4
UMBILICAL CORD BLOOD BANKS AND BANKING
The centers that collect, process, and store umbilical cord blood differ in their organization and governance as well as in the cord blood processing methods that they use. The absence of a standard, generally accepted search algorithm and differences in cord blood bank quality make it difficult for transplant physicians to know when it is most appropriate to use cord blood instead of an alternative sources of hematopoietic progenitor cells (HPCs). Furthermore, difficulties with human leukocyte antigen (HLA) typing of HPCs to the level required for cells from adult and cord blood donors make it difficult to compare and contrast the results of a search for HPCs from these two types of HPC donor sources.
Federal and state laws and regulations govern the operation of cord blood banks, and many are accredited through a variety of mechanisms. Yet the rules are not standardized and a more consistently applied set of regulations would benefit both the cord blood banks and end users. Finally, existing cord blood collections lack sufficient ethnic and racial diversity to ensure adequate probability of finding HLA-matched units for some ethnic and racial groups, which is a particularly important barrier to the fundamental goal of unimpeded access to needed treatment. Thus, donor recruitment efforts should include greater attempts at outreach to populations whose HLA types are underrepresented in cord blood collections and the development of innovative approaches to donor recruitment.
This chapter discusses the results of a survey that the committee conducted to assess current cord blood banking practices; describes procedures for the collecting and processing of cord blood; discusses the procedures used to access units; and delineates the current status of laws, regulations,
and professional standards as they are applied to cord blood banking. The committee also makes recommendations on how to improve the system of cord blood collection, processing, and storage.
DEFINITION OF A CORD BLOOD BANK
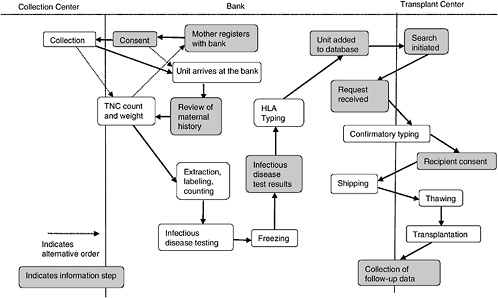
A cord blood bank is a center whose central mission is to maintain a supply of cord blood for therapeutic use in transplantation. Figure 4-1 depicts the various aspects of the collection, storage, search and transplantation processes.
In most cases, a mother will register with a bank prior to giving birth. Upon adequate completion of the informed consent (more on the informed consent process can be found in Chapter 5), the mother’s obstetrician is informed of her wish to be a donor, and arrangements are made to collect the cord blood. This is either by providing the mother with a kit or, if the delivery hospital is affiliated with a bank, by noting it in the mother’s chart. In most cases, the collected blood is then transported to the bank in order to obtain volume and cell count. If both are high enough, the unit is processed, and samples are taken for infectious disease testing. If the review of the maternal history and infectious disease testing are both within the bounds established by the bank, another sample is sent for HLA testing. Because obtaining HLA type is the most expensive part of the process, this is generally not done until the unit meets the bank’s requirements in all other ways and is the last step before listing in a database.
Cord blood banks have been established in response to the needs of different patient populations, and the motivation to store cord blood varies from person to person. For some, there might be an immediate need within the family to treat a disease amenable to transplantation, for example to a sibling or a closely matched family member. Alternatively, a woman or a couple might donate altruistically (as with blood donation) by offering the cord blood as a public resource to be available for others with the need for an immediate transplant. Finally, some donors choose to contribute to research. Table 4-1 summarizes the primary banking options available to new mothers.
For the purposes of this report, the committee has classified cord blood banks into three main categories. Public banks store unrelated cord blood units that are philanthropically donated for transplantation or research purposes. The relevant information (e.g. HLA types, cell counts, and in some cases, the donor’s medical history) is then stored in a database made available to transplant centers searching for a cord blood unit for patients. Some public banks also store a limited number of units for autologous or family use when a disease that is treatable by cord blood transplantation is known to exist within the donor’s family. In these circumstances, the blood
TABLE 4-1 Cord Blood Banking Options
|
|
Type |
Purpose Fee |
|
Option 1: autologous banking (banking for future use by the infant from whom the cord blood was obtained or immediate family members) |
Maintain a stored unit in the event that donor or a family member needs a hematopoietic stem cell transplant |
Variable; involves an initial banking cost ($1,000 to $2,000) and then a yearly maintenance fee ($50 to $150)a |
|
Option 2: philanthropic donation |
Unit will be saved for potential use by the general public |
No fee |
|
Option 3: research donation |
Procurement directed to an institution researching the properties of umbilical cord blood units |
No fee to family but some research organizations are charged |
|
Option 4: directed donation b |
Collection to serve a patient in need, usually a sibling or other family member |
Usually offered by the transplant centers no cost to the family; also offered by some public and private banks; transplant centers may be charged to procure the unit |
|
aIf there is a history of a genetic disease or condition or a malignancy within the family, it is possible that insurance will cover these costs. Select banks waive the fees for individuals with a family history of a disease or a condition or a malignancy. bCord blood banking options should be presented to the family if a family member has a current or a potential need to undergo a stem cell transplant. |
||
is often stored for a short time and the bank provides the necessary processing and testing to aid the transplant physician Units that do not meet standards for clinical use may be used for quality improvement or research.
Private banks store cord blood units only for autologous or family use. These banks generally charge a fee for the collection, processing, and storage of the cord blood and leave any decisions regarding the use of the unit to the donor or the donor’s family. These banks tend to describe their service as offering “biological life insurance” that provides peace of mind to families that might be concerned about future health conditions in the child or a close relative for which cord blood transplantation might be a possible treatment. If the family decides to discontinue banking the unit, with their consent it may be made available for research.
Mixed banks not only collect unrelated units donated for transplantation to unrelated recipients but also operate facilities for cord blood bank-
ing for autologous use and use by family members. The money received from private banking activities can help to offset the costs of public banking activities at these facilities. Both public and private units are processed and stored at the same facility; however, as with fully private banks, the private units are the property of the donor’s family and are not available for general use. As with fully public banks, units that do not meet quality standards may be used for quality improvement or research. If a family decides that it no longer wishes to maintain ownership of a unit, it is conceivable that the unit would then become available to the public for unrelated transplantation to patients unrelated to the family or for research, if the family consents.
Some cord blood companies exist on a for-profit basis by obtaining unrelated units, expanding them, and making the same unit available to multiple recipients. However, this very small field was one that the committee chose not to focus on.
As an expectant mother’s primary source of information about banking options is her obstetrician, it is very important that obstetricians fully understand the different options available, and be able to effectively convey that information to the mothers. Further, as the obstetricians are not reimbursed by the public banking system the way they are with many private banks, it is also important that they fully “buy in” to the advantages of the public system.
STATUS OF CURRENT BANKS: RESULTS OF A SURVEY
The committee identified 40 cord blood banks in the United States (see Appendix C). Based on the responses (both full and partial) of 21 of those 40 banks to a survey that the IOM committee distributed in the summer of 2004, the committee found that 9 banks store both public and private units, 8 store only public units, and 4 store only private units. However, among those banks that store both types of units, most banks predominantly stored public units, with small private programs on the site.
The committee’s survey asked banks to indicate their accreditation status (e.g., American Association of Blood Banks [AABB] and Foundation for the Accreditation of Cellular Therapy [FACT]), although the committee could not always verify this information when it consulted with the agencies providing accreditation to confirm the information. Slightly more responding banks (n = 11) self-reported AABB accreditation than FACT accreditation (n = 6). Only LifeCord indicated that it is accredited by both AABB and FACT. One bank indicated pending FACT accreditation, and two other banks responded that they will be or are in the process of accreditation. Four of the banks also indicated accreditation by the National Marrow Donor Program, which is not considered an accrediting agency. Thus,
the accreditation status of the banks varies substantially, which also reflects the diverse practices and goals that exist among the banks.
One practical example of this diversity is the different standards for banking cord blood units. Most banks require at least 40 to 50 ml for banking, although the volumes range from 10 to 50 ml.1 The total nucleated cell (TNC) count dose requirement was most often 8 × 108 or 9 × 108 cells, but that, too, varied among the banks.2 Some private banks indicated that they may store units that are smaller than what is currently considered clinically useful after they inform the parents of the donor and receive confirmation of their wishes.
Without a single system of accreditation, the banks’ standards can be expected to remain quite variably enforceable.
Recommendation 4.1: The Health Resources and Services Administration should identify a Cord Blood Accrediting Organization by means of an open, competitive request for proposal process. This organization should be charged with the delineation of standards for any cord blood bank, collection center, or transplant center desiring to participate in the National Cord Blood Stem Cell Bank Program.
Size of Identified Banks
The committee’s survey found that the number of units collected by banks also varied considerably and were as follows: Viacord, >60,000 units; St. Louis Cord Blood Bank, >40,000 units; the National Cord Blood Program of the New York Blood Center, 29,525 units; the American Red Cross Cord Blood Program, 18,680 units; Lifebank USA, 17,228 units; the Carolinas Cord Blood Bank, 17,000 units; Cryobanks International, Inc., 15,429 units; and StemCyte International Cord Blood Bank and Cord Blood Family Trust, 13,566 units.
Collection Processes
All but three of the cord blood banks indicated that obstetricians are among those who perform the cord blood collection. Staff nurses and designated collectors from the cord blood bank were also among the collectors at most delivery centers. No banks selected the “researcher” or the “other” category for this question, which was phrased as “Who collects the blood?”
Variation in this arena, including training of the cord blood collection staff, may account for the differences in the volume collected and the rates of disqualification of units for contamination and other reasons.
Storage Methods
Most cord blood banks store units as a red blood cell-depleted product (also referred to as a mononuclear cell3 product) with the exceptions of ITxM Cord Blood services, the Sibling Donor Cord Blood Program, and StemCyte International Cord Blood Banks and Cord Blood Family Trust. The most popular anticoagulant and cryoprotectant agents were citrate-phosphate-dextrose (CPD) and dimethyl sulfoxide (DMSO), respectively. The majority of banks also indicated that they store the units in the vapor phase of liquid nitrogen.
There are limited data available relating to the viability of cord blood units stored long-term. Though research suggests that units can be stored for extended time frames (as many as 12 years) with no reduction in viability, proof of this concept is needed, and research into this area is critical (Broxmeyer, 1995).
Although some researchers argue that the vapor phase of liquid nitrogen is inadequate because the temperatures are slightly higher than those of liquid nitrogen itself and also allow for temperature variations when the lid of the storage container is opened, use of the vapor phase is substantially more economical, given the reduced liquid nitrogen needs, and this method also assists in the prevention of the spread of potential contaminants.
PROCESSING PROCEDURES
Screening Maternal Donors and Cord Blood
Cord blood acquisition must be done carefully because of the potential presence of transmissible diseases or pre-existing genetic conditions and possible contamination with maternal cells and microbial agents. As mentioned above, the collection and processing practices and procedures vary substantially among the cord blood banks. Both banks and transplant centers should use current best practices to ensure that the transplanted unit is safe and that everything possible has been done to ensure the success of the graft as well as the health of the patients who have received the graft.
Once consent for the collection of cord blood has been obtained (see the discussion of consent issues in Chapter 5), an extensive behavioral
history is undertaken to determine whether the mother is likely to belong to a group that engages in a behavior that might pose a health risk to the recipient of the banked cord blood unit (e.g., risky sexual behaviors or illicit drug use). In addition, maternal and family histories for inherited genetic disorders are taken. The unit is screened for bacterial, viral, and fungal infections through testing of specimens obtained from the unit before it is frozen. A portion of the cord blood specimen is generally frozen as separate segments that may or may not be attached to the main unit to allow further, more complete testing for infectious diseases or genetic disorders if the unit is identified to be useful for transplantation or is able to fulfill some other need.
Collection of Cord Blood Units
Cord blood can be collected from the placenta at two different times. It can be collected after delivery of the baby but before the placenta has been delivered, or it can be collected in a separate room after the placenta has been delivered. In either case, after sterilization of the umbilical cord to minimize the possibility of contamination, a large-bore needle is used to drain the cord blood, which is placed into a closed bag containing an isotonic anticoagulant at a neutral pH (Rubinstein et al., 1995).4 The former method is generally performed by obstetrical staff (e.g. the physician, nurse, or midwife) as part of the delivery procedure, whereas the latter method is generally performed by trained technicians or nurses outside of the delivery room.
Collection methods that rely on individuals other than cord blood bank staff can offer the possibility of cord blood collection in remote locations, which could greatly expand the donor pool. However, means for the ongoing training of the collection personnel and the promotion of standard protocols would have to be developed to ensure the quality of the units.
Wall et al. (1997) found few differences in either the volumes collected or in the total cell counts of the units collected either before or after delivery of the placenta. Other studies reported higher volumes and CD34+ cell counts if collection was performed before the delivery of the placenta (Surbek et al., 2000; Solves et al., 2003). These variations may be due to the additional time involved in the ex utero collections, which allow the formation of microscopic clots, thereby reducing the number of cells available in the unit (Wong et al., 2001). Alternatively, uterine contractions after delivery of the fetus may enhance drainage of placental blood. Collection after delivery of the placenta results in no difference in the volumes of cord blood
recovered. However, higher rates of microbial contamination in the units collected ex utero have been reported, possibly as a result of the additional handling of the placenta before cord blood collection (Solves et al., 2003).
Several factors have been shown to affect the CD34+ counts and the numbers of colony forming units of granulocytes-macrophages (CFU-GM) in the cord blood units, including gestational age, length of labor, time of cord clamping after delivery, birth weight, placental weight, and birth order (Donaldson et al., 1999; Ballen et al., 2001). The first baby in the birth order, an extended duration of labor, and increased neonatal weight are all associated with a higher overall cell count, whereas more advanced gestational age has been noted to increase the number CD34+ cells until 37 weeks of gestation, at which point they begin to decline. Smoking has been shown to have a negative effect on the CD34+ cell count because smokers tend to have lower birth weight babies (Ballen et al., 2001). Grisaru et al. (1999) reported that placement of the infant on the mother’s abdomen after delivery significantly increases the number of cells in the cord blood units collected. Attempts to increase the number of units successfully collected should not be undertaken, however, if the collection of cord blood will change normal obstetrical attention to the health of the mother and infant.
All of those factors will need to be taken into account when considering the standards for the collection of cord blood. In addition, it is very important that the accrediting organization contact those currently in the practice of both collecting and storing cord blood in order to ensure that the standards are both reasonable and effective.
Recommendation 4.2: Uniform standards for the collection of cord blood units without alteration of safe obstetrical practice should be established by the Cord Blood Accrediting Organization suggested in Recommendation 4.1 and should be required of all banks participating in the National Cord Blood Stem Cell Bank Program.
Transport of Cord Blood and Cell Viability
A decentralized system has certain cost benefits because the infrastructure needed to support the processing, screening, and storage of cord blood units is expensive and requires significant space and resources. However, the transport of cord blood from the remote collection site to the processing and storage site is a quality concern because of the lack of temperature control and the potential for a decline in cell viability.
Through regression analysis, a 1 percent drop in cell viability was demonstrated for every 4-hour increase in transit time (Wada et al., 2004). That study also detected a noticeable decline in the viabilities of the cells in lower-volume units, although this was hypothesized to be the result of a
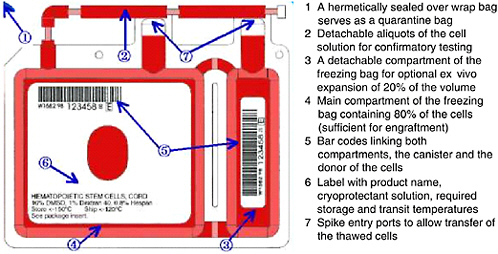
FIGURE 4-2 Example of storage container for cryopreservation of cord blood units.
SOURCE: Courtesy of Thermogenesis Corp.
greater effect of the anticoagulant on the pH of the lower-volume units; the higher volume units were not as affected (Wada et al., 2004). Hubel et al. (2004) found that the cells in units stored for up to 24 hours at room temperature before processing and cryopreservation exhibited very little loss of viability.
Storage
Cryopreservation5 is the standard practice for long-term storage of cord blood units for transplantation. Engraftment of stem cells from cord blood has been shown to be successful from cryopreserved units that were subsequently thawed by careful, specific procedures (Galmes et al., 1995, 1999; Rubinstein et al., 1995; Rubinstein, 2001, 2004; Timeus et al., 2003). Additional studies have examined the proliferative potential of cells stored for up to 10 and 15 years and reported good results with in vitro expansion of these samples (Broxmeyer and Cooper, 1997; Kobylka et al., 1998). During the processing and freezing of the unit, alterations in the rates of cooling during the initial stages of cryopreservation have been shown to affect the recovery of cord blood after thawing. Rates of cooling in excess of −5°C per minute were found to significantly reduce the colony-forming cell (CFC) activity after thawing (Creer, 2004). An example of a storage bag is shown in Figure 4-2.
The entire, unprocessed unit is generally not frozen, as storage in a liquid nitrogen freezer is quite expensive and the maximization of storage space is important. Various processing methods that reduce the volume required for storage but that maintain the viability of the cells in the finished product have been identified. These processes have been designed to remove the cells not needed for successful engraftment, leaving the desired transplantable stem cell material available for storage.
A plasma volume expanding medium (e.g., Hespan or Pentaspan) is added to the unit to facilitate separation of the red cells from the white cells containing the progenitor cells needed for engraftment. The units are then centrifuged to remove excess red blood cells and to reduce the unit to a volume more suitable for efficient long-term storage (Alonso et al., 2001). After additional centrifugation, the plasma is extracted to reduce the volume to about 20 ml.
A 10 percent DMSO solution is added as a cryopreservative to protect the cells from damage as a result of the freezing process. The units are then frozen before they are stored in liquid nitrogen, where they remain until they are ready to be used for transplantation.
Studies have shown that cross-contamination among units in a freezer is possible even at the extremely low temperatures provided by liquid nitrogen (Tedder et al., 1995; Fountain et al., 1997). For this reason, it is also important to protect against the possibility of transmission of such pathogens as hepatitis B virus (HBV) and contamination with other microbes during storage of the cord blood unit. Banks should use protective measures to prevent any inadvertent transmission of an infectious agent from occurring during the storage of their units.
Before the units are used for transplantation, they are thawed in ambient air or a water bath, or both. The cryopreservative agent (DMSO) is often diffused out of the cells by use of an isotonic salt solution (5 percent Dextran 40 and 2.5 percent human albumin), centrifuged, and then resuspended in a albumin-dextran solution for infusion (Rubinstein et al., 1995). Rubinstein et al. (1995) have reported that failure to wash the cryopreservative off of the cells may result in a 65 percent reduction in the viabilities of the leukocytes (white blood cells) and a 40 percent reduction in the viability of the cord blood units compared to units that have been washed. There is also a concern of DMSO toxicity in some patients, especially very young recipients.
Matsumoto et al. (2002) have attempted to further simplify the process of short-term storage of cord blood units by using supercooling instead of freezing the units in liquid nitrogen. Although this technique results in significantly better cell survival rates than cryopreservation, it has been studied only for time intervals up to 72 hours; thus, at present, long-term storage of cells is probably best achieved with cryopreservation.
Before the use of a cord blood unit, additional confirmatory testing is often required to ensure the accuracy of the HLA type and the unit selection. This is usually performed by detaching a segment from the stored unit and then submitting it for either high- or low-resolution typing at the request of the transplant center. This operation requires the removal of the unit from storage and can subject the whole unit to unintentional warming. Transient warming events (TWE) can occur not only during this process but also during shipment to the transplant center; thus, the number of times that the unit is subjected to temperature fluctuation should be kept to a minimum. Multiple transient warming events of up to 1 minute have relatively little effect on CFC recovery, although relatively few data for longer duration events are available (Creer, 2004). Another study reported that units can be frozen and thawed up to three times without a significant reduction in cell viability (Timeus et al., 2003).
It is thus apparent that cord blood processing is a complex process necessary for such a product. For this reason, standards should be consistent, scientifically validated, and designed with the input of current cord blood bankers and cord blood experts.
Recommendation 4.3: Uniform quality assurance standards and criteria should be established by the proposed Cord Blood Accrediting Organization for the collection, processing, and storage of cord blood, and adherence to these standards should be required of all banks participating in the National Cord Blood Stem Cell Bank Program. In addition, a system for the frequent performance of compliance reviews should be established.
RACIAL AND ETHNIC COMPOSITIONS OF THE UNITS IN CORD BLOOD BANKS
One goal of cord blood banking is to increase the HLA diversity in the inventory, particularly the HLA types of ethnic and racial minorities. However, early efforts aimed at increasing the recruitment of minority populations as donors of cord blood encountered difficulties similar to those faced in the early days of the bone marrow registries, resulting in lower than desired representations of units from members of minority populations.
In one study, the racial and ethnic compositions of the units in five cord blood banks in the NMDP network were compared with those of the individuals who signed up for bone marrow donor registries from the same geographical areas (Ballen et al., 2002). That study examined 9,020 cord blood donors and the racial and ethnic characteristics of the hospitals where collections were performed and compared them with the characteristics of 417,676 bone marrow donors and the census data of the donors’
geographical area. The California, Florida, and Massachusetts cord blood banks recruited a lower percentage of minorities than the corresponding bone marrow donor centers. In four of the five areas studied, cord blood banks recruited a lower percentage of minorities (in comparison with the census data for the corresponding collection hospitals).
A more recent study from the American Red Cross showed that racial diversity can be achieved in a national network of cord blood banks (Ballen et al., 2004). The population of that network is 64 percent Caucasian, 16 percent African-American, 12 percent Hispanic, 4 percent Asian, 1 percent Native American, and 3 percent other. Diversity was achieved by focusing collections in specific geographic areas; Detroit, for example, had the highest percentage of African-American donors and San Diego had the highest percentage of Hispanic donors.
Individual sites reported wide ranges in the distributions of their units by race and ethnicity. For example, the Karmanos Cancer Institute/JP McCarthy Cord Blood Bank in Detroit is a smaller, minority-focused bank that stores about 442 cord blood units; 81 percent of these units are from African-American donors, and less than 10 percent are from Caucasian donors. Duke University provided information on 3,870 banked units; 59 percent of these are from Caucasian donors, 19 percent are from African-American donors, and 9 percent are from Hispanic donors. Fifty-six units from this bank have been transplanted, but a disproportionately high percentage (14 percent) of units from Hispanic donors were chosen for transplantation.
ACCESSING UNITS
Information Flow
One of the most challenging aspects of cord blood acquisition is the selection of an appropriate unit for transplantation. The lack of an agreed-upon search algorithm creates a challenge for transplant physicians searching for treatment options for their patients. At present, physicians must search several unrelated databases to identify all units that might be compatible. They must also compare these results of these searches with information on potential donors in adult bone marrow registries to see whether a suitable marrow match is available. Figures 4-3 and 4-4 show outlines of the typical decision-making procedures that a physician must perform when he or she is searching for HPCs for transplantation. Multiple factors, such as cell dose requirements, HLA match requirements, and the geography of the transplant center are important in the search process.
An ideal search algorithm would encompass a scalable system that is capable of searching every available cord blood unit banked by all accred-
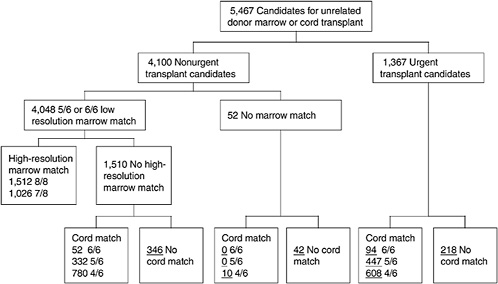
FIGURE 4-3 Algorithm for selection of bone marrow versus umbilical cord blood for patients more than 20 years of age. Underlined numbers = number of patients in the scenario with 50,000 cord blood units and a minimum cell dose of 3.0 × 107 cells.
SOURCE: Howard et al. (2005). See Appendix E.
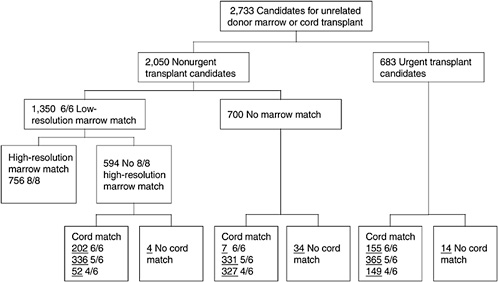
FIGURE 4-4 Algorithm for selection of bone marrow versus umbilical cord blood for patients less than 20 years of age. Underlined numbers = number of patients in the scenario with 50,000 cord blood units and a minimum cell dose of 3.0 × 107 cells.
SOURCE: Howard et al. (2005). See Appendix E.
ited facilities (see Recommendation 4.1). The searches would be based on HLA matching, and results would indicate whether the HLA-typing was high- or low-resolution and score each of these depending on the degree of match, cell dose, and availability. The algorithm should also be flexible enough so that transplant physicians could modify it depending on their own search criteria or ones the physician feels are important for a particular patient.
The availability of a unit depends on the overlapping demands of multiple clinicians with patients with similar needs. Although the requesting physician should be notified that a potentially matching unit has been identified, the bank and the cord blood center should assist with making the ultimate decision to use a particular unit for transplantation in a particular individual. Units “held” for a particular patient should not be reserved by or shipped to any other transplant centers. However, units should not be able to be reserved indefinitely, and thus it is important that the bank and cord blood center work with transplant centers to ensure that reserved units are shipped or cleared within a reasonable amount of time.
All parameters in the search should be traceable and should be maintained by the cord blood center. The units available from different banks should be comparable to one another, and thus standardization of the processes and the procedures used for the collection and storage of the units is essential. A set of protocols (e.g., infectious disease testing, steps in processing, storage guidance, and matching requirements) for all banks supplying information to the cord blood center is required to ease the processes used to search for and identify cord blood units. Lack of compliance with these protocols would result in punitive measures by the cord blood center (see Chapter 7).
Ideally, with any search algorithm, the available cord blood units and adult donors would appear in the same search results, although listed separately, ranked by availability and HLA match. This would enable transplant physicians to compare all possible options for their patients and select the best match for their patients’ needs.
Probability of Finding a Donor
At the request of the Institute of Medicine committee, the New York Blood Center (NYBC) completed a voluntary simulated search of cord blood unit matches for 9,970 patients within its inventory of 20,444 cord blood units for which high resolution HLA typing had been completed. A 4/6 HLA match or better could not be found for 94 patients (1 percent). Fifty-seven of these patients were non-Caucasian.
Cord blood banks have been able to recruit a diverse donor population, but it is not fully consistent with the census data on the racial and ethnic
diversity of the American population. Data on HLA diversity in the American population requires further investigation. Of further interest and concern are the lower CD34+ counts among cord blood units from African-Americans reported by several banks.
Physician Support in the Search and Transplant Process
At present, as described above, a transplant physician seeking a cord blood unit is required to search the cord blood unit databases of several organizations. The resolution and accuracy of HLA testing, the method of cord blood processing and even the quality of the unit itself can vary widely among these different banks. For this reason, some transplant physicians are unwilling to use units from banks with which they are not familiar or even to use cord blood in an HPC transplant. Organizations such as NYBC have attempted to make this process easier by implementing programs that facilitate the use of cord blood and increase the referrals for transplantation. Cord blood banks rely on the use of these units, as reimbursement comes with the clinical transplantation of a cord blood unit and not with the donation.
Banking organizations, patient advocacy groups, and adult donor registries like the National Marrow Donor Program have been hosting physician education programs and providing updates through publications and Web-based resources to increase the chances that a transplant physician will quickly be able to identify a suitable match for his or her patient. The registries also provide live support in the search process by providing access to qualified individuals with expertise in the relevant fields (e.g., HLA compatibility) to assist the physicians with selecting the best unit. The registries also often assist with the financial aspects of the search by offering suggestions for reimbursement and by educating the insurance companies as much as possible on the issues surrounding HPC transplantation.
Patient Support in the Search and Transplant Process
The responsibility of caring for a patient waiting for an HPC transplant falls on the transplant physician and the staff at the transplant center. Registries and banks often assist these health care providers by providing informational brochures, videos, and audio programs that educate the patient and the patient’s family on the process, risks, and outcomes. These are designed to accommodate individuals who speak a variety of languages other than English and individuals with different comprehension levels to ensure that no matter what the health literacy level of the patient and his or her family, they will have a good understanding of the process. The registries also provide counseling services and act as a support mechanism in this very difficult process.
Registries also have an opportunity to provide outreach programs for patients in underserved areas who might not otherwise enter the transplant referral system because of their remote geographical location or the lack of availability of comprehensive medical care in their area. Advertising materials directed toward the local community and continuing medical education programs directed toward local physicians can help accomplish this goal.
GOVERNMENT REGULATION
Food and Drug Administration Regulation of Human Cell, Tissue, and Cellular and Tissue-Based Products
The Food and Drug Administration (FDA) first announced its proposed approach to the regulation of cell and tissue products in 1997 (FDA, 2004b). Since then, FDA has released a series of guidelines and regulations that provide a regulatory framework for the use of human cell, tissue, cellular, and tissue-based products (HCT/Ps). FDA published a set of proposed regulations in January 2001. This proposal introduced FDA’s concept of current good tissue practices around three major goals: 1) preventing the unwitting use of contaminated tissues with the potential for transmitting infectious disease; 2) preventing improper handling or processing that might contaminate or damage tissue; and 3) ensuring that clinical safety and effectiveness is demonstrated for most tissues that are highly processed, used for nonhomologous purposes, or combined with no tissue components, or that have systemic effects on the human body (Gee and Biol, 1999; FDA, 2004a; 2004b).
A final rule put into place provisions requiring establishments that work with cells and tissues for transplantation to register with FDA and list their products was published January 19, 2001.6 Additional regulations incorporating comments on the draft regulations received from the public were incorporated into the final rule, which was released in two parts in 2004. They are scheduled to become effective in May 2005. The main focus is to ensure that all processing of HCT/Ps is controllable and accountable during the collection and processing of the units. The FDA proposal contains several exceptions involving minimally manipulated cells, including cells that are harvested for autologous or reproductive use but that are not processed and stored for commercial use, such as for the directed donation of cord blood units or ova for infertility (FDA, 2004b). FDA has assumed a role in HCT/P regulation because the manufacturing and transplantation of these products often involves interstate commerce. For example, cord blood units can be collected in one state, processed and stored in another, and
transplanted in a patient in yet another state. The FDA-regulated HCT/Ps are summarized in Table 4-2.
Despite these regulations, however, FDA has not yet licensed cord blood as a standard therapy. Its most recent discussion of the topic was at the FDA Biological Response Modifiers Advisory Committee (BRMAC) meeting on February 27, 2003, during which the committee was asked to discuss:
-
factors that FDA should consider in determining the safety and efficacy of the use of cord blood transplantation for hematopoietic reconstitution,
-
the role of the CD34+ cell count in the selection of cord blood units, and
-
other measures of quality that should be considered (BRMAC, 2003).
On the basis of data provided to BRMAC by Pablo Rubinstein and Cladd Stevens of the NYBC, it found that older recipients, as opposed to
TABLE 4-2 FDA Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products
|
Product Regulated |
Specific Product |
|
HCT/P that are or will be regulated as biological products |
|
|
HCT/Ps that are currently subject to investigational new drugs (IND) and Biologics License Application (BLA) requirements |
HCT/Ps that are
|
|
HCT/Ps that are subject to the phase-in of FDA approval requirements |
|
children, have poorer outcomes because of their higher body weights. They also noted that although the only true measure of the success of an HPC transplant is hematopoietic reconstitution in a myeloablated recipient, the CD34+ cell content is an accurate predictor of engraftment success (BRMAC, 2003).
BRMAC did agree that cord blood transplantation is an accepted approach for the treatment of a variety of diseases and that the use of bone marrow or cord blood for the treatment of particular diseases should be made on the basis of medical judgment and availability. Finally, BRMAC agreed that the general outcome parameters recommended for clinical trials of other types of HPC transplantation are suitable for clinical trials of cord blood transplantation (BRMAC, 2003).
As of January 2004, all public and private cord blood banks were required to register with FDA. However, licensure of cord blood units is still pending. The committee believes that this licensure will go a long way toward providing another layer of safety and quality assurance in the system and has the added benefit of creating requirements that are enforceable by law. Furthermore, licensure will clarify the legal status of the cord blood units to be shipped across state lines and make it easier for cord blood banks to be reimbursed at a fair market price for the units used for transplantation.
Despite of the lack of licensure, many of the public cord blood banks have voluntarily submitted investigational new drug applications (INDs) to the FDA and have actively collected clinical data to be used to support the development of product standards and licensure. The IND process requires a full application explaining the study goals and methods of a new therapy. The investigator must also file periodic reports on the progress of the trial and immediate reports upon occurrence of unexpected adverse events. This allows FDA supervision in the absence of any other control and provides a feedback mechanism that is not present in the accreditation process.
Recommendation 4.4: The Food and Drug Administration should move promptly to establish a system of licensure of cord blood units intended for clinical transplantation. As an interim measure until a licensure process is established, all banks participating in the National Cord Blood Stem Cell Bank Program should operate under an investigational new drug application.
State-Legislated Programs
A number of state laws have been recently enacted to further the development of and access to public cord blood banks. In Florida, for example, a statewide consortium called the Public Cord Blood Tissue Bank is respon-
sible for the “collection, screening for infectious and genetic disease, tissue typing, cryopreservation, and storage of cord blood as a resource to the public” (Florida State Legislature, 2004b). The banks participating in the consortium are charged with “aligning their outreach programs and activities to all geographic areas of the state, covering the entire state” (Florida State Legislature, 2004b). Interestingly, the Florida Public Health Provision identifies the need for outreach programs targeted to Hispanics, African-Americans, Native Americans, and other ethnic minorities. The law also provides for a religious exemption where “blood transfer is contrary to the moral principle the denomination considers to be an essential part of its beliefs” (Florida State Legislature, 2004b). Although the consortium is allowed to charge transplant centers, the statute requires written disclosure of any financial remuneration for collection.
The state legislatures in Maryland and Illinois have also enacted laws regarding the collection of cord blood for public use. In those states, the responsibility for public cord blood collection is largely that of the hospitals. Under these laws, a hospital “shall allow a pregnant patient to arrange for the donation of the blood extracted from the umbilical cord of the patient’s newborn child to a certified7 public cord blood bank” (State of Illinois, 2004; State of Maryland, 2004a, 2004b). Under both statutes, a patient who agrees to donate cord blood to a public bank “may not be charged for the costs of collection, storing, or transporting the cord blood.” As with the Florida statutes, exceptions to this general rule are provided in cases in which blood collection conflicts with the religious denomination of the hospital or a hospital employee or if cord blood collection would threaten the health of the mother or the newborn child.
In contrast to the statutes described above, Oklahoma has also passed legislation pertaining to cord blood, but it does not specify a precise structure for a program. Rather, the Danielle Martinez Act requires that an Advisory Council on Cord Blood Donation be established (Oklahoma State Legislature, 2004). That advisory council is charged with providing recommendations on a cord blood donor program to the legislature by an original deadline of November 1, 2004, which was recently extended to December 1, 2006 (Oklahoma State Legislature, 2005).
Although New Jersey does not have a public cord blood program like those in Florida, Maryland, or Illinois, or an advisory council like that in Oklahoma, the state is considering legislation that would offer the Coriell Institute for Medical Research a $5 million loan to provide additional funds to expand collection efforts of the New Jersey Cord Blood Bank, which the Coriell Institute maintains (Quinn, 2004; Anonymous, 2004). The legisla-
tion in Texas is similar to the New Jersey bill and provides a $1 million grant to the Texas Cord Blood Bank to start public cord blood collection efforts (Foy, 2003). In addition, the state is offering to match every dollar donated to the project up to a maximum of $3.5 million.
In New Mexico, the Umbilical Cord Blood Banking Act, introduced in February 2005 by State Senator Nancy Rodriguez, would provide $25,000 to the New Mexico Department of Health for the publishing and distribution of pamphlets on cord blood donation (Associated Press, 2005).
Sates have also passed statutes that pertain to the encouragement and promotion of cord blood research. For example, the Florida legislature recently established the Florida Center for Universal Research to Eradicate Disease (Florida State Legislature, 2004a). Under this legislation, the center is responsible for coordinating voluntary donations of cord blood as necessary to maintain an adequate supply for research.
ACCREDITATION
American Association of Blood Banks (AABB)
AABB first included standards relating to HPCs and bone marrow in 1991 as part of the 14th edition of Standards for Blood Banks and Transfusion Services (Section Q). In 1996, AABB published a separate volume of standards for HPC-related activities, Standards for Hematopoietic Progenitor Cells. AABB’s standards in this arena have evolved in conjunction with cellular therapy. In 2001, AABB published a separate volume of standards for cord blood activities, Standards for Cord Blood Services. Shortly thereafter, the AABB board of directors approved the creation of the Somatic Cell Standards Program Unit to draft requirements for facilities involved in this kind of cellular therapy. Rather than publish a third set of standards for cellular therapy, the AABB board of directors ultimately approved the merger of the publications on adult HPC, cord blood, and somatic cell therapy into a single, unified publication, Standards for Cellular Therapy Product Services, which will become effective in May 2005. This document, prepared by the Cellular Therapies Standards Program Unit, encompasses all types of cellular therapy and is intended to minimize the need for duplicative AABB assessments of facilities that collect, store, or issue different types of cellular therapy products.
AABB’s voluntary, peer-based accreditation program offers facilities a means to assess their compliance with AABB standards, including the HPC and cellular products standards and cord blood standards. Each year, AABB conducts more than 800 assessments of blood-related facilities, including more than 60 facilities involved in HPC, cord blood, and cellular product collection, processing, storage and distribution activities. Cellular therapy
facility assessments are based on AABB standards which are designed with both quality management system and technical requirements. Each assessment is customized to fit the activities of the facility. Reassessment documents are required for a “desk assessment” before the assessors visit. Areas of concern are identified in advance so that the assessors can carry on a dialogue with a facility to allow it to make necessary changes or additions. An AABB lead assessor and a cell therapy subject matter expert assessor perform the assessments. Any questions concerning the assessment are referred to the AABB Standards Committee before accreditation is given. Otherwise, accreditation is awarded without further board review. At present, AABB has approximately 60 trained assessors with expertise in HPCs and cord blood. AABB assessments are intended to ensure compliance with AABB requirements, provide education, improve the efficiency of operations and ensure consistency in the provision of safe and efficacious products.
Foundation for the Accrediatation of Cellular Therapy
FACT is a nonprofit organization founded in 1996 by the International Society for Cellular Therapy (ISCT) and the American Society of Blood and Marrow Transplantation (ASBMT). ISCT is a professional society established in 1992 to represent scientists and physicians working in the area of hematopoietic stem cell graft manipulation. ASBMT was formed in 1993 as a professional organization to represent physicians and investigators involved in the clinical aspect of HPC transplantation. The two societies established FACT to develop standards and a voluntary inspection and accreditation program.
The primary purpose of FACT is to develop standards for hematopoietic stem cell collection, processing and transplantation. FACT’s major objective is to promote high-quality patient care and high-quality laboratory performance in the belief that accreditation must assess the clinical aspects of transplantation as well as collection and laboratory practices. FACT standards are comprehensive and include quality management; facility design and operations; policies and procedures; donor evaluation, selection, and management; record keeping; labeling; processing; storage; transportation; the issue and release of HPCs; adverse event reporting; auditing; and outcomes analysis for the purposes of voluntary inspection and accreditation in the field of HPC therapy.
FACT has developed a voluntary inspection program that, if it is successfully completed, leads to a 3-year accreditation in the field of HPC therapy. FACT inspection teams always include a team leader, a physician trained in stem cell transplantation, and professional experts in the areas of stem cell collection and laboratory practices. The inspection team assesses the compliance of a facility with the standards and reports back to an
accreditation board. The accreditation board reviews all facility reports, thus maintaining consistency in the interpretation of the standards. Final accreditation is awarded after approval from the FACT board of directors. This comprehensive process helps to ensure a consistently high quality among facilities. There are currently 130 FACT-accredited HPC transplant facilities in the United States. FACT currently has 160 trained and active inspectors.
In 2002 FACT joined forces with NetCord to develop international standards for maternal donor screening and cord blood collection, processing, testing, banking, selection, and release. The standards are modeled after, but independent of, the HPC-related standards to ensure that they address the specific and sometimes different issues related to cord blood. The FACT/NetCord collaboration was intended to ensure consistently high- quality cord blood units for transplantation not only in the United States but also internationally. FACT/NetCord recognizes that global standardization of cord blood banking will facilitate the availability of quality cord blood units for a greater number of U.S. recipients of all ethnic backgrounds. The FACT/NetCord standards have since been adopted by socie-ties in a large number of countries outside the United States, including countries in Europe and Canada, Asia, and Australia. FACT/NetCord standards for cord blood are comprehensive and were developed with the same philosophy as the HPC-related standards: that the assessment of the quality of clinical transplantation of cord blood is as important as the assessment of collection and laboratory practices. Inspection teams include a physician team leader knowledgeable about cord blood transplantation and experts in collection and laboratory practices. Inspections generally occur over a 2-day period, and a FACT/NetCord accreditation board reviews all inspection reports. Three-year accreditation is awarded following after approval by the FACT and NetCord boards of directors. Currently, 36 cord blood banks around the world have applied for FACT/NetCord accreditation. Of those, 11 banks have been inspected and 5 have been awarded accreditation.
All accredited programs should have in place a quality management program, including quality audits; a system for detecting, evaluating, and reporting errors, accidents, and suspected reactions; documentation; review and reporting; and safety.
Recognizing the importance of developing consensus graft processing standards, in 1994 the North American Task Force (NATF) was formed, consisting of all the major professional organizations interested in hematopoietic cell therapy, including International Society for Hematotherapy and Graft Engineering (ISHAGE), ASBMT, and FACT. There was general consensus that the FACT standards were of sufficiently high quality to serve as
a template for the other organizations involved in this field. Thus, there should be no conflict between the FACT Standards and those of other standard-setting organizations that had joined the NATF. The first edition of the FACT Standards was published in September 1996. The first inspections began in September of 1997.
NMDP Standards
In July 2004, NMDP published a revised edition of its standards, intended to outline the most basic guidelines for facilities involved in the transplantation of HPCs (NMDP, 2004). These guidelines were not intended to be a comprehensive list encompassing all requirements; rather, they were intended to serve as a standard of care for patients in such facilities. They apply to all activities related to donor screening, collection, processing, release, and transplantation of bone marrow, peripheral blood, and cord blood progenitor cells facilitated through the NMDP network of banks and transplant centers.
Recommended Direction
In contrast to adult marrow and peripheral blood donors, who can be examined immediately prior to harvest, the umbilical cord blood donor is not available for additional testing. For this reason, it is critical that the transplant physicians be assured that a thorough screen for genetic and infectious diseases has been performed and be aware of any risk factors prior to final selection and shipment.
Because cord blood transplantation is a dynamic area of clinical research, standards should be stringent yet flexible enough to allow for the incorporation of new advances in the field. Many issues in the methodology of cord blood banking and transplantation are not fully resolved and require ongoing investigation, including procedures for processing, storage, and thawing. Numerous steps in the transplantation process ought to be considered, including:
-
donor selection and consent;
-
collection of units;
-
processing, testing, and storage of units;
-
selection of units for transplantation;
-
release and shipment of units to transplant centers;
-
thawing and infusion;
-
transplantation; and
-
outcomes monitoring by the transplant site.
Each of these steps should be optimized to ensure the success of the transplant. As part of the accreditation process, cord blood banks and collection sites should be considered an integrated unit. Centers performing transplants should also be accredited to ensure proper unit selection, infusion, follow-up, and outcomes reporting. Ideally, all four components should be accredited by the same organization to ensure consistency.
Cord blood banks are responsible for providing high-quality, HLA-typed units for transplantation to patients in need. The quality of the cells is critical, since the transplant must restore hematopoiesis and immunity in the recipient. Patients have a very low chance of achieving long-term survival if the cells do not engraft.8
Collection Site
The collection process represents the first step in ensuring a high-quality supply of cord blood units. The collection facility should meet minimum standard requirements; that is, it must routinely provide units that are of adequate volume and that test negative for bacterial, viral, or fungal contamination. A designated medical director should be responsible for overseeing the activities of the collection facility.
The collection facility staff should be trained in all aspects of the collection procedure and this training should be documented. The personnel collecting cord blood should be trained in screening and obtaining informed consent from the donors and in the proper methods of effective, sterile collection. Personnel should receive regular evaluation of their performance as well as ongoing training.
A quality management plan that incorporates all aspects of the collection facility’s operations, including personnel training, deviations, adverse event reporting, and internal audits to document compliance with standards, should be in place. The facility should maintain standard operating procedures to ensure an effective sterile collection process, including procedures for the collection, storage, and transportation of units.
Cord Blood Bank
Cord blood units are transferred from the collection site to the cord blood bank where they are processed, tested, and cryopreserved for long-term storage until they are retrieved for transplantation. Each step in this process must be performed properly to ensure a satisfactory outcome for
the transplant recipient. The standards should be flexible and allow the incorporation of new advances in the field; however, cord blood banks must always be able to provide units that meet established minimum standards. Like the collection sites, banks should have a designated director and a medical director who are suitably qualified to supervise all operations and oversee a quality management program that includes scientifically validated methods for processing, storage, thawing, and transportation of the cord blood units. The thawing method should be provided to the transplant facility before or at the time that the unit is shipped. The cord blood bank personnel should be trained in unit receipt, processing, storage, and shipment; and competency assessments should be conducted on an ongoing basis. To ensure adherence to standards, internal audits should be performed at regular intervals. Deviations and adverse events should be monitored and reported appropriately to regulatory and oversight boards. In order to provide another layer of safety and supervision, all banks providing units for allogeneic transplant should have an IND on file with FDA. The IND annual report should be shared with the accrediting agency and oversight board.
Transplant Facility
Transplant centers that receive cord blood units from the proposed national program should be accredited and should adhere to standards. They should demonstrate competency in the selection, handling, thawing, and transplantation of cord blood units. Facilities should have appropriately trained physicians, nurses, and staff, as well as a qualified, appropriately trained medical director. The transplant center should have a demonstrated quality management program for ongoing monitoring of the facility itself, the clinical unit, the experience and training of transplant center personnel, policies and procedures, patient evaluation and selection, administration, data management, and record keeping. Facilities should have a proven record of successful cord blood transplantation or, at a minimum, demonstrated competence with marrow and peripheral blood and a willingness to participate in a mentoring process in order to gain familiarity with cord blood. The time to engraftment after cord blood transplantation should be monitored, and engraftment failures and infections should be reported to the appropriate regulatory agencies. Transplant facilities should report patient outcomes to the accrediting agency, and patient outcomes should also be shared with the cord blood bank and the collection facilities that provided the unit.
Accrediting Organization
Cord blood collection facilities, banks, and transplant centers should be accredited by a central accrediting organization to participate in the proposed National Cord Blood Program. The central accrediting agency should adopt or develop consensus standards and establish a program of inspection and accreditation. This agency should monitor ongoing compliance with standards and the outcomes of transplants. The accrediting agency should report to the policy board of the proposed National Cord Blood Stem Cell Bank Program. Collection centers, banks, and transplant centers should maintain their accreditation and should report the required data to continue to participate in the National Cord Blood Stem Cell Bank Program. The accrediting agency should have a policy in place to detect facilities that become noncompliant in the interim between inspections. The accrediting agency should have a mechanism to withdraw the accreditation for any facility that is found to be noncompliant.
FDA recently issued standards for current good tissue practice which address general issues related to cellular therapies but which are not specific to cord blood banking or transplantation. At present, FDA does not license cord blood.
As mentioned above, two existing organizations, AABB and FACT/NetCord, have developed standards and are accrediting cord blood banks in the United States. In addition, NMDP, while not an accrediting organization, has developed a process to define the minimum acceptable criteria for units to be stored by member banks and listed in their search databases. These criteria do not address all of the quality management issues described above.
The Health Resources and Services Administration should issue a request for proposals to select or create the proposed accrediting body. The organization should meet the standards for the functions for ensuring quality in cord blood collection centers, banks, and transplant centers described above. The organization should demonstrate that it has a comprehensive process, documented by standard operating procedures for standards development and implementation, thorough on-site evaluation of facilities, consistent and comprehensive review of inspection reports, board or oversight committee approval for accreditation, and follow-up procedures throughout the accreditation period. The organization should demonstrate the expertise of the inspectorate, the mechanism for training and competency of the inspectors, ongoing quality control of inspectors, a mechanism for investigating problems uncovered during an inspection, and criteria for retaining or dismissing inspectors. The accrediting organization should define a mechanism for assessing foreign cord blood banks that is comparable to the mechanism for assessing U.S. facilities to guarantee quality and allow those inventories to be available to U.S. recipients.
PRIVATE BANKS
Private cord blood banks have inherently different objectives from public banks, and most of this report is focused on the public banks that might become a part of a national program. However, the committee was explicitly asked whether the standards developed for a national program should also apply to private banks. Although the committee did not want to explicitly address the differences between the two systems of banking or the merits of one over the other, there was no question that individuals storing units with private banks should be assured of the quality of the banks. For this reason, all quality standards adopted by the proposed National Cord Blood Stem Cell Bank Program should apply to both public and private cord blood banks.
Recommendation 4.5: The committee strongly recommends that all cord blood banks, regardless of public or private status or participation in the national program, adhere to the established quality standards.
REFERENCES
Alonso JM III, Regan DM, Johnson CE, Oliver DA, Fegan R, Lasky LC, Wall DA. 2001. A simple and reliable procedure for cord blood banking, processing, and freezing: St Louis and Ohio Cord Blood Bank experiences. Cytotherapy 3(6):429–433.
Anonymous. 2004. Bill would aid Coriell. Philadelphia Business Journal 22(49):3.
Associated Press. February 3, 2005. Lawmaker Pushes Umbilical Cord Donation.
Ballen KK, Wilson M, Wuu J, Ceredona AM, Hsieh C, Stewart FM, Popovsky MA, Quesenberry PJ. 2001. Bigger is better: Maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplantation 27(1):7–14.
Ballen KK, Hicks J, Dharan B, Ambruso D, Anderson K, Bianco C, Bemiller L, Dickey W, Lottenberg R, O’Neill M, Popovsky M, Skerrett D, Sniecinski I, Wingard JR. 2002. Racial and ethnic composition of volunteer cord blood donors: Comparison with volunteer unrelated marrow donors. Transfusion 42(10):1279–1284.
Ballen KK, Kurtzberg J, Lane TA, Lindgren BR, Miller JP, Nagan D, Newman B, Rupp N, Haley NR. 2004. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biology of Blood and Marrow Transplantation 10(4):269–275.
BRMAC (Biological Response Modifiers Advisory Committee, Food and Drug Administration). 2003. Meeting No. 34 Summary Minutes. Fourth Annual Somatic Cell Therapy Symposium, October 1–3, 2004, Gaithersburg, MD.
Broxmeyer HE (Walther Oncology Center, Indiana University School of Medicine, Indianapolis 46202-5121, USA.). 1995. Cord blood as an alternative source for stem and progenitor cell transplantation. [Review] [80 refs]. Current Opinion in Pediatrics 7(1): 47–55.
Broxmeyer HE, Cooper S. 1997. High-efficiency recovery of immature haematopoietic progenitor cells with extensive proliferative capacity from human cord blood cryopreserved for 10 years. Clinical and Experimental Immunology 107(Suppl. 1):45–53.
Creer MH. 2004. Cryopreservation of Cord Blood Products: Impact on Product Quality and Transplant Outcome. Presentation at the 2nd Annual International Cord Blood Transplantation Symposium. May 14–15, Los Angeles, CA.
Donaldson C, Armitage WJ, Laundy V, Barron C, Buchanan R, Webster J, Bradley B, Hows J. 1999. Impact of obstetric factors on cord blood donation for transplantation. British Journal of Haematology 106(1):128–132.
FDA (Food and Drug Administration). 2004a. Eligibility determination for donors of human cells, tissues, and cellular and tissue-based products. Final rule. Federal Register 69(101):29785–29834.
FDA. 2004b. FDA Approach to the Regulation of Hematopoietic Progenitor/Stem Cells Derived from Cord Blood. Presentation by WJ Hartzler at the Workshop of the Institute of Medicine Committee on Establishing a National Cord Blood Stem Cell Bank Program, June 2, Washington, DC.
Florida State Legislature. 2004a. Florida Center for Universal Research to Eradicate Disease. Chapter 2. Section 6. Florida State Legislature, Gainsville.
Florida State Legislature. 2004b. Public Cord Blood Tissue Bank. Code 29. Section 381.06015. Florida State Legislature, Gainsville.
Fountain D, Ralston M, Higgins N, Gorlin JB, Uhl L, Wheeler C, Antin JH, Churchill WH, Benjamin RJ. 1997. Liquid nitrogen freezers: A potential source of microbial contamination of hematopoietic stem cell components. Transfusion 37(6):585–591.
Foy N. December 10, 2003. Umbilical cord project gets grant. San Antonio Express-News. P. 3B.
Galmes A, Besalduch J, Bargay J, Matamoros N, Morey M, Novo A, Sampol A. 1995. A simplified method for cryopreservation of hematopoietic stem cells with −80 degrees C mechanical freezer with dimethyl sulfoxide as the sole cryoprotectant. Leukemia and Lymphoma 17(1–2):181–184.
Galmes A, Besalduch J, Bargay J, Novo A, Morey M, Guerra JM, Duran MA. 1999. Long-term storage at −80 degrees C of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide as the sole cryoprotectant. Transfusion 39(1):70–73.
Gee AP, Biol MI. 1999. Transplantation and the Food and Drug Administration—how will it affect your program? Cancer Research Therapy and Control 9(1–2):171–176.
Grisaru D, Deutsch V, Pick M, Fait G, Lessing JB, Dollberg S, Eldor A. 1999. Placing the newborn on the maternal abdomen after delivery increases the volume and CD34 cell content in the umbilical cord blood collected: An old maneuver with new applications. American Journal of Obstetrics and Gynecology 180(5):1240–1243.
Howard DH, Maiers M, Kollman C, Logan B, Gragert L, Setterholm M. 2005. A cost-benefit analysis of increasing cord blood inventory levels: An analysis prepared for the Committee on Establishing a National Cord Blood Stem Cell Bank, Institute of Medicine, Washington, DC.
Hubel A, Carlquist D, Clay M, McCullough J. 2004. Liquid storage, shipment, and cryopreservation of cord blood. Transfusion 44(4):518–525.
Kobylka P, Ivanyi P, Breur-Vriesendorp BS. 1998. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation 65(9):1275–1278.
Matsumoto N, Yoshizawa H, Kagamu H, Abe T, Fujita N, Watanabe S, Kuriyama H, Ishiguro T, Tanaka J, Suzuki E, Kobayashi K, Gemma A, Kudoh S, Gejyo F. 2002. Successful liquid storage of peripheral blood stem cells at subzero non-freezing temperature. Bone Marrow Transplantation 30(11):777–784.
NMDP (National Marrow Donor Program). 2004. Submission to the Institute of Medicine. National Marrow Donor Program 19th Edition Standards: Attachment 7. Minneapolis, MN: National Marrow Donor Program.
Oklahoma State Legislature. 2004. Danielle Martinez Act. House Bill No. 2306. Oklahoma State Legislature, Oklahoma City.
Oklahoma State Legislature. 2005. Danielle Martinez Act. House Bill No. 1695. Oklahoma State Legislature, Oklahoma City.
Quinn W. May 10, 2004. Opening the door for stem cell progress. NJBIZ 17:18.
Rubinstein P. 2001. HLA matching for bone marrow transplantation—how much is enough? New England Journal of Medicine 345(25):1842–1843.
Rubinstein P. 2004. Presentation at the Workshop of the Institute of Medicine Committee on Establishing a National Cord Blood Stem Cell Bank Program. The New York Blood Center Cord Blood Program Perspective, June 2, Washington, DC.
Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. 1995. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proceedings of the National Academy of Sciences (U. S. A.) 92(22):10119–10122.
Solves P, Moraga R, Saucedo E, Perales A, Soler MA, Larrea L, Mirabet V, Planelles D, Carbonell-Uberos F, Monleon J, Planells T, Guillen M, Andres A, Franco E. 2003. Comparison between two strategies for umbilical cord blood collection. Bone Marrow Transplantation 31(4):269–273.
State of Illinois. 2004. Umbilical cord blood donation. Code 210. Section 85-6.21. State of Illinois, Springfield.
State of Maryland. 2004a. Hospitals—Umbilical Cord Blood Donation (House). Chapter 451. Section 19-308.7. State of Maryland, Annapolis.
State of Maryland. 2004b. Hospitals—Umbilical Cord Blood Donation (Senate). Chapter 450. Section 19-308.7. State of Maryland, Annapolis.
Surbek DV, Visca E, Steinmann C, Tichelli A, Schatt S, Hahn S, Gratwohl A, Holzgreve W. 2000. Umbilical cord blood collection before placental delivery during cesarean delivery increases cord blood volume and nucleated cell number available for transplantation. American Journal of Obstetrics and Gynecology 183(1):218–221.
Tedder RS, Zuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs EM, Irwin D, Blair S, Gorman AM, Patterson KG. 1995. Hepatitis B transmission from contaminated cryopreservation tank. Lancet 346(8968):137–140.
Timeus F, Crescenzio N, Saracco P, Doria A, Fazio L, Albiani R, Cordero Di Montezemolo L, Perugini L, Incarbone E. 2003. Recovery of cord blood hematopoietic progenitors after successive freezing and thawing procedures. Haematologica 88(1):74–79.
Wada RK, Bradford A, Moogk M, Yim R, Strong DM, Drachman J, Reems JA. 2004. Cord blood units collected at a remote site: A collaborative endeavor to collect umbilical cord blood through the Hawaii Cord Blood Bank and store the units at the Puget Sound Blood Center. Transfusion 44(1):111–118.
Wall DA, Noffsinger JM, Mueckl KA, Alonso JM III, Regan DM, Johnson CE, Weinstein DL, Duarte LM, Winn HN. 1997. Feasibility of an obstetrician-based cord blood collection network for unrelated donor umbilical cord blood banking. Journal of Maternal-Fetal Medicine 6(6):320–323.
Wong A, Yuen PM, Li K, Yu AL, Tsoi WC. 2001. Cord blood collection before and after placental delivery: Levels of nucleated cells, haematopoietic progenitor cells, leukocyte subpopulations and macroscopic clots. Bone Marrow Transplantation 27(2):133–138.