8
Laryngeal Cancer and Asbestos
NATURE OF THIS CANCER TYPE
The larynx, commonly known as the voice box or Adam’s apple, is above the trachea and below the pharynx (Figures 7.1 and 8.1). It includes three anatomic subsites: the glottis, including the vocal fold or vocal cords, depicted near the middle of Figure 8.1; the supraglottis, which encompasses all tissues above the vocal folds and below the pharynx and includes the epiglottis, a fold that closes the larynx during swallowing to prevent food inhalation; and the subglottis, or area below the vocal fold.
The American Cancer Society (Jemal et al. 2006) has estimated that about 9,510 new cases of and 3,740 deaths from cancer of the larynx (ICD-9 161; ICD-O C32.0-C32.9) will occur in 2006. Laryngeal cancer ranks 16th in incidence and mortality among men in the United States, and 28th and 25th in incidence and mortality, respectively, among women. Both incidence and mortality are more than 4 times higher in men than women and are higher among blacks than whites, especially in men. The risk of developing laryngeal cancer increases with age. However, the incidence of laryngeal cancer, adjusted for age, has decreased by an average of 2.6% per year since 1988.
Most cancers of the larynx are squamous-cell carcinomas that arise from the thin, flat cells (squamous cells) that line the upper airway. Those tumors, like squamous-cell carcinomas of the oral cavity and pharynx, develop gradually as normal cells develop into clones of progressively abnormal cells. As the clones accumulate genetic damage, some may undergo malignant transformation, first into carcinoma in situ, and later into inva-
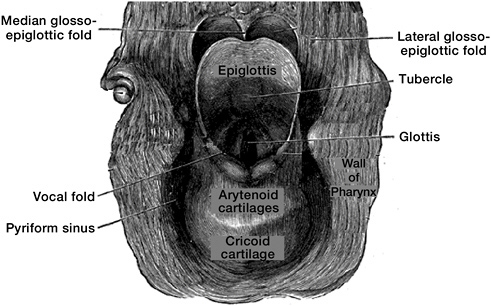
FIGURE 8.1 Larynx viewed from behind.
SOURCE: Modified from Grey’s Anatomy of the Human Body. Available at http://www.bartleby.com/107/236.html.
sive cancer. Premalignant lesions often regress after the discontinuation of tobacco use and alcohol consumption.
The most important risk factors for laryngeal cancer are tobacco-smoking (all forms) (IARC 2004) and heavy consumption of alcohol, especially when drinking and smoking occur in combination (IARC 1988). Cancer of the larynx is rare in lifelong nonsmokers, even though nonsmoking drinkers have been reported to have increased risk (Burch et al. 1981, Elwood et al. 1984). Risk increases with the number of cigarettes smoked per day and duration of smoking. The independent effect of tobacco on laryngeal cancer is greater than that of alcohol consumption. The effects of occupation on the risk of laryngeal cancer have been difficult to study, because of the powerful relationship of this cancer with tobacco use and alcohol consumption, and the little information on alcohol consumption and tobacco use in many occupational studies. Exposure to strong sulfuric acid mist is an established cause of laryngeal cancer (IARC 1987). Other factors that may increase risk, but on which current data are limited, include exposure to mustard gas (HHS 2004), steam and fumes from isopropyl alcohol (IARC 1987), metalworking fluids (Eisen et al. 1994, Zeka et al. 2004), and chronic infection with human papilloma virus (Rees et al. 2004).
The combination of tobacco-smoking and heavy drinking causes a much larger increase in laryngeal cancer risk than would be expected from the sum of the relative risk (RR) estimates associated with the separate exposures. For example, a study of laryngeal cancer published in 1976 (Wynder and Hoffmann 1976) found that, compared with men who neither smoked nor drank, those who reported both smoking (35 or more cigarettes per day) and drinking (seven or more alcoholic drinks per day) had an RR of 22.1 (95% confidence interval [CI] 7.8-62.1). Smoking alone was associated with an RR of 7.0 (95% CI 2.5-19.4), whereas the RR of this level of alcohol consumption alone could not be calculated because of the absence of cases. That study, conducted during the period when many studied occupational populations were experiencing exposure to asbestos, illustrates the strength of the association of laryngeal cancer with smoking and drinking.
EPIDEMIOLOGIC EVIDENCE CONSIDERED
The association between asbestos exposure and cancer of the larynx has been examined in many cohort and case-control studies. As discussed previously, the major strengths of the occupational cohort studies are that the magnitudes and durations of asbestos exposure tend to be substantially higher and the exposure information better documented than in case-control studies of the general population. Most of the cohort studies address death from laryngeal cancer—an imperfect surrogate of incidence because survival of laryngeal cancer is high. The case-control studies are also important with respect to laryngeal cancer because their analyses are based on incident cases rather than deaths; the number of cases is larger, thus providing greater statistical power; and some of the case-control studies collect information that can be used to adjust for or stratify on tobacco or alcohol use.
Cohort Studies
The cohorts that presented usable information about the risk of laryngeal cancer and their design properties are described in Table B.1, and the details of their results concerning cancer at this site are abstracted in Table D.2. The results of the cohort and case-control studies are summarized in Table 8.1, and Figures 8.2 and 8.3 are plots of RRs for overall exposure and for exposure-response gradients from the cohort studies reviewed.
The committee identified and included in its analyses 35 cohort populations from 29 published papers that examined the RR of a diagnosis of or death from laryngeal cancer among among people with any occupational exposure to asbestos compared with people in the general population with-
TABLE 8.1 Summary of Epidemiologic Findings Regarding Cancer of the Larynx
|
Study Type |
Figure |
Comparison |
Study Populations Included |
No. Study Populations |
Summary RR (95% CI) |
Between-Study SD |
|
Cohort |
Any vs none |
All |
35 |
1.40 (1.19-1.64) |
— |
|
|
|
High vs nonea |
Lower boundb |
11 |
2.02 (1.64-2.47) |
— |
|
|
|
|
|
Upper boundb |
11 |
2.57 (1.47-4.49) |
— |
|
Case-control |
Any vs none |
All |
15 |
1.43 (1.15-1.78) |
0.27 |
|
|
|
Any vs none |
EAM = 1 |
10 |
1.21 (1.04-1.40) |
0.02 |
|
|
|
|
|
EAM = 2 |
5 |
2.56 (1.20-5.43) |
0.65 |
|
|
Any vs none |
EAM = 1 Adjustedc |
7 |
1.18 (1.01-1.37) |
0.00 |
|
|
|
|
|
EAM = 1 Unadjustedc |
3 |
1.58 (0.86-2.91) |
0.27 |
|
|
High vs nonea |
EAM = 1 Lower boundb |
7 |
1.38 (1.02-1.86) |
0.27 |
|
|
|
|
|
EAM = 1 Upper boundb |
7 |
1.53 (1.21-1.93) |
0.07 |
|
NOTE: CI = Confidence interval; EAM = exposure-assessment method; high quality, EAM = 1; lower quality, EAM = 2; RR = relative risk; SD = standard deviation. aUsed studies that reported dose-response relationship (RR on an exposure gradient). bSome studies reported dose-response relationship on multiple gradient metrics. In computing the summary RR, “lower bound” calculation used the smallest “high vs none” RR, and “upper bound” calculation used largest “high vs none” RR. cAdjusted: RR was adjusted for both smoking and alcohol use. |
||||||
out such exposure (Table D.2 and Figure 8.2). Other reports were not included in the analysis, because they were superseded by later reports based on longer follow-up of the same cohort (e.g., Clemmensen and Hjalgrim-Jensen 1981; McDonald et al. 1986, 1993; Rubino et al. 1979), were not primarily asbestos cohorts (e.g., Magnani et al. 1986, Imbernon et al. 1995),
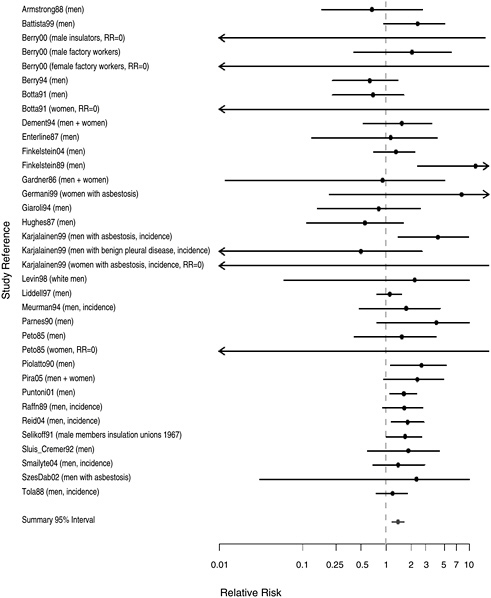
FIGURE 8.2 Cohort studies: RR of laryngeal cancer in people with “any” exposure to asbestos compared with people who report none.
did not specify the standardized mortality ratio or expected number of cases of laryngeal cancer (e.g., Djerassi et al. 1979; McDonald et al. 1983, 1984; Zhu and Wang 1993), or did not report the larynx as a separate cancer site (e.g., Seidman et al. 1986, Selikoff et al. 1979).
Figure 8.2 shows the RR estimates and 95% CI estimates in 34 cohort
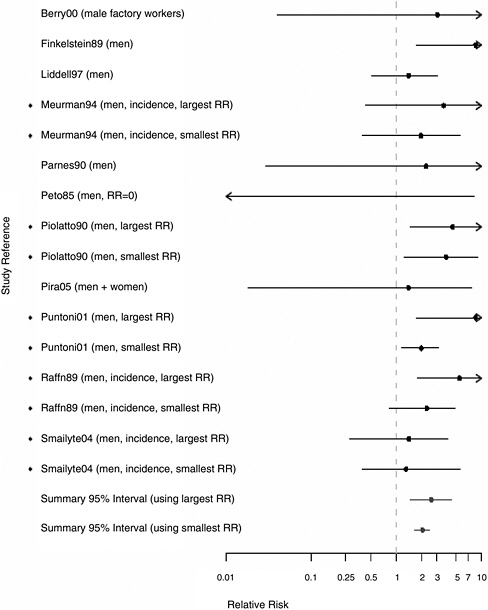
FIGURE 8.3 Cohort studies: RRs of laryngeal cancer among people in most extreme exposure category compared with those with no exposure (◆ = more than one exposure gradient reported in citation, so the plot contains both highest and lowest estimates of risk at most extreme category over all gradients).
study populations that reported “any” occupational exposure to asbestos, compared with unexposed subjects. The cohorts were drawn from a wide array of industries, including mining, textiles, and insulation. Five of the studies (Karjalainen et al. 1999, Meurman et al. 1994, Raffn et al. 1989, Reid et al. 2004, Smailyte et al. 2004) compared the incidence of laryngeal cancer in exposed and unexposed subjects; the remainder assessed mortality. The number of cases or deaths in the reports ranged from 1 (Gardner and Powell 1986, Germani et al. 1999, Szeszenia-Dabrowska et al. 2002) to 36 (Liddell et al. 1997). The RR estimates exceeded 1.0 in all cohorts with 10 or more cases of or deaths from laryngeal cancer (Finkelstein and Verma 2004, Liddell et al. 1997, Puntoni et al. 2001, Raffn et al. 1989, Reid et al. 2004, Selikoff and Hammond 1978, Selikoff and Seidman 1991, Tola et al. 1988) and in the largest study of patients with asbestosis (Karjalainen et al. 1999). Some of the heterogeneity seen in Figure 8.2 reflects the statistical imprecision of subgroup analyses, especially for women. The combined RR associated with any occupational exposure to asbestos (Figure 8.2) was 1.40 (95% CI 1.19-1.64).
Further analyses examined whether the association between asbestos exposure and laryngeal cancer was stronger among the most highly exposed subjects in a subset of 11 cohorts in which this information was available. The analysis was done in several ways to take account of the multiple indexes used by many of the studies to define the intensity or duration of exposure (duration of employment, cumulative exposure, peak exposure, probability of exposure, and so on). We plotted the highest and lowest RRs for subjects who were in one of the “most exposed” categories by any definition. Figure 8.3 presents the plots for the 11 cohorts in which this information was available. In each of the individual cohorts, the RR estimates exceeded 1.0. The aggregate RR estimate in the most highly exposed subjects was 2.57 (95% CI 1.47-4.49) for the strongest association reported and 2.02 (95% CI 1.64-2.47) for the weakest association reported; both are higher than the combined estimate associated with any exposure to asbestos 1.40 (95% CI 1.19-1.64).
Our last approach in assessing the cohort studies of asbestos exposure in relation to laryngeal-cancer risk was to examine the association in cohorts with extremely high exposure to asbestos, such as the patients with asbestosis studied by Karjalainen et al. (1999). The standardized incidence ratios (SIRs) of mesothelioma (RR = 32, 95% CI 14.4-60.0) and of lung cancer (RR = 6.7, 95%CI 5.6-7.9) were significantly increased in this cohort, compared with the incidence in the general population of Finland. The SIR of laryngeal cancer was also increased (RR = 4.2, 95% CI 1.4-9.8) in men but not women.
In summary, the larger cohort studies consistently show increased risk of laryngeal cancer in asbestos-exposed workers employed in a wide array
of industries and in a large cohort of workers with asbestosis. There is some evidence of a dose-response relationship in the meta-analyses.
Case-Control Studies
The case-control studies of laryngeal cancer that were retained for thorough evaluation after exclusion of studies that did not assess exposure to asbestos or did not meet other exclusion criteria are listed in Table 6.5 according to quality of their exposure assessment. The details of the design aspects of those studies are presented in Table C.1 and their detailed results are abstracted in Table E.2. The findings of the studies are summarized in Table 8.1 and in the plots presented in Figures 8.4-8.7.
The committee identified 18 published case-control studies that provide data on the association between risk of laryngeal cancer and exposure to asbestos or any employment in an occupation or industry where asbestos exposure was known to occur. The studies involved from 20 cases (Luce et al. 2000) to 940 cases (Elci et al. 2002). Seven of the studies had 200 or more subjects (Berrino et al. 2003, Deitz et al. 2002, Elci et al. 2002, Marchand et al. 2000, Olsen and Sabroe 1984, Wortley et al. 1992, Zheng et al. 1992), while seven more included at least 100 (Ahrens et al. 1991, Brown et al. 1988, Burch et al. 1981, De Stefani et al. 1998, Gustavsson et al. 1998, Muscat and Wynder 1992, Stell and McGill 1973). Over 97% of cases in these studies were male. The male predominance reflects the facts that about 80% of laryngeal cancers occur in men and that occupational exposures to asbestos typically occur in trades where nearly all workers have been men.
Figure 8.4 provides the RR or odds ratio estimates associated with reporting “any” exposure to asbestos in 15 studies that compared subjects with any occupational exposure to those with no exposure to asbestos. Three case-control studies are excluded from this analysis (Berrino et al. 2000, Gustavsson et al. 1998, Wortley et al. 1992), because they present results for larynx only in relation to dose. Only one (Luce et al. 2000) of the 15 studies included in Figure 8.5 has an RR estimate below 1.0. The metaanalysis, combining 15 studies, found an RR of 1.43 (95% CI 1.15-1.78) associated with “any” exposure to asbestos.
To assess whether the association between asbestos exposure and risk of laryngeal cancer was stronger in studies with higher exposure information, the committee separated the studies into those with better measures of exposure and those with more limited data, as shown in Figure 8.5. The RR from the combined analysis of 10 studies with higher-quality exposure information was 1.21 (95% CI 1.04-1.40). Among the studies considered to have more limited information on asbestos exposure were two (Shettigara and Morgan 1975, Stell and McGill 1973) in which the association with
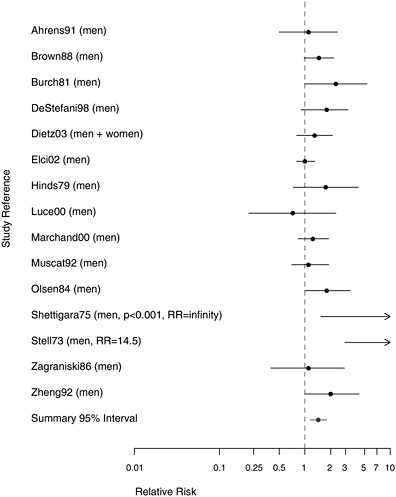
FIGURE 8.4 Case-control studies: RR of laryngeal cancer in people with “any” exposure to asbestos compared with people with none.
asbestos appeared to be the strongest. However, those small studies had a negligible influence on the summary measure of association between asbestos exposure and increased risk of laryngeal cancer. The association persisted with or without the inclusion of studies with weaker exposure data.
Most of the case-control studies made some attempt to control for tobacco and alcohol consumption in examining the association between asbestos exposure and laryngeal cancer. Two of the studies whose results are presented in Figure 8.6 with adjustment for those risk factors also gave unadjusted estimates in the citation. In Dietz et al. (2004), the association between asbestos exposure and laryngeal cancer was weakened by controlling for other covariates; but in Brown et al. (1988), controlling for tobacco
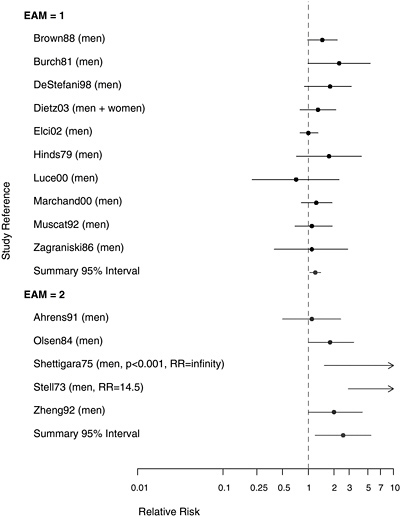
FIGURE 8.5 Case-control studies: RR of laryngeal cancer in people with “any” exposure to asbestos compared with people with none, stratified on quality of exposure assessment (top, EAM = 1: higher-quality exposure assessment; bottom, EAM = 2: lower-quality exposure assessment).
use and alcohol consumption made little difference. Overall, with adjustment for the other two prominent risk factors for laryngeal cancer, an association with asbestos exposure appears to persist (RR = 1.18, 95% CI 1.01-1.37). Given the propensity that has been demonstrated for smoking to act as an effect modifier in lung cancer rather than merely as a simple additive factor, however, it may be more appropriate to consider stratified analyses than adjusted multivariate findings.
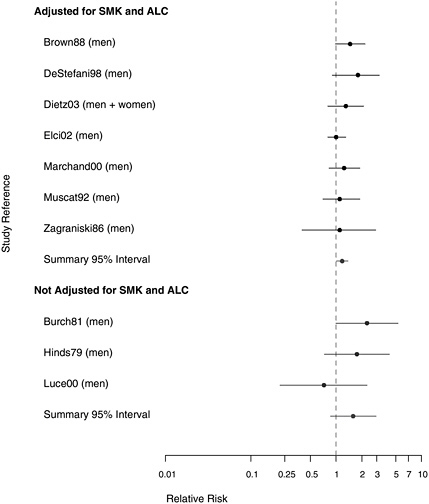
FIGURE 8.6 Case-control studies: RR of laryngeal cancer in people with “any” exposure to asbestos compared to people with none, from studies with higher-quality exposure assessment, stratified on quality of confounder assessment (top: adjusted; bottom: unadjusted).
Five of the case-control studies presented results stratified by tobacco or alcohol consumption and allowed limited consideration of whether asbestos exposure might modify the laryngeal carcinogenicity of tobacco or alcohol exposure (Burch et al. 1981, De Stefani et al. 1998, Gustavsson et al. 1998, Marchand et al. 2000, Muscat and Wynder 1992). The information presented in Burch et al. (1981) did not conform to a tabular presentation, but the results from the others are abstracted in Table 8.2.
TABLE 8.2 Effect Modification for Laryngeal Cancer Associated with Asbestos Exposure and Smoking
|
Study |
Smoking History (pack-years) |
Asbestos Exposure |
|
|
None or Low (cumulative) |
Intermediate or High (cumulative) |
||
|
Marchand et al. (2000) (adjusted for age and for alcohol consumption) |
<30 30+ |
1.0 5.3 (3.2-8.8) |
1.5 (0.9-2.5) 6.5 (3.8-10.8) |
|
|
|
Never |
Ever |
|
De Stefani et al. (1998) |
≤ 35 36+ |
1.0 6.2 (3.5-11.1) |
1.7 (0.2-14.2) 30.6 (8.4-112.1) |
|
|
|
Never |
Ever |
|
Muscat and Wynder (1992) |
not current current |
1.0 5.8 (3.4-10.00 |
1.3 (0.7-2.4) 6.3 (3.3-12.2) |
|
|
|
Never |
Ever |
|
Gustavsson et al. (1998) (adjusted for age, region, and alcohol consumption) |
not current current |
1.0 3.9 |
1.8 4.8 [vs 4.7 expected under additive model; or 7.0 under multiplicative model] |
Gustavsson et al. (1998) found the observed risk (4.8) in the combined exposure category for Swedish men closer to the prediction of an additive model (3.9 + 1.7 − 1.0 = 4.7) than of a multiplicative one (3.9 × 1.7 = 7.0). Marchand et al. (2000), reporting on a hospital-based study of 315 incident cases of laryngeal cancer in France, found risks (also adjusted for age and alcohol consumption) somewhat indicative of interaction of joint exposure to asbestos and smoking. Muscat and Wynder (1992) reported similar results in a hospital-based study of 194 white males in the United States. De Stefani et al. (1998), however, found a much stronger association with having “ever” been exposed to asbestos among Uruguayan heavy smokers than in asbestos-exposed men who had not smoked as much. A limitation of all of those studies is that, although the risk was higher among men who were exposed than those who were not unexposed to asbestos, the data were not stratified into narrowly defined combinations of asbestos exposure, tobacco-smoking, and alcohol consumption.
As in the analyses of cohort studies, the committee examined the risks in the extreme categories of exposure-related gradients (longest duration, highest probability of exposure, cumulative exposure, and so on) in the case-control studies. As depicted in Figure 8.7, the aggregate results for the weakest (RR = 1.38, 95% CI 1.02-1.86) and the strongest (RR = 1.53, 95% CI 1.21-1.93) reported associations for extreme exposure groups were both higher than the aggregated estimate for subjects with any exposure to asbestos in the studies with more reliable exposure assessment (top of Figure 8.5), and this a suggests of dose-response relationship.
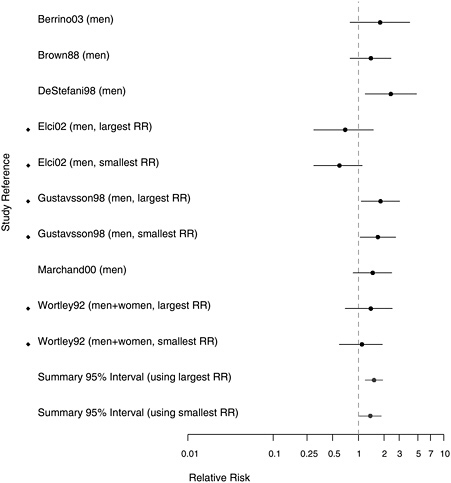
FIGURE 8.7 Case-control studies: RRs of laryngeal cancer in people in with extreme exposure to asbestos compared with those with none (◆ = more than one exposure gradient reported in citation, so the plot contains both highest and lowest estimates of risk for most extreme category over all gradients).
EVIDENCE INTERGRATION AND CONCLUSION
Evidence Considered
The evidence base included a larger number of epidemiologic studies of asbestos exposure and laryngeal cancer, particularly of the case-control design, than were available for other cancer sites. The committee reviewed the results on 35 cohort populations and 18 case-control studies. Subjects in the studies had been exposed to asbestos in a wide array of industries and occupations in North America, South America, Europe, and Japan. Many of the case-control studies collected some data with which to control for confounding by tobacco-smoking and alcohol consumption. Several case-control studies examined the association between asbestos exposure and laryngeal cancer, stratifying on tobacco use. The committee also reviewed four experimental studies in which rodents were exposed over much of their lifetime to high concentrations of asbestos through inhalation.
Consistency
Asbestos exposure was associated with increased risk of laryngeal cancer in all nine large cohort studies (those with at least 10 cases of or deaths from laryngeal cancer) and in both the cohort and case-control combined analyses. Some evidence of a dose-response relationship in risk was seen in both the cohort and the case-control studies. There was no consistent evidence of confounding in case-control studies that reported both age-adjusted and multivariate-adjusted RR estimates. Several case-control studies that stratified on tobacco-smoking observed higher risk among men who were exposed than in those not exposed to asbestos, although these analyses did not simultaneously stratify on asbestos, tobacco, and alcohol.
Strength of Association
The RR of laryngeal cancer among persons with any occupational exposure to asbestos compared with those who reported no exposure was 1.40 (95% CI 1.19-1.64) in the meta-analysis of the cohort populations and 1.43 (95% CI 1.15-1.78) in the case-control studies. There was some evidence from both cohort and case-control studies that risk increased with the intensity, duration, or likelihood of exposure; the aggregate estimates of RR in the most highly exposed subjects in either type of study ranged from 1.38 to 2.57.
Coherence
Several factors contribute to the biologic plausibility that asbestos may cause cancer of the larynx. The larynx, like the lung, is anatomically in the direct path of inhaled asbestos fibers. Inflammation or damage of the vocal folds could disrupt laminar airflow and predispose to the deposition and accumulation of asbestos fibers in the larynx. Squamous-cell carcinomas of the lung and larynx have histologic and clinical similarities. Cancers at both sites arise from the respiratory epithelium in regions of squamous metaplasia and dysplasia. Tobacco-smoking is the most important risk factor for both sites. Asbestos exposure is an established cause of lung cancer. On the basis of theoretical considerations, tobacco-smoking, alone or in combination with alcohol consumption, may predispose to the accumulation of asbestos fibers in the epithelial lining of the larynx. Aerodynamic turbulence at bifurcations of the large conducting airways is known to contribute to the deposition of long asbestos fibers in the lung (Asgharian and Yu 1988). Bronchogenic carcinomas commonly arise in those areas (Schlesinger and Lippmann 1978). The accumulation of asbestos fibers, together with smoking and/or drinking, could produce chronic irritation or inflammation and thus accelerates the progression of neoplasia.
The committee identified and considered several limitations in the evidence related to biologic plausibility. Foremost were the absence of clinical data documenting that asbestos fibers accumulate and persist in the larynx and the lack of experimental support from animal studies. The presence or absence of asbestos fibers in laryngeal tissue from occupationally exposed people has been investigated in only a few studies, in which contamination from other tissues is always a concern; Roggli et al. (1980) reported asbestos bodies and Kambic et al. (1989) reported fibers in this anatomic area. Studies in rats and Syrian hamsters found that asbestos inhalation, at levels sufficient to cause mesothelioma in both species and lung cancer in rats, did not induce chronic inflammation or increase cancer of the larynx (Hesterberg et al. 1993, 1994; McConnell 2005; McConnell et al. 1994a,b, 1999). These rodent models do not, however, reflect exposure to cofactors, such as tobacco-smoking and alcohol consumption, which may affect fiber deposition and/or persistence that may exacerbate local tissue injury and inflammation.
Conclusion
Considering all lines of evidence, the committee placed greater weight on the consistency of the epidemiologic studies and the biologic plausibility of the hypothesis than on the lack of confirmatory evidence from animal studies or documentation of fiber deposition in the larynx. The committee
concluded that the evidence is sufficient to infer a causal relationship between asbestos exposure and laryngeal cancer.
REFERENCES
Ahrens W, Jockel K, Patzak W, Elsner G. 1991. Alcohol, smoking, and occupational factors in cancer of the larynx: A case-control study. American Journal of Industrial Medicine 20(4): 477-493.
Armstrong BK, de Klerk NH, Musk AW, Hobbs MS. 1988. Mortality in miners and millers of crocidolite in Western Australia. British Journal of Industrial Medicine 45(1): 5-13.
Asgharian B, Yu C. 1988. Deposition of inhaled fibrous particles in the human lung. Journal of Aerosol Medicine 1: 37-50.
Battista G, Belli S, Comba P, Fiumalbi C, Grignoli M, Loi F, Orsi D, Paredes I. 1999. Mortality due to asbestos-related causes among railway carriage construction and repair workers. Occupation Medicine (London) 49(8): 536-539.
Berrino F, Richiardi L, Boffetta P, Esteve J, Belletti I, Raymond L, Troschel L, Pisani P, Zubiri L, Ascunce N, Guberan E, Tuyns A, Terracini B, Merletti F. 2003. Occupation and larynx and hypopharynx cancer: A job-exposure matrix approach in an international case-control study in France, Italy, Spain and Switzerland. Cancer Causes and Control 14(3): 213-223.
Berry G. 1994. Mortality and cancer incidence of workers exposed to chrysotile asbestos in the friction-products industry. Annals of Occupational Hygiene 38(4): 539-546.
Berry G, Newhouse ML, Wagner JC. 2000. Mortality from all cancers of asbestos factory workers in east London 1933-1980. Occupational and Environmental Medicine 57(11): 782-785.
Botta M, Magnani C, Terracini B, Bertolone GP, Castagneto B, Cocito V, DeGiovanni D, Paglieri P. 1991. Mortality from respiratory and digestive cancers among asbestos cement workers in Italy. Cancer Detection and Prevention 15(6): 445-447.
Brown LM, Mason TJ, Pickle LW, Stewart PA, Buffler PA, Burau K, Ziegler RG, Fraumeni JF Jr. 1988. Occupational risk factors for laryngeal cancer on the Texas Gulf Coast. Cancer Research 48(7): 1960-1964.
Burch JD, Howe GR, Miller AB, Semenciw R. 1981. Tobacco, alcohol, asbestos, and nickel in the etiology of cancer of the larynx: A case-control study. Journal of the National Cancer Institute 67(6): 1219-1224.
Clemmensen J, Hjalgrim-Jensen S. 1981. Cancer incidence among 5686 asbestos-cement workers followed from 1943 through 1976. Ecotoxicology Environment Safety 5(1): 15-23.
De Stefani E, Boffetta P, Oreggia F, Ronco A, Kogevinas M, Mendilaharsu M. 1998. Occupation and the risk of laryngeal cancer in Uruguay. American Journal of Industrial Medicine 33(6): 537-542.
Dement JM, Brown DP, Okun A. 1994. Follow-up study of chrysotile asbestos textile workers: Cohort mortality and case-control analyses. American Journal of Industrial Medicine 26(4): 431-447.
Dietz A, Ramroth H, Urban T, Ahrens W, Becher H. 2004. Exposure to cement dust, related occupational groups and laryngeal cancer risk: Results of a population based case-control study. International Journal of Cancer 108(6): 907-911.
Djerassi L, Kaufmann G, Bar-Nets M. 1979. Malignant disease and environmental control in an asbestos cement plant. Annals of the New York Academy of Sciences 330: 243-253.
Eisen EA, Tolbert PE, Hallock MF, Monson RR, Smith TJ, Woskie SR.1994. Mortality studies of machining fluid exposure in the automobile industry: III. A case-control study of larynx cancer. American Journal of Industrial Medicine 26(2): 185-202.
Elci OC, Akpinar-Elci M, Blair A, Dosemeci M. 2002. Occupational dust exposure and the risk of laryngeal cancer in Turkey. Scandinavian Journal of Work, Environment and Health 28(4): 278-284.
Elwood JM, Pearson JC, Skippen DH, Jackson SM. 1984. Alcohol, smoking, social and occupational factors in the aetiology of cancer of the oral cavity, pharynx and larynx. International Journal of Cancer 34(5): 603-612.
Enterline PE, Hartley J, Henderson V. 1987. Asbestos and cancer: A cohort followed up to death. British Journal of Industrial Medicine 44(6): 396-401.
Finkelstein MM. 1989. Mortality rates among employees potentially exposed to chrysotile asbestos at two automotive parts factories. Canadian Medical Association Journal 141(2): 125-130.
Finkelstein MM, Verma DK. 2004. A cohort study of mortality among Ontario pipe trades workers. Occupational and Environmental Medicine 61(9): 736-742.
Gardner MJ, Powell CA. 1986. Mortality of asbestos cement workers using almost exclusively chrysotile fibre. Journal of the Society of Occupational Medicine 36(4): 124-126.
Germani D, Belli S, Bruno C, Grignoli M, Nesti M, Pirastu R, Comba P. 1999. Cohort mortality study of women compensated for asbestosis in Italy. American Journal of Industrial Medicine 36(1): 129-134.
Giaroli C, Belli S, Bruno C, Candela S, Grignoli M, Minisci S, Poletti R, Ricco G, Vecchi G, Venturi G, Ziccardi A, Combra P. 1994. Mortality study of asbestos cement workers. International Archives of Occupational and Environmental Health 66(1): 7-11.
Gustavsson P, Jakobsson R, Johansson H, Lewin F, Norell S, Rutkvist LE. 1998. Occupational exposures and squamous cell carcinoma of the oral cavity, pharynx, larynx, and oesophagus: A case-control study in Sweden. Occupational and Environmental Medicine 55(6): 393-400.
Hesterberg TW, Miller WC, Mast R, McConnell EE, Bernstein DM, Anderson R. 1994. Relationship between lung biopersistence and biological effects of man-made vitreous fibers after chronic inhalation in rats. Environmental Health Perspectives 102 (Supplement 5): 133-137.
Hesterberg TW, Miller WC, McConnell EE, Chevalier J, Hadley JG, Bernstein DM, Thevenaz P, Anderson R. 1993. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundamental and Applied Toxicology 20(4): 464-476.
HHS (US Department of Health and Human Services). 2004. 11th Report on Carcinogens. Washington, DC: National Toxicology Program.
Hinds MW, Thomas DB, O’Reilly HP. 1979. Asbestos, dental X-rays, tobacco, and alcohol in the epidemiology of laryngeal cancer. Cancer 44(3): 1114-1120.
Hughes JM, Weill H, Hammad YY. 1987. Mortality of workers employed in two asbestos cement manufacturing plants. British Journal of Industrial Medicine 44(3): 161-174.
IARC (International Agency for Research on Cancer). 1987. Overall Evaluations of Carcinogenity: An Updating of IARC Monographs Volume 1 to 42. IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Man. Supplement 7. Lyon, France: World Health Organization.
IARC. 1988. Alcohol Drinking. IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Volume 44. Lyon, France: World Health Organization.
IARC. 2004. Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Volume 83. Lyon, France: World Health Organization.
Imbernon E, Goldberg M, Bonenfant S, Chevalier A, Guenel P, Vatre R, Dehaye J. 1995. Occupational respiratory cancer and exposure to asbestos: A case-control study in a cohort of workers in the electricity and gas industry. American Journal of Industrial Medicine 28(3): 339-352.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun M. 2006. Cancer statistics, 2006. CA: A Cancer Journal for Clinicians 56: 106-130.
Kambic V, Radsel Z, Gale N. 1989. Alterations in the laryngeal mucosa after exposure to asbestos. British Journal of Industrial Medicine 46(10): 717-723.
Karjalainen A, Pukkala E, Kauppinen T, Partanen T. 1999. Incidence of cancer among Finnish patients with asbestos-related pulmonary or pleural fibrosis. Cancer Causes and Control 10(1): 51-57.
Levin J, McLarty J, Hurst GA, Smith A, Frank AL. 1998. Tyler asbestos workers: Mortality experience in a cohort exposed to amosite. Occupational and Environmental Medicine 55(3): 155-160.
Liddell FD, McDonald AD, McDonald JC. 1997. The 1891-1920 birth cohort of Quebec chrysotile miners and millers: Development from 1904 and mortality to 1992. Annals of Occupational Hygiene 41(1): 13-36.
Luce D, Bugel I, Goldberg P, Goldberg M, Salomon C, Billon-Galland MA, Nicolau J, Quenel P, Fevotte J, Brochard P. 2000. Environmental exposure to tremolite and respiratory cancer in New Caledonia: A case-control study. American Journal of Epidemiology 151(3): 259-265.
Magnani C, Nardini I, Governa M, Serio A. 1986. [A cohort study of the personnel assigned to a state railroad repair shop]. Medicina del Lavoro 77(2): 154-161.
Marchand JL, Luce D, Leclerc A, Goldberg P, Orlowski E, Bugel I, Brugere J. 2000. Laryngeal and hypopharyngeal cancer and occupational exposure to asbestos and man-made vitreous fibers: Results of a case-control study. American Journal of Industrial Medicine 37(6): 581-589.
McConnell EE. 2005 (October 27). Personal Communication to Mary Paxton for the Committee on Asbestos: Selected Health Effects. Available in IOM Public Access Files.
McConnell E, Kamstrup O, Musselman R, Hesterberg T, Chevalier J, Miller W, Thevenaz P. 1994a. Chronic inhalation study of size-separated rock and slag wool insulation fibers in Fischer 344/N rats. Inhalation Toxicology 6(6): 571-614.
McConnell E, Mast R, Hesterberg T, Chevalier J, Kotin P, Bernstein D, Thevenaz P, Glass L, Anderson R. 1994b. Chronic inhalation toxicity of a kaolin-based refactory cermaic fiber in Syrian golden hamsters. Inhalation Toxicology 6(6): 503-532.
McConnell EE, Axten C, Hesterberg TW, Chevalier J, Miller WC, Everitt J, Oberdorster G, Chase GR, Thevenaz P, Kotin P. 1999. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster: Part II. Results of chronic exposure. Inhalation Toxicology 11(9): 785-835.
McDonald AD, Fry JS, Woolley AJ, McDonald JC. 1984. Dust exposure and mortality in an American chrysotile asbestos friction products plant. British Journal of Industrial Medicine 41(2): 151-157.
McDonald JC, Liddell FD. 1979. Mortality in Canadian miners and millers exposed to chrysotile. Annals of the New York Academy of Sciences 330: 1-9.
McDonald JC, McDonald AD. 1997. Chrysotile, tremolite and carcinogenicity. Annals of Occupational Hygiene 41(6): 699-705.
McDonald JC, McDonald AD, Gibbs GW, Siemiatycki J, Rossiter CE. 1971. Mortality in the chrysotile asbestos mines and mills of Quebec. Archives of Environmental Health 22(6): 677-686.
McDonald JC, Liddell FD, Gibbs GW, Eyssen GE, McDonald AD. 1980. Dust exposure and mortality in chrysotile mining, 1910-75. British Journal of Industrial Medicine 37(1): 11-24.
McDonald AD, Fry JS, Woolley AJ, McDonald J. 1983. Dust exposure and mortality in an American chrysotile textile plant. British Journal of Industrial Medicine 40(4): 361-367.
McDonald JC, McDonald AD, Armstrong B, Sebastien P. 1986. Cohort study of mortality of vermiculite miners exposed to tremolite. British Journal of Industrial Medicine 43(7): 436-444.
McDonald JC, Liddell FD, Dufresne A, McDonald AD. 1993. The 1891-1920 birth cohort of Quebec chrysotile miners and millers: Mortality 1976-88. British Journal of Industrial Medicine 50(12): 1073-1081.
Meurman LO, Pukkala E, Hakama M. 1994. Incidence of cancer among anthophyllite asbestos miners in Finland. Occupational and Environmental Medicine 51(6): 421-425.
Muscat J, Wynder E. 1992. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer 69(9): 2244-2251.
Olsen J, Sabroe S. 1984. Occupational causes of laryngeal cancer. Journal of Epidemiology and Community Health 38: 117-121.
Parnes SM. 1990. Asbestos and cancer of the larynx: Is there a relationship? Laryngoscope 100(3): 254-261.
Peto J, Doll R, Hermon C, Binns W, Clayton R, Goffe T. 1985. Relationship of mortality to measures of environmental asbestos pollution in an asbestos textile factory. Annals of Occupational Hygiene 29(3): 305-355.
Piolatto G, Negri E, La Vecchia C, Pira E, Decarli A, Peto J. 1990. An update of cancer mortality among chrysotile asbestos miners in Balangero, northern Italy. British Journal of Industrial Medicine 47(12): 810-814.
Pira E, Pelucchi C, Buffoni L, Palmas A, Turbiglio M, Negri E, Piolatto PG, La Vecchia C. 2005. Cancer mortality in a cohort of asbestos textile workers. British Journal of Cancer 92(3): 580-586.
Puntoni R, Merlo F, Borsa L, Reggiardo G, Garrone E, Ceppi M. 2001. A historical cohort mortality study among shipyard workers in Genoa, Italy. American Journal of Industrial Medicine 40(4): 363-370.
Raffn E, Lynge E, Juel K, Korsgaard B. 1989. Incidence of cancer and mortality among employees in the asbestos cement industry in Denmark. British Journal of Industrial Medicine 46(2): 90-96.
Rees L, Birchall M, Bailey M, Thomas S. 2004. A systematic review of case-control studies of human papillomavirus infection in laryngeal squamous cell carcinoma. Clinical Otolaryngology and Allied Sciences 29(4): 301-306.
Reid A, Ambrosini G, de Klerk N, Fritschi L, Musk B. 2004. Aerodigestive and gastrointestinal tract cancers and exposure to crocidolite (blue asbestos): Incidence and mortality among former crocidolite workers. International Journal of Cancer 111(5): 757-761.
Roggli VL, Greenberg SD, McLarty JL, Hurst GA, Spivey CG, Heiger LR. 1980. Asbestos body content of the larnyx in asbestos workers: A study of five cases. Archives of Otolaryngology 106(9): 533-535.
Rubino GF, Piolatto G, Newhouse ML, Scansetti G, Aresini GA, Murray R. 1979. Mortality of chrysotile asbestos workers at the Balangero Mine, northern Italy. British Journal of Industrial Medicine 36(3): 187-194.
Schlesinger RB, Lippmann M. 1978. Selective particle deposition and bronchogenic carcinoma. Environmental Research 15(3): 424-431.
Seidman H, Selikoff IJ, Gelb SK. 1986. Mortality experience of amosite asbestos factory workers: Dose-response relationships 5 to 40 years after onset of short-term work exposure. American Journal of Industrial Medicine 10(5-6): 479-514.
Selikoff IJ, Hammond EC. 1978. Asbestos-associated disease in United States shipyards. CA: A Cancer Journal for Clinicians 28(2): 87-99.
Selikoff IJ, Seidman H. 1991. Evaluation of selection bias in a cross-sectional survey. American Journal of Industrial Medicine 20(5): 615-627.
Selikoff IJ, Hammond EC, Seidman H. 1979. Mortality experience of insulation workers in the United States and Canada, 1943-1976. Annals of the New York Academy of Sciences 330: 91-116.
Shettigara PT, Morgan RW. 1975. Asbestos, smoking, and laryngeal carcinoma. Archives of Environmental Health 30(10): 517-519.
Sluis-Cremer GK, Liddell FD, Logan WP, Bezuidenhout BN. 1992. The mortality of amphibole miners in South Africa, 1946-80. British Journal of Industrial Medicine 49(8): 566-575.
Smailyte G, Kurtinaitis J, Andersen A. 2004. Cancer mortality and morbidity among Lithuanian asbestos-cement producing workers. Scandinavian Journal of Work, Environment and Health 30(1): 64-70.
Stell P, McGill T. 1973. Asbestos and laryngeal carcinoma. Lancet 2(7826): 416-417.
Szeszenia-Dabrowska N, Urszula W, Szymczak W, Strzelecka A. 2002. Mortality study of workers compensated for asbestosis in Poland, 1970-1997. International Journal Occupational Medicine and Environmental Health 15(3): 267-278.
Tola S, Kalliomaki PL, Pukkala E, Asp S, Korkala ML. 1988. Incidence of cancer among welders, platers, machinists, and pipe fitters in shipyards and machine shops. British Journal of Industrial Medicine 45(4): 209-218.
Wortley P, Vaughan TL, Davis S, Morgan MS, Thomas DB. 1992. A case-control study of occupational risk factors for laryngeal cancer. British Journal of Industrial Medicine 49(12): 837-844.
Wynder EL, Hoffmann D. 1976. Tobacco and tobacco smoke. Seminars in Oncology 3(1): 5-15.
Zagraniski RT, Kelsey JL, Walter SD. 1986. Occupational risk factors for laryngeal carcinoma: Connecticut, 1975-1980. American Journal of Epidemiology 124(1): 67-76.
Zeka A, Eisen EA, Kriebel D, Gore R, Wegman DH. 2004. Risk of upper aerodigestive tract cancers in a case-cohort study of autoworkers exposed to metalworking fluids. Occupational and Environmental Medicine 61(5): 426-431.
Zheng W, Blot WJ, Shu XO, Gao YT, Ji BT, Ziegler RG, Fraumeni JF Jr. 1992. Diet and other risk factors for laryngeal cancer in Shanghai, China. American Journal of Epidemiology 136(2): 178-191.
Zhu H, Wang Z. 1993. Study of occupational lung cancer in asbestos factories in China. British Journal of Industrial Medicine 50(11): 1039-1042.




















