Summary and Assessment
ETHICAL AND LEGAL CONSIDERATIONS IN MITIGATING PANDEMIC DISEASE
In recent public workshops and working group meetings, the Forum on Microbial Threats of the Institute of Medicine (IOM) has examined a variety of infectious disease outbreaks with pandemic potential, including those caused by influenza (IOM, 2005) and severe acute respiratory syndrome (SARS) (IOM, 2004). Particular attention has been paid to the potential pandemic threat posed by the H5N1 strain of avian influenza, which is now endemic in many Southeast Asian bird populations. Since 2003, the H5N1 subtype of avian influenza has caused 185 confirmed human deaths in 11 countries, including some cases of viral transmission from human to human (WHO, 2007). But as worrisome as these developments are, at least they are caused by known pathogens. The next pandemic could well be caused by the emergence of a microbe that is still unknown, much as happened in the 1980s with the emergence of the human immunodeficiency virus (HIV) and in 2003 with the appearance of the SARS coronavirus.
Previous Forum meetings on pandemic disease have discussed the scientific and logistical challenges associated with pandemic disease recognition, identification, and response. Participants in these earlier meetings also recognized the difficulty of implementing disease control strategies effectively and, at the
The Forum’s role was limited to planning the workshop, and the workshop summary has been prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop.
same time, with fairness and justice. Many of the proposed disease mitigation strategies may have unintended—and often undesirable—consequences, such as adverse economic effects or the restriction of civil rights and civil liberties. To focus attention on these concerns as well as on other profound ethical and legal issues that are inherent in various pandemic disease mitigation approaches being proposed domestically and internationally, the Forum convened a public workshop, Ethical and Legal Considerations in Mitigating Pandemic Disease on September 19–20, 2006. Through invited presentations and discussions, participants explored lessons learned from past pandemics, identified barriers to equitable and effective responses to future pandemics, and examined opportunities to overcome these obstacles through research, policy, legislation, communication, and community engagement.1
ORGANIZATION OF THE WORKSHOP SUMMARY
This workshop summary was prepared for the Forum membership in the name of the rapporteurs and includes a collection of individually-authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections are based on the presentations and discussions that took place during the workshop.
The workshop summary is organized into chapters as a topic-by-topic description of the presentations and discussions that took place at the workshop. Its purpose is to present lessons from relevant experience, delineate a range of pivotal issues and their respective problems, and to offer some potential responses as described by the workshop participants.
Although this workshop summary provides an account of the individual presentations, it also reflects an important aspect of the Forum philosophy. The workshop functions as a dialogue among representatives from different sectors and presents their beliefs about which areas may merit further attention. The reader should be aware, however, that the material presented here expresses the views and opinions of the individuals participating in the workshop and not the deliberations of a formally constituted IOM study committee. These proceedings summarize only what participants stated in the workshop and are not intended to be an exhaustive exploration of the subject matter or a representation of consensus evaluation.
LESSONS FROM THE PAST
Current thinking on the prevention and control of pandemic disease is informed, to a large extent, by the past century’s experiences with emerging, reemerging, and novel infectious disease threats. A careful examination of community responses to a broad range of infectious diseases, however, reveals enduring dilemmas that must be addressed anew with each pandemic threat. Workshop participants discussed a range of legal and ethical issues that were raised by the great influenza pandemic of 1918; the recent and mercifully short-lived outbreak of severe acute respiratory syndrome (SARS); the ongoing major epidemics such as HIV/AIDS and endemic diseases such as malaria; the efforts to control and eliminate poliovirus; the singular triumph over smallpox; and the threatened 1976 “swine flu” pandemic that wasn’t.2
In his keynote address (see Chapter 1), David Heymann, Executive Director for Communicable Diseases at the World Health Organization (WHO), discussed notable outbreaks of emerging and reemerging diseases in the context of several interrelated issues, each of which—either individually or collectively—carries profound ethical and legal implications:
-
The vulnerability of health workers to infectious disease and their duty to provide care
-
Each country’s responsibility to reduce the international spread of infectious diseases while simultaneously preserving trade
-
Ensuring equitable access to health-care resources
-
Balancing individual rights and the public good
Defined by past epidemics, these issues challenge our highly interconnected world with unprecedented urgency, as we anticipate the next infectious disease pandemic.
In response to this challenge, WHO has been engaged in a process of revising
the International Health Regulations (IHR).3 Heymann described recent efforts to expand the concept of “reportable diseases” (originally limited to cholera, plague, and yellow fever) to encompass all infectious diseases of global importance, including emerging microbial threats such as SARS (see below). The revised IHR will come into full effect in July 2007, but at the request of the World Health Assembly, WHO is prepared to implement the revisions sooner if an influenza pandemic should materialize before that date.
The Pandemic Response in History
Viewing the lessons of past pandemics from a historian’s perspective, speaker Howard Markel of the University of Michigan described the organization of common social responses to epidemic or pandemic disease into narrative frameworks (see Markel, page 44). One such model, described by Charles Rosenberg, portrays an epidemic as a drama in four acts (Rosenberg, 1992):
-
“Progressive revelation,” in which members of a community begin to acknowledge casualties resulting from the spread of a particular contagious disease
-
“Managing randomness,” in which community members seek explanations (often religious ones) for the seeming arbitrariness of infection
-
“Negotiating public response,” in which community members demand collective action
-
“Subsidence and retrospection,” often leading to complacency as the memory of the epidemic fades from the community
Markel’s own analysis of the social responses to epidemic or pandemic disease identifies a series of central themes, or leitmotivs, that have recurred since the Black Death (bubonic plague) of the Middle Ages:
-
The public’s understanding about how a disease is transmitted will affect the course of the epidemic.
-
The economic consequences of an epidemic influence the public’s response to the crisis.
-
The extent and speed of travel of both people and goods are major factors in the spread of pandemic disease locally and globally.
-
Microbes that kill relatively few people, but do so quickly and spectacu-
-
larly (e.g., Ebola hemorrhagic fever, anthrax) get more attention than ongoing pandemics that kill millions year after year (e.g., tuberculosis, HIV/AIDS).
-
Media coverage, which can both inform and misinform the public, influences the course of an epidemic.
-
Governments will often attempt to conceal outbreaks from the world at large, typically in an effort to protect economic assets and trade.
-
“Undesirable” social groups may be blamed for an epidemic or unfairly treated in the name of preventing disease transmission.
Escaping the 1918 Pandemic
In addition to offering insights into the legal and ethical issues afforded by a review of past infectious disease pandemics, Markel described how certain historical data—in this case, evaluating the effectiveness of past intervention efforts—can inform efforts today to reduce infectious disease transmission and its impact. As Markel pointed out, American communities produced a vast body of information between 1918 and1920 on the use of what are now termed “nonpharmaceutical interventions” (NPIs) against pandemic influenza. A critical analysis of these data may contain insights for mitigating the impacts of future pandemics.
By 1918, science was sufficiently sophisticated to characterize the most lethal infectious outbreak in recorded history and even to anticipate that such an event would occur (IOM, 2005). Nevertheless, between 50 and 100 million people perished in the global pandemic that began that year, many of them young adults. Today, although substantial efforts are under way to develop and stockpile vaccines and antiviral pharmaceuticals in anticipation of a threatened pandemic of H5N1 avian influenza, it is unlikely that enough of these measures will be available at the beginning of such a crisis, and their effectiveness is far from assured. Should influenza—or any other infectious disease with pandemic potential—strike, the initial individual and community containment measures employed will almost certainly be similar to the nonpharmaceutical interventions that were used almost a century ago: isolation of ill persons and quarantine of their suspected contacts from the “well”; social distancing; simple sanitary measures such as washing hands and wearing facemasks; and providing the public with information about the disease and its risks (Markel et al., 2006).
In his contribution to this volume, Markel observed that NPIs are generally considered to have offered little, if any, protection against the severe and fast-spreading influenza of 1918. While he conceded that “no systematic study exists on the relationship, positive or negative, between influenza case incidence and death rates during the 1918 pandemic and the various NPIs put into effect by the most-populated urban centers in the United States,” he observed that certain combinations of NPIs may have lowered death rates in some communities in the United States. His study of “escape communities”—an ongoing examination of
NPIs used in American cities in 1918—suggests that stringent sequestration measures, applied well in advance of influenza’s arrival and kept in place for extended periods, was associated with reduced influenza mortality.
While NPIs may indeed have contained influenza in some communities in 1918-1920, Markel acknowledged that implementing similar strategies in the U.S. today would present a different set of challenges. For instance, the notion that the right to civil liberty should influence public health policy—particularly with respect to minorities and the poor—is a very recent concept, he said: “The idea that you would be planning a public health policy with cultural or ethical or social or legal implications, as … we are discussing today … is quite new in the history of epidemics and medicine.”
Learning from SARS
The emergence of SARS in November 2002 gave the world an opportunity to respond to a controllable human pandemic, thanks, in large part, to the limited transmissibility of the SARS coronavirus, particularly in the early stages of the illness (IOM, 2004). The four ethical issues introduced by Heymann (see above) are prominent within this story, which he recounts in detail in Chapter 1. This experience demonstrated the feasibility of containing a pandemic through a coordinated, international effort, but it also highlighted the economic and political costs associated with reporting an outbreak and their potential to undermine efforts to protect the global community from infectious diseases.
Heymann commented that the process by which the WHO detected and responded to SARS represented an important milestone. The response to SARS gave priority to global public health over national sovereignty, and it challenged national control of communications and public health activities (Heymann, 2006a; Fidler, 2004). The experience spurred efforts to establish ethical and legal guidelines for international cooperation and collaboration through the aforementioned revision of the IHR (Gostin, 2004; Fidler and Gostin, 2006).
The revised IHR has been welcomed as a first step toward a much-needed comprehensive global plan for addressing infectious disease. However, some members of the public health community—including some workshop participants—have expressed concerns that the regulations will not be completely effective because they do little to address the economic barriers to reporting infectious disease (Cash and Narashimhan, 2000; Fidler and Gostin, 2006). These critics assert that international efforts must also compensate countries for the costs of reporting and containing infectious disease outbreaks, ensure that trade and travel restrictions are cost-effective, and support public health capacity-building in vulnerable developing countries. An example of this sort of effort is the recent U.S. decision to place part of its pre-pandemic vaccine and antiviral stockpile close to vulnerable populations in Southeast Asia.
Participants also discussed the possibility of enforcing the IHR’s infec-
tious disease reporting conventions by linking them to participation in international trade (e.g., through the World Trade Organization). While everyone acknowledged the critical influence that trade has on outbreak reporting, some expressed doubts that such global governance would be acceptable to most nations; the United States, for example, has refused to sign similar conventions that it believed might compromise its sovereignty. Heymann described efforts to end the imposition of ill-founded trade embargoes as an example of how WHO, in partnership with groups such as the Food and Agriculture Organization (FAO) of the United Nations and the World Organization for Animal Health (OIE4), is attempting to increase acceptance and improve enforcement of the IHR in the global community.
An Exceptional Case: Smallpox Eradication
In 1980, smallpox became the first—and, to date, only—human infectious disease to be eradicated from the planet. Speaker D.A. Henderson, who led the quarter-century campaign to eliminate the disease, explained that the success of WHO’s smallpox eradication program resulted in part from lessons learned from the failure of a similar campaign to eliminate malaria, which also began in the mid-1950s (Henderson, 1999). Applying these hard-learned lessons, the smallpox program took advantage of available resources in host countries, adopted broad goals that could be achieved in multiple ways, and supported a wide range of clinical, epidemiological, and operational research. Eradication of the smallpox virus was also favored by several important and unique aspects of the disease and the agent itself, including among others, the high rate of symptoms among the infected; the efficacy of the smallpox vaccine; and the absence of a nonhuman host for the virus.
Henderson also explored a series of ethical issues raised by the smallpox eradication campaign. He noted, for example, that advocates of eradication consider it to be an important element of distributive justice, since the benefits of vaccination extend to all members of a community. On the other hand, eradication also raises the possibility that individual rights will be compromised if mandatory vaccination becomes necessary. Henderson also observed that “top-down” disease eradication programs may compete for resources with “bottom-up” basic health initiatives. In the case of smallpox, he argued, providing vaccination throughout the community also served the needs of basic health services, particularly since the campaign provided a model for vaccinating against other important diseases.
Indeed, the eradication of smallpox gave birth to a new infectious disease management paradigm, and by 1990 immunization programs inspired by the success of the smallpox campaign had vaccinated 80 percent of the world’s children
against six major diseases: tuberculosis, diphtheria, pertussis, tetanus, measles, and polio. Such new approaches represent, in Henderson’s words, “key steps in revolutionizing and revitalizing public health.”
PANDEMIC PREPAREDNESS PLANNING
In their workshop presentations, representatives of the U.S. Department of Health and Human Services (HHS) and the Pan American Health Organization (PAHO) described the efforts that are now being made to prepare for pandemic influenza in the United States and in Latin America and the Caribbean (see Gellin summary and Mujica et al. in Chapter 2). From subsequent discussions it was clear that there is a growing awareness among pandemic planners of the magnitude of the task at hand as well as of the scarcity of resources that are available to accomplish it. In particular, as described below, a good deal of attention has been paid to planning for and mitigating the critical shortages that are expected in countermeasures, medical care, and essential community services. Furthermore, as workshop participants discussed, the distribution of vital but limited resources raises a number of practical, ethical, and legal issues that must be considered in planning for a pandemic.
Influenza Pandemic Planning in the Americas
Oscar Mujica, communicable disease epidemiologist for the Pan American Health Organization (PAHO), described his organization’s efforts to plan for pandemic influenza in Latin America and the Caribbean, in collaboration with partners that include the U.S. Agency for International Development (USAID), the Centers for Disease Control and Prevention (CDC), the World Bank, the Food and Agriculture Organization (FAO), and the United Nations Children’s Fund (UNICEF). In order to pursue its primary goal of strengthening pandemic preparedness in each country within the region, PAHO has conducted a series of planning workshops for national representatives, followed by tabletop simulation exercises (see Mujica et al., page 66). Guided by two key WHO documents, the Global Influenza Preparedness Plan WHO, 2005a) and the Checklist for Influenza Pandemic Preparedness Planning (WHO, 2005b), the PAHO planning workshops have addressed a series of legal issues (such as establishing a legal basis for travel restrictions, isolation, and quarantine) and ethical issues (such as access to scarce resources, compulsory vaccination, and movement restrictions) as well as other strategic and pragmatic concerns.
Countries in the Americas have made measurable progress in advancing their preparedness plans for pandemic influenza, Mujica reported, and the recognition of the likely social and economic consequences of pandemic disease has led to increased intersectoral collaboration. Mujica also said he had observed a tendency toward greater acceptance of the IHR, as national governments gained a greater
appreciation of how global disease transmission and trade are interconnected. However, as Mujica noted, considerable challenges must still be overcome before the region fully implements its pandemic plans. This is particularly true at the local level, where health-care resources are often severely constrained.
The U.S. Strategy for Pandemic Influenza
Bruce Gellin, director of the National Vaccine Program Office at HHS, introduced that agency’s pandemic influenza plan as one example of the many such plans under development at the federal level in the United States (HHS, 2006). Agency-specific pandemic influenza plans fit into the more general framework of the National Strategy for Pandemic Influenza (NSPI) created by the Homeland Security Council (Homeland Security Council, 2005, 2006). A detailed NSPI implementation plan, released in May 2006, is intended to guide all federal departments and agencies—as well as non-federal entities such as state and local governments and the private sector—as they define and carry out actions to address a potential pandemic (see Gellin summary, pages 61-65).
A critical element of the NSPI is its potential for adaptation to a broad range of pandemic scenarios. Based on the six-phase pandemic scale developed by WHO (WHO, 2005a), the NSPI is organized into “response stages” defined by events in a developing pandemic (e.g., a confirmed human outbreak overseas). It sets out a series of appropriate goals, actions, and policy decisions for each stage. The resulting catalog of more than 300 possible actions to be taken by federal departments and agencies also includes progress measures and timelines for putting each action into effect. The specific actions taken in an actual pandemic would be tailored to fit its epidemiological and sociopolitical characteristics, Gellin explained, but the NSPI implementation plan is intended to cover a broad range of contingencies.
Reflecting on the process of developing a national pandemic strategy, workshop participants applauded efforts to date but voiced concerns regarding the translation of national policies into local actions. Asserting that all responses to pandemic disease are local, Steven Bice, a former CDC infectious disease specialist now at Battelle Science and Technology International, advised planners to consult with the public health officials “who will have the fight on their shoulders” in a pandemic. The current plan, he said, represents “a lot of top-down planning and not a lot of listening up … and if I could recommend one thing above all others, [it would be to] start listening very, very carefully to the states and the locals.” State and local officials, Bice said, want guidance from the federal government on pandemic planning, such as feedback on the results of tabletop exercises or drills to test state and local preparedness. He further noted that the state and local perspective is often lacking in the evaluation of such exercises at the federal level. During the discussion, one of the meeting’s participants affiliated with a
state public health department encouraged a higher level of engagement between federal pandemic planners and state public health officials.
Addressing Shortages: Vaccines
Gellin’s own HHS program is charged with the dual tasks of creating pre-pandemic vaccine stockpiles and developing plans for vaccine access, administration, and distribution. Given the high likelihood that vaccine supplies would be limited at best in an influenza pandemic, his office is also charged with setting priorities for the distribution of limited supplies of vaccine. Gellin described the current recommendations for pandemic vaccine prioritization (see Table SA-1), with the caveat that these priorities remain under review and, in the event of a pandemic, would be subject to change based on exactly how the disease spreads in space and time.
As is the case for any distribution of scarce resources, the assignment of priorities for influenza vaccination involves, in addition to scientific and admin-
TABLE SA-1 NVAC/ACIP Recommendations for Prioritization of Pandemic Influenza Vaccine
|
Tier 1A |
Health-care workers
|
|
Tier 1B |
Highest-risk groups
|
|
Tier 1C |
Household contacts and pregnancy
|
|
Tier 1D |
Pandemic responders
|
|
Tier 2A |
Other high-risk groups
|
|
Tier 2B |
Critical infrastructure groups
|
|
Tier 3 |
|
|
Tier 4 |
|
|
SOURCE: HHS (2005). |
|
istrative considerations, implicit and explicit value judgments (Kotalik, 2005). Workshop participants discussed these particular judgments at some length, raising several concerns. The first involved the apparent low priority placed on support personnel who maintain the social and physical infrastructure of communities, such as police officers. Gellin readily acknowledged that this consideration was not addressed in the current vaccination priorities, which were developed from the point of view of public health. He maintained, however, that planned revisions to the vaccination priorities plan could take into account the preservation of public safety and civil order. Concerns were also raised about the dilemmas that influenza’s wavelike pattern of spread over space and time would pose. While a large-scale early release of vaccine would leave little in reserve for regions experiencing a later and potentially more severe wave (as has occurred in several past influenza pandemics), withholding vaccine from people exposed to influenza during the “first wave” would be difficult to justify if people were dying.
Participants also explored the potential expansion of domestic vaccine production capacity, which could reduce the need for prioritizing access to vaccine during a pandemic (or during a shortage of seasonal vaccine, as has occurred in the United States recently). While U.S. demand for seasonal influenza vaccine—a key determinant of manufacturing capacity—has increased in recent years, barely half of all U.S. health-care workers receive flu shots. Some participants said that understanding and changing the behavior of these influential workers could be an important step toward boosting production, availability, and access to seasonal— and thus also to pandemic—influenza vaccine.
The recognition of a new generation of vaccines currently under development for use against anthrax and smallpox (and possibly influenza) led some participants to question the substantial U.S. investment in stockpiling current vaccines. Unfortunately, Gellin pointed out, it would be imprudent to wait for better vaccines before accumulating stockpiles because of the immediacy of the threat posed by the various infectious agents. Gellin also noted that as this country’s experience with the 1976 “swine flu” scare demonstrated, correctly timing the switch from producing a seasonal flu vaccine to producing a strain-specific pandemic influenza vaccine is an inherently difficult task.
Addressing Shortages: Antiviral Drugs
In addition to pre-pandemic vaccines, the U.S. is also stockpiling enough of the antiviral drug oseltamivir (Tamiflu©) to treat the 25 percent of the population projected to become ill during an influenza pandemic as well as several million more doses for use in breaking the transmission cycle during a potential outbreak. Most of the antiviral stockpile is being purchased and maintained by the federal government, Gellin said; the remainder is available for states to purchase at a federally subsidized rate. Private individuals and institutions have also begun to accumulate oseltamivir, which could potentially hinder the government stock-
piling efforts. As the supply of oseltamivir begins to catch up with demand, Gellin noted, discussions about its use should shift to ethical concerns regarding fair access and the potential for increased viral resistance to the drug caused either by self-medication or by the indiscriminate use of private stockpiles.
Not only might antiviral drugs be in short supply—a likely scenario if pandemic influenza strikes in the near future—but medical researchers do not know just how effective any particular antiviral drug is likely to be against pandemic influenza. For example, as Martin Cetron, director of CDC’s Division of Global Migration and Quarantine, explained, oseltamivir could improve individual patient outcome if given when symptoms first appear and if the infection is caused by a strain of influenza that is sensitive to the drug, but it might still have only a modest effect on transmission. On the other hand, antiviral prophylaxis5 may substantially reduce virus transmission by shortening the duration of viral shedding and thereby minimizing the likelihood of infection. Promoting or stockpiling oseltamivir for H5N1 avian influenza without specific evidence of the drug’s efficacy against a pandemic strain would have a variety of legal and ethical implications, and Gellin stressed the importance of determining a drug’s efficacy as early as possible in the course of a pandemic and then using those findings to develop a treatment protocol. Gellin also voiced concerns regarding the lack of an antiviral backup plan should oseltamivir prove ineffective against a viral strain demonstrating pandemic potential.
Addressing Shortages: Medical Care
While it is widely acknowledged that an infectious disease pandemic is likely to overwhelm the U.S. medical system, the federal government has given scant attention—and even less money—to redressing this situation. “There is a great gaping gap here,” said speaker D.A. Henderson, who criticized government planners for focusing on what he believed to be “fringe things,” such as stockpiling and delivering countermeasures of questionable efficacy, rather than concentrating its efforts on “a problem which we know we are going to have.” He attributed the lack of progress toward addressing this critical and predictable need to poor communication between public health officials and hospital administrators, as well as between HHS and CDC.
Although individual hospitals are attempting to prepare themselves for pandemic influenza by conducting surge capacity trials, Henderson observed that few facilities are prepared to handle a worst-case scenario in which patients could exceed capacity by 30 to 40 percent. He predicted that under those conditions hospitals would begin to turn away patients, including some who desperately need care. In order to accommodate them, Henderson recommended the creation
of alternate regional sites staffed by volunteer caregivers. He also noted that plans for medical care during a pandemic need to address such issues as liability, the credentialing of volunteers, nonpaying patients or patients without adequate health insurance, the cancellation of elective surgical procedures, and pandemic-associated losses in hospital revenue.
Workshop participants considered a variety of gaps that exist in pandemic preparations at the hospital level. According to one estimate, if an influenza pandemic occurred today, demand for ventilators would exceed supply by nearly 200 percent (Bartlett, 2006). Furthermore, as noted below, a transportation slow-down—which would be likely during a pandemic—would probably cause oxygen supplies to dwindle. Contingency plans and standards of care will be required in order for nurses to prescribe medications, as many recommend they should; others noted that such contingencies will need to be negotiated with third-party payers.
Several audience participants regarded human resources as the most critical of the many needs that will be unmet during a public health emergency, since such a shortage already exists under “normal” conditions. It was noted, for example, that hospitals in the District of Columbia currently needed approximately 1,000 nurses. In response, one audience participant observed that “if we are going to solve the emergency problem, we need to look at the underlying daily situation” and then urge policy makers to support the hiring of hospital staff in order to fill today’s gaps as well as to anticipate tomorrow’s crises.
Addressing Shortages: Global Supply Chains
Another far-reaching concern regarding the U.S. pandemic influenza strategy is its failure to recognize America’s dependence on and interdependence with fast-moving global markets. Forum member Michael Osterholm observed, for example, that the vast majority of medicines in the U.S. are manufactured abroad or made from precursor materials that are manufactured abroad. Furthermore, critical supplies such as oxygen are delivered just in time to hospitals and other end-users and are therefore dependent upon fuel, which is also largely foreign in origin. The U.S. pandemic plan, in Osterholm’s view, needs to reflect the importance of international trade and travel to our medical system and to the national economy generally. Gellin responded that this concern about the impact of a pandemic on a global economy is being addressed to some extent through the U.S. government’s efforts toward pandemic planning with and by the private sector.
FROM PANDEMIC PLANNING TO PUBLIC HEALTH PREPAREDNESS
While workshop discussions focused on the imminent threat of pandemic influenza, many of the issues raised apply equally to preparations for public health emergencies of all kinds, from emerging infectious diseases to bioterrorism
and natural disasters. Rather than address individual threats, several participants advocated the building of public health capacity to anticipate a range of potential crises.
As one Forum member observed, shifting to this broad concept of public health preparedness would require new investments and, therefore, new considerations of cost, benefit, and risk. He added that this shift in strategy should not be driven exclusively by the local health-care needs and economic priorities that have shaped our current public health system, but should instead anticipate the global consequences of local problems.
Strategies for Disease Containment
Workshop discussion on the topic of pandemic preparedness focused on medical interventions but, as many participants noted, given the present supplies of vaccines, antiviral drugs, and ventilators, nonpharmaceutical interventions are likely to dominate the public health response to an H5N1 avian influenza pandemic. Behavioral strategies for disease containment and mitigation are thus critical to pandemic planning, but they are also fraught with legal and ethical concerns. As described below, time-tested containment measures such as quarantine are being brought into the twenty-first century by computer-modeling simulations designed to find the optimum intervention in different scenarios. Behavioral strategies for disease containment must also be compatible with modern legal thinking, with its emphasis on objective standards and fair procedures.
Isolation and Quarantine in the Twenty-First Century
After centuries of use, isolation6 and quarantine7 persist as primary public health intervention tools in the twenty-first century because of their ability to limit the spread of disease. But today the mitigation of disease spread must be done in accordance with current beliefs about individual rights and civil liberties, Cetron observed (see Cetron and Landwirth, page 99). Cetron described how quarantine and social distancing (individual and community measures that reduce the frequency of human contact) have proved effective in reducing the transmission of disease. Numerous historical examples suggest that people tend individually and collectively to respond to infectious disease threats through social distancing, Cetron observed. In order to maximize the benefits of this behavior, such measures must be planned and instituted at the beginning of a pandemic.
Modern quarantine is carried out according to ethical principles used to guide public health interventions. These ethical principles include the need to demon-
strate necessity (justifiable harms); the importance of using the least restrictive means of achieving a public good; the existence of mechanisms for notification and appeal (due process); and fairness in carrying out the intervention (Upshur, 2002). As Cetron explained, quarantine can only be justified in the case of a highly dangerous, contagious disease and only for as long as is necessary to protect the public. The quarantine need not be absolute, since even a “leaky” quarantine may be effective in disease mitigation. Cetron added that pandemic-preparedness plans should guarantee that quarantined individuals will have access to needed goods and services, and that quarantine-associated stigma should be anticipated and actively discouraged.
Modeling the Effects of Social Distancing
There is limited empirical evidence supporting the effectiveness of social distancing in fighting the spread of infectious disease. As illustrated in Figure SA-1, there are several ways that social distancing—in combination with additional infection control measures—could be expected to alter the course of an outbreak: it could can delay the peak of the outbreak (#1); it could spread the outbreak out in time, thereby easing the peak burden on hospitals and the public health infrastructure (#2); and it could reduce the overall number and severity of cases (#3).
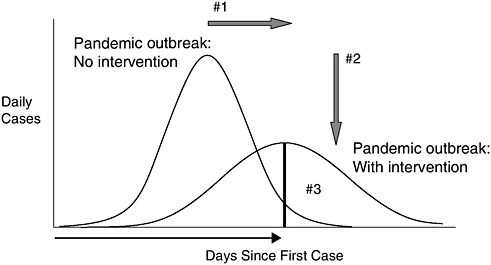
FIGURE SA-1 Community-based interventions. Figure legend: (#1) delay outbreak peak; (#2) decompress peak burden on hospitals/infrastructure; (#3) diminish overall cases and health impacts.
SOURCE: Cetron (2006).
Based on computer simulation models, specific actions that might reduce disease transmission rates include school closures; keeping children and teens at home; voluntary home isolation and quarantine; and using antiviral drugs to treat the ill and providing prophylaxis to their household contacts. As Cetron explained, these measures form part of a much broader, layered approach to behavioral intervention, which extends from individual actions (hand hygiene, cough etiquette) to global efforts (containment at the source, advisories and screening for travelers).
While social distancing measures may help slow the spread of disease, they also pose a number of other potentially far-reaching consequences, and Cetron stressed the importance of anticipating these consequences and adapting the measures accordingly. The issue of school closure is particularly contentious in this regard. While modeling indicates it to be a potent means to reduce disease transmission, its adverse consequences could be so severe and inequitable as to outweigh any benefit. D.A. Henderson of the University of Pittsburgh Medical Center cautioned against relying on models that do not take into consideration the adverse effects or practical constraints that such public health interventions would entail. Accepting such models uncritically, he warned, could result in policies that “take a perfectly manageable epidemic and turn it into a national disaster.”
While agreeing that models cannot take the place of human judgment, Joshua Epstein of the Brookings Institution described how they can inform human judgment by providing insights about how different human actions might affect the course of an epidemic. In particular, he described how the reaction of people to various disease-control measures—the so-called secondary and tertiary effects of a measure—can be incorporated into a model and evaluated prior to an event. With every biological epidemic, he predicted, “there will be a secondary epidemic, a behavioral epidemic that will affect subsequent social network patterns [and] contact behavior.” Epstein and coworkers have produced such a model for smallpox following a bioterrorist attack (see Epstein, page 105) and are currently developing such a model for pandemic influenza. Epstein’s team is also working on a global model to demonstrate the effects of international travel restrictions and other nonpharmaceutical interventions in a variety of circumstances. And the CDC, according to Cetron, is testing a model designed to identify those behavioral measures that will save the maximum number of lives in a pandemic with the minimal economic impact. Preliminary results indicate, for example, that few additional lives will be saved by reducing the disease attack rate8 below one percent or by shifting from voluntary to compulsory quarantine.
Participants noted that sound science is essential to the creation and continuous improvement of disease models. “You really have to work closely with experts in building models,” Epstein advised, illustrating this point by describing the design of his group’s model of smallpox transmission, in consultation with Henderson (Cummings et al., 2004). “We had intensive, regular meetings to arrive at reasoned assumptions about all the biomedical and critical behavioral aspects of this problem,” Epstein recalled, adding that this process forces scientists to make their assumptions explicit and to support them, and thereby identifies gaps in knowledge. James LeDuc of CDC observed that such gaps present important opportunities for scientific research to inform ethical decision making and thereby create a foundation for public health law.
Legal Preparations for Containment
Lawrence Gostin, Director of the Center on Law and the Public’s Health at Georgetown University, introduced his presentation by reviewing the legal issues surrounding nonmedical approaches to disease containment. These include the potential for infectious disease surveillance to violate individuals’ rights to privacy, the economic costs of controlling zoonoses, trade limitations imposed by international travel restrictions, and the loss of personal freedom associated with community hygiene measures, such as compulsory temperature monitoring, and the spectrum of contact-reducing interventions, ranging from voluntary sheltering in place (“snow days”) to mandatory isolation of the ill (see Gostin and Berkman, page 78). Given the lack of clear evidence that many of these disease containment strategies are effective, Gostin said that governments and institutions should carefully consider the possible adverse effects and unintended consequences that such measures can cause when they are designing and implementing them.
Gostin stressed the importance of legal preparedness for a pandemic, both nationally and internationally. Calling the revised IHR a “brave, bold, and innovative” move toward developing an international legal authority capable of addressing pandemic disease in a globalized society, he warned that the regulations would remain “vacant” until developing countries receive sufficient resources for compliance or until transnational health law is sufficiently strong to enforce the IHR. “We need to move toward more transnational governance,” Gostin asserted, because no matter how well an individual country may prepare for a pandemic, “we are all at risk unless we have some kind of coordinated approach.”
Focusing on quarantine, Gostin emphasized the importance of establishing sufficient legal authority, based on objective standards, to restrict individual freedom. If members of the public are to support such a measure, he said, they must be assured that those who are quarantined will be well taken care of—ensured a safe place to stay, provided with food and water, given adequate medical care, and so on—for the duration of the quarantine. Moreover, he contended, people subject to compulsory quarantine must have the right to a hearing to contest their confine-
ment. It is the states rather than the federal government that have the primary legal authority to mandate quarantine measures, but the federal government has drafted the Model State Emergency Health Powers Act and Model State Health Act9 to provide guidance to the states on how best to prepare for pandemic disease and other health crises. “Public health strategies require public trust and acceptance in accordance with the principles of social justice,” Gostin concluded. “We need to remember that pandemics can be deeply divisive, and the political response profoundly reflects on the kind of society we want and aspire to be.”
The Problem of Authority
Among the legal and ethical barriers to public health intervention during a pandemic, the first and most critical is the establishment of authority for decision making, according to speaker Victoria Sutton, director of the Center for Bioterrorism, Law, and Public Policy at Texas Tech University. As cruelly demonstrated in the aftermath of Hurricane Katrina, disaster response can be slowed and sometimes even brought to a halt by confusion among national, state, and local jurisdictions about who has authority and responsibility for various decisions and actions. In addition, IOM president Harvey Fineberg expressed concern that a “horizontal dimension of ambiguity” of federal public health authority has been created as a consequence of the organization of the Department of Homeland Security (DHS).
The longstanding conflict between federal and state governments concerning authority over public health is rooted, Sutton said, in decisions made early in our nation’s history,10 when disease transmission tended to be limited to communities. But because pandemic diseases are becoming recognized as posing a national threat, Sutton suggested that the federal government could pass legislation federalizing the rules for pandemic response, much as the recognition of the far-reaching and adverse effects of pollution led to federal environmental legislation. The pandemic legislation might, for example, put into effect a “cooperative federalism,” in which the federal government establishes standards for implementing pandemic measures (medical care, the distribution of therapeutic countermeasures, quarantine and isolation), which are administered by the state governments and, ultimately, implemented at the local level (Sutton, 2001).
|
9 |
See http://www.publichealthlaw.net/Resources/Modellaws.htm#MSPHPA [accessed May 17, 2007]. |
|
10 |
In U.S. history, the states’ rights doctrine is based on the Tenth Amendment to the Constitution, which states, “The powers not delegated to the United States by the Constitution, nor prohibited by it to the States, are reserved to the States respectively, or to the people.” The term embraces both the doctrine of absolute state sovereignty that was espoused by John C. Calhoun and that of the so-called strict constructionist interpretation of the U.S. Constitution, which reserves to the state governments all powers not specifically granted by that document to the federal government. See http://www. answers.com/topic/states-rights [accessed December 27, 2006]. |
This model of federal leadership was endorsed by Shelley Hearne of the Johns Hopkins University, who presented a detailed strategy for building a capacity for emergency response that would protect all U.S. citizens equally (see Hearne, page 183). At present, she reported, pandemic preparedness varies greatly from state to state and from hospital to hospital, and most health-care providers and workers are not engaged in preparedness planning or implementation activities (Trust for America’s Health, 2005). Hearne argued that, just as the federal government insists that each state meet certain minimal environmental standards, it should also define and uphold public health standards. Drawing a parallel with environmental legislation, she suggested that federal public health legislation might even allow citizens to file suits against the CDC or other federal agencies if public health standards were not enforced. LeDuc observed that until the distribution of public health authority is clarified and reformed, each community will have to identify and repair gaps in its own legal preparedness for a pandemic. He added that this process could be undertaken as a collaboration among federal, state, and local legal authorities. There has been some progress toward community engagement, Cetron said, thanks to efforts undertaken by the Association of State and Territorial Health Officers, the Department of Education, and the CDC, which issued guidelines on pandemic mitigation for communities in February 2007 (CDC, 2007). Feedback from the community level will continue an iterative process, intended to encourage communities to identify and independently address the challenges they will face in a pandemic. A number of state and local public health officials who attended the workshop commented that such federal guidance on pandemic mitigation is very much needed.
ETHICAL ISSUES IN PANDEMIC PLANNING AND RESPONSE
The various efforts at planning for an H5N1 avian influenza pandemic have created an awareness of the challenges involved in addressing infectious diseases in a highly interconnected and interdependent world. Participants in the many conferences, meetings, and workshops convened in response to this imminent threat have considered a broad range of potential effects and contingencies, of actions that could be taken, and of decisions that will need to be made prior to and during a pandemic. Accordingly, much of this workshop was devoted to identifying the logistical, legal, and ethical challenges that are likely to arise in such a public health emergency. Certain presentations and discussions, summarized below and in Chapters 3 and 4, focused on the creation of ethical guidelines for action in a pandemic, the engagement of the public in this process, and the maintenance of free and open communication about the process.
As Victoria Sutton noted, a fundamental question underlies the various ethical issues surrounding pandemic planning and response: Do ethics change during a pandemic? The answer, she said, is yes, just as is the case in any public emergency that involves a great deal of uncertainty and that threatens the well-
being of the nation (Sutton, 2005). Sutton, who sees ethics as a precursor to law, suggested that a good definition of “pandemic flu ethic” would be “a limitation on the freedom of action or the imposition of a duty to act in the pursuit of the continued existence of life and order.” This part of the workshop offered nuanced interpretations of her seemingly straightforward definition.
The Ethicist’s Role
Speaking from his experience as WHO’s former Director of Ethics, Trade, Human Rights and Health Law, medical ethicist Alexander Capron of the University of Southern California described how pandemic planning and response fit on a spectrum of ethical approaches (see Capron, page 157). A variety of ethical principles apply to the content of pandemic policies and the process by which such policies are established, he observed; further principles concern the individual’s duty in a pandemic or focus on desired outcomes (see Table SA-2). Guided by these principles, ethicists can assist pandemic planning by recognizing and raising awareness of the values that are embedded in technical decisions and by promoting an ethical process of decision making. “The key to an ethically responsible and appropriate response is advance planning, including communica-tion,” Capron said. “Part of communication is recognizing scarcity and the resulting need for collective allocation and personal responsibility.”
Capron then revisited the four primary ethical considerations raised by pandemic disease that had been discussed previously in connection with SARS and other infectious diseases (see previous section, “Learning from Pandemics Past,” and Heymann, page 33):
-
Access to health care
-
Human rights
-
Obligations of and to health-care workers
-
Obligations of countries and intergovernmental organizations
Applying these ethical considerations to the current scenarios for pandemic influenza, Capron offered a number of specific issues that need to be addressed
TABLE SA-2 Variety of Ethical Approaches/Foci
|
|
SOURCE: Capron (2006). |
TABLE SA-3 Substantive Principles
|
|
SOURCE: Capron (2006). |
in planning for a pandemic, including the need to define standards of care that result in the fair distribution of scarce resources.
Capron also described how WHO has incorporated certain ethical principles, as outlined in Table SA-3, into technical recommendations for pandemic influenza planning and response. The CDC has taken a similar route in formulating ethical guidelines for pandemic preparation and response, according to James LeDuc (see LeDuc et al., page 90). Ethicists at that agency have suggested a number of principles, including transparency, sound science, global involvement, and procedural justice, as a foundation for legal decision making and have also identified certain ethical issues, such as allocation of countermeasures and restrictions on freedom, that are inherently part of pandemic planning and response (CDC, 2007).
Pandemics tend to produce conflicts between individual entities, either persons or nations, and groups, such as communities, countries, or intergovernmental entities, which must be resolved through “choices among goods,” Capron observed. And that resolution can vary depending on the outcome of public debate and expert deliberation. Indeed, he said, rather than expecting to find an absolute “right” answer to a problem, the more viable approach is to find a solution that is both understood and accepted by the public, who ultimately must implement and abide by the decision. The public’s trust must be attained by ensuring that the decision-making process, as well as the evidence used in it, is transparent and open to public debate and that the resulting decisions must be clearly articulated and justified.
Questions of Justice
In his remarks to workshop participants, Harvey Fineberg raised an additional ethical challenge: the potential conflict between preserving society as a whole and protecting its weakest members. These considerations were further explored in presentations by ethicists Ruth Faden of the Johns Hopkins University and Bernard Lo of the University of California, San Francisco. Faden’s remarks focused on the well-founded expectation that the burdens of pandemic influ-
enza—social and economic disruption, as well as morbidity and mortality—will almost certainly fall disproportionately upon the world’s poorest people and countries and also upon the poorest inhabitants of wealthy countries. “The greatest moral challenge we face is how to respect commitments to social justice in the face of the overwhelming, systematic inequalities that form the backdrop for the harsh realities of pandemic flu,” she said.
Faden observed that some inequalities likely to be created in an influenza pandemic are more profoundly wrong than others (see Faden, page 177). And of those most egregious inequalities, some—such as limited access to technology and barriers to communication and community engagement—represent particularly feasible targets for policy, intervention, or development. Governments, she argued, should identify opportunities to mitigate, if not eliminate, the burdens imposed by such inequalities under pandemic circumstances—and perhaps, by extension, in the broader context of public health.
There are also important practical reasons for considering the interests of the disadvantaged in the course of pandemic planning. For instance, trust in public health policies—particularly among the typically poor inhabitants of regions where an influenza pandemic is expected to begin—will invite compliance, without which infectious disease will not be able to be controlled (Bellagio Group, 2006). Lo noted in his formal remarks that in public health emergencies citizens are more willing to sacrifice self-interest in favor of the common good if they believe that everyone else is doing so; conversely, he said, people who perceive that others are receiving preferential treatment (even if this is not actually the case) are less likely to act selflessly.
Duty to Care
Health-care workers on the front lines in infectious disease outbreaks (e.g., smallpox, Ebola, and SARS) have consistently fulfilled their duty to care for patients even when it has cost them their lives (see Heymann, page 33). Ruderman and colleagues report, however, that during the SARS crisis in Canada, “serious concerns arose … about the extent to which health-care providers would tolerate risk of infection,” leading to the anticipation of a potential crisis during a pandemic (Ruderman et al., 2006). Likewise, Sokol (2006) described defining the duty to care as an urgent and difficult task, “strewn with philosophical and logistical difficulties,” that must be accomplished in order to prevent “large numbers of doctors from abandoning their patients in a crisis” (Sokol, 2006).
Capron asked whether a worker’s duty to care is a product of medical training and licensure or a product of the patient’s—and the community’s—need for their skills (see also Chapter 4). If it is the former, Clark argued that the duty to treat overrides physician autonomy in social emergencies, even in cases that involve personal risk (Clark, 2005). If one accepts the latter premise, Capron said, anyone whose job is essential to the health-care system has an obligation to work during
a pandemic, and in return, society is obliged to provide these workers with the most effective protection available. “This is part of the social contract with them, that we give them special status when it comes to prophylaxis and treatment in recognition of their role,” he said.
In enforcing these dual obligations—the duty of health-care professionals to society and the reciprocal duty of society to health-care professionals— governments and professional organizations should incorporate some flexibility in the definitions of “duty to care” and “special protections,” Capron said, so that they can accommodate factors such as disease-transmission dynamics and the availability of countermeasures that will be specific to each public health emergency. Furthermore, while it is possible that during a pandemic a sufficient number of health-care workers will volunteer to put themselves at risk for the benefit of the community, this cannot be guaranteed, and thus it may be necessary for states or professional organizations to mandate worker conduct. According to Capron, if such expectations for health-care workers are going to be mandated—possibly along with specific consequences for dereliction of duty—the expectations must be agreed upon as part of pandemic planning and should be done through a transparent process that involves local and national professional associations.
Ethical Guidelines for Clinicians
An influenza pandemic is likely to produce extraordinary shortages in medical care. Hospital resources—both human and material—may be stretched beyond their limits. In order to manage the many ethical dilemmas inherent in this situation, physicians and hospital administrators will need specific guidelines, Lo said (see Lo and White, page 192). His observations were echoed by several workshop participants, some of whom spoke from a personal perspective, as they themselves will be called to play certain roles in a pandemic. Among the challenges that pandemic influenza will present to clinicians, one of the likeliest and most daunting will be a grave shortage of mechanical ventilators. Such a shortage, Lo observed, will require physicians to choose which patients will receive the life-saving use of a ventilator and which will die without respiratory therapy. There will be no time to weigh alternatives in a pandemic, Lo argued, so it will be important to develop clear criteria ahead of time for when to triage patients, along with guidelines and procedures for addressing problems that will arise as the triage system is implemented, such as handling disagreements with family members and managing patients in respiratory failure who do not receive mechanical ventilation.
Lo urged pandemic planners to anticipate the ethical and legal dilemmas that doctors and other health-care providers will face in a “worst-case” ventilator shortage and to create, with input from the public and specialists in various disciplines, guidelines and procedures for dealing with shortages of ventilators and
other medical supplies. While suggesting that rules for triage should maximize the number of lives saved, Lo also pointed out that physicians must rely on limited evidence to predict a patient’s prognosis. Triage rules, he said, should be administered by an external authority, not the physicians dealing with the patients, and they should be implemented by physicians and other health-care workers in such a way that their fairness cannot be doubted.
Fairness in allocating scare resources will be necessary to secure public trust in the process, Lo observed, but it will not be sufficient. Triage policies and priorities must also reflect popular will, he said. Moreover, the policies must be communicated clearly and in a way that people will understand. And they must be presented in a way that leads society to accept the idea that, during an infectious disease emergency, some patients will die who might otherwise have been saved under normal circumstances. Lo also stressed the importance of providing the public with ready access to the data, reasoning, and deliberative processes that support such triage guidelines. Unfortunately, Hearne observed, some states have not only failed to engage the public in pandemic planning, but they have actively excluded them from the process and have kept their plans secret, even from hospital workers and other health-care providers.
Civic Engagement
Throughout the workshop, the public perspective dominated discussions of pandemic ethics, as reflected in presentations that advocated transparent planning processes and clear communication. But transparency is not enough, Capron said. The process should also adhere to the principle of participation, which holds that stakeholders (and who is not a stakeholder in a pandemic?) should contribute to the process of formulating objectives and adopting policies (see also Chapter 4).
As previously described, public participation has been incorporated in pandemic planning efforts undertaken by both PAHO and CDC (see Mujica et al., page 66, and Cetron and Landwirth, page 99). Faden noted that among prior attempts to engage the public in health policy making (concerning such issues as resource rationing, end-of-life care, and genetic screening), some of the most successful efforts were those that occurred on a scale that was sufficiently local that people were able to voice their individual concerns. When society wrestles with issues that affect people’s daily lives, Capron said, people generally want the chance to participate in the “work of worrying.” They don’t always act on it, he said, “but they often miss it when they don’t have it.” Susan Chu, who moderates the public Internet forum FluWiki, observed that its participants exhibit a high level of engagement and sophistication. Asserting that at least “a portion of the public is teachable,” Chu encouraged policy makers to speak truthfully about the possible effects of a pandemic and to enlist citizen engagement in the planning efforts.
Acknowledging that the Internet has raised the bar for participatory communications, Capron noted that public input has influenced health-care policy in many instances, perhaps most profoundly in addressing HIV/AIDS. These experiences demonstrate that public engagement produces policies that can be accepted even by those who are not satisfied by them, he said. Conversely, exclusion often creates a heated atmosphere that leads people to attack and arbitrarily reverse decisions that may be technically sound. “Things that make people angry fall apart,” Capron observed.
Responding to Capron’s assertion that pandemic plans should be subject to revisions based on public review of their performance, Martin Cetron predicted that engaging in this process will build community resilience, as both policy makers and the public become “acculturated to being wrong” and become familiar with uncertainty. The hoped-for result of this process, he continued, is the creation of multiple contingency plans (including criteria for implementing and suspending them) as well as mechanisms to “ensure that we are going to learn from the mistakes.”
In addition to incorporating public input into pandemic planning, the federal government has been encouraging personal responsibility in emergency preparedness, and Steven Bice applauded these efforts. He briefly described the MedKit program, currently being tested by HHS, which provides families with a home supply of antimicrobial drugs as well as countermeasures for exposures to radioactive materials, which could be used in the event of a terrorist attack (CDC, 2005). Bice suggested that antiviral drugs could be provided to individuals in advance of a pandemic in much the same way, thereby providing access to medications without compromising social distancing measures.
Since the nation’s experience with the aftermath of Hurricane Katrina, many Americans have come to be extremely cynical about government efforts meant to protect them from disaster, Hearne observed. As a result, she said, broad changes in public health law will be needed to prevent a potentially disastrous breakdown in public health authority during a pandemic. She expressed the hope that such reforms could be structured to engage that public in emergency planning and response, and, as a result, transform a future pandemic into a “controlled chaos event that gives us a better chance of saving more lives and having a public that trusts its government.”
Ethics in the Midst of Uncertainty
While recognizing the ideal of public participation in pandemic planning, workshop participants nonetheless agreed that public health professionals must expect most people to be entirely unprepared when the next pandemic strikes. In order to mount an effective response, public health authorities will need to act rapidly and authoritatively on the basis of incomplete knowledge. This situation, which Gostin (2004) refers to as “the public health paradox,” raises a host of
ethical and legal issues for those who will lead the response to a threatened pandemic—scientists, public health authorities, and lawmakers (Gostin, 2004).
Fineberg warned scientists against overconfidence in assumptions based on incomplete knowledge of a few varied pandemics. “While you can be very sure that there is going to be a next time,” he said, “you have to be very, very cautious” before declaring that next time to be now. In light of this uncertainty, he posed a series of questions concerning the ethical duties of biomedical experts toward political officials. The questions were the following:
-
Are experts bound to frame evidence, based on their knowledge, so that politicians reach “correct” conclusions regarding a threatened pandemic?
-
Should experts refrain from making conclusions and merely answer questions?
-
Should experts speak directly to the media about their concerns?
Rather than offering answers, Fineberg described how various experts approached these dilemmas in the course of reacting to the appearance of “swine flu” in 1976, and he spoke of how those reactions—and their treatment in the media—shaped the nation’s response to a threatened pandemic (see Chapter 4).
Finally, Fineberg said, no matter what choices are made to address a threatened pandemic—or indeed, in response to any decision of such importance— there is going to be skepticism, criticism, and differences of opinion. “There is no way to avoid the dilemmas posed by acting without full scientific knowledge,” Gostin has observed (2004). In the face of such challenges, he concluded, “the only safeguard is the adoption of ethical values in formulating and implementing public health decisions.”
SUMMARY OF NEEDS AND OPPORTUNITIES
This section summarizes the needs and opportunities for research and for policy making that the workshop participants mentioned frequently as being important to the development of just and ethical measures for mitigating pandemic disease.
1.
A Comprehensive, Global Plan for Addressing Infectious Disease
Given the globalization of commerce, travel, and economics, and the worldwide migration of people, goods, and ideas, there needs to be a coordinated, international approach to pandemic disease mitigation which includes the following features:
-
Universal implementation of core disease surveillance and control capacities
-
International cooperation and coordination in disease surveillance
-
Protection of international trade from unnecessary embargoes related to disease reporting, and maintaining vital global supply chains
-
Compensation of citizens and governments of low-resource countries at risk for emerging infectious diseases for those sacrifices (e.g., economic consequences of disease reporting, culling of infected animals, quarantine and isolation) that benefit the global community; this would include the provision and just distribution of countermeasures, as well as economic support
2.
A Pandemic Planning Process That Tackles Ethical and Legal Issues
As Alexander Capron remarked, and many others echoed, “the key to an ethically responsible and appropriate response [to pandemic disease] is advance planning.” Important features of such a planning process would include, but not be limited to, the following:
-
Public engagement in decision-making on issues that affect personal freedom (e.g., quarantine, school closings, and other NPIs) and the allocation of limited medical resources (see also Item #4 below)
-
Adherence to ethical principles, including justifiable harms, the least restrictive means of achieving public good, procedural justice, and due process
-
Interventions of proven efficacy, implemented under conditions in which their benefits to society outweigh their well-understood and potentially far-reaching consequences
-
Establishment of clear authority for public health decision making during a pandemic (see Item #3 below)
-
Public communication prior to—as well as during—a pandemic that explains the rationale for disease control measures, establishes realistic expectations, and allows for the “emotional rehearsal” of pandemic scenarios
-
Measurable endpoints for NPIs and other disease control measures
-
Research protocols to gather evidence for the efficacy of NPIs
-
Capacity building to address a range of potential public health emergencies
3.
Improved Coordination of Federal, State, and Local Pandemic Planning Activities
Workshop participants noted several critical weaknesses in current structures and strategies for pandemic planning. It is worth noting that the application of ethical values in formulating plans may be as, or more, important than the ethical values in the plans themselves, although both are critical elements in the planning process. Confusion as to who would be in charge of the nation’s response to an unfolding pandemic is a particularly pressing concern, and it encompasses the following administrative challenges:
-
Ambiguity of federal public health authority between DHS and HHS
-
Longstanding conflicts between federal and state claims to public health authority, as demonstrated following hurricanes Katrina and Rita
-
Insufficient engagement of state public health officials by federal pandemic planners
-
Poor communication between state and local public health officials and hospital administrators
4.
Public Engagement and Communication
Transparency in policy making and clear risk communication are necessary, but not sufficient, to ensure that fairness and justice prevail in the face of a threatened pandemic. Many workshop participants suggested that there was a need for a transparent, ethical decision-making process that incorporates public debate and deliberation and that has as its goal the selection of interventions that are both understood by, and acceptable to, most people. In addition, due to the inherent uncertainty of pandemic disease, preparedness plans should be “living documents” that are subjected to constant review, testing, and revision, based on evidence and experience.
REFERENCES
ASTHO (Association of State and Territorial Health Officials). 2002. Nature’s Terrorist Attack: Pandemic Influenza: Preparedness Planning for State Health Officials. Washington, DC: Association of State and Territorial Health Officials.
Bartlett JG. 2006. Planning for avian influenza. Annals of Internal Medicine 145(2):141-144.
Bellagio Group. 2006 (August 25). Bellagio Statement. [Online]. Available: http://www.hopkinsmedicine.org/bioethics/bellagio/statement.html [accessed January 3, 2007].
Capron A. 2006 (September 19). Session II: Domestic, Regional, and International Preparedness Planning. Presentation at the Institute of Medicine’s Forum on Microbial Threats workshop entitled “Ethical and Legal Considerations in Mitigating Pandemic Disease,” Washington, DC.
Cash RA, Narashimhan V. 2000. Impediments to global surveillance of infectious diseases: Consequences of open reporting in a global economy. Bulletin of the World Health Organization 78(11):1358-1367.
CDC (Centers for Disease Control and Prevention). 2005. Advisory Committee to the Director, Meeting Summary, August 25, 2005. [Online]. Available: http://www.cdc.gov/maso/FACM/pdfs/ACDCDC/20050825%20ACDCDC%20minutes.pdf [accessed February 23, 2007].
CDC. 2007. Ethical Guidelines in Pandemic Influenza. Advisory Committee to the Director, Ethics Subcommittee. [Online]. Available: http://www.cdc.gov/od/science/phec/panFlu_Ethic_Guidelines.pdf [accessed April 13, 2007].
Cetron M. 2006 (September 20). Session III: Disease Mitigation Strategies—Quarantine, Containment and Modeling. Presentation at the Institute of Medicine’s Forum on Microbial Threats workshop entitled “Ethical and Legal Considerations in Mitigating Pandemic Disease,” Washington, DC.
Clark CC. 2005. In harm’s way: AMA physicians and the duty to treat. Journal of Medicine and Philosophy 30(1):65-87.
Cummings DAT, Chakravarty S, Singa RM, Burke DS, Epstein JM. 2004. Toward a Containment Strategy for Smallpox Bioterror: An Individual-Based Computational Approach. Washington, DC: Brookings Institution Press.
Fidler DP. 2004. SARS, Governance and the Globalization of Diseases. New York: Palgrave Macmillan. Pp. 50-60.
Fidler DP, Gostin LO. 2006. The new international health regulations: An historic development for international law and public health. Journal of Law, Medicine and Ethics 34(1):85-94.
GAO (General Accounting Office). 1977. The Swine Flu Program: An Unprecedented Venture in Preventive Medicine. GAO/HRD-77-115. Washington, DC: General Accounting Office.
GAO. 2000. Pandemic Influenza: Plan Needed for Federal and State Response. GAO-01-4. Washington, DC: General Accounting Office.
Gostin LO. 2004. Pandemic influenza: Public health preparedness for the next global health emergency. Journal of Law, Medicine and Ethics 32(4):565-573.
Henderson DA. 1999. Eradication: Lessons from the past. Morbidity and Mortality Weekly Report 48(SU01):16-22.
Heymann DL. 2006a (June 6). The sovereignty of disease. YaleGlobal. [Online]. Available: http://yaleglobal.yale.edu/display.article?id=7518 [accessed January 3, 2007].
Heymann DL. 2006b (September 19). Emerging Infectious Diseases: Past is Prologue. Keynote address at the Institute of Medicine’s Forum on Microbial Threats workshop entitled “Ethical and Legal Considerations in Mitigating Pandemic Disease,” Washington, DC.
HHS (Department of Health and Human Services). 2005. Appendix D in Pandemic Influenza Plan. Washington, DC: Department of Health and Human Services. [Online]. Available: http://www.hhs.gov/pandemicflu/plan/appendixd.html [accessed December 27, 2006].
HHS. 2006 (June 29). Pandemic Planning Update II. [Online]. Available: http://www.pandemicflu.gov/plan/pdf/panflureport2.pdf [accessed January 3, 2007].
Homeland Security Council. 2005 (November). National Strategy for Pandemic Influenza. [Online]. Available: http://www.whitehouse.gov/homeland/nspi.pdf [accessed January 3, 2007].
Homeland Security Council. 2006 (May). National Strategy for Pandemic Influenza Implementation Plan. [Online]. Available: http://www.whitehouse.gov/homeland/nspi_implementation.pdf [accessed January 3, 2007].
IOM (Institute of Medicine). 2004. Learning from SARS: Preparing for the Next Disease Outbreak. Washington, DC: The National Academies Press.
IOM. 2005. The Threat of Pandemic Influenza: Are We Ready? Washington, DC: The National Academies Press.
Kotalik J. 2005. Preparing for an influenza pandemic: Ethical issues. Bioethics 19(4):422-431. [Online]. Available: http://www.blackwell-synergy.com/doi/pdf/10.1111/j.1467-8519.2005.00453.x [accessed January 3, 2007].
Last JM, Ed. 1983. A Dictionary of Epidemiology. Oxford, UK: Oxford University Press.
Markel H, Stern AM, Navarro JA, Michalsen JR. 2006. A Historical Assessment of Nonpharmaceutical Disease Containment Strategies Employed by Selected U.S. Communities During the Second Wave of the 1918-1920 Influenza Pandemic. Ft. Belvoir, VA: Defense Threat Reduction Agency.
Neustadt RE, Fineberg HV. 1978. The Swine Flu Affair: Decision-Making on a Slippery Disease. Washington, DC: Department of Health, Education, and Welfare.
Rosenberg C. 1992. What is an epidemic? AIDS in historical perspective. In Rosenberg C, ed. 1992. Explaining Epidemics and Other Studies in the History of Medicine. New York: Cambridge University Press.
Ruderman C, Tracey CS, Bensimon CM, Bernstein M, Hawryluck L, Shaul, RZ, Upshur RE. 2006. On pandemics and the duty to care: Whose duty? Who cares? BMC Medical Ethics 7:5. [Online]. Available: http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1459179&blobtype=pdf [accessed January 3, 2007].
Sencer DJ, Millar JD. 2006. Reflections on the 1976 swine flu vaccination program. Emerging Infectious Diseases 12(1):29-33. [Online]. Available: http://www.cdc.gov/ncidod/EID/vol12no01/pdfs/05-1007.pdf [accessed January 3, 2007].
Sokol DK. 2006. Virulent epidemics and scope of healthcare workers’ duty of care. Emerging Infectious Diseases 12(8):1238-1241.
Sutton V. 2001. Bioterrorism preparation and response legislation—The struggle to protect states’ sovereignty while preserving national security. The Georgetown Public Policy Review 6:93.
Sutton V. 2005. A multidisciplinary approach to an ethic of biodefense and bioterrorism. Journal of Law, Medicine & Ethics 33(2):310-322.
Trust for America’s Health. 2005. Ready or Not? Protecting the Public’s Health from Diseases, Disasters, and Bioterrorism. [Online]. Available: http://healthyamericans.org/reports/bioterror05/ [accessed January 3, 2007].
Upshur RE. 2002. Principles for the justification of public health intervention. Canadian Journal of Public Health 93(2):101-103.
WHO (World Health Organization). 2005a. WHO Global Influenza Preparedness Plan. [Online]. Available: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5/en/index.html [accessed January 3, 2007].
WHO. 2005b. WHO Checklist for Influenza Pandemic Preparedness Planning. [Online]. Available: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_4/en/index.html [accessed January 3, 2007].
WHO (World Health Organization). 2007. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. [Online]. Available: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_05_16/en/index.html [accessed May 16, 2007].






























