1
Chlorine Dioxide1
Acute Exposure Guideline Levels (AEGLs)
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/ AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min, 1 h, 4 h, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain
asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, non-sensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Chlorine dioxide (ClO2) is a yellow to reddish-yellow gas at room temperature. It has an unpleasant odor, similar to the odor of chlorine and reminiscent of nitric acid. It is a respiratory irritant. Pure chlorine dioxide is stable in the dark and unstable in light. Inhaled (airborne) chlorine dioxide acts primarily as a respiratory tract and ocular irritant. In air, chlorine dioxide readily dissociates both thermally and photochemically and may form chlorine, oxygen, hydrogen chloride, HClO3, HClO4.ClO, chlorine peroxide, and/or chlorine trioxide, dependent on temperature and humidity. Chlorine dioxide dissociates in water into chlorite and chloride, and to a lesser extent into chlorate (Budavari et al. 1996). The major use of chlorine dioxide is that of chemical pulp bleaching. Other uses include drinking water disinfection, the bleaching of textiles, flour, cellulose, leather, fats, oils, and beeswax; taste and odor control of water; as an oxidizing agent; and the in manufacture of chlorite salts (ACGIH
2001). In 2001, chlorine dioxide was used to decontaminate public buildings in the United States after the release of anthrax spores (ATSDR 2002). The acute inhalation database for chlorine dioxide is quite sparse for both human and animal exposures.
The AEGL-1 was based on slight salivation, slight lacrimation, and slight chromodacryorrhea in rats exposed to 3 ppm chlorine dioxide for 6 h (DuPont 1955). A modifying factor of 2 was applied to account for the sparse data base. Interspecies and intraspecies uncertainty factors of 3 each were applied because chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues; this type of port-of-entry effect is not expected to vary greatly between species or among individuals. Thus, the total uncertainty/modifying factor is 20. Using the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-1 values that are not supported by the total data set by yielding a value of 0.05 ppm, which is considered excessively low in light of the fact that no irritation was noted in rats exposed to 0.1 ppm chlorine dioxide 5 h/day for 10 weeks (Dalhamn 1957) and no irritation was noted in rats exposed at 5 ppm for 15 min, 2 or 4 times/day for 1 month (Paulet and Desbrousses 1974). The AEGL-1 value was held constant across all time points because minor irritation is not likely to be time dependent.
The AEGL-2 was based on lacrimation, salivation, dyspnea, weakness, and pallor in rats exposed to 12 ppm chlorine dioxide for 6 h (DuPont 1955). Interspecies and intraspecies uncertainty factors of 3 each were applied because chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues; this type of port-of-entry effect is not expected to vary greatly between species or among individuals. A modifying factor of 2 was also applied to account for the sparse data base. Thus, the total uncertainty/modifying factor is 20. Using the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-2 values that are not supported by the total data set by yielding a 4-h AEGL-2 value of 0.23 ppm, yet rats repeatedly exposed to 3 ppm chlorine dioxide (Dupont 1955), 6 h/day for 10 days showed only minor irritation (slight salivation, slight lacrimation, and slight red ocular discharge on the first day of the study). This comparison shows that a combined uncertainty/modifying factor of 60 is excessively large. The concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al.
1986). To obtain conservative and protective AEGL values in the absence of an empirically derived chemical-specific scaling exponent, temporal scaling was performed using n = 3 when extrapolating to shorter time points (30 min, 1 h, and 4 h) and n = 1 (8 h) when extrapolating to longer time points using the Cn × t = k equation. The 30-min AEGL-2 value was also adopted as the 10-min AEGL-2 value due to the added uncertainty of extrapolating from a 6-h time point to 10-min.
The AEGL-3 was based on a study showing no deaths in rats exposed to 26 ppm chlorine dioxide for 6 h (DuPont 1955). Chlorine dioxide is highly reactive and causes serious adverse effects in the lung, including congestion and pulmonary edema. These effects are presumed to be the cause of death and are likely caused by a direct chemical effect on the tissue in the lung. As this effect is not expected to vary greatly among individuals or between species, intraspecies and interspecies uncertainty factors of 3 each were applied. A modifying factor of 2 was applied to account for the relatively sparse data base. Thus, the total uncertainty/ modifying factor is 20. Using the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-3 values that are not supported by the total data set by yielding a 4-h AEGL-3 value of 0.50 ppm. The value of 0.50 ppm is too low because it is below the 4-h AEGL-2 value of 0.69 ppm which was shown to be a reasonable lower limit of the disabling AEGL-2 value (see rationale above). The concentration-exposure time relationship for many irritant and systemically-acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain conservative and protective AEGL values in the absence of an empirically derived chemical-specific scaling exponent, temporal scaling was performed using n = 3 when extrapolating to shorter time points (30 min, 1 h, and 4 h) and n = 1 (8 h) when extrapolating to longer time points using the Cn × t = k equation. The 30-min AEGL-3 value was also adopted as the 10-min AEGL-3 value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. The proposed values appear in Table 1-1.
I.
INTRODUCTION
Chlorine dioxide (ClO2) is a yellow to reddish-yellow gas at room temperature. It has an unpleasant odor, similar to the odor of chlorine and reminiscent of nitric acid. It is very reactive and a strong oxidizing agent. Pure chlorine dioxide is stable in the dark and unstable in light (Budavari
TABLE 1-1 Summary Table of AEGL Values for Chlorine Dioxide (ppm [mg/m3])
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
Slight salivation, slight lacrimation, and slight chromodacryorrhea in rats exposed to 3 ppm for 6 h (DuPont 1955) |
|
AEGL-2 (Disabling) |
1.4 (3.9) |
1.4 (3.9) |
1.1 (3.0) |
0.69 (1.9) |
0.45 (1.2) |
Lacrimation, salivation, dyspnea, weakness, and pallor in rats exposed to 12 ppm for 6 h (DuPont 1955) |
|
AEGL-3 (Lethal) |
3.0 (8.3) |
3.0 (8.3) |
2.4 (6.6) |
1.5 (4.1) |
0.98 (2.7) |
No lethality in rats exposed to 26 ppm for 6 h (DuPont 1955) |
et al. 1996). Inhaled (airborne) chlorine dioxide acts primarily as a respiratory tract and ocular irritant. In air chlorine dioxide gas readily decomposes both thermally and photochemically. Thermal decomposition is characterized by a slow induction period followed by a rapid autocatalytic phase that may be explosive if the initial concentration is above a partial pressure of 76 mm Hg. Unstable chlorine oxide may be formed as an intermediate, and the presence of water vapor is hypothesized to extend the duration of the induction period by reacting with the chlorine oxide intermediate. When water vapor concentrations are high, explosivity is minimized and all decomposition occurs in the induction phase; the water vapor inhibits the autocatalytic phase. The products of thermal decomposition of gaseous chlorine dioxide include chlorine, oxygen, hydrogen chloride, HClO3, and HClO4. The proportions of products formed depend on the ambient temperature and concentration of water vapor (Kaczur and Cawfield 1993). Photochemical decomposition of gaseous chlorine dioxide initially involves homolytic scission of the chlorine oxygen bond to form ClO and O. These products then generate secondary products including chlorine peroxide, chlorine, oxygen, and chlo-
rine trioxide (Griese et al. 1992; Kaczur and Cawfield 1993). The chlorite ion does not persist in the atmosphere either in ionic form or as chlorite salt and is not likely to be inhaled.
In aqueous media, chlorine dioxide is relatively unstable and dissociates in water into chlorite and chloride, and to a lesser extent into chlorate (Budavari et al. 1994. Chlorine dioxide is prepared from chlorine and sodium chlorite or potassium chlorate and sulfuric acid (Budavari et al. 1996). Chlorine dioxide is always made at the place where it is used because of the risk of rapid decomposition. The production volume of chlorine dioxide was estimated from the total sodium chlorate consumption for chemical pulp bleaching, as this use accounts for > 95% of all chlorine dioxide production. The annual production of chlorine dioxide in the United States was estimated to be 79, 81, 146, 226, and 361 kilotons for the years 1970, 1975, 1980, 1985, and 1990, respectively (ATSDR 2002). As stated above, the major use of chlorine dioxide is for chemical pulp bleaching. Other uses include drinking water disinfection and the bleaching of textiles, flour, cellulose, leather, fats, oils, and beeswax; taste and odor control of water; as an oxidizing agent; and in the manufacture of chlorite salts (ACGIH 2001). In 2001, chlorine dioxide was used to decontaminate public buildings in the United States after the release of anthrax spores (ATSDR 2002). Chemical and physical properties are listed in Table 1-2.
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
A bleach tank worker died after exposure to 19 ppm chlorine dioxide for an undetermined duration; whereas, another worker exposed at the same time survived (Elkins 1959). No other details were reported.
2.2.
Nonlethal Toxicity
Elkins (1959) reported that 5 ppm chlorine dioxide was “definitely” irritating to humans. No other details were reported.
Three odor thresholds have been reported for chlorine dioxide: 0.1 ppm (Ellenhorn and Barceloux 1988), 9.4 ppm (Amoore and Hautala 1983), and 15 ppm (Vincent et al. 1946). However, there are no reliable data to support these values.
TABLE 1-2 Chemical and Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
Chlorine peroxide: Chlorine oxide; Chlorine (IV) oxide |
IPCS, 1993 |
|
Molecular formula |
ClO2 |
Budavari et al. 1996 |
|
Molecular weight |
67.45 |
Budavari et al. 1996 |
|
CAS Registry Number |
10049-04-4 |
ACGIH, 2001 |
|
Physical state |
Gas |
Budavari et al. 1996 |
|
Color |
Yellow to reddish-yellow gas bluish-white liquid |
Budavari et al. 1996 |
|
Solubility in water |
3.01 g/l at 25°C and 34 mmHg (decompose) |
Budavari et al. 1996 |
|
Vapor pressure |
760 torr at 20°C |
ACGIH, 2001 |
|
Vapor density (air = 1) |
2.3 |
ACGIH, 2001 |
|
Specific gravity |
1.642 at 0°C (liquid) |
ACGIH, 2001 |
|
Melting point |
−59°C |
ACGIH, 2001 |
|
Boiling point |
11°C |
ACGIH, 2001 |
|
Odor |
Unpleasant-similar to chlorine |
Budavari et al. 1996 |
|
Conversion factors |
1 ppm = 2.76 mg/m3 |
|
Bronchitis and emphysema were reported in a 53-year-old chemist repeatedly exposed to low concentrations of chlorine dioxide over a period of several years and to higher concentrations in conjunction with three explosions (Petry 1954). Dyspnea of increasing severity and asthmatic bronchitis were reported apparently after cessation of the exposures. No exposure concentration was reported.
A 49-year-old woman was exposed to an unknown concentration of chlorine dioxide accidentally generated while bleaching dried flowers (Exner-Friesfeld et al. 1986). She initially noticed a sharp, pungent smell and experienced coughing, pharyngeal irritation, and headache. Seven hours after exposure, she was hospitalized due to a worsening cough and dyspnea. Clinical findings included tachypnea, tachycardia, and rales on asculation. Clinical chemistry revealed marked leukocytosis. The chest x-ray was normal. The vital capacity and forced expiratory volume in 1 sec were decreased, to 73% and 70% of normal, respectively, and airway resistance was correspondingly increased. Blood gas examination revealed hypoxia despite alveolar hyperventilation. Symptoms resolved with corticosteroid treatment, and a follow-up examination two years post-exposure showed normal pulmonary function.
In another case report, Meggs et al. (1995; 1996) evaluated 13 adults (12 females and 1 male) 5 years after an occupational exposure to chlorine dioxide associated with a leak in a water purification system pipe. No exposure concentration or duration data were presented. Observed long-term effects included sensitivity to respiratory irritants (13 people), disability accompanied by loss of employment (11 people), chronic fatigue (11 people), and nasal abnormalities, including talangectasia, paleness, edema, and thick mucous (13 people). Nasal biopsies from the exposed workers showed chronic inflammation with lymphocytes and plasma cells in 11 of the 13 people. This inflammation was described as mild in two persons, moderate in eight persons, and severe in one person. Nasal biopsies of three control subjects showed mild inflammation in one subject. The number of nerve fibers in biopsies from the exposed workers was greater than in biopsies from the control group.
Gloemme and Lundgren (1957) studied 12 male employees who reported symptoms after they began work with chlorine dioxide at a sulfite-cellulose production factory. Spot samples of chlorine and chlorine dioxide during normal operations were generally <0.1 ppm. Occasional leaks from faulty vacuum lines would result in “high” levels of chlorine, chlorine dioxide, and/or sulfur dioxide. Chronic bronchitis was diagnosed in 7 of the 12 workers. The workers reported breathlessness, wheezing, irritant cough, and ocular discomfort associated with the leakages.
Ferris et al. (1967) examined 147 men employed at a pulp mill; the length of employment was not reported. The workers were exposed to sulfur dioxide or chlorine and chlorine dioxide, with average chlorine dioxide concentrations ranging from 0 to 0.25 ppm and average chlorine concentrations ranging from 0 to 7.4 ppm. (Peak chlorine dioxide concentrations reached 2 ppm, and peak chlorine concentrations reached 64 ppm.) Shortness of breath, excess phlegm, and bronchitis were noted in the workers, with workers exposed to chlorine or chlorine dioxide exhibiting more severe symptoms than those exposed only to sulfur dioxide.
Kennedy et al. (1991) compared health effects in 321 pulp mill workers exposed to chlorine dioxide and chlorine with 237 control workers at a rail yard. Personal time weighted average concentrations at the pulp mill were 5 to 14 ppm chlorine and <0.1 ppm chlorine dioxide. No air monitoring data from the rail yard were provided. Additionally, chlorine or chlorine dioxide “gassing” exposures from accidental releases were reported by 60% of the pulp mill workers. There were increased incidences of wheezing and breathlessness reported by pulp mill workers
compared to the rail yard workers; however, pulmonary function tests did not reveal any significant differences between the pulp mill workers and the rail yard controls. Airflow obstruction, as measured by FEV1, was increased (p < 0.05) in the pulp mill workers experiencing “gassing” incidents compared with those not experiencing “gassing” incidents.
2.3.
Developmental/Reproductive Effects
No data concerning developmental or reproductive effects of chlorine dioxide inhalation in humans were identified in the available literature; however, epidemiological studies of populations consuming chlorine dioxide- treated drinking water were located. A retrospective study was conducted using 1940s birth records from Chicopee, Massachusetts; this community utilized “relatively high” levels of chlorine dioxide for water disinfection (Tuthill et al. 1982). The morbidity and mortality experience of infants born in Chicopee was compared to that of infants born in Holyoke, Massachusetts, a geographically contiguous community that utilized traditional chlorination practices. There was no difference in fetal, neonatal, or infant mortality; or in birthweight, sex ratio or birth condition between infants born in the two communities. There was an apparent increase (p < 0.05) in the number of infants judged as premature by physician assessment in the chlorine-dioxide-exposed population (7.8%) compared with the control community (5.8%). However, there was no increase in prematurity when data were evaluated controlling for the age of the mother.
In another study, Kanitz et al. (1996) conducted an epidemiological study comparing 548 infants born to mothers (Genoa, Italy) who had consumed water disinfected with chlorine dioxide (<0.3 mg/mL) and/or sodium hypochlorite with 128 infants born to mothers (Chiavari, Italy) who had consumed primarily untreated well water. There was a higher frequency of infants with small (≤49.5 cm) body length in mothers exposed to chlorinated water (chlorine dioxide adjusted odds ratio [OR] = 2.0 [95% CI = 1.2-3.3]; sodium hypochlorite OR = 2.3 [95% CI = 1.3-4.2]) compared with those exposed to well water. There was also a higher frequency of infants with small (≤35 cm) cranial circumference in mothers exposed to chlorinated water (chlorine dioxide adjusted OR = 2.2 [95% CI = 1.4-3.9]; sodium hypochlorite OR = 3.5 [95% CI = 2.1-8.5]) compared with those exposed to well water. There was also an approximate doubling of cases of neonatal jaundice in infants of mothers who consumed the disinfected water. The conclusions that can be drawn
from this study are confounded by lack of quantitative exposure information, possible exposure to other chemicals in the water, and lack of consideration of nutritional and smoking habits and maternal age distribution between the two populations.
2.4.
Genotoxicity
No data concerning the genotoxicity of chlorine dioxide in humans were identified in the available literature.
2.5.
Carcinogenicity
No data concerning the carcinogenicity of chlorine dioxide in humans were identified in the available literature.
2.6.
Summary
Deaths from chlorine dioxide exposure have occurred but exposure concentrations are unknown. Exposures that failed to result in mortality suggest that chlorine dioxide is a respiratory irritant causing wheezing, cough, dyspnea, decreased pulmonary function, and nasal pathology. Specific exposure levels and/or durations for specific symptoms were not available and were confounded by concurrent exposures to other chemicals. Information on developmental/ reproductive effects was available only for the oral route of exposure from disinfected drinking water and these studies contain many confounding variables, making it impossible to definitively attribute the effects to chlorine dioxide. No genotoxicity or carcinogenicity data were located.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Lethality
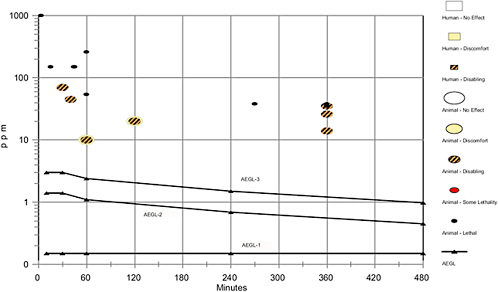
Acute lethality data are limited. A series of experiments in rats reports both lethal and nonlethal effects (DuPont 1955; Dalhamn 1957). Other lethality studies in guinea pigs, rabbits, mice, and rats are not well described. The limited data are summarized in Table 1-3.
TABLE 1-3 Summary of Acute Lethal Inhalation Data in Laboratory Animals
|
Species |
Concentration (ppm) |
Exposure Time |
Effect |
Clinical Signs/Comment |
Reference |
|
Rat |
54 |
1 h |
Death during exposure |
Cyanosis, dyspnea, salivation, lacrimation, chromodacryorrhea |
DuPont 1955 |
|
Rat |
38 |
4.5-6 h |
Death 4.5 h into exposure and 24-h post-exposure |
|
|
|
Rat |
26 |
6 h |
No mortality |
|
|
|
Rat |
260 |
4 h |
Death 1 h into exposure |
Ocular discharge, epistaxis, pulmonary edema, circulatory engorgement |
Dalhamn 1957 |
|
Rat |
10 |
4 h/day x 9 days (over 13 day period) |
Death after 10-13 days |
Decreased body weight (2nd day), respiratory infection, kidney and liver congestion |
|
|
Rat |
70 |
30 min |
No mortality |
Difficulty breathing |
Hecht 1950 |
|
Rat |
35 |
6 h |
Death |
— |
|
|
Rat |
20 |
2 h |
No mortality |
No signs reported |
|
|
Rat |
10 |
1 h |
No mortality |
No signs reported |
|
|
Species |
Concentration (ppm) |
Exposure Time |
Effect |
Clinical signs/ Comment |
Reference |
|
Mouse |
70 |
30 min |
Death |
Animals died night following exposure |
|
|
Mouse |
35 |
6 h |
Death |
— |
|
|
Mouse |
20 |
2 h |
No mortality |
No signs reported |
|
|
Mouse |
10 |
1 h |
No mortality |
No signs reported |
|
|
Guinea pig |
150 |
44 min |
Death |
Death occurred during exposure |
Haller & Northgraves1955 |
|
Guinea pig |
150 |
5-15 min |
Death |
Death occurred 5-15 min post- exposure |
|
|
Guinea pig |
1,000 |
3 min |
Death |
— |
|
|
Guinea pig |
150 |
45 min |
Death |
— |
Taylor et al. 1940 |
|
Guinea pig |
45 |
40 min |
No mortality |
— |
|
|
Guinea pig |
14-17 |
6 h |
No mortality |
— |
|
|
Guinea pig |
70 |
30 min |
No mortality |
Difficulty breathing |
Hecht 1950 |
|
Guinea pig |
35 |
6 h |
No mortality |
Difficulty breathing |
|
|
Guinea pig |
20 |
2 h |
No mortality |
Difficulty breathing |
|
|
Guinea pig |
10 |
1 h |
No mortality |
Difficulty breathing |
|
|
Rabbit |
70 |
30 min |
Death |
Death occurred during exposure |
|
|
Rabbit |
35 |
6 h |
Death |
— |
|
|
Rabbit |
20 |
2 h |
No mortality |
Difficulty breathing |
|
|
Rabbit |
10 |
1 h |
No mortality |
Difficulty breathing |
|
3.1.1.
Rats
DuPont (1955) conducted a series of acute lethality experiments in male Sprague-Dawley rats. Chlorine dioxide was generated by adding a sodium chlorite solution dropwise at a constant rate into a heated flask containing 85% phosphoric acid. Metered air was passed through the flask and then into a bell jar containing 2 or 4 rats. The chlorine dioxide concentration was determined analytically at least 3 times during each exposure period. Groups of two rats were exposed to 54 ppm chlorine dioxide for 1 h or to 38 ppm chlorine dioxide for 4.5 to 6 h. Both rats exposed to 54 ppm died during exposure. One rat exposed to 38 ppm died 4.5 h into the exposure, whereas, the other rat died within 24 h after exposure. Death was attributed to pulmonary congestion and edema observed at necropsy. No lethality was noted in a group of four rats exposed to 26 ppm chlorine dioxide for 6 h (DuPont 1955), and no pathology was observed in the one animal sacrificed 24 h post-exposure or in the remaining three animals sacrificed 10-days post-exposure. Clinical signs were similar in all treatment groups and included cyanosis, dyspnea, salivation, lacrimation, and chromodacryorrhea.
Dalhamn (1957) conducted a series of four experiments to examine both lethal and nonlethal effects of chlorine dioxide inhalation in an unspecified sex and strain of rats. The chlorine dioxide gas was generated by combining hydrochloric acid with solid sodium chlorite. The resulting chlorine and chlorine dioxide gasses were then led into a wash bottle containing sodium chlorite solution. The chlorine gas reacted with this solution to form chlorine dioxide. The chlorine dioxide was then dissolved in distilled water to avoid the presence of chlorine gas in the experiments. Compressed air was then bubbled through the pure chlorine dioxide solution to generate the chlorine dioxide for the rats to inhale. In one experiment, four rats were exposed to approximately 260 ppm chlorine dioxide for 2 h. To maintain a fairly constant concentration, the gas was changed every half-hour; the reported concentrations were 265, 264, 266, and 245 ppm, respectively. Ocular discharge and epistaxis were observed, and one rat died after exposure for 1 h. The other rats were sacrificed immediately after the 2-h exposure. Pulmonary edema and circulatory engorgement were noted in all four rats; however, no control data were presented.
In another experiment, Dalhamn (1957) exposed five rats to 0 or 10 ppm chlorine dioxide 4 h/day for 9 days over a 13 day period. The exposed rats exhibited rhinorrhea and labored respiration and weighed 20-33% less than controls from days 10 to 13. One rat died after 10 expo-
sures, two died on day 11, and the final two died on day 13. Necropsy showed respiratory infection, and acute renal and hepatic congestion in treated rats. No effects were noted in controls.
Hecht (1950) reported that rats exposed to 35 ppm chlorine dioxide for 6 h died. No deaths were reported in rats exposed to 70 ppm for 30 min, 20 ppm for 2 h, or 10 ppm for 1 h. No experimental details were described.
3.1.2.
Mice
Hecht (1950) reported that mice exposed to 70 ppm chlorine dioxide for 30 min died during the night following exposure. Mice exposed to 35 ppm for 6 h died, and no deaths were reported in mice exposed to 20 ppm for 2 h, or 10 ppm for 1 h. No experimental details were described.
3.1.3.
Guinea Pigs
Hecht (1950) reported no deaths in guinea pigs exposed to 70 ppm chlorine dioxide for 30 min, 35 ppm for 6 h, 20 ppm for 2 h, or 10 ppm for 1 h. No experimental details were described.
Taylor et al. (1940) reported lethality in guinea pigs exposed to 150 ppm chlorine dioxide for 45 min. No deaths were reported in guinea pigs exposed to 14-17 ppm for 6 h or 45 ppm for 45 min. No experimental details were described.
Haller and Northgraves (1955) reported that guinea pigs died during exposure to 150 ppm chlorine dioxide for 44 min. Deaths were also reported in guinea pigs exposed to 150 ppm for 5-15 min and 1,000 ppm for 3 min. No experimental description was provided.
3.1.4.
Rabbits
Hecht (1950) reported deaths in rabbits exposed to 70 ppm chlorine dioxide for 30 min and 35 ppm for 6 h. No deaths were reported in rabbits exposed to 20 ppm for 2 h or 10 ppm for 1 h. No experimental details were described.
3.2.
Nonlethal Toxicity
3.2.1.
Rats
DuPont (1955) conducted a series of repeated-exposure experiments in male Sprague-Dawley rats. Chlorine dioxide was generated by adding a sodium chlorite solution dropwise at a constant rate into a heated flask containing 85% phosphoric acid. Metered air was passed through the flask and then into a bell jar containing 2 or 4 rats. The chlorine dioxide concentration was determined analytically at least three times during each exposure period. A group of four rats was exposed to 12 ppm chlorine dioxide, 6 h/day for 6 or 7 days. Clinical signs observed on the first day of the study included lacrimation, salivation, dyspnea, weakness, and pallor. These signs increased in severity with repeated exposures. All of the rats survived through the sixth exposure. Two of the rats died after the sixth exposure, and two were sacrificed for pathology after the seventh exposure. Necropsy revealed acute bronchitis and emphysema, but no evidence of pulmonary edema, in all four rats.
DuPont (1955) also similarly exposed a group of four rats to 3 ppm chlorine dioxide, 6 h/day for 10 days. Clinical signs observed on the first day of the study included slight salivation, slight lacrimation, and slight chromodacryorrhea. These signs increased in severity with repeated exposures. No animals died, and no gross or microscopic pathology was observed when rats were sacrificed immediately after the tenth exposure.
As mentioned in Section 3.1.1, Dalhamn (1957) conducted a series of four experiments to examine both lethal and nonlethal effects of chlorine dioxide inhalation in an unspecified sex and strain of rats. In one experiment, three rats were exposed once a week for 3 min to decreasing concentrations of chlorine dioxide; the animals were exposed to 3435 ppm chlorine dioxide on day 1, to 1,118 ppm on day 8, and to 760 ppm on day 16. A group of three rats was exposed to compressed air and served as controls. Respiratory distress was observed and mean body weight of the exposed rats was 10% below that of controls on day 16. Necropsy revealed bronchopneumonia and hyperemia of the renal corticomedullary junction in two of the exposed rats; however, the lungs and kidneys of the third exposed rat were normal. The lungs were normal in all three control rats; however, renal hyperemia was noted in two.
In another experiment, Dalhamn (1957) exposed groups of five rats to 0 or 0.1 ppm chlorine dioxide 5 h/day for 10 weeks. No clinical signs were observed during treatment and no treatment-related effects were noted at necropsy.
Paulet and Desbrousses performed a series of repeated-exposure studies in rats. Unfortunately, for the purposes of AEGL derivation, data after single exposures were not reported. Groups of five male and five female rats were exposed to 10 ppm chlorine dioxide, 2 h/day for 30 days. Groups of 10 male and 10 female rats were exposed to 5 ppm, 2 h/day for 30 days, and groups of 10 male and 10 female rats were exposed to 2.5 ppm, 7 h/day for 30 days (Paulet and Desbrousses (1970). The strain of rat was not specified; control groups with equal numbers of rats were used for each exposure scenario. Nasal discharge, red eyes, and bronchopneumonia accompanied by desquamation of alveolar epithelium were observed at 5 and 10 ppm, with effects being more severe at 10 ppm. Increased erythrocyte and leukoctye counts were noted in animals exposed to 10 ppm chlorine dioxide. Rats exposed to 2.5 ppm exhibited lymphocytic infiltration of the alveolar spaces, alveolar vascular congestion, hemorrhagic alveoli, epithelial erosions, and inflammatory infiltrations of the bronchi. No effects were reported in control animals. In another report, Paulet and Desbrousses (1972) exposed a group of eight Wistar rats (sex not reported) to 1 ppm chlorine dioxide, 5 h/day, 5 days/week for 2 months. Vascular congestion and peribronchiolar edema were observed at necropsy.
In another study, Paulet and Desbrousses (1974) exposed groups of 10-15 rats (sex and strain not reported) to 0, 5, 10, or 15 ppm chlorine dioxide, 15 min, 2 or 4 times/day for 1 month. At 15 ppm, mortality was observed in 1/10 rats exposed 2 times/day and in 1/15 rats exposed 4 times/day. Decreased body weight, nasal and ocular inflammation and discharge, bronchitis, and peribronchiolar lesions were observed at 15 ppm; effects were more severe in animals exposed 4 times/day. Alveolar irritation and decreased body weight were observed at 10 ppm, and no effects were reported at 5 ppm.
3.2.2.
Rabbits
A group of four rabbits was exposed to 5 ppm chlorine dioxide, 2 h/day for 30 days, and a group of eight rabbits was exposed to 2.5 ppm, 4 h/day for 45 days (Paulet and Desbrousses 1970). The strain and sex were not specified; control groups with equal numbers of rabbits were used for each exposure scenario. Nasal discharge, red eyes, and bronchopneumonia accompanied by desquamation of alveolar epithelium were observed at 5 ppm. Rabbits exposed to 2.5 ppm exhibited hemorrhagic alveoli and congested capillaries in the lungs at study termination. Pul-
monary effects had resolved in animals sacrificed 15 days after exposure termination (2.5 ppm group).
3.3.
Developmental/Reproductive Effects
No information regarding developmental/reproductive effects of chlorine dioxide in animals via the inhalation route was located in the available literature. However, several oral studies were available. No developmental or reproductive effects were noted in Long-Evans rats administered daily gavage doses of 0, 2.5, 5, or 10 mg/kg chlorine dioxide in water (Carlton et al. 1991). Groups of 12 males were treated for 56 days prior to mating and throughout the 10-day mating period, and groups of 24 females were treated 14 days prior to mating, during the mating period and during gestation and lactation.
In another gavage study, Toth et al. (1990) administered daily doses of 0 or 14 mg/kg chlorine dioxide to four male and four female Long-Evans rat pups on postnatal days 1-20. Body weight of treated rats was decreased on days 11, 21, and 35, and forebrain weight was decreased on days 21 and 35. Decreased protein content and DNA content of the brain were also observed on days 21 and 35.
Taylor and Pfohl (1985) administered 0 or 100 ppm chlorine dioxide to female Sprague-Dawley rats in the drinking water 14 days prior to gestation and throughout gestation and lactation. Decreased brain weight, due mainly to a decrease in cerebellar weight, was observed in 21-day-old pups from treated dams. A decrease in total cerebellar DNA content was also noted in these pups. A decrease in exploratory behavior was observed in the 60-day-old pups of treated dams.
Taylor and Pfohl (1985) also administered 0 or 14 mg/kg chlorine dioxide to male Sprague-Dawley rat pups from untreated dams via gavage on postnatal days 5 to 20. Decreased body weight, absolute and relative whole brain and forebrain weights, and forebrain DNA content were observed in 21-day-old treated pups. Decreased home cage activity was observed on days 18-19, and wheel-running activity was decreased on day 10. No other effects were reported.
In another study, groups of six to eight female Sprague-Dawley rats were administered 0, 1, 10, or 100 ppm chlorine dioxide in the drinking water for 2.5 months prior to mating and during gestation days 0-20 (Suh, et al. 1983). There was a trend for decreasing number of implants per litter and number of live fetuses per dam. Total fetal weight and male fetal weight were increased at 100 ppm.
In another drinking water study, groups of 12 female Sprague-Dawley rats were administered 0 or 100 ppm chlorine dioxide for 10 days prior to mating and during the gestation and lactation periods (Mobley et al. 1990). The litter weight of treated animals was lower than controls at birth. Also, chlorine dioxide treated pups exhibited decreased exploratory activity on postconception days 36-38 but not on days 39-40.
3.4.
Genotoxicity
No information regarding the genotoxicity of chlorine dioxide in animals via inhalation was located in the available literature. Chlorine dioxide was positive in an in vivo micronucleus assay in mice after an i.p. injection of 3.2-25 mg/kg chlorine dioxide (Hayashi et al. 1988). Meier et al. (1985) administered 0.1 to 0.4 mg chlorine dioxide by gavage to Swiss CD-1 mice for 5 consecutive days; there was no evidence of increased incidences of micronuclei or bone marrow chromosomal aberrations and no effect on sperm head morphology. In an in vitro study, chlorine dioxide was negative for chromosome aberrations in Chinese hamster fibroblast cells (Ishidate et al. 1984). It was negative in the Salmonella typhimurium reverse mutation assay without activation and positive with activation (Ishidate et al. 1984); however, water samples disinfected with chlorine dioxide were negative both with and without activation (Miller et al. 1986).
3.5.
Chronic Toxicity/Carcinogenicity
No information regarding the carcinogenicity of chlorine dioxide in animals via the inhalation route was located in the available literature. In a dermal exposure study, the dorsal area of groups of five female SENCAR mice were shaved and the mice were placed in chambers containing 0, 1, 10, 100, 300, or 1000 ppm chlorine dioxide dissolved in water, 10 min/day for 4 days (Robinson et al. 1986). The chambers were designed to prevent inhalation of vapors. An increase in epidermal thickness, suggesting epidermal hyperplasia, was noted at 300 and 1000 ppm.
Miller et al. (1986) tested the carcinogenic potential of concentrates prepared from chlorine dioxide disinfected drinking water in several short-term assays. The concentrates did not increase the incidence of lung adenomas in strain A mice, skin tumor frequency in SENCAR mice, or gammaglutamyl transpeptidase positive foci in rat livers.
3.6.
Summary
Lethality data are very limited; no LC50 values were available. Reports of lethality were available for rats, mice, guinea pigs and rabbits; however, experimental details were generally poorly reported. Pulmonary congestion and edema were noted at necropsy in some animals after lethal exposure to chlorine dioxide. Sublethal studies were also limited and most used repeat exposure protocols; however, limited data were available describing clinical signs observed after the first exposure. Chlorine dioxide is an irritant as evidenced by lacrimation, salivation, dyspnea, weakness, and pallor, and by difficulty breathing, nasal discharge, ocular irritation and pneumonia observed in rats during or after exposure to sublethal concentrations of chlorine dioxide. Developmental delays were observed in animals following ingestion of chlorine dioxide in water. Genotoxicity studies with chlorine dioxide yielded both positive and negative results and no long-term carcinogenicity studies were available.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
No data concerning the metabolism of chlorine dioxide after inhalation were available.
Information regarding the metabolism of chlorine dioxide via ingestion is available. The chloride ion is the ultimate metabolite of chlorine dioxide after oral administration. After a single gavage dose of 100 mg/L 36ClO2 in rats, 87% of the radiolabeled chlorine in the urine was in the form of chloride ion and 80% of the label in the plasma was in the form of chloride (Abdel-Rahman et al. 1980a). Chlorite was also a major metabolite accounting for 11% and 21% of label in the urine and plasma, respectively. Approximately 2% of the urinary 36Cl was in the form of chlorate. The radioactive label was primarily excreted in the urine; during the first 24 h after dosing, 18% of the label was in the urine, and 4.5% was detected in the feces. The 72-h sample contained 31% of the label in the urine and 4.5% in the feces. No label was detected in expired air. No parent compound was detected in the urine (Abdel-Rahman et al. 1984a).
The metabolic pathways of inhaled and ingested chlorine dioxide are likely different because chlorine dioxide dissociates in air to form chlorine, oxygen, hydrogen chloride, HClO3, HClO4.ClO, chlorine per-
oxide, and/or chlorine trioxide, dependent on temperature and humidity (Griese et al. 1992; Kaczur and Cawfield 1993) and because chlorite does not persist in the atmosphere either in the ionic form or as the chlorite salt. Given the fact that gaseous chlorine dioxide readily decomposes, it is unlikely that chlorite would be formed from parent chlorine dioxide in the aqueous mucus of the upper respiratory tract; and if the chlorite were to form, it is unlikely that a sufficient amount would be absorbed to induce toxic effects similar to those noted after ingestion of chlorine dioxide in aqueous media. Therefore, use of metabolic information regarding exposure to chlorine dioxide in aqueous media is rather limited for the purposes of derivation of AEGL values for inhalation exposure.
4.2.
Mechanism of Toxicity
Inhaled (airborne) chlorine dioxide primarily acts as a respiratory tract and ocular irritant. Lacrimation, salivation, dyspnea, weakness, pallor, and pulmonary congestion and edema were noted in rats after acute exposure to chlorine dioxide (DuPont 1955). Alveolar congestion and hemorrhage, bronchial inflammation, and peribronchiolar edema have also been noted in rats and rabbits after inhalation of chlorine dioxide (Paulet and Desbrousses 1970, 1972, 1974). Limited data from human exposure also indicate respiratory irritation (Elkins 1959; Exner-Friesfeld et al. 1986; Meggs et al. 1996).
After oral exposure to sufficiently high doses, chlorine dioxide may produce hematologic effects such as methemoglobenia and Heinz Body hemolytic anemia. Due to its highly reactive nature, it is unlikely that chlorine dioxide would be absorbed in amounts great enough to produce this toxicity directly. Chlorite is produced and absorbed following oral exposure to chlorine dioxide in animals (Abdel-Rahman et al. 1980a), and is likely responsible for the hematological effects. Chlorite has been shown to be more efficient than chlorine dioxide in the production of methemoglobin, in decreasing blood glutathione, and in alteration of red blood cells (Abdel-Rhaman et al. 1980b; 1984b). Furthermore, in vitro studies have shown that ample amounts of glutathione may prevent chlorine-dioxide (chlorite)- induced osmotic fragility by prevention of the formation of disulfide bonds between hemoglobin and cell membrane components (Abdel-Rhaman et al. 1984b). Thus, it is the chlorine dioxide metabolites and byproducts (especially chlorite) that are responsible for toxicological effects from ingested chlorine dioxide. The chlorite
(CLO2-) does not persist in the atmosphere either in ionic form or chlorite salt and, thus is not likely to be inhaled (ATSDR 2002). Therefore, the use of information regarding exposure to chlorine dioxide in aqueous media is limited for the purposes of derivation of AEGL values for inhalation exposure.
4.3.
Structure-Activity Relationships
No structure-activity relationships were applicable for establishing AEGLs for chlorine dioxide.
4.4.
Other Relevant Information
4.4.1.
Species Differences
Data are sparse, inconsistent, and inadequate for comparing differential species sensitivities after chlorine dioxide inhalation.
4.4.2.
Susceptible Populations
No data were available concerning susceptible populations following inhalation of chlorine dioxide. However, chlorine dioxide is an ocular and respiratory irritant.
Developmental delays were observed in neonates following ingestion of chlorine dioxide, suggesting that they may be a sensitive subpopulation. However, the reason for this increased sensitivity is not known (EPA, 2000).
Smith and Wilhite (1990) have suggested that individuals undergoing hemodialysis may be at increased risk of erythrocyte damage from water disinfected with chlorine dioxide. Also, persons with an inherited deficiency of red blood cell glucose-6-phosphate dehydrogenase may also be more sensitive to water disinfected with chlorine dioxide than healthy people because chlorite produced in situ can induce Heinz body hemolytic anemia (HBHA) in people deficient in erythrocyte glucose-6-phosphate dehydrogenase (G-6-PD) (Calabrese et al. 1979; Moore et al. 1978). Furthermore, subpopulations with abnormal hemoglobins (HbM or HbH) are at increased risk to systemic chlorine dioxide poisoning because these hemoglobins are much more sensitive to oxidant chemicals,
and finally, newborns may be especially susceptible because of the high content of hemoglobin F and their very sluggish methemoglobin reductase. However, it is unlikely that these populations would also have an increased susceptibility to inhaled chlorine dioxide because the chlorite moiety is not present when exposure is via inhalation.
4.4.3.
Concentration-Exposure Duration Relationship
The concentration-exposure time relationship for many irritant and systemically-acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data were inadequate for derivation of an empirically derived-chemical specific scaling exponent for chlorine dioxide. To obtain conservative and protective AEGL values in the absence of an empirically derived chemical-specific scaling exponent, temporal scaling will be performed using n = 3 when extrapolating to shorter time points and n = 1 when extrapolating to longer time points using the Cn × t = k equation.
4.4.4.
Concurrent Exposure Issues
Occupational accidental exposures to chlorine dioxide have occurred with exposure to chlorine and sulfur dioxide; however, information relevant to derivation of AEGL values was not located.
5.
DATA ANALYSIS AND PROPOSED AEGL-1
5.1.
Human Data Relevant to AEGL-1
No human data were available for derivation of AEGL-1 values from chlorine dioxide. Occupational exposures were generally to a mixture of chlorine-containing chemicals.
5.2.
Animal Data Relevant to AEGL-1
Animal studies describing effects consistent with the definition were limited to one study. Slight lacrimation, slight salivation, and slight chromodacryorrhea were observed in rats exposed to 3 ppm chlorine dioxide for 6 h (DuPont 1955).
5.3.
Derivation of AEGL-1
The AEGL-1 was based on slight salivation, slight lacrimation, and slight chromodacryorrhea in rats exposed to 3 ppm chlorine dioxide for 6 h (DuPont 1955). A modifying factor of 2 will be applied to account for the sparse data base. Interspecies and intraspecies uncertainty factors of 3 each will be applied because chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues; this type of port-of-entry effect is not expected to vary greatly between species or among individuals. Thus, the total uncertainty/modifying factor is 20. Using the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-1 values that are not supported by the total data set by yielding a value of 0.05 ppm, which is considered excessively low in light of the fact that no irritation was noted in rats exposed to 0.1 ppm chlorine dioxide 5 h/day for 10 weeks (Dalhamn 1957) and no irritation was noted in rats exposed at 5 ppm for 15 min, 2 or 4 times/day for 1 month (Paulet and Desbrousses 1974). The AEGL-1 value was held constant across all time points because minor irritation is not likely to be time dependent. AEGL-1 values are presented in Table 1-4 and Appendix A.
6.
DATA ANALYSIS AND PROPOSED AEGL-2
6.1.
Human Data Relevant to AEGL-2
Elkins (1959) reported that 5 ppm chlorine dioxide was “definitely” irritating to humans; however no other details were reported. Other studies also reported effects consistent with the definition of AEGL-2; however, neither the exposure duration and/or concentration were clearly measured. Thus, these studies cannot be used for derivation of AEGL-2 values.
TABLE 1-4 AEGL-1 Values for Chlorine Dioxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.15 ppm (0.41 mg/m3) |
0.15 ppm (0.41 mg/m3) |
0.15 ppm (0.41 mg/m3) |
0.15 ppm (0.41 mg/m3) |
0.15 ppm (0.41 mg/m3) |
6.2.
Animal Data Relevant to AEGL-2
Animal studies describing effects at concentrations below those causing incapacitation or unconsciousness from single chlorine dioxide inhalation exposure were limited. Lacrimation, salivation, dyspnea, weakness, and pallor were observed in rats exposed to 12 ppm chlorine dioxide for 6 h (DuPont 1955). Difficulty breathing was noted in guinea pigs and rats exposed to 70 ppm for 30 min, guinea pigs exposed to 35 ppm for 6 h, and guinea pigs and rabbits exposed to 20 ppm for 2 h or 10 ppm for 1 h (Hecht 1950). No effects were noted in rats and mice exposed to 20 ppm chlorine dioxide for 2 h or 10 ppm for 1 h (Hecht 1950).
6.3.
Derivation of AEGL-2
The lacrimation, salivation, dyspnea, weakness, and pallor noted in rats exposed to 12 ppm chlorine dioxide for 6 h will be used to derive AEGL-2 values. Interspecies and intraspecies uncertainty factors of 3 each will be applied because chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues; this type of port-of-entry effect is not expected to vary greatly between species or among individuals. A modifying factor of 2 will also be applied to account for the sparse data base. Thus, the total uncertainty/modifying factor is 20. Using the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-2 values that are not supported by the total data set by yielding a 4-h AEGL-2 value of 0.23 ppm, yet rats repeatedly exposed to 3 ppm chlorine dioxide (Dupont 1955), 6 h/day for 10 days showed only minor irritation (slight salivation, slight lacrimation, and slight red ocular discharge on the first day of the study). This comparison shows that a combined uncertainty/modifying factor of 60 is excessively large. The concentration-exposure time relationship for many irritant and systemically-acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain conservative and protective AEGL values in the absence of an empirically derived chemical-specific scaling exponent, temporal scaling was performed using n = 3 when extrapolating to shorter time points (30 min, 1 h, and 4-h) and n = 1 (8 h) when extrapolating to longer time points using the Cn × t = k equation. The 30-min AEGL-2 value was also adopted as the 10-min AEGL-2 value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. AEGL-2 values appear in Table 1-5 below and calculations are in Appendix A.
TABLE 1-5 AEGL-2 Values for Chlorine Dioxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.4 ppm (3.9 mg/m3) |
1.4 ppm (3.9 mg/m3) |
1.1 ppm (3.0 mg/m3) |
0.69 ppm (1.9 mg/m3) |
0.45 ppm (1.2 mg/m3) |
7.
DATA ANALYSIS AND PROPOSED AEGL-3
7.1.
Human Data Relevant to AEGL-3
No human studies of sufficient exposure duration with measured concentrations and producing irreversible or life-threatening effects were located in the available literature.
7.2.
Animal Data Relevant to AEGL-3
Non-lethal concentrations of chlorine dioxide were identified in several studies. No deaths were noted in rats exposed to 26 ppm chlorine dioxide for 6 h (DuPont 1955). Taylor et al. (1940) found no deaths in guinea pigs exposed to 14-17 ppm for 6 h or 45 ppm for 45 min. No deaths were noted in guinea pigs and rats exposed to 70 ppm for 30 min (deaths were observed in rabbits and mice exposed to 70 ppm for 30 min (Hecht 1950). No guinea pigs died when exposed to 35 ppm chlorine dioxide for 6 h; however, rabbits, rats, and mice died when similarly exposed (Hecht 1950). Hecht (1950) observed no deaths in guinea pigs, rabbits, rats and mice exposed to 20 ppm chlorine dioxide for 2 h or 10 ppm for 1 h.
7.3.
Derivation of AEGL-3
The DuPont (1955) data showing no mortality in rats exposed to 26 ppm for 6 h will be utilized for the derivation of AEGL-3 values; this data set was chosen since DuPont (1955) is more robust than the other studies. Chlorine dioxide is highly reactive and causes serious adverse effects in the lung, including congestion and pulmonary edema. These effects are presumed to be the cause of death and are likely caused by a direct chemical effect on the tissue in the lung. As this effect is not expected to vary greatly among individuals or between species, intraspecies modifying factor of 2 will also be applied to account for the relatively sparse database. Thus, the total uncertainty/modifying factor is 20. Using
the default value of 10 for either intra- or interspecies variability would bring the total adjustment to 60 (total UF × MF) instead of 20. This would generate AEGL-3 values that are not supported by the total data set by yielding a 4-h AEGL-3 value of 0.50 ppm. The value of 0.50 ppm is too low because it is below the 4-h AEGL-2 value of 0.69 ppm which was shown to be a reasonable lower limit of the disabling AEGL-2 value (see rationale above). The concentration-exposure time relationship for many irritant and systemically-acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain conservative and protective AEGL values in the absence of an empirically derived chemical-specific scaling exponent, temporal scaling was performed using n = 3 when extrapolating to shorter time points (30 min, 1 h, and 4-h) and n = 1 (8 h) when extrapolating to longer time points using the Cn × t = k equation. The 30-min AEGL-3 value will also be adopted as the 10-min AEGL-3 value due to the added uncertainty of extrapolating from a 6-h time point to 10 min. AEGL-3 values appear in Table 1-6 below and calculations are in Appendix A.
8.
SUMMARY OF PROPOSED AEGLs
8.1.
AEGL Values and Toxicity End Points
The AEGL values are summarized in Table 1-7.
8.2.
Comparisons with Other Standards and Guidelines
The values appear in Table 1-8.
8.3.
Data Adequacy and Research Needs
The data base for single inhalation-exposure animal studies is very sparse and many of the studies that do exist are dated and poorly reported. There are also no clear data on human exposure concentrations for short inhalation exposure durations. The sparse data base necessitated application of a modifying factor.
TABLE 1-6 AEGL-3 Values for Chlorine Dioxide
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.0 ppm (8.3 mg/m3) |
3.0 ppm (8.3 mg/m3) |
2.4 ppm (6.6 mg/m3) |
1.5 ppm (4.1 mg/m3) |
0.98 ppm (2.7 mg/m3) |
TABLE 1-7 Summary of AEGL Values for Chlorine Dioxide (ppm [mg/m3])
|
Classification |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 (Nondisabling) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
0.15 (0.41) |
|
AEGL-2 (Disabling) |
1.4 (3.9) |
1.4 (3.9) |
1.1 (3.0) |
0.69 (1.9) |
0.45 (1.2) |
|
AEGL-3 (Lethal) |
3.0 (8.3) |
3.0 (8.3) |
2.4 (6.6) |
1.5 (4.1) |
0.98 (2.7) |
TABLE 1-8 Extant Standards and Guidelines for Chlorine Dioxide (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.15 |
0.15 |
0.15 |
0.15 |
0.15 |
|
AEGL-2 |
1.4 |
1.4 |
1.1 |
0.69 |
0.45 |
|
AEGL-3 |
3.0 |
3.0 |
2.4 |
1.5 |
0.98 |
|
ERPG-1a |
|
|
NA |
|
|
|
ERPG-2a |
|
|
0.5 |
|
|
|
ERPG-3a |
|
|
3 |
|
|
|
NIOSH RELb |
|
|
|
0.1 |
|
|
NIOSH IDLHc |
|
5 |
|
|
|
|
NIOSH STELd |
|
|
|
0.3 |
|
|
OSHA PEL-TWAe |
|
|
|
0.1 |
|
|
ACGIH-TLV TWAf |
|
|
|
0.1 |
|
|
ACGIH-TLV STELg |
|
|
0.3 (20 min, 3 times/day) |
|
|
|
MAK (German)h |
0.1 |
|
|
|
|
|
MAC (Dutch)i |
0.1 |
|
|
|
|
|
aERPG (Emergency Response Planning Guidelines, American Industrial Hygiene Association (AIHA 2002). The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for chlorine dioxide is not appropriate. The ERPG-2 is the maximum airborne concen tration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-2 for chlorine dioxide is based on human and animal experience and should protect sensitive individuals from irritant effects and accounts for the poor database. It is noted that subchronic animal data may support a higher value. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for chlorine dioxide is based on collective acute and subacute lethality data in animals. |
|||||
9.
REFERENCES
Abdel-Rahman, M.S., D. Couri and J.D. Jones. 1980a. Chlorine dioxide metabolism in rats. J. Environ. Pathol. Toxicol. 3: 421-430.
Abdel-Rahman, M.S., D. Couri and R.J. Bull. 1980b. Kinetics of CIO2, CIO2- and CIO3- in drinking water on blood glutathione and hemolysis in rat and chicken. J. Environ. Pathol. Toxicol. 3: 431-449.
Abdel-Rahman, M.S., D. Couri and R.J. Bull. 1984a. The kinetics of chlorite and chlorate in the rat. J. Am. Coll. Toxicol. 3: 261-267.
Abdel-Rahman, M.S., D. Couri and R.J. Bull. 1984b. Toxicity of chlorine dioxide in drinking water. J. Am. Coll. Toxicol. 3: 277-284.
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. Documentation of the Threshold Limit Values and Biological Exposure Indices: Chlorine Dioxide. Sixth ed., ACGIH, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 2002. Emergency Response Planning Guidelines for Chlorine Dioxide. AIHA, Fairfax, VA
Amoore, J. and Hautala, E. 1983. Odor as an aid to chemical safety. J. Appl. Toxicol. 3:272-289.
ATSDR (Agency for Toxic Substances and Disease Registry) 2002. Toxicological Profile for Chlorine Dioxide. Draft for Public Comment. U.S. Department of Health and Human Services, Public Health Service. Atlanta, GA.
Budavari, S. M.J. O’Neil, A. Smith, P.E. Heckelman and J.F. Kinneary (Eds.). 1996. The Merck Index, 12th ed. Merck & Co., Inc., Rahway, NJ.
Calabrese, E.J., G. Moore and R. Brown. 1979. Effects of environmental oxidant stressors on individuals with a G-6-PD deficiency with particular reference to an animal model. Environ. Health Perspect. 29: 49-55.
Carlton, B.D., Basaran, A.H., Mezza, L.E., George, E.L., and Smith, M.K. 1991. Reproductive effects on Long-Evans rats exposed to chlorine dioxide. Environ. Res. 56: 170-177.
D’Alessandro, A., W. Kuschner, H. Wong, H.A. Boushey, and P.D. Blanc. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109:331-337.
Dalhamn, T. (1957). Chlorine Dioxide: Toxicity in animal experiments and industrial risks. Arch. Ind. Health. 15:101-107.
DFG (Deutsche Forschungsgemeinschaft). 2000. List of MAK and BAT Values, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 35. Weinheim, Federal Republic of Germany: Wiley VCH.
DuPont (1955). Summary of Toxicological Evaluations of Chlorine Dioxide. Haskell Laboratory for Toxicology and Industrial Medicine, Haskell Lab Report No. 80-55 E.I. du Pont de Nemours and Company, Inc., Wilmington, DE.
Elkins, H.B. 1959. The Chemistry of Industrial Toxicology. 2nd Edition. John Wiley & Sons, New York. Pp. 89-90.
Ellenhorn, M.J. and Barceloux, D.G. (Eds). 1988. Medical Toxicology, diagnosis and treatment. Elsevier. New York.
Exner-Freisfeld, H., Hronenberger, H., Meier-Sydow, J., and Nerger, K.H. 1986. Bleaching agent poisoning with sodium chlorite. The toxicology and clinical course. Dtsch. Med. Wochenschr. 111: 1927-1930.
Ferris, B.G., Burgess, W.A., and Worcester, J. 1967. Prevalence of chronic respiratory diseases in a pulp mill and paper mill in the United States. Br. J. Ind. Med. 24: 26-37.
Gloemme, J., and Lundgren, K.D. 1957. Health hazards from chlorine dioxide. Arch. Ind. Health. 16: 169-176.
Griese, M.H., Kaczur, J.J, and Gordon, G. 1992. Combining methods for the reduction of oxychlorine residuals in drinking water. J. Amer. Water Works Asoc. 84:69-77.
Haller, J.F. and Northgraves, W.W. 1955. Chlorine dioxide and safety. TAPPI. 38: 199-202.
Hayashi, M., Kishi, M., Sofuni, T, Ishidate, M. 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem. Toxicol. 26: 487-500.
Hecht, A. 1950. Chlordioxyd- ein Gefahrliches Reizgas. Arch. Gewerbepath. U. Gewerbehyg. 13: 363-369. Farbwerke Bayer, Leverkusen.
IPCS (International Programme on Chemical Safety). 1993. International Chemical Safety Card. Chlorine Dioxide.
Ishidate, M., T. Sofani, K. Yoshikawa, M. Hayashi, T. Nohmi, M. Sawada and A. Matsuoka. 1984. Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol. 22: 633-636.
Kaczur, J.J., and Cawfield, D.W. 1993. Chlorine oxygen acids and salts (ClO2, HClO2). In: Kroschwitz, J.I., ed. Kirk-Othmer encyclopedia of chemical technology. Vol. 5. New York, NY: John Wiley & Sons, Inc. pp. 969-971.
Kanitz, S., Franco, Y., Patrone, V., Caltabellotta, M., Raffo, E., Riggi, C., Timitilli, D, and Ravera, G. 1996. Association between drinking water disinfection and somatic parameters at birth. Environ. Health Perspect. 104: 516-520.
Kennedy, S.M., Enarson, D.A., Janssen, R.G., and Chan-Yeung, M. 1991. Lung health consequences of reported accidental chlorine gas exposures among pulpmill workers. Am. Rev. Respir. Dis. 143: 74-79.
Meggs, W.J., Elksheikh, T., Metzger, W.J., and Albernaz, M.S. 1995.
Nasal pathology of persistent rhinitis after chlorine dioxide exposure. J. Allergy & Clinical Immunology. 95:260.
Meggs, W.J., Elksheikh, T., Metzger, W.J., Albernaz, M.S., and Bloch, R.M. 1996. Nasal pathology and ultrastructure in patients with chronic airway inflammation (RADS and RUDS) following an irritant exposure. J. Clinical Toxicology. 34: 383-396.
Meier, J.R., Bull, R.J., Stober, J.A., and Cimino, M.A. 1985. Evaluation of chemicals used for drinking water disinfection for production of chromosomal damage and sperm-head abnormalities in mice. Environ. Mutagen. 7: 210-212.
Miller, R.G., Kopler, F.C., and Condie, L.W. et al. 1986. Results of toxicological testing of Jefferson Parish pilot plant samples. Environ. Health Perspect. 69: 129-139.
Mobley, S.A., Taylor, D.H., Laurie, R.D., et al. 1990. Chlorine dioxide depresses T3 uptake and delays development of locomotor activity in young rats. In: Jolley, R.L., et al., eds. Water chlorination: chemistry, environmental impact, and health effects, vol 6. Chelsea, MI: Lewis Publications, pp. 347-358.
Moore, G.S., E.J. Calabrese, S.R. DiNardi and R.W. Tuthill. 1978. Potential health effects of chlorine dioxide as a disinfectant in potable water supplies. Med. Hypotheses 4: 481-496.
NIOSH (National Institute for Occupational Safety and Health). 1997. NIOSH Pocket Guide to Chemical Hazards. Publication 94-116, U.S. Department of Health and Human Services; U.S. Government Printing Office, Washington, DC.
Paulet, G. and Desbrousses, S. 1972. On the action of chlorine dioxide at low concentrations on laboratory animals. Arch. Mal. Med. Trav. Secur. Soc. 31: 97-106.
Paulet, G. and Desbrousses, S. 1970. On the toxicology of chlorine dioxide. Arch. Mal. Med. Trav. Secur. Soc. 33: 59-61.
Paulet, G. and Desbrousses, S. 1974. Action of discontinuous exposure to chlorine dioxide on the rat. Arch. Mal. Med. Trav. Secur. Soc. 35: 797-803.
Petry, H. 1954. Chlordioxyd- ein Gefahrliches Reizgas. Arch. Gewerbepath. U. Gewerbehyg. 13: 363-369.
Robinson, M., Bull, R.J., Schmaer, M., and Long, R.F. 1986. Epidermal hyperplasia in the mouse skin following treatment with alternate drinking water disinfectants. Environ. Health Perspect. 69: 293-300.
Rotman, H.H., M.J. Fliegelman, T. Moore, R.G. Smith, D.M. Anglen, C.J. Kowalski, and J.G. Weg. 1983. Effects of low concentration of chlorine on pulmonary function in humans. J. Appl. Physiol. 54:1120-1124.
SDU Uitgevers 2000. Nationale MAC List (under the auspices of the Ministry of Social Affairs and Employment), The Hague, The Netherlands.
Smith, R.P. and Wilhite, C.C. 1990. Chlorine dioxide and hemodialysis. Regul. Toxicol. Pharmacol. 11: 42-62.
Suh, D.H., Abdel-Rahman, M.S., and Bull, R.J. 1983. Effect of chlorine dioxide and its metabolites in drinking water on fetal development in rats. J. Appl. Toxicol. 3: 75-79.
Taylor, D.H. and Pfohl, R.J. 1985. Effects of chlorine dioxide on the neurobehavioral development of rats. In: Jolley, R.L., et al., eds. Water chlorination: chemistry, environmental impact, and health effects, vol 6. Chelsea, MI: Lewis Publications, pp. 355-364.
Taylor, M.C., White, J.F., Vincent, G.P., and Cunningham, G.L. 1940. Sodium chlorite (Properties and reactions). Indust. Engin. Chem. 32: 899-903.
ten Berge, W.F., A. Zwart and L.M. Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13:301-310.
Toth, G.P., Long, R.E., Mills, T.S., and Smith, M.K. 1990. Effects of chlorine dioxide on the developing rat brain. J. Toxicol. Environ. Health. 31: 29-44.
Tuthill, R.W., Giusti, R.A., Moore, G.S., et al. 1982. Health effects among newborns after prenatal exposure to Chlorine dioxide-disinfected water. Environ. Health Perspect. 46: 39-45.
U.S. EPA (U.S. Environmental Protection Agency). 2000. Toxicological Review of Chlorine Dioxide and Chlorite. In Support of Summary Information on the Integrated Risk Information System (IRIS). September, 2000. EPA/635/R-00/007.
Vincent, G.P., MacMahin, J.J., Synan, J.F. 1946. The use of chlorine dioxide in water treatment. Am. J. Public Health. 36: 1035-1037.
APPENDIX A
DERIVATION OF AEGL VALUES
Derivation of AEGL-1
|
Key study: |
DuPont 1955 |
|
Toxicity end point: |
Slight lacrimation, slight salivation, and slight chromodacryorrhea in rats exposed to 3 ppm for 6 h. |
|
Scaling: |
None. Value was held constant across time points since minor irritation is unlikely to be time dependent. |
|
Uncertainty factors: |
3 for interspecies |
|
|
3 for intraspecies |
|
Modifying factor: |
2 for sparse data base |
|
Total uncertainty/ modifying factor: |
20 |
|
|
10 min, 30 min, 1 h, 4 h, 8 h: 3 ppm ÷ 20 = 0.15 ppm |
Derivation of AEGL-2
|
Key study: |
DuPont 1955 |
|
Toxicity end point: |
Lacrimation, salivation, dyspnea, weakness, and pallor in rats exposed to 12 ppm for 6 h. |
|
Scaling: |
C3 × t = k (default for long- to short-time extrapolation) |
|
|
C1 × t = k (default for short- to long-time extrapolation) |
|
Uncertainty factors: |
3 for interspecies |
|
|
3 for intraspecies |
|
Modifying factor: |
2 for sparse database |
|
Total: |
20 |
|
Scaling: |
C3 × t = k (30 min, 1 h, 4 h) (12 ppm)3 × 6 h = 10,368 ppm·h |
|
|
C1 × t = k (8 h) (12 ppm)1 × 6 h = 72 ppm·h |
|
10 min AEGL-2 |
1.38 ppm (The 30-min AEGL-2 is adopted as the 10-min value) |
|
30 min AEGL-2 |
C3 × 0.5 h = 10,368 ppm·h |
|
|
C3 = 20,736 ppm |
|
|
C = 27.5 ppm |
|
|
30 min AEGL-2 = 27.5 ÷ 20 = 1.38 ppm |
|
1 h AEGL-2 |
C3 × 1 h = 10,368 ppm·h |
|
|
C3 = 10,368 ppm |
|
|
C = 21.8 ppm |
|
|
1 h AEGL-2 = 21.8 ÷ 20 = 1.09 ppm |
|
4 h AEGL-2 |
C3 × 4 h = 10,368 ppm·h |
|
|
C3 = 2592 ppm |
|
|
C = 13.7 ppm |
|
|
4 h AEGL-2 = 13.7 ÷ 20 = 0.69 ppm |
|
8 h AEGL-2 |
C1 × 8 h = 72 ppm·h |
|
|
C1 = 9 ppm |
|
|
8 h AEGL-2 = 9 ÷ 20 = 0.45 ppm |
Derivation of AEGL-3
|
Key study: |
DuPont 1955 |
|
Toxicity end point: |
No mortality in rats exposed to 26 ppm for 6 h. |
|
Scaling: |
C3 × t = k (default for long- to short-time |
|
|
extrapolation) |
|
|
C1 × t = k (default for short- to long-timeextrapolation) |
|
Uncertainty factors: |
3 for interspecies 3 for intraspecies |
|
Modifying factor: |
2 for sparse database |
|
Total: |
20 |
|
Scaling: |
C3 × t = k (30 min, 1 h, 4 h) (26 ppm)3 × 6 h = 105,456 ppm·h |
|
|
C1 × t = k (8 h) (26 ppm)1 × 6 h = 156 ppm·h |
|
10 min AEGL-3 |
2.98 ppm (The 30 min AEGL-3 is adopted as the 10-min value) |
|
30 min AEGL-3 |
C3 × 0.5 h = 105,456 ppm·h C3 = 210912 ppm C = 59.5 ppm 30 min AEGL-3 = 59.5 ÷ 20 = 2.98 ppm |
|
1 h AEGL-3 |
C3 × 1 h = 105,456 ppm·h C3 = 105,456 ppm C = 47.2 ppm 1 h AEGL-3 = 47.2 ÷ 20 = 2.36 ppm |
|
4 h AEGL-3 |
C3 × 4 h = 105,456 ppm·h C3 = 26,364 ppm C = 29.8 ppm 4 h AEGL-3 = 29.8 ÷ 20 = 1.49 ppm |
|
8 h AEGL-3 |
C1 × 8 h = 156 ppm·h C = 19.5 ppm 8 h AEGL-3 = 19.5 ÷ 20 = 0.975 ppm |
APPENDIX B
ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLORINE DIOXIDE (CAS No. 10049-04-4)
DERIVATION SUMMARY
|
AEGL-1 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.15 ppm |
0.15 ppm |
0.15 ppm |
0.15 ppm |
0.15 ppm |
|
Key Reference: DuPont (1955). Summary of Toxicological Evaluations of Chlorine Dioxide. Haskell Laboratory for Toxicology and Industrial Medicine, Haskell Lab Report No. 80-55 E.I. du Pont de Nemours and Company, Inc., Wilmington, DE. |
||||
|
Test Species/Strain/Number: Rat/Sprague-Dawley/male/4. |
||||
|
Exposure Route/Concentrations/Durations: Inhalation/ 3 ppm/ 6 h. |
||||
|
Effects: Slight lacrimation, slight salivation, slight chromodacryorrhea. |
||||
|
End point/Concentration/Rationale: Slight lacrimation, slight salivation, slight chromodacryorrhea in rats exposed to 3 ppm for 6 h. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 Intraspecies: 3 Chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues. This type of port-of-entry effect not expected to vary greatly between species or among individuals. Using the default value of 10 for either intra- or interspecies variability would generate AEGL-1 values that are not supported by the total data set by yielding a value of 0.05 ppm, which is considered excessively low in light of the fact that no irritation was noted in rats exposed to 0.1 ppm chlorine dioxide 5 h/day for 10 weeks (Dalhamn 1957) and no irritation was noted in rats exposed at 5 ppm for 15 min, 2 or 4 times/day for 1 month (Paulet and Desbrousses 1974). |
||||
|
Modifying Factor: 2—sparse database. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: AEGL-1 values were held constant across time points since minor irritation is unlikely to vary with time. |
||||
|
Data Adequacy: Data are extremely sparse. The AEGL-1 value is considered protective because no irritation was noted in rats exposed to 0.1 ppm chlorine dioxide 5 h/day for 10 weeks (Dalhamn, 1957) and no irritation was noted in rats exposed at 5 ppm for 15 min, 2 or 4 times/day for 1 month (Paulet and Desbrousses, 1974). |
|
AEGL-2 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.4 ppm |
1.4 ppm |
1.1 ppm |
0.69 ppm |
0.45 ppm |
|
Key Reference: DuPont (1955). Summary of Toxicological Evaluations of Chlorine Dioxide. Haskell Laboratory for Toxicology and Industrial Medicine, Haskell Lab Report No. 80-55 E.I. du Pont de Nemours and Company, Inc., Wilmington, DE. |
||||
|
Test Species/Strain/Sex/Number: rat/Sprague-Dawley/male/4. |
||||
|
Exposure Route/Concentrations/Durations: Inhalation, 12 ppm for 6 h. |
||||
|
Effects: Lacrimation, salivation, dyspnea, weakness, and pallor. |
||||
|
End point/Concentration/Rationale: Lacrimation, salivation, dyspnea, weakness, and pallor in rats exposed to 12 ppm for 6 h. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 Intraspecies: 3 Chlorine dioxide is highly reactive and clinical signs are likely caused by a direct chemical effect on the tissues. This type of port-of-entry effect is not expected to vary greatly between species or among individuals. Using the default value of 10 for either intra- or interspecies variability would generate AEGL-2 values that are not supported by the total data set by yielding a 4-h AEGL-2 value of 0.23 ppm, yet rats repeatedly exposed to 3 ppm chlorine dioxide (Dupont, 1955), 6 h/day for 10 days showed only minor irritation (slight salivation, slight lacrimation, and slight red ocular discharge on the first day of the study). |
||||
|
Modifying Factor: 2—sparse database. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: Cn × t = k, where the exponent, n, is the conservative default of 1 and k is 72 ppm·h (8 h) or 3 and k is 10,368 ppm·h (30 min, 1 h, 4 h). The 30-min AEGL-2 value was adopted as the 10-min value. |
||||
|
Data Adequacy: Both human and animal data are extremely sparse. |
||||
|
AEGL-3 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.0 ppm |
3.0 ppm |
2.4 ppm |
1.5 ppm |
0.98 ppm |
|
Key Reference: DuPont (1955). Summary of Toxicological Evaluations of Chlorine Dioxide. Haskell Laboratory for Toxicology and Industrial Medicine, Haskell Lab Report No. 80-55 E.I. du Pont de Nemours and Company, Inc., Wilmington, DE. |
||||
|
Test Species/Strain/Sex/Number: rat/Sprague-Dawley/male/2 or 4 |
||||
|
Exposure Route/Concentrations/Durations: Inhalation, 54 ppm for 1 h, 38ppm for 4.5-6 h, 26 ppm for 6 h. |
||||
|
Effects: 54 ppm, 1 h: death of 2/2 rats 38 ppm, 4.5-6 h: death of 2/2 rats 26 ppm, 6 h: No deaths of 4/4 rats |
||||
|
End point/Concentration/Rationale: No mortality/26 ppm for 6 h. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 Intraspecies: 3 Chlorine dioxide is highly reactive and causes serious adverse effects in the lung, including congestion and pulmonary edema. These effects are presumed to be the cause of death and are likely caused by a direct chemical effect on the tissue in the lung. This effect is not expected to vary greatly between species or among individuals. Using the default value of 10 for either intra- or interspecies variability would generate AEGL-3 values that are not supported by the total data set by yielding a 4-h AEGL-3 value of 0.50 ppm. The value of 0.50 ppm is too low because it is below the 4-h AEGL-2 value of 0.69 ppm which was shown to be a reasonable lower limit for the disabling AEGL-2 value. |
||||
|
Modifying Factor: 2—sparse database. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: Cn × t = k, where the exponent, n, is the conservative default of 1 and k is 156 ppm·h (8 h) or 3 and k is 105,456 ppm·h (30 min, 1 h, 4 h). The 30 min AEGL-3 value was adopted as the 10-min value. |
||||
|
Data Adequacy: Both human and animal data are extremely sparse. |
||||