2
U.S. and International Biomonitoring Efforts
Human monitoring of occupational exposures was conducted beginning in the 1890s through a variety of blood lead monitoring programs (Sexton et al. 2004). Population-based biomonitoring is more recent and has been implemented at various levels within the United States (both federally and among states) and internationally. Only with the recent advent of the National Health and Nutrition Examination Survey (NHANES) have population-based biomonitoring1 studies expanded on measurements of lead, cadmium, and cotinine in clinical specimens (Burke and Sexton 1995). With the additional exposure surveillance data provided by NHANES and a variety of international biomonitoring efforts, regulators now have an improved understanding of how widespread some chemical exposures are in the general population. Biomonitoring data will improve our understanding of population and individual exposures to chemicals and will help regulatory agencies to set priorities for toxicologic and environmental-health research. Litt et al. (2004) noted that “new technologies in biomonitoring have the potential to transform the nation’s capacity to track exposure to pollutants and understand their impacts on health.”
Current biomonitoring efforts can be categorized as survey projects and research projects. The objective of survey projects typically is to advance public health by producing information about the prevalence of exposure to environmental toxicants based on periodic monitoring (European
|
1 |
As stated in Chapter 1, biomonitoring in the context of this report is focused on biomarkers of exposure, that is, it is limited to the early stages in the process: internal dose, biologically effective dose, and early biologic effect. |
Commission 2004; Knudsen 2004, as cited in ECETOC 2005). Research projects typically are hypothesis-driven and geared to the collection of data to link health outcomes causally to exposures (ECETOC 2005).
Selected historical and current U.S. and international large-scale, population-based efforts to monitor environmental toxicants in human tissues are summarized below. Also included is a brief discussion of biomonitoring by private organizations and laboratories. This chapter is meant not to be a comprehensive summary of biomonitoring efforts but to provide context on the history of biomonitoring and on current and planned efforts in the field. This overview illustrates the diversity in current biomonitoring efforts, particularly with respect to study population size and analytes measured. Because of the differences in biomonitoring studies, including the type of studies conducted, when the studies were performed, and the various applications for the biomarkers, the committee explicitly did not address the duration of the biomonitoring studies or their respective costs in this chapter.
HUMAN BIOMONITORING IN THE UNITED STATES
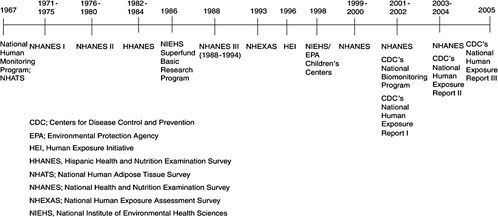
Population-based biomonitoring programs in the United States have been in place since the late 1960s and have evolved substantially (see Figure 2-1). Such initial efforts as the National Human Monitoring Program, administered by the U.S. Environmental Protection Agency (EPA), and NHANES, administered by the Centers for Disease Control and Prevention (CDC), monitored for only a few chemicals, primarily pesticides and metals. Later efforts, including the National Human Exposure Assessment Survey (NHEXAS) and NHANES (1999-2000), began monitoring for many more chemicals. In addition to monitoring for a greater variety of chemicals, some studies (NHEXAS and the Agricultural Health Study) began to include environmental sampling to quantify personal exposure better. Although the larger population-based efforts (such as NHANES) have not been able to incorporate environmental monitoring, they have been instrumental in collecting background information on exposure to upwards of 140 chemicals. In the future, the National Children’s Study (NCS), if funded, would have the potential to collect biomonitoring data and link them to environmental monitoring data.
CDC has been a major player in funding both state and national biomonitoring programs. NHANES and the National Reports on Human Exposure to Environmental Chemicals have provided regulators with a comprehensive overview of exposures in the general population to selected chemicals.
Improvements in analytic techniques for sampling, including lower detection limits, will probably change how biomonitoring data are used. It
also appears that there may be more focus on state and local monitoring through federal funding (the CDC National Biomonitoring Program, NBP) and legislated efforts (as in California and Minnesota). And the monitoring of chemicals in children could be a priority if the NCS is funded.
A majority of U.S. biomonitoring efforts measure such analytes as heavy metals, pesticides, cotinine, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polychlorinated dibenzodioxins, phthalates, and volatile organic compounds (VOCs). Future population-based studies (such as NHANES) will include such chemicals as perfluorinated compounds, polybrominated diphenyl ethers (PBDEs), and perchlorate, on which little exposure information is available.
The National Human Monitoring Program and the National Human Adipose Tissue Survey
EPA has been involved in a number of biomonitoring efforts, including the National Human Adipose Tissue Survey (NHATS). One of the earliest biomonitoring efforts was the National Human Monitoring Program (NHMP). NHMP was established in 1967 by the U.S. Public Health Service to study pesticide exposures in the population. A primary component of NHMP was the NHATS. NHATS, inherited by EPA in 1970, measured pesticides in human adipose tissue to identify chemicals to which a representative sample of the U.S. population was being exposed and to set priorities for reducing risk posed to at-risk groups (NRC 1991). Since its inception, NHATS has collected nearly 12,000 samples of human tissue, primarily from cadavers and some from patients, and provided adequate data to document the extent of exposure of the U.S. population to over 130 pesticides (NRC 1991).
In 1990, the National Research Council Committee on National Monitoring of Human Tissues reviewed and evaluated the uses and effectiveness of NHMP (NRC 1991). In its review, the committee noted that NHATS was effective in documenting a “widespread and significant prevalence of pesticide residues in the general population” and showed “that reductions in use of PCBs, DDT, and dieldrin have been followed by a decline in measured concentrations of these compounds” (NRC 1991). The committee’s review of the program, with its discussion of the need for improved monitoring, was the impetus for the development of NHEXAS.
The National Human Exposure Assessment Survey
NHEXAS was established in 1993, as a follow on to NHATS, to evaluate comprehensive human exposure to multiple chemicals on a community and regional scale (EPA 2005). NHEXAS expanded on NHATS by moni-
toring chemicals (including lead, other metals, pesticides, and organic chemicals) in blood and urine and by surveying personal exposures to chemicals through environmental sampling of air, water, and soil and dust and personal monitoring of air, food and beverages, and uptake (EPA 2005; NRC 1991). Three pilot surveys were conducted: in Arizona, in Maryland, and in a sample population of people in Illinois, Indiana, Michigan, Minnesota, Ohio, and Wisconsin (EPA 2005). NHEXAS measured 46 chemicals in about 460 people (NRC 1991). An EPA Science Advisory Board (SAB) report reviewing NHEXAS noted that among the major strengths of its design were that it was possible to “track many of the exposure pathways back to sources of exposure and [that it] provides a sound scientific basis for exposure and risk reduction” (EPA SAB 1999). The SAB report also recommended that NHEXAS be linked with NHANES data.
Other current federal efforts that incorporate biomonitoring include the National Institute of Environmental Health Sciences (NIEHS)-U.S. EPA Centers for Children’s Environmental Health and Disease Prevention Research, the NIEHS Superfund Basic Research Program, and CDC’s NHANES and the Reports on Human Exposure to Environmental Chemicals. NHANES remains the largest and most comprehensive effort to study chemicals in the U.S. population. If funded, future national biomonitoring efforts would include the NCS, a longitudinal study of children from when they are in utero to the age of 21 years that would incorporate regular biomonitoring for environmental chemicals. CDC’s NHANES and the Reports on Human Exposure to Environmental Chemicals, the NIEHS-EPA Centers for Children’s Environmental Health and Disease Prevention Research, and the NCS are highlighted below. Details on other federal programs, including NIEHS’s Superfund Basic Research Program, are presented in Table 2-1.
National Institute of Environmental Health Sciences-Environmental Protection Agency Centers for Children’s Environmental Health and Disease Prevention Research
The NIEHS-EPA Centers for Children’s Environmental Health and Disease Prevention Research conduct an array of observational studies of environmental-related diseases in children. The program, initiated in 1998, was designed to study environmental exposures of infants and young children, link the exposures to health effects, and develop intervention strategies for reducing the exposures. Funded in two phases, the program includes 12 centers (Kimmel et al. 2005; Landrigan and Tamburlini 2005). Each center has a unique and varied research focus and includes studies of respiratory diseases, childhood learning issues, and developmental disabilities, among others (Kimmel et al. 2005).
TABLE 2-1 Examples of Current U.S. and International Biomonitoring Efforts
|
Agency/Organization/State |
Biomonitoring Program |
|
United States |
|
|
National Institute of Environmental Health Sciences-Environmental Protection Agency Centers for Children’s Environmental Health and Disease Prevention Research |
Twelve centers established to conduct observational studies of environmental exposures of children. Centers are collecting a variety of human tissue and samples, including urine, breast milk, peripheral blood, cord blood, meconium, vernix, saliva, hair, placental tissue (Eskenazi et al. 2005). |
|
Centers for Disease Control and Prevention—National Health and Nutrition Examination Surveys (NHANES); National Reports on Human Exposure to Environmental Chemicals |
Provides continuing assessment of U.S. population’s exposure to environmental chemicals using biomonitoring data from NHANES. First National Report on Human Exposure to Environmental Chemicals (First Report) was issued in March 2001. Second Report, released in January 2003, presents biomonitoring exposure data on 116 environmental chemicals for noninstitutionalized, civilian U.S. population in 1999-2000. Third report was released in July 2005 and includes data on 148 chemicals (CDC 2005). |
|
Centers for Disease Control and Prevention—State funding through National Biomonitoring Program (see discussion of state biomonitoring efforts below) |
In 2001, CDC’s Environmental Health Laboratory launched planning grant program (National Biomonitoring Program) to support biomonitoring capacity building for state public-health laboratories (CDC 2005). |
|
Agency for Toxic Substances and Disease Registry (ATSDR) |
ATSDR conducts site-specific exposure investigations, many of which use biomonitoring to assess individual exposures. For example, ATSDR’s Great Lakes Human Health Effects Research Program works to characterize exposure to persistent contaminants via consumption of Great Lakes fish, to investigate potential adverse health effects, and to identify vulnerable subpopulations. Exemplary biomonitoring-based investigations include effects of Great Lakes fish consumption on body burdens of dioxins, furans, and PCBs (Anderson et al. 1998; Falk et al. 1999) on motor functioning in ageing fish-eaters (Schantz et al. 1999), on reproduction-related endpoints (Persky et al. 2001; Karmaus et al. 2002; Buck et al. 2003), and on behavior and memory (Schantz et al. 2001; Stewart et al. 2003). Vulnerable populations studied under this program include Native Americans (Dellinger et al. 1996; Fitzgerald et al. 1999), neonates (Lonky et al. 1996; Stewart et al. 2000), and the aged (Schantz et al. 1999, 2001). |
|
Chemicals Measured |
|
Mercury, lead, cotinine, pesticides, phthalates, PAHs, PAH-DNA adducts, allergens, endotoxin, antioxidant micronutrients, cytokines, immunoglobulin E, cholinesterase, thyroid hormones, DNA polymorphisms |
|
Lead, cadmium, mercury, cobalt, uranium, antimony, barium, beryllium, cesium, molybdenum, platinum, thallium, tungsten, organochlorine pesticides, organophosphorus insecticides (dialkyl phosphate metabolites), (specific metabolites), pyrethroid pesticides, other pesticides (2-isopropoyxyphenol, carbofuranphenol), herbicides, phthalates, phytoestrogens, polycyclic aromatic hydrocarbons, polychlorinated dibenzo-p-dioxins and dibenzofurans, polychlorinated biphenyls, tobacco smoke |
|
Agency/Organization/State |
Biomonitoring Program |
|
National Institute of Environmental Health Sciences Superfund Basic Research Program |
Funds peer-reviewed research in 19 university programs encompassing 70 collaborating institutions (including number of biomonitoring programs) (NIEHS 2005). For example, the Universities of North Carolina, Chapel Hill (UNC) and California, Berkeley (UCB) are conducting studies to develop and validate biomarkers of exposure to benzene and arsenic with which to investigate exposure response relationships in humans. |
|
Agricultural Health Study |
Large prospective cohort study, conducted in North Carolina and Iowa, to assess current and past agricultural exposures using interviews and environmental and biologic monitoring. Evaluating relationship between pesticide exposure and the development of specific cancers (Alavanja et al. 1996; Agricultural Health Study 2005). |
|
Farm Family Exposure Study (FFES) |
FFES was designed to study pesticide exposures of farm families by measuring urinary pesticides in applier, spouse, children (Farm Family Exposure Study 2005). |
|
California |
California Department of Health Services Environmental Health Laboratory Branch developed California Biomonitoring Plan under 2-year grant from CDC (APHL 2004). |
|
Iowa, Minnesota, North Dakota, South Dakota, and Wisconsin |
Biomonitoring consortium of five Upper Midwest states. States plan to share biomonitoring data and samples on toxicants (CDC 2005). |
|
New Hampshire |
Developing public-health laboratory capacity to biomonitor for arsenic, mercury, phthalates, polybrominated diphenyl ethers; and planning pilot studies to estimate body burden of environmental toxicants using newly developed biomonitoring analytic methods (CDC 2005). |
|
New York |
Developing capacity to monitor for polyaromatic hydrocarbons (PAHs) in urine, polybrominated diphenyl ethers (PBDEs) in serum, organochlorine pesticides in serum, volatile organic compounds (VOCs) in blood, cotinine in saliva, trace elements in blood and urine, inorganic mercury in blood; and to generate data on exposure to persistent organic pollutants (CDC 2005). |
|
Chemicals Measured |
|
Pesticides |
|
Pesticides (glyphosate, 2,4-D, 3,5,6-trichloro-2-pyridinol (chlorpyrifos)) |
|
Organochlorines (DDT), organophophorus dialkyl phosphate metabolites, pyrethroids, PCBs, PBDEs, phthalates, lead, mercury |
|
Asbestos, nitrates and nitrites, persistent organic pollutants, selenium |
|
Arsenic, mercury, phthalates, polybrominated diphenyl ethers |
|
PAHs, PBDEs, organochlorine pesticides, VOCs, cotinine, trace elements, inorganic mercury |
|
Agency/Organization/State |
Biomonitoring Program |
|
Pennsylvania |
Currently funding biomonitoring and environmental-health tracking efforts, including studies of people living in the vicinity of coal-burning power plants. |
|
Washington |
Efforts to enhance environmental monitoring and analyses of mercury and polychlorinated biphenyls, as well as other persistent toxicants. |
|
Rocky Mountain Biomonitoring Consortium (RMBC) |
RMBC includes Arizona, Colorado, Montana, New Mexico, Utah, and Wyoming in efforts to implement and expand regional laboratory-based biomonitoring program. Program will assess extent of exposure to environmental toxicants, including collecting data on background exposures (CDC 2005). |
|
American Chemistry Council Long-Range Research Initiative (LRI) |
LRI has signed a memorandum of understanding with EPA for joint grant solicitations to fund projects to identify methods and approaches for future population studies requiring exposure information, for studies that characterize exposure factors that are related to high-end exposures, and to interpret biomonitoring data in relation to the exposure data. Some current projects are: “(1) comparing biomonitoring data to exposure data in an attempt to better interpret ethylene oxide DNA adducts (i.e., ethylene oxide bound to DNA) and urine and blood levels of benzene metabolites, respectively, (2) studying the relationship between exposure to phthalates and urinary biomarkers in rats and then modeling this relationship for humans, (3) developing and applying more advanced statistical models to characterize relationships between exposures and biomonitoring data, and (4) evaluating biomarkers of in utero exposures to background levels of environmental contaminants” (LRI 2005). |
|
Canada |
|
|
Canadian Health Measures Survey |
Beginning in 2006, Statistics Canada will initiate a national survey of 5,000 people to collect data on health status and biological measurements to assess exposures to environmental chemicals, including lead and mercury. The surveys are currently in development and collection of data is expected to begin in the fall of 2006, with results released in 2009 (Statistics Canada 2006). |
|
Chemicals Measured |
|
Heavy metals (lead, arsenic, mercury) |
|
Arsenic, cotinine, DDT, dioxins, lead, PBDEs, PCBs, mercury, cholinesterase, trihalomethanes |
|
Heavy metals, arsenic speciation, mercury speciation, organophosphates, organochlorine pesticides, VOCs, dichloroethane, trichloroethylene; cotinine, nitrates and nitrites, creosote, PAHs (wood smoke), radionuclides, cyanide, dioxin-furan, disinfection byproducts, perchlorates, phthalate metabolites, thiodiglycol (mustard gas), sarin |
|
Agency/Organization/State |
Biomonitoring Program |
|
European Union |
|
|
Science, Children, Awareness-Raising, Legal Instruments and Evaluation (SCALE) |
SCALE program is designed in effort to “develop a coherent approach to biomonitoring in Europe.” SCALE Working Group aims to “(1) examine the range of policy relevant objectives for human biomonitoring and identify those which are suitable for an EU approach; (2) develop comparable protocols addressing initiation, performance and followup of biomonitoring activities; (3) develop scenarios to integrate biomonitoring results with environmental monitoring data and health monitoring data; and (4) develop communication strategies to allow for adequate responses.” (European Commission 2004)a |
|
Belgium |
|
|
Center for Environmental Health: Flemish biomonitoring program for surveillance of environmental health |
Program will include development of an early warning system based on biomarker measurements in people living in Flanders. Study population includes 1,600 people (European Commission 2004). |
|
FONIA study—follow-up study of newborns regarding immunologic development and its relation to atopy |
Assessing immunologic development and its relation to allergies in children (European Commission 2004). |
|
Croatia |
|
|
Exposure, Intake, and Effects of Toxic and Essential Elements |
Assessment of steroid hormone disruption in placenta as indicator tissue for monitoring fetal and maternal environment. Biomonitoring of metals is included with evaluation of dietary metal intake (European Commission 2004). |
|
Institute for Medical Research and Occupational Health, Zagreb: persistent organic pollutants (POPs) in human milk |
Study will monitor concentration and distribution of POPs in human milk and daily intakes by children who are breast-fed (European Commission 2004). |
|
aA number of the EU efforts discussed in this table were identified from an inventory of biomonitoring efforts in European Commission (2004) report. This inventory is currently in the process of being continuously updated. |
|
|
Agency/Organization/State |
Biomonitoring Program |
|
Denmark |
|
|
ChildrenGenoNetwork |
Study of gene-environment interactions during fetal, neonatal, and infancy periods, evaluating genotoxic exposures and environmental factors, including air pollutants (University of Copenhagen 2005). |
|
Finland |
|
|
Biomonitoring of dioxins in breast milk |
Measurements of dioxins in breast milk. |
|
France |
|
|
EDEN |
Study was initiated to identify factors associated with allergies and respiratory diseases in children, using biomarker data on heavy metals in cord blood, placenta, and hair and cotinine in cord blood and hair (European Commission 2004). |
|
Endocrine disrupters: a longitudinal study on pregnancy and child (PELAGIE) |
Study of exposure to environmental pollutants and pregnancy and postnatal development (European Commission 2004). |
|
European Prospective Investigation into Cancer and Nutrition (EPIC) |
Large study (over 500,000 participants) designed to investigate relationship between diet, nutritional status, lifestyle, and environmental factors and incidence of cancer and other chronic diseases. Blood samples are banked for future analyses (IARC 2005). |
|
ISAAC-II |
Study is evaluating health effects related to exposure to indoor pollutants in children (European Commission 2004). |
|
Germany |
|
|
Cohort study on influence of persistent organic pollutants (POPs) exposure on neurodevelopment of children |
Study will examine role of POP exposure on neurobehavioral development of children (European Commission 2004). |
|
Chemicals Measured |
|
Air pollutants, tobacco smoke, cytogenetic biomarkers |
|
Dioxins, furans, PCBs, PBDEs, heavy metals |
|
Heavy metals, cotinine |
|
Glycol ethers, trichloroacetic acid, atrazine, PCBs, dioxin-like compounds |
|
Primarily study of nutrition |
|
Allergens, air pollutants, mold, and endotoxins |
|
Lead, cadmium, PCDD/PCDF and PCBs |
|
Agency/Organization/State |
Biomonitoring Program |
|
German Environmental Specimen Bank |
Established to systematically collect, process, characterize, and store environmental samples. Blood and other human specimens have been collected since 1981 from about 100 unexposed persons (German Federal Environmental Agency 2006). |
|
German Human Biomonitoring Commission |
Commission draws up monographs on chemicals and derives reference and human biomonitoring (HBM) values. Commission has derived HBM values of lead, cadmium, mercury, and PCP. Commission is responsible for German Environmental Surveys which are representative population studies to determine the exposure of Germany’s general population to chemicals. Surveys have been conducted in 1985-1986 (GerESI), 1990-1992 (GerESII), 1998 (GerESIII), 2003-2006 (GerESIV) (GerES 2005). |
|
Influence of lifestyle factors and behavior on development of immune system and allergies in East-West settlement (LISA) Studying school beginners in Saxony-Anhalt |
Study will assess role of indoor exposures on children and associated characterization of allergies (European Commission 2004). Study will investigate impact of environment on health of schoolchildren in industrial and nonindustrial areas of Saxony-Anhalt (European Commission 2004). |
|
Luxembourg |
|
|
LNS/ALMEN/CST/AKUT: Impact of heavy metals and molds on environmentally burdened patients |
Study will evaluate immunologic biomarkers and assess heavy metals in serum and/or hair samples (European Commission 2004). |
|
Netherlands |
|
|
Association between chemical features of fine particulate air pollution and respiratory health of schoolchildren |
Study was designed to investigate whether exposure to metals and particulate air pollution is associated with airway inflammation, reduced lung function in schoolchildren (European Commission 2004). |
|
Generation R: The Rotterdam Study into Growth, Development and Health |
Study will evaluate normal and abnormal growth and development and identify biologic, social, and environmental determinants (European Commission 2004). |
|
Chemicals Measured |
|
Aluminum, arsenic, barium, cadmium, calcium, copper, chromium, iron, lead, magnesium, manganese, mercury, nickel, phosphorus, potassium, selenium, sodium, sulfur, thallium, zinc, aldrin, dichlorodiphenyldichloroethane, dichlorodiphenyldichlorethene, dieldrin, hexachlorobenzene, heptachlorepoxide, hexachlorocyclohexane, dichlorodiphenyltrichloroethane (DDT), PCBs, pentachlorophenol, P,P-DDT |
|
Lead, cadmium, mercury, PCBs, DDE, HCB, HCH, arsenic, nickel, creatinine, cotinine, nicotine, cortisol, epinephrine, norepinephrine, pentachlorophenol and other chlorophenols, metabolites of pyrethroids, PAHs, organic esters of phosphoric acid |
|
Reference ranges have been established for arsenic, lead, cadmium, mercury, platinum, nickel in blood and urine; pentachlorophenol and metabolites of organophosphorus in serum and urine; PCBs, ß-HCH, HCB, DDE in blood; organochlorine pesticides (ß-HCH, HCB, total DDT) in human milk |
|
Metabolites of benzene, toluene, nicotine |
|
Heavy metals (cadmium, mercury) |
|
Heavy metals, molds |
|
PM10, PM2.5, exhaled NO |
|
Pesticides, phthalates, bisphenol A, cotinine |
|
Agency/Organization/State |
Biomonitoring Program |
|
NEWGENERIS (Newborns and genotoxic exposure risks) |
Using 300,000 mother-child birth cohorts and stored specimens from biobanks, study will develop and apply biomarkers of dietary exposure to genotoxic and immunotoxic chemicals and biomarkers of early effects (European Commission 2006). Researchers will analyze blood samples from biobanks in Norway, Denmark, the United Kingdom, Spain, and Greece (European Union 2006). |
|
Norway |
|
|
Den norske Mor og barn undersǿǿkelsen |
Study will follow 100,000 pregnant women to assess potential exposures to mother and child as evaluated after delivery (European Commission 2004). |
|
Poland |
|
|
DNA damage in children environmentally exposed to lead with assessment of individual susceptibility to toxic effect of lead, and genetic polymorphism of lead biotransformation and mechanism of DNA repair |
Study will assess role of lead exposure in cytogenetic damage in children (European Commission 2004). |
|
Environmental Cancer Risk, Nutrition, and Individual Susceptibility (ECNIS) |
Studying use of biomarkers of exposure and susceptibility and bioindicators of disease in molecular epidemiology of cancer (ECNIS 2005). |
|
Studies of Blood Lead |
Systematic studies of blood lead in general population (Jakubowski 2004). |
|
Portugal |
|
|
Environmental Health Survey Programs (ProVEpAs) |
ProVEpAs are two regional environmental health survey programs carried out in Portugal: “1) to monitor prevalence, space and time trends of human exposure to emissions from Solid Waste Incinerators (SWI); 2) to analyze potential public health impact, either on relevant pathologies or health conditions.” Biomonitoring will be conducted in several population groups (newborn-mother pairs, children under 6 years old, adults 18-65 years old in general population) (Reis et al. 2004). |
|
Agency/Organization/State |
Biomonitoring Program |
|
Slovakia |
|
|
European Longitudinal Study of Pregnancy and Childhood (ELSPAC) |
Study of role of biologic, environmental, and social factors in survival, health, development of fetus, infant, child (European Commission 2004). |
|
Sweden |
|
|
Swedish Environmental Protection Agency: National Health Related Environmental Monitoring Program |
Projects focused on exposure through air and food, including monitoring organic pollutants in breast milk and intake of pollutants in foods. Several studies include monitoring for metal and organic pollutant concentrations in human specimens. Human specimen bank is also storing human samples. |
|
United Kingdom |
|
|
Avon Longitudinal Study of Parents and Children |
Study includes 14,000 pregnant women and their children and includes collection of environmental, dietary, personal, and socioeconomic data and clinical data and biologic samples (European Commission 2004). |
|
Placental Uptake and Transfer of Environmental Chemicals Relating to Allergy in Childhood Years (PLUTOCRACY) (Belgium, Slovakia, Romania) |
Study has been designed to “link the kinetics of the placental transfer of xenobiotics with the epidemiologic associations of allergic diseases among children” (European Commission 2004). |
|
Japan |
|
|
Ministry of Health, Labor, and Welfare |
Continuing biomonitoring projects, including study of umbilical cord blood, maternal blood, milk. |
|
New Zealand |
|
|
Ministry of Health |
Ministry of Health is biomonitoring dioxin in population. |
|
World Health Organization |
|
|
Breast-milk monitoring |
WHO has conducted global surveys of dioxins, dibenzofurans, dioxin-like PCBs in human milk. Fourth UNEP/WHO protocol includes analysis of at least 50 individual samples of breast milk in each participating country (WHO 2005). |
|
|
|
Several centers have been collecting human samples, including urine, breast milk, peripheral blood, cord blood, meconium, vernix, saliva, hair, and placental tissue (Eskenazi et al. 2005). The samples have been analyzed for the presence of numerous substances, such as mercury, lead, cotinine, pesticides, phthalates, PAHs, PAH-DNA adducts, allergens, endotoxin, antioxidant micronutrients, cytokines, immunoglobulin E, cholinesterase, and thyroid hormones. The centers have also been storing samples for future research purposes (Eskenazi et al. 2005).
Centers for Disease Control and Prevention
Since the 1960s, NHANES has been monitoring nutritional and clinical factors in the U.S. population; chemicals in blood and urine were included recently. In addition, CDC’s National Reports on Human Exposure to Environmental Chemicals, based on NHANES data, have been influential in setting priorities for future biomonitoring research (Schober 2005).
National Health and Nutrition Examination Survey
The National Health Survey Act of 1956 required the National Center for Health Statistics to begin collecting health statistics on the general U.S. population. The first health surveys were conducted from 1960 to 1962 on a small sample of the population 18-74 years old with additional surveys conducted on children, 6-17 years old (NRC 1991). Subsequent surveys, called the National Health and Nutrition Examination Surveys, included medical examinations and nutritional and dietary information on the study population.
Designed to study a probability sample of the noninstitutionalized civilian population of the United States, NHANES also conducted nutritional assessment of three high-risk populations: preschool children (6 months to 5 years old), those 60-74 years old, and the poor (persons below the poverty level) (NRC 1991). Each year, the current NHANES samples 5,000 persons, representative of the U.S. civilian household population, in 15 geographic locations. There is also an effort to oversample some demographic groups, including blacks and Mexican Americans (Schober 2005).
The first survey, NHANES I (1971-1975), did not monitor for any environmental chemicals. Later surveys, including NHANES II (1976-1980) and Hispanic HANES (1982-1984), collected data on chemicals by measuring lead and organochlorine pesticides (NCHS 1985). NHANES III measured lead, cadmium, selenium, and cotinine (Needham 2005). In the 1999-2000 NHANES, 116 chemicals were monitored; and the 2003-2004 survey monitored about 250 chemicals (Schober 2005; Needham 2005).
Needham et al. (2005a) discussed a number of limitations of NHANES, which include the following:
-
Little blood, particularly from children, is available for biomonitoring analyses, because most of the collected samples are used for clinical and nutritional testing.
-
NHANES collects only limited biomonitoring data on exposures of the fetus, infant, young and older toddler, and preschool-aged child.
-
NHANES may capture some information on highly exposed populations, but it does not target them.
National Reports on Human Exposure to Environmental Chemicals
The first National Report on Human Exposure to Environmental Chemicals was initially released in 2001 and is based on analyses of NHANES biomonitoring data on exposure to chemicals. The data have various uses: to determine which chemicals people are exposed to and at what concentrations; to establish reference ranges for assessing whether an individual or group has an unusually high exposure, including susceptible populations, such as children, the elderly, and women of childbearing age; to track exposure trends; to assess the effectiveness of public-health efforts to reduce exposure of Americans to specific chemicals; and to set priorities for research on human health effects (CDC 2005).
The first report included an analysis of 27 chemicals for 1999; the second, released in 2002, included an analysis of 116 chemicals for 1999-2000, including the 27 from the first report (CDC 2003). The third report, released in July 2005, includes 148 chemicals for 2001-2002 (CDC 2005). The analytes measured in the third report are listed in Table 2-1. The third report includes newly established biomarkers (phthalates), lower limits of detection (of dioxins, furans, and PCBs), and reference ranges for chemicals not previously monitored (pyrethroid insecticides, phthalates, additional dioxins, PCBs, and other pesticides and herbicides).
The fourth report, to be released in 2007, will include nearly 300 analytes. A high-priority dataset of chemicals—including speciated forms of arsenic, perchlorate, PBDEs, and perfluorooctane sulfonate, and other perfluorinated compounds—may be released in 2006 to assist with regulatory decisions (James Pirkle, CDC, personal commun., June 21, 2005).
The criteria for including chemicals in the National Reports on Human Exposure to Environmental Chemicals include the following considerations: (1) whether exposure is changing (increasing or decreasing) or persisting; (2) health effects of exposure, (3) the proportion of the U.S. population exposed; (4) the need to assess the efficacy of public-health actions to reduce exposure; (5) existence of an analytic method for measuring the
chemical or its metabolite in blood or urine with adequate accuracy, precision, sensitivity, specificity, and speed; and (6) incremental costs (in dollars and personnel) to perform the analyses. CDC weights those criteria; items 1-3 receive the greatest weight, items 4 and 5 receive less weights (but the same weight as each other), and item 6 receives the least weight (67 Fed. Reg. 62477 [Oct. 7, 2002]).
CDC is developing formal criteria for delisting chemicals from the National Reports on Human Exposure to Environmental Chemicals, which it plans to publish in the Federal Register. Delisting criteria will consider whether there has been a change in the concentration of a chemical; if not, the chemical may be delisted (Pirkle 2005).
The biomonitoring data presented in each of the national exposure reports include descriptive statistics on the distribution of blood or urine concentrations of each chemical, including geometric means and percentiles with confidence intervals (CDC 2003). Each report also includes brief toxicity profiles and information relating the findings to biological exposure indices and European reference values or ranges, if available. Additionally, the raw data from the reports are publicly available and serve as a valuable resource.
The use of the National Reports on Human Exposure to Environmental Chemicals has a number of limitations, including the following:
-
For most of the monitored chemicals, information for defining health-based reference values is not available.
-
Environmental-exposure monitoring is not conducted in coordination with the biomonitoring.
-
The number of ethnic groups and geographic locations sampled may limit the ability to extrapolate the data, in that exposure to chemicals may differ by ethnicity and geographic location (Schober 2005).
-
Data on susceptible populations—including infants, toddlers, and preschoolers—are limited.
-
The printed versions of the reports do not include data below the 50th percentile.
-
There appear to be only limited strategies in place for communicating the reported results.
-
The reports are based on a probability sampling of the U.S. population and cannot target “hot spots” of exposure.
Despite those limitations, the National Reports on Human Exposure to Environmental Chemicals are the most comprehensive available summaries of biomonitoring data on a representative sample of the U.S. population. The data provide reference ranges for numerous chemicals and will include, in future reports, data on chemicals which have recently become available,
such as PBDEs and perfluorinated compounds. Tests for trends in chemical exposures were not included in the most recent report (CDC 2005), because three survey periods are needed to establish these patterns; future reports will include such tests (NCHS 2005).
The National Children’s Study
The President’s Task Force on Environmental Health Risks and Safety Risks to Children was charged with “developing strategies to reduce or eliminate adverse effects on children (up to 21 years of age) caused by environmental exposures” (Needham et al. 2005b). The task force recommended that exposure be defined broadly to include biologic, chemical, physical, and psychosocial factors. The task force’s assessment included a recommendation for a longitudinal cohort study of the effects of environmental exposure on the health of the nation’s children. The Children’s Health Act of 2000 authorized the National Institute of Child Health and Human Development to conduct this study with the assistance of CDC, NIEHS, and EPA (Needham et al. 2005b). However, the administration has targeted the NCS for elimination in its fiscal year 2007 budget request, and funding for the study is being debated.
The NCS is intended to follow a representative population of 100,000 children from conception to the age of 21 years and would analyze environmental exposures in the home and biomonitoring measures (CDC 2003). Previous studies of health effects related to environmental exposures of children have been limited by small samples, collection of data on only a few chemicals at a time, an inability to examine gene-environment interactions, and a lack of detailed exposure-assessment data (Trasande and Landrigan 2004). The NCS has the potential to be unique, in that it has been designed to study an extensive cohort of children—over a long period, to collect both biomonitoring data and exposure histories, and to consecutively monitor exposure to numerous chemicals (Trasande and Landrigan 2004). In addition, children are to be screened genetically, allowing for analysis of gene-environment interactions (Transande and Landrigan 2004).
Pesticide-Exposure Studies
Two studies of pesticide exposure in farm workers that include biomonitoring are the Agricultural Health Study (AHS) and the Farm Family Exposure Study (FFES). They include biomonitoring of organochlorine pesticides (AHS), glyphosate (FFES), 2, 4-D, and chlorpyrifos (FFES) in serum, urine, and buccal cells. The studies were designed to evaluate health risks related to pesticide exposure in potentially highly exposed populations.
Agricultural Health Study
The AHS, a collaborative research effort between the National Cancer Institute of the National Institutes of Health and EPA, is a prospective occupational study of 89,658 pesticide appliers and their spouses in Iowa and North Carolina “assembled between 1993 and 1997 to evaluate risk factors for disease in rural farm populations” (Blair et al. 2005). It is being conducted in three phases—phase I (1993-1997), phase II (1999-2003), and phase III (2005)—and includes only limited biomonitoring. Data are gathered with questionnaires to determine pesticide use and exposures, work practices, and other relevant exposures; from buccal cell collection; with dietary surveys; and with interviews to determine updated pesticide exposures (Agricultural Health Study 2005).
Farm Family Exposure Study
Conducted by the University of Minnesota, the FFES is a study of pesticide workers that includes limited biomonitoring. About 95 farm families in Minnesota and South Carolina are involved in regular monitoring of pesticide exposure (Farm Family Exposure Study 2005). After pesticide exposure at the farms, urine samples are collected for 24 hours/day for 4 days. A baseline 24-hour sample is collected before pesticide application. The study is expected to improve exposure assessment in epidemiologic studies of agricultural populations (Baker et al. 2005).
State Biomonitoring Programs
The National Biomonitoring Program, launched in 2001 by CDC, was established to support a variety of state efforts to conduct biomonitoring programs to assist with environmental health tracking at the state level. Thirty-three states received grants in 2002 to initiate biomonitoring program planning. However, only eight of the 33 received grants to implement their biomonitoring plans: New Hampshire, New York, and a consortium of six midwestern states—New Mexico, Arizona, Colorado, Montana, Utah, and Wyoming—known as the Rocky Mountain Biomonitoring Consortium (RMBC).
The New Hampshire Department of Health and Human Services is determining blood mercury concentrations and related freshwater fish consumption, studying speciated arsenic in urine, and analyzing phthalates in urine and PBDEs in serum and breast milk (APHL 2004, 2006). In 2004, New Hampshire received about $300,000 to support its biomonitoring program (APHL 2004).
The New York State Department of Health has developed 10 pilot biomonitoring projects, some of which are completed or under way, includ-
ing the recently completed New York City Health and Nutrition Examination Survey, which includes measurements of mercury, other metals, and cotinine in 2000 adults in New York City; a study of mercury exposure in children in New York City; the New York State Adult Tobacco Survey, which includes an analysis of second-hand smoke exposures; and the New York Angler Cohort Study, which is analyzing exposure to PBDEs and perfluorinated compounds by measuring serum of anglers (Wadsworth Center,2 unpublished material, September 2003; George Eadon, Wadsworth Center, personal commun., July 26, 2005; APHL 2006). The state is also assessing the possibility of using newborn screening spot blood in future biomonitoring research (APHL 2006).
The RMBC assessed its regional public-health priorities and developed the following nine demonstration projects on the basis of the needs of the community: possible correlation of exposure to arsenic in drinking water and type 2 diabetes, a spot blood metals-analysis feasibility study, health-clinic samples for chemical-terrorism baselines, of relationship between urine arsenic and metal concentrations and drinking-water exposure, assessment of exposure to VOCs from subsurface volatilization, cotinine concentrations associated with environmental tobacco smoke, assessment of exposure to mercury from ingestion of fish, analysis of radionuclides in urine, and biomonitoring of organophosphorus pesticides in urine (Utah Department of Health 2006).
A number of the individual states in the RMBC have initiated biomonitoring efforts. For instance, Arizona has begun a regional arsenic study to determine the relationship between arsenic in drinking water and concentrations detected in urine (APHL 2006). Montana is conducting a regional arsenic assessment similar to that in Arizona (APHL 2006). New Mexico is integrating efforts to identify chemical terrorism through the biomonitoring of thiodyglycol, a metabolite of sulfur mustard (APHL 2006). And Utah is validating methods for biomonitoring of selenium and arsenic (APHL 2006).
In addition to those efforts funded directly by CDC, states have tried to develop biomonitoring initiatives through their state legislatures, including Minnesota (the Healthy Minnesotans Biomonitoring Program, Minnesota Senate Bill 979) and California (Healthy Californians Biomonitoring Program, California Senate Bill 600). State laboratories have also received funding from CDC since 1999 through the Laboratory Network for Chemical Terrorism to develop laboratory capacity to respond to a chemical-terrorism incident. The network supports 62 state, territorial, and metropolitan public-health laboratories in developing capacity to monitor for chemical exposures in blood and urine, including chemical-warfare agents
and a variety of metals. Participation in the program is designated by three levels of activity:
-
Level 3 laboratories actively coordinate with hospitals in clinical-specimen collection, storage, and shipment and work to develop an appropriate response plan (CDC 2006a).
-
Level 2 laboratories have the capacity to detect exposures to a number of toxic chemical agents in human blood or urine, including cyanide and metals (CDC 2006b).
-
Level 1 laboratories have the capacity to monitor for many chemicals in human blood or urine, including mustard agents and nerve agents (CDC 2006b).
States have been encouraged to apply any “unused capacity” that developed in this program to other biomonitoring projects.
Occupational Biomonitoring Efforts
Of the occupational biomonitoring programs in the United States, two are administered by the Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) but implemented primarily at the state level.
The Occupational Safety and Health Standards (29 CFR 1910.1025 [2005]) require employers to collect biomonitoring samples from workers who have been exposed to airborne lead above the current action level of 30 µg/m3. Employers must have the samples analyzed by laboratories that have met OSHA requirements for blood lead proficiency testing. State health departments often require that increased blood lead results be reported (OSHA 2005).
OSHA also requires biomonitoring of workers for cadmium exposure. Medical surveillance is required for all workers if their urine concentration of cadmium exceeds 3 µg/g creatinine, the Beta-2 microglobulin exceeds 300 µg/g creatinine, or the cadmium in whole blood exceeds 5 µg/liter (29 CFR 1910.1027 [2005]).
In addition to OSHA requirements, NIOSH has initiated the Adult Blood Lead Epidemiology and Surveillance (ABLES) program, a state-based surveillance program of laboratory-reported adult blood lead concentrations (NIOSH 2005). If blood lead is determined to exceed allowable limits, the program includes state interventions: “(1) conducting follow-up interviews with physicians, employers, and workers; (2) investigating work sites; (3) providing technical assistance; (4) providing referrals for consultation and/or enforcement; and (5) developing and disseminating educational materials and outreach programs” (NIOSH 2005). The ABLES program
funds 37 states. Participating states require that laboratories report blood lead concentrations to state health departments (NIOSH 2005).
Private Biomonitoring Efforts
A number of private organizations fund biomonitoring programs for research purposes and to assess exposure at the individual level. The efforts of the American Chemistry Council’s Long-Range Research Initiative, the International Life Sciences Institute Health and Environmental Sciences Institute (ILSI-HESI) Biomonitoring Technical Committee, and private laboratories that cater to individual requests to biomonitor for chemicals are highlighted below.
American Chemistry Council’s Long-Range Research Initiative
The International Council of Chemical Associations—made up of the American Chemistry Council, the European Chemical Industry Council, and the Japan Chemical Industry Association—is the global coordinator of the Long-Range Research Initiative (LRI), a research program that funds research in the effects of chemicals on human health and the environment (LRI 2001).
One specific subject that LRI solicits and funds research in is characterizing and estimating exposures and interpreting and using biomonitoring data (ACC 2002). Objectives of LRI’s biomonitoring program include enabling more accurate application of biomonitoring data to risk assessment, evaluating exposure models and assessments, enabling design of more realistic toxicology tests, providing leadership for governments and other organizations to fund such work, and enhancing the industry’s ability to communicate risk (LRI 2005).
Examples of biomonitoring projects that LRI is funding include comparing biomonitoring data with exposure data to interpret ethylene oxide DNA adducts (ethylene oxide bound to DNA) and urine and blood concentrations of benzene metabolites, studying the relationship between phthalate exposure and urinary biomarkers in rats and modeling the relationship for humans, developing and applying more-advanced statistical models to characterize relationships between exposures and biomonitoring data, and evaluating biomarkers of in utero exposures to compare with background concentrations of chemicals (LRI 2001).
International Life Sciences Institute Health and Environmental Sciences Institute Biomonitoring Technical Committee
Although ILSI-HESI is not directly involved in collecting biomonitoring data, it is actively researching their interpretation and potential regulatory
uses. ILSI-HESI supports scientific and educational programs dedicated to health and environmental issues that are of concern to the public, the scientific community, government agencies, and industry. Its Biomonitoring Technical Committee comprises representatives of government, industry, and academe. The technical committee’s missions are to delineate the appropriate scientific uses of biomonitoring tools and biomonitoring data needed to characterize exposure to chemicals and to define the criteria for the integration of biomonitoring and toxicology data into a robust risk assessment process (ILSI 2005). The technical committee has a number of working groups that are examining biomonitoring issues, including the application of biologic exposure indices in the occupational vs environmental settings and development of criteria for collecting, applying, and interpreting biomonitoring data. It has convened numerous meetings and workshops, including an International Biomonitoring Workshop in September 2004, which led to a series of case studies of the application of chemical-specific biomonitoring data to risk assessment (Albertini et al. in press; Barr and Angerer in press; Birnbaum and Cohen Hubal in press; Butenhoff et al. in press; Calafat and McKee in press; Hughes in press; Robison and Barr in press).
Private Laboratories
Numerous private laboratories offer biomonitoring services to individual clients. It is difficult to estimate their exact number, but a small sampling of laboratory services has revealed the following. Hundreds of laboratories offer laboratory tests to determine the presence of chemicals in various matrices (blood, urine, and hair). Clients include medical and occupational-health professionals and clinics, academic researchers, attorneys, corporations, and individuals interested in determining personal exposures.
Many of the private laboratories offer screening for heavy metals (including lead, mercury, cadmium, arsenic, aluminum, and nickel) and other chemicals, such as PCBs, chlorinated solvents, trichloroethylene, and pesticides. One such laboratory advertised testing for nearly 70 chemicals. Occupational screening was also offered at some of the laboratories. For many laboratories, people may order test and screening kits over the Internet, by fax, or by telephone. A person can send in a blood, urine, or hair sample for analysis. In some cases, a physician’s signature is required to have the sample tested.
Regarding laboratory certification for private laboratories, the Clinical Laboratory Improvement Amendments (CLIA) state the conditions that “all laboratories must meet to be certified to perform testing on human specimens under the Clinical Laboratory Improvement Amendments of 1988” (57 FR 7139, Feb. 28, 1992). The requirements do not apply to “any
facility or component of a facility that only performs testing for forensic purposes; research laboratories that test human specimens but do not report patient-specific results for the diagnosis, prevention or treatment of any disease or impairment of, or the assessment of the health of individual patients; or laboratories certified by the Substance Abuse and Mental Health Services Administration (SAMHSA), in which drug testing is performed which meets SAMHSA guidelines and regulations.”
Little concrete information is available on the proportion of private biomonitoring laboratories that have attained CLIA certification, but CLIA regulations impose substantial penalties on noncomplying laboratories (CDC 2004). Because proficiency test samples and standard reference materials for analytes not typically measured may be unavailable (CMS 2005), comparability of private-laboratory results with reference ranges developed elsewhere may be open to question.
Biomonitoring Efforts by Environmental Organizations
A number of environmental groups have been actively involved in collecting biomonitoring data in the United States, Canada, and Europe. Those organizations include the Environmental Working Group (EWG), the World Wildlife Fund (WWF), Commonweal, and Environmental Defence (based in Canada). The studies have often been conducted on a smaller scale and with samples from a few people in a select region. Examples are discussed below.
In the United States, EWG has conducted biomonitoring studies ranging from measurement of 287 chemicals in nine volunteers to monitoring specifically for perchlorate in breast milk of women in 18 states (EWG 2003a; EWG 2005). In Body Burden I, a study led by Mount Sinai School of Medicine in collaboration with EWG and Commonweal, researchers found an average of 91 chemicals in the blood and urine of nine volunteers. A total of 167 chemicals were identified in the study population (EWG 2003a). A followup study conducted by EWG (Body Burden II) in collaboration with Commonweal measured an average of 200 chemicals in cord blood from 10 babies born in August and September 2004. Over 280 chemicals were identified in the samples (EWG 2005). Chemicals tested included perfluorinated chemicals, pesticides, and PBDEs.
In addition to the studies described above, EWG has conducted biomonitoring tests for specific chemicals in breast milk, such as PBDEs (EWG 2003b).
Commonweal has conducted biomonitoring studies, primarily in Californians. Taking It All In—Documenting Chemical Pollution in Californians Through Biomonitoring (Commonweal 2005) monitored for 25 chemicals in six categories: mercury, organochlorine pesticides, perfluori-
nated compounds, PBDEs, bisphenol A, and phthalates in 11 people (Commonweal 2005).
Environmental Defence recently released Toxic Nation: A Report on Pollution in Canadians (Environmental Defence 2005). Samples were collected from 11 people for the presence of 88 chemicals, including heavy metals, PBDEs, PCBs, perfluorinated chemicals, organochlorine pesticides, organophosphorus-insecticide metabolites, and VOCs. The study objectives included determining whether pollutants were present at measurable concentrations in Canadians, identifying chemicals of concern, and creating public awareness of methods for avoiding exposure.
WWF has conducted similar biomonitoring studies in a cross-section of the population in the UK. As reported in ContamiNation, WWF took samples from 155 volunteers in 13 locations in England, Northern Ireland, Scotland and Wales and monitored for 12 organochlorine pesticides, 45 PCB congeners, and 21 PBDEs (WWF 2003).
In a similar survey (Bad Blood? A Survey of Chemicals in the Blood of European Ministers), WWF collected samples from 14 government ministers of 13 EU countries and analyzed them for 103 chemicals, including organochlorine pesticides, PCBs, synthetic musks, perfluorinated chemicals, PBDEs, phthalates, and antibacterials (WWF 2004).
INTERNATIONAL BIOMONITORING EFFORTS
Many biomonitoring studies are undertaken outside the United States, primarily in Europe. The more comprehensive efforts have been administered through the Science, Children, Awareness-Raising, Legal Instruments and Evaluation (SCALE) program, the World Health Organization, and a variety of programs in Germany, Portugal, and Belgium. (See Table 2-1 for more details on examples of biomonitoring efforts at the international level.)
Of the European studies reviewed, many measured heavy metals, cotinine, PCBs, pesticides, PAHs, dioxins, phthalates, and VOCs. Germany has taken a substantial lead in this respect through its comprehensive population-based surveys (German Environment Surveys) and concerted efforts to develop health-protective reference values for the general population. In addition European countries have been actively involved in occupational biomonitoring efforts. In fact, some countries have biomonitoring surveillance programs that have been required by law.
Science, Children, Awareness-Raising, Legal Instruments and Evaluation
Recent efforts to coordinate research and develop a “coherent approach” to biomonitoring across the European Union (EU) have led to the inception of the SCALE program (European Commission 2004). SCALE is part of a
larger European Environment and Health strategy to reduce and prevent diseases related to environmental exposures, with emphasis on exposures of susceptible populations, including children (Commission of the European Communities 2004). An initial survey conducted by SCALE of biomonitoring efforts focusing primarily on children’s exposures identified nearly 100 efforts in the EU alone, this is a preliminary effort that is being continuously updated (European Commission 2004). Nineteen projects currently examine exposures to dioxin and PCBs; 42 heavy metals; and 25 asthma or allergies (European Commission 2004). Several EU studies of exposures of children incorporated prenatal and postnatal exposures and markers of effect and susceptibility (European Commission 2004; Neri et al. 2006).
The strategy includes planning for a European Integrated Environment and Health Monitoring and Response System, which involves establishing a European Union Biomonitoring Framework that is to “assess environment and health linkages relative to children and to generate appropriate policy responses.” The strategy also addresses the need for better coordination among European biomonitoring initiatives.
The EU has developed a pilot project in an effort to coordinate biomonitoring approaches and encourage the sharing of methodologies among member states. The pilot project will test the hypothesis that human biomonitoring can be performed in a comparable way throughout Europe and that such a coordinated approach will provide better information on the relationship between health effects and exposure information. The aim of the pilot project is to establish reference values and identify reference ranges for specific biomarkers, and to evaluate the effect of policy measures if biomonitoring data are collected over time. The pilot project may elucidate the need for a full-scale European biomonitoring program, which will include harmonized procedures and comparable biomonitoring protocols (ESBIO 2006).
World Health Organization
WHO has conducted three international studies of PCBs, polychlorinated dibenzodioxins, and polychlorinated dibenzo-furans in human milk during 1987-2003 (WHO 2000). The first two surveys were conducted in 1987-1988 and 1992-1993 in a number of European countries. The third, conducted in 2000-2003, included additional countries. A fourth survey has been developed with the intent to assess the persistent organic pollutants (POPs) found in human milk so that each country can better identify and set priorities among POPs for remedial action (WHO 2000). The sample population will include at least 50 mothers from each country who are planning to breastfeed (WHO 2005). Two sampling periods have been proposed, the first to obtain a baseline sample of POPs in representative
individual and pooled samples of human milk, the second to include similarly selected participants after a 4-year period (WHO 2005).
Germany
Germany has comprehensive occupational and population-based biomonitoring programs. The German Environmental Surveys (GerESs) are a multistage probability sample of the German population that include analysis of tissues for traces of environmental chemicals (see Table 2-1 for analytes measured) (Becker et al. 2003). The surveys were conducted in 1985-1986 (GerESI), 1990-1992 (GerESII), and 1998 (GerESIII) and included 4,822 people 18-69 years old (Becker et al. 2003). The most recent survey, GerESIV (2003-2006), will include 1,800 children in 150 sampling locations (GerES 2005). Beginning in 1993, data from the GerES surveys and other epidemiologic and toxicologic studies were used to establish reference values and human biological monitoring (HBM) values (GerES 2005).
Reference values “indicate the upper margin of the current background exposure of the general population and [are used] to identify subjects with an increased level of exposure” (Jakubowski and Trzcinka-Ochocka 2005) compared with the background population level. Those values are derived from data on blood, urine, and other tissues collected from population studies (Ewers et al. 1999). Reference values may be derived differently for susceptible groups if physiologic differences are substantial (for example, children vs adults) (Ewers et al. 1999).
HBM values are derived from toxicologic and human studies and are health based (Jakubowski and Trzcinka-Ochocka 2005). Two types of HBM values exist: HBM I, “the concentration of an environmental toxin in human biological material below which there is no risk of adverse health effects”; and HBM II, “the concentration above which there is an increased risk of adverse health effects in susceptible individuals in the general population” (Jakubowski and Trzcinka-Ochocka 2005). An HBM I value serves as an alert level, and an HBM II value is an action level at which immediate efforts should be made to reduce exposure and further clinical examination should follow (Ewers et al. 1999). HBM values and reference values have been derived for a number of chemicals, including lead, cadmium, mercury, pentachlorophenol (PCP), and arsenic.
Additional examples of European biomonitoring efforts are included in Table 2-1.
HUMAN-SPECIMEN BANKING
Lee et al. (1995) defined environmental-specimen banking as “a long-term, stable storage of specimens sampled from the physical environment,
such as air, water, soil, or sediment samples, or of biological specimens sampled from human, animal, or plant populations.” If collected and stored appropriately, tissues from specimen banks can be used in retrospective and prospective cohort studies (Zenick and Griffith 1995). That allows the testing of hypotheses as the scientific community identifies them after sampling has taken place. Ideally, data provided from stored-specimen banks can be used to “relate levels of environmental contamination to health outcomes via doses” (Holzman 1996).
There are a number of large specimen banks and repositories in the United States, administered primarily by federal government. Others are administered by the military, universities, corporations, and nonprofit organizations (NBAC 1999). Similarly, the EU has specimen banks available for research purposes. A few examples from in the United States and EU are described below.
In the United States, CDC has been involved in tissue and specimen banking primarily to provide information for use in epidemiologic studies and research programs. Two larger-scale programs include specimens from NHANES and the Agency for Toxic Substances and Disease Registry (ATSDR) Specimen and Data Repository.
Regarding storage of tissue resulting from NHANES, serum, plasma, and urine have been collected and stored for future research projects. Specimens from NHANES III and NHANES 1999-2004 are available. Projected uses of the specimens include developing new analytic technologies, identifying new biomarkers, and intramural and extramural research, as approved by CDC (Gunter 1997; NCHS 2005).
In 1995, ATSDR funded the CDC-ATSDR Specimen and Data Repository to store over 6 million biologic specimens for use in various research work (Gunter 1997). Numerous specimens are stored in the repository, including serum, cells, and tissues. The repository was designed to handle a large portion of CDC’s biologic specimens (Gunter 1997).
The EU, through its Health and Environment Strategy, has stressed the importance of increased funding and capacity in addition to improved coordination among current biobanking activities (European Commission 2004). A number of European countries have established national specimen banks, including Germany and Sweden.
The German Environmental Specimen Bank, initiated in 1985, annually samples and archives specimens to determine the effectiveness of environmental regulations and to conduct retrospective monitoring (European Commission 2004). The bank collects six types of human specimens— whole blood, blood plasma, scalp hair, pubic hair, saliva, and 24-hour urine samples from people 20-29 years old in four cities (Münster, Halle/ Saale, Greifswald, and Ulm). Screening is conducted to determine the pres-
ence of a variety of metals, PCBs, hexachlorobenzene, PBDEs, phthalates, and PCPs (European Commission 2004).
The Swedish Environmental Specimen Bank is a centralized storage bank that has been operational for 20 years. It conducts annual sampling of a variety of environmental and human specimens for use in a number of studies, including retrospective analyses. Regional banks store human blood for use in these studies (European Commission 2004).
The European Prospective Investigation into Cancer and Nutrition (EPIC) is the largest study of diet and health in the EU, with over 520,000 participants in 10 European countries. The study, initiated in 1992, collects detailed information and diet and lifestyle factors in addition to blood samples, which are stored for future analyses. The study participants will be followed for the next 10 years to investigate the role of nutrition in the development of chronic disease (IARC 2005).
UK Biobank is a long-term human-specimen bank that will be initiated at full scale in 2006, although pilot projects are under way. The project will collect information, including blood (fractioned to plasma or serum) and urine samples, from 500,000 participants 40-69 years old. The study will follow participants over 20-30 years to study progression of chronic diseases.
Supporting the efforts described above, the International Society for Biological and Environmental Repositories was founded in 2000 to provide guidance on repository management, disseminate information regarding the effective management of specimen collections, and develop efforts to educate the community about related issues (ISBER 2005).
Additional information about biobanking is presented in Table 2-1 and in Chapter 4.
GENERAL OBSERVATIONS
A review of large-scale biomonitoring programs in the United States and the EU did not reveal substantial differences in the types and number of analytes measured (see Table 2-1 for discussion of examples of studies). Most studies included monitoring of heavy metals (often lead and mercury), pesticides, PAHs, PCBs, dioxins, phthalates, VOCs, and emerging chemicals (perfluorinated compounds and PBDEs).
Regarding funding, the Government Accountability Office, in its review of the long-term strategy for monitoring of exposure to chemicals in the United States, noted that, “as compared to the hundreds of millions spent on monitoring contaminants in environmental media, we estimate that $7 million was spent collectively by CDC (including ATSDR) and EPA on their respective human exposure efforts in 1999” (GAO 2000). Although funding for such efforts in the United States has increased with the
CDC National Reports on Human Exposure to Environmental Chemicals and NHANES, state biomonitoring efforts have not fared as well. For instance, funding for implementation of state biomonitoring plans has been in place in only three states or regions as of 2004. In addition, although the state planning grants were funded at $5,000,000 and $4,970,500 in FY 2002 and FY 2003, respectively, only $2,650,000 was allotted for implementation (APHL 2004). The NCS, would provide a valuable database of children’s exposures; however, its funding status is currently being debated. The burden of collecting biomonitoring data seems to fall on CDC.
Without state and local programs, valuable geographic and temporal differences in exposure cannot be examined. States are better able to target programs to serve the needs of the communities and may be able to incorporate environmental sampling to augment human monitoring data (Needham et al. 2005a). Thus, increased funding for state programs should have a high priority; as stated by the NY Wadsworth Center, a CDC grantee, “significantly higher funding is needed to support larger biomonitoring exposure investigations, ability to respond to new public health issues, and to participate in external collaborations to address emerging problems” (Wadsworth Center, unpublished material,3 September 2003; George Eadon, Wadsworth Center, personal commun., July 26, 2005).
An assessment of biomonitoring efforts reveals that the biomonitoring of chemicals in children seems to have a high priority in both the United States and the EU, as evidenced by the sheer number of programs and the aggressive agenda for future monitoring of this population.
Other issues include the monitoring of susceptible populations in the United States and Europe. In addition to current population-based biomonitoring programs, future U.S. efforts should include biomonitoring of populations that may be at higher risk of exposure to chemicals. Additional funding for state or local biomonitoring programs may provide an opportunity to evaluate exposure of those populations (for example, state HANES).
As discussed in this chapter, because biomonitoring is being conducted on an international level by numerous organizations, and there is much knowledge to be gained from understanding patterns of exposure worldwide, the committee encourages the exchange of biomonitoring information and expertise globally. This includes sharing biomonitoring data, study approaches, and tracking of trends. Such coordination will enhance national and international standardization and validation of biomonitoring techniques and provide for complementary study designs. To this end, the committee encourages the development of such information exchanges be-
tween EPA and the Organization for Economic Co-operation and Development (OECD).
CONCLUSIONS
An assessment of current U.S. and European biomonitoring efforts has yielded the following conclusions:
-
Biomonitoring is rapidly developing in the United States and Europe with comparable types and numbers of analytes being measured.
-
The biomonitoring of chemicals in children appears to have a high priority in both the United States and the European Union.
-
State and local biomonitoring programs have the potential to provide valuable biomonitoring data on the geographic and temporal differences in exposure among populations. Increased funding for such efforts in the United States is needed.
RECOMMENDATIONS
-
The committee recommends that additional funding be committed to state-level biomonitoring programs.
-
The committee encourages the sharing of biomonitoring data between the EPA and the OECD to foster international collaboration and develop the field of biomonitoring.
REFERENCES
ACC (American Chemistry Council). 2002. Long-Range Research Initiative. Research Strategy. American Chemistry Council, Arlington, VA. October 2002 [online]. Available: http://www.uslri.com/documents/cat_25/doc_427.pdf [accessed June 3, 2005].
Agricultural Health Study. 2005. Agricultural Health Study [online]. Available: http://www.aghealth.org/ [accessed Sept. 26, 2005].
Alavanja, M.C., D.P. Sandler, S.B. McMaster, S.H. Zahm, C.J. McDonnell, C.F. Lynch, M. Pennybacker, N. Rothman, M. Dosemeci, A.E. Bond, and A. Blair. 1996. The Agricultural Health Study. Environ. Health Perspect. 104(4):362-369.
Albertini, R., M. Bird, N. Doerrer, L. Needham, S. Robison, L. Sheldon, and H. Zenick. In press. The use of biomonitoring data in exposure and human health risk assessment. Environ. Health Perspect. [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9056/abstract.html [accessed Aug. 21, 2006].
Anderson, H.A., C. Falk, L. Hanrahan, J. Olson, V.W. Burse, L. Needham, D. Paschal, D. Patterson, Jr., and R.H. Hill, Jr. 1998. Profiles of Great Lakes critical pollutants: A sentinel analysis of human blood and urine. The Great Lakes Consortium. Environ. Health Perspect. 106(5):279-289.
APHL (Association of Public Health Laboratories). 2004. Biomonitoring: Measuring Chemicals in People. May 2004 [online]. Available: http://www.aphl.org/docs/Biomonitoring.pdf [accessed June 3, 2005].
APHL (American Public Health Laboratories). 2006. Recent Activities. Biomonitoring in the States: The APHL Minute 1:12-13 [online]. Available: https://www.aphl.org/docs/newsletter/january_february_3.pdf [accessed Jan. 18, 2006].
Baker, B.A., B.H. Alexander, J.S. Mandel, J.F. Acquavella, R. Honeycutt, and P. Chapman. 2005. Farm Family Exposure Study: Methods and recruitment practices for a biomonitoring study of pesticide exposure. J. Expo. Anal. Environ. Epidemiol. 15(6):491-499.
Barr, D.B., and J. Angerer. In press. Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ Health Perspect. [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9062/abstract.html [accessed Aug. 21, 2006].
Becker, K., C. Schulz, S. Kaus, M. Seiwart, and B. Seifert. 2003. German Environmental Survey 1998 (GerES III): Environmental pollutants in the urine of the German population. Int. J. Hyg. Environ. Health. 206(1):15-24.
Birnbaum, L.S., and E.A. Cohen Hubal. In press. Polybrominated diphenyl ethers: A case study for using biomonitoring data to address risk assessment questions. Environ. Health Perspect. [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9061/abstract.html [accessed Aug. 21, 2006].
Blair, A., D. Sandler, K. Thomas, J.A. Hoppin, F. Kamel, J. Coble, W.J. Lee, J. Rusiecki, C. Knott, M. Dosemeci, C.F. Lynch, J. Lubin, and M. Alavanja. 2005. Disease and injury among participants in the Agricultural Health Study. J. Agric. Saf. Health 11(2): 141-150.
Buck, G.M., P.T. Grace, E.F. Fitzgerald, J.E. Vena, J.M. Weiner, M. Swanson, and M.E. Msall. 2003. Maternal fish consumption and infant birth size and gestation: New York State Angler Cohort Study. Environ. Health 2(1):7-15.
Burke, T.A., and K. Sexton. 1995. Integrating science and policy in a National Human Exposure Assessment Survey. J. Expo. Anal. Environ. Epidemiol. 5(3):283-296.
Butenhoff, J.L., G.W. Olsen, and A. Pfahles-Hutchens. In press. The applicability of biomonitoring data for perfluorooctanesulfonate (PFOS) to the environmental public health continuum. Environ. Health Perspect. [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9060/abstract.html [accessed Aug. 21, 2006].
Calafat, A.M., and R.H. McKee. In press. Integrating biomonitoring exposure data into the risk assessment process: Phthalates (diethyl phthalate and di[2-ethylhexyl] phthalate) as a case study. Environ. Health Perspect. [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9059/abstract.html [accessed Aug. 21, 2006].
CDC (Centers for Disease Control and Prevention). 2003. Second National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.jhsph.edu/ephtcenter/Second%20Report.pdf [accessed Nov. 21, 2005].
CDC (Centers for Disease Control and Prevention). 2004. CLIA: Subpart R. Enforcement Procedures. Clinical Laboratory Improvement Amendments U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.phppo.cdc.gov/clia/regs/subpart_r.aspx. [accessed Jan. 18, 2006].
CDC (Centers for Disease Control and Prevention). 2005. Third National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.cdc.gov/exposurereport/3rd/pdf/thirdreport.pdf [accessed Sept. 26, 2005].
CDC (Centers for Disease Control and Prevention). 2006a. Laboratory Response Network. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention [online]. Available: http://www.bt.cdc.gov/lrn/pdf/lrn-overview-presentation.pdf [accessed Jan. 18, 2006].
CDC (Centers for Disease Control and Prevention). 2006b. Laboratory Network for Chemical Terrorism. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention [online]. Available: http://www.bt.cdc.gov/lrn/chemical.asp. [accessed Jan. 18, 2006].
CMS (Center for Medicare & Medicaid Services). 2005. CLIA Approved Proficiency Testing Programs 2005 [online]. Available: http://63.241.27.79/clia/ptlist.pdf. [accessed April 14, 2006].
Commission of the European Communities. 2004. The European Environment & Health Action Plan 2004-2010. Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee. COM(2004) 416 final. Brussels. June 9, 2004 [online]. Available: http://europa.eu.int/comm/environment/health/pdf/com2004416.pdf [accessed Nov. 21, 2005].
Commonweal. 2005. Taking It All In: Documenting Chemical Pollution in Californians Through Biomonitoring. Commonweal Biomonitoring Center, Bolinas, CA [online]. Available: http://www.commonweal.org/programs/download/TIAI_Lo-Rez.pdf [accessed April 17, 2006].
Dellinger, J.A., R.M. Meyers, K.J. Gebhardt, and L.K. Hansen. 1996. The Ojibwa Health Study: Fish residue comparisons for Lakes Superior, Michigan, and Huron. Toxicol. Ind. Health 12(3-4):393-402.
ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals). 2005. Guidance for the Interpretation of Biomonitoring Data. Document No. 44. Brussel, Belgium: ECETOC. Nov. 30, 2005.
ECNIS. 2005. Environmental Cancer Risk, Nutrition and Individual Susceptibility [online]. Available: http://www.ecnis.org/ [accessed Sept. 26, 2005].
Environmental Defence. 2005. Toxic Nation: A Report on Pollution in Canadians. Environmental Defence, Toronto, ON. November 2005 [online]. Available: http://www.environmentaldefence.ca/toxicnation/report/Rev_English%20Web.pdf [accessed April 17, 2006].
EPA (U.S. Environmental Protection Agency). 2005. Human Exposure Measurements: National Human Exposure Assessment Survey (NHEXAS) [online]. Available: http://www.epa.gov/heasd/edrb/nhexas.htm [accessed June 3, 2005].
EPA SAB (U.S. Environmental Protection Agency Science Advisory Board). 1999. An SAB Advisory: The National Human Exposure Assessment Survey (NHEXAS) Pilot Studies. EPA-SAB-IHEC-ADV-99-004. U.S. Environmental Protection Agency, Science Advisory Board, Washington, DC. February 1999 [online]. Available: http://www.epa.gov/sab/pdf/ihea9904.pdf [accessed Nov. 23, 2005].
ESBIO (Expert Team to Support Biomonitoring in Europe). 2006. European Pilot Project on HBM (Implementation Group on Human Biomonitoring). European Human Monitoring [online]. Available: http://www.eu-biomonitoring.org/sub/epphbm_main.htm [accessed April 14, 2006].
Eskenazi, B., E.A. Gladstone, G.S. Berkowitz, C.H. Drew, E.M. Faustman, N.T. Holland, B. Lanphear, S.J. Meisel, F.P. Perera, V.A. Rauh, A. Sweeney, R.M. Whyatt, and K. Yolton. 2005. Methodologic and logistic issues in conducting longitudinal birth cohort studies: Lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ. Health Perspect. 113(10):1419-1429.
European Commission. 2004. Baseline Report on “Biomonitoring for Children” in the Framework of the European Environment and Health Strategy (COM(2003)338 final). Prepared by the Technical Working Group on Integrated Monitoring. January 9, 2004 [online]. Available: http://www.brussels-conference.org/Download/baseline_report/BR_Biomonitoring_final.pdf [accessed June 3, 2005].
European Commission. 2006. Endocrine Disrupter Research-Ongoing 5th and 6th Framework Projects. European Commission [online]. Available: http://europa.eu.int/comm/research/endocrine/projects_ongoing_en.html [accessed April 17, 2006].
European Union. 2006. Press Release: NewGeneris: A New European Research Project on Children’s Health, Food and the Environment [online]. Available: http://www.euractiv.com/29/images/NewGeneris_pr_tcm29-152906.doc [accessed April 24, 2006].
Ewers, C., C. Krause, C. Schulz, and M. Wilhelm. 1999. Reference values and human biological monitoring values for environmental toxins. Report on the work and recommendations of the Commission on Human Biological Monitoring of the German Federal Environmental Agency. Int. Arch. Occup. Environ. Health 72(4):255-260.
EWG (Environmental Working Group). 2003a. Body Burden: The Pollution in People. Mount Sinai School of Medicine, New York, Environmental Working Group and Commonweal, Bolinas, CA [online]. Available: http://www.ewg.org/reports/bodyburden/toc.php [accessed April 17, 2004].
EWG (Environmental Working Group). 2003b. Mothers’Milk: Record Levels of Toxic Fire Retardants Found in American Mothers’ Breast Milk. Environmental Working Group [online]. Available: http://www.ewg.org/reports_content/mothersmilk/pdf/mothersmilk_final.pdf [accessed April 17, 2006].
EWG (Environmental Working Group). 2005. Body Burden: The Pollution in Newborns. A Benchmark Investigation of industrial Chemicals, Pollutants, and Pesticides in Human Umbilical Cord Blood. Environmental Working Group and Commonweal [online]. Available: http://www.ewg.org/reports/bodyburden2/ [accessed April 17, 2006].
Falk, L., L. Hanrahan, H.A. Anderson, M.S. Kanarek, L. Draheim, L. Needham, and D. Patterson, Jr. 1999. Body burdens levels of dioxins, furans, and PCBs among frequent consumers of Great Lakes sport fish. The Great Lakes Consortium. Environ. Res. 80(2 Pt 2):S19-S25.
Farm Family Exposure Study. 2005. Farm Family Exposure Study [online]. Available: http://www.farmfamilyexposure.org/ [accessed Sept. 26, 2005].
Fitzgerald, E.F., D.A. Deres, S.A. Hwang, B. Bush, B.Z. Yang, A. Tarbell, and A. Jacobs. 1999. Local fish consumption and serum PCB concentrations among Mohawk men at Akwesasne. Environ. Res. 80(2 Pt 2):S97-S103.
GAO (U.S. General Accounting Office). 2000. Toxic Chemicals: Long-Term Coordinated Strategy Needed to Measure Exposures in Humans. GAO/HEHS-00-80. U.S. General Accounting Office, Washington, DC. May 2000 [online]. Available: http://www.gao.gov/new.items/he00080.pdf [accessed June 3, 2005].
GerES (German Environmental Survey). 2005. Objectives. German Environmental Survey. Federal Environmental Agency, Dessau, Germany [online]. Available: http://www.umweltbundesamt.de/survey-e/objectives.htm [accessed June 3, 2005].
German Federal Environmental Agency. 2006. Environmental Specimen Bank [online]. Available: http://www.umweltbundesamt.de/uba-info-daten-e/daten-e/umweltprobenbank-desbundes.htm [accessed July 7, 2006].
Gunter, E.W. 1997. Biological and environmental specimen banking at the Centers for Disease Control and Prevention. Chemosphere 34(9/10):1945-1953.
Holzman, D. 1996. Banking on tissues. Environ. Health Perspect. 104(6):606-610.
Hughes, M.F. In press. Biomarkers of exposure: A case study with inorganic arsenic. Environ. Health Perspect [online]. Available: http://ehp.niehs.nih.gov/docs/2006/9058/abstract.html [accessed Aug. 21, 2006].
IARC (International Agency for Research on Cancer). 2005. European Prospective Investigation into Cancer and Nutrition. International Agency for Research on Cancer, World Health Organization, Lyon, France [online]. Available: http://www.iarc.fr/epic/Supdefault. html [accessed Sept. 26, 2005].
ILSI (International Life Science Institute). 2005. Fact Sheet: ILSI Health and Environmental Science Institute, Technical Committee on Integration of Biomonitoring Exposure Data into the Risk Assessment Process [online]. Available: http://www.hesiglobal.org/NR/rdonlyres/078BC618-01B1-41F9-814F-E36580F19449/0/IntegrationofBiomonitoringEx posureData.pdf [accessed April 19, 2006].
ISBER (International Society for Biological and Environmental Repositories). 2005. About ISBER. International Society for Biological and Environmental Repositories, Bethesda, MD [online]. Available: http://www.isber.org/aboutisber.html [accessed April 14, 2006].
Jakubowski, M. 2004. Biological monitoring of environmental exposure to lead–an example from Poland. Presentation at the Session on Biomonitoring, The European Environment and Health Action Plan 2004-2010: Implementation, December 2-3, 2004, Egmond aan Zee, The Netherlands.
Jakubowski, M., and M. Trzcinka-Ochocka. 2005. Biological monitoring of exposure: Trends and key developments. J. Occup. Health 47(1):22-48.
Karmaus. W., S. Huang, and L. Cameron. 2002. Parental concentration of dichlorodiphenyl dichloroethene and polychlorinated biphenyls in Michigan fish eaters and sex ratio in offspring. J. Occup. Environ. Med. 44(1):8-13.
Kimmel, C.A., G.W. Collman, N. Fields, and B. Eskenazi. 2005. Lessons learned for the National Children’s Study from the National Institute of Environmental Health Sciences/ U.S. Environmental Protection Agency Centers for Children’s Environmental Health and Disease Prevention Research. Environ. Health. Perspect. 113(10):1414-1418.
Knudsen, L.E. 2004. Workplace genetic screening. In Practical Ethics in Occupational Health P. Westerholm, J. Ovretveit, and T. Nilstun, eds. Oxfordshire, UK: Radcliff Medical Press (as cited in ECOTOC 2005).
Landrigan, P.J., and G. Tamburlini. 2005. Children’s health and the environment: A transatlantic dialogue. Environ. Health Perspect. 113(10):A646-A647.
Lee, L.W., J. Griffith, H. Zenick, and B.S. Hulka. 1995. Human tissue monitoring and specimen banking: Opportunities for exposure assessment, risk assessment, and epidemiologic research. Environ. Health Perspect. 103(Suppl. 3):3-8.
Litt, J., N. Tran, K.C. Malecki, R. Neff, B. Resnick, and T. Burke. 2004. Identifying priority health conditions, environmental data, and infrastructure needs: A synopsis of the Pew Environmental Health Tracking Project. Environ. Health Perspect. 112(14):1414-1418.
Lonky, E., J. Reihman, T. Darvill, J. Mather, Sr., and H. Daly. 1996. Neonatal behavioral assessment scale performance in humans influenced by maternal consumption of environmentally contaminated Lake Ontario fish. J. Great Lakes Res. 22(2):198-212.
LRI (Long-Range Research Initiative). 2001. Goals and Principles to Govern the Long-Range Research Initiative [online]. Available: http://www.uslri.com/about.cfm?id=about [accessed June 3, 2005].
LRI (Long-Range Research Initiative). 2005. Global Research Strategy [online]. Available: http://www.uslri.com/documents/cat_1/doc_276.pdf [accessed June 3, 2005].
NBAC (National Bioethics Advisory Committee). 1999. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance, Vol. 1. Report and Recommendations of the National Bioethics Advisory Commission. Rockville, MD: National Bioethics Advisory Commission.
NCHS (National Center for Health Statistics). 1985. Plan and Operation for the Hispanic Health and Nutrition Examination Survey 1982-84. Vital and Health Statistics Series 1, No. 19. DHHS (PHS) 85-1321. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Hyatts ville, MD. September 1985 [online]. Available: http://www.cdc.gov/nchs/data/series/sr_01/sr01_019.pdf [accessed June 3, 2005].
NCHS (National Center for Health Statistics). 2005. NHANES Stored Biologic Specimens. National Health and Nutrition Examination Survey. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Hyattsville, MD [online]. Available: http://www.cdc.gov/nchs/about/major/nhanes/serum1b.htm [accessed Sept. 26, 2005].
Needham, L.L. 2005. Biomonitoring in NHANES and Other Programs. Presented at the Second Meeting on Human Biomonitoring for Environmental Toxicants, April 28, 2005, Washington, DC.
Needham, L.L., D.B. Barr, and A.M. Calafat. 2005a. Characterizing children’s exposures: Beyond NHANES. Neurotoxicology 26(4):547-553.
Needham, L.L. , H. Özkaynak, R.M. Whyatt, D.B. Barr, R.Y. Wang, L. Naeher, G. Akland, T. Bahadori, A. Bradman, R. Fortmann, L.J. Liu, M. Morandi, M.K. O’Rourke, K. Thomas, J. Quackenboss, P.B. Ryan, and V. Zartarian. 2005b. Exposure assessment in the National Children’s Study: Introduction. Environ. Health Perspect. 113(8):1076-1082.
Neri, M., D. Ugolini, S. Bonassi, A. Fucic, N. Holland, L.E. Knudsen, R.J. Sram, M. Ceppi, V. Bocchini, and D.F. Merlo. 2006. Children’s exposure to environmental pollutants and biomarkers of genetic damage. II. Results of a comprehensive literature search and meta-analysis. Mutat. Res. 612(1):14-39.
NIEHS (National Institute of Environmental Health Sciences). 2005. NIEHS Superfund Basic Research Program. U.S. Department of Health and Human Services, National Institute of Health, National Institute of Environmental Health Sciences [online]. Available: http://www-apps.niehs.nih.gov/sbrp/index.cfm [accessed June 3, 2005].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Safety and Health Topic: Adult Blood Lead Epidemiology and Surveillance (ABLES). National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/topics/ABLES/ables.html. [accessed Dec. 22, 2005].
NRC (National Research Council). 1991. Monitoring Human Tissues for Toxic Substances. Washington, DC: National Academy Press.
OSHA (Occupational Safety and Health Administration). 2005. Blood Lead Laboratories Program Descriptions and Background. Salt Lake Technical Center (SLTC), U.S. Department of Labor, Occupational Safety and Health Administration [online]. Salt Lake Technical Center (SLTC), U.S. Department of Labor, Occupational Safety and Health Administration [online]. Available: http://www.osha.gov/SLTC/bloodlead/program.html [accessed Dec. 22, 2005].
Persky, V., M. Turyk, H.A. Anderson, L.P. Hanrahan, C. Falk, D.N. Steenport, R. Chatterton, Jr., and S. Freels. 2001. The effects of PCB exposure and fish consumption on endogenous hormones. Great Lakes Consortium. Environ. Health Perspect. 109(12):1275-1283.
Pirkle, J.L. 2005. Telebriefing Transcript Third National Report on Human Exposure to Environmental Chemicals. Media Relations. Centers for Disease Control and Prevention July 21, 2005 [online]. Available: http://www.cdc.gov/od/oc/media/transcripts/t050721.htm [accessed April 18, 2006].
Reis, M.F., C. Sampaio, M. Melim, and J.P. Miguel. 2004. Environmental Health Survey Programs (ProVEpAs) in Portugal. Presentation at the Session on Biomonitoring, The European Environment and Health Action Plan 2004-2010: Implementation, December 2-3, 2004, Egmond aan Zee, The Netherlands.
Robison, S.H., and D.B. Barr. In press. Use of biomonitoring data to evaluate methyl eugenol exposure and its relationship to the environmental public health continuum. Environ. Health Perspect [online]. http://ehp.niehs.nih.gov/docs/2006/9057/abstract.html [accessed Aug. 21, 2006].
Schantz, S.L., J.C. Gardiner, D.M. Gasior, A.M. Sweeney, H.E. Humphrey, and R.J. McCaffrey. 1999. Motor functioning in aging Great Lakes fisheaters. Environ. Res. 80(2 Pt 2):S46-S56.
Schantz, S.L., D.M. Gasior, E. Polverejan, R.J. McCaffrey, A.M. Sweeney, H.E. Humphrey, and J.C. Gardiner. 2001. Impairments if memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ. Health Perspect. 109(6):605-611.
Schober, S. 2005. National Health and Nutrition Examination Survey (NHANES): Environmental Biomonitoring Measures, Interpretation of Results. Presented at the Second Meeting on Human Biomonitoring for Environmental Toxicants, April 28, 2005, Washington, DC.
Sexton, K., L.L. Needham, and J.L. Pirkle. 2004. Human biomonitoring of environmental chemicals: Measuring chemicals in human tissue is the “gold standard” for assessing the people’s exposure to pollution. Am. Sci. 92(1):39-45.
Statistics Canada. 2006. Canadian Health Measures Survey [online]. Available: http://www. statcan.ca/english/survey/household/measures/measures.htm [accessed April 11, 2006].
Stewart, P., J. Reihman, E. Lonky, T.J. Darvill, and J. Pagano. 2000. Prenatal PCB exposure and neonatal behavioral assessment scale (NBAS) performance. Neurotoxicol. Teratol. 22(1):21-29.
Stewart, P.W., J. Reihman, E.I. Lonky, T.J. Darvill, and J. Pagano. 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol. 25(1):11-22.
Trasande, L., and P.J. Landrigan. 2004. The National Children’s Study: A critical national investment. Environ. Health. Perspect. 112(14):A789-A790.
Utah Department of Health. 2006. Environmental Public Health Tracking: Biomonitoring. Utah Department of Health, Office of Epidemiology [online]. Available: http://health. utah.gov/els/epidemiology/envepi/activities/EPHTP/ephtb.htm [accessed Jan. 18, 2006].
University of Copenhagen. 2005. ChildrenGenoNetwork, Background. Institute of Public Health, University of Copenhagen [online]. Available: http://cgn.pubhealth.ku.dk/child rengeno_en/background/ [accessed Sept. 26, 2005].
WHO (World Health Organization). 2000. Interlaboratory Quality Assessment on Levels of PCBs, PCDDs, and PCDFs in Human Milk and Blood Plasma: Fourth Round of WHO-coordinated study. EUR/00/5020352, E70039. WHO European Centre for Environment and Health, Bilthoven [online]. Available: http://www.who.dk/document/e70039.pdf [accessed Nov. 23, 2005].
WHO (World Health Organization). 2005. Biomonitoring of Human Milk for Persistent Organic Pollutants (POP). World Health Organization [online]. Available: http://www. who.int/foodsafety/chem/pops/en/ [accessed Dec. 20, 2005].
WWF (World Wildlife Fund). 2003. Contamination: The Results of WWF’s Biomonitoring Survey. WWF-UK. November 2003 [online]. Available: http://www.wwf.org.uk/file library/pdf/biomonitoringresults.pdf [accessed April 17, 2006].
WWF (World Wildlife Fund). 2004. Bad Blood? A Survey of Chemicals in the Blood of European Ministers. World Wildlife Fund [online]. Available: http://assets.panda.org/ downloads/badbloodoctober2004.pdf [accessed April 17, 2006].
Zenick, H., and J. Griffith. 1995. The role of specimen banking in risk assessment. Environ. Health Perspect. 103(Suppl. 3):9-12.