2
Utilizing Chemical Imaging to Address Scientific and Technical Challenges: Case Studies
This chapter provides a series of real-life “case studies” to help illustrate a grand challenge for chemical imaging. Before presenting the case studies, the grand challenge and a brief introduction to imaging techniques are discussed. More technical information about specific imaging techniques is provided in greater detail in Chapter 3.
A GRAND CHALLENGE FOR CHEMICAL IMAGING
Chemical imaging helps us to answer difficult questions, especially when these questions occur in complex chemical environments. At present, imaging lies at the heart of our high-technology industry in terms of process development and quality control. The ability to image the interior of the human body with techniques such as ultrasound and magnetic resonance imaging (MRI) has revolutionized medical diagnosis and treatment. Satellite imaging is now an indispensable tool in climate prediction and modeling. Use of remote imaging is crucial to our national security. Our capability to image will in many ways define our scientific, technological, economic, and national security future.
Clearly, advances in chemical imaging capabilities will result in more fundamental understanding of chemical processes. In this chapter, chemical imaging is addressed in the context of an overarching goal to understand and control complex chemical processes.
UNDERSTANDING AND CONTROLLING COMPLEX CHEMICAL PROCESSES
Understanding and controlling complex chemical processes requires the ability to perform multimodal imaging across all length and time scales. That is, researchers would like the capability to image a material or a process using multiple techniques, including those that can “focus” on a particular aspect of the material or process (through varying length scales), as well as capture images at appropriate time dimensions to acquire necessary information.
An overarching objective for future breakthroughs using chemical imaging techniques is to gain a fundamental understanding and control of these complex chemical structures and processes. While this is the grand challenge for chemical imaging, more specific requirements need to be addressed in order to meet this comprehensive challenge. These include: understanding and controlling self-assembly, complex biological processes, and complex materials. Each of the challenges is amplified further below.
Understanding and Controlling Self-Assembly
The self-assembly of small molecular units into larger structures is a common and important occurrence in nature. In the biological realm, proteins and RNA fold into specific functional conformations. Cells divide and communicate with each other by rearranging subcellular units. Some theorists hypothesize that the spontaneous formation of lipid vesicles is responsible for the beginning of life. Outside biology, we marvel at the growth of snowflakes. We find numerous uses for soap and liquid-crystal displays. We make materials with varying properties by tuning the degree and the nature of aggregation. Indeed, many proposed methods for creating nanomaterials are based on self-assembly.
Molecular assemblies are formed through strong and weak chemical forces. Understanding the types, magnitudes, directions, and distances associated with these interactions is thus of fundamental and practical importance. Chemical imaging can elucidate many of these processes by providing spatial and temporal relationships among the interacting units. We would like, at one extreme, to follow the rotation, formation, and breakage of individual bonds and, at the other, to investigate cooperative effects and sequences of events over extended domains. The same or different small assemblies can be tracked as they grow into larger assemblies. In addition, chemical transformations within these structures can be monitored to elucidate environmental effects on reactivity and ultimately can be controlled by the patterned exposure to electromagnetic radiation and other fields.
To gain better understanding of and to control molecular assembly processes, one needs chemical imaging techniques that can follow interactions at a broad range of length and time scales. During assembly, it would be advantageous to record inter- and intramolecular orientations and distances at picosecond to second
time scales, measure the forces between selected pairs of atoms or between selected molecular domains, and detect proximal versus long-range ordering of complexes. Once the self-assembly process is complete, imaging could be employed to follow single-molecule reactions within these structures. There are a few published examples of the monitoring of DNA synthesis and hybridization. However, major advances in imaging tools will be required to tackle the whole spectrum of molecular self-assembly processes.
Understanding and Controlling Complex Biological Processes
In the postgenomic era, there is a pressing need to functionally annotate the products of the many sequenced genes whose functions are unknown. Exploring the proteome represents a mammoth task. Although the number of genes encoded in the genomes of higher organisms has turned out to be fewer than originally thought (tens of thousands for mammals), the complexity introduced during cell development and gene expression is enormous. Combinatorial reorganization of gene fragments during immune cell development, alternate splicing pathways after transcription, and posttranslational modification of proteins and the resulting chemical heterogeneity result in millions of functionally distinct protein species. Add to this the extensive interplay of the many metabolic intermediates and connected pathways and the complexity increases even farther. This inherent complexity and heterogeneity, which is in many respects the hallmark of a living system, puts very serious limits on the utility of traditional biochemical methodologies that are based on the separation and isolation of components. A cell is much more than a list of gene products and small molecules. Just as important as the chemical formula of each component is a detailed understanding of where it is, at what time, and with what partners. Although generating a complete four-dimensional map of cellular (and ultimately organismal) complexity at the molecular level is currently beyond our capability, this is the long-range goal of chemical imaging in the realm of biology. Clearly, much has to be done to achieve this goal, but many of the fundamental concepts and tools have been or are being developed now.
From low-energy radio waves that tickle the states of nuclei, to infrared light that captures the nature and energies of chemical bonds, to visible light that probes electronic structure, to high-energy X-rays and electrons that report on electron density, spectroscopy provides detailed information and generally does so in a spatially and temporally patterned way. Scanning probe microscopy, while still largely an in vitro approach, adds an additional dimension in which mechanical and electrical probes can be applied directly. Everything from whole organisms to individual biomolecules has been imaged with these kinds of techniques. The challenge now is to come to grips with the chemical, spatial, and temporal heterogeneity involved—monitoring many molecules, molecular species, or whole cells simultaneously and thereby determining in detail the complex interactions and
networks that are the chemical essence of life. Thus, there are issues of scale and a dramatic need for multiscale approaches that allow one to place the chemistry within the context of the overarching biological system.
As our ability to probe with high resolution has improved, a number of researchers have begun to consider reversing the direction of information transfer, using the same concepts and tools inherent in chemical imaging to project information into biological systems, thereby controlling their function. A somewhat crude example of this approach is laser surgery in which specific cells, or even small parts of cells, are ablated with a focused laser beam. A more sophisticated approach that has recently become possible is to specifically turn genes on or off with light, giving complete control of gene expression within a population of cells as a function of both space and time. In general, the concept of refitting our molecular imaging probes to become “full-duplex” molecules, functioning both to report on the environment that surrounds them and to manipulate that environment in an externally controlled way, is an idea that is just taking form and provides new vistas both for fundamental research in biology and for environmental, medical, and synthetic applications.
Understanding and Controlling Complex Materials
In a high-tech society, the quality of life, economic potential, and security often rest on its ability to predict and control the properties of materials. These properties can range from the common (porous, dielectric, high-strength, magnetic, chemically reactive) to the exotic (superconductivity, superlattice, superfluidity, giant magnetoresistance). In complex materials, these properties, both exotic and common, are generally determined and controlled by the degree of coupling between the components that make up the material and their resulting level of complexity (e.g., chemical and physical heterogeneity, composition, phase, morphology). Often, the degree to which we can successfully harness a particular property or phenomenon into new technologies is based largely on our knowledge and understanding of material systems at or below the size scale of the constituents and components that constitute them. For example, the discovery of new physical phenomena such as superconductivity is only the first step in what can be a long process to bring a discovery to technological relevance or commercialization. While the properties of superconductivity hold the promise of revolutionizing everything from transportation to medicine, the technological and economic impacts will go unrealized without advances in our understanding of and improvements in superconducting materials. However, progress in understanding these systems has been hampered by the absence of chemical imaging and dynamics tools that can provide nondestructive, real-time, three-dimensional imaging with relevant resolution.
In all types of materials, phenomena such as fracture, creep, segregation, roughening, and delamination ultimately determine the utility of a material for a
given application. For example, by controlling the onset of fracture, a potato chip bag can be an effective, high-strength, low-porosity container that keeps chips from going stale, while at the same time allowing a child to rip open (fracture) the package with ease. Phenomena such as fracture mechanics are useful but not always well understood; as a consequence, these and other materials advances come by way of much trial and error. This is due in large part to the lack of suitable analytical instrumentation that can image over several length scales such things as the formation of stress (morphology contrast) and the resulting phenomena (fracture). While improved performance (e.g., high directional strength) is often an important driver in technology development, materials advances that make the technology affordable and more durable offer value and motivation as well.
In addition, complex materials can comprise several unique components (metals and nonmetals, liquids and solids, magnetic and nonmagnetic materials) that, when combined, generate a material whose properties are altered or totally distinct from those of the original. An example of this is the thin-film material systems that exhibit giant magnetoresistivity (GMR). Any of the thin films acting alone would exhibit no unique or exotic properties. However, when several materials are combined in a precise manner, the phenomenon of GMR is observed, and high-density data storage is realized.
Through advances in chemical imaging capability, we will increase both our basic understanding of the phenomena that determine the utility of complex materials and our ability to control or “tune” a material’s properties. In this way, we will go from using the inherent properties of traditional material, (e.g., the strength of steel) to programming particular properties, such as low weight and high strength, into engineered materials that are tailored for a given application.
IMAGING TECHNIQUES
The development of multiple imaging techniques provides researchers with powerful tools to probe multiple aspects of chemical problems. A more detailed discussion of these techniques is provided in Chapter 3; however, the techniques are introduced briefly here.
Optical Techniques and Magnetic Resonance
Techniques employing the ultraviolet (UV), visible, and near-infrared parts of the spectrum have the advantage of high sensitivity (single photon), high time resolution (femtoseconds), and moderate spatial resolution (on the order of 100 nm). Structural information is obtainable by infrared to radio-frequency techniques (e.g., magnetic resonance). Together, these techniques have enabled the visualization of individual molecules and the measurement of excited state dynamics from such molecules on the picosecond time scale. It is also possible to follow the time course of chemical reactions on the femtosecond time scale when
whole populations can be synchronized by light. Confocal detection and nonlinear excitation have made it possible to follow the dynamics of complex chemical systems (such as cells and tissues) using multiple probes and in three dimensions. As a whole, these technologies have also made it possible to optically pattern chemical reactivity with very high spatial resolution in three dimensions. Imaging well below the surface of an object (e.g., deep tissue imaging) remains a challenge in optical spectroscopy, but could be substantially improved with the production of labels absorbing and/or emitting farther to the red.
Vibrational imaging using Raman scattering and infrared (IR) absorption provides something like a structural “fingerprint” of matter as it is determined by the kinds of atoms, their bond strengths, and their arrangements in a specific molecule. Recent developments based on a combination of modern laser spectroscopy, scanning probe techniques, and nanotechnology provide capabilities for sensitive vibrational imaging at the single-molecule level. These developments also provide capabilities at nanoscale lateral resolution, where linear and nonlinear Raman scattering is exploited in enhanced and strongly confined local optical fields of tailored nanostructures.
Electron Microscopy, X-rays, Ions, and Neutrons
With wavelengths that are about 1,000 times smaller than that of visible light, electrons provide a high-resolution probe of chemical and structural information below surfaces of materials. Images of atomic arrangements over a large range of length scales can be obtained using electron microscopy (EM) techniques. Although significant limitations to their use exist (e.g., the need for a vacuum to produce and transmit electrons, electron beam damage to samples), EM techniques have had a tremendous impact on fields ranging from condensed matter physics to structural biology.
X-rays are able to penetrate materials more deeply than visible light or electrons and make it possible to determine the identity and local configuration of all the atoms present in a sample. Using X-rays, it is possible to image almost every conceivable sample type and gain unique insights into the deep internal molecular and atomic structure of most materials from objects as large as a shipping container to those significantly smaller than the nucleus of a single cell.
Proximal Probes (Force Microscopy, Near Field, Field Enhancement)
Proximal probe microscopes employ a variety of materials such as tungsten wire (scanning tunneling microscopy), silicon nitride pyramid and cantilever (atomic force microscopy), or optical fiber (near-field optical microscopy) in close proximity to the sample of interest for the purposes of recording an image of the sample, performing spectroscopic experiments, or manipulating the sample. All such methods were originally developed primarily for the purpose of obtaining
the highest possible spatial resolution in imaging experiments. Since then, many other unique advantages of these techniques have been realized. These methods are especially useful for understanding the chemistry of surfaces—for example, the electrophilicity of individual surface atoms, the organization of atoms or molecules at or near the surface, and the electronic properties of atomic or molecular assemblies.
Processing, Analysis, and Computation
Processing, analysis, and computation are not imaging techniques per se but, rather, play a fundamental role in enhancing their capabilities. In addition, computational methods, particularly when applied to computer modeling and simulation, extend imaging capabilities to address problems that have not or cannot be addressed using standing imaging techniques.
CASE STUDIES
A series of real-life “case studies” is presented to illustrate the importance of the technical issues that have been introduced in this chapter and show how chemical imaging can contribute to understanding them. The examples are not meant to serve as an exhaustive list of all problems that can be addressed with advances in chemical imaging. Instead, they have been included to focus on current capabilities and limitations and offer insights into where breakthroughs are needed to increase the capabilities and potential for chemical imaging. More technical information about specific imaging techniques is provided in greater detail in Chapter 3.
Case Study 1:
Mobile Crystalline Material-41 (MCM-41)
MCM-41 is an interesting self-assembled material1 that has a wide range of applications. The starting material is a monomeric surfactant, cetyltrimethyl-ammonium bromide (CTAB), similar to those used as detergents. With a long hydrophobic end and a hydrophilic head, the monomers form spherical micelles that have a hydrophobic core and a hydrophilic surface when the concentration of the monomer surpasses the critical micellar concentration. At higher concentrations, the micelles rearrange into cylindrical rods. At still higher concentrations, the rods self-assemble into hexagonal arrays. After the introduction of silicate molecules, the arrays form silica particles that possess well-defined shapes. By adding agents that alter the hydrophobicity or hydrophilicity of various parts of the structures, one can control each step of the self-assembly process. The result is a mesoporous structure with tunable pore size, variable channel length, and predictable shape (Figure 2.1 and Figure 2.2).2
MCM-41 has been employed as an industrial catalyst for many years. The assembled structure is pyrolized to become a permanent inorganic matrix.
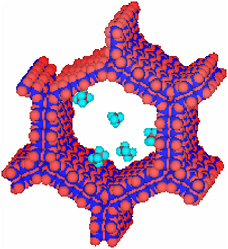
FIGURE 2.1 MCM-41 units are formed from self-assembly to create honeycomb structures that can be functionalized on the inside (light blue) to create confined catalytic sites.
SOURCE: Courtesy of Victor S. Lin, Iowa State University.
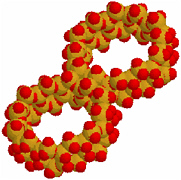
FIGURE 2.2 These honeycomb units further assemble into larger structures that can include worms, spheres, ovals, and so on, depending on preparative conditions.
SOURCE: Courtesy of Victor S. Lin, Iowa State University.
Functionalization of the matrix allows incorporation of a variety of catalytic activities into the material. Recently, procedures were developed to add functional groups that are electrostatically or hydrophobically attractive to the ammonium surfactant head groups and are able to compete with silicate anions during self-assembly. This has led to a class of mesoporous materials that are functionalized only on the inside of the pores. Highly selective polymerization and cooperative catalytic systems have been developed from these materials.3 Furthermore, by incorporating caps onto the pores, chemical reagents can be stored in the channels,
to be released simply by detaching the caps at the desired time and location.4 This scheme holds promise as a controlled drug and gene delivery protocol.
Chemical Imaging Technique(s) Involved
Current methods used to image MCM-41 include (1) analytical transmission electron microscopy (TEM) to determine structure, size, morphology, and local chemical composition; (2) energy-dispersive X-ray spectroscopy (EDXS) in a scanning electron microscope (SEM) to determine chemical composition;5 and (3) electron energy loss spectroscopy (EELS) for elemental analysis.6
Insights Obtained Using Chemical Imaging
The spatial and temporal progression of individual events involved in the formation of each type of structure can be monitored directly. A combination of imaging modes can be applied, each elucidating the process at a different length scale. Millimeter-scale variations can then be explained by nanometer-scale fluctuations. After the structures are built, single-molecule imaging can be employed to study catalytic reactions inside the nanopores.
Imaging Limitations
Limitations include the following:
-
The imaging rate of current technologies is not fast enough for continuous monitoring of microsecond transformations.
-
Single-molecule imaging techniques are not yet capable of monitoring several different chemical species simultaneously.
-
There is a lack of technologies for imaging the length scale between optical microscopy (diffraction limit) and proximal probes.
-
The need for chemical derivatization for fluorescence imaging often limits accessibility.
Opportunities for Imaging Development
Opportunities to develop imaging techniques for this application would include the following:
-
Optical imaging at microsecond to nanosecond time scales per consecutive image
-
One instrument for imaging the entire length scale from nanometers to millimeters
-
Single-molecule imaging without fluorescence labeling
Case Study 2:
Organic Electronics
Organic materials are now being employed as the active components in electronic circuitry. Perhaps the best examples of such materials are the semiconducting polymers used in polymer-based light-emitting diodes (polymer-LEDs). As a result of the successful development of extremely pure polymeric materials, polymer-LEDs are now being incorporated into commercially available display devices. Emerging applications of small-molecule, oligomeric, and polymeric organic semiconductors include their use in photovoltaics (solar cells) and organic field effect transistors. The primary benefits of such materials include the ability to manufacture moldable, flexible materials for use in large-area devices. Importantly, such materials can easily be cast as thin films, offering the potential for significant cost reductions in comparison to traditional inorganic devices.
Microscopic imaging experiments have played a key role in the development of these organic material devices and have provided detailed information on the local chemical and physical properties. They have helped researchers better understand intermolecular interactions, molecular organization within nanometer scale (and larger) domains, electronic coupling between individual molecules in the aggregate, and the mechanisms of electrical charge generation, injection, transport, and recombination. Microscopic methods will continue to provide vital information on molecular- to micrometer-length scales for both existing and emerging materials. Examples are shown below (Figures 2.3-2.5, respectively): a
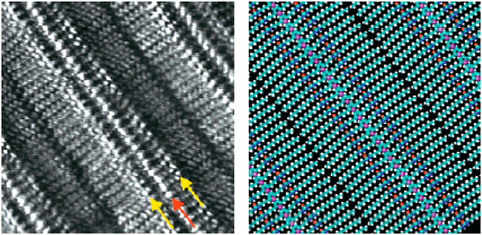
FIGURE 2.3 Left: urea-substituted thiophenes on a graphite surface. Right: a model.
SOURCE: Reprinted with permission from Gesquiere, A., M.M.S. Abdel-Mottaleb, S. De Feyter, F.C. De Schryver, F. Schoonbeek, J. van Esch, R.M. Kellogg, B.L. Feringa, A. Calderone, R. Lazzaroni, and J.L. Bredas. 2000. Molecular organization of bis-urea substituted thiophene derivatives at the liquid/solid interface studied by scanning tunneling microscopy. Langmuir 16:10385-10391. Copyright 2000 American Chemical Society.
FIGURE 2.4 Honeycomb structure formed from block copolymer films.
SOURCE: Reprinted from de Boer, B., U. Stalmach, P. F. van Hutten, C. Melzer, V.V. Krasnikov, and G. Hadziioannou. 2001. Supramolecular self-assembly and opto-electronic properties of semiconducting block copolymers. Polymer 42: 9097-9109. Copyright 2001 with permission from Elsevier.
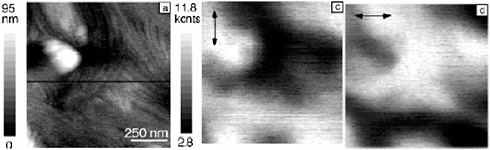
FIGURE 2.5 NSOM topography and polarized luminescence from poly(dihexylfluorene) (an organic semiconductor) film.
SOURCE: Reprinted with permission from Teetsov, J.A. and D.A. Vanden Bout. 2001. Imaging molecular and nanoscale order in conjugated polymer thin films with near-field scanning optical microscopy. J. Am. Chem. Soc. 123:3605-3606. Copyright 2001 American Chemical Society.
scanning tunneling microscopy (STM) image of and model for organized thiophene monolayers deposited on a graphite surface; an image of organized honeycomb structures formed in a film prepared from block copolymers of poly(phenylene vinylene)-poly(styrene) showing micrometer-scale phase separation of the component polymers as seen by fluorescence microscopy and SEM (inset); and near-
field scanning optical microscopy (NSOM) topography and polarized fluorescence excitation images of annealed poly(fluorene) films showing aggregated fibrous film structures.
Chemical Imaging Technique(s) Involved
Imaging of organic electronics employs conventional fluorescence and confocal microscopies, single-molecule spectroscopy,7 scanning and transmission electron microscopies,8 and several different proximal probe techniques.9,10 Optical microscopies provide direct information on spatial variations in the spectroscopic properties of the materials (i.e., due to aggregation), along with evidence for variations in the chemical composition and valuable data on their photochemical reactivities and molecular photophysics. Electron microscopy provides valuable information on nanometer and larger structures patterned within their films. Force microscopy and STM provide valuable data on organization and electronic structure on angstrom-to nanometer-scale distances, while near-field optics provides high resolution spectroscopic data with sub 50-nm spatial resolution and subnanosecond time resolution.
Insights Obtained Using Chemical Imaging
As shown in the above figures, chemical imaging has provided detailed information on molecular (self)-organization in these materials, as well as on overall film morphology and the quality of structures templated or lithographically prepared in their films. Chemical imaging methods have also provided detailed information on the optical properties of these materials, allowing a deeper understanding of the influences of inter- and intramolecular electronic coupling,11,12 and on charge carrier dynamics and trapping.13,14,15
Imaging Limitations
Direct chemical information with resolution on molecular length scales cannot yet be obtained on functioning devices or even on samples closely approximating functional materials. The vast majority of high-spatial-resolution images that have been recorded have been obtained on specially prepared samples consisting of single molecular layers on well-ordered substrates. The covering electrodes and ancillary films used in functional devices also routinely present a problem in the imaging of such materials because high-resolution proximal probes cannot then be used to image their internal surfaces directly. Although conventional optical imaging methods can “see below the surface” in such samples, the resolution of these techniques is insufficient to access direct molecular information.
Opportunities for Imaging Development
Development of new imaging methods that can probe the properties of functional and even functioning16 devices with high (molecular-scale) resolution is necessary to fully understand the organizational properties of these materials, how molecular organization influences device performance, and the detailed chemistry by which such devices fail over time. An important challenge here involves the development of methods that can image beneath the electrodes between which the materials are sandwiched, with depth discrimination capabilities. A further challenge involves implementation of techniques that provide clear chemical information (i.e., Raman, IR, or other vibrational imaging techniques) in these same imaging modalities, without sacrificing spatial resolution. Finally, for the preparation of ultimate device structures, advanced lithographic procedures based on some of these same microscopic methods will be required for the controlled fabrication of molecular architectures with optimal optical and electronic properties.
Case Study 3:
Imaging Alzheimer’s Disease: Chemical and Molecular Imaging of the Brain from Molecules to Mind
In the past ten years, we have made significant progress in our understanding of how the brain functions both in health and in disease. Much of this progress comes from discoveries of how to apply powerful existing technologies toward imaging the brain. Mass spectrometry and electron microscopy, for example, have been employed on brain tissue sections for the respective purposes of mapping on the micrometer scale both inorganic and organic compounds such as calcium, phospholipids, and proteins. These methods have also been used to image molecular events in structures as small as synapses. Novel imaging techniques have been developed for use on live specimens. In neurons, for example, it is possible to simultaneously image several cellular processes, such as calcium- and zinc-signaling pathways, through the use of multiphoton fluorescence spectroscopy. Another example is the now-routine clinical use of positron emission tomography (PET) and MRI spectrometers for low-resolution brain imaging on the millimeter scale. These instruments aid the diagnosis of lesions induced by strokes or the precise localization of gliomas. The ability to map areas of the brain that are active during complex tasks with functional MRI is revolutionizing cognitive psychology. Indeed, a whole field has developed within radiology called molecular imaging. Molecular imaging is the result of using traditional radiological imaging tools combined with the knowledge of specific biological processes gleaned from molecular biology to increase the range of information available from imaging. Results from chemical imaging are a key component in the development of molecular imaging.
Almost every problem faced in trying to understanding the normal functioning of the brain and pathophysiological processes in the brain requires the following steps. First, information must be acquired about the detailed structure and composition of key molecules in the brain. Second, by using this information, the dynamics of cell structure and function must be inferred. Finally, these inferences must then be integrated into an understanding of the complex functioning of the brain. Nowhere are the challenges greater than in trying to understand neurodegenerative diseases such as Alzheimer’s disease. Alzheimer’s is rapidly growing into a major public health problem in developed countries as their populations age. Early detection is critical, and currently, confirmation of the diagnosis relies on autopsy to detect the amyloid plaques that are the telltale sign of the disease. The detailed formation of amyloid plaques is critical to understanding and treating the disease. Finally, the detailed cellular pathology and ensuing effects on brain function are only beginning to be delineated. Thus, chemical imaging is critical to understanding Alzheimer’s due to the need to determine molecular structure, cell structure, and communication and to integrate these into obtaining information nondestructively from the human brain.
Advances in chemical imaging techniques are enabling new information to be obtained about Alzheimer’s across the full range of distance scales required. Detailed three-dimensional structures of amorphous solids that defy crystallization, such as amyloid plaques, are particularly challenging to characterize. Models for the structure have recently been deduced from solid-state nuclear magnetic resonance (NMR) studies (Figure 2.6). Detailed studies of the formation of amyloid plaques and their effect on specific neuronal structures nearby can be accomplished with multiphoton fluorescence imaging tools (Figure 2.7). New PET probes that bind specifically to amyloid plaques are promising candidates for quantifying plaque burden noninvasively (Figure 2.8). Finally, MRI can be used to show anatomical changes in the areas of the brain most affected (such as the hippocampus) as well as highlight the changes in brain function that occur in these areas during the disease (Figure 2.9).
Chemical Imaging Technique(s) Involved
A number of chemical imaging techniques are being utilized to understand Alzheimer’s disease. Nondestructive imaging techniques such as MRI and PET are at the early stages of measuring amyloid plaque and changes in brain anatomy and function that occur with disease progression. Optical imaging is important to study nondestructively the development of the disease and changes in cellular structure that occur in animal models. The full range of in vitro chemical imaging techniques (e.g., electron microscopy, mass spectrometry imaging) has been used to chemically and structurally characterize the disease at high resolution. Finally, magnetic resonance methods and X-ray crystallography for structure determina-
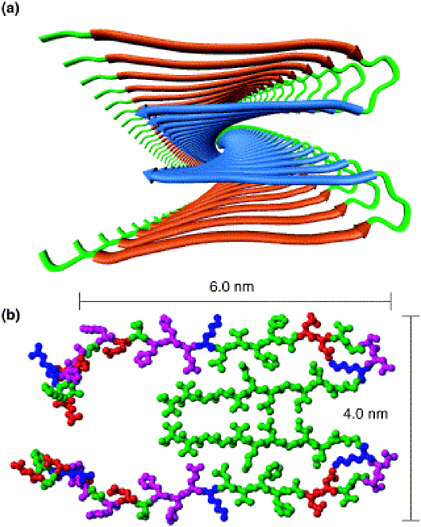
FIGURE 2.6 Solid-state NMR of the molecular structure of amyloid plaque. Model of the minimal structural unit of amyloid fibrils based primarily on solid-state NMR data. (a) Ribbon diagram of the parallel beta-sheet structure. (b) Atomic representation of the structure with colors representing side chain type (green, hydrophobic; magenta, polar; red, negatively charged; blue positively charged).
SOURCE: Reprinted from Tycko, R. 2004. Progress towards a molecular-level structural understanding of amyloid fibrils. Curr. Opin. Struct. Biol. 14:96-103. Copyright 2004, with permission from Elsevier.
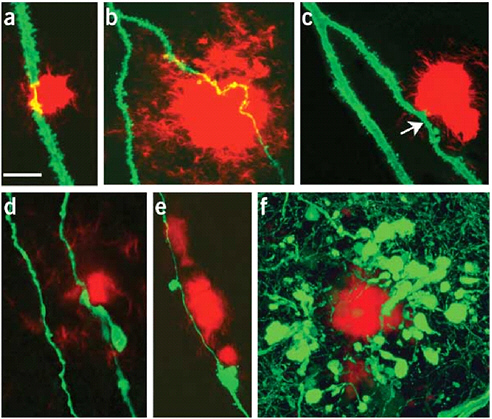
FIGURE 2.7 Two-photon fluorescence microscopy of amyloid plaque (red) and surrounding neurons (green) in the brain of a mouse model of Alzheimer’s. Numerous neuronal abnormalities, including swelling and decreased densities of spines (arrow in c), could be detected.
SOURCE: Tsai, J., J. Grutzendle, K. Duff, and W.B. Gan. 2004. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 7:1181-1183.
tion are required to understand the structure of precursors and the structure of amorphous, amyloid plaque.
Insights Obtained Using Chemical Imaging
A large amount is now known about the location of plaque formation in the brain, the pathophysiology of Alzheimer’s disease, the molecular and cellular basis of the disease, and the genetic basis of the disease. All of these developments have relied on the use of chemical imaging techniques.
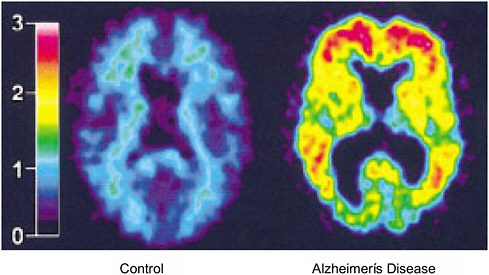
FIGURE 2.8 PET measurement of amyloid plaque in the human brain. A positron-emitting compound, Pittsburgh Compound B (PIB), binds specifically to amyloid plaque and can be used to image Alzheimer’s disease using PET. The image on the left is the brain of a normal person, and the blue colors indicate little accumulation of PIB. The image on the right is the brain of a person with Alzheimer’s disease, and the yellow to red colors indicate large accumulations of PIB and thus the presence of amyloid plaque.
SOURCE: Klunk, W.E., H. Engler, A. Nordberg, Y. Wang, G. Blomqvist, D.P. Holt, M. Bergstrom, I. Savitcheva, G.F. Huang, S. Estrada, B. Ausen, M.L. Debnath, J. Barletta, J.C. Price, J. Sandell, B.J. Lopresti, A. Wall, P. Koivisto, G. Antoni, C.A. Mathis, and B. Langstrom. 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55:306-319. Printed with permission from John Wiley & Sons, Inc.
Imaging Limitations
Despite all of the advances, we are still not able to give a definitive diagnosis or prognosis to individuals afflicted with Alzheimer’s disease. In addition, it is a major hurdle to test a large number of potential drugs that may be effective in slowing the progression. Two major challenges for chemical imaging to make more rapid progress are:
-
Development of approaches that give cellular-level resolution, nondestructively, in the human brain.
-
Development of noninvasive strategies that give chemical and structural information at the same level of detail and quality as that obtained from taking samples from living tissue.
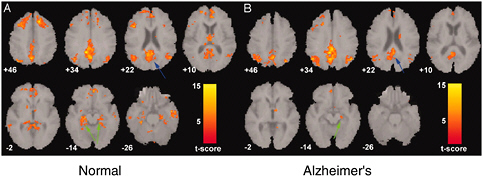
FIGURE 2.9 Functional MRI of resting or default-mode brain activity in normal and Alzheimer’s patients. Functional MRI detects fluctuations in brain activity at rest. A network of brain regions is activated in normal elderly people (left, A) as indicated by the orange-yellow regions overlaid on the MRI. This network is called the default-mode network and is altered in people with Alzheimer’s (right, B). In particular, activity in the hippocampus and entorhinal cortex is decreased (green arrows) in Alzheimer’s.
SOURCE: Greicius, M., G. Srivastava, A. Reiss, and V. Menon. 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad Sci. U.S.A. 101:4637-4642. Copyright 2004 National Academy of Sciences, U.S.A.
Opportunities for Imaging Development
Major progress can be achieved in the application of chemical imaging to Alzheimer’s disease and a wide range of other diseases with basic development of the imaging modalities. In particular, emphasis on increasing sensitivity and resolution, especially of noninvasive imaging modalities such as MRI, will enable images of the brain at resolutions comparable to histology. Along with the development of basic imaging technologies, it is critical to develop new imaging agents that will give greater chemical, molecular, and cellular specificity to imaging techniques. In particular, the development of optical imaging probes for detailed studies of animal models and new MRI and PET agents for human studies will greatly expand the capabilities of chemical imaging for the human brain.
Case Study 4:
Nonsense Suppression Techniques for Unnatural Amino Acids in Genetic Encoding
Since experimental biology often requires that many proteins be tagged in parallel during the course of a single assay or experiment, it benefits from conjugation strategies that are simple, reliable, and easily applied to many distinguishable proteins in parallel. Nonsense suppression17 techniques for the incorporation
of unnatural amino acids into proteins (Figure 2.10) are important here because they combine the specificity of placement conferred by genetic encodability with the flexibility of multiple organic and inorganic markers. At present, these tools are well developed in bacteria and yeast, but they are at the proof-of-principle stage in higher eukaryotic cells. Support for efforts to develop these tools for use in mammalian cells would be a prudent investment and would allow the more efficient conversion of newly discovered enzymes into functional reporters that can be imaged at high resolution by fluorescence microscopy.
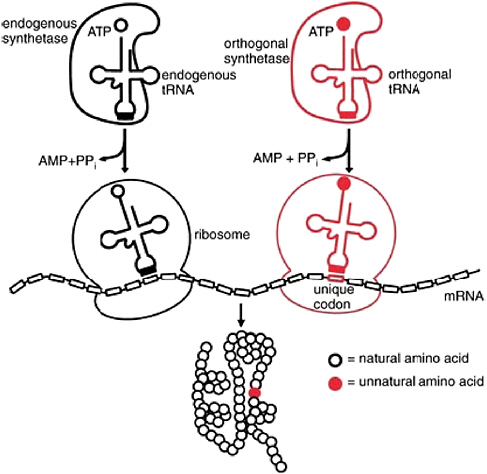
FIGURE 2.10 A general approach for site-specific incorporation of unnatural amino acids into proteins in vivo. NOTE: AMP = adenosine 5′ monophosphates; ATP = adenosine 5′-triphosphate; PPi = pyrophosphate.
SOURCE: Wang, L., and P.G. Schultz. 2005. Expanding the genetic code. Angew. Chem. Int. Ed. 44:34-66.
Chemical Imaging Technique(s) Involved
Bright fluorescent protein markers and accurate optical imaging provide nanoscopic spatial resolution over many orders of temporal magnitudes ranging from microseconds to several minutes.
Insights Obtained Using Chemical Imaging
It is possible to understand the biochemical dynamics of life processes by detecting and tracking individual macromolecules in living cell membranes and tissues.
Imaging Limitations
The limiting difficulty in utilizing the powerful approach to protein labeling for multiphoton chemical imaging is that higher cellular systems protect themselves by mechanisms of genetic nonsense suppression. To inhibit this barrier, it is necessary to devise means to inhibit natural nonsense suppression. Excellent but limited progress made in major laboratories18,19 has demonstrated the power of these methods for chemical imaging of dynamic molecular processes in living systems in cells. However, broadening the applicability of these significant improvements calls for focused research efforts to reach broad applicability.
Opportunities for Imaging Development
High-resolution chemical imaging methods utilizing these diverse fluorescent markers would strongly enhance capability in analyzing molecular patterns, mobility, and interactions important in biological and materials science research.
Case Study 5:
Imaging Nanochannels in Microfluidic Devices20
Devices based on biomolecular separation in nanochannels are expected to accelerate drug discovery, rapid diagnosis and treatment of disease, and development of vaccines. Integrated microfluidic devices are currently used to automate the generation and analysis of chemical compounds. Chemical analyses on microfluidic devices can be highly automated and can reduce the consumption of reagents by several orders of magnitude. In microchannels, the electroosmotic flow can be controlled using field effects and surface modification, but direct electrostatic manipulation of ions across the microchannels is not possible. Shrinking the dimensions of the channels down to nanometers allows direct ionic or molecular manipulation using surface charges or field effects, because the channel width then approaches the molecular diameter.
Charged solutes in electrolyte solutions that are electrokinetically driven through channels with nanoscale widths exhibit unique transport characteristics that may enable rapid and efficient separations under a variety of physiological and environmental conditions. Many biomolecules, including DNA, proteins, and peptides, are charged or can be complexed with charged surfactant molecules. Manipulating the velocity of biomolecules by variation in flow pressure or electric fields in channels of nanoscopic widths will enable efficient separations that are not possible in micro- or macroscopic channels.
Silicon-based T-chips integrate an array of parallel nanochannels with microchannels and macroscopic injection ports (Figure 2.11A). These T-chips allow the electrokinetic transport of fluorescent dyes in nanochannels to be characterized. The width of the nanochannels ranges from 35 to 200 nm, while the depth is sufficient to allow significant molecular throughput. A cross-sectional scanning electron micrograph of a small number of nanochannels in one of the T-chips is shown in Figure 2.11B. In this chip, the channels are approximately 50 nm wide by 500 nm deep and are on a 400 nm pitch. The channels are etched into a silicon wafer, which is then oxidized to present a silicon dioxide (SiO2) surface to the fluid.
Transport of molecules through the nanochannels is studied by using confocal microscopy to monitor fluorescence from the dye molecules in the fluid. Figure 2.12 shows laser-induced fluorescence micrographs that demonstrate the difference in transport of two dyes in channels with ~50 nm (left) and ~200 nm
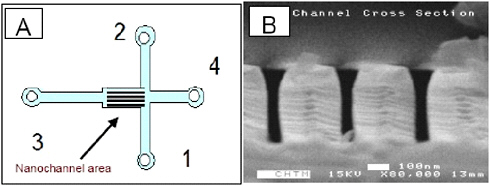
FIGURE 2.11 (A) Top view (schematic) of the integrated chips. (B) SEM image of the cross section of the nanochannel array (50 nm wide nanochannels) in a chip.
SOURCE: Courtesy of the Cancer Research Microscopy Facility, University of New Mexico Hospital; the W.M. Keck foundation; and the following individuals: Anthony L. Garcia, Linnea K. Ista, Dimiter N. Petsev, Michael J. O’Brien, Paul Bisong, Andrea A. Mammoli, Steven R.J. Brueck, and Gabriel P. Lopez.
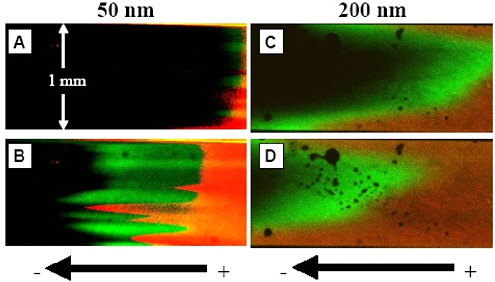
FIGURE 2.12 Sample two-color fluorescence micrographs (green = Alexa 488, red = rhodamine B) showing separation of dyes in nanochannel arrays containing channels ~50 nm wide at (A) time t = 0 and (B) t = 30 seconds, and ~200 nm wide channels at (C) time t = 0 and (D) t = 25.2 seconds.
SOURCE: Courtesy of the Cancer Research Microscopy Facility, University of New Mexico Hospital; the W.M. Keck foundation; and the following individuals: Anthony L. Garcia, Linnea K. Ista, Dimiter N. Petsev, Michael J. O’Brien, Paul Bisong, Andrea A. Mammoli, Steven R.J. Brueck, and Gabriel P. Lopez.
(right) widths. Separation of the dye fronts occurs very close to the entry to the nanochannels (in all cases less than 1 mm from the entrance). Counter to fluid flow observed in micro- and macrochannels, the negatively charged dye (Alexa 488, green fluorescence) moves more quickly toward the negatively biased electrode than the neutral dye (rhodamine B, red fluorescence).
Decreasing the nanochannel width thus leads to qualitatively new and counterintuitive behavior that can be exploited for molecular separations. Because details of the flow profiles in individual nanochannels are below the resolution limit of optical microscopy, only the average velocities of dye fronts can be monitored. Significant improvements in the lateral resolution of analytical imaging methods are required to study the transport of molecules in an individual channel.
Chemical Imaging Technique Involved
Confocal microscopy is the main imaging technique used in this research application.
Insights Obtained Using Chemical Imaging
Chemical imaging allows the behavior of molecules in nanochannels to be investigated by following the flow of fluorescent dyes. Behavior contrary to that observed in micro- and macrochannels is observed, demonstrating that this is a new and promising flow regime.
Imaging Limitations
The signal must be averaged over hundreds of nanochannels.
Opportunities for Imaging Development
Techniques that can image molecular flow in single nanochannels will enhance understanding of flow in this new regime, accelerating device development.
Case Study 6:
Biological Imaging Involving Multiple Length Scales
To observe the chemistry of the human body down to the molecular details of individual cells (Figure 2.13), imaging instruments with significantly better resolving power are needed. Approximately 300 years after Anton van Leeuwenhoek built the first light microscope, this instrument continues to be the workhorse of modern biologists. By combining the developments of modern nanotechnology, optics, and computer science, the basic light microscope has been transformed and now manifests as several advanced imaging systems, such as the confocal and multiphoton microscopes. These microscopes allow observation of individual brain cells (neurons) and their axons, many of which extend from the brain to distant regions of the body.
Tagging individual proteins with fluorescent molecules allows them to be monitored in live cells, enabling rapid discovery of many intricate details about the cellular chemistry. For example, fluorescent tags on molecules that have been packaged into small vesicles have been monitored as they travel along microtubules within the axons via complex protein machines.
Arrays of microtubules, which are long polymers of the protein tubulin, are observed using the recently developed X-ray microscope. This new imaging technology is in its infancy but is rapidly gaining importance because of its ability to produce three-dimensional computed axial tomography (CAT) scans of single cells. With the continued development of optics, a threefold increase in resolution will soon be possible. Electron microscopes, invented in the 1930s, can also yield chemical information over a range of length scales. By examining metallic replicas of fractured cells, the structural organization of microtubules within axons, and the distinct cross-bridging proteins between adjacent polymers can be visualized. The electron microscope used in the diffraction mode reveals the molecular
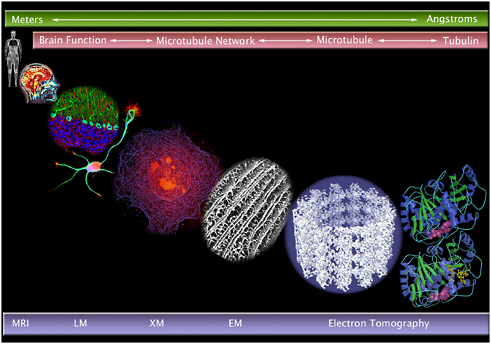
FIGURE 2.13 Instrumentation for capturing images at multiple length scales: magnetic resonance imaging (MRI), light microscopy (LM), X-ray microscopy (XM), electron microscopy (EM), and electron tomography.
SOURCE: Courtesy of Carolyn Larabell, Lawrence Berkeley National Laboratory assembled from various sources: MRI, LM, XM, microtubule network, Meyer-Ilse, W., D. Hamamoto, A. Nair, S.A. Leliévre, G. Denbeaux, L. Johnson, A.L. Pearson, D. Yager, M.A. Legros, and C.A. Larabell, 2001. High resolution protein localization using soft X-ray microscopy. J. Microsc. 201:395-403; electron tomography (cryoelectron microscopy) of a microtubule, courtesy Ken Downing, Lawrence Berkeley National Laboratory. E. Nogales, M. Whittaker, R.A. Milligan and K.H. Downing. 1999.
structure of a single microtubule, as well as the individual tubulin protein in its alpha and beta forms.
Chemical Imaging Technique Involved
Light microscopy (confocal, multiphoton), X-ray microscopy, and electron microscopy are the mainstays of imaging instrumentation for biological imaging.
Insights Obtained Using Chemical Imaging
Observation of cellular and molecular details in living cells is possible. Structural and chemical information about cellular activities within single cells can be obtained.
Imaging Limitations
Better resolving power (at or near atomic resolution) is needed in biological imaging instruments. Imaging of discrete chemical environments in living systems remains out of reach.
Opportunities for Imaging Development
Improved optics sources, more robust fluorescent probes, molecular tags that are X-ray excitable, and more sensitive detectors will contribute much to the ability to image molecular interactions within cells at desired resolutions.
Case Study 7:
Crystals and Their Structure (Data Mining and Storage)
Whether one obtains a crystal structure of material using an experimental imaging technique or a simulation, a potential problem is the large amount of information that must be stored if one chooses to retain the position of every atom for a single image. One approach to solving this complexity is to retain only the positions of those atoms that deviate significantly from the corresponding regular lattice (e.g., defects such as dislocations or disclinations in a crystal). Figure 2.14 illustrates the substantial reduction of extraneous information when only the dislocations are included. Not only is it easier to see the important features (dislocations that affect the material’s optical properties), but equally important, this represents a substantial reduction in the memory storage needed to retain all of the atomic positions in a trajectory. Such a figure represents a substantial reduction in the memory storage that would be exaggerated in a trajectory. It also allows quick assessment of the degree of dislocation under the specified conditions. Although this simulation was obtained using an atomistic molecular dynamics (MD) approach, similar information can be obtained with reduced-dimensional approaches (multiscaled), such as the phase-field model of Goldenfeld in which the dynamics are carried out at mesoscopic length scales.21,22,23 Figure 2.15 illustrates this in the case of a two-dimensional crystal. A key advantage of such a representation is that its dynamical structures can be computed so quickly that there is no need to store them; rather, one need only store the results by way of the parameterizations of the model and its parameterization for a particular material. Equally important, both sets of visual images allow the degree of heterogeneity in the samples to be seen and provide a guide to the experimentalist in harvesting information from specific samples.
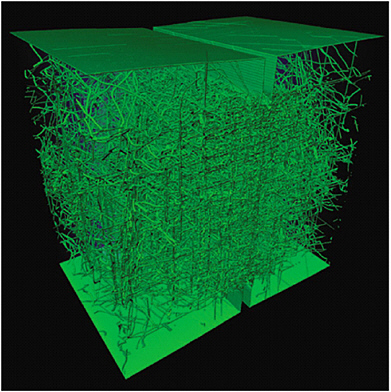
FIGURE 2.14 Two cracked tips (under stress) are pushed onto each other in an atomistic MD simulation. Only the microstructure of dislocations (comprised by a tiny percentage of the atoms) is displayed in green; all other atoms are hidden from view.
SOURCE: Yip, S. 2003. Synergistic science. Nat. Mater. 2:3-5; de Koning, M., A. Antonelli, and S. Yip. 2001. Single-simulation determination of phase boundaries: A dynamic Clausius-Clapeyron integration method. J. Chem. Phys. 115:11025–11035.
Chemical Imaging Technique Involved
-
Reductionist or projective approaches to reduce large datasets (or systems) at the atomic length scale to important components at mesoscopic or macroscopic length scales
-
Multiscaling and first-principles computational approaches to predict the structure and dynamics of materials given their atomistic or molecular composition

FIGURE 2.15 Comparison among different views of a snapshot of a subsystem satisfying the Swift-Hohenberg equation—a simplified model of convection in the absence of mean flow. Panel (a) shows the detailed flow directions, whereas panels (b) and (c) exhibit the amplitude and phase, respectively. The latter are slowly varying and allow for easier identification of the grain boundaries of the flow.
SOURCE: Courtesy of Nigel Goldenfeld’s lecture, available on-line at http://guava.physics.uiuc.edu/~nigel/articles/RG/Patterns,%20universality%20and%20computational%20algorithms.pdf.
Insights Obtained Using Chemical Imaging
Using theoretical and computational techniques, one can identify the mesoscopic structures leading to a requisite function. Once identified, these structural motifs can be used to guide experimental chemical imaging probes.
Imaging Limitations
Computers with faster processors, larger random access memory (RAM), larger disks, and better communications bandwidth are needed. In addition, new computational codes capable of easily multiscaling structures from angstroms to meters are required. The expected increases in computing power available at high-performance computing sites as well as that available on a user’s workstation will clearly make some calculations more accessible in the future. However, the major obstacles that have to be overcome lie primarily in the development of chemical theory, algorithms, and computer software. That is, the primary problem is the construction of static and dynamic structures that are simultaneously correct at resolutions ranging from the nanometer to the meter scale. This problem will not be solved easily by computing power alone.
Opportunities for Imaging Development
Development of fast, accurate, and user-friendly computer codes capable of multiscaling is necessary.
Case Study 8:
Molecular Motors
Although biology abounds with amazingly complex molecular systems, perhaps the most astounding of these are the variety of molecular motors that perform the nanoscale mechanical work of living systems. Converting the chemical energy of adenosine 51-triphosphate (ATP) to mechanical work, these motors turn, or step, or induce enzymatic reactions. A variety of imaging techniques have been applied to the investigation of molecular motors. The most revealing have been performed at the single-molecule level. Indeed, the study of molecular motors is one of the most cited successes of single-molecule imaging. Single-molecule fluorescence has been used to visualize molecules moving or being moved by molecular motors in a field. Scanning probe spectroscopies have been adapted to measure the forces and mechanical parameters of motor function. Piconewton forces and nanometer movements have been measured using these techniques, opening a world of nanomechanics that previously was entirely unknown.
Chemical Imaging Technique(s) Involved
The techniques involved are single-molecule spectroscopy including optical (fluorescence) methods and single-molecule mechanical manipulation including scanning probe techniques (e.g., force measurements).
Insights Obtained Using Chemical Imaging
Chemical imaging at the single-molecule level has led to new understanding of mechanisms of molecular motors (force, torque, etc.).
Imaging Limitations
Imaging methods used at the single-molecule level can be applied only to selected molecules or molecular machines, primarily outside a living cell. It is important to study how individual molecules work together, ultimately in living cells.
Opportunities for Imaging Development
Single-molecule imaging techniques with improved temporal and spatial resolution have to be developed. Of particular importance is the ability to follow the dynamic activities of a single molecule, such as movements, structural changes, and catalytic functions. Future single-molecule studies both in vitro and in vivo will generate new knowledge of the working of molecular motors and other macro-molecule machines and uncover mysteries in living systems (Figures 2.16 and 2.17).

FIGURE 2.16 A stylized cartoon of myosin V “walking” along an actin filament. Myosin V has the function of carrying cargo while walking along an actin tightrope and progressing in 37 nm steps. This movement has been observed using total internal reflection fluorescence (TIRF) spectroscopy of individual myosin molecules.
SOURCE: Reprinted with permission from 2003. Science (cover), 300(5628), based on Yildiz, A., J.N. Forkey, S.A. McKinney, T. Ha, Y.E. Goldman, and P.R. Selvin. 2003. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science 300:2061-2065. Copyright 2003 AAAS.
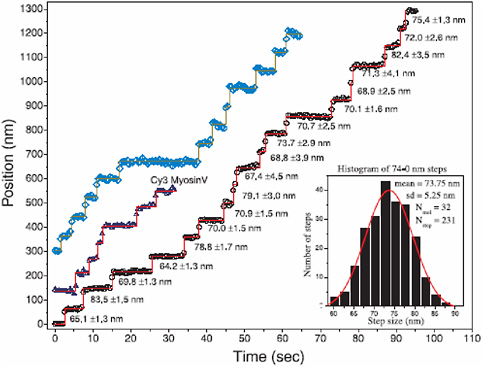
FIGURE 2.17 Results of total internal reflection fluorescence (TIRF) measurements in which the discrete and processive stepping action of myosin can be seen clearly.
SOURCE: Reprinted with permission from Yildiz, A., J.N. Forkey, S.A. McKinney, T. Ha, Y.E. Goldman, and P.R. Selvin..2003. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science 300:2061-2065. Copyright 2003 AAAS.
Case Study 9:
Reverse Imaging
In addition to using imaging as a technique to obtain spatially and temporally patterned chemical data from a sample, one can also pattern chemical reactions in space and time using similar methods. Figure 2.18 demonstrates an example in which multiphoton scanning with ultrafast laser pulses was used to polymerize a photoresist resin with about 120 nm spatial resolution in three dimensions.
Photopolymerization is only one way in which the pattern and time course of chemical reactions can be controlled using imaging instruments. Atoms can be moved around on surfaces, specific genes can be turned on in one cell and not in a neighboring cell, and large arrays of heteropolymers (DNA, protein, etc.) can be synthesized on surfaces in which the chemical identity of each molecule at each position is distinct and known. (See Chapter 3 for further details.)
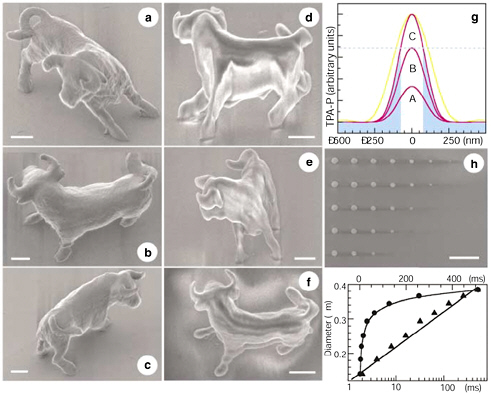
FIGURE 2.18 Example of reverse imaging in which multiphoton scanning with ultrafast laser pulses was used to polymerize a photoresist resin with about 120 nm spatial resolution. In panels a-f and h, the white bar is 2 microns.
SOURCE: Kawata, S., H.-B. Sun, T. Tanaka, and K. Takada. 2001. Finer features for microdevices. Nature 412:697-698.
Chemical Imaging Technique(s) Involved
Multiphoton microscopy has been used to initiate photopolymerization of a photoresist material in three dimensions with resolution in the hundred-nanometer range.
Insights Obtained Using Chemical Imaging
It is clearly possible to use chemical imaging not only to observe the structure and dynamics of chemical systems, but also to manipulate them at high resolution. This example presents a paradigm for the use of imaging to create much more complex chemical systems, patterned in three dimensions with extraordinary resolution.
Imaging Limitations
The wavelength of the light source employed dictates the fabrication resolution. In addition, in this case only a single chemical species, a photopolymer, is being manipulated. The potential exists for much more complex patterned chemical fabrications.
Opportunities for Imaging Development
There is no reason that chemical imaging in general cannot be turned on its head and used to manipulate chemical systems rather than just observe them. Because identifying features of chemistry can be observed in the imaging process shows that the probes used perturb this chemistry and that this perturbation can be patterned and controlled in both time and space. Considerably more could be done with high-throughput photopatterning of complex chemical systems. In addition, other techniques, such as control of individual magnetic particles in three dimensions with applied magnetic fields, should provide new vistas for fabrication and analysis as well as new opportunities for drug delivery in clinical settings.
Case Study 10:
Terahertz Imaging for Electromagnetic Materials Research
One area of imaging spectroscopy that has attracted considerable attention recently is terahertz (THz)24 radiation research. THz imaging is currently being touted in security- and defense-related applications, such as airport passenger and mailroom package screening. However, this case study focuses on the potential of time-resolved THz spectroscopy (TRTS). The THz frequency range spans the region between about 3 cm–1 (0.1 THz) to about 300 cm–1 (10 THz).25 The radiation source may be generated from either continuous wave or short-pulsed lasers; the latter source of radiation allows TRTS studies to take place with subpicosecond temporal resolution.
THz spectroscopy was born from research efforts to produce and detect ultra-short electrical currents as they traveled down a transmission line.26 In 1988-1989, it was discovered that electromagnetic radiation pulses produced by time-varying current could be propagated through free space and picked up by a detector.27 By placing a sample between a THz source and detector, one could measure the differences in radiation pulses due to scattering or absorption by the sample to understand its chemical properties.
TRTS has numerous applications in materials science, chemistry, and biological research. One such application is in the study of charge transport in titanium dioxide (TiO2), a material that is used in photovoltaic and photocatalytic systems. Scientists are currently studying electron transport in TiO2 in order to better understand its photosensitive properties and engineer a more efficient surface for harnessing solar energy. Figure 2.19 shows a Grätzel solar cell, which
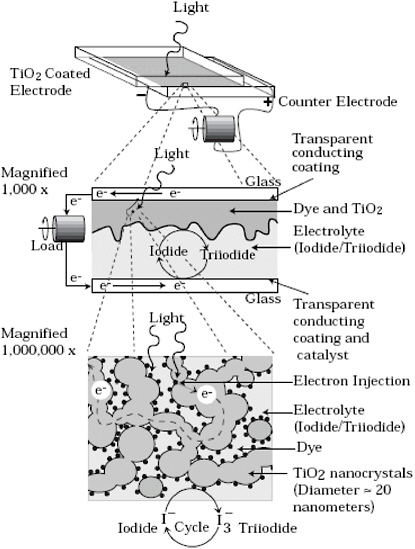
FIGURE 2.19 A schematic of the Grätzel solar cell.
SOURCE: Smestad, G.P.; M. Grätzel. 1998. Demonstrating electron transfer and nanotechnology: A natural dye-sensitized nanocrystalline energy converter. J. Chem. Educ. 75: 752-756.
utilizes dye-sensitized TiO2. Figure 2.20 shows the general scheme of dye sensitization of TiO2. The photon energy of sunlight is not strong enough to excite an electron from the TiO2 valence band to the conduction band in bulk; as a result, the surface of the TiO2 film on a photovoltaic device is coated with a monolayer of a charge-transfer dye in order to photoexcite dye molecules that then inject
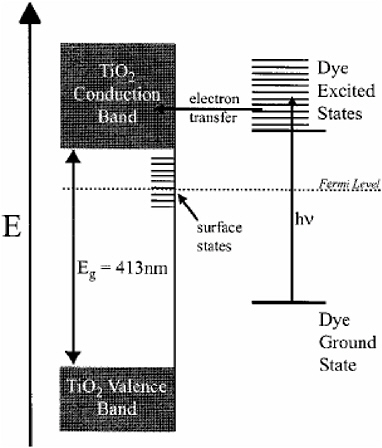
FIGURE 2.20 A schematic of the dye sensitization of TiO2.
SOURCE: Beard, M.C., G.M. Turner, and C.A. Schmuttenmaer. 2002. Terahertz spectroscopy. J. Phys. Chem. B 106:7146-7159.
electrons into the TiO2 semiconductor.28 TRTS can be used to dynamically measure the mobilized electrons on a picosecond time scale within the conduction band without being affected by the dye molecules.29
In studies of TiO2 conduction, TRTS has several advantages over fluorescence and other optical methods of spectroscopy. For example, one such advantage is that assumptions about electron behavior need not be made to analyze spectra obtained through THz spectroscopy. Characterization of photoinjected electron dynamics in dye-sensitized TiO2 (Figure 2.20) has previously been performed using the mid-infrared region of the spectrum.30 However, in these studies, it must be assumed that electron behavior follows the Drude model31 to account for transient infrared absorption of electrons. THz spectroscopy allows
complex conductivity responses to be obtained without assuming any prior model for electron behavior. In fact, TRTS revealed that the charge carriers significantly deviated from Drude behavior in colloidal, sintered TiO2.32 Furthermore, scattering responses have the greatest variation within the terahertz spectrum; thus a great deal of information regarding the dynamics of electron mobility may be obtained in this region. Finally, TRTS may be carried out at subpicosecond time resolution in order to follow the ultrafast dynamics of electron transfer within this system. Ultimately, the advantages of using TRTS to examine semiconductor materials may also be applied to spectroscopic methods for biological and medical imaging purposes.
Chemical Imaging Technique(s) Involved
Terahertz spectroscopy uses continuous wave (CW) and short pulsed laser excitation in the spectrum region between infrared and microwave frequencies. Pulsed laser excitation using pulse widths in the range of 10-100 femtoseconds has enabled the use of time-resolved terahertz spectroscopy, which is capable of capturing dynamic information at subpicosecond time scales.
Insights Obtained Using Chemical Imaging
Time-resolved terahertz imaging is capable of providing information about the dynamics of chemical reactions in materials science, chemistry, and biology.
Imaging Limitations
There exists a need for high-power pulsed CW radiation sources to enable fast switching times and high repetition rates for electromagnetic resonance experiments. In addition, commercial development of THz sources is needed so that this technology can be made more widely available to the research community. Furthermore, current detectors for THz spectroscopy have high cooling requirements to minimize noise in spectral data; further developments are needed to provide inexpensive and user-friendly detector options.
Opportunities for Imaging Development
Terahertz imaging offers the possibility of understanding complex reactions in which the chemical state of the sample under study changes with time. An extension of this ability is the control of chemical reactions in a highly specific manner; this will require the manipulation and channeling of the energy in a system such that the possible outcomes (degrees of freedom) are narrowed to those that one desires.
CONCLUSION
As demonstrated by the case studies presented in this chapter, chemical imaging has a wide variety of applications that have relevance to almost every facet of our daily lives. These applications range from medical diagnosis and treatment to the study and design of material properties in novel products. To continue receiving benefits from these technologies, sustained efforts are needed to facilitate understanding and manipulation of complex chemical structures and processes. Chemical imaging offers a means by which this can be accomplished by allowing the acquisition of direct, observable information about the nature of these chemistries.
NOTES AND REFERENCES






































