I
—The Chemistry of Carbon
The chemistry of carbon is richly variable and serves as the basis for life on Earth. Away from Earth, in the solar system and beyond, carbon and its compounds are abundant. Indeed, the range of chemical structures found on and off Earth are virtually countless. The variations in structure provide evidence of conditions that prevailed at times before Earth was formed and at cosmic distances. They record events that occurred during the formation of the solar system and during the division of materials among planets. In that sense, variations in the chemical structures of carbon compounds are some of our ultimate historical documents. The exploration and decoding of the carbon-chemical record are providing some of the best information about relationships between our planet, the solar system, and the cosmos. A major challenge, however, is to be able to differentiate biotic from abiotic molecules.
1
Biotic and Abiotic Carbon Compounds
CARBON COMPOUNDS—DEFINITIONS AND CHARACTERISTICS
Historically, chemists have referred to all compounds of carbon except the oxides, carbonates (e.g., limestone and marble), metallic carbides, and elemental forms (e.g., diamond and graphite) as “organic.” But, despite the term, organic molecules, even elaborate ones, are not necessarily produced biotically. Discussed below are several important characteristics of organic molecules that, as we shall see, are pertinent to the problem of trying to differentiate between biotic and abiotic origin:
-
Structural stability over wide ranges of temperature and pressure;
-
Aromaticity;
-
Aliphatic homologs;
-
Stereoisomerism; and
-
Structural complexity.
These characteristics are discussed in detail in subsequent sections.
Structural Stability
Carbon atoms can bond strongly to each other. Chains of atoms can extend indefinitely and, because each atom can form four bonds, with multiple branches. Chains can bend around to form rings, and rings can be fused to form sheets of atoms for which the bonding diagrams look like chicken wire. In such cases, the four bonds at each carbon atom are often arranged to provide double or even triple linkages (see the next section, “Aromaticity”). Such materials—and many smaller, simpler molecules—are capable of outliving our planet. If they are tucked away in some bit of space rock or ice, they are literally waiting to be discovered and interrogated. The chemical structure of a carbon-containing molecule (the bonding pattern and the other chemical elements that are present) provides information about the last time that molecule was warm enough to rearrange spontaneously or to react with another molecule. Depending on the molecule in question, “warm” might mean some temperature more than a hundred degrees below water’s freezing point or some temperature above the melting point of metallic zinc. The conditions monitored would be as follows:
-
What other chemical elements and compounds were present?
-
What was the pressure?
-
What mineral surfaces were available?
-
What sort of electromagnetic radiation was present?
Knowing the limits on conditions could provide clues that might reveal a biotic or an abiotic origin for compounds sampled.
Aromaticity
One of the most important characteristics of organic compounds is their ability to form molecules with a distinctive stability resulting from aromaticity, with the electrons of neighboring carbon atoms shared “in resonance.” An aromatic compound is one in which at least two equivalent structures exist. Benzene is the simplest example (Figure 1.1).
Aromatic compounds are particularly unreactive, or stable, and despite the name are not necessarily volatile. Benzene rings are easily elaborated into more complex, polycyclic structures by the one-ring build-up mechanism.1 Polycyclic aromatic hydrocarbons (PAHs) are common structural components of dyes, soot from combustion, and interstellar particles. Some simple examples are shown in Figure 1.2. PAHs in nature, particularly those in interstellar particles, can include hundreds of benzene rings fused so that most share all of their sides.
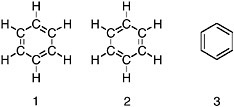
FIGURE 1.1 Three alternative views of the chemical structure of benzene, C6H6. In all structure diagrams, the lines represent chemical bonds. The letters C and H represent atoms of carbon and hydrogen, respectively. Structures 1 and 2 are identical except that the double bonds between the carbon atoms are rearranged. Because the locations of the atoms are unchanged even though the bonds have moved, the structures are said to be “in resonance,” and the actual structure of benzene is understood to be a combination of these two representations. Structure 3 is an organic-chemical-shorthand version equivalent to structures 1 and 2. In such shorthand structures, bonds to H are omitted. Each carbon is known to have four bonds and, wherever one is missing, it is understood that an unseen bond to H is present. This convention removes clutter from the drawings. Wherever a bond ends or two bonds meet and no elemental symbol is shown, it is understood that a carbon atom is present.
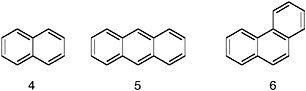
FIGURE 1.2 Shorthand representations (see Figure 1.1) of the three simplest polycyclic aromatic hydrocarbons, respectively naphthalene (4), anthracene (5), and phenanthrene (6).
Aliphatic Homologs
A comment should be made to contrast aromatic with aliphatic organic compounds. Aliphatic hydrocarbons (also called paraffins) are straight-chain alkanes (i.e., compounds having the general formula CnH2n+2, where n is an integer greater than one) starting with methane (CH4) and increasing by –CH2– units as a homologous series to high molecular weights. They can form abiotically (CH4 to typically decane, under hydrous conditions even to > C35)2 and biotically by direct synthesis or by geological degradation of lipid and cell membrane detritus.3 Lipids are aliphatic homologous compounds (e.g., fatty acids, fatty alcohols, etc.) important as membrane components and for energy storage. They are currently biologically synthesized but can also form abiotically in aqueous media at elevated temperatures and pressures.4-6 Biomarkers are organic compounds with specific structures (e.g., cholestane) that can be related back to their natural product precursors. The natural products (e.g., cholesterol) are biosynthesized from lipid precursors.7 Homologous aliphatic organic compounds and biomarkers can be distinguished by organic geochemists as being derived from abiotic or biotic sources.8 The homologous compounds with the biomarkers should be considered as the intermediary organics between the CH4 chemistry on planetary bodies and the formation of aromatics in the solar and interstellar medium as additional suitable tracers for evidence of life.
Stereoisomerism
Molecules are three-dimensional. A carbon atom with four single bonds lies at the center of a tetrahedron. The atoms to which the carbon is bonded are at the vertices of the tetrahedron. Those atoms are in turn likely to be bonded to other atoms. If the chemical structures at the four corners all differ, however slightly, the mirror images of the tetrahedron will not be superimposable (Figure 1.3).
Mirror-image stereoisomers that are not superimposable are called enantiomers; stereoisomers that are not enantiomers are called diastereomers. Enantiomers possess chirality, or handedness, and when dissolved rotate the plane of polarized light when it is passed through the solution.
Life on Earth makes use of only a limited number of diastereomers of all those that are possible.9 Moreover, biotic processes display an enantiomeric excess; e.g., left-handed amino acids and right-handed sugars almost exclusively predominate in living systems.
Carbon atoms bearing four different substituents are said to be chiral centers. If a molecule has n chiral centers it will, in most cases, have 2n stereoisomers. There will, for example, be 256 stereoisomers of a compound with eight chiral centers. Each will have exactly the same chemical formula and pattern of connectivity among its atoms (A is connected to B is connected to C and D, and so on). Only the arrangements of those atoms in space will differ, and there will be 256 variations. Life functions by using only a small subset of all possible stereoisomers.
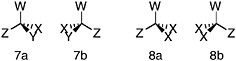
FIGURE 1.3 An illustration of stereoisomerism. In these depictions of tetrahedral carbon atoms, bonds represented by straight lines lie in the plane of the paper. Those represented by wedges project in front of the paper (the filled wedges) or to the rear (the broken wedges). W, X, Y, and Z represent different chemical groups, anything from a single atom (an H, for example) to a complex chemical substituent with many atoms in addition to the one that is bonded directly to the carbon atom. In structure 7, all four groups are different. The mirror images, 7a and 7b, cannot be rotated so that the structures are superimposable. In structure 8, by contrast, two of the groups are identical. If structure 8b is rotated 180° about its vertical axis, it can be superimposed on 8a (i.e., it is seen to be identical to 8a). Mirror images of tetrahedra will be nonsuperimposable only when all four vertices are different.
Structural Complexity and Chemical Reactivity
Descriptions of organic chemical structures focus on two distinct topics: a molecule’s “carbon skeleton” and its “functional groups” (if any). The first of these terms is practically self-defining. The carbon skeleton, a framework of chemical bonds, is usually the most important determinant of a molecule’s size and shape. The functional groups are chemical ornaments attached to that skeleton. They are usually the most important determinants of a molecule’s reactivity. Examples follow.
Carbon skeletons are best described by structural formulas like those shown in Figures 1.1 through 1.3. If the structure contains no double bonds or rings, seven carbon atoms can be assembled to produce the nine distinct carbon skeletons shown in Figure 1.4.
As drawn, the structural formulas in Figure 1.4 refer specifically to the molecule C7H16. As indicated by the elemental formula, which indicates that only carbon and hydrogen are present, it is a hydrocarbon, unadorned by functional groups. Numbers of possible structures for such compounds increase rapidly with the number of carbon atoms. The molecular formula C10H22 leads to 75 distinct structures. Doubling the number of carbon atoms (i.e., to C20H42) makes the total 366,319. Another doubling, to C40H82, yields 62,481,801,147,341 possible structures. However, as discussed above for stereoisomers, life functions by using only a small subset of all possible structures, patterns, and isomers.10 For example, a nonregular distribution of alkanes (e.g., an imbalance of even versus odd numbers of carbon atoms) and mainly linear not branched isomers occur in living systems.
The number of possible structures increases even more rapidly when multiple bonding and ring formation are considered. As an example, Figure 1.5 shows the structural formulas for the C7 hydrocarbons containing a five-membered ring and up to one double bond.
Many further structures could be drawn for C7 hydrocarbons. These would include 3-, 4-, 6-, or 7-membered rings and double and triple bonds limited only by the requirement that each carbon atom always have four bonds.
Functional groups contain elements in addition to carbon and hydrogen. The resulting polarity and presence of nonbonding electrons make them likely sites for chemical reactions. Examples are shown in Figure 1.6.
It is not practical here to review in detail the chemistry of functional groups, but the basic factors are easy to appreciate. Nitrogen and oxygen contain respectively one and two more valence electrons than does carbon. Nitrogen has to make only three chemical bonds to complete its valence octet. Oxygen can only make two. Both nitrogen and oxygen are more electronegative than carbon. C-N and C-O bonds are therefore polar. The carbon within them is susceptible to attack by nucleophiles (chemical reactants seeking to stabilize high electron density by forming a chemical bond to an electron-poor site, such as the C in a C=O bond). Simultaneously, the nonbonding electron pairs on nitrogen and oxygen (two pairs in that case) are available to participate in electronic rearrangements.
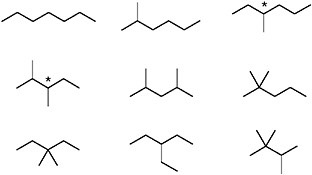
FIGURE 1.4 Shorthand representations (see caption for Figure 1.1) of the nine different carbon skeletons possible for a molecule with seven carbon atoms and no double bonds or rings. The asterisks mark chiral centers (see Figure 1.3). Each of the structures that contain a chiral center will exist as two stereoisomers.
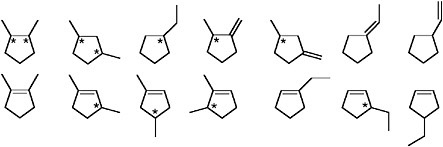
FIGURE 1.5 Structural formulas for hydrocarbons containing seven carbon atoms, one five-membered ring, and up to one double bond. The asterisks mark chiral centers (see Figure 1.3). Both of the structures containing two chiral centers happen also to contain a plane of symmetry. As a result, two of the stereoisomers will be equivalent and the molecules in question will have only three stereoisomers instead of 22 = 4.
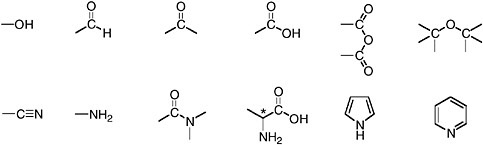
FIGURE 1.6 Functional groups. The open bonds represent points of attachment to carbon skeletons. From left to right in the top row, these examples include an alcohol, an aldehyde, a ketone, a carboxylic acid, a carboxylic acid anhydride, and an ether. Nitrogen-containing functional groups are shown in the second row. From left to right these include a nitrile (or organic cyanide), an amine, an amide, an α-amino acid, pyrrole, and pyridine.
Commonly, two carbon skeletons can be, in essence, glued together when the functional group on one attacks a functional group on the other and, as a result, a chemical bond is formed. An example is shown in Figure 1.7. In this case an alcohol with a carbon skeleton similar to those shown in Figure 1.5 reacts with a carboxylic acid having a carbon skeleton similar to one of those shown in Figure 1.4. The products are a molecule of water and an organic molecule in which the two carbon skeletons are connected by an ester linkage. Such reactions, in which two smaller molecules combine to form a larger product with the release of some small molecule, are termed “condensation reactions.”
Polymers exemplify both structural complexity (or, at least, great molecular size) and chemical reactivity. Polymers are synthesized from one or more monomers. Often, the monomers have functional groups that allow them to link their carbon skeletons together to form endless chains (addition polymers like polyethylene are the exception). Dacron and nylon are familiar polymers. The former is a polyester containing linkages like that illustrated in Figure 1.7. The latter is a polyamide (the amide structure is shown in Figure 1.6). The pertinent monomers and reactions are summarized in Figure 1.8.
Polymers are macromolecules. Natural products that are polymeric macromolecules include proteins (monomers = amino acids), nucleic acids (monomers = nucleotides), and cellulose (monomer = glucose). Coal is a natural macromolecule but does not qualify as a polymer because it has no regular, repeating structure based on a restricted set of monomers. Instead, it is a product of the condensation of cellulose, lignin, and other plant and bacterial products in the anaerobic, fermentative systems described as “coal swamps.” Another nonpolymeric
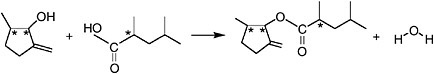
FIGURE 1.7 A typical condensation reaction. The alcohol and carboxylic acid shown as reactants are examples of carbon skeletons similar to those introduced in Figures 1.4 and 1.5 bearing two of the functional groups shown in Figure 1.6. For each carbon skeleton, the addition of the functional group has created a new chiral center by introducing asymmetry at a formerly symmetrical carbon position. In the absence of stereochemical control, the reacting alcohol will exist as a mixture of 22 = 4 stereoisomers, the carboxylic acid will be a mixture of 2 stereoisomers, and the organic product, an ester, will be a mixture of 23 = 8 stereoisomers.
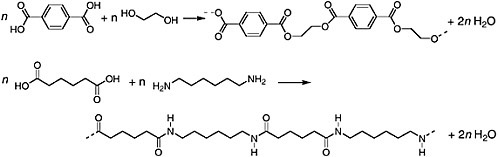
FIGURE 1.8. Chemical reactions depicting the formation of two familiar polymers, namely Dacron (top) and nylon (bottom). The dashed bonds indicate that the repeating, polymeric structures extend indefinitely. Values of n can exceed 104. The monomers used to form Dacron are a benzene dicarboxylic acid and a C2 dialcohol. The product is a polyester. Nylon is formed by polymerizing two C6 monomers, a dicarboxylic acid and a diamine. The product is a polyamide. Both polymerizations are condensation reactions. A molecule of water is released for each ester or amide link that is formed.
natural macromolecule, kerogen, forms from the condensation of algal and microbial debris in seafloor muds. The depositional environment (i.e., constant deliveries of silt, clay, lime, and other inorganic debris; ongoing metabolism of the organic matter) is such that kerogen seldom constitutes more than 10 percent of the mass of even a carbon-rich sedimentary rock like a petroleum “source rock.” Kerogen is nevertheless a familiar object of study for organic geochemists. It attracts attention because of the tendency of organic compounds to react with each other, it often constitutes 99 percent of the organic carbon in a sedimentary rock.
Kerogen, a macromolecule so large that it is not soluble in any solvent, is isolated by dissolving everything else. A sedimentary rock is ground to produce a powder. Small organic molecules are extracted using organic solvent. Inorganic material, the rocky matrix, is dissolved using hydrochloric and hydrofluoric acids. The kerogen remains. Ideas about its molecular structure (which will vary depending on the specific algal and microbial precursors and conditions, such as the abundance of H2S, in the depositional environment) can be obtained by chemical and thermal degradation of the macromolecule.
Insoluble, macromolecular, carbonaceous debris will form wherever reactive organic molecules are stored in close proximity. A terrestrial example of such material is kerogen. An extraterrestrial counterpart is found in carbon-rich meteorites. It may be called “kerogen” in written reports, but the usage is purely operational and does not require a biotic origin: The material is insoluble organic carbon.
DETERMINATION OF MOLECULAR ORIGINS
The exploration of organic cosmochemistry is not a search for life but an examination of all of the processes that have shaped the existence of life on Earth and, perhaps, elsewhere in the universe. The keys are to look in the right places, to collect and examine the right molecular mixtures, and to patiently and systematically extend the organic chemical analyses in ways that will maximize the precision with which interpretations can be made.
Each organic molecule provides information about its origins through details of its structure. Within the carbon skeleton, the number of rings and multiple bonds (which is to say the ratio of hydrogen to carbon, since each ring, double bond, or triple bond decreases the number of bonds to hydrogen) provides information about the availability of hydrogen at the site of molecular synthesis. Structures rich in multiple bonds suggest that reactive hydrogen was rare during assembly of the carbon skeleton. Some carbon skeletons, for example those containing small (3- or 4-membered) rings, incorporate quite a bit of strain (i.e., the angles between bonds are smaller than usual). If such rings have survived, they suggest that the molecule was unable to rearrange as it was formed and that the molecule has been cool since the time of its synthesis. Similarly, biotic processes synthesize many unique chemical structures.11 Such lines of evidence ramify almost endlessly, being limited only by the level of detail to which the structure is known.
Functional groups provide additional evidence about a molecule’s history. Most obviously, the presence of nitrogen and/or oxygen indicates that reactive forms of those elements must have been available at the site of molecular synthesis. The nature of the functional group carries further information. For example, alcohols, aldehydes, and carboxylic acids indicate progressively higher levels of oxidation. The survival of functional groups that would react with water (e.g., acid anhydrides or nitriles) indicates that the environments of synthesis and storage were dry. Again, the extension and elaboration of interpretations are limited only by the quality of the information about the numbers and types of functional groups.
Within mixtures of molecules, discernible patterns can be interpreted in terms of controlling mechanisms. A preference for molecules with even numbers of carbon atoms, for example, would indicate that C2 reactive units had been important in the environment of synthesis. A prevalence of two-dimensional, sheet-like structures would suggest formation on surfaces. In general, the properties of catalysts can be discerned from the extent to which some products have been favored over others. Where catalysis has been controlled precisely, the structural preferences can be absolute. At this, life, i.e., biochemistry, is the champion.
The common, biologically synthesized amino acids, the monomers used in the synthesis of proteins, provide an illustrative example. By definition, an amino acid contains both an amine group and a carboxylic acid group (see Figure 1.6). The carbon skeletons of the amino acids used in proteins contain up to 10 carbon atoms and up to six double bonds or rings. With two different functional groups, 10 carbon atoms, and varying numbers of rings and double bonds, the number of possible molecular structures is astronomical. From among those structures, each cell makes only 20 for inclusion in proteins. Each contains the particular assembly of functional groups identified in Figure 1.6 as an α-amino acid. As indicated in Figure 1.6, if the open bond is attached to anything but another H (the carbon shown as having only three bonds must have an unseen bond to H), another NH2, or another CO2H, the molecule will contain at least one chiral carbon. At that point, the biosynthetic process is even stereoselective, producing only one of the stereoisomers of each amino acid.
Encountering that mixture of materials, with only 20 structures from among the possible millions and only one chiral form of each, anyone would recognize the catalyst as being a biological process. Abiotic processes are less precisely selective, but the logical process of working backward from the analysis of an organic mixture to inferences about the mechanism of synthesis is no different.
Molecules also carry information about their origins in one rather unconventional way. The elements that are most important in organic chemistry—carbon, hydrogen, nitrogen, oxygen, and sulfur—all happen to have multiple stable isotopes (specifically, 1H and 2H; 12C and 13C; 14N and 15N; 16O, 17O, and 18O; and 32S, 33S, 34S, and 36S). Even on Earth, the relative abundances of those isotopes vary measurably (one part in 103 or, as it is usually written, 1‰) as a result of isotope effects that are associated with natural processes ranging from the evaporation of water to the fixation of carbon dioxide by photosynthetic organisms. Isotope effects associated with industrial processes such as the cracking of petroleum or electrolytic production of H2 from H2O also fractionate the stable
isotopes. In the cosmos, isotopic abundances vary not only as a result of isotope effects but also because of variations in nucleosynthetic processes.
Isotopic evidence can be used in two general ways. First, mixtures of compounds from different sources can be recognized. Compounds in one population of molecules might, for example, all be depleted in 13C. Another population might be distinguished by enrichment in 2H. In favorable cases, two or more populations can be recognized and their isotopic characteristics defined well enough that, when a particular compound happens to have multiple origins, mixing equations can be used to determine the proportion attributable to each source. Second, precursor-product and other genetic relationships can be recognized. If compound A is consistently depleted in 13C relative to compound B by a few parts per thousand, it is likely that A derives from B and that the reaction relating A and B has a kinetic isotope effect. If one family of structurally related compounds has isotopic abundances that are similar or that covary systematically, it is likely that all members of the family share a common source, and if another compound is related structurally but does not conform to the isotopic pattern, it must have a separate origin in spite of the structural relationship.
CRITERIA FOR DISTINGUISHING BETWEEN BIOTIC AND ABIOTIC COMPOUNDS
The chemistry of carbon leads to extraordinary variations in the structures of organic molecules. Factors influencing those variations include the following:
-
The abundances of reactive forms of some of the most abundant elements in the cosmos, namely hydrogen, nitrogen, and oxygen;
-
The temperatures and pressures at which the organic compounds have formed;
-
The temperatures and times of storage of the organic chemical products; and
-
The nature of any catalysts—ranging from random solid surfaces to other organic molecules—that were present in the environment of synthesis.
Knowledge of organic chemistry is so advanced, and the information content of mixtures of organic compounds (i.e., that encoded by variations in molecular compositions and structures) is so great, that inverse problems can be attacked with considerable success. Given the structures and relative abundances of organic compounds from samples of cosmochemical interest, it may be possible to work backward and to reconstruct the physical and chemical conditions prevailing at the times of their synthesis and throughout their subsequent history.
With regard to the indicators that might differentiate between a biotic and an abiotic origin for particular organic compounds, the task group found that the most compelling indicators of an abiotic origin include the following:
-
The presence of a smooth distribution of organic compounds in a sample, e.g., a balance of even versus odd numbers of carbon atoms in alkanes;
-
The presence of all possible structures, patterns, isomers, and stereoisomers in a subset of compounds such as amino acids;
-
A balance of observed entantiomers; and
-
The lack of depletions or enrichments of certain isotopes with respect to the isotopic ratio normally expected.
Likewise, the converse of the above items are indicators of possible biotic synthesis. Thus, the following are indicators of a biotic origin:
-
The presence of an irregular distribution of organic compounds in a sample, e.g., an imbalance of even versus odd numbers of carbon atoms in alkanes;
-
The presence of only a small subset of all possible structures, patterns, isomers, and stereoisomers;
-
An imbalance of observed entantiomers; and
-
The depletion or enrichment of certain isotopes with respect to the isotopic ratio normally expected.
However, some abiotic processes can mimic biotic ones and vice versa, and inferences will necessarily be based on several indicators and will, of course, be probabilistic.
NOTES
1. B.R.T. Simoneit and J.C. Fetzer, “High Molecular Weight Polycyclic Aromatic Hydrocarbons in Hydrothermal Petroleums from the Gulf of California and Northeast Pacific Ocean,” Organic Geochemistry 24: 1065-1077, 1996.
2. A.I. Rushdi and B.R.T. Simoneit, “Abiotic Condensation Synthesis of Glyceride Lipids and Wax Esters Under Simulated Hydrothermal Conditions,” Origins of Life and Evolution of Biospheres 36: 93-108, 2006.
3. See, for example, B.R.T. Simoneit, “Biomarkers (Molecular Fossils) as Geochemical Indicators of Life,” Advances in Space Research 33: 1255-1261, 2004.
4. A.I. Rushdi and B.R.T. Simoneit, “Lipid Formation by Aqueous Fischer-Tropsch-type Synthesis over a Temperature Range of 100-400º C,” Origins of Life and Evolution of the Biosphere 31: 103-118, 2001.
5. A.I. Rushdi and B.R.T. Simoneit, “Condensation Reactions and Formation of Amides, Esters, and Nitriles Under Hydrothermal Conditions,” Astrobiology 4: 211-224, 2004.
6. A.I. Rushdi and B.R.T. Simoneit, “Abiotic Condensation Synthesis of Glyceride Lipids and Wax Esters Under Simulated Hydrothermal Conditions,” Origins of Life and Evolution of Biospheres 36: 93-108, 2006.
7. B.R.T. Simoneit, “Biomarkers (Molecular Fossils) as Geochemical Indicators of Life,” Advances in Space Research 33: 1255-1261, 2004.
8. See, for example, B.R.T. Simoneit, “Biomarkers (Molecular Fossils) as Geochemical Indicators of Life,” Advances in Space Research 33: 1255-1261, 2004.
9. Roger Summons, Department of Earth, Atmospheric, and Planetary Sciences, Massachusetts Institute of Technology, “Molecular Biosignatures: Real and Potential Biomarkers, Analytical Innovations, Meteorites, and Mars,” presentation to the Committee on the Origins and Evolution of Life, January 25, 2006, Beckman Center, Irvine, California.
10. Summons, “Molecular Biosignatures,” presentation January 25, 2006.
11. See, for example, B.R.T. Simoneit, “Biomarkers (Molecular Fossils) as Geochemical Indicators of Life,” Advances in Space Research 33: 1255-1261, 2004.












