2
Source Zones
Understanding the characteristics of subsurface source zones provides a foundation for addressing source characterization, technology options, and decision making. Following the definition given in Chapter 1, source zones are volumes that have been in contact with separate phase contaminants and that act as reservoirs that sustain a contaminant plume in groundwater, surface water, or air or that act as a source for direct exposure. Within these subsurface regions, non-aqueous, sorbed, and dissolved phase contaminants in hydraulically stagnant zones can provide persistent loading of contaminants to groundwater passing through them. First, the five hydrogeologic settings that typify most hazardous waste sites are described. The chapter then turns to an examination of contaminant releases and subsequent transport, storage, and fate, describing the many processes that act on contaminants in the subsurface and how this is manifested in the field-scale distribution of contaminants. The architecture of source zones is then considered for the five hydrogeologic settings. Although many of the processes that control contaminant fate and transport in the subsurface are the same for either chlorinated solvents or chemical explosives, these contaminants are discussed separately because their release mechanisms can be significantly different from one another.
HYDROGEOLOGIC SETTINGS
Subsurface settings are a product of a set of diverse geological processes that produce an abundance of variations. Common sedimentary systems include windblown (eolian) sands, beach sands, alluvial fans, river sequences, glacial outwash,
deltaic sequences, and lake-deposited (lacustrine) clays. Common rock systems include limestone, dolomite, sandstone, shale, interbedded sandstone and shale, extrusive volcanic flow sequences, intrusive granitic bodies, and metamorphic systems of crystalline rock. To varying degrees these systems can be fractured, cemented, and/or opened by dissolution (karst). This diversity makes it challenging to develop general statements regarding the characteristics of source zones, the efficacy of remedial technologies, and what endpoints are attainable. For example, the flow of groundwater or remedial fluids (such as surfactants) is substantially different in beach sand than in karst systems, and the tools required to characterize alluvium are substantially different than tools used to characterize rock.
Five general hydrogeologic settings that are broadly representative of the common conditions of concern are illustrated in Figure 2-1. The differentiating features between the five settings are the spatial variations in permeability and porosity (see Box 2-1, which describes the terminology relevant to the following discussion). These parameters control the mechanisms by which contaminants are stored and released from source zones under natural and engineered conditions. The scale (size) of the representative hydrogeologic settings is envisioned
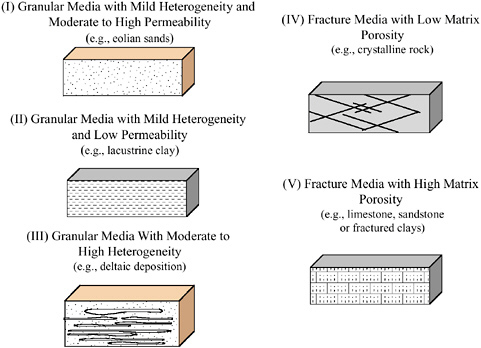
FIGURE 2-1 Five General Hydrogeologic Settings.
|
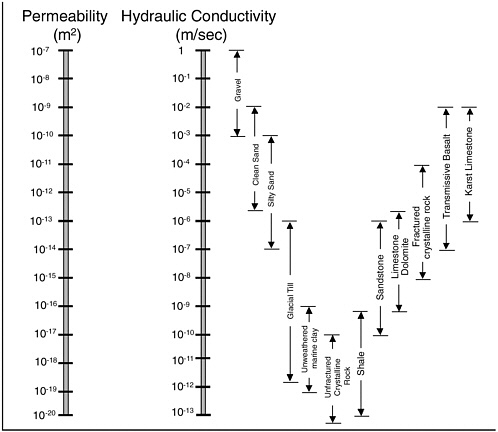
BOX 2-1 The following terms help distinguish among the five hydrogeologic settings discussed in this chapter. Consolidated vs. Unconsolidated Media: Geologic media that are cohesive as a body, firm, or secure are described as consolidated (e.g., most rock formations). Geologic media that are not cohesive as a body, are loosely arranged, and that readily separate into granular components, are described as unconsolidated. Most alluvial deposits (e.g., beach sand) are unconsolidated. Both terms are geotechnical, in that different tools are used to probe consolidated vs. unconsolidated media. The term unconsolidated may not apply to all clays, although all clays are granular. Thus, unconsolidated is a more restrictive term than granular and is used sparingly in this report. Grain Size: From Press and Siever (1974), common labels describing the sizes of granular media are: Clay < 1/256 mm < Very Fine Sand < 1/16 mm < Fine Sand < 1/8 mm < Medium Sand < 1/2 mm < Coarse Sand < 1 mm < Very Coarse Sand < 2 mm < Granule < 4mm < Pebble < 8 mm < Cobble < 256 mm < Boulder. Grain size and the degree of mixing of different grain sizes (sorting) are primary factors that control the permeability of granular porous media. Permeability: Permeability (k) is a property of a porous medium that describes its capacity to transmit fluid. Permeability is independent of the fluid or fluids present in the porous medium and has the units length squared (e.g., m2). Permeability is used in this report as the primary metric for the capacity to transmit fluid because more than one fluid (e.g., air, water, and NAPL) can coexist in the pore space of the medium of interest. Low permeability media are considered herein to be < 10−14 m2. High permeability media are considered to be > 10−10 m2. Between 10−14 and 10–10 m2 is referred to as moderate permeability media. Secondary Permeability: Secondary permeability refers to the portion of the permeability of a porous medium that can be attributed to secondary (post-emplacement) features of the matrix. Examples of secondary features include fractures, animal burrows, root casts, and solution features. In some media, such as fractured clays or crystalline rock, the dominant factor controlling fluid transmission is commonly secondary permeability. Effective Porosity: Porosity is defined as the volume of void space in the medium divided by the total volume of the medium. In hydrogeology the more important term is the effective porosity of a porous medium, |
|
For fractured media, the components of porosity include the matrix porosity, Hydraulic Conductivity: Within the field of groundwater hydrology, the term hydraulic conductivity (K) describes a porous medium’s capacity to transmit water. In contrast to permeability, conductivity is dependent on the properties of the porous medium and the fluid in the porous medium and has the units of length divided by time. Hydraulic conductivity is described as: where k is permeability, g is the gravitational constant, ρwater is the density of water, and µwater is the viscosity of water. K values are included at relevant points in this report for those more familiar with hydraulic conductivity. The relationship between permeability and hydraulic conductivity, and their values for common geologic media, are described in Figure 2-2. In all cases these values are based on the assumption that water is the fluid of interest and that water fully saturates the porous media. Low hydraulic conductivity is considered to be less than 10−7 m/sec, high is considered to be greater than 10−3 m/sec, and moderate would fall in between those two values. Heterogeneity: Heterogeneity is used to describe spatial variations in permeability. Heterogeneity can exist over a variety of scales and can be reflected in abrupt changes in permeability at discrete interfaces (caused, for example, by low-permeability inclusions) or by continuous variations in permeability over some length scale (caused, for example, by periodic gradations in grains size). Heterogeneity is of interest down to the scale of centimeters. In terms of the extent of heterogeneity, media with spatial variations in permeability of less than three orders of magnitude are referred to as mildly heterogeneous. This builds on (1) the classification of the Borden Aquifer (Canadian Forces Base Borden, Ontario) as “mildly heterogeneous” (Domenico and Schwartz, 1998) and (2) the observation of nearly three orders of magnitude variation in the permeability in the Borden Aquifer (Sudicky, 1986). Media with greater than three orders of magnitude spatial variation in permeability are described as having either moderate or high heterogeneity. Anisotropy refers to the condition in which the permeability of a geologic formation varies with the direction of measurement about a point. This commonly occurs in layered sedimentary deposits where vertical permeability is often less than 1/10th of the horizontal permeability. This anisotropy tends to foster lateral spreading and horizontal flow. |
in the range of a few meters, whereas the size of an entire source zone can be on the order of tens of meters. Source zones can occur within a single hydrogeologic setting (e.g., a sand dune deposit) or can include multiple hydrogeologic settings (e.g., alluvium overlying fractured crystalline rock). The latter case can be challenging in that the mechanism of contaminant storage and release can be substantially different in adjacent portions of a single source zone.
The following section describes the hydraulic characteristics (primarily permeability and porosity) of the five general settings. The likely distribution of contaminants in each of these settings is developed in subsequent sections. Although they do not entirely capture the diversity of hydrogeologic systems, these five settings are useful for highlighting major differences in how source zones store and release contaminants. The taxonomy used in this chapter is purposefully general and could easily be expanded to more rigorously reflect the range of hydrogeologic conditions that exist.
Type I – Granular Media with Mild Heterogeneity and Moderate to High Permeability
Type I media include systems with porosities that are consistent with typical granular media (e.g., 5 percent to 40 percent), with permeabilities that are consistent with sand or gravel deposits (>10–14 m2 or hydraulic conductivity >10–7 m/s), and mild heterogeneity (less than three orders of magnitude). As conceptualized here, this material is about as uniform as it can be in nature and thus is relatively uncommon. Deposits of this nature are encountered in association with windblown sands and beach deposits. Examples include beach sands at the Canadian Forces Base Borden, Canada, and dune deposits at Great Sand Dunes National Park, Colorado (Figure 2-3). Due to its mild heterogeneity and moderate to high permeability, all portions of this media type can transmit groundwater.
Type II – Granular Media with Low Heterogeneity and Low Permeability
Type II settings have porosities that are consistent with typical granular media (e.g., 5 percent to 40 percent), low spatial variation in permeability (less than three orders of magnitude), low permeability consistent with silt or clay deposits (k < 10–14 m2), and low hydraulic conductivity (K < 10–7 m/s). An example is a clay deposit with no significant secondary permeability features (such as fractures, root holes, animal borrows, or slickenslides). These systems are somewhat uncommon (especially in the near-surface environment where releases typically occur), although some examples include TCE-contaminated clays at the Department of Energy’s Savannah River Site in South Carolina. More typically, low-permeability materials contain significant secondary permeability features and thus fit better into the Type V setting description (see below).

FIGURE 2-3 Example of Type I media from Great Sand Dunes National Monument.
SOURCE: http://www.nps.gov/grsa
Type III – Granular Media with Moderate to High Heterogeneity
Type III encompasses systems with moderate to large variations in permeability (greater than three orders of magnitude) and porosities that are consistent with granular media (e.g., 5 percent to 40 percent). Given large spatial variations in permeability (at the scale of centimeters to meters), portions of the zone are comparatively transmissive while others contain mostly stagnant fluids. As an example, an interbedded sandstone and shale is shown in Figure 2-4. For the purpose of this report, the more transmissive zones in Type III media have a permeability greater than 10–14 m2 (K > 10–7 m/s). Near-surface deposits of this nature are common due to the abundance of alluvium with large spatial variations in permeability and are encountered in either rock or alluvium associated with deltaic, fluvial, alluvial fan, and glacial deposits. Examples include the Garber-Wellington Aquifer in central Oklahoma, the Chicot Aquifer in Texas and Louisiana, and varved sediments near Searchmont, Ontario (Figure 2-5).
Type IV – Fractured Media with Low Matrix Porosity
Fractured media with low matrix porosity are common in crystalline rock including granite, gneiss, and schist. Examples include bedrock in the Piedmont and Blue Ridge Mountain region of the southeastern United States and plutonic cores of mountain ranges in the western United States (see Figure 2-6 for an example). The primary transmissive feature in Type IV settings is secondary permeability caused by fractures, because little to no void space exists in the unfractured matrix. The permeability of the unfractured matrix is considered to

FIGURE 2-4 Interbedded sandstone and shale, shown as an example of Type III media.
SOURCE: Reprinted, with permission, from http://geology.about.com. © 2004 About.com.
be less than 10–17 m2 (K < 10–10 m/s). However, the bulk permeability of the media is dependent on the frequency, aperture size, and degree of interconnection of the fractures, such that the anticipated range of bulk permeability values is 10–15–10–11 m2 (K = 10–8–10–4 m/s). The porosity of both the matrix and the fractures is typically small—less than 1 percent. However, in regions where crystalline rock has been extensively weathered (e.g., at the top of bedrock), the bulk media can behave more like a porous medium than what would be expected from a fractured rock type setting. A primary feature that differentiates Type IV from Type I is that contaminants in Type IV will occur in a sparse network of rock fractures that may or may not be hydraulically interconnected. In general, sources zones in fractured media with low matrix porosity are less commonly encountered than sources zones in Type III and Type V settings. This reflects the fact that many surface releases never reach bedrock and, in the United States, crystalline bedrock occurs less frequently than sedimentary bedrock (Back et al., 1988).
Type V – Fractured Media with High Matrix Porosity
This setting includes systems where fractures (secondary permeability) are the primary transmissive feature and there is large void space in the matrix. The permeability of the unfractured matrix is considered to be less than 10–17 m2 (K <10–10 m/s). The anticipated range of bulk permeability values is 10–16–10–13 m2 (K = 10–9–10–6 m/s). The porosity of the fractures relative to the total unit

FIGURE 2-5 Interbedded sand and silt layers associated with annual depositional cycles from the Varved Sediments, near Searchmont, Ontario, shown as an example of Type III media. SOURCE: Reprinted, with permission, from http://geology.lssu.edu/NS102/images/varves.html. © 2004 Department of Geology and Physics, Lake Superior State University.
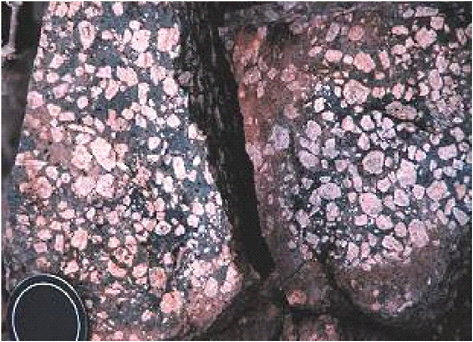
FIGURE 2-6 Fractured crystalline rock shown as an example of Type IV media. Photo taken near Kitt Peak Observatory, Arizona. SOURCE: Reprinted, with permission, from http://geology.asu.edu/~reynolds/glg103/rock_textures_crystalline.htm. © 2004 Department of Geological Sciences, Arizona State University.
volume is small (e.g., <1 percent). However, unlike Type IV, in Type V hydrogeologic settings the porosity of the unfractured matrix is anticipated to fall in the range of 1 percent to 40 percent. Fractured media with high matrix porosity are commonly encountered in sedimentary rock (e.g., limestone, dolomite, shale, and sandstone) and fractured clays. Examples include the Niagara Escarpment in the vicinity of the Great Lakes (see Figure 2-7) and fractured lake-deposited clay in Sarnia, Ontario, Canada.
An important variant of the Type V setting is karst, which is common in carbonates (e.g., limestone or dolomite). In this scenario, transmissive zones include sinkholes, caves, and other solution openings that vary widely in aperture and have the potential to store and transport significant contaminant mass (see Figure 2-8). Permeability in karst terrains varies over tens of orders of magnitude from low permeabilities between fractures to open channel flow in channels and caves (Teutsch and Sauter, 1991; White, 1998, 2002). Karst is characterized by both rapid transport along sparse dissolution features and a high ratio of stagnant to transmissive zones. As such, it is one of the most challenging hydrogeologic settings to characterize and manage.

FIGURE 2-7 Fractured limestone, Door County, Wisconsin, shown as an example of Type V media. SOURCE: Reprinted, with permission, from http://www.uwgb.edu/dutchs/GeoPhotoWis/WI-PZ-NE/BayshorePark/bayshcp3.jpg. © 2004 Natural and Applied Sciences, University of Wisconsin-Green Bay.
Relating the Hydrogeologic Settings to Specific Sites
The five hydrogeologic settings defined above represent distinct members in the continuum of settings observed at actual sites. Type I, with mild heterogeneity and moderate to high permeability, grades gradually into Type III as heterogeneity increases. With an increasing clay fraction, Type III grades to Type II. Natural systems range from clean sands to clayey sands to sandy clays to clays in a continuum. In a similar manner, the degree of importance of fractures may vary from insignificant in Type III to dominant in Type V. Because of these gradients, the presence of stagnant zones and the degree of diffusion and sorption vary continually.
Source zones, especially those above a certain size, may also encompass more than one hydrogeologic setting. This commonly occurs in the instance of shallow alluvium over bedrock. For example, in the Piedmont of the southeastern United States, one can find fluvial deposits (Type III) and saprolite (Type V) overlying fractured crystalline rock (Type IV) (Figure 2-9). Selecting characterization tools and source management technologies is challenging under these conditions, because although contamination may exist throughout, the appropriate

FIGURE 2-8 Large- and small-scale solution features in karst limestone, Redstone Arsenal.
SOURCE: Courtesy of De la Paz and Zondlo, Shaw E&I (2003).
tools for one hydrogeologic setting may not work in the adjacent hydrogeologic setting.
In the face of these complexities, the Army was nonetheless asked to estimate the percentage of its DNAPL sites that exist in the five hydrogeologic settings, for the purposes of providing context to the above discussion. Of the Army’s 43 active and BRAC (base realignment and closure) installations potentially thought to have DNAPLs (out of a total of 120), 26 percent are located in hydrogeologic setting I, 16 percent are located in hydrogeologic setting II, 16 percent are located in hydrogeologic setting III, 14 percent are located in hydrogeologic setting IV, and 28 percent are located in hydrogeologic setting V (Laurie Haines, Army Environmental Center, personal communication).
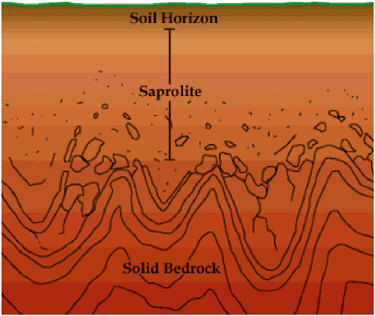
FIGURE 2-9 Mixed hydrologic settings of highly weathered saprolite overlying crystalline bedrock. SOURCE: Reprinted, with permission, from http://web.wm.edu/geology/virginia. © 2004 The Geology of Virginia, Department of Geology, College of William and Mary.
DNAPLS
How chlorinated solvents are distributed in the subsurface depends on the particular hydrogeologic setting, described above; on the chemical and physical properties of the solvents, the amount, mode, and timing of initial release of the solvents, and their fate and transport processes in the subsurface; and on human activities that may subsequently alter the source zone architecture (such as excavation). Table 2-1 lists several properties of chemicals commonly found in DNAPLs (including both chlorinated solvents and other organic compounds). As discussed below, these properties play important roles in shaping the migration capacity and eventual distribution of DNAPLs, and they can be used to predict the potential for chemical transformation or phase transition processes.
Contaminant Releases
Contaminants are typically released to the subsurface as constituents of a liquid phase, such as a dilute aqueous solution, a concentrated aqueous solution
TABLE 2-1 Properties of Organic Chemicals Found in DNAPLs
|
|
CAS # |
Aqueous Solubility (mg/L) |
Density (g/cm3) |
Vapor Pressure (mm Hg) |
Absolute Viscosity (cP) |
Log Kow |
Henry’s Law Coefficient (103 atm m3/mol) |
|
Chlorinated Solvents |
|||||||
|
Tetrachloroethene (perchloroethylene, PCE) |
127-18-4 |
150 |
1.62 |
20 |
0.89 |
2.88 |
15 |
|
Trichloroethene (trichloroethylene, TCE) |
79-01-6 |
1,100 |
1.46 |
74 |
0.57 |
2.53 |
9.1 |
|
cis-1,2-Dichloroethene (cis-1,2-DCE) |
156-59-2 |
3,500 |
1.28 |
200 |
0.48 |
1.86 |
3.4 |
|
1,1-Dichloroethene (1,1-DCE) |
75-35-4 |
400 |
1.21 |
590 |
0.36 |
2.18 |
15 |
|
1,1,1-Trichloroethane (1,1,1-TCA) |
71-55-6 |
1,300 |
1.34 |
120 |
0.87 |
2.48 |
15 |
|
1,2-Dichloroethane (1,2-DCA) |
107-06-2 |
8,300 |
1.23 |
87 |
0.80 |
1.48 |
1.1 |
|
Tetrachloromethane (carbon tetrachloride, CT) |
56-23-5 |
800 |
1.59 |
120 |
0.97 |
2.83 |
30 |
|
Trichloromethane (chloroform, CF) |
67-66-3 |
8,200 |
1.49 |
200 |
0.58 |
1.95 |
3.4 |
|
Dichloromethane, DCM (methylene chloride) |
75-09-2 |
13,000 |
1.33 |
440 |
0.43 |
1.25 |
2.0 |
|
Other Hydrocarbons |
|||||||
|
Naphthalene |
91-20-3 |
32 |
1.14 |
8.2×10–2 |
solid |
3.36 |
0.46 |
|
Benzo[a]pyrene |
50-32-8 |
0.004 |
1.35 |
5.6×10–9 |
solid |
6.04 |
3.4×10–4 |
|
Aroclor 1254 (PCB mixture) |
11097-69-1 |
51 |
1.50 |
7.7×10–5 |
700 |
6.5 |
2.7 |
|
Aroclor 1260 (PCB mixture) |
11096-82-5 |
80 |
1.56 |
4.0×10–7 |
resin |
6.9 |
0.34 |
|
Units and abbreviations: CAS # is a unique identifier used by the American Chemical Society to identify chemicals in databases. Aqueous solubility in milligrams per liter at 25°C; Density in grams per cubic centimeter at 20°C; Vapor pressure in millimeters of mercury at 25°C; Absolute viscosity in centipoise at 20°C; Log Kow = log of the octanol–water partition coefficient (unitless); Henry’s Law coefficient is in atmospheres meters cubed per mole at 25°C. SOURCE: Montgomery (2000), except as noted. Mackay et al. (1993) for properties of cis-1,2-DCE; Cohen et al. (1993) for absolute viscosity. |
|||||||
(leachate), or an organic liquid (nonaqueous phase liquid or NAPL) that is not miscible with water. The characteristics of the released liquid will greatly influence the migration pathways, extent of travel, and persistence of the released contaminants. For example, a considerable mass of a contaminant that is only slightly soluble in water may travel large distances in the subsurface as a constituent of an organic liquid.
Sources of NAPLs are numerous and include surface spills, leaking drums, pipelines, storage tanks, and liquid waste disposal operations. Many NAPLs encountered in the subsurface tend to be mixtures of several different chemical compounds, due to their use history prior to release, the sequence of chemical releases, and biotic or abiotic reactions within the subsurface environment subsequent to release. A NAPL consisting of more than one compound is a multicomponent NAPL. Volatile organic compounds (VOCs), polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) are often found as components of NAPLs. The two most prevalent classes of NAPL components are chlorinated solvents and petroleum hydrocarbons (including gasoline and fuel oils) (Mercer and Cohen, 1990). Other common NAPLs include coal tars, transformer oils (primary carriers of PCBs), and creosote (a primary carrier of PAHs and phenols).
NAPLs that have a density greater than that of water are commonly known as dense NAPLs (DNAPLs). Of the classes of compounds cited above, chlorinated solvents are the most common DNAPL components (e.g., PCE, TCE, cis-1,2-DCE, 1,1-DCE, 1,1,1-TCA, 1,2-DCA, carbon tetrachloride, chloroform, and methylene chloride). PCBs, PAHs, creosote, and coal tar often form or are constituents of DNAPLs. Petroleum hydrocarbons and transformer oils tend to form light NAPLs (LNAPLs), with densities less than that of water. It should be noted that it is not uncommon for multicomponent DNAPLs to contain significant concentrations of petroleum hydrocarbons such as benzene, toluene, ethylbenzene, and xylene (BTEX). Thus, it is important to make a distinction between a chemical component of a DNAPL, which may be denser or lighter than water in its pure liquid form, and the DNAPL itself, which is a separate phase organic liquid composed of various chemical species.
DNAPL Fate and Transport in the Subsurface
Immiscible Flow
Bulk NAPL migration within a formation is governed by properties of the organic fluid, including density, viscosity, and interfacial tension with the pore water, as well as properties of the formation solids, including texture and surface characteristics. As a NAPL enters the subsurface, it will first encounter the unsaturated zone, which contains natural pore water and air. In this zone, NAPL migration (in which the NAPL displaces air) is driven primarily by gravity and
thus will tend to be vertically downward. Depending upon its solubility and volatility, the NAPL may dissolve into the pore water and/or volatilize into the pore air as it moves within the unsaturated zone. As the NAPL continues its downward migration, it will eventually encounter the saturated zone in which water completely fills the pore space. Given its density, a DNAPL will tend to continue to migrate vertically, displacing the groundwater until a less permeable stratum is reached or the volume of DNAPL is depleted or a sufficient vertical hydraulic gradient is met. Alternatively, a LNAPL will tend to spread preferentially within the capillary fringe zone at the top of the saturated domain, migrating primarily in the direction of natural groundwater flow.
Representative specific gravities (densities) of DNAPL constituents, which play a role in their tendency to migrate downward through the saturated zone, are given in Table 2-1 (along with several other parameters of interest). Note that chlorinated solvents are characterized by densities of 1.2 g/cm3 or more and hence exhibit substantial propensity for vertical flow (Pankow and Cherry, 1996). Creosote, coal tar, and other PAH-based DNAPLs, however, tend to have densities (e.g., 1.1 g/cm3) much closer to that of water and thus may not experience as large a driving force for vertical migration.
While the density of a NAPL influences its propensity to migrate vertically within the saturated groundwater zone, its viscosity influences its rate of migration. In general, fluids of lower viscosity tend to migrate more rapidly due to reduced resistance to flow. Table 2-1 reveals that many DNAPLs, including chlorinated solvents, have viscosities smaller than that of water (1 cP). Thus, these fluids will tend to flow readily. Creosotes and coal tars, however, generally have a much higher viscosity and tend to migrate more slowly under similar hydraulic gradients.
A third phenomenon that influences the migration of a DNAPL in the subsurface is known as capillarity. Capillarity is the physical manifestation of the interfacial forces that occur between phases—either liquid/liquid or solid/liquid. The nature and extent of these interfacial forces exert primary control on migration pathways, the extent of DNAPL spreading, and DNAPL entrapment in a saturated formation. Capillarity is controlled by both by the geometry of the pores and two interfacial properties: interfacial tension and wettability. Interfacial tension is a property of the aqueous–DNAPL interface and is defined as the energy per unit interfacial area required to create a new surface (Hiemenz and Rajagopalan, 1997). DNAPL–water interfacial tensions are typically in the range of 20–50 dynes/cm (Mercer and Cohen, 1990) for pure phase organic liquids. However, interfacial tension can be significantly affected by co-contaminants or additives in the DNAPL phase, including organic acids and bases and surfactants, and by dissolved pore water constituents, such as natural humic substances. Such compounds can behave as surface-active agents, substantially reducing interfacial tension (Adamson and Gast, 1997). A reduction in interfacial tension tends to (1) decrease the spread of a DNAPL transverse (perpendicular) to its primary
direction of migration and (2) decrease the force required for the DNAPL to displace water from a saturated pore.
Wettability refers to the tendency of one fluid to spread on or adhere to a solid surface in the presence of another immiscible fluid. It controls the distribution of fluids within the pores. In water/NAPL/solid systems, the liquid having the higher affinity for the solid surface coats the solid and is referred to as the wetting phase, while the other liquid is known as the nonwetting phase. The contact angle, a measure of wettability, is the angle the liquid–liquid interface makes with the solid surface (Hiemenz and Rajagopalan, 1997). For many natural minerals, including quartz and carbonates, water is more strongly attracted to the mineral surface than are common DNAPL constituents. Thus, in such media, water generally is the wetting phase, distributing itself along the solid surfaces and in small-aperture pore regions and fractures. A solid is said to be water-wet if the contact angle is between 0° and 60°, as measured through the aqueous phase. Conversely, as the contact angle approaches 180°, the surface is said to be strongly NAPL wetting. A surface is termed intermediate-wet if the contact angle ranges from approximately 70° to 120° (Morrow, 1976). The condition of mixed wettability, in which the larger pores are organic-wetting and the smaller pores are water-wetting, has long been recognized in the petroleum industry (e.g., Salathiel, 1973). The term fractional wettability is generally used to describe media with surfaces of varying wettability (Anderson, 1987). Water-, intermediate-, and organic-wetting conditions can exist simultaneously in the subsurface due to natural variations (Anderson, 1987) or through the interaction of the released NAPLs with the solids. For example, contact with NAPL mixtures containing surface-active constituents can render a porous medium intermediate- to organicwet (e.g., Powers et al., 1996). These and other studies suggest that variations in wettability may be common in the contaminated subsurface. Such variations may influence NAPL migration and persistence in natural settings.
In water-wet media, the DNAPL, which is the nonwetting phase, tends to be concentrated in the center of the pores and in larger pores and fractures. The wetting phase is able to easily enter new pore spaces, but the nonwetting phase has to overcome capillary forces to do so. The required displacement force is a function of the pore geometry, the interfacial tension, and the contact angle. In a cylindrical pore, the pressure differential required to displace the wetting phase (ΔP) is given by the Laplace-Young equation:
(1)
where
θ = contact angle
σ = interfacial tension
r =pore radius.
This required pressure differential can be supplied by an applied pressure on the nonwetting phase or by the weight of the accumulating nonwetting phase above the pore. For a nonwetting DNAPL, the height (h) that must accumulate prior to pore penetration is thus related to the pressure differential by:
(2)
where
Δρ is the difference in density between the DNAPL and water (the nonwetting and wetting phases)
g is the gravitational constant
Pnw is the nonwetting liquid pressure
Pw is the wetting liquid pressure.
Once this pressure differential is achieved, the pore will be invaded by the DNAPL.
Although capillary forces are well understood for simple pore geometry (e.g., cylindrical pores), the complex pore structure of natural porous media makes precise predictions of interface positions difficult. Thus, a macroscopic relation between the liquid pressure differential and the degree of saturation of the wetting fluid, known as a capillary pressure–saturation curve, is typically used in practice to describe the capillary behavior of a particular medium. The critical pressure differential that must be achieved for any of the wetting fluid to be displaced is known as the entry pressure. Although the relationship shown in Equation (1) must be modified for pore geometry and surface roughness considerations in natural porous media, the general form of this relationship is still valid. Equation (1) thus suggests that the existence of capillary forces tends to create a (capillary) barrier to the movement of a nonwetting DNAPL into fine pore water-saturated media.
The process of nonwetting DNAPL migration is thus one of vertical migration until a finer-grained layer is encountered, at which point the DNAPL spreads horizontally either until a sufficient thickness of DNAPL accumulates to overcome the entry pressure or until a path with lower entry pressure, perhaps from lithologic variation, is encountered during the horizontal migration. Subtle textural variations that create differences in entry pressure sufficient to affect DNAPL flow can occur even in apparently homogeneous units (Kueper et al., 1993), and DNAPL migration in saturated sandy media has been shown to be sensitive to small-scale variation in permeability and capillary characteristics (Poulsen and Kueper, 1992; Brewster et al., 1995). Such small-scale entry-pressure variations can cause uneven DNAPL penetration of a macroscopically uniform subsurface layer, leading to the formation of narrow vertical preferential flow pathways, commonly known as fingers, that can serve as rapid conduits for DNAPL migration deep into the subsurface. Because small-scale textural variability is not easily quantifiable, these preferential pathways appear to be somewhat random in their
distribution (e.g., Rathfelder et al., 2003). This fingering behavior can make it very difficult to locate a DNAPL in situ, due to the small lateral dimensions of the fingers. The propagation of fingers is more common in coarser-textured media and under conditions of low interfacial tension, since capillary forces will tend to oppose the formation of extensive fingers.
The resistance to downward flow by finer-grained layers typically results in horizontal spreading over a distance that is large relative to the lateral extent of the vertical pathways. Thus, DNAPL often follows a highly irregular path, resulting in a source zone that contains narrow vertical pathways connected to thin, laterally extensive horizontal lenses. This will be particularly true of a Type III geologic setting that contains persistent finer-textured horizontal layers of high permeability contrast or of Type IV or V media with extensive horizontal fractures.This could also be characteristic of a Type I setting, if fine-scale layering were present. When the finer-textured or impermeable layers are dipping, the DNAPL will also tend to flow down-dip, even if the hydraulic gradient is in another direction. This combination of horizontal spreading until a more permeable path is found and of down-dip migration may displace a DNAPL a large distance from its point of entry. Variations in the topography of fine-grained layers may trap a portion of the DNAPL, creating isolated pools (as shown in Figure 2-10 for a DNAPL spill in a fractured rock system).
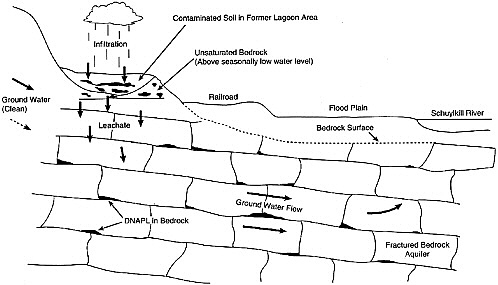
FIGURE 2-10 Schematic of DNAPL pooling in a fractured rock aquifer (Type V) in Pennsylvania. The site is contaminated with 1,2,3-trichloropropane, a DNAPL, and it has moved along bedding plane fractures. SOURCE: EPA (1992).
The DNAPL migration characteristics discussed above are illustrated in Figures 2-11 and 2-12, which contain photos of laboratory sand box experiments exploring PCE release in quartz sand media (representative of Type I media with permeability changes of 1–2 orders of magnitude). Figure 2-11(A) demonstrates the influence of a capillary barrier on PCE migration. Here the PCE was released within a coarse water-saturated sand with a permeability of 1.2 × 10–10 m2. It migrated downward under the influence of gravity, until it encountered a layer of finer-textured material with a permeability of 8.2 × 10–12 m2. At the textural interface, the PCE spread laterally and was able to cascade around this fine layer before the entry pressure was exceeded, again finding a path through the coarser medium. The PCE then pooled at another textural interface at the bottom of the tank. Inspection of the figure reveals the small thickness of the pool perched on the finer lens and the presence of vertical DNAPL fingers that are barely discernable paths to the lower capillary barrier. As noted above, these are typical characteristics of DNAPL migration behavior. Figure 2-11(B) illustrates the effect of reduced interfacial tension on PCE migration. Here the physical system is identical to that shown in (A), but the resident pore (wetting) fluid includes a surfactant that has lowered the interfacial tension between the PCE and aqueous phases (from 47.8 to 0.5 dynes/cm). Notice that the entry pressure height is now exceeded as the PCE spreads on the finer sand, and the PCE is able to penetrate
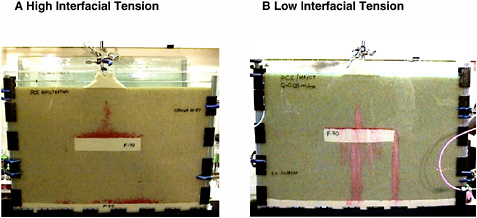
FIGURE 2-11 The influence of interfacial tension (IFT) on DNAPL migration. Photographs of aquifer cell experiments (~60 cm length by ~35 cm height) capture the final DNAPL (dark dye) distribution in (A) water-saturated sand (IFT ~ 48 dyne/cm) and (B) sand saturated with an aqueous solution containing a mixture of surfactants selected to reduce IFT to 0.5 dyne/cm. SOURCE: Reprinted, with permission, from Rathfelder et al. (2003). © 2003 Elsevier Science.
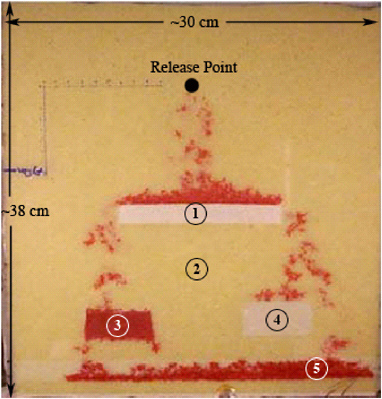
FIGURE 2-12 Effect of wettability on PCE (dark dye) migration in a sand box. Wettability and permeability of sands: (1) water-wet, 4.7 × 10–12 m2; (2) water-wet, 4.0 × 10–10 m2; (3) organic-wet, 6.4 × 10–11 m2; (4) water-wet, 6.4 × 10–11 m2; (5) organic-wet, 4 × 10–10 m2. SOURCE: Reprinted, with permission, from O’Carroll et al. (2004). © 2004 Elsevier Science.
the finer-textured material. Also note the presence of preferential flow paths through the fine-grained material, attributable to small-scale variations in lens packing that are not easily discernable.
Figure 2-12 illustrates the influence of wettability on DNAPL migration. Here, again, a release of PCE has occurred in a coarse sandy medium. This medium is embedded with finer-textured lenses of varying properties. Those labeled as organic wet sands in the figure have been treated with an octadecyltrichlorosilane coating that has rendered the quartz surface NAPL-wetting. Water is now easily displaced from these treated lenses, since capillary forces act to “pull” the DNAPL into the organic-wet material.
Residual Entrapment
As a DNAPL migrates through the subsurface, small globules or ganglia of this organic phase are retained within the pores due to the presence of capillary forces. This entrapped NAPL is frequently quantified as a residual saturation—the volumetric ratio of entrapped organic phase to the total pore volume. Entrapment occurs when capillary forces are sufficiently large to overcome the forces exerted by flowing water and gravity. Residual saturations are thus a function of pore geometry, organic phase properties (including interfacial tension, viscosity, and density), flow velocity, and porous medium wettability. The entrapped residual is also a function of the maximum DNAPL saturation reached prior to drainage of the organics and consequently of the release history—that is, higher residuals are found in media that experienced larger release rates or within areas where NAPL had pooled. Furthermore, because larger amounts of an organic compound are entrapped when the release rate is higher, a released volume of DNAPL will tend to migrate further and deeper in a formation if it is released more slowly. Thus, release rate tends to affect the migration patterns of a DNAPL spill. It should be noted that pooled organic can be mobilized by a subsequent change in the groundwater flow field or a breach of the capillary barrier on which the pool is perched. At groundwater velocities commonly encountered under natural or pump-and-treat conditions, however, residual saturations are expected to be essentially independent of velocity (Powers et al., 1992).
Local maximum residual saturations of DNAPLs measured in field-scale and laboratory experiments are typically in the range of 10 percent to 35 percent in saturated, unconsolidated media, with levels as high as 50 percent in materials of low permeability (Conrad et al., 1987; Schwille, 1988). Average residual saturations reported at typical DNAPL field sites, however, are usually much smaller, on the order of 0.1 percent to 1.0 percent (Meinardus et al., 2002) of the affected pore volume. This apparent discrepancy relates to the scale of the saturation measurement, that is, the volume over which the organic mass is averaged. For example, consider the PCE release scenario shown in Figure 2-11(A). Here quantification of the average DNAPL saturation in the tank (the total volume released divided by the tank pore volume) yields a value of 0.1 percent, while saturations measured on selected 2-cm3 subsamples ranged from 0.8 percent to 19 percent (Rathfelder et al., 2003). The first saturation value (0.1%) thus may be more characteristic of an average field-scale value, but it should be recognized that this average value is not representative of the actual distribution of DNAPL within the volume.
NAPL entrapment is illustrated in Figure 2-13(A), in which residual PCE entrapment can be observed in a sand column packed with a coarse quartz sand. Figure 2-13(B) shows a range of entrapped ganglia, polymerized in a similar sand column experiment that entrapped an LNAPL (styrene) in a more graded sand.
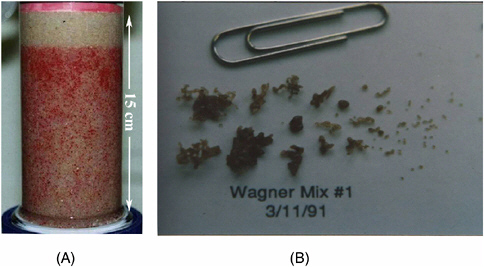
FIGURE 2-13 PCE entrapment in coarse sand (A) and representative ganglia from a graded sand (B). SOURCE: Reprinted, with permission, from Powers et al. (1992). © (1992) American Geophysical Union.
Other Processes Influencing DNAPL Persistence
The sections above have described the processes controlling the migration and entrapment of DNAPL in geologic materials to form a DNAPL source. Once the DNAPL is emplaced, the long-term persistence of this source zone will be controlled by the rates of dissolution of the DNAPL mass to the flowing pore water and by any alternations in the DNAPL properties due to chemical weathering or microbial transformations.
Local DNAPL dissolution has been the subject of much investigation. Researchers have found that the rates of dissolution are controlled by a number of variables, including the solubility of the DNAPL constituents, the local groundwater velocity, the textural heterogeneity of the geologic media, the wettability characteristics of the solid, and the saturation of the DNAPL (e.g., Powers et al., 1992, 1994; Bradford et al., 1999). In general, mass transfer rates tend to increase with groundwater velocity, grain size uniformity, and DNAPL solubility. Because DNAPL solubilities (see Table 2-1) and groundwater velocities are typically very small, dissolution rates tend to be very slow in DNAPL source zones under natural flow conditions. DNAPLs at residual saturation can thus persist for decades (Lemke et al., 2004). As would be anticipated from thermodynamic equilibrium considerations, for DNAPL mixtures the more soluble constituents tend to dissolve faster, resulting in a change in DNAPL composition over time.
Such preferential dissolution can potentially alter DNAPL solubility, viscosity, density, interfacial tension, and toxicity.
In zones of stagnation or very slow flow, dissolution is further limited by the diffusion of the organic solute away from the DNAPL interface (diffusive flux). According to Fick’s law of diffusion, diffusive flux is the product of the concentration gradient and the diffusivity of the DNAPL constituent in water. Typical aqueous diffusivities of DNAPL constituents are on the order of 10–5 cm2/s. This small value, coupled with the low solubility of these compounds, makes diffusion an extremely slow process. Thus, if the DNAPL is able to enter a stagnant zone of a formation or if water flow bypasses the DNAPL region (as will occur for high-saturation pools), dissolution rates tend to be extremely small, and the DNAPL may persist for centuries under natural conditions.
The process of molecular diffusion will also tend to spread dissolved contaminant mass into stagnant regions of a formation. Thus, even if a capillary barrier or low-permeability zone has prevented the downward propagation of a DNAPL, the low-permeability layer underlying a DNAPL pool may become a reservoir for dissolved organic mass. If the DNAPL pool is subsequently removed, the direction of the concentration gradient will be reversed, and this dissolved organic mass will slowly diffuse out of the low-permeability zone over time (reverse diffusion), serving as a persistent source of contamination to flowing groundwater in the overlying stratum.
The potential reservoir of organic mass for reverse diffusion from stagnant regions of a formation is also strongly influenced by the sorptive capacity of the solids for the DNAPL constituents. This sorbed portion of the contaminant mass is not generally quantifiable from groundwater sampling alone and can only be assessed through aquifer coring. Sorbed contamination is the organic mass that is associated with the solid matrix material. For organic compounds, the sorption capacity is generally related to the fraction and character of the solid phase organic carbon, the surface area of the solids, and the compound’s octanol–water partition coefficient (Kow) (see Table 2-1). In general, larger sorptive capacity is associated with a higher fraction of organic carbon, a higher surface area, and a larger octanol–water partition coefficient. The extent of diagenesis of the organic solid fraction will tend to affect its sorption rates and sorption reversibility (e.g., Huang and Weber, 1998; Weber et al., 1999).
Under equilibrium conditions, there is a quantifiable relationship between sorbed organic and aqueous phase organic concentrations. This relationship is known as an equilibrium isotherm. In the vicinity of a DNAPL, the persistent high aqueous concentrations, coupled with diffusion, tend to promote sorption. Conversely, if the DNAPL is removed, desorption will serve as a continuing source of contaminant mass, feeding the reverse diffusion process. Thus, in heterogeneous formations, reverse diffusion from stagnant zones that contain aqueous phase or sorbed mass can sustain plume concentrations after the DNAPL has been depleted either through remediation efforts or natural processes.
Prediction and assessment of contaminant storage and elution from low-permeability zones is a complex task because groundwater flow velocities vary by orders of magnitude within different interbeds of natural sediments (e.g., sand and silt). If groundwater flow is relatively slow, as is typical under natural flow conditions, the sorption is typically considered as an equilibrium process; that is, these solid–water exchanges are assumed to occur instantaneously as the water flows through the solids. However, when flow is relatively fast, such as may occur when groundwater is extracted by pumping, these exchanges may lag behind the flow and lead to (1) earlier-than-expected contaminant arrival or (2) later-than-expected contaminant extraction, when a plume is arriving at or passing an observation point, respectively. Biological and chemical contaminant transformation in the aqueous phase may similarly be limited by desorption, especially where those transformations are rapid. To date, the process of reverse diffusion has been studied in only simple geologic scenarios (Sudicky et al., 1985; Parker et al., 1994, 1997; Ball et al., 1997; Liu and Ball, 1998; Mackay et al., 2000).
Until recently, it was believed that biotransformation processes could not occur near a chlorinated solvent source due to the toxicity of high contaminant concentrations associated with the presence of NAPL (Robertson and Alexander, 1996). A number of studies, however, have recently documented microbial activity at concentrations at or near the aqueous solubility of PCE (e.g., Yang and McCarty, 2000; Cope and Hughes, 2001; Sung et al., 2003). Recent studies have suggested that microbial activity can enhance DNAPL dissolution by a factor of 5 or more in the laboratory by increasing local concentration gradients and enhancing aqueous solubility (Yang and McCarty, 2002; Cope and Hughes, 2001), although enhanced dissolution has not yet been well documented in the field. Under natural subsurface conditions, the absence of electron donors typically limits microbial activity.
Field-Scale Distribution of Contaminants in Source Zones
In view of the many processes affecting DNAPL distribution discussed above, it is not surprising that only a small fraction of the subsurface volume at a contaminated site actually contains DNAPL. Within this volume, the DNAPL will be irregularly distributed both horizontally and vertically, with the distribution not easily predicted by the position of the release point. The DNAPL mass will be distributed within both residual ganglia and more saturated pools. During or immediately after a release, DNAPL will be the largest component of contaminant mass in the source zone. With time, diffusive mass transfer to the aqueous, gas, and solid phases depletes the DNAPL. As this occurs, dissolved mass in stagnant aqueous zones (generally in the rock matrix) and mass sorbed to solids can become the dominant fractions of the source mass, depending on the hydro-
geologic setting. Figure 2-14 shows a conceptual distribution of contaminant mass in these various phases.
The tendency for the source to exist as DNAPL, sorbed, or dissolved phase mass is discussed below for each of the five hydrogeologic settings introduced earlier.
Type I Settings
In instances of granular media with mild heterogeneity and moderate to high permeability, all of the source zone can be viewed as transmissive. Where the media has a low sorptive capacity (e.g., where organic matter content is low), little mass will be retained on the solids. Thus, there are typically no stagnant zones or persistent elution areas in Type I settings, and the most likely source of the dissolved phase plume is DNAPL. Note, however, that extensive DNAPL pooling may occur in Type I settings at boundaries with other less-permeable units (see Figure 2-12), and such pools may be difficult to treat.
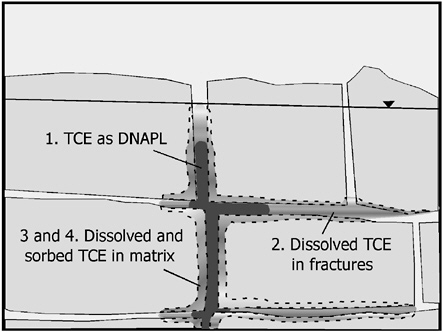
FIGURE 2-14 Conceptual diagram of contaminant mass in the subsurface showing (1) DNAPL, (2) mass dissolved in a transmissive zone (and thus part of the plume as discussed later), and (3) and (4) mass sorbed to the solid matrix or existing as a dissolved phase in stagnant zones within the matrix.
Type II Settings
More rare are source zones comprised of granular media with mild heterogeneity and low permeability. Given the absence of secondary permeability features that characterizes this setting, Type II media are difficult for contaminants to invade due to high NAPL displacement pressures and/or low conductivities to fluids. An exception to this is where DNAPL preferentially wets the fine-grained media and is drawn into the material by capillary forces. An example of this preferential wetting behavior in a lower-permeability material is shown in Figure 2-12. Here the finer-grained lens on the left near the bottom of the tank “absorbs” the DNAPL, while the lens on the right side is not penetrated. Such preferential wetting in low-permeability media can be the result of differences in mineralogy or, more commonly, the result of previous contact with groundwater containing co-contaminants that are surface-active, such as organic acids and bases.
Type III Settings
This setting involves interbedded transmissive and stagnant zones with high porosity, which is common in alluvium where low-permeability interbeds are often horizontally oriented and laterally extensive. Initial migration of DNAPL through this type of setting is along the pathways in the transmissive layers due to relatively low displacement pressures. Low-permeability layers, with high displacement pressures, act as capillary barriers. Often, DNAPL within the source zone will reside at the contact between transmissive and stagnant zones (e.g., DNAPL pools perched on clay layers). With time the DNAPL will partition into the aqueous phase where it will either be flushed out of the source zone (through transmission in flowing groundwater) or driven into the stagnant zones of low-permeability layers via diffusion. A large fraction of contaminant mass may also be adsorbed to the solids in the low-permeability layers (Parker et al., 1994). Once all of the NAPL is depleted (either by natural or engineered processes), reverse diffusion of contaminants from stagnant zones can sustain concentrations of contaminants in the transmissive layer for extended periods. This can be a primary factor driving contaminant rebound after application of source control measures that largely deplete contaminants in transmissive zones (e.g., Sudicky et al., 1985; Parker et al., 1997; Liu and Ball, 2002; Sale et al., 2004). Schematic contaminant fluxes about a DNAPL in a simplified Type III setting are illustrated in Figure 2-15.
Type IV Settings
Type IV settings involve fractured media with low matrix porosity, such that the primary space in which contaminants can be stored are fractures. Critical attributes of fracture networks are that they typically represent a small fraction of
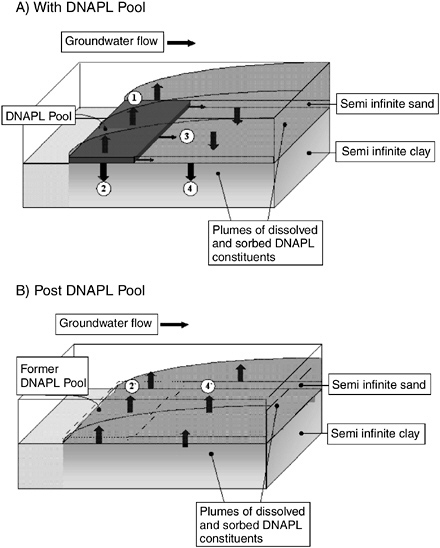
FIGURE 2-15 Contaminant fluxes in a Type III setting. (A) Contaminant fluxes (1–4) and distribution about a DNAPL pool at a contact between a sand and a clay layer, which typifies Type III settings, and (B) reverse diffusion fluxes and contaminant distribution following DNAPL depletion. Fluxes in (A) include (1) diffusion into the overlying transmissive layer, (2) diffusion into the underlying stagnant zones (3) advection through the DNAPL pool, and (4) diffusion into the stagnant zone downgradient of the DNAPL pool. In (B), the 2`and 4` fluxes are back diffusion into the transmissive zone post DNAPL dissolution. SOURCE: Sale et al. (2004).
the total rock matrix volume, and the fractures can be either well or only poorly interconnected (Parker et al., 1996). Because of low overall porosity (including the fracture zones) of Type IV settings, contaminants released into these settings tend to create a relatively large source zone. In addition, the rates of aqueous phase contaminant transport can be large due to small cross-sectional areas of flow and an absence of contaminant attenuation via diffusion into stagnant zones. Because matrix porosity in this hydrogeologic setting is low, little if any contaminant is stored as sorbed or stagnant dissolved phase mass in the source zone. However, in instances where the fracture networks are poorly connected, a subset of the fractures may behave as stagnant zones, and DNAPLs in dead-end fractures may act as persistent sources of dissolved phase contaminants that are difficult to remediate. Lastly, characterization of this type of system can be difficult due to the potentially sparse network of fractures and the limitations of characterization tools in crystalline rock.
Type V Settings
Type V settings involve fractured media (rock or low-permeability alluvium) with high matrix porosity. Thus, unlike Type IV settings, stagnant zones in Type V settings have the potential to store contaminants in the dissolved and sorbed phases and can represent a large fraction of the source mass. DNAPL itself tends to flow through the fracture networks (along paths of low displacement pressure) and is typically precluded from the matrix blocks (due to high displacement pressure) (Kueper and McWhorter, 1991). Given a finite release of moderate- to high-solubility DNAPL, complete transfer of DNAPL from the transmissive fractures into stagnant zones through dissolution and sorption is possible (Parker et al., 1994). The challenges of managing contamination in this hydrogeologic setting include describing the extent of the source zone, characterizing the fracture network, delivering remedial solutions to the targeted areas in some cases, and understanding the potential for reverse diffusion to sustain contaminant concentrations in the transmissive fractures after depletion of DNAPL.
* * *
Figure 2-16 is a conceptual diagram of a DNAPL in a system of clay and sand layers that depicts the hypothetical DNAPL distribution given the known processes described above. Depending on the thickness of the beds and the size of the spill, this site can be conceptualized as either a Type II setting or as a combination Type I (sand)–Type V (fractured clay) setting. In either case, the DNAPL migrates vertically through the upper sand aquifer to the upper clay layer. Based on field studies (Poulsen and Kueper, 1992; Kueper et al., 1993) and numerical simulations (Kueper et al., 1991a,b), the DNAPL in the sand layer is most likely
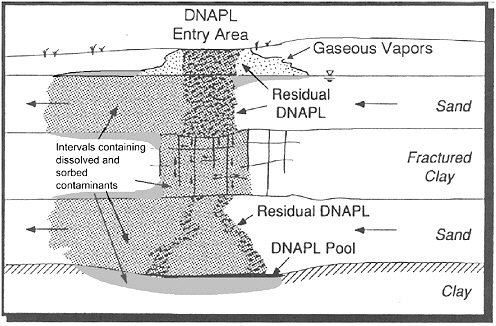
FIGURE 2-16 Hypothetical DNAPL source zone. In addition to residual and pooled DNAPL, the figure depicts a vapor plume in the unsaturated zone and a halo of dissolved and sorbed contamination in the saturated zone about the DNAPL. The plumes of dissolved and sorbed contaminants extend downgradient of the DNAPL in the sand layers, into the stagnant clay beds about the DNAPL, and into the clay beds about the plumes in the sand. Note that the residual DNAPL is more likely to occur in sparse pools and fingers, rather than in the massive bodies inferred in the picture. SOURCE: Adapted from Cohen et al. (1993).
to occur in sparse horizontal lenses and vertical fingers that have drained to near residual saturations. Upon reaching the upper fractured clay layer, the DNAPL continues to migrate downward through secondary permeability features. Large aqueous phase concentration gradients would likely drive contaminants into the clay matrix via diffusion (Parker et al., 1994, 1997). In the second sand layer, the DNAPL forms sparse horizontal lenses and vertical fingers that have drained to near residual saturations. Vertical migration ceases at the lower clay layer, where substantial pooling occurs. The pathway leaves residual DNAPL in the unsaturated soil zone and in sand and clay layers, sorbed and dissolved contaminants on the aquifer sediments that may serve as a long-term source, a pool of DNAPL deep in the subsurface, and two contaminant plumes containing dissolved compounds.
The Contaminant Plume
Given the definition of “source zone” in Chapter 1, it is important to differentiate between source zones and the contaminant plume. Contaminant plumes develop downgradient of the source material in cases where the DNAPLs are moderately soluble in water and are resistant to natural biodegradation. For example, chlorinated solvents are not easily broken down by oxidative microbial processes (being more likely to be biodegraded, at least partially, through reductive dechlorination or cometabolic oxidative processes), and they sorb weakly on aquifer materials. Thus, chlorinated solvents can form extensive plumes downgradient of the source. Depending on the extent of various biogeochemical processes (discussed previously), the chemical composition and concentration range of the contaminant suite in the plume may be quite different than in the source zone.
In general, groundwater plumes tend to have larger spatial extents and to be more continuous in nature in comparison with contaminant mass distributions within source zones. For a finite, uniform, aqueous phase contaminant release, the textbook plume shape is generally a three-dimensional ellipsoid characteristic of idealized homogeneous sandy aquifers. This plume shape is created under uniform, unidirectional flow conditions, assuming three-dimensional Gaussian mixing or dispersion. Such a regular plume concentration distribution, however, is only possible in the most idealized cases. Closer to the source zone, plumes will tend to take on spatial characteristics that mimic the irregular saturation distributions upgradient. The existence of DNAPL pools and the small vertical mixing under natural flow conditions tend to create highly irregular and stratified plumes (see Box 4-1). Furthermore, in more complex hydrogeologic regimes [e.g., highly heterogeneous granular settings (Type III), fractured bedrock or karst systems (Types IV and V)], plume shape may be dominated by macroscopic velocity variations, non-Darcian (fracture-based) flow, and matrix diffusion, and may bear little resemblance to the textbook Gaussian shapes.
The shape and extent of a groundwater plume may vary with time if the groundwater flow conditions are subject to changes, such as may stem from seasonally fluctuating infiltration. However, under reasonably steady flow conditions, and given sufficient time to satisfy the sorption capacity of the aquifer solids, groundwater plumes will appear to be fairly stationary. Under such conditions, the plume is fed by the source zone, which is losing mass at a sufficiently slow rate to appear constant over the time period of observation (perhaps several years). On the plume periphery, mass is lost or, more accurately, attenuated to below detection limits by groundwater dilution processes (advection and dispersion) or by transformation processes such as biodegradation.
It should be noted that sorption or diffusion of contaminants from the plume onto aquifer solids (and subsequent reverse diffusion) is common in many hydrogeologic settings. This phenomenon, which is shown schematically in Figure 2-17, may support the long-term contamination of groundwater. Indeed, it is thought
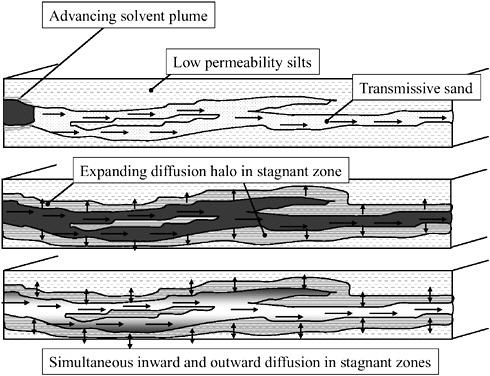
FIGURE 2-17 Conceptualization of reverse diffusion of contaminants from stagnant to transmissive zones after contact with a solvent-containing plume. SOURCE: Modified from Sale et al. (2004).
that the chronic low-level concentrations of chlorinated solvents produced by pump-and-treat systems can have more to do with reverse diffusion of this dispersed non-DNAPL mass than with discrete DNAPL or mass in the stagnant areas of the source zone (Liu and Ball, 2002). Although this sorbed or stagnant-zone mass can be a chronic supply of aqueous phase DNAPL constituents, it does not constitute a source zone as defined in Chapter 1, because it does not exist where the NAPL once was. Therefore, not all groundwater contaminant plumes imply the presence of a source zone.
CHEMICAL EXPLOSIVES
The chemical explosives that have caused the greatest environmental impact, and which are of greatest concern to the Army, are the organic explosives 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX),
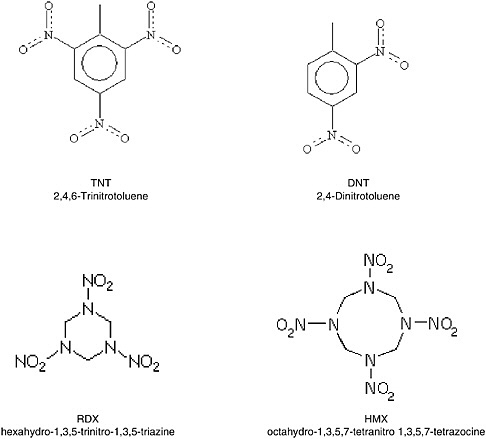
FIGURE 2-18 Structural diagrams of TNT, DNT, RDX, and HMX.
whose structures are shown in Figure 2-18.1 As with DNAPLs, the extent of subsurface contamination by chemical explosives is governed by the nature of the contaminant release and the physics of soil–chemical interactions in the subsurface. However, much less is known about how chemical explosive source material interacts with soil compared to chlorinated solvents. Hence, this section primarily describes the nature of the contaminant releases, highlighting several unique features that make sites contaminated with these compounds particularly challenging to remediate.
Table 2-2 shows the key properties of the predominant chemical explosives found as environmental pollutants. These materials are characterized by low
TABLE 2-2 Properties of Common Chemical Explosives
|
Explosive |
CAS # |
Aqueous Solubility @ 20°C (mg/L) |
Melting Point |
Log Kow |
|
TNT |
118-96-7 |
108 |
80°C |
1.86 |
|
2,4-DNT |
121-14-2 |
170 |
72°C |
1.98 |
|
RDX |
121-82-4 |
45 |
204°C |
0.86 |
|
HMX |
2691-41-0 |
3 |
286°C |
0.06 |
|
SOURCE: Rosenblatt et al. (1991). |
||||
aqueous solubilities, and they exist as solids at environmental temperatures. While the extent of sorption of explosives onto aquifer solids is dependent on many factors, the Kow values for each compound and the fraction and type of organic carbon present are particularly important and are used below to illustrate how the extent of sorption varies for the explosive compounds highlighted in this report. Given the Kow values in Table 2-2, sorption controls the fate and transport of TNT and DNT to a much greater degree than it does RDX and HMX, as observed at Department of Defense (DoD) sites that contain multiple contaminants (e.g., Louisiana AAP and Cornhusker AAP) in which groundwater plumes are most extensive for HMX, followed by RDX and then DNT and TNT. HMX is included in this discussion for completeness, although it rarely drives cleanup efforts due to its low toxicity, its less strict drinking water advisory levels, and its typically low concentrations found in the groundwater.
Explosives Releases
Environmental contamination by organic explosives at Army sites can be categorized by three generalized release mechanisms: (1) production process discharges, (2) manufacturing process discharges, and (3) military training and testing operations. More explosives problems are derived from historic production process discharges and from manufacturing processes (milling/machining or in demilitarization of ordnance) than from end-use detonations. This differentiates explosives sites from chlorinated solvent sites, most of which have arisen from spills or discharges of the final product during end-use applications.
Production Process Discharges
Explosives production operations have been performed at numerous facilities to support wartime needs and to create national security stockpiles. The explosives produced in the highest volume include TNT, RDX, and DNT. Within the Army, the principal production facilities for TNT were Volunteer AAP, Joliet
|
BOX 2-2 The Badger Army Ammunition Plant, built in 1942, was the principal DNT production facility in the United States until it changed to standby status in 1977. At this facility, waste pits were used to burn organic solvents, propellant wastes, and lumber. Three waste pits received up to 500 gallons (1893 L) per day of DNTs, solvents, and other constituents. Investigation of the waste pits showed concentrations of DNTs up to 28 percent. Interim remedial actions led to the excavation and incineration of the top 13–20 ft (4–6 m) of material from each waste pit. In addition, six soil vapor extraction wells installed at each waste pit removed 1,600 pounds (726 kg) of solvents. However, subsurface soils 15–25 ft (4.6–7.6 m) below the bottom of the waste pits still contained DNT well over 1 percent. Source treatment continues today at Badger with some success using in situ bioremediation along with in situ wetting to induce solid phase DNT mass transfer to soil pore water; however, delivery of nutrients and management of pH and nitrite are necessary to optimize field-scale biodegradation (Fortner et al., 2003). |
AAP, Radford AAP, Louisiana AAP, Longhorn AAP, Cornhusker AAP, and Iowa AAP. DNT was produced primarily at Badger AAP (see Box 2-2), while RDX was made at Holston AAP.
Trinitrotoluene (TNT). TNT is the most prevalent explosive used in military ordnance. TNT production in the United State occurs solely at military arsenals and peaked at 65 tons per day during World War II (Kaye, 1980). In a refined form, TNT is one of the most stable explosives and can be stored for long periods of time.
Commercial TNT production begins with a batch process through the sequential nitration of toluene. The first unit process produces mononitrotoluene (MNT or mono-oil) by the addition of nitric and sulfuric acids to toluene under heated conditions. The mono-oil is converted to dinitrotoluene (DNT or bi-oil) using nitric acid-fortified waste acid. In the final conversion to TNT, bi-oil was heated with oleum2-fortified sulfuric and nitric acids. In each step, the batch process produces a mixture of compounds. Thus, mono-oil and bi-oil are terms that include MNT and DNT, respectively, but also other manufacturing byproducts. Pre-product TNT is melted and washed with soda ash solution and then washed with sodium sulfite to separate 2,4,6-TNT from the other less desir-
able isomers. Waste acids from each process are routed for use in the previous batch step. Wash water from the final purification steps is routed to a “redwater” (meaning explosives-contaminated water) treatment area by a network of flumes (Urbanski, 1967a). In 1968, continuous manufacturing of TNT began at Radford AAP, where the nitric acid and oleum were introduced countercurrent in a six-stage process (Kaye, 1980). Both batch and continuous TNT production use water-filled drown tanks at each production house to stop the process if an out-of-control reaction begins. Leaks in material transfers between production houses and from storage in holding tanks, other spills, and discharges of material to the drown tanks could be significant sources of explosives contamination in the subsurface.
Historic literature uses the term “nitrobody” to represent the wide assortment of nitroaromatic molecules present in the production process prior to completion of the final product (TNT). The composition of the nitrobody in each stage of TNT production varies widely, as shown in Table 2-3 for the Radford AAP continuous production line.
The nitrobody production materials discharged to the drown tanks contained mono-oil, bi-oil, and TNT in mixtures of nitric and sulfuric acids and residual toluene, often at elevated temperatures. Evaluations have shown that the drown-tank material from stage 1 of the Joliet AAP batch process and from stages 1 and 2 of the Radford AAP continuous process were liquids containing 75 percent to 85 percent MNT (mostly 2-MNT and 4-MNT) (Persurance, 1974) that could possibly behave like DNAPLs. However, the specific gravity of MNTs ranges from 1.155 to 1.160 g/cm3 depending on the isomer, which is much less dense than chlorinated solvents (see Table 2-1). The nitrobody material from later stages in both processes was found to be a solid at ambient temperature. Unfortunately, there is no information on the frequency with which drown tanks were used among the batch or continuous operations nor on the intervals at which
TABLE 2-3 Composition of Nitrobody during Continuous TNT Production (percent).
|
|
Composition of Nitrobody, % |
|
||
|
Process |
MNT |
DNT |
TNT |
Temp (°C) |
|
1 |
77 |
18 |
4 |
50-55 |
|
2 |
0 |
71 |
29 |
70 |
|
3 |
0 |
30 |
69 |
80-85 |
|
4 |
0 |
10 |
90 |
90 |
|
5 |
0 |
2 |
98 |
95 |
|
6 |
0 |
0 |
100 |
100 |
|
SOURCE: Kaye (1980). |
||||
drown-tank materials were removed and destroyed. The physical–chemical properties of the TNT production-process discharge materials are further complicated by the high sulfuric acid content, which alters the density of the material and the solubility of the explosives compounds. The density of 100 percent sulfuric acid is 1.84 g/cm3 and 78 percent sulfuric acid has a density of 1.71 g/cm3. MNT isomers have a solubility of 34 percent in 90 percent sulfuric acid at 50°C; DNT isomer solubility is 20 percent in 90 percent sulfuric acid at 70°C; and TNT isomer solubility is 10 percent in 90 percent sulfuric acid at 80°C (Urbanski, 1967a). All these complexities make it very difficult to understand the miscibility of nitrobody production materials and to determine whether they consist of emulsions of separate nonaqueous phase liquids.
Thus, the physical–chemical properties of explosives material that might be released at TNT production facilities vary depending on the process stage and on the mixed acid content of the material, the completeness of the reactions in that stage, and the extent of dilution in drown tanks or waste lagoons. In some situations, a separate phase NAPL containing mostly MNTs may be present, and in others, a dense miscible phase liquid (DMPL) containing very high concentrations of MNT, DNT, and TNT may be present. At most explosives sites, there is limited information on these factors, making it difficult to assess the distribution of explosives in various hydrogeologic settings.
Dinitrotoluene (DNT). In general, the production of DNT mimics that of TNT, but the process stops after the second nitration. Thus, the factors that control the physical–chemical properties of any release material from DNT production are similar to those for TNT production. Nitration of MNT isomers produces various DNT isomers. For example, nitration of o-nitrotoluene produces 2,4-DNT and 2,6-DNT, while nitration of p-nitrotoluene produces only 2,4-DNT. Nitration of m-nitrotoluene produces 3,4-DNT, 2,3-DNT, and 3,6-DNT, all of which are undesirable in the production of 2,4,6-TNT. DNT production in the Army and the resulting subsurface contamination are discussed in Box 2-2.
RDX (Royal Demolition eXplosive/Research Demolition eXplosive). Cyclotrimethylenetrinitramine, hexahydro-1,3,5-trinitro-sym-triazine, and Cyclonite are all synonyms for RDX, which is a white crystalline solid with a nitrogen content of 37.84 percent. RDX is usually used in mixtures with other explosives, oils, or waxes. It has a high degree of stability in storage and is considered one of the most brisant3 of the military high explosives. Pure RDX is used in press-loaded projectiles. Cast loading is accomplished by blending RDX with a relatively low melting point substance. RDX is also used as a base charge in detonators and in blasting caps.
Just prior to and during World War II, numerous methods to synthesize RDX emerged. The first method was through the direct nitration of hexamine with nitric acid. The yield on this process was low, and in 1941 Americans and Germans simultaneously developed a method where hexamine dinitrate is reacted with ammonium dinitrate in the presence of acetic anhydride (Urbanski, 1967b). This process is the principal one used in the United States today, and it contains a constant impurity of 8 percent to 12 percent HMX (see below).
RDX was principally produced at the Holston Ordnance Works in Kingsport, Tennessee. Initial characterization efforts at this location have shown RDX in the groundwater, but only two sites located below production buildings have concentrations that imply a subsurface source zone (~ 2 percent to 4 percent of RDX’s aqueous solubility) (USACHPPM, 2003). RDX groundwater contamination is much more prevalent at other facilities where manufacturing process discharges occurred (as described below).
HMX (High Melt eXplosive or Her Majesty’s eXplosive). Cyclotetra-methylenetrinitramine, octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, and Octagen are all synonyms for HMX. HMX is used in military ordnance where the greatest explosive power per mass is needed. HMX is formed by the nitration of hexamine (or hexamethylenetetramine) in the presence of glacial acetic acid, acetic anhydride, ammonium nitrate, and nitric acid. The reaction produces a mixture of RDX and HMX, and the RDX is selectively destroyed by base hydrolysis. HMX is also a byproduct of production of RDX and has been produced at the Holston Ordnance Works. The groundwater investigations at Holston have found HMX in the same wells that RDX was found in, but at much lower concentrations and none over drinking water health advisory limits (USACHPPM, 2003).
Manufacturing Process Discharges
Manufacturing processes are defined here as post-production operations that operate with the solid phase explosive material. Examples include load, assemble, and pack facilities and demilitarization operations that remove the explosive fill from expired munitions. The most prevalent explosive fill material contains TNT and Comp B (60% RDX/40% TNT). Both operations typically use hot water or steam as a washdown or washout material. Spent cleaning solutions are typically filtered with coarse fabric to remove the suspended particulates and then are discharged via pipelines, open flumes, or ditches to infiltration or evaporation ponds. Continuous releases of aqueous solutions containing explosives as solutes can infiltrate into soils, causing large areas of contamination.
The Umatilla Army Depot provides an example, where from about 1955 to 1965 a munitions washout facility was operated where hot water and steam were used to remove explosives from munitions bodies. An estimated 85 million gallons (322 million L) of wash water containing TNT and RDX was discharged to
two surface impoundments that covered about half an acre (0.2 hectares). Source control measures began by excavating the top 20 feet (6 m) of soil for treatment by composting. The remaining 30 feet (9 m) of soil above the water table is being treated with in situ flushing using groundwater pump and granular activated carbon treatment.
Military Training and Testing Operations
Military training and testing operations relevant to the release of explosive materials include live fire exercises that use military ordnance. Detonation of military ordnance typically consumes the majority of the explosive fill. Experimental characterization of detonations of 81- and 120-mm mortars and 105-mm artillery showed only trace residues of TNT, RDX, and HMX present on the ground surface (Jenkins et al., 2001). However, a low-order detonation can distribute solid phase energetic material onto and into near-surface soil (DeLaney et al., 2003).
The low-order detonation process is very ill-defined, but it is generally characterized by initiation of detonation, which recedes before consumption of all of the explosive fill. Rupture of the case distributes large chunks to small particulates, and it can occur above the soil surface or in ground after impact. In addition, Explosive Ordnance Disposal methods (e.g., open detonation) to safely dispose of unexploded ordnance can also cause incomplete detonation of target objects (Lewis et al., 2003). These detonation methods have not been fully characterized for energetic material releases.
Chemical Explosives Fate in the Subsurface
The above sections have noted the various materials that might be released from military operations involving chemical explosives. For production and manufacturing process discharges, aqueous solutions are the most common type of waste. Under circumstances where the environmental temperatures are significantly lower than the discharge water temperatures, explosives may precipitate out of solution and create a separate solid phase material in the soil. For example, the aqueous solubility of TNT drops from 250 mg/L at 40°C to 110 mg/L at 20°C. Similarly, the solubility of RDX is 115 mg/L at 40°C and 45 mg/L at 20°C. Precipitation of explosive solids following production and manufacturing process discharges most likely occurs in the near soil surface (< 20 feet or 6 m), such that surface excavation and treatment (e.g., via incineration or composting) are effective remediation strategies. It is possible that leaks or spills from pipelines and flumes, and infiltration from unlined ditches, could lead to contaminant accumulation in unsaturated soil pores and fracture matrices and thus provide a long-term reservoir of contamination to groundwater.
As mentioned previously, some highly concentrated wastes in production
process discharges might act like DNAPLs or DMPLs. However, even heated and concentrated production materials that might initially behave like a DNAPL are likely to undergo significant change once introduced into soil, where environmental conditions would tend to decrease both temperature and acidity, promoting the creation of a separate solid phase material. Of course, recharge of the subsurface during rain events can lead to dissolution of solid phase explosives and subsequent transfer of explosives mass to soil pore water and perhaps groundwater. Subsequent dilution of the more soluble, and/or degradation of the more labile, compounds could over long periods of time (decades) result in a mixture with a very different signature than the original release materials.
Rainfall can dissolve the solid phase energetic material from the detonation of military ordnance and transfer the mass to soil pore water. The potential contaminant threat from detonation of military ordnance is determined by the depth to groundwater and the various contributions of recharge, dissolution, sorption, and degradation rates. The nature and the impact of this type of source are only beginning to be understood, and current programs are in progress to improve scientific understanding and develop mitigation approaches (SERDP, 2003). Management of these source areas is compounded by the presence of unexploded ordnance and continued operations.
Once in the subsurface, biotic and abiotic redox reactions are the principal processes that degrade the chemical explosives discussed above, although there are significant differences within this group. TNT is rapidly transformed to mono-and then di-aminonitrotoluenes by naturally occurring microorganisms and soil minerals under both aerobic and anaerobic conditions (Ahmad and Hughes, 2000). TNT biodegradation appears to be mostly cometabolic. Reduction rates decrease with the successive reduction of each nitro group due to the destabilization of the aromatic ring and a decrease in the electrophilic nature of the remaining nitro groups. A portion of the aminonitrotoluenes can continue to participate in reactions with soil organic matter, becoming covalently bound in a multistep humification process (Thorne and Leggett, 1999). 2,4-DNT and the 2,6-DNT manufacturing impurity can be biodegraded under aerobic conditions where specific bacteria use these materials for carbon, nitrogen, and energy sources (Fortner et al., 2003). Environmental reactions with RDX are strongest under reducing conditions that sequentially reduce the nitro groups to mono-, di-, and trinitroso products of RDX, followed by ring cleavage to produce a variety of short-chain compounds (Hawari, 2000). Very little work has been done to understand the fate of HMX in the subsurface, although a similar sequential reduction of the nitro groups followed by ring cleavage, as with RDX, is hypothesized.
Natural attenuation mechanisms favor the loss of TNT>DNT>RDX>HMX in the environment. TNT sorption and aerobic degradation provides for continuous elimination reactions. However, DNT appears to require nutrients and nitrite byproduct elimination to support significant biodegradation. RDX and HMX both sorb poorly to soils and require strongly reducing conditions for natural
elimination reactions. For this reason, and considering the toxicity of RDX, RDX has become one of the more challenging organic explosives for environmental remediation.
Field-Scale Distribution of Explosives in Source Zones
At this time, characterization of chemical explosive source zones is immature compared to the knowledge base for DNAPLs, primarily because when the major production operations ceased 20–30 years ago, the knowledge of past waste management practices and the physicochemical properties of the explosive production mixtures faded. Distinct source zones of explosives generally contain solid phase material, although for TNT production, the presence of mono-oil and bi-oil material as a significant source material has been speculated. Explosive debris distributed on or in surface soils by detonations is emerging as a potential source material at military training and testing ranges.
How chemical explosives might be distributed in the five hydrogeologic settings described earlier is difficult to determine at this time. The presence of reprecipitated solid phase explosive compounds in granular media is suspected, based on phase partitioning laws and maximum aqueous phase limits. But the dynamics of explosive material reprecipitation, dissolution, and transport that would define source zone architecture are not well understood. In addition, no work has been performed to understand the miscible/immiscible flow characteristics of production process wastes in drown tanks or disposed of in surface impoundments.
SUMMARY
This chapter has outlined important physical and chemical features of contaminated sites that should be understood (or at a minimum discussed) prior to any site remediation. First and foremost, it is imperative to be able to categorize the hydrogeologic setting of a site, as this plays a significant role in determining the overall subsurface distribution of contamination. Furthermore, the existing hydrogeologic setting limits both the types of tools that can be used to characterize the source zone and the technologies that might achieve reductions in source mass. Given the combination of heterogeneity in hydrogeology and in physical-chemical properties, complex sites are the norm rather than the exception.
Chlorinated solvents that exist as DNAPLs in the subsurface are the primary concern of the Army and many other potentially responsible parties, and thus constitute the major focus of this report. Among the many distinguishing features of DNAPL sites is the fact that the distribution of DNAPL in the subsurface is typically sparse and highly heterogeneous (depending on the site hydrogeology). Furthermore, depending on a site’s porosity, permeability, and sorption capacity, a substantial portion of the contaminant mass that might have been released to the
subsurface as a DNAPL may transition into stagnant zones as either sorbed or dissolved phase contamination. These sources have the potential to be a chronic supply of contamination to groundwater plumes.
In comparison to DNAPLs, the state of the art for explosives source zone characterization is quite immature. Most explosives are released to the environment as aqueous mixtures, from which chemical explosives precipitate out. The source zone architecture created by production process discharges, manufacturing process discharges, and military training and testing operations requires scientific investigation before remediation technologies can be considered, designed, and deployed with confidence. In addition, an important constraint not found with DNAPLs is explosives safety. Management of detonation hazards, especially the drilling and handling of source material, will require additional resources and technologies.
Even though five hydrogeologic settings are discussed in the chapter, there are many more than five typical contaminant distributions. A site’s contaminant distribution will be influenced by transformation and transport processes, by the nature of the contaminant release, and by the hydrogeologic setting (or combination of settings). Thus, this chapter should not be viewed as a cookbook for how to categorize sites and determine their contaminant distribution. Rather, source characterization is necessary, as discussed in the next chapter.
REFERENCES
Adamson, A. W., and A. P. Gast. 1997. Physical Chemistry of Surfaces, 6th ed. New York: Wiley.
Ahmad, F, and J. B. Hughes. 2000. Anaerobic Transformation of TNT by Clostridium. In: Biodegradation of Nitroaromatic Compounds and Explosives. J. C. Spain, J. B. Hughes, and H. Knackmuss (eds.). Boca Raton, FL: Lewis Publishers.
Anderson, W. G. 1987. Wettability literature survey—part 4: effects of wettability on capillary pressure. J. Pet. Technol. 39:1283–1300.
Back, W., J. S. Rosenshein, and P. R. Seaber. 1988. Hydrogeology—The Geology of North America Volume O-2. Boulder, CO: Geological Society of America.
Ball, W. P., C. Liu, G. Xia, and D. F. Young. 1997. A diffusion-based interpretation of tetrachloroethene and trichloroethene concentration profiles in a groundwater aquitard. Water Resources Research 33(12):2741–2758.
Bradford, S. A., R. Vendlinski, and L. M. Abriola. 1999. The entrapment and long-term dissolution of tetrachloroethylene in fractional wettability porous media. Water Resources Research 35(10):2955–2964.
Brewster, M. L., A. P. Annan, J. P. Grenhouse, B. H. Kueper, G. R. Olhoeft, J. D. Redman, and K. A. Sander. 1995. Observed migration of a controlled DNAPL release by geophysical methods. Groundwater 33(6):977–987.
Cohen, R. M., J. W. Mercer, and J. Matthews. 1993. DNAPL Site Evaluation. C. K. Smoley (ed.). Boca Raton, FL: CRC Press.
Conrad, S. H., E. F. Hagan, and J. L. Wilson. 1987. Why are residual saturation of organic liquids different above and below the water table? In: Proceedings—Petroleum Hydrocarbons and Organic Chemicals in Groundwater: Prevention, Detection and Restoration. Worthington, OH: National Water Well Association.
Cope, N., and J. B. Hughes. 2001. Biologically-enhanced removal of PCE from NAPL source zones. Environ. Sci. Tech. 35(10):2014–2021.
De la Paz, T., and T. Zondlo. 2003. DNAPLs in Karst the Redstone Experience. Presentation to the Committee on Source Removal of Contaminants in the Subsurface. January 30, 2003, San Antonio, Texas.
DeLaney, J. E., M. Hollander, H. Q. Dinh, W. Davis, J. C. Pennington, S. Taylor, and C. A. Hayes. 2003. Characterization of explosives residues from controlled detonations: low-order detonations. Chapter 5, Distribution and fate of energetics on DoD test and training ranges: Report 3, ERDC TR-03-2. Vicksburg, MS: U. S. Army Engineer Research and Development Center, Environmental Laboratory.
Domenico, P. A., and F. W. Schwartz. 1998. Physical and Chemical Hydrogeology, 2nd ed. New York: John Wiley & Sons.
Environmental Protection Agency (EPA). 1992. Evaluation of Ground-Water Extraction Remedies: Phase II, Volume I—Summary Report. Publication 9355.4-05. Washington, DC: EPA Office of Emergency and Remedial Response.
Fortner, J. D, C. Zhang, J. C. Spain, and J. B. Hughes. 2003. Soil Column Evaluation of Factors Controlling Biodegradation of DNT in the Vadose Zone. Environ. Sci. Technol. 37(15):3382–3391.
Freeze, R. A., and J. A. Cherry. 1979. Groundwater. New Jersey: Prentice-Hall.
Hawari, J. 2000. Biodegradation of RDX and HMX: from basic research to field application. In: Biodegradation of Nitroaromatic Compounds and Explosives. J. C. Spain, J. B. Hughes, and H. Knackmuss (eds.). Boca Raton, FL: Lewis Publishers.
Hiemenz, P. C., and R. Rajagopalan. 1997. Principles of Colloid and Surface Chemistry, 3rd ed. New York: Marcel Dekker.
Huang, W. L., and W. J. Weber. 1998. A distributed reactivity model for sorption by soils and sediments. 11. Slow concentration dependent sorption rates. Environ. Sci Technol. 32(22):3549–3555.
Jenkins, T. F., J. C. Pennington, T. A. Ranney, T. E. Berry, P. H. Miyares, M. E. Walsh, A. D. Hewitt, N. M. Perron, L. V. Parker, C. A. Hayes, and E. G. Wahlgren. 2001. Characterization of Explosives Contamination at Military Firing Ranges. ERDC TR-01-5. Vicksburg, MS: U. S. Army Engineer Research and Development Center.
Kaye, S. M. 1980. Encyclopedia of Explosives and Related Items. PATR 2700, Volume 9. Dover, New Jersey: U.S. Army Armament Research and Development Command, Large Caliber, Weapon Systems Laboratory.
Kueper, B. H., and D. B. McWhorter. 1991. The behavior of dense nonaqueous phase liquids in fractured clay and rock. Journal of Ground Water 29(5):716–728.
Kueper, B. H., D. Redman, R. C. Starr, S. Reitsma, and M. Mah. 1993. A field experiment to study the behavior of tetrachloroethylene below the water table: spatial distribution of pooled DNAPL. Groundwater 31:756–766.
Kueper, B. H., and E. O. Frind. 1991a. Two phase flow in heterogeneous porous media. 1. Model development. Water Resources Research 27(6):1049–1057.
Kueper, B. H., and E. O. Frind. 1991b. Two phase flow in heterogeneous porous media. 2. Model application. Water Resources Research 27(6):1058–1070.
Lemke, L. D., L. M. Abriola, and J. R. Lang. 2004. DNAPL source zone remediation: Influence of hydraulic property correlation on predicted source zone architecture, DNAPL recovery, and contaminant mass flux. Water Resources Research. In press.
Lewis, J., S. Thiboutot, G. Ampleman, S. Brochu, P. Brousseau, J. C. Pennington, and T. A. Ranney. 2003. Open detonation of military munitions on snow: An investigation of energetic material residues produced. Chapter 4, Distribution and fate of energetics on DoD test and training ranges: Report 3, ERDC TR-03-2. Vicksburg, MS: U.S. Army Engineer Research and Development Center, Environmental Laboratory.
Liu, C., and W. P. Ball. 1998. Analytical modeling of diffusion-limited contamination and decontamination in a two-layer porous medium. Advances in Water Resources 24(4):297–313.
Liu, C., and W. P. Ball. 2002. Back diffusion of chlorinated solvents from a natural aquitard to a remediated aquifer under well-controlled field conditions: predictions and measurements. Journal of Groundwater 40(2):175–184.
Mackay, D. M., W. Y. Shiu, and K. C. Ma. 1993. Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, Vol. III. Chelsea, MI: Lewis Publishers.
Mackay, D. M., R. D. Wilson, M. P. Brown, W. P. Ball, G. Xia, and D. P. Durfee. 2000. A controlled field evaluation of continuous versus pulsed pump-and-treat remediation of a VOC-contaminated aquifer: site characterization, experimental setup, and overview of results. Journal of Contaminant Hydrology 41:81–131.
Meinardus, H. W., V. Dwarakanath, J. Ewing, G. J. Hirasaki, R. E. Jackson, M. Jin, J. S. Ginn, J. T. Londergan, C. A. Miller, and G. A. Pope. 2002. Performance assessment of NAPL remediation in heterogeneous alluvium. Journal of Contaminant Hydrology 54:173–193.
Mercer, J. W., and R. M. Cohen. 1990. A review of immiscible fluids in the subsurface: properties, models, characterization and remediation. Journal of Contaminant Hydrology 6:107–163.
Montgomery, J. H. 2000. Groundwater Chemicals, 3rd ed. Boca Raton, FL: Lewis Publishers and CRC Press.
Morrow, N. R. 1976. Capillary-pressure correlations for uniformly wetted porous media. Journal of Canadian Petroleum Technology 15(4):49–69.
O’Carroll, D. M., S. A. Bradford, and L. M. Abriola. 2004. Infiltration of PCE in a system containing spatial wettability variations. Journal of Contaminant Hydrology 73:39-69.
Pankow, J. F., and J. A. Cherry (eds.). 1996. Dense Chlorinated Solvents and other DNAPLs in Groundwater. Portland, OR: Waterloo Press.
Parker, B. L., D. B, McWhorter, and J. A. Cherry. 1997. Diffusive loss of non-aqueous phase organic solvents from idealized fracture networks in geologic media. Ground Water 35(6):1077–1088.
Parker, B. L., J. A. Cherry, and R. W. Gillham. 1996. The effect of molecular diffusion on dnapl behavior in fractured porous media. Chapter 12 In: Dense Chlorinated Solvents and Other DNAPLs in Groundwater. J. F. Pankow and J. A. Cherry (eds.). Portland, OR: Waterloo Press.
Parker, B. L., R. W. Gillham, and J. A. Cherry. 1994. Diffusive disappearance of immiscible–phase organic liquids in fractured geologic media. Journal of Groundwater 32(5):805–820.
Persurance, R. 1974. Explosion Hazard Classification of Drowning Tank Material from TNT Manufacturing Process. Picatinny Arsenal Technical Report 4613. Dover, NJ.
Poulsen, M. M., and B. H. Kueper. 1992. A field experiment to study the behavior of tetrachloroethylene in unsaturated porous media. Environ. Sci. Technol. 26(5):889–895.
Powers, S. E., L. M. Abriola, and W. J. Weber, Jr. 1994. An experimental investigation of NAPL dissolution in saturated subsurface systems: transient mass transfer rates. Water Resources Research 30(2):321–332.
Powers, S. E., W. H. Anckner, and T. F. Seacord. 1996. Wettability of NAPL-contaminated sands. Journal of Environmental Engineering 122:889–896.
Powers, S. E., L. M. Abriola, and W. J. Weber, Jr. 1992. An experimental investigation of NAPL dissolution in saturated subsurface systems: steady-state mass transfer rates. Water Resources Research 28(10):2691–2705.
Press, F., and R. Siever. 1974. Earth. San Francisco, CA: W. H. Freeman and Company.
Rathfelder, K. M., L. M. Abriola, M. A. Singletary, and K. D. Pennell. 2003. Influence of surfactant-facilitated interfacial tension reduction on organic liquid migration in porous media: observations and numerical simulation. J. Contaminant Hydrology 64(3-4):227–252.
Robertson, B. K., and M. Alexander. 1996. Mitigating toxicity to permit bioremediation of constituents of nonaqueous-phase liquids. Environ. Sci. Tech. 30:2066–2070.
Rosenblatt, D. H., E. P. Burrows, W. K. Mitchell, and D. L. Parmer. 1991. Organic Explosives and Related Compounds. In The Handbook of Environmental Chemistry, Vol 3. G. O. Hutzinger (ed.). Berlin and Heidelberg: Springer-Verlag.
Salathiel, R. A.. 1973. Oil recovery by surface film drainage in mixed-wettability rocks. J. Pet. Technol. 25(OCT):1216–1224.
Sale, T., T. Illangasekare, F. Marinelli, B. Wilkins, D. Rodriguez and B. Twitchell. 2004. AFCEE Source Zone Initiative—Year One Progress Report. Colorado State University and Colorado School of Mines, Prepared for the Air Force Center for Environmental Excellence.
Schwille, F. 1988. Dense Chlorinated Solvents in Porous and Fractured Media. Translated by J. F. Pankow. Chelsea, MI: Lewis Publishers.
SERDP. 2003. Annual Report to Congress—Fiscal Year 2002, from the Strategic Environmental Research and Development Program. Arlington, VA: SERDP Program Office.
Sudicky, E. A. 1986. A natural gradient experiment on solute transport in a sand aquifer: spatial variability of hydraulic conductivity and its role in the dispersion process. Water Resources Research 22(13):2069–2082.
Sudicky, E. A., R. W. Gillham, and E. O. Frind. 1985. Experimental investigations of solute transport in stratified porous media: (1) the non reactive case. Water Resource Research 21(7):1035–1041.
Sung, Y., K. M. Ritalahti, R. A. Sanford, J. W. Urbance, S. J. Flynn, J. M. Tiedje, and F. E. Loffler. 2003. Characterization of two tetrachloroethene (PCE)-reducing, acetate-oxidizing anaerobic bacteria, and their description as Desulfuromonas michiganensis sp. nov. Applied and Environmental Microbiology 69:2694–2974.
Teutsch, G., and M. Sauter. 1991. Groundwater modeling in karst terranes: scale effects, data acquisition and field validation. Pp. 17–35 In: Proceedings of the Third Conference on Hydrogeology, Ecology, Monitoring, and Management of Ground Water in Karst Terranes, Nashville, TN.
Thorne, P. G., and D. C. Leggett. 1999. Investigations of explosives and their conjugated transformation products in biotreatment matrices. Special Report 99-3. U.S. Army Corps of Engineers, Cold Regions Research and Engineering Laboratory. Hanover, NH: Army Corps of Engineers.
Urbanski, T. 1967a. Chemistry and Technology of Explosives, Vol. 1. Oxford: Pergamon Press.
Urbanski, T. 1967b. Chemistry and Technology of Explosives, Vol. 3. Oxford: Pergamon Press.
USACHPPM. 2003. Interim Measures Report, Site-Wide Groundwater, Area B (Explosives Production Area), 28 May through 13 June 2003, Holston Army Ammunition Plant, Kingsport, Tennessee. Aberdeen Proving Ground, MD: U.S. Army Center for Health Promotion and Preventative Medicine, Ground Water and Solid Waste Program.
Weber, W. J., W. L. Huang, and E. J. LeBoeuf. 1999. Geosorbent organic matter and its relationship to the binding and sequestration of organic contaminants. Colloids and Surfaces A-Physicochemical and Engineering Aspects 151 (1–2):167–17.
White, W. B. 2002. Karst hydrology: recent developments and open questions. Engineering Geology 65(2–3):85–105
White, W. B. 1998. Groundwater flow in karstic aquifers. Pp. 18–36 In: The Handbook of Groundwater Engineering. J. W. Delleur (ed.). Boca Raton, FL: CRC Press.
Yang, Y., and P. L. McCarty. 2000. Biologically enhanced dissolution of tetrachloroethene DNAPL. Environ. Sci. Tech. 34(14):2979–2984.
Yang, Y., and P. L. McCarty. 2002. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environ. Sci. Tech. 36:3400–3404.