6
Compelling Opportunities in Global Cancer Control
Where cancer control is currently limited and resources are scarce for all health and social expenditures, decisions about expanding services has to be pragmatic and focus on interventions that are guaranteed to provide substantial benefit. Priority setting among the sectors, within the health sector, and within cancer, all require consideration of economic, political, and ethical perspectives, as well as the qualities of equity and fairness. Different countries with similar circumstances may make very different decisions. This stream of priority setting is not a focus of this report, although the report does attempt, through presentation of evidence, to make a strong case for considering the expansion of cancer control in every country. This chapter discusses interventions that would provide such benefit in most, if not all, countries.
In this report, we have seconded the already-strong efforts in tobacco control, for all the reasons discussed in Chapter 5. Palliative care, the subject of Chapter 7, represents a set of services that will benefit large numbers of people at reasonable cost, and will never become obsolete. In this chapter we identify three additional areas of opportunity with the potential to save lives now and in the future and to build capacity in cancer control where it is currently limited. They are:
-
Increased coverage with hepatitis B virus (HBV) vaccine to prevent most liver cancer globally
-
Cervical cancer prevention through cost-effective screening and treatment, and planning for the expeditious adoption of human papillomavirus
-
(HPV) vaccine to prevent infection with the viral agents that cause cervical cancer
-
Expansion of global capacity to treat the highly curable cancers of children and young adults
HBV vaccines have been available for 20 years and are now inexpensive, but still not being used in areas with some of the highest liver cancer rates. The reasons are detailed in the first section of this chapter. This is the most straightforward and obvious cancer control intervention that requires added support from the global community. One vaccine for HPV just entered the market in 2006 and another is soon to follow. A global consortium has given this intervention high visibility, and it, too, deserves continued support toward implementation. In the meantime, advances in understanding the natural history of cervical cancer have led to approaches to screen for and treat precancerous changes in adult women who will not benefit from vaccines. These approaches have proven feasible in some low- and middle-income countries (LMCs), and should be expanded. The final opportunity is to expand the availability of treatment for highly curable cancers of children and young adults. The number of children with cancer is small, but the lives saved can be long and productive. Of all the interventions described, treating children with cancer will give immediate positive results, demonstrating the curability of cancer.
REDOUBLED EFFORTS TO INCREASE THE UPTAKE OF HEPATITIS B VACCINATION
Liver cancer—hepatocellular carcinoma (HCC)—is the cause of more than 500,000 deaths each year worldwide, making it the third most frequent cause of cancer deaths in LMCs. It is currently the most preventable cancer caused by an infectious agent, chronic infection with HBV. Chronic HBV also causes significant numbers of deaths from liver cirrhosis and liver failure (Lavanchy, 2004), and an estimated 40,000 worldwide die from acute hepatitis infection (Goldstein et al., 2005). The prevalence of HBV varies widely among regions. About 45 percent of the world’s population lives where HBV prevalence is high, with the highest endemicity being in Asia, sub-Saharan Africa, and the Pacific. Other areas where infection rates are high include the southern parts of Eastern and Central Europe, the Amazon basin, the Middle East, and the Indian subcontinent. About 350 million chronic carriers are alive today, of whom 15 to 40 percent will die as a result of HBV, many in middle age. HBV is the 10th leading cause of death worldwide, and HCC is the 5th leading cause of cancer deaths, of which about 80 percent occur in developing countries (Lavanchy, 2004).
Infection with HBV and Other Hepatitis Viruses
Viral hepatitis—an inflammation of the liver—can be caused by at least six mostly unrelated viruses in humans. Some cause only acute disease (e.g., hepatitis A) and others, like HBV, can cause acute and chronic disease (although an acute phase is not a prerequisite for chronic infection). HBV is the predominant cause of chronic infection and chronic liver disease in the world, but the hepatitis C virus (HCV) is also responsible for one-quarter or more cases. HBV, because of its importance and the existence of an effective vaccine, is the focus of the remainder of this section. HCV vaccine development is ongoing and, if successful, would provide a way to prevent another part of the liver cancer burden.
Where HBV is widespread, babies may be infected perinatally by their mothers in the period shortly before and after birth, or during early childhood from contact with other children. Where HBV is less prevalent, more new infections occur among adults, from needle sharing among infected individuals, unprotected sexual contact with an infected person, and blood transfusions of infected blood. HBV is highly infectious and robust, and can survive outside the body.
Acute clinical hepatitis may or may not develop at the time of HBV infection. Few babies (about 1 percent of those infected perinatally) develop acute disease, but it becomes more common at older ages (about 30 percent of new infections). Regardless of the development of clinically apparent disease, people who clear their infections become immune for life. However, the earlier the infection occurs, the more likely it is to become chronic. As many as 90 percent of babies infected perinatally, and 30 percent of children infected before age 5, become chronically infected carriers, while the same is true of only about 6 percent of those infected as adults.
The Role of Co-Carcinogens in the Development of HCC
People with chronic HBV infection are at much higher risk of HCC when they are also exposed to a co-carcinogen that is synergistic with the virus. The most widespread known co-carcinogens are “aflatoxins,” which are chemicals produced by a genus of fungus (Aspergillus) that grows on many types of stored grains and other foods. Groundnuts (peanuts) and corn, dietary staples for millions of people, are particularly susceptible. People with exposures to both HBV and certain common aflatoxins have about a 60-fold increased risk of HCC compared with exposure to neither (Kensler et al., 2003). Other mycotoxins (products of other fungi) contaminate stored foods, mainly in developing countries with hot, humid climates. Up to one-quarter of the world’s food supply may be contaminated with mycotoxins (Turner et al., 2002). Aflatoxin contamination can be reduced by low technology techniques such as drying crops in the sun, discarding
moldy kernels before storing, and storing in natural fiber sacks on wooden pallets. Such efforts may be worthwhile, although they are more complex and difficult to achieve than vaccination (Hall and Wild, 2003).
The relationship of HBV, aflatoxins, and HCC is probably the best studied example of a virus–chemical interaction acting synergistically to vastly increase the risk of a cancer. The relationships have been established definitively in epidemiologic studies and in animal models. Both HBV and exposure to aflatoxin (through ingesting contaminated food) are detectable in blood samples, a factor that has added to the ability to study their relationship to HCC and other liver diseases (Turner et al., 2002).
Preventing Deaths from Hepatocellular Carcinoma (and Other HBV-Related Liver Diseases)
If new HBV infections could be prevented, most deaths from HCC and other HBV-related liver diseases would be avoided. Of the 350 million living HBV carriers, some proportion of deaths could also be avoided by modifying exposure to co-carcinogens. There are also treatments for chronic HBV, but they are expensive, toxic, and only partially effective. The intervention with the greatest potential for controlling HBV-related cancer and other deaths is HBV vaccination. For people already infected, reducing exposure to aflatoxin or modifying its effect through diet and reducing excessive alcohol consumption can help.
HBV Vaccination
An HBV vaccine suitable for widespread public use has been available for more than 20 years. Currently, both a plasma-derived and a recombinant DNA vaccine are available for $0.25–.50 per dose ($0.75–1.50 for the series). The three-dose series of HBV vaccine is 90 to 95 percent efficacious in preventing infection (Centers for Disease Control and Prevention, 2003). The first nationwide vaccination program began in Taiwan in 1984. It was a phased program that first vaccinated babies of carrier mothers, then all newborns, then unvaccinated preschool and elementary children. Since 1991, catch-up vaccinations have been given to unvaccinated children in first grade. Overall HBV prevalence (as measured by hepatitis B surface antigen, or HBsAg, in blood) declined from about 10 percent in 1984 to less than 1 percent in 1999 (Lavanchy, 2004). Similar declines where chronic infection rates were historically high have been documented in the Gambia, China, Indonesia, Senegal, Thailand, and among Alaska natives.
Strategies for vaccine use and vaccination schedules may vary by HBV endemicity levels (Table 6-1). The higher the prevalence, the higher the perinatal transmission; this means that giving a first dose within 24 hours
TABLE 6-1 Preferred Strategies Based on Economic Evaluation, According to the Level of Chronic Hepatitis B Endemicity
|
Level of Endemicity |
Preferred Strategy Based on Recent Economic Evaluationsa |
Cost Saving to the Health Care Sectora |
Cost Saving to Societya |
|
High |
Universal neonatal vaccination |
NDb |
Yes |
|
Intermediate |
Universal neonatal, infant, or adolescent vaccinationc |
No |
Yes |
|
Low |
Universal adolescent or infant vaccinationc |
No |
Yes |
|
Very low |
Selective risk groupd |
No |
ND |
|
ND = not determined. aGeneralizations, based on baseline calculations of recent evaluations. bDependent on the level of available treatment. cDependent on local economic and epidemiologic situation and efficiency of vaccine delivery system. dMost likely more cost-effective than universal vaccination, provided coverage in risk groups is sufficiently high. SOURCE: Reprinted, with permission, from Beutels (2001). Copyright 2001 by John Wiley & Sons Limited. |
|||
of birth is most important in high-prevalence areas. Other doses can coincide with regular childhood vaccine schedules. Where transmission is less intense, all three doses can be given along with the other routine childhood vaccines. The United States, a low-endemicity country, adopted universal infant vaccination in 1991, supplemented with targeted vaccinations for older children and adults at highest risk of infection. The recommendations have been revised over time toward ever greater coverage, with the aim of eliminating HBV transmission entirely.
Cost-Effectiveness of Hepatitis B Immunization
Hepatitis B immunization has been the subject of many economic evaluations, nearly all in industrialized countries. These countries also tend to have relatively low prevalence of HBV, with relatively little perinatal or early childhood transmission, but the availability of hepatitis B vaccines has meant that policy makers have had to make active decisions about what should be recommended. A comprehensive review of studies published worldwide between 1994 and 2000 identified 16 cost-effectiveness analyses, 4 cost-benefit analyses, and 3 combined cost-effectiveness and cost-benefit analyses. Among these were only one study in a middle-income country,
Romania, and one in a low-income country, China. Romania is categorized as “intermediate endemicity” and China, “high endemicity.”
The Romanian analysis was carried out from two perspectives: the health care payer only, and society as a whole. The main analysis used a relatively high discount rate, 7 percent. In a country like Romania—similar to other middle-income countries and certainly all low-income countries—there is little potential to “save” health care costs because virtually no treatment is offered for either the acute or chronic effects of hepatitis B. Not surprisingly, the Romanian analysis found that the vaccination program was not cost saving to the health care system. It was very sensitive to the discount rate, however. At discount rates lower than 4 percent, universal infant immunization would be cost saving for the health care system, and at rates lower than 6.3 percent, it would be cost saving to society.
How Many Deaths Could Be Prevented with HBV Vaccination?
Using information on infection rates by age, Goldstein and colleagues (Goldstein et al., 2005) developed a relatively simple model to estimate HBV infection rates and mortality from both acute and chronic disease, and the effect that HBV vaccination would have on these outcomes. The model is electronically accessible and is set up to produce estimates for individual countries (Centers for Disease Control and Prevention, 2003).
The model In the model, infection was assumed to occur in one of three age periods: perinatal (at birth); early childhood (after birth through 5 years); and late (>5 years). Deaths from cirrhosis and HCC deaths among chronic HBV carriers were presumed to be HBV related, and were estimated from age-specific, HBV-related cirrhosis and HCC mortality curves, adjusted for background mortality.
A complete HBV vaccination series (≥3 doses of hepatitis B vaccine, including the first dose within 24 hours of birth) was estimated to be 95 percent effective in preventing perinatal HBV infection (postexposure immunization) and early childhood and late infection (preexposure immunization), and was assumed to provide lifelong protection. Where the first dose was given more than 24 hours after birth, infants were considered susceptible to perinatal infection, but protected from early childhood and late infection. Values were varied in several sensitivity analyses. The central estimates are discussed here.
Calculations using the model Results based on the 2000 birth cohort were calculated. With complete global immunization, including a birth dose, it should be possible to prevent 95 percent of all HBV-related deaths. Without a birth dose, the estimate is 75 percent of HBV-related deaths prevented.
With coverage increasing from 50 to 80 to 90 percent, the proportion of deaths prevented increased from 38 to 60 to 68 percent. With 90 percent complete vaccine series coverage, administration of a birth dose to 50 percent and 90 percent of the vaccinated birth cohort increased the proportion of deaths prevented to 77 percent and 84 percent, respectively.
This is basically a static model looking at a single birth cohort. As immunization rates rise and prevalence falls, the effect of vaccination will be greater in later cohorts.
Results by World Health Organization (WHO) region (Table 6-2) show the greatest potential gains in the Western Pacific, Southeast Asia, and Africa. At the highest coverage, including a birth dose, more than 1 million premature deaths of the 2000 birth cohort could be prevented. Most of these would be deaths from HCC.
Global Vaccine Coverage
As the price of HBV vaccine declined, universal vaccination became a realistic goal. In 1992, WHO recommended that all countries with a high hepatitis B disease burden introduce HBV vaccine into their routine immunization programs by 1995, and that all countries do so by 1997. These targets were not met, however. The greatest shortfalls were in the poorest countries, most of which have high HBV burdens. Before 2000, only seven
TABLE 6-2 Reduction in Future Hepatitis B-Related Deaths: Hepatitis B Disease Burden Model
|
|
|
Proportional Reduction in Deaths with Three Doses of Hepatitis B Vaccine |
||
|
WHO Region |
Number of Deaths in 2000 Birth Cohort without Vaccination |
No Birth Dose |
50% Birth Dosea |
90% Birth Dosea |
|
Africa |
276,000 |
70% |
78% |
84% |
|
Americas |
28,000 |
66% |
6% |
84% |
|
Eastern Mediterranean |
96,000 |
74% |
80% |
84% |
|
Europe |
56,000 |
72% |
79% |
84% |
|
Southeast Asia |
368,000 |
71% |
78% |
84% |
|
Western Pacific |
581,000 |
63% |
74% |
83% |
|
Global |
1,405,000 |
68% |
77% |
84% |
|
aProportion of the vaccinated cohort receiving the first dose of vaccine within 24 hours of birth. SOURCE: Reprinted, with permission, from Goldstein et al. (2005). Copyright 2005 by the International Epidemiological Association. |
||||
|
BOX 6-1 The Global Alliance for Vaccines and Immunization and the Vaccine Fund The Global Alliance for Vaccines and Immunization (GAVI) was established in 2000 with the goal of increasing immunization rates in the poorest countries and reversing widening global disparities in access to vaccines. Countries with gross national income (GNI) levels below $1,000 are eligible for assistance from GAVI through the associated Vaccine Fund. GAVI partners include governments in industrialized and developing countries, UNICEF, the World Health Organization, the World Bank, nongovernmental organizations, foundations, vaccine manufacturers, and public health and research institutions. The Vaccine Fund has been financed by the Bill & Melinda Gates Foundation and by 10 governments to date—Canada, Denmark, France, Ireland, Luxembourg, the Netherlands, Norway, Sweden, the United Kingdom, and the United States—as well as the European Union and private contributors. Countries eligible on the basis of GNI must submit proposals to GAVI for Vaccine Fund support. Proposals are reviewed by a panel of experts from around the world. Currently, The Vaccine Fund offers the following support to qualifying governments:
As of December 2003, more than 42 million children had been vaccinated with GAVI-supported HBV vaccine. |
(less than 10%) of the poorest countries were using HBV vaccine in their routine immunization programs. When the Global Alliance for Vaccines and Immunization (GAVI) (Box 6-1) was established in 2000, HBV vaccine was one of the underused vaccines in its portfolio. The GAVI partners set a new milestone, which is for HBV vaccine to be introduced in all countries with adequate delivery systems by 2007.
Childhood HBV vaccination has now been adopted by many countries, but there is still a wide gap between rich and poor countries. In 2001, 137 of 191 WHO Member States had universal infant or childhood HBV vaccination programs. An estimated 32 percent of infants were fully vac-
cinated, with a range of 65 percent in the Western Pacific, to 58 percent in the Americas, to less than 10 percent in Southeast Asia and Africa. By May 2003, the number of countries with a universal childhood HBV vaccination policy had risen to 151, but that included only about half (24 out of 46) of the African countries (Centers for Disease Control and Prevention, 2003). Eighty-nine WHO Member States have historically high HBV prevalence (HBsAg ≥ 8%), where infant vaccination is of particular importance. Of these, 64 have adopted universal infant vaccination, and of these, 34 have a policy to administer the first dose at birth, which is the best way to prevent perinatal transmission.
Fifty of the more than 70 countries eligible for support from the Vaccine Fund, the financing arm of GAVI, had approval for HBV vaccine funding as of December 2004 (although implementation varies). Coverage of at least 50 percent for the basic infant vaccines (three doses of DTP: diphtheria, tetanus, polio) is required before a country can request support for HBV vaccine. For countries with less than 50 percent DTP3 coverage, GAVI offers assistance to improve the immunization infrastructure and boost basic coverage.
The Vaccine Fund will cover the purchase of hepatitis B vaccine and safe injection equipment for 5 years, together with a single payment of $100,000 to facilitate the introduction of the new vaccine. GAVI will then work with countries to develop a financial sustainability plan to ensure continued financing for hepatitis B vaccine once Vaccine Fund support ends.
Can More Be Done to Increase HBV Vaccine Coverage?
The global community can continue to encourage countries to include HBV vaccination with their childhood immunization programs, and particularly in high-prevalence countries, to start with a birth dose. Both a financing mechanism (at least in the short term) and technical assistance are on offer from GAVI and the Vaccine Fund. If some countries have been reluctant to request funds to begin because of the longer term cost, GAVI could extend financing for a longer period (as they are doing with the Hib vaccine in some places). Overall, further gains will depend on improving the vaccination infrastructure and implementation, both of which are likely to be slow processes in the countries that are already lagging. With poor coverage of even the standard childhood vaccines, HBV will not be the main driver for improvement.
REDUCING THE TOLL OF CERVICAL CANCER IN LMCS
Nearly half a million women around the world develop cervical cancer each year and 270,000 die from it. More than 80 percent of the cases and
TABLE 6-3 Incidence and Mortality from Cervical Cancer by Income Group of Countries
|
Country Income Group |
Cases Per Year |
Deaths Per Year |
|
High income |
54,000 |
22,000 |
|
Upper middle income |
37,000 |
17,000 |
|
Lower middle income |
161,000 |
87,000 |
|
Low income |
238,000 |
147,000 |
|
World |
490,000 |
273,000 |
|
SOURCE: Barton et al. (2005). |
||
a slightly higher percentage of the deaths are women in LMCs (Table 6-3) (IARC, 2004). The burden of disease is highest in Africa, Latin America, and South and Southeast Asia.
Virtually all cases of cervical cancer are caused by persistent infection with certain oncogenic strains of HPV, a very common sexually transmitted virus. For most women initially infected with HPV, the infection clears with no intervention; these women are no longer at risk for cervical cancer. For those who remain infected, cervical cancer can develop through a long—usually decades-long—process of cellular change. Even before the details of this progression were understood completely, most cervical cancer in high-income countries was being prevented by frequent screening for abnormal (but not yet cancerous) cells on the surface of the cervix, which can be removed using relatively simple, minimally invasive procedures.
The more detailed understanding of how cervical cancer develops, and the role of HPV, has led to new screening and treatment approaches, as well as the development of vaccines to protect against HPV infection, one of which just entered the market in 2006. These new approaches, still relatively early in development, are discussed later in this section.
Cervical Cancer and HPV
The epidemiologic study of cervical cancer dates to the 18th century and Bernardino Ramazzini’s observation that “cancer of the womb” was uncommon among Catholic nuns, but common among married women (American Cancer Society, 2005). A link with sexual activity was long suspected, eventually leading to the discovery that cervical cancer is caused by a sexually transmitted infection.
It is now understood that 90 percent of women infected with HPV will clear their infections within a few years. HPV infection will persist in the remaining 10 percent, who make up the population at risk of cervical
TABLE 6-4 Distribution of HPV Types in Invasive Cervical Cancer in All Studies
|
HPV Type |
Estimated Number of Cases Observed |
Percentage of All Cases Observed |
Percentage of Cases (cumulative) |
|
16 |
251,200 |
53.5 |
53.5 |
|
18 |
80,900 |
17.2 |
70.7 |
|
45 |
31,600 |
6.7 |
77.4 |
|
31 |
13,700 |
2.9 |
80.3 |
|
33 |
12,100 |
2.6 |
82.9 |
|
52 |
11,000 |
2.3 |
85.2 |
|
58 |
10,000 |
2.2 |
87.4 |
|
35 |
6,600 |
1.4 |
88.8 |
|
59 |
6,100 |
1.3 |
90.1 |
|
56 |
5,800 |
1.2 |
91.3 |
|
51 |
4,600 |
1.0 |
92.3 |
|
39 |
3,200 |
0.7 |
93.0 |
|
68 |
2,700 |
0.6 |
93.6 |
|
SOURCE: Adapted from Munoz et al. (2004). |
|||
cancer (Bosch and Muñoz, 2002). A vast body of evidence supports this understanding and is now fully accepted.
To summarize, studies worldwide have consistently found HPV in 95 to 100 percent of cervical cancer cells, and in virtually all cases of cervical intraepithelial neoplasia (CIN) (Bosch et al., 2002). More than 90 percent of cervical cancers have the same 10 to 15 types of HPV (Table 6-4) (Bosch et al., 1995), and metastases contain the same types as in primary sites (Lancaster et al., 1986). Morphological changes in oncogenic HPV studied in cells in the laboratory (in vitro) closely resemble the changes seen during the progression from normal to cancerous tissue in women (Meyers and Laimins, 1994; Steenbergen et al., 1996). In cervical lesions, the HPV viral genome is always active, increasing in viral numbers as the lesion increases in severity (Stoler et al., 1992). In 1995, the International Agency for Research on Cancer (IARC) categorized HPV (types 16 and 18; see below) as “carcinogenic to humans” (“Group 1”), which denotes IARC’s highest level of evidence for a causal association (IARC, 1995).
Transmission of HPV
HPV is transmitted almost entirely through sexual intercourse. The risk of acquiring HPV increases with the number of sexual partners and with early age at first sexual activity (IARC Working Group, 2005). A history of sexually transmitted infections (STIs) of women and their partners, male circumcision, and the presence of penile HPV have also been shown to be
significant risk factors in acquiring HPV (Bosch et al., 1994; Juarez-Figueroa et al., 2001; Kjaer et al., 1991; Palacio et al., 1993; Thomas et al., 2001b, cited by IARC Working Group, 2005). The natural history and persistence of HPV in men has not been well documented; however, a 4-year National Institutes of Health-funded study with that goal is under way, with results expected in 2008.
HPV Types and Their Association with Cervical Cancer
Only a handful of the 100 known types of human papillomaviruses are associated with a high risk of cervical cancer (IARC Working Group, 2005). Types 16 and 18 have accounted for 70 percent of cervical cancer cases in studies around the world (Table 6-4). Thirteen high-risk carcinogenic HPV types account for almost 95 percent of all cases studied (Muñoz et al., 2004, cited by IARC Working Group, 2005).
Once a firm causal link was drawn between HPV and cervical cancer, it became clear that many of the risk factors observed for cervical cancer— such as the number of sexual partners and age at first intercourse—were actually the risk factors for acquiring HPV. It was also recognized that certain factors might, in the presence of HPV infection, increase the chances of cancer developing, and this has been borne out by studies of infection and these risk factors. Cigarette smoking raises the risk of cervical cancer two- to three-fold among HPV-positive women (IARC Working Group, 2005; Plummer et al., 2003; Szarewski and Cuzick, 1998). HPV infections are maintained significantly longer and are less likely to clear in smokers compared to nonsmokers (Giuliano et al., 2002, cited by IARC Working Group, 2005).
Both Herpes simplex virus type 2 (HSV2) and the bacterium Chlamydia trachomatis, two common STIs, both may enhance the oncogenic potential of HPV (IARC Working Group, 2005). HIV infection appears to potentiate the risk of cervical cancer among HPV-positive women, and invasive cervical cancer is an AIDS-defining illness. Women infected with HIV are more likely to be infected with oncogenic types of HPV and have higher rates of progression to cervical dysplasia than those without HIV (IARC Working Group, 2005; Massad et al., 1999; Thomas et al., 2001b).
The number of pregnancies a woman has had may also increase the risk of cervical cancer. The risk among HPV-positive women rises with the number of full-term pregnancies, reportedly as much as four times higher with seven or more full-term pregnancies, compared with no children (IARC Working Group, 2005).
The evidence linking oral contraceptives (OCs) to cervical cancer in HPV-positive women is inconclusive. Several studies have found weak to no associations (Kruger-Kjaer et al., 1998; Lacey et al., 1999; Thomas et al.,
2001a), and others have found strong associations with long-term OC use (Bosch et al., 2002; Castellsague and Munoz, 2003; Moreno et al., 2002; Smith et al., 2003). Studies of this topic continue (Bosch et al., 2002; IARC Working Group, 2005; Smith et al., 2003).
Natural History of HPV and Cervical Cancer
In 1908, Schanenstein proposed the idea that invasive cervical cancer develops only after progressing through preinvasive lesions (Chirenje, 2004). Two decades later, Papanicolaou and Babes introduced a method to examine cells from the surface of the cervix (the Papanicolaou, or “Pap,” smear) to identify women with early-stage invasive cancer, with the aim of providing curative treatment. They recognized the long period during which preinvasive lesions could exist, and that they regressed in many women. This meant that women could be treated while the condition was still in a preinvasive lesion stage, preventing the development of cervical cancer.
As now understand, the progression toward cervical cancer begins with prolonged HPV infection, i.e., an HPV infection that is not eliminated by an immune response. After HPV clearance, infections have rarely been detected during the follow-up period in cohort studies, suggesting that immunity is acquired against reinfection with the same HPV type (Shah et al., 1997, cited by IARC Working Group, 2005). However, this evidence is incomplete and the question continues to be studied (IARC Working Group, 2005).
HPV Transience
Average levels of HPV prevalence vary and several distinct patterns of age-specific prevalence have been found in different areas of the world. In many areas, including most high-income countries (but also including some LMCs), HPV prevalence declines sharply after 30 years of age (Molano et al., 2002; Pham et al., 2003; Ronco et al., 2005). In other areas, prevalence is more constant across age groups (Shin et al., 2003; Thomas et al., 2004; Pham et al., 2003). In at least one place (Shania Province, China), prevalence was low in young women and rose with age (Dai et al., 2006). Repeated sampling of younger women in follow-up studies where prevalence declines with age has shown a median duration of transient HPV infection of 8 months for high-risk types, and 4.8 months for low-risk types. Approximately 90 percent of infections are eliminated within about 2 years (IARC Working Group, 2005). Of the remaining 10 percent of women with persistent infection with a high-risk type of HPV, some will develop abnormal cervical cells, most of which will revert to normal. A small percentage will progress to advanced-stage cervical neoplasia and cancer (Bosch et al., 2002; IARC Working Group, 2005).
TABLE 6-5 International Federation of Gynecology and Obstetrics Staging for Cervical Cancers
|
Stage |
Description |
|
Stage 0 |
Carcinoma in situ, preinvasive carcinoma (advanced CIN 3) |
|
Stage I |
Invasive carcinoma strictly confined to cervix |
|
Stage II |
Carcinoma extending beyond cervix but not to pelvic sidewall; carcinoma involves vagina, but not lower third |
|
Stage III |
Carcinoma extending onto pelvic wall; tumor involves lower third of vagina |
|
Stage IV |
Carcinoma extends beyond true pelvis or clinically involves mucosa of bladder or rectum |
|
SOURCE: IARC Working Group on the Evaluation of Cancer Preventive Strategies (2005). |
|
Stages of Cervical Dysplasia and Cancer
Several classification systems are used to describe the stages of cervical changes leading to cervical cancer. Cervical intraepithelial neoplasia 1 (CIN 1), also known as low-grade squamous intraepithelial lesion (LSIL) and mild dysplasia, tends to be benign and transient, and detected as borderline or mild cytological abnormalities (IARC Working Group, 2005). CIN 2, known also as high-grade squamous intraepithelial lesion (HSIL) and moderate dysplasia, represents a true premalignant lesion (Schiffman and Brinton, 1995), usually associated with high-risk HPV types. CIN 3 is also known as severe dysplasia, carcinoma in situ, and HSIL. CIN 3 lesions are virtually always associated with high-risk HPV types. CIN 3 is considered Stage 0 in the commonly accepted cervical cancer staging system (Table 6-5) and lasts an average of 5 to 11 years (IARC Working Group, 2005). About half of women with CIN 3 lesions will progress to invasive cervical cancer if not treated (<1 percent of all original HPV infections) (IARC Working Group, 2005).
Stage I invasive cervical cancer has several phases, depending on the rate of growth of the lesion(s), but are small (less than 5 mm) with minimal invasion. Cervical cancer at this stage is highly curable. Stage II is considered advanced cervical cancer, with extension of the carcinoma beyond the cervix to the pelvic wall but not beyond. Stage III carcinoma extends into the lower vagina. Stage IV carcinoma involves the spread of cancer to adjacent organs, including the bladder and rectum.
Intervening to Prevent Cervical Cancer
The important message from the current understanding of the natural history of cervical cancer is this: Cervical cancer is the end stage of a series of cellular changes that occur over a period of decades (usually), in the presence of specific strains of HPV. Eliminating the HPV and the altered cells when
they are still precancerous through one of a few relatively simple procedures stops the progression to cancer. At every stage, however, only a portion of women testing positive (i.e., with cellular changes or HPV present) would eventually develop cervical cancer. This means that the earlier in the course of progression treatment is given, the greater the number of women treated who would never have developed cancer, but also the simpler the treatment and the higher the success rate.
The challenges in designing prevention programs are in selecting the most appropriate detection and treatment methods and defining the target population in terms of age at screening, as well as screening intervals. The opportunities for doing this today are unprecedented because of the more complete scientific knowledge about the development of cervical cancer and because the newer methods have been studied systematically, alongside the older ones. Data are still emerging from a variety of studies, which means that not all questions can be answered immediately, but substantial information is available already.
Methods for Detecting Cervical Precancer and Cancer and Providing Treatment
Three basic methods are used to detect precancerous (or cancerous) cellular changes. The Pap smear and related laboratory examination of cells (cytology) is the oldest and the current standard in high-income countries. The second approach is some form of direct visualization, in which the examiner looks directly at the cervix with the naked eye (or with magnification) to search for patches of abnormal cells, which have been made evident by the use of a chemical, either dilute acetic acid or iodine. The third approach is to test for the presence of HPV through DNA testing.
Treatment of all precancerous stages of cervical cancer involves either excision (cutting out) or ablation (destruction in place) of suspect lesions. The most frequent excision technique is loop electrosurgical excision procedure (LEEP), which uses an electrified wire to excise the abnormal area. A skilled provider is needed, as is a reliable electrical supply. The excised tissue can be evaluated for a specific diagnosis, where laboratory services are available. The most commonly used ablative procedure is cryotherapy, which involves freezing the areas that appear abnormal using compressed carbon dioxide or nitrous oxide as the refrigerant. A wide range of health workers can be trained in cryotherapy. Because the tissue is destroyed, it cannot be further analyzed.
The detection test that is used dictates certain aspects of follow-up and treatment, including the number of visits needed and the technologies used. A Pap smear traditionally requires a minimum of three visits for a positive test. The first is for the sample to be taken during a pelvic examination. If the
Pap smear is read in the laboratory as positive, colposcopy will be carried out during a second visit. This involves a direct examination at the cervix with magnification, with and without acetic acid, during which biopsies are taken of abnormal areas. If the biopsied material is positive, treatment to eliminate the abnormality is provided at a third visit.
With direct visualization, detection and treatment can be carried out in a single visit, or in two visits. In the single-visit approach, if the examiner finds a lesion, it can be treated immediately with cryotherapy, or that can be carried out at a second visit (possibly at a different site). HPV testing requires two visits because samples must be processed in specialized laboratories. Women with positive tests are called back and a direct visualization procedure of some sort is used to localize the lesion, which is then treated.
These variations—the numbers of visits, the types of procedures, and infrastructure and personnel needs—take on greater significance in resource-poor settings. Each major screening approach is described briefly in the next sections.
Pap Smear and Cervical Cytology
“Conventional cervical cytology”1 is the process of collecting cell specimens from two areas of the cervix: the transformation zone, using a specially shaped wooden or plastic spatula; and the endocervix (endocervical canal), using a conical cervical brush. The cells are then spread onto a glass slide and stained for examination under the microscope.
Liquid-based cytology (LBC), a more expensive process, was introduced in the 1990s to improve test performance. Following conventional cytology methods to the point of cell transfer, LBC allows the cells to be transferred to a liquid preservative for transport to the laboratory, where a slide is then prepared for microscopic reading. Both of these methods are largely automated. Results are classified according to one of several systems to distinguish the different stages of dysplasia and cancer, described above. A laboratory is required for cytology, including internal and external quality controls, for processing slides and microscopy. These systems include continuous monitoring of recordkeeping, review of abnormal cases by a cytopathologist, and review of negative cases by rapid rescreening of all cases or a percentage of randomly chosen samples, with correlation of cytological and histological results when possible, as well as proficiency programs (IARC Working Group, 2005).
Visual Inspection with Acetic Acid or Lugol’s Iodine
Before the advent of the Pap smear and routine cytology-based screening in the 1950s, direct visualization of the cervix with the naked eye and no enhancement was used to detect precancerous lesions (Ferreccio and Gage, 2003). When it became clear that the Pap smear and cytology was better for this purpose, direct visualization was largely abandoned (Sehgal et al., 1991). The application of an agent—acetic acid or iodine—to the cervix, however, greatly improves detection with direct visual inspection (DVI), also known as cervicoscopy, and aided visual inspection (WHO, 2002). The immediate excision of areas of abnormal cells, using cryotherapy or LEEP, allows screening and treatment in a single visit.
With visual inspection of any kind, results are interpreted solely by the screener based on what is seen during the examination; no permanent record exists to verify or review interpretations (except in study situations, where photographs may be taken). Accurate interpretation with either technique requires training and experience.
Visual inspection with acetic acid (VIA) In VIA dilute acetic acid (vinegar) is applied to the cervix. The acetic acid causes areas of CIN to glow white temporarily, an effect called acetowhitening (Ferreccio and Gage, 2003). No laboratory processing is required and results are immediate (ACCP, 2004). VIA has been used as both a triage method for colposcopy and as a primary screening method (IARC Working Group, 2005).
A study published in 1982 laid the groundwork for further development of VIA. In the study, 2,400 women were examined both with colposcopy and VIA. Using colposcopy as the gold standard, VIA had a high sensitivity and specificity (i.e., it correctly categorized most women either positively or negatively). With colposcopy, 312 patients were identified as having abnormalities; 98 percent were identified similarly with VIA. Of the 1,584 women diagnosed as normal by colposcopy, 99 percent were also normal by VIA (Ottaviano and La Torre, 1982, cited by Ferreccio and Gage, 2003).
In most studies of VIA, nurses or nurse-midwives have conducted the screening, although in one study (Sankaranarayanan et al., 1998), assessments were made by cytotechnicians, and in two studies (Ottaviano and La Torre, 1982; Slawson et al., 1992), the screeners were physicians. Paramedical workers, such as traditional birth attendants and community health workers, also can be trained in VIA techniques (Ferreccio and Gage, 2003).
Visual inspection with Lugol’s iodine (VILI) Application of iodine solution to the normal cervix results in dark staining of mature squamous epithelium, which has a high glycogen content. Areas of neoplasia contain very little
glycogen and do not stain, instead taking on a bright mustard or saffron yellow color. Although described as early as 1933 by Schiller (“Schiller’s iodine test”), VILI fell into disuse as the Pap smear and cytology became standard (IARC Working Group, 2005). Interest has recently been renewed, although it has not yet been studied as extensively as has VIA.
Screening for HPV
Research on HPV DNA assays as a cervical cancer screening tool began in the late 1980s (IARC Working Group, 2005). “Hybrid Capture” nucleic acid hybridization (HC) and polymerase chain reaction (PCR) methods have been used to detect HPV DNA in screening studies over the past decade. Currently, the most commonly used commercial test includes 13 high-risk HPV types and is approved by the U.S. Food and Drug Administration (FDA) (Sankaranarayanan et al., 2005). The assay indicates that an included type is present, but does not identify the specific type. The sample to be tested is collected in much the same way as a liquid-based Pap smear sample, with cells stored in specimen transport medium (STM). Specimens in STM can be stored at room temperature for up to 2 weeks, for an additional week at 4°C, and up to 3 months at −20°C (IARC Working Group, 2005).
Accuracy and Reliability of the Screening Methods
How well do these methods work as primary screening tests? The question is more complex than simply asking whether a particular lesion is detected by a single test. It involves the natural history of the disease, including how strongly the endpoint being measured predicts eventual cancer without intervention, and the likelihood that cancer will occur in the absence of the endpoint the test is designed to detect. These, in turn, are influenced by the age of the person being screened and other individual factors. The outcome also depends on the quality of the service, and the competence of the practitioners involved.
Pap Smears and Cytology
The accuracy of Pap smears and cytology based on recent work was reviewed in 2005 (IARC Working Group, 2005) and is summarized in Table 6-6. The studies varied in design, particularly the proportion of negative tests that were verified, ranging from verification of all negative tests to verification of a sample of variable size. For a screening method that is used so widely, little evaluative information is available. This can be explained by the fact that the tests evolved and came into use before the type of evaluation
TABLE 6-6 Performance of Cervical Cytology in Various Large Research Studies
|
Year |
Country |
Age Range |
Study Size |
Sensitivitya (percentage) |
Specificityb (percentage) |
Histological Cut-off |
|
1999 |
United Kingdom |
34+ |
2,988 |
86 |
98 |
CIN 2+ |
|
1999 |
Costa Rica |
18+ |
8,636 |
55 |
98 |
CIN 2+ |
|
2000 |
Canada |
18–69 |
2,098 |
56 |
62 |
CIN 2+ |
|
2000* |
South Africa |
35–65 |
2,944 |
70 |
85 |
CIN 2+ |
|
2002* |
South Africa |
35–65 |
2,754 |
40 |
96 |
CIN 1+ |
|
2003 |
United Kingdom |
30–60 |
11,085 |
77 |
96 |
CIN 1+ |
|
2003 |
Germany |
30+ |
8,466 |
44 |
98 |
CIN 2+ |
|
2003 |
Mexico |
15–85 |
7,868 |
59 |
98 |
CIN 1+ |
|
2004 |
India |
25–65 |
10,591 |
65 |
92 |
CIN 2+ |
|
NOTE: Threshold for referral was ASCUS cytology except the South Africa studies (*), where it was LSIL. aSensitivity is defined as the proportion of truly diseased persons, as measured by the gold standard, who are identified as diseased by the test under study. It is calculated by this formula: True Positives/(True Positives + False Negatives). bSpecificity is the proportion of truly nondiseased persons, as measured by the gold standard, who are so identified by the diagnostic test under study. It is calculated by this formula: True Negatives/(False Positives + True Negatives). SOURCE: Adapted from IARC Working Group on the Evaluation of Cancer Preventive Strategies (2005). |
||||||
expected today was routine, and at a much earlier stage in the understanding of cervical cancer.
Results vary from a sensitivity of 40 percent to 86 percent. Several large meta-analyses have indicated that cytology sensitivity and specificity are lower than previously thought (Fahey et al., 1995; McCrory et al., 1999; Nanda et al., 2000, cited by IARC Working Group, 2005). However, a reevaluation of these meta-analyses concluded that sensitivities as low as 60–70 percent were unlikely in a modern cytological screening practice (IARC Working Group, 2005).
A critical factor for the lack of success or suboptimal performance of cytology-based screening in less developed countries is poor quality of testing. Test performance of cytology in routine conditions in many laboratories is likely to be inferior to that observed under study conditions.
Visual Inspection (VIA and VILI)
Visual inspection methods have had similar or better sensitivity than cytology for detecting CIN 2 or 3 or invasive cancer in most studies, but
specificity in most studies is lower than cytology (Table 6-7) (Ferreccio and Gage, 2003; IARC Working Group, 2005). This means that a greater proportion of abnormalities are detected correctly, but that many women without abnormalities are identified as having them.
The sensitivity of VIA to detect high-grade precancerous lesions and invasive cervical cancer varied widely, from 29 to 95 percent, and specificity varied from 68 to 98 percent (IARC Working Group, 2005).
VILI has been tested in fewer studies than VIA, but they suggest a possibly improved sensitivity over VIA. In a review of cross-sectional studies involving 49,080 women aged 25–65 years in several African nations and India (Sankaranarayanan et al., 2004b), the sensitivity of VILI was 92 percent and of VIA, 77 percent for CIN 2 or 3 lesions. Specificity was about 85 percent for both techniques.
TABLE 6-7 A Comparison of VIA and Cytology Accuracy in Published Studies
|
|
|
Positivity Rate (percentage)a |
Sensitivity (percentage) |
Specificity (percentage) |
|||
|
Location, Year |
Sample Size |
VIA |
Cytology |
VIA |
Cytology |
VIA |
Cytology |
|
United States, 1992 |
2,690 |
3 |
6 |
29 |
87 |
97 |
95 |
|
Italy, 1993 |
2,036 |
25 |
4 |
88 |
63 |
75 |
96 |
|
South Africa, 1996 |
2,426 |
3 |
13 |
65 |
100 |
98 |
88 |
|
India, 1997 |
372 |
53 |
6 |
78 |
22 |
49 |
95 |
|
India, 1998 |
2,935 |
10 |
10 |
87 |
86 |
91 |
91 |
|
Zimbabwe, 1999 |
2,148 |
40 |
13 |
77 |
44 |
64 |
91 |
|
India, 1999 |
1,268 |
36 |
16 |
95 |
62 |
68 |
87 |
|
South Africa, 2000 |
2,944 |
18 |
15 |
67 |
80 |
83 |
87 |
|
South Africa, 2001 |
6,298 |
18 |
2 |
50 |
19 |
84 |
99 |
|
India, 2001 |
402 |
42 |
42 |
87 |
81 |
82 |
79 |
|
South Africa, 2002 |
2,754 |
25 |
70 |
57 |
79 |
96 |
n/a |
|
Philippines, 2003 |
3,316 (VIA) 3,195 (Pap) |
10 |
2 |
37 |
14 |
91 |
98 |
|
Pakistan, 2003 |
501 |
31 |
16 |
94 |
47 |
78 |
89 |
|
South Africa, 2003 |
1,093 |
53 |
9 |
79 |
53 |
49 |
95 |
|
India, 2004 |
22,663 |
17 |
9 |
72 |
65 |
84 |
92 |
|
NOTES: Cytology threshold: ASCUS+. Outcome threshold: CIN 2–3. aPositivity rate is defined by IARC as the proportion of diagnoses of cancer in all positive results of the screening test: A process measure. SOURCE: Reprinted, with permission, from IARC Working Group on the Evaluation of Cancer Preventive Strategies (2005). Copyright 2005 by the International Agency for Research on Cancer. |
|||||||
Wide variability in test performance characteristics for visual inspection methods and the lack of universally accepted definitions of VIA or VILI test results have made their evaluation difficult. Standardization of test result definitions would increase the ability to interpret results, and would likely increase reproducibility, a problem that has also hampered the use of cytology in comparison studies (IARC Working Group, 2005).
HPV DNA Testing
As a primary screening method in women older than 30 years of age, HPV testing has a sensitivity of about 95 percent for detecting CIN 2 or more severe lesions compared with 75 percent for cytology at the ASCUS cut-off level, and 70 percent for cytology at the LSIL cut-off level (IARC Working Group, 2005). HPV testing specificity is about 94 percent for women over 30 years of age, compared with 95 percent for cytology at the ASCUS level, and 98 percent at the LSIL level. The specificity of HPV testing in younger women, not surprisingly, is lower. No studies have yet prospectively investigated the impact of HPV DNA testing on subsequent cancer rates (IARC Working Group, 2005).
Comparing Testing Methods
Accuracy
HPV testing and the visual inspection methods are more sensitive than Pap smears and cytology at a single screening, at least under study conditions. However, both also have a lower specificity. In a single screening, more of the women actually at risk will be picked up, at a cost of identifying as positive more women who would not develop cancer. Where screening is readily available and carried out regularly, as in most wealthy countries, low sensitivity may not have major health consequences Lesions develop very slowly and can be detected in any of several years, still at a preclinical, curable stage. Overtreatment because of low specificity is most worrisome when the treatment carries significant risk. With cryotherapy, short-term complications are minimal, but less is known about possible long-term sequelae. No red flags have yet appeared but the issue will require continued monitoring.
Conditions of Use
Pap smear, HPV testing, and DVI each require basic essential facilities and supplies to examine women in a private space and collect samples safely
and correctly. The equipment needs for DVI are simpler and less expensive than for the other methods. For VILI, Lugol’s iodine may not be easily found in most developing countries, but acetic acid for VIA is available everywhere (ACCP, 2004). The conditions under which DVI can be performed are variable, and need not be in a clinic setting. HPV testing and Pap smear require laboratory facilities or transport systems to take samples to a laboratory, and a stable supply of electricity, while visual inspection techniques do not. In addition to these requirements is the need to make arrangements for treating conditions detected, from precancerous conditions to invasive cancer. For DVI, treatment of early lesions must be at or very close to the site of screening if treatment is to be provided at the same visit. For the other methods, treatment must be accessible to all women screened, which could involve referral and transport to another area.
Some problems of access could be alleviated with the use of mobile clinics, as has been tried in Thailand, for example (Swaddiwudhipong et al., 1995). A 6-year study in 54 Thai villages was successful in raising the proportion of women who had ever had a Pap smear from 20 percent in 1991, to 58 percent in 1994, to 70 percent in 1997 (Swaddiwudhipong et al., 1999). Women were invited to be screened and village health communicators provided information about screening and cervical cancer before the study started (Swaddiwudhipong et al., 1999). Mobile services are an option for all screening methods.
Acceptability of Methods
Key factors in patient acceptability of any screening program include distance to travel, number of visits, and cost. Other important factors include a good patient–physician relationship, a female screening provider, and a screening setting that assures patient privacy (IARC Working Group, 2005).
Cytology and HPV testing require facilities and personnel that make implementation difficult in most LMCs, except in some urban areas. Women in rural areas may be compelled to travel long distances, at personal expense, to be screened, and may have to return for test results and treatment. These factors are cited as contributors to a failure to decrease cervical cancer incidence and mortality (Lazcano-Ponce et al., 1999).
HPV testing presents a unique acceptability issue, distinct from the other screening methods, because it involves testing for a sexually transmitted infection. STIs can be stigmatizing in any country, developed or developing. Several studies have found significant patient dropout after initial testing; when contacted, patients indicated fear of finding out that they had contracted an STI (IARC Working Group, 2005). This points to a
need for health professionals to be trained in HPV counseling of test-positive patients and their sexual partners to ensure treatment and follow-up (IARC Working Group, 2005).
Cervical Cancer Prevention Implementation
Experience with Pap Smear and Cytology Programs
By the 1960s, Pap smears were available in many developed countries from physicians on a case-by-case basis. Later, screening programs were organized, notifying women at 1- to 5-year intervals. An exception is the United States, where Pap smear services are widely available, but notification is not mandatory. Cervical cancer incidence has been reduced by as much as 80 percent in developed countries through these programs (IARC Working Group, 2005). Attempts to establish Pap smear and cytology programs have not been successful in LMCs, with a few exceptions, mainly in urban areas with appropriate hospitals and laboratory facilities.
Mexico’s national screening program, for example, initiated in 1974, offers annual cytology smears to women 25 to 65 years of age (Sankaranarayanan et al., 2001). In some Mexican states, fewer than 30 percent of women have ever been screened, and no systematic organization exists for call, recall, and follow-up of screened women. Evaluations of cytology test results within Mexico’s program found wide variations in test performance across screening facilities, and a random sample review indicated 64 percent of the smears were of poor quality (Lazcano-Ponce et al., 1997). Cervical cancer mortality has not declined in Mexico as a result of the program (Lazcano-Ponce et al., 1996, cited by Sankaranarayanan et al., 2001 and Arillo-Santillan et al., 2001).
Other countries have been somewhat more successful. The Colombian National League Against Cancer, a component of the public health system, has offered cytology screening alongside private organizations in several regions since the 1970s (Sankaranarayanan et al., 2001). Costa Rica has provided nationwide cytology services to women older than 15 years since 1970, with 85 percent of eligible women having been screened at least once. In Cuba, where a biennial cytology screening program was implemented as part of primary health care services in 1968, more than 80 percent of women aged 20–60 years have been screened at least once (Garrote et al., 1996).
Experience with Visual Inspection Techniques
No national or large-scale programs using visual inspection techniques have yet been established. It is unlikely that the (mainly) wealthy countries that already have functioning Pap smear and cytology programs will re-
vamp the systems responsible for what might be the most successful cancer prevention efforts in history. However, in LMCs where the primary health care infrastructure is weak or fragile (including limited laboratory capacity) and basic health care needs are not being met, visual inspection programs may be more feasible than Pap smear-based programs. This reality is the driver behind efforts to determine the value and feasibility of alternative screening methods.
A recent multicountry study was organized by the Alliance for Cervical Cancer Prevention (ACCP), a consortium supported by the Bill & Melinda Gates Foundation to assess the feasibility and acceptability of introducing a VIA screen-and-treat (with cryotherapy) program into reproductive health services in India, Kenya, Peru, South Africa, and Thailand (ACCP, 2003; 2004). The visual inspections were performed by qualified nurses. Women who came to district hospital and local health centers for other reasons were invited to participate, and others were recruited through local publicity. The services were offered successfully everywhere, and women were generally pleased with them. The study did not compare visual inspection with other types of screening programs, so it is not a source of information on the accuracy of the method. (However, see the next section for a model that uses the data from the study.)
Work is continuing by ACCP partners to complete research and demonstration projects in Ghana, India, Peru, South Africa, and Thailand, and to summarize all ACCP findings when country projects are complete, expected in 2007 (ACCP, 2006).
Cost-Effectiveness of Different Screening Strategies
Goldie and colleagues (Goldie et al., 2005) used the results of the ACCP multicountry screen-and-treat study in India, Kenya, Peru, South Africa, and Thailand, supplemented with data from the literature, to model the cost-effectiveness of several cervical cancer screening and treatment strategies. The analysis was improved over earlier modeling studies by including more actual costs (e.g., laboratory equipment and supplies, transportation of specimens, training, administration, and others measured directly) and allowing direct comparisons among countries at different economic levels of development.
The computer-based model simulates the natural history of HPV infection through the development of cervical cancer, and superimposes on it various screening strategies. The costs (in year 2000 international dollars) and the gains in life expectancy generated by the model were used to calculate cost-effectiveness ratios. Costs and benefits were discounted by 3 percent.
The screening tests included in the model were:
-
Visual inspection of the cervix with acetic acid
-
Pap smear and cytologic examination of cervical cells
-
HPV testing with hybrid-capture method
-
HPV testing followed by visual inspection for positive tests
Each test was modeled according to a minimum and a higher number of visits, from one to three visits, varying according to test characteristics. Age at screening and number of lifetime screenings (at 5-year intervals) were also investigated. The base case was a single, lifetime screening at age 35. Key variables used in the model are in Table 6-8.
In the model, screening and most treatment took place at a primary care-level facility. More extensive cytologic abnormalities, cancers, or women with anatomic abnormalities of the cervix were referred to secondary-level facilities for further diagnosis and treatment. Cryosurgery was used to treat women with abnormal findings at the primary facilities.
Costs included in the analysis are:
-
Direct medical costs (e.g., staff, disposable supplies, equipment, specimen transport)
-
Women’s time (time traveling and waiting for and receiving care)
-
Direct transportation costs
-
Program-related costs
Costs of false-positive results and the costs incurred by women in the model who were referred to higher level facilities were also included.
Results
Effectiveness All strategies reduced the lifetime risk of invasive cervical cancer. The effect of a single screening, at age 35, was greatest with HPV one- and two-visit screening (30 to 36 percent reduction in lifetime cervical cancer risk), followed by one- and two-visit visual inspection (25 to 31 percent reduction), and finally two- and three-visit Pap smear and cytology (18 to 22 percent reduction). Two screenings, at ages 35 and 40, brought all strategies up to about 40 percent reduction in lifetime cervical cancer risk, and an additional screening at age 45 reduced the risk a further 15 percent.
The number of visits was a critical factor: the more visits needed, the poorer the follow-through with all strategies. Single-visit HPV (followed closely by visual inspection) was most effective. The least effective strate-
TABLE 6-8 Selected Variables of the Models Used in the Comparative Analysis for the Five Countries
|
Variablea |
Base Case (%) |
Range (%) |
|
Characteristics of Screening Tests |
|
|
|
VIA |
|
|
|
Sensitivityb |
76 |
60–90 |
|
Specificity |
81 |
66–96 |
|
HPV DNA |
|
|
|
Sensitivityb |
88 |
65–95 |
|
Specificity |
93 |
70–96 |
|
Cytology (Pap smear) |
|
|
|
Sensitivityb |
63 |
45–85 |
|
Specificity |
94 |
80–98 |
|
Characteristics of Screening Program |
|
|
|
Participationc |
100 |
25–100 |
|
Loss to follow-up (per visit)d |
15 |
0–50 |
|
Criterion for Ineligibility for Cryosurgery According to Disease Statuse |
|
|
|
DNA is normal or positive for HPV, without CIN |
5 |
0–50 |
|
CIN, grade 1 |
15 |
0–50 |
|
CIN, grade 2 or 3 |
25 |
0–50 |
|
Cryosurgeryf |
|
|
|
Effectiveness in women with CIN, grade 1 |
85 |
50–90 |
|
Effectiveness in women with CIN, grade 2 or 3 |
75 |
50–90 |
|
Major complications |
1 |
0–3 |
|
Minor complications |
5 |
0–15 |
|
aThe variables shown represent only those values used in the comparative analysis for all five countries; see original paper, for additional variables. Each variable used in the base case analysis was varied over the range of values shown for the sensitivity analysis. bSensitivity is defined as the probability of a positive test given the presence of CIN 2 or higher. cIn order to compare results with those of other published analyses, the authors assumed that screening participation was 100 percent in the base case. Sensitivity analyses varied coverage according to the assumption that the population has either a homogeneous or a heterogeneous risk of cervical cancer (i.e., women who are not screened are at higher risk than women who receive screening). dThe authors assumed that loss to follow-up occurred for each clinical contact. For example, for a three-visit strategy, there would be an overall loss to follow-up of approximately 45 percent. eThe values are the proportions of women in each underlying disease category who would be ineligible for cryosurgery on the basis of visual inspection of the cervix and would be referred to a district or tertiary clinical care site for appropriate evaluation. Among those with grade CIN 1, one-third would undergo a loop electrosurgical excision procedure, one-third a cold-knife conization, and one-third a simple hysterectomy; among those with CIN 2 or 3, half would undergo cold-knife conization and half a simple hysterectomy. fTreatment for CIN 2 or 3 with cryosurgery resulted in a rate of immediate failure of 10 percent; a 10 percent recurrence of CIN after 6 months; and a 5 percent recurrence after 1 year. SOURCE: Reprinted, with permission, from Goldie et al. (2005). Copyright 2005 by the Massachusetts Medical Society. All rights reserved. |
||
gies were two- and three-visit Pap smear cytologic examinations, and the two-visit HPV testing and visual inspection sequence.
Costs The total costs (discounted) for a single lifetime screening varied by country. India had the lowest costs and South Africa, the highest. Among the strategies, single-visit visual inspection was the least expensive and three-visit Pap smear cytology the most expensive. For every strategy, most of the cost was attributable to cancer treatment, so additional screenings tended to raise costs only minimally.
Cost-effectiveness Single lifetime screening with either visual inspection or HPV testing was most cost-effective, depending on the country. One-visit visual inspection at age 35 cost $10 per year of life saved in India and $134 in Kenya. In South Africa, single-visit HPV testing with same-day treatment was most cost-effective, at $467 per year of life saved. In Peru and Thailand, one-visit visual inspection ($124 and $109 per year of life saved, respectively) and one-visit HPV testing ($152 and $170 per year of life saved, respectively) were most cost-effective. The least attractive strategies were three-visit Pap smear cytology or HPV testing, which cost more and gave poorer results than strategies that involved fewer visits.
Sensitivity analysis The model was tested for sensitivity to values for a number of variables. All strategies were sensitive to the costs of treating invasive cancers and to the age at screening. Screening women in their mid-30s was always optimal. When choosing among screening strategies, the results were most sensitive to the costs and characteristics (sensitivity, specificity, predictive value) of the tests. This is particularly relevant to HPV testing because the test materials and laboratory facilities constitute a larger proportion of the total costs than for visual inspection or Pap smear with cytology. If the price of HPV testing—the approach with the best performance—comes down, it would become an even more attractive option.
Conclusions from the Modeling
According to this model, a single lifetime screening of women in their mid-30s with either visual inspection or HPV testing in one or two visits should reduce the lifetime risk of developing cervical cancer by 25 to 36 percent. A second screen after 5 years would increase the benefit to about a 40 percent reduction in lifetime cervical cancer risk.
Both costs and cost-effectiveness vary several-fold across the five countries, but in all cases, the best strategies (including screening two or three times in a lifetime, depending on country) had cost-effectiveness ratios in a range generally accepted as “very cost-effective” (WHO, 2001) in the con-
text of per-capita gross domestic product. These approaches also compare favorably, in terms of cost-effectiveness, with widely adopted interventions, including hepatitis B vaccination in India, second-line treatment for chronic tuberculosis in Peru, and the use of insecticide-treated bed netting to prevent malaria in Kenya.
HPV VACCINES
GlaxoSmithKline (GSK) and Merck & Co., Inc. have each developed vaccines to prevent infection with the most prevalent types of HPV associated with cervical cancer—types 16 and 18 (associated with 70 percent of cervical cancers). The Merck vaccine is also designed to protect against types 6 and 11, the most common agents of genital warts (responsible for 90 percent of genital warts). Merck’s vaccine, GARDASIL®, was approved by the FDA in June 2006, based on clinical trials that included about 21,000 women and 4,000 men around the world. GSK, whose vaccine has been tested in 30,000 women in Phase III trials, is seeking approval first by the European Union, also anticipated before the end of 2006.
Both the Merck and GSK vaccines have shown complete efficacy in preventing persistent infection by HPV types 16 and 18 (Harper et al., 2004; Koutsky et al., 2002; Villa et al., 2005). Currently, there are no predictions regarding prevention of cancer among women who have already experienced an infection to determine whether the vaccines might have some effectiveness (WHO, 2005).
Vaccine Targeting and Scheduling
Young women aged 15–25 have been the target audience of most vaccine studies, though immunogenicity and safety studies are also being conducted in groups down to 9 years of age to demonstrate tolerability in younger girls and boys (WHO, 2005). Large trials are also planned by both Merck and GSK to determine efficacy against CIN in women aged 24 to 45 years.
Merck has also begun trials among HIV-infected people in the United States, as well as in young men aged 16 to 23 years to evaluate immunogenicity and efficacy against genital warts, HPV infection, and anal precancer (WHO, 2005). Vaccinating males, who would benefit from the protection against genital warts, would also protect women from transmission of the cervical cancer-causing types of HPV.
HPV vaccination is likely to be most effective if given prior to the onset of sexual activity, before possible exposure to HPV. Delivery of the vaccine would be simplest if it could be given in conjunction with other childhood vaccines. Determining the potential to integrate HPV vaccine into routine
infant or childhood immunization schedules is a long-term objective stated by WHO. However, safety data supporting integration will take many years to generate (WHO, 2005). There are theoretical reasons to expect that the response to vaccination would be better in infants than in adults, though response data for adults cannot be extrapolated to infants. Inclusion of one or two doses of HPV vaccine in infant immunization schedules might mean that only one dose (rather than three anticipated) is needed in the preteen or early teen years, which would be simpler to deliver programmatically (WHO, 2005).
Information about immunization schedules is still forthcoming. The companies have not made known their intentions regarding spacing of doses and the need for booster doses. Safety and immunogenicity in pregnant women also has not been established (WHO, 2005). Some of this information will emerge over time.
Vaccine Financing and Delivery
No reliable estimates of cost of HPV immunization have been made public yet. Both Merck and GSK have confirmed that they will offer tiered pricing, with lower prices for developing countries. A $12.9 million grant from the Bill and Melinda Gates Foundation was recently awarded to WHO, IARC, the Program on Appropriate Technology for Health (PATH), and Harvard University toward a collaborative effort to bring HPV vaccines to developing countries. WHO’s Initiative for Vaccine Research is investigating the potential of HPV vaccine delivery to girls under the age of 10, and conducting demonstration projects in low- and middle-income countries on the feasibility of vaccinating adolescent girls. PATH will likely oversee the demonstration projects and secure funding, currently planned for four LMCs. PATH has also begun early planning to enter into vaccine price negotiations with Merck and GlaxoSmithKline on behalf of the world’s poorest countries.
Most LMCs are likely to need external financial support to purchase the vaccine and possibly to develop delivery programs (particularly if the vaccine cannot be incorporated into the existing infant and childhood schedules). Mechanisms that could be used for this purpose exist, such as the Global Alliance for Vaccines and Immunization, the new International Finance Facility for Immunization (launched in 2005 and supported by the United Kingdom, France, and Sweden), or the Vaccines for the New Millennium Act of 2005, launched by the U.S. Congress. These or other avenues should be found to help LMCs to implement HPV vaccination where cervical cancer is of major concern.
SAVING CHILDREN WITH CANCER
The ability to cure most children with cancer stands as a great medical achievement of the last half of the 20th century. Within oncology, it is even more remarkable, given the far smaller gains against most cancers of adults. Another contrast with cancers in adults is that the gains are almost exclusively attributable to treatment alone, because no strategies for preventing childhood cancers have yet been identified (with the exception of rare childhood cancers of the liver, prevented by hepatitis B vaccination). For some relatively common cancers of childhood (e.g., certain brain tumors), treatment results remain poor, and for others, adverse effects occurring decades after treatment prod the research enterprise to find treatments that cure but do less harm (Hewitt et al., 2003). Nonetheless, in the United States and other resource-rich countries, 75–80 percent of children with cancer survive for 5 years and most live out full lifetimes. But nearly 80 percent of the world’s children who develop cancer are in resource-poor countries. Those without access to treatment, which is most in poor countries, do not survive.
In high-income countries, cancer is the leading cause of disease-related death (i.e., excluding accidents) in children, even given the high cure rates. In low-resource countries, the rank order varies, with cancer becoming more important as the level of economic development rises. In some middle-income countries, cancer is the leading cause of death from disease among children and young adults (e.g., in Mexico, where cancer is second to accidents as a cause of death among young people age 5–14), although in others, infectious diseases may still predominate (Figure 6-1).
Why Should Pediatric Cancer Be Given Priority in LMCs?
The numbers of childhood cancers and deaths (below age 15) are modest in the poorest countries, and low compared with the corresponding figures for cancer in adults. In 2002, 160,000 children worldwide developed cancer, 134,000 of them in less developed countries (IARC, 2004). Including 15- to 19-year-olds increases the numbers by 25–33 percent, and adding the 20- to 24-year age group adds a similar percentage. The proportion of children with cancer who die is much higher in less developed countries (Figure 6-2), but still amounts to fewer than 100,000 per year.
Nonetheless, the number of childhood cancers, and in particular, the proportion of childhood deaths due to cancer, is rising in low-income countries as the number of children increases and other causes of childhood death diminish. Data from China illustrate this point: Between 1960 and 2002, the death rate among children under 5 from all causes had fallen from 225 to 39 per 1,000 live births, with cancer assuming greater importance as deaths from infectious diseases declined. With more than 300 million children un-
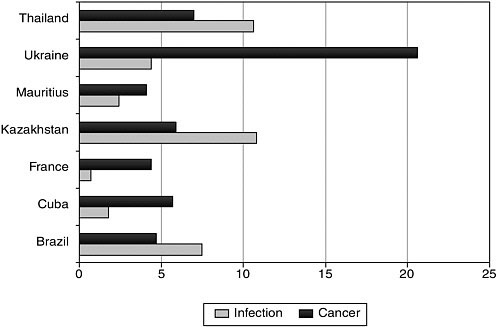
FIGURE 6-1 Number of registered deaths per 100,000 among ages 1–24 years, selected countries, in 2000.
SOURCE: Personal communication, I. Magrath, INCTR, May 2006.
Data available at http://www3.who.int/whosis/mort/table1.
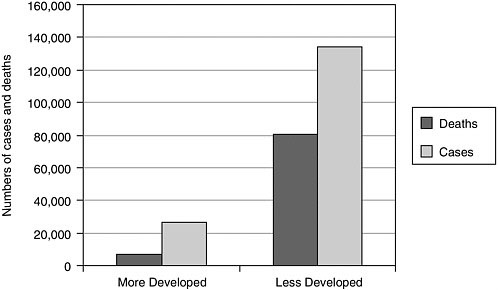
FIGURE 6-2 Annual numbers of cases and deaths from cancer, ages 0–14.
SOURCE: IARC (2004).
der 15 now in China, on the order of 45,000 new cases of childhood cancer can be expected annually (Ribeiro and Pui, 2005).
The relatively small numbers of childhood cancers in low-income countries could argue against giving them priority, or alternatively, the argument can be made that for a relatively modest investment, most of these children could be saved. There are additional benefits of a successful, visible cancer treatment program for children above and beyond curing individual children. In countries where cancer is often seen as a death sentence—because most adults and children do, in fact, die of their cancers, even if they eventually seek treatment at an advanced stage—demonstrating to the public and to health professionals that healthy survival is possible would boost the chances of adopting further achievable cancer treatment goals.
Children have long been the focus of global health efforts, but the major international organizations dealing with child health—mainly WHO and UNICEF—have not yet focused on cancer. Other parts of this report argue for effective interventions to save middle-aged and older people, and to provide comfort through palliative care for everyone, children included. But children are also a compelling focus for the reasons given: assured success for a large number if given appropriate treatment, which can be relatively uncomplicated and relatively inexpensive; living cancer survivors; and some development of cancer treatment infrastructure that forms a core for expanded services.
Differences Between Childhood and Adult Cancers
The cancers that arise in children are, by and large, different from cancers of adults, everywhere in the world. In general, they are different entities clinically and biologically, and each has its own age-specific pattern of incidence. Tumors of infants are extremely rare in adults, particularly above the age of 30 years, and vice versa, but in adolescents and young adults, there is a more mixed picture.
About 90 percent of adult cancers are carcinomas that arise from epithelial tissue. These include the familiar cancers of the prostate, breast, lung, colon and rectum, uterus, and ovary. In children, leukemias and lymphomas alone comprise some 40–50 percent of cancers. The rest are mainly cancers arising in the central nervous system; embryonal cells of the eye, kidney, and adrenal glands; and sarcomas of bone and soft tissues. These differences in tissue origin affect cancer development and the response to treatment.
Even cancers with the same name in children and adults can be significantly different in their biology and in the response to treatment. The 5-year survival rate for children with acute lymphoblastic leukemia (ALL), the most common childhood malignancy in much of the world, is about 80 percent. Among adults under age 65 with the same diagnosis, only 20 to 30 percent
survive for 5 years (Ries et al., 2002). Better outcomes in children are likely due to differences in the underlying molecular abnormalities responsible for the cancer’s biological characteristics, and possibly in the physiology and immune system of the patient. For example, a factor associated with poor prognosis is the “Philadelphia chromosome,” a cytogenetic defect found in 30 to 40 percent of adults with ALL but less than 5 percent of children with that diagnosis (Look and Kirsch, 2002).
Patterns of Childhood Cancer in Less and More Developed Countries
While leukemias and lymphomas comprise about 40–50 percent of all new cases of cancer in children worldwide, the frequencies of other cancers—and of the specific types of leukemias and lymphomas—have distinct geographic distributions. The pattern is particularly distinct in sub-Saharan Africa, where the incidence of Burkitt’s lymphoma and Kaposi’s sarcoma (the latter related to HIV infection) are much higher than anywhere else in the world (Parkin et al., 2003). Retinoblastoma is also much more common in developing countries generally, and brain tumors less common than in high-income countries. The incidence of leukemias in sub-Saharan Africa is also remarkably low, in the few places in which it can be accurately measured, and the pattern of immunological subtypes differs from that observed in countries of higher socioeconomic status. The differences in the patterns of cancer are almost certainly due to environmental and lifestyle differences associated with lower socioeconomic status. Infectious causes that are more common in low-income countries (e.g., Epstein-Barr virus and malaria in Burkitt’s lymphoma; HIV in Kaposi’s sarcoma) may be of particular importance. These descriptions of the broad patterns of cancer in children throughout the world are relatively clear despite scanty data (Parkin et al., 2003).
Children with cancer in developing countries are much more likely than their counterparts in the United States and other wealthy countries to have late-stage cancer when diagnosed and to have additional health problems. A report from the Shaukat Khanum Memorial Cancer Hospital in Pakistan illustrates this. Of the children in the mid-1990s with ALL, 71 percent met the WHO criteria for malnutrition. One-quarter were positive for hepatitis B surface antigen or hepatitis C, and 40 percent presented with high-risk ALL (hyperleukocytosis and massive hepato-splenomegaly). Even with treatment, 40 percent of the children did not survive 5 years, most because of infection, hepatitis, and intracranial hemorrhage, a pattern similar to that reported from a number of developing countries (Usmani, 2001).
Barriers to Childhood Cancer Treatment in Low-Income Countries
Many of the factors that limit children’s access to cancer care are similar to those in adults, but some are unique to children, or even to specific cancers. The major factors are discussed below.
Socioeconomic Factors
Socioeconomic factors often override all others. Particularly in rural areas, children and their parents must travel long distances to reach a facility able to treat childhood cancers, which are fewer even than those where an adult might receive treatment. Even proximity to a center, located mainly in urban areas, does not equal access. The expense of what would be considered a very modestly priced cancer treatment in a high-resource setting is out of reach for poor families, where health insurance is nonexistent and traditional treatment is less expensive and more available. As is true for adult cancers, no traditional treatments are known to be effective against childhood cancers. In addition to the purely economic and practical constraints, social and psychological support for children and families going through the arduous and prolonged process of cancer treatment is limited. Illiteracy and a general lack of understanding of the complex process further alienate people from seeking treatment in the first place, and from completing treatment and follow-up. A finding from studying retinoblastoma may be generalizable: the less educated the father, the more likely the child is to have advanced disease and delay in diagnosis (International Network for Cancer Treatment and Research, 2004).
Social and Cultural Factors
Other social and cultural factors may weigh against a child receiving treatment for a curable cancer. Cancer is stigmatized in many cultures, and patients may be rejected, even after cure. Where cure alters the body in visible ways—such as loss of an eye to retinoblastoma—children may pay an even higher price. A girl who has lost an eye may not be marriageable. Culture may also dictate consulting a traditional healer, delaying formal medical care until a cancer is far advanced. The family may be dissuaded from seeking treatment at all by some healers.
To put this in human terms, a mother may have to choose between staying with her child with cancer in a distant hospital or being with her family who may, without her, have very limited ability to fend for themselves. She may choose to provide food for her healthy children, or the family choice may be stark—money for anticancer treatment, or food and schooling for the other children.
Lack of Cancer Treatment Infrastructure
The lack of infrastructure for cancer management is pervasive in LMCs. Many children probably die from cancer without it ever being recognized and without receiving even palliative care. This is especially likely for leukemias, the early symptoms of which are fever, swollen lymph glands, and anemia, which may easily be mistaken for infectious conditions. Indeed, children in early stages of leukemia are particularly susceptible to infections that lead to death without the underlying leukemia being recognized (Usmani, 2001). Both leukemias and lymphomas in young children can progress rapidly to death in the absence of appropriate medical attention (Parkin et al., 2003).
Lack of Medical Professionals with Pediatric Cancer Training
The differences between childhood cancers and adult cancers mean that treating them appropriately requires specialized knowledge as well as drugs and other supplies and equipment. There are few trained pediatric oncologists in low-income countries, and that situation is not likely to improve significantly given the numbers of children affected and the reality of medical care in poor economies. Compounding the problem is a lack of knowledge about the signs and symptoms of childhood cancer among nonspecialists, including pediatricians, family practitioners, and others on the medical front lines. Even once in the system, other skills may be lacking. Pathologists often have not had the training needed for pediatric cancers; there may be no pediatric or specialist surgeons experienced in the diagnosis or treatment of eye and bone cancers, or even specialized pediatric surgeons, or radiotherapists with pediatric cancer training or facilities suitable for the treatment of children.
Importance of Locally Relevant Research
Treatments for childhood cancers have been optimized for the patients and infrastructure of high-income countries. The basics—key drugs and procedures—should be applicable in most settings, but the details, if treatment is to be optimized to the setting, are almost sure to differ. One feature of childhood cancer treatment in the United States and other high-income countries is worth emulating: the highly collaborative and cooperative character of the pediatric oncology community. Since the 1960s, when treatments began to make a real difference in the survival of children with cancer, the majority of pediatric oncologists and their patients have participated in clinical trials, and patients have benefited. The Children’s Oncology Group (organized through the U.S. National Cancer Institute) is, in this sense,
the envy of those who conduct adult cancer clinical trials (Adamson et al., 2005).
Clinical trials are needed in LMCs not only to adapt the treatments developed in high-income countries, but also to address clinical questions of local relevance. Children in high-income countries rarely present with advanced retinoblastoma or Kaposi’s sarcoma, yet these are common in sub-Saharan Africa, in particular. Adapting treatment is also important. Treatment protocols from high-income countries are designed for an advanced infrastructure that does not exist in resource-poor countries. Finally, patients in LMCs may differ: in disease biology, in the way drugs are metabolized (e.g., because of nutritional differences), and because of different co-morbidities. Through clinical trials in a variety of low-resource settings, it can be determined which of the differences are important clinically, and how treatment can be modified accordingly (Personal communication, I. Magrath, International Network for Cancer Treatment and Research, November 2005).
The benefits of locally relevant clinical trials go beyond an answer to a research question. Children treated in clinical trials are likely to get treatment more in line with some current recommendations, and staff receive training and education through participation in clinical trials. Pragmatically, limited resources may be more accessible as funds are more likely to be made available for research than for routine treatment.
Programs and Alliances for Childhood Cancers Relevant to LMCs
Two global initiatives for childhood cancer (described below) have recently begun. In addition, the International Network for Cancer Treatment and Research (INCTR) has established networks of various types around several childhood cancers: acute lymphocytic leukemia, retinoblastoma (Box 6-2), and Burkitt’s lymphoma. INCTR also has helped to establish “cooperative groups” that have agreed to work together toward specific goals. These include the Leukemia Study Group of India, the Middle East Children’s Cancer Group, and the Retinoblastoma Group of Mexico.
Global Alliance for the Cure of Children with Cancer
A “Global Alliance for the Cure of Children with Cancer” was formed in 2001 at a meeting organized by INCTR. The meeting included the European Organization for Research and Treatment of Cancer, the International Agency for Research on Cancer, the International Society of Pediatric Oncology (SIOP), the National Cancer Institute, the Monza International School of Pediatric Oncology (MISPHO), the International Consortium for the Cure of Childhood Cancer in China, and the Oncology Center,
|
BOX 6-2 International Network for Cancer Treatment and Research: Public and Professional Awareness Programs for Retinoblastoma: Recent Examples As part of a retinoblastoma awareness campaign in Brazil, a public service announcement was broadcast on television throughout the country and has been made available in multiple languages. The public responded with telephone calls, letters, and photographs of children, asking for guidance. In addition to the television campaign, 1 million telephone cards with information about retinoblastoma—including pictures—were distributed in Brazil, where such cards are used by a high fraction of the population. The results of the two campaigns are under evaluation. The Mexican Retinoblastoma Group has formed, with ophthalmologists and pediatric oncologists working together to study individual cases, better understand the pattern of the disease in Mexico, develop uniform treatment approaches, and develop and standardize methods for better early detection of retinoblastoma. To increase public awareness, a poster about retinoblastoma has been distributed widely and a public service announcement has aired on a popular TV channel. SOURCE: International Network for Cancer Treatment and Research (2004). |
Antwerp, with other organizations expressing interest in joining. A project on Burkitt’s lymphoma in Africa was the first undertaking, but the alliance otherwise has not been activated. As of late 2006, plans to revitalize the effort are under way.
“My Child Matters”
“My Child Matters” was launched in 2005 by the International Union Against Cancer (known as the UICC), in collaboration with drug manufacturer Sanofi-Aventis, as part of UICC’s World Cancer Campaign to improve the treatment of children with cancer in resource-poor countries. The three major activities of the campaign are: (1) funding projects in 10 countries in the first year, based on competitively selected proposals; (2) a worldwide mobilization and awareness campaign to highlight the effects of childhood cancers; and (3) a comprehensive report on childhood cancers, which was released on World Cancer Day, February 4, 2006 (International Union Against Cancer, 2006).
Proposals for the first projects were solicited from Bangladesh, Egypt, Honduras, Morocco, the Philippines, Senegal, Tanzania, Ukraine, Venezuela, and Vietnam, as pilot countries. The list for the second year will be expanded. Project goals were:
-
Raising awareness and disseminating timely information about childhood cancers to health professionals, parents, children’s organizations, and the general public;
-
Improving early diagnosis and access to care and treatment for children living with cancer; and
-
Strengthening the social welfare aspects of caring and support for children living with cancer and their families.
Fourteen projects, which began in early 2006, were selected from more than 80 applications, funded at a total of $700,000 (with additional support from the U.S. National Cancer Institute).
Improving Childhood Cancer Treatment in LMCs
Children with cancer in LMCs can be treated and cured—and appropriate psychosocial and family support provided—under conditions that are feasible even in resource-poor settings. The pediatric cancer unit (PCU) model that has worked well in high-income countries is also considered the best approach in LMCs (see Box 6-3, which lists SIOP recommendations for the organization of a PCU). An example is the success of dedicated PCU within a pediatric hospital established in Recife, Brazil beginning in the mid-1990s. The 5-year event-free survival rate for children with ALL rose from 32 percent before the PCU to 63 percent in the most recent period (Howard et al., 2004).
The initial challenges in improving pediatric cancer care are gaining public recognition of childhood cancer as an important and addressable health problem, and sustainable funding to support treatment. The “twinning” approach, which has been successful in a number of LMCs both in establishing good treatment and financing mechanisms, is described in the next part of this chapter.
“Twinning”: An Approach to Developing PCUs in Resource-Poor Settings
The “twinning” approach—partnerships between institutions in high-resource and low-resource settings—has been particularly effective in pediatric cancer treatment. Twinning programs usually involve the development or upgrading of dedicated units within cancer centers, pediatric hospitals, or other major health care institutions for the purpose of expanding access
|
BOX 6-3 International Society for Pediatric Oncology (SIOP) Working Committees on Standards of Care and Training, and Psychosocial Issues in Pediatric Oncology Recommendations for the Organization of a Pediatric Cancer Unit (PCU)
|
to care for children with cancer and improving cure rates. A long-term commitment of both partners is essential and must include all aspects of a successful program: trained personnel; basic drugs, equipment, and supplies; and relationships with parents’ groups, the extended medical community, and the community at large.
Twinning programs have been in place around the world, a few for as long as 10 or 15 years. Their success is measured in the number of children treated, the proportion who complete treatment—abandonment of treatment is a problem in most low-income countries, where families cannot afford the medical and nonmedical costs of having a child in treatment—and finally, the number successfully treated and going on to live full lives. All these factors have been demonstrated in successful programs (Ribeiro and Pui, 2005). Two major twinning programs are described below.
A PCU comprises the following facilities: A ward for in-patients sufficiently staffed and equipped (including proper isolation facilities) to:
A day-clinic for short-term investigations, short-term infusions, or short-term surveillance of patients; An outpatient clinic closely linked to imaging and laboratory facilities, providing rapid service for ambulatory treatment and control; and An administration for organizing and coordinating the long-term follow-up and evaluation of former cancer patients. |
La Mascota Hospital (Managua, Nicaragua) and Monza PCU (Milan, Italy)
In the mid-1980s, most children with cancer in Nicaragua died from lack of appropriate treatment, even in the country’s one pediatric hospital, Hospital Infantil Manuel de Jesus Rivera “La Mascota.” At that time, La Mascota was, in the words of a new director, “inundated beyond capacity with health issues at the primary and secondary levels.” Children with cancer were largely abandoned. The Director resolved to establish a PCU and sought technical assistance to do so (Masera et al., 2004).
A physician at the Mario Negri cancer research organization in Milan responded, and eventually brought The Hemato-Oncology Center of Milan-Bicocca University at Saint Gerardo Hospital, Monza, Italy (Monza
PCU) into a collaboration with La Mascota. The stated objective from the beginning was to begin “a comprehensive, long term program to establish a national PCU capable of offering a reasonable possibility of cure to Nicaraguan children with cancer.”
Staff development La Mascota initially had no staff with training in pediatric oncology. From the beginning, pediatricians, and later nurses and other specialists (urologists, nephrologists, pulmonologists, surgeons, laboratory scientists), were sent to Monza and other Italian centers for training. The relationships have been maintained by support to attend international meetings and periodic short training stints in Italy. The reality of low health care salaries led to physicians’ (and later nurses’) salaries being supplemented to reduce the need for them to practice outside the hospital.
Sources of funding La Mascota now has some support from the Nicaraguan government and other governments (Italy, Japan, Luxembourg), but the program was begun without such support. The initial budget came from two small philanthropic groups in Monza. A “National League Against Leukemia and Cancer in Children” was established jointly by the Directors of the Managua and Monza centers, and the Oncology Department at S. Giovanni Hospital in Bellinzona, Switzerland, which has also played a continuing role in the twinning program. Most of the financing comes from private institutions and private citizens. Between 1986 and 2001, the annual investment from the Italian and Swiss partners totaled $150,000–200,000. The share of local funding in Nicaragua has grown over the years.
Results The number of children treated annually at La Mascota has increased from about 50 in 1990 to more than 150 in 2001, estimated at 50 to 60 percent of all children with cancer in Nicaragua. Many more of them are completing treatment than previously. Early on, 30 to 40 percent of children who were diagnosed with cancer at the center either were not treated or abandoned treatment before it was completed, largely because the family could not bear the economic hardship of the nonmedical costs involved. A program begun in 1995 paired newly diagnosed children and their families with a family in Italy or Switzerland, which provides financial support for these nonmedical costs, including travel and other expenses. More than 750 children and their families have been part of this program, which has brought down the rate of treatment refusal or abandonment to less than 10 percent. The parents’ associations of the institutions have played an indispensable role in this and other aspects of the program.
Long-term survival for acute lymphocytic leukemia patients (the most frequent diagnosis) rose from 20 percent before the program to 40 to 50 percent in the late 1990s. Overall, the 3-year survival rate was 56 percent
for children treated at La Mascota through the late 1990s, which compares favorably with countries in the region at higher economic levels, and is better than in similarly resourced countries.
Results for Wilms’ tumor and Hodgkin’s disease are more impressive, and represent treatment advances developed in clinical trials at La Mascota, demonstrating excellent results without radiotherapy.
Key elements for success The La Mascota–Monza twinning program has succeeded for a number of reasons, beginning with the long-term commitment of cooperation on both sides, based on mutual respect of “autonomy, culture, and local traditions” and “assuring an active but noninvasive role of supervision and scientific advice” from the high-income institution (Masera et al., 2004).
The collaboration involves all aspects of treatment, as described. A therapeutic alliance has developed among the physicians, nurses, psychosocial workers, and parents’ associations. Successful continued financing has been possible because of the diversity of organizations and individuals providing support, without total reliance on a single entity.
Outgrowths of La Mascota The success of La Mascota encouraged Monza to create a structured program to foster additional twinning relationships between Latin American countries and other centers in Italy. At least four other collaborations were spawned in the first few years of effort, involving Bolivia, Cuba, Paraguay, and the Dominican Republic in Latin America, and Bergamo, Parma, Padova, Modena, and Bologna, in addition to Monza, in Italy. Health professionals from these as well as a number of other countries have been trained at Monza.
St. Jude Children’s Research Hospital International Outreach Program
St. Jude Children’s Research Hospital (“St. Jude”), opened in 1962, is well known in the United States as the leading institution in advancing the treatment of children with cancer and other catastrophic diseases. It serves mainly children from the United States, but children from all over the world are admitted, regardless of ability to pay (St. Jude Children’s Research Hospital, 2006). St. Jude’s research and treatment are supported by the third largest health care charity in the United States. The International Outreach Program (IOP) was formally established in 1991 after years of growing interest in children from poor countries (St. Jude Children’s Research Hospital, 2003).
The IOP develops twinning partnerships with (mainly) public hospitals in middle-income countries and with other local agencies and organizations. The purpose of the partnerships is to build regional capacity in diagnosis
and treatment of pediatric cancer. The aim is to create a critical mass of professionals who can support existing and developing programs. At the same time, relationships are developed with local fundraising organizations to support the programs. Current program and fundraising affiliates, predominantly in Latin America, are listed in Table 6-9 (St. Jude Children’s Re-
TABLE 6-9 St. Jude International Outreach Program Partners
|
Country and City |
Institutions |
Fundraising Foundation |
|
Brazil: Recife |
Centro de Hematologia e Oncologia Pedatrica, Instituto Materno Infantil de Pernambuco |
Nucleo de Apoio as Crianças com Cancer |
|
Chile: Santiago |
Hospital Luis Calvo Mackenna, Universidad de Chile |
|
|
China: Shanghai |
Shanghai Children’s Medical Center, Shanghai 2nd Medical University |
Partner in HOPE Foundation, Limited, Hong Kong, China |
|
China: Beijing |
Beijing Children’s Hospital |
|
|
Costa Rica: San Jose |
Hospital Nacional de Niños, Centro De Ciencias Médicas de la C.C.S.S. |
Asociación Lucha Contra el Cáncer Infantil |
|
Ecuador: Quito |
Centro Médico Meditropoli |
Foundation SOLCA: La Sociedad de Lucha Control el Cancer |
|
El Salvador: San Salvador |
Hospital de Niños Benjamín Bloom |
Fundacion Ayudame a Vivir |
|
Guatemala: Guatemala City |
Unidad Nacional de Oncologia Pediatrica, Hospital Roosevelt |
Fundacion Ayudame a Vivir |
|
Honduras: Tegucigalpa |
Hospital Escuela Bloque Materno Infantil, Hospital Viera |
Fundacion Hondureña Para El Niño con Cancer |
|
Jordan: Amman |
King Hussein Cancer Centre |
King Hussein Cancer Foundation |
|
Lebanon: Beirut |
The Children’s Cancer Center of Lebanon, Faculty of Medicine and Medical Center, American University of Beirut |
The Children’s Cancer Center of Lebanon |
|
Mexico: Sinaloa |
Hospital Pediatrico de Sinaloa, Culiacan |
Amigos de Niños Afectados de Cancer A.C. |
|
Mexico: Guadalajara |
Hospital Civil de Guadalajara |
|
|
Morocco: Rabat |
Hospital d’Enfants Rabat-Maroc |
de L’Avenir, Association des Parents et Amis de Enfants |
|
Morocco: Casablanca |
Hospital 20 Aout 1953 |
Agir, Association Marocaine de Soutien |
|
Russia: Moscow |
Research Institute of Pediatric Hematology |
|
|
Venezuela: Caracas |
Hospital de Ninos J.M. de Los Rios |
Asociacion Venezolana de Padres de Niños con Cancer |
|
Venezuela: Maracaibo |
Hospital de Especialidades Pediatricas |
Fundacion Amigos del Niño con Cáncer |
|
SOURCE: St. Jude Children’s Research Hospital (2006). |
||
search Hospital, 2003). IOP partners are treating about 3,200 new patients per year (with about 10,000 under treatment) (St. Jude Children’s Research Hospital, 2005), with good results. In El Salvador, the 5-year survival for ALL rose from 10 percent to 65 percent between 1993 and 2001, and in Brazil, from 29 percent to 60 percent between 1997 and 2001.
Two new IOP initiatives, relying on advanced telecommunications technologies, were begun in 1999: Cure4Kids and the International Training Center for Hematology-Oncology Nurses in Central America, in collaboration with the professional nursing society in El Salvador, to train nurses in the pediatric subspecialty of hematology/oncology.
Cure4Kids
Cure4Kids is an Internet-based distance-learning program provided free to physicians, nurses, scientists, and health care workers who treat children with catastrophic illnesses. The Internet tools include:
-
Online education about catastrophic childhood illnesses
-
Collaborative work spaces for document sharing and online meetings
-
Access to consultation and mentoring by St. Jude faculty
-
Technology and training for better management of patient information
The education component includes a digital library of reference material (full-text access to medical journals and papers), a discussion area for physician exchange of advice and information, and access to online seminars and lectures. The technology is also being used for live meetings and lectures through the Internet. Discussion of research protocols and specific cases being treated also take place. Partners from Brazil, Lebanon, Morocco, Guatemala, El Salvador, Honduras, and Mexico regularly participate in these virtual meetings.
More than 8,400 professionals in 155 countries (as of November 2006) are currently registered Cure4Kids users (St. Jude Children’s Research Hospital, 2006). Its 200 online seminars about catastrophic childhood illnesses are available in seven languages.
Regional Initiatives in Latin America
With the network of partners having grown in a number of countries, the IOP has developed more regional programs, including workshops for medical professionals and fundraisers. A Central American Pediatric Oncology Infrastructure Program has also been proposed through the Association
of Central American Pediatric Hematologists-Oncologists. The program would involve collaboration with other international partners already working in the region, including MISPHO and others.
The Role of Local Foundations
Local nonprofit foundations play two vital roles for IOP partners: they raise funds to support the work of the partners and they run public awareness campaigns about childhood cancers. Governments do not typically provide the resources needed for childhood cancer care, so funds must be supplemented. The IOP, in collaboration with the fundraising arm of St. Jude, sponsors training for members of the local foundations to help ensure their success. The foundations typically develop and support:
-
Care, treatment, and psychosocial support of patients
-
Salaries and training for key personnel
-
Construction and renovation of facilities
-
Efforts to increase government support for childhood cancer treatment
-
Activities to raise public awareness that childhood cancers are curable at early stages
The 19 foundations affiliated with IOP partners have raised a total of $12.5 million over the past several years through donations and by soliciting funds from grant-making organizations. These funds have been used to provide housing for patients and families at or near the hospitals, to support salaries of key personnel, and to pay for medications. Paying for medications has proven to be a continuing challenge in most centers. Six foundations reported that some children went without some scheduled treatment because of a lack of funding for medications.
Recent Progress
St. Jude compiles reports from each IOP partner every year to assess progress. The 2005 report shows progress in a number of major areas for which goals had been set, including the following:
-
Nurse training had been provided to all partner clinical programs over the previous 3 years
-
18 of 19 programs report a functioning infection control program
-
All partners have immunophenotyping tests (tests that help determine the origin of leukemic cells) routinely available, partially paid for by St. Jude IOP
-
All programs report treatment teams that include four or more subspecialties (pediatric oncologists, oncology nurses, social workers, psychologists, surgeons, dietitians or nutritionists, pharmacists, intensivists)
-
Nearly all report that the hospital has an ethics committee or review board
-
79 percent of the programs instituted measures to reduce abandonment of treatment (guest houses, food supplements, subsidized transportation, parent support groups, satellite clinics, home visits by social workers)
-
Most sites report active ALL protocols and more than half also have active protocols for a number of other cancers
-
1,543 patients were treated on protocols from May 2004 to May 2005
-
Solid tumor diagnosis has been improved in most programs through tumor boards or other special programs
The IOP has also encouraged other institutions around the world to collaborate with the IOP-associated programs.
New Initiatives
Major new initiatives of the IOP include the Central American Retinoblastoma Program to improve survival rates, the Joint ALL Protocol for Beijing and Shanghai, and the Pediatric Oncology Networked Database (POND, a shared electronic database for programs in 10 countries).
Improving the Quality of Pediatric Cancer Treatment Through Clinical Trials and Centers of Excellence
By the early 1980s, major advances had already raised long-term survival from ALL in the United States and Europe to 70 percent. In LMCs, this was not the case. By way of example, at the Cancer Institute, Madras (now Chennai), India, fewer than 20 percent of the children and adolescents with ALL achieved long-term survival. As a means to improve this situation, a collaboration was established between WIA and the U.S. NCI, and later the NCI-funded INCTR (Shanta, 2000).
In the case of the NCI–India collaboration, a more intensive treatment protocol than what had been in use at the time was designed. Although this carried a risk of increased toxicity, in view of the poor results and the extensive disease in most patients, the added risks appeared worth taking. In addition, treatment elements believed to be difficult to administer or particularly costly in India (e.g., high-dose methotrexate) were avoided. An initial trial confirmed that the regimen was feasible and likely to result in much
better long-term survival, with high but manageable toxicity. The protocol was taken to two additional major hospitals in India in 1986 and 1992.
The process has worked with the use of a locally affordable protocol that has manageable toxicity. During the 1990s, 60 percent of children with ALL treated at the Tata Memorial Hospital (in Mumbai, formerly Bombay) and 41–43 percent of those treated at the All India Institute of Medical Sciences (New Delhi) and the Cancer Institute of Chennai were cured. In addition, deaths from drug toxicity have gradually been reduced as the medical staffs have learned to better manage toxicities. The treatments used do not include unavailable or expensive technologies and could be replicated throughout the country. Results from the three centers differ, and those differences are the basis of further study. The work suggests that not only are there significant differences between Indian populations and patients in the United States and Europe, but among centers in India as well.
The India ALL experience demonstrates that although the general principles learned from clinical research in developed countries provide a foundation for treatment strategies, the differences in the populations treated, both genetic and environmental, differences in leukemia cell biology, and differences in the quality of care received can be expected to bring about differences in results when using a standard regimen. It is clear that therapies developed through clinical trials in the relevant populations are essential to quality care.
Clinical trials in these settings result in immediate patient benefits. They also contribute to basic scientific knowledge, including gene expression profiling of leukemic cells from patients in these settings, which lead to a better understanding of the genetic and environmental factors relevant both to the pathogenesis of ALL and to the identification of prognostic factors. In the process, three centers of excellence for pediatric cancer have been developed in India that form a strong nucleus for expanding treatment to other centers.
Examples of centers of excellence improving the success of pediatric cancer treatment can be found elsewhere. In centers of excellence in Brazil, the 5-year survival rate for childhood ALL is higher than 60 percent, but much lower outside these centers. Another successful model is the national pediatric oncology program in Chile. The Chilean government requires that patients receive their diagnosis and initial treatment in a certified pediatric cancer unit, with follow-up care from more numerous satellite clinics.
SUMMARY AND RECOMMENDATIONS
Three compelling opportunities to reduce the cancer burden in LMCs have been presented. The first two opportunities, vaccination against HBV to prevent liver cancer and cervical cancer prevention by screening
adult women and vaccinating young girls, address two of the three major infection-related cancers. The first, which takes place through existing childhood vaccination programs, is the simplest to implement and is already in place in many countries. The focus there is on many of the poorest countries, where coverage is still poor and liver cancer burdens are high. Cervical cancer prevention, in contrast, is not widely practiced in LMCs. Screening does require a significant infrastructure, not only for the screening itself, but to provide treatment for women who are found with advanced cancers. In some countries, however, the availability of single-visit or two-visit screening (either visual inspection or HPV) may make the opportunity more attractive than it had been.
The third opportunity is to establish or improve treatment for the curable cancers of children and young adults. Doing so will build cancer management capacity more generally, and if done properly, will result in a rare class in many LMCs—cancer survivors, whose survival belies an all-too-common belief that cancer is inevitably fatal. Experience has shown that childhood cancer treatment can be financed through a combination of local and foreign sources in countries where it has been seriously attempted. As treatment for children with cancer becomes available, outreach to the public and the medical community can be promoted to develop awareness of childhood cancer and the positive outlook with treatment.
RECOMMENDATION 6-1. GAVI and other international partners should continue to assist countries to incorporate HBV vaccination into their childhood immunization programs as quickly as possible, with support from the global cancer community.
RECOMMENDATION 6-2. Countries with a high liver cancer burden and significant aflatoxin contamination of foodstuffs should examine the options for aflatoxin exposure reduction. Development partners should help to implement those measures that are feasible and cost-effective.
RECOMMENDATION 6-3. Countries should actively plan for the introduction of HPV vaccination as more information becomes available about the vaccines and as they become affordable. The international community should support a global dialogue on HPV vaccine policy and pricing.
RECOMMENDATION 6-4. Countries and global partners should follow the evolving information on newer screening approaches and determine the feasibility of adoption, given local resources and infrastructure.
RECOMMENDATION 6-5. Countries should aim to provide access to treatment and psychosocial services for children and young adults with highly curable cancers in pediatric cancer units in cancer centers or children’s hospitals.
REFERENCES
ACCP (Alliance for Cervical Cancer Prevention). 2003. Effectiveness, safety, and acceptability of cryotherapy: A systematic literature review. Cervical Cancer Prevention Issues in Depth. Volume 1. [Online]. Available: http://www.path.org/files/RH_cryo_white_paper.pdf [accessed 11/12/05].
ACCP. 2004. Planning and Implementing Cervical Cancer Prevention and Control Programs. Seattle, WA: ACCP.
ACCP. 2006. Home Page. [Online]. Available: http://www.alliance-cxca.org/ [accessed June 13, 2006].
Adamson PC, Weiner SL, Simone JV, Gelband H. 2005. Making Better Drugs for Children With Cancer. Washington, DC: The National Academies Press.
American Cancer Society. 2005. The History of Cancer. [Online]. Available: http://www.cancer.org/docroot/cri/content/cri_2_6x_the_history_of_cancer_72.asp?sitearea=cri [accessed June 5, 2005].
Arillo-Santillan E, Nigenda G, Sanchez-Prado VM, Alonso De Ruiz P, Najera-Aguilar P, Lazcano-Ponce EC. 2001. Mexico City physicians’ awareness about cervical cancer prevention: Implications for cancer screening. Journal of Cancer Education 16(2):75–79.
Association of Reproductive Health Professionals. HIV Infection and Cervical Intraepithelial Neoplasia. [Online]. Available: http://www.arhp.org/healthcareproviders/cme/onlinecme/hpvcp/infection.cfm [accessed March 15, 2005].
Barton M, Frommer M, Shafiq J. 2005. The Role of Radiotherapy in Cancer Control in Low- and Middle-Income Countries. Background paper commissioned by the Institute of Medicine.
Beutels P. 2001. Economic evaluations of hepatitis B immunization: A global review of recent studies (1994–2000). Health Economics 10(8):751–774.
Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. 2002. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology 55(4):244–265.
Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV, Alihonou E, Bayo S, Mokhtar HC, Chichareon S, Daudt A, De los Rios E, Ghadirian P, Kitinya JN, Koulibaly M. 1995. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. Journal of the National Cancer Institute 87(11):796–802.
Bosch FX, Muñoz N. 2002. The viral etiology of cervical cancer. Virus Research 89(2): 183–190.
Bosch FX, Muñoz N, De Sanjose S, Guerrerro E, Chaffari AM, Kaldor J, Castellsague X, Shah KV. 1994. Importance of human papillomavirus endemicity in the incidence of cervical cancer: An extension of the hypothesis on sexual behavior. Cancer Epidemiology, Biomarkers & Prevention 3(5):375–379.
Burd EM. 2003. Human papillomavirus and cervical cancer. Clinical Microbiology Reviews 16(1):1–17.
Castellsague X, Muñoz N. 2003. Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. Journal of National Cancer Institute Monographs (31):20–28.
Centers for Disease Control and Prevention. 2003. Global progress toward universal childhood hepatitis B vaccination, 2003. Morbidity & Mortality Weekly Report 52(36):868–870.
Chi DS, Perez CA, Lanciano RM, Kavanagh J. 2005. Cervical Cancer. In: Pazdur R, Coia LR, Hoskins WJ, Wagman LD, Editors. Cancer Management: A Multidisciplinary Approach. 9th ed. New York: CMP Healthcare Media. Pp. 445–476.
Chirenje ZM. 2004. Cervical Cancer Screening and Treatment Strategies. [Online]. Available: http://64.233.179.104/search?q=cache:qQQKmb6dqzsJ:www.hptn.org/research_studies/HPTN035MeetingsAndTrainings.htm+%22bervical+cancer+screening+and+treatment+strategies%22&hl=en&start=2. [accessed March 15. 2005].
Dai M, Bao YP, Clifford GM, Vaccarella S, Snijders PJF, Huang RD, Sun LX, Meijer CJLM, Qiao YL, Franceschi S. 2006. Human papillomavirus infection in Shanxi Province, People’s Republic of China: A population-based study. British Journal of Cancer 95:96–101.
DeVita VT Jr., Hellman S, Rosenberg SA. 2001. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins.
Durst M, Gissmann L, Ikenberg H, Zur Hausen H. 1983. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proceedings of the National Academy of Sciences of the United States of America 80(12 I):3812–3815.
Fahey MT, Irwig L, Macaskill P. 1995. Meta-analysis of Pap test accuracy. American Journal of Epidemiology (141):680–689.
Ferreccio C, Gage J. 2003. Visual Inspection of the Uterine Cervix with Acetic Acid (VIA): A Critical Review and Selected Articles. Washington, DC: Pan American Health Organization.
Ferreccio C, Prado RB, Luzoro AV, Ampuero SL, Snijders PJ, Meijer CJ, Vaccarella SV, Jara AT, Puschel KI, Robles SC, Herrero R, Franceschi SF, Ojeda JM. 2004. Population-based prevalence and age distribution of human papillomavirus among women in Santiago, Chile. Cancer Epidemiology, Biomarkers & Prevention 13(12):2271–2276.
Franceschi S, Dal Maso L, Arniani S, Crosignani P, Vercelli M, Simonato L, Falcini F, Zanetti R, Barchielli A, Serraino D, Rezza G. 1998. Risk of cancer other than Kaposi’s sarcoma and non-Hodgkin’s lymphoma in persons with AIDS in Italy. British Journal of Cancer 78(7):966–970.
Garrote LF, Anta JJL, Cruz EC, Romero T, Camacho R. 1996. Evaluation of the cervical cancer control program in Cuba. Bulletin of the Pan American Health Organization 30(4):387–391.
Giuliano AR, Sedjo RL, Roe DJ, Harris R, Baldwin S, Papenfuss MR, Abrahamsen M, Inserra P. 2002. Clearance of oncogenic human papillomavirus (HPV) infection: Effect of smoking (United States). Cancer Causes & Control 13(9):839–846.
Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, Wright TC, Alliance for Cervical Cancer Prevention Cost Working Group. 2005. Cost-effectiveness of cervical-cancer screening in five developing countries. New England Journal of Medicine 353(20):2158–2168.
Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. 2005. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. International Journal of Epidemiology 34(6):1329.
Hall AJ, Wild CP. 2003. Liver cancer in low and middle income countries. BMJ 326(7397): 994–995.
Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 364(9447):1757–1765.
Hewitt M, Weiner SL, Simone JV. 2003. Child Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: The National Academies Press.
Hildesheim A, Schiffman MH, Gravitt PE. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. Journal of Infectious Diseases 169:235–240.
Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH, Ribeiro RC, Pedrosa F. 2004. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA 291(20):2471–2475.
IARC (International Agency for Research on Cancer). 1995 (updated in 1997). Human Papillomaviruses/IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: IARC.
IARC. 2004. GLOBOCAN 2002. Lyon, France: IARC
IARC Working Group on the Evaluation of Cancer Preventive Strategies. 2005. Cervix Cancer Screening. IARC Handbooks of Cancer Prevention. Oxford, England: Oxford University Press.
International Network for Cancer Treatment and Research. 2004. Meeting of The Retinoblastoma Strategy Group, INCTR, Brussels, Belgium, April 29–30. NETWORK 4(4).
International Union Against Cancer. 2006. Making a World of Difference. [Online]. Available: http://uicc.org/index.php?id=516 [accessed 7/03/06].
Juarez-Figueroa LA, Wheeler CM, Uribe-Salas FJ, Conde-Glez CJ, Zamilpa-Mejia LG, Garcia-Cisneros S, Hernandez-Avila M. 2001. Human papillomavirus: A highly prevalent sexually transmitted disease agent among female sex workers from Mexico City. Sexually Transmitted Diseases 28(3):125–130.
Kensler TW, Qian GS, Chen JG, Groopman JD. 2003. Translational strategies for cancer prevention in liver. Nature Reviews. Cancer 3(5):321–329.
Kjaer SK, De Villiers EM, Dahl C, Engholm G, Bock JE, Vestergaard BF, Lynge E, Jensen OM. 1991. Case-control study of risk factors for cervical neoplasia in Denmark. Role of the “male factor” in women with one lifetime sexual partner. International Journal of Cancer 48(1):39–44.
Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB. 2002. A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 347(21):1645–1651.
Kruger-Kjaer S, Van Den Brule AJC, Svare EI, Engholm G, Sherman ME, Poll PA, Walboomers JMM, Bock JE, Meijer CJLM. 1998. Different risk factor patterns for high-grade and low-grade intraepithelial lesions on the cervix among HPV-positive and HPV-negative young women. International Journal of Cancer 76(5):613–619.
Lacey JV Jr, Brinton LA, Abbas FM, Barnes WA, Gravitt PE, Greenberg MD, Greene SM, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Silverberg SG, Hildesheim A. 1999. Oral contraceptives as risk factors for cervical adenocarcinomas and squamous cell carcinomas. Cancer Epidemiology, Biomarkers & Prevention 8(12):1079–1085.
Lancaster WD, Castellano C, Santos C. 1986. Human papillomavirus deoxyribonucleic acid in cervical carcinoma from primary and metastatic sites. American Journal of Obstetrics & Gynecology 154(1):115–119.
Lavanchy D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis 11(2):97–107.
Lazcano-Ponce EC, De Ruiz PA, Lopez-Carrillo L, Najera-Aguilar P, Avila-Ceniceros R, Escandon-Romero C, Cisneros MT, Hernandez-Avila M. 1997. Validity and reproducibility of cytologic diagnosis in a sample of cervical cancer screening centers in Mexico. Acta Cytologica 41(2):277–284.
Lazcano-Ponce EC, Moss S, Alonso de Ruiz P, Salmeron Castro J, Hernandez Avila M. 1999. Cervical cancer screening in developing countries: Why is it ineffective? The case of Mexico. Archives of Medical Research 30(3):240–250.
Lazcano-Ponce EC, Rascon-Pacheco RA, Lozano-Ascencio R, Velasco-Mondragon HE. 1996. Mortality from cervical carcinoma in Mexico: Impact of screening, 1980–1990. Acta Cytologica 40(3):506–512.
Look AT, Kirsch IR. 2002. Molecular basis of childhood cancer. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins. Pp. 45–88.
Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, Ransley JE, Fetterman BJ, Hartinger JS, McIntosh KM, Pawlick GF, Hiatt RA. 1999. Identifying women with cervical neoplasia: Using human papillomavirus DNA testing for equivocal Papanicolaou results. Journal of the American Medical Association 281(17):1605–1610.
Masera G, Baez F, Biondi A, Cavalli F, Chiesa R, Conter V, Fossati-Bellani F, Marinoni M, Sala A, Valsecchi MG, Tognini G. 2004. Bridging the childhood cancer mortality gap between economically developed and low-income countries: Lessons from the MISPHO experience. In: Tannenberger S, Cavalli F, Pannuti F, Editors. Cancer in Developing Countries: The Great Challenge for Oncology in the 21st Century. Munich, Germany: W. Zuckschwerdt Verlag GmbH. Pp. 42–60.
Massad LS, Riester KA, Anastos KM, Fruchter RG, Palefsky JM, Burk RD, Burns D, Greenblatt RM, Muderspach LI, Miotti P. 1999. Prevalence and predictors of squamous cell abnormalities in Papanicolaou smears from women infected with HIV-1. Journal of Acquired Immune Deficiency Syndromes & Human Retrovirology 21(1):33–41.
McCrory DC, Matchar DB, Bastian L, Datta S, Hasselblad V, Hickey J, Meyers E, Nanda K. 1999. Evaluation of Cervical Cytology—Evidence Report/Technology Assessment Number 5. AHCPR Publication No. 99-E010. Durham, NC: Agency for Healthcare Research and Policy.
Meyers C, Laimins LA. 1994. In vitro systems for the study and propagation of human papillomaviruses. Current Topics in Microbiology & Immunology 186:199–215.
Molano M, Posso H, Weiderpass E, van den Brule AJ, Ronderos M, Franceschi S, Meijer CJ, Arslan A, Munoz N, HPV Study Group. 2002. Prevalence and determinants of HPV infection among Colombian women with normal cytology. British Journal of Cancer 87(3):324–333.
Moreno V, Bosch FX, Muñoz N, Meijer CJLM, Shah KV, Walboomers JMM, Herrero R, Franceschi S. 2002. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet 359(9312):1085–1092.
Moscicki AB, Shiboski S, Broering J. 1998. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. Journal of Pediatrics 132:277–284.
Muñoz N, Xavier Bosch F, Castellsague X, Diaz M, De Sanjose S, Hammouda D, Shah KV, Meijer CJLM. 2004. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. International Journal of Cancer 111(2):278–285.
Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB. 2000. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Annals of Internal Medicine 132(10):810–819.
Oguchi M, Komura J, Tagami H. 1981. Ultrastructural studies of spontaneously regressing plane warts: Macrophages attack verruca-epidermal cells. Archives of Dermatological Research 270:403–411.
Ottaviano M, La Torre P. 1982. Examination of the cervix with the naked eye using acetic acid test. American Journal of Obstetrics & Gynecology 143(2):139–142.
Palacio V, De Sanjose S, Vazquez S, Puente M, Vazquez F, Bosch FX. 1993. Cervical neoplasia and sexually transmitted diseases among prostitutes in Oviedo, Spain. International Journal of STD & AIDS 4(2):121–122.
Parkin DM, Ferlay J, Hamdi-Cherif M, Sitas F, Thomas JO, Wabinga H, Whelan SL. 2003. Cancer in Africa: Epidemiology and Prevention. Lyon, France: IARC Press.
Pham TH, Nguyen TH, Herrero R, Vaccarella S, Smith JS, Nguyen Thuy TT, Nguyen HN, Nguyen BD, Ashley R, Snijders PJ, Meijer CJ, Munoz N, Parkin DM, Franceschi S. 2003. Human papillomavirus infection among women in South and North Vietnam. International Journal of Cancer 104(2):213–220.
Plummer M, Herrero R, Franceschi S, Meijer CJLM, Snijders P, Bosch FX, De Sanjose S, Muñoz N. 2003. Smoking and cervical cancer: Pooled analysis of the IARC multi-centric case-control study. Cancer Causes & Control 14(9):805–814.
Ribeiro RC, Pui CH. 2005. Saving the children—improving childhood cancer treatment in developing countries. New England Journal of Medicine 352(21):2158–2160.
Ries LAG, Eisner MP, Kosary KL, Hankey BF, Miller BA, Clegg L, Edwards BK, eds. 2002. SEER Cancer Statistics Review, 1973–1999. Bethesda, MD: National Cancer Institute, SEER Program.
Ronco G, Ghisetti V, Segnan N, Snijders PJ, Gillio-Tos A, Meijer CJ, Merletti F, Franceschi S. 2005. Prevalence of human papillomavirus infection in women in Turin, Italy. European Journal of Cancer 41(2):297–305.
Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CCG, Sharma R, Dolo A, Shastri SS, Nacoulma M, Nayama M, Somanathan T, Lucas E, Muwonge R, Frappart L, Parkin DM. 2004a. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. International Journal of Cancer 110(6):907–913.
Sankaranarayanan R, Budukh AM, Rajkumar R. 2001. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bulletin of the World Health Organization 79(10):954–962.
Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. 2005. A critical assessment of screening methods for cervical neoplasia. International Journal of Gynecology & Obstetrics 89(Suppl 2):S4–S12.
Sankaranarayanan R, Thara S, Sharma A, Roy C, Shastri S, Mahe C, Muwonge R, Fontaniere B. 2004b. Accuracy of conventional cytology: Results from a multicentre screening study in India. Journal of Medical Screening 11(2):77–84.
Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, Parkin DM, Nair MK. 1998. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer 83(10):2150–2156.
Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, Chithrathara K, Parkin DM, Nair MK. 2003. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol’s iodine (VILI) in cervical cancer screening in Kerala, India. International Journal of Cancer 106(3):404–408.
Schiffman MH, Brinton LA. 1995. The epidemiology of cervical carcinogenesis. Cancer 76(10 Suppl):1888–1901.
Sehgal A, Singh V, Bhambhani S, Luthra UK. 1991. Screening for cervical cancer by direct inspection. Lancet 338(8762):282.
Serraino D, Carrieri P, Pradier C, Bidoli E, Dorrucci M, Ghetti E, Schiesari A, Zucconi R, Pezzotti P, Dellamonica P, Franceschi S, Rezza G. 1999. Risk of invasive cervical cancer among women with, or at risk for, HIV infection. International Journal of Cancer 82(3):334–337.
Shah KV, Viscidi RP, Alberg AJ, Helzlsouer KJ, Comstock GW. 1997. Antibodies to human papillomavirus 16 and subsequent in situ or invasive cancer of the cervix. Cancer Epidemiology Biomarkers and Prevention 6(4):233–237.
Shanta V. 2000. Partner Profile: Cancer Institute (WIA), Chennai, India. [Online]. Available: http://www.inctr.org/publications/2000_v01_n01_a07.shtml [accessed January 25, 2006].
Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, Park S, Touze A, Munoz N, Snijders PJ, Meijer CJ, Coursaget P, Franceschi S. 2003. Prevalence of human papillomavirus infection in women in Busan, South Korea. International Journal of Cancer 103(3):413–421.
Slawson DC, Bennett JH, Herman JM. 1992. Are Papanicolaou smears enough? Acetic acid washes of the cervix as adjunctive therapy: A HARNET study. Journal of Family Practice 35(3):271–277.
Smith JS, Green J, Berrington De Gonzalez A, Appleby P, Peto J, Plummer M, Franceschi S, Beral V. 2003. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet 361(9364):1159–1167.
Sodhani P, Gupta S, Singh V, Sehgal A, Mitra AB. 2004. Eliminating the diagnosis atypical squamous cells of undetermined significance: Impact on the accuracy of the Papanicolaou test. Acta Cytologica 48(6):783–787.
St. Jude Children’s Research Hospital. 2003. About International Outreach. [Online]. Available: http://www.stjude.org/international-outreach/0,2564,455_3206_5160,00.html [accessed February 8, 2006].
St. Jude Children’s Research Hospital. 2005. International Outreach Program—I.O.P. Annual Report 2005. Memphis, TN: St. Jude Children’s Research Hospital.
St. Jude Children’s Research Hospital. 2006. About St. Jude. [Online]. Available: http://www. stjude.org/aboutus [accessed February 8, 2006].
Steenbergen RDM, Walboomers JMM, Meijer CJLM, Van der Raaij-Helmer EMH, Parker JN, Chow LT, Broker TR, Snijders PJF. 1996. Transition of human papillomavirus type 16 and 18 transfected human foreskin keratinocytes towards immortality: Activation of telomerase and allele losses at 3p, 10p, 11q and/or 18q. Oncogene 13(6):1249–1257.
Stoler MH, Rhodes CR, Whitbeck A, Wolinsky SM, Chow LT, Broker TR. 1992. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Human Pathology 23(2):117–128.
Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC Jr. 1997. Human papillomavirus infection in women infected with the human immunodeficiency virus. New England Journal of Medicine 337(19):1343–1349.
Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Lerdlukanavonge P, Koonchote S. 1995. Effect of a mobile unit on changes in knowledge and use of cervical cancer screening among rural Thai women. International Journal of Epidemiology 24(3):493–498.
Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Tatip Y, Boonmak C. 1999. A mobile unit: An effective service for cervical cancer screening among rural Thai women. International Journal of Epidemiology 28(1):35–39.
Szarewski A, Cuzick J. 1998. Smoking and cervical neoplasia: A review of the evidence. Journal of Epidemiology and Biostatistics 3:229–256.
Thomas DB, Ray RM, Koetsawang A, Kiviat N, Kuypers J, Qin Q, Ashley RL, Koetsawang S. 2001a. Human papillomaviruses and cervical cancer in Bangkok. Risk factors for invasive cervical carcinomas with human papillomavirus types 16 and 18 DNA. American Journal of Epidemiology 153(8):723–731.
Thomas DB, Ray RM, Kuypers J, Kiviat N, Koetsawang A, Ashley RL, Qin Q, Koetsawang S. 2001b. Human papillomaviruses and cervical cancer in Bangkok. The role of husbands and commercial sex workers. American Journal of Epidemiology 153(8):740–748.
Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, Oladepo O, Smith JS, Arslan A, Munoz N, Snijders PJ, Meijer CJ, Franceschi S. 2004. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. British Journal of Cancer 90(3):638–645.
Turner PC, Sylla A, Diallo MS, Castegnaro JJ, Hall AJ, Wild CP. 2002. The role of aflatoxins and hepatitis viruses in the etiopathogenesis of hepatocellular carcinoma: A basis for primary prevention in Guinea-Conakry, West Africa. Journal of Gastroenterology & Hepatology 17(Suppl):S441–S448.
Usmani GN. 2001. Pediatric oncology in the third world. Current Opinion in Pediatrics 13(1):1–9.
Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncology 6(5):271–278.
WHO (World Health Organization). 2001. Macroeconomics and Health: Investing in Health for Economic Development: Report of the Commission on Macroeconomics and Health. Geneva, Switzerland: WHO.
WHO. 2002. Cervical Cancer Screening in Developing Countries: Report of a WHO Consultation. Geneva, Switzerland, WHO.
WHO. 2005. Report on the Consultation on Human Papillomavirus Vaccines (DRAFT). Unpublished.
Zielinski GD, Snijders PJF, Rozendaal L, Voorhorst FJ, Van der Linden HC, Runsink AP, De Schipper FA, Meijer CJLM. 2001. HPV presence precedes abnormal cytology in women developing cervical cancer and signals false negative smears. British Journal of Cancer 85(3):398–404.























































