2
State and Quality of the Current System
INTRODUCTION
The committee was charged to review, summarize, and evaluate the state and quality of the current animal health framework. This review is organized into the following categories:
-
Components of the Animal Health Framework
-
Technological Tools for Preventing, Detecting, and Diagnosing Animal Diseases
-
Scientific Preparedness for Diagnosing Animal Diseases: Laboratory Capacity and Capability
-
Animal Health Research
-
International Issues
-
Addressing Future Animal Disease Risks
-
Education and Training
-
Improving Awareness of the Economic, Social, and Human Health Effects of Animal Diseases.
COMPONENTS OF THE ANIMAL HEALTH FRAMEWORK
The animal health framework comprises organizations and participants in the public and private sectors directly responsible for maintaining the healthy status of all animals and those who are impacted by animal health or are influencers of forces affecting animal health. The essential components of the framework for addressing animal disease, beginning with the affected animal, are listed in Box 2-1.
|
BOX 2-1
|
Front Lines
The front lines contain multifarious actors and components: from intensive, large-scale, highly technical food animal facilities, monitored by well-trained livestock managers and veterinarians, to disparate clusters of companion animals within individual homes observed with differing degrees of intensity by their owners, to wildlife populations without any kind of regular monitoring contact by humans. It is a sine qua non that the first signs of a disease outbreak are small abnormalities in behavior. The sooner a new disease is recognized, the greater the likelihood that it will be effectively controlled and cause minimal damage.
In this context, an effective framework for animal health is most highly developed for agricultural animals. In today’s livestock industry, producers are encouraged to adopt herd health programs and focus on prevention rather than dealing with case-by-case problems (Gary Weber, National Cattlemen’s Beef Association, presentation to committee, April 6, 2004). As front-line responders, animal attendants and caretakers may have variable levels of training and motivation for recognizing and reporting abnormalities and sounding an alert when abnormalities are noted.
Farm animals are also raised by individual “hobbyists” who might lack the training of paid animal attendants but who potentially have the luxury to be more observant of their animals than do large-scale animal producers. They might also have expendable income with which to seek out veterinary services when needed. Because the number of hobbyists is growing, a better picture of the animal care practices of this community is
needed to evaluate the knowledge of this group of owners and their likely motivation for reporting suspected disease outbreaks.
For companion animals and wildlife, the situation is even more uncertain. With the exception of some large charismatic and commercially viable species, there is little economic incentive to survey animal health, and in some cases, an absence of financially remunerated attendants responsible for monitoring husbandry. In these cases, recognition of a disease abnormality by people not associated with the immediate habitat is due to both diligence and chance. An astute owner may seek advice on first blush of a disorder in a companion animal, or alternatively, a group of companion animals may become quite ill prior to any abnormality being reported outside of the immediate surrounding. For wildlife, especially wildlife outside the oversight of zoo veterinarians and handlers, the situation can be even more uneven. For large and charismatic species (e.g., chimpanzees, giraffes, dolphins), detection of anomalies may occur at the early stages of disease development; however, with the majority of wild species (e.g., rodents, small birds, reptiles), disease may become widespread before it is recognized by people not associated with the immediate habitat.
Veterinary Medical Profession
The goals of the veterinary profession in the United States, as embodied in the oath taken by its members, are to protect animal health, relieve animal suffering, conserve animal resources, promote public health, and advance medical knowledge. In 1994, 56,000 veterinarians were active in the profession. In 2004, that number had grown to 65,000, a 16 percent increase. The profession is expected to grow another 25 percent in the next 10 years. The Bureau of Labor Statistics expects 28,000 job openings by 2012 due to growth and net replacements—a turnover of nearly 38 percent (AAVMC, 2004). Present employment of veterinarians is described in Table 2-1. Each state is responsible for licensing veterinarians and for regulating private veterinary practice (AVMA, 2004a).
The American Veterinary Medical Association (AVMA), established in 1863, serves as the lead professional body for veterinarians in the United States. It is an organization largely driven by private practitioners, the majority of whom are in companion animal practice and AVMA’s primary activities are a reflection of the membership. It has a significant influence on veterinary education through its accreditation process administered by the Council on Education (COE). The AVMA also promulgates many and varied policy statements and guidelines that bear on animal health and welfare and on public health.
The United States Animal Health Association (USAHA) is another key organization dealing with agricultural animal health and disease is-
sues. USAHA works with state and federal governments, universities, veterinarians, livestock producers, national livestock and poultry organizations, research scientists, the extension service, and seven foreign countries to control livestock diseases in the United States (USAHA, 2005). This coalition of government, academic, and industry animal health professionals has operated for more than 100 years and serves to discuss prominent issues and deliver resolutions to appropriate organizations and government for consideration.
The nature of veterinary employment is changing (Table 2-1). Over the past 15 years, there has been a 35 percent increase in the number of veterinarians engaged in small animal practice, a 13 percent decrease in the number of veterinarians in food-animal and mixed practice, and a 47 percent decrease in the number of veterinarians in public practice (i.e., government employment). Currently over half the profession is employed in small animal practice and only about 16 percent serves the livestock industry and food system, assuming that all the work of government employees is related to this domain (AVMA, 2005b).
The veterinary medical profession and its branches have been the subject of several in-depth assessments over the past 35 years (NRC, 1972, 1982, 2004b; Pritchard, 1988; Brown and Silverman, 1999). The KPMG megastudy conducted by Brown and Silverman (1999), entitled The Current and Future Market for Veterinarians and Veterinary Medical Services in the United States, examined the profession’s income disparities, the increasing demand of services in new areas, and the critical shortage of trained professionals, and concluded that a series of strategic and substantive changes are needed in the veterinary profession to meet evolving societal needs and demands. One of the most comprehensive reviews, the Pew Veterinary Education Program, concluded: “Veterinary medicine is being threatened as never before by powerful forces of change in society, rapid advances in science and technology, and by the changing needs and expectations of almost every constituency it serves. Decisive steps must be taken at this time to make corrections in the way that the profession is trying to fulfill its responsibilities, to bring them more in line with the changing needs of society. Although it can not yet be defined as a crisis, the veterinary profession is not adapting rapidly enough to changing needs and is encountering substantial problems” (Pritchard, 1988). More recently, a 2004 National Research Council report on the veterinary medical profession found, among other key factors negatively impacting the supply of comparative medicine veterinarians, a lack of qualified applicants for all types of postgraduate training programs and the lack of commitment by veterinary medical schools and institutions that offer postgraduate training programs to prepare and train veterinary students and postgraduates for veterinary careers other than private clinical practice
TABLE 2-1 Employment of U.S. Veterinarians Who Are AVMA Members
|
Private Clinical Practice |
2004 |
1986 |
||
|
Number |
Percentage |
Number |
Percentage |
|
|
Large animal exclusive |
1,887 |
4.0 |
1,936 |
5.7 |
|
Large animal predominant |
2,596 |
5.4 |
4,570 |
13.5 |
|
Mixed animal |
3,868 |
8.2 |
3,397 |
10.1 |
|
Small animal predominant |
5,507 |
11.7 |
4,722 |
14.0 |
|
Small animal exclusive |
29,951 |
63.4 |
17,276 |
51.1 |
|
Equine |
2,257 |
4.8 |
1,888 |
5.6 |
|
Other |
1,198 |
2.5 |
|
|
|
Subtotal |
47,264 |
100 |
33,789 |
100 |
|
Public and Corporate Employment |
2004 |
1986 |
||
|
Number |
Percentage |
Number |
Percentage |
|
|
College or university |
3,961 |
46.7 |
3,713 |
39.5 |
|
Federal government |
641 |
7.6 |
2,212 |
23.5 |
|
State or local government |
542 |
6.4 |
756 |
8.0 |
|
Uniformed services |
474 |
5.6 |
586 |
6.2 |
|
Industrial |
1,566 |
18.5 |
2,128 |
22.7 |
|
Other |
1,294 |
15.2 |
|
|
|
Subtotal |
8,478 |
100 |
9,395 |
100 |
|
Grand Total |
64,867 |
|
43,184 |
|
|
SOURCE: Pritchard, 1988; AVMA, 2005b. |
||||
(NRC, 2004b). While it is too early to tell whether the recommendations from the 2004 NRC report have had an effect, the employment demographics of veterinarians over the last 15 years (Table 2-1) suggest that many of the Pew report recommendations have not been realized, due largely to the limited amount of funding provided and the complete lack of follow-up and continuity.
Private Veterinarians
Veterinarians in private practices, generally supported by veterinary technicians, are among the front-line health professionals dealing with animal disease. They constitute about 80 percent of the veterinary workforce (ca. 47,000, as shown in Table 2-1). Fewer than 10,000 derive a significant portion of their income from food-animal practice, and the number is declining (AVMA, 2005b). Rural demographic changes, inten-
sification and specialization in the livestock industry, lifestyle issues, veterinary college entrance selection, and perhaps shifts in gender balance have led to circumstances where fewer veterinary graduates opt for careers in rural food animal practice (AVMA, 2004b).
Veterinarians working in small animal and exotic practice can also play key roles in the detection of emerging disease problems. Examples of successful recognition of early incursions include the diagnosis of West Nile virus by a veterinary pathologist at the Bronx Zoo and screw worm incursions halted by small animal and equine practitioners in two different states (Nolen, 1999; Thurmond and Brown, 2002).
Federal and State Animal Health Agencies
Federal Animal Health Agencies
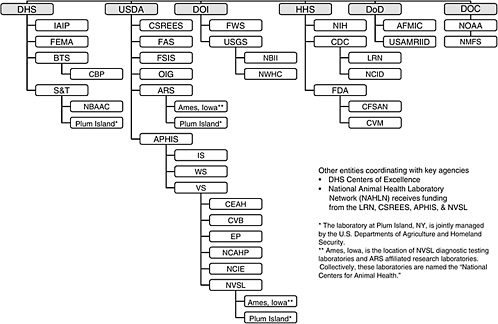
This section briefly summarizes the legal authorities and functions of the federal government for preventing, detecting, and diagnosing animal diseases. Appendix C contains a more detailed summary prepared by Nga L. Tran, entitled “Existing Federal System for Addressing Animal Diseases.” Figure 2-1 illustrates the large number of federal entities involved in addressing animal health issues. International, state, and private entities involved in animal health issues are not included in Figure 2-1.
The USDA Animal and Plant Health Inspection Service (APHIS) plays the lead role in protecting the health of domestic animals. Within APHIS, the majority of the responsibility to protect animal health resides in Veterinary Services (VS). The USDA’s programs addressing animal health cover a wide range of functions, including deterrence (the elimination or reduction of factors conducive to the potential import, transport, or transmission of disease from suspected sources of pathogens) and prevention, detection and diagnosis, monitoring and surveillance, emergency response, research, education and training, and communication (see Table C-3). A summary of deterrence and prevention efforts as they relate to reducing a potential threat before it reaches U.S. borders are described later in this chapter in the section on International Issues.
The APHIS-VS division shares responsibility for some animal health issues with the Food and Drug Administration’s (FDA) Center for Veterinary Medicine (CVM). The CVM regulates and approves the manufacture and distribution of food additives and drugs that will be given to animals. APHIS-VS’s Center for Veterinary Biologics (CVB) regulates veterinary biologics, including vaccines, bacterins, antisera, and diagnostic kits that are used to prevent, treat, or diagnose animal diseases and ensure that these products are pure, safe, potent, and effective,
Glossary of Acronyms and Abbreviations for Figure 2-1
AFMIC – Armed Forces Medical Intelligence Center
APHIS – Animal and Plant Health Inspection Service
ARS – Agricultural Research Service
BTS – Border and Transportation Security
CBP – Customs and Border Protection Bureau
CDC – Centers for Disease Control and Prevention
CEAH – Centers for Epidemiology and Animal Health
CFSAN – Center for Food Safety and Applied Nutrition
CSREES – Cooperative State Research, Education, and Extension Service
CVB – Center for Veterinary Biologics
CVM – Center for Veterinary Medicine
DHS – U.S. Department of Homeland Security
DOC – U.S. Department of Commerce
DoD - U.S. Department of Defense
DOI - U.S. Department of the Interior
EP – Emergency Programs
FAS – Foreign Agricultural Service
FDA – Food and Drug Administration
FEMA – Federal Emergency Management Agency
FSIS – Food Safety and Inspection Service
FWS – Fish and Wildlife Service Bureau
HHS – U.S. Department of Health and Human Services
IAIP – Information Analysis and Infrastructure Protection Directorate
IS – International Services
LRN – Laboratory Response Network
NBII – National Biological Information Infrastructure
NBAAC – National Biodefense Analysis Countermeasure Center
NCAHP – National Center for Animal Health Programs
NCID – National Center for Infectious Diseases
NCIE – National Center for Import and Export
NIH – National Institutes of Health
NMFS – National Marine Fisheries Service
NOAA – National Oceanic and Atmospheric Administration
NVSL – National Veterinary Services Laboratories
NWHC – National Wildlife Health Center
OIG – Office of the Inspector General
S&T – Science and Technology Directorate
USAMRIID – U.S. Army Medical Research Institute for Infectious Diseases
USDA – U.S. Department of Agriculture
USGS – U.S. Geological Survey
VS – Veterinary Services
WS – Wildlife Services
based on the Virus-Serum-Toxin Act (37 Stat. 832-833; as amended December 23, 1985, Pub. L. 99-198, 99 Stat. 1654-1655; 21 U.S.C. 151-159).
The APHIS-VS division also administers the National Veterinary Accreditation Program (NVAP). This voluntary program certifies private veterinary practitioners to work cooperatively with federal and state animal health officials. Nationally, more than 60,000 active accredited veterinarians are in the NVAP database. These veterinarians are instrumental in performing examinations and issuing health certificates critical to the safe movement of animals, assisting in disease eradication campaigns, and maintaining extensive animal disease detection and surveillance functions. NVAP work must be consistent with international requirements to safeguard animal health.
The USDA Food Safety and Inspection Service (FSIS) is responsible for ensuring the safe, wholesome, and correctly labeled and packaged commercial supply of meat, poultry, and egg products that move within interstate commerce, are imported into the United States, or exported to other countries. Over the years, FSIS has transitioned into a public health role and has especially focused on food safety and security. Through its inspection system, which involves inspection of individual animal carcasses at slaughter, FSIS plays an important disease detection function. For instance, FSIS assists APHIS in identifying tuberculous cattle carcasses for the national bovine TB eradication program. The FSIS inspection system is further enhanced through its use of toxicological, pathological, and microbiological analyses. In this capacity, the agency is able to help prevent the dissemination of pathogens and diseases to people and animals further along the commodity stream. FSIS employs approximately 7,600 inspectors and is the largest employer of veterinarians in the federal government.
The Fish and Wildlife Service (FWS) of the U.S. Department of the Interior (DOI) is responsible for the protection and enhancement of wildlife populations, safeguarding habitat for wildlife, including endangered species, and the inspection of wildlife shipments imported into the United States to ensure compliance with laws and treaties and detect illegal trade (FWS, 2001). DOI’s National Wildlife Health Center (NWHC) was established in 1975 as a biomedical laboratory dedicated to assessing the impact of disease on wildlife and identifying the role of various pathogens contributing to wildlife losses (USGS, 2004). The center provides a multidisciplinary, integrated program of disease diagnosis, field investigation and disease management, research, and training. It also maintains extensive databases on disease findings in animals and on wildlife mortality events. Other DOI programs include the National Biological Information Infrastructure (NBII), a broad collaborative program providing
increased access to data and information on the nation’s biological resources.
HSPD-9
On January 30, 2004, the White House issued a policy directive, Homeland Security Presidential Directive-9 (HSPD-9), which makes the U.S. Department of Homeland Security responsible for coordinating federal programs aimed at protecting U.S. agriculture and food from diseases, pests, and toxins. Veterinary medicine is a critical component of HSPD-9, which significantly expands federal animal health-related initiatives. For instance, the policy calls for creation of a national stockpile of animal drugs and vaccines to respond to serious animal diseases; grants to veterinary colleges for expanding training in exotic animal diseases, epidemiology, and public health; and inclusion of veterinary diagnostic laboratories in national networks of federal and state laboratories (The White House, 2004).
Over the course of 2004, federal response to HSPD-9 and related Homeland Security presidential directives was initiated and included in a USDA Agriculture Emergency Response Training session targeting APHIS animal health personnel and a scientific conference targeting development and use of rapid detection technologies. In January 2005, the Department of Homeland Security released its National Response Plan in response to HSPD-5 (Management of Domestic Incidents), which includes elements supportive of HSPD-9 efforts. The National Response Plan serves to “align federal coordination structures, capabilities, and resources into a unified, all-discipline, and all-hazards approach for incident management” (DHS, 2004d) and includes notation that annexes specific to food and agriculture will be published in subsequent versions of the plan.
State Animal Health Agencies
With few exceptions, states have the greatest responsibilities for animal health, whether for agricultural animals, companion animals, or wildlife. Local authorities will quickly become involved in an animal health emergency, but as soon as resources are overwhelmed, the state will assume responsibility. The federal government oversees issues involving foreign animal and programmatic diseases, veterinary biologics, and national identification and surveillance systems. It also monitors animals at U.S. borders, serves as a reference laboratory, and regulates imported and exported animals and animal products. Most all other animal health issues are dealt with at the state level or as a part of a cooperative state-federal program.
The state departments of agriculture play a vital role in the animal health framework. Through their departments of agriculture, each state assumes responsibility to provide services and regulations regarding the health of agricultural animals. States maintain a list of reportable diseases and require all veterinarians to report disease occurrences. State veterinarians spearhead and direct the efforts of state animal health officials who have intrastate authority for disease reporting, detection, and often, diagnosis. These same officials also serve as key cooperators with their federal government counterparts in the prevention, detection, and eradication of a number of foreign and domestic diseases associated with national animal disease programs. In addition to state veterinarians, a large majority of states also have state public health veterinarians, whose positions and offices are usually associated with departments of public or community health. These officials are responsible for dealing with zoonoses and many other dimensions of veterinary and human public health. State agencies license veterinarians, regulate the intrastate movement of animals, organize emergency response, and are responsible for wildlife. States typically provide regulatory, laboratory, epidemiological, and programmatic support to their livestock, companion animal, and wildlife industries by working through veterinary practitioners, directly with producers, with relevant industries, and with local and federal animal and public health agencies.
A major contribution of the states is the maintenance of animal health diagnostic laboratories. In most states, animal health diagnostic laboratories are associated with state departments of agriculture and, depending on the state, are located at veterinary colleges, land grant university departments of veterinary science, or state agencies for public health. Some states have multiple laboratories. These facilities handle or forward the majority of specimens for diagnosis and monitoring of disease. Private laboratories also play an increasing role in the diagnosis of animal diseases, especially for companion animal species. More information about diagnostic laboratories is described in the section of this chapter entitled “Scientific Preparedness for Diagnosing Animal Diseases: Laboratory Capacity and Capability.”
International Organizations
Many international organizations are involved with issues related to animal disease. Given the increasingly global nature of disease outbreaks, these agencies, the most important of which are highlighted here, play a key role with respect to the animal health framework in the United States. The agencies involved in prevention, detection, and diagnosis of animal diseases consist of several multilateral groups that have different mandates and functions but do not have national regulatory authority.
Nevertheless, as a member of many of these international organizations, the United States is committed to the responsibilities of membership. For example, in the case of the World Trade Organization (WTO), member countries are obligated to bring national laws in conformity with the WTO agreements and adhere to the dispute resolution procedures and outcomes.
The WTO deals with the global rules of trade between nations. Its overriding objective is to help trade flow smoothly, freely, fairly, and predictably. It achieves these objectives by administering trade agreements, acting as a forum for trade negotiations, settling trade disputes, and reviewing national trade policies. The WTO has nearly 150 member countries, which account for over 97 percent of world trade, and approximately 30 other countries are currently negotiating membership. Decisions are made by a consensus of the member countries. Agreements are the legal ground rules for international commerce and are essentially contracts, guaranteeing member countries important trade rights. They also bind governments to keep their trade policies within agreed limits to the benefit of all. While the agreements are negotiated and signed by governments, their true intent is to help the producers of goods and services, exporters, and importers conduct their business and to improve the welfare of the peoples of the member countries.
Critically important for the animal health framework is the Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement), which concerns the application of food safety and animal and plant health standards while allowing countries to set their own science-based standards. Member countries are encouraged to use international standards, guidelines, and recommendations where they exist: “The basic aim of the SPS Agreement is to maintain the sovereign right of any government to provide the level of health protection it deems appropriate, but to ensure that these sovereign rights are not misused for protectionist purposes and do not result in unnecessary barriers to international trade. The standards are developed by leading scientists in the field and governmental experts on health protection and are subject to international scrutiny and review” (WTO, 1998). For example, members may set more stringent standards if there is scientific justification or if it is based on an appropriate assessment of risks and the approach is not arbitrary.
In establishing the WTO/SPS Agreement, three international standard setting bodies were specifically referenced: the World Organization for Animal Health (OIE) for animal health and food safety issues of animal production, the International Plant Protection Convention for plant health, and the Codex Alimentarius for food safety. Of these so-called “three sisters,” the OIE is the most relevant for monitoring animal health. The importance of the OIE in the animal health framework is to promote trans-
parency in the global animal health situation through the collection, analysis, and dissemination of disease and health information; to encourage a coordinated approach to disease outbreaks; to safeguard world trade through animal health standards; to help define and support animal welfare and animal production food safety initiatives; and to improve national veterinary services through the determination of standards and levels of performance.
The OIE is an intergovernmental organization created in 1924 with 28 original member countries; it now has 167 member countries. Through the efforts of scientific commissions and participation of member countries, the OIE determines, revises, and publishes standards contained in the Terrestrial Animal Health Code and Manual and the Aquatic Animal Health Code and Manual. The OIE also collects and disseminates information on animal diseases, including changes in disease status and programs. The OIE has established 157 reference laboratories in 30 countries that are able to diagnose over 80 diseases and address related topics. The OIE also has 15 collaborating centers in 7 countries.
The World Health Organization (WHO), the program for food and agriculture within the International Atomic Energy Agency (IAEA), and the Food and Agriculture Organization (FAO) are branches of the United Nations. In terms of the animal health framework, the FAO focuses on food and animal health in developing countries. FAO activities include emerging and “transboundary” disease problems, i.e., those diseases that move with few barriers from one country to another and significantly hamper animal protein production and trade. Other forms of FAO technical assistance are technical advice, professional information, fielding of experts and consultants, provision of technical documentation, training, and preparation and execution of field projects in close cooperation with animal health services of member countries.
The IAEA program for food and agriculture contains a subprogram on animal health and disease, which is carried out in collaboration with the FAO. The subprogram promotes improved disease management through the application of nuclear and related biotechnologies. In this regard, much effort is focused on diagnostic and surveillance methods and strategies for priority livestock and poultry diseases in developing countries such as foot–and-mouth, exotic Newcastle, and African swine fever.
The WHO deals with diseases affecting humans, including zoonotic diseases. It contributes to animal health wherever human health is affected on an international scale. This regionalized organization has individual country, regional, and worldwide programs and responsibilities. The Veterinary Public Health (VPH) section, which deals with zoonoses and food hygiene, has access to the expertise of the many specialized WHO agencies.
The WHO regional office for the Americas is the Pan American Health Organization (PAHO), whose mission “is to strengthen national and local health systems and improve the health of the peoples of the Americas, in collaboration with Ministries of Health, other government and international agencies, nongovernmental organizations, universities, social security agencies, community groups, and many others.” The PAHO assists the countries of Latin America and the Caribbean in dealing with health issues through their scientific and technical experts located in the United States, country offices, and scientific centers. For example, PANAFTOSA, a regional laboratory located in Brazil, was originally established to provide diagnoses of specific diseases, such as foot-and-mouth disease. The PAHO also provides support for disease eradication efforts in infected regions and neighboring countries.
Another organization operating in the Americas is the Inter-American Institute for Cooperation in Agriculture (IICA). IICA supports national veterinary services’ efforts to: (1) develop regulatory mechanisms, science-based technical capacity, and sustainable institutional infrastructures; (2) apply the provisions of the Sanitary and Phytosanitary Agreements of the WTO as well as the decisions taken in the international reference organizations: OIE, IPPC, and Codex Alimentarius; and (3) assist countries with early recognition of emerging diseases and issues.
The International Regional Organization on Agriculture Health (OIRSA) works with the seven countries of Central America, Mexico, and the Dominican Republic (OIRSA, 2004). OIRSA provides support for the modernization of national services and related sanitary actions. Specific actions follow the disciplines outlined in the WTO/SPS Agreement and include harmonization, risk analysis, equivalence, and regionalization. It also seeks to strengthen inspection and quarantine control related especially to interregional trade and programs of prevention and control assistance with the harmonization of norms, risk analysis methodologies, surveillance, inspection controls, and support for disease eradication.
A discussion of the role of international developmental agencies, such as the U.S. Agency for International Development (USAID), foundations, nonprofit organizations, and regional banks, is beyond the scope of this report. Worth mentioning because of its relation to the WTO/SPS Agreement is the Standards Trade Development Facility (STDF), which is coordinated by the WTO/SPS Secretariat to assist countries in improving their sanitary status. Initial funding for the STDF was provided by the World Bank, one of the world’s largest sources of development assistance, providing low-interest loans, grants, and interest-free credit to governments in developing countries for infrastructure improvements. The STDF is a global program providing technical assistance and capacity building to developing countries in implementing the measures contained in the SPS
Agreement. The STDF is both a financing and coordinating mechanism working with countries to improve their sanitary status and thus comply with and benefit from the SPS Agreement (STDF, 2004).
The subject of the role of international organizations in helping to secure animal health in the United States and globally is one that merits attention in greater detail in future examinations of the animal health framework.
Supporting Institutions, Industries, and Organizations
Supporting institutions, industries, and organizations also play a role in preventing, detecting, and diagnosing animal diseases. These include educational and research institutions, professional societies, and animal commodity groups. An in-depth examination of all of these entities is not presented here, but like international organizations, they play a role that should be examined more closely in future analyses.
TECHNOLOGICAL TOOLS FOR PREVENTING, DETECTING, AND DIAGNOSING ANIMAL DISEASES
Early detection, identification, and diagnosis are critical for limiting the extent of an animal disease outbreak and protecting the public from potential zoonotic disease exposures. Despite recent advances in technologies—including DNA-based techniques, novel sampling approaches, and more rapid, automated, and nonsubjective analytic tools—the classic laboratory techniques, which have changed little in the past 50 years, remain the most common means of identifying animal disease agents. Traditional bacteriological, fungal, viral, toxicological, and serological testing methods, though tried and true for several generations, require considerable investments in time (hours to days), extensive technical training and scientific judgment, and the prior recognition of a clinical problem in order to trigger testing of the animal or animal population. Sometimes the only material available for analysis is a dead or dying animal, which is frequently the case with wildlife and occasionally with food-animals. In those cases, immunohistopathological methods are needed to examine cellular changes caused by infections, such as the presence of inflammatory cells, viruses, or antibodies.
State-of-the-art scientific approaches that would enhance early detection and diagnosis of human disease are often developed by and for basic research and military applications and then rapidly adopted by first-responder and public health communities. The same technologies have been significantly slower to transition into the animal health arena. As noted in a prior NRC report, technological advances that speed and increase the
reliability of the detection and diagnostic process have not been aggressively applied to agriculturally important pathogens, nor have they been inexpensive or field-deployable (NRC, 2003a). The same situation applies to virtually all animal disease agents, whether affecting wildlife, livestock, or companion animal species.
Transitional or applied research and federal funding sources to support the development, validation, and/or implementation of technological tools specifically for animal health applications are limited. Furthermore, economic incentives for the private sector do not traditionally support these development efforts. Federal and state laboratories across the country often have difficulties in acquiring advanced technologies such as robotics to increase the numbers of specimens or tests that could be processed with minimal human intervention (surge capacity), instrumental analyses (e.g., gas chromatography, mass spectrometry) for high resolution toxin and protein detection, and DNA-based tools that provide for rapid and sensitive agent detection or identification. There are multiple reasons for this situation, such as constraints on space, the lack of technical know-how or trained staff, or adequate numbers of samples to justify the acquisition of expensive equipment. Homeland security initiatives related to bioterror preparedness have improved both federal and state laboratory access to rapid DNA-based diagnostic tools such as realtime or quantitative polymerase chain reaction (PCR); however, as an industry, animal health lags years behind the military, first-responder, and public health communities in its implementation and use of advancing technologies.
In recent years, the movement of diagnostic assays out of the confines of the laboratory and into the field, closer to the source of the disease, has been made possible by scientific advances that provide the technology to shrink laboratory equipment by orders of magnitude (see Box 2-2 for “Examples of Evolving Technologies”). These technological advances, including miniaturization and microfluidics, allow use of increasingly smaller fluid volumes and microscopically thin equipment components and wiring, all of which allow chemical and physical reactions to occur faster and more uniformly. Devices that once required feet of laboratory space, relatively large volumes of clinical material, and large quantities of expensive assay components are now available in high-speed, portable, and in some cases hand-held forms. Sophisticated real-time PCR equipment, available just a decade ago only in high-tech laboratories, is now accessible to buyers in portable handheld or backpack versions targeting the first-responder and security communities. Access to size-reduced laboratory equipment has allowed fully functional mobile high-tech laboratories to be moved on site for immediate human health response capability, as seen in 2001 in Washington, D.C., during the anthrax letter scare and in 2002 in Salt Lake City for the Olympics. Similar portable laboratory approaches
|
BOX 2-2 PREVENTION
DETECTION AND DIAGNOSIS
|
have been proposed for rapid on-site response to critical animal health issues, such as for a potential foot-and-mouth disease (FMD) outbreak in an area not immediately accessible to regional laboratory services.
Advances in biostabilization—essentially freeze-drying of unstable assay components—have also allowed movement of assays from the traditional laboratory to the field, as well as provided the foundation for fully automated on-site detection systems that continuously sample the air (termed “sniffers”) and monitor high-risk environments. In 2000, author Richard Preston envisioned a portable environmental “sniffer” paired with PCR for genome-based detection of a bioterror agent, compacted into the size of a briefcase (Preston, 1997). Preston’s novel, a fictionalized account of bioterrorism, was based on real-world developments that ultimately led to the U.S. Department of Homeland Security’s BioWatch program. Since 2003, BioWatch and prototype commercial environmental sampling and test systems have continuously monitored selected public venues, including subways, banks, and post offices, for human biothreat agents (CDC, 2005; OSTP, 2005). Similar automated sampling and monitoring of high-risk animal environments for high-economic risk pathogens have not received equal attention.
The local public health community can choose to use a broad array of diagnostic tools ranging from simple rapid detection tests, such as at-
home pregnancy kits, to more sophisticated assay formats, such as PCR. These formats are common, readily available, and standardized for the public health community, but not so for the community of veterinary laboratories and clinics. Nanotechnology, the ability to build at a scale of a billionth of a meter, is being described as the next technical revolution and may allow the development of electronic circuitry 1,000 times smaller than current microchips. Among others, one application for human and animal health includes the potential for embedded medical monitoring (chips inserted under the skin).
SCIENTIFIC PREPAREDNESS FOR DIAGNOSING ANIMAL DISEASES: LABORATORY CAPACITY AND CAPABILITY
Overview
The nation’s animal health laboratory system is composed of federal, state, university, and commercial laboratories. The federal component is referred to as the National Veterinary Services Laboratories (NVSL). The NVSL, which is part of USDA-APHIS, provides diagnostic services through two testing facilities located in Ames, Iowa, and the Foreign Animal Disease Diagnostic Laboratory (FADDL) on Plum Island, New York. These laboratories perform the following functions: oversee and conduct laboratory testing in conjunction with federally mandated eradication programs for diseases such as brucellosis, pseudorabies, and tuberculosis; screen samples for the presence of exotic diseases at the request of federal and state regulatory staff; assist in investigating unusual agricultural animal disease occurrence in the United States; perform tests to meet animal export requirements; conduct testing for routine support of national and state animal health management; and serve as reference laboratories for certain infectious diseases (USDA, 2004b). However, the vast majority of routine diagnostic and animal health management analyses on domestic animals in the nation are conducted daily by state and university-affiliated veterinary diagnostic laboratories. The same is true for wildlife diseases. With few exceptions (for example, the U.S. Geological Survey’s [USGS] National Wildlife Health Laboratory in Madison, Wisconsin; the Southeast Cooperative Wildlife Disease Study [SECWDS] in Athens, Georgia, which is a federal-state partnership; the FWS Forensics Laboratory in Ashland, Oregon; and scattered state wildlife agency-affiliated laboratories), routine investigation of wildlife diseases in the United States occurs in state/university diagnostic laboratories. Diagnostic work on zoo and exotic animal species is performed by laboratories associated with large municipal zoos and wildlife parks and by private zoo consultants who generally are board-certified pathologists. State, university, and commer-
cial veterinary diagnostic laboratories also investigate diseases in zoo and exotic animals, often in response to requests from smaller zoos. Taxon advisory groups and species survival plans within the American Zoo and Aquarium Association may recommend particular laboratories for certain tests for the sake of consistency. A zoo and exotic animal laboratory network was recently established based largely on initial detection of West Nile virus infections in that environment (Nolen, 1999; Ludwig et al., 2002). This small but rather active network of diagnosticians and laboratories seeks to expand and integrate its activities with other veterinary and public health laboratory networks and is a key partner in detecting diseases that often emerge at the interface of exotic animals, domestic animals, and humans. Toward that goal, the International Species Information System is in the process of developing an information technology tool, termed the Zoological Information Management System (ZIMS), to replace the limited database software for zoo species.
The classical approaches of diagnosing diseases in agriculture cannot be transposed onto wildlife diseases. Whereas domestic animal populations can be manipulated and individual animals can be examined with relative ease to determine the prevalence or incidence, neither can be done with wildlife. Thus field work requires specialized expertise that is supported by research in techniques such as modeling, application of technologies such as GIS, and knowledge of pathology for field necropsy and/ or collection of specimens. Diagnostic laboratory methodology, in contrast, is essentially the same as for domestic animals. Information about current wildlife disease issues is available through the Wildlife Diseases Information Node of the National Biological Information Infrastructure (NBII).
Laboratory Networks
National Animal Health Laboratory Network (NAHLN)
In June 2002, the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 was signed into law. Section 335 authorizes the Secretary of Agriculture to develop an agricultural early warning surveillance system enhancing capacity and coordination between state veterinary diagnostic laboratories, federal and state facilities, and public health agencies, and provides authorization for Congress to appropriate funding to the National Animal Health Laboratory Network (NAHLN) (McElwain, 2003). The NAHLN addresses diagnostic needs for early diagnosis of exotic and emerging diseases and for routine animal disease surveillance, as well as diagnostic capacity for disease investigations, response and control, and eradication programs. The national labo-
ratory concept was first developed in discussions between the American Association of Veterinary Laboratory Diagnosticians (AAVLD) and NVSL, resulting in a Memorandum of Understanding signed by NVSL and AAVLD in 2001 to “cooperatively improve animal health diagnostic services in the United States.” In addition, the Safeguarding Review, commissioned by USDA to comprehensively review the federal system for safeguarding animal health in the United States, emphasized the need for a comprehensive and coordinated network (NASDARF, 2001). Initial support through cooperative agreements to 12 state diagnostic laboratories to establish the pilot NAHLN with NVSL was provided through emergency appropriation of Department of Defense (DoD) funds for Homeland Security through the USDA Cooperative State Research, Education, and Extension Service (CSREES) (USAHA, 2002).
The philosophy behind the design and implementation of NAHLN is that animal disease surveillance functions most effectively as a shared responsibility between federal and state animal health agencies. During a disease outbreak, state veterinary diagnostic laboratories would provide early diagnosis and significant surge capacity. State labs would assist and define herds for depopulation, delimit the extent of the outbreak, and conduct follow-up surveillance to determine a “disease-free status” (USDA, 2004d). The development of the NAHLN heralded a fundamental change in the animal health laboratory infrastructure in the United States. For the first time, the need for state laboratories to test for exotic pathogens was acknowledged. Perhaps more important, there was recognition of the responsibility of state laboratories to test in service to their stakeholders.
The main goals of the NAHLN are to expand detection and response measures for pathogens that threaten animal agriculture and bolster laboratory capability for select agents with support for personnel, equipment, testing, training, and information technology. Among the elements being implemented in the NAHLN are development and deployment of standard diagnostic approaches for identification of select agents; rapid diagnostic techniques, modern equipment, and experienced personnel trained in the detection of emergent, foreign, and bioterror agents; a national training program that ensures competency and consistency in diagnostic testing using new equipment and reagents; proficiency testing and quality assurance; and upgraded facilities that meet biocontainment requirements. The NAHLN is also developing an information technology tool to facilitate data sharing among animal health agencies through secure, automated, two-way communications to create a national repository for animal health data; bolstering cooperation and communication among animal health officials through maintenance of confidentiality of source data; and providing alerts at an appropriate response level (USAHA, 2002).
The pilot NAHLN involved 12 state/university diagnostic laboratories and was charged with developing capacity and surveillance programs for eight high-priority foreign animal diseases considered to be of bioterrorist threat (FMD, hog cholera, African swine fever, rinderpest, contagious bovine pleuropneumonia, lumpy skin disease, highly pathogenic influenza, exotic Newcastle disease). Other agents of interest, such as vesicular stomatitis, West Nile encephalitis, Rift Valley fever, Nipah encephalitis, Hendra encephalitis, scrapie, CWD, and BSE, will be added in the future (USAHA, 2002). NAHLN laboratory personnel have been trained in the standard nomenclature used in reporting laboratory results. It is anticipated that the number of NAHLN laboratories and the breadth of diseases covered in the NAHLN will increase significantly by FY 2009, creating a broader pool of expertise that can be tapped for surge testing capacity in an outbreak (USDA, 2004d). USDA APHIS and the CSREES recently agreed to expand the NAHLN definition to include, in addition to the original 12 laboratories in the pilot program, all federal and state laboratories currently contracting with USDA for scrapie, CWD, and AI/END surveillance testing. These additional laboratories, however, did not receive any additional funding beyond appropriate equipment to address infrastructure needs as discussed above.
The NAHLN administrative structure includes a national coordinator who reports to the director of the NVSL and a steering committee. In addition to the NVSL director and the NAHLN coordinator, the steering committee has representation from the state laboratories through AAVLD (two NAHLN laboratory directors, one non-NAHLN laboratory director, and the current president of AAVLD), a state veterinarian, and CSREES national program leader for homeland security.
Laboratory Response Network (LRN)
USDA APHIS and AAVLD are also partnering with the Centers for Disease Control and Prevention (CDC) to enlist state veterinary diagnostic laboratories into the CDC Laboratory Response Network (LRN). The LRN was established in 1999 to prepare the U.S. response to bioterrorism. The mission of LRN and its partners is to maintain an integrated national and international network of laboratories that is fully equipped to quickly respond to chemical or biological acts of terrorism, emerging infectious diseases, and public health threats and emergencies (Gilchrist, 2001). CDC runs the LRN program with direction and recommendations provided by the Association of Public Health Laboratories, the Federal Bureau of Investigation (Department of Justice), the AAVLD, the American Society for Microbiology, EPA, USDA, DoD, FDA, and DHS (CDC, 2005).
LRN is a consortium of 137 laboratories that can provide immediate and sustained laboratory testing and communication in the event of public health emergencies, particularly bioterrorism-related events. The network includes the following types of laboratories:
-
Federal—laboratories at CDC, USDA, FDA, and other facilities run by the federal agencies.
-
State and local public health—laboratories run by state and local departments of health.
-
Military—laboratories operated by the DoD, including the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID) at Fort Detrick, Maryland.
-
Food testing—FDA laboratories and others that are responsible for ensuring the safety of the food supply.
-
Environmental—laboratories that are capable of testing water and other environmental samples.
-
Veterinary—some LRN labs, such as those run by USDA and state veterinary diagnostic laboratories, that are responsible for animal testing. Some diseases can be shared by humans and animals, and animals often provide the first sign of disease outbreak.
-
International—laboratories located in Canada, the United Kingdom, and Australia.
The LRN laboratories are designated as either national, reference, or sentinel. National laboratories (including those at the CDC and USAMRIID) have the unique resources to handle highly infectious agents and the ability to identify specific agent strains (CDC, 2005). Reference laboratories, sometimes referred to as confirmatory reference, can perform tests to detect and confirm the presence of a threat agent. These laboratories ensure a timely local response, rather than having to rely on confirmation from CDC labs. Sentinel laboratories represent the thousands of hospital-based and some veterinary labs that are in the front lines. Their responsibility is to refer a suspicious sample to the right reference laboratory. For instance, in the case of severe acute respiratory syndrome (SARS), CDC laboratories identified the unique DNA sequence of the virus that causes the disease. LRN then developed tests and materials needed to support these tests and gave LRN members access to the tests and materials (CDC, 2005).
Veterinary Diagnostic Laboratories
Each state has a publicly funded veterinary diagnostic laboratory. The sizes and diagnostic capabilities of these laboratories vary widely, rang-
ing from a few states with rudimentary laboratories that perform only serology for one or more eradication program diseases such as brucellosis to state systems that offer a complete range of diagnostic services for all economically important agricultural animal species, companion animals, and wildlife in multiple locations within the state. Some laboratories specialize by species, primarily serving the local needs within a specified geographic area, but the majority of state laboratories cover a broad range of species and conditions. Many are directly affiliated and co-located with a university-based college of veterinary medicine or veterinary science department.
The state laboratory system is represented nationally by the AAVLD. One of AAVLD’s cardinal activities is to accredit publicly funded veterinary diagnostic laboratories. The accreditation program meets international standards established by the World Animal Health Organization (through the OIE). While the OIE does not conduct an accreditation program, the Standards Commission does provide standards to member countries as a guide for accrediting laboratories conducting assays for infectious diseases on the OIE lists (“OIE Quality Standard and Guidelines for Veterinary Laboratories: Infectious Diseases”). The OIE reference standards, with minor exceptions, reflect ISO17025 laboratory accreditation standards.
There currently are 38 AAVLD accredited, full-service laboratories/ systems in the United States, located in 34 states, and two AAVLD accredited laboratories in two Canadian provinces. Accredited laboratories undergo a site visit every 5 years unless major changes in funding or personnel warrant an earlier visit. Importantly, accredited laboratories maintain a full cadre of disciplinary specialists and laboratory sections that include pathology, bacteriology, virology, parasitology, and toxicology. In some cases, a few of these services are contracted to another accredited laboratory. Nearly all accredited laboratories have board-certified specialists who head laboratory sections. Specialized molecular assays such as PCR are in common use, and complete workup of unusual and challenging diagnostic cases is routine. The broad capability of these laboratories was an underappreciated resource for both diagnostic testing capacity and capabilities. One example is the wealth of expertise and equipment in toxicology. As the nation’s public health laboratories struggle to prepare for the potential challenge of toxins, whether biological or chemical, intentionally introduced in environmental or food matrices, the board-certified veterinary toxicologists, analytical chemists, sophisticated equipment, and familiarity with many of these potential toxins extant in accredited veterinary diagnostic laboratories simply must not be overlooked.
Although state laboratories offer a rich resource of diagnostic services and data, and the accreditation process ensures the quality of these ser-
vices and data, a lack of uniformity among laboratories limits the value of compiled data. This is primarily due to the use of different standard operating procedures and assays that, although withstanding the test of time, have not undergone rigorous validation to meet current international standards. To that end, the AAVLD recently began a process of compiling a table of approved methods to use as a standard for accreditation. Once completed, standard operating procedures for each approved method will be available to all, helping to promote standardization nationwide and to increase the value of diagnostic data routinely generated in state laboratories.
The size and quality of capital assets in the state laboratories vary widely, from aging buildings in which it is difficult to meet current standards for security and biocontainment, to recently completed new buildings incorporating the latest standards for good laboratory practice, security, biocontainment, and waste disposal. Capital infrastructure in many of the state laboratories is in need of modernization, but most state budgets currently struggle to maintain the current buildings and cannot begin to address new capital investments required to meet contemporary standards. In an unpublished 2003 informal survey conducted by the AAVLD to assess Biosafety Level 3 (BSL-3) laboratory and necropsy capacity in state laboratories, 23 laboratories of 35 responding (from 33 states and most accredited laboratories) had a BSL-3 laboratory, ranging from 200 to 2,000 net square feet (Terry McElwain, AAVLD, unpublished data, 2003). However, there were no BSL-3 necropsy suites in any of the state laboratories that responded. Some had remote access to a BSL-3 necropsy laboratory in another location, primarily associated with Animal Biosafety Level 3 (ABSL-3) animal housing. In 2004, one state laboratory completed a new facility that has a large BSL-3 necropsy suite, and at least one other new state laboratory, to be completed in 2006, will also have a BSL-3 necropsy suite. For additional information on BSL-3 laboratories, see the section on Containment Facilities later in this chapter.
Commercial Laboratories
Over the past 10 years, commercial for-profit laboratories have moved forcefully into the realm of animal diagnostics. Initially the primary focus of these laboratories was on companion animal pathology, both clinical and anatomical. More recently, commercial laboratories have captured an increasing share of the routine serological and microbiological work that in the past was performed almost entirely by federal and state laboratories. The availability of approved assay kits for diagnostic work in animals and the USDA policy of approving small laboratories in veterinary practices and other private labs to perform testing for reportable diseases such as equine infectious anemia has facilitated this change. Few of these
laboratories have the capability to perform routine necropsies and few offer viral culture or toxicology services. This has placed state laboratories in an interesting paradox, because necropsy, toxicology, and virology have not been profitable services for state laboratories. Thus, like their commercial counterparts, state laboratories have relied on high-volume services such as serology to enhance their revenues. In addition, commercial laboratories may not always have operational relationships with regulatory agencies as seamless as those of state laboratories. As a consequence, test results in these laboratories may not be as readily available for analysis in passive surveillance programs (except for results on reportable or notifiable diseases).
Development of private laboratories in corporate food animal production systems has also impacted animal disease diagnostics. This trend is due, at least in part, to the technological advances that have made commercial kits widely available, but it is also driven by the development of hazard analysis and critical control point (HACCP) and other quality assurance programs and by the confidentiality necessary in the competitive world of food production. The development of in-house laboratories has been most notable in the swine and poultry industries, but to some extent it is also practiced in commercial aquaculture and other industries. Again, laboratory results are not available publicly, and the development of in-house diagnostics in some production systems has replaced consultations with health professionals. For example, a 2000 study revealed that over 20 percent of swine production units (primarily small operations) reported no veterinary visits in the previous 12 months (USDA APHIS-VS, 2001). Rapid recognition, diagnosis, and reporting of health problems arising from the introduction of exotic agents are absolutely essential for limiting the spread of infectious diseases. The development of vertical integration in laboratory analysis thus provides a special challenge in disease control. The extent and effectiveness of quality assurance programs in private laboratories is difficult to assess. Opportunities for outside review and oversight of these laboratories are limited unless they utilize the International Organization for Standardization (ISO) accreditation or some other system of assessment by auditors, since AAVLD offers accreditation only for publicly funded laboratories. The USDA does assess laboratory practices before approving laboratories to run assays for reportable diseases.
ANIMAL HEALTH RESEARCH
Research on animal health issues is funded by a variety of means and at a range of levels, usually depending on three main factors: the affected species, the degree of relevance to human health, and the economic impact of the animal disease. At one end of the spectrum, disease issues
impacting only companion animals or wildlife and with no relevance to human health are traditionally very poorly supported. Examples here might include coronavirus infection in cats (prior to the outbreak of SARS) or parasitic infections of wildlife. In the absence of federal support, private charities and foundations, academic institutions, and industry typically fund research on these issues, and usually at a very modest level (Eisner, 1991). Companion animal research is conducted primarily by pharmaceutical and food companies that have research and development units; this research is usually product-oriented research that covers the span from basic research all the way to clinical trials.
At the other extreme, animal health issues that have direct relevance to human health, as well as significance to animal populations, are usually funded by federal human health sources (National Institutes of Health), and often at munificent levels. Examples are bovine spongiform encephalopathy and highly pathogenic avian influenza, two diseases that impact very economically important agricultural species, affect international trade, but also spell possibly fatal outcomes in humans. Falling in between these two ends of the spectrum are the majority of animal health problems, with USDA, and more recently DHS, supporting most of the research.
In 1994, the Federal Crop Insurance Reform and Department of Agriculture Reorganization Act authorized the Secretary of Agriculture to appoint an undersecretary for research, education, and economics (REE). Four agencies were brought into the REE mission, including the Agricultural Research Service (ARS), the CSREES, the Economic Research Service (ERS), and the National Agricultural Statistics Service (NASS). The REE agencies are also required to work with the USDA action and regulatory agencies in support of their operations and missions (U.S. Congress, 1994).
The most recent REE Strategic Plan identifies five key outcomes as goals of its research effort: (1) a highly competitive global agricultural system; (2) a safe and secure food and fiber system; (3) healthy, well-nourished children, youth, and families; (4) greater harmony between agriculture and the environment; and (5) enhanced economic opportunity and quality of life for citizens and communities (USDA, 2002d).
In 2002, ARS, CSREES, and ERS allocated $120 million, $39 million, and $0.65 million, respectively, for animal health research (Karen Lawson, personal communication, 2005). In 2001, REE agencies collectively employed 4,132 science-related technical staff, with the largest portion (approximately 75 percent) employed by ARS (USDA, 2001). In 2004, ARS employed 282 scientists in its animal health and protection programs (USDA ARS, 2004).
As the largest REE agency in overall program and staff budget (approximately $1 billion) and as the principal in-house research agency for
the USDA, the ARS uses its funds to support a blend of basic and applied research activities (NRC, 2003a). Through its 22 national programs, the ARS has created a substantial infrastructure of research laboratories across the United States with 244 ARS laboratories at 103 locations and 41 work sites. The laboratories, over 100 of which are used for research on agricultural animals, include over 3,000 buildings and the agency covers 400,478 acres of land dedicated to research (GAO, 2000).
The major source of funding for university-based research is CSREES, which in 2002 invested approximately $29 million in animal health research at universities out of $1.04 billion appropriated for all research and other activities (Peter J. Johnson, personal communication, 2005). In 2001, CSREES employed 162 science-related technical staff to oversee the funding of these extramural research activities. The research and education activities of CSREES were originally authorized under the Hatch Act of 1887. Under its current authorities, CSREES assists research and education programs at state institutions, including state agricultural experiment stations, schools of forestry, 1890 colleges, land-grant institutions, colleges of veterinary medicine, and other eligible institutions.
CSREES is also charged with implementing USDA’s higher education mission in the food and agricultural sciences. The cooperative extension system is a national educational network of partners from CSREES, land-grant university cooperative extension services, and cooperative extension services in the 3,150 counties of the United States. The work of the cooperative extension service was originally authorized by the Smith-Lever Act of 1914 (U.S. Congress, 1914). The educational arm of CSREES represents an important function in transferring knowledge produced by researchers to agriculturalists who could then apply research results to improve production and resolve problems. CSREES has identified 59 programs that span the biological, physical, and social sciences and that are related to agricultural research, economic analysis, statistics, extension, and higher education.
In 2004, CSREES received funding of $1.124 billion. Just under half of the research funds administered by CSREES are “formula funds,” based on formulas related to the size of rural and farm populations and distributed to state agricultural experiment stations. Under formula funds, the Animal Health Research and Disease Program (Section 1433) received only $4.5 million in 2004. Competitive, peer-reviewed grants for research and education programs make up approximately 25 percent of CSREES research funds. The National Research Initiative (NRI) grants are part of this external funding and in 2004 were appropriated at $164 million. The NRI is a competitive grant and peer-review research program. Some of these funds were used for research to protect natural resources including
wildlife, optimize livestock health and productivity, and protect human health and food safety. Special grants are also used for selected projects and are largely based on congressional directives.
Since the establishment of DHS, additional funding has become available for animal health issues that are considered of national security interest. This includes most foreign animal diseases, as a deliberate introduction of one of these diseases could have severe economic consequences. Recently, two different DHS Centers of Excellence, a Center for Foreign Animal and Zoonotic Diseases, and a Center for Food Safety, were established at land-grant universities.
A forthcoming NRC report Critical Needs for Research in Veterinary Science (NRC, 2005) will examine the broad range of fields in which veterinary researchers can contribute, including research in comparative medicine. Diseases cause a significant amount of morbidity and mortality in both people and animals. To help alleviate this suffering, biomedical research has included the use of animals as one component of research to understand, treat, and cure many human and animal diseases. The parallels between animal physiology, genetics, and pathology have been noted for a long time, and the study of animals to understand human disease is also an accepted area of biomedical research. Animals develop many of the same diseases as humans and are susceptible to many of the same bacteria, viruses, and parasites. Animal models have been used successfully for research when they share similar and relevant characteristics with people. Comparative medical research uses animals to simulate biological functions and help link molecular, cellular, tissue, organ systems, and organism systems (NRC, 2004d). Unlike discipline-oriented researchers, comparative medical scientists bridge the interface between fundamental and basic science and human health. Beyond the benefits to both of these research areas are the direct benefits to animals themselves.
However, the present involvement of veterinarians in comparative medicine is insufficient, and current trends to support that participation are disconcerting. Federal funds for animal research have been relatively static and the prospects for significant increases in the future seem unlikely. While the National Institutes of Health (NIH) and National Science Foundation (NSF) have enjoyed substantial increases in funding, animal health has largely benefited as a by-product from the study of animal models and comparative medical systems. Concern persists about the lack of new animal scientists and researchers for both animal and biomedical research in the future. The contemporary problems of food safety, zoonotic diseases, emerging diseases, and agro- and bioterrorism have stimulated interest in these fields of research, but accelerated interdisciplinary and new intergovernmental programs have been slow to develop.
If the contemporary problems brought about by the convergence of human and animal health are to be adequately addressed, comparative studies will need a national focus and sustained attention.
Scientific research and the investigators who carry out that work must be a central part of the animal health framework. Such research is critical to reducing animal disease and suffering and to the development of new products, treatments, and techniques for animals that indirectly benefit society. The efficiency and productivity of animal agriculture over the years has been a function of successful research on animal nutrition, effective production systems, and reducing the incidence of animal diseases.
Containment Facilities
Of particular note in the context of discussing research on animal health is the issue of containment facilities. Studies of infectious diseases, whether of interest solely for animal health or as animal models of human disease, need to be undertaken in a manner that ensures safety for the operator as well as the general public. As such, there are specified containment levels for the various organisms that mandate certain structural and procedural necessities. Containment facilities are classified as Biosafety Levels 1 through 4, with 4 being the most restrictive (HHS, 1999). Biosafety level 3 (BSL-3 or BSL-3 Ag) provides the high degree of containment that is needed when studying a variety of organisms with a recognized potential for significant detrimental impact on animal or human health or on natural ecosystems (Box 2-3).
This level of containment requires stringent measures such as protective clothing and respirators; filtered air supply and exhaust; sterilization of materials originating from the facility, including animal waste; and strictly controlled entry and exit. The number of BSL-3 laboratories in the United States is limited; in particular, there are very few BSL-3 Ag entities due to their demanding and expensive engineering and construction requirements (USDA, 2002b). Consequently, even with full institutional volition and funding to undertake research with certain agents, such as classical swine fever, monkeypox, or tularemia, studies can only be conducted if the building meets the design standards required. In 2003, the National Institute of Allergy and Infectious Disease announced awards to 11 universities to build biosafety laboratories as part of a regional and national network for infectious disease research under its Biodefense Research Agenda. Of those selected, two of the regional centers are associated with veterinary science: the University of Missouri-Columbia and Colorado State University. Each plans to build a BSL-3 facility.
|
BOX 2-3 Animal Biosafety Level 3 (ABSL-3): Involves practices suitable for work with animals infected with indigenous or exotic BSL-3 agents that present the potential of aerosol transmission and of causing serious or potentially lethal disease. ABSL-3 builds upon the standard practices, procedures, containment equipment, and facility requirements of ABSL-2. Biosafety Level 3 (BSL-3): Used with agents that may be indigenous or exotic to the United States that can be contracted by the respiratory route and may cause serious or lethal diseases to humans or animals or cause moderate economic loss to the animal industries. The BSL-3 facility is designed to support research activities with serious or potentially lethal biohazardous materials or infectious substances. Biosafety Level 3 Agriculture (BSL-3Ag): Designation for animal facilities in which research involves BSL-3 biological agents that present a risk of causing great economic harm should they infect the indigenous animal population (e.g., foot-and-mouth disease). Using the containment features of the standard BSL-3 facility as a starting point, BSL-3Ag facilities are specifically designed to protect the environment by including almost all of the features ordinarily used for BSL-4 facilities as enhancements. All BSL-3Ag containment spaces must be designed, constructed, and certified as primary containment barriers. Colloquially, they may be referred to as ABSL-3 Ag. SOURCE: Biosafety in Microbiological and Biomedical Laboratories, 4th edition. Available at http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm |
INTERNATIONAL ISSUES
Deterrence and prevention of animal disease in the United States involve global strategies that are directed at reducing a potential threat before it reaches the U.S. borders and a border strategy that focuses on interdicting a threat agent at U.S. ports of entry (NRC, 2003a). An overview of international organizations involved in prevention, detection, and diagnosis is provided earlier in this chapter, so the discussion below focuses on components of the framework responsible for interdicting threat agents at U.S. ports as well as during the sale and transport of animals (particularly exotic animals) once they have entered the country.
Importation, Sale, and Transport of Animals
In 2003, the United States exported 125,000 head of cattle and imported about 1.52 million head; there were 134,000 live hogs exported and 7.25 million live hogs imported (Beghin et al., 2004). Every year, a variety of sources provides millions of animals to the exotic companion animal trade. Animals are captured from their native habitat and transported to various countries to be sold as companion animals. Others are surplus animals from zoos or their offspring. Backyard breeders also supply exotic companion animals (API, 2003). Consequently, the importation of animals is an important concern of the animal health framework.
In 2002, more than 22 federal agencies were consolidated into the Department of Homeland Security (DHS), including components of APHIS that conduct inspection and animal quarantine activities at U.S. ports and the Plum Island Animal Disease Center (PIADC). Approximately 2,600 employees from the APHIS Agriculture Quarantine and Inspection (AQI) force became part of the DHS Border and Transportation Security’s Bureau of Customs and Border Protection (CBP) on March 1, 2003 (USDA APHIS, 2003a).
Although DHS is now responsible for protecting the nation’s borders, USDA APHIS, continues to set agricultural policy through risk assessment, pathway analysis, and rule making, including specific quarantine, testing, and other conditions under which animals, animal products, and veterinary biologics can be imported. These policies are then implemented by DHS (USDA APHIS, 2003a). USDA APHIS-VS port veterinarians inspect live animals at border ports and place animals in quarantine until testing is completed. They are located at 43 VS areas and report to the veterinarian in charge of the VS-Area Office (Joseph Annelli, personal communication, April 2004). With agricultural border inspectors now a part of DHS, VS has identified a need for developing new protocols for training and interacting with these inspectors, as well as a need to work with DHS to implement improvements recommended in the Animal Health Safeguarding Review regarding pest exclusion activities at U.S. borders in its strategic plan (USDA, 2004d).
The Secretary of the Department of Health and Human Services (HHS), through the CDC, has the authority to make and enforce regulations to prevent transmission of infectious disease from foreign countries into the United States (42 CFR70 and 71). Under these regulatory authorities, CDC has established embargoes on prairie dogs and other animals that could carry the monkeypox virus and on birds from specified Southeast Asian countries (CDC, 2003d; CDC, 2004b). Table 2-2 provides a summary of agencies and functions involved in border control and a review of the events related to their organization.
TABLE 2-2 DHS Border and Transportation Security (BTS), Bureau of Custom and Border Protection (CBP), and Other Components Addressing Animal Diseases
|
Agency |
Agency Description, Responsibilities, & Major Events |
|
Border and Transportation Security (BTS) |
• The largest of the 5 DHS directorates. • Includes former U.S. Customs Service, border security function/enforcement division of INS, APHIS, Federal Law Enforcement Training Center, and the Transportation Security Administration. • Responsible for securing the nation’s air, land, and sea borders. • Responsible for securing the nation’s transportation systems and enforcing the nation’s immigration laws. |
|
Bureau of Custom and Border Protection (CBP) |
• March 1, 2003, approximately 42,000 employees were transferred from U.S. Customs Service, INS, and APHIS to the new CBP, a new agency under the BTS directorate within the DHS. • Approximately 2,700 former USDA employees from the AQI program and APHIS were transferred into DHS. • Former APHIS-PPQ personnel at ports of entry (POEs) who were directly involved in terminal/plane inspections (100% time) were transferred to DHS; those with 60-70% time not doing inspection at terminals/ planes were not transferred. • The agricultural import and entry inspection functions that were transferred include: reviewing passenger declarations and cargo manifests to target high-risk agricultural passengers or cargo shipments. • The new CBP also carries out the traditional missions of the predecessor agencies making up CBP (seizing illegal drugs and other contraband at the U.S. border; apprehending people who attempt to enter the U.S. illegally; detecting counterfeit entry documents; determining the admissibility of people and goods; protecting U.S. agricultural interests from harmful pests or diseases; regulating and facilitating international trade; collecting duties and fees; enforcing all laws of the United States at borders). |
|
Office Field Operations (OFO) |
• Oversees over 25,000 employees at 20 field operation offices (OFOs), 317 POEs, and 14 preclearance stations in Canada and the Caribbean. • Responsible for enforcing customs, immigration, and agriculture laws and regulations at U.S. borders. • Manages core custom and border protection programs |
|
Agency |
Agency Description, Responsibilities, & Major Events |
|
Border and Transportation |
• The largest of the 5 DHS directorates. (i.e., border security and facilitation, interdiction and security, passenger operations, targeting analysis and canine enforcement; trade compliance and facilitation, trade risk management, enforcement, and seizures and penalties as well as examine trade operations to focus on antiterrorism). • Administer Agricultural Inspection Policy and Programs (agricultural quarantine inspection, AQI, at all ports of entry in order to protect the health of U.S. plant and animal resources). • Administer immigrations policy programs. • Annual operating budget of $1.1 billion. • Each OFO is run by a Director of Field Operations (DFO) |
|
OFO - Associate Commissionerof Agricultural Inspection Policy and Programs |
• Policy advisor to the Office of the Commissioner on all agricultural issues. |
|
CBP Port Director |
• On March 1, 2003, CBP designated one port director at each port of entry in charge of all federal inspection services establishing a single, unified chain of command. |
|
CBP Ag. Specialist |
• Enforce USDA regulations and seize any articles in violation of regulations • Conduct prearrival risk analysis. • Cargo examination for quarantine disease and pests. • Collection, preparation, and submission of pest and disease samples to USDA. • Seizures, safeguarding, destruction, or reexportation of inadmissible cargo. • Negotiation of compliance agreements with importers of regulated commodities. • Stationed only at ports of entry with large volumes of cargo and only to support the CBP officers. • As of October 4, 2003, there are 1,471 full-time permanent agricultural inspectors on board. • New CBP officers will be trained at the Federal Law Enforcement Training Center (FLETC) in Glynco, Ga., and agricultural specialists will continue to learn their trade at PPQ Professional Development Center in Frederick, Md. • Agricultural training of CBP officers highlighted as a concern. |
|
CBP and FDA |
• In October 2003, CBP and FDA entered into an agreement to further protect U.S. food supply. |
|
|
• At ports of entry, CBP inspectors now carry out special inspection and sampling of foreign food imports and make referrals back to FDA for further testing and analysis. • CBP and FDA work side by side in targeting efforts, making joint decisions about any food shipments that could pose a potential threat to the United States. |
|
National Targeting Center (NTC) |
• Part of CBP’s OFO, the NTC provides tactical targeting and analytical research support for antiterrorism efforts to DHS and its Operations Center. NTC has representatives from all CBP disciplines. |
|
CBP Laboratories and Scientific Sciences Division (LLS) |
• On December 8, 2003, LLS moved its Radiation Portal Monitor to the NTC. |
|
SOURCES: DHS, March 2004b; Bonner, 2004; USAHA, 2003a; CBP Today, March 2003; US CBP website press release, January 2004; Khawaja Ahmad, USDA-APHIS-VS, personal communication, April 2004; DHS 2004e. |
|
ADDRESSING FUTURE ANIMAL DISEASE RISKS
A critical tool for informing decisions about how to prevent or respond to animal disease is the evaluation of risk related to the potential occurrence, transmission, or establishment of animal diseases. In the context of animal disease, risk analysis is the framework for understanding the impact of a wide variety of variables on animal health, and particularly, of the transmission of disease through the movement of animals, animal products, and vectors.
With increased globalization and increasing access to foreign animal markets, the avenue of contamination through importation of animals and products that harbor infectious agents requires constant attention. The WTO/SPS Agreement described earlier in this chapter emphasizes the use of scientific principles as a basis for the implementation of animal and human health-related protection measures in trade. Signatory nations must document the risk that is posed by importing another country’s products in order to justify trade barriers or sanitation requirements erected to safeguard domestic animals. The agreement employs the term “risk assessment” (one component of the risk analysis process) as “the evaluation of the likelihood of entry, establishment or spread of a pest or disease within the territory of an importing member according to the sanitary and phytosanitary measures which might be applied, and of the associated potential biological and economic consequences; or the evaluation of the potential for adverse effects on human or animal health arising
from the presence of additives, contaminants, toxins, or disease-causing organisms in food, feedstuffs and beverages.”
According to the OIE (2003), identifying the pathogenic agents associated with the importation of a commodity that could potentially produce adverse consequences is the first component of risk analysis. The second component is risk assessment, described as a process of four interrelated steps:
Step 1—Release Assessment: Description of the biological pathway(s) necessary for an importation activity to “release” pathogenic agents into a particular environment, and estimating the probability of that process occurring, either qualitatively and/or quantitatively.
Step 2—Exposure Assessment: Qualitative and/or quantitative description of the biological pathway(s) necessary for exposure of animals and humans in the importing country to the hazards released from a given source and estimating the probability of exposure.
Step 3—Consequence Assessment: Description of the potential consequences of a given exposure and estimates the probability of them occurring. Examples of direct consequences include animal infection, disease, and production losses, and examples of an indirect consequence would be potential trade losses or compensation losses.
Step 4—Risk Characterization or Risk Estimation: Integration of all of the information gathered during the risk assessment process is integrated to produce overall measures of risks associated with the hazards identified at the outset. An example of a final output might be estimated numbers of herds, flocks, animals, or people likely to experience health impacts over time.
The OIE framework is useful for considering and integrating the complexities of risk assessment into logical steps that can be better analyzed both qualitatively and quantitatively. Two additional components of risk analysis are (1) risk management, the process by which the results of risk assessment are integrated with other information, such as political, social, economic, and engineering considerations, for example, to arrive at decisions about the need and methods for risk reduction; and (2) risk communication, the explanation of findings from the risk assessment to risk managers, consumers, industry, and other interested parties in an interactive dialogue about risk-related factors and perceptions (NRC, 1994b). While other tools and frameworks continue to emerge, this systematic approach is important for making decisions, setting priorities, planning interventions, and evaluating prevention and control strategies.
The nation’s animal and public health could benefit more fully from the potentially powerful analytic capabilities of risk analysis and risk assessment if there were more widespread understanding of and participation in the way in which mathematical models of disease incidence and spread are used to determine risk, and the assumptions inherent in those models. By formalizing and strengthening links and communication between risk assessment modelers, who understand risk modeling methodologies, and biologists, who understand the agents, animals, biology, and pathways of the disease, the biological accuracy of pathways being modeled could be improved. These interactions might help, for example, to clarify uncertainty in the prevailing knowledge of the disease and in the pathways being modeled, which are never known with absolute certainty, in order to understand the confidence that should be applied to reported risk estimates.
In general, there is a need to promote the education of risk assessment methods for decision-makers, biologists, diagnosticians, and others who will be called upon to use, or to respond to, risk assessment reports. New ways to communicate key findings and conclusions of each of the four steps of risk assessment to those who are neither risk modelers nor experts in the disease are needed, so that those who must apply the results of risk assessments to policy or action can better interpret the bases for risk assessment results and can have confidence in their understanding of the strengths and weaknesses of the methods used.
That goal might be accomplished, in part, by incorporating the specific goals and objectives of decision-makers and animal health planners into initial stages of risk assessment design so that risk assessments can be more focused and directed, and thus more precise, in addressing specific animal health issues.
EDUCATION AND TRAINING
Education and Training of Veterinarians
Veterinary Schools
Veterinary medicine comprises several distinct fields of practice, including the care of various species of food-animals, small animals, equids, general or rural practice (mixed domestic animals), ecosystem health (including wildlife disease and conservation biology), public health, and biomedical science. Not surprisingly, veterinary schools face a difficult challenge in producing sufficient graduates for all of these fields with the appropriate depth of competence across the full range of veterinary practices.
The United States has 28 schools of veterinary medicine that graduate approximately 2,000 individuals each year with a Doctor of Veterinary
Medicine (DVM) degree (AAVMC, 2003). Attaining this degree requires a minimum of 2–4 years of university preparation followed by a professional curriculum that normally extends over 4 years. This pattern of education emulates human medical education, with a key difference being that internship is not required prior to licensing for veterinarians. The system has served veterinary medicine reasonably well in the past, but it has not changed in about 50 years despite recent enormous changes in society that have generated markedly altered production systems and disease patterns.
The students entering veterinary schools and their decisions to specialize are also changing. For example, veterinary students are increasingly from urban environments and are women. Another trend is that, with more disposable income and greater expectations for the level of care and services for their animals, companion animal owners have demanded greater sophistication and improved health care delivery that has resulted in specialization into services such as oncology, critical care, internal medicine, and ophthalmology. These dramatic increases in specialization in companion animal services and practices, and improved financial rewards, have influenced student decision making to enter these fields.
DVM programs are uniformly subjected to accreditation by the American Veterinary Medical Association (AVMA), which sets “standard requirements of an accredited or approved college of veterinary medicine” (AVMA, 2004a). These standards include those relating to organization, finances, physical facilities and equipment, clinical resources, library and information resources, students, admission, faculty, curriculum, research programs, and outcomes assessments. The AVMA’s Council on Education reviews each veterinary school every 7 years.
The Association of American Veterinary Medical Colleges (AAVMC) provides a collective voice for the veterinary schools (AAVMC, 2004). It publishes the Journal of Veterinary Medical Education, sponsors biennial symposia, manages a national veterinary student application process, and provides leadership in addressing current issues in veterinary education and research.
Licensing
State agencies license veterinarians. U.S graduates must be from an AVMA accredited school and have passed a standard North American Veterinary Licensing Examination (NAVLE) to enter private practice. While there is some opportunity for students to focus their undergraduate clinical training in one of the specific fields of veterinary medicine, accreditation requirements and the broad range of subject matter covered
by the NAVLE puts a limitation on the practical extent of such training. As a consequence, specialization in the various fields of veterinary medicine occurs at the postgraduate level. The relatively modest incomes that are the norm in veterinary medicine (with means that range from $84,000 to $92,000/year for different fields in 2002), together with high levels of student indebtedness (a mean of $71,000 in 2002) may deter new graduates from opting for postgraduate training. This has led to the suggestion that veterinary educators should consider an engineering model of undergraduate professional education in which veterinary students elect a curriculum track with the depth of study in different disciplines appropriate to the field of their choice (Eyre, 2002; Radostits, 2003; Nielsen, 2003). This would require a change in licensing policies, which has been advocated by some (Karg, 2000).
Training in Population Health/Food Systems
An adequate education in population health is essential for veterinarians on the first line of defense in dealing with animal diseases in the livestock industry as private practitioners and as employees of government agencies or commercial enterprises. It is also essential for those involved in the food system, public health, and ecosystem health. However, since the objective of about 75 percent of students is to enter companion animal practice or a related specialty, present curricula emphasize individual animal medicine.
A symposium of U.S. veterinary educators (Hird et al., 2002) held in 2002 concluded that:
A crisis in veterinary medicine exists that requires urgent action from veterinary educators, veterinary associations and organizations, and public and private practitioners. The convergence of animal, human, and environmental health issues has created the need for veterinarians with a level of knowledge and skills that is not being achieved by either new graduates or the current pool of veterinarians. Unprecedented changes in food animal production and health, human and animal demographics, diseases, concern for animal well-being and welfare, antibiotic resistance, and biotechnology are occurring. In addition increasing threats to animal populations from the introduction of exotic animal diseases, either accidentally or intentionally, require a much larger cadre of veterinarians with training in population health concepts if the US is to manage exotic disease outbreaks and maintain the security of the of the US food supply.
The conclusions that emerged from this symposium echoed similar ones made in the 1972 NRC report New Horizons in Veterinary Medicine and the 1988 Pew National Veterinary Education Program (NRC, 1972; Pritchard, 1988).
Training in Public Health
A recent survey of education in public health in 27 (of the 28) U.S. veterinary schools found that the curricula of all 27 required at least one course in public health; when epidemiology was included, the contact hours assigned to these subjects ranged from 30 to 120 (mean of 67). Only four schools, however, have required clinical rotations in public health (Riddle et al., 2004). Twenty-four of the 27 schools offer from one to six elective courses varying from a total of 15 to 288 hours. Eight schools offer elective clinical rotations of 3–4 weeks in length. Twenty-three schools offer some form of advanced training in public health or epidemiology, four offering a dual DVM/Masters of Public Health program. Fifteen schools offer or are about to offer some form of DVM program combined with an advanced degree related to public health. Statistics describing first-year employment of new graduates (Table 2-3) indicate that few, if any, opt for careers in public health or have the opportunity without further education. Leaders in veterinary education have called for the profession and its educational establishment to give much more attention to meeting societal needs in this field (Hoblet et al., 2003).
TABLE 2-3 First-Year Employment, 2004 Veterinary Graduates in Various Fields
|
|
Percent |
|
Private Clinical Practice |
68.2 |
|
Large animal exclusive |
2.5 |
|
Large animal predominant |
2.8 |
|
Mixed animal |
9.0 |
|
Small animal exclusive |
40.4 |
|
Small animal predominant |
10.0 |
|
Equid |
3.4 |
|
Public or Corporate Employment |
1.9 |
|
College/university |
0.1 |
|
Uniformed services |
1.2 |
|
Federal government |
0.1 |
|
State/local government |
0.1 |
|
Industry/commercial |
0.1 |
|
Not-for-profit |
0.2 |
|
Other |
0.6 |
|
Unknown |
3.7 |
|
Advanced study programs |
25.7 |
|
SOURCE: AVMA, 2004b |
|
Ecosystem Health
Ecosystem health provides a broad context for veterinary education to address wildlife diseases and conservation biology at the level of multiple populations that share the same environment (Van Leeuwen et al., 1998; Deem, 2004). While some schools offer undergraduates the opportunity to choose elective courses or rotation in wildlife diseases or zoological medicine, most new graduates who wish to specialize in wildlife diseases undertake postgraduate studies to this end. Veterinary schools in Canada have jointly created an innovative elective undergraduate rotation in ecosystem health (Ribble et al., 1997).
Veterinary Technology Programs
Veterinary technicians are important members of veterinary practice teams, government agencies, biomedical research laboratories, diagnostic laboratories, and commercial enterprises. Opportunities to make rural practice more attractive could depend on having veterinary technicians who are better suited and empowered to provide appropriate support to veterinary practitioners. There are 104 programs in veterinary technology in the United States accredited by the AVMA; 15 offer baccalaureate degrees, 2 of which are at a veterinary college.
Postgraduate Studies
Although data on the total number of graduate students in the veterinary sciences are unknown, a 2004 AVMA survey indicated that of 2,225 College of Veterinary Medicine (CVM) graduates, roughly 25 percent responded they were entering graduate studies at CVMs and elsewhere (Shepherd, 2004). In 2002, 27.7 percent of all female graduates and 23 percent of all male graduates directly entered advanced studies, including internships, residencies, and graduate training programs (Wise and Shepherd, 2004).
In order to encourage more veterinary students to opt for postgraduate training, at least 10 veterinary schools offer combined DVM/graduate degree programs, such as DVM/PhD and DVM/Master’s programs, not counting schools with joint MPH programs (Riddle et al., 2004). Several colleges have recently offered new DVM/MPH dual degree programs that can be completed in 4 years.
Postgraduate training can be of several types: (1) clinical training, leading to certification as a specialist (or diplomate); (2) research training, to prepare the veterinarian to be an independent research scientist in a specific area, such as immunology, physiology, epidemiology, microbiol-
ogy and toxicology; this training may or may not lead to a PhD, although individuals seriously interested in a research career typically pursue a PhD, followed by postdoctoral training; and (3) a combination of research and clinical training—for example, veterinary pathology or laboratory animal medicine.
Clinical Training
Many, if not most, new veterinary graduates seeking formal postgraduate education elect residency training (which may be in conjunction with a M.Sc. degree) and board certification in a medical discipline with a view to becoming a clinical specialist, often in the companion animal health discipline.
In the United States, the AVMA guides and regulates the formal processes for clinical specialization in a veterinary medical discipline, a process that began in 1949 with the pathology specialty. There are now 20 specialty colleges (See Table 2-4). Veterinarians who wish to achieve the status of a specialist in a medical discipline must undertake an approved residency program and subsequently pass a rigorous examination set by a recognized specialty college. Those who successfully complete a program become registered “diplomates” in the college or board they choose. It normally requires about 3–5 years for a new graduate to achieve this goal. Specialization by species was resisted for many years, except in the case of laboratory animals, where the American College of Laboratory Animal Medicine has existed since 1957. The American College of Poultry Veterinarians was established in 1991. The American Board of Veterinary Practitioners (established in 1976) recently provided categories for specialization in avian practice, beef cattle practice, dairy practice, and swine health management. The number of diplomates in each of these categories is modest, ranging from 11 to 107.
Diplomate status in a specialty college has become a required qualification for faculty in clinical departments of many of the nation’s faculties of veterinary medicine and has greatly enhanced the quality of clinical education. The diplomate status in one of several disciplines is the preferred qualification for section heads in diagnostic laboratories that opt for accreditation by the AAVLD. Increasing the strength of the nation’s animal diagnostic laboratory and field investigative network will depend in part on having adequate numbers of veterinarians with specialist qualifications in pathology, epidemiology, microbiology, toxicology, and wildlife diseases, as well as other laboratory professionals.
TABLE 2-4 Active, Board-Certified Diplomates (as of December 2004)
|
Field |
No. of Diplomates |
|
|
All Fields |
7,970 |
|
|
Anesthesiologists |
148 |
|
|
Animal Behaviorists |
36 |
|
|
Dentistry |
75 |
|
|
Dermatologists |
158 |
|
|
Emergency and Critical Care |
156 |
|
|
Internal Medicine |
1,478 |
|
|
Cardiology |
|
120 |
|
Internal Medicine, Small Animal |
|
788 |
|
Internal Medicine, Large Animal |
|
357 |
|
Neurology |
|
126 |
|
Oncology |
|
151 |
|
Laboratory Animal Medicine |
677 |
|
|
Microbiologists |
164 |
|
|
Bacteriology/Mycology |
|
33 |
|
Immunology |
|
43 |
|
Microbiology |
|
85 |
|
Virology |
|
50 |
|
Nutrition |
47 |
|
|
Ophthalmologists |
264 |
|
|
Pathologists |
1,411 |
|
|
Anatomical Pathology |
|
1,210 |
|
Clinical Pathology |
|
255 |
|
Toxicological Pathology |
|
38 |
|
Pharmacology |
43 |
|
|
Poultry |
247 |
|
|
Practitioners |
740 |
|
|
Avian |
|
107 |
|
Beef Cattle |
|
11 |
|
Canine and Feline |
|
408 |
|
Dairy |
|
30 |
|
Equine |
|
74 |
|
Feline Exclusive |
|
71 |
|
Food Animal |
|
20 |
|
Swine Health Management |
|
18 |
|
Preventive Medicine |
531 |
|
|
Epidemiology |
|
64 |
|
Radiology |
264 |
|
|
Radiation Oncology |
|
34 |
|
Veterinary Surgeons |
1,041 |
|
|
Small Animal |
|
43 |
|
Large Animal |
|
23 |
|
Theriogenologists |
306 |
|
|
Toxicology |
98 |
|
|
Zoological Medicine |
83 |
|
|
SOURCE: AVMA, 2004c. |
||
Research Training and Combination Training
Currently, students who seek board certification are encouraged to pursue a PhD if they have an interest in research. Unlike MD equivalents, who often enter postdoctoral training in a research environment, opportunities for rigorous DVM postdoctoral research training are few. While it is not unusual for an MD involved in research not to hold a PhD, it is still expected by veterinary colleges that a veterinarian hold a PhD to undertake independent research. The extended period of time needed to become a biomedical investigator might significantly discourage students from pursuing this path. But despite the additional 4–5 years of effort, some DVMs pursue a PhD and postdoctoral training. Most typically, veterinarians entering the field of biomedical science and research do so through graduate degree(s) and postdoctoral training in a medical discipline. Some combine this with specialty training in clinical disciplines, such as laboratory animal medicine or veterinary pathology, leading to certification as a diplomate in the American College of Laboratory Animal Medicine (ACLAM) or the American College of Veterinary Pathologists (ACVP), respectively.
Data compiled by the NRC study National Need and Priorities for Veterinarians in Biomedical Research in 2004 indicate a strong but unfilled demand for veterinarians with proven research skills. The NRC report documented the rising number of position announcements for laboratory animal medicine veterinarians, which increased from less than 20 in 1995 to 50 in 2001. At the same time, animal use in the NIH grant portfolio is at an all-time high, a reflection of the continuing importance of animal based research (NRC, 2004b). Nearly 5,500 grants, or about 40 percent of all NIH competing grants, involved the use of live vertebrate animals (NRC, 2004b). However, NIH grants usually do not support animal disease research except as models for human disease. Veterinarians need to be trained in biomedical research to take active roles as principal investigators on NIH grants related to animal models for human disease and other grants for animal disease research, including investigations of the role of animals in zoonoses. The NRC report’s review of Research Project (RO1) funded NIH grants in 2001 indicated that only 4.7 percent of NIH-funded competitive grants utilizing animals were awarded to veterinary principal investigators. The number of RO1s awarded to DVMs was small even during the period of doubling of the NIH budget (1997-2001): 76 RO1 awards to DVMs in 2001 (NRC, 2004b). The report concluded that the current number of veterinary investigators is not adequate to capitalize on the unique potential of comparative medicine to contribute to biomedical research.
The ACVP has provided further evidence of the future shortfall in biomedical scientists (ACVP, 2002). It studied the national needs for veterinary pathologists by surveying potential employers for the period 2002–2007 and compared this estimate to the expected output of trainees from existing training programs for the same period. It concluded there would be a shortfall of 336 pathologists or 50 percent of the predicted demand.
In summary, these facts point to a critical need for colleges of veterinary medicine to reexamine the nature of training provided to students relative to national needs. Although a more detailed examination of factors that impede veterinary students and veterinarians from pursuing research careers is beyond the scope of this report, these issues are the subject of a forthcoming NRC report entitled Critical Needs for Research in Veterinary Science (NRC, 2005).
Continuing Veterinary Medical Education (CVME)
In 2002, the AVMA Council on Education removed the Continuing Education Standard as essential for veterinary college accreditation. Hence this body no longer reviews college CVME programs. At present, no organization sets CVME national standards, as is the case for continuing medical education (Moore, 2003).
CVME is delivered by schools of veterinary medicine, various professional associations and societies, employers, and government agencies. Forty-one states, in one form or another, have mandated requirements for CVME to maintain licensure (Moore et al., 2003).
APHIS-VS administers the National Veterinary Accreditation Program (NVAP) (USDA-APHIS-VS, 2004) and plans to make regular CVME a mandatory requirement for the accreditation of private veterinary practitioners who wish to participate in federal and state regulatory programs (Torres and Bowman, 2002). It is anticipated that accreditation will be designated in two separate categories: one for companion animals and one for food-animals. Proposed rule changes are expected to be available for public comment in the winter of 2005 (Lawrence Miller, personal communication, June 2005). Currently, 80 percent of practicing veterinarians are accredited. The current accreditation program does not require veterinarians to maintain, through continuing education, their knowledge of foreign animal diseases. Under the proposed new program, foreign animal disease training will be available to complete CVME requirements to maintain accreditation status (Lawrence Miller, personal communication, June 2005).
Education and Training of Others on the Front Lines
Most animal handlers and others working and living with animals on a day-to-day basis are not health professionals and acquire their knowledge about animal disease through one or more means, such as from their veterinarian, employer, the Internet, industry magazines, commodity organizations, and extension programs offered by universities, government, or producer organizations. By definition, extension agencies are well positioned to take the initiative to provide appropriate training programs, but would probably require additional support to develop such instruction, given competing priorities and a challenging budgetary environment.
Wildlife agencies are expected to keep staff biologists and technicians adequately informed about disease issues. Hunters and naturalists can get information from societies dedicated to their interest or hobby through print, meetings, and the Internet.
AWARENESS OF THE ECONOMIC, SOCIAL, AND HUMAN HEALTH EFFECTS OF ANIMAL DISEASES
An outbreak of animal disease can have significant economic, social, and human health effects, although these effects vary considerably depending on the nature of the disease and the specific outbreak. Some animal diseases can have significant effects on markets. These include direct impacts on lost production and farm income, unintended costs to adjust from lost output, sector and community losses in welfare, and impacts on markets (prices) and trade. Consumers may lose confidence in the safety of meat and other food products, and this loss of confidence can contribute to a decrease in prices as well as lack of trust in public authorities. The potential for market and other impacts of an actual or threatened animal disease outbreak points to the importance of accurate and ongoing communication with consumers, producers, and the general public. Increasing dependence on trade can increase the volatility of prices. With the confirmed cases BSE in Canada in May 2003 and the Canadian-U.S. border closed to live cattle trade and only limited meat trade, U.S. beef prices rose by over 26 percent in 2003. After discovery of a BSE case in the United States in December 2003, U.S. beef prices fell by nearly 11 percent. The world beef trade declined by an estimated 2.5 percent in 2004 (Beghin et al., 2004). A recent review of studies of the economic impact of transboundary animal diseases indicates significant losses caused by the perceived threat of transboundary animal disease and control efforts. The studies include losses to Uruguay of added trade revenue estimated up to $90 million per year from the presence of FMD (1996) and losses in the
United Kingdom in 2000 related to BSE (lost trade, production, and other financial costs) of €5 billion (Otte et al., 2004). USDA estimates losses to the U.K. economy of $3.6–11.6 billion for FMD and $5.8 billion for BSE (USDA ERS, 2001). BSE is linked to variant Creutzfeldt-Jakob disease (vCJD) known to have caused 147 human deaths in the United Kingdom as of December 2004 (CJD Statistics, 2004).
In addition to known animal diseases from naturally occurring exposure is the added risk of disease that is spread with malicious intent (NRC, 2003a). Also, diseases associated with environmental disturbance or degradation are becoming more important. The effect of environmental contamination can affect domestic animal production, as well as the health of wildlife, and the value of hunting and fishing for recreation or livelihood.