2
Status of Pollinators
A definitive assessment of the status of pollinator populations in North America will hinge on the quality and availability of data from a variety of well-corroborated sources, and such information is not available for every taxon. Because of their economic importance, actively managed pollinators are more likely than are wild pollinators to be closely and systematically monitored. But even when standardized data are available, interpretation of patterns of population change can be difficult. Ascertaining a pattern of decline in wild pollinator species involves consideration of a broader range of sources of information, including historical accounts, natural history collections, recently published observations, and comparative analyses. For some species, population data that are sufficient to inform an assessment of pollinator status simply do not exist.
POLLINATORS AND THE CONCEPT OF DECLINE
Identifying population declines, particularly for insects, is problematic primarily because, for many species, there are no historical data on absolute abundance. Historical accounts (for example, Jones and Kimball, 1943) often described abundance not quantitatively but qualitatively—a species might be called “common,” “uncommon,” or “rare”—so the information is difficult to interpret or compare. There are, however, numerous reports of declines of pollinating insects that have been documented according the strict criteria of federal or state law or regulations or by nongovernmental organizations. A case in point is the Massachusetts Endangered Species Act (MESA; 321 CMR 8:00), which requires demonstration of habitat
threat and population decline before an animal or plant can be listed as endangered, threatened, or of special concern—terms that themselves are suggestive of particular patterns of population change. However, different jurisdictions can define terms differently, and that causes difficulty for comparative studies of decline or endangerment. Some species also have inherently small populations and restricted ranges, and their relative rarity might not be the result of declining population.
In determining whether pollinator populations are declining, it is important to acknowledge the distinction between a “decline” and a “shortage.” An economically driven shortage of pollinators that occurs as a result of increased demand could be entirely independent of the condition of pollinator populations. In this report, the term “decline” is applied to populations for which the number of individuals is decreasing over time; “shortage” means that the supply of pollinators or their services is insufficient to meet demand. The status of pollinator populations and assemblages can be assessed in many ways, both direct and indirect (see Appendix G for examples of methods for analyzing pollinator status).
POPULATION TRENDS
Insect Pollinators
Although more than 750,000 insect species have been described (Grimaldi and Engel, 2005), possibly as many as 30 million more await discovery and formal description (Erwin, 1982; Stork, 1988, 1996; see also May, 1999, and Erwin, 2004). Insects comprise the most diverse assemblage of terrestrial animals, including within their ranks some of the most economically important pollinators and the dominant pollinators in a variety of natural systems. In some communities, insects pollinate as many as 93 percent of the flowering plants (Bawa, 1974, 1990; Kato, 2000). Unfortunately, the available taxonomic expertise does not exist to document fully the Earth’s insect biodiversity (Box 2-1); it is a virtual certainty that many insect pollinators have yet to be discovered and identified. Notwithstanding the existence of taxonomic impediments, a substantial body of information is available on pollinator population trends. The quality of this information, however, varies with taxon as, accordingly, do conclusions about the status of pollinators in these groups.
Ants, Bees, and Wasps (Order Hymenoptera)
The order Hymenoptera is a diverse and economically important group of approximately 125,000 described species comprising plant-feeding sawflies, parasitic and nonparasitic wasps, ants, and bees (Zayed and Packer,
|
BOX 2-1 Diversity and the “Taxonomic Impediment” Insects account for more than half of the estimated 1,586,800 species that have been formally described by scientists (Grimaldi and Engel, 2005). The most current estimates of species undescribed or unknown to science range from 10 million to 30 million (Grimaldi and Engel, 2005; Stork, 1988, 1996); and many of the most species-rich groups are among the least thoroughly characterized. Because of a lack of available expertise, it is often impossible to identify (or “determine”) specimens. Taxonomy and its applied interface, identification, are fundamental to continuing the study and conservation of organisms. As knowledge of living systems grows more comprehensive, the scientific community demands more from taxonomy than simply identifying which species to avoid and which are edible or otherwise useful. That the rate at which species are becoming extinct appears to exceed the rate at which new species are described (Hambler and Speight, 1996) poses not merely an academic problem but a daunting challenge to understand biodiversity with economic potential before it disappears. The problem applies to the study of plant-pollinator interactions in North America as some pollinating insects, particularly beetles and flies, are yet to be discovered and described. The Global Taxonomic Initiative is attempting to reduce the bottleneck in taxonomic research resources in the face of what has been called the greatest extinction crisis in roughly 60 million years (J.A. Thomas et al., 2004). Under |
2005). The order includes within its ranks the principal managed pollinators of the world, bees in the genera Apis, Bombus, Megachile, Osmia, and Melipona, as well as numerous unmanaged species of bees (Box 2-2) and wasps that represent a variety of families.
Honey Bees (Apis mellifera)
Nearly 17,000 species of bees have been formally described, and as many as 30,000 are estimated worldwide (Michener, 2000; T. Griswold, U.S. Department of Agriculture [USDA] Bee Biology and Systematics Laboratory, presentation to the committee, October 18, 2005). Although other species are often more efficient pollinators than are honey bees on a flower-by-flower basis, honey bees are, for many reasons, the pollinator of choice for most North American crops. A. mellifera is highly suitable as a commercial pollinator because of its biology (Hoopingarner and Waller, 1992;
|
the leadership and authority of the Convention on Biological Diversity, the initiative has two aims: (1) to increase the efficiency of biological systematics, and (2) to bolster the number of practicing, professional systematists. Critical to the development of greater understanding is a supply of professional taxonomists, usually university-trained scientists with doctorates in their disciplines. The taxonomic impediment is far from an insoluble problem. The Consortium for the Barcode of Life is an international initiative to develop DNA barcoding as a global standard for identifying specimens. DNA barcoding uses a short gene sequence from a specific region of a genome as an identifying marker for a species (http://barcoding.si.edu). DNA barcoding promises to provide a rapid and inexpensive means of identifying specimens by matching barcode sequences with those of taxonomically validated vouchers. In the United States, steps to ameliorate the shortage of professionals include the highly successful program of the National Science Foundation (NSF) Partnerships for Enhancing Expertise in Taxonomy, which supports taxonomic research and training (Rodman and Cody, 2003). Assembling the Tree of Life—another NSF effort—involves advanced molecular and optical technology, readily disseminated Web-based initiatives, and increasingly advanced analytical software. Whether the federal govenment will continue to support and expand such programs is an open question. Hence, the first challenges to solving the taxonomic impediment in North America and globally are to assess available resources and identify the support and resources needed to reduce or eliminate taxonomic impediments. |
Winston, 1987). In contrast to most other species of bees that have annual nests founded by individual, overwintered females each spring, honey bee colonies are perennial. Honey bee populations range between 10,000 and 30,000 individual worker bees, even at their nadir in late winter and early spring. Thus, honey bee colonies are able to muster large numbers of pollinators when they are needed for late winter and early spring blooms, as well as throughout the rest of the growing season. As a generalist, the honey bee can pollinate many agricultural crops, including almond and blueberry. Because it forages over long distances (up to 14 km from its nest), it is useful in expansive monocultures where wild bees of other species with more limited foraging ranges are restricted to field margins. In addition, honey bees exhibit sophisticated communication, which increases foraging efficiency, and floral constancy; individuals repeatedly visit a single plant species during each foraging trip and can recruit nestmates to flowers of that species (von Frisch, 1967). Thus, honey bees’ behavior increases the
|
BOX 2-2 Sociality and Bee Pollination Of the nearly 17,000 described species of bees (Michener 2000), the vast majority are solitary. Each female makes her own nest and cares for her own offspring. Among the species of pollinators that are actively managed, Megachile rotundata, Nomia melanderi, Osmia cornifrons, and Osmia lignaria all exhibit this solitary lifestyle. The other species of bees that are actively managed for pollination in North America, Apis mellifera and various species of Bombus, are “eusocial.” Eusociality is defined by three traits: (1) cooperative care of young by members of the same colony; (2) reproductive division of labor, with more or less sterile individuals (“workers”) working on behalf of fecund colony members (“queens”); and (3) an overlap of at least two generations of adults in the same colony (Michener, 1969; Wilson, 1971). Eusociality is the most extreme form of social organization in the animal kingdom (Wilson, 1971). It is relatively rare, limited to termites (order Isoptera), several groups of Hymenoptera (all ant species and several lineages of bees and wasps), and a few species of aphids, thrips, beetles, shrimp, and mammals (Crespi and Yanega, 1995; Sherman et al., 1995). Eusociality plays a prominent role in pollinator behavior, especially in the case of the honey bee. Division of labor for reproduction lies at the heart of eusociality. Hymenoptera display the haplodiploid mode of sex determination; fertilized diploid eggs develop into females and unfertilized haploid eggs develop into males. Females can develop into either queens or workers. Queens specialize in reproduction, laying up to several thousand worker eggs per day. Workers engage in little if any personal reproduction, and perform all tasks related to colony maintenance and growth, including foraging. Eusocial species are divided into two groups: primitively eusocial and advanced eusocial. In most primitively eusocial species, colonies have annual life cycles and populations are relatively small, typically a few dozen to a few hundred individuals. There are no morphological differences between queens and workers, but there can be differences in physiology and size. Division of labor for reproduction is achieved by a dominance hierarchy that is established and maintained by direct behavioral mechanisms, including pushing, biting, and physical prevention of egg laying. Aggression is a common occurrence in a primitively eusocial colony. Bumble bee species exhibit a primitively eusocial lifestyle. In advanced eusocial species, colonies are typically perennial and populations number in the thousands to even millions of individuals. Queens and workers are distinguished by striking morphological differences. In advanced eusocial species, queen inhibition of worker reproduction is achieved by chemical communication—queen pheromones—rather than by direct physical aggression. In advanced eusocial species, the fate of an individual—queen |
|
or worker—is determined before adulthood, and there is far less dominance-related aggression among individuals than in other animal societies. This sets the stage for natural selection, acting on the phenotypes of colonies, to fashion systems of division of labor among groups of highly specialized workers and intricate forms of communication to integrate their activities. Honey bees exhibit an advanced eusocial lifestyle. Several aspects of eusociality contribute to the value of the honey bee as a commercial pollinator: (1) Perennial colonies result in large forces of foraging worker bees, especially early in the growing season, when pollination is required for many crops. Noneusocial species, with annual population cycles, have far smaller populations early in the growing season. (2) Foraging in honey bee colonies is based on division of labor. There is an age-related division of labor among worker honey bees, which is based on a process of behavioral maturation (Robinson, 1992). After working in the hive for 2 to 3 weeks, worker honey bees specialize in foraging for the remainder of their 4- to 7-week adult life. They take about 10 foraging trips per day and log up to 800 km over the course of their foraging career (Winston, 1987). Workers become more efficient at foraging with experience (Dukas and Visscher, 1994), which likely increases their efficacy as pollinators. (3) Foraging in honey bee colonies also is enhanced by communication. Foragers communicate the location of particularly rewarding food sources by means of the famous “dance language,” elucidated by Nobel laureate Karl von Frisch (1967), the only nonprimate symbolic language. Honey bees are thus able to rapidly and effectively direct their foraging force toward a particular field or orchard in bloom. This can enhance pollination by mobilizing a large group of foragers during what is sometimes a relatively short window of opportunity. Pollination often is constrained temporally by floral phenology or adverse weather conditions that limit bee flight (Delaplane and Mayer, 2000). Other traits that enhance the value of A. mellifera as a pollinator are described in the section entitled “Honey Bees (Apis mellifera)” in this chapter. Other species of bees display levels of sociality that are intermediate between solitary and eusocial. “Communal” species nest in aggregations but do not display any of the three defining traits of eusociality. Megachile rotundata nests in aggregation, which facilitates their use as an actively managed pollinator. “Quasisocial” species display cooperative brood care, but no reproductive castes or generational overlap. “Semisocial” species display cooperative brood care and reproductive castes, but no generational overlap. Some species exhibit different levels of sociality during different phases of the colony lifecycle. A bumble bee colony, for example, is established by a single individual, acting in a solitary manner. When the first brood emerges and assumes responsibility for all colony activities except egg laying, the colony then becomes primitively eusocial. Bumble bee colonies are most valuable for pollination during the eusocial phase, when they have an active group of worker foragers. |
efficiency of pollination by ensuring that compatible pollen is transferred among conspecific flowers when needed.
Perhaps of greatest significance to the economic importance of A. mellifera is that apiculture—the management of honey bees—is a highly developed discipline that has made bees and beekeeping equipment widely available. Honey bees have been used in North Amercia to provide pollination services for crops in bloom in extensive areas. Typically, one-quarter to one-third of workers in a colony during flight season are foragers. Honey bees can be concentrated in very high densities, which are required for effective pollination in large monocultures with extremely high floral densities, and they can be transported by truck to any location at any time crops are in bloom. Finally, because honey bees can be cared for and maintained by humans, they are buffered to some extent from declines in environmental quality.
Honey bee populations have followed different trends in the three North American nations. In the United States, data from the USDA National Agricultural Statistics Service (NASS) reveal declines in the number of honey bee colonies producing honey during 1947–1972 and 1989–1996 (Figure 2-1) (USDA-NASS, 1995, 1999, 2004a, 2005, 2006a). Overall, the number of managed colonies dropped from 5.9 million in 1947 to 2.6 million in 1996–2004. That number fell again in 2005 to 2.4 million. The decline from 1985 to 1996 is likely linked to the occurence of the tracheal mite,
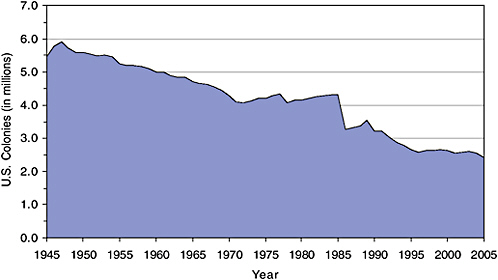
FIGURE 2-1 U.S. honey bee colonies, 1945–2005. Data compiled from USDA-NASS (1995, 1999, 2004a, 2005, 2006a).
Acarapis woodi (first detected in 1984) and to the Varroa destructor mite (first detected in 1987) (Chapter 3). The precipitous drop between 1985 and 1986 also is attributable to a change in NASS survey methods that could represent a statistical artifact. After not collecting data from 1982 to 1985 (official data were based on estimates only), NASS changed its surveys to exclude beekeepers who had fewer than five colonies.
Assuming that estimates of honey bee pollination activity in agricultural crops would be improved by more accurate information on total commercial honey bee colony numbers, the U.S. data have four limitations, most of them linked to the NASS focus on honey production. First, the surveys count only honey bee colonies from which commercial honey is harvested; those that exclusively provide pollination services are not counted. Second, the same hives can be counted in several states if commercial honey is harvested in more than one state. Third, annual data are no longer collected on the number of colonies held by beekeepers who own fewer than five hives. Finally, no data are collected on colony health, a factor that has become more important since the parasitic mite invasions of the 1980s (Chapter 3). NASS also surveys beekeeping operations every 5 years for its census of agriculture (USDA-NASS, 2004a). The 2002 census included all honey bee colonies and reported them “in the county where the owner of the colonies’ largest value of agricultural products was raised or produced” (USDA-NASS, 2004a), thus addressing the first three limitations above. However, the agricultural census data are taken less frequently and the variable definitions are incompatible with the annual honey survey data.
In contrast to the declines in the United States, Canada had important periods of growth in honey bee colony numbers between 1955 and 1986 and from 1996 to 2005 (Figure 2-2) (Statistics Canada, raw data and 2006). As in the United States, there was a decline after the period of mite invasions in the late 1980s, and the Canada-U.S. border was closed to the importation of live bees in 1987 to prevent the spread of mites from the United States to Canada (Saskatchewan Agriculture and Food, 2004). Statistics Canada collects data on honey bees kept for pollination and on those that produce honey (Statistics Canada, 2006), but there are some inconsistencies in data collection practices across provinces.
Honey bee colony data from Mexico, available only for 1990–2003, show a decline in the total from 2.1 million colonies to 1.7 million colonies between 1990 and 1997 (SIAP, 2005). With minor fluctuations, colony numbers remained stable at 1.7 million during 1997–2003. Mexican honey production data are available for a longer period, 1980–2002, but those data do not show any clear trend. Honey production in the principal states of Yucatán, Campeche, Veracruz, Jalisco, Guerrero, and Quintana Roo has fluctuated from 42,000 to 75,000 metric tons, leveling off at 57,000– 59,000 metric tons in 2000–2004 (Ortega-Rivas and Ochoa-Bautista,

FIGURE 2-2 Canadian honey bee colonies, 1945–2005. Data compiled from StatisticsYear Canada (raw data and 2006).
2004; SAGARPA, 2005). Details on data collection procedures were not available.
In contrast to honey bees reared for commercial pollination, feral honey bees are not well studied (Buchmann and Nabhan, 1996; Hoopingarner, 1991). Because honey bees are not native to North America, feral honey bee populations (like those that are actively managed) represent races introduced to the United States from eastern and western Europe and from Africa since the 1620s (Schiff et al., 1994). Schiff et al. (1994) studied the genetic diversity of feral honey bee populations in the southern United States and found that 61 percent of the 692 colonies assessed were maternal descendants of the European races most commonly used for commercial pollination.
Few studies have examined the population status of feral honey bees over time. The USDA Carl Hayden Bee Research Center has data on the survival of feral honey bee colonies in southern Arizona that span 19 years (Loper et al., 2006). Loper found that feral honey bee colonies in this area were decimated by tracheal mites in 1990 and varroa mites in 1995 (Loper, 1995, 1996, 1997). In 2002, Seeley (2003) repeated a survey of feral honey bee colonies of Arnot Forest, New York, that he conducted with Visscher in 1978 (Visscher and Seeley, 1982). He found that the number of honey bee colonies was about the same in 1978 and 2002. Kraus and Page (1995) studied the spread of varroa mites within California’s population of feral honey bees. In 1990, a sample of bees from 208 colonies located in feral hives revealed no varroa mites. In 1993, a survey of 124 of the same
feral hives revealed 75 percent of these colonies no longer existed, and all surviving colonies were infested with varroa. The proximity of these feral colonies relative to commercial apiaries led Kraus and Page (1995) to suggest that the mites moved from commercial to feral colonies. Other than these studies, the committee is not aware of other surveys of feral honey bee colonies in North America.
Bumble Bees (Bombus spp.)
Approximately 239 bumble bee species are known worldwide (Williams, 1998), 49 of them in the United States. The 41 species in Canada are also all found in the United States. Twenty species are known in Mexico, nine of them also present in the contiguous United States (R.W. Thorp, University of California, Davis, presentation to the committee, January 14, 2006; personal communication, March 2006). Some species of bumble bees (Bombus impatiens and B. occidentalis) have been managed primarily for pollinating greenhouse tomatoes (Dogterom et al., 1998). In contrast with managed honey bee colonies (Apis mellifera) in the United States and Canada—for which agricultural monitoring agencies often have long-term records of honey bee colonies (Figure 2-1)—data on managed bumble bee colonies are not collected in the United States, Canada, or Mexico.
Native bumble bees pollinate wild flowers and serve as alternative or complementary pollinators for some crops, such as watermelon and cucumber (Stanghellini et al., 1996a,b). Although many native bumble bee species in the United States were once common, entomologists and naturalists have been noting declines and regional absences of some species within the past decade. The Xerces Society for Invertebrate Conservation has placed four bumble bee species (Appendix H) on its Red List of at-risk pollinator insects of North America (Shepherd et al., 2005).
Bombus (Bombus) franklini (Frison, 1921), the Franklin bumble bee, is (or was) an endemic species with the most restricted geographic range of any bumble bee in North America and possibly the world (Williams, 1998). Its range, known at one time to span from southwest Oregon to northwest California, encompasses a distance of 241 km north to south and 112 km east to west. Within that area, B. franklini could be found at elevations from 162 m in the north to above 2,340 m in the southern portion of its historic range. B. frankilini is thought to have become extinct recently in its native range of the U.S. Pacific Northwest (Buchmann and Ascher, 2005; Shepherd et al., 2003, 2005). Thorp (2003, 2005) first began to notice and document a precipitous decline in B. franklini at numerous localities in 1988. Extensive searching by R.W. Thorp and his colleagues over the last 4 years has failed to re-locate B. franklini populations or individuals across that region (Thorp, 2003). B. franklini is now treated as a “species of concern” or a “special
status species” by the U.S. Fish and Wildlife Service, the California Natural History Data Base, and the Oregon Natural Heritage Information Center. It also appears on the Xerces Society Red List (Appendix H; Shepherd et al., 2005).
B. occidentalis, at one time commonly observed in central California, began in the late 1990s to disappear from most of its known geographic range. Thorp (2003, 2005) conducted extensive searches for B. occidentalis and reported that it is now extremely rare in habitats where it was formerly common. The species is still present in some parts of its range, such as the Colorado Rocky Mountains; it is still relatively common near the Rocky Mountain Biological Laboratory as of 2006 (D. Inouye, University of Maryland, personal observation, 2006).
B. affinis apparently disappeared from northern New York state about 1998 and from southern New York before 2004 (Day, in preparation; J. Ascher, American Museum of Natural History, personal communication, March 2006). John Ascher of the American Museum of Natural History reports that B. affinis was common on the Cornell University campus between 1996 and 1998, but that he and other entomology students and faculty have not observed it since 2001. Despite collecting more than 1,200 bumble bees in the Black Rock Forest of New York during 2003, Giles and Ascher (2006) failed to find any specimens of B. affinis.
Because there is no long-term monitoring or corresponding baseline data for bumble bees or other species of wild non-Apis bees in the United States, Canada, or Mexico, the population status of bumble bees cannot be determined definitively in North America. The United Kingdom, in contrast, has a long and well-established tradition of monitoring by scientists and naturalists. Extensive standardized monitoring protocols are followed across a grid system covering the entire United Kingdom. The Bees, Wasps and Ants Recording Society was established in 1978 expressly to allow “anyone of any age or experience with an interest in aculeates” (ants, bees, and stinging wasps) to contribute to a recording scheme designed to obtain “proper, well coordinated data on the distribution and habitats of many species in order to support conservation programmes, ecological research, and to promote effective conservation strategies on a national basis” (http://www.searchnbn.net/organisation/organisation.jsp?orgKey=222). The ALARM project (Assessing Large Scale Risks for Biodiversity with Tested Methods) was established in 2004 with the objective of assessing changes in the richness, abundance, and distribution of pollinators across Europe (Box 2-3). This project and several other studies show that decline in species richness, frequency, and distribution of bees is evident (Box-2-4; Goulson et al., 2005; Westrich 1989, 1996) if these parameters are carefully monitored or observed. Records of species richness, frequency, and distribution of bees in North America are few in number.
|
BOX 2-3 The ALARM Project Changes in Bee and Flower Fly Richness, Abundance, and Distribution Documented In 2004, the ALARM project (Assessing Large Scale Risks for Biodiversity with Tested Methods) was initiated and funded as part of the United Nations Food and Agriculture Organization. Details of the program are presented in Chapter 5, but one component relevant to this discussion is the effort to quantify distribution shifts in keystone pollinator groups across Europe. ALARM researchers conducted an extensive before-and-after 1980 repeat survey of native bees and flower flies in the United Kingdom and the Netherlands (Biesmeijer et al., 2006). Each country was divided into 10 × 10 km grid cells and species richness and abundance were analyzed on the basis of more than 500,000 authenticated records. A landscape-level rarefaction analysis (Colwell et al., 2004; Krebs, 1999; Magurran, 2004) was conducted from the United Kingdom and Netherlands data sets and analyzed with EstimateS 7.5 software (Colwell, 2005). The results of these first comprehensive national surveys of pollinators show widespread decline of bees and syrphid flies. Species richness of bees declined in about 40 percent and 60 percent of the grid cells in the United Kingdom and the Netherlands, respectively. In the United Kingdom, 18 percent of the grid cells showed increases and 45 percent had the same species richness. The national data from the two countries allowed researchers to ask whether individual pollinator species were changing in distribution and abundance. Of the 229 bee species assessed in the United Kingdom and the 201 species assessed in the Netherlands, more showed declines in abundance from before to after 1980 (based on the total number of times each species was recorded across all grid cells in which they were present in those two periods) than stayed stable or decreased. In the Netherlands, the number of species that showed area loss (range contraction) was substantially higher than the number that showed area gain (range expansion), with an overall significant decrease in occupancy. In contrast, no clear trends in range changes were observed in the United Kingdom. When abundance and range changes were considered together, there were statistically significant declines in native bees in the United Kingdom and the Netherlands (Biesmeijer et al., 2006). The findings are consistent with studies of status for butterflies (Asher et al., 2001; J.A. Thomas et al., 2004), for bumble bees (Rasmont et al., 2006), and for native European bees (Banaszak and Kosior, unpublished data; Sárospataki et al., 2005). SOURCE: Biesmeijer et al., 2006. |
|
BOX 2-4 European Bee Surveys and Population Declines A United Nations Food and Agriculture Organization report, The Survey of Wild Bees (Hymenoptera, Apoidea) in Belgium and France (Rasmont et al., 2006), presents evidence of pollinator declines among diverse taxa of bees native to those countries during the past 30 years. The Belgian Mons and Gembloux government laboratories published their first report on native bees of Belgium and France in 1980 (Leclercq et al., 1980), listing 13 species of the superfamily Apoidea at risk in Belgium, Luxembourg, and northern France. A subsequent report (Rasmont and Mersch, 1988) presented information on the faunistic drift of Belgian bumble bee species; that report cites the first red list of threatened insects in the Belgian fauna. The 1988 report was updated and revised in 1993 (Rasmont et al., 1993) to include general findings about the entire Belgian bee fauna of more than 300 species. After 1993, research teams at the two Belgian laboratories expanded their bee survey efforts to other areas and countries, including France (Pauly, 1999; Rasmont and Adamski, 1995; Rasmont and Gaspar, 2002; Rasmont et al., 1995), Morocco (Rasmont and Barbier, 2003), Turkey (Rasmont and Flagothier, 1996), and even Madagascar (Pauly et al., 2001). Rasmont and his colleagues have prepared biogeographic, faunistic surveys and taxonomic revisions of bee genera at continental scales (Andrenidae: Patiny, 1998; Patiny and Gaspar, 2000; Anthophoridae: Terzo and Rasmont, 2004; Melittidae: Michez et al., 2004a,b; Terzo and Ortiz-Sanchez, 2004). To date, 360 species of bees have been reported in Belgium; of these, 330 species of solitary bees (Apoidea) were documented as occurring within Belgium between 1900 and 1992 (Rasmont et. al., 2006). In that period, 91 species (25.2 percent) were decreasing in abundance, 145 (40.2 percent) remained relatively stable, 39 (10.8 percent) had expanded their ranges, and 85 species (23.5 percent) were placed in an undetermined situation as rare species. These data were collected from more than 48,000 records and 79,765 specimens. Results from the study published by Rasmont and colleagues (2006) confirmed that 13 of the same species were decreasing as had been reported by Leclercq et al. (1980). Rasmont and colleagues (2006) also reported a sharp contraction in most cleptoparasitic (cuckoo bee) species and hypothesized that their bee host species had declined. The report also discussed a decline among mason bees (Osmia spp.) that use snail shells as nesting substrates. The same observation had been reported earlier by Westrich (1990). With respect to the overall pattern of change, Rasmont and colleagues (2006) state: |
|
In an untouched wild bee fauna, the isolated regression of a long-tongued species could probably be ecologically compensated for by the spontaneous substitution of competing species. However, in the present situation, it is the whole guild that is threatened. Therefore, we may fear that the linked regression of all species will not allow a spontaneous replacement. It is likely that the density and the diversity of these pollinators fall under the population level needed to insure the pollination requirement of many agricultural and horticultural productions. Moreover, the regression of key species would lead to the disappearance of great parts of the wild flora. Rasmont and colleagues (2006) make a special case to discuss the plight of bumble bees in Europe. They reported a continental decline in abundance in several bumble bee species. The most dramatic case was for Bombus cullumanus (Kirby). At the start of the 20th century, B. cullumanus was seen as far north as southern Sweden and was abundant in northern Germany, the Netherlands, and England. It had been observed in large cities, such as Brussels and Paris, since the 1950s. By the 1990s, it had disappeared from most of its former range. Today, it can be found only in a few dozen localities in the West and East Pyrénées and in western portions of the Massif Central mountains, and it is now rare wherever it is found (Rasmont et al., 1993). The authors reported that other bumble bees—B. confusus Schenck, B. distinguendus Morawitz, and B. sylvarum (L.), all long-tongued species associated with leguminous floral hosts—also experienced contractions within their respective ranges. A few, however, have actually expanded their ranges. B. semenoviellus (Skorikov), originally known from Moscow, can be now found in northern Germany and is expected to colonize the Netherlands and Belgium in the next few years. Another formerly common, now rare, species is the leafcutting bee Megachile parietina (Goeffroy) (= Chalicodoma muraia Retzius) in France. Toward the end of the 19th century, when Jean-Henri Fabre was writing his famed Souvenirs Entomologiques, this leafcutting bee was common enough to be considered a pest in buildings. It has become rare throughout France, for unknown reasons (Rasmont and Barbier, 2003). M. parietina is a visitor to legume flowers, so its population decline could be symptomatic of a larger problem among native leguminous plants and their pollinators in France and Belgium. A rare carpenter bee, Xylocopa cantabrita Lepeletier, was formally known only from a few localities in France and Spain. Terzo and Rasmont (2004) relocated that rare bee during recent faunal surveys. |
Mexican Stingless Bees (Melipona and Trigona)
Stingless bees, species in the tribe Meliponini, comprise about 400 species found in neotropical and paleotropical forests (Roubik, 1989). These social bees form long-lived colonies, make and store honey, and are important pollinators of forest trees and crops. For millenia, ancient and modern Mayan peoples of southernmost Mexico and neighboring countries have kept the meliponine bees of their tropical forest environments in hollow log hives (“jobones”) in or near villages. Stingless bees have supplied the Maya with honey for food and for medicine and with beeswax as a sealant and art material (Crane, 1999).
Mayan beekeepers have traditionally searched for stingless bee colonies in the low tropical forests of the Yucatán, traveling several kilometers from their villages to locate wild colonies of Melipona and Trigona. Today, beekeepers must travel even farther into smaller patches of remnant forests of large-diameter trees (used by stingless bees for nesting cavities) to find Melipona colonies (Villanueva, personal communication, March 2006). In the past, bee houses with traditional log hives contained dozens or hundreds of colonies, and beekeepers knew how to divide and reproduce colonies (Villanueva-Gutierrez et al., 2005). It is now uncommon to find a beekeeping operation with more than five colonies of Melipona in villages near Felipe Carillo Puerto, Quintana Roo (Vilanueva-Gutierrez and Roubik, personal communication, March 2006).
Ancient Mayan beekeeping is disappearing because of habitat fragmentation and intensive apiculture. A recent survey (Villanueva-Gutierrez et al., 2005) documented that 93 percent of the managed Melipona and Trigona stingless bee colonies in the Yucatán Peninsula have been lost during the past quarter-century (Table 2-1). Hurricanes in the past two decades have also decimated feral and managed colonies of Yucatecan stingless bees. Villanueva-Gutierrez and colleagues predict there will be no managed colonies of Melipona and Trigona in Yucatecan Mayan lands by 2008 unless action is taken to maintain the bee colonies (Villanueva-Gutierrez et al., 2005).
Sixteen species of gentle native bees are found within the Yucatán Peninsula, where they pollinate forest trees, Mayan dooryard gardens, and milpa agricultural lands. There are no studies on population trends of feral meliponines, although Búrquez (2003) examined the distributional limits of meliponines in northwestern Mexico. They are not present in the United States or Canada.
Orchid Bees (Euglossa, Eufriesea, Exaerete, Eulaema)
Orchid bees (family Apidae, tribe Euglossini) constitute a natural group of approximately 250 brilliant metallic-green, blue, and red species from
TABLE 2-1 Number of Melipona Hives That Beekeepers from the Mayan Zone (near Felipe Carillo Puerto, Quintana Roo, Mexico) Have Kept During the Past 54 Years
|
Name of Mayan Community |
Name of Beekeepers or Institutions |
Number of Hives Kept Between 1950 and End of 1981 |
Number of Hives Kept at End of 1990 |
Number of Hives Kept at End of 2003 |
|
Chan Santa Cruz |
Delfino Naal |
Unknown |
8 |
2 |
|
Chan Santa Cruz |
Nemesio Pot |
Unknown |
12 |
8 |
|
Chancá de Repente |
Bernardo Peña |
42 |
25 |
8 |
|
Chancá de Repente |
Anastasio Perez |
Unknown |
10 |
0 |
|
Chancá de Repente |
Eduardo Yam |
Unknown |
5 |
0 |
|
Chunyá |
Patricio Canul |
45 |
30 |
8 |
|
Felipe Carrillo Puerto |
Instituto Nacional Indigenista |
0 |
40 |
11 |
|
Miztequilla |
Santiago Pat |
Unknown |
6 |
2 |
|
Miztequilla |
Fernando Yam |
40 |
19 |
8 |
|
Naranjal |
Francisco Cimá |
25 |
15 |
3 |
|
Naranjal |
Juán Mena |
26 |
12 |
6 |
|
Nueva Loría |
Celestino Camal |
Unknown |
7 |
2 |
|
Nuevo Israel |
Ponciano Tun |
Unknown |
6 |
0 |
|
Palmas |
Margarito Tuz |
220 |
5 |
0 |
|
Presidente Juárez |
Bernabé Kantún |
Unknown |
16 |
4 |
|
San Hermenegildo |
Humberto Ku Cauichl |
60 |
40 |
0 |
|
Santa María |
Francisco HuiCab |
50 |
37 |
7 |
|
Señor |
Doroteo Pech |
22 |
18 |
0 |
|
Señor |
José Pott |
Unknown |
6 |
0 |
|
Tihosuco |
Pedro Cahun Uh |
Unknown |
5 |
15 |
|
Tuzic |
Isidro Peña Tuz |
200 |
40 |
8 |
|
X hazil |
Modesto Chuc |
10 |
7 |
0 |
|
X hazil |
Isaías Cahuich |
15 |
10 |
0 |
|
Yo Actún |
Rancho San Martín |
Unknown |
10 |
4 |
|
Totals |
|
Likely >1000 |
389 |
96 |
|
SOURCE: Villanueva-Gutierrez et al., 2005. |
||||
the neotropical forests of Mexico south into Central and South America (Cameron, 2004; Michener, 2000; Roubik and Hanson, 2004). Although an errant Eulaema male was discovered in the United Status (Minckley and Reyes, 1996), orchid bees live in neotropical forests and can be found in Sonora and Sinaloa (Alamos region) and farther south, including the rainforests of Quintana Roo, Mexico. Euglossines historically have comprised up to 25 percent of the total bee fauna in many intact neotropical forests, and they are thought to be excellent indicators of disturbance (Roubik and Hanson, 2004; Roubik, personal communication, March 2006). Within intact forests, however, euglossine populations are extremely stable, and some species might even be increasing (Roubik, 2001).
TABLE 2-2 Examples of Bees in North America and Some of the Plants They Visit and Pollinate
|
Common Name |
Scientific Name |
Examples of Crop Plants Pollinated |
|
Alkali bee |
Nomia melanderi |
Alfalfa, clover, mint |
|
Blueberry bee |
Habropoda laboriosa |
Blueberry |
|
Carpenter bee |
Xylocopa spp. |
Passion flower, eggplant, pepper |
|
Digger bee |
Andrena, Colletes, and Melissodes spp. |
Cotton, fruit trees |
|
Alfalfa leafcutting bee |
Megachile rotundata |
Alfalfa |
|
Blue Orchard bee (a mason bee) |
Osmia lignaria |
Almond, apple, sweet cherry |
|
Squash and gourd bee |
Peponapis pruinosa other Peponapis and Xenoglossa spp. |
Squash, pumpkin, gourds |
|
Sunflower bee |
Eumegachile pugnata |
Sunflower |
Other Bees
Other than honey bees and bumble bees, more than 3,500 species of solitary bees pollinate crops and wild plants in North America. Examples of native bee pollinators include alkali bees, squash bees, and leafcutting bees (Table 2-2). A number of native bee species are rare and have narrow geographic ranges or have not been collected for many decades. The Xerces Society Red List for bees is presented in Appendix H, Table H-2.
Ants
All 11,844 named ant species are eusocial, ecologically important insects (Agosti and Johnson, 2005; Hölldobler and Wilson, 1990, 1994; Wilson and Hölldobler, 2005). Common in most plant communities, ants visit plants and collect sugars. Interactions with flowering plants involve mutualisms that include dispersing seeds, visiting extra-floral nectarines, serving as guards against seed predators or herbivores, and in some cases pollinating flowers (Beattie, 1985; Boucher, 1985; Bronstein, 1944a,b; Bronstein and McKey, 1998; Buckley, 1982; Huxley and Cutler, 1991; Janzen, 1985; Rico-Gray and Oliveira, 2006; Thompson, 1982, 1994, 2005; Wilson and Hölldobler, 2005). Although ants are collectively involved in thousands of interactions with plants and are commonly observed on flowers, they pollinate few species (about 20) of angiosperms (Table 2-2; Beattie, 1985; Peakall et al., 1991; Rico-Gray and Oliveira, 2006; Thien and Rico-Gray, 2004). Nevertheless, many ant-plant mutualisms other than pollination greatly increase the reproductive success of plants and ants.
The 20 flowering plants (18 genera, 9 families) pollinated by ants (Table 2-3) occur in a variety of habitats throughout the world. Of the
TABLE 2-3 Plant Species in North America Pollinated by Ants
|
Species |
References |
|
Caryophyllaceae |
|
|
Arenaria tetraquetra |
Gómez et al., 1996 |
|
Gypsophyla |
Gómez et al. 1996 |
|
Paronychia pulvinata |
Puterbaugh, 1998 |
|
Crassulaceae |
|
|
Diamorpha smalli |
Gomez et al., 1996; Wyatt, 1981; Wyatt and Stoneburner, 1981; |
|
Brassicaceae |
|
|
Lobularia maritime |
Gómez, 2000 |
|
Euphorbiaceae |
|
|
Euphorbia cyparissias |
Schurch et al., 2000 |
|
Orchidaceae |
|
|
Mirabilis nyctaginea |
Cruden, 1973 |
|
Epipactis palustris |
Brantjes, 1981; Nilsson, 1978 |
|
Maxillaria parviflora |
Singer, 2003 |
|
Polygonaceae |
|
|
Eriogonum pelinophilum |
Bowlin et al., 1993 |
|
Polygonum cascadense |
Hickman, 1974 |
|
Orthocarpus pusillus |
Kincaid, 1963 |
16 subfamilies of ants, the Ponerinae, Myrmicinae, Formicidae, and Dolichoderinae (Grimaldi and Engel, 2005; Wilson and Hölldobler, 2005) are commonly involved in plant interactions (more than 33 genera; antagonistic and mutualistic) (Rico-Gray and Oliveira, 2006).
There are no databases that monitor fluctuations in ant populations. Recent studies, however, have documented population shifts of groups of organisms that include ants (Christian, 2001; Forys and Allen, 2005; Haugaasen et al., 2003; Morrison, 2002; Richardson et al., 1996; Roberts et al., 2000; Torres and Snelling, 1997). Currently, the Conservation International Tropical Ecology, Assessment, and Monitoring project (http://www.teaminitiative.org) tracks litter and ant diversity in Costa Rica (Agosti et al., 2000; J.T. Longino, Evergreen State College, personal communication, October 2005). An excellent source of information on ant databases in general is http://antbase.org, a website maintained jointly by the American Museum of Natural History and the Ohio State University.
Wasps
Most of the approximately 100,000 described wasp species worldwide are carnivorous, preying on other insects. Many others are parasites, laying their eggs on or inside immature insects or other living hosts. The large
“digger wasp” family (Sphecidae) contains approximately 9,550 species, most of which are carnivorous, seeking out insect prey to provision their larval cells (http://www.calacademy.org/research/entomology/Entomology_Resources/Hymenoptera/sphecidae/Number_of_Species.htm). Although sphecids are not always hairy or good pollen vectors, many adults that visit flowers to search for nectar might serve as pollinators. Similarly, some species within the large spider wasp family (Pompilidae) seek out flowers for nectar. Their spiny legs pick up and transport the specialized pollinia of milk-weed plants (Asclepias spp.) and they can be effective pollinators (Kephart and Theiss, 2003). One group of large wasps that has adopted a flower-visiting, pollen-collecting, larval-provisioning lifestyle includes the subfamily Masarinae within the family Vespidae. Two genera (Pseudomasaris, with 14 species limited to the western United States, and Euparagia with 6 species) are found in the United States. Euparagia species visit Eriogonum blossoms for nectar, but provision nests with paralyzed weevil prey items. Unlike bees, Pseudomasaris wasps actively harvest pollen and nectar and then provision their underground brood cells with pollen or nectar instead of live or paralyzed arthropod prey (spiders, pompilid wasps, weevils). Many masarid species have elongate mouthparts that are specialized for extracting nectar from long tubular blossoms. In the southwestern United States, species of Pseudomasaris are oligolectic (their pollen foraging is restricted to particular genera or to a single genus within a plant family), and they provision their nests with pollen and nectar largely from flowers of plants within the families Hydrophyllaceae, Polygonaceae, and Scrophulariaceae. They are principal pollinators of species of beardstongue (Penstemon spp.), including the endangered P. grahamii (http://www.epa.gov/fedrgstr/EPA-SPECIES/2006/January/Day-19/e363.htm), Phacelia, Hydrophyllum, and Eriodictyon. Pseudomasarines are important to the ecology of these wildflowers, and they play ecosystem service roles in the southwestern United States and Mexico. Within the southwestern United States, several masarid species are restricted to highly localized habitats or have not been collected in decades (Richards, 1963, 1966). Pseudomasaris micheneri is known only from the Inyo Mountains of Inyo County, California (Westgard Pass), and only from collections made during the 1930s and 1940s. It has not been collected since then. Pseudomasaris macneilli is known from only two locations in northern California (Trinity Alps) and one location in Utah. Those collections were made during 1951 and 1961. Similarly, Pseudomasaris macswaini has a localized distribution and could be at risk (Richards, 1963, 1966). Further exploration and collection in known habitats when Phacelia and other floral hosts are in bloom will be necessary to determine the current status of masarine populations in North America.
An example of pollinator-plant interdependence involves the so-called fig wasps. The term “fig wasp” has been broadly applied to many plant-
feeding chalcid wasps (Chalcidoidea) that have mutualistic relationships in inflorescences (synconia) of fig trees (Ficus, Moraceae). There could be several hundred such species, although many are still undescribed (Weiblen, 2002). Formerly, these wasps were all placed within the family Agaonidae. Molecular studies and DNA sequences indicate that the five families containing fig wasps (Agaonidae, Eurytomidae, Pteromalidae, Ormyridae, and Torymidae and the subfamilies Epichrysomallinae and the Sycophaginae) are not closely related (Campbell et al., 2000; Machado et al., 1996; Rasplus et al., 1998). The Sycoecinae, Otitesellinae, and Sycorctinae have been placed in the Pteromalidae, which leaves only the truly fig-pollinating wasps in the Agaonidae. Forty-seven genera, comprising approximately 800 species worldwide of fig-loving wasps are recognized as pollinators of Ficus species. There are 21 species of Ficus in Mexico, two in the United States (Florida), and none in Canada. Unlike most flowering plants, which can be pollinated by a guild of pollinators and floral visitors, each Ficus species typically is primarily pollinated by one, or sometimes two, species of agaonid fig wasps (Weiblen, 2002). Some wasps associated with figs, but not typically thought of as pollinators, can enter through fig ostioles—small openings in the fruit—to effect pollination (Jousselin et al., 2001).
Originally native to western Asia and the Mediterranean, the edible fig (Ficus carica) is the only species cultivated commercially for fruit production. In the United States, California dominates, with 98 percent of U.S. fig production (60,000 tons), although according to the USDA Economic Research Service (ERS), figs are grown commercially in at least 14 states (USDA-ERS, 2004).
Twenty-one species of Ficus are indigenous to Mexico; none of these tropical species can survive unaided in Canada. Two native Ficus species are known in the continental United States, occurring naturally in southern Florida (F. aurea and F. citrifolia). Only five species of agaonid fig wasp are known from Mexico, although unreported species could occur there (Noyes, 1998; Rasplus et al., 1988; J.Y. Rasplus, personal communication, January 2006). Despite the importance of fig wasps to pollination of Ficus, there are no published studies documenting population trends in this group.
Beetles (Order Coleoptera)
With about 350,000 described species, beetles (Coleoptera) constitute the largest insect order (Grimaldi and Engel, 2005). Beetles often are ignored as pollinators because both the larvae and the adults of so many species destroy the reproductive organs of wild and domesticated seed plants (Borror et al., 1989). However, beetle pollination is believed to have contributed to the pollination of different lineages of flowering plants for over 120 million years (Bernhardt, 1999, 2000) as shown by the preponderance of beetle pol-
lination in surviving species of basal (magnoliid) angiosperms (Bernhardt and Thien, 1987) and in reconstructions of flower and beetle fossils (insects that belonged to still extant lineages associated with pollen consumption).
In a review of the international scientific literature published between 1906 and 1999, Bernhardt (2000) reported that 34 families (representing some 170 discrete species) of flowering plant contain at least one species pollinated primarily by beetles. Another 79 generalist species distributed within 22 families of flowering plants are pollinated by beetles with other animals, including flies, bees, and birds. Pollinating beetles come from at least 27 families within the order. Beetle behavior on flowers varies according to insect species and sex, and some effect pollination while searching for edible rewards (nectar, pollen, starchy food bodies).
The number of pollinating beetle species cannot be estimated—another result of the acknowledged taxonomic impediment (Box 2-1). Knowledge of known beetle species as effective pollinators of U.S. and Canadian vegetation continues to lag behind information about native bees, bats, and birds in the two countries. An estimated 52 U.S. and Canadian plant species are pollinated by beetles (Table 2-4). However, similar estimates are not available in Mexico, and the role of North American flower-visiting beetles as pollinators has yet to be assessed.
Beetle pollination is usually associated with tropical latitudes (reviewed by Bernhardt, 2000). The extent of beetle pollination within the flora of the United States, Canada, and northern Mexico is open to speculation; few studies confirm that beetles contact both receptive pistil tips and pollenshedding anthers while they forage or find mates (Table 2-3). The role of beetles as pollinators of temperate, North American flora differs by plant geography so that the role a beetle species plays in pollination of temperate, North American flora could change with the distribution of the plant species. In the Southeast, most yellow pond lilies (Nuphar) are pollinated by the chrysomelid beetle, Donacia crassipes (Schneider et al, 1977). As this genus of water lily has expanded its distribution northwards (it also has been introduced into European water ways), it is pollinated by insects in other orders (Herring, 2003; Lippok and Renner, 1997; Renner and Johanson, 1995).
There are no beetle-pollinated crops in Canada or the United States, unless the recent attempts to domesticate and commercialize fruit production in American species of paw paw (Asimina spp.; Norman and Clayton, 1986) are considered. Most Annona spp. sold as custard apples, sugar apples, or soursops are beetle pollinated (Gottsberger, 1989a,b), as is Myristica fragrans (Armstrong, 1986), the source of the commercial “sister” species, nutmeg and mace.
In the United States and Canada, beetle pollination is economically important in the context of significant numbers of annual and bulbous or-
TABLE 2-4 An Illustrative List of Indigenous Beetle-Pollinated Plants of Canada and the United States (Common Names of Plants are in Parentheses)
|
Plant Genus |
Taxa of Pollinating Beetles |
Other Pollinators |
References |
|
Asimina (paw paws, 8) |
Euphoria sepulchralis Trichius spp. Trichotinius lunulatus T. piger T. rufobrunneus Typocerus zebra |
Bombus, flies |
Kral, 1960; Norman and Clayton, 1986 |
|
Calycanthus (W. spice bush, 1) |
Calopterus truncatus |
Staphylinid beetles |
Grant, 1950 |
|
Calochortus (mariposa lilies, 26) |
Acanthoscelides sp. Acmaeodera sp. Anastranglia sp. Anthaxia sp. Anthremus sp. Brachysomida sp. Cryptorhorpalum sp. Diabrotica sp. Emmenotarsus sp. Eschatocrepis sp. Eutrichopterus sp. Hippodomia sp. Judolia sp. Listrus sp. Mordella sp. Mordellistena sp. Nemognatha sp. Tricochrous sp. Trichodes sp. |
Bees, flies, and moths |
Dilley, 2000 |
|
Ipomopsis (gilia, 1) |
Trichochrous sp. |
|
Grant and Grant, 1965 |
|
Linanthus (linanthus, 1) |
Trichochrous sp. |
|
Grant and Grant, 1965 |
|
Plant Genus |
Taxa of Pollinating Beetles |
Other Pollinators |
References |
|
Magnolia (magnolias, 10) |
Acmaeodera pulchella Aleochara lata A. sp. Allecula punctulata Amaspis rufa Arthromacra aenea Collops tricolor Copidita thoracica Conotelus sp. Derelomus bicolor Diabrotica duodecimpunctata Epuraea ovata E. duryi Gaurotes cyanipennis Goes debilis Gyrophaena sp. Leptaura sp. Macrodactylos angustatus Mordella discoidea M. melaena M. octopunctata M. sp. Nitidula sp. Ophistomis bicolor Phyllopaga fervida Prionomerus calceatus Satira gagatina Sitophilus oryzae Strangalina luteicornis Staphylinus sp. Trichiotinus innulatus T. piger T. trinotata Typoceros zebra |
Bees |
Thien, 1974 |
|
Nuphar (yellow pond lilies, 3) |
Donacia crassipes |
Bees, flies |
Lippok and Renner, 1997; Schneider et al., 1977 |
|
Saururus (lizard’s tail, 1) |
Trichiotinus spp. |
Bees, flies, wasp, and wind |
Thien et al., 1999 |
|
Xerophyllum (bear grass, 1) |
Anastrangalia laetifica Cosmosalia chrysocoma Epicauta sp. Leptaura propinqua Trichodes ornatus |
Flies |
Vance et al., 2004 |
|
Total Plant Species |
52 |
|
|
|
Total Pollinating Beetles Identified to Species |
36 |
|
|
namental flowers—garden favorites on hybrids and cultivars derived from wild species native to the Mediterranean basin and southern Africa, whose ancestors are beetle pollinated (Dafni et al., 1990; Goldblatt et al., 1998). At least 4 species in the genus Amphicoma and the 26 monkey beetle species in 9 genera (Anisochelus, Anisonyx, Heterochelis, Khoina, Lepisia, Lepithix, Pachynema, Peritichia, and Scelophysa) may be involved in pollinating the St. Brigid or poppy anemone (Anenome coronaria), orange buttercup (Ranunculus asiaticus), red tulip (Tulipa agenensis), corn poppy (Papaver rhoeas), and the more recently marketed peacock moraeas (Moraea), ixias (Ixia) and romuleas (Romulea). The committee is not aware of any studies on population trends of pollinating beetles.
Flies (Order Diptera)
The true flies (order Diptera) are among the most diverse of the insects, with more than 150,000 species described (Thompson, 2006). Flies are ancient—the oldest fossils are known to have come from the Permian (250 million years ago), and flies might have been the first pollinators (Labandeira, 1998). The group, however, underwent its greatest diversification along with flowering plants from the late Cretaceous onward. Higher flies (suborder Cyclorrhapha) are the result of this radiation and now account for about two-thirds of the extant Diptera (Grimaldi and Engel, 2005). Extant flies are classified into 153 families and 148,416 species, with 124 families and 24,219 species found in Canada, Mexico, and the continental United States (Thompson, 2006).
The state of taxonomic knowledge for higher categories (families and genera) is summarized in the Manual of Nearctic Diptera (McAlpine et al., 1981, 1989). The last assessment of the Nearctic Diptera fauna was done in 1988 (Thompson, 1990) and, according to the results, about two-thirds of all the flies known to occur in America north of Mexico have been named. Unfortunately, fewer than 1 percent of these flies are treated comprehensively in monographs, and fewer than one-quarter of the species have been thoroughly revised. No field guides exist exclusively for flies, and only one digital key has been developed (Carroll et al., 2005). The assessment also noted a decline in specialists in the ranks of graduate teachers at universities and among museum curators.
Most higher flies are flower visitors and many have been documented as pollinators. All of the world’s floristic provinces contain at least one plant species pollinated primarily or exclusively by flies, and fly pollination is regarded as second only to bee pollination in the evolution of flowering-plant diversity (reviewed by Larson et al., 2001). Flies as flower visitors (anthophiles) and pollinators have been reviewed by Larson and colleagues (2001). Following from their work (and table) and combined with the number of
species for each group identified as containing pollinators, it is possible to estimate that 17,460 species in North America are flower visitors and likely pollinators (Table 2-5).
Some fly species forage actively in cool, wet climates, so fly pollination could dominate among the flora of the Arctic tundra and the montane-alpine zones of North America (Kearns, 1990; Kevan, 1972). Research published within the past 15 years demonstrates that fly pollination dominates in plant species that are characterized by small flowers that bloom under shade and in seasonally moist habitats. Cacao (Theobroma cacao, from which chocolate is obtained) is the best-known example (Young, 1994), but others are in the Iridaceae (Goldblatt et al., 2005) and Saxifragaceae (Goldblatt et al., 2004).
In association with bees, flies appear to contribute to the pollination of several fruit and vegetable crops (Free, 1970), but cacao is the only domesticated plant of major economic significance pollinated exclusively by flies (tropical midges). Cacao is a tropical shrub that cannot be grown in Canada or in most of the United States, but it has been commercially and culturally important to Mexico since the days of the Aztec Empire (Young, 1994).
The dominant pollinators of beargrass (Xerophyllum tenax), a montane herb native to the American Pacific Northwest, consist of more than 20 species of flower fly. Licensed collection of beargrass leaves occurs annually for exportation to Europe for the floral craft trade. Harvesters in the Pacific Northwest were paid over $11.5 million for beargrass in 1989 (Schlosser et al., 1991). A number of garden and potted plants are derived from flypollinated species, including birthwort (Aristolochia), starfish flower (Stapelia), tuberous aroids including the jack-in-the-pulpits (Araceae), and the ancestors of some hybrid gladioli (Gladiolus; Bernhardt and Thien, 1987; Goldblatt and Manning, 1999; Proctor et al., 1996). No flies have been truly “managed,” but some flower flies (Syrphidae, genera Eristalis, Syritta; Jarlan et al., 1997) have been used to pollinate crops in greenhouses.
Knowledge of the distribution and population sizes of North American flies is virtually nonexistent. Although the few published monographs and revisions usually contain summary statements of material examined and, frequently, distribution maps, there are no publicly available databases of substantive specimen data. Similarly, there could be just one long-term program to collect population data for flies, using a Malaise trap at the Rocky Mountain Biological Laboratory in Crested Butte, Colorado. In Europe, however, some groups of flies are actively studied by citizen-scientists. Flower flies (Syrphidae) are very popular with citizen-scientists; syrphids, for example, have been included in the ALARM project (Box 2-2 and Chapter 6).
Population trends cannot be determined without population data for North American flies, although in Europe the ALARM project has
TABLE 2-5 Total Number of Identified Diptera Species and the Estimated Number of Diptera Species That Are Pollinators
documented shifts and declines for several flower fly species in the United Kingdom and the Netherlands (Box 2-3; Biesmeijer et al., 2006). One North American species, the Delhi Sands flower-loving fly (Rhaphiomidas terminatus abdominalis; Kingsley, 1996), has been placed on the U.S. Endangered Species List.
Butterflies and Moths (Order Lepidoptera)
With approximately 150,000 species described worldwide (Grimaldi and Engel, 2005), Lepidoptera are among the most species-rich orders of insects, as measured by formally described and documented species. The Lepidoptera include many of the most easily recognized insects, and they are relevant to understanding pollination systems and their origins.
As with most herbivorous insects, butterflies and moths interact with plants during both larval and adult stages, the latter of which are those usually involved in pollination. Although some of the best-studied examples of plant-insect mutualisms involve moths and butterflies (Heliconius butterfly, Boggs et al., 1981; Estrada and Jiggins, 2002; yucca moth, Pellmyr et al., 1996; Powell, 1992), obligatory mutualisms are exceptional in Lepidoptera. Although many flowering plants rely primarily on butterflies or moths, most Lepidoptera visit a wide variety of nectar sources. Although lepidopteran pollinators largely are generalists and often transfer only small amounts of pollen, they tend to move longer distances to visit flowers of the same species than do other pollinating insects, such as bees, and thus are important in maintaining gene flow within and among populations (Herrera, 1987, 2000).
The earliest documented North American extinctions of insects involved Lepidoptera (the xerces blue, Glaucopsyche xerces), and the Lepidoptera were the bellwethers of the earliest observations of invertebrate species decline in North America (Tilden, 1956). At least some of these extinctions occurred before any information on pollinating capacity of these butterflies was obtained. Butterflies and moths account for a high number of species currently regarded as threatened or endangered in various North American regions and tracked by various state heritage programs (http://www.natureserve.org). Numerous butterfly species are protected under the terms of the Endangered Species Act (Chapter 1) and by state legislation.
Massachusetts, for example, lists 48 Lepidoptera species—nearly half of the invertebrates on its list—as endangered, threatened, or of special concern under MESA (http://www.mass.gov/dfwele/dfw/nhesp/nhrare.htm). Most, if not all, of those butterflies and moths have declined significantly in the past 100 years. MESA also lists 10 beetles, 31 dragonflies and damselflies, 8 crustaceans, 7 mussels, 7 snails, and 4 other invertebrates. The large proportion of Lepidoptera listed could be more a reflection of the generally
greater knowledge about them than their larger propensity toward decline (May et al., 1996). Although threatened species legislation varies from state to state, similar trends are widely recognized by scientific and academic communities.
Butterflies
Butterflies and moths often have precise habitat and life history requirements. As is the case for most herbivorous insects, the majority of Lepidoptera generally specialize on three or fewer host plant families for larvae (Bernays and Graham 1988; Farrell and Mitter, 1993; Mitter and Farrell, 1993; Powell et al., 1998). Yet there are significant information gaps about the extent to which plants—economically important and otherwise—rely exclusively on particular lepidopteran species for pollination. Although butterfly larvae tend to be host-specific for food, their activities as adult pollinators are likely to be general. There are notable exceptions; the wild carnation Dianthus carthusianorum in Europe, for example, appears to be specialized for butterfly pollination and is visited by five butterfly species, of which two, due to visitation frequency, are principal pollinators (Bloch et al., 2006).
The bay checkerspot butterfly, Euphydryas editha bayensis (Nymphalidae), pollinates native plants in North America. Variation in population size of this butterfly has been meticulously documented. It has been the focus of regular census efforts in the Jasper Ridge Biological Preserve near Palo Alto, California, for more than 40 years (Ehrlich and Hanski, 2004), and local population extinctions have been documented for 1991 and 1998 (McLaughlin et al., 2002). Although it is known from other localities (there is a large population in Morgan Hills and a small population near Redwood City), the bay checkerspot was designated as threatened in 1987 and included on the Federal Endangered Species List. It was almost certainly more extensively distributed before the invasion of its habitat by Eurasian grasses (McLaughlin et al., 2002). Local population extinctions of the sort observed for the bay checkerspot have also been documented in the Rhone Valley of Switzerland for Satyrus ferulae, which has disappeared from 31 of 62 sites, and Melanargia galathea, which has disappeared from 29 of 67 sites, since 1970 (Bloch et al., 2006).
The monarch butterfly, Danaus plexippus, pollinates many milkweed and other plant species. It has been the subject of intense interest, in part because of its dramatic ecology (sequestering cardenolides from asclepiaceous host plants and serving as the central model in an extensive mimicry system) and migratory behavior (Halpern, 2001). The Fourth of July butterfly count (known as 4JC in short), an annual monitoring effort undertaken by a broad cross-section of amateurs and professionals, provides insight about fluctua-
tions in monarch populations (Swengel, 1990). Swengel (1995) described population fluctuations.
From 1977 to 1986, the mean number of monarchs per hour in eastern North America varied significantly in only one of nine pairs of consecutive years. From 1986 to 1994, eastern monarchs varied significantly in five of eight year-pairs, with a nearly equal number of increases and decreases. However, the amount of variation within each year-pair was similar for 1977–1986 and 1986–1994 (median difference ca. a factor of 2). Although the Atlantic and midwestern subregions of the eastern population covaried in four of eight year-pairs from 1986–1994, differences between the two subregions sometimes damped fluctuations in the eastern population overall. Data from independent transect surveys in Wisconsin for 1990–1994 agreed with the direction of fluctuations in Wisconsin 4JCs. In 4JCs from 1987–1994 in the Pacific coastal states, monarchs varied less than the eastern population, with a significant increase in 1989 and significant decrease in 1988. However, a nonsignificant decline of an order of magnitude occurred in 1993, and a considerable but smaller increase occurred in 1994. Fluctuations in monarch abundance in eastern North America during 1977–1994 often, but not always, coincided with years affected by major widespread perturbations of typical weather such as the El Nino-Southern Oscillation, major volcanic eruptions, droughts and floods.
Since Swengel’s study, other groups have collected data on monarch populations in various locations, and they also have reported fluctuating numbers from year to year (Gibbs et al., 2006; O. Taylor, University of Kansas, presentation to the committee, October 18, 2005).
Results from another Fourth of July Butterfly Count, carried out since 1977 by Arthur M. Shapiro (University of California, Davis), provide information about the variations in populations of 36 butterfly species in the Sacramento Valley. During the 29-year study, 39,614 butterflies were counted; the number of species observed each year ranged from 17 to 30 and the number of inviduals from 618 to 2613. The site is a partly channelized perennial stream, its floodplain, and adjacent levees, and it is surrounded by agricultural lands. The results from Shapiro’s annual census have been used in a recent analysis (O’Brien et al., in review) to investigate the usefulness of different statistical methods for identifying trends in overall species diversity and in the probability of the presence of individual species. Species diversity declined 38 percent over the study period. Although the decline was detectable by the 13th year, it did not become statistically significant until the 23rd year of the study. Of the 23 species analyzed, 8 have declined significantly and 11 more show a negative (statistically nonsignificant) trend. Neither abundance nor diversity was significantly correlated with any weather patterns examined, although the weather at the study site has warmed significantly over the past 30 years. Thus, the cause of the decline
is still unknown, but species that overwinter as eggs or larvae were more likely to decline than those that overwinter as pupae or adults. Most of the species reported as declining also are declining regionally. The results published by O’Brien and colleagues (in review) point out the importance of systematic, long-term monitoring. Because of statistical limitations, the dramatic decline in species numbers reported in the study would have gone undocumented in a census that lasted less than 22 years.
Moths
Moths are underappreciated as pollinators because most of their pollinating activity takes place at night. They are known to pollinate a diverse suite of plants, ranging from cacti (Clark-Tapia and Molina-Freaner, 2004) to orchids (Little et al., 2005) to trees (Lin and Bernardello, 1999). Some moth species have specialized morphological features, such as long proboscides, and behavior that make them excellent pollinators of some plants (Barth, 1985; Proctor et al., 1996). Some plants, such as various species of Yucca (Thompson, 1994), are icons of specialized pollination biology. Yucca moths (Tegeticula and Pronuba spp.) are highly specialized pollinators of Yucca spp. within desert and chaparral habitats of the United States and Mexico. The adult yucca moth does not feed, but it uses specially modified palps (“tentacles”) to gather up a ball of pollen that it places on a receptive floral stigma, ensuring the production of the seeds that larvae eat (Bogler et al., 1995; Faegri and van der Pijl, 1979; Pellmyr et al., 1996; Proctor et al., 1996). Similarly, Gaura and Calylophus species (Clinebell et al., 2004) and the senita cactus (Pachycereus schottii; Fleming and Holland, 1998) are in plant-specific moth pollinator systems that have been intensively studied.
Although a variety of moths are known to be pollinators, the families Geometridae (geometer moths), Noctuidae (owlet moths), and Sphingidae (sphinx moths) are among the best studied. The Sphingidae (Gregory, 1963– 1964) and the highly diverse Noctuidae are among the most efficient of the lepidopteran pollinators. Within the deserts of the southwestern United States and Mexico, large fast-flying hawkmoths or sphingids (Sphingidae) are coadapted pollinators of night-blooming Datura spp. (Solanaceae) and night-blooming cacti in the genus Peniocereus (Raguso et al., 2003). At least 106 species in the temperate North American flora are known to be visited by sphinx moths, including many from the Onagraceae (Grant, 1985). Migratory noctuids feed on the floral nectar of many species of plants (Kevan and Kendall, 1997). Geometrids have been recorded as pollinators of orchids (Thien and Utech, 1970) among other species. There are no data on population trends of moth species.
Thrips (Order Thysanoptera)
The thrips (Thysanoptera) are slender, small insects (generally no more than one millimeter long), arranged into nine families of living species distributed worldwide, largely in the tropics and temperate regions, with a few species in Arctic regions (Lewis, 1997; Mound, 1997). Checklists of adult thrips have been produced by Stannard (1957, 1968) for North America.
Thrips feed on a variety of plant tissues, including pollen, fungal mycelia, and spores, and they also are predatory (Grimaldi and Engel, 2005; Kirk, 1993, 1997). When they feed on pollen, thrips puncture the coat and drain the grains (Kirk, 1984, 1985, 1997). Grimaldi and Engel (2005) note that pollen feeding evolved several times in thrips; they are so numerous on flowers that they can be effective pollinators of a wide variety of plants in nature and agriculture (Ananthakrishnan, 1993; Endress, 1994; Kirk, 1988; Lewis, 1973, 1997; Terry, 1997). Generally, however, they are regarded as minor or secondary pollinators (Kirk, 1997; Lewis, 1973, 1997; Terry, 2001).
As minor pollinators, thrips also pollinate such agricultural plants as beets, beans, onions, and cacao (Kirk, 1997; Lewis, 1973). Although thrips can pollinate plants in the absence of other pollinators, their importance in open-pollinated crops depends on whether other insects pollinate the flowers first (Kirk, 1997). Thrips can enter unopened buds (Mackie and Smith, 1935), but the peak number of thrips can occur after peak visits by other insects (Kirk, 1984). The grooming behavior of thrips contributes to both self- and cross-pollination in plants (Kirk, 1997). As thrips arrange the fringe hairs before and after flight, pollen grains are shed from their bodies (Kirk, 1997). The stigma is prominent in many flowers and because it is used by thrips for take-off and landing, the pollinator thus places pollen directly on the stigma (Kirk, 1997).
Populations of thrips on crops grown in greenhouses and shade houses depend on breeding within the crop (Kirk, 1997). For example, young chrysanthemum plants are rooted from older plants, and when adult female Frankliniella occidentalis (western flower thrips) oviposit in apical leaves, growers can inadvertently raise their own pest populations and transport them to other sites in the cuttings (Kirk, 1997). The flower trade is responsible for the worldwide distribution of that thrips species, as well as others (Table 6.2 of Kirk, 1997).
In part because of their size and their more frequent role as herbivorous plant pests and disease vectors (Ullman et al. 1997), North American thrips have not generally been the focus of concern about population decline; no thrips species is currently protected under the provisions of the Endangered Species Act (ESA). Because of restrictions on ESA, it is unlikely that any species that has had an adverse economic impact on a crop species would
be eligible for listing, even if it could be shown that thrips provide essential pollination services (Chapter 6).
Mammalian Pollinators
Bats
Estimates of the number of bat-pollinated plants species in the Americas range from 600 (Neuweiler, 2000) to 1,000 species (Winter and von Helversen, 2001). Most bat-pollinated flowers have intense scents that are different from those pollinated by other animals (von Helversen and Winter, 2003). Sulphur-based compounds are more common in bat-pollinated species than they are in other pollination systems (von Helversen et al., 2000). Given that the scents are produced in many phylogenetically unrelated species of plants, they are likely the result of long coevolutionary associations (Knudsen et al., 1993).
The color of bat-pollinated flowers is normally inconspicuous, from whitish to green or brown. The color reflectance of the flower itself probably would not be a strong attractant for bats, because most bats are considered color-blind (Jacobs, 1992), although some species might see some color (von Helversen and Winter, 2003). Ultraviolet clues in several bat-pollinated flowers (notably on columnar cacti) prompted studies of bats’ ability to detect ultraviolet radiation. Von Helversen and Winter (2003) reported that Glossophaga soricina is highly sensitive to ultraviolet light. Other characteristics of bat-pollinated flowers include an outward-facing position at the edge or away from the plant’s foliage, thereby facilitating the bats’ access. Most bat-pollinated flowers are large, with sturdy petals and exposed stamens and pistil. They generally open at night, and many open only for a single night. The protein content in the nectar of bat-pollinated flowers is greater than it is in flowers pollinated by insects, and those plants generally have more nectar (Neuweiler, 2000). Plant families recognized for their many bat-pollinated species include Agavaceae, Bignoniaceae, Bombacaceae, Cactaceae, Caesalpiniaceae, Chrysobalanaceae, Convolvulaceae, Cucurbitaceae, Fabaceae, Malvaceae, Marcgraviaceae, Mimosaceae, Musaceae, Pandanaceae, and Tiliaceae (Neuweiler, 2000), although as many as 27 plant families in the New World have bat-pollinated species (Vogel, 1969). Most of those groups are tropical, and the number of bat-pollinated species increases as latitude decreases (Heithaus, 1982; von Helversen and Winter, 2003).
There are at least 12 species of pollinating bats in North America, including southern Mexico (Baker et al., 2003; Ceballos et al., 1997; Medellín et al., 1997). The most prominently recognized—by virtue of their conservation status—are the three long-distance migratory species: the
lesser long-nosed bat (Leptonycteris curasoae), the Mexican long-nosed bat (L. nivalis), and the hog-nosed bat (Choeronycteris mexicana). Few population data are available for pollinating bats in North America, given that populations are difficult to survey, few people are qualified to survey them, and few people study them. Most population data take the form of isolated reports that indicate local trends in abundance rather than precise estimates of population size. Information on both species of Leptonycteris (Ceballos et al., 1997; Fleming, 2004; Fleming and Nassar, 2002; Fleming et al., 2001a; Galindo et al., 2004; Moreno-Valdez et al., 2004; Stoner et al., 2003) covers regions of western, northwestern, and central Mexico and the researchers have presented data on population dynamics that cover only relatively short periods. An effort to monitor and assess the status of L. curasoae is being centralized by the Arizona Game and Fish Department (http://www.azgfd.gov/w_c/edits/documents/Leptcuye.fi.pdf), and the Program for Conservation of Mexican Bats has been compiling information on this and other species for several years (Medellín, 2003; Medellín et al., 2004).
Pollinating bats can be divided into two behaviorally functional groups: species restricted to the tropical regions of southern Mexico (Glossophaga, Hylonycteris, Choeroniscus, Anoura, Lichonycteris, Musonycteris) and those that migrate, moving from central and southern Mexico to northern Mexico and the southern United States (Ceballos and Oliva, 2005; Reid, 1997). All pollinating bats provide important services for many species of North American plants. Many species of columnar cacti, species of Agave, trees in the family Bombacaceae, and many other plants rely heavily on bats to carry out sexual reproduction. Agaves are economically important throughout Mexico, but particularly in western regions where they are used for the production of tequila. Tequila production does not, however, depend on pollination by bats. By virtue of their common asexual mode of reproduction, agaves are planted from shoots associated with adult plants, and their flowering is prevented by premature harvest. Nevertheless, bats could be necessary to promote genetic diversity for the long-term viability of commercially grown agaves (Arizaga et al., 2002; Dalton, 2005; Rocha et al., 2005; Valenzuela-Zapata and Nabhan, 2004).
Some other economically important plants linked to bat pollination are the balsa tree (Bombacaceae: Ochroma pyramidale), ceiba (Ceiba pentandra), and many columnar cacti whose fruits are used and commercialized fresh or dried, or processed into jams, jellies, and candies (Neobuxbaumia spp., Pachycereus spp., and others) (Bawa, 1990; Watson and Dallwitz, 1992). Ecologically important plants, such as the cardon (Pachycereus pringlei), saguaro (Carnegiea gigantea), and other columnar cacti, vary in their reliance on bats (Fleming et al., 2001b; Grant and Grant, 1979; Valiente-Banuet et al., 1996). Some populations of these cacti are frequently pollinated by white-winged doves (Zenaida asiatica), several species
of hummingbirds, sphingid moths, bees, or beetles (Fleming et al., 2001b; Grant and Grant, 1979; Valiente-Banuet et al., 1996) on the day after their nocturnal anther dehiscence.
The genus Glossophaga has four North American species, all present in tropical Mexico. Three of them have wider distribution, extending into Central and South America, and one, G. morenoi, is endemic to the dry tropical forest of western Mexico. Two species, G. commissarissi and G. soricina, are widespread and common and do not seem to face any threat; the other two tend to be locally rare (Ceballos and Oliva, 2005; Reid, 1997). No population estimates or trends have been obtained, but neither is considered to be facing conservation threats by the Mexican government and neither appears on the International Union for the Conservation of Nature and Natural Resources/The World Conservation Union Red List of threatened species (http://www.iucnredlist.org/).
Leptonycteris curasoae and L. nivalis are migratory species considered threatened in Mexico and endangered in the United States. Their listing in the United States was prompted by surveys in some known roosts that indicated severe declines (U.S. Fish and Wildlife Service, 1988, 1994; Wilson et al., 1985), although subsequent studies suggested that the declines might not have been as severe as originally thought (Cockrum and Petryszyn, 1991). In Mexico, the species was listed as threatened when some winter maternity roosts (the species has a summer and a winter reproductive pulse) were found severely depleted; much of its original habitat in western and central Mexico has been destroyed for tourism and agricultural development (SEMARNAT, 2002). The Program for Conservation of Mexican Bats has monitored between 7 and 20 roosts per year, documenting population stability or growth, and noting temporary declines in some years (Medellín, 2003; Medellín et al., 2004).
Another migratory species listed as threatened in Mexico, but not in the United States, is the hog-nosed bat, Choeronycteris mexicana (SEMARNAT, 2002). Although there are no reliable population estimates, since 1906, fewer than 1,500 individuals have been documented throughout the species’ range (Cryan and Bogan, 2003). In contrast with the long-nosed bats, this species roosts in small numbers, typically about 12 bats per roost. Because roosts tend to be scattered widely over landscapes, surveys are difficult (Arroyo-Cabrales et al., 1987; Cryan and Bogan, 2003; Tuttle, 2000).
Two of the four North American Glossophaga species are very abundant (Ceballos and Oliva, 2005; Reid, 1997), and there is no evidence of decline. Although the one species endemic to Mexico appears to be less common, there are no population estimates that permit a firm assessment of status.
The banana bat (Musonycteris harrisoni), a rare species that appears to be highly specialized, as evidenced by its extremely long snout and tongue,
is endemic to western Mexico, from Jalisco to Oaxaca. Fewer than 70 individuals have ever been observed, and only 3 roosts with 3 individuals or fewer have been reported (Téllez and Ortega, 1999). The species is considered threatened by the Mexican federal government (SEMARNAT, 2002).
The tailless bat (Anoura geoffroyi) is widespread in the southern half of Mexico and occurs frequently at medium elevations—1,000–2,500 m above sea level—and also, rarely, at lower elevations. Although this species is not very common, it is not thought to be facing any threats (Ceballos and Oliva, 2005; Reid, 1997). No population estimates are available.
Three well-recognized pollinator species, Hylonycteris underwoodi, Choeroniscus godmani, and Lichonycteris obscura, tend to be rare. The former two have a wide distribution over the southern half of Mexico; the third is known only from the state of Chiapas south to South America. No population estimates or trends are detectable through peer-reviewed literature. The three species are associated with primary tropical forests, both dry and wet (Ceballos and Oliva, 2005; Reid, 1997), and none is on the Mexican list of species at risk of extinction.
Three migratory bat species are considered threatened or endangered by the Mexican (SEMARNAT, 2002) and U.S. (U.S. Fish and Wildlife Service, 1988) federal governments. Conservation and recovery programs have been initiated and the bat populations are being monitored; surveys in recent years in several colonies suggest that the populations of at least two (L. nivalis and L. curasoae) of these three species in the genus Leptonycteris are stable. However, taking those species off the threatened or endangered species list may be premature. More local evidence is required before a firm conclusion can be drawn (Medellín, 2003; Medellín et al., 2004). Other pollinating bats might not be in decline, but those associated with primary habitats have long been considered rare, and their biology and importance are virtually unknown.
Nonflying Mammalian Pollinators
Among nonflying mammals, at least two species of opossum (Caluromys derbianus and Didelphis marsupialis; Tschapka and von Helversen, 1999) visit the flowers of Marcgravia in Central America. Coatis (Nasua nasua; Mora et al., 1999) and kinkajous (Potos flavus; Kays, 1999) have been documented as consistent flower visitors and potential or realized pollinators of various trees, including Ochroma, Pseudobombax, Tetrathylacium, and others. Janson et al. (1981) suggested that several primates (including the spider monkey, Ateles), opossums, and procyonids could be pollinators of several tree species in the rainforests, although at least the spider monkey has been shown to damage virtually all flowers it visits, apparently substantially decreasing fruit set (Riba-Hernandez and Stoner, 2005).
These mammals’ habitats span southern Mexico to central Mexico and from the northern coatis range to southwestern United States. Opossums can also be found in southern Canada. The woolly opossum (Caluromys derbianus) is considered under special protection, and two of its subspecies are considered endangered. An endemic species of coati is threatened, and the kinkajou is under special protection (SEMARNAT, 2002). Pollination by nonflying mammals is reported more often for other continents (Carthew and Goldingay 1997; Goldingay et al. 1991).
Avian Pollinators
Pollination by birds is well known and recognized in North America, largely because of hummingbirds (Faegri and van der Pijl, 1966). Population data for avian pollinators are available from a variety of sources, including the North American Breeding Bird Survey (BBS) (see http://www.mbr-pwrc.usgs.gov/bbs/genintro.html for a history of this effort), which is now coordinated through the U.S. Geological Survey (USGS) Patuxent Wildlife Research Center. Bird banding data collected by individuals or at bird banding stations are compiled by the North American Bird Banding Laboratory, (http://www.pwrc.usgs.gov/bbl/) which also is part of the Patuxent Wildlife Research Center. Some summary BBS statistics are available from a USGS website (Sauer et al., 2005); some results are presented here. Cautious interpretation is necessary, however, because at least for hummingbirds, the BBS methodology is less than ideal.
Hummingbirds
Eighteen hummingbird species are known in the United States, 9 are known from Canada (although some of these are rare visitors from Mexico; Sibley, 2000), and 63 are known from Mexico. Most hummingbirds do not sing (even though they vocalize in aggressive interactions), so they can be more difficult to detect. Males and females can occupy different habitats, and males are polygynous—that is, they mate with more than one female in a breeding season.
Although a few species overwinter in the United States, most migrate southward, depending on migration corridors or nectar corridors (Nabhan et al., 2004). It can be difficult to assess hummingbird populations because some surveys (such as the Christmas Bird Counts) are conducted when individuals might have left for wintering grounds. There is evidence that a high percentage of rufous hummingbirds (Selasphorus rufus) lose body weight during migration, requiring longer stopover times if floral resources are scarce (Russell et al., 1994).
The relationship between hummingbirds and the flowers they visit
is well studied (Arizmendi and Ornelas, 1990; Calder, 2004; Grant and Grant, 1967), and hummingbirds are important pollinators in much of North America. Although hummingbirds visit a wide variety of flowers, many hummingbird-pollinated flowers share some general characteristics. The flowers tend to be tubular, brightly colored (red, orange, bright yellow), and relatively odorless, and their nectar is often more diluted than that of bee-pollinated flowers (Baker, 1975; Pyke and Waser, 1981) but could contain higher levels of sucrose (Baker et al., 1998). Hummingbirds display variation in bill shape and length (Stiles and Skutch, 1989), and some studies indicate that all hummingbirds can extract nectar from long-tubed, wide-opening flowers, but that only long-billed hummingbirds do so from long-tubed, narrow-opening flowers (Temeles et al., 2002). The list of species of plants that are visited and pollinated by hummingbirds is extensive (Bertin, 1982; Grant and Grant, 1968), and it encompasses plants in many families: Acanthaceae, Asteraceae, Bromeliaceae, Campanulaceae, Ericaceae, Fabaceae, Gentianaceae, Heliconiaceae, Loranthaceae, Malvaceae, Onagraceae, Polemoniaceae, Rubiaceae, Zingiberaceae, and many others (Knudsen et al., 2004; McDade and Weeks, 2004).
Although hummingbirds might be minor as pollinators of agricultural crops (cacti; Griffith, 2004), many species of wildflowers have coevolved with hummingbirds and exhibit morphological, phenological, or other traits that facilitate interaction (Fenster et al., 2004). Data from BBS with a high credibility index (at least 14 samples in the long term, of moderate precision, and of moderate abundance on routes) are available for 8 hummingbird species. Data cited below come from the BBS website (http://www.mbr-pwrc.usgs.gov/bbs/bbs.html).
In the states where the credibility index is high (such as North Carolina, Oklahoma, and West Virginia), the trend (percentage change per year) shown for the ruby-throated hummingbird (Archilochus colubris) from 1966 to 2005 is positive. Overall, trends in the United States (2.5 percent per year) and Canada (2.5 percent per year) are positive (http://www.mbr-pwrc.usgs.gov/cgi-bin/atlasa99.pl?04280&1&05). For the black-chinned hummingbird (Archilochus alexandri), for one site (Edward’s Plateau) for which the credibility index is high, the trend from 1966 to 2005 is positive (1.2 percent per year). Overall, the trend for A. alexandri is positive in the United States (1.6 percent per year), and negative in Canada (−3.2 percent per year). For Anna’s hummingbird (Calypte anna), in the states where the credibility index is high, the trend from 1966 to 2005 is positive; the data set includes California, a state with a few regions—California, Southern California grasslands, foothills, and Fish and Wildlife Service Region 1. Overall, the trend is positive (1.2 percent per year) in the United States. For the broad-tailed hummingbird (Selasphorus platycercus), in the states where the credibility index is high, the trend from 1966 to 2005 is mixed;
it is slightly negative in Colorado and slightly positive in New Mexico. Overall, the trend is slightly negative (–0.2 percent per year) in the United States. Calder et al. (1983) reported that, over a 10-year period at the Rocky Mountain Biological Laboratory, the population of S. platycercus appeared to be declining, although nest counts of breeding females remained fairly constant. In the years since that study, the population has remained variable, but with no discernable long-term trend (D. Inouye, University of Maryland, and Rocky Mountain Biological Laboratory, Colorado, personal observation). For the rufous hummingbird (Selasphorus rufus), in Oregon and Washington, the two states where the credibility index is high, the trend from 1966 to 2005 is negative. Overall, trends in the United States (–2.0 percent per year) and Canada (–2.1 percent per year) are negative. An iteresting development over the past decade is that rufous hummingbirds are commonly found in the eastern United States, where they previously were thought to be absent.
The status of several species, because of a lack of information, is more difficult to determine. For the Costa hummingbird (Calypte costae), there are no states where the credibility index is high, but the trend from 1966 to 2005 is positive for one state with a few regions—the Great Basin deserts and Mexican highlands. Overall, the trend is positive (0.5 percent per year) in the United States. Similarly, for the calliope hummingbird (Stellula calliope), there are no states where the credibility index is high, but the trend from 1966 to 2005 is positive in Idaho and Wyoming and negative in California, Montana, Oregon, and Washington. Overall, the trend is negative (–0.9 percent per year) for the United States and slightly positive (0.8 percent per year) for Canada. There is no statistically significant detectable trend. Finally, for the Allen hummingbird (Selasphorus sasin) there are no states with a high credibility index, but in the southern Pacific rainforest region—the only region with a high index—the trend from 1966 to 2005 is negative (–1.2 percent per year) but edging upward over the past several years. Overall, the trend for S. sasin in the United States is negative (–2.0 percent per year). This species is on the Audubon Society Watch List because of its very restricted range in the United States. There are two subspecies, identified primarily through their distribution (mainland or the Channel Islands off the coast of Southern California), and although the subspecies with the wider range (coastal Mexico to Oregon) appears to be in decline, the other appears to be spreading.
Unfortunately, there do not appear to be any long-term data about population trends of species of hummingbirds that are distributed in Mexico exclusively. Although these species could be under pressure from habitat alteration and fragmentation, there is no equivalent of the BBS data and no systematic banding efforts that the committee could discover.
Nonhummingbird Avian Pollinators
Although not typically thought of as pollinators, at least one dove species, the white-winged dove (Zenaida asiatica), is an important pollinator of saguaro and possibly other cacti (Fleming, 2000). The population trend for that species is slightly negative from 1966 to 2005 in Arizona, the Sonoran Desert, and the Mexican highlands. Overall, in the United States and in the survey area, the trend is positive (1.6 percent per year). A few other nonhummingbird avian species also pollinate North American plants, and there is evidence that they also affect flower morphology. The genus Erythrina (Fabaceae), a pantropical leguminous small tree, shows two distinct flower and nectar types: hummingbird-pollinated species have upright inflorescences with tubular, radially arranged flowers and nectar that has relatively high concentrations of sugar. The species pollinated by passerine birds (including swallows) have horizontal inflorescences held upright, with the flowers arranged radially along the axis, the narrow standard petal folded to form a pseudotube, and relatively dilute nectar (Bruneau, 1997). Several species have been identified: verdins (pollinating ocotillo, Fouquieria splendens; Waser, 1979), oriole (Icterus spp.: Etcheverry and Aleman, 2005; Toledo and Hernandez, 1979), parrot (Aratinga), woodpecker (Centurus), tityra (Tityra), warbler (Dendroica), wren (Campylorhyncus), jay (Psilorhinus), vireo (Vireo), blackbird (Dives), grackle (Cassidix), oropensola (Psarocolius), honeycreeper (Cyanerpes), tanager (Thraupis, Piranga), euphonia (Euphonia), mockingbird (Mimus), thrasher (Toxostoma), and finch (Carpodacus) visit flowers of a diverse array of species including Bernoullia, Ceiba, Tabebuia, Spathodea, and Agave (Ornelas et al., 2002; Toledo, 1977).
Some bird species of these genera are on the Mexican federal list of endangered species: six vireo species are under special protection, two species are threatened, and one is endangered. Three orioles and two oropendolas are under special protection; two parrots of the genus Aratinga are under special protection, two more are threatened, and one subspecies of one threatened species is considered endangered. Two species of warbler are threatened, and one wren species is under special protection, one is threatened, and one is endangered. Two euphonias are under special protection, one thrasher is endangered, and two subspecies of finch are endangered (SEMARNAT, 2002).
Information Needs
As the number of individual hummingbird banders in the United States has grown, so has the amount of information about the birds’ abundance and migration paths. Additional data might have been collected at some
long-term bird banding stations or by long-term individual banders that could add to the incomplete data for hummingbirds. Long-term monitoring of bird-pollinated plants also could provide useful information on the stability of the ecosystem services they provide, particularly if plants pollinated solely by the birds are chosen.
CONCLUSIONS
Despite the paucity of long-term data, collectively there is reliable evidence that some North American pollinator species have become extinct or locally extirpated, or have exhibited decreases in population size (Table 2-6). At least two bumble bee species could face imminent extinction, and several other pollinators have declined significantly (honey bees and U.S. and Mexi-
TABLE 2-6 Illustrative Examples of Pollinators in North America for Which Evidence of Decline Is Available
|
Common Name |
Species Name |
Location |
|
Species for Which Quantitative Data Are Available |
||
|
|
Hymenoptera |
|
|
Honey bee |
Apis mellifera |
United States |
|
Honey bee |
A. mellifera |
Mexico |
|
Franklin’s bumble bee |
Bombus franklini |
Pacific Northwest of the United States |
|
Western bumble bee |
B. occidentalis |
Central California |
|
Bumble bee |
B. affinis |
New York |
|
|
Lepidoptera |
|
|
Bay checkerspot butterfly |
Euphydryas editha bayensis |
Palo Alto, California and other localities |
|
|
Chiroptera |
|
|
Long-nosed bat |
Leptonycteris curasoae |
United States and Mexico |
|
Long-nosed bat |
L. nivalis |
United States and Mexico |
|
|
Apodiformes |
|
|
Rufous hummingbird |
Selasphorus rufus |
United States and Canada |
|
Allen’s hummingbird |
S. sasin |
United States |
|
Species for Which Quantitative Data Are Not Available |
||
|
|
Hymenoptera |
|
|
Stingless bees |
Melipona spp. |
Southern Mexico |
|
|
Trigona spp. |
|
|
Pollen wasps |
Pseudomasaris micheneri |
Inyo County, California |
|
Pollen wasps |
P. macswaini |
|
|
|
Chiroptera |
|
|
Hog-nosed bat |
Choeronycteris mexicana |
Mexico |
|
Banana bat |
Musonycteris harrisoni |
Mexico |
can pollinating bats), although many have populations that are stable or perhaps even increasing, as are a few of the hummingbird species. It should be noted that there is no evidence of population decline for some species merely because their populations have not been monitored over time. Overall, whether there is a “pollinator crisis” is difficult to ascertain inasmuch as there is no definition of “crisis” that is universally accepted; however, if “decline” is defined as a systematic decrease in population size over time, then there is evidence that some pollinators in North America representing a diversity of taxa are, in fact, in decline. It is accordingly important to ascertain the causes and the consequences of those declines as a step toward informed decision making about action to be taken and what would most likely ensure successful reversal.









































