5
Expanding the Population of Potential Donors
Much of this report has focused on increasing the rates of donation by the small and relatively static population of deceased donors for whom there has been neurologic determination of death. Of the more than 2 million deaths that occur each year in the United States, it is estimated that there are only 10,500 to 16,800 eligible donors with neurologic determination of death (Guadagnoli et al., 2003; Sheehy et al., 2003). This chapter focuses on expanding the opportunities for donation to the much larger segment of U.S. deaths, those with circulatory determination of death. Because most Americans support organ donation and many have designated their consent for donation by signing their driver’s license or a donor card or by joining a donor registry, it is important to identify ways in which these individuals can have the opportunity to be organ donors after they die.
This chapter builds on the work of two previous Institute of Medicine (IOM) reports (IOM, 1997, 2000) that emphasized the importance of developing the nation’s capabilities for donation after circulatory determination of death (DCDD). It is not possible for this chapter to provide in-depth coverage of the many issues that must be addressed on this topic; however, the committee urges that its recommendations be actively pursued, as there is an opportunity to significantly expand the number of organ donors and thereby provide improved health and lifelong benefits for transplant recipients.
The chapter begins with an overview of some of the key terminology that is involved and presents a brief synopsis of the clinical issues that present both challenges and opportunities for DCDD. Since DCDD has been a topic of considerable discussion, the chapter presents the highlights
of recent conferences and major reports. It then examines general ethical considerations, before focusing on the ethical issues specific to controlled and to uncontrolled DCDD. Because uncontrolled DCDD has not been fully explored, the chapter provides an estimate of the potential number of donors as well as outlining major challenges in moving forward in this area. An additional group of potential donors are those with age or medical characteristics outside of the standard criteria for organ donation. Issues relevant to expanded criteria donors are discussed. The chapter concludes with the committee’s recommendations on DCDD and expanded criteria donation.
BACKGROUND AND ISSUES
Determination of Death
The fundamental tenet of organ donation is the dead donor rule, that is, that organ donation should not cause or hasten death (Robertson, 1999). As discussed in Chapter 1, the advent of external ventilation technologies and other technological advances led to the development of criteria for neurologic determination of death (based on the irreversible loss of function of the whole brain, including the brain stem) and to clarification of criteria for circulatory determination of death (irreversible cessation of circulatory function) (Report of the Ad Hoc Committee, 1968; President’s Commission, 1981).
However, the terminology that has arisen, specifically, the terms brain death and cardiac death, has often been misunderstood and thought to imply only the death of an organ—either the brain or the heart—and not the death of the human being. The committee believes that it is particularly important to clarify the terms that are used so that it is fully understood that death can be declared or determined by a physician either by the use of neurologic criteria or by the use of circulatory criteria.
When it is necessary to distinguish between the ways in which death is determined, the committee suggests using the terms neurologic determination of death (NDD) or circulatory determination of death (CDD). In either case, organ donation occurs only after death. Because different kinds of protocols and procedures are used to recover organs from individuals who have died according to these two criteria, it is useful to distinguish between donation in these different contexts. The committee recommends a change in terminology to donation after neurologic determination of death (DNDD) and donation after circulatory determination of death (DCDD).1 The com-
mittee believes that current misperceptions justify the shift to a more detailed terminology to clarify important concepts that are particularly relevant to organ donation and transplantation. The change in terminology emphasizes that the starting point for organ donation is death—death that is determined by using one of two sets of criteria. For the most part, it is not necessary to qualify the way in which the determination of death is made, but when the distinction is made, it must be clearly stated.
Defining Controlled and Uncontrolled Death
The circumstances of death because of cardiac arrest or other causes leading to circulatory determination of death vary widely. Many cardiac arrests occur in the home and at other sites outside of the hospital setting. As a result, a number of factors (such as the duration of time before emergency care is provided and the availability of healthcare professionals and medical equipment) can affect the viability of the organs and the potential that they can be transplanted. In some other circumstances of death, the patient is on ventilatory support and the discussion of donation occurs only after an independent decision to withdraw supportive technologies.
In 1995, a categorization schema was developed to address the various circumstances of circulatory determination of death. The Maastricht categories (Box 5-1) outline the circumstances of controlled (or expected) death (Maastricht Category III), in which death is anticipated and occurs after medical supportive therapy is withdrawn, usually in an operating room or hospital intensive care unit, and within a closely monitored time frame. Unexpected or uncontrolled death (Maastricht Categories I, II, and IV) occurs in circumstances in which cardiopulmonary function ceases spontaneously, often with death occurring in an unanticipated fashion, frequently outside the hospital setting or in a situation in which less is known about the viability of the organs; these deaths often involve the loss of circulatory function before the neurologic determination of death (Kootstra et al., 2002;
|
BOX 5-1 Maastricht Categories (as described in Kootstra et al., 1995, p. 2893)
|
Doig and Rocker, 2003). For Maastricht Categories I, II, and IV, there is a greater likelihood of a long interval between the cessation of circulatory function and the opportunity for the initiation of organ preservation or removal.
The terms controlled and uncontrolled are subject to misinterpretation. Donation after either controlled or uncontrolled circumstances of death occurs only after a series of carefully delineated procedures and medical protocols have been followed. Controlled (or expected) circumstances of death typically occur after the family or surrogate has made the decision to withdraw support measures. In uncontrolled (unexpected) circumstances, death occurs after emergency medical procedures and resuscitation measures have been exhausted and are no longer productive. The committee is concerned that the term uncontrolled has been misunderstood on some occasions to imply a lack of procedure or protocols. Other terms, such as unexpected, unanticipated, or unplanned, may be better descriptors of the circumstances of death.
Organ donation in cases of expected or controlled death can differ from donation in cases of unanticipated or uncontrolled death in several ways: in the time to initiation of the preservation of the organs to be transplanted, in the psychological preparation of the family, and in the ascertainment by the healthcare team of the potential for organ donation. In controlled-death situations, the family can decide when and where life-sustaining measures will be discontinued (Edwards et al., 1999). In situations involving unanticipated or uncontrolled deaths, there may be many more unknowns; healthcare professionals may arrive after the patient has collapsed and the length of time with hypotension, cardiovascular shock, or cardiac dysrhythmia may be unknown. In general, the kidneys, lungs, liver, and pancreas can be recovered after circumstances of controlled death because the organs can continue to receive oxygenated blood until nearly the time of recovery. Kidneys and lungs are the primary organs recovered after death in uncontrolled situations, since their viability is maintained for longer periods of time (Egan, 2004). Because individuals needing kidney transplants comprise more than 70 percent of the individuals on the transplant waiting list as of March 2006 (OPTN, 2006), it is important to explore the potential of DCDD in both controlled and uncontrolled circumstances as a means to achieve increased donation rates.
Controlled DCDD2 is the area that has been the most developed by U.S. programs; uncontrolled DCDD has been utilized primarily in Europe and in
a few pilot programs in the United States (see below). It is important to note that in the early days of transplantation, DCDD protocols were frequently used, and in some organ procurement organizations (OPOs), it remained a common practice into the 1980s. In recent years, organ recovery from DCDD has again become more commonplace.
General Considerations
As with many aspects of organ transplantation (Chapter 6), there have been misperceptions about DCDD. In particular, there have been concerns that death is hastened or that the patient’s best chance for survival is compromised in some other way by measures that are used to preserve the viability of the organs for transplantation. Therefore, it is important to clarify the practical aspects of this process. Although it is uncomfortable to discuss the issues around death and, particularly, those that deal with the body after death, openness and clarity about the events that occur in the process of DCDD are very important. Furthermore, healthcare and transplantation professionals must ensure that all aspects of the planning and development of DCDD protocols are transparent and open to a wide range of patient and stakeholder inputs.
Successful transplantation of an organ from a deceased donor requires that the organ be viable, a goal that can be met by minimizing the ischemic injury caused by a lack of bloodflow carrying oxygen and other nutrients to the organ(s). The length of time in which the organ can be deprived of oxygen (ischemic) and still be successfully transplanted varies among types of organs. Thus, measures to ensure organ viability for transplantation (and decrease ischemia) must be initiated as rapidly as possible after the patient’s death.
In cases of neurologic determination of death, after death is declared—and when there is consent for organ donation—continuous cardiopulmonary function is achieved with artificial assistance until the organs are removed, permitting high-quality circulation of oxygenated blood to maintain organ viability. For individuals who have had cardiac arrest, advanced cardiac life support protocols are followed (Box 5-2) and may be continued as the patient is transported to the hospital. In some cases, all resuscitation measures are administered in the home or other field setting; and if they are found to be nonproductive in regaining heart function, death can be declared in the field by emergency medical personnel, in consultation with a physician. In cases of circulatory determination of death, individuals are generally not on ventilatory support at the time of death, so there is an immediate need to proceed with organ-preserving measures to limit the damage from warm ischemia and improve the viability of the organs for transplantation (Bos, 2005).
|
BOX 5-2 Common Criteria for Termination of Advanced Cardiac Life-Support Efforts Termination of advanced cardiac life-support efforts occurs if there is no response after the following interventions have been performed:
SOURCE: Kern et al. (2001). |
For cases of controlled DCDD, measures for preservation and recovery may include the administration of medications to improve organ viability, including heparin, or the use of intravenous cannulation or cardiopulmonary assist devices (bypass procedures). After organ removal, pulsatile perfusion may be used for the kidneys to help determine if the patient’s organs will be eligible for transplantation. For cases of cardiac arrest in uncontrolled circumstances, there is not time to plan in advance to maintain organ viability, but certain organ preservation measures can be implemented after death is declared.
The outcomes of controlled DCDD transplantation have been found to be similar to those for DNDD transplantation for most organs. The University of Wisconsin has performed DCDD transplantations since 1974, with DCDD donors making up 10 to 15 percent of the total donations annually (Lewis et al., 2003). Researchers at that university reported on 568 organs transplanted from DCDD donors between 1984 and 2003 and found that the patient and graft survival rates for patients receiving kidney, pancreas, and lung transplants were similar for organs from DCDD and DNDD donors (D’Alessandro et al., 2004). Delayed graft function has been found to be higher in kidneys transplanted from DCDD donors, but long-term graft survival is similar to DNDD organs (Weber et al., 2002; Droupy et al., 2003; Cooper et al., 2004; D’Alessandro et al., 2004). Researchers have found that the liver is more susceptible to warm ischemic injury (Abt et al., 2004; Foley et al., 2005).
Much remains to be learned about methods to improve organ viability and to preserve organs recovered through both DCDD and DNDD. Re-
search efforts in these areas continue (see, for example, Magliocca et al., 2005) and need to be bolstered.
Prior Reports and Recommendations
IOM has released two previous reports on DCDD, formerly known as non-heart-beating organ donation (NHBD) and donation after cardiac death. The first report, Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement, undertaken at the request of the U.S. Department of Health and Human Services and published in 1997, noted that organ donations from living donors and DNDD donors would not bridge the widening gap between organ supply and demand in the United States (IOM, 1997). Despite the clear need to increase the rates of organ donation, the report found that the majority of transplantation programs in the United States did not have a mechanism or protocol for organ recovery after DCDD. The small number of programs with active involvement in DCDD concentrated virtually all of their efforts on controlled DCDD (Cooper et al., 2004).
Although implementation of uncontrolled DCDD protocols and programs is recognized as an opportunity to greatly increase the number of potential organ donors (especially kidney donors), the need to limit the warm ischemia time by means of early postmortem cannulation and cooling has raised the fundamental ethical issue of initiating organ preservation before informed consent is obtained from family members. At the time that the IOM report was published, enabling legislation existed in several other Western democratic countries and in three jurisdictions in the United States (Washington, D.C., Virginia, and Florida) that allowed this practice while the next of kin were being sought for permission for organ recovery (IOM, 2000).
Strong opposition to this position was taken by the American Society of Transplant Physicians (ASTP), who articulated the following reservation: “Uncontrolled NHBDs do not meet the principles of the ASTP, particularly in the area of consent, and therefore, the ASTP does not support the widespread use of this organ source” (IOM, 1997, p. 80). Perhaps in consideration of this testimony, which was concordant with several surveys of public sentiment conducted at the time, the IOM committee concluded: “Cannulation and cooling without consent may be a situation in which a decision based on deference to what the public is prepared to accept may be the wisest policy at the moment” (IOM, 1997, p. 55).
Despite this constraint placed on uncontrolled DCDD, the IOM committee concluded that “the recovery of organs from NHBDs is an important, medically effective, and ethically acceptable approach to reducing the gap that exists now and will exist in the future between the demand for and
the available supply of organs for transplantation” (IOM, 1997, p. 1). That report’s recommendations focused on controlled DCDD and suggested that attention be given to greater consistency in DCDD policies and better support for patients and their families, sustaining the integrity of organ procurement efforts, and maintaining public confidence in the organ transplantation system (Box 5-3) (IOM, 1997).
Following publication of the 1997 IOM report, the U.S. Department of Health and Human Services again sought IOM’s assistance in facilitating the adoption of protocols for DCDD, consistent with prior IOM recommendations, by all OPOs in the United States. This resulted in the publication in 2000 of Non-Heart-Beating Organ Transplantation: Practice and Protocols (IOM, 2000) (Box 5-4). The report’s recommendations were congruent with the transplant community’s long-standing orientation toward obtaining organs from DNDD donors in a planned, orderly, and controlled fashion. Subsequently, the addition of DCDD donors to the potential donor pool naturally evolved in a parallel direction that favored an emphasis on controlled DCDD. Many of the same challenges discussed in these two reports still confront the widespread adoption and implementation of DCDD. The present report further expands on those recommendations and emphasizes the need for ongoing efforts to ensure that DCDD is a priority for hospitals and OPOs.
A national conference in 2005 examined the essential actions needed to expand the practice of DCDD in the continuum of quality end-of-life care
|
BOX 5-3 Recommendations of Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement
SOURCE: IOM (1997). |
|
BOX 5-4 Recommendations of Non-Heart-Beating Organ Transplantation: Practice and Protocols Recommendation 1: All OPOs should explore the option of non-heart-beating organ transplantation, in cooperation with local hospitals, healthcare professionals, and communities. A protocol must be in place in order for non-heart-beating organ and tissue donation to proceed. Recommendation 2: The decision to withdraw life-sustaining treatment should be made independently of and prior to any staff-initiated discussion of organ and tissue donation. Recommendation 3: As recommended in the 1997 IOM report, statistically valid observational studies of patients after the cessation of cardiopulmonary function need to be undertaken by appropriate experts. Recommendation 4: Like all care at the end of life, non-heart-beating organ and tissue donation should focus on the patient and the family. Recommendation 5: Efforts to develop voluntary consensus on non-heart-beating donation practices and protocols should be continued. Recommendation 6: Adequate resources must be provided to sustain non-heart-beating organ and tissue donation. Adequate resources are required to cover (1) the costs of outreach, education and support for OPOs, providers, and the public, and (2) any increased costs associated with non-heart-beating organ and tissue recovery. Recommendation 7: Data collection and research should be undertaken to evaluate the impact of non-heart-beating donation on families, care providers, and the public. SOURCE: IOM (2000). |
and identified a number of ways to increase opportunities for increasing the opportunities for DCDD (Bernat et al., 2006). As discussed at that conference, there is an ongoing need to ensure that all OPOs and hospitals have policies, organizational structures, and institutional support for DCDD and that there is adequate professional education on organ donation under these circumstances.
Furthermore, all DCDD-related efforts must have a strong component of public education, and include measures to strengthen and sustain trust in the medical system. Clear messages and transparent actions are essential for promoting DCDD as an organ donation option. Any episode of inadequate or inappropriate information being conveyed to the public can negatively
|
BOX 5-5 National Conference on Donation After Cardiac Death (DCD): Specific Actions Proposed for Agencies and Organizations Association of Organ Procurement Organizations (AOPO)
U.S. DHHS Advisory Committee on Organ Transplantation
OPTN-United Network for Organ Sharing (UNOS)
|
affect public perception and damage the understanding and trust of the healthcare system regarding organ donation (Chapter 7).
The committee concurs with the actions recommended in earlier IOM reports (Boxes 5-3 and 5-4) and at the National Conference on Donation after Cardiac Death (Box 5-5) and encourages further efforts to overcome the general inertia regarding the full implementation of the policies and practices that will facilitate and expand DCDD. It will be important to focus on the systems changes at the hospital and the OPO levels and will also require that the public and healthcare professionals be engaged in discussions about the complexities of DCDD.
Learning from Past Experience and International Models
Several European countries have developed and have used the infrastructures of both the emergency medical services and the transplantation services to provide optimal emergency care and to be available as needed to
NATCO (The Organization for Transplant Professionals)
American Society of Transplant Surgeons and American Society of Transplantation
Joint Commission on Accreditation of Healthcare Organizations
Centers for Medicare & Medicaid Services
SOURCE: Bernat et al. (2006). |
respond to demands for DCDD (Alvarez et al., 2002; Sanchez-Fructuoso et al., 2003; del Rio Gallegos et al., 2004). Furthermore, in the early 1990s efforts were made in Washington, D.C., to develop a program for uncontrolled DCDD.
Washington, D.C.
In the early 1990s, the Washington Hospital Center in Washington, D.C., developed a rapid organ recovery program that focused on DCDD, particularly organ donation from victims of fatal trauma (Kowalski et al., 1996; Light et al., 1996). The emphasis on DCDD donors (termed “non-heart-beating donors” at the time) included a strong component of community education and input (WHC, 1993). A Community Oversight Committee was formed to provide input into the development of the DCDD protocol (Appendix F) and its implementation. Furthermore, the Office of Decedent
Affairs was established and provided continuous, 24-hour-a-day support to donor families and provided the link between the District of Columbia government (particularly, the medical examiner’s office), the hospital medical staff, and the families. The office was staffed by family advocates with experience in counseling and crisis management and trained in organ and tissue donation.
The medical criteria established for donors for this program were as follows: the donor had to be 18 to 55 years of age, the time of asystole had to be known, asystole had to be for less than 30 minutes, the donor could have no infection or cancer, the donor had to have negative test results for human immunodeficiency virus and hepatitis B virus, and the donor had to have a low-risk medical history (Kowalski et al., 1996). The program used two procedures to preserve the organs: cannulation of the femoral arterial-venous system to perfuse the kidneys with preservative solution combined with iced peritoneal lavage for cooling (Kowalski et al., 1996). Forty-five minutes was the maximum length of time allowed between cardiac arrest and the beginning of in situ organ preservation. The number of solid-organ donors in the hospital increased from 9 in 1993–1994 to 15 in 1994–1995, with 60 percent of the donors in 1995 coming from the rapid recovery program. Ninety-one deaths met the criteria for the program but the family could not be reached within the 45-minute window needed to begin solid organ preservation. Families of 29 of the 91 decedents were located within 4 hours and 10 of those families consented to tissue donation.
The District of Columbia government amended its Anatomical Gift Act to permit the initiation of organ preservation methods after death and pending consent for donation. If the family could not be reached, the preservation methods were discontinued.
The Washington Hospital Center’s program was successful in increasing organ donation rates and in galvanizing community support through public education and extensive opportunities for public input. The program illustrates the potential for communities to come together to support organ donation efforts; the challenges in financing this program, however, led to its discontinuation and point out the necessity of sustained funding sources for such efforts to be fully developed and implemented. Furthermore, there needs to be a broader appreciation of the impact that DCDD could have in terms of the numbers of lives that could be saved as well as the healthcare cost savings that could be achieved by reducing the numbers of people receiving dialysis.
Europe
Several European countries have explored and implemented DCDD programs focusing on patients with unanticipated cardiac arrests. The Hos-
pital Clinico San Carlos in Madrid, Spain, has developed extensive policies and criteria regarding uncontrolled DCDD (Box 5-6). Cardiac arrest is considered irreversible after a minimum 30-minute resuscitation period without a return of the circulation (Sanchez-Fructuoso et al., 2003). The criteria used to identify potential organ donors include no evidence of drug dependency or death by physical violence, and the onset of external cardiac massage and mechanical ventilation within 15 minutes of cardiac arrest. This onset of care within 15 minutes means that acceptable potential do-
|
BOX 5-6 Modified Madrid Criteria Criteria used to assess eligibility for kidney and other organ preservation among individuals suffering out-of-hospital sudden cardiac-related death:
SOURCES: Gomez et al. (1993); Alvarez et al. (1997, 2000, 2002). |
nors include almost all individuals with in-hospital uncontrolled cardiac-related deaths and most individuals with out-of-hospital uncontrolled cardiac-related deaths.
Deceased potential donors are transported by the emergency medical services personnel to the hospital. The transplantation laws in Spain allow perfusion of the organs through cardiopulmonary bypass while the family is being located and a determination regarding donation is being made (Alvarez et al., 2002). Unique features that facilitate uncontrolled DCDD in Madrid include the use of intensive care ambulances that are staffed by a prehospital physician and nurse who deliver patient care in the field (Alvarez et al., 2002).
Examination of the 1996–1999 data from the Hospital Clinico San Carlos reveals that among 111 potential uncontrolled DCDD donors, 62 patients met the criteria for donation and 53 became actual donors (9 were lost because of an inability to obtain either family or judicial consent for donation after cold perfusion had been instituted) (Alvarez et al., 2000). From these 53 consenting donors, 72 kidneys were transplanted, 80 percent of which had the expected delayed function characteristic of DCDD grafts but had long-term graft survival rates comparable to those of kidneys from DNDD donors (Alvarez et al., 2000). It is important to note that about 40 percent of the 53 donors died of trauma. When the 36-month cumulative probability of graft survival was evaluated as a subset analysis of the same patients in a separate paper, not only were the DCDDs comparable to the DNDDs, but also both within and across these two donor groups, long-term graft functioning was similar whether death was a primary cardiac event or secondary to trauma (Sanchez-Fructuoso et al., 2000).
DCDD efforts in multiple countries (Table 5-1) show the potential for increasing the number of organ transplants, particularly kidney transplants.
TABLE 5-1 Kidneys Obtained as a Result of DCDD
|
Country |
Year |
Number of DCDD Donors |
Number of DCDD Kidneys Transplanted |
Percentage of All Kidneys Transplanted |
|
The Netherlands |
2003 |
87 |
158 |
39 |
|
Spain |
2003 |
56 |
80 |
4 |
|
United Kingdom |
2003 |
66 |
112 |
9 |
|
United States |
2003 |
264 |
501 |
4 |
|
Japan |
1995–2003 |
867 |
1,279 |
25 |
|
SOURCE: Adapted from Bos (2005). |
||||
ASSESSMENT OF DCDD STRATEGIES IN THE UNITED STATES
Data from more than a decade of assessments are now available to evaluate the effectiveness of augmenting the donor pool by increasing the number of controlled DCDD donors. Between 1994 and 2004 there was a marked proportionate increase in DCDD donors (Table 5-2). However, when viewed in absolute terms, which are the numbers that matter to patients awaiting organs, there were just fewer than 400 DCDD donors in 2004, up from about 60 in 1994. Only 5 percent of all deceased donors in 2004 were DCDD donors (Table 5-2). As might be anticipated, among this relatively small number of DCDD donors, only about 6 percent were the result of uncontrolled DCDD, and most of these were Maastricht Category IV donors (Table 5-2). Data from 2004 show that 1,038 organs were recovered from DCDD donors (Table 5-3), a small fraction of the 26,539 organ transplants in 2004 (HRSA and SRTR, 2006).
In 2004, only 21 of the 59 OPOs in the United States completed five or more DCDD cases. Eighteen OPOs did not have a DCDD case that year (Table 5-4). The potential for DCDD can be seen in the fact that in 2004 only seven donation service areas accounted for 58 percent of all cases of DCDD (HRSA and SRTR, 2006).
Efforts to increase the number of OPOs and transplant centers that are actively engaged in DCDD are ongoing. The Organ Donation Breakthrough Collaboratives (Chapter 4) are working with transplant hospitals and OPOs to increase the number of hospitals with DCDD policies and capabilities.
Because of the large and growing gap between the number of organs available for transplantation and the number of individuals on the transplant waiting list in the United States (Chapter 2), it is important to explore any scientifically credible and ethically acceptable proposal that might increase the organ supply. This may, of necessity, require a reexamination of the sources of organs and strategies for their acquisition that were rejected in the past at a time when the crisis was less acute.
To assess progress in DCDD, it is important for the conversion rate measures to include both DCDD and DNDD cases in the denominator of eligible donors. Currently, the donation rate (also termed the conversion rate) is calculated as the number of actual donors (i.e., the organs are removed for transplantation) per the total number of donor-eligible individuals, with donor eligibility defined as “any heartbeating individuals meeting, or imminently meeting, the criteria for neurological death (brain death), age 70 years or under, who have not been diagnosed with exclusionary medical conditions published by the Health Resources and Services Administration” (HRSA and SRTR, 2005, p. X-2). This focus on DNDD hinders the assessment of DCDD efforts and does not place a priority on DCDD.
TABLE 5-2 Deceased Donors, 1994 to 2004
|
|
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
|
|
Number of Donors |
||||||||||
|
Controlled DCDD |
11 |
46 |
49 |
60 |
48 |
64 |
100 |
151 |
154 |
236 |
366 |
|
Uncontrolled DCDD |
3 |
13 |
18 |
13 |
23 |
4 |
10 |
11 |
22 |
17 |
22 |
|
Unknown |
43 |
5 |
4 |
5 |
4 |
19 |
8 |
7 |
13 |
17 |
3 |
|
Total DCDD |
57 |
64 |
71 |
78 |
75 |
87 |
118 |
169 |
189 |
270 |
391 |
|
DNDD |
4,017 |
5,282 |
5,282 |
5,354 |
5,666 |
5,700 |
5,848 |
5,905 |
5,994 |
6,178 |
6,751 |
|
Unknown |
1,025 |
16 |
63 |
46 |
52 |
37 |
19 |
6 |
7 |
9 |
8 |
|
Total deceased donors |
5,099 |
5,362 |
5,416 |
5,478 |
5,793 |
5,824 |
5,985 |
6,080 |
6,190 |
6,457 |
7,150 |
|
DCDD as percentageof totala |
1.1 |
1.2 |
1.3 |
1.4 |
1.3 |
1.5 |
2.0 |
2.8 |
3.1 |
4.2 |
5.5 |
|
NOTE: OPTN data indicate that most of the uncontrolled DCDDs were Maastricht Category IV deaths. aDCDD donors as a percentage of total deceased donors. SOURCE: Based on OPTN data as of September 8, 2005. |
|||||||||||
TABLE 5-3 Organs Recovered from DCDD Donors in the United States, 2004
|
Cause of Death |
Number of Organs Recovered |
|||
|
Kidney |
Liver |
Lung |
Pancreas |
|
|
Controlled DCDD |
689 |
233 |
10 |
47 |
|
Uncontrolled DCDD |
42 |
8 |
0 |
1 |
|
Unknown |
6 |
1 |
0 |
1 |
|
Total DCDD |
737 |
242 |
10 |
49 |
|
SOURCE: Based on OPTN data as of September 8, 2005. |
||||
TABLE 5-4 DCDD Cases Reported by OPOs, 1994 to 2004
|
Number of DCDD Cases |
Number of OPOs Reporting |
||||||||||
|
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
|
|
0 |
45 |
44 |
45 |
45 |
47 |
42 |
31 |
26 |
29 |
24 |
18 |
|
1 to 4 |
17 |
17 |
16 |
13 |
11 |
14 |
22 |
23 |
16 |
18 |
20 |
|
5 to 10 |
5 |
5 |
4 |
5 |
4 |
5 |
5 |
4 |
7 |
9 |
10 |
|
>10 |
0 |
0 |
1 |
1 |
1 |
1 |
3 |
6 |
7 |
8 |
11 |
|
Number of OPOs |
67 |
66 |
66 |
64 |
63 |
62 |
61 |
59 |
59 |
59 |
59 |
|
SOURCE: Based on OPTN data as of September 8, 2005. |
|||||||||||
GENERAL ETHICAL CONSIDERATIONS
When the IOM committees examined DCDD in both 1997 and 2000, they acknowledged that DCDD raises numerous potential ethical issues, and they presented both general ethical principles and specific rules to guide DCDD. The present IOM committee concurs with the general ethical principles established in the 1997 IOM report, and these have subsequently been embraced by both U.S. and Canadian multistakeholder consensus conferences on DCDD (Canadian Council, 2005; Bernat et al., 2006):
-
Organ donors must be dead at the time of organ removal.
-
Active euthanasia is absolutely prohibited.
-
There is complete openness about policies and protocols.
-
There is a commitment to informed consent.
-
The donor’s and the family’s wishes are respected.
-
Enhancing rates of organ donation is of value to society.
The present IOM committee also endorses many of the specific ethical recommendations that these bodies have made:
-
DCDD protocols must be written, locally approved, and publicly accessible.
-
Informed consent for all premortem interventions (such as cannulation or heparinization) must be undertaken for the purposes of organ donation.
-
Safeguards against conflicts of interest must be taken, including the use of separate times and separate personnel for important decisions.
-
Determination of death may be made only after circulation has permanently been lost.3
-
Family wishes to be present at the time mechanical supports are withdrawn should be honored, and families should not incur expenses related to donation.
Nevertheless, despite the identification of general ethical principles and some specific ethical rules to guide the practice, DCDD has remained controversial in many circles and has not achieved its full potential, in part because of the controversy surrounding the subject (Bernat et al., 2006). Although the present IOM committee acknowledges that society will never enjoy complete consensus on ethical matters and that some individuals and families will choose not to donate, it is also convinced that some obstacles to DCDD are primarily due to the inadequate education of families, communities, and healthcare professionals. Therefore, although a complete analysis of the ethical issues surrounding DCDD is beyond the scope of this report, this committee believes that it is worth examining why three IOM committees and at least two international consensus conferences have all concluded that both controlled and uncontrolled DCDD can proceed in an ethical manner yet so little has changed in clinical practice.
Controlled DCDD in the United States: From Ethical Controversy to an Emerging Consensus
In the United States, transplant centers have almost exclusively focused on developing protocols for controlled DCDD. This section seeks to develop two main theses regarding controlled DCDD:
-
Although DCDD has been controversial in some circles, a consensus is emerging that controlled DCDD can proceed in accordance with widely shared ethical commitments.
-
Controlled DCDD has been successful and should be pursued by all OPOs to the fullest extent possible. To the extent that a fear of controversy presents an obstacle to DCDD, OPOs should engage in hospital and community education.
Anecdotally, DCDD is frequently more controversial among healthcare workers than it is among the general public, a situation quite the reverse of that for the recovery of organs from DNDD donors. This section explores points of controversy surrounding DCDD and presents the standards of practice and ethical resolutions that are emerging in the United States.
Controversy over Irreversibility
The Uniform Determination of Death Act (UDDA) states that “An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain, including the brain stem, is dead. A determination of death must be made in accordance with accepted medical standards” (President’s Commission, 1981, p. 73).
Whether death is pronounced by the use of circulatory or neurologic criteria, irreversibility is part of the legal definition of death. At the same time, under normal circumstances, organs quickly deteriorate when circulatory or neurologic functions are lost. Therefore, to enable the transplantation of organs, it is necessary to declare death as soon as possible.
In 1997, an IOM committee recommended that death be pronounced at least 5 minutes after the cessation of cardiopulmonary function. Some considered this waiting time inadequate because they believe that circulatory functions are not yet irreversibly lost and that with aggressive resuscitative efforts, some level of functioning might be restored (Cole, 1993; Menikoff, 1998).
Both in 1997 and in 2000, IOM committees presented a response to this concern that has subsequently been embraced by multistakeholder consensus conferences and several ethicists (DeVita, 2001; DuBois, 2002; Canadian Council, 2005; Bernat et al., 2006). This response comprises two key points. First, the best available data and expert judgment indicate that
individuals do not spontaneously resume circulation once it has been lost for 2 minutes (IOM, 2000; DeVita, 2001; Wijdicks and Diringer, 2004). Second, all controlled DCDD donors have “do not resuscitate” orders in place; organ recovery occurs only when an independent decision is made to discontinue all life-sustaining treatments. For these reasons, the National Conference and the Society of Critical Care Medicine have recommended that “at least 2 minutes of observation is required, and more than 5 minutes is not recommended” (SCCM, 2001; Bernat et al., 2006, p. 282).
Although this reliance on “permanent” loss of function may seem like a shift from a strict notion of “irreversibility,” it is a reasonable interpretation of the concept of “irreversibility” and is compatible with the probable intentions of the Commission that formulated the UDDA definition (President’s Commission, 1981, p. 76; Cole, 1993; DeVita, 2001) and with the UDDA’s reference to “accepted medical standards,” which may evolve, particularly in the light of expert consensus. The committee urges that further observational studies be conducted on spontaneous resumption of circulation.
Controversy over Brain Function
Although the concept of neurologic determination of death is foreign to many laypeople, medical and nursing students are taught that the brain supports consciousness, the respiratory effort, and the integrated functioning of the organism and that when the brain ceases to function the organism is dead. Therefore, it is not surprising that some medical personnel have objected to the use of circulatory criteria to pronounce death when the permanent loss of neurologic function is in question (Lynn, 1993; Menikoff, 1998).
However, here, too, a consensus on what is legally and ethically permissible is emerging. First, UDDA clearly allows the use of circulatory-respiratory criteria to determine death. Second, by requiring a 2- to 5-minute waiting period, DCDD actually exceeds requirements of ordinary medical practice, in which there is no fixed observation period from the time that circulation arrests (DeVita, 2001). Third, once circulation is permanently lost, so, too, is neurologic function permanently lost. Consciousness is lost and brain function ceases approximately 15 seconds after circulation to the brain ceases. If the circulation does not resume, neither will neurologic functions resume.
Controversy over Premortem Interventions
As noted above, heparin is an anticoagulant that is frequently administered to potential donors shortly before mechanical support is removed. The purpose is to prevent blood from clotting in the organs that will be transplanted; the omission of heparin could negatively affect organ recov-
ery and hinder the distribution of the recovered organs (Bernat et al., 2006). The use of heparin has been controversial on the basis of theoretical concerns that it could contribute to active cerebral bleeding and thereby hasten death. However, there is no evidence that heparin in fact has such an effect. Although it can successfully prevent clots from occurring, it is unlikely to dissolve clots or exacerbate active bleeding, particularly in a patient expected to die within minutes of the withdrawal of mechanical supports. Moreover, the effects of heparin can be reversed in the rare patient who might recover cardiac function within the permitted window of time allowed by DCDD protocols.
Some have further objected that medications that are not meant to benefit the donors themselves should not be administered. However, it is important to note that consent is obtained for the use of premortem medications to facilitate donation and that the entire procedure of organ donation is meant to benefit someone other than the donor.
End-of-Life Care
Careful attention to ensuring quality end-of-life care is paramount (see Chapter 4). The option to donate organs by using DCDD is a specialized form of end-of-life care. Although some previously used DCDD protocols prohibited the administration of medications that might be viewed as suppressing the respiratory effort and thereby hastening death, current practice places a priority on ensuring that standard methods for ensuring the patient’s comfort (such as the administration of morphine or other comfort measures prior to the withdrawal of ventilation) are used in the context of DCDD; patients should not receive substandard comfort care because of their decision to become donors (DeVita, 1996; IOM, 2000). These practices are consistent with national standards for end-of-life care and offer reassurance that care is not oriented toward hastening death but, rather, is oriented toward honoring the patient’s choices and providing comfort (SCCM, 2001; National Consensus Project, 2004). Professional education of nurses, physicians, and other healthcare providers should emphasize the central role of providing quality end-of-life care, regardless of a decision to donate organs.
Withdrawal of Treatment
The medical and ethical literature on controlled DCDD rarely discusses the fact that donation follows the withdrawal of mechanical ventilatory support. Yet, surveys indicate that among the general public the acceptability of DCDD is directly tied to the acceptability of withdrawing artificial ventilation (Keenan et al., 2002; DuBois and Schmidt, 2003).
The law clearly permits the withdrawal of life-sustaining treatments.
The U.S. Supreme Court has embraced widely accepted distinctions between foreseeing and intending death and between causing and permitting death (Vacco v. Quill). It is common practice to withhold or withdraw treatments that are considered unwanted, medically ineffective, or overly burdensome.
Confusion over the boundaries of ethically permissible withdrawal of life-supporting treatment in conjunction with DCDD is evident in the literature about DCDD. For example, articles on DCDD have mentioned “causing death in a controlled environment” (Spike, 2000) or have referred to the fact that “more [DCDD] donors could be survivors” if aggressive support measures were continued (Burke, 2003). Such statements indicate confusion about the cause of death (the patient’s irreversible disease process) and the reasons why mechanical ventilation is discontinued (not for the sake of donation but because it is deemed unwanted, medically ineffective, or overly burdensome by those involved in the care of the patient).
Moreover, anecdotal data indicate that the connection between DCDD and the withdrawal of ventilatory support causes psychological distress for some healthcare workers involved in the transplantation process (Spike, 2000). This suggests that such confusion can have a detrimental effect on the implementation of DCDD protocols and on the healthcare professionals themselves.
Clearly, to the extent that controlled DCDD depends upon a decision to withdraw life-sustaining treatments, it is important that the legal and ethical justifications for withdrawing treatments are made explicit through education, practice, protocols, and professional standards. All individuals involved in the process must understand that the decision to withdraw life-sustaining treatment is independent of the decision to donate and that the withdrawal of life-sustaining treatment will proceed even if the patient is ineligible to donate.
Finally, whenever life-sustaining treatments are discontinued, it is important to distinguish withdrawing unwanted or ineffective medical interventions from withdrawing care for the patient or family. Standard end-of-life care for the patient continues until death is declared even though efforts to sustain the patient’s life have ceased, and all families should receive proper bereavement care during and after the process of making these difficult decisions (see Chapter 4).
Conflicts of Interest
Of all the ethical issues that DCDD presents, the topic of conflicts of interest has generated the least controversy. Although potential conflicts exist between the best interests of donors and recipients, an IOM committee observed in 1997 that the matter of conflicts of interests “has generated
the most consistent approaches in protocols reviewed by the IOM. All procurement organizations and transplant programs appear to understand the need for strong safeguards to ensure that conflicts of interest do not lead to violations of prevailing medical and ethical standards” (IOM, 1997, p. 55). Chief among the specific recommendations regarding conflicts of interest is the separation of “major decisions and discussions in patient care (withdrawal of life support, discontinuing cardiopulmonary resuscitation [CPR], and declaration of death) from major decisions and discussions in organ donation and transplantation (obtaining consent for donation and other transplant-related procedures and involvement in the actual process of organ retrieval)” (IOM, 1997, p. 55). These decisions and discussions frequently require separate staff (which is always the case in the determination of death) and separation in time. Although the present IOM committee has recommended against offering financial incentives for organ donation (Chapter 8), it bears stating that under no circumstances should financial incentives for organ donation be offered to families who need to make decisions regarding the continuation or discontinuation of life-sustaining treatments.
Family Interests and Consent
As the 2000 IOM report emphasized in its fourth recommendation, DCDD “should focus on the patient and the family.” Specifically, DCDD should
-
follow patient and family wishes as closely as possible;
-
meet family needs for information, support, and follow-up;
-
recognize and respect the patient’s and the family’s social, economic, and racial or ethnic diversity; and
-
follow clear mechanisms for identifying and covering all organ procurement costs.
Families of dying patients often want to be present when death is determined. Increasingly, protocols permit families to be present in the operating room until death is pronounced; some protocols even permit death to be declared outside of the operating room when it is feasible to subsequently quickly transport the donor to the operating room. These practices are consistent with professional standards (SCCM, 2001; Emergency Nurses Association, 2005). The committee encourages the use of such practices and the allocation of sufficient institutional and human resources and support systems to implement them.
This committee has supported the trend within the transplantation community to honor documented donor wishes. However, when donation is coupled with a decision to withdraw life-sustaining treatments—a deci-
sion typically made by family members unless there is a definitive advance directive or designated surrogate—it seems appropriate to solicit family permission for donation even in the face of first-person (donor) consent. This is primarily because DCDD directly affects the timing and environment for the withdrawal of treatment. Moreover, when families oppose donation, DCDD could introduce a conflict of interest for families as they decide whether or when to withdraw mechanical ventilation. Nevertheless, families should always be informed about the patient’s wishes for donation and encouraged to honor those wishes.
Although the committee acknowledges that controlled DCDD has been controversial (Box 5-7), it also supports the direction in which standards of care for controlled DCDD are developing and encourages OPOs to pursue controlled DCDD with greater intensity.
Ethical Issues Pertinent to Uncontrolled DCDD
When IOM committees considered DCDD in 1997 and 2000, they clearly addressed both controlled and uncontrolled (unanticipated) DCDD. Nevertheless, most of their recommendations addressed only controlled DCDD, and the 2000 report included only one uncontrolled DCDD protocol, the Washington Hospital Center’s Protocol for the Rapid Organ Recovery Program (Appendix F). The 2005 National Conference focused exclusively on controlled situations (Bernat et al., 2006).
It is beyond the scope of work of the present IOM committee to investigate thoroughly the obstacles to uncontrolled DCDD in the United States. This is also a complex and often misunderstood area (Box 5-8). However, given the significant potential of uncontrolled DCDD to increase the number of organs available for transplantation, this committee believes that it is imperative to further explore the essential issues and opportunities. This section briefly examines some of the ethical issues that surround uncontrolled DCDD. The discussion explores the potential impact that uncontrolled DCDD could have on the number of organs recovered and offers specific recommendations.
Because DCDD has primarily occurred in controlled settings in the United States, the unique ethical issues that arise regarding uncontrolled DCDD have not been as thoroughly examined by ethicists and policy makers. These ethical issues fall into two broad categories: concerns about resuscitation efforts and concerns about informed consent.
Resuscitation
As in all forms of DCDD, death is declared after an individual’s circulatory functions are permanently lost. Likewise, as in all forms of DCDD,
|
BOX 5-7 Correcting Myths and Misperceptions About Controlled DCDD Controlled DCDD protocols enable individuals who are currently on mechanical ventilation to become organ donors following a decision by the family to remove mechanical ventilation because it no longer benefits the individual. Myth: Patients would continue to live on the ventilator if they did not decide to become organ donors. Fact: The family’s or the patient’s decision to remove a ventilator is completely independent from the decision to donate organs. Myth: Taking patients off a ventilator is euthanasia. It kills them. Fact: Removing artificial ventilation is not homicide or euthanasia and is legal in all states. It allows the patient to die of a medical illness when further treatment is considered medically ineffective or overly burdensome or is not desired by the patient. Myth: Patients are not really dead when their organs are removed. Fact: Organs are not removed until an independent physician pronounces the patient dead. Patients are not pronounced dead until their heart, lungs, and brain have permanently stopped functioning. Myth: Medications are given to organ donors that will cause their death. Fact: No medications are given to cause a patient’s death. Patients may be given medications while they are on the ventilator to keep them comfortable. They may also be given medications to improve the quality of their organs for transplantation. However, experts consider these medications safe for patients, and families are told about the use of these medicines as part of the consent process. Myth: Families cannot be with their loved one when he or she dies. Fact: Families can request to be with their loved one until death is pronounced. Most hospitals have mechanisms to respond to these requests. Myth: Organs removed by using DCDD protocols do not work well for transplantation. Fact: Most organs removed by using a DCDD protocol work as well or nearly as well as other donated organs. DCDD has the potential to vastly increase the number of donated organs in the United States. |
medical caregivers may make decisions that affect the time of death and its determination. In controlled settings, decisions are made about the discontinuation of mechanical ventilatory support. In uncontrolled settings, decisions are made about the discontinuation of CPR.
At what point should resuscitative efforts be discontinued, and who should be allowed to make such decisions? The 1997 IOM committee observed the following: “The circumstantial events and medical status of
|
BOX 5-8 Correcting Myths and Misperceptions About Uncontrolled DCDD Uncontrolled DCDD protocols enable individuals to become donors after they die after full efforts to resuscitate them from cardiopulmonary arrest have been made. Myth: Only patients on ventilators can become organ donors. Fact: Many people who die unexpectedly can become organ donors if DCDD protocols are used. Myth: If you register as an organ donor, doctors will not do as much to save your life. Fact: The doctors who try to resuscitate patients work independently of the doctors involved with organ removal. Uncontrolled DCDD protocols require that resuscitation efforts meet or exceed current standards of practice. Myth: Uncontrolled DCDD will not work well in the United States because getting consent is difficult in an emergency situation. Fact: Individuals who have signed a donor card or joined a donor registry have given permission for organ donation and can become donors by the use of uncontrolled DCDD protocols. When individuals have not given prior permission, the use of organ preservation techniques while families are contacted for permission to donate is ethically permissible and explicitly allowed by law in some states. Myth: Organs removed by using an uncontrolled DCDD protocol are not really good for transplantation. Fact: In Europe, uncontrolled DCDD is more common, and very good outcomes are obtained when best practices are followed. Myth: Opportunities for uncontrolled DCDD are rare. Fact: Uncontrolled DCDD protocols can be used for a significantly broader population of potential donors than any other protocols. |
those who suffer unexpected cardiopulmonary arrest are enormously varied. It is difficult, therefore, to design a uniform approach to the determination of death in uncontrolled NHBDs” (IOM, 1997, p. 60). Although this is true when one reaches the level of specific rules, the committee nevertheless acknowledges that some valuable guidelines are commonly found in active protocols in Europe:
-
Those making the decision to discontinue CPR should not be affiliated with the organ recovery team.
-
To ensure a separation between the resuscitation and the transplant teams, a “hands-off” period is often observed. This period is currently between 5 and 10 minutes in European countries (Koffman and Gambaro, 2003; Bos, 2005). The committee believes that although a separation be-
-
tween teams is essential, the hands-off period could be very brief and may even be unnecessary.
-
Solid knowledge of resuscitative evidence and the best interests of the patient who has had a cardiac arrest must guide decisions to terminate resuscitative efforts. Decisions to discontinue resuscitative efforts must be based on medical futility in restoring circulation or breathing (Box 5-2). For example, in Spain, resuscitation is attempted for 30 minutes, in accord with the standards of the European Resuscitation Council and the American Heart Association (del Rio Gallegos et al., 2004). The present IOM committee recommends that protocols be regularly updated to reflect current best practices in resuscitative medicine.
-
Transplant centers that proceed with uncontrolled DCDD should do so only after a publicly created protocol that specifies resuscitative guidelines with concrete protections is developed. Appendix F provides the protocol developed by the Washington Hospital Center for uncontrolled DCDD.
Informed Consent
As this report has already observed, the National Organ Transplant Act and, more recently, specific state laws direct OPOs to honor an individual’s documented wishes regarding organ donation. In the view of the present IOM committee, when an individual has signed a donor card or joined a donor registry, OPOs have adequate permission to proceed with the recovery of organs from patients after death is declared. Permission to remove organs presupposes that the donor has been declared dead by the use of accepted medical standards; it does not presuppose a specific circumstance of death or a determination of death by the use of neurologic criteria.
However, the issue of consent to donate becomes more complex when an individual has not documented his or her wishes. When neurologic criteria are used to declare death, potential donors are typically maintained on artificial ventilation. This provides OPOs with the opportunity to contact families and to devote considerable time to the process of informing families and obtaining permission. However, when death occurs unexpectedly, potential donors are not maintained on artificial ventilation and families are frequently not available (Bos, 2005).
The use of cold preservation techniques can preserve organ viability and the option for donation while the families are contacted. However, cold preservation techniques themselves are invasive procedures, and consent must either be given or presumed. In nations that rely on an opt-out system of permission, consent for cannulation and cold perfusion may be presumed. The United States uses an opt-in system of consent for donation, and the committee recommends that this approach be retained (Chapter 7).
However, the committee regards the use of preservation techniques while families are contacted—for the purpose of preserving the family’s opportunity to make their own informed decision regarding donation—to be ethically acceptable in principle. In cases in which the family will be making the decision regarding donation, organ preservation interventions are a component of proper medical practice.
Surveys and community meetings conducted by the Washington Hospital Center preceding the implementation of its rapid recovery program indicate that the public believes—and the committee concurs—that a presumption of consent to use preservation techniques enhances rather than limits autonomy by enabling a decision about whether to donate; absent such presumed permission, the opportunity to donate is irretrievably lost (Light et al., 1996).
Some nations that rely on an opt-in system, like The Netherlands, have enacted specific legislation permitting the use of preservation techniques until families can be contacted for consent (Bos, 2005). Similarly, the District of Columbia, Virginia, and Florida have passed legislation permitting perfusion and cooling without consent to enable a decision regarding donation (IOM, 1997, p. 26), although no transplantation programs in these jurisdictions are currently making use of the authority conferred by such legislation. The committee is not convinced that such legislation is necessary; courts might very well regard postmortem cannulation to be permissible as a potentially life-saving medical practice, in the absence of legislation explicitly prohibiting it. On the other hand, although specific legislation may not be legally necessary, some OPOs may want such legislation to remove all doubt regarding the legal permissibility of the procedure. (Either way, it is incumbent upon hospitals and physicians to develop the necessary protocols in accord with ethical principles bearing on respect for the remains of the deceased, to be transparent, and to consult relevant communities.)
REEXAMINATION OF UNCONTROLLED DCDD
Uncontrolled DCDD typically involves individuals who have collapsed suddenly out of hospital and arrive in the emergency department without spontaneous vital signs after having received CPR from emergency medical services (EMS) personnel. The American Heart Association estimates that each year in the United States about 335,000 deaths are due to sudden cardiac arrest (AHA, 2005). Although reports of the rates of survival vary among EMS systems (Eisenberg et al., 1991), about 95 percent of sudden cardiac arrest victims die before they reach the hospital (AHA, 2005). At present, virtually all of these individuals and their families are denied the opportunity to be organ donors.
Estimation of Potential Donors
Application of the Modified Madrid Criteria displayed in Box 5-6 to the estimated cohort of 335,000 cardiac arrest deaths in the United States may be the best means available of estimating the number of potential uncontrolled DCDD donors in the United States (this approximation considers potential donors to be Maastricht Category I and II decedents who had a sudden cardiac arrest outside of the hospital). Because criteria drawn from a relatively small data set are being applied to a much larger one, there will be a substantial margin of error in these estimates. In the interest of arriving at an estimate and driving this error in the direction of underestimation rather than overestimation of organ yield, the Modified Madrid Criteria can be applied to the 335,000 individuals who die of cardiac arrest in the United States each year as if all of these deaths occurred in New York City.
One advantage of this approach is that an analysis using the New York City Pre-Hospital Arrest Survival Evaluation (PHASE) data set (Lombardi et al., 1994) should provide a conservative estimate of the number of potential uncontrolled DCDD donors who meet the Modified Madrid Criteria. This assertion is based on several features of this data set that tend to make most cardiac arrest patients ineligible to meet the Modified Madrid Criteria, thus minimizing the estimated number of potential qualifying donors: (1) the median age of the PHASE cohort was 70 years (interquartile range, 60 to 79 years), which exceeds the upper age limit of the criteria by 20 years; (2) about one-third of the patients had an unwitnessed cardiac arrest, which disqualifies them from further consideration as donors because their warm ischemia time is unknown; and (3) about two-thirds of the patients did not receive bystander CPR, which, combined with lengthy EMS response times, placed many individuals outside the 15-minute time window postcollapse during which CPR must be initiated to meet the donation criteria.
Two additional advantages to the use of the PHASE data are that they constitute the largest consecutive series of data on out-of-hospital cardiac arrests in the United States with complete follow-up, and raw data from the study are available to perform the requisite analyses.
Of 2,329 consecutive patients with out-of-hospital cardiac arrests occurring in New York City over a 6-month period on whom resuscitation was attempted, 14 percent, or 326 patients, were under 50 years of age. Of these 326 patients with cardiac arrest, 44 percent, or 143 patients, received bystander CPR, plus an additional 31 percent, or 101 patients, received CPR from EMS within 15 minutes of collapse. Thus, 143 + 101 = 244 patients who experienced cardiac arrest, who were under age 50 years, and who received CPR within 15 minutes of collapse. CPR was continued for a median of 31 minutes.
The median time that elapsed from the time of collapse to the time of the pronouncement of death was 53 minutes for these 244 patients with cardiac arrest, 73 percent of whom were pronounced dead within 60 minutes. Thus, 73 percent (178/244) of the patients with cardiac arrest were pronounced dead within an hour of collapse. If one allows a 10-minute hands-off period with an absent heartbeat after the pronouncement of death and the cessation of CPR, the transplantation team would be left with 50 minutes, on average, to perform cannulation and cooling of the kidneys (which is well within the requisite window of total warm ischemia time).
Thus, 178 of 2,329, or about 7.6 percent (95 percent confidence interval, 6.6 to 8.7 percent), of all patients with out-of-hospital cardiac arrests could theoretically meet the criteria for donor eligibility. If one then applies the lower limit of the confidence interval to the American Heart Association figure of 335,000 cardiac arrest deaths in the United States each year, the result is a conservative estimate of about 22,000 decedents who meet the Modified Madrid Criteria for uncontrolled DCDD kidney donation each year. This is significantly higher than the current pool of 10,500 to 16,800 eligible donors for whom death is determined by neurologic criteria (Guadagnoli et al., 2003; Sheehy et al., 2003) and does not include potential controlled DCDD donors.
NEXT STEPS FOR DCDD
Because the vast majority of Americans die as a result of the loss of circulatory function, many individuals who during their lifetimes expressed a desire to be an organ donor are not currently able to have that wish carried out upon their death for merely technical reasons and not medical reasons of exclusion. A well-established DCDD program would meet their end-of-life wishes. DCDD is not widely practiced in the United States, and at present the circumstances for its use are largely limited to controlled situations in which artificial ventilation is withdrawn. The committee believes that a concerted effort is needed to implement DCDD in uncontrolled situations and thereby provide the opportunity for organ donation to a greater number of people. In addition to the current focus on increasing the number of controlled DCDD donors, the committee believes that the possibility of uncontrolled DCDD should be fully explored to see if this might be realistic, particularly in urban areas with extensive trauma and emergency care systems.
It is important to acknowledge the challenges and the level of effort that will be needed to ensure that DCDD is a feasible option. Trust in the healthcare system is the prime consideration. Patients and their families must have complete confidence that all emergency and resuscitative efforts
will be made and that organ donation will be considered only in the event of a loss of life after every appropriate measure has been attempted.
Furthermore, it is incumbent on the healthcare system to commit the resources needed for implementation of DCDD protocols. It is acknowledged that deaths due to cardiac arrest occur at all hours and that there is often a more time-sensitive urgency with DCDD cases as compared with DNDD. Healthcare systems that are already heavily stressed and thinly stretched may be resistant to changes that will impose additional demands, albeit for lifesaving reasons (Chapter 4).
The committee has identified several actions that are needed to increase the rates of DCDD and offers a proposal to fully evaluate the potential for uncontrolled DCDD. These are outlined below.
-
Provide excellent emergency and resuscitative care. The first and foremost action in implementing a DCDD program is the use of excellent emergency and resuscitative care. State-of-the-art guidelines must be followed, and all efforts should be made to ensure that the patient has every opportunity for survival. The priority is to focus all possible medical care on the individual’s survival.
-
Provide public education. An informed public can make the assessments that are needed to provide input into the planning and development of DCDD protocols. As with the development of the Washington Hospital Center’s uncontrolled DCDD program (described earlier), a transparent and open process is essential, as is the substantive and ongoing involvement of the community in the planning, development, and implementation of a DCDD program. Because deaths due to cardiac arrest occur more frequently than the types of deaths that are determined by neurologic criteria, the general public is probably familiar with what it means to ascertain whether cardiac and pulmonary functions have permanently ceased and is likely to understand, and to be receptive to, public education messages regarding the conditions necessary to facilitate organ donation in that event. Uncontrolled DCDD is admittedly a complex issue, but given the potential for a dramatic increase in the number of available organs for transplantation the committee believes that high priority should be given to these public education efforts as an essential component of an aggressive effort to implement uncontrolled DCDD protocols.
-
Provide professional education. For healthcare professionals, it is particularly important to sponsor educational efforts clarifying the elements of high-quality end-of-life care and explaining the steps and protocols needed to implement DCDD in all settings. Support for DCDD is particularly needed from professional associations. Additionally, the urgent need to implement DCDD protocols should be thoroughly discussed in continuing education and other professional education settings in order to
-
demystify the process and to address legitimate concerns. The overall message is that implementing DCDD protocols will offer many more individuals the opportunity to donate organs at the end of their lives. This conceptual integration of end-of-life care and organ recovery in DCDD cases will help create a more rational comprehensive clinical approach to patient and family care in these difficult circumstances (Chapter 4).
-
Ensure the opportunity for donation. Steps can to be taken to increase opportunities for DCDD. Professional societies, such as the American Heart Association, should add steps for preparation for such donations to the end of their standard resuscitation protocols. This would prompt emergency services personnel to take various actions, such as administer organ preservation medications or search for documentation of the individual wishes (e.g., a driver’s license). Furthermore, it will be important to develop standards by which organ preservation measures (such as cannulation) for DCDD can be taken. Registration with an organ donor registry or other forms of donor consent (an organ donor card or a driver’s license) should be considered as the necessary documentation for beginning appropriate medical processes for organ donation after death. In cases of death without that documentation, organ preservation methods could be started to allow the family the opportunity to donate their loved one’s organs if they choose to do so.
-
Mentor and evaluate. Current variations among hospitals and OPOs in the number of DCDD cases need to be addressed through mentoring programs and other efforts by the Organ Donation Breakthrough Collaboratives and professional organizations, which should encourage the development of DCDD programs. As programs are being evaluated, it is important that potential DCDD donors be considered part of the denominator in all measures used to evaluate actual conversion rates.
-
Clarify regulatory and statutory requirements. Because preservation efforts to maintain the viability of the organs must be initiated soon after circulatory determination of death to preserve the opportunity for organ donation, statutory criteria for the determination of death by either neurologic or circulatory criteria should be clearly specified. Furthermore, the regulation requiring Medicare-funded hospitals to refer all deaths and imminent deaths to an OPO (ACOT, 2005) must be strengthened and expanded to encompass imminent circulatory-related deaths. Despite this requirement, many referrals are being made after the patient has been declared dead by neurologic criteria, cardiopulmonary resuscitation has been stopped, mechanical support has been withdrawn, or the decision has been made to withdraw support. These late referrals are much less likely to result in successful organ donation. In fact, premature removal of mechanical support can be a major barrier to organ donation.
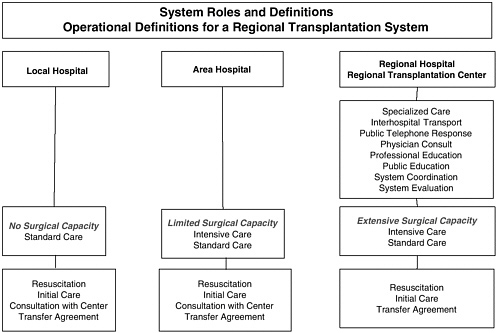
FIGURE 5-1 Regional transplantation system.
-
Develop a regionalized infrastructure. In urban areas, particularly those with extensive EMS and trauma centers, a regionalized system of organ donation and transplantation care could be developed that would focus organ retrieval and transplantation efforts in regional transplant centers (Figure 5-1). These regional centers would centralize the expertise and would provide the capacity needed to be prepared for DCDD cases 24 hours a day. This approach is consistent with the current categorization and regionalization of EMS systems currently used in the United States for cardiac care, trauma, neurosurgery, etc. Such a regionalized system would allow EMS workers to reduce the time of transport directly to a regional transplant center or regional hospital that can perform the essential steps needed.
Economic Considerations
Careful consideration must be given to the economic impact of emphasizing uncontrolled DCDD. There are potential savings in the value of the organs procured. However, because there are multiple healthcare payers, the savings will be seen by multiple entities and would not have a direct impact on any one system. Economic questions to be considered include the
impacts on emergency department staffing and space, the impacts on operating room capacity, costs required to implement educational programs and develop community consensus, as well as costs of in situ preservation equipment and personnel.
Demonstration Projects
The committee recommends that HRSA, other federal agencies, states, and local entities fund demonstration projects to examine the feasibility of implementing donation programs focused on uncontrolled DCDD. These projects would best be developed within defined geographic areas with well-established EMS systems, particularly those served by a Level I trauma and transplant center, which is the principal receiving hospital for a single EMS system. The demonstration projects would involve the development of processes, in conjunction with hospital and prehospital personnel, to transport all patients with cardiac arrest, whether it is due to primary heart disease or secondary to trauma, to the emergency department of the hospital.
The hospital, OPO, and the EMS system must be well respected and trustworthy entities in the surrounding community that they serve. The single most important feature of the agreement reached about a DCDD protocol is that it meets with public approval and satisfies the community. Community input would be needed during all phases of the project; and public education, as well as professional education, would be important. Extensive stakeholder collaboration involving community members and hospital, OPO, and EMS staff would be needed to develop the protocols for postmortem cannulation and cooling, pending consent for donation from the next of kin. Whether or not such an agreement will also require legal sanction can be determined only by input from the various stakeholders. All organs would be considered appropriate for retrieval as circumstances permit, but the principal target would be kidney donation.
Demonstration projects would allow and enable exploration of the challenges and opportunities for uncontrolled DCDD and should be evaluated carefully. If deemed successful, donation programs focused on uncontrolled DCDD should be scaled up and disseminated to other cities and regions.
EXPANDED CRITERIA FOR ORGAN DONATION
Organ quality is a primary determinant of the likelihood of successful organ transplantation. For this reason, potential organ donors are carefully assessed. In general, the suitability of organs for transplantation has traditionally been based on donor age, the circumstances of death, the absence
of infection and malignancy, the health of the prospective donor immediately before death, and the function of the organ at the time of death (Modlin et al., 2001). Historically, the typical organ donor has been a person less than 55 years of age who has suffered a head trauma.
Over the past 5 to 10 years the methods used to evaluate organ quality and to decide organ allocation have been changing. Changes in organ donation criteria have occurred, at least in part, because of the growing number of patients awaiting organ transplantation. These changes in practice have been associated with at least a fivefold increase in the use of organs that would not have met traditional standard criteria but that have proven to be not only transplantable but also safe and associated with enhanced rates of survival and, likely, an improved quality of life (Whiting et al., 2000; Weill, 2002; Kawut et al., 2005).
Increasingly, organs from older donors and from donors with some medical comorbidities (e.g., hypertension, positive viral serology for patients infected with the same virus, and evidence of some prior organ dysfunction) are being used. Various investigators have assessed the efficacies of organs from pediatric deceased donors; organs from donors with abnormal renal function, prolonged ischemia times, diabetes, and hypertension; organs with vascular or other anatomic abnormalities; or organs retrieved from living unrelated donors and DCDD donors (IOM, 1997; Rudich et al., 2002; Tan et al., 2004).
It is apparent that there is great variation in the extent to which transplant surgeons, transplant centers, and OPOs have been interested in using expanded-criteria organs. Expanded criteria have been most thoroughly investigated and delineated for potential kidney donors (Metzger et al., 2003). Earlier investigations commented on the lack of a uniform definition of what constituted an ideal kidney for transplantation, although there was some understanding of some of the factors that were associated with poorer outcomes, e.g., age, diabetes mellitus, hypertension, and prolonged cold ischemia time (Ojo et al., 2001). These efforts resulted in a more uniform definition accepted by UNOS (Table 5-5). Factors subsequently identified through national registry data that independently predicted a significantly higher risk of graft loss by the use of expanded-criteria kidneys compared with the use of standard kidneys were ultimately included in the UNOS policy (Port et al., 2002).
The current OPTN-UNOS policy change includes the allocation of expanded-criteria donor organs solely by recipient waiting time. Candidates on the standard waiting list may also choose to be added to the expanded-criteria waiting list (HRSA and SRTR, 2006). Since expanded-criteria candidates are preidentified, preprocurement tissue typing can be performed. Human leukocyte antigen matching (for determination of the level of match between the donor and the recipient) is not an allocation
TABLE 5-5 UNOS Definition of Expanded-Criteria Kidney Donors
issue for expanded-criteria donor kidneys, as long as the mismatch is not zero.
Use of these expanded-criteria kidneys is associated with a significant increase in the number of kidneys recovered and transplanted (Sung et al., 2005). Early reports suggested that the donation of expanded-criteria kidneys would be associated with survival benefits compared with those obtained by the use of maintenance hemodialysis (Ojo et al., 2001), although it was understood that the magnitude of this effect could vary according to the referent group of patients (Merion et al., 2005). As noted by Whiting and colleagues (2000), a key question was not whether transplantation with an expanded-criteria donor kidney was preferable to a lifetime of dialysis but, rather, when and under what circumstances would a policy of transplantation with expanded-criteria donor kidneys be preferable to a policy of using only “ideal” kidneys in the context of the longer waiting times for transplantation that would result.
Some centers have reported that the use of expanded-criteria organs yields results that are comparable to those obtained by using standard-criteria organs (Stratta et al., 2004). Further investigations of the potential impact of the change in OPTN-UNOS policy on expanded-criteria donation suggested that the new policy might be significantly advantageous only for specific subsets of patients, e.g., elderly individuals, awaiting transplantation of a kidney from a deceased donor unless the use of expanded-criteria kidneys dramatically reduced the waiting time for transplantation (Schnitzler et al., 2003). Recent studies also provide some evidence that
expanded-criteria organs could be expected to function less effectively and that recipients might have more postoperative complications (Sellers et al., 2004).
Merion and colleagues (2005) found that the long-term relative risk of mortality was 17 percent lower for expanded-criteria recipients (compared to standard therapy, including dialysis) and for specific patient subgroups (including patients older than 40 years of age or those with diabetes and hypertension) the benefit of receiving an expanded-criteria kidney transplant was significantly higher. The analysis suggests that expanded-criteria kidney transplants should be offered to patients who are older than 40 years of age and who face long waiting times. For OPOs with shorter waiting times, in which non-expanded-criteria kidneys for transplantation are more readily available, a survival benefit from expanded-criteria kidney transplantation was observed only in patients with diabetes. It should be noted that complication rates and the likelihood of a survival benefit are likely diminished in patients who require kidney retransplantation (Sellers et al., 2004).
Comparatively fewer studies exist on expanded-criteria considerations for other solid organs, especially the pancreas and the heart. Therefore, the recommendations for the use of expanded-criteria organs for these patients are less clearly established. Much remains to be learned about the defining characteristics of ideal potential donors, donated organs, and potential recipients so that expanded-criteria allocation and utilization policies can become more evidence based. Outcomes data from multiple centers that include quality-of-life assessments, extended follow-up data, and more rigorous examinations of recipient status and its effect on outcomes are needed.
Weill and colleagues (2002) observed that the donor criteria used for lung transplantation were developed on the basis of the experience of a few institutions in the early 1980s and 1990s. More recent investigations have suggested that the standard selection criteria currently in use4 lead to the exclusion of some otherwise acceptable donor lungs (Fisher et al., 2004). It
is especially important to address donor suitability, because only a minority of multiorgan donors are ultimately believed to be suitable lung donors. Recent studies have questioned the relationship between sputum Gram stain results and patient outcomes, now that broad-spectrum prophylactic antibiotic coverage is routinely available. In those studies, no predictive value was found in the numbers or the types of bacteria seen or the presence or absence of white blood cells in donor sputum (Ciulli et al., 1993; Gabbay et al., 1999; Weill et al., 2002). Kawut and colleagues (2005) found that patients receiving expanded-criteria lungs with specific characteristics including age of 55 years or greater, extensive smoking history, or evidence of chest trauma had acceptable rates of survival but tended to spend more time in the intensive care unit, had longer hospitalizations, and had decreased pulmonary function after one year. The use of extended-criteria donor lungs might be acceptable to improve survival because donors are in such short supply and because of the high risk of death for some patients who do not receive lung transplants (Pierre et al., 2002; Kawut et al., 2005).
A universally accepted definition of expanded criteria for liver donation has also not yet been established (Busuttil and Tanaka, 2003). Nevertheless, surgeons are beginning to transplant livers that would previously have been considered unacceptable (Fukumori et al., 2003). For example, therapeutic use of inotropic drugs, donor age, and the presence of moderate to severe hepatic steatosis independently influence the severity of liver preservation injury and the duration of stay in the intensive care unit for liver transplant recipients (Briceno et al., 2002). OPTN-UNOS’s organ-sharing liver transplant registry database has been used to identify predictors of death or retransplantation in liver transplant recipients. In a multivariate regression model, donor age, recipient age, recipient creatinine concentration greater than 2 milligrams per deciliter, and ventilator dependency for the recipient were associated with graft failure at 1 year, especially in liver transplant recipients infected with hepatitis C virus (Condron et al., 2005). Various investigators have confirmed that biological factors can be used to predict liver preservation injury, because, as with other organs, ischemia time is an important determinant of organ function and transplant efficacy (He et al., 2004; Cuende et al., 2005). Renz and colleagues (2005) recently evaluated the effect of the use of extended donor liver allografts by comparing extended-criteria donor livers allocated by transplant center instead of by regional waiting list priority. Expanded-criteria liver allografts can be transferred out of the region only if they are declined by all of the transplant centers within reasonable geographic proximity.
The expanded criteria used included donor age greater than 65 years, DCDD, positive viral serology, split liver grafts, hypernatremia, or a history of cancer, steatosis, or high-risk behavior in donors. The rate of patient access to liver transplantation increased by 77 percent and the
pretransplantation mortality rate was reduced by 50 percent compared with the rates obtained by the traditional allograft allocation system. The waiting times, complications, and lengths of hospitalization were similar; and there were no significant differences in patient or graft survival, although the causes of death were different. This study confirms the need to establish and update guidelines to include all current and prospective variables that might influence donor selection, graft function, and patient and graft survival.
Ethical Considerations for Expanded-Criteria Organ Donation
The use of expanded-criteria organs raises a number of ethical issues. On the one hand, their use has the potential to save or significantly improve lives. As noted already, many expanded-criteria organs have been used and the outcomes achieved have been good; they have shortened waiting times, enabled patients to move off of dialysis, and have enabled some people who might have died waiting for an organ to live. On the other hand, because the use of some expanded-criteria organs is associated with greater risks of morbidity and mortality, there is a risk that some patients will be harmed. Moreover, given these risks, many patients will view expanded-criteria organs as less desirable, thus generating questions of the fair allocation of standard-criteria versus expanded-criteria organs. Without attempting a complete ethical analysis of the use of expanded-criteria organs, the committee offers the following guidance:
-
Because expanded-criteria organ donation has the potential of increasing the number of donated organs and thereby saving and improving lives, the use of expanded-criteria organs should be further explored.
-
Consent for the use of expanded-criteria organs should be obtained from transplant candidates. The consent information should distinguish between expanded-criteria organs with known heightened associated risks and expanded-criteria organs with unknown heightened associated risks. So-called expanded-criteria organs that are known to function as well as standard organs (as is the case with some categories of DCDD organs) should not be labeled or treated as expanded-criteria organs.
-
Practices associated with the use of expanded-criteria organs must be evidence based. Accordingly, ongoing collaborative research is needed.
-
Expanded-criteria organs should be allocated in ways that minimize potential harms, for example, by matching organs infected with a particular agent with patients infected with the same agent.
-
Cost-effectiveness should be considered in the development of practice guidelines.
Next Steps for Expanded Criteria
The potential exists to expand the pool of potential donors while also maximizing the quality of the transplanted organ and protecting the safety of the recipient. The development of nomenclature that denotes different categories of organ quality is important to clinical practice. Terminology that can provide separate designations for organ quality and organ screening for disease status is needed. Less precise terms such as “expanded criteria” or “marginal” should be replaced with terms or categories of terms that convey information about the organ’s quality or laboratory screening results. These categories of organ quality and designations for organ screening will evolve with new knowledge about graft survival.
Organ Quality
Much remains to be learned about the spectrum of organ variability, particularly for organs other than kidneys, and there is a need to develop the quantitative measures that can inform patients and clinicians. Although there have been age restrictions in the past, it is apparent that there is wide variability in the suitability of organs obtained from donors of all ages.
It is important to develop an evidence-based approach to the characterization of the quality of organs. Ensuring the accuracy and integrity of registry data is fundamental in this process; and a uniformly accepted, scientific method-quality, multicenter database should be supported. Such an effort would likely accelerate the development of guidelines that define acceptable outcomes after expanded-criteria organ donation and may help refine competency standards and qualifications for transplant surgeons. In addition, such an effort, in which OPOs, donor service areas, and transplant centers are more closely linked around data collection and reporting standards, will provide a unique opportunity for OPOs and transplant centers to interface with other national programs investigating the relationships between clinical effectiveness, quality, and safety. Evaluations of cost-effectiveness should be included as new practice guidelines are developed; and such evaluations are likely to be more rigorous as all data elements (e.g., age, disease state, resuscitation status, and waiting time) that could potentially influence outcome are considered.
Targeted Research Needs
The cellular changes that correlate with or predict graft function and survival are not well described. Efforts to further the understanding of warm and cold ischemia and reperfusion injury at the cellular level are needed. Such research will likely identify important molecular determinants
of viability before the organs are collected and transplanted and may identify better methods of storing and maintaining recovered organs.
Organ Screening
It is important that, in addition to and separately from organ quality, organs should be denoted as positive or negative for diseases, disorders, and infectious disease agents for which they are screened, such as hepatitis viruses and human immunodeficiency virus. This designation should affect clinical practice, including consent procedures and allocation criteria. Organs should be allocated to minimize harm, e.g., by matching infected organs with similarly infected patients, and detailed, specific informed consent should be obtained when organs are expected to perform below “gold standard” quality.
SUMMARY AND RECOMMENDATIONS
In 2004, approximately 95 percent of deceased donors were individuals declared dead by neurologic criteria (Table 5-2). However, because deaths determined by neurologic criteria constitute only a small percentage of total deaths in the United States, there is the potential to significantly increase organ donation rates by carefully reexamining and expanding the nation’s ability and infrastructure to transplant organs from deceased individuals with circulatory determination of death, deaths that occur in both anticipated and unanticipated (controlled and uncontrolled) conditions. Research is needed to more fully explore the graft survival rates of other organs and to develop optimum methods for organ preservation and for categorizing the viability of organs.
It is acknowledged that DCDD can be a more complex and less facile process than DNDD. Expansion of the nation’s capabilities, particularly in large urban areas with excellent emergency medical care, could provide the opportunity for donation to larger numbers of individuals and families. One set of conservative estimates suggests that at least 22,000 of out-of-hospital cardiac arrest deaths annually in the United States would meet Modified Madrid Criteria for unanticipated DCDD if important ethical and practical matters could be resolved. Before proceeding further, demonstration projects to assess the feasibility of undertaking such a strategy within a defined community should be considered. This activity should complement current ongoing efforts to expand the number of controlled DCDD and DNDD donors and should be accompanied by thorough research on the nature and extent of impediments to the implementation of DCDD protocols and on the effects of efforts to remove them.
Recommendation 5.1 Implement Initiatives to Increase Rates of Donation after Circulatory Determination of Death.
HRSA, the National Institutes of Health (NIH), OPTN, OPOs, donor hospitals, transplant centers, and professional societies should implement initiatives to increase rates of DCDD. Particular emphasis should be given to
-
funding of interdisciplinary research by NIH and HRSA to understand and remove institutional, professional, and community barriers and resistance to DCDD;
-
enhancing public and professional education, disseminating best practices, and monitoring and evaluating DCDD efforts;
-
clarifying required referral regulations to ensure that all potential donors are considered; and
-
adding preparation for organ donation to the end of standard resuscitation protocols.
Recommendation 5.2 Encourage and Fund DCDD Demonstration Projects.
The U.S. Department of Health and Human Services, states, and local entities should encourage and fund demonstration projects to determine the feasibility of increasing the rates of uncontrolled DCDD in cities with established and extensive trauma centers and emergency response systems. Such demonstration projects should include extensive public and professional education, including an emphasis on donor registry efforts, and participation by all relevant stakeholders in the development of protocols and processes.
Recommendation 5.3 Maintain Opportunities for Organ Donation.
OPOs should work with relevant stakeholders to obtain community authorization for the use of postmortem organ preservation techniques during the time needed to seek family consent for donation when the deceased person’s donation intention is unknown.
Recommendation 5.4 Increase Research on Organ Quality and Enhanced Organ Viability.
NIH should request and allocate funds for the purpose of determining the characteristics that modify and define organ quality. NIH should fund further research on enhancing the viability of organs for transplantation, including improved methods of organ preservation and improved criteria (with appropriate point-of-care testing) for determining the viability of organs.
REFERENCES
Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF. 2004. Survival following liver transplantation from non-heart-beating donors. Annals of Surgery 239(1):87–92.
ACOT (Advisory Committee on Organ Transplantation), U.S. Department of Health and Human Services. 2005. Recommendations. [Online]. Available: http://www.organdonor.gov/acotrecs.html [accessed March 13, 2006].
AHA (American Heart Association). 2005. CPR Facts and Statistics. [Online]. Available: http://www.americanheart.org/presenter.jhtml?identifier=3034352 [accessed November 28, 2005].
Alvarez J, Iglesias J, Pulido O, Maldonado L, San Juan G, Sanchez P, Corral E, Medina JC. 1997. Type I non-heart-beating donors: Policy and results. Transplantation Proceedings 29(8):3552.
Alvarez J, del Barrio R, Arias J, Ruiz F, Iglesias J, de Elias R, Yebenes C, Matesanz J, Caniego C, Elvira J. 2000. Non-heart-beating donors from the streets: An increasing donor pool source. Transplantation 70(2):314–317.
Alvarez J, del Barrio MR, Arias J, Gonzalez M, Cordoba L, Moreno F, Corpas R, Nieto M, Oglesias J, Corral E, Barra C, Elvira J, Ibarguren C. 2002. Five years of experience with non-heart-beating donors coming from the streets. Transplantation Proceedings 34(7): 2589–2590.
Bernat JL, D’Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, Rosenbaum SH, DeVita MA, Gaston RS, Merion RM, Barr ML, Marks WH, Nathan H, O’Connor K, Rudow DL, Leichtman AB, Schwab P, Ascher NL, Metzger RA, McBride V, Graham W, Wagner D, Warren J, Delmonico FL. 2006. Report of a national conference on donation after cardiac death. American Journal of Transplantation 6(2):281–291.
Bos MA. 2005. Ethical and legal issues in non-heart-beating organ donation. Transplantation 79(9):1143–1147.
Briceno J, Marchal T, Solorzano G, Pera C. 2002. Influence of marginal donors on liver preservation injury. Transplantation 74(4):522–526.
Burke WJ. 2003 (June 13). More donors could be survivors. St. Louis Review. [Online]. Available: http://www.stlouisreview.com/article.php?id=4179 [accessed February 28, 2006].
Busuttil RW, Tanaka K. 2003. The utility of marginal donors in liver transplantation. Liver Transplantation 9(7):651–663.
Canadian Council for Donation and Transplantation. 2005 (February 17–20). Donation after Cardiocirculatory Death: A Canadian Forum. Vancouver, British Columbia, Canada.
Ciulli F, Tamm M, Dennis C, Biocina B, Mullins P, Wells FC, Large SR, Wallwork J. 1993. Donor-transmitted bacterial infection in heart-lung transplantation. Transplantation Proceedings 25(1):1155–1156.
Cole D. 1993. Statutory definitions of death and management of terminally ill patients who may become organ donors after death. Kennedy Institute of Ethics Journal 3(2):145–155.
Condron SL, Heneghan MA, Patel K, Dev A, McHutchison JG, Muir AJ. 2005. Effect of donor age on survival of liver transplant recipients with hepatitis C virus infection. Transplantation 80(1):145–148.
Cooper JT, Chin LT, Krieger NR, Fernandez LA, Foley DP, Becker YT, Odorico JS, Knechtle SJ, Kalayoglu M, Sollinger HW, D’Alessandro AM. 2004. Donation after cardiac death: The University of Wisconsin experience with renal transplantation. American Journal of Transplantation 4(9):1490–1494.
Cuende N, Miranda B, Canon JF, Garrido G, Matesanz R. 2005. Donor characteristics associated with liver graft survival. Transplantation 79(10):1445–1452.
D’Alessandro AM, Fernandez LA, Chin LT, Shames BD, Turgeon NA, Scott DL, Di Carlo A, Becker YT, Odorico JS, Knechtle SJ, Love RB, Pirsch JD, Becker BN, Musat AI, Kalayoglu M, Sollinger HW. 2004. Donation after cardiac death: The University of Wisconsin experience. Annals of Transplantation 9(1):68–71.
del Rio Gallegos F, Nunez Pena JR, Soria Garcia A, Moreno Roy MA, Varela A, Calatayud J. 2004. Non heart beating donors. Successfully expanding the donor’s pool. Annals of Transplantation 9(2):19–20.
DeVita MA. 1996. Reflection on non-heartbeating organ donation: How three years of experience affected the University of Pittsburgh’s ethics committee’s actions. Cambridge Quarterly of Healthcare Ethics 5(2):285–292.
DeVita MA. 2001. The death watch: Certifying death using cardiac criteria. Progress in Transplantation 11(1):58–66.
Doig CJ, Rocker G. 2003. Retrieving organs from non-heart-beating organ donors: A review of medical and ethical issues. Canadian Journal of Anesthesia 50(10):1069–1076.
Droupy S, Blanchet P, Eschwege P, Hammoudi Y, Joseph L, Kriaa F, Bedossa P, Duranteau J, Charpentier B, Benoit G. 2003. Long-term results of renal transplantation using kidneys harvested from non-heartbeating donors: A 15-year experience. Journal of Urology 169(1):28–31.
DuBois J. 2002. Is organ procurement causing the death of patients? Issues in Law & Medicine 18(1):21–41.
DuBois J, Schmidt T. 2003. Does the public support organ donation using higher brain-death criteria? Journal of Clinical Ethics 14(1–2):26–36.
Edwards JM, Hasz RD, Robertson VM. 1999. Non-heart-beating organ donation: Process and review. AACN Clinical Issues: Advanced Practice in Acute Critical Care 10(2): 293–300.
Egan TM. 2004. Non-heart-beating donors in thoracic transplantation. Journal of Heart and Lung Transplantation 23(1):3–10.
Eisenberg MS, Cummins RO, Larsen MP. 1991. Numerators, denominators, and survival rates: Reporting survival from out-of-hospital cardiac arrest. American Journal of Emergency Medicine 9(6):544–546.
Emergency Nurses Association. 2005. End of Life Care in the Emergency Department. [Online]. Available: http://www.ena.org/about/position/PDFs/End-of-LifeCare.PDF [accessed March 10, 2006].
Fisher AJ, Donnelly SC, Pritchard G, Dark JH, Corris PA. 2004. Objective assessment of criteria for selection of donor lungs suitable for transplantation. Thorax 59(5):434–437.
Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, Shames BD, Becker YT, Odorico JS, Knechtle SJ, Sollinger HW, Kalayoglu M, D’Alessandro AM. 2005. Donation after cardiac death: The University of Wisconsin experience with liver transplantation. Annals of Surgery 242(5):724–731.
Fukumori T, Kato T, Levi D, Olson L, Nishida S, Ganz S, Nakamura N, Madariaga J, Ohkohchi N, Satomi S, Miller J, Tzakis A. 2003. Use of older controlled non-heart-beating donors for liver transplantation. Transplantation 75(8):1171–1174.
Gabbay E, Williams TJ, Griffiths AP. 1999. Maximizing the utilization of donor organs offered for lung transplantation. American Journal of Respiratory and Critical Care Medicine 160(1):265–271.
Gomez M, Alvarez J, Arias J, Barrio R, Muguerza J, Balibrea JL, Martin F. 1993. The use of kidneys from non-heart-beating donors for transplantation. Transplantation Proceedings 25(1):1501–1502.
Guadagnoli E, Christiansen CL, Beasley CL. 2003. Potential organ-donor supply and efficiency of organ procurement organizations. Health Care Financing Review 24(4): 101–110.
He XS, Ma Y, Wu LW, Ju WQ, Wu JL, Hu RD, Chen GH, Huang JF. 2004. Safe time to warm ischemia and posttransplant survival of liver graft from non-heart-beating donors. World Journal of Gastroenterology 10(21):3157–3160.
HRSA (Health Resources and Services Administration) and SRTR (Scientific Registry of Transplant Recipients). 2005. 2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. [Online]. Available: http://www.optn.org/AR2004/ [accessed January 31, 2006].
HRSA and SRTR. 2006. 2005 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1995–2004. [Online]. Available: http://www.optn.org/AR2005/default.htm [accessed March 3, 2006].
IOM (Institute of Medicine). 1997. Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement. Washington, DC: National Academy Press.
IOM. 2000. Non-Heart-Beating Organ Transplantation: Practice and Protocols. Washington, DC: National Academy Press.
Kawut SM, Reyentovich A, Wilt JS, Anzeck R, Lederer DJ, O’Shea MK, Sonett JR, Arcasoy SM. 2005. Outcomes of extended donor lung recipients after lung transplantation. Transplantation 79(3):310–316.
Keenan S, Hoffmaster B, Rutledge F, Eberhard J, Chen L, Sibbald W. 2002. Attitudes regarding organ donation from non-heart-beating donors. Journal of Critical Care 17(1):29–36.
Kern KB, Halperin HR, Field J. 2001. New guidelines for cardiopulmonary resuscitation and emergency cardiac care: Changes in the management of cardiac arrest. Journal of the American Medical Association 285(10):1267–1269.
Koffman G, Gambaro G. 2003. Renal transplantation from non-heart-beating donors: A review of the European experience. Journal of Nephrology 16(3):334–341.
Kootstra G, Daemen JH, Oomen AP. 1995. Categories of non-heart-beating donors. Transplantation Proceedings 27(5):2893–2894.
Kootstra G, Kieveit J, Nederstigt A. 2002. Organ donors: Heartbeating and non-heartbeating. World Journal of Surgery 26(2):181–184.
Kowalski AE, Light JA, Ritchie WO, Sasaki TM, Callender CO, Gage F. 1996. A new approach for increasing the organ supply. Clinical Transplantation 10(6 Pt 2):653–657.
Lewis J, Peltier J, Nelson H, Snyder W, Schneider K, Steinberger D, Anderson M, Krichevsky A, Anderson J, Ellefson J, D’Alessandro A. 2003. Development of the University of Wisconsin donation after cardiac death evaluation tool. Progress in Transplantation 13(4):265–273.
Light JA, Kowalski AE, Ritchie WO, Gage F, Sasaki TM, Aquino A, Callender CO. 1996. New profile of cadaveric donors: What are the kidney donor limits? Transplantation Proceedings 28(1):17–20.
Lombardi G, Gallagher EJ, Gennis P. 1994. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. Journal of the American Medical Association 271(9):678–683.
Lynn J. 1993. Are the patients who become organ donors under the Pittsburgh protocol for ‘non-heart-beating donors’ really dead? Kennedy Institute of Ethics Journal 3(2): 167–178.
Magliocca JF, Magee JC, Rowe SA, Gravel MT, Chenault RH 2nd, Merion RM, Punch JF, Bartlett RH, Hemmila MR. 2005. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. Journal of Trauma 58(6):1095–1101.
Menikoff J. 1998. Doubts about death: The silence of the Institute of Medicine. Journal of Law, Medicine & Ethics 26(2):157–165.
Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK. 2005. Deceased-donor characteristics and the survival benefit of kidney transplantation. Journal of the American Medical Association 294(21):2726–2733.
Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. 2003. Expanded criteria donors for kidney transplantation. American Journal of Transplantation 3(Suppl 4): 114–125.
Modlin CS, Goldfarb DA, Novick AC. 2001. The use of expanded criteria cadaver and live donor kidneys for transplantation. Urologic Clinics of North America 28(4):1–30.
National Consensus Project Steering Committee. 2004. Clinical Practice Guidelines for Quality Palliative Care. [Online]. Available: http://www.nationalconsensusproject.org/Guideline.pdf [accessed March 20, 2006].
Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK. 2001. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. Journal of the American Society of Nephrology 12(3):589–597.
OPTN (Organ Procurement and Transplantation Network). 2006. National Data Reports. [Online]. Available: http://www.optn.org/latestData/step2.asp? [accessed March 29, 2006].
Pierre AF, Sekine Y, Hutcheon MA, Waddell TK, Keshavjee SH. 2002. Marginal donor lungs: A reassessment. Journal of Thoracic and Cardiovascular Surgery 123(3):421–427.
Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ. 2002. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74(9):1281–1286.
President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. 1981. Defining Death: Medical, Legal, and Ethical Issues in the Determination of Death. Washington, DC: U.S. Government Printing Office.
Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, Brown R Jr, Emond JC. 2005. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Annals of Surgery 242(4):556–565.
Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. 1968. A definition of irreversible coma. Journal of the American Medical Association 205(6):85–88.
Robertson J. 1999. The dead donor rule. Hastings Center Report 29(6):6–14.
Rudich SM, Kaplan B, Magee JC, Arenas JD, Punch JD, Kayler LK, Merion RM, Meier-Kriesche HU. 2002. Renal transplantations performed using non-heart-beating organ donors: Going back to the future? Transplantation 74(12):1715–1720.
Sanchez-Fructuoso AI, Prats D, Torrente J, Perez-Contin MJ, Fernandez C, Alvarez J, Barrientos A. 2000. Renal transplantation from non-heart-beating donors: A promising alternative to enlarge the donor pool. Journal of the American Society of Nephrology 11(2):350–358.
Sanchez-Fructuoso AI, Marques M, Prats D, Battientos A. 2003. Non-heart-beating donors: Experience from the Hospital Clinico of Madrid. Journal of Nephrology 16(3):387–392.
SCCM (Society of Critical Care Medicine). 2001. Recommendations for nonheartbeating organ donation. A position paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Critical Care Medicine 29(9):1826–1831.
Schnitzler MA, Whiting JF, Brennan DC, Lin G, Chapman W, Lowell J, Boxerman S, Hardinger KL, Kalo Z. 2003. The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation 75(12):1940–1945.
Sellers MT, Velidedeoglu E, Bloom RD, Grossman RA, Markmann JW, Naji A, Frank AM, Kass AB, Nathan HM, Hasz RD, Abrams JD, Markmann JF. 2004. Expanded-criteria donor kidneys: A single-center clinical and short-term financial analysis—cause for concern in retransplantation. Transplantation 78(11):1670–1675.
Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, Schkade L, Hunsicker L. 2003. Estimating the number of potential organ donors in the United States. New England Journal of Medicine 349(7):667–674.
Spike J. 2000. Controlled NHBD protocol for a fully conscious person: When death is intended as an end in itself and it has its own end. Journal of Clinical Ethics 11(1):73–77.
Stratta RJ, Rohr MS, Sundberg AK, Armstrong G, Hairston G, Hartmann E, Farney AC, Roskopf J, Iskandar SS, Adams PL. 2004. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Annals of Surgery 239(5):688–697.
Sung RS, Guidinger MK, Lake CD, McBride MA, Greenstein SM, Delmonico FL, Port FK, Merion RM, Leichtman AB. 2005. Impact on the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation 79(9):1257–1261.
Tan JC, Alfrey EJ, Dafoe DC, Millan MT, Scanding JD. 2004. Dual-kidney transplantation with organs from expanded criteria donors: A long-term follow-up. Transplantation 78(5):692–696.
UNOS (United Network for Organ Sharing). 2005. UNOS Policy 3.5.1. [Online]. Available: http://www.unos.org/PoliciesandBylaws2/policies/pdfs/policy_7.pdf [accessed March 13, 2006].
Weber M, Dindo D, Demartines N, Ambuhl PM, Clavien PA. 2002. Kidney transplantation from donors without a heartbeat. New England Journal of Medicine 347(4):248–255.
Weill D. 2002. Donor criteria in lung transplantation—an issue revisited. Chest 121(6):2029–2031.
Weill D, Dey GC, Hicks RA, Young KR Jr, Zorn GL Jr, Kirklin JK, Early L, McGiffin DC. 2002. A positive donor Gram stain does not predict outcome following lung transplantation. Journal of Heart and Lung Transplantation 21(5):555–558.
WHC (Washington Hospital Center), MRI (Medlantic Research Institute), WRTC (Washington Regional Transplant Consortium. 1993 (October 7–8). Consensus Conference on Trauma Victims and Organ Donation. Consensus Report. Washington, DC: WHC.
Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, Lowell JA, First MR, Brennan DC, Schnitzler MA. 2000. Economic cost of expanded criteria donors in cadaveric renal transplantation: Analysis of Medicare payments. Transplantation 70(5): 755–760.
Wijdicks EFM, Diringer MN. 2004. Electrocardiographic activity after terminal cardiac arrest in neurocatastrophes. Neurology 62(4):673–674.
















































