Immobilization of High-Level Waste: Analysis of Appropriate Synthetic Waste Forms*
S. V. Yudintsev
Institute of the Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry
Russian Academy of Sciences
The immobilization matrix is the key element in the system of engineering barriers. It must ensure the isolation of radioisotopes after they are placed in geological repositories under conditions affected by underground water. The glasses that are presently being used have a low capacity to incorporate plutonium and a low chemical stability. This article presents the results of a selection of crystalline matrices for the immobilization of actinide wastes. Pyrochlore-type phases could be used for the fixation of the actinide-zirconium-rare earth fraction of high-level wastes, while ferrites with a garnet structure could be used for the immobilization of complex wastes, including corrosion products, for example, Fe, Al, and Ga.
The reprocessing of irradiated fuel from nuclear power plants produces a large quantity of radioactive wastes, including high-level wastes. These high-level wastes represent a small portion of the total by volume but are the source of the bulk of the total activity level. As shown in Table 1, they contain fission products, activated corrosion products, and actinides, including those formed as a result of nuclear transformations in the reactor, as well as numerous nonradioactive elements, such as isotopes of fission products and technological additives.1 The most dangerous radionuclides in high-level wastes are the actinides and certain long-lived products of their fission (for example, 93Zr, 99Tc, 126Sn). Plans call for introducing them into stable immobilization matrices that would subsequently be deposited underground.2 Selecting optimal materials is a key point in ensuring the safe management of high-level wastes from the nuclear fuel cycle. The quest for high-level waste immobilization matrices began in the 1950s
TABLE 1 Basic Radioisotopes in High-Level Wastes from the Reprocessing of Spent Nuclear Fuel and Their Half-Lives
|
Basic Fission Products and Corrosion Elements (β- and γ-emitters) |
Transuranic Actinides (α-emitters) |
||
|
Radionuclide |
T1/2, in years |
Radionuclide |
T1/2, in years |
|
90Sr |
29 |
237Np |
2.1 × 106 |
|
93Zr |
1.5 × 106 |
238Pu |
89.9 |
|
99Tc |
2.1 × 105 |
239Pu |
2.4 × 104 |
|
126Sn |
105 |
240Pu |
6.5 ×103 |
|
129I |
1.7 × 107 |
241Pu |
14 |
|
137Cs |
30 |
242Pu |
3.78 × 105 |
|
147Pm |
2.6 |
241Am |
433 |
|
151Sm |
93 |
242Am |
152 |
|
154Eu |
16 |
243Am |
7.3 × 103 |
|
Activated corrosion products |
243Cm |
28 |
|
|
59Ni |
7.5 × 104 |
244Cm |
17.9 |
|
60Co |
5.3 |
245Cm |
8.5 × 103 |
|
63Ni |
96 |
246Cm |
4.76 × 103 |
with the study of various vitreous and crystalline materials based on silicates, phosphates, and titanates.3 For industrial-scale operations for this purpose, glasses are currently used—borosilicate glasses abroad4 and aluminophosphate glasses in Russia.5 The shortcoming of glasses is their limited capacity to incorporate actinides (especially plutonium) and their low chemical stability.6 The interaction of glass-like matrices with underground water is accompanied by the formation of colloidal particles7 in which radionuclides could migrate over great distances. In addition, glasses crystallize over time, further reducing the stability of radionuclide fixation due to the appearance of soluble phases—silicates or phosphates of the alkali and alkaline earth metals, molybdates, and so forth.
To manage them more efficiently wastes may be separated into radionuclide fractions.8 In one of these fractions the actinide content totals tens of percent by mass, and there is a significant amount of zirconium and lanthanides. The ratio of quantities of these elements in liquid high-level wastes from the reprocessing of spent fuel from various reactors is characterized as follows: actinides 10–15 percent by mass; lanthanides 60–65 percent by mass; and zirconium 20–25 percent by mass.9 Predominant among the actinides are uranium, neptunium, plutonium, and americium, while the lanthanide group is represented by neodymium, cerium, lanthanum, and praseodymium. Another group of wastes with high actinide content is created during the conversion of metallic weapons-grade plutonium into nuclear fuel.10
Besides glass, crystalline matrices represent an alternative waste form with high actinide content.11 More than 20 phases with various capacities for the
given elements and different levels of chemical and radiation stability have already been proposed.12 A matrix based on pyrochlore [(Ca,Gd,U,Pu,Hf)2Ti2O7] has been developed in the United States to immobilize excess plutonium.13 Zircon, zirconolite, cubic zirconium dioxide, perovskite, yttrium-aluminum garnet, britholite, monazite, and others have also been suggested for use in immobilizing plutonium-containing wastes.14 The final selection of an actinide matrix has not yet been made and remains an urgent question.
Since 1994, researchers at the Institute of the Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry (IGEM) of the Russian Academy of Sciences have been studying artificial materials intended for the immobilization of various types of radioactive wastes. These studies are carried out in cooperation with specialists from the Radon Research and Production Association in Moscow, the A. A. Bochvar All-Russian Scientific Research Institute of Inorganic Materials, the Institute of Physics and Power Engineering, the V. I. Vernadsky Institute of Geochemistry and Analytical Chemistry of the Russian Academy of Sciences, Moscow State University, and a number of others, including foreign organizations (the University of Michigan, the Australian Nuclear Science and Technology Organization). These activities are part of a larger overall research effort aimed at creating a basis for the safe burial of high-level radioactive wastes that has been under way since the early 1990s on the initiative and under the leadership of Academician Nikolai P. Laverov of the Russian Academy of Sciences.
From a structural standpoint the advantage lies with those phases having lattices that maintain stability even with wide variations in waste content. They may be used for the immobilization of both excess plutonium and more complex wastes, for example, actinide-zirconium-rare earth element fractions of high-level wastes, actinides and fission products, such as 93Zr, 99Tc, and 126Sn, and wastes from the conversion of plutonium into nuclear fuel. The process of seeking high-level waste matrices may be optimized by doing a preliminary analysis of the characteristics of the crystal structure of promising phases. Using the results of this analysis it is possible to reduce the number of potential compounds, and this significantly simplifies the further experimental testing of the selected material. The efficiency of this sort of approach has been demonstrated using the example of the titanates and aluminates and the structures of zirconolite, perovskite, hibonite, and hollandite.15 I will now review the possible applications of this analytical method using the example of a matrix with a pyrochlore and garnet lattice.
GENERAL DATA ON THE STRUCTURE OF PYROCHLORE
The structure of pyrochlore (Fd3m, Z = 8) may be considered as having twice the lattice parameter of fluorite with half the polyhedrons lacking two
diagonal anions.16 As a result, instead of a cube, it forms a distorted octahedron, while the formula is transformed from A4X8 (the fourfold formula for fluorite) to VIIIA2VIB2IVY6IVX. A and B represent the cations in two structural positions while Y and X are the anions, one of which (Y, represented by O2− ions) is included in the octahedral polyhedron, while the others (X = O2−, F−, Cl−, OH−) are located in the interstices and are not involved in the structural framework.
The structural-chemical characteristics of pyrochlore-type phases are interrelated.17 To ensure the stability of the pyrochlore lattice, another requirement besides the general condition of electroneutrality is the correlation of the sizes of the cations in the two structural positions. Based on the results of a geometric analysis it was concluded that the phases (A3+)2(B4+)2O7 crystallize in the pyrochlore structural type if the ratio of (RA + RO) to (RB + RO) is in the 1.08–1.22 range.18 Here RA and RB signify the sizes of the ions in positions A and B, while RO is the ratio of the oxygen anion. In the case of strongly polarized ions with a coordination number equaling 8—Bi3+, for example—the maximum value of the ratio could rise to 1.33. Similar values are cited by Wang et al., who state their view that the (RA + RO) to (RB + RO) ratio must lie between 1.10 and 1.24 if the pyrochlore lattice is to be maintained.19 Therefore, the limits of variations in the correlations between the sizes of the cations in the two structural positions in pyrochlore can be assessed as 1.3–1.8. A narrower range (1.46–1.80) is given in other works.20 Either increasing or decreasing the value of this ratio leads to the destabilization of the lattice and the appearance of a phase with a different crystalline structure instead of pyrochlore. Based on these data for various ions (B) in sixfold coordination, we may determine those elements in position (A) with a coordination number of 8 (including actinides) with which they form (A3+)2(B4+)2O7 phases with a pyrochlore lattice (see Table 2). Thus, for titanate pyrochlores ([B]VI = Ti4+, RVI = 0.605 Å), the radii of the ions must be between 0.8 and 1.0 Å, while stabilization of the zirconate phases (RVI = 0.72 Å) requires larger ions with a radius of 0.9–1.2 Å.
Pyrochlore Matrices for Actinides
The pyrochlores CaUTi2O7 and CaCeTi2O7 (Ce serves as an imitator of Pu) were synthesized previously.21 Vance et al. have also obtained the phases Ca(Pu,U,Zr) Ti2O7 and Ca(Np,Zr) Ti2O7 containing up to 45 percent by mass of PuO2 and NpO2 (or 0.8–1 atom of Pu or Np in the formula).22 Titanate pyrochlore is the fundamental phase in matrices for wastes with high actinide content, for example, spent nuclear fuel.23 By analogy with other materials, Synroc-F was named as another promising ceramic. Matrices with 80–90 percent pyrochlore content are considered in the United States to be promising forms for the fixation of excess plutonium. They are manufactured using cold pressing and then caking. More than 1000 samples of ceramics with cerium, thorium, uranium, and
TABLE 2 Pyrochlore-Type Phases [(REE)VIII2BVI2O7] and [VIII(CaAn4+)VI(B4+)2O7]
even plutonium have been produced by this method. The composition of the material with the latter element is a solid solution of CaUTi2O7, CaPuTi2O7, and Gd2Hf2O7 in a molar ratio of 2:1:1.24
SYNTHESIS AND STUDY OF NEW PYROCHLORE MATRICES FOR ACTINIDES
We have obtained new varieties of actinide pyrochlores. The basic composition [(A2+1A24+)2(B4+)2O7], where A2+1 = Ca or Sr, A24+ = U or Th, and B4+ = Ti, Sn, or Zr, was prepared from oxides of elements ground to 20–30 µm. The powders were pressed at 200–400 MPa into tables 12–20 mm in diameter and 4–5 mm in height. These tablets were placed in aluminum oxide crucibles baked at 1300–1550°C for 0.5 to 50 hours. Achievement of equilibrium was established based on the stability of the phase content of the end product as the duration of the experiments was increased. The samples were studied using X-ray phase analysis methods as well as scanning and transmission electron microscopy.
The rate of transformation was highest for pyrochlores containing titanium and tin, while synthesis proceeded most slowly in zirconate systems.25 In the majority of samples studied, pyrochlore is the main or only phase (see Table 3). In titanate matrices, brannerite, perovskite, and fluorite-type oxides are also present. When thorium is replaced by uranium in the content of the zirconates, a fluorite-type oxide is formed instead of pyrochlore (see Table 3).
Pyrochlores were obtained for the first time ever except for those phases in the systems Ca-U-Ti-O and Ca-Ce-Ti-O. There are no reports about them in X-ray databases. The common point in their formulas is the deficit of cations in positions with coordination numbers equal to 8. It is possible that part of the Zr4+ could also be in positions with coordination numbers equal to 8. It is preferable that the positions with this coordination number be filled with bivalent calcium ions instead of quadrivalent actinides. The replacement of thorium by uranium in the matrices CaThZr2O7 or (Ca0.5GdTh0.5) Zr2O7 makes the pyrochlore structure unstable (see Table 3). This is probably associated with the fact that the radius of U4+ (1.0 Å) is less than that of Th4+ (1.05 Å). As a result the ratio between the sizes of ions in positions VIII[A] and VI[B] is reduced and goes beyond the bounds of the range characteristic for pyrochlore-type phases. The replacement of zirconium with smaller titanium ions leads to an increase in this ratio, which stabilizes the pyrochlore structure for the phases (CaU)(ZrTi)O7 and (Ca0.5GdU0.5)(ZrTi)O7. Sr-Th pyrochlores were not obtained even with large Sn4+ and Zr4+ ions in positions with the coordination number = 6, despite the
TABLE 3 Pyrochlore Phases and Formulas Discovered in Samples
|
Calculated Composition |
Phases Discovered |
Pyrochlore Formula |
|
CaUTi2O7 |
Pyr > Br |
Ca1.06U0.72Ti2.22O6.94 |
|
CaUZr2O7 |
KO |
Pyrochlore not found |
|
CaCeTi2O7 |
Pyr > Per > KO |
Ca1.03Ce0.99Ti1.98O6.98 |
|
CaThSn2O7 |
Pyr ≫ KO |
Ca0.92Th0.92Sn2.0(Fe0.08)*O6.96 |
|
SrThSn2O7 |
Cas > KO > Per |
Pyrochlore not found |
|
CaThZr2O7 |
Pyr > Per ~ KO |
Ca0.91Th0.84Zr2.25O7.09 |
|
SrThZr2O7 |
Per > KO |
Pyrochlore not found |
|
(Ca0.5GdTh0.5)Zr2O7 |
Pyr ≫ KO |
(Ca0.44GdTh0.42)Zr2.13O7.05 |
|
(Ca0.5GdU0.5)Zr2O7 |
KO |
Pyrochlore not found |
|
(Ca0.5GdU0.5)(ZrTi)O7 |
Pyr > KO |
(Ca0.62Gd0.97U0.23)(Zr0.84Ti1.34)O6.90 |
|
(Ca0.5GdTh0.5)(ZrTi)O7 |
Pyr ≫ KO |
(Ca0.47Gd0.95Th0.4)(Zr1.29Ti0.89)O7.05 |
|
*Contamination during formula attrition. NOTE: Pyr—target phase with pyrochlore structure; Br—uranium titanate (brannerite); KO—oxide with a fluorite lattice; Per—phase with perovskite structure; Cas—tin oxide (cassiterite); >—primary and secondary phases; ≫—primary and secondary phases with the secondary phase present in a much smaller amount. |
||
fact that the ratio of the ion radii is in accordance with conditions of geometric stability. With a base composition of SrThSn2O7 and SrThZr2O7, oxides with a fluorite or perovskite structure as well as tin oxide were formed instead of pyrochlore.
The data obtained on the correlation of composition and structure must be taken into account in selecting matrices for complex actinide wastes, for example, actinide-zirconium-rare earth element fractions of high-level wastes. The octahedral ions of the pyrochlore phases of rare earth elements and trivalent actinides could vary in size from 0.55 to 0.75 Å. Ti4+, Sn4+, Hf4+, and Zr4+ meet this condition. As for other elements that could be located in octahedral positions in the lattice, we may note Tc4+ with a radius of 0.65 Å. Its long-lived isotope 99Tc, which is formed during the fission of 235U, could also it seems be included in a pyrochlore matrix. The capacity of the pyrochlore lattice with regard to quadrivalent actinides increases as a result of the conjugate inclusion of Ca2+ cations, which serve as compensators for the excess charge.
While pyrochlore-type phases have a high solubility with regard to actinides, they have a limited capacity for other high-level waste components such as iron, aluminum, and others. This could give rise to the appearance of additional phases, thus reducing the isolation capabilities of the matrix. Therefore, it is important to select crystalline phases for the immobilization of complex wastes resulting from the processing of spent fuel. Compounds with a garnet lattice are promising phases for the immobilization of these materials.
GENERAL DATA ON THE STRUCTURE OF GARNET
The structure of garnet, AVIII3BVI2X3O12 (la3d, Z = 8), is a three-dimensional shell made up of alternating XO4 tetrahedrons and BO6 octahedrons linked by common vertices.26 The spaces in between, which have the form of distorted cubes, hold large A cations. The presence of three structural positions—A, B, and X, with coordination numbers 8, 6, and 4—makes it possible to include various elements in the lattice. Position AVIII holds bivalent (Ca, Mn, Mg, Fe, Co, Cd) and trivalent (Y, rare earth elements) cations. Trivalent (Fe, Al, Ga, Cr, Mn, In, Sc, V) and quadrivalent (Zr, Ti, Sn) ions fill the BVI positions. In the XVI position we find trivalent (Al, Ga, Fe), quadrivalent (Ge, Si), or pentavalent (V, As) cations.
Relation Between Structural and Chemical Properties of Garnet Phases
The formation of the garnet structure is possible given a certain combination of charges and sizes of ions in various positions.27 The cation-anion composition must satisfy the general rule of electroneutrality, while another factor in lattice stability is the relation between the sizes of the ions. Based on the example of 200 combinations of cation pairs, the field of stability for silicate garnets has
been defined according to the coordinates of the radii of the ions in positions A and B,28 and it has been shown that if the size of the cation in either of the two positions is increased, the radius of the ion in the other position must also increase. Violation of these relations will lead to the formation of compounds with a different structure. However, by no means can all garnets that may be formed based on the results of this structural analysis be synthesized at atmospheric pressure. This is especially true of compounds at the extreme ranges of the identified area of stability. Obtaining many of these compounds requires increased pressure, with more pressure being required as the variety of sizes of ions in positions with coordination numbers 8 and 6 increases.29
The structure of garnet may be stabilized by adding components that change the effective ionic radius in this or that position. Thus, grossular (Ca3Al3Si3O12) cannot be obtained at atmospheric pressure and at temperatures greater than 800°C; however, it was possible to synthesize garnet [Ca3(Al1.33Cr0.67)Si3O12] by replacing one-third of the aluminum atoms with chromium.30 The same effect occurs when yttrium oxide and iron oxide are added.31 At one atmosphere and 1400°C in an interval from 90 molar percent Ca3Al2(SiO4)3 plus 10 molar percent Y3Fe2(FeO4)3 to 100 molar percent Y3Fe2(FeO4)3, there is an uninterrupted solid solution with a garnet structure. Replacing the yttrium with gadolinium reduces the solubility of the grossular minal to 10 molar percent. This is probably associated with the larger size of the Gd3+ ion (1.05 Å) in comparison with Y3+ (1.02 Å), which makes it less efficient as a lattice stabilizer.
On Possible Garnet-Type Actinide Phases
Due to their sizes the ions of trivalent and quadrivalent actinides and rare earths predominantly occupy large dodecahedral spaces in the lattice. The way the polyhedrons are joined in the crystalline lattice determines the relation between the sizes of the ions in the various structural positions. For rare earth garnets the effect of the structural factor on the chemical composition of the compounds is obvious.32 These compounds include phases with aluminate, gallate, and ferrite composition (REE3B2X3O12), B = X = Al3+, Ga3+, or Fe3+ (see Table 4). In this group there is an increase in the ion radius in positions with coordination numbers 4 and 6: Al3+—0.39 Å (IV) and 0.54 Å (VI); Ga3+—0.47 Å (IV) and 0.62 Å (VI); or Fe3+—0.49 Å (IV) and 0.65 Å (VI). In order to ensure the stability of the lattice the increase in ion size in positions BVI and XIV must be accompanied by an increase in the radii of ions in the AVIII structural positions. Therefore, in this group of compounds one should expect an increase in the maximum size of the ion in the dodecahedral position. In aluminate systems one observes the formation of garnets of rare earths from Lu3+ to Dy3+ with a radius from 0.98 to 1.03 Å. For gallates the garnet-type structure is stable up to Nd3+ (1.09Å). If the radius of the ion falls outside these limits, a phase with a perovskite structure and an oxide will form instead of a garnet: REE3B5O12 = 3
TABLE 4 Stability of Garnet Structure for Phases [(REEVIII)3(BVI)2(BIV)3O12], Where B = Al3+, Ga3+, or Fe3+
|
Lanthanides (rVIII, Å) |
Al3+ (rIV = 0.39 Å, rVI = 0.545 Å) |
Ga3+ (rIV = 0.47 Å, rVI = 0.62 Å) |
Fe3+ (rIV = 0.49 Å, rVI = 0.65 Å) |
Actinides (rVIII, Å) |
|
La (1.16) |
− |
− |
? |
|
|
Pr (1.13) |
− |
? |
? |
|
|
Nd (1.11) |
− |
+ |
? |
Am3+ (1.09) |
|
Sm (1.08) |
− |
+ |
+ |
Cm3+ (1.08) |
|
Eu (1.07) |
− |
+ |
+ (1460** °C) |
|
|
Gd (1.05) |
− |
+ (1740* °C) |
+ (1460** °C) |
|
|
Dy (1.03) |
+ (1920* °C) |
+ |
+ |
|
|
Y (1.02) |
+ |
|||
|
Ho (1.02) |
+ (1950* °C ) |
+ |
+ |
|
|
Er (1.00) |
+ (1960* °C) |
+ |
+ |
|
|
Yb (0.99) |
+ (2000* °C) |
+ |
+ |
|
|
Lu (0.98) |
+ (2060* °C) |
+ |
+ |
|
|
*Garnet melt temperature **Eutectic temperature of garnet-oxide mixture (Al2O3, Ga2O3, or Fe3O4) NOTE: + means garnet structure stable; − means garnet structure unstable; ? means no data available. SOURCE: N. A. Toropov, V. L, Barzakovsky, V. V. Lapin, and N. N. Kurtseva. Diagrams of the status of silicate systems. In Dual Systems (1st ed.). Leningrad: Nauka Publishers, 1969. |
||||
REEBO3 + B2O3, where B = Al3+, Ga3+, or Fe3+. The melt temperature of ferrites is 400°C lower than that of aluminates and 200°C lower than that of gallates (see Table 4). The eutectic temperature of ferrite garnet and spinel, at which the amount of garnet is about 35 molar percent (70 percent by mass), is even lower (approximately 1460°C). This makes it easier to develop a technology for obtaining high-level waste matrices by melting and crystallization. Spinel accumulates many activated corrosion products (cobalt, nickel, manganese, chromium, iron). All of this makes it possible to regard a biphase garnet-spinel association as a promising matrix for high-level actinide wastes with increased content of the above elements.
Based on the similarity of the ion radii of Am3+ and Cm3+ on the one hand and Gd3+ and Nd3+ on the other, we might expect these elements to behave in a similar manner during the synthesis of a garnet matrix. The formation of phases with a garnet structure is most likely for ferrites and gallates of trivalent actinides. For the quadrivalent actinides inclusion in garnet has been experimentally confirmed only for thorium.33 Garnet was synthesized from the mixture of (Ca2.5Th0.5)Zr2Fe3O12 and (Ca2Th)(ZrFe)Fe3O12 at temperatures of 1050 and 1200°C and pressure of 1 atmosphere. The composition of the products
of the experiments were studied using X-ray methods. The basic formula (Ca2.5Th0.5)Zr2Fe3O12 led to the creation of a monophase garnet ceramic. The mixture with (Ca2Th)(ZrFe)Fe3O12 at 1050°C also formed a monophase garnet ceramic, but at 1200°C, thorium dioxide or thorianite also appeared along with the garnet. Researchers also synthesized a garnet in which the thorium was replaced by cerium: (Ca2.5Ce4+0.5)Zr2Fe3O12.
We must emphasize that the published data on the composition of thorium and cerium garnets are not based on direct determinations but rather are established indirectly based on suppositions about the correspondence of the stoichiometry of the synthesized garnet with the composition of the base formula. This approach is based on the absence of other crystalline phases besides garnet among the synthetic products. From this the conclusion is drawn that the correlation of components in the initial mixture characterizes the composition of the end product. This product requires testing, using local analytical methods. We also note that the above experiments were not aimed at studying the properties of garnet as a possible matrix material for the immobilization of actinide-containing wastes. Only in the past 15 years have special studies been conducted to address this problem.
EXPERIMENTAL STUDY OF GARNET MATRICES FOR HIGH-LEVEL WASTES
Specialists from the Institute of Inorganic Materials were the first in Russia to propose garnet for the immobilization of high-level radioactive wastes.34 Using the induction melt method in a cold crucible, they synthesized samples similar in composition to andradite (Ca3Fe2Si3O12). However, the data on the phase and chemical compositions of the samples and consequently the proof of the formation of garnet phases were not cited. These studies were later continued, which led to the creation of a matrix with a garnet content of more than 80 percent.35 The content of oxides of rare earth elements and zirconium in the garnet exceeds 20 percent by mass, but the possibility of including actinides has not yet been studied.
Specialists from the Radium Institute are studying garnet ceramics as potential actinide matrices.36 They have focused on a rare earth (Y, Gd) aluminum-gallate garnet as a base. Taking the place of actual high-level wastes in these studies are uranium and cerium oxides or a complex mixture representing wastes from plutonium production. The products synthesized in these experiments are polyphase ceramics consisting of garnet-, perovskite-, and hibonite-type phases as well as rarer oxides of zirconium and aluminum. The researchers have discovered an effect of the chemical composition on the phase composition of the synthesized samples: in the system Gd2O3—Al2O3, perovskite is predominant, while garnet becomes the leading phase in the systetm Gd2O3—Ga2O3. Cerium content in the garnet reaches 6 percent by mass, while for uranium the figure is
less than 0.1 percent by mass. Adding 4 percent by mass of metallic tin to the initial mixture leads to an increase in the uranium content of the garnet to 5.5 percent by mass. But even in the latter case, the garnet contains less than 10 percent of the total quantity of uranium in the sample. A similar solubility value (about 6 percent by mass) in garnet has also been established for plutonium.37 Thus, rare earth Al-Ga garnets have a low capacity for incorporating uranium, plutonium, and cerium, and evidently cannot be recommended for immobilizing high-level wastes with high quadrivalent actinide content. These results do not mean that garnet ceramics should be ruled out as actinide matrices. They only indicate that a detailed study of their structure is required in order to identify compounds that are suitable for this purpose.
It has been shown that the highest quadrivalent actinide capacity should be expected in ferrite garnets.38 The size of the Fe3+ ion is greater than that of Al3+, which ensures the incorporation of larger actinide ions in the garnet lattice, especially with the conjugate inclusion of Ca2+ as a charge compensator. The radii of Th4+ and Ce4+ equal 1.05 and 0.97 Å. The radius of Pu4+ (0.96 Å) is close to that of Ce4+, while the sizes of the ions of Np4+ (0.98 Å) and U4+ (1.0 Å) lie between the values for Th4+ and Ce4+. Taking previously collected data into account, we may expect that garnets of the type (Ca2.5An4+0.5)Zr2Fe3O12 and (Ca2An4+)(ZrFe)Fe3O12, where An = Pu, Np, or U, may be obtained experimentally.39
In order to test this hypothesis, phases formed in the system CaO—Al2O3—SiO2—Fe2O3—ZrO2—Gd2O3—CeO2—UO2—ThO2—PuO2 were studied. The initial mixtures corresponding in content to the stoichiometry of garnet (see Table 4) were pressed at 200–400 MPa into tablets 10 mm in diameter and 2–3 mm in height. The synthesis was carried out in an aluminum oxide crucible in a regular air environment (for cerium compounds, in an oxygen environment) as follows. The mixture was heated to the necessary temperature (1300, 1400, or 1500°C) at a rate of 10 degrees per minute, held for a period of from 1 to 20 hours, and cooled to room temperature. The time necessary for achieving equilibrium totaled one or two hours even at the lowest temperature of 1300°C. Those samples that were heated to 1500°C melted to form a layer 3–5 mm thick on the bottom of the crucible. The other samples maintained their initial form, indicating their higher melting points.
The content of the target garnet phase in the samples ranged from 70 to 100 percent (see Table 5). In one of the experiments (13-Th) two different types of garnet formed. The main spikes on the diffractograms were located in the ranges 3.05–3.15 Å (d400), 2.72–2.82 Å (d420), and 2.49–2.57 Å (d422), which illustrates the significant variations in the parameters of the elemental cell depending on the content. Along with garnet the samples contained other phases, such as fluorite-type actinide oxides, a Ca-Zr oxide with a perovskite structure, and oxides of Ca, Fe, and Al (hibonite). The baked and melted garnet ceramics with a similar composition also share a similar phase structure. The basic difference lies in the
TABLE 5 Garnet Formulas Tested and Composition of Products of Experiments
larger size (10–100 µm as opposed to 1–20 µm) and the regular form of the grains in the samples obtained by melting and subsequent crystallization.
The results of the study of the composition of the phases (see Table 6) attest to the significant capacity of the ferrite garnets to incorporate the rare earth elements (Gd, Ce), Zr, and the actinides. The content of the latter reaches 16–18 percent by mass in Ca-Zr-Fe garnet and is reduced with the addition of aluminum and especially silicon. The concentration of actinides is reduced by a decrease in the size of the structural positions when Fe3+ ions are replaced with smaller Al3+ and Si4+ cations. Compression of the structural polyhedrons prevents the incorporation of larger actinide ions in the lattice. This is clearly illustrated in the composition of the product of experiment 13-Th, which included two garnets with different amounts of actinides. In the phase with the higher silicon content the thorium content was 80 percent lower. The excess quantity of thorium is included in the composition of thorianite.
In the plutonium sample only garnet, calcium oxide, and iron oxide with a hibonite structure were found. All of the plutonium incorporated into this sample (16 percent by mass) was found in the garnet phase (see Figure 1). Considering that the relative amount of garnet in the sample equaled approximately 80 percent, the concentration of plutonium in it may be assessed to be on the order of 20 percent by mass.
CONCLUSION
Using the examples of pyrochlore- and garnet-type phases, the possibilities offered by structural analysis were evaluated as part of efforts to find optimal matrices for the immobilization of high-level actinide wastes. The presence of several positions (two for pyrochlore and three for garnet) filled with cations of
TABLE 6 Compositions (percent by mass) and Calculated Formulas of Garnet in Samples
various charges and sizes creates conditions for the inclusion in them of various waste components, including actinides.
Only a small part of the actinides can be incorporated into the pyrochlore A2B2O7 in its quadrivalent form. In order to increase their solubility it is necessary to introduce Ca2+ ions as charge compensators. An example would be compounds in which the trivalent ions are replaced by a pair of bi- and quadrivalent cations according to the following formula: 2REE3+ = Ca2+ + Ce4+ (U4+, Th4+, Np4+, Pu4+). By comparing the radii of these pairs and the REE3+ ions one may suppose the existence of several phases of quadrivalent actinides with a pyrochlore lattice. The average radii of the pairs (Ca2+ + U4+) and (Ca2+ + Np4+) is close to the size of Gd3+. For the pair (Ca2+ + Th4+), the analog would be Pm3+ or Sm3+, while for pairs involving Ce4+ or Pu4+ it would be Tb3+.
As confirmation of this, actinide phases with a pyrochlore structure were synthesized as follows: CaThSn2O7, CaThZr2O7, (Ca0.5GdTh0.5)Zr2O7, (Ca0.5
GdU0.5)(ZrTi)O7, and (Ca0.5GdTh0.5)(ZrTi)O7. Attempts to synthesize the phases CaUZr2O7, SrThSn2O7, SrThZr2O7, and (Ca0.5GdU0.5)Zr2O7 were unsuccessful, evidently due to the lack of correspondence between the sizes of the ions with the lattice stability conditions for pyrochlore. Instead of pyrochlore, phases with fluorite- and perovskite-type structures were formed along with tin dioxide.
Conditions required for the formation of high-level waste matrices with a garnet-type lattice were also studied. The incorporation of actinide ions (U4+, Th4+, Np4+, Pu4+) into the garnet was promoted by the filling of positions XIV and BVI with large low-valence Fe3+ cations (rIV = 0.49 Å, rVI = 0.65 Å). It has been experimentally established that the actinide content in garnet varies from 0.6–0.8 percent by mass to 16–18 percent by mass depending on its composition.
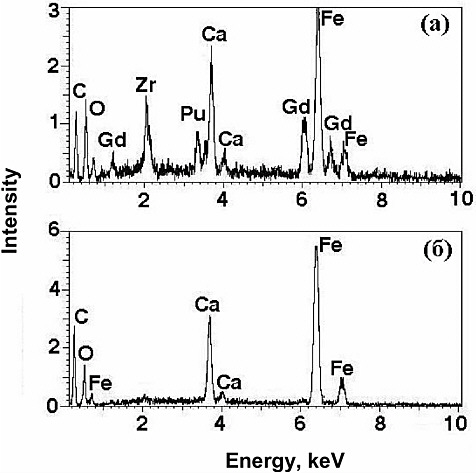
FIGURE 1 Energy dispersion spectra for garnet (a) and Ca-Fe oxide (hibonite), (b) in Sample 1-Pu. The carbon peak (C) is associated with the graphite film on the surface.
The highest actinide concentrations are characteristic of ferrites, while the lowest are observed in silicon- and aluminum-containing garnets.
Based on data that has been collected it has been concluded that it is possible to use pyrochlore matrices to incorporate the actinide and actinide-zirconium-rare earth fractions of high-level radioactive wastes. A phase with a garnet structure represents a more universal matrix. This material may be used for immobilizing both actinide and actinide-zirconium-rare earth fractions and actinide wastes with a more complex composition containing high levels of corrosion products (Al, Ga, Fe, Cr, Ni, Mn, Co). A biphase garnet-spinel association is a promising matrix for such wastes. Research on the radiation resistance and chemical stability of samples of garnet matrices under the influence of heated waters is a top priority for further study.
ACKNOWLEDGMENTS
The author is grateful to A. G. Ptashkin (Radon Research and Production Association, Moscow) and M. I. Lapina, A. V. Mokhov, and A. V. Sivtsov (IGEM) for their help in synthesizing and studying samples. This research was carried out with the partial financial support of the U.S. Department of Energy (project RC0-20002-SC14).
NOTES

















