2
Cancer Causes and Risk Factors and the Elements of Cancer Control
A logical way to identify cancer control opportunities is to consider what we know about the causes and risk factors for common cancers, and then to consider how easy or difficult it is to eliminate or modify them in ways that would reduce cancer incidence. Where the causes or risk factors are not well understood, are only weak, or cannot readily be changed, the opportunities lie in how easy or difficult it is to “cure” people who get cancer or to care for those with cancer who cannot be cured.
This chapter starts with a review of the causes and known risk factors for the most common cancers in low- and middle-income countries (LMCs), with discussion of the modifiability of these causes and risk factors. The latter part of the chapter is a general review of the approaches to cancer control, beginning with cancer control planning, then prevention and the elements of cancer management (diagnosis and treatment): psychosocial care, radiotherapy, chemotherapy, and surgery. Chapter 7 is devoted entirely to palliative care, the other major pillar of cancer management, so that area is not covered in this chapter.
CAUSES OF AND RISK FACTORS FOR CANCER IN LMCS
Knowing the causes of cancer provides a basis for understanding the potential for preventing cancer. If a cause is known, it is much easier to know whether it can (e.g., tobacco use) or cannot (e.g., ionizing radiation in the atmosphere) be avoided easily. An accounting of the causes also shows up the gaps in knowledge—for example, few specific causes of colon cancer are known. Table 2-1 lists the major risk factors for the top 10 causes
TABLE 2-1 Leading Risk Factors for Cancer Deaths in LMCs (or Developing Countries) and Primary Prevention Strategies
|
Cancer Type (number of deaths in LMCs in 2002) |
Main Risk Factors |
Theoretical Minimum Exposure Distribution |
Primary Prevention: Currently Available Strategies |
PAF % |
|
Lung, trachea, and bronchus (770,938) |
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in Framework Convention on Tobacco Control (FCTC) |
60 |
|
|
Low fruit and vegetable intake |
600 grams/day fruit and vegetable intake for adults |
Dietary improvements |
13 |
|
|
Urban air pollution |
7.5 μg/m3 for particles with aerodynamic diameters <2.5 microns 15 μg/m3 for particles with aerodynamic diameters <10 microns |
Regulation of automobile exhaust and industrial combustion products |
7 |
|
|
Indoor smoke from cooking and heating |
Zero exposure possible |
Ventilation and improved low-technology heating and cooking |
2 |
|
|
Radon in buildings (from the earth) |
NGE |
Building regulations to avoid radon seepage into enclosed buildings, mainly in cold climates |
NGE |
|
|
Various occupational exposures |
NGE |
Workplace regulation and controls |
9b |
|
Cancer Type (number of deaths in LMCs in 2002) |
Main Risk Factors |
Theoretical Minimum Exposure Distribution |
Primary Prevention: Currently Available Strategies |
PAF % |
|
Stomach (695,426) |
Chronic infection with Helicobacter pylori |
Zero exposure possible |
Improved living conditions (nonspecific) Future: Vaccine to prevent infection? Future: Drugs to clear infection? |
74a |
|
|
Low fruit and vegetable intake |
600 grams/day fruit and vegetable intake for adults |
Dietary improvements |
19 |
|
|
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
11 |
|
Liver (504,407) |
Chronic hepatitis B (HBV), mainly from infection in infancy and childhood; co-factors, such as fungal toxins (e.g., aflatoxin) |
Zero exposure possible |
Hepatitis B vaccination in infancy Reduced fungal contamination of stored grains Future: Cure of chronic HBV? |
59a |
|
|
Chronic hepatitis C (HCV) from contaminated blood and unsafe injections, and person to person; co-factors such as fungal toxins (e.g., aflatoxin) |
Zero exposure possible |
Blood supply and injection safety and measures Future: Cure of chronic HCV? |
33a |
|
|
Alcohol use |
Zero exposure possible |
Reduced alcohol use |
23 |
|
|
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
11 |
|
Cancer Type (number of deaths in LMCs in 2002) |
Main Risk Factors |
Theoretical Minimum Exposure Distribution |
Primary Prevention: Currently Available Strategies |
PAF % |
|
Colon and rectum (356,949) |
Physical inactivity |
At least 2.5 hours/ week of moderate-intensity activity or equivalent (4,000 KJ/week) |
Lifestyle changes |
15 |
|
|
Overweight and obesity |
BMI (weight/height2) of 21 |
Dietary improvements and exercise |
9 |
|
|
Low fruit and vegetable intake |
600 grams/day fruit and vegetable intake for adults |
Dietary improvements |
2 |
|
Esophagus (379,760) |
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
37 |
|
|
Alcohol use |
Zero exposure possible |
Reduced alcohol use |
24 |
|
|
Low fruit and vegetable intake |
600 grams/day fruit and vegetable intake for adults |
Dietary improvements and exercise |
19 |
|
Breast (317,195) |
Physical inactivity |
At least 2.5 hours/ week of moderate-intensity activity or equivalent (4,000 KJ/week) |
Exercise |
10 |
|
|
Overweight and obesity |
BMI (weight/height2) of 21 |
Dietary improvements and exercise |
7 |
|
|
Alcohol use |
Zero exposure possible |
Reduced alcohol use |
4 |
|
Mouth and oropharynx (271,074) |
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
37 |
|
|
Alcohol use |
Zero exposure possible |
Reduced alcohol use |
14 |
|
Uterine cervix (218,064) |
Chronic infection with specific strains of human papillomavirus (HPV) |
Zero exposure possible |
Screening for precancerous stages Vaccination against HPV in infancy or adolescence |
100 |
|
|
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
2 |
|
Cancer Type (number of deaths in LMCs in 2002) |
Main Risk Factors |
Theoretical Minimum Exposure Distribution |
Primary Prevention: Currently Available Strategies |
PAF % |
|
Lymphomas and multiple myelomaa |
Burkitt’s lymphoma (7,800 cases in children in less developed countries): chronic infection with Epstein-Barr virus |
Very low exposure possible |
None apparent |
NGE |
|
Leukemia (190,059) |
Ionizing radiation (natural and medical) |
Low medical exposure possible; lower radon exposure possible |
Improved medical practices Improved building practices to exclude radon |
NGE |
|
|
Tobacco use |
Zero exposure possible |
Tobacco control as outlined in FCTC |
6 |
|
|
Various occupational exposures |
Lower exposures possible (varying by exposure) |
Workplace regulation and controls |
2b |
|
NOTE: PAFs from the Global Burden of Disease and Risk Factors (Lopez et al., 2006) unless otherwise noted. The Institute of Medicine did not independently recalculate these figures. These PAFs refer to fractions of deaths, and the denominator is all cancer deaths in LMCs (as defined by the World Bank)—about 5 million deaths. The fractions reported from Parkin (Parkin, 2005) refer to cases in developing countries (as defined by the World Health Organization), which total about 6 million. These two types of figures are approximately, but not strictly, comparable. For purposes of this table, however, together they provide a relatively accurate overview of the major risk factors for cancers and cancer deaths. PAF = Population attributable fraction, which is the proportional reduction in disease that would occur if population exposure to the risk factor were reduced to the theoretical minimum risk level. Many cancers are affected by more than one risk factor (e.g., tobacco and alcohol combining to greatly increase the risk of esophageal cancer); elimination of one or another could prevent a particular cancer from occurring. This means that the PAFs are not mutually exclusive, and added together will generally overestimate the cancer reduction possible by eliminating all identified risk factors. NGE = No global estimate available. aData from Parkin (2005). bData from Rosenstock et al. (2006); refers to worldwide PAF, compared with zero exposure, with no breakdown by country economic level. SOURCES: Lopez et al. (2006); Parkin (2005); and Institute of Medicine. |
||||
of cancer death in LMCs, with their “population attributable fractions” (PAFs). Table 2-2 summarizes the impact of these risk factors for all cancers in LMCs. The PAF represents the proportion of cancer deaths that could be prevented if the risk factor or exposure were reduced to a theoretical minimum. For a number of major risk factors, a zero level of exposure is at least theoretically possible. These include: tobacco use, alcohol use (but see below), indoor smoke from cooking, most carcinogenic infectious agents (if not zero exposure, vastly reduced from current levels). For other factors, zero exposure is not a relevant concept, such as for obesity or for inactivity. The values for those risk factors (from Global Burden of Disease and Risk Factors, Lopez et al., 2006) are based on an achievable level that would minimize the risk of all major diseases associated with that risk factor.
Heart disease, stroke, cancer, chronic respiratory diseases, and diabetes—the main chronic diseases—share some common risk factors (Epping-Jordan et al., 2005; WHO, 2005a). Tobacco smoking is overwhelmingly the most significant risk factor for cancer and across the board for chronic diseases. Diet, exercise, and alcohol use also cut across the diseases, and they are significant contributors to cancer, but more significant to other conditions. Of lesser global importance (but in some cases, very important focally), are factors such as occupational exposures to asbestos, coal, and other substances; indoor smoke from cooking and heating; and air pollution, which can cause cancer and a larger burden of chronic respiratory diseases. Beyond these factors, the common cancers in LMCs do not share significant risk factors with other chronic diseases. What stands out is the role of infections in common cancers of LMCs (discussed below). Cancer control will benefit from integrated approaches to chronic disease control, but cancer-specific strategies—which will have little or no impact on other chronic diseases—are also needed.
Table 2-1 describes the risk factors associated with current deaths from cancer. Because of the time scale of cancer, the pattern of deaths today reflects the distribution of risk factors some decades ago. For smoking, the peak in smoking-related deaths follows the peak in smoking rates by about 30 years (Figure 2-1). Today’s smoking rates will determine the cancer rates 30 years hence. It is important, therefore, to know about levels and trends in risk factors for cancer control planning (see Chapter 3 for a discussion about monitoring risk factors).
Tobacco
Tobacco use is the largest single contributor to cancer mortality: It was a cause of nearly one-fifth of all cancer deaths in LMCs in 2002. It is also the leading cause of death from the totality of major noncommunicable diseases. Chapter 5 goes into considerable detail on the effects of tobacco
and its control. What should be noted from Figure 2-1 is where the LMCs are on the epidemic curve. The low-income countries are all in either stage 1 or 2 (tobacco use still low but rising, first among males; tobacco-related deaths low and just beginning to rise), and the middle-income countries in stage 2 or 3 (tobacco use rising steeply or having peaked; mortality following). The challenge to tobacco control is to alter the trajectories in those countries by encouraging adults to quit smoking (benefits that appear in a very short timeframe) and discouraging young people from starting (the long-term solution). They do not have to follow the trajectory we now see in retrospect for the high-income countries. If they do, however, the number of deaths from tobacco will continue to increase steeply. In the year 2000, about 4.9 million deaths from all causes were related to tobacco, about half in developed and half in developing countries. In 2020, if current trends continue, 9 million people will die from tobacco-related causes, and 7 million of these will be in developing countries.
Infections
The three leading types of cancer-causing infections—hepatitis B virus (HBV) and hepatitis C virus, human papillomavirus (HPV), and Helicobacter pylori—follow tobacco in importance as risk factors for cancer incidence in developing countries. It is clear from their much lesser importance in high-income countries (responsible for an estimated 8 percent of all cancers, compared with 26 percent in developing countries) (Parkin, 2005) that the prevalence of these infections can be greatly reduced, although the means to do so differ. HBV is readily preventable by immunization. Preventing the spread of hepatitis C requires blood bank screening and safe injection practices, which is a more difficult set of interventions to implement. HPV-related cancers have been prevented in high-income countries largely through screening and treatment of precancerous lesions, but infection prevention is newly possible by vaccination as well. HBV- and HPV-related cancers represent immediate opportunities for preventing cancer in current generations.
H. pylori is one major cause of stomach cancer, a cancer that is poorly responsive to treatment. The prevalence of H. pylori (and stomach cancer) has declined dramatically without targeted measures in much of the world, suggesting the possibility of developing interventions for places where it is not declining, which includes most LMCs. At present, this is a research question focused on treatment of infected individuals with antibiotics that eradicate the bacteria. Work on vaccines is also reported to be progressing (Parkin, 2005). Progress depends on continued support of promising leads. H. pylori research is not pursued further in this report, however.
Other types of infection are locally important and account for about
TABLE 2-2 Summary of Major Risk Factors for All Cancers in LMCs (or Developing Countries)
|
PAF % |
Importance of Risk Factor to Other Serious Health Conditions |
|
18 |
|
|
26a |
|
|
8.2a |
Significant burden from other serious liver diseases from HBV and HCV |
|
8a |
Very small burden from other conditions |
|
6.9a |
|
|
6 |
Much greater burden of cardiovascular disease |
|
5 |
Much greater burden of injuries, neuropsychiatric conditions, cardiovascular conditions, and other causes |
|
2 |
Much greater burden of cardiovascular and other noncommunicable diseases |
|
1 |
Much greater burden of cardiovascular and other noncommunicable diseases |
|
1 |
Greater burden of chronic respiratory disease |
|
<0.5 |
Much greater burden of cardiovascular and chronic respiratory conditions |
|
risk level. Many cancers are affected by more than one risk factor (e.g., tobacco and alcohol combining to greatly increase the risk of esophageal cancer); elimination of one or another could prevent a particular cancer from occurring. This means that the PAFs are not mutually exclusive, and added together will generally overestimate the cancer reduction possible by eliminating all identified risk factors. NGE = No global estimate available. aData from Parkin (2005). SOURCES: Lopez et al. (2006); Parkin (2005); and Institute of Medicine. |
|
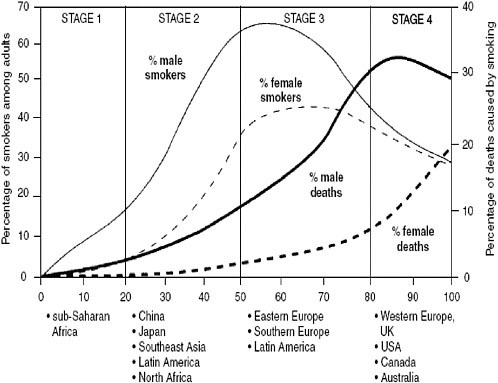
FIGURE 2-1 Four stages of the tobacco epidemic.
SOURCE: Reprinted, with permission, from Lopez et al. (1994). Copyright 1994 by BMJ Publishing Group Ltd.
4 percent of all cancers (Parkin, 2005). The most significant is Epstein-Barr virus, a risk factor for Burkitt’s lymphoma and cancers of the nasopharynx, to which 2 percent of cancers in LMCs are attributable. Both of these cancers are much more common in developing than developed countries. Next in importance, the human immunodeficiency virus (HIV) is responsible for an estimated 62,500 cases of Kaposi’s sarcoma in developing countries (about 1 percent of all cancers). Somewhat less than 1 percent of cancers are attributable to helminth (worm) infections, including bladder and possibly colorectal cancers (“limited” evidence, according to the International Agency for Research on Cancer, or IARC) (IARC, 1997) caused by schistosomes, and liver cancers caused by liver flukes. Finally, human T-cell lymphotropic virus type 1 (HTLV-1) is the cause of some non-Hodgkin’s lymphomas.
It is worth noting that other infectious causes of cancer may be discovered. Current knowledge has been developed largely in the past 30 years. An organism as significant as H. pylori was not recognized until the 1980s, originally as the primary cause of gastritis and stomach ulcers, and it was
classified as a human carcinogen by IARC only about a decade ago (IARC, 1997).
Diet, Overweight and Obesity, and Physical Inactivity
Diet, body weight, and activity levels are interrelated and seem to act in complex ways to either promote or reduce the risk of cancer. Global Burden of Disease and Risk Factors (Lopez et al., 2006) made separate estimates for the three components, based on the best available quantitative evidence and focusing on low fruit and vegetable intake as the best established specific dietary factor. Nine percent of cancer deaths in LMCs are attributable to these three factors, which could be the focus of interventions. As significant as these risk factors are for cancer, they are responsible for considerably greater disability and death from other causes: heart disease, hypertension and stroke, diabetes, and arthritis most important among them. These other conditions are at least five times as important as cancer in terms of disability-adjusted life-years (DALYs) (Lopez et al., 2006).
A high intake of fresh fruits and vegetables (generally excluding root vegetables and pulses (peas and beans), which have a higher carbohydrate content than other types of vegetables), has consistently been found in epidemiologic studies to be associated with lower cancer rates. Fruits and vegetables are generally low in calories (supply less than 5 percent of calories in most countries) and high in fiber, vitamins, minerals, and other active molecules. Studies that have looked at vegetables individually or in classes (categorized several different ways, either botanically or culinarily) have not been able to pinpoint more precisely which vegetables or fruits are more or less beneficial, or which components cause which effects. Eventually, this may be possible, but given the state of the science today, it is best to consider fruits and vegetables collectively (World Cancer Research Fund, 1997).
The evidence is convincing that an adequate intake of fruits and vegetables (for some cancers, mainly vegetables) lowers the risk of the following cancers: colon and rectum, lung, stomach, esophagus, and mouth and pharynx (IOM, 2003; World Cancer Research Fund, 1997). The risk of a number of other cancers also may be lowered, but the evidence is not as strong. Those with the strongest evidence are cancers of the larynx, pancreas, breast, and bladder. The strength of the totality of the research literature underlies the estimate that 6 percent of cancer deaths are attributable to low fruit and vegetable intake (Lopez et al., 2006).
Other estimates of cancers related to “diet and nutrition” are higher. IARC’s 2003 World Cancer Report (Stewart and Kleihues, 2003) states: “Up to 30 percent of human cancers are probably related to diet and nutrition,” using an expansive definition of diet and nutrition. The estimate also refers to the world as a whole, with no separate estimates for countries by
income level. In addition to the effects of fruit and vegetable consumption and body weight, salt and salt-preserved food increase the risk of stomach and nasopharyngeal cancers (more important in LMCs than in high-income countries); red meat consumption appears to increase the risk of colorectal cancer (more important in high-income countries than in LMCs); and other factors, such as food additives, have a small overall effect.
Diet and physical activity are clearly very important determinants of cancer (although the specifics of the associations are not clear), and even more so for other major chronic diseases. However, interventions that are known to have a substantial effect on diet and exercise habits are not well established in high-income countries, where virtually all the documented efforts have been made (e.g., changing dietary composition, losing weight, or increasing exercise) (IOM, 2003). In LMCs, food availability, agriculture policies, and the much different lifestyles make this a difficult, though important, area to address in the context of integrated chronic disease control. WHO’s “Global Strategy on Diet, Physical Activity and Health,” which was endorsed by the May 2004 World Health Assembly, has made a series of recommendations based on the following principles (WHO, 2004):
-
Stronger evidence for policy: synthesize existing knowledge, science and interventions on the relationship between diet, physical activity, and chronic disease.
-
Advocacy for policy change: inform decision-makers and stakeholders of the problem, determinants, interventions, and policy needs.
-
Stakeholder involvement: agree on the roles of stakeholders in implementing the global strategy.
-
A strategic framework for action: propose appropriately tailored policies and interventions for countries.
This report concentrates on measures that are mainly or exclusively cancer related, so contains no specific recommendations in this area. Clearly, cancer control authorities in LMCs should join efforts to improve diet and encourage appropriate physical activity in their own countries.
Alcohol Use
Cancers of the oral cavity, pharynx, larynx, esophagus, liver, and breast can be caused by heavy alcohol drinking, with the risk varying by cancer site, but increasing for all sites with greater consumption and resulting in an estimated 5 percent of attributable cancer deaths in LMCs (Lopez et al., 2006). Similar to diet and physical activity, the disease burden from heavy alcohol consumption is several times greater for other conditions—cardiovascular disease, neuropsychiatric illness, and intentional and unintentional
injuries—than for cancer in LMCs. What is different is that moderate alcohol consumption has the benefit of reducing cardiovascular risks, although this is documented only in high-income countries. The many acute social and psychological problems caused by excessive drinking must be added to the tally of harm from chronic conditions.
Certain interventions to curb heavy drinking have been effective in high-income countries, but have rarely been implemented in LMCs and even more rarely evaluated. Increased excise taxes, reduced access to stores selling alcoholic beverages, advertising bans, random breath testing for motor vehicle drivers, and brief advice to heavy drinkers from physicians are among the effective and cost-effective interventions known (Rehm et al., 2006). Applying these interventions in LMCs, however, will require tailoring to national and local cultural, social, and economic environments.
Occupational Exposures
Workplaces can be hazardous, whether they are factories, family farms, city streets, or any other formal or informal place of work. The International Labor Organization (ILO) estimates that 2 million deaths per year worldwide, among 2.7 billion workers, are attributable to workplace exposures. The burden of disease and disability is enormous, but data to support quantitative estimates are not available for most of the world (Rosenstock et al., 2006).
About 25 chemicals or mixed chemical exposures for which exposures are mostly occupational have been identified by IARC as established human carcinogens, and an equal number are probably carcinogenic (Stewart and Kleihues, 2003). The numbers of workers exposed and exposure levels are largely unknown, however. Certain patterns suggest that at least some hazardous exposures are becoming more frequent in LMCs, and that in general, with industrialization and globalization, occupational health problems are rising in these countries. An example is the relocation of manufacturing involving asbestos from high-income countries to countries such as Brazil, India, Pakistan, and the Republic of Korea, where occupational health control may be less stringent (Stewart and Kleihues, 2003). These operations are also more likely to be on a smaller scale than in high-income countries, often using older machinery and with limited protection for workers.
The World Health Organization (WHO) undertook a thorough assessment of worldwide data for five occupational risk factors, one of which was carcinogens (the others were risks for injuries, airborne particulates, ergonomic risks for back pain, and noise). All five together accounted for about 800,000 annual workplace-related deaths (about 40 percent of the 2 million estimated by ILO). Of these, 100,000 were lung cancers and 2,000 were leukemias, the most prevalent (but not the only) types of occupation-
ally related cancers. By comparison, chronic obstructive pulmonary disease was responsible for about 318,000 deaths per year, behind injuries, the leading cause of occupational death.
The technical means exist to prevent a substantial portion of occupationally related cancers, as has happened in high-income countries. Implementing these preventive measures requires both the knowledge and a regulatory framework to enforce lower exposures. International (e.g., ILO and WHO) and national or state governments, as well as industry, have roles to play in this process. However, many impediments stand in the way of effective protection against workplace exposures to carcinogens and other hazards in LMCs, discussed in detail by Rosenstock and colleagues (Rosenstock et al., 2006). Improvements in occupational health go far beyond cancer control, and the activities necessary for it are largely outside the general health infrastructure.
Pollution
Pollution of the air (indoor and outdoor), water, and soil causes an estimated 1 to 4 percent of all cancers worldwide (Stewart and Kleihues, 2003). Even though the risk levels per person are quite low, the large numbers of people exposed—involuntarily—can mean a substantial population effect. For indoor and outdoor air pollution, lung cancer is the main cancer type implicated. Ambient air pollution is caused mainly by vehicular exhaust, which is a mixture of chemicals. While emission levels in high-income countries have tended to decline over recent decades, they are level or increasing in many LMCs. In poor rural areas of sub-Saharan Africa, and South Asia, East Asia, and the Pacific in particular, indoor air is polluted by heating and cooking fires, and fumes from cooked food. Coal and biomass, the smokiest fuels, are those used most frequently in these places. Much more widespread (but far less hazardous per person exposed) is indoor tobacco smoke. The number of cancer deaths caused by indoor and outdoor air pollution is exceeded by deaths from respiratory and some cardiovascular conditions in adults and deaths from acute respiratory infections in children.
Poor water quality is associated mainly with infectious diseases. Some cancers are probably caused by the by-products of chlorination used to purify water, and by arsenic, which occurs naturally in high concentrations in the soil in certain areas. Focal exposure to a variety of harmful compounds from industrial waste may also cause some cancers in local populations, but these are difficult to sort out and more difficult to quantify.
As with occupational exposures, the main means of reducing exposure to outdoor pollution is through regulation. Reduction of motor vehicle emissions and industrial emissions in air and water have been successful in high-income countries, and have probably produced health benefits, although the
cancer prevention benefits have probably been modest. Reducing exposure to indoor air pollution from stoves requires specific policy actions. Those most affected are the poorest and most vulnerable in the population who have no financial means to improve their living conditions, including the use of cleaner fuels and better stoves. In a recent in-depth analysis, Bruce and colleagues (Bruce et al., 2006) reviewed programs over the past few decades aimed at reducing indoor air pollution. Lessons have been learned, but the situation globally has improved little. Various technologies are available, but making them available to those at highest risk, in a sustainable fashion, has proven very difficult.
Other Causes and Risk Factors
Food Contaminants
Apart from the substantial influence of macro- and micronutrients and energy balance in affecting the risk of cancer, food can be contaminated with naturally occurring or manmade carcinogens. The burden of cancer from food contaminants has not been estimated, but it is thought to be quite small globally (Stewart and Kleihues, 2003). The best known (and possibly most important) natural carcinogenic contaminants are mycotoxins produced by fungi, the best characterized of which are aflatoxins. Aflatoxins are produced by fungi of the genus Aspergillus, which live on grains and groundnuts (peanuts). Aflatoxins accumulate on these foods particularly if they are stored in hot, moist conditions. Certain aflatoxins and chronic infection with HBV together are the main risk factors for liver cancer in parts of Africa, Asia, and South America. Improved storage conditions can greatly reduce aflatoxin contamination (see Chapter 6).
Food can become contaminated with pesticides and other industrial chemicals either directly or as they move up the food chain. Possibly carcinogenic chemicals are also produced during cooking (e.g., polycyclic aromatic hydrocarbons, N-Nitroso compounds). Any of these could be locally important, but are difficult to detect and quantify, and no broad strategies are available for controlling them.
Medical Drugs
A small number of drugs—often intentionally powerful compounds— have been found to be carcinogenic. The largest group is, ironically, drugs used to treat cancer. Although they increase the risk of a cancer decades later, their immediate benefits outweigh that risk, which may be accepted knowingly. When carcinogenicity has been suspected or proven, other drugs for less serious conditions have been withdrawn (e.g., diethylstilbestrol [DES])
or restricted (e.g., phenacetin). Drugs with hormonal actions may increase some cancer risks (while decreasing others). Drugs with immunosuppressive effects may also increase the risk of certain cancers. The total global cancer burden attributable to medical drugs is thought to be small, and in LMCs, where drug use is limited, probably lower than the global average (Stewart and Kleihues, 2003).
Hormonal and Reproductive Factors
Sex steroid hormones (androgens, estrogens, progestogens) affect the development and growth of certain cancers in women, specifically, cancers of the breast, endometrium, and ovary. While oral contraceptives and hormone replacement therapy contribute to both increased and decreased risks, endogenous hormonal differences related to age at menarche and menopause, and reproductive history also contribute. It is well established that an early age at first birth protects women against cancers of the breast, endometrium, and ovary, and that having no children is associated with higher risks. Even obesity, which alters the circulating levels of various hormones, increases the risk of breast cancer in post-menopausal women, apparently at least in part through this mechanism (Stewart and Kleihues, 2003).
In men, the prostate is responsive to the hormone testosterone, the levels of which may vary and be associated with increased prostate cancer risk. Obesity is also associated with prostate cancer, and at least some of the risk may be mediated by an increase in an insulin-like growth factor (Stewart and Kleihues, 2003).
Risks posed by medical hormones can be reduced, if they are understood. The composition of birth control pills has, in fact, changed markedly both in dosages and combinations of hormones in response to health problems, including cancer, as they were identified. The burden of cancer attributable to these factors must be quite small, however.
Ionizing Radiation from Natural, Industrial, and Medical Sources
Everyone is exposed to ionizing radiation in the form of gamma rays from outer space and from global distribution of manmade gamma and X-radiation from nuclear weapons testing and nuclear reactor accidents (e.g., Chernobyl). Radon gas, which is a natural decay product of uranium from the earth, diffuses through the earth into the atmosphere, where it is ubiquitous. Outdoors, it disperses and is of little consequence. Where radon is concentrated and emerges into buildings that are relatively airtight, the exposure of residents can be substantial. Radon itself is inert, but its decay products include radioactive alpha particles that can lodge in the lung, causing damage near where they are deposited, and resulting in lung cancer
in some people. A recent analysis of the available evidence from studies in Europe concluded that 9 percent of all lung cancer deaths—and 2 percent of all deaths from cancer in Europe—are attributable to residential radon. Most of these deaths are among smokers, whose risk is about 26 times that of nonsmokers (i.e., most of these deaths would be prevented by elimination of exposure to either smoking or radon) (Darby et al., 2005). No estimate of the importance of radon in LMCs has been made. In tropical, lower income countries, where houses are less likely to be tightly enclosed, exposure is likely to be lower than in colder cities. Radon can be largely excluded from buildings by relatively simple measures during construction, and by remedial measures in existing buildings. Most European and other high-income countries have guidelines for doing so (Darby et al., 2001). Although probably not a major problem in most LMCs, it could be locally important in certain areas. It is not a focus of this report, however.
Medical radiation from diagnostic X-rays and radiotherapy represents almost 40 percent of the global human exposure to ionizing radiation worldwide, but is likely to be substantially less in LMCs, where the use of these technologies is far lower.
Ultraviolet (UV) Radiation
Natural UV radiation from sunlight causes most cases of melanoma in the world (about 160,000 per year) (IARC, 2004) about 80 percent of which occur in North America, Europe, Australia, and New Zealand (Stewart and Kleihues, 2003). It is also the cause of most of the more numerous non-melanoma skin cancers, also predominantly in light-skinned populations and generally highly curable by excision. The obvious interventions to prevent UV-related cancer are sun avoidance, protective clothing and sunglasses, and sunscreen, measures that require public awareness and access to the physical items. UV-related cancer is a relatively small problem in most LMCs, and is not considered further in this report. However, some LMCs, e.g., in Eastern and Central Europe, do have significant light-skinned populations and may wish to prioritize UV exposure measures. The U.N. INTERSUN Programme provides comprehensive information and guidance for countries to protect their populations from UV-related cancers (WHO, 2003; 2006b).
Immunosuppression
The body’s immune response plays a role in defending the body against cancer, a concept known mainly because certain cancers occur at a greatly increased prevalence among people whose immune systems are suppressed persistently. Medical drugs and certain infections are the most frequent
immunosuppressants, with infections being more important in LMCs. The best known is HIV, which is associated with Kaposi’s sarcoma, an otherwise rare cancer that is an AIDS-defining condition, and some non-Hodgkin’s lymphomas. Immunosuppressive drugs given to organ transplant patients, to prevent rejection of the organ, are also associated with these cancers and some others. Transplant-associated cancers do not constitute a major problem globally, and are not preventable given current transplant standards. Cancers associated with AIDS, however, are largely preventable with standard antiretroviral therapies. In this case, AIDS control can make a significant contribution to cancer control.
Genetic Susceptibility
An increased risk of developing certain cancers can be inherited in the genetic material passed from generation to generation, accounting for up to 4 percent of all cancers worldwide (Stewart and Kleihues, 2003) (there is no separate estimate for LMCs). In some cases, a specific gene mutation can mean an extraordinarily high risk of cancer: women with certain mutations in the BRCA1 gene have a lifetime risk of about 70 percent of developing either breast or ovarian cancer. Fortunately, these and other mutations with such high penetrance are rare in the population as a whole. About 20 cancer syndromes caused by single gene defects are known. Probably more important, however, are susceptibilities—the genetic details of which are largely unknown—that increase cancer risks by much smaller amounts, but occur widely in the population. It is likely that interactions with the environment that cause additional chance mutations are key in whether cancer develops in people with these genetic traits. Strategies to prevent cancer or detect it early in people with genetic susceptibility are limited currently and do not represent major opportunities for cancer control in LMCs.
BASIC ELEMENTS OF CANCER CONTROL
The basic elements of cancer control are the same everywhere in the world: Cancer planning, cancer prevention and early detection (screening), cancer management—diagnosis and treatment, including palliative care delivered by a multidisciplinary team—and psychosocial support for patients and families. Details of what is most important and what is feasible vary according to the cancer burden and the resources available. The opportunities described in the body of this report include elements of each approach. The basic approaches are described briefly here. They are all discussed in the context of breast cancer in Chapter 4. Discussion of cancer control elements is prefaced by a section about national cancer control planning and programs.
National Cancer Control Planning and Programs
For cancer control to advance systematically, it requires priority setting and budgeting, at a minimum, which require an understanding of the cancer problems that exist in a country and the means available to address them. The process by which this logically occurs is cancer control planning. Because of the differences among countries, cancer control planning and programs must be tailored to individual country situations, recognizing the realities of time and place. In the end, an appropriate cancer plan and program may be limited to a single or a few activities, or can be more comprehensive. Plans and programs should be reviewed every few years and should change as needs and resources dictate.
WHO has been the global leader in promoting and providing guidance for cancer control planning and programs. In 2001, fewer than half of 167 WHO Member States responding to a survey reported having a plan to target cancer or other chronic diseases. Eighty percent of the African countries lacked national plans (Alwan et al., 2001). To address this, WHO produced an updated edition of National Cancer Control Programs: Policy and Managerial Guidelines (WHO, 2002) in 2002. It provides guidance on planning, implementing, managing, and evaluating cancer programs to help policy makers and program managers make the most efficient use of their available resources. It outlines the scientific basis for cancer prevention, early detection, cure, and care; discusses the appropriateness of particular technologies; and describes how to manage national programs tailored to different resource settings.
Building on the 2002 report, a six-volume “how-to” series, Cancer Control: Knowledge Into Action. WHO Guide for Effective Programmes (WHO, forthcoming), will appear in 2006–2007. The impetus for this expanded series is the World Health Assembly resolution on Cancer Prevention and Control. In May 2005, the 192 member countries of the 58th World Health Assembly, the governing body of WHO, approved a resolution that calls on all WHO Member States to develop national cancer programs covering preventive measures, early detection and screening, and improved treatment and palliative care (Box 2-1).
The forthcoming series includes the following modules:
-
Planning: “A practical guide for programme managers on how to plan overall cancer control effectively, according to available resources and integrating cancer control with programmes for other chronic diseases and related problems.”
-
Prevention: “A practical guide for programme managers on how to implement effective cancer prevention by controlling major avoidable cancer risk factors.”
|
BOX 2-1 World Health Organization Resolution on Cancer Prevention and Control (Summary)
|
SOURCE: World Health Assembly (2005). |
-
Early detection: “A practical guide for programme managers on how to implement effective early detection of major types of cancer that are amenable to early diagnosis and screening.”
-
Diagnosis and treatment: “A practical guide for programme managers on how to implement effective cancer diagnosis and treatment, particularly linked to early detection programmes or curable cancers.”
-
Palliative care: “A practical guide for programme managers on how to implement effective palliative care for cancer, with a particular focus on community-based care.”
-
Policy and advocacy: “A practical guide for medium level decision-makers and programme managers on how to advocate for policy development and effective programme implementation for cancer control.”
This Institute of Medicine report endorses the concept of cancer control planning and programs, and the guidance provided by WHO and others (in particular, the International Union Against Cancer, also known as UICC). We endorse the idea that countries revisit their cancer control plans every few years to reassess and fine tune priorities and programs. As with the operational aspects of cancer control, the role of global partners can be key in helping to organize local stakeholders and providing support to begin the process. An example is the current development of a new cancer control plan in Peru (Box 2-2).
The challenge, of course, lies in moving beyond planning to implementation. The process described by WHO, if followed, would involve a wide range of stakeholders—public and private sectors, including the advocacy community—and forward movement toward action.
Cancer Prevention and Early Detection
Preventing cancer from occurring in the first place—primary prevention—is the most definitive way to lessen the burden of cancer. Developing primary prevention strategies requires knowing something about the causes or risk factors associated with the cancer. If the cause or risk factor can be eliminated or reduced, prevention is possible through behavior modification, modification of the environment, or in the case of infectious agents, vaccination or treatment. Not surprisingly, most of the evidence about cancer prevention and early detection relates to high-income countries. Much of this information may be applicable directly to LMCs, although prevention strategies may have to be modified to fit the conditions in those countries. For a thorough review of the world’s literature on cancer prevention and early detection, see the Institute of Medicine report Fulfilling the Potential of Cancer Prevention and Early Detection (IOM, 2003).
|
BOX 2-2 Development of a New National Cancer Control Plan for Peru A new National Cancer Control Plan and program is under development in Peru, a process initiated in 2002 by the Instituto Nacional de Enfermedades Neoplasicas (National Institute for Neoplastic Illnesses, INEN), the central cancer institute for the country (Coalición Multisectorial Peru Contra el Cáncer, 2006). INEN is collaborating with a large number of Peruvian partners as well as the American Cancer Society, U.S. National Cancer Institute, the Pan American Health Organization, and the International Union Against Cancer (also known as UICC). Support from the American Cancer Society has been made available not only to develop the plan, but to help defray staff costs of the program for the first 2 years of start-up. A major task during the early phase is to secure the future of the program by raising internal funds. This arrangement of support and the centralization of the work in a major cancer center is the first such effort of its kind in Latin America (Personal communication, E. Huerta, Cancer Preventorium, Washington Cancer Institute, 2006). |
The one modifiable cause of cancer that dwarfs all others is tobacco use. Fortunately, specific interventions are known to work at reducing tobacco use. Tobacco control is the subject of Chapter 5 of this report. In LMCs, because of the large burden of cancer from infectious agents, prevention through vaccination or treatment is a major focus. When it comes to other preventive measures, much is known about what could be done—eating a healthy diet, maintaining a healthy weight, exercising, not drinking too much alcohol—but how to motivate these changes in people is still a developing field in high-income countries.
Screening for early stages of cancer or precancerous states is the other strategy for reducing the cancer death rate, assuming appropriate management is available when a treatable condition is detected. The cancers for which screening is widely recommended in high-income countries are breast, cervical, and colon. Controversy persists over the value of screening for prostate cancer using the prostate specific antigen (PSA) test. Screening that reaches a substantial proportion of the population requires significant infrastructure for the screening itself as well as the capacity for treatment. Whether or not a country embarks on a screening program will depend on the state of readiness to deal with both aspects—screening and management.
Cancer Management
When cancer is suspected in an individual, either because of a screening test or because of signs and symptoms that lead the person to seek care, a host of services may be needed. Ideally the person would have access to diagnostic services and if a cancer is, in fact, diagnosed, services appropriate to the type and stage of cancer. For some people, this means potentially curative treatment with surgery, radiotherapy, or chemotherapy, or much more frequently, some combination of these modalities, applied by a multidisciplinary medical team working together. Even at early treatable stages, and often as a result of treatment, palliative care for symptom control can be beneficial. For difficult-to-treat cancers, the many cancers of all types not seen until they are advanced beyond probable cure, and other cancers that advance despite treatment, palliative care alone may be most appropriate. Psychosocial services to help deal with the psychological and social impacts of cancer can be appropriate for virtually all people with cancer, and for the survivors of those who die from cancer. These approaches all fall into the “cancer management” category.
Cancer Management: Diagnosis and Staging
An accurate diagnosis is key to receiving appropriate care for cancer. Diagnostic tests include imaging, laboratory, and pathology techniques, in addition to physical examination. The same techniques are applicable to the initial diagnosis and staging and when reassessments are needed to determine a patient’s state at later time points. As is the case for other aspects of cancer management, new and more sophisticated (usually more expensive) diagnostic techniques have been added to those available traditionally, requiring choices to be made where resources are limited. A diagnosis may require one or more tests in sequence.
Imaging includes conventional X-rays, as well as ultrasonography, computed tomography (CT) scanning, and magnetic resonance imaging (MRI). These methods are used to visualize the anatomy of tumors. Two types of nuclear imaging techniques have been added more recently to cancer imaging modalities: Positron emission tomography (PET) and single photon emission computed tomography (SPECT). These techniques detect metabolic activity in cells, and can differentiate cancer cells by their different levels of activity.
Laboratory tests include tests on blood, urine, other fluids, and tissues. Specimens are collected by phlebotomy (blood drawing), fine-needle aspiration cytology or fine-needle-biopsy, and surgical procedures. In addition to tests looking directly for cancerous cells, other types of tests, e.g., to assess liver function or look for tumor markers (biological or chemical compounds
that may increase when cancer is present) can provide information about the status of the cancer.
Pathologic examination for most solid tumors requires surgically excising a sample of the tumor, a biopsy. Cells found in body fluids are also evaluated by pathologic techniques. Microscopic evaluation of the tissue is carried out to determine the size of the tumor, its growth into other tissues and organs, the type of cancer cells, and the grade of the tumor (how closely the cancer cells resemble normal tissue).
Additional information about cancers is found during surgery. Surgical reports describe the size and appearance of tumors and may include observations about lymph nodes and nearby organs.
Cancer Staging
Once a cancer diagnosis is confirmed, further testing (using the same techniques) may be needed to determine the extent of the cancer. This information is captured in “staging systems,” which have developed over time. The principles are common to all cancers, but the details vary depending on the specific cancer. The common elements of staging are:
-
location of primary tumor
-
tumor size and number (if multiple)
-
spread into lymph nodes
-
cell type and grade of tumor (i.e., how closely the cancer cells resemble normal tissue)
-
spread to distant sites (i.e., metastases)
The “TNM” system is a widely used staging system (although not the only one). The UICC is instrumental in updating and disseminating the TNM system. The initials T, N, and M stand for:
-
T, tumor (extent of primary tumor)
-
N, nodes (extent of spread to regional lymph nodes)
-
M, metastases (presence or absence)
Numbers modify each letter indicating the size or extent of the feature (Table 2-3).
A typical example is a breast cancer classified as T3 N2 M0. This refers to a large tumor that has spread to nearby lymph nodes but has not metastasized to other parts of the body. TNM classifications also correspond to numerical stages 0 through IV (expressed as Roman numerals) (Table 2-4).
Another set of terms is also used to denote cancer stage (National Cancer Institute, 2004):
TABLE 2-3 TNM Staging
|
T—Primary Tumor |
|
|
TX |
Primary tumor cannot be evaluated |
|
T0 |
No evidence of primary tumor |
|
Tis |
Carcinoma in situ (early cancer that has not spread to neighboring tissue) |
|
T1, T2, T3, T4 |
Size and/or extent of the primary tumor (increasing from T1 to T4 |
|
N—Regional Lymph Nodes |
|
|
NX |
Regional lymph nodes cannot be evaluated |
|
N0 |
No regional lymph node involvement (no cancer found in the lymph nodes) |
|
N1, N2, N3 |
Involvement of regional lymph nodes (number and/or extent of spread) |
|
M—Distant Metastasis |
|
|
MX |
Distant metastasis cannot be evaluated |
|
M0 |
No distant metastasis (cancer has not spread to other parts of the body) |
|
M1 |
Distant metastasis (cancer has spread to distant parts of the body) |
|
SOURCE: National Cancer Institute (2004). |
|
TABLE 2-4 Cancer Stages
|
Stage |
Definition |
|
0 |
Carcinoma in situ (early cancer that is present only in the layer of cells in which it began). |
|
I, II, III |
Higher numbers indicate more extensive disease: greater tumor size, and/or spread of the cancer to nearby lymph nodes and/or organs adjacent to the primary tumor. |
|
IV |
The cancer has spread to another organ. |
|
SOURCE: National Cancer Institute (2004). |
|
-
In situ cancer is confined to the layer of cells in which it arose
-
Localized cancer is limited to the organ in which it arose
-
Regional cancer has spread beyond the primary site to nearly lymph nodes, or organs or tissues
-
Distant cancer has spread from the primary site to distant parts of the body
Special classification systems are used for certain types of cancer, including cancers of the brain and spinal cord, leukemias and lymphomas, and other cancers in some circumstances (e.g., childhood cancers).
Cancer Management: Surgery
Surgery was the earliest form of cancer treatment and remains its mainstay where the range of cancer treatment is available. Until the middle of the 20th century, when chemotherapy and radiotherapy were developed as treatment modalities, surgical resection of tumors was the only available approach. For solid tumors today, long-term survival is usually dependent on surgical removal of the primary tumor (and a margin of normal tissue) and regional lymph nodes, often with additional treatment modalities. In the United States, about 90 percent of cures of solid tumors are through surgery alone or with other modalities. Cancers in which surgical resection is a major factor in cure include melanomas and cancers of the breast, colon, rectum, thyroid, stomach, and lung (Fleming et al., 1995). The surgeries involved range from basic to highly complex, which bears on the types of settings in which they can be performed. The trend is toward less radical surgery than previously, using radiotherapy and/or chemotherapy to augment surgery. For example, bone or soft-tissue sarcomas of the extremities used to be treated by surgical amputation of the affected limb. Now, limbs are routinely spared by adding other treatment modalities to more conservative surgery.
Higher technology surgical techniques are common where resources are available. This includes the use of laparoscopic surgery and the extensive use of imaging, such as ultrasound, during surgery.
Not all surgery is done with curative intent (although intent might not be known until surgery is begun). When complete removal of a tumor is not possible, surgery is often still used to reduce (debulk) the tumor, which can prolong life and in some cases reduce symptoms (e.g., if the tumor is interfering with vital functions).
Surgical Settings
Debas and colleagues (Debas et al., 2006) have described a “coordinated model system for surgical care” appropriate for LMCs, consisting of community clinics, district hospitals, and tertiary hospitals. They also analyzed, for the first time, the burden of disease (expressed as disability-adjusted life years) of surgical conditions, defined as conditions requiring surgery for management. The analysis was based on expert opinion of 18 surgeons in different parts of the world. “Surgical DALYs” attributed to cancer are less important as a percentage of total surgical DALYs in LMCs than in high-income countries, but still represent a substantial fraction. In Europe, malignancies are the leading cause of surgical DALYs, at 36 percent, and in the Americas (a mixture of mainly high- and middle-income countries), at 22 percent; in Africa, 8 percent. The level of surgery (i.e., the complexity) is not captured in these estimates. How much could realistically be addressed would have to be critically assessed in each country.
Cancer Management: Radiotherapy
Radiotherapy refers to the application of ionizing radiation (X-rays, γ-rays [gamma rays], or radioactive particles) for treatment. Radiation oncology is the medical discipline of treating malignant disease with radiation. Radiotherapy can be used curatively, as a single modality, or in conjunction with surgery, chemotherapy, or both. It can also relieve symptoms (palliate) in patients with incurable cancer. Radiotherapy has some limited medical uses in noncancerous conditions (e.g., keloid or “heaped-up” scars), but it is overwhelmingly a cancer treatment modality.
How Radiotherapy Works
Different types of cells—normal and malignant—vary in their susceptibility to ionizing radiation. Clinical radiotherapy schedules are designed to exploit the differences between normal tissues and tumors, so that as many malignant cells as possible are killed, while damage to normal tissue is minimized. In radical curative treatments, total radiation doses may be close to the tolerance of normal tissues. In palliative treatments, lower doses are the norm.
Some tumors, such as seminoma of the testis and lymphoma, are very sensitive to radiotherapy and can be cured with relatively low doses. Others, such as glioblastoma multiforme in the brain, are notoriously resistant, even to large doses.
A course of radiotherapy may be spread over days or weeks. This is known as fractionating, and the radiation delivered to a patient in a single
treatment session is called a fraction. Fractionating allows normal tissues to repair much of the radiation damage, while tumor cells, which are less efficient at repair, do not recover. Each fraction of radiotherapy kills a certain proportion of the cancer cells in the irradiated region. A beam of radiation is called a field. A fraction consists of one or more fields delivered sequentially.
External-beam radiotherapy can be delivered by cobalt machines or linear accelerators (“linacs”), collectively known as “megavoltage machines.” Cobalt machines and linacs deliver very high-energy, highly focused beams that can reach deeper tumor tissues while depositing relatively small doses in the normal tissues through which they pass. Linacs produce the same intensity of radiation throughout operation, but the machines are more complex than cobalt machines and require greater manpower and attention to maintain them. These factors make cobalt machines—with replaceable cobalt sources—more appropriate in many LMCs. As the cobalt source decays, treatment time increases, decreasing the number of patients that can be treated per day. The half-life of cobalt is 5.3 years, so a source that is 5.3 years old will take twice as long to deliver the prescribed dose to a patient as a new source. An 11-year-old source will take four times as long as a new source to deliver the same dose.
Side Effects of Radiotherapy
Both early (acute) and late (chronic) side effects can occur after radiotherapy. The occurrence and severity depend on the body site being treated, the volume of normal tissue irradiated (the larger the volume, the higher the risk and severity of side effects), the total dose, and the rate of dose accumulation (the amount per week).
Early side effects result from damage to proliferating tissues (i.e., cells that continually divide and replace old cells with new ones) such as the lining of the gastrointestinal tract, or the skin. For example, radiotherapy to an abdominal tumor may damage the mucosa of the small bowel, causing malabsorption and diarrhea, but most patients recover completely within a few weeks when new cells have replaced the damaged ones.
Late reactions, which are much less common than acute side effects, occur months or years after treatment ends. They may result from damage to nonproliferating differentiated tissues, which cannot compensate for cell death by dividing to replace lost cells. These effects may be difficult to reverse and can be permanent or progressive. Examples include fibrosis of the skin, spinal cord damage, scarring of the lungs, and radiation-induced liver disease.
Side effects can be minimized by meticulous planning and delivery of radiotherapy. Late-reacting tissues are particularly sensitive to the size of
each radiation dose, so they can be protected by giving a greater number of smaller fractions of radiation, provided the total dose is not too high.
Second cancers are an even rarer type of late effect. Especially in children, even relatively low doses of radiation increase the risk of developing another malignancy, unrelated to the one that was treated originally. Leukemias appear on average 7 years after exposure and solid tumors after 10 or 20 years.
Curative Radiotherapy
In high-income countries, at least half of all cancer patients treated with radiotherapy—alone or with surgery, chemotherapy, or both—are treated with the goal of achieving a cure. Radiotherapy is used by itself when it has the highest cure rate or because it is likely to have fewer side effects. Examples include treatment of advanced cervical cancer, pituitary tumors, deep-seated gliomas, nasopharyngeal cancer, and early-stage, low-grade lymphomas, including Hodgkin’s lymphoma.
Radiotherapy is preferred over surgery when surgery will result in the loss of an organ and the control of the tumor is similar. Examples include laryngeal cancer and prostate cancer. Surgery alone can be effective for small tumors. For large tumors, radiotherapy is often used with surgery to reduce tumor size or reduce the risk of tumor recurrence so that the whole tumor site can be treated with the least effect on the patient’s normal functioning. In general, radiotherapy is used along with surgery when:
-
Organ preservation is desirable; an example is breast-conserving surgery (lumpectomy);
-
The tumor is advanced with a high risk of local recurrence after surgery, such as rectal cancer;
-
An inoperable cancer can be rendered operable, such as when advanced rectal cancers that are adhered to other organs, preventing complete excision, can be shrunk by radiation;
-
The surgery included too small a margin of normal tissue around the tumor to preclude a high likelihood of local recurrence; radiotherapy reduces that likelihood.
Chemotherapy may improve the results of radiotherapy in the treatment of some cancers (Table 2-5). Radiotherapy can treat large primary tumors, and chemotherapy can work on disseminated micrometastases. The dose-limiting toxicities of radiotherapy and chemotherapy are different, which means that it is possible to deliver a higher overall dose of “treatment” with the two modalities than with one or the other. Other mechanisms are
TABLE 2-5 Beneficial Interactions Between Radiotherapy and Chemotherapy
|
Mechanism |
Benefits |
Examples of Cancer |
|
Spatial cooperation |
Radiotherapy cures the high-volume local cancer and chemotherapy cures micrometastases |
Hodgkin’s lymphoma Rectal cancer |
|
Independent toxicity |
Because radiotherapy and chemotherapy have different dose-limiting toxicities, it is possible to deliver a higher antitumor dose with fewer side effects than with radiotherapy alone |
Cervical cancer Esophageal cancer |
|
Enhanced tumor response |
Even if the effects of radiotherapy and chemotherapy are only additive, the steep dose response of tumors means that there can be greater rates of cure than with radiotherapy by itself |
Anal cancer |
|
Protection of normal tissues |
Some dose-limiting normal tissues can be protected by chemical modifiers such as amifostine, resulting in fewer side effects |
Head and neck cancers |
|
SOURCE: Barton et al. (2006).Barton et al. (2006). |
||
enhanced tumor response with two modalities, and the use of chemical agents to protect normal tissues from radiation damage, allowing a greater radiation dose.
Palliative Radiotherapy
Growing tumors cause symptoms by their physical presence, e.g., by pressing on adjacent organs or blocking passages or orifices. Radiotherapy can be used to shrink tumors directly causing symptoms. In many cases, it may reduce or eliminate the need for analgesics (including opioids).
Radiotherapy is effective for people with incurable lung cancer, alleviating shortness of breath, cough, and hemoptysis (coughing up blood). For breast cancer, radiotherapy can control fungating masses (large, rapidly growing tissue), and for prostate cancer it can be used to relieve urinary obstruction. Short-course radiotherapy (sometimes just a single treatment, or for more extensive disease, a few treatments) is effective in relieving pain from bone and brain metastases and compression of the spinal cord and various nerves (Roos et al., 2005). Radiotherapy can reverse the effects of spinal cord compression and prevent paraplegia.
With longer courses than those used for symptom relief, radiotherapy can prolong life for patients with some incurable cancers such as high-grade gliomas (Laperriere and Bernstein, 1994).
Infrastructure Needs and Costs of Radiotherapy
Providing a safe and effective radiation oncology service requires an initial capital investment in radiotherapy equipment and specially designed buildings, as well as an ongoing investment in consumable items and maintenance of the equipment; an expert team of doctors, therapists, and physicists; and good access to engineering support. The necessary medical, scientific, and technical expertise is in short supply in many countries and is an even bigger constraint in many LMCs than the shortage of radiotherapy equipment. A shortage of trained staff may limit the number of patients who can be treated, to the point of underutilization of even the existing scarce equipment (Tatsuzaki and Levin, 2001; Radiation Oncology Inquiry, 2002).
The introduction or expansion of radiation oncology services in any health care system inevitably has implications for other services. These include surgical and medical oncology, pathology, imaging, general and specialist medical and surgical services, and nursing and psychosocial support services.
The costs of radiotherapy include the capital costs of the building and equipment, maintenance costs, and staff salaries. Buildings can be relatively expensive, but are durable, so amortized costs are small. Cobalt machines are considerably cheaper than linacs because they are mechanically and electronically simpler. Capital costs also include equipment for planning treatment, including simulators and computers. Staff costs are for radiation oncologists, physicists, and technologists, each of whom is necessary to assess and treat patients.
The cost of establishing a radiotherapy facility in an LMC is about $1 million. If used 12 hours per day, it could deliver half a million doses of radiotherapy over its lifetime, with an amortized cost of about $2 per fraction. Adding the costs of consumables and salaries, each fraction of radiotherapy would cost a few dollars in an LMC. In a study of 11 countries of differing economic status, median costs per treatment dose of radiotherapy were US$11 for linear accelerators and US$4.87 for cobalt machines, with a range from US$1.29 to US$39.59 (Van Der Giessen et al., 2004). One fraction is often enough for producing pain relief for several weeks or months, while 20 to 40 fractions are typically required for curing a cancer such as laryngeal cancer. Radiotherapy is clearly a beneficial technology but is limited in where it can be delivered, in addition to limitations imposed by resources. In general, facilities are limited to urban areas with infrastructure and transportation, and may never be accessible to the largely rural population of much of the developing world.
Cancer Management: Chemotherapy
Chemotherapy refers broadly to the use of drugs to treat cancer with the intention of producing long-term survival (or “cure”), or at least a substantial increase in the length and possibly quality of life. (Drugs that improve the quality of life of cancer patients during their illness and at the end of life by controlling pain and other symptoms are considered separately in the discussion of palliative care in Chapter 8.) Medical oncologists are the medical professionals trained in the use of chemotherapy.
“Cytotoxic” drugs kill cancer cells by several mechanisms. Other drugs—referred to more specifically as hormonal therapy—add, block, or remove hormones to slow or stop the growth of certain cancers (mainly cancers of the breast and prostate). Typically, drugs are used in combinations, not infrequently including three or four drugs, given on a schedule that may be months long. Hormonal therapy with tamoxifen, for breast cancer, may be taken for at least several years. The main classes of cancer chemotherapy drugs are listed in Table 2-6.
WHO Model List of Essential Medicines
The WHO Model List of Essential Medicines was developed and is periodically revised as a guide for the development of national and local essential medicine lists that “satisfy the priority health care needs of the population” (WHO, 2006a), tailored to each specific situation. The list, organized by category of use, includes a core list and a complementary list. The core list includes the “minimum medicine needs for a basic health care system.” The most efficacious, safe, and cost-effective medicines for “priority conditions”—selected on the basis of current and estimated future public health relevance, and potential for safe and cost-effective treatment—are included. Cancer chemotherapeutic agents are on a complementary list (Table 2-7), acknowledging that cancer is a priority condition, but that “specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training” are needed for the appropriate use of the drugs. A full menu of drugs for palliative care, however, is on the core list (see Chapter 7). These are, according to WHO, “intended to be available within the context of functioning health systems at all times in adequate amounts … at a price the individual and the community can afford” (WHO, 2006a). Countries, of course, are free to select the drugs that best meet their needs and budgets, and are available to them. What is actually available may differ considerably from the WHO lists.
TABLE 2-6 Major Classes of Chemotherapeutic Drugs for Cancer
|
Drug Type |
Mode of Action and Examples |
|
Alkylating agents |
|
|
Nitrosoureas |
|
|
Antimetabolites |
|
|
Antitumor antibiotics |
|
|
Plant (vinca) alkaloids |
|
|
Hormonal agents |
|
The Development of Cancer Chemotherapy
Development of chemotherapy began in the 1940s and progressed rapidly through the 1960s. Leukemias and lymphomas were the first major classes of cancer to respond to chemotherapy. Early successes led to extensive screening programs of agents both of biological origin (e.g., plants and sea creatures) and synthesized molecules to find agents with anticancer properties. Early on, it was discovered that cancers easily develop resistance to single agents, leading to the common use of drugs in combination, and a continuing search for more effective combinations. “Responses” to single and multiple agents, leading to a temporary reprieve from the cancer, which may last months or longer, are always more common than long-term free-
TABLE 2-7 WHO Model List of Essential Medicines: Antineoplastic Drugs
|
Cytotoxic Medicines (complementary lista) |
|
|
Asparaginase |
Powder for injection |
|
Bleomycin |
Powder for injection |
|
Calcium folinate |
Tablet; injectable liquid |
|
Chlorambucil |
Tablet |
|
Chlormethine |
Powder for injection |
|
Cisplatin |
Powder for injection |
|
Cyclophosphamide |
Tablet; powder for injection |
|
Cytarabine |
Powder for injection |
|
Dacarbazine |
Powder for injection |
|
Dactinomycin |
Powder for injection |
|
Daunorubicin |
Powder for injection |
|
Doxorubicin |
Powder for injection |
|
Etoposide |
Capsule; injectable liquid |
|
Fluorouracil |
Injectable liquid |
|
Levamisole |
Tablet |
|
Mercaptopurine |
Tablet |
|
Methotrexate |
Tablet; powder for injection |
|
Procarbazine |
Capsule |
|
Vinblastine |
Powder for injection |
|
Vincristine |
Powder for injection |
|
Hormones and Antihormones (complementary lista) |
|
|
Dexamethasone |
Injectable liquid |
|
Hydrocortisone |
Powder for injection |
|
Prednisolone |
Tablet |
|
Tamoxifen |
Tablet |
|
Medicines Used in Palliative Careb |
|
|
The WHO Expert Committee on the Selection and Use of Essential Medicines recommended that all the drugs mentioned in the second edition of the WHO publication Cancer Pain Relief (WHO, 1996) be considered essential. The drugs are included in the relevant sections of the Model List, according to their therapeutic use, such as analgesics. |
|
|
aThe complementary list includes essential medicines for priority diseases for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed. bSee Chapter 7, “Palliative Care.” SOURCE: Reprinted, with permission, from WHO (2005b). Copyright 2005 by WHO. |
|
dom (“cure”) from cancer after treatment. A caveat of particular importance in LMCs is that chemotherapy (and other treatments) are more effective in the earlier stages of cancer. Cancers that have spread only locally are often curable; those with distant metastases are much less so (with some exceptions). In this discussion, effective treatment refers mainly to earlier stage cancers. While palliative care and pain control medications can be beneficial
to patients with late-stage cancers, even the most effective chemotherapy is unlikely to prolong life significantly.
Through the 1980s, effective drugs for testicular cancer and other malignancies of children and young adults were found. Many of these treatments involve only drugs. Drugs are also used as an “adjuvant” to cancer surgery. Adjuvant chemotherapy is given either before or after surgery (or both) to kill both remaining cancer cells at the primary site and cells that are circulating or lodged at distant sites where, left unchecked, they may proliferate into metastatic lesions. Successful adjuvant treatments that produce long-term survival in a large proportion of patients are available for some cancers, including cancers of the breast and colon, among the common cancers. For other common cancers, including cancers of the liver, pancreas, brain, and melanomas, no currently available drugs produce large benefits for a large proportion of patients. For still others, such as stomach cancer, the diagnosis is most often made (outside of screening programs) at a late stage, when treatment is unlikely to be effective. A new era has begun with the advent of “targeted” agents for cancer chemotherapy (Box 2-3).
Delivery of Chemotherapy
The way chemotherapy is administered has a bearing on the settings in which it can be given. The most common routes of administration are by mouth, intravenously, intramuscularly, and topically. Less commonly, drugs are infused directly into body cavities, such as the abdomen (intraperitoneal), the lung (intrapleural), or the central nervous system (intrathecal). It is not uncommon for more than one route to be used for a chemotherapy regimen that involves several drugs. Depending on the route of administration and other factors (e.g., need for monitoring), treatments can be given in a medical office or clinic, or in a hospital as an inpatient or outpatient. Laboratory tests are usually carried out periodically to monitor the blood and organs for side effects (the specifics depend on the cancer and the treatment).
Treatment regimens not uncommonly stretch over 6 months or more, with treatments occurring in cycles (periods of active treatment and rest). In a cycle, treatment may be daily, weekly, or at some other interval.
Side Effects
The predominant strategy with cytotoxic drugs is to give them at the maximum possible total dosage, but in individual increments and over time. Experience in clinical trials over decades has shown that success rates are best with highest doses. However, dose intensity is limited by side effects that occur because of damage to normal tissues. Certain side effects—such as
|
BOX 2-3 Targeted Cancer Therapies Cytotoxic cancer drugs exploit the rapid growth and division of cancer cells compared with normal cells, preferentially killing cancer cells. But cancer cells may not differ so much from normal cells, so significant damage to normal tissue may occur. Newer “targeted” therapies block cancer cells’ ability to grow, divide, repair, and/or communicate with other cells by interfering with specific molecules associated with cancer cells, but not found on normal cells (or found in very small numbers). The successful targeted therapy homes in on the cancer-specific target. There are, theoretically, many targets in the pathway of a single cancer cell, involved with the development, growth, and spread of cancer. Unlike traditional cancer drugs, targeted therapies are taken over a long period of time, possibly for life. In the United States, the dozen or so approved targeted therapies can cost thousands or tens of thousands of dollars per year. Targeted therapies can be a variety of drug types. Some synthetic small molecule drugs work inside cancer cells to disrupt their function. Monoclonal antibodies target receptors on the cell surface. Antiangiogenesis agents block formation of blood vessels within tumors, causing the cells to die for lack of oxygen. Many of the targeted therapies that have been or are being developed target proteins that are involved in cell signalling, interfering with the molecular signals that instruct cells to grow and divide as cancer cells. These include Herceptin (trastuzumab), a monoclonal antibody; the small molecules Gleevec (imatinib) for some types of myelogenous leukemia (CML) and gastrointestinal stromal tumors; and Iressa (erlotinib), for some cases of lung cancer. Other targeted therapies cause cancer cells to die (undergo apoptosis), including Velcade (bortezomib) to treat multiple myeloma. Avastin (bevacizumab) blocks the formation of blood vessels. About a dozen targeted therapies have been approved in one or another high-income country, most for limited indications. Many more are in clinical trials. The hope is that eventually, an individual’s tumor will be characterized by the particular set of molecular markers present, and treatments will be targeted to interact with them. The specificity of the interactions should mean less damage to normal tissue, hence, fewer side effects. |
hair loss—can be distressing, but are usually temporary. More serious are effects on proliferating cells in the digestive tract, bone marrow, or elsewhere, which may result in suppression of blood cell formation, debilitating nausea, and other effects (Stewart and Kleihues, 2003). Little information is avail-
able on the impacts of chemotherapy on patients in LMCs, who may have different profiles than those in high-income countries in their co-morbidities and nutritional status, at a minimum.
Implications for LMCs
The facilities and trained personnel for prescribing an appropriate regimen, for administering chemotherapy, for conducting laboratory testing, and for managing side effects are bare necessities where chemotherapy is going to be used. The protracted schedule of chemotherapy regimens means that patients must be able to get to the treatment site and have financial access to treatment. A reliable supply of drugs, which in most LMCs must be imported, is implicit. Based on information assembled for this report, these conditions cannot be met in a large proportion of LMCs. A global view is not available, but in Latin America and the Caribbean, chemotherapy drugs are often not available, out-of-pocket costs for patients are high because few governments cover the costs, and there are few public cancer centers (Eisenchlas, 2006). We know from pediatric cancer treatment experiences in LMCs around the globe that abandonment of treatment—stopping before a long regimen is complete—is among the top problems identified (see Chapter 6).
Cancer Management: Psychosocial Services
People with cancer may experience psychosocial distress at any point from diagnosis through treatment, during advanced illness, and even during long-term survival. Psychosocial distress is defined as an unpleasant emotional experience that may be psychological, social, or spiritual in nature. These feelings may become severe and disabling, and may result eventually in a diagnosis of major depression. Transition points in treatment (time of diagnosis, awaiting treatment, completion of treatment) often trigger worsening distress (IOM, 2004). Psychosocial support enables patients with cancer to cope and deal with the disease, its impact, and with life after cancer. This form of support can be made available at all stages of the cancer experience—at diagnosis, during treatment, and beyond, including during palliative care. Support may also be needed by family members and others close to the patient.
Psychosocial interventions are accepted as an essential component of cancer care, although they are often not well developed. Little information is available about these services in LMCs. At the most basic level, people with cancer need help coping with their illness through personal interaction and empathy from caregivers and other knowledgeable individuals. Social and emotional support focuses on adjusting to the diagnosis, apprehension
regarding treatment, and existential concerns (IOM, 2004). In addition to information about the treatment, a wide range of specific interventions have been described, including the following:
-
Psychotherapeutic interventions, including brief crisis counseling, group therapy and counseling, pastoral counseling, family therapy and counseling, grief therapy, and sexual counseling
-
Psychopharmacologic interventions
-
Complementary therapies, such as yoga, massage, exercise, acupuncture, art, music, and dance therapy, and others
A variety of providers can deliver psychosocial services. In many places, nurses are on the front lines of cancer care, and this includes psychosocial support. Physicians, including primary care physicians and specialists, also can provide support, if the patient has access to them. Social workers, psychologists, counselors, and religious workers also can give the needed support. Patient support groups organized by cancer survivors are among the most common sources of support. Community-based traditional resource systems can be tapped to offer psychosocial support to patients and family members.
Reach to Recovery International (RRI) is an example of psychosocial support from breast cancer survivors, under the auspices of the International Union Against Cancer. RRI affiliates have been established in every region of the world, but are all run locally. The program is built on the premise that one woman who has experienced breast cancer herself and received specialized training gives of her time and experience to support another woman facing the same challenges.
The types of services most needed by cancer patients are likely to be similar around the world, but the providers who are available and have sufficient training will vary according to the health care system, as will the resources available, and other social and cultural factors.
SUMMARY AND RECOMMENDATION
The review of causes and risk factors and of cancer control elements sets the stage for the latter chapters of this report, where the greatest opportunities for cancer control are identified. What comes from this review is that a few causes and risk factors are prominent in cancers common in LMCs: tobacco use; infectious agents, particularly hepatitis viruses, HPV, and H. pylori; and dietary factors, too little exercise, and too much body mass. A lot of factors have smaller, yet not insignificant, effects. The factors vary with how easily they can be modified. Tobacco smoking is addictive and difficult for people to stop once started, yet certain interventions are
effective (more in Chapter 5). We have the tools now to prevent nearly all hepatitis B and most cancer-causing HPV infections. Changing behavior related to diet and exercise has proved exceptionally challenging in the high-income countries where work has been done, and there is little to offer in this regard to LMCs at the moment. In 10 years, new knowledge about behavior change and about the causes of cancer could allow greater scope for direct intervention to prevent cancers.
Cancer management—the mix of treatment and support interventions for people with cancer—has been developed and continues to evolve in high-income countries. While all the elements of cancer management could be applied in LMCs (not only for the rich minorities in LMCs), they may need modification for best effect (as basic as adjusted doses of chemotherapy for populations with different co-morbidities and nutritional status). However, in all cases, they require careful consideration of the circumstances in which they are being used, which may be vastly different from those in high-income countries. The point is made that cancer management often requires multiple interventions, necessitating a mix of medical expertise and support services, and appropriate facilities. The discussion of how this can best be accomplished continues in Chapter 8, which is about cancer centers in LMCs.
Cancer prevention includes an array of activities, some outside the health care system (e.g., increasing tobacco taxes), some in the primary care system (e.g., infant vaccination against HBV), and others under more specialized conditions (e.g., screening for precancerous changes in the cervix). Which prevention activities any country can and will adopt will depend on their specific circumstances.
These decisions and others about cancer control at a national level are best approached through a formal process that weighs the opportunities against the costs within the country context. National cancer control planning and the development of national cancer control programs is the obvious means for making such decisions. In this area, we defer the details to WHO, UICC, and other organizations that provide guidelines and recommendations referenced in this chapter. No other specific recommendations come out of this chapter directly, but the discussion here leads to recommendations in the remainder of the report.
RECOMMENDATION 2-1. Cancer control plans should be developed, or updated, in each country every 3 to 5 years through a process that involves all major stakeholders, public and private sectors, as described by WHO, UICC, and others. Cancer control plans should be promoted and supported financially and programmatically through both government action and public advocacy.
In both the planning and implementation phases, global partners should provide necessary guidance and financial support.
REFERENCES
Alwan A, Maclean D, Mandil A. 2001. Assessment of National Capacity for Noncommunicable Disease Prevention and Control; the Report of a Global Survey. Geneva, Switzerland: World Health Organization.
Barton MB, Frommer M, Shafiq J. 2006. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncology 7(7):584–595.
Bruce N, Rehfuess E, Mehta S, Hutton G, Smith K. 2006. Indoor air pollution. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press. Pp. 793–815.
Coalición Multisectorial Peru Contra el Cáncer. 2006. Documento De Consenso. Lima, Peru: Ministerio de Salud.
Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Makelainen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Rosario AS, Tirmarche M, Tomasek L, Whitley E, Wichmann HE, Doll R. 2005. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 330(7485):223.
Darby S, Hill D, Doll R. 2001. Radon: A likely carcinogen at all exposures. Annals of Oncology 12(10):1341–1351.
Debas HT, Gosselin R, McCord C, Thind A. 2006. Surgery. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press. Pp. 1245–1259.
Eisenchlas J. 2006. Cancer Prevention and Management in Latin America. Unpublished paper commissioned by IOM.
Epping-Jordan JE, Galea G, Tukuitonga C, Beaglehole R. 2005. Preventing chronic diseases: Taking stepwise action. Lancet 366(9497):1667–1671.
Fleming ID, Brady LW, Mieszkalski GB, Cooper MR.1995. Basis for major current therapies for cancer. In: Murphy GP, Lawrence W, Lenhard RE, eds. American Cancer Society Textbook of Clinical Oncology. 2nd ed. Atlanta, GA: American Cancer Society. Pp. 96 ff.
IARC (International Agency for Research on Cancer). 1997. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter pylori. Lyon, France: IARC.
IARC. 2004. GLOBOCAN 2002. Lyon, France: IARC.
IOM (Institute of Medicine). 2003. Fulfilling the Potential of Cancer Prevention and Early Detection. Curry SJ, Byers T, Hewitt M, eds. Washington, DC: The National Academies Press.
IOM. 2004. Meeting Psychosocial Needs of Women with Breast Cancer. Hewitt M, Herdman R, Holland J, eds. Washington, DC: The National Academies Press.
Laperriere NJ, Bernstein M. 1994. Radiotherapy for brain tumors. CA: A Cancer Journal for Clinicians 44(2):96–108.
Lopez AD, Collishaw NE, Piha T. 1994. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control 1994(3):242–247.
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, eds. 2006. Global Burden of Disease and Risk Factors. New York: Oxford University Press.
National Cancer Institute. 2004. Staging: Questions and Answers. [Online] Available: http://www.cancer.gov/cancertopics/factsheet/Detection/staging [accessed October 17, 2006].
Parkin DM. 2005. The global health burden of infection-associated cancers in the year 2002. International Journal of Cancer 118 (12):3030–3044.
Radiation Oncology Inquiry. 2002. A Vision for Radiotherapy. Canberra, Australia: Commonwealth of Australia.
Rehm J, Chisholm D, Room R, Lopez AD. 2006. Alcohol. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press. Pp. 887–906.
Roos DE, Turner SL, O’Brien PC, Smith JG, Spry NA, Burmeister BH, Hoskin PJ, Ball DL, Trans-Tasman Radiation Oncology Group. 2005. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases. Radiotherapy & Oncology 75(1):54–63.
Rosenstock L, Cullen M, Fingerhut M. 2006. Occupational health. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press. Pp. 1127–1145.
Stewart BW, Kleihues P. 2003. World Cancer Report. Lyon, France: IARC Press.
Tatsuzaki H, Levin CV. 2001. Quantitative status of resources for radiation therapy in Asia and Pacific region. Radiotherapy & Oncology 60(1):81–89.
Van Der Giessen PH, Alert J, Badri C, Bistrovic M, Deshpande D, Kardamakis D, Van Der Merwe D, Da Motta N, Pinillos L, Sajjad R, Tian Y, Levin V. 2004. Multinational assessment of some operational costs of teletherapy. Radiotherapy & Oncology 71(3):347–355.
World Health Assembly. 2005. Cancer prevention and control. WHA Resolution 58.22. Geneva, Switzerland: WHO.
WHO (World Health Organization). 1996. Cancer Pain Relief, 2nd edition. Geneva, Switzerland: WHO.
WHO. 2002. National Cancer Control Programmes: Policies and Managerial Guidelines, 2nd edition. Geneva, Switzerland: WHO.
WHO. 2003. INTERSUN: The Global UV Project, A Guide and Compendium. Geneva, Switzerland: WHO.
WHO. 2004. Global Strategy on Diet, Physical Activity and Health. [Online]. Available: http://www.who.int/dietphysicalactivity/goals/en/ [accessed October 17, 2006].
WHO. 2005a. Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: WHO.
WHO. 2005b. Essential Medicines: WHO Model List, 14th edition. [Online]. Available: http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf [accessed January 28, 2006].
WHO. 2006a. Essential Medicines. [Online]. Available: http://www.who.int/medicines/services/essmedicines_def/en/index.html [accessed July 5, 2006].
WHO. 2006b. Ultraviolet Radiation and the INTERSUN Programme. [Online]. Available: http://www.who.int/uv/intersunprogramme/en/ [accessed October 16, 2006].
WHO. Forthcoming. Cancer Control: Knowledge Into Action. WHO Guide for Effective Programmes (Six Volumes). Geneva, Switzerland: World Health Organization.
World Cancer Research Fund. 1997. Food, Nutrition, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research.










































