Page 139
6—
Calories: Total Macronutrient Intake, Energy Expenditure, and Net Energy Stores
Carbohydrates, protein, fats, and alcohol—the dietary macrocomponents—are the sources of energy in the diet. Under normal circumstances, more than 95% of this food energy is digested and absorbed from the gastrointestinal tract to provide the body's energy needs. Studies of normal and overweight subjects have not shown any significant differences in the proportion of food energy absorbed. In some underweight subjects, however, malabsorption of nutrients is an important factor. Food energy is used to meet the body's needs, including protein synthesis; maintenance of body temperature, cardiac output, respiration, and muscle function; and storage and metabolism of food sources of energy. When more energy is consumed than is needed for metabolism and physical activity, the excess is stored, primarily as adipose tissue.
Energy is the capacity to do work. In biologic systems it is usually measured in kilocalories (kcal) or kilojoules (kJ). One kilocalorie (equivalent to 4.184 kJ) is the amount of heat required to raise the temperature of 1 kg of water 1°C (e.g., from 15 to 16°C) at standard atmospheric pressure (760 mm Hg).
Energy balance refers to the relationship of energy intake to energy expenditure and energy storage. Less energy expenditure than energy intake results in a positive energy balance and storage of energy primarily as body fat. Increased fat storage is appropriate during pregnancy and lactation, during some periods of growth and development, and during recovery from trauma or malnutrition, but it may not be desirable under other conditions. When energy expenditure exceeds energy intake, energy balance is negative and leads to weight loss. When intake equals expenditure, equilibrium results and body fat is maintained, regardless of whether the body weight is at, above, or below normal. Even at stable body weight, however, the percentage of body fat frequently increases with age unless regular physical activity is maintained. At the same body weight, some sedentary people have relatively more body fat than those who exercise. Thus even at normal weights, these individuals may have more adiposity than desirable.
Prolonged insufficient energy intake results in malnutrition, which is observed in many developing countries and is also a public health concern for a minority of the U.S. population. In most cases, insufficient food intake results from a lack of economic ability to obtain food, from an illness, or from physical or mental disorders that prevent sufficient ingestion or utilization of food to meet energy expenditure, or in some cases from voluntary restriction of food intake and dieting.
Energy Intake
In general, carbohydrates and fats are completely oxidized in the body. In contrast, protein is
Page 140
only partially oxidized, resulting in the excretion of urea and other nitrogenous products. Oxidation of 1 g of dietary carbohydrate and 1 g of dietary protein (which is oxidized to urea) each yield approximately 4 kcal, whereas oxidation of 1 g of alcohol yields approximately 7 kcal and oxidation of 1 g of dietary fat yields approximately 9 kcal. The energy cost of storing dietary fats as triglycerides is lower than that of converting protein or carbohydrates into fat. Donato and Hegsted (1985) have suggested that in growing animals, dietary fat can be stored as body fat with little energy expenditure and, therefore, that dietary fat stored as adipose tissue fat still yields approximately 9 kcal per gram. In contrast, energy is required to store dietary carbohydrates as body fat and 4 kcal per gram of dietary carbohydrate yields only approximately 3.27 kcal when stored as fat and subsequently oxidized for energy. Therefore, the ratio of the energy required to store dietary fat as body fat relative to the energy cost to store dietary carbohydrates or protein as body fat may be close to 9 to 3.27. That is, the conversion of fats in food to body fat (triglycerides) is more efficient than the conversion of carbohydrates or protein in food to body fat.
Historical Trends
Food disappearance data indicate that energy available in the food supply had not changed from 1909 to 1982, averaging 3,500 kcal/day per person during 1909-1913 and 3,600 kcal/day per person in 1985 (see Table 3-3). However, these amounts are considerably higher than actual intakes reported by individuals (see below). The percentage of total calories available from carbohydrates decreased from 57% during 1909-1913 to 46% in 1985, whereas the proportion from fats increased from 32% during 1909-1913 to 43% in 1985 (see Figure 3-3).
Recent Surveys of Energy Intake
Caloric intake by men and women has been reported in several recent surveys (Beaton et al., 1979; Braitman et al., 1985; Goor et al., 1985; USDA, 1987). Using the 24-hour recall method, Beaton et al. (1979) found that the mean daily energy intake was 2,639 kcal/day for men and 1,793 kcal/day for women. Using data from the National Center for Health Statistics (NCHS), which were based on 24-hour recall, Braitman et al. (1985) reported intakes of 2,359 kcal/day for men and 1,639 kcal/day for women.
Data from the Nationwide Food Consumption Survey (NFCS) can be used to compare energy intakes in 1965 and in 1977. Data from a single 24-hour recall plus 2-day records indicate that the energy intakes of males 9 to 64 years of age in 1977 were 10 to 17% lower than in 1965. Females ages 23 to 50 consumed 8.5 to 9% fewer calories in 1977 than in 1965 (USDA, 1984). The highest intakes were found among younger people. In 1965, males ages 15 to 18 consumed 3,008 kcal/ day compared to 2,698 kcal/day in 1977. 'The highest intake for females in 1965 was 2,146 kcal/ day among 12- to 14-year-old girls. This declined to 1,903 kcal/day by 1977. With advancing age, the average caloric intake declines for men and women (Goor et al., 1985). Among 20-year-old men, the intake reported between 1972 and 1978 in the Lipid Research Clinics Prevalence Study (LRC, 1980) ranged from 2,800 to 3,500 kcal/day, but for 59-year-old men, this range was 1,900 to 2,600 kcal/day. Intakes for 20-year-old women during the same period ranged from 2,000 to 2,200 kcal/day. Intakes had fallen to a range of 1,500 to 1,700 kcal/day by age 59. These data suggest that energy intake has declined for both sexes by approximately 10% during the past 10 to 20 years.
Factors Influencing Energy Intake
Caloric intake is influenced by many variables, including age, sex, environmental temperature, energy expenditure, pregnancy, hormonal status, and dieting behaviors. Figure 6-1 shows the effect of age on caloric intake among Americans based on three studies: the NFCS (USDA, 1984), the Health and Nutrition Examination Survey (HANES I), and studies of the Lipid Research Clinics Prevalence Study (Braitman et al., 1985; Goor et al., 1985). For both sexes, caloric intake peaked in the second decade of life and declined thereafter. At all ages, males had higher total caloric intakes than females and higher intakes for all three classes of macronutrients—fats, carbohydrates, and protein.
Caloric intake is also modestly affected by environmental temperature: at high and low ambient temperature, energy intake increases. Energy expenditure also affects caloric intake. For example, long-distance runners have high caloric intakes. More moderate levels of physical activity may increase food intake by lean but not by obese subjects. In controlled metabolic ward studies, Woo et al. (1985) compared the effect of three levels of physical expenditure on food intake by
Page 141
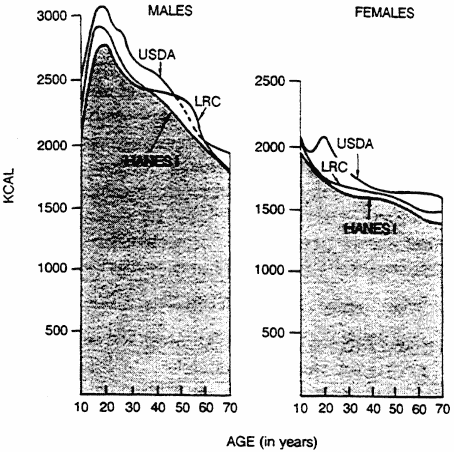
FIGURE 6-1
Relationship of caloric intake to age for males and females.
Based on data from three studies—the Lipid Research Clinics Prevalence
Study (LRC, 1980), the Nationwide Food Consumption Survey of the
U.S. Department of Agriculture (USDA, 1980), and the Health and
Nutrition Examination Survey (HANES I) of the National
Center for Health Statistics (Carroll et al., 1983).
normal-weight and obese subjects. They noted that as energy expenditure was increased by increasing the time spent in physical activity, lean women increased their food intake in proportion to the increase in energy expenditure. Obese women, on the other hand, did not (Woo et al., 1985).
During pregnancy, a new life with its own energy stores is created. Thus, it is usually suggested that pregnant women should increase their food intake (NRC, 1980). Recent measurements from several countries indicate that increases in energy needs and food intake during pregnancy may be less than previously suggested (Durnin, 1986). (See section on Pregnancy, below, under Energy Balance in Special Situations.)
Energy Expenditure
Measurement of Energy Expenditure
Energy expenditure can be measured in a variety of ways (McLean and Tobin, 1988). In the classic procedure, a calorimeter is used to measure heat production. In direct calorimetry, heat produced is measured as temperature changes through thermal gradients or as heat added to the ambient environment in an insulated chamber. Indirect calorimetry can be used to determine energy expenditure by measuring oxygen consumption and carbon dioxide production (e.g., in ventilated hoods) and then relating these measurements to the energy value of the oxygen consumed and the carbon dioxide produced. In ambulatory subjects, energy expenditure can be measured indirectly by collecting samples of expired air in which oxygen consumption and carbon dioxide production are determined and related to work activity. A recently developed technique is the use of doubly labelled water (2H218O or D218O) for measuring energy expenditure in ambulatory subjects. The heavy oxygen (18O) can be incorporated into carbon dioxide C18O2 during metabolism or excreted as water (H218O). Deuterium, on the other hand, can only be excreted as water (D2O). Thus the rate at which 18O declines relative to the decline in deuterium is a measurement of metabolic rate. This method holds promise for resolving issues related to the stoichiometry of energy expenditure and intake in ambulatory subjects (Lifson, 1966; Schoeller et al., 1986).
Other methods of estimating energy expenditure include diaries of physical activity, which are widely used in epidemiologic studies, and measurement of heart rate, because it varies directly with activity level. However, basal heart rate is a poor index of resting energy expenditure.
Components of Energy Expenditure
Energy expenditure can be subdivided into resting metabolic rate (RMR), thermic effects of food, physical activity, and growth. RMR is the quantity of energy needed to maintain body temperature, repair internal organs, support cardiac function, maintain ionic gradients across cells, and support respiration. This constitutes approximately two-thirds of total energy expenditure. The second largest component of energy expenditure is required for physical work. The energy expenditure required to move the body is related directly to body weight, to the distance that weight is moved, and to the state of physical fitness.
The heat produced following ingestion of a meal is usually termed the thermic effect of food (TEF) or diet-induced thermogenesis (DIT). (It was formerly called specific dynamic action.) This effect can be produced by any food, but the consumption of protein or carbohydrates results in much larger thermic effects than does consumption of fat.
There is an adaptive component to energy expenditure (adaptative thermogenesis). An acute
Page 142
large increase or decrease in energy intake lasting more than a few days is accompanied by a corresponding change in total energy expenditure. Thus, the overall RMR decreases during food restriction or starvation and increases with overfeeding; this may act in a counterregulatory way to decrease energy loss (Forbes, 1987; Garrow, 1978; Woo et al., 1985).
Historical Trends
The transition from an agricultural economy to a manufacturing economy and more recently to an information-gathering sedentary society has been associated with a steady decline in energy intake, presumably because of decreased caloric expenditure. Average energy intake has been used as a surrogate measurement for energy expenditure, since it is assumed that over time, individuals are in energy balance. Between 1965 and 1977, average caloric intake for all people dropped from 2,060 to 1,865 kcal/day. Protein intake dropped from 81.5 to 75.5 g/day, fat intake decreased from 99.3 to 85.3 g/day, and carbohydrates, from 209.8 to 195.7 g/day (USDA, 1984). In 1985, the mean daily energy intake by women over 4 days was 1,528 kcal (USDA, 1987). There are no direct surveys of trends in energy expenditure.
Factors Influencing Energy Expenditure
Resting energy expenditure is influenced by age, sex, body weight, pregnancy, and hormonal status. The highest rates of energy expenditure per unit of body weight occur during infancy and decline through childhood. In adult life, the decline continues at approximately 2% per decade because of a decline in lean body mass. Females have a lower energy expenditure per unit of weight than do males, probably because of the higher proportion of body fat in women. When expressed on the basis of fat-free mass, however, the differences between men and women and younger and older adults disappear. Resting energy expenditure is directly related to body weight and fat-free mass in men as well as women. People with high body weight on average also have high resting energy expenditure. Pregnancy increases energy expenditure to support fetal growth and the increase in maternal tissues. Several hormones, including thyroxin, catecholamines, and insulin, also increase energy expenditure.
Obese people have a modestly, but significantly, higher 24-hour energy expenditure than do normal-weight subjects (James, 1983). There is a positive and significant relationship between energy expenditure and fat-free mass, body surface area, or body weight (Garrow, 1978; Jequier, 1984; Owen et al., 1986, 1987; Ravussin et al., 1988; de Boer et al., 1987). Since body weight is more readily determined than specific components of body composition, basal energy needs can be estimated from body weight using the formulas derived by Owen et al. (1986, 1987). One can estimate total energy needs from an assessment of overall activity level (high, moderate, or low) as follows:
Basal energy needs = RMR x activity factor,
where RMR for men is 900 + 10 x body weight (kg) and for women is 800 + 7 x body weight (kg), and activity factors are 1.2 for low levels of activity (sedentary), 1.4 for moderate activity, and 1.6 for high levels of activity (regular exercise or manual labor).
The data of Bogardus et al. (1986) and Bouchard and his colleagues (1986) have shown a significant genetic component for resting metabolic rate (Fontaine et al., 1985) and endurance performance, although there is less evidence of a heredity effect on maximal oxygen consumption (VO2 max) (Bouchard et al., 1986). This suggests that important genetic determinants influence metabolic activity. For example, a decreased metabolic rate may predict the onset of weight gain in some adults (Ravussin et al., 1988) and infants (Roberts et al., 1988).
Using time-lapse motion pictures, Bullen et al. (1964) showed lower levels of physical activity in obese as compared to nonobese adolescents swimming or playing volleyball or tennis. Others, however, have found little difference between the activity level of obese and control subjects (Stefanik et al., 1959; Tryon, 1987; Wilkinson et al., 1977). Brownell et al. (1980) observed that when confronted with a choice between escalators and stairs, more lean than obese subjects used the stairs.
Several, but not all, studies suggest that obese people are less physically active; however, to interpret this observation as an indication that obese subjects expend less energy, one must know the energy cost of the activity. Since overweight people must do more physical work to move their bodies, smaller total amounts of movements may add up to comparable or greater total energy expenditure.
Waxman and Stunkard (1980) compared activity levels of lean and obese boys within the same families. In this setting, total energy expenditure,
Page 143
when corrected for energy costs for specific activities, was comparable between the paired individuals. Bray (1983) observed that the efficiency of energy expenditure in muscles of obese and lean subjects is comparable, but because the obese subjects carry more weight, their total energy expenditure may be higher. One important difference in energy expenditure among individuals may be the extent and degree of fidgeting. Bogardus et al. (1986) in their studies of the Pima Indians observed important differences between different groups of people in the amount of time spent in fidgeting when they were studied in a metabolic chamber.
The efficiency of energy transfer from foods to adenosine triphosphate (ATP) and of ATP utilization may also influence energy needs. In some forms of obesity, the obese may be more efficient than lean subjects in utilizing nutrients for resting metabolic requirements and, thus, may have more energy to store as fat (Sims et al., 1973). A number of mechanisms have been suggested for this metabolic efficiency, including reduced sodium pump activity, lower protein turnover, altered nutrient partitioning, faulty thermogenic mediation by the sympathetic nervous system, impaired function of brown adipose tissue, and reduced efficiency of muscular contraction (Bray, 1983). The thermogenic effect of a meal usually amounts to approximately 10% of the caloric value of the ingested food Jequier, 1984) and is higher for carbohydrates and protein than for fats. It may also differ with the type of food eaten (Forbes, 1987). It is not known if such thermogenesis is higher in obese people, but energy requirements do appear to decrease with weight loss (Geissler et al., 1987; Leibel and Hirsch, 1984). A number of biochemical changes, including alterations in oxygen consumption, increased lipoprotein lipase (LPL) levels in adipose tissue, and altered pancreatic insulin kinetics, have been demonstrated in the first week of life in genetically obese rodents, but definitive biochemical lesions have yet to be found in humans. There is increasing evidence, however, that metabolic mechanisms relevant to efficiency play a role in human energy balance as well.
Energy Balance
Measurement of Body Fat
Body fat is the primary energy store in humans. Because direct chemical analysis of fat is impossible in living human beings, indirect methods have been developed to assess body composition and thus measure body fat. These methods are summarized below. Anthropometric methods are noninvasive, are quick, and require inexpensive equipment. They include measurements of height, weight, body circumferences, body diameters, and skinfold thickness. The coefficient of variation for height and weight is very small (1 to 2%) but is greater for the other measures: 5 to 10% for circumferences and up to 20% for skinfold thickness of obese individuals, whose skinfolds are often too thick to be measured with commonly used calipers (Bray et al., 1978). Many equations have been developed for estimating body fat from measurements of skinfold thickness at one or more sites. These equations are usually influenced by the sex, age, and ethnic group from which they were developed. Skinfolds have frequently been used in epidemiologic studies of normal populations, but their limitations must be kept in mind. In general, circumference measures are less subject to investigator bias and are more helpful in assessing both total body fat and fat distribution.
Measurement of body density is one of the better and most widely accepted methods for estimating total body fat and is often used as the reference standard. By determining body weight in air and under water, and correcting for lung volume, the body can be subdivided into its fat and fat-free compartments. It is assumed that the density of the fat-free compartment does not vary, but this assumption may require modification for children, well-trained athletes, and the elderly. Various radioactive materials (3H2O, 40K, 82Br, 24Na) and nonradioactive substances (stable isotopes, cyclopropane, krypton, and antipyrine) can also be used to estimate body compartments; several recent methods have extended the range of tools available for taking these measurements.
Following is a list of techniques used to determine body composition:
Direct Carcass Analysis
Indirect Analysis of Body Composition
· Visual Observation (somatotypes)
· Anthropomorphic Measurements
Height and Weight
Circumferences and Diameters
Skinfold Thicknesses
· Isotope or Chemical Dilution
Body Water (3H2O, antipyrine)
Body Potassium (40K, 42K)
Body Fat (cyclopropane, krypton)
Page 144
· Body Density and Body Volume
· Conductivity
Total Body Electrical Conductivity (TOBEC)
Bioelectric Impedance Analysis (BIA)
· Neutron Activation
· Imaging Techniques
Ultrasound
Computed Tomography (CT)
Nuclear Magnetic Resonance Spectroscopy (NMRS)
Using the differences in electrical conductivity between water and fat, one can estimate the fat and nonfat compartments by TOBEC or BIA. The latter method is likely to be particularly useful (Segal et al., 1988), since it correlates highly with body fat estimated by density and it is highly reproducible between laboratories. The thickness of fat can be measured with ultrasound, CT (Sjöström et al., 1986), and magnetic resonance imaging. All these methods can be used to assess body fat as a function of body weight as well as its regional and intraabdominal distribution.
As noted in Chapter 21, it is not only the total quantity of fat (high or low) that is associated with health risks but also the location of fat. Abdominal or central body fat carries a higher risk of coronary heart disease (CHD), diabetes mellitus, hypertension, stroke, endometrial cancer in women, and overall mortality compared to peripheral or lower-body gluteal fat. This risk can be assessed by determining the ratio of abdominal-to-gluteal circumference or by the ratio of central-to-peripheral skinfolds.
The Association of Caloric Intake with Body Weight and Energy Stores
Epidemiologic Data
There is little question that changes in food intake can produce corresponding changes in body weight (Bray, 1976; Forbes, 1987; Garrow, 1978). Increased body fatness during adult life has been observed with increasing frequency during this century. This appears to be associated primarily with a reduction in physical activity and a corresponding reduction in lean body mass. An increase in energy intake may also be associated with this phenomenon.
In studies of Army recruits, Edholm et al. (1970) observed a wide range of daily variations in food intake among normal-weight individuals. However, he also showed a correlation between energy intake and expenditure over 7 days. There was an intraindividual correlation between intake and expenditure only over periods longer than 7 days. Body composition and physical activity have been examined in ''big eaters" and "small eaters" who have similar levels of fat-free mass. Male big eaters consumed 48.8 kcal per kilogram of body weight per day (204 kJ/kg per day) compared to 19.8 kcal/kg per day (83 kJ/kg per day) for the small eaters. Body fat, on the other hand, was 31.5% in the small eaters and 21.1% in the large eaters (George, in press).
In several cross-sectional studies, overweight subjects have often been found to eat the same or smaller amounts of food compared to normal-weight subjects (Baecke et al., 1983; Braitman et al., 1985; Keen et al., 1979; Kromhout, 1983b; Lincoln, 1972; Noppa, 1980; Romieu et al., 1988). (See Table 6-1.) In all cases, except the study of "research patients" by Beaudoin and Mayer (1953), the mean intake by the overweight subjects was less than that of the controls (for details on the control groups, see footnotes to Table 6-1). In the studies of females alone, this difference was statistically significant, but it was only significant in one of three studies of men. Keen et al. (1979) also found a negative correlation between energy intake and body mass index. Romieu et al. (1988) reported that obese women tended to be older, to exercise less, and to drink less alcohol than younger women. After correcting for these variables, the inverse correlation between energy intake and relative weight was significantly reduced from R = -.11 to R = -.02, showing almost no correlation between these two variables. Of particular interest is that in the obese women there was a positive correlation between relative weight and total fat intake as well with the intake of saturated fat.
These cross-sectional findings have been confirmed in longitudinal studies (Kromhout, 1983a; Lapidus et al., 1986). In a cohort study of 871 men in Zutphen, the Netherlands, energy intake decreased approximately 450 kcal/day over a 10-year period, during which body weight increased by 3.5 kg (Kromhout, 1983a). Food intake has declined over the past decade when body weight and presumably fat stores have, on average, increased. From the epidemiologic data, it appears that increased caloric intake in the population cannot explain the positive energy balance observed in adult life in the United States, the Netherlands, or Sweden.
Clinical Data
The clinical data on the effect of over- and undereating on changes in fat stores clearly show that overeating can increase body fat stores,
Page 145
| TABLE 6-1 Caloric Intake of Overweight and Normal-Weight (Control) Subjects | |||||||
| Control Subjects | Overweight Subjects | ||||||
| N | Sex | (kcal/day) | N | Sex | (kcal/day) | p | Reference |
| 58a | F | 2,198±587b | 59 | F | 1,964±594b | <.05 | Beaudoin and Mayer (1953) |
| 20c | F | 2,201±475b | 33 | F | 2,829±674b | <.01 | |
| 98d | M&F | 3,319 | 101 | M&F | 3,144 | NSe | Lincoln (1972) |
| 47f | M | 3,070±105b | 27 | M | 2,983±167b | NS | Baecke et al. (1983) |
| 50 | F | 2,280±65b | 45 | F | 2,045± 64b | <.05 | |
| 202g | M | 3,193±606b | 202 | M | 2,916±652b | <.001 | Kromhout (1983b) |
| 708h | M | 2,359 | 79 | M | 2,411 | NS | Braitman et al. (1985) |
| 1,246 | F | 1,639 | 245 | F | 1,525 | <.001 | |
| 45i | F | 1,684±315j | 47 | F | 1,635±340j | NS | Romieu et al. (1988) |
| a A population-based study in New York. | |||||||
whereas undereating can result in a decrease of body fat stores. (See Chapter 21 for details.)
Animal Studies
There are sex-specific responses to the effects of voluntarily increased food intake by animals. In general, female animals respond to calorie-dense diets with an increased intake and a higher percent increment of body fat than males (Jen et al., 1981). Whether this effect is related to the increment in total caloric intake or to the amount or type of fat in the diet is presently unclear. In pregnant and lactating animals of most species, food intake increases voluntarily during the latter part of pregnancy and throughout lactation. In animal studies, these are among the few times when increased voluntary intake of the usual diet occurs. During pregnancy, food efficiency—i.e., weight gained per gram of food eaten—is also enhanced. Much of the weight gain in later pregnancy is associated with growth of the fetus; during early pregnancy, the enhanced metabolic efficiency contributes to fat accumulation, which is regionally specific (in either the inguinal or omental region). One of the few natural examples of increased voluntary food intake with decreasing or stabilized weight gain is noted during lactation. Under these conditions, the nearly doubled or tripled caloric intake is utilized primarily for milk synthesis to supply the lactational demands of the suckling young. At the end of lactation, despite the large increase in food intake, the body fat stores of the rat are reduced below prepregnant and prelactating levels.
In spontaneously obese mutants, particularly among rodents, hyperphagia is a correlate of obesity. Thus, it appears that overeating is an important feature of the obesity; however, obesity in these mutants cannot be attributed solely to increased total caloric intake. Pair-feeding of the obese or preobese animal to a lean control leads to a reduction in the body weight of these spontaneously obese rodents but has little effect on body composition (Cleary et al., 1980; Cox and Powley, 1977). That is, genetically obese animals pair-fed to lean controls maintain as much as 60% body fat compared to 20% in lean animals of equivalent body weight. Thus, obesity in normal animals is induced primarily by overingestion, whereas in genetically obese animal models, hyperphagia is not a prerequisite for obesity.
Association Between Energy Expenditure and Net Energy Stores
Epidemiologic Data
Changes in net energy stores are a sensitive indicator of the relationship between energy intake
Page 146
and expenditure. The increase in body fat stores with age indicates imbalance. Since the data show that energy intake declines with age in adults, the most probable explanation is that energy expenditure declines as people grow older (Kromhout, 1983a).
Clinical Data
Physical exercise may reduce the total quantity of body fat and body weight. In a review of training programs lasting from 7 to 22 weeks, Wilmore (1983) found that changes in body composition were surprisingly small. On average, body fat decreased by only 1.6%, and in 5 of 55 studies there was actually an increase in body fat after training. Moreover, lean body mass dropped in 17 of these 55 studies.
A decrease in body fat after exercise can be demonstrated more readily in moderately obese subjects than in normal-weight subjects. In 12 studies of overweight men and women, the mean decrease in body weight ranged from 2.6 to 13.3 kg, most of which was accounted for by a decrease in body fat. In contrast to data on moderately obese people, a reduction in body fat after physical training is difficult to demonstrate in massively obese subjects. Frequently, this can be attributed to the fact that only modest amounts of weight are lost. Thus, the decrease in fat may not be readily detectable. Reduction in body fat content during physical training is accompanied by a decrease in the average size of fat cells. People with larger fat cells (hypertrophic obesity) may lose more fat during an exercise program than those with a larger number of relatively small fat cells (Krotkiewski et al., 1977).
Both acute exercise and prolonged physical training modify the metabolism of glucose, insulin, and lipids in obese and normal subjects. During physical training, glucose tolerance is improved and insulin levels decrease, suggesting reduced resistance to insulin. Exercise by normal-weight subjects can be associated with an increase in levels of high-density lipoproteins (HDLs). In obese people, however, changes in lipid metabolism that occur with exercise are more complex (Bray and Gray, 1988).
Obesity is usually associated with reduced levels of HDL cholesterol. Brownell et al. (1982) reported that a 10.7 kg weight loss in men was associated with a 5% increase in HDL cholesterol, a 15.8% decrease in low-density lipoprotein (LDL) cholesterol, and, thus, a 30% increase in the ratio of HDL to LDL cholesterol. In women, on the other hand, a weight loss of 8.9 kg produced no change in HDL cholesterol.
Animal Studies
The concept that some cases of obesity result from an energy imbalance related primarily to decreased caloric expenditure rather than to increased caloric intake has received much attention over the past decade. In rats spontaneously ingesting a cafeteria diet (mixed human food), thermogenesis increased to compensate for increased food intake (Stock and Rothwell, 1981). There has been considerable debate in the literature concerning the basis of this effect. Since many of these studies were conducted in rodents that possess brown adipose tissue (which is highly thermogenic), Himms-Hagen (1985) examined the ability of brown adipose tissue to uncouple the mitochondrial proton conductance pathway and thus to produce heat without producing equivalents for ATP synthesis. The effects on thermogenesis may be related to dietary effects on the sympathetic nervous system, which in turn may be related to other potentially thermogenic effects in tissues such as liver and muscle. This area requires further research.
It has been well demonstrated in several species of normally lean animals (males, not females) that increased physical activity in the absence of increased caloric intake leads to a decrease in body weight and a change in body composition. The amount of caloric expenditure is proportional to these effects. In addition, spontaneous or genetic inter- and intraspecies effects on RMR may underlie the animals' responsiveness to exercise. The concept has been advanced that there is a set point—a weight level that is maintained within a small margin—that regulates energy balance in animals. In support of this concept, Boyle et al. (1978), Keesey (1980), and Keesey and Powley (1986) have proposed that caloric consumption can be predicted from RMR. (See also Kleiber, 1947.)
Some investigators have reasoned that a thermogenic defect in genetically obese or experimentally manipulated obese animals provides evidence that defective themogenesis plays a role in the genesis of obesity (Himms-Hagen, 1985). In fact, genetically obese adult rodents of several species exhibit decreased overall thermogenesis, diet-induced thermogenesis, and poor responsiveness to cold. In addition, there is frequently decreased guanosine 5'-diphosphate (GDP) binding in their brown adipose tissue (Himms-Hagen, 1985); how-
Page 147
| TABLE 6-2 Energy Expenditure During Pregnancy in Four Different Countriesa | ||||
| Energy Cost (kcal) | ||||
| Source of Energy | ||||
| Expenditure | Scotland | Holland | Thailand | Philippines |
| Fetus | 8,110 | 8,230 | 7,140 | 6,900 |
| Placenta | 730 | 740 | 600 | 600 |
| Uterus and blood volume | 2,890 | 2,950 | 2,480 | 2,410 |
| Maternal fat | 25,230 | 14,300 | 15,400 | 14,300 |
| Basal metabolic rate | 30,100 | 34,500 | 24,000 | 19,000 |
| TOTAL | 67,060 | 60,720 | 49,620 | 43,210 |
| a Adapted from Durnin (1986). | ||||
ever, extirpation of brown adipose tissue does not promote the development of obesity (Horwitz et al., 1985), and proliferation of such tissue as a consequence of exposure to cold does not promote leanness (Miller and Faust, 1982). In at least some species, alterations in body fat content clearly precede any changes in the metabolism of brown adipose tissue (Greenwood et al., 1981). In obese animals, alterations in substrate utilization lead to alterations in body composition (i.e., increased fatness and decreased lean tissue) and may reduce the thermogenic contribution of skeletal and other muscle tissue. Thus, it is not clear how thermogenic defects per se may contribute to the etiology of obesity, but it is becoming evident that they may be important factors to delineate in order to understand different types of obesity.
Energy Balance in Special Situations
As noted earlier in this chapter, positive energy balance occurs normally during growth and pregnancy. During lactation, food energy increases as does energy expenditure. This results in negative energy balance.
Periods of Rapid Growth
Estimated nutrient requirements for human growth are 117 kcal/kg body weight at 0 to 0.5 year of age, 108 kcal/kg at 0.5 to 1.0 year, and 1,300 kcal at 1 to 3 years (NRC, 1980). It is not yet possible to precisely specify the macronutrient composition of this caloric requirement. The best indication that an infant is growing properly is normal linear growth.
Energy requirements peak during adolescence—a time of rapid growth and changes in body composition. The adolescent growth spurt begins at about 10 years of age in girls and at about 12 years of age in boys. Energy requirements increase to support this rapid rate of growth. In boys, estimated energy intake is 2,500 to 3,200 kcal/day between the ages of 10 and 20. Girls between the ages of 10 and 20 require an estimated 1,800 to 2,000 kcal/day (see Figure 6-1).
Pregnancy
The amounts of extra energy intake recommended during pregnancy vary from 240 to 285 kcal/day and are based on the classic calculations of Hytten and Leitch (1971). In a recent multinational study, Durnin (1986) reexamined these assumptions using measurements of changing body weight and basal metabolic rate during pregnancy. The energy cost of pregnancy from data on four countries in that study is shown in Table 6-2. All the values in the table are lower than the 80,000 kcal calculated by Hytten and Leitch (1971). The most unexpected finding by Durnin (1986) was that food intake increased little or not at all during pregnancy, suggesting that much of the net energy needed for pregnancy comes from altered metabolic efficiency.
Lactation
Approximately 900 kcal of energy are required to produce 1 liter of milk in women. The 2 to 4 kg of body fat that are stored during pregnancy can be mobilized to supply a portion of the additional energy needed for lactation. Additional energy needs during the first 3 months of lactation are derived by ingesting an additional 500 kcal/day.
The Elderly
Studies in several countries show that energy intake declines with advancing age (Figure 6-1). In a cross-sectional study of 20- to 93-year-old male executives in Baltimore, McGandy et al. (1966) found a steady decline in energy intake from 2,700 kcal/day at 30 years to 2,100 kcal/day at 80 years. Approximately 200 kcal of this decline was due to the decrease of lean body mass with
Page 148
advancing age; the remaining 400 kcal appears to be due to a decline in physical activity. The second National Health and Nutrition Examination Survey (NHANES II) showed that men reduce their daily energy intake from 2,700 kcal at 23 to 24 years of age to 1,800 kcal at 65 to 74 years and that women reduce their daily energy consumption from 1,600 kcal to 1,300 kcal over the same period (Carroll et al., 1983). The NFCS indicated that elderly men averaged 1,700 kcal/day, whereas elderly women averaged 1,330 kcal (USDA, 1980). The energy requirements estimated in the Food and Nutrition Board's Recommended Dietary Allowances for a 70-kg man are 2,400 kcal at 51 to 75 years and 2,050 for those over 75 years. For 55-kg women, the requirements are 1,800 and 1,600 kcal, respectively, for the same age groups (NRC, 1980). These estimated requirements are well above the measured values noted above. It is possible that the RDAs are too high or that elderly people are inactive.
Evidence Associating Total Energy Intake with Chronic Diseases
Atherosclerotic Cardiovascular Diseases
Epidemiologic Studies
The relationship of total energy intake to cardiovascular diseases has been examined in several studies. Subjects who develop heart disease have a history of lower total caloric intake on the average compared to those who remain free of the disease (Gordon et al., 1981). In the Honolulu Heart Study, crude caloric intake and the intake of 8 of 11 other nutrients were lower for those who subsequently developed CHD; for the remaining three nutrients, there were no associations with CHD (Willett and Stampfer, 1986). The authors proposed expressing the data in the form of the nutrient density of caloric intake. "Because coronary heart disease is associated with low caloric intake, nutrient density expressed as a percentage of total calories will tend to be positively associated with disease" (Willett and Stampfer, 1986, p. 21). However, for some nutrients there may be a negative association with CHD, and for others there may be no association. When this approach was used, the ratio of specific nutrients to total calories was positive for protein and for total, saturated, and monounsaturated fats but was negative for carbohydrates.
In the Göteborg longitudinal study, Lapidus et al. (1986) reported that energy intake was inversely correlated with death from all causes and with several variables, including age, systolic blood pressure, body mass index, and ratio of waist-to-hip circumference. Percentage of energy intake derived from fats was positively correlated with serum triglycerides. Although there was a negative relationship between energy intake and risk of myocardial infarction, there was no correlation between energy intake and 12-year overall mortality from or incidence of stroke. Energy intake was also inversely correlated with the risk of myocardial infarction when such factors as obesity, smoking habits, serum cholesterol concentration, serum triglyceride concentration, diabetes, systolic blood pressure, and physical activity were taken into account.
Clinical Studies
In studies of experimental overfeeding, Sims and his colleagues (1973) demonstrated an increase in plasma triglycerides, insulin, and glucose. These same factors are observed to change during spontaneous alterations in body weight (Ashley and Kannel, 1974). A reduction in food intake by obese subjects under experimental conditions is associated with a marked decrease in triglycerides and an initial reduction in serum total cholesterol, which may subsequently increase even though caloric intake remains reduced (Henry et al., 1986).
Therefore, although a direct relationship of total caloric intake with morbidity and mortality cannot be shown in population studies, it is possible to demonstrate that several salient cardiovascular disease risk factors can be manipulated in individuals by changing their overall caloric intake. In general, many studies have shown that short-term weight loss—however achieved—results in lowered blood lipids except for HDL cholesterol, which is increased, and lowered blood pressure, blood glucose, and insulin levels.
Animal Studies
In animals, it has been difficult to demonstrate any specific effect of total calories, other than their contribution to body weight and body composition as a correlate of cardiovascular diseases. Several species of animals with high levels of body fat have high cardiovascular risk indices. Although increased caloric intake in laboratory animals leads to increased adiposity, most of the effects of diet on cardiovascular risk factors, such as altered plasma
Page 149
lipid profiles, stroke, and hypertension, have been attributed to changes in the macronutrient composition of the diet rather than to the increments in total calories (see Chapter 7). In addition, overingestion, especially of carbohydrates, leads to increased blood insulin levels. This in turn leads to increased norepinephrine turnover in several tissues (Young and Landsberg, 1977), which may have direct effects on adipose tissue blood flow and blood pressure. Thus, increased total caloric intake by obese and nonobese animals may alter hemodynamics and blood pressure and may influence the development of hypertension.
In the spontaneously hypertensive rat (SHR), decreased overall caloric intake resulted in lower blood pressure, whereas overfeeding elevated it (Young and Landsberg, 1977). However, this effect may be related more to the intake of sucrose than to total caloric intake, since equicaloric intake of high-fat diets did not provoke the same response in the sympathetic nervous system. Furthermore, even moderate caloric restriction frequently can effect changes in blood pressure or blood lipids in animals already identified as being at risk.
Diabetes Mellitus
Epidemiologic Studies
Decreased death rates from diabetes were observed during World War I in Berlin, Paris, and London but not in New York or Tokyo (Himsworth, 1935). During World War II, there was a drop in the incidence of diabetes in Japan (Goto et al., 1958). After both wars, the incidence of and mortality from diabetes returned to prewar levels as caloric intake increased.
In contrast to the decline in diabetes incidence observed in epidemiologic studies of starvation, evidence that total caloric intake is related to the development of noninsulin-dependent diabetes mellitus (NIDDM) within a population with adequate supplies of food is weak. Himsworth (1935) analyzed dietary intakes in several countries and found that those with the highest death rates tended to have a relatively low proportion of dietary fats. Whether caloric intake, independent of obesity, plays a role in the development of NIDDM is unclear. West and Kalbfleisch (1971) established that there is a strong relationship between the prevalence of diabetes and relative weight, but they could not show a relationship with total caloric intake. Acculturation is often associated with a change in the quality of the diet and with an increased incidence of diabetes, obesity, hypertension, and heart disease. Physical activity is also often reduced during acculturation, further confounding the interpretation of the relationship between diet and chronic diseases. In a cross-sectional study, Japanese living in Hiroshima have been compared with those who migrated to Hawaii (Kawate et al., 1979). Obesity was approximately seven times more prevalent among those living in Hawaii, and the incidence of diabetes was nearly double. The total quantity of calories consumed by these two groups did not differ, although more fats and more simple carbohydrates were consumed by those living in Hawaii. Over a 5-year period, investigators conducting the Israeli Heart Study also failed to find a relationship between energy intake and the risk of developing NIDDM (Medalie et al., 1975). In summary, there is no evidence to indicate that total caloric intake is related to the onset of NIDDM.
Clinical Studies
Restriction of caloric intake in both inpatient and outpatient settings improves glucose tolerance in most obese people (Arky, 1976). This effect and the detrimental consequences of subsequent weight regain were clearly shown by Drenick et al. (1972). (See Chapter 21 for more detail.)
Metabolic pathways, including those concerned with glucose metabolism, can be disturbed by overeating. Sims et al. (1973) found that glucose levels following an oral glucose load were higher after weight gain in normal-weight volunteers. Insulin was also slightly increased and associated with the development of insulin resistance in adipose tissue. These deleterious effects of forced overeating were reversed with weight loss.
Animal Studies
There is no evidence that total caloric intake is a cause of either NIDDM or insulin-dependent diabetes mellitus (IDDM) in animals (Glinsmann et al., 1986). However, there is little question that when animals increase their total food intake they develop adiposity (see Animal Studies subsection of The Relationship of Caloric Intake to Body Weight and Energy Stores, above), and adiposity is usually associated with the development of insulin resistance (Stern et al., 1975; Susini and Lavau, 1978). Accompanying the insulin resistance and impaired glucose tolerance are impaired pancreatic responses to oral glucose tolerance tests and hyperglycemia. Nonetheless, frank diabetes with marked hyperglycemia and glycosuria is rarely found in
Page 150
studies of animals in which increased total caloric intake is used as the variable. Even studies that focus on overingestion of specific nutrients, such as simple sugars, can often be criticized for failing to include pair-fed controls or for feeding the nutrient at levels unlikely to be encountered by humans or animals.
The metabolic responses associated with increased total caloric intake usually appear only after a long period of overeating. Thus, in addition to the associated adiposity, findings in many animal models of diet-induced diabetes are further confounded by the effects of aging inherent in most long-term feeding studies. In most cases, weight reduction and restoration of a more normal body composition reverse the effects of increased caloric intake on insulin resistance. Thus, it becomes exceedingly difficult to identify any specific effect of calories, independent of the effects on overall adiposity, on the development of diabetes.
There are several genetic models of both NIDDM and IDDM, for example, the female Yucatan miniature swine (Phillips et al., 1982), a strain of spiny mouse (Acomy cahirihup) (Obell, 1974), the desert sand rat (Kalderon et al., 1986; Rice and Robertson, 1980), and the Wistar fatty rat (WDF/Ta-fa/fa) (see Chapter 24 and Salans and Graham, 1982, for further details). Although there is some evidence that overingestion of specific nutrients may enhance (Kava et al., 1989) or provoke (Ikeda et al., 1981) the incipient diabetic condition and exacerbate specific pathologies, there is little evidence that the condition depends upon hyperphagia. One exception to this general statement is the development of diabetes in the sand rat. This strain of rat normally remains lean and nondiabetic in the wild, but when housed under laboratory conditions, it becomes hyperphagic and diabetic (Salans and Graham, 1982).
Cancer
Epidemiologic Studies
As reviewed in detail by the National Research Council's Committee on Diet, Nutrition, and Cancer (NRC, 1982), few investigators have specifically studied the association of total caloric intake with cancer risk. Different results were obtained in three case-control studies of colorectal cancer—one in Canada (Jain et al., 1980), one in Australia (Potter and McMichael, 1986), and one in Belgium (Tuyns et al., 1987). A higher caloric intake by cancer patients than by controls was reported in the Canadian and Australian studies, but the opposite was found in the Belgian study. A recent reanalysis of the Canadian data (Howe et al., 1986) very clearly indicates that the risk in this study was associated with total fat and saturated fat intake for both males and females and that the relative risks associated with caloric intake were very close to unity.
In earlier studies, Gregor et al. (1969) concluded that as the per-capita food intake for gross national products increases, the mortality rates for gastric cancers fall and those for intestinal cancer rise. Lew and Garfinkel (1979) examined the relationship between mortality from cancer and other diseases and variations in weight among 750,000 men and women selected from the general population. Cancer mortality was significantly elevated in both sexes only among those 40% or more overweight. (See NRC for a detailed analysis of this and other studies up to 1981.)
Animal Studies
The early studies of Tannenbaum (1947, 1959) on diet and cancer were concerned mainly with effects of caloric restriction on carcinogenesis. His experiments and those in other laboratories showed that a variety of murine tumors, including skin tumors, mammary tumors, hepatomas, lung adenomas, sarcomas, and leukemia, are inhibited by reducing caloric intake (Carroll, 1975). In those experiments, food intake was usually restricted to between one-half and two-thirds of normal, in some cases by underfeeding and thus reducing the intake of all nutrients and in other cases by reducing either carbohydrates or fats alone. The inhibitory effect seemed to be exerted at later stages of tumor development rather than at initiation and was evidently not due to inanition, since the restricted animals were generally active and healthy and tended to live longer than animals fed ad libitum. Since many different types of tumors are inhibited by caloric restriction, the effect may be due simply to lack of energy intake or the metabolites required for tumor development, but other more specific factors such as pituitary hypofunction (Mulinos and Pomerantz, 1940), adrenal hyperfunction, lower insulin levels, and levels of a variety of growth factors may also be involved (Boutwell et al., 1948; Pariza, 1987).
Over the past few years, there has been a revival of interest in experiments in animals to study the influence of caloric intake on carcinogenesis (Kritchevsky, 1986; Kritchevsky and Klurfeld, 1987; Pariza, 1987). From the results of these experiments, it is clear that caloric restriction
Page 151
markedly inhibits tumors of the mammary gland and colon in rats, even when the diet is high in fat (Boissonneault et al., 1986; Klurfeld et al., 1987; Kritchevsky et al., 1984, 1986; Thompson et al., 1985). In most of these studies, dietary intake was restricted by 25% or more, and the body weights of restricted animals were substantially lower than those fed ad libitum. In one experiment, a 10% caloric restriction had no effect on mammary tumor incidence but reduced the tumor weights (Kritchevsky, 1986).
The effect of overfeeding on tumorigenesis is more difficult to investigate experimentally. However, animal species fed high-fat diets tend to gain more weight than those fed low-fat diets, even when the caloric intake is similar. This may be because less energy is required to store dietary fats as body fat than to convert dietary carbohydrates or protein to fat for storage (Pariza, 1987). The promoting effect of dietary fats may thus be due in part to increased caloric intake or to more efficient utilization of dietary calories (Carroll, 1986; Reddy, 1986). There is some evidence that cancer develops more readily in obese animals (Carroll, 1975).
Evidence Associating Energy Expenditure with Health and Chronic Diseases
Atherosclerotic Cardiovascular
Epidemiologic Studies
In reasonably homogeneous populations, people with jobs requiring high levels of physical activity cardiovascular diseases. Aerobic physical train(1975) reported lower mortality rates from all causes and from CHD in longshoremen, who do physically demanding work, compared to those who engage in moderate or light activity. Morris et al. (1980) reported that British civil servants with high levels of physical activity during leisure experienced fewer coronary events and had fewer abnormalities in their electrocardiograms compared to those who exercised less. They emphasized the and improved glucose tolerance. importance of bursts of increased physical activity, which at times exceeded 7.5 kcal/min (31 kJ/min or 5 METs/min). Paffenbarger et al. (1986) related leisure-time physical activity to the first heart attack in a follow-up of a large cohort of Harvard University alumni. For the entire range of energy expenditure, the risk of a first heart attack seemed to be lower in those who regularly participated in strenuous sports than in those with more casual activities. Siscovick et al. (1984) used a case-control technique to examine the relationship between habitual leisure-time activity and sudden death among people without a history of heart disease. The overall risk of primary cardiac arrest was increased among those who were habitually less active, compared to people who were more vigorous.
Sobolski et al. (1987) examined the relationship of physical fitness and physical activity to the development of CHD in approximately 1,734 men given a test to measure work capacity and followed for more than 5 years. They observed no association between physical activity on the job or at leisure and the development of CHD; however, there was an inverse relationship between work capacity(to produce a heart rate of 150) divided by body weight (in kilograms) to the risk of systemic heart disease. This variable, as well as HDL cholesterol and smoking, remained significant mediators of heart disease in multivariate analyses. The authors noted that physical fitness and personal estimates of physical activity may be poorly correlated.
The epidemiologic data presented here clearly suggest that there is an inverse association between the level of habitual, vigorous physical activity and the overall risk of CHD. Furthermore, the data indicate that the benefit results from exercise and that there are few who would not benefit from increased physical activity.
Clinical Studies
Clinical studies of people during physical training reveal a variety of physiologic changes relevant to cardiovascular diseases. Aerobic physical training improves cardiovascular function manifested by increased maximal oxygen uptake and a reduced heart rate. In many studies, physical training is associated with increased lean body mass as well as a reduction in body fate stores (Wilmore, 1983); the latter are related to the degree of negative energy balance during physical training. Physical training is also associated with a reduction in insulin levels and improved glucose tolerance.
Animal Studies
There is a substantial literature on the effects of physical fitness on cardiovascular function in animals. Similar to studies in humans, studies in animals have shown that cardiovascular fitness can be enhanced by regular exercise (Brooks and
Page 152
White, 1978; Gleeson and Baldwin, 1981; Mazzeo et al., 1984; Starnes et al., 1983). It is also known that food intake by animals may increase to maintain energy balance over a wide range of energy expenditures.
Dynamic exercise lowers heart rate response (Frick et al., 1967; Ordway et al., 1982; Tipton, 1965) and may diminish maximal heart rate. These effects may be mediated through regulation of b-adrenergic receptor number or through modulation of the sympathetic nervous system input into cardiovascular dynamics (Hammond et al., 1987). Since the sympathetic nervous system may play an important role in the regulation of energy expenditure during physical activity as well as during rest, it may be that total energy expenditure and fitness over time are reflected in predictable changes in cardiovascular diseases, but the committee could identify no specific long-term studies in animals that specifically supported this hypothesis.
Diabetes Mellitus
Epidemiologic Studies
Jarrett et al. (1986) examined the relationship of physical activity levels to the risk of developing NIDDM in the Whitehall study. They found no association between blood glucose level and a physical activity score ascertained through questionnaires. There was also no evident relationship in this study between glucose tolerance or NIDDM and the time reportedly spent in leisure-time physical activity, or between glucose tolerance and energy expenditure. In the Tecumseh Study, physical activity was divided into three categories based on time spent in leisure and occupation-related physical activity (Montoye et al., 1977). As in the Whitehall study, there was no correlation between glucose tolerance and physical activity among 1,300 men ages 16 to 64 years.
Clinical Studies
In contrast to the epidemiologic studies, there is clear evidence from clinical studies that increasing physical activity can improve glucose tolerance and that physical inactivity can worsen it. Björntorp and colleagues (1973) placed overweight subjects in an extended physical training program under careful supervison and demonstrated that glucose tolerance, especially the secretion of insulin following an oral glucose tolerance test, is substantially improved by physical training. In contrast, after a few days of bed rest, glucose tolerance deteriorated in healthy subjects.
Animal Studies
Some studies in humans suggest that diet-induced thermogenesis is impaired in subjects with NIDDM. The few good animal models for NIDDM include the diabetic mouse, the Wistar fatty rat, and the sand rat. Studies of obese monkeys and dogs have not provided sufficient data to determine whether a reduction in diet-induced thermogenesis enhances the risk of adiposity and thus increases the incidence of diabetes. In a comparison of two diabetic mouse strains, Coleman (1979) concluded that the impairment in thermogenesis attributable to the mutant strains could not account for most of the observed metabolic efficiency. It is possible that increased adiposity contributes to the insulin resistance associated with diabetes. It is uncertain whether decreased thermogenesis contributes to the adiposity.
The effects of exercise on diabetic animals seem to be related primarily to effects on body weight and composition. Exercise training of obese, glucose-intolerant rodents leads to substantial reduction in plasma insulin (Walberg et al., 1982) and glucose. Exercise of sufficient intensity and endurance to affect muscle mass nevertheless affects glucose disposal rates and improves overall glucose tolerance (Ivy and Holloszy, 1981; Wardzala et al., 1982). Thus, it appears that increased energy expenditure may lessen the effects of incipient diabetes, but the data regarding prevention of initiation are inconclusive.
Cancers
Epidemiologic Studies
There are no reports of epidemiologic studies based on precise measurements of energy expenditure to determine if there is an association with cancer risk. In studies of college alumni, Frisch et al. (1985) found that formerly athletic women had a lower risk of cancers of the breast and reproductive organs than did nonathletes, and Paffenbarger et al. (1987) reported that people most active in college were subsequently at lower risk of rectal cancer but at a somewhat higher risk of prostate cancer. After college, leisure-time activity level was slightly positively associated in a dose-dependent manner with risk of colon and rectal cancers.
Paffenbarger et al. (1987) in their study of longshoremen found no association between estimated levels of energy expenditure and cancer risk. In a second group of dock workers, however, they found somewhat higher risks for colorectal cancer
Page 153
and lower risks for lung and prostate cancer in men engaged in heavy work as compared to men engaged in lighter work.
In two case-control studies of large bowel cancer (Garabrant et al., 1984; Vena et al., 1985), people with sedentary jobs or light workloads had a higher risk of colon cancer but not of rectal cancer compared to people who held more active jobs. A similar inverse relationship with job activity was found by Gerhardsson et al. (1986) in a retrospective cohort analysis.
The findings of these studies are inconsistent and permit no clear conclusion about the association of physical activity and cancer risk. Future studies based on better estimates of energy output are needed to clarify the relationship of energy expenditure to the risks of different types of cancer.
Animal Studies
A relationship between caloric restriction and reduced tumorigenesis has been observed in some rodent strains with specific types of tumors, as mentioned earlier. Reductions in tumor growth or burden have not been associated only with caloric restriction, however. They have also been noted under various conditions of energy store depletion, e.g., during physical activity. Only a few experiments directly examined the effects of energy expenditure on carcinogenesis, and these have provided limited data. For example, rats injected with dimethylbenzanthracene were subjected to food restriction or exercise or were pair-gained (i.e., weight gain of one animal was limited to the same level as that of the second animal in a pair) to the exercise group; however, all groups of rats lost weight and did not have the expected tumor growth (Moore and Tittle, 1973). Consequently, it was not possible to attribute the probable effect to either physical activity or caloric restriction per se.
In an experiment by Hoffman et al. (1962), Walker 256 tumors were transplanted by injection into Wistar rats who were either strenuously exercised or confined to small cages. The exercised group had smaller tumors than controls, but no pair-fed or pair-gained controls were included, which makes it difficult to attribute the decreased tumor load directly to exercise. In several more recent but preliminary reports (e.g., Bennink et al., 1986; Cohen, 1987; Thompson et al., 1988), comparisons of exercise and food restriction suggest that exercise interacts with either total caloric intake or dietary fats to determine the outcome (Yedinak et al., 1987). Cohen et al. (1988) recently reported that voluntary exercise inhibits mammary carcinogenesis in rats. They have also reviewed earlier studies on forced exercise, but found the results to be inconsistent. The authors concluded that the promoting effects of high-fat diets fed ad libitum and the antipromoting effects of energy restriction and exercise are not due to alterations in energy homeostasis per se, but may be mediated indirectly, and perhaps independently, by endocrine, paracrine, or neurohormonal pathways. To date, the nature of this association or interaction remains to be elucidated. Therefore, the role of energy expenditure in the prevention or promotion of tumorigenesis remain unclear.
Summary
Clinical and animal data indicate that increased energy intake leads to an increase in body energy stores and that reduced caloric intake lowers energy stores. In cross-sectional population studies, however, total caloric intake has no direct correlation with body weight and generally shows an inverse correlation with body weight. Thus, body fat acquired during adult life cannot be solely attributed to increased caloric intake, but probably also results from reduced energy expenditure. The association between total energy intake and energy expenditure and the risk of other chronic diseases, including diabetes and cancers, is unclear. For example, the results of epidemiologic studies regarding measurements of energy expenditure and risk of cancer are inconsistent. Animal studies have also yielded inconsistent results. No correlation has been reported between glucose tolerance and physical activity in epidemiologic studies; however, results of clinical studies suggest that increasing physical activity can improve glucose tolerance. Animal studies also suggest that increased energy expenditure may lessen the effects of incipient diabetes.
Total energy intake is influenced by age, sex, body weight, and ambient temperature. The highest caloric intake estimated for males and females occurs during the second decade of life and declines thereafter in both sexes. Total energy expenditure is influenced by age, sex, hormonal, and nutritional status and is related directly to body weight. RMR most likely has a familial component. Energy expenditure is directly associated with total energy intake in normal-weight subjects, but not necessarily in obese subjects. Increased energy expenditure resulting from increased physical activity has been associated with an overall decreased risk of CHD.
Page 154
Directions for Research
· The genetic basis for RMR, metabolic efficiency, and other components of energy balance is unknown and deserves further research.
· More information is needed about the relationship of physical activity to food intake. Increased physical activity may increase energy intake in lean subjects but not necessarily in overweight people. There is no current explanation for this phenomenon.
· The increased energy needed for fetal growth and development may not always stimulate a corresponding increase in food intake, suggesting recruitment of mechanisms for enhancing metabolic efficiency. The basis for this efficiency is unknown and should be studied.
· The proportion of fats in the diet may be a factor in the development of positive energy balance, but the validity of this claim and the possible mechanisms of action require elucidation.
· Lean body mass declines with age in most people. The relationship between energy expenditure, RMR, and lean body mass in the elderly warrants further investigation.
· The mechanism of the effects of exercise on carbohydrate tolerance and on HDL cholesterol levels is not adequately understood and requires further study.
References
Arky, R.A. 1976. Environmental factors influencing the development of the diabetic state. Pp. 89-105 in S.S. Fajans, ed. Diabetes Mellitus. DHEW Publ. No. (NIH) 76-854, National Institutes of Health, Public Health Service, U.S. Department of Health, Education, and Welfare, Bethesda, Md.
Ashley, F.W., Jr., and W.B. Kannel. 1974. Relation of weight change to changes in atherogenic traits: the Framingham Study. J. Chronic Dis. 27:103-114.
Baecke, J.A., W.A. van Staveren, and J. Burema. 1983. Food consumption, habitual physical activity, and body fatness in young Dutch adults. Am. J. Clin. Nutr. 37:278-286.
Beaton, G.H., J. Milner, P. Corey, V. McGuire, M. Cousins, E. Stewart, M. de Ramos, D. Hewitt, P.V. Grambsch, N. Kassim, and J.A. Little. 1979. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am. J. Clin. Nutr. 32:2546-2559.
Beaudoin, R., and J. Mayer. 1953. Food intakes of obese and non-obese women. J. Am. Diet. Assoc. 29:29-33.
Bennink, M.R., H.J. Palmer, and M.J. Messina. 1986. Exercise and caloric restriction modify rat mammary carcinogenesis. Fed. Proc. 45:1087.
Björntorp, P., K. de Jounge, M. Krotkiewski, L Sullivan, L. Sjöström, and J. Stenberg. 1973. Physical training in human obesity. 3. Effects of long-term physical training on body composition. Metabolism 22:1467-1475.
Bogardus, C., S. Lillioja, E. Ravussin, W. Abbott, J.K. Zawadzk, A. Young, W.C. Knowler, R. Jacobowitz, and P.P. Moll. 1986. Familial dependence of the resting metabolic rate. N. Engl. J. Med. 315:96-100.
Boissonneault, G.A., C.E. Elson, and M.W. Pariza. 1986. Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J. Natl. Cancer Inst. 76:335-338.
Bouchard, C., R. Lesage, G. Lorrie, J.A. Simoneau, P. Hamel, M.R. Boulay, L. Perusse, G. Theriault, and C. Leblanc. 1986. Aerobic performance in brothers, dizygotic and monozygotic twins. Med. Sci. Sports Exercise 18:639-646.
Boutwell, R.K., M.K. Brush, and H.P. Rusch. 1948. Some physiological effects associated with chronic caloric restriction. Am. J. Physiol. 154:517-524.
Boyle, P.C., L.H. Storlein, and R.E. Keesey. 1978. Increased efficiency of food utilization following weight loss. Physiol. Behav. 21:261-264.
Braitman, L.E., E.V. Adlin, and J.L. Stanton, Jr. 1985. Obesity and caloric intake: the National Health and Nutrition Examination Survey of 1971-1975 (NHANES I). J. Chronic Dis. 38:727-732.
Bray, G.A. 1976. The obese patient. Pp. 1-450 in Major Problems in Internal Medicine, Vol. 9. W.B. Saunders, Philadelphia.
Bray, G.A. 1983. The energetics of obesity. Med. Sci. Sports Exercise 15:32-40.
Bray, G.A., and D.S. Gray. 1988. Obesity. Part I—Pathogenesis. West. J. Med. 149:429-441.
Bray, G.A., F.L. Greenway, M. Molitch, W.T. Dahms, R.L. Atkinson, and K. Hamilton. 1978. Use of anthropometric measures to assess weight loss. Am. J. Clin. Nutr. 31:769773.
Brooks, G.A., and T.P. White. 1978. Determination of metabolic and heart rate responses of rats to treadmill exercises. J. Appl. Physiol. 45:1009-1015.
Brownell, K.D., A.J. Stunkard, and J.M. Albaum. 1980. Evaluation and modification of exercise patterns in the natural environment. Am. J. Psychiatry 137:1540-1545.
Brownell, K.D., P.S. Bachorik, and R.S. Ayerle. 1982. Changes in plasma lipid and lipoprotein levels in men and women after a program of moderate exercise. Circulation 65: 477-484.
Bullen, B.A., R.B. Reed, and J. Mayer. 1964. Physical activity of obese and nonobese adolescent girls appraised by motion picture sampling. Am. J. Clin. Nutr. 14:211-223.
Carroll, K.K. 1975. Experimental evidence of dietary factors and hormone-dependent cancers. Cancer Res. 35:3374-3383.
Carroll, K.K. 1986. Dietary fat and cancer: specific action or caloric effect? J. Nutr. 116:1130-1132.
Carroll, M.D., S. Abraham, and C.M. Dresser. 1983. Dietary Intake Source Data: United States, 1976-1980. Vital and Health Statistics, Series 11, No. 231. DHHS Publ. No. (PHS) 83-1681. National Center for Health Statistics, Public Health Service, U.S. Department of Health and Human Services, Hyattsville, Md. 483 pp.
Cleary, M.P., J.R. Vasselli, and M.R. Greenwood. 1980. Development of obesity in the Zucker obese (fafa) rat in absence of hyperphagia. Am. J. Physiol. 238:E284-E292.
Cohen, LA. 1987. Fat and endocrine-responsive cancer in animals. Prev. Med. 16:468474.
Cohen, LA., K.W. Choi, and C.X. Wang. 1988. Influence of
Page 155
dietary fat, caloric restriction, and voluntary exercise on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Res. 48:4276-4283.
Coleman, D.L. 1979. Obesity genes: beneficial effects in heterozygous mice. Science 203:663-665.
Cox, J.E., and T.L. Powley. 1977. Development of obesity in diabetic mice pair-fed with lean siblings. J. Comp. Physiol. Psychol. 91:347-358.
de Boer, J.O., A.J. van Es, J.M. van Raaij, and J.G. Hautvast. 1987. Energy requirements and energy expenditure of lean and overweight women, measured by indirect calorimetry. Am. J. Clin. Nutr. 46:13-21.
Donato, K., and D.M. Hegsted. 1985. Efficiency of utilization of various sources of energy for growth. Proc. Natl. Acad. Sci. U.S.A. 82:4866-4870.
Drenick, E.J., A.S. Brickman, and E.M. Gold. 1972. Dissociation of the obesity-hyperinsulinism relationship following dietary restriction and hyperalimentation. Am. J. Clin. Nutr. 25:746-755.
Durnin, J.V.G.A. 1986. Energy requirements of pregnancy. An integration of the longitudinal data from the 5-country study. Pp. 147-154 in Nestlé Foundation for the Study of the Problems of Nutrition in the World, Annual Report 1986. Nestlé Foundation, Lausanne, Switzerland.
Edholm, O.G., J.M. Adam, M.J. Healy, H.S. Wolff, R. Goldsmith, and T.W. Best. 1970. Food intake and energy expenditure of army recruits. Br. J. Nutr. 24:1091-1107.
Fontaine, E., R. Savard, A. Tremblay, J.P. Despres, E. Poehlman, and C. Bouchard. 1985. Resting metabolic rate in monozygotic and dizygotic twins. Acta Genet. Med. Gemellol. 34:41-47.
Forbes, G.B. 1987. Lean body mass-body fat interrelationships in humans. Nutr. Rev. 45:225-231.
Frick, M.H., R.O. Elovainio, and T. Somer. 1967. The mechanism of bradycardia evoked by physical training. Cardiologia 51:46-54.
Frisch, R.E., G. Wyshak, N.L. Albright, T.E. Albright, I. Schiff, K.P. Jones, J. Witschi, E. Shiang, E. Koff, and M. Marguglio. 1985. Lower prevalence of breast cancer and cancers of the reproductive system among former college athletes compared to non-athletes. Br. J. Cancer 52:885-891.
Garabrant, D.H., J.M. Peters, T.M. Mack, and L. Bernstein. 1984. Job activity and colon cancer risk. Am. J. Epidemiol. 119:1005-1014.
Garrow, J.S. 1978. Energy Balance and Obesity in Man, 2nd ed. Elsevier/North-Holland, Amsterdam. 243 pp.
Geissler, C.A., D.S. Miller, and M. Shah. 1987. The daily metabolic rate of the post-obese and the lean. Am. J. Clin. Nutr. 45:914-920.
George, V., A. Tremblay, J.P. Després, C. Leblanc, L Pérusse, and C. Bouchard. In press. Evidence for the existence of small eaters and large eaters of similar fat-free mass and activity level. Int. J. Obes.
Gerhardsson, M., S.E. Norell, H. Kiviranta, N.L Pedersen, and A. Ahlbom. 1986. Sedentary jobs and colon cancer. Am. J. Epidemiol. 123:775-780.
Gleeson, T.T., and K.M. Baldwin. 1981. Cardiovascular response to treadmill exercise in untrained rats. J. Appl. Physiol. 50:1206-1211.
Glinsmann, W.H., H. Irausquin, and Y.K. Park. 1986. Evaluation of health aspects of sugars contained in carbohydrate sweeteners: report of Sugars Task Force, 1986. J. Nutr. 116:S1-S216.
Goor, R., J.D. Hosking, B.H. Dennis, K.L. Graves, G.T. Waldman, and S.G. Haynes. 1985. Nutrient intakes among selected North American populations in the Lipid Research Clinics Prevalence Study: composition of fat intake. Am. J. Clin. Nutr. 41:299-311.
Gordon, T., A. Kagan, M. Garcia-Palmieri, W.B. Kannel, W.J. Zukel, J. Tillotson, P. Sorlie, and M. Hjortland. 1981. Diet and its relation to coronary heart disease and death in three populations. Circulation 63:500-515.
Goto, Y., Y. Nakayama, and T. Yagi. 1958. Influence of the World War II food shortage on the incidence of diabetes mellitus in Japan. Diabetes 7:133-135.
Greenwood, M.R.C., M.P. Cleary, L. Steingrimsdottir, and J.R. Vasselli. 1981. Adipose tissue metabolism and genetic obesity: the LPL hypothesis. Recent Adv. Obesity Res. III: 75-79.
Gregor, O., R. Toman, and F. Prusova. 1969. Gastrointestinal cancer and nutrition. Gut 10:1031-1034.
Hammond, H.K., F.C. White, L.L. Brunton, and J.C. Longhurst. 1987. Association of decreased myocardial b-receptors and chronotropic response to isoproterenol and exercise in pigs following chronic dynamic exercise. Circ. Res. 60: 720-726.
Henry, R.R., T.A. Wiest-Kent, L. Scheaffer, O.G. Kolterman, and J.M. Olefsky. 1986. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 35: 155-164.
Himms-Hagen, J. 1985. Brown adipose tissue metabolism and thermogenesis. Annu. Rev. Nutr. 5:69-94.
Himsworth, H.P. 1935. Diet and incidence of diabetes mellitus. Clin. Sci. 2:117-148.
Hoffman, S.A., K.E. Paschkis, D.A. Debias, A. Cantarow, and T.L. Williams. 1962. The influence of exercise on the growth of transplanted rat tumors. Cancer Res. 22:597-599.
Horwitz, B.A., T. Inokuchi, B.J. Moore, and J.S. Stern. 1985. The effect of brown fat removal on the development of obesity in Zucker and Osborne-Mendel rats. Int. J. Obesity 2 suppl. 9:43-48.
Howe, G.R., A.B. Miller, and M. Jain. 1986. Re: ''Total energy intake: implications for epidemiologic analyses." Am. J. Epidemiol. 124:157-159.
Hytten, F.E., and I. Leitch. 1971. The Physiology of Human Pregnancy, 2nd ed. Blackwell, Oxford. 599 pp.
Ikeda, H., A. Shino, T. Matsuo, H. Iwatsuka, and Z. Suzuoki. 1981. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes 30:1045-1050.
Ivy, J.L., and J.O. Holloszy. 1981. Persistent increase in glucose uptake by rat skeletal muscle following exercise. Am. J. Physiol. 241:C200-C203.
Jain, M., G.M. Cook, F.G. Davis, M.G. Grace, G.R. Howe, and A.B. Miller. 1980. A case-control study of diet and colo-rectal cancer. Int. J. Cancer 26:757-768.
James, W.P. 1983. Energy requirements and obesity. Lancet 2: 386-389.
Jarrett, R.J., M.J. Shipley, and R. Hunt. 1986. Physical activity, glucose tolerance, and diabetes mellitus: the Whitehall Study. Diabetic Med. 3:549-551.
Jen, K.L., M.R.C. Greenwood, and S.A. Brasel. 1981. Sex differences in the effects of high-fat feeding on behavior and carcass composition. Physiol. Behav. 27:161-166.
Jequier, E. 1984. Energy expenditure in obesity. Clin. Endocrinol. Metab. 13:563-580.
Kalderon, B., A. Gutman, E. Levy, E. Shafrir, and J.H.
Page 156
Adler. 1986. Characterization of stages in development of obesity—diabetes syndrome in sand rat (Psammomys obesus). Diabetes 35:717-724.
Kava, R.A., D.B. West, V.A. Lukasik, and M.R.C. Greenwood. 1989. Sexual dimorphism of hyperglycemia and glucose tolerance in Wistar fatty rat. Diabetes 38:159-163.
Kawate, R., M. Yamakido, Y. Nishimoto, P.H. Bennett, R.F. Hamman, and W.C. Knowler. 1979. Diabetes mellitus and its vascular complications in Japanese migrants on the island of Hawaii. Diabetes Care 2:161-170.
Keen, H., B.J. Thomas, R.J. Jarrett, and J.H. Fuller. 1979. Nutrient intake, adiposity, and diabetes. Br. Med. J. 1:655-658.
Keesey, R.E. 1980. Set point analysis of the regulation of body weight. Pp. 144-165 in A.J. Stunkard, ed. Obesity. W.B. Saunders, Philadelphia.
Keesey, R.E., and T.L. Powley. 1986. The regulation of body weight. Annu. Rev. Psychol. 37:109-133.
Kleiber, M. 1947. Body size and metabolic rate. Physiol. Rev. 15:511-541.
Klurfeld, D.M., M.M. Weber, and D. Kritchevsky. 1987. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res. 47:2759-2762.
Kritchevsky, D. 1986. Fat, calories and fiber. Pp. 495-515 in C. Ip, D.F. Birt, A.E. Roges, and C. Mettlin, eds. Progress in Clinical and Biological Research, Vol. 222. Dietary Fat and Cancer. Alan R. Liss, New York.
Kritchevsky, D., and D.M. Klurfeld. 1987. Caloric effects in experimental mammary tumorigenesis. Am. J. Clin. Nutr. 45:236-242.
Kritchevsky, D., M.M. Weber, and D.M. Klurfeld. 1984. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenzanthracene-induced mammary tumorigenesis in rats. Cancer Res. 44:3174-3177.
Kritchevsky, D., M.M. Weber, C.L. Buck, and D.M. Klurfeld. 1986. Calories, fat and cancer. Lipids 21:272-274.
Kromhout, D. 1983a. Changes in energy and macronutrients in 871 middle-aged men during 10 years of follow-up (the Zutphen Study). Am. J. Clin. Nutr. 37:287-294.
Kromhout, D. 1983b. Energy and macronutrient intake in lean and obese middle-aged men (the Zutphen Study). Am. J. Clin. Nutr. 37:295-299.
Krotkiewski, M., L. Sjöström, P. Björntorp, G. Carlgren, G. Garellick, and U. Smith. 1977. Adipose tissue cellularity in relation to prognosis for weight reduction. Int. J. Obes. 1: 395-416.
Lapidus, L., H. Andersson, C. Bengtsson, and I. Bosaeus. 1986. Dietary habits in relation to incidence of cardiovascular disease and death in women: a 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Am. J. Clin. Nutr. 44:444-448.
Leibel, R.L., and J. Hirsch. 1984. Diminished energy requirements in reduced-obese patients. Metabolism 33:164-170.
Lew, E.A., and L. Garfinkel. 1979. Variations in mortality by weight among 750,000 men and women. J. Chronic Dis. 32: 563-576.
Lifson, N. 1966. Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 12:46-74.
Lincoln, J.E. 1972. Calorie intake, obesity, and physical activity. Am. J. Clin. Nutr. 25:390-394.
LRC (Lipid Research Clinic). 1980. Lipid Research Clinics Population Studies Data Book, Vol. I: The Prevalence Study. NIH Publ. No. 80-1527. National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Bethesda, Md. 136 pp.
Mazzeo, R.S., G.A. Brooks, and S.M. Horvath. 1984. Effects of age on metabolic responses to endurance training in rats. J. Appl. Physiol. 57:1369-1374.
McGandy, R.B., C.H. Barrows, Jr., A. Spanias, A. Meredith, J.L. Stove, and A.H. Norris. 1966. Nutrient intakes and energy expenditure in men of different ages. J. Gerontol. 21: 581-587.
McLean J.A., and G. Tobin. 1988. Animal and Human Calorimetry. Cambridge University Press, New York. 352 pp.
Medalie, J.H., C.M. Papier, U. Goldbourt, and J.D. Herman. 1975. Major factors in the development of diabetes mellitus in 10,000 men. Arch. Intern. Med. 135:811-817.
Miller, W.H., Jr., and I.M. Faust. 1982. Alterations in rat adipose tissue morphology induced by a low-temperature environment. Am. J. Physiol. 242:E93-E96.
Montoye, H.J., W.D. Block, H. Metzner, and J.B. Keller. 1977. Habitual physical activity and glucose tolerance. Males age 16-64 in a total community. Diabetes 26:172-176.
Moore, C., and P.W. Tittle. 1973. Muscle activity, body fat, and induced rat mammary tumors. Surgery 73:329-332.
Morris, J.N., M.G. Everitt, R. Pollard, S.P. Chave, and A.M. Semmence. 1980. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 2:1207-1210.
Mulinos, M.G., and L. Pomerantz. 1940. Pseudo-hypophysectomy; condition resembling hypophysectomy produced by malnutrition. J. Nutr. 19:493-504.
Noppa, H. 1980. Body weight change in relation to incidence of ischemic heart disease and change in risk factors for ischemic heart disease. Am. J. Epidemiol. 111:693-704.
NRC (National Research Council). 1980. Recommended Dietary Allowances, 9th ed. Report of the Food and Nutrition Board, Assembly of Life Sciences. National Academy Press, Washington, D.C. 185 pp.
NRC (National Research Council). 1982. Diet, Nutrition, and Cancer. Report of the Committee on Diet, Nutrition, and Cancer, Assembly of Life Sciences. National Academy Press, Washington, D.C. 478 pp.
Obell, A.E. 1974. Recent advances in mechanisms of causation of diabetes mellitus in man and Acomy cahirinus. East Afr. Med. J. 51:425-428.
Ordway, G.A., J.B. Charles, D.C. Randall, G.E. Billman, and D.R. Wekstein. 1982. Heart rate adaptation to exercise training in cardiac denervated dogs. J. Appl. Physiol. 52: 1586-1590.
Owen, O.E., E. Kavle, R.S. Owen, M. Polansky, S. Caprio, M.A. Mozzoli, ZV. Kendrick, M.C. Bushman, and G. Boden. 1986. A reappraisal of caloric requirements in healthy women. Am. J. Clin. Nutr. 44:1-19.
Owen, O.E., J.L Holup, D.A. D'Alessio, E.S. Craig, M. Polansky, K.J. Smalley, E.C. Kavle, M.C. Bushman, LR.
Owen, M.A. Mozzoli, Z.V. Kendrick, and G.H. Boden. 1987. A reappraisal of the caloric requirements of men. Am. J. Clin. Nutr. 46:875-885.
Paffenbarger, R.S., and W.E. Hale. 1975. Work activity and coronary heart mortality. N. Engl. J. Med. 292:545-550.
Paffenbarger, R.S., Jr., R.T. Hyde, A.L. Wing, and C.C. Hsieh. 1986. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 314-605-613.
Paffenbarger, R.S., Jr., R.T. Hyde, and A.L. Wing. 1987.
Page 157
Physical activity and incidence of cancer in diverse populations: a preliminary report. Am. J. Clin. Nutr. 45:312-317.
Pariza, M.W. 1987. Fat, calories, and mammary carcinogenesis: net energy effects. Am. J. Clin. Nutr. 45:261-263.
Phillips, R.W., N. Westmoreland, L. Panepinto, and G.L. Case. 1982. Dietary effects on metabolism of Yucatan miniature swine selected for low and high glucose utilization. J. Nutr. 112:104-111.
Potter, J.D., and A.J. McMichael. 1986. Diet and cancer of the colon and rectum: a case-control study. J. Natl. Cancer Inst. 76:557-569.
Ravussin, E., S. Lillioja, W.C. Knowler, L. Christin, D. Freymond, W.G. Abbott, V. Boyce, B.V. Howard, and C. Bogardus. 1988. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 318:467-472.
Reddy, B.S. 1986. Dietary fat and cancer: specific action or caloric effect. J. Nutr. 116:1132-1135.
Rice, M.G., and R.P. Robertson. 1980. Reevaluation of the sand rat as a model for diabetes mellitus. Am. J. Physiol. 239:340E-345E.
Roberts, S.B., J. Savage, W.A. Coward, B. Chew, and A. Lucas. 1988. Energy expenditure and intake in infants born to lean and overweight mothers. N. Engl. J. Med. 318:461-466.
Romieu, I., W.C. Willett, M.J. Stampfer, G.A. Colditz, L. Sampson, B. Rosner, C.H. Hennekens, and F.E. Speizer. 1988. Energy intake and other determinants of relative weight. Am. J. Clin. Nutr. 47:406-412.
Salans, L., and B. Graham, eds. 1982. Proceedings of a task force on animals appropriate for studying diabetes mellitus and its complications. Diabetes 31 suppl. 1:1-102.
Schoeller D.A., E. Ravussin, Y. Schutz, K.J. Acheson, P. Baertschi, and E. Jequier. 1986. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am. J. Physiol. 250:R823-R830.
Segal, K.R., M. Van Loan, P.I. Fitzgerald, J.A. Hodgdon, and T.B. Van Itallie. 1988. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am. J. Clin. Nutr. 47:7-14.
Sims, E.A., E. Danforth, Jr., E.S. Horton, G.A. Bray, J.A. Glennon, and L.B. Salans. 1973. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 29:457-496.
Siscovick, D.S., N.S. Weiss, R.H. Fletcher, V.J. Schoenbach, and E.H. Wagner. 1984. Habitual vigorous exercise and primary cardiac arrest: effect of other risk factors on the relationship. J. Chronic Dis. 37:625-631.
Sjöström, L, H. Kvist, Å. Cederblad, and U. Tylén. 1986. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am. J. Physiol. 250:E736-E745.
Sobolski, J., M. Kornitzer, G. De Backer, M. Dramaix, M. Abramowicz, S. Degre, and H. Denolin. 1987. Protection against ischemic heart disease in the Belgian Physical Fitness Study: physical fitness rather than physical activity? Am. J. Epidemiol. 125:601-610.
Starnes, J.W., R.E. Beyer, and D.W. Edington. 1983. Myocardial adaptations to endurance exercise in aged rats. Am. J. Physiol. 245:H560-H566.
Stefanik, P.A., F.P. Heald, Jr., and J. Mayer. 1959. Caloric intake in relation to energy output of obese and non-obese adolescent boys. Am. J. Clin. Nutr. 7:55-62.
Stern, J.S., P.R. Johnson, B.R. Batchelor, L.M. Zucker, and J. Hirsch. 1975. Pancreatic insulin release and peripheral tissue resistance in Zucker obese rats fed high- and low-carbohydrate diets. Am. J. Physiol. 228:543-548.
Stock, M.J., and N.J. Rothwell. 1981. Sympathetic control of brown adipose tissue in the regulation of body weight. Biochem. Soc. Trans. 9:525-527.
Susini, C., and M. Lavau. 1978. In-vitro and in-vivo responsiveness of muscle and adipose tissue to insulin in rats rendered obese by a high-fat diet. Diabetes 27:114-120.
Tannenbaum, A. 1947. The role of nutrition in the origin and growth of tumors. Pp. 96-127 in F.R. Moulton, ed. Approaches to Tumor Chemotherapy. American Association for the Advancement of Science, Washington, D.C.
Tannenbaum, A. 1959. Nutrition and cancer. Pp. 517-562 in F.F. Hornburger, ed. The Physiopathology of Cancer, 2nd ed. Hoeber-Harper, New York.
Thompson, H.J., L.D. Meeker, A.R. Tagliaferro, and J.S. Roberts. 1985. Effect of energy intake on the promotion of mammary carcinogenesis by dietary fat. Nutr. Cancer 7:37-41.
Thompson, H.J., A.M. Ronan, K.A. Ritacco, A.R. Tagliaferro, and L.D. Meeker. 1988. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res. 48: 2720-2723.
Tipton, C.M. 1965. Training and bradycardia in rats. Am. J. Physiol. 209:1089-1094.
Tryon, W.W. 1987. Activity as a function of body weight. Am. J. Clin. Nutr. 46:451-455.
Tuyns, A.J., M. Haelterman, and R. Kaaks. 1987. Colorectal cancer and the intake of nutrients: oligosaccharides are a risk factor, fats are not. A case control study in Belgium. Nutr. Cancer 10:181-196.
USDA (U.S. Department of Agriculture). 1980. Nationwide Food Consumption Survey 1977-78. Food and Nutrient Intakes of Individuals in 1 Day in the United States, Spring 1977. Preliminary Report No. 2. Consumer Nutrition Center, Human Nutrition, Science and Education Administration. Hyattsville, Md. 121 pp.
USDA (U.S. Department of Agriculture). 1984. Nationwide Food Consumption Survey. Nutrient Intakes: Individuals in 48 States, Year 1977-78. Report No. 1-2. Consumer Nutrition Division, Human Nutrition Information Service, Hyattsville, Md. 439 pp.
USDA (U.S. Department of Agriculture). 1987. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes of Individuals. Women 19-50 Years and Their Children 1-5 Years, 1 Day, 1986. Report No. 86-1. Nutrition Monitoring Division, Human Nutrition Information Service, Hyattsville, Md. 98 pp.
Vena, J.E, S. Graham, M. Zielezny, M.K. Swanson, R.E Barnes, and J. Nolan. 1985. Lifetime occupational exercise and colon cancer. Am. J. Epidemiol. 122:357-365.
Walberg, J.L., P.A. Mole, and J.S. Stem. 1982. Effect of swim training on development of obesity in the genetically obese rat. Am. J. Physiol. 242:R204-R211.
Wardzala, L.J., M. Crettaz, E.D. Horton, B. Jeanrenaud, and E.S. Horton. 1982. Physical training of lean and genetically obese Zucker rats: effect on fat cell metabolism. Am. J. Physiol. 243:E418-E426.
Waxman, M., and A.J. Stunkard. 1980. Caloric intake and expenditure of obese boys. J. Pediatr. 96:187-193.
West, K.M., and J.M. Kalbfleisch. 1971. Influence of nutritional factors on prevalence of diabetes. Diabetes 20:99-108.
Wilkinson, P.W., J.M. Parkin, G. Pearlson, M. Strong, and
Page 158
P. Sykes. 1977. Energy intake and physical activity in obese children. Br. Med. J. 1:756.
Willett, W., and M.J. Stampfer. 1986. Total energy intake: implications for epidemiologic analyses. Am. J. Epidemiol. 124:17-27.
Wilmore, J.H. 1983. Body composition in sport and exercise: directions for future research. Med. Sci. Sports Exercise 15: 21-31.
Woo, R., R. Daniels-Kush, and E.S. Horton. 1985. Regulation of energy balance. Annu. Rev. Nutr. 5:411-433.
Yedinak, R.A., D.K. Layman, and J.A. Milner. 1987. Influences of dietary fat and exercise on DMBA-induced mammary tumors. Fed. Proc. 46:436.
Young, J.B., and L. Landsberg. 1977. Stimulation of the sympathetic nervous system during sucrose feeding. Nature 269:615-617.




















