple sclerosis. Again this raises the tough question of causation versus an epiphenomenon secondary to the immunological changes in the disease.
Chlamydia commonly causes chronic infection of macrophages, so its recovery from an inflammatory lesion may only reflect the ingress of macrophages. Similarly Epstein-Barr is latent in B cells, and in a disease such as multiple sclerosis, where intrathecal antibody synthesis is taking place, finding it in spinal fluid or brain by PCR is not surprising. Human herpesvirus 6 has similar latency and may be nonspecifically activated by a disease exacerbation. Endogenous retrovirus sequences are present in all our cells, but again nonspecific activation of macrophages increases the translation of these sequences.
In conclusion, patients with multiple sclerosis have abnormally active immune responses to many viruses, and these responses include intrathecal responses. Viral infections precede exacerbations of disease more often than can be explained by chance. The pathogenetic role of viruses in the cause of multiple sclerosis and the precipitation of exacerbations remain a mystery.
REFERENCES
These references are not specifically cited in text.
Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, van Der Meche FG, van Doorn PA, Hintzen RQ. 2002. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125:952–960.
Gieffers J, Pohl D, Treib J, Dittmann R, Stephan C, Klotz K, Hanefeld F, Solbach W, Haass A, Maass M. 2001. Presence of Chlamydia pneumoniae DNA in the cerebral spinal fluid is a common phenomenon in a variety of neurological diseases and not restricted to multiple sclerosis. Annals of Neurology 49:585–589.
Gonzalez-Scarano F and Rima B. 1999. Infectious etiology in multiple sclerosis: the database continues. Trends in Microbiology 7:475–477.
Johnson RT. 1994. The virology of demyelinating diseases. Annals of Neurology 36:S54–S60.
Johnson RT. 1998. Viral Infections of the Nervous System 2nd Ed. Philadelphia: Lippincott-Raven.
Johnston JB, Silva C, Holden J, Warren KG, Clark AW, Power C. 2001. Monocyte activation and differentiation augments human retrovirus expression: implications for inflammatory brain diseases. Annals of Neurology 50:434–442.
Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, Wessel K, Kirchner H, Hennig H. 2000. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 55:178–184.
Yao SY, Stratton CW, Mitchell WM, Sriram S. 2000. CSF oligoclonal bands in MS include antibodies against Chlamydophila antigens. Neurology 56:1168–1176.
COMMON INFECTIONS AND UNCOMMON DISEASE: ELUSIVE ASSOCIATIONS OF ENTEROVIRUSES AND TYPE I DIABETES MELLITUS
Mark A. Pallansch, Ph.D.; and M. Steven Oberste, Ph.D.
Respiratory and Enteric Viruses Branch,
Division of Viral and Rickettsial Diseases
National Center for Infectious Diseases,
Centers for Disease Control and Prevention, Atlanta, GA
Host genetic determinants have a major influence on an individual’s risk of developing Type 1 diabetes mellitus (T1DM). At the same time, such environmental factors as foods and infectious agents are thought to play a role in the genesis of prediabetic autoimmunity or in the progression from persistent beta-cell autoimmunity to clinical diabetes (Yoon, 1990; See and Tilles, 1998). Immunity to one or more beta cell autoantigens, such as insulin, GAD65, or IA-2, may lead to destruction of beta cells and a loss of the capacity to produce insulin, ultimately resulting in clinical insulin-dependent diabetes mellitus. Postulated mechanisms by which infectious agents may trigger T1DM include:
-
direct cytolytic infection of beta cells, resulting in destruction of beta cells and loss of capacity to synthesize insulin;
-
a virus-induced immune response against infected beta cells, such as T-cell induced killing of virus-infected cells;
-
non-specific “innocent bystander” killing of beta cells through activation of non-specific immune mediators; and
-
induction of an autoimmune response to islet antigens by cross-reactivity with viral antigens (molecular mimicry) or disruption of normal immune tolerance mechanisms.
Several viruses have been proposed as infectious triggers of diabetes, but the enteroviruses (family Picornaviridae, genus Enterovirus) are the subject of the most intense scrutiny at present (Leinikki, 1998; Hyöty et al., 1998). Numerous studies have provided evidence for an association between enterovirus infection and prediabetic autoimmunity or clinical diabetes. Diabetes incidence has been epidemiologically linked to the incidence of enteroviral meningitis or enterovirus outbreaks (Karvonen et al., 1993). Serologic studies have shown that there is a correlation between enterovirus seroprevalence in patients with prediabetic autoimmunity or diabetes, compared to unaffected control individuals (Hiltunen et al., 1997; Helfand et al., 1995). Direct enterovirus detection in pancreas, blood, serum, or stool has suggested a temporal correlation between enterovirus infection and onset of diabetes (Yoon et al., 1979; Andreoletti et al., 1997; Clements et al., 1995).
Enteroviruses are among the most common of human viruses, infecting an estimated 50 million people annually in the United States and possibly a billion or more annually worldwide (Morens and Pallansch, 1995; Pallansch and Roos, 2001). Most infections are inapparent, but enteroviruses may cause a wide spectrum of acute disease, including mild upper respiratory illness (common cold), febrile rash (hand, foot, and mouth disease and herpangina), aseptic meningitis, pleurodynia, encephalitis, acute flaccid paralysis (paralytic poliomyelitis), and neonatal sepsis-like disease. Enterovirus infections result in 30,000 to 50,000 hospitalizations per year in the United States, with aseptic meningitis cases accounting for the vast majority of the hospitalizations (Pallansch and Roos, 2001). In addition to these acute illnesses, enteroviruses have also been associated with severe chronic diseases such as myocarditis (Martino et al., 1995; Kim et al., 2001), Type 1 diabetes mellitus (Leinikki, 1998; Rewers and Atkinson, 1995), and neuromuscular diseases (Dalakas, 1995). Enteroviruses are transmitted primarily by the fecal-oral route but respiratory transmission to close contacts may also be important. The incubation period between infection and onset of symptoms is usually 4–7 days. The intestinal mucosa or upper respiratory tract is the site of primary infection, with secondary spread to the central nervous system and other tissues. Viremia is usually short-lived, often waning before the onset of symptoms, except in very young children. Virus is excreted in the stool for up to 8 weeks (average 2–4 weeks) but maximal virus shedding occurs before the onset
of symptoms. The maximum virus titer in stool is approximately 104 infectious virus particles per gram.
Of the 89 recognized enterovirus serotypes, 64 are known to infect humans (Pallansch and Roos, 2001). In addition to the human enteroviruses, human pathogenic viruses are found in four other picornavirus genera: Rhinovirus (human rhinoviruses), Hepatovirus (human hepatitis A virus), Parechovirus (human parechoviruses 1 and 2, formerly echoviruses 22 and 23, respectively), and Kobuvirus (aichivirus, an agent of gastroenteritis). Most of the human enterovirus serotypes were discovered and described between 1947 and 1963 as a result of the application of cell culture and suckling mouse inoculation to the investigation of cases of infantile paralysis (paralytic poliomyelitis) and other central nervous system diseases (Committee on Enteroviruses, 1962; Panel for Picornaviruses, 1963). The human enteroviruses were originally classified on the basis of human disease (polioviruses), replication and pathogenesis in newborn mice (coxsackie A and B viruses), and growth in cell culture without causing disease in mice (echoviruses), but they have recently been reclassified, based largely on molecular properties, into four species, A through D (King et al., 2000). Sequences in various portions of the enterovirus coding-region correlate with species, but only capsid sequence correlates with serotype.
The neutralization test, long the gold standard for enterovirus typing, is generally reliable, but it is labor-intensive and time-consuming, and may fail to identify an isolate because of aggregation of virus particles or antigenic drift (the widely used standardized typing antisera were raised against prototype strains that were isolated 40 to 50 years ago [Lim and Benyesh-Melnick, 1960]). Antisera to all serotypes are not generally available and isolates that are not of a known human enterovirus serotype (new serotypes or serotypes that normally infect animals other than humans) would obviously also present difficulties in identification by antigenic means, as the neutralization method requires the use of serotype-specific reagents. In addition, neutralization requires virus isolation, which may require the use of multiple cell lines and adds to the time required to make an identification.
The application of PCR has improved the speed and accuracy of general enterovirus detection (Rotbart and Romero, 1995; Rotbart et al., 1997), and has found wide acceptance in the clinical diagnostic laboratory. Since the enterovirus serotype is rarely relevant to clinical case management, many clinical virology laboratories are bypassing virus isolation entirely, in favor of PCR detection of viral nucleic acid directly in clinical specimens such as cerebrospinal fluid, nasopharyngeal swabs, or tissue specimens (Rotbart and Romero, 1995). This approach uses genus-specific primers targeted to the 5′ non-translated region (see Figure 1-6), often coupled to probe-hybridization and detection of product in a microplate format (Rotbart and Romero, 1995). Specimens of choice for the direct detection of enteroviruses by RT-PCR are stool or rectal swab (stool is preferred because it contains a larger amount of fecal material and, hence, virus);
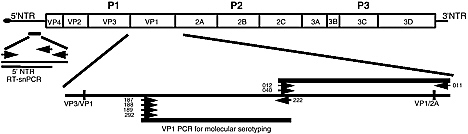
FIGURE 1-6 Schematic representation of the enterovirus genome, indicating regions that have been targeted for development of PCR diagnostics. The genome is a positive-stranded, polyadenylated RNA of ~7400 nucleotides, with a viral protein (3B/VPg) covalently linked to the 5′ end. The genome is divided into five functional regions: the 5′ non-translated region (NTR) (control of viral translation initiation and initiation of positive-strand RNA synthesis); P1 (encodes the structural proteins that comprise the virus capsid); P2 and P3 (encode the non-structural proteins involved in RNA replication, proteolytic processing of polyprotein, and host cell shut-down); and 3′ NTR (involved in initiation of negative-strand RNA synthesis).
oro- or nasopharyngeal specimens (throat swab, nasopharyngeal swab or aspirate, saliva); cerebrospinal fluid (if there is concomitant CNS disease); fresh-frozen or formalin-fixed tissue; and serum/plasma. Serum and plasma are generally only useful for RT-PCR in infants because viremia may still be present after onset of symptoms. If virus is detected only in a non-sterile site, such as stool or nasopharynx, a large number of patients are needed to establish the association between infection and disease.
Despite the advantages of enterovirus detection by RT-PCR, challenges remain. In the case of chronic diseases, the virus may act indirectly (e.g., through immune-mediated pathology). The virus may be cleared well before disease onset or virus may be present in the patient but not in the diseased tissue. Even in acute illnesses, the titer is relatively low in all specimens. As a result, a conventional single-step RT-PCR amplification may not be sensitive enough for direct detection from the original clinical specimen. Designing a prospective study and collecting multiple specimens, at multiple time points throughout the duration of the study, may overcome some of these problems; however, the only way to solve the sensitivity problem is by increasing the sensitivity of the detection method. To address this issue, we have developed an enterovirus-specific semi-nested RT-PCR assay (5′ NTR RT-snPCR) that targets the conserved regions of the 5′ NTR (see Figure 1-6). Figure 1-7 shows the sensitivity of our standard, conventional RT-PCR (Yang et al., 1992) compared with that of the 5′ NTR RT-snPCR. Ten-fold serial dilutions of a virus isolate (10–1 to 10–10) were prepared with uninfected cell extract as diluent. RNA was extracted using the QIAamp viral RNA mini-kit (Qiagen Inc., Valencia, CA) and reverse-transcribed using the
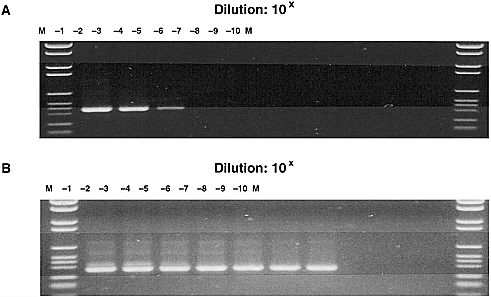
FIGURE 1-7 Sensitivity of pan-enterovirus RT-PCR methods. M-molecular weight marker. Virus dilutions are shown at the top of each panel. A. Titration of conventional two-primer RT-PCR. B. Titration of RT-semi-nested (three-primer) PCR.
antisense primer. PCR was performed using a single round of amplification (conventional PCR) or two rounds of amplification (semi-nested PCR). The second round of the semi-nested amplification used the same primers as the conventional PCR. Amplification products were visualized by polyacrylamide gel electrophoresis and staining with ethidium bromide. The RT-snPCR method (see Figure 1-7B) was approximately 10,000-fold more sensitive than the conventional RT-PCR (see Figure 1-7A). The 10–7 dilution corresponds to less than 20 infectious virus particles.
Enterovirus infection elicits a serotype-specific immune response directed against epitopes on the surface of the viral capsid. Mucosal immunity is most important. Antibody alone fully protects from disease, probably by limiting virus spread from the gut, but antibody does not necessarily protect from infection. The virus-specific T-cell response, directed against epitopes on both the structural and non-structural proteins, is probably involved in virus clearance but it is not needed for protection. Antigenic sites are located in each of the three enterovirus structural proteins, VP1, VP2, and VP3 (Minor, 1990; Mateu, 1995), but the epitopes responsible for serotype specificity have not been identified. Since the picornavirus VP1 protein contains a number of immunodominant neutralization domains, we hypothesized that VP1 sequence should correspond with neutralization properties (serotype) (Oberste et al., 1999b). Due to the high frequency of recombination among picornaviruses (Kopecka et al., 1995; King, 1988; Santti et al., 1999),
sequence information from non-capsid regions is of little value in characterizing new serotypes within known genera.
Practical criteria must be established before molecular sequence information can be applied routinely to picornavirus identification. A partial or complete VP1 nucleotide sequence identity of at least 75 percent (minimum 85 percent amino acid sequence identity) between a clinical enterovirus isolate and serotype prototype strain may be used to establish the serotype of the isolate (Oberste et al., 1999a,b, 2000). These criteria also appear to apply to comparisons among isolates of foot-and-mouth-disease virus (family Picornaviridae, genus Aphthovirus) (Vosloo et al., 1992), but a study directly comparable to the enterovirus studies has not yet been performed. A best-match nucleotide sequence identity of between 70 percent and 75 percent or a second-highest score of greater than 70 percent may provide a tentative identification, pending confirmation by other means, such as neutralization with monospecific antisera (Oberste et al., 2000) or more extensive sequencing. A best-match nucleotide sequence identity below 70 percent (less than 85 percent amino acid sequence identity) may indicate that the isolate represents an unknown serotype (Oberste et al., 2000, 2001). Sequencing of the complete capsid-coding region may be useful in confirming this result, but complete capsid sequences are available for less than half of the known enterovirus serotypes, limiting the utility of complete capsid sequence comparisons until more sequence becomes available. More extensive characterization, possibly including complete genome sequences, may be required for viruses that appear to represent previously unknown genera (Hyypiä et al., 1992; Marvil et al., 1999; Niklasson et al., 1999; Yamashita et al., 1998).
Recognizing the technical difficulties and limitations inherent in the classic approach to enterovirus identification, we developed RT-PCR and sequencing primers that target the VP1 capsid gene and may be used to determine enterovirus serotype by sequencing of the amplicon and comparison to a database of the VP1 sequences of all enterovirus serotypes (Oberste et al., 1999a,b, 2000). These molecular detection and typing methods, when coupled with well-designed prospective studies, will be useful in addressing the potential causal relationship between enterovirus infection and development of prediabetic autoimmunity or progression from persistent autoimmunity to clinical diabetes.
REFERENCES
Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyghem MC, Lefebvre J, Wattre P. 1997. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. Journal of Medical Virology 52:121–127.
Clements GB, Galbraith DN, Taylor KW. 1995. Coxsackie B virus infection and onset of childhood diabetes. Lancet 346:221–223.
Committee on Enteroviruses. 1962. Classification of human enteroviruses. Virology 16:501–504.
Dalakas MC. 1995. Enteroviruses and human neuromuscular diseases. Pp. 387–398 in Human Enterovirus Infections, HA Rotbart, ed. Washington, DC: ASM Press.
Helfand RF, Gary HE Jr, Freeman CY, Anderson LJ, Pallansch MA. 1995. Serologic evidence of an association between enteroviruses and the onset of type 1 diabetes mellitus. Pittsburgh Diabetes Research Group. The Journal of Infectious Diseases 172:1206–1211.
Hiltunen M, Hyoty H, Knip M, Ilonen J, Reijonen H, Vahasalo P, Roivainen M, Lonnrot M, Leinikki P, Hovi T, Akerblom HK. 1997. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. Childhood Diabetes in Finland (DiMe) Study Group. The Journal of Infectious Diseases 175:554–560.
Hyöty H, Hiltunen M, Lonnrot M. 1998. Enterovirus infections and insulin dependent diabetes mellitus—evidence for causality. Clinical and Diagnostic Virology 9:77–84.
Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. 1992. A distinct picornavirus group identified by sequence analysis. Proceedings of the National Academy of Sciences 89:8847–8851.
Karvonen M, Tuomilehto J, Libman I, LaPorte R. 1993. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organisation DIAMOND Project Group. Diabetologia 36:883–892.
Kim KS, Hufnagel G, Chapman NM, Tracy S. 2001. The group B coxsackieviruses and myocarditis. Reviews in Medical Virology 11:355–368.
King AMQ. 1988. Genetic recombination in positive strand RNA viruses. Pp. 149–165 in RNA Genetics, E Domingo, JJ Holland, and P Ahlquist, eds. Boca Raton, FL: CRC Press, Inc.
King AMQ et al. 2000. Picornaviridae. Pp. 657–678 in Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses, MH Van Regenmortel et al., eds. San Diego: Academic Press.
Kopecka H, Brown B, Pallansch M. 1995. Genotypic variation in coxsackievirus B5 isolates from three different outbreaks in the United States. Virus Research 38:125–136.
Leinikki P. 1998. Viruses and type 1 diabetes: elusive problems and elusive answers. Clinical and Diagnostic Virology 9:65–66.
Lim KA and Benyesh-Melnick M. 1960. Typing of viruses by combinations of antiserum pools. Application to typing of enteroviruses (coxsackie and ECHO). Journal of Immunology 84:309–317.
Martino TA et al. 1995. Enteroviral myocarditis and cardiomyopathy: a review of clinical and experimental studies. Pp. 291–351 in Human Enterovirus Infections, HA Rotbart, ed. Washington, DC: ASM Press.
Marvil P, Knowles NJ, Mockett AP, Britton P, Brown TD, Cavanagh D. 1999. Avian encephalomyelitis virus is a picornavirus and is most closely related to hepatitis A virus. Journal of General Virology 80:653–662.
Mateu MG. 1995. Antibody recognition of picornaviruses and escape from neutralization. Virus Research 38:1–24.
Minor PD. 1990. Antigenic structure of picornaviruses. Current Topics in Microbiology and Immunology 161:121–154.
Morens DM and Pallansch MA. 1995. Epidemiology. Pp. 3–23 in Human Enterovirus Infections, HA Rotbart, ed. Washington, DC: ASM Press.
Niklasson B, Kinnunen L, Hornfeldt B, Horling J, Benemar C, Hedlund KO, Matskova L, Hyypia T, Winberg G. 1999. A new picornavirus isolated from bank voles (Clethrionomys glareolus). Virology 255:86–93.
Oberste MS, Maher K, Kilpatrick DR, Flemister MR, Brown BA, Pallansch MA. 1999a. Typing of human enteroviruses by partial sequencing of VP1. Journal of Clinical Microbiology 37:1288–1293.
Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. 1999b. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. Journal of Virology 73:1941–1948.
Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA. 2000. Comparison of classic and molecular approaches for the identification of “untypable” enteroviruses. Journal of Clinical Microbiology 38:1170–1174.
Oberste MS, Schnurr D, Maher K, al-Busaidy S, Pallansch M. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. Journal of General Virology 82:409–416.
Pallansch MA and Roos RP. 2001. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. Pp. 723–775 in Fields Virology, DM Knipe and PM Howley, eds. Philadelphia: Lippincott Williams and Wilkins.
Panel for Picornaviruses. 1963. Picornaviruses: classification of nine new types. Science 141:153–154.
Rewers M and Atkinson M. 1995. The possible role of enteroviruses in diabetes mellitus. Pp. 353–385 in Human Enterovirus Infections, HA Rotbart, ed. Washington, DC:ASM Press.
Rotbart HA and Romero JR. 1995. Laboratory diagnosis of enteroviral infections. Pp. 401–418 in Human Enterovirus Infections, HA Rotbart, ed. Washington, DC: ASM Press.
Rotbart HA, Ahmed A, Hickey S, Dagan R, McCracken GH Jr, Whitley RJ, Modlin JF, Cascino M, O’Connell JF, Menegus MA, Blum D. 1997. Diagnosis of enterovirus infection by polymerase chain reaction of multiple specimen types. The Pediatric Infectious Disease Journal 16:409–411.
Santti J, Hyypia T, Kinnunen L, Salminen M. 1999. Evidence of recombination among enteroviruses. Journal of Virology 73:8741–8749.
See DM and Tilles JG. 1998. The pathogenesis of viral-induced diabetes. Clinical and Diagnostic Virology 9:85–88.
Vosloo W, Knowles NJ, Thomson GR. 1992. Genetic relationships between southern African SAT-2 isolates of foot-and-mouth-disease virus. Epidemiology and Infection 109:547–558.
Yamashita T, Sakae K, Tsuzuki H, Suzuki Y, Ishikawa N, Takeda N, Miyamura T, Yamazaki S. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. Journal of Virology 72:8408–8412.
Yang CF, De L, Yang SJ, Ruiz Gomez J, Cruz JR, Holloway BP, Pallansch MA, Kew OM. 1992. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Research 24:277–296.
Yoon JW. 1990. The role of viruses and environmental factors in the induction of diabetes. Current Topics in Microbiology and Immunology 164:95–123.
Yoon JW, Austin M, Onodera T, Notkins AL. 1979. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. New England Journal of Medicine 300:1173–1179.
INFECTIOUS AGENTS AND SCHIZOPHRENIA*
Robert H. Yolken
Johns Hopkins School of Medicine, Baltimore, MD
E. Fuller Torrey
Stanley Medical Research Institute, Bethesda, MD
Schizophrenia is a pervasive neuropsychiatric disorder of worldwide importance. This disease and related serious psychiatric diseases exact an enormous cost in terms of medical resources, lost productivity, and social ills such as crime and homelessness (see Box 1-2). Despite more than 100 years of extensive research, the causes of schizophrenia remain obscure. Much of the recent research
|
Box 1-2 Positive symptoms:
Negative symptoms:
Impairment in cognitive and social functioning Structural and functional brain abnormalities Lifetime prevalence = ~1 percent Peak onset of symptoms in young adulthood Significant societal consequences worldwide Characteristics of available medicines:
|
in schizophrenia has focused on possible genetic etiologies. The rationale for this approach is based on numerous studies indicating a strong risk associated with having a biological parent with this disease. Extensive genetic analyses of families with schizophrenia have led to the identification of a number of broad genomic regions which appear to be inherited in a non-random fashion by individuals with schizophrenia. However, despite intensive searches, no genes of major, or even minor effect, have been consistently linked to the schizophrenia phenotype (see Box 1-3).
Due to the limited success of genetic investigations, there has been renewed interest in the role of environmental factors in the etiopathogenesis of schizophrenia. This approach derives its rationale from a number of epidemiological studies which indicate that environmental factors may contribute to the risk of schizophrenia in some individuals. Many of these studies identify environmental events occurring during fetal development and early infancy as risk factors for the development of schizophrenia in adult life. Risk factors which have been identified
|
Box 1-3 Increased incidence in biological first-degree relatives:
Most individuals with schizophrenia lack a first degree relative with the disease. Genetic factors have a large relative risk but a small risk (5 percent) in the overall population. Intensive search for genes using molecular methods:
|
include infections, nutritional deprivation, and animal exposures in pregnancy (Yolken and Torrey, 1995; Torrey et al., 2000). Additional studies have documented an association between risk of schizophrenia with place and season of birth (Torrey et al., 1997; Torrey and Yolken, 1998). While the relative risks associated with these factors are relatively modest, the common nature of these exposures indicates that they may have a large effect on a population basis (Mortensen et al., 1999).
Based on this background, we have devised the working hypothesis that most cases of schizophrenia are caused by infections and other environmental events occurring in genetically susceptible individuals (Torrey and Yolken, 2000). It is of note that infections relating to schizophrenia occurring in this context would not be expected to follow satisfy Koch’s postulates since they would not lead to disease in individuals who did not have the appropriate genetic susceptibility. It is also likely that different microbial agents could lead to a similar disease process in individuals who share common genetic predispositions. It will thus be necessary to move beyond Koch’s postulates in order to analyze the interaction between genetic and environmental factors as causative agents of schizophrenia and other serious psychiatric diseases.
We have applied a number of laboratory techniques to the examination of these hypotheses (Johnston-Wilson et al., 2001). These techniques have been used to analyze brain tissues obtained postmortem from individuals with psychiatric diseases and control conditions. These brains were obtained by the Stanley Foun-
dation from a consortium of neuropathologists located in several different regions of the United States and are available to researchers with an interest in the studies of these disorders.
One of the most informative techniques which we have applied to these samples has been that of differential display. This method has indicated that there are several differences in RNA transcription in the brains of individuals with schizophrenia as compared to unaffected controls (Yee and Yolken, 1997). Sequence analysis of differentially expressed transcripts has indicated that many have a high degree of homology with a range of endogenous retroviruses. These elements are components of the human genome which arose from retrotransposition of infectious retroviruses in our evolutionary past. Endogenous retroviruses are integrated into the human genome. Upon activation, they can modulate the transcription of genes located upstream or downstream from the site of chromosomal integration (see Figure 1-8). Since they share properties of both genes and infectious agents, they are a potential link between genetic and environmental causes of human disease (Yolken et al., 2000).
Further studies were performed on cerebrospinal fluids (CSFs) obtained from living individuals with early symptoms of schizophrenia. Amplification of RNA extracted from these fluids indicated an increased rate of transcription of an endogenous retrovirus called HERV-W. This endogenous retrovirus was found in the CSFs of approximately 30 percent of individuals with recent-onset schizophrenia and 5 percent of individuals with chronic forms of the disease (see Figure 1-9). HERV-W transcription was not detected in the CSF of individuals without psychiatric disorders (Karlsson et al., 2001). HERV-W is of interest since its
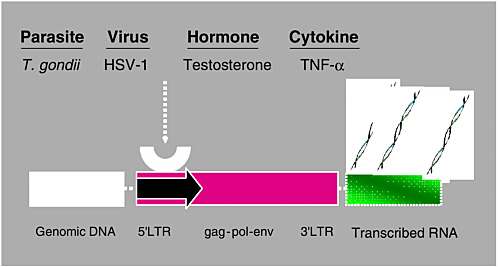
FIGURE 1-8 Integration and transcription of endogenous retroviruses.
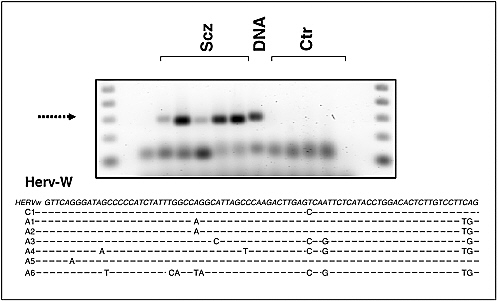
FIGURE 1-9 Endogenous retrovirus was found in the CSFs of approximately 30 percent of individuals with recent-onset schizophrenia and 5 percent of individuals with chronic forms of the disease.
SOURCE: Karlsson et al. (2001).
transcription has also been found to be increased in the CSFs of individuals with multiple sclerosis (Perron et al., 1997). It has also been demonstrated to be active during human fetal development and to encode a protein with syncytium forming activity in the human placenta (Mi et al., 2000). Furthermore, the envelope protein of HERV-W is capable of causing polyclonal T-lymphocyte activation (Perron et al., 2001). HERV-W may thus also provide a link between environmental events active both during fetal development and adult life.
The transcription of endogenous retroviruses can be activated by a number of infectious agents and other environmental factors. We have examined the prevalence of potential activating infections in different stages of schizophrenia. We have found an increased level of antibodies to Toxoplasma gondii in individuals with the recent onset of schizophrenia (see Figure 1-10) (Yolken et al., 2001). This finding is consistent with epidemiological studies documenting an increased rate in schizophrenia in individuals who were exposed to cats in early life (Torrey et al., 2000). We have also found that serological evidence of infection with Herpes Simplex Virus Type 1 and Toxoplasma gondii are associated with increased levels of cognitive and memory impairments in individuals with established forms of schizophrenia (Dickerson et al., 2003b). We also examined the possible association between infections in pregnancy in the occurrence of schizophrenia in
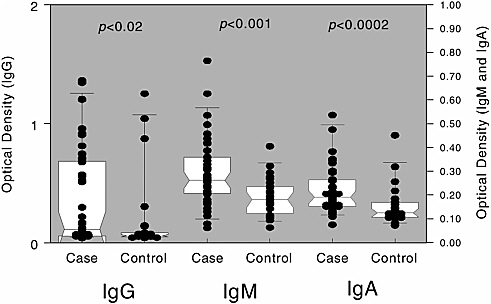
FIGURE 1-10 An increased level of antibodies to Toxoplasma gondii is found in individuals with recent onset of schizophrenia.
SOURCE: Yolken et al. (2001).
later life. These analyses were accomplished by the testing of sera which had been obtained from healthy pregnant women as part of the National Collaborative Perinatal Study performed in the United States during the 1950s and 1960s. Initial analyses of this cohort indicates that the offspring of mothers who had evidence of infection, as indicated by increased levels of IgG, IgM, and of IgG antibodies to Herpes Simplex Virus type 2, have higher rates of schizophrenia in adult life (see Figure 1-11) (Buka et al., 2001). There was also a risk of schizophrenia associated with IgM antibodies to Toxoplasma gondii, although the antigenic source of these antibodies is still under investigation.
These studies indicate that infectious agents play a role in the generation of schizophrenia in some individuals. The activation of endogenous retroviruses within the central nervous system is likely to be one of several mechanisms by means of which infections can lead to disease. If this is the case, it is possible that the treatment of infectious agents which activate retroviral transcription may be capable of modulating the course of disease at different times in the lifelong course of disease. For example, the treatment of active infection with herpes simplex virus might prevent endogenous retrovirus activation due to this organism. It is of note in this regard that several of the medications which are commonly used for the treatment of schizophrenia also have the ability to inhibit the replication of infectious agents (Jones-Brando et al., 1997). Preliminary analysis of a clinical
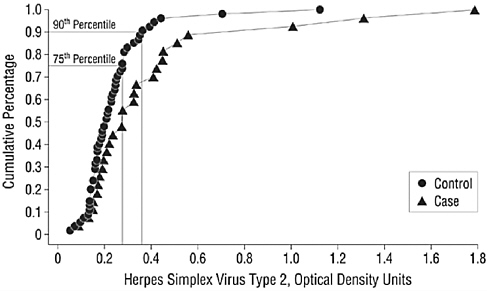
FIGURE 1-11 Association between HSV infections in pregnant women and the occurrence of schizophrenia in their adult offspring. The adult offspring of mothers whose sera showed evidence of HSV infection during pregnancy have higher rates of schizophrenia than the adult offspring of mothers whose sera did not show such evidence during pregnancy.
SOURCE: Reprinted from Buka et al. (2001).
trial of the anti-herpesvirus medication valacyclovir indicates that it is effective in reducing the symptoms of some individuals with schizophrenia (Dickerson et al., 2003a). Ongoing studies are directed at the further evaluation of the role of anti-viral and anti-parasitic agents in the treatment of schizophrenia. The definitive establishment of the role of infectious agents in the etiopathogenesis of schizophrenia may lead to new methods for the diagnosis, prevention, and treatment of this devastating disease.
REFERENCES
Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. 2001. Maternal infections and subsequent psychosis among offspring. Archives of General Psychiatry 58:1032–1037.
Dickerson F, Boronow JJ, Stallings C, Origoni A, Yolken R. 2003a. Valacyclovir reduces symptoms in individuals with schizophrenia who are seropositive for cytomegalovirus. Paper presented at the International Congress on Schizophrenia Research, Colorado Springs, March 2003.
Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. 2003b. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Archives of General Psychiatry 60:466–472.
Johnston-Wilson NL, Bouton CM, Pevsner J, Breen JJ, Torrey EF, Yolken RH. 2001. Emerging technologies for large-scale screening of human tissues and fluids in the study of severe psychiatric disease. The International Journal of Neuropsychopharmacology 4:83–92.
Jones-Brando LV, Buthod JL, Holland LE, Yolken RH, Torrey EF. 1997. Metabolites of the antipsycotic agent clozapine inhibit the replication of human immunodeficiency virus type 1. Schizophrenia Research 25:63–70.
Karlsson H, Bachmann S, Schroder J, McArthur J, Torrey EF, Yolken RH. 2001. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proceedings of the National Academy of Sciences 98:4634–4639.
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC Jr, McCoy JM. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789.
Mortensen PB, Pederson CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M. 1999. Effects of family history and place and season of birth on the risk of schizophrenia. New England Journal of Medicine 340:603–608.
Perron H, Garson JA, Beden F, Beseme F, Paranhos-Baccala G, Komurian-Pradel F, Mallet F, Tuke PW, Voisset C, Blond JL, Lalande B, Seigneurin JM, Mandrand B. 1997. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proceedings of the National Academy of Sciences 94:7583–7588.
Perron H, Jouvin-Marche E, Michael M, Quanonian-Paroz A, Camelo S, Dumon A, Jolivet-Reynaud C, Marcel F, Souillet Y, Barel E, Gebeihrer L, Santoro L, Marcel S, Seigreurin JM, Marche PN, Lafon M. 2001. Multiple sclerosis retrovirus particles and recombinant envelope trigger on abnormal immune response in vitro, by inducing polyclonal Vbetal 6 T-lymphocyte activation. Virology 287:321–332.
Torrey EF and Yolken RH. 1998. At issue: is household crowding a factor for schizophrenia and bipolar disorder. Schizophrenia Bulletin 24:321–324.
Torrey EF and Yolken RH. 2000. Familial and genetic mechanisms in schizophrenia. Brain Research Reviews 31:113–117.
Torrey EF, Miller J, Rawlings R, Yolken RH. 1997. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophrenia Research 28:1–38.
Torrey EF, Rawlings R, Yolken RH. 2000. The antecedents of psychoses: a case-control study of selected risk factors. Schizophrenia Research 46:17–23.
Yee F and Yolken RH. 1997. Identification of differentially expressed RNA transcripts in neuropsychiatric disorders. Biological Psychiatry 41:759–761.
Yolken RH and Torrey EF. 1995. Viruses, schizophrenia and bipolar disorder. Clinical Microbiology Reviews 8:131–145.
Yolken RH, Karlsson H, Yee F, Johnston-Wilson NL, Torrey EF. 2000. Endogenous retroviruses and schizophrenia. Brain Research Reviews 31:193–199.
Yolken RH, Bachmann S, Rouslanova I, Lillehoj E, Ford G, Torrey EF, Schroeder J. 2001. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clinical Infectious Diseases 32:842–844.
OVINE PULMONARY ADENOCARCINOMA: IDENTIFYING THE CAUSATIVE AGENT FOR A NEOPLASTIC DISEASE AND IMPLICATIONS FOR HUMAN LUNG CANCER
Hung Fan, Ph.D.
Department of Molecular Biology and Biochemistry
Cancer Research Institute, University of California, Irvine, CA
Cancer is a collection of diseases that result from uncontrolled cell growth. Progression from a normal cell to a fully malignant one is a multi-step process,
and numerous factors can contribute. Over the years, it has been shown that environmental factors (e.g., radiation and heavy metals) and lifestyle habits (e.g., tobacco smoking) can increase the rates of particular cancers. In some cases, infectious agents have been identified as causative to certain cancers. In animal model systems, several classes of viruses have been shown to cause cancers, including retroviruses (e.g., avian sarcoma/leukemia viruses and murine leukemia viruses) and small DNA viruses (e.g., polyoma and SV40). Viruses associated with human cancers include retroviruses (human T-cell leukemia virus—adult T-cell leukemia), human papillomavirus (cervical cancer), hepatitis virus types B and C (hepatocellular carcinoma) and gamma herpes viruses (EBV—lymphomas and nasopharyngeal carcinoma; HHV8—Kaposi’s sarcoma). In addition, the bacterium Helicobacter pylori has been associated with stomach cancer. Proving the involvement of viruses and bacteria in human cancers has typically taken many years. Steps involved include appreciating an epidemiological pattern of the particular cancer suggesting an infectious agent, identification of an infectious agent whose distribution fits the epidemiological pattern, and ultimately demonstrating in an animal model or in vitro culture system that the putative infectious agent is carcinogenic.
Lung cancer is one of the most common human neoplasms. While a substantial portion of lung cancer can be attributed to tobacco smoking, other factors may also contribute to development of disease. Moreover, in some cases tobacco smoking is not involved in causation of the tumor. Human adenocarcinoma of the lung represents neoplasms of secretory epithelia cells. In the distal airways (alveoli and bronchioles), the targets of transformation are Type II pneumocytes and Clara cells. Bronchiolo alveolar carcinoma (BAC) is a sub-classification of lung adenocarcinoma in which the tumor cells line the alveoli or bronchioles and spread in a sideways (lepidic) fashion (Mornex et al., 2003). BAC does not appear to be tightly associated with tobacco smoking, and the incidence of this cancer may be rising. Thus the possibility that an infectious agent may be involved in BAC or human lung adenocarcinoma in general has been suggested by a number of investigators (Jackson et al., 2000; Koyi et al., 2001; Laurila et al., 1997).
A very interesting animal model for human lung adenocarcinoma exists: ovine pulmonary adenocarcinoma (OPA), a contagious lung cancer of sheep (Fan, 2003). The disease was first described in the late 1800s in South Africa, and was named jaagsiekte—“driving sickness” in Afrikaans (York and Querat, 2003). The disease is prevalent worldwide, and is particularly well-documented in Europe and Africa. It is estimated that the lifetime risk of developing OPA in high incidence flocks is approximately 25 percent (Sharp and DeMartini, 2003). The spread of OPA may result from inhalation of aerosols. A noteworthy feature of the disease is production of excess surfactant by the tumor cells—the normal function of Type II pneumocytes and Clara cells is to produce lung surfactant and other molecules important for lung physiology. As a result, animals with end-stage OPA exhibit respiratory distress; “tipping” of OPA animals results in lung
fluid (excess surfactant) draining from the nose. In the mid-1970s, researchers in the United Kingdom showed that filtered OPA lung fluid could transfer the disease to unaffected animals, indicating a viral etiology of OPA (York and Querat, 2003). In the 1960s and 1970s, further evidence was obtained that supported this notion. In particular, OPA lung fluid was shown to contain reverse transcriptase activity (characteristic of retroviruses), and OPA lung fluid was shown to contain antigens that cross-react with two retroviruses—Mason Pfizer monkey virus (MPMV) and murine mammary tumor virus (MMTV) (Sharp and Herring, 1983). MPMV and MMTV are relatively closely related viruses, belonging to the betaretrovirus class. However, the experiments were complicated by the presence of another retrovirus, an ovine lentivirus, that was also present in many of the animals with OPA. This confounded experiments to isolate and purify the causative agent of OPA. Moreover, attempts to propagate the OPA-inducing virus from lung fluid in tissue culture were unsuccessful. A major advance was made in 1990, when York et al. deduced the presence and sequence of a novel retrovirus in OPA lung fluid (York et al., 1991, 1992). This was accomplished by first developing a technique that partially removed the contaminating ovine lentivirus from the OPA lung fluid (treatment with a fluorocarbon). The treated lung fluid was then banded to equilibrium in a sucrose density gradient, and RNA was extracted from the peak of reverse transcriptase activity. This RNA was then reverse transcribed in vitro using purified reverse transcriptase and an oligodT primer, and a series of overlapping partial cDNA clones was obtained. Sequencing of the cDNA clones and overlapping the resulting sequences revealed a novel complete retroviral sequence; this retrovirus was designated jaagsiekte sheep retrovirus or JSRV. Consistent with the previous serology, sequence homology analyses indicated that JSRV is also a beta retrovirus, with sequence similarities to both MPMV and MMTV. A diagram of the JSRV sequence is shown in Figure 1-12. Disappointingly, attempts to isolate a replication-competent retrovirus from assembled cDNA clones were unsuccessful.
The availability of the JSRV sequence allowed generation of important molecular reagents for detection of the putative virus. Initial Southern blot experiments indicated that, as for many other retroviruses, endogenous JSRV-related proviruses are present in the germ line of all sheep and goats (DeMartini et al., 2003; Hecht et al., 1996; York et al., 1991). There are 15–20 endogenous JSRV-related proviruses in most sheep. On an evolutionary scale, these endogenous viruses entered the sheep germ line relatively recently—1–5 million years ago (Palmarini et al., 2000a). The existence of endogenous proviruses complicated experiments, in that it was necessary to distinguish the endogenous proviruses from the exogenous JSRV present in lung fluid. PCR-based assays were developed that allowed distinguishing exogenous from endogenous JSRV’s (Palmarini et al., 1996). This allowed demonstration that tumor samples from OPA animals consistently contain exogenous JSRV DNA above and beyond the endogenous JSRV-related sequences. The JSRV cDNA clones were also used for production
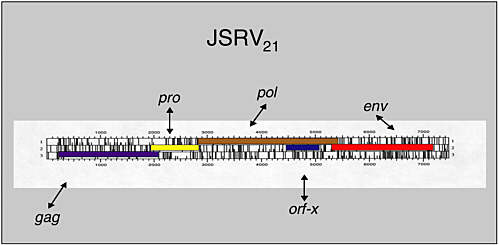
FIGURE 1-12 Genetic organization of the genome of jaagsiekte sheep retrovirus. NOTE: The acronyms and abbreviations in the diagram stand for genes that code for the following: gag = viral packaging protein; pro = promoter; pol = polymerase; orf = open reading frame; env = viral envelope protein.
of bacterial expression plasmids, for development of anti-JSRV antisera. A rabbit polyclonal antibody to JSRV capsid (CA) has been particularly useful. Immunohistochemistry with this antiserum indicated that in OPA animals, the tumor cells consistently show the presence of JSRV CA antigens, while normal cells of the lung do not. In addition, lung cells from uninfected animals do not show JSRV CA protein. These results further strengthened the likelihood that JSRV is the causative agent of OPA.
A major goal was isolation of an infectious and oncogenic molecular clone of JSRV. We undertook such experiments (Palmarini et al., 1999), taking advantage of the prior work. Most notably, the availability of the complete JSRV sequence, and the PCR-based diagnosis for exogenous JSRV were important. A lambda phage genomic library was prepared from a naturally occurring OPA tumor from the United Kingdom. The library was screened by a combination of sib-selection and PCR diagnosis for exogenous JSRV recombinants, followed by standard filter hybridizations with JSRV-specific probes. One lambda phage recombinant was obtained that contained a complete JSRV provirus integrated into adjacent cellular sequences, JSRV21. Sequencing of this clone revealed a genome with very high (~95 percent) homology to the previously deduced South African JSRV sequence. All of the open reading frames in the initial sequence deduced by York et al. were present, and there were no other additional frames. Thus the failure of previous attempts to obtain infectious virus from the assembled cDNA clones did not reflect the absence of sequences in the partial cDNA clones. To test whether the JSRV21 genome was infectious, we carried out in vivo DNA
transfections in newborn lambs. In vivo transfection of retroviral DNA was first demonstrated for bovine leukemia virus (Willems et al., 1993). In vivo transfection of JSRV DNA was accomplished by incorporating a plasmid form of JSRV21, pJS21, into liposomes containing a lipid that favors DNA transfer into lung epithelial cells. The pJS21 liposomes were injected intratracheally into newborn lambs, and PBMCs were tested for the presence of JSRV DNA at different times post-transfection. Nested PCR amplifications revealed the presence of exogenous JSRV DNA in PBMCs from the transfected animals at various times up to nine months, when the animals were sacrificed. These results indicated that the JSRV21 was an infectious provirus. However, at necropsy (9 months), no tumors were observed, so this experiment did not indicate if JSRV21 was an oncogenic clone.
The failure to observe tumors in the pJS21-transfected animals might have been due to the relative inefficiency of in vivo DNA transfection. Therefore, a method for generating genuine JSRV virus from the pJS21 clone was developed. Ultimately, we were able to prepare significant amounts of JSRV virus from a version of the pJS21 plasmid in which the human cytomegalovirus immediate early promoter drives expression of the JSRV sequences. Transient transfection of pJS21 into human 293T cells resulted in the production of JSRV particles. When concentrated JSRV stocks prepared in this way were inoculated intratracheally into four newborn lambs, two lambs developed classic OPA within four months. The resulting tumors were positive for viral CA antigen and DNA (Palmarini et al., 1999). This proved that JSRV is the causative agent of OPA. The availability of an infectious and oncogenic molecular clone of JSRV has opened up several avenues of research that are being pursued.
Two features of JSRV molecular biology are particularly noteworthy. First, JSRV is unusual among retroviruses in that its expression is highly restricted in vivo. In infected animals, JSRV DNA sequences can be detected in various cells, including different lineages of hematopoietic cells in the PBMCs (Holland et al., 1999). The level infection is low—detection requires a nested PCR—and this infection is apparently not productive, since CA antigen-positive cells cannot be detected in PBMCs. Even in lungs of animals with end-stage OPA, CA antigen is only detected in the tumor cells. In particular, other lung cells (even normal lung epithelial cells) do not typically express the CA antigen (Sharp and DeMartini, 2003). Thus, lung epithelial cells may be the only cell types in which JSRV infection is productive. The basis for the expression specificity is the enhancer sequences in the JSRV long terminal repeat (LTR). Retroviral LTRs containing enhancer sequences in the U3 region that are responsible for driving transcription of the provirus. We showed that the JSRV LTR is quite specific for lung epithelial cells in transient transfection assays using a JSRV LTR-driven luciferase reporter gene in mouse cell lines of different differentiation lineages (Palmarini et al., 2000b). Deletional analysis indicated that the JSRV enhancers function in lung epithelial-derived cell lines, while they are inactive in most other cell types.
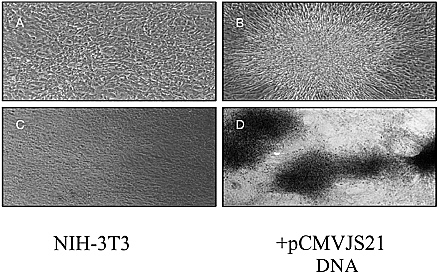
FIGURE 1-13 Foci of transformation induced by the transfection of clone JSRV DNA into mouse NIH 3T3 cells. Such transfection is a standard assay for detecting viral and cellular oncogenes. Panels (a) and (c) show untransfected NIH3T3 cells. Panel (b) shows a focus of transformed cells resulting from transfection with CMV-driven plasmid DNA (pCMV2JS21). Panel (d) shows a pCMV2JS21 transfected culture that had been passaged several times prior to plating under focus-forming conditions.
Detailed analysis of the factors responsible for the lung epithelial-specific expression is in progress.
The second interesting feature of JSRV biology is that the viral genome appears to contain a transforming gene. In fact, JSRV is an extremely potent carcinogen in the laboratory setting. Experimentally inoculated newborn animals develop end-stage OPA with a mean time of six weeks, and in some cases tumors have been observed as early as 10 to 14 days. The rapid oncogenesis, coupled with the multi-focal pattern of the tumors is consistent with a direct transforming function (oncogene) in the virus. We showed that transfection of clone JSRV DNA into mouse NIH 3T3 cells could induce foci of transformation, a standard assay for detection of viral and cellular oncogenes (see Figure 1-13) (Maeda et al., 2001). Further studies indicated that the envelope gene of JSRV is responsible for the transformation. Transformation appears to occur through the cytoplasmic tail of the envelope transmembrane (TM) protein (Palmarini et al., 2001a). The cytoplasmic tail contains a docking site for PI 3 kinase, an important cellular kinase involved in signal transduction and oncogenic transformation. Mutation of the critical tyrosine for methionine residues in the PI3K docking site led to loss of transformation. It is noteworthy that all exogenous JSRV envelopes sequenced so far contain the PI3K binding domain, while endogenous JSRV-related envelope genes do not.
The finding that the JSRV envelope gene contains oncogenic potential is unusual for replication-competent retroviruses. Most other replication-competent retroviruses do not normally cause tumors by a direct mechanism (i.e., oncogenes). More typically, oncogenesis is a byproduct of the replication cycle (e.g., insertional activation of cellular proto-oncogenes). However, the fact that all exogenous JSRVs have a transforming envelope suggests that this property is important for replication of the virus. We have proposed a hypothesis to explain this. In studies of the endogenous JSRV-related proviruses, we found that the endogenous JSRV LTRs do not show transcriptional specificity for lung epithelial cells (Palmarini et al., 2000a). The endogenous viruses provide a view into the primordial progenitor of JSRV, since they reflect the JSRV progenitor from 1 million to 5 million years ago. (Mutation rates of retroviral DNAs decrease markedly when they are transmitted in the proviral [DNA] form.) Thus the progenitor to exogenous JSRV likely replicated through different cells in the animals than lung epithelial cells. Indeed, the endogenous JSRV proviruses in current day sheep are not expressed in lung epithelial cells, but they are expressed in cells of the female reproductive tract (Palmarini et al., 2001b). During evolution of exogenous JSRV, presumably alterations in the enhancer sequences in the LTR arose that conferred transcriptional specificity for lung epithelial cells. However, in the normal adult lung there is relatively little division and growth of Type II pneumocytes and close cells. Most retroviruses require cell division for efficient infection and production. Thus during evolution of exogenous JSRV, the mutation in the cytoplasmic tail of the envelope TM protein would allow for more efficient infection and expression in lung epithelial cells, to which JSRV is transcriptionally restricted.
As mentioned above, JSRV-induced OPA is histologically very similar to human adenocarcinoma and BAC. In light of the lack of association of human BAC with tobacco smoking and its increasing incidence, the possibility of a viral involvement in human lung adenocarcinoma has also been raised. Several investigators have specifically explored whether a human virus related to JSRV might be associated with human lung cancer. In particular, De las Heras and colleagues recently reported a study in which they screened a series of human lung cancers and other tumors for immunological staining with a polyclonal antibody to JSRV CA protein (De las Heras et al., 2000). They found that approximately 30 percent of human BACs and nearly 25 percent of human lung adenocarcinomas showed immunohistochemical staining with the JSRV CA antibody. In contrast, little or no reactivity was detected in squamous cell carcinomas of the lung and other tumors. Thus the reactivity appears to be rather specific for human lung adenocarcinomas and BACs. Another laboratory has been able to replicate these immunohistochemistry findings (J. DeMartini, personal communication). On the other hand, several investigators have attempted to clone a JSRV-related retrovirus from these human tumors by using PCR amplification with degenerative oligonucleotide primers. So far no one has succeeded.
The antigenic cross-reactivity to JSRV observed in some human lung adenocarcinomas might result from two possibilities. First, it could reflect infection with an exogenous human retrovirus with some relationship to JSRV. Alternatively, it could reflect expression of a human endogenous retrovirus (HERV) with antigenic cross-reactivity to JSRV. The human genome contains many copies of HERVs, divided into several classes. It has been shown that different HERVs are expressed in normal versus malignant tissues. It has been noted that the JSRV sequence has some similarity to the HERV-K class, so it is possible that these tumors are expressing a HERV-K (J. DeMartini, personal communication). A third possibility is that the antigenic cross-reactivity could reflect a human cellular protein unrelated to a retrovirus. Identification of the nucleic acid encoding the JSRV antigenic cross-reactivity in the human lung adenocarcinomas and BACs is a goal of primary importance, and several laboratories are actively pursuing this. Once the genetic material encoding the cross-reactivity is identified, if it corresponds to an exogenous or endogenous human retrovirus, the next important issue will be to ascertain whether it has a causal role in lung carcinogenesis. For such experiments, the JSRV OPA system and sheep will provide a valuable framework for designing experiments to address this question.
REFERENCES
De las Heras M, Barsky SH, Hasleton P, Wagner M, Larson E, Egan J, Ortin A, Gimenez-Mas JA, Palmarini M, Sharp JM. 2000. Evidence for a protein related immunologically to the jaagsiekte sheep retrovirus in some human lung tumors. The European Respiratory Journal 15:330–332.
DeMartini J, Carlson J, Leroux C, Spencer T, Palmarini M. 2003. Endogenous retroviruses related to jaagsiekte sheep retrovirus. Pp. 117–137 in Jaagsiekte Sheep Retrovirus and Lung Cancer, H Fan, ed. Berlin: Springer-Verlag.
Fan H, ed. 2003. Jaagsiekte Sheep Retrovirus and Lung Cancer, Vol. 275. Berlin: Springer-Verlag.
Hecht SJ, Stedman KE, Carlson JO, DeMartini JC. 1996. Distribution of endogenous type B and type D sheep retrovirus sequences in ungulates and other mammals. Proceedings of the National Academy of Sciences 93:3297–3302.
Holland MJ, Palmarini M, Garcia-Goti M, Gonzalez L, de las Heras M, McKendrick I, Sharp JM. 1999. Jaagsiekte retrovirus is widely distributed both in T and B lymphocytes and in mononuclear phagocytes of sheep with naturally and experimentally acquired pulmonary adenomatosis. Journal of Virology 73:4004–4008.
Jackson LA, Wang SP, Nazar-Stewart V, Grayston JT, Vaughan TL. 2000. Association of Chlamydia pneumoniae immunoglobulin A seropositivity and risk of lung cancer. Cancer Epidemiology Biomarkers and Prevention 9:1263–1266.
Koyi H, Branden E, Gnarpe J, Gnarpe H, Steen B. 2001. An association between chronic infection with Chlamydia pneumoniae and lung cancer. A prospective 2-year study. APMIS 109:572–580.
Laurila AL, Anttila T, Laara E, Bloigu A, Virtamo J, Albanes D, Leinonen M, Saikku P. 1997. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. International Journal of Cancer 74:31–34.
Maeda N, Palmarini M, Murgia C, Fan H. 2001. Direct transformation of rodent fibroblasts by jaasiekte sheep retrovirus DNA. Proceedings of the National Academy of Sciences 98:4449–4454.
Mornex JF, Thivolet F, de las Heras M, Leroux C. 2003. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Pp. 225–248. In Fan H, editor. Jaagsiekte Sheep Retrovirus and Lung Cancer. Berlin: Springer-Verlag.
Palmarini M, Cousens C, Dalziel RG, Bai J, Stedman K, DeMartini JC, Sharp JM. 1996. The exogenous form of Jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. Journal of Virology 70:1618–1623.
Palmarini M, Sharp JM, de las Heras M, Fan H. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. Journal of Virology 73:6964–6972.
Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. 2000a. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous jaagsiekte sheep retrovirus. Journal of Virology 74:8065–8076.
Palmarini M, Datta S, Omid R, Murgia C, Fan H. 2000b. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. Journal of Virology 74:5776–5787.
Palmarini M, Maeda N, Murgia C, De-Fraja C, Hofacre A, Fan H. 2001a. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. Journal of Virology 75:11002–11009.
Palmarini M, Gray CA, Carpenter K, Fan H, Bazer FW, Spencer TE. 2001b. Expression of endogenous betaretroviruses in the ovine uterus: effects of neonatal age, estrous cycle, pregnancy and progersterone. Journal of Virology 75:11319–11327.
Sharp J and DeMartini J. 2003. Natural history of JSRV in sheep. Pp. 55–79. In Fan H, editor. Jaagsiekte Sheep Retrovirus and Lung Cancer. Berlin: Springer-Verlag.
Sharp JM and Herring AJ. 1983. Sheep pulmonary adenomatosis: demonstration of a protein which cross-reacts with the major core proteins of Mason-Pfizer monkey virus and mouse mammary tumour virus. The Journal of General Virology 64:2323–2327.
Willems L, Kettmann R, Dequiedt F, Portetelle D, Voneche V, Cornil I, Kerkhofs P, Burny A, Mammerickx M. 1993. In vivo infection of sheep by bovine leukemia virus mutants. Journal of Virology 67:4078–4085.
York D and Querat G. 2003. A history of ovine pulmonary adenocarcinoma (Jaagsiekte) and experiments leading to the deduction of the JSRV nucleotide sequence. Pp. 1–23. In Fan H, editor. Jaagsiekte Sheep Retrovirus and Lung Cancer. Berlin: Springer-Verlag.
York DF, Vigne R, Verwoerd DW, Querat G. 1991. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. Journal of Virology 65:5061–5067.
York DF, Vigne R, Verwoerd DW, Querat G. 1992. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. Journal of Virology 66:4930–4939.
PROPIONIBACTERIUM ACNES AND CHRONIC DISEASES
Ajay Bhatia, Ph.D.; Jean-Francoise Maisonneuve, Ph.D.; and
David H. Persing, M.D., Ph.D.
Corixa Corporation, Seattle, WA
Propionibacterium acnes is a gram-positive human skin commensal that prefers anaerobic growth conditions and is involved in the pathogenesis of acne (Kirschbaum and Kligman, 1963). Acne is one of the most common skin diseases, affecting more than 45 million individuals in the United States. It is esti-
mated that nearly 20 percent of all visits to dermatologists are related to the treatment of acne. Acne often debuts during changes in hormonal levels in pre-teens; however, it is also very common as an adult-onset condition, often associated with hormonal fluctuation during the menstrual cycle and pregnancy. While not life-threatening, acne can persist for years and is known to have serious psychosocial effects such as decreased self-esteem, depression, frustration, and social withdrawal. In addition to dermatological pathology, P. acnes is also suspected to be discreetly involved in post-operative infections, prostheses failure, and more recently, in inflammation of lumbar nerve roots leading to sciatica.
P. acnes, previously known by the name Corynebacterium parvum, has been studied extensively by immunologists for its ability to stimulate the reticuloendothelial system (Adlam and Scott, 1973). Not too long ago, an important cytokine, interleukin (IL)-18 was cloned from the liver of mice primed with P. acnes followed by challenge with LPS (Okamura et al., 1995). In the early eighties, certain bacteria, including BCG and P. acnes, were commonly used to stimulate the innate immune response against cancer in mice and human cells (Cantrell and Wheat, 1979; Davies, 1982). One of the great ironies of this organism is that it is a powerful nonspecific immune stimulant that resides naturally in the skin; its role as an immunostimulant in humans is appreciated when cases of severe acne also develop adjuvant-type arthritis.
Some investigators have gone so far as to suggest that severe acne, by virtue of the nonspecific immunostimulatory effects of P. acnes, might have played a role in natural protection against life-threatening diseases such as malaria and plague. In contrast, the acquired immune response to P. acnes has received little attention in humans.
Pathogenesis of Acne
Chronic inflammatory acne cannot be defined as an infectious disease, since the bacteria are normally present on the skin of a vast majority of individuals, irrespective of the presence of acne lesions. P. acnes apparently only triggers the disease when it meets favorable dermatophysiological terrain; P. acnes colonization of the skin is therefore necessary but not sufficient for the establishment of the pathology. The 4 major recognized pathophysiological features of acne include androgen stimulated seborrhea, hyperkeratinization and obstruction of the follicular epithelium, proliferation of P. acnes, and then inflammation.
Comedogenesis, the transformation of the pilosebaceous follicle into the primary acne lesion, the comedone, is the product of abnormal follicular keratinization related to excessive sebum secretion. During this process, P. acnes often gets trapped in layers of corneocytes and sebum and rapidly colonizes the comedonal kernel, resulting in a microcomedone, a structure invisible to the naked eye (Plewig and Kligman, 2000). A microcomedone can develop into larger structures, called comedones. Comedones can be a closed structure (whitehead) that
appears like a colored bump on the skin or an open structure (blackhead). Unlike open comedones, closed comedones cannot evacuate the thread-looking conglomerate of cell debris, sebum, P. acnes and its products to the skin surface, and this makes them more prone to inflammation and rupture. In inflammatory acne, comedones rupture and the follicular material becomes dispersed in the dermis rather than on the skin surface. Depending on the extent of the damage to the comedone wall, various types of inflammatory lesions are produced and these are classified as papules, pustules, or nodules. Nodules are the most severe types of acne lesions and scarring may be associated with any form of severe inflammatory acne.
A break in the lining of the comedone was initially attributed to free fatty acids generated by P. acnes-mediated triglyceride hydrolysis, but for several reasons, it is now thought that substances produced by P. acnes are directly involved in the rupture the comedone epithelial lining (Holland et al., 1981). The bacteria secrete many polypeptides, among which are numerous extracellular enzymes such as proteases, hyaluronidases, neuraminidases, and others that could be involved in epithelium permeabilization and inflammatory infiltration (Noble, 1984). P. acnes is also known to produce chemotactic factors (Puhvel and Sakamoto, 1977), proinflammatory cytokine inducing-factors (Vowels et al., 1995), and to activate both the direct and indirect complement pathways (Webster et al., 1978). The infiltrate of an early inflamed lesion consists of polymorphonuclear cells that certainly contribute to the lining breakage, but eventually, as time goes by and infection becomes chronic, these cells attract and are replaced by mononuclear cells, predominantly T-cells of the CD4 phenotype (Norris and Cunliffe, 1988; Layton et al., 1994). As the inflammation propagates to the lining of adjacent sebaceous follicules, it can start a chain reaction that results in multiple lesions connected together and called a sinus. Studies by Hoffler et al. (1985) have revealed differences in the production of various enzymes by Propionibacterium isolates of acne lesions versus bacteria isolated from healthy controls. These studies are important for differentiating bacterial antigens that lead healthy controls to generate a protective immune response and those that might be involved in pathogenesis.
Antibody against P. acnes antigenic determinants are found in the blood of most adults, whether they have had acne or not (Ingham et al., 1987); amounts may vary between the two populations, and possibly the nature of the determinants the antibodies recognize (Holland et al., 1993). Recent investigations by our group suggest that differential recognition might involve surface molecules with physiological functions. P. acnes specific IgG and IgA are also found at the level of the follicular infudibulum (Knop et al., 1983); these antibodies might be of great importance in limiting or preventing P. acnes proliferation, and maybe more importantly, in preventing comedonal lining destruction by P. acnes-derived soluble factors. Our preliminary data suggests that a robust P. acnes specific T-cell response is also common in adult donors, but its specificity at the
antigen level is currently under investigation. We like to think that there possibly exists a P. acnes-specific protective immunity against acne. This hypothesis is supported by the fact that some people never get acne, as well as by the observation that acne is mostly a disease of young people, (although there are numerous exceptions), and that even in countries where people are unable to afford sophisticated medications, chronic disease of adolescents eventually resolves with age. Finally, there have been successful human trials of therapeutic vaccination against P. acnes, and although the rate of success has not been high, some individuals refractory to conventional approaches experienced remission (Goldman et al., 1979; Vymola et al., 1970).
Role of P. acnes in Chronic Inflammation and Systemic Infections
The chronic inflammatory condition of the pilosebaceous follicle caused by P. acnes is generally considered non-pathogenic. However, there is a growing body of evidence that point to the bacterium as being low virulence pathogen in several types of postoperative infections and other chronic conditions. P. acnes have been associated with endocarditis of prosthetic (Lazar and Schulman, 1992) and native aortic valves (Mohsen et al., 2001), corneal infections (Underdahl et al., 2000) and postoperative endophthalmitis (Clark et al., 1999). It has also been recognized as a source of infection in focal intracranial infections (Chu et al., 2001) and various cerebrospinal fluid shunt infections (Thompson and Albright, 1998).
A recent study from Japan (Ishige et al., 1999) has shown that P. acnes DNA can be detected in lymph nodes of Japanese individuals with sarcoidosis. Sarcoidosis is a granulomatous disease that results in the inflammation of lymph nodes, lungs, eyes, liver, and other tissues. P. acnes have also been implicated in sciatica, a chronic inflammatory condition of the lower back. Stirling et al. (2001) have isolated P. acnes from intervertebral disc material of patients with severe sciatica and they hypothesize that low virulent organisms such as P. acnes can gain access to the injured spinal disc and initiate chronic inflammation. However, until confirmatory data is available, the proposed role of P. acnes in sarcoidosis and sciatica should be considered intriguing but preliminary.
It also appears to be significant that P. acnes have been isolated from several orthopedic infections, silicone breast prosthesis, and prosthetic joint infections (Yu et al., 1997; Tunney et al., 1999). The infected prostheses have been shown to contain bacterial biofilms of P. acnes and/or Staphylococcus epidermidis. The adhesion of P. acnes to the surface of the prostheses has been postulated to be a result of binding of propionibacterial cell surface proteins or adhesion molecules to host plasma or connective tissue proteins such as fibronectin (Yu et al., 1997). Evidence for this hypothesis comes from the studies of Herrmann et al. (1988), who show that fibronectin, fibrinogen, and laminin are mediators of adherence of staphylococcal isolates to polymer surfaces in intravenous device infection.
Corixa Acne Vaccine Program
The gamut of acne treatments range from topical and systemic antibiotics to oral and topical isotretinoins, chemicals like benzoyl peroxide, oral contraceptives and corticosteroids. Antibiotics have been in use for several decades as one of the most common treatments for acne. Antibiotics, both topical and systemic, take a relatively long time to reduce the numbers of P. acnes bacteria in the skin and do not address other causative factors of acne. More recently, vitamin A derivatives called retinoids have been used effectively for acne treatment since these drugs help unclog pores, reduce sebum production and help normalize skin shedding and growth. However, oral isotretinoins are also known to cause severe side effects including elevated serum triglyceride levels, acute pancreatitis, hepatotoxicity, clinical depression, and birth defects in pregnant women.
To help identify components of P. acnes involved in pathogenesis or a protective immune response and develop a therapeutic vaccine for acne, we recently sequenced the genome of P. acnes. The genome is approximately 2.6 Mb and organized into 100 contigs. It shares similarity with the genomes of other bacteria, including Streptomyces coelicor, Mycobacterium tuberculosis, and other gram-positive cocci. Numerous homologues to virulence factors of other grampositive pathogens have been found in the P. acnes genome, including homologues of known vaccine targets.
Whole genome sequencing of microbial pathogens has been used successfully to predict vaccine candidates in Streptococcus pneumoniae and Haemophilus influenzae (Adamou et al., 2001; Wizemann et al., 2001; Chakravarti et al., 2000). We are using a multifaceted approach that combines traditional immunological and biochemical antigen discovery strategies along with a genomics approach to identify antigens for use as vaccine targets. This approach includes serological expression cloning, proteomics, and CD4 T-cell expression cloning. We are further enhancing antigen discovery methods by using in-silico approaches to predict targets for antibody-based vaccines and antimicrobial agents. The products of these various research strategies provide attractive antigen candidates, i.e., a polypeptide that is detected by serum from adult individuals who never suffered acne, and predicted to be extracellular and involved in P. acnes metabolism, or an immunogenic extracellular enzyme potentially involved in epithelial destruction. Such antigens may prove to be valuable vaccine candidates for the other chronic diseases associated with P. acnes as well.
Knowing the physiological function of our targets allows us to tailor in-vitro and in-vivo assays to evaluate the potential of specific immune components to limit or abolish the events that lead to inflammatory acne. Since the antigens of choice will be delivered under a recombinant protein format, they will require a strong adjuvant that induces an adequate immune response at the correct site. Recent data indicates that Corixa’s proprietary adjuvants, MPL® and AGPs (aminoalkyl glucosaminide phosphates), induce strong mucosal and systemic
immunity when administered mucosally. Adjuvants such as these would be useful to prime a local immune system against P. acnes at the pilosebaceous level.
Lastly, the molecules discovered by immunological methods could be used in immunodiagnostic assays. For example, we might be able to develop serological markers to predict in early adolescence the likelihood of future acne flares. In addition, since many of the studies of the involvement of P. acnes outside of the skin have so far relied on culture-based and molecular techniques that are prone to false positive results, future studies of disease associations of P. acnes might be facilitated by the availability of a specific immunoassay comprising recombinant P. acnes proteins.
REFERENCES
Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infection and Immunity 69:949–958.
Adlam C and Scott MT. 1973. Lympho-reticular stimulatory properties of Corynebacterium parvum and related bacteria. Journal of Medical Microbiology 6:261–274.
Cantrell JL and Wheat RW. 1979. Antitumor activity and lymphoreticular stimulation properties of fractions isolated from Corynebacterium parvum. Cancer Research 39:3554–3563.
Chakravarti DN, Fiske MJ, Fletcher LD, Zagursky RJ. 2000. Application of genomics and proteomics for identification of bacterial gene products as potential vaccine candidates. Vaccine 19:601–612.
Chu RM, Tummala RP, Hall WA. 2001. Focal intracranial infections due to Propionibacterium acnes: report of three cases. Neurosurgery 49:717–720.
Clark WL, Kaiser PK, Flynn HW Jr, Belfort A, Miller D, Meisler DM. 1999. Treatment strategies and visual acuity outcomes in chronic postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:1665–1670.
Davies M. 1982. Bacterial cells as anti-tumour agents in man. Reviews on Environmental Health 4:31–56.
Goldman L, Michael JG, Riebel S. 1979. The immunobiology of acne. A polyvalent proprionibacteria vaccine. Cutis 23:181–184.
Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacher-Perdreau F, Peters G, Waldvogel FA. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. The Journal of Infectious Diseases 158:693–701.
Hoffler U, Gehse M, Gloor M, Pulverer G. 1985. Enzyme production of propionibacteria from patients with acne vulgaris and healthy persons. Acta Dermato-Venereologica 65:428–432.
Holland KT, Ingham E, Cunliffe WJ. 1981. A review, the microbiology of acne. The Journal of Applied Bacteriology 51:195–215.
Holland KT, Holland DB, Cunliffe WJ, Cutcliffe AG. 1993. Detection of Propionibacterium acnes polypeptides which have stimulated an immune response in acne patients but not in normal individuals. Experimental Dermatology 2:12–16.
Ingham E, Gowland G, Ward RM, Holland KT, Cunliffe WJ. 1987. Antibodies to P. acnes and P. acnes exocellular enzymes in the normal population at various ages and in patients with acne vulgaris. The British Journal of Dermatology 116:805–812.
Ishige I, Usui Y, Takemura T, Eishi Y. 1999. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 354:120–123.
Kirschbaum JO and Kligman AM. 1963. The pathogenic role of Corynebacterium acnes in acne vulgaris. Archives of Dermatology 88:832–833.
Knop J, Ollefs K, Frosch PJ. 1983. Anti-P. acnes antibody in comedonal extracts. The Journal of Investigative Dermatology 80:9–12.
Layton AM, Henderson CA, Cunliffe WJ. 1994. A clinical evaluation of acne scarring and its incidence. Clinical and Experimental Dermatology 19:303–308.
Lazar JM and Schulman DS. 1992. Propionibacterium acnes prosthetic valve endocarditis: a case of severe aortic insufficiency. Clinical Cardiology 15:299–300.
Mohsen AH, Price A, Ridgway E, West JN, Green S, McKendrick MW. 2001. Propionibacterium acnes endocarditis in a native valve complicated by intraventricular abscess: a case report and review. Scandinavian Journal of Infectious Diseases 33:379–380.
Noble WC. 1984. Skin microbiology: coming of age. Journal of Medical Microbiology 17:1–12
Norris JF and Cunliffe WJ. 1988. A histological and immunocytochemical study of early acne lesions. The British Journal of Dermatology 118:651–659.
Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infection and Immunity 63:3966–3972.
Plewig G and Kligman AM, eds. 2000. Acne and Rosacea, 3rd ed., 744 pages. New York: Springer-Verlag.
Puhvel SM and Sakamoto M. 1977. Chemoattractant properties of Corynebacterium parvum and pyran copolymer for human monocytes and neutrophils. Journal of the National Cancer Institute 58:781–783.
Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TS. 2001. Association between sciatica and Propionibacterium acnes. Lancet 357:2024–2025.
Thompson TP and Albright AL. 1998. Propionibacterium [correction of Proprionibacterium] acnes infections of cerebrospinal fluid shunts. Childs Nervous System 14:378–380.
Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. Journal of Clinical Microbiology 37:3281–3290.
Underdahl JP, Florakis GJ, Braunstein RE, Johnson DA, Cheung P, Briggs J, Meisler DM. 2000. Propionibacterium acnes as a cause of visually significant corneal ulcers. Cornea 19:451–454.
Vymola F, Buda J, Lochmann O, Pillich J. 1970. Successful treatment of acne by immunotherapy. Journal of Hygiene, Epidemiology, Microbiology, and Immunology 14:135–138.
Vowels BR, Yang S, Leyden JJ. 1995. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infection and Immunity 63:3158–3165.
Webster GF, Leyden JJ, Norman ME, Nilsson UR. 1978. Complement activation in acne vulgaris: in vitro studies with Propionibacterium acnes and Propionibacterium granulosum. Infection and Immunity 22:523–529.
Wizemann TM, Heinrichs JH, Adamou JE, Erwin AL, Kunsch C, Choi GH, Barash SC, Rosen CA, Masure HR, Tuomanen E, Gayle A, Brewah YA, Walsh W, Barren P, Lathigra R, Hanson M, Langermann S, Johnson S, Koenig S. 2001. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infection and Immunity 69:1593–1598.
Yu JL, Mansson R, Flock JI, Ljungh A. 1997. Fibronectin binding by Propionibacterium acnes. FEMS Immunology and Medical Microbiology 19:247–253.




































































