Twelfth Interim Report of the Subcommittee on Acute Exposure Guideline Levels
BACKGROUND
In 1991, the U.S. Environmental Protection Agency (EPA) and the Agency for Toxic Substances and Disease Registry (ATSDR) asked the National Research Council (NRC) to provide technical guidance for establishing community emergency exposure levels (CEELs) for extremely hazardous substances (EHSs) pursuant to the Superfund Amendments and Reauthorization Act of 1986. In response to that request, a subcommittee of the NRC Committee on Toxicology prepared a report titled “Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances” (NRC 1993). That report provides step-by-step guidance for the derivation of CEELs for EHSs.
In 1995, EPA, several other federal and state agencies, and several private organizations, academia convened an advisory committee—the National Advisory Committee on Acute Exposure Guideline Levels (AEGLs) for Hazardous Substances (referred to as the NAC)—to develop, review, and approve AEGLs (similar to CEELs) for up to 400 EHSs. AEGLs developed by the NAC have a broad array of potential applications for federal, state, and local governments, and for the private sector. AEGLs are needed for prevention and emergency response planning for potential releases of EHSs, either from accidents or as a result of terrorist activities.
THE CHARGE TO THE SUBCOMMITTEE
The NRC convened the Subcommittee on Acute Exposure Guideline Levels to review the AEGL documents approved by the NAC. The subcommittee members were selected for their expertise in toxicology, pharmacology, medicine, industrial hygiene, biostatistics, risk assessment, and risk communication.
The charge to the subcommittee is to (1) review AEGLs developed by the NAC for scientific validity, completeness, and conformance to the NRC (1993) guidelines report, (2) identify priorities for research to fill data gaps, and (3) identify guidance issues that may require modification or further development based on the toxicological database for the chemicals reviewed.
This interim report presents the subcommittee’s comments concerning the NAC’s draft AEGL documents for 15 chemicals: toluene, xylenes, ammonia, bromine, aniline, methyl ethyl ketone, hydrazine, iron pentacarbonyl, phosphine, chlorine trifluoride, ethyleneimine, propylenimine, allyl alcohol, ethylene oxide, and nickel carbonyl.
COMMENTS ON TOLUENE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on toluene. The presentation was made by Sylvia Talmage of Oak Ridge National Laboratory. The subcommittee recommends a number of revisions. The subcommittee will review the revised AEGLs draft at its next meeting.
General Comments
The TSD appears too long. It should be condensed.
The consideration to use PBPK modeling is appropriate. After review by NAC, the NAS/COT Subcommittee on AEGLs will review this approach. Some specific points to the PBPK presentation are listed below under Specific Comments.
In a recent publication (Tanaka et al., 2003. J. Med. Sci. 49:129-39), 19 symptoms related to CNS and autonomic nervous system are reported to occur upon exposure to low (15.3-31.5 ppm) concentrations of toluene. It is recommended to evaluate whether these include some symptoms which may serve as starting points for AEGL derivation and to include this study in Section 2.2.1 (page 20, line 22).
It would be desirable and hopefully possible for the justifications of the AEGL values to be more explicit. Thus, on one hand, the use of 200 ppm for AEGL-1 (page 82, line 12) appears too conservative. This exposure level is a threshold for altered performance of an extended series of certain complex psychophysiological tests by humans. If the AEGL-1 values are to be based on CNS depression, the 15-min 300 ppm NOAEL of Baelum et al. (1990) could be used in conjunction with PBPK modeling to extrapolate the shorter and longer exposure periods. Once a decision is reached about factoring exercise into derivation of AEGLs, this can also be accommodated by PBPK modeling.
On the other hand, an intraspecies uncertainty factor (UF) of 1 was used to derive AEGL-1, yet insufficient scientific support appears to be provided to justify a reduction of the standard UF of 10 to 1 (as if there were zero intraspecies uncertainty left).
The text says that the “preponderance of data as a weight of the evidence consideration indicates that an 8-hr exposure to 200 ppm would be without adverse effects for the general population” (page 7, lines 33-34). The discussion in the text (page 81, Section 5.3 Derivation of AEGL-1) is even more vague.
The study used as the basis for deriving the AEGL-2 values is weak and not supported well by the studies cited in the text. The primary study is by Gamberale and Hultengren (1972), where people were exposed to 700 ppm toluene for 20 min. It says that at this level, “only a very subtle effect on the CNS was observed during this short exposure” (page 83, lines 5-6). Given the numerous studies on toluene, it is surprising that this is the best study that could be found to derive AEGL-2.
Also the number taken as the basis for the AEGL-2 (page 85, lines 15-18) merits reconsideration.
The 20-min 700-ppm exposure cannot be considered a true 20-min exposure. As noted in line 17, it immediately followed successive 20-min 100-, 300- and 500-ppm sessions. PBPK modeling should be used to establish what the subjects’ blood level would have been after such a four-part regimen. This blood level would serve as the basis from which to extrapolate (by PBPK) to other time periods to predict the magnitude of exposures that would be required to produce the same blood level.
In addition, there was considerable loading of blood and tissues with toluene before Gamberale and Hultengren’s (1972) 20-min, 700-ppm exposure. Near steady-state was therefore likely achieved during this 20 min of exposure (page 86, lines 22-27). Thus, reduction of 30-min AEGL-2 to 570 ppm is probably excessive. PBPK modeling should give a more accurate value.
Also, the basis for “slightly lowering” the 1 hr AEGL-2 value from 570 to 510 ppm is not well stated or clear. Why 510 and not 500 ppm? Why such precision to a value judgment? The only reason given is that the “steady-state in the blood and brain may not be reached at the 30 min time point” (page 86, lines 25-26).
An intraspecies UF of 1 was used to derive AEGL-2. Again, little specific support is provided to justify moving away from a default UF of 10. The text says only that the “observed effects are below the definition of an AEGL-2 (which should always be the case since the AEGL values are defined as the concentration above which it is predicted that…), and this value is conservative as the exposure followed a 20-min exposure to 500 ppm,” (which bears no relation to the expected magnitude of intraspecies variation) (page 8, lines 24-25).
The supporting analyses in both cases (AEGL-1 and AEGL-2) appear quite arbitrary as opposed to being consistent, scientifically logical, and defensible. This is apparent in the analysis of the supporting data at higher concentrations of AEGL-1 and AEGL-2. In both cases, the minimum alveolar concentration (MAC) for volatile organics in humans is said to vary by about 2-3-fold, though no citation is provided (page 8, lines 2-3; page 77, lines 1-7). In the case of AEGL-1, the high exposure was divided by a factor of 3 to show that there was close agreement with the selected AEGL-1 value. In the case of AEGL-2, the same argument is given, but in this case the higher concentration is divided by a factor of 2, apparently because it gives a better agreement with the selected AEGL value than dividing it by 3 would. The decision to divide by 3 versus 2 provides no insight or justification for moving away (let alone for a quantification of how much to move away) from a default intraspecies UF of 10.
An interspecies UF of 1 was used to derive AEGL-3, even though a study of rats was used to evaluate exposures. The support given to justify a UF of 1 appears insufficient. The Executive Summary argues that a UF of 1 is “sufficient because toluene uptake is more rapid and tissue toluene levels are higher in the more rapidly respiring rodents than in humans” (page 9, lines 30-31). That factor may be sufficient to justify using a UF of 3, but it is not sufficient to justify
using a UF of 1. The text where this is discussed is confusing and needs to be rewritten to clarify the rationale for selecting both the intra- and interspecies UFs (see page 88, lines 3-9).
It is recommended that in the Executive Summary a statement that the AEGL-3 values are all greater than 10% of the lower explosive limit be included.
Specific Comments
The following comments are in regard to the PBPK-Modeling Based Derivation of AEGL Values for Toluene, preliminary draft, August 21, 2004.
Some background should be given on why PBPK was used. Will this tool be used for other chemicals?
What is the point of the time to steady-state statement on page 2 and then showing a simulated time to steady-state later (Figure A-13)? Clarify and discuss in the text. Were dosimetrics measured under steady state conditions for each exposure scenario? One deficiency in the report is on the details of how the simulations (of the NOAELs) were carried out. If the NAC did not evaluate the blood concentration levels at steady state or for multiple days (or weeks), what are the implications? Single exposure, non-steady state?
The subcommittee believes that the review of blood/air partition coefficients and the list of values in a table format are appropriate. This should also be carried out for metabolic constants such as KM, Vmax, and first-order rate constants used in published human and rodent PBPK models. This will provide more insight into the selection of metabolic constants for use in this exercise.
Is there a lung compartment? Clarify the lung blood volume in Table A-2.
Figures do not show up in black and white print very well.
It would help to show more figures that demonstrate the effect of exercise on the model-predicted blood and breath concentration levels, such as those in Figure A-12.
The subcommittee agrees that the early time points with blood and breath can be problematic without going to a more complicated description of the lung. Exhaled breath can be problematic, even for longer time points, in part, because of methods used to collect breath samples. The subcommittee emphasizes the blood as the important dosimetric in comparison to exhaled breath.
Sensitivity analysis: The subcommittee usually looks for a 1:1 correspondence between model parameter change (1%) and the change in the outcome of interest (blood concentration of toluene). If this is the case, everything over a sensitivity coefficient value of 0.1 (absolute value) would be sensitive. Elaborate on sensitivity analysis and how it is important for the modeling papers. Perhaps, for the few most sensitive parameters, running simulations and
demonstrating the effect on model-predicted toluene concentrations would be beneficial in gaining acceptability of the model.
Again, to better understand exactly what was done with the simulations for the AEGL values, it would be good to specify the details.
Comment on Figure A-14, where the standard approach (ten Berge et al.) may underpredict the blood concentration levels at rest and overpredict with exercise relative to PBPK model predictions. What are the implications of using modified CxT calculations vs. PBPK?
For AEGLs, how many blood concentration measurements are there?
Derivation of “n”: When using an AEGL-3 effect for the derivation of AEGL-2, for completeness, state that the mechanisms are the same. If this is not true, then per the SOP use the default values of 1/3.
Page 6, lines 5-6. Range 0.16 to 100 ppm? (see page 14, line 23).
Page 8, lines 2-3; page 77, lines 1-7. A specific reference is needed for the statement repeated several times in the text that “among humans, the minimum alveolar concentration (MAC) for volatile anesthetics typically varies by about 2-3 fold.”
Page 9, lines 16-17. The statement, “because of the long-term exposures to 800 ppm in the supporting studies of von Oettingen et al. (1942) and Carpenter et al. (1944),” is incomplete.
Page 11, lines 12-16. Unclear: a) “the primary use is for production of chemicals” and b) “accounts for about 14%.” Do these refer to toluene or solvents in general?
Page 11, lines 30-34. Move this paragraph to begin at line 6, and make a summary statement of this paragraph at line 5 on page 6.
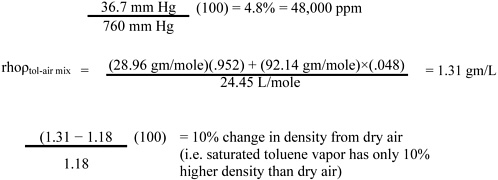
Page 11, line 31. Since toluene vapor (saturated) is only slightly (about 10%) more dense than dry air (see calculation below), it is rapidly dispersed with normal eddy currents. It may therefore be advisable to limit the warning by adding, “and in still air (confined space) may travel”) after “The vapor is heavier than air.”
Calculation: change in density with toluene vapor concentration (compared with dry air) is
1 mole at NTP (normal temperature and pressure, 25 °C and 760 mm Hg) = 24.45 L MW dry air = 28.96 gm/mole
MW toluene = 92.14 gm/mole
At 30,000 ppm toluene = 30,000 of a million molecules (i.e., 3% are toluene)
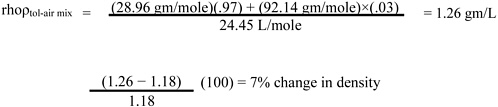
Similarly, at 20,000 ppm toluene = 20,000 of a million molecules (i.e., 2% are toluene)
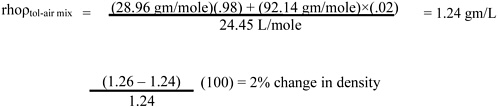
At the saturated toluene vapor pressure of 36.7 mm Hg:
Page 12, line 24. Define the term “low.” As written, the statement implies that toluene release into ambient air results in a “euphoric community.”
Page 13, line 3. Adverse effects on liver, kidneys, lungs, and heart are limited to acute and chronic exposures to very high vapor concentrations.
Page 13, line 7. Bruckner and Warren (2001) have also recently reviewed toluene toxicity.
Page 13, line 22. Delete the speculation, “and thereby provides a built-in safety mechanism.” The conclusion suggests that toluene abuse at 10,000 ppm can be considered safe.
Page 13, line 34. Probably section 3.1.1 is meant.
Page 14, line 16. The statement refers to the total range, that is, to 0.16-100 ppm (see lines 18 and 23).
Page 14, line 27; page 15, line 2. It should be pointed out that solvent abusers repeatedly inhale anesthetizing concentrations on a daily basis during most of their waking hr.
Page 14, line 30. “…metabolic acidosis, often with “anion gap…”. Is “…often with an increase in anion gap…” what does this mean?
Page 15, lines 21-23. The discussion of the exposure of the workers is unclear. Which and how many employees were grouped according to concentration of toluene?
Page 15, line 20 to the end. The Wilson (1943) study is weak due to the highly suspect concentrations. These concentrations were measured using a combustible gas indicator (CGI). With an LEL of 1.4% v/v, 200 ppm is only 1.4% LEL. This is typically much lower than the measuring range of the CGI (generally > 5% and more reliably 10%), and that is with today’s technology. There is also no indication of confounding chemicals. The article states it was commercial toluene. A CGI measures all combustibles. Further, there is no indication the CGI was calibrated to toluene. In fact, this is unlikely because most CGIs are calibrated to a combustible gas (methane, propane, etc.). In summary, the reported atmospheric concentrations are suspect at best. Apply an appropriate disclaimer.
Page 15, lines 37-38. Why is it stated that the results of this study are troubling,” when it has been stated previously in the document that toluene was contaminated with benzene during these early years?
Page 16, lines 20-27. Details are needed on exposure for this study (Ukai et al., 1993). If they are not available, say so.
Page 17, lines 1-12. Is there anything that can be stated about the sampling and analysis (for example, NIOSH analytical method, charcoal tubes with GC/FID, or GC/MS analysis)? It would be helpful to know something about these.
Page 19, line 16; page 20, lines 5-6. How does the subcommittee know that there were no permanent or persistent effects? Was a follow-up study done that led to this conclusion?
Page 22, Table 2 (cont’d), 2nd column. Add that the workers, exposures ranged from 9 to 25 years.
Page 33, lines 18-19. Add and evaluate Svensson et al. (1992), Am. J. Ind. Med. 22:99-107, 1992, study which found the opposite.
Page 35, lines 6-10. Use new IARC evaluation (1999) that concludes “there is no evidence that toluene is a potential human carcinogen based on animal studies.”
Page 36, line 7. It is preferable to state that exercise results in “increased,” rather than “maximum,” uptake.
Page 36, lines 22-27. Include some of the developmental effects from page 31, lines 35-37, in this summary.
Page 36, line 32. Drop “two” because there are several.
Page 40, lines 21-22. Should the value of 1,000 ppm for 2 weeks be higher?
Page 49, line 20. Should 80,000 be 8,000 ppm?
Page 51, line 6. What was the duration of the exposure in the rabbit study?
Page 54, lines 32-33. The major metabolites.. are nongenotoxic while the minor metabolite o-cresol is clearly genotoxic.
Page 55, line 25. Add, “while the great majority of tests showed no genotoxicity of toluene, some were positive (reviewed in IARC Monograph 1999).”
Page 56, line 18. Add, “the IARC (1999) concluded that there is evidence suggesting lack of carcinogenicity of toluene in experimental animals.”
Page 57, line 35. Systemic uptake of inhaled toluene is dependent upon cardiac output, as well as respiratory rate. Both increase with exercise.
Page 59, line 15. Which species?
Page 60, line 38. Replace IIC11 and IIE1 with 2C11 and 2E1.
Page 61, line 1. Add that CYP2B1 is induced by toluene concentrations as low as 500 ppm and is important for the formation of the genotoxic o-cresol (Wang et al., 1993). Biochem. Pharmacol. 46:413-9. The Wang et al. (1993) reference should be added in the reference section.
Page 61, line 4. Add the information on the human CYPs responsible for toluene metabolism (Nakajima et al. 1997, given in the list of references, but the information is not given in the text).
Page 61, lines 14-16. This sentence is awkward and needs to be rewritten (what are “times of metabolites”? rates of formation? times of persistence?). It seems to be saying that there is a wide variability among humans in their metabolism of toluene. If this is right, then doesn’t that contradict the general reasoning used to support the use of a UF of only 1 for intraspecies variations?
Page 64, lines 18, 22-25. Did Carlsson (1982) actually report blood levels in mg/kg?
Page 69, lines 11, 27, 31, 33; page 72, line 25. Since the parent molecule and several toluene metabolites appear in the circulation after toluene exposure, please indicate which compound(s) was (were) measured here.
Page 72, line 9. General statement or referring to toluene?
Page 72, line 11. Exposure to toluene?
Page 73, line 1. It is more accurate to state that toluene produces CNS “depression” or “inhibition” rather than “toxicity.”
Page 73, lines 7-8. It is not clear whether the last three values are tissue:air or tissue:blood partition coefficients. Also, one value appears to be lacking.
Page 73, line 12. The paper by DeJongh and Blaauboer (1996) is not included in the references. Did these authors compare their model predictions with empirical data to validate their model?
Page 75, lines 26-29. Xylene(s) would be expected to be somewhat more potent CNS depressants than toluene, because the additional methyl group makes xylene(s) more lipid soluble.
Page 75, lines 38-39. The higher respiratory rate and cardiac output of mice produce greater systemic uptake of toluene. This should result in substantially greater CNS depression in mice than in rats. These species differences, however, are offset to some degree by more rapid toluene metabolism by the mouse.
Page 77, line 16; page 78, line 4. It would be worthwhile to point out the relative merits of PBPK modeling vs. the ten Berge et al. (1986) approach for time scaling.
Page 78, lines 23-34. It should be related here that toluene and a number of other VOCs are competitive metabolic inhibitors, as they are oxidized by some of the same P450 isozymes. The net effect is an increase in the blood and tissue (for example, brain concentration) levels of each parent compound (despite some increase in exhalation) and an increase in the degree and duration of CNS depression.
Page 80, line 13. Are subtle manifestations of CNS depression indicative of “neurotoxicity” or reversible “inhibition”?
Page 81, lines 21-22. What is meant by “neurobehavioral effects were subtle and reversible, also below the definition of an AEGL-1”? Clarify.
Page 81, lines 24-25. It is incorrectly stated that 700 ppm was a NOAEL in the study of Gamberale and Hultengren (1972). Their subjects exhibited a decrease in perceptual speed, as well as apparent decreases in simple and complex reaction times at this exposure level.
Page 81, lines 24-30. It is not clear what is meant by the statement that “the concentrations would effectively approach a doubling value, i.e., 400 ppm in the former study and 600 ppm during the latter due to exercise.” If exercise produces an approximate two-fold
increase in toluene uptake/blood concentration levels, exercising subjects would only have to inhale half as much (that is, 100 and 150 ppm) in order to exhibit the same blood concentration levels as the sedentary subjects inhaling 200 and 300 ppm.
Page 81, lines 26-27. It is not accurate to state that exercise in the studies of Astrand et al. (1972) and Baelum et al. (1990) takes into account the stress that may occur during an emergency situation. Although an emergency would likely involve increased exercise, stress would also likely result in increased release of catecholamines. Toluene can sensitize the myocardium to catecholamines, but predisposition to arrhythmias requires inhalation of ≥5,000 ppm toluene.
Page 81, lines 31-32. What is meant by, “the preponderance of data as a weight-of-evidence consideration indicates that an 8-hr exposure to 200 ppm would be without an effect that exceeds the definition on and AEGL-1”? Clarify.
Page 81, line 38. “Dividing the 700 ppm.” Explain to what study “the 700 ppm” refers.
Page 82, lines 5-7. Increases in blood and brain toluene concentration levels are asymptotic once near-steady-state is reached. It would be better to say that these increases are “relatively modest” rather than “minimal.”
Page 82, lines 21-25. It is true that the systemic uptake (and CNS depression) of/by toluene is (are) greater in rodents than in humans. One cannot measure subjective complaints (for example, headache, dizziness, irritation) in rodents. Our measures of more pronounced manifestations of CNS depression in rodents are insensitive. Thus, rodent toluene NOAELs are often considerably higher than corresponding human NOAELs.
Page 85, line 13. In light of the foregoing, “difficult” should be replaced by “not possible.”
Page 88, lines 6-8. Another important factor that contributes to lower blood toluene concentration levels in humans is their lower blood:air partition coefficient (PC). Although Gargas et al. (1989) do not include human and rat blood:air PCs for toluene in their Table 8, the PCs for F-344 rats are higher for benzene and o- and m-xylene.
Page 88, lines 21-24. It appears preferable to use PBPK modeling for time scaling from 2 to 4 and 8 hr.
Page 88, lines 26-27. Near-steady-state is reached within 60 min in rats.
Minor Points
Page 6, line 8-9. Remove the bolding.
Page 13, line 18-22. Remove the bolding.
Page 16, line 27. Typo: should be “essentially.”
Page 31, line 37. Delete “fetal effects.”
Page 32, line 2. Insert to read, “other chemicals or drugs (especially ethanol)…”
Page 68, line 26. The abbreviation PBTK should be defined the first time it appears in the text. PBPK is used on pages 72 and 73. Choose one of these abbreviations for sake of consistency.
Page 72, lines 13-16. The blood concentration values of Benignus et al. (1998) in mL/L should be stated in mg/L in the text, so they may be readily compared to the blood concentrations reported in mg/L by other investigators.
Page 74, line 34. Typo: should be demyelinization.
Page 76, line 15. It is not clear here whether the volume of the blood sample was “smaller than usual” or whether the third dog was smaller than the other two dogs studied by von Oettingen (1942).
Page 81, lines 34. What is meant by, “Although these concentrations do not approach gross CNS effects”?
Page 85, line 15. Delete the phrase, “of the general population.” As written, the conclusion implies that community exposures to 700 ppm are “appropriate”?
Page 97, line 21. Complete the author names in accord with NRC style directions. This entry appears to be a repeat of that at line 18, and one of the Donald references can be deleted.
COMMENTS ON XYLENES
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on xylenes. The document was presented by Claudia Troxel of Oak Ridge National Laboratory. The subcommittee recommends a number of revisions. The subcommittee will review the revised xylene AEGLs at its next meeting.
Overall Comments
Of major importance is the fact that the AEGL-2 and AEGL-3 values are greater than 10% of the lower explosive limit. This fact should not be hidden in the footnotes to Table 4 (page 42) and the footnote in the Executive Summary on page xi. The fact that the xylene AEGLs represent an explosion hazard should be highlighted on line 8 of the Executive Summary (page ix) and should appear in the first sentence of Section 8.
Since the authors included results of rodent behavioral testing with xylene, some explanation of the relevance of those data to the AEGLs is in order here. The absence of any comment as
to whether the six full pages (18-24) of diverse rodent behavioral findings associated with inhaled xylene leaves the reader at a loss to understand why those data were included. For example, how does intracranial self-stimulation in male rats (page 20 and Table 8) relate to humans and to AEGLs? How does performance on a Morris water maze test or rat “swimming length” (page 25) relate to humans and to AEGLs (page 33, lines 11-17)? If an authoritative review of these observations has been published which interprets the diverse findings (for example, ATSDR 1995), those conclusions should be brought forward here. If no such review has been published, and the authors elect to include these numerous and diverse end points and text descriptions (which seem to have no bearing on AEGL derivation), a summary paragraph should be included to address the relative importance of these findings to the AEGL process. Why is it necessary to devote such effort to studies that show “no clear effect” and demonstrate no dose response (page 26)? Since none of these observations are used in any aspect of AEGL derivation, consideration should be given (especially in future documents) to presenting such voluminous data only in summary tables.
The summary on page 36 (lines 9-11) adequately captures the differences (none) between the isomers. Why is it necessary to repeat here all the same information on pages 36 and 37 as discussed previously in the text? If necessary, the authors can simply list the numbers of the text sections which support the conclusions made at lines 9-11.
The document is comprehensive, and it is generally well written. The text should be prepared with line numbers on each page. At each mention of ortho-, meta-, para-, m-, o-, and p-, it is proper to show these entries in italics.
Page 39, lines 22-25. 1) AEGL-2 is the concentration above which impaired ability to escape is expected, not an inability to escape. Clarify this definition and note that poor coordination many impair the ability to escape. (2) The 1,300 ppm for 1 2-hr exposure may not represent a no-effect level for AEGL-2.
General Comments
The authors used an interspecies UF of 1 to derive both the AEGL-2 and AEGL-3 values. The justification for this decision is based on the PBPK modeling, which “eliminated the toxicokinetic component of the uncertainty factor, and the pharmacodynamic component was assigned a 1 based on similar exposure effects (central nervous system effects) in humans compared to animals” (page x). One subcommittee member disagreed with the use of an interspecies UF of 1 in this instance due to the failure in addressing the pharmacodynamic aspects of CNS depression across species.
Although a PBPK model was used, there are inherent uncertainties in PBPK models that need to be itemized, and uncertainty in the xylene pharmacodymamics may preclude a total interspecies using UF of 1. While there may be similar CNS effects in humans and animals, it is not clear from the text as written whether for the same internal dose, animals and humans will respond the same way. In other words, will a rat respond in the same way as a human to the same internal xylene dose? To address this question, the text should include authoritative
references to the biochemical modes of solvent and anesthetic gas actions at the molecular level. In the absence of some discussion about CNS depression across species, it appears that uncertainty remains, and that an interspecies UF greater than 1 may be needed.
As written, there are questions about the use of PBPK models to extrapolate animal data to humans and about whether the xylene PBPK model is sufficient and appropriate to eliminate an interspecies UF greater than 1. The text provides no justification for why this model should be used (compared to other approaches), and there is no discussion of why the PBPK-scaled dose is an appropriate surrogate for evaluating the differences in xylene metabolism and toxicity between animals and humans. The NAC may wish to consult previous NRC publications (for example, Methods for Developing Spacecraft Water Exposure Guidelines [2000], Drinking Water and Health, Vol. 8, Pharmacokinetics [1987]) about the use of PBPK modeling and revise the SOP before incorporating the results of PBPK dose scaling into the AEGL process.
Specific Comments
Page ix, Executive Summary. There is no mention in the opening summary paragraph that sufficiently high xylene exposures can affect the liver. This should be included in this summary.
Page ix, Executive Summary. “This concentration represents the threshold for reversible equilibrium disturbances and the no-effect level for the inability to escape.” This is a strong statement that may not be categorically true. Qualify this conclusion in a rigorous manner and expand the explanation which supports this conclusion.
Page x, line 2. The editorializations detract from the science presented and should be deleted from the text.
Page x, lines 4 and 25. The text states, “the values at 4- and 8-hr were at equal.” To which term (delivered dose to brain or the corresponding AEGL) do these sentences refer?
Page x, lines 7 and 28. The word “eliminated” should be replaced with the words “accounted for.”
Page x, lines 11-12, lines 32-33. Delete. Is the sentence, “The values at 4 and 8 hr are still protective of human health,” necessary? If the NAC supports the authors’ proposed AEGL values, is not this taken at face value?
Page x, lines 40-41. Delete. Why is it necessary to include the sentence, “The AEGL values should be protective of human health”? It appears that the AEGL values have simply been developed in accord with the SOP.
Pages x, lines 45-46; and page xi, lines 1-4. Last sentence on page running over to next page (p. xi) does not follow. Something is missing: “Numerous human studies…”
Page xi, lines 4-7. The statement, “AEGL-3 levels … are protective as supported by human data” may not be true. The NAC judgment is that the AEGL-3 values are protective, but the magnitude of protection afforded by these recommendations for all members of the population are subject to debate. Therefore, the statement should be qualified.
Page xi, Table, footnote a; page 42, lines 26 and 31; page 40, lines 16-17. The AEGL-2 for a single time point cannot be listed as two different concentrations. Fix or delete the “a” footnote.
Page 3, line 5. Were adult males involved in this episode? As written, the descriptions of a boy suggest perhaps that young humans are more susceptible to inhaled xylenes than mature humans.
Page 3. Did the incident described by Klaucke (1982) involve both males and females? Did Carpenter (1975) list the sex of his 21- to 60-year-old volunteers?
Page 5, 1st full paragraph (Hastings et al. 1986 study). This paragraph is not well written. There should be a transitional link to make it clear that the data reflect the results from the Hastings et al. study described in the prior paragraph. The sentence that begins, “No definitive increase in the percentage of exposed subjects experiencing nose or throat irritation was observed as compared to controls,” is unclear. What is meant by “No definitive increase”? Does this mean there were was an increase, but that it was not statistically significant? Also, in the prior sentence, what is meant by, “as compared to the high control percentage”? This statement is confusing as written.
Page 6, 1st paragraph, last sentence. Explain the abbreviation “100W.”
Page 6, 3rd paragraph (Nelson et al. 1943 study). Why comment that the “majority of subjects stated that they thought exposure to 100 ppm xylene for an 8-hr exposure would be tolerable”? This statement should be noted as a quote from the original paper, and the AEGL text should note it was highly subjective since the volunteers had such short exposures (3 to 5 min).
Page 9. The text should point out the differences between the Savolainene et al. protocol and the Laine et al. 1993 protocol, which is that the afternoon exposure in the second study group was 1 hr compared to the 40 min of the first study group.
Page 10, 3rd paragraph. Clarify that when the concentration of the afternoon exposure was “doubled” that the exposure was 400 ppm (stated twice). This will also help the reader understand where the 400 ppm concentration discussed in the next paragraph originated.
Page 11, Section 2.3, last sentence. Explain how “small sample sizes” are considered by NAC as a limitation in these studies.
Page 12, 1st paragraph, last sentence. Same comment as above.
Page 15, bottom 11 lines; page 16, top 3 lines. As these two pages are written, it is not clear whether the rats were pretreated with these various chemicals (3-methylcholanthrene is not a drug) or whether the rats received ip injections following xylene exposure? Page 15 suggests the ip injections were given prior to xylene, but the table is not clear on this point. On page 15 and 16, there is no interpretation of the results of the study—in fact, it appears from Table 5 that the LC50 values are not different regardless of prior phenobarbital, chlorpromazine, or 3-methylcholanthrene exposure.
Page 19, top 10 lines. Should the authors elect to include studies on rodent flavor aversion in the AEGL documentation, some interpretation of the significance or relevance of these findings to the AEGL process and to human beings should be included at some point in the documentation. As written, it is not clear whether humans exposed to xylene vapors are at risk for the development of anosmia or other decrements in sensory ability to taste and detect odors.
Page 25, line 10 from bottom; page 26, lines 1-6. What is a rat “swimming length”? Does this refer to duration of the ability to swim?
Page 27, first paragraph. The last sentence in this paragraph does not make sense: “…and/or inadequate sample sizes were available.” Clarify.
Page 28, Table 9; page 29, Table 10. Add units to duration column (hr).
Page 31, line 5 from bottom. Specify the species to which the xylene excretion half-times refer.
Page 34, Section 4.3.1., lines 31-32. Text states that, “A similar effect has been proposed for humans.” Who has proposed this? What is the citation to support this statement?
Page 34, lines 23-25. Two other very important reasons that rats achieve higher blood xylene concentrations than humans are the rats’ higher respiratory (alveolar ventilation) rate and higher cardiovascular output/tissue blood flow rates. The higher blood and brain xylene concentrations result in more pronounced CNS depression in rats than in humans subjected to equivalent inhalation exposures. The subcommittee commends the NAC for the application of PBPK modeling to interspecies extrapolation and time scaling for scientifically based derivation of AEGLs. It is important to note that the SOP lacks direction on the use and verification of PBPK scaling, and the SOP should be revised as soon as practical.
Page 35, Section 4.3.2. The discussions that intraspecies differences in response to xylene exposure are no more than 2-3-fold need further documentation. The data provided describe, in general, results from exposure to anesthetics, and no specific data related to xylenes, toluene, benzene or other similar VOCs were cited. In particular, no specific data are presented to support the statements that “all available data point to a 2-3-fold difference in interindividual sensitivity to xylenes” (page 34, line 34) or “the total range of sensitivity is 2-3-fold” (page 35, lines 9-10).
Page 35, lines 25-29. It is worthwhile mentioning here that xylenes readily diffuse bidirectionally between the blood and brain, rapidly attaining and striving to maintain an equilibrium between the two compartments. The blood:brain partition coefficient is the ratio of the xylene concentrations in blood and brain under near-steady-state conditions. Thus, the arterial or venous blood concentration of xylene is a reliable index of the brain level, and in turn, the magnitude of the CNS depression that is due to the parent compound.
Page 38. Check the terms “slight” or “mild” used by Hastings. These terms are mixed in the text and may mean different things to different readers.
Page 38, lines 17-35. An AEGL-1 is defined as “the vapor concentration above which notable discomfort, irritation,…” Thus, it is not reasonable to apply a 3-fold intraspecies UF to a vapor level (400 ppm) at which mild eye irritation was reported by some subjects, although they did not exhibit an increase in eye blinks nor experience nose or throat irritation. Note that the AEGL-1 of 130 ppm is lower than the NOAEL of 200 ppm reported by other investigators for 3-, 4-, and 5.5-hr exposures.
Page 40, lines 4-5. It is reasonable to assume 50w of work for the shorter (10-, 30-, and 60-min) AEGL-2s to account for increases in physical activity likely during emergency evacuations. It is important that this concept be considered during future revisions of the SOP.
Page 40, lines 1-12. As noted in lines 24 and 25 of page 39, exposure to 1,300 ppm xylenes is a threshold for equilibrium disturbances in rats and represents a no-effect-level for the inability of rats to escape. Thus, it does not follow to apply a 3-fold intraspecies UF, resulting in a 1-hr AEGL-2 of 400 ppm, which has been shown experimentally to be a threshold for minimal effects on some sensitive tests of psychophysiological and visual-evoked potential parameters in exercising human subjects (Hastings et al. 1986; Savolainen et al. 1981; Seppalainen et al. 1984, 1985, 1989).
Page 42, line 11; page 45. The statement, “The proposed AEGL values should be protective of human health,” would seem to go without restatement here. As written, the statement begs the question, why would the NAC recommend AEGL values that are not protective of the public? The text continues with the similar bizarre statement that “the key study was an acceptable study.” Why would NAC recommend a key study that was not acceptable? Revise the text to note the xylene AEGLs were derived in accord with the SOP. Also note that the SOP shall be revised to address the practical application of PBPK methodology.
Page 44. The section here comparing the AEGLs with other standards lacks the text explanation found in other AEGL documents. For example, the 130 ppm AEGL-1 is almost identical to the ACGIH 15-min STEL (150 ppm), and it is remarkably close to the 8-hr TWA (100 ppm).
Page 44, Table 15. Include an explanation for EEL.
Page 45, Data Adequacy and Research Needs. Xylene represents one of the most robust data sets considered by the AEGL program. To suggest that the AEGL derivations were based
on “limited” data is erroneous. The section can be condensed to reduce the editorial comment and to list only the major missing end points (lack of a chronic inhalation carcinogenicity bioassay) that could aid in derivation of the AEGLs. The sentence at lines 30-31 could be misleading in that many nongenotoxic carcinogens are well recognized.
Page 45, Section 8.3. Data Adequacy and Research Needs. This section does not say much about data adequacy and offers no suggestions about research needs. The last two paragraphs (starting on the bottom of page 45) are irrelevant in that they only discuss the consistency of the data with the derived for AEGL values. The statement on line 37 (page 45), “The AEGL-2 values are protective,” should be struck as it is irrelevant to the section on data gaps.
Minor Points
Page x, 2nd line of 2nd paragraph. Carpenter et al. 1975a or 1975b or both? Same for page 41, line 25.
Page x, 2nd line of 2nd paragraph. Should read “2,800 ppm for 4 hr.”
Page 14; last 6 lines on page 15; last 2 lines of Table 4; page 28, Table 9. Figures “6700 ppm” and “6011 ppm” are differently cited to “Carpenter et al. 1975b” and to “calculated by probit for this document” in text and tables.
Page 28, line 1. There is a new evaluation by IARC (1999). The conclusions remain the same, but quote and cite the most recent evaluation.
Page 30, line 3 from bottom. Xylene has also been detected.
Page 34, lines 15-16. “350 ppm p-xylene resulted in hyperinnervation or degeneration of noradrenergic nerves.” This is a serious (AEGL-2) health effect.
COMMENTS ON AMMONIA
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on ammonia. The document was presented by Kowetha Davidson of Oak Ridge National Laboratory. The document can be finalized after the subcommittee’s recommended revisions have been made appropriately.
General Comments
Page 35, Section 5.3. Proposed value of 30 ppm to derive the AEGL-1 is justified, although the rationale for not applying an intraspecies UF is flawed. Please provide a better rationale.
Page 38, Section 6.3. Similar comment regarding asthmatics as above. Basis concentration justified.
Page 40, Section 7.3. While there is good rationale presented for using the mouse studies to derive AEGL-3, there is one problem relating to the interspecies UF of 1 used for this AEGL. While the mouse is relatively sensitive to ammonia compared to the rat, we do not know the relationship between mouse and human. Furthermore, the nasal passages of the mouse are more efficient in scrubbing inhaled ammonia that would be the nasal passages of humans. Thus, in an exposure atmosphere, the relative percentage between the URT and LRT would likely differ, with a greater percentage reaching the LRT in humans than in mice. Thus, some reconsideration of the interspecies UF should be given.
The rationale for using an intraspecies UF of 3 for AEGL-3, where there would likely be little difference in response between sensitive and nonsensitive people due to highly irritating properties of ammonia at AEGL-3 levels, while the UF is only 1 for AEGLs 1 and 2 is not sound. From the discussion presented, it appears that the UF for AEGL-3 should be less than that for 1 and 2.
On page 41, one of the reasons for using the stated inter- and intraspecies UFs is that larger UF values would lower one of the AEGL-3 values to a concentration inconsistent with the definition of AEGL-3. Cite the specific section of the SOP that describes this situation.
Specific Comments
Add line numbers to all documents.
Page 7, line 12. Is it standard practice to derive a 5-min AEGL? The SOP (page 95) points out that it is inappropriate to extrapolate to the time periods shorter than 10 min. Should a 5-min AEGL be derived, using the very short duration studies would be a better basis.
Pages 11-13. The discussion on these pages of the Houston and Potchefstroom accidents should be shorted to 1-2 paragraphs at most that succinctly state: the accident, resultant human lethality, that concentrations causing lethality are unknown but that several different models (WHAZEN, HGSYSTEM, RAM TRAC) have been applied to estimate concentrations at various distances from the accident and relate these to lethality, and that these models have limitations. Additional details of the models or the modelers’ interpretation of the output do not constitute human lethality data appropriate for the AEGLs and should be deleted.
Page 13, line 14-25; Page 15, line 10-14. Delete the report of Henderson and Haggard (1943) as it appears to be a review paper and not a primary research report.
Page 15, line 38. Minor editorial corrections will need to be done for misspellings (e.g., “atropine”). The NAC should do a spell check.
Page 18, Table 3. Combining the results for the two groups of subjects (i.e., experts and student/non-experts) is not appropriate as one of the key findings is that the expert group generally scored lower than the student non-experts. Further, this is the key study for AEGL-2, based upon increased concentration and duration-response in the non-experts. The table needs to be revised to better support the effects for the AEGL derivation.
Page 35, line 42. AEGL1 intraspecies UF is 1- why? Why is 3 not appropriate? The data suggest variability between the elderly subpopulation and the general population. Is it because in Erskine et al. (1993) a response was elicited only at a high concentration (~600 ppm), or because the response was considered protective (pg. 19 TSD)? The rationale for this UF needs to be better explained.
Page 37, last line. Additional explanation is needed as to why 100 ppm at 1 hr (Verbeck, 1977) was chosen as the point of departure for AEGL-2. Why not 140 ppm or why not at 30 min or 2 hr? The effect appears to be the same at 100 and 140 ppm for 30 min to 2 hr (see Table 9).
Page 38, line 9. Are there sufficient data to support an intraspecies UF of 1?
Page 38, line 25. Where are the data supporting the statement that irritant response is not expected to change for up to 8 hr?
Page 41, lines 1-3. Are the intra-species and inter-species UF confused in this paragraph?
Page 41, lst line before Table 12. 5-min AEGL values are not routinely derived (see SOP page 95); these should be deleted.
Page 42, lines 1-4; Appendix A, page 54. The BMD text about Alexeeff and Guth (1996) is not relevant for the TSD and both should be deleted. The discussion in the appendix raises more questions (particularly regarding uncertainty factors) than it answers for the AEGL.
Page 42, Table 13. Delete 5 min values.
Page 43, Table 14. AEGL-1 values should be 30 ppm, not 25 ppm (see also TSD page 36).
Pages 54-57. The BMD comparison and the dose reconstruction models do not add to the TSD and should be deleted.
Page 58, Appendix B. The page for derivation of AEGL-1 values is missing.
Minor Issues
Page 6, line 40. State the AEGL-2 values in this summary paragraph.
Page 6, last line. Change “or” to “of”.
Page 8, line 7. Clarify the sentence that begins “Ammonia and air will explode….”
Page 14, line 4. Note the concentration; state if unknown.
Page 14, line 10. Note exposure route and concentration.
Page 14, line 21-24. The two sentences about the accident should be moved to the second and third sentences of the paragraph beginning on line 13.
Page 14, line 37. Note exposure route in O’Kane (1983).
Page 15, line 17. Delete “the” at the end of the line.
Page 15, line 24. Change “immediately” to “immediate”.
Page 15, line 30. Change “test” to “tests”.
Page 15, line 43. The study design for Bio-Test Laboratories, Inc. (1973) is not clear. Were the same subjects exposed to all concentrations?
Page 16, line 11. Add a comma and space in between perceptible and the number 3.
Page 16, lines 18-19. The study results should be rephrased to indicate both an increase in the number of subjects responding as well as an increase in the intensity of the irritation reported.
Page 16, line 37. Convert µg to ppm.
Page 18, line 20. Change “and” to “or” when listing concentrations (mg/m3 and ppm).
Page 18, line 21. What is w? Watts?
Page 18, Table 3, footnote b. Correct scale is 0= No sensation.
Page 19, line 22. Provide duration of exposure for the workers in Holness et al. (1989).
Page 19, line 24. Change “of” to “or”.
Page 19, line 29. Change “small” to “low”.
Page 20, lines 5-18. Delete the paragraph with Pedersen and Selig (1989) assessment of Markham (1986); their assessment is not relevant and the Markham paper appears to be a review and not a primary source.
Page 20, line 23. Delete comma after Reynolds.
Page 20, line 27. Exposure data (to ammonia?) from OSHA Monitoring Data (1997) are not relevant and should be deleted without additional information such as what industries, health effects or duration of exposure. This does not appear to be an epidemiologic study.
Page 20, line second from bottom (no number). Change Section 5.1 to 7.1.
Page 21, line 1. Why are children considered a sensitive subgroup? There is no discussion in the TSD about this.
Page 21, line 21. Change “particular” to “particularly”.
Page 22, Table 4. This table needs the following revisions: 1) add “in Humans” to the table title, 2) the effects listed for the Verbeck study should retain the descriptions of the effects as reported by the author and presented properly elsewhere in the TSD text (e.g., just perceptible, distinctly perceptible, nuisance, offensive). The effects in the table now appear to have been re-categorized into mild, moderate, severe by the TSD author, and may misrepresent Verbeck’s findings. Are these results only for the students? 3) add McLean (1979).
Page 23, line 1 in Section 3.1.1. Add “or” after 5468.
Page 24, line 6 of Paragraph 1. Did Appleman et al. (1982) report mouth breathing? Page 24, last line, Paragraph 1. What was the difference in LC50 values between males and females? If it’s worth noting, the reader should be provided with both values.
Page 25, Table 5. Where does footnote b go on the table? What values are in parentheses, as stated in the footnotes?
Page 26, lines 3-5. Add exposure durations for 375, 651 and 672 ppm exposures.
Page 26, line 34. Delete comma after exposure.
Page 27, line 23. Delete “postexposure”. Add “s” to observation.
Page 28, line 2-3. Clarify activity patterns observed. The paragraph appears to indicate both increased and decreased activity.
Page 28, line 15. Mycoplasma pulmonis should be italicized.
Page 28, line 23. Add comma after et al.
Page 30, line 12. Add “to ammonia” after exposure concentrations are listed.
Page 30, line 28. Change “were” to “was”.
Page 30, line 30. Delete “were observed”.
Page 32, line 3. Dalhamn (1956) should be cited.
Page 32, line 5. Tepper et al. (1985) should be cited.
Page 32, lines 20-30. Are the study results reported in this paragraph attributable to Coon et al. (1970)? Please clarify.
Page 35, line 1. Add “region” between the words “respiratory” and “until”.
Page 36, line 2. Delete parentheses before 1979.
Page 36, line 5. Delete “of” before adaptation.
Page 36, line 9. Is the source for this statement Verbeck (1977)?
Page 38, line 27. Delete “reported”.
Page 40, Section 7.3. Clarify that BMD was applied to the mouse data sets.
Page 40, Table 11, first rat column. The footnote should be for Appleman et al. (1982) study. In the footnotes, to what does n=2 refer?
Page 44, EEGL (footnote b). Complete the last sentence.
Page 45, line 21. Change “considerate” to “considered”.
COMMENTS ON BROMINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the revised AEGL document on bromine. The document was presented by Sylvia Talmage of Oak Ridge National Laboratory. The subcommittee recommends a number of revisions. The revised document will be reviewed by the subcommittee at its next meeting.
General Comments
Overall, the data for derivation of the AEGLs are limited. The use of defaults for time scaling for AEGL-1 and -2 seems more appropriate. As written, the text can be interpreted that the AEGL-3 should be reduced by a factor of 3. There is a very sparse database for bromine, and this leaves the reader with the impression that the proposed AEGLs are not very “solid.”
AEGL-1. The reliance on bromine-induced eye irritation in healthy humans is reasonable as the starting point. The description of the results of the key study needs to be elucidated and
reconciled with the data in the tables in the paper. The use of a mouse-lethality study as the basis of time scaling for human eye irritation is not justified. The use of defaults would be more appropriate. The rationale for a 3 intraspecies UF based on the response of workers does not appear appropriate, since the response of healthy workers does not account for potentially sensitive populations. A robust rationale for derivation of the AEGL-1 values should be provided.
AEGL-2. The reliance on throat irritation in humans seems appropriate as the starting point, but the use of mouse-lethality data to time scale for human throat irritation may not be justified. The rationale for a intraspecies of UF based on the response of healthy workers does not appear appropriate to account for all populations described in the SOP.
AEGL-3. The data for derivation of the AEGL-3 values are very few. The resulting values may be too high. For example, the proposed values are 10 times lower than the estimated LC50 in vulnerable humans (page 8, line 19) at a given ventilation rate. Given the possibility that ventilation rates may be higher, and the fact that lethality increased with exposure time in the Bitron and Aharonson study, the AEGL-3 values appear to be too high. Consideration should be given to using an additional interspecies UF or using a database UF.
The review of the bromine literature should be summarized in a table to illustrate the discrepancies between studies. Since investigators have reported differing effects of bromine for short-term and low concentrations, it may be easier to see this in a table.
Make reference to the clinical use of bromine as a medication and the numerous effects of its usage (see the 4th edition of The Pharmacological Basis of Therapeutics, L.S. Goodman and A. Gilman, eds. MacMillan, New York). In this regard, bromide is the oldest (1857) of the anti-epileptic agents where it is effective against generalized tonic-clonic seizures (effective oral daily dose is 3-5 g/ person).
Specific Comments
Page 8, line 18. Expand the description of the estimated lethal concentrations as the terminology and basis of the statement is not clear.
Page 9, Section 2.2.3. The experimental details of the Rupp and Henschler study do not agree with the data in Figure 4. It appears from Figure 4 that eye irritation starts to be noted at concentrations below 0.1 ppm and also for times approximately 17 min. Reconcile the paper with the text. The translation states that 0.006 ppm is irritating to the eyes. The letter from the author states that concentrations below 0.01 were not tested. This needs to be evaluated in the AEGL document. Is some of the confusion due to the actual vs. nominal concentrations?
Page 10, line 4. Clarify the duration of the exposures. It appears they were conducted for up to only 30 min and not 1 hr.
Page 10, lines 17-19. The actual concentration is less than the nominal, but it is not clear why the nominal concentration is used and the actual concentration was not used. The rationale should be expanded.
Page 17, lines 18-19. This statement should be clarified because it appears incorrect since concentrations over 1 ppm are also irritating. State the specific low doses that are irritating, or alternatively, state where above a certain concentration there is irritation. Add details about the duration of bromine exposure at which 0.5 ppm is irritating.
Page17, lines 20-22. Rewrite this sentence because the two findings do not appear to be as different as implied. Add that the Rupp and Henschler study was carried out with healthy young adults and that it was a single exposure. Make the second part of the sentence on OSHA monitoring data into another sentence because these are chronic exposures and, there could be acclimatization occurring over time.
Page 18. Use of mouse-lethality data does not seem relevant for time scaling human irritation. Use defaults or justify its use.
Page 18, line 9. Basing the intraspecies UF on the response of workers does not seem appropriate. Use alternative justification or explain in detail the reasons for use of these occupational studies.
Page 18, Section 6.3. The derivation of the AEGL-2 comes across as particularly weak given the lack of adequate data. What data are available to support the justification to make it more convincing?
Page 21, Section 7.3. If, as noted, effects are not expected to differ greatly among individuals, then why is the UF 3 rather than 1?
Page 24, Section 8.3. As written, it is not clear how the irritation data in mice are available, and how this would be obtained since most of the human data are subjective.
Table 10. Values for AEGLs 1 and 2 are much lower than ERPGs 1 and 2. Explain.
COMMENTS ON ANILINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on aniline. The document was presented by Sylvia Talmage of Oak Ridge National Laboratory. The subcommittee recommends minor revisions to the document. A revised draft can be finalized if the recommended revisions are made appropriately.
General Comment
The aniline AEGL document was revised to add 10-min AEGL values. The revised document was reviewed during the July 2003 AEGL Committee meeting. The comments from the NAS were considered and each one was addressed. The present document is acceptable. It is recommended that the 10-min AEGL values be made available rather then republishing the entire document.
Specific Comments
The request to ensure that the current literature was searched and new information was added has been addressed. All references were updated to reflect the most current documentation.
The rationale for derivation of the 10-min AEGL from the available data has been added.
Limitations of the study of Kakkar et al. 1992 have been noted and added. This study is only a supporting study.
Statements were added that methemoglobin would not reach steady state during a 10-min exposure.
COMMENTS ON METHYL ETHYL KETONE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on methyl ethyl ketone. The document was presented by Sylvia Talmage of Oak Ridge National Laboratory. The subcommittee recommends the following revisions. A revised draft should be reviewed by the subcommittee at its next meeting.
Overall Comments
The use of an interspecies uncertainty factor (UF) of 1 in deriving AEGL-2 values is inappropriate and is not supported by the data presented in the document. The rationale given for selecting an interspecies UF of 1 is “because of the subchronic nature and mild endpoint of the key study and because rodents have a higher respiratory rate and cardiac output than humans, resulting in more rapid uptake of chemicals” (page 37, lines 35-37). It is not clear what the “subchronic nature” and “mild endpoint of the key study” tell us about differences or similarities between humans and animals in responding to MEK exposure. Furthermore, the fact that rodents have higher respiratory and cardiac output rates than humans is not a new one and do not necessarily guarantee that humans and animals will respond identically to exposure to MEK. At best, this argument might justify moving from the default value of 10 to 3, but the text as written does not justify moving from the default of 10 to 1. An interspecies UF of 3 should be used in this case.
The use of an interspecies UF of 1 in deriving AEGL-3 values may also be inappropriate. The rationale is not supported by the data cited in the document. No rationale is provided in the text or in the Executive Summary (ES) for why an interspecies UF of 1 was chosen. The text (page 37, lines 20-21) and the ES (page 7, lines 22-23) both state that the “application of inter-and intraspecies uncertainty factors of 1 and 3, respectively, would be sufficient in the support study of Hansen et al. (1992).” The meaning of this sentence is not clear, and as written, it appears subjective. The Hansen study generated a “projected” RD50 value in mice of 32,145 ppm even though the highest concentration tested was 26,000 ppm. As is the case with using an interspecies UF of 1 for deriving the AEGL-2 values, the arguments presented here for using an interspecies UF of 1 might justify moving from the default value of 10 to 3, but they do not necessarily justify moving from the default of 10 to 1. An interspecies UF of 3 should be used.
Some reviewers have the opinion that the inclusion of MCS patients as a sensitive group is a good starting point for deriving the AEGL-1 with an UF of 1. One subcommittee member, however, disagrees with that. According to that member, the use of an intraspecies UF of 1 in deriving AEGL-1 values is not supported by the data in the document. The rational provided for this opinion is “because effects were not greater at the higher concentrations of 380 ppm, and because subjects with sMCS, a hypersensitive population, did not report enhanced sensory effects compared to controls.” In fact, the sMCS group did report a “weak dose response increase in nasal symptoms” (page 13, lines 24-25). Furthermore, if this group were truly chemically sensitive, they would not have participated in this study. At best, this is a single questionable study, and it is not sufficient to establish that there is no sensitivity or variability in the general population. In addition, the study by Nakaaki 1974 (page14, line 37) shows variability in response. The rationale provided is sufficient to justify moving the intraspecies UF from the default of 10 to 3, but not sufficient for moving the default of 10 to 1.
The appropriateness of the study selected for the derivation of AEGL-2 is questionable. AEGL-2 was derived using the Cavender et al. (1983) study that exposed 15 rats to several MEK concentrations 6 hr/day for 90 days. This a subchronic (repeated exposure) study, which is generally not preferred for deriving AEGLs, as they are intended to provide exposure guidelines for short-term acute exposures.
Other studies that should be reconsidered include Patty (1935) in which guinea pigs exposed to 10,000 ppm developed incoordination in 90 min and narcosis within 4-4.7 hr and Glowa and Davis (1987) study in which most mice exposed to 5,600 ppm for 9.5 min ceased responding in a scheduled controlled-response experiment. All mice ceased responding at 10,000 ppm. In DeCeaurriz (1983) study, most mice exposed to 2,065 ppm MEK developed a 50% decrease in mobility during a 3-min “behavior despair” swimming test. While these studies may have their weaknesses, they may actually be more appropriate than the selected Cavender study.
In addition, the RD50 studies (Stone 1981; DeCeaurriz 1983; Hansen 1992) are dismissed without explaining why they are not suitable for the derivation of the AEGL-2 values.
The data on neurotoxicity should be grouped together for proper evaluation of this issue (see pages 14 and 21-22). It should be stressed that it is not PNS neurotoxicity that is of major
concern, because this is usually a result of chronic exposure. Therefore, CNS neurotoxicity should be discussed as a risk factor of acute exposure. It is not only CNS depression that we are afraid of (not only because it impairs the ability to escape but also creates the subclinical effects of cognitive functions, which may influence the decision of whether or not to escape from a dangerous situation? The effects are usually difficult to interpret because many neuropsychological methods are not properly validated. The observation of Nakaaki et al., (1974) (see page 14, lines 30-34) that “males tended to underestimate times” is sufficient evidence that MEK affects CNS function. This effect was already observed at 150 ppm, below the proposed AEGL-1 value of 200 ppm. This might be a reason to consider a UF of 1 for AEGL-1 to be inappropriate.
Finally, the subcommittee does not agree with the conclusion that MEK is not acutely neurotoxic because it “only” potentiates the neurotoxic action of some solvents (page 33, line 6). This potentiation has to be considered a neurotoxic effect.
General Comments
Make it clear that the AEGL-3 values were derived using different studies. The 10- and 30-min time periods were derived using the studies by Klimisch (1998) and Zakhari (1977) with support from Hansen (1992) (page 39, lines 13-17). The 1-hr, 4-hr, and 8-hr values were derived from the studies by Fowles 1999 using data from La Belle (1955).
The subjects in the Nelson study were exposed to several concentrations of MEK for 3 to 5 min. Yet the AEGL document states that “mild eye irritation was reported by some subjects at 200 ppm, and 350 ppm was considered objectionable for an 8-hr exposure (page10, lines 25-27). The majority of subjects considered 200 ppm “satisfactory for an 8-hr exposure” (page 10, lines 26-27). This statement should be clarified as being that of the investigators’, or it should be eliminated from the text. As written, the statement is subjective, given that the volunteers were exposed for 3 to 5 min. Whether they think that exposure to 350 ppm for 8 hr was “objectionable” and that 200 was “satisfactory” may represent conjecture or simplistic opinion, and it does not appear to be based on objective criteria. This statement is made several times in the text in support of the “safety” and appropriateness of the 200 ppm AEGL-1 values (also see page 34, lines 24-25 and page 35, line 8).
The statement, “Subjects with self-reported multiple chemical sensitivity also found concentrations of 200 ppm practically nonirritating (Seeber et al. 2002)” (page 17, lines 14-15) is not supported by data presented in the text (see page 15, lines 15-29). The discussion on page 15 says nothing about what these people reported feeling at 200 ppm (perhaps there are more details in the original report?). The text describes a weak concentration-response increase in nasal symptoms in the MCS group (page 13, lines 24-25).
Provide a citation to support the statement, “the susceptibility of the general population to central nervous system anesthetics varies by no more than 2- to 3-fold as indicated by the minimum alveolar concentration (MAC), the concentration of an anesthetic that produces
immobility in 50% of patients” (page 32, lines 9-14). This statement is not properly referenced, and as such, it is not sufficient to justify using an intraspecies UF of 1.
Was the fact that MEK “potentiates the neurotoxic action of structurally related solvents such as n-hexane and methyl-n-butyl ketone” (page 33, line 6) taken into consideration in any way in the development of the AEGLs? Is this consideration necessary given the context in which AEGLs are developed and applied?
The document states, “Several recent studies indicate that strong odor rather than irritation was responsible for symptoms complaints in earlier studies (studies listed)” (page 17, lines 11-12). It is not clear how recent studies were used to reinterpret results from earlier studies and what the proper interpretation was in the earlier studies. As written, this conclusion may be conjecture. At a minimum, the text should read, “Several recent studies indicate that strong odor rather than irritation was likely responsible for symptoms complaints in earlier studies.”
Specific Comments
Page 6, line 28. Insert “and was used to derive AEGL-1” at the end of the sentence that ends, “for sensory irritation.”
Page 6, line 31. Delete “not greater” and replace with “not different.”
Page 7, line 7. Include citation for the statement, “Because the threshold for narcosis differs by no more than 2-3-fold among the general population” (see comment above).
Page 8, Table of Summary of Proposed AEGL Values for MEK. Insert the number “10,000” in the 10- and 30-min columns for AEGL-3.
Page 8, lines 19-27. This paragraph is inconsistent with the same paragraph in the Executive Summary. These paragraphs should be consistent.
Page 10, line 5. Is the citation for the statement, “Odor thresholds were similar for male and female control subjects, 8.2 and 8.1, and male and female subjects with multiple chemical sensitivities, 5.7 and 7.6 ppm,” from Devos and Laffort (1990)? If this is not the proper citation for this statement, include the appropriate citation.
Page 10, line 7. Change the last word in this line from “parameter” to “value.”
Page 10, lines 29-38. This paragraph summarizes a study by Shibata et al. (2002) that is referred to as a metabolism study (page 10, line 15; and Table 2, page 11). This description does not indicate that any evaluation of metabolism was part of this study. It may be; the description is not clear.
Page 13, line 14 and 21. Correct typo: “hardly at all.”
Page 13, lines 31-36. Add an introductory sentence that describes the full range of exposures and time frames that were evaluated in the study by Patty et al. (1935).
Page 14, Section 2.2.4. Is there any information on the exposure duration for the report by Smith and Mayers (1994)? The exposure in this case report was to ketone vapors (MEK and acetone), with MEK measured at 398-561 ppm. The workers suffered from “episodes of CNS depression and loss of consciousness.” What does this case study say about the AEGL-2 values that range from 1,700 to 4,900 ppm?
Page 17, line 1. The word “most” should be added between the words “of” and “ketones.” Methyl butyl ketone (MEK), a major metabolite of n-hexane, is a more potent neurotoxin than the parent n-hexane.
Page 17, line 10. Include the average for the study that is given by the authors as 150 ppm (page 14, line 32).
Page 17, line 13. To what studies does the sentence that begins, “The subjects in these studies,” refer?
Page 17, line 17. Delete “most probably” and replace with “most likely.”
Page 17, line 20. Insert the phrase, “specific to methyl ethyl ketone,” in the sentence starting, “No conclusion could be drawn from a developmental study.”
Page 17, line 21. Add a sentence that briefly describes the results reported in the developmental study with mixed solvents including MEK.
Page 18, Table 3. Why was Carpenter et al. (1949) not included in this summary table?
Page 19, line 12. Insert “(See Table 3)” at the end of the sentence that ends with “exposure concentrations.”
Page 19, Section 3.2. Nonlethal Toxicity. At some points, the text refers to nonlethal toxicity and at other times to sublethal toxicity. These terms can have different meanings to different people and should not be used interchangeably. Be consistent.
Page 22, lines 12-14. It is stated here that a 7-day exposure of rats to MEK reduced hexabarbital sleeping times, indicating a “stimulatory” effect. MEK, like other ketones, induces cytochrome P450s. An increase in P450 activities would increase the rate of hexabarbital metabolism, thereby enhancing its metabolic clearance and reducing its hypnotic action.
Page 32, lines 1-7. Add a sentence at the end of this paragraph that describes the specific developmental abnormalities that were found in studies of animals exposed to MEK.
Page 34, lines 9-10 and 20. Add the fact that the average MEK exposure in the Seeber et al. (2002) study was 150 pm.
Page 35, line 11. The use of the word “safety” is inappropriate. Safety is not something that can be assured by the AEGL values regardless of supporting data. Perhaps the authors are referring to the margin of exposure between the proposed AEGL and frank adverse health outcomes.
Page 35, lines 11-13; page 40, lines 11-14. The comment that AEGL-1 is “supported by numerous behavioral and metabolic studies” can be considered misleading. As the review is written, none of the metabolic studies addressed “sensory irritation or neurotoxic effects.” It is inappropriate to cite these studies as supportive the absence of effects at 200 ppm when the authors did not look for any signs or symptoms of these effects.
Page 40, line 15. Add the fact that the average MEK exposure in the Seeber et al. (2002) study was 150 pm.
AEGL-1
On page 35, lines 3-19, several studies that show MEK to be a very weak human sensory irritant or CNS depressant upon 4-hr exposures of up to 380-400 ppm are cited here. Although 200 ppm is a NOAEL, significantly higher exposure levels are very likely to be NOAELs. Findings in the animal studies summarized in lines 36-39 of page 34 demonstrate that rodents must inhale much higher concentrations than humans in order to exhibit CNS depression. Rodents receive a greater systemic dose and are thus more susceptible to MEK vapor-induced CNS depression than humans upon equivalent exposures. This is because of the animals’ more rapid respiration (alveolar ventilation) and cardiac output (tissue perfusion) rates. Therefore, the selection of a higher NOAEL is recommended in order for the actual threshold to be more closely approached. Data included in the draft AEGL document support a value of at least 400 ppm.
It is stated in lines 16-18 of page 35 that the same value was used for all exposure durations, because steady-state would be approached within 4 hr. This is probably not the case. MEK is a relatively water-soluble volatile organic compound (VOC) with a relatively low air:blood partition coefficient. Thus, its exhalation rate is slow for a VOC. MEK’s rate of metabolism is also slow, particularly at exposure levels that saturate its metabolism in humans (that is, >50-100 ppm) (Liira et al. 1990a). As a result, blood MEK concentrations typically continue to increase under these conditions rather than reaching near-steady-state. A validated PBPK model for MEK can and should be used to forecast the time-course of this material in blood.
There is concern that the individuals with self-reported multiple chemical sensitivity (sMCS) may not be a population that is truly sensitive to MEK. While they may be more sensitive than normal to sensory irritation, it appears unlikely they would necessarily be more sensitive to CNS depression. This should be distinguished in the draft AEGL documentation.
As described above, rodents will receive a greater systemic dose than humans upon equivalent inhalation exposures to VOCs. This negates the pharmacokinetic component of the
classical 10-fold interspecies uncertainty factor. There is uncertainty, however, about the remaining pharmacodynamic component of the factor. It is generally accepted at present that VOCs depress neuronal function by the same mechanism in (all) mammals (that is, the lipophilic VOCs partition into the lipids of myelin sheaths and neuronal membranes, and inhibit propagation of action potentials due to their physical presence). A number of research groups are currently investigating molecular mechanisms (for example, effects of VOCs on membrane receptors and binding of neurotransmitters to receptors). These investigations could reveal interspecies pharmacodynamic differences. The subcommittee requests that the NAC search the recent biomedical literature to learn whether there is information on the relative CNS-depressant potency of VOC anesthetics in rodents vs. humans and to evaluate the state-of-the-art mode of action of CNS anesthetics across mammalian species.
AEGL-2
There is concern that humans subjected to 4,900 ppm for 10 min or 3,400 ppm for 30 min may experience serious irritation of ocular and respiratory mucus membranes that could impair ability to escape. Therefore, it is recommended that the 10- and 30-min AEGL-2s be set at 1/5 of the mean RD50 of 10,000 ppm (that is, at 2,000 ppm). The 1-, 4-, and 8-hr values can be based upon CNS depression, as was proposed in the interim document. PBPK modeling should be utilized to extrapolate across time. Such extrapolation from longer to shorter exposure periods typically results in lower AEGL values for the shorter exposures (Bruckner et al. 2004. J. Toxicol. Environ. Health A 67:621-634), than are obtained with the ten Berge et al. (1986) approach.
AEGL-3
On page 39, lines 3-35, there is some concern that the 10- and 30-min AEGL-3s (1) were based upon variable casual observations in a menagerie of studies, and (2) may be high. La Belle and Brieger (1955) observed deaths of rats inhaling 9,090 or 9,260 ppm for 4 hr. It would be preferable to base all of the AEGL-3s upon the 4-hr MLE01 of 7,500 ppm calculated by Fowler et al. (1999) from La Belle and Brieger’s data. PBPK modeling should be utilized for interspecies dose extrapolation.
Concern was again expressed that the interspecies UF of 1 may not take into account uncertainty about the molecular mechanism of action (pharmacodynamic component). As mentioned previously, rodents receive significantly greater systemic doses of VOCs than do humans upon equivalent exposures, and mice are expected to receive the greatest systemic dose. In absorbed dose, rats are expected to be between mice and humans exposed to the same administered air concentration. As a result, the pharmacokinetic component of the interspecies UF would actually range from 0.3- to 0.7-fold. In practice, this should offset a potential 3-fold pharmacodynamic interspecies difference, and some discussion of these differences should be offered in the discussion of UF.
COMMENTS ON HYDRAZINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on hydrazine. The document was presented by Robert Young of Oak Ridge National Laboratory. The subcommittee recommends the following revisions. A revised draft should be reviewed by the subcommittee at its next meeting.
General Comments
The document has been improved substantially, but the text omitted the critical observations of Leakakos and Shank (1994). These investigators found that hepatic DNA methylation (presumably a requirement for oral and parenteral hydrazine-induced liver cancer in rodents) was detectable only when the dose of hydrazine was necrogenic. Thus, overt tissue damage is an apparent prerequisite to both hydrazine-induced liver and nasal cancer in rodents. Those observations are important in regards to the mechanism of hydrazine-induced cancers (Section 4.2) and to the assessment of hydrazine’s carcinogenic potential (Appendix B). The NRC has published a report on hydrazine in the report Emergency and Continuous Guidance Levels for Selected Submarine Contaminants (2004). The NRC should review the document to determine whether further revisions need to be made to this AEGL document.
Discuss the AEGL-1 in relation to the human occupational exposure study (Koizumi et al. 1968) and explain the monkey study as supporting it (House 1964). Analytical methods from 1964 are questionable (at less than 1 ppm) compared to later analytical methods, and while no confirmatory information is available, it may be that the monkeys were exposed to higher concentrations than reported.
Check 2003/2004 papers on hydrazine DNA binding to determine if necrosis precedes binding. This is an important concept, and if recent papers question it, the reader should know. The document will benefit from an expanded discussion of the DNA adducts observed after hydrazine exposure and from references to authoritative reviews (for example, ATSDR 1997). At least 19 chronic oral bioassays of hydrazine (generally as the sulfate) in mice, rats, and hamsters have demonstrated its unequivocal carcinogenic activity in rodents (reviewed in ACGIH 2001 and IARC 1999). Dose-dependent hepatocellular carcinomas in hamsters (Bosan et al. 1987), hepatocellular adenocarcinomas, carcinomas, spindle cell carcinomas, and cholangiomas in rats (Severi and Biancifiori 1968; Steinhoff and Mohr 1988) and pulmonary adenomas, pulmonary carcinomas, hepatocarcinomas, myeloid leukemia, lymphomas, and reticulum cell sarcomas in mice are typical consequences of chronic hydrazine ingestion (summarized in ACGIH 2001). On the basis of the animal bioassay data, hydrazine was classified by IARC (1999) as possibly carcinogenic to humans (Group 2B) and by EPA (1991) as a probable human carcinogen (B2).
Shank (1987) proposed that hydrazine carcinogenesis is related to indirect DNA methylation; hydrazine adduct formation is associated with hydrazine condensation with endogenous formaldehyde to produce the highly reactive tetraformyltrisazine (Lambert et al. 1986; Bosan et al. 1986) and formaldehyde hydrazone (CH2 = N-NH2) (Lambert and Shank
1988; FitzGerald and Shank 1996). Biancifiori and Severi (1966) suggested the latter may be converted in situ to the reactive methylating agent diazomethane (CH2 - N = N). After hydrazine exposures sufficient to induce hepatic necrosis, two of every 10,000 guanine bases were methylated at each position (Bosan and Shank 1983). These site-specific adducts develop as the damage progressed at or near genes for γ-glutamyl transpeptidase and cytochrome P450 IIB1 (Leakakos and Shank 1994) with hypomethylation at the p53 and c-jun proto-oncogenes and hypermethylation at c-Ha-ras and DNA methyltransferase. Hydrazine-induced changes in p53 are thought to be late events associated with the appearance of adenomas and carcinomas (Zheng and Shank 1996). Rodent hepatocarcinogenesis induced by hydrazine is associated with increased DNA methyltransferase expression, K-ras oncogene activation, and a propensity at high necrogenic doses toward formation of methylguanine adducts (FitzGerald and Shank 1994).
Rat and hamster inhalation data show consistent damage to the dorsal lateral, middle lateral, and ventral meatuses of the nasal passages with the most severe lesions found in the dorsal medial and superior ventral meatuses (Latendresse et al. 1995; Vernot et al. 1985). The target transitional epithelium in those areas has high metabolic capability (Bogdanffy 1990). Acute or subchronic exposure to airborne hydrazine produced degeneration and necrosis in the olfactory transitional epithelium. Vernot et al. (1985) noted the similarity of the hydrazine-induced pathology in lateral aspects of the naso- and maxilloturbinates and in the lateral wall of the anterior part of the nasal cavity to that seen after formaldehyde exposure. These areas of transitional epithelium (Harkema 1991) from which rat nasal hyperplasia and adenomas stem receive high inspiratory air flows which result in high local xenobiotic delivered dose (Kimball et al. 1993). Latendresse et al. (1995) found only minimal to mild rhinitis and concluded that hydrazine-induced local inflammation likely played a minimal role in rat hydrazine nasal tulmorigenesis; Vernot et al. (1985) found the rat nasal tumors produced after 6 hr/day, 5 day/week exposures were associated with chronic irritation. The promotion and progression of hydrazine-induced rat nasal cancer is similar to that seen after formaldehyde exposure, except that hydrazine-induced benign nasal adenomatous polyps were associated with the nasal transitional epithelium where formaldehyde-induced squamous carcinoma was associated with DNA-protein crosslinks and damage in transitional and respiratory epithelia. The regenerative proliferation in target tissues induced by inhaled formaldehyde appears to increase mutagenic frequency, and the nonlinearity observed in the formaldehyde concentration-response relationship is consistent with its genotoxicity and consequences of regenerative cell proliferation (Mathison et al. 1997).
Hydrazine apparently presents mixed modes of action, resulting from its marked corrosive and irritant properties and selective DNA methylation. Upper respiratory tract tumors after inhalation exposure in rodents are associated with high local-delivered dose and overt cytotoxicity in the target tissue. Systemic hydrazine intoxication is characterized by hepatic, renal, brain, and pulmonary damage where the parent compound, metabolic products, and reactions between hydrazine and endogenous formaldehyde appear to all play contributory roles.
A number of reactive intermediates including acetyl, hydroxyl, and hydrogen radicals are formed over the course of hydrazine biotransformation (Sinha 1987). Hydrazine exposure inhibits glutamine synthase (Kaneo et al. 1984; Noda et al. 1987), and succinate dehydrogenase (Ghatineh et al. 1992), depletes ATP formation (Preece et al. 1990), and induces formation of
megamitochondria (Teranishi et al. 2000; Wakabayashi et al. 2000). Hydrazine inhibits the hepatic urea cycle (Roberge et al. 1971), depletes stores of reduced glutathione (GSH) (Jenner and Timbrell 1994), generates reactive oxygen species, reduces the mitochondrial membrane potential, and inhibits catalase activity in rodent livers (Hussain and Frazier 2002). Available data point to the conclusion that hemoglobin damage (Rung-Morris et al. 1988) and hepatocellular toxicity (Hussain and Frazier 2002) are related primarily to hydrazine-induced oxidative stress.
Modulation of hydrazine biotransformation by phenobarbital pretreatment reduced the incidence and severity of hepatic lipid accumulation. Prior piperonyl butoxide exposure increased hepatic fatty deposition (Scales and Timbrell 1982). These data suggest that the parent material and products of its metabolism contribute to the liver damage.
NRC (1985) reviewed the rodent hepatic DNA N-7 and O-6 methylation data in relation to the carcinogenic activity of oral hydrazine. Liver concentrations of the intermediary methyl donor S-adenosylmethionine were altered, and there was no difference in [3H] methionine incorporation into S-adenosylmethionine after hydrazine treatment, but concentrations of 5-methylcytosine increased shortly after hydrazine challenge (Barrows et al. 1983). These data suggest that de novo 5-methylcytosine synthesis increased initially, followed by formation of the N-7 and O-6 methyl guanines (Barrows et al. 1983). Methylguanines were detectable in rat livers only after necrogenic doses, and O6-methylation occurs only after near-lethal exposure (Leakakos and Shank 1994).
While there are potential differences in location and type of tumors between those induced with formaldehyde and those induced with hydrazine, the relationships between early cytotoxic changes (epithelial degeneration, necrosis, exfoliation, and subsequent regenerative hyperplasia and metaplasia) observed in rodents subchronically exposed to airborne hydrazine (Latendresse et al. 1995), and the development of hydrazine-induced tumors in the rodent nasal transitional epithelium have not been conducted.
Despite the similarities in response between hydrazine and formaldehyde in the rodent upper respiratory tract, the fundamental mechanistic studies of hydrazine tumorigenesis in the nasal transitional epithelium recommended by Latendresse et al. (1995) have not been forthcoming. The absence of species-specific hydrazine molecular dosimetry in target tissues precludes rigorous conclusions concerning the applicability of the Appendix B default linear models to the rodent nasal response to estimate hydrazine oncogenic risk. The absence of those key data should be acknowledged in a final revised hydrazine AEGL document.
Specific Comments
Page 18, line 35; page 37, line 17. It is unlikely that the eyeballs per se were swollen as a result of atmospheric hydrazine. Rather, the statement appears to refer to “swollen eyelids” or “mucous membranes around the eyes.”
Page 20, lines 1-8. On line 8, it is not clear whether the statement relates to the groups of rats exposed for 2 hr to 19 hr (line 1)—was this a single exposure—or to the groups of rats exposed for 7 months (line 3)? To what does the phrase, “allowing for possible detoxification and excretion of hydrazine and/or its metabolites” refer? Why is the speculation on lines 6-8 necessary?
Page 23, line 37. To what species does the statement refer?
Page 27, line 34. Cite the reference(s) to the “early reports” (line 33) to which the sentence refers.
Page 37, lines 36-37. Indicate the section of the SOP containing the text, “alternative approach whereby AEGL-2 values are derived by a 3-fold reduction in AEGL-3 values”? The subcommittee was unable to locate that SOP guidance and should this be an NAC addition not described in the SOP, the sentence on lines 36 and 37 should be deleted.
Page 37, lines 42-44. Delete. Where are the Benchmark Dose calculations? Since the AEGL-3 was based on the HRC (1993) data, the HRC (1993) data were not subjected to the Benchmark calculations and the Benchmark Dose approach was not used in AEGL-3 derivation (page A-4). Why is this circuitous information included here?
Page 38, line 32; page 40, line 14. Does the A2 entry refer to the current ACGIH carcinogen classification? If so, indicate that here and modify footnote h on page 40 to reflect that classification.
Page 38, last sentence. The statement is inaccurate. First, the OSHA Permissible Exposure Limits have nothing to do with the AEGL exposure duration. Second, define the term “more conservative.” As written, the statement does not appear to be supported by empirical data.
Page 40, line 39; page 44, line 26. Insert NRC (2001) Standing Operating Procedures reference and include the NRC (2001) citation on page 44, line 26.
Pages B-1 to B-3. There is no statement in Appendix B which matches the key conclusion appearing on page 26, lines 46-47. As written, pages B1-B3 focus only on a default linear treatment of the rodent inhalation data and fail to take the overt tissue damage and regenerative hyperplasia in rodent nasal epithelium into account. The Appendix should be consistent with the body of the document and should acknowledge the fundamental limitations of the underlying Appendix B linear dose-response assumption in the present situation.
The presentation of the UF remains a problem. For irritants and direct-acting chemicals (and the case is made for hydrazine-induced irritation as the primary basis for AEGL-1 and AEGL-2), interspecies and intraspecies UF adjustments have generally applied 3 for each for a total of 10. This was done for AEGL-1. However, for AEGL-2, “An uncertainty factor of 10 for interspecies variability was applied to account for the high degree of variability in
the data due to the extreme reactivity of hydrazine that compromised exposure concentration measurements.” What does this have to do with interspecies variability, especially when hydrazine appears to be a direct acting material? While an interspecies UF of 10 may be reasonable, the rationale of uncertainty of the data is not a UF issue. Uncertainty in the data should be addressed with a modifying factor. The response provided to the comment in Section 6.3, that the nasopharyngeal area of rats and humans are different, should be discussed here. This concept is very helpful in distinguishing hydrazine from a simple irritant.
Regarding LOA, move it to between AEGL-2 and AEGL-3. If it is true that the LOA is at the AEGL-3, this should be clearly stated.
The text should include a general statement regarding exposure concentrations. In addition to being highly reactive, it is the general understanding that hydrazine also adsorbs to everything, including Teflon. The text should focus on the more recent studies, which have presumably solved these problems, and use the older reports as supporting data.
Section 2.2.1. What is meant by the phrase “Nonlethal toxicity of the who person died”? Also, the document needs to confirm whether this was a six-week or six-month exposure. It’s been stated differently in two locations: once per week for 6 weeks (§2.2.1) and 6 months (§7.1).
Section 2.2.2. This section is disjointed. For example, not all of the studies are associated with propellant manufacturing. Consider combining the first and last paragraphs which would aid in supporting the conclusions.
Section 5.2. If hydrazine is a direct-acting chemical and irritation is the effect, why is it discussed as a cumulative exposure? How does the NAC use a cumulative exposure and derive an AEGL-1 that is constant across time? This was answered in the meeting, but the observation was made at the end of a 24-hr period. Add this information to anwser questions before they get asked. At the end of this paragraph, add the human exposure level (not stated other than below the TLV of 0.1 at the time). The previous response does not address the question.
Section 5.3, 2nd paragraph. Is the discussion on geometric mean employing the proper terminology and rationale? Cite the section of the SOP where 3.16 as the square root of 10 is discussed. Provide a very brief summary for the reason 3.16 is used. Other than SOP, this is the first time 3 (3.16) has been discussed in a chemical dossier. This addition is commended; just make sure the rationale is understandable. The SOP indicates 3.16 is the geometric mean of 1 and 10, not just 10. Correct the text.
Section 7.1. There is no mention of the concentration associated with the human exposure described here.
Section 7.3. The statement is made that the lethal effects of hydrazine appear to be more dependent on concentration than duration; therefore, exponential scaling was used. If the
lethality is more dependent on concentration than total dose, then time scaling is not as important. Delete the reference to scaling and just use the discussion on “n” as usual.
Section 7.3, 5th paragraph. It may not be that the highly reactive nature of hydrazine resulting in a compromised exposure concentration measurement is a good rationale for using a species UF of 10. This would be better addressed as a modifying factor.
Page 10, 11, and 34. Rewrite the description of the paper by Sotaniemi et al. (1971). If the airborne concentration derived from the simulation of the fatality were likely 0.05 ppm as estimated, then the AEGL derivations could be incorrect. Since this study is not relied upon for the AEGL calculations, the reasons for its exclusion should be more fully stated. The actual dose received from a once-a-week, 6-month exposure is not known, and was likely variable and highly uncertain. This should be stated. Can dermal exposure in this case report be ruled out from the author’s description of events, or was it possible?
Additional Reference
Leakakos, T., and R.C. Shank. 1994. Hydrazine genotoxicity in the neonatal rat. Toxicol. Appl. Pharmacol. 126:295-300.
COMMENTS ON IRON PENTACARBONYL
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on pentacarbonyl (Fe5CO). The document was presented by Robert Young of Oak Ridge National Laboratory. The subcommittee recommends minor revisions. The document can be finalized if the recommended revision is made appropriately.
Overall Comment
The iron pentacarbonyl AEGL document was generally revised to address comments by NAS. However, the following comments revolve around the use of the scaling factors and public exposure to this material. While the exposure issue was addressed at the August 31-September 2, 2004, meeting, those conclusions must be included in the TSD.
General Comments
The time-scaling value needs to be either derived from the data or defaulted to the SOP values of n = 1 or n = 3. The first and revised draft used n = 1, and that value was not changed. The relationship was described as near linear, but the data described do not consistently fit with this conclusion. The rationale was based on the Sunderman and Biodynamics studies where CT = 59 and 40, respectively. However, the key BASF study had a 6-hr LC50 of 3; therefore, CT = 18. Thus, from the data presented, there is not consistent support for n = 1. To address this problem, the NAC may make use of defaults.
The rationale for iron pentacarbonyl exposures to the general public was not added. The substance may ignite but would require an ignition source. It would be helpful to add a few sentences on page 1 that explain the need for an AEGL for this material.
If iron pentacarbonyl is pyrophoric in air, and is light sensitive, decomposing to iron nonacarbonyl and CO, what is its half-life at STP? Is there a need for an 8-hr. AEGL? Refer to page 1; the footnotes to Table 8 on page 12; Table 10 on page15; page 17, lines 33-34; page B-3, lines 27-28; and page B-4, lines 44-46 in addressing this request.
The footnote under Table 8 regarding photochemical decomposition under ambient conditions should be reworded because it contradicts the rationale for the development of the AEGL on page 1.
On page B-4, line 32 refers to “possible variability in metabolism and disposition” as one of the factors supporting an interspecies UF of 3. On page 14, lines 35-36 refer to portal of entry effects and limited variability due to dosimetric factors. In what way are these statements consistent?
Specific Comments
Page ii, line 17. Add a space before 30.
Page ii, lines 18-19. To improve clarity, add the phrase “For mice, a 1.35-fold increase in the LC50exposure concentration resulted in …” (addition italicized). This would then be consistent with the phrasing used on line 21. A similar change should be made on page 6, line 19, and on page 8, line 25.
Page ii, line 21. To improve clarity, expand the parenthetical, “…in exposure concentration (from 86 to 244 ppm) …” (additions italicized). A similar change should be made on page 8, line 28.
Page ii, line 40. In the sentence, “… while a single exposure to 2.91 ppm for 6 hr caused …”
Page iii, line 24. To avoid ambiguity, add the exposure concentration to the following: “(6 hr/day at 1 ppm for 28 days)” (addition italicized). Earlier in the paragraph, at lines 11-15, reference is made to exposures at both 2.91 ppm and 1 ppm for the specified duration.
Page 1, lines 8-9. Does this reaction occur at STP, or are special conditions required for its formation?
Page 1, line 23, Table 1. The chemical formula normally used is the structural form on line 6.
Page 2, line 6. “Hepatization” is a colorful term; is it the one used by pathologists?
Page 2, lines 14-16. This sentence notes similarities in signs and symptoms between iron pentacarbonyl intoxication and nickel carbonyl intoxication. While the Stokinger reference may not mention it, it might be helpful to note here similarities with metal fume fever as well, including the time course of the effects.
Page 3, line 32. What was the interval between the two 5.5 hr exposures to 15 ppm?
Page 4, lines 5-6. The phrasing of this sentence is peculiar, “Although the … concentrations did not remain constant …, there was no definitive relationship.” It would be clearer to separately describe the variation in chamber concentrations within each exposure group and their relation to the nominal concentrations and then address the dose-response effect.
Page 5, line 25. The rat studies are described in Section 3.1.1. not 3.1.2.
Page 7, lines 29-31 (and similar recommendations for page 10, lines 18-20). For clarity, delete “also” on line 30. This sentence (and perhaps the following one) really belongs immediately after the description of post-exposure clinical observations on lines 21-24.
Page 8, line 28. For clarity, change to read: “… ppm) results in a increases the mortality rate in rats of from 4/12” (additions italicized).
Page 8, lines 33-36. The previous paragraph cites the significant studies being summarized. For consistency, this paragraph should cite the study or studies referred to.
Page 9, line 10 (also page 14, line 35; page A-4, lines 17 and 27; and page B-4, line 23). Should this not be “portal of entry”? The phrase used, port-of-entry, is a term used in international commerce to indicate the location where goods enter a country and have to pay taxes or tariffs.
Page 9, line 32. The sentence is incomplete; the phrasing requires a comparison species for the 4-hr LC50 in rats.
Page 11, line 25. Appropriate conjunction is missing to link the two clauses of the sentence: “… pentacarbonyl, but the effects …” [addition italicized]
Page 11, line 40. For clarity, add a word and a comma : “… after only one exposure, and significant …” (addition italicized).
Page 13, Table 9, line 22. For clarity, rewrite: “Number of animals dying at 2.91 ppm for 6 hr.”
Page 13, line 30. Insert missing word: “Due to insufficient data …”
Page 13, lines 35-36. To improve clarity, reword sentence : “In the absence of human data, and because there is some variability among the laboratory species tested, some uncertainty exists regarding inter-species variability” (additions italicized).
Page 14, line 32. Change the parenthetical word “mouse” to “rat” (see page 13, lines 37-38).
Page 14, lines 32-34. To improve clarity, rewrite: “an interspecies UF of 3 is supportable. The intraspecies variability UF of 3 is supported by two points.”
Page 14, line 33. Add a space and a period before “The.”
Page 14, lines 40-43. To improve clarity, reword sentence to read: “Finally, the total uncertainty factor of 10 resulted in AEGL-3 values that were consistent with the acute exposure data and the data from multiple-exposure animal studies.”
Page 15, line 14. Insert missing word: “in the BASF, 1995 study, rats died …” (addition italicized).
Page 19, line 28. The date is missing.
Page 20, lines 1-2. Reference is incomplete.
Page 20, line 8. Author and date are missing.
Page 20, line 10. Inconsistent capitalization in journal title.
Page 20, line 21. Missing publication number.
References, pages 19-20. The ACGIH documents and Patty’s Industrial Hygiene and Toxicology all have more recent editions published. Is there material in these editions that did not appear in the newer editions?
Page A-2, line 7. Delete comma.
COMMENTS ON PHOSPHINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on phosphine. The document was presented by Cheryl Bast of Oak Ridge National Laboratory. The subcommittee recommends the following revisions.
General Comment
Page 17, Section 6.3, line 31. Red mucoid nasal discharge may seem to be less severe than effects defined by the AEGL-2 definition, but it may be a symptom of haemolysis, which is one of the (serious) symptoms of the chemically and toxicologically related arsine.
Specific Comments
Page 5, line 14. Change “patients” to “victims.”
Page 7, line 39. Delete “respiratory”, as salivation and lacrimation are not signs of respiratory irritation.
Page 17, line 31. Typo: replace “severe that effects” with “severe than effects.”
Page 21, line 7. Replace “Argeitsplatzkonzentration” with proper German word “rbeitsplatzkonzentration” (in all other documents as well).
Page 21, line 10. Replace “aanvaaarde” with the proper Dutch word “aanvaarde” (in all other documents as well).
COMMENTS ON CHLORINE TRIFLUORIDE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on chlorine trifluoride (ClF3). The document was presented by Sylvia Talmage of Oak Ridge National Laboratory. The subcommittee recommends the following revisions.
General Comment
This document had been reviewed previously by the committee in July 2003. All substantive comments were addressed. The document was considered acceptable with minor revisions.
Specific Comments
Page 12. Recommend wording on line 35 be changed to read: “Gas flow rates were measured with mass flow meters, exposure chamber ClF3 concentrations were verified by infrared spectral analysis.”
Page 9, lines 42-43; page 13, lines 25-26; page 17, lines 11-12. It is recommended that the text not include confidence limits with MacEwen and Vernot’s monkey and rat data. It is not possible to substantiate these confidence limits using the published data and contemporary probit analysis programs.
Page 20. Should the point of departure on line 23 be 5 or 6 hr?
COMMENTS ON ETHYLENEIMINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on ethyleneimine. The document was presented by Kowetha Davidson of Oak Ridge National Laboratory. The subcommittee recommends the following revisions. A revised draft should be reviewed by the subcommittee at its next meeting.
Specific and Minor Points
Page v, line 1. Change to “Executive Summary.”
Page vi, Executive Summary. The LOA is not mentioned in the text of the document.
Page vi, line 24. To what does “both species” refer, guinea pig and human?
Page vii, line 10; page 24, line 11. While topical administration of the neat material has been associated with fatalities in rabbits, and some workers handling this material have developed dermatitis and skin sensitization, the statement “toxic levels” may be absorbed through the skin” here in the context of concentrations in the air (mg/m3) suggests that exposure to ethylene imine vapor (page 1, lines 8-9) can produce systemic toxicity in human beings. As described on page 11, lines 19-25, quite the opposite appears to be true.
Page 1, 1st paragraph. Include introductory discussion of LOA here.
Page 1, line 9. It would be useful to add, “It polymerizes explosively in contact with silver, aluminum, or acid” (IARC Monographs 71:337-344, 1999).
Page 1, line 25. Does the word “domestic” refer to the United States? If so, say so.
Page 1, line 29. Is this sentence missing the word “no” (no current information)? Otherwise, provide citation for the comment that “current information on shipping quantities was found in the literature.”
Page 3, line 23. Change to: “no additional information.”
Page 3, line 29. Change to: “conclusion can be drawn.”
Page 3, line 33. Sentence needs verb, add “was reported” to end of sentence.
Page 4, line 9. Rewrite the sentence to read, “The symptoms experienced by these volunteers cannot be attributed entirely to ethylene imine since these exposures involved both ethylene imine and N-ethylethylene imine.”
Page 4, lines 14-16 and 28. The text on page 3 (line 40) indicates that the initial exposure trial with ammonia and isopentane was concluded prior to the ethylene imine and N-
ethylethylene imine exposure trial and that the ammonia and isopentane were removed by a “ventilation fan in 5 to 10 min.” What is this about a “broken window” (page 4, line 28)? Does the page 4 entry here refer to any residual airborne ammonia in the room air after the ventilation period?
Page 4, lines 21-26; page 19, lines 32-34; page 23, lines 7-8. It is not clear what author(s) made these assumptions and speculative calculations. Why is this section necessary? Since no actual concentration data were reported by Weightman and Hoyle (1964), it is appropriate to note that fact as is done on page 19, line 34. Since these calculated values are not used in AEGL-1, AEGL-2, or AEGL-3 derivations, the page 4, 19, and 23 statements can be deleted. None of the calculated concentration results are considered reliable (page 19, lines 34-35).
Page 5, lines 4-9. The description here is at odds with the TLV (1991) documentation description of the Danehy and Pflaum (1938) report. The TLV text states that a “2-3 min exposure to the vapor produced no symptoms until after 3 hr, when vomiting occurred and irritation to the eyes and nose was evident.” Please check the original report to determine whether there is a description by Danehy and Pflaum of an occupational exposure to the vapor of ethylene imine.
Page 5, line 21. Typo: “human” should be plural.
Page 5, lines 21-22; page 6, line 28. The statements appear to refer to published accounts of the carcinogenic potential of chronic occupational exposure to ethylene imine in the peer-reviewed literature. As written, the text ignores the TLV description from D.J. Kilian (October 17, 1973) of Dow Chemical that “an epidemiologic study of Badische Anilin and Soda Fabrik of 144 ethylene imine workers some of whom had 40 years experience, revealed no evidence that ethylenimine was carcinogenic.” This appears to be an important point since other than this entry, there are no chronic inhalation data in animals or humans. It is worthwhile to contact ACGIH directly, contact Dow and obtain a copy of that report, and note the existence of this finding and the range of occupational exposures of these 144 workers (if available).
Page 5, line 30. Add “in” between induced and cultured.
Page 5, line 30. Typo: “significantly” missing last letter.
Page 6, lines 7-9. The new explanation for why the odor of ethylenimine can be confused with ammonia is not as good as the explanation provided in the response from Oak Ridge to the NRC comments in the 10th Interim Report (see page 36). Incorporate most of the explanation included in the response to the original NRC comment into the text here.
Page 6, line 21. “Hemoconcentration” is an unusual word. Is there a simpler word/phrase that can be used?
Page 8, line 11. Add “s” to “concentration.”
Page 9, line 25. Delete “during exposure” at end of sentence.
Page 10, line 22. Replace “mixed sex” with “both sexes.”
Page 10, line 33. The context implies that “Respiratory difficulty was not observed at 10 ppm” over 3 hr (the last mentioned time). From the Executive Summary (page vi, line 18) and from the derived AEGL-2 value, it seems that it was actually 4 hr. Clarify since the calculation of the AEGL-2 relies on this.
Page 11, line 39. Indicate strain of rat (if available).
Page 12, lines 13-14. “There was no statistically significant increase in the incidence of tumors at any site in animals injected with ethylene imine.” However, subcutaneous injection to suckling mice produced lung tumors in males (IARC Monograph 1999). This is worth mentioning since it proves systemic availability after subcutaneous injection.
Page 12, line 22. Typo: should be “Kirsch-Volders 1990.”
Page 13, after last line. A conclusion from these data with respect to human carcinogenicity and germ-cell mutagenicity would be useful to the reader, for example, “Taken together, these data make it probable that ethylene imine is genotoxic in any mammalian species. The IARC (1999) concluded that ethylene imine is possibly carcinogenic to humans (group 2B). The results from the dominant lethal tests show that ethylene imine is mutagenic to mammalian germ cells.”
Page 15, line 27. Rewrite: “and phosphate as well as amino groups on nucleic acids (Trochimowicz et al. 1994; Hemminki, 1994).”
Page 16, line 8. Typo: place “of” between “alklyation” and “DNA.”
Page 16, line 12. Typo: add first parenthesis before “1999.”
Page 16, line 12. Add a comma after Hemminki.
Page 19, line 35. The controlled inhalation study of Weightman and Hoyle (1964) is not a “case report.” Delete lines 32-34 as noted above.
Page 20, line 13. The non-lethal 25 ppm concentration in guinea pigs exposed for 3 hr cannot be considered “life-threatening” for human beings, particularly in light of the Carpenter et al. (1948) observation that irritation does not occur in humans exposed to less than 100 ppm (page 19, lines 24-25). Delete the conjecture found in the AEGL-2 derivation here concerning death.
Page 20, lines 21-22. The subcommittee agrees that the 3 is appropriate for interspecies differences in AEGL-2 and the AEGL-3 for intraspecies variability, but they still need clarification, and the reasons from the SOPs should be cited.
Page 20, lines 21-23. The rationale for the interspecies UF (page 20, line 18) is proposed as identical to that used for the intraspecies UF here (page 20, lines 21-23). As written, line 23 is not consistent with the rationale used for AEGL-2 intraspecies uncertainty for other irritants (for example, allyl alcohol on page 19, lines 17-22). Based on the rather consistent response reported by Weightman and Hoyle (1964) among the five young adult male volunteers (pages 3 and 4) and the statement on page 17, lines 13-14, it appears that the intraspecies UF of 3 can be justified.
Page 20, lines 35-36. Delete. What difference does it make in AEGL-2 derivation what the opinion of any regulatory agency on any toxicological end point is at any particular time?
Page 20, line 37. The entry here to Appendix A is not correct; should this entry refer the reader to Appendix B?
Page 20, lines 36-37. The statement as written is incomplete. No acute or chronic inhalation bioassay data in animals are available to identify ethylene imine as carcinogenic by the route of exposure relevant to the AEGL. Furthermore, the available epidemiologic report failed to demonstrate a carcinogenic response among 144 ethylene imine workers exposed for up to 40 years. The conclusion should be written: “Available data are not adequate for determining the carcinogenic potential of airborne ethylene imine or deriving theoretical excess cancer risk estimates (Appendix B).”
Page 21, line 23: Typo: add “of” after “showed signs.”
Page 22, line 2. Say why the data were “unsuitable” for probit analysis.
Page 24, lines 17-18. The NAC is mistaken. The skin designation is assigned to material because data are available showing that skin contact is associated with absorption sufficient to contribute to systemic toxicity, not sensitization (which carries the sensitizer designaton) or dermatitis. Correct the entry. Typo in comment,“which carries the sensitizer designation” (not designaton).
Page 24, lines 24-29. Delete. Why is it necessary to list organizations that have not established values for this or any other material? One could also include a great many other organizations that have not assigned occupational exposure limits for this or any other material considered under the AEGL program.
Page 29, line 1. Not in alphabetical order (should be on page 28, line 27).
Page 72, Section 2.5.3.2.3. The interspecies UF value of 3 is consistent with the SOP. The explanation for the intraspecies UF of 3 suggests that the mode of action is known (direct alkylation) and that the response is likely to be similar across the population, although
some variability is still expected (e.g., in repair), so that a full UF of 10 is not needed. Again, this is consistent with the SOPs (page 90, section 2.5.3.4.4), but not well articulated. The rationale for decreasing the interspecies and intraspecies UFs from 10 appear to be the same for both factors.
Section 8.3. The statement at line 17 is in direct contradiction to that made at lines 20-23. How is it that AEGL-2 values can be derived (page 20) when Section 8.3 states, “The data for deriving the AEGL-2 value was less than adequate (line 27)?”
Appendix B. The text ignores the Dow report (Kilian 1973, see above comment to page 5, lines 21-22).
COMMENTS ON PROPYLENIMINE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on propylenimine. The document was presented by Kowetha Davidson of Oak Ridge National Laboratory. The subcommittee recommends the following revisions.
General Comment
The document has been improved substantially; however, minor problems remain.
Major Comment
The rationale for selecting a relative potency of 5 for AEGL-2 needs to be clearly explained.
Specific Comments
Section 2.2, line 30. The statement on line 31 is not entirely correct in that duration of human ethyleneimine exposure has been described on at least one occasion. It is worthwhile to note the report by Danehy and Pflaum (Ind. Eng. Chem. Res. 30:778, 1938) who found that a single exposure to ethyleneimine vapor for 2-3 min resulted in vomiting that was delayed some 3 hr after the incident. While no concentration data were presented, the nausea and vomiting continued for “a few days.”
Page 4, line 27. Use and reference the IARC (1999) evaluation.
Page 7, line 23; page 8, line 35; page 9, bottom line; page 10, line 5; page 10, line 34. No data concerning percutaneous toxicity associated with topical or airborne propylenimine are presented in the document. It appears that the NAC relied upon the ACGIH skin designation assigned in 1991. Quoting from ACGIH (1991), “Because propylenimine resembles ethylenimine in its physiologic action, a skin notation is also recommended.” As
written, the draft AEGL document suggests that airborne propylenimine contributes to systemic toxicity due to its absorption through intact human skin. No data are cited in the present document to support that contention. The erroneous statements listed above should be deleted from the document.
Page 8, line 1. Add “by inhalation” after ethylenimine, since the toxicity difference on the skin is smaller than given here.
Page 10, line 13. It is stated that a relative toxicity approach compared to ethylenimine was used to develop AEGLs 1 and 2; however, there is no AEGL-1. AEGL-3 is based upon a single concentration over time. Since the relative toxicity was developed on mortality data, it would seem the relative potency approach would work best for AEGL-3. Based upon ethylenimine what is the AEGL-3?
Page 10, line 40. No references to ACGIH (1991) or ACGIH (1997) appear in the page 12 bibliography.
COMMENTS ON ALLYL ALCOHOL
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on allyl alcohol. The document was presented by Claudia Troxel of Oak Ridge National Laboratory. The subcommittee recommends the following revisions. A revised draft should be reviewed by the subcommittee at its next meeting.
Overall Comments
The NAC used an interspecies UF of 1 in deriving an AEGL-3 based on data from an animal study. The rationale provided for this determination is that “these data suggest little difference between species in response to allyl alcohol exposure” (page vii, line 28; page 17, lines 25-26). However, no data are provided in the Executive Summary to support this claim. The text (page 17, Section 4.3 Species) discusses some data, but it is not sufficient to conclude that all species (including humans) respond similarly to the effects resulting from exposure to allyl alcohol.
The data discussed in Section 4.3 are mostly lethality data, and no data on humans were presented that are comparable to the animal data. In addition, the text states that “the lethality data summarized in Table 5 lack LC50 values suitable for direct comparisons of species sensitivity” (page 17, lines 19-20). In addition, the data presented on nonlethal effects come from a study in which all the animal data were grouped together such that the reader cannot determine which specific effects occurred in which specific species. The text states that these results were “discussed in general terms for all species” (page 9, line 33). For these reasons, selecting an interspecies UF of 1 for AEGL-3 may not be justified, and a UF of 3 could be used to derive AEGL-3.
The argument for selecting an intraspecies UF of 3 for AEGL-3 is weak and not scientifically based; the values would be “inconsistent with available empirical data” (page vii, line 33). Inconsistency between the results and other established values is not sufficient reason to alter the UFs. It is illogical to make a scientific judgment about what the UF should be based on the data and available information, and if the end result values seem inconsistent with other values, go back and adjust the UFs. The UFs should remain the same and then, if there is a strong reason to change the resulting numbers, an adjustment should be made. There needs to be a solid scientific basis for moving away from the default value of 10. This should not be done in order to “make the numbers work.”
The basis for selecting an intraspecies UF of 3 for AEGL-1 is that “irritants are not likely to vary greatly among individuals” (page vii, lines 11-12). No data were presented to support this statement in the Executive Summary or in the text. While it may be true, it needs to be discussed and documented here or by reference to the exact section of the SOP. The statement should be precise in discussing nasal irritation, not ocular or other forms of irritation. Does this protect the individual from other adverse health effects as well? Are children more sensitive to irritants than adults?
General Comments
Explain how the NAC justified a 30 min AEGL-3 nearly 7 times greater than the IDLH. At a minimum, the text on page 22, line 3, should provide some rationale for the marked difference—perhaps by providing a one- or two-sentence critique of the 20-ppm IDLH for this material.
The authors should refrain from statements like those made on page 10 (“Thus, the NAC inferred…”), page 17 (“The NAC committee recommended…”), and page 23 (“The NAC recognizes…”). As written, the text begs the questions, if NAC recognizes the potential carcinogenicity of allyl alcohol, why don’t the AEGL values recognize that conclusion accordingly?
As written, it is not clear why the experimentally derived n = 0.8 in Section 4.4 was not used for time scaling since page 94 of the SOP lists TCE as one example of a substance with n = 0.8. There is nothing in Section 2.7 of the SOP that states empirical n values < 1.0 shall be assumed equal to the default n value of 1; SOP page 103 states, “The lowest value of n was 0.8 and the highest value of n was 3.5.” Therefore, additional justification for n = 1 (page 20, lines 28-29) is needed unless the empirical n = 0.8 is used in time scaling.
As written, lines 2-11 on page 21 are not understandable. On line 1, it is not clear whether the “default” to which the author refers relates to n or possibly to the various uncertainty factors. This section should either be deleted or rewritten.
In discussion of the intraspecies UF on page 20, lines 15-17, there is no consideration of those with clinical, bacterial, or other infections. Given that allyl alcohol-treated rats pretreated with bacterial endotoxin experienced enhanced hepatic damage as compared to rats given ally
alcohol alone (Sneed et al. 1997), it is not clear that the justification provided for the 3-fold intraspecies UF can be supported.
The regression analysis yielded n = 0.78, and the NAC rounded it to 1. As discussed at the meeting, rationale must be provided for why the value was changed (not rounded) based upon the data. Reference should be made to the appropriate section in SOP to support treatment of the data.
Specific Comments
Page vii, line 8, Executive Summary. What is meant by “the model”? Clarify the intent of this phrase.
Page 3, Table 2. Fix spacing typo in 5th column.
Page 9, Section 3.2.2., lines 2-14. This paragraph is redundant because earlier text describes the same study (page 6 Lines 16-38). Is it necessary to describe both studies in detail? Should these results only be included in the lethality section since some animals died, although others, at different exposure levels, survived.
Page 9, Section 3.2.2., lines 15-20. This paragraph (lines 15 to 20) is redundant. Same comment as above.
Page 9, lines 31 to 33. The comment that the “effects reported by the study authors were not separated by species (dogs, guinea pigs, rabbits [Section 3.2.1.], and rats), but were discussed in general terms for all four species” should be included in the discussion of the same data on page 8, lines 26-39.
Page 15. The document will be improved if the mode of action (depletion of reduced glutathione [GSH], loss of protein sulfhydryls, and peroxidation of lipid membranes) was explained. It is important to explain that studies with inhibitors of alcohol dehydrogenase (such as 4-methylpyrazole) abolish allyl alcohol-induced hepatotoxicity and that pretreatment with inactivators of Kupffer cells (such as gadolinium chloride) can attenuate allyl alcohol-induced hepatoxicity. As written, the text leaves the reader with the impression that not much is known about the mode of allyl alcohol action when this is really not the case at all.
Page 16, lines 8-9. The statement, “there was no delay in the appearance, development, or disappearance of the measured irritant response,” does not follow the previous four lines on the role of acrolein in allyl alcohol toxicity.
Page 17, Section 4.3. This section should include a discussion on potentially susceptible populations, especially children. As written, the AEGL document does not consider whether the proposed AEGL values would be protective of children or those with compromised medical conditions.
Page 17, lines 5-12. It is not clear from the text here whether allyl alcohol is considered a reactive or a nonreactive alcohol.
Page 17, line 36. What does allyl amine have to do with the present document on alcohol? Delete.
Page 18, lines 18-19. Should this sentence state “nasal irritation is not likely to vary greatly among individuals” (as opposed to irritation in general)?
Page 19, Section 6.2. This section states that “no single exposure inhalation study reported effects consistent with the AEGL-2 definition.” This may be true, but there were several single-exposure mouse studies that should be mentioned in this section.
Page 20, lines 10-12. The comment is made here that AEGL-3 values were based on the “highest concentration causing mortality in mice, rats, and rabbits,” but it is not clear which species was used to derive the AEGL-3 values. As is stated in the next paragraph, there were deaths in these same species at higher exposures. The difference is that these higher exposures occurred for different periods of time—longer in the rabbit and shorter in the mouse (see Table 5). This should be noted in the text. The reason for selecting the 200 ppm exposure values, as opposed to the 500 ppm exposure, to derive AEGL-3 needs to be made clear in the text.
Page 21, line 2. Should this read AEGL-3 and not AEGL-2?
Page 21, lines 6-7. What is meant by the statement, “The AEGL-2 values help to serve as a baseline: they are based on a multiple scenario in which rats exposed to 40 ppm for 7 hr/d exhibited reversible signs of irritation”? The current AEGL-2 is based on data from human exposure, not on animal data. To what does this statement refer?
Page 21, lines 16-17. Why is this sentence necessary?
Additional References
Belinsky, S.A., T. Matsumura, F.C. Kaufman, and R.G. Thurman. 1984. Rates of allyl alcohol metabolism in periportal and pericentral regions of the liver lobule. Mol. Pharmacol. 25:158-164.
Hormann, V.A., D.R. Moore, and L.E. Rikans. 1989. Relative contributions of protein sulfhydryl loss and lipid peroxidation to allyl alcohol-induced cytotoxicity in isolated rat hepatocytes. Toxicol. Appl. Pharmacol. 98:375-384.
Maellaro, E., A.F. Casini, B. Del Bello, and M. Comporti. 1990. Lipid peroxidation and antioxidant systems in the liver injury produced by glutathione depleting agents. Biochem. Pharmacol. 39:1513-1521.
Przybocki, J.M., K.R. Reuhl, R.G. Thurman, and F.C. Kaufman. 1992. Involvement of nonparencyhmal cells in oxygen-dependent hepatic injury by allyl alcohol. Toxicol. Appl. Pharmacol. 115:57-63.
Sneed, R.A., S.D. Grimes, A.E. Schultze, and P.E. Ganey. 1997. Bacterial endotoxin enhances the hepatotoxicity of allyl alcohol. Toxicol. Appl. Pharmacol. 144:77-87.
COMMENTS ON ETHYLENE OXIDE
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on ethylene oxide. The document was presented by Kowetha Davidson of Oak Ridge National Laboratory. The subcommittee recommends the following revisions. A revised draft should be reviewed by the subcommittee at its next meeting.
General Comments
The subcommittee understands the argument that dose to the tissue in rats is likely to be higher than in humans. This should have been succintly stated for the non-toxicologist who may read this TDS.
The subcommittee is not convinced of the correctness of the inter- and intra-species UFs used. They seem low and not sufficiently justified. Is the ossification issue expected to be the same in rats and humans? The subcommittee believes the answer is no. If no, the inter-species UF should be higher.
The use of 100 ppm (from the Snellings et al. 1982a study) as a starting point for the development of the AEGL-2 value needs a convincing argument saying that (consistent with the definition of the AEGL values as “concentrations above which it is predicted that…”) the effects seen at this concentration are not serious health effects and those at the next higher concentrations are (or else the proposed values should be changed).
Specific Comments
The reasons for choosing the older and analytically less refined study (Jacobson et al. 1956) as the basis for deriving the AEGL-3 values (instead of Nachreiner 1991, 1992) should be explained better. The addition of the argument that this leads to more conservative values may be useful. It is stated that the reason for using the Jacobson study is that it is a clear dose response, and if this is the case, showing the plots may be helpful.
Page 1, 2nd paragraph. The major use of ethylene glycol is not in the production of antifreeze, but rather as an intermediate in the production of polyesters (IARC monograph 1994).
Pages 43-45. It should be reevaluated whether the contribution of glutathione S-transferase to the total disposition of ethylene oxide is really only 10%-20%, and whether at the same time, the contribution of glutathione S-transferase polymorphism(s) to ethylene oxide toxicity is really significant. Could it be that the two statements refer to different concentrations of ethylene oxide (10%-20% contribution at high saturating concentrations; significant contribution to toxicitiy at low, nonsaturating concentrations)?
Page 44, lines 11-14. It is probably not accurate to say that EtO is metabolized by hydrolysis. The hydrolysis of EtO is reportedly not catalyzed by epoxide hydrolase or any other enzyme. It would be better to replace “metabolized by hydrolysis” with “hydrolyzed.” Similarly, the word “metabolism” in line 4 should be replaced with “clearance.”
Page 45, line 5. Change “adduct level” to “adducts” at the end of the line.
Page 45, lines 6-9. Specify which GST isozyme the authors are referring to here.
Page 46, lines 1-5. Have any more recent papers been published that address the mechanism(s) of EtO-induced neurotoxicity?
Page 47, Section 4.4.3 and Figure 1. The text should point out that the data base for using n = 1.2 to extrapolate from 4 hr to other time points is slim but considered to be better than just using a default factor.
Page 49, lines 4-6. It is stated in paragraph 1 that “the reproduction study of Snellings et al. (1982b) cannot be used because it is difficult to attribute the observed effects to a single exposure to EtO.” This reasoning appears inconsistent with that utilized in paragraph 2 to justify basing the AEGL-2s on the developmental study of Snellings et al. (1982a).
Page 49, lines 5-17. It is correctly stated that there would not be an accumulation of EtO upon daily exposure to the chemical. There could, however, be an accumulation of EtO adducts to proteins, RNA, and DNA in the fetus. This is an argument against use of a repeated-dose study’s results to derive AEGL-2 values. It should be pointed out here that EtO is a direct-acting alkylating agent that has been shown to be a reproduction toxicant. The mention of two anticancer agents at line 9 may imply that the next mentioned agent, chlorpromazine, is one of these. Chlorpromazine’s major clinical applications have been as a tranquilizer and a skeletal muscle relaxant may be preferable to name the two anticancer agents.
Despite the foregoing, manifestations of EtO’s fetotoxic effects in a substantial number of studies are limited to growth retardation. EtO has not been shown to cause fetal malformations (that is, be a teratogen). Modest delays in ossification of bone(s) are consistent with ongoing/prolonged nutritional deficits or chemical effects that retard cell growth and replication. Adverse developmental outcomes of a single/acute dose of toxicant would more likely result in fetal malformation(s). Therefore, the AEGL-2 values derived by use of the repetitive dosage study of Snellings et al. (1982a) should be supported by results of another investigation. Division of the AEGL-3 values by 3 yields
AEGL-2 values that are similar to those based on the repeated-dose study of Snellings et al. (1982a).
Page 49, last 3 lines; page 50, line 1. An interspecies UF of 3 is advocated despite (a) the aforementioned simulations of Fennell and Brown (2001) showing comparable blood EtO concentrations in rats and humans inhaling EtO at 100 ppm for 4 hr; and (b) the statement that the potential mechanism of toxicity is not expected to differ across species. Is the magnitude of alkylation/toxicity known to be dependent upon the peak blood EtO concentration or the area under the blood concentration versus time curve (AUC)? The PBPK modeling (see their Figure 6) of Fennell and Brown (2001) indicates that the post-exposure rate of elimination is slower in humans. This implies that a 100-ppm exposure would result in a larger AUC in humans than in rats.
Page 50, lines 2-8. The reasoning appears to be illogical. Change to a more logical deduction or conclusion or improve the wording (or omit).
Page 50, lines 8-13. It should be noted here that both Muller et al. (1998) and Farmer et al. (1996. Environ. Health Perspec. 104(Suppl. 3):449-452) found no effect of glutathione S-transferase M1 genotype on levels of HEV hemoglobin adducts in a human study population. Muller et al. did find a 2-fold higher HEV level in persons deficient in the T1 isozyme.
Page 50, lines 13-15. The question of whether EtO is passed from the mother to the fetus is critical to determining the propriety of using the results of the Snellings et al. (1982a) study as the basis for deriving AEGL-2s. EtO has such a short half-life and is so reactive that it might not be expected to cross the placental barrier and reach the fetus in amounts adequate to retard its growth. No study was located in which a pregnant animal was dosed with EtO and her fetuses analyzed for EtO or HEV adducts. Tavares et al. (1994) and Farmer et al. (1996) did find elevated HEV hemoglobin adducts in the newborns of smoking mothers. Cigarette smoke contains substantial concentrations of ethylene, but very small amounts of EtO. Ethylene is very lipid soluble and, therefore, readily crosses the placenta. Ethylene is metabolized by hepatic cytochrome P450s in the fetus to EtO. Thus, ethylene rather than EtO may be responsible for Tavares et al. finding of elevated neonatal HEV hemoglobin adducts. Alternatively, the mother metabolizes some of the ethylene she inhales to EtO (though ethylene metabolism is slow in humans [Filser et al. 1992], followed by possible transfer to the fetus. Thus, there is considerable uncertainty about transplacental passage of EtO. The findings of Tavares et al. (1994) are not strong supporting evidence for such transfer. These investigators’ publication in Carcinogenesis (66:157-163, 1994) should be cited rather than their abstract (?) in Human Experimental Toxicology.
Page 50, 2nd paragraph. If possible, PBPK modeling should be conducted to extrapolate from rats to humans, and from 6 hr to the shorter exposure periods. Such modeling sometimes results in lower short-term AEGLs than does the ten Berge et al. (1986) method. The ten Berge et al. paper should be included in the references if their method is utilized.
Page 52, 2nd paragraph. PBPK modeling should be utilized if possible for time scaling and interspecies extrapolation in the AEGL-3 derivations.
Minor Points
Numbering of the pages is from 1-8 and then starts again with 1. When it is referred here to one of the pages 1-8 as they occur for the first time, this is marked by (1) in parenthesis following the page number.
Page 6 (1). Title should be “Executive Summary”
Page 7 (1), line 11 from bottom. Typos: “lower the. AEGL-3 values.”
Page 3, line 22. Typo: add i to “distinct.”
Page 3, line 23. Should this be “to be 1625 ppm”?
Page 3, line 25. Change to “The derivation of the LOA.”
Page 27, line 3. Capitalize “gd”; define at first occurrence, add to list of abbreviations, or write out in full.
Page 43, lines 22-23. Change to “ethylene oxide metabolizing activity.”
Page 43, lines 29-30. Change to “demonstrated that glutathione in various.”
Page 43, line 3 from bottom. Do the three different depletion levels refer to the three different doses or to the three different organs?
Page 49, line 13. Put “too” in front of “mild.”
Page 49, line 22. Change to “The developmental study in rats by Snellings.”
Page 49, line 3 from bottom. Typo: “3 for interspecies sensitivity and 3 for intraspecies.”
Page 51, line 9 from bottom. Change to “being lower.”
Page 51, line 5 from bottom. Put “it” between “because” and “presented.”
COMMENTS ON NICKEL CARBONYL
At its August 31-September 2, 2004, meeting, the subcommittee reviewed the AEGL document on nickel carbonyl. The document was presented by Robert Young of Oak Ridge National Laboratory. The subcommittee recommends the following revisions.
General Comments
The document has improved substantially; however, relatively minor problems remain. While page ii, lines 26-30, address the developmental toxicity of inhaled nickel carbonyl in relation to maternal health status, page 15 and page 19, line 5, provide no corresponding statement. It is worthwhile to repeat the data described on page 22, lines 15-16, at this point.
On page 25, what is the meaning of the phrase, “the total uncertainty adjustment of 10 is weighted towards the uncertainty in individual sensitivity to nickel carbonyl exposure”? Page 23 suggests that information is not adequate to assess rigorously an intraspecies UF. In that respect, what is the meaning of this page 25 conclusion?
Specific Comments
Page ii, lines 27-28. What data are available on the maternal health status of the rat dams? If none are available, this should be so stated.
Page 16, Table 12. While the table is titled, “Maternal Lethality and…,” no data on maternal deaths are included in the table.
Page 16, lines 23-41; page 17, lines 4-5. The discussion should indicate which groups experienced maternal deaths. To what does the statement, “increased mortality in some treatment groups,” refer? Refer to Table 13, line 4.
Page 16, line 35; page 17, line 22. In general, data on prenatal deaths are presented as either resorptions (early embryonic demise) or as late fetal deaths rather than the number of live fetuses per implantation site.
Page 17, line 1. As written, it is not clear whether the total numbers of malformations (that is, the numbers of malformations per fetus) or an increase in the total numbers of abnormal fetuses is the intent.
Page 17, line 7. It is not clear whether the “parenteral” administration includes intravenous nickel injection. It is probably wise to state the precise route (for example, intraperitoneal injection), since bioavailability is complete after intravenous injection.
Page 17, line 16. To what does the table entry “0.16 AEGL” refer?
Page 22, line 16. Into which body cavity (thoracic, peritoneum) was the serous hemorrhage observed?
Page 22, lines 13-20. Refer the reader to (a revised) Table 12.
Page 22, lines 28-29. This is an incomplete sentence. What happened to the 9 of 19 and 9 of 14 rat dams? Include a description of maternal toxicity. If that parameter was not presented by Sunderman, indicate that.
Page 22, lines 35-37. The text reads as speculation. If no empirical data are available for direct support of this NAC conclusion, it should be deleted.
Page 24, lines 15-17. The text should mention the 80% mortality in the hamster dams and the fact that the hamsters were afflicted with serous hemorrhage (page 22, line 16).
Page 24, line 42. Does this sentence refer to a general practice or to a specific section of the SOP?
























































