Summary and Assessment1
Most infectious disease experts believe that the world stands on the verge of an influenza pandemic (Chen et al., 2004; WHO, 2004a; Webby and Webster, 2003). Yet despite the legacy of the 1918 “Spanish flu,” estimated to have killed at least 20 million people,2 and the additional deaths, social disruption, and economic losses that resulted from pandemics in 1957 and 1968, the general public appears relatively unconcerned about the next “killer flu.” Considerably more attention has been focused on protecting the public from terrorist attacks than from the far more likely and pervasive threat of pandemic influenza—an event conservatively expected to cause between 2 and 8 million deaths (WHO, 2004a).
Meanwhile, the danger mounts as the world’s capacity to produce vaccines shrinks and H5N1 reaches endemic levels in poultry in many parts of Asia. A recent expert consultation convened by the World Health Organization (WHO) concluded that “the unpredictability of influenza viruses and the speed with which transmissibility can improve means that the time for preparedness planning is now” (WHO, 2004a).
To address these urgent concerns, the Institute of Medicine’s (IOM) Forum on Microbial Threats convened the workshop Pandemic Influenza:
|
1 |
The assessments contained in the summary are based on the presentations and discussion periods of the workshop. They reflect the assessments of individuals and the editors and cannot be construed as the deliberations, consensus, or recommendations of a formally constituted study committee of the Institute of Medicine. |
|
2 |
For a more detailed description of how estimates have been determined for the numbers of deaths caused by the 1918 influenza outbreak, see Barry’s section in Chapter 1. |
Assessing Capabilities for Prevention and Response on June 16 and 17, 2004. Participants discussed the history of influenza pandemics and the potentially valuable lessons it holds; the 2003–2004 H5N1 avian influenza outbreak in Asia and its implications for human health; ongoing pandemic influenza preparedness planning at global, regional, national, state, and local levels; strategies for preventing and controlling avian influenza and its transmission within bird and animal populations; and a broad range of medical, technical, social, economic and political opportunities for pandemic preparedness, as well as the many obstacles that stand in the way of this goal.
ORGANIZATION OF WORKSHOP SUMMARY
This workshop summary report is prepared for the Forum membership in the name of the editors as a collection of individually authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the editors and not those of the Forum on Microbial Threats, its sponsors, or the Institute of Medicine. The contents of the unattributed sections are based on the presentations and discussions that took place during the workshop.
The workshop summary is organized within chapters as a topic-by-topic description of the presentations and discussions. Its purpose is to present lessons from relevant experience, delineate a range of pivotal issues and their respective problems, and put forth some potential responses as described by the workshop participants.
Although this workshop summary provides an account of the individual presentations, it also reflects an important aspect of the Forum philosophy. The workshop functions as a dialogue among representatives from different sectors and presents their beliefs on which areas may merit further attention. However, the reader should be aware that the material presented here expresses the views and opinions of the individuals participating in the workshop and not the deliberations of a formally constituted IOM study committee. These proceedings summarize only what participants stated in the workshop and are not intended to be an exhaustive exploration of the subject matter or a representation of consensus evaluation.
SECURING THE FUTURE
Over the course of 2 days of wide-ranging, intense, and detailed discussion, several themes recurred and were elaborated upon from multiple perspectives. By the end of the proceedings, many of these ideas were surrounded by considerable clarity and a sense of urgency. These pervasive observations, described below, are grouped according to their ability to be
accomplished in the near term or, following additional research or resolution, in the future. What can be said and was echoed throughout the discussions—if the question is: “Are we ready for a pandemic influenza?,” the answer is “no.”
Addressing Unmet Needs
Close Gaps in Global Surveillance3
Many countries lack infectious disease surveillance capabilities. Disturbingly, some of the most glaring gaps in surveillance occur in Asia, where H5N1 avian influenza has infected and killed scores of people since 1997. Developed countries’ interests would be well served by funding improved influenza surveillance in such flu “hot spots.” In addition to increasing surveillance capacity, replacing the current economic disincentives to early reporting of disease with incentives for surveillance, timely disease detection, and access to vaccines and antivirals will greatly increase the chance of catching and containing an emerging pandemic strain before or soon after it emerges.
U.S. data on severe illness and death from influenza are also inadequate. Improved data would more effectively inform priorities for prevention and treatment investments and strategies made at the local, state, regional, and national levels (e.g., immunization and preparedness planning). Importantly, improved real-time surveillance and disease reporting could provide an early warning for an emerging pandemic outbreak.
Integrate Animal and Public Health Communities4
Influenza surveillance, research, and pandemic response planning should reflect the zoonotic nature of the disease. Improved communication and the development of professional relationships among veterinary and medical researchers and agriculture and public health officials would encourage a greater appreciation in both communities for the implications of animal diseases in human populations, and for human practices that promote or prevent zoonoses. Current lack of integrated funding for influenza surveillance within the animal and human populations collectively now
works against such integration. Better coordination between public and private funders of research and disease surveillance will be necessary.
Explore Compensation for Preemptive Culling of Animals5
As is the case with surveillance, encouraging farmers (or even entire countries) to curtail or prevent a human pandemic by sacrificing their poultry or livestock is in the interests of global public health. A variety of options should be explored to support this outcome in a variety of settings, from individual farmers in low-resource settings to industrial poultry and livestock producers in wealthy countries.
Promote the Use of Rapid, Inexpensive Influenza Diagnostics6
Cheap, simple diagnostic tests would improve influenza surveillance in animals and humans. Polymerase chain reaction (PCR) testing is the best current option, but the international veterinary community has yet to adopt PCR. As a result, the first farm to be culled in the recent H7N7 outbreak in The Netherlands was delayed 4 days as officials waited for virus isolation results. Increased use and improved diagnostics for influenza will also promote more prudent and effective use of both vaccines and antiviral drugs.
Increase Demand for Annual Influenza Immunization and Antiviral Therapy and Prophylaxis7
Demand for influenza vaccine drives supply. After last year’s severe flu season and this year’s unanticipated vaccine shortages, the public may respond well to a pro-immunization campaign, perhaps one that introduces the hazards of pandemic influenza. It will be important to include in that message the distinction between the protective effect of an antiviral influenza vaccine and additional vaccination that would be necessary to respond to a pandemic strain. A similar argument can be made for increasing interpandemic demand for antiviral drugs, which to date have low demand. More interpandemic use of antivirals means the greater production and greater supply of them for use in an outbreak situation. Moreover, increasing physician experience with and public awareness of antiviral medications should support their effective use in responding to a pandemic.
|
5 |
Buranathai (2004); Meltzer (2004); Soebandrio (2004); Webster (2004a). |
|
6 |
Koch (2004); Nguyen (2004); Swayne (2004); Webster (2004a); see Perdue in Chapter 5. |
|
7 |
Brown (2004); Fedson (2004a); Gellin (2004); Hosbach (2004); Nowak (2004). |
Create International Stockpiles of Antiviral Drugs and Vaccines8
A dedicated supply of vaccines and antiviral drugs is necessary for a rapid response to the first cases of a potential pandemic influenza strain (e.g., through ring immunization and/or targeted antiviral prophylaxis). This plan would probably require a smaller investment, and possibly offers greater benefit in relation to cost, than the aforementioned strategy of compensating farmers for preemptive culling of poultry or livestock in areas affected by avian influenza. However, this strategy is unlikely to work unless an international agreement to create such stockpiles is in place when the next pandemic arrives; otherwise, stockpiles and production of vaccines and antiviral drugs are expected to be nationalized. Additionally, these antiviral stockpiles need to be placed in geographically high-volume points of care (e.g., outpatient clinics, emergency rooms, occupational health sites, student health facilities, nursing homes, pharmacies) for rapid access to therapy that does not rely on a visit to a physician for an effective pandemic response. If stockpiles of vaccines are to be developed and relied upon, it is clear that the range of factors contributing to the recent crises in seasonal influenza production and deployment will need to be overcome.
Establish Protocols for Research During a Pandemic9
When the next influenza pandemic emerges, it will be essential to gain a greater understanding of the clinical, epidemiological, and biological nature of influenza—but this will only be possible if research protocols and the laboratory networks to pursue them are established before a pandemic strikes. As Klaus Stöhr of WHO observed, “We have to invest more into planning research, into having protocols ready, and having networks of scientists in place and eager to contribute before the next pandemic virus emerges.” For example, protocols to estimate vaccine efficacy could be implemented immediately upon the commencement of immunization in response to a pandemic, and could even be conducted during the annual flu season.
Goals for Research
Determine the Molecular Basis of Influenza Pathogenesis10
Much remains to be understood about the molecular basis of influenza pathogenesis, host immune response, immune protection, immune enhance-
|
8 |
Brown (2004); Gellin (2004); Hosbach (2004); Longini (2004a); Stohr (2004). |
|
9 |
Grundy (2004); see Hayden in Chapter 3. |
|
10 |
Taubenberger (2004); Webster (2004a). |
ment, virulence, and transmissibility. H5N1 variants provide an opportunity to study all of these phenomena. Breakthroughs in these areas of scientific understanding could rapidly lead to more effective and more easily produced countermeasures to an influenza pandemic.
Predict Pandemic Potential of Influenza Isolates11
As knowledge of the molecular pathology of influenza expands, it should become possible to predict the threat posed by a particular strain by analyzing key sequences in its genome. While there has been one probable case of human-to-human transmission (ProMED-mail, 2004e) to date, the fact that H5N1 has not yet accomplished infectious human-to-human transmission begs the question, “why not?” Risk assessment tools based on influenza viral genomics may one day provide an answer—and perhaps prevent the unnecessary culling of poultry or livestock following outbreaks of avian influenza.
Increase the Efficacy of Influenza Vaccines12
Limited supplies of vaccine could go further if their antigen content could be adjusted to provide the lowest effective dose to each recipient, and if they could be safely made more effective with an adjuvant. Several participants suggested the need for the United States and Europe to view this problem as a joint effort and work together to assure that the entire set of needs for improving influenza vaccines is addressed and shared.
An atmosphere of 11th-hour urgency surrounded many of the workshop presentations and participant discussions. The potential for catastrophe is immense, but that potential has been evident, and largely ignored, since 1918. The power of vaccines to prevent influenza is well proven, but the capacity to produce them—as recent events confirm—is limited so as to put them out of reach of the vast majority of the global population. If the initial cases of an emerging human influenza strain are detected, and if antiviral drugs were quickly administered to the close contacts of index cases, transmission could be stifled—but those are big “ifs” in a world where early reporting of influenza carries dire economic consequences and where nations are expected to nationalize stockpiles and production of antiviral drugs and vaccines in response to a threatened pandemic. What should be done to prevent the loss of millions of lives, and the evidence for doing it, is quite clear. What is missing—as evidenced by the clarion calls of
workshop presenters—is the political will to support such efforts before the next pandemic renders them futile. However, developments during the writing of this report might suggest that the tide is changing. The World Health Organization has called for an unprecedented summit of national public health leaders, vaccine manufacturers, and leading researchers to expand the plans and possibilities for responding to a pandemic influenza threat—now a growing concern among many nations and leaders (see, http://www.who.int/en/) (Marchione, 2004).
THE STORY OF INFLUENZA: 1918 AND BEYOND
To expand on some of the key messages described above, the following text summarizes workshop presentations and discussions concerning preparedness for influenza outbreaks at every level of government and society and the prospects for preventing or mitigating the next pandemic.
Although historical evidence of probable encounters with virulent influenza date back to the 16th century, chronicles of the disease often begin with the 1918 pandemic (see Barry in Chapter 1). By that time, science was sufficiently sophisticated to characterize the most lethal infectious outbreak in recorded history, and even to anticipate that such an event would occur. As a result of its staggering mortality, the brunt of which was borne by young adults, the 1918 influenza pandemic remains a focus of scientific inquiry; the origin of the virus remains to be determined. Most recently, the “source” of its exceptional virulence has been discovered, and these findings suggest it is due to the hemagglutinin (HA) gene (Kobasa et al., 2004). Workshop participants discussed progress to date in addressing these critical issues. They also considered the consequences of deeply flawed public and official responses to the 1918 flu and their implications for the management of future pandemics and other public health crises.
Current estimates place the death toll from the approximately year-long 1918 pandemic at 50 to 100 million.13 A “herald wave” of influenza in the spring of that year produced a relatively mild disease, as described in Western medical journals (Taubenberger, 2004). The second pandemic wave struck violently in early autumn, spreading and killing with astounding rapidity. The unusually severe symptoms of this so-called Spanish flu included cyanosis, internal and external hemorrhage, and intense pain (see Barry in Chapter 1). Limited reliable mortality statistics from the United States show that the highest number of flu deaths occurred in people aged 25 to 29 years and that more than twice as many people aged 20 to 34 died
|
13 |
For a more detailed description of how estimates have been determined for the numbers of deaths caused by the 1918 influenza outbreak, see Barry’s section in Chapter 1. |
than did people older than 50 (see Barry in Chapter 1). Pregnant women had the highest case fatality (the number of deaths among people with clinically diagnosed illness) of any group in this country, a phenomenon that has been reported in other influenza outbreaks (see Barry in Chapter 1). Local estimates of case fatality varied widely across the globe and in some circumstances (e.g., among populations never before challenged by influenza and troops stationed in close quarters) reportedly exceeded 20 percent.
Patterns of Pandemic-Associated Mortality
Despite the devastation caused by the 1918 virus, it produced what was in many ways a typical influenza pandemic (see Taubenberger in Chapter 1) (Taubenberger, 2004). Most pandemics arrive in waves, albeit generally separated by years, rather than months. In the United States, with an aggregate case fatality of 2.5 percent, more than 97 percent of people with clinically reported influenza recovered from the disease; serological studies, conducted in the 1930s on people alive during the pandemic, suggest that less than 1 percent of people exposed to the virus died of flu. Prior exposure to pandemics in the mid-1850s and around 1890 apparently provided protection against the 1918 virus, resulting in relatively low mortality in people aged 35 and older. Thus the crucial uniqueness of the 1918 pandemic lay not in its virulence, but in the disproportionate number of deaths it caused among young adults, as reflected in its famously “W-shaped” pattern of mortality (Figure S-1).
Several workshop participants are studying this trend, described by presenter Jeffery Taubenberger as “the one issue that desperately needs to have a biological explanation before we can actually draw any lessons from 1918” (Taubenberger, 2004). Hypotheses under investigation include a genetic feature of the virus that targeted young adults; an intrinsic characteristic of their immune systems that produced a deadly response to viral infection; and—perhaps most likely—a deadly interaction between this virus and the young adult immune system.
Epidemiological analyses of the 1918 pandemic further highlight the dramatic shift in age-adjusted mortality as compared with subsequent years in which influenza was epidemic (Simonsen, 2004). Such studies also show that the profound impact of the 1918 flu on young adults was not limited to the second, autumnal wave of the disease, but could be detected in the initial herald wave and in influenza seasons for several years after the pandemic’s peak. Similar age shifts in mortality also marked the two subsequent influenza pandemics in 1957 and 1968, which caused far fewer deaths than the 1918 flu.
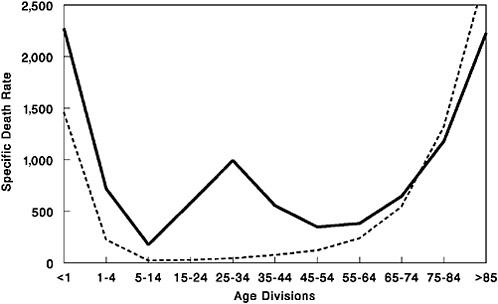
FIGURE S-1 Influenza and pneumonia mortality by age, United States. Influenza and pneumonia specific mortality by age, including an average of the inter-pandemic years 1911–1915 (dashed line) and the pandemic year 1918 (solid line). Specific death rate is per 100,000 of the population in each age division.
SOURCES: Grove and Hetzel (1968); Linder and Grove (1943); U.S. Department of Commerce (1976).
Clues to Lethality and Adaptation
It remains unclear why the 1918 influenza virus was so deadly to otherwise healthy young adults. Historical data suggest its virulence was due in part to its novelty to people under the age of 30, who were not exposed to similar viral antigens during the apparent pandemics of the mid-and late 19th century. Children between the ages of 5 and 10 years were diagnosed with flu at higher than average rates, yet had the lowest mortality rates of any age group; that outcome may reflect a weak T-cell response to the virus, which is known to spare this age group from mortality due to measles (Taubenberger, 2004). By contrast, young adults may have mounted an unusual—and deadly—immune response to the 1918 virus. This possibility is supported by death records from Kentucky which, when analyzed on a year-by-year basis, reveal a precipitous rise to a peak in flu deaths beginning at age 17 and ending with a more gradual drop beginning around age 30 (Taubenberger, 2004). Researchers have also found epidemiological evidence that in the United States, people infected with tuberculosis (TB)—a relatively common infection in 1918, particularly among young males—
were more likely than others to die of influenza (Noymer and Garenne, 2000). However, contradictory evidence from 120 autopsy reports of influenza victims showed that none had evidence of TB in the lungs, leading to the speculation that TB could have had a protective effect (Taubenberger, 2004).
Genetic features of the 1918 virus have also been examined for clues to its deadliness, but none of the mutations identified have been shown to correlate with virulence (see Taubenberger in Chapter 1). Such genomic studies are, however, revealing the genetic basis of viral adaptation to human infection and transmission. For example, research on the 1918 hemagglutinin gene and its product suggest that a single amino acid change allowed the avian-like virus to bind to a human receptor (Kobasa et al., 2004); it was speculated that a similar change in the avian H5 gene—now circulating among birds infected with pandemic H5N1 influenza—would make it easier for the avian virus to infect humans.
Applying Lessons Learned from Past Pandemics
There is particular pressure to recognize and heed the lessons of past pandemics in the shadow of the worrisome 2003–2004 flu season. At the time of this report’s release, 44 confirmed human cases of H5N1 avian influenza occurred in Thailand and Vietnam; 32 (72 percent) of these patients, mostly children and young adults, have died of the disease (ProMED-mail, 2004a; WHO, 2004d). Six of those confirmed deaths have occurred in Vietnam during a resurgence of the avian flu epidemic since July 2004, as this report was being prepared (ProMED-mail, 2004b,c). Concurrently, Thailand has confirmed four deaths since July 2004 (ProMED-mail, 2004d,v), with one case possibly having been transmitted from daughter to mother (ProMED-mail, 2004e).14,15
In addition, an early-onset, severe form of influenza A (H3N2) made headlines when it claimed the lives of many children in the United States in late 2003. As a result, stronger than usual demand for annual flu vaccine outstripped the vaccine supply, of which 10 to 20 percent typically goes unused (Hosbach, 2004). Because statistics on pediatric flu deaths had not been collected previously, it is unknown if the 2003–2004 season witnessed a significant change in mortality patterns. However, in response to these deaths, the Centers for Disease Control and Prevention’s (CDC’s) Advisory
Committee on Immunization Practices now recommends that beginning in 2004–2005, children aged 6 to 23 months (and their close contacts) receive the annual flu vaccine (Harper et al., 2004).
During the writing of this report, another vaccine shortage was making headlines. On October 5, 2004, British authorities suspended Chiron Corporation’s license for vaccine production due to contamination problems during the manufacturing process (ProMED-mail, 2004f). Currently one of only two suppliers of the influenza vaccine to the United States, Chiron was expected to provide approximately half of the supply of vaccines to the United States this flu season. As a result, the U.S. Department of Health and Human Services is urging healthy adults to forego the shot this year in an effort to conserve the remaining doses for the youngest, oldest, and sickest Americans, who are the most vulnerable to influenza (CDC, 2004b). However, problems with distribution of vaccine supply to providers well placed to serve the at-risk populations and the unwillingness of many healthy adults to sacrifice on behalf of these at-risk individuals continue to complicate this public health strategy. Difficulty finding the vaccines, long lines, and frustration have caused many to even cross the border into Canada to be immunized (Americans cross border for flu shots, 2004).
Both of these shortages reveal the historic lack of adequate attention and preparedness to the threat of influenza and the complications presented by the vaccine development and production process. This continued crisis demonstrates how fragile the method of vaccine production is and has brought to light our lack of investments for alternative forms of vaccine production. Most importantly, these shortages raise questions about our ability to respond to an influenza crisis or pandemic if we cannot provide routine influenza vaccine in a typical influenza season. The outrage expressed and extreme measures taken recently by some individuals do not suggest that the population is adequately prepared to respond rationally to a future crisis.
A series of recent avian influenza epidemics, discussed in detail below, can be seen to foreshadow pandemic human influenza in an age of globalization. In several cases, the virus has spread rapidly across entire countries, necessitating the destruction and disposal of millions of domestic birds. None of these viruses has proven readily transmissible among humans, but several workshop participants recognized that this development—a recipe for pandemic influenza—may be only a few mutations away (Figure S-4). It was also noted that although there is no historical precedent for an influenza pandemic spawned by highly pathogenic avian influenza virus in poultry, flu “does something different every time” (Taubenberger, 2004). To more reliably predict the threat posed by emerging avian influenza strains, the complex, and largely unknown, spectrum of genetic variability among these viruses must be better understood.
On the other hand, as pointed out by presenter Lone Simonsen, there may be predictive value in features shared by the three 20th-century influenza pandemics (see Simonsen in Chapter 1). Mortality data from the three pandemics provide important insights into how the pandemic evolves over time, and shows that younger age groups (ages 64 and younger) are at the highest risk for severe outcomes. Similar observations in future influenza surveillance may suggest an emerging pandemic. Several workshop participants noted a need for further historical epidemiological research, particularly toward extending our understanding of the two probable 19th-century pandemics for use in preparing for future outbreaks.
Public Communication: A Cautionary Tale
The 1918 influenza pandemic also has much to teach, by negative example, about public communication in times of crisis (see Barry in Chapter 1). Because the pandemic struck when the world was caught up in its first global war, public officials and the media were particularly reluctant to diminish public morale by announcing the arrival of a plague. Indeed, the pandemic’s nickname, “Spanish flu,” is unlikely to reflect the origin of the pandemic, but rather the fact that it was first announced in the relatively uncensored press of Spain, a noncombatant nation.
In the United States (and probably in other Western countries as well), public officials and the media played down—and in some cases, lied about—the pandemic’s approach, its severity, and its probable course. The public responded to this breach of trust with inaction: uncharacteristically, throughout the United States, calls for public assistance and sacrifice for the sake of the common good went unheeded. As the credibility of public authorities crumbled, so did social order. There is no more powerful demonstration of the need for clear and truthful communications in a public health emergency (the subject of additional discussion below) than the social chaos visited on the United States during the 1918 influenza pandemic.
TODAY’S PANDEMIC THREAT: H5N1 INFLUENZA
The past decade has seen increasingly frequent and severe outbreaks of highly pathogenic avian influenza (Webby and Webster, 2003) (see Li et al., in Chapter 2, p. 116). The current, ongoing epidemic of H5N1 avian influenza in Asia is unprecedented in its scale, in its geographical distribution, and in the economic losses it has caused (WHO, 2004b). But the prospect for the future is far more worrisome: recent evidence suggests that H5N1 has accumulated mutations that have made it increasingly infectious and deadly in mammals (Chen et al., 2004).
The first documented direct infection of humans by H5N1 occurred in 1997, by a virus that originated among Chinese geese and found its way into Hong Kong poultry markets (Webster, 2004b). There it re-sorted with other viral subtypes in both quail and duck to produce a strain that killed 6 of 18 people it was known to have infected. By acting quickly to cull every domestic bird in the country—about 1.5 million animals—Hong Kong thwarted the continued progress of this deadly strain, which has not since been detected. The parental H5N1 strain continued to evolve in geese and recombine with other avian influenza viruses, however, yielding more novel viruses that infected additional bird species and, eventually, humans. In late 2002, coincident with the arrival of migratory birds, an emergent H5N1 variant began to kill resident waterfowl in two Hong Kong parks (Guan et al., 2004). In February 2003, H5N1 virus was isolated from a 33-year-old man and his 9-year-old son in Hong Kong. They had become ill with a pneumonia-like disease upon returning from a trip to Fujian Province in China to celebrate the lunar New Year; the man’s 8-year-old daughter had died of a similar illness while abroad, and the man died as well.
Reports from the 2003–2004 Asian Epidemic
This reemergence of a species-jumping, highly pathogenic H5N1 strain foreshadowed the next year’s epidemic. In late 2003, H5N1 began to appear in domestic poultry and spread rapidly across Asia; by mid-February, outbreaks had been confirmed in South Korea, Vietnam, Japan, Thailand, Cambodia, China, Laos, and Indonesia (CDC, 2004a). The highly integrated poultry industry that connects farms and markets throughout China, Vietnam, Thailand, and Indonesia provided ample opportunity for widespread viral transmission, but several species of birds that migrate long distances across the epidemic area may also have spread the virus (Li et al., 2004; Webster, 2004b). As a result, tens of millions of birds died of influenza and hundreds of millions were culled to protect humans after 34 confirmed human cases of H5N1 influenza in Thailand and Vietnam resulted in 23 deaths. Among the first 10 human cases, which occurred in Vietnam in December 2003 and January 2004, none had a pre-existing medical condition, and all but one were known to have been in direct contact with poultry within 3 days before their symptoms appeared (Tran et al., 2004). Eight of these 10 patients died (Figure S-2).
Recent evidence also indicates that H5N1 has infected pigs in Vietnam (but are not yet established in the population) (Webster, 2004a), a white tiger and a clouded leopard (both in captivity) in Thailand, and domestic cats; all of the felines had eaten raw chicken (ProMED-mail, 2004g; Lovgren, 2004). During the preparation of this report, further confirmation of H5N1 infection has been shown in tigers (Keawcharoen et al., 2004;
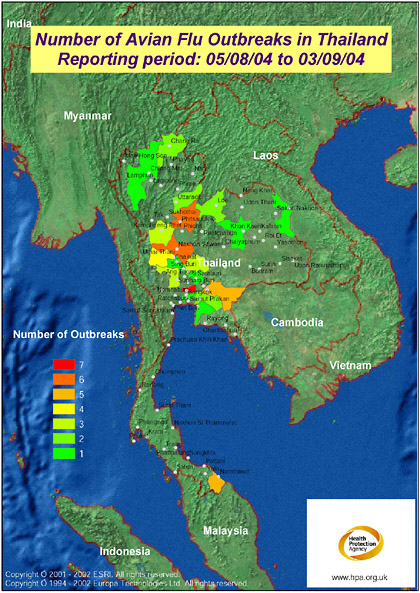
FIGURE S-2 The map displays the most recent reporting of avian influenza outbreaks in Thailand as published by the Office International des Epizooties (OIE, 2004). The mapping has been produced on a provincial basis, requiring “Bangkok Province” (as named in the data source: OIE, 2004) to be mapped to Krung Thep Province. Access to improved geographical data, e.g., sub province, would enable the mapping to be more precise, making full use of the level of detail published by OIE. Produced by: Microbial Risk Assessment Team, HPA Porton Down. In collaboration with colleagues at ProMED-mail, Oracle Corporation, and Environmental Systems Institute, Inc. (UK) Health Protection Agency are developing a mapping front end to the ProMED website.
SOURCE: Health Protection Agency (2004).
ProMED-mail, 2004h) and domestic cats (Kuiken et al., 2004) through the ingestion of raw chicken. The Thailand Zoo tiger outbreak killed more than 140 tigers, causing health officials to make the decision to cull all the sick tigers in an effort to stop the zoo from becoming a reservoir for H5N1 influenza (ProMED-mail, 2004i; ProMED-mail, 2004w). A study of domestic cats showed H5N1 virus infection by ingestion of infected poultry and also by contact with other infected cats (Kuiken et al., 2004).
Throughout Asia, affected countries responded to the avian flu epidemic with time-tested strategies: surveillance to detect the outbreak and monitor the progress of control efforts; culling potentially infected birds; disinfection of affected facilities, including the safe disposal of dead and culled birds; and educating poultry farmers and the general public about the threat posed by H5N1 avian influenza. Yet each country’s circumstances and their handling of the epidemic were unique, as illustrated by workshop presentations by agricultural and public health officials from Indonesia, Vietnam, and Thailand. The diversity of these responses, and their resulting outcomes, offer important lessons for the control of future avian flu outbreaks—a key protection against a human pandemic.
Indonesia
Although Indonesia was one of the first Asian countries to experience cases of H5N1, which was identified in Central Java in August 2003, a comprehensive control strategy was not initiated until November (Soebandrio, 2004). By that time, the disease had spread throughout Java and had also been detected on the islands of Bali, Sumatra, and Borneo. This late start, along with the fact that the epidemic had spread widely and affected many small farms, made disease control a daunting challenge. Nevertheless, a strong effort was made to cull all domestic birds on all farms and facilities where H5N1 was detected. Surveillance for human infection was also conducted among more than 1,000 people (80 percent of whom had direct contact with poultry; the others as controls) in affected provinces; no positive cases were found by Reverse Transcriptase (RT)-PCR.
Unlike most other affected countries, Indonesia also instituted mass vaccination of healthy domestic birds against H5N1, followed by routine vaccination (China has a similar policy; other Asian countries are considering it [ProMED-mail, 2004j]) (Soebandrio, 2004). This is a risky strategy, because vaccinated birds can develop asymptomatic infections that allow virus to spread, mutate, and recombine (ProMED-mail, 2004j). Intensive surveillance is required to detect these “silent epidemics” in time to curtail them. In Mexico, for example, mass vaccination of chickens against epidemic H5N2 influenza in 1995 has had to continue in order to control a persistent and evolving virus (Lee et al., 2004). The prospects, advantages,
and limitations of vaccination as a means to control avian influenza (and prevent the infection of other animals, including humans) are further discussed below. Indonesia’s decision to vaccinate poultry against H5N1 was, however, strongly influenced by the fact that illegal vaccine of questionable quality, some of which may have harbored live influenza virus, was already being used throughout the country (ProMED-mail, 2004k; Soebandrio, 2004). To accompany its offer of a free, safer alternative to illegal vaccine, the Indonesian government launched a multimedia public awareness campaign on avian influenza (Soebandrio, 2004).
Vietnam
In Vietnam, the avian influenza outbreak was recognized comparatively quickly, but several factors hindered effective action to control influenza (see Nguyen in Chapter 2; Nguyen, 2004). These included an initial lack of engagement of the highest levels of government in the institution and enforcement of control measures; a delay in imposing a ban on the movement and consumption of poultry; and disagreement within the country’s scientific community as to how to gauge the threat influenza posed to human health. However, once the grave danger of H5N1 was recognized, the Vietnamese government took action to bring the outbreak rapidly under control through culling in infected premises and a ban on the movement and consumption of poultry. Vietnam’s political structure and media were instrumental in educating the public and gaining popular support for infectious control efforts. CDC also played a key role in quelling the epidemic by providing the Vietnamese government with training and materials for the rapid diagnosis of H5N1.
Vietnam was, however, widely criticized for announcing that H5N1 was controlled on March 30, only 15 days after its last human victim died (ProMED-mail, 2004l,m). Successful eradication of avian influenza is generally believed to take at least 2 to 3 years. Nevertheless it is easy to understand why a country where the poultry industry is important not only to economic stability, but as an affordable source of protein for a growing population would be powerfully motivated to pronounce the end of this devastating epidemic (Nguyen, 2004). Nor is it surprising that expert predictions of a resurgence of influenza in Vietnam were realized, with devastating consequences, as described below (ProMED-mail, 2004l).
Thailand
Two features of Thailand’s response to the avian flu epidemic merit particular attention: the degree to which the country was prepared to address the outbreak, and its willingness to compensate farmers for their losses. When surveillance for highly pathogenic H5N1 first detected the virus in
Thailand in January 2004, officials adapted the country’s established emergency response plan, which specifies chains of command and communications, to address the threat (see Buranathai in Chapter 2; Buranathai, 2004).
Approximately 75,000 cloacal samples from poultry in every village in the country were tested for the virus within a 2-week period, followed by culling and disinfection of the 160 premises found to be infected. In addition, all poultry within 5 kilometers of each of the infected farms were preemptively culled, and the movement of all poultry within a 50 to 60 kilometer radius was controlled. A second round of active surveillance and culling was performed from mid-February through early March, when the epidemic was deemed to be under control. The country then reverted to passive agricultural surveillance while maintaining active clinical surveillance for human cases. Thailand is currently investigating the possibility of using vaccination against future avian flu outbreaks. The country has the necessary manufacturing capacity, but at present prohibits livestock vaccination due to the aforementioned risks.
Thailand’s generous emergency compensation policy, also in place prior to the recent epidemic, became even more generous in response to farmers’ losses (Buranathai, 2004). Rather than the standard 75 percent of market price, farmers whose infected flocks were culled received their full market value. This strategy backfired, however, when struggling farmers infected their flocks so as to recoup losses sustained as a result of decreased demand for poultry products. While many workshop participants identified compensation for farmers’ losses as a key strategy in the control of avian influenza, this example highlights the difficulty of designing a compensation policy that truly supports the goal of infection control.
The Puzzling Present and Worrisome Future of Avian Flu
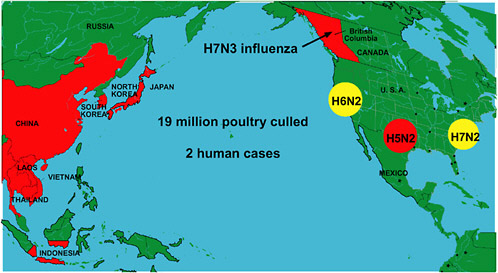
In addition to the Asian epidemic, unprecedented numbers of outbreaks of diverse subtypes of avian influenza arose during the 2003–2004 flu season in locations including British Columbia and three separate regions of the United States (Figure S-3) (Webster, 2004a). In several of these instances, a few nonfatal cases of human infection were also identified (ProMED-mail, 2004n). Meanwhile, the Asian H5N1 epidemic continued to smolder. In July 2004 it reignited, resulting in multiple outbreaks in Vietnam and Thailand and a single outbreak in China; hundreds of thousands of birds were culled in both Vietnam and Thailand in an attempt to contain the epidemic (ProMED-mail, 2004o). Since July, Vietnam has confirmed that six more people have died from H5N1 influenza (ProMED-mail, 2004b,c), and Thailand has confirmed four more deaths (ProMED-mail, 2004d,v), with one case possibly having been transmitted from human to human (ProMED-mail, 2004e).
The possibility that H5N1 is gaining momentum is especially troubling in light of recent evidence that the virus has become increasingly pathogenic toward mammals (Chen et al., 2004). Between 1999 and 2002, researchers periodically isolated samples of the virus from asymptomatically infected southern Chinese ducks (a natural reservoir for the H5N1 and other avian influenzas) and tested each isolate for its ability to infect a mouse model. Recently, a new study reported that domestic ducks infected with H5N1 shed more virus for longer periods and the majority do so asymptomatically. This suggests that ducks might now be acting as a “silent” reservoir for the virus and may play an increased role in transmitting H5N1 to both poultry and humans (WHO, 2004c). The results reveal a progressive increase in virulence (based on the virus’s ability to replicate in mice) and lethality over time. Viewed in light of the proliferation of H5N1 influenza in Asia and the numerous concurrent outbreaks of other avian flu subtypes, these findings led one of the investigators, workshop presenter Robert Webster, to the ominous conclusion that “flu has got something going at the moment that we don’t fully understand.”
THE PLANNED RESPONSE TO PANDEMIC THREAT
The odds of detecting, controlling, and even preventing the spread of an influenza virus with pandemic potential have improved dramatically since 1918, when the disease was recognized by symptoms alone; it was not until 1933 that a virus was determined to cause influenza (Noymer and Garenne, 2000). In 1957 and 1968, although surveillance of new viral subtypes was theoretically possible, pandemic viruses were not identified until after outbreaks had occurred in Asia (WHO, 1999). Today, international programs permit the characterization of thousands of viral isolates each year and support worldwide surveillance and communications networks. These efforts are informed by expanding scientific understanding of viral molecular biology and evolution, and bolstered by simultaneous preparations against the threat of bioterrorism.
Yet major challenges to pandemic preparedness remain to be overcome. The world’s growing—and increasingly urbanized—population and the speed and volume of international travel create abundant opportunities for widespread viral transmission. A recent example illustrating these vulnerabilities was reported when two eagles were smuggled into Belgium from Thailand. Customs officials at Brussels airport found and seized the birds, which were then discovered to be infected with H5N1 and immediately culled at the quarantined holding zone. For precautionary measures, the other birds being held in the quarantine zone were also destroyed and all the people in direct contact with the eagles were monitored and tested for H5N1 infection. To date, no one has tested positive for the virus (ProMED-mail, 2004p).
Some countries will respond to a pandemic with abundant resources and expertise, but many others remain essentially defenseless. Even populations wealthy enough to obtain vaccine are unlikely to get enough to prevent significant morbidity and mortality from pandemic influenza, unless more rapid vaccine production methods or novel prophylactic vaccines can be introduced before the next pandemic strikes (see also upcoming section on vaccines).
Global Preparations
WHO plays a central role among the many international and nongovernmental organizations that contribute to global preparations against pandemic influenza. Its 1999 Influenza Pandemic Plan provides a model of flexible contingency planning and outlines “the separate but complementary roles and responsibilities for WHO and for national authorities when an influenza pandemic appears possible or actually occurs” (WHO, 1999). The basic precepts of that plan were tested and proved effective during the global response to severe acute respiratory syndrome in 2003, which WHO coordinated (IOM, 2004). Many countries have based their pandemic influenza plans on the 1999 WHO document, which provides guidance on the issues to be addressed and actions to be undertaken by each nation in the event of a threatened or actual pandemic (Stöhr, 2004).
The WHO influenza pandemic plan has two main objectives:
-
To assess the risk posed by new viruses, primarily the responsibility of WHO; and
-
To manage risk when a virus appears capable of causing widespread and serious disease, an authority that rests largely with national governments (see below) (WHO, 1999).
Recognizing that new influenza strains may infect and even kill humans without causing a pandemic—as was the case with swine flu in the United States in 1976 and avian influenza in Hong Kong in 1997—the plan presents a range of responses (“preparedness levels”) appropriate to each prepandemic and pandemic phase. WHO’s specific contributions to influenza pandemic preparedness and response are summarized in Box S-1. Parts of the 1999 document have been revised to reflect knowledge gained from the recent lethal transmission of H5N1 avian influenza to humans and the recommendations and conclusions of a consultation convened by WHO in March 2004 in response to these cases (WHO, 2004a).
In addition to the activities listed in Box S-1, which can largely be characterized as reactive to a threatened pandemic, WHO has begun to pursue strategies to avert an influenza pandemic—a goal long considered to
|
BOX S-1 Specific contributions by WHO to influenza pandemic preparedness and response include:
SOURCE: Stöhr (2004). |
be impossible (Stöhr, 2004). To stop, or even slow, a pandemic would require an internationally coordinated, “all-out” response in the early stages of human-to-human transmission (WHO, 2004a). Such an effort would necessitate balancing agricultural and public health interests—which frequently conflict with regard to infection control measures for zoonoses—and a coordinated approach by animal and human health authorities to influenza surveillance and reporting (Stöhr, 2004).
National Preparations
While global preparations against pandemic influenza focus on detecting and defining risk, national governments must determine how to manage the threat posed by an actual or potential pandemic. National governments must be prepared to respond to a developing—and largely unpredictable—pandemic, and in “an atmosphere of considerable scientific uncertainty and fragile public confidence,” as described in the report of the recent WHO
consultation (WHO, 2004a). In order to face these challenges, several countries have adopted WHO’s model of contingency planning; Canada’s plan, for example, stresses the documentation of response activities and outcomes so that timely adjustments can be made (Health Canada, 2004). Complete, partial, and draft pandemic influenza plans from several countries can be accessed through the WHO website (http://www.who.int/csr/disease/influenza/nationalpandemic/en/print.html).
In the United States, managing the risk of a pandemic influenza entails not only addressing key national issues through the Department of Health and Human Services (DHHS) and the Department of Homeland Security, but also the harmonization and coordination of state, local, and private-sector plans (GAO, 2000; Slater, 2004). The first U.S. pandemic preparedness plan was organized in 1978, following the emergence of H1N1 swine flu in 1976. The federal government’s experience in responding to this threat revealed the importance of developing agreements with private- and public-sector players to assure the timely purchase, distribution, and administration of vaccines and drugs in advance of future infectious outbreaks (GAO, 2000; Millar, 1977). In a sense, the latest version of the U.S. pandemic plan has been under construction since the release of its predecessor nearly 25 years ago, but looming threats of avian influenza and bioterrorism, along with recent sobering estimates of the potential impact of pandemic influenza on the United States (Table S-1) have raised the plan’s profile considerably (Gellin, 2004).
The DHHS National Vaccine Program Office is specifically responsible for the U.S. pandemic preparedness plan. Director Bruce Gellin described the contents of the latest version of this plan, which at the time of the meeting was nearing release in draft form. During the development of this report, it was posted on the Internet for a 60-day comment period, beginning on August 26, 2004 (see Chapter 3 for Executive Summary of the plan) (DHHS, 2004a). Beyond revisions to the draft plan based on comments received during this time, DHHS expects future updates based on advancements in the understanding of influenza biology and of the effectiveness of various control measures.
TABLE S-1 U.S. Impact Estimates for the Next Influenza Pandemic
|
|
Number of People |
Percentage |
|
Deaths |
89,000-207,000 |
.03-.07 |
|
Hospitalizations |
314,000-733,000 |
.1-.3 |
|
Outpatient care |
18,000,000-42,000,000 |
6-15 |
|
Total infected |
43,000,000-100,000,000 |
15-35 |
|
Absent vaccination, health-related economic impacts = $71 to $166 billion |
||
The three main objectives of the U.S. pandemic plan parallel those of other national pandemic preparedness plans, and of the WHO plan: to decrease the burden of disease, minimize social disruption, and reduce the economic impact associated with a pandemic (CDC, 2004c). It addresses surveillance; development and licensure of a vaccine against the strain; production of sufficient vaccine for the U.S. population and provision for its delivery; targeted distribution strategies for limited supplies of vaccine and antiviral medications; coordination with international, state, and local authorities; maintenance of medical care and other community services; and communication with community leaders, medical care providers, the public, and the media (Gellin, 2004). The plan includes the following elements (Gellin, 2004):
-
A core plan, which describes the protocol for national coordination and decision-making, reviews key preparedness issues, and outlines response at national, state, and local levels.
-
Two guides to aid planning by (1) state and local health departments and (2) public and private health care systems.
-
Twelve annexes providing detailed and technical information on preparedness and response issues.
Additional efforts by the federal government to prepare for pandemic influenza at the national level include a $100 million DHHS initiative in 2003 to build U.S. vaccine production. Several agencies within DHHS—including the Office of the Secretary, the Food and Drug Administration (FDA), CDC, and the National Institute of Allergy and Infectious Diseases (NIAID)—are in the process of working with vaccine manufacturers to facilitate production of pilot vaccine lots for both H5N1 and H9N2 strains as well as contracting for the manufacturing of 2 million doses of an H5N1 vaccine. This H5N1 vaccine production will provide a critical pilot test of the pandemic vaccine system; it will also be used for clinical trials to evaluate dose and immunogenicity and can provide initial vaccine for early use in the event of an emerging pandemic. Other efforts include the introduction into the U.S. Senate of the Flu Protection Act of 2004, which aims to increase safeguards against both annual influenza and epidemic/pandemic preparation. Two Senate bills introduced in 2003 also address key influenza preparedness issues: boosting vaccine production16 and promoting immunization against several diseases, including influenza.17
State and Local Preparations
The most direct, most primary response to pandemic influenza will come from state and local authorities, public health officials, and providers of medical and other public services. Unlike a typical, localized public health emergency or natural disaster, a pandemic disease cannot be managed from outside the affected community; each community must face the possibility of responding to influenza with minimal external resources or support—or none at all (Gensheimer et al., 2003; Health Canada, 2004; Perrotta, 2004). If a pandemic is confirmed, governors will make most state-level decisions on infection control and case management; however, state health officials are generally responsible for overseeing pandemic preparations and resolving potential conflicts between state and federal governments on issues such as strategies for mass vaccination and disease containment (ASTHO, 2002). Logistical models for local response to pandemic influenza include the widely used Incident Command System, which has been adapted in some areas specifically to respond to infectious disease outbreaks, including pandemic influenza (ASTHO, 2002).
State and local health departments rely heavily on guidance from the federal government in formulating pandemic influenza plans (see Gensheimer in Chapter 3). According to a recent survey conducted by the Council of State and Territorial Epidemiologists (CSTE), 29 states have complete drafts or final plans to address pandemic influenza, and 14 have earlier drafts of a plan (Personal communication, Kristine Morris). In addition to the federal pandemic plan, resources available for state and local planning include CDC’s guide for state and local officials and its online modeling tool, FluAid (CDC, 2000), which uses state-specific statistics to approximate the impact of a pandemic on an area (ASTHO, 2002). Tabletop exercises are being developed to help state and local officials rehearse and refine strategies for coping with a pandemic (DHHS, 2004a), much as simulations of bioterrorist attacks have been used to assess federal, state, and local preparedness (ASTHO, 2002; Vastag, 2002).
Several states, including Texas, Wisconsin, and California, are preparing for the next influenza pandemic as a complement or adjunct to preparations against bioterrorism (Perrotta, 2004; Shult, 2004; State of California, 2003). Wisconsin, for example, is establishing a common infrastructure for coping with bioterrorism and infectious disease outbreaks—and managing two or more such events simultaneously. Such efforts are made possible by federal funding for bioterrorism preparedness. Since September 11, 2001, DHHS has provided more than $3.7 billion to improve state public health emergency preparedness for bioterrorism, infectious disease outbreaks, and public health emergencies (DHHS, 2004b). These funds enable states and communities to conduct influenza-related activities including surveillance, planning, drills, and tabletop exercises.
Hospitals are a key focus of state and local influenza preparedness because a pandemic is likely to result in increased demand for health care services, staff shortages, and limited access to critical equipment and supplies (above predicted shortfalls in vaccines and antiviral medications) (DHHS, 2004b). Hospital surge capacities are extremely limited; for example, medical care capacity in a major urban center (Los Angeles County) recently proved insufficient even to address severe seasonal influenza (Glaser et al., 2002). In addition, of course, hospitals and hospital workers themselves become the sieves for infection spread as sick health care workers are required to report to work due to staff shortages, and spread their illness to immunocompromised patients, particularly the elderly, who visit emergency rooms and occupy beds on the wards.
Workshop participants expressed concern that much work remains to be done to establish pandemic planning at state and local levels. The presenters particularly noted a gap in planning and coordination between public health agencies and hospital administrators. Moreover, while most people who contract influenza will not require hospital care, many will need other support services such as home health care, delivery of prescription drugs, and meals (DHHS, 2004b). These needs, along with those for other essential community services (e.g., police, fire, utility, sanitary services) should be addressed in local pandemic planning and in infection control.
State and Local Surveillance
In addition to serving on the “front lines” of the response to pandemic influenza, state and local public health officials and health care providers are also largely responsible for implementing influenza surveillance in the United States. Surveillance programs and capacity vary considerably from state to state. The program in Texas, described by state epidemiologist Dennis Perrotta as “better than most,” was modeled on a program run by Baylor University Medical School that tracks year-round respiratory illness in the Houston area (Perrotta, 2004). In the statewide program, participating physicians and hospitals collect swabs from people presenting with febrile illness during flu season. The swabs are analyzed by the state public health laboratory, which uses these results to create a statewide “picture” of respiratory viruses.
In Wisconsin, influenza surveillance is conducted through a network of public and private laboratories that track all emerging diseases and provide isolates to CDC for further testing (Shult, 2004). Small private laboratories and clinicians that conduct rapid diagnostic tests for flu also send their samples to the state laboratory for confirmation. Additional samples and patient information are obtained by clinicians who serve populations where
new influenza strains are likeliest to emerge: preschool and school-age children in rural areas where swine and poultry are raised. The data are shared and compared with surveillance of animal respiratory diseases by the University of Wisconsin’s veterinary diagnostic laboratory.
TOWARD PREPAREDNESS: OPPORTUNITIES AND OBSTACLES
Addressing Avian Influenza
Considerations of the pandemic threat posed by H5N1 avian influenza in Asia were augmented and enriched by further discussion of the global phenomenon of avian influenza, its impact on the poultry industry, and possible strategies for preventing and controlling its spread among birds and mammals, including humans. Participants noted the importance of surveillance to the effective control of influenza, as well as the limitations of predominant models of surveillance that focus on a single species or industry. In recognition of the need for a broader understanding of influenza behavior, the Office International des Épizooties (OIE), an international and intergovernmental organization that promotes worldwide solidarity in animal disease control, is developing influenza surveillance guidelines that encompass birds, domestic mammals, wildlife, and humans (see Sibartie in Chapter 4; Sibartie, 2004). Weeks after the workshop, the OIE, the Food and Agriculture Organization (FAO), and WHO announced plans to launch a jointly sponsored regional veterinary influenza network intended to strengthen surveillance and speed the diagnosis and reporting of emergent strains (ProMED-mail, 2004q).
Obstacles to Early Reporting
In the absence of a comprehensive surveillance network in place, the rapid reporting of early cases is essential to controlling an emergent infectious disease. All OIE member countries are therefore required to report certain diseases—including avian influenza—within 24 hours of their detection in animals. After an outbreak of H7N7 avian influenza in ducks in 2003, The Netherlands established its own early warning system for the disease. Unfortunately, the system’s utility is limited by the fact that avian influenza has been relatively rare in The Netherlands, and is thus unlikely to be recognized by veterinarians (Koch, 2004).
Given existing obstacles to surveillance and early reporting, it is not surprising that in many instances, infection control for avian influenza has entailed mass culling of poultry. However, according to Dewan Sebartie of the OIE, that organization “recognizes that culling is no longer a viable option for certain countries for social, economic, technical, ethical and
ecological reasons.” Small farmers, for example, are unlikely to comply with culling policies because their flocks provide a lifeline of daily income; they cannot help but focus on their immediate and pressing need to sell their birds (Soebandrio, 2004). On a larger scale, the economic consequences of early reporting—to a country or region if many animals must be culled, or to a corporation raising millions of infected, and therefore potentially unprofitable, animals—present a massive barrier to disease control. One country, for example, experienced outbreaks of H5N1 over more than 6 months before admitting the situation to the OIE (Sibartie, 2004). In California, poultry producers kept their knowledge of a recent H6N2 avian influenza outbreak to themselves due to their fear of public rejection of poultry products; meanwhile, the disease spread across the western United States and has since become endemic (Box S-2).
The need to remove economic disincentives to the timely discovery and control of emergent avian influenza strains is clearly established. Providing compensation for culled animals could, at least in theory, remove a major disincentive to reporting for farmers in developing countries (however, see earlier discussion of Thailand’s problematic compensation program). Several participants urged the creation of a fund by developed countries to compensate for culling of infected flocks in developing countries, as well as for the quarantine and isolation of humans should transmission occur (Meltzer, 2004). In developed countries, government-run mandated insurance policies, similar to policies currently in use to encourage reporting of Salmonella in eggs and poultry in the United States, could compensate the losses of poultry producers who report suspected or confirmed cases of avian influenza (Meltzer, 2004). Another option proposed by the OIE is to allow demonstrably biosecure regions of a country where avian influenza has been reported—or even biosecure farms within an affected area—to continue to export poultry products, because avian influenza is not a foodborne disease (Sibartie, 2004). This could, however, also be a disincentive for farms to certify the presence of avian influenza in their flocks and possibly their workers. It was also suggested that, given increased public interest in avian influenza, poultry from producers who can certify their chickens to be “influenza free” and their workers to be “influenza safe” through protection programs may be more desirable to consumers (Cardona, 2004).
Immunization of Poultry
Avian influenza vaccines increasingly are being viewed as a means of reducing the necessity for massive poultry culls, particularly in Asia. Together with culling, immunization can speed the eradication of avian influenza and, by decreasing the amount of virus shed by infected animals,
|
BOX S-2 In February 2000, a low pathogenic H6N2 virus was detected for the first time in commercial egg layers in southern California, an area with a population of approximately 15 million chickens. At that time fewer than 10 farms were found to be infected with this low-pathogenic virus in an area with about 60 large farms ranging in size from about 30,000 to 1.2 million birds. The infection was asymptomatic—egg production and mortality were not affected—thus little was done to eradicate the virus. The state performed some vaccination of layer farms, but because it was not accompanied by depopulation or stamping-out, the measure proved futile. Over the next 2 years, the virus persisted on some farms where it found a continual stream of naïve hosts. Sporadic infections occurred in additional farms in the area, bringing the total number of affected (H6N2 antibody positive) farms to 15. Three different genotypes of H6N2 virus were found to be circulating; in January 2002, one form emerged to cause clinical disease in San Diego County, where an infected flock suffered a 50 percent decrease in egg production and a 10-fold increase in mortality. The farmer had his birds tested, but during a 2-week period before the diagnosis was made, chicken manure was removed from the farm, young hens were brought in, and eggs continued to be taken to be processed. The virus immediately spread to approximately 10 farms and the outbreak continued to expand over the next 2 months. A major expansion of the outbreak subsequently occurred in Turlock, a town in northern California where layer hens from several states were slaughtered and processed. Infected layer hens from southern California were sent to the Turlock plant, into the heart of a densely populated poultry region. Shortly thereafter, three egg-laying flocks in the Turlock region were found to be positive for avian influenza detected through passive surveillance; a drop in egg production prompted the producers to have the birds tested. One week later a broiler flock was found to be positive for avian influenza at slaughter. Once the virus got into broilers, it spread very rapidly among turkeys and layers as well. This acceleration resulted from the unfortunate coincidence |
reduce human exposure as well (ProMED-mail, 2004r; Sibartie, 2004). However, as several conference participants stressed, vaccination must be accompanied by strong surveillance to prevent the spread of asymptomatic infection among vaccinated birds. This can be accomplished either through the use of unvaccinated sentinel birds or of recombinant vaccines that elicit a distinct “marker” antibody (ProMED-mail, 2004s). Should a “silent epidemic” of influenza manage to erupt under these conditions, it could serve as an incubator for the evolution of a more deadly viral strain. This apparently occurred in Mexico after chickens were vaccinated against highly pathogenic H5N2 influenza in 1995; today, antigenically distinct variants of the vaccine strain are spreading among the country’s poultry flocks (Lee et al., 2004).
|
that broilers are sent to slaughter at an optimal age for viral shedding, as well as from the large numbers of broilers that traveled to that facility. Millions of birds shedding viruses traveling in trucks to Turlock easily spread the infection to farms along the route. That is when the Turlock region, which is bound by three major highways, became known as the Triangle of Doom: a bird couldn’t enter the area without becoming infected with H6N2. An estimated 35 million birds in California became infected with this H6N2 virus during a 4-month period beginning in March 2002. Perhaps more astonishing than the extent and speed of this outbreak was the fact that no one outside the region heard much about it. The virus was not a feared H5 or H7 subtype, but the Triangle of Doom was also kept quiet by corporate decision-makers who feared that consumer demand would plummet if the public knew they were buying infected meat and eggs, safe though they may be to eat. Thus, other than the initial diagnosis of the broiler flock, all other diagnoses were made by corporate veterinarians, and the results were not released—not to the state or to other potentially affected states, not to the Office International des Épizooties, not even to neighboring farmers, who might have better protected their flocks from infection had they known about it. H6N2 has since become endemic in California, following its spread to farms that raise birds for the state’s live fowl markets. Eventually, the poultry producers in the Triangle of Doom developed a biosecurity plan to curtail the spread of the virus, and thereby restore egg and poultry production. The plan does not penalize farms that test positive for influenza, and it provides for the safe movement of eggs and broilers to market from infected facilities. These sorts of protections need to be offered to the industries that raise much of the poultry (and swine) in the United States in order to achieve complete surveillance and their cooperation in addressing avian influenza. Similar dense poultry and swine populations exist throughout the country. Any one of them could be the site of the next outbreak of an emergent influenza virus. SOURCE: Cardona (2004). |
Since the height of the Asian avian influenza in February 2004, the FAO has recommended vaccination against influenza “where appropriate and practical,” but the practice remains prohibited in several Asian nations (ProMED-mail, 2004r). Japan and Korea, the only Asian countries that successfully controlled and eradicated H5N1 following the recent epidemic, did so through culling alone. Thailand is currently considering the possibility of immunization following a resurgence of H5N1 in July 2004, although as a major poultry exporter, it will surely take into consideration the European Union’s ban on poultry imports from countries where chickens have been vaccinated against avian influenza. Vaccination against avian influenza is not widely practiced in the United States due to its high cost relative
to profit margins for most of the poultry industry (see Swayne and Sibartie in Chapter 4; Swayne, 2004).
Preventing Interspecies Transmission
The intersecting and sometimes conflicting interests of commerce and public health were also evident in discussions on preventing transmission of avian influenza from wild to domestic birds, and from poultry to domestic animals and humans (for transmission pathways between species, see Figure S-4). Because wild waterfowl can carry the influenza A virus without developing signs of infection, influenza cannot realistically be considered an
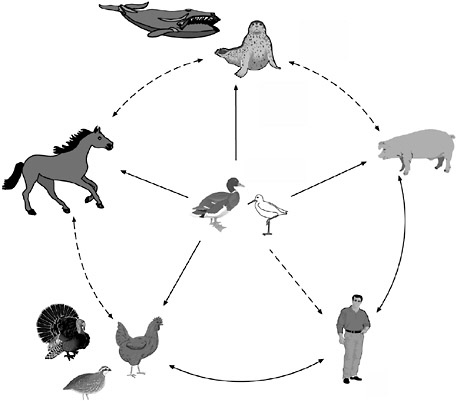
FIGURE S-4 The reservoir of influenza A viruses. The working hypothesis is that wild aquatic birds are the primordial reservoir of all influenza viruses for avian and mammalian species. Transmission of influenza has been demonstrated between pigs and humans and between chickens and humans but not between wild birds and humans (dotted lines). There is extensive evidence for transmission of influenza viruses between wild ducks and other species (solid lines). The five different host groups are based on phylogenetic analysis of the nucleoprotein genes of a large number of different influenza viruses.
eradicable disease (Webster, 1998). During the development of this report, it was reported that H5N1 avian influenza had been discovered in migratory birds in the Novosibirskaya region of Russia (ProMED-mail, 2004t). Preliminary data indicate that the virus was brought from south east Asia by migratory birds, such as ducks and geese (ProMED-mail, 2004t). Workshop discussants concurred that the best chance of averting a pandemic lies in stopping the further spread of epidemics in poultry populations, thereby reducing human exposure to the virus and limiting its opportunities for reassortment (Trampuz et al., 2004; Barclay and Zambon, 2004).
Live poultry markets provide near-optimal conditions for amplifying and perpetuating viruses due to the continuous movement of many bird species through the market (Webster, 2004b). These risks are multiplied when livestock and poultry are kept in close proximity and in crowded conditions, as is often the case in “wet markets” and in livestock (especially swine) and poultry feeding operations (Liu et al., 2003). Because neither practice is likely to end soon, participants agreed, the immediate focus of preventive efforts should be on making farms and markets safer through measures and regulations that reduce the possibility of contact between domestic and wild animals and between domestic mammals and poultry, as well as through efforts to protect workers from infection (see Swayne and Sibartie in Chapter 4).
Surveillance
Influenza surveillance at all levels—from global to local—has a common, practical goal: to detect events indicating unusually large or severe outbreaks of influenza as early as possible, and to determine the intensity and impact of influenza on populations. The first step toward this goal is to determine normal conditions, so that an unusual event can be recognized for what it is. Thus influenza surveillance must be stable, ongoing, and representative of populations on the basis of geography, demography, and severity of disease. The vital data that emerge determine vaccine strain selection and public health resource allocation and drive influenza prevention and control policy and planning for pandemic preparedness.
Surveillance findings also contribute to epidemiological research, most often as a springboard for more detailed investigation. In the case of influenza, surveillance has clarified essential questions regarding the clinical epidemiology of pandemics and the biological causes of epidemiological phenomena. Pursuing answers to these questions will require investment in planning research, designing protocols, and establishing networks of scientists ready to engage in these studies in advance of the next pandemic (Stöhr, 2004).
Global Surveillance
WHO has spearheaded global influenza surveillance efforts since 1948 and now coordinates a network of 110 national influenza centers in more than 80 countries (Cox, 2004; Stöhr, 2004). These laboratories isolate and characterize influenza viruses and collect epidemiological information; they also submit certain viruses for characterization to one of four WHO international collaborating centers in Atlanta, London, Melbourne, and Tokyo. These facilities analyze and compare thousands of viral strains each year in order to determine the antigen content of the three annual influenza vaccines, then prepare and distribute the candidate vaccine strain to manufacturers. While this network is strong in Western countries and was characterized as “sufficient” in Eastern Europe and the Middle East, it is riddled with strategic gaps in Africa as well as in Asia—an area of particular concern due to H5N1 (Stöhr, 2004). Laos and Cambodia, for example, lack national influenza centers and did not conduct routine influenza surveillance during the recent H5N1 epidemic; thus the extent of infection in human populations in those countries is unknown (Cox, 2004). Even in countries participating in WHO surveillance, the surveillance of the H5N1 epidemic was hindered by limited access to virus isolates (in part because laboratories in developing countries could not afford to ship isolates to international collaboration centers) and poor communication between veterinary and public health officials.
As a result of this experience, DHHS recently launched a $5.5 million initiative to create and enhance infrastructure for influenza surveillance in Asia (Cox, 2004). While this investment is likely to lead to immediate improvements in virologic surveillance in this important region, participants noted that it will be equally necessary—but much more difficult—to obtain epidemiological evidence of disease impact. The United States was described as a possible model for collecting both types of information on a national scale. Here, a network of 2,000 sentinel physicians monitor more than 8 million patient visits per year and submit reports and specimens to CDC; additional reports are provided by state and territorial epidemiologists and public health authorities in 122 U.S. cities.
Integration and Collaboration
It was frequently noted that influenza surveillance efforts at all governmental levels would benefit immensely from better integration between the animal and human health communities. While acknowledging that formal collaboration between the OIE and WHO has been limited to date, a forthcoming meeting promised to discuss information and strain sharing and the harmonization of surveillance methods between the two international orga-
nizations and their collaborators (Sibartie, 2004). The previously mentioned plan for a regional veterinary influenza network, jointly sponsored by the OIE, WHO, and the FAO, was announced weeks after the workshop (ProMED-mail, 2004u). The recent DHHS initiative will also support study of the animal–human interface in influenza, particularly as it enables predictions of human risk from animal influenza viruses (Cox, 2004).
Workshop participants also emphasized the need to strengthen international influenza surveillance through the sharing of:
-
Timely information,
-
Viral isolates,
-
Reagents,
-
Funding, and
-
Expertise.
The global threat posed by influenza necessitates international collaboration that balances the health and economic needs of developing countries—essential participants in influenza surveillance—with the medical, scientific, and financial resources of developed countries.
Vaccines
Widely accepted as the most effective intervention against influenza in humans, vaccines significantly reduce morbidity and mortality during annual (interpandemic) flu seasons (Fedson, 2004a; Hosbach, 2004; Nichol, 2003). Considerable obstacles hinder the production of a vaccine against a pandemic strain of influenza, however. Planning and production of interpandemic flu vaccine require nearly a year to complete; experts estimate that if a pandemic were to strike today, 6 to 8 months would elapse from the identification of the viral strain to the initial release of vaccine if it were produced by standard methods (GAO, 2000). Moreover, given existing manufacturing capacity, vaccine availability would fall far short of projected demand, especially in countries without vaccine manufacturing facilities (Fedson, 2004b). Workshop participants considered the critical role of vaccine manufacturers in addressing a pandemic; methods and logistics for the development and production of a pandemic vaccine; and the challenges of equitably distributing it given the likelihood that demand will far out-strip supply (see Fedson in Chapter 3).
Supply and Demand
Above all, several workshop participants stressed the importance of building capacity to manufacture pandemic vaccine by accelerating demand
for interpandemic influenza vaccines (Fedson, 2004a; Gellin, 2004; Hosbach, 2004; Nowak, 2004). Although annual influenza immunization rates have increased sharply over the past decade, from 135 to 292 million doses of flu vaccine worldwide (Fedson, 2004b), demand remains too weak for manufacturers to make investment in preparations for a rapid ramp-up of pandemic vaccine production. Currently only 75 to 85 million Americans (about one-quarter of the population) are immunized annually against influenza, and only 38 percent of health care workers receive influenza vaccination (see Hosbach in Chapter 3).
Because last year’s early and virulent flu season, and particularly news of several child fatalities, ratcheted up vaccine demand in the United States, participants suggested that the country may be ripe for a pro-immunization campaign. In addition, the flu immunization season—now largely limited to October and November—could be extended into January, when peak rates of infection generally occur. This might have been possible in the United States during the 2003–2004 flu season if more vaccine doses had been preordered; instead, as rarely occurs, demand for vaccine exceeded supply. Because influenza vaccine cannot be stockpiled (due to antigenic shift and drift), it was also suggested that the federal government share the risk of investing in producing vaccine reserves, in preparation for either a severe annual flu season or in response to a threatened pandemic. This arrangement would help ensure that influenza vaccine supplies will meet a sharply increased demand (Hosbach, 2004) (see Figure S-5).
The subject of liability protection was also noted as crucial to the production of a pandemic vaccine. Manufacturers are loath to repeat the experience of the 1976 swine flu, when they produced 150 million doses of vaccine for a threatened pandemic that never occurred; only 45 million doses were used, and the immunization campaign was suspended after the vaccine was linked with Guillain-Barré syndrome (ASTHO, 2002). Participants also observed there is some likelihood that a pandemic vaccine could be offered as an Investigational New Drug as befits an emergency situation, demanding advance consideration of the complexities of administering an unlicensed vaccine in this context (Hosbach, 2004).
The effort to produce a pandemic vaccine promises to send shock waves beyond the drug manufacturing sector. Demand for syringes, vials, and other vaccine-related materials are likely to skyrocket, as will demand for the attention and resources of the FDA’s Center for Biologics Evaluation and Research (CBER). That agency will need to significantly increase its own capacity in order to speed the testing of candidate vaccines. Efficient delivery of the vaccine must also be considered; currently, 85 percent of influenza vaccine in the United States is sold, distributed, and administered by private health care providers. Although participants recognized that this system is not perfect, they advised that in the midst of a pandemic
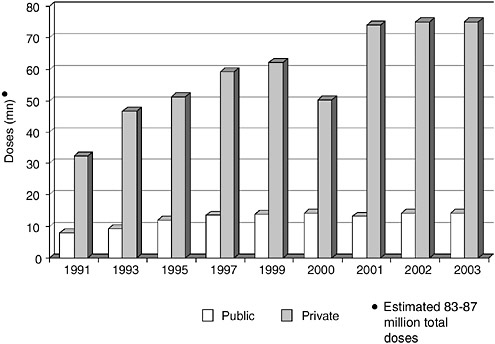
FIGURE S-5 The influenza vaccine market is increasingly a private purchase system. Public versus private demand.
it would be better to build on and improve current distribution networks rather than attempt to replace them. Pandemic vaccine production is expected to displace production of all other vaccines for 1 to 2 years; thus pediatric and other routinely used vaccines would require stockpiling to ensure their uninterrupted availability.
H5N1 “Pandemic-Like” Vaccines
In response to the threat posed by H5N1 in Asia, both the European Union (under the auspices of the European Medicines Evaluation Agency) and the United States (under the auspices of NIAID) have embarked on efforts to produce, and test in clinical trials, a vaccine against the viruses that infected and killed humans. However, divergent approaches are being taken to the initial development of these vaccines on either side of the Atlantic: While the U.S. researchers have given highest priority to assuring that the vaccine is safe and capable of protecting individual recipients against severe disease, the Europeans seek to balance safety with an antigen-sparing dosage level intended to maximize pandemic vaccine supply (see Fedson in Chapter 3).
Some workshop participants raised concerns that in the event of an imminent pandemic, the NIAID approach would provide enough vaccine to supply only a fraction of the U.S. population (Fedson, 2004a). Earlier European trials with other monovalent pandemic-like vaccines had successfully added adjuvant to gain immunogenicity at low doses, it was observed, and it was suggested that such a formulation would assure the largest possible vaccine supply. Americans and Europeans were also urged to develop a common process for funding the clinical trials needed to develop candidate pandemic vaccines. One workshop participant proposed that the candidate H5N1 vaccine, after validation for immunogenicity and reactogenicity, should be deployed as soon as possible in the at-risk populations of south east Asia and data should be collected and analyzed on its effectiveness.
The program officer for the National Institutes of Health (NIH) clinical trials for the H5N1 vaccine responded to these concerns by stating that NIAID, which is part of NIH, will initially focus on the safety and dose-related antigenicity of vaccines produced by currently licensed manufacturing processes. Although influenza vaccines formulated with adjuvants have not consistently enhanced immune responses in previous studies and have in some cases shown increased reactogenicity, NIAID expects to conduct direct comparisons of pandemic-like vaccines with and without adjuvants in the future.
Technical Innovations
Without the use of reverse genetics—the cloning of inactivated hemagglutinin and neuraminidase genes and their expression in a recombinant virus in cell culture—the seed strain for the H5N1 vaccine currently in clinical trials might never have been produced. Avian influenza viruses often grow poorly—and some kill—the embryonated eggs in which commercial viral seed stocks are grown (see Fedson in Chapter 3). During a pandemic, the use of reverse genetics would be expected to speed vaccine production significantly as compared with conventional egg-based methods. MedImmune Incorporated, the major patent holder for reverse genetic technology, permitted WHO to employ the method, free of charge, to prepare H5N1 seed strains and provide them to vaccine manufacturers in the United States and Europe (see MedImmune in Chapter 3; Coelingh, 2004). If a company eventually produces pandemic vaccine for profit, however, MedImmune and possibly other parties must be paid a royalty on its patented technology. Although this arrangement does not in theory restrict research on the use of reverse genetics in vaccine production, it was suggested that the necessity to license the technology discourages its use by pharmaceutical companies, few of whom make vaccines due to their already low profit margins. Moreover, MedImmune is not the sole patent
holder in reverse genetic technology (Fedson, 2004b). In Europe, which produces more than 70 percent of the world’s vaccine supply, reverse genetic vaccines also encounter significant regulatory hurdles due to their designation as genetically modified organisms (GMOs). In response to these challenges, European vaccine manufacturers have called for publicly funded international trials of all pilot lots of potential pandemic vaccines made by its companies that produce vaccine before the next pandemic occurs.
Workshop participants also discussed the possible replacement of egg-based viral propagation with mammalian cell culture methods that may lead to more rapid vaccine production. While cell culture may eventually prove viable, and sufficiently cost effective for vaccine manufacturers, participants recognized that egg-based production is the only proven and rigorously tested method of large-scale vaccine manufacture and is likely to remain so for several years to come. Fortunately, promising new methods for boosting antigen yields in egg-based vaccine systems, coupled with adjuvants and delivery devices to enhance vaccine supply and performance, could increase vaccine availability in the near term (Hosbach, 2004; Stöhr, 2004). Participants also learned of recent progress in a baculovirus-derived antigen production system for a recombinant, trivalent influenza vaccine that is expected to enter Phase III trials within months (Jones et al., 2003). In 1997, this method produced 1,700 doses of a vaccine against “Hong Kong” avian influenza in a total of 8 weeks.
Coping with Limited Vaccine Supplies
While encouraged by the variety of ways in which influenza vaccine may be made increasingly available, participants also emphasized the need to make just and optimal use of limited vaccine stocks in an imminent pandemic—and the challenges that could prevent this from happening. Chief among these hurdles is the fact that 95 percent of the world’s vaccine is produced by countries accounting for only 12 percent of its population (see Fedson in Chapter 3). Thus in the widely expected event that vaccine-producing countries nationalize vaccine production in response to pandemic influenza, nearly 90 percent of the world’s population would be denied timely access to vaccine. Participants therefore urged the negotiation of international agreements to ensure equitable vaccine distribution in advance of a pandemic. WHO also stresses the importance of developing and evaluating non-medical interventions for use in populations without access to a pandemic vaccine (Stöhr, 2004). At the national level, as discussed previously (with regard to pandemic planning) and subsequently (with regard to mathematical and economic models of pandemic impact), the probability of vaccine shortages necessitates the prioritization of recipients. Such a scenario is currently unfolding with the U.S. vaccine shortages for the
2004–2005 influenza season. The United States is attempting to prioritize the vaccine by urging healthy adults to forego the shot this year so that the remaining doses can go to the youngest, oldest, and sickest Americans, who are the most vulnerable to influenza. The two difficulties with this prioritization method are that the prioritization is voluntary and not enforced, allowing anyone to receive it, and it has been difficult for the health care industry to effectively distribute the limited supplies to individuals and facilities that need it most in different regions of the country.
Antiviral Drugs
Vaccines provide the best protection against influenza, but due to their limitations, as detailed in the previous section, they are not likely to protect the vast majority of people, particularly in the early phases of a pandemic. This breach could be filled by antiviral drugs, which have been shown to be effective in influenza treatment and prophylaxis (Longini et al., 2004; Monto, 2003); evidence indicates that antivirals also inhibit bacterial superinfection, a significant cause of influenza-related mortality in the elderly and other high-risk individuals (Petola and McCullers, 2004; Kaiser et al., 2000, 2003). Unlike vaccines, antivirals currently can be stockpiled in advance of a pandemic. Ensuring adequate supplies of antivirals and methods for effective mass distribution to higher-risk populations would be critical to any containment of a pandemic that could be achieved by antiviral prophylaxis (especially in lieu of adequate vaccine supply).
Of the two classes of antiviral agents, M2 inhibitors (amantidine and rimantidine) and neuraminidase inhibitors (zanamivir and oseltamivir), only the latter appear to be effective against current H5N1 avian influenza, considered to be the likeliest source of the next pandemic. Unfortunately, similar obstacles to those hindering vaccine use stand in the way of fighting flu with neuraminidase inhibitors. Only two manufacturers produce these compounds, and their existing surge capacity is unlikely to meet pandemic demand. One means to increase the availability of neuraminidase inhibitors is similar to that proposed for vaccines: to make greater use of the product in treating and preventing influenza in interpandemic seasons, with the expectation that capacity for its production will rise with demand; currently only 1.5 million oseltamivir treatments are produced per year.
Timely access to antiviral drugs is also a concern, because they would need to be administered early in a pandemic to provide effective prophylaxis against influenza. Thus in addition to setting priorities for the distribution of limited supplies of antiviral drugs, including how much of an emphasis to place on prophylaxis versus therapeutic use, pandemic preparations should provide for the rapid and efficient distribution of prophylactic doses. The draft U.S. pandemic plan assigns priority for antiviral pro-
phylaxis to health care workers and other public service providers who are likely to be exposed to the virus and to workers who cull infected animals (Gellin, 2004). A plea was also made to award high priority to employees of pharmaceutical companies who are engaged in producing pandemic vaccine (Hosbach, 2004).
Stockpiles
Unlike vaccines, antiviral drugs can be stockpiled in anticipation of a pandemic. The strategic U.S. national stockpile has made an initial purchase of oseltamivir, and the federal government is studying various stockpiling options and distribution strategies for the future (Gellin, 2004). Oseltamivir may be stockpiled either as “active pharmaceutical ingredient” (API) or after formulation into pills or capsules; each model offers advantages and disadvantages (Brown, 2004). Reserves of oseltamivir, an oral suspension that can be used for either adults or children, are relatively inexpensive and have a shelf life of at least 5 years; however, the drug is relatively difficult to distribute and would require regulatory review because it is currently approved for sale only in capsule form. Oseltamivir capsules, sold under the name Tamiflu™, take longer to produce than the API and are not approved for use in people who weigh less than 88 pounds. Because much about the U.S. plan for pandemic use of antiviral drugs (and oseltamivir in particular) remains to be determined, manufacturers cannot predict the extent to which they can recoup the costs of increasing production capacity. Moreover, unlike vaccine manufacturers, antiviral drug producers recognize that stockpiling their product means that this expanded capacity may be used only once.
Participants also considered the possibility of creating an international stockpile (either as a single entity controlled by WHO, or a virtual stockpile contributed by participating countries from their national stockpiles) that could be made available to slow or contain a potential pandemic—or any emerging zoonosis—at an early stage. The effectiveness of such targeted antiviral prophylaxis has been suggested by studies of influenza transmission within families, and has also been explored through mathematical models of infectious disease transmission (see subsequent discussion on emerging technical tools). Several participants emphasized that negotiations to build such a stockpile should be undertaken immediately, given the likelihood that the production of antiviral drugs, as with vaccines, will be nationalized in the event of a pandemic. The need for such a stockpile was also demonstrated by the recent experience of WHO, which attempted to introduce antiviral prophylaxis early in the recent Asian H5N1 outbreak. The organization’s order for neuraminidase inhibitors was not delivered for
2 weeks, and it was limited to 6,000 doses—too little and too late to avert a pandemic, if one had been imminent (Stöhr, 2004).
Inappropriate Use
Workshop participants were alerted to the danger posed by the inappropriate use of antiviral medications during a pandemic, as apparently occurred during the recent Asian H5N1 epidemic (Brown, 2004). Patients who take the drug for only 2 or 3 days gain significant relief from flu symptoms, but continue to shed virus (Treanor et al., 2000)—and virus that is potentially resistant to oseltamivir—if they do not complete the prescribed 5-day course. This is an especially worrisome trend and should be an area of future investigation given the scarcity of antiviral medications in the drug development pipeline that potentially could be substituted for oseltamivir should resistance to the drug develop during or before a pandemic.
Antiviral Resistance Surveillance
As antivirals are increasingly used for treatment of the Asian H5N1 epidemic, surveillance in both humans and animals (i.e., poultry, swine) for emergence of a drug-resistant strain is essential. Concerns are loss of drug efficacy, spread of resistant variants, and possible increased virulence or transmissibility of resistant variants. Recent reports have raised concerns that the frequency of antiviral resistance might be higher than previously observed when Tamiflu™ is used for treatment of influenza in infants and young children (Kiso et al., 2004). Further data on the frequency of resistance emergence, its relationship to dosing regimens and its consequences are needed for anti-influenza agents (NISN, 2004). It was suggested during discussion that a need exists for continued surveillance of antiviral susceptibility patterns in community isolates and for resistance transmission in high-risk epidemiological settings.
The Role of the Private Medical System
Although the American health care system is overwhelmingly privatized, little attention has been paid to private medicine’s potential role in preparing for pandemic influenza. Thus the workshop presentation and discussion led by Gordon Grundy—a regional medical director for Aetna, one of the country’s largest managed care insurance corporations—introduced a novel perspective and several new ideas to discussions of pandemic planning (see Chapter 3). Workshop participants considered how partnerships could be fostered between government and managed care organizations to better prepare the United States to cope with an influenza pan-
demic, the resources each partner could bring to the table, and some key issues that must be addressed by private health care organizations to prepare themselves for pandemic influenza.
Approximately two thirds of the U.S. population is insured by the more than 1,300 private health care plans that operate in this country (Grundy, 2004). A key mission of such plans is to control the expense of members’ medical care; this is accomplished in part through “gatekeeping” measures that include requirements for referrals and restrictions on formularies. Although these policies are generally effective in holding down health care costs, health plan administrators realize these policies would hinder the medical response to a public health crisis such as pandemic influenza. Aetna and other insurers have therefore waived certain cost-containment measures in response to emergencies including the September 11, 2001, terrorist attacks, the 2003 blackout of the northeastern United States, and various natural disasters, and they could be expected to do so in the event of an influenza pandemic.
But the private health system has done little toward planning—or even considering—a coordinated response to pandemic influenza (Grundy, 2004). Likewise, few federal, state, or local officials have attempted to engage representatives of the private medical system in the pandemic planning process. In considering how such a partnership for pandemic planning might be initiated, participants advised that it represent a broad spectrum of health plans, perhaps under the aegis of an industry trade association. One such group, the Council for Affordable Quality Health Care, has taken on other public health initiatives, including a partnership with CDC to encourage appropriate antibiotic use.
Potential Contributions to Preparedness
Private health care organizations could contribute to pandemic preparedness in several ways. Their broad outreach capabilities could be used to educate their members about the risks posed by influenza, and they could provide incentives to encourage annual immunization, particularly for individuals at high risk for complications. Health plans could also offer financial incentives to physicians for providing services that prevent or mitigate the impact of pandemic influenza. The vast patient databases maintained by health plans could be used to contact and alert vulnerable members to an impending pandemic and instruct them in protective measures. Health plans could also help identify and establish facilities for mass immunizations in nontraditional sites (e.g., shopping malls). These same medical record database systems could also be utilized to provide surveillance data on emerging outbreaks of influenza to the public health system as a part of an early warning system to engage an effective response.
Managed care providers also have much to offer to the pandemic planning process (Grundy, 2004). Their detailed knowledge of the capacity and limitations of most community health care could be brought to bear on local or regional planning to accommodate a pandemic surge. Health plans could also recommend strategies to reduce this burden based on their expertise in providing cost-effective alternatives to intensive care and in optimizing the use of other health care resources. In return, health plans can best support the government’s response to pandemic influenza if plan managers receive clear statements, directives, and recommendations as to their industry’s role and responsibilities in that response. For example, health plan managers will need to know the priority targets for immunization and the rationale governing these choices, whether it is reducing overall morbidity and mortality, minimizing lost productivity, or safeguarding health care workers. Both partners also need to understand the financial consequences to private medicine of responding to (or simply weathering) pandemic influenza, and particularly how health care providers will be compensated for the services they provide during a pandemic.
In addition to encouraging participation by the private medical system in pandemic planning at all levels of government, workshop participants also urged private care providers—particularly hospitals and hospital systems—to make their own preparations for pandemic influenza. Issues that might be addressed by such plans include what to do when hospital resources are exhausted (send patients away? make arrangements for home health care for as many patients as possible?) and strategies for maintaining services when significant numbers of hospital workers and health care providers contract influenza. “To my knowledge, health plans do not have a comparable pandemic plan … to what is being discussed in the public sector,” Dr. Grundy observed. “We do have contingency plans [for delivering critical messages to members], but I can tell you, we need a [pandemic] plan in my shop.”
Emerging Technical Tools
Targeting Broadly Conserved Viral Features
Strain-specific immunization offers the best protection against influenza, but as previously discussed, is unlikely to be widely available in time to have a significant impact on the course of an imminent pandemic. It has long been recognized that people who have had influenza tend to have less severe symptoms upon subsequent infections with immunologically distinct viruses. This phenomenon, known as heterosubtypic immunity (Het-I), was first characterized in animal studies that began in the 1960s. Het-I is a non-sterilizing immunity—that is, it reduces symptoms but does not eliminate
viral replication—induced by one influenza A subtype that protects against another (see Epstein in Chapter 5; Epstein, 2004). It can be induced in mouse models with live, wild-type viruses or inactivated viruses given mucosally, but has not been studied for the live attenuated viruses sometimes used in influenza vaccines. Although Het-I has not been demonstrated to occur in humans, accounts of the 1957 pandemic suggest that it occurred and that additional historical epidemiological investigation would reveal further evidence of its existence.
Researchers thus reasoned that if Het-I could be induced in humans through mass or routine immunization, there is the possibility that they would gain broad cross-protection against all influenza A subtypes, which should at least reduce mortality in a pandemic until a matched vaccine became widely available (Epstein, 2004). Vaccines could be made in advance and administered to prime immunity, and they could be used “off the shelf” in the event of a pandemic to reduce symptoms until a matched vaccine became widely available. Several proteins that are relatively conserved among all subtypes have proved promising targets for such a strategy, and have been shown to induce immunity that greatly reduced morbidity and mortality in mouse influenza models (Epstein, 2004; Epstein et al., 2002). These antigens were delivered in the form of DNA vaccines, which offer several advantages: They can be preserved at ambient temperatures, removing the need for a cold chain; because they are produced in bacterial cell culture, not in eggs or mammalian cell cultures, they might eventually be cheaper or faster to produce; and they permit investigation of the roles of individual viral proteins in immunity. However, it must be stated that although a DNA vaccine strategy may be effective in addressing pandemic influenza, it is years away from clinical use because no DNA vaccine has been shown to provide effective immune protection from disease (any disease) nor has one been registered with the FDA for future approval.
Another weapon that could be aimed at broadly conserved features among influenza A strains is RNA interference (RNAi) technology, which consists of short, complementary RNA sequences that inhibit protein expression. In this case, the target proteins are necessary for influenza A replication, of which several are relatively conserved among known viral subtypes. Prior studies on this technology have examined viral systems in vitro and in vivo, but few disease models have been explored. Recently, researchers from the CBER and CDC demonstrated that RNAi could protect against lethal virus challenge by H5N1, H1N1, and H7N7 in mice (Tompkins et al., 2004). A second group from the Massachusetts Institute of Technology produced similar results in mice against H1N1 using an intranasal plasmid delivery system that could be adapted for clinical use (Ge et al., 2004).
Although these initial results appear promising, workshop participants raised important challenges that must be resolved before taking this tar-
geted approach to the clinic. Foremost among them is the need to examine the potential risk that immunization against conserved viral features could under some circumstances result in immunopathology (e.g., immune enhancement) rather than protection (Epstein, 2004). The cost of these measures—particularly RNAi, which would be classified as a drug therapy—is also prohibitively expensive as currently delivered.
Transgenic Suppression of Influenza Virus Replication in Chickens
Given that the next influenza pandemic is widely expected to be avian in origin and to emerge from domestic poultry (though perhaps detouring through pigs or another mammalian species on the way), it would seem desirable to inhibit the replication of influenza viruses in chickens, greatly reducing the danger of transmission to humans or other livestock. Recent developments in transgenic technologies and inhibitory strategies make possible the engineering of disease-resistant livestock, including influenza A-resistant chickens; meanwhile, research on the influenza virus has revealed promising strategies for inhibiting influenza replication (see Tiley and Sang in Chapter 5). In addition to RNAi, described above, influenza A replication potentially could be suppressed through the introduction of Mx genes—which block the expression of incoming viral genomes in several mammal and bird species, but not in chickens—and through the presence of RNA decoys, short sequences that mimic the binding sites of RNA proteins and thereby act as competitive inhibitors for transcription. By combining these strategies, researchers hope to achieve complete blockage of influenza replications and prevent the development of resistant viral strains; if they fall short of this goal, dangerous “silent epidemics” of sub-clinical infection could occur (see the earlier discussion of this phenomenon in relation to avian influenza vaccines).
Until recently, the lack of a delivery system suitable for engineering the chicken genome posed a major roadblock to developing influenza virus-resistant poultry. Thanks to the advent of lentiviral vectors, which can be prepared to very high concentrations and can successfully infect and integrate into the chromosomes of virtually any cell type, the first attempts to produce influenza-resistant transgenic birds are currently underway. If these efforts prove successful, researchers must then face the far more daunting challenge of demonstrating the system’s long-term efficacy, and perhaps more importantly, its lack of detrimental effects on chickens, humans who consume poultry products, and the environment. While acknowledging that many people hold negative attitudes toward genetically modified organisms, presenter Laurence Tiley (see Chapter 5) observed that “even the direst GMO scare-mongering scenario” pales in comparison to that of another 1918.
Mathematical Models of Pandemic Containment
Mathematical modeling of pandemic scenarios added another dimension to a recurring topic of discussion: how to manage likely shortages of vaccines and antiviral medications (see Medema et al. in Chapter 5). Although models are not evidence of effectiveness, it was suggested by several presenters that they could be helpful decision-making tools during the crisis of a pandemic outbreak. One presentation described the use of a stochastic model that simulates daily contacts by a cross-section of Americans to examine the effects of epidemic influenza under various regimens of antiviral prophylaxis and/or vaccination (Longini, 2004; Longini et al., 2004; Monto, 2003). A strategy known as targeted antiviral prophylaxis (TAP), in which antiviral medications are given to people believed to be in close contact with index cases, was found to temporarily contain influenza transmission when 80 percent of all identifiable contacts were treated prophylactically for 4 weeks; similar results were obtained in simulations when 50 percent of the population in the model was immunized (Longini et al., 2004). The epidemic was extinguished when the TAP treatment was extended to 8 weeks, much as it was when 80 percent of the children in the model population were vaccinated. Vaccinating children, as opposed to adults, was found to increase the effectiveness of vaccination in this model (Longini et al., 2004).
Beyond the Biomedical Response
A comprehensive response to pandemic influenza should address far more than the disease itself. As several of the previously discussed pandemic plans have anticipated, a pandemic will introduce a plethora of legal and ethical dilemmas and political and economic consequences. It will also take place in a social context in which public perception of and reaction to an emergency strongly influences its impact. In light of these profound influences, workshop participants considered a variety of social perspectives on the coming pandemic: economic, legal, and ethical implications of various response options; opportunities for collaboration between public and private sectors; and public communication strategies to address both interpandemic and pandemic influenza.
“Insuring” the Pandemic Response
Pandemic preparations can be viewed as an insurance policy: an investment, accumulated over time, in anticipation of an eventual crisis (see Chapter 6) (Meltzer, 2004). The annual rate of investment in pandemic preparedness, the “premium” on the insurance policy, depends on the potential impact of pandemic influenza and its probability of occurrence in
any given year. Using a relatively simple, conservative model, presenter Martin Meltzer and colleagues determined the total costs of a moderate (15 percent attack rate) and severe (35 percent attack rate) influenza pandemic, then calculated annual “premiums” to be paid on preparations against these losses based on the cost of vaccination (Meltzer et al., 1999).
The cost of vaccination, however, depends on the population segment that first receives the projected limited quantities of pandemic vaccine. Given the expected pattern of higher mortality in the elderly, it would be most cost effective to vaccinate high-risk people of working age, but more deaths would be prevented if the high-risk elderly are given priority (Meltzer et al., 1999). Only one of these outcomes can be maximized, so decision-makers must make the difficult choice among them. This requirement highlights the need for a system by which such a choice could be made, as well as a means to gain public understanding and support for the decision-making process (and thereby, for its outcome). Having chosen a goal, public health officials must be vigilant for changes in patterns of pandemic mortality, and be ready to adapt interventions to support the desired outcome.
To make optimal use of funds set aside for pandemic preparedness, decision-makers were advised to invest in activities that both ensure a strong pandemic response and enhance the response to annual influenza (Meltzer, 2004). These include improvements in surveillance (including support for surveillance in low-resource countries where a pandemic strain is likely to emerge), increasing vaccination rate among high-risk individuals (and perhaps providing a financial incentive to do so), and conducting planning and preparedness exercises to strengthen the response to a broad range of possible public health emergencies.
The Legal and Ethical Context
Legal authority should be brought to bear on nearly every facet of pandemic preparedness, from measures designed to reduce the risk of animal-to-human transmission of disease; to surveillance and detection procedures; to medical interventions to prevent or control the spread of infection; to the imposition of voluntary or mandatory quarantine and/or isolation measures; to travel limitations, trade restrictions, and border closures (see Gostin in Chapter 6) (Gostin, 2004b). Each of these interventions, while potentially beneficial to society, also imposes a burden on at least some of its members in the form of economic disadvantage, loss of political power, or sacrifice of human rights. Moreover, if these measures are to be effective, they should be imposed early in the course of a pandemic, before it can be scientifically ascertained whether they are actually warranted. Thus, it was argued, decision makers must undertake transpar-
ent and ethical deliberations in order to safeguard against the possibility of an unjustified and burdensome response to an apparent pandemic threat.
But public health law at many levels is not sufficiently robust to meet this daunting challenge. At the international level, efforts to ensure strong surveillance and response to infectious disease outbreaks are hampered by the outdated International Health Regulations, which are currently undergoing revision (Gostin, 2004a). Strong national infrastructures for public health law also need to be developed to support the delivery of essential services, assign public health powers, and safeguard human rights; unfortunately, no such foundation currently exists, because most public health statutes have been enacted in response to a specific (and therefore limited) threat or crisis. In response to this need, model public health laws are currently being developed for the United States (Center for Law and the Public’s Health, 2001), and WHO is studying the development of a national public health law toolkit for worldwide distribution (Gostin, 2004b).
In addition to this legal framework, workshop participants were also urged to evaluate the ethics of public health interventions against pandemic influenza. Compelling ethical considerations include the need for transparency, the importance of anticipating and addressing stigmatization, the pursuit of human rights and social justice, and the fair distribution of scarce resources.
Coordinating Public and Private Sectors
The example of the Department of Veterans Affairs (VA) was used to illustrate a variety of opportunities for public–private partnership in addressing pandemic influenza, including the guaranteed advanced purchase of vaccine, the establishment of coordinated risk communications, the delivery of prophylaxis and treatment in home-based and other non-medical settings, and the removal of disincentives to sheltering in place for ill and exposed workers. Health policy within the VA system focuses on the use of information systems and outcomes measurement in clinical decision-making. This paradigm also supports the collection of standardized data that, if replicated in hospitals nationwide, could be used to build a national health information database—a possibility that has come one step closer to reality with the recent launch of a 10-year federal initiative to develop electronic medical records for most Americans (DHHS, 2004c).
Increasing Immunization Uptake
The previously described confluence of events that led to exceptionally high demand for immunization during the 2003–2004 flu season can be likened to a worst case scenario in order to increase uptake: let the disease
come early, at the beginning of immunization season; let it be severe, striking otherwise healthy people—even lethally, so that it makes headlines and causes medical experts to issue warnings of dire consequences. Unfortunately, a large component of this success depended on the timing and severity of the disease and so may not result in continued high immunization rates; however, this experience could also be seen as a dress rehearsal for communication during an influenza pandemic, with its attendant shortages of vaccine and dire media reports.
Clear communication will be essential to obtaining adherence to mass vaccination campaigns during a pandemic, but it faces several challenges, particularly among people who consistently refuse annual flu shots. Mixed messages will be hard to avoid, particularly given the fact that only a minority (less than 40 percent) of health care workers are immunized each year. On the one hand, people with high priority to receive vaccine will need to be encouraged (and perhaps made afraid enough) to get it; others will need to be encouraged to wait calmly for their opportunity to receive vaccine while using non-medical measures to reduce their exposure to infection. Unless public messages are tailored to gain the attention of specific segments of our racially and culturally diverse society, they are likely to be ignored.
The most effective way that public officials can avoid a damaging credibility problem in a pandemic, participants advised, is by sharing the dilemmas of pandemic control with the public in a productive and effective way—that is, by doing more than simply furnishing facts and figures. More research is needed to learn how to do this well; in the meantime, public health officials are advised to invest in targeted (as opposed to nuanced) and widely dispersed communications in order to sway as many “undecideds” as possible to the cause of influenza prevention and control (see Nowak in Chapter 6).
Public Communication Strategies
The history of public response to a variety of natural disasters demonstrates that people are capable of effective collective action in these circumstances, and conversely, that failure to involve the general public in crisis response can increase the likelihood of social disruption (Glass and Schoch-Spana, 2002). Although it is widely believed that public support for a pandemic response can only be won through good communication, it is important to recognize that “good communication” is perceived differently by the communicator, who wants public cooperation and understanding in a time of crisis, and members of the public, who want inclusion, consideration, respect, expert guidance, and proof that officials have justly consid-
ered the public (see Schoch-Spana in Chapter 6). Risk communication,18 a key component of the WHO pandemic plan and several national plans, attempts to bridge this gap by providing individuals and communities with information that allows them to make the best possible decisions about their well-being.
Systemic challenges make it difficult to get such messages out, however. Due to the multiplicity of contemporary media outlets, the significant decline in newspaper readership, and the lack of a public broadcast system (in the United States) public health messages should be broadly directed and more effectively coordinated among agencies and official channels that often compete for resources and notoriety. Although a more coordinated effort at the federal level is paramount, it was suggested that important information could be disseminated effectively through trusted sources in communities, such as physicians, neighborhood leaders, schools, or places of worship. It was also noted that official attitudes that equate overwhelming public requests for information with “panic” obscure an important opportunity for communication. Rather than view these demands as a distraction, communicators were advised to address the public’s increased need for information as part of an emergency response (Schoch-Spana, 2000). Both the quality and quantity of information available to the public should address the fears as well as the facts about disease spread, and are key to promoting public security as well as more effective public health. Chapter 6 (see Schoch-Spana) describes the development of analytical tools for decision-makers to help them forge collaborations with the public during health crises.
REFERENCES
Americans cross border for flu shots. 2004. CNN. [Online]. Available: http://www.cnn.com/2004/HEALTH/conditions/10/20/fluvaccine.canada.ap/ [accessed November 10, 2004].
ASTHO (Association of State and Territorial Health Officials). 2002. Nature’s Terrorist Attack: Pandemic Influenza; Preparedness Planning for State Health Officials. Washington, DC: ASTHO.
Barclay WS, Zambon M. 2004. Pandemic risks from bird flu. BMJ 328(7434):238–239.
Brown P. 2004 (June 16). Ensuring an Adequate Stockpile of Antivirals. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
|
18 |
Defined by CDC as the attempt by science or public health professionals to provide information that allows individuals, stakeholders, or an entire community to make the best possible decisions about their well-being, under nearly impossible time constraints, and to communicate those decisions, while accepting the imperfect nature of their choices (source: http://www.cdc.gov/communication/emergency/ercoverview.htm). |
Buranathai C. 2004 (June 17). Avian Influenza Outbreak in Thailand 2004. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Cardona C. 2004 (June 17). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
CDC (Centers for Disease Control and Prevention). 2000. FluAid Home. [Online]. Available: http://www2.cdc.gov/od/fluaid/ [accessed November 10, 2004].
CDC. 2004a. Outbreaks of avian influenza A (H5N1) in Asia and interim recommendations for evaluation and reporting of suspected cases—United States. MMWR 53(05):97–100.
CDC. 2004b. Interim Influenza Vaccination Recommendations—2004–05 Influenza Season. [Online]. Available: http://www.cdc.gov/flu/protect/whoshouldget.htm [accessed November 10, 2004].
CDC. 2004c. Pandemic Influenza Planning. [Online]. Available: http://www.cdc.gov/programs/immun5.htm [accessed November 10, 2004].
Center for Law and the Public’s Health. 2001. Model State Emergency Health Powers Act and the Turning Point Model State Public Health Act. [Online]. Available: http://www.publichealthlaw.net [accessed November 10, 2004].
Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P, Zhang L, Liu Z, Webster RG, Yu K. 2004. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci USA 101(28):10452–10457.
Coelingh K. 2004 (June 17). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Cox N. 2004 (June 17). Enhancing Influenza Surveillance: From the Global to Local Perspective. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
DHHS (Department of Health and Human Services). 2004a. Pandemic Influenza Preparedness and Response Plan. [Online]. Available: http://www.hhs.gov/nvpo/pandemicplan [accessed November 10, 2004].
DHHS. 2004b. HHS Awards $849 Million to Improve Public Health Preparedness. [Online]. Available: http://www.hhs.gov/news/press/2004pres/20040617.html [accessed November 10, 2004].
DHHS. 2004c. The decade of health information technology: Delivering consumer-centric and information-rich health care. HHS Fact Sheet—HIT Report At-A-Glance. [Online]. Available: http://www.hhs.gov/news/press/2004pres/20040721.html [accessed November 10, 2004].
Epstein SL. 2004 (June 16). Strategies for Control of Influenza by Targeting Broadly Conserved Viral Features. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, Renshaw M, Sambhara S, Katz JM. 2002. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. EID 8(8):796–801.
Fedson DS. 2004a (June 16). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Fedson DS. 2004b. Vaccination for pandemic influenza: A six point agenda for interpandemic years. Pediatr Infect Dis J 23:S74–S77.
GAO (General Accounting Office). 2000. Influenza Pandemic: Plan Needed for Federal and State Response. [Online]. Available: http://www.gao.gov/new.items/d014.pdf [accessed December 20, 2004].
Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. 2004. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA 101(23):8676–8681.
Gellin B. 2004 (June 16). U.S. Government Pandemic Influenza Preparedness Plan. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Gensheimer KF, Meltzer MI, Postema AS, Strikas RA. 2003. Influenza pandemic preparedness. EID 9(12):1645–1648.
Glaser CA, Gilliam S, Thompson WW, Dassey DE, Waterman SH, Saruwatari M, Shapiro S, Fukuda K. 2002. Medical care capacity for influenza outbreaks, Los Angeles. EID 8(6):569–574.
Glass TA, Schoch-Spana M. 2002. Bioterrorism and people: How to vaccinate a city against panic. Clinical Infectious Diseases 34:217–223.
Gostin LO. 2004a. International infectious disease law: Revision of the World Health Organization’s International Health Regulations. JAMA 291(21):2623–2627.
Gostin LO. 2004b (June 17). Preparing the Legal System for Pandemic Influenza. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Grove RD, Hetzel AM. 1968. Vital Statistics Rates in the United States: 1940–1960. Washington, DC: Government Printing Office.
Grundy G. 2004 (June 16). Partnering with the Private Medical System. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Guan Y, Poon LL, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Sturm-Ramirez KM, Cheung CL, Leung YH, Yuen KY, Webster RG, Peiris JS. 2004. H5N1 influenza: A protean pandemic threat. Proc Natl Acad Sci USA 101(21):8156–8161.
Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB; Centers for Disease Control and Prevention Advisory Committee on Immunization Practices. 2004. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports 53(RR-6):1–40.
Health Canada. 2004. Canadian Pandemic Influenza Plan. [Online]. Available: http://www.hc-sc.gc.ca/pphb-dgspsp/cpip-pclcpi/ [accessed December 20, 2004].
Health Protection Agency. 2004. Number of Avian Flu Outbreaks in Thailand Reporting Period: 05/08/04 to 03/09/04. [Online]. Available: http://www.hpa.org.uk/infections/topics_az/avianinfluenza/pdfs/Thailand_outbreak_map0904.pdf [accessed November 18, 2004].
Hosbach P. 2004 (June 16). Vaccine Development and Production Issues. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
IOM (Institute of Medicine). 2004. Learning from SARS. Washington, DC: The National Academies Press.
Jones T, Allard F, Cyr SL, Tran SP, Plante M, Gauthier J, Bellerose N, Lowell GH, Burt DS. 2003. A nasal proteosome influenza vaccine containing baculovirus-delivered hemagglutinin induces protective mucosal and systemic immunity. Vaccine 21(25–26):3706–3712.
Kaiser L, Keene ON, Hammond JM, Elliott M, Hayden FG. 2000. Impact of zanamivir on antibiotic use for respiratory events following acute influenza in adolescents and adults. Arch Intern Med 160(21):3234–3240.
Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. 2003. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 163(14):1667–1672.
Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RAM, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theamboonlers A, Tantilertcharoen R, Pattanarangsan R, Arya N, Ratanakorn P, Osterhaus A, Poovorawan Y. 2004 (December). Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases. [Online]. Available: http://www.cdc.gov/ncidod/EID/vol10no12/04-0759.htm [accessed December 10, 2004].
Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y. 2004. Resistant influenza A viruses in children treated with oseltamivir: Descriptive study. Lancet 364(9436):759–765.
Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, Usui T, Murata T, Maeda Y, Watanabe S, Suresh M, Suzuki T, Suzuki Y, Feldmann H, Kawaoka Y. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431(7009):703–707.
Koch G. 2004 (June 17). Some Lessons Learned from the Dutch Avian Influenza Outbreak 2003. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Osterhaus A. 2004. Avian H5N1 influenza in cats. Science 306:241.
Lee CW, Senne DA, Suarez DL. 2004. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J Virol 78(15):8372–8381.
Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430(6996):209–213.
Linder FE, Grove RD. 1943. Vital Statistics Rates in the United States: 1900–1940. Washington, DC: Government Printing Office.
Liu M, Guan Y, Peiris M, He S, Webby RJ, Perez D, Webster RG. 2003. The quest of influenza A viruses for new hosts. Avian Diseases 47:849–856.
Longini IM. 2004 (June 16). Mathematical Modeling: Containing Pandemic Influenza with Vaccines and Antivirals. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Longini IM Jr, Halloran ME, Nizam A, Yang Y. 2004. Containing pandemic influenza with antiviral agents. Am J Epidemiol 159:623–633.
Lovgren S. 2004. Cats can catch and spread bird flu, study says. Stefan Lovgren for National Geographic News. [Online]. Available: http://news.nationalgeographic.com/news/2004/09/0902_040902_birdflu.html#main [accessed November 10, 2004].
Marchione M. 2004. Pandemic Risk Spurs Flu Vaccine Planning. [Online]. Available: http://www.washingtonpost.com/wp-dyn/articles/A13922-2004Oct31.html [accessed November 10, 2004].
Meltzer M. 2004 (June 16). “Insuring” a Better Response. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Meltzer MI, Cox NJ, Fukuda K. 1999. The economic impact of pandemic influenza in the United States: Priorities for intervention. Emerging Infectious Diseases 5(5):659–671.
Millar JD. 1977 (June 27). The Swine Flu Program: An unprecedented venture in preventive medicine. Report to the Congress by the Comptroller General of the United States. HRD-77-115. Washington, DC: Department of Health, Education, and Welfare.
Monto AS. 2003. The role of antivirals in the control of influenza. Vaccine 21(16):1796–1800.
Nguyen TD. 2004 (June 17). Retrospection into Avian Influenza Outbreak in Vietnam during 2003-04. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Nichol KL. 2003. The efficacy, effectiveness, and cost-effectiveness of inactivated influenza vaccines. Vaccine 2003:1769–1775.
NISN (Neuraminidase Inhibitor Susceptibility Network). 2004 (August 13). Neuraminidase Inhibitor Susceptibility Network statement. Weekly Epidemiological Record 79:306–308. [Online]. Available: http://www.who.int/wer/2004/en/wer7933/en/ [accessed November 10, 2004].
Nowak G. 2004 (June 17). Increasing Awareness and Uptake of Influenza Immunization. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Noymer A, Garenne M. 2000. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Population and Development Review 26(3):565–581.
OIE (Office International des Epizooties). 2004. Disease Information. 17(36). [Online]. Available: http://www.oie.int/eng/info/hebdo/a_current.htm#Sec0 [accessed December 21, 2004].
Perrotta D. 2004 (June 16). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Petola VT, McCullers JA. 2004. Respiratory viruses predisposing to bacterial infections: Role of neuraminidase. Pediatr Infect Dis J 23:S87–S97.
ProMED-mail. 2004a. PRO/AH/EDR> Avian influenza, human—East Asia (52): update. Published date: October 4, 2004. Archive number: 20041004.2738. Available at http://www.promedmail.org.
ProMED-mail. 2004b (August 31). PRO/AD/EDR> Avian influenza, human—East Asia (38). Published date: August 31, 2004. Archive number: 20040813.2249. Available at http://www.promedmail.org. Source: WHO Outbreak News, 13 August 2004 [edited]: http://www.who.int/csr/don/2004_08_13/en/print.html. “Human cases of avian influenza: situation in Viet Nam.”
ProMED-mail. 2004c (September 29). PRO/AH/EDR> Avian influenza, human—East Asia (49): Viet Nam. Archive number: 20040929.2692. Available at http://www.promedmail.org.
ProMED-mail. 2004d. PRO/AH/EDR> Avian influenza—Eastern Asia (98): Viet Nam, Thailand. Published date: July 27, 2004. Archive number: 20040727.2058. Available at http://www.promedmail.org.
ProMED-mail. 2004e. PRO/AH/EDR> Avian influenza, human—East Asia (46): Thailand, susp. Published date: September 25, 2004. Archive number: 20040925.2647. Available at http://www.promedmail.org.
ProMED-mail. 2004f. PRO/AH/EDR> Influenza vaccine 2004/2005—N. hemisphere (02): supply. Published date: October 5, 2004. Archive number: 20041005.2742. Available at http://www.promedmail.org.
ProMED-mail. 2004g. Avian influenza—eastern Asia (78): Thailand, cats. Published date: June 17, 2004. Archive number: 20040617.1614. Available at http://www.promedmail.org. Source: The Messybeast Cat Resource Archive: 16 June 2004 [edited]: http://www.messybeast.com/zoonoses.htm. “Asian Bird Flu.” Byline: Sarah Hartwell.
ProMED-mail. 2004h. PRO/AH/EDR> Avian influenza—Eastern Asia (127): Thailand, tigers. Published date: Oct 19, 2004. Archive number: 20041019.2838. Available at http://www.promedmail.org.
ProMED-mail. 2004i. PRO/AD/EDR> Avian influenza—Eastern Asia (128): Thailand, tigers. Published date: October 22, 2004. Archive number: 20041022.2862. Available at http://www.promedmail.org.
ProMED-mail. 2004j. PRO/AD/EDR> Avian influenza, poultry vaccines (08). Published date: March 24, 2004. Archive number: 20040325.0829. Available at http://promedmail.org. Source: New Scientist, 24 Mar 04 [edited]: http://www.newscientist.com/news/news.jsp?id=ns99994810. “Bird vaccination could lead to new strains.” Byline: Debora MacKenzie.
ProMED-mail. 2004k (July 16). PRO/AH/EDR> Avian influenza—Eastern Asia (92). Published date: July 16, 2004. Archive number: 20040716.1925. Available at http://www.promedmail.org. Source: The Jakarta Post, 16 July 2004 [edited]: http://www.thejakartapost.com/detailcity.asp?fileid=20040716.G04&i. “IPB, Japan to produce bird flu super vaccine.” Byline: Theresia Sufa.
ProMED-mail. 2004l (July 16). PRO/AH/EDR> Avian influenza—Eastern Asia (92). Published date: July 16, 2004. Archive number: 20040716.1925. Available at http://www.promedmail.org. Source: ABC Online [Australia]: 16 July 2004 [edited]: http://www.abc.net.au/news/newsitems/200407/s1155030.htm. “More bird flu outbreaks in Viet Nam.”
ProMED-mail. 2004m (July 2). PRO/AH/EDR> Avian influenza—Eastern Asia (83): Viet Nam. Published date: July 2, 2004. Archive number: 20040702.1771. Available at http://www.promedmail.org. Source: Taipei Times: http://www.taipeitimes.com/News/world/archives/2004/07/02/20031773. “Mekong bird flu may be spreading to nearby province.”
ProMED-mail. 2004n (July 3). PRO/AH/EDR> Influenza activity update 2003/2004—worldwide. Published date: July 3, 2004. Archive number: 20040703.1776. Available at http://www.promedmail.org.
ProMED-mail. 2004o (August 9). PRO/AH/EDR. Avian influenza—Eastern Asia (103): FAO. Published date: August 9, 2004. Archive number: 20040809.2196. Available at http://www.promedmail.org.
ProMED-mail. 2004p. PRO/AH/EDR> Avian influenza—Belgium ex Thailand: smuggled birds (02): OIE. Published date: October 27, 2004. Archive number: 20041027.2907. Available at http://www.promedmail.org.
ProMED-mail 2004q (July 30). PRO/AH/EDR> Avian influenza—Eastern Asia (100). Published date: July 30, 2004. Archive number: 20040730.2078. Available at http://www.promedmail.org. Source: WHO: http://www.who.int/csr/don/2004_07_30/en/. “Avian influenza—assessment of the current situation.”
ProMED-mail. 2004r (July 29). PRO/AH/EDR> Avian influenza—Eastern Asia (99). Published date: July 29, 2004. Archive number: 20040729.2069. Available at http://www.promedmail.org. Source: Sapa-AFP via IOL South Africa, 28 July 2004 [edited]: http://www.iol.co.za/index.php?set_id=1&click_id=31&art_id=qw109099908550B221. “UN to recommend wider use of bird flu vaccine.”
ProMED-mail. 2004s. PRO/AD/EDR> Avian influenza, poultry vaccines (08). Published date: March 24, 2004. Archive number: 20040325.0829. Available at http://promedmail.org. Source: New Scientist, 24 Mar 2004 [edited]: http://newscientest.com/news/news.jsp?id=ns99994810. “Bird flue vaccination could lead to new strains.”
ProMED-mail. 2004t. PRO/AD/EDR> Avian influenza A/H5N1, migratory birds—Russia (Siberia). Published date: October 28, 2004. Archive number: 20041028.2911. Available at http://promedmail.org.
ProMED-mail. 2004u. (July 30). PRO/AH/EDR> Avian influenza—Eastern Asia (100). Published date: July 30, 2004. Archive number: 20040730.2078. Available at http://www.promedmail.org. Source: WHO: http://www.who.int/csr/don/2004_07_30/en/. “Avian influenza—assessment of the current situation.”
ProMED-mail. 2004v (October 25). PRO/AH/EDR> Avian influenza, human—Thailand (08). Published date: October 25, 2004. Archive number: 20041025.2886. Available at http://www.promedmail.org.
ProMED-mail. 2004w. Avian influenza—Eastern Asia (137): Viet Nam. Published date: November 19, 2004. Archive number: 20041119.3103. Available at http://www.promedmail.org.
ProMED-mail. 2005. PRO/AH/EDR> Avian influenza—Eastern Asia (14). Published date: February 1, 2005. Archive number: 20050201.0348. Available at http://www.promedmail.org.
Schoch-Spana M. 2000. Implications of pandemic influenza for bioterrorism response. Clin Infect Dis. 31(6):1409–1413.
Shult P. 2004 (June 16). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Sibartie D. 2004 (June 17). Strategies for Preventing and Controlling Avian Influenza and its Transmission Within the Bird and Animal Population. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Simonsen L. 2004 (June 17). Pandemic Influenza and Mortality: Past Evidence and Projections for the Future. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Slater E. 2004. Industry and government perspective in influenza control. Texas Heart Institute Journal 31(1):42–44.
Soebandrio A. 2004 (June 17). Avian Influenza: Indonesian Experience. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
State of California. 2003. State of California Hospital Bioterrorism Preparedness Program: 2003 Implementation Plan. Emergency Medical Services Authority, Department of Health Services, State of California. Pp. 1–6, 35–36.
Stöhr K. 2004 (June 16). Priority Public Health Interventions Before and During an Influenza Pandemic. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Swayne D. 2004 (June 17). Panel Discussion at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Taubenberger J. 2004 (June 16). Chasing the Elusive Virus: Preparing for the Future by Examining the Past. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Tompkins SM, Lo C-Y, Tumpey TM, Epstein SL. 2004. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA 101(23):8682–8686.
Trampuz A, Prabhu RM, Smith TF, Baddour LM. 2004. Avian influenza: A new pandemic threat? Mayo Clinic Proc. 79:523–530.
Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J; World Health Organization International Avian Influenza Investigative Team. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 350(12):1179–1188.
Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. U.S. Oral Neuraminidase Study Group. JAMA 283(8):1016–1024.
Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 352(4):333–340.
U.S. Department of Commerce. 1976. Historical Statistics of the United States: Colonial Times to 1970. Washington, DC: Government Printing Office.
Vastag B. 2002. Experts urge bioterrorism readiness. JAMA 285(1):30–32.
Webby RJ, Webster RG. 2003. Are we ready for pandemic influenza? Science 302:1519–1522.
Webster R. 2004a (June 17). A Report from the Field: The 2003/2004 H5N1 Outbreak. Presentation at the Institute of Medicine Workshop on Pandemic Influenza: Assessing Capabilities for Prevention and Response. Washington, DC: Institute of Medicine Forum on Microbial Threats.
Webster R. 2004b. Wet markets—a continuing source of Severe Acute Respiratory Syndrome and influenza? Lancet 363:234–236.
Webster RG. 1998. Influenza: An emerging infectious disease. Emerg Infect Dis 4:436–441.
WHO (World Health Organization). 1999. Influenza Pandemic Plan. WHO/CDS/CSR/EDC/ 99.1. [Online]. Available: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_EDC_99_1/en/ [accessed November 10, 2004].
WHO. 2004a. WHO Consultation on Priority Public Health Interventions Before and During an Influenza Pandemic. [Online]. Available: http://www.who.int/csr/disease/avian_influenza/consultation/en/ [accessed November 10, 2004].
WHO. 2004b. Development of a vaccine effective against avian influenza H5N1 infection in humans. Wkly Epidemiol Rec 79:25–26.
WHO. 2004c (October 29). Avian Influenza—Situation in Asia: Altered Role of Domestic Ducks. [Online]. Available: http://www.who.int/csr/don/2004_10_29/en/print.html [accessed November 10, 2004].
WHO. 2004d. Cumulative Number of Confirmed Human Cases of Avian Influenza A (H5N1) Since 28 January 2004. [Online]. Available: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2004_10_25/en/ [accessed November 10, 2004].