5
Interpretation of Biomonitoring Results
INTRODUCTION
Finding chemicals in bodily fluids is evidence of contact with them through inhalation, dermal exposure, or ingestion, and it typically leads to two questions that pose important challenges in interpreting biomonitoring results and are the focus of this chapter:
-
Is the biomonitoring result in a range that is typical of the general, non-occupationally exposed population?
-
Does the biomonitoring result indicate a health risk?
This chapter describes various options for interpreting biomonitoring results with respect to those two questions and discusses how the analysis and interpretation can be used in different biomonitoring settings. The settings in which biomonitoring results may need interpretation include the workplace, the doctor’s office, screening of the general population, and study of specific subpopulations. The purpose and use of biomonitoring data may vary among those scenarios, but the options for interpreting the data are generally similar.
Other questions that are alluded to but not addressed in detail in this chapter include, how did the exposures occur? Are there means to decrease the exposures? These questions involve interpretation of biomonitoring data but also extend into risk-management issues.
Figure 5-1 is a flow diagram of the information provided in this chapter. When biomonitoring data become available, one must determine the
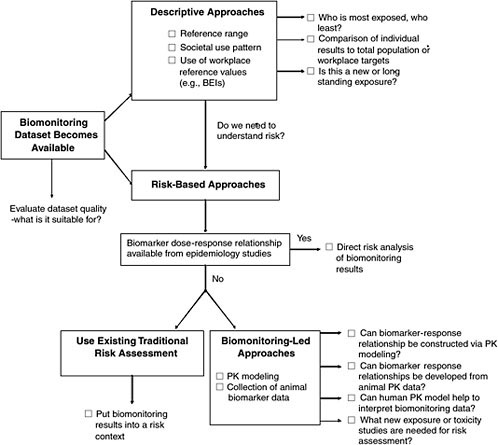
FIGURE 5-1 Overview of interpretive options for biomonitoring data.
interpretive options for evaluating them. The options include descriptive approaches that involve comparisons among biomonitoring datasets and risk-based approaches that describe the degree of risk associated with a given biomonitoring result. Throughout this chapter and in Appendix B, case studies are used to illustrate the applications of biomonitoring data to understanding of risk. The case studies are intended solely as illustrations and are not judgments about the data or risks associated with the chemicals discussed.
INITIAL REVIEW OF BIOMONITORING DATA
Interpreting biomonitoring results depends on the availability of various types of information, including data on exposure, toxicity, and toxicokinetics. If toxicity information is unavailable, the results cannot be put into a risk
context. If exposure information is unavailable, it may not be possible to determine where and how the exposures that produced the measured biomarker concentrations occurred. However, the starting point for interpreting biomonitoring data is an assessment of the quality of the biomonitoring data. If they are of low quality, there is little point in considering whether exposure or toxicity information is available. But high-quality biomonitoring data may be applied to a variety of interpretive options, as outlined below. Chapter 4 reviews the considerations relevant to the design of biomonitoring studies to ensure scientific quality and integrity.
OVERVIEW OF INTERPRETIVE OPTIONS FOR BIOMONITORING DATA
Two main options for interpreting biomonitoring results—descriptive and risk-based approaches—appear in Figure 5-1. This figure is organized from simplest to most complex approaches, with the potential for interpreting health risks also increasing from top to bottom. The expectation is that the quicker, descriptive approaches would be used first and then, depending on the level of concern and data availability, risk-based approaches would be used. The final interpretation of biomonitoring data would probably have elements of both.
Descriptive Approaches
The first level of analysis is purely descriptive, presenting a statistical review of the data, typically in the form of a data distribution from which percentiles of the population (such as 10th, 25th, 50th, 75th, and 90th percentiles) are easily obtained. That establishes a reference range with which individual or subgroup results can be compared. The range offers a point of comparison; individuals or subgroups may be within the range or may be subject to more or less exposure or vulnerability. A number of interpretive issues in this approach are described in this chapter. For the most part, the Centers for Disease Control and Prevention (CDC) analysis of biomonitoring results from its National Health and Nutrition Examination Survey (NHANES) is focused on the reference-range approach (CDC 2005).
Another descriptive approach characterizes a chemical’s use pattern in society at large. The information is used to interpret biomonitoring data in terms of how long the chemical may have been detected in bodily fluids and whether its concentration may be going up or down with changing use. It is not uncommon for the public to consider a new biomarker as evidence of new exposure. But it is possible that exposure has been going on for de-
cades and the biomarker became available only recently. Because new or increasing exposure generally prompts greater concern, the context is important. This question is best answered by analysis of biomonitoring results that span several years of sample collection. However, if biomonitoring results are available only for a single sampling round, temporal trends cannot be known. In such a case, historical data on chemical production rates and trends may be useful (if they are available).
Workplace biologic reference values are another descriptive option for interpreting biomonitoring results in the general population. Such values as the Biological Exposure Index (BEI) of the American Conference of Governmental Industrial Hygienists (ACGIH) are workplace standards used to evaluate whether individual workers have received exposures that exceed a workplace air standard, such as a Threshold Limit Value (TLV). A blood or urinary biomarker is a better indication of personal exposure than an area air sample. BEIs have been used as points of reference for biomonitoring results in the general public (CDC 2005). However, because BEIs do not take into account the differing exposure patterns (continuous vs 8-hour workshift exposure) and vulnerability of the general public (including children, pregnant women, the elderly, and the ill) compared with healthy workers, using BEIs to judge community exposure and risk raises numerous interpretive issues. This chapter reviews those issues and outlines major limitations in applying adjustment factors to BEIs to derive biomarker targets relevant to the general public.
Risk-Based Approaches
The most data-intensive approaches are those which evaluate the risk associated with a biomonitoring result. Evaluation of risk may be a desirable outcome, given the importance of the “How risky is this blood concentration” question and the fact that the descriptive approaches only provide relative information and do not assess risk. Figure 5-2 illustrates the various risk-based options discussed in the report.
In the most straightforward risk-based approach, epidemiologic studies have developed exposure-response relationships based on biomarker measurements in hair, blood, urine, or other matrices (e.g., mercury, lead) (see Figure 5-2a). The relationships can be applied directly to new biomonitoring data to determine where on the exposure-response curve any person is. That may facilitate an understanding of risk, but it does not analyze sources of exposure, so other techniques (such as environmental sampling and behavioral surveys) may be needed to assess where the exposure came from.
Because human biomarkers are rarely the basis of exposure-response relationships, practitioners generally rely on more traditional risk assess-
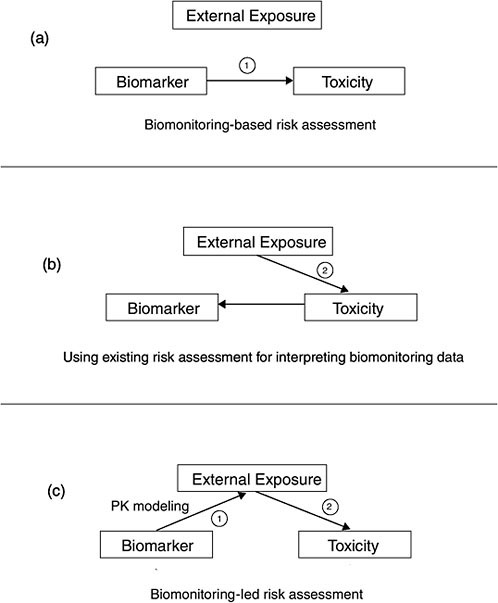
FIGURE 5-2 Illustration of the interpretative risk-based options.
ments. Those assessments characterize human exposure with a pathways analysis, accounting for concentrations in air, food, water, and soil to estimate human dose in milligrams per kilogram per day. The dose is then used to calculate risk on the basis of reference doses or cancer slope factors (see Figure 5-2b). Using existing risk assessments for interpreting biomonitoring data can help to put biomonitoring results into a broad risk
context that makes maximal use of the underlying exposure and toxicology data but falls short of actually calculating risk.
Another option attempts to convert biomonitoring results into a form that is directly useful for risk assessment. The chapter describes both the human pharmacokinetic (PK) modeling used to relate internal concentration to dose and the development of exposure-response relationships in animal studies that use biomarker concentrations rather than applied dose (see Figure 5-2c). Finally, the chapter describes how biomonitoring studies can augment and help to interpret traditional risk assessments.
Many communication challenges stem from collection, interpretation, and reporting of biomonitoring results. This chapter indicates where communication issues arise in relation to the interpretation of biomonitoring results; these issues are explored more fully in Chapter 6.
Case Examples Used in This Chapter
A number of case examples are used to illustrate the feasibility of the interpretive options described in this chapter. Some of the examples are presented in the chapter, and others are presented in Appendix B. Generally, examples were selected because they have the requisite data from epidemiology, PK, or animal toxicology studies to facilitate the risk interpretation of biomonitoring results. For many other chemicals that may be the subjects of biomonitoring, those types of data are not available and thus constitute biomarker-specific data gaps. Such data gaps need to be filled case by case on the basis of the type of biomarker and the underlying database to improve our interpretation of biomonitoring results. As exemplified by the examples presented, it may be most expeditious in some cases to obtain animal PK and in others to use human PK modeling or epidemiology studies (Table 5-1). However, obtaining data may take months. Some of the recommendations presented by the committee in Chapter 7 attempt to address the biomarker data gaps through a research agenda.
When data gaps are filled, there may be disagreement about how to apply the data for interpreting biomonitoring results. For example, the biomarker–toxicity relationship for methylmercury has been controversial because of the differences in results among major epidemiology studies (Appendix B). Although a national consensus has emerged after the National Research Council review of methylmercury (NRC 2000), there may not be an opportunity for such a comprehensive analysis of other biomarkers as data gaps are addressed and risk assessors use existing data.
The case studies in this chapter and in Appendix B are presented to illustrate particular points and are not intended to be exhaustive in their review or analysis of a chemical.
TABLE 5-1 Overview of Major Biomarker Case Examples Used to Illustrate Interpretive Options
|
Chemical |
Biomarker |
Interpretive Option Exemplified |
Where Presented |
|
PBDE |
PBDEs in blood and breast milk |
Biomonitoring studies demonstrate key data gaps; need to obtain new toxicity and exposure information |
|
|
Organophosphates |
Various metabolites |
Comparison of subpopulation with reference range |
|
|
Glyphosate |
Urinary glyphosate |
Use of existing risk assessment to put biomonitoring results into risk context |
|
|
Permethrin |
Urinary carboxylic |
Use of existing risk assessment to put biomonitoring results into risk context acid metabolite |
|
|
TCE |
Blood TCE |
Use of Bayesian techniques and bounding approaches to estimate exposure dose from non-steady-state blood concentration |
|
|
PFOA |
Serum PFOA |
Use of animal toxicology and physiologically based pharmacokinetic modeling to develop biomarker-response relationship in animals |
|
|
Lead |
Blood lead |
Use of epidemiology studies to develop biomarker-response relationship in humans |
|
|
Mercury |
Blood mercury |
Use of epidemiology studies to develop biomarker-response relationship in humans |
|
|
Chlorpyrifos |
Urinary TCP |
Use of pharmacokinetic modeling to estimate exposure dose from amount excreted in urine |
|
|
Phthalates |
Urinary monoester metabolites |
Use of pharmacokinetic modeling to estimate exposure dose from amount excreted in urine |
|
|
Dioxin |
Dioxin in blood or lipid |
Use of pharmacokinetic modeling to estimate body burden and daily dose |
|
|
Styrene |
Urinary metabolites |
Use of worker urinary metabolite-exposure information to develop pharmacokinetic model applicable to general public |
|
|
Abbreviations: PBDE = polybrominated diphenyl ether; TCE = trichloroethylene; PFOA = perfluorooctanoic acid; TCP = trichloro-2-pyridinol. |
|||
REFERENCE RANGES
Biomonitoring results can be interpreted at different levels of complexity (Figure 5-1). The reference-range approach represents the least complex level. It is only descriptive, offering a statistical presentation of data (Tables 5-2 and 5-3) for comparison with data from other populations or individuals but with no conclusions about risk potential. However, this approach is often the first stage in the more complex risk-related analyses discussed in the remainder of the chapter.
In the reference-range approach, reference ranges (or intervals),1 are established, and biomonitoring values from individuals or subgroups are compared with them. The validity and utility of biomonitoring values for use as reference ranges depends on study design and data quality, with special attention to the availability and comparability of data on the reference population in relation to the study population.
The overview below focuses on two fundamental elements of the reference-range approach: establishing a reference range and interpreting biomonitoring data in comparison with it. The remainder of this section details methods, principles, and issues related to data quality and reference-population selection and comments on regulatory uses of this approach and related cautions.
Overview
Establishing Reference Ranges
Recent biomonitoring efforts in the United States and Europe have placed a high priority on establishing reference ranges. For example, a central purpose of the Third National Report on Human Exposure to Environmental Chemicals (CDC 2005) is “to establish reference ranges that can be used by physicians and scientists to determine whether a person or group has an unusually high exposure.” The report updates and supplements two earlier reports (CDC 2001, 2003). As documented in Chapter 2, other nations and international organizations are developing comparable information.
The CDC sampling plan follows a “complex, stratified, multistage, probability cluster design to select a representative sample of the civilian noninstitutionalized population of the United States.” Relevant details are
TABLE 5-2 Blood Concentrations for Cadmium in the U.S. Population Aged 1 Year and Older
|
|
Survey Years |
Geometric Mean (95% confidence interval |
Selected Percentilesa |
|
50th |
|||
|
Total, age 1 year and older |
1999-2000 |
0.412 (0.378-0.449) |
0.300 (0.300-0.400) |
|
|
2001-2002 |
0.300 (<LOD-0.300) |
|
|
Age group 1-5 years |
1999-2000 |
<LOD |
|
|
|
2001-2002 |
<LOD |
|
|
6-11 years |
1999-2000 |
<LOD |
|
|
|
2001-2002 |
<LOD |
|
|
12-19 years |
1999-2000 |
0.333 (0.304-0.336) |
0.300 (<LOD-0.300) |
|
|
2001-2002 |
<LOD |
|
|
20 years and older |
1999-2000 |
0.468 (0.426-0.513) |
0.400 (0.300-0.400) |
|
|
2001-2002 |
0.300 (0.300-0.400) |
|
|
Sex |
|
|
|
|
Male |
1999-2000 |
0.403 (0.368-0.441) |
0.400 (0.300-0.400) |
|
|
2001-2002 |
0.300 (<LOD-0.300) |
|
|
Female |
1999-2000 |
0.421 (0.386-0.460) |
0.300 (0.300-0.400) |
|
|
2001-2002 |
0.300 (0.300-0.400) |
|
|
Race or ethnicity Mexican Americans |
1999-2000 |
0.395 (0.367-0.424) |
0.400 (0.300-0.400) |
|
|
2001-2002 |
<LOD |
|
|
Non-Hispanic blacks |
1999-2000 |
0.393 (0.361-0.427) |
0.300 (0.300-0.400) |
|
|
2001-2002 |
<LOD |
|
|
Non-Hispanic whites |
1999-2000 |
0.420 (0.376-0.470) |
0.400 (0.300-0.400) |
|
|
2001-2002 |
<LOD |
|
|
aLOD = limit of detection, which may vary for some chemicals by year and by individual sample. bNot calculated. Proportion of results below limit of detection was too high to provide valid result. Source: CDC 2005. |
|||
developed in Chapter 4. The monitored populations are in broad groups defined by age, sex, and race or ethnicity. Data are analyzed and presented in eight main categories: 6-11 years old, 12-19 years old, over 20 years old; males, females, Mexican Americans, non-Hispanic blacks, and non-Hispanic whites. Other racial groups are sampled as part of the total population but do not make up a large enough proportion of the total to provide valid estimates. Newborns and infants are not included, because of difficulties (such as parental resistance and sample size) in obtaining biomonitoring data for these age groups.
|
(in mg/L) (95% confidence interval) |
|
||
|
75th |
90th |
95th |
Sample Size |
|
0.600 (0.500-0.600) |
1.00 (0.900-1.00) |
1.30 (1.20-1.40) |
7,970 |
|
0.400 (0.400-0.500) |
0.900 (0.900-1.10) |
1.30 (1.20-1.60) |
8,945 |
|
0.300 (<LOD-0.300) |
0.400 (0.300-0.400) |
0.400 (0.300-0.400) |
723 |
|
<LOD |
<LOD |
0.300 (<LOD-0.300) |
898 |
|
0.300 (<LOD-0.300) |
0.400 (0.300-0.400) |
0.400 (0.400-0.500) |
905 |
|
<LOD |
<LOD |
0.400 (0.300-0.400) |
1,044 |
|
0.300 (0.300-0.400) |
0.800 (0.600-0.900) |
1.10 (0.900-1.10) |
2,135 |
|
0.300 (<LOD-0.300) |
0.400 (0.400-0.500) |
0.800 (0.600-1.10) |
2,231 |
|
0.600 (0.600-0.700) |
1.00 (1.00-1.10) |
1.50 (1.40-1.60) |
4,207 |
|
0.600 (0.500-0.600) |
1.10 (0.900-1.20) |
1.60 (1.30-1.80) |
4,772 |
|
0.600 (0.500-0.600) |
1.00 (0.900-1.10) |
1.30 (1.20-1.50) |
3,913 |
|
0.400 (0.400-0.500) |
0.900 (0.900-1.10) |
1.40 (1.20-1.80) |
4,339 |
|
0.600 (0.500-0.600) |
1.00 (0.800-1.00) |
1.30 (1.10-1.40) |
4,057 |
|
0.500 (0.500-0.600) |
1.00 (0.900-1.10) |
1.40 (1.20-1.60) |
4,606 |
|
0.400 (0.400-0.500) |
0.700 (0.700-0.900) |
1.10 (0.900-1.30) |
2,742 |
|
0.300 (0.300-0.400) |
0.600 (0.500-0.700) |
1.00 (0.700-0.900) |
2,268 |
|
0.600 (0.500-0.600) |
1.00 (0.800-1.10) |
1.40 (1.10-1.50) |
1,842 |
|
0.400 (0.400-0.500) |
1.00 (0.900-1.00) |
1.40 (1.20-1.50) |
2,219 |
|
0.500 (0.500-0.600) |
1.00 (0.900-1.10) |
1.30 (1.20-1.40) |
2,716 |
|
0.500 (0.500-0.600) |
0.900 (0.900-1.10) |
1.40 (1.20-1.80) |
3,806 |
As shown in Tables 5-2 and 5-3, the data on each group include survey period, geometric mean, population sample size, and the biomarker concentration at the 50th, 75th, 90th, and 95th percentiles of the population distribution.
Comparison with a Reference Population
At the simplest level of interpretation of biomonitoring data, a biomarker concentration found in an individual or group under study is com-
TABLE 5-3 Urine Concentrations for Cadmium in the U.S. Population Aged 6 Years and Older
|
|
Survey Years |
Geometric Mean (95% confidence interval |
Selected Percentilesa |
|
50th |
|||
|
Total, age 6 years and older |
1999-2000 |
0.193 (0.169-0.220) |
0.232 (0.214-0.249) |
|
|
2001-2002 |
0.210 (0.189-0.235) |
0.229 (0.207-0.255) |
|
Age group |
|
|
|
|
6-11 years |
1999-2000 |
0.078 (0.061-0.101) |
|
|
|
2001-2002 |
0.061 (<LOD-0.081 |
0.077 (0.067-0.092) |
|
12-19 years |
1999-2000 |
0.092 (0.067-0.126) |
0.128 (0.107-0.148) |
|
|
2001-2002 |
0.109 (0.087-0.136) |
0.135 (0.114-0.157) |
|
20 years and older |
1999-2000 |
0.281 (0.253-0.313) |
0.306 (0.261-0.339) |
|
|
2001-2002 |
0.273 (0.249-0.299) |
0.280 (0.261-0.308) |
|
Sex |
|
|
|
|
Male |
1999-2000 |
0.199 (0.165-0.241) |
0.227 (0.193-0.263) |
|
|
2001-2002 |
0.201 (0.177-0.229) |
0.223 (0.191-0.257) |
|
Female |
1999-2000 |
0.187 (0.153-0.229) |
0.239 (0.220-0.255) |
|
|
2001-2002 |
0.219 (0.192-0.251) |
0.234 (0.202-0.265) |
|
Race or ethnicity |
|
|
|
|
Mexican Americans |
1999-2000 |
0.191 (0.157-0.233) |
0.202 (0.167-0.221) |
|
|
2001-2002 |
0.160 (0.135-0.189) |
0.181 (0.171-0.198) |
|
Non-Hispanic blacks |
1999-2000 |
0.283 (0.208-0.387) |
0.312 (0.243-0.412) |
|
|
2001-2002 |
0.277 (0.229-0.336) |
0.302 (0.257-0.354) |
|
Non-Hispanic whites |
1999-2000 |
0.175 (0.148-0.206) |
0.220 (0.194-0.246) |
|
|
2001-2002 |
0.204 (0.179-0.231) |
0.221 (0.191-0.255) |
|
aNot calculated. Proportion of results below limit of detection was too high to provide valid result. Source: CDC 2005. |
|||
pared with that in a reference population. That approach depends on a suitable reference population and a body of biomonitoring data collected in comparable fashion that can serve as a reference range. (For discussion of an appropriate comparison population, see Chapter 4.)
Figure 5-3 illustrates the distribution of biomarker concentrations in a generic reference population, expressed as cumulative frequency. As is commonly done in a clinical test, the 95th percentile of the distribution can be used to determine the upper limit value of this test result. However, a different percentile may be chosen, depending on the circumstances, the characteristics of the reference population, the distribution of the results, and the intended purpose of the study. It is important to be aware that a particular cut point does not represent a bright line that automatically separates the population into typical vs highly exposed, or no risk vs high risk (when, for example, BEIs or risk-based targets are used). Rather, it is a guideline to point out where in the population distribution exposures may require more detailed analysis of sources and health risks.
|
(in mg/L) (95% confidence interval) |
Sample Size |
||
|
75th |
90th |
95th |
|
|
0.475 (0.436-0.519) |
0.858 (0.763-0.980) |
1.20 (1.06-1.33) |
2,257 |
|
0.458 (0.423-0.482) |
0.839 (0.753-0.919) |
1.20 (1.07-1.28) |
2,690 |
|
0.141 (0.115-0.173) |
0.219 (0.178-0.233) |
0.279 (0.211-0.507) |
310 |
|
0.140 (0.112-0.160) |
0.219 (0.184-0.262) |
0.282 (0.260-0.326) |
368 |
|
0.202 (0.183-0.232) |
0.329 (0.272-0.372) |
0.424 (0.366-0.596) |
648 |
|
0.210 (0.189-0.247) |
0.327 (0.289-0.366) |
0.442 (0.366-0.480) |
762 |
|
0.551 (0.510-0.621) |
0.979 (0.836-1.13) |
1.31 (1.13-1.57) |
1,299 |
|
0.545 (0.493-0.607) |
0.955 (0.855-1.06) |
1.28 (1.20-1.43) |
1,560 |
|
0.462 (0.381-0.539) |
0.892 (0.748-1.15) |
1.41 (0.980-1.83) |
1,121 |
|
0.445 (0.393-0.481) |
0.870 (0.741-1.03) |
1.22 (1.12-1.38) |
1,335 |
|
0.492 (0.456-0.540) |
0.806 (0.705-0.980) |
1.10 (1.01-1.19) |
1,136 |
|
0.466 (0.433-0.519) |
0.817 (0.733-0.886) |
1.17 (0.918-1.36) |
1,355 |
|
0.438 (0.351-0.551) |
0.813 (0.686-0.977) |
1.12 (0.886-1.38) |
780 |
|
0.321 (0.285-0.362) |
0.559 (0.430-0.733) |
0.766 (0.633-1.15) |
683 |
|
0.633 (0.498-0.806) |
1.22 (0.892-1.38) |
1.48 (1.30-1.72) |
546 |
|
0.580 (0.476-0.713) |
1.04 (0.843-1.38) |
1.51 (1.28-1.74) |
667 |
|
0.455 (0.388-0.510) |
0.797 (0.714-1.01) |
1.17 (0.963-1.47) |
760 |
|
0.445 (0.394-0.479) |
0.813 (0.717-0.875) |
1.17 (0.989-1.24) |
1,132 |
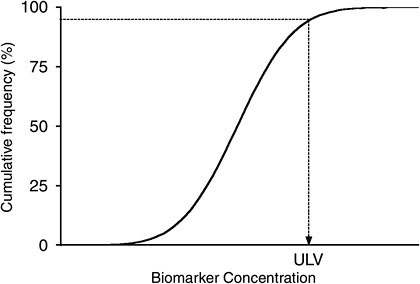
FIGURE 5-3 Distribution of biomarker concentrations in generic reference population. ULV is the upper limit value.
Thus, although the 95th percentile is commonly used and provides a convenient reference value, a variety of factors recommend case-by-case attention to cut-point selection. In a distribution influenced by unique but low percentage subgroups, the 95th percentile may include a unique subgroup that is distinct from the remainder of the population in PK factors. Those factors may lead to much higher internal concentrations than the central tendency. To include such a group in the reference range would encourage an analyst to overlook important heterogeneity in the population. If a subgroup is large enough to cause the overall distribution to be bimodal, a reference range for each group may be needed to characterize the population fully. In that case, the internodal or inflection point is a critical value that distinguishes subpopulations.
Finally, if the 95th percentile is highly unstable or uncertain because of high variability (for example, if the distribution has a long tail), a more stable percentile would be preferable.
Comparison with a reference range is useful for all applications in which a reference population is available. In the workplace, one may be able to identify high-exposure job categories by using biomonitoring results and evaluate whether measures to mitigate exposure are working to bring subgroups back toward the reference range. Air monitoring may also be helpful in that regard. Establishing a reference range for the general population can help in interpreting the results on an individual subject by assessing where on the results distribution the person lies. Results near or below the median suggest that exposure is not particularly high in relation to everyone else’s. Some people will be at the upper end of the distribution and lie outside the reference range. It may be important to identify and study them further to determine the characteristics (such as location of dwelling, product use, and other personal behaviors) and physiologic factors (such as genetic polymorphisms) that caused the high exposures. It may be equally valuable to understand why other people lie at the lower end of the distribution; such information may offer lessons in controlling exposure to some chemicals. Unfortunately the tendency is for biomonitoring studies to highlight central tendency and upper-bound statistics and not to report the lower end of the distribution. Therefore, researchers may overlook individuals who are important to evaluate because of either low exposures or genetic factors that favor chemical clearance.
Pertinent to the above comparisons is the assumption that the data are from a single study that used a uniform sampling design and method, which initially treated the sampled group as a single population. However, biomonitoring can also study subgroups suspected of having unique exposures—for example, because they lived next to a potential source—in a cross-sectional design. Those results can be informative when compared with the reference range and can help to answer the question of whether a
|
BOX 5-1 Case Example: Organophosphorus Metabolites in Pregnant Farm Workers A recent report illustrates the utility of the reference-range approach. In a study designed to collect descriptive information on urinary concentrations of organophosphorus (OP) metabolite during and soon after pregnancy, Bradman et al. (2005) collected samples from 600 pregnant low-income women living in California’s Salinas Valley. Some 28% were employed as farm field workers during pregnancy. For 81% of these women, at least one member of their household worked in agriculture. In addition to providing comparative antepartum and postpartum OP metabolite concentrations in the study population, the study compared data in these women with data on women 19-40 years old in the general U.S. population surveyed for the Second National Exposure Report (NHANES) (CDC 2003). The investigators reported that pregnant women living in an agricultural area had higher concentrations of the metabolites than the general U.S. population. Used in that way, the biomarker exemplifies the type of comparative information that can be obtained with what Chapter 3 classifies as a category II biomarker (a reliable method for indexing internal exposure and useful for characterizing reference ranges and comparisons among population groups). |
suspected contaminant source is, in fact, leading to increased exposures and body burden. Box 5-1 presents a case example of the utility and limitations of reference ranges.
Methodology, Principles, and Issues
Ideally, reference ranges consist of biomonitoring values developed according to scientifically rigorous study design and quality-control procedures (see Chapter 4). The utility of reference ranges depends on attention to reference populations and data quality.
Reference Populations
The reference-range approach depends on data availability and data comparability for both the reference and test populations. Ideally, the two populations are comparable in age, race or ethnicity, sex, and other demographic factors and were analyzed for the same end point in the same tissues or fluids (see introduction to this chapter and Chapter 4).
Interpreting biomonitoring data from individuals or groups depends on the characteristics of the reference group (Viau et al. 2000). The importance of comparability cannot be overstated. For example, comparative data on
urinary 1-hydroxypyrene (1-OHP) in a rural population in Burundi suggest that it might be more relevant to compare this population with other inhabitants of Burundi—even urban residents living in the capital, Bujumbura— than with an urban population in North America. There may be any number of reasons for this, such as more comparable lifestyle and exposures within a country than between continents and greater relevance for interpreting the opportunity for public-health intervention within a country. As shown in Figure 5-4, the rural population of this study (no occupational exposure) excreted considerably more 1-OHP than did the in-country urban reference group and as much urinary 1-OHP as some populations of workers in the creosote industry (data not shown). The rural population had no occupational exposure but was heavily exposed to polycyclic aromatic hydrocarbons (PAHs) through the use of indoor wood-burning for cooking purposes. A different type of comparison could be made among
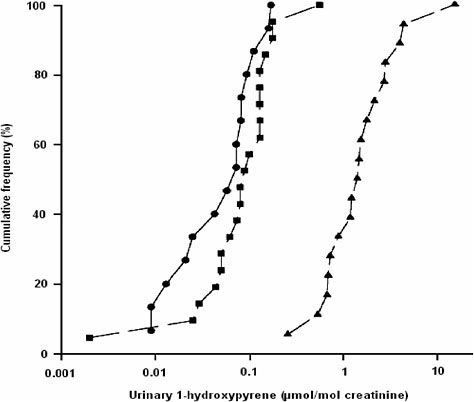
FIGURE 5-4 Cumulative frequency distribution of the urinary excretion of 1-hydroxypyrene in people living in two rural districts of Burundi (▲), in Bujumbura (•) and in a reference group at the University of Montreal (■). Source: Viau et al. 2000. Reprinted with permission; copyright 2000, International Archives of Occupational and Environmental Health.
urban residents between continents (Bujumbura vs North American populations). In other words, the reference group must always be selected on the basis of the study’s objectives and a priori knowledge of exposure of the reference group.
In addition, information is needed on the history of exposure to the parent chemical leading to the biomarker of interest and on potential confounders. In constructing a reference range, the sampled population is important to consider. Interindividual variability in biomonitoring results will be a function of differences not only in exposure but also in pharmacokinetics with regard to metabolic and excretory clearance. Such host factors as age, genetic polymorphisms, clinical disease, medication and alcohol use, and nutritional status can affect pharmacokinetics. The blending of those factors throughout the population generally creates a broad population distribution.
If some segments of the population are underrepresented, the full population distribution may not be adequately represented. For example, if the frequency of a metabolic genotype that slows chemical clearance occurs naturally at a rate of 30% in the population but were only sampled at a rate of 5% in the reference population, the reference range will be statistically biased low. A person in the slow-clearance group would have a biomonitoring result well above the central tendency, which would suggest high exposure. However, a reference population that is more representative of the subgroup would show that the individual result is not so different from the expected value. Developing reference ranges representative of the general population, often desirable, is sometimes difficult because sources of variability are unknown and it is impossible to try to account for key groups in their proper percentage. The hope is that the reference population will be representative if it includes large numbers of people and sampling is directed toward obvious groups (for example, of particular ethnicities, ages, and geographic areas).
Although the reference range should be inclusive and representative of the general population, it may be desirable to exclude a group from the reference population to make comparisons between the group and the reference population more meaningful. In such a case, care should be taken to describe which groups are included and which are excluded from the reference population. A reference population that excludes any particular group has the advantage of allowing comparison of that group with the remainder of the population to identify statistical differences. In the case of the slow metabolizer genotype mentioned above, keeping this group separate from the general population would allow evaluation of the impact of the genotype on biomarker levels and potentially also risk. But, including the group in the reference population allows one to determine where a particular result falls in the overall population distribution.
As another example, if biomonitoring results on people who live near a contamination source (proximity subgroup) are being evaluated, these results can be lumped in with those on the remainder of the population to create an overall distribution. However, the contribution of the group may shift the distribution, and unless there are large exposure differences (as in a bimodal distribution), it may be difficult to distinguish the proximity group from the rest of the population. Keeping the proximity group separate would allow more direct comparisons with the reference population and more relevant interpretation of study results.
Data Quality
Ideally, reference ranges are developed from biomonitoring data that conform to the study design and to data-quality considerations (see Chapter 4). For example, valid comparisons depend on comparable sampling methods for collecting data from reference and test populations. Similarly, comparisons are meaningful only if comparable statistical methods are applied in analyzing and reporting the data.
Comparison with a reference range, or with a cut point from a reference range, can be influenced by the type of data qualifiers that introduce uncertainty into any biomonitoring result. For example, short half-life is important because it detracts from how well a biomarker can represent a long-term exposure pattern. Biomarker results for a rapidly eliminated chemical are indicative only of the most recent exposure. That may skew an individual result high or low depending on the timing of sample collection relative to the last exposure. For a short-lived biomarker, the reference range would need to be developed from a large population to capture the variability contributed by sample timing in addition to the other variabilities inherent in the population (behaviors that affect exposure and pharmacokinetics).
Even with an adequate reference sample, comparison of an individual result with the reference range presents considerable uncertainty for short-lived biomarkers because of variability in a single result from a single person. Repeat sampling of a given person or other forms of exposure assessment (such as an exposure questionnaire) may be needed to improve the ability to compare an individual result with a reference range. In the case of a long-lived biomarkers, a single sampling event and a moderately sized sample population may be sufficient for useful comparisons.
It is important to note that short half-life chemicals do have the potential to be useful biomarkers for establishing the reference range if exposure is fairly frequent at about the same concentrations. In this case, rapid clearance can be balanced by frequent exposure to yield a stable blood or urinary concentration. Biomonitoring examples with cotinine and phthalates, as described in Chapter 4, illustrate this point.
Some cautions require attention. Reference ranges do not provide information on health status or risk. The primary issue is the extent to which the sampled population represents the total population. Although volumes of biomonitoring data are available from different periods and populations, the data must be reviewed to clarify the extent to which they can be used as reliable reference values for the general population and defined subpopulations. Poulsen et al. (1994) observe, for example, that most of the available data on trace elements in the Danish population are “of limited use as reliable baseline data” on that population because the studies give insufficient attention to important aspects of establishing reliable reference intervals—specifically, definition and characterization of the reference population, preanalytic factors and quality assurance of analytic methods, and statistical treatment and presentation of data. Regarding characterization of the reference population, less than half the papers reviewed by Poulsen et al. (1994) gave sufficient detail on sex, age, residence, health status, lifestyle, and occupation of the reference population. Preanalytic factors requiring attention included sample contamination during collection and instability during storage.
The ability to define a reference population can be a substantial limitation. There may be insufficient information regarding the exposure history of the reference population to a particular contaminant of interest. It is important to know the range of values of a given biomarker in a “normal” population (NRC 1991), where normal is regarded as without occupational exposure or without observed or hypothetical environmental exposure. However, Schulte and Talaska (1995) point out that pristine populations are rare, so “nonexposed” populations generally have some exposure of widely varied extent.
Appropriate reference populations are not always available. For example, recruiting children, especially newborns and infants, is difficult. When it is possible, the samples are usually small (European Commission 2004).
Use in the Regulatory Context
Reference-range information permits officials to compare exposures in specific geographic or demographic groups with those in the general (reference) population. The reference range may be chosen at a fixed time to facilitate evaluation of temporal trends and the effects of regulatory interventions. Because, a reference range can be critical in deciding whether public-health action is warranted (Box 5-2) or in evaluating the effectiveness of regulatory initiatives, informed attention to the construction of statistically valid and representative reference ranges is imperative (GAO 2000).
In the same vein, the absence of reliable reference ranges limits the
|
BOX 5-2 Reference Ranges Encourage Public-Health Action In one community where citizens were concerned about exposure to dioxins from nearby chemical manufacturing plants, Agency for Toxic Substance and Disease Registry (ATSDR) officials had CDC’s laboratory analyze blood samples and found that some residents had concentrations of several dioxins above the highest in a CDC-ATSDR-developed reference range. In response, ATSDR helped residents to obtain assistance from medical professionals expert in dioxins and, working with state and federal environmental agencies, began environmental testing to locate exposure sources (GAO 2000). |
utility of biomonitoring data. Over 60% of state officials responding to a General Accounting Office (now Government Accountability Office) survey said that the lack of reference-range data prevented them from using human exposure data in their work (GAO 2000). That situation can arise when datasets are biased in some way, background exposures in the control population are not well characterized, or other sampling requirements are not fully met.
In appropriate cases, reference ranges established in other studies can provide helpful information. For example, in biomonitoring studies around hazardous-waste sites, industrial emission sources, and other point sources, it is not always possible to have a control population of sufficient size to yield an adequate reference or comparison range (Pirkle et al. 1995). As pointed out in the OP-pesticide example in Box 5-3, those types of studies often rely on a reference range developed by other investigators who worked with a broad population sample that may be unrelated to the group under investigation. If that is done, the cautions noted earlier regarding similarity
|
BOX 5-3 Potential Utility of Pilot Data from “Other” Populations As part of NHANES III, a subsample of about 1,000 people provided blood and urine to determine reference ranges for 32 volatile organic compounds (blood) and 12 pesticides (urine). Demographic subgroups were defined by urban-rural status, region of the country, age, sex, race or ethnicity, and so on. The urinary measurements included metabolites of pesticides, such as carbaryl, naphthlalene, propoxur, carbofuran, parathion, and chlorpyriphos (Pirkle et al. 1995). The datasets demonstrate the feasibility of developing reference ranges for emerging analytes. |
in sampling design and overall methodology between reference and subpopulation datasets are especially relevant.
As stated at the outset, reference ranges do not provide conclusions on safety or risk. Presenting that fact and other limitations is an essential aspect of communicating reference-range information to individuals, the general public, and organizational decision-makers—a topic developed more fully in Chapter 6.
ADAPTING WORKPLACE BIOLOGIC REFERENCE VALUES FOR INTERPRETING BIOMONITORING RESULTS
Comparing Occupational Reference Values with Results of the National Health and Nutrition Examination Survey
The use of reference ranges has been considered as a way to compare the exposure in an individual or group against a reference group, generally taken to mean a random sample of the general population. Another type of comparison group is workers, who represent a convenient point of reference because a number of biologic reference values have been established for this population; these values are biomonitoring criteria, typically blood or urinary concentrations, that if exceeded indicate worker overexposure to an occupational toxicant.
The frequently cited sources of biomarker reference values include ACGIH (2005), the Deutsche Forschungsgemeinschaft (DFG) (DFG 2004), and Lauwerys and Hoet (2001). In the ACGIH sourcebook on TLVs and BEIs (ACGIH 2005), BEIs were proposed for 42 substances or groups of substances (such as methemoglobin inducers). Because a number of the substances have more than one determinant (biomarker), a total of 71 determinants were reported.
Table 5-4 compares ACGIH BEIs with the NHANES median and 95th percentile for the same determinants in the July 2005 CDC report (CDC 2005). The purpose of this comparison, in conjunction with the discussions below, is to objectively assess the utility and limitations of using BEIs to interpret biomonitoring results in the general population. To make results as comparable as possible between limit values set for workers and those observed in the general population, only the distribution in subjects 20 years old and older was considered in the NHANES results. It should be noted that specifications regarding sampling time are included with the BEIs and are an integral part of the ACGIH recommendations.
As shown in Table 5-4, the NHANES median values observed for the four metals in the U.S. population correspond, on the average, to about 4% of the BEIs, whereas the 95th percentile values reach up to over 30% of the BEI. Mercury is a special case because occupational exposures to mercury
TABLE 5-4 Comparison of Biomarker Reference Values Proposed by ACGIH (2005) and Observed Concentrations in Adults for Same Determinants from NHANES 1999-2002 (CDC 2005)
|
Substance |
Tissue |
BEI |
NHANES Median |
% of BEI |
NHANES 95th percentile |
% of BEI |
|
Cadmium |
Urinary cadmium |
5 µg/g of creatinine |
0.27 µg/g of creatinine |
5.5 |
0.98 µg/g of creatinine |
19.6 |
|
Cadmium |
Blood cadmium |
5 µg/L |
0.3 µg/L |
6.0 |
1.6 µg/L |
32.0 |
|
Cobalt |
Urinary cobalt |
15 µg/L |
0.37 µg/L |
2.5 |
1.15 µg/L |
7.7 |
|
Lead |
Blood lead |
300 µg/L |
16 µg/L |
5.3 |
46 µg/L |
15.3 |
|
Mercurya |
Urinary mercury |
35 µg/g of creatinine |
0.65 µg/g of creatinine |
1.9 |
3.0 µg/g of creatinine |
8.6 |
|
Mercury |
Blood mercury |
15 µg/L |
0.7 µg/L |
4.7 |
4.6 µg/L |
30.7 |
|
Parathionb |
Urinary p-nitrophenol |
0.5 mg/g of creatinine |
<LOD3 |
— |
0.00289 mg/g of creatinine |
0.6 |
|
Pentachlorophenol |
Urinary pentachlorophenol |
2 mg/g of creatinine |
<LOD |
— |
0.00206 of mg/g creatinine |
0.1 |
|
aNHANES results for mercury are for women 16-49 years old. BEIs for mercury apply only to exposure to inorganic forms, whereas main population exposure is usually to organic forms. bNHANES results are for parathion and other organophosphorous pesticides. |
||||||
concern its inorganic forms almost exclusively and the BEIs apply only to these forms. In contrast, the general population is exposed largely to the organic forms. The comparisons presented in Table 5-4 are therefore of limited value for this specific metal. For parathion and pentachlorophenol, the 95th percentile values are below 1% of the BEI. PAHs are also assessed in NHANES and ACGIH proposes only one biomarker, 1-hydroxypyrene. However, ACGIH does not recommend a specific limit value for this determinant.
Comparing Biological Exposure Index and Biologischer Arbeitsstoff-Toleranz Wert
Morgan and Schaller (1999) analyzed the differences between the bases for setting Biologischer Arbeitsstoff-Toleranz Wert (BAT, Biologic Tolerance Value for Occupational Exposures) and BEI values. The BEI generally corresponds to the mean biomarker concentration that would result from inhalation-only exposure to the parent chemical at its TLV. Some workers would be expected to be able to exceed that value without harm because of inherent interindividual variability. BAT values are health-based and are conceived of as ceiling values for healthy people. As a result, BAT values are expected to be higher than BEI values. That difference underscores the importance of carefully examining the basis of a given occupational reference value before making simple arithmetic adjustments to obtain a reference value applicable to the general population.
Considerations in Deriving Reference Values for the General Population
The committee cannot make a generic recommendation on applying occupational limit values to the general population. Specifically, ACGIH explicitly states that “the values are inappropriate to use for the general population or for nonoccupational exposures” (ACGIH 2003). Similarly, DFG indicates that “BAT values are not suitable for the derivation, by means of fixed conversion factors, of biological threshold values for long-term nonoccupational exposure such as from air pollution or contaminants in food” (DFG 2004).
Thus, although it may be tempting to lower a BEI by time-weighting and uncertainty factors that extrapolate from workers to the general public, the factors listed below must be carefully considered, and such extrapolations should be used with caution. They may be appropriate in specific situations.
Route of Entry
In the occupational setting, inhalation is the main route of entry of numerous chemical substances. For some solvents (such as dimethylformamide), dermal exposure may also be a major route of entry. Ingestion usually comes last, although poor hygiene in the workplace may result in substantial exposure to aerosolized or particulate contaminants by this route. In comparison, ingestion is often the principal exposure pathway for the general population. Assuming that an objective is to protect the general population from the same systemic toxic effects as workers, the importance of potentially different routes of entry must be examined. For example, the liver first-pass effect on ingested substances might alter the proportion of metabolites formed from a parent chemical. The pharmacokinetics of a biomarker may also be modified by different rates of absorption after exposure by various routes.
Dose-Response Relationships
An occupational limit value may be set to protect workers from, for example, central nervous system (CNS) effects that might occur when exposures are near the maximal acceptable concentrations in the workplace. But CNS effects might be irrelevant for the general environmental exposure situations where a different effect might be more critical. The slopes of the dose-response relationships may be different for those various effects. In such cases, attempting to adapt the occupational limit value to environmental exposure situations by using a fixed conversion factor might be inappropriate. While evaluating the potential for the general public to develop the workplace critical effect (CNS effects may be of interest), it is important to evaluate the full toxicologic profile and determine whether other effects may present greater risks to the general public. Considerations bearing on the level of protection applied (uncertainty factors, safety factors for children, or low-dose linear approaches for carcinogens) also may differ between the occupational and the public-health setting. Additionally, when chronic exposure begins in childhood, children have many more years to live during which slowly developing adverse health effects might occur.
The workplace airborne concentration of a substance and the biomarker concentration in exposed workers typically vary as illustrated in Figure 5-5. Although such relationships aid in interpreting occupational biomonitoring data on a group basis, the effect of such variability (see Chapter 4) must be considered, as must the shape of the dose–biomarker concentration relationship at the low-dose end of the correlation. The dispersion of data points around the regression line may be due to biologic variability and partial inadequacy of air-concentration measurements for inferring actual exposure
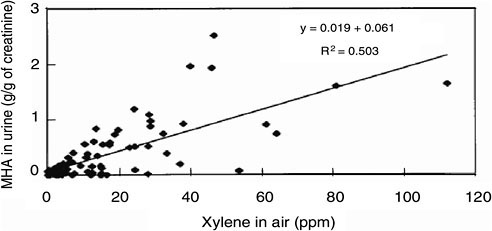
FIGURE 5-5 Daily average workplace xylene air and urinary methylhippuric acid (MHA) in exposed workers. Source: Jang et al. 2001. Reprinted with permission; copyright 2001, International Archives of Occupational and Environmental Health.
(Viau 2005). Indeed, such factors as varying ventilation rates associated with varying workloads and dermal exposure are unaccounted for by the air measurement; this might make the biomarker a better metric of the actual exposure than the air-concentration measurement of the parent chemical.
Pharmacokinetic Factors
In addition to differences in absorption pharmacokinetics—possibly due to differences in the major route of entry—physical activity, inhalation rate, and cardiac output affect absorbed and distributed doses. Greater amounts of a chemical are inhaled when respiration rate is increased, and greater cardiac output can increase chemical absorption and delivery to key excretory and metabolic organs. Those PK factors can modify the relationship between inhaled concentration and biomarker concentration, especially of metabolites detected in blood or urine. Sampling time is important. For biomarkers having a long half-life, such as urinary cadmium, sampling time is not considered critical. However, for short-lived biomarkers, such as urinary methanol, or those with a specific sampling time, such as urinary pentachlorophenol (before the last shift of the workweek), the PK rationale for setting the BEI must be considered before using this value in setting a general population reference.
Furthermore, whereas biologic limit values are set for workers exposed 8 hours/day, 5 days/week for a working lifetime, the potential exposure of the general population is generally assumed to be 24 hours/day, 7 days/ week for an entire lifetime. ACGIH (2003) addresses that point as follows:
“although modified work schedules are sometimes used in various occupations, ACGIH does not recommend that any adjustment or correction factor be applied to the BEIs (i.e., the BEIs should be used as listed, regardless of the work schedule).”
Occupational exposure to chemical substances almost invariably involves multiple chemicals. That situation may result in PK interactions, which may affect the relationship between the atmospheric concentration of the parent chemical and the associated biomarker concentration (Viau 2002). For example, such an interaction is known to occur between ethylbenzene and the xylene isomers (Jang et al. 2001). Commercial xylene contains about 20% ethylbenzene, which modifies the slope of the relationship between urinary methylhippuric acid (MHA) and airborne xylene concentrations. That kind of interaction is unlikely at the subparts-per-million exposure concentrations seen in the general population. But because the BEI for MHA was obtained from the relationship observed after exposure to commercial xylene, thereby taking the interaction into account, the slope of the relationship cannot be extrapolated to the subparts-per-million range. Similar PK interactions have been observed for other mixtures but only at concentrations nearing or exceeding the occupational exposure limits (Viau 2002), so it would be a priori reasonable to consider extrapolation of the relationship between biomarker concentrations and those of their parent chemicals. For example, Tardif et al. (1991) demonstrated that, provided inhalation exposure to a mixture of toluene and xylene was kept below their airborne occupational exposure limits, there were no PK interactions between the compounds that affected the linear relationship between airborne parent-chemical exposure and urinary-metabolite concentrations. However, such an interaction was apparent at higher concentrations.
Weighing Advantages and Limitations of Using Occupational Limit Values
One of the main advantages of using occupational limit values to derive reference points for the general population is that the former apply to humans, although animal data are sometimes used in their derivation. One might therefore consider that occupational limit values alleviate the need for interspecies extrapolations. The other advantage is that the values have been used for preventing work-related diseases for years and so offer some degree of validation of the reference values.
Some of the limitations presented above can be given appropriate consideration in setting a reference value for the general population. For example, biomarkers with long half-lives are less prone to limitations in sampling time and are also probably reasonably related to cumulative exposure. In such cases, it might be tempting to apply a correction factor of 4
(rounded) to account for workweek (40-hour) vs continuous (168-hours/ week) exposures and another factor that takes into account interindividual susceptibility. The workforce is usually composed of “selected, healthy” people, whereas the general population includes infants, the elderly, and the infirm. Typically, this second factor has a value of 10 in risk assessment. However, applying a total factor of 40 to the urinary cadmium BEI, for example, gives a value of 5 µg/g of creatinine ÷ 40 = 0.125 µg/g of creatinine. This would clearly be an inapplicable value for environmental exposure since it is below the median reported for the general U.S. population (CDC 2005).
The reason that time adjustment and uncertainty factors do not seem to be appropriate for extrapolation of BEIs in these cases is unclear, but it may be that internal concentrations do not scale according to Haber’s law (concentration × time of exposure = a constant value) at steady state. If exposure was long enough in workplace biomarker studies to have achieved steady state (for example, at the end of the last workshift of the week), the biomarker concentration is determined more by ambient concentration rather than by time of exposure; that is, longer exposure will not achieve a higher steady-state concentration). Therefore, a simple extrapolation to the general public based on longer exposure may not be appropriate. Furthermore, although the BEIs are set to protect workers from deleterious effects of exposure to chemicals for a working lifetime, their toxicologic basis is often protection against acute effects that are not likely to be seen in the general population, whose members are exposed at much lower concentrations of the same substances and for whom long-term chronic effects are of greater concern.
Although use of BEI or an adjusted BEI to evaluate biomonitoring results in the general population is problematic, the BEI derivation may provide useful information on the relationship between biomarker and external dose. That information could be the basis of deriving a human pharmacokinetic model that relates biomarker concentration to an environmental exposure. Appendix B uses the biomarker for styrene exposure in the workplace to illustrate the limitations of BEIs with respect to the general public and to show that the data supporting the BEI can be used to construct a modeling approach that could be relevant to the general public. Styrene is a pertinent case example because the Integrated Risk Information System reference concentration (RfC) is based on the relationship between urinary biomarker and toxicity found in workers, with extrapolation back to workplace air concentration and then further extrapolation to the general public. Thus, even though the BEI itself is not directly useful for estimating risks in the general population associated with biomonitoring results, the data used to derive the BEI may become part of an RfC determination
or be used in developing a PK model. Those approaches, particularly the latter, can be useful in interpreting population biomonitoring data.
In conclusion, workplace biomarker targets (BEIs, BATs, and so on) provide a useful frame of reference for considering general-population biomonitoring results. The NHANES reports have referred to workplace biomarker targets in this manner. Comparison with workplace standards indicates whether the results found in the general population are in a range that would be of concern if found in workers. On the basis of the comparisons shown previously (Table 5-4), general-population biomarker concentrations do not typically approach workplace targets. However, for the reasons described above, it is a very inexact comparison and cannot be used to make firm judgments about the risks experienced by the general population. Furthermore, simple adjustment factors based on exposure time or sensitivity (uncertainty) factors do not appear to be appropriate for deriving biomarker concentrations for the general public. The database supporting the derivation of a BEI might be applicable to the development of human PK models that could be used to interpret biomonitoring results in the general population.
As developed in Chapter 6, workplace biomarker targets also provide context and a frame of reference for communicating general-population biomonitoring results. However, this raises a number of communication issues, given that workplace biomarker criteria are not directly relevant to the general public, for the reasons described previously and because the standards may be established with a different level of health protection than would be suitable for the general public.
USING BIOMONITORING RESULTS TO ESTIMATE RISK
Introduction
The approaches described previously can be used to relate biomonitoring results to a reference population or to workplace exposures, but they do not evaluate the risk associated with the amount of a chemical found in the body. To do that, one needs to develop a relationship between biomarker concentration and toxic response, a relationship that is not commonly derived in standard toxicologic practice. The following sections outline methods for deriving such a relationship. The approaches include the ideal case of existing risk assessments based on biomarker-response relationships established in epidemiologic research. Lead and mercury are used as examples of cases in which exposure was quantified according to hair or blood biomarkers and dose-response associations were developed on this basis.
Although applying biologic markers to risk assessment has long been
lauded as a way of reducing uncertainty (Goldstein 1996; Schulte and Waters 1999; Perera 2000; Vainio 2001; WHO 2001; Maier et al. 2004), there are relatively few such cases.
Alternatively, traditional risk assessments may help to put biomonitoring results into a risk context. Those assessments combine animal toxicology studies with human exposure assessments to estimate risks to the general population and selected groups. Biomonitoring results from those groups can then be understood on the basis of the range of risks projected in the traditional assessment. For some chemicals, exposure pathways are ill defined, and it is not possible to estimate human exposure or risk with traditional methods. In such cases, the best—perhaps only—exposure information may be the biomonitoring dataset itself. Alternative techniques, which we have termed biomonitoring-led risk-assessment approaches, will then be needed. Biomonitoring data can also inform risk assessment by identifying data gaps, replacing default assumptions, reducing exposure misclassification, or elucidating factors that affect exposure variability in a population.
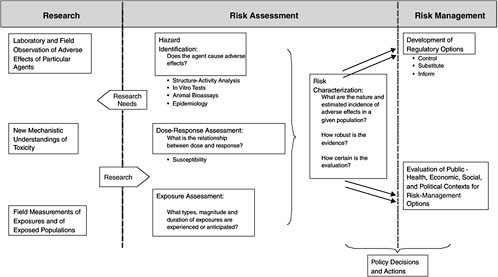
Overview of Risk Assessment
Figure 5-6 outlines the classical risk-assessment paradigm along with research needs and risk management (Omenn 2003). The steps of risk assessment include hazard characterization (hazard identification and dose-response assessment), exposure assessment, and risk characterization. Risk assessment is an iterative process; conclusions derived at each step inform and refine the succeeding steps. Exposure assessment traditionally involves a pathway analysis in which chemical concentrations in various media are combined with information on human contact rates to calculate human dose in milligrams per kilogram per day. The dose is normally used in risk characterization with the reference dose (RfD), cancer slope factor, or some other estimate of potency to provide a quantitative risk estimate. Risk characterization calls for presenting the quantitative estimate with its uncertainties. The uncertainties can arise from inadequacies in toxicity data, gaps in understanding of mechanisms of action, gaps in knowledge of factors that modulate interspecies and intraspecies variability in response, or inadequacies in exposure information.
BIOMONITORING-BASED RISK ASSESSMENT
Although biomonitoring data constitute a key body of knowledge about the distribution of exposure, relatively few risk assessments have been based on biomarker-response relationships established in epidemiologic studies (WHO 2001). In a recent informal survey of leading risk-assessment prac-
titioners, only a handful of cases were identified; mercury and lead were the only examples cited consistently (Maier et al. 2004). Other examples were cadmium, ethanol, arsenic, dioxin, and formaldehyde (Maier et al. 2004); of these, only cadmium and ethanol have biomarkers suitable for population screening and well-developed dose-response relationships. The best examples remain mercury and lead because of the detailed human biomarker-response information available and because of their application in public health. A sentinel feature that makes them ideal biomarkers for risk assessment is the powerful epidemiologic (prospective cohort) design that established the basis of the biomarker-response relationships. That study design is considered the pre-eminent standard in biomarker validation (WHO 2001). Pathway analyses were also reasonably complete so sources of human exposure could be identified. That was facilitated by the specificity of biomarkers of both chemicals to the exposure. The epidemiologic design and the relatively complete pathway analysis enabled the use of traditional exposure assessment involving applied dose and biomarker-based approaches in assessing risk.
Another advantage of the use of lead and mercury biomarkers is that exposure to both chemicals is more readily quantified through measurement of the biomarkers than through collection of questionnaire data or environmental measures (WHO 2001). In addition, both compounds have relatively long half-lives and therefore provide relatively stable metrics that integrate dose over long exposure periods. The lead example, which follows, details the development and utility of this blood biomarker. Similar information on mercury is presented in Appendix B.
Lead Case Study
Consistent epidemiologic and experimental data have documented deleterious effects in children associated with blood lead greater than 10 µg/ dL. The data include results of eight prospective cohort studies conducted in the United States (Bellinger et al. 1992; Canfield et al. 2003; Dietrich et al. 1993; Ernhart et al. 1989), Mexico (Rothenberg et al. 2000), Australia (Baghurst et al. 1992; Cooney et al. 1989) and Yugoslavia (Wasserman et al. 1997). Effects include lowered intelligence, behavioral problems, and diminished school performance (Lanphear 2005). That body of evidence led to the identification of a blood lead concentration of 10 µg/dL or greater as the level of concern in children by both CDC and the World Health Organization (WHO) (CDC 1991; WHO 1995). The CDC and WHO determinations followed a series of reductions in the concentration of lead in blood thought to be deleterious from 60 to 40 µg/dL in 1971, to 30 µg/ dL in 1978, to 25 µg/dL in 1985, and finally to 10 µg/dL in 1991 (Lanphear 2005).
However, more recent epidemiologic investigations suggest that there may not be a threshold for the adverse neurodevelopmental effects of lead in children and that the dose-response curve may in fact be steeper at blood lead concentrations less than 10 µg/dL, than at higher concentrations (Lanphear 2005; Bellinger and Needleman 2003; Canfield et al. 2003). In 2005, CDC concluded that “since no safe blood lead level in children has been identified, emphasis should be placed on efforts to control or eliminate lead in children’s environment before children are exposed” (CDC 2005).
Lead is an example in which risk assessors lack an RfD for evaluating exposure doses with traditional pathway analysis. Therefore, one must use a model, the Integrated Exposure Uptake Biokinetic model (EPA 1994) for lead in children, to convert exposure doses in milligrams per kilogram per day into blood concentrations. The model takes into account numerous sources of variability and presents a population distribution of blood lead results for a given intake dose. A core feature of the model is the biokinetic slope factor, which converts exposure dose to blood concentration on the basis of empirical data on this relationship in children. The biomarker-based risk target for lead and the associated biokinetic model constitute an excellent example of the type of information and tools needed to use biomonitoring data in risk assessment maximally. This is an example of a category VII biomarker as described in the Chapter 3 categorization scheme.
The application of biologic markers to environmental epidemiology provides an optimal approach for determining whether or not biomonitoring results indicate a health risk. Chapter 7 has recommendations for leveraging of existing or planned research to assess biomarker-response relationships in a cost-effective manner within ongoing epidemiologic study designs. Many excellent reviews have been written on the specific design issues that should be considered when incorporating biologic markers into epidemiologic research (for example, Schulte and Perera 1993; Hulka 1991, Bonassi and Au 2002; Schulte and Waters 1999; Rothman et al. 1995). However, several key points illustrated by the lead and mercury case examples should be emphasized. The biomarkers must have low limits of detection. The biomarkers should be specific to the exposure of interest and reflect exposure over the hypothesized window of susceptibility. Reliable measures of the potential toxicologic end points must be a key component of the epidemiologic study design. This will require at least preliminary evaluation of potential mechanisms of toxicity from human or experimental studies or from structure activity relationships.
Comparing Biomarker-Based Risk with Population-Based Biomonitoring Results
One can compare the biomarker-based risk derived for lead with population-based biomonitoring results. Data from NHANES 2000-2001 can be used to estimate the number of children in the United States who have increased blood lead (CDC 2005). Blood lead in U.S. children declined dramatically after the removal of lead from gasoline—from a median of 15 µg/dL in 1978 to 2 µg/dL in 1999 (Rogan and Ware 2003). Nonetheless, an estimated 1.6% of U.S. children 1-5 years old had blood lead greater than 10 µg/dL in 1999-2002, according to NHANES data (CDC 2005). The major exposure sources of lead for U.S. children are deterioration of lead-based paint and the resulting dust and soil contamination (CDC 2005).
Communication issues may arise with the use of biomarker-toxicity relationships, in part because of the high level of confidence that investigators place in the results. Researchers may be tempted to extrapolate from a biomarker concentration to a health effect in an individual or group. For example, biomonitoring data have been used to extrapolate to the number of pregnant women in the general population who may beat increased risk from methyl mercury exposure (NRC 2000). As described in Chapter 6, risk communication for such extrapolations needs to capture the uncertainties in the numerical estimates (number of people with increased mercury concentrations), and the health significance of surpassing a particular “bright line” biomarker concentration.
USING EXISTING RISK ASSESSMENTS FOR INTERPRETING BIOMONITORING DATA
Interpretation of biomonitoring results can be enhanced by existing risk assessments of a specific chemical. Traditional risk assessment calculates the dose associated with various exposure pathways, cumulates the pathways into a total dose, and then compares the total dose with the RfD or uses it to estimate cancer risk. A comprehensive exposure and risk assessment for the general population, if available, can be a useful starting point for interpreting biomonitoring data. For example, if the risk assessment indicates that the general public, including high-end individuals, is exposed on average to levels less than the RfD, then biomonitoring results showing widespread population exposure may still not raise a health concern. Alternatively, if the risk assessment indicates that a typical exposure can increase risk, then biomonitoring data that show widespread exposure may lead to health concerns for the general population, especially for those in the upper percentiles of exposure. Ideally, the risk assessment will provide an analysis
of scenarios that involve high-end exposures and susceptible populations (such as young children) to point out whether particular groups are important to include in biomonitoring studies.
Appendix B provides brief case studies of two pesticides, glyphosate and permethrin, for which a pre-existing risk assessment can help to put biomonitoring results into perspective. In both cases, the Environmental Protection Agency (EPA) has evaluated risks for a wide array of exposure scenarios as part of the reregistration process, and there are biomonitoring data whose interpretation could benefit from these risk assessments.
BIOMONITORING-LED RISK-ASSESSMENT APPROACHES
Biomonitoring-led risk assessment is needed when the biomonitoring and toxicology databases are robust but epidemiologic data are not adequate to establish the biomarker-response relationship and there are few exposure data. In such cases, biomonitoring results may raise important health questions that cannot be answered without knowing more about exposure. There are three main options for converting biomonitoring data into a format that can be used as exposure information in risk assessments:
-
Use human PK modeling (Box 5-4) to convert the biomonitoring data into a human-exposure dose that can be related to an RfD, cancer potency value, or other toxicity value.
-
Use animal PK modeling to convert the dose-response relationship seen in toxicity studies (applied dose) to a dose-response relationship based on internal dose, using a dose metric derived from human biomonitoring data. This approach fosters the development of a biomarker-response relationship and biomarker-based toxicity values.
-
Collect sufficient biomarker data in animals to express the dose-response relationship in key toxicology studies in terms of a biomarker-response relationship, in addition to an applied dose-response relationship.
Using Biomarker-Led Approaches to Assess Risks Associated with Biomonitoring Results
Option 1: Conversion of Biomonitoring Data to Exposure Dose with Human Pharmacokinetic Modeling
In the sections below, four different cases for converting biomonitoring data to exposure dose using pharmacokinetic modeling are considered: lipid-soluble, bioaccumulative chemicals at steady state; lipid-soluble, bioaccumulative chemicals not at steady state; shorter-half-life chemicals at
|
BOX 5-4 Brief Overview of Pharmacokinetic Models (See Appendix C for Expanded Discussion) PK modeling can take the form of relatively simple models that treat the body as one or two compartments. The compartments have no precise physiologic meaning but provide sites into which a chemical can be distributed and from which a chemical can be excreted. Transport rates into (absorption and redistribution) and out of (excretion) these compartments can simulate the buildup of chemical concentration, achievement of a steady state (uptake and elimination rates are balanced), and washout of a chemical from tissues. The one- and two-compartment models typically use first-order linear rate constants for chemical disposition. That means that such processes as absorption, hepatic metabolism, and renal excretion are assumed to be directly related to chemical concentration without the possibility of saturation. Such models constitute the classical approach to PK analysis of therapeutic drugs (Dvorchik and Vesell 1976) and have also been used in selected cases for environmental chemicals (such as hydrazine, dioxins and methyl mercury) (Stern 1997; Lorber and Phillips 2002). As described below, these models can be used to relate biomonitoring results to exposure dose under some circumstances. Physiologically based pharmacokinetic (PBPK) models have been seen as an advance in that they describe physiologically relevant compartments into which a chemical is taken up and eliminated on the basis of blood flow, partitioning properties, and clearance mechanisms. PBPK models have the advantage of being able to simulate chemical concentration in specific target tissues, such as brain, fetus, and thyroid (Rao and Brown 1993; Thrall et al. 2002; Gentry et al. 2003); nonlinear kinetics, such as metabolic saturation; protein and macromolecule binding; route-specific differences in chemical disposition; formation of primary and secondary metabolites and their concentrations in specific tissues; blood flow limitations of metabolism and clearance (Kedderis and Lipscomb 2001); intake routes and exposure scenarios; population variability through the interface with probabilistic Monte Carlo techniques; and interactions with chemical mixtures. PBPK models rely on a series of simultaneous differential equations that simulate chemical delivery to tissues via the arterial circulation and removal via the venous circulation. The models are run in time steps such that the entire course of chemical disposition can be presented for calculation of the area-under-the-curve (AUC) dose, often a key metric for chronic risk assessment. The physiologic parameters can be adapted for different species, sexes, age groups, and genetic variants to facilitate extrapolation from one type of receptor to another. The classical compartmental and more complex PBPK models require actual pharmacokinetic data to calibrate some parameters such as metabolic rate constants. However, PBPK models are more data-intensive and require greater numbers of chemical-specific and receptor-specific inputs. Although PBPK models have been used extensively in the last 20 years to address cross-species differences and other uncertainties, there are cases in which simpler one- or two-compartment models have been sufficient for risk assessment, for example for methyl mercury (EPA 2001). |
pseudosteady state; and short-half-life chemicals that do not approach steady state.
Human Modeling Case 1: Lipid-Soluble, Bioaccumulative Chemicals at Steady State
The extensive body-burden work done with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and its congeners (Lorber and Phillips 2002; EPA 2003) has provided a model for converting biomonitoring data into dose estimates. Although some aspects pertain specifically to highly lipid-soluble, bioaccumulative chemicals, there are elements that may be suitable to other chemicals. Figure 5-7 shows the general framework for a one-compartment model for interpreting biomonitoring serum data for lipid-soluble chemicals under the assumption of steady state.
The construction of this type of model and the requisite calculations are illustrated in Appendix B on the basis of the work done with TCDD. A one-compartment model can yield estimates of intake dose that can be used in risk assessment, but it should be considered a screening-level analysis that is applicable to long-term average exposure. More complete physiologic models that take into account not only lipid partitioning but also protein binding, the induction of metabolizing enzymes, blood flows to lipid and other compartments, and non-steady-state kinetics (for example, due to changes in intake dose) are advances that should be used to simulate shorter-term fluctuations in biomarker concentration (Emond et al. 2005a,b).
Human Modeling Case 2: Lipid-Soluble, Bioaccumulative Chemicals Not at Steady State
Even though chemicals with long half-lives and high storage in lipid tend to have stable concentrations, they may not be at steady state. For example, dioxin body burdens apparently increased in the early part of the 20th century and then declined over the last 2 decades. That pattern repre-
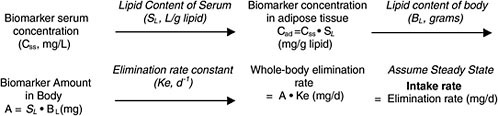
FIGURE 5-7 Conversion of biomonitoring data to daily dose on the basis of one-compartment (body-burden) model.
sents a nonuniform exposure and thus a non-steady-state condition. It can still be addressed with simple one-compartment modeling as described above, but the exposure dose is varied to match the temporal trends in the biomonitoring data (Pinsky and Lorber 1998). The dose profile that best matches the biomonitoring data can then be used for risk assessment.
Human Modeling Case 3: Shorter-Half-Life Chemicals at Pseudosteady State
Chemicals that are not highly lipid-soluble and that have relatively short half-lives (hours to days) do not build up a stable long-term reservoir. However, a pseudosteady state can be attained if exposure is nearly uniform and constant (Figure 5-8). Pseudosteady state refers to the case in which blood or tissue concentrations are changing and therefore are not completely stable. These blood or tissue concentrations fluctuate slightly and in a regular pattern around the average concentration.
If that is the case, blood concentrations may be relatively stable, and there is a potential to convert biomonitoring results into exposure estimates in a manner analogous to that described above for TCDD. The difference is that starting from a blood or serum concentration, one needs the volume of distribution (Vd) to estimate the total amount in the body. The general framework is as shown in Figure 5-9.
FIGURE 5-8 Blood concentrations of rapidly cleared chemical to which there is frequent and nearly uniform exposure. Highlighted line (≈—≈) is mean blood concentration. Under these exposure conditions, biomarker concentration will be within a factor of 2 of mean after first several hours. Simplifying assumption of pseudosteady state (mean concentration is approximated by concentration found at any sampling time) may suffice for estimating exposure dose from blood concentration under these circumstances.
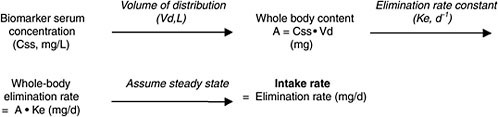
FIGURE 5-9 Conversion of biomonitoring data to daily dose on basis of one-compartment model for non-lipid-soluble chemicals at steady state.
Vd is a characteristic of the chemical that depends on its water and lipid solubility, protein-binding properties, and affinity for storage depots, such as bone. It is largely independent of dose unless transport and storage mechanisms are saturable. In general, the more water-soluble or plasma-protein-bound the chemical, the smaller will be its Vd. (The more lipid-soluble, the higher the Vd.) Chemicals that bind extensively to receptors in tissues may have a larger Vd than expected simply on the basis of partitioning principles. The Vd of a chemical can be determined experimentally in animals and extrapolated to humans or derived from physicochemical properties (lipid solubility and protein-binding capacity). For a one-compartment model the Vd is simply the adminstered dose divided by the initial plasma concentration.
Derivation of Vd relies on the ratio of dose and blood concentration immediately after intravenous administration. Two-compartment models involve more complex calculations to estimate Vd at steady state that reflect the transport of chemical between compartments as well as out of the body.
In summary, conversion of biomonitoring data to exposure dose requires knowledge of chemical elimination rate and Vd and requires that conditions be approximately pseudosteady state. It may be useful to estimate dose from body burden; however, this cannot be used to interpret an individual’s biomonitoring result, because the elimination rate and Vd would not be known. Reasonable bounds on elimination rate and Vd could be used to calculate an upper end of daily dose that is still compatible with the biomonitoring results (for example, when both Vd and elimination rate are high).
Methylmercury is a primary example of this type of model in humans. A human mercury one-compartment PK model has been developed on the basis of several exposure datasets in which blood or hair concentrations have been measured at various times after methyl mercury exposure (Stern 1997; Kershaw et al. 1980; Sherlock et al. 1984). The one-compartment model takes into account the elimination half-life of mercury from blood and methylmercury volume distribution (in blood) to back-calculate the
exposure dose needed to achieve a mercury concentration in blood. That is the basis of the methyl mercury RfD (EPA 2001). Although it is intended primarily for use under steady-state conditions, the model is predictive of non-steady-state conditions and in fact was used to assess the single fish-meal mercury intake needed to exceed the blood concentration associated with the RfD (Ginsberg and Toal 2000). That example and the one described above for TCDD demonstrate the potential utility of simple one-compartment models in interpreting biomonitoring data and in converting blood or tissue concentrations to applied doses in humans.
Case Example: Pharmacokinetic Calculations to Interpret Phthalate Urinary Biomarker Data. The previous descriptions focused on blood or adipose biomarker concentrations that were converted to body burden to yield estimates of daily dose based on chemical half-life. A modified form of that is conversion of urinary biomarker data to daily exposure dose via simple model calculations as described for phthalates.
The biomonitoring of phthalates in urine has been of increasing interest as methods to detect the metabolites of specific phthalates have improved in recent years. Human exposure to phthalates comes from food via leaching from plastic packaging, from personal-care products, from children’s toys, and from medical procedures that involve the storage of fluids in plastic bags or their delivery via flexible tubing (Hauser and Calafat 2005). The CDC dataset on samples collected in 2001-2002 (CDC 2005) included analysis of 12 phthalate metabolites reflecting exposure to eight phthalates, including the most commonly used phthalates: diethylhexylphthalate (DEHP), diethylphthalate, two forms of dibutylphthalate, butylbenzylphthalate, di-iso-nonylphthalate and di-n-octylphthalate. The samples were collected from a subsample of the total NHANES population (N = 2,780) and constituted a followup of rounds of phthalate-metabolite collection involving about 2,500 subjects in 1999 and 289 subjects in 1988-1994 (Blount et al. 2000; Silva et al. 2004). A much smaller sampling of phthalate metabolites in nonoccupationally exposed German subjects (N = 85) has been reported (Koch et al. 2003).
The widespread detection of phthalate metabolites in human urine has produced questions about public-health risks, especially with regard to antiandrogen effects that can influence male gonadal development (Gray et al. 2000; Parks et al. 2000). The extrapolation from urinary biomonitoring results to exposure and risk assessment has been facilitated by calculations that convert urinary metabolite concentrations to intake dose of the parent phthalate (Koo et al. 2002; Koch et al. 2003; Kohn et al. 2000; David 2000). The parent diester phthalates are rapidly and completely metabolized to the monoester metabolites, which are rapidly cleared by the kidney. Those features allow one to assume that the daily excretion rate of metabolite is equal to the daily intake rate of the parent chemical. Furthermore,
because phthalates are ubiquitous and exposure occurs daily, one can assume pseudosteady-state conditions. Preliminary evidence that that is the case has been presented in several human studies (Hauser et al. 2004; Hoppin et al. 2002).
Biomonitoring data have been converted to daily exposure dose (e.g., Koch et al. 2003) by calculating the amount of metabolite excreted per day (amount in urine per gram of creatinine times creatinine excretion per day); adjusting for the fractional excretion of metabolite (some of the phthalate metabolites account for as little as 1 or 2% of total chemical excreted, whereas others account for 70%); and adjusting for the difference in molecular weight between metabolite and parent chemical. That approach was used to convert urinary biomonitoring data from the NHANES 1988-1994 dataset to the daily intake rate (in micrograms per kilogram per day) for seven phthalates (Kohn et al. 2000). Confidence in the resulting exposure estimates was provided by comparison with the results of exposure analyses conducted on the general population with traditional pathway analysis, taking into account the major sources of exposure (phthalates in food and indoor air) and minor sources (CERHR 2000; Kohn et al. 2000). Table 5-5 shows the estimated exposures of the general population (Kohn et al. 2000).
Table 5-5 shows that the biomonitoring-based exposure estimate for DEHP had a median of 0.71, a 95th percentile value of 3.6, and a maxi-
TABLE 5-5 Estimated Exposures (µg/kg/day) to the General Population Based on Extrapolated Intake from Urinary Metabolites in 289 Individuals Measured by Blount et al. (2000)
|
Monoester |
Diester |
Mini- mum |
Median |
95th percentile |
Maximum |
CERHRa |
|
Ethyl |
Diethyl |
<LOD |
12 |
110 |
320 |
NA |
|
n-Butyl |
Di-n-butyl |
0.084 |
1.5 |
7.2 |
110 |
2- 10b |
|
Benzyl |
n-Butyl benzyl |
0.094 |
0.88 |
4.0 |
29 |
2c |
|
Cydohexyl |
Dicyclohexyl |
<LOD |
0.026 |
0.25 |
2.3 |
NA |
|
2-Ethylhexyl |
Di(2-ethylhexyl) |
<LOD |
0.71 |
3.6 |
46 |
3- 30 |
|
n-Octyl |
Di-n-octyl |
<LOD |
0.0096 |
0.96 |
13 |
<3- <30d |
|
i-Nonyl |
Di-i-nonyl |
<LOD |
<LOD |
1.7 |
22 |
<3- <30d |
|
aThe CERHR Phthalates Expert Panel held its third and final meeting 12-13 July 2000 in Arlington, Virginia; the CERHR final reports on the seven phthalates evaluated along with a full description of the center and its activities are available on the CERHR Web site. bThe upper bound for occupational exposure was estimated as 286 µg/kg/day: the estimate of 2 µg/kg/day is at the 84th percentile of our calculated values. cThe CERHR estimate for n-butyl benzyl phthalate is at the 11th percentile of our calculated values. dDi-n-octyl and di-i-nonyl phthalate estimates from the CERHR were reported as less than for di(2-ethylhexyl) phthalate. Source: Adapted from Kohn et al. 2000. |
||||||
mum of 46 µg/kg per day (N = 289). The exposure-pathway analysis yielded a range of 3-30 µg/kg/d, demonstrating reasonable concordance, given the vast difference in approaches. A similar degree of concordance between biomonitoring-based and exposure-pathway-based estimates of phthalate daily doses was reported by David (2000).
There are several interesting features of the phthalate biomarker-exposure dose analyses. First, women and children have greater exposure to several phthalates than male adults. That may be due to the use of cosmetics and personal-care products in the case of women and due to higher food intake rate per body weight and exposure to plasticizers in toys in children. Data on children younger than 6 years old are not available, and this may be an important data gap. The other feature of note is that the relatively small German study found exposures to some phthalates that were 3-20 times above those found in the United States, whereas for other phthalates the exposure differential was smaller and concentrations in the United States were greater (Koch et al. 2003). That implies a different exposure pattern in different countries.
The risk associated with phthalate biomarker results has not been formally analyzed. However, exposure doses estimated from the German biomonitoring data suggest a potential concern about DEHP in that the range of dose estimates was 2-185 µg/kg per day, whereas the EPA RfD is 20 µg/ kg per day and the tolerable daily intake of the European Commission Scientific Committee on Toxicity, Ecotoxicity and the Environment is 37 µg/kg per day (Koch et al. 2003). The lower exposure doses estimated in the Kohn et al. analysis suggest that most people in the United States are below the RfD for DEHP. However, caution is needed regarding this risk conclusion, in part because the RfD was established in 1991 on the basis of increased liver weight in rodents after DEHP exposure (EPA 1991). More recent research indicates that reproductive effects, particularly structural and functional changes in the testes, constitute an important toxicologic end point (Kavlock et al. 2002). Furthermore, testing of effects during the late gestational and neonatal periods, thought to be times of particularly high sensitivity, is incomplete (Kavlock et al. 2002). A recent epidemiologic study of male postnatal measures in relation to maternal prenatal urinary concentrations of four phthalate metabolites suggests that biomarker results within the reference range are associated with altered male reproductive development (Swan et al. 2005). Although results from that study should be considered preliminary, in part because of the small sample and the use of a novel index (anogenital distance) to assess reproductive development, they highlight the need for additional epidemiologic research in light of the widespread phthalate exposures (Kaiser 2005). Future research involving biomarker-response relationships for phthalate metabolites may obviate the extrapolation of biomarker results to exposure dose followed
by comparison with the traditional RfD. Nevertheless, the case study demonstrates the utility of estimating exposure dose from urinary biomarker data for potential application to risk assessment.
The phthalate example illustrates the utility of a Group VI biomarker as described in the Chapter 3 framework. Phthalate urinary metabolites are reliable biomarkers of parent-chemical exposure, there is sufficient information to extrapolate exposure dose from biomarker concentrations (assuming that near steady-state conditions apply), and exposure dose-toxicity relationships in animals are available. If biomarker-toxicity relationships are more firmly established in humans (as in a followup to the Swan et al. 2005 study), it might be possible to recategorize phthalate metabolites as Group VII biomarkers.
One caveat in basing exposure dose on urinary-biomarker data is that the percentage conversion of the parent chemical to the biomonitored metabolite needs to be well established and not highly variable. For example, for some of the phthalates, the biomarker accounts for a very small percentage of total chemical fate; numerous other pathways account for the remainder. That necessitates a large numerical adjustment in going from the urinary measurement to the exposure-dose estimate—an adjustment that is subject to interindividual variability in the percentage disposition via the measured biomarker. In fact, there is some disagreement as to the fraction of DEHP excreted as the urinary biomarker; the conflicting estimates ranging from 2.4% to 13% (Koch et al. 2004). Each urinary-metabolite result in the population actually represents a range of exposure doses governed by the degree of intersubject variability in the fraction of biomarker excreted. A probabilistic analysis of the phthalate urinary dataset may be appropriate for displaying the full range of exposure-dose estimates.
Another important caveat has to do with how the urinary concentration of a biomarker is expressed. If it is expressed simply per volume of urine, then the estimate of daily biomarker output can be misinterpreted because urine volume is variable from day to day and over the course of a day. Biomonitoring results from spot urine samples can be greatly influenced by changes in the water content of urine when expressed on a volume basis. To correct for that, urinary biomonitoring results are often also expressed as per gram of creatinine (CDC 2005) on the assumption that the creatinine-excretion rate is less variable than the water-excretion rate. In the phthalate example described above, the creatinine adjustment is used not only to express the biomarker result but also to convert it to a milligrams-per-kilogram-per-day dose by multiplying by the grams of creatinine excreted per day. That is a convenient way to derive an estimate of exposure, but it carries the uncertainty that creatinine-excretion rate can also be a substantial source of variability (Barr et al. 2005). For example, analysis of NHANES III data on 3,400 men (20-29 years old) found a 6-
fold spread in urinary creatinine concentrations across the 10th to 90th percentiles of this population. The distributions in boys 6-11 years old and elderly men (for example, over 70 years old) were shifted to the left in such a way that the means were about 60% of the means for men 20-29 years old. Other important age, sex, and race or ethnicity differences in urinary creatinine concentrations were also evident (Barr et al. 2005). The differences in total amount of creatinine excretion per day would be somewhat larger than that described above for creatinine concentration because the groups with low creatinine (children and the elderly) also have less urine output per day. Thus, creatinine-excretion rate is a substantial source of variability in calculations that convert a urinary biomarker concentration to a dose of parent chemical. One could simulate that variability and its influence on estimates of intake dose with Monte Carlo modeling techniques.
The chlorpyrifos example described in Appendix B illustrates another caveat related to biomarkers that are urinary metabolites. A metabolite can sometimes appear in urine not only as a result of parent-chemical uptake and metabolism but also as a result of uptake of the metabolite from environmental media (Lu et al. 2005; Wilson et al. 2003). Thus, the biomarker for chlorpyrifos, 3,5,6-trichloro-2-pyridinol (TCP), occurs in a wide variety of environmental media, and the concentration in foods surpasses that of the parent chemical (Morgan et al. 2005). If the intake of the metabolite from environmental sources is substantial in comparison with that of the parent chemical, as in the case of chlorpyrifos and TCP, the extrapolation of urinary biomarker concentration to parent-chemical exposure dose is uncertain.
Human Modeling Case 4: Short-Half-Life Chemicals That Do Not Approach Steady State
The last case is that of chemicals that are not highly lipid-soluble or long-lived and are unlikely to be at steady state. Population biomonitoring data, however, may be able to present a reasonable distribution of internal concentration if the sample size is sufficient. A one-compartment model may be used, with knowledge of elimination rate and Vd, to estimate exposure dose of the average person. As above, there is uncertainty with respect to how variability in elimination rate and Vd may interact to affect dosimetry. There is also uncertainty in sample timing, inasmuch as the sample may have been taken at any point along the elimination phase of the most recent exposure. Those factors preclude a definitive calculation of daily dose, but approaches described in Appendix B for chlorpyrifos and trichloroethylene (TCE) can yield screening-level estimates that are useful in initial risk assessments for biomonitored concentrations of these chemicals. The chlorpyrifos example illustrates the use of urinary-metabolite data
to estimate intake dose, which when augmented with human activity and exposure models can simulate exposure patterns that could have produced the biomonitoring result. The chlorpyrifos biomarker, TCP, is available for intake in environmental media, so not all of what is measured in urine comes from the metabolism of the parent chemical.
Studies which involved controlled human exposure to volatile organic compounds (VOCs) combined with repeated blood sampling have enabled researchers to evaluate the utility of PBPK models for interpreting biomonitoring results taken under non-steady-state conditions (Canuel et al. 2000; Tan et al. 2005; Sohn et al. 2004).
The TCE example demonstrates the utility of Bayesian inference techniques and bounding approaches for estimating the relationship between blood concentration and exposure pattern. Additional illustrations involving toluene and chloroform have shown the potential utility of PBPK modeling approaches for VOC biomonitoring data (Canuel et al. 2000; Tan et al. 2005). In the case of toluene, a PBPK model run in reverse was able to use exposure time and biomonitoring results as inputs to accurately predict the ambient exposure concentration (Canuel et al. 2000). With population-based biomonitoring, the exposure time relative to the sampling time is not known. Therefore, Tan et al. (2005) used Monte Carlo uncertainty analysis together with PBPK modeling to evaluate a range of potential exposure scenarios (for example, water concentration and timing of exposure) resulting from chloroform blood levels found in tap water exposure studies.
Option 2: Use of Animal Pharmacokinetic Modeling to Derive Biomarker-Based Dose-Response Relationship
Human biomonitoring data can be interpreted through animal biomarker-response relationships in a manner parallel to that with which human exposure information is interpreted through applied dose-response relationships. The latter relationship is typically used to derive an RfD or a cancer potency value that can be expressed as risk. A number of steps are used to convert the animal dose-response relationship to an RfD or cancer slope factor that is expressed in units of applied dose (mg/kg/d) and thus is not directly relevant for interpreting the internal exposure data obtained in biomonitoring studies. However, a number of steps can be used to convert the animal dose-response relationship in applied dose units to the corresponding RFD or cancer slope factor in units of internal dose (biomarker). The same steps used in setting an RFD (application of uncertainty factors and extrapolation to low dose) would be used in deriving the biomarker-
based toxicity value, which would be of direct use in interpreting risks associated with biomonitoring results.
The biomarker-response relationship may be constructed through PBPK modeling as described in this section or through direct measurements of biomarker concentrations in animals as described in the next section.
One issue in deriving biomarker-based toxicity values is that the typical exposure-response relationship is in the form of total daily dose vs toxic effect. The applied dose metric, although crude, integrates exposure over 24 hours. However, a biomarker measurement at a single time often is not a time-integrated dose but merely an isolated data point. The ideal is to express the internal concentration as an integrated internal dose, also called the area under the concentration-time curve (AUC). That can be accomplished by simulating chemical fate over 24 hours (or longer) and estimating the AUC for each dose that was used in the toxicology studies. The AUC-response relationships can then be used to derive the biomarker-based equivalent of an RfD or cancer potency value.
For long-half-life chemicals, the internal concentration can generally be considered stable and the human concentration measured at a single time can be multiplied by 24 to estimate the 24-hour AUC (EPA 2005).2 That approach can also be used for shorter-half-life chemicals if exposure can be assumed to be relatively uniform and continuous, so that the biomonitored concentration reflects the long-term average internal concentration. If those conditions do not apply, it may not be possible to estimate human AUC from a single biomonitoring result. In such a case, the animal biomarker-response relationship may need to be based on a single point on the internal-concentration-response curve. If one picks the lowest concentration (for example, at the end of the time course after much washout has occurred), most of the exposure will be missed. If one picks the peak internal concentration, the blood concentrations required to produce an effect will be higher, and the risk assessment will be less conservative than otherwise. Picking the lowest or highest internal concentration is an arbitrary decision, so it may be most practical to pick the average concentration achieved over 24 hours as a reasonable correlate to the toxic effects. In that case, the biomonitoring concentrationss would be assumed also to represent an average for that day of exposure and thus could be compared directly with the animal biomarker-based dose-response relationship. Although that involves an assumed course in human blood, it can provide a reasonable first approximation of the blood concentrations and of its significance with respect to biomarker-based toxicity values.
Pharmacokinetic Modeling in Animals: PFOA Case Example
Biomonitoring data on perfluorooctanoic acid (PFOA) has led to questions of exposure and risk, and efforts to address these questions have involved pharmacokinetic modeling in animals. A draft risk assessment of PFOA evaluates the potential risks associated with concentrations found in human serum (EPA 2005). The main form of exposure assessment in the analysis is biomonitoring data on the U.S. general population. PFOA biomonitoring datasets involve serum samples from blood-bank specimens from adults (N = 645), from a prospective study of an elderly population (N = 238; age, 65-96 years), and from a clinical trial in children (N = 598; age, 2-12 years). The data yielded geometric means of 4-5 µg/L for all three age groups, but regression analysis of the children’s data showed that age was inversely related to serum PFOA concentration (Olsen et al. 2004). The risk assessment used a margin-of-exposure (MOE) approach in which serum concentrations associated with no-observed-adverse-effect level (NOAELs) or lowest observed-adverse-effect levels (LOAELs) in animal toxicology studies were divided by the geometric mean or 90th percentile of biomonitoring serum results. The animal serum concentrations were derived with two methods: for toxicity end points in monkeys, serum PFOA measurements were available throughout the toxicity studies, and the steady-state concentration associated with the LOAEL was used in MOE calculations; and for toxicity end points in rats, a PK model was used to estimate serum concentrations at LOAEL or NOAEL doses. The PK model runs were informed by limited plasma data on rats; these data were used to calibrate the model and extrapolate across individual points to develop AUC estimates. A one-compartment PK model was selected for predicting serum concentrations by using linear (first-order) rate constants to describe chemical absorption and elimination. The backfit elimination rate constants reflected the large sex-dependent difference in clearance; the female elimination rate was 37 times greater than that of the male.
Figure 5-10 provides evidence that the PFOA model developed by EPA is predictive when tested against male rat plasma PFOA data from 90-day dietary studies. The data represent steady-state PFOA concentrations. Figure 5-10 also shows that the model was successful in simulating the limited data available for non-steady-state conditions for plasma concentrations in pregnant rats. Those validation runs suggest that the model is suitable for estimating LOAEL and NOAEL internal doses across a range of PFOA toxicity end points in rats (subchronic, chronic, reproductive, and developmental). The internal LOAELs and NOAELs could then be used for comparison with biomarker data on humans.3
represent a steady state rather than a peak, so the AUC rat data were more relevant for comparison with human exposure. In addition, PFOA toxic effects may be more likely to be related to the cumulative internal dose than to peaks. The biomonitoring data were converted from the single data point to AUC by multiplying the PFOA concentration by 24 to reflect the expectation that the measured concentration would be constant over the entire day. That is a safe assumption, given the long half-life of PFOA in humans (about 4 years). Those modeling predictions and calculations allowed for a direct comparison between rat and human AUC, which resulted in the MOE analysis.
The PFOA risk assessment showed that the MOE for several toxicity end points was 1,000-fold or higher but was somewhat smaller in the adult female rat NOAEL (200- to 400-fold). The MOEs for end points relevant to children (such as, maturation of juvenile rats) were large, around 10,000-fold. However, as stated above, it was not possible to model rat lactational AUCs; furthermore, the human biomonitoring age group does not include lactating children. Given that PFOA is excreted into breast milk and that there was an inverse correlation between age and serum PFOA in the human dataset, measurement or modeling of nursing infants may be an important addition to the risk assessment.
Another uncertainty in the children’s MOE analysis is that the biomonitoring data showed that perfluorooctanoates related to PFOA—perfluorohexansulfonate and N-methyl perfluorooctanesulfonamidoacetate— were considerably higher in children than in adults. The reason is unknown, but the original authors suggest that it may reflect a different exposure pattern in children (Olsen et al. 2004). Toxicology and exposure data on these analytes are not sufficient to enable a separate risk assessment to evaluate the children’s exposures.
A final consideration is that there is a large PK difference between rats and humans with respect to half-life: in rats, it is hours (in the female) to days (in the male), whereas in humans, it is about 4 years (EPA 2005). The steady-state AUC modeling approach used in the MOE analyses takes half-life in rats into account and is independent of human half-life because of the likelihood that the biomonitoring data represent steady-state concentrations. However, the large cross-species half-life difference raises the possibility of other PK and pharmacodynamic cross-species differences that are not adequately captured in the MOE analysis.
In summary, the PFOA risk assessment is a good example of biomonitoring-led risk assessment. There is no attempt to calculate exposure dose with pathway analysis, because the sources of human PFOA exposure are too uncertain. Instead, the biomonitoring data served as the sole source of human exposure information. Those data could be interpreted in a risk-assessment framework with the aid of PK mod-
eling of rat dosimetry in key toxicology studies and with the aid of limited biomarker measurements in monkey and rat studies. Thus, although an RfD is not available for PFOA, these types of animal-human biomarker comparisons are useful to put the degree of human exposure into a risk context. Uncertainties exist in the PFOA analysis with respect to cross-species extrapolation of PK and toxicologic results, other perfluoro biomarkers in humans have not been tested in animals (the aggregate-risk question), and key PFOA toxicity end points could not be fully compared with the human biomonitoring data (because of limitations in early-life analyses). Those uncertainties are indicative of what one can expect to encounter when conducting similar biomonitoring-led analyses.
Option 3: Obtaining Sufficient Animal Pharmacokinetic Data to Develop a Biomarker-Response Relationship
In Option 3, the biomarker-response relationship would be developed from actual measurements rather than (or in addition to) PK models. The biomarker-toxicity relationship would be quantified directly from results of the experimental bioassay by investing additional effort in gathering biomarker data (such as, blood and urinary concentrations) at doses relevant to key toxicology studies. A parallelogram approach similar to that derived from the work in the 1970s by Sobels (1977) could be used to extrapolate experimental biomarker results in animals to human health risk assessment as is now commonly done with exposure dose-response relationships (Sobels 1977; WHO 2001; Perera 1986; Sutter 1995). Adding biomarker measurements to 90-day and 2-year bioassay designs may not require substantial cost but may be instrumental in interpreting human biomonitoring data in the future. Because biomarker measurements are not part of standard animal toxicology protocols, there are relatively few examples of this approach. Even after toxicity studies are completed, it is possible to recreate the conditions of such animal tests and obtain biomarker information useful for relating biomarker concentration to toxicity.
The approach for collecting biomarker data on animals that is relevant to human risk should initially involve animal PK studies to determine possible sampling media (blood, plasma, and urine) and biomarkers (such as, parent chemical, metabolites, and hemoglobin adducts). Consideration should be given to the toxic mechanism and to half-life. The former is important in selecting the most relevant biomarkers. The latter is important in making the biomarker reliable for screening human populations even under conditions of sporadic exposure. If biomonitoring data on humans
already exist, the human biomarker should be applied to the animal PK testing to the greatest extent possible.
Once a suitable biomarker is selected, the biomarker-toxicity relationship can be generated through biomarker-data collection during toxicology studies, possibly requiring a satellite group of animals to generate the biomarker data. If the critical dose-response studies have already been conducted, one can recreate the design (species, dose route, and dose) for a limited number of animals to generate biomarker data that could be related to the previous toxicity findings. Alternatively, it may be possible to construct a PBPK model based on the PK data already collected that could simulate the key toxicology studies and generate a biomarker-toxicity relationship. Chemical-specific partition coefficients would also be needed to develop the model (Gargas et al. 1989). The World Health Organization has outlined ways in which biomarker data can assist in such modeling efforts (WHO 2001).
In a number of cases, PK data have been collected as part of toxicity testing in animals. For example, the National Toxicology Program (NTP) has added PK studies to its test battery of short-term, long-term, and genotoxicity studies (Buchanan et al. 1997). That information is used to evaluate the potential for nonlinearities in the dose-response relationship due to such phenomena as metabolic saturation. It is helpful in selecting doses for NTP’s chronic bioassays, learning about a chemical’s mechanism of action, and interpreting the results of toxicity studies. The PK studies are not designed specifically for the interpretation of human biomonitoring data, but that may be possible in a post hoc fashion. It may require PBPK modeling in which a model would be constructed for the species used for toxicity testing, and it would be calibrated to the PK data developed as part of the animal testing. The calibrated model could then be used to convert the dose-response relationship found in toxicity studies to an internal dose (biomarker-response) relationship, and the latter could be extrapolated to humans to interpret biomonitoring data. In the PFOA example described, biomarker measurements in animals were used to help to interpret human biomonitoring data. Although there are few examples of this type of approach, it should become more applicable if biomarker measurements are routinely incorporated into experimental bioassays.
As in other risk-assessment approaches (e.g., NRC 1994), scientific uncertainties are a predictable feature of any new biomonitoring-led risk assessments. As shown above and discussed more fully in Chapter 6, identifying and communicating those uncertainties—such as the effect of interindividual variation in elimination rate and limits on extrapolating adult PK data to children—are critical in communicating the risk results.
Hemoglobin Adducts as Biomarkers of Exposure
This chapter has focused on the interpretation of biomarkers of exposure, specifically biomarkers circulating in blood or excreted in urine or breast milk. Hemoglobin adducts are an additional type of exposure biomarker. They are unlike the more traditional biomarkers in that they represent integrated exposure, the cumulative dose of an agent that can irreversibly bind to hemoglobin. They depend not only on the intensity of exposure but also on the length of exposure, the reaction rate with hemoglobin, and the lifespan of red blood cells. Methods have been developed for converting hemoglobin-adduct concentrations to daily exposure to acrylamide and ethylene oxide (Calleman 1996; Tornqvist and Landin 1995). That approach is useful for chemicals that form reactive metabolites that would otherwise be too transient to measure in bodily fluids. Thus, it is one step closer to the biologically effective dose and so is a potentially important type of biomarker in risk assessments of reactive chemicals (Tornqvist and Ehrenberg 2001).
One hemoglobin adduct, carboxyhemoglobin, is a special case in that it is both an indicator of exposure and an effect. Carboxyhemoglobin is the key biochemical derangement caused by carbon monoxide, so its concentration is directly related to health risk. For other biomarkers that utilize hemoglobin adducts, hemoglobin is not the biochemical target.
Other types of internal adducts (DNA and RNA adducts) are further in the direction of biomarkers of effect and pose interpretive challenges in addition to the challenges that spring from exposure and PK factors. For example, rates of DNA repair can differ widely between tissues and types of adducts, so its use as a biomarker of exposure or effect is more complex.
Interpretation of BM in Terms of Exposure Assessment
Chapter 5 has provided an overview of approaches to assess the biomonitoring-response relationship. However, exposure assessment is also a critical component of risk assessment, since if risks are determined to be excessive, pathway analyses must be carried out to identify the major sources of exposure. This includes not only the immediate sources such as house dust, water, food, indoor air, or soil, but also the initial sources from which human exposure pathways originate (for example, industrial emissions, transportation sources, or consumer products). While dose reconstruction can be difficult, the NHEXAS approach referenced in Chapter 2 should be more broadly applied (Pang et al. 2002). This involves obtaining detailed environmental sampling and survey information at the same time that biomonitoring samples are collected. Such data-intensive biomonitoring efforts may not be feasible for large numbers of subjects but can be conducted on a representative subset
of the population of interest. Exposure pathways found to be key contributors to internal dose can then be explored to determine the initial steps of contaminant entry into the environment.
It may be possible to use Geographic Information Systems (GIS) to map biomonitoring results to determine whether there is a spatial pattern in exposure concentrations. This could be overlaid with GIS maps of environmental data (for example, air or water pollution or distribution of waste sites) to determine whether biomonitoring results correspond to specific environmental sources. However, mapping techniques are generally not useful for sporadic, localized sources such as food or consumer products. In such cases, survey questionnaires and sampling of the home environment are of more direct use in understanding exposure sources.
Chapter 7 presents the committee’s recommendations on approaches that should be utilized to obtain data on exposure-biomarker relationships within ongoing large-scale biomonitoring studies.
Utility of Biomonitoring Data to Inform Risk Assessments
Biomonitoring can facilitate risk assessments in many ways. These are discussed below.
-
It demonstrates that exposure of the general population or specific groups is occurring. Classical exposure pathway analyses are hypothetical constructs with many assumptions about human behaviors that lead to exposure. The finding of a chemical in blood or urine documents that exposure has occurred and may indicate whether exposure is widespread or occurs only in isolated cases.
-
It demonstrates the range of exposures, the degree of interindividual variability, and the potential for highly exposed subpopulations. Classical pathway assessments are typically limited in their analysis of variability, perhaps estimating dose for a central tendency and an upper-bound case. Application of probabilistic Monte Carlo techniques can provide a hypothetical population distribution of exposure doses. However, the distribution depends on robust input of human metrics and behaviors (such as soil ingestion rates), which may not be available. The tails of such distributions can be particularly uncertain. Biomonitoring data on a broad, representative sample can provide a distribution of internal doses that can improve on Monte Carlo estimates of variability and have the potential to identify subpopulations that may be more exposed. That information can be used in risk assessment to estimate exposures and risks at upper tails of the distribution. It may also enable future assessments to focus on the subpopulations identified. Such future assessments might include pharmacogenetic probes to determine whether biomarker concentrations are high because of
-
high intake or because of metabolic traits that lead to enhanced retention. Given that young children are often more highly exposed to environmental chemicals than adults (EPA 2000), it is important to include them in biomonitoring studies.
-
It can establish population exposure baseline values and status and trends. Risk assessment may lead to risk-management decisions to intervene and thus decrease exposure. Biomonitoring data can document the baseline exposure and how it is affected by risk-management interventions in multiyear status and trends biomonitoring programs. Biomonitoring studies have demonstrated the success of public-health and regulatory programs in decreasing exposure to lead, environmental tobacco smoke (with serum cotinine biomonitoring) (CDC 2005), and chlorpyrifos (Whyatt et al. 2003; Whyatt et al. 2005).
-
It can identify chemicals and risk-assessment questions for which there are key research gaps. Biomonitoring data on widespread human exposure raise questions about risk and indicate where data gaps exist. Addressing the gaps will be instrumental in developing a risk assessment. A case in point is polybrominated diphenyl ethers (PBDEs). Biomonitoring studies of PBDEs in breast milk found an increasing trend that correlated with societal use of flame retardants in consumer products during the 1980s and 1990s (Birnbaum and Staskal 2004). Figure 5-11 shows that PBDE concentrations in breast milk in North America exceed those found in Europe, with a steep rise in the Canadian (and presumably also U.S.) concentrations in the last decade (Schecter et al. 2003). However, there is still little understanding of the exposure sources and pathways that have led to the biomonitoring results. Furthermore, the toxicology database is only in the initial stages of development; key end points (such as, hormonal and neurodevelopmental effects) need further exploration (Birnbaum and Staskal 2004). The rising trend in PBDE bomonitoring results and their implications for exposure of nursing infants have led to a major research focus on exposure sources, toxic effects, and health risks.
-
It can estimate exposure via breast milk. Biomonitoring of breast milk provides information directly applicable to assessing infant exposure and risk from nursing. The measured breast-milk concentration is the starting point for exposure calculations that include breast-milk intake rate per unit body weight. The nursing dose estimates can then be used to assess risks during the postnatal period on the basis of toxicity end points developed from developmental studies or from adults and extrapolated to the postnatal period. Exposures via breast milk may be especially important for persistent organic pollutants that bioaccumulate in fat, because they are efficiently transferred through the milk to the breastfeeding infant (Landrigan et al. 2002). Examples include DDT, polychlorinated biphenyls, and PBDEs. However, water-soluble nonpersistent chemicals may also par-
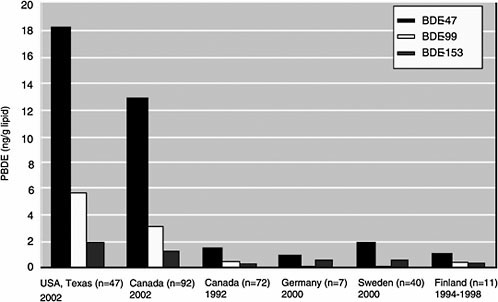
FIGURE 5-11 Median concentrations (ng/g lipid) of BDE-47, BDE-99, and BDE-153 in human milk from different countries. Data from Ryan et al. (2002) and Ryan and Patry (2001) for Canada, from Schroeter-Kermani et al. (2000) for Germany, from Noren and Merionyte (2000) for Sweden, and from Strandman et al. (2000) for Finland. Source: Schecter et al. 2003.
-
tition to some degree into the water fraction of breast milk. Models that predict the degree to which a chemical will partition into breast milk (Fisher et al. 1997) can be used to help to determine which contaminants should be the focus of breast-milk biomonitoring studies.
-
It can assess in utero exposure. Any substance in the maternal circulation can be transferred across the placenta to the developing fetus unless it is first metabolized and eliminated (Ginsberg et al. 2004). Risk assessment of the fetal period typically relies on maternal dose. However, biomonitoring of cord blood relative to maternal blood may be important to document whether there are substantial maternal-fetal differences in exposure. Evidence on methylmercury suggests that it concentrates in the fetus (Stern and Smith 2003), whereas an evaluation of 29 pesticides suggests similar concentrations across the maternal-fetal unit (Whyatt et al. 2003).
-
It can assess risks posed by multiple exposures. Large-scale biomonitoring studies illustrate the need for developing cumulative risk-assessment approaches for biomonitoring data because exposures are typically to mixtures rather than to single toxicants. An examination of the Third National Report on Human Exposure to Environmental Chemicals, for example,
-
gives some indication of the extent of mixed exposures in the United States. Concentrations of 148 chemicals or chemical isomers in 12 broad chemical classes were measured in blood or urinary samples collected from a representative sample of the U.S. population; biomarker concentrations at the 50th, 75th, 90th, and 95th percentiles of the population distribution were presented (CDC 2005). In almost all cases, multiple compounds in each chemical class were detected in at least half the urinary or blood samples collected. For example, all but two of the 13 metals measured in urine were detected in at least 50% of samples. Similarly, when nine phthalates were assessed in urinary samples in the 2001-2002 NHANES (the first year in which phthalates were measured), metabolites of six were detected in 50% or more of samples: dimethyl phthalate, diethyl phthalate, dibutyl phthalate, butylbenzyl phthalate, di-2-ethylhexyl phthalate, and di-n-octyl phthalate. Metabolites of multiple organophosphorous insecticides were also detected in 50% or more of samples. Although mixed exposures to compounds in the other chemical classes did not appear to be as extensive, biomarkers of one or more chemical in each class were found in at least 50% of the samples. Given the current paucity of data on approaches for assessing risks associated with biomarker mixtures, research into risk-assessment techniques for these types of mixtures should be used (see Chapter 7). Several recent small-scale epidemiologic studies have evaluated risks associated with combinations of biomarkers and can provide illustrations of various potential approaches (see for example, Swan et al. 2005; Whyatt et al. 2004; Castorina et al. 2003). In addition, multiple contaminant exposures can be addressed by designing animal bioassays in which the animal exposures mimic a human environmental exposure pattern. (Examples of such mixture bioassays are discussed in Chapter 7.) The mixture bioassays in animals, with PBPK modeling approaches (also discussed in Chapter 7), may address some of the multiple-contaminant issues that are presented by population-based biomonitoring data.
The committee recommends that CDC’s National Reports on Exposure to Environmental Chemicals present population distributions showing the number of chemicals (and chemical classes) detected per person. For both mixtures and single chemicals, the committee recommends that the data in the national exposure reports be presented for the full range of the distribution (including the 10th and 25th percentiles of the distribution), rather than just at the 50th percentile and above.
SUMMARY
Biomonitoring data often provide an indication of human internal exposure to environmental contaminants, and this becomes the springboard
for important public-health or clinical questions. As defined at the outset of this chapter, these questions are
-
Is the biomonitoring result in a range that is typical of the general population?
-
Does the biomonitoring result indicate a health risk?
The answer to the first question can be obtained with descriptive statistics and straightforward comparisons between the individual result and the reference range. That assumes that sufficient data are available to define a reference range and that it has been properly constructed for the general population. The answer to the second question is more complex and requires information from diverse sources, including toxicology, epidemiology, exposure studies, pharmacokinetics, and risk assessment. Those are all well-established fields, but only recently have they developed a focus on the interpretation of biomonitoring data. Therefore, there are a number of data gaps, uncertainties, and other limitations in developing risk assessments that can explain biomonitoring results. Risk communication needs to recognize those limitations in developing messages about the meaning of chemical concentrations in human blood, urine, or other media.
This chapter has provided a variety of options for interpreting biomonitoring data, all of which have utility for specific situations and applications. There are wide differences in the amount of information available on different chemicals and biomarkers, so the approach to answering the second question will vary between chemicals. A useful way to summarize the different approaches is with the case studies provided throughout the chapter and in Appendix B. Case-study chemicals were selected because they provide the type of information needed for interpreting biomonitoring data and thus are useful to illustrate the options discussed in this chapter. However, data on many chemicals that are evaluated in biomonitoring studies are not available; this represents a data gap that hinders the interpretation of biomonitoring data on most of the chemicals currently sampled.
Summary of Case Studies: Exemplifying the Interpretation of Biomonitoring Data
The case studies presented in this chapter and in Appendix B exemplify a variety of interpretive options. Table 5-6 and the associated text summarize the case-study biomarkers in terms of their utility, supporting data, and the interpretive option that they exemplify.
Uncertainties and Limitations in Interpreting Biomonitoring Data
Throughout this chapter, in connection with each of the several approaches to interpreting biomonitoring data, points of confidence (strengths) and limitations or uncertainties (weaknesses) have been identified. For example, such studies as NHANES are strengths, providing a nationally representative survey and a wealth of data on a large number and variety of biomarkers. The lead and mercury case studies demonstrate a strength, showing that biomonitoring data can be interpreted directly from powerful epidemiology datasets in which the collection of biomarker data was central to the study. The PFOA case is important to show that the increased use of animal PBPK modeling can be a strength for the interpretation of biomonitoring results. The increasing availability of PK data and analyses points to new possibilities for using biomarker data in the risk-assessment process.
As in classical risk assessment, there are numerous uncertainties in interpreting biomonitoring data: cross-species extrapolations, interindividual variability, extrapolation from external to internal dose, high-dose-to-low-dose extrapolations, and the effect of mixtures as opposed to single chemicals. How those uncertainties would affect interpretation of a particular biomonitoring result depends on the question being asked and the robustness of the biomarker’s database. Key uncertainties in the realm of reference range include the potential to misrepresent the general population because of undersampling of some groups and oversampling of others and the potential to misclassify an important group as part of the general population. Increased attention to enrollment criteria in population biomonitoring studies and potential future uses of genetic markers indicative of metabolic capability can help to inform these kinds of uncertainty.
The risk interpretation of biomonitoring results will tend to have additional uncertainties. That is because, in addition to the standard uncertainties encountered in risk assessment, there is the uncertainty of extrapolating from a blood or urinary concentration to an external dose. There will be variability both in the timing between sample draw and most recent exposure and in the relationship between blood concentration and dose. Those kinds of variability are compounded by uncertainty in the ability of a PK calculation or model to convert biomarker to dose accurately. For example, reliance on urinary biomarker results expressed per gram of urinary creatinine leads to an uncertain calculation of total chemical excretion per day because of the considerable variability in creatinine clearance per day. That complicates an otherwise simple approach to estimating dose. Furthermore, the conversion requires knowledge of fractional excretion via various pathways, which may not be present for a large sample of humans. The uncertainties created by these factors can be bounded via sensitivity and Monte
TABLE 5-6 Properties of Biomarkers Used as Examples in Chapter 5
|
Chemical |
Biomarker |
Type of Information Available |
Possible Applications |
|
Glyphosate |
Urinary glyphosate |
Exposure pathways risk assessment; external dose to toxic effect in animals; limited analysis of biomarker to external dose |
Biomarker results can be put into risk context by using existing risk assessment |
|
Permethrin |
Urinary carboxylic acid metabolites |
Exposure pathways risk assessment; external dose to toxic effect in animals |
Biomarker results can be put into risk context by using existing risk assessment |
|
PBDE |
PBDE congeners in blood and breast milk |
Emerging exposure and toxicity database |
Biomarker results useful for demonstrating need for research and establishing reference range |
|
PFOA |
Serum PFOA |
External dose to toxic effect in animals; biomarker to animal external dose; therefore, biomarker to toxic effect in animals |
Biomarker results can estimate human risk; need to extrapolate biomarker-response relationship across species |
|
Lead |
Blood lead |
Biomarker to toxic effect in humans; biomarker to external dose in humans |
Biomarker results can be used directly for estimation of human risk; exposure apportionment and intervention possible |
|
Mercury |
Blood mercury |
Biomarker to toxic effect in humans, although this relationship is for cord blood; biomarker to external dose in humans |
Biomarker results can be used directly for estimation of human risk; exposure apportionment and intervention possible |
|
Chlorpyrifos |
Urinary TCP |
Biomarker to external dose in humans; external dose to toxic effect in animals; therefore, biomarker to toxic effect in animals |
Biomarker results can estimate human risk; need to extrapolate external dose-toxicity relationship across species |
|
Dioxin |
Dioxin in blood or lipid |
Biomarker to body burden and external dose in humans; body burden and external dose to toxicity in animals |
Biomarker results can estimate human risk; exposure intervention possible |
|
Styrene |
Urinary metabolites |
Biomarker to external dose (air concentration) in workers; external dose to toxicity in animals biomarker to toxicity in workers |
Biomarker results can estimate risk in workers but not directly applicable to general population. |
|
TCE |
Blood TCE |
External dose to toxic effect in animals; biomarker to animal external dose; biomarker to external dose in humans |
Biomarker too transient to be a reliable index of exposure of general population. |
|
Phthalates |
Urinary monoester metabolites |
Biomarker to external dose in humans; external dose to toxic effect in animals; therefore, biomarker to toxic effect in animals; preliminary biomarker to effects in humans |
Biomarker results can estimate human risk; need to extrapolate external dose-toxicity relationship across species |
Carlo analysis, but ultimately the variability in fractional excretion and creatinine clearance needs to be understood to characterize population exposure to urinary biomarkers.
A major factor governing variability in biomonitoring results is inter-individual differences in metabolic clearance. Genetic polymorphisms can affect the activity or inducibility of Phase I and Phase II metabolic enzymes, potentially affecting both the activation and detoxification of xenobiotics (Perera 2000; Eaton 2000). Biomonitoring results for parent compounds in blood or metabolites in urine or blood will be influenced by these differences. This can be a large factor if the enzyme systems involved are highly variable across the population. For example, a polymorphism in the CYP2D6 gene has a large influence on the clearance of certain drugs, CYP2E1 is inducible by exposure to alcohol, and glutathione conjugation to epoxides can be affected by null polymorphisms in several glutathione transferases (Thier et al. 2003; Ingelman-Sundberg 2005; Kessova and Cederbaum 2003). The design of biomonitoring studies should include an evaluation of the dominant clearance pathways for the chemical being monitored. If these pathways are modulated by genetic polymorphisms, then genotype probes should be considered when collecting the biomonitoring samples. This would be consistent with the increasing use of genotyping methods in environmental epidemiology studies (Nebert et al. 1996). This can decrease uncertainty and assist in data interpretation, pointing out whether a high biomonitoring result may have been from high intake or slow clearance. These can have very different risk implications.
Another kind of uncertainty is related to the utility of occupational reference values for comparisons with general population biomonitoring results. The workplace targets are inappropriate for a general population that includes infants, the elderly, and the infirm.
The committee’s attention to those limitations and uncertainties is important for two reasons. First, full disclosure of limiting factors gives scientists and the public a fuller understanding of the reliability and credibility of biomonitoring results. It provides risk assessors with information needed to “characterize” risk conclusions fully, as called for by the National Research Council risk-assessment paradigm (NRC 1983; 1994). Second, and equally important, the kinds of uncertainty define data gaps for immediate attention and related long-term research needs.
CONCLUSIONS
This chapter identifies a variety of approaches for interpreting biomonitoring results, ranging from descriptive to risk-based. The descriptive approaches are useful as a first step in analyzing biomonitoring data, but they do not describe the level of risk. That requires the risk-based approaches
described in the chapter. Although the methods presented are feasible, minimum data are required to exercise the various interpretive options. These minimal data are lacking in the case of numerous chemicals, so priorities need to be set in selecting biomarkers for expansion of the database to enable assessment of risk.
The committee drew the following conclusions about descriptive approaches:
-
Descriptive approaches are important in laying a foundation that risk-based approaches can build from, and in some cases they are the only type of analysis needed.
-
The reference-range approach is a critical data layer that summarizes the biomonitoring dataset and enables comparisons between segments of the population and times. Although they do not provide information about risk, simple comparisons between an individual’s biomarker concentration and the population distribution may be all that is needed to answer key questions about the need for personal action.
-
Workplace biologic exposure targets (such as BEIs) provide another point of reference that may be of some use in assessing the relative degree of individual or group exposure outside the workplace.
Risk-based approaches try to determine how much risk is associated with a given biomarker result. Those approaches and their interpretive power vary widely with the extent of information available on a chemical and its biomarker.
The committee drew the following conclusions about risk-based approaches:
-
The biomarkers of greatest utility for interpreting risk are those for which biomarker-toxicity relationships have been developed in humans, as in the case of lead and mercury.
-
If such relationships are not available, biomonitoring data may be interpreted by converting them to human exposure dose with the aid of PK models. That can be done in different ways depending on the chemical and the type of biomarker (for example, parent chemical and metabolite).
-
For persistent lipid-soluble compounds, conversion of blood or adipose tissue biomonitoring results to body burden and intake dose is feasible even with simple one-compartment models, although multicompartment physiologic models can provide a more flexible and improved tool for estimating dose.
-
Approaches for less lipid-soluble and nonpersistent chemicals can depend on whether a blood or urinary biomarker is available.
-
Urinary biomarkers can be related to exposure dose in a straightforward manner for chemicals that are excreted rapidly in urine. This approach requires the collection of data describing the percentage of dose excreted each day in urine and percentages excreted by different metabolic and elimination pathways. There can be important variability and uncertainty in those factors and in the normalization of the biomarker result (per gram of creatinine). Furthermore, there may be environmental sources of the urinary biomarker that can confound an estimation of parent-chemical dose based on the metabolite in urine. It is also possible that the urinary metabolites may exist as breakdown products in the environment.
-
An alternative interpretive approach is to leave the human biomonitoring result as is but develop applied dose-biomarker relationships in animals. That requires obtaining animal PK data to support PBPK modeling or the collection of animal biomarker information in study designs that mimic key toxicology datasets.
RECOMMENDATIONS
Improved interpretation of biomonitoring results will require the expansion of the database typically available on many chemicals. The following recommendations will help in the evaluation of exposure and risk associated with biomonitoring results in general. More specific recommendations can be made case by case after an individual chemical’s database is reviewed.
-
Increase the use of biomarkers in environmental epidemiology studies.
-
Develop biomarkers suitable for determining internal dose-response or excreted dose-response relationships in animal studies with confirmation of biomarker applicability to humans.
-
Improve animal toxicology study designs to incorporate use of validated biomarkers to characterize biomarker-response relationships that can be used to interpret human biomonitoring data.
-
Expand use of exposure assessment in the biomonitoring study protocol to identify exposure sources and allow a pathway-exposure analysis that could help to interpret biomonitoring data.
-
Research is needed on various aspects of chemicals mixtures beginning with better reporting from population-based biomonitoring studies on the number and diversity of chemicals found in subjects. New bioassays are needed that explore the health outcomes of environmentally relevant mixtures (that is chemicals and amounts found in human tissues). PBPK models also need to be expanded to better understand chemical-chemical interactions.
-
Research the factors governing human excretion of chemicals in urine and breast milk and how it can affect biomarker results:
-
How breast-milk content changes over the course of the lactational period can affect excretion of toxicants into breast milk.
-
How uncertainties and variability in creatinine clearance can affect urinary biomarker results and their extrapolation to external dose.
-
-
Identify to what extent exposures to chemical degradation products in the environment contribute to metabolite levels measured in urine samples, as certain urinary metabolites may exist as breakdown products in the environment.
-
Add a wider variety of media to biomonitoring studies, especially media that will provide information about early life stages. For example, biomonitoring of breast milk can inform about exposures during infancy, and biomarkers in cord blood and meconium can inform about fetal exposure.
-
Include in utero exposures and young children in biomonitoring designs because they are a substantial source of population variability in exposure and susceptibility.
-
Improve human dosimetry models to simulate life stages and population groups (for example, those with polymorphisms) that have not been biomonitored; this may allow extension of biomonitoring results to vulnerable groups that are difficult to identify or sample.
-
Incorporate metabolic-trait determination into biomonitoring studies (for example, genotyping or phenotyping of metabolic traits) to understand how the traits can affect biomonitoring results.
-
Expand modeling approaches and case examples in which non-steady-state biomonitoring data are simulated to explore the exposure conditions responsible for biomonitoring results; this may provide exposure estimates that can be used in risk assessment (for example, Bayesian inference techniques and population behavior-exposure models).
-
Increase research emphasis on the low and high ends of the biomarker distribution to discover what leads to these tails and thus enhance the development of exposure interventions if warranted.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2003. TLVs and BEIs: Threshold Limit Values for Chemical Substances and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2005. TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
Baghurst, P.A., A.J. McMichael, N.R. Wigg, G.V. Vimpani, E.F. Robertson, R.J. Roberts, and S.L. Tong. 1992. Environmental exposure to lead and children’s intelligence at the age of seven years. The Port Pirie cohort study. N. Engl. J. Med. 327(18):1279-1284.
Barr, D.B., L.C. Wilder, S.P. Caudill, A.J. Gonzalez, L.L. Needham, and J.L. Pirkle. 2005. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 113(2):192-200.
Bellinger, D.C., and H.L. Needleman. 2003. Intellectual impairment and blood lead levels. N. Engl. J. Med. 349(5):500-502.
Bellinger, D.C., K.M. Stiles, and H.L. Needleman. 1992. Low level lead exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics 90(6):855-861.
Birnbaum, L.S., and D.F. Staskal. 2004. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 112(1):9-17.
Blount, B.C., M.J. Silva, S.P. Caudill, L.L. Needham, J.L. Pirkle, E.J. Sampson, G.W. Lucier, R.J. Jackson, and J.W. Brock. 2000. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 108(10):979-982.
Bonassi, S., and W.W. Au. 2002. Biomarkers in molecular epidemiology studies for health risk prediction. Mutat. Res. 511(1):73-86.
Bradman, A., B. Eskenazi, D.B. Barr, R. Bravo, R. Castorina, J.U. Chevrier, K. Kogut, M.E. Harnly, and T.E. McKone. 2005. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ. Health Perspect. 113(12):1802-1807.
Buchanan, J.R., L.T. Burka, and R.L. Melnick. 1997. Purpose and guidelines for toxicokinetic studies within the National Toxicology Program. Environ. Health Perspect. 105(5):468-471.
Calleman, C.J. 1996. The metabolism and pharmacokinetics of acrylamide: Implications for mechanisms of toxicity and human risk estimation. Drug Metab. Rev. 28(4): 527-590.
Canfield, R.L., C.R. Henderson, D.A. Cory-Slechta, C. Cox, T.A. Jusko, and B.P. Lanphear. 2003. Intellectual impairment in children with blood lead concentrations below 10 µg per deciliter. N. Engl. J. Med. 348(16):1517-1526.
Canuel, G., C. Viau, and K. Krishnan. 2000. A modeling framework for back-calculating ambient concentrations of volatile organic chemicals from biomarker data. Pp. 97-102 in Proceedings of the International Conference on Health Sciences Simulation, J.G. Anderseon, and M. Katzper, eds. San Diego, CA: Society for Computer Simulation International.
Castorina, R., A. Bradman, T.E. McKone, D.B. Barr, M.E. Harnley, and B. Eskenazi. 2003. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: A case study from the CHAMACOS cohort. Environ. Health Perspect. 111(13):1640-1648.
CDC (Centers for Disease Control and Prevention). 1991. Preventing Lead Poisoning in Young Children: A Statement by the Centers for Disease Control. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://aepo-xdv-www.epo.cdc.gov/wonder/prevguid/p0000029/p0000029.asp [accessed Jan. 19, 2006].
CDC (Centers for Disease Control and Prevention). 2001. National Report on Human Exposure to Environmental Chemicals. National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA.
CDC (Centers for Disease Control and Prevention). 2003. Second National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.jhsph.edu/ephtcenter/Second%20Report.pdf [accessed Nov. 16, 2005].
CDC (Centers for Disease Control and Prevention). 2005. Third National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.cdc.gov/exposurereport/3rd/ [accessed Nov. 16, 2005].
CERHR (Center for the Evaluation of Risks to Human Reproduction). 2000. NTP-CERHR Expert Panel Report on Di-2-ethylhexylphthalate. NTP-CERHR-DINP-00. National Toxicology Program, U.S. Department of Health and Human Services, Research Triangle Park, NC. October, 2000.
Cooney, G.H., A. Bell, W. McBride, and C. Carter. 1989. Low-level exposures to lead: The Sydney lead study. Dev. Med. Child. Neurol. 31(5):640-649.
David, R.M. 2000. Exposure to phthalate esters [letter]. Environ. Health Perspect. 108(10): 440.
DFG (Deutsche Forschungsgemeinschaft). 2004. List of MAK- and BAT-Values 2004, 1st Ed. Report No. 40. Weinheim: Wiley-VCH.
Dietrich, K.N., O.G. Berger, P.A. Succop, P.B. Hammond, and P.L. Bornschein. 1993. The developmental consequences of low to moderate prenatal and postnatal lead exposure: Intellectual attainment in the Cincinnati lead study cohort following school entry. Neurotoxicol Teratol 15(1):37-44.
Dvorchik, B.H., and E.S. Vesell. 1976. Pharmacokinetic interpretation of data gathered during therapeutic drug monitoring. Clin. Chem. 22(6):868-878.
Eaton, D.L. 2000. Biotransformation enzyme polymorphisms and pesticide susceptibility. Neurotoxicology 21(1-2):101-111.
Emond, E., M. Charbonneau, and K. Krishnan. 2005a. Physiologically based modeling of the accumulation in plasma and tissue lipids of a mixture of PCB congeners in female Sprague-Dawley rats. J. Toxicol. Environ. Health A 68(16):1393-1412.
Emond, C, J.E. Michalek, L.S. Birnbaum, and M.J. DeVito. 2005b. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessment. Environ. Health Perspect. 113(12):1666-1668.
EPA (U.S. Environmental Protection Agency). 1991. Di(2-ethylhexyl) phthalate (DEHP) (CASRN 117-81-7). Integrated Risk Information System, U.S. Environmental Protection Agency [online]. Available: http://cfpub.epa.gov/iris/quickview.cfm?substance_nmbr=0014 [accessed Feb. 26, 2006].
EPA (U.S. Environmental Protection Agency). 1994. Guidance Manual for the Integrated Exposure Uptake Biokinetic Model for Lead in Children. EPA/540/R-93/081. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 2000. Summary Report of the Technical Workshop on Issues Associated with Considering Developmental Changes in Behavior and Anatomy when Assessing Exposure to Children, July 26-27, 2000, Washington, DC. EPA/630/R-00/005. Risk Assessment Forum, U.S. Environmental Protection Agency [online]. Available: http://cfpub.epa.gov/ncea/raf/recordisplay.cfm?deid=20680 [accessed Jan. 19, 2006].
EPA (U.S. Environmental Protection Agency). 2001. Methylmercury (MeHg) (CASRN 22967-92-6). Integrated Risk Information System, U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/iris/subst/0073.htm [accessed Jan. 20, 2006].
EPA (U.S. Environmental Protection Agency). 2003. Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds National Academy Sciences (NAS) Review Draft, Dec, 2003 [online]. Available: http://www.epa.gov/ncea/pdfs/dioxin/nas-review/ [accessed Jan. 20, 2006].
EPA (U.S. Environmental Protection Agency). 2005. Draft Risk Asssessment of the Potential Human Health Effects Associated with Exposure to Perfluorooctanoic Acid and its Salts, January 2005 [online]. Available: http://www.epa.gov/opptintr/pfoa/pfoarisk.pdf [accessed January 24, 2006].
Ernhart, C.B., M. Morrow-Tlucak, A.W. Wolf, D. Super, and D. Drotar. 1989. Low level lead exposure in the prenatal and early preschool periods: Intelligence prior to school entry. Neurotoxicol. Teratol. 11(2):161-170.
European Commission. 2004. Baseline Report on “Biomonitoring of Children.” in the Frame work of the European Environment and Health Stategy (COM(2003)338final). Prepared by the Technical Working Group on Integrated Monitoring, Subgroup Biomonitoring of Children. January 9, 2004 [online]. Available: http://www.eu-human biomonitoring.org/documents/br_bio_final.pdf [accessed Jan. 20, 2006].
Fisher, J., D. Mahle, L. Bankston, R. Greene, and J. Gearhart. 1997. Lactational transfer of volatile chemicals in breast milk. Am. Ind. Hyg. Assoc. J. 58(6):425-431.
GAO (U.S. General Accounting Office). 2000. Toxic Chemicals: Long-term Coordinated Strategy Needed to Measure Exposures in Humans. GAO/HEHS-00-80. U.S. General Accounting Office, Washington, DC. May 2000 [online]. Available: http://www.gao.gov/new.items/he00080.pdf. [accessed Nov. 16, 2005].
Gargas, M.L., R.J. Burgess, D.E. Voisard, G.H. Cason, and M.E. Andersen. 1989. Partition coefficients of low-molecular-weight volatile chemicals in various liquids and tissues. Toxicol. Appl. Pharmacol. 98(1):87-99.
Gentry, P.R., T.R. Covington, and H.J. Clewell. 2003. Evaluation of the potential impact of pharmacokinetic differences on tissue dosimetry in offspring during pregnancy and lactation. Regul. Toxicol. Pharmacol. 38(1):1-16.
Ginsberg, G.L., and B.F. Toal. 2000. Development of a single meal fish consumption advisory for methyl mercury. Risk Anal. 20(1):41-47.
Ginsberg, G., W. Slikker Jr., J. Bruckner, and B. Sonawane. 2004. Incorporating children’s toxicokinetics into a risk framework. Environ. Health Perspect. 112(2):272-283.
Goldstein, B.D. 1996. Biological markers and risk assessment. Drug Metab. Rev. 28(1-2): 225-233.
Gray, L.E. Jr., J. Ostby, J. Furr, M. Price, D.N. Veeramachaneni, and L. Parks. 2000. Perinatal exposure to the phthalates DEHP, BBP and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation in the male rat. Toxicol. Sci. 58(2):350-365.
Han, X. 2003. Ammonium Perfluorooctanoate: Age Effect on the Plasma Concentration in Post-Weanling Rats Following Oral Gavage. Study No. Dupont 13267. EPA AR226-1553. Haskell Laboratory for Health and Environmental Sciences. December 15, 2003.
Hauser, R. and A.M Calafat. 2005. Phthalates and human health. Occup. Environ. Med. 62(11):806-818.
Hauser, R., J.D. Meeker, S. Park, M.J. Silva, and A.M Calafat. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 112(17):1734-1740.
Hoppin, J.A. J.W. Brock, B.J. Davis, and D.D. Baird. 2002. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect. 110(5):515-518.
Hulka, B.S. 1991. Epidemiological studies using biological markers: Issues for epidemiologists. Cancer Epidemiol. Biomarkers Prev. 1(1):13-19.
Ingelman-Sundberg, M. 2005. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects, and functional diversity. Pharmacogenomics J. 5(1):6-13.
Jang, J.Y., P.O. Droz, and S. Kim. 2001. Biological monitoring of workers exposed to ethylbenzene and co-exposed to xylene. Int. Arch. Occup. Environ. Health 74(1):31-37.
Kaiser J. 2005. Panel finds no proof that phthalates harm infant reproductive system. Science 310(5747):422-423.
Kavlock, R., K. Boekelheide, R. Chapin, M. Cunningham, E. Faustman, P. Foster, M. Golub, R. Henderson, I. Hinberg, R. Little, J. Seed, K. Shea, S. Tabacova, R. Tyl, P. Williams, and T. Zacharewski. 2002. NTP Center for the Evaluation of Risk to Human Reproduction: Phthalates expert panel report on the reproductive and development toxicity of di(2-ehtylhexyl) phthalate. Reprod. Toxicol. 16(5):529-653.
Kedderis, G.L., and J.C. Lipscomb. 2001. Application of in vitro biotransformation data and pharmacokinetic modeling to risk assessment. Toxicol. Ind. Health 17(5-10):315-321.
Kershaw T.G., T.W. Clarkson, and P.H. Dhahir. 1980. The relationship between blood levels and dose of methylmercury in man. Arch. Environ. Health 35(1):28-36.
Kessova, I., and A.I. Cederbaum. 2003. CYP2E1: Biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr. Mol. Med. 3(6):509-518.
Koch, H.M., H. Drexler, and J. Angerer. 2003. An estimation of the daily intake of di-(2ethyhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 206(2):77-83.
Koch, H.M., H. Drexler, and J. Angerer. 2004. Response to the letter of R.M. David, Int. J. Hyg. Environ. Health 207(1):75-76. Int. J. Hyg. Environ. Health 207(1):77-78.
Kohn, M.C., F. Parham, S.A. Masten, C.J. Portier, M.D. Shelby, J.W. Brock, and L.L. Needham. 2000. Human exposure estimates for phthalates. Environ. Health Perspect. 108(10):A440-A442.
Koo, J.W., F. Parham, M.C. Kohn, S.A. Masten, J.W. Brock, L.L. Needham, and C.J. Portier. 2002. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environ. Health Perspect. 110(4): 405-410.
Landrigan, P.J., B. Sonawane, D. Mattison, M. McCally, and A. Garg. 2002. Chemical contaminants in breast milk and their impacts on children’s health: An overview. Environ. Health Perspect. 110(6):A313-A3155.
Lanphear, B.P. 2005. Origins and evolution of children’s environmental health. Pp. 24-31 in Essays on the Future of Environmental Health Research: A Tribute to Dr. Kenneth Olden, T.J. Goehl, ed. Research Triangle Park, NC: Environmental Health Perspectives/ National Institute of Environmental Health Sciences [online]. Available: http://ehp.niehs.nih.gov/docs/2005/8003/8003.html [accessed Jan. 20, 2006].
Lauwerys, R.R., and P. Hoet. 2001. Industrial Chemical Exposure: Guidelines for Biological Monitoring, 3rd Ed. Boca Raton, FL: Lewis.
Lorber, M., and L. Phillips. 2002. Infant exposure to dioxin-like compounds in breast milk. Environ. Health Perspect. 110(6):A325-A332.
Lu, C., R. Bravo, L.M. Caltabiano, R.M. Irish, G. Weerasekera, and D.B. Barr. 2005. The presence of dialkylphosphates in fresh fruit juices: Implications for organophosphate pesticide exposure and risk assessment. J. Toxicol. Environ. Health 68(3):209-227.
Maier, A., R.E. Savage Jr., and L.T. Haber. 2004. Assessing biomarker use in risk assessment—a survey of practitioners. J. Toxicol. Environ. Health A 67(8-10):687-695.
Morgan, M.K., L.S. Sheldon, C.W. Croghan, P.A. Jones, G.L. Robertson, J.C. Chuang, N.K. Wilson, and C.W. Lyu. 2005. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 15(4):297-309.
Morgan, M.S., and K.H. Schaller. 1999. An analysis of criteria for biological limit values developed in Germany and in the United States. Int. Arch. Occup. Environ. Health 72(4):195-204.
Nebert, D.W., R.A. McKinnon, and A. Puga. 1996. Human drug-metabolizing enzyme polymorphisms: Effects on risk of toxicity and cancer. DNA Cell Biol. 15(4):273-280.
Noren, K., and D. Merionyte. 2000. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20-30 years. Chemosphere 40(9-11):1111-1123.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press.
NRC (National Research Council). 1991. Monitoring Human Tissues for Toxic Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
NRC (National Research Council). 2000. Toxicological Effects of Methylmercury. Washington, DC: National Academy Press.
Olsen, G.W., T.R. Church, K.J. Hansen, J.M. Burris, J.L. Butenhoff, J.H. Mandel, and L.R. Zobel. 2004. Quantitatitive evaluation of perfluorooctanesulfonate (PFOS) and other fluorochemicals in the serum of children. J. Children’s Health 2:53-76.
Omenn, G. 2003. On the significance of the Red Book in the evolution of risk assessment and risk management. Hum. Ecol. Risk Assess. 9(5):1155-1168.
Pang, Y., D.L. MacIntosh, D.E. Camann, and P.B. Ryan. 2002. Analysis of aggregate exposure to chlorpyrifos in the NHEXAS—Maryland investigation. Environ. Health Perspect. 110(3):235-240.
Parks, L.G., J.S. Ostby, C.R. Lambright, B.D. Abbott, G.R. Klinefelter, N.J. Barlow, and L.E. Gray Jr. 2000. The plasticizer DEHP induces malformations by decreasing fetal test-osterone syntheseis during sexual differentiation in the male rat. Toxicol. Sci. 58(2): 339-349.
Perera, F.P. 1986. New approaches in risk assessment for carcinogens. Risk Anal. 6(2): 195-201.
Perera, F.P. 2000. Molecular epidemiology: On the path to prevention? J. Natl. Cancer Inst. 92(8):602-612.
Pinsky, P.F., and M.N. Lorber. 1998. A model to evaluate past exposure to 2,3,7,8-TCDD. J Expo. Anal. Environ. Epidemiol. 8(2):187-206.
Pirkle, J.L., L.L. Needham, and K. Sexton. 1995. Improving exposure assessment by monitoring human tissues for toxic chemicals. J. Expo. Anal. Environ. Epidemiol. 5(3): 405-424.
Poulsen, O.M., J.M. Christensen, E. Sabbioni, and M.T. Van der Venne. 1994. Trace element reference values in tissues from inhabitants of the European Community. V. Review of trace elements in blood, serum and urine and critical evaluation of reference values for the Danish population. Sci. Total Environ. 141(1-3):197-215.
Rao, H.V., and D.R. Brown. 1993. A physiologically based pharmacokinetic assessment of tetrachloroethylene in groundwater for a bathing and showering determination. Risk Anal. 13(1):37-49.
Rogan, W., and J.H. Ware. 2003. Intellectual impairment in children with blood lead concentrations below 10 µg per deciliter. J. Pediatr. 143(5):687-688.
Rothenberg, S.J., A. Poblano, and L. Schnaas. 2000. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicol. Teratol. 22(4):503-510.
Rothman, N., W.F. Stewart, and P.A. Schulte. 1995. Incorporating biomarkers into cancer epidemiology: A matrix of biomarker and study design categories. Cancer Epidemiol. Biomarkers Prev. 4(4):301-311.
Ryan, J.J., and B. Patry. 2001. Body burdens and food exposure in Canada for polybrominated diphenyl ethers (BDEs). Organohalogen Compounds 51:226-229.
Ryan, J.J., B. Patry, P. Mills, and G. Beaudoin. 2002. Recent trends in levels of brominated diphenyl ethers in human milks from Canada. Organohalogen Compounds 58:173-176.
Schecter, A., M. Pavuk, O. Papke, J.J. Ryan, L. Birnbaum, and R. Rosen. 2003. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ. Health Perspect. 111(14): 1723-1729.
Schroeter-Kermani, C., D. Hel, T. Hermann, and O. Papke. 2000. The German environmental specimen bank—application in trend monitoring of PBDEs in human blood. Organohalogen Compounds. 47:49-52.
Schulte, P.A., and F.P. Perera. 1993. Molecular Epidemiology: Principle and Practices. New York: Academy Press.
Schulte, P.A., and G. Talaska. 1995. Validity criteria for the use of biological markers of exposure to chemical agents in environmental epidemiology. Toxicology 101(1-2):73-88.
Schulte, P.A., and M. Waters. 1999. Using molecular epidemiology in assessing exposure for risk assessment. Ann. NY Acad. Sci. 895:101-111.
Sherlock, J., J. Hislop, D. Newton, G. Topping, and K. Whittle. 1984. Elevation of mercury in human blood from controlled chronic ingestion of methylmercury in fish. Hum. Toxicol. 3(2):117-131.
Silva, M.J., D.B. Barr, J.A. Reidy, N.A. Malek, C.C. Hodge, S.P. Caudill, J.W. Brock, L.L. Needham, and A.M. Calafat. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Study (NHANES) 1999-2000. Environ. Health Perspect. 112(3):331-338.
Sobels, F.H. 1977. Some problems associated with the testing for environmental mutagens and a perspective for studies in “comparative mutagenesis”. Mutat. Res. 46(4):245-260.
Sohn, M.D., T.E. McKone, and J.N. Blancato. 2004. Reconstructing population exposures from dose biomarkers: Inhalation of trichloroethylene (TCE) as a case study. J. Expo. Anal. Environ. Epidemiol. 14(3):204-213.
Stern, A.H. 1997. Estimation of the interindividual variability in the one-compartment pharmacokinetic model for methylmercury: Implications for the derivation of a reference dose. Regul. Toxicol. Pharmacol. 25(3):277-288.
Stern, A.H., and A.E. Smith. 2003. An assessment of the cord blood: Maternal blood methylmercury ratio: Implications for risk assessment. Environ. Health Perspect. 111(12):1465-1470.
Strandman, T., J. Koistinen, and T. Vartiainen. 2000. Polybrominated diphenyl ethers (PBDEs) in placenta and human milk. Organohalogen Compounds 47:61-64.
Sutter, T.R. 1995. Molecular and cellular approaches to extrapolation for risk assessment. Environ. Health Perspect. 103(4):386-389.
Swan, S.H., K.M. Main, F. Liu, S.L. Stewart, R.L. Kruse, A.M. Calafat, C.S. Mao, J.B. Redmon, C.L. Ternand, S. Sullivan, and J.L. Teague. 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113(8):1056-1061.
Tan, C., K. Liao, and H.J. Clewell. 2005. Physiologically based pharmacokinetic modeling as a tool to interpret human biomonitoring data. CIIT Activities 25(4):1-6.
Tardif, R., S. Laparé, G.L. Plaa, and J. Brodeur. 1991. Effect of simultaneous exposure to toluene and xylene on their respective biological exposure indices in humans. Int. Arch. Occup. Environ. Health 63(4):279-284.
Thier, R., T. Bruning, P.H. Roos, H.P. Rihs, K. Golka, Y. Ko, and H.M. Bolt. 2003. Markers of genetic susceptibility in human environmental hygiene and toxicology: The role of selected CYP, NAT, and GST genes. Int. J. Hyg. Environ. Health. 206(3):149-171.
Thrall, K.D., R.A. Gies, J. Muniz, A.D. Woodstock, and G. Higgins. 2002. Route-of-entry and brain tissue partition coefficients for common superfund contaminants. J. Toxicol. Environ. Health A. 65(24):2075-2086.
Tornqvist, M., and L. Ehrenberg. 2001. Estimation of cancer risk caused by environmental chemicals based on in vivo dose measurement. J. Environ. Pathol. Toxicol. Oncol. 20(4):263-271.
Tornqvist, M., and H.H. Landin. 1995. Hemoglobin adducts for in vivo dose monitoring and cancer risk estimation. J. Occup. Environ. Med. 37(9):1077-1085.
Vainio, H. 2001. Use of biomarkers in risk assessment. Int. J. Hyg. Environ. Health 204 (2-3):91-102.
Viau, C., G. Hakizimana, and M. Bouchard. 2000. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int. Arch. Occup. Environ. Health 73(5):331-338.
Viau, C. 2002. Biological monitoring of exposure to mixtures. Toxicol. Lett. 134(1-3):9-16.
Viau, C. 2005. Biomonitoring in occupational health: Scientific, socio-ethical, and regulatory issues. Toxicol. Appl. Pharmacol. 207(suppl. 2):S347-S353.
Wasserman, G.A., X. Liu, N.J. Lolacono, P. Factor-Litvak, J.K. Kline, D. Popovac, N. Morina, A. Musabegovic, N. Vrenezi, S. Capuni-Paracka, V. Lekic, E. Preteni-Redjepi, S. Hadzialjevic, V. Slavkovich, and J.H. Graziano. 1997. Lead exposure and intelligence in 7-year-old children: The Yugoslavia Prospective Study. Environ. Health Perspect. 105(9): 956-962.
WHO (World Health Organization). 1995. Inorganic Lead. Environmental Health Criteria 165. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc165.htm [accessed Jan. 20, 2006].
WHO (World Health Organization). 2001. Biomarkers in Risk Assessment: Validity and Validation. Environmental Health Criteria 222. Geneva: World Health Organization. 238pp [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc222.htm [accessed Nov. 21, 2005].
Whyatt, R.M., D.B. Barr, D.E. Camann, P.L. Kinney, J.R. Barr, H.F. Andrews, L.A. Hoepner, R. Garfinkel, Y. Hazi, A. Reyes, J. Ramirez, Y. Cosme, and F.P. Perera. 2003. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect. 111(5):749-756.
Whyatt, R.M., V. Rauh, D.B. Barr, D.E. Camann, H.F. Andrews, R. Garfinkel, L.A. Hoepner, D. Diaz, J. Dietrich, A. Reyes, D. Tang, P.L. Kinney, and F.P. Perera. 2004. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 112(10):1125-1132.
Whyatt, R.M., D. Camann, F.P. Perera, V.A. Rauh, D. Tang, P.L. Kinney, R. Garfinkel, H. Andrews, L. Hoepner, and D.B. Barr. 2005. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol. 206(2):246-254.
Wilson, N.K., J.C. Chuang, C. Lyu, R. Menton, and M.K. Morgan. 2003. Aggregate exposures of nine preschool children to persistent organic pollutant at day care and at home. J. Expo. Anal. Environ. Epidemiol. 13(3):187-202.