1
HIV/AIDS in Injecting Drug Users
At the end of 2005, some 38.6 million people were living with HIV (UNAIDS, 2006). An estimated 4.1 million people were newly infected with HIV in 2005 (UNAIDS, 2006). While sub-Saharan Africa remains hardest hit by the HIV/AIDS epidemic, major epidemics are also emerging in other parts of the world, mainly as a result of injecting drug use. This report focuses on high-risk1 countries—namely in Eastern Europe, the Commonwealth of Independent States, and significant parts of Asia—where injecting drug use is, or is on the verge of becoming, the primary driver of the HIV epidemic.
This chapter begins by exploring the epidemiology of HIV among injecting drug users (IDUs) in those regions, including prevalence of injecting drug use, prevalence of HIV among IDUs, and transmission routes. The chapter next focuses on the wide range of individual and structural (or environmental) factors that affect IDUs’ risk of HIV infection. Examples of individual-level factors discussed in this chapter include severity of dependence, type of drug used, and existence and severity of co-occurring psychiatric disorders. Examples of structural-level factors include proximity to overland trafficking routes, drug laws and enforcement practices, injecting environment and culture, and stigma and discrimination.
The chapter then describes specific interventions to prevent HIV among
IDUs. The committee has grouped these interventions into three broad categories: drug dependence treatment (including both pharmacotherapies and psychosocial interventions), sterile needle and syringe access, and outreach and education. Other important HIV prevention and treatment strategies that are not specific to IDUs, such as voluntary counseling and testing, antiretroviral treatment, and prevention and treatment of sexually transmitted diseases, are also briefly discussed, but are not part of the committee’s evidence review. The chapter ends with a brief discussion of the global coverage of HIV prevention services for IDUs.
EPIDEMIOLOGY OF HIV IN INJECTING DRUG USERS
Data on the size of the IDU population and HIV prevalence among IDUs are scarce. Estimating the size of the IDU population is difficult because drug use is an illegal and stigmatized activity. IDUs are often hidden and avoid settings where researchers might obtain data for fear of arrest or stigmatization (Magnani et al., 2005; Des Jarlais et al., 2001). HIV prevalence is difficult to estimate because many areas also lack the capacity to systematically monitor HIV infections among IDUs (Des Jarlais et al., 2001). Areas with routine HIV surveillance collect most of the data at institutions such as prisons, jails, and drug abuse treatment and outreach centers, which do not necessarily represent the IDU population at large (Dehne et al., 2002).
The United Nations Reference Group on HIV/AIDS Prevention and Care among IDUs recently developed estimates of the prevalence of injecting drug use, prevalence of HIV infection among IDUs, and the availability of prevention services worldwide, with a focus on developing and transitional countries (Aceijas et al., 2004). These estimates were compiled from a comprehensive review of published and unpublished documents for the period 1998–2003. Estimates were based on information available for 130 countries and territories.2 The authors assigned a strength of evidence rating to each source based on the type of information and methods used in calculating the estimate. The strength of evidence supporting estimates of both IDU prevalence and HIV prevalence among IDUs was generally poor. Approximately 95 percent of the estimates of IDU prevalence and 64 percent of HIV prevalence estimates received a rating of “low,” meaning that estimates lacked any supporting technical information (Aceijas et al., 2004). As a result of these limitations, estimates of IDU population size and HIV
prevalence among IDUs should be interpreted with caution (Aceijas et al., 2004).
Based on these data, the UN Reference Group estimated that there are 13.2 million injecting drug users worldwide (Aceijas et al., 2004). Of those, an estimated 8.8 million live in Eastern Europe and Central, South, and Southeast Asia (Aceijas et al., 2004; UNAIDS, 2006), and an estimated 10.3 million (or 78 percent) live in developing or transitional countries (see Figure 1.1). A major driver of the rapid expansion of HIV in these and other areas is injecting drug use, accounting for about one-third of new infections outside sub-Saharan Africa. Worldwide, an estimated 10 percent of all HIV infections are related to injecting drug use, although that proportion is estimated to be much higher in certain regions of the world (UNODC, 2005; WHO, 2005a; Aceijas et al., 2004).
Primarily because of injecting drug use, Eastern Europe, Central Asia, and the Commonwealth of Independent States have witnessed as much as a 20-fold increase in the number of people living with HIV in less than a decade (UNAIDS, 2006). The majority of these individuals live in Ukraine and the Russian Federation. In Russia, an estimated 940,000 people were living with HIV at the end of 2005, and unsafe injecting practices are the main cause of HIV infection among people under the age of 30 (UNAIDS, 2006). In Ukraine, unsafe injecting practices and unprotected sex are both responsible for alarming increases in HIV infection. In some cities in Ukraine, 58 percent of IDUs are HIV-seropositive (UNAIDS, 2006). Young people are especially affected by the increase in HIV transmission among IDUs in the Commonwealth of Independent States, as many IDUs are below the age of 25 and began injecting before the age of 20 (UNAIDS, 2005).
Many other countries in Eastern Europe, Central Asia, and the Commonwealth of Independent States are also experiencing growing HIV epidemics. Currently, injecting drug use fuels the HIV epidemic in Uzbekistan, Kazakhstan, and Armenia (UNAIDS, 2006). Tajikistan is witnessing a smaller, yet rapidly evolving epidemic, illustrated by a study in its capital Dushanbe, which found an HIV prevalence of 12 percent among IDUs (Stachowiak et al., 2006). Sexual transmission continues to drive the epidemics of countries such as Belarus, Azerbaijan, Georgia, and Romania. However, both the continued increase in the number of injecting drug users and the rising HIV prevalence rates among both injecting drug users and sex workers could signal that a more generalized epidemic is looming (UNAIDS, 2006).
In Asia, an estimated 8.3 million people were living with HIV at the end of 2005, with India home to more than two-thirds of these individuals (UNAIDS, 2006). While sexual transmission is still the predominant route of transmission in India, injecting drug use is driving the epidemic in the
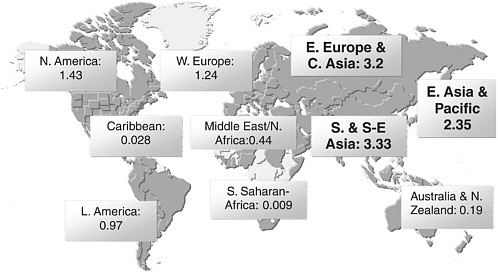
FIGURE 1.1 Estimates of IDU populations by region (in millions).
NOTE: Estimates are based on data collected from 130 countries from 1998–2003.
SOURCE: Reprinted, with permission, from the United Nations Office on Drugs and Crime, 2004 and the UN Reference Group on HIV/AIDS Prevention and Care among IDUs in Developing and Transitional Countries, 2004.
northeast states of Manipur, Mizoram, and Nagaland, and increasingly in the major cities of Chennai, Mumbai, and New Delhi (UNAIDS, 2006).
In Vietnam, injecting drug use and sex work are the main drivers of the HIV epidemic, with prevalence among IDUs rising from 9 percent in 1996 to 32 percent in 2003 (UNAIDS, 2006). Although Thailand has witnessed dramatic declines in the levels of HIV and sexually transmitted infections since the late 1990s, HIV among IDUs remains a major problem (UNAIDS, 2006).
As of September 2004, Malaysia had approximately 61,000 cases of HIV infection (Chawarski et al., 2006), with some 76 percent resulting from injecting drug use (WHO, 2003a). While the sharing of contaminated injecting equipment remains a large risk factor for HIV, sexual transmission, particularly in northern peninsular Malaysia, accounts for an estimated 12–56 percent of HIV infections among heroin users (Lye et al., 1994; Singh and Crofts, 1993). Other countries such as Indonesia and Bangladesh report low HIV prevalence among the general population, but the potential remains for explosive epidemics among high-risk groups such as IDUs and sex workers. Although data are very limited for Myanmar, an estimated one in three IDUs was HIV-seropositive in 2004 (UNAIDS, 2006).
In China, injecting drug users accounted for almost half of the people living with HIV in 2005 (UNAIDS, 2006). Sexual transmission has also grown substantially in the past few years, and evidence shows HIV infection spreading to the general population.
Injecting drug use is also driving the HIV epidemic in Iran, accounting for two-thirds of infections (Razzaghi et al., 2006). A recent study found that 15 percent of male IDUs attending drug treatment centers in Tehran were HIV-seropositive (UNAIDS, 2006).
Injecting drug use also accounts for a significant portion of HIV transmission in some countries outside these high-risk regions. In South America and the Caribbean, Brazil and Puerto Rico also report HIV prevalence among IDUs greater than 20 percent, as do some areas of Argentina and Uruguay (UNAIDS, 2006). Recent studies also suggest that injecting drug use is a growing problem that accounts for a small but increasing proportion of new HIV cases in Kenya, Nigeria, South Africa, Tanzania, Mauritius, and Egypt (Dewing et al., 2006; McCurdy et al., 2006; Beckerleg et al., 2005). In North America, approximately 20 percent of new infections in the United States in 2004 were attributed to injecting drug use (CDC, 2005). Canada’s small HIV epidemic is driven by unprotected sex between men although HIV infection among women is rising, mostly due to unprotected sex and unsafe injecting drug use (UNAIDS, 2006). In Western and Central Europe, heterosexual transmission remains the driver of the HIV epidemic, while the epidemic in Australia and New Zealand is mainly driven by unprotected sex between men (UNAIDS, 2006).
|
BOX 1-1 Amphetamine-Type Stimulants Methamphetamine and related amphetamine compounds (amphetamine-type stimulants, or ATS) are among the most commonly abused drugs, with an estimated 35 million users worldwide (Colfax and Shoptaw, 2005). These drugs target the central nervous system, increasing energy, alertness, disinhibition, and feelings of euphoria while decreasing appetite (Colfax and Shoptaw, 2005). Methamphetamine use can cause neurological toxicity, cardiovascular problems, dental decay, and skin infections (Colfax and Shoptaw, 2005). Chronic use is also associated with severe neurotoxicity and long-term cognitive impairment and mood disorders, although some cognitive functions can return after prolonged abstinence from the drug (Colfax and Shoptaw, 2005). Methamphetamine use is also associated with increased high-risk sexual behavior. Use of ATS has grown rapidly in recent years, perhaps because ingredients are readily available in over-the-counter medicines (such as cough medicine), and large quantities can be easily produced in small, mobile laboratories (UNODC, 2002). Increases in ATS use are being reported in many areas of the world, with the most rapid expansions occurring in countries of Southeast Asia, including Hong Kong SAR, Indonesia, Thailand, Myanmar, Malaysia, Singapore, the Philippines, China, Lao People’s Democratic Republic, Cambodia, and Vietnam. A major expansion of “yaba”—as amphetamines in tablet form are known in Thailand—prompted an intensive crackdown by the Thai government in its War on Drugs to decrease both supply and demand (Poshyachinda et al., 2005) (see Appendix C). ATS can be snorted, ingested, smoked, or injected, depending on the form in which the drug is available and cultural practices (UNODC, 2002). Most countries in Southeast Asia report that the preferred routes of administration are smoking, sniffing, and inhaling. However, the Philippines and Vietnam are reporting the injection of ATS (UNODC, 2002). |
In addition to opiates3 which are commonly injected, amphetamine-type stimulants and cocaine are also major injectable drugs of abuse in many high-risk countries (UNAIDS, 2006). Regions and countries vary widely in the types of drugs people use and their injecting behavior. In Latin America, for example, cocaine is the most commonly injected drug, although opium derivatives are increasingly available (Magis-Rodriguez et al., 2002). In Southeast Asia, methamphetamine production, trafficking, and use are rising dramatically, with an unknown percentage of users transitioning to injecting amphetamine use (see Box 1.1) (Kulsudjarit, 2004).
In South Asia, besides heroin, IDUs commonly inject synthetic painkillers, benzodiazepines, and other pharmaceuticals (Ghys, 2005).
ROUTES OF HIV TRANSMISSION
The sharing of contaminated injecting equipment is the primary mode of HIV transmission among IDUs, accounting for up to 80 percent of all HIV infections among IDUs in Eastern Europe and Central Asia (UNAIDS, 2006; UNODC, 2005). Sexual transmission from HIV-infected drug injectors to their sex partners is becoming an important secondary route of spread (Grassly et al., 2003), as is perinatal transmission from HIV-infected female IDUs or HIV-infected female sex partners of IDUs to their children.
Transmission Through Contaminated Injecting Equipment
HIV epidemics driven by injecting drug use tend to spread more rapidly than epidemics spread by sexual transmission, because exposure to the virus occurs more frequently, and because needles are more efficient at transmitting it than sex. In many parts of the world, HIV prevalence reached 40 percent and above among IDUs just 1 to 2 years after HIV entered the IDU population (Rhodes et al., 1999a). For example, in Edinburgh, an HIV outbreak started in 1983 among IDUs who had been injecting for only a year or two and spread rapidly through the IDU population, skyrocketing from 5 to 57 percent within 2 years (Robertson et al., 1986).
Examples of this trend also exist throughout Southeast Asia and Eastern Europe (Crofts et al., 1998; Rhodes et al., 1999b). A recent review concluded that HIV had spread rapidly in Belarus, Kazakhstan, Moldova, Russia, and Ukraine by the late 1990s, with 50–90 percent of new HIV infections occurring among IDUs (Rhodes et al., 1999b). While not the focus of this report, contaminated injecting equipment is also a mode of transmission for viral hepatitis (see Box 1.2).
Sexual Transmission
Sexual transmission from HIV-infected IDUs to their sex partners is becoming an important route of HIV transmission. Drug use is highly correlated with unsafe sexual practices, including unprotected sex, multiple partners, or exchanging sex for money or drugs (UNODC, 2005).
Many studies have found links between injecting drug use, commercial sex, and risky sexual behavior, resulting in high rates of HIV prevalence among sex workers who are also IDUs. Studies have shown that drug injecting sex workers are more willing to engage in unprotected sex, and
|
BOX 1-2 Viral Hepatitis There are five identified types of viral hepatitis (A-E) and each one is caused by a different virus (CDC, 2003). Hepatitis C and B are the two most common types found among injecting drug users. Hepatitis C is an inflammation of the liver caused by the hepatitis C virus (HCV). Although the infection can be asymptomatic or mild, it can become chronic in over half of those infected. Among these, about half will eventually develop cirrhosis (scarring) or liver cancer (CDC, 2003). It is spread primarily by contact with the blood of an infected person, e.g., through receipt of contaminated blood or blood products, sharing needles or other injecting equipment, and through accidental needle-sticks or sharps exposures (CDC, 2003). Far less frequently it is spread sexually or from an infected mother to her baby. Recent studies show that HCV may survive on environmental surfaces at room temperature at least 16 hours, but not longer than 4 days (CDC, 2003). While there is regional variation, studies show that HCV prevalence among IDUs is often as high as 60 percent (Hagan, 1998; Garfein et al., 1998; Shapatava et al., 2006; Zhao et al., 2006). In addition to the sharing of needles and syringes, the sharing of other injecting and drug preparation equipment such as cookers used to melt drugs, cotton used to filter out particles when drawing the drug into the syringe, and water used to rinse syringes, has been associated with HCV infection (Diaz et al., 2001; Hagan et al., 1999, 2001; Hahn et al., 2002; Thorpe et al., 2002). There is no vaccine available for the prevention of HCV infection. Hepatitis B is a disease caused by another bloodborne pathogen, the hepatitis B virus (HBV). HBV also produces liver inflammation and can cause lifelong infection, cirrhosis of the liver, liver cancer, liver failure, and death (CDC, 2003). Besides the potential for transmission through receipt of contaminated blood and blood products and through contact with other infectious bodily fluids (such as saliva or semen) and tissues, HBV is also spread through having unprotected sex with an infected person, by sharing drugs, needles, or other injecting equipment, through accidental needle-sticks or sharps exposures, or from an infected mother to her baby (CDC, 2003). It is stable on environmental surfaces for at least 7 days and has been transmitted between children living closely together in household settings. There is a vaccine to prevent HBV infection. |
more likely to have a non-paying sex partner who is an IDU (Pisani et al., 2003; Paone et al., 1999).
National survey data also reveal a link between injecting drug use and high-risk sexual activity among commercial sex workers. In Ho Chi Minh City, Vietnam, 49 percent of injecting sex workers are infected with HIV, compared with 19 percent of those who use drugs but do not inject, and 8 percent of those who do not use drugs at all (MAP, 2005a). In Manipur, India, HIV prevalence was found to be 57 percent among sex workers who were also IDUs, compared with 20 percent among sex workers who did not inject drugs (Panda et al., 2001).
There are reports of high percentages of sex workers who also inject drugs. Estimates of the proportion of female sex workers who inject drugs include:
-
From 25 to 80 percent in the Russian Federation (Lowndes et al., 2003).
-
Some 30 percent across the Commonwealth of Independent States of the former Soviet Union (UNODC, 2005).
-
Between 20 and 50 percent in Eastern Europe (UNODC, 2005).
-
Between 10 and 25 percent in Central Asia (UNODC, 2005).
In addition, many IDUs sell sex as a means to obtain drugs or money to buy drugs (Lowndes et al., 2003). Data from several cities in countries such as Kazakhstan, Uzbekistan, and Kyrgyzstan show that while only a relatively small percentage of female sex workers in these cities inject drugs (6–14 percent), most female IDUs sell sex (56–67 percent) (MAP, 2005b). Studies in different Russian cities show that 15–50 percent of female IDUs are involved in sex work (Lowndes et al., 2003; Dehne and Kobyshcha, 2000).
Finally, some non-injecting women are infected with HIV by their injecting sexual partner or husband. In a study in Sao Paulo, 40 percent of non-injecting HIV-infected females had acquired the virus through unsafe sexual activity with IDUs (UNODC, 2005). In another study in India, 45 percent of non-injecting wives of HIV-seropositive IDUs were themselves infected with HIV, with 97 percent reporting sexual activity only with their husbands (Panda et al., 2000).
Perinatal Transmission
Perinatal transmission from infected female IDUs and infected partners of IDUs to their children is another growing concern. Transmission from a mother to a child can occur during pregnancy, labor and delivery, or breastfeeding (WHO, 2004a). The magnitude of IDU-associated perinatal transmission has not been systematically examined, but some studies suggest that it is a major problem. For example, according to one report, most HIV-infected infants born between 1996 and 2001 in the Russian Federation apparently had mothers who were either IDUs or sexual partners of IDUs (UNODC, 2005).
FACTORS INFLUENCING HIV RISK AMONG IDUS
A range of individual and structural factors can affect an IDU’s risk of contracting HIV. Examples of factors specific to the individual include severity of drug dependence, preferred drug, and existence and severity of
co-occurring psychiatric disorders, among others. Structural factors contribute to the “risk environment” for injecting drug users, affecting their HIV-related risks and health outcomes by creating an environment in which HIV is more likely to be transmitted (Rhodes et al., 2005). Examples discussed in the following section include proximity to drug trafficking routes, drug laws and law enforcement practices, socioeconomic and political stability, injecting environment and culture, and stigma and discrimination.4 Individual and structural factors converge to affect the likelihood that an IDU will engage in high-risk behavior, such as sharing of injecting equipment, more frequent injecting, commercial sex work, unprotected sex, and multiple sex partners.
Individual-Level Risk Factors
Severity of Dependence
Severity of dependence can influence the likelihood that someone will inject, the frequency of injection, and the sharing of contaminated equipment. Two studies by Gossop and colleagues using the same sample (n=408) examined the association between severity of heroin dependence with sharing injecting behavior and sexual behavior. One study (Gossop et al., 1993a) found that severity of heroin dependence was positively related to the occurrence and frequency of sex-for-money and sex-for-drugs transactions. The other study (Gossop et al., 1993b) found that more severely dependent heroin users were more likely to have shared injecting equipment. More dependent users also appeared to use heroin in private settings and to be at greater risk of sharing with dealers, possibly because of their urgent need during drug withdrawal. Other studies also found that severity of drug use, as measured by frequency of injection and injection of drug combinations, is significantly associated with sharing of equipment (Klee et al., 1990; Watters et al., 1994).
Type of Drug Used
The type of drug an IDU uses influences the frequency of injection and the risk of HIV transmission. An early epidemiological study in San Francisco found that injection cocaine use significantly increased the risk of HIV infection (Chaisson et al., 1989). Because cocaine has a relatively short half-
life and is highly addictive, people may inject it more frequently, with reports of 10 times or more a day, compared with 1 to 3 injections per day among heroin-dependent IDUs (Chaisson et al., 1989). Even after controlling for frequency of use, Chaisson and colleagues found that cocaine injectors had a higher prevalence of HIV. They found that cocaine injection was associated with other behaviors that increased the risk of HIV: cocaine injectors were more likely to report sharing injecting equipment, using drugs in shooting galleries, and “booting” drugs (withdrawing blood into the syringe before injecting). Other studies in Montreal, Vancouver, and Toronto also found that cocaine use was positively associated with HIV infection (Bruneau et al., 2001; Strathdee et al., 2001, Lamothe et al., 1993).
Methamphetamine increases sexual drive and decreases inhibitions, leading to high-risk sexual behaviors (Colfax and Shoptaw, 2005). Methamphetamine use also increases the likelihood of engaging in high-risk sexual behavior such as unprotected sex and increased number of partners, and the acquisition of HIV and other sexually transmitted infections (Colfax and Shoptaw, 2005; Molitor et al., 1998; Molitor et al., 1999).
Presence and Severity of Co-Occurring Psychiatric Disorders
Co-occurring psychiatric disorders are common among drug-dependent individuals. Some psychiatric disorders precede the onset of drug dependence, while others are precipitated by chronic drug use (O’Brien et al., 2004). Opioid addicts have high rates of depression and antisocial personality disorder (Kosten and Rounsaville, 1986; Brooner et al., 1997). Similarly, cocaine abusers have high rates of affective and anxiety disorders, attention deficit disorder in childhood, and personality disorders (Schottenfeld et al., 1993). Methamphetamine users have high levels of depression, anxiety, and personality disorders (Chen et al., 2003; Zweben et al., 2004). Long-term methamphetamine use can also lead to psychosis (Chen et al., 2003), and amphetamine withdrawal commonly results in symptoms of severe depression (Urbina and Jones, 2004).
Studies have demonstrated a consistent positive association between psychiatric problems, particularly depressive disorders, and sharing of injecting equipment (Hawkins et al., 1998; Mandell at al., 1999). Depression may influence IDUs’ risk of HIV infection by altering their perception of the threat of HIV infection, reducing their ability to judge the consequences of their decisions, decreasing their ability to cope with stressful events, and increasing the likelihood of careless behavior (Stein et al., 2003). Research has found that greater severity of depression is associated with increased sharing of injecting equipment, and the risk of acquiring or transmitting HIV (Stein et al., 2003).
Other psychopathologies also increase the risk for HIV infection. For example, in a study of cocaine users entering treatment (n=174), those with antisocial personality disorder (ASPD)5 (35 percent of the sample) reported higher levels of sexual risk behaviors, and had more severe problems on the legal, alcohol, and psychiatric components of the Addiction Severity Index, than non-ASPD patients (Ladd and Petry, 2003).
Structural-Level Risk Factors
Proximity to Drug Trafficking Routes
Drug trafficking routes are also tightly linked with injecting drug use and HIV epidemics (Beyrer et al., 2000; Beyrer, 2002; Quan et al., 2000; Yu et al., 1999). The most apparent links lie along trafficking routes originating from the Golden Triangle of Southeast Asia (Myanmar, Laos, and Thailand) and from the Golden Crescent of Central Asia (Afghanistan, Pakistan, and Iran) (Chelala and Beyrer, 1999; Crofts et al., 1998; Poshyachinda, 1993; Qian et al., 2006). This factor is closely tied to shifts in drug production, which can lead to changes in drug transit routes. For example, increasing opium production in Afghanistan since 1989 has had an enormous impact on heroin availability throughout Central Asia (Todd et al., 2005).
Trafficking in methamphetamine accounts for the bulk of trafficking in amphetamine-type stimulants, and has been clearly shifting toward East and Southeast Asia. Myanmar is a major supplier of methamphetamine and serves as a source for Thailand and China. Seizures of methamphetamine are on the rise in China, mostly along its border with Myanmar (UNODC, 2004). Some reports indicate that ephedrine—used to illicitly manufacture methamphetamine in Southeast Asia—is diverted and smuggled out of China and India, whereas caffeine, used in making methamphetamine tablets, is mainly smuggled into Myanmar through its border with Thailand (Kulsudjarit, 2004). Growing demand for ecstasy (3,4-methylenedioxy methamphetamine), and the availability of precursor chemicals from China and Vietnam, provide evidence that nations in Southeast Asia may become havens for large-scale ecstasy manufacture. Significant laboratories have been discovered in China, Hong Kong, Taiwan, Malaysia, and, most notably, Indonesia (Kulsudjarit, 2004).
Drug Laws and Law Enforcement
Drug laws and law enforcement practices have a complex influence on the HIV risk environment for IDUs. Burris and colleagues (2004) define law as having four components: (1) the law on the books—that is, statutes, constitutions, and regulations; (2) the management tools of criminal justice and law enforcement agencies, including training, policies, and standard operating procedures; (3) the “practices, knowledge, attitudes, and beliefs” of frontline personnel who enforce the laws (such as police officers); and (4) people’s understanding of the laws (in this case the knowledge, attitudes, and beliefs of injecting drug users regarding laws).
Drug paraphernalia and drug possession laws have the most widespread impact on IDUs. Drug possession laws forbid the possession of a certain amount of illicit drugs. They can often be interpreted to include any measurable amount of drugs, including drug residue left in a used syringe (Burris et al., 2003). Drug paraphernalia is defined to include any equipment, product, or material that is intended for use in introducing controlled substances into the body. Drug paraphernalia laws ban the manufacture, sale, distribution, or possession of a wide range of such devices (Gostin, 1991). These laws do not prohibit the sale of equipment if it will not be used for injecting illicit drugs. For example, a diabetic could possess a needle/syringe if he/she could prove there is a valid medical purpose for possessing the equipment (Gostin, 1991).
Needle and syringe exchanges (NSEs) are illegal under general drug paraphernalia laws (Gostin et al., 1997) unless specific legislation exists to allow them or special permission is granted from local law enforcement authorities (Burris et al., 2002). Often the legal status of NSEs remains uncertain and consequently staff and participants may be arrested and legal action may be brought to close the NSE (Gostin et al., 1997).
Other laws that affect access to sterile needles and syringes include syringe prescription laws and pharmacy regulations. Syringe prescription laws prohibit persons from dispensing or possessing syringes without a valid medical prescription (Gostin et al., 1997). In addition, doctors must have a legitimate medical reason to prescribe syringes. Some prescription laws require pharmacists to maintain sales records (Gostin et al., 1997). Pharmacy regulations are not technically legally binding, but failure to comply may leave a pharmacist open to professional sanction (Gostin et al., 1997). Examples of pharmacy regulations could include requirements to maintain sales records or limits on the number of syringes sold at one time (Burris et al., 2003). In many cases, the decision to sell syringes is left to the discretion of the pharmacist and that decision may be influenced by uncertainty or lack of knowledge regarding the law (Burris et al., 2002). Furthermore, despite the legality of a pharmacist selling a syringe, the purchaser
may be violating syringe prescription or paraphernalia laws (Burris et al., 2002).
A number of studies have shown that drug laws and law enforcement practices can adversely affect HIV risks for IDUs, by limiting access to prevention services and deterring IDUs from participating (Rhodes et al., 2005; Burris et al., 2004; Hammett et al., 2005). If drug users do not have a sterile syringe when purchasing or injecting drugs, they are more likely to share injecting equipment (Gostin et al., 1997). A study in Vancouver found that the number of sterile syringes reaching IDUs declined more than 26 percent during a police operation that placed a constant and highly visible police presence near an NSE funded by the local health authority (Wood et al., 2003).
Laws and enforcement practices also shape the perceptions of IDUs and may increase their risk behaviors. For instance, fear of arrest for possessing needles and syringes reduces IDUs’ participation in sterile needle and syringe access programs (Kral and Bluthenthal, 2004). Studies show that IDUs often fail to carry sterile syringes for fear of detection of such equipment by law enforcement (Gostin et al., 1997). Often drug users fear that if they are found carrying paraphernalia, the police have cause to search for illicit drugs which, if found, could lead to arrest and incarceration. In Russia, reluctance to carry needles and syringes because of fear of arrest for possessing drug paraphernalia was associated with a higher risk of needle and syringe sharing (Rhodes et al., 2003, 2004).
Because of syringe prescription laws or pharmacy regulations, persons purchasing syringes at pharmacies often have to present identification and sign a register (Burris et al., 2003). Drug users may avoid pharmacies because of the intrusive questioning and pharmacists may be reluctant to sell syringes because of fear of criminal penalties and professional sanctions. In China, even though IDUs have legal pharmacy access to syringes, they often fear that police monitor pharmacies (Singer et al., 2003).
In some countries, drug users are required to register with officials in order to receive drug treatment and other health and social services. These registries are sometimes shared with the police (ODCCP and UNAIDS, 2001), raising fears among and IDUs that registration will increase the chances of police detainment (Platt et al., 2004). For example, in Russia, registration could affect an IDU’s ability to gain employment and secure housing (Platt et al., 2004) and could result in consequences related to employment status, citizenship, and residency rights (ODCCP and UNAIDS, 2001; Rhodes et al., 2003).
Many countries maintain a delicate balance between criminal justice approaches to combat drug use and public health programs to prevent HIV among IDUs (Hammett et al., 2005). An inherent tension exists between policies designed to enforce laws against illicit drug use and illegal activities
to support continued drug use, and public health approaches to mitigate health harms from drug use. For example, some may view HIV prevention programs that include sterile needle and syringe access as encouraging drug use or undermining police efforts to control access to drugs (NRC and IOM, 1995).
This tension suggests the need for more collaboration between public health and law enforcement officials to address the twin problems of drug use and HIV transmission (Hammett et al., 2005; Kozlov, 2006). As discussed in Chapter 4, public health and drug control officials need to work together to harmonize their policies while balancing their respective missions, and to increase communication and collaboration between police and health officials on the ground. This tension also points to the need to identify common ground and overlapping goals between these two approaches. Reconciling these roles can be difficult, but it is critical if HIV prevention efforts for IDUs are to succeed (Hammett et al., 2005).
Appendix C provides several case studies that illustrate how these two approaches to IDUs—criminal justice and public health—have played out at the national level in several high-risk countries.
Economic and Political Instability
Rapidly changing socioeconomic conditions, governmental transitions, and changes in transnational mobility stemming from border openings and new trade agreements can make populations more vulnerable to IDU outbreaks. For example, economic and social changes after Ukraine’s independence left many people unemployed or earning only US$30–50 a month (Barcal et al., 2005). Selling hanka, a homemade opium solution, for US$0.95 per 1-milliliter dose during the poppy season, and US$1.50 out of season, quickly became a prosperous way to make a living, encouraging the spread of injecting opioid use (Barcal et al., 2005). Wars and other armed conflict can also spur drug production and alter or enhance drug distribution routes (Hankins et al., 2002).
Injecting Environment and Culture
The risk of HIV transmission is correlated with the context in which people inject drugs. IDUs who frequent shooting galleries, for example, are more likely to engage in risky behavior (such as needle sharing), and have been shown to have a higher risk of acquiring HIV (Magis-Rodriguez et al., 2005; Fuller et al., 2003; Hien et al., 2001). Shooting galleries are locations where IDUs can rent injecting equipment and use drugs. The equipment is usually not disinfected or cleaned before it is rented to the next user (McCoy and Inciardi, 1995). Professional injectors are IDUs who give injections to
other IDUs, often using the same needle on multiple clients, increasing the risk of HIV transmission (Kral et al., 1999).
The prevalence and burden of HIV/AIDS and other infectious diseases are higher in correctional facilities than in the general community (Hammett, 2006; Brown, 2005; Maruschak, 2002). A study in Tehran, Iran, recruited 213 IDUs to determine the prevalence and correlates of HIV infection (Zamani et al., 2006). In multivariate analyses, the study found that a history of sharing injecting equipment in prison (odds ratio [OR]=2.45; 95% confidence interval [CI] 1.01, 5.97) and multiple incarcerations (OR=3.13; 95% CI 1.08, 9.09) were associated with significantly higher prevalence of HIV infection.
Drug users are often over-represented in prisons, and, once incarcerated, many may continue using drugs (UNODC, 2005). IDUs in prisons share injecting equipment significantly more often than IDUs in the general community (Small et al., 2005). In prisons where possession of needles and syringes is prohibited, drug users often circulate their limited supply of injecting equipment, or even craft their own equipment out of common objects and pieces of old syringes (Small et al., 2005). Although coverage of HIV prevention interventions in prisons is limited, some prisons distribute condoms, bleach, and needles and syringes, or offer drug dependence treatment, risk reduction education, and voluntary counseling and testing (WHO, 2004b).
In addition to high-risk environments, a “culture of sharing” among IDUs can increase a user’s likelihood of infection. Sharing is sometimes viewed as showing solidarity among IDUs, and is more common within tight-knit social networks, and between “running partners” and sexual partners (Des Jarlais et al., 1992). In addition to the social solidarity that sharing implies, drugs and injecting equipment are sometimes shared when users pool money to purchase larger quantities of drugs at cheaper prices. Other common reasons for sharing drugs and injecting equipment are the prevention of withdrawal, mediation of conflicts, or to reconcile financial debts (Grund et al., 1996). IDUs who are in the same social network often share food, money, information, and clothing, as well as provide each other with social support (Grund et al., 1996). Evidence shows that when knowledge of HIV/AIDS risks improves, and supplies of readily available sterile injecting equipment increase, syringe sharing falls, in accordance with a new ethics of “informed altruism” and “partner restriction.” In such cases, sharing, if it occurs at all, will be more and more limited to sexual partners and other intimate acquaintances (Des Jarlais et al., 2004).
Stigma and Discrimination
Stigma occurs when an undesirable attribute reduces an individual’s status in the eyes of society (Goffman, 1963). Stigma can result in discrimi-
nation, rejection, prejudice, and discounting of an individual or group. Stigma toward drug users can exist in many forms. A study by Dean and Rud (1984) found that the term “drug addict” evoked images of disorientated, thin, and unhealthy people with behavioral problems. Attitudes of health care professionals can discourage IDUs from seeking treatment (Ritson, 1999), as can attitudes of pharmacists toward IDUs seeking to purchase clean injecting equipment (Taussig et al., 2002). In Russia, IDUs who use drug treatment facilities are registered and then monitored for 5 years, and this registration can have everyday consequences such as restrictions on employment and social discrimination. Drug users cite stigma as a reason to avoid registration and drug treatment (Bobrova et al., 2006).
Drug-related stigma is often layered on top of other stigmas associated with specific groups, such as people living with HIV and commercial sex workers. Consequences of stigma can be viewed along a continuum from reactions including silence and denial to ostracism and violence. Research has shown that stigma can have a variety of negative effects on people’s willingness to be tested for HIV, disclose their HIV status, and seek health care, as well as the quality of the health care and social support they solicit and receive (King, 1989; Malcolm et al., 1998; Raveis et al., 1998; Sowell et al., 1997).
HIV PREVENTION STRATEGIES
This section provides a brief overview of the HIV prevention strategies for IDUs. Because drug dependence is a chronic disease, most drug users experience repeated cycles of remission and resumption over extended periods of time (Hser et al., 1993, 2001). The chronic nature of drug dependence poses an enormous challenge to developing intervention policies and programs that effectively reduce HIV transmission—not just in the short term but also in the long term—among and from IDUs.
The most effective way for IDUs to reduce their risks of HIV infection is to stop using drugs. However, not all drug users are ready or able to stop using drugs. If they are not, a variety of strategies is available for preventing HIV infection, each designed for drug users at different points (see Box 1.3).
As noted, an individual IDU’s risk of contracting and transmitting HIV is mediated by both individual-level and structural-level risk factors. The vast majority of HIV prevention efforts target the risk behavior of individual drug users, attempting to get them to: stop using drugs (through drug treatment); stop injecting (through drug treatment or education); use a clean needle (through sterile needle and syringe access programs); stop sharing equipment (through sterile needle and syringe access and education); disinfect each time (through disinfection programs and education); and to know their HIV status (through voluntary counseling and testing).
|
BOX 1-3 Hierarchy of Steps IDUs Can Take to Reduce HIV Risk
SOURCE: Adapted (with modifications by the Committee) from NIDA (2002) and Des Jarlais (2005). |
Outreach and education complements each step of the hierarchy by serving to engage and foster the participation of IDUs. Structural-level interventions, which attempt to create an environment supportive of individual behavioral change, have not received sufficient attention from researchers and policymakers (Rhodes et al., 2005; Burris et al., 2004). Examples of structural-level interventions include law reform or programs to reduce stigma and discrimination against HIV-infected people or drug users While not the focus of the Committee’s report, structural interventions are an important component of an effective HIV prevention response.
Although definitions vary, many health policy and research organizations recommend a comprehensive HIV prevention strategy for IDUs. According to the World Health Organization (WHO), a comprehensive HIV prevention program for IDUs includes outreach, information, education and communication, risk reduction counseling, HIV testing and counseling, disinfection programs, sterile needle and syringe access programs, disposal of used injecting equipment, drug treatment services, agonist pharmacotherapy programs, HIV/AIDS treatment and care, primary health care, and peer education (WHO, 2005a). According to the U.S. National Institute on Drug Abuse (NIDA, 2002), comprehensive programs encompass three approaches: community-based outreach, drug abuse treatment, and sterile needle and syringe access. These three approaches include a voluntary HIV counseling and testing component and may include many components cited by WHO. Although HIV prevention programs for IDUs rarely include just a single intervention, truly comprehensive programs are rare. Descriptions
of various specific components of a comprehensive approach are outlined below.
Treatment for Drug Dependence
Treatment for drug dependence can occur in a variety of settings including inpatient, outpatient, and residential venues, and often blends different approaches, including pharmacotherapies and psychosocial or behavioral interventions (WHO, 2005b). The efficacy and effectiveness of these strategies are discussed in Chapter 2.
Pharmacotherapies
No pharmacotherapies have been found to be consistently efficacious in treating stimulant dependence. Pharmacological agents are available, however, to treat opioid dependence. There are two primary types of opioid pharmacotherapies: agonist and antagonist medications.
Opioid agonist medications: These medications work by preventing withdrawal symptoms and reducing opiate cravings—and therefore the need to use illicit drugs—and also by diminishing the effects of opioid use by creating cross-tolerance to their effects (IOM, 1995). Agonist medications have two primary clinical applications; they can be used on a limited basis to facilitate opioid detoxification,6 or they can be administered over a longer period as a maintenance treatment (IOM, 1995). When used to assist with detoxification, the agonist agent helps to relieve patient discomfort during withdrawal and the dosage is slowly tapered over time until the person reaches a drug-free state (IOM, 1990, 1995). Studies have demonstrated high rates of relapse when detoxification is not followed by further therapeutic intervention (IOM, 1990). Furthermore, because it lowers tolerance, detoxification can raise the risk of fatal overdose among individuals who resume opioid use (Strang et al., 2003).
In maintenance therapy, the agonist agent is administered at high doses for a sustained period. The goal of maintenance treatment is to reduce illicit drug use and high-risk behavior by building cross-tolerance to the effects of other opioids, thereby allowing patients to stabilize physiologically and psychologically, so they can reengage in normal life activities (IOM, 1990;
WHO et al., 2004). Due to their long half life and resulting steady state, opioid agonists are not intoxicating and do not impair function when used at clinically appropriate and stable doses over time (IOM, 1990, 1995).
Methadone is the most widely used and researched agonist maintenance medication for the treatment of opioid dependence (WHO et al., 2004). Methadone is a synthetic, full opioid agonist; initially developed as an analgesic, it became a treatment for heroin dependence in the 1960s (IOM, 1995). Methadone is typically administered orally in liquid (syrup) form, but is also available in tablet form in some countries. Methadone can be administered once a day and prevents opioid withdrawal and provides cross-tolerance to the effects of other opioids for 24 hours in most patients (IOM, 1995). Methadone has a low incidence of side-effects and most patients entering treatment respond well (WHO et al., 2004). Because overdose is possible and methadone may be abused, it is generally administered to patients in controlled medical settings (e.g., specialized methadone clinics) (IOM, 1995).
Buprenorphine is a partial opioid agonist that is used increasingly as an alternative to methadone. Because buprenorphine tablets are not well absorbed if swallowed, it is administered sublingually (under the tongue) (WHO et al., 2004). Buprenorphine tablets take 3 to 15 minutes to dissolve in the mouth (Compton et al., 2006). Buprenorphine has long-lasting effects on opiate receptors, and can be administered on alternate days or three times a week (Fudala et al., 1990; Johnson et al., 1995). Buprenorphine has relatively few side effects (WHO et al., 2004). Buprenorphine has been widely used in France since 19967 and has been used in over 20 other countries (WHO, 2003b). It was recently approved by the U.S. Food and Drug Administration for distribution in office-based settings outside of dedicated drug abuse treatment programs (WHO, 2005b). Both methadone and buprenorphine are classified as psychotherapeutic medicines for substance dependence treatment programs on the WHO list of essential medicines (WHO, 2005c).
Opioid antagonist medications: An alternative to opioid agonists are antagonist agents which block the effects of opiates. Naltrexone, the most commonly used opioid antagonist drug, is used to help patients maintain long-term abstinence from opiates (WHO, 2005b). Oral naltrexone provides relatively long-lasting (up to 1–3 days depending on dose) blockades
|
7 |
In response to concerns about diversion and misuse of buprenorphine, France is considering a controversial proposal to reclassify buprenorphine as a narcotic. The effect that this measure, if implemented, would have on treatment access and availability is unclear. [Online]. Available: http://opiateaddictionrx.info/whatsnew.asp?id=1186 [accessed July 31, 2006]. |
of euphoric or rewarding effects of heroin or other opioids, and thus may help prevent resumption of opiate use (O’Brien and Kampman, 2004). New long-acting, injectable formulations of naltrexone produce adequate opioid blockade for up to one month (Comer and Collins, 2002). Before beginning naltrexone treatment, patients must be detoxified (medically withdrawn from heroin or other opioids), because naltrexone will precipitate severe withdrawal symptoms in people physically dependent on opioids (O’Brien and Kampman, 2004). Naltrexone binds tightly to opiate receptors, but does not activate them or have any rewarding, mood-altering, or euphoric effects, or lead to withdrawal symptoms when it is discontinued (O’Brien and Kampman, 2004). Because naltrexone’s blockade of opiate effects can be overridden by sufficiently large doses of opioids, naltrexone decreases but does not eliminate the risk of opioid overdose (O’Brien and Kampman, 2004). Patients who discontinue naltrexone are at greater risk for overdose if they resume opioid use (O’Brien and Kampman, 2004; Digiusto et al. 2004).
Psychosocial Interventions
A second major approach to drug treatment involves psychosocial interventions, which include a broad range of psychological and behavioral strategies, used either alone or in combination with pharmacotherapies and other medical or social interventions (Mayet et al., 2004). These interventions may be provided with varying levels of intensity, frequency, and duration using different approaches including outpatient, partial hospital, hospital, or residential-based programs. Psychosocial interventions may be delivered in individual or group settings, and may also include family members in order to address family functioning (e.g., through behavioral family therapy). Examples of psychosocial interventions include specific behavioral interventions (e.g., cognitive behavioral therapy, contingency management) as well as collection of program models (e.g., therapeutic communities, 12-step programs) (see Chapter 2, Box 2.2 for a description of these interventions).
Sterile Needle and Syringe Access
Several HIV prevention approaches provide IDUs with access to sterile needle and syringes, including needle and syringe exchange (NSE), disinfection programs, safe injecting facilities, syringe sales through pharmacies and vending machines, and syringe prescriptions from physicians. The efficacy and effectiveness of these strategies are discussed in Chapter 3.
Sterile needle and syringe access aims to increase the availability of sterile injecting equipment, remove contaminated needles from circulation
among IDUs, and prevent drug users from discarding used needles in the community, where others might use them or suffer needle sticks. Sterile needle and syringe access may also offer activities or referrals designed to encourage IDUs to stop using drugs.
Needle and syringe exchange is rarely, if ever, conducted as an isolated intervention—nearly all programs combine NSE with one or more of the following prevention strategies: outreach, risk reduction education, condom distribution, bleach distribution and education on needle disinfection, and referrals to substance-abuse treatment and other health and social services. As a result, the Committee refers to these combined programs as “multi-component HIV prevention programs that include needle and syringe exchange.”
Prevention programs that include NSE may operate in fixed or mobile sites (e.g., mobile van). Rules on the number of needles and syringes that IDUs can exchange at one time vary—although recent trends are to ease such limits and shift to need-based distribution. Recent evidence from several countries suggests that secondary needle and syringe exchange is growing: that is, a participant redistributes supplies from an NSE to other IDUs in his or her social network who are unwilling or unable to access the NSE directly. Such practices have the potential to increase the number of IDUs with access to supplies of sterile injection equipment as well as expand the reach of peer education, risk reduction information, and referrals to other services (Stopka, 2006; Irwin et al., 2006).
NSEs may distribute other injecting equipment, such as cotton, sterile cookers, sterile water bottles, and alcohol wipes, as well as bleach, condoms, and health pamphlets (Lurie et al., 1993). Such programs often include a variety of other services, such as HIV counseling and testing, referrals to drug treatment and other medical and social services, and information on reducing the risk of HIV infection.
Outreach and Education
Outreach relies on peers and local health workers to identify IDUs, provide education on preventing HIV infection, and serve as guides to health and social services (WHO, 2004c). Outreach is particularly useful in targeting hard-to-reach IDUs (WHO, 2004c). Outreach workers may distribute information on HIV/AIDS, bleach kits for disinfecting injection equipment, and condoms. While some programs are linked to needle and syringe exchanges or drug treatment programs, outreach efforts often occur outside clinical settings and separate from other interventions. Personal interactions between outreach workers and clients can occur in various venues in a community, as workers gain the trust of IDUs and become recognized as a source of information on reducing HIV risk (WHO, 2004c).
The efficacy and effectiveness of outreach and education is discussed in Chapter 3.
Other HIV Prevention Strategies
Other HIV prevention programs are not specific to IDUs, but may provide important sources of information or links to health and social services for drug users. Examples of these interventions include voluntary counseling and testing, antiretroviral therapy, and prevention and treatment of sexually transmitted infections. While there is a large body of evidence evaluating the effectiveness and efficacy of these interventions, it was beyond the Committee’s scope to review this evidence. Instead, a brief overview of these interventions is presented below.
Voluntary HIV Counseling and Testing
Voluntary counseling and testing (VCT) involves providing counseling to an individual to help make an informed choice about HIV testing. It also provides HIV education and is increasingly seen as an entry to other HIV/AIDS and drug abuse services (Liechty, 2004). VCT is among the most common type of HIV education program worldwide, and is often the entry point to other HIV/AIDS prevention programs for IDUs and other at-risk groups. The efficacy of HIV VCT has been clearly associated with decreases in risk behavior among individuals and couples, and among both HIV-infected and non-infected people (Voluntary HIV-1 Counseling and Testing Efficacy Study Group, 2000; Kamenga et al., 2000). According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), data from more than 70 countries show the number of people using these services grew from an estimated 4 million in 2001 to 16.5 million in 2005 (UNAIDS, 2006).
Despite being a common and effective HIV prevention method, developing countries have been somewhat slow to implement VCT (Motuvu et al., 2005). Factors limiting access to VCT include the stigma associated with HIV/AIDS and drug use, fear of prosecution for illicit drug use, low awareness of the risks of HIV infection and thus the relevance of VCT, and the distance, time, and cost required to engage in VCT. However, the removal of barriers to knowing one’s HIV serostatus by providing free, anonymous, and rapid testing with same-day results, can substantially enhance the uptake of VCT services. (Morin et al., 2006). Many agencies and governments have urged the use of rapid tests for HIV (Arthur et al., 2005; Metcalf et al., 2005), with same-day results to encourage testing (Morin et al., 2006; Liang et al., 2005). Providing anonymous HIV VCT has been shown to lead to higher testing rates (Rennie and Behets, 2006).
Antiretroviral Therapy
Antiretroviral therapy (ART), when used appropriately, can reduce HIV-related morbidity and mortality, improve the quality of life, restore and preserve immunologic function, and suppress viral load (DHHS, 2006). Globally, antiretroviral drugs reach only one in five who need them, and only about 1.3 million people received ART in low- and middle-income countries in 2005 (UNAIDS, 2006).
Treating IDUs with ART poses unique treatment challenges due to the high rate of co-occurring medical and psychiatric conditions, limited access to HIV care, increased likelihood of medication side effects and toxicities, and interactions between ART and opioid agonist maintenance treatments (DHHS, 2006). IDUs typically have a high rate of morbidity and mortality that can be unrelated or related to HIV disease. For example, IDUs have increased rates of tuberculosis, infections, endocarditis, hepatitis B and C infection, and neurologic and renal disease (DHHS, 2006). The high rate of co-occurring mental illness among IDUs also complicates treatment (DHHS, 2006).
Efficacy of ART among IDUs is comparable to other populations. IDUs are often thought to be poor candidates for ART because of concerns about adherence during active drug use and underlying medical complications resulting from drug use (WHO, 2005d). IDUs are more likely to experience ART-related side-effects and toxicities perhaps due to the high degree of underlying medical problems (DHHS, 2006). Furthermore, clinical care of IDUs with HIV enrolled in opioid agonist treatment is complicated by the numerous pharmokinetic interactions between many ARTs and opioid agonist medications that can decrease the efficacy of one or the other treatments (DHHS, 2006). As a result, regimens need to be carefully selected and patients monitored for such interactions.
Studies have found that IDUs have limited access to and are less likely than other populations to receive ART (DHHS, 2006). In a study in Baltimore, factors associated with IDUs who report no ART include active drug use, lack of involvement in drug treatment, and recent incarceration (Celentano et al., 1998). In Vancouver, younger IDUs, females, IDUs not currently enrolled in drug treatment, and IDUs with inexperienced physicians were less likely to be receiving ART (Strathdee et al., 1998). Some studies show that IDUs are less likely to seek ART when it is available compared with non-IDUs (Strathdee et al., 1998; Celentano et al., 2001), especially when they are not participating in drug dependence treatment.
However, provision of successful ART to IDUs is possible. The success of HIV treatment is increased by provision of drug treatment, supportive providers who are familiar with unique health and social needs of IDUs, and an awareness of increased likelihood of ART side-effects and potential interactions with other therapies (DHHS, 2006).
HIV-seropositive pregnant women may receive ART both to treat their HIV infection and to prevent HIV infection in their infants (WHO, 2004a). The risk of mother-to-child transmission can be greatly reduced by providing antiretroviral drugs to women during pregnancy and labor and to the infant during the first weeks of life (WHO, 2004a). WHO has a set of recommendations regarding ART use during pregnancy to prevent mother-to-child transmission (WHO, 2004a). In resource-rich settings, WHO recommends triple-combination regimens to prevent perinatal transmission when the woman does not yet require ART. However, these regimens have not been fully evaluated in resource-limited settings. Short course antiretroviral drugs are currently recommended in resource-limited settings during late pregnancy or during labor, as well as for the infant after childbirth (WHO, 2004a).
Prevention and Treatment of Sexually Transmitted Infections
Early detection and treatment of sexually transmitted infections (STIs) greatly reduces the likelihood of sexual transmission of HIV (IOM, 1997). STIs increase the concentration of HIV in genital secretions and lesions and could also spark a more infectious variant of HIV (Cohen, 2004). In terms of susceptibility, STIs can cause lesions, inflammation, and produce a vaginal environment that is more conducive to HIV transmission (Cohen, 2004, 2006).
In particular, genital ulcers, especially genital herpes (herpes simplex virus type 2), syphilis (Treponema pallidum), and chancroid (Haemophilus ducreyi) greatly increase the risk of HIV infection (Wasserheit, 1992; Nelson et al., 1997; Serwadda et al., 2003). HIV infection has also been associated with reactivation of herpes simplex virus type 2. Herpes lesions provide a convenient entry point for HIV. A study in India found that individuals with newly acquired herpes simplex virus type2—the most common cause of genital ulcers—are at greatest risk for acquiring HIV, compared with those who do not have herpes simplex virus and those who have prevalent herpes simplex virus type 2 infection (Reynolds et al., 2003).
Non-ulcerative STIs are also associated with HIV transmission, perhaps by increasing the recruitment and activation of CD4+ cells (Laga et al., 1993; Royce et al., 1997). Vaginal infections are important risk factors for HIV acquisition. Both trichomonas and bacterial vaginosis have been associated with increased risk of HIV infection throughout sub-Saharan Africa (Gregson et al., 2001; Buve 2002).
The effectiveness of STI treatment for HIV prevention has been tested in Tanzania and Uganda. The study in Tanzania found that intensive clinical management of symptomatic STIs was associated with a 38 percent reduction in HIV incidence over 2 years (Grosskurth et al., 1995), while the
study in Uganda found that mass periodic treatment of STIs did not reduce HIV incidence (Wawer et al., 1999). Finally, Quinn and colleagues (2000) have shown that plasma viral load in one partner is highly associated with the sexual transmission of HIV to the uninfected partner, but antiretroviral therapy can reduce transmission risks substantially (Palella et al., 1998). Thus, the detection and appropriate medical management of common STIs remains one of the principal prevention strategies for reducing HIV risk in sexually active adults.
GLOBAL COVERAGE OF PREVENTION INTERVENTIONS
Coverage is a measure of the extent to which the services rendered cover the potential need for these services in a community (Last, 1995). Effective interventions could fail to prevent, control, or reduce the problem it seeks to address if a significant proportion of the population is not reached (Burrows, 2006). Estimates of the coverage of prevention interventions among IDUs are generally unreliable because of the uncertainty and limitations of the underlying data. As noted, estimates of the IDU population size (the denominator) often have a large margin of error. Data sources used to estimate the number of people reached by the service(s) (the numerator) also have limitations. National population surveys and facility-based surveys are the most reliable methods for estimating utilization, but are time-consuming and expensive to conduct (USAID et al., 2004). Service statistics can also be used, but data are often incomplete and less accurate than other sources. Furthermore, obtaining unduplicated estimates of program attendees from service statistics is difficult if service information systems are not designed to capture unique users (USAID et al., 2004).
Various organizations have set different coverage targets for HIV prevention programs. For example, the United Nations agencies recommend a minimum coverage target of 60 percent (i.e., the program should reach 60 percent of IDUs) for syringe distribution programs. A recent modeling study highlights the problem of setting universal coverage targets (Vickerman et al., 2006). While the model suggests that a coverage “threshold” exists which, if attained, can reduce HIV risk, the threshold depends on many factors such as frequency of injection, reuse of syringes, or the efficacy of disinfection.
Several studies have attempted to quantify the coverage of HIV prevention services for IDUs. According to a recent study of coverage of HIV/AIDS prevention, care, and support programs in low- and middle-income countries, an estimated 4.3 percent of the estimated 9–10 million IDUs living in these countries had access to HIV prevention programs (USAID et al., 2004). The most common type of program was risk reduction education (320,000 IDUs), followed by needle and syringe exchange (150,000
IDUs), and opioid agonist maintenance treatment (20,000 IDUs) (see Table 1-1). These estimates, however, are extrapolated from data in 24 countries that reported having prevention programs for IDUs in 2003. The remaining 64 countries included in the study (total n=88) did not report any coverage data because either injecting drug use was not prevalent or data were missing on program coverage or the size of the IDU population (USAID et al., 2004).8 As a result, these estimates should be treated with caution.
A variety of other sources provide estimates of HIV prevention coverage for IDUs. The information below was compiled from multiple sources that do not use consistent definitions or timeframes, and therefore are not complete or comparable. However, they all suggest that coverage of prevention services for IDUs is limited.
Based on data collected by the UN Reference Working Group, 65 of 130 countries reporting injecting drug use have at least one sterile needle and syringe access program. However, programs have not been widely implemented in every country. Even when NSE does exist, the coverage is often poor. Results from a survey suggest that 65 percent of needle and syringe exchanges in Russia reach less than 1 percent of the local IDU population, and that fewer than 5 percent of projects reach more than 5 percent of their local IDU population (Burrows, 2001).
At least eight countries have reported NSEs in prisons (Switzerland, Germany, Spain, Armenia, Moldova, Kyrgyzstan, Belarus, and Iran) (Canadian HIV/AIDS Legal Network, 2004; Dolan et al., 2003; OSI, 2005). Programs are pending in Kazakhstan, Tajikistan, and Ukraine (Canadian HIV/AIDS Legal Network, 2004).
More than 19 developing and transitional countries have approved opioid agonist maintenance treatment (Hankins, 2005). However, the United States, United Kingdom, Spain, Germany, Italy, Canada, Australia, and Iran accounted for more than 85 percent of methadone consumption in 2004 (INCB, 2005). One recent study estimated that out of some 3 million IDUs, approximately 2,000 patients were receiving methadone or buprenorphine in 16 high-risk countries9 in Central and Eastern Europe and the former Soviet Union. It also estimated that about 10,850 patients were receiving methadone or buprenorphine in 5 high-risk countries10 in Asia out of roughly 2.2 million IDUs (OSI, 2005).
TABLE 1-1 Percent of Injecting Drug Users Covered by HIV Prevention Services in 2003, by Region
China is rapidly scaling up access to methadone maintenance treatment, with plans to treat over 300,000 patients in the next few years (Office of the State Council Working Committee on AIDS in China, 2005). On the other side of the spectrum, federal law in Russia prohibits the treatment of drug dependence with opioid agonist agents such as methadone. There is widespread opinion among the Russian narcotics community that methadone substitutes one addiction for another. Thus drug dependence treatment in Russia focuses on abstinence-based programs (Personal communication, V.N. Krasnov, Russian Society of Psychiatrists, June 16, 2006).
Some countries, such as Australia, Moldova, Indonesia, Poland, the United States, among others, have successfully provided methadone treatment, often on a pilot basis, in prison settings (OSI, 2005; Dolan et al., 2003). One example of a prison-based methadone program is at the New York City Correctional Facility at Rikers Island in the United States. The prison has operated a methadone maintenance program there since 1986 (Joseph et al., 1988). Eligible inmates are voluntarily enrolled from the detoxification wards into the KEEP (Key Extended Entry Process) program which stabilizes patients on methadone while in jail and then refers them to community-based KEEP methadone programs to continue their treatment upon release (Joseph, 1988). The goals of the Rikers Island program are to prevent inmate relapse into drug abuse upon release, to reduce the spread of HIV, and to initiate long-term treatment (Joseph, 1988).
Community-based outreach has been successful in making contact with large numbers of IDUs in North America, Western Europe, and Australia. However, few programs in developing and transitional countries are reaching the majority of IDUs through outreach services. Exceptions include Bangladesh, the Czech Republic, Kyrgyzstan, and Lithuania (WHO, 2004c). India has also implemented large-scale outreach programs in connection with needle and syringe exchange in some states, and Nepal has been conducting outreach activities for more than a decade in connection with its national NSE program (WHO, 2004c). While the Russian Federation experienced some early success with an outreach program in Kazan, which at one time extended across 101 sites and reached an estimated 7,700 IDUs, police activities have forced most of those sites to close (WHO, 2004c). In Latin America, Brazil and Argentina are the only countries that have achieved widespread IDU coverage through outreach. No countries in Africa or the Middle East have reported providing community-based outreach services to IDUs (WHO, 2004c).
CONCLUSION
Injecting drug use is a major factor in the continuing spread of HIV. This report focuses on high-risk countries—namely in Eastern Europe, the
Commonwealth of Independent States, and significant parts of Asia—where injecting drug use is, or is on the verge of becoming, the primary driver of the HIV epidemic. There are an estimated 13.2 million IDUs worldwide. Of those, an estimated 8.8 million live in Eastern Europe, Central, South, and Southeast Asia (Aceijas et al., 2004; UNAIDS, 2006), and an estimated 10.3 million (or 78 percent) live in developing or transitional countries. Primarily because of injecting drug use, Eastern Europe, Central Asia, and the Commonwealth of Independent States have witnessed as much as a 20-fold increase in the number of people living with HIV in less than a decade (UNAIDS, 2006). In Asia, an estimated 8.3 million people were living with HIV at the end of 2005, with rapidly expanding epidemics in both Central and particularly Southeast Asia from injecting drug use (UNAIDS, 2006).
Drug use behavior and the type of drugs used vary substantially across regions. HIV epidemics driven by injecting drug use tend to spread more rapidly than epidemics spread by sexual transmission, because exposure to the virus occurs more often, and because needles are more efficient at transmitting it than sex. In many parts of the world, HIV prevalence reached 40 percent and above among IDUs just 1 to 2 years after HIV entered the IDU population (Rhodes et al., 1999a). While sharing of contaminated injecting equipment is the primary mode of HIV transmission among IDUs, sexual transmission from HIV-infected drug injectors to their sex partners is becoming an important secondary route of spread (Grassly et al., 2003), as is perinatal transmission from HIV-infected female IDUs or HIV-infected partners of IDUs to their children.
Both individual-level factors (severity of dependence, preferred drug, presence and severity of psychiatric problems) and structural-level factors (proximity to drug trafficking routes, drug laws and enforcement practices, and stigma and discrimination) affect a drug user’s risk of acquiring or transmitting HIV. Understanding these risk factors is critical to designing effective prevention programs. While many interventions focus on individual-level risk factors, few focus on structural-level factors (Rhodes et al., 2005).
There are many approaches to HIV prevention for IDUs available, and there are many permutations in their application. Three major categories of HIV prevention interventions for IDUs that are reviewed in this report include: drug dependence treatment programs, which include both pharmacotherapies and psychosocial interventions; sterile needle and syringe access programs; and outreach and education programs. Data on the coverage of these interventions are quite limited, but in general, estimates suggest that coverage is inadequate in many areas.
The next two chapters review the effectiveness regarding drug dependence treatment, sterile needle and syringe access, and outreach and education. By reviewing this evidence, the Committee hopes to provide up-to-
date information that policymakers in high-risk countries can use to decide how to best adopt and implement interventions to prevent HIV infection among IDUs.
REFERENCES
Aceijas C, Stimson GV, Hickman M, Rhodes T. 2004. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 18(17):2295–2303.
American Psychiatric Association. 1994. Diagnostic and Statistical Manual of Mental Disorders (4th Ed.). Washington, DC: American Psychiatric Association.
Arthur GR, Ngatia G, Rachier C, Mutemi R, Odhiambo J, Gilks CF. 2005. The role for government health centers in provision of same-day voluntary HIV counseling and testing in Kenya. Journal of Acquired Immune Deficiency Syndromes. 40(3):329–335.
Barcal K, Schumacher JE, Dumchev K, Moroz LV. 2005. A situational picture of HIV/AIDS and injection drug use in Vinnitsya, Ukraine. Harm Reduction Journal. 2(1):16.
Beckerleg S, Telfer M, Hundt GL. 2005. The rise of injecting drug use in East Africa: A case study from Kenya. Harm Reduction Journal. 2:12.
Beyrer C. 2002. Human immunodeficiency virus (HIV) infection rates and heroin trafficking: Fearful symmetries. Bulletin on Narcotics. 44(1-2):103–116.
Beyrer C, Razak M, Lisam K, Chen J, Lui W, Yu XF. 2000. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 14:75–83.
Bobrova N, Rhodes T, Power R, Alcorn R, Neifeld E, Krasiukov N, Latyshevskaia N, Maksimova S. 2006. Barriers to accessing drug treatment in Russia: A qualitative study among injecting drug users in two cities. Drug and Alcohol Dependence. 82(Suppl 1):S57–S63.
Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. 1997. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Archives of General Psychiatry. 54(1):71–80.
Brown K. 2005. Managing STIs in jails. Infectious Diseases in Corrections Report. 8:1–4. [Online]. Available: http://www.idcronline.org/archives/april05/article.html [accessed June 30, 2006].
Bruneau J, Lamothe F, Soto J, Lachance N, Vincelette J, Vassai A, Franco EL. 2001. Sex-specific determinants of HIV infection among injection drug users in Montreal. Canadian Medical Association Journal. 164(6): 767–773.
Burris S, Vernick J, Ditzler A, Strathdee S. 2002. The legality of selling or giving syringes to injection drug users. Journal of the American Pharmaceutical Association. 42(Suppl 2):S13–S18.
Burris S, Strathdee S, Vernick J. 2003. Lethal injections: The law, science, and politics of syringe access for injection drug users. University of San Francisco Law Review. 37: 813–885.
Burris S, Blankenship KM, Donoghoe M, Sherman S, Vernick JS, Case P, Lazzarini Z, Koester S. 2004. Addressing the “risk environment” for injection drug users: The mysterious case of the missing cop. Milbank Quarterly. 82(1):125–156.
Burrows D. 2001. A Best Practice Model of Harm Reduction in the Community and in Prisons in the Russian Federation: Final Project Report. Washington, DC: World Bank.
Burrows D. 2006. Rethinking coverage of needle exchange programs. Substance Use and Misuse. 41:1045–1048.
Buve A. 2002. HIV epidemics in Africa: What explains the variations in HIV prevalence? International Union of Biochemistry and Molecular Biology: Life. 53:193–195.
Canadian HIV/AIDS Legal Network. 2004. Prison Needle Exchange: Lessons from a Comprehensive Review of International Evidence and Experience. Toronto, Canada: Canadian HIV/AIDS Legal Network.
CDC (Centers for Disease Control and Prevention). 2003. Viral Hepatitis. [Online]. Available: http://www.cdc.gov/ncidod/diseases/hepatitis/index.htm [accessed August 24, 2006].
CDC. 2005. HIV/AIDS Surveillance Report. 16. [Online]. Available: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004report/default.htm [accessed August 11, 2006].
Celentano D, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. 1998. Self-reported antiretroviral therapy in injection drug users. Journal of the American Medical Association. 280:544–546.
Celentano D, Galai N, Sethi A, Shah N, Strathdee S, Vlahov D, Gallant J. 2001. Time to initiate highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 15:1707–1715.
Chaisson RE, Bachetti P, Osmond D, Brodie B, Sande MA, Moss AR. 1989. Cocaine use and HIV infection in intravenous drug users in San Francisco. Journal of the American Medical Association. 261(4):61–65.
Chawarski MC, Mazlan M, Schottenfeld RS. 2006. Heroin dependence and HIV infection in Malaysia. Drug and Alcohol Dependence. 82(Suppl. 1):S39–S42.
Chelala C, Beyrer C. 1999. Drug use and HIV/AIDS in Burma. Lancet. 354(9184):1119.
Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chiang YL, Ree SC, Lee CH, Murray RM. 2003. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine. 33(8):1407–1414.
Cohen M. 2004. HIV and sexually transmitted diseases: Lethal synergy. Topics in HIV Medicine. 12(4): 104–107.
Cohen M. 2006. Thomas Parran Award Lecture: Transmission and prevention of transmission of HIV-1. Sexually Transmitted Diseases. 33(6):338–341.
Colfax G, Shoptaw S. 2005. The methamphetamine epidemic: Implications for HIV prevention and treatment. Current HIV/AIDS Reports. 2(4):194–199.
Comer SD, Collins ED. 2002. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. Journal of Pharmacology and Experimental Therapeutics. 303(2):695–703.
Compton P, Ling W, Moody D, Chiang N. 2006. Pharmacokinetics, bioavailability and opioid effects of liquid versus tablet buprenorphine. Drug and Alcohol Dependence 82(1):25-31.
Crofts N, Reid G, Deany P. 1998. Injecting drug use and HIV infection in Asia: The Asian Harm Reduction Network. AIDS. 12(Suppl B):S69–S78.
Dean JC, Rud F. 1984. The drug addict and the stigma of addiction. International Journal of Addiction. 19:859–869.
Dehne K, Kobyshcha Y. 2000. The HIV/AIDS Epidemic in Central and Eastern Europe: Update 2000. Geneva, Switzerland: UNAIDS.
Dehne K, Adelekan M, Chatterjee A, Weiler G. 2002. The need for a global understanding of epidemiological data to inform human immunodeficiency virus (HIV) prevention among injection drug users. Bulletin on Narcotics. 44(1-2):117–130.
Des Jarlais DC. 2005 (December 20). HIV Prevention for Injecting Drug Users: Lessons from North America. Presentation at the Institute of Medicine Workshop on the Prevention of HIV Among Injecting Drug Users in High-Risk Countries, Geneva, Switzerland. Institute of Medicine Committee on the Prevention of HIV Infection Among Injecting Drug Users in High-Risk Countries.
Des Jarlais DC, Friedman SR, Choopanya K, Vanichseni S, Ward TP. 1992. International epidemiology of HIV and AIDS among injecting drug users. AIDS. 6:1053–1068.
Des Jarlais DC, Dehne K, Casabona J. 2001. HIV surveillance among injecting drug users. AIDS. 15(Suppl 3):S13–22.
Des Jarlais DC, Perlis T, Arasteh K, Hagan H, Milliken J, Braine N, Yancovitz S, Mildvan D, Perlman DC, Maslow C, Friedman SR. 2004. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. Journal of Acquired Immune Deficiency Syndromes. 35:158–166.
Dewing S, Pluddemann A, Myers B, Parry C. 2006. Review of injection drug use in six African countries: Egypt, Kenya, Mauritius, Nigeria, South Africa and Tanzania. Drugs: Education, Prevention and Policy. 13(2):121–137.
DHHS (Department of Health and Human Services). 2006. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Online]. Available: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [accessed August 3, 2006].
Diaz T, Des Jarlais DC, Vlahov D, Perlis T, Edwards V, Friedman S, Rockwell R, Hoover D, Williams I, Monterroso E. 2001. Factors associated with prevalent hepatitis C: Differences among young adult injection drug users in lower and upper Manhattan, New York City. American Journal of Public Health. 91(1):23–30.
Digiusto E, Shakeshaft A, Ritter A, O’Brien S, Mattick RP, NEPOD Research Group. 2004. Serious adverse events in the Australian national evaluation of pharmacotherapies for opioid dependence (NEPOD). Addiction. 99:450–460.
Dolan K, Rutter S, and Wodak AD. 2003. Prison-based syringe exchange programmes: A review of international research and development. Addiction. 98:153–158.
Fudala PJ, Jaffe JH, Dax EM, Johnson RE. 1990. Use of buprenorphine in the treatment of opioid addiction II: Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clinical Pharmacology and Therapeutics. 47:525–534.
Fuller CM, Vlahov D, Latkin CA, Ompad DC, Celentano DD, Strathdee SA. 2003. Social circumstances of initiation of injection drug use and early shooting gallery attendance: Implications for HIV intervention among adolescent and young adult injection drug users. Journal of Acquired Immune Deficiency Syndromes. 32(1):86–93.
Garfein RS, Doherty MC, Monterrosso ER, Thomas DL, Nelson KE, Vlahov D. 1998. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 18(Suppl 1):S11–19.
Ghys P. 2005 (December 19). Epidemiology of HIV Infection and AIDS among Injecting Drug Users. Presentation at the Institute of Medicine Workshop on the Prevention of HIV Among Injecting Drug Users in High-Risk Countries, Geneva, Switzerland. Institute of Medicine Committee on the Prevention of HIV Infection Among Injecting Drug Users in High-Risk Countries.
Goffman, E. 1963. Stigma: Notes on the Management of Spoiled Identity. New York: Simon and Schuster.
Gossop M, Griffiths P, Powis B, Strang J. 1993a. Severity of heroin dependence and HIV risk. I. Sexual behaviour. AIDS Care. 5(2):149–157.
Gossop M, Griffiths P, Powis B, Strang J. 1993b. Severity of heroin dependence and HIV risk. II. Sharing injecting equipment. AIDS Care. 5(2):159–168.
Gostin L. 1991. An alternative public health vision for a national drug strategy: “Treatment works.” Houston Law Review. 28(1):285–308.
Gostin L, Lazzarini Z, Jones S, Flaherty K. 1997. Prevention of HIV/AIDS and other blood-borne diseases among injection drug users: A national survey on the regulation of syringes and needles. Journal of the American Medical Association. 277:53–62.
Grassly NC, Lowndes CM, Rhodes T, Judd A, Renton A, Garnett GP. 2003. Modeling emerging HIV epidemics: The role of injecting drug use and sexual transmission in the Russian Federation, China, and India. International Journal of Drug Policy. 14:25–43.
Gregson S, Mason PR, Garnett GP, Zhuwau T, Nyamukapa CA, Anderson RM, Chandiwana SK. 2001. A rural HIV epidemic in Zimbabwe? Findings from a population-based survey. International Journal of STDs and AIDS. 12:89–96.
Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Swekoro K, et al. 1995. Impact of improved treatment of sexually transmitted disease on HIV infection in rural Tanzania: Randomised controlled trial. Lancet. 346:530–536.
Grund JP, Friedman S, Stern LS, Benny J, Neaigus A, Curtis R, Des Jarlais DC. 1996. Syringe-mediated drug sharing among injecting drug users: Patterns, social context and implications for transmission of blood-borne pathogens. Social Science Medicine. 42(5): 691–703.
Hagan H. 1998. Hepatitis C virus transmission dynamics in injection drug users. Substance Use and Misuse. 33:1197–1212.
Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. 1999. Syringe exchange and risk of infection with hepatitis B and C viruses. American Journal of Epidemiology. 149:203–213.
Hagan H, Thiede H, Weiss N, Hopkins S, Duchin J, Alexander ER. 2001. Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal of Public Health. 91(1):42–46.
Hahn JA, Page-Shafer K, Lum PJ, Bourgois P, Stein E, Evans J, Busch M, Tobler L, Phelps B, Moss AR. 2002. Hepatitis C virus seroconversion among young injection drug users: Relationships and risks. Journal of Infectious Diseases. 186:1558–1564.
Hammett TM. 2006. HIV/AIDS and other infectious disease among correctional inmates: Transmission, burden, and appropriate response. American Journal of Public Health. 96(3):13–17.
Hammett TM, Bartlett NA, Chen Y, Ngu D, Cuong DD, Phuong NM, Tho NH, Van LK, Donghua M, Shaomi X, Chen H, Quyen HN, Broadhead RS, Des Jarlais DC. 2005. Law enforcement influences on HIV prevention for injection drug users: observations from a cross-border project in China and Vietnam. International Journal of Drug Policy. 16: 235–245.
Hankins C. 2005 (December 19). The Global Response to HIV and Injecting Drug Use. Presentation at the Institute of Medicine Workshop on the Prevention of HIV Among Injecting Drug Users in High-Risk Countries, Geneva, Switzerland. Institute of Medicine Committee on the Prevention of HIV Infection Among Injecting Drug Users in High-Risk Countries.
Hankins CA, Friedman SR, Zafar T, Strathdee SA. 2002. Transmission and prevention of HIV and sexually transmitted infections in war settings: Implications for current and future armed conflicts. AIDS. 16:2245–2252.
Hawkins WE, Latkin C, Chowdury D, Hawkins MJ. 1998. Depressive symptoms and HIV-risk behaviour in inner-city users of drug injections. Psychological Reports. 82: 137–138.
Hien NT, Giang LT, Binh PN, Deville W, van Ameijden EJC, Wolffers I. 2001. Risk factors of HIV infection and needle sharing among injecting drug users in Ho Chi Minh City, Vietnam. Journal of Substance Abuse. 13:45–58.
Hser YI, Anglin D, Powers K. 1993. A 24-year follow-up of California narcotics addicts. Archives of General Psychiatry. 50:577–584.
Hser YI, Grella CE, Hubbard RL, Hsieh SC, Fletcher BW, Brown BS, Anglin MD. 2001. An evaluation of drug treatments for adolescents in 4 U.S. cities. Archives of General Psychiatry. 58(7):689–695.
INCB (International Narcotics Control Board). 2005. Part Four: Statistical Information on Narcotic Drugs. [Online]. Available: http://www.incb.org/pdf/e/tr/nar/2004/narcotics_ part4.pdf [accessed June 30, 2006].
IOM (Institute of Medicine). 1990. Treating Drug Problems: Volume 1. Washington, DC: National Academy Press.
IOM. 1995. Federal Regulation of Methadone Treatment. Washington, DC: National Academy Press.
IOM. 1997. The Hidden Epidemic: Confronting Sexually Transmitted Diseases. Washington, DC: National Academy Press.
Irwin K, Karchevsky E, Heimer R, Badrieva L. 2006. Secondary syringe exchange as a model for HIV prevention programs in the Russian Federation. Substance Use and Misuse. 41: 979–999.
Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. 1995. Buprenorphine treatment of opioid dependence: Clinical trial of daily versus alternate-day dosing. Drug and Alcohol Dependence. 40(1):27-35.
Joseph H. 1988. The criminal justice system and opiate addiction: A historical perspective. NIDA Research Monograph. 86:106–125.
Joseph H, Appel P, Marx R, Perez J, Tardalo F, Watts L. 1988. Evaluation of Pre-KEEP in Three Facilities of the New York City Department of Corrections on Rikers Island. Treatment Issues Report. New York: New York State Division of Substance Abuse Services.
Kamenga MC, Sweat MD, De Zoysa I, et al. 2000. The Voluntary HIV-1 Counseling and Testing Efficacy Study: Design and methods. AIDS and Behavior. 4(1):5–13.
King MB. 1989. Prejudice and AIDS: The views and experience of people with HIV infection. AIDS Care. 1(2):137–143.
Klee H, Faugier J, Hayes C, Boulton T, Morris J. 1990. Factors associated with risk behaviour among injecting drug users. AIDS Care. 2:133–145.
Kosten TR, Rounsaville BJ. 1986. Psychopathology in opioid addicts. The Psychiatric Clinics of North America. 9(3):515–532.
Kozlov AP. 2006. Uncertainty in descriptions of biosocial phenomena and the schism between preventionists and moralists. Medical Hypotheses. 67:662–665.
Kral A, Bluthenthal R. 2004. The Pmpact of Police Practices on the Health of IDUs in the San Francisco Bay Area. Abstract No. 1140. Presented at the 15th International Conference on the Reduction of Drug-Related Harm, Melbourne, 20–24 April.
Kral AH, Bluthenthal RN, Erringer E, Lorvick J, Edlin BR. 1999. Risk factors among IDUs who give injections to or receive injections from other drug users. Addiction. 94(5): 675–683.
Kulsudjarit K. 2004. Drug problem in southeast and southwest Asia. Annals of the New York Academy of Sciences. 1025:446–457.
Ladd GT, Petry NM. 2003. Antisocial personality in treatment-seeking cocaine abusers: Psychological functioning and HIV risk. Journal of Substance Abuse Treatment. 24: 323–330.
Laga M, Manoka A, Kivuvu M, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: Results from a cohort study. Journal of Infectious Diseases. 7:95–102.
Lamothe F, Bruneau J, Coates R, Rankin JG, Soto J, Arshinoff R, Brabant M, Vincelette J, Fauvel M. 1993. Seroprevalence of and risk factors for HIV-1 infection in injection drug users in Montreal and Toronto: A collaborative study. Canadian Medical Association Journal. 149(7):945–951.
Last J. 1995. A Dictionary of Epidemiology. 3rd edition. New York: Oxford University Press.
Liang TS, Erbelding E, Jacob CA, Wicker H, Christmyer C, Brunson S, Richardson D, Ellen JM. 2005. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care and STDs. 19(4):253–257.
Liechty CA. 2004. The evolving role of HIV counseling and testing in resource-limited settings: HIV prevention and linkage to expanding HIV care access. Current HIV/AIDS Reports. 1(4):181–185.
Lowndes C, Alary M, Platt L. 2003. Injection drug use, commercial sex work, and the HIV/ STI epidemic in the Russian Federation. Sexually Transmitted Diseases. 30(1):46–48.
Lurie P, Reingold AL, Bowser B, Chen D, Foley J, Guydish J, Kahn JG, Lane S, Sorensen J. 1993. The Public Health Impact of Needle Exchange Programs in the United States and Abroad. Volume 1. San Francisco: University of California.
Lye MS, Archibald C, Ghazali AA, Low BT, Sinniah M, Rus SC, Singh J, Nair RC. 1994. Patterns of risk behavior for patients with sexually transmitted diseases and surveillance for HIV in Kuala Lumpur, Malaysia. International Journal of STDs and AIDS. 5: 124–129.
Magis-Rodriguez C, Marques LF, Touze G. 2002. HIV and injection drug use in Latin America. AIDS. 16(Suppl 3):S34–S41.
Magis-Rodriguez C, Brouwer KC, Morales S, Gayet C, Lozada R, Ortiz-Mondragon R, Ricketts EP, Strathdee SA. 2005. HIV prevalence and correlates of receptive needle sharing among injection drug users in the Mexican-U.S. border city of Tijuana. Journal of Psychoactive Drugs. 37(3):333–339.
Magnani R, Sabin K, Saidel T, Heckathorn D. 2005. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 19(Suppl 2):S67–S72.
Malcolm A, Aggleton P, Bronfman M, Galvao J, Mane P, Verrall J. 1998. HIV-related stigmatization and discrimination: Its forms and contexts. Critical Public Health. 8(4): 347–370.
Mandell W, Joohyng K, Latkin C, Suh T. 1999. Depressive symptoms, drug networks, and their synergistic effect on needle-sharing behavior among street injection drug users. American Journal of Drug and Alcohol Abuse. 25(1):117–127.
MAP (Monitoring the AIDS Pandemic). 2005a. Sex Work and HIV/AIDS in Asia. Washington, DC: MAP.
MAP. 2005b. Drug Injection and HIV/AIDS in Asia. Washington, DC: MAP.
Maruschak LM. 2002. HIV in Prisons, 2000. Washington, DC: Bureau of Justice Statistics.
Mayet S, Farrell M, Ferri M, Amato L, Davoli M. 2004. Psychosocial treatment for opiate abuse and dependence. Cochrane Database of Systematic Reviews. (4):CD004330.
McCoy CB, Inciardi J. 1995. Sex, Drugs, and the Continuing Spread of AIDS. Los Angeles, CA: Roxbury.
McCurdy SA, Ross MW, Kilonzo GP, Leshabari MT, Williams ML. 2006. HIV/AIDS and injection drug use in the neighborhoods of Dar es Salaam, Tanzania. Drug and Alcohol Dependence. 82(Suppl 1):S23–S27.
Metcalf CA, Douglas JM Jr, Malotte CK, Cross H, Dillon BA, Paul SM, Padilla SM, Brookes LC, Lindsey CA, Byers RH, Peterman TA. 2005. Relative efficacy of prevention counseling with rapid and standard HIV testing: A randomized, controlled trial (RESPECT-2). The RESPECT-2 Study Group. Sexually Transmitted Diseases. 32(2):130–138.
Molitor F, Traux S, Ruiz J, Sun R. 1998. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. The Western Journal of Medicine. 168:93–97.
Molitor F, Ruiz J, Flynn N, Mikanda J, Sun R, Anderson R. 1999. Methamphetamine use and sexual and injection risk behaviors among out-of-treatment injection drug users. American Journal of Drug and Alcohol Abuse. 25(3):475–493.
Morin SF, Khumalo-Sakutukwa G, Charlebois ED, Routh J, Fritz K, Lane T, Vaki T, Fiamma A, Coates TJ. 2006. Removing barriers to knowing HIV status: Same-day mobile HIV testing in Zimbabwe. Journal of Acquired Immune Deficiency Syndromes. 41(2): 218–224.
Motuvu JK, Gray RH, Makumbi F, Wawer MJ, Serwadda D, Kigozi G, Sewankambo NK, Nalugoda F. 2005. Voluntary HIV counseling and testing acceptance, sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS. 19(5):503–511.
Nelson KE, Celentano DD, Eiumtrakul S, et al. 1997. The association of herpes simplex-type 2, Haemophilus ducreyi, and syphilis infections with genital ulcer disease and HIV infection among young men in northern Thailand. Journal of Acquired Immune Deficiency Syndromes. 16:293–300.
NIDA (National Institute on Drug Abuse). 2002. Principles of HIV Prevention in Drug-Using Populations: A Research-Based Guide. Washington, DC: NIDA. [Online]. Available: http://www.nida.nih.gov/POHP/FAQ_1.html [accessed June 22, 2006].
NRC (National Research Council) and IOM (Institute of Medicine). 1995. Preventing HIV Transmission: The Role of Sterile Needles and Bleach. Washington, DC: National Academy Press.
O’Brien C, Kampman K. 2004. Opioids: Antagonists and partial agonists. In: The American Psychiatric Association Textbook of Substance Abuse Treatment. 3rd edition. Washington, DC: American Psychiatric Press, Inc. Pp. 305–319.
O’Brien CP, Charney DS, Lewis L, Cornish JW, Post RM, Woody GE, Zubieta JK, Anthony JC, Blaine JD, Bowden CL, Calabrese JR, Carroll K, Kosten T, Rounsaville B, Childress AR, Oslin DW, Pettinati HM, Davis MA, Demartino R, Drake RE, Fleming MF, Fricks L, Glassman AH, Levin FR, Nunes EV, Johnson RL, Jordan C, Kessler RC, Laden SK, Regier DA, Renner JA Jr, Ries RK, Sklar-Blake T, Weisner C. 2004. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: A call to action. Biological Psychiatry. 56(10):703–713.
ODCCP (Office of Drug Control and Crime Prevention) and UNAIDS. 2001. Drug Abuse and HIV/AIDS: Lessons Learned—Case Studies in Central Eastern Europe and Central Asia. New York: United Nations.
Office of the State Council Working Committee on AIDS in China. 2005. Progress on Implementing UNGASS Declaration of Commitment in China 2005. [Online]. Available: http://data.unaids.org/pub/Report/2006/2006_country_progress_report_china_en.pdf [accessed June 30, 2006].
OSI (Open Society Institute). 2005. Harm Reduction Developments 2005. Countries with Injection-Driven HIV Epidemics. New York: Open Society Institute.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. New England Journal of Medicine. 338:853–860.
Panda S, Chatterjee A, Bhattacharya SK, Manna B, Singh PN, Sarkar S, Naik TN, Chakrabarti S, Detels R. 2000. Transmission of HIV from injection drug users to their wives in India. International Journal of STDs and AIDS. 11:468–473.
Panda S, Bijaya L, Sadhana Devi N, Foley E, Chatterjee A, Banerjee D, Naik TN, Saha MK, Bhattacharya SK. 2001. Interface between drug use and sex work in Manipur. National Medical Journal of India. 14(4):209–211.
Paone D, Cooper H, Alperen J, Shi Q, Des Jarlais DC. 1999. HIV risk behaviour of current sex workers attending syringe exchange: The experiences of women in five US cities. AIDS Care. 11(3):269–280.
Pisani E, Dadun, Sucahya PK, Kamil O, Jazan S. 2003. Sexual behavior among injection drug users in 3 Indonesian cities carries a high potential for HIV spread to noninjectors. Journal of Acquired Immune Deficiency Syndromes. 34(4):403–406.
Platt L, Hickman M, Rhodes T, Mikhailova L, Karavashkin V, Vlasov A, Tilling K, Hope V, Khutorksoy M, Renton A. 2004. The prevalence of injecting drug use in a Russian city: Implications for harm reduction and coverage. Addiction. 99:1430–1438.
Poshyachinda V. 1993. Drug injecting and HIV infection among the population of drug abusers in Asia. Bulletin on Narcotics. 45(1):77–90.
Poshyachinda V, Ayudhya SNA, Aramrattana A, Kanato M, Assanangkornchai S, Jitipiromsri S. 2005. Illicit substance supply and abuse in 2000–2004: An approach to assess the outcome of the war on drug operation. Drug and Alcohol Review. 24:461–466.
Qian H, Schumacher J, Chen H, Ruan Y. 2006. Injection drug use and HIV/AIDS in China: Review of current situation, prevention and policy implications. Harm Reduction Journal. 3:4.
Quan VM, Chung A, Long HT, Donder TJ. 2000. HIV in Vietnam: The evolving epidemic and the prevention response, 1996–1999. Journal of Acquired Immune Deficiency Syndromes. 25:360–369.
Quinn TC, Wawer MJ, Sewankambo N, Servadda D, Li C, Wabwire-Mangen et al. 2000. Viral load and heterosexual transmission of Human Immunodeficiency Virus Type 1. Rakai Project Study Group. New England Journal of Medicine. 342:921–929.
Raveis VH, Siegel K, Gorey E. 1998. Factors associated with HIV-infected women’s delay in seeking medical care. AIDS Care. 10(5):549–562.
Razzaghi EM, Movaghar AR, Green TC, Khoshnood K. 2006. Profiles of risk: A qualitative study of injecting drug users in Tehran, Iran. Harm Reduction Journal. 3:12.
Rennie S, Behets F. 2006. Desperately seeking targets: The ethics of routine HIV testing in low-income countries. Bulletin of the World Health Organization. 84:52–57.
Reynolds S, Risbud A, Shepherd M, Zenilman J, Brookmeyer R, Paranjape R, Divekar A, Gangakhedkar R, Ghate M, Bolllinger R, Mehendale S. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. Journal of Infectious Diseases. 187:1513–1521.
Rhodes T, Stimson GV, Crofts N, Ball A, Dehne K, Khodakevich L. 1999a. Drug injecting, rapid HIV spread, and the “risk environment”: Implications for assessment and response. AIDS. 13 (Suppl A):S259–S269.
Rhodes T, Ball A, Stimson GV, Kobyshcha Y, Fitch C, Pokrovsky V, Bezruchenko-Novachuk M, Burrows D, Renton A, Andrushckak L. 1999b. HIV infection associated with drug injecting in the newly independent states, eastern Europe: The social and economic context of epidemics. Addiction. 94(9):1323–1336.
Rhodes T, Mikhailova L, Sarang A, Lowndes CM, Rylkov A, Khutorskoy M, Renton A. 2003. Situational factors influencing drug injecting, risk reduction and syringe exchange in Togliatti City, Russian Federation: A qualitative study of micro risk environment. Social Science Medicine. 57(1):39–54.
Rhodes T, Sarang A, Bobrik A, Bobkov E, Platt L. 2004. HIV transmission and HIV prevention among injecting drug users in Russia. International Journal of Drug Policy. 15(1): 1–16.
Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. 2005. The social structural production of HIV risk among injecting drug users. Social Science Medicine. 61(5):1026– 1044.
Ritson EB. 1999. Alcohol, drugs, and stigma. International Journal of Clinical Practice. 53:549–551.
Robertson JR, Bucknall AB, Welsby PD, Roberts JJ, Inglis JM, Peutherer JF, Brettle RP. 1986. Epidemic of AIDS-related virus (HTLV-III/LAV) infection among intravenous drug users. British Medical Journal. 292(6519):527–529.
Royce RA, Sena A, Cates W, Cohen MS. 1997. Sexual transmission of HIV. New England Journal of Medicine. 336(15):1072–1078.
Schottenfeld R, Carroll K, Rounsaville B. 1993. Comorbid psychiatric disorders and cocaine abuse. NIDA Research Monograph. 135:31–47.
Serwadda D, Gray RH, Sewankambo N, Wabwire-Mangen F, Chen MZ, Quinn TC, Lutalo T, Kiwanuka N, Kigozi G, Nalugoda F, Meehan MP, Morrow AR, Wawer MJ. 2003. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: A nested case-control study in Rakai, Uganda. Journal of Infectious Diseases. 188(10):1492–1497.
Shapatava E, Nelson KE, Tsertsvadze T, del Rio C. 2006. Risk behaviors and HIV, hepatitis B, and hepatitis C seroprevalence among injection drug users in Georgia. Drug Alcohol Dependence. 82(Suppl 1):S35–S38.
Singer M, Li J, Duke M. 2003. Drug Use Patterns and AIDS Risk in China. Portland, Oregon: Society for Applied Anthropology.
Singh S, Crofts N. 1993. HIV infection among drug users in north-east Malaysia. AIDS Care. 5:273–281.
Small W, Kain S, Laliberte N, Schechter MT, O’Shaughnessy MV, Spittal PM. 2005. Incarceration, addiction and harm reduction: Inmates experience injecting drugs in prison. Substance Use & Misuse 40:831–843.
Sowell RL, Lowenstein A, Moneyham L, Demi A, Mizuno Y, Seals BF. 1997. Resources, stigma, and patterns of disclosure in rural women with HIV Infection. Public Health Nursing. 14(5):302–312.
Stachowiak JA, Tishkova FK, Strathdee SA, Stibich MA, Latypov A, Mogilnii V, Beyrer C. 2006. Marked ethnic differences in HIV prevalence and risk behaviors among injection drug users in Dushanbe, Tajikistan, 2004. Drug and Alcohol Dependence. 82(Suppl 1):S7–S14.
Stein MD, Solomon DA, Herman DS, Anderson BJ, Miller I. 2003. Depression severity and drug injection HIV risk behaviors. American Journal of Psychiatry. 160:1659–1662.
Stopka A. 2006. Editor’s introduction to this special issue on syringe access and secondary syringe exchange: International perspectives and future directions. Substance Use and Misuse. 41:771–776.
Strang J, McCambridge J, Best D, Beswick T, Bearn J, Rees S, Gossop M. 2003. Loss of tolerance and overdose mortality after inpatient opiate detoxification: Follow up study. British Medical Journal. 326:9589–9960.
Strathdee S, Palepu A, Cornelisse P, Yip B, O’Shaughnessy M, Montaner J, Schechter M, Hogg R. 1998. Barriers to use of free antiretroviral therapy in injection drug users. Journal of the American Medical Association. 280:547–549.
Strathdee SA, Galai N, Safaiean M, Celentano DD, Vlahov D, Johnson L, Nelson KE. 2001. Sex differences in risk factors for HIV seroconversion among injection drug users: A 10-year perspective. Archives of Internal Medicine. 161(10):1281–1288.
Taussig J, Junge B, Burris S, Jones TS, Sterk CE. 2002. Individual and structural influences shaping pharmacists’ decisions to sell syringes to injection drug users in Atlanta, Georgia. Journal of the American Pharmaceutical Association. 42(6 Suppl 2):S40–S45.
Thorpe L, Ouellet L, Hershow R, Bailey S, Williams I, Williamson J, Monterroso E, Garfein R. 2002. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. American Journal of Epidemiology. 155(7):645–653.
Todd CS, Safi N, Strathdee SA. 2005. Drug use and harm reduction in Afghanistan. Harm Reduction Journal. 2:13.
UNAIDS (Joint United Nations Programme on HIV/AIDS). 2005. HIV/AIDS Prevention, Treatment and Care Among Injecting Drug Users and in Prison. Ministerial meeting on “Urgent Response to the HIV/AIDS Epidemics in the Commonwealth of Independent States,” Moscow, March 31 to April 1, 2005. [Online]. Available: http://www.unodc.org/pdf/event_2005-03-31_prisons.pdf [accessed June 30, 2006].
UNAIDS. 2006. 2006 Report on the Global AIDS Epidemic: A UNAIDS 10th Anniversary Special Edition. Geneva: UNAIDS.
UNODC (United Nations Office on Drugs and Crime). 2002. Amphetamine Type Stimulants Threaten East Asia: East Asia Now Has the Most Serious ATS Problems in the World. [Online]. Available: http://www.unodc.un.or.th/factsheet/ATSissuesplans310102.htm [accessed June 25, 2006].
UNODC. 2004. Law Enforcement. [Online]. Available: http://www.unodc.un.or.th/law/ [accessed June 30, 2006].
UNODC. 2005. 2005 World Drug Report. Vienna, Austria: UNODC.
Urbina A, Jones K. 2004. Crystal methamphetamine, its analogues, and HIV infection: Medical and psychiatric aspects of a new epidemic. Clinical Infectious Diseases. 38(6): 890–894.
USAID, UNAIDS, WHO, UNICEF, and POLICY Project. 2004. Coverage of Selected Services for HIV/AIDS Prevention, Care, and Support in Low- and Middle-Income Countries in 2003. Washington, DC: Futures Group, POLICY Project.
Vickerman P, Hickman M, Rhodes T, Watts C. 2006. Model projections on the required coverage of syringe distribution to prevent HIV epidemics among injecting drug users. Journal of Acquired Immune Deficiency Syndromes. 42(3):355–361.
Voluntary HIV-1 Counseling and Testing Efficacy Study Group. 2000. Efficacy of voluntary HIV-1 counseling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: A randomised trial. Lancet. 356(9224):103–112.
Wasserheit JN. 1992. Epidemiologic synergy: Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually Transmitted Diseases. 19:61–77.
Watters JK, Estilo MJ, Clark GL, Lorvick J. 1994. Syringe and needle exchange as HIV/AIDS prevention for injection drug users. Journal of the American Medical Association. 271:115–120.
Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. 1999. Control of sexually transmitted diseases for AIDS prevention in Uganda: A randomised community trial. Lancet. 353:525–535.
WHO (World Health Organization). 2003a. HIV/AIDS in Asia and the Pacific Region. Annual report. Manila, Philippines: WHO Western Pacific Regional Office.
WHO. 2003b. WHO Collaborative Study on Substitution Therapy of Opioid Dependence and HIV/AIDS. Geneva, Switzerland: WHO.
WHO. 2004a. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Guidelines on Care, Treatment and Support for Women Living with HIV/AIDS and Their Children in Resource-Constrained Settings. Geneva, Switzerland, WHO.
WHO. 2004b. Policy Brief: Reduction of HIV Transmission in Prisons. Geneva, Switzerland: WHO.
WHO. 2004c. Evidence for Action: Effectiveness of Community-Based Outreach in Preventing HIV/AIDS Among Injecting Drug Users. Geneva, Switzerland: WHO.
WHO. 2005a. Policy and Programming Guide for HIV/AIDS Prevention and Care Among Injecting Drug Users. Geneva, Switzerland: WHO.
WHO. 2005b. Evidence for Action: Effectiveness of Drug Dependence Treatment in Preventing HIV among Injecting Drug Users. Geneva, Switzerland: WHO.
WHO. 2005c. WHO Model List of Essential Medicine. 14th edition. [Online]. Available: http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf [accessed August 11, 2006].
WHO. 2005d. Policy Brief: Antiretroviral Therapy and Injecting Drug Users. Geneva, Switzerland: WHO.
WHO, UNODC, UNAIDS. 2004. Substitution Maintenance Therapy in the Management of Opioid Dependence and HIV/AIDS Prevention: Position Paper. Geneva, Switzerland: WHO.
Wood E, Kerr T, Small W, Jones J, Schechter M, Tyndall M. 2003. The impact of a police presence on access to needle exchange programs. Journal of Acquired Immune Deficiency Syndromes. 34(1):116–118.
Yu XF, Chen J, Scao Y, Beyrer C, Liu B, Wang Z, Liu W, Yang J, Liang S, Viscidi RP, Gu J, Gurrie-Glass, G, Lai S. 1999. Emerging HIV infections with distinct subtypes of HIV-1 infection among injection drug users from geographically separate locations in Guangxi Province, China. Journal of Acquired Immune Deficiency Syndromes. 22(2):180–188.
Zamani S, Kihara M, Gouya MM, Vazirian M, Nassirimanesh B, Ono-Kihara M, Ravari SM, Safaie A, Ichikawa S. 2006. High prevalence of HIV infection associated with incarceration among community-based injecting drug users in Tehran, Iran. Journal of Acquired Immune Deficiency Syndromes. 42(3):342–346.
Zhao M, Du J, Lu GH, Wang QU, Xu H, Zhu M, McCoy CB. 2006. HIV sexual risk behaviors among injection drug users in Shanghai. Drug Alcohol Dependence. 82(Suppl 1):S43–S47.
Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M, Methamphetamine Treatment Project. 2004. Psychiatric symptoms in methamphetamine users. American Journal of Addiction. 13(2):181–190.









































