4
Primitive Bodies
Primitive bodies are those bodies that have undergone the least amount of change (chemically, thermally, and physically) since their formation in the solar nebula. These objects include main belt asteroids, Trojan asteroids, small satellites, comets, Centaurs,1 and Kuiper belt objects (KBOs).2 Because of their small size, and their often low albedos and large distances from the Sun and Earth that contribute to their faintness, the observation and understanding of their organic components are challenging.
FORMATION AND DYNAMICAL REGIMES
Asteroids orbit the Sun mainly in the region between Mars and Jupiter and represent inner solar system planetesimals that were unable to accrete into a planet because of the gravitational perturbations of Jupiter.
Comets are icy bodies that probably formed primarily in the Uranus-Neptune region and beyond (from ~20 to 50 AU); however, some of the material may have formed as close to the Sun as Jupiter. Many of the comets formed near Uranus-Neptune (~20 AU) were perturbed outward in what is now known as the Oort cloud, via gravitational interactions with the giant planets. Subsequent stellar perturbations can inject these Oort cloud comets into the inner solar system where they may become visible as either short- or long-period comets. Objects that originally formed farther out, in the vicinity of the Kuiper belt (30 to 50 AU), are sometimes perturbed inward and become short-period comets. As they migrate inward, they are observed as Centaurs. The importance of the different formation locations is that bodies from different dynamical regimes will have formed at different temperatures, will have had different thermal histories, and may, therefore, have different chemistries. The small outer planet satellites and Trojan asteroid populations probably represent planetesimals that formed in the outer solar system near the orbit of Jupiter and were subsequently captured into dynamically stable niches,3 and thus may share similarities with other outer solar system icy bodies such as KBOs, Centaurs, and comets. The interiors of Trojan asteroids are expected to be rich in H2O and other volatile materials.
The precursor cometary material may be a combination of unaltered interstellar grains/ices and volatiles that would have sublimated or vaporized as the material fell through accretion shock fronts in the protoplanetary disk, and then subsequently recondensed. Water ice can exist in either an amorphous or a crystalline phase depending on its condensation temperature and thermal history. If water ice condenses from the gas phase at temperatures below 100 K, then it will not have enough energy for an orderly crystalline structure and will be present in the amorphous phase. Laboratory experiments have shown that amorphous ice has the ability to trap large amounts of gases in
spaces between the water molecules,4 with gas/ice ratios as high as 3 to 3.5 (by number) at 30 K. As the temperature of condensation increases above 30 K the amount of gas that can be trapped drops quickly by 6 orders of magnitude at the temperature of 100 K, and different species are preferentially trapped, resulting in fractionation. When the ices are warmed, the ice structure reorganizes and the spaces between the molecules get smaller (annealing), and volatiles can be released. In addition, the amorphous ice will undergo an exothermic phase transition to its crystalline form, peaking near 137 K, also resulting in the release of trapped volatiles. Once the phase transition has occurred, the ice will not revert to the amorphous form if the temperature is lowered, unless there is some process (such as cosmic-ray irradiation, or vaporization and recondensation at low temperature) that destroys the structure in the ice.
MEASUREMENT TECHNIQUES—ORGANICS
The compositions of the primitive, low-albedo bodies are only broadly understood, whereas researchers have more information for the gaseous comas (the cloud of gas and dust that extends from the nucleus) of comet nuclei. Comets have been measured photometrically in the optical and spectroscopically in the near-infrared, and volatiles escaping from comet nuclei have been observed at radio wavelengths. In addition, several space missions have made in situ measurements. The materials expected on the surfaces of these bodies include minerals, and with increasing distance from the Sun water-ice, other volatiles, and solid organic materials.
While optical spectroscopy has been used to identify dissociation fragments (daughters) in cometary comas for nearly 100 years, it is often difficult to deduce their parent species. The inherent difficulty is that the volatiles sublime from the nucleus and shortly after entering the coma can experience photolysis and photodissociation. Many species can be seen as both a nuclear (parent) and a dissociation product. Models are then used to infer from the observed spectroscopic band strengths the abundance of various parent or original species coming from the nucleus. One of the surprising things learned from spectroscopic observations of recent bright comets, such as C/1995 O1 (Hale Bopp), is that it is very likely that chemical reactions may have been occurring in the inner coma, and thus these types of observations may not reflect the primordial composition in the cometary interior and may not be entirely useful indicators of the preservation of interstellar material. With recently observed bright comets such as C/1995 O1 (Hale Bopp), C/1996 B2 (Hyakutake), and C/1999 H1 (Lee), it was possible to make direct observations of the parent molecules in the infrared and radio wavelengths.
Spectroscopy of the solid bodies is more difficult owing to their faintness, making it very expensive (i.e., requiring considerable time) in terms of telescope time. In the optical wavelength region the most prominent spectral features come from mineral assemblages, whereas the signatures of organic material lie in the near-infrared region between 1 and 5 µm. Water ice also has strong solid-state absorption bands in this region, as do other volatiles of interest. Good near-infrared spectrometers have been installed only recently on large telescopes to facilitate the search for organic signatures in this wavelength region. The identification of organic species in the spectra requires the use of spectral models that apply Hapke scattering theory using the complex refractive indices of minerals, volatiles, and organic materials in combination to match the features in the spectrum. One of the problems is that the models are very complex and non-unique. In addition, optical constants (complex refractive indices), which must be measured in the laboratory, are known for only a relatively small number of the compounds that are likely to be present.
SYNTHESIS AND DESTRUCTION OF ORGANIC MATERIALS
Organic molecules can be both synthesized and destroyed on outer solar system solids by irradiation. The surface materials on small bodies or grains are typically exposed to charged-particle and ultraviolet radiation, producing radiolysis and photolysis. There is abundant laboratory evidence that carbon-containing frozen mixtures will form complex organics when exposed to radiation and that complex organics break down under irradiation. Under the influence of radiation, these surfaces will grow progressively redder and darker as hydrogen is lost.5-7 However, there is as yet no definitive observational evidence that radiation processing plays an important role in forming organics on solar system surfaces (note that there is abundant evidence now for radiation processing).
Although there is considerable speculation that the red-spectral character of many outer solar system surfaces is due to organics and/or to the processing of these materials, again there is no direct evidence. Determining if such processes occur is important, because exposure to radiation affects the reflectance properties of optical surfaces, which in turn impacts observations made by remote sensing.
The laboratory evidence for chemistry induced by radiolysis and photolysis in low-temperature solids is clear. However, detecting chemical products in a solid by remote sensing is more difficult than it is for the gas phase. As a result, there has been much less progress in both understanding solid-state radiation chemistry and applying that to remote sensing observations. While there has been considerable improvement in reflectance studies of outer solar system surfaces, it is now realized that vertical mixing does occur on geologically “young” surfaces like that of Europa. It is also clear that tenuous atmospheres on small objects, as well as certain gas-phase species in the interstellar medium, are produced by stimulated desorption and sputtering. Therefore, it is now important to understand the chemistry induced by the radiolysis and photolysis of ice containing a variety of carbon species in the solid state. Further, with the developing plans to explore organic environments in the outer solar system, there is a need to understand the effect of radiation on any indigenous or delivered organics.
As in the gas phase, the chemical reactions of interest in solids are driven primarily by bond breaking and ionization due to ultraviolet photons and energetic charged particles. This process creates free radicals (as well as ions and electrons) that can react with neighboring molecules. The resulting chemistry is different from that in the gas-phase chemistry in three significant ways. First, the reactive species produced are often immobile, leading to enhanced geminate recombination and hot-atom/insertion interactions. As trapped species (including electrons) are often stable for long periods, a later heat pulse or further irradiation may be needed to drive reactions. A second difference from gas-phase chemistry is that energetic charged particles produce a locally dense set of excitations in a solid. Therefore, the chemical pathways can be very different from those in photolysis of a gas or a solid and different from charged-particle irradiation in a gas. Finally, because solid objects with low gravity lose their hydrogen preferentially, the surfaces of interest are typically oxidizing, so that the organic chemistry occurring there can differ significantly from that in hydrogen-dominated regions of space.
In the outer solar system galactic cosmic rays, solar particles (solar wind and solar energetic particles), charged particles trapped in the magnetic fields of the giant planets, and solar ultraviolet and extreme-ultraviolet radiation are all of interest because each is capable of providing energy for radiolysis or photolysis to take place on these surfaces. These various forms of radiation act over very different time scales and depths into a surface. Typically the relevant time scales increase with depth into a solid. That is, on a stable surface the optical layer is often modified in periods of months to hundreds of years, whereas depths of the order of centimeters at unit density are modified in millions of years and depths of the order of a meter are modified in the lifetime of the solar system. In addition, there is evidence in the early solar system for intense solar particle radiation near the edge of the accretion disk or during the Sun’s T-Tauri phase, so that materials incorporated into objects, particularly refractory organics, may have been pre-irradiated.
The major sources of carbon in interstellar ices are CO, CO2, and CH3OH and, possibly, condensed carbon, PAHs, or fullerenes. In contrast, in the vicinity of giant-planet formation CH4 is a principal source of carbon, as is the case in the solar nebula where chemical equilibrium has occurred. Therefore, the thermal and radiation processing of these species in the presence of H2O, NH3, N2, and others, can, in principle, lead to organic chemical pathways on planetary satellites that are different from those on relatively unprocessed bodies, such as Kuiper belt objects. However, even if different initial chemical conditions occurred, the exchange of materials by impacts and by plasma transport can homogenize the cometary bodies and icy satellites. Therefore, a key to understanding the evolution of outer solar system objects is to understand their organic inventory in detail, particularly the trace organic molecules, and to understand the inventory as a function of distance from the Sun. A critical question is whether there is any evidence, other than the deuterium/hydrogen ratios, that any outer solar system body contains unprocessed interstellar organics.
Comets and Centaurs can have highly elliptical orbits and thus can pass through very different thermal environments. This environmental variability leads to significant evolution in their interiors and possible chemical processing. While residing in the distant reaches of the solar system, comets will undergo irradiation of the surface layers, and in addition may be heated to at least 30 K to a depth of 20 to 60 m by the passage of luminous stars, and
it is believed that most comets may have been heated as high as 45 K to a depth of 1 m from stochastic supernova events. This heating may result in depletion of volatiles in the upper layers. The evolution of meteorites provides evidence of an early heat source in the solar system; while many researchers believe that radioactive 26Al was likely responsible for radiogenic heating of large bodies, strong arguments can be made that other radionuclides (e.g., 60Fe) also played important roles. Prialnik et al. have examined the role of 26Al in the possible evolution of cometary interiors.8 Because there is evidence for amorphous ice in comets, they cannot have been heated above 137 K. However, for larger comet nuclei, such as Kuiper belt objects, models have shown that it is possible under certain interior conditions (which include specific porosities, the fraction of other volatiles present, and so on) to have melting in the interior creating reservoirs of liquid water.9 This possibility has interesting implications in view of recent experiments on organic compounds that show self-organizing behavior in the presence of liquid water. During the active phase, when a comet passes within the inner solar system and experiences significant solar heating, the comet’s surface and subsurface layers (up to a few meters in depth) will be depleted in volatile material and also may lose the highly volatile radicals that were created from galactic cosmic-ray processing.
The phase transition and subsequent gas release are sensitive to the physical and chemical properties of the nucleus, as well as the orbital evolution of the comet. As volatiles are depleted when heated, a dust mantle will form on the surface, which may erode during periods of high activity and the thickness of which will be a function of orbital evolution (Figure 4.1). These types of effects have been observed in comets, ranging from production of vastly different amounts of volatile material between comets coming close to the Sun for the first time and comets that have
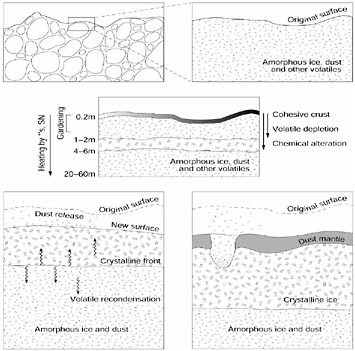
FIGURE 4.1 Diagram showing the sequence of aging processes in the upper layers of a comet nucleus from (a) the pristine state, consisting of primordial planetesimals (upper left, enlargement at upper right), (b) to the alterations it undergoes while stored in the Oort cloud including a possible crystalline core caused by radioactive heating from 26Al (central diagram) to (c) the changes in the surface during the active phase (lower left) and (d) near the end of its evolution as a dust mantle builds up (lower right). SOURCE: K.J. Meech, “Physical Aging of Comets,” pp. 195-210 in Evolution and Source Regions of Asteroids and Comets (J. Svoren, E.M. Pittich, and H. Rickman, eds.), IAU colloquium No. 173, Tatranska Lomnica, Slovakia, August 24-28, 1998, Astronomical Institute of the Slovak Academy of Sciences, Tatranska Lomnica, The Slovak Republic, 1999.
spent a large amount of time in the inner solar system, to observations of carbon-chain species (e.g., C2, C3) in comets dynamically associated with Jupiter. It has been suggested that some process in the solar nebula may have preferentially produced or destroyed the carbon chain molecules at the distance of the Kuiper belt, the source region for most of the Jupiter-family comets,10 although observationally researchers cannot yet distinguish this from an evolutionary effect in comets. Therefore, comets are unusual because of their passage through very different thermal regimes, their possible preservation of pre-solar materials, and their small sizes, all of which create different physical and chemical processing conditions compared to those associated with planets and their major satellites.
ORGANIC INVENTORY
Surfaces of small primitive bodies are composed of varying fractions of refractory material and frozen volatiles. Many of these surfaces, in particular in the outer solar system, exhibit abundant evidence for dark materials that are generally spectrally neutral (i.e., they are equally bright at visual, red, and infrared wavelengths) to red (i.e., they are brighter at red and, in particular, infrared wavelengths than they are at visual wavelengths). The redness of these objects has led to the suggestion that their surfaces contain organics or a carbon-rich material, possibly frozen in an icy matrix, and therefore may be of interest in studies of abiotic chemical processes. However, the presence of specific organics on most of these bodies remains speculative because of the lack of large numbers of spectra and because models are limited by lack of laboratory measurements.
Asteroids
Asteroids can be classified by their surface spectral properties, colors, and albedos. S-type asteroids have relatively high albedos, significant spectral features associated with anhydrous silicates, and a general “reddish” spectral slope.11 These are primarily assemblages of anhydrous silicates, NiFe metal and iron sulfide, analogous to ordinary chondrites, primitive achondrites, stony-iron meteorites, and silicate-bearing iron meteorites. C-type asteroids tend to have a very low albedo and are neutral in color, with subdued features indicating the presence of phyllosilicates (clay minerals). Most C-type asteroids appear analogous to CI1- or CM2-type carbonaceous chondrites. Other types of asteroids have been defined, including the common P- and D-types in the outer parts of the asteroid belt, but little is known about their composition and origin. D-type objects have low albedos and are characterized by featureless spectra with neutral to slightly red slopes shortward of 0.55 µm, and very red slopes longward of 0.55 µm.12 S-class asteroids are those that in general are closest to the Sun and contain little carbon, while the C-type asteroids (based on their meteorite analogs) have carbon compounds that have been transformed by heat and water. The more distant P- and D-type asteroids do not appear to have been heated, and so their organics have not been subjected to alteration by high temperatures and liquid water. Presumably the materials in the P- and D-type asteroids contain organics whose structures are similar to those in comets. The C-, P-, and D-type asteroids are believed to be the source of both the carbonaceous meteorites and a large fraction of the carbon-containing interplanetary dust particles deposited on the inner planets. Indeed, the reflectance spectrum of the Tagish Lake meteorite is similar to the observed spectra of D-type asteroids.13 On the other hand, there has been no direct observation of organic material on any class of asteroids in the main belt. Among the outer belt asteroids and the Trojans there is no evidence for ices, and even evidence for specific minerals and organic material is indirect. However, some researchers have argued that the only plausible material that can provide both the low albedo and the spectrally red slope of their reflectivities is complex organic solid material.14 Caution is needed, however, because albedo and color are highly nonlinear functions of composition, grain size, and shapes (see the section “Laboratory Opportunities” below in this chapter).
Comets Within the Inner Solar System
The first analog for the composition of comets began with the “dirty snowball” model of Whipple.15 Whipple proposed that cometary nuclei were made predominantly of water ice with an admixture of rocky particles and large organic entities or CHON (carbon, hydrogen, oxygen, and nitrogen) particles.16,17 The chemical composition
of comet nuclei has been constrained by Greenberg, who proposed that 26 percent of the mass is incorporated in silicates, 23 percent in refractory material, 9 percent in small carbonaceous molecules, and the remainder in water ice and trace gases (e.g., CO, CH4, C2H2, and others).18 These trace elements are important, however, as they provide clues about the environment of formation of these and more complex organics.
It is uncertain how much the original interstellar material has been altered in comets. There is some evidence of preservation from deuterium/hydrogen ratios in comets that are higher than nebular values (e.g., solar), and more representative of values in hot molecular cloud cores. But there is also evidence of chemical processing in comets.19
With the exception of in situ observations of organics in comet 1P/Halley, most of the organics known in comets have been detected as parent molecules in spectra obtained at millimeter, submillimeter, and infrared wavelengths. HCN was first detected in comet 1P/Halley in the radio region of the electromagnetic spectrum by several telescopes, and it was post-Halley (i.e., in the last 10 years) that other species were detected in comets. The field of comet parent-molecule radio spectroscopy has blossomed only recently, with the advent of new low-noise receivers. A summary of carbon-bearing species in cometary comas is shown in Table 4.1. While the approximate abundances are given for comets in general, these are based on the observations of only a few comets. In some cases, there are significant differences in the amount of organic compounds with different volatilities between comets, and this variability has implications concerning the location in the solar nebula where they were formed.20
Overall, it is estimated that perhaps as much as one-third of the comet nucleus by mass consists of organic solids, and carbon may be present at the 1 to 10 percent level in other volatiles such as CH3OH, CO, CO2, and others. Some of these molecules originate from the comet nucleus in the coma, but others have extended sources, originating presumably on the surfaces of the CHON grains. These include CO, H2CO, OCS, HCN, and CS.
The neutral gas mass spectrometer aboard the European Space Agency’s Giotto provided measurements of several native species, including the retrospective identification of ethane.21 The so-called IKS infrared spectrometer on the former Soviet Union’s Vega 1 spacecraft achieved detections of H2O, CO2, H2CO, and possibly CO.22 This instrument also detected a spectral signature in the 3.2- to 3.6-µm region attributed primarily to C-H stretching vibrations in one or more organics. The Particle Impact Analyser dust composition experiment on the Giotto spacecraft discovered a population of dust particles composed of an organic material containing CHON grains. These detections are included in Table 4.1. Most of the carbon found in comet 1P/Halley from mass spectrometry consisted of oxidized compounds, e.g., CO, CO2, and H2CO, and the refractory materials can be interpreted as being the following types of carbonaceous matter: pure carbon, polycyclic aromatic and highly branched aliphatic hydrocarbons, and polymers of carbon suboxide and of cyanopolyynes.23,24 Many of the grains contained mixtures of complex organic compounds consistent with the presence of alcohols, aldehydes, ketones, acids, and amino acids. Other organic compounds included PAHs, highly branched aliphatic hydrocarbons, and unsaturated hydrocarbon chains, including alkynes, dienes, various large nitriles, and analogous species with imino and amino end groups. Several nitrogen heterocycles may also have been identified, including pyrrole, pyrroline, pyridine, pyrimidine, imidazole, purine and adenine.
The abundances listed in Table 4.1 are consistent with the abundances seen in interstellar ices or in hot molecular cloud cores where the molecules may have evaporated off ices. However, not all of the species listed are considered to be parent molecules (e.g., direct nucleus sublimation products), or unaltered molecules preserved from the interstellar medium. It is very important to note that the data in Table 4.1 are from a very small sample of comets: primarily from comets C/1996 B2 (Hyakutake) and C/1995 O1 (Hale-Bopp). The significance of these two objects is that they were the first really bright comets discovered far enough in advance to be observable over a range of distances following the development of adequate technology for the detection of parent molecules.
The study of cometary chemical composition has evolved rapidly in recent years, mainly owing to advances in instrumentation, especially high-dispersion cryogenic spectrometers at infrared wavelengths. High-resolution (λ/Δλ > 104) infrared spectroscopy enables individual rotational-vibrational lines to be resolved and differentiated from telluric absorptions (i.e., absorptions in Earth’s atmosphere), other molecular emissions, and the continuum, making accessible species that are not observable with low resolution (Figure 4.2). The discovery of methane (CH4), ethane (C2H6), and acetylene (C2H2) in comet C/1996 B2 (Hyakutake)25 established the importance of these
TABLE 4.1 Carbon-Bearing Species Abundances in Comets Relative to Water
|
Observed |
Inferred |
||||
|
Molecule |
Abundance |
Technique |
Molecule |
Abundance |
Technique |
|
H2O |
100 |
UV, IR, radio |
CH3CHO |
0.005 |
Mass speca |
|
CO |
1-30 |
UV, IR, radio |
C3H2 |
0.001 |
Mass speca |
|
CO2 |
3-10 |
IR |
C2H5CN |
0.00028 |
Mass speca |
|
HCO |
|
Radio |
CH3NH2 |
<0.0015 |
Mass speca |
|
H2CO |
0.1-1.1 |
IR, radio |
|
|
|
|
CH3OH |
1-7 |
IR, radio |
|
|
|
|
HCOOH |
0.08 |
Radiob |
|
|
|
|
HNCO |
0.1 |
Radiob |
|
|
|
|
HCONH2 |
0.01 |
Radiob |
|
|
|
|
HCO2CH3 |
0.08 |
Radiob |
|
|
|
|
CH3CHO |
0.02 |
Radiob |
|
|
|
|
CH2 |
0.0027 |
Mass speca |
|
|
|
|
CH4 |
0.6 |
IRb |
|
|
|
|
C2H2 |
0.1 |
IR |
|
|
|
|
C2H4 |
0.003 |
Mass speca |
|
|
|
|
C2H6 |
0.1-0.5 |
IR |
|
|
|
|
CH3C2H |
<0.045 |
Radiob |
|
|
|
|
HCN |
0.05-0.2 |
IR, radio |
|
|
|
|
HNC |
0.04 |
Radio |
|
|
|
|
CH3CN |
0.02 |
Radiob |
|
|
|
|
HC3N |
0.03 |
Radiob |
|
|
|
|
NH2CHO |
0.01 |
Radiob |
|
|
|
|
CS |
0.2 |
UV, radio |
|
|
|
|
CS2 |
0.1-0.2 |
Radiob |
|
|
|
|
H2CS |
0.02 |
Radiob |
|
|
|
|
OCS |
0.4-0.5 |
IR, radiob |
|
|
|
|
H2CS |
0.02 |
Radiob |
|
|
|
|
OCS |
0.4-0.5 |
IR, radiob |
|
|
|
|
OCS |
0.4-0.5 |
IR, radiob |
|
|
|
|
OCS |
0.4-0.5 |
IR, radiob |
|
|
|
|
NOTE: This table does not include species for which only upper limits for nondetections are known. aComet Halley mass spectrometer data. bMeasurement from comet C/1995 O1 (Hale-Bopp) only. SOURCE: Data from W.M. Irvine and E.A. Bergin, “Molecules in Comets: An ISM-Solar System Connection?,” p. 447 in Astrochemistry: From Molecular Clouds to Planetary Systems, Proceedings of IAU Symposium 197 (Y.C. Minh and E.F. van Dishoeck, eds.), Publications of the Astronomical Society of the Pacific, San Francisco, Calif., 2000; K. Altwegg, H. Balsiger, and J. Geiss, “Composition of the Volatile Material in Halley’s Coma from In Situ Measurements,” Space Science Reviews 90: 3-18, 1999; D. Bockelé-Morvan, D.C. Lis, J.E. Wink, D. Despois, J. Crovisier, R. Bachiller, D.J. Benford, N. Biver, P. Colom, J.K. Davies, E. Gérard, B. Germain, M. Houde, D. Mehringer, R. Moreno, G. Paubert, T.G. Phillips, and H. Rauer, “New Molecules Found in Comet C/1995 O1 (Hale-Bopp): Investigating the Link Between Cometary and Interstellar Material,” Astronomy and Astrophysics 353: 1101-1114, 2000; and P. Ehrenfreund, W. Irvine, L. Becker, J. Blank, J.R. Brucato, L. Colangeli, S. Derenne, D. Despois, A. Dutrey, H. Fraaije, A. Lazcano, T. Owen, and F. Robert, “Astrophysical and Astrochemical Insights into the Origin of Life,” Reports on Progress in Physics 65: 1427-1487, 2002. |
|||||
hydrocarbons in comets. Relative abundances provide crucial information about the link between interstellar and cometary ices. A larger-format high-efficiency array allowing simultaneous spectral order sampling from 1 to 5.5 µm became available as a facility instrument on the Keck II 10-m telescope in 1999 (NIRSPEC), and this capability is revolutionizing the characterization of the distribution of organics in small bodies. With only three grating settings, NIRSPEC permits a nearly complete high-dispersion survey of the spectral region from 2.9 to 3.7 µm, which is key for investigating the organic composition of primitive bodies. However, intense observing
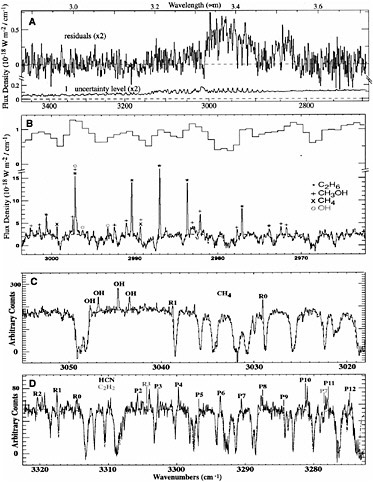
FIGURE 4.2 A comparison of high- and moderate-resolution cometary spectra. (A) Shown is an extracted spectrum of comet C/1999 H1 (Lee) taken in the moderate dispersion mode (λ/Δλ ~ 2000) on UT on August 20, 1999. The broad feature centered at 3.52 µm is due to CH3OH ν3 and shows emission from the P-, Q-, and R-branches. The broad feature between 3.3 and 3.45 µm is due mainly to unresolved organic emission lines. The channel-by-channel noise amplitude residuals (1σ) are also shown. (B) High-dispersion spectra (λ/Δλ ~ 25,000) of comet C/1999 H1 (Lee) acquired on UT August 21, 1999. Shown for comparison at the top of the panel is the moderate-resolution spectrum from (A) in this wavelength range. Ethane (C2H6), methanol (CH3OH), methane (CH4), and OH prompt emission, clearly detected in the high-dispersion spectrum (λ/Δλ ~ 25,000), are not resolved at λ/Δλ ~ 2000. (C) High-dispersion spectra (λ/Δλ ~ 25,000) of comet C/2000 WM1 (LINEAR) acquired on UT November 23, 2001. Methane and OH prompt emission were detected. (D) High-dispersion spectra (λ/Δλ ~ 25,000) of comet C/2000 WM1 (LINEAR) acquired on UT November 23, 2001. Rovibrational lines of hydrogen cyanide (HCN) and acetylene (C2H2) were detected. Symmetric hydrocarbons C2H2, C2H6, and CH4 can be detected only at infrared wavelengths and were not detected until high-resolution spectroscopy became available. (See T.Y. Brooke, A.T. Tokunaga, H.A. Weaver, J. Crovisier, D. Bockelé-Morvan, and D. Crisp, “Detection of Acetylene in the Infrared Spectrum of Comet Hyakutake,” Nature 383: 606-608, 1996; and M.J. Mumma, M.A. DiSanti, N. Dello Russo, M. Fomenkova, K. Magee-Sauer, C.D. Kaminski, and D.X. Xie, “Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin,” Science 272: 1310-1314, 1996.) This illustrates the difficulty of detecting and isolating organic species with low or moderate spectral resolution, and the need for high-resolution spectroscopic studies to provide a more complete picture of cometary chemistry. SOURCE: Image courtesy Michael J. Mumma and Neil Dello Russo, NASA.
pressure for this limited resource has meant that progress in infrared spectroscopic detection of parent species has been slow.
A’Hearn and colleagues have undertaken an extensive study of abundances and molecular production rates for C2, C3, and several noncarbon-bearing species.26 They found that most comets were similar in overall chemical composition. However, there was a group of comets depleted in the carbon-chain molecules. This depletion correlates strongly with dynamical age, which is related to location of formation in the solar nebula; i.e., only the Jupiter-family short-period comets show the depletion. A’Hearn and colleagues argue that this correlation is probably a consequence of primordial rather than evolutionary difference, with some process destroying carbon-chain molecules in the vicinity of the Kuiper belt (the source region of the Jupiter-family short-period comets).
Carbon Isotopes
Time-of-flight mass spectrometry for comet 1P/Halley found that the isotopic composition of carbon had an unusual signature. The 12C/13C ratios in some of the grains were up to 5000, suggesting that some of the grains may have originated from different interstellar environments (similar to what is observed for some chondrites).27
Delivery to Earth
Cometary impacts with Earth today are rare, but they may have been more important on primitive Earth when they were ejected into the Oort cloud in the first few 107 years of the solar system’s history. While some researchers maintain that comets could have played an important role in the origins of life by delivering water and biologically relevant organic molecules to the primitive Earth, not all agree. Many researchers argue that the origin of life likely requires simple mixtures of a few relatively pure compounds and not the complex mixtures of highly processed carbon compounds likely to be found on comets.28 Even if small amounts of biologically important prebiotic compounds are transported to Earth by comets, it is not known if these organics survived the collision with Earth’s atmosphere. Fullerenes, formed in the interstellar medium, were detected in the Sudbury impact site in Canada.29 While some researchers argue that the Sudbury structure is the result of a cometary impact,30 the detection of fullerenes remains controversial. But, in addition to not being a good starting point for the origins of life, fullerenes, polycyclic aromatic hydrocarbons, and other complex organic molecules are not even good foodstuffs for biochemistry. Indeed, the conversion of cometary carbon compounds into biomass may require their oxidation to carbon dioxide and subsequent fixation by preexisting biochemical processes.
Centaurs and Kuiper Belt Objects
Kuiper belt objects and Centaurs (with few exceptions) never get close enough to the Sun to be active and bright enough for direct detection of carbon-bearing species. The presence of organics must be inferred from solid-state infrared spectra of the nuclei or from the colors of the surface materials. Measurements of the thermal emission from some of the Centaurs show that they have generally low albedos.
Centaurs and Kuiper belt objects display a range of surface colors from neutral (solar) to very red for nearly 150 objects that have been measured. Near-infrared spectroscopy of several dozen Centaurs and KBOs has revealed a similarly large diversity. Water-ice absorption features are present on a few, and spectral models suggest surfaces probably rich in complex organic solid materials such as amorphous carbon, methanol ice, other light hydrocarbons, and Titan and Triton tholins31 in the presence of olivine. Table 4.2 lists the Centaurs and KBOs for which spectra exist, along with the possible identification of the surface compositions as derived from spectral models.
This diversity seen in the surface properties is likely to be the result of several processes. One possibility is a competition between a reddening of the surface by high-energy particles and collisions that can excavate more neutral material. However, statistical work on the distribution of the colors has led to contradictions with this model. For instance, the dispersion of colors over all the objects is much larger than the dispersion of colors for any given object (over rotational phase), suggesting that the bodies have uniform colors, whereas the collision/
TABLE 4.2 Carbon Compounds Inferred on Surfaces of Centaurs and Kuiper Belt Objects
|
Object |
Spectral Features |
Typea |
Object |
Spectral Features |
Typea |
|
(15789) 1993 SC |
Hydrocarbons |
KBO |
(15874) 1996 TL66 |
Featureless |
KBOS |
|
(26181) 1996 GQ21 |
Flat, Titan tholin, H2O, C |
KBOS |
1996 TS66 |
Featureless |
KBO |
|
(19308) 1996 TO66 |
Strong H2O |
KBO |
(28978) 2001 KX76 |
Featureless |
KBO |
|
(47171) 1999 TC36 |
Titan tholin, amorphous C, H2O |
KBO |
(2060) Chiron |
Featureless; H2O |
Cent |
|
(26375) 1999 DE9 |
H2O, organic, C |
KBOS |
5145 Pholus |
Titan tholins, olivine, H2O, CH3OH, amorphous C |
Cent |
|
(38628) 2000 EB173 |
Featureless, H2O? |
KBO |
(52872) Okyrhoe |
H2O, kerogen, olivine |
Cent |
|
(20000) 2000 Varuna |
H2O? |
KBO |
(32532) PT13 |
Titan tholin, ice tholin, olivine, amorphous C |
Cent |
|
(54598) 2000 QC243 |
H2O, kerogen, olivine |
Cent |
(8405) Asbolus |
Featureless; Triton tholin, Titan tholin, amorphous C, ice tholin |
Cent |
|
(63252) 2001 BL41 |
Triton tholin, ice tholin, amorphous C |
Cent |
(10199) Chariklo |
Titan, Triton tholins, amorphous C, H2O |
Cent |
|
(31824) 1999 UG35 |
H2O, amorphous C, Titan tholin, CH3OH, olivine |
Cent |
1996 TQ66 |
1.9-µm feature— identity unknown |
KBO |
|
1996 TS66 |
1.9-µm feature— identity unknown |
KBO |
|
|
|
|
NOTE: Review papers that summarize the near-infrared spectra and spectral model results are quoted in K.J. Meech, O.R. Hainaut, H. Boehnhardt, and A. Delsanti, “Search for Cometary Activity in KBO (24952) 1997 QJ4,” Earth, Moon, and Planets 92: 169-181, 2003; and E. Dotto, M.A. Barucci, and C. de Bergh, “Colours and Composition of the Centaurs,” Earth, Moon, and Planets 92: 157-167, 2003. a KBO, low-eccentricity Kuiper belt object; KBOS, high-eccentricity KBO; Cent, Centaur. |
|||||
reddening model predicts variegated surfaces. Another suggestion, based on the observation of a color trend with inclination,32 is that the high-inclination objects form a dynamically “hot” (excited) population of objects that were scattered during planet migration from smaller heliocentric distances. In this scenario, the color/compositional diversity might simply reflect different source regions.
Cometary-like volatile activity could cause a change in surface albedo by removing some of the older, reddened surface and replacing it with bluer material. This hypothesis has been suggested as the mechanism to explain the change in the color and rotational light curve for object (19308) 1996 TO66.33
Trojan Asteroids, Small Outer Solar System Satellites, and Rings
Small Jovian Satellites and Trojans
There is only sparse multispectral data for the jovian ring and inner satellites, with observations from the Hubble Space Telescope’s Near-Infrared Camera and Multi-Object Spectrometer (NICMOS) showing similar positive spectral slopes through the near-infrared, suggesting similar dusty compositions possibly due to contamination by Io. The minor outer satellites (likely captured asteroids) of Jupiter are spectrally dark and neutral to red in the visible, although they lack the ultra-red material seen on some of the Centaurs. In the near-infrared, the
retrograde satellites show more spectral heterogeneity than do the prograde satellites, and have spectra and presumably compositions similar to D-type asteroids, whereas the prograde satellites are compositionally more similar to C-type asteroids. The Trojan asteroids have low mean geometric albedos and flat optical reflection spectra with a range of spectral slopes from neutral to reddish, implying surfaces rich in complex organic material. Their spectra are very similar to those of comet nuclei and to that of the Tagish Lake meteorite.34 A summary of the outer solar system satellite carbon-bearing materials is given in Table 4.3.
Phoebe, Hyperion, Iapetus, and Enceladus
The outermost of Saturn’s major satellites, Phoebe, has a relatively flat spectrum thought to be composed of carbonaceous chondritic material. Hyperion shares spectral properties with the uranian satellites. The best laboratory fit to the spectrum of Hyperion obtained with Earth-based telescopes comes from a mixture of water ice and spectrally neutral charcoal. The latter suggests the presence of dark, carbon-bearing materials.
Iapetus has the most striking large-scale albedo contrast of any solar system body. While its trailing hemisphere has an albedo of ~0.5 and is composed of nearly pure ice, material in its leading hemisphere has an albedo of <0.05 with an especially steep red spectral slope through the visible. The spectrum has been modeled with Murchison organic residue, polymeric HCN, and water ice.35 Visible and near-infrared spectra suggest a spectral match to the organic fraction of carbonaceous chondrites along with iron-rich hydrated minerals, i.e., clays. The source of the dark material being exogenic (e.g., impacted dust) or endogenic (perhaps concentrated in the leading hemisphere by impacts) is debated.
Observations of Enceladus from the Cassini spacecraft have recently revealed the presence of an atmospheric plume and coma dominated by water, with significant amounts of carbon dioxide, methane, and other constituents, possibly carbon monoxide or molecular nitrogen. Trace quantities (<1 percent) of acetylene and propane also appear to be present.36 The plume, or rather plumes, of material appears to be venting from a series of anomalously
TABLE 4.3 Carbon Compounds Inferred on Surfaces of Small Outer Solar System Bodies
warm, trenchlike rifts in Enceladus’s southern polar region. Additional remote sensing and in situ observations of the venting regions, the plumes, and their organic content will be a high priority for the remainder of Cassini’s prime mission and will likely be an even higher priority during an extended mission.
The Uranian and Neptunian Systems
The uranian satellites are notably dark. The five major satellites (Miranda, Ariel, Umbriel, Titania, and Oberon) have average normal reflectances ranging from a high of ~33 percent for Miranda down to ~19 percent for Oberon and Umbriel. There is a general anticorrelation of visible geological activity and albedo on their surfaces, with the overall brightest (Miranda) showing the most geological activity. The floor of a large basin on Oberon shows the darkest material observed on these five satellites, with normal reflectance of ~10 percent. Some of darkest materials on Miranda are along prominent scarps interpreted as normal faults, perhaps exposing a non-uniform subsurface layer of dark material. The satellites’ Earth-based telescopic spectra have been modeled with mixtures of spectrally neutral charcoal and water ice, and possibly ammonium hydrate for Miranda.37 Voyager observations show that they are somewhat red, notably on the leading hemispheres of the outer four, suggesting accumulation of meteoritic dust.
The small satellite Puck, and potentially the other small inner uranian satellites, have albedos of ~0.07, but their spectral characteristics are uncertain. The narrow uranian rings appear to be spectrally neutral and dark, suggesting carbon as an important component. The spectroscopic and geological evidence leaves open the question of whether dark surfaces in the uranian system include primordial silicate and/or carbon-bearing dark materials, or have darkened in situ due to energetic processing and polymerization of carbon-bearing ices.
Little is known of the composition of Neptune’s satellite Nereid and its six small inner satellites. These satellites have relatively flat visible spectra and low albedos.
SUMMARY OF PAST, PRESENT, AND PLANNED MISSIONS: IMPLICATIONS FOR CARBON STUDIES
Comets have been proposed as a potential source of organics that were delivered to the inner planets. As noted previously, the organics in comets are probably more closely related structurally to the interstellar molecules than any other organics in the solar system. Currently three missions to primitive bodies are in progress or under development, and six have successfully completed their primary missions:
-
Galileo. The Galileo spacecraft flew by asteroid 951 Gaspra on October 29, 1991, and by 243 Ida and its moon, Dactyl, on August 28, 1993. Near-Infrared Mapping Spectrometer (NIMS) data in the range from 0.7 to 5.3 µm showed that Gaspra is olivine-rich with a high olivine/pyroxene abundance ratio, and that Ida has an orthopyroxene/(orthopyroxene + olivine) ratio consistent with LL chondrites. Both are S-type asteroids. The spectrum of Dactyl also features a relatively deep 0.97-µm absorption.38 No organic features were found.
-
NEAR. The Near-Earth Asteroid Rendezvous (NEAR) spacecraft was launched on February 17, 1996, and carried an instrument package optimized to examine asteroid mineralogy and elemental abundances. Its instruments included an x-/gamma-ray spectrometer, a multispectral imager, and a near-infrared spectrometer that could provide spatially resolved spectra between 0.8 and 2.5 µm. On June 27, 1997, NEAR flew by the C-type asteroid 253 Mathilde and obtained spatially resolved spectra of one side of this 66 km by 48 km by 46 km body. The color of Mathilde is consistent with that of CM carbonaceous chondrites.
On February 14, 2000, NEAR entered orbit about the S-type asteroid 433 Eros. For the next year it conducted a global survey of this 34 km by 11 km by 11 km body before finally landing on its surface on February 12, 2001. Resolved spectra showed mineral absorption features at 1 and 2 µm that were characteristic of orthopyroxene and olivine, and spectral models suggested that the red color of the surface was due to space weathering of nanophase iron.39 The infrared spectral data and results of the x-ray spectrometer suggested a composition similar to that of a relatively primitive ordinary chondrite.40,41
-
Deep Space 1. The Deep Space 1 mission was a technology validation mission that was launched in October 1998 carrying a camera and ultraviolet (0.08 to 0.18 µm) and infrared (1.2 to 2.4 µm) spectrophotometers. The main purpose of the mission was to test a solar-electric propulsion system. The spacecraft had a close encounter with asteroid 9969 Braille on July 28, 1999. Initial indications were that the spectrum of Braille was similar to that of the asteroid Vesta. This interpretation proved untenable because the center of the 1-µm absorption band is at much too long a wavelength, and it is now accepted that the spectrum of Braille is a good match to that of a Q-type asteroid.42 Subsequent studies by the Deep Space 1 science team have confirmed this classification and have also suggested that Braille’s spectrum closely matches that of the most commonly found meteorites, the ordinary chondrites.43 Following the encounter, the spacecraft then embarked on its extended science mission to comet 19P/Borrelly (see Figure 4.3). The first in situ images were obtained of a Jupiter-family short-period comet and its surrounding environment on September 21, 2001, revealing a low-albedo (i.e., very black) nucleus.44 The mission ended in December 2001.
-
Stardust. The Stardust spacecraft was launched by NASA in 1999 and successful encountered the nucleus of the short-period comet 81P/Wild 2 (see Figure 4.3) on January 2, 2004. In situ images of the nucleus at closest approach showed a low-albedo nucleus covered in crater-like features. The spacecraft captured particles coming from the coma of the comet and successfully returned them to Earth for analysis on January 15, 2006. In addition to the aerogel used to capture the dust grains, the spacecraft carried a Cometary and Interstellar Dust Analyzer (CIDA), a dust flux monitor, and a navigation camera. CIDA, a mass spectrometer, provided some information about possible organic compounds. It is estimated that several thousand individual particles were collected on the back-to-back aerogel collecting trays as the spacecraft passed through the coma. The collectors were withdrawn into a reentry capsule that parachuted to Earth. The particles were then transferred to a laboratory for analysis. The samples will ultimately provide data on the amounts and classes of organics present in comets. To date, the most perplexing result from the initial analysis of the cometary samples is the abundance of mineral grains that could only have originated in the hottest, innermost regions of the solar nebula. Meanwhile, the Stardust spacecraft (minus its sample-return canister) continues to operate, and it may be redirected to encounter additional comets or asteroids.
-
Hayabusa. The Hayabusa mission, formerly known as MUSES-C, is a Japanese undertaking to rendezvous with 25143 Itokawa (a small near-Earth S-type asteroid, with a semimajor axis of 1.32 AU) and collect a surface sample for return to Earth. The spacecraft was launched on May 9, 2003, performed an Earth gravity-assist maneuver in May 2004, and used its solar-electric propulsion system to match orbits with the ~700 m by 300 m by 300 m body on September 12, 2005. Hayabusa’s instrumentation consists of a LIDAR (operating at 1.064 µm and used for ranging, gravitational field and surface reflectivity measurements, and topographic mapping), an x-ray fluorescence spectrometer (to determine the global abundances of iron, solium, magnesium, aluminum, silicon, sulfur, calcium, and titanium on the asteroid’s surface), a multiband imager (to enable topographic mapping and multiband polarimetry in eight spectral bands between 0.30 and 1.10 µm), and a near-infrared spectrometer (to obtain high-resolution mineralogical maps at wavelengths from 0.85 to 2.10 µm).
These instruments were used successfully to conduct a global survey of the asteroid from an altitude as low as 10 km. But then things began to go wrong. The attempt to deploy Minerva, a 0.55-kg lander equipped with three camera systems and temperature sensors, failed. Instead of using a pair of torquers to enable it to hop across the surface of Itokawa, Minerva drifted off into space and was lost. On November 19, 2005, and again 6 days later, Hayabusa attempted to perform touch-and-go landing at different sites on the asteroid’s surface.45 During each momentary touch down, the spacecraft was supposed to fired a 5-gram tantalum projectile into Itokawa’s surface. The debris thrown up from the surface was to be directed into a sample-return canister via a 1-m-long aluminum and fabric collecting funnel. It is not clear if any of these actions actually occurred. Multiple problems appear to have arisen during both attempts to conduct the complex, autonomously directed sample-collection process. These problems, combined with the failure of other spacecraft subsystems, ultimately delayed Hayabusa’s departure from Itokawa, and the return of the samples to Earth, if any were actually collected. It is now expected to reach Earth in 2010. In the
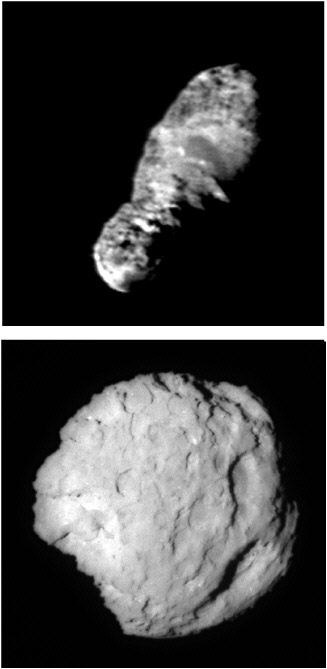
FIGURE 4.3 Image of the nucleus 19P/Borrelly taken by the Deep Space 1 mission on September 25, 2001, from a distance of 3417 km. The nucleus is 8 km long (nuclei range in size between 0.1 and 100 km). The albedo varies between 0.007 and 0.03 across the surface. The closest short exposure of the comet Wild 2, which NASA’s Stardust spacecraft flew by on January 2, 2004, taken at an 11.4-degree angle, the angle between the camera, comet, and the Sun. SOURCE: Images courtesy of NASA/JPL.
-
meantime, the Japan Aerospace Exploration Agency is investigating the possibility of building an improved version of Hayabusa and sending it to another asteroid sometime in the future.
-
Deep Impact. The main goal of the Deep Impact mission was to examine the pristine interior of a comet by creating a controlled experiment to excavate beneath the evolved surface layers. The Deep Impact spacecraft was launched on January 12, 2005, and released a 360-kg impactor on July 2, 2005. Two days later, while the Deep Impact spacecraft remained at a safe distance, the impactor collided with the nucleus of the Jupiter-family comet 9P/Tempel 1. The impact was observed by an imager on the impactor, as well as by the medium- and high-resolution optical cameras and near-infrared spectrometer (1.0 to 4.8 µm) on Deep Impact. The spectroscopic capabilities were designed to cover the organically interesting region of the near-infrared spectrum. The team was particularly interested in measuring the abundances of H2O, H2CO, CO2, CO, and other organic species before, during, and after impact (Figure 4.4). Deep Impact continues to operate and may be redirected to encounter additional objects in the future.
-
Dawn. The goal of the solar-electric-powered Dawn mission, currently scheduled for launch in June 2007, is to sequentially orbit the large, main-belt asteroids 1 Ceres and 4 Vesta to study their basic structures and compositions. Despite numerous technical and programmatic issues during its development, including cancellation in February 2006 and reinstatement a few months later, Dawn is still scheduled to arrive at Vesta in October 2011 and at Ceres in February 2015. The Dawn mission instrumentation includes an imaging camera, gamma-ray and neutron spectrometer, and a visible and infrared mapping spectrometer (0.35 to 0.9 µm, 0.8 to 2.5 µm, and 2.4 to 5.0 µm, respectively). One of the top-level questions addressed will be to assess the role of size and water in the evolution of planetary bodies. The 960-km-diameter Ceres is a low-albedo G-type asteroid, which exhibits an infrared reflectance spectrum possibly associated with the effects of aqueous alteration. The 520-km-diameter Vesta is a V-type asteroid and likely source of the so-called Howardite, Eucrite, and Diogenite (HED) meteorites.46
-
Rosetta. The European Space Agency launched Rosetta to comet 67P/Churyumov-Gerisimenko in March 2004, and it is expected to arrive at its target in November 2014. The mission goals are to undertake a long-
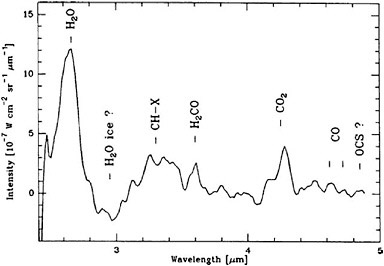
FIGURE 4.4 Infrared spectrum of comet 1P/Halley showing emission lines in the region of the spectrum of interest for Deep Impact. SOURCE: Reprinted from M. Combes, J. Crovisier, T. Encrenaz, V.I. Moroz, and J.-P. Bibring, “The 2.5-12 Micron Spectrum of Comet Halley from the IKS-VEGA Experiment,” Icarus 76: 404-436, copyright 1988, with permission from Elsevier.
-
term in situ exploration of the comet from large heliocentric distances through perihelion. The scientific payload of Rosetta includes a visual infrared spectral and thermal spectrometer (VIRTIS), which will be fundamental for characterizing specific spectral bands of minerals and organic molecules arising from surface components and from materials dispersed in the coma. Their identification is a primary goal of the Rosetta mission.47 In addition, the orbiter mission instrumentation includes an ultraviolet spectrometer, a nucleus sounder, an ion mass analyzer, a grain impact analyzer, a microwave system, an imaging system, a plasma experiment, and a radio science experiment. The lander will also be well instrumented, including the cometary sampling and composition (COSAC) experiment and an evolved gas analyzer (PTOLEMY). COSAC is designed to identify complex organic molecules, and PTOLEMY will make accurate measurements of the isotopic ratios of light elements. The team is also planning to fly by 1 to 2 asteroids en route (the targets are still to be identified).
-
New Horizons. The first of the New Frontiers mission line, and the NRC solar system exploration decadal survey’s highest recommendation for a new medium-class mission,48 New Horizons is designed to characterize the primitive icy bodies in the far reaches of the solar system. The spacecraft was launched on schedule on January 19, 2006, and will reach the Pluto-Charon system in July 2015. After a flyby reconnaissance of the Pluto-Charon system, the spacecraft will continue outward to examine one or two KBOs. The primary objectives include characterization of the global geology and surface composition (H2O, CO, CO2, and CH4) of Pluto and Charon, and the neutral atmosphere of Pluto. The primary instrument payload includes an ultraviolet/visible/infrared imaging and spectroscopy system, and a radio science/radiometry package. Low-resolution infrared spectroscopy is available between 1.25 and 2.50 µm.
FUTURE RESEARCH DIRECTIONS
Although there clearly is a wealth of recent, ongoing, and upcoming missions to primitive bodies, they have not all been optimized for the study of organics. Within the primitive small-body population there exists a preserved reservoir of organic material and some small bodies are trapped in dynamically stable niches spread over a wide range of nebular heliocentric distances. A rich research opportunity exists to explore these different chemical and thermal regimes, thus enabling an understanding of the distribution and history of primordial carbon in the solar system. Support for key existing and new capabilities in several areas related to research on small bodies can have a large impact toward furthering knowledge of organic matter in the solar system.
Laboratory Opportunities
The spectrally red colors of these primitive solar system bodies are often interpreted to be caused by the presence of organic materials. However, the spectral reflectance of Hektor, a D-type Trojan asteroid, can be modeled with a magnesium-rich pyroxene and a serpentine, and organic solids are not required to match the low albedo and red color of this object. Spectral models can account for the full range of colors of KBO and Centaur reflectance spectra as well as their low albedos from combinations of tholins and amorphous carbon.49 These models also allow for (but do not require) the presence of space-weathered (reddened) igneous rock-forming minerals, and can incorporate the presence of volatiles in sufficient quantities that the absorption bands are not completely quenched by the coexistence of other low-albedo materials. Caution should be used within this type of model in inferring the organic inventory of these bodies. Observed changes in spectral slopes or broad, weak bumps or hollows can have no analytical relevance because color and albedo are highly nonlinear functions of composition and grain sizes and shapes. Thus the surface composition of mixtures (geometric and intimate) can be strongly influenced by the details of how the components are mixed. However, by adding information about the albedo of the object, many of the ambiguities can be reduced.50 The Spitzer Space Telescope will undertake to measure the albedos of a large number of these outer solar system bodies, and thus researchers will soon be extremely well situated to investigate the organic compositions of these bodies with a combination of infrared spectroscopy and spectral modeling.
Additional laboratory spectral measurements of model compounds and materials are needed to provide further information concerning organic materials possibly found on Triton, Pluto, Charon, Centaurs, and Kuiper
belt objects. The spectral properties of the ices of relevant hydrocarbons should also be studied in order to facilitate the detection of the various organics that may exist on these bodies. These laboratory-based investigations should utilize mixtures of candidate organic compounds alone and together with water ice to examine effects of mixing. Effects of irradiation of these ices should be examined to determine what organic compounds are formed.
At present, the relevant optical constants have been measured for only a few of the organic and inorganic compounds that are likely to be present in primitive bodies of interest. Without a suite of materials with known constants to incorporate in the spectral models, the identification of many of the observed spectral features remains challenging. With modest support for laboratory work of this kind, great progress could be made in understanding the organic components of primitive solar system bodies.
Recommendation: The physical, chemical, and spectroscopic properties of ices of potential hydrocarbon species should be studied to facilitate the detection of organic materials.
Ground-Based Observing Facilities and Opportunities
High-resolution infrared spectroscopy of comets is playing an increasing role in characterizing the link between cometary and interstellar ices and is providing insights into the chemistry of the protoplanetary nebula. While the science is exciting, progress in this area is likely to be slow because of the limited access to ground-based facilities capable of performing such observations (at present Keck with NIRSPEC, and Subaru with IRCS). Having more planetary science access to these facilities (Keck in particular), or a larger, modern replacement for the NASA Infrared Telescope Facility, would significantly increase the rate of progress in studies of the organic inventory in the solar system’s most primitive objects. In addition, information is just beginning to accumulate about the organics on the Kuiper belt objects. The limit on gathering such information is access to large facilities capable of performing the low-resolution near-infrared spectroscopy of the solid surfaces (combined with spectral modeling).
Recommendation: The task group reiterates the call made in the 2003 report of the National Research Council’s Solar System Exploration Decadal Survey Committee, New Frontiers in the Solar System,51 that NASA’s support for planetary observations with ground-based astronomical instruments, such as the Infrared Telescope Facility and the Keck telescopes, be continued and upgraded as appropriate, for as long as they provide significant scientific return and/or mission-critical support services.
Space Missions
The current and upcoming space missions are targeted to active comets, Pluto/Charon, and perhaps one or two KBOs, as well as to asteroids with a variety of spectral types. Many of the missions have at least limited capability to search for organics and carbon-bearing compounds, but only Deep Impact and Rosetta can obtain spectra out to 5 µm, and only Rosetta is truly optimized for organic studies.
Recommendation: Every opportunity should be taken to direct space missions to small bodies to do infrared spectral studies of these targets, especially a D- or P-type asteroid, to determine if these dark bodies contain an appreciable amount of carbon compounds and, if so, whether they are the sources of the carbonaceous meteorites and dust reaching Earth.
In this regard, a possible opportunity to conduct such studies is as an adjunct to the Trojan Asteroid/Centaur Reconnaissance flyby mission described in the solar system exploration decadal survey. Although this mission was not ranked in the survey’s final list of priorities, the possibility of using a single spacecraft to make a sequential flyby of three different classes of primitive bodies—i.e., a D- or P-type main-belt asteroid, a jovian
Trojan asteroid, and a Centaur—has sufficient merit to warrant additional study for possible implementation as a New Frontiers mission at some time in the future.
The successful landing of the NEAR spacecraft on the asteroid Eros demonstrated the feasibility of sending a probe to land on an asteroid. In situ analyses as well as sample-return missions should be performed for both asteroids and comets. Such missions will provide direct information on the nature of the organics present in asteroids and comets and information about whether or not the asteroids are the sources of meteorites and dust reaching Earth. They will also supply data on the structural information on the compounds coming from asteroids as compared to the organics derived from comets. If there are major structural differences between the cometary and asteroidal organics, that information will provide insight into the chemical reactions that took place in forming the organics in each reservoir. These data will also provide insight into the differences in conditions present where the cometary and asteroidal organics were formed. It may be possible to suggest what organic structures in comets or asteroids were the more likely contributors of chemical precursors of life to the early Earth. At present there is no consensus as to which starting materials initiated the origin of life or even if extraterrestrial organic compounds even played a significant role in life’s origin.
Recommendation: In situ analyses as well as sample-return missions should be performed for both asteroids and comets. The task group points to the solar system exploration decadal survey report’s recommendation for a New Frontiers-class Comet Surface Sample Return mission as an example of an activity that would greatly enhance understanding of the organic constituents of the solar system’s primitive bodies.52
Not all existing and planned missions to primitive bodies have been optimized for the study of organic materials. The population of primitive small bodies, however, may preserve organic materials from a wide range of nebular heliocentric distances. A rich research opportunity exists to explore these different chemical and thermal regimes, thus enabling an understanding of the distribution and history of organic materials in the solar system.
NOTES
1. Centaurs are minor solar system bodies whose heliocentric orbit lies between Jupiter and Neptune and typically crosses the orbits of one of the other outer giant planets (Saturn, Uranus, Neptune). The orbits of the Centaurs are dynamically unstable due to interactions with the giant planets and are probably dynamically evolving from the Kuiper belt into short-period comet orbits.
2. The Kuiper belt is a region at a heliocentric distance of approximately 30 to 50 AU in the solar system populated by small (up to a few times 100-km diameter) bodies believed to be remnants from the formation of the solar system. This region is believed to be the source of some of the short-period comets.
3. A. Carusi, L. Kresak, E. Perozzi, and G.B. Valsecchi, Long-term Evolution of Short-period Comets, Adam Hilger, Ltd., Bristol, England, 1985.
4. D. Laufer, E. Kochavi, and A. Bar-Nun “Structure and Dynamics of Amorphous Water Ice,” Physical Review B 36: 9219-9227, 1987.
5. R.E. Johnson, “Irradiation Effects in a Comet’s Outer Layers,” Journal of Geophysical Research 96: 17553-17557, 1991.
6. G. Strazzulla and R.E. Johnson, “Irradiation Effects on Comets and Cometary Debris,” pp. 243-275 in Comets in the Post-Halley Era (R.L. Newburn, M. Neugebauer, and Jürgen H. Rahe, eds.), Kluwer Academic Publishers, Dordrecht, The Netherlands, 1991.
7. M.H. Moore, R.L. Hudson, and R.F. Ferrante, “Radiation Products in Processed Ices Relevant to Edgeworth-Kuiper Belt Objects,” Earth, Moon, and Planets 92: 291-306, 2003.
8. D. Prialnik, A. Bar-Nun, and M. Podolak, “Radiogenic Heating of Comets by 26Al and Implications for their Time of Formation,” Astrophysical Journal 319: 993-1002, 1987.
9. M. Podolak and D. Prialnik, “Conditions for the Production of Liquid Water in Comet Nuclei,” pp. 231-234 in A New Era in Bioastronomy (G. Lemarchand and K. Meech, eds.), ASP Conference Series, Vol. 213, 2000.
10. M.F. A’Hearn, R.L. Millis, D.G. Schleicher, D.J. Osip, and P.V. Birch, “The Ensemble Properties of Comets: Results from Narrowband Photometry of 85 Comets, 1976-1992,” Icarus 118: 223-270, 1995.
11. The color of an object refers to the variation of reflectivity as a function of wavelength, and those objects that have increasing reflectance at longer wavelengths are termed “red.”
12. M.A. Barucci, D.P. Cruikshank, S. Mottola, and M. Lazzarin, “Physical Properties of Trojan and Centaur Asteroids,” pp. 273-287 in Asteroids III (W.F. Bottke, Jr., A. Cellino, P. Paolicchi, and R.P. Binzel, eds.), University of Arizona Press, Tucson, Ariz., 2002.
13. T. Hiroi, M.E. Zolensky, and C.M. Pieters, “The Tagish Lake Meteorite: A Possible Sample from a D-Type Asteroid,” Science 293: 2234-2236, 2001.
14. D.P. Cruikshank and C.M. Dalle Ore, “Spectral Models of Kuiper Belt Objects and Centaurs,” Earth, Moon, and Planets 92: 313-330, 2003.
15. F.L. Whipple, “A Comet Model. I. The Acceleration of Comet Encke,” Astrophysical Journal 111: 375-394, 1950.
16. M.J. Mumma., P.R. Weissman, and S.A. Stern, “Comets and the Origin of the Solar System—Reading the Rosetta Stone,” pp. 1177-1252 in Protostars and Planets III (E. Levy and J.I. Lunine, eds.), University of Arizona Press, Tucson, Ariz., 1993.
17. W.M. Irvine, F.P. Schloerb, J. Crovisier, B. Fegley, Jr., and M.J. Mumma, “Comets: A Link Between Interstellar and Nebular Chemistry,” p. 1159 in Protostars and Planets IV (V. Mannings, A.P. Boss, and S.S. Russell, eds.), University of Arizona Press, Tucson, Ariz., 2000.
18. J.M. Greenberg, “Making a Comet Nucleus,” Astronomy and Astrophysics 330: 375-380, 1998.
19. M.J. Mumma, N. Dello Russo, M.A. DiSanti, K. Magee-Sauer, R.E. Novak, S. Brittain, T. Rettig, I.S. McLean, D.C. Reuter, and Li-H. Xu, “Organic Composition of C/1999 S4 (LINEAR): A Comet Formed Near Jupiter?” Science 292: 1334-1339, 2001.
20. N. Biver, D. Bockelé-Morvan, P. Colom, J. Crovisier, B. Germain, E. Lellouch, J.K. Davies, W.R.F. Dent, R. Moreno, G. Paubert, J. Wink, D. Despois, D.C. Lis, D. Mehringer, D. Benford, M. Gardner, T.G. Phillips, M. Gunnarsson, H. Rickman, A. Winnberg, P. Bergman, L.E.B. Johansson, and H. Rauer, “Long-term Evolution of the Outgassing of Comet Hale-Bopp from Radio Observations,” Earth, Moon, and Planets 78: 5-11, 1999.
21. P. Eberhardt, “Comet Halley’s Gas Composition and Extended Sources: Results from the Neutral Mass Spectrometer on Giotto,” Space Science Reviews 90: 45-52, 1999.
22. M.J. Mumma and D.C. Reuter, “On the Identification of Formaldehyde in Halley’s Comet,” Astrophysical Journal 344: 940-948, 1989.
23. K. Altwegg, H. Balsiger, and J. Geiss, “Composition of the Volatile Material in Halley’s Coma from In Situ Measurements,” Space Science Reviews 90: 3-18, 1999.
24. M.N. Fomenkova, “On the Organic Refractory Component of Cometary Dust,” Space Science Reviews 90: 109-114, 1999.
25. M.J. Mumma, M.A. Disanti, N. dello Russo, M. Fomenkova, K. Magee-Sauer, C.D. Kaminski, and D.X. Xie, “Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin,” Science 272: 1310-1314, 1996.
26. M.F. A’Hearn, R.L. Millis, D.G. Schleicher, D.J. Osip, and P.V. Birch, “The Ensemble Properties of Comets: Results from Narrowband Photometry of 85 Comets, 1976-1992,” Icarus 118: 223-270, 1995.
27. E.K. Jessberger, “Rocky Cometary Particulates: Their Elemental Isotopic and Mineralogical Ingredients,” Space Science Reviews 90:91-97, 1999.
28. National Research Council, The Astrophysical Context of Life, The National Academies Press, Washington, D.C., 2005, pp. 41-42.
29. L. Becker and R.J. Poreda, “Fullerene and Mass Extinctions in the Geologic Record,” Meteoritics and Planetary Science 36: A17, 2001.
30. K.O. Pope, S.W. Kieffer, and D.E. Ames, “Empirical and Theoretical Comparisons of the Chicxulub and Sudbury Impact Structures,” Meteoritics and Planetary Science 39: 97-116, 2004.
31. Tholins are organic solids produced by the irradiation of cosmically abundant reducing gases (C. Sagan and B. Khare, “Tholins: Organic Chemistry of Interstellar Grains and Gas,” Nature 277, 102-107, 1979). “Tholin” is not a specific term, since one will generate a wide array of different solids depending on the type of irradiation used and the composition of the mixture of gases irradiated. It was coined from the Greek word “tholos,” meaning “muddy.”
32. C.A. Trujillo and M.E. Brown, “A Correlation Between Inclination and Color in the Classical Kuiper Belt,” Astrophysical Journal Letters 566: 125-128, 2002.
33. O.R. Hainaut, C.E. Delahodde, H. Boehnhardt, E. Dotto, M.A. Barucci, K.J. Meech, J.M. Bauer, R.M. West, and A. Doressoundiram, “Physical Properties of TNO 1996 TO66,” Astronomy and Astrophysics 356: 1076-1088, 2000.
34. T. Hiroi, M.E. Zolensky, and C.M. Pieters, “The Tagish Lake Meteorite: A Possible Sample from a D-type Asteroid,” Science 293:2234-2236, 2001.
35. P.D. Wilson and C. Sagan, “Spectrophotometry and Organic Matter on Iapetus. 1: Composition Models,” Journal of Geophysical Research 100: 7531-7537, 1995.
36. J. Hunter Waite, Jr., M.R. Combi, W-H. Ip, T.E. Cravens, R.L. McNutt, Jr., W. Kasprzak, R. Yelle, J. Luhman, H. Niemann, D. Gell, B. Magee, G. Flecther, J. Lunine, and W-L. Tseng, “Cassini Ion and Neutral Mass Spectrometer: Enceladus Plume Composition and Structure,” Science 311: 1419-1422, 2006.
37. J.M. Bauer, T.L. Roush, T.R. Gaballe, K.J. Meech, T.C. Owen, W.D. Vacca, J.T. Rayner, and K.T.C. Jim, “The Near-infrared Spectrum of Miranda: Evidence of Crystalline Water Ice,” Icarus 158: 178-190, 2002.
38. J. Granahan, “A Compositional Study of Asteroid 243 Ida and Dactyl from Galileo NIMS and SSI Observations,” Journal of Geophysical Research 107: 20-21, 2002.
39. J.F. Bell, N.I. Izenberg, P.G. Lucey, B.E. Clark, C. Peterson, M.J. Gaffey, J. Joseph, B. Carcich, A. Harch, M.E. Bell, J. Warren, P.D. Martin, L.A. McFadden, D. Wellnitz, S. Murchie, M. Winter, J. Veverka, P. Thomas, M.S. Robinson, M. Malin, and A. Cheng, “Near-infrared Reflectance Spectroscopy of 433 Eros from the NIS instrument on the NEAR Mission. I. Low Phase Angle Observations,” Icarus 155: 119-144, 2002.
40. J. Veverka, M. Robinson, P. Thomas, S. Murchie, J.F. Bell III, N. Izenberg, C. Chapman, A. Harch, M. Bell, B. Carcich, A. Cheng, B. Clark, D. Domingue, D. Dunham, R. Farquhar, M.J. Gaffey, E. Hawkins, J. Joseph, R. Kirk, H. Li, P. Lucey, M. Malin, P. Martin, L.
McFadden, W.J. Merline, J.K. Miller, W.M. Owen, Jr., C. Peterson, L. Prockter, J. Warren, D. Wellnitz, B.G. Williams, and D.K. Yeomans, “NEAR at Eros: Imaging and Spectral Results,” Science 289: 2088-2097, 2000.
41. J.I. Trombka, S.W. Squyres, J. Brückner, W.V. Boynton, R.C. Reedy, T.J. McCoy, P. Gorenstein, L.G. Evans, J.R. Arnold, R.D. Starr, L.R. Nittler, M.E. Murphy, I. Mikheeva, R.L. McNutt, Jr., T.P. McClanahan, E. McCartney, J.O. Goldsten, R.E. Gold, S.R. Floyd, P.E. Clark, T.H. Burbine, J.S. Bhangoo, S.H. Bailey, and M. Petaev, “The Elemental Composition of Asteroid 433 Eros: Results from the NEAR-Shoemaker X-ray Spectrometer,” Science 289: 2101-2105, 2000.
42. R.P. Binzel, A.W. Harris, S.J. Bus, and T.H. Burbine, “Spectral Properties of Near-Earth Objects: Palomar and IRTF Results for 48 Objects Including Spacecraft Targets (9969) Braille and (10302) 1989 ML,” Icarus 151: 139-149, 2001.
43. B.J. Buratti, D.T. Britt, L.A. Soderblom, M.D. Hicks, D.C. Boice, R.H. Brown, R. Meier, R.M. Nelson, J. Oberst, T.C. Owen, A.S. Rivkin, B.R. Sandel, S.A. Stern, N. Thomas, and R.V. Yelle, “9969 Braille: Deep Space 1 Infrared Spectroscopy, Geometric Albedo, and Classification,” Icarus 167: 129-135, 2004.
44. D.C. Boice, L.A. Soderblom, D.T. Britt, R.H. Brown, B.R. Sandel, R.V. Yelle, B.J. Buratti, M.D. Hicks, R.M. Nelson, M.D. Rayman, J. Oberst, and N. Thomas, “The Deep Space 1 Encounter with Comet 19P/Borrelly,” Earth, Moon, and Planets 89: 301-324, 2002.
45. For a more complete description of touch-and-go sampling see, for example, Space Studies Board, National Research Council, A Scientific Rationale for Mobility in Planetary Environments, National Academy Press, Washington, D.C., 1999, p. 37.
46. A.S. Rivkin, Observations of Main-belt Asteroids in the 3-Micron Region, Ph.D. Thesis, University of Arizona, 1997.
47. A. Coradini, F. Capaccioni, P. Drossart, A. Semery, G. Arnold, U. Schade, F. Angrilli, M.A. Barucci, G. Bellucci, G. Bianchini, J.P. Bibring, A. Blanco, M. Blecka, D. Bockelee-Morvan, R. Bonsignori, M. Bouye, E. Bussoletti, M.T. Capria, R. Carlson, U. Carsenty, P. Cerroni, L. Colangeli, M. Combes, M. Combi, J. Crovisier, M. Dami, M.C. DeSanctis, A.M. DiLellis, E. Dotto, T. Encrenaz, E. Epifani, S. Erard, S. Espinasse, A. Fave, C. Federico, U. Fink, S. Fonti, V. Formisano, Y. Hello, H. Hirsch, G. Huntzinger, R. Knoll, D. Kouach, W.H. Ip, P. Irwin, J. Kachlicki, Y. Langevin, G. Magni, T. McCord, V. Mennella, H. Michaelis, G. Mondello, S. Mottola, G. Neukum, V. Orofino, R. Orosei, P. Palumbo, G. Peter, B. Pforte, G. Piccioni, J.M. Reess, E. Ress, B. Saggin, B. Schmitt, D. Stefanovitch, A. Stern, F. Taylor, D. Tiphene, and G. Tozzi, “Virtis: An Imaging Spectrometer for the Rosetta Mission,” Planetary and Space Science 46: 1291-1304, 1998.
48. National Research Council, New Frontiers in the Solar System: An Integrated Exploration Strategy, The National Academies Press, Washington, D.C., 2003, pp. 31-32, 115, 136, 145, and 206-207.
49. D.P. Cruikshank and C.M. Dalle Ore, “Spectral Models of Kuiper Belt Objects and Centaurs,” Earth, Moon, and Planets 92: 313-330, 2003.
50. W.M. Grundy and J.A. Stansberry, “Mixing Models, Colors and Thermal Emissions,” Earth, Moon, and Planets 92: 331-336, 2003.
51. National Research Council, New Frontiers in the Solar System: An Integrated Exploration Strategy, The National Academies Press, Washington, D.C., 2003, pp. 206-207.
52. National Research Council, New Frontiers in the Solar System: An Integrated Exploration Strategy, The National Academies Press, Washington, D.C., 2003, p. 195.




















