Chemical Treatment of High-Level Waste for Utilization
Boris F. Myasoedov
Vernadsky Institute of Geochemistry and Analytical Chemistry
Russian Academy of Science
Russia is managing its irradiated nuclear fuel by reprocessing it and extracting useful isotopes. These activities will benefit the national economy as well as minimize the disposal of radioactive waste.
The RT-1 Plant at the Mayak Production Association was put into operation in 1976 within the framework of the Russian concept of preparation and transition to a closed fuel cycle. Over its 25 years of operation the RT-1 Plant has progressed from the trial reprocessing of a few kinds of nuclear fuel to the powerful and steady processing of a wide range of irradiated nuclear fuel from water-moderated water-cooled power (VVER-440), fast neutron (BN-600), transport, and research reactors. The RT-1 is the only pilot production plant in Russia that is able to rationally combine production and research activities to demonstrate the possibility of a closed nuclear fuel cycle. As a result of the development of an atomic industry, nuclear power engineering, and a nuclear powered fleet, a considerable amount of radioactive waste with total activity of about 1.5 × 109 Ci, and spent nuclear fuel (SNF) with total activity of about 4.65 × 109 Ci are accumulated in Russia at various enterprises belonging to different departments of the federal executive authority.
In order to achieve a closed fuel cycle, proper management of long-lived radionuclides contained in spent nuclear fuel, optimization of existing high-level waste reprocessing methods, and separation technologies for new and existing reprocessing plants have been studied over the last 20 years. These studies have been carried out at several Russian institutes (for example, the Khlopin Radium Institute, Institute of Chemical Technology, Institute of Physical Chemistry, and Institute of Geochemistry and Analytical Chemistry) in collaboration with the radiochemical plants of Mayak Production Association and the Mining-Chemi-
cal Association. Basic operation of the technology of separation of long-lived radionuclides involves the selective recovery from high-level waste of cesium, strontium, technetium, rare earth elements, and transplutonium elements, as well as the residues of uranium, neptunium, and plutonium remaining after the PUREX process. The objective of this presentation is to consider those developments that are now most feasible for application, are already in service at Mayak Production Association, or are soon to be introduced there.
FERROCYANIDE SORBENTS FOR CESIUM RECOVERY
An example of the successful use of sorption processes for recovery of radionuclides from high-level waste is the method of cesium recovery by inorganic ferrocyanide-containing sorbents developed by the Institute of Physical Chemistry in collaboration with Mayak Production Association.1 In practical application, preference was given to copper-nickel ferrocyanide, which has the highest stability in the cycle of sorption-desorption-regeneration. Systematic studies on the use of this sorbent enabled the development of technology for cesium recovery from high-level waste during SNF reprocessing. When testing the technology at Mayak Production Association, a 120 liter sorption column was used. The recovery degree of cesium at sorption stage was more than 98 percent; the cesium yield into desorbate was 98–99 percent; and the concentration ratio attained was 100 percent. After 15 cycles, the sorption properties of the sorbent remained unaffected. In the course of pilot industrial tests, about 7 millicuries of Cs were separated from high-level waste.
CROWN ETHERS FOR RECOVERY OF STRONTIUM AND CESIUM
A method using macrocyclic polyethers (crown ethers) has successfully been developed for strontium recovery from high-level waste. Specifically, an elaboration of the Institute of Chemistry’s technology using dicyclohexyl-18-crown-6 (DCH-6) was brought to the level of pilot industrial tests at Mayak Production Association. As a result of these tests, about 90 m3 of high-level waste were reprocessed and more than 0.5 mCi of radiostrontium were recovered with a sixfold degree of concentration.2 The degree of strontium recovery was 96 percent. A special advantage of the DCH-6 method is the simplicity and efficiency of conducting the strontium stripping operation using water.
RECOVERY OF CESIUM AND STRONTIUM USING CHLORINATED COBALT DICARBOLLIDE
Fundamental studies on the extraction of Cs and Sr by cobalt(III) dicarbollide have been carried out primarily in the Czech Republic. In Russia dicarbollide technology has been applied to high-level waste processing. Figure 1 shows the
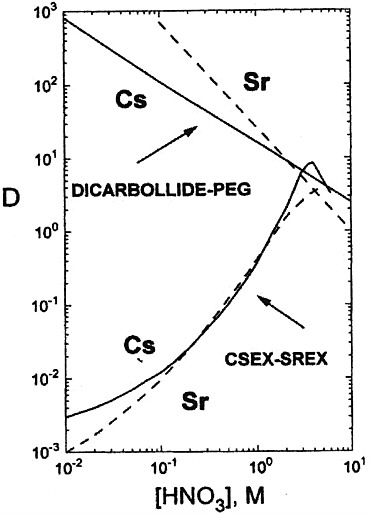
FIGURE 1 Nitric acid dependences of Cs and Sr extraction (D is the distribution coefficient) using dicarbollide and crown ether extractants (see Horwitz and Schulz, 1998). Dicarbollide (0.01M cobalt (III) dicarbollide and 0.01M PEG − 400 in nitrobenzene), CSEX-SREX (0.1 M Cs extractant − 0.005M Sr extractant − 1.2M TBP -Isopar-L − 5 vol percent lauronitrile).
nitric acid dependencies for the extraction of Sr and Cs using a 0.01 M solution of cobalt(III) dicarbollide and 0.01 M polyethylene glycol (PEG-400) in nitrobenzene. The flowsheet shows the combined recovery of cesium and strontium, as well as the extraction of barium and lead from high-level waste with HNO3. The resulting content has no more than 3.0 M and an overall content of nitrate-ion of no more than 5.0 M. The combined stripping of cesium and strontium is carried out by means of 5.0–6.0 M HNO3; the extractant is regenerated
TABLE 1 Industrial Operation Results of the UE-35 Separation Facility at Mayak Production Association
|
Operation Year |
Duration Operation, Months |
Characteristics of Reprocessed High-Level Waste |
|
|
Volume, m3 |
Total Activity, kCi |
||
|
1996 |
3 |
210 |
11,886 |
|
1998 |
2.5 |
95 |
6,539 |
|
1999 |
~1 |
62 |
1,498 |
|
2000 |
~3 |
254 |
6,156 |
|
2001 |
11 |
558 |
23,436 |
|
Total |
20.5 |
1,179 |
49,515 |
|
See Dzekun et al. (1991). |
|||
by a solution of 8 M HNO3, and 20 g/L hydrazine, which is then used for the stripping operation. This extraction mixture recovers more than 99.5 percent of the cesium and strontium. The most notable achievement in the use of the ChCoDiC process in Russia relates to the reprocessing of high-level waste of varying composition at Mayak Production Association.3 Using this technology, the first commercial facility in the world (UE-35) for the recovery of radionuclides began operation in August 1996. The first line of this facility was planned for the selective recovery of cesium and strontium from high-level waste. By the time UE-35 was put into operation in 13 storage tanks at Mayak Production Association there were 4315 m3 of highly salted aged wastes that cannot be subject to direct vitrification. The operating experience of UE-35 shows that three tanks with high-level waste can be reprocessed yearly. This will allow for the reprocessing of the most hazardous high-level waste of Mayak Production Association within several years (see Table 1).
RECOVERY OF ACTINIDES USING NEUTRAL ORGANOPHOSPHOROUS COMPOUNDS
Neutral bifunctional extractants have been studied in Russia for three to four years. Parallel investigations have been carried out in the United States.4 To extract TRU (transuranium) from high-level waste Russian chemists adopted a different carbamoylmethylphosphine oxide (CMPO) derivative from the one used in the United States. The TRUEX (transuranium extraction) process, namely, diphenyl-N, N-di-n-butyl CMPO, is abbreviated DPhDBCMPO. The diphenyl CMPO derivative is insufficiently soluble in paraffinic hydrocarbon diluents, even in the presence of excess TBP (tributyl phosphate), to be of practical use.
Diphenyl CMPO derivatives have a strong propensity toward third phase formation. However, Russian chemists have found that use of a fluoroether called Fluoropol-732 as a dilutent for the diphenyl CMPO eliminated the unfavorable solubility and third phase formation properties of this derivative. The DAm versus aqueous HNO3 concentration curve using a 0.05 M DPhDBCMPO solution in fluoropol-732 is shown in Figure 2. These data show that the values of DAm obtained with the DPhDBCMPO-fluoropol system are significantly higher, using only one-fourth the concentration of CMPO of those obtained with the TRUEX process solvent over the entire nitric acid concentration range. The Russian TRU extraction process uses a 0.1 M solution of DPhDBCMPO in
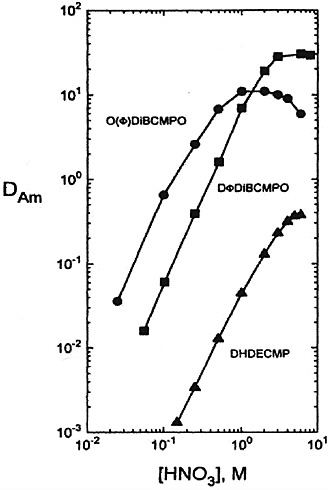
FIGURE 2 Comparison of octyl(phenyl)- and diphenyl-DiBuCMPO and DHDECMP in the presence of TBP at 25°C as extractants for Am(III) in nitric acid (see Horwitz and Schulz, 1998). 0.25M CMP or CMPO − 0.75M TBP − CCI4.
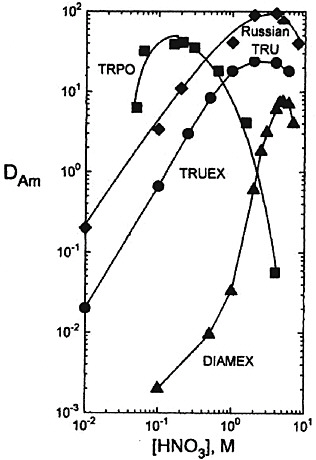
FIGURE 3 Comparison of TRUEX, Russian TRU, DIAMEX, and TRPO process solvents as extractants for Am(III) in nitric acid (see Horwitz and Schulz, 1998). TRUEX process solvent (0.02M O(0)DiBCMPO − 1.4M TBP − Conoco (C12–C14)), t=30°C. Russian TRU process solvent (0.05M D (ø) DBCMPO − − 1.4M TBP − Conoco (C12–C14)), t=30°C. Russian TRU process solvent (0.05M D (ø) DBCMPO -Fluoropol-732), t=23°C. DIAMEX process solvent (0.5M DMDBTDMA-TPH), t=25°C. TRPO process solvent (30 vol percent TRPO − kerosene), t=25°C.
fluoropol-732 as the process solvent (see Figure 3). An 18-stage bank of centrifugal contactors was used to test the TRU extraction. The feed solution consisted of a high-level waste simulation, 5 M in HNO3, containing more than 13 g/L of lanthanides and actinides. An interesting feature of the flowsheet is the use of acetohydroxamic acid (AHA) to strip Fe(III), Zr(IV), and Mo(VI), which also extracts the transplutonium elements (TPE). A solution of 2 M HNO3 10 g/L AHA was employed for this purpose. The AHA strip solution is con-
tacted with fresh process solvent to remove any traces of TPE and possibly Pu. TPEs and lanthanides were stripped from the process solvent using 0.01 M HNO3. More than 99.5 percent of the actinides and lanthanides were recovered and concentrated by a factor of four to six. The reduction of Fe, Zr, and Mo from the TPE fraction was >50 percent. Efforts are currently underway to apply the process to a plant-scale operation. A number of the favorable features outlined for TRUEX also apply to the Russian TRU process, namely, efficient extraction of Am(III) over a wide range of HNO3 concentrations, and the ability to strip, using low concentrations of acid. The Russian TRU process has the added advantage of using a lower concentration of a less expensive extractant. Because of the absence of TBP in the DPhDBCMPO-fluoropol system, radiolytic and hydrolytic degradation is probably less than with the TRUEX process solvent. The technology of the modified TRUEX process was confirmed by recent tests under static conditions and for industrial high-level waste from the Mayak Production Association facility.
SEPARATION OF ACTINIDES AND LANTHANIDES BY COUNTERCURRENT CHROMATOGRAPHY
The most effective method for TPE separation from highly radioactive wastes is extraction based on the use of bidentate neutral organophosphorus compounds (BNOC).5 A technological scheme of TPE removal with diphenyl (dibutyl-carbamoylmethyl) phosphine oxide (Ph2Bu2) in fluorine-containing polar solvent using an 18-step set of extractors has been proposed. The possibility of isolating >99.5 percent TPE as well as purifying the TPE from accompanying elements other than lanthanides has been demonstrated. The TPE + lanthanides fraction contains a low concentration of nitric acid (about 0.02–0.03 M) that makes it possible to choose other water systems for group separation of these elements. It is possible to increase the separation factors for the elements in the BNOC-based systems by (1) improvement of reagent selectivity by changing the reagent structure mainly by introduction of hard bridge fragments (arresting the arrangement of the donor atoms) into the extractant molecule and (2) addition of complex-forming agents to the aqueous phase that are able to react selectively with TPE or lanthanides in acid solutions. Neither the first nor the second method allows single-stage separation of TPE from lanthanides, as they have very similar properties. A multistage liquid-liquid extraction separation is needed to separate the TPE and lanthanides, in order to increase the separation factors.
Separation of actinides and lanthanides can be carried out by partition countercurrent chromatography (CCC), which is also called liquid chromatography with a free stationary phase. CCC is a relatively new method of separation. This method, suggested by the U.S. scientist Yoichiro Ito is based on the retention of the stationary organic phase in a rotating column under the action of centrifugal force while the mobile aqueous phase is pumped through continuously. Sub-
TABLE 2 Precipitation of Am (III) and Eu (III) Taken Separately from Nitric Acid Solutions Containing Potassium Ferro- and Ferricyanide
|
Conditions of Precipitation |
|||||
|
Precipitant |
Element |
M |
Mg/mL |
Percent Found in the Sediment |
Percent Found in the Solution |
|
[K4Fe(CN)6] |
Am(III) |
4.1 × 10−3 |
1.00 |
99 |
1 |
|
Eu(III) |
8.6 × 10−3 |
1.31 |
98 |
2 |
|
|
[K3Fe(CN)6] |
Am(III) |
6.4 × 10−3 |
1.55 |
92 |
8 |
|
Eu(III) |
8.6 × 10−3 |
1.31 |
0 |
100 |
|
|
See Kulyako et al. (1996). |
|||||
stances are separated due to differences in their distribution coefficients when the mixture to be separated moves with the flow of the aqueous phase through the column. It was shown that TPE and lanthanides can be separated in various BNOC-based systems using CCC.6
SEPARATION OF ACTINIDES AND LANTHANIDES BY COUNTERCURRENT CHROMATOGRAPHY
The search for new complex-forming and oxidizing systems for TPE and rare earth elements (REE) has opened up new possibilities in the chemistry and technology of these elements. Ferrocyanide ions are known to form a multitude of salts with, in particular, cations of various elements and rare earths. The formation of salts with the same cations occurs also with ferricyanide ions but to a lesser degree. Use of the ferri/ferrocyanide system has been described in various redox reactions. Study of the behavior of some TPE and REE in weakly acid and alkaline solutions containing ions of tri- and bivalent iron is of immediate interest. When potassium ferrocyanide is added to an americium solution in 0.1 M nitric acid, a white precipitate forms, just as in the case of REE. When potassium ferricianide is added, an americium species precipitates from the acidic solution, contrary to REE (see Table 2). TPEs and REEs are separated at the stage of TPE ferricyanide precipitation. In this case REEs remain in acidic solution and can be easily separated from precipitated TPEs. Americium is isolated from curium at the stage of its alkaline-ferricyanide dissolution.
CONCLUSION
Development of efficient technologies for recovery of long-lived radionuclides from high-level wastes is urgent for implementation of promising new
management methods (transmutation and disposal), as well as for the existing practice of high-level waste management. In Russia at the Mayak radiochemical plant, the UE-35 industrial facility, which recovers cesium and strontium from high-level waste, has been in operation since 1996. The next stage is aimed at development and implementation of actinide separation technology from high-level waste. For this purpose the following four processes are being studied and tested: (1) processes based on chlorinated cobalt dicarbollide (ChCoDiC-process); (2) isoamyldialkyl-phosphine oxide (POR-process); (3) diphenyldibutyl-carbamoylphosphine oxide (modified TRUEX-process); and (4) a combination of ChCoDiC, carbamoylphosphine oxide (CMPO), and polyethylene glycol (PEG) (UNEX-process).
ADDITIONAL REFERENCES
Dzekun, E. G., A. S. Scobtsov, et al. Proceedings of Conference in Ozersk (Zav. Lab.) pp. 29–36. [in Russian]
Horowitz, E. P., and W. W. Schulz. 1998. Metal Ion Separation and Preconcentration: Progress and Opportunities, Chapter XX, eds. A. H. Bond, M. L. Dietz, and R. D. Rogers. Cary, N.C.: American Nuclear Society.
Kulyako, Yu. M., D. A. Malikov, T. I. Trofimov, and B. F. Myasoedov. 1996. Behavior of transplutonium and rare earth elements in acidic and alkaline solutions of potassium ferricyanide. Mendeleev Communications 5:173–174.
Myasoedov, B. F., M. K. Chmutova, A. Yu. Shadrin, V. N. Romanovskiy, I. V. Smirnov, V. A. Babain. 1993. Proceedings of the International Conference and Technology Exhibition on Future Nuclear Systems: Emerging Fuel Cycles and Waste Disposal Options, GLOBAL ‘93, September 12–17, 1993, Seattle, Washington 1:581–587.
NOTES









