4
Ethylenediamine1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/ AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min, 1 h, 4 h, and 8 h) and
are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, non-sensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
EXECUTIVE SUMMARY
Ethylenediamine (EDA) is a hygroscopic, flammable liquid and a strong base (pKa1 = 10.7; pKa2 = 7.6). EDA is a high production volume chemical, and is used to stabilize rubber latex, as an inhibitor in anti-freeze solutions, and in the preparation of dyes, insecticides, and fungicides. EDA is an eye, mucous membrane, and respiratory irritant and a known respiratory and skin sensitizer. Occupational inhalation exposure
has resulted in an asthmatic response (rhinitis, coughing, wheezing, shortness of breath, and bronchospasm).
EDA-sensitized individuals may experience more severe and/or different effects at a given exposure concentration or duration than nonsensitized people. The qualitative and quantitative differences in the response of the two groups to EDA are undefined. The derived AEGL values are for a once-in-a lifetime exposure and do not consider previous sensitization.
The level of distinct odor awareness (LOA) for EDA is 2.1 ppm (see Appendix B for LOA derivation). The LOA represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception.
AEGL-1 values were not recommended due to insufficient data. Absence of AEGL-1 values does not imply that exposure to concentrations below the AEGL-2 is without adverse effects.
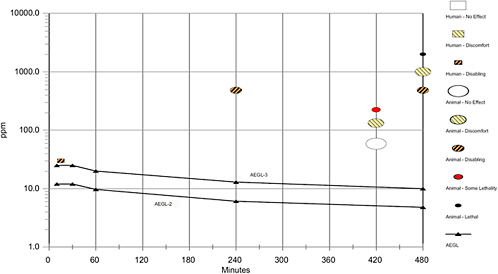
AEGL-2 values were based on a study in which rats and guinea pigs (6/group) were exposed to approximately 484 ppm EDA (1,000 ppm nominal) for 30 min to 8 h. Both species exposed for 8 h had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney” (Carpenter et al., 1948). [The same laboratory showed in another study that the analytical concentration was approximately 50% of the nominal concentration, 1,000 ppm nominal corresponding to 484 ppm analytical (Pozzani and Carpenter 1954).] This was the only single-exposure study adequate for AEGL-2 derivation. No data were available to determine the concentration-time relationship for EDA toxic effects. The concentration-time relationship for many irritant and systemically acting vapors and gases was described by ten Berge et al. (1986) with the equation Cn × t = k, where the exponent n ranged from 1 to 3 for 90% of the chemicals examined. To obtain AEGL-2 values, scaling from 8 h to 30, 60, and 240 min was performed using n = 3. The 30-min value was adopted as the 10-min value because scaling from 8 h to 10 min was associated with unacceptably large uncertainty. An uncertainty factor of 3 was used for interspecies variability because a similar response was seen in two species, and a modifying factor of 3 was used because the key study did not specify the severity of the bronchiolar edema. An intraspecies uncertainty factor of 10 was applied because the data were insufficient to determine the mode of lung and kidney lesions (or which was the more
sensitive end point) in the key study and consequently the potential variability of the human response to EDA. The AEGL-2 values are supported by a study in which 1/26 rats had unspecified lesions but no mortality after 30 exposures to 132 ppm EDA for 7 h/day (Pozzani and Carpenter, 1954).
AEGL-3 values were derived from a range-finding test (Smyth et al. 1951) in which 0/6 rats died from exposure for 8 h to ~1,000 ppm but 6/6 died from 8-h exposure to ~2,000 ppm (stated as 2000 ppm and 4,000 ppm nominal, respectively; analytical estimates based on Pozzani and Carpenter 1954). Toxic effects (other than death) were not described, and 1,000 ppm was considered to be the lethality threshold. This was the only single-exposure study adequate for AEGL-3 derivation. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the equation Cn × t = k and n = 3, as was done for AEGL-2. A total uncertainty factor of 100 was applied: 10 for interspecies variability (cause of death was undefined and there were no studies using other species) and 10 for intraspecies variability (lack of toxicity data in key study precludes definition of the mode or variability of the toxic response in humans). Target organs (liver and kidneys) were identified in a study where rats received 225 ppm EDA 7 h/day for up to 30 days (first deaths on exposure day 4), although the mode of toxicity was unclear (Pozzani and Carpenter 1954).
The values appear in Table 4-1.
TABLE 4-1 Summary of AEGL Values for Ethylenediamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End point (Reference) |
|
AEGL-1a (Nondisabling) |
Not recommended due to insufficient |
|||||
|
AEGL-2data. (Disabling) |
12 ppm (30 mg/m3) |
12 ppm (30 mg/m3) |
9.7 ppm (24 mg/m3) |
6.1 ppm (15 mg/m3) |
4.8 ppm (12 mg/m3) |
Bronchiolar edema, kidney swelling (Carpenter et al. 1948) |
|
AEGL-3 (Lethal) |
25 ppm (62 mg/m3) |
25 ppm (62 mg/m3) |
20 ppm (49 mg/m3) |
13 ppm (32 mg/m3) |
10 ppm (25 mg/m3) |
Lethality threshold (Smyth et al. 1951) |
1.
INTRODUCTION
Ethylenediamine (EDA) is a very basic, hygroscopic and fuming liquid (25% solution has a pH of 11.9 at 25°C; pKa1 = 10.7; pKa2 = 7.6). It has a low flash point (93°F; open cup) and is very flammable (Benya and Harbison 1994). EDA vapor is an eye, mucous membrane, and respiratory irritant and a well-known respiratory and skin sensitizer (Beard and Noe 1981). EDA liquid is corrosive and produces chemical burns in the skin and eyes (Carpenter and Smyth 1946; Smyth et al. 1951). The EDA odor threshold has been reported as 1.0 ppm (Verschueren 1996; Amoore and Hautala 1983) and 1-11 ppm (Ruth 1986). Occupational inhalation exposure has resulted in both immediate and delayed asthmatic symptoms including rhinitis, coughing, wheezing, shortness of breath, and bronchospasm. In animal studies, EDA vapor caused hair loss and lung, kidney, and liver damage.
EDA is used to stabilize rubber latex, as an inhibitor in antifreeze solutions, as a pharmaceutic aid (aminophylline stabilizer), in the preparation of dyes, synthetic resins, insecticides, carbamate fungicides, and asphalt wetting agents (HSDB 2005). EDA vapor readily absorbs CO2 from the air to form a non-volatile carbonate (Budavari et al. 1996). EDA is manufactured mainly by reacting ethylene chloride with aqueous or liquid ammonia at about 100°C (HSDB 2005). EDA use in chemical synthesis is in closed systems (Cary et al. 1999). EDA is a high production volume chemical: U.S. production was >58 million pounds in 1993 (HSDB 2005). Some of the chemical and physical properties of EDA are listed in Table 4-2.
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
A 36-year-old worker in France was accidentally splashed on the chest with EDA liquid and also breathed in EDA vapors for a “few minutes” (Niveau and Painchaux 1973). The exposure concentration was not determined. The man quickly removed his clothes and washed up but, nevertheless, 4 h after exposure had red-brown generalized erythema, anuria, tachycardia (up to 100/min), and symptoms of hemolysis: increased blood potassium (275 mg/L), and lowered RBC count (5.16 × 106/mm3). Twelve hours after exposure, the man had elevated body tem-
TABLE 4-2 Chemical and Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
1,2-ethanediamine; 1,2-diaminoethane |
Budavari et al. 1996 |
|
Chemical formula |
C2H8N2 |
Budavari et al. 1996 |
|
Molecular weight |
60.10 |
Budavari et al. 1996 |
|
CAS Registry Number |
107-15-3 |
Benya and Harbison 1994 |
|
Physical state |
Thick liquid |
Budavari et al. 1996 |
|
Color |
Colorless, clear |
Budavari et al. 1996 |
|
Solubility in water |
Freely soluble, forming a hydrate |
Budavari et al. 1996 |
|
Acid ionization constant, pKa |
pKa1 = 10.7; pKa2 = 7.6 |
HSDB 2005 |
|
Vapor pressure |
10.7 mm Hg at 20°C |
Parmeggiani 1983 |
|
|
10 mm Hg at 21.5°C |
Benya and Harbison 1994 |
|
|
12.1 mm Hg at 25°C |
HSDB 2005 |
|
Vapor density (air = 1) |
2.07 |
Benya and Harbison 1994 |
|
Liquid density (water = 1) |
0.898 at 25°C |
Budavari et al. 1996 |
|
Melting point |
8.5°C |
Benya and Harbison 1994 |
|
Boiling point |
116-117°C at 760 mm |
Budavari et al. 1996 |
|
Flammability/explosive limits |
2.5-12% (at 212°F) |
NIOSH 2005a |
|
Conversion factors |
1 mg/m3 = 0.407 ppm; 1 ppm = 2.46 mg/m3 |
Benya and Harbison 1994 |
perature (39°C) and pulse (120/min), coughing with expectoration, abdominal cramps, diarrhea, blackish vomiting, and anuria resulting in an increased blood urea (0.8 g/L). His condition continued to worsen and he died from cardiac collapse 55 h after exposure.
2.2.
Nonlethal Toxicity
Studies were conducted with EDA-sensitized and non-sensitized individuals. In many cases there was incomplete information about the actual EDA exposure concentration or time that elicited the human responses. Studies in which quantitative data were provided for air EDA concentration and/or exposure duration are summarized in Table 4-3.
Several secondary sources (Cary et al. 1999; IPCS 2005) list effects potentially caused in humans by EDA inhalation as irritation of the respiratory tract (a burning sensation, cough, dyspnea, a sore throat), lung edema, and an asthmatic response. It is noted that symptoms of lung edema often do not become manifest until a few hours after exposure,
TABLE 4-3 Summary of Quantitative Human Ethylenediamine (EDA) Inhalation Studies
|
Exposure Concentration |
Exposure Time |
End Point |
Reference |
|
1.0-11 ppm |
Unknown |
Odor threshold |
Hellman and Small 1974a |
|
100 ppm |
5-10 sec |
Inoffensive |
Pozzani and Carpenter 1954 |
|
200 ppm |
5-10 sec |
Slight tingling of face and nasal mucosa |
|
|
400 ppm |
5-10 sec |
Intolerably irritating to nasal mucosa |
|
|
0.4 ppm |
Not specified (≤8 h) |
Background maximal concentration; effects on workers were not addressed. |
Hansen et al. 1984 |
|
<1 to >10 ppm |
<8 h |
Asthmatic symptoms in 38 EDA-sensitized workers |
Aldrich et al. 1987 |
|
Unknown (TLV=10 ppm) |
20 min |
Delayed (2.5-4 h) asthmatic symptoms in 2 sensitized workers from workplace exposure |
Nakazawa and Matsui 1990 |
|
30 ppm |
15 min |
Severe asthmatic reaction 3 and 12 h after exposure of sensitized worker (24-33% ↓ in FEV1) |
Ng et al. 1991 |
|
Not stated; in area had 4.8, 10.5 ppm |
<8 h |
Cough, phlegm, wheezing in 12 sensitized workers; diurnal expiratory flow rate variation |
Ng et al. 1995 |
|
0.804 mg/m3 (vapor/aerosol) |
1 min |
Irritation threshold for the most“sensitive” individuals tested. |
Dubinina et al. 1997 |
|
aSame values were reported in Amoore and Hautala 1983, Verschueren 1996, and Ruth 1986. |
|||
and may mask an asthmatic reaction. However, neither source cites specific studies from which this information was obtained. Cary et al. (1999) conclude that there is insufficient data to define the dose-response for an EDA-induced asthmatic response or an exposure level “without adverse effect.”
2.2.1.
Odor Threshold/Odor Awareness
The odor detection threshold for ethylenediamine was reported to be 1.0 ppm and the 50% and 100% odor recognition thresholds were given as 3.4 ppm and 11.2 ppm, respectively by Hellman and Small (1974). The same values were listed subsequently by Amoore and Hautala (1983), Verschueren (1996), and Ruth (1986). The latter also listed the odor detection threshold as 1-11 ppm and the human irritation threshold alternately as 250 and 500 mg/m3 (102 and 204 ppm). Union Carbide Corp. (1971) reported that “workers will not stay in concentrations of 2,000 ppm.”
2.2.2.
Occupational Exposure
Four laboratory personnel intentionally sniffed 100, 200, or 400 ppm EDA vapor for 5-10 sec (Pozzani and Carpenter 1954). It was not specified if these were nominal or analytical concentrations or how the test atmospheres were generated, although in another experiment described in the same study (rat 7-h exposure), EDA atmospheres were generated using liquid EDA and an evaporator. The test subjects indicated that 100 ppm was inoffensive, 200 ppm caused a slight tingling sensation in the face and slight irritation of the nasal mucosa, and 400 ppm was intolerably irritating to the nasal mucosa.
Air EDA concentration in a Swedish petrochemical plant producing amines in a closed system was 0.4 ppm “only at a site for tanking” (Hansen et al. 1984). The number of samplings was not specified; presumably all other samples were below practical limits of detection (~0.04 ppm for same volume collected by impinger sampling method and analyzed by isotachophoreis).
Air monitoring data was conducted in a manufacturing plant where 38/337 employees who worked with EDA became sensitized: they had rhinitis, coughing, and wheezing that cleared after removal from EDA
exposure (Aldrich et al. 1987). EDA concentrations eliciting the worker responses were not provided. The vast majority of the 1,053 EDA monitoring measurements were <1 ppm (actual data were not given). The exposure period before sensitization occurred was shortest in current smokers (7.0 months) and longest for employees with no previous symptoms (37.3 months). Coater machine operators had the greatest incidence of EDA sensitization (14/54). Aldrich et al. (1987) concluded that “increased risk of EDA sensitization might be expected when workplace air concentrations of EDA exceed 1 ppm” and that perhaps the present 10 ppm EDA TLV should be reconsidered.
Nakazawa and Matsui (1990) described two cases of occupational exposure to EDA in a Japanese chemical factory. An 18-year-old man with a history of urticaria and a 37-year-old man with a history of rhinitis developed symptoms of asthma after 4 and 7 months, respectively, of exposure to unspecified concentrations of EDA (Japanese workplace limits for EDA were 10 ppm at the time). Provocative exposure tests were done on these two men when they were symptom-free: they went to work as usual and after 20 min of exposure to EDA their reactions were monitored. Wheeze and rhonchi were audible in their lungs 2.5-4 h after the 20-min exposure, and both men had ~20-40% decreased FEV1 (forced expiratory volume in 1 sec) approximately 4 h after exposure. The 18-year-old additionally had cough, wheezing, and chronic dyspnea for ~7 days, and the 37-year-old man had additional symptoms 10-18 h after exposure. A non-sensitized subject did not develop any of these symptoms upon similar EDA exposure. Upon transfer to a new work environment, neither patient showed any asthmatic symptoms. Both men had elevated peripheral blood IgE antibodies to EDA but IgG and plasma histamine levels were unaffected.
A 31-year-old non-smoking male chemical worker in Singapore without a history of asthma developed symptoms of bronchial asthma (frequent coughing, wheezing, and breathlessness) after approximately 3 months of EDA vapor exposure (Ng et al. 1991). He was also exposed to lesser amounts of other amines and organic chemicals. Measurement of his peak expiratory flow every 3 h while awake over 2 weeks showed reduced flow rates in the late mornings and afternoons (~17-24% lower FEV1), except on Saturdays and Sundays. In a bronchial provocation test, the worker inhaled 30 ppm EDA for 15 min from a respirator. He had no effects immediately after exposure, but 3 h later his peak flow rate fell by about 24% below baseline and he had chest tightness. Twelve hours after exposure, he had a severe bout of coughing, wheezing, and breathless-
ness and his peak flow rate fell another 10%. (He was administered nebulized Ventolin and his peak flow improved.) A histamine challenge test (not described) showed he had a high degree of non-specific bronchial hyperreactivity. His chest x-ray and eosinophil counts were normal. His asthmatic attacks became less frequent and severe when he reduced his EDA exposure.
Twelve men who worked in a Singapore factory manufacturing polyamide resin for a mean of 2.5 years and were exposed primarily to EDA vapor (also to other polyamines and organics) reported significantly more frequent symptoms of chronic cough, chronic phlegm, wheezing, and exertional breathlessness than unexposed workers (Ng et al. 1995). The EDA air concentration and exposure duration that elicited the stated symptoms were not specified. EDA analytical air concentrations of 10.5 and 4.8 ppm were measured in two air samples taken in areas where EDA was manually handled. The four workers who developed wheezing after beginning work at the factory had significantly greater diurnal variation in peak expiratory flow rates than the control group (DV-PEFR; measured every 3 waking hours for a week) but the FEV1, FVC, and FEV/FVC were unaffected.
Dubinina et al. (1997) determined that the irritation threshold for a 1-min exposure in humans was 0.804 mg/m3 for the most sensitive individuals tested (not stated whether these were EDA-sensitized workers). The EDA vapor concentration is unknown because it was administered as a mixture of vapor and aerosol.
Several other studies lacked sufficient EDA exposure information but provided useful descriptions of the effects of EDA exposure on humans. A 30-year-old male photography chemical mixer with late-onset asthma challenged for 15 min with an unknown concentration of EDA vapor developed asthmatic symptoms 4 h later (Lam and Chan-Yeung 1980). He had chest tightness, coughing, wheezing, and a 26% decrease in the FEV1 for 24 h after exposure. Results of a skin test (prick or intradermal with 1:100 EDA) were negative for immediate or type III reaction and precipitating antibodies to EDA were not found (Ouchterlony method). Plasma histamine levels in venous blood were not increased during bronchoconstriction. Dernehl (1951) and Lewinsohn and Ott (1991) examined medical records of approximately 200 workers exposed primarily to ethylene amines at a large chemical company (1947-1983). The concentration of EDA in the air was not reported. The employees had eye, skin, and respiratory symptoms, the latter consisting of rhinitis, congestion, coughing, wheezing, and dyspnea. The workers’ pulmonary
function (FEV1 and FVC) was not related to EDA exposure duration or sensitization status after accounting for height, age, race, cigarette smoking, and examination date. Symptoms resolved in workers transferred from the amines unit. Grant (1986) reported that industrial exposure to EDA vapors for several hours at concentrations too low to cause discomfort or disability (exposure undefined) caused reversible edema of the corneal epithelium that was generally painless and caused colored halos to be seen around lights.
Popa et al. (1969) found that 4/6 workers with EDA-induced bronchial asthma (no prior history of respiratory ailments) had bronchoconstriction immediately following a 5-min challenge with nebulized EDA. The EDA exposure concentration was 2 to 10-fold below concentrations that were non-irritating to control (non-sensitized) asthmatics, although no actual EDA concentrations were reported. The four workers had a 62% reduction in the FEV1 and a 44% increase in respiratory resistance compared to non-sensitized asthmatic controls when examined 30 or 60 min after exposure, a positive Prausnitz-Kustner IgE test, and eosinophilia in the sputum but no precipitating antibodies to EDA. The other two workers had dyspnea 1-2 h after exposure but all inhalation and immunological tests were negative. None of the workers reacted to common allergens, indicating that EDA induced a state of hypersensitivity in the airways that was specific to EDA.
2.3.
Neurotoxicity
No human neurotoxicity studies were located for EDA exposure by any route.
2.4.
Developmental/Reproductive Toxicity
No human developmental or reproductive EDA studies were found.
2.5.
Genotoxicity
No human genotoxicity data were located.
2.6.
Carcinogenicity
No human carcinogenicity studies were located with ethylenediamine exposure by any route. The ACGIH (2004) and EPA (2005) conclude that there is insufficient evidence to implicate EDA as either a human or animal carcinogen (see Section 3.5.)
2.7.
Summary
Respiratory irritation and asthma-like symptoms were described in EDA-sensitized individuals exposed to EDA concentrations ranging from <1 ppm during a workday (Aldrich et al. 1987) to 30 ppm for 15 min (Ng et al. 1991). An unusually large fraction of workers exposed to EDA vapor became sensitized and experienced asthmatic symptoms: 33% in a Singapore chemical manufacturing plant (Ng et al. 1995), 11% in a modern U.S. manufacturing facility where it was used as a process chemical (Aldrich et al. 1987), and up 17% at a large U.S. chemical company (Dernehl 1951; Lewinsohn and Ott 1991). No human genotoxicity or oncogenicity studies were located.
3.
ANIMAL TOXICITY DATA
The available single- and multiple-exposure animal studies in which the exposure concentration and duration were both specified are summarized in Table 4-4.
3.1.
Acute Lethality
3.1.1.
Rats
Using the range-finding test that their laboratory developed, Smyth et al. (1951) reported that 0/6 rats exposed to 2,000 ppm for 8 h died but 6/6 died after an 8-h exposure to 4,000 ppm EDA. No experimental details or other results were given in the study report, but subsequent publications by the same laboratory indicated that the observation period was two weeks, that the exposure concentrations were nominal and not ana-
TABLE 4-4 Summary of Quantitative Animal Ethylenediamine Inhalation Studies
|
Species |
Exposure Time |
Exposure Conc. (ppm) |
End point and Comments |
Reference |
|
Single-exposure studies |
||||
|
Rat |
30, 60, 120, 240, 480 min |
484 [1,000]a |
0/6 mortality for each exposure time; kidney cloudy swelling and lung edema seen after 8 h |
Carpenter et al. 1948 |
|
Rat |
8 h |
1,000 [2,000]a |
0/6 mortality; no effects data |
Smyth et al. 1951 |
|
|
8 h |
2,000 [4,000]a |
6/6 mortality; no effects data |
|
|
Guinea pig |
30, 60, 120, 240, 480 min |
484 [1,000]a |
0/6 mortality for each exposure time; kidney cloudy swelling and lung edema seen after 8 h |
Carpenter et al. 1948 |
|
Multiple-exposure studies |
||||
|
Rat |
7 h/day for up to30 days |
59 |
No effects noted |
Pozzani and Carpenter 1954 |
|
|
|
132 |
Hair loss, small increase in microscopic lesions |
|
|
|
|
225 |
16/20 toxic deaths (mean 17.4 days); lower body weights; liver and kidney lesions; alopecia |
|
|
|
|
484 |
27/30 toxic deaths (mean 11.4 days); liver, kidney, lung, adrenal effects; alopecia |
|
|
aStudy provided nominal concentrations, which are in brackets. The analytical concentrations listed are ~50% of the nominal concentration, based on another study by the same laboratory (Pozzani and Carpenter, 1954). |
||||
lytically verified, and that the rats could be either males or females (Smyth et al. 1962).
Sherman rats (15/sex) were exposed 7 h/day for up to 30 days to 484, 225, 132, or 59 ppm EDA (nominal concentrations of 1,000, 500, 250, and 125 ppm, respectively) (Pozzani and Carpenter 1954). Formation of a solid white reactant product on the inlet and outlet pipes and the walls of the exposure chamber was noted by the study authors, who proposed this was the reaction product of EDA with atmospheric CO2, and was responsible for the 50% discrepancy between the measured and nominal EDA concentrations (20% was a typical discrepancy for other chemicals tested by this laboratory). The EDA atmospheres were generated using liquid EDA and an evaporator and EDA concentration was determined by titration. The four exposure groups were not run concurrently, and a separate control group was provided for each exposure group. Food and water were withheld from all animals during exposure. Animals that survived the entire 30 days were killed immediately after the last exposure and their liver and kidneys were weighed. Microscopic examination was performed on the lungs, heart, liver, kidney, adrenal gland, and spleen in the three highest dose groups, and on the kidneys, lungs, and liver in the 59 ppm group.
At 484 ppm, the earliest deaths occurred on days 3 and 5 (one rat each), and all rats died within 20 days of the first exposure due to compound toxicity (11.4 days mean time to death); no controls died. Hair loss was almost complete by 10-15 exposure days. Most of the animals examined histologically had cloudy swelling in the liver and in the kidney convoluted tubules (some had kidney degeneration), and congested lungs (17/28), and some had congestion of the adrenal cortex (5/28). Of the 30 rats exposed to 225 ppm EDA, 16 had “toxic deaths,” 4 survived for 30 days, and 10 deaths were due to lung infections and were considered by the study author to be unrelated to treatment (although only 2 rats in the concurrent control group had lung infections; it was not specified whether these animals died). The mean time to death was 17.4 days, with the first animals dying on exposure days 4, 5, and 9 (2, 1, and 2 rats per day, respectively). The four surviving rats had a significantly lower weight gain and increased liver and kidney weights after 30 days than the controls, some hair loss, and most rats had cloudy swelling of the liver and kidney convoluted tubules. About 1/3 of the rats had congested lungs, however, a similar fraction of the control rats also had congested lungs. Animals exposed to 132 ppm had slight depilation and 1/26 rats (vs. 0/27 for controls) had “major” unspecified histopathological find-
ings; the 4 deaths at 132 ppm were attributed to lung infections and not considered “toxic deaths” (3 control animals had infections; death not specified). All 59 ppm rats survived the 30 exposures with no reported toxic effects.
3.1.2.
Mice
Izmerov et al. (1982) reported an inhalation LC50 of 300 ppm for the mouse. The exposure duration and other experimental details were not provided.
3.2.
Nonlethal Toxicity
3.2.1.
Rats
In the multiple-exposure study of Pozzani and Carpenter (1954), and described in section 3.1.1., Sherman rats exposed to 59 ppm 7 h/day for up to 30 days had no toxic effects, those exposed to 132 ppm had hair loss and a slight increase in the incidence of microscopic lesions, and rats exposed to 225 or 484 ppm died and/or had hair loss and liver, kidney, and lung lesions.
Male Wistar albino rats exposed to a nominal concentration of 1,000 ppm EDA for 30, 60, 120, 240, or 480 min (6 rats/exposure time) all survived the 2-week observation period (Carpenter et al. 1948). Histopathological examination of rats exposed for 8 h revealed “light cloudy swelling of the kidney” and bronchiolar edema (results for shorter exposure periods were not given).
Several published rat inhalation studies were poorly reported but help provide an overall picture of EDA acute toxicity. Dubinina et al. (1997) conducted acute and multiple-exposure rat studies in which EDA was administered as a mixture of vapor and aerosol for an unspecified number of hours/day. A single exposure to 1.94 mg/m3 caused a change in the respiration frequency of rats (faster/ slower not specified), 6.36 mg/m3 led to changes in blood catalase and peroxidase activities, 20.75 mg/m3 increased body temperature and lung lesions, and 430 mg/m3 caused mortality. Rats inhaling 2.43 mg/m3 EDA for ≥4 months had lowered body weight gains, altered CNS activity, increased eosinophil counts, catalase activity, and liver, lung, and kidney lesions; rats inhaling
0.814 mg/m3 EDA had less frequent changes in behavior, transiently elevated eosinophils and gamma-globulins, and reversible alterations in the organ histology; and rats inhaling 0.2 mg/m3 had no toxicity. Fukalova and Dubinina (1992) found that male rats exposed to 0.7 mg/m3 EDA for 2 weeks to 4 months had altered substrate specificity of monoamine oxygenase (MAO) enzymes after 2 months but no pronounced signs of toxicity.
3.2.2.
Guinea Pigs
Guinea pigs (mixed sex) were exposed to a nominal concentration of 1,000 ppm EDA for 30, 60, 120, 240, or 480 min (six pigs/exposure time) by Carpenter et al. (1948) (study described in Section 3.2.1.). All the animals survived the 2-week observation period, and microscopic examination of animals exposed for 8 h revealed “light cloudy swelling of the kidney” and bronchiolar edema of unspecified severity.
Dubinina et al. (1997) exposed guinea pigs to 2.43, 0.814, or 0.2 mg/m3 EDA vapor/aerosol for ≥4 months (hours/day not given), as described for rats in Section 3.2.1. High-dose animals had lower total body weight gain, increased relative lung and kidney weights, and microscopic lesions in the liver, lungs, and kidneys. Mid-dose animals had reversible histopathological changes, and the low-dose animals had no toxicity. A one-month exposure to 1.21 mg/m3 EDA (hours/day not given) caused “significant reorganization of the immune system,” as characterized by skin tests and by in vitro assays for immune cells (protocols not described).
3.3.
Neurotoxicity
No animal neurotoxicity studies were located with EDA exposure by any route.
3.4.
Developmental/Reproductive Toxicity
In the study by Dubinina et al. (1997) in which rats were exposed for ≥4 months to 2.43, 0.814, or 0.2 mg/m3 EDA vapor/aerosol (procedure and numerous deficiencies of this study were described in Sections
3.2.1.), the reproductive and embryotoxicity of EDA were also assessed. A statistically significant decrease in the number of spermatogonia was observed in the high-dose males (p<0.01). There were no changes in ovarian function, or in the pre- and postimplantation fetal morbidity, the number of progeny per female, or the body weight of the offspring. However, the offspring of exposed males and unexposed females had changes in leukocyte counts, whereas offspring of exposed females and unexposed males had a delay in body mass increase, changes in CNS characteristics, and decreased levels of peripheral blood hemoglobin, erythrocytes and leukocytes. Exposure to 0.814 mg/m3 EDA led to no gonad morphofunctional changes, although minor changes in the progeny of the experimental animals (behavior, levels of blood eosinophils and gamma-globulins) were observed. The lowest exposure concentration caused no toxicological effects.
Several developmental or reproductive studies were conducted on animals by oral EDA exposure. No teratogenic effects were found in fetuses of pregnant female F344 rats given 50, 250, or 1,000 mg EDA-2HCl/kg/day in the diet during gestation days 6-15 in a conventional teratogenicity study, or given 0 or 1,000 mg EDA/kg/day in a pair-feeding study (DePass et al. 1987). No reproductive toxicity was seen in a two-generation study in which F344 rats were given 50, 150, or 500 mg EDA dihydrochloride/kg/day in the diet (Yang et al. 1984a). Parameters examined included the fertility index, days from mating to parturition, the fraction of pregnancies resulting in litters with live pups, fraction of pups alive at birth, litter size, and 0-4 day, 4-14 day, and 4-21 day pup survival indices and body weight. Both sexes of the high dose F0 and F1 parents, however, had toxic effects (lowered weight gain, decreased liver weight, increased kidney weight, and hepatocellular pleiomorphism). No maternal or fetal toxicity occurred at gestational day 30 in pregnant NZW rabbits gavaged with 0, 10, 40, or 80 mg EDA/kg/day (as aqueous EDA-2HCl) on gestational days 6-19 (Price et al. 1993). Conversely, EDA (400 mg/kg/day) given to 50 pregnant CD-1 mice by gavage on days 6-13 of gestation caused reduced birth weights and weight gains in the offspring, but no maternal toxicity (Hardin et al. 1987).
3.5.
Genotoxicity
EDA caused a weakly positive response in Salmonella typhimurium TA100 and TA1535, with or without addition of rat liver S9
homogenate (Hedenstedt 1978; Hulla et al. 1981; Haworth et al. 1983). Leung (1994), however, obtained a negative response in the Salmonella mutagenicity assay using strains TA98, TA100, TA1535, TA1537, and TA1538 (±S9 homogenate). EDA did not induce sister chromatid exchanges or HGPRT mutations in CHO cells with or without rat liver S9 activation and did not induce unscheduled DNA synthesis in primary rat hepatocytes (Slesinski et al. 1983). EDA was negative in a dominant lethal assay in which male Fischer 344 rats were given 0.05-0.5 mg/kg/day EDA-2HCl in the diet for 23 weeks, and then mated for 3 weeks (Slesiniski et al. 1983). EDA was negative in the Drosophila sex-linked recessive lethal assay when administered to adult Canton-S wild-type males in the diet (10,000 or 20,000 ppm) or by injection (1,500 ppm) (Zimmering et al. 1985).
3.6.
Carcinogenicity
No inhalation-exposure carcinogenicity studies were located in the literature. No neoplasms were seen in a multi-generation carcinogenicity study in which F344 rats were given 50, 150, or 500 mg EDA dihydrochloride/kg/day in the diet (Yang et al. 1984b). The F0 parents were given the compound for 100 days before mating, and the F1 offspring were fed the same dietary concentrations of EDA dihydrochloride. No evidence of epidermal tumors (or life shortening) was seen in a lifetime skin application assay in male C3H/HeJ mice in which 25 μl of 1% EDA in water was applied 3× per week until death (DePass et al. 1984).
The ACGIH (2004) has concluded that there is inadequate evidence in humans and in experimental animals to establish the carcinogenicity of ethylenediamine and places it in carcinogenicity group A4 (“not classifiable as a human carcinogen”). The Environmental Protection Agency (EPA) classifies EDA as carcinogenicity weight-of-evidence group D: not classifiable as to human carcinogenicity, based on no human data and inadequate animal data (EPA 2005).
3.7.
Summary
The database for EDA inhalation animal studies is very limited, with many studies missing critical information. Carpenter et al. (1948) showed that one 8-h exposure of rats or guinea pigs to a nominal concen-
tration of 1,000 ppm resulted in no deaths but did cause lung edema and kidney swelling. [A nominal concentration of 1,000 ppm was found to be 484 ppm analytical in another study by the same laboratory (Pozzani and Carpenter, 1954), which showed that analytical EDA concentration is approximately 50% of the nominal concentration.] In a subsequent range-finding study, Smyth et al. (1951) determined that an 8-h exposure to 2,000 ppm EDA (nominal; analytical was likely ~1,000 ppm) caused no deaths whereas 6/6 rats died at 4,000 ppm (nominal; analytical was likely ~2,000 ppm); no effects other than death were described. Pozzani and Carpenter (1954) found that rats exposed 7 h/day for up to 30 days to 59 ppm had no effects, at 132 ppm had hair loss and a slight increase in the incidence of “major” microscopic lesions (types of lesions were not specified), whereas most or all rats exposed to 225 or 484 ppm died and had liver, kidney, and lung lesions. Dubinina et al. (1997) and Fukalova and Dubinina (1992) conducted several acute and multiple-exposure inhalation studies using rats and guinea pigs, although the significance of their results is questionable due to numerous study deficiencies.
EDA showed little genotoxic activity, as most assays yielded negative or weakly positive responses. No inhalation-exposure carcinogenicity studies were located, and animal dietary and skin painting studies yielded negative results (Yang et al. 1984b; DePass et al. 1984).
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
No human or animal studies were located that described the metabolism or disposition of ethylenediamine following inhalation exposure. Animal and human studies have shown that amines are well absorbed from the gut, respiratory tract, and skin (Benya and Harbison 1994).
The metabolism and pharmacokinetics of EDA were studied in male Hilltop Wistar rats. They were given a single dose of 5, 50, or 500 mg/kg [14C]EDA-2HCl solution orally, intravenously, or endotracheally for 24 or 48 h (Yang and Tallant 1982). The vast majority of radiolabel was excreted within 24 h by all exposure routes, the urine being the primary excretion route and accounting for 42-65% of the given radioactivity. The feces accounted for about 10-12% of the endotracheally administered radiolabel, and for 4.5-16% and 12-31% of the radioactivity given
orally and intravenously, respectively. A substantial amount of radioactivity was found in the expired air as 14CO2 (5-8%) and in the major organs and carcass (1.7-2.7% and 9.1-19%, respectively) for all three exposure routes. The thyroid, liver, kidneys, and bone marrow contained the greatest amount of radioactivity on a per gram basis. Bioavailability (AUCoral/endo/AUCiv), total clearance, terminal half-life, and AUC were similar for the three exposure routes; minor differences in parameter values were seen among the three doses. AG 50W cation exchange chromatography identified N-acetylethylenediamine as the major metabolite in the urine and the feces by all three exposure routes. Yang and Tallant (1982) proposed that N-acetylation is the primary metabolic pathway for EDA, with aminoacetaldehyde and ethanolamine also being formed as intermediates before final conversion to CO2. Based on the pharmacokinetic and metabolic results, the study authors concluded that the fate of EDA was similar following oral and endotracheal administration at 5 and 50 mg/kg.
Hilltop Swiss Webster mice dosed orally with 5 mg/kg [14C]EDA excreted approximately 70% of the radiolabel in the urine, 5% in the feces, and 12% as 14CO2 at 48 h after dosing (Yang et al. 1978). The major organs contained a small amount of radioactivity.
Pharmacokinetic studies were conducted using Fischer 344 rats that were part of a two-year chronic toxicity dietary study with EDA dihydrochloride (Yang et al. 1984b). Male and female rats (43 days old) were initially given a single per os dose of 50 mg [14C]EDA-2HCl per kg body weight on day 0, prior to EDA dietary treatment. After 6 and 18 months, rats receiving 0 (control) or 350 mg EDA/kg/day (high-dose) in the diets were given a single per os dose of 50 mg [14C]EDA-2HCl per kg body weight. The rats showed no sex-related, age-related, or chronic dosing-related differences in the absorption rate or terminal half-life. However, the older rats had 2-3 times greater AUC than the younger rats, which correlated with their smaller volume of distribution (1/4 to 1/2 that of day 0 rats), and the 14CO2 production rate constant (from 14C-EDA) was slightly (≤18%) but statistically significantly greater in the females than males. Approximately 10-22% of the administered radiolabel appeared as expired 14CO2, and urinary and fecal excretion accounted for 39-51% and 11-30% of the administered dose, respectively. Most of the excreted radioactivity was as metabolites.
4.2.
Mechanism of Toxicity
Ethylenediamine is highly alkaline, water soluble and lipid-soluble skin and respiratory sensitizer and irritant. Its alkalinity is likely responsible for the corneal and skin lesions described in humans and animals, and for respiratory irritation leading to lung edema that may occur in humans. However, respiratory irritation as the sole end point was not reported in any human studies, which only examined asthmatic symptoms in EDA-sensitized workers. Animal inhalation studies also did not report EDA-induced irritation but found liver, kidney, and lung lesions. The mechanism by which EDA sensitizes humans and causes internal organ lesions is unknown.
Several studies examined the mechanism of EDA-induced asthma in humans. Workers with EDA-induced bronchial asthma had notable bronchoconstriction immediately after exposure to EDA at concentrations below those that were non-irritating to unsensitized asthmatics (Popa et al. 1969). A delayed asthmatic response (several hours after exposure) was seen in several studies in EDA-sensitized workers (Lam and Chan-Yeung 1980, Nakazawa and Matsui 1990, and Ng et al. 1991). Histamine did not appear to be an important mediator because plasma histamine levels were unchanged in venous blood during bronchoconstriction in occupationally exposed workers (Lam and Chan-Yeung 1980; Nakazawa and Matsui 1990). Evidence for an immunological mechanism was inconclusive because precipitable EDA antibody was not found in sensitized workers although IgE and eosinophil levels were increased (Popa et al. 1969, Lam and Chan-Yeung 1980, Nakazawa and Matsui 1990).
4.3.
Structure-Activity Relationships
Inhalation toxicity information about chemicals related structurally to EDA was very limited. Repeated exposure of rabbits to 100 ppm ethylamine (C2H7N) caused lung, liver, and kidney damage, lung irritation, and corneal injury (Benya and Harbison 1994). One worker exposed to up to 28 ppm hexamethylene diamine (C6H16N2) developed acute hepatitis and dermatitis following an unspecified number of exposures (Benya and Harbison 1994). Asthmatic symptoms were associated with occupational exposure to a TWA of approximately 0.085 and 0.34 ppm piperizine (C4H10N2) (Hagmar et al. 1982).
Leung and Auletta (1997) compared the allergic contact skin sensitization and cross-reaction potential of EDA and eight other alkyleneamines using the guinea pig maximization test (10 animals/sex). Sensitizing potency was inversely correlated with the number of amine groups. EDA was the most potent skin sensitizer and skin irritant, and elicited the greatest cross-reaction in guinea pigs originally sensitized with the other amines, when tested as either the inducing or challenge agent.
4.4.
Other Relevant Information
4.4.1.
Species Variability
EDA toxicity in a species other than the rat was examined in only one inhalation study, in which rats and guinea pigs exposed for 8 h to 1,000 ppm EDA (nominal; analytical approximately 484 ppm) did not die but had lung edema and kidney swelling (Carpenter et al. 1948). No differences in the response of the two species were reported, although only a very brief description of the experimental results was provided.
4.4.2.
Susceptible Populations
A susceptible human subpopulation exists, consisting of persons who have become sensitized to EDA either through work or by living in a community near a plant that uses EDA. Workers have reported symptoms including chronic cough, phlegm, wheezing, and exertional breathlessness when exposed to EDA, which typically disappear upon cessation of EDA exposure. Aldrich et al. (1987) showed that persons exposed to <1 ppm EDA became sensitized in an occupational setting after exposure for approximately 7 months (smokers) to 37.3 months (nonsmokers). In the case of community residents, people may become sensitized to EDA over time from periodic but persistent exposures resulting from fugitive or routine emissions.
EDA-sensitized people may experience more severe and/or idiosyncratic response to a given concentration and exposure duration compared to non-sensitized people. Popa et al. (1969) showed that EDA-sensitized individuals had an asthmatic response to EDA at concentrations not irritating to unsensitized asthmatics, although exposure concen-
trations were not stated. Because the qualitative and quantitative differences in the response of nonsensitized and sensitized people to EDA are undefined, an uncertainty factor to specifically account for previously sensitized people cannot be determined. The derived AEGL values are for a once-in-a-lifetime exposure and do not consider previous sensitization.
4.4.3.
Concentration-Exposure Duration Relationship
No data were available from which to determine the concentration-time relationship for EDA toxic effects. Ten Berge et al. (1986) determined that the concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5, and n ranged from 1 to 3 for 90% of the chemicals examined. To obtain protective AEGL 30-, 60-, and 240-min values, scaling across time was performed using n = 3 and the ten Berge equation, except that the 10-min value was not extrapolated from 8 h (exposure duration in the key studies) because extrapolating from ≥4 h to 10 min is associated with unacceptably large inherent uncertainty, and the 30-min value was adopted for 10 min to be protective of human health (NRC 2001).
4.4.4.
Concurrent Exposure Issues
Workers may be exposed to other dermal and/or respiratory sensitizers which could potentially increase susceptibility to EDA, although the degree of cross-sensitization in humans is not defined. EDA-sensitized workers exposed to EDA dermally or by inhalation did not cross-react to aminophylline (molecular combination of EDA and theophylline), ethylenediamine tetraacetate, or procaine (4-aminbenzoic acid-2(diethylanimo)ethyl ester) (Popa et al. 1969). In a guinea pig maximization test (Leung and Auletta 1997), a comparison of the allergic contact skin sensitization and cross-reaction potential of EDA and eight other alkyleneamines showed that EDA was the most potent skin sensitizer and skin irritant. EDA elicited the greatest cross-reaction in guinea pigs originally sensitized with the other alkyleneamines.
5.
RATIONALE FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
No human studies were located with end points consistent with the definition of AEGL-1. In the available studies, the exposure time was either too short (5-10 sec exposure by Pozzani and Carpenter 1954), not given (Aldrich et al. 1987; Ng et al. 1995), or the exposure concentration was not specified (Nakazawa and Matsui 1990).
5.2.
Summary of Animal Data Relevant to AEGL-1
In the multiple-exposure study of Pozzani and Carpenter (1954), Sherman rats (15/sex/dose) exposed 7 h/day for up to 30 days to 59 ppm had no toxic effects, rats exposed to 132 ppm had hair loss and a slight increase in the incidence of microscopic lesions, and those exposed to 225 or 484 ppm died and/or had hair loss and liver, kidney, and lung lesions.
5.3.
Derivation of AEGL-1
AEGL-1 values, as shown in Table 4-5, were not recommended because none of the available human or animal data were considered adequate. The multiple-exposure study of Pozzani and Carpenter (1954), in which rats exposed to 59 ppm 7 h/day for up to 30 days had no toxic effects, was not used because it was not associated with a specific end point within the scope of the AEGL-1 definition. Absence of AEGL-1 values does not imply that exposure to concentrations below the AEGL-2 is without adverse effects.
TABLE 4-5 AEGL-1 Values for Ethylenediamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not recommended due to insufficient data. |
||||
6.
RATIONALE FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
The only human study for which both the exposure concentration and duration were defined was the bronchial provocation test in which a 31-year-old male EDA-sensitized chemical worker exposed to 30 ppm EDA for 15 min had a delayed asthmatic response (Ng et al. 1991). He had decreased peak flow rate 3 h after exposure and coughed, wheezed, was breathless and had a further fall in peak flow rate 12 h after exposure. He improved after treatment with nebulized ventolin (bronchodilator). However, because an asthmatic response can encompass either AEGL-2 or AEGL-3 effects and the response of non-sensitized persons to the same exposure scenario is unknown, this study was not considered appropriate for derivation of AEGL-2 values.
6.2.
Summary of Animal Data Relevant to AEGL-2
Two animal studies are potentially useful for AEGL-2 derivation: (1) the single-exposure study in which rats and guinea pigs exposed for 30 min to 8 h to 0 or ~484 ppm EDA (1,000 ppm nominal) all survived and had “light cloudy swelling of the kidney” and bronchiolar edema of unspecified severity (Carpenter et al. 1948), and (2) the 30-day study (7 h/day) by Pozzani and Carpenter (1954) in which rats (15/sex/dose) exposed to 59 ppm had no toxic effects, rats exposed to 132 ppm had hair loss, and one rat had an unspecified microscopic lesion; rats exposed to 225 ppm had fractional mortality (earliest death, day 4) and kidney and liver lesions; and rats exposed to 484 ppm all died from ≤20 exposures (earliest death, day 3) and most had liver, kidney, and/or lung lesions.
6.3.
Derivation of AEGL-2
AEGL-2 values were based on the Carpenter et al. (1948) study in which rats and guinea pigs (6/group) exposed for 8 h to approximately 484 ppm EDA (1,000 ppm nominal) had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney” but none died (Carpenter et al. 1948). No studies were available from which to determine the EDA concentration-time relationship, so scaling to exposure
times <8 h was performed with the ten Berge et al. (1986) equation Cn × t = k, where n = 3 was used to obtain AEGL values for 30, 60, and 240 min and the 30-min value was adopted as the 10-min value, as discussed in section 4.4.3. An uncertainty factor of 3 was used for interspecies variability because a similar response was seen in two species, and a modifying factor of 3 because the key study did not specify the severity of the bronchiolar edema. An intraspecies uncertainty factor of 10 was applied because the data were insufficient to determine the mode of lung and kidney lesions (or which was the more sensitive end point) in the key study and consequently the potential variability of the human response to EDA. Note that UF (30) × MF (3) is rounded to 100 for simplicity, per Section 2.9.2. of the SOP (NRC 2001). The developed AEGL-3 values are shown in Table 4-6; calculations are detailed in Appendix A. The AEGL-2 values are supported by the Pozzani and Carpenter (1954) study, in which 1/26 rats had unspecified lesions but no mortality after 30 exposures to 132 ppm EDA for 7 h/day.
EDA-sensitized individuals may experience more severe and/or different effects at a given exposure concentration or duration than nonsensitized people. The qualitative and quantitative differences in the response of the two groups are undefined.
7.
RATIONALE FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
No quantitative information on lethal EDA exposure in humans was located. An EDA-sensitized chemical worker challenged with 30 ppm EDA for 15 min had a delayed asthmatic response (Ng et al. 1991) that was ameliorated by the administration of a bronchodilator. This study was not used for derivation of AEGL-3 values because it is unclear what would have happened to this individual without medical intervention, and an asthmatic response can encompass either AEGL-2 or AEGL-3 effects. Additionally, the quantitative and qualitative differences in the
TABLE 4-6 AEGL-2 Values for Ethylenediamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
12 ppm (30 mg/m3) |
12 ppm (30 mg/m3) |
9.7 ppm (24 mg/m3) |
6.1 ppm (15 mg/m3) |
4.8 ppm (12 mg/m3) |
response of non-sensitized persons to the same exposure scenario is unknown.
7.2.
Summary of Animal Data Relevant to AEGL-3
Two studies are relevant for deriving AEGL-3 values: (1) the range-finding test of Smyth et al. (1951) in which 0/6 rats exposed to approximately 1,000 ppm (2,000 ppm nominal) for 8 h died but 6/6 died after an 8-h exposure to 2,000 ppm EDA (4,000 ppm nominal). Few experimental details were provided and the effects on the animals (besides death) were not described, and (2) the 30-exposure study (7 h/day) by Pozzani and Carpenter (1954) in which rats (15/sex/dose) exposed to 59 ppm had no toxic effects, rats exposed to 132 ppm had hair loss and one rat had an unspecified microscopic lesion, rats exposed to 225 ppm had fractional mortality (earliest death day 4) and kidney, and lung lesions, and rats exposed to 484 ppm all died from ≤20 exposures (earliest death day 3) and most had liver, kidney, and/or lung lesions.
7.3.
Derivation of AEGL-3
AEGL-3 derivation was based on the range-finding study in which 0/6 rats died after an 8-h exposure to ~1,000 ppm (2,000 ppm nominal) but 6/6 died at 4,000 ppm (nominal) (Smyth et al. 1951). Toxic effects (other than death) were not described, and 1,000 ppm was considered to be the lethality threshold. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the equation Cn × t = k and n = 3, as was done for AEGL-2 and is discussed in Section 4.4.3. A total uncertainty factor of 100 was applied: 10 for interspecies variability (cause of death was undefined and there were no studies using other species) and 10 for intraspecies variability (lack of toxicity data in key study precludes definition of the mode or variability of the toxic response in humans). Target organs (liver and kidneys) were identified in a study where rats received 225 ppm EDA 7 h/day for up to 30 days (first deaths on exposure day 4), although the mode of toxicity was unclear (Pozzani and Carpenter 1954). The developed AEGL-3 values are shown in Table 4-7; calculations are detailed in Appendix A.
EDA-sensitized individuals may experience more severe and/or different effects at a given exposure concentration or duration than nonsensitized people. The qualitative and quantitative differences in the response of the two groups are undefined.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
A summary of the AEGL values for EDA and their relationship to one another are shown in Table 4-8. AEGL-1 values were not developed due to insufficient data. Absence of AEGL-1 values does not imply that exposure to concentrations below the AEGL-2 is without adverse effects. AEGL-2 values were based on the Carpenter et al. (1948) study in which rats and guinea pigs (6/group) exposed for 8 h to approximately 484 ppm EDA (1,000 ppm nominal) had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney” but none died (Carpenter et al. 1948). No studies were available from which to determine the EDA concentration-time relationship, but scaling to exposure times <8 h was performed with the ten Berge et al. (1986) equation Cn × t = k where n = 3 was used obtain protective AEGL values for 30, 60, and 240 min and the 30-min value was also adopted for 10 min, as discussed in section 4.4.3. An uncertainty factor of 3 was used for interspecies variability because a similar response was seen in two species, and a modifying factor of 3 because the key study did not specify the severity of the bronchiolar edema. An intraspecies uncertainty factor of 10 was applied because the data were insufficient to determine the mode of lung and kidney lesions (or which was the more sensitive end point) in the key study and consequently the potential variability of the human response to EDA.
The AEGL-3 was based on a range-finding study in which 0/6 rats died after an 8-h exposure to ~1,000 ppm (2,000 ppm nominal) but 6/6 died at 4,000 ppm (nominal) (Smyth et al. 1951). Toxic effects (other
TABLE 4-7 AEGL-3 Values for Ethylenediamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
25 ppm (62 mg/m3) |
25 ppm (62 mg/m3) |
20 ppm (49 mg/m3) |
13 ppm (32 mg/m3) |
10 ppm (25 mg/m3) |
TABLE 4-8 Summary of AEGL Values for Ethylenediamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (Nondisabling) |
Not recommended due to insufficient data. |
||||
|
AEGL-2 (Disabling) |
12 ppm (30 mg/m3) |
12 ppm (30 mg/m3) |
9.7 ppm (24 mg/m3) |
6.1 ppm (15 mg/m3) |
4.8 ppm (12 mg/m3) |
|
AEGL-3 (Lethal) |
25 ppm (62 mg/m3) |
25 ppm (62 mg/m3) |
20 ppm (49 mg/m3) |
13 ppm (32 mg/m3) |
10 ppm (25 mg/m3) |
than death) were not described, and 1,000 ppm was considered to be the lethality threshold. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the equation Cn × t = k and n = 3, as was done for AEGL-2. A total uncertainty factor of 100 was applied: 10 for interspecies variability (cause of death was undefined and there were no studies using other species) and 10 for intraspecies variability (lack of toxicity data in key study precludes definition of the mode or variability of the toxic response in humans). Kidney and liver toxicity and death occurred in rats given 4 to 30 exposures of 225 ppm EDA for 7 h/day in another study, although the mode of toxicity was unclear (Pozzani and Carpenter 1954).
8.2.
Comparison with Other Standards and Guidelines
The existing standards and guidelines for EDA are summarized in Table 4-9.
The ACGIH TLV-TWA of 10 ppm (25 mg/m3; skin notation) is based on a rat 90-day oral exposure study in which the NOEL was 23 mg/kg/day (Yang et al. 1978) and a 30-day rat inhalation study in which the NOEL was 59 ppm (Pozzani and Carpenter 1954). ACGIH defines the critical toxic EDA effects as irritation, asthma, and sensitization (ACGIH 2004). The OSHA PEL-TWA and NIOSH REL-TWA are also 10 ppm (25 mg/m3), intended to avert EDA toxic effects including irritation of nose and respiratory system, dermal sensitization, asthma, liver and kidney damage (NIOSH 2005b; OSHA 2005). The NIOSH IDLH for ethylenediamine was lowered from 2,000 ppm to 1,000 ppm in 1994, NIOSH noting that 1,000 ppm may be a conservative value due to the
lack of relevant acute toxicity data for occupational exposure between 1,000 and 2,000 ppm (NIOSH 2005b).
Aldrich et al. (1987) suggested that because there was evidence that EDA sensitization occurred (in coater machine operator) when the EDA concentrations were <1 ppm, the present TLV of EDA of 10 ppm should be reconsidered (study described in Section 2.2).
The 10-ppm occupational exposure limit is also used in other countries including Australia, Belgium, Denmark, Finland (20 ppm STEL), France (15 ppm STEL), Germany, Japan, the Netherlands, the Phillippines, Russia, Sweden (15 ppm STEL), Switzerland (20 ppm STEL), Turkey, and the U.K. (RTECS 2005).
TABLE 4-9 Extant Standards and Guidelines for Ethylenediamine (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
Not recommended due to insufficient data. |
||||
|
AEGL-2 |
12 |
12 |
9.7 |
6.1 |
4.8 |
|
AEGL-3 |
25 |
25 |
20 |
13 |
10 |
|
PEL-TWA (OSHA)a |
|
|
|
|
10 |
|
IDLH (NIOSH)b |
|
1,000 |
|
|
|
|
REL-TWA (NIOSH)c |
|
|
|
|
10 |
|
TLV-TWA (ACGIH)d |
|
|
|
|
10 |
|
MAK (Germany)e |
|
|
|
|
10 |
|
MAK Peak Limit (Germany)f |
20 (15 min) |
|
|
|
|
|
MAC (Netherlands)g |
|
|
|
|
7 |
|
LLV (Sweden)h |
|
|
|
|
10 |
|
STV (Sweden)i |
15 |
|
|
|
|
|
aOSHA PEL-TWA (Occupational Safety and Health Administration, Permissible Exposure Limits - Time Weighted Average) (OSHA 2005) is defined analogous to the ACGIH-TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week. bIDLH (Immediately Dangerous to Life and Health, National Institute of Occupational Safety and Health) (NIOSH 2005b) represents the maximum concentration from which one could escape within 30 minutes without any escape- impairing symptoms, or any irreversible health effects. The IDLH for EDA is based on a study in which rats exposed to 2,000 ppm (~1,000 ppm analytical; see text) for 8 h had 0/6 deaths but exposure to 4,000 ppm (~2,000 ppm analytical; see text) for 8 h caused 6/6 deaths (Smyth et al. 1951). |
|||||
8.3.
Data Quality and Research Needs
Although EDA is a high production volume chemical, very few inhalation toxicity studies were available for deriving AEGL values, and data were insufficient to determine the mode of EDA toxicity. In addition to causing lesions in the lungs, as is typical for respiratory irritants, EDA caused systemic effects including liver and kidney lesions. Studies were not available, and are needed, to determine the most sensitive target organ, including whether irritation occurs at concentrations below those
causing kidney and liver lesions. Studies are also needed that can be used to derive the EDA concentration-time relationship (n in Cnt = k), which will ideally include exposure times of ≤1 h. The small database, lack of mechanistic information, and shortcomings of the available studies led to the use of large uncertainty factors in developing AEGL values for EDA.
Studies are needed in which effects within the scope of AEGL-1 occurred, as no adequate human or animal studies were available to derive AEGL-1 values. Only three animal studies (conducted by the same laboratory) were located for the development of AEGL-2 and AEGL-3 values, and additional studies are needed to confirm these values. In the one single-exposure study adequate for AEGL-2 derivation, rats and guinea pigs were exposed for 30 min to 8 h to only one test concentration (~484 ppm EDA). Both species had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney” (Carpenter et al. 1948). Because the key study did not specify the severity of the bronchiolar edema, a modifying factor of 3 was applied in addition to the interspecies UF of 3 (similar response in two species). Because the most sensitive end point and mode of toxicity were unknown, the potential variability of the human response to EDA could not be predicted, and an intraspecies UF of 10 was used. Only one single-exposure study was adequate for AEGL-3 derivation as well, which was a sparsely reported range-finding test (Smyth et al. 1951) in which 0/6 rats died from exposure for 8 h to ~1,000 ppm but 6/6 died from 8 h exposure to ~2,000 ppm. The toxic effects on the animals were not described, which led to the use of a total UF of 100 (10 each for interspecies and intraspecies UF) because the mode and variability of the toxic response in animals and humans was undefined.
Although the key studies used for derivation of AEGL-2 and AEGL-3 values had shortcomings, they were mutually supportive and were consistent with the Pozzani and Carpenter (1954) a multiple-exposure rat study. The consistency between these three studies, together with the use of large uncertainty factors, provides a reasonable degree of confidence in the developed AEGL-2 and AEGL-3 values.
EDA is a respiratory (and skin) sensitizer, but no studies were found to determine the qualitative and quantitative differences in the response of non-sensitized and sensitized people. This lack of data is not considered relevant to the development of AEGL values for EDA because AEGL values are intended for a once-in-a lifetime exposure and do not consider previous sensitization.
9.
REFERENCES
ACGIH. 1996. American Conference of Government Industrial Hygienists. Ethylenediamine. In: Documentation of the threshold limit values and biological exposure indices. Supplements to the sixth edition, p. 603-605. ACGIH, Cincinnati, OH.
ACGIH. 2004. American Conference of Government Industrial Hygienists: Ethylenediamine. In: Threshold Limit Values and Biological Exposure Indices for Chemical Substances and Physical Agents, p. 32. ACGIH, Cincinnati, OH.
Aldrich, F.D., A.W. Stange, and R.E. Geesaman. 1987. Smoking and ethylene diamine sensitization in an industrial population. J. Occup. Med. 29: 311-314.
Amoore, J.E. and E. Hautala. 1983. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272-290.
Beard, R.R. and J.T. Noe. 1981. Aliphatic and alicyclic amines. In: Patty’s Industrial Hygiene and Toxicology, 3rd ed, Vol. 2B, Clayton and Clayton, Eds., pp. 3135-3173.
Benya, T.J. and R.D. Harbison. 1994. Aliphatic and alicyclic amines. In: Patty's Industrial Hygiene and Toxicology, 4th ed., Vol. 2B, pp. 1087-1175. Clayton G.D. and Clayton F.E. (Eds.), John Wiley & Sons, New York.
Budavari S., M.J. O'Neil, A. Smith, P.E. Heckelman, J.F. Kinneary (Eds.). 1996. The Merck Index, 12th ed. Merck & Co., Inc., Whitehouse Station, NJ., p. 646.
Carpenter, C.P. and H.F. Smyth. 1946. Chemical burns of the rabbit cornea. Am. J. Ophthalmol. 29: 1363-1372.
Carpenter, C.P., H.F. Smyth, Jr., and C.B. Shaffer. 1948. The acute toxicity of ethylene imine to small animals. J. Ind. Hyg. Toxicol. 30: 2-6.
Cary, R., S. Dobson, and J. Delic. 1999. Concise International Chemical Assessment Document number 15: 1,2-Diaminoethane (Ethylenediamine). First draft. United Kingdom. Online at http://www.inchem.org/.
DePass, L.R., E.H. Fowler, and R.S.H. Yang. 1984. Dermal oncogenicity studies on ethylenediamine in male C3H mice. Fund. Appl. Toxicol. 4: 641-645.
DePass, L.R., R.S.H. Yang, and M.D. Woodside. 1987. Evaluation of the teratogenicity of ethylenediamine dihydrochloride in Fischer 344 rats by conventional and pair-feeding studies. Fundam. Appl. Toxicol. 9: 687-697.
Dernehl, C.U. 1951. Clinical experiences with exposures to ethylene amines. Ind. Med. Surg. 20: 541-546.
DFG (Deutsche Forschungsgemeinschaft). 2002. List of MAK and BAT Values, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 35. Weinheim, Federal Republic of Germany: Wiley VCH.
Dubinina, O.N., L.R. Galeeva, L.I. Trubnikova, et al. 1997. Experimental data for potential correction of ethylenediamine MAC in air of work environment. Meditsina Truda I Promyshlennaya Ekologiya 1: 38-41.
Fukalova, L.A. and O.N. Dubinina. 1992. Deamination of biological amines and of other nitro compounds after a prolonged exposure to ethylenediamine in rats. Vopr. Med. Khim. 38: 23-25.
Grant, W.M. 1986. Amines. In: Toxicology of the Eye, C.C. Thomas, Pub., pp. 75-76.
Hagmar, L., B. Bergoo, and B.G. Simonsson. 1982. Piperazine-induced occupational asthma. J. Occup. Med. 24: 193-197.
Hansen, L., B. Kristiansson, and J. Sollenberg. 1984. A method for the determination of ethylenediamine in workroom air. Scand. J. Work Environ. Health 10: 95-98.
Hardin B.D., R.L. Schuler, J.R. Burg, et al. 1987. Evaluation of 60 chemicals in a preliminary developmental toxicity test. Terat. Carcinog. Mutagen. 7: 29-48.
Haworth, S., T. Lawlor, M. Mortelmans, et al. 1983. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. Suppl. 1: 10-11, 94-95.
Hedenstedt, A. 1978. Mutagenicity screening of industrial chemicals: seven aliphatic amines and one amide tested in the Salmonella/microsomal assay. Mutat. Res. 53: 198-199.
Hellman, T.M. and F.H. Small. 1974. Characterization of the odor properties of 101 petrochemicals using sensory methods. J. Air Pollut. Control 24: 979-982.
HSDB. 2005. Hazardous Substances Data Bank. MEDLARS Online Information Retrieval System, National Library of Medicine (http://toxnet.nlm.nih.gov retrieved 7/2005).
Hulla, J.E., S.J. Rogers, and G.R. Warren. 1981. Mutagenicity of a series of polyamines. Environ. Mutagen. 3: 332-333.
IPCS (International Programme on Chemical Safety). 2005. International Chemical Safety Card – Ethylenediamine. Online at http://www.inchem.org/.
Izmerov, N.F., I.V. Santosky, and K.K. Sidorov. 1982. Toxicometric parameters of industrial toxic chemicals under single exposure, p. 66. USSR Commission for the United Nations Environment Programme, International Registry of Potentially Toxic Chemicals.
Lam, S. And M. Chan-Yeung. 1980. Ethylenediamine-induced asthma. Am. Rev. Resp. Dis. 121: 151-155.
Leung, H-W. 1994. Evaluation of the genotoxic potential of alkyleneamines. Mutat. Res. 320: 31-43.
Leung, H-W. and Auletta, C.S. 1997. Evaluation of skin sensitization and cross-reaction of nine alkyleneamines in the guinea pig maximization test. J. Toxicol.-Cut. & Ocular Toxicol., 16:189-195.
Lewinsohn, H.C. and M.G. Ott. 1991. A review of medical surveillance records of employees exposed to ethyleneamines. J. Occup. Med. 33:148-154.
Nakazawa, T. And S. Matsui. 1990. Ethylenediamine-induced late asthmatic responses. J. Asthma. 27: 207-212.
Ng, T.P., H.S. Lee, F. Lee, et al. 1991. Occupational asthma due to ethylene diamine. Ann. Acad. Med. Singapore 20: 399-402.
Ng, T.P., H.S. Lee, M.A. Malik , et al. 1995. Asthma in chemical workers exposed to aliphatic polyamines. Occup. Med. 45: 45-48.
NIOSH. 2005a. National Institute for Occupational Safety and Health. Ethylenediamine. In: Pocket Guide to Chemical Hazards. U.S. Department of Health and Human Services, Public Health Service, Cincinnati, OH. Online at http://www.cdc.gov/niosh/npg/npgd0269.html/; retrieved 7/2005.
NIOSH. 2005b. National Institute for Occupational Safety and Health. Ethylenediamine. In: Documentation for immediately dangerous to life or health concentrations. U.S. Department of Health and Human Services, Public Health Service, Cincinnati, OH. Online at http://www.cdc.gov/niosh/idlh/107153.html; retrieved 7/2005.
Niveau, J. and J. Painchaux. 1973. Fatal poisoning by ethylenediamine. Arch. Mal. Prof. 34: 523-528.
OSHA. 2005. Occupational Safety and Health Administration. Code of Federal Regulations, Table Z-1 - Limits for air contaminants. Ethylenediamine. Online http://www.osha.gov/; retrieved 7/2005].
U.S. Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1000.
Parmeggiani, L. 1983. Ethylenediamine. In: Encyclopedia of Occupational Safety and Health, 3rd Ed., Geneva, Switzerland, International Labour Office p. 141.
Popa, V., D. Teculescu, D. Stănescu, and N. Gavrilescu. 1969. Bronchial asthma and asthmatic bronchitis determined by simple chemicals. Dis. Chest 56: 395-404.
Pozzani, U.C. and C.P. Carpenter. 1954. Response of rats to repeated inhalation of ethylenediamine vapors. Arch. Ind. Hyg. Occup. Med. 9: 223-226. [The day on which the animals died was provided as a personal communication by R. Myers, Union Carbide Corporation, January 1999.]
Price, C.J., J.D. George, M.C. Marr, et al. 1993. Development toxicity evaluation of ethylenediamine EDA in New Zealand white NZW rabbits. Teratology 47: 432-433.
RTECS. 2005. Registry of Toxic Effects of Chemical Substances. Ethylenediamine. MEDLARS Online Information Retrieval System, National Library of Medicine. Online at http://www.cdc.gov/niosh/rtecs/kh82d818.html; retrieved 7/2005.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: a review. Am. Ind. Hyg. Assoc. 47: A142-A151.
SDU Uitgevers. 2000. Dutch National MAC list 2000. The Hague, The Netherlands (under the auspices of the Ministry of Social Affairs and Employment).
Slesinski, R.S., P.J. Guzzie, W.C. Hengler et al. 1983. Assessment of genotoxic potential for ethylenediamine: in vitro and in vivo studies. Mutat. Res. 124: 299-314.
Smyth, H.F., C.P. Carpenter, and C.S. Weil. 1951. Range-finding toxicity data: List IV. AMA Arch. Ind. Hyg. Occup. Med. 4: 119-122.
Smyth, H.F., C.P. Carpenter, C.S. Weil et al. 1962. Range-finding toxicity data: List VI. Am. Ind. Hyg. Assoc. J. 23: 95-107.
Swedish National Board of Occupational Safety and Health. 2000. Swedish Occupational Exposure Limits: LLV (Level Limit Values), CLV (Ceiling Limit Values), and STV (Short-Term Values), Adopted 28th July, 2000 by Ordinance of the Swedish National Board of Occupational Safety and Health.
ten Berge, W.F., A. Zwart and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Materials. 13:302-309.
Union Carbide Corporation. 1971. Information cited in NIOSH 2005b.
U.S. EPA. 2005. Integrated Risk Information System (IRIS) Online [http://www.epa.gov/iris/; retrieved 7/2005]. Ethylenediamine [CAS No. 107-15-3]. Office of Health and Environmental Assessment, Cincinnati, OH.
Van Doorn, R., M. Ruijten and T. Van Harreveld. 2002. Guidance for the application of odor in 22 chemical emergency response. Version 2.1, 29.08.2002.
Verschueren, K. (Ed.) 1996. Ethylenediamine. In: Handbook of Environmental Data on Organic Chemicals, Third Edition. Van Nostrand Reinhold Co., New York, pp. 956-958.
Yang, R. SH., M.J. Tallant, D.N. O’Malley, and L.J. Sullivan. 1978. Metabolism of carbon-14 ethylenediamine in animals following various routes of administration. Toxicol. Appl. Pharmacol. 45: 240. (Abstract)
Yang, R. SH. and M.J. Tallant. 1982. Metabolism and pharmacokinetics and ethylenediamine in the rat following, oral, endotracheal or intravenous administration. Fund. Appl. Toxicol. 2: 252-260.
Yang, R. SH., R.H. Garman, E.V. Weaver, and M.D. Woodside. 1984a. Two-generation reproduction study of ethylenediamine in Fischer 344 rats. Fund. Appl. Toxicol. 4: 539-546.
Yang, R. SH., M.J. Tallant, and J.A. McKelvey. 1984b. Age-dependent pharmacokinetic changes of ethylenediamine in Fischer 344 rats parallel to a two-year chronic toxicity study. Fund. Appl. Toxicol. 4: 663-670.
Zimmering, S., J.M Mason, R. Valencia and R.C. Woodruff. 1985. Chemical mutagenesis testing in Drosophila. 2. Results of 20 coded compounds tested for the National Toxicology Program. Environ. Mutagen. 7: 87-100.
APPENDIX A
Derivation of AEGL Values
Derivation of AEGL-1
AEGL-1 values are not recommended due to insufficient data. Absence of AEGL-1 values does not imply that exposure to concentrations below the AEGL-2 is without adverse effects.
Derivation of AEGL-2
Key study:
Carpenter et al. 1948. Rats and guinea pigs (6/group) were exposed for 30 min to 8 h to approximately 484 ppm EDA (1,000 ppm nominal). Rats exposed for 8 h had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney.”
Toxicity end point:
Bronchiolar edema and kidney swelling. (Note that EDA-sensitized individuals may experience more severe effects at a given exposure concentration and/or duration.)
Scaling:
Cn × t = k (ten Berge et al. 1986); no data were available to derive n; used n = 3 to extrapolate to <8 h to obtain protective AEGL values, except the 30-min value was adopted as the 10-min value because extrapolating from 8 h to 10 min is associated with unacceptably large inherent uncertainty.
Total uncertainty factor: 30
Interspecies: 3: A similar response was seen in two species in the key study.
Intraspecies: 10: Data were insufficient to determine the mode of lung and kidney lesions (or which was the more sensitive end point)
in the key study and consequently the potential variability of the human response to EDA.
Modifying factor: 3: The key study did not specify the severity of the bronchiolar edema.
Calculations for <8 h:

Calculations for 8 h:
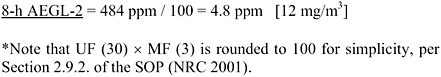
Derivation of AEGL-3
Key study: Smyth et al. (1951). No rats (0/6) died after an 8-h exposure to 1,000 ppm (2,000 ppm nominal) but 6/6 died at 2,000 ppm (4,000 ppm nominal). Toxic effects (other than death) were not described.
Toxicity end point: Lethality threshold at 1,000 ppm. (Note that EDA-sensitized individuals may experience more severe effects at a given exposure concentration and/or duration.)
Scaling: Cn × t = k (ten Berge et al. 1986); no data were available to derive n; used n = 3 to extrapolate to <8 h to obtain protective AEGL values, except the 30-min value was adopted as the 10-min value because extrapolating from 8 h to 10 min is associated with unacceptably large inherent uncertainty.
Total uncertainty factor: 100
Interspecies: 10: The cause of death was not defined in the key study, and there were no supporting data with AEGL-3 end points from other species.
Intraspecies: 10: Lack of toxicity data in key study precludes definition of the mode or variability of the toxic response in humans.
Calculations for <8 h:
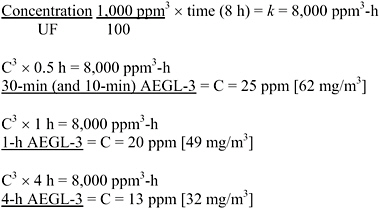
Calculations for 8 h:
8-h AEGL-3 = 1,000 ppm / 100 = 10 ppm [25 mg/m3]
APPENDIX B
Derivation of the Level of Distinct Odor Awareness
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, about 10 % of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
An odor detection threshold (OT50, i.e., concentration at which 50% of the odor panel observed an odor without necessarily recognizing it) of 1.0 ppm was obtained for EDA from Hellman and Small (1974). The same citation listed an OT50 of 0.30 for n-butanol, as compared to the reference value of 0.04 ppm as the odor threshold provided by van Doorn et al (2002). Based on the differences in n-butanol values from the two sources, an “inter-laboratory” correction factor is applied to EDA as follows:

The concentration (C) leading to an odor intensity (I) of distinct odor detection (I = 3) is derived using the Fechner function:
For the Fechner coefficient, the default of kw = 2.33 will be used due to the lack of chemical-specific data:
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in every day life, factors such as sex, age, sleep, smoking, upper airway infections and allergies, as well as distraction, increase the odor detection threshold by a factor of 4. In addi-
tion, it takes into account that odor perception is very fast (about 5 sec) which leads to the perception of concentration peaks. Based on the current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustment for distraction and peak exposure lead to a correction factor of 4/3 = 1.33.
The LOA for EDA is 2.1 ppm.
APPENDIX C
ACUTE EXPOSURE GUIDELINES FOR ETHYLENEDIAMINE (107-15-3)
DERIVATION SUMMARY
|
AEGL-1 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
Not recommended due to insufficient data. |
||||
|
Key Reference: Not applicable. |
||||
|
Test Species/Strain/Number: Not applicable. |
||||
|
Exposure Route/Concentrations/Durations: Not applicable. |
||||
|
Effects: Not applicable. |
||||
|
End point/Concentration/Rationale: Not applicable. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: Not applicable. Interspecies: Intraspecies: |
||||
|
Modifying Factor: Not applicable. |
||||
|
Animal to Human Dosimetric Adjustment: Not applicable. |
||||
|
Time Scaling: Not applicable. |
||||
|
Data Adequacy: AEGL-1 values were not recommended because none of the available human or animal data were considered adequate. Absence of AEGL-1 values does not imply that exposure to concentrations below the AEGL-2 is without adverse effects. |
||||
|
AEGL-2 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
12 ppm |
12 ppm |
9.7 ppm |
6.1 ppm |
4.8 ppm |
|
Key Reference: Carpenter, C.P., H.F. Smyth, Jr., and C.B. Shaffer. 1948. The acute toxicity of ethylene imine to small animals. J. Ind. Hyg. Toxicol. 30: 2-6. |
||||
|
Test Species/Strain/Sex/Number: Rats and guinea pigs, 6/group, sex unspecified. |
||||
|
Exposure Route/Concentrations/Durations: Rats and guinea pigs were exposed to 0 or to approximately 484 ppm EDA (1,000 ppm nominal) for 1/2, 1, 2, 4, or 8 h. |
||||
|
Effects: Animals exposed for 8 h had bronchiolar edema of unspecified severity and “light cloudy swelling of the kidney” but none died. Effects for shorter exposure durations were not specified. |
||||
|
End point/Concentration/Rationale: Bronchiolar edema and kidney swelling from 8-h exposure to approximately 484 ppm EDA. Note that persons previously sensitized to EDA may experience more severe effects at a given exposure concentration and/or duration. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 30 Interspecies: 3: A similar response was seen in two species in the key study. Intraspecies: 10: Data were insufficient to determine the mode of lung and kidney lesions and consequently the potential variability of the human response to EDA. |
||||
|
Modifying Factor: 3: The key study did not specify the severity of the organ lesions. |
||||
|
Animal to Human Dosimetric Adjustment: Not performed. |
||||
|
Time Scaling: Cn × t = k; no data were available to derive n, so used n = 3 to extrapolate to <8 h to obtain protective AEGL values, except the 30-min values were adopted as 10-min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
||||
|
Data Adequacy: Key study tested only one EDA concentration but at a number of time intervals. AEGL values are supported by a study in which 1/26 rats had unspecified lesions after 30 exposures of 7 h/day but none died (Pozzani and Carpenter, 1954). |
|
AEGL-3 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
25 ppm |
25 ppm |
20 ppm |
13 ppm |
10 ppm |
|
Key Reference: Smyth, H.F., C.P. Carpenter, and C.S. Weil. 1951. Range-finding toxicity data: List IV. AMA Arch. Ind. Hyg. Occup. Med. 4: 119-122. |
||||
|
Test Species/Strain/Sex/Number: Sprague-Dawley rats; 6/concentration (sex not specified). |
||||
|
Exposure Route/Concentrations/Duration: Inhalation for 8 h to ~1,000 ppm (2,000 ppm nominal). |
||||
|
Effects: Death was the only stated effect: 0/6 deaths at 2,000 ppm; 6/6 deaths at 4,000 ppm. |
||||
|
End point/Concentration/Rationale: 1,000 ppm (2,000 ppm nominal) is the estimated lethality threshold for an 8-h exposure in rats. Note that EDA-sensitized persons may experience more severe and/or different effects at a given exposure concentration and/or duration. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 100 Interspecies: 10: The cause of death was not defined in the key study, and there were no supporting data with AEGL-3 end points from other species. Intraspecies: 10: Lack of toxicity data in key study precludes definition of the mode or variability of the toxic response in humans. |
||||
|
Modifying Factor: None. |
||||
|
Animal to Human Dosimetric Adjustment: Not performed. |
||||
|
Time Scaling: Cn × t = k; no data were available to derive n, so used n = 3 to extrapolate to <8 h to obtain protective AEGL values, except the 30-min values were adopted as 10-min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
||||
|
Data Adequacy: Key study lacked a description of toxic effects other than death. An uncertainty factor of 100 is intended to account for the lack of supporting data from other species and an unknown mode of toxicity. Target organs (liver and kidneys) are identified in another rat study in which fractional mortality resulted from 30 exposures of 7 h/day to 225 ppm (first deaths on exposure day 4; Pozzani and Carpenter, 1954). |