3
Basic Concepts in Radiation Physics, Biology, and Epidemiology
The scientific issues related to radiation and associated health effects are complex and may be confusing for persons not professionally involved with them. The topics are even more complicated in the context of the Radiation Exposure Compensation Act (RECA) and the Radiation Exposure Screening and Education Program (RESEP). This chapter will give concerned readers an opportunity to become familiar with the terminology and concepts used in the radiological sciences. It is limited to scientific topics directly related to the basic charge presented to the committee. The chapter is divided into three sections. The first presents the principles of physics related to ionizing radiation. The second presents the biology necessary for understanding how radiation affects cells and the mechanisms of radiation injury and repair. The third section describes the methods used to identify and measure the risks to persons who are exposed to radiation.
RADIATION PHYSICS
Definition of Radiation
Observable matter is made up of discrete components known as atoms and molecules. Atoms are divisible into particles, such as electrons, protons, and neutrons. Other elementary particles are part of the fabric of nature, but they are more elusive and do not directly form stable atoms or molecules. When a particle or group of particles is accelerated, it can reach high energies and travel a large distance in a very short time. Radiation can be defined as any collection of
elementary particles that have sufficient energy to interact with and transfer some of their energy to objects or materials that intercept their path.
Ionizing Radiation
Many different types of interactions can take place when radiation strikes an object. For instance, atoms in an irradiated object are neutral; they each consist of a positively charged nucleus (made up of protons and neutrons) surrounded by negatively charged electrons. The process of removing an orbital electron from an atom is called ionization.
Some types of radiation can transfer energy in a manner that creates ionization in the object. X rays and gamma rays are particles called photons that can create ionization. Microwaves, ultraviolet radiation, visible light, and infrared are also photons, but they do not result in ionization and are referred to as nonionizing radiation.
Ionization created by radiation in living systems can have unique biologic consequences that are different from those caused by nonionizing radiation. RECA is related specifically to diseases found to have an association with exposure to ionizing radiation.
The process that accelerates particles to form radiation can occur naturally. For example, the sun continuously emits particles that reach the atmosphere and result in a continuous shower of elementary particles on the surface of the earth. Some sources of radiation are man-made, such as x-ray machines, particle accelerators used for cancer therapy, and nuclear power reactors used to generate electricity.
Radioactivity
Radioactivity is another important source of ionizing radiation. Every element such as hydrogen, oxygen, or iron are defined by the number of protons in the nucleus. However, atoms of the same element can have a different number of neutrons in the nucleus. These are called isotopes. Isotopes are identified by the name of the element and the total number of protons and neutrons in the nucleus. For example, the element hydrogen has one proton, 1H. There is another isotope of hydrogen with one proton and one neutron, 2H, called deuterium and also one proton and two neutrons, 3H, called tritium. Some nuclei are unstable, and these can transform (decay) into more stable nuclei by emitting particles—a process called radioactive decay. The emitted particles are a form of radiation originating from radioactivity.
Every element in the periodic chart has at least one isotope that is radioactive. For instance, sodium-23 (23Na) is stable, but sodium-22 (22Na) and sodium-24 (24Na) are radioactive; similarly, iodine-127 (127I) is stable, and iodine-131 (131I) is radioactive. A salt containing natural potassium will always contain some radioactive potassium-40 (40K). Potassium is an essential mineral in our
diet. Some of the ingested potassium is absorbed in tissue. That process is not limited to potassium, but can occur with iodine, sodium, radium, and so on. Therefore, all persons contain some radioactivity.
Each radioactive isotope has unique properties. One property is the type of particles emitted, and another is the energy of the particles emitted. No two radioactive isotopes emit the same combination of particles and energies. Therefore, one can identify the presence of a specific isotope at a given location by measuring the types and energies of the emitted particles.
Radioactive decay is a random process: it is impossible to determine when a given nucleus will decay. However, it is possible to estimate how many nuclei in a group will decay during a given period. The half-life of an isotope is the time it takes for half the nuclei in a group or sample to decay. Thus, isotopes with short half-lives decay rapidly and those with long half-lives decay more slowly. No two isotopes have the same half-life. For example, the half-life of nitrogen-16 (16N) is 7.3 seconds; that of radon 222 (222Rn), 3.8 days; that of 131I, 8 days; and that of uranium-238 (238U), 4.5 billion years.
Radioactivity specifically refers to the rate at which decays occur. The amount of radioactivity present depends on the number of radioactive atoms and their corresponding half-life. The rate at which atoms are decaying is proportional to the number of atoms divided by the half-life. This decay rate is described in units of either Becquerels (Bq) in the International System, SI, of units or Curies (Ci) in the traditional system of units used in the United States; 1 Bq is equal to 1 decay per second, and 1 Ci is equal to 37 billion decays per second. The amount of radioactivity is often stated in terms of a millicurie (mCi), which is one thousand times smaller than a Curie. One microcurie (µCi) is one million times smaller than a Curie and one picocurie is one trillion times smaller than a Curie. The amount of radioactivity at any time is reduced by one-half in a period of time equal to one half-life.
Radioactivity generates radiation by emitting particles. Radioactive materials outside the body are called external emitters, and radioactive materials located within the body are called internal emitters.
Types of Ionizing Radiations
Radioactive nuclei can emit several kinds of particles, but there are three primary types: alpha particles (α), beta particles (β), and photons that are either x rays or gamma rays (γ). Several properties distinguish those particles from one another. One is electric charge; alpha particles are emitted with a positive charge of 2, beta particles are emitted with either 1 negative charge (electron) or 1 positive charge (positron), and x rays and gamma rays have no charge and are thus neutral.
Another important property is penetration of the particles through matter. Alpha particles lose energy rapidly and stop in a very short distance. Most travel
no more than 3-5 centimeters in air and only about 30-50 microns in water or tissue. They cannot penetrate clothes or skin. Alpha particles must be emitted very close to biologic targets to produce an effect. External alpha emitters therefore are generally not considered to pose a health hazard. However, radioactive materials can enter the body through inhalation, ingestion, or transfer through cuts and wounds. Some of this radioactive material passes through the body and is eliminated, and some remains in tissues that might contain radiosensitive cells. The distribution of the radioactive material in the body depends on the chemistry of the radioactive element. For example, radium has chemical properties similar to those of calcium, and the alpha-particle emitter radium-226 (226Ra) will accumulate with calcium in bone.
Beta particles are electrons that lose energy rather slowly when passing through materials. A high-energy beta particle can travel several centimeters through water and tissue. Lower-energy beta particles travel some fraction of that distance. External emission of low-energy beta particles, as in the decay of tritium, which is an isotope of hydrogen (3H), or carbon-14 (14C) is not considered a health hazard, whereas external emission of high-energy beta particles from strontium-90 (90Sr) reach some regions of the body that are sensitive to radiation. As in the case of alpha-emitters, the distribution of internal beta-emitters depends on the chemistry of the radioactive element. Strontium has chemistry similar to that of calcium, and 90Sr will accumulate in bone. Most of the iodine in the body that is not excreted will accumulate in the thyroid. Beta particles from 131I can originate in the thyroid and deposit most of their energy there.
Photons can be very penetrating. High-energy x rays and gamma rays travel many meters in air and through many centimeters of concrete, iron, and tissue. Thus, external gamma rays can penetrate and deposit energy throughout the body. The distribution of internal gamma-emitters depends on the chemistry of the radioactive element. Internally emitted gamma rays can deposit energy in the tissue of residence or neighboring tissues. For example, cesium-137 (137Cs) deposited in soft tissues, and the entire body is exposed uniformly to gamma rays.
Radiation Measurements and Units
Radiation can be described and measured in many ways. For purposes of radiobiology and radiation protection, the concept of absorbed dose, D, is most commonly used. It does not measure each particle but describes the energy deposited in a specified region. Absorbed dose is the energy absorbed in a volume of material divided by the mass of the material. It is the result of the physical interactions of the ionizing radiation within the volume of material. An absorbed dose can be delivered by any type or combination of types of radiation in any type of material.
The units of absorbed dose are the gray (Gy) in the SI and the rad in the traditional system often still used in the United States; 1 Gy is equivalent to 100
rad. The centigray (cGy) is a unit of convenience often used in cancer therapy that is equivalent to 1 rad.
Dose rate refers to the distribution of dose as a function of time. It can be expressed as Gy per second (Gy s−1), per minute (Gy min−1), per hour (Gy h−1), and per year (Gy y−1). A protracted dose is one received over a long period of time. A given dose delivered within 1 h often will have different consequences than the same total dose delivered over a period of one year. In some cases, if the dose rate is constant for long periods, it is referred to as continuous exposure to radiation. A dose rate can change with time; radiation could occur in the form of random pulses or vary periodically.
Dose fractionation describes the case in which a dose is delivered in segments or fractions over a specified period. For example, in radiation therapy for cancer, a total dose of 50 Gy might be delivered at a high dose rate of 2 Gy min−1 for only 1 minute per day over a period of 25 days (5 weeks, excluding weekends).
Equivalent Dose
The concept of absorbed dose, D, was created to estimate biologic effects of ionizing radiation. Scientists hoped that absorbed dose could serve as a universal predictor of biologic effects and corresponding risks to humans from exposure to ionizing radiation. However, it was soon discovered that similar doses of radiation from different particles produced different amounts of biologic damage. In some cases, up to 1 Gy of gamma rays is needed to produce the same effect as 0.1 Gy of alpha particles. That was observed for many biologic systems and was ultimately referred to as relative biological effectiveness (RBE).
RBE is related to the density or rate of ionization produced by a particle as it passes through matter. Linear energy transfer, LET, is a measure of the rate of energy loss and therefore ionization along the track of a particle. Alpha particles have short tracks, but create large amounts of ionization along the track and are referred to as high LET radiation. Electrons and beta particles are sparsely ionizing and are referred to as low LET radiation. X rays and gamma rays create electrons when they interact in materials and are also considered to be low LET radiation. To a first approximation, RBE increases with LET.
Rules for and regulation of radiation protection of humans must be related to the risks associated with exposure to ionizing radiation. RBE makes it impossible to base a system of regulations on absorbed dose alone. It was necessary to include the type of radiation in a consistent manner that reflected changes in the biology as well as the physics. For this reason, the concept of equivalent dose was established for purposes of radiation protection. Equivalent dose (HT) in a tissue or organ, T, is the product of absorbed dose averaged within a tissue (DT) and a radiation weighting factor (wR), and thus HT = DT × wR.
The radiation weighting factor is used to adjust the absorbed dose to reflect the RBE for radiation of type R. It is thus related to LET. Alpha particles have
a wR of 20. Beta particles, x rays and gamma rays have a wR of 1.0. Equivalent dose is described in sievert (Sv) or rem.
Effective Dose
Some tissues and organs are more sensitive to radiation than others. When the entire body is irradiated uniformly, all organs receive a dose and contribute to the total risk of a health effect, such as cancer. In some cases, particularly with internal emitters, only one or two organs receive a dose, and the other organs are not at risk. When one needs to know the combined risk for such a case, it is necessary to include a factor that is related to the risk to each of the exposed organs. The equivalent dose, HT, in each tissue, T, is multiplied by a tissue-weighting factor, wT. The effective dose, E, is then the sum of HTwT for all exposed tissues. Effective dose is a risk averaged dose that serves as a measure of risk including adjustments for both the type of radiation, wR, and the tissues exposed, wT. Effective dose is expressed in sievert (Sv) when the absorbed dose is measured in Gy, or in rem when the dose is measured in rads; 1 Sv = 100 rem.
The International Commission on Radiological Protection (ICRP, 1991) has made recommendations for values of wT on the basis of the occurrence of cancer and hereditary effects observed in exposed populations. The currently accepted values are shown in Table 3.1.
One way to interpret Table 3.1 is for a large population of persons irradiated uniformly. Some people might develop cancer as a result of the absorbed dose received. The types of cancer associated with radiation would be distributed according to the fraction represented by wT in Table 3.1. ICRP makes recom-
TABLE 3.1 Currently Recommended Tissue Weighting Factors, wTa
|
Tissue |
wT |
|
Gonads |
0.20 |
|
Bone marrow |
0.12 |
|
Colon |
0.12 |
|
Lung |
0.12 |
|
Stomach |
0.12 |
|
Bladder |
0.05 |
|
Breast |
0.05 |
|
Liver |
0.05 |
|
Esophagus |
0.05 |
|
Thyroid |
0.05 |
|
Skin |
0.01 |
|
Bone surfaces |
0.01 |
|
Remaindera |
0.05 |
|
Total |
1.00 |
|
awT for the remainder is divided equally between adrenals, brain, upper large intestine, small intestine, kidney, muscle, pancreas, spleen, thymus, and uterus. |
|
mendations for revising the values as new evidence on cancer incidence and tissue sensitivity becomes available.
Natural Background Radiation
All persons are exposed to ionizing radiation from natural sources. Sources of background radiation can be outside the body (external radiation) or inside the body (internal radiation). The primary contributions to external radiation from natural background are cosmic rays and penetrating gamma rays emitted by radioactive materials in rocks and soil, in particular 40K, 232Th and 238U. The primary contributions to internal radiation from natural background are radioactive materials that enter the body through the diet—40K, carbon-14 (14C), 226Ra—and inhaled radioactivity originating from 222Rn.
Natural background radiation can have large variations. Exposure rates around the world depend on geography, geology, and housing environments. Table 3.2 shows a summary of the average annual effective dose received from natural background radiation by persons in the United States and the average received by persons residing near the mountains in the western part of the country (NCRP, 1987).
There are other exposures to ionizing radiation. The most common sources are medical examinations that prescribe diagnostic x rays and computed tomography (CT) scans. Table 3.3 shows the effective dose received from several types of diagnostic examinations (NCRP, 1987).
In addition to medical examinations, the general population may be exposed to radiation from industrial applications and consumer products. Figure 3.1 shows the relative contribution to effective dose for an average person in the United States from natural background and man-made sources (NCRP, 1987).
In Figure 3.1, cosmic refers to the contribution from external radiation from penetrating particles originating in the atmosphere. Terrestrial refers to the contribution from external gamma rays originating in radioactivity in soil, rocks, and building materials. Internal radiation refers to the contribution from radioactivity deposited throughout the body from diet and inhalation. Radon represents
TABLE 3.2 Average Annual Effective Dose Received by People in the United States from Natural Background Radiation
|
Source |
|
United States (mSv/year) |
Mountains (mSv/year) |
|
External |
Cosmic rays |
0.3 |
0.6 |
|
External |
Radioactivity |
0.3 |
0.6 |
|
Internal |
Radioactivity |
0.4 |
0.4 |
|
Radon |
Inhalation |
2.0 |
3.4 |
|
|
Total |
3.0 |
5.0 |
TABLE 3.3 Effective Dose Received from Diagnostic Examinations of Specific Organs and Tissues
|
Examination |
mSv |
|
Arms and legs |
0.10 |
|
Chest |
0.08 |
|
Pelvis |
0.44 |
|
Upper gastrointestinal tract |
2.40 |
|
Mouth (Dental) |
0.03 |
|
Breast (mammography) |
0.40 |
|
Head and body (CT) |
1.11 |
the contribution from inhalation and deposition of radioactivity in the lung that originates from radon gas. Medical represents the contribution from diagnostic medical examinations. Other represents the contribution from man-made sources of ionizing radiation, such as the nuclear-power industry and consumer products (for example, smoke detectors, CRT monitors, porcelain, and tobacco).
Uranium
The original nuclear-weapons program depended on exploration, mining, and milling of natural uranium. At that time, most of the known uranium deposits were deep underground and required extensive mining operations that were labor-intensive. As mentioned earlier, uranium is radioactive and has a very long half-life. When uranium decays, it emits an alpha particle. The remaining nucleus, thorium, is also radioactive. It promptly decays by emitting a beta particle. That radioactive sequence continues for 13 decays until a stable isotope of lead is
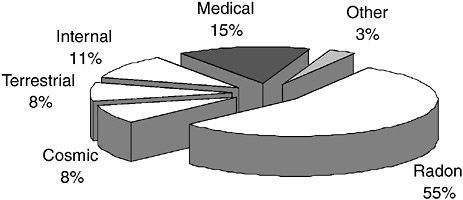
FIGURE 3.1 Relative contribution to average effective dose received by persons living in the United States. The striped sections are from man-made sources of ionizing radiation. The other sections are from natural background radiation.
formed. Thus, alpha (α), beta (β), and gamma (γ) radiations are present in underground mines. Their presence can result in external exposure to gamma rays and internal exposure to alpha, beta, and gamma radiations from inhalation and unintentional ingestion of ore dust.
Risk to Miners
In general, the most hazardous exposure pathway for underground miners is not related to the ore dust itself or external gamma rays. About halfway through the uranium decay process, 226Ra decays into 222Rn. Radon is an inert gas that escapes from the rocks and begins to accumulate in the mine. It ultimately decays. This initiates a prompt series of decays that occur within a matter of minutes:
Those four short-lived radioactive descendants or decay products of radon have historically been called radon daughters. They can become suspended in air and are respirable. Inhaled radon is rapidly exhaled whereas radon decay products can deposit in the airways. Alpha particles emitted by 218Po and 214Po can deliver a large amount of energy and result in a large dose to cells in the airways. Those processes have been directly associated with the development of lung cancer in uranium miners. They are also the reasons for concern in family dwellings that have high concentrations of indoor radon from natural background.
The concentration of the short-lived decay products of radon is measured in working level (WL). A person’s exposure at a given location is based on the concentration of decay products and the amount of time the person spends at the location. That exposure is expressed in working level month (WLM). For the purposes of this definition, 1 month is considered to be 170 h. Thus, 1 WLM is equivalent to 1 WL for 170 h, 2 WL for 85 h, 5 WL for 34 h, and so on. The risk of radiation-induced lung cancer is related to the exposure in WLM. For comparison purposes, 1 WLM delivers an effective dose of about 10 mSv (1 rem) to the trachea bronchial region of the respiratory tract.
Risk to Ore Transporters and Millers
After uranium ore is extracted from the mine, it is shipped to a mill, where it is crushed into fine sand and subjected to a chemical process to remove uranium selectively from the ore. The final product, uranium oxide (U3O8), often called yellowcake, is used for the production of weapons or as fuel for nuclear reactors. The remaining sands, called uranium mill tailings, are placed into a tailings pile close to the mill.
Yellowcake has a much higher concentration of uranium than the original ore removed from the mine. However, because uranium has an extremely long half-life, the radioactivity is not the principal hazard. The most serious hazard is heavy-metal chemical toxicity because of ingestion or inhalation.
Risk from Mill Tailings
The fine silt and sands in mill tailings contain all the other radioactive isotopes in the ore except the uranium. In effect, that represents all the radioactivity in the uranium decay series, including radon. Because radon is a noble gas, it can escape from the sands and is a potential route of exposure of persons residing near the mill or of exposure later when the tailings are used for construction or landfill around homes. Exposure to external radiation and radon decreases rapidly with distance from the tailings.
Nuclear Weapons and Fallout
Yellowcake is an oxide of natural uranium. Natural uranium consists of the isotopes 238U (99.3%), 235U (0.7%), and 234U (trace). 235U is necessary for a nuclear weapon. Thus, the yellowcake must undergo another process to increase the proportion of 235U. That is called enrichment, and the desired product is enriched uranium. The remaining byproduct is called depleted uranium and is almost exclusively 238U.
When a nuclear weapon is detonated, energy is released through a process called fission. Fission occurs when a heavy nucleus absorbs an additional neutron and then violently splits into two pieces and a few extra neutrons. If neutrons survive to produce another fission, the process can sustain itself. Weapons are designed to generate enough fissions to initiate an explosion in a fraction of a second following detonation. The first nuclear weapons released energy equivalent to 15,000 tons (15 kiloton) of TNT. Later versions used either 235U or plutonium-239 (239Pu) to produce fission yields over 1,000 kilotons.
The two nuclear fragments that remain after fission are called fission products. Many possible combinations of fragments can occur. One or both of the fission products can be radioactive. Some have very short half-lives and so decay within seconds or minutes. Others have half-lives of days (for example, 131I) or years (for example, 90Sr, and 137Cs).
Fission products are propelled into the atmosphere by the force of the explosion. They can remain suspended and transported by winds. Eventually, the radioactive fission products settle back toward the surface of the earth and are called fallout. Fallout can be increased locally by precipitation wash-out (Beck, 2002; Bennett, 2002). Fallout can be responsible for both external and internal exposures of people in the vicinity. More than 150 fission products have half-
lives greater than 1 h. Some of the important radioactive fission products in fallout and their principal exposure pathway are listed in Table 3.4.
Dosimetry
Dosimetry is the process of determining the effective dose received by persons exposed to ionizing radiation. The most accurate way to determine dose to an individual is to make measurements with a dosimeter assigned to each person. That is required today for radiation workers that might be exposed during routine occupational activities. Area monitors measure external radiation or radioactivity suspended in the air at specific locations. No dosimeter can directly measure the dose to the lung from the inhalation of radioactive materials, so area monitors are the principal instruments used for measuring and controlling internal exposure in underground mines.
Personal dosimeters were not available for all persons who might have been affected by fallout from atmospheric weapons testing. The US Atomic Energy Commission collected fallout on gummed film at more than 100 locations in the United States and its territories. The film was collected regularly and analyzed to estimate radioactivity deposited on the ground (Beck et al., 1990). The resulting data and weather patterns were used to create maps of fallout across the country.
Dose reconstruction is a computational process for estimating the dose to persons in situations when direct measurements are incomplete or unavailable. The National Cancer Institute has developed maps that show concentrations of radioactivity deposited in the United States from fallout during the period of atmospheric
TABLE 3.4 Some Important Fission Products in Fallout and Their Exposure Pathways. They are Ordered with Increasing Atomic Mass
|
Fission Product |
Symbol |
Half-Life |
Emissions |
External Exposure |
Exposure Internal |
|
Manganese |
54Mn |
300 days |
γ |
|
|
|
Strontium |
89Sr |
52 days |
β |
|
|
|
Strontium |
90Sr |
28 years |
β |
|
|
|
Zirconium |
95Zr |
64 days |
β,γ |
|
|
|
Zirconium |
97Zr |
17 hours |
β,γ |
|
|
|
Ruthenium |
103Ru |
39 days |
β,γ |
|
|
|
Ruthenium |
106Ru |
368 days |
β |
|
|
|
Iodine |
131I |
8 days |
β,γ |
|
|
|
Iodine |
133I |
22 hours |
β,γ |
|
|
|
Cesium |
136Cs |
13 days |
β,γ |
|
|
|
Cesium |
137Cs |
300 days |
β,γ |
|
|
|
Barium |
140Ba |
13 days |
β,γ |
|
|
|
Neptunium |
239Np |
2.4 days |
β,γ |
|
|
|
Plutonium |
241Pu |
14.4 years |
α |
|
|
weapons testing. These data can be used to estimate the dose from internal and external radiation to persons living downwind of a test site. A more extensive description of these maps and dose calculators is presented in Chapter 4.
RADIATION BIOLOGY
When people are exposed to ionizing radiation from sources outside or inside the body, the radiation may interact with molecules in cells in their path. As described earlier in this chapter, some ionizing radiation can travel through a few or several layers of cells (beta-particle radiation) or through many cell layers into and through tissues deep within the body (x and gamma radiation), whereas alpha-particle radiation has short paths or tracks. The rate at which radiation loses energy along its tracks is referred to as linear energy transfer (LET) and depends on its track length. Thus, beta-particle radiation and the electrons associated with x and gamma rays, which are sparsely ionizing, are described as low-LET radiation, and alpha-particle radiation, which is densely ionizing, as high-LET radiation.
Biologic Actions of Ionizing Radiations
The main target of importance with respect to radiation damage is the deoxyribonucleic acid (DNA) in the cell’s nucleus. The interactions between ionizing radiation and DNA can be direct or indirect.
Direct interactions occur when the radiation is deposited or transfers its energy directly to DNA. However, the probability of direct interactions is low because the volume of DNA is small relative to the total volume of the cell. Direct interactions occur more commonly when the radiation is of the densely ionizing type, such as alpha- or beta-particle radiation, than when it is less densely ionizing, such as gamma and x radiation.
Radiation interacts indirectly with DNA by first interacting with water molecules in the vicinity of the DNA, causing ionizations that result in the formation of free hydroxyl radicals. The free radicals can then diffuse to the vicinity of the DNA and can cause alterations in it. About 60% of the DNA damage caused by radiation is the result of indirect interactions. However, few of the many interactions that occur result in DNA damage, because most of the free radicals disperse and deposit their energy without interacting with DNA.
Biologic Sensitivity to Ionizing Radiations
An important concept in radiation biology is that the most rapidly dividing cells are the least well differentiated and are the most sensitive to radiation and thus are the most vulnerable to radiation-induced death and injury. The concept of radiosensitivity was formulated by Bergonie and Triboneau (1906). Some
proliferative cells in the testis, red bone marrow, and intestinal mucosa, are among the most radiosensitive. Cells that divide more slowly, if at all, and cells that are highly differentiated, such as mature red blood cells and muscle and nerve cells, typically are relatively insensitive to radiation. Large lymphocytes (a type of white blood cell) divide more frequently than do small lymphocytes, but they are both highly sensitive to radiation. One of the earliest clinical effects of an acute whole-body dose of radiation—over about 250 mSv (25 rem)—in humans is a rapid fall in the number of large lymphocytes, beginning within 24 h. Because small lymphocytes divide infrequently radiation-induced changes in their DNA are more persistent, so aberrations in them can persist for many years after a large radiation dose (Goans et al., 2001).
A radiation dose delivered all at once or within a short period has a greater biologic effect than the same total dose delivered in small amounts over a period of weeks (fractionation) or in very small amounts continuously over a long period (protraction). In the latter cases, fewer cells are likely to be killed or lethally damaged at one time. DNA repair can proceed in the intervals between the successive exposures of a single cell from a fractionated exposure, or may be sufficient to counteract damage occurring during a protracted exposure, so that low dose rates allow for cell recovery or replacement.
Radiation-Induced Biological Damage
External exposure of the whole body or a substantial part of the body to penetrating radiation, such as gamma and x rays, can damage DNA in the cells of tissues deep within the body. External radiation dose is deposited independent of differential uptake in cells and sub-cellular regions due to ongoing local metabolic processes. Inhomogeneous dose distribution is more characteristic of internal emitters than external radiation sources. When an exposed person leaves the vicinity of an external source of radiation, no further dose is received from that source.
High-energy alpha- or beta-particles deposited on or close to the skin can penetrate the outer layers of dead and aging skin cells to reach the deeper or germinal layer in which cells are actively dividing. Radioactive particles that enter the body are distributed through many organs according to the nature of the metabolism of the particles, and the functions of the different organs. Only rarely are they distributed uniformly throughout the body; most are deposited in target tissues or organs; for example, 131I, like stable iodine, targets the thyroid gland. The dose deposited in different organs is the best measure of radiation to use in correlations of internal dose with observed and expected effects. Doses to different organs from radioactive particles in the body are likely to be quite heterogeneous; large differences between organs are based on metabolic factors. Radioactivity taken into the body persists until it decays away or the radioactive element is eliminated from the body.
Repair of Radiation-Induced Damage
Repair of DNA damage caused by radiation from sources outside or inside the body is an effective, normal biologic process. This highly efficient repair process, which has evolved over many millennia, enables organisms, including humans, to survive and thrive despite constant exposure to background levels of radiation in the environment that in earlier millennia were much higher than they are now. However, ionizing radiation is more likely to damage both strands of DNA simultaneously than are normal metabolic processes. That is because ionizations may occur close together along the tracks of charged particles (electrons, protons, and alpha particles), thereby damaging both DNA strands and producing DNA double-strand breaks or other damage affecting both DNA strands in close proximity.
Repair of radiation-induced damage is usually complete and accurate, restoring damaged DNA to its full function. But if the damage is irreparable and the cells die immediately or are unable to divide to produce new cells of the same type, cell systems become depleted; and if the rate of depletion exceeds the rate at which the body can replace the lost cells, the underlying radiation-induced biologic damage is likely to become clinically evident in the form of adverse health effects. Radiation biologists describe such effects as deterministic effects because their type and severity are determined by the nature and magnitude of the radiation dose received. DNA repair also can be incomplete or inaccurate, in which case cells survive and divide but with some probability of changes, or mutations, in some of their genes. In time, such mutations may result in other adverse health effects, primarily cancer. Radiation biologists describe these late or delayed-onset effects as stochastic effects because their occurrence follows some random probability distribution or pattern; that is, they are effects that occur at random with some degree of probability that is related to a person’s radiation dose.
Human Health Effects of Radiation-Induced Biologic Damage
The onset of deterministic health effects may be acute or delayed, depending on the type.
Acute or Early Deterministic Effects
Acute or early deterministic effects become clinically evident within minutes up to about 2 months after an acute radiation exposure of the whole body or partial body of sufficient magnitude to cause a critical number of cells in individual tissue systems, such as the blood-forming tissues, to die prematurely or to lose their ability to divide. The higher the acute radiation dose, the earlier the deterministic effects occur after the exposure and the more severe they are. Clini-
TABLE 3.5 Estimated Threshold Absorbed Doses for Selected Deterministic Effects of Acute Exposure to low LET Radiationa
cal, epidemiologic, and animal studies have shown that threshold doses of radiation are required to cause specific deterministic effects, that is, dose thresholds below which specific types of deterministic effects are not seen (Mettler and Upton, 1995). The minimum or threshold doses necessary to cause specific deterministic effects depend on the radiation sensitivities of the exposed cell systems. Estimated threshold absorbed doses for selected deterministic effects of acute exposure to low-LET radiation are shown in Table 3.5.
The spectrum of early signs and symptoms observed after a whole- or partial-body dose of 0.5-1.0 Gy or more is known as the acute radiation syndrome (ARS). The clinical features of the ARS have been described in detail by Young (1987). On the basis of the committee’s review of information about reconstructed radiation dose estimates of downwinders and onsite participants, it is considered highly unlikely that people in the RECA populations received acute whole- or partial-body doses of gamma radiation of sufficient magnitude to cause deterministic effects, including the ARS (Lloyd et al., 1990; Henderson and Smale, 1990; Till et al., 1995; Caldwell et al., 1983).
Exposure to ionizing radiation at natural background levels normally present in the environment does not result in discernible deterministic health effects in humans.
Late Deterministic Effects
Some types of deterministic effects may appear many months or years after an exposure to a relatively high dose of radiation; these effects result from cell death or injury that occurred at the time of the exposure but which do not become clinically evident until a long period has passed. This category includes radiation-induced cataract, fibrosis, fibrovascular atrophy, thyroid dysfunction, and effects in an exposed embryo or fetus.
Cataract is one of the few health effects of radiation exposure that essentially is pathologically characteristic, at least in its early stages, of radiation injury.
Cataracts of the posterior subcapsular type have been described as being clinically detectable and distinguishable from cataracts due to other causes after doses to the lens of about 2 Gy of low LET radiation and a minimum latent period of about 10-12 months.
The threshold doses of radiation to localized areas of the body sufficient to result in radiation-induced fibrosis, fibrovascular atrophy, and thyroid dysfunction are considerably higher than the threshold dose for cataract induction.
Exposure of a pregnant woman to radiation may cause nonspecific deterministic effects in the embryo or fetus. Such in utero effects may be expressed clinically in the embryo or fetus or after the child’s birth. The nature of these effects and their severity are related to the radiation dose to the embryo or fetus and the period of the pregnancy (gestation) in which the exposure occurred (Brent, 1999) (see Chapter 7).
Stochastic Effects
Radiation-induced damage that is incompletely or incorrectly repaired increases the probability of genetic mutations in affected cells. If the affected cells are of the somatic type, that is, the type of cell that is not handed on to a person’s offspring, the probability is increased for stochastic (late) effects such as cancer, appearing in irradiated people years or even decades after exposure. If the affected cells are of the reproductive type—that is, they are transmitted to the next generation—there also is a small probability of radiation-induced heritable genetic effects in the progeny of those exposed. Such effects, which are not peculiar to radiation, occur randomly with frequencies and probabilities that increase with increasing dose. Their severity is unrelated to dose. In the absence of definitive biologic or epidemiologic data to the contrary, it is assumed that there is no dose threshold below which the risk of stochastic effects is zero.
Cancer and the Carcinogenic Effect of Radiation Cancer is a collective term used to describe many types of malignant diseases. Their induction and development follow a multistage process that is not yet fully understood but is known to be influenced by many factors inside and outside the body. Cancer occurs mainly in older people. The American Cancer Society estimates that 40-45% of the US population develop some form of cancer during their lifetime and that cancer accounts for about 25% of deaths in the United States (Jemal et al., 2004). Exposure to radiation has been shown to increase the cancer risk in the exposed population by some amount that is often related to the dose and to the normal or background risk in the nonexposed population.
After exposure to radiation, mutations induced in somatic cells (cells whose genes are not passed on to the next generation) of an exposed person may alter cell proliferation and result in benign or noncancerous tumors. Additional mutations may then cause malignant changes whereby a benign tumor becomes malig-
nant. Theoretically, radiation-induced mutations in a single somatic cell can eventually result in the cell and its progeny becoming malignant or cancerous; this progression is complex and depends on a variety of factors, only some of which have been characterized. On the basis of animal and epidemiologic studies, factors known to influence radiation induction of tumors include age at the time of exposure, sex, genetic background, and immune status; these host factors and other known factors are discussed in more detail in Chapters 4 and 7.
In the absence of definitive data, scientists generally assume that all types of cancers are susceptible to induction by ionizing radiation. However, animal and epidemiologic studies have shown some cancers to be more likely to have been caused by radiation than others. Various types of cancer grouped by the strength of their statistical association with radiation and available risk estimates obtained in analyses of data from epidemiologic studies of populations at risk of exposure are shown in Table 3.6.
The time between the induction of any disease and its clinical detection or diagnosis is known epidemiologically as the latent period. Because we do not know precisely when a tumor is induced after a radiation exposure, the latent period of a radiation-induced tumor in an exposed person generally is taken to be the time between exposure and detection or diagnosis of the tumor. On the basis of epidemiologic data, the minimum latent periods for radiation-induced leukemia and most solid cancers usually are taken to be about 2 years and 10 years, respectively. For thyroid and bone cancers, the minimum latent periods are estimated to be about 5 years. Age-at-exposure and the magnitude of the radiation dose have been shown in epidemiologic studies to influence the latent periods of some specific tumor types that have been causally associated with radiation exposure.
The relative risk (RR) of developing leukemia (all types except CLL) after radiation exposure appears to rise to a plateau about 15 years after exposure and then about 25 years after exposure to begin a gradual decline toward the risk in the general, or nonexposed, population. The RRs for solid cancers appear to increase to a plateau at about 25 years after exposure and to remain at that level for an extended period—possibly for life, depending on the type of cancer.
Radiogenic cancers, cancers that can be attributed to radiation exposure, are histopathologically and clinically indistinguishable from spontaneous, or naturally occurring, cancers in nonexposed populations. As is discussed later in this chapter, attribution of cancer in general or of specific cancer types to radiation therefore must depend on the observation of statistical differences between their frequencies in populations exposed and those not exposed to radiation (other than background exposures).
When a specific type of cancer is described as radiogenic it does not mean that every cancer of that type was caused by radiation; rather, it means that it is a type of cancer that has been statistically associated with radiation exposure in studies of exposed populations. Similar findings for a specific cancer type in
TABLE 3.6 Susceptibility of Cancers to Induction by Radiation, Grouped by Strength of Association with Radiationa
several epidemiologically valid population studies confirm the association as causal.
Most populations for which strong associations have been found between increased risks of specific cancers and radiation, were exposed to moderate to high doses of radiation at high dose rates. The findings of updated studies of several of those populations are discussed in Chapter 4.
Heritable Genetic Effects. Radiation-induced mutations in the reproductive cells of exposed people may lead to increases in the risk of genetic diseases in their children or descendants. That effect was observed originally in Drosophila (fruit flies) by Muller (1928) and later in other animal species (Russell et al., 1960). However, in the absence of measurable increases in the risk of genetic diseases in the offspring of the atomic-bomb survivors (Schull et al., 1981), estimates of the risk of radiation-induced heritable genetic effects in humans are based largely on data from laboratory animal studies (UNSCEAR, 2001).
RADIATION EPIDEMIOLOGY
Much of what is known about the long-term effects of ionizing radiation in humans has been learned from epidemiologic studies of exposed populations at risk of exposure. Epidemiology is the study of determinants and distributions of disease in human populations. Humans cannot be exposed to radiation under the same conditions as are used in experimental studies or clinical trials. Thus, investigators who want to conduct studies of radiation effects on human health have had to take advantage of situations in which groups or populations of humans have already been exposed to radiation under conditions over which the investigators have had no control. Such situations are described as natural experiments, and the studies are described as observational. Radiation-epidemiology studies conducted to date have involved populations previously exposed to radiation accidentally, in military operations, medically, or occupationally.
Like epidemiologic studies in general, radiation-epidemiology studies can be “descriptive” or “analytic.” Descriptive studies are conducted to generate hypotheses to evaluate cause-effect relationships as a basis for risk estimation. Analytic studies can then be used to test the hypotheses and to estimate exposure-specific risks of disease or death. Radiation epidemiology’s primary objective is to estimate risks as related to radiation exposure or doses, and its risk estimates take several forms.
Epidemiologic Study Designs
The epidemiologic studies discussed in later chapters are of several types. It is important to understand the strengths and limitations of each type of study design in evaluating the relative importance of studies. The four types of epidemiologic studies commonly used in radiation research are cohort, case-control, cross-sectional, and ecologic designs. We briefly describe the designs in decreasing order of importance and reliability.
Cohort Study
A cohort study is an observational study of a defined group of people who are followed for the purpose of comparing outcomes (usually death or disease)
between exposed subsets and unexposed or low-exposed subsets. These studies generally involve large samples, compare multiple outcomes (different causes of death or disease), are the least susceptible to bias, and examine changes in outcome patterns over time, so they are considered the most informative studies. When individual exposures or doses are available, cohort studies typically use a dose-response model and report estimates of relative or absolute risk as a function of exposure or dose. When individual estimates of dose are not available, mortalities often compared with that in an external referent population, usually the US or state rates, and the result is a standardized mortality ratio (SMR). When incidence is used, the analogous metric is the standardized incidence ratio (SIR). Although the cohort study is often described as prospective, there are also retrospective or historical cohort studies where the cohort and its exposures are defined from records. True prospective studies are generally preferable because all data regarding cohort characteristics needed for the eventual analysis can be collected at the beginning of the study, and changes in subject characteristics are added as they occur. This design, however, is expensive, and it generally takes a long time for cases to accumulate, particularly in a cancer study. The atomic-bomb survivors study and the Colorado Plateau Uranium Miners study are examples of prospective cohort studies.
The historical cohort has the advantage that a cohort is identified many years in the past and is followed forward from that time with existing records. This type of cohort study can be completed in much less time and for less money, but with much the same research strength as the true prospective study. The studies of workers exposed to radiation while engaged in nuclear-weapons development in Department of Energy facilities are examples of historical, or retrospective, cohort studies. The disadvantage of this study is that exposure records not originally designed for epidemiologic research must be used to estimate exposures or doses for individual cohort members.
Case-Control Study
A case-control study is an observational study that identifies the persons with a disease of interest (cases), such as lung cancer, and a suitable control group without the disease. Comparisons are made between cases and controls with respect to their exposure. The comparisons are calculated as odds ratios (OR) which are discussed in the next section. If the disease is rare, these studies provide an unbiased estimate of the RR with a major gain in efficiency over a cohort study because far fewer subjects who do not develop the disease of interest are needed to obtain an estimate of RR. Another advantage is that case-control studies are retrospective (cases occurred in the past), whereas cohort studies are often prospective so that the investigator must wait for cases to occur. Accordingly, case-control studies can generally be done much more quickly than prospective cohort studies. Case-control studies have two major disadvantages: they
can be used to study only one disease at a time, and, when the disease is not rare, the OR overestimates the RR that would have been obtained with a cohort study (unless it is corrected).
Cross-Sectional Study
A cross-sectional study is an observational study in which exposure and disease are determined simultaneously at one time. This design is also referred to as a prevalence study. When the study is designed primarily to estimate the association of the presence or absence of disease and exposure to a hazardous agent, the risk measure most commonly used is the prevalence OR. The principal disadvantage of the cross-sectional design is that temporal order of exposure and disease cannot be determined; therefore, causal relationships cannot be determined, only associations between disease and exposure. Another weakness of this design is that the duration of the disease has a substantial effect on the relationship between the prevalence and the incidence of the disease. The prevalent cases may not be representative of all cases that developed in the population over some period, either because the condition is detectable for only a short time, or because it is lethal and affected persons are removed from the study by premature death.
Ecologic Study
The least reliable study for estimating risk posed by an exposure is the ecologic study. An ecologic study is an investigation of the association of a disease and an exposure in groups. For example, Cohen (Cohen, 1995) has attempted to link average radon exposures in US counties to county lung-cancer rates. Using an ecologic design, he has reported negative exposure-response relationships; that is, the higher the county radon average, the lower the lung-cancer rate. The problem with ecologic studies is that they cannot determine whether the people who have the disease also had higher exposures. This error in ascribing to the members of a group characteristics that they do not have is known as the ecologic fallacy. These studies are often highly biased, so the committee has chosen to avoid citing them in discussions of radiation risk estimates.
Measures of Risk
Relative Risk
The term relative risk (RR) is used in several ways in epidemiologic studies. In general, RR is the ratio of the risk of disease or death among the exposed population to the risk of disease among the unexposed. In practice, RR can be
estimated in a number of ways, depending primarily on study design. In a cohort study, which compares the overall or cause-specific mortality with that in an external referent population, such as the US population, the estimate of average RR is often the SMR. The SMR is the ratio of the number of observed deaths due to some disease to the number expected in the referent population after stratification on such factors as age, calendar year, race, and sex. The SMR is usually reported as a summary RR without regard to individual exposure levels. The summary ratio is reported as either the ratio of the observed to the expected number of deaths or as that ratio multiplied by 100. Since this inconsistency may be confusing to the reader of the committee’s literature review, we will adopt the convention of reporting the SMR as a ratio, regardless of the metric used by the author(s). When mortality or morbidity is examined in a cohort study with RR models, the RR is estimated on the basis of the ratio of mortality or incidence among those exposed to a given level of exposure to that among the unexposed or low-exposed group. In such analyses, the referent group is often a subset of the cohort (internal comparison).
In case-control studies or cross-sectional studies, the RR is usually estimated on the basis of the OR. The OR is defined as the ratio of the odds of an event in one group to the odds of the event in another group. The odds is the number of times (or proportion) that the event occurred divided by the number of times (or proportion) that it did not occur. That is in contrast with a probability, which is the number of times the event occurred divided by the total number of times that it could have occurred. The OR is an accurate estimate of the RR when the disease or condition being studied is rare in both the exposed and unexposed populations, for example, when the incidence or prevalence is less than 10%. When the prevalence is high, the OR will overestimate the RR when the RR is over 1 and underestimate the RR when the RR is less than 1. In this report, the cancers and diseases are sufficiently rare that any cited OR is considered to be a good estimate of RR.
Although the term relative risk is sometimes used in a general sense, in this report the committee attempts to specify the type of RR estimate for any study cited.
Absolute Risk
Absolute risk (AR) is the expression of risk in terms of the proportion of persons with disease in some defined population or the number of cases of the disease in a population of some defined size. In epidemiologic studies, AR is often discussed in terms of the difference in risks or rates, in contrast with RR which is a ratio of risks or rates. AR is generally reported as either a difference in proportions (difference in percentages) or a difference in rates (difference in number of cases per population size per unit time). Understanding the nature of those various measures of comparative risk is important for comprehending epi-
demiologic study results. When a disease is relatively rare, the RR can be large and the AR reasonably small. For example, if the annual baseline rate of leukemia is 1 per 10,000 persons and the rate among a radiation-exposed population is 2 per 10,000, the RR is 2.0, but the AR is only one additional case per 10,000 persons or 0.0001 (10−4). In contrast, common diseases may appear to have a low RR in an epidemiologic study, but the number of additional cases may be large. For example, a study of cardiovascular mortality may report a RR of 1.2, which appears to be rather small, but the AR (often not reported) may indicate hundreds of excess deaths in a large study population.
Excess Relative Risk vs Excess Absolute Risk
In discussing the results of epidemiologic studies, the terms excess relative risk (ERR) or excess absolute risk (EAR) are often useful. These are estimates of the amount of risk due to the exposure of interest when the effects of all other exposures are removed. When RR estimates are used, ERR is defined as
When AR estimates are used, EAR is defined as
where AR1 = total number of observed deaths or cases of disease per population at risk in a specified period, and AR0 = number of deaths or cases of disease in the unexposed population at risk in the same period.
For example, if the estimated RR is 2.5 for lung cancer in a group of uranium miners, their ERR is 2.5 − 1 = 1.5. If the AR is 200 deaths from leukemia per 10,000 persons up to the age of 70 years in a group exposed to external radiation and AR0 is 150 per 10,000 persons up to the age of 70 years among those exposed only to background radiation, then
EAR = 200 − 150 = 50 additional leukemia deaths per 10,000 persons up to the age of 70 years
Most radiation studies have reported their results in ERR, but the importance of EAR in complementing the ERR estimates has been widely recognized (Preston et al., 2003). The latest publications of risk estimates for the atomic-bomb survivors report both types of estimates. In considering the results of radiation studies cited in Chapters 5 and 6, it is useful to examine how different the patterns of ERR and EAR can be in the same population. Figures 3.2 and 3.3 show hypothetical examples of patterns in lung-cancer rates by age. Lung cancer mortality in the US population in 1990-1994 by age at death was used as the referent population. Figure 3.2 illustrates a hypothetical situation where the number of additional lung-cancer deaths due to radiation (EAR) was a constant
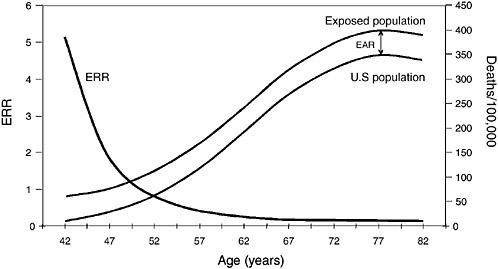
FIGURE 3.2 Lung-cancer decreasing ERR vs constant EAR.
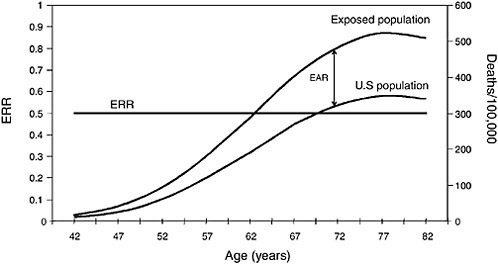
FIGURE 3.3 Lung-cancer constant ERR vs increasing EAR.
50 deaths per 10,000 at each year of age. The background (overall US lung-cancer death rate) rises sharply with advancing age, so the constant-EAR scenario results in ERRs that decrease rapidly with age. In contrast, Figure 3.3 illustrates a hypothetical situation where ERR is a constant 0.50; that is, RR is 1.5 for all ages above 40 y, before which lung cancer is extremely rare. In this scenario, EAR would rise dramatically with age because the excess number of
deaths would have to increase rapidly to maintain a 50% increase above the increasing background rate. That is indicated by the widening gap between the US rate and the exposed-population rate in Figure 3.3.
It is important to understand the relationship between ERR and EAR because many of the studies discussed in later chapters report that ERR decreases with age. Few studies report both ERR and EAR, so it is often impossible to discuss both types of estimates when describing study results in later chapters. The decreasing ERR estimates, however, do not necessarily imply that EAR is also decreasing with age. As can be seen by examining Figures 3.2 and 3.3, a decreasing ERR could be present in a population in which EAR is constant or even increasing with age. In short, when the mortality rate in the unexposed population rises with age, as is the case with most health outcomes, a constant ERR results in an increasing EAR, while a constant EAR implies a decreasing ERR.
Mortality and Incidence Studies
Many of the studies discussed in this report use death from a particular cancer as the end point. Virtually all lung-cancer and pancreatic-cancer studies are mortality studies, because these cancers are often rapidly fatal. Few incident cases of pancreatic and lung cancer are missed in a longitudinal study. In general, mortality studies provide a good estimate of disease incidence when the case-fatality rate is high and the duration of the disease is relatively short. For diseases that are not commonly fatal, the most useful end point is incidence (diagnosis) of the disease. An example of the latter type of disease in radiation research is thyroid cancer. A mortality study of thyroid cancer could be biased and have marginal statistical power because most of the incident cases would be missed.
Cancer-incidence studies measure directly the rate at which cancers occur in a population over time, so they would seem to be the design of choice. There are difficulties, however, in conducting cancer-incidence studies in the United States. To conduct an incidence study, one has to be able to identify each new diagnosis of the disease in the study population. That is possible only in states where cancer registries are available and have been in place throughout the period of study. Because many states do not have registries or the registries are relatively new, cancer-incidence studies are often difficult to implement. Even if a study is done in a state with an existing registry, cases may be missed because of emigration to states without registries. Accordingly, mortality studies are more commonly used in the United States because of the ease of identifying cases nationwide through the National Death Index.
Uncertainty in Risk Estimates
Risk estimates are based on limited information that reflects a lack of perfect knowledge concerning the factors used to calculate the estimate. For
that reason, risk estimates are not always precise and always have an element of uncertainty.
Uncertainty in a risk estimate may be the result of the amount of data available, the quality of the data, or both. For example, in an epidemiologic study of the relationship between radiation dose from an identified source and disease frequency, little information might be available on the doses that people received. In addition, the number of exposed persons in a study might be too small to produce precise risk estimates. Each of those situations increases the uncertainty of the estimates of risk per unit dose that investigators can develop.
Uncertainty Intervals and Confidence Intervals
Because of the uncertainty in risk estimates that are made for different radiation dose levels, scientists often include an interval surrounding a risk estimate. The intervals can be confidence intervals or uncertainty intervals. Confidence intervals provide an upper and lower bound on the point estimate of risk that accounts for sampling variability that causes error in the point estimate. For example, a 95% confidence interval (CI) associated with an estimate of RR from a particular study means that if a study of the same size and age and dose distributions were replicated 100 times, we would expect 95 of the intervals to contain the true RR and 5 not to. The width of a confidence interval decreases with increasing sample size.
Uncertainty intervals are much wider because they attempt to account for the uncertainty in all the factors that are used to estimate the risk. These intervals are also referred to as credibility intervals, and Bayesian methods are often used to calculate the credibility bounds. That is done by specifying uncertainty distributions for all risk factors and then drawing random samples from this family of distributions in a Monte Carlo analysis using hundreds or thousands of realizations. The credibility interval is then defined by percentiles of the Monte Carlo sample.
Challenges for Studies on Radiation Health Effects
Several challenges arise in designing, conducting, and evaluating studies of possible links between radiation exposure and specific illnesses in a selected population. They include the following
-
Health effects can have causes other than radiation exposure. Although studies have established a strong link between radiation and some types of cancer, radiation is not the only cause of these cancers. Lung cancer is a good example: smoking is a stronger risk factor than all but the highest radon exposures in miners.
-
Generally, dose estimates are not available for individuals. Without accurate estimates, assessing reliably whether a detected health problem is connected directly to radiation exposure is difficult.
-
People are exposed to a variety of radiation sources. In addition to specifically identified radiation exposures, people are exposed to natural sources of radiation and radiation from medical and dental procedures, consumer goods (such as tobacco products), and fallout from global nuclear-weapons testing.
People are different, and their circumstances change. People eat different foods, have different lifestyles, and change their habits as they age. All those factors, and many others can directly or indirectly influence the probability of radiogenic disease in individuals and populations.
-
Health effects of low-level radiation exposure cannot be detected immediately. The delay between the time of exposure and the time when a health effect occurs can be long. That period of time, the latent period, varies among diseases and among individuals. For leukemia, the latent period can be as short as about 2-5 years. Thyroid cancer generally takes at least 5 years to grow enough to be diagnosed. Most thyroid cancers would be expected to appear within 10-20 years after exposure; in some people, the delay could be much longer.
Derivation of Radiation Risk Estimates
General approaches for estimating health risks (most specifically cancer risks) posed by exposure to ionizing radiation have been developed by ICRP and in the United States by the National Council on Radiation Protection and Measurements (NCRP). Those and other bodies have used the risk estimates as input data to develop dose limits for radiation-protection purposes (ICRP, 1991; NCRP, 1997).
The epidemiologic data that have been used for estimating tumor risks posed by low-dose exposures (a few mSv) have been obtained for much higher doses (10s of mSv) as well. The sources of the data are survivors of the atomic-bombs at Hiroshima and Nagasaki and people exposed occupationally and medically.
To use those data on medium to high doses to estimate responses at much lower doses, scientists have developed extrapolation methods. The generally accepted model for such an extrapolation for solid tumors is the linear nonthreshold (LNT) model (NCRP, 2001), which assumes that from very low doses to much higher doses the stochastic cancer response is linear and that there is no radiation dose that poses no risk of inducing cancer, however small it might be. The best fit curve using the data for solid tumors at high and intermediate doses for the atomic-bomb survivors is linear and the extrapolation to low doses is considered to be conservative such that it does not underestimate the low-dose risk (Pierce and Preston, 2000). A similar conclusion holds when the new dosimetry system of the atomic-bomb survivors, DS02, is used (Preston et al., 2004).
Given the relative paucity of human data and the extrapolation approaches used, some degree of uncertainty is inevitable in the risk estimates calculated. For leukemia, the dose-response curve based on the atomic-bomb survivor data is nonlinear over the dose range studied. In general terms, the slope of the curve increases as the dose increases—an upwardly curving response (Preston et al., 1994).
The slope of the line that is the best fit to the total solid-cancer mortality data on the Japanese atomic-bomb survivors in the Life Span Study (LSS) has been used to derive the risk coefficient for solid cancers in an exposed population (ICRP, 1991). The coefficient is presented as fatal cancers per 10,000 persons per Sv of external radiation; we discuss this more fully below. A similar approach has been used for developing risk coefficients for specific tumor types, and these values are presented in Table 3.7. An effort as part of the LSS is under way to develop a similar set of risk coefficients for cancer incidence.
The total risk in a population exposed to external radiation is generally considered in terms of a population of working age (adults) and the whole population (adults and children). For a population of working age composed of both sexes, the lifetime risk of death from cancer is 8 × 10−2 per sievert for high doses and high dose rates of external radiation and 4 × 10−2 per sievert for low doses and low dose rates (ICRP, 1991). The comparable values for the whole population are slightly higher because of the increased sensitivity of young persons: they are 10 × 10−2 per sievert for high doses and dose rates and 5 × 10−2 per sievert for low doses and dose rates. Those values need to be considered in the context of a background lifetime risk of about 15% of dying as a result of one of the cancers listed as being radiogenic in RECA.
TABLE 3.7 Contributions of Organs to Total Cancer Risk for Populationa
The need to conduct epidemiologic studies for low exposure levels (in the range of a few mSv) is clear. Such work can yield a more direct measure of the risk posed by low doses of radiation. Such low-dose studies have been conducted with cohorts of workers involved in the development of nuclear weapons in the United States and several other countries.
In addition to the risk estimates described above for external radiation exposure, scientists have developed separate estimates for specific exposure conditions. For example, the risk of lung cancer from radon exposure (for example, from uranium mining) has been calculated (NRC, 1999). Similar approaches have also been used to calculate the thyroid-tumor risk from exposure to 131I (discussed in NCRP, 1999, pages 155-162).
In general, risk assessments for deterministic noncancer effects (effects resulting from tissue injury) are estimated on the basis of a threshold dose-response such that, over the range of several hundred mSv, the radiation-induced response does not differ from the response to background levels for any particular adverse health outcome (ICRP, 1991). Thus, for the range of doses estimated for the great majority of environmental and occupational exposures, no increase in deterministic noncancer effects is expected.
An informative general source that provides an overview of risk-assessment practices is Radiation Carcinogenesis (Hall, 1994, chapter 19).
Probability of Causation/Assigned Share
In 2003, the National Cancer Institute and the Centers for Disease Control and Prevention (NCI-CDC) produced an updated set of radioepidemiological tables that associate a given dose of ionizing radiation with the likelihood that exposure caused a given type of cancer (NCI-CDC, 2003). The tables make a broad set of assumptions of susceptibility and exposure that are applied more properly to a subpopulation than to an individual. Such an association has been termed the probability of causation (PC), although it expresses strength of association rather than a mechanism of causation. The tables generate PCs as a function of a person’s estimated dose, sex, age at exposure, and age at diagnosis. The tables are available as Web-based calculators for 22 specific cancers at http://irep.nci.nih.gov.
In an exposed population of people who have developed a specific type of cancer, PC is the ratio of their excess risk of that cancer to their overall risk of that cancer. More specifically,
The concept of PC has been applied to people (for example, in the Energy Employees Occupational Illness Compensation Program Act) in much the same way that clinical-prediction decision rules are routinely applied to individual
patients in the clinical arena. It differs, however, in that most clinical rules are applied prospectively to predict the likelihood of an outcome or event whereas the PC is applied retrospectively to analyze the likelihood that a known cancer was caused by radiation. In that respect it is akin to the statistical concept of attributable risk. In other words, clinical-prediction rules are conditioned on a set of factors and a prior probability to predict a future event whereas the PC is conditioned on the event’s having already occurred. In some sense, clinical predictions are a priori chances, whereas PCs are a posteriori chances. Both are probabilities, and both range from 0 to 1. In recent years, when these analytic likelihoods (PCs) have been applied to individuals, they have been called the assigned share (AS) of the risk, and the sum of all such assignments (base plus excess) equals unity. The PC/AS concept is developed in detail in Chapter 5.






























