F
SUPPLEMENTARY INFORMATION
LIST OF BOX, FIGURES, AND TABLES
|
Box F-1 |
Chronology of Statutes Pertaining to the Definition of WIC Supplemental Foods, |
|
Table F-1 |
Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Elements, |
|||
|
Table F-2 |
Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Vitamins, |
|||
|
Table F-3 |
Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Selected Macronutrients, |
|||
|
Table F-4 |
Dietary Reference Intakes and Other Dietary Guidance Used for Assessing Intakes of WIC-Eligible Subgroups, Selected Fats, |
|
BOX F-1 September 26, 1972: Public Law No. 92-433. The term supplemental foods is defined in the original WIC statute, Child Nutrition Act, as amended. § 17(f)(3): “Supplemental foods” shall mean those foods containing nutrients known to be lacking in the diets of populations at nutritional risks and, in particular, those foods and food products, containing high-quality protein, iron, calcium, vitamin A, and vitamin C. Such term may also include (at the discretion of the Secretary) any food product commercially formulated preparation specifically designed for infants. July 11, 1973: In what appears to be the first WIC rule (Fed Reg p. 18447): § 246.2(v): “Supplemental food” means any food authorized to be made available under the WIC program. October 7, 1975: Public Law No. 94-105. Child Nutrition Act §17(f)(3) is amended to include a new, final sentence: The contents of the food package shall be made available in such a manner as to provide flexibility, taking into account medical and nutritional objectives and cultural eating patterns. January 12, 1976: Interim “Revision, Reorganization, and Republication” (Fed Reg p. 1743) reads: § 246.2(t): “Supplemental foods” means the foods authorized by FNS in this part to be made available under the WIC program. August 26, 1977: Final “Revision, Reorganization, and Republication” (Fed Reg p. 43206) reads: § 246.2 (no “letter” designation): “Supplemental foods” means foods which meet the specifications of this part. November 10, 1978: Public Law No. 95-627, the Child Nutrition Amendments of 1978, completely revised Child Nutrition Act § 17. In the revision, definitions were moved to subsection (b), with supplemental foods found at § 17(b)(14). The reference to nutrients of particular interest was deleted and additional direction was included at (f)(11). § 17(b)(14): “Supplemental foods” means those foods containing nutrients determined by nutritional research to be lacking in the diets of pregnant, breastfeeding, and postpartum women, infants, and children, as prescribed by the Secretary. State agencies may, with the approval of the Secretary, substitute different foods providing the nutritional equivalent of foods prescribed by the Secretary, to allow for different cultural eating patterns. In subparagraph (f)(11): The Secretary shall prescribe by regulation the supplemental foods to be made available in the program under this section. To the de- |
|
gree possible, the Secretary shall assure that the fat, sugar, and salt content of the prescribed foods is appropriate. January 9, 1979: Proposed Rule, to comply with section 3 of Public Law No. 95-627 § 3 (beginning Fed Reg p. 2114) deletes the definition of supplemental foods (no explanation is provided for this change): § 246.2 (no “letter” designation): “Supplemental foods” [Reserved] July 27, 1979: Final Rule, to comply with Public Law No. 95-627 § 3 (beginning Fed Reg p. 44422): § 246.2 (no “letter” designation): “Supplemental foods” [Reserved]. July 8, 1983: Proposed Rule (beginning on Fed Reg p. 31502) issued to “reduce the regulatory burden on State and local agencies.” It states: A definition of “supplemental foods” was reserved in the 1979 regulations because of the pending issuance of the proposed food package Regulations. A definition consistent with the legislative definition and past regulatory definitions is proposed in this rulemaking. § 246.2 (no “letter” designation): “Supplemental foods” means those foods containing nutrients determined to be beneficial for pregnant, breastfeeding, and postpartum women, infants and children, as prescribed by the Secretary in section 246.10. November 10, 1989: Public Law No. 101-147. Child Nutrition and WIC Reauthorization Act of 1989 continues the statutory emphasis on providing nutrients for which WIC participants are most vulnerable to deficiencies and adds concern regarding nutrient density and how to effectively provide the priority nutrients June 30, 2004: Public Law No. 108-265. Child Nutrition and WIC Reauthorization Act of 2004 continues the statutory emphasis on nutrients that are lacking. It also adds language about foods to the definition, still at (b)(14), and adds material to (f)(11) without altering the sentences inserted in 1978. The new (b)(14) reads: (b)(14): “Supplemental foods” means those foods containing nutrients determined by nutritional research to be lacking in the diets of pregnant, breastfeeding, and postpartum women, infants, and children, and those foods that promote the health of the population served by the program authorized by this section, as indicated by relevant nutrition science, public health concerns, and cultural eating patterns, as prescribed by the Secretary. State agencies may, with the approval of the Secretary, substitute different foods providing the nutritional equivalent of foods prescribed by the Secretary, to allow for different cultural eating patterns. Child Nutrition Act § 17, includes the following relevant provisions in a paragraph primarily addressing state operations: “(f)(11) SUPPLEMENTAL FOODS— (A) IN GENERAL—The Secretary shall prescribe by regulation the supplemental foods to be made available in the program under this section. (B) APPROPRIATE CONTENT—To the degree possible, the Secretary shall assure that the fat, sugar, and salt content of the prescribed foods is appropriate.” |
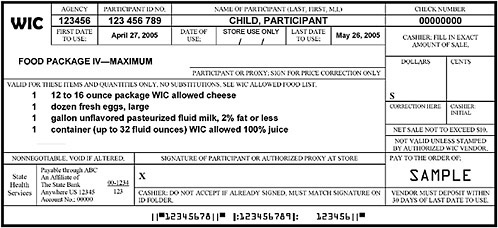
FIGURE F-1A Representation of a standard WIC food instrument (net sale not to exceed $10).
NOTE: This sample of a standard food instrument is one of set that would be issued to the participant with a sum total equal to the entire prescription of food per month for this child participant. Some WIC state agencies currently issue a series of standard food instruments to be used throughout the month. This representation was constructed using food instruments received from a number of WIC state agencies; this sample illustrates features drawn from various food instruments and does not reflect the food instruments issued by a specific WIC state agency.
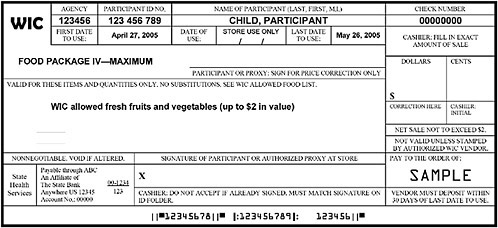
FIGURE F-1B Representation of a cash-value WIC food instrument (net sale not to exceed $2).
NOTE: This sample of a cash-value food instrument for fresh fruits and vegetables is one of a set that would be issued to the participant with a sum total cash-value of $8 per month for this child participant. Some WIC state agencies currently issue a series of standard food instruments to be used throughout the month. This example is representative of a WIC state agency in which food instruments are issued as four sets per month, easily accommodating participants who obtain groceries on an average of once per week. The cash-value voucher for fresh produce is a workable procedure in other scenarios; however, in situations were WIC foods are obtained on an average of once per month, the fresh fruit and vegetable option may not be optimal. In these situations, the processed fruit and vegetable option or a combination of the fresh and processed options may be more workable. From the committee’s discussion with representatives of grocery vendors, an important feature is that the fresh produce is obtained on a food instrument separate from other grocery items.
TABLE F-1 Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Elements
|
|
Dietary Component |
||
|
Participant Category |
Calcium (mg/d) |
Iron (mg/d) |
Zinc (mg/d) |
|
Infants, 0 through 5 mo |
|||
|
AI*a |
210* (breast-fed) 320* (formula-fed) |
0.27* |
2.0* |
|
UL |
NDb |
40.0 |
4.0 |
|
Infants, 6 through 11 mo |
|||
|
EAR |
— |
6.9 |
2.5 |
|
RDA or AI* |
270* (breast-fed) 340* (formula-fed) |
11.0 |
3.0 |
|
UL |
ND |
40.0 |
5.0 |
|
Children, 1 through 3 y |
|||
|
EAR |
— |
3.0 |
2.5 |
|
RDA or AI* |
500* |
7.0 |
3.0 |
|
UL |
2,500 |
40.0 |
7.0 |
|
Children, 4 y |
|||
|
EAR |
— |
4.1 |
4.0 |
|
RDA or AI* |
800* |
10.0 |
5.0 |
|
UL |
2,500 |
40.0 |
12.0 |
|
Females, 14 through 18 y |
|||
|
EAR |
— |
7.9 |
7.3 |
|
RDA or AI* |
1,300* |
15.0 |
9.0 |
|
UL |
2,500 |
45.0 |
34.0 |
|
Females, 19 through 30 y |
|||
|
EAR |
— |
8.1 |
6.8 |
|
RDA or AI* |
1,000* |
18.0 |
8.0 |
|
UL |
2,500 |
45.0 |
40.0 |
|
Females, 31 through 44 y |
|||
|
EAR |
— |
8.1 |
6.8 |
|
RDA or AI* |
1,000* |
18.0 |
8.0 |
|
UL |
2,500 |
45.0 |
40.0 |
|
Pregnant females, < 19 y |
|||
|
EAR |
— |
23.0 |
10.5 |
|
RDA or AI* |
1,300* |
27.0 |
12.0 |
|
UL |
2,500 |
45.0 |
34.0 |
|
Selenium (mcg/d) |
Magnesium (mg/d) |
Phosphorus (mg/d) |
Sodium (mg/d) |
Potassium (mg/d) |
|
15* |
30* |
100* |
120* |
400* |
|
45 |
NDb |
NDb |
NDb |
NDb |
|
— |
— |
— |
— |
— |
|
20* |
75* |
275* |
370* |
700* |
|
60 |
ND |
ND |
ND |
ND |
|
17 |
65 |
380 |
— |
— |
|
20 |
80 |
460 |
1,000* |
3,000* |
|
90 |
65c |
3,000 |
1,500 |
ND |
|
23 |
110 |
405 |
— |
— |
|
30 |
130 |
500 |
1,200* |
3,800* |
|
150 |
110c |
3,000 |
1,900 |
ND |
|
45 |
300 |
1055 |
— |
— |
|
55 |
360 |
1,250 |
1,500* |
4,700* |
|
400 |
350c |
4,000 |
2,300 |
ND |
|
45 |
255 |
580 |
— |
— |
|
55 |
310 |
700 |
1,500* |
4,700* |
|
400 |
350c |
4,000 |
2,300 |
ND |
|
45 |
265 |
580 |
— |
— |
|
55 |
320 |
700 |
1,500* |
4,700* |
|
400 |
350c |
4,000 |
2,300 |
ND |
|
49 |
335 |
1,055 |
— |
— |
|
60 |
400 |
1,250 |
1,500* |
4,700* |
|
400 |
350c |
3500 |
2,300 |
ND |
|
Selenium (mcg/d) |
Magnesium (mg/d) |
Phosphorus (mg/d) |
Sodium (mg/d) |
Potassium (mg/d) |
|
49 |
290 |
580 |
— |
— |
|
60 |
350 |
700 |
1,500* |
4,700* |
|
400 |
350c |
3,500 |
2,300 |
ND |
|
49 |
300 |
580 |
— |
— |
|
60 |
360 |
700 |
1,500* |
4,700* |
|
400 |
350c |
3,500 |
2,300 |
ND |
|
59 |
300 |
1,055 |
— |
— |
|
70 |
360 |
1,250 |
1,500* |
5,100* |
|
400 |
350c |
4,000 |
2,300 |
ND |
|
59 |
265 |
580 |
— |
— |
|
70 |
320 |
700 |
1,500* |
5,100* |
|
400 |
350c |
4,000 |
2,300 |
ND |
|
NOTES FOR TABLE F-1: AI = Adequate Intake, used when necessary, indicated by an asterisk (*); EAR = Estimated Average Requirement, used when available; ND = not determined, UL not determined due to lack of data of adverse effects RDA = Recommended Dietary Allowance; UL = Tolerable Upper Intake Level. DATA SOURCES: Institute of Medicine (IOM, 1997, 2000b, 2001, 2005a) (see IOM, 2005b). |
||||
TABLE F-2 Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Vitamins
|
|
Dietary Component |
||
|
Participant Category |
Vitamin Aa (mcg/d) |
Vitamin D (mcg/d) |
Vitamin Eb (mg AT/d) |
|
Infants, 0 through 5 mo |
|||
|
AI* |
400* |
5* |
4* |
|
UL |
600e |
25 |
ND |
|
Infants, 6 through 11 mo |
|||
|
AI* |
500* |
5* |
5* |
|
UL |
600e |
25 |
ND |
|
Children, 1 through 3 y |
|||
|
EAR |
210 |
— |
5 |
|
RDA or AI* |
300 |
5* |
6 |
|
UL |
600e |
50 |
200 |
|
Children, 4 y |
|||
|
EAR |
275 |
— |
6 |
|
RDA or AI* |
400 |
5* |
7 |
|
UL |
900e |
50 |
300 |
|
Females, 14 through 18 y |
|||
|
EAR |
485 |
— |
12 |
|
RDA or AI* |
700 |
5* |
15 |
|
UL |
2,800e |
50 |
800 |
|
Females, 19 through 44 y |
|||
|
EAR |
500 |
— |
12 |
|
RDA or AI* |
700 |
5* |
15 |
|
UL |
3,000e |
50 |
1,000 |
|
Pregnant females, < 19 y |
|||
|
EAR |
530 |
— |
12 |
|
RDA or AI* |
750 |
5* |
15 |
|
UL |
2,800e |
50 |
800 |
|
Pregnant females, 19 through 44 y |
|||
|
EAR |
550 |
— |
12 |
|
RDA or AI* |
770 |
5* |
15 |
|
UL |
3,000e |
50 |
1,000 |
|
Lactating females, < 19 y |
|||
|
EAR |
885 |
— |
16 |
|
RDA or AI* |
1,200 |
5* |
19 |
|
UL |
2,800e |
50 |
800 |
|
Lactating females, 19 through 44 y |
|||
|
EAR |
900 |
— |
16 |
|
RDA or AI* |
1,300 |
5* |
19 |
|
UL |
3,000e |
50 |
1,000 |
|
a The EAR and AI for vitamin A are expressed as retinol activity equivalents (RAEs) per day. 1 RAE = 1 mcg retinol, 12 mcg -carotene, 24 mcg -carotene, or 24 mcg -cryptoxanthin. b The EAR and AI for vitamin E are expressed as mg -tocopherol (AT) per day. The EAR and AI for vitamin E include RRR--tocopherol, the only form of -tocopherol that occurs naturally in foods, and the 2R-stereoisomeric forms of -tocopherol that occur in fortified foods and dietary supplements. The UL for vitamin E applies to any form of -tocopherol used in dietary supplements or added to foods as a fortificant or antioxidant. Note that the CSFII data used elsewhere in this report were originally calculated as mg -tocopherol equivalents (ATE) per day, an older unit of measure for vitamin E. |
|||
|
Vitamin C (mg/d) |
Thiamin (mg/d) |
Riboflavin (mg/d) |
Niacinc (mg/d) |
Vitamin B6 (mg/d) |
Vitamin B12 (mcg/d) |
Folated (mcg/d) |
|
40* |
0.2* |
0.3* |
2* |
0.1* |
0.4* |
65* |
|
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
50* |
0.3* |
0.4* |
4* |
0.3* |
0.5* |
80* |
|
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
13 |
0.4 |
0.4 |
5 |
0.4 |
0.7 |
120 |
|
15 |
0.5 |
0.5 |
6 |
0.5 |
0.9 |
150 |
|
400 |
ND |
ND |
10 |
30.0 |
ND |
300 |
|
22 |
0.5 |
0.5 |
6 |
0.5 |
1.0 |
160 |
|
25 |
0.6 |
0.6 |
8 |
0.6 |
1.2 |
200 |
|
650 |
ND |
ND |
15 |
40.0 |
ND |
400 |
|
56 |
0.9 |
0.9 |
11 |
1.0 |
2.0 |
330 |
|
65 |
1.0 |
1.0 |
14 |
1.2 |
2.4 |
400f |
|
1,800 |
ND |
ND |
30 |
80.0 |
ND |
800 |
|
60 |
0.9 |
0.9 |
11 |
1.1 |
2.0 |
320 |
|
75 |
1.1 |
1.1 |
14 |
1.3 |
2.4 |
400f |
|
2,000 |
ND |
ND |
35 |
100.0 |
ND |
1,000 |
|
66 |
1.2 |
1.2 |
14 |
1.6 |
2.2 |
520 |
|
80 |
1.4 |
1.4 |
18 |
1.9 |
2.6 |
600f |
|
1,800 |
ND |
ND |
30 |
80.0 |
ND |
800 |
|
70 |
1.2 |
1.2 |
14 |
1.6 |
2.2 |
520 |
|
85 |
1.4 |
1.4 |
18 |
1.9 |
2.6 |
600f |
|
2,000 |
ND |
ND |
35 |
100.0 |
ND |
1,000 |
|
96 |
1.2 |
1.3 |
13 |
1.7 |
2.4 |
450 |
|
115 |
1.4 |
1.6 |
17 |
2.0 |
2.8 |
500 |
|
1,800 |
ND |
ND |
30 |
80.0 |
ND |
800 |
|
100 |
1.2 |
1.3 |
13 |
1.7 |
2.4 |
450 |
|
120 |
1.4 |
1.6 |
17 |
2.0 |
2.8 |
500 |
|
2,000 |
ND |
ND |
35 |
100.0 |
ND |
1,000 |
|
c The AI for infants 0 through 5 months is expressed as preformed niacin (not niacin equivalents, NE).The EAR and AI for niacin for individuals above the age of 5 months are expressed as niacin equivalents (NE) per day. 1 mg of niacin = 60 mg of tryptophan. The UL for niacin is in mg/d and applies to synthetic forms obtained from fortified foods or dietary supplements. d The EAR and AI for folate are expressed as dietary folate equivalents (DFE) per day. 1 DFE = 1 mcg food folate = 0.6 mcg of folic acid from fortified food or as a supplement |
||||||
|
consumed with food = 0.5 mcg of a supplement taken on an empty stomach. The UL for folate is expressed as mcg per day and applies to synthetic forms (i.e., folic acid) obtained from fortified foods or dietary supplements. e The UL applies only to preformed vitamin A (i.e., retinol). f In view of evidence linking folate intake with neural tube defects in the fetus, it is recommended that all women capable of becoming pregnant consume 400 mcg of folate as folic acid from fortified foods or supplements in addition to intake of food folate from a varied diet. NOTES FOR TABLE F-2: AI = Adequate Intake, used when necessary, indicated by an asterisk (*); AT = -tocopherol; DFE = dietary folate equivalents; EAR = Estimated Average Requirement, used when available; ND = not determined, UL not determined due to lack of data of adverse effects; RDA = Recommended Dietary Allowance; UL = Tolerable Upper Intake Level. DATA SOURCES: Institute of Medicine (IOM, 1997, 1998, 2000b, 2001) (see IOM, 2005b). |
TABLE F-3 FOLLOWS
TABLE F-3 Dietary Reference Intakes Used for Assessing Intakes of WIC-Eligible Subgroups, Selected Macronutrients
|
|
Dietary Component |
||
|
Participant Category |
Food Energy (kcal/d) |
Protein (% of food energy) |
|
|
Infants, 0 through 5 mo |
|||
|
EER or AI* |
570 (3 mo M) 520 (3 mo F) |
ND |
|
|
Infants, 6 through 11 mo |
|||
|
EAR |
|
|
|
|
EER, RDA, or AI* |
743 (9 mo M) 676 (9 mo F) |
ND |
|
|
Children, 1 through 3 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
1046 (2 y M) 992 (2 y F) |
5–20† |
|
|
Children, 4 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
1742 (6 y M) 1642 (6 y F) |
10–30† |
|
|
Females, 14 through 18 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2368 (16 y) |
10–30† |
|
|
Females, 19 through 44 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2403 (19 y) |
10–35† |
|
|
Pregnant females, < 19 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2368 (1st trimester) 2708 (2nd trimester) 2820 (3rd trimester) |
10–30† |
|
|
Pregnant females, 19 through 44 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2403 (1st trimester) 2743 (2nd trimester) 2855 (3rd trimester) |
10–35† |
|
|
Lactating females, < 19 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2698 (1st 6 mo) 2768 (2nd 6 mo) |
10–30† |
|
|
Lactating females, 19 through 44 y |
|||
|
EAR |
|
|
|
|
EER, RDA, AI* or AMDR† |
2733 (1st 6 mo) 2803 (2nd 6 mo) |
10–35† |
|
|
Total Carbohydrate (% of food energy) |
Total Fat (% of food energy) |
Proteina (g/d) |
Total Carbohydrate (g/d) |
Added Sugarsb (% of food energy) |
Fiber, total dietary (g/d) |
|
ND |
55‡ (31 g/d*) |
9.1* |
60* |
<25 |
ND |
|
ND |
40‡ (30 g/d*) |
11.0 |
95* |
<25 |
ND |
|
|
|
|
100 |
|
|
|
45–65† |
30–40†c |
13.0 |
130 |
<25 |
19* |
|
|
|
|
100 |
|
|
|
45–65† |
25–35† |
19.0 |
130 |
<25 |
25* |
|
|
|
|
100 |
|
|
|
45–65† |
25–35† |
46.0 |
130 |
<25 |
26* |
|
|
|
|
100 |
|
|
|
45–65† |
20–35† |
46.0 |
130 |
<25 |
25* |
|
|
|
|
135 |
|
|
|
45–65† |
25–35† |
71.0 |
175 |
<25 |
28* |
|
|
|
|
135 |
|
|
|
45–65† |
20–35† |
71.0 |
175 |
<25 |
28* |
|
|
|
|
160 |
|
|
|
45–65† |
25–35† |
71.0 |
210 |
<25 |
29* |
|
|
|
|
160 |
|
|
|
45–65† |
20–35† |
71.0 |
210 |
<25 |
29* |
|
a The Dietary Reference Intakes (DRIs) for protein include an AI of 1.52 g/kg body weight/d for infants age 0 through 5 months and EARs of 1.2 g/kg body weight/d for infants age 6 through 11 months, 0.87 g/kg body weight/d for children ages 1 through 3 years, 0.76 g/kg body weight/d for children ages 4 through 8 years, 0.71 g/kg body weight/d for adolescent women (nonpregnant, nonlactating) ages 14 through 18 years, and 0.66 g/kg body weight/d for adult women (nonpregnant, nonlactating) ages 19 through 50 years. The EAR for protein intake per day is 0.88 g/kg body weight plus 21 g for pregnant women of all age groups and 1.05 g/kg body weight plus 21.2 g for lactating women of all age groups (IOM, 2002/2005). b The DRI reports establish some dietary guidance for macronutrient intake beyond the AMDR. Part of this dietary guidance is that added sugars be limited to no more than 25% of total energy intake (IOM, 2002/2005). c The American Academy of Pediatrics recommends that whole milk, rather than reduced fat milk, be consumed by children ages 13 through 23 mo (AAP, 2004). Dietary guidance from AAP to avoid atherogenic diets during childhood were applied to children 2 years of age and older (AAP, 1992b, 1998). The AAP recommendations, when taken out of context, might be interpreted that there should be no restriction of fat intake for children age 1 y. However, the AAP recommendation is not in conflict with the DRI reports that recommend a transitioning of dietary fat from the high fat diet of infancy (55% of energy from fat for ages 0 through 5 mo; 40% of energy from fat for ages 6 through 11 mo) to the moderate fat diet of childhood (25 to 35% of energy from fat) (IOM, 2002/2005). Thus it is appropriate to follow the AMDR recommendations for dietary fat to contribute 30 to 40% of food energy intake for children ages 13 through 23 mo (IOM, 2002/2005). NOTES FOR TABLE F-3: AI = Adequate Intake, used when necessary, indicated by an asterisk (*); AMDR = Acceptable Macronutrient Distribution Range, indicated by a dagger (†); EAR = Estimated Average Requirement, used when available; EER = Estimated Energy Requirement; F = female; kcal = kilocalories; M = male; ND = not determined; RDA = Recommended Dietary Allowance. An AMDR for total fat has not been set for infants; however, the AIs for total fat (indicated by an asterisk (*) represent a high fat diet as indicated by the usual intake of total fat as the percentage of food energy intake for breast-fed infants (indicated by a double dagger [‡]). DATA SOURCES: The American Heart Association (Krauss et al., 1996; AHA, 2004); and the Institute of Medicine (IOM, 2002/2005) (see IOM, 2005b). |
TABLE F-4 FOLLOWS
TABLE F-4 Dietary Reference Intakes and Other Dietary Guidance Used for Assessing Intakes of WIC-Eligible Subgroups, Selected Fats
|
|
Dietary Component |
|
|
Participant Category |
Total Fat (% of food energy) |
Saturated Fata (% of food energy) |
|
Infants, 0 through 5 mo |
||
|
AI* |
55‡ (31 g/d*) |
<10 |
|
UL |
ND |
ND |
|
Infants, 6 through 11 mo |
||
|
AI* |
40‡ (30 g/d*) |
<10 |
|
UL |
ND |
ND |
|
Children, 1 through 3 y |
||
|
AI* or AMDR† |
30–40†g |
<10 |
|
UL |
ND |
ND |
|
Children, 4 y |
||
|
AI* or AMDR† |
25–35† |
<10 |
|
UL |
ND |
ND |
|
Females, 14 through 18 y |
||
|
AI* or AMDR† |
25–35† |
<10 |
|
UL |
ND |
ND |
|
Females, 19 through 44 y |
||
|
AI* or AMDR† |
20–35† |
<10 |
|
UL |
ND |
ND |
|
Pregnant females, < 19 y |
||
|
AI* or AMDR† |
25–35† |
<10 |
|
UL |
ND |
ND |
|
Pregnant females, 19 through 44 y |
||
|
AI* or AMDR† |
20–35† |
<10 |
|
UL |
ND |
ND |
|
Lactating females, < 19 y |
||
|
AI* or AMDR† |
25–35† |
<10 |
|
UL |
ND |
ND |
|
Lactating females, 19 through 44 y |
||
|
AI* or AMDR† |
20–35† |
<10 |
|
UL |
ND |
ND |
|
aThe dietary guidance for saturated fat presented in Table F-1D is from the American Heart Association (Krauss et al., 1996; AHA, 2004) and the Dietary Guidelines for Americans (USDA/DHHS, 2000; DHHS/USDA, 2005). The dietary guidance for saturated fat from the DRI report is to consume amounts as low as possible while consuming a nutritionally adequate diet (IOM, 2002/2005). bThe dietary guidance for monounsaturated fatty acids presented in Table F-1D is from the American Heart Association (Krauss et al., 1996). cThe AIs for n-6 fatty acids shown in Table F-1D are for linoleic acid (18:2, n-6). The AMDR for total n-6 fatty acids is 5 to 10% of food energy intake with at least 90% as linoleic acid and up to 10% from longer-chain n-6 fatty acids (IOM, 2002/2005). For n-6 |
||
|
Monounsaturated Fatty Acidsb (% of food energy) |
Polyunsaturated Fatty Acids (g/d) |
n-6 Fatty Acidsc (g/d) |
n-3 Fatty Acidsd (g/d) |
Trans Fatty Acidse |
Cholesterolf (mg/d) |
|
≤ 15 |
4.4* |
4.4* |
0.5* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
4.6* |
4.6* |
0.5* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
7.0* |
7.0* |
0.7* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
10.0* |
10.0* |
0.9* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
11.0* |
12.0* |
1.1* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
12.0* |
12.0* |
1.1* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
13.0* |
13.0* |
1.4* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
13.0* |
13.0* |
1.4* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
13.0* |
13.0* |
1.3* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
≤ 15 |
13.0* |
13.0* |
1.3* |
limit |
<300 mg |
|
ND |
ND |
ND |
ND |
ND |
ND |
|
polyunsaturated fatty acids, the first double bond from the methyl end is at the sixth carbon atom. dThe AIs for n-3 fatty acids shown in Table F-1D are for (alpha)-linolenic acid (18:3, n-3). The AMDR for total n-3 fatty acids is 0.6 to 1.2% of food energy intake with at least 90% as (alpha)-linolenic acid and up to 10% from longer-chain n-6 fatty acids (IOM, 2002/2005). For n-3 fatty acids, the first double bond from the methyl end is at the third carbon atom. eThe dietary guidance from the DRI report for trans fatty acids is to consume in amounts as low as possible while consuming a nutritionally adequate diet (IOM, 2002/2005). The term trans fatty acids refers to unsaturated fatty acids that contain at least one double bond in the |
|||||
|
trans configuration (that is, with carbon atoms on opposite sides of the longitudinal axis of the double bond). fThe dietary guidance for cholesterol presented in Table F-1D is from the American Heart Association (Krauss et al., 1996; AHA, 2004) and the Dietary Guidelines (USDA/DHHS, 2000; DHHS/USDA, 2005). The dietary guidance for cholesterol from the DRI report is to consume an amount as low as possible while consuming a nutritionally adequate diet (IOM, 2002/2005). gThe American Academy of Pediatrics recommends that whole milk, rather than reduced fat milk, be consumed by children ages 13 through 23 mo (AAP, 2004). Dietary guidance from AAP to avoid atherogenic diets during childhood were applied to children 2 years of age and older (AAP, 1992b, 1998). The AAP recommendations, when taken out of context, might be interpreted that there should be no restriction of fat intake for children age 1 y. However, the AAP recommendation is not in conflict with the DRI reports that recommend a transitioning of dietary fat from the high fat diet of infancy (55% of energy from fat for ages 0 through 5 mo; 40% of energy from fat for ages 6 through 11 mo) to the moderate fat diet of childhood (25 to 35% of energy from fat) (IOM, 2002/2005). Thus it is appropriate to follow the AMDR recommendations for dietary fat to contribute 30 to 40% of food energy intake for children ages 13 through 23 mo (IOM, 2002/2005). NOTES FOR TABLE F-4: AI = Adequate Intake, used when necessary, indicated by an asterisk (*); AMDR = Acceptable Macronutrient Distribution Range, indicated by a dagger (†); ND = not determined; UL = Tolerable Upper Intake Level. An AMDR for total fat has not been set for infants; however, the AIs for total fat (indicated by an asterisk [*]) represent a high fat diet as indicated by the usual intake of total fat as the percentage of food energy intake for breast-fed infants (indicated by a double dagger [‡]). DATA SOURCES: The American Heart Association (Krauss et al., 1996; AHA, 2004); and the Institute of Medicine (IOM, 2002/2005) (see IOM, 2005b). |




















