2
PLENARY SPEAKERS, DAY 1
OPENING REMARKS
The Honorable Michael Leavitt, Secretary of the Department of Health and Human Services
Thank you, Harvey, for that kind introduction (Harvey Fineberg, President of the Institute of Medicine).
This meeting is a tribute to a man who was a friend to many in this room, Dr. John La Montagne. You all know how his brilliant work helped save people from many diseases. While working on one of these projects, he told a colleague, “It’s good that we’re doing this. But if anything is going to get us, it will be the flu.”
I am also especially pleased to have the opportunity to share the podium with Dr. Fineberg this morning. Because, as he knows, I have learned many lessons from his excellent book: The Epidemic that Never Was: Policymaking and the Swine Flu Scare.
I know that many of you have dedicated your careers to this field. In the short time that I have been Secretary of Health and Human Services, I have become acutely aware of the disastrous public health impact that an influenza pandemic could have throughout the world. This is one of the most urgent health challenges we face, and I’ve made it a top HHS priority. Recently, I increased my briefing frequency on the flu to daily.
While much of our attention is focused on the H5N1 virus in Asia, I know very well that it is not the only flu threat we face. Many of the lessons that we learn from it will prepare us for annual influenza as well as for other potentially pandemic influenza viruses that may emerge in the future.
President Bush also understands the gravity of our situation. In fact, the United States government has made significant progress on pandemic influenza since he took office. We have increased spending on influenza tenfold over the past 5 years. We have added flu vaccine and flu drugs to the stockpile and made influenza part of regular public health discussions.
In order to increase our readiness against a pandemic strain of influenza, last Friday, on my recommendation, President Bush added pandemic influenza to the list of quarantinable events. This gives HHS the authority to take steps to prevent people with a new or reemerging influenza virus from infecting others by stopping them at our borders.
As we learned from CDC in last week’s Morbidity and Mortality Weekly Report, there was a silver lining in last season’s influenza vaccine situation. Despite the fact that we lost nearly half of our expected influenza vaccine supply, careful management of the available supply allowed this vaccine to be directed to the most vulnerable members of our population. We also sought out additional vaccine produced by foreign manufacturers and made arrangements to use
it if needed. I applaud the remarkable effort that this took, and the close working relationship between our agencies, the vaccine companies, state and local health officials, and healthcare providers that made it possible.
In spite of such challenges as that one, we’ve made great progress on influenza preparedness over the past few years. Flu preparation is an international responsibility, and I know many of you are involved in projects around the world.
My study of this matter has been short in duration by intensive, and the best in the world. Flu virus is a networked enemy. We must fight it with a networked army.
The United States will take precautions necessary to protect this country but we know our success is dependent on others protecting their own countries.
When you fight a networked enemy, a mainframe response will not do. Let me just mention a few steps we’ve taken here in the United States:
-
HHS is working to bring more influenza vaccine manufacturers into the domestic market through the joint efforts of CDC, FDA, NIH, our National Vaccine Program Office (NVPO), and the Office of Public Health Emergency Preparedness.
-
We’re working to accelerate the development of new influenza vaccine formulation and production techniques that will allow us to have a flexible surge capacity to make the doses of vaccine that we would need in a pandemic.
-
We’re devoting an unprecedented amount of resources to vaccine research, development, and procurement, and we want to increase the routine seasonal use of influenza vaccine for all who would benefit from it.
-
On Friday, I was delighted to announce a contract with Sanofi Pasteur for the development of an influenza vaccine produced in cell culture rather than eggs.
We’re doing all we can to ensure that Americans are healthy and protected against the flu. And everything we do to improve our approach to seasonal influenza prepares us to respond to an influenza pandemic.
In the past century, the world experienced three global outbreaks, or pandemics, of influenza. The recent emergence and persistence of a new influenza virus in birds in Asia and its infection of a limited number of humans with a high mortality rate has raised concern among scientists and public health professionals about the possibility of another pandemic influenza.
Dr. Julie Gerberding will talk more about this situation later this morning.
I am sure that most of you have seen the HHS draft Pandemic Influenza Preparedness and Response Plan we released last August, and I know that many of you have submitted comments. We're grateful for all of your input. I expect we will have the next revision out in the next few months. I am hopeful that the discussions and deliberations at this important meeting will feed into this effort.
And as part of our commitment to preparedness against the possibility of a pandemic, I am pleased to report that NIH has very recently begun clinical trials of a vaccine specifically designed against the H5N1 strain of avian influenza that is currently circulating in Asia. We have also gone ahead and produced 2 million doses of this vaccine in bulk. You will hear more about these efforts from Dr. Fauci later this morning.
Since we don’t know where or when a pandemic may originate, we have enhanced our surveillance network across the globe, but especially in east and southeast Asia, where we at HHS have people on the ground who are working with local researchers, clinicians, and governments. We are also in daily contact with the World Health Organization Secretariat in Geneva and its regional offices in Manila and New Delhi. We at HHS have experts on short- and long-term assignments to W.H.O. headquarters and the W.H.O. Country Office in Vietnam.
I've begun meeting with health ministers and ambassadors from affected countries, and soon I will begin to visit their countries. In May, I will also travel to the World Health Assembly, where pandemic influenza preparedness is on the agenda; I am convening a special meeting of health ministers from affected and donor countries to coordinate planning on influenza, followed by a technical meeting of experts the next day. Influenza will continue to be an important topic in all my discussions with my counterparts.
Needless to say, I’ve gained a much greater appreciation for how important your work is. We have learned so much in recent years about how to assess and respond to flu outbreaks, but we also have much more work to do. I am glad that all of you are engaged in these research and public health activities, and glad that you’ve come together today to compare notes and help us reexamine and reset the direction of our collective efforts.
While pandemics have happened several times in the past, never before have we had all of the tools of today. Never before have we possessed the wealth of knowledge on the problem and the ability to prepare. The challenge is immense, but so is our will to protect and preserve.
The outcome of this conference will be extremely important and will help guide us all in our work toward improving our ability to prepare ourselves. I look forward to being able to present a brief report on this symposium to my fellow health ministers when we meet at the World Health assembly next month.
MEETING OBJECTIVES
Dr. Bruce Gellin, Director, National Vaccine Program Office
As you just heard from Secretary Leavitt, the Department of Health and Human Service is devoting unprecedented focus on pandemic influenza preparedness. In addition to our work and the many activities that you'll hear about during this meeting, other countries and international organizations are also stepping up to the plate. As individual nations and as a global public health community, we are now better prepared to detect and respond to an influenza pandemic, but we clearly have to do more.
Secretary Leavitt also reminded us that the draft pandemic influenza plan issued last summer is now being revised to more clearly articulate the roles and responsibilities at each stage of an emerging pandemic and provide clearer guidance to state and local health departments, the healthcare sector and the public. In addition, the updated plan will conform to the new format proposed by the World Health Organization (WHO) which will facilitate international communication before and during a pandemic.
Like the pandemic influenza plans of many countries, our plan has had a long incubation period. In 1995, under John La Montagne’s leadership, the National Institutes of Health convened an international meeting to examine available data, identify critical scientific issues, and frame research questions to address gaps in knowledge vital to controlling pandemic influenza. Many of you participated in that meeting in December 1995, and much has happened since, but it is worth reviewing a few of the nearly 20 recommendations as they appeared in the 1997 supplement to the Journal of Infectious Disease (JID):
-
Improve or sustain international surveillance efforts, particularly in Asia and the Pacific Rim.
-
Improve our understanding of the role of humeral, cellular and mucosal immunity in protection against exposure to influenza, especially in immunologically naive populations.
-
Determine immunologic correlates of protection for live attenuated influenza virus vaccines.
-
Improve our understanding of the molecular basis of pathogenesis of pandemic strains.
-
Manufacture and clinically test new, inactivated vaccines made from selected novel influenza viruses that have pandemic potential.
-
Evaluate the effectiveness of using less than 15 micrograms of the current inactivated vaccine.
In addition to the specific research priorities was the overarching recommendation to establish a mechanism to facilitate collaboration among international laboratories—to share reagent strains and new technological advances and to enhance overall capacity and capability. Some of those ideas that helped us respond to severe acute respiratory syndrome (SARS). The international composition of this meeting acknowledges the need for a coordinated global response.
By the time the proceedings of that 1995 NIH symposium were published in 1997, we were facing the outbreak of H5N1 in Hong Kong that was challenging some existing assumptions. We expect that the road ahead will include additional twists and turns.
Before turning our attention to the task ahead over the next two days, I would like to offer one final quote from Dr. La Montagne that appeared in the 1997 JID supplement. “The ability to initiate the tasks outlined above is beyond the responsibility and resources of the NIH or any single government agency. This scope of action requires international organizations and the vaccine and pharmaceutical companies.”
Acknowledging the need for a coordinated global response, HHS and WHO organized this meeting to refocus our collective efforts on the scientific underpinnings of preparedness. Within this venue of the National Academy of Sciences, and with Institute of Medicine (IOM) president Dr. Fineberg presiding, we have assembled the top scientific leaders to seek your individual and collective input on critical scientific and epidemiological questions.
The goals of this meeting are to describe the state of the sciences relevant to pandemic influenza, identify and prioritize scientific and technical questions that will have the greatest impact on our ability to identify and respond to a pandemic, and develop an action plan for addressing those gaps. This meeting also provides an opportunity set a course that will strengthen our international collaborations. For this we will need your input, and have constructed the meeting with substantial time for breakout sessions that focus on specific scientific areas.
Last fall, Dr. John La Montagne speculated to a small group of us that pandemics might not necessarily be the virologic equivalent of the Big Bang, wherein a spark instantly becomes a raging fire. Rather, he hypothesized, that our strengthened surveillance systems and new diagnostic tools allow us to watch pandemics slowly unfold. If that is the case, then now is the time to advance our preparedness, because the only thing that is more difficult than planning for an emergency is explaining why you didn’t. We have a large task ahead.
CURRENT STATUS OF AVIAN INFLUENZA AND PANDEMIC THREAT
Dr. Julie Louise Gerberding, Director, Centers for Disease Control and Prevention
Secretary Leavitt, in his opening remarks, used a metaphor—the network model—that is absolutely appropriate for this meeting. CDC is a highly connected hub in the network of disease preparedness and response, and we are here to exchange ideas and information with our colleagues from the Department of Health and Human Services, (DHHS), the Department of Defense, the Department of State, other federal agencies, state and local agencies, private sector organizations, academics, and our key global partner, the World Health Organization. We thank Dr. Fineberg and IOM because you are also a highly connected node in this network, and much of the scientific work we do would not happen without your facilitation.
Every time I have been in this room in the last four years, it has been in the context of some horrifying public health threat—anthrax, smallpox, SARS, and now influenza. Anthrax, smallpox, and SARS are threatening situations where the risk calculation is relatively low yet the terror threat is high. In the case of influenza, the risk calculation suggests that we will certainly experience a pandemic sooner or later, yet most people perceive a very low threat. How do we prepare our society and the world for a likely threat amid growing complacency?
The fact that we are here today speaks to growing scientific recognition that influenza is an urgent menace, and that the time for action is now. I'm going to talk about we know about avian influenza, highlight what we don't we know, and mention a few steps we are taking to do something about it.
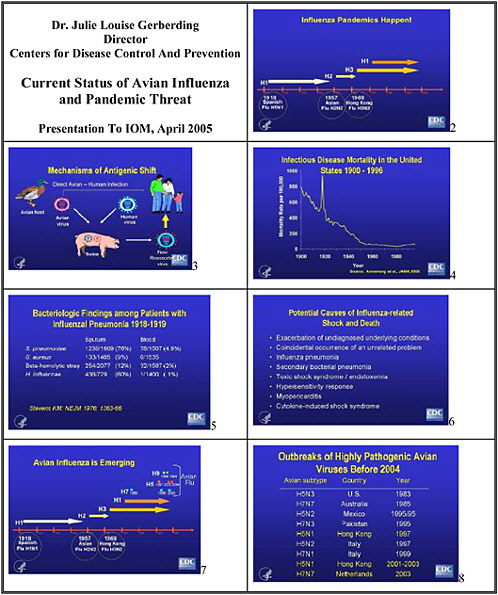
One thing we know for sure is that influenza epidemics and pandemics do happen—the three large pandemics in the last century attest to that. But as Mike Osterholm will tell you, many other influenza pandemics have also occurred throughout recorded history, some as large if not larger than the 1918–1919 epidemic. It doesn't take a scientist to appreciate that the clock is ticking, and that another pandemic is due.
We also have some understanding of how antigenic shift occurs in influenza viruses, and why pandemics may emerge. There are at least two mechanisms. One is through reassortment of viruses—typically avian and human viruses in swine, which create a new strain which can infect people who lack immunity to the new antigens. The second mechanism, direct avian-to-human transmission, may also have accounted for some of the past pandemics.
The picture becomes more complicated in the context of the current avian influenza outbreak, because we also have the possibility of an avian virus and a human virus reassorting in people and/or other host species. We need to know much more about influenza virus strain evolution before we can predict whether any of these mechanisms would allow this or any other avian strain to emerge and become more efficiently transmitted to people.
We also know that pandemics are brutal on their impact on human mortality. The spike in mortality in 1918 and 1919 is a sober reminder of what happened when global connectivity was unusually high, given the movement of people that occurred at the end of the world war. But that situation was nothing compared with the connectivity and complex global networks in which we
live and move today, and the increasing connectivity between humans and animal reservoirs of influenza viruses.
We have only to think about SARS, as it moved from “Hotel M” in Hong Kong to global distribution in just a few short weeks, to be sobered by how quickly a problem in one corner of the world can reach other backyards literally overnight. So while we can be optimistic about advances in medical care and vaccine development, the potential for a pandemic with high mortality in this very small world is great.
We know of avian influenza have been identified in people living in Thailand, Vietnam, and Cambodia. Why haven’t we seen human cases in other countries? Is this surveillance bias? Is it virologically determined? Is it host determined? What exactly is the explanation for the relative paucity of infections among people, given that the virus is much more prevalent in avian species than these human cases would suggest? What is the full spectrum of illness? We must do more research to understand the relationships among the virus, its virulence factors, the host’s immune response, and the clinical outcomes of infection.
We know that the people with H5N1 influenza reported to the WHO in the current outbreak have a high mortality rate. To date, 74 cases and 49 deaths in Asia have been reported to the WHO, yielding case fatality rate of 66 percent. We don’t know if this fatality rate is accurate. Does it represent detection bias—in that sicker people are being diagnosed? Is the reported number of cases the tip of the iceberg, in that many less severe or asymptomatic cases have gone unrecognized? We do not know why so many young people died from influenza in 1918–1919. Certainly the stereotypical explanation has cited complications—particularly bacterial complications, although a 1976 review in the New England Journal of Medicine suggests that they might not have been the reason for so many deaths. Case reports, a review of the pathology literature, and recent experiments with influenza virus constructs containing genes from that pandemic strain suggest other potential explanations for the high incidence of shock and death associated with that pandemic.
Most of the affected individuals in the current epidemic have been young and healthy. Why young and healthy people? Does this reflect exposure bias or a susceptibility in young people that perhaps reflects lack of prior exposure? Does age, ethnicity, nutrition status, or viral strain affect the case mortality rate? We need to address these very important questions about the clinical presentation and outcome of avian influenza through careful epidemiologic, laboratory, and clinical investigations.
We also lack information on the relationship between treatment and the outcome of these infections. We know that the avian viruses currently causing human infections are resistant to amantadines and susceptible to neuraminadase inhibitors, and that some of the patients who succumbed had been treated. But whether treatment, alone or in combination of antivirals, offers any virologic or outcome advantage is unknown.
We know that exposure to infected birds is a major source of infection among people, but what are the specific modes of transmission? Recent case reports suggest exposure to contaminated water and eating uncooked chicken could be risks as well. If we look back at the H5N1 influenza virus outbreak in Hong Kong in 1997, some information could direct us to the studies that we need to do today. We learned from case-control studies that the primary risk factor in the last outbreak was exposure to live poultry, and that the prevalence of the virus in chickens was very high. The prevalence of antibody to H5 in a cohort of the exposed population
was about 10 percent, and those who butchered high risk birds were at higher risk for seropositivity. Targeted research—designed to understand the relationship between exposure, transmission modes, immunity, and disease outcome, as well as opportunities for intervention in environmental, occupational, household, and healthcare settings—is critical in affected countries. We must conduct these studies and many others in people, birds, swine, other mammals, as well as water and other potential environmental reservoirs to develop a comprehensive understanding of the epidemiology of this infection.
Although we know that avian H5N1 influenza virus is widespread in Asia today, some places are not represented in the surveillance network or have very weak connectivity, and those weak links create vulnerability. Research needs to address how to strengthen the network and obtain information from these missing nodes on the network.
Other critical questions include: Are all virus isolates among the poultry strains alike, and are they evolving? If so, what selection pressures are encouraging that evolution? Where are the carriers of these viruses from one country to another or one population to another or one species to another? How does the ecology of avian influenza virus affect its mobility to other parts of the world through migratory bird vectors or other movements of people, animals, birds, or fish?
Unlike the 2003 isolates, which were homogeneous, these H5N1 influenza viruses now circulating in Asia are expressing some degree of heterogeneity. We don’t know how immunogenic these viruses are. We also don’t know what implications heterogeneity will have for vaccine development. We need a much more comprehensive understanding of the differences between the human and the avian isolates and their geographic distribution.
Given the relative paucity of what we know compared with what we need to know, what are we doing about it? At DHHS, Secretary Leavitt received a comprehensive briefing on influenza even before his Senate confirmation, and CDC has since had several face-to-face briefings with the DHHS team as well as a daily update. We could not have better departmental leadership and commitment. Besides our support for WHO as the lead for international preparedness, we are also investing resources in specific Asian countries to improve their ability to detect emerging influenza strains and transport them to laboratories for reliable evaluation. Our other regional activities include our International Emerging Infections Program in Thailand and our collaboration with the US Naval Medical Research Unit laboratories of the U.S. Department of Defense (DOD) in Jakarta and Cairo.
Our laboratory and epidemiologic scientists are hard at work to study the genetic determinants of pathogenicity and transmissibility, the genomic bases of drug resistance and binding properties, tracking and monitoring the antigenic evolution of virus isolates in time and place, and understanding the epidemiology of the current epizootic in more detail. This meeting will undoubtedly incubate other scientific questions.
We are taking steps to enhance communication between public health and veterinary agencies. Dr. Lonnie King, former dean of the College of Veterinary Medicine at Michigan State University, is heading CDC’s Office of Strategy and Innovation. Dr. King is forging better connectivity between CDC and the veterinary community—both domestically and internationally—and the academic animal health community.
Through investments in influenza preparedness as well as global detection of emerging threats, CDC is building a comprehensive international health protection network to connect all
relevant capabilities and assets. The private sector is a key partner that we have engaged through our global roundtable of senior business leaders to understand how the international business community can benefit from our preparedness efforts and provide relevant information as another hub in our preparedness and response network.
Secretary Leavitt mentioned that President Bush has authorized us to use our quarantine powers, if needed, for a novel or re-emergent strain of influenza with the potential to cause a pandemic. We are also moving domestically to expand our capacity from 8 quarantine stations to 30. Those are important steps, but they also highlight the fact that preparedness takes time – and time is of the essence if we hope to optimize quarantine and isolation capacity on a global scale. We had some practice with SARS. That experience taught us that with the right framework, people can do the seemingly impossible. But SARS was a relatively easy problem compared with the global challenges that an influenza virus strain with a high reproductive number (Ro) would create. We need to investigate the human aspects of isolation and quarantine, and what we need to do to prepare people and engage our leaders and our population in appropriate isolation and quarantine responsibilities.
Anthrax and SARS taught us that we need solid communication science if we are going to have any hope of managing a major influenza outbreak. That science needs to address the content and credibility of communication to diverse populations. That effort isn’t just about translating science into messages that ordinary people can understand. It’s about translating ordinary messages into hopeful and helpful information that people of multiple cultural and linguistic backgrounds can use, and about transmitting information through a variety of channels on which we do not usually rely in the Western Hemisphere. I would urge this meeting not to lose sight of the human side of the research agenda, and to grapple with the communication sciences that are essential to our ability to prepare for and respond to an influenza outbreak or pandemic.
The biggest lesson we have learned from other public health threats is that the most important enemy is complacency. I do not know how to develop a research agenda around preventing complacency, but I would submit that doing so is urgent. My fear is that although the lens may be shining on avian influenza right now, if the H5N1 strain does not become more transmissible to people, we will falsely assume that the threat is over. Worse, we could be accused of inappropriately revving up our preparedness efforts without a scientific basis. I do not believe we have done that, but it reminds us about the importance of credible communication so that the public understands the need to prepare and what is at stake if we don’t. We must strike the right balance between action and reassurance. The stimulus to effective research that this conference promises to foster is an essential step toward evidence-based policy decisions, effective public health action, and credible communication in the context of this global threat.
MEETING THE CHALLENGE OF PANDEMIC VACCINE PREPAREDNESS: AN FDA PERSPECTIVE
Dr. Jesse Goodman, Director, Center for Biologics Evaluation and Review U.S. Food and Drug Administration
My presentation will focus on how FDA can and is approaching meeting the challenges of pandemic vaccine preparedness including the more applied science needed to support assessment of safety and efficacy as well as quality in manufacturing. I will also focus on steps to increase manufacturing diversity and capacity. While the drivers for investment are primarily economic, FDA can help by developing and defining the needed and efficient pathways and regulatory processes to speed vaccine development, assessment and availability. Assuring safety and public confidence is absolutely critical as a pandemic vaccine will impact millions of individuals. Finally, and perhaps most important, I also want to touch briefly on the idea of considering whether there are pathways to prevent a pandemic, such as incorporating preparedness into routine immunization, and to emphasize the need for thinking and working globally. I'm not going to emphasize antivirals, as doing so would require an entire workshop, but I do think they have a role to play.
The Secretary and Dr. Gerberding made analogies to bioterrorism threats, and I want to reiterate that pandemic flu poses similar and equally serious challenges and demands some of the same kinds of approaches. As a result, we are not treating this as business as usual. We have had extensive interactions with sponsors to encourage them to develop new products, and have turned things around very rapidly. We have taken proactive trips to look at manufacturing facilities and have participated in multiple product development teams to ensure expedited reviews. We used such approaches to combat last year’s supply problems with flu vaccine. These approaches are appropriate for pandemic preparedness.
Markets—that is demand and sales—are the main drivers of manufacturing. No one is going to build factories just for a possible pandemic. In the last two or three years, growing yearly vaccine use in the United States—prompted by CDC and its public health partners—has helped to stimulate interest in the U.S. vaccine market among global manufacturers. This past year’s problems at Chiron in producing flu vaccine, and the growing concerns about, and investments in, pandemic preparedness, have also accelerated commercial interest and the development plans of potential manufacturers.
Our interactions with industry to respond to the problems this past year included extensive review of clinical and manufacturing data and multiple facility inspections of foreign manufacturers, which made an additional 5 million doses of investigational vaccine potentially available if needed in 2004. I’m proud of the contributions of the people in our department, and appreciative of the cooperation of manufacturers, although fortunately we did not experience a bad flu season. Several manufacturers have expressed interest in becoming licensed to supply flu vaccine to the U.S. market, and, as I will discuss, FDA has defined an accelerated approval mechanism that can help speed their availability to meet this important public health need.
Several of the most important lessons learned regarding the manufacturing infrastructure for flu vaccine are relevant to other critical vaccines, many of which also have only one or a few
manufacturers and an overall fragility in their supply. While many vaccines can be stored from year to year, this is not the case for influenza vaccine, which changes in composition almost every year. We should not lose this opportunity to teach our colleagues in the policy arena more about the fragility of vaccines—and steps that can be taken to support the infrastructure, an area where IOM has provided leadership.
We now have a global pharmaceutical marketplace as well as a global disease marketplace—we are fully globalized on both ends of the equation. FDA has realized the need for better international information sharing, much as CDC has recognized the need for better surveillance. We have therefore completed new confidentiality and information-sharing agreements with regulatory authorities in other countries, so that we can obtain or share important product manufacturing and safety information both pre- and post-licensure. I have also been trying for some time to encourage global plans to develop vaccines: why develop a vaccine for just one country? Regulatory cooperation and harmonization will help, and we are starting to see companies develop vaccines for a global market.
Also in response to last year’s flu vaccine shortfall, FDA has decided to move from biannual inspections of flu manufacturers to annual inspections in the hope of catching problems sooner or, even better, wherever possible encouraging the prevention of problems through robust quality systems and high quality manufacturing processes. The full swing of manufacturing of flu vaccine occurs in summer. If problems develop then, we may not detect them early enough to respond. We want to address these issues, consistent with FDA’s good manufacturing practices (GMP) initiative to strengthen the communications with companies around vaccine GMPs.
I would like to talk a bit about how we handle annual availability of flu vaccine in the United States. Each year, any of the three strains that made up the previous year’s vaccine can be replaced with a new strain. We base the determination on surveillance, working with CDC, WHO, and our advisory committees, among others. We do not view this as a major change to the vaccine, so we require a manufacturer with an existing license for inactivated influenza vaccine to submit only a prior-approval manufacturing supplement, which basically describes the strain and its characteristics—a routine, straightforward process. We do not require licensed manufacturers of inactivated influenza vaccine to provide clinical data to gain approval of these annual supplements.
To meet current deficits in capacity and to make licensure of other flu vaccines faster and more efficient, we have turned to our accelerated approval authorities. Accelerated approval can be used to approve a product that provides a meaningful therapeutic benefit for a serious or life-threatening condition when there is a lack or shortage of available alternative therapies.We determined that influenza vaccines qualify for accelerated approval, as the number of individuals who could benefit and for whom vaccination is recommended far exceeds the current supply. For pandemic strains as well, we would certainly find an unmet medical need, in that no vaccines currently exist for those strains. Accelerated approval allows approval of a product under a surrogate endpoint—a marker reasonably likely to predict clinical benefit—rather than requiring completion of all clinical efficacy studies before licensure. Clinical endpoint studies would later confirm this benefit.
Considering flu vaccine to be in short supply, we have stated that we consider hemagglutinating inhibiting anti-HA antibody levels as a likely surrogate marker for efficacy. We commonly approve vaccines based on proven surrogate markers such as hepatitis-B anti-
surface antibody levels, but this would be the first or one of the first accelerated approvals based on likely markers.
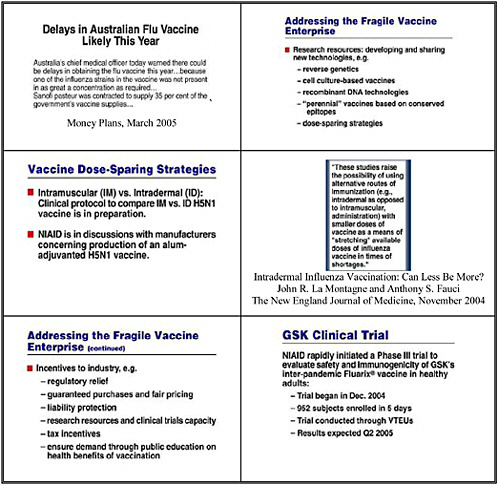
We have thus told manufacturers that they can seek accelerated approval based on immunogenicity-provided validated assays are used, and complete manufacturing data and control, and satisfactory safety data are provided, followed by post-approval studies of efficacy. In considering the vaccines that other manufacturers offered or expressed interest in bringing to the U.S. market—vaccines licensed in other countries with competent regulatory authorities—we have said that well performed clinical trials and data from use under foreign licensure can contribute to U,S, licensure. At least two firms, GSK and ID Biomedical, have indicated they will seek U.S. licensure for their flu vaccines under this approval mechanism. We believe we have shortened the time to approval by one to two years.
The kind of immunogenicity data we are looking for can also be useful when we try to bridge efficacy of flu vaccine to other populations. For example, these data could help us allow a manufacturer to look at immune response in different populations as a surrogate for efficacy in those populations, making it easier to perform certain clinical studies. The information obtained from immunogenicty studies will also be important in looking at and establishing appropriate dosing for novel strains such as pandemic strains.
How can we facilitate the rapid availability of vaccine needed to prevent or respond to a pandemic? First, we will view a pandemic strain used in a licensed manufacturing process as a strain change. Biologically, a new hemagglutinin antigen is just that: another hemagglutinin antigen such as we use in a routine strain change. For licensed manufacturers using licensed processes, we wouldn’t treat this as a new vaccine but as a supplement. This will significantly reduce unneeded costs and speed availability. However, having some clinical data are important because of the differences in immunogenicity in a naive population are likely to affect the doses needed to protect the population and because there should be assurance that the vaccine has the excellent safety profile typical of routine flu vaccines. We also have no problem with the use of recombinant or cell-culture–based technologies, including reverse genetics, in strain production, as long as manufacturers use adequate controls and characterization. We don't want to immunize potentially billions of people with a vaccine strain if there is any substantive concern about its origin or safety.
Conducting the needed clinical studies for candidate pandemic vaccines during the inter-pandemic period is very important, and real progress is being made here. Studies by the National Institute of Allergy and Infectious Diseases (NIAID) of H5N1 will provide critical information on dose and schedule. However, it is important not to get too overconfident. Just when we think we understand something, it changes, and right now we don't have the experience to generalize from one pandemic strain to another.
Whenever we undertake important public health programs, ensuring product safety to the extent we can and engaging in effective communication are critical, as is full transparency about the potential risks and uncertainties of a pandemic vaccine versus the pandemic itself. Where time permits, we think it is a good idea to obtain an additional safety database on several thousand individuals before licensure and wide use of the pandemic vaccine, even though there is no particular reason to suggest that the vaccine should behave differently. Establishing a system for surveillance and reporting of adverse events before the use of such a product is important, as is public communication that we are taking that step.
Applied science, such as the preparation of qualified seed strains representing major known and evolving pandemic antigens, can facilitate vaccine manufacturing and availability a The flu community, including CDC, academic researchers, WHO, and our regulatory and public health counterparts around the world, have done a great job, but we need more such efforts, and they need to be well supported and coordinated.
We also need much more information on the biological basis for strain cross-protection among evolving pandemic types, and on whether we can predict whether one vaccine strain will be protective against another without extensive studies. We also need to prepare in advance reagents for manufacturing, such as antigens and antisera. Our lab and others have been very engaged in that, but the needs of a pandemic are an order of magnitude greater. We need to not only evaluate and standardize assays but also consider new approaches to improve assays of potency, antibodies, and sterility to speed the regulatory and manufacturing processes.
Many people have noted that even if we had a decent interpandemic vaccine infrastructure and supply, manufacturing capacity will still most likely be inadequate to meet U.S. needs in a pandemic—if we needed a higher antigen dose or multiple doses, for example. And, it is very clear that inadequate global vaccine capacity currently exists to meet global needs.
Many questions have therefore been asked about adjuvants and whether they could reduce the requirements for antigen used in each dose and therefore enable us to stretch supplies in a pandemic. There have been some promising reports and if safe adjuvants could reduce dose requirements and/or enhance vaccine immunogenicity, they could play an important role in pandemic preparedness. However, it is important to recognize that both published and unpublished results have been conflicting. We need adequate studies before we assume adjuvants will be effective and, if they are, to determine how best to use them. These studies should be a very high priority and are now being undertaken with HHS support.
Such an approach would be considered a new product because it would require different formulation and manufacturing, and we would need to assess the safety and efficacy of the product. The simplest adjuvant would be aluminum, with which we have extensive experience and safety records in licensed vaccines. Early studies should demonstrate significant increases in immunogenicity with an acceptable safety profile. More novel adjuvants, especially any with reactogenicty or safety signals in early use, would obviously require more safety data. If proof of concept and other studies are favorable—and recent informal and published presentations have suggested potential benefits from adjuvant approaches—researchers should pursue controlled studies in the inter-pandemic period. Accelerated approval mechanisms could be used, so long as adequate safety and immunogenicity data were provided.
Other potential antigen-sparing strategies for vaccine delivery should be on the table. The simplest change—as indicated in provocative papers in the New England Journal of Medicine in the last year—might include intradermal vaccines using needle and syringe, but this raises practicality and delivery issues. Other means of intradermal or transdermal delivery are also available. Again, safety and efficacy would need to be determined, especially immunogenicity in the population using the vaccine. Immune stimulators, such as patches with added cytokines or other stimulants, also look promising in small numbers of patients, but again we would need data before adopting such measures on a public health basis.
Non-egg-based technologies such as cell culture and recombinant vaccines offer very significant potential advantages, such as flexibility, potential for rapid scale up and production of
large quantities, and the fact that they could be primarily sterile processes from the start. Despite problems, egg-based manufacturing has been successful and cost-effective, and other technologies have not been widely used or marketed yet despite early promise.
We have licensed other cell-culture–derived and recombinant vaccines, and we have no special regulatory concerns with those technologies if used for flu. We are working hard to help people with development processes, though the scientific and technical challenges have not been trivial. For cell-based vaccines, these include the usual assessment of tumorgenicity, advantageous agents, and residual DNA. These are critical but addressable scientific issues for a vaccine that, as stated before, could be given to hundreds of millions or billions of people. FDA is providing guidance for manufacturers on cell lines and adventitious agents. However, the biggest hurdle has been obtaining enough yield, manufacturing scale, and cost-effectiveness. Still, there is no reason in the long-term or even medium-term why a cell culture-based technology couldn’t succeed. With recombinant vaccines, some of these issues are less pressing. However, in that case we have to address antigenicity and ensure that the immune response elicited to a recombinant protein is indeed a protective one.
Other new technologies worth considering include vaccination with potentially cross-protective antigens to build immunity against influenza strains in the population more generally, and live attenuated vaccines, one of which is licensed. The latter provide multiple immunogens, some of which may offer some cross-protection. They may also enhance more rapid development of immunity, and potentially require only a single dose, an advantage especially in the immune naive, but they raise potential containment issues, at least in the pre-pandemic period.
Can we consider other pathways to pandemic preparedness, and other approaches to how we use vaccines? Obviously, for a pandemic to occur, not only does the viral strain need to be lead to transmissible and virulent, but, by definition, the population must lack immunity. Can we conceptualize pandemic preparedness as routine prevention rather than as something that must occur only during a crisis? Some of the tremendously challenging public health communication problems that occurred around the swine flu, anthrax, and smallpox reflected a crisis mode of communications and intervention. Should we consider building immunity against evolving virulent pandemic-threat strains through more routine and earlier immunization against those strains? Should we produce and make vaccine against pandemic threat strains available before a pandemic? Should we even integrate antigens protective against pandemic threat strains into routine influenza immunization? What are the risks and benefits? Transparency and public dialogue would be essential in considering such approaches.
We are acting locally, but we should also be acting globally. CDC is taking the initiative to work with global partners in surveillance, as is WHO, and FDA is similarly work with industry worldwide to build vaccine capacity.
Regulatory cooperation can facilitate the potential transnational use of vaccines in both pandemic and inter-pandemic settings. We, as a global community, should also consider vaccinating people at geographic sites of evolving virulent pandemic strains. Such an approach could save lives at the sites of pandemic strain emergence and, potentially, help slow or halt a pandemic. Antivirals could also play a role in such settings, even before widespread human-to-human spread, to slow or halt a pandemic.
To summarize, we are working with partners, many of whom are in this room, to help diversify and strengthen manufacturing and provide flexible, rapid regulatory pathways. I think
we are making progress. Pandemic vaccines licensed as supplements rather than new vaccines can help speed and reduce the burden and cost of pandemic response, but we need to ensure that the vaccines are high quality.
Advance preparation and improvement of strains, reagents, assays, and standards would be beneficial. Many here are engaged in that, but we can do more. We can best address scientific needs, create manufacturing capacity, and evaluate the safety and effectiveness of antigen-sparing new vaccines and delivery methods as well as non-egg-based technologies before a pandemic. Those studies are now under way and should be expanded and accelerated as results indicate.
Finally, we need to consider the risks and benefits of earlier intervention against virulent potential pandemic strains, including potentially integrating them into routine medical and public health preparedness as we do for annual influenza. Can we learn from experiences such as that with swine flu and still meet the challenge of pandemic preparedness? Enhanced surveillance and increased awareness along with improved vaccine and antiviral manufacturing capacity and global regulatory and public health infrastructure and cooperation, can provide mankind with the potential opportunity to effectively intervene to prevent or reduce the catastrophic consequences of the inevitable, the next influenza pandemic.
GLOBAL PANDEMIC PREPAREDNESS RESEARCH EFFORTS
Dr. Klaus Stöhr, Project Leader, Global Influenza Programme, World Health Organization
I would like to thank the Department of Health and Human Service and the Institute of Medicine for their vision and leadership in organizing this meeting—not only because it is 10 years since the National Institutes of Health and the World Health Organization convened a meeting in Washington to figure out what would happen in a 1918-style pandemic, a meeting that John La Montagne was critical in organizing, but also given the evolving situation in Asia and the need to consider strategies to reduce morbidity and mortality from the next pandemic.
Medical and public health interventions must be built on the best science and evidence. But the world is changing, and that includes the influenza world. The current situation requires a thorough understanding not only of the research landscape but also of the most important public health and research priorities that inform policy. I regard research as generating knowledge for action.
From the perspective of an ever-changing world, influenza has been extremely interesting over the last few years. I hope that the influenza virus will slow down a bit and make fewer changes. On the other hand, it is certainly exciting to live and work during this extraordinarily dynamic time.
WHO collaborates with many partners world wide. I would like to particularly mention colleagues at HHS, CDC, NIH, FDA, and many academic institutions with which we have the privilege to work so closely. I would like to thank them for not only the direct support they provide to WHO but also for their international leadership.
I was asked to talk today about global pandemic preparedness efforts. I would like to look at only a small fraction of pandemic preparedness and research during this inter-pandemic period. We will have to confront a pandemic at some point, and then we will return to the next inter-pandemic period. I hope that we do not have to find out during the next pandemic that complacency during the current inter-pandemic period slowed our preparations. I would like to look at medium-term applied research linked to medical and public health interventions, addressing current needs, especially the situation in Asia. I would also like to cover two smaller areas that can make a profound difference in our ability to reduce morbidity and mortality from the next influenza pandemic.
In my view, we are all overwhelmed with responding to the current situation and have not yet had time to prepare for the research necessary DURING the next pandemic. For targeted public health interventions during the next pandemic we will have to understand the natural history of the disease and the risk groups. We do not yet know the incubation period of the new virus or the duration of infectivity. We are considering quarantining, but how long will we quarantine someone if we don’t know how long he or she is going to excrete the virus? We have to develop standardized protocols for the next pandemic to obtain a critical mass of knowledge to react very quickly and precisely.
We have to inform policy, and that will be impossible unless we can assess the medical impact of the disease. Many interventions that we are recommending now are based on the best science, but that science may be limited. The evidence may have come from good studies conducted many years ago, perhaps with viruses that are different from those now circulating or that might cause the next pandemic. That caveat also applies to vaccines and antivirals. We have to anticipate the economic impacts of the next pandemic and consider how can we convince people that strengthening the communicable disease infrastructure now, makes sense and is economically sound.
We certainly need to do virologic research. Because a pandemic virus will change very rapidly, we must better understand the factors that influence the genetic and antigenic evolution of influenza viruses. Preparing not only for control and response during a pandemic but also for the research needed to inform policy will require vision, leadership, and the ideas of the participants in the symposium over the next two days.
I consider research during the pandemic and pre-pandemic phase as deserving particular attention, along with risk assessment and communication. Medical interventions—vaccines, antivirals, treatment with antimicrobials—as well as non-pharmaceutical interventions are also critical. The combination of these measures will vary within countries. Many potential interventions such as vaccines and antivirals will be only suboptimally effective or not available.
We consider risk assessment very important. There are intense efforts from a global perspective to control—and possibly even eradicate—the disease in animals. This will require long-term control measures and an enormous investment. The government of Thailand has invested $120 million to compensate farmers for sacrificing chickens infected with bird flu. That country can afford such an effort and is capable of doing it because it is the fourth largest poultry exporter, and poultry production is critical to its gross national product. On the one hand we are encouraging considerable investments by the agricultural industry for the control of H5N1 in animals. On the other hand, we can not precisely quantify the pandemic risk from H5N1. As an example, we have not seen evidence of reassortment since 2004, even though the virus is widespread and has been transmitted thousands of times to humans. We are certainly seeing only the tip of the iceberg in Vietnam and Thailand: many more cases are likely occurring.
The question that needs to be answered fast is: what is the likelihood and the outcome of a reassortment between an H5N1 and human as well as perhaps pig viruses? One-third of the pig population in China has human H3 influenza viruses. This might be a good time to look at pig viruses, as we have no understanding of whether H5N1 has already gained a foothold in pigs, because studies have not yet even begun to assess this. Fortunately, CDC has embarked on a laboratory project to assess outcome of reassortment. These studies are now in the second phase, and a second laboratory from Europe might join these activities.
There is certainly a need to conduct studies on the H5N1 infection rate in the general population of an affected country. Experiences from studying other diseases show that such research can be relatively simple and inexpensive. Such research would help us understand the true prevalence of mild and severe H5N1 infection in the general population against the background noise of 100 of thousand influenza-like- illnesses every year and help assess epidemiological significance of the few severe cases that turn up in hospitals.
The second area with numerous opportunities for researcher is the control of pathways of transmission. Laboratory data and recent studies from Thailand and Vietnam suggest that ducks
are a potent reservoir for highly pathogenic avian influenza for a relatively short period of time. H5N1 has an increased pathogenicity in poultry and mice and is found in a growing number of mammalian species. But what is the role of these animals and birds in the epidemiology of influenza viruses of pandemic potential? Vector studies in domestic and wild animals and birds are very important and could be of relatively low cost. A few colleagues are conducting such serological and biological studies, and some funding agencies are supporting them, but the research is too limited to provide the knowledge necessary to guide policy decisions. Another area for research in countries now affected by H5N1 and which have developed control strategies in response to this outbreak in animals, would but the assessment of the effectiveness of the interventions.
Case management and control of infections in hospitals also present opportunities for research. For rapid detection of the emergence of a pandemic virus, the number of reported cases is less important than immediately investigating every suspicious case in Vietnam, Thailand, and elsewhere in Asia. If we do not make such an effort, the narrow window of opportunity to stop an emerging pandemic virus or virus with greater human-to-human transmissibility will close.
We have seen only very few publications on the clinical course of H5N1. We still have very little understanding of H5N1 key clinical epidemiological and virological parameters, incubation period, duration of infectivity, duration of transmission, and excretion kinetics. And we are talking about a disease that has been around for a relatively long period of time. We have decent data on the usefulness of antivirals, and we are finding out more about the duration of treatment and size of dose, but how will we use that knowledge?
We need coordinated clinical research and case management, as well as a network of hospitals in affected countries, not only for avian influenza but also for emerging infectious diseases. Such a mechanism for coordinating clinical research, exchanging samples and information can enable affected countries to rely on standardized treatments and research protocols and support effective communication. NIH is supporting an initiative to establish an international clinical research network on emerging infectious diseases in Asia. We believe this is vitally important so we can investigate diseases in a standardized way at a very early stage.
Doing so will not only strengthen national capacity and resources but will also facilitate international collaboration and exchange. With NIH, WHO has developed a concept paper on such a network, and we have identified international research and funding partners. The next step is to engage national partners and enroll hospitals. It’s not going to be easy sailing, but we believe this is a step in the right direction.
For the first time we have the possibility of detecting a pandemic virus early, and of stockpiling H5N1 vaccines as well as antivirals. But could massive prophylactic use of antivirals and possibly vaccines in and around an epicenter extinguish an emerging new subtype, or at least buy time, is another unanswered question.
Modeling of a pandemic is ongoing, and we will hear the first results at this meeting. We have also looked at data from other groups, and they are very encouraging. However, we need to take conditions in developing countries into account and review the applicability of the models.
We also have to factor in the time it would take to detect a pandemic virus. Today a diagnosis and possible field investigation of an individual case takes more than 20 days. We also have to look at the accessibility of populations in developing countries, particularly in remote
areas. More than 80 percent of the territory in Vietnam may only be reached by four-wheel drive. We have also to look at the challenges of enforcing control measures, such as quarantine time, over large areas in developing countries.
We certainly need more research on an H5N1 vaccine stockpile, which could make a difference for countries that could afford one. A stockpile could be a complementary tool in a large armamentarium of pandemic interventions, allowing vaccination of certain critical groups before a pandemic virus arrives. A stockpile would also provide an incentive for pharmaceutical companies to invest in a pandemic vaccine, although clinical trials are not complete. WHO is looking forward to receiving data from the modeling, because without them it is difficult to think about the appropriate size of a possible international stockpile of antivirals and the intensity with which we should be pursuing this strategy.
If antigen-sparing strategies and low-antigen dosage for stockpiled vaccines are chosen, will they remain fully effective after storage over 6 or 10 months, when the vaccines may have degraded? We also need to investigate vaccination liability before a pandemic starts.
WHO has developed recommendations for slowing down local spread and using non-pharmaceutical measures at international or national levels during different phases of an influenza pandemic. They are built on the best available science, but assessing their potential impact is difficult. We will need a research protocol during a pandemic, or we will be relying on incomplete data generated during the last pandemic.
One incompletely understood area is the pathways of transmission of seasonal influenza virus and their relative importance. Patients in Asia now excrete large amounts of H5N1 virus in stool, perhaps even enough to infect other persons or chickens. We don’t know the infectious dose for humans. What role would fomites play in transmitting this disease now, and perhaps in the future? How should we change hospital infection control, knowing that there are different pathways of transmission?
There is also a need to establish permanently the immunogenicity of the available H5N1 vaccine against newly emerging H5N1 strains. Although that will not tell us everything on the expected effectiveness of available H5N1 vaccines against the future pandemic virus, up-dating of the antigen content will contribute to increased vaccine effectiveness.
Global production capacity of normal seasonal vaccines over eight months is roughly 3 million doses of a trivalent vaccine with 15 micrograms of antigen. This information is based on an estimate prepared by the Influenza Vaccine Supply Task Force of the International Federation of Pharmaceutical Manufacturers Association. Manufacturers could produce three times that amount of a monovalent vaccine. Manufacturers could produce even more whole-virus vaccine, because splitting the virus and developing a subunit eliminates some of the virus in the original liquid. Vaccine output could be further increased, perhaps up to four times, if immuneenhancers are used. Under such circumstance, the global daily vaccine production capacity could be in the range of 13 million doses, Smart vaccine design and use of immuneenhancers could thus make a big difference—not only for one country but for the world population. Such an effort would require coordination of vaccine research. WHO will organize a meeting in November for all pharmaceutical companies to compare data and recommend next steps.
Since November 2004, two companies are working on the preparation of clinical trials in the United States. Ten more companies in Australia, Canada, France, Germany, Japan, and the
United States will have begun or finished clinical trials before the end of the year. All will use antigen-sparing strategies, which is very encouraging. Every company which wants to start pandemic vaccine production rapidly after the begin of a pandemic—whether it uses H5 or another strain- will have to license a pandemic vaccine beforehand.
If a pandemic virus were to emerge today, companies would require about two months to prepare their production sites, although some might need more time and some less time. Manufacturers would require another two months for pilot production. By that time a pandemic virus emerging in Asia might already have reached Europe or Australia. Clinical testing would require another two months, and by then the virus might be globally distributed. That means that vaccine production might start only after the virus had already circumvented the globe—a scenario we all would consider inappropriate in view of expected morbidity and mortality. Companies need to update and test vaccines now so they can begin production much more quickly.
Another problem is that manufacturers have little or no surge capacity for seasonal and pandemic influenza vaccine. We need to look now at improving existing production systems and devising alternative systems. Dr. Goodman has already mentioned cell-culture vaccine and recombinant vaccine—a very promising development. Improved antigen harvest from eggs is also a possibility. None of these options is going to be a silver bullet—each is only one component.
The epidemiology, natural history, and public health risk of emerging avian and mammalian influenza viruses are very difficult to evaluate. Surveillance in animals and constant characterization of viruses would be ideal, as they would allow us to better determine whether these emerging strains pose a public health risk. Then we would not need to invest into outbreak response from an H7 virus in a given country, because we could tell from the beginning that a highly pathogenic strain had little or no pathogenicity for humans.
The determinants of human pathogenicities of animal and bird influenza viruses and the role of migratory birds are not well understood. We also need studies of the ecology and molecular biology of animal influenza viruses, to understand the genetic foundation for specificity and pathogenicity. And we certainly need virological and serological studies on the prevalence and molecular evolution of influenza viruses in animals and birds. That would require a long-term investment which will be profoundly important in anticipating the next pandemic.
About 40 countries use seasonal influenza vaccine in humans. Most have vaccination policies, this means that at the end it is tax payers money that governments or health care providers are investing in disease control. Many countries can not afford influenza vaccines. Per capita spending on health in Vietnam is $3. The wholesale price of influenza vaccine in South America might be $3; perhaps in some countries it is lower. In the United States it's much more than $10, depending on the vaccine.
Global expenditures on influenza vaccine are considerable. If we assume 300 million doses per year at a wholesale price of $7.50, and a 5 percent increase over time, we can expect annual expenditures on influenza vaccine of around $3 billion per year in 2015. So over the next 10 years, public health institutions will spend at least $28.3 billion to buy vaccine to reduce the impact of seasonal influenza. That represents a considerable amount of money. If we could invest only 5 percent of those funds on research or add 5 percent, after 10 years, we would have $1.4 billion to invest in research for a vaccine that could fundamentally change the landscape of
influenza control. Such a vaccine would be cross-subtype specific, could be stockpiled, might not need to be given annually, and would be affordable for developing countries.
In 10 years’ time, many of us may look back and say, yes, we have made a big difference. I know many of you might be raising your eyebrows and shrugging your shoulders, thinking about insurmountable obstacles. But we must try to find solutions to address both pandemic and epidemic dilemmas. I would also remind you that the estimated global investment in the antiviral stockpile is exceeds already by far $2 billion, and 23 countries have orders in. Such spending might not be necessary if we had a vaccine.
Prioritizing research is very important, but that does not equal international coordination of research efforts. That should come next. This meeting is very important in that regard, and will provide us with better insight into priorities for influenza research. Some research projects already exist, initiated by governments, academia, national research and philanthropic institutions, the U.N. Food and Agriculture Organization, and Office International des Epizooties (OIE). The WHO Global Influenza Network, including the WHO Animal Influenza Network, has been supporting operational research and also providing direct support to influenza control efforts. However, we have to think more about coordinating research in addition to identifying research priorities.
WHO is also seriously considering organizing an international meeting to coordinate international support for surveillance and control of avian influenza in Asia. That will be a donor meeting, bringing together everyone who is interested in investing in influenza control in Asia, as well as everyone who has already invested. A meeting to coordinate research and identify the gaps could accompany such an event.
In summary, urgent, short-term research needs to include risk assessment for possible reassortment of H5N1 viruses with other circulating influenza A viruses. Assessing the effectiveness of non-pharmaceutical interventions is becoming increasingly important. We have also overlooked preparing for research during a pandemic. Regarding long-term research, we believe the best bang for the buck will come from investing in a subtype-specific influenza vaccine with long-lasting immunity.
One major priority is rapid exchange of information during operational research, and the time to think about stronger international coordination is now. As an example, last August, WHO received a telephone call from a colleague in this room who said that ducks might play a much greater role in the epidemiology of the disease. That finding has shaped global as well as national intervention policies. Many countries since have embarked on control programs in ducks, as field investigation has confirmed the laboratory results. If this scientific group had held on to that information and we had delayed developing control strategies, many more people would have been infected, and many more would probably have died. We need to create an environment for translating scientific evidence into immediate public health action without negative consequences for scientists.
More research will initially cost money, but we will save lives and money in the end. And fundamental research is the foundation for any applied research. If we do not want to invest now in research, let's put money aside to spend when the disease comes.
THE ROLE OF NIH RESEARCH IN PANDEMIC INFLUENZA PREPAREDNESS
Dr. Anthony S. Fauci, Director, National Institute of Allergy and Infectious Disease, National Institutes of Health
I am going to talk about one component of pandemic influenza preparedness: the role of NIH research endeavors in complementing the activities of CDC, FDA, and other U.S. agencies as well as international collaboration.
As this is the John La Montagne Symposium, I would like to remind us not only of the extraordinary loss we all felt when John passed away on November 2, 2004, but also of his extraordinary impact at both a scientific level and at the level of human interactions throughout the international and national community. As many of you know, John died at the airport in Mexico City, the city of his birth. John loved Mexico and Mexico City. He had returned there for one of the important meetings at which he so well represented not only NIH but the U.S. Government.
I could probably use my entire time to talk about his accomplishments, but I would like to point out some highlights of his career, most importantly in vaccinology. He loved vaccinology. He loved global health, and he played a major role personally and administratively in the development of vaccines for acellular pertussis, in supporting malaria initiatives with international colleagues, and in leading research endeavors in pneumococcus, rotovirus, influenza, and TB.
He was an advisor to virtually every important international organization, and he had a special interest in the Children’s Vaccine Initiative. He was a major player in the U.S.-Japan Program. He was our representative on the Multilateral Initiative on Malaria (MIM), and he enticed Harold Varmus, when he was director of NIH, to become heavily involved as well.
John’s greatest love, as we all know, was influenza research. John had been talking for many years about the dangers not only of seasonal flu but also the potential for a pandemic influenza that we are discussing here. He also focused on emerging infectious diseases and the threat of bioterrorism—all long before they became fashionable.
However, as important as his scientific and administrative accomplishments was the fact that John was one of the finest individuals I have ever met. He was the most self-effacing, highly competent person that I have dealt with in my professional career. When I called then-Secretary of Health and Human Services, Tommy Thompson the morning after I had learned in the middle of the night that John had died, Secretary Thompson said something that all of us feel: that John was a true public health hero whose leadership—especially in the realm of infectious diseases—truly left the world a healthier place. I am very pleased that we have the opportunity to dedicate this symposium to such an extraordinary scientist, science administrator, and human being.
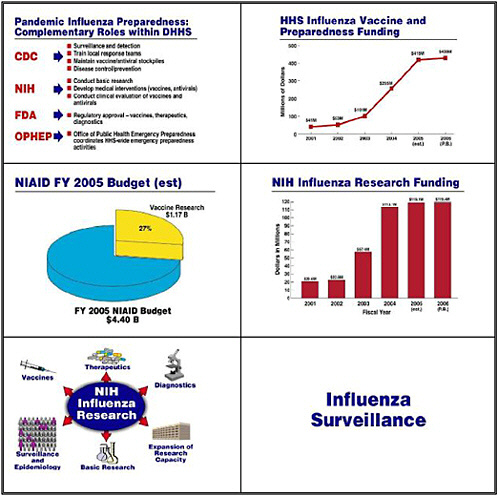
Let me move on now to pandemic influenza preparedness. In the United States, CDC heads a multiagency endeavor that encompasses surveillance, detection, training, maintaining the stockpile, and importantly, disease control and prevention. FDA guides us through the regulatory approval process for vaccines, therapeutics, and diagnostics. NIH is involved not only in basic research but also in developing medical interventions and conducting clinical trials. HHS’s
Office of Public Health Emergency Preparedness, under the leadership of Stewart Simonson, coordinates all of these efforts.
The HHS commitment and allocation of resources to influenza has grown dramatically over the past several years, starting with about $41 million in the 2001 Budget and rising to $430 million in the President’s request for the FY 2006 budget. Of NIAID’s 2005 budget of $4.4 billion, the agency is spending over $1 billion on vaccine research—about 27 percent of its endeavors. Funding for influenza research has similarly risen from about $20 million in 2001 to about $120 million in the President's 2006 budget request.
NIH divides those funds into several components. Although the main player in surveillance and epidemiology is CDC, some of our grantees monitor the molecular evolution of bird and other animal influenza viruses as they evolve into viruses that can infect humans. Our basic research looks at the pathophysiology of viral diseases, in this case influenza. We are building not only physical but also intellectual research capacity by training people in the development of countermeasure: diagnostics, therapeutics, and vaccines.
In the influenza surveillance component, one of our main players is Robert Webster at St. Jude’s Children’s Research Hospital. He pursues the surveillance of animal influenza in Asia, and plays a major role in generating candidates for vaccines against a pandemic influenza strain, as well as studying emerging strains that are infecting swine in Asia. He is playing a key role in generating the H5N1 vaccine reference virus now being used by both Sanofi Pasteur and Chiron. He has also collaborated with others to study the spectrum of the host range of H5N1 influenza virus. One important resulting observation was the fact that ducks can serve as a silent reservoir for H5N1.
Basic research provides the fundamental matrix for developing countermeasures. The NIH portfolio includes a substantial effort to understand the pathogenesis of pandemic influenza viruses, including virulence factors, and the transmissibility of H5N1 among different animal species as well as the molecular evolution that allows for the host range to expand. Our research also includes studies on the mechanisms of animal-to-human transmission and the development and propagation of animal models to study pandemic influenza viruses.
Also important are attempts to understand better the extraordinary pathogenicity and virulence of the 1918 pandemic flu, known as H1N1. Current studies include sequencing the 1918 influenza viral genes, trying to identify signature sequences responsible for the virulence, determining the molecular mechanisms that led to the emergence of this virus, and understanding the contribution of the hemagglutinin and the neuraminidase genes to the unprecedented clinical virulence. Four important papers have probed the pathogenicity, immunogenicity, and virulence of this very important viral strain, as we are obviously concerned about the possibility of a repeat of this phenomenon.
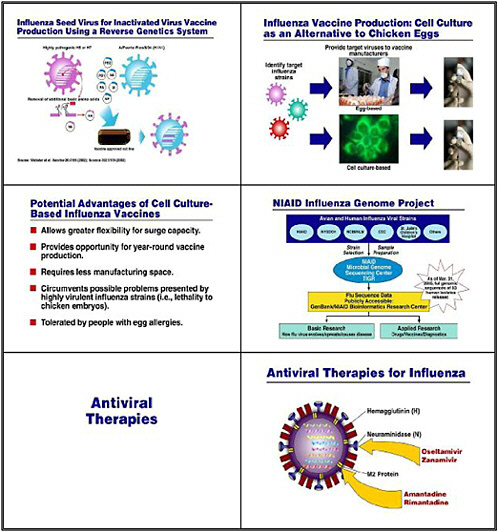
Other basic research and applied research involves the growing use of reverse genetics, whereby we eliminate some of the uncertainty when trying to develop a seed virus for our vaccine. Generally, researchers would put two different viruses together in culture and wait for the inevitable reassortment of genes between them. In this case, we would co-cultivate either an H5 or an H7 influenza virus with a familiar strain such as the Puerto Rico strain, which we use regularly in our egg-based cultures. Reverse genetics, in contrast, deliberately takes the relevant genes from each strain, and actually creates the hybrid virus strain that expresses the H and N from the potentially pandemic virus together with the 6 other influenza genes from the benign
and easy-to-grow strain. This approach has been used to develop the seed virus for the H5N1 vaccine, which has just recently entered clinical trials.
Multiple HHS agencies, including CDC, as well as some of our grantees and pharmaceutical and biotechnology companies are also doing research to develop a sound, consistent, cell-culture–based capability. The goal is to take some of the uncertainty out of egg-based culture and to give us greater surge capacity so we can have the year-round capability to produce vaccine. These cell-culture–based strains are also important because if we confront a virulent influenza strain that might prove lethal to chicken embryos, we can use reverse genetics to splice out the virulence factor. Also, a vaccine that is not grown in eggs is important to individuals who have allergies to egg proteins.
Another important project that NIAID supports is the influenza genome project in collaboration with CDC, the New York State Department of Health, the National Center for Biotechnology Information, St. Jude’s Hospital in Memphis, and others. This project collects the full genomic sequences of a large number of isolates of influenza from humans and, in some cases, animals. The goal is to make these sequences available for basic as well as applied researchers, so that they may be helpful in the development of drugs, vaccines, and diagnostics.
As of a couple of days ago, we have released the sequences of about 93 human isolates—an important research resource for our grantees and collaborators.
What about antiviral therapies? Four separate anti-influenza antivirals are available. Among these, only Oseltamivir (Tamiflu) is adequate for treatment of H5N1 influenza. We are putting about 2.3 million treatment courses of Tamiflu into the national stockpile,
NIAID and NIH research projects are developing and testing long-acting, next-generation neuraminidase inhibitors. We need targeted antiviral efforts like those for other disease such as HIV/AIDS, for which we have made remarkable advances, with more than 20 separate antiviral drugs now available.
Animal studies are examining the effectiveness of combination therapies—a new approach for influenza. Other non-traditional pharmacological approaches include inhaled polyclonal IgG as an immunoprophylactic and the evaluation of new targets—not just the M protein and the neuraminidase, but also viral entry, replication and maturation.
Protocols for the use of oseltamivir in infants are evolving. Oseltamivir is licensed for individuals older than 1 year, but we do not have enough information on people 1–2 years of age or younger to feel totally confident in the use of this drug in these subjects. That is why we are developing protocols to try to identify the optimal use of oseltamivir, particularly in children.
Our main weapon in the armamentarium against influenza is a vaccine. As we have done in developing other countermeasures, particularly in the arena of biodefense, we are collaborating with at least two companies to develop a new vaccine for H5N1.
Last spring we signed contracts with Sanofi Pasteur and Chiron to develop an H5N1 vaccine, as well as with Chiron for an H9N2 vaccine. Progress has been quite good. In fact, for H5N1, we contracted to produce pilot lots of 18,000 doses—10,000 from Chiron and 8,000 from Sanofi Pasteur—for a phase I-II clinical trial. The trial of the Sanofi product, which has accrued about 450 individuals thus far, will examine safety and immunogenicity first in healthy adults, then in the elderly population, and ultimately in the pediatric population.
We are also contracting for 2 million doses of the H5N1 vaccine from Sanofi Pasteur for the strategic national stockpile. We also have an H9N2 vaccine, and one might ask why we would want that. Although it is not now as important as the H5N1 virus in Asia, particularly in Vietnam and Thailand, we need to learn more about the body’s immune response and the safety of the vaccines that are derived avian viruses. The H9N2 has also started its clinical trial. The live attenuated version of both vaccines is very important. Studies on that version are taking place in the NIAID intramural laboratory of Brian Murphy and Kanta Subbarao, but other individuals in the outside community are also looking at this.
We all know from experience with live attenuated vaccines that this approach induces a broader range of serum and local mucosal antibodies. It induces cellular immune responses as well as humeral, and immunity develops rapidly, usually with a single dose. This is important because there is a question of whether we will have to use multiple doses of the killed H5N1 vaccine.
The attenuated vaccine has a wider breadth of cross-reactivity, as do most live attenuated vaccines. This becomes critical when dealing with H5N1. If it mutates to become much more efficient in its transmissibility from human to human, will that mutation affect the ability of the vaccine to protect against it? Not necessarily, but broader cross-reactivity would be very helpful. The other advantage is that such a vaccine does not require needle injection, and it is more effective in infants and children than are inactivated virus vaccines.
Vaccine clinical trials traditionally occur in our vaccine and treatment evaluation units. The three involved in the H5N1 vaccine are located in Los Angeles, Baltimore, and Rochester, New York, but other units will also be involved in the second and third components of the clinical trial.
I want to finish by commenting on the fragility of the vaccine enterprise in general, but particularly the influenza vaccine enterprise, and the role of the research enterprise in addressing this fragility. We all were faced with a rude awakening when we discovered the contamination of half of the U.S. supply of influenza vaccine last year. However, other nations that rely on influenza vaccine have also experienced delays. For example, Australia found that one of the influenza strains in a vaccine was not present in as great a concentration as required, which created somewhat of an analogous situation to the one that we experienced last fall.
How can we strengthen the fragile vaccine enterprise? One approach is by targeting research resources. I have already mentioned several research efforts, such as the use of reverse genetics to add consistency and reproducibility to our ability to obtain a seed virus. We can also gradually transition to cell-based vaccines and the use of recombinant-DNA technologies to express hemagglutinin and neuraminidase and other proteins. Developing a perennial vaccine based on conserved epitopes is particularly problematic with influenza, because humans are continually infected each year, yet we do not seem to have adequate cross-protection against small changes in connection with the yearly antigenic drifts.
We also lack dose-sparing strategies. Toward that end NIH is looking at using intradermal vaccines, and comparing intradermal with intramuscular vaccines. A clinical protocol to compare intramuscular to intradermal approaches in H5N1 vaccination is in preparation. We are also discussing production of an adjuvant-aided H5N1 vaccine with manufacturers, although the trial that has just started is not adjuvant-aided.
Because this is the John La Montagne symposium, I want to mention that one of his last scientific efforts was to co-author with me an invited commentary in the New England Journal of Medicine, which was published on November 3, 2004, the day after his death. He was hired as one of our first influenza program officers, and his last scientific publication was on influenza.
The fragility of the influenza enterprise is real, and we must address it with incentives to industry as well as with basic research that improves the process of vaccine development. Potential incentives are now being discussed at HHS. NIAID is also trying to help industry make products that might supplement our normal supply of influenza vaccine—that is, to obtain the necessary information for as rapid FDA approval as possible. Such efforts are occurring with GlaxoSmithKline’s inter-pandemic Fluarix vaccine. One of the most rapidly accrued trials that we have ever experienced—it enrolled almost 1,000 people in 5 days—began in December 2004. People were interested in getting into the trial given the shortage of influenza vaccine. We are expecting the results shortly.
I want to close by mentioning that when we deal with emerging diseases, we see the extraordinary capability of pathogens to persist, emerge, and reemerge. None does it better than influenza. To address this threat, we must balance public health measures, biomedical research, and technological advances. Staying on top of what could be an extraordinary public health problem is extraordinarily important.
John was co-author of a review of the last such meeting in 1995, published in the Journal of Infectious Diseases, called “Pandemic Influenza: Confronting a Re-emerging Threat.” What he said then holds true today. Success in controlling a pandemic will benefit from new advances by the scientific community, which provides the pool of solutions to combating emerging threats.