II
Ideal Objectives and Real-World Challenges
The committee believes that as a starting point for analyzing options, developing plans, and making waste management decisions, it is useful to examine the life cycle of the wastes, identifying both their current condition and the desired end points. The choices and decisions that represent the different paths from the current condition to the desired end points can then be delineated. In this section, the committee examines what would be the ideal objectives or end points for the tank wastes and what real-world obstacles make those objectives difficult to achieve.
IDEAL OBJECTIVES FOR THE TANK WASTES
In an ideal world—that is, if waste retrieval and processing facilities worked ideally and at an acceptable cost—the objectives would be the following:
-
Remove tank waste. The first priority for most, if not all, of the interested parties is to retrieve the waste from the tanks,36 particularly the noncompliant tanks. If all of the waste could be retrieved, tank closure would be a minor concern because there would be virtually no residual radiological hazards. No real waste retrieval system, however, will retrieve all of the waste.
-
Separate the radioactive constituents from the salt solutions and sludge. Separations are carried out primarily to reduce the volume of high-level radioactive waste that must be immobilized, stored, and ultimately shipped to a repository for disposal. The radionuclides constitute only a small volume of waste. The more voluminous but less radioactive bulk would be disposed in a manner appropriate for the lower hazard it poses. It should be noted that in any real separation system, complete separation of the radioactive components is not possible.
-
Immobilize radioactive waste for disposal. The highly radioactive wastes must be immobilized in a manner suitable for a high-level waste repository (vitrified, in this case), and the less contaminated bulk salt wastes must be immobilized in a form that will prevent unacceptable accidental direct exposures and also inhibit leaching of contaminants.
-
Minimize public and occupational hazards. Worker safety is the top priority in the near term, before radionuclides and hazardous chemicals move into the accessible
-
environment. Regulations require DOE to keep exposures as low as reasonably achievable (ALARA), with economic and social considerations taken into account.
-
Minimize residual hazards to reduce long-term maintenance. The ideal situation for waste staying on-site, whether grouted waste residues in the tanks or saltstone in vaults (or even the tank structure, piping, and other internals), would be if the wastes were left in a condition that would not require institutional controls or long-term monitoring and maintenance to prevent unacceptable exposures to workers, the public, and the environment.
REAL-WORLD CHALLENGES: STATUS AND DIFFICULTIES IN ACHIEVING IDEAL OBJECTIVES
While DOE and others may strive toward these ideal outcomes, reality makes it quite difficult to achieve them. This is not to say that anything less than the ideal is unacceptable. Rather, thorough examination of the disposition options, including technological capabilities, relative risks, costs, and other trade-offs, is needed to select an appropriate plan of action.
Remove Tank Waste
The South Carolina Department of Health and Environmental Control and DOE have developed a list of common goals and values to guide tank waste cleanup activities. At the top of that list is to “reduce operational risk and the risk of high-level waste (HLW) leaks to the environment by removing waste from tanks” (Mahoney and Chew, 2004). The top priority is to remove waste safely from the noncompliant tanks that are more likely to leak. Some of these wastes will be sent to compliant tanks and some to the DWPF.
The waste removal process consists of two stages: a bulk waste removal stage and a “heel removal” stage.37 The bulk waste removal phase entails the removal of the majority of waste from the tanks. It consists of several cycles of adding water to the tanks, mixing the waste, and pumping it out. DOE’s goal for the bulk removal stage is to leave no more than 76 m3 (20,000 gallons) of settled sludge and residual liquids in tanks that have a significant amount of sludge (Hintze, 2005). This remainder is called the “heel” and corresponds to about 1.5 to 2.7 percent of the tank volume, depending on the tank design. For the heel removal stage, DOE uses jet pumps to dislodge the sludge and pump the slurry from the tank. This method was used to clean out four Type IV tanks (Tanks 17 through 20).38 It is also possible to use acid to dissolve the heels, a method called chemical cleaning.
DOE has previously tested several different acids with sludge simulants, tested oxalic acid with real waste, and demonstrated chemical cleaning of a waste tank heel using oxalic acid (Adu-Wusu et al., 2003). The demonstration used two water washes, three oxalic acid washes, and a final water rinse to clean Tank 16, a Type II tank (West, 1980). This method yielded thorough tank waste removal: DOE reports that 99.98 percent of the waste by radioactivity, and 99.8 percent by volume, was removed from the tank (see Table 2). DOE regards oxalic acid to be the most promising of the chemical cleaning options, but it believes that there are some difficulties with the approach. These problems are associated
with both in situ chemical cleaning (e.g., corrosion of the tanks)39 and subsequent processing (e.g., foaming, precipitation, nuclear criticality safety40) of solutions containing metal oxalates and oxalic acid (Adu-Wusu et al., 2003).
Because the chemical composition of the waste in each tank is somewhat different, the problems listed above may have to be addressed anew for each tank, just as the chemical state of each waste batch is adjusted before processing and immobilization. Without chemical cleaning, it may be quite difficult to remove the heels from Types I, II, III, and IIIa tanks, each of which has more than a thousand meters of cooling coils snaking through it vertically and horizontally. Figure 4 is a photograph of the interior of Tank 4 (a Type I tank) before it was brought into service, which illustrates the dense piping. As noted previously, access to the interior of the tanks is limited. There are no plans to remove the cooling coils, which are attached to both the roofs and the floors of the tanks. The Type IV tanks are the simplest tanks, with no cooling coils, and only minor obstructions on their bottoms. These tanks were selected to be the first ones for cleaning and closure at least in part because they posed fewer technological challenges. DOE estimates that the residual heels left in the Type I, II, and IV tanks after heel removal efforts will range in volume between 7.6 and 26.4 m3 (2,000 to 7,000 gallons, or about 2.5 to 5 centimeters [1 to 2 inches] if spread uniformly over the bottom of a tank). The smaller of the two quantities is of the same order of magnitude as the experience gained in closing two tanks: DOE reports the Tank 17 residues as comprising approximately 2,400 Ci in about 9 m3 of sludge (d’Entremont et al., 1997), and Tank 20 residues as comprising less than 500 Ci in about 3.8 m3 (d’Entremont and Hester, 1997). Volume estimates are based on systematic measurements across the floors of those tanks. Radioactivity estimates are extrapolations based on process knowledge and concentrations in two samples multiplied by the volume of the heels. Inhomogeneities in the heels make the radioactivity estimates much less likely to be accurate than the volume estimates.
TABLE 2 Tank 16 Waste Removal Process and Curies Removed with Each Sequential Step
|
Sequential Waste Removal Step |
Curies Removed |
Percentage of Curies |
Cumulative Curies Removed |
Cumulative Percentage Curies Removed |
|
Bulk waste removal |
2.74 × 106 |
97 |
2.74 × 106 |
97 |
|
Spray water washing |
2.78 × 104 |
0.98 |
2.77 × 106 |
97.98 |
|
Oxalic acid wash and rinse |
5.82 × 104 |
2 |
2.83 × 106 |
99.98 |
|
SOURCE: (DOE, 2002a) |
||||

FIGURE 4 Photograph of the interior of a Type I tank (Tank 4) prior to receipt of wastes. SOURCE: Caldwell (2005a).
There will soon be experience upon which to base estimates for cleaning out the heel from a tank that has cooling coils without using oxalic acid: the site will start work to clean out the heel from Tank 11 (a Type I tank) in the summer of 2005. Figure 5 is a photograph of Tank 11 after recent bulk waste removal. The photograph shows little residual waste, although it should be noted that the area displayed is below a riser, where removal equipment is situated and where one would expect removal efforts to be most effective. The committee did not receive data showing how far the clean bottom extends laterally but notes that it may be possible to use other risers in the same tank to increase the overall efficiency of heel removal. The waste that is difficult to retrieve tends to be consolidated sludge that has high concentrations of radioactivity, and is difficult to mobilize.
Savannah River Site personnel also indicated that a few tanks have zeolite in them. Zeolite, a class of hydrated alumino-silicate minerals with an ability to “trap” cesium (and other cations), was used to remove cesium from the condensed steam recovered from an evaporator (a heated tank that evaporates water from waste). The zeolite has now agglo-merated and is difficult to slurry out of the tank. For example, zeolite remains in Tank 19 despite attempts to retrieve it. Also, oxalic acid proved ineffective at removing zeolite from Tank 24, although the temperatures and quantities of oxalic acid used may have been too low (Adu-Wusu et al., 2003).
Finally, in tanks that have a history of leakage, there is waste in the tank annulus (the space between the inner tank wall and the outer containment shell; see Figure 1). DOE told the committee at its April 2005 meeting that DOE does not yet have a plan to remove waste from the annuli and the committee was given inconsistent opinions concerning how difficult removal of waste from the annuli will be. The waste in the annulus is salt waste and, presumably, readily dissolved, but the geometry of the annulus and the presence of obstructions such as ventilation ducts make access difficult. As previously mentioned, the interior of Tank 16 has been thoroughly cleaned, but the committee was told at its May 2005 meeting that removal of waste from the annulus was unsuccessful because of the difficulty of

FIGURE 5 A Type I tank (Tank 11) after undergoing bulk waste removal in March 2005. SOURCE: Caldwell (2005b).
access and because the technology used to clean the annulus wall made the waste less soluble.
It is often reported that the waste projected to remain in the tanks after cleanup will be less than a few percent of the initial inventory. However, the committee notes that, common to many remediation projects, it is not the fraction that is removed from a source that determines the long-term risk, but what is left behind. Risks depend on radionuclide quantities and concentrations, their conditioning, and their interactions with the environment, not just the volume of the heels. It is difficult to predict the amount of waste that will be left behind before the removal efforts are actually undertaken. Most of the tanks have unique design features and waste may have different characteristics (although the variation in waste characteristics is not as great at the Savannah River Site as at the Hanford Site).
Characterization of waste residuals is important to make the determination that waste has been retrieved to the maximum extent practical and to meet performance objectives. During the first meeting at the Savannah River Site, the committee received a presentation on waste characterization but it was not specific to residual waste (see Appendix B); therefore, the committee has to gather additional information on this topic for its final report.
Tank Space (usable storage volume)
Capacity in compliant tanks is needed to prepare batches of salt waste for processing through the interim and high-capacity salt waste processing facilities. In addition, sludge waste processing activities result in a net increase in the volume of liquid in the tank system. Also, newly generated waste will continue to be produced by ongoing operations in the H Canyon and other projected missions at the Savannah River Site. As a result,
compliant tank space is scarce and in great demand. It is somewhat counterintuitive that retrieving sludge consumes rather than frees up tank space during this phase of cleanup, but DOE reports that for each liter of sludge retrieved from a tank and immobilized in glass, 1.3 liters of concentrated waste is added to compliant tanks. Even this 1:1.3 ratio is achieved only because of waste reduction efforts undertaken to conserve tank space (DNFSB, 2004). After sludge waste is retrieved from noncompliant tanks, the sludge is washed with fresh water to remove soluble species. In addition, liquid waste is generated in the DWPF and then recycled back into Type IV tanks (see Figure 3). What remains after these wastes are run through an evaporator is stored in the compliant waste tanks until it can be processed for disposal.
To carry out waste retrieval and other operations, DOE must track the space in the tanks closely and often must make multiple transfers among tanks, and even between tank farms, to ensure that there is sufficient open tank space where needed to support operations. This is a complex problem compounded by the continuing introduction of waste into the tanks.
The Defense Nuclear Facilities Safety Board (DNFSB) has raised concerns about tank space problems, the risks of accidents, and worker exposures incurred as a result of these operations (DNFSB, 2001, 2004). A 2001 DNFSB letter recommends a set of actions to relieve tank space problems, including (DNFSB, 2001, p. 5):
Develop and implement an integrated plan for HLW tank space management that emphasizes continued safe operation of the Tank Farms throughout its life cycle. This plan should include enough margin to accommodate contingencies and reduce overall programmatic risk. The plan should also restore operating margin to the Tank Farms by including action to:
-
reduce or eliminate the DWPF recycle stream,
-
recover former ITP [in-tank precipitation] tanks for Tank Farm operations,
-
assess the desirability of adding an additional HLW evaporator to support Tank Farm operations,
-
assess the feasibility of constructing new HLW tanks, and
-
resolve waste compatibility and equipment degradation problems to allow unconstrained operation of the three existing evaporators.
The committee discussed these options with DOE. DWPF recycle is a particular concern because it contributes more than half of the annual waste input to the tanks, adding approximately 5,700 m3 (1.5 million gallons) of unconcentrated waste (prior to evaporation) to the tank farms every year. Sludge washing contributes about 5,700 m3 to 7,600 m3 (1.5 million to 2 million gallons) every one to two years, and ongoing operations at the H Canyon add approximately 760 m3 to 1,150 m3 (200,000 to 300,000 gallons) of waste to the tanks annually (Mahoney, 2005). The DNFSB letter explains that DOE has long considered, but never pursued, installing an evaporator at DWPF. DOE informed the committee that conceptual design for an evaporator has begun, but on the current schedule the evaporator would not be brought on-line until around 2010.
The DNFSB’s action “b” refers to tanks used in the in-tank precipitation (ITP) process described in the next subsection. Only one tank, Tank 48, now contains ITP waste. Because of chemical compatibility and safety problems associated with this waste, DOE has concluded that other wastes cannot simply be added to utilize the open space in Tank 48.
Evaporator operations are becoming constrained by the buildup of salt in the tanks that receive evaporator concentrates and by the buildup of hydroxides in the tanks that feed the evaporators. It is not clear that adding an evaporator to one of the tank farms without additional open tank space would alleviate the space problem. The compatibility problem
referred to in the DNFSB’s action “e” above is that some of the tank wastes are high in silica and others are high in aluminum. If they are mixed, they form hydrous alumino-silicates (zeolite), which can clog evaporators and ancillary systems and are difficult to remove. DOE lost the use of an evaporator for two years, starting in 1999, because of this problem. Since then, it has instituted a policy of not mixing these wastes, which limits its ability to transfer waste between some tanks. This further constrains management of space in the tank farms.
At its April 2005 meeting, the committee asked representatives from DOE and the South Carolina Department of Health and Environmental Control whether a new tank might alleviate the tank space crisis. These parties have agreed to avoid, if possible, creating new tanks that will themselves eventually require remediation and closure. Further, DOE estimates that bringing a new tank into service would require five years, so it is not a solution to the near-term tank space crisis.
DOE has gone to great lengths to find tank space but says that it has run out of options in the tank farm operations. Tank space crises have loomed at the Savannah River Site for 15-20 years. However, recent actions to free up tank space, including the numerous tank waste transfers across the tank farms, illustrate that available options are diminishing. Until recently, DOE held open the equivalent of a full tank of compliant tank space in each tank farm as contingency space for leaks and other problems (DNFSB, 2004). DOE informed the committee that it has decided to reduce the contingent space to the equivalent of one tank overall. DOE now plans to address the tank space problem in large part by passing the waste through the interim salt processing facilities, described below.
Separate Radioactive Constituents from the Salts
Once waste has been removed from a storage tank, DOE plans to separate it into high- and low-activity fractions. During the late 1980s and 1990s, DOE planned to use the ITP process to separate radionuclides from the salt waste. This process was to be implemented in parallel with sludge processing so that radionuclides from the sludge and from the salt waste could be immobilized together in the DWPF, which began production operations in 1996. By 1999, DOE concluded that the ITP approach could not meet processing demands and safety requirements. This conclusion led to two actions. First, DOE selected a new separation technology and is designing and building a new facility (NRC, 2000a). Second, DOE is managing the products from its failed attempt to implement the ITP process. DOE has concluded that no practical means of processing the ITP products exists, so it has proposed to mix this waste with other liquids and immobilize it in saltstone (see Sidebar 1).
DOE now proposes to process its salt wastes utilizing three different processes41 that will be available at different times and have different capabilities (see Figure 6). Two low-capacity processes are expected to be available sooner and are referred to as “interim” processing by DOE (Phase 1, Figure 6). These are the deliquification, dissolution, and adjustment process (DDA), which could begin immediately upon approval of the waste determination by the Secretary of Energy in accordance with Section 3116 of the 2005 National Defense Authorization Act, consultation with the USNRC, and permitting by the State of South Carolina; and the actinide removal, modular caustic-side solvent extraction process (ARP/MCU), which is expected to begin operations in 2007.
|
SIDEBAR 1 TANK 48 In the early 1980s, DOE demonstrated the in-tank precipitation (ITP) process in tank 48. The ITP process was designed to separate the high- and low-activity fractions of the supernate by adding sodium tetraphenylborate and monosodium titanate. Sodium tetraphenylborate reacted with cesium to form cesium tetraphenylborate precipitate, and monosodium titanate targeted the separation of strontium and actinides. After this and a subsequent test in 1995, however, DOE determined that the ITP process could not be operated safely at the required production rates because the tetraphenylborate anion degraded into potentially flammable benzene (NRC, 2000a). Since then, DOE has concluded that no practical processing option for the waste in Tank 48 exists given the current design of the Savannah River Site tank system (DOE, 2005a). DOE proposes to dilute the contents of Tank 48 with DWPF recycle waste or other low-activity waste and immobilize the mixture in saltstone without radionuclide separations (DOE, 2005a). DOE states that Tank 48 contains “relatively low-activity salt solution” (DOE, 2005a). DOE’s current understanding of the radionuclide content of 900 m3 of liquid waste and 16,000 kg of sludge in Tank 48 is summarized in a February 2005 document (Ketusky, 2005). DOE sampled the contents of Tank 48 in September 2003 and August 2004. In each case, DOE stirred up the contents of the tank by operating four slurry pumps in the tank for several hours and then drew a grab sample of the mixture within minutes after pump shutdown. DOE analyzed the slurry sample and then filtered the suspended solids and analyzed the supernate remainder. Based on these data, DOE has estimated the cesium-137 concentration (apparently without its decay product) in the slurry to be 455 Ci/m3 and in the supernate to be 12.4 Ci/m3. Joining the supernate concentration with the volume of liquid waste in the tank and comparing that to the total radioactivity in the tank, one finds that more than 97 percent of the cesium radioactivity is in the solids. The committee has not evaluated the practicality of the processing options available for the Tank 48 wastes. On one hand, it will be unfortunate if the precipitated cesium compound were to be mixed with the supernate and other low-activity salt waste for disposal as saltstone. On the other hand, processing the 910 m3 (240,000 gallons) of waste as DOE suggests would free a compliant 4,900 m3 (1.3-million-gallon) tank located in a particularly useful place, which would significantly help the tank capacity problem at the Savannah River Site. |

FIGURE 6 Time line for salt waste processing at the Savannah River Site as described by DOE (two-phase, three-step approach).
The high-capacity chemical processing facility, called the Salt Waste Processing Facility, is scheduled to be available in 2009 and could be supplemented by the ARP, if needed. DOE’s current plan for such retrieval and processing is shown in Figures 7 and 8 (DOE, 2005a).
The first low-capacity process, the DDA process (Step 1, Figure 6), selectively retrieves salt waste that has relatively low concentrations of cesium. The process begins with removing the overlying supernate, draining the interstitial liquid from the saltcake (DOE estimates that this step will be 50 percent effective), and storing the liquid for later processing. Liquids are then added to the tank to dissolve the remaining saltcake and insoluble constituents are allowed to settle out.42
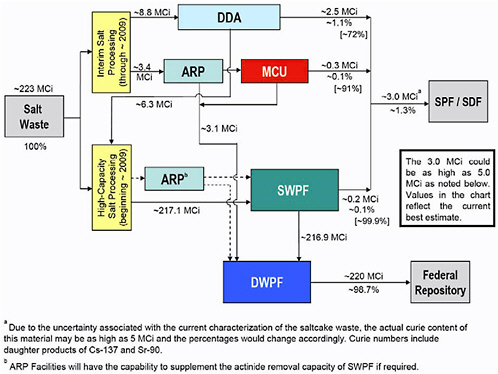
FIGURE 7 Radioactivity flows in DOE’s salt waste processing plans. Percentages in brackets are the radioactivity separation efficiencies of the processes. NOTES: DDA = Deliquification, Dissolution, and Adjustment; ARP = Actinide Removal Process; MCU = Modular Caustic-Side Solvent Extraction Unit; SWPF = Salt Waste Processing Facility; DWPF = Defense Waste Processing Facility; SPF = Saltstone Production Facility; SDF = Saltstone Disposal Facility. SOURCE: Adapted from DOE (2005a).

FIGURE 8 Volume flows in DOE’s salt waste processing plans. NOTES: 1 Mgal = approximately 3,800 m3. DDA = Deliquification, Dissolution, and Adjustment; ARP = Actinide Removal Process; MCU = Modular Caustic-Side Solvent Extraction Unit; SWPF = Salt Waste Processing Facility; SPF = Saltstone Production Facility; SDF = Saltstone Disposal Facility. SOURCE: Adapted from DOE (2005a)
Finally, after adjusting the sodium concentration in dissolved salt waste, DOE sends this waste to the Saltstone Production Facility for immobilization in the Saltstone Vaults. Thus, the DDA process uses physical rather than chemical means to accomplish cesium separation. A discussion of the effectiveness of the DDA removal process compared to the ARP/MCU and the SWPF follows (see Finding and Recommendation 2). The physical separation method used in DDA cannot achieve degrees of separation similar to those in the chemical processes used in the other facilities, described below. DDA is scheduled to operate from October 2005 until approximately 2009.
In the second low-capacity process (Step 2, Figure 6), DOE plans to apply two chemical processes in sequence: an actinide removal process (ARP) and a modular caustic-side solvent extraction unit (MCU). In the ARP, monosodium titanate is added to a tank to sorb strontium and the actinides. The monosodium titanate is recovered by filtration. The MCU will use a solvent extraction process to recover cesium from the salt waste. The ARP/MCU processing facility will have a smaller throughput than the other two processes and is designed to operate on its own for two years and, then in conjunction with the high-capacity facility thereafter, if needed. The recovered products from both ARP and MCU will be sent to the DWPF to be incorporated into glass logs, and the processed salt waste will be incorporated into saltstone. ARP/MCU is scheduled to begin operation in 2007 and could run until the end of the waste processing campaign (projected to be 2019).
The high-capacity processing will apply the actinide and cesium removal processes described above on a larger scale and in facilities designed for a much greater salt waste throughput, the Salt Waste Processing Facility. The ARP may continue to be used for additional recovery of strontium and actinides from selected wastes. Recovered products will be sent the DWPF to be incorporated into glass logs, and the processed salt waste will be incorporated into saltstone. This larger-volume treatment phase is scheduled to operate from 2009 until the salt waste processing is completed in approximately 2019.
The approach shown in Figure 6, using different processes at different times, was conceived for two reasons: (1) to allow DOE to continue tank remediation and operation of the DWPF during the time required to construct and permit the SWPF and (2) to free up tank space to support site operations and batch preparation for the SWPF (Hintze, 2005; Spears, 2005).
Table 3 presents DOE’s estimates of the amounts of salt waste processed, saltstone produced, and concentrations of radionuclides relative to concentration limits for Class C waste given in Title 10, Part 61.55 of the Code of Federal Regulations (10 CFR 61.55; DOE, 2005a). The concentration limits in 10 CFR 61.55 define the maximum concentrations generally acceptable for near-surface disposal in a disposal facility meeting the other requirements of the regulation.
DOE plans to send what it has identified as the least radioactive salt wastes from the tanks through the DDA process. Yet because of the limited cesium separation achieved by DDA, the saltstone from this process is still expected to contain cesium concentrations that are two orders of magnitude (i.e., a factor of 100) higher than the waste from the chemical processes to be used in SWPF. Although DDA generates only 8 percent of the volume of the low-activity waste to be generated during salt waste processing, its waste contains 80-90 percent of the total radioactivity that is projected to be sent to the Saltstone Disposal Vaults.43
No separations process is perfect, and using processes that are tailored to the demands imposed by the characteristics of the wastes is, in principle, consistent with the recommendations of a previous National Academies report (NRC, 2000a). The earlier report found that given the varied nature of the wastes in the tank farms, DOE need not use the same radionuclide separations process for all of the wastes, and some of the least contaminated wastes may be suitable for disposal without separations. Deciding how much effort to expend in both retrieving wastes from tanks and avoiding sending radionuclides to saltstone requires balancing technical factors, such as long-term risks to the public and occupational exposures, as well as nontechnical factors, such as costs and stakeholder views. From a technical perspective, to have an impact on long-term risks the separations must reduce concentrations of particular radionuclides. Both the low-activity stream and the residual waste in the tanks are to be grouted and disposed on site, either in the tanks or in the saltstone facility. If the separations processes are ineffective at removing radionuclides from the salt waste and instead leave them for on-site disposal as saltstone, aggressive efforts to retrieve tank heels may not reduce the overall long-term risks. An overall risk assessment comparing the risk of release from the residual radionuclides in the grouted tanks to those of release from saltstone is an essential tool to evaluate the trade-off of immobilizing radionuclides in the tanks or in the saltstone. Such a risk assessment was not available to the committee when it prepared this report.
TABLE 3 Estimated Saltstone Volume and Radionuclide Contents for Salt Waste Processing
|
Saltstone Contents |
Salt Waste Processing Phase |
||
|
DDA |
ARP/MCU |
ARP/SWPF |
|
|
Contained volume of liquid salt waste, thousands of m3 |
31 |
10 |
348 |
|
Volume of saltstone, thousands of m3 |
49 |
16 |
546 |
|
Radionuclide |
Radionuclide Concentration in Saltstone Ci/m3 (Fraction of Class C Limit) |
||
|
Carbon-14 |
0.00009 (0.001%) |
0.00003 (0.0003%) |
0.00003 (0.0003%) |
|
Technetium-99 |
0.002 (0.06%) |
0.00004 (0.001%) |
0.00004 (0.002%) |
|
Iodine-129 |
0.00001 (0.02%) |
0.000006 (0.008%) |
0.00003 (0.04%) |
|
Alpha-emitting transuranic nuclides with a half-life greater than 5 yearsa |
64 nCi/g (64%) |
8 nCi/g (8%) |
<10 nCi/g (<10%) |
|
Plutonium-241 |
19 nCi/g (0.5%) |
10 nCi/g (0.3%) |
8 nCi/g (0.2%) |
|
Curium-242 |
0.005 nCi/g (0.00003%) |
0.005 nCi/g (0.00003%) |
0.005 nCi/g (0.00003%) |
|
Nickel-63 |
0.00008 (0.00001%) |
0.00003 (0.000004%) |
0.00003 (0.000004%) |
|
Strontium-90 |
3 (0.04%) |
0.0002 (0.000003%) |
0.0002 (0.000003%) |
|
Cesium-137 |
27 (0.6%) |
9 (0.2%) |
0.01 (0.0002%) |
|
a Primarily plutonium-238 but also includes other isotopes of plutonium, neptunium-237, americium-241 and -243, and curium-244. NOTES: DDA = Deliquification, Dissolution, and Adjustment; ARP = Actinide Removal Process; MCU = Modular Caustic-Side Solvent Extraction Unit; SWPF = Salt Waste Processing Facility. SOURCE: Adapted from DOE (2005a) |
|||
Immobilization
After DOE has completed its separation of radionuclides, the inherent radiological hazards of the low-activity waste and the waste left in tanks will not be reduced further. Thus, all that can be done to reduce the risks posed by the material is to limit the potential exposures of humans and the environment. For the high- and low-activity wastes, DOE plans to solidify the material itself (in glass and grout, respectively), with the high-activity waste planned for disposal in a deep geologic repository off-site and the low-activity waste planned for disposal on-site in the Saltstone Vaults. For the tanks and their residual waste, DOE plans to fill the tanks with cementitious materials as described in Section I.
Site personnel have explained that in previous tank grouting, an engineered grout (Smart Grout) was placed by tremie44 on top of the tank heel.45 The engineered grout was designed to provide near- and long-term reducing capabilities to maintain the radionuclides and toxic heavy metals in their least mobile chemical forms (i.e., low-oxidation state or reduced form); to minimize the flow of water through the material (and the consequent leaching of radionuclides and metals from the grout); and to be pumped through grout lines into the tank and flow in the tank without segregation.
There is effectively no mixing of the grout with the insoluble tank heel due to the nature of tremie placement and the differences in density and viscosity between the Smart Grout and the tank heel. However, in Tanks 17 and 20 a series of tremie placements was made around the circumference of the tank to lay down the first grout layer and contain the tank residues rather than displace them toward the walls. Documents indicate that the small areas of incomplete grout coverage were at the intersections of different tremie deposits (USNRC, 1997). The engineered grout covered the fixed insoluble waste particles (containing primarily the actinides and strontium) and displaced the liquids. The liquids, which contain most of the technetium, other soluble radionuclides, and some suspended insoluble particles, were largely displaced to the top of the grout. They were absorbed by a second layer of dry grout placed on top to provide further immobilization of the waste. An improved version of a "controlled low-strength material" was then added above to provide structural stability. Finally, a third layer of a higher-strength grout material was used to fill the voids around the risers and to act as an intruder barrier. Plans for grouting tanks for future closures are still evolving as DOE learns from previous closures and factors in other considerations.
DOE’s estimates of grout behavior over time do not assume that the waste is mixed in the grout, but they do assume that the grout maintains its structural integrity46 for 1,000 years and its chemical integrity47 for 10,000 years. Despite the considerable amount of work performed by DOE contractors, the committee received only conceptual bases for evaluating how long the grout might last. Although requested, little quantitative (experimental or other) basis for the 1,000-year and 10,000-year assumptions was provided to the committee. The committee is aware of a qualitative analysis of the tank waste grout from 1992 (Lokken et al., 1992). Langton and co-authors describe the different needs and challenges for waste tanks at each DOE site, tank fill materials placement requirements,
leaching and durability properties, and technology needs to demonstrate tank fill physical and leaching properties (Langton et al., 2001). The committee is aware of only one recent experimental study on the leach properties of grout with respect to technetium-99 (Harbour et al., 2004). The committee believes that the short- and long-term performance of Smart Grout warrant further research to bridge a knowledge gap (see Recommendation 4).
Minimize Public and Occupational Hazards
Some occupational hazards are unavoidable: working with radioactive waste is potentially hazardous. Workers incur exposures during any operations that open the risers, including sampling, inserting equipment for cleaning, and inspecting the tanks. DOE reports that collecting and analyzing a single salt waste sample results in 2 person-millisieverts (200 person-millirem) of worker dose. DOE tries to keep workers’ exposure below 5 millisieverts per person per year at most of its sites.48 Exposures to radiation and other worker hazards are incurred in any waste management option to some degree. In selecting among options, there often are trade-offs between reduction of near-term committed dose incurred by workers and reduction of long-term risks to human health and the environment.
Minimize Residual Hazards to Minimize Long-Term Maintenance
What long-term monitoring, maintenance, and controls are required at a site is determined in large part by the magnitude, duration, and type of hazard that remains. The hazards that are left on-site are determined by the effectiveness of the tank waste removal operations and radionuclide separation operations described above. Reducing reliance on the use of long-term monitoring, maintenance, and control could help DOE select performance requirements for these operations.
A previous National Academies study (NRC, 2005) found that recovery of every last gram of high-level waste from the tanks is technically impractical and unnecessary. At this committee’s April 2005 meeting, DOE reported that the estimated effort involved in dismantling and removing a tank would take five years and cost about $100 million. DOE has argued that it is impractical to dismantle and remove tanks because of the exposures incurred by workers from radioactive residues and because of the overall prohibitive costs of exhuming such large structures. If a tank were very clean, little, if any, reduction in long-term risk would be achieved by removing the tank, its cooling coils, and any other waste material stored in it.
If the impetus for removing a tank were that substantial waste remained after attempts at heel removal, and removing the tank afforded greater access for further waste retrieval, the worker exposures entailed would probably be very high. Although highly radioactive waste recovered from a tank could be immobilized for disposal in a repository, exhumed tank pieces and much of the waste from the tank likely would simply be reburied in
another waste facility on-site. The committee has seen no formal analysis of DOE’s claims about the difficulty of tank removal.
The federal government plans to continue operations at portions of the Savannah River Site indefinitely. DOE assumes that the Savannah River Site will remain under federal government ownership within its current boundaries in perpetuity. Even if operations at the site are discontinued, South Carolina DHEC staff explained to committee staff that the federal government would have to maintain institutional controls at the General Separations Area (including the tank farms; see Appendix E for a map) because of extensive contamination from the seepage basins and other contaminant sources in the area (SCDHEC, 2005). DOE intends to restrict the area around the facilities where tank waste will remain on-site, namely the F- and H-Tank Farms and the Z-area saltstone storage facility, from residential use for 10,000 years.49
DOE’s assumptions of institutional control at the site have been translated into assumptions about future human activity and, thus, future exposures. Previous National Academies studies have pointed out the pitfalls of such assumptions (NRC, 2000b, 2003, 2005), noting the likelihood of failure of institutional controls over the long term, even in a location such as the Savannah River Site, which has a continuing national security mission. It is, therefore, prudent to plan for such failures and to assess their consequences. The committee will address this topic further in its final report.
Based on USNRC guidance, active institutional controls that prevent inadvertent human intrusion are assumed to endure for 100 years. DOE has considered in its facility performance assessments50 the possibility that after this time an inadvertent intruder could construct a residence on top of one of the storage facilities (DOE, 2005; Ross, 2005).51 According to recent DOE analyses (DOE, 2002a, Table C.4.1-4), radiological doses from drinking water from the water-table aquifer 1 meter from the H-Tank Farm facility boundary (after the tanks are grouted, but without an engineered cap) are expected to peak at 1 sievert per year (100 rem per year) 175 years following tank farm closure.52 (Figure E.3 shows the water table and the main flow directions of the groundwater.) The same analyses report that the maximum drinking water dose from water taken from the seepline (where the groundwater meets the surface and where the performance objectives were applied, called the point of compliance) is 25 microsieverts per year (2.5 mrem per year) 455 years after tank farm closure (DOE, 2002a). Thus, failure to maintain groundwater controls in the tank farm area for a period stretching into centuries could expose an intruder who draws drinking water from a well to high dose rates.53 Comparable estimates for the Saltstone Vaults were not part of the saltstone performance assessment.
The difference between the dose rates quoted above is dramatic. From this difference, it is clear that the point of compliance (i.e., the location at which compliance with the performance objectives is measured) may determine whether DOE can comply with the
performance objectives. The point of compliance for the tanks was selected by DOE and the South Carolina DHEC for the tank closure assessments for Tanks 17 and 20.
Similarly, the exposure scenario is crucial to the calculated dose results. The U.S. Environmental Protection Agency (EPA) approach to protecting human health and the environment from such contaminants differs from that used by the USNRC. The EPA uses a lifetime cancer risk criterion to determine acceptable levels of residual soil contamination, whereas the USNRC and most states use an annual dose criterion. The two criteria cannot be compared in a meaningful way without also examining each agency’s entire system for the protection of public health and the environment, including methods of site characterization, assumptions about future land use, and methods assessing dose and risk, as well as uncertainties in determining levels of residual contamination and uncertainties in dose and risk assessments (NCRP, 2004).
Selection of an intruder scenario and the point of compliance involve policy and technical choices. A number of different intruder scenarios and points of compliance have been used in performance assessments and accepted by regulatory authorities. However, good risk management practice requires that the intruder doses and the rest of the spectrum of risks and trade-offs associated with future scenarios be considered with other assumptions in the context of the entire decision-making process, not in isolation. The committee is exploring what it can say further on these matters in its final report.
















